User login
Proximal Periprosthetic Femur Fractures: Strategies for Internal Fixation
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
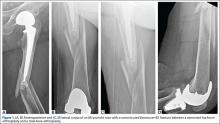
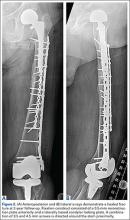
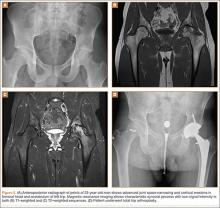
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
Active Robotics for Total Hip Arthroplasty
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.
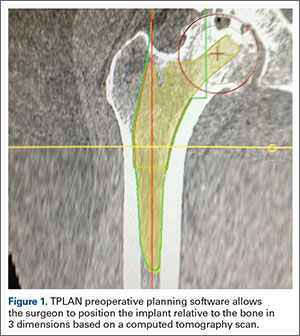
In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
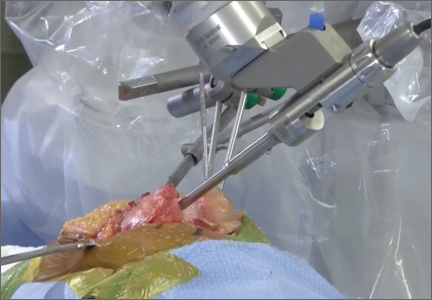
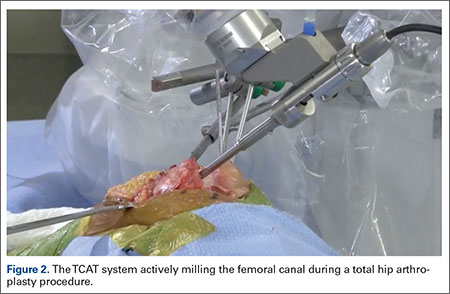
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.
A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).
Outcomes
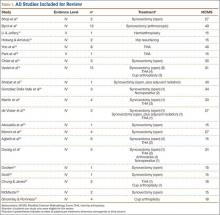
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.
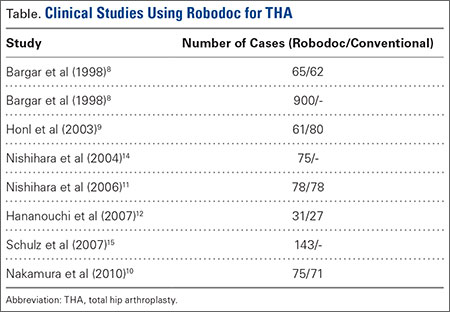
Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.

In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.

A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).

Outcomes
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.

Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.

In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.

A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).

Outcomes
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.

Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
Guideline change advocated on using acetaminophen for OA
AMSTERDAM – Further evidence that acetaminophen has limited benefits in patients with osteoarthritis was presented at the World Congress on Osteoarthritis, with authors of a systematic review calling for reconsideration of guidelines recommending the common analgesic as a first-line option.
“[Acetaminophen] provides minimal short-term benefits for people with hip or knee OA,” said presenting author and rheumatologist Dr. David J. Hunter of the University of Sydney. The treatment effects for both pain relief and for improving physical function were smallest in people with knee OA, he said. “In general, the small effect sizes are unlikely to be clinically relevant,” Dr. Hunter observed.
“These are mean differences across large populations in the clinical trials, and there may be certain individuals with knee or hip osteoarthritis that this may not necessarily apply to,” he conceded during a discussion following his presentation, “but I think from the perspective of the recommendations that come from guidelines, we have got to think about what would be do-able in the general population.”
The findings come shortly after the publication of a large meta-analysis of 74 trials evaluating pain-relieving medications that highlighted the ineffectiveness of acetaminophen for OA pain, particularly when compared against diclofenac and other nonsteroidal anti-inflammatory drugs (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(16)30002-2).
Dr. Hunter and coworkers searched clinical trial and medical databases from inception to September 2015 for records relating to acetaminophen use in patients with hip or knee OA. Only placebo-controlled, randomized trials were included, and nine records were found that reported 10 trials involving 3,541 patients. Part of the analysis was published in the BMJ last year (BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225). The last prior systematic review on the topic was published in 2004 (Ann Rheum Dis. 2004;Aug;63[8]:901–7).
Pain scores were converted to a common 0-100 scale with 0 signifying no pain or disability and 100 the worst possible pain or disability and then expressed as a mean difference between the acetaminophen and placebo groups. Physical function scores were pooled to give a standardized mean difference.
There was high-quality evidence that acetaminophen given at a dose of 3-4 g per day had a significant effect on pain and physical function during a short period of more than 2 weeks to less than 3 months and a more immediate time frame of 2 weeks or less, but it was unlikely to be clinically significant, with a mean difference of just –3.14 for pain and a standardized mean difference of –0.12 to –0.15 for physical function. Differences would need to be at least 9 points for pain and greater than 0.2 for physical function to be clinically significant, Dr. Hunter explained.
Four of the trials considered knee OA only. The mean and standardized mean differences between the acetaminophen and placebo groups in those trials was just –1.09 for pain and –0.06 for physical function.
Similar numbers of patients reported being adherent to their assigned treatment group, with less rescue analgesic use in the acetaminophen-treated patients. Although no differences in adverse events, serious adverse events, or withdrawals because of adverse events were seen, there was a higher risk of liver function test (LFT) abnormalities in the acetaminophen-treated patients. The relative risk for abnormal LFTs was 3.79, but the clinical significance of this is uncertain according to the review’s authors.
“Current guidelines consistently recommend [acetaminophen] as the first line of analgesic medication for this condition,” Dr. Hunter said at the meeting, sponsored by the Osteoarthritis Research Society International. “But these results call for reconsideration of these recommendations.”
The results highlight the importance of using other, nonpharmacologic means to manage pain and physical function, the authors conclude, such as lifestyle changes, weight control, and regular physical exercise.
Dr. Hunter had no disclosures relevant to his comments.
AMSTERDAM – Further evidence that acetaminophen has limited benefits in patients with osteoarthritis was presented at the World Congress on Osteoarthritis, with authors of a systematic review calling for reconsideration of guidelines recommending the common analgesic as a first-line option.
“[Acetaminophen] provides minimal short-term benefits for people with hip or knee OA,” said presenting author and rheumatologist Dr. David J. Hunter of the University of Sydney. The treatment effects for both pain relief and for improving physical function were smallest in people with knee OA, he said. “In general, the small effect sizes are unlikely to be clinically relevant,” Dr. Hunter observed.
“These are mean differences across large populations in the clinical trials, and there may be certain individuals with knee or hip osteoarthritis that this may not necessarily apply to,” he conceded during a discussion following his presentation, “but I think from the perspective of the recommendations that come from guidelines, we have got to think about what would be do-able in the general population.”
The findings come shortly after the publication of a large meta-analysis of 74 trials evaluating pain-relieving medications that highlighted the ineffectiveness of acetaminophen for OA pain, particularly when compared against diclofenac and other nonsteroidal anti-inflammatory drugs (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(16)30002-2).
Dr. Hunter and coworkers searched clinical trial and medical databases from inception to September 2015 for records relating to acetaminophen use in patients with hip or knee OA. Only placebo-controlled, randomized trials were included, and nine records were found that reported 10 trials involving 3,541 patients. Part of the analysis was published in the BMJ last year (BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225). The last prior systematic review on the topic was published in 2004 (Ann Rheum Dis. 2004;Aug;63[8]:901–7).
Pain scores were converted to a common 0-100 scale with 0 signifying no pain or disability and 100 the worst possible pain or disability and then expressed as a mean difference between the acetaminophen and placebo groups. Physical function scores were pooled to give a standardized mean difference.
There was high-quality evidence that acetaminophen given at a dose of 3-4 g per day had a significant effect on pain and physical function during a short period of more than 2 weeks to less than 3 months and a more immediate time frame of 2 weeks or less, but it was unlikely to be clinically significant, with a mean difference of just –3.14 for pain and a standardized mean difference of –0.12 to –0.15 for physical function. Differences would need to be at least 9 points for pain and greater than 0.2 for physical function to be clinically significant, Dr. Hunter explained.
Four of the trials considered knee OA only. The mean and standardized mean differences between the acetaminophen and placebo groups in those trials was just –1.09 for pain and –0.06 for physical function.
Similar numbers of patients reported being adherent to their assigned treatment group, with less rescue analgesic use in the acetaminophen-treated patients. Although no differences in adverse events, serious adverse events, or withdrawals because of adverse events were seen, there was a higher risk of liver function test (LFT) abnormalities in the acetaminophen-treated patients. The relative risk for abnormal LFTs was 3.79, but the clinical significance of this is uncertain according to the review’s authors.
“Current guidelines consistently recommend [acetaminophen] as the first line of analgesic medication for this condition,” Dr. Hunter said at the meeting, sponsored by the Osteoarthritis Research Society International. “But these results call for reconsideration of these recommendations.”
The results highlight the importance of using other, nonpharmacologic means to manage pain and physical function, the authors conclude, such as lifestyle changes, weight control, and regular physical exercise.
Dr. Hunter had no disclosures relevant to his comments.
AMSTERDAM – Further evidence that acetaminophen has limited benefits in patients with osteoarthritis was presented at the World Congress on Osteoarthritis, with authors of a systematic review calling for reconsideration of guidelines recommending the common analgesic as a first-line option.
“[Acetaminophen] provides minimal short-term benefits for people with hip or knee OA,” said presenting author and rheumatologist Dr. David J. Hunter of the University of Sydney. The treatment effects for both pain relief and for improving physical function were smallest in people with knee OA, he said. “In general, the small effect sizes are unlikely to be clinically relevant,” Dr. Hunter observed.
“These are mean differences across large populations in the clinical trials, and there may be certain individuals with knee or hip osteoarthritis that this may not necessarily apply to,” he conceded during a discussion following his presentation, “but I think from the perspective of the recommendations that come from guidelines, we have got to think about what would be do-able in the general population.”
The findings come shortly after the publication of a large meta-analysis of 74 trials evaluating pain-relieving medications that highlighted the ineffectiveness of acetaminophen for OA pain, particularly when compared against diclofenac and other nonsteroidal anti-inflammatory drugs (Lancet. 2016 Mar 17. doi: 10.1016/S0140-6736(16)30002-2).
Dr. Hunter and coworkers searched clinical trial and medical databases from inception to September 2015 for records relating to acetaminophen use in patients with hip or knee OA. Only placebo-controlled, randomized trials were included, and nine records were found that reported 10 trials involving 3,541 patients. Part of the analysis was published in the BMJ last year (BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225). The last prior systematic review on the topic was published in 2004 (Ann Rheum Dis. 2004;Aug;63[8]:901–7).
Pain scores were converted to a common 0-100 scale with 0 signifying no pain or disability and 100 the worst possible pain or disability and then expressed as a mean difference between the acetaminophen and placebo groups. Physical function scores were pooled to give a standardized mean difference.
There was high-quality evidence that acetaminophen given at a dose of 3-4 g per day had a significant effect on pain and physical function during a short period of more than 2 weeks to less than 3 months and a more immediate time frame of 2 weeks or less, but it was unlikely to be clinically significant, with a mean difference of just –3.14 for pain and a standardized mean difference of –0.12 to –0.15 for physical function. Differences would need to be at least 9 points for pain and greater than 0.2 for physical function to be clinically significant, Dr. Hunter explained.
Four of the trials considered knee OA only. The mean and standardized mean differences between the acetaminophen and placebo groups in those trials was just –1.09 for pain and –0.06 for physical function.
Similar numbers of patients reported being adherent to their assigned treatment group, with less rescue analgesic use in the acetaminophen-treated patients. Although no differences in adverse events, serious adverse events, or withdrawals because of adverse events were seen, there was a higher risk of liver function test (LFT) abnormalities in the acetaminophen-treated patients. The relative risk for abnormal LFTs was 3.79, but the clinical significance of this is uncertain according to the review’s authors.
“Current guidelines consistently recommend [acetaminophen] as the first line of analgesic medication for this condition,” Dr. Hunter said at the meeting, sponsored by the Osteoarthritis Research Society International. “But these results call for reconsideration of these recommendations.”
The results highlight the importance of using other, nonpharmacologic means to manage pain and physical function, the authors conclude, such as lifestyle changes, weight control, and regular physical exercise.
Dr. Hunter had no disclosures relevant to his comments.
AT OARSI 2016
Key clinical point: Acetaminophen has minimal effects on pain and physical function in patients with hip and knee osteoarthritis.
Major finding: Doses of 3-4 g of acetaminophen resulted in a mean difference of just –3.14 for pain and a standardized mean difference of –0.12 to –0.15 for physical function versus placebo.
Data source: Cochrane systematic review of 10 trials involving 3,541 patients with hip or knee OA.
Disclosures: Dr. Hunter had no disclosures relevant to his comments.
Can the Mediterranean Diet Reduce the Risk of Hip Fracture?
Eating a Mediterranean diet full of fruits, vegetables, fish, nuts, legumes, and whole grains is associated with a slightly lower risk of hip fracture in women, according to a study published online ahead of print in JAMA Internal Medicine.
Researchers analyzed data on diet and fracture risk in more than 90,000 postmenopausal women (average age, 63.6 years) who were followed for an average of almost 16 years. Diet quality and adherence were assessed by scores on 4 scales: the alternate Mediterranean Diet (aMED); the Healthy Eating Index 2010 (HEI-2010); the Alternate Healthy Eating Index 2010 (AHEI-2010); and the Dietary Approaches to Stop Hypertension (DASH) diet.
Women who scored the highest for adherence to a Mediterranean diet were at lower risk for hip fractures, although the absolute risk reduction was 0.29%. There was no association between a Mediterranean diet and total fracture risk.
A higher HEI-2010 or DASH score was inversely related to the risk of hip fracture, but the finding was not statistically significant. There was no association between HEI-2010, DASH and total fracture risk. The highest scores for AHEI-2010 were not significantly associated with hip or total fracture risk.
Suggested Reading
Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the women's health initiative. JAMA Intern Med. 2016 Mar 28. [Epub ahead of print]
Eating a Mediterranean diet full of fruits, vegetables, fish, nuts, legumes, and whole grains is associated with a slightly lower risk of hip fracture in women, according to a study published online ahead of print in JAMA Internal Medicine.
Researchers analyzed data on diet and fracture risk in more than 90,000 postmenopausal women (average age, 63.6 years) who were followed for an average of almost 16 years. Diet quality and adherence were assessed by scores on 4 scales: the alternate Mediterranean Diet (aMED); the Healthy Eating Index 2010 (HEI-2010); the Alternate Healthy Eating Index 2010 (AHEI-2010); and the Dietary Approaches to Stop Hypertension (DASH) diet.
Women who scored the highest for adherence to a Mediterranean diet were at lower risk for hip fractures, although the absolute risk reduction was 0.29%. There was no association between a Mediterranean diet and total fracture risk.
A higher HEI-2010 or DASH score was inversely related to the risk of hip fracture, but the finding was not statistically significant. There was no association between HEI-2010, DASH and total fracture risk. The highest scores for AHEI-2010 were not significantly associated with hip or total fracture risk.
Eating a Mediterranean diet full of fruits, vegetables, fish, nuts, legumes, and whole grains is associated with a slightly lower risk of hip fracture in women, according to a study published online ahead of print in JAMA Internal Medicine.
Researchers analyzed data on diet and fracture risk in more than 90,000 postmenopausal women (average age, 63.6 years) who were followed for an average of almost 16 years. Diet quality and adherence were assessed by scores on 4 scales: the alternate Mediterranean Diet (aMED); the Healthy Eating Index 2010 (HEI-2010); the Alternate Healthy Eating Index 2010 (AHEI-2010); and the Dietary Approaches to Stop Hypertension (DASH) diet.
Women who scored the highest for adherence to a Mediterranean diet were at lower risk for hip fractures, although the absolute risk reduction was 0.29%. There was no association between a Mediterranean diet and total fracture risk.
A higher HEI-2010 or DASH score was inversely related to the risk of hip fracture, but the finding was not statistically significant. There was no association between HEI-2010, DASH and total fracture risk. The highest scores for AHEI-2010 were not significantly associated with hip or total fracture risk.
Suggested Reading
Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the women's health initiative. JAMA Intern Med. 2016 Mar 28. [Epub ahead of print]
Suggested Reading
Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the women's health initiative. JAMA Intern Med. 2016 Mar 28. [Epub ahead of print]
Progressive Cardiomyopathy in a Patient With Elevated Cobalt Ion Levels and Bilateral Metal-on-Metal Hip Arthroplasties
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
Cryo-Compression Therapy
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
The Epidemiology of Hip and Groin Injuries in Professional Baseball Players
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
Does Medication Use Decrease After Hip-Replacement Surgery?
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].
Incidence, Risk Factors, and Outcome Trends of Acute Kidney Injury in Elective Total Hip and Knee Arthroplasty
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
Pigmented Villonodular Synovitis of the Hip: A Systematic Review
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.