User login
Pain starting in knee later arises in other joints
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:People with frequently painful knees often develop pain in joints outside the knee, and the sites vary from person to person.
Major finding: The odds of patients with new knee pain to later develop joint pain outside the knee were 30% higher than for those without knee pain.
Data source: A study of 693 persons with index visit knee pain and 2,793 without it from two community-based cohorts.
Disclosures: The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
United States an expensive place for knee, hip replacement
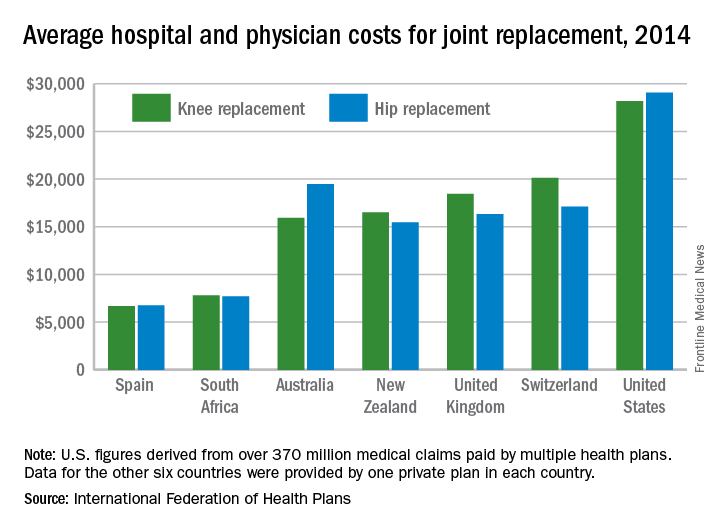
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.
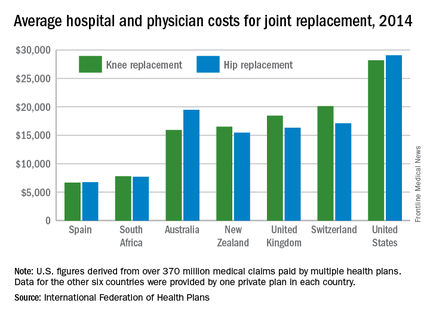
“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.

“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.

“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.
Thigh Injuries in American Football
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
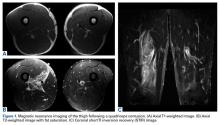
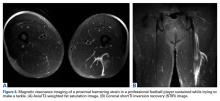
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
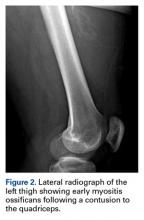
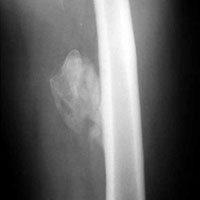
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
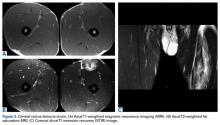
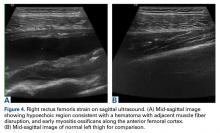
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
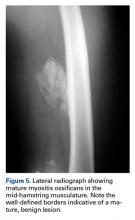
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
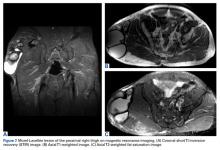
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
No VTE prophylaxis needed after joint surgery in patients with hemophilia
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
AT WFH 2016 WORLD CONGRESS
Key clinical point: Prophylaxis against thromboembolic events after orthopedic surgery in patients with hemophilia may not be necessary.
Major finding: There were no thromboembolic events after joint surgery without anticoagulant prophylaxis in patients with hemophilia A or B.
Data source: Cohort study of 50 patients with hemophilia A or B undergoing major joint replacement surgery.
Disclosures: The study was internally funded. The investigators reported no conflicts of interest.
Postop delirium linked to greater long-term cognitive decline
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
FROM ALZHEIMER’S & DEMENTIA
How Do Age, Sex Affect Outcomes After Arthroscopy for Hip Impingement?
Although both men and women generally do well after having arthroscopic surgery for hip impingement, patients over age 45, particularly women over 45, don’t fare quite as well, according to a study published May 18 in The Journal of Bone and Joint Surgery.
Researchers examined 150 men and women of various ages, who underwent hip arthroscopy to treat femoroacetabular impingement (FAI). Patients were divided into groups based on age and sex. Outcomes were evaluated based on results from several instruments, include the Hip Outcome Score Activities of Daily Living Subscale, Hip Outcome Score Sport-Specific Subscale, and modified Harris hip score, as well as by clinical improvement at follow-up.
Researchers found that while all patients had significant improvements after hip arthroscopy for FAI, patients under age 45 had better overall results and fewer complications compared with people over age 45. Women older than age 45 had lower outcome scores than their male counterparts.
Suggested Reading
Frank MR, Lee S, Bush-Joseph C, et al. Outcomes for hip arthroscopy according to sex and age. J Bone Joint Surg Am. 2016;98(10):797-804.
Although both men and women generally do well after having arthroscopic surgery for hip impingement, patients over age 45, particularly women over 45, don’t fare quite as well, according to a study published May 18 in The Journal of Bone and Joint Surgery.
Researchers examined 150 men and women of various ages, who underwent hip arthroscopy to treat femoroacetabular impingement (FAI). Patients were divided into groups based on age and sex. Outcomes were evaluated based on results from several instruments, include the Hip Outcome Score Activities of Daily Living Subscale, Hip Outcome Score Sport-Specific Subscale, and modified Harris hip score, as well as by clinical improvement at follow-up.
Researchers found that while all patients had significant improvements after hip arthroscopy for FAI, patients under age 45 had better overall results and fewer complications compared with people over age 45. Women older than age 45 had lower outcome scores than their male counterparts.
Although both men and women generally do well after having arthroscopic surgery for hip impingement, patients over age 45, particularly women over 45, don’t fare quite as well, according to a study published May 18 in The Journal of Bone and Joint Surgery.
Researchers examined 150 men and women of various ages, who underwent hip arthroscopy to treat femoroacetabular impingement (FAI). Patients were divided into groups based on age and sex. Outcomes were evaluated based on results from several instruments, include the Hip Outcome Score Activities of Daily Living Subscale, Hip Outcome Score Sport-Specific Subscale, and modified Harris hip score, as well as by clinical improvement at follow-up.
Researchers found that while all patients had significant improvements after hip arthroscopy for FAI, patients under age 45 had better overall results and fewer complications compared with people over age 45. Women older than age 45 had lower outcome scores than their male counterparts.
Suggested Reading
Frank MR, Lee S, Bush-Joseph C, et al. Outcomes for hip arthroscopy according to sex and age. J Bone Joint Surg Am. 2016;98(10):797-804.
Suggested Reading
Frank MR, Lee S, Bush-Joseph C, et al. Outcomes for hip arthroscopy according to sex and age. J Bone Joint Surg Am. 2016;98(10):797-804.
Prevention of Periprosthetic Joint Infections of the Hip and Knee
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
New fragility fracture recommendations emphasize coordination of care
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
AT THE EULAR 2016 CONGRESS
AAOS Introduces New Apps for Patient Education
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
Total Hip Arthroplasty After Proximal Femoral Osteotomy: A Technique That Can Be Used to Address Presence of a Retained Intracortical Plate
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.