User login
FDA okays prophylactic Pradaxa for VTE in hip replacement
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
Do Heavier Patients Require Fewer Blood Transfusions In Hip, Knee Replacement Surgery?
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
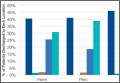
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
Risk Factors for Discharge to Rehabilitation Among Hip Fracture Patients
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
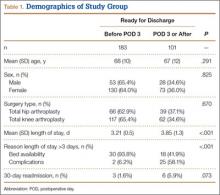
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
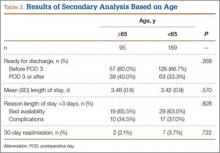
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
Reinforcing a Spica Cast With a Fiberglass Bar
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
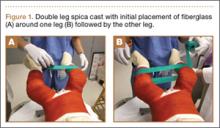
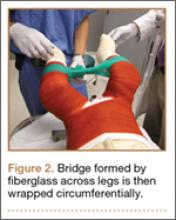
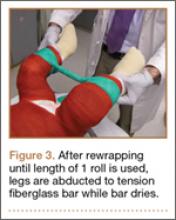
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
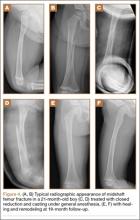
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Is There a Greater Risk of Mortality Following Hip Fracture Surgery Compared With Hip Replacement Surgery?
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.
Current Evidence Does Not Support Medicare’s 3-Day Rule in Primary Total Joint Arthroplasty
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Is There a Link Between Diabetes and Bone Health?
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Are Knee and Hip Replacements Bad For the Heart?
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Hip Fracture and the Weekend Effect: Does Weekend Admission Affect Patient Outcomes?
Weekend admission has been hypothesized to be a risk factor for increased patient mortality and complications during hospital stays—commonly referred to as the weekend effect.1 Reduced hospital staffing on weekends, particularly of senior-level physicians and ancillary nursing services, may affect the quality of diagnosis and management for patients admitted for traumatic and emergent conditions. Investigators have found increased mortality in weekend admissions for stroke,2 subdural hematoma,3 gastrointestinal bleeding,4,5 atrial fibrillation,6 and pulmonary embolism.7 Investigators have not found increased mortality in weekend admissions for hip fracture, though the majority of the data was derived from European patient populations, which may be subject to management and staffing strategies different from those for US patients.8-10 Furthermore, data on this topic in US patients are limited to a multispecialty study of 50 different admission diagnoses, which used 1 year of data from a single US state.1
We conducted a study to comprehensively assess the effect of weekend admission on adverse outcomes during hospital stays. The literature suggests that surgery for hip fracture can be delayed up to 48 hours without significant additional risk of death,11-13 allowing orthopedic departments to stabilize routine hip fracture admissions on weekends and operate whenever limited surgical teams become available. Surgical delay has not been thoroughly analyzed by day of admission among US patients,14 but the combined potential of more conservative preoperative management and the availability of fewer senior physicians and ancillary providers may result in worse outcomes for weekend versus weekday admissions.
Materials and Methods
Study Population
Part of the Healthcare Cost and Utilization Project, the Nationwide Inpatient Sample (NIS) provides a 20% representative sample of annual US hospital admissions.15 For these admissions, the NIS includes data related to demographic and clinical variables, such as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, as well as descriptive variables for the hospitals where the patients were admitted. The NIS is publicly available to researchers. As its health information is deidentified, we did not have to obtain institutional review board approval for this study.
Ascertainment of Cases
Our initial study population, drawn from the period 1998–2010, consisted of 821,531 patients with a principal ICD-9-CM diagnosis of femoral neck fracture (820.0-820.9). To best capture the typical presentation of hip fracture, we excluded:
- Patients with open femoral neck fractures (820.1, 820.3, 820.9).
- Patients who did not have open reduction and internal fixation (ORIF) (79.35), hemiarthroplasty (81.52), closed reduction and internal fixation (CRIF) (79.15), internal fixation (78.55), or total hip arthroplasty (THA) (81.51) as their primary surgical procedure.
- Patients admitted from sources other than the emergency department.
- Patients who underwent surgery before admission.
- Patients whose admission type was not classified as emergency or urgent.
Ascertainment of Covariates
For all patients, we extracted data on exposure of interest, day of admission (weekend or weekday), and demographic variables including age, sex, race (white, black, Hispanic, other, missing), and insurance (Medicare, Medicaid, private, other). We used the Elixhauser method to determine 30 different comorbidities from ICD-9-CM diagnosis coding16 and sorted patients by total number of comorbidities (0, 1, 2, 3 or 4, ≥5). As has been done before,17 we excluded blood loss anemia, coagulopathy, and fluid and electrolyte disorders from this comorbidity calculation, as these conditions can be secondary to trauma. We also extracted data on the admission itself, including hospital region (Northeast, Midwest, South, West), hospital bed size (small, medium, large), hospital teaching status (nonteaching, teaching), and hospital location (rural, urban). We used diagnosis codes to categorize fracture location as “not otherwise specified” (820.8), intracapsular (820.0), or extracapsular (820.2).
Because of low frequencies, we collapsed 2 race designations (Native American, Asian or Pacific Islander) into the “other race” category and 2 insurance designations (self-pay, no charge) into the “other insurance” category. For a substantial number of patients, race information was missing, so we included “missing” as its own category in analyses. Patients who were missing data on day of admission, age, sex, insurance, or hospital characteristics were excluded from our final cohort, as missing frequencies for each variable were small.
Ascertainment of Outcomes
For all patients, we extracted data on death status at discharge and length of hospital stay. We log-transformed length of stay because of its right skew, assigning the value of 12 hours to patients admitted and discharged the same day. Perioperative complications were calculated using ICD-9-CM codes as defined by a recent study of orthopedics-related complications by Lin and colleagues.18 There were 14 possible complications, including acute renal failure (584.5-9), tachycardia (427), wound hemorrhage (719.15, 998.31-2), wound disruption (998.3, 998.31-2), wound infection (682.6, 686.9, 891, 891.1-2, 894, 894.1-2, 998.5, 998.51, 998.6, 998.83, 998.59), deep vein thrombosis (453.4, 453.41-2, 453.9), acute myocardial infarction (410, 410.01, 410.11, 410.2, 410.21, 410.3, 410.31, 410.4, 410.41, 410.5, 410.51, 410.6, 410.9, 410.91, 997.1), pneumonia (480-480.9, 481, 482-482.9, 483, 483.1, 483.8, 484, 484.1, 484.3, 484.5-8, 485, 486, 487, 507), pulmonary embolism (415.11, 415.19), sepsis (995.91-2), stroke (997.02), urinary tract infection (599, 997.5), implant infection (996.66-7, 996.69), and incision and débridement (86.04, 86.09, 86.22, 86.28, 86.3). In our statistical analyses, we examined both the risk of having a complicated admission (≥1 perioperative complication) and the risk of having each specific complication.
Statistical Analysis
To assess similarity between weekend and weekday admissions, we used the Fisher exact test and χ2P values. Logistic regression was used to calculate the odds ratios (ORs) of mortality and perioperative complications for weekend versus weekday admissions. Linear regression was used to calculate parameter estimates for length of hospital stay for weekend versus weekday admissions. We interpreted parameter estimates as percentage differences using the formula 100(eb–1), where b is the estimated standardized regression coefficient of a log-transformed outcome variable.19 All regression models were controlled for age, sex, race, insurance, number of comorbidities, fracture location, hospital region, hospital bed size, hospital teaching status, and hospital location. We also stratified our study population by surgical delay in hours (<24, 24-48, 49-72, 73-120, ≥121) and by surgery performed (ORIF, hemiarthroplasty, CRIF, internal fixation only, THA, multiple procedures) to examine the effect of weekend admission on mortality, perioperative complications, and length of stay within each stratum. We did not control for these variables in our regression models because they were potential mediators of mortality, complications, and length of stay. All statistical analyses in this study were performed using SAS Version 9.1 (SAS Institute), and P < .05 was interpreted as statistically significant.
Results
After exclusions, our study population consisted of 96,892 weekend admissions and 248,097 weekday admissions. Among all admissions, mean age was 79.3 years (range, 0-113 years), with patients primarily being female and white, paying with Medicare, and having 1 to 4 comorbidities. Admissions were primarily for extracapsular femoral neck fractures and occurred most often in the South region, in hospitals with large beds, in nonteaching hospitals, and in urban locations. Table 1 lists details of baseline characteristics for weekend and weekday admissions.
Hospital stay details, including surgical delay and procedure performed, were examined for weekend and weekday admissions. Mean delay to surgery was 31.0 hours for weekend admissions and 30.2 hours for weekday admissions (P < .0001). The difference was driven by a higher proportion of weekend admissions in which surgery was performed 24 to 120 hours after admission. Patients admitted on the weekend also underwent more ORIF procedures and fewer hemiarthroplasties. Table 2 is a full list of hospital stay characteristics.
In regression analyses, weekend OR of mortality was 0.94 (95% CI, 0.89-0.99), weekend OR of having at least 1 complication was 1.00 (95% CI, 0.98-1.02), and weekend mean hospital stay was 3.74% shorter (95% CI, 3.40-4.08) in comparison with weekday figures. Within our models, risk of mortality and complications and mean length of stay increased as the number of patient comorbidities increased. Table 3 lists selected results from our regression models. Comprehensive tables for each outcome’s model are presented in Appendices 1 to 3.
In our analyses of specific complications, there were no significant associations between weekend admissions and risk of acute renal failure, wound hemorrhage, wound disruption, wound infection, deep vein thrombosis, myocardial infarction, pneumonia, pulmonary embolism, sepsis, urinary tract infection, implant infection, or incision and débridement. In addition, we found a lower risk of tachycardia (OR, 0.90; 95% CI, 0.82-1.00) and a higher risk (P < .10) of stroke (OR, 1.16; 95% CI, 0.99-1.35). Table 4 is a full list of the specific complications and their risks for weekend versus weekday admissions.
According to stratified analyses involving surgical delay, weekend admissions in which patients had surgery the same day as admission had decreased risk of mortality (OR, 0.81; 95% CI, 0.72-0.91) and perioperative complications (OR, 0.96; 95% CI, 0.92-0.99). In addition, hospital stay was shorter for weekend admissions with surgical delay of less than 24 hours (4.89% shorter; 95% CI, 4.22-5.55), 24 to 48 hours (5.93% shorter; 95% CI, 5.51-6.35), and 49 to 72 hours (3.50% shorter; 95% CI, 2.80-4.20). When admissions were stratified by procedure performed, patients who were admitted on the weekend and underwent ORIF, hemiarthroplasty, CRIF, internal fixation only, and THA had shorter stays than patients admitted on weekdays. For all surgeries performed, the risk of both mortality and complications did not significantly differ by day of admission. Table 5 lists the comprehensive results of all our stratified analyses.
Discussion
In this large, multiyear analysis of patients admitted for hip fracture in the United States, risk of mortality was slightly lower for weekend versus weekday admissions, hospital stay was significantly shorter, and risk of perioperative complications was not significantly different between admission types. In secondary analyses, shorter hospital stay was limited to patients who were admitted on weekends and underwent surgery within 48 hours. Our results therefore suggest that the weekend effect does not apply to hip fracture patients in the United States.
Our results are largely consistent with the literature on the topic.11-14 An Australian study of 4183 patients with acute hip fracture found no significant difference in 2- or 30-day mortality among weekend and weekday admissions.11 Similarly, 2 Danish studies did not find a difference in hospital-stay or 30-day mortality between weekend and weekday admissions among samples of 600 and 38,020 patients with hip fracture, respectively.12,13 In US patients, a cross-specialty study that included hip fractures did not find a difference in hospital-stay mortality among 22,001 admissions in the state of California in 1998.14 Our analysis significantly extended the findings of these studies by using comprehensive admission data from 46 US states over a 13-year period (1998–2010) and by examining outcomes other than mortality, including perioperative complications and length of hospital stay.
Our study had several limitations. First, the clinical data on fracture diagnoses and surgical procedures were based on ICD-9-CM codes, limiting our ability to account for the full details of fracture severity and subsequent management. Second, our analyses were limited to outcomes during the hospital stay, and we could not examine the effect of weekend admission on readmission and long-term mortality. Third, because of the dichotomization of admission day in the NIS database, we could not selectively examine the effect of Friday, Saturday, or Sunday admission on our outcomes. Fourth, we excluded admissions that were missing demographic and clinical data, potentially creating a complete-case bias. However, these exclusions were needed to accurately capture the common presentation of acute hip fracture, and there is no reason to believe that differences in record coding were nonrandom. Last, our study was observational, and we cannot rule out the effect of residual confounding on our results.
Our results failed to show a weekend effect on mortality, perioperative complications, or length of hospital stay in US patients with hip fracture. The reason for this, as suggested before,12 may be that hip fractures are becoming easier to diagnose. Furthermore, the observation that hospital stay was shorter for weekend admissions suggests that, despite decreased staffing of nursing and rehabilitation services, the lower volume of elective surgeries on weekends may actually increase staff availability to hip fracture patients.
1. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157.
2. Saposnik G, Baibergenova A, Bayer N, Hachinski V. Weekends: a dangerous time for having a stroke? Stroke. 2007;38(4):1211-1215.
3. Busl KM, Prabhakaran S. Predictors of mortality in nontraumatic subdural hematoma. J Neurosurg. 2013;119(5):1296-1301.
4. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296e1-302e1.
5. Shaheen AA, Kaplan GG, Myers RP. Weekend versus weekday admission and mortality from gastrointestinal hemorrhage caused by peptic ulcer disease. Clin Gastroenterol Hepatol. 2009;7(3):303-310.
6. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211.
7. Aujesky D, Jiménez D, Mor MK, Geng M, Fine MJ, Ibrahim SA. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119(7):962-968.
8. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783.
9. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, Van der mark S. Is mortality after hip fracture associated with surgical delay or admission during weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop. 2012;83(6):609-613.
10. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during weekends and holidays. Br J Anaesth. 2006;96(4):450-4514.
11. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis and meta-regression. Can J Anaesth. 2008;55(3):146-154.
12. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
13. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
14. Ho V, Hamilton BH, Roos LL. Multiple approaches to assessing the effects of delays for hip fracture patients in the United States and Canada. Health Serv Res. 2000;34(7):1499-1518.
15. Steiner C, Elixhauser A, Schnaier J. The Healthcare Cost and Utilization Project: an overview. Eff Clin Pract. 2002;5(3):143-151.
16. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27.
17. Brasel KJ, Guse CE, Layde P, Weigelt JA. Rib fractures: relationship with pneumonia and mortality. Crit Care Med. 2006;34(6):1642-1646.
18. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
19. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer-Verlag; 2012. Statistics for Biology and Health.
Weekend admission has been hypothesized to be a risk factor for increased patient mortality and complications during hospital stays—commonly referred to as the weekend effect.1 Reduced hospital staffing on weekends, particularly of senior-level physicians and ancillary nursing services, may affect the quality of diagnosis and management for patients admitted for traumatic and emergent conditions. Investigators have found increased mortality in weekend admissions for stroke,2 subdural hematoma,3 gastrointestinal bleeding,4,5 atrial fibrillation,6 and pulmonary embolism.7 Investigators have not found increased mortality in weekend admissions for hip fracture, though the majority of the data was derived from European patient populations, which may be subject to management and staffing strategies different from those for US patients.8-10 Furthermore, data on this topic in US patients are limited to a multispecialty study of 50 different admission diagnoses, which used 1 year of data from a single US state.1
We conducted a study to comprehensively assess the effect of weekend admission on adverse outcomes during hospital stays. The literature suggests that surgery for hip fracture can be delayed up to 48 hours without significant additional risk of death,11-13 allowing orthopedic departments to stabilize routine hip fracture admissions on weekends and operate whenever limited surgical teams become available. Surgical delay has not been thoroughly analyzed by day of admission among US patients,14 but the combined potential of more conservative preoperative management and the availability of fewer senior physicians and ancillary providers may result in worse outcomes for weekend versus weekday admissions.
Materials and Methods
Study Population
Part of the Healthcare Cost and Utilization Project, the Nationwide Inpatient Sample (NIS) provides a 20% representative sample of annual US hospital admissions.15 For these admissions, the NIS includes data related to demographic and clinical variables, such as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, as well as descriptive variables for the hospitals where the patients were admitted. The NIS is publicly available to researchers. As its health information is deidentified, we did not have to obtain institutional review board approval for this study.
Ascertainment of Cases
Our initial study population, drawn from the period 1998–2010, consisted of 821,531 patients with a principal ICD-9-CM diagnosis of femoral neck fracture (820.0-820.9). To best capture the typical presentation of hip fracture, we excluded:
- Patients with open femoral neck fractures (820.1, 820.3, 820.9).
- Patients who did not have open reduction and internal fixation (ORIF) (79.35), hemiarthroplasty (81.52), closed reduction and internal fixation (CRIF) (79.15), internal fixation (78.55), or total hip arthroplasty (THA) (81.51) as their primary surgical procedure.
- Patients admitted from sources other than the emergency department.
- Patients who underwent surgery before admission.
- Patients whose admission type was not classified as emergency or urgent.
Ascertainment of Covariates
For all patients, we extracted data on exposure of interest, day of admission (weekend or weekday), and demographic variables including age, sex, race (white, black, Hispanic, other, missing), and insurance (Medicare, Medicaid, private, other). We used the Elixhauser method to determine 30 different comorbidities from ICD-9-CM diagnosis coding16 and sorted patients by total number of comorbidities (0, 1, 2, 3 or 4, ≥5). As has been done before,17 we excluded blood loss anemia, coagulopathy, and fluid and electrolyte disorders from this comorbidity calculation, as these conditions can be secondary to trauma. We also extracted data on the admission itself, including hospital region (Northeast, Midwest, South, West), hospital bed size (small, medium, large), hospital teaching status (nonteaching, teaching), and hospital location (rural, urban). We used diagnosis codes to categorize fracture location as “not otherwise specified” (820.8), intracapsular (820.0), or extracapsular (820.2).
Because of low frequencies, we collapsed 2 race designations (Native American, Asian or Pacific Islander) into the “other race” category and 2 insurance designations (self-pay, no charge) into the “other insurance” category. For a substantial number of patients, race information was missing, so we included “missing” as its own category in analyses. Patients who were missing data on day of admission, age, sex, insurance, or hospital characteristics were excluded from our final cohort, as missing frequencies for each variable were small.
Ascertainment of Outcomes
For all patients, we extracted data on death status at discharge and length of hospital stay. We log-transformed length of stay because of its right skew, assigning the value of 12 hours to patients admitted and discharged the same day. Perioperative complications were calculated using ICD-9-CM codes as defined by a recent study of orthopedics-related complications by Lin and colleagues.18 There were 14 possible complications, including acute renal failure (584.5-9), tachycardia (427), wound hemorrhage (719.15, 998.31-2), wound disruption (998.3, 998.31-2), wound infection (682.6, 686.9, 891, 891.1-2, 894, 894.1-2, 998.5, 998.51, 998.6, 998.83, 998.59), deep vein thrombosis (453.4, 453.41-2, 453.9), acute myocardial infarction (410, 410.01, 410.11, 410.2, 410.21, 410.3, 410.31, 410.4, 410.41, 410.5, 410.51, 410.6, 410.9, 410.91, 997.1), pneumonia (480-480.9, 481, 482-482.9, 483, 483.1, 483.8, 484, 484.1, 484.3, 484.5-8, 485, 486, 487, 507), pulmonary embolism (415.11, 415.19), sepsis (995.91-2), stroke (997.02), urinary tract infection (599, 997.5), implant infection (996.66-7, 996.69), and incision and débridement (86.04, 86.09, 86.22, 86.28, 86.3). In our statistical analyses, we examined both the risk of having a complicated admission (≥1 perioperative complication) and the risk of having each specific complication.
Statistical Analysis
To assess similarity between weekend and weekday admissions, we used the Fisher exact test and χ2P values. Logistic regression was used to calculate the odds ratios (ORs) of mortality and perioperative complications for weekend versus weekday admissions. Linear regression was used to calculate parameter estimates for length of hospital stay for weekend versus weekday admissions. We interpreted parameter estimates as percentage differences using the formula 100(eb–1), where b is the estimated standardized regression coefficient of a log-transformed outcome variable.19 All regression models were controlled for age, sex, race, insurance, number of comorbidities, fracture location, hospital region, hospital bed size, hospital teaching status, and hospital location. We also stratified our study population by surgical delay in hours (<24, 24-48, 49-72, 73-120, ≥121) and by surgery performed (ORIF, hemiarthroplasty, CRIF, internal fixation only, THA, multiple procedures) to examine the effect of weekend admission on mortality, perioperative complications, and length of stay within each stratum. We did not control for these variables in our regression models because they were potential mediators of mortality, complications, and length of stay. All statistical analyses in this study were performed using SAS Version 9.1 (SAS Institute), and P < .05 was interpreted as statistically significant.
Results
After exclusions, our study population consisted of 96,892 weekend admissions and 248,097 weekday admissions. Among all admissions, mean age was 79.3 years (range, 0-113 years), with patients primarily being female and white, paying with Medicare, and having 1 to 4 comorbidities. Admissions were primarily for extracapsular femoral neck fractures and occurred most often in the South region, in hospitals with large beds, in nonteaching hospitals, and in urban locations. Table 1 lists details of baseline characteristics for weekend and weekday admissions.
Hospital stay details, including surgical delay and procedure performed, were examined for weekend and weekday admissions. Mean delay to surgery was 31.0 hours for weekend admissions and 30.2 hours for weekday admissions (P < .0001). The difference was driven by a higher proportion of weekend admissions in which surgery was performed 24 to 120 hours after admission. Patients admitted on the weekend also underwent more ORIF procedures and fewer hemiarthroplasties. Table 2 is a full list of hospital stay characteristics.
In regression analyses, weekend OR of mortality was 0.94 (95% CI, 0.89-0.99), weekend OR of having at least 1 complication was 1.00 (95% CI, 0.98-1.02), and weekend mean hospital stay was 3.74% shorter (95% CI, 3.40-4.08) in comparison with weekday figures. Within our models, risk of mortality and complications and mean length of stay increased as the number of patient comorbidities increased. Table 3 lists selected results from our regression models. Comprehensive tables for each outcome’s model are presented in Appendices 1 to 3.
In our analyses of specific complications, there were no significant associations between weekend admissions and risk of acute renal failure, wound hemorrhage, wound disruption, wound infection, deep vein thrombosis, myocardial infarction, pneumonia, pulmonary embolism, sepsis, urinary tract infection, implant infection, or incision and débridement. In addition, we found a lower risk of tachycardia (OR, 0.90; 95% CI, 0.82-1.00) and a higher risk (P < .10) of stroke (OR, 1.16; 95% CI, 0.99-1.35). Table 4 is a full list of the specific complications and their risks for weekend versus weekday admissions.
According to stratified analyses involving surgical delay, weekend admissions in which patients had surgery the same day as admission had decreased risk of mortality (OR, 0.81; 95% CI, 0.72-0.91) and perioperative complications (OR, 0.96; 95% CI, 0.92-0.99). In addition, hospital stay was shorter for weekend admissions with surgical delay of less than 24 hours (4.89% shorter; 95% CI, 4.22-5.55), 24 to 48 hours (5.93% shorter; 95% CI, 5.51-6.35), and 49 to 72 hours (3.50% shorter; 95% CI, 2.80-4.20). When admissions were stratified by procedure performed, patients who were admitted on the weekend and underwent ORIF, hemiarthroplasty, CRIF, internal fixation only, and THA had shorter stays than patients admitted on weekdays. For all surgeries performed, the risk of both mortality and complications did not significantly differ by day of admission. Table 5 lists the comprehensive results of all our stratified analyses.
Discussion
In this large, multiyear analysis of patients admitted for hip fracture in the United States, risk of mortality was slightly lower for weekend versus weekday admissions, hospital stay was significantly shorter, and risk of perioperative complications was not significantly different between admission types. In secondary analyses, shorter hospital stay was limited to patients who were admitted on weekends and underwent surgery within 48 hours. Our results therefore suggest that the weekend effect does not apply to hip fracture patients in the United States.
Our results are largely consistent with the literature on the topic.11-14 An Australian study of 4183 patients with acute hip fracture found no significant difference in 2- or 30-day mortality among weekend and weekday admissions.11 Similarly, 2 Danish studies did not find a difference in hospital-stay or 30-day mortality between weekend and weekday admissions among samples of 600 and 38,020 patients with hip fracture, respectively.12,13 In US patients, a cross-specialty study that included hip fractures did not find a difference in hospital-stay mortality among 22,001 admissions in the state of California in 1998.14 Our analysis significantly extended the findings of these studies by using comprehensive admission data from 46 US states over a 13-year period (1998–2010) and by examining outcomes other than mortality, including perioperative complications and length of hospital stay.
Our study had several limitations. First, the clinical data on fracture diagnoses and surgical procedures were based on ICD-9-CM codes, limiting our ability to account for the full details of fracture severity and subsequent management. Second, our analyses were limited to outcomes during the hospital stay, and we could not examine the effect of weekend admission on readmission and long-term mortality. Third, because of the dichotomization of admission day in the NIS database, we could not selectively examine the effect of Friday, Saturday, or Sunday admission on our outcomes. Fourth, we excluded admissions that were missing demographic and clinical data, potentially creating a complete-case bias. However, these exclusions were needed to accurately capture the common presentation of acute hip fracture, and there is no reason to believe that differences in record coding were nonrandom. Last, our study was observational, and we cannot rule out the effect of residual confounding on our results.
Our results failed to show a weekend effect on mortality, perioperative complications, or length of hospital stay in US patients with hip fracture. The reason for this, as suggested before,12 may be that hip fractures are becoming easier to diagnose. Furthermore, the observation that hospital stay was shorter for weekend admissions suggests that, despite decreased staffing of nursing and rehabilitation services, the lower volume of elective surgeries on weekends may actually increase staff availability to hip fracture patients.
Weekend admission has been hypothesized to be a risk factor for increased patient mortality and complications during hospital stays—commonly referred to as the weekend effect.1 Reduced hospital staffing on weekends, particularly of senior-level physicians and ancillary nursing services, may affect the quality of diagnosis and management for patients admitted for traumatic and emergent conditions. Investigators have found increased mortality in weekend admissions for stroke,2 subdural hematoma,3 gastrointestinal bleeding,4,5 atrial fibrillation,6 and pulmonary embolism.7 Investigators have not found increased mortality in weekend admissions for hip fracture, though the majority of the data was derived from European patient populations, which may be subject to management and staffing strategies different from those for US patients.8-10 Furthermore, data on this topic in US patients are limited to a multispecialty study of 50 different admission diagnoses, which used 1 year of data from a single US state.1
We conducted a study to comprehensively assess the effect of weekend admission on adverse outcomes during hospital stays. The literature suggests that surgery for hip fracture can be delayed up to 48 hours without significant additional risk of death,11-13 allowing orthopedic departments to stabilize routine hip fracture admissions on weekends and operate whenever limited surgical teams become available. Surgical delay has not been thoroughly analyzed by day of admission among US patients,14 but the combined potential of more conservative preoperative management and the availability of fewer senior physicians and ancillary providers may result in worse outcomes for weekend versus weekday admissions.
Materials and Methods
Study Population
Part of the Healthcare Cost and Utilization Project, the Nationwide Inpatient Sample (NIS) provides a 20% representative sample of annual US hospital admissions.15 For these admissions, the NIS includes data related to demographic and clinical variables, such as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, as well as descriptive variables for the hospitals where the patients were admitted. The NIS is publicly available to researchers. As its health information is deidentified, we did not have to obtain institutional review board approval for this study.
Ascertainment of Cases
Our initial study population, drawn from the period 1998–2010, consisted of 821,531 patients with a principal ICD-9-CM diagnosis of femoral neck fracture (820.0-820.9). To best capture the typical presentation of hip fracture, we excluded:
- Patients with open femoral neck fractures (820.1, 820.3, 820.9).
- Patients who did not have open reduction and internal fixation (ORIF) (79.35), hemiarthroplasty (81.52), closed reduction and internal fixation (CRIF) (79.15), internal fixation (78.55), or total hip arthroplasty (THA) (81.51) as their primary surgical procedure.
- Patients admitted from sources other than the emergency department.
- Patients who underwent surgery before admission.
- Patients whose admission type was not classified as emergency or urgent.
Ascertainment of Covariates
For all patients, we extracted data on exposure of interest, day of admission (weekend or weekday), and demographic variables including age, sex, race (white, black, Hispanic, other, missing), and insurance (Medicare, Medicaid, private, other). We used the Elixhauser method to determine 30 different comorbidities from ICD-9-CM diagnosis coding16 and sorted patients by total number of comorbidities (0, 1, 2, 3 or 4, ≥5). As has been done before,17 we excluded blood loss anemia, coagulopathy, and fluid and electrolyte disorders from this comorbidity calculation, as these conditions can be secondary to trauma. We also extracted data on the admission itself, including hospital region (Northeast, Midwest, South, West), hospital bed size (small, medium, large), hospital teaching status (nonteaching, teaching), and hospital location (rural, urban). We used diagnosis codes to categorize fracture location as “not otherwise specified” (820.8), intracapsular (820.0), or extracapsular (820.2).
Because of low frequencies, we collapsed 2 race designations (Native American, Asian or Pacific Islander) into the “other race” category and 2 insurance designations (self-pay, no charge) into the “other insurance” category. For a substantial number of patients, race information was missing, so we included “missing” as its own category in analyses. Patients who were missing data on day of admission, age, sex, insurance, or hospital characteristics were excluded from our final cohort, as missing frequencies for each variable were small.
Ascertainment of Outcomes
For all patients, we extracted data on death status at discharge and length of hospital stay. We log-transformed length of stay because of its right skew, assigning the value of 12 hours to patients admitted and discharged the same day. Perioperative complications were calculated using ICD-9-CM codes as defined by a recent study of orthopedics-related complications by Lin and colleagues.18 There were 14 possible complications, including acute renal failure (584.5-9), tachycardia (427), wound hemorrhage (719.15, 998.31-2), wound disruption (998.3, 998.31-2), wound infection (682.6, 686.9, 891, 891.1-2, 894, 894.1-2, 998.5, 998.51, 998.6, 998.83, 998.59), deep vein thrombosis (453.4, 453.41-2, 453.9), acute myocardial infarction (410, 410.01, 410.11, 410.2, 410.21, 410.3, 410.31, 410.4, 410.41, 410.5, 410.51, 410.6, 410.9, 410.91, 997.1), pneumonia (480-480.9, 481, 482-482.9, 483, 483.1, 483.8, 484, 484.1, 484.3, 484.5-8, 485, 486, 487, 507), pulmonary embolism (415.11, 415.19), sepsis (995.91-2), stroke (997.02), urinary tract infection (599, 997.5), implant infection (996.66-7, 996.69), and incision and débridement (86.04, 86.09, 86.22, 86.28, 86.3). In our statistical analyses, we examined both the risk of having a complicated admission (≥1 perioperative complication) and the risk of having each specific complication.
Statistical Analysis
To assess similarity between weekend and weekday admissions, we used the Fisher exact test and χ2P values. Logistic regression was used to calculate the odds ratios (ORs) of mortality and perioperative complications for weekend versus weekday admissions. Linear regression was used to calculate parameter estimates for length of hospital stay for weekend versus weekday admissions. We interpreted parameter estimates as percentage differences using the formula 100(eb–1), where b is the estimated standardized regression coefficient of a log-transformed outcome variable.19 All regression models were controlled for age, sex, race, insurance, number of comorbidities, fracture location, hospital region, hospital bed size, hospital teaching status, and hospital location. We also stratified our study population by surgical delay in hours (<24, 24-48, 49-72, 73-120, ≥121) and by surgery performed (ORIF, hemiarthroplasty, CRIF, internal fixation only, THA, multiple procedures) to examine the effect of weekend admission on mortality, perioperative complications, and length of stay within each stratum. We did not control for these variables in our regression models because they were potential mediators of mortality, complications, and length of stay. All statistical analyses in this study were performed using SAS Version 9.1 (SAS Institute), and P < .05 was interpreted as statistically significant.
Results
After exclusions, our study population consisted of 96,892 weekend admissions and 248,097 weekday admissions. Among all admissions, mean age was 79.3 years (range, 0-113 years), with patients primarily being female and white, paying with Medicare, and having 1 to 4 comorbidities. Admissions were primarily for extracapsular femoral neck fractures and occurred most often in the South region, in hospitals with large beds, in nonteaching hospitals, and in urban locations. Table 1 lists details of baseline characteristics for weekend and weekday admissions.
Hospital stay details, including surgical delay and procedure performed, were examined for weekend and weekday admissions. Mean delay to surgery was 31.0 hours for weekend admissions and 30.2 hours for weekday admissions (P < .0001). The difference was driven by a higher proportion of weekend admissions in which surgery was performed 24 to 120 hours after admission. Patients admitted on the weekend also underwent more ORIF procedures and fewer hemiarthroplasties. Table 2 is a full list of hospital stay characteristics.
In regression analyses, weekend OR of mortality was 0.94 (95% CI, 0.89-0.99), weekend OR of having at least 1 complication was 1.00 (95% CI, 0.98-1.02), and weekend mean hospital stay was 3.74% shorter (95% CI, 3.40-4.08) in comparison with weekday figures. Within our models, risk of mortality and complications and mean length of stay increased as the number of patient comorbidities increased. Table 3 lists selected results from our regression models. Comprehensive tables for each outcome’s model are presented in Appendices 1 to 3.
In our analyses of specific complications, there were no significant associations between weekend admissions and risk of acute renal failure, wound hemorrhage, wound disruption, wound infection, deep vein thrombosis, myocardial infarction, pneumonia, pulmonary embolism, sepsis, urinary tract infection, implant infection, or incision and débridement. In addition, we found a lower risk of tachycardia (OR, 0.90; 95% CI, 0.82-1.00) and a higher risk (P < .10) of stroke (OR, 1.16; 95% CI, 0.99-1.35). Table 4 is a full list of the specific complications and their risks for weekend versus weekday admissions.
According to stratified analyses involving surgical delay, weekend admissions in which patients had surgery the same day as admission had decreased risk of mortality (OR, 0.81; 95% CI, 0.72-0.91) and perioperative complications (OR, 0.96; 95% CI, 0.92-0.99). In addition, hospital stay was shorter for weekend admissions with surgical delay of less than 24 hours (4.89% shorter; 95% CI, 4.22-5.55), 24 to 48 hours (5.93% shorter; 95% CI, 5.51-6.35), and 49 to 72 hours (3.50% shorter; 95% CI, 2.80-4.20). When admissions were stratified by procedure performed, patients who were admitted on the weekend and underwent ORIF, hemiarthroplasty, CRIF, internal fixation only, and THA had shorter stays than patients admitted on weekdays. For all surgeries performed, the risk of both mortality and complications did not significantly differ by day of admission. Table 5 lists the comprehensive results of all our stratified analyses.
Discussion
In this large, multiyear analysis of patients admitted for hip fracture in the United States, risk of mortality was slightly lower for weekend versus weekday admissions, hospital stay was significantly shorter, and risk of perioperative complications was not significantly different between admission types. In secondary analyses, shorter hospital stay was limited to patients who were admitted on weekends and underwent surgery within 48 hours. Our results therefore suggest that the weekend effect does not apply to hip fracture patients in the United States.
Our results are largely consistent with the literature on the topic.11-14 An Australian study of 4183 patients with acute hip fracture found no significant difference in 2- or 30-day mortality among weekend and weekday admissions.11 Similarly, 2 Danish studies did not find a difference in hospital-stay or 30-day mortality between weekend and weekday admissions among samples of 600 and 38,020 patients with hip fracture, respectively.12,13 In US patients, a cross-specialty study that included hip fractures did not find a difference in hospital-stay mortality among 22,001 admissions in the state of California in 1998.14 Our analysis significantly extended the findings of these studies by using comprehensive admission data from 46 US states over a 13-year period (1998–2010) and by examining outcomes other than mortality, including perioperative complications and length of hospital stay.
Our study had several limitations. First, the clinical data on fracture diagnoses and surgical procedures were based on ICD-9-CM codes, limiting our ability to account for the full details of fracture severity and subsequent management. Second, our analyses were limited to outcomes during the hospital stay, and we could not examine the effect of weekend admission on readmission and long-term mortality. Third, because of the dichotomization of admission day in the NIS database, we could not selectively examine the effect of Friday, Saturday, or Sunday admission on our outcomes. Fourth, we excluded admissions that were missing demographic and clinical data, potentially creating a complete-case bias. However, these exclusions were needed to accurately capture the common presentation of acute hip fracture, and there is no reason to believe that differences in record coding were nonrandom. Last, our study was observational, and we cannot rule out the effect of residual confounding on our results.
Our results failed to show a weekend effect on mortality, perioperative complications, or length of hospital stay in US patients with hip fracture. The reason for this, as suggested before,12 may be that hip fractures are becoming easier to diagnose. Furthermore, the observation that hospital stay was shorter for weekend admissions suggests that, despite decreased staffing of nursing and rehabilitation services, the lower volume of elective surgeries on weekends may actually increase staff availability to hip fracture patients.
1. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157.
2. Saposnik G, Baibergenova A, Bayer N, Hachinski V. Weekends: a dangerous time for having a stroke? Stroke. 2007;38(4):1211-1215.
3. Busl KM, Prabhakaran S. Predictors of mortality in nontraumatic subdural hematoma. J Neurosurg. 2013;119(5):1296-1301.
4. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296e1-302e1.
5. Shaheen AA, Kaplan GG, Myers RP. Weekend versus weekday admission and mortality from gastrointestinal hemorrhage caused by peptic ulcer disease. Clin Gastroenterol Hepatol. 2009;7(3):303-310.
6. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211.
7. Aujesky D, Jiménez D, Mor MK, Geng M, Fine MJ, Ibrahim SA. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119(7):962-968.
8. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783.
9. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, Van der mark S. Is mortality after hip fracture associated with surgical delay or admission during weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop. 2012;83(6):609-613.
10. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during weekends and holidays. Br J Anaesth. 2006;96(4):450-4514.
11. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis and meta-regression. Can J Anaesth. 2008;55(3):146-154.
12. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
13. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
14. Ho V, Hamilton BH, Roos LL. Multiple approaches to assessing the effects of delays for hip fracture patients in the United States and Canada. Health Serv Res. 2000;34(7):1499-1518.
15. Steiner C, Elixhauser A, Schnaier J. The Healthcare Cost and Utilization Project: an overview. Eff Clin Pract. 2002;5(3):143-151.
16. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27.
17. Brasel KJ, Guse CE, Layde P, Weigelt JA. Rib fractures: relationship with pneumonia and mortality. Crit Care Med. 2006;34(6):1642-1646.
18. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
19. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer-Verlag; 2012. Statistics for Biology and Health.
1. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157.
2. Saposnik G, Baibergenova A, Bayer N, Hachinski V. Weekends: a dangerous time for having a stroke? Stroke. 2007;38(4):1211-1215.
3. Busl KM, Prabhakaran S. Predictors of mortality in nontraumatic subdural hematoma. J Neurosurg. 2013;119(5):1296-1301.
4. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296e1-302e1.
5. Shaheen AA, Kaplan GG, Myers RP. Weekend versus weekday admission and mortality from gastrointestinal hemorrhage caused by peptic ulcer disease. Clin Gastroenterol Hepatol. 2009;7(3):303-310.
6. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211.
7. Aujesky D, Jiménez D, Mor MK, Geng M, Fine MJ, Ibrahim SA. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119(7):962-968.
8. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783.
9. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, Van der mark S. Is mortality after hip fracture associated with surgical delay or admission during weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop. 2012;83(6):609-613.
10. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during weekends and holidays. Br J Anaesth. 2006;96(4):450-4514.
11. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis and meta-regression. Can J Anaesth. 2008;55(3):146-154.
12. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
13. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
14. Ho V, Hamilton BH, Roos LL. Multiple approaches to assessing the effects of delays for hip fracture patients in the United States and Canada. Health Serv Res. 2000;34(7):1499-1518.
15. Steiner C, Elixhauser A, Schnaier J. The Healthcare Cost and Utilization Project: an overview. Eff Clin Pract. 2002;5(3):143-151.
16. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27.
17. Brasel KJ, Guse CE, Layde P, Weigelt JA. Rib fractures: relationship with pneumonia and mortality. Crit Care Med. 2006;34(6):1642-1646.
18. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
19. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer-Verlag; 2012. Statistics for Biology and Health.
Commentary to "CDC Will Soon Issue Guidelines for the Prevention of Surgical Site Infection"
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.