User login
Operational Curriculum and Research Initiatives: Shaping the Future of Military Medicine
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
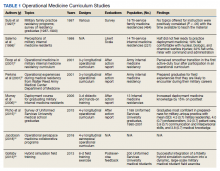
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
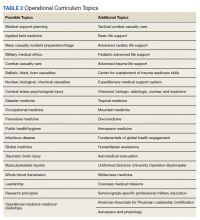
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
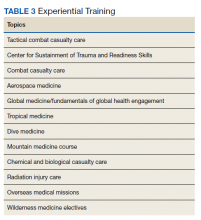
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
VA Firearm Policy Got It Half Right
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
Evaluating the Impact of a Simulated Hypersensitivity Reaction Case Study for New Fellows and Chemotherapy Nurses in an Outpatient Infusion Clinic
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers
The Transitions of Care Clinic: Demonstrating the Utility of the Single-Site Quality Improvement Study
A significant literature describes efforts to reduce hospital readmissions through improving care transitions. Many approaches have been tried, alone or in combination, targeting different points across the spectrum of discharge activities. These approaches encompass interventions initiated prior to discharge, such as patient education and enhanced discharge planning; bridging interventions, such as transition coaches; and postdischarge interventions, such as home visits or early follow-up appointments. Transitions of care clinics (TOCC) attempt to improve posthospital care by providing dedicated, rapid follow-up for patients after discharge.1
The impact of care transitions interventions is mixed, with inconsistent results across interventions and contexts. More complex, multipronged, context- and patient-sensitive interventions, however, are more likely to be associated with lower readmission rates.2,3
In this issue of the journal, Griffin and colleagues4 report on their TOCC implementation. Their focus on a high-risk, rural veteran population is different from prior studies, as is their use of in-person or virtual follow-up options. While the authors describe their intervention as a TOCC, their model serves as an organizer for an interprofessional team, including hospitalists, that coordinates multiple activities that complement the postdischarge appointments: identification of high-risk patients, pharmacist-led medication reconciliation, dietary counseling, contingency planning for potential changes, follow-up on pending tests and studies, and coordination of primary care and specialty care appointments. The multipronged, patient-sensitive nature of their intervention makes their positive findings consistent with other care transition literature.
Griffin and colleagues’ reporting of their TOCC experience is worth highlighting, as they present their experience and results in a way that maximizes our ability to learn from their implementation. Unfortunately, reports of improvement initiatives often lack sufficient detail regarding the context or intervention to potentially apply their findings. Griffin and colleagues applied the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines, a standardized framework for describing improvement initiatives that captures critical contextual and intervention elements.5Griffin and colleagues describe their baseline readmission performance and how the TOCC model was relevant to this issue. They describe the context, including their patient population, and their intervention with sufficient detail for us to understand what they actually did. Importantly, Griffin and colleagues clearly delineate the dynamic phases of the implementation, their use of Plan-Do-Study-Act cycles to assess and improve their implementation, and the specific changes they made. The Figure clearly puts their results in the context of their program evolution, and their secondary outcomes support our understanding of program growth. Their use of a committee for ongoing monitoring could be important for ongoing adaptation and sustainability.
There are several limitations worth noting. There may have been subjectivity in teams’ decisions to refer specific patients with lower
In this era of multisite collaborative studies and analyses of large administrative datasets, Griffin et al4 demonstrate that there is still much to learn from a well-done, single-site improvement study.
Funding: Drs Leykum and Penney reported funding from the Department of Veterans Affairs.
1. Nall RW, Herndon BB, Mramba LK, Vogel-Anderson K, Hagen MG. An interprofessional primary care-based transition of care clinic to reduce hospital readmission. J Am Med. 2019; 133(6):E260-E268. https://doi.org/10.1016/j.amjmed.2019.10.040
2. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608
3. Pugh J, Penney LS, Noel PH, Neller S, Mader M, Finley EP, Lanham HJ, Leykum LK. Evidence-based processes to prevent readmissions: more is better, a ten-site observational study. BMC Health Serv Res. 2021; 21:189. https://doi.org/10.1186/s12913-021-06193-x
4. Griffin BR, Agarwal N, Amberker R, et al. An initiative to improve 30-day readmission rates using a transitions-of-care clinic among a mixed urban and rural Veteran population. J Hosp Med. 2021;16(10):583-588. https://doi.org/10.12788/jhm.3659
5. Squire 2.0 guidelines. Accessed September 17, 2021. http://squire-statement.org
A significant literature describes efforts to reduce hospital readmissions through improving care transitions. Many approaches have been tried, alone or in combination, targeting different points across the spectrum of discharge activities. These approaches encompass interventions initiated prior to discharge, such as patient education and enhanced discharge planning; bridging interventions, such as transition coaches; and postdischarge interventions, such as home visits or early follow-up appointments. Transitions of care clinics (TOCC) attempt to improve posthospital care by providing dedicated, rapid follow-up for patients after discharge.1
The impact of care transitions interventions is mixed, with inconsistent results across interventions and contexts. More complex, multipronged, context- and patient-sensitive interventions, however, are more likely to be associated with lower readmission rates.2,3
In this issue of the journal, Griffin and colleagues4 report on their TOCC implementation. Their focus on a high-risk, rural veteran population is different from prior studies, as is their use of in-person or virtual follow-up options. While the authors describe their intervention as a TOCC, their model serves as an organizer for an interprofessional team, including hospitalists, that coordinates multiple activities that complement the postdischarge appointments: identification of high-risk patients, pharmacist-led medication reconciliation, dietary counseling, contingency planning for potential changes, follow-up on pending tests and studies, and coordination of primary care and specialty care appointments. The multipronged, patient-sensitive nature of their intervention makes their positive findings consistent with other care transition literature.
Griffin and colleagues’ reporting of their TOCC experience is worth highlighting, as they present their experience and results in a way that maximizes our ability to learn from their implementation. Unfortunately, reports of improvement initiatives often lack sufficient detail regarding the context or intervention to potentially apply their findings. Griffin and colleagues applied the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines, a standardized framework for describing improvement initiatives that captures critical contextual and intervention elements.5Griffin and colleagues describe their baseline readmission performance and how the TOCC model was relevant to this issue. They describe the context, including their patient population, and their intervention with sufficient detail for us to understand what they actually did. Importantly, Griffin and colleagues clearly delineate the dynamic phases of the implementation, their use of Plan-Do-Study-Act cycles to assess and improve their implementation, and the specific changes they made. The Figure clearly puts their results in the context of their program evolution, and their secondary outcomes support our understanding of program growth. Their use of a committee for ongoing monitoring could be important for ongoing adaptation and sustainability.
There are several limitations worth noting. There may have been subjectivity in teams’ decisions to refer specific patients with lower
In this era of multisite collaborative studies and analyses of large administrative datasets, Griffin et al4 demonstrate that there is still much to learn from a well-done, single-site improvement study.
Funding: Drs Leykum and Penney reported funding from the Department of Veterans Affairs.
A significant literature describes efforts to reduce hospital readmissions through improving care transitions. Many approaches have been tried, alone or in combination, targeting different points across the spectrum of discharge activities. These approaches encompass interventions initiated prior to discharge, such as patient education and enhanced discharge planning; bridging interventions, such as transition coaches; and postdischarge interventions, such as home visits or early follow-up appointments. Transitions of care clinics (TOCC) attempt to improve posthospital care by providing dedicated, rapid follow-up for patients after discharge.1
The impact of care transitions interventions is mixed, with inconsistent results across interventions and contexts. More complex, multipronged, context- and patient-sensitive interventions, however, are more likely to be associated with lower readmission rates.2,3
In this issue of the journal, Griffin and colleagues4 report on their TOCC implementation. Their focus on a high-risk, rural veteran population is different from prior studies, as is their use of in-person or virtual follow-up options. While the authors describe their intervention as a TOCC, their model serves as an organizer for an interprofessional team, including hospitalists, that coordinates multiple activities that complement the postdischarge appointments: identification of high-risk patients, pharmacist-led medication reconciliation, dietary counseling, contingency planning for potential changes, follow-up on pending tests and studies, and coordination of primary care and specialty care appointments. The multipronged, patient-sensitive nature of their intervention makes their positive findings consistent with other care transition literature.
Griffin and colleagues’ reporting of their TOCC experience is worth highlighting, as they present their experience and results in a way that maximizes our ability to learn from their implementation. Unfortunately, reports of improvement initiatives often lack sufficient detail regarding the context or intervention to potentially apply their findings. Griffin and colleagues applied the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines, a standardized framework for describing improvement initiatives that captures critical contextual and intervention elements.5Griffin and colleagues describe their baseline readmission performance and how the TOCC model was relevant to this issue. They describe the context, including their patient population, and their intervention with sufficient detail for us to understand what they actually did. Importantly, Griffin and colleagues clearly delineate the dynamic phases of the implementation, their use of Plan-Do-Study-Act cycles to assess and improve their implementation, and the specific changes they made. The Figure clearly puts their results in the context of their program evolution, and their secondary outcomes support our understanding of program growth. Their use of a committee for ongoing monitoring could be important for ongoing adaptation and sustainability.
There are several limitations worth noting. There may have been subjectivity in teams’ decisions to refer specific patients with lower
In this era of multisite collaborative studies and analyses of large administrative datasets, Griffin et al4 demonstrate that there is still much to learn from a well-done, single-site improvement study.
Funding: Drs Leykum and Penney reported funding from the Department of Veterans Affairs.
1. Nall RW, Herndon BB, Mramba LK, Vogel-Anderson K, Hagen MG. An interprofessional primary care-based transition of care clinic to reduce hospital readmission. J Am Med. 2019; 133(6):E260-E268. https://doi.org/10.1016/j.amjmed.2019.10.040
2. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608
3. Pugh J, Penney LS, Noel PH, Neller S, Mader M, Finley EP, Lanham HJ, Leykum LK. Evidence-based processes to prevent readmissions: more is better, a ten-site observational study. BMC Health Serv Res. 2021; 21:189. https://doi.org/10.1186/s12913-021-06193-x
4. Griffin BR, Agarwal N, Amberker R, et al. An initiative to improve 30-day readmission rates using a transitions-of-care clinic among a mixed urban and rural Veteran population. J Hosp Med. 2021;16(10):583-588. https://doi.org/10.12788/jhm.3659
5. Squire 2.0 guidelines. Accessed September 17, 2021. http://squire-statement.org
1. Nall RW, Herndon BB, Mramba LK, Vogel-Anderson K, Hagen MG. An interprofessional primary care-based transition of care clinic to reduce hospital readmission. J Am Med. 2019; 133(6):E260-E268. https://doi.org/10.1016/j.amjmed.2019.10.040
2. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608
3. Pugh J, Penney LS, Noel PH, Neller S, Mader M, Finley EP, Lanham HJ, Leykum LK. Evidence-based processes to prevent readmissions: more is better, a ten-site observational study. BMC Health Serv Res. 2021; 21:189. https://doi.org/10.1186/s12913-021-06193-x
4. Griffin BR, Agarwal N, Amberker R, et al. An initiative to improve 30-day readmission rates using a transitions-of-care clinic among a mixed urban and rural Veteran population. J Hosp Med. 2021;16(10):583-588. https://doi.org/10.12788/jhm.3659
5. Squire 2.0 guidelines. Accessed September 17, 2021. http://squire-statement.org
© 2021 Society of Hospital Medicine
Predictive Models for In-Hospital Deterioration in Ward Patients
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
© 2021 Society of Hospital Medicine
Black Pain Matters: Prioritizing Antiracism and Equity in the Opioid Epidemic
In 2016, a study was published that continues to shock observers today.1 Examining 200 medical trainees, researchers reported that an alarming percentage of these individuals held false beliefs about Black bodies, including 22% believing that nerve endings in Black persons are less sensitive than nerve endings in White persons and 63% believing that Black skin is thicker than White skin. Furthermore, the study found that those who held these false beliefs about biological differences between Black and White individuals were also less likely to recommend pain treatment to Black patients in a follow-up case vignette. Two years later, in an evaluation of racial differences in opioid prescribing in the United States published in Epidemiology, one of the authors suggested, “It’s an extremely rare case where racial biases actually protected the population [Black individuals] being discriminated against.”2
These studies provide the background for the analysis by Rambachan et al3 published in this issue of the Journal of Hospital Medicine. The authors examined a diverse cohort of more than 10,000 patients hospitalized on a general medicine service at an academic medical center in San Francisco from 2012 to 2018. Black patients were significantly less likely to receive an opioid prescription at discharge, and when they did, were discharged on opioids for fewer days than White patients. No other racial group experienced such a disparity, with Asian patients more likely to receive opioids at discharge. Whereas these findings align with myriad studies demonstrating racial disparities in opioid prescribing,4 the authors focus on patients admitted to a general medicine service, where most hospitalized patients receive medical care daily.
The authors concede that determining the etiology of these disparities was beyond the scope of their study, yet this is the exact question we must answer today. Why should the color of a patient’s skin continue to determine the type, and duration, of care they receive, especially when treating pain? The authors hypothesize that individual factors such as provider bias and systemic factors, including limited guidelines on pain management, may drive the observed racial inequities. This progression from individual- and institutional- to community- and policy-level determinants offers a useful framework for understanding the drivers of disparities in opioid prescribing. It also provides an agenda for future research that can guide us from simply detecting disparities to understanding and eliminating them. Furthermore, it is important to examine care team provider characteristics, including race/ethnicity, years in practice, education level (eg, resident vs attending),5 experience with implicit bias training, and differential referral to specialists, such as pain, palliative care, and addiction providers. Factors associated with the facility where a patient is hospitalized also warrant further exploration, including the diversity of medical and nonmedical staff as well as patients.6 Examining these factors will allow us to move closer toward implementing effective interventions that eliminate disparities in pain treatment.
The authors begin to provide us with possible levers to pull to address the inequities in opioid prescribing. They suggest provider-level bias training, improved institutional tracking of disparities, and policy-level solutions to address the persistent dearth of diversity in the healthcare workforce. While these broad solutions may address health disparities across the medical field, targeted solutions are needed to directly address inequities in pain treatment. First, we must explore the reasons for disparities in the prevalence, presentation, and management of pain in Black populations. These reasons may include occupational exposures or injuries, psychological stress (often associated with racism), and a disproportionate presence of chronic medical comorbidities. Second, health systems can implement a standardized system for opioid prescribing, supported by pharmacy expertise and considering clinical diagnoses, to reduce subjectivity associated with determining the appropriateness of an opioid prescription. Third, health systems must improve access to addiction, harm reduction, and pain specialty services to effectively manage comorbid conditions in at-risk patients.7 Furthermore, we must look beyond traditional measures of healthcare access, such as insurance coverage, to address social determinants of health, such as distance to pharmacy, housing security, employment status, and experience with the criminal justice system, which may influence a patient’s receipt of a prescription. Finally, as a society, we must prioritize early training of healthcare providers, long before the undergraduate and graduate medical education level, to practice medicine without stigmatizing biases and stereotypes related to drug use in communities of color.8
The pattern of racial and ethnic disparities in healthcare has been documented for decades, with an ever-increasing depth of the different ways in which minoritized patients are undertreated. Despite this breadth of research, our understanding of the etiology of these inequities and development and implementation of interventions to reduce them remain limited. Rambachan et al3 do a commendable job highlighting further racial disparities in opioid prescribing in hospitalized patients and provide another opportunity to answer the important questions plaguing health care today: Why do these disparities exist and what can be done to address them? The urgency we take towards answering these questions will confirm our commitment to achieving antiracism in medicine and prioritizing health equity. Black lives are depending on it.
1. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296-4301. https://doi.org/10.1073/pnas.1516047113
2. Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White opioid mortality in the United States, 1979-2015. Epidemiology. 2018;29(5):707-715. https://doi.org/10.1097/EDE.0000000000000858
3. Rambachan A, Fang MA, Prasad P, Iverson N. Racial and ethnic disparities in discharge opioid prescribing from a hospital medicine service. J Hosp Med. 2021;16(10):589-595. https://doi.org/10.12788/jhm.3667
4. Essien UR, Sileanu FE, Zhao X, et al. Racial/ethnic differences in the medical treatment of opioid use disorders within the VA healthcare system following non-fatal opioid overdose. J Gen Intern Med. 2020;35(5):1537-1544. https://doi.org/10.1007/s11606-020-05645-0
5. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. https://doi.org/10.1007/s11606-019-04960-5
6. Hollingsworth JM, Yu X, Yan PL, et al. Provider care team segregation and operative mortality following coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2021;14(5):e007778. https://doi.org/10.1161/CIRCOUTCOMES.120.007778
7. Sue KL, Fiellin DA. Bringing harm reduction into health policy - combating the overdose crisis. N Engl J Med. 2021;384(19):1781-1783. https://doi.org/10.1056/NEJMp2103274
8. James K, Jordan A. The opioid crisis in Black communities. J Law Med Ethics. 2018;46(2):404-421. https://doi.org/10.1038/jes.2015.55
In 2016, a study was published that continues to shock observers today.1 Examining 200 medical trainees, researchers reported that an alarming percentage of these individuals held false beliefs about Black bodies, including 22% believing that nerve endings in Black persons are less sensitive than nerve endings in White persons and 63% believing that Black skin is thicker than White skin. Furthermore, the study found that those who held these false beliefs about biological differences between Black and White individuals were also less likely to recommend pain treatment to Black patients in a follow-up case vignette. Two years later, in an evaluation of racial differences in opioid prescribing in the United States published in Epidemiology, one of the authors suggested, “It’s an extremely rare case where racial biases actually protected the population [Black individuals] being discriminated against.”2
These studies provide the background for the analysis by Rambachan et al3 published in this issue of the Journal of Hospital Medicine. The authors examined a diverse cohort of more than 10,000 patients hospitalized on a general medicine service at an academic medical center in San Francisco from 2012 to 2018. Black patients were significantly less likely to receive an opioid prescription at discharge, and when they did, were discharged on opioids for fewer days than White patients. No other racial group experienced such a disparity, with Asian patients more likely to receive opioids at discharge. Whereas these findings align with myriad studies demonstrating racial disparities in opioid prescribing,4 the authors focus on patients admitted to a general medicine service, where most hospitalized patients receive medical care daily.
The authors concede that determining the etiology of these disparities was beyond the scope of their study, yet this is the exact question we must answer today. Why should the color of a patient’s skin continue to determine the type, and duration, of care they receive, especially when treating pain? The authors hypothesize that individual factors such as provider bias and systemic factors, including limited guidelines on pain management, may drive the observed racial inequities. This progression from individual- and institutional- to community- and policy-level determinants offers a useful framework for understanding the drivers of disparities in opioid prescribing. It also provides an agenda for future research that can guide us from simply detecting disparities to understanding and eliminating them. Furthermore, it is important to examine care team provider characteristics, including race/ethnicity, years in practice, education level (eg, resident vs attending),5 experience with implicit bias training, and differential referral to specialists, such as pain, palliative care, and addiction providers. Factors associated with the facility where a patient is hospitalized also warrant further exploration, including the diversity of medical and nonmedical staff as well as patients.6 Examining these factors will allow us to move closer toward implementing effective interventions that eliminate disparities in pain treatment.
The authors begin to provide us with possible levers to pull to address the inequities in opioid prescribing. They suggest provider-level bias training, improved institutional tracking of disparities, and policy-level solutions to address the persistent dearth of diversity in the healthcare workforce. While these broad solutions may address health disparities across the medical field, targeted solutions are needed to directly address inequities in pain treatment. First, we must explore the reasons for disparities in the prevalence, presentation, and management of pain in Black populations. These reasons may include occupational exposures or injuries, psychological stress (often associated with racism), and a disproportionate presence of chronic medical comorbidities. Second, health systems can implement a standardized system for opioid prescribing, supported by pharmacy expertise and considering clinical diagnoses, to reduce subjectivity associated with determining the appropriateness of an opioid prescription. Third, health systems must improve access to addiction, harm reduction, and pain specialty services to effectively manage comorbid conditions in at-risk patients.7 Furthermore, we must look beyond traditional measures of healthcare access, such as insurance coverage, to address social determinants of health, such as distance to pharmacy, housing security, employment status, and experience with the criminal justice system, which may influence a patient’s receipt of a prescription. Finally, as a society, we must prioritize early training of healthcare providers, long before the undergraduate and graduate medical education level, to practice medicine without stigmatizing biases and stereotypes related to drug use in communities of color.8
The pattern of racial and ethnic disparities in healthcare has been documented for decades, with an ever-increasing depth of the different ways in which minoritized patients are undertreated. Despite this breadth of research, our understanding of the etiology of these inequities and development and implementation of interventions to reduce them remain limited. Rambachan et al3 do a commendable job highlighting further racial disparities in opioid prescribing in hospitalized patients and provide another opportunity to answer the important questions plaguing health care today: Why do these disparities exist and what can be done to address them? The urgency we take towards answering these questions will confirm our commitment to achieving antiracism in medicine and prioritizing health equity. Black lives are depending on it.
In 2016, a study was published that continues to shock observers today.1 Examining 200 medical trainees, researchers reported that an alarming percentage of these individuals held false beliefs about Black bodies, including 22% believing that nerve endings in Black persons are less sensitive than nerve endings in White persons and 63% believing that Black skin is thicker than White skin. Furthermore, the study found that those who held these false beliefs about biological differences between Black and White individuals were also less likely to recommend pain treatment to Black patients in a follow-up case vignette. Two years later, in an evaluation of racial differences in opioid prescribing in the United States published in Epidemiology, one of the authors suggested, “It’s an extremely rare case where racial biases actually protected the population [Black individuals] being discriminated against.”2
These studies provide the background for the analysis by Rambachan et al3 published in this issue of the Journal of Hospital Medicine. The authors examined a diverse cohort of more than 10,000 patients hospitalized on a general medicine service at an academic medical center in San Francisco from 2012 to 2018. Black patients were significantly less likely to receive an opioid prescription at discharge, and when they did, were discharged on opioids for fewer days than White patients. No other racial group experienced such a disparity, with Asian patients more likely to receive opioids at discharge. Whereas these findings align with myriad studies demonstrating racial disparities in opioid prescribing,4 the authors focus on patients admitted to a general medicine service, where most hospitalized patients receive medical care daily.
The authors concede that determining the etiology of these disparities was beyond the scope of their study, yet this is the exact question we must answer today. Why should the color of a patient’s skin continue to determine the type, and duration, of care they receive, especially when treating pain? The authors hypothesize that individual factors such as provider bias and systemic factors, including limited guidelines on pain management, may drive the observed racial inequities. This progression from individual- and institutional- to community- and policy-level determinants offers a useful framework for understanding the drivers of disparities in opioid prescribing. It also provides an agenda for future research that can guide us from simply detecting disparities to understanding and eliminating them. Furthermore, it is important to examine care team provider characteristics, including race/ethnicity, years in practice, education level (eg, resident vs attending),5 experience with implicit bias training, and differential referral to specialists, such as pain, palliative care, and addiction providers. Factors associated with the facility where a patient is hospitalized also warrant further exploration, including the diversity of medical and nonmedical staff as well as patients.6 Examining these factors will allow us to move closer toward implementing effective interventions that eliminate disparities in pain treatment.
The authors begin to provide us with possible levers to pull to address the inequities in opioid prescribing. They suggest provider-level bias training, improved institutional tracking of disparities, and policy-level solutions to address the persistent dearth of diversity in the healthcare workforce. While these broad solutions may address health disparities across the medical field, targeted solutions are needed to directly address inequities in pain treatment. First, we must explore the reasons for disparities in the prevalence, presentation, and management of pain in Black populations. These reasons may include occupational exposures or injuries, psychological stress (often associated with racism), and a disproportionate presence of chronic medical comorbidities. Second, health systems can implement a standardized system for opioid prescribing, supported by pharmacy expertise and considering clinical diagnoses, to reduce subjectivity associated with determining the appropriateness of an opioid prescription. Third, health systems must improve access to addiction, harm reduction, and pain specialty services to effectively manage comorbid conditions in at-risk patients.7 Furthermore, we must look beyond traditional measures of healthcare access, such as insurance coverage, to address social determinants of health, such as distance to pharmacy, housing security, employment status, and experience with the criminal justice system, which may influence a patient’s receipt of a prescription. Finally, as a society, we must prioritize early training of healthcare providers, long before the undergraduate and graduate medical education level, to practice medicine without stigmatizing biases and stereotypes related to drug use in communities of color.8
The pattern of racial and ethnic disparities in healthcare has been documented for decades, with an ever-increasing depth of the different ways in which minoritized patients are undertreated. Despite this breadth of research, our understanding of the etiology of these inequities and development and implementation of interventions to reduce them remain limited. Rambachan et al3 do a commendable job highlighting further racial disparities in opioid prescribing in hospitalized patients and provide another opportunity to answer the important questions plaguing health care today: Why do these disparities exist and what can be done to address them? The urgency we take towards answering these questions will confirm our commitment to achieving antiracism in medicine and prioritizing health equity. Black lives are depending on it.
1. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296-4301. https://doi.org/10.1073/pnas.1516047113
2. Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White opioid mortality in the United States, 1979-2015. Epidemiology. 2018;29(5):707-715. https://doi.org/10.1097/EDE.0000000000000858
3. Rambachan A, Fang MA, Prasad P, Iverson N. Racial and ethnic disparities in discharge opioid prescribing from a hospital medicine service. J Hosp Med. 2021;16(10):589-595. https://doi.org/10.12788/jhm.3667
4. Essien UR, Sileanu FE, Zhao X, et al. Racial/ethnic differences in the medical treatment of opioid use disorders within the VA healthcare system following non-fatal opioid overdose. J Gen Intern Med. 2020;35(5):1537-1544. https://doi.org/10.1007/s11606-020-05645-0
5. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. https://doi.org/10.1007/s11606-019-04960-5
6. Hollingsworth JM, Yu X, Yan PL, et al. Provider care team segregation and operative mortality following coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2021;14(5):e007778. https://doi.org/10.1161/CIRCOUTCOMES.120.007778
7. Sue KL, Fiellin DA. Bringing harm reduction into health policy - combating the overdose crisis. N Engl J Med. 2021;384(19):1781-1783. https://doi.org/10.1056/NEJMp2103274
8. James K, Jordan A. The opioid crisis in Black communities. J Law Med Ethics. 2018;46(2):404-421. https://doi.org/10.1038/jes.2015.55
1. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296-4301. https://doi.org/10.1073/pnas.1516047113
2. Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White opioid mortality in the United States, 1979-2015. Epidemiology. 2018;29(5):707-715. https://doi.org/10.1097/EDE.0000000000000858
3. Rambachan A, Fang MA, Prasad P, Iverson N. Racial and ethnic disparities in discharge opioid prescribing from a hospital medicine service. J Hosp Med. 2021;16(10):589-595. https://doi.org/10.12788/jhm.3667
4. Essien UR, Sileanu FE, Zhao X, et al. Racial/ethnic differences in the medical treatment of opioid use disorders within the VA healthcare system following non-fatal opioid overdose. J Gen Intern Med. 2020;35(5):1537-1544. https://doi.org/10.1007/s11606-020-05645-0
5. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. https://doi.org/10.1007/s11606-019-04960-5
6. Hollingsworth JM, Yu X, Yan PL, et al. Provider care team segregation and operative mortality following coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2021;14(5):e007778. https://doi.org/10.1161/CIRCOUTCOMES.120.007778
7. Sue KL, Fiellin DA. Bringing harm reduction into health policy - combating the overdose crisis. N Engl J Med. 2021;384(19):1781-1783. https://doi.org/10.1056/NEJMp2103274
8. James K, Jordan A. The opioid crisis in Black communities. J Law Med Ethics. 2018;46(2):404-421. https://doi.org/10.1038/jes.2015.55
© 2021 Society of Hospital Medicine
Leadership & Professional Development: New Team? No Problem. Creating Teams From Strangers
“Well begun is half done.” — Aristotle
In the clinical environment, team composition changes frequently and time is limited. As a result, teams often jump directly into patient care, addressing issues related to interpersonal dynamics only after they arise. Team leaders can accelerate the process of forming highly effective teams by deliberately leveraging principles of teaming, or the process of “how to turn a group of strangers into a team.”1
Setting the Stage
On the first day with a new team, a common misconception is that teaming will take away time, when in fact it will save time. Investing a few minutes before rounds to clarify roles and expectations can streamline subsequent shared work. For example, an attending might request to accompany residents and medical students for new admissions in the last 2 hours of the workday, rather than following the usual pattern of discussing the case after the team completes a full evaluation on their own. Importantly, attendings should clarify their intent—to preserve learning opportunities while helping teams wrap up on time—and their role, which is to provide real-time feedback, facilitate decision-making, or assist with documentation. This 2-minute upfront investment results in improved team camaraderie, better task coordination, and fewer late days in the hospital.
Uncovering Connections and Skills
By integrating a few positively framed, thoughtful questions into introductions, teams may also discover surprising expertise or valuable perspectives that positively impact team performance.2 For example, in lieu of questions about level of training or hometown, you might ask, “What is an experience outside the hospital that helps you inside the hospital?” or “What skills allow you to contribute best on teams?” These questions might lead, for example, a medical student to leverage her background in computer science to help her team design new electronic health record shortcuts. Or, they might enable a resident with a personal history of leukemia to help the team communicate with a young patient facing a prolonged hospitalization for a newly diagnosed serious illness. With typical introductions, these opportunities and unexpected solutions can easily be missed.
Creating Mutual Understanding and Focus
As part of teaming, members should also explicitly share individual work-style preferences to avoid misunderstandings that may adversely affect subsequent work. On new teams, members—especially trainees—expend considerable energy scrutinizing subtle behaviors, such as a clarifying question or a blank stare, to assess whether their performance is perceived favorably. That energy can be reallocated to more important tasks by encouraging each person to state nuances of their work style that may be misinterpreted. For example, an attending might share, “I ask questions to identify what to teach, not to judge knowledge, so don’t worry about saying you don’t know,” whereas a resident might warn, “I have trouble concentrating when I’m hungry, so I often get impatient if we don’t take a break for lunch.” Without this information, a student might feel unnecessarily embarrassed by an attending on rounds, and an attending might incorrectly interpret a resident’s impatience around lunchtime as a reflection of low commitment. Individual work styles vary, and recognizing these differences upfront allows teams to maintain a sharper focus on more important issues, such as clinical care.
A Winning Team
In the hospital, we find ourselves in perpetual motion, with frequent transitions of care and new team members. Teaming offers a concrete method to proactively avoid predictable challenges and to enable teams to become more efficient, effective, and connected. Furthermore, teaming empowers us to substitute the uncertainty of ever-changing teams with the excitement of discovering what each new team can achieve through intentional leadership at the outset.
1. Edmondson AC. How to turn a group of strangers into a team. Accessed March 1, 2021. https://www.ted.com/talks/amy_edmondson_how_to_turn_a_group_of_strangers_into_a_team?language=en
2. Edmondson AC. Teamwork on the fly. Harvard Business Review. Published April 2012. Accessed July 26, 2021. https://hbr.org/2012/04/teamwork-on-the-fly-2
“Well begun is half done.” — Aristotle
In the clinical environment, team composition changes frequently and time is limited. As a result, teams often jump directly into patient care, addressing issues related to interpersonal dynamics only after they arise. Team leaders can accelerate the process of forming highly effective teams by deliberately leveraging principles of teaming, or the process of “how to turn a group of strangers into a team.”1
Setting the Stage
On the first day with a new team, a common misconception is that teaming will take away time, when in fact it will save time. Investing a few minutes before rounds to clarify roles and expectations can streamline subsequent shared work. For example, an attending might request to accompany residents and medical students for new admissions in the last 2 hours of the workday, rather than following the usual pattern of discussing the case after the team completes a full evaluation on their own. Importantly, attendings should clarify their intent—to preserve learning opportunities while helping teams wrap up on time—and their role, which is to provide real-time feedback, facilitate decision-making, or assist with documentation. This 2-minute upfront investment results in improved team camaraderie, better task coordination, and fewer late days in the hospital.
Uncovering Connections and Skills
By integrating a few positively framed, thoughtful questions into introductions, teams may also discover surprising expertise or valuable perspectives that positively impact team performance.2 For example, in lieu of questions about level of training or hometown, you might ask, “What is an experience outside the hospital that helps you inside the hospital?” or “What skills allow you to contribute best on teams?” These questions might lead, for example, a medical student to leverage her background in computer science to help her team design new electronic health record shortcuts. Or, they might enable a resident with a personal history of leukemia to help the team communicate with a young patient facing a prolonged hospitalization for a newly diagnosed serious illness. With typical introductions, these opportunities and unexpected solutions can easily be missed.
Creating Mutual Understanding and Focus
As part of teaming, members should also explicitly share individual work-style preferences to avoid misunderstandings that may adversely affect subsequent work. On new teams, members—especially trainees—expend considerable energy scrutinizing subtle behaviors, such as a clarifying question or a blank stare, to assess whether their performance is perceived favorably. That energy can be reallocated to more important tasks by encouraging each person to state nuances of their work style that may be misinterpreted. For example, an attending might share, “I ask questions to identify what to teach, not to judge knowledge, so don’t worry about saying you don’t know,” whereas a resident might warn, “I have trouble concentrating when I’m hungry, so I often get impatient if we don’t take a break for lunch.” Without this information, a student might feel unnecessarily embarrassed by an attending on rounds, and an attending might incorrectly interpret a resident’s impatience around lunchtime as a reflection of low commitment. Individual work styles vary, and recognizing these differences upfront allows teams to maintain a sharper focus on more important issues, such as clinical care.
A Winning Team
In the hospital, we find ourselves in perpetual motion, with frequent transitions of care and new team members. Teaming offers a concrete method to proactively avoid predictable challenges and to enable teams to become more efficient, effective, and connected. Furthermore, teaming empowers us to substitute the uncertainty of ever-changing teams with the excitement of discovering what each new team can achieve through intentional leadership at the outset.
“Well begun is half done.” — Aristotle
In the clinical environment, team composition changes frequently and time is limited. As a result, teams often jump directly into patient care, addressing issues related to interpersonal dynamics only after they arise. Team leaders can accelerate the process of forming highly effective teams by deliberately leveraging principles of teaming, or the process of “how to turn a group of strangers into a team.”1
Setting the Stage
On the first day with a new team, a common misconception is that teaming will take away time, when in fact it will save time. Investing a few minutes before rounds to clarify roles and expectations can streamline subsequent shared work. For example, an attending might request to accompany residents and medical students for new admissions in the last 2 hours of the workday, rather than following the usual pattern of discussing the case after the team completes a full evaluation on their own. Importantly, attendings should clarify their intent—to preserve learning opportunities while helping teams wrap up on time—and their role, which is to provide real-time feedback, facilitate decision-making, or assist with documentation. This 2-minute upfront investment results in improved team camaraderie, better task coordination, and fewer late days in the hospital.
Uncovering Connections and Skills
By integrating a few positively framed, thoughtful questions into introductions, teams may also discover surprising expertise or valuable perspectives that positively impact team performance.2 For example, in lieu of questions about level of training or hometown, you might ask, “What is an experience outside the hospital that helps you inside the hospital?” or “What skills allow you to contribute best on teams?” These questions might lead, for example, a medical student to leverage her background in computer science to help her team design new electronic health record shortcuts. Or, they might enable a resident with a personal history of leukemia to help the team communicate with a young patient facing a prolonged hospitalization for a newly diagnosed serious illness. With typical introductions, these opportunities and unexpected solutions can easily be missed.
Creating Mutual Understanding and Focus
As part of teaming, members should also explicitly share individual work-style preferences to avoid misunderstandings that may adversely affect subsequent work. On new teams, members—especially trainees—expend considerable energy scrutinizing subtle behaviors, such as a clarifying question or a blank stare, to assess whether their performance is perceived favorably. That energy can be reallocated to more important tasks by encouraging each person to state nuances of their work style that may be misinterpreted. For example, an attending might share, “I ask questions to identify what to teach, not to judge knowledge, so don’t worry about saying you don’t know,” whereas a resident might warn, “I have trouble concentrating when I’m hungry, so I often get impatient if we don’t take a break for lunch.” Without this information, a student might feel unnecessarily embarrassed by an attending on rounds, and an attending might incorrectly interpret a resident’s impatience around lunchtime as a reflection of low commitment. Individual work styles vary, and recognizing these differences upfront allows teams to maintain a sharper focus on more important issues, such as clinical care.
A Winning Team
In the hospital, we find ourselves in perpetual motion, with frequent transitions of care and new team members. Teaming offers a concrete method to proactively avoid predictable challenges and to enable teams to become more efficient, effective, and connected. Furthermore, teaming empowers us to substitute the uncertainty of ever-changing teams with the excitement of discovering what each new team can achieve through intentional leadership at the outset.
1. Edmondson AC. How to turn a group of strangers into a team. Accessed March 1, 2021. https://www.ted.com/talks/amy_edmondson_how_to_turn_a_group_of_strangers_into_a_team?language=en
2. Edmondson AC. Teamwork on the fly. Harvard Business Review. Published April 2012. Accessed July 26, 2021. https://hbr.org/2012/04/teamwork-on-the-fly-2
1. Edmondson AC. How to turn a group of strangers into a team. Accessed March 1, 2021. https://www.ted.com/talks/amy_edmondson_how_to_turn_a_group_of_strangers_into_a_team?language=en
2. Edmondson AC. Teamwork on the fly. Harvard Business Review. Published April 2012. Accessed July 26, 2021. https://hbr.org/2012/04/teamwork-on-the-fly-2
© 2021 Society of Hospital Medicine
Mobile Integrated Health: Reducing Chronic Obstructive Pulmonary Disease Hospitalizations Through Novel Outpatient Care Initiatives
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.
The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.
Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).
Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.
Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; klannutti@southshorehealth.org.
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.
The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.
Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).
Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.
Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; klannutti@southshorehealth.org.
Financial disclosures: None.
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.
The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.
Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).
Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.
Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; klannutti@southshorehealth.org.
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
Virtual Visitation: Exploring the Impact on Patients and Families During COVID-19 and Beyond
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.
Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; nfilippa@northwell.edu.
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.
Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; nfilippa@northwell.edu.
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.
Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; nfilippa@northwell.edu.
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
Improving Physicians’ Bowel Documentation on Geriatric Wards
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002