User login
Aerosolization of COVID-19 and Contamination Risks During Respiratory Treatments
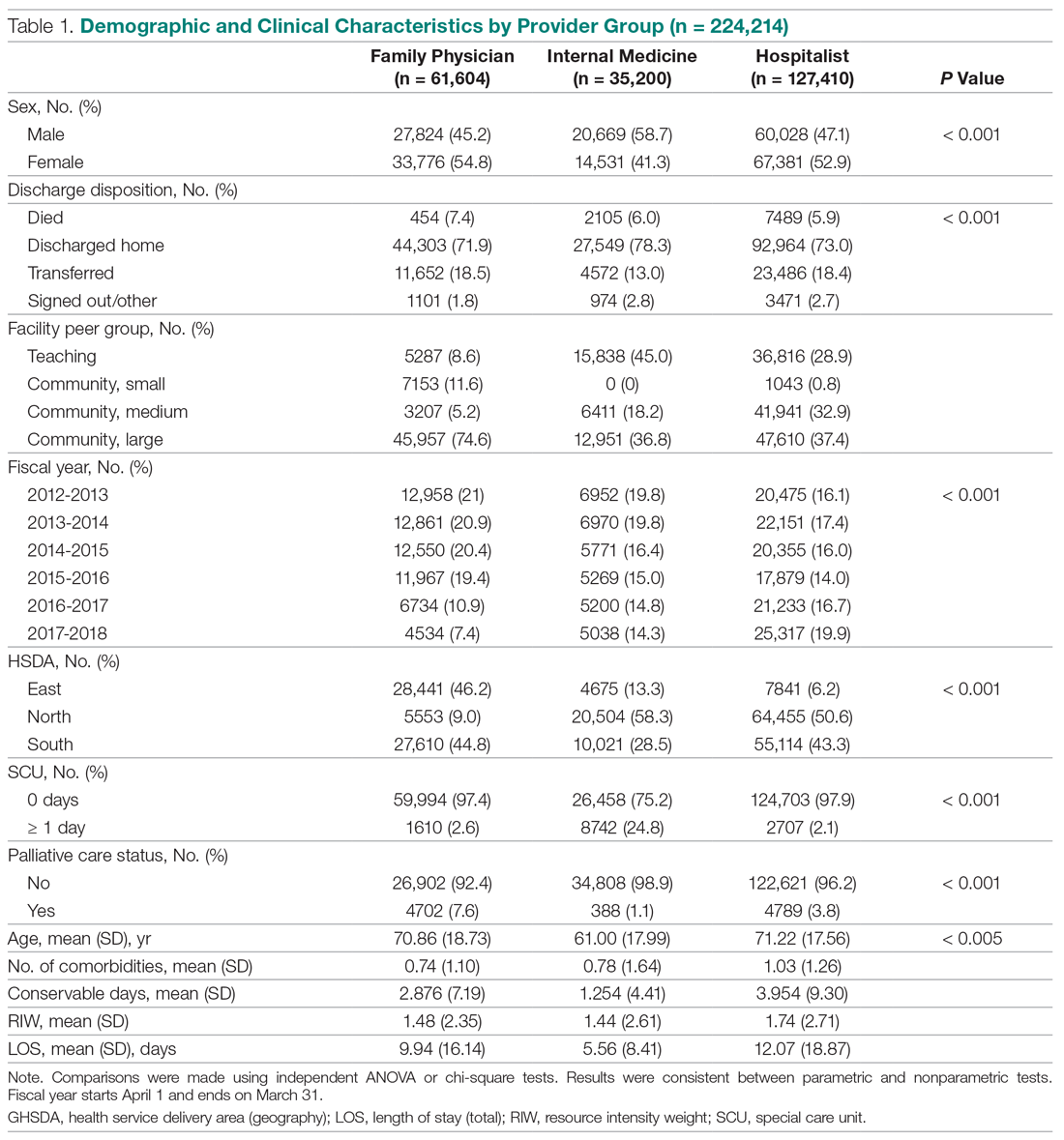
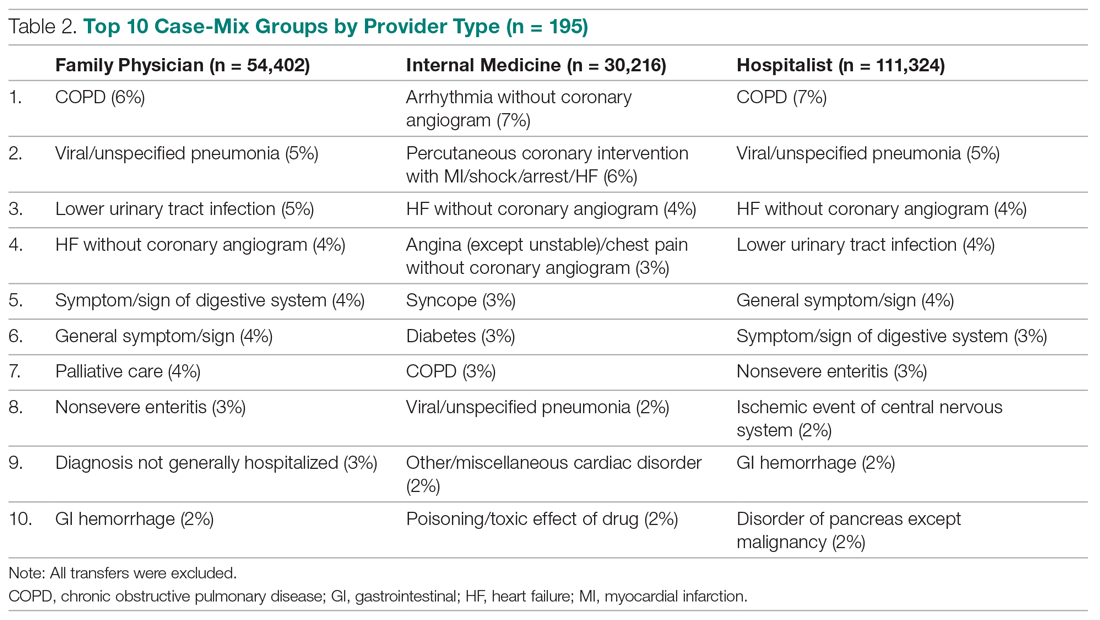
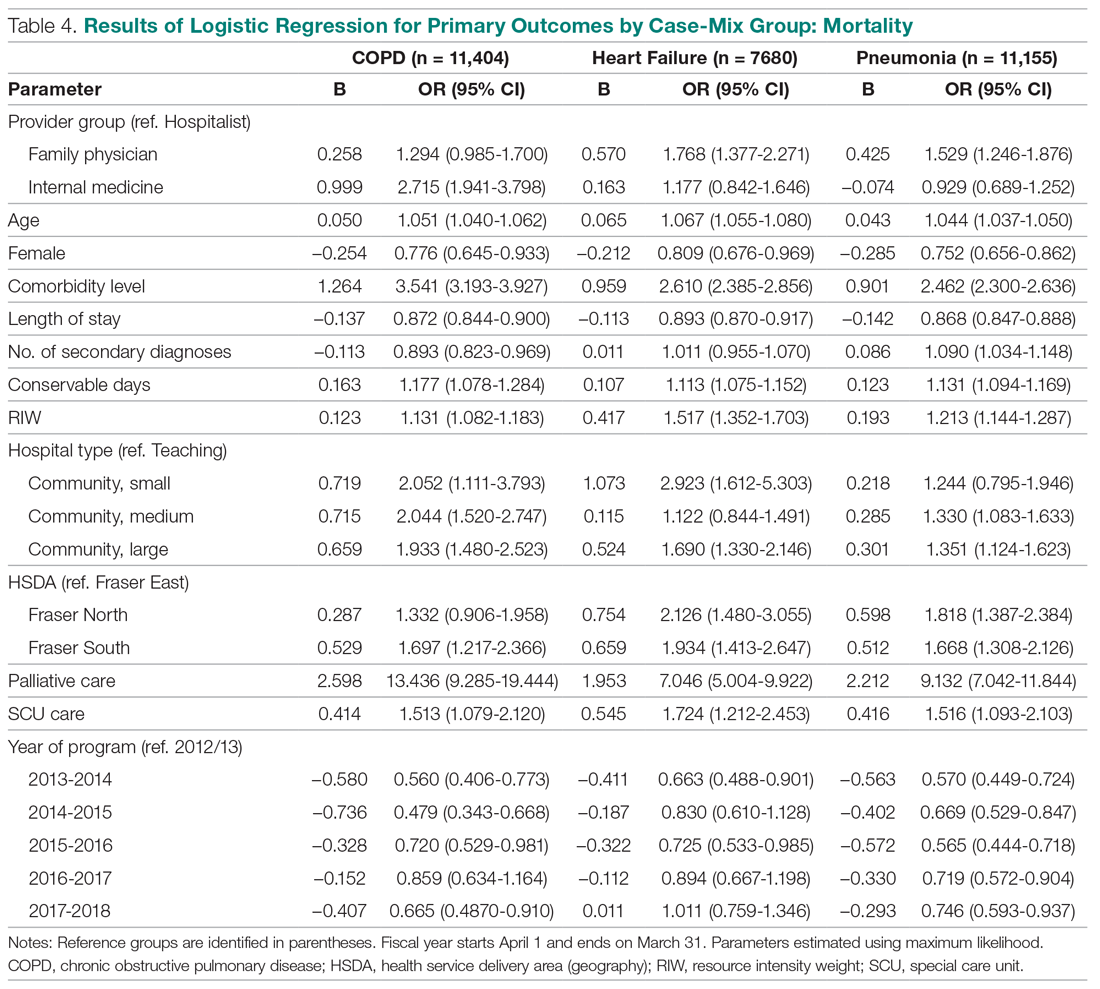
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
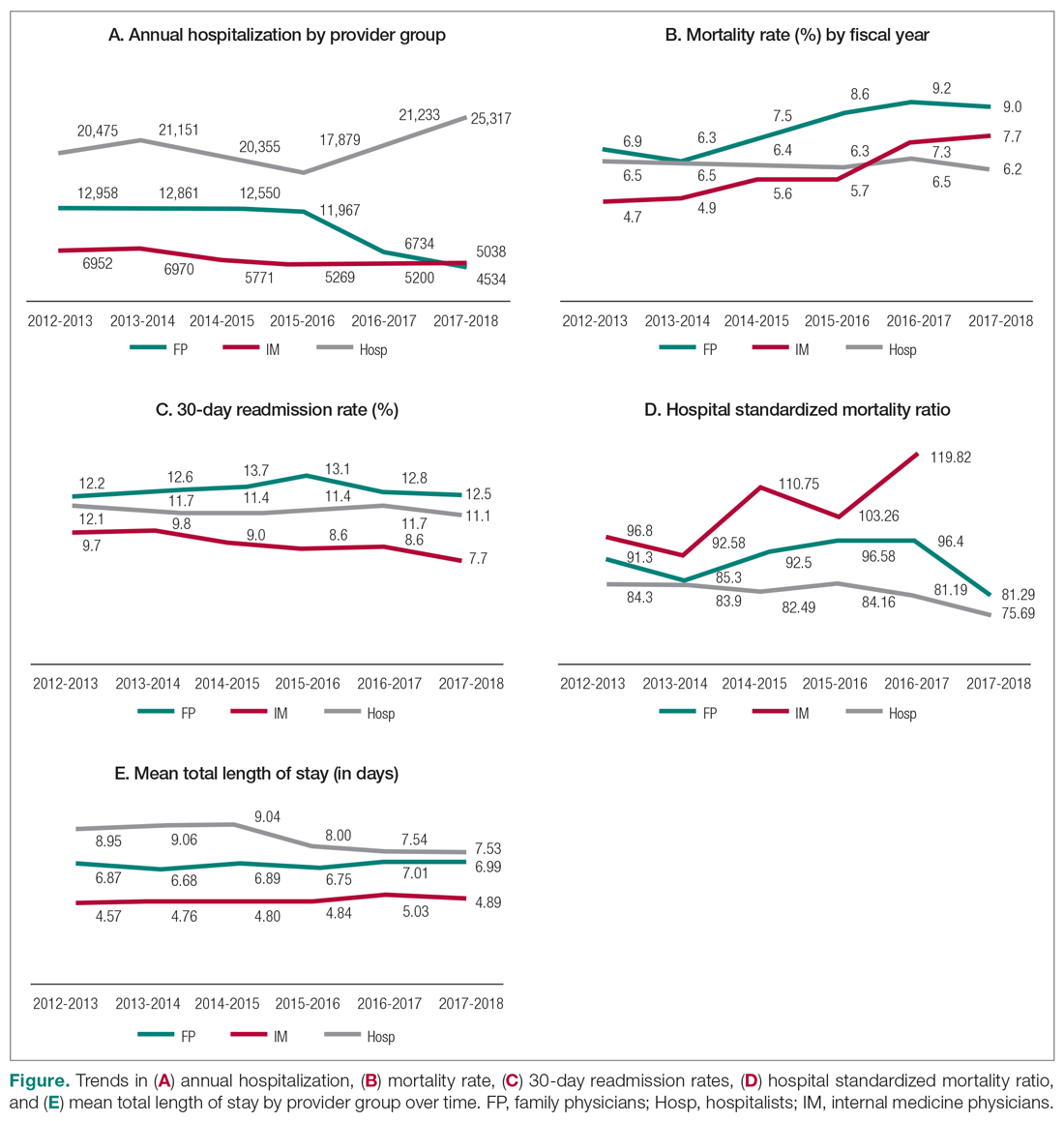
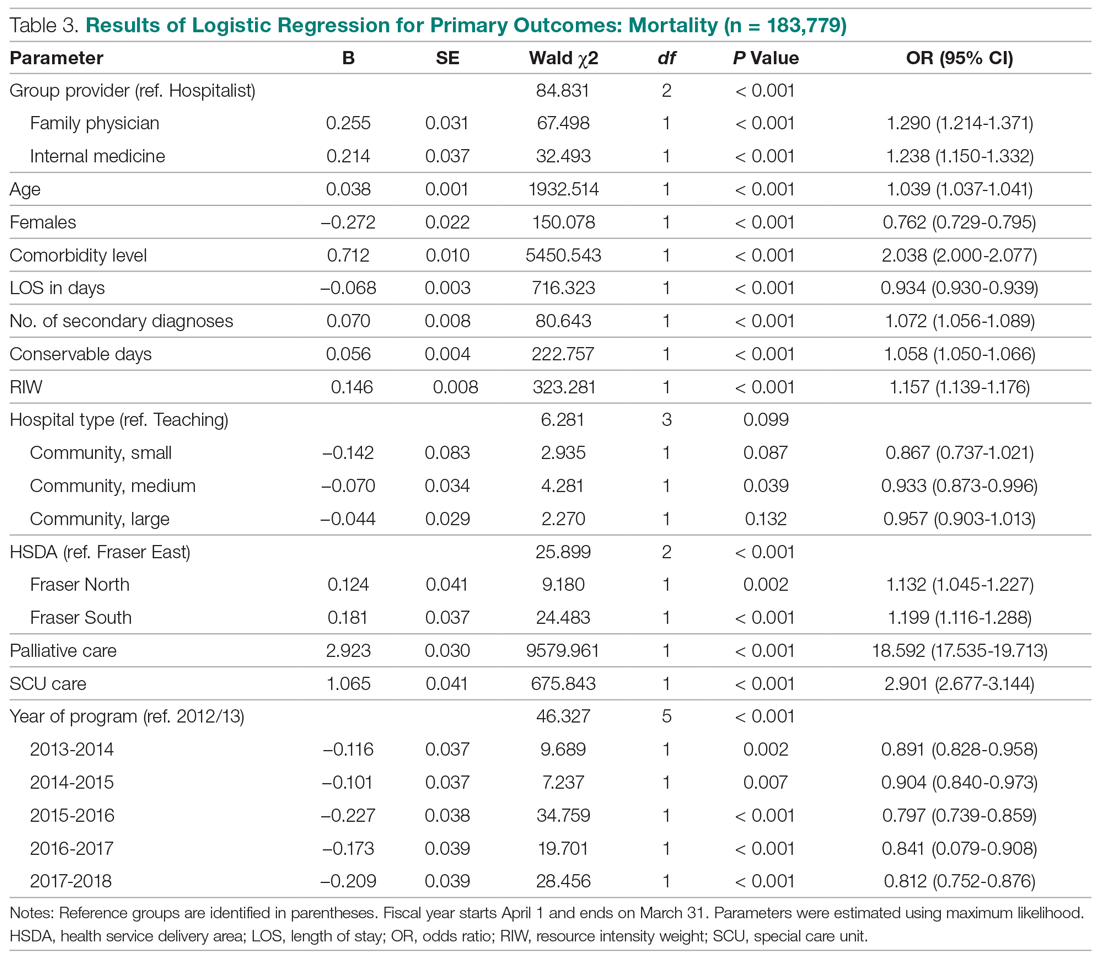
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
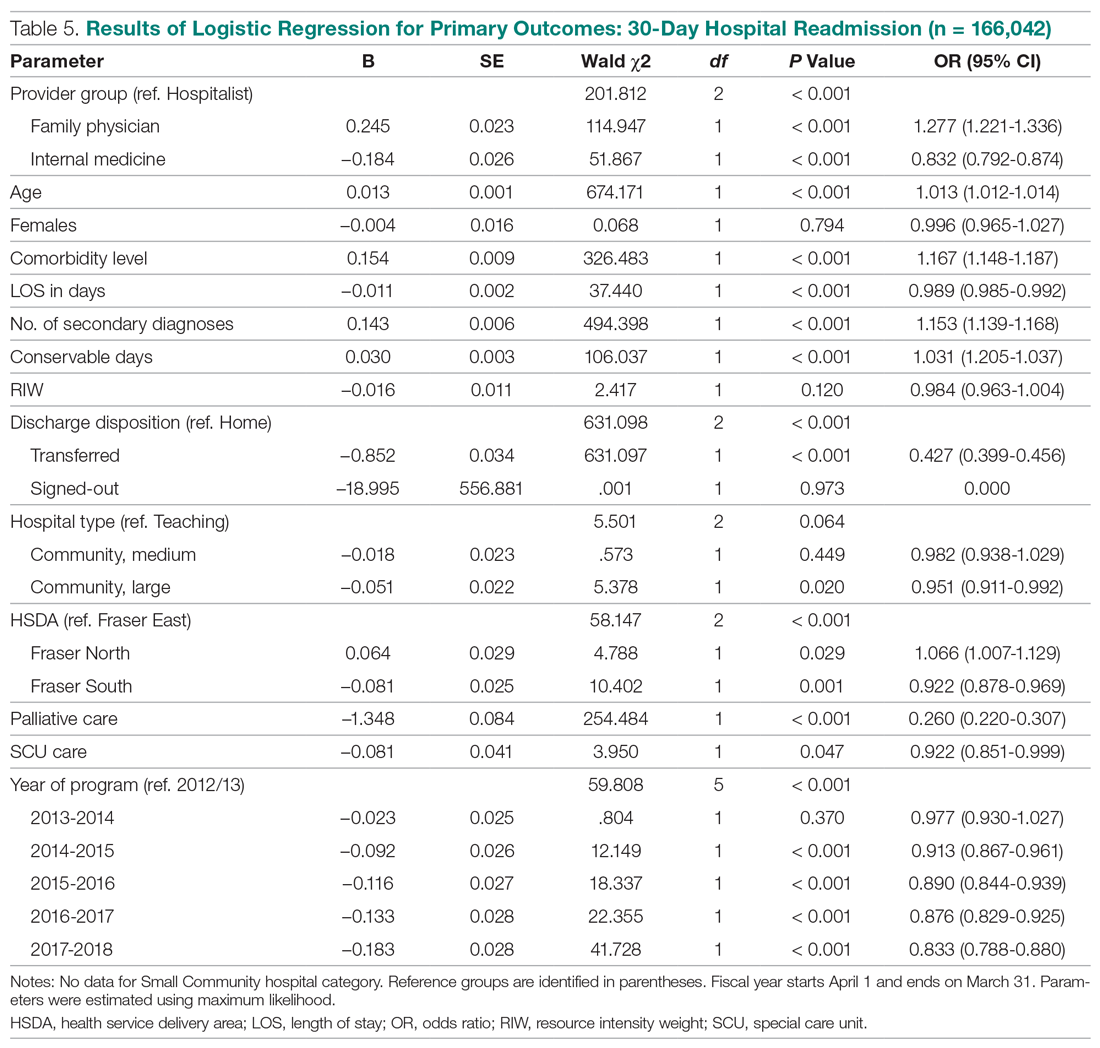
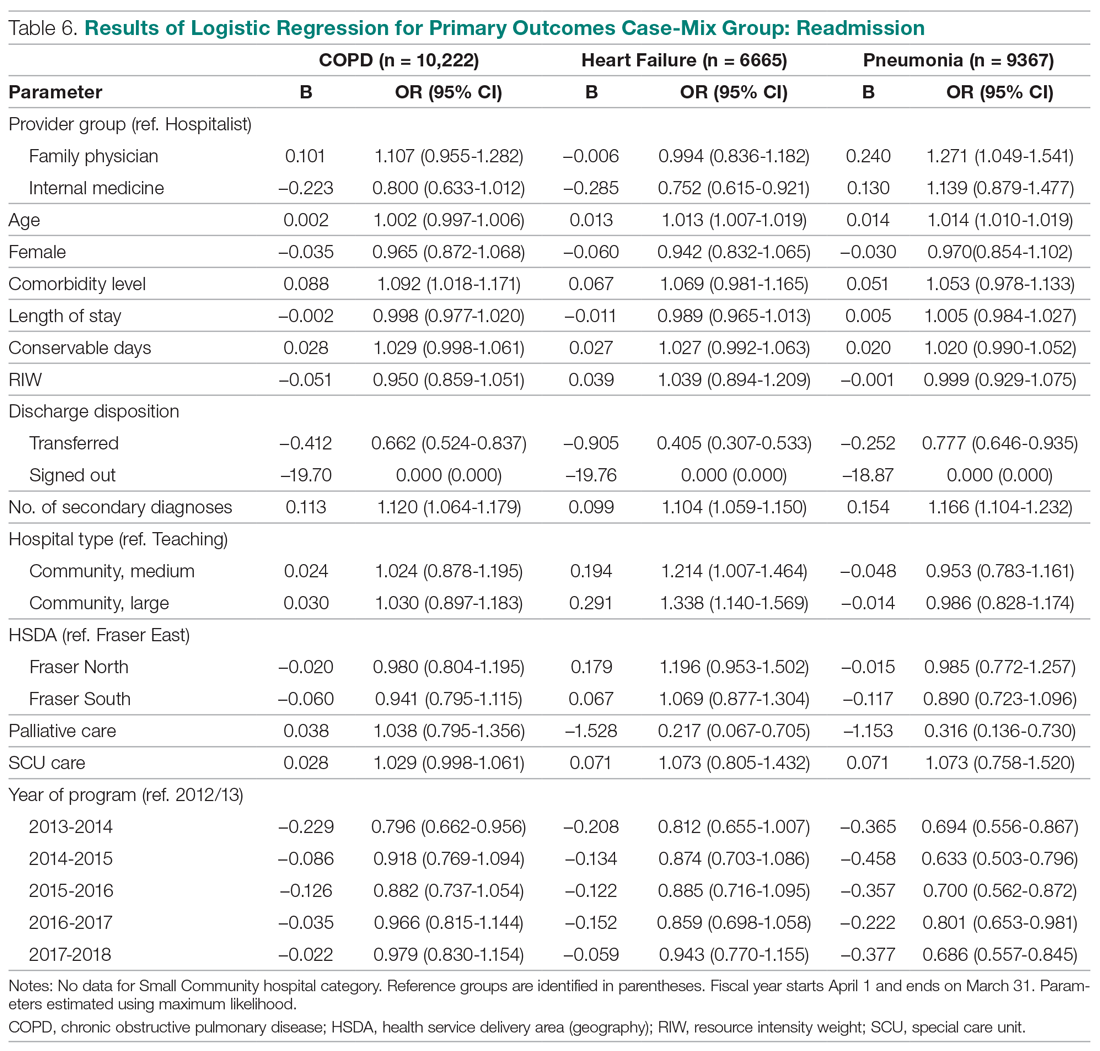
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
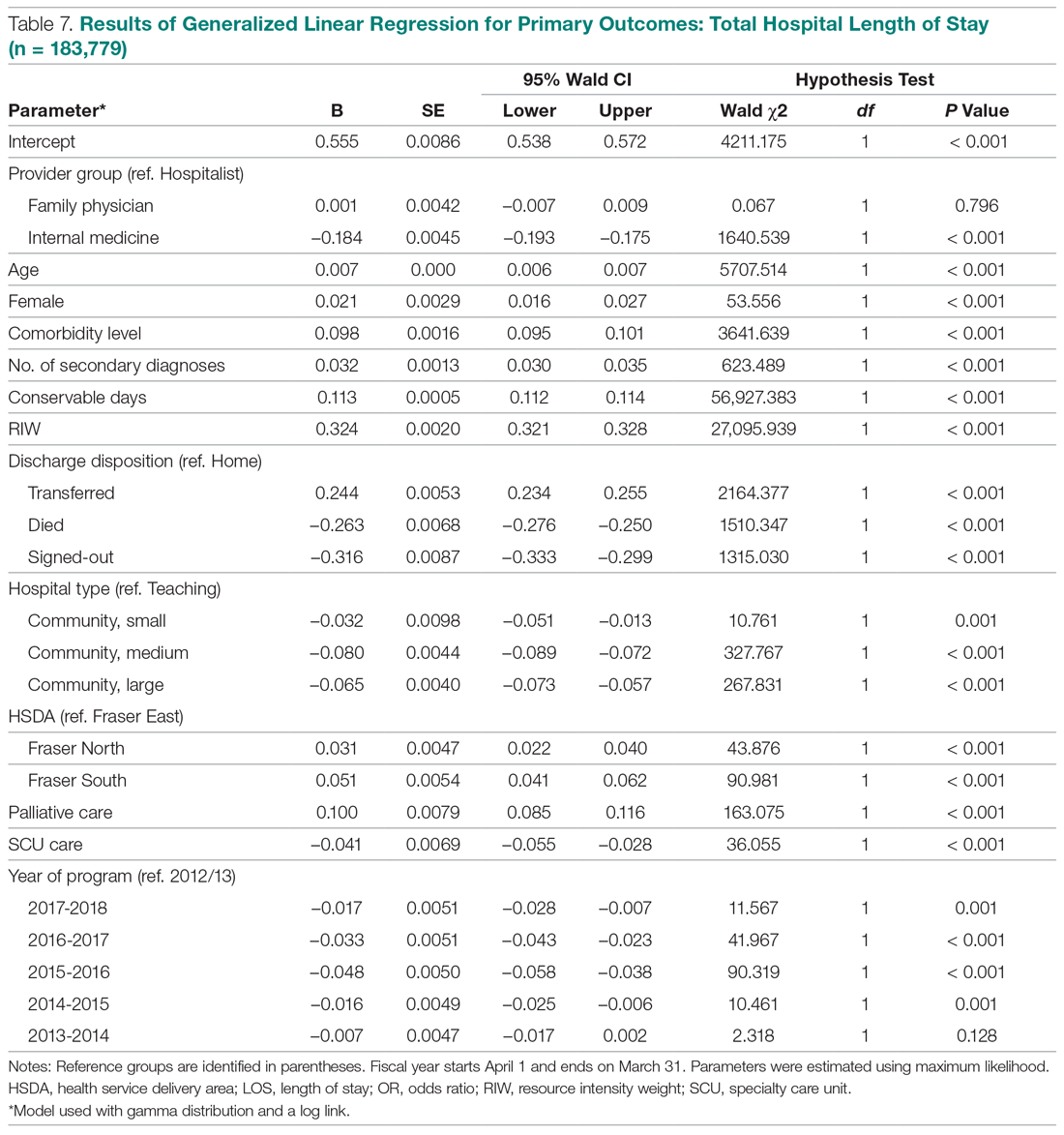
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
Conflicting Duties and Reciprocal Obligations During a Pandemic
The current COVID-19 pandemic has raised substantial anxieties and fears for healthcare workers, many of which they have not previously encountered. Important ethical issues have arisen involving the tension between their duties to their patients and their duties to themselves and to their loved ones. While these fears and duties are not unique to physicians or to members of one specialty, this article will focus on hospitalists. In general, hospitalists have an obligation to care for patients even if this puts them at risk, but duties to patients may at times be constrained by duties to others. At the same time, hospitals have correlative obligations to protect their employees and mitigate risk. Balancing these duties requires weighing benefits and risks, often in the context of considerable uncertainty. At this current time, it is conceivable that the risks may become so great that caring for patients is no longer obligatory but becomes heroic.
Conflicting duties arise in a variety of ways. Hospitalists are at increased risk of contracting the virus, given workplace exposures. The risk of complications is even higher for those who are older or have chronic medical conditions. Further, the shortage of personal protective equipment (PPE) adds to the overall risk. Hospitalists may also have concerns about transmitting the virus to family members or friends, especially to those who are elderly or have comorbidities. Hospitalists may also become physically and emotionally exhausted as work and home demands increase. Concerns for the care of dependents adds to the stress as daycares and schools close and older relatives are isolated in their homes. Hospitalists who are single parents and those whose partners are also healthcare workers are especially affected. The duty to care, encumbered by the cumulative stressors, creates an environment ripe for conflict.
DUTY TO CARE
Hospitalists have a duty to expose themselves to some, albeit not unlimited, risks. There are different ways of characterizing this obligation.1 Some base it in the knowledge and power differential between physicians and patients, a differential increased by patients’ illnesses. Others frame it as a social contract: physicians receive certain benefits and privileges and, in accepting them, incur certain duties. Physicians practicing in the 1980s may recall a similar discussion about treating patients with the human immunodeficiency virus (HIV), while those who practiced in other countries in the early 2000s faced a similar conflict during the severe acute respiratory syndrome (SARS) epidemic, caused by another coronavirus.2 The expectation of accepting risk may have been weakened in the last several decades, however, by the relative lack of risk in treating hospitalized patients in the United States.
DUTIES TO SELF AND OTHERS
Hospitalists’ duties to themselves and to their families are both intrinsically and instrumentally important. Being a hospitalist is not every hospitalist’s sole or predominant identity. They may also be adult children, spouses, and/or parents, or school board members or leaders in religious communities. Each of these roles entails its own duties and fulfilling them is also important. Effectuating them may, however, be more difficult because of the pandemic. Adult children may feel obligated to shop for their parents and parents of young children may have more childcare obligations. If no one else can fulfill these duties, they might take precedence over professional duties.
By fulfilling their duties to themselves and others, hospitalists may also be enabled to serve their patients. Unlike some discrete events, such as mass shootings or tornados, for which contingency and crisis standards of care may last for hours or days, we may be working under altered standards of care for weeks or months. (A contingency standard of care involves doing things differently in order to produce comparable clinical outcomes. A crisis standard of care is reached when it’s no longer possible to produce comparable clinical outcomes and the focus shifts from individual patient’s best interests or preferences to trying to save the most lives.3) It, therefore, is important we maintain our health and well-being by getting adequate sleep, eating well, and exercising.4 Arranging alternative child- and eldercare may reduce distractions at work and decrease the chance of needing to leave work unexpectedly.
MINIMIZING RISKS
In addressing these ethical issues, one of the key considerations is reducing the risks. We can reduce some risks ourselves while maintaining comparable outcomes to our conventional practices. I hope that it would go without saying, for example, that we should not work when we are sick. It is also important that we engage in adequate physical distancing whenever possible. It is important that physical distancing measures be applied equitably to all employees and that the actions hospitalists take to reduce their exposure do not disproportionately burden trainees or other types of providers. Consider, for example, having residents or nurse practitioners examine patients instead of the attending physician. This places subordinates at greater risk. Attending physicians, however, may have the best examination skills and their feedback is integral to trainees’ learning. Modeling a commitment to the duty to care and equitably accepting risk is exceptionally important as team members and leaders.
We can check in with one another and support each other emotionally. If some colleagues have substantially higher risks of complications, they may be assigned alternative duties with lower exposure risks. As a relatively young specialty, this may be more feasible for hospitalists than other specialties with a greater number of older practitioners. Care, however, should be taken to respect individuals’ privacy and confidentiality.
RECIPROCAL OBLIGATIONS
Minimizing risk is also a responsibility of hospitals and the local, state, and federal government. They have crucial roles in, for example, establishing adequate infection control policies and securing sufficient PPE. Many institutions have already moved to contingency standards of care to conserve PPE.5 These efforts not only support the duty of reciprocity6 but also help maintain an adequate workforce by reducing sick leave. The government’s apparent failure to fulfill its obligation to stockpile and distribute adequate equipment is currently being acutely felt.7
There are other potential actions that facilities can take, such as providing scrubs, child- and eldercare, housing, or life insurance. Individuals may be concerned about infecting family members. There is unfortunately limited data about spread on objects or asymptomatic spread, but these are reasonable possibilities. Facilities can provide scrubs to employees who do not normally wear them to provide a further barrier between the facility and the employees’ homes. They can provide child and elder care. It has been wonderful to see local community organizations and groups of medical students provide childcare for healthcare workers and other essential employees.8 Healthcare facilities could also consider providing temporary housing to staff with family members at high risk of complications. During the Ebola outbreak, some facilities provided supplemental disability and life insurance to staff who volunteered to put themselves at risk to help assure that their families would be provided for if the staff member unfortunately contracted the virus and became disabled or died.
Reciprocal duties to healthcare workers in a crisis standard of care are unresolved. Establishing ethically and clinically sound ventilator triage criteria is complex.9,10 Some argue that healthcare providers should have some degree of priority. One argument is that if they recover, they can return to work and save more lives. (Having individuals who have recovered and are theoretically immune treat patients without PPE is one proposed conservation strategy.) It is, however, unclear whether individuals are likely to recover in enough time to return to work while we are still in a crisis standard of care. A different argument is that healthcare workers should be given priority because they accepted risk. This assumes they were infected at work and not in the community. While this argument has merit, it could be influenced by or perceived to be influenced by self-interest. Prioritizing healthcare workers for scarce resources requires substantial community support.11
LIMITATIONS
While providers have a duty to accept some risks, this duty is not unlimited. The mitigation strategies may be unsuccessful, and the risks substantial. One can think of analogies in other fields. Firefighters, for example, expose themselves to risk to save lives and to protect property. They are trained to take calculated risks, considering the likelihood and type of benefit and the degree of risk, but not to be reckless. They will take greater risk to save a life than property, and less risk if the victim is unlikely to survive. Their obligation to accept risk is not unlimited. They may justifiably choose not to enter a building, which is at significant, imminent risk of collapse, to protect property. The same is true for physicians. They are obligated to expose themselves to some risk, but not at a high likelihood of serious injury or death. At some point the duty to care for patients becomes supererogatory; fulfilling the duty is no longer required but becomes optional and doing so is heroic.12 Some facilities, for example, will not perform cardiopulmonary resuscitation under a crisis standard of care due to the high risk of exposure and the low likelihood of success.13
DECISION-MAKING PROCESS
Weighing potential benefits and risk is difficult. This difficulty is exacerbated by uncertainty. Some decisions would be easier, for example, if there was better evidence regarding asymptomatic spread. Finally, the subjectivity of some of these decisions raises concerns about unconscious bias or self-interest. It is therefore valuable to make some decisions collectively rather than individually. In particular, it is important to include those with adequate situation awareness. Conversely, once decisions are made, it is valuable to communicate both the decision and its rationale, and to be open to revising them based on feedback.
Given the fear and uncertainty generated by the pandemic, some individuals may be tempted to act unethically. Individuals have, unfortunately, taken hospital supplies, such as masks and hand sanitizer, for household use, and healthcare providers have hoarded medications, such as hydroxychloroquine.14 Individuals may also be tempted to use PPE for encounters when it is not indicated. We should address these fears and anxieties in other ways, such as discussing them with colleagues, chaplains, social workers, or employee assistance programs. If you observe coworkers acting in a manner that appears to be unethical, it is important to address their behavior while still giving them the benefit of the doubt. If you do not receive a satisfactory response, you should utilize the appropriate chain of command.
CONCLUSIONS
Most hospitalists are encountering situations that they have not previously experienced in their careers. These situations generate significant fear and anxiety. Many of these situations involve tensions between our duties to our patients and our duties to ourselves and to our families and friends. This tension is heightened for individuals who are older or have chronic health conditions or have family members who are. While healthcare providers have an obligation to accept some risks, this duty is not unlimited. Hospitals, healthcare systems, and governments have reciprocal obligations to keep providers safe. It is important to think creatively about ways to minimize risk. Due to uncertainty and self-interest, it may be better to make decisions collectively and transparently.
1. Malm H, May T, Francis LP, Omer SB, Salmon DA, Hood R. Ethics, pandemics, and the duty to treat. Am J Bioeth. 2008;8(8):4-19. https://doi:10.1080/15265160802317974.
2. Dwyer J, Tsai DF. Developing the duty to treat: HIV, SARS, and the next epidemic. J Med Ethics. 2008;34(1):7-10. https://doi: 10.1136/jme.2006.018978.
3. Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 Suppl):S59–S67. https://doi:10.1097/DMP.0b013e31819f1ae2.
4. Centers for Disease Control and Prevention. Emergency Responders: Tips for Taking Care of Yourself. March 19, 2018. https://emergency.cdc.gov/coping/responders.asp. Accessed March 30, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease 2109 (COVID-19): Facemasks. March 17, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html. Accessed March 30, 2020.
6. Pandemic Influenza Working Group. Stand on Guard for Thee: Ethical Considerations in Preparedness Planning for Pandemic Influenza. Toronto: University of Toronto Joint Centre for Bioethics; 2005. http://www.jcb.utoronto.ca/people/documents/upshur_stand_guard.pdf. Accessed March 30, 2020.
7. Miroff N. Protective gear in national stockpile is nearly depleted, DHS officials say. The Washington Post. April 1, 2020. https://www.washingtonpost.com/national/coronavirus-protective-gear-stockpile-depleted/2020/04/01/44d6592a-741f-11ea-ae50-7148009252e3_story.html. Accessed April 2, 2020.
8. Lewis T. Medical students provide childcare for healthcare professionals during COVID-19 pandemic. Fox 5 DC. March 27, 2020. https://www.fox5dc.com/news/medical-students-provide-childcare-for-healthcare-professionals-during-covid-19-pandemic. Accessed March 30, 2020.
9. New York State Task Force on Life and the Law. Ventilator Allocation Guidelines. New York: New York State Department of Health; 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Accessed March 30, 2020.
10. Antommaria AH, Powell T, Miller JE, Christian MD, Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi:10.1097/PCC.0b013e318234a88b.
11. Rothstein, MA. Currents in contemporary ethics. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010; 38(2):412-419. https://doi:10.1111/j.1748-720X.2010.00499.x.
12. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5.
13. Cha AE. Hospitals consider universal do-not-resuscitate orders for coronavirus patient. The Washington Post. March 25, 2020. https://www.washingtonpost.com/health/2020/03/25/coronavirus-patients-do-not-resucitate/. Accessed March 30, 2020.
14. Sanders T, Armstrong D, Kofman A. Doctors are hoarding unproven coronavirus medicine by writing prescriptions for themselves and their families. ProPublica. March 24, 2020. https://www.propublica.org/article/doctors-are-hoarding-unproven-coronavirus-medicine-by-writing-prescriptions-for-themselves-and-their-families. Accessed March 30, 2020.
The current COVID-19 pandemic has raised substantial anxieties and fears for healthcare workers, many of which they have not previously encountered. Important ethical issues have arisen involving the tension between their duties to their patients and their duties to themselves and to their loved ones. While these fears and duties are not unique to physicians or to members of one specialty, this article will focus on hospitalists. In general, hospitalists have an obligation to care for patients even if this puts them at risk, but duties to patients may at times be constrained by duties to others. At the same time, hospitals have correlative obligations to protect their employees and mitigate risk. Balancing these duties requires weighing benefits and risks, often in the context of considerable uncertainty. At this current time, it is conceivable that the risks may become so great that caring for patients is no longer obligatory but becomes heroic.
Conflicting duties arise in a variety of ways. Hospitalists are at increased risk of contracting the virus, given workplace exposures. The risk of complications is even higher for those who are older or have chronic medical conditions. Further, the shortage of personal protective equipment (PPE) adds to the overall risk. Hospitalists may also have concerns about transmitting the virus to family members or friends, especially to those who are elderly or have comorbidities. Hospitalists may also become physically and emotionally exhausted as work and home demands increase. Concerns for the care of dependents adds to the stress as daycares and schools close and older relatives are isolated in their homes. Hospitalists who are single parents and those whose partners are also healthcare workers are especially affected. The duty to care, encumbered by the cumulative stressors, creates an environment ripe for conflict.
DUTY TO CARE
Hospitalists have a duty to expose themselves to some, albeit not unlimited, risks. There are different ways of characterizing this obligation.1 Some base it in the knowledge and power differential between physicians and patients, a differential increased by patients’ illnesses. Others frame it as a social contract: physicians receive certain benefits and privileges and, in accepting them, incur certain duties. Physicians practicing in the 1980s may recall a similar discussion about treating patients with the human immunodeficiency virus (HIV), while those who practiced in other countries in the early 2000s faced a similar conflict during the severe acute respiratory syndrome (SARS) epidemic, caused by another coronavirus.2 The expectation of accepting risk may have been weakened in the last several decades, however, by the relative lack of risk in treating hospitalized patients in the United States.
DUTIES TO SELF AND OTHERS
Hospitalists’ duties to themselves and to their families are both intrinsically and instrumentally important. Being a hospitalist is not every hospitalist’s sole or predominant identity. They may also be adult children, spouses, and/or parents, or school board members or leaders in religious communities. Each of these roles entails its own duties and fulfilling them is also important. Effectuating them may, however, be more difficult because of the pandemic. Adult children may feel obligated to shop for their parents and parents of young children may have more childcare obligations. If no one else can fulfill these duties, they might take precedence over professional duties.
By fulfilling their duties to themselves and others, hospitalists may also be enabled to serve their patients. Unlike some discrete events, such as mass shootings or tornados, for which contingency and crisis standards of care may last for hours or days, we may be working under altered standards of care for weeks or months. (A contingency standard of care involves doing things differently in order to produce comparable clinical outcomes. A crisis standard of care is reached when it’s no longer possible to produce comparable clinical outcomes and the focus shifts from individual patient’s best interests or preferences to trying to save the most lives.3) It, therefore, is important we maintain our health and well-being by getting adequate sleep, eating well, and exercising.4 Arranging alternative child- and eldercare may reduce distractions at work and decrease the chance of needing to leave work unexpectedly.
MINIMIZING RISKS
In addressing these ethical issues, one of the key considerations is reducing the risks. We can reduce some risks ourselves while maintaining comparable outcomes to our conventional practices. I hope that it would go without saying, for example, that we should not work when we are sick. It is also important that we engage in adequate physical distancing whenever possible. It is important that physical distancing measures be applied equitably to all employees and that the actions hospitalists take to reduce their exposure do not disproportionately burden trainees or other types of providers. Consider, for example, having residents or nurse practitioners examine patients instead of the attending physician. This places subordinates at greater risk. Attending physicians, however, may have the best examination skills and their feedback is integral to trainees’ learning. Modeling a commitment to the duty to care and equitably accepting risk is exceptionally important as team members and leaders.
We can check in with one another and support each other emotionally. If some colleagues have substantially higher risks of complications, they may be assigned alternative duties with lower exposure risks. As a relatively young specialty, this may be more feasible for hospitalists than other specialties with a greater number of older practitioners. Care, however, should be taken to respect individuals’ privacy and confidentiality.
RECIPROCAL OBLIGATIONS
Minimizing risk is also a responsibility of hospitals and the local, state, and federal government. They have crucial roles in, for example, establishing adequate infection control policies and securing sufficient PPE. Many institutions have already moved to contingency standards of care to conserve PPE.5 These efforts not only support the duty of reciprocity6 but also help maintain an adequate workforce by reducing sick leave. The government’s apparent failure to fulfill its obligation to stockpile and distribute adequate equipment is currently being acutely felt.7
There are other potential actions that facilities can take, such as providing scrubs, child- and eldercare, housing, or life insurance. Individuals may be concerned about infecting family members. There is unfortunately limited data about spread on objects or asymptomatic spread, but these are reasonable possibilities. Facilities can provide scrubs to employees who do not normally wear them to provide a further barrier between the facility and the employees’ homes. They can provide child and elder care. It has been wonderful to see local community organizations and groups of medical students provide childcare for healthcare workers and other essential employees.8 Healthcare facilities could also consider providing temporary housing to staff with family members at high risk of complications. During the Ebola outbreak, some facilities provided supplemental disability and life insurance to staff who volunteered to put themselves at risk to help assure that their families would be provided for if the staff member unfortunately contracted the virus and became disabled or died.
Reciprocal duties to healthcare workers in a crisis standard of care are unresolved. Establishing ethically and clinically sound ventilator triage criteria is complex.9,10 Some argue that healthcare providers should have some degree of priority. One argument is that if they recover, they can return to work and save more lives. (Having individuals who have recovered and are theoretically immune treat patients without PPE is one proposed conservation strategy.) It is, however, unclear whether individuals are likely to recover in enough time to return to work while we are still in a crisis standard of care. A different argument is that healthcare workers should be given priority because they accepted risk. This assumes they were infected at work and not in the community. While this argument has merit, it could be influenced by or perceived to be influenced by self-interest. Prioritizing healthcare workers for scarce resources requires substantial community support.11
LIMITATIONS
While providers have a duty to accept some risks, this duty is not unlimited. The mitigation strategies may be unsuccessful, and the risks substantial. One can think of analogies in other fields. Firefighters, for example, expose themselves to risk to save lives and to protect property. They are trained to take calculated risks, considering the likelihood and type of benefit and the degree of risk, but not to be reckless. They will take greater risk to save a life than property, and less risk if the victim is unlikely to survive. Their obligation to accept risk is not unlimited. They may justifiably choose not to enter a building, which is at significant, imminent risk of collapse, to protect property. The same is true for physicians. They are obligated to expose themselves to some risk, but not at a high likelihood of serious injury or death. At some point the duty to care for patients becomes supererogatory; fulfilling the duty is no longer required but becomes optional and doing so is heroic.12 Some facilities, for example, will not perform cardiopulmonary resuscitation under a crisis standard of care due to the high risk of exposure and the low likelihood of success.13
DECISION-MAKING PROCESS
Weighing potential benefits and risk is difficult. This difficulty is exacerbated by uncertainty. Some decisions would be easier, for example, if there was better evidence regarding asymptomatic spread. Finally, the subjectivity of some of these decisions raises concerns about unconscious bias or self-interest. It is therefore valuable to make some decisions collectively rather than individually. In particular, it is important to include those with adequate situation awareness. Conversely, once decisions are made, it is valuable to communicate both the decision and its rationale, and to be open to revising them based on feedback.
Given the fear and uncertainty generated by the pandemic, some individuals may be tempted to act unethically. Individuals have, unfortunately, taken hospital supplies, such as masks and hand sanitizer, for household use, and healthcare providers have hoarded medications, such as hydroxychloroquine.14 Individuals may also be tempted to use PPE for encounters when it is not indicated. We should address these fears and anxieties in other ways, such as discussing them with colleagues, chaplains, social workers, or employee assistance programs. If you observe coworkers acting in a manner that appears to be unethical, it is important to address their behavior while still giving them the benefit of the doubt. If you do not receive a satisfactory response, you should utilize the appropriate chain of command.
CONCLUSIONS
Most hospitalists are encountering situations that they have not previously experienced in their careers. These situations generate significant fear and anxiety. Many of these situations involve tensions between our duties to our patients and our duties to ourselves and to our families and friends. This tension is heightened for individuals who are older or have chronic health conditions or have family members who are. While healthcare providers have an obligation to accept some risks, this duty is not unlimited. Hospitals, healthcare systems, and governments have reciprocal obligations to keep providers safe. It is important to think creatively about ways to minimize risk. Due to uncertainty and self-interest, it may be better to make decisions collectively and transparently.
The current COVID-19 pandemic has raised substantial anxieties and fears for healthcare workers, many of which they have not previously encountered. Important ethical issues have arisen involving the tension between their duties to their patients and their duties to themselves and to their loved ones. While these fears and duties are not unique to physicians or to members of one specialty, this article will focus on hospitalists. In general, hospitalists have an obligation to care for patients even if this puts them at risk, but duties to patients may at times be constrained by duties to others. At the same time, hospitals have correlative obligations to protect their employees and mitigate risk. Balancing these duties requires weighing benefits and risks, often in the context of considerable uncertainty. At this current time, it is conceivable that the risks may become so great that caring for patients is no longer obligatory but becomes heroic.
Conflicting duties arise in a variety of ways. Hospitalists are at increased risk of contracting the virus, given workplace exposures. The risk of complications is even higher for those who are older or have chronic medical conditions. Further, the shortage of personal protective equipment (PPE) adds to the overall risk. Hospitalists may also have concerns about transmitting the virus to family members or friends, especially to those who are elderly or have comorbidities. Hospitalists may also become physically and emotionally exhausted as work and home demands increase. Concerns for the care of dependents adds to the stress as daycares and schools close and older relatives are isolated in their homes. Hospitalists who are single parents and those whose partners are also healthcare workers are especially affected. The duty to care, encumbered by the cumulative stressors, creates an environment ripe for conflict.
DUTY TO CARE
Hospitalists have a duty to expose themselves to some, albeit not unlimited, risks. There are different ways of characterizing this obligation.1 Some base it in the knowledge and power differential between physicians and patients, a differential increased by patients’ illnesses. Others frame it as a social contract: physicians receive certain benefits and privileges and, in accepting them, incur certain duties. Physicians practicing in the 1980s may recall a similar discussion about treating patients with the human immunodeficiency virus (HIV), while those who practiced in other countries in the early 2000s faced a similar conflict during the severe acute respiratory syndrome (SARS) epidemic, caused by another coronavirus.2 The expectation of accepting risk may have been weakened in the last several decades, however, by the relative lack of risk in treating hospitalized patients in the United States.
DUTIES TO SELF AND OTHERS
Hospitalists’ duties to themselves and to their families are both intrinsically and instrumentally important. Being a hospitalist is not every hospitalist’s sole or predominant identity. They may also be adult children, spouses, and/or parents, or school board members or leaders in religious communities. Each of these roles entails its own duties and fulfilling them is also important. Effectuating them may, however, be more difficult because of the pandemic. Adult children may feel obligated to shop for their parents and parents of young children may have more childcare obligations. If no one else can fulfill these duties, they might take precedence over professional duties.
By fulfilling their duties to themselves and others, hospitalists may also be enabled to serve their patients. Unlike some discrete events, such as mass shootings or tornados, for which contingency and crisis standards of care may last for hours or days, we may be working under altered standards of care for weeks or months. (A contingency standard of care involves doing things differently in order to produce comparable clinical outcomes. A crisis standard of care is reached when it’s no longer possible to produce comparable clinical outcomes and the focus shifts from individual patient’s best interests or preferences to trying to save the most lives.3) It, therefore, is important we maintain our health and well-being by getting adequate sleep, eating well, and exercising.4 Arranging alternative child- and eldercare may reduce distractions at work and decrease the chance of needing to leave work unexpectedly.
MINIMIZING RISKS
In addressing these ethical issues, one of the key considerations is reducing the risks. We can reduce some risks ourselves while maintaining comparable outcomes to our conventional practices. I hope that it would go without saying, for example, that we should not work when we are sick. It is also important that we engage in adequate physical distancing whenever possible. It is important that physical distancing measures be applied equitably to all employees and that the actions hospitalists take to reduce their exposure do not disproportionately burden trainees or other types of providers. Consider, for example, having residents or nurse practitioners examine patients instead of the attending physician. This places subordinates at greater risk. Attending physicians, however, may have the best examination skills and their feedback is integral to trainees’ learning. Modeling a commitment to the duty to care and equitably accepting risk is exceptionally important as team members and leaders.
We can check in with one another and support each other emotionally. If some colleagues have substantially higher risks of complications, they may be assigned alternative duties with lower exposure risks. As a relatively young specialty, this may be more feasible for hospitalists than other specialties with a greater number of older practitioners. Care, however, should be taken to respect individuals’ privacy and confidentiality.
RECIPROCAL OBLIGATIONS
Minimizing risk is also a responsibility of hospitals and the local, state, and federal government. They have crucial roles in, for example, establishing adequate infection control policies and securing sufficient PPE. Many institutions have already moved to contingency standards of care to conserve PPE.5 These efforts not only support the duty of reciprocity6 but also help maintain an adequate workforce by reducing sick leave. The government’s apparent failure to fulfill its obligation to stockpile and distribute adequate equipment is currently being acutely felt.7
There are other potential actions that facilities can take, such as providing scrubs, child- and eldercare, housing, or life insurance. Individuals may be concerned about infecting family members. There is unfortunately limited data about spread on objects or asymptomatic spread, but these are reasonable possibilities. Facilities can provide scrubs to employees who do not normally wear them to provide a further barrier between the facility and the employees’ homes. They can provide child and elder care. It has been wonderful to see local community organizations and groups of medical students provide childcare for healthcare workers and other essential employees.8 Healthcare facilities could also consider providing temporary housing to staff with family members at high risk of complications. During the Ebola outbreak, some facilities provided supplemental disability and life insurance to staff who volunteered to put themselves at risk to help assure that their families would be provided for if the staff member unfortunately contracted the virus and became disabled or died.
Reciprocal duties to healthcare workers in a crisis standard of care are unresolved. Establishing ethically and clinically sound ventilator triage criteria is complex.9,10 Some argue that healthcare providers should have some degree of priority. One argument is that if they recover, they can return to work and save more lives. (Having individuals who have recovered and are theoretically immune treat patients without PPE is one proposed conservation strategy.) It is, however, unclear whether individuals are likely to recover in enough time to return to work while we are still in a crisis standard of care. A different argument is that healthcare workers should be given priority because they accepted risk. This assumes they were infected at work and not in the community. While this argument has merit, it could be influenced by or perceived to be influenced by self-interest. Prioritizing healthcare workers for scarce resources requires substantial community support.11
LIMITATIONS
While providers have a duty to accept some risks, this duty is not unlimited. The mitigation strategies may be unsuccessful, and the risks substantial. One can think of analogies in other fields. Firefighters, for example, expose themselves to risk to save lives and to protect property. They are trained to take calculated risks, considering the likelihood and type of benefit and the degree of risk, but not to be reckless. They will take greater risk to save a life than property, and less risk if the victim is unlikely to survive. Their obligation to accept risk is not unlimited. They may justifiably choose not to enter a building, which is at significant, imminent risk of collapse, to protect property. The same is true for physicians. They are obligated to expose themselves to some risk, but not at a high likelihood of serious injury or death. At some point the duty to care for patients becomes supererogatory; fulfilling the duty is no longer required but becomes optional and doing so is heroic.12 Some facilities, for example, will not perform cardiopulmonary resuscitation under a crisis standard of care due to the high risk of exposure and the low likelihood of success.13
DECISION-MAKING PROCESS
Weighing potential benefits and risk is difficult. This difficulty is exacerbated by uncertainty. Some decisions would be easier, for example, if there was better evidence regarding asymptomatic spread. Finally, the subjectivity of some of these decisions raises concerns about unconscious bias or self-interest. It is therefore valuable to make some decisions collectively rather than individually. In particular, it is important to include those with adequate situation awareness. Conversely, once decisions are made, it is valuable to communicate both the decision and its rationale, and to be open to revising them based on feedback.
Given the fear and uncertainty generated by the pandemic, some individuals may be tempted to act unethically. Individuals have, unfortunately, taken hospital supplies, such as masks and hand sanitizer, for household use, and healthcare providers have hoarded medications, such as hydroxychloroquine.14 Individuals may also be tempted to use PPE for encounters when it is not indicated. We should address these fears and anxieties in other ways, such as discussing them with colleagues, chaplains, social workers, or employee assistance programs. If you observe coworkers acting in a manner that appears to be unethical, it is important to address their behavior while still giving them the benefit of the doubt. If you do not receive a satisfactory response, you should utilize the appropriate chain of command.
CONCLUSIONS
Most hospitalists are encountering situations that they have not previously experienced in their careers. These situations generate significant fear and anxiety. Many of these situations involve tensions between our duties to our patients and our duties to ourselves and to our families and friends. This tension is heightened for individuals who are older or have chronic health conditions or have family members who are. While healthcare providers have an obligation to accept some risks, this duty is not unlimited. Hospitals, healthcare systems, and governments have reciprocal obligations to keep providers safe. It is important to think creatively about ways to minimize risk. Due to uncertainty and self-interest, it may be better to make decisions collectively and transparently.
1. Malm H, May T, Francis LP, Omer SB, Salmon DA, Hood R. Ethics, pandemics, and the duty to treat. Am J Bioeth. 2008;8(8):4-19. https://doi:10.1080/15265160802317974.
2. Dwyer J, Tsai DF. Developing the duty to treat: HIV, SARS, and the next epidemic. J Med Ethics. 2008;34(1):7-10. https://doi: 10.1136/jme.2006.018978.
3. Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 Suppl):S59–S67. https://doi:10.1097/DMP.0b013e31819f1ae2.
4. Centers for Disease Control and Prevention. Emergency Responders: Tips for Taking Care of Yourself. March 19, 2018. https://emergency.cdc.gov/coping/responders.asp. Accessed March 30, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease 2109 (COVID-19): Facemasks. March 17, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html. Accessed March 30, 2020.
6. Pandemic Influenza Working Group. Stand on Guard for Thee: Ethical Considerations in Preparedness Planning for Pandemic Influenza. Toronto: University of Toronto Joint Centre for Bioethics; 2005. http://www.jcb.utoronto.ca/people/documents/upshur_stand_guard.pdf. Accessed March 30, 2020.
7. Miroff N. Protective gear in national stockpile is nearly depleted, DHS officials say. The Washington Post. April 1, 2020. https://www.washingtonpost.com/national/coronavirus-protective-gear-stockpile-depleted/2020/04/01/44d6592a-741f-11ea-ae50-7148009252e3_story.html. Accessed April 2, 2020.
8. Lewis T. Medical students provide childcare for healthcare professionals during COVID-19 pandemic. Fox 5 DC. March 27, 2020. https://www.fox5dc.com/news/medical-students-provide-childcare-for-healthcare-professionals-during-covid-19-pandemic. Accessed March 30, 2020.
9. New York State Task Force on Life and the Law. Ventilator Allocation Guidelines. New York: New York State Department of Health; 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Accessed March 30, 2020.
10. Antommaria AH, Powell T, Miller JE, Christian MD, Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi:10.1097/PCC.0b013e318234a88b.
11. Rothstein, MA. Currents in contemporary ethics. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010; 38(2):412-419. https://doi:10.1111/j.1748-720X.2010.00499.x.
12. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5.
13. Cha AE. Hospitals consider universal do-not-resuscitate orders for coronavirus patient. The Washington Post. March 25, 2020. https://www.washingtonpost.com/health/2020/03/25/coronavirus-patients-do-not-resucitate/. Accessed March 30, 2020.
14. Sanders T, Armstrong D, Kofman A. Doctors are hoarding unproven coronavirus medicine by writing prescriptions for themselves and their families. ProPublica. March 24, 2020. https://www.propublica.org/article/doctors-are-hoarding-unproven-coronavirus-medicine-by-writing-prescriptions-for-themselves-and-their-families. Accessed March 30, 2020.
1. Malm H, May T, Francis LP, Omer SB, Salmon DA, Hood R. Ethics, pandemics, and the duty to treat. Am J Bioeth. 2008;8(8):4-19. https://doi:10.1080/15265160802317974.
2. Dwyer J, Tsai DF. Developing the duty to treat: HIV, SARS, and the next epidemic. J Med Ethics. 2008;34(1):7-10. https://doi: 10.1136/jme.2006.018978.
3. Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 Suppl):S59–S67. https://doi:10.1097/DMP.0b013e31819f1ae2.
4. Centers for Disease Control and Prevention. Emergency Responders: Tips for Taking Care of Yourself. March 19, 2018. https://emergency.cdc.gov/coping/responders.asp. Accessed March 30, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease 2109 (COVID-19): Facemasks. March 17, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html. Accessed March 30, 2020.
6. Pandemic Influenza Working Group. Stand on Guard for Thee: Ethical Considerations in Preparedness Planning for Pandemic Influenza. Toronto: University of Toronto Joint Centre for Bioethics; 2005. http://www.jcb.utoronto.ca/people/documents/upshur_stand_guard.pdf. Accessed March 30, 2020.
7. Miroff N. Protective gear in national stockpile is nearly depleted, DHS officials say. The Washington Post. April 1, 2020. https://www.washingtonpost.com/national/coronavirus-protective-gear-stockpile-depleted/2020/04/01/44d6592a-741f-11ea-ae50-7148009252e3_story.html. Accessed April 2, 2020.
8. Lewis T. Medical students provide childcare for healthcare professionals during COVID-19 pandemic. Fox 5 DC. March 27, 2020. https://www.fox5dc.com/news/medical-students-provide-childcare-for-healthcare-professionals-during-covid-19-pandemic. Accessed March 30, 2020.
9. New York State Task Force on Life and the Law. Ventilator Allocation Guidelines. New York: New York State Department of Health; 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Accessed March 30, 2020.
10. Antommaria AH, Powell T, Miller JE, Christian MD, Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi:10.1097/PCC.0b013e318234a88b.
11. Rothstein, MA. Currents in contemporary ethics. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010; 38(2):412-419. https://doi:10.1111/j.1748-720X.2010.00499.x.
12. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5.
13. Cha AE. Hospitals consider universal do-not-resuscitate orders for coronavirus patient. The Washington Post. March 25, 2020. https://www.washingtonpost.com/health/2020/03/25/coronavirus-patients-do-not-resucitate/. Accessed March 30, 2020.
14. Sanders T, Armstrong D, Kofman A. Doctors are hoarding unproven coronavirus medicine by writing prescriptions for themselves and their families. ProPublica. March 24, 2020. https://www.propublica.org/article/doctors-are-hoarding-unproven-coronavirus-medicine-by-writing-prescriptions-for-themselves-and-their-families. Accessed March 30, 2020.
© 2020 Society of Hospital Medicine
Ten Tips for a Crisis: Lessons from a Soldier
A few days ago, I had a heartfelt conversation with my good friend Dr Omayra Mansfield. Dr Mansfield has been an Emergency Department Physician for more than 12 years. She is also the wife of another physician and the mother of two young children, the recently appointed Chief Medical Officer at a hospital at AdventHealth, and one of the first graduates of the Physician Leader Development Course I teach.
“During the leadership course, you always provided examples of how physicians are like soldiers,” she began. She reminded me of my words describing how both doctors and soldiers are part of a professional body, how both have a cherished ethos and a set of directing values to guide both their path and their actions as a very special part of our society, and how of all the professions in our society, the military and medicine are the only two that deal in life and death, albeit in very different ways.
She had certainly paid attention in our seminars. Now, as she and her team faced the COVID-19 pandemic, she realized their daily challenges are expanding and they are now going to war. The leadership discussions that had sparked so much debate in our colloquia had now become real.
Dr Mansfield explained that beyond caring for patients, one of her key concerns was the physical and emotional well-being of the clinical staff at her hospital: the physicians, nurses, technicians, and clinicians under her care. Getting to her point, she asked if I might have any suggestions based on my time and experiences in combat that might be helpful to her as she “cared for her troops” as they faced the battle ahead.
Her request was a good one. Lessons from my military past immediately rushed to my mind. I started scribbling and came up with a Top Ten list of recommendations for anyone going into a tough fight. Here’s what I sent to her:
- Find a battle-buddy. On the first day of Army basic training, drill sergeants pair new recruits with one another. That’s primarily for accountability purposes throughout the weeks of training—to ensure soldiers hold each other responsible for getting to the right place, at the right time, in the right uniform—but it’s also part of a larger psychological dynamic related to building teams and mutual support within organizations. Your battle-buddy is charged with keeping you out of trouble, having your back, and being there when you need it most. In combat, battle-buddies do all those things and then some; they protect you from harm in so many other ways. Healthcare providers during this crisis will sometimes feel all alone, and they need to rely on someone else to help them when times get really tough. Having a battle-buddy—for those at the healthcare team level, of course, but also among those at the level of clinical director, hospital administrator, CMO, or even CEO—will help get you through the tough times and provide sanity when you need it the most. So my first piece of advice: Find a battle-buddy.
- Plan and prepare for things you don’t expect will happen. During a preparatory training exercise for our unit’s deployment to Iraq during the surge, when we thought the exercise was about to end, the trainers surprised us with a final crisis we had to solve. According to the scenario, Al Qaida had blown up a major bridge in our area, causing dire logistical problems for the security forces and challenges to the population as they brought their goods to market. I remember my initial reaction: “They don’t have the strength to do that. This’ll never happen,” I said to the Chief of Staff under my breath, as we started developing the required drill to counter the action and please the trainers. I quickly forgot about that lesson, until after we deployed. Two weeks into our 15-month tour in Iraq, the enemy blew the exact bridge that was part of the scenario, causing the exact problems that were predicted. Because we had prepared for the unexpected, we were able to quickly repair the bridge, reestablish the logistics flow, and satisfy the worried population. The lesson: Teams can hope for the best, but it’s always important to prepare for the worst—a lack of equipment, a key member of the team not being available to contribute, an overwhelming surge of patients—and then develop a plan to mitigate it. Take time to reflect, and ask yourself, What is the worst that can happen, what can the “enemy” do to disrupt our lives, and how do we prepare to counter it?
- Get everyone into the fight. In every organizations, it’s often true that some people take on too much and try and do it all themselves, others do only what they’re told to do, there’s the unique few who want to contribute but don’t know how they can to help, and then there’s some who even attempt to avoid contributing at all. It’s important for leaders to know who on their team fits each of these categories. It’s even more critical for leaders to be able to find ways to relieve the overworked, assign tasks to those who might not know their role, bring those who want to contribute into the fold, and cross-train teams to help relieve those who are exhausted. Leaders must look across their “battlespace” and ensure everyone is contributing. Leaders assign everyone tasks and do their best to level—and lighten—the load of the overworked.
- “Fatigue makes cowards of us all.” During any type of crisis, the body and mind will rapidly break down from lack of sleep, emotional strain, or overwhelming stress. While a 12-hour shift in a hospital is exceedingly tough even during normal operations, the COVID-19 crisis will demand dramatically more of all the members of any healthcare team. For that reason, leaders must incorporate rest cycles, team rotations, and half-days away from the hospitals even when all hands are on deck, as well as consider reducing shift times, if possible. Many who have experienced the disease in hot spots say this is really tough, but not attempting to plan for this will cause eventual breakdown and dysfunction. Take a break, do all you can to maintain a modicum of balance, and get away for a while.
- Take time to huddle. Communication and information are always key, but especially critical during any crisis. One technique that has proven valuable, beyond meetings and shift changes, is a preshift and postshift huddle. Different from the formal passing of critical information, the huddle is a brief opportunity for teams to pass informal information, look each other in the eye, and perhaps even pray together. As a two-star general, I did that every morning in combat with my small team of sergeants, captains, and privates before we left the headquarters to visit units, and it gave us all the power of knowing we had shared information, and we had a common operating picture. It gave us strength. During a crisis, all kinds of communication, formal and informal, are key.
- This ain’t peacetime. In a crisis, the enemy gets a vote. If leaders don’t find ways to counter the enemy’s action (and fast!), they’ll be behind the curve! It’s important to find the techniques and procedures that are bureaucratic (or even dumb) and overturn or eliminate them quickly. Decisions must be made with alacrity and with an understood flow, and people must be assigned responsibilities and held accountable to make things happen. In a crisis, speed in action will almost always trump perfection in understanding. Stay calm but ensure that those who might not understand this come around to the dynamics associated with the threat. A crisis isn’t the time for business as usual.
- Force adaptation—don’t wait ’til things are over to adjust. In a crisis, faults and disconnects in techniques and procedures often bubble to the surface and cause consternation. Don’t wait for a break in the action to adjust and find new ways to do things because a break in the action will usually never happen. The military has an expression: “Those who adapt the fastest on the battlefield win.” Find ways to look for and then publicize your methods of adaptation to the team, pin the rose on someone to ensure the changes are made, and then have someone make a historical record so other teams might also learn from your scar tissue. Lessons from the fight must be incorporated by the organization, or they’re not “lessons learned.”
- Talkin’ ain’t fightin’. During a crisis, it’s important to establish techniques of verbal shorthand between the members of a team, and everyone must know their responsibilities and required actions. In the military, this is called a battle drill; in medicine, you know it as a code. In these situations, leaders must find ways to pass information quickly, and the reaction should be immediate response. In a crisis, normal process must take on the dynamics of a “code.” All members of the team must understand that there are just times when things can’t be explained, but it’s also important that leaders know when to use this abbreviated format. Explain when you can, but act when you must.
- Cherish your teams. Every single team will experience things that human beings aren’t designed or meant to handle—even those in the medical profession, who likely thought they had seen it all. There will be repeated and overwhelming trauma, with the expected emotional reactions. The approach during these situations requires empathy, humility, emotional understanding, and validation. Praise your team at every opportunity, find ways to turn mistakes into learning opportunities, but most importantly be human and find ways to provide memories that your team can cherish and look back upon. Give them memories.
- Leaders don’t have the right to have a bad day. In 2004, after a 12-month deployment in Iraq, our unit was on our way home. We had been a long time away from our families, and we had experienced some tough fighting. A third of our unit had already returned to their families in Germany when we were told we would be extended because of a changing situation on the ground. A wave of frustration went through our 18,000 soldiers. Our commander then pulled us together, communicated our new mission, and told us he was also disappointed, but it was time we had to show our grit by getting those soldiers who had already returned to Europe back, unpack our equipment, and return to the fight. Then he said something I will always remember: “It’s tough, but understand your soldiers are looking at you to lead in this crisis … and leaders don’t have the right to have a bad day.” He didn’t mean we couldn’t be frustrated, or disappointed, or emotional, or even pissed off. He meant we just couldn’t show it when others were around. That’s one of the toughest things about leading during a crisis: The unimaginable is expected of leaders. And leaders have to be ready to lead.
All this advice may seem like philosophical musings rather than pragmatic thoughts for a crisis, but hopefully this advice will make a difference as healthcare providers tackle the issues ahead. Stay healthy, mitigate risks, but know that the calm provided by leaders will make a difference.
A few days ago, I had a heartfelt conversation with my good friend Dr Omayra Mansfield. Dr Mansfield has been an Emergency Department Physician for more than 12 years. She is also the wife of another physician and the mother of two young children, the recently appointed Chief Medical Officer at a hospital at AdventHealth, and one of the first graduates of the Physician Leader Development Course I teach.
“During the leadership course, you always provided examples of how physicians are like soldiers,” she began. She reminded me of my words describing how both doctors and soldiers are part of a professional body, how both have a cherished ethos and a set of directing values to guide both their path and their actions as a very special part of our society, and how of all the professions in our society, the military and medicine are the only two that deal in life and death, albeit in very different ways.
She had certainly paid attention in our seminars. Now, as she and her team faced the COVID-19 pandemic, she realized their daily challenges are expanding and they are now going to war. The leadership discussions that had sparked so much debate in our colloquia had now become real.
Dr Mansfield explained that beyond caring for patients, one of her key concerns was the physical and emotional well-being of the clinical staff at her hospital: the physicians, nurses, technicians, and clinicians under her care. Getting to her point, she asked if I might have any suggestions based on my time and experiences in combat that might be helpful to her as she “cared for her troops” as they faced the battle ahead.
Her request was a good one. Lessons from my military past immediately rushed to my mind. I started scribbling and came up with a Top Ten list of recommendations for anyone going into a tough fight. Here’s what I sent to her:
- Find a battle-buddy. On the first day of Army basic training, drill sergeants pair new recruits with one another. That’s primarily for accountability purposes throughout the weeks of training—to ensure soldiers hold each other responsible for getting to the right place, at the right time, in the right uniform—but it’s also part of a larger psychological dynamic related to building teams and mutual support within organizations. Your battle-buddy is charged with keeping you out of trouble, having your back, and being there when you need it most. In combat, battle-buddies do all those things and then some; they protect you from harm in so many other ways. Healthcare providers during this crisis will sometimes feel all alone, and they need to rely on someone else to help them when times get really tough. Having a battle-buddy—for those at the healthcare team level, of course, but also among those at the level of clinical director, hospital administrator, CMO, or even CEO—will help get you through the tough times and provide sanity when you need it the most. So my first piece of advice: Find a battle-buddy.
- Plan and prepare for things you don’t expect will happen. During a preparatory training exercise for our unit’s deployment to Iraq during the surge, when we thought the exercise was about to end, the trainers surprised us with a final crisis we had to solve. According to the scenario, Al Qaida had blown up a major bridge in our area, causing dire logistical problems for the security forces and challenges to the population as they brought their goods to market. I remember my initial reaction: “They don’t have the strength to do that. This’ll never happen,” I said to the Chief of Staff under my breath, as we started developing the required drill to counter the action and please the trainers. I quickly forgot about that lesson, until after we deployed. Two weeks into our 15-month tour in Iraq, the enemy blew the exact bridge that was part of the scenario, causing the exact problems that were predicted. Because we had prepared for the unexpected, we were able to quickly repair the bridge, reestablish the logistics flow, and satisfy the worried population. The lesson: Teams can hope for the best, but it’s always important to prepare for the worst—a lack of equipment, a key member of the team not being available to contribute, an overwhelming surge of patients—and then develop a plan to mitigate it. Take time to reflect, and ask yourself, What is the worst that can happen, what can the “enemy” do to disrupt our lives, and how do we prepare to counter it?
- Get everyone into the fight. In every organizations, it’s often true that some people take on too much and try and do it all themselves, others do only what they’re told to do, there’s the unique few who want to contribute but don’t know how they can to help, and then there’s some who even attempt to avoid contributing at all. It’s important for leaders to know who on their team fits each of these categories. It’s even more critical for leaders to be able to find ways to relieve the overworked, assign tasks to those who might not know their role, bring those who want to contribute into the fold, and cross-train teams to help relieve those who are exhausted. Leaders must look across their “battlespace” and ensure everyone is contributing. Leaders assign everyone tasks and do their best to level—and lighten—the load of the overworked.
- “Fatigue makes cowards of us all.” During any type of crisis, the body and mind will rapidly break down from lack of sleep, emotional strain, or overwhelming stress. While a 12-hour shift in a hospital is exceedingly tough even during normal operations, the COVID-19 crisis will demand dramatically more of all the members of any healthcare team. For that reason, leaders must incorporate rest cycles, team rotations, and half-days away from the hospitals even when all hands are on deck, as well as consider reducing shift times, if possible. Many who have experienced the disease in hot spots say this is really tough, but not attempting to plan for this will cause eventual breakdown and dysfunction. Take a break, do all you can to maintain a modicum of balance, and get away for a while.
- Take time to huddle. Communication and information are always key, but especially critical during any crisis. One technique that has proven valuable, beyond meetings and shift changes, is a preshift and postshift huddle. Different from the formal passing of critical information, the huddle is a brief opportunity for teams to pass informal information, look each other in the eye, and perhaps even pray together. As a two-star general, I did that every morning in combat with my small team of sergeants, captains, and privates before we left the headquarters to visit units, and it gave us all the power of knowing we had shared information, and we had a common operating picture. It gave us strength. During a crisis, all kinds of communication, formal and informal, are key.
- This ain’t peacetime. In a crisis, the enemy gets a vote. If leaders don’t find ways to counter the enemy’s action (and fast!), they’ll be behind the curve! It’s important to find the techniques and procedures that are bureaucratic (or even dumb) and overturn or eliminate them quickly. Decisions must be made with alacrity and with an understood flow, and people must be assigned responsibilities and held accountable to make things happen. In a crisis, speed in action will almost always trump perfection in understanding. Stay calm but ensure that those who might not understand this come around to the dynamics associated with the threat. A crisis isn’t the time for business as usual.
- Force adaptation—don’t wait ’til things are over to adjust. In a crisis, faults and disconnects in techniques and procedures often bubble to the surface and cause consternation. Don’t wait for a break in the action to adjust and find new ways to do things because a break in the action will usually never happen. The military has an expression: “Those who adapt the fastest on the battlefield win.” Find ways to look for and then publicize your methods of adaptation to the team, pin the rose on someone to ensure the changes are made, and then have someone make a historical record so other teams might also learn from your scar tissue. Lessons from the fight must be incorporated by the organization, or they’re not “lessons learned.”
- Talkin’ ain’t fightin’. During a crisis, it’s important to establish techniques of verbal shorthand between the members of a team, and everyone must know their responsibilities and required actions. In the military, this is called a battle drill; in medicine, you know it as a code. In these situations, leaders must find ways to pass information quickly, and the reaction should be immediate response. In a crisis, normal process must take on the dynamics of a “code.” All members of the team must understand that there are just times when things can’t be explained, but it’s also important that leaders know when to use this abbreviated format. Explain when you can, but act when you must.
- Cherish your teams. Every single team will experience things that human beings aren’t designed or meant to handle—even those in the medical profession, who likely thought they had seen it all. There will be repeated and overwhelming trauma, with the expected emotional reactions. The approach during these situations requires empathy, humility, emotional understanding, and validation. Praise your team at every opportunity, find ways to turn mistakes into learning opportunities, but most importantly be human and find ways to provide memories that your team can cherish and look back upon. Give them memories.
- Leaders don’t have the right to have a bad day. In 2004, after a 12-month deployment in Iraq, our unit was on our way home. We had been a long time away from our families, and we had experienced some tough fighting. A third of our unit had already returned to their families in Germany when we were told we would be extended because of a changing situation on the ground. A wave of frustration went through our 18,000 soldiers. Our commander then pulled us together, communicated our new mission, and told us he was also disappointed, but it was time we had to show our grit by getting those soldiers who had already returned to Europe back, unpack our equipment, and return to the fight. Then he said something I will always remember: “It’s tough, but understand your soldiers are looking at you to lead in this crisis … and leaders don’t have the right to have a bad day.” He didn’t mean we couldn’t be frustrated, or disappointed, or emotional, or even pissed off. He meant we just couldn’t show it when others were around. That’s one of the toughest things about leading during a crisis: The unimaginable is expected of leaders. And leaders have to be ready to lead.
All this advice may seem like philosophical musings rather than pragmatic thoughts for a crisis, but hopefully this advice will make a difference as healthcare providers tackle the issues ahead. Stay healthy, mitigate risks, but know that the calm provided by leaders will make a difference.
A few days ago, I had a heartfelt conversation with my good friend Dr Omayra Mansfield. Dr Mansfield has been an Emergency Department Physician for more than 12 years. She is also the wife of another physician and the mother of two young children, the recently appointed Chief Medical Officer at a hospital at AdventHealth, and one of the first graduates of the Physician Leader Development Course I teach.
“During the leadership course, you always provided examples of how physicians are like soldiers,” she began. She reminded me of my words describing how both doctors and soldiers are part of a professional body, how both have a cherished ethos and a set of directing values to guide both their path and their actions as a very special part of our society, and how of all the professions in our society, the military and medicine are the only two that deal in life and death, albeit in very different ways.
She had certainly paid attention in our seminars. Now, as she and her team faced the COVID-19 pandemic, she realized their daily challenges are expanding and they are now going to war. The leadership discussions that had sparked so much debate in our colloquia had now become real.
Dr Mansfield explained that beyond caring for patients, one of her key concerns was the physical and emotional well-being of the clinical staff at her hospital: the physicians, nurses, technicians, and clinicians under her care. Getting to her point, she asked if I might have any suggestions based on my time and experiences in combat that might be helpful to her as she “cared for her troops” as they faced the battle ahead.
Her request was a good one. Lessons from my military past immediately rushed to my mind. I started scribbling and came up with a Top Ten list of recommendations for anyone going into a tough fight. Here’s what I sent to her:
- Find a battle-buddy. On the first day of Army basic training, drill sergeants pair new recruits with one another. That’s primarily for accountability purposes throughout the weeks of training—to ensure soldiers hold each other responsible for getting to the right place, at the right time, in the right uniform—but it’s also part of a larger psychological dynamic related to building teams and mutual support within organizations. Your battle-buddy is charged with keeping you out of trouble, having your back, and being there when you need it most. In combat, battle-buddies do all those things and then some; they protect you from harm in so many other ways. Healthcare providers during this crisis will sometimes feel all alone, and they need to rely on someone else to help them when times get really tough. Having a battle-buddy—for those at the healthcare team level, of course, but also among those at the level of clinical director, hospital administrator, CMO, or even CEO—will help get you through the tough times and provide sanity when you need it the most. So my first piece of advice: Find a battle-buddy.
- Plan and prepare for things you don’t expect will happen. During a preparatory training exercise for our unit’s deployment to Iraq during the surge, when we thought the exercise was about to end, the trainers surprised us with a final crisis we had to solve. According to the scenario, Al Qaida had blown up a major bridge in our area, causing dire logistical problems for the security forces and challenges to the population as they brought their goods to market. I remember my initial reaction: “They don’t have the strength to do that. This’ll never happen,” I said to the Chief of Staff under my breath, as we started developing the required drill to counter the action and please the trainers. I quickly forgot about that lesson, until after we deployed. Two weeks into our 15-month tour in Iraq, the enemy blew the exact bridge that was part of the scenario, causing the exact problems that were predicted. Because we had prepared for the unexpected, we were able to quickly repair the bridge, reestablish the logistics flow, and satisfy the worried population. The lesson: Teams can hope for the best, but it’s always important to prepare for the worst—a lack of equipment, a key member of the team not being available to contribute, an overwhelming surge of patients—and then develop a plan to mitigate it. Take time to reflect, and ask yourself, What is the worst that can happen, what can the “enemy” do to disrupt our lives, and how do we prepare to counter it?
- Get everyone into the fight. In every organizations, it’s often true that some people take on too much and try and do it all themselves, others do only what they’re told to do, there’s the unique few who want to contribute but don’t know how they can to help, and then there’s some who even attempt to avoid contributing at all. It’s important for leaders to know who on their team fits each of these categories. It’s even more critical for leaders to be able to find ways to relieve the overworked, assign tasks to those who might not know their role, bring those who want to contribute into the fold, and cross-train teams to help relieve those who are exhausted. Leaders must look across their “battlespace” and ensure everyone is contributing. Leaders assign everyone tasks and do their best to level—and lighten—the load of the overworked.
- “Fatigue makes cowards of us all.” During any type of crisis, the body and mind will rapidly break down from lack of sleep, emotional strain, or overwhelming stress. While a 12-hour shift in a hospital is exceedingly tough even during normal operations, the COVID-19 crisis will demand dramatically more of all the members of any healthcare team. For that reason, leaders must incorporate rest cycles, team rotations, and half-days away from the hospitals even when all hands are on deck, as well as consider reducing shift times, if possible. Many who have experienced the disease in hot spots say this is really tough, but not attempting to plan for this will cause eventual breakdown and dysfunction. Take a break, do all you can to maintain a modicum of balance, and get away for a while.
- Take time to huddle. Communication and information are always key, but especially critical during any crisis. One technique that has proven valuable, beyond meetings and shift changes, is a preshift and postshift huddle. Different from the formal passing of critical information, the huddle is a brief opportunity for teams to pass informal information, look each other in the eye, and perhaps even pray together. As a two-star general, I did that every morning in combat with my small team of sergeants, captains, and privates before we left the headquarters to visit units, and it gave us all the power of knowing we had shared information, and we had a common operating picture. It gave us strength. During a crisis, all kinds of communication, formal and informal, are key.
- This ain’t peacetime. In a crisis, the enemy gets a vote. If leaders don’t find ways to counter the enemy’s action (and fast!), they’ll be behind the curve! It’s important to find the techniques and procedures that are bureaucratic (or even dumb) and overturn or eliminate them quickly. Decisions must be made with alacrity and with an understood flow, and people must be assigned responsibilities and held accountable to make things happen. In a crisis, speed in action will almost always trump perfection in understanding. Stay calm but ensure that those who might not understand this come around to the dynamics associated with the threat. A crisis isn’t the time for business as usual.
- Force adaptation—don’t wait ’til things are over to adjust. In a crisis, faults and disconnects in techniques and procedures often bubble to the surface and cause consternation. Don’t wait for a break in the action to adjust and find new ways to do things because a break in the action will usually never happen. The military has an expression: “Those who adapt the fastest on the battlefield win.” Find ways to look for and then publicize your methods of adaptation to the team, pin the rose on someone to ensure the changes are made, and then have someone make a historical record so other teams might also learn from your scar tissue. Lessons from the fight must be incorporated by the organization, or they’re not “lessons learned.”
- Talkin’ ain’t fightin’. During a crisis, it’s important to establish techniques of verbal shorthand between the members of a team, and everyone must know their responsibilities and required actions. In the military, this is called a battle drill; in medicine, you know it as a code. In these situations, leaders must find ways to pass information quickly, and the reaction should be immediate response. In a crisis, normal process must take on the dynamics of a “code.” All members of the team must understand that there are just times when things can’t be explained, but it’s also important that leaders know when to use this abbreviated format. Explain when you can, but act when you must.
- Cherish your teams. Every single team will experience things that human beings aren’t designed or meant to handle—even those in the medical profession, who likely thought they had seen it all. There will be repeated and overwhelming trauma, with the expected emotional reactions. The approach during these situations requires empathy, humility, emotional understanding, and validation. Praise your team at every opportunity, find ways to turn mistakes into learning opportunities, but most importantly be human and find ways to provide memories that your team can cherish and look back upon. Give them memories.
- Leaders don’t have the right to have a bad day. In 2004, after a 12-month deployment in Iraq, our unit was on our way home. We had been a long time away from our families, and we had experienced some tough fighting. A third of our unit had already returned to their families in Germany when we were told we would be extended because of a changing situation on the ground. A wave of frustration went through our 18,000 soldiers. Our commander then pulled us together, communicated our new mission, and told us he was also disappointed, but it was time we had to show our grit by getting those soldiers who had already returned to Europe back, unpack our equipment, and return to the fight. Then he said something I will always remember: “It’s tough, but understand your soldiers are looking at you to lead in this crisis … and leaders don’t have the right to have a bad day.” He didn’t mean we couldn’t be frustrated, or disappointed, or emotional, or even pissed off. He meant we just couldn’t show it when others were around. That’s one of the toughest things about leading during a crisis: The unimaginable is expected of leaders. And leaders have to be ready to lead.
All this advice may seem like philosophical musings rather than pragmatic thoughts for a crisis, but hopefully this advice will make a difference as healthcare providers tackle the issues ahead. Stay healthy, mitigate risks, but know that the calm provided by leaders will make a difference.
Keep Calm and Log On: Telemedicine for COVID-19 Pandemic Response
The field of telemedicine, in which clinicians use remote evaluation and monitoring to diagnose and treat patients, has grown substantially over the past decade. Its roles in acute care medicine settings are diverse, including virtual intensive care unit (ICU) care, after-hours medical admissions, cross coverage, and, most aptly, disaster management.1
At HealthPartners, a large integrated healthcare delivery and financing system based in the Twin Cities region of Minnesota, we have used provider-initiated telemedicine in hospital medicine for more than 2 years, providing evening and nighttime hospitalist coverage to our rural hospitals. We additionally provide a 24/7 nurse practitioner-staffed virtual clinic called Virtuwell.2 Because we are now immersed in a global pandemic, we have taken steps to bolster our telemedicine infrastructure to meet increasing needs.
SARS-CoV-2, the causative agent of COVID-19, is a novel coronavirus with the capability to cause severe illness in roughly 14% of those infected.3 According to some estimates, the virus may infect up to 60% of the US population in the next year.4 As the pandemic looms over the country and the healthcare community, telemedicine can offer tools to help respond to this crisis. Healthcare systems leveraging telemedicine for patient care will gain several advantages, including workforce sustainability, reduction of provider burnout, limitation of provider exposure, and reduction of personal protective equipment (PPE) waste (Table). Telemedicine can also facilitate staffing of both large and small facilities that find themselves overwhelmed with pandemic-related patient overload (PRPO). Although telemedicine holds promise for pandemic response, this technology has limitations. It requires robust IT infrastructure, training of both nurses and physicians, and modifications to integrate within hospital workflows. In this article, we summarize key clinical needs that telemedicine can meet, implementation challenges, and important business considerations.
BACKGROUND
Our organization currently uses telemedicine to provide after-hours hospital medicine coverage from 6
APPLICATIONS
Patient Triage
Limiting exposure in the community and in the acute care setting is key to “flattening the curve” in pandemics.5 Triaging patients by telephone and online surveys is an important method to prevent high-risk patients from exposing others to infection. For example, since March 9, 2020, over 20,000 patients have called in weekly for COVID-19 screening. Although our organization introduced drive-up testing to reduce exposure, patients are still presenting to our clinics and emergency rooms in need of screening and testing. In several of our clinics, patients have been roomed alone to facilitate screening in the room by use of Google Duo, a free video chat product. Rooms with telemedicine capabilities allow patients with potentially communicable infections to be evaluated and observed while avoiding the risk of viral transmission. Additional considerations could include self-administered nasal swabs; although this has comparable efficacy to staff-administered swabs,6 it has not yet been implemented in our clinics.
Direct Care
Virtual care, specifically synchronous video and audio provider-initiated services, is a well-established modality to provide direct care to patients in acute care and ambulatory settings.7 Telemedicine can be deployed to care for hospitalized patients in most locations as long as they meet the operational requirements described below. With a bedside nurse or other facilitator, patients can be interviewed and examined using a high definition camera and digital peripherals, including stethoscopes, otoscopes, ophthalmoscopes, and dermatoscopes. COVID-19 patients or patients under investigation may be seen in this manner. In-person visits should remain part of patients’ care as an important part of the provider-patient relationship8; however, telemedicine could still be deployed to provide direct care and monitoring to these patients while minimizing exposure to healthcare personnel. Additionally, telemedicine can be used for specialist consultations that are likely in high demand with COVID-19, including infectious disease, cardiology, and pulmonology.
Exposure Reduction and Resource Allocation
Currently in the United States there are concerns for shortages of PPE including surgical masks and N95 respirators. Telemedicine can reduce provider exposure, increase provider efficiency, and curtail PPE utilization by minimizing the number and frequency of in-room visits while still allowing virtual visits for direct patient care. For instance, our nursing staff is currently using telemedicine to conduct hourly rounding and limit unnecessary in-room visits.
We recommend keeping telemedicine equipment within individual isolation rooms intended for COVID-19 patients in order to eliminate the need for repeated cleaning. For other patients, a mobile cart could be used. Most commercial video software can autoanswer calls to allow for staff-free history taking. For a thorough physical exam, a bedside facilitator is need for use of digital stethoscopes and similar peripherals.
Provider Shortages and Reducing Burnout
Because SARS-CoV-2 is a highly contagious pathogen that can spread prior to symptom presentation, current CDC guidelines recommend self-monitoring at home for health care workers who have a healthcare-related exposure to a COVID-19 patient.9 This can leave significant gaps in coverage for healthcare systems. For example, in Vacaville, California, one positive case resulted in over 200 health care workers unable to work on site.10
Large volumes of acutely ill patients, coupled with the risk of ill or quarantined providers, means provider shortages due to PRPO are likely to occur and threaten hospitals’ ability to care for patients with or without COVID-19. Furthermore, given increased patient loads, frontline staff are at exceptionally high risk of burnout in pandemic situations. Hospital medicine teams will need contingency plans to meet the needs. Using telemedicine to protect the workforce and maintain staffing levels will reduce that risk.
Telehospitalists can see and examine patients, write orders, and maintain patient service lines much like in-person providers. Recently, we have used it when providers are ill or self-monitoring. In multisite systems, telehospitalists who are privileged in multiple hospitals can be efficiently deployed to meet patient care needs and relieve overburdened providers across hundreds of miles or more.
Enabling patient rooms for telemedicine allows telehospitalists and other providers to see hospitalized patients. Furthermore, quarantined hospitalists can continue to work and support in-person clinical services during PRPO. Providers in high-risk groups (eg, older, immunosuppressed, pregnant) can also continue caring for patients with telemedicine while maintaining safety. As schools close, telemedicine can help providers navigate the challenge between patient care and childcare responsibilities.
OPERATIONAL REQUIREMENTS
The basic element of telemedicine involves a computer or monitor with an internet-connected camera and a HIPAA-compliant video application, but implementation can vary.
Recent changes have allowed the use of popular video chat software such as FaceTime, Skype, or Google Duo for patient interactions; with a tablet attached to a stand, organizations can easily create a mobile telemedicine workstation. Larger monitors or mounted screens can be used in patient areas where portability is not required. A strong network infrastructure and robust IT support are also necessary; as of 2016, 24 million Americans did not have broadband access, and even areas that do can struggle with wireless connectivity in hospitals with thick concrete walls and lack of wi-fi extenders.11
With the addition of a digital stethoscope, hospitalists can perform a thorough history and physical with the aid of bedside staff. This requires dedicated training for all members of the care team in order to optimize the virtual hospitalist’s “telepresence” and create a seamless patient experience. Provider education is imperative: Creating a virtual telepresence is essential in building a strong provider-patient relationship. We have used simulation training to prepare new telehospitalists.
An overlooked, but important, operational requirement is patient education and awareness. In the absence of introduction and onboarding, telemedicine can be viewed by patients as impersonal; however, with proper implementation, high patient satisfaction has been demonstrated in other virtual care experiences.12
FINANCIAL CONSIDERATIONS
Though several health systems offer “tele-ICU” services, the number of hospital medicine programs is more limited. The cost of building a program can be significant, with outlays for equipment, IT support, provider salaries, and training. While all 50 states and the District of Columbia cover some form of fee-for-service live video with Medicaid, only 40, along with DC, have parity laws with commercial payors. Medicare has historically had more restrictions, limiting covered services to specific types of originating sites in certain geographic areas. Furthermore, growth of telehospitalist programs has been hampered by the lack of reimbursement for “primary care services.”13
With passage of the Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020, geographic and site restrictions have been waived for Medicare reimbursement.14 Providers must still demonstrate a prior relationship with patients, which requires at least one encounter with the patient in the past 3 years by the same provider or one with a similar tax identification number (TIN). All hospitalists within our group are identified with a common TIN, which helps to meet this requirement for patient with recent admissions. However, clear guidance on reimbursement for primary care services by acute care providers is still lacking. As the utility of telemedicine is demonstrated in the hospital setting, we hope further changes may be enacted.
Organizations must properly credential and privilege telehospitalists. Telemedicine services may fall under either core or “delegated” privileges depending on the individual hospital. Additionally, while malpractice insurance does typically cover telemedicine services, each organization should verify this with their particular carrier.
SUMMARY
The COVID-19 pandemic has created a systemic challenge for healthcare systems across the nation. As hospitalists continue to be on the front lines, organizations can leverage telemedicine to support their patients, protect their clinicians, and conserve scarce resources. Building out a virtual care program is intricate and requires significant operational support. Laying the groundwork now can prepare institutions to provide necessary care for patients, not just in the current pandemic, but in numerous emergency health care situations in the future.
1. Lurie N, Carr BG. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;178(6):745-74. https://doi.org/10.1001/jamainternmed.2018.1314.
2. Virtuwell. HealthPartners. 2020. https://www.virtuwell.com.
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
4. Powell A. Coronavirus screening may miss two-thirds of infected travelers entering U.S. The Harvard Gazette. 2020. https://news.harvard.edu/gazette/story/2020/03/hundreds-of-u-s-coronavirus-cases-may-have-slipped-through-screenings/. Accessed March 13, 2020.
5. Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007:104(18);7582-7587. https://doi.org/10.1073/pnas.0610941104.
6. Akmatov MK, Gatzemeier A, Schughart, K, Pessler F. Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens. PLoS One. 2012:7(11);e48508. https://doi.org/10.1371/journal.pone.0048508.
7. Centers for Medicare & Medicaid Services. Telehealth Services. 2019. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Telehealth Srvcsfctsht.pdf. Accessed March 14, 2020.
8. Daniel H, Sulmasy LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. 2015;163(10):787-789. https://doi.org/10.7326/M15-0498.
9. Centers for Disease Control and Prevention. Healthcare Personnel with Potential Exposure to COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed March 13, 2020.
10. Gold J. Surging Health Care Worker Quarantines Raise Concerns as Coronavirus Spreads. Kaiser Health News. 2020. https://khn.org/news/surging-health-care-worker-quarantines-raise-concerns-as-coronavirus-spreads/. Accessed March 12, 2020.
11. Federal Communications Commission. 2018 Broadband Deployment Report. 2018. https://www.fcc.gov/reports-research/reports/broadband-progress-reports/2018-broadband-deployment-report. Accessed March 13, 2020.
12. Martinez KA, Rood M, Jhangiani N, et al. Patterns of use and correlates of patient satisfaction with a large nationwide direct to consumer telemedicine service. J Gen Intern Med. 2018;33(10):1768-1773. https://doi.org/10.1007/s11606-018-4621-5.
13. Centers for Medicare & Medicaid Services. List of Telehealth Services. 2019. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed March 13, 2020.
14. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, H.R. 6074, 116th Cong. 2020. https://congress.gov/bill/116th-congress/house-bill/6074/. Accessed March 13, 2020.
The field of telemedicine, in which clinicians use remote evaluation and monitoring to diagnose and treat patients, has grown substantially over the past decade. Its roles in acute care medicine settings are diverse, including virtual intensive care unit (ICU) care, after-hours medical admissions, cross coverage, and, most aptly, disaster management.1
At HealthPartners, a large integrated healthcare delivery and financing system based in the Twin Cities region of Minnesota, we have used provider-initiated telemedicine in hospital medicine for more than 2 years, providing evening and nighttime hospitalist coverage to our rural hospitals. We additionally provide a 24/7 nurse practitioner-staffed virtual clinic called Virtuwell.2 Because we are now immersed in a global pandemic, we have taken steps to bolster our telemedicine infrastructure to meet increasing needs.
SARS-CoV-2, the causative agent of COVID-19, is a novel coronavirus with the capability to cause severe illness in roughly 14% of those infected.3 According to some estimates, the virus may infect up to 60% of the US population in the next year.4 As the pandemic looms over the country and the healthcare community, telemedicine can offer tools to help respond to this crisis. Healthcare systems leveraging telemedicine for patient care will gain several advantages, including workforce sustainability, reduction of provider burnout, limitation of provider exposure, and reduction of personal protective equipment (PPE) waste (Table). Telemedicine can also facilitate staffing of both large and small facilities that find themselves overwhelmed with pandemic-related patient overload (PRPO). Although telemedicine holds promise for pandemic response, this technology has limitations. It requires robust IT infrastructure, training of both nurses and physicians, and modifications to integrate within hospital workflows. In this article, we summarize key clinical needs that telemedicine can meet, implementation challenges, and important business considerations.
BACKGROUND
Our organization currently uses telemedicine to provide after-hours hospital medicine coverage from 6
APPLICATIONS
Patient Triage
Limiting exposure in the community and in the acute care setting is key to “flattening the curve” in pandemics.5 Triaging patients by telephone and online surveys is an important method to prevent high-risk patients from exposing others to infection. For example, since March 9, 2020, over 20,000 patients have called in weekly for COVID-19 screening. Although our organization introduced drive-up testing to reduce exposure, patients are still presenting to our clinics and emergency rooms in need of screening and testing. In several of our clinics, patients have been roomed alone to facilitate screening in the room by use of Google Duo, a free video chat product. Rooms with telemedicine capabilities allow patients with potentially communicable infections to be evaluated and observed while avoiding the risk of viral transmission. Additional considerations could include self-administered nasal swabs; although this has comparable efficacy to staff-administered swabs,6 it has not yet been implemented in our clinics.
Direct Care
Virtual care, specifically synchronous video and audio provider-initiated services, is a well-established modality to provide direct care to patients in acute care and ambulatory settings.7 Telemedicine can be deployed to care for hospitalized patients in most locations as long as they meet the operational requirements described below. With a bedside nurse or other facilitator, patients can be interviewed and examined using a high definition camera and digital peripherals, including stethoscopes, otoscopes, ophthalmoscopes, and dermatoscopes. COVID-19 patients or patients under investigation may be seen in this manner. In-person visits should remain part of patients’ care as an important part of the provider-patient relationship8; however, telemedicine could still be deployed to provide direct care and monitoring to these patients while minimizing exposure to healthcare personnel. Additionally, telemedicine can be used for specialist consultations that are likely in high demand with COVID-19, including infectious disease, cardiology, and pulmonology.
Exposure Reduction and Resource Allocation
Currently in the United States there are concerns for shortages of PPE including surgical masks and N95 respirators. Telemedicine can reduce provider exposure, increase provider efficiency, and curtail PPE utilization by minimizing the number and frequency of in-room visits while still allowing virtual visits for direct patient care. For instance, our nursing staff is currently using telemedicine to conduct hourly rounding and limit unnecessary in-room visits.
We recommend keeping telemedicine equipment within individual isolation rooms intended for COVID-19 patients in order to eliminate the need for repeated cleaning. For other patients, a mobile cart could be used. Most commercial video software can autoanswer calls to allow for staff-free history taking. For a thorough physical exam, a bedside facilitator is need for use of digital stethoscopes and similar peripherals.
Provider Shortages and Reducing Burnout
Because SARS-CoV-2 is a highly contagious pathogen that can spread prior to symptom presentation, current CDC guidelines recommend self-monitoring at home for health care workers who have a healthcare-related exposure to a COVID-19 patient.9 This can leave significant gaps in coverage for healthcare systems. For example, in Vacaville, California, one positive case resulted in over 200 health care workers unable to work on site.10
Large volumes of acutely ill patients, coupled with the risk of ill or quarantined providers, means provider shortages due to PRPO are likely to occur and threaten hospitals’ ability to care for patients with or without COVID-19. Furthermore, given increased patient loads, frontline staff are at exceptionally high risk of burnout in pandemic situations. Hospital medicine teams will need contingency plans to meet the needs. Using telemedicine to protect the workforce and maintain staffing levels will reduce that risk.
Telehospitalists can see and examine patients, write orders, and maintain patient service lines much like in-person providers. Recently, we have used it when providers are ill or self-monitoring. In multisite systems, telehospitalists who are privileged in multiple hospitals can be efficiently deployed to meet patient care needs and relieve overburdened providers across hundreds of miles or more.
Enabling patient rooms for telemedicine allows telehospitalists and other providers to see hospitalized patients. Furthermore, quarantined hospitalists can continue to work and support in-person clinical services during PRPO. Providers in high-risk groups (eg, older, immunosuppressed, pregnant) can also continue caring for patients with telemedicine while maintaining safety. As schools close, telemedicine can help providers navigate the challenge between patient care and childcare responsibilities.
OPERATIONAL REQUIREMENTS
The basic element of telemedicine involves a computer or monitor with an internet-connected camera and a HIPAA-compliant video application, but implementation can vary.
Recent changes have allowed the use of popular video chat software such as FaceTime, Skype, or Google Duo for patient interactions; with a tablet attached to a stand, organizations can easily create a mobile telemedicine workstation. Larger monitors or mounted screens can be used in patient areas where portability is not required. A strong network infrastructure and robust IT support are also necessary; as of 2016, 24 million Americans did not have broadband access, and even areas that do can struggle with wireless connectivity in hospitals with thick concrete walls and lack of wi-fi extenders.11
With the addition of a digital stethoscope, hospitalists can perform a thorough history and physical with the aid of bedside staff. This requires dedicated training for all members of the care team in order to optimize the virtual hospitalist’s “telepresence” and create a seamless patient experience. Provider education is imperative: Creating a virtual telepresence is essential in building a strong provider-patient relationship. We have used simulation training to prepare new telehospitalists.
An overlooked, but important, operational requirement is patient education and awareness. In the absence of introduction and onboarding, telemedicine can be viewed by patients as impersonal; however, with proper implementation, high patient satisfaction has been demonstrated in other virtual care experiences.12
FINANCIAL CONSIDERATIONS
Though several health systems offer “tele-ICU” services, the number of hospital medicine programs is more limited. The cost of building a program can be significant, with outlays for equipment, IT support, provider salaries, and training. While all 50 states and the District of Columbia cover some form of fee-for-service live video with Medicaid, only 40, along with DC, have parity laws with commercial payors. Medicare has historically had more restrictions, limiting covered services to specific types of originating sites in certain geographic areas. Furthermore, growth of telehospitalist programs has been hampered by the lack of reimbursement for “primary care services.”13
With passage of the Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020, geographic and site restrictions have been waived for Medicare reimbursement.14 Providers must still demonstrate a prior relationship with patients, which requires at least one encounter with the patient in the past 3 years by the same provider or one with a similar tax identification number (TIN). All hospitalists within our group are identified with a common TIN, which helps to meet this requirement for patient with recent admissions. However, clear guidance on reimbursement for primary care services by acute care providers is still lacking. As the utility of telemedicine is demonstrated in the hospital setting, we hope further changes may be enacted.
Organizations must properly credential and privilege telehospitalists. Telemedicine services may fall under either core or “delegated” privileges depending on the individual hospital. Additionally, while malpractice insurance does typically cover telemedicine services, each organization should verify this with their particular carrier.
SUMMARY
The COVID-19 pandemic has created a systemic challenge for healthcare systems across the nation. As hospitalists continue to be on the front lines, organizations can leverage telemedicine to support their patients, protect their clinicians, and conserve scarce resources. Building out a virtual care program is intricate and requires significant operational support. Laying the groundwork now can prepare institutions to provide necessary care for patients, not just in the current pandemic, but in numerous emergency health care situations in the future.
The field of telemedicine, in which clinicians use remote evaluation and monitoring to diagnose and treat patients, has grown substantially over the past decade. Its roles in acute care medicine settings are diverse, including virtual intensive care unit (ICU) care, after-hours medical admissions, cross coverage, and, most aptly, disaster management.1
At HealthPartners, a large integrated healthcare delivery and financing system based in the Twin Cities region of Minnesota, we have used provider-initiated telemedicine in hospital medicine for more than 2 years, providing evening and nighttime hospitalist coverage to our rural hospitals. We additionally provide a 24/7 nurse practitioner-staffed virtual clinic called Virtuwell.2 Because we are now immersed in a global pandemic, we have taken steps to bolster our telemedicine infrastructure to meet increasing needs.
SARS-CoV-2, the causative agent of COVID-19, is a novel coronavirus with the capability to cause severe illness in roughly 14% of those infected.3 According to some estimates, the virus may infect up to 60% of the US population in the next year.4 As the pandemic looms over the country and the healthcare community, telemedicine can offer tools to help respond to this crisis. Healthcare systems leveraging telemedicine for patient care will gain several advantages, including workforce sustainability, reduction of provider burnout, limitation of provider exposure, and reduction of personal protective equipment (PPE) waste (Table). Telemedicine can also facilitate staffing of both large and small facilities that find themselves overwhelmed with pandemic-related patient overload (PRPO). Although telemedicine holds promise for pandemic response, this technology has limitations. It requires robust IT infrastructure, training of both nurses and physicians, and modifications to integrate within hospital workflows. In this article, we summarize key clinical needs that telemedicine can meet, implementation challenges, and important business considerations.
BACKGROUND
Our organization currently uses telemedicine to provide after-hours hospital medicine coverage from 6
APPLICATIONS
Patient Triage
Limiting exposure in the community and in the acute care setting is key to “flattening the curve” in pandemics.5 Triaging patients by telephone and online surveys is an important method to prevent high-risk patients from exposing others to infection. For example, since March 9, 2020, over 20,000 patients have called in weekly for COVID-19 screening. Although our organization introduced drive-up testing to reduce exposure, patients are still presenting to our clinics and emergency rooms in need of screening and testing. In several of our clinics, patients have been roomed alone to facilitate screening in the room by use of Google Duo, a free video chat product. Rooms with telemedicine capabilities allow patients with potentially communicable infections to be evaluated and observed while avoiding the risk of viral transmission. Additional considerations could include self-administered nasal swabs; although this has comparable efficacy to staff-administered swabs,6 it has not yet been implemented in our clinics.
Direct Care
Virtual care, specifically synchronous video and audio provider-initiated services, is a well-established modality to provide direct care to patients in acute care and ambulatory settings.7 Telemedicine can be deployed to care for hospitalized patients in most locations as long as they meet the operational requirements described below. With a bedside nurse or other facilitator, patients can be interviewed and examined using a high definition camera and digital peripherals, including stethoscopes, otoscopes, ophthalmoscopes, and dermatoscopes. COVID-19 patients or patients under investigation may be seen in this manner. In-person visits should remain part of patients’ care as an important part of the provider-patient relationship8; however, telemedicine could still be deployed to provide direct care and monitoring to these patients while minimizing exposure to healthcare personnel. Additionally, telemedicine can be used for specialist consultations that are likely in high demand with COVID-19, including infectious disease, cardiology, and pulmonology.
Exposure Reduction and Resource Allocation
Currently in the United States there are concerns for shortages of PPE including surgical masks and N95 respirators. Telemedicine can reduce provider exposure, increase provider efficiency, and curtail PPE utilization by minimizing the number and frequency of in-room visits while still allowing virtual visits for direct patient care. For instance, our nursing staff is currently using telemedicine to conduct hourly rounding and limit unnecessary in-room visits.
We recommend keeping telemedicine equipment within individual isolation rooms intended for COVID-19 patients in order to eliminate the need for repeated cleaning. For other patients, a mobile cart could be used. Most commercial video software can autoanswer calls to allow for staff-free history taking. For a thorough physical exam, a bedside facilitator is need for use of digital stethoscopes and similar peripherals.
Provider Shortages and Reducing Burnout
Because SARS-CoV-2 is a highly contagious pathogen that can spread prior to symptom presentation, current CDC guidelines recommend self-monitoring at home for health care workers who have a healthcare-related exposure to a COVID-19 patient.9 This can leave significant gaps in coverage for healthcare systems. For example, in Vacaville, California, one positive case resulted in over 200 health care workers unable to work on site.10
Large volumes of acutely ill patients, coupled with the risk of ill or quarantined providers, means provider shortages due to PRPO are likely to occur and threaten hospitals’ ability to care for patients with or without COVID-19. Furthermore, given increased patient loads, frontline staff are at exceptionally high risk of burnout in pandemic situations. Hospital medicine teams will need contingency plans to meet the needs. Using telemedicine to protect the workforce and maintain staffing levels will reduce that risk.
Telehospitalists can see and examine patients, write orders, and maintain patient service lines much like in-person providers. Recently, we have used it when providers are ill or self-monitoring. In multisite systems, telehospitalists who are privileged in multiple hospitals can be efficiently deployed to meet patient care needs and relieve overburdened providers across hundreds of miles or more.
Enabling patient rooms for telemedicine allows telehospitalists and other providers to see hospitalized patients. Furthermore, quarantined hospitalists can continue to work and support in-person clinical services during PRPO. Providers in high-risk groups (eg, older, immunosuppressed, pregnant) can also continue caring for patients with telemedicine while maintaining safety. As schools close, telemedicine can help providers navigate the challenge between patient care and childcare responsibilities.
OPERATIONAL REQUIREMENTS
The basic element of telemedicine involves a computer or monitor with an internet-connected camera and a HIPAA-compliant video application, but implementation can vary.
Recent changes have allowed the use of popular video chat software such as FaceTime, Skype, or Google Duo for patient interactions; with a tablet attached to a stand, organizations can easily create a mobile telemedicine workstation. Larger monitors or mounted screens can be used in patient areas where portability is not required. A strong network infrastructure and robust IT support are also necessary; as of 2016, 24 million Americans did not have broadband access, and even areas that do can struggle with wireless connectivity in hospitals with thick concrete walls and lack of wi-fi extenders.11
With the addition of a digital stethoscope, hospitalists can perform a thorough history and physical with the aid of bedside staff. This requires dedicated training for all members of the care team in order to optimize the virtual hospitalist’s “telepresence” and create a seamless patient experience. Provider education is imperative: Creating a virtual telepresence is essential in building a strong provider-patient relationship. We have used simulation training to prepare new telehospitalists.
An overlooked, but important, operational requirement is patient education and awareness. In the absence of introduction and onboarding, telemedicine can be viewed by patients as impersonal; however, with proper implementation, high patient satisfaction has been demonstrated in other virtual care experiences.12
FINANCIAL CONSIDERATIONS
Though several health systems offer “tele-ICU” services, the number of hospital medicine programs is more limited. The cost of building a program can be significant, with outlays for equipment, IT support, provider salaries, and training. While all 50 states and the District of Columbia cover some form of fee-for-service live video with Medicaid, only 40, along with DC, have parity laws with commercial payors. Medicare has historically had more restrictions, limiting covered services to specific types of originating sites in certain geographic areas. Furthermore, growth of telehospitalist programs has been hampered by the lack of reimbursement for “primary care services.”13
With passage of the Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020, geographic and site restrictions have been waived for Medicare reimbursement.14 Providers must still demonstrate a prior relationship with patients, which requires at least one encounter with the patient in the past 3 years by the same provider or one with a similar tax identification number (TIN). All hospitalists within our group are identified with a common TIN, which helps to meet this requirement for patient with recent admissions. However, clear guidance on reimbursement for primary care services by acute care providers is still lacking. As the utility of telemedicine is demonstrated in the hospital setting, we hope further changes may be enacted.
Organizations must properly credential and privilege telehospitalists. Telemedicine services may fall under either core or “delegated” privileges depending on the individual hospital. Additionally, while malpractice insurance does typically cover telemedicine services, each organization should verify this with their particular carrier.
SUMMARY
The COVID-19 pandemic has created a systemic challenge for healthcare systems across the nation. As hospitalists continue to be on the front lines, organizations can leverage telemedicine to support their patients, protect their clinicians, and conserve scarce resources. Building out a virtual care program is intricate and requires significant operational support. Laying the groundwork now can prepare institutions to provide necessary care for patients, not just in the current pandemic, but in numerous emergency health care situations in the future.
1. Lurie N, Carr BG. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;178(6):745-74. https://doi.org/10.1001/jamainternmed.2018.1314.
2. Virtuwell. HealthPartners. 2020. https://www.virtuwell.com.
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
4. Powell A. Coronavirus screening may miss two-thirds of infected travelers entering U.S. The Harvard Gazette. 2020. https://news.harvard.edu/gazette/story/2020/03/hundreds-of-u-s-coronavirus-cases-may-have-slipped-through-screenings/. Accessed March 13, 2020.
5. Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007:104(18);7582-7587. https://doi.org/10.1073/pnas.0610941104.
6. Akmatov MK, Gatzemeier A, Schughart, K, Pessler F. Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens. PLoS One. 2012:7(11);e48508. https://doi.org/10.1371/journal.pone.0048508.
7. Centers for Medicare & Medicaid Services. Telehealth Services. 2019. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Telehealth Srvcsfctsht.pdf. Accessed March 14, 2020.
8. Daniel H, Sulmasy LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. 2015;163(10):787-789. https://doi.org/10.7326/M15-0498.
9. Centers for Disease Control and Prevention. Healthcare Personnel with Potential Exposure to COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed March 13, 2020.
10. Gold J. Surging Health Care Worker Quarantines Raise Concerns as Coronavirus Spreads. Kaiser Health News. 2020. https://khn.org/news/surging-health-care-worker-quarantines-raise-concerns-as-coronavirus-spreads/. Accessed March 12, 2020.
11. Federal Communications Commission. 2018 Broadband Deployment Report. 2018. https://www.fcc.gov/reports-research/reports/broadband-progress-reports/2018-broadband-deployment-report. Accessed March 13, 2020.
12. Martinez KA, Rood M, Jhangiani N, et al. Patterns of use and correlates of patient satisfaction with a large nationwide direct to consumer telemedicine service. J Gen Intern Med. 2018;33(10):1768-1773. https://doi.org/10.1007/s11606-018-4621-5.
13. Centers for Medicare & Medicaid Services. List of Telehealth Services. 2019. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed March 13, 2020.
14. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, H.R. 6074, 116th Cong. 2020. https://congress.gov/bill/116th-congress/house-bill/6074/. Accessed March 13, 2020.
1. Lurie N, Carr BG. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;178(6):745-74. https://doi.org/10.1001/jamainternmed.2018.1314.
2. Virtuwell. HealthPartners. 2020. https://www.virtuwell.com.
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
4. Powell A. Coronavirus screening may miss two-thirds of infected travelers entering U.S. The Harvard Gazette. 2020. https://news.harvard.edu/gazette/story/2020/03/hundreds-of-u-s-coronavirus-cases-may-have-slipped-through-screenings/. Accessed March 13, 2020.
5. Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007:104(18);7582-7587. https://doi.org/10.1073/pnas.0610941104.
6. Akmatov MK, Gatzemeier A, Schughart, K, Pessler F. Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens. PLoS One. 2012:7(11);e48508. https://doi.org/10.1371/journal.pone.0048508.
7. Centers for Medicare & Medicaid Services. Telehealth Services. 2019. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Telehealth Srvcsfctsht.pdf. Accessed March 14, 2020.
8. Daniel H, Sulmasy LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. 2015;163(10):787-789. https://doi.org/10.7326/M15-0498.
9. Centers for Disease Control and Prevention. Healthcare Personnel with Potential Exposure to COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed March 13, 2020.
10. Gold J. Surging Health Care Worker Quarantines Raise Concerns as Coronavirus Spreads. Kaiser Health News. 2020. https://khn.org/news/surging-health-care-worker-quarantines-raise-concerns-as-coronavirus-spreads/. Accessed March 12, 2020.
11. Federal Communications Commission. 2018 Broadband Deployment Report. 2018. https://www.fcc.gov/reports-research/reports/broadband-progress-reports/2018-broadband-deployment-report. Accessed March 13, 2020.
12. Martinez KA, Rood M, Jhangiani N, et al. Patterns of use and correlates of patient satisfaction with a large nationwide direct to consumer telemedicine service. J Gen Intern Med. 2018;33(10):1768-1773. https://doi.org/10.1007/s11606-018-4621-5.
13. Centers for Medicare & Medicaid Services. List of Telehealth Services. 2019. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed March 13, 2020.
14. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, H.R. 6074, 116th Cong. 2020. https://congress.gov/bill/116th-congress/house-bill/6074/. Accessed March 13, 2020.
© 2020 Society of Hospital Medicine
Use of an Electronic Alert Tool to Prevent Readmissions Following Coronary Artery Bypass Graft Surgery
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; smithsd@uncw.edu.
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; smithsd@uncw.edu.
Funding disclosures: None.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; smithsd@uncw.edu.
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
Impact of Hospitalists on Care Outcomes in a Large Integrated Health System in British Columbia
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
The Society of Hospital Medicine’s Commitment to Increasing Academic Representation for Women and Underrepresented Groups in Medicine: A Good Start
Documentation of gender-based disparities in medicine often focus on lower numbers of women in prominent positions as evidence of inequality and inequity; examples include lower proportion of women physicians as conference speakers,1 first and last authors of manuscripts,2 invited editorials,3 award recipients,4 grant recipients,5 medical society leadership,6 editorial boards,7 and presenters at grand rounds.8 Notably, these disparities are likely greater for intersectional physicians, who experience bias through multiple lenses of disadvantage.9 While the scarcity of women and marginalized populations in leadership roles in medicine provides convincing evidence that inequality exists, the underrepresentation of women and other marginalized physicians in prominent positions is also a cause of continued disparity. Fewer academic opportunities for women physicians and other underrepresented physician groups in medicine may perpetuate slower career advancement10 and contribute to less availability of mentors and sponsors.11 Less obviously, underrepresentation also unintentionally and explicitly signals to junior faculty from marginalized groups that they are not welcome and are unlikely to be successful.9,12
Improving representation of women in other fields has been demonstrated to reduce implicit and explicit sexism.13,14 Increasing diversity in academic leadership is likely to further improve diversity at all levels,9,15 which may in turn reduce gaps in health outcomes seen for marginalized patients.16-18 Measuring and eliminating bias that disadvantages underrepresented physicians in academic opportunities is a moral imperative for institutions and organizations. For this reason, the Society of Hospital Medicine (SHM) has been attempting to address this issue within its organizational structure, publications, and conference presenters.19
The first step for an organization that aims to increase representation of women and other marginalized groups in medicine is to assess the current representation of leadership and opportunities.20 If data are available, this review should include intersectional measurement of other axes of discrimination. Rapid analysis of large data sets of names is feasible using freely available computer algorithms, for example.21 Only once a baseline understanding of representation within an organization is established can identification of goals and areas of improvement and evaluation of efforts to increase representation begin. Reporting this data to the organization’s membership should be undertaken to increase the accountability of leadership to reduce gaps. This work is currently underway at the Journal of Hospital Medicine and within the Society of Hospital Medicine.19
This month’s issue of the Journal of Hospital Medicine includes an article written by Northcutt, et al that describes one such attempt, focusing on representation of conference speakers at SHM’s Annual Meeting. In this study, authors performed a pre- and postintervention analysis of an open call system for selecting didactic speakers for the SHM Annual Meeting. The open call system, implemented for the 2019 SHM Annual Meeting, invited all members to apply for a didactic session. The planning committee then utilized a standardized evaluation form to determine the final speaker list. In previous years, didactic speakers did not apply but were invited and were not formally evaluated. Northcutt et al report that this intervention was associated with a significant increase in the proportion of women conference speakers.22
The Northcutt article and the open call and evaluation system is one example of an intentional adjustment to the speaker selection process aimed at recruiting more diverse presenters. Other examples of intentional efforts to increase diversity within conferences include using curated lists designed to improve representation or contacting other national organizations for recommendations. 20 Efforts such as these are necessary because men in medicine are more likely to volunteer for prominent positions than women,23 meaning that any system of recruitment or allocation of academic opportunities that relies on self-promotion is likely to perpetuate underrepresentation. Using pre-existing speakers list or previous programs will also support ongoing disparities, because men have traditionally represented the majority of speakers.
Of course, conferences are an important and public representation of a society, but are only the starting point for working towards equity within a large organization such as SHM. Similar efforts must be directed towards authorship in SHM publications, representation on editorial boards, society leadership and employment opportunities. Once organizations have an established baseline around publications, leadership recruitment, and employment representation, a review of recruitment policies (for articles, speakers, leaders, and employees) should then be conducted, looking for areas that lead to bias.
Planning committees, editorial boards, and society leadership groups should also intentionally increase their own diversity, as increasing the proportion of women on a convening committee has been demonstrated to increase the number of invited women speakers.15,24 In addition, committees can adopt a mandate to increase diversity in invited speakers, editorials, and authorship; for example, direct instruction to avoid all-male panels led a conference planning committee to invite more women and increased the numbers of women speakers.25 A speaker, authorship, or editorial policy that emphasizes diversity and inclusion should be developed and made available to the organization’s membership.26
Finally, there is evidence that implicit bias training for editorial boards and conference planning committees may be effective.27 Implicit bias training emphasizes that judgements of merit and skill are often subjective and based on in-group membership rather than the quality of applicants.9 For example, underrepresentation of women at a neuroimmunology conference was not explained by quantity or impact of previous publications,28 and evaluation scores for the Society for Hospital Medicine’s Annual Meeting have increased as the proportion of women speakers has increased, suggesting that the presence of women presenters was associated with better presentations. To address concerns about how diversity and inclusion efforts may influence the quality of speakers and authors,29 objective criteria could be developed in advance of a selection process and candidates should be held to the same standard.30 The use of objective evaluation criteria in the selection of conference speakers has also been associated with increasing the proportion of women conference speakers. All in all, SHM’s efforts (and Northcutt’s work) should be lauded but also recognized as what they are: a good start. Continued vigilance focused on equity is the only way to ensure that the move towards greater representation continues.
1. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/ 10.1001/jamanetworkopen.2019.2103.
2. Penn CA, Ebott JA, Larach DB, Hesson AM, Waljee JF, Larach MG. The gender authorship gap in gynecologic oncology research. Gynecol Oncol Rep. 2019;29:83-84. https://doi.org/10.1016/j.gore.2019.07.011.
3. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682.https://doi.org/10.1001/jamanetworkopen.2019.13682.
4. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/ 10.1016/j.pmrj.2017.06.001.
5. Burns KEA, Straus SE, Liu K, Rizv, L, Guyatt G. Gender differences in grant and personnel award funding rates at the Canadian Institute of Health Research based on research content area: a retrospective analysis. PLoS Med. 2019;16(10):e1002935. https://doi.org/ 10.1371/journal.pmed.1002935.
6. Silver JK, Ghalib R, Poorman JA, Al-Assi D, Parangi S, Bhargava H, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Internal Medicine. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
7. Erren TC, Groß JV, Shaw DM, Selle B. Representation of women as authors, reviewers, editors in chief, and editorial board members at 6 general medical journals in 2010 and 2011. JAMA Intern Med. 2014;174(4):633-635. https://doi.org/ 10.1001/jamainternmed.2013.14760.
8. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. https://doi.org/ 10.1089/jwh.2016.6044.
9. Price EG, Gozu A, Kern DE, et al. The role of cultural diversity climate in recruitment, promotion, and retention of faculty in academic medicine. J Gen Intern Med. 2005;20(7):565-571. https://doi.org/10.1111/j.1525-1497.2005.0127.x.
10. Carr PL, Gunn CM, Kaplan SA, Raj A, Freund KM. Inadequate progress for women in academic medicine: findings from the National Faculty Study. J Womens Health (Larchmt). 2015;24(3):190-199. https://doi.org/10.1089/jwh.2014.4848.
11. Farkas AH, Bonifacino E, Turner R, Tilstra SA, Corbelli JA. Mentorship of women in academic medicine: a systematic review. J Gen Intern Med. 2019;34(7):1322-1329. https://doi.org/10.1007/s11606-019-04955-2.
12. Pololi L, Cooper LA, Carr P. Race, disadvantage and faculty experiences in academic medicine. J Gen Intern Med. 2010;25(12):1363-1369. https://doi.org/10.1007/s11606-010-1478-7.
13. Beaman L CR, Duflo E, Pande R, Topalova P. Powerful women: does exposure reduce bias? Q J Econ. 2009;124(4):1497-1540.
14. Mansbridge J. Should Blacks represent Blacks and women represent women? A contingent “Yes”. J Polit. 1999;61(3):628-657. https://doi.org/ https://doi.org/10.2307/2647821.
15. Lithgow KC, Fletcher, S., Earp, M.E., Bharwani, A., Ruzycki, S.M. Association between the proprtion of women on a conference planning committee and the proportion of women conference speakers at medical conferences. JAMA Netw Open. 2020; In press.
16. Alsan M, Garrick, O., Graziani, G.C. Does diversity matter for health? Experimental evidence from Oakland. National Bureau of Economic Research. 2018.
17. Greenwood BN, Carnahan, S., Huang, L. Patient–physician gender concordance and increased mortality among female heart attack patients. Proc Natl Acad Sci USA. 2018;115(34):8569-8574. https://doi.org/10.1073/pnas.1800097115.
18. Silver JK, Bean AC, Slocum C, et al. Physician Workforce Disparities and Patient Care: A Narrative Review. Health Equity. 2019;3(1):360-777. https://doi.org/10.1089/heq.2019.0040.
19. Shah SS, Shaughnessy, E.E., Spector, N.D. Leading by example: How medical journals can improve representation in academic medicine. J Hos Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
20. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/ 10.1371/journal.pcbi.1003903.
21. Sumner J. The Gender Balance Assessment Tool (GBAT): a web-based tool for estimating gender balance in syllabi and bibliographies. Polit Sci Polit. 2018;2(51):396-400. https://doi.org/10.1017/S1049096517002074.
22. Northcutt N, Papp S, Keniston A, et al; on behalf of the Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: A Follow-UP Study of Gender Equity for Conference Speakers from 2015 to 2019. The SPEAK Up Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401.
23. Wayne NL, Vermillion M, Uijtdehaage S. Gender differences in leadership amongst first-year medical students in the small-group setting. Acad Med. 2010;85(8):1276-1281. https://doi.org/10.1097/ACM.0b013e3181e5f2ce
24. Casadevall A, Handelsman J. The presence of female conveners correlates with a higher proportion of female speakers at scientific symposia. MBio. 2014;5(1):e00846-13. https://doi.org/10.1128/mBio.00846-13.25. Casadevall A. Achieving speaker gender equity at the American Society for Microbiology General Meeting. MBio. 2015;6(4):e01146. https://doi.org/10.1128/mBio.01146-15.
26. Health NIo. Guidelines for the inclusion of women, minorities, and persons with disabilities in NIH-supported conference grats 2003. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-066.html. Accessed March 12, 2019.
27. Devine PG, Forscher PS, Cox WTL, Kaatz A, Sheridan J, Carnes M. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215. https://doi.org/10.1016/j.jesp.2017.07.002.
28. Klein RS, Voskuhl, R, Segal BM, et al. Speaking out about gender imbalance in invited speakers improves diversity. Nat Immunol. 201;18(5):475-478. https://doi.org/10.1038/ni.3707.
29. Borrero-Mejias C, Starling AJ, Burch R, Loder E. Ten (Eleven) things not to say to your female colleagues. Headache. 2019;59(10):1846-1854. https://doi.org/10.1111/head.13647.
30. Bandiera G, Abrahams C, Ruetalo M, Hanson MD, Nickell L, Spadafora S. Identifying and promoting best practices in residency application and selection in a complex academic health network. Acad Med. 2015;90(12):1594-1601. https://doi.org/10.1097/ACM.0000000000000954.
Documentation of gender-based disparities in medicine often focus on lower numbers of women in prominent positions as evidence of inequality and inequity; examples include lower proportion of women physicians as conference speakers,1 first and last authors of manuscripts,2 invited editorials,3 award recipients,4 grant recipients,5 medical society leadership,6 editorial boards,7 and presenters at grand rounds.8 Notably, these disparities are likely greater for intersectional physicians, who experience bias through multiple lenses of disadvantage.9 While the scarcity of women and marginalized populations in leadership roles in medicine provides convincing evidence that inequality exists, the underrepresentation of women and other marginalized physicians in prominent positions is also a cause of continued disparity. Fewer academic opportunities for women physicians and other underrepresented physician groups in medicine may perpetuate slower career advancement10 and contribute to less availability of mentors and sponsors.11 Less obviously, underrepresentation also unintentionally and explicitly signals to junior faculty from marginalized groups that they are not welcome and are unlikely to be successful.9,12
Improving representation of women in other fields has been demonstrated to reduce implicit and explicit sexism.13,14 Increasing diversity in academic leadership is likely to further improve diversity at all levels,9,15 which may in turn reduce gaps in health outcomes seen for marginalized patients.16-18 Measuring and eliminating bias that disadvantages underrepresented physicians in academic opportunities is a moral imperative for institutions and organizations. For this reason, the Society of Hospital Medicine (SHM) has been attempting to address this issue within its organizational structure, publications, and conference presenters.19
The first step for an organization that aims to increase representation of women and other marginalized groups in medicine is to assess the current representation of leadership and opportunities.20 If data are available, this review should include intersectional measurement of other axes of discrimination. Rapid analysis of large data sets of names is feasible using freely available computer algorithms, for example.21 Only once a baseline understanding of representation within an organization is established can identification of goals and areas of improvement and evaluation of efforts to increase representation begin. Reporting this data to the organization’s membership should be undertaken to increase the accountability of leadership to reduce gaps. This work is currently underway at the Journal of Hospital Medicine and within the Society of Hospital Medicine.19
This month’s issue of the Journal of Hospital Medicine includes an article written by Northcutt, et al that describes one such attempt, focusing on representation of conference speakers at SHM’s Annual Meeting. In this study, authors performed a pre- and postintervention analysis of an open call system for selecting didactic speakers for the SHM Annual Meeting. The open call system, implemented for the 2019 SHM Annual Meeting, invited all members to apply for a didactic session. The planning committee then utilized a standardized evaluation form to determine the final speaker list. In previous years, didactic speakers did not apply but were invited and were not formally evaluated. Northcutt et al report that this intervention was associated with a significant increase in the proportion of women conference speakers.22
The Northcutt article and the open call and evaluation system is one example of an intentional adjustment to the speaker selection process aimed at recruiting more diverse presenters. Other examples of intentional efforts to increase diversity within conferences include using curated lists designed to improve representation or contacting other national organizations for recommendations. 20 Efforts such as these are necessary because men in medicine are more likely to volunteer for prominent positions than women,23 meaning that any system of recruitment or allocation of academic opportunities that relies on self-promotion is likely to perpetuate underrepresentation. Using pre-existing speakers list or previous programs will also support ongoing disparities, because men have traditionally represented the majority of speakers.
Of course, conferences are an important and public representation of a society, but are only the starting point for working towards equity within a large organization such as SHM. Similar efforts must be directed towards authorship in SHM publications, representation on editorial boards, society leadership and employment opportunities. Once organizations have an established baseline around publications, leadership recruitment, and employment representation, a review of recruitment policies (for articles, speakers, leaders, and employees) should then be conducted, looking for areas that lead to bias.
Planning committees, editorial boards, and society leadership groups should also intentionally increase their own diversity, as increasing the proportion of women on a convening committee has been demonstrated to increase the number of invited women speakers.15,24 In addition, committees can adopt a mandate to increase diversity in invited speakers, editorials, and authorship; for example, direct instruction to avoid all-male panels led a conference planning committee to invite more women and increased the numbers of women speakers.25 A speaker, authorship, or editorial policy that emphasizes diversity and inclusion should be developed and made available to the organization’s membership.26
Finally, there is evidence that implicit bias training for editorial boards and conference planning committees may be effective.27 Implicit bias training emphasizes that judgements of merit and skill are often subjective and based on in-group membership rather than the quality of applicants.9 For example, underrepresentation of women at a neuroimmunology conference was not explained by quantity or impact of previous publications,28 and evaluation scores for the Society for Hospital Medicine’s Annual Meeting have increased as the proportion of women speakers has increased, suggesting that the presence of women presenters was associated with better presentations. To address concerns about how diversity and inclusion efforts may influence the quality of speakers and authors,29 objective criteria could be developed in advance of a selection process and candidates should be held to the same standard.30 The use of objective evaluation criteria in the selection of conference speakers has also been associated with increasing the proportion of women conference speakers. All in all, SHM’s efforts (and Northcutt’s work) should be lauded but also recognized as what they are: a good start. Continued vigilance focused on equity is the only way to ensure that the move towards greater representation continues.
Documentation of gender-based disparities in medicine often focus on lower numbers of women in prominent positions as evidence of inequality and inequity; examples include lower proportion of women physicians as conference speakers,1 first and last authors of manuscripts,2 invited editorials,3 award recipients,4 grant recipients,5 medical society leadership,6 editorial boards,7 and presenters at grand rounds.8 Notably, these disparities are likely greater for intersectional physicians, who experience bias through multiple lenses of disadvantage.9 While the scarcity of women and marginalized populations in leadership roles in medicine provides convincing evidence that inequality exists, the underrepresentation of women and other marginalized physicians in prominent positions is also a cause of continued disparity. Fewer academic opportunities for women physicians and other underrepresented physician groups in medicine may perpetuate slower career advancement10 and contribute to less availability of mentors and sponsors.11 Less obviously, underrepresentation also unintentionally and explicitly signals to junior faculty from marginalized groups that they are not welcome and are unlikely to be successful.9,12
Improving representation of women in other fields has been demonstrated to reduce implicit and explicit sexism.13,14 Increasing diversity in academic leadership is likely to further improve diversity at all levels,9,15 which may in turn reduce gaps in health outcomes seen for marginalized patients.16-18 Measuring and eliminating bias that disadvantages underrepresented physicians in academic opportunities is a moral imperative for institutions and organizations. For this reason, the Society of Hospital Medicine (SHM) has been attempting to address this issue within its organizational structure, publications, and conference presenters.19
The first step for an organization that aims to increase representation of women and other marginalized groups in medicine is to assess the current representation of leadership and opportunities.20 If data are available, this review should include intersectional measurement of other axes of discrimination. Rapid analysis of large data sets of names is feasible using freely available computer algorithms, for example.21 Only once a baseline understanding of representation within an organization is established can identification of goals and areas of improvement and evaluation of efforts to increase representation begin. Reporting this data to the organization’s membership should be undertaken to increase the accountability of leadership to reduce gaps. This work is currently underway at the Journal of Hospital Medicine and within the Society of Hospital Medicine.19
This month’s issue of the Journal of Hospital Medicine includes an article written by Northcutt, et al that describes one such attempt, focusing on representation of conference speakers at SHM’s Annual Meeting. In this study, authors performed a pre- and postintervention analysis of an open call system for selecting didactic speakers for the SHM Annual Meeting. The open call system, implemented for the 2019 SHM Annual Meeting, invited all members to apply for a didactic session. The planning committee then utilized a standardized evaluation form to determine the final speaker list. In previous years, didactic speakers did not apply but were invited and were not formally evaluated. Northcutt et al report that this intervention was associated with a significant increase in the proportion of women conference speakers.22
The Northcutt article and the open call and evaluation system is one example of an intentional adjustment to the speaker selection process aimed at recruiting more diverse presenters. Other examples of intentional efforts to increase diversity within conferences include using curated lists designed to improve representation or contacting other national organizations for recommendations. 20 Efforts such as these are necessary because men in medicine are more likely to volunteer for prominent positions than women,23 meaning that any system of recruitment or allocation of academic opportunities that relies on self-promotion is likely to perpetuate underrepresentation. Using pre-existing speakers list or previous programs will also support ongoing disparities, because men have traditionally represented the majority of speakers.
Of course, conferences are an important and public representation of a society, but are only the starting point for working towards equity within a large organization such as SHM. Similar efforts must be directed towards authorship in SHM publications, representation on editorial boards, society leadership and employment opportunities. Once organizations have an established baseline around publications, leadership recruitment, and employment representation, a review of recruitment policies (for articles, speakers, leaders, and employees) should then be conducted, looking for areas that lead to bias.
Planning committees, editorial boards, and society leadership groups should also intentionally increase their own diversity, as increasing the proportion of women on a convening committee has been demonstrated to increase the number of invited women speakers.15,24 In addition, committees can adopt a mandate to increase diversity in invited speakers, editorials, and authorship; for example, direct instruction to avoid all-male panels led a conference planning committee to invite more women and increased the numbers of women speakers.25 A speaker, authorship, or editorial policy that emphasizes diversity and inclusion should be developed and made available to the organization’s membership.26
Finally, there is evidence that implicit bias training for editorial boards and conference planning committees may be effective.27 Implicit bias training emphasizes that judgements of merit and skill are often subjective and based on in-group membership rather than the quality of applicants.9 For example, underrepresentation of women at a neuroimmunology conference was not explained by quantity or impact of previous publications,28 and evaluation scores for the Society for Hospital Medicine’s Annual Meeting have increased as the proportion of women speakers has increased, suggesting that the presence of women presenters was associated with better presentations. To address concerns about how diversity and inclusion efforts may influence the quality of speakers and authors,29 objective criteria could be developed in advance of a selection process and candidates should be held to the same standard.30 The use of objective evaluation criteria in the selection of conference speakers has also been associated with increasing the proportion of women conference speakers. All in all, SHM’s efforts (and Northcutt’s work) should be lauded but also recognized as what they are: a good start. Continued vigilance focused on equity is the only way to ensure that the move towards greater representation continues.
1. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/ 10.1001/jamanetworkopen.2019.2103.
2. Penn CA, Ebott JA, Larach DB, Hesson AM, Waljee JF, Larach MG. The gender authorship gap in gynecologic oncology research. Gynecol Oncol Rep. 2019;29:83-84. https://doi.org/10.1016/j.gore.2019.07.011.
3. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682.https://doi.org/10.1001/jamanetworkopen.2019.13682.
4. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/ 10.1016/j.pmrj.2017.06.001.
5. Burns KEA, Straus SE, Liu K, Rizv, L, Guyatt G. Gender differences in grant and personnel award funding rates at the Canadian Institute of Health Research based on research content area: a retrospective analysis. PLoS Med. 2019;16(10):e1002935. https://doi.org/ 10.1371/journal.pmed.1002935.
6. Silver JK, Ghalib R, Poorman JA, Al-Assi D, Parangi S, Bhargava H, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Internal Medicine. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
7. Erren TC, Groß JV, Shaw DM, Selle B. Representation of women as authors, reviewers, editors in chief, and editorial board members at 6 general medical journals in 2010 and 2011. JAMA Intern Med. 2014;174(4):633-635. https://doi.org/ 10.1001/jamainternmed.2013.14760.
8. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. https://doi.org/ 10.1089/jwh.2016.6044.
9. Price EG, Gozu A, Kern DE, et al. The role of cultural diversity climate in recruitment, promotion, and retention of faculty in academic medicine. J Gen Intern Med. 2005;20(7):565-571. https://doi.org/10.1111/j.1525-1497.2005.0127.x.
10. Carr PL, Gunn CM, Kaplan SA, Raj A, Freund KM. Inadequate progress for women in academic medicine: findings from the National Faculty Study. J Womens Health (Larchmt). 2015;24(3):190-199. https://doi.org/10.1089/jwh.2014.4848.
11. Farkas AH, Bonifacino E, Turner R, Tilstra SA, Corbelli JA. Mentorship of women in academic medicine: a systematic review. J Gen Intern Med. 2019;34(7):1322-1329. https://doi.org/10.1007/s11606-019-04955-2.
12. Pololi L, Cooper LA, Carr P. Race, disadvantage and faculty experiences in academic medicine. J Gen Intern Med. 2010;25(12):1363-1369. https://doi.org/10.1007/s11606-010-1478-7.
13. Beaman L CR, Duflo E, Pande R, Topalova P. Powerful women: does exposure reduce bias? Q J Econ. 2009;124(4):1497-1540.
14. Mansbridge J. Should Blacks represent Blacks and women represent women? A contingent “Yes”. J Polit. 1999;61(3):628-657. https://doi.org/ https://doi.org/10.2307/2647821.
15. Lithgow KC, Fletcher, S., Earp, M.E., Bharwani, A., Ruzycki, S.M. Association between the proprtion of women on a conference planning committee and the proportion of women conference speakers at medical conferences. JAMA Netw Open. 2020; In press.
16. Alsan M, Garrick, O., Graziani, G.C. Does diversity matter for health? Experimental evidence from Oakland. National Bureau of Economic Research. 2018.
17. Greenwood BN, Carnahan, S., Huang, L. Patient–physician gender concordance and increased mortality among female heart attack patients. Proc Natl Acad Sci USA. 2018;115(34):8569-8574. https://doi.org/10.1073/pnas.1800097115.
18. Silver JK, Bean AC, Slocum C, et al. Physician Workforce Disparities and Patient Care: A Narrative Review. Health Equity. 2019;3(1):360-777. https://doi.org/10.1089/heq.2019.0040.
19. Shah SS, Shaughnessy, E.E., Spector, N.D. Leading by example: How medical journals can improve representation in academic medicine. J Hos Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
20. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/ 10.1371/journal.pcbi.1003903.
21. Sumner J. The Gender Balance Assessment Tool (GBAT): a web-based tool for estimating gender balance in syllabi and bibliographies. Polit Sci Polit. 2018;2(51):396-400. https://doi.org/10.1017/S1049096517002074.
22. Northcutt N, Papp S, Keniston A, et al; on behalf of the Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: A Follow-UP Study of Gender Equity for Conference Speakers from 2015 to 2019. The SPEAK Up Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401.
23. Wayne NL, Vermillion M, Uijtdehaage S. Gender differences in leadership amongst first-year medical students in the small-group setting. Acad Med. 2010;85(8):1276-1281. https://doi.org/10.1097/ACM.0b013e3181e5f2ce
24. Casadevall A, Handelsman J. The presence of female conveners correlates with a higher proportion of female speakers at scientific symposia. MBio. 2014;5(1):e00846-13. https://doi.org/10.1128/mBio.00846-13.25. Casadevall A. Achieving speaker gender equity at the American Society for Microbiology General Meeting. MBio. 2015;6(4):e01146. https://doi.org/10.1128/mBio.01146-15.
26. Health NIo. Guidelines for the inclusion of women, minorities, and persons with disabilities in NIH-supported conference grats 2003. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-066.html. Accessed March 12, 2019.
27. Devine PG, Forscher PS, Cox WTL, Kaatz A, Sheridan J, Carnes M. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215. https://doi.org/10.1016/j.jesp.2017.07.002.
28. Klein RS, Voskuhl, R, Segal BM, et al. Speaking out about gender imbalance in invited speakers improves diversity. Nat Immunol. 201;18(5):475-478. https://doi.org/10.1038/ni.3707.
29. Borrero-Mejias C, Starling AJ, Burch R, Loder E. Ten (Eleven) things not to say to your female colleagues. Headache. 2019;59(10):1846-1854. https://doi.org/10.1111/head.13647.
30. Bandiera G, Abrahams C, Ruetalo M, Hanson MD, Nickell L, Spadafora S. Identifying and promoting best practices in residency application and selection in a complex academic health network. Acad Med. 2015;90(12):1594-1601. https://doi.org/10.1097/ACM.0000000000000954.
1. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/ 10.1001/jamanetworkopen.2019.2103.
2. Penn CA, Ebott JA, Larach DB, Hesson AM, Waljee JF, Larach MG. The gender authorship gap in gynecologic oncology research. Gynecol Oncol Rep. 2019;29:83-84. https://doi.org/10.1016/j.gore.2019.07.011.
3. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682.https://doi.org/10.1001/jamanetworkopen.2019.13682.
4. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/ 10.1016/j.pmrj.2017.06.001.
5. Burns KEA, Straus SE, Liu K, Rizv, L, Guyatt G. Gender differences in grant and personnel award funding rates at the Canadian Institute of Health Research based on research content area: a retrospective analysis. PLoS Med. 2019;16(10):e1002935. https://doi.org/ 10.1371/journal.pmed.1002935.
6. Silver JK, Ghalib R, Poorman JA, Al-Assi D, Parangi S, Bhargava H, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Internal Medicine. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
7. Erren TC, Groß JV, Shaw DM, Selle B. Representation of women as authors, reviewers, editors in chief, and editorial board members at 6 general medical journals in 2010 and 2011. JAMA Intern Med. 2014;174(4):633-635. https://doi.org/ 10.1001/jamainternmed.2013.14760.
8. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. https://doi.org/ 10.1089/jwh.2016.6044.
9. Price EG, Gozu A, Kern DE, et al. The role of cultural diversity climate in recruitment, promotion, and retention of faculty in academic medicine. J Gen Intern Med. 2005;20(7):565-571. https://doi.org/10.1111/j.1525-1497.2005.0127.x.
10. Carr PL, Gunn CM, Kaplan SA, Raj A, Freund KM. Inadequate progress for women in academic medicine: findings from the National Faculty Study. J Womens Health (Larchmt). 2015;24(3):190-199. https://doi.org/10.1089/jwh.2014.4848.
11. Farkas AH, Bonifacino E, Turner R, Tilstra SA, Corbelli JA. Mentorship of women in academic medicine: a systematic review. J Gen Intern Med. 2019;34(7):1322-1329. https://doi.org/10.1007/s11606-019-04955-2.
12. Pololi L, Cooper LA, Carr P. Race, disadvantage and faculty experiences in academic medicine. J Gen Intern Med. 2010;25(12):1363-1369. https://doi.org/10.1007/s11606-010-1478-7.
13. Beaman L CR, Duflo E, Pande R, Topalova P. Powerful women: does exposure reduce bias? Q J Econ. 2009;124(4):1497-1540.
14. Mansbridge J. Should Blacks represent Blacks and women represent women? A contingent “Yes”. J Polit. 1999;61(3):628-657. https://doi.org/ https://doi.org/10.2307/2647821.
15. Lithgow KC, Fletcher, S., Earp, M.E., Bharwani, A., Ruzycki, S.M. Association between the proprtion of women on a conference planning committee and the proportion of women conference speakers at medical conferences. JAMA Netw Open. 2020; In press.
16. Alsan M, Garrick, O., Graziani, G.C. Does diversity matter for health? Experimental evidence from Oakland. National Bureau of Economic Research. 2018.
17. Greenwood BN, Carnahan, S., Huang, L. Patient–physician gender concordance and increased mortality among female heart attack patients. Proc Natl Acad Sci USA. 2018;115(34):8569-8574. https://doi.org/10.1073/pnas.1800097115.
18. Silver JK, Bean AC, Slocum C, et al. Physician Workforce Disparities and Patient Care: A Narrative Review. Health Equity. 2019;3(1):360-777. https://doi.org/10.1089/heq.2019.0040.
19. Shah SS, Shaughnessy, E.E., Spector, N.D. Leading by example: How medical journals can improve representation in academic medicine. J Hos Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
20. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/ 10.1371/journal.pcbi.1003903.
21. Sumner J. The Gender Balance Assessment Tool (GBAT): a web-based tool for estimating gender balance in syllabi and bibliographies. Polit Sci Polit. 2018;2(51):396-400. https://doi.org/10.1017/S1049096517002074.
22. Northcutt N, Papp S, Keniston A, et al; on behalf of the Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: A Follow-UP Study of Gender Equity for Conference Speakers from 2015 to 2019. The SPEAK Up Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401.
23. Wayne NL, Vermillion M, Uijtdehaage S. Gender differences in leadership amongst first-year medical students in the small-group setting. Acad Med. 2010;85(8):1276-1281. https://doi.org/10.1097/ACM.0b013e3181e5f2ce
24. Casadevall A, Handelsman J. The presence of female conveners correlates with a higher proportion of female speakers at scientific symposia. MBio. 2014;5(1):e00846-13. https://doi.org/10.1128/mBio.00846-13.25. Casadevall A. Achieving speaker gender equity at the American Society for Microbiology General Meeting. MBio. 2015;6(4):e01146. https://doi.org/10.1128/mBio.01146-15.
26. Health NIo. Guidelines for the inclusion of women, minorities, and persons with disabilities in NIH-supported conference grats 2003. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-066.html. Accessed March 12, 2019.
27. Devine PG, Forscher PS, Cox WTL, Kaatz A, Sheridan J, Carnes M. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215. https://doi.org/10.1016/j.jesp.2017.07.002.
28. Klein RS, Voskuhl, R, Segal BM, et al. Speaking out about gender imbalance in invited speakers improves diversity. Nat Immunol. 201;18(5):475-478. https://doi.org/10.1038/ni.3707.
29. Borrero-Mejias C, Starling AJ, Burch R, Loder E. Ten (Eleven) things not to say to your female colleagues. Headache. 2019;59(10):1846-1854. https://doi.org/10.1111/head.13647.
30. Bandiera G, Abrahams C, Ruetalo M, Hanson MD, Nickell L, Spadafora S. Identifying and promoting best practices in residency application and selection in a complex academic health network. Acad Med. 2015;90(12):1594-1601. https://doi.org/10.1097/ACM.0000000000000954.
© 2020 Society of Hospital Medicine
Leadership & Professional Development: Evidence-Based Strategies to Make Team Meetings More Effective
“Without meeting leadership skills, one joins the ranks of so many others who bear the responsibility for the meeting ‘problem’ and are the cause of so much frustration in the workplace.”1 Physicians, like so many others, often feel that team meetings are inefficient, a waste of time, and mentally draining. It does not have to be this way. There are evidence-based strategies that can make meetings truly work and actually enjoyable to attend.2 This is particularly important because eliminating meetings is a false solution. Hospitals need team meetings to promote coordination, collaboration, communication, and consensus decision-making. While no one individual can solve the meetings problem, each of us can find a meeting we lead and make it work better.
First, recognize that, as a leader, you are a steward of others’ time. As a steward, be intentional when designing meetings. Think carefully about who needs to be there, how much time to spend on the meeting, and how the meeting should be run. Dysfunction increases with meeting size, so invite attendees wisely; include only those essential to the meeting. For individuals not in the core group, offer them the opportunity to share their input premeeting if desired, share good meeting minutes with them, and welcome them to attend future meetings if desired. Consider “representative voices”—openly asking certain attendees to represent a group of stakeholders. Use a timed agenda to invite certain people for part, but not all, of the meeting.
Keep your meetings lean and deliberate. Avoid defaulting to one-hour meetings out of habit. Parkinson’s Law suggests that people will fill the time allotted to a particular task. If a meeting can be done in 30 minutes but is scheduled for 60 minutes, chances are that people will use the full hour. If a decision is reached faster than anticipated, end the meeting early. Refer back to your steward mindset and schedule meeting time with intention.
Meetings are often experienced psychologically like we experience interruptions. Thus, when attendees arrive at a meeting, express gratitude. Your job is to keep attendees active and engaged; therefore, facilitate the meeting actively and creatively. Try out different techniques such as devoting a few minutes to silent, written brainstorming. Leveraging silence gives attendees the opportunity to think on their own before contributing to the discussion and results in nearly twice the number of ideas.3 Perhaps members can be assigned explicit roles such as devil’s advocate, or each attendee can be assigned a specific agenda item, invoking responsibility and participation. If you always sit during meetings, try standing. If you have never tried a walking meeting, give it a go. Attendees appreciate mixing things up.
Lastly, remember to check-in with attendees to see how things are going. Never get too comfortable as a meeting leader, especially since meeting frustration abounds. Asking your team for feedback will carry over to other aspects of your role. You will be seen as a conscientious leader, open to exploration and professional development. This builds trust and creates a positive, collaborative work environment.
While you cannot control how others run their meetings, you do have the ability to make a meeting that you lead truly work. Be intentional with your role as a meeting facilitator and focus on the whole experience. Evaluate and learn from your team, show others that you care about your meetings so that they begin to care about theirs too.
1. Rogelberg SG. The Surprising Science of Meetings: How You Can Lead your Team to Peak Performance. Oxford University Press; 2019.
2. Rogelberg SG. Why your meetings stink–and what to do about it. Harvard Business Review. 2019;140-143. https://hbr.org/2019/01/why-your-meetings-stink-and-what-to-do-about-it. Accessed March 6, 2020.
3. Rogelberg SG, Kreamer L. The case for more silence in meetings. Harvard Business Review. https://hbr.org/2019/06/the-case-for-more-silence-in-meetings. Accessed August 2, 2019.
“Without meeting leadership skills, one joins the ranks of so many others who bear the responsibility for the meeting ‘problem’ and are the cause of so much frustration in the workplace.”1 Physicians, like so many others, often feel that team meetings are inefficient, a waste of time, and mentally draining. It does not have to be this way. There are evidence-based strategies that can make meetings truly work and actually enjoyable to attend.2 This is particularly important because eliminating meetings is a false solution. Hospitals need team meetings to promote coordination, collaboration, communication, and consensus decision-making. While no one individual can solve the meetings problem, each of us can find a meeting we lead and make it work better.
First, recognize that, as a leader, you are a steward of others’ time. As a steward, be intentional when designing meetings. Think carefully about who needs to be there, how much time to spend on the meeting, and how the meeting should be run. Dysfunction increases with meeting size, so invite attendees wisely; include only those essential to the meeting. For individuals not in the core group, offer them the opportunity to share their input premeeting if desired, share good meeting minutes with them, and welcome them to attend future meetings if desired. Consider “representative voices”—openly asking certain attendees to represent a group of stakeholders. Use a timed agenda to invite certain people for part, but not all, of the meeting.
Keep your meetings lean and deliberate. Avoid defaulting to one-hour meetings out of habit. Parkinson’s Law suggests that people will fill the time allotted to a particular task. If a meeting can be done in 30 minutes but is scheduled for 60 minutes, chances are that people will use the full hour. If a decision is reached faster than anticipated, end the meeting early. Refer back to your steward mindset and schedule meeting time with intention.
Meetings are often experienced psychologically like we experience interruptions. Thus, when attendees arrive at a meeting, express gratitude. Your job is to keep attendees active and engaged; therefore, facilitate the meeting actively and creatively. Try out different techniques such as devoting a few minutes to silent, written brainstorming. Leveraging silence gives attendees the opportunity to think on their own before contributing to the discussion and results in nearly twice the number of ideas.3 Perhaps members can be assigned explicit roles such as devil’s advocate, or each attendee can be assigned a specific agenda item, invoking responsibility and participation. If you always sit during meetings, try standing. If you have never tried a walking meeting, give it a go. Attendees appreciate mixing things up.
Lastly, remember to check-in with attendees to see how things are going. Never get too comfortable as a meeting leader, especially since meeting frustration abounds. Asking your team for feedback will carry over to other aspects of your role. You will be seen as a conscientious leader, open to exploration and professional development. This builds trust and creates a positive, collaborative work environment.
While you cannot control how others run their meetings, you do have the ability to make a meeting that you lead truly work. Be intentional with your role as a meeting facilitator and focus on the whole experience. Evaluate and learn from your team, show others that you care about your meetings so that they begin to care about theirs too.
“Without meeting leadership skills, one joins the ranks of so many others who bear the responsibility for the meeting ‘problem’ and are the cause of so much frustration in the workplace.”1 Physicians, like so many others, often feel that team meetings are inefficient, a waste of time, and mentally draining. It does not have to be this way. There are evidence-based strategies that can make meetings truly work and actually enjoyable to attend.2 This is particularly important because eliminating meetings is a false solution. Hospitals need team meetings to promote coordination, collaboration, communication, and consensus decision-making. While no one individual can solve the meetings problem, each of us can find a meeting we lead and make it work better.
First, recognize that, as a leader, you are a steward of others’ time. As a steward, be intentional when designing meetings. Think carefully about who needs to be there, how much time to spend on the meeting, and how the meeting should be run. Dysfunction increases with meeting size, so invite attendees wisely; include only those essential to the meeting. For individuals not in the core group, offer them the opportunity to share their input premeeting if desired, share good meeting minutes with them, and welcome them to attend future meetings if desired. Consider “representative voices”—openly asking certain attendees to represent a group of stakeholders. Use a timed agenda to invite certain people for part, but not all, of the meeting.
Keep your meetings lean and deliberate. Avoid defaulting to one-hour meetings out of habit. Parkinson’s Law suggests that people will fill the time allotted to a particular task. If a meeting can be done in 30 minutes but is scheduled for 60 minutes, chances are that people will use the full hour. If a decision is reached faster than anticipated, end the meeting early. Refer back to your steward mindset and schedule meeting time with intention.
Meetings are often experienced psychologically like we experience interruptions. Thus, when attendees arrive at a meeting, express gratitude. Your job is to keep attendees active and engaged; therefore, facilitate the meeting actively and creatively. Try out different techniques such as devoting a few minutes to silent, written brainstorming. Leveraging silence gives attendees the opportunity to think on their own before contributing to the discussion and results in nearly twice the number of ideas.3 Perhaps members can be assigned explicit roles such as devil’s advocate, or each attendee can be assigned a specific agenda item, invoking responsibility and participation. If you always sit during meetings, try standing. If you have never tried a walking meeting, give it a go. Attendees appreciate mixing things up.
Lastly, remember to check-in with attendees to see how things are going. Never get too comfortable as a meeting leader, especially since meeting frustration abounds. Asking your team for feedback will carry over to other aspects of your role. You will be seen as a conscientious leader, open to exploration and professional development. This builds trust and creates a positive, collaborative work environment.
While you cannot control how others run their meetings, you do have the ability to make a meeting that you lead truly work. Be intentional with your role as a meeting facilitator and focus on the whole experience. Evaluate and learn from your team, show others that you care about your meetings so that they begin to care about theirs too.
1. Rogelberg SG. The Surprising Science of Meetings: How You Can Lead your Team to Peak Performance. Oxford University Press; 2019.
2. Rogelberg SG. Why your meetings stink–and what to do about it. Harvard Business Review. 2019;140-143. https://hbr.org/2019/01/why-your-meetings-stink-and-what-to-do-about-it. Accessed March 6, 2020.
3. Rogelberg SG, Kreamer L. The case for more silence in meetings. Harvard Business Review. https://hbr.org/2019/06/the-case-for-more-silence-in-meetings. Accessed August 2, 2019.
1. Rogelberg SG. The Surprising Science of Meetings: How You Can Lead your Team to Peak Performance. Oxford University Press; 2019.
2. Rogelberg SG. Why your meetings stink–and what to do about it. Harvard Business Review. 2019;140-143. https://hbr.org/2019/01/why-your-meetings-stink-and-what-to-do-about-it. Accessed March 6, 2020.
3. Rogelberg SG, Kreamer L. The case for more silence in meetings. Harvard Business Review. https://hbr.org/2019/06/the-case-for-more-silence-in-meetings. Accessed August 2, 2019.
© 2020 Society of Hospital Medicine
SPEAKers at the National Society of Hospital Medicine Meeting: A Follow-UP Study of Gender Equity for Conference Speakers from 2015 to 2019. The SPEAK UP Study
Persistent gender disparities exist in pay,1,2 leadership opportunities,3,4 promotion,5 and speaking opportunities.6 While the gender distribution of the hospitalist workforce may be approaching parity,3,7,8 gender differences in leadership, speakership, and authorship have already been noted in hospital medicine.3 Between 2006 and 2012, women constituted less than a third (26%) of the presenters at the national conferences of the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM).3
The SHM Annual Meeting has historically had an “open call” peer review process for workshop presenters with the goal of increasing the diversity of presenters. In 2019, this process was expanded to include didactic speakers. Our aim in this study was to assess whether these open call procedures resulted in improved representation of women speakers and how the proportion of women speakers affects the overall evaluation scores of the conference. Our hypothesis was that the introduction of an open call process for the SHM conference didactic speakers would be associated with an increased proportion of women speakers, compared with the closed call processes, without a negative impact on conference scores.
METHODS
The study is a retrospective evaluation of data collected regarding speakers at the annual SHM conference from 2015 to 2019. The SHM national conference typically has two main types of offerings: workshops and didactics. Workshop presenters from 2015 to 2019 were selected via an open call process as defined below. Didactic speakers (except for plenary speakers) were selected using the open call process for 2019 only.
We aimed to compare (1) the number and proportion of women speakers, compared with men speakers, over time and (2) the proportion of women speakers when open call processes were utilized versus that seen with closed call processes. Open call included workshops for all years and didactics for 2019; closed call included didactics for 2015 to 2018 and plenary sessions 2015 to 2019 (Table). The speaker list for the conferences was obtained from conference pamphlets or agendas available via Internet searches or obtained through attendance at the conference.
Speaker Categories and Identification Process
We determined whether each individual was a featured speaker (one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), whether they spoke in a group format, and whether the speaking opportunity type was a workshop or a didactic session. Numbers of featured and plenary speakers were combined because of low numbers. SHM provided deidentified conference evaluation data for each year studied. For the purposes of this study, we analyzed all speakers which included physicians, advanced practice providers, and professionals such as nurses and other interdisciplinary team members. The same speaker could be included multiple times if they had multiple speaking opportunities.
Open Call Process
We defined the “open call process” (referred to as “open call” here forward) as the process utilized by SHM that includes the following two components: (1) advertisements to members of SHM and to the medical community at large through a variety of mechanisms including emails, websites, and social media outlets and (2) an online submission process that includes names of proposed speakers and their topic and, in the case of workshops, session objectives as well as an outline of the proposed workshop. SHM committees may also submit suggestions for topics and speakers. Annual Conference Committee members then review and rate submissions on the categories of topic, organization and clarity, objectives, and speaker qualifications (with a focus on institutional, geographic, and gender diversity). Scores are assigned from 1 to 5 (with 5 being the best score) for each category and a section for comments is available. All submissions are also evaluated by the course director.
After initial committee reviews, scores with marked reviewer discrepancies are rereviewed and discussed by the committee and course director. A cutoff score is then calculated with proposals falling below the cutoff threshold omitted from further consideration. Weekly calls are then focused on subcategories (ie tracks) with emphasis on clinical and educational content. Each of the tracks have a subcommittee with track leads to curate the best content first and then focus on final speaker selection. More recently, templates are shared with the track leads that include a location to call out gender and institutional diversity. Weekly calls are held to hone the content and determine the speakers.
For the purposes of this study, when the above process was not used, the authors refer to it as “closed call.” Closed call processes do not typically involve open invitations or a peer review process. (Table)
Gender
Gender was assigned based on the speaker’s self-identification by the pronouns used in their biography submitted to the conference or on their institutional website or other websites where the speaker was referenced. Persons using she/her/hers pronouns were noted as women and persons using he/him/his were noted as men. For the purposes of this study, we conceptualized gender as binary (ie woman/man) given the limited information we had from online sources.
ANALYSIS
REDCap, a secure, Web-based application for building and managing online survey and databases, was used to collect and manage all study data.9
All analyses were performed using SAS Enterprise Guide 8.1 (SAS Institute, Inc., Cary, North Carolina) using retrospectively collected data. A Cochran-Armitage test for trend was used to evaluate the proportion of women speakers from 2015 to 2019. A chi-square test was used to assess the proportion of women speakers for open call processes versus that seen with closed call. One-way analysis of variance (ANOVA) was used to evaluate annual conference evaluation scores from 2015 to 2019. Either numbers with proportions or means with standard deviations have been reported. Bonferroni’s correction for multiple comparisons was applied, with a P < .008 considered statistically significant.
RESULTS
Between 2015 and 2019, a total of 709 workshop and didactic presentations were given by 1,261 speakers at the annual Society of Hospital Medicine Conference. Of these, 505 (40%) were women; 756 (60%) were men. There were no missing data.
From 2015 to 2019, representation of women speakers increased from 35% of all speakers to 47% of all speakers (P = .0068). Women plenary speakers increased from 23% in 2015 to 45% in 2019 (P = .0396).
The proportion of women presenters for workshops (which have utilized an open call process throughout the study period), ranged from 43% to 53% from 2015 to 2019 with no statistically significant difference in gender distribution across years (Figure).
A greater proportion of speakers selected by an open call process were women compared to when speakers were selected by a closed call process (261 (47%) vs 244 (34%); P < .0001).
Of didactics or workshops given in a group format (N = 299), 82 (27%) were given by all-men groups and 38 (13%) were given by all-women groups. Women speakers participating in all-women group talks accounted for 21% of all women speakers; whereas men speakers participating in all-men group talks account for 26% of all men speakers (P = .02). We found that all-men group speaking opportunities did decrease from 41% of group talks in 2015 to 21% of group talks in 2019 (P = .0065).
We saw an average 3% annual increase in women speakers from 2015 to 2019, an 8% increase from 2018 to 2019 for all speakers, and an 11% increase in women speakers specific to didactic sessions. Overall conference ratings increased from a mean of 4.3 ± 0.24 in 2015 to a mean of 4.6 ± 0.14 in 2019 (n = 1,202; P < .0001; Figure).
DISCUSSION
The important findings of this study are that there has been an increase in women speakers over the last 5 years at the annual Society of Hospital Medicine Conference, that women had higher representation as speakers when open call processes were followed, and that conference scores continued to improve during the time frame studied. These findings suggest that a systematic open call process helps to support equitable speaking opportunities for men and women at a national hospital medicine conference without a negative impact on conference quality.
To recruit more diverse speakers, open call and peer review processes were used in addition to deliberate efforts at ensuring diversity in speakers. We found that over time, the proportion of women with speaking opportunities increased from 2015 to 2019. Interestingly, workshops, which had open call processes in place for the duration of the study period, had almost equal numbers of men and women presenting in all years. We also found that the number of all-men speaking groups decreased between 2015 and 2019.
A single process change can impact gender equity, but the target of true equity is expected to require additional measures such as assessment of committee structures and diversity, checklists, and reporting structures (data analysis and plans when goals not achieved).10-13 For instance, the American Society for Microbiology General Meeting was able to achieve gender equity in speakers by a multifold approach including ensuring the program committee was aware of gender statistics, increasing female representation among session convener teams, and direct instruction to try to avoid all-male sessions.11
It is important to acknowledge that these processes do require valuable resources including time. SHM has historically used committee volunteers to conduct the peer review process with each committee member reviewing 20 to 30 workshop submissions and 30 to 50 didactic sessions. While open processes with peer review seem to generate improved gender equity, ensuring processes are in place during the selection process is also key.
Several recent notable efforts to enhance gender equity and to increase diversity have been proposed. One such example of a process that may further improve gender equity was proposed by editors at the Journal of Hospital Medicine to assess current representation via demographics including gender, race, and ethnicity of authors with plans to assess patterns in the coming years.14 The American College of Physicians also published a position paper on achieving gender equity with a recommendation that organizational policies and procedures should be implemented that address implicit bias.15
Our study showed that, from 2015 to 2019, conference evaluations saw a significant increase in the score concurrently with the rise in proportion of women speakers. This finding suggests that quality does not seem to be affected by this new methodology for speaker selection and in fact this methodology may actually help improve the overall quality of the conference. To our knowledge, this is one of the first studies to concurrently evaluate speaker gender equity with conference quality.
Our study offers several strengths. This study took a pragmatic approach to understanding how processes can impact gender equity, and we were able to take advantage of the evolution of the open call system (ie workshops which have been an open call process for the duration of the study versus speaking opportunities that were not).
Our study also has several limitations. First, this study is retrospective in nature and thus other processes could have contributed to the improved gender equity, such as an organization’s priorities over time. During this study period, the SHM conference saw an average 3% increase annually in women speakers and an increase of 8% from 2018 to 2019 for all speakers compared to national trends of approximately 1%,6 which suggests that the open call processes in place could be contributing to the overall increases seen. Similarly, because of the retrospective nature of the study, we cannot be certain that the improvements in conference scores were directly the result of improved gender equity, although it does suggest that the improvements in gender equity did not have an adverse impact on the scores. We also did not assess how the composition of selection committee members for the meeting could have impacted the overall composition of the speakers. Our study looked at diversity only from the perspective of gender in a binary fashion, and thus additional studies are needed to assess how to improve diversity overall. It is unclear how this new open call for speakers affects race and ethnic diversity specifically. Identifying gender for the purposes of this study was facilitated by speakers providing their own biographies and the respective pronouns used in those biographies, and thus gender was easier to ascertain than race and ethnicity, which are not as readily available. For organizations to understand their diversity, equity, and inclusion efforts, enhancing the ability to fairly track and measure diversity will be key. Lastly, understanding of the exact composition of hospitalists from both a gender and race/ethnicity perspective is lacking. Studies have suggested that, based upon those surveyed or studied, there is a fairly equal balance of men and women albeit in academic groups.3
CONCLUSIONS
An open call approach to speakers at a national hospitalist conference seems to have contributed to improvements regarding gender equity in speaking opportunities with a concurrent improvement in overall rating of the conference. The open call system is a potential mechanism that other institutions and organizations could employ to enhance their diversity efforts.
Acknowledgments
Society of Hospital Medicine Diversity, Equity, Inclusion Special Interest Group
Work Group for SPEAK UP: Marisha Burden, MD, Daniel Cabrera, MD, Amira del Pino-Jones, MD, Areeba Kara, MD, Angela Keniston, MSPH, Keshav Khanijow, MD, Flora Kisuule, MD, Chiara Mandel, Benji Mathews, MD, David Paje, MD, Stephan Papp, MD, Snehal Patel, MD, Suchita Shah Sata, MD, Dustin Smith, MD, Kevin Vuernick
1. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400.
2. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284.
3. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340.
4. Silver JK, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
5. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680.
6. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the Proportion of Female Speakers at Medical Conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
7. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
8. Today’s Hospitalist 2018 Compensation and Career Survey Results. https://www.todayshospitalist.com/salary-survey-results/. Accessed September 28, 2019.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. Association of American Medical Colleagues (AAMC) Group on Women in Medicine and Science. Recruitment Toolkit: https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf. Accessed July 27, 2019.
11. Casadevall A. Achieving speaker gender equity at the american society for microbiology general meeting. MBio. 2015;6:e01146. https://doi.org/10.1128/mBio.01146-15.
12. Westring A, McDonald JM, Carr P, Grisso JA. An integrated framework for gender equity in academic medicine. Acad Med. 2016;91(8):1041-1044. https://doi.org/10.1097/ACM.0000000000001275.
13. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/10.1371/journal.pcbi.1003903.
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
15. Butkus R, Serchen J, Moyer DV, et al. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168:721-723. https://doi.org/10.7326/M17-3438.
Persistent gender disparities exist in pay,1,2 leadership opportunities,3,4 promotion,5 and speaking opportunities.6 While the gender distribution of the hospitalist workforce may be approaching parity,3,7,8 gender differences in leadership, speakership, and authorship have already been noted in hospital medicine.3 Between 2006 and 2012, women constituted less than a third (26%) of the presenters at the national conferences of the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM).3
The SHM Annual Meeting has historically had an “open call” peer review process for workshop presenters with the goal of increasing the diversity of presenters. In 2019, this process was expanded to include didactic speakers. Our aim in this study was to assess whether these open call procedures resulted in improved representation of women speakers and how the proportion of women speakers affects the overall evaluation scores of the conference. Our hypothesis was that the introduction of an open call process for the SHM conference didactic speakers would be associated with an increased proportion of women speakers, compared with the closed call processes, without a negative impact on conference scores.
METHODS
The study is a retrospective evaluation of data collected regarding speakers at the annual SHM conference from 2015 to 2019. The SHM national conference typically has two main types of offerings: workshops and didactics. Workshop presenters from 2015 to 2019 were selected via an open call process as defined below. Didactic speakers (except for plenary speakers) were selected using the open call process for 2019 only.
We aimed to compare (1) the number and proportion of women speakers, compared with men speakers, over time and (2) the proportion of women speakers when open call processes were utilized versus that seen with closed call processes. Open call included workshops for all years and didactics for 2019; closed call included didactics for 2015 to 2018 and plenary sessions 2015 to 2019 (Table). The speaker list for the conferences was obtained from conference pamphlets or agendas available via Internet searches or obtained through attendance at the conference.
Speaker Categories and Identification Process
We determined whether each individual was a featured speaker (one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), whether they spoke in a group format, and whether the speaking opportunity type was a workshop or a didactic session. Numbers of featured and plenary speakers were combined because of low numbers. SHM provided deidentified conference evaluation data for each year studied. For the purposes of this study, we analyzed all speakers which included physicians, advanced practice providers, and professionals such as nurses and other interdisciplinary team members. The same speaker could be included multiple times if they had multiple speaking opportunities.
Open Call Process
We defined the “open call process” (referred to as “open call” here forward) as the process utilized by SHM that includes the following two components: (1) advertisements to members of SHM and to the medical community at large through a variety of mechanisms including emails, websites, and social media outlets and (2) an online submission process that includes names of proposed speakers and their topic and, in the case of workshops, session objectives as well as an outline of the proposed workshop. SHM committees may also submit suggestions for topics and speakers. Annual Conference Committee members then review and rate submissions on the categories of topic, organization and clarity, objectives, and speaker qualifications (with a focus on institutional, geographic, and gender diversity). Scores are assigned from 1 to 5 (with 5 being the best score) for each category and a section for comments is available. All submissions are also evaluated by the course director.
After initial committee reviews, scores with marked reviewer discrepancies are rereviewed and discussed by the committee and course director. A cutoff score is then calculated with proposals falling below the cutoff threshold omitted from further consideration. Weekly calls are then focused on subcategories (ie tracks) with emphasis on clinical and educational content. Each of the tracks have a subcommittee with track leads to curate the best content first and then focus on final speaker selection. More recently, templates are shared with the track leads that include a location to call out gender and institutional diversity. Weekly calls are held to hone the content and determine the speakers.
For the purposes of this study, when the above process was not used, the authors refer to it as “closed call.” Closed call processes do not typically involve open invitations or a peer review process. (Table)
Gender
Gender was assigned based on the speaker’s self-identification by the pronouns used in their biography submitted to the conference or on their institutional website or other websites where the speaker was referenced. Persons using she/her/hers pronouns were noted as women and persons using he/him/his were noted as men. For the purposes of this study, we conceptualized gender as binary (ie woman/man) given the limited information we had from online sources.
ANALYSIS
REDCap, a secure, Web-based application for building and managing online survey and databases, was used to collect and manage all study data.9
All analyses were performed using SAS Enterprise Guide 8.1 (SAS Institute, Inc., Cary, North Carolina) using retrospectively collected data. A Cochran-Armitage test for trend was used to evaluate the proportion of women speakers from 2015 to 2019. A chi-square test was used to assess the proportion of women speakers for open call processes versus that seen with closed call. One-way analysis of variance (ANOVA) was used to evaluate annual conference evaluation scores from 2015 to 2019. Either numbers with proportions or means with standard deviations have been reported. Bonferroni’s correction for multiple comparisons was applied, with a P < .008 considered statistically significant.
RESULTS
Between 2015 and 2019, a total of 709 workshop and didactic presentations were given by 1,261 speakers at the annual Society of Hospital Medicine Conference. Of these, 505 (40%) were women; 756 (60%) were men. There were no missing data.
From 2015 to 2019, representation of women speakers increased from 35% of all speakers to 47% of all speakers (P = .0068). Women plenary speakers increased from 23% in 2015 to 45% in 2019 (P = .0396).
The proportion of women presenters for workshops (which have utilized an open call process throughout the study period), ranged from 43% to 53% from 2015 to 2019 with no statistically significant difference in gender distribution across years (Figure).
A greater proportion of speakers selected by an open call process were women compared to when speakers were selected by a closed call process (261 (47%) vs 244 (34%); P < .0001).
Of didactics or workshops given in a group format (N = 299), 82 (27%) were given by all-men groups and 38 (13%) were given by all-women groups. Women speakers participating in all-women group talks accounted for 21% of all women speakers; whereas men speakers participating in all-men group talks account for 26% of all men speakers (P = .02). We found that all-men group speaking opportunities did decrease from 41% of group talks in 2015 to 21% of group talks in 2019 (P = .0065).
We saw an average 3% annual increase in women speakers from 2015 to 2019, an 8% increase from 2018 to 2019 for all speakers, and an 11% increase in women speakers specific to didactic sessions. Overall conference ratings increased from a mean of 4.3 ± 0.24 in 2015 to a mean of 4.6 ± 0.14 in 2019 (n = 1,202; P < .0001; Figure).
DISCUSSION
The important findings of this study are that there has been an increase in women speakers over the last 5 years at the annual Society of Hospital Medicine Conference, that women had higher representation as speakers when open call processes were followed, and that conference scores continued to improve during the time frame studied. These findings suggest that a systematic open call process helps to support equitable speaking opportunities for men and women at a national hospital medicine conference without a negative impact on conference quality.
To recruit more diverse speakers, open call and peer review processes were used in addition to deliberate efforts at ensuring diversity in speakers. We found that over time, the proportion of women with speaking opportunities increased from 2015 to 2019. Interestingly, workshops, which had open call processes in place for the duration of the study period, had almost equal numbers of men and women presenting in all years. We also found that the number of all-men speaking groups decreased between 2015 and 2019.
A single process change can impact gender equity, but the target of true equity is expected to require additional measures such as assessment of committee structures and diversity, checklists, and reporting structures (data analysis and plans when goals not achieved).10-13 For instance, the American Society for Microbiology General Meeting was able to achieve gender equity in speakers by a multifold approach including ensuring the program committee was aware of gender statistics, increasing female representation among session convener teams, and direct instruction to try to avoid all-male sessions.11
It is important to acknowledge that these processes do require valuable resources including time. SHM has historically used committee volunteers to conduct the peer review process with each committee member reviewing 20 to 30 workshop submissions and 30 to 50 didactic sessions. While open processes with peer review seem to generate improved gender equity, ensuring processes are in place during the selection process is also key.
Several recent notable efforts to enhance gender equity and to increase diversity have been proposed. One such example of a process that may further improve gender equity was proposed by editors at the Journal of Hospital Medicine to assess current representation via demographics including gender, race, and ethnicity of authors with plans to assess patterns in the coming years.14 The American College of Physicians also published a position paper on achieving gender equity with a recommendation that organizational policies and procedures should be implemented that address implicit bias.15
Our study showed that, from 2015 to 2019, conference evaluations saw a significant increase in the score concurrently with the rise in proportion of women speakers. This finding suggests that quality does not seem to be affected by this new methodology for speaker selection and in fact this methodology may actually help improve the overall quality of the conference. To our knowledge, this is one of the first studies to concurrently evaluate speaker gender equity with conference quality.
Our study offers several strengths. This study took a pragmatic approach to understanding how processes can impact gender equity, and we were able to take advantage of the evolution of the open call system (ie workshops which have been an open call process for the duration of the study versus speaking opportunities that were not).
Our study also has several limitations. First, this study is retrospective in nature and thus other processes could have contributed to the improved gender equity, such as an organization’s priorities over time. During this study period, the SHM conference saw an average 3% increase annually in women speakers and an increase of 8% from 2018 to 2019 for all speakers compared to national trends of approximately 1%,6 which suggests that the open call processes in place could be contributing to the overall increases seen. Similarly, because of the retrospective nature of the study, we cannot be certain that the improvements in conference scores were directly the result of improved gender equity, although it does suggest that the improvements in gender equity did not have an adverse impact on the scores. We also did not assess how the composition of selection committee members for the meeting could have impacted the overall composition of the speakers. Our study looked at diversity only from the perspective of gender in a binary fashion, and thus additional studies are needed to assess how to improve diversity overall. It is unclear how this new open call for speakers affects race and ethnic diversity specifically. Identifying gender for the purposes of this study was facilitated by speakers providing their own biographies and the respective pronouns used in those biographies, and thus gender was easier to ascertain than race and ethnicity, which are not as readily available. For organizations to understand their diversity, equity, and inclusion efforts, enhancing the ability to fairly track and measure diversity will be key. Lastly, understanding of the exact composition of hospitalists from both a gender and race/ethnicity perspective is lacking. Studies have suggested that, based upon those surveyed or studied, there is a fairly equal balance of men and women albeit in academic groups.3
CONCLUSIONS
An open call approach to speakers at a national hospitalist conference seems to have contributed to improvements regarding gender equity in speaking opportunities with a concurrent improvement in overall rating of the conference. The open call system is a potential mechanism that other institutions and organizations could employ to enhance their diversity efforts.
Acknowledgments
Society of Hospital Medicine Diversity, Equity, Inclusion Special Interest Group
Work Group for SPEAK UP: Marisha Burden, MD, Daniel Cabrera, MD, Amira del Pino-Jones, MD, Areeba Kara, MD, Angela Keniston, MSPH, Keshav Khanijow, MD, Flora Kisuule, MD, Chiara Mandel, Benji Mathews, MD, David Paje, MD, Stephan Papp, MD, Snehal Patel, MD, Suchita Shah Sata, MD, Dustin Smith, MD, Kevin Vuernick
Persistent gender disparities exist in pay,1,2 leadership opportunities,3,4 promotion,5 and speaking opportunities.6 While the gender distribution of the hospitalist workforce may be approaching parity,3,7,8 gender differences in leadership, speakership, and authorship have already been noted in hospital medicine.3 Between 2006 and 2012, women constituted less than a third (26%) of the presenters at the national conferences of the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM).3
The SHM Annual Meeting has historically had an “open call” peer review process for workshop presenters with the goal of increasing the diversity of presenters. In 2019, this process was expanded to include didactic speakers. Our aim in this study was to assess whether these open call procedures resulted in improved representation of women speakers and how the proportion of women speakers affects the overall evaluation scores of the conference. Our hypothesis was that the introduction of an open call process for the SHM conference didactic speakers would be associated with an increased proportion of women speakers, compared with the closed call processes, without a negative impact on conference scores.
METHODS
The study is a retrospective evaluation of data collected regarding speakers at the annual SHM conference from 2015 to 2019. The SHM national conference typically has two main types of offerings: workshops and didactics. Workshop presenters from 2015 to 2019 were selected via an open call process as defined below. Didactic speakers (except for plenary speakers) were selected using the open call process for 2019 only.
We aimed to compare (1) the number and proportion of women speakers, compared with men speakers, over time and (2) the proportion of women speakers when open call processes were utilized versus that seen with closed call processes. Open call included workshops for all years and didactics for 2019; closed call included didactics for 2015 to 2018 and plenary sessions 2015 to 2019 (Table). The speaker list for the conferences was obtained from conference pamphlets or agendas available via Internet searches or obtained through attendance at the conference.
Speaker Categories and Identification Process
We determined whether each individual was a featured speaker (one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), whether they spoke in a group format, and whether the speaking opportunity type was a workshop or a didactic session. Numbers of featured and plenary speakers were combined because of low numbers. SHM provided deidentified conference evaluation data for each year studied. For the purposes of this study, we analyzed all speakers which included physicians, advanced practice providers, and professionals such as nurses and other interdisciplinary team members. The same speaker could be included multiple times if they had multiple speaking opportunities.
Open Call Process
We defined the “open call process” (referred to as “open call” here forward) as the process utilized by SHM that includes the following two components: (1) advertisements to members of SHM and to the medical community at large through a variety of mechanisms including emails, websites, and social media outlets and (2) an online submission process that includes names of proposed speakers and their topic and, in the case of workshops, session objectives as well as an outline of the proposed workshop. SHM committees may also submit suggestions for topics and speakers. Annual Conference Committee members then review and rate submissions on the categories of topic, organization and clarity, objectives, and speaker qualifications (with a focus on institutional, geographic, and gender diversity). Scores are assigned from 1 to 5 (with 5 being the best score) for each category and a section for comments is available. All submissions are also evaluated by the course director.
After initial committee reviews, scores with marked reviewer discrepancies are rereviewed and discussed by the committee and course director. A cutoff score is then calculated with proposals falling below the cutoff threshold omitted from further consideration. Weekly calls are then focused on subcategories (ie tracks) with emphasis on clinical and educational content. Each of the tracks have a subcommittee with track leads to curate the best content first and then focus on final speaker selection. More recently, templates are shared with the track leads that include a location to call out gender and institutional diversity. Weekly calls are held to hone the content and determine the speakers.
For the purposes of this study, when the above process was not used, the authors refer to it as “closed call.” Closed call processes do not typically involve open invitations or a peer review process. (Table)
Gender
Gender was assigned based on the speaker’s self-identification by the pronouns used in their biography submitted to the conference or on their institutional website or other websites where the speaker was referenced. Persons using she/her/hers pronouns were noted as women and persons using he/him/his were noted as men. For the purposes of this study, we conceptualized gender as binary (ie woman/man) given the limited information we had from online sources.
ANALYSIS
REDCap, a secure, Web-based application for building and managing online survey and databases, was used to collect and manage all study data.9
All analyses were performed using SAS Enterprise Guide 8.1 (SAS Institute, Inc., Cary, North Carolina) using retrospectively collected data. A Cochran-Armitage test for trend was used to evaluate the proportion of women speakers from 2015 to 2019. A chi-square test was used to assess the proportion of women speakers for open call processes versus that seen with closed call. One-way analysis of variance (ANOVA) was used to evaluate annual conference evaluation scores from 2015 to 2019. Either numbers with proportions or means with standard deviations have been reported. Bonferroni’s correction for multiple comparisons was applied, with a P < .008 considered statistically significant.
RESULTS
Between 2015 and 2019, a total of 709 workshop and didactic presentations were given by 1,261 speakers at the annual Society of Hospital Medicine Conference. Of these, 505 (40%) were women; 756 (60%) were men. There were no missing data.
From 2015 to 2019, representation of women speakers increased from 35% of all speakers to 47% of all speakers (P = .0068). Women plenary speakers increased from 23% in 2015 to 45% in 2019 (P = .0396).
The proportion of women presenters for workshops (which have utilized an open call process throughout the study period), ranged from 43% to 53% from 2015 to 2019 with no statistically significant difference in gender distribution across years (Figure).
A greater proportion of speakers selected by an open call process were women compared to when speakers were selected by a closed call process (261 (47%) vs 244 (34%); P < .0001).
Of didactics or workshops given in a group format (N = 299), 82 (27%) were given by all-men groups and 38 (13%) were given by all-women groups. Women speakers participating in all-women group talks accounted for 21% of all women speakers; whereas men speakers participating in all-men group talks account for 26% of all men speakers (P = .02). We found that all-men group speaking opportunities did decrease from 41% of group talks in 2015 to 21% of group talks in 2019 (P = .0065).
We saw an average 3% annual increase in women speakers from 2015 to 2019, an 8% increase from 2018 to 2019 for all speakers, and an 11% increase in women speakers specific to didactic sessions. Overall conference ratings increased from a mean of 4.3 ± 0.24 in 2015 to a mean of 4.6 ± 0.14 in 2019 (n = 1,202; P < .0001; Figure).
DISCUSSION
The important findings of this study are that there has been an increase in women speakers over the last 5 years at the annual Society of Hospital Medicine Conference, that women had higher representation as speakers when open call processes were followed, and that conference scores continued to improve during the time frame studied. These findings suggest that a systematic open call process helps to support equitable speaking opportunities for men and women at a national hospital medicine conference without a negative impact on conference quality.
To recruit more diverse speakers, open call and peer review processes were used in addition to deliberate efforts at ensuring diversity in speakers. We found that over time, the proportion of women with speaking opportunities increased from 2015 to 2019. Interestingly, workshops, which had open call processes in place for the duration of the study period, had almost equal numbers of men and women presenting in all years. We also found that the number of all-men speaking groups decreased between 2015 and 2019.
A single process change can impact gender equity, but the target of true equity is expected to require additional measures such as assessment of committee structures and diversity, checklists, and reporting structures (data analysis and plans when goals not achieved).10-13 For instance, the American Society for Microbiology General Meeting was able to achieve gender equity in speakers by a multifold approach including ensuring the program committee was aware of gender statistics, increasing female representation among session convener teams, and direct instruction to try to avoid all-male sessions.11
It is important to acknowledge that these processes do require valuable resources including time. SHM has historically used committee volunteers to conduct the peer review process with each committee member reviewing 20 to 30 workshop submissions and 30 to 50 didactic sessions. While open processes with peer review seem to generate improved gender equity, ensuring processes are in place during the selection process is also key.
Several recent notable efforts to enhance gender equity and to increase diversity have been proposed. One such example of a process that may further improve gender equity was proposed by editors at the Journal of Hospital Medicine to assess current representation via demographics including gender, race, and ethnicity of authors with plans to assess patterns in the coming years.14 The American College of Physicians also published a position paper on achieving gender equity with a recommendation that organizational policies and procedures should be implemented that address implicit bias.15
Our study showed that, from 2015 to 2019, conference evaluations saw a significant increase in the score concurrently with the rise in proportion of women speakers. This finding suggests that quality does not seem to be affected by this new methodology for speaker selection and in fact this methodology may actually help improve the overall quality of the conference. To our knowledge, this is one of the first studies to concurrently evaluate speaker gender equity with conference quality.
Our study offers several strengths. This study took a pragmatic approach to understanding how processes can impact gender equity, and we were able to take advantage of the evolution of the open call system (ie workshops which have been an open call process for the duration of the study versus speaking opportunities that were not).
Our study also has several limitations. First, this study is retrospective in nature and thus other processes could have contributed to the improved gender equity, such as an organization’s priorities over time. During this study period, the SHM conference saw an average 3% increase annually in women speakers and an increase of 8% from 2018 to 2019 for all speakers compared to national trends of approximately 1%,6 which suggests that the open call processes in place could be contributing to the overall increases seen. Similarly, because of the retrospective nature of the study, we cannot be certain that the improvements in conference scores were directly the result of improved gender equity, although it does suggest that the improvements in gender equity did not have an adverse impact on the scores. We also did not assess how the composition of selection committee members for the meeting could have impacted the overall composition of the speakers. Our study looked at diversity only from the perspective of gender in a binary fashion, and thus additional studies are needed to assess how to improve diversity overall. It is unclear how this new open call for speakers affects race and ethnic diversity specifically. Identifying gender for the purposes of this study was facilitated by speakers providing their own biographies and the respective pronouns used in those biographies, and thus gender was easier to ascertain than race and ethnicity, which are not as readily available. For organizations to understand their diversity, equity, and inclusion efforts, enhancing the ability to fairly track and measure diversity will be key. Lastly, understanding of the exact composition of hospitalists from both a gender and race/ethnicity perspective is lacking. Studies have suggested that, based upon those surveyed or studied, there is a fairly equal balance of men and women albeit in academic groups.3
CONCLUSIONS
An open call approach to speakers at a national hospitalist conference seems to have contributed to improvements regarding gender equity in speaking opportunities with a concurrent improvement in overall rating of the conference. The open call system is a potential mechanism that other institutions and organizations could employ to enhance their diversity efforts.
Acknowledgments
Society of Hospital Medicine Diversity, Equity, Inclusion Special Interest Group
Work Group for SPEAK UP: Marisha Burden, MD, Daniel Cabrera, MD, Amira del Pino-Jones, MD, Areeba Kara, MD, Angela Keniston, MSPH, Keshav Khanijow, MD, Flora Kisuule, MD, Chiara Mandel, Benji Mathews, MD, David Paje, MD, Stephan Papp, MD, Snehal Patel, MD, Suchita Shah Sata, MD, Dustin Smith, MD, Kevin Vuernick
1. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400.
2. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284.
3. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340.
4. Silver JK, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
5. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680.
6. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the Proportion of Female Speakers at Medical Conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
7. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
8. Today’s Hospitalist 2018 Compensation and Career Survey Results. https://www.todayshospitalist.com/salary-survey-results/. Accessed September 28, 2019.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. Association of American Medical Colleagues (AAMC) Group on Women in Medicine and Science. Recruitment Toolkit: https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf. Accessed July 27, 2019.
11. Casadevall A. Achieving speaker gender equity at the american society for microbiology general meeting. MBio. 2015;6:e01146. https://doi.org/10.1128/mBio.01146-15.
12. Westring A, McDonald JM, Carr P, Grisso JA. An integrated framework for gender equity in academic medicine. Acad Med. 2016;91(8):1041-1044. https://doi.org/10.1097/ACM.0000000000001275.
13. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/10.1371/journal.pcbi.1003903.
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
15. Butkus R, Serchen J, Moyer DV, et al. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168:721-723. https://doi.org/10.7326/M17-3438.
1. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400.
2. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284.
3. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340.
4. Silver JK, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
5. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680.
6. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the Proportion of Female Speakers at Medical Conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
7. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
8. Today’s Hospitalist 2018 Compensation and Career Survey Results. https://www.todayshospitalist.com/salary-survey-results/. Accessed September 28, 2019.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. Association of American Medical Colleagues (AAMC) Group on Women in Medicine and Science. Recruitment Toolkit: https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf. Accessed July 27, 2019.
11. Casadevall A. Achieving speaker gender equity at the american society for microbiology general meeting. MBio. 2015;6:e01146. https://doi.org/10.1128/mBio.01146-15.
12. Westring A, McDonald JM, Carr P, Grisso JA. An integrated framework for gender equity in academic medicine. Acad Med. 2016;91(8):1041-1044. https://doi.org/10.1097/ACM.0000000000001275.
13. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/10.1371/journal.pcbi.1003903.
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
15. Butkus R, Serchen J, Moyer DV, et al. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168:721-723. https://doi.org/10.7326/M17-3438.
© 2020 Society of Hospital Medicine
What I Learned From SARS in 2003 That Will Help Me Cope With COVID-19 in 2020
On March 25, 2003, I was in Vancouver at my niece’s bat mitzvah when I saw a picture of my hospital in Toronto on the television news; a story about SARS patients in Toronto. Until then, SARS had been a distant event happening in mainland China and Hong Kong; it had been something that seemed very far away and theoretical. When I returned to Toronto, we had clusters of cases in several hospitals and healthcare workers were falling ill. I was the Physician in Chief at one of those hospitals and was responsible for the clinical care delivered by physicians in the Department of Medicine. So the burden of figuring out what we were going to do fell on me and the other members of the hospital leadership team.
SARS IN 2003
As the outbreak evolved, we only knew a few things. It was a respiratory infection, likely viral, with a very high mortality rate, compared with most other viral respiratory infections. We learned the hard way that, while it was mostly transmitted by droplets, some patients were able to widely transmit it through the air, and therefore likely through ventilation systems. We knew that most infections were occurring in hospitals but there was also community spread at events like funerals. We had no test to confirm the presence of the virus and, indeed, only figured out it was a coronavirus well into the outbreak. Diagnoses were made using clinical criteria; this uncertainty was a major source of anxiety about potential community spread without direct links to known cases. We had no idea how long it was going to last, nor did we know how it would end. We were entering uncharted territory.
Decisions had to be made. Which patients needed isolation, and which did not? We made mistakes early on that caused hundreds of healthcare workers and people to be quarantined (complete isolation) for 10 days; this was a difficult situation for them, their families, and the people who had to replace them in the workplace.
Within a very short period we changed our way of life in hospitals. We screened everyone who entered with questionnaires and measured their temperatures. Once entering the hospital, we all wore N95 masks in public spaces and when in a room with another person—not just patients. We all got sore throats from wearing the masks 10 hours a day. All patients were placed in respiratory precautions, which meant that, any time we entered their rooms, we had to don all the personal protective equipment (PPE). Yet we didn’t run out of supplies. When a member of a provincial leadership team fell ill with SARS shortly after attending an in-person meeting of the committee, all the other members went into quarantine. As a result, we stopped having leadership team meetings in person, and mostly stayed in our own offices, communicating by phone and email.
The hospital took on a bizarre atmosphere: everyone in masks and little face-to-face contact. Yet outside the hospital, life went on mostly as normal. Some people wore masks on the street, but public events and businesses stayed open. Some healthcare workers were shunned in the community out of fear. But I went to another bat mitzvah and even a Stanley Cup playoff game at the height of the outbreak. Only healthcare workers were asked to stop meeting in large groups. The contrast for me was striking.
The Ontario Ministry of Health started a daily noon hour phone conference call; one physician and one administrator from every hospital in the province were on the call. I attended those for my hospital and, because I knew or taught many of the people on the line, was quickly asked to chair the calls. They were incredibly important and were a source of information exchange and emotional support for all of us. Before each call, I spoke with a person from Toronto Public Health who updated me on the number of cases and deaths. I needed to absorb that information before the calls to maintain my composure when she told the rest of the group. At times I could hear the fear in people’s voices as they described the clinical course of their patients.
Because I chaired the calls, I was asked to coordinate the study that documented the clinical outcomes of all the patients in the hopes that we could distinguish it from other common respiratory syndromes. With the help of my colleagues in the 11 hospitals that treated SARS patients, the ethics review boards, medical records personnel who copied the charts, Christopher Booth, MD, (a second- year resident at the time who headed the study), and a few medical students we were able to go from the idea to do the study to electronic publication in JAMA in 30 days.1 It was JAMA’s first experience with rapid review, and the editors there were very helpful. Working on this study was very therapeutic; it allowed me to feel I was doing something that could help.
I was scared—both for my own health and the health of my family, but also terribly frightened for the health of the people who worked here. When I went home every night, I looked at the people on the street and wondered how many would still be there a few months later. And then it all ended. (Actually, it ended twice; we let up a bit too early because we so wanted it to be over.)
COVID-19 IN 2020
The COVID-19 pandemic has many similarities, but there are also significant differences. The most obvious is that because there is more community spread, life outside the hospital is much more severely disrupted. Countries have responded by sliding into more and more practices that try to limit person-to-person spread. First travel restrictions from other countries, then moral suasion to promote social distancing (which is really just physical distancing), then closing schools and nonessential businesses, and finally complete lock downs.
These events have spurred panic buying of some items (hand sanitizer, toilet paper, masks), and the fear of major disruptions of the supply chain for things like food. SARS was much more limited in its overall economic effect, though the WHO precautionary travel advisory against nonessential travel to Toronto, which lasted for only 1 week, resulted in a long-lasting reduction in tourism and a hit to the theatre business in our city.
The internet and social media have made it easier to disseminate valuable information and instructions, while at the same time easier to spread false information. But we had a lot of false information during SARS, too. One of the biggest differences for the United States (which was almost unaffected by SARS) is that the current extreme political divide creates two separate tracks of information and beliefs. A united message is very important.
Finally, the shortage of PPE in some jurisdictions, which was not an issue in Toronto during SARS, has severely heightened the fear for healthcare workers. In 2003, we also had lots of discussion about the tension between our professional duty and the safety of healthcare workers and their families (many of us separated ourselves from our families in our own homes while working clinically). To my recollection, two nurses and one physician died of SARS in Toronto. But when hospitals actually run out of PPE—something that is happening with COVID-19—those discussions take on a much more ominous tone.
LESSONS LEARNED
In my opinion, SARS was a dry run for us in Toronto and the other places in the world that it affected (Taiwan, Hong Kong, Singapore); one that helped us prepare in advance and will help us cope with COVID-19. But what did I personally learn from my SARS experience?
First, I learned that accurate information in these kinds of situations is hard to come by. We heard lots of rumors from people all over the world. But when I found that it was very difficult for me to figure out exactly what was going on in my own hospital (eg, who was in contact with people who fell ill or went into quarantine, how patients were faring), I realized that figuring out what was happening half way around the world from news reports was near impossible. I learned to wait for official announcements.
Second, I learned that talking to my colleagues was both therapeutic—providing emotional support and an outlet for feelings—and anxiety provoking when we overreacted to rumors.
Third, I learned that, like others, I was susceptible to exhibiting obsessive behaviors in an attempt to establish control over uncertainty. Constantly washing my hands, checking my temperature, and seeking reassuring facts from others only worked to calm me for a few minutes. And then I felt the need to do it again. This time I find myself checking my twitter account constantly; half afraid I will see something frightening, half looking for good news from people I trust. I now recognize this behavior and it helps me contain it.
Fourth, I learned that events that occurred remotely had much less effect on everyone than those that occurred close by. Having two people I knew get SARS, and then learning they recovered was perhaps the most meaningful event for me during the entire episode.
Finally, I learned that in the end I and the people I care about survived—nothing bad happened to us. The world did not end after SARS. It took me about a year, including some time with a terrific psychiatrist, to realize I was safe after all. And that realization is what I am most hanging on to today.
Acknowledgments
Sanjay Saint (University of Michigan), Christopher Booth (Queens University), and Sagar Rohailla (University of Toronto) provided comments on an earlier draft. None were compensated for doing so.
1. Booth C, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801-2809. https://doi.org/10.1001/jama.289.21.JOC30885.
On March 25, 2003, I was in Vancouver at my niece’s bat mitzvah when I saw a picture of my hospital in Toronto on the television news; a story about SARS patients in Toronto. Until then, SARS had been a distant event happening in mainland China and Hong Kong; it had been something that seemed very far away and theoretical. When I returned to Toronto, we had clusters of cases in several hospitals and healthcare workers were falling ill. I was the Physician in Chief at one of those hospitals and was responsible for the clinical care delivered by physicians in the Department of Medicine. So the burden of figuring out what we were going to do fell on me and the other members of the hospital leadership team.
SARS IN 2003
As the outbreak evolved, we only knew a few things. It was a respiratory infection, likely viral, with a very high mortality rate, compared with most other viral respiratory infections. We learned the hard way that, while it was mostly transmitted by droplets, some patients were able to widely transmit it through the air, and therefore likely through ventilation systems. We knew that most infections were occurring in hospitals but there was also community spread at events like funerals. We had no test to confirm the presence of the virus and, indeed, only figured out it was a coronavirus well into the outbreak. Diagnoses were made using clinical criteria; this uncertainty was a major source of anxiety about potential community spread without direct links to known cases. We had no idea how long it was going to last, nor did we know how it would end. We were entering uncharted territory.
Decisions had to be made. Which patients needed isolation, and which did not? We made mistakes early on that caused hundreds of healthcare workers and people to be quarantined (complete isolation) for 10 days; this was a difficult situation for them, their families, and the people who had to replace them in the workplace.
Within a very short period we changed our way of life in hospitals. We screened everyone who entered with questionnaires and measured their temperatures. Once entering the hospital, we all wore N95 masks in public spaces and when in a room with another person—not just patients. We all got sore throats from wearing the masks 10 hours a day. All patients were placed in respiratory precautions, which meant that, any time we entered their rooms, we had to don all the personal protective equipment (PPE). Yet we didn’t run out of supplies. When a member of a provincial leadership team fell ill with SARS shortly after attending an in-person meeting of the committee, all the other members went into quarantine. As a result, we stopped having leadership team meetings in person, and mostly stayed in our own offices, communicating by phone and email.
The hospital took on a bizarre atmosphere: everyone in masks and little face-to-face contact. Yet outside the hospital, life went on mostly as normal. Some people wore masks on the street, but public events and businesses stayed open. Some healthcare workers were shunned in the community out of fear. But I went to another bat mitzvah and even a Stanley Cup playoff game at the height of the outbreak. Only healthcare workers were asked to stop meeting in large groups. The contrast for me was striking.
The Ontario Ministry of Health started a daily noon hour phone conference call; one physician and one administrator from every hospital in the province were on the call. I attended those for my hospital and, because I knew or taught many of the people on the line, was quickly asked to chair the calls. They were incredibly important and were a source of information exchange and emotional support for all of us. Before each call, I spoke with a person from Toronto Public Health who updated me on the number of cases and deaths. I needed to absorb that information before the calls to maintain my composure when she told the rest of the group. At times I could hear the fear in people’s voices as they described the clinical course of their patients.
Because I chaired the calls, I was asked to coordinate the study that documented the clinical outcomes of all the patients in the hopes that we could distinguish it from other common respiratory syndromes. With the help of my colleagues in the 11 hospitals that treated SARS patients, the ethics review boards, medical records personnel who copied the charts, Christopher Booth, MD, (a second- year resident at the time who headed the study), and a few medical students we were able to go from the idea to do the study to electronic publication in JAMA in 30 days.1 It was JAMA’s first experience with rapid review, and the editors there were very helpful. Working on this study was very therapeutic; it allowed me to feel I was doing something that could help.
I was scared—both for my own health and the health of my family, but also terribly frightened for the health of the people who worked here. When I went home every night, I looked at the people on the street and wondered how many would still be there a few months later. And then it all ended. (Actually, it ended twice; we let up a bit too early because we so wanted it to be over.)
COVID-19 IN 2020
The COVID-19 pandemic has many similarities, but there are also significant differences. The most obvious is that because there is more community spread, life outside the hospital is much more severely disrupted. Countries have responded by sliding into more and more practices that try to limit person-to-person spread. First travel restrictions from other countries, then moral suasion to promote social distancing (which is really just physical distancing), then closing schools and nonessential businesses, and finally complete lock downs.
These events have spurred panic buying of some items (hand sanitizer, toilet paper, masks), and the fear of major disruptions of the supply chain for things like food. SARS was much more limited in its overall economic effect, though the WHO precautionary travel advisory against nonessential travel to Toronto, which lasted for only 1 week, resulted in a long-lasting reduction in tourism and a hit to the theatre business in our city.
The internet and social media have made it easier to disseminate valuable information and instructions, while at the same time easier to spread false information. But we had a lot of false information during SARS, too. One of the biggest differences for the United States (which was almost unaffected by SARS) is that the current extreme political divide creates two separate tracks of information and beliefs. A united message is very important.
Finally, the shortage of PPE in some jurisdictions, which was not an issue in Toronto during SARS, has severely heightened the fear for healthcare workers. In 2003, we also had lots of discussion about the tension between our professional duty and the safety of healthcare workers and their families (many of us separated ourselves from our families in our own homes while working clinically). To my recollection, two nurses and one physician died of SARS in Toronto. But when hospitals actually run out of PPE—something that is happening with COVID-19—those discussions take on a much more ominous tone.
LESSONS LEARNED
In my opinion, SARS was a dry run for us in Toronto and the other places in the world that it affected (Taiwan, Hong Kong, Singapore); one that helped us prepare in advance and will help us cope with COVID-19. But what did I personally learn from my SARS experience?
First, I learned that accurate information in these kinds of situations is hard to come by. We heard lots of rumors from people all over the world. But when I found that it was very difficult for me to figure out exactly what was going on in my own hospital (eg, who was in contact with people who fell ill or went into quarantine, how patients were faring), I realized that figuring out what was happening half way around the world from news reports was near impossible. I learned to wait for official announcements.
Second, I learned that talking to my colleagues was both therapeutic—providing emotional support and an outlet for feelings—and anxiety provoking when we overreacted to rumors.
Third, I learned that, like others, I was susceptible to exhibiting obsessive behaviors in an attempt to establish control over uncertainty. Constantly washing my hands, checking my temperature, and seeking reassuring facts from others only worked to calm me for a few minutes. And then I felt the need to do it again. This time I find myself checking my twitter account constantly; half afraid I will see something frightening, half looking for good news from people I trust. I now recognize this behavior and it helps me contain it.
Fourth, I learned that events that occurred remotely had much less effect on everyone than those that occurred close by. Having two people I knew get SARS, and then learning they recovered was perhaps the most meaningful event for me during the entire episode.
Finally, I learned that in the end I and the people I care about survived—nothing bad happened to us. The world did not end after SARS. It took me about a year, including some time with a terrific psychiatrist, to realize I was safe after all. And that realization is what I am most hanging on to today.
Acknowledgments
Sanjay Saint (University of Michigan), Christopher Booth (Queens University), and Sagar Rohailla (University of Toronto) provided comments on an earlier draft. None were compensated for doing so.
On March 25, 2003, I was in Vancouver at my niece’s bat mitzvah when I saw a picture of my hospital in Toronto on the television news; a story about SARS patients in Toronto. Until then, SARS had been a distant event happening in mainland China and Hong Kong; it had been something that seemed very far away and theoretical. When I returned to Toronto, we had clusters of cases in several hospitals and healthcare workers were falling ill. I was the Physician in Chief at one of those hospitals and was responsible for the clinical care delivered by physicians in the Department of Medicine. So the burden of figuring out what we were going to do fell on me and the other members of the hospital leadership team.
SARS IN 2003
As the outbreak evolved, we only knew a few things. It was a respiratory infection, likely viral, with a very high mortality rate, compared with most other viral respiratory infections. We learned the hard way that, while it was mostly transmitted by droplets, some patients were able to widely transmit it through the air, and therefore likely through ventilation systems. We knew that most infections were occurring in hospitals but there was also community spread at events like funerals. We had no test to confirm the presence of the virus and, indeed, only figured out it was a coronavirus well into the outbreak. Diagnoses were made using clinical criteria; this uncertainty was a major source of anxiety about potential community spread without direct links to known cases. We had no idea how long it was going to last, nor did we know how it would end. We were entering uncharted territory.
Decisions had to be made. Which patients needed isolation, and which did not? We made mistakes early on that caused hundreds of healthcare workers and people to be quarantined (complete isolation) for 10 days; this was a difficult situation for them, their families, and the people who had to replace them in the workplace.
Within a very short period we changed our way of life in hospitals. We screened everyone who entered with questionnaires and measured their temperatures. Once entering the hospital, we all wore N95 masks in public spaces and when in a room with another person—not just patients. We all got sore throats from wearing the masks 10 hours a day. All patients were placed in respiratory precautions, which meant that, any time we entered their rooms, we had to don all the personal protective equipment (PPE). Yet we didn’t run out of supplies. When a member of a provincial leadership team fell ill with SARS shortly after attending an in-person meeting of the committee, all the other members went into quarantine. As a result, we stopped having leadership team meetings in person, and mostly stayed in our own offices, communicating by phone and email.
The hospital took on a bizarre atmosphere: everyone in masks and little face-to-face contact. Yet outside the hospital, life went on mostly as normal. Some people wore masks on the street, but public events and businesses stayed open. Some healthcare workers were shunned in the community out of fear. But I went to another bat mitzvah and even a Stanley Cup playoff game at the height of the outbreak. Only healthcare workers were asked to stop meeting in large groups. The contrast for me was striking.
The Ontario Ministry of Health started a daily noon hour phone conference call; one physician and one administrator from every hospital in the province were on the call. I attended those for my hospital and, because I knew or taught many of the people on the line, was quickly asked to chair the calls. They were incredibly important and were a source of information exchange and emotional support for all of us. Before each call, I spoke with a person from Toronto Public Health who updated me on the number of cases and deaths. I needed to absorb that information before the calls to maintain my composure when she told the rest of the group. At times I could hear the fear in people’s voices as they described the clinical course of their patients.
Because I chaired the calls, I was asked to coordinate the study that documented the clinical outcomes of all the patients in the hopes that we could distinguish it from other common respiratory syndromes. With the help of my colleagues in the 11 hospitals that treated SARS patients, the ethics review boards, medical records personnel who copied the charts, Christopher Booth, MD, (a second- year resident at the time who headed the study), and a few medical students we were able to go from the idea to do the study to electronic publication in JAMA in 30 days.1 It was JAMA’s first experience with rapid review, and the editors there were very helpful. Working on this study was very therapeutic; it allowed me to feel I was doing something that could help.
I was scared—both for my own health and the health of my family, but also terribly frightened for the health of the people who worked here. When I went home every night, I looked at the people on the street and wondered how many would still be there a few months later. And then it all ended. (Actually, it ended twice; we let up a bit too early because we so wanted it to be over.)
COVID-19 IN 2020
The COVID-19 pandemic has many similarities, but there are also significant differences. The most obvious is that because there is more community spread, life outside the hospital is much more severely disrupted. Countries have responded by sliding into more and more practices that try to limit person-to-person spread. First travel restrictions from other countries, then moral suasion to promote social distancing (which is really just physical distancing), then closing schools and nonessential businesses, and finally complete lock downs.
These events have spurred panic buying of some items (hand sanitizer, toilet paper, masks), and the fear of major disruptions of the supply chain for things like food. SARS was much more limited in its overall economic effect, though the WHO precautionary travel advisory against nonessential travel to Toronto, which lasted for only 1 week, resulted in a long-lasting reduction in tourism and a hit to the theatre business in our city.
The internet and social media have made it easier to disseminate valuable information and instructions, while at the same time easier to spread false information. But we had a lot of false information during SARS, too. One of the biggest differences for the United States (which was almost unaffected by SARS) is that the current extreme political divide creates two separate tracks of information and beliefs. A united message is very important.
Finally, the shortage of PPE in some jurisdictions, which was not an issue in Toronto during SARS, has severely heightened the fear for healthcare workers. In 2003, we also had lots of discussion about the tension between our professional duty and the safety of healthcare workers and their families (many of us separated ourselves from our families in our own homes while working clinically). To my recollection, two nurses and one physician died of SARS in Toronto. But when hospitals actually run out of PPE—something that is happening with COVID-19—those discussions take on a much more ominous tone.
LESSONS LEARNED
In my opinion, SARS was a dry run for us in Toronto and the other places in the world that it affected (Taiwan, Hong Kong, Singapore); one that helped us prepare in advance and will help us cope with COVID-19. But what did I personally learn from my SARS experience?
First, I learned that accurate information in these kinds of situations is hard to come by. We heard lots of rumors from people all over the world. But when I found that it was very difficult for me to figure out exactly what was going on in my own hospital (eg, who was in contact with people who fell ill or went into quarantine, how patients were faring), I realized that figuring out what was happening half way around the world from news reports was near impossible. I learned to wait for official announcements.
Second, I learned that talking to my colleagues was both therapeutic—providing emotional support and an outlet for feelings—and anxiety provoking when we overreacted to rumors.
Third, I learned that, like others, I was susceptible to exhibiting obsessive behaviors in an attempt to establish control over uncertainty. Constantly washing my hands, checking my temperature, and seeking reassuring facts from others only worked to calm me for a few minutes. And then I felt the need to do it again. This time I find myself checking my twitter account constantly; half afraid I will see something frightening, half looking for good news from people I trust. I now recognize this behavior and it helps me contain it.
Fourth, I learned that events that occurred remotely had much less effect on everyone than those that occurred close by. Having two people I knew get SARS, and then learning they recovered was perhaps the most meaningful event for me during the entire episode.
Finally, I learned that in the end I and the people I care about survived—nothing bad happened to us. The world did not end after SARS. It took me about a year, including some time with a terrific psychiatrist, to realize I was safe after all. And that realization is what I am most hanging on to today.
Acknowledgments
Sanjay Saint (University of Michigan), Christopher Booth (Queens University), and Sagar Rohailla (University of Toronto) provided comments on an earlier draft. None were compensated for doing so.
1. Booth C, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801-2809. https://doi.org/10.1001/jama.289.21.JOC30885.
1. Booth C, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801-2809. https://doi.org/10.1001/jama.289.21.JOC30885.
© 2020 Society of Hospital Medicine