User login
POCUS in hospital pediatrics
PHM 2021 Session
Safe and (Ultra)sound: Why you should use POCUS in your Pediatric Practice
Presenter
Ria Dancel, MD, FAAP, FACP
Session summary
Dr. Ria Dancel and her colleagues from the University of North Carolina at Chapel Hill presented a broad overview of point-of-care ultrasound (POCUS) applications in the field of pediatric hospital medicine. They discussed its advantages and potential uses, ranging from common scenarios to critical care to procedural guidance. Using illustrative scenarios and interactive cases, she discussed the bedside applications to improve care of hospitalized children. The benefits and risks of radiography and POCUS were reviewed.
The session highlighted the use of POCUS in SSTI (skin and soft tissue infection) to help with differentiating cellulitis from abscesses. Use of POCUS for safer incision and drainages and making day-to-day changes in management was discussed. The ease and benefits of performing real-time lung ultrasound in different pathologies (like pneumonia, effusion, COVID-19) was presented. The speakers discussed the use of POCUS in emergency situations like hypotension and different types of shock. The use of ultrasound in common bedside procedures (bladder catheterization, lumbar ultrasound, peripheral IV placement) were also highlighted. Current literature and evidence were reviewed.
Key takeaways
- Pediatric POCUS is an extremely valuable bedside tool in pediatric hospital medicine.
- It can be used to guide clinical care for many conditions including SSTI, pneumonia, and shock.
- It can be used for procedural guidance for bladder catheterization, lumbar puncture, and intravenous access.
Dr. Patra is a pediatric hospitalist at West Virginia University Children’s Hospital, Morgantown, and associate professor at West Virginia University School of Medicine. He is interested in medical education, quality improvement and clinical research. He is a member of the Executive Council of the Pediatric Special Interest Group of the Society of Hospital Medicine.
PHM 2021 Session
Safe and (Ultra)sound: Why you should use POCUS in your Pediatric Practice
Presenter
Ria Dancel, MD, FAAP, FACP
Session summary
Dr. Ria Dancel and her colleagues from the University of North Carolina at Chapel Hill presented a broad overview of point-of-care ultrasound (POCUS) applications in the field of pediatric hospital medicine. They discussed its advantages and potential uses, ranging from common scenarios to critical care to procedural guidance. Using illustrative scenarios and interactive cases, she discussed the bedside applications to improve care of hospitalized children. The benefits and risks of radiography and POCUS were reviewed.
The session highlighted the use of POCUS in SSTI (skin and soft tissue infection) to help with differentiating cellulitis from abscesses. Use of POCUS for safer incision and drainages and making day-to-day changes in management was discussed. The ease and benefits of performing real-time lung ultrasound in different pathologies (like pneumonia, effusion, COVID-19) was presented. The speakers discussed the use of POCUS in emergency situations like hypotension and different types of shock. The use of ultrasound in common bedside procedures (bladder catheterization, lumbar ultrasound, peripheral IV placement) were also highlighted. Current literature and evidence were reviewed.
Key takeaways
- Pediatric POCUS is an extremely valuable bedside tool in pediatric hospital medicine.
- It can be used to guide clinical care for many conditions including SSTI, pneumonia, and shock.
- It can be used for procedural guidance for bladder catheterization, lumbar puncture, and intravenous access.
Dr. Patra is a pediatric hospitalist at West Virginia University Children’s Hospital, Morgantown, and associate professor at West Virginia University School of Medicine. He is interested in medical education, quality improvement and clinical research. He is a member of the Executive Council of the Pediatric Special Interest Group of the Society of Hospital Medicine.
PHM 2021 Session
Safe and (Ultra)sound: Why you should use POCUS in your Pediatric Practice
Presenter
Ria Dancel, MD, FAAP, FACP
Session summary
Dr. Ria Dancel and her colleagues from the University of North Carolina at Chapel Hill presented a broad overview of point-of-care ultrasound (POCUS) applications in the field of pediatric hospital medicine. They discussed its advantages and potential uses, ranging from common scenarios to critical care to procedural guidance. Using illustrative scenarios and interactive cases, she discussed the bedside applications to improve care of hospitalized children. The benefits and risks of radiography and POCUS were reviewed.
The session highlighted the use of POCUS in SSTI (skin and soft tissue infection) to help with differentiating cellulitis from abscesses. Use of POCUS for safer incision and drainages and making day-to-day changes in management was discussed. The ease and benefits of performing real-time lung ultrasound in different pathologies (like pneumonia, effusion, COVID-19) was presented. The speakers discussed the use of POCUS in emergency situations like hypotension and different types of shock. The use of ultrasound in common bedside procedures (bladder catheterization, lumbar ultrasound, peripheral IV placement) were also highlighted. Current literature and evidence were reviewed.
Key takeaways
- Pediatric POCUS is an extremely valuable bedside tool in pediatric hospital medicine.
- It can be used to guide clinical care for many conditions including SSTI, pneumonia, and shock.
- It can be used for procedural guidance for bladder catheterization, lumbar puncture, and intravenous access.
Dr. Patra is a pediatric hospitalist at West Virginia University Children’s Hospital, Morgantown, and associate professor at West Virginia University School of Medicine. He is interested in medical education, quality improvement and clinical research. He is a member of the Executive Council of the Pediatric Special Interest Group of the Society of Hospital Medicine.
In all-comer approach, FFR adds no value to angiography: RIPCORD 2
Study confirms selective application
In patients with coronary artery disease scheduled for a percutaneous intervention (PCI), fractional flow reserve (FFR) assessment at the time of angiography significantly improves outcome, but it has no apparent value as a routine study in all CAD patients, according to the randomized RIPCORD 2 trial.
When compared to angiography alone in an all comer-strategy, the addition of FFR did not significantly change management or lower costs, but it was associated with a longer time for diagnostic assessment and more complications, Nicholas P. Curzen, BM, PhD, reported at the annual congress of the European Society of Cardiology.
As a tool for evaluating stenotic lesions in diseased vessels, FFR, also known as pressure wire assessment, allows interventionalists to target those vessels that induce ischemia without unnecessarily treating vessels with lesions that are hemodynamically nonsignificant. It is guideline recommended for patients with scheduled PCI on the basis of several randomized trials, including the landmark FAME trial.
“The results of these trials were spectacular. The clinical outcomes were significantly better in the FFR group despite less stents being placed and fewer vessels being stented. And there was significantly less resource utilization in the FFR group,” said Dr. Curzen, professor of interventional cardiology, University of Southampton, England.
Hypothesis: All-comers benefit from FFR
This prompted the new trial, called RIPCORD 2. The hypothesis was that systematic FFR early in the diagnosis of CAD would reduce resource utilization and improve quality of life relative to angiography alone. Both were addressed as primary endpoints. A reduction in clinical events at 12 months was a secondary endpoint.
The 1,136 participants, all scheduled for angiographic evaluation for stable angina or non-ST elevated myocardial infarction (NSTEMI), were randomized at 17 participating centers in the United Kingdom. All underwent angiography, but the experimental arm also underwent FFR for all arteries of a size suitable for revascularization.
Resource utilization evaluated through hospital costs at 12 months was somewhat higher in the FFR group, but the difference was not significant (P =.137). There was also no significant difference (P = 0.88) between the groups in quality of life, which was measured with EQ-5D-5L, an instrument for expressing five dimensions of health on a visual analog scale.
No impact from FFR on clinical events
Furthermore, there was no difference in the rate of clinical events, whether measured by a composite endpoint of major adverse cardiac events (MACE) (P = .64) or by the components of death, stroke, myocardial infarction, and unplanned revascularization, according to Dr. Curzen.
Finally, FFR did not appear to influence subsequent management. When the intervention and control groups were compared, the proportions triaged to optimal medical therapy, optimal medical therapy plus PCI, or optimal medical therapy plus bypass grafting did not differ significantly.
Given the lack of significant differences for FFR plus angiography relative to angiography alone for any clinically relevant outcome, the addition of FFR provides "no overall advantage" in this all comer study population, Dr. Curzen concluded.
However, FFR was associated with some relative disadvantages. These included significantly longer mean procedure times (69 vs. 42.4 minutes; P < .001), significantly greater mean use of contrast (206 vs. 146.3 mL; P < .001), and a significantly higher mean radiation dose (6608.7 vs. 5029.7 cGY/cm2; P < .001). There were 10 complications (1.8%) associated with FFR.
RIPCORD 1 results provided study rationale
In the previously published nonrandomized RIPCORD 1 study, interventionalists were asked to develop a management plan on the basis of angiography alone in 200 patients with stable chest pain. When these interventionalists were then provided with FFR results, the new information resulted in a change of management plan in 36% of cases.
According to Dr. Curzen, it was this study that raised all-comer FFR as a “logical and clinically plausible question.” RIPCORD 2 provided the answer.
While he is now conducting an evaluation of a subgroup of RIPCORD 2 patients with more severe disease, “it appears that the atheroma burden on angiography is adequate” to make an appropriate management determination in most or all cases.
The invited discussant for this study, Robert Byrne, MD, BCh, PhD, director of cardiology, Mater Private Hospital, Dublin, pointed out that more angiography-alone patients in RIPCORD 2 required additional evaluation to develop a management strategy (14.7% vs. 1.8%), but he agreed that FFR offered “no reasonable benefit” in the relatively low-risk patients who were enrolled.
Results do not alter FFR indications
However, he emphasized that the lack of an advantage in this trial should in no way diminish the evidence of benefit for selective FFR use as currently recommended in guidelines. This was echoed strongly in remarks by two other interventionalists who served on the same panel after the RIPCORD 2 results were presented.
“I want to make sure that our audience does not walk away thinking that FFR is useless. This is not what was shown,” said Roxana Mehran, MD, director of interventional cardiovascular research at Icahn School of Medicine at Mount Sinai, New York. She emphasized that this was a study that found no value in a low-risk, all-comer population and is not relevant to the populations where it now has an indication.
Marco Roffi, MD, director of the interventional cardiology unit, Geneva University Hospitals, made the same point.
“These results do not take away the value of FFR in a more selected population [than that enrolled in RIPCORD 2],” Dr. Roffi said. He did not rule out the potential for benefit from adding FFR to angiography even in early disease assessment if a benefit can be demonstrated in a higher-risk population.
Dr. Curzen reports financial relationships with Abbott, Beckman Coulter, HeartFlow, and Boston Scientific, which provided funding for RIPCORD 2. Dr. Byrne reported financial relationships with the trial sponsor as well as Abbott, Biosensors, and Biotronik. Dr. Mehran reports financial relationships with more than 15 medical product companies including the sponsor of this trial. Dr. Roffi reports no relevant financial disclosures.
Study confirms selective application
Study confirms selective application
In patients with coronary artery disease scheduled for a percutaneous intervention (PCI), fractional flow reserve (FFR) assessment at the time of angiography significantly improves outcome, but it has no apparent value as a routine study in all CAD patients, according to the randomized RIPCORD 2 trial.
When compared to angiography alone in an all comer-strategy, the addition of FFR did not significantly change management or lower costs, but it was associated with a longer time for diagnostic assessment and more complications, Nicholas P. Curzen, BM, PhD, reported at the annual congress of the European Society of Cardiology.
As a tool for evaluating stenotic lesions in diseased vessels, FFR, also known as pressure wire assessment, allows interventionalists to target those vessels that induce ischemia without unnecessarily treating vessels with lesions that are hemodynamically nonsignificant. It is guideline recommended for patients with scheduled PCI on the basis of several randomized trials, including the landmark FAME trial.
“The results of these trials were spectacular. The clinical outcomes were significantly better in the FFR group despite less stents being placed and fewer vessels being stented. And there was significantly less resource utilization in the FFR group,” said Dr. Curzen, professor of interventional cardiology, University of Southampton, England.
Hypothesis: All-comers benefit from FFR
This prompted the new trial, called RIPCORD 2. The hypothesis was that systematic FFR early in the diagnosis of CAD would reduce resource utilization and improve quality of life relative to angiography alone. Both were addressed as primary endpoints. A reduction in clinical events at 12 months was a secondary endpoint.
The 1,136 participants, all scheduled for angiographic evaluation for stable angina or non-ST elevated myocardial infarction (NSTEMI), were randomized at 17 participating centers in the United Kingdom. All underwent angiography, but the experimental arm also underwent FFR for all arteries of a size suitable for revascularization.
Resource utilization evaluated through hospital costs at 12 months was somewhat higher in the FFR group, but the difference was not significant (P =.137). There was also no significant difference (P = 0.88) between the groups in quality of life, which was measured with EQ-5D-5L, an instrument for expressing five dimensions of health on a visual analog scale.
No impact from FFR on clinical events
Furthermore, there was no difference in the rate of clinical events, whether measured by a composite endpoint of major adverse cardiac events (MACE) (P = .64) or by the components of death, stroke, myocardial infarction, and unplanned revascularization, according to Dr. Curzen.
Finally, FFR did not appear to influence subsequent management. When the intervention and control groups were compared, the proportions triaged to optimal medical therapy, optimal medical therapy plus PCI, or optimal medical therapy plus bypass grafting did not differ significantly.
Given the lack of significant differences for FFR plus angiography relative to angiography alone for any clinically relevant outcome, the addition of FFR provides "no overall advantage" in this all comer study population, Dr. Curzen concluded.
However, FFR was associated with some relative disadvantages. These included significantly longer mean procedure times (69 vs. 42.4 minutes; P < .001), significantly greater mean use of contrast (206 vs. 146.3 mL; P < .001), and a significantly higher mean radiation dose (6608.7 vs. 5029.7 cGY/cm2; P < .001). There were 10 complications (1.8%) associated with FFR.
RIPCORD 1 results provided study rationale
In the previously published nonrandomized RIPCORD 1 study, interventionalists were asked to develop a management plan on the basis of angiography alone in 200 patients with stable chest pain. When these interventionalists were then provided with FFR results, the new information resulted in a change of management plan in 36% of cases.
According to Dr. Curzen, it was this study that raised all-comer FFR as a “logical and clinically plausible question.” RIPCORD 2 provided the answer.
While he is now conducting an evaluation of a subgroup of RIPCORD 2 patients with more severe disease, “it appears that the atheroma burden on angiography is adequate” to make an appropriate management determination in most or all cases.
The invited discussant for this study, Robert Byrne, MD, BCh, PhD, director of cardiology, Mater Private Hospital, Dublin, pointed out that more angiography-alone patients in RIPCORD 2 required additional evaluation to develop a management strategy (14.7% vs. 1.8%), but he agreed that FFR offered “no reasonable benefit” in the relatively low-risk patients who were enrolled.
Results do not alter FFR indications
However, he emphasized that the lack of an advantage in this trial should in no way diminish the evidence of benefit for selective FFR use as currently recommended in guidelines. This was echoed strongly in remarks by two other interventionalists who served on the same panel after the RIPCORD 2 results were presented.
“I want to make sure that our audience does not walk away thinking that FFR is useless. This is not what was shown,” said Roxana Mehran, MD, director of interventional cardiovascular research at Icahn School of Medicine at Mount Sinai, New York. She emphasized that this was a study that found no value in a low-risk, all-comer population and is not relevant to the populations where it now has an indication.
Marco Roffi, MD, director of the interventional cardiology unit, Geneva University Hospitals, made the same point.
“These results do not take away the value of FFR in a more selected population [than that enrolled in RIPCORD 2],” Dr. Roffi said. He did not rule out the potential for benefit from adding FFR to angiography even in early disease assessment if a benefit can be demonstrated in a higher-risk population.
Dr. Curzen reports financial relationships with Abbott, Beckman Coulter, HeartFlow, and Boston Scientific, which provided funding for RIPCORD 2. Dr. Byrne reported financial relationships with the trial sponsor as well as Abbott, Biosensors, and Biotronik. Dr. Mehran reports financial relationships with more than 15 medical product companies including the sponsor of this trial. Dr. Roffi reports no relevant financial disclosures.
In patients with coronary artery disease scheduled for a percutaneous intervention (PCI), fractional flow reserve (FFR) assessment at the time of angiography significantly improves outcome, but it has no apparent value as a routine study in all CAD patients, according to the randomized RIPCORD 2 trial.
When compared to angiography alone in an all comer-strategy, the addition of FFR did not significantly change management or lower costs, but it was associated with a longer time for diagnostic assessment and more complications, Nicholas P. Curzen, BM, PhD, reported at the annual congress of the European Society of Cardiology.
As a tool for evaluating stenotic lesions in diseased vessels, FFR, also known as pressure wire assessment, allows interventionalists to target those vessels that induce ischemia without unnecessarily treating vessels with lesions that are hemodynamically nonsignificant. It is guideline recommended for patients with scheduled PCI on the basis of several randomized trials, including the landmark FAME trial.
“The results of these trials were spectacular. The clinical outcomes were significantly better in the FFR group despite less stents being placed and fewer vessels being stented. And there was significantly less resource utilization in the FFR group,” said Dr. Curzen, professor of interventional cardiology, University of Southampton, England.
Hypothesis: All-comers benefit from FFR
This prompted the new trial, called RIPCORD 2. The hypothesis was that systematic FFR early in the diagnosis of CAD would reduce resource utilization and improve quality of life relative to angiography alone. Both were addressed as primary endpoints. A reduction in clinical events at 12 months was a secondary endpoint.
The 1,136 participants, all scheduled for angiographic evaluation for stable angina or non-ST elevated myocardial infarction (NSTEMI), were randomized at 17 participating centers in the United Kingdom. All underwent angiography, but the experimental arm also underwent FFR for all arteries of a size suitable for revascularization.
Resource utilization evaluated through hospital costs at 12 months was somewhat higher in the FFR group, but the difference was not significant (P =.137). There was also no significant difference (P = 0.88) between the groups in quality of life, which was measured with EQ-5D-5L, an instrument for expressing five dimensions of health on a visual analog scale.
No impact from FFR on clinical events
Furthermore, there was no difference in the rate of clinical events, whether measured by a composite endpoint of major adverse cardiac events (MACE) (P = .64) or by the components of death, stroke, myocardial infarction, and unplanned revascularization, according to Dr. Curzen.
Finally, FFR did not appear to influence subsequent management. When the intervention and control groups were compared, the proportions triaged to optimal medical therapy, optimal medical therapy plus PCI, or optimal medical therapy plus bypass grafting did not differ significantly.
Given the lack of significant differences for FFR plus angiography relative to angiography alone for any clinically relevant outcome, the addition of FFR provides "no overall advantage" in this all comer study population, Dr. Curzen concluded.
However, FFR was associated with some relative disadvantages. These included significantly longer mean procedure times (69 vs. 42.4 minutes; P < .001), significantly greater mean use of contrast (206 vs. 146.3 mL; P < .001), and a significantly higher mean radiation dose (6608.7 vs. 5029.7 cGY/cm2; P < .001). There were 10 complications (1.8%) associated with FFR.
RIPCORD 1 results provided study rationale
In the previously published nonrandomized RIPCORD 1 study, interventionalists were asked to develop a management plan on the basis of angiography alone in 200 patients with stable chest pain. When these interventionalists were then provided with FFR results, the new information resulted in a change of management plan in 36% of cases.
According to Dr. Curzen, it was this study that raised all-comer FFR as a “logical and clinically plausible question.” RIPCORD 2 provided the answer.
While he is now conducting an evaluation of a subgroup of RIPCORD 2 patients with more severe disease, “it appears that the atheroma burden on angiography is adequate” to make an appropriate management determination in most or all cases.
The invited discussant for this study, Robert Byrne, MD, BCh, PhD, director of cardiology, Mater Private Hospital, Dublin, pointed out that more angiography-alone patients in RIPCORD 2 required additional evaluation to develop a management strategy (14.7% vs. 1.8%), but he agreed that FFR offered “no reasonable benefit” in the relatively low-risk patients who were enrolled.
Results do not alter FFR indications
However, he emphasized that the lack of an advantage in this trial should in no way diminish the evidence of benefit for selective FFR use as currently recommended in guidelines. This was echoed strongly in remarks by two other interventionalists who served on the same panel after the RIPCORD 2 results were presented.
“I want to make sure that our audience does not walk away thinking that FFR is useless. This is not what was shown,” said Roxana Mehran, MD, director of interventional cardiovascular research at Icahn School of Medicine at Mount Sinai, New York. She emphasized that this was a study that found no value in a low-risk, all-comer population and is not relevant to the populations where it now has an indication.
Marco Roffi, MD, director of the interventional cardiology unit, Geneva University Hospitals, made the same point.
“These results do not take away the value of FFR in a more selected population [than that enrolled in RIPCORD 2],” Dr. Roffi said. He did not rule out the potential for benefit from adding FFR to angiography even in early disease assessment if a benefit can be demonstrated in a higher-risk population.
Dr. Curzen reports financial relationships with Abbott, Beckman Coulter, HeartFlow, and Boston Scientific, which provided funding for RIPCORD 2. Dr. Byrne reported financial relationships with the trial sponsor as well as Abbott, Biosensors, and Biotronik. Dr. Mehran reports financial relationships with more than 15 medical product companies including the sponsor of this trial. Dr. Roffi reports no relevant financial disclosures.
FROM ESC CONGRESS 2021
Use of point-of-care ultrasound (POCUS) for heart failure
Case
A 65-year-old woman presents to the emergency department with a chief complaint of shortness of breath for 3 days. Medical history is notable for moderate chronic obstructive pulmonary disorder, systolic heart failure with last known ejection fraction (EF) of 35% and type 2 diabetes complicated by hyperglycemia when on steroids. You are talking the case over with colleagues and they suggest point-of-care ultrasound (POCUS) would be useful in her case.
Brief overview of the issue
Once mainly used by ED and critical care physicians, POCUS is now a tool that many hospitalists are using at the bedside. POCUS differs from traditional comprehensive ultrasounds in the following ways: POCUS is designed to answer a specific clinical question (as opposed to evaluating all organs in a specific region), POCUS exams are performed by the clinician who is formulating the clinical question (as opposed to by a consultative service such as cardiology and radiology), and POCUS can evaluate multiple organ systems (such as by evaluating a patient’s heart, lungs, and inferior vena cava to determine the etiology of hypoxia).
Hospitalist use of POCUS may include guiding procedures, aiding in diagnosis, and assessing effectiveness of treatment. Many high-quality studies have been published that support the use of POCUS and have proven that POCUS can decrease medical errors, help reach diagnoses in a more expedited fashion, and complement or replace more advanced imaging.
A challenge of POCUS is that it is user dependent and there are no established standards for hospitalists in POCUS training. As the Society of Hospital Medicine position statement on POCUS points out, there is a significant difference between skill levels required to obtain a certificate of completion for POCUS training and a certificate of competency in POCUS. Therefore, it is recommended hospitalists work with local credentialing committees to delineate the requirements for POCUS use.
Overview of the data
POCUS for initial assessment and diagnosis of heart failure (HF)
Use of POCUS in cases of suspected HF includes examination of the heart, lungs, and inferior vena cava (IVC). Cardiac ultrasound provides an estimated ejection fraction. Lung ultrasound (LUS) functions to examine for B lines and pleural effusions. The presence of more than three B lines per thoracic zone bilaterally suggests cardiogenic pulmonary edema. Scanning the IVC provides a noninvasive way to assess volume status and is especially helpful when body habitus prevents accurate assessment of jugular venous pressure.
Several studies have addressed the utility of bedside ultrasound in the initial assessment or diagnosis of acute decompensated heart failure (ADHF) in patients presenting with dyspnea in emergency or inpatient settings. Positive B lines are a useful finding, with high sensitivities, high specificities, and positive likelihood ratios. One large multicenter prospective study found LUS to have a sensitivity of 90.5%, specificity of 93.5%, and positive and negative LRs of 14.0 and 0.10, respectively.1 Another large multicenter prospective cohort study showed that LUS was more sensitive and more specific than chest x-ray (CXR) and brain natriuretic peptide in detecting ADHF.2 Additional POCUS findings that have shown relatively high sensitivities and specificities in the initial diagnosis of ADHF include pleural effusion, reduced left ventricular ejection fraction (LVEF), increased left ventricular end-diastolic dimension, and jugular venous distention.
Data also exists on assessments of ADHF using combinations of POCUS findings; for example, lung and cardiac ultrasound (LuCUS) protocols include an evaluation for B lines, assessment of IVC size and collapsibility, and determination of LVEF, although this has mainly been examined in ED patients. For patients who presented to the ED with undifferentiated dyspnea, one such study showed a specificity of 100% when a LuCUS protocol was used to diagnose ADHF while another study showed that the use of a LuCUS protocol changed management in 47% of patients.3,4 Of note, although each LuCUS protocol integrated the use of lung findings, IVC collapsibility, and LVEF, the exact protocols varied by institution. Finally, it has been established in multiple studies that LUS used in addition to standard workup including history and physical, labs, and electrocardiogram has been shown to increase diagnostic accuracy.2,5
Using POCUS to guide diuretic therapy in HF
To date, there have been multiple small studies published on the utility of daily POCUS in hospitalized patients with ADHF to help assess response to treatment and guide diuresis by looking for reduction in B lines on LUS or a change in IVC size or collapsibility. Volpicelli and colleagues showed that daily LUS was at least as good as daily CXR in monitoring response to therapy.6 Similarly, Mozzini and colleagues performed a randomized controlled trial of 120 patients admitted for ADHF who were randomized to a CXR group (who had a CXR performed on admission and discharge) and a LUS group (which was performed at admission, 24 hours, 48 hours, 72 hours, and discharge).7 This study found that the LUS group underwent a significantly higher number of diuretic dose adjustments as compared with the CXR group (P < .001) and had a modest improvement in LOS, compared with the CXR group. Specifically, median LOS was 8 days in CXR group (range, 4-17 days) and 7 days in the LUS group (range, 3-10 days; P < .001).
The impact of POCUS on length of stay (LOS) and readmissions
There is increasing data that POCUS can have meaningful impacts on patient-centered outcomes (morbidity, mortality, and readmission) while exposing patients to minimal discomfort, no venipuncture, and no radiation exposure. First, multiple studies looked at whether performing focused cardiac US of the IVC as a marker of volume status could predict readmission in patients hospitalized for ADHF.8,9 Both of these trials showed that plethoric, noncollapsible IVC at discharge were statistically significant predictors of readmission. In fact, Goonewardena and colleagues demonstrated that patients who required readmission had an enlarged IVC at discharge nearly 3 times more frequently (21% vs. 61%, P < .001) and abnormal IVC collapsibility 1.5 times more frequently (41% vs. 71%, P = .01) as compared with patients who remained out of the hospital.9
Similarly, a subsequent trial looked at whether IVC size on admission was of prognostic importance in patients hospitalized for ADHF and showed that admission IVC diameter was an independent predictor of both 90-day mortality (hazard ratio, 5.88; 95% confidence interval, 1.21-28.10; P = .025) and 90-day readmission (HR, 3.20; 95% CI, 1.24-8.21; P = .016).10 Additionally, LUS heart failure assessment for pulmonary congestion by counting B lines also showed that having more than 15 B lines prior to discharge was an independent predictor of readmission for ADHF at 6 months (HR, 11.74; 95% CI, 1.30-106.16).11
A challenge of POCUS: Obtaining competency
As previously noted, there are not yet any established standards for training and assessing hospitalists in POCUS. The SHM Position Statement on POCUS recommends the following criteria for training5: the training environment should be similar to the location in which the trainee will practice, training and feedback should occur in real time, the trainee should be taught specific applications of POCUS (such as cardiac US, LUS, and IVC US) as each application comes with unique skills and knowledge, clinical competence must be achieved and demonstrated, and continued education and feedback are necessary once competence is obtained.12 SHM recommends residency-based training pathways, training through a local or national program such as the SHM POCUS certificate program, or training through other medical societies for hospitalists already in practice.
Application of the data to our original case
Targeted POCUS using the LuCUS protocol is performed and reveals three B lines in two lung zones bilaterally, moderate bilateral pleural effusions, EF 20%, and a noncollapsible IVC leading to a diagnosis of ADHF. Her ADHF is treated with intravenous diuresis. She is continued on her chronic maintenance chronic obstructive pulmonary disorder regimen but does not receive steroids, avoiding hyperglycemia that has complicated prior admissions. Over the next few days her respiratory and cardiac status is monitored using POCUS to assess her response to therapy and titrate her diuretics to her true dry weight, which was several pounds lower than her previously assumed dry weight. At discharge she is instructed to use the new dry weight which may avoid readmissions for HF.
Bottom line
POCUS improves diagnostic accuracy and facilitates volume assessment and management in acute decompensated heart failure.
Dr. Farber is a medical instructor at Duke University and hospitalist at Duke Regional Hospital, both in Durham, N.C. Dr. Marcantonio is a medical instructor in the department of internal medicine and department of pediatrics at Duke University and hospitalist at Duke University Hospital and Duke Regional Hospital. Dr. Stafford and Dr. Brooks are assistant professors of medicine and hospitalists at Duke Regional Hospital. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor at Duke University. Dr. Menon is a hospitalist at Duke University. Dr. Sharma is associate medical director for clinical education at Duke Regional Hospital and associate professor of medicine at Duke University.
References
1. Pivetta E et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur J Heart Fail. 2019 Jun;21(6):754-66. doi: 10.1002/ejhf.1379.
2. Pivetta E et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: A SIMEU multicenter study. Chest. 2015;148(1):202-10. doi: 10.1378/chest.14-2608.
3. Anderson KL et al. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31:1208-14. doi: 10.1016/j.ajem.2013.05.007.
4. Russell FM et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea: A lung and cardiac ultrasound (LuCUS) protocol. Acad Emerg Med. 2015;22(2):182-91. doi:10.1111/acem.12570.
5. Maw AM et al. Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: A systematic review and meta-analysis. JAMA Netw Open. 2019 Mar 1;2(3):e190703. doi:10.1001/jamanetworkopen.2019.0703.
6. Volpicelli G et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008 Jun;26(5):585-91. doi:10.1016/j.ajem.2007.09.014.
7. Mozzini C et al. Lung ultrasound in internal medicine efficiently drives the management of patients with heart failure and speeds up the discharge time. Intern Emerg Med. 2018 Jan;13(1):27-33. doi: 10.1007/s11739-017-1738-1.
8. Laffin LJ et al. Focused cardiac ultrasound as a predictor of readmission in acute decompensated heart failure. Int J Cardiovasc Imaging. 2018;34(7):1075-9. doi:10.1007/s10554-018-1317-1.
9. Goonewardena SN et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595-601. doi:10.1016/j.jcmg.2008.06.005.
10. Cubo-Romano P et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016 Nov;11(11):778-84. doi: 10.1002/jhm.2620.
11. Gargani L et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc Ultrasound. 2015 Sep 4;13:40. doi: 10.1186/s12947-015-0033-4.
12. Soni NJ et al. Point-of-care ultrasound for hospitalists: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2;14:E1-6. doi: 10.12788/jhm.3079.
Key points
- Studies have found POCUS improves the diagnosis of acute decompensated heart failure in patients presenting with dyspnea.
- Daily evaluation with POCUS has decreased length of stay in acute decompensated heart failure.
- Credentialing requirements for hospitalists to use POCUS for clinical care vary by hospital.
Additional reading
Maw AM and Soni NJ. Annals for hospitalists inpatient notes – why should hospitalists use point-of-care ultrasound? Ann Intern Med. 2018 Apr 17;168(8):HO2-HO3. doi: 10.7326/M18-0367.
Lewiss RE. “The ultrasound looked fine”: Point of care ultrasound and patient safety. AHRQ’s Patient Safety Network. WebM&M: Case Studies. 2018 Jul 1. https://psnet.ahrq.gov/web-mm/ultrasound-looked-fine-point-care-ultrasound-and-patient-safety.
Quiz: Testing your POCUS knowledge
POCUS is increasingly prevalent in hospital medicine, but use varies among different disease processes. Which organ system ultrasound or lab test would be most helpful in the following scenario?
An acutely dyspneic patient with no past medical history presents to the ED. Chest x-ray is equivocal. Of the following, which study best confirms a diagnosis of acute decompensated heart failure?
A. Brain natriuretic peptide
B. Point-of-care cardiac ultrasound
C. Point-of-care lung ultrasound
D. Point-of-care inferior vena cava ultrasound
Answer
C. Point-of-care lung ultrasound
Multiple studies, including three systematic reviews, have shown that point-of-care lung ultrasound has high sensitivity and specificity to evaluate for B lines as a marker for cardiogenic pulmonary edema. Point-of-care ultrasound of ejection fraction and inferior vena cava have not been evaluated by systematic review although one randomized, controlled trial showed that an EF less than 45% had 74% specificity and 77% sensitivity and IVC collapsibility index less than 20% had an 86% specificity and 52% sensitivity for detection of acute decompensated heart failure. This same study showed that the combination of cardiac, lung, and IVC point-of-care ultrasound had 100% specificity for diagnosing acute decompensated heart failure. In the future, health care providers could rely on this multiorgan evaluation with point-of-care ultrasound to confirm a diagnosis of acute decompensated heart failure in a dyspneic patient.
Case
A 65-year-old woman presents to the emergency department with a chief complaint of shortness of breath for 3 days. Medical history is notable for moderate chronic obstructive pulmonary disorder, systolic heart failure with last known ejection fraction (EF) of 35% and type 2 diabetes complicated by hyperglycemia when on steroids. You are talking the case over with colleagues and they suggest point-of-care ultrasound (POCUS) would be useful in her case.
Brief overview of the issue
Once mainly used by ED and critical care physicians, POCUS is now a tool that many hospitalists are using at the bedside. POCUS differs from traditional comprehensive ultrasounds in the following ways: POCUS is designed to answer a specific clinical question (as opposed to evaluating all organs in a specific region), POCUS exams are performed by the clinician who is formulating the clinical question (as opposed to by a consultative service such as cardiology and radiology), and POCUS can evaluate multiple organ systems (such as by evaluating a patient’s heart, lungs, and inferior vena cava to determine the etiology of hypoxia).
Hospitalist use of POCUS may include guiding procedures, aiding in diagnosis, and assessing effectiveness of treatment. Many high-quality studies have been published that support the use of POCUS and have proven that POCUS can decrease medical errors, help reach diagnoses in a more expedited fashion, and complement or replace more advanced imaging.
A challenge of POCUS is that it is user dependent and there are no established standards for hospitalists in POCUS training. As the Society of Hospital Medicine position statement on POCUS points out, there is a significant difference between skill levels required to obtain a certificate of completion for POCUS training and a certificate of competency in POCUS. Therefore, it is recommended hospitalists work with local credentialing committees to delineate the requirements for POCUS use.
Overview of the data
POCUS for initial assessment and diagnosis of heart failure (HF)
Use of POCUS in cases of suspected HF includes examination of the heart, lungs, and inferior vena cava (IVC). Cardiac ultrasound provides an estimated ejection fraction. Lung ultrasound (LUS) functions to examine for B lines and pleural effusions. The presence of more than three B lines per thoracic zone bilaterally suggests cardiogenic pulmonary edema. Scanning the IVC provides a noninvasive way to assess volume status and is especially helpful when body habitus prevents accurate assessment of jugular venous pressure.
Several studies have addressed the utility of bedside ultrasound in the initial assessment or diagnosis of acute decompensated heart failure (ADHF) in patients presenting with dyspnea in emergency or inpatient settings. Positive B lines are a useful finding, with high sensitivities, high specificities, and positive likelihood ratios. One large multicenter prospective study found LUS to have a sensitivity of 90.5%, specificity of 93.5%, and positive and negative LRs of 14.0 and 0.10, respectively.1 Another large multicenter prospective cohort study showed that LUS was more sensitive and more specific than chest x-ray (CXR) and brain natriuretic peptide in detecting ADHF.2 Additional POCUS findings that have shown relatively high sensitivities and specificities in the initial diagnosis of ADHF include pleural effusion, reduced left ventricular ejection fraction (LVEF), increased left ventricular end-diastolic dimension, and jugular venous distention.
Data also exists on assessments of ADHF using combinations of POCUS findings; for example, lung and cardiac ultrasound (LuCUS) protocols include an evaluation for B lines, assessment of IVC size and collapsibility, and determination of LVEF, although this has mainly been examined in ED patients. For patients who presented to the ED with undifferentiated dyspnea, one such study showed a specificity of 100% when a LuCUS protocol was used to diagnose ADHF while another study showed that the use of a LuCUS protocol changed management in 47% of patients.3,4 Of note, although each LuCUS protocol integrated the use of lung findings, IVC collapsibility, and LVEF, the exact protocols varied by institution. Finally, it has been established in multiple studies that LUS used in addition to standard workup including history and physical, labs, and electrocardiogram has been shown to increase diagnostic accuracy.2,5
Using POCUS to guide diuretic therapy in HF
To date, there have been multiple small studies published on the utility of daily POCUS in hospitalized patients with ADHF to help assess response to treatment and guide diuresis by looking for reduction in B lines on LUS or a change in IVC size or collapsibility. Volpicelli and colleagues showed that daily LUS was at least as good as daily CXR in monitoring response to therapy.6 Similarly, Mozzini and colleagues performed a randomized controlled trial of 120 patients admitted for ADHF who were randomized to a CXR group (who had a CXR performed on admission and discharge) and a LUS group (which was performed at admission, 24 hours, 48 hours, 72 hours, and discharge).7 This study found that the LUS group underwent a significantly higher number of diuretic dose adjustments as compared with the CXR group (P < .001) and had a modest improvement in LOS, compared with the CXR group. Specifically, median LOS was 8 days in CXR group (range, 4-17 days) and 7 days in the LUS group (range, 3-10 days; P < .001).
The impact of POCUS on length of stay (LOS) and readmissions
There is increasing data that POCUS can have meaningful impacts on patient-centered outcomes (morbidity, mortality, and readmission) while exposing patients to minimal discomfort, no venipuncture, and no radiation exposure. First, multiple studies looked at whether performing focused cardiac US of the IVC as a marker of volume status could predict readmission in patients hospitalized for ADHF.8,9 Both of these trials showed that plethoric, noncollapsible IVC at discharge were statistically significant predictors of readmission. In fact, Goonewardena and colleagues demonstrated that patients who required readmission had an enlarged IVC at discharge nearly 3 times more frequently (21% vs. 61%, P < .001) and abnormal IVC collapsibility 1.5 times more frequently (41% vs. 71%, P = .01) as compared with patients who remained out of the hospital.9
Similarly, a subsequent trial looked at whether IVC size on admission was of prognostic importance in patients hospitalized for ADHF and showed that admission IVC diameter was an independent predictor of both 90-day mortality (hazard ratio, 5.88; 95% confidence interval, 1.21-28.10; P = .025) and 90-day readmission (HR, 3.20; 95% CI, 1.24-8.21; P = .016).10 Additionally, LUS heart failure assessment for pulmonary congestion by counting B lines also showed that having more than 15 B lines prior to discharge was an independent predictor of readmission for ADHF at 6 months (HR, 11.74; 95% CI, 1.30-106.16).11
A challenge of POCUS: Obtaining competency
As previously noted, there are not yet any established standards for training and assessing hospitalists in POCUS. The SHM Position Statement on POCUS recommends the following criteria for training5: the training environment should be similar to the location in which the trainee will practice, training and feedback should occur in real time, the trainee should be taught specific applications of POCUS (such as cardiac US, LUS, and IVC US) as each application comes with unique skills and knowledge, clinical competence must be achieved and demonstrated, and continued education and feedback are necessary once competence is obtained.12 SHM recommends residency-based training pathways, training through a local or national program such as the SHM POCUS certificate program, or training through other medical societies for hospitalists already in practice.
Application of the data to our original case
Targeted POCUS using the LuCUS protocol is performed and reveals three B lines in two lung zones bilaterally, moderate bilateral pleural effusions, EF 20%, and a noncollapsible IVC leading to a diagnosis of ADHF. Her ADHF is treated with intravenous diuresis. She is continued on her chronic maintenance chronic obstructive pulmonary disorder regimen but does not receive steroids, avoiding hyperglycemia that has complicated prior admissions. Over the next few days her respiratory and cardiac status is monitored using POCUS to assess her response to therapy and titrate her diuretics to her true dry weight, which was several pounds lower than her previously assumed dry weight. At discharge she is instructed to use the new dry weight which may avoid readmissions for HF.
Bottom line
POCUS improves diagnostic accuracy and facilitates volume assessment and management in acute decompensated heart failure.
Dr. Farber is a medical instructor at Duke University and hospitalist at Duke Regional Hospital, both in Durham, N.C. Dr. Marcantonio is a medical instructor in the department of internal medicine and department of pediatrics at Duke University and hospitalist at Duke University Hospital and Duke Regional Hospital. Dr. Stafford and Dr. Brooks are assistant professors of medicine and hospitalists at Duke Regional Hospital. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor at Duke University. Dr. Menon is a hospitalist at Duke University. Dr. Sharma is associate medical director for clinical education at Duke Regional Hospital and associate professor of medicine at Duke University.
References
1. Pivetta E et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur J Heart Fail. 2019 Jun;21(6):754-66. doi: 10.1002/ejhf.1379.
2. Pivetta E et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: A SIMEU multicenter study. Chest. 2015;148(1):202-10. doi: 10.1378/chest.14-2608.
3. Anderson KL et al. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31:1208-14. doi: 10.1016/j.ajem.2013.05.007.
4. Russell FM et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea: A lung and cardiac ultrasound (LuCUS) protocol. Acad Emerg Med. 2015;22(2):182-91. doi:10.1111/acem.12570.
5. Maw AM et al. Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: A systematic review and meta-analysis. JAMA Netw Open. 2019 Mar 1;2(3):e190703. doi:10.1001/jamanetworkopen.2019.0703.
6. Volpicelli G et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008 Jun;26(5):585-91. doi:10.1016/j.ajem.2007.09.014.
7. Mozzini C et al. Lung ultrasound in internal medicine efficiently drives the management of patients with heart failure and speeds up the discharge time. Intern Emerg Med. 2018 Jan;13(1):27-33. doi: 10.1007/s11739-017-1738-1.
8. Laffin LJ et al. Focused cardiac ultrasound as a predictor of readmission in acute decompensated heart failure. Int J Cardiovasc Imaging. 2018;34(7):1075-9. doi:10.1007/s10554-018-1317-1.
9. Goonewardena SN et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595-601. doi:10.1016/j.jcmg.2008.06.005.
10. Cubo-Romano P et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016 Nov;11(11):778-84. doi: 10.1002/jhm.2620.
11. Gargani L et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc Ultrasound. 2015 Sep 4;13:40. doi: 10.1186/s12947-015-0033-4.
12. Soni NJ et al. Point-of-care ultrasound for hospitalists: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2;14:E1-6. doi: 10.12788/jhm.3079.
Key points
- Studies have found POCUS improves the diagnosis of acute decompensated heart failure in patients presenting with dyspnea.
- Daily evaluation with POCUS has decreased length of stay in acute decompensated heart failure.
- Credentialing requirements for hospitalists to use POCUS for clinical care vary by hospital.
Additional reading
Maw AM and Soni NJ. Annals for hospitalists inpatient notes – why should hospitalists use point-of-care ultrasound? Ann Intern Med. 2018 Apr 17;168(8):HO2-HO3. doi: 10.7326/M18-0367.
Lewiss RE. “The ultrasound looked fine”: Point of care ultrasound and patient safety. AHRQ’s Patient Safety Network. WebM&M: Case Studies. 2018 Jul 1. https://psnet.ahrq.gov/web-mm/ultrasound-looked-fine-point-care-ultrasound-and-patient-safety.
Quiz: Testing your POCUS knowledge
POCUS is increasingly prevalent in hospital medicine, but use varies among different disease processes. Which organ system ultrasound or lab test would be most helpful in the following scenario?
An acutely dyspneic patient with no past medical history presents to the ED. Chest x-ray is equivocal. Of the following, which study best confirms a diagnosis of acute decompensated heart failure?
A. Brain natriuretic peptide
B. Point-of-care cardiac ultrasound
C. Point-of-care lung ultrasound
D. Point-of-care inferior vena cava ultrasound
Answer
C. Point-of-care lung ultrasound
Multiple studies, including three systematic reviews, have shown that point-of-care lung ultrasound has high sensitivity and specificity to evaluate for B lines as a marker for cardiogenic pulmonary edema. Point-of-care ultrasound of ejection fraction and inferior vena cava have not been evaluated by systematic review although one randomized, controlled trial showed that an EF less than 45% had 74% specificity and 77% sensitivity and IVC collapsibility index less than 20% had an 86% specificity and 52% sensitivity for detection of acute decompensated heart failure. This same study showed that the combination of cardiac, lung, and IVC point-of-care ultrasound had 100% specificity for diagnosing acute decompensated heart failure. In the future, health care providers could rely on this multiorgan evaluation with point-of-care ultrasound to confirm a diagnosis of acute decompensated heart failure in a dyspneic patient.
Case
A 65-year-old woman presents to the emergency department with a chief complaint of shortness of breath for 3 days. Medical history is notable for moderate chronic obstructive pulmonary disorder, systolic heart failure with last known ejection fraction (EF) of 35% and type 2 diabetes complicated by hyperglycemia when on steroids. You are talking the case over with colleagues and they suggest point-of-care ultrasound (POCUS) would be useful in her case.
Brief overview of the issue
Once mainly used by ED and critical care physicians, POCUS is now a tool that many hospitalists are using at the bedside. POCUS differs from traditional comprehensive ultrasounds in the following ways: POCUS is designed to answer a specific clinical question (as opposed to evaluating all organs in a specific region), POCUS exams are performed by the clinician who is formulating the clinical question (as opposed to by a consultative service such as cardiology and radiology), and POCUS can evaluate multiple organ systems (such as by evaluating a patient’s heart, lungs, and inferior vena cava to determine the etiology of hypoxia).
Hospitalist use of POCUS may include guiding procedures, aiding in diagnosis, and assessing effectiveness of treatment. Many high-quality studies have been published that support the use of POCUS and have proven that POCUS can decrease medical errors, help reach diagnoses in a more expedited fashion, and complement or replace more advanced imaging.
A challenge of POCUS is that it is user dependent and there are no established standards for hospitalists in POCUS training. As the Society of Hospital Medicine position statement on POCUS points out, there is a significant difference between skill levels required to obtain a certificate of completion for POCUS training and a certificate of competency in POCUS. Therefore, it is recommended hospitalists work with local credentialing committees to delineate the requirements for POCUS use.
Overview of the data
POCUS for initial assessment and diagnosis of heart failure (HF)
Use of POCUS in cases of suspected HF includes examination of the heart, lungs, and inferior vena cava (IVC). Cardiac ultrasound provides an estimated ejection fraction. Lung ultrasound (LUS) functions to examine for B lines and pleural effusions. The presence of more than three B lines per thoracic zone bilaterally suggests cardiogenic pulmonary edema. Scanning the IVC provides a noninvasive way to assess volume status and is especially helpful when body habitus prevents accurate assessment of jugular venous pressure.
Several studies have addressed the utility of bedside ultrasound in the initial assessment or diagnosis of acute decompensated heart failure (ADHF) in patients presenting with dyspnea in emergency or inpatient settings. Positive B lines are a useful finding, with high sensitivities, high specificities, and positive likelihood ratios. One large multicenter prospective study found LUS to have a sensitivity of 90.5%, specificity of 93.5%, and positive and negative LRs of 14.0 and 0.10, respectively.1 Another large multicenter prospective cohort study showed that LUS was more sensitive and more specific than chest x-ray (CXR) and brain natriuretic peptide in detecting ADHF.2 Additional POCUS findings that have shown relatively high sensitivities and specificities in the initial diagnosis of ADHF include pleural effusion, reduced left ventricular ejection fraction (LVEF), increased left ventricular end-diastolic dimension, and jugular venous distention.
Data also exists on assessments of ADHF using combinations of POCUS findings; for example, lung and cardiac ultrasound (LuCUS) protocols include an evaluation for B lines, assessment of IVC size and collapsibility, and determination of LVEF, although this has mainly been examined in ED patients. For patients who presented to the ED with undifferentiated dyspnea, one such study showed a specificity of 100% when a LuCUS protocol was used to diagnose ADHF while another study showed that the use of a LuCUS protocol changed management in 47% of patients.3,4 Of note, although each LuCUS protocol integrated the use of lung findings, IVC collapsibility, and LVEF, the exact protocols varied by institution. Finally, it has been established in multiple studies that LUS used in addition to standard workup including history and physical, labs, and electrocardiogram has been shown to increase diagnostic accuracy.2,5
Using POCUS to guide diuretic therapy in HF
To date, there have been multiple small studies published on the utility of daily POCUS in hospitalized patients with ADHF to help assess response to treatment and guide diuresis by looking for reduction in B lines on LUS or a change in IVC size or collapsibility. Volpicelli and colleagues showed that daily LUS was at least as good as daily CXR in monitoring response to therapy.6 Similarly, Mozzini and colleagues performed a randomized controlled trial of 120 patients admitted for ADHF who were randomized to a CXR group (who had a CXR performed on admission and discharge) and a LUS group (which was performed at admission, 24 hours, 48 hours, 72 hours, and discharge).7 This study found that the LUS group underwent a significantly higher number of diuretic dose adjustments as compared with the CXR group (P < .001) and had a modest improvement in LOS, compared with the CXR group. Specifically, median LOS was 8 days in CXR group (range, 4-17 days) and 7 days in the LUS group (range, 3-10 days; P < .001).
The impact of POCUS on length of stay (LOS) and readmissions
There is increasing data that POCUS can have meaningful impacts on patient-centered outcomes (morbidity, mortality, and readmission) while exposing patients to minimal discomfort, no venipuncture, and no radiation exposure. First, multiple studies looked at whether performing focused cardiac US of the IVC as a marker of volume status could predict readmission in patients hospitalized for ADHF.8,9 Both of these trials showed that plethoric, noncollapsible IVC at discharge were statistically significant predictors of readmission. In fact, Goonewardena and colleagues demonstrated that patients who required readmission had an enlarged IVC at discharge nearly 3 times more frequently (21% vs. 61%, P < .001) and abnormal IVC collapsibility 1.5 times more frequently (41% vs. 71%, P = .01) as compared with patients who remained out of the hospital.9
Similarly, a subsequent trial looked at whether IVC size on admission was of prognostic importance in patients hospitalized for ADHF and showed that admission IVC diameter was an independent predictor of both 90-day mortality (hazard ratio, 5.88; 95% confidence interval, 1.21-28.10; P = .025) and 90-day readmission (HR, 3.20; 95% CI, 1.24-8.21; P = .016).10 Additionally, LUS heart failure assessment for pulmonary congestion by counting B lines also showed that having more than 15 B lines prior to discharge was an independent predictor of readmission for ADHF at 6 months (HR, 11.74; 95% CI, 1.30-106.16).11
A challenge of POCUS: Obtaining competency
As previously noted, there are not yet any established standards for training and assessing hospitalists in POCUS. The SHM Position Statement on POCUS recommends the following criteria for training5: the training environment should be similar to the location in which the trainee will practice, training and feedback should occur in real time, the trainee should be taught specific applications of POCUS (such as cardiac US, LUS, and IVC US) as each application comes with unique skills and knowledge, clinical competence must be achieved and demonstrated, and continued education and feedback are necessary once competence is obtained.12 SHM recommends residency-based training pathways, training through a local or national program such as the SHM POCUS certificate program, or training through other medical societies for hospitalists already in practice.
Application of the data to our original case
Targeted POCUS using the LuCUS protocol is performed and reveals three B lines in two lung zones bilaterally, moderate bilateral pleural effusions, EF 20%, and a noncollapsible IVC leading to a diagnosis of ADHF. Her ADHF is treated with intravenous diuresis. She is continued on her chronic maintenance chronic obstructive pulmonary disorder regimen but does not receive steroids, avoiding hyperglycemia that has complicated prior admissions. Over the next few days her respiratory and cardiac status is monitored using POCUS to assess her response to therapy and titrate her diuretics to her true dry weight, which was several pounds lower than her previously assumed dry weight. At discharge she is instructed to use the new dry weight which may avoid readmissions for HF.
Bottom line
POCUS improves diagnostic accuracy and facilitates volume assessment and management in acute decompensated heart failure.
Dr. Farber is a medical instructor at Duke University and hospitalist at Duke Regional Hospital, both in Durham, N.C. Dr. Marcantonio is a medical instructor in the department of internal medicine and department of pediatrics at Duke University and hospitalist at Duke University Hospital and Duke Regional Hospital. Dr. Stafford and Dr. Brooks are assistant professors of medicine and hospitalists at Duke Regional Hospital. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor at Duke University. Dr. Menon is a hospitalist at Duke University. Dr. Sharma is associate medical director for clinical education at Duke Regional Hospital and associate professor of medicine at Duke University.
References
1. Pivetta E et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur J Heart Fail. 2019 Jun;21(6):754-66. doi: 10.1002/ejhf.1379.
2. Pivetta E et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: A SIMEU multicenter study. Chest. 2015;148(1):202-10. doi: 10.1378/chest.14-2608.
3. Anderson KL et al. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31:1208-14. doi: 10.1016/j.ajem.2013.05.007.
4. Russell FM et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea: A lung and cardiac ultrasound (LuCUS) protocol. Acad Emerg Med. 2015;22(2):182-91. doi:10.1111/acem.12570.
5. Maw AM et al. Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: A systematic review and meta-analysis. JAMA Netw Open. 2019 Mar 1;2(3):e190703. doi:10.1001/jamanetworkopen.2019.0703.
6. Volpicelli G et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008 Jun;26(5):585-91. doi:10.1016/j.ajem.2007.09.014.
7. Mozzini C et al. Lung ultrasound in internal medicine efficiently drives the management of patients with heart failure and speeds up the discharge time. Intern Emerg Med. 2018 Jan;13(1):27-33. doi: 10.1007/s11739-017-1738-1.
8. Laffin LJ et al. Focused cardiac ultrasound as a predictor of readmission in acute decompensated heart failure. Int J Cardiovasc Imaging. 2018;34(7):1075-9. doi:10.1007/s10554-018-1317-1.
9. Goonewardena SN et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595-601. doi:10.1016/j.jcmg.2008.06.005.
10. Cubo-Romano P et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016 Nov;11(11):778-84. doi: 10.1002/jhm.2620.
11. Gargani L et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc Ultrasound. 2015 Sep 4;13:40. doi: 10.1186/s12947-015-0033-4.
12. Soni NJ et al. Point-of-care ultrasound for hospitalists: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2;14:E1-6. doi: 10.12788/jhm.3079.
Key points
- Studies have found POCUS improves the diagnosis of acute decompensated heart failure in patients presenting with dyspnea.
- Daily evaluation with POCUS has decreased length of stay in acute decompensated heart failure.
- Credentialing requirements for hospitalists to use POCUS for clinical care vary by hospital.
Additional reading
Maw AM and Soni NJ. Annals for hospitalists inpatient notes – why should hospitalists use point-of-care ultrasound? Ann Intern Med. 2018 Apr 17;168(8):HO2-HO3. doi: 10.7326/M18-0367.
Lewiss RE. “The ultrasound looked fine”: Point of care ultrasound and patient safety. AHRQ’s Patient Safety Network. WebM&M: Case Studies. 2018 Jul 1. https://psnet.ahrq.gov/web-mm/ultrasound-looked-fine-point-care-ultrasound-and-patient-safety.
Quiz: Testing your POCUS knowledge
POCUS is increasingly prevalent in hospital medicine, but use varies among different disease processes. Which organ system ultrasound or lab test would be most helpful in the following scenario?
An acutely dyspneic patient with no past medical history presents to the ED. Chest x-ray is equivocal. Of the following, which study best confirms a diagnosis of acute decompensated heart failure?
A. Brain natriuretic peptide
B. Point-of-care cardiac ultrasound
C. Point-of-care lung ultrasound
D. Point-of-care inferior vena cava ultrasound
Answer
C. Point-of-care lung ultrasound
Multiple studies, including three systematic reviews, have shown that point-of-care lung ultrasound has high sensitivity and specificity to evaluate for B lines as a marker for cardiogenic pulmonary edema. Point-of-care ultrasound of ejection fraction and inferior vena cava have not been evaluated by systematic review although one randomized, controlled trial showed that an EF less than 45% had 74% specificity and 77% sensitivity and IVC collapsibility index less than 20% had an 86% specificity and 52% sensitivity for detection of acute decompensated heart failure. This same study showed that the combination of cardiac, lung, and IVC point-of-care ultrasound had 100% specificity for diagnosing acute decompensated heart failure. In the future, health care providers could rely on this multiorgan evaluation with point-of-care ultrasound to confirm a diagnosis of acute decompensated heart failure in a dyspneic patient.
Intracranial atherosclerosis finding on MRA linked to stroke
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
No prehydration prior to contrast-enhanced CT in patients with stage 3 CKD
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Analysis supports CAC for personalizing statin use
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
FROM JAMA CARDIOLOGY
Abnormal exercise EKG in the setting of normal stress echo linked with increased CV risk
Background: Exercise EKG is often integrated with stress echocardiography, but discordance with +EKG/–Echo has unknown significance.
Study design: Observational cohort study.
Setting: Duke University Medical Center, Durham, N.C.
Synopsis: 47,944 patients without known coronary artery disease underwent exercise stress echocardiogram (Echo) with stress EKG. Of those patients, 8.5% had +EKG/–Echo results, which was associated with annualized event rate of adverse cardiac events of 1.72%, which is higher than the 0.89% of patients with –EKG/–Echo results. This was most significant for composite major adverse cardiovascular events less than 30 days out, with an adjusted hazard ratio of 8.06 (95% confidence interval, 5.02-12.94). For major adverse cardiovascular events greater than 30 days out, HR was 1.25 (95% CI 1.02-1.53).
Bottom line: Patients with +EKG/–Echo findings appear to be at higher risk of adverse cardiac events, especially in the short term.
Citation: Daubert MA et al. Implications of abnormal exercise electrocardiography with normal stress echocardiography. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6958.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Exercise EKG is often integrated with stress echocardiography, but discordance with +EKG/–Echo has unknown significance.
Study design: Observational cohort study.
Setting: Duke University Medical Center, Durham, N.C.
Synopsis: 47,944 patients without known coronary artery disease underwent exercise stress echocardiogram (Echo) with stress EKG. Of those patients, 8.5% had +EKG/–Echo results, which was associated with annualized event rate of adverse cardiac events of 1.72%, which is higher than the 0.89% of patients with –EKG/–Echo results. This was most significant for composite major adverse cardiovascular events less than 30 days out, with an adjusted hazard ratio of 8.06 (95% confidence interval, 5.02-12.94). For major adverse cardiovascular events greater than 30 days out, HR was 1.25 (95% CI 1.02-1.53).
Bottom line: Patients with +EKG/–Echo findings appear to be at higher risk of adverse cardiac events, especially in the short term.
Citation: Daubert MA et al. Implications of abnormal exercise electrocardiography with normal stress echocardiography. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6958.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Exercise EKG is often integrated with stress echocardiography, but discordance with +EKG/–Echo has unknown significance.
Study design: Observational cohort study.
Setting: Duke University Medical Center, Durham, N.C.
Synopsis: 47,944 patients without known coronary artery disease underwent exercise stress echocardiogram (Echo) with stress EKG. Of those patients, 8.5% had +EKG/–Echo results, which was associated with annualized event rate of adverse cardiac events of 1.72%, which is higher than the 0.89% of patients with –EKG/–Echo results. This was most significant for composite major adverse cardiovascular events less than 30 days out, with an adjusted hazard ratio of 8.06 (95% confidence interval, 5.02-12.94). For major adverse cardiovascular events greater than 30 days out, HR was 1.25 (95% CI 1.02-1.53).
Bottom line: Patients with +EKG/–Echo findings appear to be at higher risk of adverse cardiac events, especially in the short term.
Citation: Daubert MA et al. Implications of abnormal exercise electrocardiography with normal stress echocardiography. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6958.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Fact or fiction? Intravascular contrast and acute kidney injury
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.
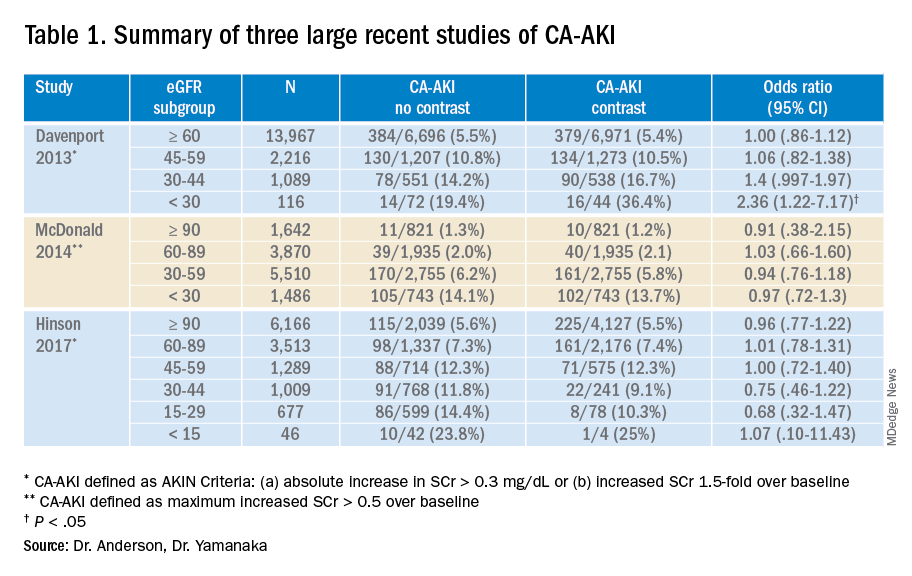
Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Withholding contrast may be the greater risk
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Are left atrial thrombi that defy preprocedure anticoagulation predictable?
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
In acute lower GI bleeding, there may be no benefit to early colonoscopy
Background: Current U.S. guidelines recommend colonoscopy within 24 hours for patients presenting with high-risk or severe acute lower gastrointestinal bleeding. However, prior meta-analyses of the timing of colonoscopy relied primarily on observational studies, and a recent multicenter randomized, controlled trial suggests no substantial benefit for early colonoscopy.
Study design: Systematic review and meta-analysis of randomized, clinical trials.
Setting: English language literature search from MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials, performed in July 2019.
Synopsis: The authors identified four randomized, controlled trials that compared early colonoscopy (defined as within 24 hours) with elective colonoscopy (defined as beyond 24 hours) and/or other diagnostic tests for patients presenting with acute lower GI bleeding. They performed a meta-analysis, including 463 patients, which showed no significant difference in risk of persistent or recurrent bleeding for early versus elective colonoscopy. The authors also found no significant differences in secondary outcomes of mortality, endoscopic intervention, primary hemostatic intervention, or identification of bleeding source. Limitations of this research include the relatively small number of studies included, and potential for selection bias in the original studies. Notably two of the four studies included were prematurely terminated before their planned sample sizes were reached.
Bottom line: In patients hospitalized with acute lower GI bleeding, colonoscopy within 24 hours may not reduce further bleeding or mortality when compared with elective colonoscopy.
Citation: Tsay C et al. Early colonoscopy does not improve outcomes of patients with lower gastrointestinal bleeding: Systematic review of randomized trials. Clin Gastroenterol Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.061.
Dr. Hu is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Current U.S. guidelines recommend colonoscopy within 24 hours for patients presenting with high-risk or severe acute lower gastrointestinal bleeding. However, prior meta-analyses of the timing of colonoscopy relied primarily on observational studies, and a recent multicenter randomized, controlled trial suggests no substantial benefit for early colonoscopy.
Study design: Systematic review and meta-analysis of randomized, clinical trials.
Setting: English language literature search from MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials, performed in July 2019.
Synopsis: The authors identified four randomized, controlled trials that compared early colonoscopy (defined as within 24 hours) with elective colonoscopy (defined as beyond 24 hours) and/or other diagnostic tests for patients presenting with acute lower GI bleeding. They performed a meta-analysis, including 463 patients, which showed no significant difference in risk of persistent or recurrent bleeding for early versus elective colonoscopy. The authors also found no significant differences in secondary outcomes of mortality, endoscopic intervention, primary hemostatic intervention, or identification of bleeding source. Limitations of this research include the relatively small number of studies included, and potential for selection bias in the original studies. Notably two of the four studies included were prematurely terminated before their planned sample sizes were reached.
Bottom line: In patients hospitalized with acute lower GI bleeding, colonoscopy within 24 hours may not reduce further bleeding or mortality when compared with elective colonoscopy.
Citation: Tsay C et al. Early colonoscopy does not improve outcomes of patients with lower gastrointestinal bleeding: Systematic review of randomized trials. Clin Gastroenterol Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.061.
Dr. Hu is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Current U.S. guidelines recommend colonoscopy within 24 hours for patients presenting with high-risk or severe acute lower gastrointestinal bleeding. However, prior meta-analyses of the timing of colonoscopy relied primarily on observational studies, and a recent multicenter randomized, controlled trial suggests no substantial benefit for early colonoscopy.
Study design: Systematic review and meta-analysis of randomized, clinical trials.
Setting: English language literature search from MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials, performed in July 2019.
Synopsis: The authors identified four randomized, controlled trials that compared early colonoscopy (defined as within 24 hours) with elective colonoscopy (defined as beyond 24 hours) and/or other diagnostic tests for patients presenting with acute lower GI bleeding. They performed a meta-analysis, including 463 patients, which showed no significant difference in risk of persistent or recurrent bleeding for early versus elective colonoscopy. The authors also found no significant differences in secondary outcomes of mortality, endoscopic intervention, primary hemostatic intervention, or identification of bleeding source. Limitations of this research include the relatively small number of studies included, and potential for selection bias in the original studies. Notably two of the four studies included were prematurely terminated before their planned sample sizes were reached.
Bottom line: In patients hospitalized with acute lower GI bleeding, colonoscopy within 24 hours may not reduce further bleeding or mortality when compared with elective colonoscopy.
Citation: Tsay C et al. Early colonoscopy does not improve outcomes of patients with lower gastrointestinal bleeding: Systematic review of randomized trials. Clin Gastroenterol Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.061.
Dr. Hu is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.