User login
No matter the valve, protective device cuts post-TAVR stroke risk
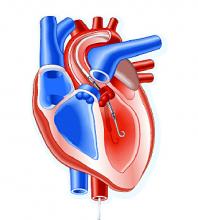
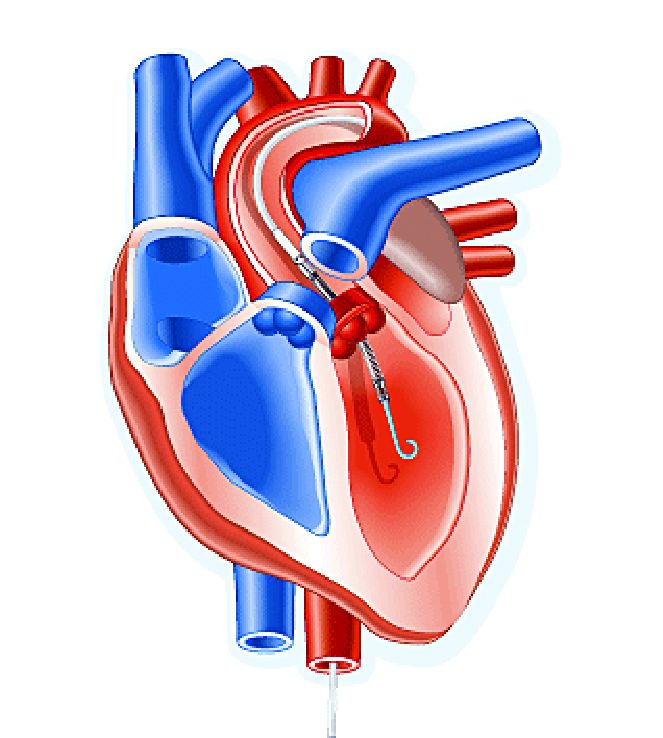
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
REPORTING FROM TCT 2018
In Medicare population, carotid revascularization has declined
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: During 1999-2014, the rate of carotid endarterectomy per 100,000 beneficiaries fell from 291 to 128 (57%; P less than .001).
Study details: Retrospective database review.
Disclosures: Dr. Lal reported having no financial conflicts relevant to the study.
Redo carotid endarterectomy is more risky than previously estimated
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: The odds ratio for stroke is 3.71 times higher (P = .002) with redo than with primary carotid endarterectomy.
Study details: Multivariate retrospective database analysis.
Disclosures: Dr. Siracuse reported he had no financial relationships relevant to this study.
RAS inhibitors improve outcomes in TAVR patients
After transcatheter aortic valve replacement (TAVR), treatment with a renin-angiotensin system (RAS) inhibitor at hospital discharge is associated with lower risk of mortality and heart failure–related readmission, according to an analysis of Medicare patients.
RAS inhibitors may reverse left ventricular remodeling and improve function, which could explain the association, noted lead investigator Taku Inohara, MD.
The researchers analyzed data from consecutive Medicare patients who underwent TAVR, drawn from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. They included 15,896 propensity-matched patients from 417 U.S. centers.
At 1 year, a RAS inhibitor prescription at discharge was linked to a statistically significant relative 18% reduction in all-cause mortality (12.5% vs. 14.9%) and a 16% drop in heart failure readmissions (12.0% vs. 13.8%).
The researchers conducted a propensity-scored analysis of 12,942 patients with preserved left ventricular ejection fraction (greater than 40%) and 2,954 with LVEF up to 40%. In the preserved LVEF group, RAS inhibitor prescription was associated with a significant 22% reduction in mortality (11.1% vs. 13.9%), but there was no statistically significant association in patients with reduced LVEF (18.8% vs. 19.5%).
There was no clinically meaningful difference in quality of life between those who received a RAS inhibitor and those who did not, but the subgroup analysis could be performed on only 30% of the overall cohort, Dr. Inohara and his colleagues at the Duke Clinical Research Center in Durham, N.C., wrote.
They added that most patients undergoing TAVR are eligible for RAS inhibitors because of frequently comorbid hypertension, coronary artery disease, and renal dysfunction.
The study was funded by the American College of Cardiology Foundation’s National Cardiovascular Data Registry and the Society of Thoracic Surgeons. Study authors have wide-ranging financial relationships with pharmaceutical companies.
SOURCE: Inohara T et al. JAMA. 2018 Dec 4;320(21):2231-41.
After transcatheter aortic valve replacement (TAVR), treatment with a renin-angiotensin system (RAS) inhibitor at hospital discharge is associated with lower risk of mortality and heart failure–related readmission, according to an analysis of Medicare patients.
RAS inhibitors may reverse left ventricular remodeling and improve function, which could explain the association, noted lead investigator Taku Inohara, MD.
The researchers analyzed data from consecutive Medicare patients who underwent TAVR, drawn from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. They included 15,896 propensity-matched patients from 417 U.S. centers.
At 1 year, a RAS inhibitor prescription at discharge was linked to a statistically significant relative 18% reduction in all-cause mortality (12.5% vs. 14.9%) and a 16% drop in heart failure readmissions (12.0% vs. 13.8%).
The researchers conducted a propensity-scored analysis of 12,942 patients with preserved left ventricular ejection fraction (greater than 40%) and 2,954 with LVEF up to 40%. In the preserved LVEF group, RAS inhibitor prescription was associated with a significant 22% reduction in mortality (11.1% vs. 13.9%), but there was no statistically significant association in patients with reduced LVEF (18.8% vs. 19.5%).
There was no clinically meaningful difference in quality of life between those who received a RAS inhibitor and those who did not, but the subgroup analysis could be performed on only 30% of the overall cohort, Dr. Inohara and his colleagues at the Duke Clinical Research Center in Durham, N.C., wrote.
They added that most patients undergoing TAVR are eligible for RAS inhibitors because of frequently comorbid hypertension, coronary artery disease, and renal dysfunction.
The study was funded by the American College of Cardiology Foundation’s National Cardiovascular Data Registry and the Society of Thoracic Surgeons. Study authors have wide-ranging financial relationships with pharmaceutical companies.
SOURCE: Inohara T et al. JAMA. 2018 Dec 4;320(21):2231-41.
After transcatheter aortic valve replacement (TAVR), treatment with a renin-angiotensin system (RAS) inhibitor at hospital discharge is associated with lower risk of mortality and heart failure–related readmission, according to an analysis of Medicare patients.
RAS inhibitors may reverse left ventricular remodeling and improve function, which could explain the association, noted lead investigator Taku Inohara, MD.
The researchers analyzed data from consecutive Medicare patients who underwent TAVR, drawn from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. They included 15,896 propensity-matched patients from 417 U.S. centers.
At 1 year, a RAS inhibitor prescription at discharge was linked to a statistically significant relative 18% reduction in all-cause mortality (12.5% vs. 14.9%) and a 16% drop in heart failure readmissions (12.0% vs. 13.8%).
The researchers conducted a propensity-scored analysis of 12,942 patients with preserved left ventricular ejection fraction (greater than 40%) and 2,954 with LVEF up to 40%. In the preserved LVEF group, RAS inhibitor prescription was associated with a significant 22% reduction in mortality (11.1% vs. 13.9%), but there was no statistically significant association in patients with reduced LVEF (18.8% vs. 19.5%).
There was no clinically meaningful difference in quality of life between those who received a RAS inhibitor and those who did not, but the subgroup analysis could be performed on only 30% of the overall cohort, Dr. Inohara and his colleagues at the Duke Clinical Research Center in Durham, N.C., wrote.
They added that most patients undergoing TAVR are eligible for RAS inhibitors because of frequently comorbid hypertension, coronary artery disease, and renal dysfunction.
The study was funded by the American College of Cardiology Foundation’s National Cardiovascular Data Registry and the Society of Thoracic Surgeons. Study authors have wide-ranging financial relationships with pharmaceutical companies.
SOURCE: Inohara T et al. JAMA. 2018 Dec 4;320(21):2231-41.
REPORTING FROM JAMA
Key clinical point:
Major finding: Treatment was linked to reductions in 1-year mortality and heart failure of 18% and 16%, respectively.
Study details: Propensity matched, retrospective analysis of 15,896 Medicare patients.
Disclosures: The study was funded by the American College of Cardiology Foundation’s National Cardiovascular Data Registry and the Society of Thoracic Surgeons. Study authors have wide-ranging financial relationships with pharmaceutical companies.
Source: Inohara T et al. JAMA. 2018 Dec 4;320(21):2231-41.
EARLY: Angiography within 2 hours of acute non-ST event cut recurrent ischemic events
CHICAGO – Coronary angiography within 2 hours of a diagnosis of non–ST-segment elevation acute coronary syndrome (NSTE-ACS) significantly reduced the risk of recurrent ischemic events as compared to angiography delayed for 12 hours or more, based on the results of the EARLY trial presented at the American Heart Association scientific sessions.
EARLY examined the impacts of not pretreating with a P2Y12-ADP antagonist and of delay before coronary angiography; all study participants received the loading dose of a P2Y12-ADP antagonist at the time of intervention. The early group received angiography within 2 hours, and a delayed group received angiography 12 or more hours after NSTE-ACS.
“Regarding the primary endpoint at 30 days, which is a composite of cardiovascular death and recurrent ischemic event, there is a fivefold lower rate of MACE [major adverse cardiovascular events] in the very-early [group as] compared to the control group,” said Laurent Bonello, MD, PhD, of University Hospital North in Marseilles, France.
The MACE rate was 4.4% in the early group and 21.3% in the delayed group. However, the reduction in MACE was largely because of a reduction in recurrent ischemic events; death rates were similar in the two groups.
The EARLY trial randomized 740 patients at 16 hospitals in France with NSTE-ACS to one of two timing strategies for intervention: within 2 hours of diagnosis, the early-intervention group, and between 12 and 72 hours after diagnosis, the delayed group. Intermediate- and high-risk patients did not receive pretreatment with a P2Y12-ADP antagonist such as clopidogrel before angiography; they received the loading dose at the time of the intervention.
On average, angiography was done within 1 hour in the early group and at 18 hours in the delayed group. Percutaneous coronary intervention (PCI) was performed on 75% of the study population; 3% underwent coronary artery bypass grafting; and 20% received medical therapy.
Dr. Bonello said the purpose of the trial was to settle some uncertainties over the management of NSTE-ACS patients regarding the benefit of pretreatment with P2Y12-ADP antagonists – namely, to evaluate the impact of the lack of pretreatment on the optimal timing of the intervention. “There are no randomized clinical trials available on this specific group of non-ST elevation acute coronary syndrome patients not pretreated for the timing of the invasive strategy,” he said.
Both groups had similar baseline characteristics, such as history of MI, PCI, and aspirin and P2Y12-ADP use, although the delayed group had a higher rate of diabetes (35% vs. 28.3%).
Regarding secondary endpoints, rates of recurrent ischemic events were 2.9% for the early group and 19.8% for the delayed group during hospitalization, and 4.1% vs. 20.7% at 30 days.
Dr. Bonello noted that rates of cardiovascular death were similar for both groups: 0.3% and 0.8% in-hospital deaths, and 0.6% and 1.1% deaths at 30 days.
The disparity in MACE between all subgroups paralleled that of the overall results with two exceptions, Dr. Bonello said: The positive effect of early intervention was less pronounced in women, and there were no differences in MACE rates among those who had interventions other than PCI.
In his discussion of the trial, Gilles Montalescot, MD, PhD, of the Institute of Cardiology at Pitié-Salpêtrière Hospital in Paris, said the EARLY trial with no P2Y12-ADP pretreatment confirms findings of studies before the ACCOAST trial (N Engl J Med. 2013;369:999-1010), that early angiography has no benefit on survival, recurrent MI, revascularization, or bleeding. While the ACCOAST trial, of which Dr. Montalescot was a principal investigator, found no benefit of pretreatment with prasugrel in patients with NTSE-ASC, the EARLY trial extends those findings to other P2Y12-ADP antagonists. “With the immediate angiography strategy, there is a trivial benefit on recurrent ischemia and length of stay, like in the previous studies, thus not related to pretreatment,” he said.
Dr. Montalescot cautioned against embracing this early-intervention strategy with no P2Y12-ADP pretreatment in all situations.
“If you have a conservative strategy for managing the NTSE-ASC patient or if you are in a center far away from a cath lab and your patients have to wait days for a test, yes, you should consider administration of the P2Y12-ADP antagonist,” Dr. Montalescot said.
Dr. Montalescot disclosed receiving grants or honoraria from ADIR, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, and Action Coeur Academic Research Organization.
Dr. Bonello reported financial relationships with AstraZeneca, Boston Scientific, Abbott, and Biotronik. The EARLY trial received funding from the French Ministry of Health.
SOURCE: Bonello B et al. AHA scientific sessions, Session LBS.04 19343.
CHICAGO – Coronary angiography within 2 hours of a diagnosis of non–ST-segment elevation acute coronary syndrome (NSTE-ACS) significantly reduced the risk of recurrent ischemic events as compared to angiography delayed for 12 hours or more, based on the results of the EARLY trial presented at the American Heart Association scientific sessions.
EARLY examined the impacts of not pretreating with a P2Y12-ADP antagonist and of delay before coronary angiography; all study participants received the loading dose of a P2Y12-ADP antagonist at the time of intervention. The early group received angiography within 2 hours, and a delayed group received angiography 12 or more hours after NSTE-ACS.
“Regarding the primary endpoint at 30 days, which is a composite of cardiovascular death and recurrent ischemic event, there is a fivefold lower rate of MACE [major adverse cardiovascular events] in the very-early [group as] compared to the control group,” said Laurent Bonello, MD, PhD, of University Hospital North in Marseilles, France.
The MACE rate was 4.4% in the early group and 21.3% in the delayed group. However, the reduction in MACE was largely because of a reduction in recurrent ischemic events; death rates were similar in the two groups.
The EARLY trial randomized 740 patients at 16 hospitals in France with NSTE-ACS to one of two timing strategies for intervention: within 2 hours of diagnosis, the early-intervention group, and between 12 and 72 hours after diagnosis, the delayed group. Intermediate- and high-risk patients did not receive pretreatment with a P2Y12-ADP antagonist such as clopidogrel before angiography; they received the loading dose at the time of the intervention.
On average, angiography was done within 1 hour in the early group and at 18 hours in the delayed group. Percutaneous coronary intervention (PCI) was performed on 75% of the study population; 3% underwent coronary artery bypass grafting; and 20% received medical therapy.
Dr. Bonello said the purpose of the trial was to settle some uncertainties over the management of NSTE-ACS patients regarding the benefit of pretreatment with P2Y12-ADP antagonists – namely, to evaluate the impact of the lack of pretreatment on the optimal timing of the intervention. “There are no randomized clinical trials available on this specific group of non-ST elevation acute coronary syndrome patients not pretreated for the timing of the invasive strategy,” he said.
Both groups had similar baseline characteristics, such as history of MI, PCI, and aspirin and P2Y12-ADP use, although the delayed group had a higher rate of diabetes (35% vs. 28.3%).
Regarding secondary endpoints, rates of recurrent ischemic events were 2.9% for the early group and 19.8% for the delayed group during hospitalization, and 4.1% vs. 20.7% at 30 days.
Dr. Bonello noted that rates of cardiovascular death were similar for both groups: 0.3% and 0.8% in-hospital deaths, and 0.6% and 1.1% deaths at 30 days.
The disparity in MACE between all subgroups paralleled that of the overall results with two exceptions, Dr. Bonello said: The positive effect of early intervention was less pronounced in women, and there were no differences in MACE rates among those who had interventions other than PCI.
In his discussion of the trial, Gilles Montalescot, MD, PhD, of the Institute of Cardiology at Pitié-Salpêtrière Hospital in Paris, said the EARLY trial with no P2Y12-ADP pretreatment confirms findings of studies before the ACCOAST trial (N Engl J Med. 2013;369:999-1010), that early angiography has no benefit on survival, recurrent MI, revascularization, or bleeding. While the ACCOAST trial, of which Dr. Montalescot was a principal investigator, found no benefit of pretreatment with prasugrel in patients with NTSE-ASC, the EARLY trial extends those findings to other P2Y12-ADP antagonists. “With the immediate angiography strategy, there is a trivial benefit on recurrent ischemia and length of stay, like in the previous studies, thus not related to pretreatment,” he said.
Dr. Montalescot cautioned against embracing this early-intervention strategy with no P2Y12-ADP pretreatment in all situations.
“If you have a conservative strategy for managing the NTSE-ASC patient or if you are in a center far away from a cath lab and your patients have to wait days for a test, yes, you should consider administration of the P2Y12-ADP antagonist,” Dr. Montalescot said.
Dr. Montalescot disclosed receiving grants or honoraria from ADIR, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, and Action Coeur Academic Research Organization.
Dr. Bonello reported financial relationships with AstraZeneca, Boston Scientific, Abbott, and Biotronik. The EARLY trial received funding from the French Ministry of Health.
SOURCE: Bonello B et al. AHA scientific sessions, Session LBS.04 19343.
CHICAGO – Coronary angiography within 2 hours of a diagnosis of non–ST-segment elevation acute coronary syndrome (NSTE-ACS) significantly reduced the risk of recurrent ischemic events as compared to angiography delayed for 12 hours or more, based on the results of the EARLY trial presented at the American Heart Association scientific sessions.
EARLY examined the impacts of not pretreating with a P2Y12-ADP antagonist and of delay before coronary angiography; all study participants received the loading dose of a P2Y12-ADP antagonist at the time of intervention. The early group received angiography within 2 hours, and a delayed group received angiography 12 or more hours after NSTE-ACS.
“Regarding the primary endpoint at 30 days, which is a composite of cardiovascular death and recurrent ischemic event, there is a fivefold lower rate of MACE [major adverse cardiovascular events] in the very-early [group as] compared to the control group,” said Laurent Bonello, MD, PhD, of University Hospital North in Marseilles, France.
The MACE rate was 4.4% in the early group and 21.3% in the delayed group. However, the reduction in MACE was largely because of a reduction in recurrent ischemic events; death rates were similar in the two groups.
The EARLY trial randomized 740 patients at 16 hospitals in France with NSTE-ACS to one of two timing strategies for intervention: within 2 hours of diagnosis, the early-intervention group, and between 12 and 72 hours after diagnosis, the delayed group. Intermediate- and high-risk patients did not receive pretreatment with a P2Y12-ADP antagonist such as clopidogrel before angiography; they received the loading dose at the time of the intervention.
On average, angiography was done within 1 hour in the early group and at 18 hours in the delayed group. Percutaneous coronary intervention (PCI) was performed on 75% of the study population; 3% underwent coronary artery bypass grafting; and 20% received medical therapy.
Dr. Bonello said the purpose of the trial was to settle some uncertainties over the management of NSTE-ACS patients regarding the benefit of pretreatment with P2Y12-ADP antagonists – namely, to evaluate the impact of the lack of pretreatment on the optimal timing of the intervention. “There are no randomized clinical trials available on this specific group of non-ST elevation acute coronary syndrome patients not pretreated for the timing of the invasive strategy,” he said.
Both groups had similar baseline characteristics, such as history of MI, PCI, and aspirin and P2Y12-ADP use, although the delayed group had a higher rate of diabetes (35% vs. 28.3%).
Regarding secondary endpoints, rates of recurrent ischemic events were 2.9% for the early group and 19.8% for the delayed group during hospitalization, and 4.1% vs. 20.7% at 30 days.
Dr. Bonello noted that rates of cardiovascular death were similar for both groups: 0.3% and 0.8% in-hospital deaths, and 0.6% and 1.1% deaths at 30 days.
The disparity in MACE between all subgroups paralleled that of the overall results with two exceptions, Dr. Bonello said: The positive effect of early intervention was less pronounced in women, and there were no differences in MACE rates among those who had interventions other than PCI.
In his discussion of the trial, Gilles Montalescot, MD, PhD, of the Institute of Cardiology at Pitié-Salpêtrière Hospital in Paris, said the EARLY trial with no P2Y12-ADP pretreatment confirms findings of studies before the ACCOAST trial (N Engl J Med. 2013;369:999-1010), that early angiography has no benefit on survival, recurrent MI, revascularization, or bleeding. While the ACCOAST trial, of which Dr. Montalescot was a principal investigator, found no benefit of pretreatment with prasugrel in patients with NTSE-ASC, the EARLY trial extends those findings to other P2Y12-ADP antagonists. “With the immediate angiography strategy, there is a trivial benefit on recurrent ischemia and length of stay, like in the previous studies, thus not related to pretreatment,” he said.
Dr. Montalescot cautioned against embracing this early-intervention strategy with no P2Y12-ADP pretreatment in all situations.
“If you have a conservative strategy for managing the NTSE-ASC patient or if you are in a center far away from a cath lab and your patients have to wait days for a test, yes, you should consider administration of the P2Y12-ADP antagonist,” Dr. Montalescot said.
Dr. Montalescot disclosed receiving grants or honoraria from ADIR, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, and Action Coeur Academic Research Organization.
Dr. Bonello reported financial relationships with AstraZeneca, Boston Scientific, Abbott, and Biotronik. The EARLY trial received funding from the French Ministry of Health.
SOURCE: Bonello B et al. AHA scientific sessions, Session LBS.04 19343.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Coronary angiography within 2 hours of non–ST-segment elevation acute coronary syndrome yielded improved outcomes.
Major finding: Rates of major cardiovascular events were 4.4% with early intervention and 21.3% with delayed intervention.
Study details: Prospective, multicenter, randomized clinical trial of 709 patients.
Disclosures: Dr. Bonello reported financial relationships with AstraZeneca, Boston Scientific, Abbott, and Biotronik. The trial received funding from the French Ministry of Health.
Source: Bonello B et al. AHA scientific sessions, Session LBS.04 19343.
Open AAA repair mortality rates doubled for very-low-volume surgeons
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: In-hospital mortality is approximately double (OR 2.09; P less than .001) for very low-volume relative to high-volume surgeon.
Study details: Retrospective database review.
Disclosures: Dr. Cronenwett reports no conflicts of interest.
Source: Cronenwett JL et al. 2018; 45th VEITHsymposium.
Impella heart pump may enable 30-minute reperfusion delay
CHICAGO – An investigative heart pump for unloading the left ventricle in patients who had an ST-elevation myocardial infarction (STEMI) yielded similar safety and efficacy outcomes with a 30-minute delay in reperfusion or the standard approach of immediate reperfusion.
That’s according to results of a pilot feasibility trial presented at the American Heart Association scientific sessions.
The trial, titled the DTU (Door to Unload)–STEMI trial, evaluated the Impella CP (Abiomed) device used for unloading the left ventricle (LV). “No prohibitive safety signals that would preclude proceeding to a larger pivotal study of left ventricle unloading and delaying reperfusion for 30 minutes were identified,” said principal investigator Navin Kapur, MD, of Tufts Medical Center.
The trial evaluated 50 patients who received the Impella device in two different groups: one that underwent immediate reperfusion after LV unloading, the other that had a 30-minute delay before reperfusion. The study found no significant difference in major adverse cardiovascular or cerebral events between the two groups (there were two in the delayed group vs. none in the immediate group), and no difference in infarct size increase as a percentage of LV mass at 30 days between the groups, Dr. Kapur said.
Door-to-balloon times averaged 73 minutes in the immediate reperfusion group and 97 minutes in the delayed reperfusion group, with door-to-unload times averaging around 60 minutes in both groups. “We were able to see successful enrollment and distribution across multiple sites and multiple operators, suggesting the feasibility of this approach,” Dr. Kapur said.
He noted “one of the most important messages” of the study was that no patients in either arm required percutaneous coronary intervention. “What this suggests is that, when we look at operator behavior, operators were comfortable with initiating LV unloading and waiting 30 minutes,” Dr. Kapur said.
The primary endpoint of the trial was to determine if delayed reperfusion led to an increase in infarct size. “We did not see that,” he noted. “And among patients with an anterior ST-segment elevation sum in leads V1-V4 of more than 6 mm Hg, infarct size normalized to the area at risk was significantly lower with 30 minutes of LV unloading before reperfusion, compared to LV unloading with immediate reperfusion.”
The next step is to initiate a pivotal trial of the device, Dr. Kapur said. “The findings from the DTU-STEMI pilot trial will inform the pivotal trial based on preclinical data showing that LV unloading attenuates myocardial ischemia and also preconditions the myocardium to allow it to be more receptive to reperfusion with a reduction in reperfusion injury,” he said. The pivotal trial will have two similar arms: one using the standard of care of immediate reperfusion and the other utilizing the 30-minute delay.
In his discussion of the DTU-STEMI trial, Holger Thiele, MD, of the Leipzig (Germany) Heart Institute and the University of Leipzig, expressed concern with the lack of a standard-of-care group in the trial. “Thus, the primary efficacy endpoint on infarct size cannot be reliably compared,” he said. “Based on the small sample size, there’s no reliable information on safety.”
Dr. Kapur reported financial relationships with Abiomed, Boston Scientific, Abbott, Medtronic, and MD Start. Dr. Thiele had no financial disclosures.
SOURCE: Kapur NK et al. AHA scientific sessions, LBCT-19578
CHICAGO – An investigative heart pump for unloading the left ventricle in patients who had an ST-elevation myocardial infarction (STEMI) yielded similar safety and efficacy outcomes with a 30-minute delay in reperfusion or the standard approach of immediate reperfusion.
That’s according to results of a pilot feasibility trial presented at the American Heart Association scientific sessions.
The trial, titled the DTU (Door to Unload)–STEMI trial, evaluated the Impella CP (Abiomed) device used for unloading the left ventricle (LV). “No prohibitive safety signals that would preclude proceeding to a larger pivotal study of left ventricle unloading and delaying reperfusion for 30 minutes were identified,” said principal investigator Navin Kapur, MD, of Tufts Medical Center.
The trial evaluated 50 patients who received the Impella device in two different groups: one that underwent immediate reperfusion after LV unloading, the other that had a 30-minute delay before reperfusion. The study found no significant difference in major adverse cardiovascular or cerebral events between the two groups (there were two in the delayed group vs. none in the immediate group), and no difference in infarct size increase as a percentage of LV mass at 30 days between the groups, Dr. Kapur said.
Door-to-balloon times averaged 73 minutes in the immediate reperfusion group and 97 minutes in the delayed reperfusion group, with door-to-unload times averaging around 60 minutes in both groups. “We were able to see successful enrollment and distribution across multiple sites and multiple operators, suggesting the feasibility of this approach,” Dr. Kapur said.
He noted “one of the most important messages” of the study was that no patients in either arm required percutaneous coronary intervention. “What this suggests is that, when we look at operator behavior, operators were comfortable with initiating LV unloading and waiting 30 minutes,” Dr. Kapur said.
The primary endpoint of the trial was to determine if delayed reperfusion led to an increase in infarct size. “We did not see that,” he noted. “And among patients with an anterior ST-segment elevation sum in leads V1-V4 of more than 6 mm Hg, infarct size normalized to the area at risk was significantly lower with 30 minutes of LV unloading before reperfusion, compared to LV unloading with immediate reperfusion.”
The next step is to initiate a pivotal trial of the device, Dr. Kapur said. “The findings from the DTU-STEMI pilot trial will inform the pivotal trial based on preclinical data showing that LV unloading attenuates myocardial ischemia and also preconditions the myocardium to allow it to be more receptive to reperfusion with a reduction in reperfusion injury,” he said. The pivotal trial will have two similar arms: one using the standard of care of immediate reperfusion and the other utilizing the 30-minute delay.
In his discussion of the DTU-STEMI trial, Holger Thiele, MD, of the Leipzig (Germany) Heart Institute and the University of Leipzig, expressed concern with the lack of a standard-of-care group in the trial. “Thus, the primary efficacy endpoint on infarct size cannot be reliably compared,” he said. “Based on the small sample size, there’s no reliable information on safety.”
Dr. Kapur reported financial relationships with Abiomed, Boston Scientific, Abbott, Medtronic, and MD Start. Dr. Thiele had no financial disclosures.
SOURCE: Kapur NK et al. AHA scientific sessions, LBCT-19578
CHICAGO – An investigative heart pump for unloading the left ventricle in patients who had an ST-elevation myocardial infarction (STEMI) yielded similar safety and efficacy outcomes with a 30-minute delay in reperfusion or the standard approach of immediate reperfusion.
That’s according to results of a pilot feasibility trial presented at the American Heart Association scientific sessions.
The trial, titled the DTU (Door to Unload)–STEMI trial, evaluated the Impella CP (Abiomed) device used for unloading the left ventricle (LV). “No prohibitive safety signals that would preclude proceeding to a larger pivotal study of left ventricle unloading and delaying reperfusion for 30 minutes were identified,” said principal investigator Navin Kapur, MD, of Tufts Medical Center.
The trial evaluated 50 patients who received the Impella device in two different groups: one that underwent immediate reperfusion after LV unloading, the other that had a 30-minute delay before reperfusion. The study found no significant difference in major adverse cardiovascular or cerebral events between the two groups (there were two in the delayed group vs. none in the immediate group), and no difference in infarct size increase as a percentage of LV mass at 30 days between the groups, Dr. Kapur said.
Door-to-balloon times averaged 73 minutes in the immediate reperfusion group and 97 minutes in the delayed reperfusion group, with door-to-unload times averaging around 60 minutes in both groups. “We were able to see successful enrollment and distribution across multiple sites and multiple operators, suggesting the feasibility of this approach,” Dr. Kapur said.
He noted “one of the most important messages” of the study was that no patients in either arm required percutaneous coronary intervention. “What this suggests is that, when we look at operator behavior, operators were comfortable with initiating LV unloading and waiting 30 minutes,” Dr. Kapur said.
The primary endpoint of the trial was to determine if delayed reperfusion led to an increase in infarct size. “We did not see that,” he noted. “And among patients with an anterior ST-segment elevation sum in leads V1-V4 of more than 6 mm Hg, infarct size normalized to the area at risk was significantly lower with 30 minutes of LV unloading before reperfusion, compared to LV unloading with immediate reperfusion.”
The next step is to initiate a pivotal trial of the device, Dr. Kapur said. “The findings from the DTU-STEMI pilot trial will inform the pivotal trial based on preclinical data showing that LV unloading attenuates myocardial ischemia and also preconditions the myocardium to allow it to be more receptive to reperfusion with a reduction in reperfusion injury,” he said. The pivotal trial will have two similar arms: one using the standard of care of immediate reperfusion and the other utilizing the 30-minute delay.
In his discussion of the DTU-STEMI trial, Holger Thiele, MD, of the Leipzig (Germany) Heart Institute and the University of Leipzig, expressed concern with the lack of a standard-of-care group in the trial. “Thus, the primary efficacy endpoint on infarct size cannot be reliably compared,” he said. “Based on the small sample size, there’s no reliable information on safety.”
Dr. Kapur reported financial relationships with Abiomed, Boston Scientific, Abbott, Medtronic, and MD Start. Dr. Thiele had no financial disclosures.
SOURCE: Kapur NK et al. AHA scientific sessions, LBCT-19578
REPORTING FROM AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Door-to-balloon times averaged 73 minutes in the immediate reperfusion group and 97 minutes in the delayed reperfusion group.
Study details: A phase 1, randomized, exploratory safety and feasibility trial in 50 patients with anterior STEMI to left ventricle unloading using the Impella CP followed by immediate reperfusion versus delayed reperfusion after 30 minutes of unloading.
Disclosures: Dr. Kapur reported financial relationships with Abiomed, Boston Scientific, Abbott, Medtronic and MD Start/Precardia.
Source: Kapur NK et al. AHA scientific sessions, LBCT-19578.
Prolonged DAPT doesn’t help left main CAD
SAN DIEGO – Among patients who receive a drug-eluting stent (DES) in their left main coronary artery (LMCA), continuation of dual antiplatelet therapy (DAPT) past 1 year appears to provide no benefit.
The results come from a subanalysis of the EXCEL trial, which compared the Xience DES to coronary artery bypass graft (CABG) surgery in LMCA lesions.
The LMCA is worrisome to a lot of physicians because of the large amount of myocardial tissue it contains, and they often prescribe DAPT past the generally recommended 1 year. “It’s this magic thing, that ‘Well, it’s left main stenting, and you’d better protect the patient.’ But it turns out that it doesn’t,” said Sorin Brener, MD, director of the catheterization lab at New York Methodist Hospital, who presented the results of the study at the Transcatheter Cardiovascular Therapeutics annual meeting.
The researchers compared patients in both groups who opted to stop DAPT after 1 year versus those who continued therapy out to 3 years, and the news was not favorable for continuation. A composite of death, myocardial infarction, or stroke was higher among patients who continued DAPT, though the results did not reach statistical significance.
It’s possible that patients who continued DAPT were more ill on average. “Obviously, there could be cases where the doctor decided they deserved more prolonged therapy, but it’s not measurable, so I don’t think they were sicker,” said Dr. Brener.
The study was also underpowered. Those worse trends probably don’t represent a real signal, according to Dr. Brener. Rather, they suggest that there is no significant difference between the approaches. “The signal just tells you that there is no difference, and that prolonging DAPT probably just induces some minor bleeding, but it doesn’t protect you. So the message would be that you should treat all your patients the same way regardless of where you put the stent,” said Dr. Brener.
The researchers compared data from 497 patients in the EXCEL trial who continued DAPT out to 3 years with that from 136 who stopped DAPT early (115 stopped in year 1-2; 21 stopped in year 2-3). The baseline characteristics of the two groups were similar except for a higher incidence of recent MI in the group that stopped DAPT early (21.3% vs. 13.7%; P = .03).
At 3 years, death, MI, or stroke occurred in 7.8% of the continuation group and in 5.2% of the patients who stopped DAPT. All-cause mortality was 5.8% in the continuation group compared with 2.3% of those who stopped. When the researchers restricted the analysis to patients who presented with acute coronary syndrome, 7.6% and 3.6%, respectively, met the primary endpoint. None of these differences reached statistical significance.
The study was limited by a high dropout rate from DAPT in the first year: 152 patients stopped taking the medication even though they experienced no events, and they were excluded from the analysis.
The EXCEL trial was funded by Abbott. Dr. Brener has been a consultant or received honoraria or speaker’s fees for AstraZeneca.
SOURCE: Brener S. TCT 2018, Abstract TCT-1.
SAN DIEGO – Among patients who receive a drug-eluting stent (DES) in their left main coronary artery (LMCA), continuation of dual antiplatelet therapy (DAPT) past 1 year appears to provide no benefit.
The results come from a subanalysis of the EXCEL trial, which compared the Xience DES to coronary artery bypass graft (CABG) surgery in LMCA lesions.
The LMCA is worrisome to a lot of physicians because of the large amount of myocardial tissue it contains, and they often prescribe DAPT past the generally recommended 1 year. “It’s this magic thing, that ‘Well, it’s left main stenting, and you’d better protect the patient.’ But it turns out that it doesn’t,” said Sorin Brener, MD, director of the catheterization lab at New York Methodist Hospital, who presented the results of the study at the Transcatheter Cardiovascular Therapeutics annual meeting.
The researchers compared patients in both groups who opted to stop DAPT after 1 year versus those who continued therapy out to 3 years, and the news was not favorable for continuation. A composite of death, myocardial infarction, or stroke was higher among patients who continued DAPT, though the results did not reach statistical significance.
It’s possible that patients who continued DAPT were more ill on average. “Obviously, there could be cases where the doctor decided they deserved more prolonged therapy, but it’s not measurable, so I don’t think they were sicker,” said Dr. Brener.
The study was also underpowered. Those worse trends probably don’t represent a real signal, according to Dr. Brener. Rather, they suggest that there is no significant difference between the approaches. “The signal just tells you that there is no difference, and that prolonging DAPT probably just induces some minor bleeding, but it doesn’t protect you. So the message would be that you should treat all your patients the same way regardless of where you put the stent,” said Dr. Brener.
The researchers compared data from 497 patients in the EXCEL trial who continued DAPT out to 3 years with that from 136 who stopped DAPT early (115 stopped in year 1-2; 21 stopped in year 2-3). The baseline characteristics of the two groups were similar except for a higher incidence of recent MI in the group that stopped DAPT early (21.3% vs. 13.7%; P = .03).
At 3 years, death, MI, or stroke occurred in 7.8% of the continuation group and in 5.2% of the patients who stopped DAPT. All-cause mortality was 5.8% in the continuation group compared with 2.3% of those who stopped. When the researchers restricted the analysis to patients who presented with acute coronary syndrome, 7.6% and 3.6%, respectively, met the primary endpoint. None of these differences reached statistical significance.
The study was limited by a high dropout rate from DAPT in the first year: 152 patients stopped taking the medication even though they experienced no events, and they were excluded from the analysis.
The EXCEL trial was funded by Abbott. Dr. Brener has been a consultant or received honoraria or speaker’s fees for AstraZeneca.
SOURCE: Brener S. TCT 2018, Abstract TCT-1.
SAN DIEGO – Among patients who receive a drug-eluting stent (DES) in their left main coronary artery (LMCA), continuation of dual antiplatelet therapy (DAPT) past 1 year appears to provide no benefit.
The results come from a subanalysis of the EXCEL trial, which compared the Xience DES to coronary artery bypass graft (CABG) surgery in LMCA lesions.
The LMCA is worrisome to a lot of physicians because of the large amount of myocardial tissue it contains, and they often prescribe DAPT past the generally recommended 1 year. “It’s this magic thing, that ‘Well, it’s left main stenting, and you’d better protect the patient.’ But it turns out that it doesn’t,” said Sorin Brener, MD, director of the catheterization lab at New York Methodist Hospital, who presented the results of the study at the Transcatheter Cardiovascular Therapeutics annual meeting.
The researchers compared patients in both groups who opted to stop DAPT after 1 year versus those who continued therapy out to 3 years, and the news was not favorable for continuation. A composite of death, myocardial infarction, or stroke was higher among patients who continued DAPT, though the results did not reach statistical significance.
It’s possible that patients who continued DAPT were more ill on average. “Obviously, there could be cases where the doctor decided they deserved more prolonged therapy, but it’s not measurable, so I don’t think they were sicker,” said Dr. Brener.
The study was also underpowered. Those worse trends probably don’t represent a real signal, according to Dr. Brener. Rather, they suggest that there is no significant difference between the approaches. “The signal just tells you that there is no difference, and that prolonging DAPT probably just induces some minor bleeding, but it doesn’t protect you. So the message would be that you should treat all your patients the same way regardless of where you put the stent,” said Dr. Brener.
The researchers compared data from 497 patients in the EXCEL trial who continued DAPT out to 3 years with that from 136 who stopped DAPT early (115 stopped in year 1-2; 21 stopped in year 2-3). The baseline characteristics of the two groups were similar except for a higher incidence of recent MI in the group that stopped DAPT early (21.3% vs. 13.7%; P = .03).
At 3 years, death, MI, or stroke occurred in 7.8% of the continuation group and in 5.2% of the patients who stopped DAPT. All-cause mortality was 5.8% in the continuation group compared with 2.3% of those who stopped. When the researchers restricted the analysis to patients who presented with acute coronary syndrome, 7.6% and 3.6%, respectively, met the primary endpoint. None of these differences reached statistical significance.
The study was limited by a high dropout rate from DAPT in the first year: 152 patients stopped taking the medication even though they experienced no events, and they were excluded from the analysis.
The EXCEL trial was funded by Abbott. Dr. Brener has been a consultant or received honoraria or speaker’s fees for AstraZeneca.
SOURCE: Brener S. TCT 2018, Abstract TCT-1.
REPORTING FROM TCT 2018
Key clinical point: Prolonged DAPT was not associated with better outcomes.
Major finding: Composite death, myocardial infarction, and stroke was similar between the two groups.
Study details: A post hoc analysis of 633 patients in the EXCEL trial.
Disclosures: The EXCEL trial was funded by Abbott. Dr. Brener has been a consultant or received honoraria or speaker’s fees for AstraZeneca.
Source: Brener S. TCT 2018, Abstract TCT-1.
Invasive strategy increased bleeding risk in frail older AMI patients
Frail older patients with acute myocardial infarction (AMI) may be at increased bleeding risk if managed with an invasive strategy, results of a large U.S. registry study suggest.
The increased bleeding risk was seen among frail older AMI patients who underwent cardiac catheterization, but it was not seen in those treated with more conservative medical management, according to study results.
That finding highlights the conundrum with invasive management strategies for frail patients with AMI, wrote John A. Dodson, MD, MPH, of New York University and study coinvestigators.
“Awareness of vulnerability and greater utilization of evidence-based strategies to reduce bleeding, including radial access and properly dose-adjusted anticoagulant therapies, may mitigate some bleeding events,” they wrote in JACC: Cardiovascular Interventions.
Results of this study, the first large U.S. registry analysis evaluating in-hospital bleeding risk in frail older adults with AMI, confirm findings from several previous small cohort studies linking frailty in AMI patients to in-hospital bleeding, investigators reported.
The analysis included a total of 129,330 AMI patients in the ACTION (Acute Coronary Treatment and Intervention Outcomes Network) registry who were aged at least 65 years in 2015 or 2016.
About one in six of these older patients were frail, as defined by a composite score based on impaired walking, cognition, and activities of daily living, investigators reported.
The bleeding rate was significantly higher among frail patients undergoing cardiac catheterization, at 9.4% for patients rated as having vulnerable/mild frailty and 9.9% for patients with moderate to severe frailty (P less than.001), compared with fit/well patients, whose rate was 6.5%, investigators wrote. By contrast, there was no significant difference in bleeding rates for frail versus nonfrail patients managed conservatively, they said.
After adjusting for bleeding risk factors, frailty was independently associated with increased risk of bleeding, compared with fit/well status, with odds ratios of 1.33 for vulnerable/mild frailty and 1.40 for moderate to severe frailty. Again, no association was found between frailty and bleeding risk in patients managed conservatively, according to investigators.
Frail patients in the ACTION registry were more often older and female and less likely to undergo cardiac catheterization when compared with fit or well patients, they added in the report.
Like the small cohort studies that preceded it, this large U.S. registry study shows that frailty is an “important additional risk factor” among older adults with AMI who are managed with an invasive strategy, investigators said.
“When applicable, estimation of bleeding risk in frail patients before invasive care may facilitate clinical decision making and the informed consent process,” they wrote.
The ACTION registry, an ongoing quality improvement initiative sponsored by the American College of Cardiology and the American Heart Association, started collecting frailty characteristics among hospitalized AMI patients in 2015, investigators noted.
Dr. Dodson reported support from the National Institutes of Health/National Institute on Aging and from the American Heart Association. Study coauthors provided disclosures related to Bayer, Janssen, Abbott Vascular, Jarvik Heart, LifeCuff Technologies, and Ancora Heart. JACC Cardiovasc Interv. 2018 Nov 26;11:2287-96
SOURCE: Dodson JA et al. JACC Cardiovasc Intv. 2018;11:2287-96.
This analysis is important and has clinical implications beyond those of previous analyses linking frailty to poor outcomes in patients with cardiovascular disease, according to John A. Bittl, MD.
“The present study helps to transform the rote recording of frailty from a mere quality metric in the medical record into an actionable diagnosis,” Dr. Bittl said in an editorial comment on the findings.
Results of the study show an association between invasive cardiac procedures and increased bleeding in frail patients with AMI.
The benefits of an invasive procedure might be outweighed by the incremental risk added by frailty in older AMI patients at low to moderate risk of poor outcomes, Dr. Bittl suggested.
By contrast, frail older AMI patients at high risk for poor outcomes might be better candidates for an invasive procedure if they can undergo a transradial approach, he said, noting that in the study by Dodson and colleagues, only 26% of frail patients received radial access despite randomized trials showing the approach reduces risk of bleeding.
“In this way, diagnosing frailty in a patient with AMI facilitates clinical decision making and helps to personalize an approach to optimize outcomes,” Dr. Bittl concluded.
Dr. Bittl is with the Interventional Cardiology Group, Florida Hospital Ocala (Fla.) He reported no relationships relevant to his editorial comment ( JACC Cardiovasc Interv. 2018 Nov 26;11[2];2297-93 ).
This analysis is important and has clinical implications beyond those of previous analyses linking frailty to poor outcomes in patients with cardiovascular disease, according to John A. Bittl, MD.
“The present study helps to transform the rote recording of frailty from a mere quality metric in the medical record into an actionable diagnosis,” Dr. Bittl said in an editorial comment on the findings.
Results of the study show an association between invasive cardiac procedures and increased bleeding in frail patients with AMI.
The benefits of an invasive procedure might be outweighed by the incremental risk added by frailty in older AMI patients at low to moderate risk of poor outcomes, Dr. Bittl suggested.
By contrast, frail older AMI patients at high risk for poor outcomes might be better candidates for an invasive procedure if they can undergo a transradial approach, he said, noting that in the study by Dodson and colleagues, only 26% of frail patients received radial access despite randomized trials showing the approach reduces risk of bleeding.
“In this way, diagnosing frailty in a patient with AMI facilitates clinical decision making and helps to personalize an approach to optimize outcomes,” Dr. Bittl concluded.
Dr. Bittl is with the Interventional Cardiology Group, Florida Hospital Ocala (Fla.) He reported no relationships relevant to his editorial comment ( JACC Cardiovasc Interv. 2018 Nov 26;11[2];2297-93 ).
This analysis is important and has clinical implications beyond those of previous analyses linking frailty to poor outcomes in patients with cardiovascular disease, according to John A. Bittl, MD.
“The present study helps to transform the rote recording of frailty from a mere quality metric in the medical record into an actionable diagnosis,” Dr. Bittl said in an editorial comment on the findings.
Results of the study show an association between invasive cardiac procedures and increased bleeding in frail patients with AMI.
The benefits of an invasive procedure might be outweighed by the incremental risk added by frailty in older AMI patients at low to moderate risk of poor outcomes, Dr. Bittl suggested.
By contrast, frail older AMI patients at high risk for poor outcomes might be better candidates for an invasive procedure if they can undergo a transradial approach, he said, noting that in the study by Dodson and colleagues, only 26% of frail patients received radial access despite randomized trials showing the approach reduces risk of bleeding.
“In this way, diagnosing frailty in a patient with AMI facilitates clinical decision making and helps to personalize an approach to optimize outcomes,” Dr. Bittl concluded.
Dr. Bittl is with the Interventional Cardiology Group, Florida Hospital Ocala (Fla.) He reported no relationships relevant to his editorial comment ( JACC Cardiovasc Interv. 2018 Nov 26;11[2];2297-93 ).
Frail older patients with acute myocardial infarction (AMI) may be at increased bleeding risk if managed with an invasive strategy, results of a large U.S. registry study suggest.
The increased bleeding risk was seen among frail older AMI patients who underwent cardiac catheterization, but it was not seen in those treated with more conservative medical management, according to study results.
That finding highlights the conundrum with invasive management strategies for frail patients with AMI, wrote John A. Dodson, MD, MPH, of New York University and study coinvestigators.
“Awareness of vulnerability and greater utilization of evidence-based strategies to reduce bleeding, including radial access and properly dose-adjusted anticoagulant therapies, may mitigate some bleeding events,” they wrote in JACC: Cardiovascular Interventions.
Results of this study, the first large U.S. registry analysis evaluating in-hospital bleeding risk in frail older adults with AMI, confirm findings from several previous small cohort studies linking frailty in AMI patients to in-hospital bleeding, investigators reported.
The analysis included a total of 129,330 AMI patients in the ACTION (Acute Coronary Treatment and Intervention Outcomes Network) registry who were aged at least 65 years in 2015 or 2016.
About one in six of these older patients were frail, as defined by a composite score based on impaired walking, cognition, and activities of daily living, investigators reported.
The bleeding rate was significantly higher among frail patients undergoing cardiac catheterization, at 9.4% for patients rated as having vulnerable/mild frailty and 9.9% for patients with moderate to severe frailty (P less than.001), compared with fit/well patients, whose rate was 6.5%, investigators wrote. By contrast, there was no significant difference in bleeding rates for frail versus nonfrail patients managed conservatively, they said.
After adjusting for bleeding risk factors, frailty was independently associated with increased risk of bleeding, compared with fit/well status, with odds ratios of 1.33 for vulnerable/mild frailty and 1.40 for moderate to severe frailty. Again, no association was found between frailty and bleeding risk in patients managed conservatively, according to investigators.
Frail patients in the ACTION registry were more often older and female and less likely to undergo cardiac catheterization when compared with fit or well patients, they added in the report.
Like the small cohort studies that preceded it, this large U.S. registry study shows that frailty is an “important additional risk factor” among older adults with AMI who are managed with an invasive strategy, investigators said.
“When applicable, estimation of bleeding risk in frail patients before invasive care may facilitate clinical decision making and the informed consent process,” they wrote.
The ACTION registry, an ongoing quality improvement initiative sponsored by the American College of Cardiology and the American Heart Association, started collecting frailty characteristics among hospitalized AMI patients in 2015, investigators noted.
Dr. Dodson reported support from the National Institutes of Health/National Institute on Aging and from the American Heart Association. Study coauthors provided disclosures related to Bayer, Janssen, Abbott Vascular, Jarvik Heart, LifeCuff Technologies, and Ancora Heart. JACC Cardiovasc Interv. 2018 Nov 26;11:2287-96
SOURCE: Dodson JA et al. JACC Cardiovasc Intv. 2018;11:2287-96.
Frail older patients with acute myocardial infarction (AMI) may be at increased bleeding risk if managed with an invasive strategy, results of a large U.S. registry study suggest.
The increased bleeding risk was seen among frail older AMI patients who underwent cardiac catheterization, but it was not seen in those treated with more conservative medical management, according to study results.
That finding highlights the conundrum with invasive management strategies for frail patients with AMI, wrote John A. Dodson, MD, MPH, of New York University and study coinvestigators.
“Awareness of vulnerability and greater utilization of evidence-based strategies to reduce bleeding, including radial access and properly dose-adjusted anticoagulant therapies, may mitigate some bleeding events,” they wrote in JACC: Cardiovascular Interventions.
Results of this study, the first large U.S. registry analysis evaluating in-hospital bleeding risk in frail older adults with AMI, confirm findings from several previous small cohort studies linking frailty in AMI patients to in-hospital bleeding, investigators reported.
The analysis included a total of 129,330 AMI patients in the ACTION (Acute Coronary Treatment and Intervention Outcomes Network) registry who were aged at least 65 years in 2015 or 2016.
About one in six of these older patients were frail, as defined by a composite score based on impaired walking, cognition, and activities of daily living, investigators reported.
The bleeding rate was significantly higher among frail patients undergoing cardiac catheterization, at 9.4% for patients rated as having vulnerable/mild frailty and 9.9% for patients with moderate to severe frailty (P less than.001), compared with fit/well patients, whose rate was 6.5%, investigators wrote. By contrast, there was no significant difference in bleeding rates for frail versus nonfrail patients managed conservatively, they said.
After adjusting for bleeding risk factors, frailty was independently associated with increased risk of bleeding, compared with fit/well status, with odds ratios of 1.33 for vulnerable/mild frailty and 1.40 for moderate to severe frailty. Again, no association was found between frailty and bleeding risk in patients managed conservatively, according to investigators.
Frail patients in the ACTION registry were more often older and female and less likely to undergo cardiac catheterization when compared with fit or well patients, they added in the report.
Like the small cohort studies that preceded it, this large U.S. registry study shows that frailty is an “important additional risk factor” among older adults with AMI who are managed with an invasive strategy, investigators said.
“When applicable, estimation of bleeding risk in frail patients before invasive care may facilitate clinical decision making and the informed consent process,” they wrote.
The ACTION registry, an ongoing quality improvement initiative sponsored by the American College of Cardiology and the American Heart Association, started collecting frailty characteristics among hospitalized AMI patients in 2015, investigators noted.
Dr. Dodson reported support from the National Institutes of Health/National Institute on Aging and from the American Heart Association. Study coauthors provided disclosures related to Bayer, Janssen, Abbott Vascular, Jarvik Heart, LifeCuff Technologies, and Ancora Heart. JACC Cardiovasc Interv. 2018 Nov 26;11:2287-96
SOURCE: Dodson JA et al. JACC Cardiovasc Intv. 2018;11:2287-96.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Key clinical point: Frail older patients with acute myocardial infarction may be at increased bleeding risk if managed with an invasive strategy.
Major finding: Frailty was associated with increased risk of bleeding, with odds ratios of 1.33 and 1.40, compared with fit or well patients.
Study details: Analysis including 129,330 AMI patients in a U.S. registry who were at least 65 years of age.
Disclosures: Researchers reported support from the National Institutes of Health/National Institute on Aging, as well as other disclosures related to Bayer, Janssen, Abbott Vascular, Jarvik Heart, LifeCuff Technologies, and Ancora Heart.
Source: Dodson JA et al. JACC Cardiovasc Interv. 2018 Nov 26;11:2287-96.
Embolic protection devices advocated in some lower limb endovascular surgery
NEW YORK – Embolic protection devices to prevent debris from causing complications in percutaneous cardiac procedures also have a role when treating occlusions in the lower extremities, according to an expert who reviewed data and spoke about his experience at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
In this video interview, Peter Schneider, MD, chief of the division of vascular therapy at the Hawaii Kaiser Permanente Medical Group, Honolulu, provided an expert’s opinion about when filters can be helpful to reduce risk of complications.
According to Dr. Schneider, filters do have relative disadvantages so he does not advocate their use when risk is low. Among these disadvantages, the wire used to place the filter can make the endovascular procedure more difficult.
However, he said that there are well-documented cases in which debris in the runoff of a lower limb endovascular procedure resulted in adverse clinical consequences. Importantly, these may not be acute clinical events. Rather, occlusions in the tibial or pedal collaterals may remain asymptomatic for months or years.
Outlining those clinical situations that pose the greatest risk, Dr. Schneider identified several specific patient groups for whom he would advocate filters, particularly those with a large thrombotic burden. Based on his own experience, he provided some tips about situations in which he now employs embolic protection devices routinely.
NEW YORK – Embolic protection devices to prevent debris from causing complications in percutaneous cardiac procedures also have a role when treating occlusions in the lower extremities, according to an expert who reviewed data and spoke about his experience at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
In this video interview, Peter Schneider, MD, chief of the division of vascular therapy at the Hawaii Kaiser Permanente Medical Group, Honolulu, provided an expert’s opinion about when filters can be helpful to reduce risk of complications.
According to Dr. Schneider, filters do have relative disadvantages so he does not advocate their use when risk is low. Among these disadvantages, the wire used to place the filter can make the endovascular procedure more difficult.
However, he said that there are well-documented cases in which debris in the runoff of a lower limb endovascular procedure resulted in adverse clinical consequences. Importantly, these may not be acute clinical events. Rather, occlusions in the tibial or pedal collaterals may remain asymptomatic for months or years.
Outlining those clinical situations that pose the greatest risk, Dr. Schneider identified several specific patient groups for whom he would advocate filters, particularly those with a large thrombotic burden. Based on his own experience, he provided some tips about situations in which he now employs embolic protection devices routinely.
NEW YORK – Embolic protection devices to prevent debris from causing complications in percutaneous cardiac procedures also have a role when treating occlusions in the lower extremities, according to an expert who reviewed data and spoke about his experience at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
In this video interview, Peter Schneider, MD, chief of the division of vascular therapy at the Hawaii Kaiser Permanente Medical Group, Honolulu, provided an expert’s opinion about when filters can be helpful to reduce risk of complications.
According to Dr. Schneider, filters do have relative disadvantages so he does not advocate their use when risk is low. Among these disadvantages, the wire used to place the filter can make the endovascular procedure more difficult.
However, he said that there are well-documented cases in which debris in the runoff of a lower limb endovascular procedure resulted in adverse clinical consequences. Importantly, these may not be acute clinical events. Rather, occlusions in the tibial or pedal collaterals may remain asymptomatic for months or years.
Outlining those clinical situations that pose the greatest risk, Dr. Schneider identified several specific patient groups for whom he would advocate filters, particularly those with a large thrombotic burden. Based on his own experience, he provided some tips about situations in which he now employs embolic protection devices routinely.
REPORTING FROM VEITHSYMPOSIUM