User login
Hinged-Knee External Fixator Used to Reduce and Maintain Subacute Tibiofemoral Coronal Subluxation
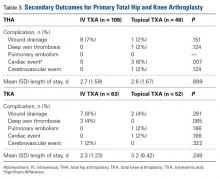
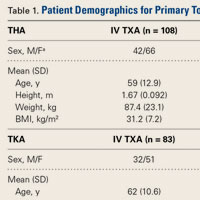
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
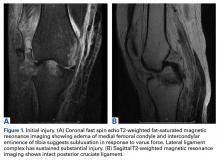
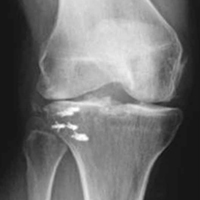
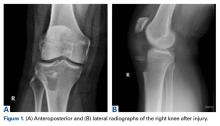
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
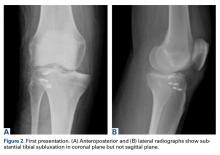
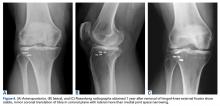
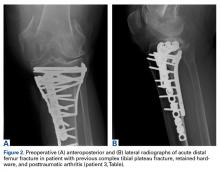
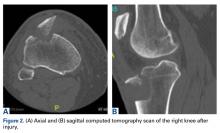
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
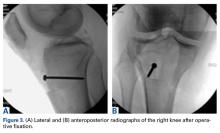
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
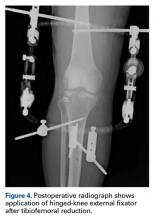
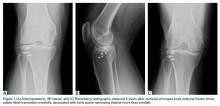
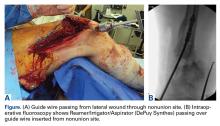
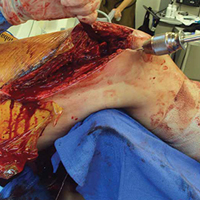
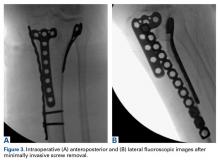
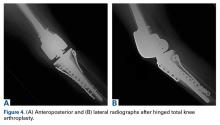
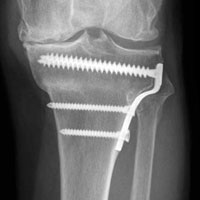
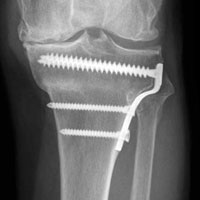
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
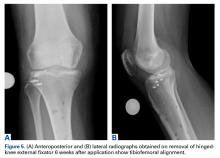
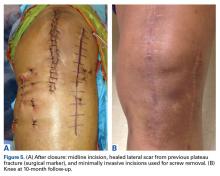
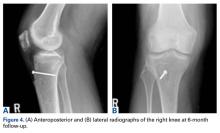
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
Fat Embolism Syndrome With Cerebral Fat Embolism Associated With Long-Bone Fracture
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
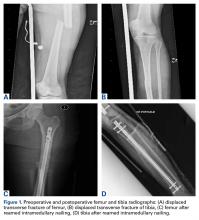
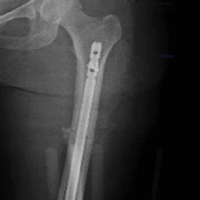
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
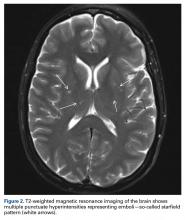
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
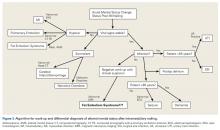
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
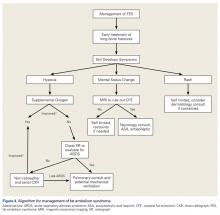
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
A New Technique for Obtaining Bone Graft in Cases of Distal Femur Nonunion: Passing a Reamer/Irrigator/Aspirator Retrograde Through the Nonunion Site
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
Can a Total Knee Arthroplasty Perioperative Surgical Home Close the Gap Between Primary and Revision TKA Outcomes?
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
Comparing Cost, Efficacy, and Safety of Intravenous and Topical Tranexamic Acid in Total Hip and Knee Arthroplasty
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
Total Knee Arthroplasty With Retained Tibial Implants: The Role of Minimally Invasive Hardware Removal
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
Robotic Technology Produces More Conservative Tibial Resection Than Conventional Techniques in UKA
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
Tibial Tubercle Fracture After Bone–Patellar Tendon–Bone Autograft
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
Perceived Leg-Length Discrepancy After Primary Total Knee Arthroplasty: Does Knee Alignment Play a Role?
Leg-length discrepancy (LLD) is common in the general population1 and particularly in patients with degenerative joint diseases of the hip and knee.2 Common complications of LLD include femoral, sciatic, and peroneal nerve palsy; lower back pain; gait abnormalities3; and general dissatisfaction. LLD is a concern for orthopedic surgeons who perform total knee arthroplasty (TKA) because limb lengthening is common after this procedure.4,5 Surgeons are aware of the limb lengthening that occurs during TKA,4,5 and studies have confirmed that LLD usually decreases after TKA.4,5
Despite surgeons’ best efforts, some patients still perceive LLD after surgery, though the incidence of perceived LLD in patients who have had TKA has not been well documented. Aside from actual, objectively measured LLD, there may be other factors that lead patients to perceive LLD. Study results have suggested that preoperative varus–valgus alignment of the knee joint may correlate with how much an operative leg is lengthened after TKA4,5; however, the outcome investigated was objective LLD measurements, not perceived LLD. Understanding the factors that may influence patients’ ability to perceive LLD would allow surgeons to preoperatively identify patients who are at higher risk for postoperative perceived LLD. This information, along with expected time to resolution of postoperative perceived LLD, would allow surgeons to educate their patients accordingly.
We conducted a study to determine the incidence of perceived LLD before and after primary TKA in patients with unilateral osteoarthritis and to determine the correlation between mechanical axis of the knee and perceived LLD before and after surgery. Given that surgery may correct mechanical axis misalignment, we investigated the correlation between this correction and its ability to change patients’ preoperative and postoperative perceived LLD. We hypothesized that a large correction of mechanical axis would lead patients to perceive LLD after surgery. The relationship of body mass index (BMI) and age to patients’ perceived LLD was also assessed. The incidence and time frame of resolution of postoperative perceived LLD were determined.
Methods
Approval for this study was received from the Institutional Review Board at our institution, Rush University Medical Center in Chicago, Illinois. Seventy-three patients undergoing primary TKA performed by 3 surgeons at 2 institutions between February 2010 and January 2013 were prospectively enrolled. Inclusion criteria were age 18 years to 90 years and primary TKA for unilateral osteoarthritis; exclusion criteria were allergy or intolerance to the study materials, operative treatment of affected joint or its underlying etiology within prior month, previous surgeries (other than arthroscopy) on affected joint, previous surgeries (on unaffected lower extremity) that may influence preoperative and postoperative leg lengths, and any substance abuse or dependence within the past 6 months. Patients provided written informed consent for total knee arthroplasty.
All surgeries were performed by Dr. Levine, Dr. Della Valle, and Dr. Sporer using the medial parapatellar or midvastus approach with tourniquet. Similar standard postoperative rehabilitation protocols with early mobilization were used in all cases.
During clinical evaluation, patient demographic data were collected and LLD surveys administered. Patients were asked, before surgery and 3 to 6 weeks, 3 months, 6 months, and 1 year after surgery, if they perceived LLD. A patient who no longer perceived LLD after surgery was no longer followed for this study.
At the preoperative clinic visit and at the 3-month or 6-week postoperative visit, standing mechanical axis radiographs were viewed by 2 of the authors (not the primary surgeons) using PACS (picture archiving and communication system software). The mechanical axis of the operative leg was measured with ImageJ software by taking the angle from the center of the femur to the middle of the ankle joint, with the vertex assigned to the middle of the knee joint.
We used a 2-tailed unpaired t test to determine the relationship of preoperative mechanical axis to perceived LLD (or lack thereof) before surgery. The data were analyzed for separate varus and valgus deformities. Then we determined the relationship of postoperative mechanical axis to perceived LLD (or lack thereof) after surgery. The McNemar test was used to determine the effect of surgery on patients’ LLD perceptions.
To determine the relationship between preoperative-to-postoperative change in mechanical axis and change in LLD perceptions, we divided patients into 4 groups. Group 1 had both preoperative and postoperative perceived LLD, group 2 had no preoperative or postoperative perceived LLD, group 3 had preoperative perceived LLD but no postoperative perceived LLD, and group 4 had postoperative perceived LLD but no preoperative perceived LLD. The absolute value of the difference between preoperative and postoperative mechanical axis was then determined, relative to 180°, to account for changes in varus to valgus deformity before and after surgery and vice versa. Analysis of variance (ANOVA) was used to detect differences between groups. This analysis was then stratified based on BMI and age.
Results
Of the 73 enrolled patients, 2 were excluded from results analysis because of inadequate data—one did not complete the postoperative LLD survey, and the other did not have postoperative standing mechanical axis radiographs—leaving 71 patients (27 men, 44 women) with adequate data. Mean (SD) age of all patients was 65 (8.4) years (range, 47-89 years). Mean (SD) BMI was 35.1 (9.9; range, 20.2-74.8).
Of the 71 patients with adequate data, 18 had preoperative perceived LLD and 53 did not; in addition, 7 had postoperative perceived LLD and 64 did not. All 7 patients with postoperative perceived LLD noted resolution of LLD, at a mean of 8.5 weeks (range, 3 weeks-3 months). There was a significant difference between the 18 patients with preoperative perceived LLD and the 7 with postoperative perceived LLD (P = .035, analyzed with the McNemar test).
Table 1 lists the mean preoperative mechanical axis measurements for patients with and without preoperative perceived LLD.
Table 2 lists the mean postoperative mechanical axis measurements for patients with and without postoperative perceived LLD.
Table 3 lists the mean absolute values of mechanical axis correction (preoperative to postoperative) for the 4 patient groups described in the Methods section.
Discussion
In this study, 18 patients (25%) had preoperative perceived LLD, proving that perceived LLD is common in patients who undergo TKA for unilateral osteoarthritis. Surgeons should give their patients a preoperative survey on perceived LLD, as survey responses may inform and influence surgical decisions and strategies.
Of the 18 patients with preoperative perceived LLD, only 1 had postoperative perceived LLD. That perceived LLD decreased after surgery makes sense given the widely accepted notion that actual LLD is common before primary TKA but in most cases is corrected during surgery.4,5 As LLD correction during surgery is so successful, surgeons should tell their patients with preoperative perceived LLD that in most cases it will be fixed after TKA.
Although the incidence of perceived LLD decreased after TKA (as mentioned earlier), the decrease seemed to be restricted mostly to patients with preoperative perceived LLD, and the underlying LLD was most probably corrected by the surgery. However, surgery introduced perceived LLD in 6 cases, supporting the notion that it is crucial to understand which patients are at higher risk for postoperative perceived LLD and what if any time frame can be expected for resolution in these cases. In our study, all cases of perceived LLD had resolved by a mean follow-up of 8.5 weeks (range, 3 weeks-3 months). This phenomenon of resolution may be attributed to some of the physical, objective LLD corrections that naturally occur throughout the postoperative course,4 though psychological factors may also be involved. Our study results suggest patients should be counseled that, though about 10% of patients perceive LLD after primary TKA, the vast majority of perceived LLD cases resolve within 3 months.
One study goal was to determine the relationship between the mechanical axis of the knee and perceived LLD both before and after surgery. There were no significant relationships. This was also true when cases of varus and valgus deformity were analyzed separately.
Another study goal was to determine if a surgical change in the mechanical alignment of the knee would influence preoperative-to-postoperative LLD perceptions. In our analysis, patients were divided into 4 groups based on their preoperative and postoperative LLD perceptions (see Methods section). ANOVA revealed no significant differences in absolute values of mechanical axis correction among the 4 groups. Likewise, there were no correlations between BMI and age and mechanical axis correction among the groups, suggesting LLD perception is unrelated to any of these variables. Ideally, if a relationship between a threshold knee alignment value and perceived LLD existed, surgeons would be able to counsel patients at higher risk for perceived LLD about how their knee alignment may contribute to their perception. Unfortunately, our study results did not show any significant statistical relationships in this regard.
The problem of LLD in patients undergoing TKA is not new, and much research is needed to determine the correlation between perceived versus actual discrepancies, and why they occur. Our study results confirmed that TKA corrects most cases of preoperative perceived LLD but introduces perceived LLD in other cases. Whether preoperative or postoperative LLD is merely perceived or is in fact an actual discrepancy remains to be seen.
One limitation of this study was its lack of leg-length measurements. Although we studied knee alignment specifically, it would have been useful to compare perceived LLD with measured leg lengths, either clinically or radiographically, especially since leg lengths obviously play a role in any perceived LLD. We used mechanical alignment as a surrogate for actual LLD because we hypothesized that alignment may contribute to patients’ perceived discrepancies.
Another limitation was the relatively small sample. Only 24 cases of perceived LLD were analyzed. Given our low rates of perceived LLD (25% before surgery, 10% after surgery), it is difficult to study a large enough TKA group to establish a statistically significant number of cases. Nevertheless, investigators may use larger groups to establish more meaningful relationships.
A third limitation was that alignment was measured on the operative side but not the contralateral side. As we were focusing on perceived discrepancy, contralateral knee alignment may play an important role. Our study involved patients with unilateral osteoarthritis, so it would be reasonable to assume the nonoperative knee was almost neutral in alignment in most cases. However, given that varus/valgus misalignment is a known risk factor for osteoarthritis,6 many of our patients with unilateral disease may very well have had preexisting misalignment of both knees. The undetermined alignment of the nonoperative side may be a confounding variable in the relationship between operative knee alignment and perceived LLD.
Fourth, not all patients were surveyed 3 weeks after surgery. Some were first surveyed at 6 weeks, and it is possible there were cases of transient postoperative LLD that resolved before that point. Therefore, our reported incidence of postoperative LLD could have missed some cases. In addition, our mean 8.5-week period for LLD resolution may not have accounted for these resolved cases of transient perceived LLD.
Am J Orthop. 2016;45(7):E429-E433. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
2. Noll DR. Leg length discrepancy and osteoarthritic knee pain in the elderly: an observational study. J Am Osteopath Assoc. 2013;113(9):670-678.
3. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
4. Chang MJ, Kang YG, Chang CB, Seong SC, Kim TK. The patterns of limb length, height, weight and body mass index changes after total knee arthroplasty. J Arthroplasty. 2013;28(10):1856-1861.
5. Lang JE, Scott RD, Lonner JH, Bono JV, Hunter DJ, Li L. Magnitude of limb lengthening after primary total knee arthroplasty. J Arthroplasty. 2012;27(3):341-346.
6. Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940-1945.
Leg-length discrepancy (LLD) is common in the general population1 and particularly in patients with degenerative joint diseases of the hip and knee.2 Common complications of LLD include femoral, sciatic, and peroneal nerve palsy; lower back pain; gait abnormalities3; and general dissatisfaction. LLD is a concern for orthopedic surgeons who perform total knee arthroplasty (TKA) because limb lengthening is common after this procedure.4,5 Surgeons are aware of the limb lengthening that occurs during TKA,4,5 and studies have confirmed that LLD usually decreases after TKA.4,5
Despite surgeons’ best efforts, some patients still perceive LLD after surgery, though the incidence of perceived LLD in patients who have had TKA has not been well documented. Aside from actual, objectively measured LLD, there may be other factors that lead patients to perceive LLD. Study results have suggested that preoperative varus–valgus alignment of the knee joint may correlate with how much an operative leg is lengthened after TKA4,5; however, the outcome investigated was objective LLD measurements, not perceived LLD. Understanding the factors that may influence patients’ ability to perceive LLD would allow surgeons to preoperatively identify patients who are at higher risk for postoperative perceived LLD. This information, along with expected time to resolution of postoperative perceived LLD, would allow surgeons to educate their patients accordingly.
We conducted a study to determine the incidence of perceived LLD before and after primary TKA in patients with unilateral osteoarthritis and to determine the correlation between mechanical axis of the knee and perceived LLD before and after surgery. Given that surgery may correct mechanical axis misalignment, we investigated the correlation between this correction and its ability to change patients’ preoperative and postoperative perceived LLD. We hypothesized that a large correction of mechanical axis would lead patients to perceive LLD after surgery. The relationship of body mass index (BMI) and age to patients’ perceived LLD was also assessed. The incidence and time frame of resolution of postoperative perceived LLD were determined.
Methods
Approval for this study was received from the Institutional Review Board at our institution, Rush University Medical Center in Chicago, Illinois. Seventy-three patients undergoing primary TKA performed by 3 surgeons at 2 institutions between February 2010 and January 2013 were prospectively enrolled. Inclusion criteria were age 18 years to 90 years and primary TKA for unilateral osteoarthritis; exclusion criteria were allergy or intolerance to the study materials, operative treatment of affected joint or its underlying etiology within prior month, previous surgeries (other than arthroscopy) on affected joint, previous surgeries (on unaffected lower extremity) that may influence preoperative and postoperative leg lengths, and any substance abuse or dependence within the past 6 months. Patients provided written informed consent for total knee arthroplasty.
All surgeries were performed by Dr. Levine, Dr. Della Valle, and Dr. Sporer using the medial parapatellar or midvastus approach with tourniquet. Similar standard postoperative rehabilitation protocols with early mobilization were used in all cases.
During clinical evaluation, patient demographic data were collected and LLD surveys administered. Patients were asked, before surgery and 3 to 6 weeks, 3 months, 6 months, and 1 year after surgery, if they perceived LLD. A patient who no longer perceived LLD after surgery was no longer followed for this study.
At the preoperative clinic visit and at the 3-month or 6-week postoperative visit, standing mechanical axis radiographs were viewed by 2 of the authors (not the primary surgeons) using PACS (picture archiving and communication system software). The mechanical axis of the operative leg was measured with ImageJ software by taking the angle from the center of the femur to the middle of the ankle joint, with the vertex assigned to the middle of the knee joint.
We used a 2-tailed unpaired t test to determine the relationship of preoperative mechanical axis to perceived LLD (or lack thereof) before surgery. The data were analyzed for separate varus and valgus deformities. Then we determined the relationship of postoperative mechanical axis to perceived LLD (or lack thereof) after surgery. The McNemar test was used to determine the effect of surgery on patients’ LLD perceptions.
To determine the relationship between preoperative-to-postoperative change in mechanical axis and change in LLD perceptions, we divided patients into 4 groups. Group 1 had both preoperative and postoperative perceived LLD, group 2 had no preoperative or postoperative perceived LLD, group 3 had preoperative perceived LLD but no postoperative perceived LLD, and group 4 had postoperative perceived LLD but no preoperative perceived LLD. The absolute value of the difference between preoperative and postoperative mechanical axis was then determined, relative to 180°, to account for changes in varus to valgus deformity before and after surgery and vice versa. Analysis of variance (ANOVA) was used to detect differences between groups. This analysis was then stratified based on BMI and age.
Results
Of the 73 enrolled patients, 2 were excluded from results analysis because of inadequate data—one did not complete the postoperative LLD survey, and the other did not have postoperative standing mechanical axis radiographs—leaving 71 patients (27 men, 44 women) with adequate data. Mean (SD) age of all patients was 65 (8.4) years (range, 47-89 years). Mean (SD) BMI was 35.1 (9.9; range, 20.2-74.8).
Of the 71 patients with adequate data, 18 had preoperative perceived LLD and 53 did not; in addition, 7 had postoperative perceived LLD and 64 did not. All 7 patients with postoperative perceived LLD noted resolution of LLD, at a mean of 8.5 weeks (range, 3 weeks-3 months). There was a significant difference between the 18 patients with preoperative perceived LLD and the 7 with postoperative perceived LLD (P = .035, analyzed with the McNemar test).
Table 1 lists the mean preoperative mechanical axis measurements for patients with and without preoperative perceived LLD.
Table 2 lists the mean postoperative mechanical axis measurements for patients with and without postoperative perceived LLD.
Table 3 lists the mean absolute values of mechanical axis correction (preoperative to postoperative) for the 4 patient groups described in the Methods section.
Discussion
In this study, 18 patients (25%) had preoperative perceived LLD, proving that perceived LLD is common in patients who undergo TKA for unilateral osteoarthritis. Surgeons should give their patients a preoperative survey on perceived LLD, as survey responses may inform and influence surgical decisions and strategies.
Of the 18 patients with preoperative perceived LLD, only 1 had postoperative perceived LLD. That perceived LLD decreased after surgery makes sense given the widely accepted notion that actual LLD is common before primary TKA but in most cases is corrected during surgery.4,5 As LLD correction during surgery is so successful, surgeons should tell their patients with preoperative perceived LLD that in most cases it will be fixed after TKA.
Although the incidence of perceived LLD decreased after TKA (as mentioned earlier), the decrease seemed to be restricted mostly to patients with preoperative perceived LLD, and the underlying LLD was most probably corrected by the surgery. However, surgery introduced perceived LLD in 6 cases, supporting the notion that it is crucial to understand which patients are at higher risk for postoperative perceived LLD and what if any time frame can be expected for resolution in these cases. In our study, all cases of perceived LLD had resolved by a mean follow-up of 8.5 weeks (range, 3 weeks-3 months). This phenomenon of resolution may be attributed to some of the physical, objective LLD corrections that naturally occur throughout the postoperative course,4 though psychological factors may also be involved. Our study results suggest patients should be counseled that, though about 10% of patients perceive LLD after primary TKA, the vast majority of perceived LLD cases resolve within 3 months.
One study goal was to determine the relationship between the mechanical axis of the knee and perceived LLD both before and after surgery. There were no significant relationships. This was also true when cases of varus and valgus deformity were analyzed separately.
Another study goal was to determine if a surgical change in the mechanical alignment of the knee would influence preoperative-to-postoperative LLD perceptions. In our analysis, patients were divided into 4 groups based on their preoperative and postoperative LLD perceptions (see Methods section). ANOVA revealed no significant differences in absolute values of mechanical axis correction among the 4 groups. Likewise, there were no correlations between BMI and age and mechanical axis correction among the groups, suggesting LLD perception is unrelated to any of these variables. Ideally, if a relationship between a threshold knee alignment value and perceived LLD existed, surgeons would be able to counsel patients at higher risk for perceived LLD about how their knee alignment may contribute to their perception. Unfortunately, our study results did not show any significant statistical relationships in this regard.
The problem of LLD in patients undergoing TKA is not new, and much research is needed to determine the correlation between perceived versus actual discrepancies, and why they occur. Our study results confirmed that TKA corrects most cases of preoperative perceived LLD but introduces perceived LLD in other cases. Whether preoperative or postoperative LLD is merely perceived or is in fact an actual discrepancy remains to be seen.
One limitation of this study was its lack of leg-length measurements. Although we studied knee alignment specifically, it would have been useful to compare perceived LLD with measured leg lengths, either clinically or radiographically, especially since leg lengths obviously play a role in any perceived LLD. We used mechanical alignment as a surrogate for actual LLD because we hypothesized that alignment may contribute to patients’ perceived discrepancies.
Another limitation was the relatively small sample. Only 24 cases of perceived LLD were analyzed. Given our low rates of perceived LLD (25% before surgery, 10% after surgery), it is difficult to study a large enough TKA group to establish a statistically significant number of cases. Nevertheless, investigators may use larger groups to establish more meaningful relationships.
A third limitation was that alignment was measured on the operative side but not the contralateral side. As we were focusing on perceived discrepancy, contralateral knee alignment may play an important role. Our study involved patients with unilateral osteoarthritis, so it would be reasonable to assume the nonoperative knee was almost neutral in alignment in most cases. However, given that varus/valgus misalignment is a known risk factor for osteoarthritis,6 many of our patients with unilateral disease may very well have had preexisting misalignment of both knees. The undetermined alignment of the nonoperative side may be a confounding variable in the relationship between operative knee alignment and perceived LLD.
Fourth, not all patients were surveyed 3 weeks after surgery. Some were first surveyed at 6 weeks, and it is possible there were cases of transient postoperative LLD that resolved before that point. Therefore, our reported incidence of postoperative LLD could have missed some cases. In addition, our mean 8.5-week period for LLD resolution may not have accounted for these resolved cases of transient perceived LLD.
Am J Orthop. 2016;45(7):E429-E433. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Leg-length discrepancy (LLD) is common in the general population1 and particularly in patients with degenerative joint diseases of the hip and knee.2 Common complications of LLD include femoral, sciatic, and peroneal nerve palsy; lower back pain; gait abnormalities3; and general dissatisfaction. LLD is a concern for orthopedic surgeons who perform total knee arthroplasty (TKA) because limb lengthening is common after this procedure.4,5 Surgeons are aware of the limb lengthening that occurs during TKA,4,5 and studies have confirmed that LLD usually decreases after TKA.4,5
Despite surgeons’ best efforts, some patients still perceive LLD after surgery, though the incidence of perceived LLD in patients who have had TKA has not been well documented. Aside from actual, objectively measured LLD, there may be other factors that lead patients to perceive LLD. Study results have suggested that preoperative varus–valgus alignment of the knee joint may correlate with how much an operative leg is lengthened after TKA4,5; however, the outcome investigated was objective LLD measurements, not perceived LLD. Understanding the factors that may influence patients’ ability to perceive LLD would allow surgeons to preoperatively identify patients who are at higher risk for postoperative perceived LLD. This information, along with expected time to resolution of postoperative perceived LLD, would allow surgeons to educate their patients accordingly.
We conducted a study to determine the incidence of perceived LLD before and after primary TKA in patients with unilateral osteoarthritis and to determine the correlation between mechanical axis of the knee and perceived LLD before and after surgery. Given that surgery may correct mechanical axis misalignment, we investigated the correlation between this correction and its ability to change patients’ preoperative and postoperative perceived LLD. We hypothesized that a large correction of mechanical axis would lead patients to perceive LLD after surgery. The relationship of body mass index (BMI) and age to patients’ perceived LLD was also assessed. The incidence and time frame of resolution of postoperative perceived LLD were determined.
Methods
Approval for this study was received from the Institutional Review Board at our institution, Rush University Medical Center in Chicago, Illinois. Seventy-three patients undergoing primary TKA performed by 3 surgeons at 2 institutions between February 2010 and January 2013 were prospectively enrolled. Inclusion criteria were age 18 years to 90 years and primary TKA for unilateral osteoarthritis; exclusion criteria were allergy or intolerance to the study materials, operative treatment of affected joint or its underlying etiology within prior month, previous surgeries (other than arthroscopy) on affected joint, previous surgeries (on unaffected lower extremity) that may influence preoperative and postoperative leg lengths, and any substance abuse or dependence within the past 6 months. Patients provided written informed consent for total knee arthroplasty.
All surgeries were performed by Dr. Levine, Dr. Della Valle, and Dr. Sporer using the medial parapatellar or midvastus approach with tourniquet. Similar standard postoperative rehabilitation protocols with early mobilization were used in all cases.
During clinical evaluation, patient demographic data were collected and LLD surveys administered. Patients were asked, before surgery and 3 to 6 weeks, 3 months, 6 months, and 1 year after surgery, if they perceived LLD. A patient who no longer perceived LLD after surgery was no longer followed for this study.
At the preoperative clinic visit and at the 3-month or 6-week postoperative visit, standing mechanical axis radiographs were viewed by 2 of the authors (not the primary surgeons) using PACS (picture archiving and communication system software). The mechanical axis of the operative leg was measured with ImageJ software by taking the angle from the center of the femur to the middle of the ankle joint, with the vertex assigned to the middle of the knee joint.
We used a 2-tailed unpaired t test to determine the relationship of preoperative mechanical axis to perceived LLD (or lack thereof) before surgery. The data were analyzed for separate varus and valgus deformities. Then we determined the relationship of postoperative mechanical axis to perceived LLD (or lack thereof) after surgery. The McNemar test was used to determine the effect of surgery on patients’ LLD perceptions.
To determine the relationship between preoperative-to-postoperative change in mechanical axis and change in LLD perceptions, we divided patients into 4 groups. Group 1 had both preoperative and postoperative perceived LLD, group 2 had no preoperative or postoperative perceived LLD, group 3 had preoperative perceived LLD but no postoperative perceived LLD, and group 4 had postoperative perceived LLD but no preoperative perceived LLD. The absolute value of the difference between preoperative and postoperative mechanical axis was then determined, relative to 180°, to account for changes in varus to valgus deformity before and after surgery and vice versa. Analysis of variance (ANOVA) was used to detect differences between groups. This analysis was then stratified based on BMI and age.
Results
Of the 73 enrolled patients, 2 were excluded from results analysis because of inadequate data—one did not complete the postoperative LLD survey, and the other did not have postoperative standing mechanical axis radiographs—leaving 71 patients (27 men, 44 women) with adequate data. Mean (SD) age of all patients was 65 (8.4) years (range, 47-89 years). Mean (SD) BMI was 35.1 (9.9; range, 20.2-74.8).
Of the 71 patients with adequate data, 18 had preoperative perceived LLD and 53 did not; in addition, 7 had postoperative perceived LLD and 64 did not. All 7 patients with postoperative perceived LLD noted resolution of LLD, at a mean of 8.5 weeks (range, 3 weeks-3 months). There was a significant difference between the 18 patients with preoperative perceived LLD and the 7 with postoperative perceived LLD (P = .035, analyzed with the McNemar test).
Table 1 lists the mean preoperative mechanical axis measurements for patients with and without preoperative perceived LLD.
Table 2 lists the mean postoperative mechanical axis measurements for patients with and without postoperative perceived LLD.
Table 3 lists the mean absolute values of mechanical axis correction (preoperative to postoperative) for the 4 patient groups described in the Methods section.
Discussion
In this study, 18 patients (25%) had preoperative perceived LLD, proving that perceived LLD is common in patients who undergo TKA for unilateral osteoarthritis. Surgeons should give their patients a preoperative survey on perceived LLD, as survey responses may inform and influence surgical decisions and strategies.
Of the 18 patients with preoperative perceived LLD, only 1 had postoperative perceived LLD. That perceived LLD decreased after surgery makes sense given the widely accepted notion that actual LLD is common before primary TKA but in most cases is corrected during surgery.4,5 As LLD correction during surgery is so successful, surgeons should tell their patients with preoperative perceived LLD that in most cases it will be fixed after TKA.
Although the incidence of perceived LLD decreased after TKA (as mentioned earlier), the decrease seemed to be restricted mostly to patients with preoperative perceived LLD, and the underlying LLD was most probably corrected by the surgery. However, surgery introduced perceived LLD in 6 cases, supporting the notion that it is crucial to understand which patients are at higher risk for postoperative perceived LLD and what if any time frame can be expected for resolution in these cases. In our study, all cases of perceived LLD had resolved by a mean follow-up of 8.5 weeks (range, 3 weeks-3 months). This phenomenon of resolution may be attributed to some of the physical, objective LLD corrections that naturally occur throughout the postoperative course,4 though psychological factors may also be involved. Our study results suggest patients should be counseled that, though about 10% of patients perceive LLD after primary TKA, the vast majority of perceived LLD cases resolve within 3 months.
One study goal was to determine the relationship between the mechanical axis of the knee and perceived LLD both before and after surgery. There were no significant relationships. This was also true when cases of varus and valgus deformity were analyzed separately.
Another study goal was to determine if a surgical change in the mechanical alignment of the knee would influence preoperative-to-postoperative LLD perceptions. In our analysis, patients were divided into 4 groups based on their preoperative and postoperative LLD perceptions (see Methods section). ANOVA revealed no significant differences in absolute values of mechanical axis correction among the 4 groups. Likewise, there were no correlations between BMI and age and mechanical axis correction among the groups, suggesting LLD perception is unrelated to any of these variables. Ideally, if a relationship between a threshold knee alignment value and perceived LLD existed, surgeons would be able to counsel patients at higher risk for perceived LLD about how their knee alignment may contribute to their perception. Unfortunately, our study results did not show any significant statistical relationships in this regard.
The problem of LLD in patients undergoing TKA is not new, and much research is needed to determine the correlation between perceived versus actual discrepancies, and why they occur. Our study results confirmed that TKA corrects most cases of preoperative perceived LLD but introduces perceived LLD in other cases. Whether preoperative or postoperative LLD is merely perceived or is in fact an actual discrepancy remains to be seen.
One limitation of this study was its lack of leg-length measurements. Although we studied knee alignment specifically, it would have been useful to compare perceived LLD with measured leg lengths, either clinically or radiographically, especially since leg lengths obviously play a role in any perceived LLD. We used mechanical alignment as a surrogate for actual LLD because we hypothesized that alignment may contribute to patients’ perceived discrepancies.
Another limitation was the relatively small sample. Only 24 cases of perceived LLD were analyzed. Given our low rates of perceived LLD (25% before surgery, 10% after surgery), it is difficult to study a large enough TKA group to establish a statistically significant number of cases. Nevertheless, investigators may use larger groups to establish more meaningful relationships.
A third limitation was that alignment was measured on the operative side but not the contralateral side. As we were focusing on perceived discrepancy, contralateral knee alignment may play an important role. Our study involved patients with unilateral osteoarthritis, so it would be reasonable to assume the nonoperative knee was almost neutral in alignment in most cases. However, given that varus/valgus misalignment is a known risk factor for osteoarthritis,6 many of our patients with unilateral disease may very well have had preexisting misalignment of both knees. The undetermined alignment of the nonoperative side may be a confounding variable in the relationship between operative knee alignment and perceived LLD.
Fourth, not all patients were surveyed 3 weeks after surgery. Some were first surveyed at 6 weeks, and it is possible there were cases of transient postoperative LLD that resolved before that point. Therefore, our reported incidence of postoperative LLD could have missed some cases. In addition, our mean 8.5-week period for LLD resolution may not have accounted for these resolved cases of transient perceived LLD.
Am J Orthop. 2016;45(7):E429-E433. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
2. Noll DR. Leg length discrepancy and osteoarthritic knee pain in the elderly: an observational study. J Am Osteopath Assoc. 2013;113(9):670-678.
3. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
4. Chang MJ, Kang YG, Chang CB, Seong SC, Kim TK. The patterns of limb length, height, weight and body mass index changes after total knee arthroplasty. J Arthroplasty. 2013;28(10):1856-1861.
5. Lang JE, Scott RD, Lonner JH, Bono JV, Hunter DJ, Li L. Magnitude of limb lengthening after primary total knee arthroplasty. J Arthroplasty. 2012;27(3):341-346.
6. Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940-1945.
1. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
2. Noll DR. Leg length discrepancy and osteoarthritic knee pain in the elderly: an observational study. J Am Osteopath Assoc. 2013;113(9):670-678.
3. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
4. Chang MJ, Kang YG, Chang CB, Seong SC, Kim TK. The patterns of limb length, height, weight and body mass index changes after total knee arthroplasty. J Arthroplasty. 2013;28(10):1856-1861.
5. Lang JE, Scott RD, Lonner JH, Bono JV, Hunter DJ, Li L. Magnitude of limb lengthening after primary total knee arthroplasty. J Arthroplasty. 2012;27(3):341-346.
6. Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940-1945.
VIDEO: Biologics: Proposed guideline addresses perioperative management
WASHINGTON – Biologic agents should be stopped prior to elective total knee or hip arthroplasty in patients with rheumatic diseases, according to a draft guideline developed by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
The guideline, which address the perioperative management of antirheumatic medications in patients with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, juvenile idiopathic arthritis (JIA), or lupus who are undergoing such surgery, is currently under review, Dr. Susan Goodman, MD, coprincipal investigator, reported at the annual meeting of the American College of Rheumatology.
The draft guideline was created because “guidance was needed for common clinical situations, even where data were sparse. We didn’t want to configure treatment mandates – that’s not what these are,” Dr. Goodman of Cornell University, New York, said.
The recommendations are conditional, she said, meaning that the benefits probably outweigh the harms, that the recommendations apply to most but not all patients, and that future research may lead to changes.
“They’re also preference sensitive,” she said, explaining that patients’ values and preferences should be carefully considered, as they might differ from those of the patient panel consulted during guideline development; the panel expressed greater concern about the risk of infection following surgery than about perioperative flares resulting from medication discontinuation.
Based on agreement by at least 80% of a voting panel which considered available evidence in the context of their clinical experience along with the input from the patient panel, the draft guideline states that:
• Current doses of methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine should be continued in patients with rheumatic diseases undergoing elective hip and knee replacement. This recommendation is based on an extensive literature review that showed the infection rate is decreased in patients who continue these medications, Dr. Goodman said.
• All biologics should be withheld prior to surgery in patients with inflammatory arthritis, and surgery should be planned for the end of the dosing cycle. This matter wasn’t specifically addressed in the literature; however, numerous randomized controlled trials outside of the surgical setting demonstrate an increased risk of infection associated with their use, she noted.
“All of the biologic medications were found to be associated with an increased risk of infection,” she said. “Because of this and the level of importance patients place on minimizing infection risk, we’ve recommended that biologics be withheld prior to surgery.”
• Tofacitinib, which was considered in a separate oral, targeted therapy category, should be withheld for at least 7 days prior to surgery in patients with RA, spondyloarthritis, and JIA. Data from systematic reviews and meta-analyses showed an increased risk of infection with tofacitinib, although more research is needed in order to “firm up” this recommendation, Dr. Goodman said.
• In lupus patients, rituximab and belimumab should be withheld prior to surgery, and surgery should be planned for the end of the dosing period.
“Again, this was not answered in the literature. We depended on observational studies that we reviewed that did show that patients with severe active lupus were at much higher risk for adverse events. But since rituximab isn’t approved by the [Food and Drug Administration] for use in lupus, and belimumab isn’t approved for use in severe lupus – and those seem to be the high-risk patients – we thought withholding them was more prudent,” she said.
• Patients with severe lupus should continue on current doses of methotrexate, mycophenolic acid, azathioprine, mizoribine, cyclosporine, and tacrolimus through surgery. This recommendation is based on indirect data from experience in organ transplant patients.
• All medications should be discontinued in patients whose lupus is not severe.
“Our recommendation is to withhold for 7 days to 2-5 days after surgery in the absence of any wound healing complications or any other complications,” she said, noting that the literature does not directly address this; the recommendation is based on indirect evidence in patients with either active infection or who are at risk for infection.
“We thought that careful monitoring of the patient would permit us to identify flare and intervene quickly. … and that, for mild cases of lupus, the morbidity associated with infection might not be greater than the morbidity associated with the disease flare,” she said.
• Biologics should be restarted once surgical wounds show evidence of healing and there is no clinical evidence of infection. The literature does not directly address this; the recommendation is based on the rationale for use of these medications in patients with either active infection or risk for infection.
• Current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with RA, lupus, or inflammatory arthritis. A meta-analysis and systematic review of randomized controlled trial data and observational data showed no hemodynamic difference between daily doses and stress doses.
“In addition, there are abundant observational data demonstrating an increase in infection in patients on chronic steroids greater than 15 mg, and we thought that part of the optimization of the patient would be getting them on the lowest possible steroid dose,” she said, stressing that this refers only to adults receiving glucocorticoids for their rheumatic disease, and not to those with a history of JIA who may have received steroids during development, or to those receiving glucocorticoids for primary, adrenal, or hypothalamic disease.
According to Dr. Goodman, the time is right for the introduction of these recommendations, because the increased use of disease-modifying drugs and biologics means that most patients coming in for these surgeries will be taking these medications.
Further, despite the widespread use of the medications, the rate of total knee and hip arthroplasty surgeries among patients with rheumatic diseases is about the same as it was 20 or 30 years ago – and their risk for devastating complications, including infections, remains high, she said, noting that appropriate medication management provides an opportunity to mitigate risk.
Coprincipal investigator, Bryan Springer, MD, further emphasized the importance of the guideline, noting that the 5-year survival among rheumatic disease patients who develop certain perioperative complications is lower than for many common cancers, and that the literature offers little guidance on managing medications in the perioperative period.
“We now have a document that’s based on the available evidence, and also based on expert opinion, to help us manage these patients much more thoroughly in the perioperative period,” Dr. Springer, an orthopedic surgeon in Charlotte, N.C., said during a press briefing on the guideline.
Dr. Springer highlighted the value of the unique collaboration between the ACR and the AAHKS, calling the effort a win both for patients, and for “collaborative efforts, collaborative research, which we just really don’t do enough of,” he said. “I hope this is a huge step towards that direction.”
This guideline development process was funded by the ACR and AAHKS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Biologic agents should be stopped prior to elective total knee or hip arthroplasty in patients with rheumatic diseases, according to a draft guideline developed by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
The guideline, which address the perioperative management of antirheumatic medications in patients with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, juvenile idiopathic arthritis (JIA), or lupus who are undergoing such surgery, is currently under review, Dr. Susan Goodman, MD, coprincipal investigator, reported at the annual meeting of the American College of Rheumatology.
The draft guideline was created because “guidance was needed for common clinical situations, even where data were sparse. We didn’t want to configure treatment mandates – that’s not what these are,” Dr. Goodman of Cornell University, New York, said.
The recommendations are conditional, she said, meaning that the benefits probably outweigh the harms, that the recommendations apply to most but not all patients, and that future research may lead to changes.
“They’re also preference sensitive,” she said, explaining that patients’ values and preferences should be carefully considered, as they might differ from those of the patient panel consulted during guideline development; the panel expressed greater concern about the risk of infection following surgery than about perioperative flares resulting from medication discontinuation.
Based on agreement by at least 80% of a voting panel which considered available evidence in the context of their clinical experience along with the input from the patient panel, the draft guideline states that:
• Current doses of methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine should be continued in patients with rheumatic diseases undergoing elective hip and knee replacement. This recommendation is based on an extensive literature review that showed the infection rate is decreased in patients who continue these medications, Dr. Goodman said.
• All biologics should be withheld prior to surgery in patients with inflammatory arthritis, and surgery should be planned for the end of the dosing cycle. This matter wasn’t specifically addressed in the literature; however, numerous randomized controlled trials outside of the surgical setting demonstrate an increased risk of infection associated with their use, she noted.
“All of the biologic medications were found to be associated with an increased risk of infection,” she said. “Because of this and the level of importance patients place on minimizing infection risk, we’ve recommended that biologics be withheld prior to surgery.”
• Tofacitinib, which was considered in a separate oral, targeted therapy category, should be withheld for at least 7 days prior to surgery in patients with RA, spondyloarthritis, and JIA. Data from systematic reviews and meta-analyses showed an increased risk of infection with tofacitinib, although more research is needed in order to “firm up” this recommendation, Dr. Goodman said.
• In lupus patients, rituximab and belimumab should be withheld prior to surgery, and surgery should be planned for the end of the dosing period.
“Again, this was not answered in the literature. We depended on observational studies that we reviewed that did show that patients with severe active lupus were at much higher risk for adverse events. But since rituximab isn’t approved by the [Food and Drug Administration] for use in lupus, and belimumab isn’t approved for use in severe lupus – and those seem to be the high-risk patients – we thought withholding them was more prudent,” she said.
• Patients with severe lupus should continue on current doses of methotrexate, mycophenolic acid, azathioprine, mizoribine, cyclosporine, and tacrolimus through surgery. This recommendation is based on indirect data from experience in organ transplant patients.
• All medications should be discontinued in patients whose lupus is not severe.
“Our recommendation is to withhold for 7 days to 2-5 days after surgery in the absence of any wound healing complications or any other complications,” she said, noting that the literature does not directly address this; the recommendation is based on indirect evidence in patients with either active infection or who are at risk for infection.
“We thought that careful monitoring of the patient would permit us to identify flare and intervene quickly. … and that, for mild cases of lupus, the morbidity associated with infection might not be greater than the morbidity associated with the disease flare,” she said.
• Biologics should be restarted once surgical wounds show evidence of healing and there is no clinical evidence of infection. The literature does not directly address this; the recommendation is based on the rationale for use of these medications in patients with either active infection or risk for infection.
• Current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with RA, lupus, or inflammatory arthritis. A meta-analysis and systematic review of randomized controlled trial data and observational data showed no hemodynamic difference between daily doses and stress doses.
“In addition, there are abundant observational data demonstrating an increase in infection in patients on chronic steroids greater than 15 mg, and we thought that part of the optimization of the patient would be getting them on the lowest possible steroid dose,” she said, stressing that this refers only to adults receiving glucocorticoids for their rheumatic disease, and not to those with a history of JIA who may have received steroids during development, or to those receiving glucocorticoids for primary, adrenal, or hypothalamic disease.
According to Dr. Goodman, the time is right for the introduction of these recommendations, because the increased use of disease-modifying drugs and biologics means that most patients coming in for these surgeries will be taking these medications.
Further, despite the widespread use of the medications, the rate of total knee and hip arthroplasty surgeries among patients with rheumatic diseases is about the same as it was 20 or 30 years ago – and their risk for devastating complications, including infections, remains high, she said, noting that appropriate medication management provides an opportunity to mitigate risk.
Coprincipal investigator, Bryan Springer, MD, further emphasized the importance of the guideline, noting that the 5-year survival among rheumatic disease patients who develop certain perioperative complications is lower than for many common cancers, and that the literature offers little guidance on managing medications in the perioperative period.
“We now have a document that’s based on the available evidence, and also based on expert opinion, to help us manage these patients much more thoroughly in the perioperative period,” Dr. Springer, an orthopedic surgeon in Charlotte, N.C., said during a press briefing on the guideline.
Dr. Springer highlighted the value of the unique collaboration between the ACR and the AAHKS, calling the effort a win both for patients, and for “collaborative efforts, collaborative research, which we just really don’t do enough of,” he said. “I hope this is a huge step towards that direction.”
This guideline development process was funded by the ACR and AAHKS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Biologic agents should be stopped prior to elective total knee or hip arthroplasty in patients with rheumatic diseases, according to a draft guideline developed by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
The guideline, which address the perioperative management of antirheumatic medications in patients with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, juvenile idiopathic arthritis (JIA), or lupus who are undergoing such surgery, is currently under review, Dr. Susan Goodman, MD, coprincipal investigator, reported at the annual meeting of the American College of Rheumatology.
The draft guideline was created because “guidance was needed for common clinical situations, even where data were sparse. We didn’t want to configure treatment mandates – that’s not what these are,” Dr. Goodman of Cornell University, New York, said.
The recommendations are conditional, she said, meaning that the benefits probably outweigh the harms, that the recommendations apply to most but not all patients, and that future research may lead to changes.
“They’re also preference sensitive,” she said, explaining that patients’ values and preferences should be carefully considered, as they might differ from those of the patient panel consulted during guideline development; the panel expressed greater concern about the risk of infection following surgery than about perioperative flares resulting from medication discontinuation.
Based on agreement by at least 80% of a voting panel which considered available evidence in the context of their clinical experience along with the input from the patient panel, the draft guideline states that:
• Current doses of methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine should be continued in patients with rheumatic diseases undergoing elective hip and knee replacement. This recommendation is based on an extensive literature review that showed the infection rate is decreased in patients who continue these medications, Dr. Goodman said.
• All biologics should be withheld prior to surgery in patients with inflammatory arthritis, and surgery should be planned for the end of the dosing cycle. This matter wasn’t specifically addressed in the literature; however, numerous randomized controlled trials outside of the surgical setting demonstrate an increased risk of infection associated with their use, she noted.
“All of the biologic medications were found to be associated with an increased risk of infection,” she said. “Because of this and the level of importance patients place on minimizing infection risk, we’ve recommended that biologics be withheld prior to surgery.”
• Tofacitinib, which was considered in a separate oral, targeted therapy category, should be withheld for at least 7 days prior to surgery in patients with RA, spondyloarthritis, and JIA. Data from systematic reviews and meta-analyses showed an increased risk of infection with tofacitinib, although more research is needed in order to “firm up” this recommendation, Dr. Goodman said.
• In lupus patients, rituximab and belimumab should be withheld prior to surgery, and surgery should be planned for the end of the dosing period.
“Again, this was not answered in the literature. We depended on observational studies that we reviewed that did show that patients with severe active lupus were at much higher risk for adverse events. But since rituximab isn’t approved by the [Food and Drug Administration] for use in lupus, and belimumab isn’t approved for use in severe lupus – and those seem to be the high-risk patients – we thought withholding them was more prudent,” she said.
• Patients with severe lupus should continue on current doses of methotrexate, mycophenolic acid, azathioprine, mizoribine, cyclosporine, and tacrolimus through surgery. This recommendation is based on indirect data from experience in organ transplant patients.
• All medications should be discontinued in patients whose lupus is not severe.
“Our recommendation is to withhold for 7 days to 2-5 days after surgery in the absence of any wound healing complications or any other complications,” she said, noting that the literature does not directly address this; the recommendation is based on indirect evidence in patients with either active infection or who are at risk for infection.
“We thought that careful monitoring of the patient would permit us to identify flare and intervene quickly. … and that, for mild cases of lupus, the morbidity associated with infection might not be greater than the morbidity associated with the disease flare,” she said.
• Biologics should be restarted once surgical wounds show evidence of healing and there is no clinical evidence of infection. The literature does not directly address this; the recommendation is based on the rationale for use of these medications in patients with either active infection or risk for infection.
• Current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with RA, lupus, or inflammatory arthritis. A meta-analysis and systematic review of randomized controlled trial data and observational data showed no hemodynamic difference between daily doses and stress doses.
“In addition, there are abundant observational data demonstrating an increase in infection in patients on chronic steroids greater than 15 mg, and we thought that part of the optimization of the patient would be getting them on the lowest possible steroid dose,” she said, stressing that this refers only to adults receiving glucocorticoids for their rheumatic disease, and not to those with a history of JIA who may have received steroids during development, or to those receiving glucocorticoids for primary, adrenal, or hypothalamic disease.
According to Dr. Goodman, the time is right for the introduction of these recommendations, because the increased use of disease-modifying drugs and biologics means that most patients coming in for these surgeries will be taking these medications.
Further, despite the widespread use of the medications, the rate of total knee and hip arthroplasty surgeries among patients with rheumatic diseases is about the same as it was 20 or 30 years ago – and their risk for devastating complications, including infections, remains high, she said, noting that appropriate medication management provides an opportunity to mitigate risk.
Coprincipal investigator, Bryan Springer, MD, further emphasized the importance of the guideline, noting that the 5-year survival among rheumatic disease patients who develop certain perioperative complications is lower than for many common cancers, and that the literature offers little guidance on managing medications in the perioperative period.
“We now have a document that’s based on the available evidence, and also based on expert opinion, to help us manage these patients much more thoroughly in the perioperative period,” Dr. Springer, an orthopedic surgeon in Charlotte, N.C., said during a press briefing on the guideline.
Dr. Springer highlighted the value of the unique collaboration between the ACR and the AAHKS, calling the effort a win both for patients, and for “collaborative efforts, collaborative research, which we just really don’t do enough of,” he said. “I hope this is a huge step towards that direction.”
This guideline development process was funded by the ACR and AAHKS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING