User login
The Role of Medial Patellofemoral Ligament Repair and Imbrication
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
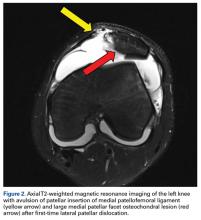
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
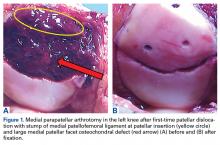
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
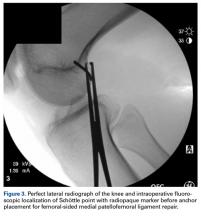
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
Patellofemoral Pain: An Enigma Explained by Homeostasis and Common Sense
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
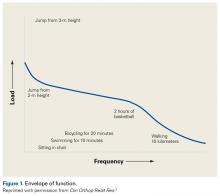
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
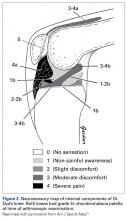
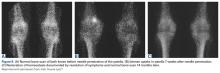
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
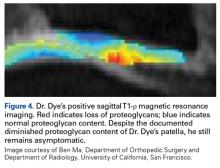
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
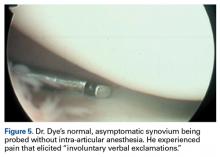
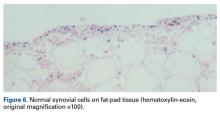
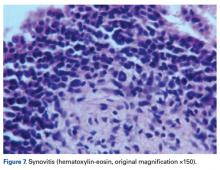
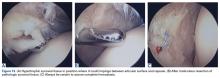
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
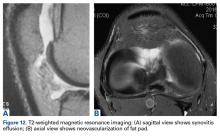
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
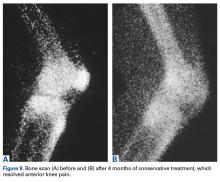
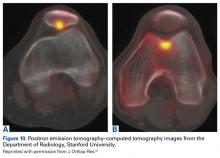
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
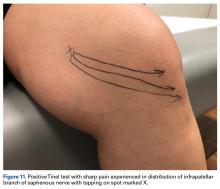
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
A Practical Guide to Understanding and Treating Patellofemoral Pain
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
Robotic-Assisted Total Knee Arthroplasty
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Antibiotic prophylaxis for artificial joints
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
Perioperative infliximab does not increase serious infection risk
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Subjects on glucocorticoids had an OR of 2.11 (95% CI 1.30-3.40) for serious infection within 30 days and an HR of 2.70 (95% CI 1.30-5.60) for prosthetic joint infection within 1 year.
Data source: Retrospective cohort study of 4,288 elective knee and hip arthroplasties in Medicare patients with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis during 2007-2013.
Disclosures: The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Rates of Deep Vein Thrombosis Occurring After Osteotomy About the Knee
Take-Home Points
- DVT and PE are uncommon complications following osteotomies about the knee.
- Use of oral contraceptives can increase the risk of a patient sustaining a postoperative DVT and PE following osteotomies about the knee.
- In the absence of significant risk factors, postoperative chemical DVT prophylaxis may be unnecessary in patients undergoing osteotomies about the knee.
High tibial osteotomy (HTO), distal femoral osteotomy (DFO), and tibial tubercle osteotomy (TTO) are viable treatment options for deformities about the knee and patella maltracking.1-4 Although TTO can be performed in many ways (eg, anteriorization, anteromedialization, medialization), the basic idea is to move the tibial tubercle to improve patellar tracking or to offload a patellar facet that has sustained trauma or degenerated.2 DFO is a surgical option for treating a valgus knee deformity (the lateral tibiofemoral compartment is offloaded) or for protecting a knee compartment after cartilage or meniscal restoration (medial closing wedge or lateral opening wedge).1 Similarly, HTO is an option for treating a varus knee deformity or isolated medial compartment arthritis; the diseased compartment is offloaded, and any malalignment is corrected. Akin to DFO, HTO is often performed to protect a knee compartment, typically the medial tibiofemoral compartment, after cartilage or meniscal restoration.2-4
Compared to most arthroscopic knee surgeries, these osteotomies are much more involved, have longer operative times, and restrict postoperative weight-bearing and range of motion.2-4 The rates of deep vein thrombosis (DVT) and pulmonary embolism (PE) after these osteotomies are not well documented. In addition, there is no documentation of the risks in patients who smoke, are obese, or are using oral contraceptives (OCs) at time of surgery, despite the increased DVT and PE risks posed by smoking, obesity, and OC use in other surgical procedures.5-7 Although the American Academy of Orthopaedic Surgeons (AAOS) issued clinical practice guidelines for DVT/PE prophylaxis after hip and knee arthroplasty, there is no standard prophylaxis guidelines for DVT/PE prevention after HTO, DFO, or TTO.8,9 Last, rates of DVT after total knee arthroplasty (TKA) are well defined; they range from 2% to 12%.10,11 These rates may be surrogates for osteotomies about the knee, but this is only conjecture.
We conducted a study to determine the rates of symptomatic DVT and PE after HTO, DFO, or TTO in patients who did not receive postoperative DVT/PE prophylaxis. We also wanted to determine if age, body mass index (BMI), and smoking status have associations with the risk of developing either DVT or PE after HTO, DFO, or TTO. We hypothesized that the DVT and PE rates would both be <1%.
Methods
After this study was approved by our university’s Institutional Review Board, we searched the surgical database of Dr. Cole, a sports medicine fellowship–trained surgeon, to identify all patients who had HTO, DFO, or TTO performed between September 1, 2009 and September 30, 2014. Current Procedural Terminology (CPT) codes were used for the search. The code for HTO was 27457: osteotomy, proximal tibia, including fibular excision or osteotomy (includes correction of genu varus [bowleg] or genu valgus [knock-knee]); after epiphyseal closure). The code for DFO was 27450: osteotomy, femur, shaft or supracondylar; with fixation. Last, the code for TTO was 27418: anterior tibial tubercleplasty (eg, Maquet-type procedure). The 141 patients identified in the search were treated by Dr. Cole at a single institution and were included in the study. Study inclusion did not require a minimum follow-up. Follow-up duration was defined as the time between surgery and the final clinic note in the patient chart. No patient was excluded for lack of follow-up clinic visits, and none was lost to follow-up.
Age, BMI, smoking status, and OC use were recorded for all patients. For each procedure, the surgeon’s technique remained the same throughout the study period: HTO, medial opening-wedge osteotomy with plate-and-screw fixation; DFO, lateral opening-wedge osteotomy with plate-and-screw fixation; and TTO, mostly anteromedialization with screw fixation (though this was dictated by patellar contact pressures). A tourniquet was used in all cases. Each patient’s hospital electronic medical record and outpatient office notes were reviewed to determine if symptomatic DVT or PE developed after surgery. The diagnosis of symptomatic DVT was based on clinical symptoms and confirmatory ultrasound, and the PE diagnosis was based on computed tomography. Doppler ultrasound was performed only in symptomatic patients (ie, it was not routinely performed).
Per surgeon protocol, postoperative DVT prophylaxis was not administered. Patients were encouraged to begin dorsiflexion and plantar flexion of the ankle (ankle pumps) immediately and to mobilize as soon as comfortable. Each patient received a cold therapy machine with compression sleeve. Patients were allowed toe-touch weight-bearing for 6 weeks, and then progressed 25% per week for 4 weeks to full weight-bearing by 10 weeks. After surgery, each patient was placed in a brace, which was kept locked in extension for 10 days; when the brace was unlocked, the patient was allowed to range the knee.
Continuous variable data are reported as weighted means and weighted standard deviations. Categorical variable data are reported as frequencies and percentages.
Results
Our database search identified 141 patients (44% male, 56% female) who underwent HTO (47 patients, 33.3%), DFO (13 patients, 9.2%), or TTO (81 patients, 57.5%). Mean (SD) age was 34.28 (9.86) years, mean (SD) BMI was 26.88 (5.11) kg/m2, and mean (SD) follow-up was 17.1 (4.1) months. Of the female patients, 36.7% were using OCs at time of surgery. Of all patients, 13.48% were smokers.
Two patients (1.42%) had clinical symptoms consistent with DVT. In each case, the diagnosis was confirmed with Doppler ultrasound. The below-knee DVT was unilateral in 1 case and bilateral in the other.
The unilateral DVT occurred in a patient who underwent anteromedialization of the tibial tubercle and osteochondral allograft transfer to the lateral femoral condyle for patellar maltracking and a focal trochlear defect. The DVT was diagnosed 8 days after surgery and was treated with warfarin. Low-molecular-weight heparin (LMWH) was used as a bridge until the warfarin level was therapeutic (4 days). This male patient had no significant medical history.
The bilateral DVT with PE occurred in a patient who underwent a medial opening-wedge HTO for a varus deformity with right medial compartment osteoarthritis and a meniscal tear. The DVT and PE were diagnosed 48 hours after surgery, when the patient complained of lightheadedness and lost consciousness. She had no medical problems but was using OCs at time of surgery. The patient died 3 days after surgery and subsequently was found to have a maternal-side family history of DVT (the patient and her family physician had been unaware of this history).
Discussion
As the rates of DVT and PE after osteotomies about the knee have not been well studied, we wanted to determine these rates after HTO, DFO, and TTO in patients who did not receive postoperative DVT prophylaxis. We hypothesized that DVT and PE rates would both be <1%, and this hypothesis was partly confirmed: The rate of PE after HTO, DFO, and TTO was <1%, and the rate of symptomatic DVT was >1%. Similarly, the patients who developed these complications were nonsmokers and had a BMI no higher than that of the patients who did not develop DVT or PE. In addition, only 1 patient developed DVT and PE, and she was using OCs and had a family history of DVT. Last, the patients who developed these complications were on average 14 years older than the patients who did not develop DVT or PE.
Although there is a plethora of reports on the incidence of DVT and PE after TKA, there is little on the incidence after osteotomies about the knee.8,12 The rate of DVT after TKA varies, but many studies place it between 2% and 12%, and routinely find a PE rate of <0.5%.10,11,13,14 Although the AAOS issued a clinical practice guideline for postoperative DVT prophylaxis after TKA, and evaluated the best available evidence, it could not reach consensus on a specific type of DVT prophylaxis, though the workgroup did recommend that patients be administered postoperative DVT prophylaxis of some kind.8,9 Similarly, the American College of Chest Physicians (ACCP) issued clinical practice guidelines for preventing DVT and PE after elective TKA and total hip arthroplasty.15 According to the ACCP guidelines, patients should receive prophylaxis—LMWH, fondaparinux, apixaban, dabigatran, rivaroxaban, low-dose unfractionated heparin, adjusted-dose vitamin K antagonist, aspirin, or an intermittent pneumatic compression device—for a minimum of 14 days. Unfortunately, though there are similarities between TKAs and peri-knee osteotomies, these procedures are markedly different, and it is difficult to extrapolate and adapt recommendations and produce a consensus statement for knee arthroplasties. In addition, guidelines exist for hospitalized patients who are being treated for medical conditions or have undergone surgery, but all the patients in the present study had their osteotomies performed on an outpatient basis.
Martin and colleagues16 reviewed 323 cases of medial opening-wedge HTO and found a DVT rate of 1.4% in the absence of routine DVT prophylaxis, except in patients with a history of DVT. Their rate is almost identical to ours, but we also included other osteotomies in our study. Miller and colleagues17 reviewed 46 cases of medial opening-wedge HTO and found a 4.3% DVT rate, despite routine prophylaxis with once-daily 325-mg aspirin and ankle pumps. This finding contrasts with our 1.42% DVT rate in the absence of postoperative chemical DVT prophylaxis. Motycka and colleagues18 reviewed 65 HTO cases in which DVT prophylaxis (oral anticoagulant) was given for 6 weeks, and they found a DVT rate of 9.7%. Turner and colleagues19 performed venous ultrasound on 81 consecutive patients who underwent HTO and received DVT prophylaxis (twice-daily subcutaneous heparin), and they found a DVT rate of 41% and a PE rate of 1.2%, though only 8.6% of the DVT cases were symptomatic. Of note, whereas the lowest postoperative DVT rate was for patients who did not receive postoperative DVT prophylaxis, the rate of symptomatic DVT after these osteotomies ranged from 1.4% to 8.6% in patients who received prophylaxis.16,19 Given this evidence and our study results, it appears routine chemical DVT prophylaxis after osteotomies about the knee may not be necessary, though higher level evidence is needed in order to make definitive recommendations.
In the present study, the 2 patients who developed symptomatic DVT (1 subsequently developed PE) were nonsmokers in good health. The female patient (DVT plus PE) was using OCs at time of surgery. Studies have shown that patients who smoke and who use OCs are at increased risk for developing DVT or PE after surgery.5,6,12 Given that only 2 of our patients developed DVT/PE, and neither was a smoker, smoking was not associated with increased DVT or PE risk in this study population, in which 13.48% of patients were smokers at time of surgery. In addition, given that the 1 female patient who developed DVT/PE was using OCs and that 36.7% of all female patients in the study were using OCs, it is difficult to conclude whether OC use increased the female patient’s risk for DVT or PE. Furthermore, neither the literature nor the AAOS consensus statement supports discontinuing OCs for this surgical procedure.
Patients in this study did not receive chemical or mechanical DVT prophylaxis after surgery. Regarding various post-TKA DVT prophylaxis regimens, aspirin is as effective as LMWH in preventing DVT, and the risk for postoperative blood loss and wound complications is lower with aspirin than with rivaroxaban.20,21 Given that the present study’s postoperative rates of DVT (1.42%) and PE (0.71%) are equal to or less than rates already reported in the literature, routine DVT prophylaxis after osteotomies about the knee may be unnecessary in the absence of other significant risk factors.16,19 However, our study considered only symptomatic DVT and PE, so it is possible that the number of asymptomatic DVT cases is higher in this patient population. Definitively answering our study’s clinical question will require a multicenter registry study (prospective cohort study).
Study Limitations
The strengths of this study include the large number of patients treated by a single surgeon using the same postoperative protocol. Limitations of this study include the lack of a control group. Although we found a DVT rate of 1.42% and a PE rate of 0.71%, the literature on the accepted risks for DVT and PE after HTO, DFO, and TTO is unclear. With our results stratified by procedure, the DVT rate was 2% in the HTO group, 0% in the DFO group, and 1% in the TTO group. However, we were unable to reliably stratify these results by each specific procedure, as the number of patients in each group would be too low. This study involved reviewing charts; as patients were not contacted, it is possible a patient developed DVT or PE, was treated at an outside facility, and then never followed up with the treating surgeon. Patients were identified by CPT codes, so, if a patient underwent HTO, DFO, or TTO that was recorded under a different CPT code, it is possible the patient was missed by our search. All patients were seen after surgery, and we reviewed the outpatient office notes that were taken, so unless the DVT or PE occurred after a patient’s final postoperative visit, it would have been recorded. Similarly, the DVT and PE rates reported here cannot be extrapolated to overall risks for DVT and PE after osteotomies about the knee in all patients—only in patients who did not receive DVT prophylaxis after surgery.
Conclusion
The rates of DVT and PE after HTO, DFO, and TTO in patients who did not receive chemical prophylaxis are low: 1.42% and 0.71%, respectively. After these osteotomies, DVT/PE prophylaxis in the absence of known risk factors may not be warranted.
Am J Orthop. 2017;46(1):E23-E27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Rossi R, Bonasia DE, Amendola A. The role of high tibial osteotomy in the varus knee. J Am Acad Orthop Surg. 2011;19(10):590-599.
2. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
3. Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13(4):279-289.
4. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015.
5. Ng WM, Chan KY, Lim AB, Gan EC. The incidence of deep venous thrombosis following arthroscopic knee surgery. Med J Malaysia. 2005;60(suppl C):14-16.
6. Platzer P, Thalhammer G, Jaindl M, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77(5):755-760.
7. Wallace G, Judge A, Prieto-Alhambra D, de Vries F, Arden NK, Cooper C. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage. 2014;22(7):918-927.
8. Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(19):1801-1811.
9. Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19(12):777-778.
10. Kim YH, Kulkarni SS, Park JW, Kim JS. Prevalence of deep vein thrombosis and pulmonary embolism treated with mechanical compression device after total knee arthroplasty in Asian patients. J Arthroplasty. 2015;30(9):1633-1637.
11. Kim YH, Yoo JH, Kim JS. Factors leading to decreased rates of deep vein thrombosis and pulmonary embolism after total knee arthroplasty. J Arthroplasty. 2007;22(7):974-980.
12. Raphael IJ, Tischler EH, Huang R, Rothman RH, Hozack WJ, Parvizi J. Aspirin: an alternative for pulmonary embolism prophylaxis after arthroplasty? Clin Orthop Relat Res. 2014;472(2):482-488.
13. Won MH, Lee GW, Lee TJ, Moon KH. Prevalence and risk factors of thromboembolism after joint arthroplasty without chemical thromboprophylaxis in an Asian population. J Arthroplasty. 2011;26(7):1106-1111.
14. Bozic KJ, Vail TP, Pekow PS, Maselli JH, Lindenauer PK, Auerbach AD. Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty. 2010;25(7):1053-1060.
15. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
16. Martin R, Birmingham TB, Willits K, Litchfield R, Lebel ME, Giffin JR. Adverse event rates and classifications in medial opening wedge high tibial osteotomy. Am J Sports Med. 2014;42(5):1118-1126.
17. Miller BS, Downie B, McDonough EB, Wojtys EM. Complications after medial opening wedge high tibial osteotomy. Arthroscopy. 2009;25(6):639-646.
18. Motycka T, Eggerth G, Landsiedl F. The incidence of thrombosis in high tibial osteotomies with and without the use of a tourniquet. Arch Orthop Trauma Surg. 2000;120(3-4):157-159.
19. Turner RS, Griffiths H, Heatley FW. The incidence of deep-vein thrombosis after upper tibial osteotomy. A venographic study. J Bone Joint Surg Br. 1993;75(6):942-944.
20. Jiang Y, Du H, Liu J, Zhou Y. Aspirin combined with mechanical measures to prevent venous thromboembolism after total knee arthroplasty: a randomized controlled trial. Chin Med J (Engl). 2014;127(12):2201-2205.
21. Zou Y, Tian S, Wang Y, Sun K. Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis. 2014;25(7):660-664.
Take-Home Points
- DVT and PE are uncommon complications following osteotomies about the knee.
- Use of oral contraceptives can increase the risk of a patient sustaining a postoperative DVT and PE following osteotomies about the knee.
- In the absence of significant risk factors, postoperative chemical DVT prophylaxis may be unnecessary in patients undergoing osteotomies about the knee.
High tibial osteotomy (HTO), distal femoral osteotomy (DFO), and tibial tubercle osteotomy (TTO) are viable treatment options for deformities about the knee and patella maltracking.1-4 Although TTO can be performed in many ways (eg, anteriorization, anteromedialization, medialization), the basic idea is to move the tibial tubercle to improve patellar tracking or to offload a patellar facet that has sustained trauma or degenerated.2 DFO is a surgical option for treating a valgus knee deformity (the lateral tibiofemoral compartment is offloaded) or for protecting a knee compartment after cartilage or meniscal restoration (medial closing wedge or lateral opening wedge).1 Similarly, HTO is an option for treating a varus knee deformity or isolated medial compartment arthritis; the diseased compartment is offloaded, and any malalignment is corrected. Akin to DFO, HTO is often performed to protect a knee compartment, typically the medial tibiofemoral compartment, after cartilage or meniscal restoration.2-4
Compared to most arthroscopic knee surgeries, these osteotomies are much more involved, have longer operative times, and restrict postoperative weight-bearing and range of motion.2-4 The rates of deep vein thrombosis (DVT) and pulmonary embolism (PE) after these osteotomies are not well documented. In addition, there is no documentation of the risks in patients who smoke, are obese, or are using oral contraceptives (OCs) at time of surgery, despite the increased DVT and PE risks posed by smoking, obesity, and OC use in other surgical procedures.5-7 Although the American Academy of Orthopaedic Surgeons (AAOS) issued clinical practice guidelines for DVT/PE prophylaxis after hip and knee arthroplasty, there is no standard prophylaxis guidelines for DVT/PE prevention after HTO, DFO, or TTO.8,9 Last, rates of DVT after total knee arthroplasty (TKA) are well defined; they range from 2% to 12%.10,11 These rates may be surrogates for osteotomies about the knee, but this is only conjecture.
We conducted a study to determine the rates of symptomatic DVT and PE after HTO, DFO, or TTO in patients who did not receive postoperative DVT/PE prophylaxis. We also wanted to determine if age, body mass index (BMI), and smoking status have associations with the risk of developing either DVT or PE after HTO, DFO, or TTO. We hypothesized that the DVT and PE rates would both be <1%.
Methods
After this study was approved by our university’s Institutional Review Board, we searched the surgical database of Dr. Cole, a sports medicine fellowship–trained surgeon, to identify all patients who had HTO, DFO, or TTO performed between September 1, 2009 and September 30, 2014. Current Procedural Terminology (CPT) codes were used for the search. The code for HTO was 27457: osteotomy, proximal tibia, including fibular excision or osteotomy (includes correction of genu varus [bowleg] or genu valgus [knock-knee]); after epiphyseal closure). The code for DFO was 27450: osteotomy, femur, shaft or supracondylar; with fixation. Last, the code for TTO was 27418: anterior tibial tubercleplasty (eg, Maquet-type procedure). The 141 patients identified in the search were treated by Dr. Cole at a single institution and were included in the study. Study inclusion did not require a minimum follow-up. Follow-up duration was defined as the time between surgery and the final clinic note in the patient chart. No patient was excluded for lack of follow-up clinic visits, and none was lost to follow-up.
Age, BMI, smoking status, and OC use were recorded for all patients. For each procedure, the surgeon’s technique remained the same throughout the study period: HTO, medial opening-wedge osteotomy with plate-and-screw fixation; DFO, lateral opening-wedge osteotomy with plate-and-screw fixation; and TTO, mostly anteromedialization with screw fixation (though this was dictated by patellar contact pressures). A tourniquet was used in all cases. Each patient’s hospital electronic medical record and outpatient office notes were reviewed to determine if symptomatic DVT or PE developed after surgery. The diagnosis of symptomatic DVT was based on clinical symptoms and confirmatory ultrasound, and the PE diagnosis was based on computed tomography. Doppler ultrasound was performed only in symptomatic patients (ie, it was not routinely performed).
Per surgeon protocol, postoperative DVT prophylaxis was not administered. Patients were encouraged to begin dorsiflexion and plantar flexion of the ankle (ankle pumps) immediately and to mobilize as soon as comfortable. Each patient received a cold therapy machine with compression sleeve. Patients were allowed toe-touch weight-bearing for 6 weeks, and then progressed 25% per week for 4 weeks to full weight-bearing by 10 weeks. After surgery, each patient was placed in a brace, which was kept locked in extension for 10 days; when the brace was unlocked, the patient was allowed to range the knee.
Continuous variable data are reported as weighted means and weighted standard deviations. Categorical variable data are reported as frequencies and percentages.
Results
Our database search identified 141 patients (44% male, 56% female) who underwent HTO (47 patients, 33.3%), DFO (13 patients, 9.2%), or TTO (81 patients, 57.5%). Mean (SD) age was 34.28 (9.86) years, mean (SD) BMI was 26.88 (5.11) kg/m2, and mean (SD) follow-up was 17.1 (4.1) months. Of the female patients, 36.7% were using OCs at time of surgery. Of all patients, 13.48% were smokers.
Two patients (1.42%) had clinical symptoms consistent with DVT. In each case, the diagnosis was confirmed with Doppler ultrasound. The below-knee DVT was unilateral in 1 case and bilateral in the other.
The unilateral DVT occurred in a patient who underwent anteromedialization of the tibial tubercle and osteochondral allograft transfer to the lateral femoral condyle for patellar maltracking and a focal trochlear defect. The DVT was diagnosed 8 days after surgery and was treated with warfarin. Low-molecular-weight heparin (LMWH) was used as a bridge until the warfarin level was therapeutic (4 days). This male patient had no significant medical history.
The bilateral DVT with PE occurred in a patient who underwent a medial opening-wedge HTO for a varus deformity with right medial compartment osteoarthritis and a meniscal tear. The DVT and PE were diagnosed 48 hours after surgery, when the patient complained of lightheadedness and lost consciousness. She had no medical problems but was using OCs at time of surgery. The patient died 3 days after surgery and subsequently was found to have a maternal-side family history of DVT (the patient and her family physician had been unaware of this history).
Discussion
As the rates of DVT and PE after osteotomies about the knee have not been well studied, we wanted to determine these rates after HTO, DFO, and TTO in patients who did not receive postoperative DVT prophylaxis. We hypothesized that DVT and PE rates would both be <1%, and this hypothesis was partly confirmed: The rate of PE after HTO, DFO, and TTO was <1%, and the rate of symptomatic DVT was >1%. Similarly, the patients who developed these complications were nonsmokers and had a BMI no higher than that of the patients who did not develop DVT or PE. In addition, only 1 patient developed DVT and PE, and she was using OCs and had a family history of DVT. Last, the patients who developed these complications were on average 14 years older than the patients who did not develop DVT or PE.
Although there is a plethora of reports on the incidence of DVT and PE after TKA, there is little on the incidence after osteotomies about the knee.8,12 The rate of DVT after TKA varies, but many studies place it between 2% and 12%, and routinely find a PE rate of <0.5%.10,11,13,14 Although the AAOS issued a clinical practice guideline for postoperative DVT prophylaxis after TKA, and evaluated the best available evidence, it could not reach consensus on a specific type of DVT prophylaxis, though the workgroup did recommend that patients be administered postoperative DVT prophylaxis of some kind.8,9 Similarly, the American College of Chest Physicians (ACCP) issued clinical practice guidelines for preventing DVT and PE after elective TKA and total hip arthroplasty.15 According to the ACCP guidelines, patients should receive prophylaxis—LMWH, fondaparinux, apixaban, dabigatran, rivaroxaban, low-dose unfractionated heparin, adjusted-dose vitamin K antagonist, aspirin, or an intermittent pneumatic compression device—for a minimum of 14 days. Unfortunately, though there are similarities between TKAs and peri-knee osteotomies, these procedures are markedly different, and it is difficult to extrapolate and adapt recommendations and produce a consensus statement for knee arthroplasties. In addition, guidelines exist for hospitalized patients who are being treated for medical conditions or have undergone surgery, but all the patients in the present study had their osteotomies performed on an outpatient basis.
Martin and colleagues16 reviewed 323 cases of medial opening-wedge HTO and found a DVT rate of 1.4% in the absence of routine DVT prophylaxis, except in patients with a history of DVT. Their rate is almost identical to ours, but we also included other osteotomies in our study. Miller and colleagues17 reviewed 46 cases of medial opening-wedge HTO and found a 4.3% DVT rate, despite routine prophylaxis with once-daily 325-mg aspirin and ankle pumps. This finding contrasts with our 1.42% DVT rate in the absence of postoperative chemical DVT prophylaxis. Motycka and colleagues18 reviewed 65 HTO cases in which DVT prophylaxis (oral anticoagulant) was given for 6 weeks, and they found a DVT rate of 9.7%. Turner and colleagues19 performed venous ultrasound on 81 consecutive patients who underwent HTO and received DVT prophylaxis (twice-daily subcutaneous heparin), and they found a DVT rate of 41% and a PE rate of 1.2%, though only 8.6% of the DVT cases were symptomatic. Of note, whereas the lowest postoperative DVT rate was for patients who did not receive postoperative DVT prophylaxis, the rate of symptomatic DVT after these osteotomies ranged from 1.4% to 8.6% in patients who received prophylaxis.16,19 Given this evidence and our study results, it appears routine chemical DVT prophylaxis after osteotomies about the knee may not be necessary, though higher level evidence is needed in order to make definitive recommendations.
In the present study, the 2 patients who developed symptomatic DVT (1 subsequently developed PE) were nonsmokers in good health. The female patient (DVT plus PE) was using OCs at time of surgery. Studies have shown that patients who smoke and who use OCs are at increased risk for developing DVT or PE after surgery.5,6,12 Given that only 2 of our patients developed DVT/PE, and neither was a smoker, smoking was not associated with increased DVT or PE risk in this study population, in which 13.48% of patients were smokers at time of surgery. In addition, given that the 1 female patient who developed DVT/PE was using OCs and that 36.7% of all female patients in the study were using OCs, it is difficult to conclude whether OC use increased the female patient’s risk for DVT or PE. Furthermore, neither the literature nor the AAOS consensus statement supports discontinuing OCs for this surgical procedure.
Patients in this study did not receive chemical or mechanical DVT prophylaxis after surgery. Regarding various post-TKA DVT prophylaxis regimens, aspirin is as effective as LMWH in preventing DVT, and the risk for postoperative blood loss and wound complications is lower with aspirin than with rivaroxaban.20,21 Given that the present study’s postoperative rates of DVT (1.42%) and PE (0.71%) are equal to or less than rates already reported in the literature, routine DVT prophylaxis after osteotomies about the knee may be unnecessary in the absence of other significant risk factors.16,19 However, our study considered only symptomatic DVT and PE, so it is possible that the number of asymptomatic DVT cases is higher in this patient population. Definitively answering our study’s clinical question will require a multicenter registry study (prospective cohort study).
Study Limitations
The strengths of this study include the large number of patients treated by a single surgeon using the same postoperative protocol. Limitations of this study include the lack of a control group. Although we found a DVT rate of 1.42% and a PE rate of 0.71%, the literature on the accepted risks for DVT and PE after HTO, DFO, and TTO is unclear. With our results stratified by procedure, the DVT rate was 2% in the HTO group, 0% in the DFO group, and 1% in the TTO group. However, we were unable to reliably stratify these results by each specific procedure, as the number of patients in each group would be too low. This study involved reviewing charts; as patients were not contacted, it is possible a patient developed DVT or PE, was treated at an outside facility, and then never followed up with the treating surgeon. Patients were identified by CPT codes, so, if a patient underwent HTO, DFO, or TTO that was recorded under a different CPT code, it is possible the patient was missed by our search. All patients were seen after surgery, and we reviewed the outpatient office notes that were taken, so unless the DVT or PE occurred after a patient’s final postoperative visit, it would have been recorded. Similarly, the DVT and PE rates reported here cannot be extrapolated to overall risks for DVT and PE after osteotomies about the knee in all patients—only in patients who did not receive DVT prophylaxis after surgery.
Conclusion
The rates of DVT and PE after HTO, DFO, and TTO in patients who did not receive chemical prophylaxis are low: 1.42% and 0.71%, respectively. After these osteotomies, DVT/PE prophylaxis in the absence of known risk factors may not be warranted.
Am J Orthop. 2017;46(1):E23-E27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- DVT and PE are uncommon complications following osteotomies about the knee.
- Use of oral contraceptives can increase the risk of a patient sustaining a postoperative DVT and PE following osteotomies about the knee.
- In the absence of significant risk factors, postoperative chemical DVT prophylaxis may be unnecessary in patients undergoing osteotomies about the knee.
High tibial osteotomy (HTO), distal femoral osteotomy (DFO), and tibial tubercle osteotomy (TTO) are viable treatment options for deformities about the knee and patella maltracking.1-4 Although TTO can be performed in many ways (eg, anteriorization, anteromedialization, medialization), the basic idea is to move the tibial tubercle to improve patellar tracking or to offload a patellar facet that has sustained trauma or degenerated.2 DFO is a surgical option for treating a valgus knee deformity (the lateral tibiofemoral compartment is offloaded) or for protecting a knee compartment after cartilage or meniscal restoration (medial closing wedge or lateral opening wedge).1 Similarly, HTO is an option for treating a varus knee deformity or isolated medial compartment arthritis; the diseased compartment is offloaded, and any malalignment is corrected. Akin to DFO, HTO is often performed to protect a knee compartment, typically the medial tibiofemoral compartment, after cartilage or meniscal restoration.2-4
Compared to most arthroscopic knee surgeries, these osteotomies are much more involved, have longer operative times, and restrict postoperative weight-bearing and range of motion.2-4 The rates of deep vein thrombosis (DVT) and pulmonary embolism (PE) after these osteotomies are not well documented. In addition, there is no documentation of the risks in patients who smoke, are obese, or are using oral contraceptives (OCs) at time of surgery, despite the increased DVT and PE risks posed by smoking, obesity, and OC use in other surgical procedures.5-7 Although the American Academy of Orthopaedic Surgeons (AAOS) issued clinical practice guidelines for DVT/PE prophylaxis after hip and knee arthroplasty, there is no standard prophylaxis guidelines for DVT/PE prevention after HTO, DFO, or TTO.8,9 Last, rates of DVT after total knee arthroplasty (TKA) are well defined; they range from 2% to 12%.10,11 These rates may be surrogates for osteotomies about the knee, but this is only conjecture.
We conducted a study to determine the rates of symptomatic DVT and PE after HTO, DFO, or TTO in patients who did not receive postoperative DVT/PE prophylaxis. We also wanted to determine if age, body mass index (BMI), and smoking status have associations with the risk of developing either DVT or PE after HTO, DFO, or TTO. We hypothesized that the DVT and PE rates would both be <1%.
Methods
After this study was approved by our university’s Institutional Review Board, we searched the surgical database of Dr. Cole, a sports medicine fellowship–trained surgeon, to identify all patients who had HTO, DFO, or TTO performed between September 1, 2009 and September 30, 2014. Current Procedural Terminology (CPT) codes were used for the search. The code for HTO was 27457: osteotomy, proximal tibia, including fibular excision or osteotomy (includes correction of genu varus [bowleg] or genu valgus [knock-knee]); after epiphyseal closure). The code for DFO was 27450: osteotomy, femur, shaft or supracondylar; with fixation. Last, the code for TTO was 27418: anterior tibial tubercleplasty (eg, Maquet-type procedure). The 141 patients identified in the search were treated by Dr. Cole at a single institution and were included in the study. Study inclusion did not require a minimum follow-up. Follow-up duration was defined as the time between surgery and the final clinic note in the patient chart. No patient was excluded for lack of follow-up clinic visits, and none was lost to follow-up.
Age, BMI, smoking status, and OC use were recorded for all patients. For each procedure, the surgeon’s technique remained the same throughout the study period: HTO, medial opening-wedge osteotomy with plate-and-screw fixation; DFO, lateral opening-wedge osteotomy with plate-and-screw fixation; and TTO, mostly anteromedialization with screw fixation (though this was dictated by patellar contact pressures). A tourniquet was used in all cases. Each patient’s hospital electronic medical record and outpatient office notes were reviewed to determine if symptomatic DVT or PE developed after surgery. The diagnosis of symptomatic DVT was based on clinical symptoms and confirmatory ultrasound, and the PE diagnosis was based on computed tomography. Doppler ultrasound was performed only in symptomatic patients (ie, it was not routinely performed).
Per surgeon protocol, postoperative DVT prophylaxis was not administered. Patients were encouraged to begin dorsiflexion and plantar flexion of the ankle (ankle pumps) immediately and to mobilize as soon as comfortable. Each patient received a cold therapy machine with compression sleeve. Patients were allowed toe-touch weight-bearing for 6 weeks, and then progressed 25% per week for 4 weeks to full weight-bearing by 10 weeks. After surgery, each patient was placed in a brace, which was kept locked in extension for 10 days; when the brace was unlocked, the patient was allowed to range the knee.
Continuous variable data are reported as weighted means and weighted standard deviations. Categorical variable data are reported as frequencies and percentages.
Results
Our database search identified 141 patients (44% male, 56% female) who underwent HTO (47 patients, 33.3%), DFO (13 patients, 9.2%), or TTO (81 patients, 57.5%). Mean (SD) age was 34.28 (9.86) years, mean (SD) BMI was 26.88 (5.11) kg/m2, and mean (SD) follow-up was 17.1 (4.1) months. Of the female patients, 36.7% were using OCs at time of surgery. Of all patients, 13.48% were smokers.
Two patients (1.42%) had clinical symptoms consistent with DVT. In each case, the diagnosis was confirmed with Doppler ultrasound. The below-knee DVT was unilateral in 1 case and bilateral in the other.
The unilateral DVT occurred in a patient who underwent anteromedialization of the tibial tubercle and osteochondral allograft transfer to the lateral femoral condyle for patellar maltracking and a focal trochlear defect. The DVT was diagnosed 8 days after surgery and was treated with warfarin. Low-molecular-weight heparin (LMWH) was used as a bridge until the warfarin level was therapeutic (4 days). This male patient had no significant medical history.
The bilateral DVT with PE occurred in a patient who underwent a medial opening-wedge HTO for a varus deformity with right medial compartment osteoarthritis and a meniscal tear. The DVT and PE were diagnosed 48 hours after surgery, when the patient complained of lightheadedness and lost consciousness. She had no medical problems but was using OCs at time of surgery. The patient died 3 days after surgery and subsequently was found to have a maternal-side family history of DVT (the patient and her family physician had been unaware of this history).
Discussion
As the rates of DVT and PE after osteotomies about the knee have not been well studied, we wanted to determine these rates after HTO, DFO, and TTO in patients who did not receive postoperative DVT prophylaxis. We hypothesized that DVT and PE rates would both be <1%, and this hypothesis was partly confirmed: The rate of PE after HTO, DFO, and TTO was <1%, and the rate of symptomatic DVT was >1%. Similarly, the patients who developed these complications were nonsmokers and had a BMI no higher than that of the patients who did not develop DVT or PE. In addition, only 1 patient developed DVT and PE, and she was using OCs and had a family history of DVT. Last, the patients who developed these complications were on average 14 years older than the patients who did not develop DVT or PE.
Although there is a plethora of reports on the incidence of DVT and PE after TKA, there is little on the incidence after osteotomies about the knee.8,12 The rate of DVT after TKA varies, but many studies place it between 2% and 12%, and routinely find a PE rate of <0.5%.10,11,13,14 Although the AAOS issued a clinical practice guideline for postoperative DVT prophylaxis after TKA, and evaluated the best available evidence, it could not reach consensus on a specific type of DVT prophylaxis, though the workgroup did recommend that patients be administered postoperative DVT prophylaxis of some kind.8,9 Similarly, the American College of Chest Physicians (ACCP) issued clinical practice guidelines for preventing DVT and PE after elective TKA and total hip arthroplasty.15 According to the ACCP guidelines, patients should receive prophylaxis—LMWH, fondaparinux, apixaban, dabigatran, rivaroxaban, low-dose unfractionated heparin, adjusted-dose vitamin K antagonist, aspirin, or an intermittent pneumatic compression device—for a minimum of 14 days. Unfortunately, though there are similarities between TKAs and peri-knee osteotomies, these procedures are markedly different, and it is difficult to extrapolate and adapt recommendations and produce a consensus statement for knee arthroplasties. In addition, guidelines exist for hospitalized patients who are being treated for medical conditions or have undergone surgery, but all the patients in the present study had their osteotomies performed on an outpatient basis.
Martin and colleagues16 reviewed 323 cases of medial opening-wedge HTO and found a DVT rate of 1.4% in the absence of routine DVT prophylaxis, except in patients with a history of DVT. Their rate is almost identical to ours, but we also included other osteotomies in our study. Miller and colleagues17 reviewed 46 cases of medial opening-wedge HTO and found a 4.3% DVT rate, despite routine prophylaxis with once-daily 325-mg aspirin and ankle pumps. This finding contrasts with our 1.42% DVT rate in the absence of postoperative chemical DVT prophylaxis. Motycka and colleagues18 reviewed 65 HTO cases in which DVT prophylaxis (oral anticoagulant) was given for 6 weeks, and they found a DVT rate of 9.7%. Turner and colleagues19 performed venous ultrasound on 81 consecutive patients who underwent HTO and received DVT prophylaxis (twice-daily subcutaneous heparin), and they found a DVT rate of 41% and a PE rate of 1.2%, though only 8.6% of the DVT cases were symptomatic. Of note, whereas the lowest postoperative DVT rate was for patients who did not receive postoperative DVT prophylaxis, the rate of symptomatic DVT after these osteotomies ranged from 1.4% to 8.6% in patients who received prophylaxis.16,19 Given this evidence and our study results, it appears routine chemical DVT prophylaxis after osteotomies about the knee may not be necessary, though higher level evidence is needed in order to make definitive recommendations.
In the present study, the 2 patients who developed symptomatic DVT (1 subsequently developed PE) were nonsmokers in good health. The female patient (DVT plus PE) was using OCs at time of surgery. Studies have shown that patients who smoke and who use OCs are at increased risk for developing DVT or PE after surgery.5,6,12 Given that only 2 of our patients developed DVT/PE, and neither was a smoker, smoking was not associated with increased DVT or PE risk in this study population, in which 13.48% of patients were smokers at time of surgery. In addition, given that the 1 female patient who developed DVT/PE was using OCs and that 36.7% of all female patients in the study were using OCs, it is difficult to conclude whether OC use increased the female patient’s risk for DVT or PE. Furthermore, neither the literature nor the AAOS consensus statement supports discontinuing OCs for this surgical procedure.
Patients in this study did not receive chemical or mechanical DVT prophylaxis after surgery. Regarding various post-TKA DVT prophylaxis regimens, aspirin is as effective as LMWH in preventing DVT, and the risk for postoperative blood loss and wound complications is lower with aspirin than with rivaroxaban.20,21 Given that the present study’s postoperative rates of DVT (1.42%) and PE (0.71%) are equal to or less than rates already reported in the literature, routine DVT prophylaxis after osteotomies about the knee may be unnecessary in the absence of other significant risk factors.16,19 However, our study considered only symptomatic DVT and PE, so it is possible that the number of asymptomatic DVT cases is higher in this patient population. Definitively answering our study’s clinical question will require a multicenter registry study (prospective cohort study).
Study Limitations
The strengths of this study include the large number of patients treated by a single surgeon using the same postoperative protocol. Limitations of this study include the lack of a control group. Although we found a DVT rate of 1.42% and a PE rate of 0.71%, the literature on the accepted risks for DVT and PE after HTO, DFO, and TTO is unclear. With our results stratified by procedure, the DVT rate was 2% in the HTO group, 0% in the DFO group, and 1% in the TTO group. However, we were unable to reliably stratify these results by each specific procedure, as the number of patients in each group would be too low. This study involved reviewing charts; as patients were not contacted, it is possible a patient developed DVT or PE, was treated at an outside facility, and then never followed up with the treating surgeon. Patients were identified by CPT codes, so, if a patient underwent HTO, DFO, or TTO that was recorded under a different CPT code, it is possible the patient was missed by our search. All patients were seen after surgery, and we reviewed the outpatient office notes that were taken, so unless the DVT or PE occurred after a patient’s final postoperative visit, it would have been recorded. Similarly, the DVT and PE rates reported here cannot be extrapolated to overall risks for DVT and PE after osteotomies about the knee in all patients—only in patients who did not receive DVT prophylaxis after surgery.
Conclusion
The rates of DVT and PE after HTO, DFO, and TTO in patients who did not receive chemical prophylaxis are low: 1.42% and 0.71%, respectively. After these osteotomies, DVT/PE prophylaxis in the absence of known risk factors may not be warranted.
Am J Orthop. 2017;46(1):E23-E27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Rossi R, Bonasia DE, Amendola A. The role of high tibial osteotomy in the varus knee. J Am Acad Orthop Surg. 2011;19(10):590-599.
2. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
3. Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13(4):279-289.
4. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015.
5. Ng WM, Chan KY, Lim AB, Gan EC. The incidence of deep venous thrombosis following arthroscopic knee surgery. Med J Malaysia. 2005;60(suppl C):14-16.
6. Platzer P, Thalhammer G, Jaindl M, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77(5):755-760.
7. Wallace G, Judge A, Prieto-Alhambra D, de Vries F, Arden NK, Cooper C. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage. 2014;22(7):918-927.
8. Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(19):1801-1811.
9. Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19(12):777-778.
10. Kim YH, Kulkarni SS, Park JW, Kim JS. Prevalence of deep vein thrombosis and pulmonary embolism treated with mechanical compression device after total knee arthroplasty in Asian patients. J Arthroplasty. 2015;30(9):1633-1637.
11. Kim YH, Yoo JH, Kim JS. Factors leading to decreased rates of deep vein thrombosis and pulmonary embolism after total knee arthroplasty. J Arthroplasty. 2007;22(7):974-980.
12. Raphael IJ, Tischler EH, Huang R, Rothman RH, Hozack WJ, Parvizi J. Aspirin: an alternative for pulmonary embolism prophylaxis after arthroplasty? Clin Orthop Relat Res. 2014;472(2):482-488.
13. Won MH, Lee GW, Lee TJ, Moon KH. Prevalence and risk factors of thromboembolism after joint arthroplasty without chemical thromboprophylaxis in an Asian population. J Arthroplasty. 2011;26(7):1106-1111.
14. Bozic KJ, Vail TP, Pekow PS, Maselli JH, Lindenauer PK, Auerbach AD. Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty. 2010;25(7):1053-1060.
15. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
16. Martin R, Birmingham TB, Willits K, Litchfield R, Lebel ME, Giffin JR. Adverse event rates and classifications in medial opening wedge high tibial osteotomy. Am J Sports Med. 2014;42(5):1118-1126.
17. Miller BS, Downie B, McDonough EB, Wojtys EM. Complications after medial opening wedge high tibial osteotomy. Arthroscopy. 2009;25(6):639-646.
18. Motycka T, Eggerth G, Landsiedl F. The incidence of thrombosis in high tibial osteotomies with and without the use of a tourniquet. Arch Orthop Trauma Surg. 2000;120(3-4):157-159.
19. Turner RS, Griffiths H, Heatley FW. The incidence of deep-vein thrombosis after upper tibial osteotomy. A venographic study. J Bone Joint Surg Br. 1993;75(6):942-944.
20. Jiang Y, Du H, Liu J, Zhou Y. Aspirin combined with mechanical measures to prevent venous thromboembolism after total knee arthroplasty: a randomized controlled trial. Chin Med J (Engl). 2014;127(12):2201-2205.
21. Zou Y, Tian S, Wang Y, Sun K. Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis. 2014;25(7):660-664.
1. Rossi R, Bonasia DE, Amendola A. The role of high tibial osteotomy in the varus knee. J Am Acad Orthop Surg. 2011;19(10):590-599.
2. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
3. Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13(4):279-289.
4. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015.
5. Ng WM, Chan KY, Lim AB, Gan EC. The incidence of deep venous thrombosis following arthroscopic knee surgery. Med J Malaysia. 2005;60(suppl C):14-16.
6. Platzer P, Thalhammer G, Jaindl M, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77(5):755-760.
7. Wallace G, Judge A, Prieto-Alhambra D, de Vries F, Arden NK, Cooper C. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage. 2014;22(7):918-927.
8. Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(19):1801-1811.
9. Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19(12):777-778.
10. Kim YH, Kulkarni SS, Park JW, Kim JS. Prevalence of deep vein thrombosis and pulmonary embolism treated with mechanical compression device after total knee arthroplasty in Asian patients. J Arthroplasty. 2015;30(9):1633-1637.
11. Kim YH, Yoo JH, Kim JS. Factors leading to decreased rates of deep vein thrombosis and pulmonary embolism after total knee arthroplasty. J Arthroplasty. 2007;22(7):974-980.
12. Raphael IJ, Tischler EH, Huang R, Rothman RH, Hozack WJ, Parvizi J. Aspirin: an alternative for pulmonary embolism prophylaxis after arthroplasty? Clin Orthop Relat Res. 2014;472(2):482-488.
13. Won MH, Lee GW, Lee TJ, Moon KH. Prevalence and risk factors of thromboembolism after joint arthroplasty without chemical thromboprophylaxis in an Asian population. J Arthroplasty. 2011;26(7):1106-1111.
14. Bozic KJ, Vail TP, Pekow PS, Maselli JH, Lindenauer PK, Auerbach AD. Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty. 2010;25(7):1053-1060.
15. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
16. Martin R, Birmingham TB, Willits K, Litchfield R, Lebel ME, Giffin JR. Adverse event rates and classifications in medial opening wedge high tibial osteotomy. Am J Sports Med. 2014;42(5):1118-1126.
17. Miller BS, Downie B, McDonough EB, Wojtys EM. Complications after medial opening wedge high tibial osteotomy. Arthroscopy. 2009;25(6):639-646.
18. Motycka T, Eggerth G, Landsiedl F. The incidence of thrombosis in high tibial osteotomies with and without the use of a tourniquet. Arch Orthop Trauma Surg. 2000;120(3-4):157-159.
19. Turner RS, Griffiths H, Heatley FW. The incidence of deep-vein thrombosis after upper tibial osteotomy. A venographic study. J Bone Joint Surg Br. 1993;75(6):942-944.
20. Jiang Y, Du H, Liu J, Zhou Y. Aspirin combined with mechanical measures to prevent venous thromboembolism after total knee arthroplasty: a randomized controlled trial. Chin Med J (Engl). 2014;127(12):2201-2205.
21. Zou Y, Tian S, Wang Y, Sun K. Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis. 2014;25(7):660-664.
Plesiomonas shigelloides Periprosthetic Knee Infection After Consumption of Raw Oysters
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
Bariatric surgery or total joint replacement: which first?
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
AT OBESITY WEEK 2016
Key clinical point:
Major finding: When total joint replacement in obese patients was performed after bariatric surgery, mean hospital length of stay was a full day less than when the orthopedic surgery preceded the bariatric surgery.
Data source: This retrospective observational study included 102 obese patients who underwent bariatric surgery and total knee or hip replacement.
Disclosures: The study presenter reported having no financial conflicts of interest.
Potential Operating Room Fire Hazard of Bone Cement
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.