User login
VIDEO: Surgery use declines for non–small cell lung cancer
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
agallegos@frontlinemedcom.com
On Twitter @legal_med
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
agallegos@frontlinemedcom.com
On Twitter @legal_med
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
agallegos@frontlinemedcom.com
On Twitter @legal_med
AT THE AATS ANNUAL MEETING
FDA approves brigatinib for second-line advanced ALK-positive NSCLC
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.
ASCO updates NSCLC guidelines for adjuvant therapy
Adjuvant cisplatin-based chemotherapy is recommended for routine use in non–small cell lung cancer patients with stage IIA, IIB, or IIIA disease who have undergone complete surgical resections, according to updated guidelines from the American Society of Clinical Oncology.
In patients with stage IA disease, the guidelines recommend against cisplatin-based chemotherapy. The new guidelines are based on a systematic review current to January 2016 and an American Society for Radiation Oncology guideline and systematic review, which ASCO had already endorsed, which formed the basis for recommendations on adjuvant radiation therapy, Mark G. Kris, MD, and panel authors said (J Clin Oncol. 2017 April 26. doi: 10.1200/JCO.2017.72.4401).
The guidelines, available online, also recommend against routine cisplatin-based chemotherapy in patients with stage IB disease, but they suggest an evaluation of these patients to explore the pros and cons of adjuvant chemotherapy.
With respect to radiation, the authors recommend against it for patients with resected stage I or stage II disease, and they recommend against it for routine use in patients with stage IIIA. However, in patients with IIIA N2 disease, patients should be evaluated postoperatively, in consultation with a medical oncologist, for the potential risks and benefits of adjuvant radiation.
The authors also provide some recommendations for communicating with patients regarding treatment decisions. There are few studies examining this question, so the recommendations are based on low-quality evidence.
Non–small cell lung cancer patients may have complex social, psychological, and medical issues, and discussions should be carried out with this in mind. Pain, impaired breathing, and fatigue are common after surgery, and smoking cessation can lead to short-term stress as a result of nicotine withdrawal. Older patients may have a range of comorbidities.
Studies show that patients are most satisfied if they feel that physicians allow them to share in the decision making, and if they are given sufficient time to choose. To that end, the authors recommend a session dedicated to discussion of adjuvant therapy.
Communicating risk of death is challenging for many reasons. Patients should be asked how they would like to hear about their risks: some prefer general terms, while others may opt for numbers, charts, or graphs. The guidelines include a risk chart that can help patients understand the potential benefits and risks of chemotherapy.
The study authors report financial relationships with numerous pharmaceutical companies.
Adjuvant cisplatin-based chemotherapy is recommended for routine use in non–small cell lung cancer patients with stage IIA, IIB, or IIIA disease who have undergone complete surgical resections, according to updated guidelines from the American Society of Clinical Oncology.
In patients with stage IA disease, the guidelines recommend against cisplatin-based chemotherapy. The new guidelines are based on a systematic review current to January 2016 and an American Society for Radiation Oncology guideline and systematic review, which ASCO had already endorsed, which formed the basis for recommendations on adjuvant radiation therapy, Mark G. Kris, MD, and panel authors said (J Clin Oncol. 2017 April 26. doi: 10.1200/JCO.2017.72.4401).
The guidelines, available online, also recommend against routine cisplatin-based chemotherapy in patients with stage IB disease, but they suggest an evaluation of these patients to explore the pros and cons of adjuvant chemotherapy.
With respect to radiation, the authors recommend against it for patients with resected stage I or stage II disease, and they recommend against it for routine use in patients with stage IIIA. However, in patients with IIIA N2 disease, patients should be evaluated postoperatively, in consultation with a medical oncologist, for the potential risks and benefits of adjuvant radiation.
The authors also provide some recommendations for communicating with patients regarding treatment decisions. There are few studies examining this question, so the recommendations are based on low-quality evidence.
Non–small cell lung cancer patients may have complex social, psychological, and medical issues, and discussions should be carried out with this in mind. Pain, impaired breathing, and fatigue are common after surgery, and smoking cessation can lead to short-term stress as a result of nicotine withdrawal. Older patients may have a range of comorbidities.
Studies show that patients are most satisfied if they feel that physicians allow them to share in the decision making, and if they are given sufficient time to choose. To that end, the authors recommend a session dedicated to discussion of adjuvant therapy.
Communicating risk of death is challenging for many reasons. Patients should be asked how they would like to hear about their risks: some prefer general terms, while others may opt for numbers, charts, or graphs. The guidelines include a risk chart that can help patients understand the potential benefits and risks of chemotherapy.
The study authors report financial relationships with numerous pharmaceutical companies.
Adjuvant cisplatin-based chemotherapy is recommended for routine use in non–small cell lung cancer patients with stage IIA, IIB, or IIIA disease who have undergone complete surgical resections, according to updated guidelines from the American Society of Clinical Oncology.
In patients with stage IA disease, the guidelines recommend against cisplatin-based chemotherapy. The new guidelines are based on a systematic review current to January 2016 and an American Society for Radiation Oncology guideline and systematic review, which ASCO had already endorsed, which formed the basis for recommendations on adjuvant radiation therapy, Mark G. Kris, MD, and panel authors said (J Clin Oncol. 2017 April 26. doi: 10.1200/JCO.2017.72.4401).
The guidelines, available online, also recommend against routine cisplatin-based chemotherapy in patients with stage IB disease, but they suggest an evaluation of these patients to explore the pros and cons of adjuvant chemotherapy.
With respect to radiation, the authors recommend against it for patients with resected stage I or stage II disease, and they recommend against it for routine use in patients with stage IIIA. However, in patients with IIIA N2 disease, patients should be evaluated postoperatively, in consultation with a medical oncologist, for the potential risks and benefits of adjuvant radiation.
The authors also provide some recommendations for communicating with patients regarding treatment decisions. There are few studies examining this question, so the recommendations are based on low-quality evidence.
Non–small cell lung cancer patients may have complex social, psychological, and medical issues, and discussions should be carried out with this in mind. Pain, impaired breathing, and fatigue are common after surgery, and smoking cessation can lead to short-term stress as a result of nicotine withdrawal. Older patients may have a range of comorbidities.
Studies show that patients are most satisfied if they feel that physicians allow them to share in the decision making, and if they are given sufficient time to choose. To that end, the authors recommend a session dedicated to discussion of adjuvant therapy.
Communicating risk of death is challenging for many reasons. Patients should be asked how they would like to hear about their risks: some prefer general terms, while others may opt for numbers, charts, or graphs. The guidelines include a risk chart that can help patients understand the potential benefits and risks of chemotherapy.
The study authors report financial relationships with numerous pharmaceutical companies.
Complex Malignancies: A Diagnostic and Therapeutic Trilemma
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
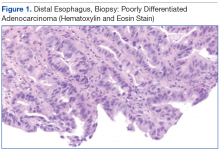
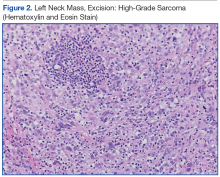
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of highgrade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
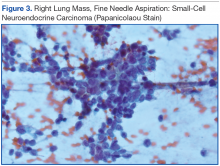
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of highgrade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Mr. F. is a 67-year-old man with a medical history significant for hypertension, hyperlipidemia, gastroesophageal reflux disease, abdominal aortic aneurysm repair, and lung nodules (8-mm nodules in his right lower lobe and left upper lobe) since 2009 for which he had been followed in the pulmonary clinic. His last radiologic imaging had been performed in November 2010. The patient had failed to show for a serial scan and follow-up pulmonary appointments. He had seen his primary care practitioner annually. He reporteded a 60 pack-year smoking history and drank up to 10 to 15 beers a day, 3 days a week. He also reported exposure to asbestos while in the U.S. Navy as well as having worked in a sheet metal yard. He reported no family history of lung cancer.
In January 2014, Mr. F. presented to his primary care doctor with a nonpainful neck lump noted by the patient for a few months. On physical examination, the patient appeared healthy and without change in weight, loss of appetite, dysphagia, hemoptysis, or any other remarkable symptoms except for the pain in the left-sided neck mass. On oral examination, the patient did not have any oral lesions but was noted to have poor dentition. He had decreased breath sounds on the right side but no adventitious breath sounds. His cardiac status was within normal limits with a regular heart rate, warm skin, and well-perfused extremities. His abdomen was soft and nontender with good bowel sounds.
A neck computed tomography (CT) done on January 17, 2014, showed 2 left neck masses in the tail of the parotid gland or in level 2 adjacent to the parotid gland. The ear, nose, and throat (ENT) clinic noted that he had a 3-cm level 2 mass, which was mobile and nontender. A fine needle aspiration biopsy showed poorly differentiated rare malignant cells, but the small size of the biopsy precluded identification of the cells. For that reason, the patient was scheduled for an operative laryngoscopy.
A combined positron emission tomography and CT (PET/CT) scan on February 5, 2014, showed multiple areas of hypermetabolic activity. A highly metabolic 2.6 x 1.9-cm mass was noted in the left neck inseparable from the inferior border of the left parotid gland; whether this represented a parotid neoplasm or neoplastic lymph node could not be determined. Positive level 5 neck lymph nodes were suggestive of metastatic disease. There were also 2 foci of mild nonspecific metabolic activity in the right parotid gland. A spiculated mass in the right lower lung, measuring 2.3 x 3.5 cm, demonstrated intense fludeoxyglucose F 18 (FDG) uptake with maximum standardized uptake value (SUV) of 12.7, which was consistent with neoplasm. Thickening of the middistal esophageal wall with intense FDG uptake (SUV of 7.9) was suggestive of esophageal carcinoma.
The patient underwent an endoscopic ultrasound (EUS) esophageal biopsy, which demonstrated poorly differentiated adenocarcinoma (Figure 1). The tumor seemed to have originated at this site, as the biopsy fragments also contained foci of Barrett’s metaplasia and in situ carcinoma. He was presented in the hospital tumor board about whether he should undergo a high-risk lung biopsy due to the location of the mass or have his left neck mass rebiopsied, assuming that he had metastatic esophageal cancer in both the lung and neck. The safer procedure of redoing the neck biopsy in the Ear, Nose, and Throat clinic was pursued, and the patient underwent a biopsy and surgical excision of his left neck mass with positive pathology of highgrade sarcoma (Figure 2).
The tumor consisted of spindle and epithelioid cell proliferation within a lymph node. The neoplastic cells exhibited prominent nuclear atypia, multinucleation, frequent mitoses, and focal necrosis. By immunohistochemistry, the tumor cells were diffusely positive for vimentin, CD68, and CD34 and were negative for pankeratin, CK7, CAM 5.2, CD30, CD15, tyrosine, melan-a, HMB-45, epithelial membrane antigen, keratin 903, desmin protein, CD56, CD99, actin protein, and CD1a. A differential diagnosis included malignant fibrous histiocytoma and histiocytic sarcoma.
Knowing the patient had 2 tumors, a percutaneous lung biopsy was scheduled. It was assumed that the lung mass would be metastatic from esophagus vs sarcoma, but the biopsy showed small cell cancer (Figure 3). The tumor was composed of small cells with finely granular chromatin and a sparse amount of cytoplasm. By immunohistochemistry, the tumor cells were positive for TTF-1, CK7, CAM 5.2, CD56, and synaptophysin and were negative for CK5, CK6, and p63. The patient was represented at the tumor board with diagnostic recommendations to undergo a magnetic resonance imaging of the brain (negative for metastasis) and a PET/CT scan (stable disease).
A lengthy discussion ensued regarding treatment for the 3 cancers. The AJCC Cancer Staging Manual, 7th edition, was used as the framework for all staging.1 The patient had a stage IIA (T1bN0MX), grade 3 sarcoma, which could be removed by neck dissection. He had a stage IIIB esophageal carcinoma (T3N2MX), which would require concurrent chemoradiation. His limited stage small cell lung cancer might have been amenable to a lobectomy if a mediastinoscopy was negative. The standard of care for limited stage small cell lung cancer is that if the cancer is precisely staged and the patient is a good surgical candidate, then surgery can be considered. The rationale for considering resection in this case was that if his other cancers could be cured, then surgery would be an excellent option.2 These options were presented to the patient, who opted for chemotherapy. The oncologist treating the patient elected to target the small cell cancer initially, because it was likely the fastest growing cancer. The patient started treatment with etoposide/carboplatin shortly thereafter. Mr. F. was also referred to palliative care.
Discussion
When a patient presents with several tumor masses detected either clinically or radiologically, it is reasonable to assume, at least initially, that all the lesions can be attributed to the same disease (ie, a primary neoplasm and distant metastases). Based on this assumption, it is rational to establish a tissue diagnosis by sampling the most accessible lesion. In the correct clinical context, this may be sufficient to initiate an appropriate therapy. However, health care providers should be aware that unrelated neoplasms can develop simultaneously, and about 8% of patients have > 1 malignancy at presentation.3
This patient presented with 3 different malignant neoplasms: esophageal adenocarcinoma, pulmonary small-cell carcinoma, and high-grade sarcoma. These types of tumors have completely different histogenesis. Esophageal adenocarcinoma develops from glandular cells of Barrett’s esophagus, small-cell carcinoma develops from neuroendocrine Kulchitsky cells, and sarcoma arises from mesenchymal cells. This constellation of neoplasms does not represent any known multicancer syndrome, such as Li-Fraumeni syndrome or Lynch syndrome. Whether these concomitant tumors represent coincidental neoplasms or have common underlying molecular alterations remains unknown.
The treatment for each of this patient’s cancers was very different and required a multidisciplinary, staged approach for management. This could have been arranged for him had he wanted to pursue multiple resections of the neck and lung plus have chemotherapy and radiation for the esophageal cancer. The patient opted for chemotherapy for the most aggressive cancer, but other options would have been possible had he wanted to pursue them.
Conclusion
This complex case demonstrates the role for careful review of all data and the importance of not making assumptions when analyzing images and history despite all the procedures and time to diagnosis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.
1. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
2. Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30.
3. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127(4):1119-1125.
Nivolumab boosts 5-year survival in advanced NSCLC
Early data show that treatment with the immune checkpoint inhibitor nivolumab (Opdivo) resulted in a 5-year overall survival rate of 16% among patients with advanced non–small-cell lung cancer (NSCLC).
In comparison, the 5-year survival rate for patients with advanced lung and bronchus cancer, according to SEER data, is 4.3%, and for those with advanced NSCLC, 4.9%.
“This is the first report of the long-term survival rate in patients with metastatic NSCLC treated with an immune checkpoint inhibitor,” said Julie Brahmer, MD, of the Bloomberg Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Baltimore.
For a small subset of patients, immunotherapy can work for a very long time, explained Dr. Brahmer, who discussed her findings during a presscast at the annual meeting of the American Association for Cancer Research.
The 5-year overall survival rate that was reported in this study was much higher than what has been seen for this patient population who receive the standard of care. Statistics show that the majority of patients with advanced disease will die within a year of their diagnosis, Dr. Brahmer pointed out.
The findings presented at the meeting are updated results from the phase Ib CA209-003 dose-escalation cohort expansion trial that comprised 129 patients with heavily pretreated, advanced NSCLC . The cohort was randomized to receive nivolumab once every 2 weeks for up to 2 years at one of three dose levels: 1 mg/kg, 3 mg/kg, or 10 mg/kg.
A previous analysis of the data showed promising activity, and findings from subsequent clinical trials led to the approval of nivolumab for use in the second line setting of advanced NSCLC.
Dr. Brahmer now reported findings based on 5-year results of this phase Ib trial. “This analysis is based on a minimum follow up of 58 months,” she said.
The overall 5-year survival rates for squamous NSCLC were 16%, and the rates for nonsquamous were 15%.
At 1 year, overall survival was 42%. At 2 years, it was 24%, and at 3 years, 18%.
“After 3 years, the survival curve has plateaued out, which is similar to what has been seen in the past in other diseases treated with immunotherapy,” Dr. Brahmer noted.
Within the cohort, there were 16 patients who had survived for at least 5 years. Of this group, 12 achieved a partial response, 2 patients had stable disease, and 2 had progressive disease.
Dr. Brahmer pointed out that there was nothing different or unusual among the 16 patients who survived for 5 years, compared with the rest of the cohort. Their characteristics were similar to others in the study, most of them were former smokers, and they had very similar rates of different histologies.
One interesting note was that within that group, there were two patients with EGFR mutations. “We usually don’t expect them to do well with immunotherapy,” she said.
Dr. Brahmer received research funding from, and is an adviser to, Bristol-Myers Squibb, which funded the study.
Early data show that treatment with the immune checkpoint inhibitor nivolumab (Opdivo) resulted in a 5-year overall survival rate of 16% among patients with advanced non–small-cell lung cancer (NSCLC).
In comparison, the 5-year survival rate for patients with advanced lung and bronchus cancer, according to SEER data, is 4.3%, and for those with advanced NSCLC, 4.9%.
“This is the first report of the long-term survival rate in patients with metastatic NSCLC treated with an immune checkpoint inhibitor,” said Julie Brahmer, MD, of the Bloomberg Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Baltimore.
For a small subset of patients, immunotherapy can work for a very long time, explained Dr. Brahmer, who discussed her findings during a presscast at the annual meeting of the American Association for Cancer Research.
The 5-year overall survival rate that was reported in this study was much higher than what has been seen for this patient population who receive the standard of care. Statistics show that the majority of patients with advanced disease will die within a year of their diagnosis, Dr. Brahmer pointed out.
The findings presented at the meeting are updated results from the phase Ib CA209-003 dose-escalation cohort expansion trial that comprised 129 patients with heavily pretreated, advanced NSCLC . The cohort was randomized to receive nivolumab once every 2 weeks for up to 2 years at one of three dose levels: 1 mg/kg, 3 mg/kg, or 10 mg/kg.
A previous analysis of the data showed promising activity, and findings from subsequent clinical trials led to the approval of nivolumab for use in the second line setting of advanced NSCLC.
Dr. Brahmer now reported findings based on 5-year results of this phase Ib trial. “This analysis is based on a minimum follow up of 58 months,” she said.
The overall 5-year survival rates for squamous NSCLC were 16%, and the rates for nonsquamous were 15%.
At 1 year, overall survival was 42%. At 2 years, it was 24%, and at 3 years, 18%.
“After 3 years, the survival curve has plateaued out, which is similar to what has been seen in the past in other diseases treated with immunotherapy,” Dr. Brahmer noted.
Within the cohort, there were 16 patients who had survived for at least 5 years. Of this group, 12 achieved a partial response, 2 patients had stable disease, and 2 had progressive disease.
Dr. Brahmer pointed out that there was nothing different or unusual among the 16 patients who survived for 5 years, compared with the rest of the cohort. Their characteristics were similar to others in the study, most of them were former smokers, and they had very similar rates of different histologies.
One interesting note was that within that group, there were two patients with EGFR mutations. “We usually don’t expect them to do well with immunotherapy,” she said.
Dr. Brahmer received research funding from, and is an adviser to, Bristol-Myers Squibb, which funded the study.
Early data show that treatment with the immune checkpoint inhibitor nivolumab (Opdivo) resulted in a 5-year overall survival rate of 16% among patients with advanced non–small-cell lung cancer (NSCLC).
In comparison, the 5-year survival rate for patients with advanced lung and bronchus cancer, according to SEER data, is 4.3%, and for those with advanced NSCLC, 4.9%.
“This is the first report of the long-term survival rate in patients with metastatic NSCLC treated with an immune checkpoint inhibitor,” said Julie Brahmer, MD, of the Bloomberg Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Baltimore.
For a small subset of patients, immunotherapy can work for a very long time, explained Dr. Brahmer, who discussed her findings during a presscast at the annual meeting of the American Association for Cancer Research.
The 5-year overall survival rate that was reported in this study was much higher than what has been seen for this patient population who receive the standard of care. Statistics show that the majority of patients with advanced disease will die within a year of their diagnosis, Dr. Brahmer pointed out.
The findings presented at the meeting are updated results from the phase Ib CA209-003 dose-escalation cohort expansion trial that comprised 129 patients with heavily pretreated, advanced NSCLC . The cohort was randomized to receive nivolumab once every 2 weeks for up to 2 years at one of three dose levels: 1 mg/kg, 3 mg/kg, or 10 mg/kg.
A previous analysis of the data showed promising activity, and findings from subsequent clinical trials led to the approval of nivolumab for use in the second line setting of advanced NSCLC.
Dr. Brahmer now reported findings based on 5-year results of this phase Ib trial. “This analysis is based on a minimum follow up of 58 months,” she said.
The overall 5-year survival rates for squamous NSCLC were 16%, and the rates for nonsquamous were 15%.
At 1 year, overall survival was 42%. At 2 years, it was 24%, and at 3 years, 18%.
“After 3 years, the survival curve has plateaued out, which is similar to what has been seen in the past in other diseases treated with immunotherapy,” Dr. Brahmer noted.
Within the cohort, there were 16 patients who had survived for at least 5 years. Of this group, 12 achieved a partial response, 2 patients had stable disease, and 2 had progressive disease.
Dr. Brahmer pointed out that there was nothing different or unusual among the 16 patients who survived for 5 years, compared with the rest of the cohort. Their characteristics were similar to others in the study, most of them were former smokers, and they had very similar rates of different histologies.
One interesting note was that within that group, there were two patients with EGFR mutations. “We usually don’t expect them to do well with immunotherapy,” she said.
Dr. Brahmer received research funding from, and is an adviser to, Bristol-Myers Squibb, which funded the study.
Key clinical point: Treatment with nivolumab resulted in a 5-year overall survival rate that is much higher than what is reported for this patient population receiving standard-of-care treatment.
Major finding: Nivolumab yielded a 5-year survival rate of 16% in a cohort of patients with advanced NSCLC.
Data source: Updated results from a phase Ib study that included 129 patients with advanced NSCLC.
Disclosures: Dr. Brahmer received research funding from, and is an adviser to, Bristol-Myers Squibb, which funded the study.
Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
Background Morbidity related to cancer and its treatment remains a significant source of human suffering and a challenge to the delivery of high-quality care.
Objective To develop and apply quality indicators to evaluate quality of supportive care for advanced lung cancer in the Veterans Health Administration (VHA) and examine facility-level predictors of quality.
Methods We evaluated supportive care quality using 12 quality indicators. Data were taken from VHA electronic health records for incident lung cancer cases occurring during 2007. Organizational characteristics of 111 VHA facilities were examined for association with receipt of care.
Results Rates of care-receipt were high, especially in the treatment toxicity (89%) and pain management (79%-98%) domains, but were lower in the palliative cancer treatment (60%-90%) and hospice (75%) domains, with substantial facility-level variation. Presence of a care tracking method that was monitored by a midlevel practitioner seemed to be associated with better quality for treatment toxicity (OR, 3.38; 95% CI, 1.87-6.10) and referral to hospice (OR, 1.60; 95% CI, 1.22-2.28); having a psychologist for cancer patients was associated with higher odds for pain management (OR, 1.76; 95% CI, 1.16-2.66).
Limitations Not all supportive care was evaluated. Care processes identified as present at facilities may not have been applied to cohort patients. Facility-level results may be influenced by errors in attributing a patient’s care to the correct facility.
Conclusions Quality indicators for supportive cancer care can be developed and applied in large evaluations using electronic health record review. This study confirmed high-quality supportive care, while identifying significant facility-level variation in VHA.
Funding Veterans Health Administration Office of Informatics and Analytics.
Morbidity related to cancer and its treatment remains a significant source of human suffering and a national challenge to delivery of high-quality cancer care. Quality care refers to the delivery of state-of-the-art treatments intended to achieve cure or prolong life as well as the supportive processes that address the disease- and treatment-related burdens of living with cancer. These processes span the cancer care continuum from diagnosis to end of life, and include pain-, symptom-, and side effect-management; psychosocial support; communication needs; and support for caregivers.1-5
A 2006 report from the Agency for Healthcare Research and Quality concluded that “a large number of measures are available for addressing palliative cancer care, but testing them in relevant populations is urgently needed.”6 Since then, evidence-based standards have been translated into “quality indicators” that may be used to identify outcome targets indicative of quality care, such as patient reports of pain reduction; or, they may specify facility-level care processes associated with these outcomes, such as pain screening, treatment, and follow-up assessment.1,7-9 Quality indicators can be used as the basis for tools to
measure processes that are critical to ensuring highquality supportive cancer care and identifying specific processes and practice sites that should be targeted for quality improvement efforts.10,11 Such tools can help characterize care quality for patient populations served by a health care system as a whole, while revealing important variations at individual facilities within the system.
Quality indicators for supportive and end-of-life care have been successfully applied to electronic health record (EHR) data in research studies assessing care quality in patients with different cancer types and showing supportive care for cancer to be generally in need of quality improvement.10-15 In this paper, we examine supportive care findings from the first largescale application of quality indicators in a system-wide evaluation of lung cancer care in the Veterans Health Administration (VHA). The evaluation was part of a series of operationally motivated projects to identify targets for cancer care improvement in VHA. Lung cancer, the most common malignancy in the United States, accounts for one-fifth of all tumors diagnosed each year within this large, integrated health care system and is responsible for a significant proportion of the system’s supportive care needs. Recently demonstrated benefits of early palliative care on the quality of life and end-of-life care in patients with advanced lung cancer point to the particular importance of assessing supportive care in this population.16 We report VHA evaluation findings and subsequent research analyses seeking to identify patient and facility characteristics associated with better quality supportive lung cancer care.
Methods
Quality Indicators
Quality indicators for supportive cancer care were identified from a systematic review of existing measures and expert guidelines. A panel of 9 national palliative and supportive cancer care experts rated the validity and feasibility of the indicators for use in the VHA using a modified Delphi panel method adapted from the RAND-UCLA appropriateness method17 and prioritized candidate indicators in a ranking exercise. Those with low validity or feasibility scores, or low priority rankings, were excluded, resulting in a final set of 12 quality indicators (8 supportive care, 4 end-of-life care) for use in the national evaluation.
Evaluation Population
All incident non-small-cell (NSCLC) and small-cell (SCLC) lung cancer cases diagnosed within the VHA during 2007 (N = 7,816) were identified through the Veterans Affairs Central Cancer Registry (VACCR). Cases were eligible for the study if the patient had advanced cancer (extensive small-cell or metastatic non-small-cell lung cancer) and had lived long enough to be eligible for the supportive care that was being evaluated. Patients were excluded if the health record did not confirm the lung cancer diagnosis (eg, no pathologic diagnosis or diagnosis outside the VHA, n = 1,297); they had a preexisting or concurrent diagnosis of metastatic neoplasm other than lung cancer (n = 540); had died or enrolled in hospice ≤ 30 days after diagnosis (n = 947); had documented
“comfort measures only” ≤ 30 days after diagnosis (n = 91); had documented life expectancy of ≤ 6 months at time of diagnosis (n = 39); or enrolled in a clinical trial (as care received as part of a trial may not be completely captured in the VHA record (n = 57). Of the patients meeting inclusion criteria, 2,969 were eligible for at least one of the supportive care quality indicators.
Data Collection
EHRs were reviewed remotely by abstractors from the West Virginia Medical Institute, the VHA’s contractor for its external peer-review program. Data were collected retrospectively from the year before to the 2007 diagnosis, with follow-up through 2009. Stage was determined through the VACCR or by abstraction if no VACCR stage was available. The VACCR also provided sociodemographic information including race, which is abstracted from patient charts by cancer registrars. Urban or rural residence was determined by using the rural-urban commuting area codes. Case-level quality indicator results were provided to each facility for review to identify any missed documentation, and abstractors updated data as appropriate. Facility characteristics for the Veterans Administration Medical Centers (VAMCs) at which patients received care were obtained from the 2009 Veterans Health Administration (VHA) Oncology Services
Survey.18 The study was approved by the Veterans Administration Greater Los Angeles Healthcare System Institutional Review Board Subcommittee on Human Studies.
Dependent Variables
For each quality indicator, cases eligible for the indicator were categorized as having received the specified care or not. Patient refusals of specified care or documentation of a contraindication were also considered to have met criteria for receipt of appropriate care. For bivariate and multivariate analyses, indicators were grouped into 4 domains: treatment toxicity (1 indicator), pain screening and management (6), palliative cancer treatment (4), and hospice (1). For these domain analyses, cases were considered to have met criteria for receipt of recommended supportive care if they received the care specified
for all of the indicators for which they were eligible in the domain.
Independent Variables
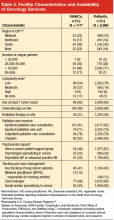
Patient characteristics included stage of disease, age, gender, race (white, black, other/unknown), marital status (married/living with partner, other), urban/rural residence, and performance status (poor/not poor), as shown in Table 1. Performance status was abstracted from the medical record as ECOG (Eastern Cooperative Oncology Group) or Karnofsky when present, but most of the time it was recorded in patients’ charts qualitatively (eg, “poor performance status”). Cases were attributed to facilities based on the VACCR attribution, which is usually the VHA facility where treatment is initiated. Facility characteristics included geographic region; number of unique patients; facility complexity level; chemotherapy availability on-site; radiation therapy availability on-site; and types of palliative care services, psychosocial support, and patient tracking and case management available on-site (Table 2).
Statistical Analyses
Bivariate analyses were performed to determine which patient and facility characteristics were associated with quality of care for treatment toxicity, pain, palliative treatment, and hospice. Multilevel logistic regression models were developed for each of these 4 domains to examine the relationship between indicator care receipt and patient and facility characteristics. Collinearity was assessed by examining Pearson correlations between independent variables, and highly collinear variables were not included in multivariate analyses.
Independent variables significantly associated with dependent variables in bivariate analyses or considered necessary for adjustment from conceptual standpoints were included in the multivariate models (cancer type [NSCLC or SCLC], age, sex, race, marital status, urban/rural classification, poor performance status, Veterans Integrated Service Network location, facility complexity level, facility region, as well as the facility having the following cancer-related services: tumor board, palliative care unit, palliative care services, chaplain, method for tracking patients through treatment and posttreatment care (to ensure receipt of timely and appropriate care), and presence of a case manager]. Testing yielded no significant interactions between any of the variables; thus interaction terms were not included in the final regressions. All analyses were performed using SAS statistical software, version 9.1 (Cary, NC).
Results
Patient Characteristics
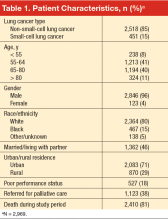
Table 1 summarizes patient characteristics of this elderly (mean age, 67 years; SD, 9), mostly white, and mostly male veteran cohort. In all, 15% of the cohort was black, nearly one-third lived in a rural area, and 46% were married or living with a partner. Eighteen percent had poor performance status, 38% were referred for palliative care, and 81% died during the study period.
Organizational Characteristics
Patients in the cohort were cared for at one or more of 111 VAMC facilities (Table 2), with the largest proportion (45%) seen at facilities located in the South. Most patients (76%) were seen in low-complexity facilities (54% of facilities) and almost all (95%) used a facility reporting having at least 1 tumor board (76% of facilities) in the 2009 VHA Oncology Services Survey. Most facilities (90%) reported having chemotherapy on-site, with 99% of patients in our cohort receiving care at one of these facilities. Radiation therapy at most sites was provided by referral to a non-VA facility, although 31% of facilities had radiation therapy available on-site and these VAMCs provided care to 40% of the patients. A substantial proportion of facilities offered inpatient (91%) and outpatient (76%) palliative care consultation, serving the majority of patients, 95% and 78%, respectively. Various forms of psychosocial support were reported at facilities serving over half of the study patients. Forty-five percent of veterans were seen at facilities that provided care tracking for lung cancer patients (41% of facilities) and at 14% of facilities (12% of patients) the tracking method was monitored by a nurse practitioner or physician assistant. Sixty-eight percent of patients were cared for at facilities reporting the presence of a case manager (69%). (The fact that a site has chemotherapy or any of the other aforementioned services in-house does not imply that patients receiving care at this site were eligible for or received these particular services, but rather that they were cared for at a facility reporting presence of the service.)
About 8% of patients were diagnosed at a VAMC different from the VACCR attribution. Six percent received specialty service consultations or treatment at multiple VAMCs, and about 25% received at least one specialty service consultation or treatment at a non-VHA facility.
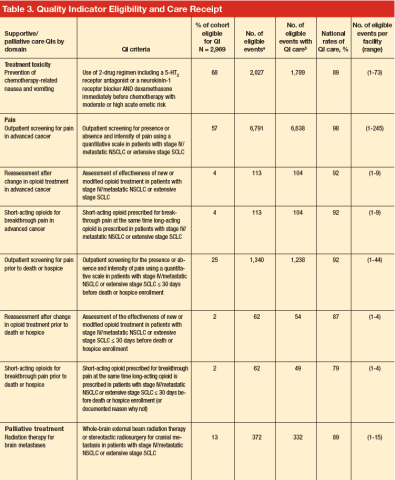
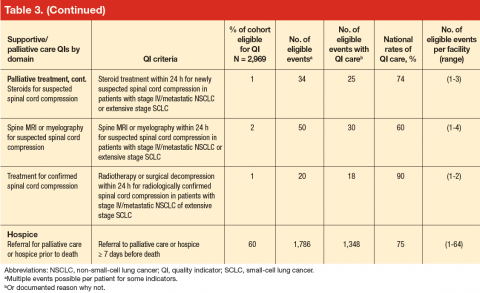
Quality Indicator Eligibility and Rates of Receiving Recommended Care
Patient eligibility for the quality indicators (Table 3) ranged from 1% (treatment of spinal cord compression) to 68% (prevention of chemotherapy-related nausea and vomiting). Few patients were eligible for palliative treatment indicators (palliative radiation therapy and treatment of spinal cord compression), with only 2% of patients (50 individuals) eligible for the imaging indicator. Eligibility for the two indicators related to use of shortacting opioids for break-through pain was also low (2%-4%).
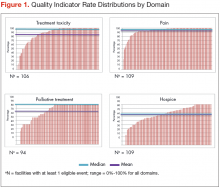
Mean national rates for recommended support ive care were generally high (above 85% for 8 of the 12 indicators), ranging from 98% for outpatient pain screening to a low of 60% for spine MRI or myelography within 24 hours for suspected spinal cord compression (Table 3). There was pronounced variability across facilities, with facility rates in every domain ranging from 0%-100% (Figure 1). Many facilities scored 100% in at least 1 domain (eg, 44 of 109 facilities for pain screening; 51 of 94 for palliative cancer therapy). Eight (of 111) facilities did not provide recommended care in 1 domain and 1 did not provide recommended care in 2 domains (ie, 0% of recommended care).
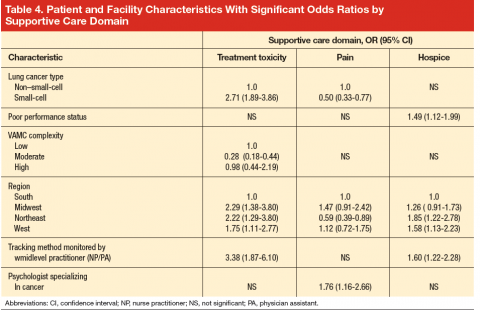
In bivariate analyses (data not shown), referral to palliative care was associated with better care for treatment toxicity (P = .04) but not the other domains. Patients with poor performance status were more likely to be referred to hospice (81% vs 74% for those with good performance status, P = .005) as were patients who lived in an urban zip code area (77% vs 71% for rural zip code, P = .03). In multivariate analyses (Table 4), small-cell cancer type, though not significant in the bivariate analyses, was associated with a higher odds of receiving recommended care [OR, 2.71; 95% CI, 1.89-3.86)] in the
treatment toxicity domain and lower odds in the pain domain [OR, 0.50; 95% CI, 0.33-0.77)]; and poor performance remained associated with higher odds of referral to hospice [OR, 1.49; 95% CI, 1.12-1.99)] after adjusting for other patient and facility characteristics.
In multivariate analysis, moderate facility complexity was associated with lower quality in the toxicity domain than was low facility complexity [OR, 0.28; 95% CI, 0.18-0.44)]; however there was no such difference between the low- and high-complexity facilities. The presence of a patient tracking method appeared to be associated with better quality of care in the treatment toxicity domain [OR, 3.38; 95% CI, 1.87-6.10)] and a higher likelihood of referral to hospice [OR, 1.60; 95% CI, 1.22-2.28)] when the method was monitored by a midlevel practitioner (nurse practitioner or physician assistant), but no association was found for methods monitored by other types of staff. In the pain domain, having a psychologist specializing in cancer was associated with a higher odds of receiving recommended care [OR, 1.76; 95% CI, 1.16-2.66)]. No patient or facility characteristics were associated with receiving recommended palliative cancer therapy, although statistical power was limited by the small numbers of eligible events.
Discussion
Use of evidence-based measures to assess care quality proved feasible in this evaluation of supportive care for advanced lung cancer in a large national integrated health care system and confirmed that gaps found in smaller studies represent critical unmet needs for patients. The methods permitted individualized feedback to facilities, accompanied by support for quality improvement efforts. The effectiveness of providing facility feedback with quality improvement strategies will be evaluated in follow-up studies.
Although quality of supportive care as measured by this specific set of indicators was quite high compared to previous reports,10,13,15 substantial variation across facilities was observed even for measures with high overall performance. Comparatively high levels of supportive care may reflect greater emphasis on supportive measures for advanced lung cancer patients whose expectation of cure is low (other studies evaluated cohorts with mixed cancer types), or the fact that previous studies did not account for documentation of a reason for forgoing indicated care. Tough high quality of supportive care for lung cancer in VHA nationally is encouraging, variability across individual facilities is of concern. Such variation may well exist in any large health care system, with patients potentially experiencing different standards of care depending on where they live.
In this study, facilities located in the South, treating 45% of advanced lung cancer patients in the cohort, were associated with worse care in the treatment toxicity and hospice domains, and those in the Northeast appeared to generally have worse care for pain. Although reasons for these differences are unclear, geographic variation in Medicare hospice use has been related to a variety of determinants,20-23 some of which may also apply to VHA. Referral to palliative care or hospice was among the lowest scoring quality indicators in the evaluation, with a 75% national rate and 43% of facilities documenting referral for less than 75% of eligible cases. Although our measures determined only referral to palliative care or hospice (versus actual receipt of services), low rates may in part reflect provider anticipation of low local availability of hospice. Although the VHA Hospice and Palliative Care program has made progress in reducing variability in hospice access for Veterans, surveys conducted by Hospice-Veteran Partnerships showed lack of shared knowledge about the different systems of benefits and health care available for veterans, misunderstandings about referral processes among health care providers and payment for hospice services, and difficulties in caring for veterans across multiple care settings to be barriers to Veteran access to community services.24 Targeted inquiry at lowreferral facilities may elucidate which of these and other possible challenges may suggest points of intervention for improvement in hospice referral patterns and support of appropriate hospice use.
Attempting to identify facility-related explanations for overall variability, we explored the potential role of a number of structural variables, including presence of a tumor board, a palliative care unit, outpatient palliative care services, chaplain services, presence of a cancer care tracking system, and presence of a case manager. Tough facilities with a psychologist specializing in cancer provided better care for pain, and those with midlevel practitioners (nurse practitioner or physician assistant) monitoring patient tracking through care were more likely to appropriately refer to hospice and have better care for treatment toxicity, many facilities without such designated professionals also provided quality care in these domains. Because of limited methodological feasibility of demonstrating patient receipt of psychosocial/spiritual support and care tracking using medical record review, the final set of quality indicators did not include measures for these forms of care; however, psychosocial care is considered integral to supportive care in cancer25 and care tracking has been associated with improved cancer care in non-VA settings.26 The presence of appropriate professionals providing cancer-specific psychological support and tracking may be directly associated with improved care, not only in the domains noted above, but also in the important domain of existential and emotional well-being not evaluated here. These services may also be part of larger facility-level quality improvement mechanisms contributing to cancer care quality that are worth identifying in future research. Lack of consistent explanations for the overall facility-level variation in this study suggests the need to identify data sources that can be used to measure additional organizational characteristics in future research.
Our study has several limitations. The scope of the national evaluation did not include indicators for all aspects of supportive care, including care for dyspnea, depression, and psychosocial distress; support for care givers; and care planning assistance. Small numbers of eligible events per facility for some indicators limit interpretation, particularly in the palliative treatment domain, where the largest range was 1-15 eligible events per facility. Facility characteristics used for analyses were identified from a surveyand may be subject to respondent error. Potentially useful processes, such as a midlevel practitioner monitored tracking system were identified as “present” at the facility, but use for cohort patients was not possible to confirm, limiting direct linkage with better care. Some facility-level results may be influenced by errors in VACCR attribution to a facility that was not directly involved with the patient’s care, specialty service receipt at multiple VAMCs, or through contract care at non-VHA facilities. Quality-of-care coordination between different VHA and non-VHA facilities for services not provided at the diagnosing facility, or to reduce travel burden if the VAMC was far from the patient’s home may be associated with better or worse care quality and should be evaluated in future work.
In conclusion, use of quality indicators to evaluate quality of supportive lung cancer care in a large integrated health care system proved feasible, confirmed
provision of overall high-quality supportive care, but identified a high degree of variability across individual facilities in the system. Facility characteristics examined did not explain this variation; however, the data suggested that, while not accounting for the overall variation, having professionals providing cancer-specific psychological support and care-tracking may contribute to better quality of care in some domains. Difficulties in identifying predictors of quality suggest that future research should include qualitative comparisons of facilities with varying rates of providing recommended supportive care to identify potentially impactful organizational factors not examined in this study.
Disclaimer and Disclosures
Accepted for publication February 24, 2014. Correspondence: Sabine Oishi, PhD, MSPH; sabine.oishi@va.gov. Disclosures: Dr Ryoo was supported in part by the Robert Wood Johnson Foundation Clinical Scholars Program and by the UCLA Jonsson Comprehensive Cancer Center R25 grant. The authors have no direct conflicts of interest to disclose. Dr Karl Lorenz is a consultant as a member of the Data Monitoring Committee for a Phase II trial of Sativex being conducted by Otsuka Pharmaceuticals. The current study has no direct financial implications related to his participation and was conducted entirely outside of the timeframe of his consultancy; however, it may be perceived as indirectly related. The views and opinions of authors expressed herein do not necessarily state or reflect that of the Department of Veterans Affairs, the United States Government, Kaiser Permanente, Stanford University, or the University of California.
Click here to read the digital edition.
1. Lorenz KA, Dy SM, Naeim A, et al. Quality measures for supportive cancer care: the Cancer Quality-ASSIST Project. J Pain Symptom Manage. 2009;37(6):943-964.
2. Dy SM, Asch SM, Naeim A, Sanati H, Walling A, Lorenz KA. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
3. Gordon, DB, Dahl, JL, Miakowski, C. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med. 2005;165(14):1574-1580.
4. Lorenz, KA, Lynn, J, Dy, SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
5. Seow, H, Snyder, CF, Shugarman, LR, et al. Developing quality indicators for cancer end-of-life care: proceedings from a national symposium. Cancer. 2009;115(17):3820-3829.
6. Lorenz KA, Lynn J, Dy SM, et al. Agency for Healthcare Research and Quality (US). Cancer care quality measures: symptoms and end-of-life care. http://www.ncbi.nlm.nih.gov/books/NBK38028/. Published 2006. Accessed December 17, 2012.
7. Lorenz KA, Lynn J, Dy S, et al. Quality measures for symptoms and advance care planning in cancer: a systematic review. J Clin Oncol. 2006;24(30):4933-4938.
8. Lorenz KA, Rosenfeld K, Wenger N. Quality indicators for palliative and end-of-life care in vulnerable elders. JAGS. 2007;55(suppl 2):S318-S326.
9. Dy SM, Lorenz KA, O’Neill SM, et al. Cancer Quality-ASSIST supportive oncology quality indicator set. Cancer. 2010;116(13):3267-3275.
10. Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26(11):1893-1898.
11. Jacobsen PB, Shibata D, Siegel E, et al. Evaluating the quality of psychosocial care in outpatient medical oncology settings using performance indicators. Psycho-oncology. 2010; 20(11):1221-1227.
12. Walling AM, Asch SM, Lorenz KA, et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170(12):1057-1063.
13. Dy SM, Asch SM, Lorenz KA, et al. Quality of end-of-life care for patients with advanced cancer in an academic medical center. J Palliat Med. 2011;14(4):451-457.
14. Gonsalves WI, Tsewang T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
15. Malin JL, O’Neill SM, Asch SM, et al. Quality of supportive care for patients with advanced cancer in a VA medical center. J Palliat Med. 2011;14(5):573-577.
16. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326.
17. Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2(1):53-63.
18. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2009 VHA Oncology Services Survey. April, 2010. Accessed November 1, 2010.
19. US Census Bureau. Census Bureau regions and divisions with state FIPS codes. Available from URL: https://www.census.gov/geo/ maps-data/maps/pdfs/reference/us_regdiv.pdf. Accessed December 17, 2012.
20. Virnig BA. Toward a better understanding of the role of geography in intensity of end-of-life care: must we first come to an understanding of end-of-life care? Medical Care. 2007;45(5):374-376.
21. Virnig BA, Kind S, McBean M, Fisher E. Geographic variation in hospice use prior to death. JAGS. 2000;48(9):117-11.
22. McCarthy EP, Burns RB, Davis RB, Phillips RS. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21(4): 728-735.
23. Virnig BA, Ma H, Hartman LK, Moscovice I, Carlin B. Access to home-based hospice care for rural populations: identification of areas lacking service. J Palliat Med. 2006;9(6):1292-1299.
24. Jones D, Edes T, Shreve S. You won’t know if you’re improving unless you measure: recommendations for evaluating hospice-veteran partnerships. J Pain Symptom Manage. 2006;32(5):488-496.
25. Surbone A, Baider L, Weitzman TS, Brames MJ, Rittenberg CN, Johnson J, et al. Psychosocial care for patients and their families is integral to supportive care in cancer: MASCC position statement. Support Care Cancer. 2010;18(2):255-263.
26. Callahan M, Sanderson, J. A breast cancer tracking system. The Permanente Journal. 2000;4:36-39. http://www.thepermanentejournal.org/files/Fall2000/BreastCancer.pdf. Accessed June 1, 2012.
Background Morbidity related to cancer and its treatment remains a significant source of human suffering and a challenge to the delivery of high-quality care.
Objective To develop and apply quality indicators to evaluate quality of supportive care for advanced lung cancer in the Veterans Health Administration (VHA) and examine facility-level predictors of quality.
Methods We evaluated supportive care quality using 12 quality indicators. Data were taken from VHA electronic health records for incident lung cancer cases occurring during 2007. Organizational characteristics of 111 VHA facilities were examined for association with receipt of care.
Results Rates of care-receipt were high, especially in the treatment toxicity (89%) and pain management (79%-98%) domains, but were lower in the palliative cancer treatment (60%-90%) and hospice (75%) domains, with substantial facility-level variation. Presence of a care tracking method that was monitored by a midlevel practitioner seemed to be associated with better quality for treatment toxicity (OR, 3.38; 95% CI, 1.87-6.10) and referral to hospice (OR, 1.60; 95% CI, 1.22-2.28); having a psychologist for cancer patients was associated with higher odds for pain management (OR, 1.76; 95% CI, 1.16-2.66).
Limitations Not all supportive care was evaluated. Care processes identified as present at facilities may not have been applied to cohort patients. Facility-level results may be influenced by errors in attributing a patient’s care to the correct facility.
Conclusions Quality indicators for supportive cancer care can be developed and applied in large evaluations using electronic health record review. This study confirmed high-quality supportive care, while identifying significant facility-level variation in VHA.
Funding Veterans Health Administration Office of Informatics and Analytics.
Morbidity related to cancer and its treatment remains a significant source of human suffering and a national challenge to delivery of high-quality cancer care. Quality care refers to the delivery of state-of-the-art treatments intended to achieve cure or prolong life as well as the supportive processes that address the disease- and treatment-related burdens of living with cancer. These processes span the cancer care continuum from diagnosis to end of life, and include pain-, symptom-, and side effect-management; psychosocial support; communication needs; and support for caregivers.1-5
A 2006 report from the Agency for Healthcare Research and Quality concluded that “a large number of measures are available for addressing palliative cancer care, but testing them in relevant populations is urgently needed.”6 Since then, evidence-based standards have been translated into “quality indicators” that may be used to identify outcome targets indicative of quality care, such as patient reports of pain reduction; or, they may specify facility-level care processes associated with these outcomes, such as pain screening, treatment, and follow-up assessment.1,7-9 Quality indicators can be used as the basis for tools to
measure processes that are critical to ensuring highquality supportive cancer care and identifying specific processes and practice sites that should be targeted for quality improvement efforts.10,11 Such tools can help characterize care quality for patient populations served by a health care system as a whole, while revealing important variations at individual facilities within the system.
Quality indicators for supportive and end-of-life care have been successfully applied to electronic health record (EHR) data in research studies assessing care quality in patients with different cancer types and showing supportive care for cancer to be generally in need of quality improvement.10-15 In this paper, we examine supportive care findings from the first largescale application of quality indicators in a system-wide evaluation of lung cancer care in the Veterans Health Administration (VHA). The evaluation was part of a series of operationally motivated projects to identify targets for cancer care improvement in VHA. Lung cancer, the most common malignancy in the United States, accounts for one-fifth of all tumors diagnosed each year within this large, integrated health care system and is responsible for a significant proportion of the system’s supportive care needs. Recently demonstrated benefits of early palliative care on the quality of life and end-of-life care in patients with advanced lung cancer point to the particular importance of assessing supportive care in this population.16 We report VHA evaluation findings and subsequent research analyses seeking to identify patient and facility characteristics associated with better quality supportive lung cancer care.
Methods
Quality Indicators
Quality indicators for supportive cancer care were identified from a systematic review of existing measures and expert guidelines. A panel of 9 national palliative and supportive cancer care experts rated the validity and feasibility of the indicators for use in the VHA using a modified Delphi panel method adapted from the RAND-UCLA appropriateness method17 and prioritized candidate indicators in a ranking exercise. Those with low validity or feasibility scores, or low priority rankings, were excluded, resulting in a final set of 12 quality indicators (8 supportive care, 4 end-of-life care) for use in the national evaluation.
Evaluation Population
All incident non-small-cell (NSCLC) and small-cell (SCLC) lung cancer cases diagnosed within the VHA during 2007 (N = 7,816) were identified through the Veterans Affairs Central Cancer Registry (VACCR). Cases were eligible for the study if the patient had advanced cancer (extensive small-cell or metastatic non-small-cell lung cancer) and had lived long enough to be eligible for the supportive care that was being evaluated. Patients were excluded if the health record did not confirm the lung cancer diagnosis (eg, no pathologic diagnosis or diagnosis outside the VHA, n = 1,297); they had a preexisting or concurrent diagnosis of metastatic neoplasm other than lung cancer (n = 540); had died or enrolled in hospice ≤ 30 days after diagnosis (n = 947); had documented
“comfort measures only” ≤ 30 days after diagnosis (n = 91); had documented life expectancy of ≤ 6 months at time of diagnosis (n = 39); or enrolled in a clinical trial (as care received as part of a trial may not be completely captured in the VHA record (n = 57). Of the patients meeting inclusion criteria, 2,969 were eligible for at least one of the supportive care quality indicators.
Data Collection
EHRs were reviewed remotely by abstractors from the West Virginia Medical Institute, the VHA’s contractor for its external peer-review program. Data were collected retrospectively from the year before to the 2007 diagnosis, with follow-up through 2009. Stage was determined through the VACCR or by abstraction if no VACCR stage was available. The VACCR also provided sociodemographic information including race, which is abstracted from patient charts by cancer registrars. Urban or rural residence was determined by using the rural-urban commuting area codes. Case-level quality indicator results were provided to each facility for review to identify any missed documentation, and abstractors updated data as appropriate. Facility characteristics for the Veterans Administration Medical Centers (VAMCs) at which patients received care were obtained from the 2009 Veterans Health Administration (VHA) Oncology Services
Survey.18 The study was approved by the Veterans Administration Greater Los Angeles Healthcare System Institutional Review Board Subcommittee on Human Studies.
Dependent Variables
For each quality indicator, cases eligible for the indicator were categorized as having received the specified care or not. Patient refusals of specified care or documentation of a contraindication were also considered to have met criteria for receipt of appropriate care. For bivariate and multivariate analyses, indicators were grouped into 4 domains: treatment toxicity (1 indicator), pain screening and management (6), palliative cancer treatment (4), and hospice (1). For these domain analyses, cases were considered to have met criteria for receipt of recommended supportive care if they received the care specified
for all of the indicators for which they were eligible in the domain.
Independent Variables
Patient characteristics included stage of disease, age, gender, race (white, black, other/unknown), marital status (married/living with partner, other), urban/rural residence, and performance status (poor/not poor), as shown in Table 1. Performance status was abstracted from the medical record as ECOG (Eastern Cooperative Oncology Group) or Karnofsky when present, but most of the time it was recorded in patients’ charts qualitatively (eg, “poor performance status”). Cases were attributed to facilities based on the VACCR attribution, which is usually the VHA facility where treatment is initiated. Facility characteristics included geographic region; number of unique patients; facility complexity level; chemotherapy availability on-site; radiation therapy availability on-site; and types of palliative care services, psychosocial support, and patient tracking and case management available on-site (Table 2).
Statistical Analyses
Bivariate analyses were performed to determine which patient and facility characteristics were associated with quality of care for treatment toxicity, pain, palliative treatment, and hospice. Multilevel logistic regression models were developed for each of these 4 domains to examine the relationship between indicator care receipt and patient and facility characteristics. Collinearity was assessed by examining Pearson correlations between independent variables, and highly collinear variables were not included in multivariate analyses.
Independent variables significantly associated with dependent variables in bivariate analyses or considered necessary for adjustment from conceptual standpoints were included in the multivariate models (cancer type [NSCLC or SCLC], age, sex, race, marital status, urban/rural classification, poor performance status, Veterans Integrated Service Network location, facility complexity level, facility region, as well as the facility having the following cancer-related services: tumor board, palliative care unit, palliative care services, chaplain, method for tracking patients through treatment and posttreatment care (to ensure receipt of timely and appropriate care), and presence of a case manager]. Testing yielded no significant interactions between any of the variables; thus interaction terms were not included in the final regressions. All analyses were performed using SAS statistical software, version 9.1 (Cary, NC).
Results
Patient Characteristics
Table 1 summarizes patient characteristics of this elderly (mean age, 67 years; SD, 9), mostly white, and mostly male veteran cohort. In all, 15% of the cohort was black, nearly one-third lived in a rural area, and 46% were married or living with a partner. Eighteen percent had poor performance status, 38% were referred for palliative care, and 81% died during the study period.
Organizational Characteristics
Patients in the cohort were cared for at one or more of 111 VAMC facilities (Table 2), with the largest proportion (45%) seen at facilities located in the South. Most patients (76%) were seen in low-complexity facilities (54% of facilities) and almost all (95%) used a facility reporting having at least 1 tumor board (76% of facilities) in the 2009 VHA Oncology Services Survey. Most facilities (90%) reported having chemotherapy on-site, with 99% of patients in our cohort receiving care at one of these facilities. Radiation therapy at most sites was provided by referral to a non-VA facility, although 31% of facilities had radiation therapy available on-site and these VAMCs provided care to 40% of the patients. A substantial proportion of facilities offered inpatient (91%) and outpatient (76%) palliative care consultation, serving the majority of patients, 95% and 78%, respectively. Various forms of psychosocial support were reported at facilities serving over half of the study patients. Forty-five percent of veterans were seen at facilities that provided care tracking for lung cancer patients (41% of facilities) and at 14% of facilities (12% of patients) the tracking method was monitored by a nurse practitioner or physician assistant. Sixty-eight percent of patients were cared for at facilities reporting the presence of a case manager (69%). (The fact that a site has chemotherapy or any of the other aforementioned services in-house does not imply that patients receiving care at this site were eligible for or received these particular services, but rather that they were cared for at a facility reporting presence of the service.)
About 8% of patients were diagnosed at a VAMC different from the VACCR attribution. Six percent received specialty service consultations or treatment at multiple VAMCs, and about 25% received at least one specialty service consultation or treatment at a non-VHA facility.
Quality Indicator Eligibility and Rates of Receiving Recommended Care
Patient eligibility for the quality indicators (Table 3) ranged from 1% (treatment of spinal cord compression) to 68% (prevention of chemotherapy-related nausea and vomiting). Few patients were eligible for palliative treatment indicators (palliative radiation therapy and treatment of spinal cord compression), with only 2% of patients (50 individuals) eligible for the imaging indicator. Eligibility for the two indicators related to use of shortacting opioids for break-through pain was also low (2%-4%).
Mean national rates for recommended support ive care were generally high (above 85% for 8 of the 12 indicators), ranging from 98% for outpatient pain screening to a low of 60% for spine MRI or myelography within 24 hours for suspected spinal cord compression (Table 3). There was pronounced variability across facilities, with facility rates in every domain ranging from 0%-100% (Figure 1). Many facilities scored 100% in at least 1 domain (eg, 44 of 109 facilities for pain screening; 51 of 94 for palliative cancer therapy). Eight (of 111) facilities did not provide recommended care in 1 domain and 1 did not provide recommended care in 2 domains (ie, 0% of recommended care).
In bivariate analyses (data not shown), referral to palliative care was associated with better care for treatment toxicity (P = .04) but not the other domains. Patients with poor performance status were more likely to be referred to hospice (81% vs 74% for those with good performance status, P = .005) as were patients who lived in an urban zip code area (77% vs 71% for rural zip code, P = .03). In multivariate analyses (Table 4), small-cell cancer type, though not significant in the bivariate analyses, was associated with a higher odds of receiving recommended care [OR, 2.71; 95% CI, 1.89-3.86)] in the
treatment toxicity domain and lower odds in the pain domain [OR, 0.50; 95% CI, 0.33-0.77)]; and poor performance remained associated with higher odds of referral to hospice [OR, 1.49; 95% CI, 1.12-1.99)] after adjusting for other patient and facility characteristics.
In multivariate analysis, moderate facility complexity was associated with lower quality in the toxicity domain than was low facility complexity [OR, 0.28; 95% CI, 0.18-0.44)]; however there was no such difference between the low- and high-complexity facilities. The presence of a patient tracking method appeared to be associated with better quality of care in the treatment toxicity domain [OR, 3.38; 95% CI, 1.87-6.10)] and a higher likelihood of referral to hospice [OR, 1.60; 95% CI, 1.22-2.28)] when the method was monitored by a midlevel practitioner (nurse practitioner or physician assistant), but no association was found for methods monitored by other types of staff. In the pain domain, having a psychologist specializing in cancer was associated with a higher odds of receiving recommended care [OR, 1.76; 95% CI, 1.16-2.66)]. No patient or facility characteristics were associated with receiving recommended palliative cancer therapy, although statistical power was limited by the small numbers of eligible events.
Discussion
Use of evidence-based measures to assess care quality proved feasible in this evaluation of supportive care for advanced lung cancer in a large national integrated health care system and confirmed that gaps found in smaller studies represent critical unmet needs for patients. The methods permitted individualized feedback to facilities, accompanied by support for quality improvement efforts. The effectiveness of providing facility feedback with quality improvement strategies will be evaluated in follow-up studies.
Although quality of supportive care as measured by this specific set of indicators was quite high compared to previous reports,10,13,15 substantial variation across facilities was observed even for measures with high overall performance. Comparatively high levels of supportive care may reflect greater emphasis on supportive measures for advanced lung cancer patients whose expectation of cure is low (other studies evaluated cohorts with mixed cancer types), or the fact that previous studies did not account for documentation of a reason for forgoing indicated care. Tough high quality of supportive care for lung cancer in VHA nationally is encouraging, variability across individual facilities is of concern. Such variation may well exist in any large health care system, with patients potentially experiencing different standards of care depending on where they live.
In this study, facilities located in the South, treating 45% of advanced lung cancer patients in the cohort, were associated with worse care in the treatment toxicity and hospice domains, and those in the Northeast appeared to generally have worse care for pain. Although reasons for these differences are unclear, geographic variation in Medicare hospice use has been related to a variety of determinants,20-23 some of which may also apply to VHA. Referral to palliative care or hospice was among the lowest scoring quality indicators in the evaluation, with a 75% national rate and 43% of facilities documenting referral for less than 75% of eligible cases. Although our measures determined only referral to palliative care or hospice (versus actual receipt of services), low rates may in part reflect provider anticipation of low local availability of hospice. Although the VHA Hospice and Palliative Care program has made progress in reducing variability in hospice access for Veterans, surveys conducted by Hospice-Veteran Partnerships showed lack of shared knowledge about the different systems of benefits and health care available for veterans, misunderstandings about referral processes among health care providers and payment for hospice services, and difficulties in caring for veterans across multiple care settings to be barriers to Veteran access to community services.24 Targeted inquiry at lowreferral facilities may elucidate which of these and other possible challenges may suggest points of intervention for improvement in hospice referral patterns and support of appropriate hospice use.
Attempting to identify facility-related explanations for overall variability, we explored the potential role of a number of structural variables, including presence of a tumor board, a palliative care unit, outpatient palliative care services, chaplain services, presence of a cancer care tracking system, and presence of a case manager. Tough facilities with a psychologist specializing in cancer provided better care for pain, and those with midlevel practitioners (nurse practitioner or physician assistant) monitoring patient tracking through care were more likely to appropriately refer to hospice and have better care for treatment toxicity, many facilities without such designated professionals also provided quality care in these domains. Because of limited methodological feasibility of demonstrating patient receipt of psychosocial/spiritual support and care tracking using medical record review, the final set of quality indicators did not include measures for these forms of care; however, psychosocial care is considered integral to supportive care in cancer25 and care tracking has been associated with improved cancer care in non-VA settings.26 The presence of appropriate professionals providing cancer-specific psychological support and tracking may be directly associated with improved care, not only in the domains noted above, but also in the important domain of existential and emotional well-being not evaluated here. These services may also be part of larger facility-level quality improvement mechanisms contributing to cancer care quality that are worth identifying in future research. Lack of consistent explanations for the overall facility-level variation in this study suggests the need to identify data sources that can be used to measure additional organizational characteristics in future research.
Our study has several limitations. The scope of the national evaluation did not include indicators for all aspects of supportive care, including care for dyspnea, depression, and psychosocial distress; support for care givers; and care planning assistance. Small numbers of eligible events per facility for some indicators limit interpretation, particularly in the palliative treatment domain, where the largest range was 1-15 eligible events per facility. Facility characteristics used for analyses were identified from a surveyand may be subject to respondent error. Potentially useful processes, such as a midlevel practitioner monitored tracking system were identified as “present” at the facility, but use for cohort patients was not possible to confirm, limiting direct linkage with better care. Some facility-level results may be influenced by errors in VACCR attribution to a facility that was not directly involved with the patient’s care, specialty service receipt at multiple VAMCs, or through contract care at non-VHA facilities. Quality-of-care coordination between different VHA and non-VHA facilities for services not provided at the diagnosing facility, or to reduce travel burden if the VAMC was far from the patient’s home may be associated with better or worse care quality and should be evaluated in future work.
In conclusion, use of quality indicators to evaluate quality of supportive lung cancer care in a large integrated health care system proved feasible, confirmed
provision of overall high-quality supportive care, but identified a high degree of variability across individual facilities in the system. Facility characteristics examined did not explain this variation; however, the data suggested that, while not accounting for the overall variation, having professionals providing cancer-specific psychological support and care-tracking may contribute to better quality of care in some domains. Difficulties in identifying predictors of quality suggest that future research should include qualitative comparisons of facilities with varying rates of providing recommended supportive care to identify potentially impactful organizational factors not examined in this study.
Disclaimer and Disclosures
Accepted for publication February 24, 2014. Correspondence: Sabine Oishi, PhD, MSPH; sabine.oishi@va.gov. Disclosures: Dr Ryoo was supported in part by the Robert Wood Johnson Foundation Clinical Scholars Program and by the UCLA Jonsson Comprehensive Cancer Center R25 grant. The authors have no direct conflicts of interest to disclose. Dr Karl Lorenz is a consultant as a member of the Data Monitoring Committee for a Phase II trial of Sativex being conducted by Otsuka Pharmaceuticals. The current study has no direct financial implications related to his participation and was conducted entirely outside of the timeframe of his consultancy; however, it may be perceived as indirectly related. The views and opinions of authors expressed herein do not necessarily state or reflect that of the Department of Veterans Affairs, the United States Government, Kaiser Permanente, Stanford University, or the University of California.
Click here to read the digital edition.
Background Morbidity related to cancer and its treatment remains a significant source of human suffering and a challenge to the delivery of high-quality care.
Objective To develop and apply quality indicators to evaluate quality of supportive care for advanced lung cancer in the Veterans Health Administration (VHA) and examine facility-level predictors of quality.
Methods We evaluated supportive care quality using 12 quality indicators. Data were taken from VHA electronic health records for incident lung cancer cases occurring during 2007. Organizational characteristics of 111 VHA facilities were examined for association with receipt of care.
Results Rates of care-receipt were high, especially in the treatment toxicity (89%) and pain management (79%-98%) domains, but were lower in the palliative cancer treatment (60%-90%) and hospice (75%) domains, with substantial facility-level variation. Presence of a care tracking method that was monitored by a midlevel practitioner seemed to be associated with better quality for treatment toxicity (OR, 3.38; 95% CI, 1.87-6.10) and referral to hospice (OR, 1.60; 95% CI, 1.22-2.28); having a psychologist for cancer patients was associated with higher odds for pain management (OR, 1.76; 95% CI, 1.16-2.66).
Limitations Not all supportive care was evaluated. Care processes identified as present at facilities may not have been applied to cohort patients. Facility-level results may be influenced by errors in attributing a patient’s care to the correct facility.
Conclusions Quality indicators for supportive cancer care can be developed and applied in large evaluations using electronic health record review. This study confirmed high-quality supportive care, while identifying significant facility-level variation in VHA.
Funding Veterans Health Administration Office of Informatics and Analytics.
Morbidity related to cancer and its treatment remains a significant source of human suffering and a national challenge to delivery of high-quality cancer care. Quality care refers to the delivery of state-of-the-art treatments intended to achieve cure or prolong life as well as the supportive processes that address the disease- and treatment-related burdens of living with cancer. These processes span the cancer care continuum from diagnosis to end of life, and include pain-, symptom-, and side effect-management; psychosocial support; communication needs; and support for caregivers.1-5
A 2006 report from the Agency for Healthcare Research and Quality concluded that “a large number of measures are available for addressing palliative cancer care, but testing them in relevant populations is urgently needed.”6 Since then, evidence-based standards have been translated into “quality indicators” that may be used to identify outcome targets indicative of quality care, such as patient reports of pain reduction; or, they may specify facility-level care processes associated with these outcomes, such as pain screening, treatment, and follow-up assessment.1,7-9 Quality indicators can be used as the basis for tools to
measure processes that are critical to ensuring highquality supportive cancer care and identifying specific processes and practice sites that should be targeted for quality improvement efforts.10,11 Such tools can help characterize care quality for patient populations served by a health care system as a whole, while revealing important variations at individual facilities within the system.
Quality indicators for supportive and end-of-life care have been successfully applied to electronic health record (EHR) data in research studies assessing care quality in patients with different cancer types and showing supportive care for cancer to be generally in need of quality improvement.10-15 In this paper, we examine supportive care findings from the first largescale application of quality indicators in a system-wide evaluation of lung cancer care in the Veterans Health Administration (VHA). The evaluation was part of a series of operationally motivated projects to identify targets for cancer care improvement in VHA. Lung cancer, the most common malignancy in the United States, accounts for one-fifth of all tumors diagnosed each year within this large, integrated health care system and is responsible for a significant proportion of the system’s supportive care needs. Recently demonstrated benefits of early palliative care on the quality of life and end-of-life care in patients with advanced lung cancer point to the particular importance of assessing supportive care in this population.16 We report VHA evaluation findings and subsequent research analyses seeking to identify patient and facility characteristics associated with better quality supportive lung cancer care.
Methods
Quality Indicators
Quality indicators for supportive cancer care were identified from a systematic review of existing measures and expert guidelines. A panel of 9 national palliative and supportive cancer care experts rated the validity and feasibility of the indicators for use in the VHA using a modified Delphi panel method adapted from the RAND-UCLA appropriateness method17 and prioritized candidate indicators in a ranking exercise. Those with low validity or feasibility scores, or low priority rankings, were excluded, resulting in a final set of 12 quality indicators (8 supportive care, 4 end-of-life care) for use in the national evaluation.
Evaluation Population
All incident non-small-cell (NSCLC) and small-cell (SCLC) lung cancer cases diagnosed within the VHA during 2007 (N = 7,816) were identified through the Veterans Affairs Central Cancer Registry (VACCR). Cases were eligible for the study if the patient had advanced cancer (extensive small-cell or metastatic non-small-cell lung cancer) and had lived long enough to be eligible for the supportive care that was being evaluated. Patients were excluded if the health record did not confirm the lung cancer diagnosis (eg, no pathologic diagnosis or diagnosis outside the VHA, n = 1,297); they had a preexisting or concurrent diagnosis of metastatic neoplasm other than lung cancer (n = 540); had died or enrolled in hospice ≤ 30 days after diagnosis (n = 947); had documented
“comfort measures only” ≤ 30 days after diagnosis (n = 91); had documented life expectancy of ≤ 6 months at time of diagnosis (n = 39); or enrolled in a clinical trial (as care received as part of a trial may not be completely captured in the VHA record (n = 57). Of the patients meeting inclusion criteria, 2,969 were eligible for at least one of the supportive care quality indicators.
Data Collection
EHRs were reviewed remotely by abstractors from the West Virginia Medical Institute, the VHA’s contractor for its external peer-review program. Data were collected retrospectively from the year before to the 2007 diagnosis, with follow-up through 2009. Stage was determined through the VACCR or by abstraction if no VACCR stage was available. The VACCR also provided sociodemographic information including race, which is abstracted from patient charts by cancer registrars. Urban or rural residence was determined by using the rural-urban commuting area codes. Case-level quality indicator results were provided to each facility for review to identify any missed documentation, and abstractors updated data as appropriate. Facility characteristics for the Veterans Administration Medical Centers (VAMCs) at which patients received care were obtained from the 2009 Veterans Health Administration (VHA) Oncology Services
Survey.18 The study was approved by the Veterans Administration Greater Los Angeles Healthcare System Institutional Review Board Subcommittee on Human Studies.
Dependent Variables
For each quality indicator, cases eligible for the indicator were categorized as having received the specified care or not. Patient refusals of specified care or documentation of a contraindication were also considered to have met criteria for receipt of appropriate care. For bivariate and multivariate analyses, indicators were grouped into 4 domains: treatment toxicity (1 indicator), pain screening and management (6), palliative cancer treatment (4), and hospice (1). For these domain analyses, cases were considered to have met criteria for receipt of recommended supportive care if they received the care specified
for all of the indicators for which they were eligible in the domain.
Independent Variables
Patient characteristics included stage of disease, age, gender, race (white, black, other/unknown), marital status (married/living with partner, other), urban/rural residence, and performance status (poor/not poor), as shown in Table 1. Performance status was abstracted from the medical record as ECOG (Eastern Cooperative Oncology Group) or Karnofsky when present, but most of the time it was recorded in patients’ charts qualitatively (eg, “poor performance status”). Cases were attributed to facilities based on the VACCR attribution, which is usually the VHA facility where treatment is initiated. Facility characteristics included geographic region; number of unique patients; facility complexity level; chemotherapy availability on-site; radiation therapy availability on-site; and types of palliative care services, psychosocial support, and patient tracking and case management available on-site (Table 2).
Statistical Analyses
Bivariate analyses were performed to determine which patient and facility characteristics were associated with quality of care for treatment toxicity, pain, palliative treatment, and hospice. Multilevel logistic regression models were developed for each of these 4 domains to examine the relationship between indicator care receipt and patient and facility characteristics. Collinearity was assessed by examining Pearson correlations between independent variables, and highly collinear variables were not included in multivariate analyses.
Independent variables significantly associated with dependent variables in bivariate analyses or considered necessary for adjustment from conceptual standpoints were included in the multivariate models (cancer type [NSCLC or SCLC], age, sex, race, marital status, urban/rural classification, poor performance status, Veterans Integrated Service Network location, facility complexity level, facility region, as well as the facility having the following cancer-related services: tumor board, palliative care unit, palliative care services, chaplain, method for tracking patients through treatment and posttreatment care (to ensure receipt of timely and appropriate care), and presence of a case manager]. Testing yielded no significant interactions between any of the variables; thus interaction terms were not included in the final regressions. All analyses were performed using SAS statistical software, version 9.1 (Cary, NC).
Results
Patient Characteristics
Table 1 summarizes patient characteristics of this elderly (mean age, 67 years; SD, 9), mostly white, and mostly male veteran cohort. In all, 15% of the cohort was black, nearly one-third lived in a rural area, and 46% were married or living with a partner. Eighteen percent had poor performance status, 38% were referred for palliative care, and 81% died during the study period.
Organizational Characteristics
Patients in the cohort were cared for at one or more of 111 VAMC facilities (Table 2), with the largest proportion (45%) seen at facilities located in the South. Most patients (76%) were seen in low-complexity facilities (54% of facilities) and almost all (95%) used a facility reporting having at least 1 tumor board (76% of facilities) in the 2009 VHA Oncology Services Survey. Most facilities (90%) reported having chemotherapy on-site, with 99% of patients in our cohort receiving care at one of these facilities. Radiation therapy at most sites was provided by referral to a non-VA facility, although 31% of facilities had radiation therapy available on-site and these VAMCs provided care to 40% of the patients. A substantial proportion of facilities offered inpatient (91%) and outpatient (76%) palliative care consultation, serving the majority of patients, 95% and 78%, respectively. Various forms of psychosocial support were reported at facilities serving over half of the study patients. Forty-five percent of veterans were seen at facilities that provided care tracking for lung cancer patients (41% of facilities) and at 14% of facilities (12% of patients) the tracking method was monitored by a nurse practitioner or physician assistant. Sixty-eight percent of patients were cared for at facilities reporting the presence of a case manager (69%). (The fact that a site has chemotherapy or any of the other aforementioned services in-house does not imply that patients receiving care at this site were eligible for or received these particular services, but rather that they were cared for at a facility reporting presence of the service.)
About 8% of patients were diagnosed at a VAMC different from the VACCR attribution. Six percent received specialty service consultations or treatment at multiple VAMCs, and about 25% received at least one specialty service consultation or treatment at a non-VHA facility.
Quality Indicator Eligibility and Rates of Receiving Recommended Care
Patient eligibility for the quality indicators (Table 3) ranged from 1% (treatment of spinal cord compression) to 68% (prevention of chemotherapy-related nausea and vomiting). Few patients were eligible for palliative treatment indicators (palliative radiation therapy and treatment of spinal cord compression), with only 2% of patients (50 individuals) eligible for the imaging indicator. Eligibility for the two indicators related to use of shortacting opioids for break-through pain was also low (2%-4%).
Mean national rates for recommended support ive care were generally high (above 85% for 8 of the 12 indicators), ranging from 98% for outpatient pain screening to a low of 60% for spine MRI or myelography within 24 hours for suspected spinal cord compression (Table 3). There was pronounced variability across facilities, with facility rates in every domain ranging from 0%-100% (Figure 1). Many facilities scored 100% in at least 1 domain (eg, 44 of 109 facilities for pain screening; 51 of 94 for palliative cancer therapy). Eight (of 111) facilities did not provide recommended care in 1 domain and 1 did not provide recommended care in 2 domains (ie, 0% of recommended care).
In bivariate analyses (data not shown), referral to palliative care was associated with better care for treatment toxicity (P = .04) but not the other domains. Patients with poor performance status were more likely to be referred to hospice (81% vs 74% for those with good performance status, P = .005) as were patients who lived in an urban zip code area (77% vs 71% for rural zip code, P = .03). In multivariate analyses (Table 4), small-cell cancer type, though not significant in the bivariate analyses, was associated with a higher odds of receiving recommended care [OR, 2.71; 95% CI, 1.89-3.86)] in the
treatment toxicity domain and lower odds in the pain domain [OR, 0.50; 95% CI, 0.33-0.77)]; and poor performance remained associated with higher odds of referral to hospice [OR, 1.49; 95% CI, 1.12-1.99)] after adjusting for other patient and facility characteristics.
In multivariate analysis, moderate facility complexity was associated with lower quality in the toxicity domain than was low facility complexity [OR, 0.28; 95% CI, 0.18-0.44)]; however there was no such difference between the low- and high-complexity facilities. The presence of a patient tracking method appeared to be associated with better quality of care in the treatment toxicity domain [OR, 3.38; 95% CI, 1.87-6.10)] and a higher likelihood of referral to hospice [OR, 1.60; 95% CI, 1.22-2.28)] when the method was monitored by a midlevel practitioner (nurse practitioner or physician assistant), but no association was found for methods monitored by other types of staff. In the pain domain, having a psychologist specializing in cancer was associated with a higher odds of receiving recommended care [OR, 1.76; 95% CI, 1.16-2.66)]. No patient or facility characteristics were associated with receiving recommended palliative cancer therapy, although statistical power was limited by the small numbers of eligible events.
Discussion
Use of evidence-based measures to assess care quality proved feasible in this evaluation of supportive care for advanced lung cancer in a large national integrated health care system and confirmed that gaps found in smaller studies represent critical unmet needs for patients. The methods permitted individualized feedback to facilities, accompanied by support for quality improvement efforts. The effectiveness of providing facility feedback with quality improvement strategies will be evaluated in follow-up studies.
Although quality of supportive care as measured by this specific set of indicators was quite high compared to previous reports,10,13,15 substantial variation across facilities was observed even for measures with high overall performance. Comparatively high levels of supportive care may reflect greater emphasis on supportive measures for advanced lung cancer patients whose expectation of cure is low (other studies evaluated cohorts with mixed cancer types), or the fact that previous studies did not account for documentation of a reason for forgoing indicated care. Tough high quality of supportive care for lung cancer in VHA nationally is encouraging, variability across individual facilities is of concern. Such variation may well exist in any large health care system, with patients potentially experiencing different standards of care depending on where they live.
In this study, facilities located in the South, treating 45% of advanced lung cancer patients in the cohort, were associated with worse care in the treatment toxicity and hospice domains, and those in the Northeast appeared to generally have worse care for pain. Although reasons for these differences are unclear, geographic variation in Medicare hospice use has been related to a variety of determinants,20-23 some of which may also apply to VHA. Referral to palliative care or hospice was among the lowest scoring quality indicators in the evaluation, with a 75% national rate and 43% of facilities documenting referral for less than 75% of eligible cases. Although our measures determined only referral to palliative care or hospice (versus actual receipt of services), low rates may in part reflect provider anticipation of low local availability of hospice. Although the VHA Hospice and Palliative Care program has made progress in reducing variability in hospice access for Veterans, surveys conducted by Hospice-Veteran Partnerships showed lack of shared knowledge about the different systems of benefits and health care available for veterans, misunderstandings about referral processes among health care providers and payment for hospice services, and difficulties in caring for veterans across multiple care settings to be barriers to Veteran access to community services.24 Targeted inquiry at lowreferral facilities may elucidate which of these and other possible challenges may suggest points of intervention for improvement in hospice referral patterns and support of appropriate hospice use.
Attempting to identify facility-related explanations for overall variability, we explored the potential role of a number of structural variables, including presence of a tumor board, a palliative care unit, outpatient palliative care services, chaplain services, presence of a cancer care tracking system, and presence of a case manager. Tough facilities with a psychologist specializing in cancer provided better care for pain, and those with midlevel practitioners (nurse practitioner or physician assistant) monitoring patient tracking through care were more likely to appropriately refer to hospice and have better care for treatment toxicity, many facilities without such designated professionals also provided quality care in these domains. Because of limited methodological feasibility of demonstrating patient receipt of psychosocial/spiritual support and care tracking using medical record review, the final set of quality indicators did not include measures for these forms of care; however, psychosocial care is considered integral to supportive care in cancer25 and care tracking has been associated with improved cancer care in non-VA settings.26 The presence of appropriate professionals providing cancer-specific psychological support and tracking may be directly associated with improved care, not only in the domains noted above, but also in the important domain of existential and emotional well-being not evaluated here. These services may also be part of larger facility-level quality improvement mechanisms contributing to cancer care quality that are worth identifying in future research. Lack of consistent explanations for the overall facility-level variation in this study suggests the need to identify data sources that can be used to measure additional organizational characteristics in future research.
Our study has several limitations. The scope of the national evaluation did not include indicators for all aspects of supportive care, including care for dyspnea, depression, and psychosocial distress; support for care givers; and care planning assistance. Small numbers of eligible events per facility for some indicators limit interpretation, particularly in the palliative treatment domain, where the largest range was 1-15 eligible events per facility. Facility characteristics used for analyses were identified from a surveyand may be subject to respondent error. Potentially useful processes, such as a midlevel practitioner monitored tracking system were identified as “present” at the facility, but use for cohort patients was not possible to confirm, limiting direct linkage with better care. Some facility-level results may be influenced by errors in VACCR attribution to a facility that was not directly involved with the patient’s care, specialty service receipt at multiple VAMCs, or through contract care at non-VHA facilities. Quality-of-care coordination between different VHA and non-VHA facilities for services not provided at the diagnosing facility, or to reduce travel burden if the VAMC was far from the patient’s home may be associated with better or worse care quality and should be evaluated in future work.
In conclusion, use of quality indicators to evaluate quality of supportive lung cancer care in a large integrated health care system proved feasible, confirmed
provision of overall high-quality supportive care, but identified a high degree of variability across individual facilities in the system. Facility characteristics examined did not explain this variation; however, the data suggested that, while not accounting for the overall variation, having professionals providing cancer-specific psychological support and care-tracking may contribute to better quality of care in some domains. Difficulties in identifying predictors of quality suggest that future research should include qualitative comparisons of facilities with varying rates of providing recommended supportive care to identify potentially impactful organizational factors not examined in this study.
Disclaimer and Disclosures
Accepted for publication February 24, 2014. Correspondence: Sabine Oishi, PhD, MSPH; sabine.oishi@va.gov. Disclosures: Dr Ryoo was supported in part by the Robert Wood Johnson Foundation Clinical Scholars Program and by the UCLA Jonsson Comprehensive Cancer Center R25 grant. The authors have no direct conflicts of interest to disclose. Dr Karl Lorenz is a consultant as a member of the Data Monitoring Committee for a Phase II trial of Sativex being conducted by Otsuka Pharmaceuticals. The current study has no direct financial implications related to his participation and was conducted entirely outside of the timeframe of his consultancy; however, it may be perceived as indirectly related. The views and opinions of authors expressed herein do not necessarily state or reflect that of the Department of Veterans Affairs, the United States Government, Kaiser Permanente, Stanford University, or the University of California.
Click here to read the digital edition.
1. Lorenz KA, Dy SM, Naeim A, et al. Quality measures for supportive cancer care: the Cancer Quality-ASSIST Project. J Pain Symptom Manage. 2009;37(6):943-964.
2. Dy SM, Asch SM, Naeim A, Sanati H, Walling A, Lorenz KA. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
3. Gordon, DB, Dahl, JL, Miakowski, C. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med. 2005;165(14):1574-1580.
4. Lorenz, KA, Lynn, J, Dy, SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
5. Seow, H, Snyder, CF, Shugarman, LR, et al. Developing quality indicators for cancer end-of-life care: proceedings from a national symposium. Cancer. 2009;115(17):3820-3829.
6. Lorenz KA, Lynn J, Dy SM, et al. Agency for Healthcare Research and Quality (US). Cancer care quality measures: symptoms and end-of-life care. http://www.ncbi.nlm.nih.gov/books/NBK38028/. Published 2006. Accessed December 17, 2012.
7. Lorenz KA, Lynn J, Dy S, et al. Quality measures for symptoms and advance care planning in cancer: a systematic review. J Clin Oncol. 2006;24(30):4933-4938.
8. Lorenz KA, Rosenfeld K, Wenger N. Quality indicators for palliative and end-of-life care in vulnerable elders. JAGS. 2007;55(suppl 2):S318-S326.
9. Dy SM, Lorenz KA, O’Neill SM, et al. Cancer Quality-ASSIST supportive oncology quality indicator set. Cancer. 2010;116(13):3267-3275.
10. Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26(11):1893-1898.
11. Jacobsen PB, Shibata D, Siegel E, et al. Evaluating the quality of psychosocial care in outpatient medical oncology settings using performance indicators. Psycho-oncology. 2010; 20(11):1221-1227.
12. Walling AM, Asch SM, Lorenz KA, et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170(12):1057-1063.
13. Dy SM, Asch SM, Lorenz KA, et al. Quality of end-of-life care for patients with advanced cancer in an academic medical center. J Palliat Med. 2011;14(4):451-457.
14. Gonsalves WI, Tsewang T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
15. Malin JL, O’Neill SM, Asch SM, et al. Quality of supportive care for patients with advanced cancer in a VA medical center. J Palliat Med. 2011;14(5):573-577.
16. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326.
17. Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2(1):53-63.
18. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2009 VHA Oncology Services Survey. April, 2010. Accessed November 1, 2010.
19. US Census Bureau. Census Bureau regions and divisions with state FIPS codes. Available from URL: https://www.census.gov/geo/ maps-data/maps/pdfs/reference/us_regdiv.pdf. Accessed December 17, 2012.
20. Virnig BA. Toward a better understanding of the role of geography in intensity of end-of-life care: must we first come to an understanding of end-of-life care? Medical Care. 2007;45(5):374-376.
21. Virnig BA, Kind S, McBean M, Fisher E. Geographic variation in hospice use prior to death. JAGS. 2000;48(9):117-11.
22. McCarthy EP, Burns RB, Davis RB, Phillips RS. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21(4): 728-735.
23. Virnig BA, Ma H, Hartman LK, Moscovice I, Carlin B. Access to home-based hospice care for rural populations: identification of areas lacking service. J Palliat Med. 2006;9(6):1292-1299.
24. Jones D, Edes T, Shreve S. You won’t know if you’re improving unless you measure: recommendations for evaluating hospice-veteran partnerships. J Pain Symptom Manage. 2006;32(5):488-496.
25. Surbone A, Baider L, Weitzman TS, Brames MJ, Rittenberg CN, Johnson J, et al. Psychosocial care for patients and their families is integral to supportive care in cancer: MASCC position statement. Support Care Cancer. 2010;18(2):255-263.
26. Callahan M, Sanderson, J. A breast cancer tracking system. The Permanente Journal. 2000;4:36-39. http://www.thepermanentejournal.org/files/Fall2000/BreastCancer.pdf. Accessed June 1, 2012.
1. Lorenz KA, Dy SM, Naeim A, et al. Quality measures for supportive cancer care: the Cancer Quality-ASSIST Project. J Pain Symptom Manage. 2009;37(6):943-964.
2. Dy SM, Asch SM, Naeim A, Sanati H, Walling A, Lorenz KA. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
3. Gordon, DB, Dahl, JL, Miakowski, C. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med. 2005;165(14):1574-1580.
4. Lorenz, KA, Lynn, J, Dy, SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
5. Seow, H, Snyder, CF, Shugarman, LR, et al. Developing quality indicators for cancer end-of-life care: proceedings from a national symposium. Cancer. 2009;115(17):3820-3829.
6. Lorenz KA, Lynn J, Dy SM, et al. Agency for Healthcare Research and Quality (US). Cancer care quality measures: symptoms and end-of-life care. http://www.ncbi.nlm.nih.gov/books/NBK38028/. Published 2006. Accessed December 17, 2012.
7. Lorenz KA, Lynn J, Dy S, et al. Quality measures for symptoms and advance care planning in cancer: a systematic review. J Clin Oncol. 2006;24(30):4933-4938.
8. Lorenz KA, Rosenfeld K, Wenger N. Quality indicators for palliative and end-of-life care in vulnerable elders. JAGS. 2007;55(suppl 2):S318-S326.
9. Dy SM, Lorenz KA, O’Neill SM, et al. Cancer Quality-ASSIST supportive oncology quality indicator set. Cancer. 2010;116(13):3267-3275.
10. Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26(11):1893-1898.
11. Jacobsen PB, Shibata D, Siegel E, et al. Evaluating the quality of psychosocial care in outpatient medical oncology settings using performance indicators. Psycho-oncology. 2010; 20(11):1221-1227.
12. Walling AM, Asch SM, Lorenz KA, et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170(12):1057-1063.
13. Dy SM, Asch SM, Lorenz KA, et al. Quality of end-of-life care for patients with advanced cancer in an academic medical center. J Palliat Med. 2011;14(4):451-457.
14. Gonsalves WI, Tsewang T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
15. Malin JL, O’Neill SM, Asch SM, et al. Quality of supportive care for patients with advanced cancer in a VA medical center. J Palliat Med. 2011;14(5):573-577.
16. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326.
17. Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2(1):53-63.
18. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2009 VHA Oncology Services Survey. April, 2010. Accessed November 1, 2010.
19. US Census Bureau. Census Bureau regions and divisions with state FIPS codes. Available from URL: https://www.census.gov/geo/ maps-data/maps/pdfs/reference/us_regdiv.pdf. Accessed December 17, 2012.
20. Virnig BA. Toward a better understanding of the role of geography in intensity of end-of-life care: must we first come to an understanding of end-of-life care? Medical Care. 2007;45(5):374-376.
21. Virnig BA, Kind S, McBean M, Fisher E. Geographic variation in hospice use prior to death. JAGS. 2000;48(9):117-11.
22. McCarthy EP, Burns RB, Davis RB, Phillips RS. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21(4): 728-735.
23. Virnig BA, Ma H, Hartman LK, Moscovice I, Carlin B. Access to home-based hospice care for rural populations: identification of areas lacking service. J Palliat Med. 2006;9(6):1292-1299.
24. Jones D, Edes T, Shreve S. You won’t know if you’re improving unless you measure: recommendations for evaluating hospice-veteran partnerships. J Pain Symptom Manage. 2006;32(5):488-496.
25. Surbone A, Baider L, Weitzman TS, Brames MJ, Rittenberg CN, Johnson J, et al. Psychosocial care for patients and their families is integral to supportive care in cancer: MASCC position statement. Support Care Cancer. 2010;18(2):255-263.
26. Callahan M, Sanderson, J. A breast cancer tracking system. The Permanente Journal. 2000;4:36-39. http://www.thepermanentejournal.org/files/Fall2000/BreastCancer.pdf. Accessed June 1, 2012.
Osimertinib receives full approval for advanced EGFR-mutated NSCLC
The Food and Drug Administration has converted accelerated approval of osimertinib to full approval for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation–positive non–small cell lung cancer (NSCLC), as detected by an FDA-approved test.
Also included in the indication, disease must have progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy, the FDA said in a statement.
Full approval was based on an improvement in progression-free survival (PFS) in the phase III AURA3 study, which randomized 419 patients (2:1) to receive osimertinib (n = 279) 80 mg orally once daily or platinum-based doublet chemotherapy (n = 140). The hazard ratio for the investigator-assessed PFS was .30 (95% confidence interval: 0.23, 0.41; P less than .001).
The estimated median PFS was 10.1 months in the osimertinib arm and 4.4 months in the chemotherapy arm. Confirmed ORR was 65% (95% CI: 59%, 70%) and 29% (95% CI: 21%, 37%) in the osimertinib and chemotherapy arms, respectively (P less than .0001). Estimated median response durations were 11 months (95% CI: 8.6, 12.6) and 4.2 months (95% CI: 3.9, 5.9) in the osimertinib and chemotherapy arms, respectively, according to the FDA statement.
Overall survival data are immature, the FDA said.
All patients had metastatic EGFR T790M mutation–positive NSCLC, identified by the cobas EGFR mutation test performed in a central laboratory, and progressive disease following first-line EGFR TKI therapy. Patients in the chemotherapy arm received either pemetrexed, 500 mg/m2 with carboplatin AUC5, or pemetrexed, 500mg/m2 with cisplatin 75 mg/m2), on day 1 of every 21-day cycle for up to six cycles followed by pemetrexed maintenance therapy.
The most serious adverse reactions, evaluated in 833 patients receiving osimertinib, were interstitial lung disease/pneumonitis (3.5%), QTc interval prolongation (0.7%), cardiomyopathy (1.9%), and keratitis (0.7%). The most common adverse reactions were diarrhea, rash, dry skin, nail toxicity, and fatigue.
The recommended dose of osimertinib, to be marketed as Tagrisso by AstraZeneca, is 80 mg orally once daily, with or without food, until disease progression or unacceptable toxicity. The presence of an EGFR T790M mutation in a tumor specimen, or plasma specimen (if tumor tissue is unavailable), should be confirmed by an FDA-approved test prior to initiation of treatment.
Full prescribing information is available here.
The Food and Drug Administration has converted accelerated approval of osimertinib to full approval for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation–positive non–small cell lung cancer (NSCLC), as detected by an FDA-approved test.
Also included in the indication, disease must have progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy, the FDA said in a statement.
Full approval was based on an improvement in progression-free survival (PFS) in the phase III AURA3 study, which randomized 419 patients (2:1) to receive osimertinib (n = 279) 80 mg orally once daily or platinum-based doublet chemotherapy (n = 140). The hazard ratio for the investigator-assessed PFS was .30 (95% confidence interval: 0.23, 0.41; P less than .001).
The estimated median PFS was 10.1 months in the osimertinib arm and 4.4 months in the chemotherapy arm. Confirmed ORR was 65% (95% CI: 59%, 70%) and 29% (95% CI: 21%, 37%) in the osimertinib and chemotherapy arms, respectively (P less than .0001). Estimated median response durations were 11 months (95% CI: 8.6, 12.6) and 4.2 months (95% CI: 3.9, 5.9) in the osimertinib and chemotherapy arms, respectively, according to the FDA statement.
Overall survival data are immature, the FDA said.
All patients had metastatic EGFR T790M mutation–positive NSCLC, identified by the cobas EGFR mutation test performed in a central laboratory, and progressive disease following first-line EGFR TKI therapy. Patients in the chemotherapy arm received either pemetrexed, 500 mg/m2 with carboplatin AUC5, or pemetrexed, 500mg/m2 with cisplatin 75 mg/m2), on day 1 of every 21-day cycle for up to six cycles followed by pemetrexed maintenance therapy.
The most serious adverse reactions, evaluated in 833 patients receiving osimertinib, were interstitial lung disease/pneumonitis (3.5%), QTc interval prolongation (0.7%), cardiomyopathy (1.9%), and keratitis (0.7%). The most common adverse reactions were diarrhea, rash, dry skin, nail toxicity, and fatigue.
The recommended dose of osimertinib, to be marketed as Tagrisso by AstraZeneca, is 80 mg orally once daily, with or without food, until disease progression or unacceptable toxicity. The presence of an EGFR T790M mutation in a tumor specimen, or plasma specimen (if tumor tissue is unavailable), should be confirmed by an FDA-approved test prior to initiation of treatment.
Full prescribing information is available here.
The Food and Drug Administration has converted accelerated approval of osimertinib to full approval for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation–positive non–small cell lung cancer (NSCLC), as detected by an FDA-approved test.
Also included in the indication, disease must have progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy, the FDA said in a statement.
Full approval was based on an improvement in progression-free survival (PFS) in the phase III AURA3 study, which randomized 419 patients (2:1) to receive osimertinib (n = 279) 80 mg orally once daily or platinum-based doublet chemotherapy (n = 140). The hazard ratio for the investigator-assessed PFS was .30 (95% confidence interval: 0.23, 0.41; P less than .001).
The estimated median PFS was 10.1 months in the osimertinib arm and 4.4 months in the chemotherapy arm. Confirmed ORR was 65% (95% CI: 59%, 70%) and 29% (95% CI: 21%, 37%) in the osimertinib and chemotherapy arms, respectively (P less than .0001). Estimated median response durations were 11 months (95% CI: 8.6, 12.6) and 4.2 months (95% CI: 3.9, 5.9) in the osimertinib and chemotherapy arms, respectively, according to the FDA statement.
Overall survival data are immature, the FDA said.
All patients had metastatic EGFR T790M mutation–positive NSCLC, identified by the cobas EGFR mutation test performed in a central laboratory, and progressive disease following first-line EGFR TKI therapy. Patients in the chemotherapy arm received either pemetrexed, 500 mg/m2 with carboplatin AUC5, or pemetrexed, 500mg/m2 with cisplatin 75 mg/m2), on day 1 of every 21-day cycle for up to six cycles followed by pemetrexed maintenance therapy.
The most serious adverse reactions, evaluated in 833 patients receiving osimertinib, were interstitial lung disease/pneumonitis (3.5%), QTc interval prolongation (0.7%), cardiomyopathy (1.9%), and keratitis (0.7%). The most common adverse reactions were diarrhea, rash, dry skin, nail toxicity, and fatigue.
The recommended dose of osimertinib, to be marketed as Tagrisso by AstraZeneca, is 80 mg orally once daily, with or without food, until disease progression or unacceptable toxicity. The presence of an EGFR T790M mutation in a tumor specimen, or plasma specimen (if tumor tissue is unavailable), should be confirmed by an FDA-approved test prior to initiation of treatment.
Full prescribing information is available here.
Rare Cancer Gets Timely Right Treatment
Be careful not to assume multiple cancerous lesions are advanced stage metastatic cancer, caution clinicians from H Plus Yangji Hospital in Seoul, and Inje University Haeundae Paik Hospital in Busan, both in the Republic of Korea. They came close to making that mistake.
Related: A Mysterious Massive Hemorrhage
The researchers reported on a patient who was on the verge of getting only palliative care for advanced laryngeal cancer with multiple lung metastases, when in fact he was a candidate for curative-aim chemotherapy. Thanks to a “meticulous approach,” the researchers switched their plan in time.
The patient was referred to their hospital because of symptoms such as “foreign body sensation” and voice change. A smoker for 45 years, he stopped 5 years before. A physical examination revealed that lymph nodes in the neck, axillary, and inguinal areas were not enlarged. Blood count and laboratory data were all normal, but a computed tomography (CT) scan and MRI revealed that the patient had an unusual constellation of simultaneous triple primary cancers: laryngeal supraglottic cancer, small cell lung cancer (SCLC), and squamous cell lung cancer.
Head and neck cancer with synchronous or metachronous lung cancers during follow-up isn’t rare, the clinicians say. But their patient’s case is uncommon because the coexistence of SCLC and squamous cell lung cancer has rarely been reported.
Related: Finding Synchronous Cancers
Supraglottic laryngeal cancer is usually diagnosed in the advanced stages with cervical lymph node metastasis because of its nonspecific presenting symptoms and its anatomic characteristics, including a rich lymphatic network, the researchers say. That was why at the initial presentation they presumed the patient had advanced metastatic cancer. If there had not been “high suspicion” or effort to confirm the 2 distinct lung masses by invasive diagnostic procedures, the patient would have received the expected palliative treatment and not the curative-aim treatment.
After the patient received concurrent chemoradiation therapy with weekly cisplatin, the supraglottic laryngeal cancer was “markedly decreased” without newly developed cervical lymph node metastasis. A follow-up chest CT showed partial response for the SCLC in the left upper lobe; the squamous cell lung cancer in the right lower lobe remained stable. The patient was given additional chemotherapy with etoposide and cisplatin. He has survived without recurrence.
Related: Timeliness of Lung Cancer Diagnosis and Treatment
Source:
Kim EK, Kim JY, Kim BM, Lim SN. BMJ Case Rep. 2017;2017.
doi: 10.1136/bcr-2016-216305.
Be careful not to assume multiple cancerous lesions are advanced stage metastatic cancer, caution clinicians from H Plus Yangji Hospital in Seoul, and Inje University Haeundae Paik Hospital in Busan, both in the Republic of Korea. They came close to making that mistake.
Related: A Mysterious Massive Hemorrhage
The researchers reported on a patient who was on the verge of getting only palliative care for advanced laryngeal cancer with multiple lung metastases, when in fact he was a candidate for curative-aim chemotherapy. Thanks to a “meticulous approach,” the researchers switched their plan in time.
The patient was referred to their hospital because of symptoms such as “foreign body sensation” and voice change. A smoker for 45 years, he stopped 5 years before. A physical examination revealed that lymph nodes in the neck, axillary, and inguinal areas were not enlarged. Blood count and laboratory data were all normal, but a computed tomography (CT) scan and MRI revealed that the patient had an unusual constellation of simultaneous triple primary cancers: laryngeal supraglottic cancer, small cell lung cancer (SCLC), and squamous cell lung cancer.
Head and neck cancer with synchronous or metachronous lung cancers during follow-up isn’t rare, the clinicians say. But their patient’s case is uncommon because the coexistence of SCLC and squamous cell lung cancer has rarely been reported.
Related: Finding Synchronous Cancers
Supraglottic laryngeal cancer is usually diagnosed in the advanced stages with cervical lymph node metastasis because of its nonspecific presenting symptoms and its anatomic characteristics, including a rich lymphatic network, the researchers say. That was why at the initial presentation they presumed the patient had advanced metastatic cancer. If there had not been “high suspicion” or effort to confirm the 2 distinct lung masses by invasive diagnostic procedures, the patient would have received the expected palliative treatment and not the curative-aim treatment.
After the patient received concurrent chemoradiation therapy with weekly cisplatin, the supraglottic laryngeal cancer was “markedly decreased” without newly developed cervical lymph node metastasis. A follow-up chest CT showed partial response for the SCLC in the left upper lobe; the squamous cell lung cancer in the right lower lobe remained stable. The patient was given additional chemotherapy with etoposide and cisplatin. He has survived without recurrence.
Related: Timeliness of Lung Cancer Diagnosis and Treatment
Source:
Kim EK, Kim JY, Kim BM, Lim SN. BMJ Case Rep. 2017;2017.
doi: 10.1136/bcr-2016-216305.
Be careful not to assume multiple cancerous lesions are advanced stage metastatic cancer, caution clinicians from H Plus Yangji Hospital in Seoul, and Inje University Haeundae Paik Hospital in Busan, both in the Republic of Korea. They came close to making that mistake.
Related: A Mysterious Massive Hemorrhage
The researchers reported on a patient who was on the verge of getting only palliative care for advanced laryngeal cancer with multiple lung metastases, when in fact he was a candidate for curative-aim chemotherapy. Thanks to a “meticulous approach,” the researchers switched their plan in time.
The patient was referred to their hospital because of symptoms such as “foreign body sensation” and voice change. A smoker for 45 years, he stopped 5 years before. A physical examination revealed that lymph nodes in the neck, axillary, and inguinal areas were not enlarged. Blood count and laboratory data were all normal, but a computed tomography (CT) scan and MRI revealed that the patient had an unusual constellation of simultaneous triple primary cancers: laryngeal supraglottic cancer, small cell lung cancer (SCLC), and squamous cell lung cancer.
Head and neck cancer with synchronous or metachronous lung cancers during follow-up isn’t rare, the clinicians say. But their patient’s case is uncommon because the coexistence of SCLC and squamous cell lung cancer has rarely been reported.
Related: Finding Synchronous Cancers
Supraglottic laryngeal cancer is usually diagnosed in the advanced stages with cervical lymph node metastasis because of its nonspecific presenting symptoms and its anatomic characteristics, including a rich lymphatic network, the researchers say. That was why at the initial presentation they presumed the patient had advanced metastatic cancer. If there had not been “high suspicion” or effort to confirm the 2 distinct lung masses by invasive diagnostic procedures, the patient would have received the expected palliative treatment and not the curative-aim treatment.
After the patient received concurrent chemoradiation therapy with weekly cisplatin, the supraglottic laryngeal cancer was “markedly decreased” without newly developed cervical lymph node metastasis. A follow-up chest CT showed partial response for the SCLC in the left upper lobe; the squamous cell lung cancer in the right lower lobe remained stable. The patient was given additional chemotherapy with etoposide and cisplatin. He has survived without recurrence.
Related: Timeliness of Lung Cancer Diagnosis and Treatment
Source:
Kim EK, Kim JY, Kim BM, Lim SN. BMJ Case Rep. 2017;2017.
doi: 10.1136/bcr-2016-216305.
Real-world EGFR and ALK testing of NSCLC falls short
ORLANDO – A large proportion of patients with advanced non–small cell lung cancer (NSCLC) are not being tested for tumor associated–epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations according to national guidelines. This situation may be leading to suboptimal treatment, a large retrospective cohort study suggests.
Guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend testing before first-line therapy for all treatment-eligible patients with nonsquamous histology and for those patients with squamous histology who are nonsmokers or who have mixed cell types or small tumor samples. Additionally, the guidelines recommend that results be made available within 2 weeks of the lab’s receipt of the sample so that they can be used to inform treatment decisions.
Overall, 22% of patients with nonsquamous tumors had no evidence of EGFR and ALK testing in their records. The large majority of patients with squamous tumors did not have any evidence of testing either, and it was unclear how well testing corresponded with the criteria.
In roughly a third of cases in which testing was done, the time between diagnosis of advanced disease and availability of test results exceeded 4 weeks. Among patients with positive test results, those whose results came back after the start of first-line therapy, were about half as likely to appropriately receive a therapy that targeted their tumor’s molecular aberration.
“We observed variation in adherence to [the American Society of Clinical Oncology] and [the National Comprehensive Cancer Network] guidelines around biomarker testing in advanced NSCLC, and we saw significant variation in testing in the squamous population and the nonsquamous population across practices,” presenting author Jay Rughani, manager of Life Sciences at Flatiron Health, New York, commented in an interview. Observed delays in availability of test results were mainly driven by delays between diagnosis and submission of samples to the lab for testing.
“There may be an opportunity to educate the oncology community around testing, certainly for all nonsquamous patients, because this is a case where they all should have been tested,” he said. “And there is also an opportunity to ensure testing of the appropriate squamous cell patients, while discouraging the testing of the majority who aren’t candidates, so there may be an opportunity for education around smoking status.”
Slow uptake of the national guidelines is unlikely to explain the observed variations in testing, according to Mr. Rughani. “Since we looked at patients diagnosed after Jan. 1, 2014, our impression was that the guidelines were sort of disseminated enough and widely known enough by that point, particularly around EGFR and ALK, that we wouldn’t expect any lag there. If we had done this for PD-L1 [programmed death ligand 1] testing, perhaps we might have thought about some lag in adoption.”
The impact of variations in testing and receipt of inappropriate initial therapy on clinical outcomes is yet to be determined. “As a follow-on, some of the work we have been doing is trying to understand, for these separate cohorts of patients, depending on what they received in the front line, what their overall survival was and what their surrogate endpoints were,” Mr. Rughani concluded.
Study details
For the study, the investigators identified 16,316 patients with advanced NSCLC from 206 community clinics across the United States participating in the Flatiron Network. All patients were treated between 2014 and 2016.
Cross-checking of the total Flatiron population against the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results databases suggested that it is a good national representation, according to Mr. Rughani.
A record review showed that the rate of EGFR and ALK testing among study patients ranged widely across clinics, from 0% to 100% for both the nonsquamous cases and the squamous cases, according to results reported in a poster session. The median was 79% for the former and 16% for the latter.
Overall, 22% of the nonsquamous cohort and 79% of the squamous cohort did not have any evidence of testing in their records. For the latter, a sampling of records was unable to verify whether testing was appropriately matched to eligibility criteria.
When testing was performed, 35% of EGFR test results and 37% of ALK test results were not available to the treating clinician until more than 4 weeks after the date of the advanced cancer diagnosis.
“The delays were mostly attributed to nonlab factors. When we isolated the time that the lab took to turn it around, it was under 2 weeks for the vast majority of patients,” Mr. Rughani reported. Possible nonlab culprit factors include clinic work flows, insurance-related issues, and families’ and patients’ hesitancy to be tested, he said.
Delays in receipt of positive test results appeared to influence choice of first-line therapy. Among patients in whom these results were available before first-line therapy, 80% of those found to have an EGFR-mutated tumor received an EGFR–tyrosine kinase inhibitor, and 77% of those found to have ALK-rearranged tumors received an ALK inhibitor.
In sharp contrast, among patients in whom positive test results did not become available until after the start of first-line therapy, respective values were just 43% and 42%.
“Anecdotally, we saw that some patients would go on to Avastin [bevacizumab] in the front line when the results were delayed, and then, ultimately, they would have the opportunity to receive an EGFR[–tyrosine kinase inhibitor] or something like that in later lines,” commented Mr. Rughani. “So, that impacted treatment decisions there.”
Mr. Rughani disclosed stock and other ownership interests in Flatiron Health.
ORLANDO – A large proportion of patients with advanced non–small cell lung cancer (NSCLC) are not being tested for tumor associated–epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations according to national guidelines. This situation may be leading to suboptimal treatment, a large retrospective cohort study suggests.
Guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend testing before first-line therapy for all treatment-eligible patients with nonsquamous histology and for those patients with squamous histology who are nonsmokers or who have mixed cell types or small tumor samples. Additionally, the guidelines recommend that results be made available within 2 weeks of the lab’s receipt of the sample so that they can be used to inform treatment decisions.
Overall, 22% of patients with nonsquamous tumors had no evidence of EGFR and ALK testing in their records. The large majority of patients with squamous tumors did not have any evidence of testing either, and it was unclear how well testing corresponded with the criteria.
In roughly a third of cases in which testing was done, the time between diagnosis of advanced disease and availability of test results exceeded 4 weeks. Among patients with positive test results, those whose results came back after the start of first-line therapy, were about half as likely to appropriately receive a therapy that targeted their tumor’s molecular aberration.
“We observed variation in adherence to [the American Society of Clinical Oncology] and [the National Comprehensive Cancer Network] guidelines around biomarker testing in advanced NSCLC, and we saw significant variation in testing in the squamous population and the nonsquamous population across practices,” presenting author Jay Rughani, manager of Life Sciences at Flatiron Health, New York, commented in an interview. Observed delays in availability of test results were mainly driven by delays between diagnosis and submission of samples to the lab for testing.
“There may be an opportunity to educate the oncology community around testing, certainly for all nonsquamous patients, because this is a case where they all should have been tested,” he said. “And there is also an opportunity to ensure testing of the appropriate squamous cell patients, while discouraging the testing of the majority who aren’t candidates, so there may be an opportunity for education around smoking status.”
Slow uptake of the national guidelines is unlikely to explain the observed variations in testing, according to Mr. Rughani. “Since we looked at patients diagnosed after Jan. 1, 2014, our impression was that the guidelines were sort of disseminated enough and widely known enough by that point, particularly around EGFR and ALK, that we wouldn’t expect any lag there. If we had done this for PD-L1 [programmed death ligand 1] testing, perhaps we might have thought about some lag in adoption.”
The impact of variations in testing and receipt of inappropriate initial therapy on clinical outcomes is yet to be determined. “As a follow-on, some of the work we have been doing is trying to understand, for these separate cohorts of patients, depending on what they received in the front line, what their overall survival was and what their surrogate endpoints were,” Mr. Rughani concluded.
Study details
For the study, the investigators identified 16,316 patients with advanced NSCLC from 206 community clinics across the United States participating in the Flatiron Network. All patients were treated between 2014 and 2016.
Cross-checking of the total Flatiron population against the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results databases suggested that it is a good national representation, according to Mr. Rughani.
A record review showed that the rate of EGFR and ALK testing among study patients ranged widely across clinics, from 0% to 100% for both the nonsquamous cases and the squamous cases, according to results reported in a poster session. The median was 79% for the former and 16% for the latter.
Overall, 22% of the nonsquamous cohort and 79% of the squamous cohort did not have any evidence of testing in their records. For the latter, a sampling of records was unable to verify whether testing was appropriately matched to eligibility criteria.
When testing was performed, 35% of EGFR test results and 37% of ALK test results were not available to the treating clinician until more than 4 weeks after the date of the advanced cancer diagnosis.
“The delays were mostly attributed to nonlab factors. When we isolated the time that the lab took to turn it around, it was under 2 weeks for the vast majority of patients,” Mr. Rughani reported. Possible nonlab culprit factors include clinic work flows, insurance-related issues, and families’ and patients’ hesitancy to be tested, he said.
Delays in receipt of positive test results appeared to influence choice of first-line therapy. Among patients in whom these results were available before first-line therapy, 80% of those found to have an EGFR-mutated tumor received an EGFR–tyrosine kinase inhibitor, and 77% of those found to have ALK-rearranged tumors received an ALK inhibitor.
In sharp contrast, among patients in whom positive test results did not become available until after the start of first-line therapy, respective values were just 43% and 42%.
“Anecdotally, we saw that some patients would go on to Avastin [bevacizumab] in the front line when the results were delayed, and then, ultimately, they would have the opportunity to receive an EGFR[–tyrosine kinase inhibitor] or something like that in later lines,” commented Mr. Rughani. “So, that impacted treatment decisions there.”
Mr. Rughani disclosed stock and other ownership interests in Flatiron Health.
ORLANDO – A large proportion of patients with advanced non–small cell lung cancer (NSCLC) are not being tested for tumor associated–epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations according to national guidelines. This situation may be leading to suboptimal treatment, a large retrospective cohort study suggests.
Guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend testing before first-line therapy for all treatment-eligible patients with nonsquamous histology and for those patients with squamous histology who are nonsmokers or who have mixed cell types or small tumor samples. Additionally, the guidelines recommend that results be made available within 2 weeks of the lab’s receipt of the sample so that they can be used to inform treatment decisions.
Overall, 22% of patients with nonsquamous tumors had no evidence of EGFR and ALK testing in their records. The large majority of patients with squamous tumors did not have any evidence of testing either, and it was unclear how well testing corresponded with the criteria.
In roughly a third of cases in which testing was done, the time between diagnosis of advanced disease and availability of test results exceeded 4 weeks. Among patients with positive test results, those whose results came back after the start of first-line therapy, were about half as likely to appropriately receive a therapy that targeted their tumor’s molecular aberration.
“We observed variation in adherence to [the American Society of Clinical Oncology] and [the National Comprehensive Cancer Network] guidelines around biomarker testing in advanced NSCLC, and we saw significant variation in testing in the squamous population and the nonsquamous population across practices,” presenting author Jay Rughani, manager of Life Sciences at Flatiron Health, New York, commented in an interview. Observed delays in availability of test results were mainly driven by delays between diagnosis and submission of samples to the lab for testing.
“There may be an opportunity to educate the oncology community around testing, certainly for all nonsquamous patients, because this is a case where they all should have been tested,” he said. “And there is also an opportunity to ensure testing of the appropriate squamous cell patients, while discouraging the testing of the majority who aren’t candidates, so there may be an opportunity for education around smoking status.”
Slow uptake of the national guidelines is unlikely to explain the observed variations in testing, according to Mr. Rughani. “Since we looked at patients diagnosed after Jan. 1, 2014, our impression was that the guidelines were sort of disseminated enough and widely known enough by that point, particularly around EGFR and ALK, that we wouldn’t expect any lag there. If we had done this for PD-L1 [programmed death ligand 1] testing, perhaps we might have thought about some lag in adoption.”
The impact of variations in testing and receipt of inappropriate initial therapy on clinical outcomes is yet to be determined. “As a follow-on, some of the work we have been doing is trying to understand, for these separate cohorts of patients, depending on what they received in the front line, what their overall survival was and what their surrogate endpoints were,” Mr. Rughani concluded.
Study details
For the study, the investigators identified 16,316 patients with advanced NSCLC from 206 community clinics across the United States participating in the Flatiron Network. All patients were treated between 2014 and 2016.
Cross-checking of the total Flatiron population against the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results databases suggested that it is a good national representation, according to Mr. Rughani.
A record review showed that the rate of EGFR and ALK testing among study patients ranged widely across clinics, from 0% to 100% for both the nonsquamous cases and the squamous cases, according to results reported in a poster session. The median was 79% for the former and 16% for the latter.
Overall, 22% of the nonsquamous cohort and 79% of the squamous cohort did not have any evidence of testing in their records. For the latter, a sampling of records was unable to verify whether testing was appropriately matched to eligibility criteria.
When testing was performed, 35% of EGFR test results and 37% of ALK test results were not available to the treating clinician until more than 4 weeks after the date of the advanced cancer diagnosis.
“The delays were mostly attributed to nonlab factors. When we isolated the time that the lab took to turn it around, it was under 2 weeks for the vast majority of patients,” Mr. Rughani reported. Possible nonlab culprit factors include clinic work flows, insurance-related issues, and families’ and patients’ hesitancy to be tested, he said.
Delays in receipt of positive test results appeared to influence choice of first-line therapy. Among patients in whom these results were available before first-line therapy, 80% of those found to have an EGFR-mutated tumor received an EGFR–tyrosine kinase inhibitor, and 77% of those found to have ALK-rearranged tumors received an ALK inhibitor.
In sharp contrast, among patients in whom positive test results did not become available until after the start of first-line therapy, respective values were just 43% and 42%.
“Anecdotally, we saw that some patients would go on to Avastin [bevacizumab] in the front line when the results were delayed, and then, ultimately, they would have the opportunity to receive an EGFR[–tyrosine kinase inhibitor] or something like that in later lines,” commented Mr. Rughani. “So, that impacted treatment decisions there.”
Mr. Rughani disclosed stock and other ownership interests in Flatiron Health.
Key clinical point:
Major finding: Overall, 22% of patients with nonsquamous advanced NSCLC had no evidence of EGFR and ALK tumor testing in their records.
Data source: A retrospective cohort study of 16,316 community oncology patients with advanced NSCLC.
Disclosures: Mr. Rughani disclosed that he is an employee of and has stock or other ownership interests in Flatiron Health.
Hold your breath
“Exercising my ‘reasoned judgment,’ I have no doubt that the right to a climate system capable of sustaining human life is fundamental to a free and ordered society.”
– U.S. District Judge Ann Aiken in Kelsey Cascadia Rose Juliana vs. United States of America, et al.
In many areas of the world, the simple act of breathing has become hazardous to people’s health.
According to the World Health Organization, more people die every day from air pollution than from HIV/AIDS, tuberculosis, and road injuries combined. In China, more than 1 million deaths annually are linked to polluted air (76/100,000); in India the number of deaths is more than 600,000 annually (49/100,000); and in the United States, that figure comes to more than 38,000 (12/100,000).
And yet, nonpolluting, alternative options – such as sun and wind power – are readily available.
Dirty air is visible on a hot summer day – when, mixed with other substances, it forms smog. Higher temperatures can then speed up the chemical reactions that form smog. We breathe in that polluted air, especially on days when the air is stagnant or there is temperature inversion.
The health effects of climate change
Black carbon found in air pollution leads to drug-resistant bacteria and alters antibiotic tolerance.1 The pollution also is associated with multiple cancers: lung, liver, ovarian, and, possibly, breast.2,3,4,5 It causes inflammation linked to the development of coronary artery disease (seen even in children!) and plaque formation leading to heart attacks and cardiac arrhythmias – including atrial fibrillation. Air pollution causes, triggers, or worsens respiratory illnesses – chronic obstructive pulmonary disease, emphysema, asthma, infections – and is responsible for lifelong diminished lung volume in children (a reason families are leaving Beijing.) Exponentially increased rates of autism are linked to bad air quality, as are autoimmune diseases, which also are on the rise.6,7 Polluted air causes brain inflammation – living near sources of air pollution increases the risk of dementia – and other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s, and amyotrophic lateral sclerosis.8 The blood brain barrier protects the brain from most foreign matter, but particulate matter, especially ultrafine particulate matter of less than 1 mcm such as magnetite, can cross directly into the brain via the olfactory nerve. (Magnetite has been identified in the brain tissue of residents living in areas where the substance is produced as a result of industrial waste.) While particulate matter of 2.5 mcmis measured in the United States, ultrafine particulate matter is not.
Psychiatric symptoms and chronic psychiatric disorders also are associated with polluted air: On days with poor air quality, a statistically significant increase is seen in suicide threats and visits to emergency departments for panic attacks.9,10
A rise in aggression occurs when there are abnormally high temperatures and significant changes in rainfall. More assaults, murders, suicides, domestic violence, and child abuse can be expected, and a rise in unrest around the world should come as no surprise.
As a consequence of increased CO2 in the atmosphere, temperatures have already risen by 2° F: Sixteen of the hottest years on record have occurred in the last 17 years, with 2016 as the hottest year ever recorded. In Iraq and Kuwait, the temperature last summer reached 129.2° F.
We are experiencing more frequent and extreme weather events, chronic climate conditions, and the cascading disruption of ecosystems. Drought and sea level rise are leading to physical and psychological impacts – both direct and indirect. Some regions of the world have become destabilized, triggering migrations and the refugee crisis.
Along with these psychological impacts, CO2 affects cognition: A recent study by the Harvard School of Public Health, Boston, shows that the indoor levels of CO2 to which American workers typically are exposed impair cognitive functioning, particularly in the areas of strategic thinking, information processing, and crisis management.11
What do we do about it?
As mental health professionals, we know that aggression can be overt or passive (from inaction). Overwhelming evidence shows harm to public health from burning fossil fuels, and yet, though we are making progress, resistance still exists in the transition to clean, renewable energy critical for the health of our families and communities. When political will is what stands between us and getting back on a path to breathing clean air, how can inaction be understood as anything but an act of aggression?
This issue has reached U.S. courts: In a landmark case, 21 youths aged 9-20 years represented by “Our Children’s Trust” are suing the U.S. government in the Oregon U.S. District Court for failure to act on climate. The case, heard by Judge Ann Aiken, is now headed to trial.
All of us have a duty to collectively, repeatedly, and forcefully call on policy makers to take action.
That leads me to what we can do as doctors. In this effort to quickly transition to safe, clean renewable energy, we all have a role to play. The notion that we can’t do anything as individuals is no more credible than saying “my vote doesn’t matter.” Just as our actions as voters in a democracy demonstrate the collective civic responsibility we owe one another, so too do our actions on climate. As global citizens, all actions that we take to help us live within the planet’s means are opportunities to restore balance.
What we do collectively drives markets and determines the social norms that powerfully influence the decisions of others – sometimes even unconsciously.
As doctors, we have a unique role to play in the places we work – urging hospitals, clinics, academic centers, and other organizations and facilities to lead by example, become role models for energy efficiency, and choose clean renewable energy sources over the ones harming our health. We can start by choosing wind and solar to power our homes and influencing others to do the same.
We are the voices because this is a health message.
Dr. Van Susteren is a practicing general and forensic psychiatrist in Washington. She serves on the advisory board of the Center for Health and the Global Environment at Harvard T.H. Chan School of Public Health, Boston. Dr. Van Susteren is a former member of the board of directors of the National Wildlife Federation and coauthor of group’s report, “The Psychological Effects of Global Warming on the United States – Why the U.S. Mental Health System is Not Prepared.” In 2006, Dr. Van Susteren sought the Democratic nomination for a U.S. Senate seat in Maryland. She also founded Lucky Planet Foods, a company that provides plant-based, low carbon foods.
References
1. Environ Microbiol. 2017 Feb 14. doi: 10.1111/1462-2920.13686.
2. Environ Health Perspect. 2017 Mar;125[3]:378-84.
3. J Hepatol. 2015;63[6]:1397-1404.
4. J Toxicol Environ Health A. 2012;75[3]:174-82.
5. Environ Health Perspect. 2012 Nov; 118[11]:1578-83.
6. J Child Psychol Psychiatry. 2016; 57[3]:271-92.
7. Curr Opin Pediatr. 2010;22[2]219-25.
8. Inhal Toxicol. 2008;20[5]:499-506.
9. J Psychiatr Res. 2015 Mar;62:130-5.
10. Schizophr Res. 2016 Oct 5. doi: 10.1016/j.schres.2016.10.003.
11. Environ Health Perspect. 2016 Jun;124[6]:805-12.
“Exercising my ‘reasoned judgment,’ I have no doubt that the right to a climate system capable of sustaining human life is fundamental to a free and ordered society.”
– U.S. District Judge Ann Aiken in Kelsey Cascadia Rose Juliana vs. United States of America, et al.
In many areas of the world, the simple act of breathing has become hazardous to people’s health.
According to the World Health Organization, more people die every day from air pollution than from HIV/AIDS, tuberculosis, and road injuries combined. In China, more than 1 million deaths annually are linked to polluted air (76/100,000); in India the number of deaths is more than 600,000 annually (49/100,000); and in the United States, that figure comes to more than 38,000 (12/100,000).
And yet, nonpolluting, alternative options – such as sun and wind power – are readily available.
Dirty air is visible on a hot summer day – when, mixed with other substances, it forms smog. Higher temperatures can then speed up the chemical reactions that form smog. We breathe in that polluted air, especially on days when the air is stagnant or there is temperature inversion.
The health effects of climate change
Black carbon found in air pollution leads to drug-resistant bacteria and alters antibiotic tolerance.1 The pollution also is associated with multiple cancers: lung, liver, ovarian, and, possibly, breast.2,3,4,5 It causes inflammation linked to the development of coronary artery disease (seen even in children!) and plaque formation leading to heart attacks and cardiac arrhythmias – including atrial fibrillation. Air pollution causes, triggers, or worsens respiratory illnesses – chronic obstructive pulmonary disease, emphysema, asthma, infections – and is responsible for lifelong diminished lung volume in children (a reason families are leaving Beijing.) Exponentially increased rates of autism are linked to bad air quality, as are autoimmune diseases, which also are on the rise.6,7 Polluted air causes brain inflammation – living near sources of air pollution increases the risk of dementia – and other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s, and amyotrophic lateral sclerosis.8 The blood brain barrier protects the brain from most foreign matter, but particulate matter, especially ultrafine particulate matter of less than 1 mcm such as magnetite, can cross directly into the brain via the olfactory nerve. (Magnetite has been identified in the brain tissue of residents living in areas where the substance is produced as a result of industrial waste.) While particulate matter of 2.5 mcmis measured in the United States, ultrafine particulate matter is not.
Psychiatric symptoms and chronic psychiatric disorders also are associated with polluted air: On days with poor air quality, a statistically significant increase is seen in suicide threats and visits to emergency departments for panic attacks.9,10
A rise in aggression occurs when there are abnormally high temperatures and significant changes in rainfall. More assaults, murders, suicides, domestic violence, and child abuse can be expected, and a rise in unrest around the world should come as no surprise.
As a consequence of increased CO2 in the atmosphere, temperatures have already risen by 2° F: Sixteen of the hottest years on record have occurred in the last 17 years, with 2016 as the hottest year ever recorded. In Iraq and Kuwait, the temperature last summer reached 129.2° F.
We are experiencing more frequent and extreme weather events, chronic climate conditions, and the cascading disruption of ecosystems. Drought and sea level rise are leading to physical and psychological impacts – both direct and indirect. Some regions of the world have become destabilized, triggering migrations and the refugee crisis.
Along with these psychological impacts, CO2 affects cognition: A recent study by the Harvard School of Public Health, Boston, shows that the indoor levels of CO2 to which American workers typically are exposed impair cognitive functioning, particularly in the areas of strategic thinking, information processing, and crisis management.11
What do we do about it?
As mental health professionals, we know that aggression can be overt or passive (from inaction). Overwhelming evidence shows harm to public health from burning fossil fuels, and yet, though we are making progress, resistance still exists in the transition to clean, renewable energy critical for the health of our families and communities. When political will is what stands between us and getting back on a path to breathing clean air, how can inaction be understood as anything but an act of aggression?
This issue has reached U.S. courts: In a landmark case, 21 youths aged 9-20 years represented by “Our Children’s Trust” are suing the U.S. government in the Oregon U.S. District Court for failure to act on climate. The case, heard by Judge Ann Aiken, is now headed to trial.
All of us have a duty to collectively, repeatedly, and forcefully call on policy makers to take action.
That leads me to what we can do as doctors. In this effort to quickly transition to safe, clean renewable energy, we all have a role to play. The notion that we can’t do anything as individuals is no more credible than saying “my vote doesn’t matter.” Just as our actions as voters in a democracy demonstrate the collective civic responsibility we owe one another, so too do our actions on climate. As global citizens, all actions that we take to help us live within the planet’s means are opportunities to restore balance.
What we do collectively drives markets and determines the social norms that powerfully influence the decisions of others – sometimes even unconsciously.
As doctors, we have a unique role to play in the places we work – urging hospitals, clinics, academic centers, and other organizations and facilities to lead by example, become role models for energy efficiency, and choose clean renewable energy sources over the ones harming our health. We can start by choosing wind and solar to power our homes and influencing others to do the same.
We are the voices because this is a health message.
Dr. Van Susteren is a practicing general and forensic psychiatrist in Washington. She serves on the advisory board of the Center for Health and the Global Environment at Harvard T.H. Chan School of Public Health, Boston. Dr. Van Susteren is a former member of the board of directors of the National Wildlife Federation and coauthor of group’s report, “The Psychological Effects of Global Warming on the United States – Why the U.S. Mental Health System is Not Prepared.” In 2006, Dr. Van Susteren sought the Democratic nomination for a U.S. Senate seat in Maryland. She also founded Lucky Planet Foods, a company that provides plant-based, low carbon foods.
References
1. Environ Microbiol. 2017 Feb 14. doi: 10.1111/1462-2920.13686.
2. Environ Health Perspect. 2017 Mar;125[3]:378-84.
3. J Hepatol. 2015;63[6]:1397-1404.
4. J Toxicol Environ Health A. 2012;75[3]:174-82.
5. Environ Health Perspect. 2012 Nov; 118[11]:1578-83.
6. J Child Psychol Psychiatry. 2016; 57[3]:271-92.
7. Curr Opin Pediatr. 2010;22[2]219-25.
8. Inhal Toxicol. 2008;20[5]:499-506.
9. J Psychiatr Res. 2015 Mar;62:130-5.
10. Schizophr Res. 2016 Oct 5. doi: 10.1016/j.schres.2016.10.003.
11. Environ Health Perspect. 2016 Jun;124[6]:805-12.
“Exercising my ‘reasoned judgment,’ I have no doubt that the right to a climate system capable of sustaining human life is fundamental to a free and ordered society.”
– U.S. District Judge Ann Aiken in Kelsey Cascadia Rose Juliana vs. United States of America, et al.
In many areas of the world, the simple act of breathing has become hazardous to people’s health.
According to the World Health Organization, more people die every day from air pollution than from HIV/AIDS, tuberculosis, and road injuries combined. In China, more than 1 million deaths annually are linked to polluted air (76/100,000); in India the number of deaths is more than 600,000 annually (49/100,000); and in the United States, that figure comes to more than 38,000 (12/100,000).
And yet, nonpolluting, alternative options – such as sun and wind power – are readily available.
Dirty air is visible on a hot summer day – when, mixed with other substances, it forms smog. Higher temperatures can then speed up the chemical reactions that form smog. We breathe in that polluted air, especially on days when the air is stagnant or there is temperature inversion.
The health effects of climate change
Black carbon found in air pollution leads to drug-resistant bacteria and alters antibiotic tolerance.1 The pollution also is associated with multiple cancers: lung, liver, ovarian, and, possibly, breast.2,3,4,5 It causes inflammation linked to the development of coronary artery disease (seen even in children!) and plaque formation leading to heart attacks and cardiac arrhythmias – including atrial fibrillation. Air pollution causes, triggers, or worsens respiratory illnesses – chronic obstructive pulmonary disease, emphysema, asthma, infections – and is responsible for lifelong diminished lung volume in children (a reason families are leaving Beijing.) Exponentially increased rates of autism are linked to bad air quality, as are autoimmune diseases, which also are on the rise.6,7 Polluted air causes brain inflammation – living near sources of air pollution increases the risk of dementia – and other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s, and amyotrophic lateral sclerosis.8 The blood brain barrier protects the brain from most foreign matter, but particulate matter, especially ultrafine particulate matter of less than 1 mcm such as magnetite, can cross directly into the brain via the olfactory nerve. (Magnetite has been identified in the brain tissue of residents living in areas where the substance is produced as a result of industrial waste.) While particulate matter of 2.5 mcmis measured in the United States, ultrafine particulate matter is not.
Psychiatric symptoms and chronic psychiatric disorders also are associated with polluted air: On days with poor air quality, a statistically significant increase is seen in suicide threats and visits to emergency departments for panic attacks.9,10
A rise in aggression occurs when there are abnormally high temperatures and significant changes in rainfall. More assaults, murders, suicides, domestic violence, and child abuse can be expected, and a rise in unrest around the world should come as no surprise.
As a consequence of increased CO2 in the atmosphere, temperatures have already risen by 2° F: Sixteen of the hottest years on record have occurred in the last 17 years, with 2016 as the hottest year ever recorded. In Iraq and Kuwait, the temperature last summer reached 129.2° F.
We are experiencing more frequent and extreme weather events, chronic climate conditions, and the cascading disruption of ecosystems. Drought and sea level rise are leading to physical and psychological impacts – both direct and indirect. Some regions of the world have become destabilized, triggering migrations and the refugee crisis.
Along with these psychological impacts, CO2 affects cognition: A recent study by the Harvard School of Public Health, Boston, shows that the indoor levels of CO2 to which American workers typically are exposed impair cognitive functioning, particularly in the areas of strategic thinking, information processing, and crisis management.11
What do we do about it?
As mental health professionals, we know that aggression can be overt or passive (from inaction). Overwhelming evidence shows harm to public health from burning fossil fuels, and yet, though we are making progress, resistance still exists in the transition to clean, renewable energy critical for the health of our families and communities. When political will is what stands between us and getting back on a path to breathing clean air, how can inaction be understood as anything but an act of aggression?
This issue has reached U.S. courts: In a landmark case, 21 youths aged 9-20 years represented by “Our Children’s Trust” are suing the U.S. government in the Oregon U.S. District Court for failure to act on climate. The case, heard by Judge Ann Aiken, is now headed to trial.
All of us have a duty to collectively, repeatedly, and forcefully call on policy makers to take action.
That leads me to what we can do as doctors. In this effort to quickly transition to safe, clean renewable energy, we all have a role to play. The notion that we can’t do anything as individuals is no more credible than saying “my vote doesn’t matter.” Just as our actions as voters in a democracy demonstrate the collective civic responsibility we owe one another, so too do our actions on climate. As global citizens, all actions that we take to help us live within the planet’s means are opportunities to restore balance.
What we do collectively drives markets and determines the social norms that powerfully influence the decisions of others – sometimes even unconsciously.
As doctors, we have a unique role to play in the places we work – urging hospitals, clinics, academic centers, and other organizations and facilities to lead by example, become role models for energy efficiency, and choose clean renewable energy sources over the ones harming our health. We can start by choosing wind and solar to power our homes and influencing others to do the same.
We are the voices because this is a health message.
Dr. Van Susteren is a practicing general and forensic psychiatrist in Washington. She serves on the advisory board of the Center for Health and the Global Environment at Harvard T.H. Chan School of Public Health, Boston. Dr. Van Susteren is a former member of the board of directors of the National Wildlife Federation and coauthor of group’s report, “The Psychological Effects of Global Warming on the United States – Why the U.S. Mental Health System is Not Prepared.” In 2006, Dr. Van Susteren sought the Democratic nomination for a U.S. Senate seat in Maryland. She also founded Lucky Planet Foods, a company that provides plant-based, low carbon foods.
References
1. Environ Microbiol. 2017 Feb 14. doi: 10.1111/1462-2920.13686.
2. Environ Health Perspect. 2017 Mar;125[3]:378-84.
3. J Hepatol. 2015;63[6]:1397-1404.
4. J Toxicol Environ Health A. 2012;75[3]:174-82.
5. Environ Health Perspect. 2012 Nov; 118[11]:1578-83.
6. J Child Psychol Psychiatry. 2016; 57[3]:271-92.
7. Curr Opin Pediatr. 2010;22[2]219-25.
8. Inhal Toxicol. 2008;20[5]:499-506.
9. J Psychiatr Res. 2015 Mar;62:130-5.
10. Schizophr Res. 2016 Oct 5. doi: 10.1016/j.schres.2016.10.003.
11. Environ Health Perspect. 2016 Jun;124[6]:805-12.