User login
Examining the No. 1 Preventable Cause of Cancer
When tobacco and cancer are mentioned, many people automatically assume lung cancer. But the 70-plus carcinogens identified in tobacco smoke cause at least 12 types of cancer, according to a Vital Signs report, including cancers of the stomach, kidney and renal pelvis, urinary bladder, and cervix. “Smokeless” products such as chewing tobacco carry 28 identified carcinogens.
Related: Quitting Smoking During Substance Abuse Treatment
Researchers from the CDC and National Cancer Institute analyzed data from the U.S. Cancer Statistics 2004-2013 to assess incidence and death rates for cancers caused by tobacco use. During that time, the incidence of tobacco-related cancer went down 1.3% per year, and mortality dropped 1.6% each year. Tobacco-related cancer incidence declined significantly in 44 states. Rates were lowest in the West and dropped the slowest in the Midwest.
However, the burden remains high, the researchers note, and disparities persist among certain groups. For instance, the incidence of tobacco-related cancer rose with age: Two-fifths of deaths were among people aged ≥ 75 years. And the incidence of cancer and death rates were highest but decreased fastest among blacks.
Related: Impact of a Drop-in Group Medical Appointment on Tobacco Quit Rates
There’s no question that lung cancer remains the cancer most linked to smoking. Lung cancer accounted for about one-third of tobacco-related cancer cases and almost one-half of tobacco-related cancer deaths. Between 2009 and 2013, about 101,300 men and 65,700 women died each year of cancers attributable to cigarette smoking—most died of lung cancer. Exposure to secondhand smoke accounted for another 7,300 deaths by lung cancer among nonsmokers.
Roughly 6 million people may die prematurely of a tobacco-related cancer unless they can take advantage of more sustained, comprehensive, and evidence-based interventions, the researchers say. States that have invested in smoking prevention and control programs, they point out, have seen larger declines in youth and adult smoking, decreases in lung cancer, and reduced tobacco-related health care costs.
Related: CDC Media Campaign Helps Americans Quit Smoking
The researchers also emphasize the benefits of smoking-cessation counseling and treatment as well as screening for cervical, colorectal, and lung cancers, to help detect them at earlier more easily treatable stages. Vaccination against hepatitis B virus and human papillomavirus can prevent liver and cervix cancer, to which tobacco can contribute.
According to Vital Signs, the CDC funds 65 comprehensive cancer control programs to prevent cancer, increase access to early detection and care for tobacco-related cancers, help cancer survivors quit using tobacco, and improve cancer outcomes, especially in communities with higher tobacco-related rates of cancer and deaths. The CDC also urges health care providers to encourage patients to quit—or not start—smoking, to be aware of screenings, and to remember that there’s no “risk-free” level of secondhand smoke.
Source:
Henley SJ, Thomas CC, Sharapova SR, et al. MMWR. 2016;65(44):1212-1218.
When tobacco and cancer are mentioned, many people automatically assume lung cancer. But the 70-plus carcinogens identified in tobacco smoke cause at least 12 types of cancer, according to a Vital Signs report, including cancers of the stomach, kidney and renal pelvis, urinary bladder, and cervix. “Smokeless” products such as chewing tobacco carry 28 identified carcinogens.
Related: Quitting Smoking During Substance Abuse Treatment
Researchers from the CDC and National Cancer Institute analyzed data from the U.S. Cancer Statistics 2004-2013 to assess incidence and death rates for cancers caused by tobacco use. During that time, the incidence of tobacco-related cancer went down 1.3% per year, and mortality dropped 1.6% each year. Tobacco-related cancer incidence declined significantly in 44 states. Rates were lowest in the West and dropped the slowest in the Midwest.
However, the burden remains high, the researchers note, and disparities persist among certain groups. For instance, the incidence of tobacco-related cancer rose with age: Two-fifths of deaths were among people aged ≥ 75 years. And the incidence of cancer and death rates were highest but decreased fastest among blacks.
Related: Impact of a Drop-in Group Medical Appointment on Tobacco Quit Rates
There’s no question that lung cancer remains the cancer most linked to smoking. Lung cancer accounted for about one-third of tobacco-related cancer cases and almost one-half of tobacco-related cancer deaths. Between 2009 and 2013, about 101,300 men and 65,700 women died each year of cancers attributable to cigarette smoking—most died of lung cancer. Exposure to secondhand smoke accounted for another 7,300 deaths by lung cancer among nonsmokers.
Roughly 6 million people may die prematurely of a tobacco-related cancer unless they can take advantage of more sustained, comprehensive, and evidence-based interventions, the researchers say. States that have invested in smoking prevention and control programs, they point out, have seen larger declines in youth and adult smoking, decreases in lung cancer, and reduced tobacco-related health care costs.
Related: CDC Media Campaign Helps Americans Quit Smoking
The researchers also emphasize the benefits of smoking-cessation counseling and treatment as well as screening for cervical, colorectal, and lung cancers, to help detect them at earlier more easily treatable stages. Vaccination against hepatitis B virus and human papillomavirus can prevent liver and cervix cancer, to which tobacco can contribute.
According to Vital Signs, the CDC funds 65 comprehensive cancer control programs to prevent cancer, increase access to early detection and care for tobacco-related cancers, help cancer survivors quit using tobacco, and improve cancer outcomes, especially in communities with higher tobacco-related rates of cancer and deaths. The CDC also urges health care providers to encourage patients to quit—or not start—smoking, to be aware of screenings, and to remember that there’s no “risk-free” level of secondhand smoke.
Source:
Henley SJ, Thomas CC, Sharapova SR, et al. MMWR. 2016;65(44):1212-1218.
When tobacco and cancer are mentioned, many people automatically assume lung cancer. But the 70-plus carcinogens identified in tobacco smoke cause at least 12 types of cancer, according to a Vital Signs report, including cancers of the stomach, kidney and renal pelvis, urinary bladder, and cervix. “Smokeless” products such as chewing tobacco carry 28 identified carcinogens.
Related: Quitting Smoking During Substance Abuse Treatment
Researchers from the CDC and National Cancer Institute analyzed data from the U.S. Cancer Statistics 2004-2013 to assess incidence and death rates for cancers caused by tobacco use. During that time, the incidence of tobacco-related cancer went down 1.3% per year, and mortality dropped 1.6% each year. Tobacco-related cancer incidence declined significantly in 44 states. Rates were lowest in the West and dropped the slowest in the Midwest.
However, the burden remains high, the researchers note, and disparities persist among certain groups. For instance, the incidence of tobacco-related cancer rose with age: Two-fifths of deaths were among people aged ≥ 75 years. And the incidence of cancer and death rates were highest but decreased fastest among blacks.
Related: Impact of a Drop-in Group Medical Appointment on Tobacco Quit Rates
There’s no question that lung cancer remains the cancer most linked to smoking. Lung cancer accounted for about one-third of tobacco-related cancer cases and almost one-half of tobacco-related cancer deaths. Between 2009 and 2013, about 101,300 men and 65,700 women died each year of cancers attributable to cigarette smoking—most died of lung cancer. Exposure to secondhand smoke accounted for another 7,300 deaths by lung cancer among nonsmokers.
Roughly 6 million people may die prematurely of a tobacco-related cancer unless they can take advantage of more sustained, comprehensive, and evidence-based interventions, the researchers say. States that have invested in smoking prevention and control programs, they point out, have seen larger declines in youth and adult smoking, decreases in lung cancer, and reduced tobacco-related health care costs.
Related: CDC Media Campaign Helps Americans Quit Smoking
The researchers also emphasize the benefits of smoking-cessation counseling and treatment as well as screening for cervical, colorectal, and lung cancers, to help detect them at earlier more easily treatable stages. Vaccination against hepatitis B virus and human papillomavirus can prevent liver and cervix cancer, to which tobacco can contribute.
According to Vital Signs, the CDC funds 65 comprehensive cancer control programs to prevent cancer, increase access to early detection and care for tobacco-related cancers, help cancer survivors quit using tobacco, and improve cancer outcomes, especially in communities with higher tobacco-related rates of cancer and deaths. The CDC also urges health care providers to encourage patients to quit—or not start—smoking, to be aware of screenings, and to remember that there’s no “risk-free” level of secondhand smoke.
Source:
Henley SJ, Thomas CC, Sharapova SR, et al. MMWR. 2016;65(44):1212-1218.
Tumor markers may predict anti–PD-L1 treatment response
NATIONAL HARBOR, MD. – Density in tumors of two key immune cells may be a biomarker for response to an inhibitor of programmed death–ligand 1 (PD-L1) among patients with non–small cell lung cancer (NSCLC).
Patients with NSCLC whose tumors bore high densities of both CD8-positive cytotoxic T lymphocytes and PD-L1 had significantly better outcomes when treated with the investigational PD-L1 inhibitor durvalumab than patients with high densities of either cell type alone, reported Sonja Althammer, PhD, a scientist at Definiens AG, maker of the anti–PD-L1 compound.
Dr. Althammer and her colleagues assessed the use of an automated image analysis and pattern recognition system to determine whether tumor-infiltrating CD8-positive cytotoxic T lymphocytes and PD-L1 densities could identify patients most likely to respond to the investigational PD-L1 inhibitor durvalumab.
“The hypothesis that we had in mind was that the interaction between both cell populations is important, so both cell populations should be present,” she said.
The investigators analyzed archived or fresh tumor biopsy samples from 163 patients with untreated or previously treated NSCLC (median 3 prior lines of therapy) who were enrolled in a nonrandomized phase I/II trial evaluating durvalumab in advanced NSCLC and other solid tumors.
They matched CD8 and PD-L1 immunohistochemistry-stained sections from tissues blocks. High PD-L1 expression was defined as a 25% or higher proportion of PD-L1–positive tumor cells with membrane staining at any intensity.
The images were then evaluated with an automated system, with results matched to clinical outcomes based on the densities of CD8-positive and PD-L1–positive cells, and PD-L1 alone from pre-treatment biopsy samples using a discovery set (84 samples) and validation set (79). The datasets were matched on baseline PD-L1 status, histology, Eastern Cooperative Oncology Group performance status, lines of therapy, and response.
In a training set looking at overall response rate (ORR), they found evidence to suggest that the combination of CD8 and PD-L1 positivity was associated with a higher ORR: 42%, compared with 31% for CD8-positive expression alone, 27% for PD-L1–positive alone, and 7% for CD8-positive PD-L1–negative (less than 25% expression) alone.
They then examined the predictive ability of the combined markers, and found that over approximately 28 months of follow-up, patients with CD8-positive and PD-L1–positive dense tumors had better overall survival (median overall survival, 24.3 months), compared with PD-L1–positive only patients (median overall survival, 17.1 months), CD8-positive only patients (median overall survival, 17.8 months), or the entire patient sample (median overall survival, 11.1 months).
Similarly, progression-free survival was also better among patients with high expression of both markers (respective median progression-free survival was 7.3, 3.6, 5.3, and 2.8 months).
Dr. Althammer acknowledged that the findings were preliminary and needed to be confirmed independently in larger studies.
The study was supported by MedImmune. Sonja Althammer is an employee of Definiens AG, which is a subsidiary of MedImmune/AstraZeneca.
NATIONAL HARBOR, MD. – Density in tumors of two key immune cells may be a biomarker for response to an inhibitor of programmed death–ligand 1 (PD-L1) among patients with non–small cell lung cancer (NSCLC).
Patients with NSCLC whose tumors bore high densities of both CD8-positive cytotoxic T lymphocytes and PD-L1 had significantly better outcomes when treated with the investigational PD-L1 inhibitor durvalumab than patients with high densities of either cell type alone, reported Sonja Althammer, PhD, a scientist at Definiens AG, maker of the anti–PD-L1 compound.
Dr. Althammer and her colleagues assessed the use of an automated image analysis and pattern recognition system to determine whether tumor-infiltrating CD8-positive cytotoxic T lymphocytes and PD-L1 densities could identify patients most likely to respond to the investigational PD-L1 inhibitor durvalumab.
“The hypothesis that we had in mind was that the interaction between both cell populations is important, so both cell populations should be present,” she said.
The investigators analyzed archived or fresh tumor biopsy samples from 163 patients with untreated or previously treated NSCLC (median 3 prior lines of therapy) who were enrolled in a nonrandomized phase I/II trial evaluating durvalumab in advanced NSCLC and other solid tumors.
They matched CD8 and PD-L1 immunohistochemistry-stained sections from tissues blocks. High PD-L1 expression was defined as a 25% or higher proportion of PD-L1–positive tumor cells with membrane staining at any intensity.
The images were then evaluated with an automated system, with results matched to clinical outcomes based on the densities of CD8-positive and PD-L1–positive cells, and PD-L1 alone from pre-treatment biopsy samples using a discovery set (84 samples) and validation set (79). The datasets were matched on baseline PD-L1 status, histology, Eastern Cooperative Oncology Group performance status, lines of therapy, and response.
In a training set looking at overall response rate (ORR), they found evidence to suggest that the combination of CD8 and PD-L1 positivity was associated with a higher ORR: 42%, compared with 31% for CD8-positive expression alone, 27% for PD-L1–positive alone, and 7% for CD8-positive PD-L1–negative (less than 25% expression) alone.
They then examined the predictive ability of the combined markers, and found that over approximately 28 months of follow-up, patients with CD8-positive and PD-L1–positive dense tumors had better overall survival (median overall survival, 24.3 months), compared with PD-L1–positive only patients (median overall survival, 17.1 months), CD8-positive only patients (median overall survival, 17.8 months), or the entire patient sample (median overall survival, 11.1 months).
Similarly, progression-free survival was also better among patients with high expression of both markers (respective median progression-free survival was 7.3, 3.6, 5.3, and 2.8 months).
Dr. Althammer acknowledged that the findings were preliminary and needed to be confirmed independently in larger studies.
The study was supported by MedImmune. Sonja Althammer is an employee of Definiens AG, which is a subsidiary of MedImmune/AstraZeneca.
NATIONAL HARBOR, MD. – Density in tumors of two key immune cells may be a biomarker for response to an inhibitor of programmed death–ligand 1 (PD-L1) among patients with non–small cell lung cancer (NSCLC).
Patients with NSCLC whose tumors bore high densities of both CD8-positive cytotoxic T lymphocytes and PD-L1 had significantly better outcomes when treated with the investigational PD-L1 inhibitor durvalumab than patients with high densities of either cell type alone, reported Sonja Althammer, PhD, a scientist at Definiens AG, maker of the anti–PD-L1 compound.
Dr. Althammer and her colleagues assessed the use of an automated image analysis and pattern recognition system to determine whether tumor-infiltrating CD8-positive cytotoxic T lymphocytes and PD-L1 densities could identify patients most likely to respond to the investigational PD-L1 inhibitor durvalumab.
“The hypothesis that we had in mind was that the interaction between both cell populations is important, so both cell populations should be present,” she said.
The investigators analyzed archived or fresh tumor biopsy samples from 163 patients with untreated or previously treated NSCLC (median 3 prior lines of therapy) who were enrolled in a nonrandomized phase I/II trial evaluating durvalumab in advanced NSCLC and other solid tumors.
They matched CD8 and PD-L1 immunohistochemistry-stained sections from tissues blocks. High PD-L1 expression was defined as a 25% or higher proportion of PD-L1–positive tumor cells with membrane staining at any intensity.
The images were then evaluated with an automated system, with results matched to clinical outcomes based on the densities of CD8-positive and PD-L1–positive cells, and PD-L1 alone from pre-treatment biopsy samples using a discovery set (84 samples) and validation set (79). The datasets were matched on baseline PD-L1 status, histology, Eastern Cooperative Oncology Group performance status, lines of therapy, and response.
In a training set looking at overall response rate (ORR), they found evidence to suggest that the combination of CD8 and PD-L1 positivity was associated with a higher ORR: 42%, compared with 31% for CD8-positive expression alone, 27% for PD-L1–positive alone, and 7% for CD8-positive PD-L1–negative (less than 25% expression) alone.
They then examined the predictive ability of the combined markers, and found that over approximately 28 months of follow-up, patients with CD8-positive and PD-L1–positive dense tumors had better overall survival (median overall survival, 24.3 months), compared with PD-L1–positive only patients (median overall survival, 17.1 months), CD8-positive only patients (median overall survival, 17.8 months), or the entire patient sample (median overall survival, 11.1 months).
Similarly, progression-free survival was also better among patients with high expression of both markers (respective median progression-free survival was 7.3, 3.6, 5.3, and 2.8 months).
Dr. Althammer acknowledged that the findings were preliminary and needed to be confirmed independently in larger studies.
The study was supported by MedImmune. Sonja Althammer is an employee of Definiens AG, which is a subsidiary of MedImmune/AstraZeneca.
AT SITC 2016
Key clinical point: Tumor expression of two cell types may predict responses to anti–PD-L1 immunotherapy.
Major finding: High tumor densities of CD8-postive cytotoxic T lymphocytes and the programmed death-ligand 1 were associated with improved overall response rates and survival in patients with advanced non–small cell lung cancer treated with a PD-L1 inhibitor.
Data source: Imaging study of samples from 163 patients enrolled in a nonrandomized phase I/II trial.
Disclosures: The study was supported by MedImmune. Sonja Althammer is an employee of Definiens AG, which is a subsidiary of MedImmune/AstraZeneca.
Tobacco-related cancer incidence, mortality drop
Tobacco-related cancer incidence and mortality rates dropped from 2004 to 2013, the Centers for Disease Control and Prevention reported in the Morbidity and Mortality Weekly Report published on Nov. 11, 2016.
Overall, tobacco-related invasive cancer incidence decreased from 206 cases per 100,000 during 2004-2008 to 193 cases per 100,000 during 2009-2013.
The announcement of this data marks a continuation of the downward trend that has been observed since the 1990s, lead author S. Jane Henley, MSPH, and her associates at the CDC wrote in the report (MMWR. 2016 Nov 11;65[44]:1212-8).
Despite this continued decline in tobacco-related cancer incidence and mortality rates, the tobacco-related cancer burden remains high, and disparities in the rates and decline of tobacco-related cancer persists.
“Tobacco use remains the leading preventable cause of disease and death in the United States,” reported Henley and her associates. For each year between 2009 and 2013, an estimated 660,000 Americans were diagnosed with tobacco-related cancer, and an estimated 343,000 people died from those cancers, according to the investigators’ analysis of data collected by the United States Cancer Statistics working group, which compiles data from multiple nationwide sources including the National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Tobacco-related cancer incidence and deaths were higher among men than women and higher among blacks than any other ethnic group. However, the cancer incidence and mortality rates also declined the fastest among men and blacks, compared with women and other ethnic groups, respectively.
Cancer incidence and death were also highest and decreased the most slowly in counties with lower educational attainment or highest poverty. Conversely, cancer incidence and mortality was the lowest and decreased the most quickly in metropolitan areas with populations greater than 1 million people.
Given that an estimated 40% of cancers diagnosed in the country and 3 in 10 cancer deaths are attributable to cigarette smoking and the use of smokeless tobacco, it is imperative that the CDC implement programs to help the almost 6 million smokers quit, CDC director Tom Frieden, MD, said in an associated telebriefing. Most people who smoke want to quit, and the health care system should do all it can to help them, Dr. Frieden said. At the same time, he echoed a claim from Henley’s paper, which said many tobacco-related cancers could be prevented by reducing tobacco use through implementation of evidence-based tobacco prevention and control interventions, such as increasing tobacco product prices, enforcing smoke-free laws, and promoting anti-tobacco mass media campaigns. These programs should be tailored to local geographic areas and demographics given the continued inconsistent progress and persistent disparities in tobacco-related cancer incidence and mortality, Dr. Frieden added.
jcraig@frontlinemedcom.com
On Twitter @jessnicolecraig
Tobacco-related cancer incidence and mortality rates dropped from 2004 to 2013, the Centers for Disease Control and Prevention reported in the Morbidity and Mortality Weekly Report published on Nov. 11, 2016.
Overall, tobacco-related invasive cancer incidence decreased from 206 cases per 100,000 during 2004-2008 to 193 cases per 100,000 during 2009-2013.
The announcement of this data marks a continuation of the downward trend that has been observed since the 1990s, lead author S. Jane Henley, MSPH, and her associates at the CDC wrote in the report (MMWR. 2016 Nov 11;65[44]:1212-8).
Despite this continued decline in tobacco-related cancer incidence and mortality rates, the tobacco-related cancer burden remains high, and disparities in the rates and decline of tobacco-related cancer persists.
“Tobacco use remains the leading preventable cause of disease and death in the United States,” reported Henley and her associates. For each year between 2009 and 2013, an estimated 660,000 Americans were diagnosed with tobacco-related cancer, and an estimated 343,000 people died from those cancers, according to the investigators’ analysis of data collected by the United States Cancer Statistics working group, which compiles data from multiple nationwide sources including the National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Tobacco-related cancer incidence and deaths were higher among men than women and higher among blacks than any other ethnic group. However, the cancer incidence and mortality rates also declined the fastest among men and blacks, compared with women and other ethnic groups, respectively.
Cancer incidence and death were also highest and decreased the most slowly in counties with lower educational attainment or highest poverty. Conversely, cancer incidence and mortality was the lowest and decreased the most quickly in metropolitan areas with populations greater than 1 million people.
Given that an estimated 40% of cancers diagnosed in the country and 3 in 10 cancer deaths are attributable to cigarette smoking and the use of smokeless tobacco, it is imperative that the CDC implement programs to help the almost 6 million smokers quit, CDC director Tom Frieden, MD, said in an associated telebriefing. Most people who smoke want to quit, and the health care system should do all it can to help them, Dr. Frieden said. At the same time, he echoed a claim from Henley’s paper, which said many tobacco-related cancers could be prevented by reducing tobacco use through implementation of evidence-based tobacco prevention and control interventions, such as increasing tobacco product prices, enforcing smoke-free laws, and promoting anti-tobacco mass media campaigns. These programs should be tailored to local geographic areas and demographics given the continued inconsistent progress and persistent disparities in tobacco-related cancer incidence and mortality, Dr. Frieden added.
jcraig@frontlinemedcom.com
On Twitter @jessnicolecraig
Tobacco-related cancer incidence and mortality rates dropped from 2004 to 2013, the Centers for Disease Control and Prevention reported in the Morbidity and Mortality Weekly Report published on Nov. 11, 2016.
Overall, tobacco-related invasive cancer incidence decreased from 206 cases per 100,000 during 2004-2008 to 193 cases per 100,000 during 2009-2013.
The announcement of this data marks a continuation of the downward trend that has been observed since the 1990s, lead author S. Jane Henley, MSPH, and her associates at the CDC wrote in the report (MMWR. 2016 Nov 11;65[44]:1212-8).
Despite this continued decline in tobacco-related cancer incidence and mortality rates, the tobacco-related cancer burden remains high, and disparities in the rates and decline of tobacco-related cancer persists.
“Tobacco use remains the leading preventable cause of disease and death in the United States,” reported Henley and her associates. For each year between 2009 and 2013, an estimated 660,000 Americans were diagnosed with tobacco-related cancer, and an estimated 343,000 people died from those cancers, according to the investigators’ analysis of data collected by the United States Cancer Statistics working group, which compiles data from multiple nationwide sources including the National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Tobacco-related cancer incidence and deaths were higher among men than women and higher among blacks than any other ethnic group. However, the cancer incidence and mortality rates also declined the fastest among men and blacks, compared with women and other ethnic groups, respectively.
Cancer incidence and death were also highest and decreased the most slowly in counties with lower educational attainment or highest poverty. Conversely, cancer incidence and mortality was the lowest and decreased the most quickly in metropolitan areas with populations greater than 1 million people.
Given that an estimated 40% of cancers diagnosed in the country and 3 in 10 cancer deaths are attributable to cigarette smoking and the use of smokeless tobacco, it is imperative that the CDC implement programs to help the almost 6 million smokers quit, CDC director Tom Frieden, MD, said in an associated telebriefing. Most people who smoke want to quit, and the health care system should do all it can to help them, Dr. Frieden said. At the same time, he echoed a claim from Henley’s paper, which said many tobacco-related cancers could be prevented by reducing tobacco use through implementation of evidence-based tobacco prevention and control interventions, such as increasing tobacco product prices, enforcing smoke-free laws, and promoting anti-tobacco mass media campaigns. These programs should be tailored to local geographic areas and demographics given the continued inconsistent progress and persistent disparities in tobacco-related cancer incidence and mortality, Dr. Frieden added.
jcraig@frontlinemedcom.com
On Twitter @jessnicolecraig
FROM MMWR
Key clinical point:
Major finding: Tobacco-related cancer mortality dropped from 108 deaths per 100,000 during 2004-2008 to 100 per 100,000 during 2009-2013.
Data source: Retrospective analysis of United States Cancer Statistics data for 2004 to 2013.
Disclosures: This study was sponsored by the Centers for Disease Control and Prevention. The authors’ disclosures were not reported.
Optimal MPE management requires early pulmonology referral
LOS ANGELES – About half of patients with symptomatic malignant pleural effusions at McGill University Health Centre in Montreal had unnecessary procedures and hospital admissions before definitive treatment with chemical pleurodesis or indwelling pleural catheters, according to researchers.
Instead of chest taps to relieve symptoms followed by referrals for definitive treatment, some patients got chest tubes – without pleurodesis – after presenting to the emergency department and being referred to radiology; they were then admitted to the hospital for a few days while the tubes were in place. In short, cancer patients were wasting what time they had left on medical care they didn’t need, and incurring unnecessary costs, said lead investigator Benjamin Shieh, MD, formerly at McGill but now an interventional pulmonology fellow at the University of Calgary.
McGill is a tertiary care center able to perform both definitive procedures, so “we should be a center of excellence. I imagine there are similar situations” at other hospitals, especially those without the resources of McGill, Dr. Shieh said at the annual meeting of the American College of Chest Physicians.
McGill has taken several steps to address the problem, including early ED referral to the pulmonology service and discouraging radiology from placing chest tubes for malignant pleural effusions (MPE). “I think we can avoid a big proportion of hospitalizations for MPE, and certainly a proportion of repeat [ED] visits,” said senior author Anne Gonzalez, MD, an attending pulmonologist at McGill.
The investigators looked into the issue after noting that a lot of their MPE cases had been hospitalized with chest tubes. They reviewed 72 symptomatic MPE cases in 69 patients treated in 2014 and 2015. Management was ideal in 36 cases (50%), meaning that, prior to definitive treatment, patients had no more than two pleural taps for symptom relief, no more than one ED visit, no chest tubes without pleurodesis, and no hospitalizations. “We thought this would be reasonable to try to achieve for MPE,” since there’s no definition of ideal management, Dr. Shieh said.
Nonideal patients had a mean of 2.5 pleural procedures – almost twice the number in the ideal group – before definitive palliation, with no respiratory consult beforehand. Chest tubes were placed in 27 cases (38%) for an average of 3.7 days; 28 cases (39%) were hospitalized. Nonideal patients were far more likely to present first to the ED, and ED presentations were more likely to get chest tubes and be admitted. All the cases were eventually treated definitively, 68 with indwelling pleural catheters and 4 by thoracoscopic talc insufflation. Time from initial presentation to definitive palliation was about 1 month in both groups. The investigators didn’t consider rate of effusion recurrence, which might help explain why the ideal group wasn’t treated sooner; they might not have needed it. The higher number of ED visits in the nonideal group suggests that they may have had quicker recurrences, and should have been treated sooner, Dr. Gonzalez said.
The patients were 70 years old, on average, and about 60% were women. Lung and breast were the most common cancers.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – About half of patients with symptomatic malignant pleural effusions at McGill University Health Centre in Montreal had unnecessary procedures and hospital admissions before definitive treatment with chemical pleurodesis or indwelling pleural catheters, according to researchers.
Instead of chest taps to relieve symptoms followed by referrals for definitive treatment, some patients got chest tubes – without pleurodesis – after presenting to the emergency department and being referred to radiology; they were then admitted to the hospital for a few days while the tubes were in place. In short, cancer patients were wasting what time they had left on medical care they didn’t need, and incurring unnecessary costs, said lead investigator Benjamin Shieh, MD, formerly at McGill but now an interventional pulmonology fellow at the University of Calgary.
McGill is a tertiary care center able to perform both definitive procedures, so “we should be a center of excellence. I imagine there are similar situations” at other hospitals, especially those without the resources of McGill, Dr. Shieh said at the annual meeting of the American College of Chest Physicians.
McGill has taken several steps to address the problem, including early ED referral to the pulmonology service and discouraging radiology from placing chest tubes for malignant pleural effusions (MPE). “I think we can avoid a big proportion of hospitalizations for MPE, and certainly a proportion of repeat [ED] visits,” said senior author Anne Gonzalez, MD, an attending pulmonologist at McGill.
The investigators looked into the issue after noting that a lot of their MPE cases had been hospitalized with chest tubes. They reviewed 72 symptomatic MPE cases in 69 patients treated in 2014 and 2015. Management was ideal in 36 cases (50%), meaning that, prior to definitive treatment, patients had no more than two pleural taps for symptom relief, no more than one ED visit, no chest tubes without pleurodesis, and no hospitalizations. “We thought this would be reasonable to try to achieve for MPE,” since there’s no definition of ideal management, Dr. Shieh said.
Nonideal patients had a mean of 2.5 pleural procedures – almost twice the number in the ideal group – before definitive palliation, with no respiratory consult beforehand. Chest tubes were placed in 27 cases (38%) for an average of 3.7 days; 28 cases (39%) were hospitalized. Nonideal patients were far more likely to present first to the ED, and ED presentations were more likely to get chest tubes and be admitted. All the cases were eventually treated definitively, 68 with indwelling pleural catheters and 4 by thoracoscopic talc insufflation. Time from initial presentation to definitive palliation was about 1 month in both groups. The investigators didn’t consider rate of effusion recurrence, which might help explain why the ideal group wasn’t treated sooner; they might not have needed it. The higher number of ED visits in the nonideal group suggests that they may have had quicker recurrences, and should have been treated sooner, Dr. Gonzalez said.
The patients were 70 years old, on average, and about 60% were women. Lung and breast were the most common cancers.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – About half of patients with symptomatic malignant pleural effusions at McGill University Health Centre in Montreal had unnecessary procedures and hospital admissions before definitive treatment with chemical pleurodesis or indwelling pleural catheters, according to researchers.
Instead of chest taps to relieve symptoms followed by referrals for definitive treatment, some patients got chest tubes – without pleurodesis – after presenting to the emergency department and being referred to radiology; they were then admitted to the hospital for a few days while the tubes were in place. In short, cancer patients were wasting what time they had left on medical care they didn’t need, and incurring unnecessary costs, said lead investigator Benjamin Shieh, MD, formerly at McGill but now an interventional pulmonology fellow at the University of Calgary.
McGill is a tertiary care center able to perform both definitive procedures, so “we should be a center of excellence. I imagine there are similar situations” at other hospitals, especially those without the resources of McGill, Dr. Shieh said at the annual meeting of the American College of Chest Physicians.
McGill has taken several steps to address the problem, including early ED referral to the pulmonology service and discouraging radiology from placing chest tubes for malignant pleural effusions (MPE). “I think we can avoid a big proportion of hospitalizations for MPE, and certainly a proportion of repeat [ED] visits,” said senior author Anne Gonzalez, MD, an attending pulmonologist at McGill.
The investigators looked into the issue after noting that a lot of their MPE cases had been hospitalized with chest tubes. They reviewed 72 symptomatic MPE cases in 69 patients treated in 2014 and 2015. Management was ideal in 36 cases (50%), meaning that, prior to definitive treatment, patients had no more than two pleural taps for symptom relief, no more than one ED visit, no chest tubes without pleurodesis, and no hospitalizations. “We thought this would be reasonable to try to achieve for MPE,” since there’s no definition of ideal management, Dr. Shieh said.
Nonideal patients had a mean of 2.5 pleural procedures – almost twice the number in the ideal group – before definitive palliation, with no respiratory consult beforehand. Chest tubes were placed in 27 cases (38%) for an average of 3.7 days; 28 cases (39%) were hospitalized. Nonideal patients were far more likely to present first to the ED, and ED presentations were more likely to get chest tubes and be admitted. All the cases were eventually treated definitively, 68 with indwelling pleural catheters and 4 by thoracoscopic talc insufflation. Time from initial presentation to definitive palliation was about 1 month in both groups. The investigators didn’t consider rate of effusion recurrence, which might help explain why the ideal group wasn’t treated sooner; they might not have needed it. The higher number of ED visits in the nonideal group suggests that they may have had quicker recurrences, and should have been treated sooner, Dr. Gonzalez said.
The patients were 70 years old, on average, and about 60% were women. Lung and breast were the most common cancers.
There was no industry funding for the work, and the investigators had no disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Those patients had a mean of 2.5 pleural procedures before definitive palliation, with no respiratory consult beforehand. Chest tubes were placed for an average of 3.7 days.
Data source: Review of 72 MPE cases in 69 patients.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
Blood assay rapidly identifies lung cancer mutations
LOS ANGELES – A newer blood test (GeneStrat from Biodesix) identified genetic mutations in lung tumors in about 24 hours, allowing for an early start of mutation-specific chemotherapy, in an investigation from Gundersen Health System in La Crosse, Wis.
Interventional pulmonologists drew blood samples when they performed biopsies on 84 patients with highly suspicious lung nodules and submitted both blood and tissue for mutation analysis. The blood was analyzed by Biodesix, the maker of GeneStrat, a commercially available digital droplet polymerase change reaction assay launched in 2015. The company sent the results back in an average of 24.1 hours, and all within 72 hours. The mutation results from tissue analysis took 2-3 weeks.
Fifteen patients (18%) had actionable epidermal growth factor receptor, anaplastic lymphoma kinase, or K-Ras protein gene mutations, and were candidates for targeted therapy. Compared with tissue testing, the blood assay had a sensitivity of 88% and a specificity of 99%. The tissue testing picked up two mutations missed by blood testing. One of the two mutations is rare and was not included in the blood assay. Meanwhile, the assay caught a mutation missed on tissue analysis.
She and her colleagues are now routinely using GeneStrat to guide initial lung cancer therapy. “The turnaround time is fantastic. It allows us to have [the mutation status] when oncologists meet with patients for the very first time,” she said.
It “definitely” makes a difference. “If you have an actionable mutation and there’s a targeted chemotherapy” – such as erlotinib (Tarceva) for epidermal growth factor receptor mutation patients – it can be started right out of the gate. “Time to treatment is very important,” not just psychologically for patients but also for them to have the best chance against the tumor. The sooner “we can start a targeted therapy,” the better outcomes are likely to be, Dr. Mattingley said.
When mutation status is delayed, patients might be started “on the wrong therapy upfront, and it’s really hard to back up and start over again,” she said.
“Once we give patients a diagnosis of lung cancer, the next thing they should hear right away is how we are going to attack it. We felt strongly [that there was a] need to look at this to see if we could truly expedite the time from diagnosis to treatment. We believe our patients should have no sleepless nights,” Dr. Mattingley said.
There’s also usually not much tissue left after genetic work-up to send into a clinical trial. Using blood to identify mutations “may allow us to conserve our tissue block for future trials, but still get the genetic information we need to start treatment plans for our patients,” she said.
There was no company funding for the work, but Dr. Mattingley is a speaker for GeneStrat’s maker, Biodesix.
The results of this study are very promising. The high concordance between liquid biopsies and tissue biopsies as well as the short turn-around time for the results of the liquid biopsies makes a big difference in terms of getting patients started on appropriate therapy sooner rather than later. We need additional studies to find out if liquid biopsies will be good for detection of other molecular alterations such as ROS-1 and EGFR acquired mutation T790M.
The results of this study are very promising. The high concordance between liquid biopsies and tissue biopsies as well as the short turn-around time for the results of the liquid biopsies makes a big difference in terms of getting patients started on appropriate therapy sooner rather than later. We need additional studies to find out if liquid biopsies will be good for detection of other molecular alterations such as ROS-1 and EGFR acquired mutation T790M.
The results of this study are very promising. The high concordance between liquid biopsies and tissue biopsies as well as the short turn-around time for the results of the liquid biopsies makes a big difference in terms of getting patients started on appropriate therapy sooner rather than later. We need additional studies to find out if liquid biopsies will be good for detection of other molecular alterations such as ROS-1 and EGFR acquired mutation T790M.
LOS ANGELES – A newer blood test (GeneStrat from Biodesix) identified genetic mutations in lung tumors in about 24 hours, allowing for an early start of mutation-specific chemotherapy, in an investigation from Gundersen Health System in La Crosse, Wis.
Interventional pulmonologists drew blood samples when they performed biopsies on 84 patients with highly suspicious lung nodules and submitted both blood and tissue for mutation analysis. The blood was analyzed by Biodesix, the maker of GeneStrat, a commercially available digital droplet polymerase change reaction assay launched in 2015. The company sent the results back in an average of 24.1 hours, and all within 72 hours. The mutation results from tissue analysis took 2-3 weeks.
Fifteen patients (18%) had actionable epidermal growth factor receptor, anaplastic lymphoma kinase, or K-Ras protein gene mutations, and were candidates for targeted therapy. Compared with tissue testing, the blood assay had a sensitivity of 88% and a specificity of 99%. The tissue testing picked up two mutations missed by blood testing. One of the two mutations is rare and was not included in the blood assay. Meanwhile, the assay caught a mutation missed on tissue analysis.
She and her colleagues are now routinely using GeneStrat to guide initial lung cancer therapy. “The turnaround time is fantastic. It allows us to have [the mutation status] when oncologists meet with patients for the very first time,” she said.
It “definitely” makes a difference. “If you have an actionable mutation and there’s a targeted chemotherapy” – such as erlotinib (Tarceva) for epidermal growth factor receptor mutation patients – it can be started right out of the gate. “Time to treatment is very important,” not just psychologically for patients but also for them to have the best chance against the tumor. The sooner “we can start a targeted therapy,” the better outcomes are likely to be, Dr. Mattingley said.
When mutation status is delayed, patients might be started “on the wrong therapy upfront, and it’s really hard to back up and start over again,” she said.
“Once we give patients a diagnosis of lung cancer, the next thing they should hear right away is how we are going to attack it. We felt strongly [that there was a] need to look at this to see if we could truly expedite the time from diagnosis to treatment. We believe our patients should have no sleepless nights,” Dr. Mattingley said.
There’s also usually not much tissue left after genetic work-up to send into a clinical trial. Using blood to identify mutations “may allow us to conserve our tissue block for future trials, but still get the genetic information we need to start treatment plans for our patients,” she said.
There was no company funding for the work, but Dr. Mattingley is a speaker for GeneStrat’s maker, Biodesix.
LOS ANGELES – A newer blood test (GeneStrat from Biodesix) identified genetic mutations in lung tumors in about 24 hours, allowing for an early start of mutation-specific chemotherapy, in an investigation from Gundersen Health System in La Crosse, Wis.
Interventional pulmonologists drew blood samples when they performed biopsies on 84 patients with highly suspicious lung nodules and submitted both blood and tissue for mutation analysis. The blood was analyzed by Biodesix, the maker of GeneStrat, a commercially available digital droplet polymerase change reaction assay launched in 2015. The company sent the results back in an average of 24.1 hours, and all within 72 hours. The mutation results from tissue analysis took 2-3 weeks.
Fifteen patients (18%) had actionable epidermal growth factor receptor, anaplastic lymphoma kinase, or K-Ras protein gene mutations, and were candidates for targeted therapy. Compared with tissue testing, the blood assay had a sensitivity of 88% and a specificity of 99%. The tissue testing picked up two mutations missed by blood testing. One of the two mutations is rare and was not included in the blood assay. Meanwhile, the assay caught a mutation missed on tissue analysis.
She and her colleagues are now routinely using GeneStrat to guide initial lung cancer therapy. “The turnaround time is fantastic. It allows us to have [the mutation status] when oncologists meet with patients for the very first time,” she said.
It “definitely” makes a difference. “If you have an actionable mutation and there’s a targeted chemotherapy” – such as erlotinib (Tarceva) for epidermal growth factor receptor mutation patients – it can be started right out of the gate. “Time to treatment is very important,” not just psychologically for patients but also for them to have the best chance against the tumor. The sooner “we can start a targeted therapy,” the better outcomes are likely to be, Dr. Mattingley said.
When mutation status is delayed, patients might be started “on the wrong therapy upfront, and it’s really hard to back up and start over again,” she said.
“Once we give patients a diagnosis of lung cancer, the next thing they should hear right away is how we are going to attack it. We felt strongly [that there was a] need to look at this to see if we could truly expedite the time from diagnosis to treatment. We believe our patients should have no sleepless nights,” Dr. Mattingley said.
There’s also usually not much tissue left after genetic work-up to send into a clinical trial. Using blood to identify mutations “may allow us to conserve our tissue block for future trials, but still get the genetic information we need to start treatment plans for our patients,” she said.
There was no company funding for the work, but Dr. Mattingley is a speaker for GeneStrat’s maker, Biodesix.
AT CHEST 2016
Key clinical point:
Major finding: Compared with tissue testing, the blood assay had a sensitivity of 88% and a specificity of 99%.
Data source: Eighty-four patients with highly suspicious lung nodules.
Disclosures: There was no company funding for the work, but the presenter is a paid speaker for GeneStrat’s maker, Biodesix.
Few non-ICU patients receive palliative care consults
LOS ANGELES – A significant percentage of patients who meet criteria for palliative care consultations do not receive a consult during their hospital stay, results from a single-center retrospective analysis showed.
“Physicians need to recognize the palliative care needs of patients with chronic illnesses other than malignancy before they get admitted to the ICU, especially when these patients are admitted repeatedly for the same problem [and] have a significant decline in functional status with a large symptom burden,” Mohleen Kang, MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians. “There is a potential missed opportunity for these conversations to occur with the patients and their family prior to their decompensation and crisis.”
Twenty-nine percent (132) of the patients studied met an indication for a palliative care consult (PCC), with only 35 (27%) of such patients having received a PCC. Patients with metastatic cancer were significantly more likely to have received a PCC, compared with non-cancer patients (64% vs. 21%, respectively; P less than .001), while patients with New York Heart Association Class III or IV congestive heart failure were less likely to receive a PCC, compared with those who did not have congestive heart failure (5.6% vs. 29.8%; P = .014).
Criteria for PCC on admission include a life-limiting diagnosis and more than one admission in the past 3 months, decline in function, or complex care requirements. Criteria for PCC during hospitalization include life-limiting diagnosis and uncertainty about decisions, an ICU stay greater than 7 days, or lack of goals of care.
Dr. Kang, chief resident in the department of medicine at New Jersey Medical School, Newark, presented the results, which were of patients admitted to the department of medicine at University Hospital in Newark in 2015. Those admitted to the ICU within 24 hours of admission were excluded from the analysis, leaving 461 patient charts that were screened for PCC needs based on the consensus report from the Center to Advance Palliative Care.
The patients who met an indication for PCC had a mean age of 60 years and an average length of stay of 7 days. The percentages of these patients who were female, African American, and Hispanic were 45%, 40%, and 21%, respectively.
On multivariate analysis, patients who had a PCC within 72 hours of admission were eight times more likely to have a hospital length of stay less than 7 days (P = .019), while those who had a PCC within 48 hours of admission were 20 times more likely to have a hospital length of stay less than 7 days (P = .017). “So if we intervened early, we were able to decrease their length of stay to less than 7 days,” Dr. Kang said at the meeting.
She acknowledged certain limitations of the study, including its small sample size, retrospective design, and lack of follow-up. “This study also has a lot of confounding socioeconomic factors that do not make it applicable to every hospital across the country,” she said. “This is not a homogeneous patient population.”
The study’s principal investigator was Anne Sutherland, MD, medical intensive care unit director at University Hospital. Dr. Kang reported having no financial disclosures.
LOS ANGELES – A significant percentage of patients who meet criteria for palliative care consultations do not receive a consult during their hospital stay, results from a single-center retrospective analysis showed.
“Physicians need to recognize the palliative care needs of patients with chronic illnesses other than malignancy before they get admitted to the ICU, especially when these patients are admitted repeatedly for the same problem [and] have a significant decline in functional status with a large symptom burden,” Mohleen Kang, MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians. “There is a potential missed opportunity for these conversations to occur with the patients and their family prior to their decompensation and crisis.”
Twenty-nine percent (132) of the patients studied met an indication for a palliative care consult (PCC), with only 35 (27%) of such patients having received a PCC. Patients with metastatic cancer were significantly more likely to have received a PCC, compared with non-cancer patients (64% vs. 21%, respectively; P less than .001), while patients with New York Heart Association Class III or IV congestive heart failure were less likely to receive a PCC, compared with those who did not have congestive heart failure (5.6% vs. 29.8%; P = .014).
Criteria for PCC on admission include a life-limiting diagnosis and more than one admission in the past 3 months, decline in function, or complex care requirements. Criteria for PCC during hospitalization include life-limiting diagnosis and uncertainty about decisions, an ICU stay greater than 7 days, or lack of goals of care.
Dr. Kang, chief resident in the department of medicine at New Jersey Medical School, Newark, presented the results, which were of patients admitted to the department of medicine at University Hospital in Newark in 2015. Those admitted to the ICU within 24 hours of admission were excluded from the analysis, leaving 461 patient charts that were screened for PCC needs based on the consensus report from the Center to Advance Palliative Care.
The patients who met an indication for PCC had a mean age of 60 years and an average length of stay of 7 days. The percentages of these patients who were female, African American, and Hispanic were 45%, 40%, and 21%, respectively.
On multivariate analysis, patients who had a PCC within 72 hours of admission were eight times more likely to have a hospital length of stay less than 7 days (P = .019), while those who had a PCC within 48 hours of admission were 20 times more likely to have a hospital length of stay less than 7 days (P = .017). “So if we intervened early, we were able to decrease their length of stay to less than 7 days,” Dr. Kang said at the meeting.
She acknowledged certain limitations of the study, including its small sample size, retrospective design, and lack of follow-up. “This study also has a lot of confounding socioeconomic factors that do not make it applicable to every hospital across the country,” she said. “This is not a homogeneous patient population.”
The study’s principal investigator was Anne Sutherland, MD, medical intensive care unit director at University Hospital. Dr. Kang reported having no financial disclosures.
LOS ANGELES – A significant percentage of patients who meet criteria for palliative care consultations do not receive a consult during their hospital stay, results from a single-center retrospective analysis showed.
“Physicians need to recognize the palliative care needs of patients with chronic illnesses other than malignancy before they get admitted to the ICU, especially when these patients are admitted repeatedly for the same problem [and] have a significant decline in functional status with a large symptom burden,” Mohleen Kang, MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians. “There is a potential missed opportunity for these conversations to occur with the patients and their family prior to their decompensation and crisis.”
Twenty-nine percent (132) of the patients studied met an indication for a palliative care consult (PCC), with only 35 (27%) of such patients having received a PCC. Patients with metastatic cancer were significantly more likely to have received a PCC, compared with non-cancer patients (64% vs. 21%, respectively; P less than .001), while patients with New York Heart Association Class III or IV congestive heart failure were less likely to receive a PCC, compared with those who did not have congestive heart failure (5.6% vs. 29.8%; P = .014).
Criteria for PCC on admission include a life-limiting diagnosis and more than one admission in the past 3 months, decline in function, or complex care requirements. Criteria for PCC during hospitalization include life-limiting diagnosis and uncertainty about decisions, an ICU stay greater than 7 days, or lack of goals of care.
Dr. Kang, chief resident in the department of medicine at New Jersey Medical School, Newark, presented the results, which were of patients admitted to the department of medicine at University Hospital in Newark in 2015. Those admitted to the ICU within 24 hours of admission were excluded from the analysis, leaving 461 patient charts that were screened for PCC needs based on the consensus report from the Center to Advance Palliative Care.
The patients who met an indication for PCC had a mean age of 60 years and an average length of stay of 7 days. The percentages of these patients who were female, African American, and Hispanic were 45%, 40%, and 21%, respectively.
On multivariate analysis, patients who had a PCC within 72 hours of admission were eight times more likely to have a hospital length of stay less than 7 days (P = .019), while those who had a PCC within 48 hours of admission were 20 times more likely to have a hospital length of stay less than 7 days (P = .017). “So if we intervened early, we were able to decrease their length of stay to less than 7 days,” Dr. Kang said at the meeting.
She acknowledged certain limitations of the study, including its small sample size, retrospective design, and lack of follow-up. “This study also has a lot of confounding socioeconomic factors that do not make it applicable to every hospital across the country,” she said. “This is not a homogeneous patient population.”
The study’s principal investigator was Anne Sutherland, MD, medical intensive care unit director at University Hospital. Dr. Kang reported having no financial disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Patients with metastatic cancer were significantly more likely to have received a PCC, compared with non-cancer patients (64% vs. 21%, respectively; P less than .001).
Data source: A retrospective study of 132 patients admitted to the department of medicine at University Hospital in Newark, N.J., in 2015.
Disclosures: Dr. Kang reported having no financial disclosures.
FDA expands indication for pembrolizumab in NSCLC
The Food and Drug Administration has approved pembrolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 as determined by an FDA-approved test. This is the first approval of a checkpoint inhibitor for first-line treatment of the disease.
Pembrolizumab (Keytruda) is now approved to treat patients with metastatic NSCLC whose tumors have high PD-L1 expression (Tumor Proportion Score [TPS] greater than or equal to 50%), with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC, the FDA said in a written statement.
The FDA based its approval on improvement in overall survival in two trials comparing treatment with pembrolizumab to treatment from chemotherapy. In one trial of 305 patients who had no prior treatment for metastatic NSCLC and TPS greater than or equal to 50%, those who received pembrolizumab (200 mg every 3 weeks) had a statistically significant improvement in overall survival, compared with patients randomized to receive chemotherapy (hazard ratio, 0.60; 95% confidence interval, 0.41-0.89; P less than .005). There was also significant improvement in progression-free survival for those receiving the checkpoint inhibitor (HR, 0.50; 95% CI, 0.37-0.68; P less than .001).
In the second trial, a three-arm trial of 1,033 patients who were previously treated for metastatic NSCLC with a TPS greater than or equal to 1%, those randomized to pembrolizumab 2 mg/kg every 3 weeks (HR, 0.71; 95% CI, 0.58-0.88; P less than .001) or pembrolizumab 10 mg/kg every 3 weeks (HR, 0.61; 95% CI, 0.49-0.75; P less than .001) had an improved overall survival, compared with patients receiving docetaxel. The median survival was 10.4 months in the pembrolizumab 2 mg/kg arm, 12.7 months in the pembrolizumab 10 mg/kg arm, and 8.5 months in the docetaxel arm.
The most common side effects of treatment with pembrolizumab included decreased appetite, fatigue, nausea, dyspnea, cough, and constipation. Rare but serious adverse events included immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis, the FDA said.
The recommended dose and schedule of pembrolizumab for NSCLC is 200 mg intravenously every 3 weeks. Full prescribing information is available here.
The Food and Drug Administration has approved pembrolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 as determined by an FDA-approved test. This is the first approval of a checkpoint inhibitor for first-line treatment of the disease.
Pembrolizumab (Keytruda) is now approved to treat patients with metastatic NSCLC whose tumors have high PD-L1 expression (Tumor Proportion Score [TPS] greater than or equal to 50%), with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC, the FDA said in a written statement.
The FDA based its approval on improvement in overall survival in two trials comparing treatment with pembrolizumab to treatment from chemotherapy. In one trial of 305 patients who had no prior treatment for metastatic NSCLC and TPS greater than or equal to 50%, those who received pembrolizumab (200 mg every 3 weeks) had a statistically significant improvement in overall survival, compared with patients randomized to receive chemotherapy (hazard ratio, 0.60; 95% confidence interval, 0.41-0.89; P less than .005). There was also significant improvement in progression-free survival for those receiving the checkpoint inhibitor (HR, 0.50; 95% CI, 0.37-0.68; P less than .001).
In the second trial, a three-arm trial of 1,033 patients who were previously treated for metastatic NSCLC with a TPS greater than or equal to 1%, those randomized to pembrolizumab 2 mg/kg every 3 weeks (HR, 0.71; 95% CI, 0.58-0.88; P less than .001) or pembrolizumab 10 mg/kg every 3 weeks (HR, 0.61; 95% CI, 0.49-0.75; P less than .001) had an improved overall survival, compared with patients receiving docetaxel. The median survival was 10.4 months in the pembrolizumab 2 mg/kg arm, 12.7 months in the pembrolizumab 10 mg/kg arm, and 8.5 months in the docetaxel arm.
The most common side effects of treatment with pembrolizumab included decreased appetite, fatigue, nausea, dyspnea, cough, and constipation. Rare but serious adverse events included immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis, the FDA said.
The recommended dose and schedule of pembrolizumab for NSCLC is 200 mg intravenously every 3 weeks. Full prescribing information is available here.
The Food and Drug Administration has approved pembrolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 as determined by an FDA-approved test. This is the first approval of a checkpoint inhibitor for first-line treatment of the disease.
Pembrolizumab (Keytruda) is now approved to treat patients with metastatic NSCLC whose tumors have high PD-L1 expression (Tumor Proportion Score [TPS] greater than or equal to 50%), with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC, the FDA said in a written statement.
The FDA based its approval on improvement in overall survival in two trials comparing treatment with pembrolizumab to treatment from chemotherapy. In one trial of 305 patients who had no prior treatment for metastatic NSCLC and TPS greater than or equal to 50%, those who received pembrolizumab (200 mg every 3 weeks) had a statistically significant improvement in overall survival, compared with patients randomized to receive chemotherapy (hazard ratio, 0.60; 95% confidence interval, 0.41-0.89; P less than .005). There was also significant improvement in progression-free survival for those receiving the checkpoint inhibitor (HR, 0.50; 95% CI, 0.37-0.68; P less than .001).
In the second trial, a three-arm trial of 1,033 patients who were previously treated for metastatic NSCLC with a TPS greater than or equal to 1%, those randomized to pembrolizumab 2 mg/kg every 3 weeks (HR, 0.71; 95% CI, 0.58-0.88; P less than .001) or pembrolizumab 10 mg/kg every 3 weeks (HR, 0.61; 95% CI, 0.49-0.75; P less than .001) had an improved overall survival, compared with patients receiving docetaxel. The median survival was 10.4 months in the pembrolizumab 2 mg/kg arm, 12.7 months in the pembrolizumab 10 mg/kg arm, and 8.5 months in the docetaxel arm.
The most common side effects of treatment with pembrolizumab included decreased appetite, fatigue, nausea, dyspnea, cough, and constipation. Rare but serious adverse events included immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis, the FDA said.
The recommended dose and schedule of pembrolizumab for NSCLC is 200 mg intravenously every 3 weeks. Full prescribing information is available here.
Lung cancer screening found effective in a community hospital
LOS ANGELES – Lung cancer screening with low-dose CT scans in a community hospital setting replicates results from international and multicenter trials when it comes to diagnosing early-stage lung cancer, findings from a single-center study showed.
“It’s too early in our experience to say that we’re saving lives, but the fact that we’re detecting early lung cancers in the predicted percentages is good for community hospitals that are wondering, ‘Is it worth it to screen for lung cancer? Can we do it?’ ” Richard P. Salzano Jr., MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians.
In July 2013, the 130-bed Griffin Hospital launched a lung cancer screening program codirected by a pulmonologist and a cardiothoracic surgeon. All low-dose CT scans were read by two designated radiologists. Dr. Salzano reported results from 514 patients enrolled in the program between July 2013 and December 2015. A total of nine lung cancers were detected. Seven (78%) were stage I or II lung cancers, and the remaining two (22%) were stage II or IV, results that are in line with data from the I-ELCAP and NLST trials.
In another component of the study, the researchers randomly selected 101 patients from the lung cancer screening program to answer questions by telephone intended to quantify their anxiety about lung cancer before and after participating in the program, attitudes about smoking behaviors, and general impressions of the screening process. On a scale of 0-10, with 10 being “very anxious,” Dr. Salzano reported that the mean anxiety level about lung cancer fell from a level of 4.69 before screening to 3.87 afterward, a difference that reached statistical significance, with a P value of .014. “None of the patients reported negative impacts of the program,” he added. “They reported a general improvement in their well-being as a result of participating in the program.” In addition, of the 53 respondents who were current smokers upon enrolling in the screening program, five quit after intake, and the remaining 48 indicated that they were “more likely to quit” as a result of being enrolled.
“Community hospitals need to embrace lung screening,” Dr. Salzano concluded. “The findings from the large studies are transferable. It’s helping your patients in terms of their attitudes about lung cancer, about smoking cessation, and about improving their wellness.”
He reported having no relevant financial disclosures.
LOS ANGELES – Lung cancer screening with low-dose CT scans in a community hospital setting replicates results from international and multicenter trials when it comes to diagnosing early-stage lung cancer, findings from a single-center study showed.
“It’s too early in our experience to say that we’re saving lives, but the fact that we’re detecting early lung cancers in the predicted percentages is good for community hospitals that are wondering, ‘Is it worth it to screen for lung cancer? Can we do it?’ ” Richard P. Salzano Jr., MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians.
In July 2013, the 130-bed Griffin Hospital launched a lung cancer screening program codirected by a pulmonologist and a cardiothoracic surgeon. All low-dose CT scans were read by two designated radiologists. Dr. Salzano reported results from 514 patients enrolled in the program between July 2013 and December 2015. A total of nine lung cancers were detected. Seven (78%) were stage I or II lung cancers, and the remaining two (22%) were stage II or IV, results that are in line with data from the I-ELCAP and NLST trials.
In another component of the study, the researchers randomly selected 101 patients from the lung cancer screening program to answer questions by telephone intended to quantify their anxiety about lung cancer before and after participating in the program, attitudes about smoking behaviors, and general impressions of the screening process. On a scale of 0-10, with 10 being “very anxious,” Dr. Salzano reported that the mean anxiety level about lung cancer fell from a level of 4.69 before screening to 3.87 afterward, a difference that reached statistical significance, with a P value of .014. “None of the patients reported negative impacts of the program,” he added. “They reported a general improvement in their well-being as a result of participating in the program.” In addition, of the 53 respondents who were current smokers upon enrolling in the screening program, five quit after intake, and the remaining 48 indicated that they were “more likely to quit” as a result of being enrolled.
“Community hospitals need to embrace lung screening,” Dr. Salzano concluded. “The findings from the large studies are transferable. It’s helping your patients in terms of their attitudes about lung cancer, about smoking cessation, and about improving their wellness.”
He reported having no relevant financial disclosures.
LOS ANGELES – Lung cancer screening with low-dose CT scans in a community hospital setting replicates results from international and multicenter trials when it comes to diagnosing early-stage lung cancer, findings from a single-center study showed.
“It’s too early in our experience to say that we’re saving lives, but the fact that we’re detecting early lung cancers in the predicted percentages is good for community hospitals that are wondering, ‘Is it worth it to screen for lung cancer? Can we do it?’ ” Richard P. Salzano Jr., MD, said in an interview in advance of the annual meeting of the American College of Chest Physicians.
In July 2013, the 130-bed Griffin Hospital launched a lung cancer screening program codirected by a pulmonologist and a cardiothoracic surgeon. All low-dose CT scans were read by two designated radiologists. Dr. Salzano reported results from 514 patients enrolled in the program between July 2013 and December 2015. A total of nine lung cancers were detected. Seven (78%) were stage I or II lung cancers, and the remaining two (22%) were stage II or IV, results that are in line with data from the I-ELCAP and NLST trials.
In another component of the study, the researchers randomly selected 101 patients from the lung cancer screening program to answer questions by telephone intended to quantify their anxiety about lung cancer before and after participating in the program, attitudes about smoking behaviors, and general impressions of the screening process. On a scale of 0-10, with 10 being “very anxious,” Dr. Salzano reported that the mean anxiety level about lung cancer fell from a level of 4.69 before screening to 3.87 afterward, a difference that reached statistical significance, with a P value of .014. “None of the patients reported negative impacts of the program,” he added. “They reported a general improvement in their well-being as a result of participating in the program.” In addition, of the 53 respondents who were current smokers upon enrolling in the screening program, five quit after intake, and the remaining 48 indicated that they were “more likely to quit” as a result of being enrolled.
“Community hospitals need to embrace lung screening,” Dr. Salzano concluded. “The findings from the large studies are transferable. It’s helping your patients in terms of their attitudes about lung cancer, about smoking cessation, and about improving their wellness.”
He reported having no relevant financial disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Of nine lung cancers detected, seven (78%) were stage I or II lung cancers and the remaining two (22%) were stage II or IV.
Data source: Results from 514 patients enrolled in a community hospital–based lung cancer screening program between July 2013 and December 2015.
Disclosures: Dr. Salzano reported having no relevant financial disclosures.
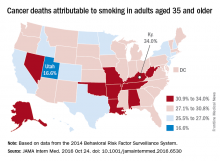
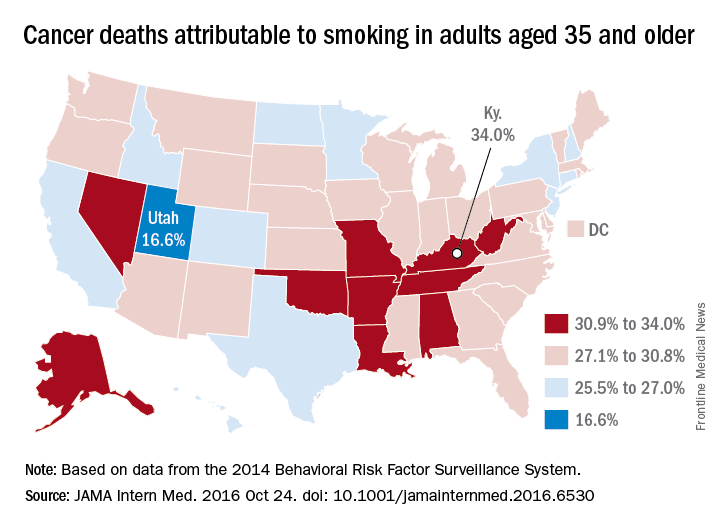
Smoking-attributable cancer mortality highest in Kentucky
Almost 29% of cancer deaths among U.S. adults aged 35 years and older were attributable to cigarette smoking in 2014, according to investigators from the American Cancer Society.
Of the 585,000 cancer deaths among those 35 years and older that year, more than 167,000 (28.6%) were estimated to be the result of cigarette smoking, reported Joannie Lortet-Tieulent, MSc, and her associates at the ACS in Atlanta (JAMA Intern Med. 2016 Oct 24. doi: 10.1001/jamainternmed.2016.6530).
Among men aged 35 years and older, 33.7% of U.S. cancer deaths were attributable to cigarette smoking, compared with 22.9% for women. Arkansas had the highest rate (39.5%) for men and Kentucky had the highest rate (29.0%) for women. Not surprisingly, Utah had the lowest rate for both men (21.8%) and women (11.1%), according to the analysis of data from the 2014 Behavioral Risk Factor Surveillance System. For men, Utah was the only state with a rate below 30%.
The investigators did not report any conflicts of interest. The study was supported by the intramural research department of the American Cancer Society.
Almost 29% of cancer deaths among U.S. adults aged 35 years and older were attributable to cigarette smoking in 2014, according to investigators from the American Cancer Society.
Of the 585,000 cancer deaths among those 35 years and older that year, more than 167,000 (28.6%) were estimated to be the result of cigarette smoking, reported Joannie Lortet-Tieulent, MSc, and her associates at the ACS in Atlanta (JAMA Intern Med. 2016 Oct 24. doi: 10.1001/jamainternmed.2016.6530).
Among men aged 35 years and older, 33.7% of U.S. cancer deaths were attributable to cigarette smoking, compared with 22.9% for women. Arkansas had the highest rate (39.5%) for men and Kentucky had the highest rate (29.0%) for women. Not surprisingly, Utah had the lowest rate for both men (21.8%) and women (11.1%), according to the analysis of data from the 2014 Behavioral Risk Factor Surveillance System. For men, Utah was the only state with a rate below 30%.
The investigators did not report any conflicts of interest. The study was supported by the intramural research department of the American Cancer Society.
Almost 29% of cancer deaths among U.S. adults aged 35 years and older were attributable to cigarette smoking in 2014, according to investigators from the American Cancer Society.
Of the 585,000 cancer deaths among those 35 years and older that year, more than 167,000 (28.6%) were estimated to be the result of cigarette smoking, reported Joannie Lortet-Tieulent, MSc, and her associates at the ACS in Atlanta (JAMA Intern Med. 2016 Oct 24. doi: 10.1001/jamainternmed.2016.6530).
Among men aged 35 years and older, 33.7% of U.S. cancer deaths were attributable to cigarette smoking, compared with 22.9% for women. Arkansas had the highest rate (39.5%) for men and Kentucky had the highest rate (29.0%) for women. Not surprisingly, Utah had the lowest rate for both men (21.8%) and women (11.1%), according to the analysis of data from the 2014 Behavioral Risk Factor Surveillance System. For men, Utah was the only state with a rate below 30%.
The investigators did not report any conflicts of interest. The study was supported by the intramural research department of the American Cancer Society.
FDA approves atezolizumab for advanced NSCLC
The Food and Drug Administration has approved the programmed death-ligand 1 (PD-L1) blocking antibody atezolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose disease has progressed during or following platinum-containing chemotherapy.
The FDA previously approved atezolizumab (Tecentriq) for the treatment of locally advanced or metastatic urothelial carcinoma that has progressed after platinum-containing chemotherapy.
Approval for treatment of NSCLC was based on results from the phase III OAK and phase II POPLAR trials that enrolled a total of 1,137 patients with NSCLC whose disease had progressed on platinum-containing chemotherapy. In OAK, median overall survival for patients assigned to atezolizumab was 13.8 months, compared with 9.6 months for patients assigned to docetaxel, as recently reported at the European Society for Medical Oncology Congress.
In POPLAR, overall survival was 12.6 months for patients receiving atezolizumab versus 9.7 months for those assigned to docetaxel, as reported at the European Cancer Congress in 2015.
The most common (greater than or equal to 20%) adverse reactions in patients treated with atezolizumab were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation, according to the FDA website. The most common (greater than or equal to 2%) grade 3-4 adverse events in patients treated with atezolizumab were dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, anemia, musculoskeletal pain, AST increase, ALT increase, dysphagia, and arthralgia. Clinically significant immune-related adverse events for patients receiving atezolizumab have included pneumonitis, hepatitis, colitis, and thyroid disease.
The recommended dose is 1,200 mg administered as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
Patients with EGFR or ALK genomic tumor aberrations should not receive atezolizumab before having disease progression on FDA-approved therapy for these aberrations, the FDA said.
Full prescribing information is available on the FDA website.
The Food and Drug Administration has approved the programmed death-ligand 1 (PD-L1) blocking antibody atezolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose disease has progressed during or following platinum-containing chemotherapy.
The FDA previously approved atezolizumab (Tecentriq) for the treatment of locally advanced or metastatic urothelial carcinoma that has progressed after platinum-containing chemotherapy.
Approval for treatment of NSCLC was based on results from the phase III OAK and phase II POPLAR trials that enrolled a total of 1,137 patients with NSCLC whose disease had progressed on platinum-containing chemotherapy. In OAK, median overall survival for patients assigned to atezolizumab was 13.8 months, compared with 9.6 months for patients assigned to docetaxel, as recently reported at the European Society for Medical Oncology Congress.
In POPLAR, overall survival was 12.6 months for patients receiving atezolizumab versus 9.7 months for those assigned to docetaxel, as reported at the European Cancer Congress in 2015.
The most common (greater than or equal to 20%) adverse reactions in patients treated with atezolizumab were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation, according to the FDA website. The most common (greater than or equal to 2%) grade 3-4 adverse events in patients treated with atezolizumab were dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, anemia, musculoskeletal pain, AST increase, ALT increase, dysphagia, and arthralgia. Clinically significant immune-related adverse events for patients receiving atezolizumab have included pneumonitis, hepatitis, colitis, and thyroid disease.
The recommended dose is 1,200 mg administered as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
Patients with EGFR or ALK genomic tumor aberrations should not receive atezolizumab before having disease progression on FDA-approved therapy for these aberrations, the FDA said.
Full prescribing information is available on the FDA website.
The Food and Drug Administration has approved the programmed death-ligand 1 (PD-L1) blocking antibody atezolizumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose disease has progressed during or following platinum-containing chemotherapy.
The FDA previously approved atezolizumab (Tecentriq) for the treatment of locally advanced or metastatic urothelial carcinoma that has progressed after platinum-containing chemotherapy.
Approval for treatment of NSCLC was based on results from the phase III OAK and phase II POPLAR trials that enrolled a total of 1,137 patients with NSCLC whose disease had progressed on platinum-containing chemotherapy. In OAK, median overall survival for patients assigned to atezolizumab was 13.8 months, compared with 9.6 months for patients assigned to docetaxel, as recently reported at the European Society for Medical Oncology Congress.
In POPLAR, overall survival was 12.6 months for patients receiving atezolizumab versus 9.7 months for those assigned to docetaxel, as reported at the European Cancer Congress in 2015.
The most common (greater than or equal to 20%) adverse reactions in patients treated with atezolizumab were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation, according to the FDA website. The most common (greater than or equal to 2%) grade 3-4 adverse events in patients treated with atezolizumab were dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, anemia, musculoskeletal pain, AST increase, ALT increase, dysphagia, and arthralgia. Clinically significant immune-related adverse events for patients receiving atezolizumab have included pneumonitis, hepatitis, colitis, and thyroid disease.
The recommended dose is 1,200 mg administered as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
Patients with EGFR or ALK genomic tumor aberrations should not receive atezolizumab before having disease progression on FDA-approved therapy for these aberrations, the FDA said.
Full prescribing information is available on the FDA website.