User login
FDA takes more steps to keep kids off tobacco products
New regulations from the Food and Drug Administration ban the sale of all tobacco products – including e-cigarettes – to minors.
The final rule announced May 5 extends FDA regulatory authority to e-cigarettes, cigars, hookah tobacco, pipe tobacco, and other similar products. Prior to this, FDA regulated cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco under the Tobacco Control Act of 2009.
The new regulations requires manufacturers of all newly regulated products to show that their products meet applicable public health standards and that makers receive marketing authorization from the FDA. The agency now has the authority to evaluate ingredients, product design, and health risks of these products.
U.S. Department of Health & Human Services Secretary Sylvia Burwell said the move was critical to improve public health and protect future generations from the dangers of tobacco.
“As cigarette smoking among those under 18 has fallen, the use of other nicotine products, including e-cigarettes, has taken a drastic leap,” Secretary Burwell said in a statement. “All of this is creating a new generation of Americans who are at risk of addiction. Today’s announcement is an important step in the fight for a tobacco-free generation – it will help us catch up with changes in the marketplace, put into place rules that protect our kids and give adults information they need to make informed decisions.”
Before the rule, no federal law prohibited stores and websites from selling e-cigarettes, hookah tobacco, and cigars to minors, according to an FDA fact sheet. The new rule aims to deter youth access to the products by barring sales to persons under 18, requiring age verification of purchasers, preventing the selling of covered tobacco products in vending machines, and prohibiting the distribution of free samples.
In addition, manufacturers, importers, and retailers of newly regulated tobacco products must follow provisions, such as registering their manufacturing establishments and providing product listings to the FDA, reporting ingredients and potentially harmful constituents to the agency, and placing health warnings on packages and advertisements, among other guidelines. Manufacturers of newly regulated products must meet the applicable public health standards, unless the product was on the market as of Feb. 15, 2007.
FDA Commissioner Dr. Robert M. Califf said the rule marks a milestone in patient protection.
“As a physician, I’ve seen first hand the devastating health effects of tobacco use,” Dr. Califf said in a statement. “At the FDA, we must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”
It’s about time that e-cigarettes are regulated like other tobacco products, said Dr. Roy Herbst, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital at Yale-New Haven (Conn).
“It’s been quite some time that e-cigarettes have been gaining momentum in our communities and now to finally have them under the purview of the FDA is huge,” Dr. Herbst said in an interview. “It will force more science and research into these products. ... If these are so safe, they should go through clinical studies to prove that.”
Dr. Herbst noted that within 90 days of the published rule, retailers can no longer sell the products to minors, and that within 3 years, the products will be regulated like other tobacco products.
Manufacturers will be allowed to continue selling their products for up to 3 years while they submit a new tobacco product application and the FDA reviews the application, according to the FDA.
Physicians and other health advocates expressed support for the regulations.
“The AMA supports the FDA’s new rule and its efforts to ensure the public – especially young people – is aware of and protected from these harmful products,” AMA President Dr. Steven J. Stack said in a statement. “The new FDA rule, which fills the gap in federal regulations on purchasing, labeling, and packaging of e-cigarettes; cigars; and other tobacco products, is a notable and important step that will ban the sale of these products to minors and improve public health. However, we urge FDA to issue further regulations addressing marketing of these products and banning flavored e-cigarettes, which are particularly enticing to minors.”
The American Academy of Pediatrics also suggested more steps to curb tobacco use.
“The rule is a welcomed starting point, but it is only a framework upon which to build meaningful regulation to end the tobacco epidemic in the United States once and for all,” American Academy of Pediatrics President Dr. Benard P. Dreyer said in a statement. “Today’s action marks an historic step forward in helping to alleviate the threat of lifelong nicotine addiction for our youth, and should serve as a foundation for further progress when it comes to keeping children safe from dangerous tobacco products.”
The rule will be published in the Federal Register on May 10.
On Twitter @legal_med
New regulations from the Food and Drug Administration ban the sale of all tobacco products – including e-cigarettes – to minors.
The final rule announced May 5 extends FDA regulatory authority to e-cigarettes, cigars, hookah tobacco, pipe tobacco, and other similar products. Prior to this, FDA regulated cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco under the Tobacco Control Act of 2009.
The new regulations requires manufacturers of all newly regulated products to show that their products meet applicable public health standards and that makers receive marketing authorization from the FDA. The agency now has the authority to evaluate ingredients, product design, and health risks of these products.
U.S. Department of Health & Human Services Secretary Sylvia Burwell said the move was critical to improve public health and protect future generations from the dangers of tobacco.
“As cigarette smoking among those under 18 has fallen, the use of other nicotine products, including e-cigarettes, has taken a drastic leap,” Secretary Burwell said in a statement. “All of this is creating a new generation of Americans who are at risk of addiction. Today’s announcement is an important step in the fight for a tobacco-free generation – it will help us catch up with changes in the marketplace, put into place rules that protect our kids and give adults information they need to make informed decisions.”
Before the rule, no federal law prohibited stores and websites from selling e-cigarettes, hookah tobacco, and cigars to minors, according to an FDA fact sheet. The new rule aims to deter youth access to the products by barring sales to persons under 18, requiring age verification of purchasers, preventing the selling of covered tobacco products in vending machines, and prohibiting the distribution of free samples.
In addition, manufacturers, importers, and retailers of newly regulated tobacco products must follow provisions, such as registering their manufacturing establishments and providing product listings to the FDA, reporting ingredients and potentially harmful constituents to the agency, and placing health warnings on packages and advertisements, among other guidelines. Manufacturers of newly regulated products must meet the applicable public health standards, unless the product was on the market as of Feb. 15, 2007.
FDA Commissioner Dr. Robert M. Califf said the rule marks a milestone in patient protection.
“As a physician, I’ve seen first hand the devastating health effects of tobacco use,” Dr. Califf said in a statement. “At the FDA, we must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”
It’s about time that e-cigarettes are regulated like other tobacco products, said Dr. Roy Herbst, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital at Yale-New Haven (Conn).
“It’s been quite some time that e-cigarettes have been gaining momentum in our communities and now to finally have them under the purview of the FDA is huge,” Dr. Herbst said in an interview. “It will force more science and research into these products. ... If these are so safe, they should go through clinical studies to prove that.”
Dr. Herbst noted that within 90 days of the published rule, retailers can no longer sell the products to minors, and that within 3 years, the products will be regulated like other tobacco products.
Manufacturers will be allowed to continue selling their products for up to 3 years while they submit a new tobacco product application and the FDA reviews the application, according to the FDA.
Physicians and other health advocates expressed support for the regulations.
“The AMA supports the FDA’s new rule and its efforts to ensure the public – especially young people – is aware of and protected from these harmful products,” AMA President Dr. Steven J. Stack said in a statement. “The new FDA rule, which fills the gap in federal regulations on purchasing, labeling, and packaging of e-cigarettes; cigars; and other tobacco products, is a notable and important step that will ban the sale of these products to minors and improve public health. However, we urge FDA to issue further regulations addressing marketing of these products and banning flavored e-cigarettes, which are particularly enticing to minors.”
The American Academy of Pediatrics also suggested more steps to curb tobacco use.
“The rule is a welcomed starting point, but it is only a framework upon which to build meaningful regulation to end the tobacco epidemic in the United States once and for all,” American Academy of Pediatrics President Dr. Benard P. Dreyer said in a statement. “Today’s action marks an historic step forward in helping to alleviate the threat of lifelong nicotine addiction for our youth, and should serve as a foundation for further progress when it comes to keeping children safe from dangerous tobacco products.”
The rule will be published in the Federal Register on May 10.
On Twitter @legal_med
New regulations from the Food and Drug Administration ban the sale of all tobacco products – including e-cigarettes – to minors.
The final rule announced May 5 extends FDA regulatory authority to e-cigarettes, cigars, hookah tobacco, pipe tobacco, and other similar products. Prior to this, FDA regulated cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco under the Tobacco Control Act of 2009.
The new regulations requires manufacturers of all newly regulated products to show that their products meet applicable public health standards and that makers receive marketing authorization from the FDA. The agency now has the authority to evaluate ingredients, product design, and health risks of these products.
U.S. Department of Health & Human Services Secretary Sylvia Burwell said the move was critical to improve public health and protect future generations from the dangers of tobacco.
“As cigarette smoking among those under 18 has fallen, the use of other nicotine products, including e-cigarettes, has taken a drastic leap,” Secretary Burwell said in a statement. “All of this is creating a new generation of Americans who are at risk of addiction. Today’s announcement is an important step in the fight for a tobacco-free generation – it will help us catch up with changes in the marketplace, put into place rules that protect our kids and give adults information they need to make informed decisions.”
Before the rule, no federal law prohibited stores and websites from selling e-cigarettes, hookah tobacco, and cigars to minors, according to an FDA fact sheet. The new rule aims to deter youth access to the products by barring sales to persons under 18, requiring age verification of purchasers, preventing the selling of covered tobacco products in vending machines, and prohibiting the distribution of free samples.
In addition, manufacturers, importers, and retailers of newly regulated tobacco products must follow provisions, such as registering their manufacturing establishments and providing product listings to the FDA, reporting ingredients and potentially harmful constituents to the agency, and placing health warnings on packages and advertisements, among other guidelines. Manufacturers of newly regulated products must meet the applicable public health standards, unless the product was on the market as of Feb. 15, 2007.
FDA Commissioner Dr. Robert M. Califf said the rule marks a milestone in patient protection.
“As a physician, I’ve seen first hand the devastating health effects of tobacco use,” Dr. Califf said in a statement. “At the FDA, we must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”
It’s about time that e-cigarettes are regulated like other tobacco products, said Dr. Roy Herbst, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital at Yale-New Haven (Conn).
“It’s been quite some time that e-cigarettes have been gaining momentum in our communities and now to finally have them under the purview of the FDA is huge,” Dr. Herbst said in an interview. “It will force more science and research into these products. ... If these are so safe, they should go through clinical studies to prove that.”
Dr. Herbst noted that within 90 days of the published rule, retailers can no longer sell the products to minors, and that within 3 years, the products will be regulated like other tobacco products.
Manufacturers will be allowed to continue selling their products for up to 3 years while they submit a new tobacco product application and the FDA reviews the application, according to the FDA.
Physicians and other health advocates expressed support for the regulations.
“The AMA supports the FDA’s new rule and its efforts to ensure the public – especially young people – is aware of and protected from these harmful products,” AMA President Dr. Steven J. Stack said in a statement. “The new FDA rule, which fills the gap in federal regulations on purchasing, labeling, and packaging of e-cigarettes; cigars; and other tobacco products, is a notable and important step that will ban the sale of these products to minors and improve public health. However, we urge FDA to issue further regulations addressing marketing of these products and banning flavored e-cigarettes, which are particularly enticing to minors.”
The American Academy of Pediatrics also suggested more steps to curb tobacco use.
“The rule is a welcomed starting point, but it is only a framework upon which to build meaningful regulation to end the tobacco epidemic in the United States once and for all,” American Academy of Pediatrics President Dr. Benard P. Dreyer said in a statement. “Today’s action marks an historic step forward in helping to alleviate the threat of lifelong nicotine addiction for our youth, and should serve as a foundation for further progress when it comes to keeping children safe from dangerous tobacco products.”
The rule will be published in the Federal Register on May 10.
On Twitter @legal_med
Neighborhood demographics linked with NSCLC treatment and survival
The demographic characteristics of neighborhoods are associated with the odds of receiving surgical treatment for early non–small-cell lung cancer (NSCLC), according to a study published in Cancer Epidemiology, Biomarkers & Prevention.
Living in areas with higher economic deprivation was associated with lower odds of receiving surgery for both black and white patients, in a retrospective study of 8,322 patients with early-stage NSCLC.
“The results of this study are intended to bring importance to segregation and other characteristics as determinants of lung cancer outcomes. As a result, what is learned from these epidemiologic findings can be applied to interventions and public policies to improve patient outcomes and contribute to the difficult and complex task of reducing racial health disparities,” wrote Dr. Asal M. Johnson of Stetson University and her associates (Cancer Epidemiol Biomarkers Prev; 25[5];750-8).
The early-stage NSCLC patients were identified in the Georgia Comprehensive Cancer Registry from 2000 to 2009 to determine the effects of residential segregation and other neighborhood characteristics on the odds of receiving surgical treatment and the risk of death based on 5-year survival.
Three separate multilevel models were employed: A, economic deprivation; B, segregation; and C, segregation and economic deprivation. Individual-level variables (age, sex, and tumor grade) and area-level variables (place of residence, educational attainment, and elderly concentration) were controlled for in all models.
Regarding odds of surgical intervention, model A showed that living in areas with higher economic deprivation was associated with lower odds of receiving surgery for both black and white patients. Model B demonstrated that living in highly segregated areas was associated with lower odds of receiving surgery among black patients only. For white patients, no significant associations were observed between living in areas with combined segregation-deprivation and receipt of surgery using model C; however, living in segregated areas, regardless of the level of economic deprivation, was associated with decreased odds of receiving surgery for black patients.
As for 5-year survival, all three models indicated no effects of economic deprivation, segregation, or the combination of segregation and deprivation on survival among white patients. Models A and B showed no effects of economic deprivation or segregation on survival in black patients. In model C, however, the combination of high residential segregation and high economic deprivation was associated with a 31% higher risk of death in these patients, even after surgery.
Funding was provided by Stetson University. The authors reported no conflicts of interest.
The demographic characteristics of neighborhoods are associated with the odds of receiving surgical treatment for early non–small-cell lung cancer (NSCLC), according to a study published in Cancer Epidemiology, Biomarkers & Prevention.
Living in areas with higher economic deprivation was associated with lower odds of receiving surgery for both black and white patients, in a retrospective study of 8,322 patients with early-stage NSCLC.
“The results of this study are intended to bring importance to segregation and other characteristics as determinants of lung cancer outcomes. As a result, what is learned from these epidemiologic findings can be applied to interventions and public policies to improve patient outcomes and contribute to the difficult and complex task of reducing racial health disparities,” wrote Dr. Asal M. Johnson of Stetson University and her associates (Cancer Epidemiol Biomarkers Prev; 25[5];750-8).
The early-stage NSCLC patients were identified in the Georgia Comprehensive Cancer Registry from 2000 to 2009 to determine the effects of residential segregation and other neighborhood characteristics on the odds of receiving surgical treatment and the risk of death based on 5-year survival.
Three separate multilevel models were employed: A, economic deprivation; B, segregation; and C, segregation and economic deprivation. Individual-level variables (age, sex, and tumor grade) and area-level variables (place of residence, educational attainment, and elderly concentration) were controlled for in all models.
Regarding odds of surgical intervention, model A showed that living in areas with higher economic deprivation was associated with lower odds of receiving surgery for both black and white patients. Model B demonstrated that living in highly segregated areas was associated with lower odds of receiving surgery among black patients only. For white patients, no significant associations were observed between living in areas with combined segregation-deprivation and receipt of surgery using model C; however, living in segregated areas, regardless of the level of economic deprivation, was associated with decreased odds of receiving surgery for black patients.
As for 5-year survival, all three models indicated no effects of economic deprivation, segregation, or the combination of segregation and deprivation on survival among white patients. Models A and B showed no effects of economic deprivation or segregation on survival in black patients. In model C, however, the combination of high residential segregation and high economic deprivation was associated with a 31% higher risk of death in these patients, even after surgery.
Funding was provided by Stetson University. The authors reported no conflicts of interest.
The demographic characteristics of neighborhoods are associated with the odds of receiving surgical treatment for early non–small-cell lung cancer (NSCLC), according to a study published in Cancer Epidemiology, Biomarkers & Prevention.
Living in areas with higher economic deprivation was associated with lower odds of receiving surgery for both black and white patients, in a retrospective study of 8,322 patients with early-stage NSCLC.
“The results of this study are intended to bring importance to segregation and other characteristics as determinants of lung cancer outcomes. As a result, what is learned from these epidemiologic findings can be applied to interventions and public policies to improve patient outcomes and contribute to the difficult and complex task of reducing racial health disparities,” wrote Dr. Asal M. Johnson of Stetson University and her associates (Cancer Epidemiol Biomarkers Prev; 25[5];750-8).
The early-stage NSCLC patients were identified in the Georgia Comprehensive Cancer Registry from 2000 to 2009 to determine the effects of residential segregation and other neighborhood characteristics on the odds of receiving surgical treatment and the risk of death based on 5-year survival.
Three separate multilevel models were employed: A, economic deprivation; B, segregation; and C, segregation and economic deprivation. Individual-level variables (age, sex, and tumor grade) and area-level variables (place of residence, educational attainment, and elderly concentration) were controlled for in all models.
Regarding odds of surgical intervention, model A showed that living in areas with higher economic deprivation was associated with lower odds of receiving surgery for both black and white patients. Model B demonstrated that living in highly segregated areas was associated with lower odds of receiving surgery among black patients only. For white patients, no significant associations were observed between living in areas with combined segregation-deprivation and receipt of surgery using model C; however, living in segregated areas, regardless of the level of economic deprivation, was associated with decreased odds of receiving surgery for black patients.
As for 5-year survival, all three models indicated no effects of economic deprivation, segregation, or the combination of segregation and deprivation on survival among white patients. Models A and B showed no effects of economic deprivation or segregation on survival in black patients. In model C, however, the combination of high residential segregation and high economic deprivation was associated with a 31% higher risk of death in these patients, even after surgery.
Funding was provided by Stetson University. The authors reported no conflicts of interest.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Key clinical point: Neighborhood characteristics are linked with lung cancer treatment and outcomes.
Major finding: Patients with non–small-cell lung cancer living in areas with high levels of racial segregation had decreased odds of receiving surgery.
Data sources: Patients in the Georgia Comprehensive Cancer Registry diagnosed with non–small-cell lung cancer from January 2000 to December 2009.
Disclosures: Funding was provided by Stetson University. The authors reported no conflicts of interest.
An unusual case of non-small-cell lung cancer presenting as spontaneous cardiac tamponade
Hemorrhagic pericardial effusion with associated cardiac tamponade as a de novo sign of malignancy is seen in about 2% of patients.1 Consequently, cardiac tamponade is an oncologic emergency and considered a unique presentation of a malignancy.2 Cancer emergency is defined as an acute condition that is caused directly by the cancer itself or its treatment and requires intervention to avoid death or significant morbidity.3 The mechanism by which cardiac tamponade is classified as a life-threatening emergency stems from its impairment of right ventricular filling, resulting in ventricular diastolic collapse and decreased cardiac output, which can ultimately lead to death.4
Click on the PDF icon at the top of this introduction to read the full article.
Hemorrhagic pericardial effusion with associated cardiac tamponade as a de novo sign of malignancy is seen in about 2% of patients.1 Consequently, cardiac tamponade is an oncologic emergency and considered a unique presentation of a malignancy.2 Cancer emergency is defined as an acute condition that is caused directly by the cancer itself or its treatment and requires intervention to avoid death or significant morbidity.3 The mechanism by which cardiac tamponade is classified as a life-threatening emergency stems from its impairment of right ventricular filling, resulting in ventricular diastolic collapse and decreased cardiac output, which can ultimately lead to death.4
Click on the PDF icon at the top of this introduction to read the full article.
Hemorrhagic pericardial effusion with associated cardiac tamponade as a de novo sign of malignancy is seen in about 2% of patients.1 Consequently, cardiac tamponade is an oncologic emergency and considered a unique presentation of a malignancy.2 Cancer emergency is defined as an acute condition that is caused directly by the cancer itself or its treatment and requires intervention to avoid death or significant morbidity.3 The mechanism by which cardiac tamponade is classified as a life-threatening emergency stems from its impairment of right ventricular filling, resulting in ventricular diastolic collapse and decreased cardiac output, which can ultimately lead to death.4
Click on the PDF icon at the top of this introduction to read the full article.
Targeting gene rearrangements shows promise in early study
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.
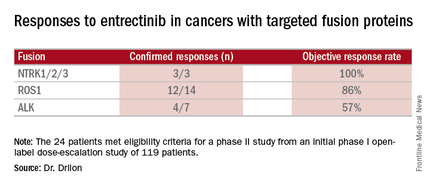
Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.

Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.

Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
FROM THE AACR ANNUAL MEETING
Key clinical point: Entrectinib showed antitumor activity including intracranial responses.
Major finding: Patients with the targeted abnormalities had a 79% response rate.
Data source: Twenty-four patients meeting eligibility criteria for a phase II study from an initial phase I open-label dose-escalation study of 119 patients.
Disclosures: Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
FDA grants priority review for additional use of atezolizumab
The Food and Drug Administration has granted priority review for atezolizumab for the treatment of people with locally advanced or metastatic non–small-cell lung cancer expressing the programmed death ligand–1 protein (PD-L1) who progressed on or after platinum-containing chemotherapy.
“In a study of atezolizumab in people with previously treated advanced lung cancer, PD-L1 expression correlated with how well they responded to the medicine,” said Dr. Sandra Horning, chief medical officer and head of global product development for Roche and Genentech, in a press release. “The goal of PD-L1 as a biomarker is to identify people most likely to benefit form atezolizumab alone.”
In February 2015, the FDA also granted the drug breakthrough therapy designation for the treatment of people with non–small-cell lung cancer expressing PD-L1 that progressed during or after standard treatments.
Earlier this year, the FDA granted a priority review of atezolizumab for the treatment of people with locally advanced or metastatic urothelial carcinoma, who had disease progression during or following platinum-based chemotherapy in the metastatic stetting or disease worsening within 12 months of receiving platinum-based chemotherapy before or after surgery.
The Food and Drug Administration has granted priority review for atezolizumab for the treatment of people with locally advanced or metastatic non–small-cell lung cancer expressing the programmed death ligand–1 protein (PD-L1) who progressed on or after platinum-containing chemotherapy.
“In a study of atezolizumab in people with previously treated advanced lung cancer, PD-L1 expression correlated with how well they responded to the medicine,” said Dr. Sandra Horning, chief medical officer and head of global product development for Roche and Genentech, in a press release. “The goal of PD-L1 as a biomarker is to identify people most likely to benefit form atezolizumab alone.”
In February 2015, the FDA also granted the drug breakthrough therapy designation for the treatment of people with non–small-cell lung cancer expressing PD-L1 that progressed during or after standard treatments.
Earlier this year, the FDA granted a priority review of atezolizumab for the treatment of people with locally advanced or metastatic urothelial carcinoma, who had disease progression during or following platinum-based chemotherapy in the metastatic stetting or disease worsening within 12 months of receiving platinum-based chemotherapy before or after surgery.
The Food and Drug Administration has granted priority review for atezolizumab for the treatment of people with locally advanced or metastatic non–small-cell lung cancer expressing the programmed death ligand–1 protein (PD-L1) who progressed on or after platinum-containing chemotherapy.
“In a study of atezolizumab in people with previously treated advanced lung cancer, PD-L1 expression correlated with how well they responded to the medicine,” said Dr. Sandra Horning, chief medical officer and head of global product development for Roche and Genentech, in a press release. “The goal of PD-L1 as a biomarker is to identify people most likely to benefit form atezolizumab alone.”
In February 2015, the FDA also granted the drug breakthrough therapy designation for the treatment of people with non–small-cell lung cancer expressing PD-L1 that progressed during or after standard treatments.
Earlier this year, the FDA granted a priority review of atezolizumab for the treatment of people with locally advanced or metastatic urothelial carcinoma, who had disease progression during or following platinum-based chemotherapy in the metastatic stetting or disease worsening within 12 months of receiving platinum-based chemotherapy before or after surgery.
Feds advance cancer moonshot with expert panel, outline of goals
Federal officials took the next step in their moonshot to end cancer by announcing on April 4 a blue ribbon panel to guide the effort.
A total of 28 leading researchers, clinicians, and patient advocates have been named to the panel charged with informing the scientific direction and goals of the National Cancer Moonshot Initiative, led by Vice President Joe Biden.
“This Blue Ribbon Panel will ensure that, as [the National Institutes of Health] allocates new resources through the Moonshot, decisions will be grounded in the best science,” Vice President Biden said in a statement. “I look forward to working with this panel and many others involved with the Moonshot to make unprecedented improvements in prevention, diagnosis, and treatment of cancer.”
The key goals of the initiative were set out simultaneously in a perspective from Dr. Francis S. Collins, NIH director, and Dr. Douglas R. Lowy, director of the National Cancer Institute. The editorial was published in the New England Journal of Medicine.
“Fueled by an additional $680 million in the proposed fiscal year 2017 budget for the NIH, plus additional resources for the Food and Drug Administration, the initiative will aim to accelerate progress toward the next generation of interventions that we hope will substantially reduce cancer incidence and dramatically improve patient outcomes,” Dr. Collins and Dr. Lowy wrote. “The NIH’s most compelling opportunities for progress will be set forth by late summer 2016 in a research plan informed by the deliberations of a blue-ribbon panel of experts, which will provide scientific input to the National Cancer Advisory Board. Some possible opportunities include vaccine development, early-detection technology, single-cell genomic analysis, immunotherapy, a focus on pediatric cancer, and enhanced data sharing.”
To read the full editorial, click here.
On Twitter @denisefulton
Federal officials took the next step in their moonshot to end cancer by announcing on April 4 a blue ribbon panel to guide the effort.
A total of 28 leading researchers, clinicians, and patient advocates have been named to the panel charged with informing the scientific direction and goals of the National Cancer Moonshot Initiative, led by Vice President Joe Biden.
“This Blue Ribbon Panel will ensure that, as [the National Institutes of Health] allocates new resources through the Moonshot, decisions will be grounded in the best science,” Vice President Biden said in a statement. “I look forward to working with this panel and many others involved with the Moonshot to make unprecedented improvements in prevention, diagnosis, and treatment of cancer.”
The key goals of the initiative were set out simultaneously in a perspective from Dr. Francis S. Collins, NIH director, and Dr. Douglas R. Lowy, director of the National Cancer Institute. The editorial was published in the New England Journal of Medicine.
“Fueled by an additional $680 million in the proposed fiscal year 2017 budget for the NIH, plus additional resources for the Food and Drug Administration, the initiative will aim to accelerate progress toward the next generation of interventions that we hope will substantially reduce cancer incidence and dramatically improve patient outcomes,” Dr. Collins and Dr. Lowy wrote. “The NIH’s most compelling opportunities for progress will be set forth by late summer 2016 in a research plan informed by the deliberations of a blue-ribbon panel of experts, which will provide scientific input to the National Cancer Advisory Board. Some possible opportunities include vaccine development, early-detection technology, single-cell genomic analysis, immunotherapy, a focus on pediatric cancer, and enhanced data sharing.”
To read the full editorial, click here.
On Twitter @denisefulton
Federal officials took the next step in their moonshot to end cancer by announcing on April 4 a blue ribbon panel to guide the effort.
A total of 28 leading researchers, clinicians, and patient advocates have been named to the panel charged with informing the scientific direction and goals of the National Cancer Moonshot Initiative, led by Vice President Joe Biden.
“This Blue Ribbon Panel will ensure that, as [the National Institutes of Health] allocates new resources through the Moonshot, decisions will be grounded in the best science,” Vice President Biden said in a statement. “I look forward to working with this panel and many others involved with the Moonshot to make unprecedented improvements in prevention, diagnosis, and treatment of cancer.”
The key goals of the initiative were set out simultaneously in a perspective from Dr. Francis S. Collins, NIH director, and Dr. Douglas R. Lowy, director of the National Cancer Institute. The editorial was published in the New England Journal of Medicine.
“Fueled by an additional $680 million in the proposed fiscal year 2017 budget for the NIH, plus additional resources for the Food and Drug Administration, the initiative will aim to accelerate progress toward the next generation of interventions that we hope will substantially reduce cancer incidence and dramatically improve patient outcomes,” Dr. Collins and Dr. Lowy wrote. “The NIH’s most compelling opportunities for progress will be set forth by late summer 2016 in a research plan informed by the deliberations of a blue-ribbon panel of experts, which will provide scientific input to the National Cancer Advisory Board. Some possible opportunities include vaccine development, early-detection technology, single-cell genomic analysis, immunotherapy, a focus on pediatric cancer, and enhanced data sharing.”
To read the full editorial, click here.
On Twitter @denisefulton
FROM NEJM
Gefitinib missed noninferiority endpoint vs. erlotinib in lung cancer trial
Gefitinib failed to meet its noninferiority endpoint, compared with erlotinib, but was significantly less likely to cause grade 3 rash in a multicenter, randomized phase III trial of patients with advanced, pretreated lung adenocarcinoma.
“Subset analysis including mutation status did not reveal any populations with noteworthy differences in clinical efficacy between gefitinib and erlotinib,” said Dr. Yoshiko Urata at Hyogo Cawncer Center in Akashi, Japan, and associates. Nonetheless, gefitinib could be a therapeutic option, given its milder adverse effects in this patient population, the investigators reported online in the Journal of Clinical Oncology.
Both gefitinib and erlotinib are first-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. Gefitinib had a higher response rate in Japanese patients than in non-Japanese patients in a prior phase II trial. Erlotinib yielded better survival results than gefitinib in subsequent trials, but the two agents had never been compared directly in a phase III trial, according to the investigators (J. Clin. Oncol. 2016 Mar. 28. doi: 10.1200/JCO.2015.63.4154).
Their study included 561 patients, of whom 401 had the EGFR mutation. Baseline factors except performance status were balanced between the randomization arms. Erlotinib was dosed at 150 mg/day, and gefitinib at 250 mg/day. In the event of toxicities, erlotinib could be lowered to 100 mg and 50 mg, and gefitinib to 250 mg on alternate days and 250 mg once every 3 days.
The median progression-free survival (PFS) was 6.5 months for gefitinib and 7.5 months for erlotinib (hazard ratio, 1.1; 95% confidence interval, 0.9-1.4; P = .26). The median PFS also did not statistically differ according to treatment in patients with the EGFR mutation (8.3 and 10.0 months, respectively; HR, 1.1; 95% CI, 0.9-1.4). The median overall survival was 22.8 months for gefitinib and 24.5 months for erlotinib (HR, 1.04; 95% CI, 0.833-1.29). The response rates were 45.9% and 44.1%, respectively.
The most common grade 3-4 toxicities included rash and increases in liver enzymes, said the researchers. Notably, grade 3 rash affected 18% of erlotinib patients and only 2% of gefitinib patients; no patients had grade 4 rash. Gefitinib was linked to three to four times the rate of increased liver enzymes, but an overall analysis of adverse effects nonetheless favored gefitinib (P less than .001).
Gefitinib failed to meet its noninferiority endpoint, compared with erlotinib, but was significantly less likely to cause grade 3 rash in a multicenter, randomized phase III trial of patients with advanced, pretreated lung adenocarcinoma.
“Subset analysis including mutation status did not reveal any populations with noteworthy differences in clinical efficacy between gefitinib and erlotinib,” said Dr. Yoshiko Urata at Hyogo Cawncer Center in Akashi, Japan, and associates. Nonetheless, gefitinib could be a therapeutic option, given its milder adverse effects in this patient population, the investigators reported online in the Journal of Clinical Oncology.
Both gefitinib and erlotinib are first-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. Gefitinib had a higher response rate in Japanese patients than in non-Japanese patients in a prior phase II trial. Erlotinib yielded better survival results than gefitinib in subsequent trials, but the two agents had never been compared directly in a phase III trial, according to the investigators (J. Clin. Oncol. 2016 Mar. 28. doi: 10.1200/JCO.2015.63.4154).
Their study included 561 patients, of whom 401 had the EGFR mutation. Baseline factors except performance status were balanced between the randomization arms. Erlotinib was dosed at 150 mg/day, and gefitinib at 250 mg/day. In the event of toxicities, erlotinib could be lowered to 100 mg and 50 mg, and gefitinib to 250 mg on alternate days and 250 mg once every 3 days.
The median progression-free survival (PFS) was 6.5 months for gefitinib and 7.5 months for erlotinib (hazard ratio, 1.1; 95% confidence interval, 0.9-1.4; P = .26). The median PFS also did not statistically differ according to treatment in patients with the EGFR mutation (8.3 and 10.0 months, respectively; HR, 1.1; 95% CI, 0.9-1.4). The median overall survival was 22.8 months for gefitinib and 24.5 months for erlotinib (HR, 1.04; 95% CI, 0.833-1.29). The response rates were 45.9% and 44.1%, respectively.
The most common grade 3-4 toxicities included rash and increases in liver enzymes, said the researchers. Notably, grade 3 rash affected 18% of erlotinib patients and only 2% of gefitinib patients; no patients had grade 4 rash. Gefitinib was linked to three to four times the rate of increased liver enzymes, but an overall analysis of adverse effects nonetheless favored gefitinib (P less than .001).
Gefitinib failed to meet its noninferiority endpoint, compared with erlotinib, but was significantly less likely to cause grade 3 rash in a multicenter, randomized phase III trial of patients with advanced, pretreated lung adenocarcinoma.
“Subset analysis including mutation status did not reveal any populations with noteworthy differences in clinical efficacy between gefitinib and erlotinib,” said Dr. Yoshiko Urata at Hyogo Cawncer Center in Akashi, Japan, and associates. Nonetheless, gefitinib could be a therapeutic option, given its milder adverse effects in this patient population, the investigators reported online in the Journal of Clinical Oncology.
Both gefitinib and erlotinib are first-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. Gefitinib had a higher response rate in Japanese patients than in non-Japanese patients in a prior phase II trial. Erlotinib yielded better survival results than gefitinib in subsequent trials, but the two agents had never been compared directly in a phase III trial, according to the investigators (J. Clin. Oncol. 2016 Mar. 28. doi: 10.1200/JCO.2015.63.4154).
Their study included 561 patients, of whom 401 had the EGFR mutation. Baseline factors except performance status were balanced between the randomization arms. Erlotinib was dosed at 150 mg/day, and gefitinib at 250 mg/day. In the event of toxicities, erlotinib could be lowered to 100 mg and 50 mg, and gefitinib to 250 mg on alternate days and 250 mg once every 3 days.
The median progression-free survival (PFS) was 6.5 months for gefitinib and 7.5 months for erlotinib (hazard ratio, 1.1; 95% confidence interval, 0.9-1.4; P = .26). The median PFS also did not statistically differ according to treatment in patients with the EGFR mutation (8.3 and 10.0 months, respectively; HR, 1.1; 95% CI, 0.9-1.4). The median overall survival was 22.8 months for gefitinib and 24.5 months for erlotinib (HR, 1.04; 95% CI, 0.833-1.29). The response rates were 45.9% and 44.1%, respectively.
The most common grade 3-4 toxicities included rash and increases in liver enzymes, said the researchers. Notably, grade 3 rash affected 18% of erlotinib patients and only 2% of gefitinib patients; no patients had grade 4 rash. Gefitinib was linked to three to four times the rate of increased liver enzymes, but an overall analysis of adverse effects nonetheless favored gefitinib (P less than .001).
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Gefitinib failed to meet its noninferiority endpoint, compared with erlotinib, in patients with advanced, pretreated lung adenocarcinoma.
Major finding: The median PFS was 6.5 months for gefitinib and 7.5 months for erlotinib (hazard ratio, 1.1; 95% confidence interval, 0.9-1.4; P = .26).
Data source: A multicenter, randomized, phase III trial of 561 patients with advanced, pretreated lung adenocarcinoma.
Disclosures: The study was funded by West Japan Oncology Group, a nonprofit organization supported by unrestricted donations from several pharmaceutical companies. Dr. Urata reported receiving honoraria from AstraZeneca, Chugai Pharma, Eli Lilly, Astellas Pharma, and Novartis. Sixteen coinvestigators reported financial relationships with numerous pharmaceutical companies.
Crizotinib bests chemo at controlling brain metastases in advanced NSCLC
Crizotinib achieved better control of brain metastases then chemotherapy in a manufacturer-sponsored international open-label phase III randomized trial involving 343 patients with advanced non–small-cell lung cancer (NSCLC), according to a report published March 28 in the Journal of Clinical Oncology.
Even though targeted therapies have greatly improved outcomes for patients whose NSCLC is associated with oncogenic driver mutations, brain metastases remain “a significant clinical problem, resulting in considerable physical and neurocognitive morbidity as well as mortality.” In addition, as patients live longer because of these targeted treatments, the proportion who have brain metastases rises, “such that up to 50% of patients alive at 2 years with EGFR- [epidermal growth factor receptor]–mutated or ALK- [anaplastic lymphoma kinase]–rearranged lung cancer will have brain metastases,” according to Dr. Benjamin J. Solomon of the department of medical oncology, Peter MacCallum Cancer Centre, Melbourne, and his associates.
They compared the efficacy of intracranial disease control between oral crizotinib and IV pemetrexed-platinum chemotherapy in patients who had advanced nonsquamous ALK-positive NSCLC. A total of 79 of these patients (23%) had brain metastases at baseline.
At 12 weeks, a significantly higher percentage of patients achieved intracranial disease control with crizotinib (85%) than with chemotherapy (45%), and the same was true at 24 weeks (56% vs. 25%). Median progression-free survival also was longer with crizotinib, regardless of whether patients had brain metastases at baseline (9 months vs. 4 months) or did not have brain metastases at baseline (11 months vs. 7 months), Dr. Solomon and his associates said (J Clin Oncol. 2016 Mar 28. doi: 10.1200/JCO.2015.63.5888).
Similarly, the overall response rate was significantly higher with crizotinib, regardless of whether patients had brain metastases at baseline (77% vs. 28%) or did not have brain metastases at baseline (74% vs. 50%). In addition, disease progression was less frequent with crizotinib than with chemotherapy in the overall patient population (52% vs. 77%), in the subgroup of patients who had brain metastases at baseline (54% vs. 75%), and in the subgroup of patients who did not have brain metastases at baseline (52% vs. 78%).
These findings indicate that crizotinib should be the standard first-line therapy for patients who have advanced ALK-positive NSCLC, whether or not they have brain metastases, the investigators said.
Crizotinib achieved better control of brain metastases then chemotherapy in a manufacturer-sponsored international open-label phase III randomized trial involving 343 patients with advanced non–small-cell lung cancer (NSCLC), according to a report published March 28 in the Journal of Clinical Oncology.
Even though targeted therapies have greatly improved outcomes for patients whose NSCLC is associated with oncogenic driver mutations, brain metastases remain “a significant clinical problem, resulting in considerable physical and neurocognitive morbidity as well as mortality.” In addition, as patients live longer because of these targeted treatments, the proportion who have brain metastases rises, “such that up to 50% of patients alive at 2 years with EGFR- [epidermal growth factor receptor]–mutated or ALK- [anaplastic lymphoma kinase]–rearranged lung cancer will have brain metastases,” according to Dr. Benjamin J. Solomon of the department of medical oncology, Peter MacCallum Cancer Centre, Melbourne, and his associates.
They compared the efficacy of intracranial disease control between oral crizotinib and IV pemetrexed-platinum chemotherapy in patients who had advanced nonsquamous ALK-positive NSCLC. A total of 79 of these patients (23%) had brain metastases at baseline.
At 12 weeks, a significantly higher percentage of patients achieved intracranial disease control with crizotinib (85%) than with chemotherapy (45%), and the same was true at 24 weeks (56% vs. 25%). Median progression-free survival also was longer with crizotinib, regardless of whether patients had brain metastases at baseline (9 months vs. 4 months) or did not have brain metastases at baseline (11 months vs. 7 months), Dr. Solomon and his associates said (J Clin Oncol. 2016 Mar 28. doi: 10.1200/JCO.2015.63.5888).
Similarly, the overall response rate was significantly higher with crizotinib, regardless of whether patients had brain metastases at baseline (77% vs. 28%) or did not have brain metastases at baseline (74% vs. 50%). In addition, disease progression was less frequent with crizotinib than with chemotherapy in the overall patient population (52% vs. 77%), in the subgroup of patients who had brain metastases at baseline (54% vs. 75%), and in the subgroup of patients who did not have brain metastases at baseline (52% vs. 78%).
These findings indicate that crizotinib should be the standard first-line therapy for patients who have advanced ALK-positive NSCLC, whether or not they have brain metastases, the investigators said.
Crizotinib achieved better control of brain metastases then chemotherapy in a manufacturer-sponsored international open-label phase III randomized trial involving 343 patients with advanced non–small-cell lung cancer (NSCLC), according to a report published March 28 in the Journal of Clinical Oncology.
Even though targeted therapies have greatly improved outcomes for patients whose NSCLC is associated with oncogenic driver mutations, brain metastases remain “a significant clinical problem, resulting in considerable physical and neurocognitive morbidity as well as mortality.” In addition, as patients live longer because of these targeted treatments, the proportion who have brain metastases rises, “such that up to 50% of patients alive at 2 years with EGFR- [epidermal growth factor receptor]–mutated or ALK- [anaplastic lymphoma kinase]–rearranged lung cancer will have brain metastases,” according to Dr. Benjamin J. Solomon of the department of medical oncology, Peter MacCallum Cancer Centre, Melbourne, and his associates.
They compared the efficacy of intracranial disease control between oral crizotinib and IV pemetrexed-platinum chemotherapy in patients who had advanced nonsquamous ALK-positive NSCLC. A total of 79 of these patients (23%) had brain metastases at baseline.
At 12 weeks, a significantly higher percentage of patients achieved intracranial disease control with crizotinib (85%) than with chemotherapy (45%), and the same was true at 24 weeks (56% vs. 25%). Median progression-free survival also was longer with crizotinib, regardless of whether patients had brain metastases at baseline (9 months vs. 4 months) or did not have brain metastases at baseline (11 months vs. 7 months), Dr. Solomon and his associates said (J Clin Oncol. 2016 Mar 28. doi: 10.1200/JCO.2015.63.5888).
Similarly, the overall response rate was significantly higher with crizotinib, regardless of whether patients had brain metastases at baseline (77% vs. 28%) or did not have brain metastases at baseline (74% vs. 50%). In addition, disease progression was less frequent with crizotinib than with chemotherapy in the overall patient population (52% vs. 77%), in the subgroup of patients who had brain metastases at baseline (54% vs. 75%), and in the subgroup of patients who did not have brain metastases at baseline (52% vs. 78%).
These findings indicate that crizotinib should be the standard first-line therapy for patients who have advanced ALK-positive NSCLC, whether or not they have brain metastases, the investigators said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Crizotinib achieved better control of brain metastases than chemotherapy in advanced NSCLC.
Major finding: A significantly higher percentage of study participants achieved intracranial disease control with crizotinib (85%) than with chemotherapy (45%) at 12 weeks and at 24 weeks (56% vs. 25%).
Data source: An ongoing international open-label phase III randomized trial involving 343 patients.
Disclosures: This study was supported by Pfizer. Dr. Solomon reported ties to Pfizer and other industry sources; his associates also reported ties to numerous industry sources.
An uncommon presentation of non-small-cell lung cancer with acrometastases to the great toe and index finger
Case presentation and summary
A 71-year-old white woman was referred to the emergency department by her primary care physician for necrosis and swelling of the left great toe for work-up of possible osteomyelitis (Figure 1). Before she presented to her physician, she had been complaining of severe pain, swelling, and erythema of the left great toe that had lasted for 1-2 months. Infection was initially suspected. She completed 2 courses of oral antibiotics with no improvement. She was also complaining of similar symptoms on the left index finger and attributed her symptoms to an injury a month earlier (Figure 2). The pain was so severe that she was not able to bear weight on her left foot. An outpatient X-ray of her left great toe raised her physician’s concerns that it might be osteomyelitis so she was referred to the emergency department.
Click on the PDF icon at the top of this introduction to read the full article.
Case presentation and summary
A 71-year-old white woman was referred to the emergency department by her primary care physician for necrosis and swelling of the left great toe for work-up of possible osteomyelitis (Figure 1). Before she presented to her physician, she had been complaining of severe pain, swelling, and erythema of the left great toe that had lasted for 1-2 months. Infection was initially suspected. She completed 2 courses of oral antibiotics with no improvement. She was also complaining of similar symptoms on the left index finger and attributed her symptoms to an injury a month earlier (Figure 2). The pain was so severe that she was not able to bear weight on her left foot. An outpatient X-ray of her left great toe raised her physician’s concerns that it might be osteomyelitis so she was referred to the emergency department.
Click on the PDF icon at the top of this introduction to read the full article.
Case presentation and summary
A 71-year-old white woman was referred to the emergency department by her primary care physician for necrosis and swelling of the left great toe for work-up of possible osteomyelitis (Figure 1). Before she presented to her physician, she had been complaining of severe pain, swelling, and erythema of the left great toe that had lasted for 1-2 months. Infection was initially suspected. She completed 2 courses of oral antibiotics with no improvement. She was also complaining of similar symptoms on the left index finger and attributed her symptoms to an injury a month earlier (Figure 2). The pain was so severe that she was not able to bear weight on her left foot. An outpatient X-ray of her left great toe raised her physician’s concerns that it might be osteomyelitis so she was referred to the emergency department.
Click on the PDF icon at the top of this introduction to read the full article.
Impact of trimodality treatment on patient quality of life and arm function for superior sulcus tumors
Background Trimodality treatment leads to improved survival for superior sulcus tumor (SST) patients. Not much is known about the impact of this treatment on arm function and patient quality of life.
Objective To analyze arm function and quality of life in SST patients undergoing trimodality treatment.
Methods This was a prospective cohort study of consecutive SST patients treated with trimodality treatment that was conducted between April 1, 2010 and October 31, 2012. We obtained informed consent for 20 of 22 eligible patients. The 36-item Short Form Health Survey (SF-36) and disabilities of the arm, shoulder, and hand (DASH) questionnaires were used to asses patient quality of life and subjective arm function at 0 (preoperative day), 3, and 12 months after trimodality treatment.
Results DASH scores were significantly lower at 3 and 12 months (P = .024 and P = .011) compared with preoperative scores. Significantly lower scores were reported for the SF-36 domains of physical functioning at 12 months (P = .020) and of physical role functioning at 3 months (P = .041), and significantly more pain was reported at 3 and 12 months (P = .006 and P = .019, respectively). Patients who underwent T1 nerve root resection had lower scores for the SF-36 domain health change at 3 months (P = .037) compared with those in whom the T1 root was spared. For all other domains no differences were found.
Limitations Small sample size; patient pre-chemoradiation function and quality of life unknown.
Conclusion Subjective arm function and patient quality of life is reduced following trimodality treatment. Resection of the T1 nerve root has no significant long-term effect on the subjective arm function and quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Background Trimodality treatment leads to improved survival for superior sulcus tumor (SST) patients. Not much is known about the impact of this treatment on arm function and patient quality of life.
Objective To analyze arm function and quality of life in SST patients undergoing trimodality treatment.
Methods This was a prospective cohort study of consecutive SST patients treated with trimodality treatment that was conducted between April 1, 2010 and October 31, 2012. We obtained informed consent for 20 of 22 eligible patients. The 36-item Short Form Health Survey (SF-36) and disabilities of the arm, shoulder, and hand (DASH) questionnaires were used to asses patient quality of life and subjective arm function at 0 (preoperative day), 3, and 12 months after trimodality treatment.
Results DASH scores were significantly lower at 3 and 12 months (P = .024 and P = .011) compared with preoperative scores. Significantly lower scores were reported for the SF-36 domains of physical functioning at 12 months (P = .020) and of physical role functioning at 3 months (P = .041), and significantly more pain was reported at 3 and 12 months (P = .006 and P = .019, respectively). Patients who underwent T1 nerve root resection had lower scores for the SF-36 domain health change at 3 months (P = .037) compared with those in whom the T1 root was spared. For all other domains no differences were found.
Limitations Small sample size; patient pre-chemoradiation function and quality of life unknown.
Conclusion Subjective arm function and patient quality of life is reduced following trimodality treatment. Resection of the T1 nerve root has no significant long-term effect on the subjective arm function and quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Background Trimodality treatment leads to improved survival for superior sulcus tumor (SST) patients. Not much is known about the impact of this treatment on arm function and patient quality of life.
Objective To analyze arm function and quality of life in SST patients undergoing trimodality treatment.
Methods This was a prospective cohort study of consecutive SST patients treated with trimodality treatment that was conducted between April 1, 2010 and October 31, 2012. We obtained informed consent for 20 of 22 eligible patients. The 36-item Short Form Health Survey (SF-36) and disabilities of the arm, shoulder, and hand (DASH) questionnaires were used to asses patient quality of life and subjective arm function at 0 (preoperative day), 3, and 12 months after trimodality treatment.
Results DASH scores were significantly lower at 3 and 12 months (P = .024 and P = .011) compared with preoperative scores. Significantly lower scores were reported for the SF-36 domains of physical functioning at 12 months (P = .020) and of physical role functioning at 3 months (P = .041), and significantly more pain was reported at 3 and 12 months (P = .006 and P = .019, respectively). Patients who underwent T1 nerve root resection had lower scores for the SF-36 domain health change at 3 months (P = .037) compared with those in whom the T1 root was spared. For all other domains no differences were found.
Limitations Small sample size; patient pre-chemoradiation function and quality of life unknown.
Conclusion Subjective arm function and patient quality of life is reduced following trimodality treatment. Resection of the T1 nerve root has no significant long-term effect on the subjective arm function and quality of life.
Click on the PDF icon at the top of this introduction to read the full article.