User login
CT of chest, extremity effective for sarcoma follow-up
BOSTON – CT scans appear to be effective for detecting local recurrences and pulmonary metastases in patients treated for soft-tissue sarcomas of the extremities, for about a third less than the cost of follow-up with MRI.
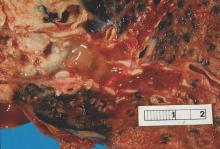
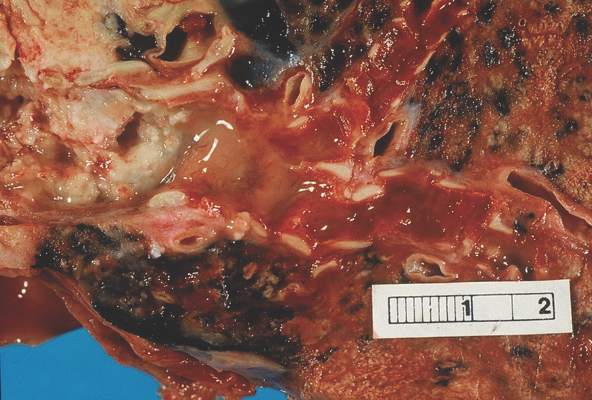
In a retrospective study by Dr. Allison Maciver and her colleagues, among 91 patients with soft-tissue sarcomas of the extremity followed with CT, 11 patients had a total of 14 local recurrences detected on CT, and 11 of the recurrences were in patients who were clinically asymptomatic.
Surveillance CT also identified 15 cases of pulmonary metastases, and 4 incidental second primary malignancies, Dr. Maciver of the Roswell Park Cancer Institute in Buffalo, N.Y., and her coinvestigators found, and there was only one false-positive recurrence.
The benefits of CT over extremity MRI in this population include decreased imaging time, lower cost, and a larger field of view, allowing for detection of second primary malignancies, she noted in a poster session at the annual Society of Surgical Oncology Cancer Symposium.
Many sarcomas of the extremities are highly aggressive, and timely detection of local recurrences could improve chances for limb-sparing salvage therapies. Although MRI has typically been used to follow patients with sarcomas, it is expensive and has a limited field of view, Dr. Maciver said.
In addition, the risk of pulmonary metastases with some soft-tissue sarcomas is high, necessitating the use of chest CT as a surveillance tool.
To see whether CT scans of the chest and extremities could be a cost-effective surveillance strategy for both local recurrences and pulmonary metastases, the investigators did a retrospective study of a prospective database of patients who underwent surgical resection for soft-tissue sarcomas of the extremities from 2001 through 2014 and who had CT as the primary follow-up imaging modality.
They identified a total of 91 high-risk patients followed for a median of 50.5 months. The patients had an estimated 5-year freedom from local recurrence of 82%, and from distant recurrence of 80%. Five-year overall survival was 76%.
Of the 15 patients found on CT to have pulmonary metastases, there were 4 incidentally discovered second primary cancers, including 1 each of non–small cell lung cancer, pancreatic adenocarcinoma, Merkel cell carcinomatosis, and myxofibrosarcoma. There were no false-positive pulmonary metastases.
The estimated cost of 10 years of surveillance, based on 2014 gross technical costs, was $64,969 per patient for chest CT and extremity MRI, compared with $41,595 per patient for chest and extremity CT surveillance, a potential cost savings with the CT-only strategy of $23,374 per patient.
The investigators said that the overall benefits of CT, including the cost savings in an accountable care organization model, “appear to outweigh the slightly increased radiation exposure.”
The study was internally funded. The authors reported having no relevant financial disclosures.
BOSTON – CT scans appear to be effective for detecting local recurrences and pulmonary metastases in patients treated for soft-tissue sarcomas of the extremities, for about a third less than the cost of follow-up with MRI.
In a retrospective study by Dr. Allison Maciver and her colleagues, among 91 patients with soft-tissue sarcomas of the extremity followed with CT, 11 patients had a total of 14 local recurrences detected on CT, and 11 of the recurrences were in patients who were clinically asymptomatic.
Surveillance CT also identified 15 cases of pulmonary metastases, and 4 incidental second primary malignancies, Dr. Maciver of the Roswell Park Cancer Institute in Buffalo, N.Y., and her coinvestigators found, and there was only one false-positive recurrence.
The benefits of CT over extremity MRI in this population include decreased imaging time, lower cost, and a larger field of view, allowing for detection of second primary malignancies, she noted in a poster session at the annual Society of Surgical Oncology Cancer Symposium.
Many sarcomas of the extremities are highly aggressive, and timely detection of local recurrences could improve chances for limb-sparing salvage therapies. Although MRI has typically been used to follow patients with sarcomas, it is expensive and has a limited field of view, Dr. Maciver said.
In addition, the risk of pulmonary metastases with some soft-tissue sarcomas is high, necessitating the use of chest CT as a surveillance tool.
To see whether CT scans of the chest and extremities could be a cost-effective surveillance strategy for both local recurrences and pulmonary metastases, the investigators did a retrospective study of a prospective database of patients who underwent surgical resection for soft-tissue sarcomas of the extremities from 2001 through 2014 and who had CT as the primary follow-up imaging modality.
They identified a total of 91 high-risk patients followed for a median of 50.5 months. The patients had an estimated 5-year freedom from local recurrence of 82%, and from distant recurrence of 80%. Five-year overall survival was 76%.
Of the 15 patients found on CT to have pulmonary metastases, there were 4 incidentally discovered second primary cancers, including 1 each of non–small cell lung cancer, pancreatic adenocarcinoma, Merkel cell carcinomatosis, and myxofibrosarcoma. There were no false-positive pulmonary metastases.
The estimated cost of 10 years of surveillance, based on 2014 gross technical costs, was $64,969 per patient for chest CT and extremity MRI, compared with $41,595 per patient for chest and extremity CT surveillance, a potential cost savings with the CT-only strategy of $23,374 per patient.
The investigators said that the overall benefits of CT, including the cost savings in an accountable care organization model, “appear to outweigh the slightly increased radiation exposure.”
The study was internally funded. The authors reported having no relevant financial disclosures.
BOSTON – CT scans appear to be effective for detecting local recurrences and pulmonary metastases in patients treated for soft-tissue sarcomas of the extremities, for about a third less than the cost of follow-up with MRI.
In a retrospective study by Dr. Allison Maciver and her colleagues, among 91 patients with soft-tissue sarcomas of the extremity followed with CT, 11 patients had a total of 14 local recurrences detected on CT, and 11 of the recurrences were in patients who were clinically asymptomatic.
Surveillance CT also identified 15 cases of pulmonary metastases, and 4 incidental second primary malignancies, Dr. Maciver of the Roswell Park Cancer Institute in Buffalo, N.Y., and her coinvestigators found, and there was only one false-positive recurrence.
The benefits of CT over extremity MRI in this population include decreased imaging time, lower cost, and a larger field of view, allowing for detection of second primary malignancies, she noted in a poster session at the annual Society of Surgical Oncology Cancer Symposium.
Many sarcomas of the extremities are highly aggressive, and timely detection of local recurrences could improve chances for limb-sparing salvage therapies. Although MRI has typically been used to follow patients with sarcomas, it is expensive and has a limited field of view, Dr. Maciver said.
In addition, the risk of pulmonary metastases with some soft-tissue sarcomas is high, necessitating the use of chest CT as a surveillance tool.
To see whether CT scans of the chest and extremities could be a cost-effective surveillance strategy for both local recurrences and pulmonary metastases, the investigators did a retrospective study of a prospective database of patients who underwent surgical resection for soft-tissue sarcomas of the extremities from 2001 through 2014 and who had CT as the primary follow-up imaging modality.
They identified a total of 91 high-risk patients followed for a median of 50.5 months. The patients had an estimated 5-year freedom from local recurrence of 82%, and from distant recurrence of 80%. Five-year overall survival was 76%.
Of the 15 patients found on CT to have pulmonary metastases, there were 4 incidentally discovered second primary cancers, including 1 each of non–small cell lung cancer, pancreatic adenocarcinoma, Merkel cell carcinomatosis, and myxofibrosarcoma. There were no false-positive pulmonary metastases.
The estimated cost of 10 years of surveillance, based on 2014 gross technical costs, was $64,969 per patient for chest CT and extremity MRI, compared with $41,595 per patient for chest and extremity CT surveillance, a potential cost savings with the CT-only strategy of $23,374 per patient.
The investigators said that the overall benefits of CT, including the cost savings in an accountable care organization model, “appear to outweigh the slightly increased radiation exposure.”
The study was internally funded. The authors reported having no relevant financial disclosures.
Key clinical point: Lower-cost CT scans of the extremity and chest appear to be effective for surveillance of patients following resection of soft-tissue sarcomas.
Major finding: Of 91 patients with soft-tissue sarcomas of the extremity followed with CT, 11 had a total of 14 local recurrences detected. Of the recurrences, 11 were clinically asymptomatic.
Data source: A retrospective study of a prospectively maintained surgical database.
Disclosures: The study was internally funded. The authors reported having no relevant financial disclosures.
Does sharing genetic risk change behavior?
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
Wanted: Better evidence on fast-track lung resection
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”
 |
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”
 |
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”
 |
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
Key clinical point: Well-designed clinical trials are needed to determine the effectiveness of fast-track recovery pathways in lung resection.
Major finding: Fast-track lung resection patients showed no differences in readmissions, overall complication and death rates compared to patients subjected to a traditional recovery course.
Data source: Systematic review of six studies published from 1997 to 2012 that involved 1,612 individuals who had lung resection.
Disclosures: The study authors had no financial relationships to disclose.
Serious complications after cancer surgery linked to worse long-term survival
BOSTON – The operation was a success, but the patient died.
It’s an old chestnut for sure, but there is a painful kernel of truth in it, say investigators who found that patients who undergo complex cancer surgery and have serious complications are at significantly increased risk for death for at least 6 months after surgery, compared with patients who undergo the same procedure with few or no complications.
“Our work has important implications for quality assessment. I think in cancer surgery in particular we have to get away from the short-term metrics of survival, and we have to think about the implications of complications for long-term survival, even if at a very high-quality hospital we’re good at salvaging those patients who do experience those complications,” said Dr. Hari Nathan of the University of Michigan, Ann Arbor.
In a retrospective study, results of which were presented at the annual Society of Surgical Oncology Cancer Symposium, Dr. Nathan and colleagues showed that patients who underwent surgery for cancers of the esophagus and lung who had serious complications but survived at least 30 days after surgery had a more than twofold greater risk for death than did patients who had no complications, and patients with serious complications following surgery for cancer of the pancreas had a nearly twofold greater risk.
The effects of serious complications on survival persisted out to at least 180 days after surgery for each of the three procedures.
The findings suggest that just getting the patient through the operation and keeping him or her alive in the ICU is not sufficient cause for celebration by surgeons, Dr. Nathan said.
The investigators conducted the study to examine the incidence of complications following cancer surgery in older patients, the relationship between surgical complications and long-term survival, and whether the effects of complications would diminish or “wash out” over time. They reviewed Surveillance, Epidemiology and End Results–Medicare data on patients aged 65 years and older who underwent surgery with curative intent for esophageal cancer, non–small cell lung cancer, or pancreatic adenocarcinoma from 2005 through 2009.
They defined serious complications as “the appearance of a complication associated with a hospital length of stay greater than the 75th percentile for that procedure.”
The cohort included 965 patients who underwent esophageal surgery, 12,395 who had lung surgery, and 1,966 who underwent pancreatic resection. The proportion of patients over 80 years who underwent the procedures, respectively, were 12%, 18%, and 19%.
Serious complications occurred in 17% of patients with esophageal cancer, 10% of those with lung cancer, and 12% of those with cancer of the pancreas. The respective 30-day mortality rates were 6.%, 3.3%, and 3.9%.
Looking only at those patients with lung cancer who survived at least 30 days after surgery, the investigators found that median survival among those who had no complications was 79 months, compared with 60 months for those who had mild complications, and 33 months for patients who had serious complications (P less than .001)
“And indeed, when we performed adjusted survival analyses looking at all three disease sites, we saw a very consistent story: that those patients who had serious complications had decreased long-term survival for all three malignancies we looked at,” Dr. Nathan said.
Specifically, in survival analyses adjusted for sex, age, and procedure code, hazard ratios for patients with serious complications compared with those who had no complications were 2.55 for esophageal cancer patients, 2.13 for lung cancer patients, and 1.57 for pancreatic cancer patients (all comparisons significant as shown by 95% confidence intervals).
The investigators questioned whether the differences in mortality were due to the late effects of perioperative complications.
“In modern ICUs, we can keep virtually anybody alive for 30 days, and there has been a lot interest in longer-term metrics for perioperative mortality, for example, at 30 or 90 days, so we thought maybe that’s what we were seeing here,” he said. To test this idea, the investigators looked at the effects of complications on patient who survived lung cancer surgery for at least 90 days, and those who lived for at least 180 days after surgery, and they saw that the survival curves were similar to those seen with the 30-day survivors, showing significantly and persistently worse survival for patients with serious complications (P less than .001).
For each of the disease states, patients with serious complications were also significantly less likely than were those with no or mild complications to receive adjuvant chemotherapy, even after adjustment for patient age and cancer stage, two significant determinants of the likelihood of receiving chemotherapy.
And even when the effect of chemotherapy for those who did receive it was added into the survival models, patients with serious complications still had significantly worse overall survival, Dr. Nathan noted.
“Serious complications after these three cancer resections are common and they are associated with dramatically inferior long-term survival. Thirty, 60, 90, and even 180-day measures of mortality do not capture the full impact of complications on long-term survival,” he said.
Asked whether it may be possible to identify those patients at higher risk for serious complications due to comorbidities or other factors, and perhaps suggest withholding surgery from such patients, Dr. Nathan agreed, but added that “the best chance for survival for all of these patients is a high-quality surgical resection, so it’s hard to deny a patient that chance unless you think they have a really high risk of perioperative death.”
The study was internally funded. Dr. Nathan reported no significant disclosures.
BOSTON – The operation was a success, but the patient died.
It’s an old chestnut for sure, but there is a painful kernel of truth in it, say investigators who found that patients who undergo complex cancer surgery and have serious complications are at significantly increased risk for death for at least 6 months after surgery, compared with patients who undergo the same procedure with few or no complications.
“Our work has important implications for quality assessment. I think in cancer surgery in particular we have to get away from the short-term metrics of survival, and we have to think about the implications of complications for long-term survival, even if at a very high-quality hospital we’re good at salvaging those patients who do experience those complications,” said Dr. Hari Nathan of the University of Michigan, Ann Arbor.
In a retrospective study, results of which were presented at the annual Society of Surgical Oncology Cancer Symposium, Dr. Nathan and colleagues showed that patients who underwent surgery for cancers of the esophagus and lung who had serious complications but survived at least 30 days after surgery had a more than twofold greater risk for death than did patients who had no complications, and patients with serious complications following surgery for cancer of the pancreas had a nearly twofold greater risk.
The effects of serious complications on survival persisted out to at least 180 days after surgery for each of the three procedures.
The findings suggest that just getting the patient through the operation and keeping him or her alive in the ICU is not sufficient cause for celebration by surgeons, Dr. Nathan said.
The investigators conducted the study to examine the incidence of complications following cancer surgery in older patients, the relationship between surgical complications and long-term survival, and whether the effects of complications would diminish or “wash out” over time. They reviewed Surveillance, Epidemiology and End Results–Medicare data on patients aged 65 years and older who underwent surgery with curative intent for esophageal cancer, non–small cell lung cancer, or pancreatic adenocarcinoma from 2005 through 2009.
They defined serious complications as “the appearance of a complication associated with a hospital length of stay greater than the 75th percentile for that procedure.”
The cohort included 965 patients who underwent esophageal surgery, 12,395 who had lung surgery, and 1,966 who underwent pancreatic resection. The proportion of patients over 80 years who underwent the procedures, respectively, were 12%, 18%, and 19%.
Serious complications occurred in 17% of patients with esophageal cancer, 10% of those with lung cancer, and 12% of those with cancer of the pancreas. The respective 30-day mortality rates were 6.%, 3.3%, and 3.9%.
Looking only at those patients with lung cancer who survived at least 30 days after surgery, the investigators found that median survival among those who had no complications was 79 months, compared with 60 months for those who had mild complications, and 33 months for patients who had serious complications (P less than .001)
“And indeed, when we performed adjusted survival analyses looking at all three disease sites, we saw a very consistent story: that those patients who had serious complications had decreased long-term survival for all three malignancies we looked at,” Dr. Nathan said.
Specifically, in survival analyses adjusted for sex, age, and procedure code, hazard ratios for patients with serious complications compared with those who had no complications were 2.55 for esophageal cancer patients, 2.13 for lung cancer patients, and 1.57 for pancreatic cancer patients (all comparisons significant as shown by 95% confidence intervals).
The investigators questioned whether the differences in mortality were due to the late effects of perioperative complications.
“In modern ICUs, we can keep virtually anybody alive for 30 days, and there has been a lot interest in longer-term metrics for perioperative mortality, for example, at 30 or 90 days, so we thought maybe that’s what we were seeing here,” he said. To test this idea, the investigators looked at the effects of complications on patient who survived lung cancer surgery for at least 90 days, and those who lived for at least 180 days after surgery, and they saw that the survival curves were similar to those seen with the 30-day survivors, showing significantly and persistently worse survival for patients with serious complications (P less than .001).
For each of the disease states, patients with serious complications were also significantly less likely than were those with no or mild complications to receive adjuvant chemotherapy, even after adjustment for patient age and cancer stage, two significant determinants of the likelihood of receiving chemotherapy.
And even when the effect of chemotherapy for those who did receive it was added into the survival models, patients with serious complications still had significantly worse overall survival, Dr. Nathan noted.
“Serious complications after these three cancer resections are common and they are associated with dramatically inferior long-term survival. Thirty, 60, 90, and even 180-day measures of mortality do not capture the full impact of complications on long-term survival,” he said.
Asked whether it may be possible to identify those patients at higher risk for serious complications due to comorbidities or other factors, and perhaps suggest withholding surgery from such patients, Dr. Nathan agreed, but added that “the best chance for survival for all of these patients is a high-quality surgical resection, so it’s hard to deny a patient that chance unless you think they have a really high risk of perioperative death.”
The study was internally funded. Dr. Nathan reported no significant disclosures.
BOSTON – The operation was a success, but the patient died.
It’s an old chestnut for sure, but there is a painful kernel of truth in it, say investigators who found that patients who undergo complex cancer surgery and have serious complications are at significantly increased risk for death for at least 6 months after surgery, compared with patients who undergo the same procedure with few or no complications.
“Our work has important implications for quality assessment. I think in cancer surgery in particular we have to get away from the short-term metrics of survival, and we have to think about the implications of complications for long-term survival, even if at a very high-quality hospital we’re good at salvaging those patients who do experience those complications,” said Dr. Hari Nathan of the University of Michigan, Ann Arbor.
In a retrospective study, results of which were presented at the annual Society of Surgical Oncology Cancer Symposium, Dr. Nathan and colleagues showed that patients who underwent surgery for cancers of the esophagus and lung who had serious complications but survived at least 30 days after surgery had a more than twofold greater risk for death than did patients who had no complications, and patients with serious complications following surgery for cancer of the pancreas had a nearly twofold greater risk.
The effects of serious complications on survival persisted out to at least 180 days after surgery for each of the three procedures.
The findings suggest that just getting the patient through the operation and keeping him or her alive in the ICU is not sufficient cause for celebration by surgeons, Dr. Nathan said.
The investigators conducted the study to examine the incidence of complications following cancer surgery in older patients, the relationship between surgical complications and long-term survival, and whether the effects of complications would diminish or “wash out” over time. They reviewed Surveillance, Epidemiology and End Results–Medicare data on patients aged 65 years and older who underwent surgery with curative intent for esophageal cancer, non–small cell lung cancer, or pancreatic adenocarcinoma from 2005 through 2009.
They defined serious complications as “the appearance of a complication associated with a hospital length of stay greater than the 75th percentile for that procedure.”
The cohort included 965 patients who underwent esophageal surgery, 12,395 who had lung surgery, and 1,966 who underwent pancreatic resection. The proportion of patients over 80 years who underwent the procedures, respectively, were 12%, 18%, and 19%.
Serious complications occurred in 17% of patients with esophageal cancer, 10% of those with lung cancer, and 12% of those with cancer of the pancreas. The respective 30-day mortality rates were 6.%, 3.3%, and 3.9%.
Looking only at those patients with lung cancer who survived at least 30 days after surgery, the investigators found that median survival among those who had no complications was 79 months, compared with 60 months for those who had mild complications, and 33 months for patients who had serious complications (P less than .001)
“And indeed, when we performed adjusted survival analyses looking at all three disease sites, we saw a very consistent story: that those patients who had serious complications had decreased long-term survival for all three malignancies we looked at,” Dr. Nathan said.
Specifically, in survival analyses adjusted for sex, age, and procedure code, hazard ratios for patients with serious complications compared with those who had no complications were 2.55 for esophageal cancer patients, 2.13 for lung cancer patients, and 1.57 for pancreatic cancer patients (all comparisons significant as shown by 95% confidence intervals).
The investigators questioned whether the differences in mortality were due to the late effects of perioperative complications.
“In modern ICUs, we can keep virtually anybody alive for 30 days, and there has been a lot interest in longer-term metrics for perioperative mortality, for example, at 30 or 90 days, so we thought maybe that’s what we were seeing here,” he said. To test this idea, the investigators looked at the effects of complications on patient who survived lung cancer surgery for at least 90 days, and those who lived for at least 180 days after surgery, and they saw that the survival curves were similar to those seen with the 30-day survivors, showing significantly and persistently worse survival for patients with serious complications (P less than .001).
For each of the disease states, patients with serious complications were also significantly less likely than were those with no or mild complications to receive adjuvant chemotherapy, even after adjustment for patient age and cancer stage, two significant determinants of the likelihood of receiving chemotherapy.
And even when the effect of chemotherapy for those who did receive it was added into the survival models, patients with serious complications still had significantly worse overall survival, Dr. Nathan noted.
“Serious complications after these three cancer resections are common and they are associated with dramatically inferior long-term survival. Thirty, 60, 90, and even 180-day measures of mortality do not capture the full impact of complications on long-term survival,” he said.
Asked whether it may be possible to identify those patients at higher risk for serious complications due to comorbidities or other factors, and perhaps suggest withholding surgery from such patients, Dr. Nathan agreed, but added that “the best chance for survival for all of these patients is a high-quality surgical resection, so it’s hard to deny a patient that chance unless you think they have a really high risk of perioperative death.”
The study was internally funded. Dr. Nathan reported no significant disclosures.
AT SSO 2016
Key clinical point: Thirty-day postoperative survival may not be an adequate measure of success of complex cancer surgeries.
Major finding: Patients with serious complications from esophageal, lung, and pancreatic cancer operations had significantly worse survival out to 180 days ,compared with those with mild or no complications.
Data source: Retrospective review of SEER-Medicare data from 2005-2009.
Disclosures: The study was internally funded. Dr. Nathan reported no significant disclosures.
Quitting smoking plus low-dose helical CT reduces lung cancer death risk
Smoking abstinence for 7 years results in a 20% reduction in death from lung cancer – a benefit that is comparable to three rounds of annual screening with low-dose helical computed tomography (LDCT) – in asymptomatic individuals with at least a 30–pack-year smoking history, based on a secondary analysis of 50,263 participants in the National Lung Screening Trial (NLST).
Not smoking for 7 years plus screening for lung cancer with LDCT conferred an additional 10% reduction in lung cancer mortality. Similar patterns for smoking cessation benefits were noted for overall mortality, as well.
“This study is the first to quantify the benefit of smoking cessation coupled with lung cancer screening in a cohort that is asymptomatic,” wrote Dr. Nichole T. Tanner of the Medical University of South Carolina, Charleston, and her colleagues (Am J Respir Crit Care. 2016 March 1. doi: 10.1164/rccm.201507-1420OC). “[Its] findings highlight the importance of integrating smoking cessation efforts into lung cancer screening programs.”
The NLST subset study included 47,902 participants who self-identified as non-Hispanic white and 2,361 who self-identified as non-Hispanic black; 24,190 were current smokers and 26,073 were former smokers who had quit within the 15 years prior to entering the study. Participants ranged in age from 55 to 74 years at the time of randomization and had a 30–pack-year or more history of cigarette smoking.
All participants were screened for lung cancer with either LDCT or a chest radiograph examination. A 20% reduction in death from lung cancer was seen in those who had abstained from smoking for 7 years and were screened for lung cancer with a chest radiograph and in those who had undergone three rounds of annual screening for lung cancer with LDCT and continued to smoke.
For former smokers screened with LDCT, the risk of dying of lung cancer decreased at a faster rate than it did for those screened with chest radiographs. For each additional year an individual abstained from smoking and had an LDCT screen, the risk of dying of lung cancer decreased by 9%. For those individuals who abstained from smoking and had been screened with a chest x-ray, the risk of dying of lung cancer decreased by 3%.
In contrast, there was a 10% increase in lung cancer mortality for each additional 10 pack-years smoked for those screened with LDCT (HR, 1.10; 95% CI, 1.08-1.13). This “did not vary significantly in the chest radiograph group,” according to the researchers.
In both screening groups, “an additional 6% risk of death from all causes (was seen) for each additional 10 pack-years smoked.”
In addition, black study participants who had quit smoking at trial entry had “a more pronounced benefit” from having done so, compared with the white study participants (HR, 0.53, 95% CI, 0.28-1.0).
The NLST was sponsored by the National Cancer Institute. Dr. Nichole T. Tanner, one of this secondary analysis’s authors reported receiving grants from ACCP OneBreath Foundation for her work on this project and grants from Olympus America, Cook, and the American Cancer Society for other work. Disclosures for all investigators are available at atsjournals.org.
This secondary analysis was limited by the fact that the National Lung Screening Trial “does not have information about smoking cessation or persistence during the trial.”
The finding of black former smokers having a hazard rate for lung cancer mortality of 0.53, compared with white former smokers, was reassuring, because “there is evidence that African Americans are at higher risk for lung cancer at lower smoking intensities than whites.”
While this secondary analysis suggests that screening for lung cancer can reduce lung cancer death risk, lung cancer screening alone is not adequate for preventing the disease. Screening must be “linked to smoking cessation efforts in those who are current smokers” and may need to follow criteria that are different from those used in the NLST.
“Implementation of lung cancer screening will be a serious challenge that must be linked to smoking cessation efforts in those who are current smokers at the time they enter a screening program, both for Centers for Medicare & Medicaid Services reimbursement and for medical appropriateness.”
Dr. Christine D. Berg is with Johns Hopkins Medicine, Baltimore, and the division of cancer epidemiology and prevention at the National Cancer Institute, Bethesda, Md. She made these remarks in an editorial accompanying Dr. Tanner’s report (J Respir Crit Care. 2016 March 1. doi: 10.1164/rccm.201511-2270ED). She reported receiving personal fees from Medial CS, and she was the study director of the NLST.
This secondary analysis was limited by the fact that the National Lung Screening Trial “does not have information about smoking cessation or persistence during the trial.”
The finding of black former smokers having a hazard rate for lung cancer mortality of 0.53, compared with white former smokers, was reassuring, because “there is evidence that African Americans are at higher risk for lung cancer at lower smoking intensities than whites.”
While this secondary analysis suggests that screening for lung cancer can reduce lung cancer death risk, lung cancer screening alone is not adequate for preventing the disease. Screening must be “linked to smoking cessation efforts in those who are current smokers” and may need to follow criteria that are different from those used in the NLST.
“Implementation of lung cancer screening will be a serious challenge that must be linked to smoking cessation efforts in those who are current smokers at the time they enter a screening program, both for Centers for Medicare & Medicaid Services reimbursement and for medical appropriateness.”
Dr. Christine D. Berg is with Johns Hopkins Medicine, Baltimore, and the division of cancer epidemiology and prevention at the National Cancer Institute, Bethesda, Md. She made these remarks in an editorial accompanying Dr. Tanner’s report (J Respir Crit Care. 2016 March 1. doi: 10.1164/rccm.201511-2270ED). She reported receiving personal fees from Medial CS, and she was the study director of the NLST.
This secondary analysis was limited by the fact that the National Lung Screening Trial “does not have information about smoking cessation or persistence during the trial.”
The finding of black former smokers having a hazard rate for lung cancer mortality of 0.53, compared with white former smokers, was reassuring, because “there is evidence that African Americans are at higher risk for lung cancer at lower smoking intensities than whites.”
While this secondary analysis suggests that screening for lung cancer can reduce lung cancer death risk, lung cancer screening alone is not adequate for preventing the disease. Screening must be “linked to smoking cessation efforts in those who are current smokers” and may need to follow criteria that are different from those used in the NLST.
“Implementation of lung cancer screening will be a serious challenge that must be linked to smoking cessation efforts in those who are current smokers at the time they enter a screening program, both for Centers for Medicare & Medicaid Services reimbursement and for medical appropriateness.”
Dr. Christine D. Berg is with Johns Hopkins Medicine, Baltimore, and the division of cancer epidemiology and prevention at the National Cancer Institute, Bethesda, Md. She made these remarks in an editorial accompanying Dr. Tanner’s report (J Respir Crit Care. 2016 March 1. doi: 10.1164/rccm.201511-2270ED). She reported receiving personal fees from Medial CS, and she was the study director of the NLST.
Smoking abstinence for 7 years results in a 20% reduction in death from lung cancer – a benefit that is comparable to three rounds of annual screening with low-dose helical computed tomography (LDCT) – in asymptomatic individuals with at least a 30–pack-year smoking history, based on a secondary analysis of 50,263 participants in the National Lung Screening Trial (NLST).
Not smoking for 7 years plus screening for lung cancer with LDCT conferred an additional 10% reduction in lung cancer mortality. Similar patterns for smoking cessation benefits were noted for overall mortality, as well.
“This study is the first to quantify the benefit of smoking cessation coupled with lung cancer screening in a cohort that is asymptomatic,” wrote Dr. Nichole T. Tanner of the Medical University of South Carolina, Charleston, and her colleagues (Am J Respir Crit Care. 2016 March 1. doi: 10.1164/rccm.201507-1420OC). “[Its] findings highlight the importance of integrating smoking cessation efforts into lung cancer screening programs.”
The NLST subset study included 47,902 participants who self-identified as non-Hispanic white and 2,361 who self-identified as non-Hispanic black; 24,190 were current smokers and 26,073 were former smokers who had quit within the 15 years prior to entering the study. Participants ranged in age from 55 to 74 years at the time of randomization and had a 30–pack-year or more history of cigarette smoking.
All participants were screened for lung cancer with either LDCT or a chest radiograph examination. A 20% reduction in death from lung cancer was seen in those who had abstained from smoking for 7 years and were screened for lung cancer with a chest radiograph and in those who had undergone three rounds of annual screening for lung cancer with LDCT and continued to smoke.
For former smokers screened with LDCT, the risk of dying of lung cancer decreased at a faster rate than it did for those screened with chest radiographs. For each additional year an individual abstained from smoking and had an LDCT screen, the risk of dying of lung cancer decreased by 9%. For those individuals who abstained from smoking and had been screened with a chest x-ray, the risk of dying of lung cancer decreased by 3%.
In contrast, there was a 10% increase in lung cancer mortality for each additional 10 pack-years smoked for those screened with LDCT (HR, 1.10; 95% CI, 1.08-1.13). This “did not vary significantly in the chest radiograph group,” according to the researchers.
In both screening groups, “an additional 6% risk of death from all causes (was seen) for each additional 10 pack-years smoked.”
In addition, black study participants who had quit smoking at trial entry had “a more pronounced benefit” from having done so, compared with the white study participants (HR, 0.53, 95% CI, 0.28-1.0).
The NLST was sponsored by the National Cancer Institute. Dr. Nichole T. Tanner, one of this secondary analysis’s authors reported receiving grants from ACCP OneBreath Foundation for her work on this project and grants from Olympus America, Cook, and the American Cancer Society for other work. Disclosures for all investigators are available at atsjournals.org.
Smoking abstinence for 7 years results in a 20% reduction in death from lung cancer – a benefit that is comparable to three rounds of annual screening with low-dose helical computed tomography (LDCT) – in asymptomatic individuals with at least a 30–pack-year smoking history, based on a secondary analysis of 50,263 participants in the National Lung Screening Trial (NLST).
Not smoking for 7 years plus screening for lung cancer with LDCT conferred an additional 10% reduction in lung cancer mortality. Similar patterns for smoking cessation benefits were noted for overall mortality, as well.
“This study is the first to quantify the benefit of smoking cessation coupled with lung cancer screening in a cohort that is asymptomatic,” wrote Dr. Nichole T. Tanner of the Medical University of South Carolina, Charleston, and her colleagues (Am J Respir Crit Care. 2016 March 1. doi: 10.1164/rccm.201507-1420OC). “[Its] findings highlight the importance of integrating smoking cessation efforts into lung cancer screening programs.”
The NLST subset study included 47,902 participants who self-identified as non-Hispanic white and 2,361 who self-identified as non-Hispanic black; 24,190 were current smokers and 26,073 were former smokers who had quit within the 15 years prior to entering the study. Participants ranged in age from 55 to 74 years at the time of randomization and had a 30–pack-year or more history of cigarette smoking.
All participants were screened for lung cancer with either LDCT or a chest radiograph examination. A 20% reduction in death from lung cancer was seen in those who had abstained from smoking for 7 years and were screened for lung cancer with a chest radiograph and in those who had undergone three rounds of annual screening for lung cancer with LDCT and continued to smoke.
For former smokers screened with LDCT, the risk of dying of lung cancer decreased at a faster rate than it did for those screened with chest radiographs. For each additional year an individual abstained from smoking and had an LDCT screen, the risk of dying of lung cancer decreased by 9%. For those individuals who abstained from smoking and had been screened with a chest x-ray, the risk of dying of lung cancer decreased by 3%.
In contrast, there was a 10% increase in lung cancer mortality for each additional 10 pack-years smoked for those screened with LDCT (HR, 1.10; 95% CI, 1.08-1.13). This “did not vary significantly in the chest radiograph group,” according to the researchers.
In both screening groups, “an additional 6% risk of death from all causes (was seen) for each additional 10 pack-years smoked.”
In addition, black study participants who had quit smoking at trial entry had “a more pronounced benefit” from having done so, compared with the white study participants (HR, 0.53, 95% CI, 0.28-1.0).
The NLST was sponsored by the National Cancer Institute. Dr. Nichole T. Tanner, one of this secondary analysis’s authors reported receiving grants from ACCP OneBreath Foundation for her work on this project and grants from Olympus America, Cook, and the American Cancer Society for other work. Disclosures for all investigators are available at atsjournals.org.
FROM AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Key clinical point: Smoking cessation and lung cancer screening with low-dose helical computed tomography reduces lung cancer mortality.
Major finding: Combining 15 years of smoking cessation with LDCT screening for lung cancer resulted in a 38% risk reduction in lung cancer death.
Data source: A secondary analysis of a 50,263-person subset of the randomized, controlled National Lung Screening Trial.
Disclosures: The NLST was sponsored by the National Cancer Institute. Dr. Nichole T. Tanner, one of this secondary analysis’s authors reported receiving grants from ACCP OneBreath Foundation for her work on this project and grants from Olympus America, Cook, and the American Cancer Society for other work. Disclosures for all investigators are available at atsjournals.org.
Occult micrometastases in N2 lymph nodes correlated with shorter survival in NSCLC
In patients with non–small cell lung cancer (NSCLC) designated stage I by conventional histopathology, occult metastases were detected by immunohistochemistry (IHC) staining of cytokeratin in 14% of patients and by reverse transcriptase polymerase chain reaction (RT-PCR) for carcinoembryonic antigen in 69% of patients; however, only IHC-positivity within N2 nodes was correlated with overall survival.
Patients who were IHC-positive within an N2 node had worse overall survival than did IHC-negative patients (HR, 2.04; 95% CI, 1.14 to 3.66); 5-year survival for N2 IHC-positive patients compared with IHC-negative patients was 50% (95% CI, 29.1% to 67.8%) vs. 66.9% (60.9% to 72.2%), P = .017. Patients who were IHC-positive within N1 nodes had survival similar to that of IHC-negative patients.
Although the majority of patients in the study (69%) had occult metastases by RT-PCR, no relationship between PCR status and overall survival or disease-free survival emerged from the data.
Surgical resection in early stage NSCLC yields unpredictable outcomes, and one explanation for this is the presence of occult metastases in regional nodes.
“The presence of (occult metastases) is a logical explanation for tumors that are classified as stage I by conventional histopathology to demonstrate a worse prognosis. However, the current rigorously designed and executed prospective study only showed a significant difference in survival when N2 nodes demonstrated positivity by IHC, but not by RT-PCR,” wrote Dr. Linda W. Martin of the University of Maryland, Baltimore, and colleagues (J Clin Oncol. 2016 Feb 29. doi: 10.1200/JCO.2015.63.4543).
“Clearly RT-PCR is more sensitive, but perhaps (carcinoembryonic antigen) is not as specific for NSCLC and thus the clinical impact is insignificant,” they wrote. RT-PCR data showed poor concordance with IHC data in the study, and did not correlate with outcomes, pointing to a lack of clinical utility for RT-PCR in NSCLC, the authors stated.
The Cancer and Leukemia Group B (CALGB) 9761 trial accrued 501 patients from 1997 to 2002, 304 of whom had stage 1A or 1B NSCLC. Median follow up was 8.4 years (range: 0.97 to 11.4 years). Local only recurrence occurred in 24 patients, local and distant in 18, and distant only in 27.
Dr. Martin and coauthors had no disclosures.
In patients with non–small cell lung cancer (NSCLC) designated stage I by conventional histopathology, occult metastases were detected by immunohistochemistry (IHC) staining of cytokeratin in 14% of patients and by reverse transcriptase polymerase chain reaction (RT-PCR) for carcinoembryonic antigen in 69% of patients; however, only IHC-positivity within N2 nodes was correlated with overall survival.
Patients who were IHC-positive within an N2 node had worse overall survival than did IHC-negative patients (HR, 2.04; 95% CI, 1.14 to 3.66); 5-year survival for N2 IHC-positive patients compared with IHC-negative patients was 50% (95% CI, 29.1% to 67.8%) vs. 66.9% (60.9% to 72.2%), P = .017. Patients who were IHC-positive within N1 nodes had survival similar to that of IHC-negative patients.
Although the majority of patients in the study (69%) had occult metastases by RT-PCR, no relationship between PCR status and overall survival or disease-free survival emerged from the data.
Surgical resection in early stage NSCLC yields unpredictable outcomes, and one explanation for this is the presence of occult metastases in regional nodes.
“The presence of (occult metastases) is a logical explanation for tumors that are classified as stage I by conventional histopathology to demonstrate a worse prognosis. However, the current rigorously designed and executed prospective study only showed a significant difference in survival when N2 nodes demonstrated positivity by IHC, but not by RT-PCR,” wrote Dr. Linda W. Martin of the University of Maryland, Baltimore, and colleagues (J Clin Oncol. 2016 Feb 29. doi: 10.1200/JCO.2015.63.4543).
“Clearly RT-PCR is more sensitive, but perhaps (carcinoembryonic antigen) is not as specific for NSCLC and thus the clinical impact is insignificant,” they wrote. RT-PCR data showed poor concordance with IHC data in the study, and did not correlate with outcomes, pointing to a lack of clinical utility for RT-PCR in NSCLC, the authors stated.
The Cancer and Leukemia Group B (CALGB) 9761 trial accrued 501 patients from 1997 to 2002, 304 of whom had stage 1A or 1B NSCLC. Median follow up was 8.4 years (range: 0.97 to 11.4 years). Local only recurrence occurred in 24 patients, local and distant in 18, and distant only in 27.
Dr. Martin and coauthors had no disclosures.
In patients with non–small cell lung cancer (NSCLC) designated stage I by conventional histopathology, occult metastases were detected by immunohistochemistry (IHC) staining of cytokeratin in 14% of patients and by reverse transcriptase polymerase chain reaction (RT-PCR) for carcinoembryonic antigen in 69% of patients; however, only IHC-positivity within N2 nodes was correlated with overall survival.
Patients who were IHC-positive within an N2 node had worse overall survival than did IHC-negative patients (HR, 2.04; 95% CI, 1.14 to 3.66); 5-year survival for N2 IHC-positive patients compared with IHC-negative patients was 50% (95% CI, 29.1% to 67.8%) vs. 66.9% (60.9% to 72.2%), P = .017. Patients who were IHC-positive within N1 nodes had survival similar to that of IHC-negative patients.
Although the majority of patients in the study (69%) had occult metastases by RT-PCR, no relationship between PCR status and overall survival or disease-free survival emerged from the data.
Surgical resection in early stage NSCLC yields unpredictable outcomes, and one explanation for this is the presence of occult metastases in regional nodes.
“The presence of (occult metastases) is a logical explanation for tumors that are classified as stage I by conventional histopathology to demonstrate a worse prognosis. However, the current rigorously designed and executed prospective study only showed a significant difference in survival when N2 nodes demonstrated positivity by IHC, but not by RT-PCR,” wrote Dr. Linda W. Martin of the University of Maryland, Baltimore, and colleagues (J Clin Oncol. 2016 Feb 29. doi: 10.1200/JCO.2015.63.4543).
“Clearly RT-PCR is more sensitive, but perhaps (carcinoembryonic antigen) is not as specific for NSCLC and thus the clinical impact is insignificant,” they wrote. RT-PCR data showed poor concordance with IHC data in the study, and did not correlate with outcomes, pointing to a lack of clinical utility for RT-PCR in NSCLC, the authors stated.
The Cancer and Leukemia Group B (CALGB) 9761 trial accrued 501 patients from 1997 to 2002, 304 of whom had stage 1A or 1B NSCLC. Median follow up was 8.4 years (range: 0.97 to 11.4 years). Local only recurrence occurred in 24 patients, local and distant in 18, and distant only in 27.
Dr. Martin and coauthors had no disclosures.
Key clinical point: Micrometastasis detected by immunohistochemistry (IHC) staining for cytokeratin (AE1/AE3) in the lymph nodes of patients with non–small cell lung cancer (NSCLC), designated stage I or N0 by standard techniques, correlated with decreased overall survival.
Major finding: Patients who were IHC-positive within an N2 node had worse overall survival than did IHC-negative patients (HR, 2.04; 95% CI, 1.14 to 3.66); 5-year survival for N2 IHC-positive compared with IHC-negative patients was 50% (95% CI, 29.1% to 67.8%) vs. 66.9% (60.9% to 72.2%), P = .017.
Data source: The Cancer and Leukemia Group B (CALGB) 9761 trial of 304 patients with stage 1A or 1B NSCLC.
Disclosures: Dr. Martin and coauthors had no disclosures.
NSQIP calculator shown inadequate to stratify risk in stage I non–small cell lung cancer.
A study performed to validate the National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator for use in patients receiving surgery or stereotactic body radiation therapy (SBRT) for stage I non–small cell lung cancer showed the calculator to be inadequate for both classification and risk stratification. The study was reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151;697-705).
Dr. Pamela Samson of Washington University in St. Louis and her colleagues performed a retrospective analysis of 485 patients with clinical stage I NSCLC who underwent either surgery (277) or SBRT (195) from 2009 to 2012. Surgery was either wedge resection (19.3%) or lobectomy (74.5%), with smaller percentages receiving segmentectomy (4.0%), pneumonectomy (1.5%), and bilobectomy (0.7%). A large majority of surgical patients (84.1%) underwent a video-assisted thoracoscopic surgery (VATS) approach.
The researchers calculated NSQIP complication risk estimates for both surgical and SBRT patients using the NSQIP Surgical Risk Calculator. They compared predicted risk with actual adverse events.
Compared with patients undergoing VATS wedge resection, patients receiving SBRT were older, had larger tumors, lower forced expiratory volume (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO), higher American Society of Anesthesiologist scores, higher rates of dyspnea and higher NSQIP serious complication risk estimates, all significant at P less than .05. Similar disparities were seen in comparing patients receiving SBRT vs. VATS lobectomy.
The actual serious complication rate for surgical patients was significantly higher than the NSQIP risk calculator prediction (16.6% vs. 8.8%), as was the rate of pneumonia (6.0% vs. 3.2%), both at P less than .05.
Overall, the NSQIP Surgical Risk Calculator provided a fair level of discrimination between VATS lobectomy and SBRT on receiver operating characteristic (ROC) curve analysis, but it was a poor model for differentiating between VATS wedge resection and SBRT. “Unfortunately, it is this latter population of the highest risk surgical patients (for whom a lobectomy is not a surgical option) where risk models and decision aids are needed most,” Dr. Samson and her colleagues stated.
“Counseling the high-risk but operable patient with clinical stage I NSCLC in regard to lobectomy, sublobar resection, or SBRT is challenging for both the clinician and the patient,” according to the researchers. “We believe that a model tailored to patients with clinical stage I needs to serve as both an estimator of operative risks and a patient decision aid for surgery versus SBRT, especially with projected increases in the number of early-stage lung cancers as a result of increased lung cancer screening efforts,” they added.
“Our analysis suggests that the NSQIP Surgical Risk Calculator likely does not profile the risk of a patient with lung cancer closely enough to dichotomize surgical and inoperable SBRT cases (especially when patients are being considered for a wedge resection) or adequately estimate a surgical patient’s risk of serious complications,” Dr. Samson and her colleagues concluded.
The study was supported by grants from National Institutes of Health. The authors had no relevant financial disclosures.
In their reported study, Dr. Samson and her colleagues found that the NSQIP tool underestimated morbidity. They also found that risk predicted by the NSQIP tool was not necessarily aligned with their institution’s actual treatment selection for stage I NSCLC, which they based upon a number of factors. “This study potentially has important clinical implications,” according to Dr. Xiaofei Wang and Dr. Mark F. Berry in their invited commentary (J Thorac Cardiovasc Surg. 2016 Mar;151:706-7). “This present study shows that even a robust, well-managed tool from the NSQIP does not adequately stratify surgical risk... Their analysis implies that the treatment decision made by the institutional clinicians is optimal.”
“The lackluster performance of the NSQIP score is understandable, because it was not designed to optimally differentiate patients who benefited most from surgery or SBRT. Randomized clinical trials or well-controlled prospective observations are needed to develop and validate specific predictive tools for optimal treatment selection. These models must consider not only treatment morbidity, but also the cost of possible recurrence with each therapy,” Dr. Wang and Dr. Berry stated.
“Perhaps the most important conclusion that can be drawn from this present study is that current risk assessment tools can be helpful, but cannot replace evaluation by clinicians for whom all management options are available when therapy is chosen for a specific patient,” they concluded.
Dr. Wang is from the department of biostatistics and bioinformatics at Duke University, Durham, N.C., and Dr. Berry is from the department of cardiothoracic surgery, Stanford University, Stanford, Calif. They had no relevant financial disclosures.
In their reported study, Dr. Samson and her colleagues found that the NSQIP tool underestimated morbidity. They also found that risk predicted by the NSQIP tool was not necessarily aligned with their institution’s actual treatment selection for stage I NSCLC, which they based upon a number of factors. “This study potentially has important clinical implications,” according to Dr. Xiaofei Wang and Dr. Mark F. Berry in their invited commentary (J Thorac Cardiovasc Surg. 2016 Mar;151:706-7). “This present study shows that even a robust, well-managed tool from the NSQIP does not adequately stratify surgical risk... Their analysis implies that the treatment decision made by the institutional clinicians is optimal.”
“The lackluster performance of the NSQIP score is understandable, because it was not designed to optimally differentiate patients who benefited most from surgery or SBRT. Randomized clinical trials or well-controlled prospective observations are needed to develop and validate specific predictive tools for optimal treatment selection. These models must consider not only treatment morbidity, but also the cost of possible recurrence with each therapy,” Dr. Wang and Dr. Berry stated.
“Perhaps the most important conclusion that can be drawn from this present study is that current risk assessment tools can be helpful, but cannot replace evaluation by clinicians for whom all management options are available when therapy is chosen for a specific patient,” they concluded.
Dr. Wang is from the department of biostatistics and bioinformatics at Duke University, Durham, N.C., and Dr. Berry is from the department of cardiothoracic surgery, Stanford University, Stanford, Calif. They had no relevant financial disclosures.
In their reported study, Dr. Samson and her colleagues found that the NSQIP tool underestimated morbidity. They also found that risk predicted by the NSQIP tool was not necessarily aligned with their institution’s actual treatment selection for stage I NSCLC, which they based upon a number of factors. “This study potentially has important clinical implications,” according to Dr. Xiaofei Wang and Dr. Mark F. Berry in their invited commentary (J Thorac Cardiovasc Surg. 2016 Mar;151:706-7). “This present study shows that even a robust, well-managed tool from the NSQIP does not adequately stratify surgical risk... Their analysis implies that the treatment decision made by the institutional clinicians is optimal.”
“The lackluster performance of the NSQIP score is understandable, because it was not designed to optimally differentiate patients who benefited most from surgery or SBRT. Randomized clinical trials or well-controlled prospective observations are needed to develop and validate specific predictive tools for optimal treatment selection. These models must consider not only treatment morbidity, but also the cost of possible recurrence with each therapy,” Dr. Wang and Dr. Berry stated.
“Perhaps the most important conclusion that can be drawn from this present study is that current risk assessment tools can be helpful, but cannot replace evaluation by clinicians for whom all management options are available when therapy is chosen for a specific patient,” they concluded.
Dr. Wang is from the department of biostatistics and bioinformatics at Duke University, Durham, N.C., and Dr. Berry is from the department of cardiothoracic surgery, Stanford University, Stanford, Calif. They had no relevant financial disclosures.
A study performed to validate the National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator for use in patients receiving surgery or stereotactic body radiation therapy (SBRT) for stage I non–small cell lung cancer showed the calculator to be inadequate for both classification and risk stratification. The study was reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151;697-705).
Dr. Pamela Samson of Washington University in St. Louis and her colleagues performed a retrospective analysis of 485 patients with clinical stage I NSCLC who underwent either surgery (277) or SBRT (195) from 2009 to 2012. Surgery was either wedge resection (19.3%) or lobectomy (74.5%), with smaller percentages receiving segmentectomy (4.0%), pneumonectomy (1.5%), and bilobectomy (0.7%). A large majority of surgical patients (84.1%) underwent a video-assisted thoracoscopic surgery (VATS) approach.
The researchers calculated NSQIP complication risk estimates for both surgical and SBRT patients using the NSQIP Surgical Risk Calculator. They compared predicted risk with actual adverse events.
Compared with patients undergoing VATS wedge resection, patients receiving SBRT were older, had larger tumors, lower forced expiratory volume (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO), higher American Society of Anesthesiologist scores, higher rates of dyspnea and higher NSQIP serious complication risk estimates, all significant at P less than .05. Similar disparities were seen in comparing patients receiving SBRT vs. VATS lobectomy.
The actual serious complication rate for surgical patients was significantly higher than the NSQIP risk calculator prediction (16.6% vs. 8.8%), as was the rate of pneumonia (6.0% vs. 3.2%), both at P less than .05.
Overall, the NSQIP Surgical Risk Calculator provided a fair level of discrimination between VATS lobectomy and SBRT on receiver operating characteristic (ROC) curve analysis, but it was a poor model for differentiating between VATS wedge resection and SBRT. “Unfortunately, it is this latter population of the highest risk surgical patients (for whom a lobectomy is not a surgical option) where risk models and decision aids are needed most,” Dr. Samson and her colleagues stated.
“Counseling the high-risk but operable patient with clinical stage I NSCLC in regard to lobectomy, sublobar resection, or SBRT is challenging for both the clinician and the patient,” according to the researchers. “We believe that a model tailored to patients with clinical stage I needs to serve as both an estimator of operative risks and a patient decision aid for surgery versus SBRT, especially with projected increases in the number of early-stage lung cancers as a result of increased lung cancer screening efforts,” they added.
“Our analysis suggests that the NSQIP Surgical Risk Calculator likely does not profile the risk of a patient with lung cancer closely enough to dichotomize surgical and inoperable SBRT cases (especially when patients are being considered for a wedge resection) or adequately estimate a surgical patient’s risk of serious complications,” Dr. Samson and her colleagues concluded.
The study was supported by grants from National Institutes of Health. The authors had no relevant financial disclosures.
A study performed to validate the National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator for use in patients receiving surgery or stereotactic body radiation therapy (SBRT) for stage I non–small cell lung cancer showed the calculator to be inadequate for both classification and risk stratification. The study was reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151;697-705).
Dr. Pamela Samson of Washington University in St. Louis and her colleagues performed a retrospective analysis of 485 patients with clinical stage I NSCLC who underwent either surgery (277) or SBRT (195) from 2009 to 2012. Surgery was either wedge resection (19.3%) or lobectomy (74.5%), with smaller percentages receiving segmentectomy (4.0%), pneumonectomy (1.5%), and bilobectomy (0.7%). A large majority of surgical patients (84.1%) underwent a video-assisted thoracoscopic surgery (VATS) approach.
The researchers calculated NSQIP complication risk estimates for both surgical and SBRT patients using the NSQIP Surgical Risk Calculator. They compared predicted risk with actual adverse events.
Compared with patients undergoing VATS wedge resection, patients receiving SBRT were older, had larger tumors, lower forced expiratory volume (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO), higher American Society of Anesthesiologist scores, higher rates of dyspnea and higher NSQIP serious complication risk estimates, all significant at P less than .05. Similar disparities were seen in comparing patients receiving SBRT vs. VATS lobectomy.
The actual serious complication rate for surgical patients was significantly higher than the NSQIP risk calculator prediction (16.6% vs. 8.8%), as was the rate of pneumonia (6.0% vs. 3.2%), both at P less than .05.
Overall, the NSQIP Surgical Risk Calculator provided a fair level of discrimination between VATS lobectomy and SBRT on receiver operating characteristic (ROC) curve analysis, but it was a poor model for differentiating between VATS wedge resection and SBRT. “Unfortunately, it is this latter population of the highest risk surgical patients (for whom a lobectomy is not a surgical option) where risk models and decision aids are needed most,” Dr. Samson and her colleagues stated.
“Counseling the high-risk but operable patient with clinical stage I NSCLC in regard to lobectomy, sublobar resection, or SBRT is challenging for both the clinician and the patient,” according to the researchers. “We believe that a model tailored to patients with clinical stage I needs to serve as both an estimator of operative risks and a patient decision aid for surgery versus SBRT, especially with projected increases in the number of early-stage lung cancers as a result of increased lung cancer screening efforts,” they added.
“Our analysis suggests that the NSQIP Surgical Risk Calculator likely does not profile the risk of a patient with lung cancer closely enough to dichotomize surgical and inoperable SBRT cases (especially when patients are being considered for a wedge resection) or adequately estimate a surgical patient’s risk of serious complications,” Dr. Samson and her colleagues concluded.
The study was supported by grants from National Institutes of Health. The authors had no relevant financial disclosures.
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The current NSQIP Surgical Risk Calculator does not adequately estimate risk among patients with clinical stage I non–small cell lung cancer.
Major finding: The NSQIP risk calculator significantly underestimated serious complication risk in operative patients (16.6% actual risk vs. 8.8% predicted) and did not adequately stratify risk between surgical and stereotactic body radiation therapy (SBRT) patients.
Data source: Researchers retrospectively assessed 279 NSCLC stage I lung cancer patients who underwent surgery vs. 206 patients who underwent SBRT from 2009 to 2012.
Disclosures: The study was supported by grants from the National Institutes of Health. The authors had no relevant financial disclosures.
Study evaluates which prior cancers pose a risk for developing NSCLC
PHOENIX – Patients with a history of head and neck, lung, bladder, and hematologic malignancies had increased rates of subsequent non–small cell lung cancer (NSCLC), a large analysis of national data found.
“It is unclear to what extent the higher rate of primary NSCLC in these patients may be attributed to smoking, previous cancer history, or other lung cancer risk factors,” researchers led by Dr. Geena Wu wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons. “Further research using individual smoking data may better delineate who is at increased risk of NSCLC based on prior cancer site and smoking history.”
In a study that Dr. Wu led during a research fellowship at the City of Hope National Medical Center, Duarte, Calif., she and her associates used the Surveillance, Epidemiology, and End Results (SEER) 1992-2007 dataset to identify 32,058 patients with a prior malignancy who subsequently developed primary lung cancer at 6 months or more after their initial cancer. They calculated standardized incidence ratios (SIRs) for NSCLC as a rate of observed to expected NSCLC cases adjusted by person-years at risk, age, gender, and time of diagnosis.
The researchers found that patients with a history of the following cancers had higher rates of second primary NSCLC than expected: head and neck (SIR, 4.00), colon and rectum (SIR, 1.16), pancreas (SIR, 1.44), lung (SIR, 4.88), bladder (SIR, 1.97), kidney (SIR, 1.21), breast (SIR, 1.09), and leukemia or lymphoma (SIR, 1.40).
At the same time, patients with a history of pancreatic or breast cancer who were treated with radiation had a higher incidence of second primary NSCLC (SIR of 2.54 and SIR of 1.14, respectively), while those who were not treated with radiation did not.
Although the SEER database does not contain information about patient smoking history, the researchers evaluated adult smoking rates by state by using a national map from the Centers for Disease Control and Prevention’s 2013 Behavioral Risk Factor Surveillance System. Smoking rates were low (defined as 13.7% or less) in California, Hawaii, and Utah, and were higher in all other states, especially in Southeastern states. Dr. Wu and her associates found that patients from high smoking areas who had previous cancers of the colon and rectum, pancreas, kidney, thyroid, and breast had higher rates of a primary NSCLC, while those from low smoking areas did not. Interestingly, patients from high smoking areas who previously had uterine cancer, prostate, or melanoma had did not have higher rates of a primary NSCLC.
“Just because someone has a previous history of cancer, they’re not necessarily at increased risk of a second lung cancer,” Dr. Wu, who is now a fourth-year general surgery resident at Maricopa County Hospital in Phoenix said in an interview at the meeting. “You have to look at what kind of cancer they had and what their smoking history is – whether or not they continue to smoke, because smoking is such an important risk factor.”
Another limitation of the SEER database is that it lacks details about the type of chemotherapy patients receive, “so whether or not chemotherapy plays an impact in the elevated risk of lung cancer we can’t say.”
Dr. Wu reported having no financial disclosures.
PHOENIX – Patients with a history of head and neck, lung, bladder, and hematologic malignancies had increased rates of subsequent non–small cell lung cancer (NSCLC), a large analysis of national data found.
“It is unclear to what extent the higher rate of primary NSCLC in these patients may be attributed to smoking, previous cancer history, or other lung cancer risk factors,” researchers led by Dr. Geena Wu wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons. “Further research using individual smoking data may better delineate who is at increased risk of NSCLC based on prior cancer site and smoking history.”
In a study that Dr. Wu led during a research fellowship at the City of Hope National Medical Center, Duarte, Calif., she and her associates used the Surveillance, Epidemiology, and End Results (SEER) 1992-2007 dataset to identify 32,058 patients with a prior malignancy who subsequently developed primary lung cancer at 6 months or more after their initial cancer. They calculated standardized incidence ratios (SIRs) for NSCLC as a rate of observed to expected NSCLC cases adjusted by person-years at risk, age, gender, and time of diagnosis.
The researchers found that patients with a history of the following cancers had higher rates of second primary NSCLC than expected: head and neck (SIR, 4.00), colon and rectum (SIR, 1.16), pancreas (SIR, 1.44), lung (SIR, 4.88), bladder (SIR, 1.97), kidney (SIR, 1.21), breast (SIR, 1.09), and leukemia or lymphoma (SIR, 1.40).
At the same time, patients with a history of pancreatic or breast cancer who were treated with radiation had a higher incidence of second primary NSCLC (SIR of 2.54 and SIR of 1.14, respectively), while those who were not treated with radiation did not.
Although the SEER database does not contain information about patient smoking history, the researchers evaluated adult smoking rates by state by using a national map from the Centers for Disease Control and Prevention’s 2013 Behavioral Risk Factor Surveillance System. Smoking rates were low (defined as 13.7% or less) in California, Hawaii, and Utah, and were higher in all other states, especially in Southeastern states. Dr. Wu and her associates found that patients from high smoking areas who had previous cancers of the colon and rectum, pancreas, kidney, thyroid, and breast had higher rates of a primary NSCLC, while those from low smoking areas did not. Interestingly, patients from high smoking areas who previously had uterine cancer, prostate, or melanoma had did not have higher rates of a primary NSCLC.
“Just because someone has a previous history of cancer, they’re not necessarily at increased risk of a second lung cancer,” Dr. Wu, who is now a fourth-year general surgery resident at Maricopa County Hospital in Phoenix said in an interview at the meeting. “You have to look at what kind of cancer they had and what their smoking history is – whether or not they continue to smoke, because smoking is such an important risk factor.”
Another limitation of the SEER database is that it lacks details about the type of chemotherapy patients receive, “so whether or not chemotherapy plays an impact in the elevated risk of lung cancer we can’t say.”
Dr. Wu reported having no financial disclosures.
PHOENIX – Patients with a history of head and neck, lung, bladder, and hematologic malignancies had increased rates of subsequent non–small cell lung cancer (NSCLC), a large analysis of national data found.
“It is unclear to what extent the higher rate of primary NSCLC in these patients may be attributed to smoking, previous cancer history, or other lung cancer risk factors,” researchers led by Dr. Geena Wu wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons. “Further research using individual smoking data may better delineate who is at increased risk of NSCLC based on prior cancer site and smoking history.”
In a study that Dr. Wu led during a research fellowship at the City of Hope National Medical Center, Duarte, Calif., she and her associates used the Surveillance, Epidemiology, and End Results (SEER) 1992-2007 dataset to identify 32,058 patients with a prior malignancy who subsequently developed primary lung cancer at 6 months or more after their initial cancer. They calculated standardized incidence ratios (SIRs) for NSCLC as a rate of observed to expected NSCLC cases adjusted by person-years at risk, age, gender, and time of diagnosis.
The researchers found that patients with a history of the following cancers had higher rates of second primary NSCLC than expected: head and neck (SIR, 4.00), colon and rectum (SIR, 1.16), pancreas (SIR, 1.44), lung (SIR, 4.88), bladder (SIR, 1.97), kidney (SIR, 1.21), breast (SIR, 1.09), and leukemia or lymphoma (SIR, 1.40).
At the same time, patients with a history of pancreatic or breast cancer who were treated with radiation had a higher incidence of second primary NSCLC (SIR of 2.54 and SIR of 1.14, respectively), while those who were not treated with radiation did not.
Although the SEER database does not contain information about patient smoking history, the researchers evaluated adult smoking rates by state by using a national map from the Centers for Disease Control and Prevention’s 2013 Behavioral Risk Factor Surveillance System. Smoking rates were low (defined as 13.7% or less) in California, Hawaii, and Utah, and were higher in all other states, especially in Southeastern states. Dr. Wu and her associates found that patients from high smoking areas who had previous cancers of the colon and rectum, pancreas, kidney, thyroid, and breast had higher rates of a primary NSCLC, while those from low smoking areas did not. Interestingly, patients from high smoking areas who previously had uterine cancer, prostate, or melanoma had did not have higher rates of a primary NSCLC.
“Just because someone has a previous history of cancer, they’re not necessarily at increased risk of a second lung cancer,” Dr. Wu, who is now a fourth-year general surgery resident at Maricopa County Hospital in Phoenix said in an interview at the meeting. “You have to look at what kind of cancer they had and what their smoking history is – whether or not they continue to smoke, because smoking is such an important risk factor.”
Another limitation of the SEER database is that it lacks details about the type of chemotherapy patients receive, “so whether or not chemotherapy plays an impact in the elevated risk of lung cancer we can’t say.”
Dr. Wu reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point: Not all patients with history of cancer face an increased risk of developing subsequent NSCLC.
Major finding: Patients with a history of the following cancers had higher rates of second primary NSCLC than expected: head and neck (SIR, 4.00), colon and rectum (SIR, 1.16), pancreas (SIR, 1.44), lung (SIR, 4.88), bladder (SIR, 1.97), kidney (SIR, 1.21), breast (SIR, 1.09), and leukemia or lymphoma (SIR, 1.40).
Data source: An analysis of 32,058 patients with a prior malignancy that subsequently developed primary lung cancer at 6 months or more after their initial cancer.
Disclosures: Dr. Wu reported having no financial disclosures.
Cola enhances absorption of erlotinib in NSCLC
Drinking cola significantly improved bioavailability of the orally administered tyrosine kinase inhibitor (TKI) erlotinib in patients with lung cancer who were concomitantly taking the acid-reducing agent esomeprazole, investigators reported online in the Journal of Clinical Oncology.
Mean exposure of erlotinib was significantly higher after drinking cola, compared with water in patients treated concomitantly with esomeprazole (area under the plasma concentration curve, AUC0-12h was 39% higher; range, –12% to +136%; P = .004 and Cmax was 42% higher; range, –4% to +199%; P = .019), probably due to increased solubility and absorption. In patients treated with erlotinib only (without esomeprazole), exposure was moderately increased with cola intake (AUC0-12h was 9% higher; range, –10% to +30%; P = .03 and Cmax was comparable; range, –19% to +18%; P = .75).
Use of proton pump inhibitors (PPIs) is often indicated during erlotinib therapy for patients with gastroesophageal reflux disease, or for patients treated with corticosteroids and nonsteroidal anti-inflammatory drugs.
“When erlotinib and a PPI are given concomitantly, the AUC of erlotinib steeply decreases, which suggests that lower bioavailability due to PPI use (up to 46% for erlotinib) may deprive patients from optimal therapy. Thus, in the case that the combination of a PPI and erlotinib is inevitable, the pH-lowering effects of cola may help physicians to optimize erlotinib therapy,” wrote Dr. Roelof van Leeuwen of Erasmus MC Cancer Institute, Rotterdam, the Netherlands (J Clin Oncol. 2016 Feb 7. doi: 10.1200/JCO.205.65.1158).
The researchers noted that Coca-Cola Classic has a substantially lower pH (about 2.5) than other acidic drinks, such as orange juice (pH about 4), 7-Up (pH about 3.5), and diet colas (pH about 3-4), making it well suited for use with erlotinib, since drinks with higher pH may not enhance absorption as well. Patients had 250 mL of cola, a volume that was well tolerated.
Previous studies have shown that erlotinib has significant intrasubject and intersubject variability, and intragastric pH is an important determinant. The drug’s pKa, at 5.4, is near the stomach pH range of 1 to 4, and intragastric pH changes lead to shifts toward the nonionized (less soluble) form and subsequent lower bioavailability than TKIs with higher pKa values.
The results with erlotinib might extrapolate to other TKIs with pH-dependent solubility, such as dasatinib, gefitinib, nilotinib, the authors suggested, which should be tested in future studies.
Drinking cola significantly improved bioavailability of the orally administered tyrosine kinase inhibitor (TKI) erlotinib in patients with lung cancer who were concomitantly taking the acid-reducing agent esomeprazole, investigators reported online in the Journal of Clinical Oncology.
Mean exposure of erlotinib was significantly higher after drinking cola, compared with water in patients treated concomitantly with esomeprazole (area under the plasma concentration curve, AUC0-12h was 39% higher; range, –12% to +136%; P = .004 and Cmax was 42% higher; range, –4% to +199%; P = .019), probably due to increased solubility and absorption. In patients treated with erlotinib only (without esomeprazole), exposure was moderately increased with cola intake (AUC0-12h was 9% higher; range, –10% to +30%; P = .03 and Cmax was comparable; range, –19% to +18%; P = .75).
Use of proton pump inhibitors (PPIs) is often indicated during erlotinib therapy for patients with gastroesophageal reflux disease, or for patients treated with corticosteroids and nonsteroidal anti-inflammatory drugs.
“When erlotinib and a PPI are given concomitantly, the AUC of erlotinib steeply decreases, which suggests that lower bioavailability due to PPI use (up to 46% for erlotinib) may deprive patients from optimal therapy. Thus, in the case that the combination of a PPI and erlotinib is inevitable, the pH-lowering effects of cola may help physicians to optimize erlotinib therapy,” wrote Dr. Roelof van Leeuwen of Erasmus MC Cancer Institute, Rotterdam, the Netherlands (J Clin Oncol. 2016 Feb 7. doi: 10.1200/JCO.205.65.1158).
The researchers noted that Coca-Cola Classic has a substantially lower pH (about 2.5) than other acidic drinks, such as orange juice (pH about 4), 7-Up (pH about 3.5), and diet colas (pH about 3-4), making it well suited for use with erlotinib, since drinks with higher pH may not enhance absorption as well. Patients had 250 mL of cola, a volume that was well tolerated.
Previous studies have shown that erlotinib has significant intrasubject and intersubject variability, and intragastric pH is an important determinant. The drug’s pKa, at 5.4, is near the stomach pH range of 1 to 4, and intragastric pH changes lead to shifts toward the nonionized (less soluble) form and subsequent lower bioavailability than TKIs with higher pKa values.
The results with erlotinib might extrapolate to other TKIs with pH-dependent solubility, such as dasatinib, gefitinib, nilotinib, the authors suggested, which should be tested in future studies.
Drinking cola significantly improved bioavailability of the orally administered tyrosine kinase inhibitor (TKI) erlotinib in patients with lung cancer who were concomitantly taking the acid-reducing agent esomeprazole, investigators reported online in the Journal of Clinical Oncology.
Mean exposure of erlotinib was significantly higher after drinking cola, compared with water in patients treated concomitantly with esomeprazole (area under the plasma concentration curve, AUC0-12h was 39% higher; range, –12% to +136%; P = .004 and Cmax was 42% higher; range, –4% to +199%; P = .019), probably due to increased solubility and absorption. In patients treated with erlotinib only (without esomeprazole), exposure was moderately increased with cola intake (AUC0-12h was 9% higher; range, –10% to +30%; P = .03 and Cmax was comparable; range, –19% to +18%; P = .75).
Use of proton pump inhibitors (PPIs) is often indicated during erlotinib therapy for patients with gastroesophageal reflux disease, or for patients treated with corticosteroids and nonsteroidal anti-inflammatory drugs.
“When erlotinib and a PPI are given concomitantly, the AUC of erlotinib steeply decreases, which suggests that lower bioavailability due to PPI use (up to 46% for erlotinib) may deprive patients from optimal therapy. Thus, in the case that the combination of a PPI and erlotinib is inevitable, the pH-lowering effects of cola may help physicians to optimize erlotinib therapy,” wrote Dr. Roelof van Leeuwen of Erasmus MC Cancer Institute, Rotterdam, the Netherlands (J Clin Oncol. 2016 Feb 7. doi: 10.1200/JCO.205.65.1158).
The researchers noted that Coca-Cola Classic has a substantially lower pH (about 2.5) than other acidic drinks, such as orange juice (pH about 4), 7-Up (pH about 3.5), and diet colas (pH about 3-4), making it well suited for use with erlotinib, since drinks with higher pH may not enhance absorption as well. Patients had 250 mL of cola, a volume that was well tolerated.
Previous studies have shown that erlotinib has significant intrasubject and intersubject variability, and intragastric pH is an important determinant. The drug’s pKa, at 5.4, is near the stomach pH range of 1 to 4, and intragastric pH changes lead to shifts toward the nonionized (less soluble) form and subsequent lower bioavailability than TKIs with higher pKa values.
The results with erlotinib might extrapolate to other TKIs with pH-dependent solubility, such as dasatinib, gefitinib, nilotinib, the authors suggested, which should be tested in future studies.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Taking oral erlotinib with cola significantly increased the drug’s bioavailability in patients concomitantly taking the acid-reducing agent esomeprazole.
Major finding: Patients treated with erlotinib and esomeprazole had significantly better absorption after drinking cola, compared with water (AUC0-12h was 39% higher; range, –12% to +136%; P = .004 and Cmax was 42% higher; range, –4% to +199%; P = .019).
Data source: 28 evaluable patients with lung cancer; 14 received erlotinib and esomeprazole and 14 received erlotinib only.
Disclosures: Research was supported by Stichting de Merel and Roche. Dr. van Leeuwen reported research funding from Roche. Several of his coauthors reported ties to industry.
Intense tumor lymphocytic infiltration indicates favorable prognosis in NSCLC
Tumor lymphocytic infiltration (TLI), categorized as intense or nonintense, was an independent prognostic indicator for survival in non–small cell lung cancer (NSCLC).
Patients with intense TLI had significantly longer overall survival (OS) and disease-free survival (DFS), compared with patients who had nonintense TLI. In the validation data set, 5-year OS for patients with intense TLI was 85% (95% confidence interval, 70-92), compared with 58% (95% CI, 54-62) for patients with nonintense TLI (P = .002). Five-year DFS was 79% (95% CI, 65-88) for intense and 50% (95% CI, 47-54) for nonintense TLI (P = .001).
The retrospective study evaluated data from four randomized clinical trials, separated into a discovery set of 783 patient samples and a validation set of 763 patient samples. The LACE-Bio (Lung Adjuvant Cisplatin Evaluation Biomarker) collaborative group trials examined the benefit of platinum-based adjuvant chemotherapy in NSCLC. The median follow-up for the discovery and validation sets were 4.8 and 6.0 years, respectively.
Differences in outcomes according to TLI were significant in both discovery and validation data sets. In the discovery set, hazard ratios for OS and DFS were 0.56 (95% CI, 0.39-0.81; P = .002) and 0.59 (95% CI, 0.42-0.83; P = .002), respectively. In the validation set, OS and DFS hazard ratios were 0.45 (95% CI, 0.23-0.85; P = .01) and 0.44 (95% CI, 0.24-0.78; P = .005), respectively. Differences in risk reductions between the two data sets may be a result of differences in trial populations.
“The results raise the question about whether lymphocytic infiltration should be considered a stratification factor in trials that test immunotherapy or immunomodulation. Therefore, as suggested recently for CD8 density level in NSCLC, which predicted survival independently of all other variables and within each pathologic stage, intense lymphocytic infiltration could be a good candidate marker for establishing a TNM immunoscore,” wrote Dr. Elisabeth Brambilla of Institut Albert Bonniot–Institut National de la Santé et de la Recherche Médicale, La Tronche, France, and her colleagues (J Clin Onc. 2016 Feb. 1. doi: 10.1200/JCO.2015.63.0970).
In contrast to results from breast cancer studies, TLI did not predict differential survival benefit from adjuvant chemotherapy in NSCLC.
The intensity of TLI on hematoxylin- and eosin-stained representative sections was first assigned into one of four categories (minimal, mild, moderate, and intense). The first three categories subsequently were collapsed into one to form a binary scoring system of intense and nonintense infiltration.
Tumor lymphocytic infiltration (TLI), categorized as intense or nonintense, was an independent prognostic indicator for survival in non–small cell lung cancer (NSCLC).
Patients with intense TLI had significantly longer overall survival (OS) and disease-free survival (DFS), compared with patients who had nonintense TLI. In the validation data set, 5-year OS for patients with intense TLI was 85% (95% confidence interval, 70-92), compared with 58% (95% CI, 54-62) for patients with nonintense TLI (P = .002). Five-year DFS was 79% (95% CI, 65-88) for intense and 50% (95% CI, 47-54) for nonintense TLI (P = .001).
The retrospective study evaluated data from four randomized clinical trials, separated into a discovery set of 783 patient samples and a validation set of 763 patient samples. The LACE-Bio (Lung Adjuvant Cisplatin Evaluation Biomarker) collaborative group trials examined the benefit of platinum-based adjuvant chemotherapy in NSCLC. The median follow-up for the discovery and validation sets were 4.8 and 6.0 years, respectively.
Differences in outcomes according to TLI were significant in both discovery and validation data sets. In the discovery set, hazard ratios for OS and DFS were 0.56 (95% CI, 0.39-0.81; P = .002) and 0.59 (95% CI, 0.42-0.83; P = .002), respectively. In the validation set, OS and DFS hazard ratios were 0.45 (95% CI, 0.23-0.85; P = .01) and 0.44 (95% CI, 0.24-0.78; P = .005), respectively. Differences in risk reductions between the two data sets may be a result of differences in trial populations.
“The results raise the question about whether lymphocytic infiltration should be considered a stratification factor in trials that test immunotherapy or immunomodulation. Therefore, as suggested recently for CD8 density level in NSCLC, which predicted survival independently of all other variables and within each pathologic stage, intense lymphocytic infiltration could be a good candidate marker for establishing a TNM immunoscore,” wrote Dr. Elisabeth Brambilla of Institut Albert Bonniot–Institut National de la Santé et de la Recherche Médicale, La Tronche, France, and her colleagues (J Clin Onc. 2016 Feb. 1. doi: 10.1200/JCO.2015.63.0970).
In contrast to results from breast cancer studies, TLI did not predict differential survival benefit from adjuvant chemotherapy in NSCLC.
The intensity of TLI on hematoxylin- and eosin-stained representative sections was first assigned into one of four categories (minimal, mild, moderate, and intense). The first three categories subsequently were collapsed into one to form a binary scoring system of intense and nonintense infiltration.
Tumor lymphocytic infiltration (TLI), categorized as intense or nonintense, was an independent prognostic indicator for survival in non–small cell lung cancer (NSCLC).
Patients with intense TLI had significantly longer overall survival (OS) and disease-free survival (DFS), compared with patients who had nonintense TLI. In the validation data set, 5-year OS for patients with intense TLI was 85% (95% confidence interval, 70-92), compared with 58% (95% CI, 54-62) for patients with nonintense TLI (P = .002). Five-year DFS was 79% (95% CI, 65-88) for intense and 50% (95% CI, 47-54) for nonintense TLI (P = .001).
The retrospective study evaluated data from four randomized clinical trials, separated into a discovery set of 783 patient samples and a validation set of 763 patient samples. The LACE-Bio (Lung Adjuvant Cisplatin Evaluation Biomarker) collaborative group trials examined the benefit of platinum-based adjuvant chemotherapy in NSCLC. The median follow-up for the discovery and validation sets were 4.8 and 6.0 years, respectively.
Differences in outcomes according to TLI were significant in both discovery and validation data sets. In the discovery set, hazard ratios for OS and DFS were 0.56 (95% CI, 0.39-0.81; P = .002) and 0.59 (95% CI, 0.42-0.83; P = .002), respectively. In the validation set, OS and DFS hazard ratios were 0.45 (95% CI, 0.23-0.85; P = .01) and 0.44 (95% CI, 0.24-0.78; P = .005), respectively. Differences in risk reductions between the two data sets may be a result of differences in trial populations.
“The results raise the question about whether lymphocytic infiltration should be considered a stratification factor in trials that test immunotherapy or immunomodulation. Therefore, as suggested recently for CD8 density level in NSCLC, which predicted survival independently of all other variables and within each pathologic stage, intense lymphocytic infiltration could be a good candidate marker for establishing a TNM immunoscore,” wrote Dr. Elisabeth Brambilla of Institut Albert Bonniot–Institut National de la Santé et de la Recherche Médicale, La Tronche, France, and her colleagues (J Clin Onc. 2016 Feb. 1. doi: 10.1200/JCO.2015.63.0970).
In contrast to results from breast cancer studies, TLI did not predict differential survival benefit from adjuvant chemotherapy in NSCLC.
The intensity of TLI on hematoxylin- and eosin-stained representative sections was first assigned into one of four categories (minimal, mild, moderate, and intense). The first three categories subsequently were collapsed into one to form a binary scoring system of intense and nonintense infiltration.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Intense tumor lymphocytic infiltration (TLI) predicted longer overall and disease-free survival in patients with non–small cell lung cancer.
Major finding: Five-year overall survival for patients with intense TLI was 85% (95% CI, 70-92), compared with 58% (95% CI, 54-62) for patients with nonintense TLI (P = .002).
Data source: Retrospective study using LACE-Bio (Lung Adjuvant Cisplatin Evaluation Biomarker) collaborative group data from four randomized clinical trials of platinum-based adjuvant chemotherapy, with 783 patient samples in the discovery set and 763 patient samples in the validation set.
Disclosures: Dr. Brambilla reported having no disclosures. Several of her coauthors reported ties to industry.