User login
Could nivolumab prevent oral cancer in high-risk patients?
(PL), a high-risk precancerous disease, into oral cancer, suggest the results from a phase 2 study.
“We think that immunotherapy as a preventative strategy, either as first-line or even secondary prevention, should be further explored,” said lead researcher Glenn J. Hanna, MD, director, Center for Salivary and Rare Head and Neck Cancers, Dana-Farber Cancer Institute, Boston.
The research was presented at the European Society for Medical Oncology Annual Congress in Paris.
Oral leukoplakia refers to a white plaque of “questionable cancer risk” that affects about 4% of the global population, Dr. Hanna explained. However, about 5% of leukoplakia cases develop into oral proliferative leukoplakia, an aggressive form of the disease characterized by multifocal lesions. It has a high risk of transformation to oral squamous cell carcinoma (OSCC), at approaching 10% per year, and the 5-year cancer-free survival rate is estimated to be 47%.
While there are no effective therapies to prevent progression to oral cancer, the condition does have a “rich immune microenvironment,” potentially making it amenable to programmed death (PD)-1 blockade, Dr. Hanna said.
His team conducted a single-arm, phase 2 trial involving 33 patients with proliferative leukoplakia with greater than or equal to 2 multifocal lesions, or contiguous lesions of greater than or equal to 3 cm, or a single lesion greater than or equal to 4 cm with any degree of epithelial dysplasia. The median age was 63.2 years, and 55% were women. Just over half (52%) were never smokers.
The main disease subsite was the oral tongue in 39% of participants, followed by the buccal gingiva in 30%, and 24% of patients had a prior diagnosis of OSCC.
Following a pretreatment biopsy at one to three sites, the patients received four doses of nivolumab every 28 days, followed by rebiopsy. At each visit, the patients had intraoral photographs taken of the lesions and measurements taken.
The median time from study registration to the first dose of nivolumab was 9 days. The majority (88%) of patients completed all four doses of nivolumab.
The median time from the first dose of nivolumab to the posttreatment biopsy was 115 days and ranged from 29 to 171 days.
The overall response rate, defined as a greater than or equal to 40% decrease in a composite score combining the size and degree of dysplasia between the pre- and posttreatment assessments, was observed in 36.4% of patients.
After a median follow-up of 14.7 months, the median cancer-free survival was not reached, with cancer events recorded in 21.2% of patients. The median time from the last dose of nivolumab to the first OSCC event was 3.7 months.
Cancer-free survival at 1 year was calculated to be 77.7%, which was unchanged at 2 years. At the final follow-up, all patients were still alive.
Additional analysis of the biopsies revealed that the lesions had programmed death ligand 1 (PD-L1) combined positive scores that ranged from 0 to 80, with 66.7% of patients having a score of greater than or equal to 1. A cutoff score of greater than or equal to 20 did not reveal any significant differences in cancer-free survival rates.
Turning to safety, Dr. Hanna said that nivolumab was associated with “acceptable toxicity” in this “non-cancer population,” with 21.2% of patients experiencing a grade 3-4 adverse event.
The most common adverse events of any grade were fatigue (55%), diarrhea (27%), elevated alanine transaminase levels (18%), elevated aspartate transaminase levels (18%), and other skin disorders (18%).
With a relatively low rate of adverse events and a “clinical benefit” in up to a third of patients, Dr. Hanna said that this was the “first study to our knowledge to demonstrate the potential efficacy of anti–PD-L1 blockade among patients with a high-risk oral precancerous disease.”
Discussing this study at the meeting, Amanda Psyrri, MD, PhD, professor of medical oncology, Attikon University Hospital, Athens, who was not involved in the research, said these data were “very interesting,” but she expressed some reservations over the way the study was conducted.
She said that the composite score to measure response rates was “defined arbitrarily,” its prognostic value “has not been demonstrated,” and also pointed out that mixed responses by lesions within the same patient led to changes in scores.
In addition, the time interval between the end of treatment and lesion rebiopsy was “highly variable,” and the follow-up period was short.
Consequently, Dr. Psyrri believes the importance of the findings is “unclear,” especially as several patients who responded to nivolumab went on to develop cancer anyway, a finding that needs further investigation.
The study was funded by Bristol Myers Squibb.
Dr. Hanna declared relationships with BMS, Bicara, Exicure, Gateway for Cancer Research, GSK, Kite, NantKwest, Regeneron, Sanofi Genzyme, Maverick, and Merck.
A version of this article first appeared on Medscape.com.
(PL), a high-risk precancerous disease, into oral cancer, suggest the results from a phase 2 study.
“We think that immunotherapy as a preventative strategy, either as first-line or even secondary prevention, should be further explored,” said lead researcher Glenn J. Hanna, MD, director, Center for Salivary and Rare Head and Neck Cancers, Dana-Farber Cancer Institute, Boston.
The research was presented at the European Society for Medical Oncology Annual Congress in Paris.
Oral leukoplakia refers to a white plaque of “questionable cancer risk” that affects about 4% of the global population, Dr. Hanna explained. However, about 5% of leukoplakia cases develop into oral proliferative leukoplakia, an aggressive form of the disease characterized by multifocal lesions. It has a high risk of transformation to oral squamous cell carcinoma (OSCC), at approaching 10% per year, and the 5-year cancer-free survival rate is estimated to be 47%.
While there are no effective therapies to prevent progression to oral cancer, the condition does have a “rich immune microenvironment,” potentially making it amenable to programmed death (PD)-1 blockade, Dr. Hanna said.
His team conducted a single-arm, phase 2 trial involving 33 patients with proliferative leukoplakia with greater than or equal to 2 multifocal lesions, or contiguous lesions of greater than or equal to 3 cm, or a single lesion greater than or equal to 4 cm with any degree of epithelial dysplasia. The median age was 63.2 years, and 55% were women. Just over half (52%) were never smokers.
The main disease subsite was the oral tongue in 39% of participants, followed by the buccal gingiva in 30%, and 24% of patients had a prior diagnosis of OSCC.
Following a pretreatment biopsy at one to three sites, the patients received four doses of nivolumab every 28 days, followed by rebiopsy. At each visit, the patients had intraoral photographs taken of the lesions and measurements taken.
The median time from study registration to the first dose of nivolumab was 9 days. The majority (88%) of patients completed all four doses of nivolumab.
The median time from the first dose of nivolumab to the posttreatment biopsy was 115 days and ranged from 29 to 171 days.
The overall response rate, defined as a greater than or equal to 40% decrease in a composite score combining the size and degree of dysplasia between the pre- and posttreatment assessments, was observed in 36.4% of patients.
After a median follow-up of 14.7 months, the median cancer-free survival was not reached, with cancer events recorded in 21.2% of patients. The median time from the last dose of nivolumab to the first OSCC event was 3.7 months.
Cancer-free survival at 1 year was calculated to be 77.7%, which was unchanged at 2 years. At the final follow-up, all patients were still alive.
Additional analysis of the biopsies revealed that the lesions had programmed death ligand 1 (PD-L1) combined positive scores that ranged from 0 to 80, with 66.7% of patients having a score of greater than or equal to 1. A cutoff score of greater than or equal to 20 did not reveal any significant differences in cancer-free survival rates.
Turning to safety, Dr. Hanna said that nivolumab was associated with “acceptable toxicity” in this “non-cancer population,” with 21.2% of patients experiencing a grade 3-4 adverse event.
The most common adverse events of any grade were fatigue (55%), diarrhea (27%), elevated alanine transaminase levels (18%), elevated aspartate transaminase levels (18%), and other skin disorders (18%).
With a relatively low rate of adverse events and a “clinical benefit” in up to a third of patients, Dr. Hanna said that this was the “first study to our knowledge to demonstrate the potential efficacy of anti–PD-L1 blockade among patients with a high-risk oral precancerous disease.”
Discussing this study at the meeting, Amanda Psyrri, MD, PhD, professor of medical oncology, Attikon University Hospital, Athens, who was not involved in the research, said these data were “very interesting,” but she expressed some reservations over the way the study was conducted.
She said that the composite score to measure response rates was “defined arbitrarily,” its prognostic value “has not been demonstrated,” and also pointed out that mixed responses by lesions within the same patient led to changes in scores.
In addition, the time interval between the end of treatment and lesion rebiopsy was “highly variable,” and the follow-up period was short.
Consequently, Dr. Psyrri believes the importance of the findings is “unclear,” especially as several patients who responded to nivolumab went on to develop cancer anyway, a finding that needs further investigation.
The study was funded by Bristol Myers Squibb.
Dr. Hanna declared relationships with BMS, Bicara, Exicure, Gateway for Cancer Research, GSK, Kite, NantKwest, Regeneron, Sanofi Genzyme, Maverick, and Merck.
A version of this article first appeared on Medscape.com.
(PL), a high-risk precancerous disease, into oral cancer, suggest the results from a phase 2 study.
“We think that immunotherapy as a preventative strategy, either as first-line or even secondary prevention, should be further explored,” said lead researcher Glenn J. Hanna, MD, director, Center for Salivary and Rare Head and Neck Cancers, Dana-Farber Cancer Institute, Boston.
The research was presented at the European Society for Medical Oncology Annual Congress in Paris.
Oral leukoplakia refers to a white plaque of “questionable cancer risk” that affects about 4% of the global population, Dr. Hanna explained. However, about 5% of leukoplakia cases develop into oral proliferative leukoplakia, an aggressive form of the disease characterized by multifocal lesions. It has a high risk of transformation to oral squamous cell carcinoma (OSCC), at approaching 10% per year, and the 5-year cancer-free survival rate is estimated to be 47%.
While there are no effective therapies to prevent progression to oral cancer, the condition does have a “rich immune microenvironment,” potentially making it amenable to programmed death (PD)-1 blockade, Dr. Hanna said.
His team conducted a single-arm, phase 2 trial involving 33 patients with proliferative leukoplakia with greater than or equal to 2 multifocal lesions, or contiguous lesions of greater than or equal to 3 cm, or a single lesion greater than or equal to 4 cm with any degree of epithelial dysplasia. The median age was 63.2 years, and 55% were women. Just over half (52%) were never smokers.
The main disease subsite was the oral tongue in 39% of participants, followed by the buccal gingiva in 30%, and 24% of patients had a prior diagnosis of OSCC.
Following a pretreatment biopsy at one to three sites, the patients received four doses of nivolumab every 28 days, followed by rebiopsy. At each visit, the patients had intraoral photographs taken of the lesions and measurements taken.
The median time from study registration to the first dose of nivolumab was 9 days. The majority (88%) of patients completed all four doses of nivolumab.
The median time from the first dose of nivolumab to the posttreatment biopsy was 115 days and ranged from 29 to 171 days.
The overall response rate, defined as a greater than or equal to 40% decrease in a composite score combining the size and degree of dysplasia between the pre- and posttreatment assessments, was observed in 36.4% of patients.
After a median follow-up of 14.7 months, the median cancer-free survival was not reached, with cancer events recorded in 21.2% of patients. The median time from the last dose of nivolumab to the first OSCC event was 3.7 months.
Cancer-free survival at 1 year was calculated to be 77.7%, which was unchanged at 2 years. At the final follow-up, all patients were still alive.
Additional analysis of the biopsies revealed that the lesions had programmed death ligand 1 (PD-L1) combined positive scores that ranged from 0 to 80, with 66.7% of patients having a score of greater than or equal to 1. A cutoff score of greater than or equal to 20 did not reveal any significant differences in cancer-free survival rates.
Turning to safety, Dr. Hanna said that nivolumab was associated with “acceptable toxicity” in this “non-cancer population,” with 21.2% of patients experiencing a grade 3-4 adverse event.
The most common adverse events of any grade were fatigue (55%), diarrhea (27%), elevated alanine transaminase levels (18%), elevated aspartate transaminase levels (18%), and other skin disorders (18%).
With a relatively low rate of adverse events and a “clinical benefit” in up to a third of patients, Dr. Hanna said that this was the “first study to our knowledge to demonstrate the potential efficacy of anti–PD-L1 blockade among patients with a high-risk oral precancerous disease.”
Discussing this study at the meeting, Amanda Psyrri, MD, PhD, professor of medical oncology, Attikon University Hospital, Athens, who was not involved in the research, said these data were “very interesting,” but she expressed some reservations over the way the study was conducted.
She said that the composite score to measure response rates was “defined arbitrarily,” its prognostic value “has not been demonstrated,” and also pointed out that mixed responses by lesions within the same patient led to changes in scores.
In addition, the time interval between the end of treatment and lesion rebiopsy was “highly variable,” and the follow-up period was short.
Consequently, Dr. Psyrri believes the importance of the findings is “unclear,” especially as several patients who responded to nivolumab went on to develop cancer anyway, a finding that needs further investigation.
The study was funded by Bristol Myers Squibb.
Dr. Hanna declared relationships with BMS, Bicara, Exicure, Gateway for Cancer Research, GSK, Kite, NantKwest, Regeneron, Sanofi Genzyme, Maverick, and Merck.
A version of this article first appeared on Medscape.com.
In NSCLC, not all EGFR mutations are the same
In non–small cell lung cancer (NSCLC), . However, there is a range of different EGFR mutations, and different mutation combinations can lead to different tumor characteristics that might in turn affect response to therapy.
A new real-world analysis of 159 NSCLC patients found that a combination of a mutation of the TP53 tumor suppressor gene and the EGFR Ex20 mutation is associated with worse disease outcomes, compared to patients with the EGFR Ex20 mutation alone. But the news wasn’t all bad. The same group of patients also responded better to ICB (immune checkpoint blockade) therapy than did the broader population of EGFR Ex20 patients.
The EGFR Ex20 mutation occurs in about 4% of NSCLC cases, while TP53 is quite common: The new study found a frequency of 43.9%. “We first have to mention that the findings regarding TP53 do not reach statistical significance; however, the trend is very strong, and results might be hampered due to small sample sizes. We think it is [appropriate] to exhaust more treatment options for these patients, especially targeted approaches with newer drugs that specifically target exon 20 insertions, as these drugs were not applied in our cohort,” Anna Kron, Dr. rer. medic., said in an email exchange. Dr. Kron presented the results at a poster session in Paris at the ESMO Congress. She is a researcher at University Hospital of Cologne, Germany.
The ImmunoTarget study, published in 2019, examined over 500 NSCLC patients with a range of driver mutations including EGFR and found that they responded poorly to ICIs in comparison to KRAS mutations.
But Dr. Kron’s group was not convinced. “Ex20 mutations differ clinically from other tyrosine kinase mutations in EGFR. We set out this study to rechallenge the paradigm of impaired benefit from ICI in EGFR-mutated patients, as we consider these mutations not interchangeable with other EGFR mutations,” Dr. Kron said.
“We would postulate that in EGFR Exon 20 mutations, ICI and specific inhibitors should be part of the therapeutic course. In patients with co-occurring TP53 mutations, treatment escalation could be considered,” Dr. Kron said.
The study included 159 patients with advanced NSCLC with the EGFR exon 20 insertion, who were treated between 2014 and 2020 at German hospitals. Among the patients, 37.7% were female; mean age at diagnosis was 65.87 years; 50.3% had a smoking history and 38.4% did not (data were unavailable for the rest); and 9.4% of tumors were stage I, 4.4% stage II, 8.2% stage IIIA, 3.8% stage IIIB, and 74.2% stage IV.
Over a follow-up of 4.1 years, there was a trend toward longer survival among patients with TP53 wild type (OS, 20 versus 12 months; P = .092). Sixty-six patients who received ICI therapy had better OS compared with those who did not (22 versus 10 months; P = .018). Among patients with co-occurring TP53 mutations, receipt of ICI therapy was associated with longer OS (16 versus 8 months; P = .048). There was a trend toward patients with TP53 wild type treated with ICI faring better than those who didn’t receive ICI (27.0 months versus 11.0 months; P = .109).
The researchers are continuing to study patients with EGFR Ex20 to better understand the role of TP53 and ICI therapy in these patients.
The study received no funding. Dr. Kron has no relevant financial disclosures.
In non–small cell lung cancer (NSCLC), . However, there is a range of different EGFR mutations, and different mutation combinations can lead to different tumor characteristics that might in turn affect response to therapy.
A new real-world analysis of 159 NSCLC patients found that a combination of a mutation of the TP53 tumor suppressor gene and the EGFR Ex20 mutation is associated with worse disease outcomes, compared to patients with the EGFR Ex20 mutation alone. But the news wasn’t all bad. The same group of patients also responded better to ICB (immune checkpoint blockade) therapy than did the broader population of EGFR Ex20 patients.
The EGFR Ex20 mutation occurs in about 4% of NSCLC cases, while TP53 is quite common: The new study found a frequency of 43.9%. “We first have to mention that the findings regarding TP53 do not reach statistical significance; however, the trend is very strong, and results might be hampered due to small sample sizes. We think it is [appropriate] to exhaust more treatment options for these patients, especially targeted approaches with newer drugs that specifically target exon 20 insertions, as these drugs were not applied in our cohort,” Anna Kron, Dr. rer. medic., said in an email exchange. Dr. Kron presented the results at a poster session in Paris at the ESMO Congress. She is a researcher at University Hospital of Cologne, Germany.
The ImmunoTarget study, published in 2019, examined over 500 NSCLC patients with a range of driver mutations including EGFR and found that they responded poorly to ICIs in comparison to KRAS mutations.
But Dr. Kron’s group was not convinced. “Ex20 mutations differ clinically from other tyrosine kinase mutations in EGFR. We set out this study to rechallenge the paradigm of impaired benefit from ICI in EGFR-mutated patients, as we consider these mutations not interchangeable with other EGFR mutations,” Dr. Kron said.
“We would postulate that in EGFR Exon 20 mutations, ICI and specific inhibitors should be part of the therapeutic course. In patients with co-occurring TP53 mutations, treatment escalation could be considered,” Dr. Kron said.
The study included 159 patients with advanced NSCLC with the EGFR exon 20 insertion, who were treated between 2014 and 2020 at German hospitals. Among the patients, 37.7% were female; mean age at diagnosis was 65.87 years; 50.3% had a smoking history and 38.4% did not (data were unavailable for the rest); and 9.4% of tumors were stage I, 4.4% stage II, 8.2% stage IIIA, 3.8% stage IIIB, and 74.2% stage IV.
Over a follow-up of 4.1 years, there was a trend toward longer survival among patients with TP53 wild type (OS, 20 versus 12 months; P = .092). Sixty-six patients who received ICI therapy had better OS compared with those who did not (22 versus 10 months; P = .018). Among patients with co-occurring TP53 mutations, receipt of ICI therapy was associated with longer OS (16 versus 8 months; P = .048). There was a trend toward patients with TP53 wild type treated with ICI faring better than those who didn’t receive ICI (27.0 months versus 11.0 months; P = .109).
The researchers are continuing to study patients with EGFR Ex20 to better understand the role of TP53 and ICI therapy in these patients.
The study received no funding. Dr. Kron has no relevant financial disclosures.
In non–small cell lung cancer (NSCLC), . However, there is a range of different EGFR mutations, and different mutation combinations can lead to different tumor characteristics that might in turn affect response to therapy.
A new real-world analysis of 159 NSCLC patients found that a combination of a mutation of the TP53 tumor suppressor gene and the EGFR Ex20 mutation is associated with worse disease outcomes, compared to patients with the EGFR Ex20 mutation alone. But the news wasn’t all bad. The same group of patients also responded better to ICB (immune checkpoint blockade) therapy than did the broader population of EGFR Ex20 patients.
The EGFR Ex20 mutation occurs in about 4% of NSCLC cases, while TP53 is quite common: The new study found a frequency of 43.9%. “We first have to mention that the findings regarding TP53 do not reach statistical significance; however, the trend is very strong, and results might be hampered due to small sample sizes. We think it is [appropriate] to exhaust more treatment options for these patients, especially targeted approaches with newer drugs that specifically target exon 20 insertions, as these drugs were not applied in our cohort,” Anna Kron, Dr. rer. medic., said in an email exchange. Dr. Kron presented the results at a poster session in Paris at the ESMO Congress. She is a researcher at University Hospital of Cologne, Germany.
The ImmunoTarget study, published in 2019, examined over 500 NSCLC patients with a range of driver mutations including EGFR and found that they responded poorly to ICIs in comparison to KRAS mutations.
But Dr. Kron’s group was not convinced. “Ex20 mutations differ clinically from other tyrosine kinase mutations in EGFR. We set out this study to rechallenge the paradigm of impaired benefit from ICI in EGFR-mutated patients, as we consider these mutations not interchangeable with other EGFR mutations,” Dr. Kron said.
“We would postulate that in EGFR Exon 20 mutations, ICI and specific inhibitors should be part of the therapeutic course. In patients with co-occurring TP53 mutations, treatment escalation could be considered,” Dr. Kron said.
The study included 159 patients with advanced NSCLC with the EGFR exon 20 insertion, who were treated between 2014 and 2020 at German hospitals. Among the patients, 37.7% were female; mean age at diagnosis was 65.87 years; 50.3% had a smoking history and 38.4% did not (data were unavailable for the rest); and 9.4% of tumors were stage I, 4.4% stage II, 8.2% stage IIIA, 3.8% stage IIIB, and 74.2% stage IV.
Over a follow-up of 4.1 years, there was a trend toward longer survival among patients with TP53 wild type (OS, 20 versus 12 months; P = .092). Sixty-six patients who received ICI therapy had better OS compared with those who did not (22 versus 10 months; P = .018). Among patients with co-occurring TP53 mutations, receipt of ICI therapy was associated with longer OS (16 versus 8 months; P = .048). There was a trend toward patients with TP53 wild type treated with ICI faring better than those who didn’t receive ICI (27.0 months versus 11.0 months; P = .109).
The researchers are continuing to study patients with EGFR Ex20 to better understand the role of TP53 and ICI therapy in these patients.
The study received no funding. Dr. Kron has no relevant financial disclosures.
FROM ESMO CONGRESS 2022
In early NSCLC, comorbidities linked to survival
Cardiometabolic and respiratory comorbidities are associated with worse survival in patients with non–small cell lung cancer (NSCLC), and new research suggests a potential mechanism.
Prior studies had shown mixed results when it came to these comorbidities and survival, according to study coauthor author Geoffrey Liu, MD, who is an epidemiology researcher at the University of Toronto Princess Margaret Cancer Centre. The new work represents data from multiple continents, from various ethnicities and cultures.
“We found that comorbidities had much greater impact on earlier than later stages of lung cancer, consistent with this previous study,” said Dr. Liu in an email. The study was presented by Miguel Garcia-Pardo, who is a researcher at University of Toronto Princess Margaret Cancer Centre, during a poster session at the annual meeting of the European Society for Medical Oncology.
“Deaths from [cardiometabolic] comorbidities were mainly from non–lung cancer competing causes, whereas the deaths from respiratory comorbidities were primarily driven by lung cancer specific survival, i.e., deaths from lung cancer itself. We conclude that Dr. Liu said.
Dr. Liu noted that controlling cardiometabolic risk factors like diabetes and hypertension is typically de-emphasized after diagnosis with early-stage lung cancer. The rationale is often that the lung cancer is a more acute concern than longer-term cardiometabolic risks. “The data from our analyses suggest a rethinking of this strategy. We need to pay more attention to controlling cardiovascular risk factors in early-stage lung cancer,” Dr. Liu said.
The findings also suggest that respiratory comorbidities should be managed more aggressively. That would allow more patients to undergo treatments like surgery and stereotactic radiation.
The Clinical Outcome Studies of the International Lung Cancer Consortium drew from two dozen studies conducted across five continents. It examined clinical, epidemiologic, genetic, and genomic factors and their potential influence on NSCLC outcomes. Cardiometabolic comorbidities included coronary artery disease, diabetes, vascular related diseases, and other heart diseases. Respiratory comorbidities included chronic obstructive pulmonary disease and asthma.
The analysis included 16,354 patients. Among patients with stage I NSCLC, there was an association between reduced overall survival (OS) and cardiometabolic comorbidity (adjusted hazard ratio, 1.17; P = .01) and respiratory comorbidity (aHR, 1.36; P < .001). For stage II/III patients, there was no significant association between OS and cardiometabolic comorbidities, but respiratory comorbidity was associated with worse OS (aHR, 1.15; P < .001). In stage 4, worse OS was associated with both cardiometabolic health comorbidity (aHR, 1.11; P = .03), but not respiratory comorbidity.
Among patients with stage IV NSCLC, there were no associations between overall survival or lung cancer–specific survival (LCSS) and respiratory or cardiometabolic risk factors. However, an examination of cause of death found a different pattern in patients with stage IB-IIIA disease: LCSS was worse among patients with respiratory comorbidities (aHR, 1.21; 95% CI, 1.09-1.34). Among those with cardiovascular comorbidities, the risk of non-NSCLC mortality was higher (aHR, 1.36; 95% CI, 1.15-1.63). The presence of respiratory comorbidity was associated with a reduced probability of undergoing surgical resection for both stage I (adjusted odds ratio, 0.45; 95% CI, 0.35-0.59) and stage II/III patients (aOR, 0.66; 95% CI, 0.53-0.80).
There was an association between non-NSCLC mortality and cardiometabolic comorbidities in stage IA (aHR, 1.37; 95% CI, 1.06-1.77) and in stages IB-IIIA (aHR, 1.32; 95% CI, 1.03-1.71) NSCLC. There were also associations between NSCLC mortality and respiratory comorbidity among stage IA (aHR, 1.51; 95% CI, 1.17-1.95) and stages IB-IIIA (aHR, 1.20; 95% CI, 1.06-1.36) NSCLC. There were no associations between respiratory comorbidity and non-NSCLC mortality.
Respiratory comorbidity was associated with a lower chance of undergoing surgical resection in stage IA (aHR, 0.54; 95% CI, 0.35-0.83) and stage IB-IIIA (aHR, 0.57; 95% CI, 0.46-0.70) cancers. Cardiometabolic comorbidity was associated with a lower rate of surgical resection only in stage 1B-3A patients (aHR, 0.73; 95% CI, 0.56-0.96). Among those who underwent resection, stage IA patients were less likely to die of lung cancer (aHR, 0.38; 95% CI, 0.28-0.52) but more likely to die of other causes (aHR, 1.73; 95% CI, 1.07-1.78). Stage IB-IIIA patients who underwent resection were less likely to die of lung cancer (aHR, 0.37; 95%, 0.32-0.42), but there was no significant association with non–lung cancer mortality.
The study was funded by the Lusi Wong Family Fund and the Alan Brown Chair. Dr. Liu has no relevant financial disclosures.
Cardiometabolic and respiratory comorbidities are associated with worse survival in patients with non–small cell lung cancer (NSCLC), and new research suggests a potential mechanism.
Prior studies had shown mixed results when it came to these comorbidities and survival, according to study coauthor author Geoffrey Liu, MD, who is an epidemiology researcher at the University of Toronto Princess Margaret Cancer Centre. The new work represents data from multiple continents, from various ethnicities and cultures.
“We found that comorbidities had much greater impact on earlier than later stages of lung cancer, consistent with this previous study,” said Dr. Liu in an email. The study was presented by Miguel Garcia-Pardo, who is a researcher at University of Toronto Princess Margaret Cancer Centre, during a poster session at the annual meeting of the European Society for Medical Oncology.
“Deaths from [cardiometabolic] comorbidities were mainly from non–lung cancer competing causes, whereas the deaths from respiratory comorbidities were primarily driven by lung cancer specific survival, i.e., deaths from lung cancer itself. We conclude that Dr. Liu said.
Dr. Liu noted that controlling cardiometabolic risk factors like diabetes and hypertension is typically de-emphasized after diagnosis with early-stage lung cancer. The rationale is often that the lung cancer is a more acute concern than longer-term cardiometabolic risks. “The data from our analyses suggest a rethinking of this strategy. We need to pay more attention to controlling cardiovascular risk factors in early-stage lung cancer,” Dr. Liu said.
The findings also suggest that respiratory comorbidities should be managed more aggressively. That would allow more patients to undergo treatments like surgery and stereotactic radiation.
The Clinical Outcome Studies of the International Lung Cancer Consortium drew from two dozen studies conducted across five continents. It examined clinical, epidemiologic, genetic, and genomic factors and their potential influence on NSCLC outcomes. Cardiometabolic comorbidities included coronary artery disease, diabetes, vascular related diseases, and other heart diseases. Respiratory comorbidities included chronic obstructive pulmonary disease and asthma.
The analysis included 16,354 patients. Among patients with stage I NSCLC, there was an association between reduced overall survival (OS) and cardiometabolic comorbidity (adjusted hazard ratio, 1.17; P = .01) and respiratory comorbidity (aHR, 1.36; P < .001). For stage II/III patients, there was no significant association between OS and cardiometabolic comorbidities, but respiratory comorbidity was associated with worse OS (aHR, 1.15; P < .001). In stage 4, worse OS was associated with both cardiometabolic health comorbidity (aHR, 1.11; P = .03), but not respiratory comorbidity.
Among patients with stage IV NSCLC, there were no associations between overall survival or lung cancer–specific survival (LCSS) and respiratory or cardiometabolic risk factors. However, an examination of cause of death found a different pattern in patients with stage IB-IIIA disease: LCSS was worse among patients with respiratory comorbidities (aHR, 1.21; 95% CI, 1.09-1.34). Among those with cardiovascular comorbidities, the risk of non-NSCLC mortality was higher (aHR, 1.36; 95% CI, 1.15-1.63). The presence of respiratory comorbidity was associated with a reduced probability of undergoing surgical resection for both stage I (adjusted odds ratio, 0.45; 95% CI, 0.35-0.59) and stage II/III patients (aOR, 0.66; 95% CI, 0.53-0.80).
There was an association between non-NSCLC mortality and cardiometabolic comorbidities in stage IA (aHR, 1.37; 95% CI, 1.06-1.77) and in stages IB-IIIA (aHR, 1.32; 95% CI, 1.03-1.71) NSCLC. There were also associations between NSCLC mortality and respiratory comorbidity among stage IA (aHR, 1.51; 95% CI, 1.17-1.95) and stages IB-IIIA (aHR, 1.20; 95% CI, 1.06-1.36) NSCLC. There were no associations between respiratory comorbidity and non-NSCLC mortality.
Respiratory comorbidity was associated with a lower chance of undergoing surgical resection in stage IA (aHR, 0.54; 95% CI, 0.35-0.83) and stage IB-IIIA (aHR, 0.57; 95% CI, 0.46-0.70) cancers. Cardiometabolic comorbidity was associated with a lower rate of surgical resection only in stage 1B-3A patients (aHR, 0.73; 95% CI, 0.56-0.96). Among those who underwent resection, stage IA patients were less likely to die of lung cancer (aHR, 0.38; 95% CI, 0.28-0.52) but more likely to die of other causes (aHR, 1.73; 95% CI, 1.07-1.78). Stage IB-IIIA patients who underwent resection were less likely to die of lung cancer (aHR, 0.37; 95%, 0.32-0.42), but there was no significant association with non–lung cancer mortality.
The study was funded by the Lusi Wong Family Fund and the Alan Brown Chair. Dr. Liu has no relevant financial disclosures.
Cardiometabolic and respiratory comorbidities are associated with worse survival in patients with non–small cell lung cancer (NSCLC), and new research suggests a potential mechanism.
Prior studies had shown mixed results when it came to these comorbidities and survival, according to study coauthor author Geoffrey Liu, MD, who is an epidemiology researcher at the University of Toronto Princess Margaret Cancer Centre. The new work represents data from multiple continents, from various ethnicities and cultures.
“We found that comorbidities had much greater impact on earlier than later stages of lung cancer, consistent with this previous study,” said Dr. Liu in an email. The study was presented by Miguel Garcia-Pardo, who is a researcher at University of Toronto Princess Margaret Cancer Centre, during a poster session at the annual meeting of the European Society for Medical Oncology.
“Deaths from [cardiometabolic] comorbidities were mainly from non–lung cancer competing causes, whereas the deaths from respiratory comorbidities were primarily driven by lung cancer specific survival, i.e., deaths from lung cancer itself. We conclude that Dr. Liu said.
Dr. Liu noted that controlling cardiometabolic risk factors like diabetes and hypertension is typically de-emphasized after diagnosis with early-stage lung cancer. The rationale is often that the lung cancer is a more acute concern than longer-term cardiometabolic risks. “The data from our analyses suggest a rethinking of this strategy. We need to pay more attention to controlling cardiovascular risk factors in early-stage lung cancer,” Dr. Liu said.
The findings also suggest that respiratory comorbidities should be managed more aggressively. That would allow more patients to undergo treatments like surgery and stereotactic radiation.
The Clinical Outcome Studies of the International Lung Cancer Consortium drew from two dozen studies conducted across five continents. It examined clinical, epidemiologic, genetic, and genomic factors and their potential influence on NSCLC outcomes. Cardiometabolic comorbidities included coronary artery disease, diabetes, vascular related diseases, and other heart diseases. Respiratory comorbidities included chronic obstructive pulmonary disease and asthma.
The analysis included 16,354 patients. Among patients with stage I NSCLC, there was an association between reduced overall survival (OS) and cardiometabolic comorbidity (adjusted hazard ratio, 1.17; P = .01) and respiratory comorbidity (aHR, 1.36; P < .001). For stage II/III patients, there was no significant association between OS and cardiometabolic comorbidities, but respiratory comorbidity was associated with worse OS (aHR, 1.15; P < .001). In stage 4, worse OS was associated with both cardiometabolic health comorbidity (aHR, 1.11; P = .03), but not respiratory comorbidity.
Among patients with stage IV NSCLC, there were no associations between overall survival or lung cancer–specific survival (LCSS) and respiratory or cardiometabolic risk factors. However, an examination of cause of death found a different pattern in patients with stage IB-IIIA disease: LCSS was worse among patients with respiratory comorbidities (aHR, 1.21; 95% CI, 1.09-1.34). Among those with cardiovascular comorbidities, the risk of non-NSCLC mortality was higher (aHR, 1.36; 95% CI, 1.15-1.63). The presence of respiratory comorbidity was associated with a reduced probability of undergoing surgical resection for both stage I (adjusted odds ratio, 0.45; 95% CI, 0.35-0.59) and stage II/III patients (aOR, 0.66; 95% CI, 0.53-0.80).
There was an association between non-NSCLC mortality and cardiometabolic comorbidities in stage IA (aHR, 1.37; 95% CI, 1.06-1.77) and in stages IB-IIIA (aHR, 1.32; 95% CI, 1.03-1.71) NSCLC. There were also associations between NSCLC mortality and respiratory comorbidity among stage IA (aHR, 1.51; 95% CI, 1.17-1.95) and stages IB-IIIA (aHR, 1.20; 95% CI, 1.06-1.36) NSCLC. There were no associations between respiratory comorbidity and non-NSCLC mortality.
Respiratory comorbidity was associated with a lower chance of undergoing surgical resection in stage IA (aHR, 0.54; 95% CI, 0.35-0.83) and stage IB-IIIA (aHR, 0.57; 95% CI, 0.46-0.70) cancers. Cardiometabolic comorbidity was associated with a lower rate of surgical resection only in stage 1B-3A patients (aHR, 0.73; 95% CI, 0.56-0.96). Among those who underwent resection, stage IA patients were less likely to die of lung cancer (aHR, 0.38; 95% CI, 0.28-0.52) but more likely to die of other causes (aHR, 1.73; 95% CI, 1.07-1.78). Stage IB-IIIA patients who underwent resection were less likely to die of lung cancer (aHR, 0.37; 95%, 0.32-0.42), but there was no significant association with non–lung cancer mortality.
The study was funded by the Lusi Wong Family Fund and the Alan Brown Chair. Dr. Liu has no relevant financial disclosures.
FROM ESMO CONGRESS 2022
‘Smoking gun–level’ evidence found linking air pollution with lung cancer
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
AT ESMO CONGRESS 2022
Gene mutations may drive lung cancer in never-smokers
Small cell lung cancer has traditionally been attributed almost exclusively to tobacco exposure, but some recent studies have suggested a higher than expected prevalence among nonsmokers. indicating that the subgroups may have unique disease characteristics. Key differences included a lower frequency of TP53 gene mutations and a higher frequency of epidermal growth factor receptor (EGFR) alterations in never smokers.
About 6.9% of small cell lung cancer patients in the CASPIAN study were nonsmokers, as were 3.0% in the IMpower133 study.
“Given that the pathogenesis of small cell lung cancer is often tied to the damaging effects of tobacco, we hypothesized that small cell lung cancer in never-smokers would possess distinct molecular attributes. Our data does not provide any solid evidence for any treatment implications, though it does raise therapeutic questions which we believe deserve further exploration,” said Michael Oh, MD, during a presentation of the study results at the annual meeting of the European Society for Medical Oncology. Dr. Oh is a fellow at the University of California, Los Angeles.
The topic is important clinically, according to Antonio Passaro, MD, PhD, who served as a discussant during the session. He noted that small cell lung cancer in never-smokers is the seventh-most common cause of cancer-related mortality worldwide. In non–small cell lung cancer, rates of tobacco-associated disease have been decreasing, but there are increases in diagnoses among never smokers. Nonsmoking small cell lung cancer patients do not have better prognoses, and novel therapies and advances like immunotherapy and low-dose CT lung cancer screening disproportionately benefit current or former smokers.
Potential risk factors for never-smokers include environmental exposures like radon gas, cooking oil vapors, indoor and outdoor wood burning, and genetic and viral factors. “At the present time we do not have the knowledge to identify the most important factor in development of lung cancer in never-smoking [patients],” said Dr. Passaro, who is a medical oncologist at the European Institute of Oncology in Milan.
He added that the current study results are interesting but need much more follow-up, such as “longitudinal studies combining detailed clinical annotation with tissue and blood sampling. Here there is a need for collaborative efforts.” Key questions include the roles of the genomic landscape in normal lung tissue may play, the lung micro-environment, genetic factors, and environmental exposures.
One key possibility is air pollution. “We know that lung cancer in never-smokers is frequent in some countries, for example in Asian countries and it is more frequent in the United States than in Europe, but to find an explanation to this kind of data is difficult at the present time,” Dr. Passaro said.
The researchers retrospectively analyzed data from 608 current or former smokers and 54 never-smokers with small cell lung cancer, with the latter making up 8% of the total population. 70.4% of never-smokers and 55.1% of current or former smokers were female (P = .031). There was no significant between-group difference with respect to age at diagnosis or race.
Somatic mutations were similar to what has been found in previous studies for current or former smokers. 85.2% had changes in TP53, compared with just 59.3% of never-smokers (Q < .001). Changes to EGFR were more common in never-smokers, occurring in 25.9% versus 2.6% (Q < .001). PIK3CA alterations were also more common in never-smokers (14.8% vs. 3.6%; Q = 0.022). There was no significant difference between the two groups with respect to changes in RB1.
Never smokers had tumors with less immune cell infiltration (P = .008), including fewer CD4+ T cells, CD8+ T cells, and macrophages. Their tumor mutation burden was also lower (median, 2.59 vs. 4.99; P < .001).
Dr. Oh has no relevant financial disclosures. Dr. Passaro has consulted, advised, and received research funding from a wide range of pharmaceutical companies.
Small cell lung cancer has traditionally been attributed almost exclusively to tobacco exposure, but some recent studies have suggested a higher than expected prevalence among nonsmokers. indicating that the subgroups may have unique disease characteristics. Key differences included a lower frequency of TP53 gene mutations and a higher frequency of epidermal growth factor receptor (EGFR) alterations in never smokers.
About 6.9% of small cell lung cancer patients in the CASPIAN study were nonsmokers, as were 3.0% in the IMpower133 study.
“Given that the pathogenesis of small cell lung cancer is often tied to the damaging effects of tobacco, we hypothesized that small cell lung cancer in never-smokers would possess distinct molecular attributes. Our data does not provide any solid evidence for any treatment implications, though it does raise therapeutic questions which we believe deserve further exploration,” said Michael Oh, MD, during a presentation of the study results at the annual meeting of the European Society for Medical Oncology. Dr. Oh is a fellow at the University of California, Los Angeles.
The topic is important clinically, according to Antonio Passaro, MD, PhD, who served as a discussant during the session. He noted that small cell lung cancer in never-smokers is the seventh-most common cause of cancer-related mortality worldwide. In non–small cell lung cancer, rates of tobacco-associated disease have been decreasing, but there are increases in diagnoses among never smokers. Nonsmoking small cell lung cancer patients do not have better prognoses, and novel therapies and advances like immunotherapy and low-dose CT lung cancer screening disproportionately benefit current or former smokers.
Potential risk factors for never-smokers include environmental exposures like radon gas, cooking oil vapors, indoor and outdoor wood burning, and genetic and viral factors. “At the present time we do not have the knowledge to identify the most important factor in development of lung cancer in never-smoking [patients],” said Dr. Passaro, who is a medical oncologist at the European Institute of Oncology in Milan.
He added that the current study results are interesting but need much more follow-up, such as “longitudinal studies combining detailed clinical annotation with tissue and blood sampling. Here there is a need for collaborative efforts.” Key questions include the roles of the genomic landscape in normal lung tissue may play, the lung micro-environment, genetic factors, and environmental exposures.
One key possibility is air pollution. “We know that lung cancer in never-smokers is frequent in some countries, for example in Asian countries and it is more frequent in the United States than in Europe, but to find an explanation to this kind of data is difficult at the present time,” Dr. Passaro said.
The researchers retrospectively analyzed data from 608 current or former smokers and 54 never-smokers with small cell lung cancer, with the latter making up 8% of the total population. 70.4% of never-smokers and 55.1% of current or former smokers were female (P = .031). There was no significant between-group difference with respect to age at diagnosis or race.
Somatic mutations were similar to what has been found in previous studies for current or former smokers. 85.2% had changes in TP53, compared with just 59.3% of never-smokers (Q < .001). Changes to EGFR were more common in never-smokers, occurring in 25.9% versus 2.6% (Q < .001). PIK3CA alterations were also more common in never-smokers (14.8% vs. 3.6%; Q = 0.022). There was no significant difference between the two groups with respect to changes in RB1.
Never smokers had tumors with less immune cell infiltration (P = .008), including fewer CD4+ T cells, CD8+ T cells, and macrophages. Their tumor mutation burden was also lower (median, 2.59 vs. 4.99; P < .001).
Dr. Oh has no relevant financial disclosures. Dr. Passaro has consulted, advised, and received research funding from a wide range of pharmaceutical companies.
Small cell lung cancer has traditionally been attributed almost exclusively to tobacco exposure, but some recent studies have suggested a higher than expected prevalence among nonsmokers. indicating that the subgroups may have unique disease characteristics. Key differences included a lower frequency of TP53 gene mutations and a higher frequency of epidermal growth factor receptor (EGFR) alterations in never smokers.
About 6.9% of small cell lung cancer patients in the CASPIAN study were nonsmokers, as were 3.0% in the IMpower133 study.
“Given that the pathogenesis of small cell lung cancer is often tied to the damaging effects of tobacco, we hypothesized that small cell lung cancer in never-smokers would possess distinct molecular attributes. Our data does not provide any solid evidence for any treatment implications, though it does raise therapeutic questions which we believe deserve further exploration,” said Michael Oh, MD, during a presentation of the study results at the annual meeting of the European Society for Medical Oncology. Dr. Oh is a fellow at the University of California, Los Angeles.
The topic is important clinically, according to Antonio Passaro, MD, PhD, who served as a discussant during the session. He noted that small cell lung cancer in never-smokers is the seventh-most common cause of cancer-related mortality worldwide. In non–small cell lung cancer, rates of tobacco-associated disease have been decreasing, but there are increases in diagnoses among never smokers. Nonsmoking small cell lung cancer patients do not have better prognoses, and novel therapies and advances like immunotherapy and low-dose CT lung cancer screening disproportionately benefit current or former smokers.
Potential risk factors for never-smokers include environmental exposures like radon gas, cooking oil vapors, indoor and outdoor wood burning, and genetic and viral factors. “At the present time we do not have the knowledge to identify the most important factor in development of lung cancer in never-smoking [patients],” said Dr. Passaro, who is a medical oncologist at the European Institute of Oncology in Milan.
He added that the current study results are interesting but need much more follow-up, such as “longitudinal studies combining detailed clinical annotation with tissue and blood sampling. Here there is a need for collaborative efforts.” Key questions include the roles of the genomic landscape in normal lung tissue may play, the lung micro-environment, genetic factors, and environmental exposures.
One key possibility is air pollution. “We know that lung cancer in never-smokers is frequent in some countries, for example in Asian countries and it is more frequent in the United States than in Europe, but to find an explanation to this kind of data is difficult at the present time,” Dr. Passaro said.
The researchers retrospectively analyzed data from 608 current or former smokers and 54 never-smokers with small cell lung cancer, with the latter making up 8% of the total population. 70.4% of never-smokers and 55.1% of current or former smokers were female (P = .031). There was no significant between-group difference with respect to age at diagnosis or race.
Somatic mutations were similar to what has been found in previous studies for current or former smokers. 85.2% had changes in TP53, compared with just 59.3% of never-smokers (Q < .001). Changes to EGFR were more common in never-smokers, occurring in 25.9% versus 2.6% (Q < .001). PIK3CA alterations were also more common in never-smokers (14.8% vs. 3.6%; Q = 0.022). There was no significant difference between the two groups with respect to changes in RB1.
Never smokers had tumors with less immune cell infiltration (P = .008), including fewer CD4+ T cells, CD8+ T cells, and macrophages. Their tumor mutation burden was also lower (median, 2.59 vs. 4.99; P < .001).
Dr. Oh has no relevant financial disclosures. Dr. Passaro has consulted, advised, and received research funding from a wide range of pharmaceutical companies.
AT ESMO CONGRESS 2022
Reporting Coronary Artery Calcium on Low-Dose Computed Tomography Impacts Statin Management in a Lung Cancer Screening Population
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
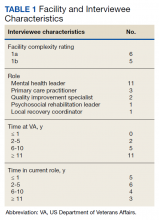
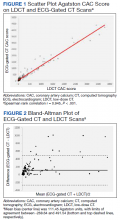
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
Evaluating Progression Free Survival Among Veteran Population With Stage IV Non-Small Cell Immunotherapy vs Chemo- Immunotherapy
Background
Use of immune checkpoint inhibitors against advanced stage NSCLC has been associated with significant reduction in overall disease morbidity and mortality. However, despite the significant survival benefit, tumors invariably relapse. It is important to understand the pattern of progression and the progression free survival (PFS) to better predict disease outcomes and modify treatment approach.
Methods
We performed a retrospective review of 74 veterans with new diagnosis of stage IV NSCLC who received 2 or more cycles of immunotherapy with/without concurrent chemotherapy between 2015-2021 at the Stratton VA Medical Center. IRB approval was obtained. Fisher exact probability test and Kaplan-Meier estimators were used to analyze data with level of significance P < .05.
Results
Out of 74 patients, 38 patients were identified who received immunotherapy alone (Group A; n = 23, 60.5%) vs chemo-immunotherapy (Group B; n = 15, 39.5%). Baseline characteristics of Group A revealed median age 70 (IQR, 65-78), adenocarcinoma (n = 10, 43.4%), squamous cell carcinoma (n = 12, 52.1%), PD-L1 > 50% expression (n = 21, 91.3%), molecular testing positive for EGFR in 1 patient, otherwise negative for ROS, ALK, EGFR and BRAF mutations in all patients. Similarly, in Group B, median age 66 (IQR, 63-72), adenocarcinoma (n = 6, 40%), squamous cell carcinoma (n = 8, 53.3%), PD-L1 > 50% expression (n = 3, 20%), no mutations noted on molecular testing. Out of 38 patients, disease progression was noted in 19 patients, 10 in Group A (progression at initial site and new site n = 5, 50%) vs 9 in Group B (progression at initial site and new site, n = 6, 66.7%). Most common sites of progression included local and distant lymph nodes, brain, bone, and liver. Using the Kaplan-Meier analysis, median progression free survival (PFS) from start of immunotherapy till evidence of progression on imaging was 11 months in Group A and 7 months in Group B, P = .22. Our study recognized widespread metastases at the time of diagnosis (P = .03) as a possible factor affecting progression of diseases in Group A compared to Group B.
Conclusion
We conclude that although no statistically significant association was noted between the progression free survival between the two groups, the increased median PFS in immunotherapy only group is worth additional investigation. We recommend further large-scale studies to explore this association.
Background
Use of immune checkpoint inhibitors against advanced stage NSCLC has been associated with significant reduction in overall disease morbidity and mortality. However, despite the significant survival benefit, tumors invariably relapse. It is important to understand the pattern of progression and the progression free survival (PFS) to better predict disease outcomes and modify treatment approach.
Methods
We performed a retrospective review of 74 veterans with new diagnosis of stage IV NSCLC who received 2 or more cycles of immunotherapy with/without concurrent chemotherapy between 2015-2021 at the Stratton VA Medical Center. IRB approval was obtained. Fisher exact probability test and Kaplan-Meier estimators were used to analyze data with level of significance P < .05.
Results
Out of 74 patients, 38 patients were identified who received immunotherapy alone (Group A; n = 23, 60.5%) vs chemo-immunotherapy (Group B; n = 15, 39.5%). Baseline characteristics of Group A revealed median age 70 (IQR, 65-78), adenocarcinoma (n = 10, 43.4%), squamous cell carcinoma (n = 12, 52.1%), PD-L1 > 50% expression (n = 21, 91.3%), molecular testing positive for EGFR in 1 patient, otherwise negative for ROS, ALK, EGFR and BRAF mutations in all patients. Similarly, in Group B, median age 66 (IQR, 63-72), adenocarcinoma (n = 6, 40%), squamous cell carcinoma (n = 8, 53.3%), PD-L1 > 50% expression (n = 3, 20%), no mutations noted on molecular testing. Out of 38 patients, disease progression was noted in 19 patients, 10 in Group A (progression at initial site and new site n = 5, 50%) vs 9 in Group B (progression at initial site and new site, n = 6, 66.7%). Most common sites of progression included local and distant lymph nodes, brain, bone, and liver. Using the Kaplan-Meier analysis, median progression free survival (PFS) from start of immunotherapy till evidence of progression on imaging was 11 months in Group A and 7 months in Group B, P = .22. Our study recognized widespread metastases at the time of diagnosis (P = .03) as a possible factor affecting progression of diseases in Group A compared to Group B.
Conclusion
We conclude that although no statistically significant association was noted between the progression free survival between the two groups, the increased median PFS in immunotherapy only group is worth additional investigation. We recommend further large-scale studies to explore this association.
Background
Use of immune checkpoint inhibitors against advanced stage NSCLC has been associated with significant reduction in overall disease morbidity and mortality. However, despite the significant survival benefit, tumors invariably relapse. It is important to understand the pattern of progression and the progression free survival (PFS) to better predict disease outcomes and modify treatment approach.
Methods
We performed a retrospective review of 74 veterans with new diagnosis of stage IV NSCLC who received 2 or more cycles of immunotherapy with/without concurrent chemotherapy between 2015-2021 at the Stratton VA Medical Center. IRB approval was obtained. Fisher exact probability test and Kaplan-Meier estimators were used to analyze data with level of significance P < .05.
Results
Out of 74 patients, 38 patients were identified who received immunotherapy alone (Group A; n = 23, 60.5%) vs chemo-immunotherapy (Group B; n = 15, 39.5%). Baseline characteristics of Group A revealed median age 70 (IQR, 65-78), adenocarcinoma (n = 10, 43.4%), squamous cell carcinoma (n = 12, 52.1%), PD-L1 > 50% expression (n = 21, 91.3%), molecular testing positive for EGFR in 1 patient, otherwise negative for ROS, ALK, EGFR and BRAF mutations in all patients. Similarly, in Group B, median age 66 (IQR, 63-72), adenocarcinoma (n = 6, 40%), squamous cell carcinoma (n = 8, 53.3%), PD-L1 > 50% expression (n = 3, 20%), no mutations noted on molecular testing. Out of 38 patients, disease progression was noted in 19 patients, 10 in Group A (progression at initial site and new site n = 5, 50%) vs 9 in Group B (progression at initial site and new site, n = 6, 66.7%). Most common sites of progression included local and distant lymph nodes, brain, bone, and liver. Using the Kaplan-Meier analysis, median progression free survival (PFS) from start of immunotherapy till evidence of progression on imaging was 11 months in Group A and 7 months in Group B, P = .22. Our study recognized widespread metastases at the time of diagnosis (P = .03) as a possible factor affecting progression of diseases in Group A compared to Group B.
Conclusion
We conclude that although no statistically significant association was noted between the progression free survival between the two groups, the increased median PFS in immunotherapy only group is worth additional investigation. We recommend further large-scale studies to explore this association.
MYO1E DNA Methylation in U.S. Military Veterans With Adenocarcinoma of the Lung Is Associated With Increased Mortality Risk
Project Purpose
The aim is to assess the role of MYO1E in survival among veterans with lung adenocarcinoma (LUAD).
Background
Veterans have a higher smoking exposure than civilians; a higher incidence of lung cancer; and a younger age at diagnosis of lung cancer. We recently showed that MYO1E DNA methylation and RNA expression in LUAD are associated with survival among civilians.
Methods
This is a retrospective cohort study involving LUAD among civilians and veterans with biopsy or pathologically proven LUAD from surgical specimens. DNA extraction and isolation from FFPE cancer tissues was performed using methylation-onbeads as previously published, followed by qMSP with bisulfite treatment to quantify DNA methylation. RNA extraction and quantification from lung tissues was obtained as described in previous publications.
Data Analysis
Differences were assessed with Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical. Two-tailed log-rank test was used to estimate overall survival differences and Cox hazard models, to quantify risk of mortality using hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
There were 91 LUAD patients, 27 veterans and 64 civilians. Veterans were older than civilians, aged 70 years vs aged 66 years (P = .003); with higher proportions of males, 93% vs 69% (P = .03); higher proportion of African Americans, 67% vs 39% (P = .03); smoking more, 50 pack-year vs 40 (0.005), and having a higher proportion of grade I, 78% vs 55% (P = .036). Survival was statistically longer for MYO1E high DNA methylation group 48 months vs 33 for low methylation (P = .049). MYO1E RNA expression did not show statistically significant differences (P = .32). Multivariate Cox regression analysis adjusted by age, veteran/civil status, gender, race, packyear, and stage showed that DNA methylation was significantly associated with mortality risk (HR 5.14; 95% CI, 1.12-23.60) (P = .035).
Conclusions/Implications
This study suggests the utility of MYO1E DNA methylation as a prognostic biomarker for veterans with LUAD. Further studies are necessary to understand the role of MYO1E in chemotherapy resistance and microenvironment immune modulation. Given the low expression of MYO1E in blood cells, MYO1E DNA methylation has the potential to be used as circulating tumor marker in liquid biopsies.
Project Purpose
The aim is to assess the role of MYO1E in survival among veterans with lung adenocarcinoma (LUAD).
Background
Veterans have a higher smoking exposure than civilians; a higher incidence of lung cancer; and a younger age at diagnosis of lung cancer. We recently showed that MYO1E DNA methylation and RNA expression in LUAD are associated with survival among civilians.
Methods
This is a retrospective cohort study involving LUAD among civilians and veterans with biopsy or pathologically proven LUAD from surgical specimens. DNA extraction and isolation from FFPE cancer tissues was performed using methylation-onbeads as previously published, followed by qMSP with bisulfite treatment to quantify DNA methylation. RNA extraction and quantification from lung tissues was obtained as described in previous publications.
Data Analysis
Differences were assessed with Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical. Two-tailed log-rank test was used to estimate overall survival differences and Cox hazard models, to quantify risk of mortality using hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
There were 91 LUAD patients, 27 veterans and 64 civilians. Veterans were older than civilians, aged 70 years vs aged 66 years (P = .003); with higher proportions of males, 93% vs 69% (P = .03); higher proportion of African Americans, 67% vs 39% (P = .03); smoking more, 50 pack-year vs 40 (0.005), and having a higher proportion of grade I, 78% vs 55% (P = .036). Survival was statistically longer for MYO1E high DNA methylation group 48 months vs 33 for low methylation (P = .049). MYO1E RNA expression did not show statistically significant differences (P = .32). Multivariate Cox regression analysis adjusted by age, veteran/civil status, gender, race, packyear, and stage showed that DNA methylation was significantly associated with mortality risk (HR 5.14; 95% CI, 1.12-23.60) (P = .035).
Conclusions/Implications
This study suggests the utility of MYO1E DNA methylation as a prognostic biomarker for veterans with LUAD. Further studies are necessary to understand the role of MYO1E in chemotherapy resistance and microenvironment immune modulation. Given the low expression of MYO1E in blood cells, MYO1E DNA methylation has the potential to be used as circulating tumor marker in liquid biopsies.
Project Purpose
The aim is to assess the role of MYO1E in survival among veterans with lung adenocarcinoma (LUAD).
Background
Veterans have a higher smoking exposure than civilians; a higher incidence of lung cancer; and a younger age at diagnosis of lung cancer. We recently showed that MYO1E DNA methylation and RNA expression in LUAD are associated with survival among civilians.
Methods
This is a retrospective cohort study involving LUAD among civilians and veterans with biopsy or pathologically proven LUAD from surgical specimens. DNA extraction and isolation from FFPE cancer tissues was performed using methylation-onbeads as previously published, followed by qMSP with bisulfite treatment to quantify DNA methylation. RNA extraction and quantification from lung tissues was obtained as described in previous publications.
Data Analysis
Differences were assessed with Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical. Two-tailed log-rank test was used to estimate overall survival differences and Cox hazard models, to quantify risk of mortality using hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
There were 91 LUAD patients, 27 veterans and 64 civilians. Veterans were older than civilians, aged 70 years vs aged 66 years (P = .003); with higher proportions of males, 93% vs 69% (P = .03); higher proportion of African Americans, 67% vs 39% (P = .03); smoking more, 50 pack-year vs 40 (0.005), and having a higher proportion of grade I, 78% vs 55% (P = .036). Survival was statistically longer for MYO1E high DNA methylation group 48 months vs 33 for low methylation (P = .049). MYO1E RNA expression did not show statistically significant differences (P = .32). Multivariate Cox regression analysis adjusted by age, veteran/civil status, gender, race, packyear, and stage showed that DNA methylation was significantly associated with mortality risk (HR 5.14; 95% CI, 1.12-23.60) (P = .035).
Conclusions/Implications
This study suggests the utility of MYO1E DNA methylation as a prognostic biomarker for veterans with LUAD. Further studies are necessary to understand the role of MYO1E in chemotherapy resistance and microenvironment immune modulation. Given the low expression of MYO1E in blood cells, MYO1E DNA methylation has the potential to be used as circulating tumor marker in liquid biopsies.
Molecular Profiling of Lung Malignancies in Veterans: What We Have Learned About the Impact of Agent Orange Exposure
Background
There are no studies in oncologic literature that report biomarker alterations in Vietnam War veterans with lung cancers. Our study elucidates genetic mutations in veterans with lung cancer exposed to Agent Orange (AO) and compares them to non-Agent Orange exposed (NAO) veterans.
Methods
We collected data of veterans with lung cancers from VA Central California Health Care System who had NGS testing via Foundation One CDx from January 2007 to January 2022. We collected data of AO versus NAO veterans including age, race, gender, smoking and exposure history, histologic subtypes, treatment modalities, PDL-1, and molecular mutations. Median PFS and OS were calculated between AO and NAO in all veterans and adenocarcinoma group after first-line therapy in months by Kaplan-Meier R log-rank test.
Results
There were total of 58 lung cancer veterans, 27 AO and 31 NAO. 33 (56.9%) veterans had adenocarcinoma (20 AO vs 13 NAO). Veterans were White (81%), male (93%) and all had tobacco exposure. The median age at diagnosis was 72 years in both groups. 65.5% had stage III-IV disease. Veterans with AO adenocarcinoma had more early stage I-II disease (50%) as compared to NAO (16%). The AO group had more PDL1 expression (TPS > 1%). 15/31 (48.4%) NAO received immunotherapy vs 7/27 (25.9%) AO. 104 molecular mutations were identified. Veterans with AO had more ROS1, MET, and NRAS while NAO had more EGFR, KRAS, and NF1 mutations. In adenocarcinoma group, AO had more MET and less KRAS while NAO has more KRAS, TP53, and EGFR. The median PFS and OS for all veterans with AO vs NAO were 8 mo vs 6 mo and 12 mo vs 10 mo, respectively (non-significant [NS]). In adenocarcinoma group the median PFS and OS for AO vs NAO veterans were 8 mo vs 4 mo and 11.75 mo vs 6 mo, respectively (NS).
Conclusions
Our study is the first to report molecular biomarkers in AO and NAO veterans with lung cancers. We found different markers between the groups. The median PFS and OS of AO and adenocarcinoma AO veterans were longer due to early stage diagnoses while NAO vetera
Background
There are no studies in oncologic literature that report biomarker alterations in Vietnam War veterans with lung cancers. Our study elucidates genetic mutations in veterans with lung cancer exposed to Agent Orange (AO) and compares them to non-Agent Orange exposed (NAO) veterans.
Methods
We collected data of veterans with lung cancers from VA Central California Health Care System who had NGS testing via Foundation One CDx from January 2007 to January 2022. We collected data of AO versus NAO veterans including age, race, gender, smoking and exposure history, histologic subtypes, treatment modalities, PDL-1, and molecular mutations. Median PFS and OS were calculated between AO and NAO in all veterans and adenocarcinoma group after first-line therapy in months by Kaplan-Meier R log-rank test.
Results
There were total of 58 lung cancer veterans, 27 AO and 31 NAO. 33 (56.9%) veterans had adenocarcinoma (20 AO vs 13 NAO). Veterans were White (81%), male (93%) and all had tobacco exposure. The median age at diagnosis was 72 years in both groups. 65.5% had stage III-IV disease. Veterans with AO adenocarcinoma had more early stage I-II disease (50%) as compared to NAO (16%). The AO group had more PDL1 expression (TPS > 1%). 15/31 (48.4%) NAO received immunotherapy vs 7/27 (25.9%) AO. 104 molecular mutations were identified. Veterans with AO had more ROS1, MET, and NRAS while NAO had more EGFR, KRAS, and NF1 mutations. In adenocarcinoma group, AO had more MET and less KRAS while NAO has more KRAS, TP53, and EGFR. The median PFS and OS for all veterans with AO vs NAO were 8 mo vs 6 mo and 12 mo vs 10 mo, respectively (non-significant [NS]). In adenocarcinoma group the median PFS and OS for AO vs NAO veterans were 8 mo vs 4 mo and 11.75 mo vs 6 mo, respectively (NS).
Conclusions
Our study is the first to report molecular biomarkers in AO and NAO veterans with lung cancers. We found different markers between the groups. The median PFS and OS of AO and adenocarcinoma AO veterans were longer due to early stage diagnoses while NAO vetera
Background
There are no studies in oncologic literature that report biomarker alterations in Vietnam War veterans with lung cancers. Our study elucidates genetic mutations in veterans with lung cancer exposed to Agent Orange (AO) and compares them to non-Agent Orange exposed (NAO) veterans.
Methods
We collected data of veterans with lung cancers from VA Central California Health Care System who had NGS testing via Foundation One CDx from January 2007 to January 2022. We collected data of AO versus NAO veterans including age, race, gender, smoking and exposure history, histologic subtypes, treatment modalities, PDL-1, and molecular mutations. Median PFS and OS were calculated between AO and NAO in all veterans and adenocarcinoma group after first-line therapy in months by Kaplan-Meier R log-rank test.
Results
There were total of 58 lung cancer veterans, 27 AO and 31 NAO. 33 (56.9%) veterans had adenocarcinoma (20 AO vs 13 NAO). Veterans were White (81%), male (93%) and all had tobacco exposure. The median age at diagnosis was 72 years in both groups. 65.5% had stage III-IV disease. Veterans with AO adenocarcinoma had more early stage I-II disease (50%) as compared to NAO (16%). The AO group had more PDL1 expression (TPS > 1%). 15/31 (48.4%) NAO received immunotherapy vs 7/27 (25.9%) AO. 104 molecular mutations were identified. Veterans with AO had more ROS1, MET, and NRAS while NAO had more EGFR, KRAS, and NF1 mutations. In adenocarcinoma group, AO had more MET and less KRAS while NAO has more KRAS, TP53, and EGFR. The median PFS and OS for all veterans with AO vs NAO were 8 mo vs 6 mo and 12 mo vs 10 mo, respectively (non-significant [NS]). In adenocarcinoma group the median PFS and OS for AO vs NAO veterans were 8 mo vs 4 mo and 11.75 mo vs 6 mo, respectively (NS).
Conclusions
Our study is the first to report molecular biomarkers in AO and NAO veterans with lung cancers. We found different markers between the groups. The median PFS and OS of AO and adenocarcinoma AO veterans were longer due to early stage diagnoses while NAO vetera
Nearly 30% of U.S. cancer deaths linked to smoking
Nearly 123,000 cancer deaths – or
That corresponds to more than 2 million person-years of lost life and nearly $21 billion in annual lost earnings.
“During the past few decades, smoking has substantially declined in the U.S., followed by great declines in mortality from lung cancer and some other smoking-related cancers,” said lead author Farhad Islami, MD, senior scientific director of cancer disparity research at the American Cancer Society.
Despite this “remarkable progress, our results indicate that smoking is still associated with about 30% of all cancer deaths and substantial lost earnings in the U.S., and that more work should be done to further reduce smoking in the country,” he said.
The study was published online in the International Journal of Cancer.
Dr. Islami and colleagues had found that lost earnings from cancer deaths in 2015 came to nearly $95 billion. Other research showed that a substantial portion of lost earnings from cancer deaths could be traced to cigarette smoking, but estimates were more than a decade old.
To provide more recent estimates and help guide tobacco control policies, Dr. Islami and colleagues estimated person-years of life lost (PYLL) and lost earnings from cigarette smoking-related cancer deaths in 2019.
Of the 418,563 cancer deaths in adults ages 25-79 years, an estimated 122,951 could be linked to cigarette smoking. That corresponds to 29.4% of all cancer deaths and roughly 2.2 million PYLL. Translated to lost earnings, the authors estimated $20.9 billion total, with average lost earnings of $170,000 per cancer death linked to smoking.
By cancer type, lung cancer accounted for about 62%, or $12.9 billion, of the total lost earnings linked to smoking, followed by esophageal cancer (7%, or $1.5 billion), colorectal cancer (6%, or $1.2 billion), and liver cancer (5%, or $1.1 billion).
Smoking-related death rates were highest in the 13 “tobacco nation” states with weaker tobacco control policies and a higher rate of cigarette smoking. These states are Alabama, Arkansas, Indiana, Kentucky, Louisiana, Michigan, Mississippi, Missouri, Ohio, Oklahoma, South Carolina, Tennessee, and West Virginia.
The lost earnings rate in all 13 tobacco nation states combined was about 44% higher, compared with other states and the District of Columbia combined, and the annual PYLL rate was 47% higher in tobacco nation states.
The researchers estimated that if PYLL and lost earnings rates in all states matched those in Utah, which has the lowest rates, more than half of the total PYLL and lost earnings nationally would have been avoided. In other words, that would mean 1.27 million PYLL and $10.5 billion saved in 2019.
Ending the ‘scourge of tobacco’
To kick the smoking habit, health providers should “screen patients for tobacco use, document tobacco use status, advise people who smoke to quit, and assist in attempts to quit,” Dr. Islami said.
Getting more people to screen for lung cancer in the United States is also important, given that only 6.6% of eligible people in 2019 received screening.
In a statement, Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said this report “further demonstrates just how critical reducing tobacco use is to ending suffering and death from cancer.”
To end the “scourge of tobacco,” local, state, and federal lawmakers need to pass proven tobacco control policies, she said.
These include regular and significant tobacco tax increases, thorough statewide smoke-free laws, and enough funding for state programs to prevent and stop smoking. It also means ensuring all Medicaid enrollees have access to all services that can help smokers quit as well as access to all FDA-approved medications that help users stop smoking.
“We have the tools to get this done, we just need lawmakers to act,” Ms. Lacasse said.
A version of this article first appeared on WebMD.com.
Nearly 123,000 cancer deaths – or
That corresponds to more than 2 million person-years of lost life and nearly $21 billion in annual lost earnings.
“During the past few decades, smoking has substantially declined in the U.S., followed by great declines in mortality from lung cancer and some other smoking-related cancers,” said lead author Farhad Islami, MD, senior scientific director of cancer disparity research at the American Cancer Society.
Despite this “remarkable progress, our results indicate that smoking is still associated with about 30% of all cancer deaths and substantial lost earnings in the U.S., and that more work should be done to further reduce smoking in the country,” he said.
The study was published online in the International Journal of Cancer.
Dr. Islami and colleagues had found that lost earnings from cancer deaths in 2015 came to nearly $95 billion. Other research showed that a substantial portion of lost earnings from cancer deaths could be traced to cigarette smoking, but estimates were more than a decade old.
To provide more recent estimates and help guide tobacco control policies, Dr. Islami and colleagues estimated person-years of life lost (PYLL) and lost earnings from cigarette smoking-related cancer deaths in 2019.
Of the 418,563 cancer deaths in adults ages 25-79 years, an estimated 122,951 could be linked to cigarette smoking. That corresponds to 29.4% of all cancer deaths and roughly 2.2 million PYLL. Translated to lost earnings, the authors estimated $20.9 billion total, with average lost earnings of $170,000 per cancer death linked to smoking.
By cancer type, lung cancer accounted for about 62%, or $12.9 billion, of the total lost earnings linked to smoking, followed by esophageal cancer (7%, or $1.5 billion), colorectal cancer (6%, or $1.2 billion), and liver cancer (5%, or $1.1 billion).
Smoking-related death rates were highest in the 13 “tobacco nation” states with weaker tobacco control policies and a higher rate of cigarette smoking. These states are Alabama, Arkansas, Indiana, Kentucky, Louisiana, Michigan, Mississippi, Missouri, Ohio, Oklahoma, South Carolina, Tennessee, and West Virginia.
The lost earnings rate in all 13 tobacco nation states combined was about 44% higher, compared with other states and the District of Columbia combined, and the annual PYLL rate was 47% higher in tobacco nation states.
The researchers estimated that if PYLL and lost earnings rates in all states matched those in Utah, which has the lowest rates, more than half of the total PYLL and lost earnings nationally would have been avoided. In other words, that would mean 1.27 million PYLL and $10.5 billion saved in 2019.
Ending the ‘scourge of tobacco’
To kick the smoking habit, health providers should “screen patients for tobacco use, document tobacco use status, advise people who smoke to quit, and assist in attempts to quit,” Dr. Islami said.
Getting more people to screen for lung cancer in the United States is also important, given that only 6.6% of eligible people in 2019 received screening.
In a statement, Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said this report “further demonstrates just how critical reducing tobacco use is to ending suffering and death from cancer.”
To end the “scourge of tobacco,” local, state, and federal lawmakers need to pass proven tobacco control policies, she said.
These include regular and significant tobacco tax increases, thorough statewide smoke-free laws, and enough funding for state programs to prevent and stop smoking. It also means ensuring all Medicaid enrollees have access to all services that can help smokers quit as well as access to all FDA-approved medications that help users stop smoking.
“We have the tools to get this done, we just need lawmakers to act,” Ms. Lacasse said.
A version of this article first appeared on WebMD.com.
Nearly 123,000 cancer deaths – or
That corresponds to more than 2 million person-years of lost life and nearly $21 billion in annual lost earnings.
“During the past few decades, smoking has substantially declined in the U.S., followed by great declines in mortality from lung cancer and some other smoking-related cancers,” said lead author Farhad Islami, MD, senior scientific director of cancer disparity research at the American Cancer Society.
Despite this “remarkable progress, our results indicate that smoking is still associated with about 30% of all cancer deaths and substantial lost earnings in the U.S., and that more work should be done to further reduce smoking in the country,” he said.
The study was published online in the International Journal of Cancer.
Dr. Islami and colleagues had found that lost earnings from cancer deaths in 2015 came to nearly $95 billion. Other research showed that a substantial portion of lost earnings from cancer deaths could be traced to cigarette smoking, but estimates were more than a decade old.
To provide more recent estimates and help guide tobacco control policies, Dr. Islami and colleagues estimated person-years of life lost (PYLL) and lost earnings from cigarette smoking-related cancer deaths in 2019.
Of the 418,563 cancer deaths in adults ages 25-79 years, an estimated 122,951 could be linked to cigarette smoking. That corresponds to 29.4% of all cancer deaths and roughly 2.2 million PYLL. Translated to lost earnings, the authors estimated $20.9 billion total, with average lost earnings of $170,000 per cancer death linked to smoking.
By cancer type, lung cancer accounted for about 62%, or $12.9 billion, of the total lost earnings linked to smoking, followed by esophageal cancer (7%, or $1.5 billion), colorectal cancer (6%, or $1.2 billion), and liver cancer (5%, or $1.1 billion).
Smoking-related death rates were highest in the 13 “tobacco nation” states with weaker tobacco control policies and a higher rate of cigarette smoking. These states are Alabama, Arkansas, Indiana, Kentucky, Louisiana, Michigan, Mississippi, Missouri, Ohio, Oklahoma, South Carolina, Tennessee, and West Virginia.
The lost earnings rate in all 13 tobacco nation states combined was about 44% higher, compared with other states and the District of Columbia combined, and the annual PYLL rate was 47% higher in tobacco nation states.
The researchers estimated that if PYLL and lost earnings rates in all states matched those in Utah, which has the lowest rates, more than half of the total PYLL and lost earnings nationally would have been avoided. In other words, that would mean 1.27 million PYLL and $10.5 billion saved in 2019.
Ending the ‘scourge of tobacco’
To kick the smoking habit, health providers should “screen patients for tobacco use, document tobacco use status, advise people who smoke to quit, and assist in attempts to quit,” Dr. Islami said.
Getting more people to screen for lung cancer in the United States is also important, given that only 6.6% of eligible people in 2019 received screening.
In a statement, Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said this report “further demonstrates just how critical reducing tobacco use is to ending suffering and death from cancer.”
To end the “scourge of tobacco,” local, state, and federal lawmakers need to pass proven tobacco control policies, she said.
These include regular and significant tobacco tax increases, thorough statewide smoke-free laws, and enough funding for state programs to prevent and stop smoking. It also means ensuring all Medicaid enrollees have access to all services that can help smokers quit as well as access to all FDA-approved medications that help users stop smoking.
“We have the tools to get this done, we just need lawmakers to act,” Ms. Lacasse said.
A version of this article first appeared on WebMD.com.
FROM THE INTERNATIONAL JOURNAL OF CANCER