User login
Lung adverse effects in patients taking trastuzumab deruxtecan
although the benefit-to-risk relationship with use of the drug is still positive, say researchers who report a review of early clinical trials with the drug.
T-DXd is a monoclonal antibody that targets HER2. It is approved for use in HER2-positive breast, gastric, and lung cancers.
In the new study, investigators analyzed data from early clinical trials that involved patients with advanced cancers who had been heavily pretreated. They found an incidence of just over 15% for interstitial lung disease (ILD)/pneumonitis associated with the drug. Most patients (77.4%) had grade 1 or 2 ILD, but 2.2% of patients had grade 5 ILD.
“Interstitial lung disease is a known risk factor in patients treated with antibody conjugates for cancer,” commented lead author Charles Powell, MD, Icahn School of Medicine at Mount Sinai, New York. This adverse effect can lead to lung fibrosis and can become severe, life threatening, and even fatal, the authors warned.
The authors also discussed management of the event, which involves corticosteroids, and recommended that any patient who develops ILD of grade 3 or higher be hospitalized.
Close monitoring and proactive management may reduce the risk of ILD, they suggested.
Indeed, the incidence of this adverse effect was lower in a later phase 3 trial of the drug (10.5% in the DESTINY-Breast03 trial) and that the adverse events were less severe in this patient population (none of these events were of grade 4 or 5).
“Increased knowledge ... and implementation of ILD/pneumonitis monitoring, diagnosis, and management guidelines” may have resulted in this adverse effect being identified early and treated before it progressed, they commented.
ILD is highlighted in a boxed warning on the product label.
The study was published online in ESMO Open.
In their review, the investigators evaluated nine early-stage monotherapy clinical trials (phases 1 and 2) involving a total of 1,150 patients (breast cancer, 44.3%; gastric cancer, 25.6%; lung cancer, 17.7%; colorectal cancer, 9.3%, other cancers, 3.0%).
These patients had advanced cancer and had been heavily pretreated with a median of four prior lines of therapy. They received one or more doses of at least 5.4 mg/kg of T-DXd.
Nearly half of the cohort were treated for more than 6 months. A total of 276 potential ILD/pneumonitis events were sent for adjudication; of those, 85% were adjudicated as ILD/pneumonitis.
The overall incidence of adjudicated ILD/pneumonitis events was 15.4%; most were low-grade events. Some 87% of patients experienced their first ILD event within 12 months of treatment. The median time to experiencing an ILD/pneumonitis event was 5.4 months.
Some of the patients who developed grade 1 ILD/pneumonitis were treated and the adverse event resolved. These patients were then rechallenged with the drug. Only 3 of the 47 rechallenged patients experienced recurrence of ILD/pneumonitis, the authors noted.
“Rechallenge with T-DXd after complete resolution of grade 1 events is possible and warrants further investigation,” they commented. They cautioned, however, that rechallenge is not recommended for all patients, at least not for those with grade 2 or higher ILD/pneumonitis.
Overall, the authors concluded that the “benefit-risk of T-DXd treatment is positive,” but they warned that some patients may be at increased risk of developing ILD/pneumonitis
Baseline factors that increase the risk of developing an ILD/pneumonitis event include the following: being younger than 65 years, receiving a T-DXd dose of more than6.4 mg/kg, having a baseline oxygen saturation level of less than 95%, having moderate to severe renal impairment, and having lung comorbidities. In addition, patients who had initially been diagnosed with cancer more than 4 years before receiving the drug were at higher risk of developing ILD/pneumonitis.
“Using learnings from the early clinical trials experience, physician education and patient management protocols were revised and disseminated by the study sponsors [and] more recent trial data in earlier lines of therapy has demonstrated lower rates of ILD events, suggesting close monitoring and proactive management of ILD/pneumonitis is warranted for all patients,” Dr. Powell said in a statement.
The T-DXd clinical trials were sponsored by AstraZeneca and Daiichi Sankyo. Dr. Powell has received fees from Daiichi Sankyo, AstraZeneca, and Voluntis.
A version of this article first appeared on Medscape.com.
although the benefit-to-risk relationship with use of the drug is still positive, say researchers who report a review of early clinical trials with the drug.
T-DXd is a monoclonal antibody that targets HER2. It is approved for use in HER2-positive breast, gastric, and lung cancers.
In the new study, investigators analyzed data from early clinical trials that involved patients with advanced cancers who had been heavily pretreated. They found an incidence of just over 15% for interstitial lung disease (ILD)/pneumonitis associated with the drug. Most patients (77.4%) had grade 1 or 2 ILD, but 2.2% of patients had grade 5 ILD.
“Interstitial lung disease is a known risk factor in patients treated with antibody conjugates for cancer,” commented lead author Charles Powell, MD, Icahn School of Medicine at Mount Sinai, New York. This adverse effect can lead to lung fibrosis and can become severe, life threatening, and even fatal, the authors warned.
The authors also discussed management of the event, which involves corticosteroids, and recommended that any patient who develops ILD of grade 3 or higher be hospitalized.
Close monitoring and proactive management may reduce the risk of ILD, they suggested.
Indeed, the incidence of this adverse effect was lower in a later phase 3 trial of the drug (10.5% in the DESTINY-Breast03 trial) and that the adverse events were less severe in this patient population (none of these events were of grade 4 or 5).
“Increased knowledge ... and implementation of ILD/pneumonitis monitoring, diagnosis, and management guidelines” may have resulted in this adverse effect being identified early and treated before it progressed, they commented.
ILD is highlighted in a boxed warning on the product label.
The study was published online in ESMO Open.
In their review, the investigators evaluated nine early-stage monotherapy clinical trials (phases 1 and 2) involving a total of 1,150 patients (breast cancer, 44.3%; gastric cancer, 25.6%; lung cancer, 17.7%; colorectal cancer, 9.3%, other cancers, 3.0%).
These patients had advanced cancer and had been heavily pretreated with a median of four prior lines of therapy. They received one or more doses of at least 5.4 mg/kg of T-DXd.
Nearly half of the cohort were treated for more than 6 months. A total of 276 potential ILD/pneumonitis events were sent for adjudication; of those, 85% were adjudicated as ILD/pneumonitis.
The overall incidence of adjudicated ILD/pneumonitis events was 15.4%; most were low-grade events. Some 87% of patients experienced their first ILD event within 12 months of treatment. The median time to experiencing an ILD/pneumonitis event was 5.4 months.
Some of the patients who developed grade 1 ILD/pneumonitis were treated and the adverse event resolved. These patients were then rechallenged with the drug. Only 3 of the 47 rechallenged patients experienced recurrence of ILD/pneumonitis, the authors noted.
“Rechallenge with T-DXd after complete resolution of grade 1 events is possible and warrants further investigation,” they commented. They cautioned, however, that rechallenge is not recommended for all patients, at least not for those with grade 2 or higher ILD/pneumonitis.
Overall, the authors concluded that the “benefit-risk of T-DXd treatment is positive,” but they warned that some patients may be at increased risk of developing ILD/pneumonitis
Baseline factors that increase the risk of developing an ILD/pneumonitis event include the following: being younger than 65 years, receiving a T-DXd dose of more than6.4 mg/kg, having a baseline oxygen saturation level of less than 95%, having moderate to severe renal impairment, and having lung comorbidities. In addition, patients who had initially been diagnosed with cancer more than 4 years before receiving the drug were at higher risk of developing ILD/pneumonitis.
“Using learnings from the early clinical trials experience, physician education and patient management protocols were revised and disseminated by the study sponsors [and] more recent trial data in earlier lines of therapy has demonstrated lower rates of ILD events, suggesting close monitoring and proactive management of ILD/pneumonitis is warranted for all patients,” Dr. Powell said in a statement.
The T-DXd clinical trials were sponsored by AstraZeneca and Daiichi Sankyo. Dr. Powell has received fees from Daiichi Sankyo, AstraZeneca, and Voluntis.
A version of this article first appeared on Medscape.com.
although the benefit-to-risk relationship with use of the drug is still positive, say researchers who report a review of early clinical trials with the drug.
T-DXd is a monoclonal antibody that targets HER2. It is approved for use in HER2-positive breast, gastric, and lung cancers.
In the new study, investigators analyzed data from early clinical trials that involved patients with advanced cancers who had been heavily pretreated. They found an incidence of just over 15% for interstitial lung disease (ILD)/pneumonitis associated with the drug. Most patients (77.4%) had grade 1 or 2 ILD, but 2.2% of patients had grade 5 ILD.
“Interstitial lung disease is a known risk factor in patients treated with antibody conjugates for cancer,” commented lead author Charles Powell, MD, Icahn School of Medicine at Mount Sinai, New York. This adverse effect can lead to lung fibrosis and can become severe, life threatening, and even fatal, the authors warned.
The authors also discussed management of the event, which involves corticosteroids, and recommended that any patient who develops ILD of grade 3 or higher be hospitalized.
Close monitoring and proactive management may reduce the risk of ILD, they suggested.
Indeed, the incidence of this adverse effect was lower in a later phase 3 trial of the drug (10.5% in the DESTINY-Breast03 trial) and that the adverse events were less severe in this patient population (none of these events were of grade 4 or 5).
“Increased knowledge ... and implementation of ILD/pneumonitis monitoring, diagnosis, and management guidelines” may have resulted in this adverse effect being identified early and treated before it progressed, they commented.
ILD is highlighted in a boxed warning on the product label.
The study was published online in ESMO Open.
In their review, the investigators evaluated nine early-stage monotherapy clinical trials (phases 1 and 2) involving a total of 1,150 patients (breast cancer, 44.3%; gastric cancer, 25.6%; lung cancer, 17.7%; colorectal cancer, 9.3%, other cancers, 3.0%).
These patients had advanced cancer and had been heavily pretreated with a median of four prior lines of therapy. They received one or more doses of at least 5.4 mg/kg of T-DXd.
Nearly half of the cohort were treated for more than 6 months. A total of 276 potential ILD/pneumonitis events were sent for adjudication; of those, 85% were adjudicated as ILD/pneumonitis.
The overall incidence of adjudicated ILD/pneumonitis events was 15.4%; most were low-grade events. Some 87% of patients experienced their first ILD event within 12 months of treatment. The median time to experiencing an ILD/pneumonitis event was 5.4 months.
Some of the patients who developed grade 1 ILD/pneumonitis were treated and the adverse event resolved. These patients were then rechallenged with the drug. Only 3 of the 47 rechallenged patients experienced recurrence of ILD/pneumonitis, the authors noted.
“Rechallenge with T-DXd after complete resolution of grade 1 events is possible and warrants further investigation,” they commented. They cautioned, however, that rechallenge is not recommended for all patients, at least not for those with grade 2 or higher ILD/pneumonitis.
Overall, the authors concluded that the “benefit-risk of T-DXd treatment is positive,” but they warned that some patients may be at increased risk of developing ILD/pneumonitis
Baseline factors that increase the risk of developing an ILD/pneumonitis event include the following: being younger than 65 years, receiving a T-DXd dose of more than6.4 mg/kg, having a baseline oxygen saturation level of less than 95%, having moderate to severe renal impairment, and having lung comorbidities. In addition, patients who had initially been diagnosed with cancer more than 4 years before receiving the drug were at higher risk of developing ILD/pneumonitis.
“Using learnings from the early clinical trials experience, physician education and patient management protocols were revised and disseminated by the study sponsors [and] more recent trial data in earlier lines of therapy has demonstrated lower rates of ILD events, suggesting close monitoring and proactive management of ILD/pneumonitis is warranted for all patients,” Dr. Powell said in a statement.
The T-DXd clinical trials were sponsored by AstraZeneca and Daiichi Sankyo. Dr. Powell has received fees from Daiichi Sankyo, AstraZeneca, and Voluntis.
A version of this article first appeared on Medscape.com.
FROM ESMO OPEN
The ‘great dynamism’ of radiation oncology
The field of radiation oncology has rapidly evolved in recent years, thanks in large part to findings from randomized clinical trials (RCTs) that have helped shift therapeutic standards, a review of the literature shows.
Highlights from this research reveal how high-tech radiotherapy, such as hypofractionation and stereotactic body radiotherapy, has improved care for many patients, how personalized radiotherapy using image-based guidance has helped tailor treatments, and how endpoints that focus on quality of life and patient satisfaction are emerging.
For instance, Charles B. Simone II, MD, FACRO, who was not involved in the current work, pointed to “a proliferation of trials assessing hypofractionation in the curative setting and stereotactic body radiation therapy in the curative and poly- and oligometastatic settings that have allowed for increased patient convenience and dose intensification, respectively.”
Dr. Simone, chief medical officer, New York Proton Center, Memorial Sloan Kettering Cancer Center, also noted that the first personalized radiotherapy trials using imaging and biological markers have “the profound potential to individualize treatment and improve patient outcomes.”
The review was published in the European Journal of Cancer.
An evolving field
Given the fast-changing landscape for cancer therapeutics and a deluge of research studies, the authors wanted to understand the most notable advances established in recent trials as well as caveats to some approaches and emerging areas to watch.
In the review, Sophie Espenel, MD, from the department of radiation oncology, Gustave Roussy Cancer Campus, Villejuif, France, and colleagues identified 1,347 radiotherapy RCTs that were conducted from January 2018 to December 2021. Of these, the authors selected 110 large phase 2 or 3 RCTs that contained data showing practice-changing or emerging concepts.
Overall, the studies showed “great dynamism” in radiation oncology research and covered a wide range of radiotherapy practices, according to Dr. Espenel and coauthors.
A central area of research has focused on radioimmunotherapy, an approach that aims to enhance the antitumor immune response. One RCT in the preoperative setting showed, for instance, that concurrent stereotactic body radiotherapy delivered at 24 Gy over eight fractions, along with the anti–PD-L1 agent durvalumab, increased major pathologic complete response rates almost eightfold in comparison with durvalumab alone for patients with early-stage lung cancer (53.3% vs. 6.7%).
Although promising, not all trials that evaluated a concurrent chemoradiotherapy-immunotherapy strategy showed positive results. One RCT of locally advanced head and neck squamous cell carcinoma, for instance, found that median progression-free survival was not reached when adding the anti–PD-L1 avelumab to chemoradiotherapy. In addition, trials in the metastatic setting have shown conflicting results, the authors note.
Another topic of interest is that of newer radiosensitizers. A trial that evaluated high-risk locoregionally advanced head and neck squamous cell carcinoma highlighted the efficacy of xevinapant, a pro-apoptotic agent that inhibits apoptosis proteins. Xevinapant was used for the first time in conjunction with a standard high-dose cisplatin chemoradiotherapy. In this study, locoregional control at 18 months was achieved for 54% of patients who received xevinapant vs. 33% of those who received standard care. The toxicity profiles were similar.
The use of high-tech radiotherapy is gaining ground. It allows patients to receive more targeted treatments at lower doses and in shorter time frames. One trial found, for instance, that a more hypofractionated adjuvant whole breast approach, using 26 Gy in five fractions over a week, is as effective and safe as 40 Gy in 15 fractions over 3 weeks. The researchers found that there was no difference in the incidence of locoregional relapses, disease-free survival, and overall survival between the regimens.
Dr. Simone also noted that advanced treatment modalities, such as intensity-modulated radiotherapy, stereotactic radiosurgery, and proton therapy, have the potential to improve patient-reported adverse events and clinical outcomes. “I have seen this both in my clinical practice and in several recent publications,” he says.
Personalization of radiotherapy is also an emerging area that may allow for more tailored treatments with improved outcomes. The authors highlighted a study that found that PMSA PET-CT was better than conventional CT for accurately staging prostate cancer. This approach was also less expensive and led to less radiation exposure.
On the basis of this research, “PMSA PET-CT has since become the [standard of care] for prostate cancer staging,” the authors explain.
Dr. Espenel and colleagues note that as patients survive longer, quality of life and patient satisfaction are increasingly becoming endpoints in RCTs. Experts are focusing more attention on sequelae of treatments and advances in technology that can spare critical organs from radiation and reduce overall treatment time.
Shared decision-making is becoming increasingly possible in many cases as well. For example, with some clinical trials that involved different treatment modalities, outcomes were equivalent, but toxicity profiles differed, allowing patients to choose therapeutic options tailored to their preferences.
Overall, these data demonstrate “a great dynamism of radiation oncology research in most primary tumor types,” the researchers write.
The study received no outside financial support. The authors have disclosed no relevant financial relationships. Dr. Simone is chair of the American Society for Radiation Oncology Lung Resource Panel and the American Society for Radiation Oncology Veteran Affairs Radiation Oncology Quality Surveillance Blue Ribbon Lung Panel and has received honorarium from Varian Medical Systems.
A version of this article first appeared on Medscape.com.
The field of radiation oncology has rapidly evolved in recent years, thanks in large part to findings from randomized clinical trials (RCTs) that have helped shift therapeutic standards, a review of the literature shows.
Highlights from this research reveal how high-tech radiotherapy, such as hypofractionation and stereotactic body radiotherapy, has improved care for many patients, how personalized radiotherapy using image-based guidance has helped tailor treatments, and how endpoints that focus on quality of life and patient satisfaction are emerging.
For instance, Charles B. Simone II, MD, FACRO, who was not involved in the current work, pointed to “a proliferation of trials assessing hypofractionation in the curative setting and stereotactic body radiation therapy in the curative and poly- and oligometastatic settings that have allowed for increased patient convenience and dose intensification, respectively.”
Dr. Simone, chief medical officer, New York Proton Center, Memorial Sloan Kettering Cancer Center, also noted that the first personalized radiotherapy trials using imaging and biological markers have “the profound potential to individualize treatment and improve patient outcomes.”
The review was published in the European Journal of Cancer.
An evolving field
Given the fast-changing landscape for cancer therapeutics and a deluge of research studies, the authors wanted to understand the most notable advances established in recent trials as well as caveats to some approaches and emerging areas to watch.
In the review, Sophie Espenel, MD, from the department of radiation oncology, Gustave Roussy Cancer Campus, Villejuif, France, and colleagues identified 1,347 radiotherapy RCTs that were conducted from January 2018 to December 2021. Of these, the authors selected 110 large phase 2 or 3 RCTs that contained data showing practice-changing or emerging concepts.
Overall, the studies showed “great dynamism” in radiation oncology research and covered a wide range of radiotherapy practices, according to Dr. Espenel and coauthors.
A central area of research has focused on radioimmunotherapy, an approach that aims to enhance the antitumor immune response. One RCT in the preoperative setting showed, for instance, that concurrent stereotactic body radiotherapy delivered at 24 Gy over eight fractions, along with the anti–PD-L1 agent durvalumab, increased major pathologic complete response rates almost eightfold in comparison with durvalumab alone for patients with early-stage lung cancer (53.3% vs. 6.7%).
Although promising, not all trials that evaluated a concurrent chemoradiotherapy-immunotherapy strategy showed positive results. One RCT of locally advanced head and neck squamous cell carcinoma, for instance, found that median progression-free survival was not reached when adding the anti–PD-L1 avelumab to chemoradiotherapy. In addition, trials in the metastatic setting have shown conflicting results, the authors note.
Another topic of interest is that of newer radiosensitizers. A trial that evaluated high-risk locoregionally advanced head and neck squamous cell carcinoma highlighted the efficacy of xevinapant, a pro-apoptotic agent that inhibits apoptosis proteins. Xevinapant was used for the first time in conjunction with a standard high-dose cisplatin chemoradiotherapy. In this study, locoregional control at 18 months was achieved for 54% of patients who received xevinapant vs. 33% of those who received standard care. The toxicity profiles were similar.
The use of high-tech radiotherapy is gaining ground. It allows patients to receive more targeted treatments at lower doses and in shorter time frames. One trial found, for instance, that a more hypofractionated adjuvant whole breast approach, using 26 Gy in five fractions over a week, is as effective and safe as 40 Gy in 15 fractions over 3 weeks. The researchers found that there was no difference in the incidence of locoregional relapses, disease-free survival, and overall survival between the regimens.
Dr. Simone also noted that advanced treatment modalities, such as intensity-modulated radiotherapy, stereotactic radiosurgery, and proton therapy, have the potential to improve patient-reported adverse events and clinical outcomes. “I have seen this both in my clinical practice and in several recent publications,” he says.
Personalization of radiotherapy is also an emerging area that may allow for more tailored treatments with improved outcomes. The authors highlighted a study that found that PMSA PET-CT was better than conventional CT for accurately staging prostate cancer. This approach was also less expensive and led to less radiation exposure.
On the basis of this research, “PMSA PET-CT has since become the [standard of care] for prostate cancer staging,” the authors explain.
Dr. Espenel and colleagues note that as patients survive longer, quality of life and patient satisfaction are increasingly becoming endpoints in RCTs. Experts are focusing more attention on sequelae of treatments and advances in technology that can spare critical organs from radiation and reduce overall treatment time.
Shared decision-making is becoming increasingly possible in many cases as well. For example, with some clinical trials that involved different treatment modalities, outcomes were equivalent, but toxicity profiles differed, allowing patients to choose therapeutic options tailored to their preferences.
Overall, these data demonstrate “a great dynamism of radiation oncology research in most primary tumor types,” the researchers write.
The study received no outside financial support. The authors have disclosed no relevant financial relationships. Dr. Simone is chair of the American Society for Radiation Oncology Lung Resource Panel and the American Society for Radiation Oncology Veteran Affairs Radiation Oncology Quality Surveillance Blue Ribbon Lung Panel and has received honorarium from Varian Medical Systems.
A version of this article first appeared on Medscape.com.
The field of radiation oncology has rapidly evolved in recent years, thanks in large part to findings from randomized clinical trials (RCTs) that have helped shift therapeutic standards, a review of the literature shows.
Highlights from this research reveal how high-tech radiotherapy, such as hypofractionation and stereotactic body radiotherapy, has improved care for many patients, how personalized radiotherapy using image-based guidance has helped tailor treatments, and how endpoints that focus on quality of life and patient satisfaction are emerging.
For instance, Charles B. Simone II, MD, FACRO, who was not involved in the current work, pointed to “a proliferation of trials assessing hypofractionation in the curative setting and stereotactic body radiation therapy in the curative and poly- and oligometastatic settings that have allowed for increased patient convenience and dose intensification, respectively.”
Dr. Simone, chief medical officer, New York Proton Center, Memorial Sloan Kettering Cancer Center, also noted that the first personalized radiotherapy trials using imaging and biological markers have “the profound potential to individualize treatment and improve patient outcomes.”
The review was published in the European Journal of Cancer.
An evolving field
Given the fast-changing landscape for cancer therapeutics and a deluge of research studies, the authors wanted to understand the most notable advances established in recent trials as well as caveats to some approaches and emerging areas to watch.
In the review, Sophie Espenel, MD, from the department of radiation oncology, Gustave Roussy Cancer Campus, Villejuif, France, and colleagues identified 1,347 radiotherapy RCTs that were conducted from January 2018 to December 2021. Of these, the authors selected 110 large phase 2 or 3 RCTs that contained data showing practice-changing or emerging concepts.
Overall, the studies showed “great dynamism” in radiation oncology research and covered a wide range of radiotherapy practices, according to Dr. Espenel and coauthors.
A central area of research has focused on radioimmunotherapy, an approach that aims to enhance the antitumor immune response. One RCT in the preoperative setting showed, for instance, that concurrent stereotactic body radiotherapy delivered at 24 Gy over eight fractions, along with the anti–PD-L1 agent durvalumab, increased major pathologic complete response rates almost eightfold in comparison with durvalumab alone for patients with early-stage lung cancer (53.3% vs. 6.7%).
Although promising, not all trials that evaluated a concurrent chemoradiotherapy-immunotherapy strategy showed positive results. One RCT of locally advanced head and neck squamous cell carcinoma, for instance, found that median progression-free survival was not reached when adding the anti–PD-L1 avelumab to chemoradiotherapy. In addition, trials in the metastatic setting have shown conflicting results, the authors note.
Another topic of interest is that of newer radiosensitizers. A trial that evaluated high-risk locoregionally advanced head and neck squamous cell carcinoma highlighted the efficacy of xevinapant, a pro-apoptotic agent that inhibits apoptosis proteins. Xevinapant was used for the first time in conjunction with a standard high-dose cisplatin chemoradiotherapy. In this study, locoregional control at 18 months was achieved for 54% of patients who received xevinapant vs. 33% of those who received standard care. The toxicity profiles were similar.
The use of high-tech radiotherapy is gaining ground. It allows patients to receive more targeted treatments at lower doses and in shorter time frames. One trial found, for instance, that a more hypofractionated adjuvant whole breast approach, using 26 Gy in five fractions over a week, is as effective and safe as 40 Gy in 15 fractions over 3 weeks. The researchers found that there was no difference in the incidence of locoregional relapses, disease-free survival, and overall survival between the regimens.
Dr. Simone also noted that advanced treatment modalities, such as intensity-modulated radiotherapy, stereotactic radiosurgery, and proton therapy, have the potential to improve patient-reported adverse events and clinical outcomes. “I have seen this both in my clinical practice and in several recent publications,” he says.
Personalization of radiotherapy is also an emerging area that may allow for more tailored treatments with improved outcomes. The authors highlighted a study that found that PMSA PET-CT was better than conventional CT for accurately staging prostate cancer. This approach was also less expensive and led to less radiation exposure.
On the basis of this research, “PMSA PET-CT has since become the [standard of care] for prostate cancer staging,” the authors explain.
Dr. Espenel and colleagues note that as patients survive longer, quality of life and patient satisfaction are increasingly becoming endpoints in RCTs. Experts are focusing more attention on sequelae of treatments and advances in technology that can spare critical organs from radiation and reduce overall treatment time.
Shared decision-making is becoming increasingly possible in many cases as well. For example, with some clinical trials that involved different treatment modalities, outcomes were equivalent, but toxicity profiles differed, allowing patients to choose therapeutic options tailored to their preferences.
Overall, these data demonstrate “a great dynamism of radiation oncology research in most primary tumor types,” the researchers write.
The study received no outside financial support. The authors have disclosed no relevant financial relationships. Dr. Simone is chair of the American Society for Radiation Oncology Lung Resource Panel and the American Society for Radiation Oncology Veteran Affairs Radiation Oncology Quality Surveillance Blue Ribbon Lung Panel and has received honorarium from Varian Medical Systems.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF CANCER
Safety Profile of Mutant EGFR-TK Inhibitors in Advanced Non–Small Cell Lung Cancer: A Meta-analysis
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
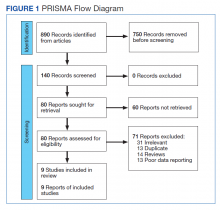
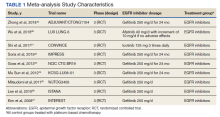
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
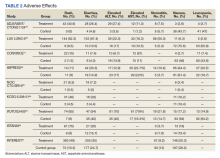
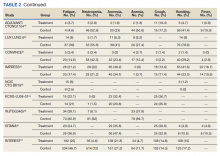
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Getting cancer research on track again may require a ‘behemoth’ effort
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
A look at lung cancer screening in resource-limited countries
Lung cancer screening has been a success story in high-income countries, leading to a shift in diagnoses to earlier stages and a reduction in mortality among eligible groups.
A new report shows that middle- and low-income countries are being left out. “We do have good screening programs and some national ones, even in smaller European countries and in Canada, but either at all or not implemented nationwide. This is a huge problem in the world,” said Milena Cavic, PhD, who presented the interim results on behalf of the diagnostics working group of the International Association for the Study of Lung Cancer early detection and screening committee at a press conference on Aug. 7 at the World Conference on Lung Cancer. Dr. Cavic is a senior research associate at the Institute for Oncology and Radiology of Serbia in Belgrade.
“It’s definitely a work in progress, and it’s also about raising awareness of the problem. In several parts of Asia, in Taiwan, in Korea, smoking is not the major, or at least, not the only reason for getting lung cancer. The other reasons are family history and also environmental factors like cooking fires, etc. So, the criteria we have for screening in Western countries are not one to one implementable in these countries,” said Rudolf Huber, MD, PhD, a respiratory physician at Ludwig Maximilian University of Munich and a coauthor of the report.
The report also pointed out the lack of recommendations for lung cancer screening in middle- and low-income countries. One approach would be to produce recommendations for countries with similar infrastructures and health resources, as well as primary risk factors such as smoking or cooking fires. “We have to adapt it to the various situations,” said Dr. Huber.
Another possibility is to rework existing recommendations for high income countries to adapt them to low- and middle-income countries. In the coming year, the working group will conduct a modeling study of Serbia, China, South Africa, and Columbia. It will look at population-specific and geographic factors from each country to produce country-specific models. “It will be interesting to see if these models will give us new recommendations for countries like this. So we can derive something from the high-income countries, but it will need to be adapted very, very much,” said Dr. Cavic.
The report highlighted some of the disparities between countries. CT scanners are far more common in high-income countries. Japan leads the way at 111.5 per million residents, followed by Australia at 70.2, Iceland at 47.6, and the United States at 44.9. At the other end is Columbia with 1.3, which trails Mexico at 5.9, Hungary at 9.4, and the United Kingdom at 9.5. However, the authors point out that there is no consensus on the optimum number of CT scanners per capita, since too few can lead to lack of access and too many can result in overuse. In fact, the greatest number of CT scans performed per capita was in the United States (278.5 per million), followed by Iceland (234.4), Japan (230.8), and Korea (228.1).
Lung cancer screening can be at odds with other health priorities, especially in low-income countries. These can include HIV, tuberculosis, and granulomatous diseases. But that could also provide an opportunity, according to Dr. Huber. “For example, in South Africa, tuberculosis programs are done by chest x-ray. We now have data that [allows us to] detect nodules by artificial intelligence, so one of the things we are thinking about is whether we could even use chest x-ray to get an earlier detection. At the end, it may be that in some countries it’s possible to do the classical CT screening, while in other countries we have to adapt to other options – probably chest x-ray using artificial intelligence or computer-aided diagnosis. And, then a consequent program for following up and managing the incidentally diagnosed nodules.”
The group is hoping to explore the environmental factors that could affect lung cancer risk in middle- and low-income countries. That is difficult to do, however, because smoking data can be hard to come by in many countries, and there is general uncertainty about what other risk factors may exist, though air pollution is a clear suspect. “It is something we are hoping to focus on in the future because there is a subgroup of individuals without a smoking history who are at high risk. It would be really good to find this high-risk population that should actually be screened in the future,” Dr. Cavic said.
Some countries have no data on lung cancer screening. For example, only South Africa is represented from Africa, and data is missing from many countries in Asia. The diagnostics working group of the IASLC early detection and screening committee has created a survey to gather information on the availability of lung cancer screening and its effect on diagnosis and treatment in countries throughout the world.
Dr. Cavic and Dr. Huber reported no relevant financial disclosures. The meeting was sponsored by the IASLC.
Lung cancer screening has been a success story in high-income countries, leading to a shift in diagnoses to earlier stages and a reduction in mortality among eligible groups.
A new report shows that middle- and low-income countries are being left out. “We do have good screening programs and some national ones, even in smaller European countries and in Canada, but either at all or not implemented nationwide. This is a huge problem in the world,” said Milena Cavic, PhD, who presented the interim results on behalf of the diagnostics working group of the International Association for the Study of Lung Cancer early detection and screening committee at a press conference on Aug. 7 at the World Conference on Lung Cancer. Dr. Cavic is a senior research associate at the Institute for Oncology and Radiology of Serbia in Belgrade.
“It’s definitely a work in progress, and it’s also about raising awareness of the problem. In several parts of Asia, in Taiwan, in Korea, smoking is not the major, or at least, not the only reason for getting lung cancer. The other reasons are family history and also environmental factors like cooking fires, etc. So, the criteria we have for screening in Western countries are not one to one implementable in these countries,” said Rudolf Huber, MD, PhD, a respiratory physician at Ludwig Maximilian University of Munich and a coauthor of the report.
The report also pointed out the lack of recommendations for lung cancer screening in middle- and low-income countries. One approach would be to produce recommendations for countries with similar infrastructures and health resources, as well as primary risk factors such as smoking or cooking fires. “We have to adapt it to the various situations,” said Dr. Huber.
Another possibility is to rework existing recommendations for high income countries to adapt them to low- and middle-income countries. In the coming year, the working group will conduct a modeling study of Serbia, China, South Africa, and Columbia. It will look at population-specific and geographic factors from each country to produce country-specific models. “It will be interesting to see if these models will give us new recommendations for countries like this. So we can derive something from the high-income countries, but it will need to be adapted very, very much,” said Dr. Cavic.
The report highlighted some of the disparities between countries. CT scanners are far more common in high-income countries. Japan leads the way at 111.5 per million residents, followed by Australia at 70.2, Iceland at 47.6, and the United States at 44.9. At the other end is Columbia with 1.3, which trails Mexico at 5.9, Hungary at 9.4, and the United Kingdom at 9.5. However, the authors point out that there is no consensus on the optimum number of CT scanners per capita, since too few can lead to lack of access and too many can result in overuse. In fact, the greatest number of CT scans performed per capita was in the United States (278.5 per million), followed by Iceland (234.4), Japan (230.8), and Korea (228.1).
Lung cancer screening can be at odds with other health priorities, especially in low-income countries. These can include HIV, tuberculosis, and granulomatous diseases. But that could also provide an opportunity, according to Dr. Huber. “For example, in South Africa, tuberculosis programs are done by chest x-ray. We now have data that [allows us to] detect nodules by artificial intelligence, so one of the things we are thinking about is whether we could even use chest x-ray to get an earlier detection. At the end, it may be that in some countries it’s possible to do the classical CT screening, while in other countries we have to adapt to other options – probably chest x-ray using artificial intelligence or computer-aided diagnosis. And, then a consequent program for following up and managing the incidentally diagnosed nodules.”
The group is hoping to explore the environmental factors that could affect lung cancer risk in middle- and low-income countries. That is difficult to do, however, because smoking data can be hard to come by in many countries, and there is general uncertainty about what other risk factors may exist, though air pollution is a clear suspect. “It is something we are hoping to focus on in the future because there is a subgroup of individuals without a smoking history who are at high risk. It would be really good to find this high-risk population that should actually be screened in the future,” Dr. Cavic said.
Some countries have no data on lung cancer screening. For example, only South Africa is represented from Africa, and data is missing from many countries in Asia. The diagnostics working group of the IASLC early detection and screening committee has created a survey to gather information on the availability of lung cancer screening and its effect on diagnosis and treatment in countries throughout the world.
Dr. Cavic and Dr. Huber reported no relevant financial disclosures. The meeting was sponsored by the IASLC.
Lung cancer screening has been a success story in high-income countries, leading to a shift in diagnoses to earlier stages and a reduction in mortality among eligible groups.
A new report shows that middle- and low-income countries are being left out. “We do have good screening programs and some national ones, even in smaller European countries and in Canada, but either at all or not implemented nationwide. This is a huge problem in the world,” said Milena Cavic, PhD, who presented the interim results on behalf of the diagnostics working group of the International Association for the Study of Lung Cancer early detection and screening committee at a press conference on Aug. 7 at the World Conference on Lung Cancer. Dr. Cavic is a senior research associate at the Institute for Oncology and Radiology of Serbia in Belgrade.
“It’s definitely a work in progress, and it’s also about raising awareness of the problem. In several parts of Asia, in Taiwan, in Korea, smoking is not the major, or at least, not the only reason for getting lung cancer. The other reasons are family history and also environmental factors like cooking fires, etc. So, the criteria we have for screening in Western countries are not one to one implementable in these countries,” said Rudolf Huber, MD, PhD, a respiratory physician at Ludwig Maximilian University of Munich and a coauthor of the report.
The report also pointed out the lack of recommendations for lung cancer screening in middle- and low-income countries. One approach would be to produce recommendations for countries with similar infrastructures and health resources, as well as primary risk factors such as smoking or cooking fires. “We have to adapt it to the various situations,” said Dr. Huber.
Another possibility is to rework existing recommendations for high income countries to adapt them to low- and middle-income countries. In the coming year, the working group will conduct a modeling study of Serbia, China, South Africa, and Columbia. It will look at population-specific and geographic factors from each country to produce country-specific models. “It will be interesting to see if these models will give us new recommendations for countries like this. So we can derive something from the high-income countries, but it will need to be adapted very, very much,” said Dr. Cavic.
The report highlighted some of the disparities between countries. CT scanners are far more common in high-income countries. Japan leads the way at 111.5 per million residents, followed by Australia at 70.2, Iceland at 47.6, and the United States at 44.9. At the other end is Columbia with 1.3, which trails Mexico at 5.9, Hungary at 9.4, and the United Kingdom at 9.5. However, the authors point out that there is no consensus on the optimum number of CT scanners per capita, since too few can lead to lack of access and too many can result in overuse. In fact, the greatest number of CT scans performed per capita was in the United States (278.5 per million), followed by Iceland (234.4), Japan (230.8), and Korea (228.1).
Lung cancer screening can be at odds with other health priorities, especially in low-income countries. These can include HIV, tuberculosis, and granulomatous diseases. But that could also provide an opportunity, according to Dr. Huber. “For example, in South Africa, tuberculosis programs are done by chest x-ray. We now have data that [allows us to] detect nodules by artificial intelligence, so one of the things we are thinking about is whether we could even use chest x-ray to get an earlier detection. At the end, it may be that in some countries it’s possible to do the classical CT screening, while in other countries we have to adapt to other options – probably chest x-ray using artificial intelligence or computer-aided diagnosis. And, then a consequent program for following up and managing the incidentally diagnosed nodules.”
The group is hoping to explore the environmental factors that could affect lung cancer risk in middle- and low-income countries. That is difficult to do, however, because smoking data can be hard to come by in many countries, and there is general uncertainty about what other risk factors may exist, though air pollution is a clear suspect. “It is something we are hoping to focus on in the future because there is a subgroup of individuals without a smoking history who are at high risk. It would be really good to find this high-risk population that should actually be screened in the future,” Dr. Cavic said.
Some countries have no data on lung cancer screening. For example, only South Africa is represented from Africa, and data is missing from many countries in Asia. The diagnostics working group of the IASLC early detection and screening committee has created a survey to gather information on the availability of lung cancer screening and its effect on diagnosis and treatment in countries throughout the world.
Dr. Cavic and Dr. Huber reported no relevant financial disclosures. The meeting was sponsored by the IASLC.
FROM WCLC 2022
How common are second primary lung cancers?
diagnosis, with about half occurring within 6 months of the first diagnosis. More than 80% of second primary cancers diagnosed within 2 years were stage 1, compared with about 25% when diagnosed more than 5 years later.
“With the growing adoption of lung cancer screening, more patients are being diagnosed with early-stage lung cancers and are able to achieve excellent long-term survival. After lung cancer diagnosis, these patients remain at high risk of developing a second primary lung cancer. The incidence, timing, and survival of second primary lung cancers is not well understood, particularly in a patient population with initial primary lung cancers detected via lung cancer screening,” said Alexandra Potter, who is a study coauthor.
The results were presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer sponsored by the International Association for the Study of Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
A 2012 study analyzed data from the SEER database and found that lung cancer survivors had a four- to sixfold increase in the risk of developing a second primary lung cancer, compared with the risk of lung cancer in the general population after adjusting for sex, age, race, and calendar year. “That study demonstrated that second primary lung cancers are an important risk among lung cancer survivors. However, it did not evaluate patients diagnosed with initial lung cancers detected via lung cancer screening. Thus, the incidence, timing, characteristics, and survival of lung cancers diagnosed among patients diagnosed with initial lung cancers detected via lung cancer screening remain unknown,” said Ms. Potter, who is a research assistant at Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
To address that question, the researchers used data from the National Lung Screening Trial, which compared low-dose computed tomography to chest x-ray and found that the former led to a 15%-20% lower risk of death. The new analysis included 1,405 patients who were diagnosed with stage I-III lung cancer and treated between 2002 and 2009. Of these patients, 5.8% went on to be diagnosed with a second primary lung cancer, at a rate of 1%-2% per year. Of the second lung cancers, 54.9% were synchronous, occurring within 6 months of the diagnosis, and 45.1% were metachronous, occurring later than 6 months; 65% of synchronous secondary cancers and 81% of metachronous cancers were diagnosed at stage I; 24% of synchronous and 14% of metachronous were stage III (P = .25). The median time to diagnosis of metachronous lung cancers was 2.7 years, and 27% of the second primary tumors were diagnosed 4 or more years after the first diagnosis.
Among those with synchronous tumors, 5- and 10-year survival rates were 55.2% and 39.5%. The rates were 90.0% and 30.8% among metachronous tumors, respectively. Ms. Potter emphasized that most patients with second primary cancer were diagnosed at stage I, suggesting that it is very possible to catch these cancers early. But patients who were diagnosed with a second primary tumor 4 or more years after their first diagnosis had a greater likelihood of later-stage second cancer. Medical societies generally recommend CT screening surveillance every 6 months for 2 years following a lung cancer diagnosis, then annually thereafter. The greater frequency of later-stage cancer detected after 4 years suggests that surveillance may be flagging as time goes on. “These data highlight the importance of lifelong follow up after initial lung cancer diagnosis,” said Ms. Potter.
She also emphasized the importance of smoking cessation and ongoing abstinence following a diagnosis of lung cancer. “About 70% of patients in the NLST who developed second primary lung cancer currently smoked at the time of entry into the trial. Smoking cessation can help reduce patients’ risk of developing second primary lung cancers,” she said. Ms. Potter has no relevant financial disclosures.
diagnosis, with about half occurring within 6 months of the first diagnosis. More than 80% of second primary cancers diagnosed within 2 years were stage 1, compared with about 25% when diagnosed more than 5 years later.
“With the growing adoption of lung cancer screening, more patients are being diagnosed with early-stage lung cancers and are able to achieve excellent long-term survival. After lung cancer diagnosis, these patients remain at high risk of developing a second primary lung cancer. The incidence, timing, and survival of second primary lung cancers is not well understood, particularly in a patient population with initial primary lung cancers detected via lung cancer screening,” said Alexandra Potter, who is a study coauthor.
The results were presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer sponsored by the International Association for the Study of Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
A 2012 study analyzed data from the SEER database and found that lung cancer survivors had a four- to sixfold increase in the risk of developing a second primary lung cancer, compared with the risk of lung cancer in the general population after adjusting for sex, age, race, and calendar year. “That study demonstrated that second primary lung cancers are an important risk among lung cancer survivors. However, it did not evaluate patients diagnosed with initial lung cancers detected via lung cancer screening. Thus, the incidence, timing, characteristics, and survival of lung cancers diagnosed among patients diagnosed with initial lung cancers detected via lung cancer screening remain unknown,” said Ms. Potter, who is a research assistant at Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
To address that question, the researchers used data from the National Lung Screening Trial, which compared low-dose computed tomography to chest x-ray and found that the former led to a 15%-20% lower risk of death. The new analysis included 1,405 patients who were diagnosed with stage I-III lung cancer and treated between 2002 and 2009. Of these patients, 5.8% went on to be diagnosed with a second primary lung cancer, at a rate of 1%-2% per year. Of the second lung cancers, 54.9% were synchronous, occurring within 6 months of the diagnosis, and 45.1% were metachronous, occurring later than 6 months; 65% of synchronous secondary cancers and 81% of metachronous cancers were diagnosed at stage I; 24% of synchronous and 14% of metachronous were stage III (P = .25). The median time to diagnosis of metachronous lung cancers was 2.7 years, and 27% of the second primary tumors were diagnosed 4 or more years after the first diagnosis.
Among those with synchronous tumors, 5- and 10-year survival rates were 55.2% and 39.5%. The rates were 90.0% and 30.8% among metachronous tumors, respectively. Ms. Potter emphasized that most patients with second primary cancer were diagnosed at stage I, suggesting that it is very possible to catch these cancers early. But patients who were diagnosed with a second primary tumor 4 or more years after their first diagnosis had a greater likelihood of later-stage second cancer. Medical societies generally recommend CT screening surveillance every 6 months for 2 years following a lung cancer diagnosis, then annually thereafter. The greater frequency of later-stage cancer detected after 4 years suggests that surveillance may be flagging as time goes on. “These data highlight the importance of lifelong follow up after initial lung cancer diagnosis,” said Ms. Potter.
She also emphasized the importance of smoking cessation and ongoing abstinence following a diagnosis of lung cancer. “About 70% of patients in the NLST who developed second primary lung cancer currently smoked at the time of entry into the trial. Smoking cessation can help reduce patients’ risk of developing second primary lung cancers,” she said. Ms. Potter has no relevant financial disclosures.
diagnosis, with about half occurring within 6 months of the first diagnosis. More than 80% of second primary cancers diagnosed within 2 years were stage 1, compared with about 25% when diagnosed more than 5 years later.
“With the growing adoption of lung cancer screening, more patients are being diagnosed with early-stage lung cancers and are able to achieve excellent long-term survival. After lung cancer diagnosis, these patients remain at high risk of developing a second primary lung cancer. The incidence, timing, and survival of second primary lung cancers is not well understood, particularly in a patient population with initial primary lung cancers detected via lung cancer screening,” said Alexandra Potter, who is a study coauthor.
The results were presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer sponsored by the International Association for the Study of Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
A 2012 study analyzed data from the SEER database and found that lung cancer survivors had a four- to sixfold increase in the risk of developing a second primary lung cancer, compared with the risk of lung cancer in the general population after adjusting for sex, age, race, and calendar year. “That study demonstrated that second primary lung cancers are an important risk among lung cancer survivors. However, it did not evaluate patients diagnosed with initial lung cancers detected via lung cancer screening. Thus, the incidence, timing, characteristics, and survival of lung cancers diagnosed among patients diagnosed with initial lung cancers detected via lung cancer screening remain unknown,” said Ms. Potter, who is a research assistant at Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
To address that question, the researchers used data from the National Lung Screening Trial, which compared low-dose computed tomography to chest x-ray and found that the former led to a 15%-20% lower risk of death. The new analysis included 1,405 patients who were diagnosed with stage I-III lung cancer and treated between 2002 and 2009. Of these patients, 5.8% went on to be diagnosed with a second primary lung cancer, at a rate of 1%-2% per year. Of the second lung cancers, 54.9% were synchronous, occurring within 6 months of the diagnosis, and 45.1% were metachronous, occurring later than 6 months; 65% of synchronous secondary cancers and 81% of metachronous cancers were diagnosed at stage I; 24% of synchronous and 14% of metachronous were stage III (P = .25). The median time to diagnosis of metachronous lung cancers was 2.7 years, and 27% of the second primary tumors were diagnosed 4 or more years after the first diagnosis.
Among those with synchronous tumors, 5- and 10-year survival rates were 55.2% and 39.5%. The rates were 90.0% and 30.8% among metachronous tumors, respectively. Ms. Potter emphasized that most patients with second primary cancer were diagnosed at stage I, suggesting that it is very possible to catch these cancers early. But patients who were diagnosed with a second primary tumor 4 or more years after their first diagnosis had a greater likelihood of later-stage second cancer. Medical societies generally recommend CT screening surveillance every 6 months for 2 years following a lung cancer diagnosis, then annually thereafter. The greater frequency of later-stage cancer detected after 4 years suggests that surveillance may be flagging as time goes on. “These data highlight the importance of lifelong follow up after initial lung cancer diagnosis,” said Ms. Potter.
She also emphasized the importance of smoking cessation and ongoing abstinence following a diagnosis of lung cancer. “About 70% of patients in the NLST who developed second primary lung cancer currently smoked at the time of entry into the trial. Smoking cessation can help reduce patients’ risk of developing second primary lung cancers,” she said. Ms. Potter has no relevant financial disclosures.
FROM WCLC 2022
FDA approves Enhertu (trastuzumab deruxtecan) for HER2 lung cancer
Patients with lung cancer now have another treatment option: If their tumors are found to carry HER2 mutations, they can now be treated with trastuzumab deruxtecan (Enhertu), a drug that specifically targets that defect.
This product is already approved for used in HER2-positive breast cancer and gastric cancer.
The FDA also approved companion diagnostic tests to detect HER2 mutations: Life Technologies Corporation’s Oncomine Dx Target Test for use in lung tissue and Guardant Health’s Guardant360 CDx for use on plasma samples. The agency notes that if no mutation is detected in a plasma specimen, the tumor tissue should be tested.
Specifically, the new indication is used in patients with unresectable or metastatic non–small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have already received a prior systemic therapy.
About 3% of nonsquamous NSCLC tumors carry mutations in the HER2 gene, and they are associated with female sex, never-smokers, and a poor prognosis.
“HER2 mutant non–small cell lung cancer is an aggressive form of disease, which commonly affects young patients who have faced limited treatment options and a poor prognosis to date,” said Dave Fredrickson, executive vice-president of the oncology business unit at AstraZeneca.
The new approval “provides these patients with the opportunity to benefit from a targeted therapy and highlights the importance of testing for predictive markers, including HER2 in lung cancer, at the time of diagnosis to ensure patients receive the most appropriate treatment for their specific disease,” he commented in a company press release.
This is an accelerated approval, based on overall response rate data from the DESTINY-Lung02 phase 2 trial, the company noted. An interim efficacy analysis in a prespecified patient cohort showed that trastuzumab deruxtecan (at 5.4 mg/kg) demonstrated a confirmed overall response rate of 57.7% (n = 52; 95% confidence interval, 43.2%-71.3%) in patients with HER2-mutant unresectable or metastatic nonsquamous NSCLC who had received one prior systemic therapy as assessed by blinded independent central review. Complete responses were seen in 1.9% of patients (n = 1) and partial responses in 55.8% of patients (n = 29), with a median duration of response of 8.7 months (95% CI, 7.1-NE).
The FDA noted that for the 52 patients in the primary efficacy population of the DESTINY-Lung02 trial, the median age was 58 years (range, 30-78 years); 69% were female; and 79% were Asian, 12% were White, and 10% were of other races.
Clinical data welcomed by experts
Clinical data are already available from the DESTINY-Lung 01 trial, and the results were welcomed enthusiastically by experts when they were published in the New England Journal of Medicine earlier this year.
“These results establish the new standard of care for patients with NSCLC harboring HER2 mutations,” Antonio Passaro, MD, PhD, from the European Institute of Oncology IRCCS, Milan, and Solange Peters, MD, PhD, from Lausanne (Switzerland) University Hospital, wrote in an accompanying editorial.
This trial involved 91 patients, all treated with trastuzumab deruxtecan (at 6.4 mg/kg of body weight every 3 weeks). The median duration of treatment was 6.9 months, and the median follow-up was 13.1 months.
The results showed a 55% centrally confirmed objective response, and median duration of response was 9.3 months.
In addition, the investigators reported a median progression-free survival of 8.2 months and a median overall survival of almost 18 months, both of which they described as “encouraging” in this patient population.
However, the results also highlighted a problem with the drug in this patient population. Notably, 26% of patients experienced interstitial lung disease, which resulted in death in two patients. The drug was also withdrawn in 16 patients and interrupted in 8 patients because of this adverse event.
Editorialists Dr. Passaro and Dr. Peters described this finding as “a concern” and note that “the incidence of interstitial lung disease is significantly higher among patients with lung cancer than among those with breast or gastric cancers, which may indicate a role of smoking-related damage.”
They also highlighted the need for an “investigation of the clinical efficacy of a reduced dose of trastuzumab deruxtecan,” and so the dose was reduced for the DESTINY-Lung02 trial.
A version of this article first appeared on Medscape.com.
Patients with lung cancer now have another treatment option: If their tumors are found to carry HER2 mutations, they can now be treated with trastuzumab deruxtecan (Enhertu), a drug that specifically targets that defect.
This product is already approved for used in HER2-positive breast cancer and gastric cancer.
The FDA also approved companion diagnostic tests to detect HER2 mutations: Life Technologies Corporation’s Oncomine Dx Target Test for use in lung tissue and Guardant Health’s Guardant360 CDx for use on plasma samples. The agency notes that if no mutation is detected in a plasma specimen, the tumor tissue should be tested.
Specifically, the new indication is used in patients with unresectable or metastatic non–small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have already received a prior systemic therapy.
About 3% of nonsquamous NSCLC tumors carry mutations in the HER2 gene, and they are associated with female sex, never-smokers, and a poor prognosis.
“HER2 mutant non–small cell lung cancer is an aggressive form of disease, which commonly affects young patients who have faced limited treatment options and a poor prognosis to date,” said Dave Fredrickson, executive vice-president of the oncology business unit at AstraZeneca.
The new approval “provides these patients with the opportunity to benefit from a targeted therapy and highlights the importance of testing for predictive markers, including HER2 in lung cancer, at the time of diagnosis to ensure patients receive the most appropriate treatment for their specific disease,” he commented in a company press release.
This is an accelerated approval, based on overall response rate data from the DESTINY-Lung02 phase 2 trial, the company noted. An interim efficacy analysis in a prespecified patient cohort showed that trastuzumab deruxtecan (at 5.4 mg/kg) demonstrated a confirmed overall response rate of 57.7% (n = 52; 95% confidence interval, 43.2%-71.3%) in patients with HER2-mutant unresectable or metastatic nonsquamous NSCLC who had received one prior systemic therapy as assessed by blinded independent central review. Complete responses were seen in 1.9% of patients (n = 1) and partial responses in 55.8% of patients (n = 29), with a median duration of response of 8.7 months (95% CI, 7.1-NE).
The FDA noted that for the 52 patients in the primary efficacy population of the DESTINY-Lung02 trial, the median age was 58 years (range, 30-78 years); 69% were female; and 79% were Asian, 12% were White, and 10% were of other races.
Clinical data welcomed by experts
Clinical data are already available from the DESTINY-Lung 01 trial, and the results were welcomed enthusiastically by experts when they were published in the New England Journal of Medicine earlier this year.
“These results establish the new standard of care for patients with NSCLC harboring HER2 mutations,” Antonio Passaro, MD, PhD, from the European Institute of Oncology IRCCS, Milan, and Solange Peters, MD, PhD, from Lausanne (Switzerland) University Hospital, wrote in an accompanying editorial.
This trial involved 91 patients, all treated with trastuzumab deruxtecan (at 6.4 mg/kg of body weight every 3 weeks). The median duration of treatment was 6.9 months, and the median follow-up was 13.1 months.
The results showed a 55% centrally confirmed objective response, and median duration of response was 9.3 months.
In addition, the investigators reported a median progression-free survival of 8.2 months and a median overall survival of almost 18 months, both of which they described as “encouraging” in this patient population.
However, the results also highlighted a problem with the drug in this patient population. Notably, 26% of patients experienced interstitial lung disease, which resulted in death in two patients. The drug was also withdrawn in 16 patients and interrupted in 8 patients because of this adverse event.
Editorialists Dr. Passaro and Dr. Peters described this finding as “a concern” and note that “the incidence of interstitial lung disease is significantly higher among patients with lung cancer than among those with breast or gastric cancers, which may indicate a role of smoking-related damage.”
They also highlighted the need for an “investigation of the clinical efficacy of a reduced dose of trastuzumab deruxtecan,” and so the dose was reduced for the DESTINY-Lung02 trial.
A version of this article first appeared on Medscape.com.
Patients with lung cancer now have another treatment option: If their tumors are found to carry HER2 mutations, they can now be treated with trastuzumab deruxtecan (Enhertu), a drug that specifically targets that defect.
This product is already approved for used in HER2-positive breast cancer and gastric cancer.
The FDA also approved companion diagnostic tests to detect HER2 mutations: Life Technologies Corporation’s Oncomine Dx Target Test for use in lung tissue and Guardant Health’s Guardant360 CDx for use on plasma samples. The agency notes that if no mutation is detected in a plasma specimen, the tumor tissue should be tested.
Specifically, the new indication is used in patients with unresectable or metastatic non–small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have already received a prior systemic therapy.
About 3% of nonsquamous NSCLC tumors carry mutations in the HER2 gene, and they are associated with female sex, never-smokers, and a poor prognosis.
“HER2 mutant non–small cell lung cancer is an aggressive form of disease, which commonly affects young patients who have faced limited treatment options and a poor prognosis to date,” said Dave Fredrickson, executive vice-president of the oncology business unit at AstraZeneca.
The new approval “provides these patients with the opportunity to benefit from a targeted therapy and highlights the importance of testing for predictive markers, including HER2 in lung cancer, at the time of diagnosis to ensure patients receive the most appropriate treatment for their specific disease,” he commented in a company press release.
This is an accelerated approval, based on overall response rate data from the DESTINY-Lung02 phase 2 trial, the company noted. An interim efficacy analysis in a prespecified patient cohort showed that trastuzumab deruxtecan (at 5.4 mg/kg) demonstrated a confirmed overall response rate of 57.7% (n = 52; 95% confidence interval, 43.2%-71.3%) in patients with HER2-mutant unresectable or metastatic nonsquamous NSCLC who had received one prior systemic therapy as assessed by blinded independent central review. Complete responses were seen in 1.9% of patients (n = 1) and partial responses in 55.8% of patients (n = 29), with a median duration of response of 8.7 months (95% CI, 7.1-NE).
The FDA noted that for the 52 patients in the primary efficacy population of the DESTINY-Lung02 trial, the median age was 58 years (range, 30-78 years); 69% were female; and 79% were Asian, 12% were White, and 10% were of other races.
Clinical data welcomed by experts
Clinical data are already available from the DESTINY-Lung 01 trial, and the results were welcomed enthusiastically by experts when they were published in the New England Journal of Medicine earlier this year.
“These results establish the new standard of care for patients with NSCLC harboring HER2 mutations,” Antonio Passaro, MD, PhD, from the European Institute of Oncology IRCCS, Milan, and Solange Peters, MD, PhD, from Lausanne (Switzerland) University Hospital, wrote in an accompanying editorial.
This trial involved 91 patients, all treated with trastuzumab deruxtecan (at 6.4 mg/kg of body weight every 3 weeks). The median duration of treatment was 6.9 months, and the median follow-up was 13.1 months.
The results showed a 55% centrally confirmed objective response, and median duration of response was 9.3 months.
In addition, the investigators reported a median progression-free survival of 8.2 months and a median overall survival of almost 18 months, both of which they described as “encouraging” in this patient population.
However, the results also highlighted a problem with the drug in this patient population. Notably, 26% of patients experienced interstitial lung disease, which resulted in death in two patients. The drug was also withdrawn in 16 patients and interrupted in 8 patients because of this adverse event.
Editorialists Dr. Passaro and Dr. Peters described this finding as “a concern” and note that “the incidence of interstitial lung disease is significantly higher among patients with lung cancer than among those with breast or gastric cancers, which may indicate a role of smoking-related damage.”
They also highlighted the need for an “investigation of the clinical efficacy of a reduced dose of trastuzumab deruxtecan,” and so the dose was reduced for the DESTINY-Lung02 trial.
A version of this article first appeared on Medscape.com.
More than 64% of younger adults with lung cancer diagnosed at later stages
Advances have been made in earlier diagnosis and better overall survival among older patients with lung cancer, but younger adults have not experienced the same benefit, according to a new study.
The improvements in patients aged 55-80 are likely associated with the introduction in 2013 of low dose computed tomography lung cancer screening.
“It was unknown whether young adults diagnosed with lung cancer, who are ineligible for screening, have experienced a similar shift to earlier stages of lung cancer. While previous studies have shown that young adults diagnosed with lung cancer have distinct tumor characteristics and survival compared to older adults diagnosed with lung cancer, no study has examined whether recent improvements in early diagnosis and survival among older adults with lung cancer extend to younger adults diagnosed with lung cancer,” study coauthor Alexandra Potter told this news organization.
The study was presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
The difference might be explained by difference in tumor biology, as younger adults are often diagnosed with more aggressive cancers. Other factors include delayed diagnosis and a lack of early detection strategies for this population. Older patients likely benefited from the onset of lung cancer screening, as well as an increase in non-screening chest CT use in hospital settings, which may lead to more incidental diagnoses, according to Ms. Potter, a research assistant Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
Investigators found that about three in four lung cancer diagnoses among adults aged 20-29 were stage IV disease, and only 8% in that group were stage I. “I was surprised” by the high frequency of stage IV cancer, said Ms. Potter. “I would also highlight that there has been no improvement in early diagnosis among patients aged 20-49 during the study period,” she added.
And : Five-year survival was 10%-15% among patients diagnosed at age 20-49 with stage IV cancer. “More research is needed to better understand the risk factors, diagnosis, treatment, and survival of lung cancer in young adults,” Ms. Potter said.
There are strategies in development, including biomarkers, machine learning analysis of CT scans, and risk prediction models, but none have yet borne fruit. “Once we are able to [identify high-risk young adults], this will allow us to offer lung cancer screening to these young adults who are at high risk for developing lung cancer,” Ms. Potter said.
Study methodology
The researchers analyzed data from the United States Cancer Statistics (USCS) database and the National Cancer Database (NCDB). They included patients aged 20-79 diagnosed with non–small cell lung cancer (NSCLC) between 2010 and 2018. The study included 1,328 individuals aged 20-29, 5,682 men and women aged 30-39, 39,323 individuals aged 40-49, 202,709 aged 50-59, 410,482 aged 60-69, and 447,366 aged 70-79.
Stage IV diagnoses were most common in the youngest group (76% versus 8% stage I), and steadily declined with age 30-39 (70% versus 10%), age 40-49 (60% versus 14%), 50-59 (52%versus 19%), 60-69 (45% versus 25%), and 70-79 (40% versus 25%; P < .001). The trend reversed among patients aged 80-89, with 45% of patients diagnosed with stage IV cancer, though the rising trend of stage I diagnoses continued at 29%. Between 2010 and 2018, there was a statistically significant increase in stage IV diagnoses among those aged 40-49, and a decrease among those aged 50-59, 60-69, and 70-79.
Five-year overall survival was lowest among patients aged 20-29 at 20%. It was 27%-28% among each 10-year age group up to age 69, then dropped to 24% among those aged 70-79 (P < .001).
The study was limited by a lack of data on disease-free or recurrence-free survival, as well as use of biomarkers or targeted therapy. Ms. Potter has no relevant financial disclosures. The conference was sponsored by the International Association for the Study of Lung Cancer.
Advances have been made in earlier diagnosis and better overall survival among older patients with lung cancer, but younger adults have not experienced the same benefit, according to a new study.
The improvements in patients aged 55-80 are likely associated with the introduction in 2013 of low dose computed tomography lung cancer screening.
“It was unknown whether young adults diagnosed with lung cancer, who are ineligible for screening, have experienced a similar shift to earlier stages of lung cancer. While previous studies have shown that young adults diagnosed with lung cancer have distinct tumor characteristics and survival compared to older adults diagnosed with lung cancer, no study has examined whether recent improvements in early diagnosis and survival among older adults with lung cancer extend to younger adults diagnosed with lung cancer,” study coauthor Alexandra Potter told this news organization.
The study was presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
The difference might be explained by difference in tumor biology, as younger adults are often diagnosed with more aggressive cancers. Other factors include delayed diagnosis and a lack of early detection strategies for this population. Older patients likely benefited from the onset of lung cancer screening, as well as an increase in non-screening chest CT use in hospital settings, which may lead to more incidental diagnoses, according to Ms. Potter, a research assistant Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
Investigators found that about three in four lung cancer diagnoses among adults aged 20-29 were stage IV disease, and only 8% in that group were stage I. “I was surprised” by the high frequency of stage IV cancer, said Ms. Potter. “I would also highlight that there has been no improvement in early diagnosis among patients aged 20-49 during the study period,” she added.
And : Five-year survival was 10%-15% among patients diagnosed at age 20-49 with stage IV cancer. “More research is needed to better understand the risk factors, diagnosis, treatment, and survival of lung cancer in young adults,” Ms. Potter said.
There are strategies in development, including biomarkers, machine learning analysis of CT scans, and risk prediction models, but none have yet borne fruit. “Once we are able to [identify high-risk young adults], this will allow us to offer lung cancer screening to these young adults who are at high risk for developing lung cancer,” Ms. Potter said.
Study methodology
The researchers analyzed data from the United States Cancer Statistics (USCS) database and the National Cancer Database (NCDB). They included patients aged 20-79 diagnosed with non–small cell lung cancer (NSCLC) between 2010 and 2018. The study included 1,328 individuals aged 20-29, 5,682 men and women aged 30-39, 39,323 individuals aged 40-49, 202,709 aged 50-59, 410,482 aged 60-69, and 447,366 aged 70-79.
Stage IV diagnoses were most common in the youngest group (76% versus 8% stage I), and steadily declined with age 30-39 (70% versus 10%), age 40-49 (60% versus 14%), 50-59 (52%versus 19%), 60-69 (45% versus 25%), and 70-79 (40% versus 25%; P < .001). The trend reversed among patients aged 80-89, with 45% of patients diagnosed with stage IV cancer, though the rising trend of stage I diagnoses continued at 29%. Between 2010 and 2018, there was a statistically significant increase in stage IV diagnoses among those aged 40-49, and a decrease among those aged 50-59, 60-69, and 70-79.
Five-year overall survival was lowest among patients aged 20-29 at 20%. It was 27%-28% among each 10-year age group up to age 69, then dropped to 24% among those aged 70-79 (P < .001).
The study was limited by a lack of data on disease-free or recurrence-free survival, as well as use of biomarkers or targeted therapy. Ms. Potter has no relevant financial disclosures. The conference was sponsored by the International Association for the Study of Lung Cancer.
Advances have been made in earlier diagnosis and better overall survival among older patients with lung cancer, but younger adults have not experienced the same benefit, according to a new study.
The improvements in patients aged 55-80 are likely associated with the introduction in 2013 of low dose computed tomography lung cancer screening.
“It was unknown whether young adults diagnosed with lung cancer, who are ineligible for screening, have experienced a similar shift to earlier stages of lung cancer. While previous studies have shown that young adults diagnosed with lung cancer have distinct tumor characteristics and survival compared to older adults diagnosed with lung cancer, no study has examined whether recent improvements in early diagnosis and survival among older adults with lung cancer extend to younger adults diagnosed with lung cancer,” study coauthor Alexandra Potter told this news organization.
The study was presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
The difference might be explained by difference in tumor biology, as younger adults are often diagnosed with more aggressive cancers. Other factors include delayed diagnosis and a lack of early detection strategies for this population. Older patients likely benefited from the onset of lung cancer screening, as well as an increase in non-screening chest CT use in hospital settings, which may lead to more incidental diagnoses, according to Ms. Potter, a research assistant Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
Investigators found that about three in four lung cancer diagnoses among adults aged 20-29 were stage IV disease, and only 8% in that group were stage I. “I was surprised” by the high frequency of stage IV cancer, said Ms. Potter. “I would also highlight that there has been no improvement in early diagnosis among patients aged 20-49 during the study period,” she added.
And : Five-year survival was 10%-15% among patients diagnosed at age 20-49 with stage IV cancer. “More research is needed to better understand the risk factors, diagnosis, treatment, and survival of lung cancer in young adults,” Ms. Potter said.
There are strategies in development, including biomarkers, machine learning analysis of CT scans, and risk prediction models, but none have yet borne fruit. “Once we are able to [identify high-risk young adults], this will allow us to offer lung cancer screening to these young adults who are at high risk for developing lung cancer,” Ms. Potter said.
Study methodology
The researchers analyzed data from the United States Cancer Statistics (USCS) database and the National Cancer Database (NCDB). They included patients aged 20-79 diagnosed with non–small cell lung cancer (NSCLC) between 2010 and 2018. The study included 1,328 individuals aged 20-29, 5,682 men and women aged 30-39, 39,323 individuals aged 40-49, 202,709 aged 50-59, 410,482 aged 60-69, and 447,366 aged 70-79.
Stage IV diagnoses were most common in the youngest group (76% versus 8% stage I), and steadily declined with age 30-39 (70% versus 10%), age 40-49 (60% versus 14%), 50-59 (52%versus 19%), 60-69 (45% versus 25%), and 70-79 (40% versus 25%; P < .001). The trend reversed among patients aged 80-89, with 45% of patients diagnosed with stage IV cancer, though the rising trend of stage I diagnoses continued at 29%. Between 2010 and 2018, there was a statistically significant increase in stage IV diagnoses among those aged 40-49, and a decrease among those aged 50-59, 60-69, and 70-79.
Five-year overall survival was lowest among patients aged 20-29 at 20%. It was 27%-28% among each 10-year age group up to age 69, then dropped to 24% among those aged 70-79 (P < .001).
The study was limited by a lack of data on disease-free or recurrence-free survival, as well as use of biomarkers or targeted therapy. Ms. Potter has no relevant financial disclosures. The conference was sponsored by the International Association for the Study of Lung Cancer.
FROM WCLC 2022
Commentary: Concomitant Therapies May Affect NSCLC Survival, August 2022
A Danish population-based cohort study by Ehrenstein and colleagues involved 21,282 patients with non–small-cell lung cancer (NSCLC), 8758 of whom received a diagnosis at stage I-IIIA. Of those, 4071 (46%) were tested for epidermal growth factor receptor (EGFR) mutations at diagnosis. Median overall survival (OS) was 5.7 years among patients with EGFR mutation–positive status (n = 361) and 4.4 years among patients with EGFR mutation–negative status (n = 3710). EGFR mutation–positive status was associated with lower all-cause mortality in all subgroups. This is not surprising, because EGFR-mutated lung cancers are associated with never or light smoking history and the patients tend to be younger and have fewer medical comorbidities. Nevertheless, the lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios (HR) ranging from 0.48 to 0.83. In addition, targeted therapies, such as osimertinib, improved OS and progression-free survival (PFS) in the metastatic setting as first-line treatment. Now that the ADAURA study has demonstrated a substantial disease-free survival benefit with osimertinib in the adjuvant setting in early-stage EGFR-mutated NSCLC (with final OS results still to come) it will be interesting to see whether this magnitude of difference in OS grows over time.
Wang and colleagues conducted a large meta-analysis of 10 retrospective studies and one prospective study including a total of 5892 patients with NSCLC who were receiving programmed cell death protein 1 (PD-1)/ programmed death-ligand 1 (PD-L1) inhibitors with the concomitant use of gastric acid suppressants (GAS). Use of PD-1/PD-L1 inhibitors with vs without GAS worsened PFS by 32% (HR 1.32; P < .001) and OS by 36% (HR 1.36; P < .001). The GAS in these studies were predominantly proton-pump inhibitors (PPI). There is still much to learn about medications that may influence outcomes to immune checkpoint blockade, such as steroids, antibiotics, GAS, and others. We are also learning that the microbiome probably plays an important role in contributing to activity of PD-1/PDL-1 antibodies and PPI may modify the microbiome. More research is needed, but it is reasonable to try and switch patients receiving PD-1/PDL-1 inhibitors from PPI to other GAS, if clinically appropriate.
Nazha and colleagues performed a SEER-Medicare database analysis evaluating 367,750 patients with lung cancer. A total of 11,061 patients had an initial prostate cancer diagnosis and subsequent lung cancer diagnosis, 3017 had an initial lung cancer diagnosis and subsequent prostate cancer diagnosis, and the remaining patients had an isolated lung cancer diagnosis. Patients who received androgen deprivation therapy (ADT) for a previously diagnosed prostate cancer showed improved survival after lung cancer diagnosis (adjusted HR for death 0.88; P = .02) and a shorter latency period to the diagnosis of lung cancer (40 vs 47 months; P < .001) compared with those who did not receive ADT. This finding applied mainly to White patients and may not apply to Black patients because there was an underrepresentation of Black patients in the study. The association of ADT for prostate cancer improving clinical outcomes in patients subsequently diagnosed with lung cancer is intriguing. There is known crosstalk between receptor kinase signaling and androgen receptor signaling that may biologically explain the findings in this study. This theoretically could apply more to certain molecular subtypes of lung cancer, such as EGFR-mutated lung cancer. However, further studies are needed to confirm this because confounding factors and immortal time bias (where patients receiving ADT may be more likely to have more frequent interactions in the healthcare system and thus receive an earlier lung cancer diagnosis) may in part explain the findings in this retrospective analysis. More research is needed to determine whether patients with prostate cancer receiving ADT had improved survival compared with those who did not receive ADT.
A Danish population-based cohort study by Ehrenstein and colleagues involved 21,282 patients with non–small-cell lung cancer (NSCLC), 8758 of whom received a diagnosis at stage I-IIIA. Of those, 4071 (46%) were tested for epidermal growth factor receptor (EGFR) mutations at diagnosis. Median overall survival (OS) was 5.7 years among patients with EGFR mutation–positive status (n = 361) and 4.4 years among patients with EGFR mutation–negative status (n = 3710). EGFR mutation–positive status was associated with lower all-cause mortality in all subgroups. This is not surprising, because EGFR-mutated lung cancers are associated with never or light smoking history and the patients tend to be younger and have fewer medical comorbidities. Nevertheless, the lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios (HR) ranging from 0.48 to 0.83. In addition, targeted therapies, such as osimertinib, improved OS and progression-free survival (PFS) in the metastatic setting as first-line treatment. Now that the ADAURA study has demonstrated a substantial disease-free survival benefit with osimertinib in the adjuvant setting in early-stage EGFR-mutated NSCLC (with final OS results still to come) it will be interesting to see whether this magnitude of difference in OS grows over time.
Wang and colleagues conducted a large meta-analysis of 10 retrospective studies and one prospective study including a total of 5892 patients with NSCLC who were receiving programmed cell death protein 1 (PD-1)/ programmed death-ligand 1 (PD-L1) inhibitors with the concomitant use of gastric acid suppressants (GAS). Use of PD-1/PD-L1 inhibitors with vs without GAS worsened PFS by 32% (HR 1.32; P < .001) and OS by 36% (HR 1.36; P < .001). The GAS in these studies were predominantly proton-pump inhibitors (PPI). There is still much to learn about medications that may influence outcomes to immune checkpoint blockade, such as steroids, antibiotics, GAS, and others. We are also learning that the microbiome probably plays an important role in contributing to activity of PD-1/PDL-1 antibodies and PPI may modify the microbiome. More research is needed, but it is reasonable to try and switch patients receiving PD-1/PDL-1 inhibitors from PPI to other GAS, if clinically appropriate.
Nazha and colleagues performed a SEER-Medicare database analysis evaluating 367,750 patients with lung cancer. A total of 11,061 patients had an initial prostate cancer diagnosis and subsequent lung cancer diagnosis, 3017 had an initial lung cancer diagnosis and subsequent prostate cancer diagnosis, and the remaining patients had an isolated lung cancer diagnosis. Patients who received androgen deprivation therapy (ADT) for a previously diagnosed prostate cancer showed improved survival after lung cancer diagnosis (adjusted HR for death 0.88; P = .02) and a shorter latency period to the diagnosis of lung cancer (40 vs 47 months; P < .001) compared with those who did not receive ADT. This finding applied mainly to White patients and may not apply to Black patients because there was an underrepresentation of Black patients in the study. The association of ADT for prostate cancer improving clinical outcomes in patients subsequently diagnosed with lung cancer is intriguing. There is known crosstalk between receptor kinase signaling and androgen receptor signaling that may biologically explain the findings in this study. This theoretically could apply more to certain molecular subtypes of lung cancer, such as EGFR-mutated lung cancer. However, further studies are needed to confirm this because confounding factors and immortal time bias (where patients receiving ADT may be more likely to have more frequent interactions in the healthcare system and thus receive an earlier lung cancer diagnosis) may in part explain the findings in this retrospective analysis. More research is needed to determine whether patients with prostate cancer receiving ADT had improved survival compared with those who did not receive ADT.
A Danish population-based cohort study by Ehrenstein and colleagues involved 21,282 patients with non–small-cell lung cancer (NSCLC), 8758 of whom received a diagnosis at stage I-IIIA. Of those, 4071 (46%) were tested for epidermal growth factor receptor (EGFR) mutations at diagnosis. Median overall survival (OS) was 5.7 years among patients with EGFR mutation–positive status (n = 361) and 4.4 years among patients with EGFR mutation–negative status (n = 3710). EGFR mutation–positive status was associated with lower all-cause mortality in all subgroups. This is not surprising, because EGFR-mutated lung cancers are associated with never or light smoking history and the patients tend to be younger and have fewer medical comorbidities. Nevertheless, the lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios (HR) ranging from 0.48 to 0.83. In addition, targeted therapies, such as osimertinib, improved OS and progression-free survival (PFS) in the metastatic setting as first-line treatment. Now that the ADAURA study has demonstrated a substantial disease-free survival benefit with osimertinib in the adjuvant setting in early-stage EGFR-mutated NSCLC (with final OS results still to come) it will be interesting to see whether this magnitude of difference in OS grows over time.
Wang and colleagues conducted a large meta-analysis of 10 retrospective studies and one prospective study including a total of 5892 patients with NSCLC who were receiving programmed cell death protein 1 (PD-1)/ programmed death-ligand 1 (PD-L1) inhibitors with the concomitant use of gastric acid suppressants (GAS). Use of PD-1/PD-L1 inhibitors with vs without GAS worsened PFS by 32% (HR 1.32; P < .001) and OS by 36% (HR 1.36; P < .001). The GAS in these studies were predominantly proton-pump inhibitors (PPI). There is still much to learn about medications that may influence outcomes to immune checkpoint blockade, such as steroids, antibiotics, GAS, and others. We are also learning that the microbiome probably plays an important role in contributing to activity of PD-1/PDL-1 antibodies and PPI may modify the microbiome. More research is needed, but it is reasonable to try and switch patients receiving PD-1/PDL-1 inhibitors from PPI to other GAS, if clinically appropriate.
Nazha and colleagues performed a SEER-Medicare database analysis evaluating 367,750 patients with lung cancer. A total of 11,061 patients had an initial prostate cancer diagnosis and subsequent lung cancer diagnosis, 3017 had an initial lung cancer diagnosis and subsequent prostate cancer diagnosis, and the remaining patients had an isolated lung cancer diagnosis. Patients who received androgen deprivation therapy (ADT) for a previously diagnosed prostate cancer showed improved survival after lung cancer diagnosis (adjusted HR for death 0.88; P = .02) and a shorter latency period to the diagnosis of lung cancer (40 vs 47 months; P < .001) compared with those who did not receive ADT. This finding applied mainly to White patients and may not apply to Black patients because there was an underrepresentation of Black patients in the study. The association of ADT for prostate cancer improving clinical outcomes in patients subsequently diagnosed with lung cancer is intriguing. There is known crosstalk between receptor kinase signaling and androgen receptor signaling that may biologically explain the findings in this study. This theoretically could apply more to certain molecular subtypes of lung cancer, such as EGFR-mutated lung cancer. However, further studies are needed to confirm this because confounding factors and immortal time bias (where patients receiving ADT may be more likely to have more frequent interactions in the healthcare system and thus receive an earlier lung cancer diagnosis) may in part explain the findings in this retrospective analysis. More research is needed to determine whether patients with prostate cancer receiving ADT had improved survival compared with those who did not receive ADT.
Air pollution contribution to lung cancer may be underestimated
There is a growing body of evidence to show that air pollution is a major risk factor for lung cancer among never-smokers, although there is less certainty about the duration of exposure to fine particulate matter in ambient air as it relates to risk for lung cancer.
But as Canadian researchers now report, even 20 years of data on cumulative exposure to air pollution may underestimate the magnitude of the effect, especially among people diagnosed with lung cancer who have migrated from regions where heavy air pollution is the norm.
In a study of Canadian women with newly diagnosed lung cancer who never smoked, Renelle Myers, MD, FRCPC, from the University of British Columbia in Vancouver and colleagues found that shorter-term assessment of cumulative exposure to ambient air particles smaller than 2.5 microns (PM2.5) may underestimate the health effects of chronic exposure to pollution, especially among those patients who had migrated to Canada after living in areas of high PM2.5 exposure for long periods of time.
“Our study points to the importance of incorporating this long-term cumulative exposure to air pollutants in the assessment of individual lung cancer risk, of course in combination with traditional risk factors, and depending on the country of residence, I think that even a 20-year cumulative exposure may underestimate the effects of PM2.5, as we’re not capturing childhood or adolescent exposure when the lung is developing, and what effect that will have,” she said in an oral abstract presented at the World Conference on Lung Cancer.
Satellite data on local pollution
With the objective of comparing cumulative 3-year vs. 20-year exposure to PM2.5 in women who had never smoked and had a new diagnosis of lung cancer, Dr. Myers and colleagues conducted a cross-sectional study.
They recruited a total of 236 women and had them fill out a detailed residential history questionnaire, and demographic details including age, race, country of birth, arrival in Canada for those born out of the country, occupations, family history of lung cancer, and exposure to second-hand smoke.
The investigators linked local addresses or postal to satellite-derived data on local PM2.5 levels, which first became available in 1996.
The median age of participants was 66.1 years. Of the 236 participants, 190 (80.5%) were born outside of Canada, and came to the country at the median age of 45. About half of all participants came from mainland China or Hong Kong, and another one-third came from elsewhere in Asia.
Tumor histologies included adenocarcinomas in 219 patients, squamous cell carcinoma in 1, and other types in 16 patients. Slightly more than half of the patients (55.%) had stage III or IV disease at diagnosis. In all, 106 of 227 evaluable patients had EGFR mutations.
3 years not enough
Among the foreign-born patients, only 4 (2%) had 3-year cumulative PM2.5 exposure greater than 10 mcg/m3, but 38 (20%) had 20-year cumulative exposure greater than 10 mcg/m3 (P < .0001).
All of the patients had cumulative PM2.5 exposures greater than 5 mcg/m3.
Comparing patients with and without EGFR mutations, the investigators found that higher 3-year cumulative PM2.5 exposure was significantly associated with EGFR mutations compared with nonmutated cancers (P = .049), but there was no significant association with higher 20-year cumulative exposures.
“The significance of this study really captures that short term or at least less than 3-year cumulative exposure risk for PM2.5 will probably underestimate the adverse effects that chronic exposure to air pollution has, especially among patients who lived elsewhere that may have had higher exposure throughout their lifetime than where you actually meet them,” Dr. Myers said in a media briefing held prior to her presentation.
Lung cancer in female nonsmokers
During the oral abstract session, invited discussant Chang-Chuan Chan, ScD, National Taiwan University, Taipei, said that the study’s focus on female patients with lung cancer is important. He pointed to a 2019 study examining the relationship between air pollution and lung cancer among nonsmokers in Taiwan, in which the authors found that, although smoking levels among women remained low over time (about 5%), the incidence of lung adenocarcinomas among women increased from 7.05 per 100,000 in 1995, to 24.22 per 100,000 in 2015.
The authors of that study also found that changes in PM2.5 levels in Taiwan were predictive of fluctuations in lung cancer prevalence in never-smokers.
“We’re moving from 50-year studies of smoking to these new issues of air pollution, asbestos, and radon, and I think it’s better that these three factors can be combined together,” he said at the meeting sponsored by the International Association for the Study of Lung Cancer.
The study was supported by the BC Cancer Foundation, Terry Fox Research Institute, and VGH-UBC Hospital Foundation. Dr. Myers and Dr. Chan reported having no financial conflicts of interest to disclose.
There is a growing body of evidence to show that air pollution is a major risk factor for lung cancer among never-smokers, although there is less certainty about the duration of exposure to fine particulate matter in ambient air as it relates to risk for lung cancer.
But as Canadian researchers now report, even 20 years of data on cumulative exposure to air pollution may underestimate the magnitude of the effect, especially among people diagnosed with lung cancer who have migrated from regions where heavy air pollution is the norm.
In a study of Canadian women with newly diagnosed lung cancer who never smoked, Renelle Myers, MD, FRCPC, from the University of British Columbia in Vancouver and colleagues found that shorter-term assessment of cumulative exposure to ambient air particles smaller than 2.5 microns (PM2.5) may underestimate the health effects of chronic exposure to pollution, especially among those patients who had migrated to Canada after living in areas of high PM2.5 exposure for long periods of time.
“Our study points to the importance of incorporating this long-term cumulative exposure to air pollutants in the assessment of individual lung cancer risk, of course in combination with traditional risk factors, and depending on the country of residence, I think that even a 20-year cumulative exposure may underestimate the effects of PM2.5, as we’re not capturing childhood or adolescent exposure when the lung is developing, and what effect that will have,” she said in an oral abstract presented at the World Conference on Lung Cancer.
Satellite data on local pollution
With the objective of comparing cumulative 3-year vs. 20-year exposure to PM2.5 in women who had never smoked and had a new diagnosis of lung cancer, Dr. Myers and colleagues conducted a cross-sectional study.
They recruited a total of 236 women and had them fill out a detailed residential history questionnaire, and demographic details including age, race, country of birth, arrival in Canada for those born out of the country, occupations, family history of lung cancer, and exposure to second-hand smoke.
The investigators linked local addresses or postal to satellite-derived data on local PM2.5 levels, which first became available in 1996.
The median age of participants was 66.1 years. Of the 236 participants, 190 (80.5%) were born outside of Canada, and came to the country at the median age of 45. About half of all participants came from mainland China or Hong Kong, and another one-third came from elsewhere in Asia.
Tumor histologies included adenocarcinomas in 219 patients, squamous cell carcinoma in 1, and other types in 16 patients. Slightly more than half of the patients (55.%) had stage III or IV disease at diagnosis. In all, 106 of 227 evaluable patients had EGFR mutations.
3 years not enough
Among the foreign-born patients, only 4 (2%) had 3-year cumulative PM2.5 exposure greater than 10 mcg/m3, but 38 (20%) had 20-year cumulative exposure greater than 10 mcg/m3 (P < .0001).
All of the patients had cumulative PM2.5 exposures greater than 5 mcg/m3.
Comparing patients with and without EGFR mutations, the investigators found that higher 3-year cumulative PM2.5 exposure was significantly associated with EGFR mutations compared with nonmutated cancers (P = .049), but there was no significant association with higher 20-year cumulative exposures.
“The significance of this study really captures that short term or at least less than 3-year cumulative exposure risk for PM2.5 will probably underestimate the adverse effects that chronic exposure to air pollution has, especially among patients who lived elsewhere that may have had higher exposure throughout their lifetime than where you actually meet them,” Dr. Myers said in a media briefing held prior to her presentation.
Lung cancer in female nonsmokers
During the oral abstract session, invited discussant Chang-Chuan Chan, ScD, National Taiwan University, Taipei, said that the study’s focus on female patients with lung cancer is important. He pointed to a 2019 study examining the relationship between air pollution and lung cancer among nonsmokers in Taiwan, in which the authors found that, although smoking levels among women remained low over time (about 5%), the incidence of lung adenocarcinomas among women increased from 7.05 per 100,000 in 1995, to 24.22 per 100,000 in 2015.
The authors of that study also found that changes in PM2.5 levels in Taiwan were predictive of fluctuations in lung cancer prevalence in never-smokers.
“We’re moving from 50-year studies of smoking to these new issues of air pollution, asbestos, and radon, and I think it’s better that these three factors can be combined together,” he said at the meeting sponsored by the International Association for the Study of Lung Cancer.
The study was supported by the BC Cancer Foundation, Terry Fox Research Institute, and VGH-UBC Hospital Foundation. Dr. Myers and Dr. Chan reported having no financial conflicts of interest to disclose.
There is a growing body of evidence to show that air pollution is a major risk factor for lung cancer among never-smokers, although there is less certainty about the duration of exposure to fine particulate matter in ambient air as it relates to risk for lung cancer.
But as Canadian researchers now report, even 20 years of data on cumulative exposure to air pollution may underestimate the magnitude of the effect, especially among people diagnosed with lung cancer who have migrated from regions where heavy air pollution is the norm.
In a study of Canadian women with newly diagnosed lung cancer who never smoked, Renelle Myers, MD, FRCPC, from the University of British Columbia in Vancouver and colleagues found that shorter-term assessment of cumulative exposure to ambient air particles smaller than 2.5 microns (PM2.5) may underestimate the health effects of chronic exposure to pollution, especially among those patients who had migrated to Canada after living in areas of high PM2.5 exposure for long periods of time.
“Our study points to the importance of incorporating this long-term cumulative exposure to air pollutants in the assessment of individual lung cancer risk, of course in combination with traditional risk factors, and depending on the country of residence, I think that even a 20-year cumulative exposure may underestimate the effects of PM2.5, as we’re not capturing childhood or adolescent exposure when the lung is developing, and what effect that will have,” she said in an oral abstract presented at the World Conference on Lung Cancer.
Satellite data on local pollution
With the objective of comparing cumulative 3-year vs. 20-year exposure to PM2.5 in women who had never smoked and had a new diagnosis of lung cancer, Dr. Myers and colleagues conducted a cross-sectional study.
They recruited a total of 236 women and had them fill out a detailed residential history questionnaire, and demographic details including age, race, country of birth, arrival in Canada for those born out of the country, occupations, family history of lung cancer, and exposure to second-hand smoke.
The investigators linked local addresses or postal to satellite-derived data on local PM2.5 levels, which first became available in 1996.
The median age of participants was 66.1 years. Of the 236 participants, 190 (80.5%) were born outside of Canada, and came to the country at the median age of 45. About half of all participants came from mainland China or Hong Kong, and another one-third came from elsewhere in Asia.
Tumor histologies included adenocarcinomas in 219 patients, squamous cell carcinoma in 1, and other types in 16 patients. Slightly more than half of the patients (55.%) had stage III or IV disease at diagnosis. In all, 106 of 227 evaluable patients had EGFR mutations.
3 years not enough
Among the foreign-born patients, only 4 (2%) had 3-year cumulative PM2.5 exposure greater than 10 mcg/m3, but 38 (20%) had 20-year cumulative exposure greater than 10 mcg/m3 (P < .0001).
All of the patients had cumulative PM2.5 exposures greater than 5 mcg/m3.
Comparing patients with and without EGFR mutations, the investigators found that higher 3-year cumulative PM2.5 exposure was significantly associated with EGFR mutations compared with nonmutated cancers (P = .049), but there was no significant association with higher 20-year cumulative exposures.
“The significance of this study really captures that short term or at least less than 3-year cumulative exposure risk for PM2.5 will probably underestimate the adverse effects that chronic exposure to air pollution has, especially among patients who lived elsewhere that may have had higher exposure throughout their lifetime than where you actually meet them,” Dr. Myers said in a media briefing held prior to her presentation.
Lung cancer in female nonsmokers
During the oral abstract session, invited discussant Chang-Chuan Chan, ScD, National Taiwan University, Taipei, said that the study’s focus on female patients with lung cancer is important. He pointed to a 2019 study examining the relationship between air pollution and lung cancer among nonsmokers in Taiwan, in which the authors found that, although smoking levels among women remained low over time (about 5%), the incidence of lung adenocarcinomas among women increased from 7.05 per 100,000 in 1995, to 24.22 per 100,000 in 2015.
The authors of that study also found that changes in PM2.5 levels in Taiwan were predictive of fluctuations in lung cancer prevalence in never-smokers.
“We’re moving from 50-year studies of smoking to these new issues of air pollution, asbestos, and radon, and I think it’s better that these three factors can be combined together,” he said at the meeting sponsored by the International Association for the Study of Lung Cancer.
The study was supported by the BC Cancer Foundation, Terry Fox Research Institute, and VGH-UBC Hospital Foundation. Dr. Myers and Dr. Chan reported having no financial conflicts of interest to disclose.
FROM WCLC