User login
Symptom burdens related to chemotherapy-induced anemia in stage IV cancer
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
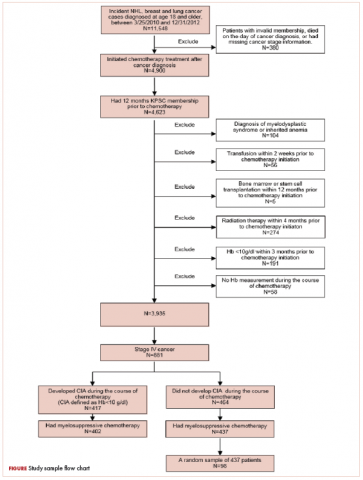
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
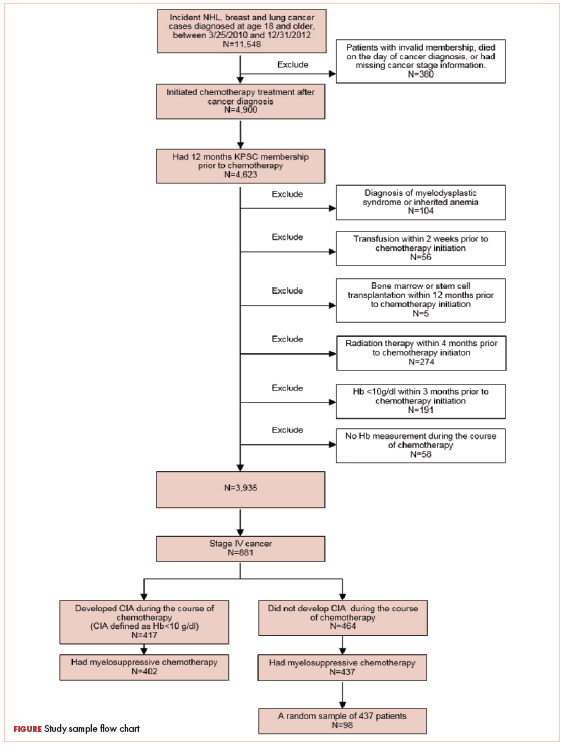
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
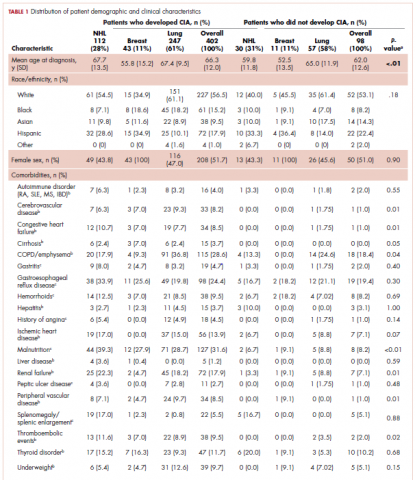
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
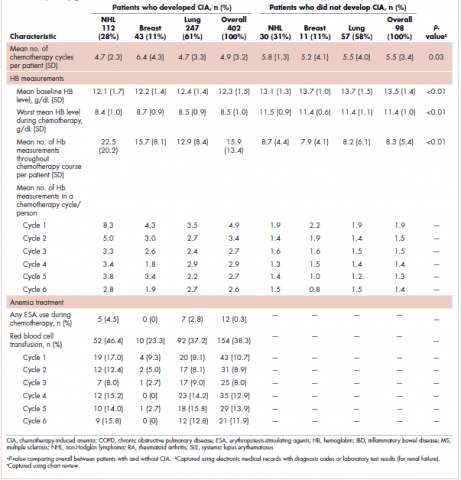
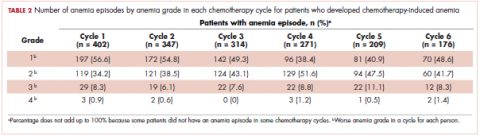
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
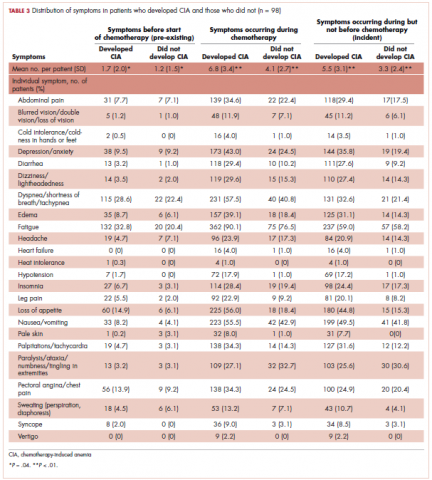
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
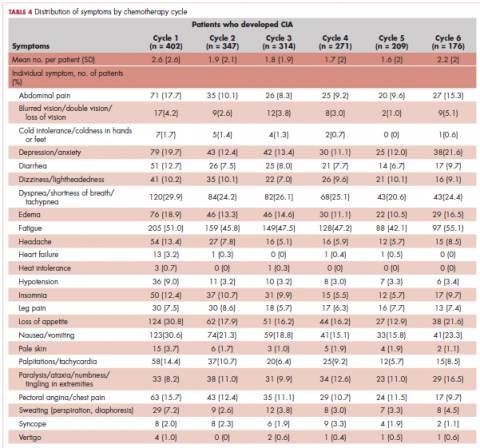
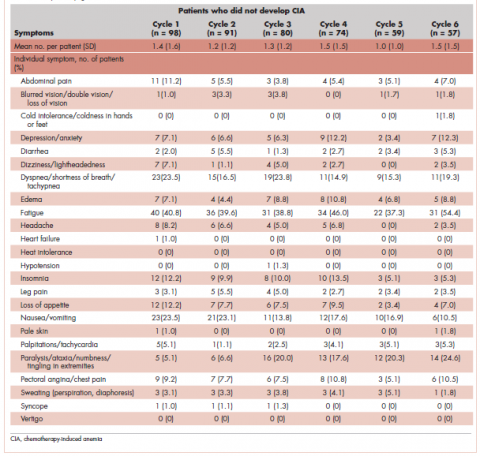
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
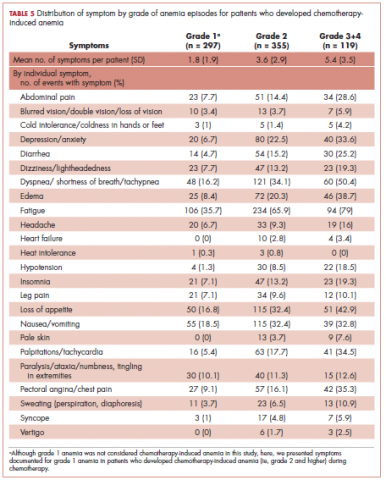
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
A novel tracer shows promise for detecting CD8 T-cells in advanced solid tumors
WASHINGTON – and reference tissue in an open-label, phase 1, first-in-human study.
The findings demonstrate the ability of the tracer–an anti-CD8 zirconium-labeled minibody–to noninvasively detect CD8 distribution in patients with metastatic solid tumors, potentially providing more information – and more quickly – than is possible with a single biopsy, Michael S. Gordon, MD, reported during a late-breaking abstract session at the annual meeting of the Society for Immunotherapy of Cancer.
During a dose escalation period (stage 1) of the study, six patients received 3 mCi of 89Zr-IAB22M2C once intravenously followed by serial PET scans over a period of 5-7 days. The patients received increasing protein doses of 0.2 through 10 mg to establish safety and determine a “recommended protein dose and scanning parameters for subsequent trials,” explained Dr. Gordon of HonorHealth Research Institute, Scottsdale, Ariz.
Stage 1 was followed by a dose expansion period (stage 2) in which an additional nine subjects were scanned to better delineate the recommended phase 2 study dose, he said.
All patients were monitored for drug-related adverse events and evaluated with blood chemistry, hematology, cytokine assay, and anti-drug antibodies. Biodistribution, radiodosimetry and semi-quantitative evaluation of CD8-tracer uptake were performed in all patients.
“We saw rapid clearance with excretion through the hepatobiliary mechanism, uptake in T-cell rich tissues, and no uptake in background normal tissues – so no uptake in muscle, heart, brain, or lungs,” he said, adding that “tumor uptake was variable and was clearly seen in 10 out of 15 patients.
“The protein dose that was considered to have favorable biodistribution was the range between 0.5 and 1.5, and based upon the analysis, the most favorable imaging time point ... was deemed to be 24 hours,” he said, noting that changes could be seen in as early as 6 hours.
The estimated mean effective radiation dose was 2.4 rem/mCi, “which is consistent with other zirconium-labeled antibody or minibody technologies,” Dr. Gordon said.
Study subjects ranged in age from 31 to 82 years and included nine men and six women with solid tumor malignancies who were eligible to receive checkpoint inhibitor therapy. Their primary cancer types were melanoma (eight patients), non–small-cell lung cancer (six patients), and hepatocellular carcinoma (one patient).
Two patients had received no prior treatment, three had discontinued prior checkpoint inhibitor therapy, and 10 were on immunotherapy.
No drug-related adverse events occurred during the course of the study, although one patient had a transient increase in anti-drug antibodies, Dr. Gordon said.
“Immunotherapy, and specifically checkpoint inhibitors (CPIs), have transformed the landscape of cancer care. Antitumor activity of CPIs is mediated by the CD8-positive T-cell cytotoxic effects, with preclinical and translational clinical studies demonstrating the importance of activated CD8-positive cells within the tumor microenvironment,” he explained, adding that currently available technology is limited in its ability to continually assess the presence of and change in the CD8 infiltrate; one biopsy may fail to capture the immunologic heterogeneity that exists among various tumors in an individual patient.
“As CPI therapy moves into front-line and earlier settings, the ability to have a noninvasive technology to assess whole body and intratumoral changes in CD8 trafficking or expansion in response to therapy is viewed as being crucial,” he said.
A phase 2 study to look closer at the potential for PET + 89Zr-IAB22M2C to fulfill that role will begin soon. The study will focus on correlating imaging with synchronous biopsies before and after primary immunotherapy to look for any predictive potential for this technology, he said.
This study was supported by ImaginAb and Parker Institute for Cancer Immunotherapy. Dr. Gordon reported having no disclosures.
SOURCE: Gordon M et al., SITC 2018: Abstract LB49.
WASHINGTON – and reference tissue in an open-label, phase 1, first-in-human study.
The findings demonstrate the ability of the tracer–an anti-CD8 zirconium-labeled minibody–to noninvasively detect CD8 distribution in patients with metastatic solid tumors, potentially providing more information – and more quickly – than is possible with a single biopsy, Michael S. Gordon, MD, reported during a late-breaking abstract session at the annual meeting of the Society for Immunotherapy of Cancer.
During a dose escalation period (stage 1) of the study, six patients received 3 mCi of 89Zr-IAB22M2C once intravenously followed by serial PET scans over a period of 5-7 days. The patients received increasing protein doses of 0.2 through 10 mg to establish safety and determine a “recommended protein dose and scanning parameters for subsequent trials,” explained Dr. Gordon of HonorHealth Research Institute, Scottsdale, Ariz.
Stage 1 was followed by a dose expansion period (stage 2) in which an additional nine subjects were scanned to better delineate the recommended phase 2 study dose, he said.
All patients were monitored for drug-related adverse events and evaluated with blood chemistry, hematology, cytokine assay, and anti-drug antibodies. Biodistribution, radiodosimetry and semi-quantitative evaluation of CD8-tracer uptake were performed in all patients.
“We saw rapid clearance with excretion through the hepatobiliary mechanism, uptake in T-cell rich tissues, and no uptake in background normal tissues – so no uptake in muscle, heart, brain, or lungs,” he said, adding that “tumor uptake was variable and was clearly seen in 10 out of 15 patients.
“The protein dose that was considered to have favorable biodistribution was the range between 0.5 and 1.5, and based upon the analysis, the most favorable imaging time point ... was deemed to be 24 hours,” he said, noting that changes could be seen in as early as 6 hours.
The estimated mean effective radiation dose was 2.4 rem/mCi, “which is consistent with other zirconium-labeled antibody or minibody technologies,” Dr. Gordon said.
Study subjects ranged in age from 31 to 82 years and included nine men and six women with solid tumor malignancies who were eligible to receive checkpoint inhibitor therapy. Their primary cancer types were melanoma (eight patients), non–small-cell lung cancer (six patients), and hepatocellular carcinoma (one patient).
Two patients had received no prior treatment, three had discontinued prior checkpoint inhibitor therapy, and 10 were on immunotherapy.
No drug-related adverse events occurred during the course of the study, although one patient had a transient increase in anti-drug antibodies, Dr. Gordon said.
“Immunotherapy, and specifically checkpoint inhibitors (CPIs), have transformed the landscape of cancer care. Antitumor activity of CPIs is mediated by the CD8-positive T-cell cytotoxic effects, with preclinical and translational clinical studies demonstrating the importance of activated CD8-positive cells within the tumor microenvironment,” he explained, adding that currently available technology is limited in its ability to continually assess the presence of and change in the CD8 infiltrate; one biopsy may fail to capture the immunologic heterogeneity that exists among various tumors in an individual patient.
“As CPI therapy moves into front-line and earlier settings, the ability to have a noninvasive technology to assess whole body and intratumoral changes in CD8 trafficking or expansion in response to therapy is viewed as being crucial,” he said.
A phase 2 study to look closer at the potential for PET + 89Zr-IAB22M2C to fulfill that role will begin soon. The study will focus on correlating imaging with synchronous biopsies before and after primary immunotherapy to look for any predictive potential for this technology, he said.
This study was supported by ImaginAb and Parker Institute for Cancer Immunotherapy. Dr. Gordon reported having no disclosures.
SOURCE: Gordon M et al., SITC 2018: Abstract LB49.
WASHINGTON – and reference tissue in an open-label, phase 1, first-in-human study.
The findings demonstrate the ability of the tracer–an anti-CD8 zirconium-labeled minibody–to noninvasively detect CD8 distribution in patients with metastatic solid tumors, potentially providing more information – and more quickly – than is possible with a single biopsy, Michael S. Gordon, MD, reported during a late-breaking abstract session at the annual meeting of the Society for Immunotherapy of Cancer.
During a dose escalation period (stage 1) of the study, six patients received 3 mCi of 89Zr-IAB22M2C once intravenously followed by serial PET scans over a period of 5-7 days. The patients received increasing protein doses of 0.2 through 10 mg to establish safety and determine a “recommended protein dose and scanning parameters for subsequent trials,” explained Dr. Gordon of HonorHealth Research Institute, Scottsdale, Ariz.
Stage 1 was followed by a dose expansion period (stage 2) in which an additional nine subjects were scanned to better delineate the recommended phase 2 study dose, he said.
All patients were monitored for drug-related adverse events and evaluated with blood chemistry, hematology, cytokine assay, and anti-drug antibodies. Biodistribution, radiodosimetry and semi-quantitative evaluation of CD8-tracer uptake were performed in all patients.
“We saw rapid clearance with excretion through the hepatobiliary mechanism, uptake in T-cell rich tissues, and no uptake in background normal tissues – so no uptake in muscle, heart, brain, or lungs,” he said, adding that “tumor uptake was variable and was clearly seen in 10 out of 15 patients.
“The protein dose that was considered to have favorable biodistribution was the range between 0.5 and 1.5, and based upon the analysis, the most favorable imaging time point ... was deemed to be 24 hours,” he said, noting that changes could be seen in as early as 6 hours.
The estimated mean effective radiation dose was 2.4 rem/mCi, “which is consistent with other zirconium-labeled antibody or minibody technologies,” Dr. Gordon said.
Study subjects ranged in age from 31 to 82 years and included nine men and six women with solid tumor malignancies who were eligible to receive checkpoint inhibitor therapy. Their primary cancer types were melanoma (eight patients), non–small-cell lung cancer (six patients), and hepatocellular carcinoma (one patient).
Two patients had received no prior treatment, three had discontinued prior checkpoint inhibitor therapy, and 10 were on immunotherapy.
No drug-related adverse events occurred during the course of the study, although one patient had a transient increase in anti-drug antibodies, Dr. Gordon said.
“Immunotherapy, and specifically checkpoint inhibitors (CPIs), have transformed the landscape of cancer care. Antitumor activity of CPIs is mediated by the CD8-positive T-cell cytotoxic effects, with preclinical and translational clinical studies demonstrating the importance of activated CD8-positive cells within the tumor microenvironment,” he explained, adding that currently available technology is limited in its ability to continually assess the presence of and change in the CD8 infiltrate; one biopsy may fail to capture the immunologic heterogeneity that exists among various tumors in an individual patient.
“As CPI therapy moves into front-line and earlier settings, the ability to have a noninvasive technology to assess whole body and intratumoral changes in CD8 trafficking or expansion in response to therapy is viewed as being crucial,” he said.
A phase 2 study to look closer at the potential for PET + 89Zr-IAB22M2C to fulfill that role will begin soon. The study will focus on correlating imaging with synchronous biopsies before and after primary immunotherapy to look for any predictive potential for this technology, he said.
This study was supported by ImaginAb and Parker Institute for Cancer Immunotherapy. Dr. Gordon reported having no disclosures.
SOURCE: Gordon M et al., SITC 2018: Abstract LB49.
REPORTING FROM SITC 2018
Key clinical point: PET with CD8-tracer 89Zr-IAB22M2C is safe, provides detailed CD8 T-cell information.
Major finding: Tumor uptake of the CD8-tracer was seen in 10 of 15 subjects.
Study details: An open-label phase 1 study of 15 patients.
Disclosures: This study was supported by ImaginAb and Parker Institute for Cancer Immunotherapy. Dr. Gordon reported having no disclosures.
Source: Gordon M et al. SITC 2018: Abstract LB49.
Lenvatinib/Pembrolizumab shows promise in previously treated metastatic NSCLC
WASHINGTON – (NSCLC), according to interim findings from a phase 1b/2 study.
Of note, the 21 patients enrolled in the multicenter, open-label study as of March 2018 were not preselected for programmed death-ligand 1 (PD-L1) tumor expression status, Marcia S. Brose, MD, reported at the annual meeting of the Society for the Immunotherapy of Cancer.
They were treated with 20 mg of oral lenvatinib daily and 200 mg of intravenous pembrolizumab every 3 weeks, and the overall response rate at 24 weeks – the primary endpoint of the study – was 33.3%, said Dr. Brose of Abramson Cancer Center of the University of Pennsylvania, Philadelphia.
One patient had a complete response, six had a partial response, 10 had stable disease, two progressed on treatment, and the outcome in two was unknown or not evaluable, for an overall clinical benefit rate of 66%, she said, adding that the median duration of response was 10.9 months and median progression-free survival (PFS) was 5.9 months.
All patients had good performance status (ECOG score of 0-1), and nine (43%) were PD-L1–positive as defined by a tumor proportion score of at least 1%, five (24%) were PD-L1-negative, and seven (33%) were not tested for PD-L1 status. Three (14%) were treatment naive, while seven (33%), 10 (48%), and one (5%) had received one, two, or three or more prior lines of systemic therapy, respectively. No prior nivolumab or pembrolizumab treatment was allowed.
“At least one of the patients who was PD-L1–negative remained on study after 40 weeks and still continuing to respond, and ... the PD-L1–positive patients were also doing well,” Dr. Brose said.
Tumor assessments were performed by study investigators using immune-related Response Evaluation Criteria in Solid Tumors (irRECIST).
Grade 3 or greater treatment-related adverse events occurred in 10 patients (48%), and mainly included hypertension, fatigue, and diarrhea, but only four were considered serious treatment-related adverse events. Nineteen patients had treatment adjustments because of adverse events, four discontinued treatment due to adverse events, and one patient died from a pulmonary hemorrhage that was thought to possibly be treatment related, Dr. Brose said.
“The toxicity is really what you would have expected from either of these drugs on their own; it didn’t seem like there was anything that happened in synergy from the two that was unexpected,” she noted.
Lenvatinib is a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor receptors (FGFR) 1-4, platelet-derived growth factor receptor (PDGFR) alpha, and the RET and c-KIT proto-oncogenes. Pembrolizumab is an anti–PD-1 antibody approved as a monotherapy for previously treated patients with metastatic PD-L1–positive NSCLC, and it has been shown to be associated with an overall response rate of 18%, she explained.
The current results are from the NSCLC cohort of an ongoing trial of lenvatinib plus pembrolizumab in patients with solid tumors.
“Further investigation of this study drug combination in patients is warranted, but we will have to think carefully about what point in the treatment paradigm these patients should be treated in order to maximize the benefit from this combination therapy,” she concluded.
Dr. Brose has received consulting fees, research grants, and honorarium from Eisai.
SOURCE: Brose M et al. SITC 2018, Abstract P392.
WASHINGTON – (NSCLC), according to interim findings from a phase 1b/2 study.
Of note, the 21 patients enrolled in the multicenter, open-label study as of March 2018 were not preselected for programmed death-ligand 1 (PD-L1) tumor expression status, Marcia S. Brose, MD, reported at the annual meeting of the Society for the Immunotherapy of Cancer.
They were treated with 20 mg of oral lenvatinib daily and 200 mg of intravenous pembrolizumab every 3 weeks, and the overall response rate at 24 weeks – the primary endpoint of the study – was 33.3%, said Dr. Brose of Abramson Cancer Center of the University of Pennsylvania, Philadelphia.
One patient had a complete response, six had a partial response, 10 had stable disease, two progressed on treatment, and the outcome in two was unknown or not evaluable, for an overall clinical benefit rate of 66%, she said, adding that the median duration of response was 10.9 months and median progression-free survival (PFS) was 5.9 months.
All patients had good performance status (ECOG score of 0-1), and nine (43%) were PD-L1–positive as defined by a tumor proportion score of at least 1%, five (24%) were PD-L1-negative, and seven (33%) were not tested for PD-L1 status. Three (14%) were treatment naive, while seven (33%), 10 (48%), and one (5%) had received one, two, or three or more prior lines of systemic therapy, respectively. No prior nivolumab or pembrolizumab treatment was allowed.
“At least one of the patients who was PD-L1–negative remained on study after 40 weeks and still continuing to respond, and ... the PD-L1–positive patients were also doing well,” Dr. Brose said.
Tumor assessments were performed by study investigators using immune-related Response Evaluation Criteria in Solid Tumors (irRECIST).
Grade 3 or greater treatment-related adverse events occurred in 10 patients (48%), and mainly included hypertension, fatigue, and diarrhea, but only four were considered serious treatment-related adverse events. Nineteen patients had treatment adjustments because of adverse events, four discontinued treatment due to adverse events, and one patient died from a pulmonary hemorrhage that was thought to possibly be treatment related, Dr. Brose said.
“The toxicity is really what you would have expected from either of these drugs on their own; it didn’t seem like there was anything that happened in synergy from the two that was unexpected,” she noted.
Lenvatinib is a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor receptors (FGFR) 1-4, platelet-derived growth factor receptor (PDGFR) alpha, and the RET and c-KIT proto-oncogenes. Pembrolizumab is an anti–PD-1 antibody approved as a monotherapy for previously treated patients with metastatic PD-L1–positive NSCLC, and it has been shown to be associated with an overall response rate of 18%, she explained.
The current results are from the NSCLC cohort of an ongoing trial of lenvatinib plus pembrolizumab in patients with solid tumors.
“Further investigation of this study drug combination in patients is warranted, but we will have to think carefully about what point in the treatment paradigm these patients should be treated in order to maximize the benefit from this combination therapy,” she concluded.
Dr. Brose has received consulting fees, research grants, and honorarium from Eisai.
SOURCE: Brose M et al. SITC 2018, Abstract P392.
WASHINGTON – (NSCLC), according to interim findings from a phase 1b/2 study.
Of note, the 21 patients enrolled in the multicenter, open-label study as of March 2018 were not preselected for programmed death-ligand 1 (PD-L1) tumor expression status, Marcia S. Brose, MD, reported at the annual meeting of the Society for the Immunotherapy of Cancer.
They were treated with 20 mg of oral lenvatinib daily and 200 mg of intravenous pembrolizumab every 3 weeks, and the overall response rate at 24 weeks – the primary endpoint of the study – was 33.3%, said Dr. Brose of Abramson Cancer Center of the University of Pennsylvania, Philadelphia.
One patient had a complete response, six had a partial response, 10 had stable disease, two progressed on treatment, and the outcome in two was unknown or not evaluable, for an overall clinical benefit rate of 66%, she said, adding that the median duration of response was 10.9 months and median progression-free survival (PFS) was 5.9 months.
All patients had good performance status (ECOG score of 0-1), and nine (43%) were PD-L1–positive as defined by a tumor proportion score of at least 1%, five (24%) were PD-L1-negative, and seven (33%) were not tested for PD-L1 status. Three (14%) were treatment naive, while seven (33%), 10 (48%), and one (5%) had received one, two, or three or more prior lines of systemic therapy, respectively. No prior nivolumab or pembrolizumab treatment was allowed.
“At least one of the patients who was PD-L1–negative remained on study after 40 weeks and still continuing to respond, and ... the PD-L1–positive patients were also doing well,” Dr. Brose said.
Tumor assessments were performed by study investigators using immune-related Response Evaluation Criteria in Solid Tumors (irRECIST).
Grade 3 or greater treatment-related adverse events occurred in 10 patients (48%), and mainly included hypertension, fatigue, and diarrhea, but only four were considered serious treatment-related adverse events. Nineteen patients had treatment adjustments because of adverse events, four discontinued treatment due to adverse events, and one patient died from a pulmonary hemorrhage that was thought to possibly be treatment related, Dr. Brose said.
“The toxicity is really what you would have expected from either of these drugs on their own; it didn’t seem like there was anything that happened in synergy from the two that was unexpected,” she noted.
Lenvatinib is a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor receptors (FGFR) 1-4, platelet-derived growth factor receptor (PDGFR) alpha, and the RET and c-KIT proto-oncogenes. Pembrolizumab is an anti–PD-1 antibody approved as a monotherapy for previously treated patients with metastatic PD-L1–positive NSCLC, and it has been shown to be associated with an overall response rate of 18%, she explained.
The current results are from the NSCLC cohort of an ongoing trial of lenvatinib plus pembrolizumab in patients with solid tumors.
“Further investigation of this study drug combination in patients is warranted, but we will have to think carefully about what point in the treatment paradigm these patients should be treated in order to maximize the benefit from this combination therapy,” she concluded.
Dr. Brose has received consulting fees, research grants, and honorarium from Eisai.
SOURCE: Brose M et al. SITC 2018, Abstract P392.
REPORTING FROM SITC 2018
Key clinical point: Lenvatinib/pembrolizumab shows promise in metastatic NSCLC.
Major finding: Overall response rate at 24 weeks was 33.3%.
Study details: Interim findings in 21 patients from a phase 1b/2 study.
Disclosures: Dr. Brose has received consulting fees, research grants, and honorarium from Eisai.Source: Brose M et al. SITC 2018, Abstract P392.
TMB measured by NGS may ID SCLC patients who will benefit from immunotherapy
WASHINGTON – and targeted next-generation sequencing may help identify those likely to benefit from immunotherapy, findings from a case series suggest.
Of 113 small cell lung cancer (SCLC) patients who had successful next-generation sequencing (NGS) with tumor mutational burden (TMB) assessment at the Dana-Farber Cancer Institute (DFCI) in Boston, 52 were treated with immune checkpoint inhibitors and 61 received chemotherapy but never received subsequent immunotherapy, Biagio Ricciuti, MD, of DFCI said at the annual meeting of the Society for the Immunotherapy of Cancer.
Median TMB for all patients was 9.68 mutations/megabase, with those with TMB above the median considered TMB high, and those with TMB below the median considered TMB low. Median progression-free survival (PFS) was significantly longer among TMB-high versus TMB-low patients (3.3 vs. 1.2 months; hazard ratio, 0.37), as was median overall survival (OS, 10.4 vs. 2.5 months; HR, 0.38), he said.
“To confirm that TMB was a predictive biomarker for immunotherapy only, we also looked at the outcome with chemotherapy according to tumor mutational burden, and as expected we found no difference in terms of median progression-free survival or median overall survival according to TMB-high versus TMB-low groups,” he said.
Additionally, patients with SCLC who were treated with immune checkpoint inhibitors and experienced at least one immune-related adverse event had significantly better median PFS and OS than did patients who experienced no immune-related adverse events (6.7 vs. 1.3 months; HR, 0.25; and 17.9 vs. 2.9 months; HR, 0.27, respectively), he said, noting that, in a 12-week landmark analysis, the differences in PFS and OS between the groups were “nearly double” but did not reach statistical significance.
TMB in the SCLC patients in this study was assessed using the DFCI NGS OncoPanel platform of more than 450 genes, and the TMB-high and TMB-low groups were similar with respect to baseline clinical and pathological features and known prognostic factors, Dr. Ricciuti said.
Prior studies have demonstrated that high TMB as assessed by whole exome sequencing correlates with benefits from immunotherapy. However, “whole exome sequencing is a very expensive technique, it’s challenging ... and it’s not really available to oncologists across countries,” he said.
Whether the more readily available targeted NGS could help identify the small fraction of SCLC patients who are likely to benefit from immunotherapy has been unclear, as has the relationship between the development of irAEs and immunotherapy response in SCLC; factors associated with clinical benefit from immunotherapy have not previously been well characterized, Dr. Ricciuti noted.
The current findings, though limited by the retrospective study design and small sample size, provide the first evidence for the use of targeted NGS panels to identify patients with advanced SCLC who are most likely to benefit from immunotherapy, he said, adding that, when compared with whole genome sequencing, TMB as assessed using targeted NGS “may offer a very useful tool for clinicians to optimize small cell lung cancer patient selection for immunotherapy.
“Our study also suggests that immune-related adverse events might be associated with improved efficacy of immunotherapy, although larger studies with longer follow-up are required to confirm this finding,” he concluded.
Dr. Ricciuti reported having no disclosures.
WASHINGTON – and targeted next-generation sequencing may help identify those likely to benefit from immunotherapy, findings from a case series suggest.
Of 113 small cell lung cancer (SCLC) patients who had successful next-generation sequencing (NGS) with tumor mutational burden (TMB) assessment at the Dana-Farber Cancer Institute (DFCI) in Boston, 52 were treated with immune checkpoint inhibitors and 61 received chemotherapy but never received subsequent immunotherapy, Biagio Ricciuti, MD, of DFCI said at the annual meeting of the Society for the Immunotherapy of Cancer.
Median TMB for all patients was 9.68 mutations/megabase, with those with TMB above the median considered TMB high, and those with TMB below the median considered TMB low. Median progression-free survival (PFS) was significantly longer among TMB-high versus TMB-low patients (3.3 vs. 1.2 months; hazard ratio, 0.37), as was median overall survival (OS, 10.4 vs. 2.5 months; HR, 0.38), he said.
“To confirm that TMB was a predictive biomarker for immunotherapy only, we also looked at the outcome with chemotherapy according to tumor mutational burden, and as expected we found no difference in terms of median progression-free survival or median overall survival according to TMB-high versus TMB-low groups,” he said.
Additionally, patients with SCLC who were treated with immune checkpoint inhibitors and experienced at least one immune-related adverse event had significantly better median PFS and OS than did patients who experienced no immune-related adverse events (6.7 vs. 1.3 months; HR, 0.25; and 17.9 vs. 2.9 months; HR, 0.27, respectively), he said, noting that, in a 12-week landmark analysis, the differences in PFS and OS between the groups were “nearly double” but did not reach statistical significance.
TMB in the SCLC patients in this study was assessed using the DFCI NGS OncoPanel platform of more than 450 genes, and the TMB-high and TMB-low groups were similar with respect to baseline clinical and pathological features and known prognostic factors, Dr. Ricciuti said.
Prior studies have demonstrated that high TMB as assessed by whole exome sequencing correlates with benefits from immunotherapy. However, “whole exome sequencing is a very expensive technique, it’s challenging ... and it’s not really available to oncologists across countries,” he said.
Whether the more readily available targeted NGS could help identify the small fraction of SCLC patients who are likely to benefit from immunotherapy has been unclear, as has the relationship between the development of irAEs and immunotherapy response in SCLC; factors associated with clinical benefit from immunotherapy have not previously been well characterized, Dr. Ricciuti noted.
The current findings, though limited by the retrospective study design and small sample size, provide the first evidence for the use of targeted NGS panels to identify patients with advanced SCLC who are most likely to benefit from immunotherapy, he said, adding that, when compared with whole genome sequencing, TMB as assessed using targeted NGS “may offer a very useful tool for clinicians to optimize small cell lung cancer patient selection for immunotherapy.
“Our study also suggests that immune-related adverse events might be associated with improved efficacy of immunotherapy, although larger studies with longer follow-up are required to confirm this finding,” he concluded.
Dr. Ricciuti reported having no disclosures.
WASHINGTON – and targeted next-generation sequencing may help identify those likely to benefit from immunotherapy, findings from a case series suggest.
Of 113 small cell lung cancer (SCLC) patients who had successful next-generation sequencing (NGS) with tumor mutational burden (TMB) assessment at the Dana-Farber Cancer Institute (DFCI) in Boston, 52 were treated with immune checkpoint inhibitors and 61 received chemotherapy but never received subsequent immunotherapy, Biagio Ricciuti, MD, of DFCI said at the annual meeting of the Society for the Immunotherapy of Cancer.
Median TMB for all patients was 9.68 mutations/megabase, with those with TMB above the median considered TMB high, and those with TMB below the median considered TMB low. Median progression-free survival (PFS) was significantly longer among TMB-high versus TMB-low patients (3.3 vs. 1.2 months; hazard ratio, 0.37), as was median overall survival (OS, 10.4 vs. 2.5 months; HR, 0.38), he said.
“To confirm that TMB was a predictive biomarker for immunotherapy only, we also looked at the outcome with chemotherapy according to tumor mutational burden, and as expected we found no difference in terms of median progression-free survival or median overall survival according to TMB-high versus TMB-low groups,” he said.
Additionally, patients with SCLC who were treated with immune checkpoint inhibitors and experienced at least one immune-related adverse event had significantly better median PFS and OS than did patients who experienced no immune-related adverse events (6.7 vs. 1.3 months; HR, 0.25; and 17.9 vs. 2.9 months; HR, 0.27, respectively), he said, noting that, in a 12-week landmark analysis, the differences in PFS and OS between the groups were “nearly double” but did not reach statistical significance.
TMB in the SCLC patients in this study was assessed using the DFCI NGS OncoPanel platform of more than 450 genes, and the TMB-high and TMB-low groups were similar with respect to baseline clinical and pathological features and known prognostic factors, Dr. Ricciuti said.
Prior studies have demonstrated that high TMB as assessed by whole exome sequencing correlates with benefits from immunotherapy. However, “whole exome sequencing is a very expensive technique, it’s challenging ... and it’s not really available to oncologists across countries,” he said.
Whether the more readily available targeted NGS could help identify the small fraction of SCLC patients who are likely to benefit from immunotherapy has been unclear, as has the relationship between the development of irAEs and immunotherapy response in SCLC; factors associated with clinical benefit from immunotherapy have not previously been well characterized, Dr. Ricciuti noted.
The current findings, though limited by the retrospective study design and small sample size, provide the first evidence for the use of targeted NGS panels to identify patients with advanced SCLC who are most likely to benefit from immunotherapy, he said, adding that, when compared with whole genome sequencing, TMB as assessed using targeted NGS “may offer a very useful tool for clinicians to optimize small cell lung cancer patient selection for immunotherapy.
“Our study also suggests that immune-related adverse events might be associated with improved efficacy of immunotherapy, although larger studies with longer follow-up are required to confirm this finding,” he concluded.
Dr. Ricciuti reported having no disclosures.
REPORTING FROM SITC 2018
Key clinical point: Next-generation sequencing may help identify small cell lung cancer patients who will benefit from immunotherapy.
Major finding: Median progression-free survival and overall survival were significantly better among tumor mutational burden–high versus tumor mutational burden–low patients (3.3 vs. 1.2 months; hazard ratio, 0.37; and 10.4 vs. 2.5 months; HR, 0.38, respectively).
Study details: A series of 113 patients.
Disclosures: Dr. Ricciuti reported having no disclosures.
Immunotherapy-related toxicities may be more common than reported in trials
SAN DIEGO – Certain immune-related adverse events related to PD1/PD-L1 treatment of patients with non–small cell lung cancer (NSCLC) may be more common than reported in clinical trials, a recent analysis of administrative claims data suggests.
Pneumonitis was seen in 10.9% of patients up to 60 days after the last dose of immunotherapy, according to the analysis of data from a large, U.S. commercial insurance database, presented at the Palliative and Supportive Care in Oncology Symposium.
By comparison, pneumonitis was reported in just 5.8% of NSCLC patients during treatment with the PD-1 (programmed cell death-1) inhibitor pembrolizumab in KEYNOTE-024, a pivotal randomized phase 3 clinical trial, said Elizabeth Jane Cathcart-Rake, MD, senior study author and an oncology fellow at the Mayo Clinic, Rochester, Minn.
Rates of immune-related adverse events in this study were generally higher than in clinical trials, both for common side effects and more rare conditions such as hypophysitis, according to Dr. Cathcart-Rake.
These new claims-based data might be considered complementary to clinical trial data, the researcher said.
“Together, they may give us a better sense of the broader implications of these adverse events,” she said in an interview.
Joe Rotella, MD, a board member of the American Academy for Hospice and Palliative Care Medicine, said results of this insurance database study provide a perspective on the real-world incidence of adverse events associated with immune checkpoint inhibitors.
“We’ve only been using these therapies for a few years, so this new analysis gives us more information on the prevalence of these side effects in patients as the therapies gain wider use,” Dr. Rotella said in a news release.
In the study, Dr. Cathcart-Rake and coinvestigators queried the OptumLabs Data Warehouse to identify 3,164 patients with NSCLC who received PD-1 or PD-L1 (programmed death-ligand 1) inhibitors between 2015 and 2017. They looked at incidence of adverse events both at the time of the last immunotherapy dose and at 60 days after the last dose.
The incidence of pneumonitis, just 4.9% on the last date of immunotherapy, increased to 10.9% at 60 days after the last dose, Dr. Cathcart-Rake reported.
Beyond pneumonitis, the most common immunotherapy-related toxicities at 60 days were hypothyroidism in 7.0%, arrhythmia in 6.1%, and nephritis or acute kidney injury in 5.4%, according to the investigators.
Dr. Cathcart-Rake also highlighted the incidence of some less common immunotherapy-related toxicities such as hypophysitis or hypothalamic-pituitary-adrenal axis toxicity, seen in 2.8% of patients by 60 days.
“That’s a small number, but hypophysitis can be really profound, and frequently leads to hospitalization,” she said. “I think this just gives us enough of a signal that providers really need to be on top of looking for these adverse events and to counsel patients beforehand.”
These data could also be helpful for advising hospitalists, emergency room physicians, and other providers who may not be attuned to the potential risks of cancer immunotherapy as compared with traditional cytotoxic chemotherapy, Dr. Cathcart-Rake said at the meeting cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
“A patient with cancer may be on immunotherapy and their risk for infection is quite low, but they may be at a huge risk for pneumonitis, which is treated completely differently,” she said. “So I think this should just raise alarms that close clinical monitoring for these conditions is really important.”
Dr. Cathcart-Rake disclosed that her institution receives research funding from Novartis. One study coinvestigator reported consulting or advisory roles with Trovagene, Genentech, Bristol-Myers Squibb, and Abbvie.
SOURCE: Cathcart-Rake EJ et al. 2018 Palliative and Supportive Care in Oncology Symposium. Abstract 184.
SAN DIEGO – Certain immune-related adverse events related to PD1/PD-L1 treatment of patients with non–small cell lung cancer (NSCLC) may be more common than reported in clinical trials, a recent analysis of administrative claims data suggests.
Pneumonitis was seen in 10.9% of patients up to 60 days after the last dose of immunotherapy, according to the analysis of data from a large, U.S. commercial insurance database, presented at the Palliative and Supportive Care in Oncology Symposium.
By comparison, pneumonitis was reported in just 5.8% of NSCLC patients during treatment with the PD-1 (programmed cell death-1) inhibitor pembrolizumab in KEYNOTE-024, a pivotal randomized phase 3 clinical trial, said Elizabeth Jane Cathcart-Rake, MD, senior study author and an oncology fellow at the Mayo Clinic, Rochester, Minn.
Rates of immune-related adverse events in this study were generally higher than in clinical trials, both for common side effects and more rare conditions such as hypophysitis, according to Dr. Cathcart-Rake.
These new claims-based data might be considered complementary to clinical trial data, the researcher said.
“Together, they may give us a better sense of the broader implications of these adverse events,” she said in an interview.
Joe Rotella, MD, a board member of the American Academy for Hospice and Palliative Care Medicine, said results of this insurance database study provide a perspective on the real-world incidence of adverse events associated with immune checkpoint inhibitors.
“We’ve only been using these therapies for a few years, so this new analysis gives us more information on the prevalence of these side effects in patients as the therapies gain wider use,” Dr. Rotella said in a news release.
In the study, Dr. Cathcart-Rake and coinvestigators queried the OptumLabs Data Warehouse to identify 3,164 patients with NSCLC who received PD-1 or PD-L1 (programmed death-ligand 1) inhibitors between 2015 and 2017. They looked at incidence of adverse events both at the time of the last immunotherapy dose and at 60 days after the last dose.
The incidence of pneumonitis, just 4.9% on the last date of immunotherapy, increased to 10.9% at 60 days after the last dose, Dr. Cathcart-Rake reported.
Beyond pneumonitis, the most common immunotherapy-related toxicities at 60 days were hypothyroidism in 7.0%, arrhythmia in 6.1%, and nephritis or acute kidney injury in 5.4%, according to the investigators.
Dr. Cathcart-Rake also highlighted the incidence of some less common immunotherapy-related toxicities such as hypophysitis or hypothalamic-pituitary-adrenal axis toxicity, seen in 2.8% of patients by 60 days.
“That’s a small number, but hypophysitis can be really profound, and frequently leads to hospitalization,” she said. “I think this just gives us enough of a signal that providers really need to be on top of looking for these adverse events and to counsel patients beforehand.”
These data could also be helpful for advising hospitalists, emergency room physicians, and other providers who may not be attuned to the potential risks of cancer immunotherapy as compared with traditional cytotoxic chemotherapy, Dr. Cathcart-Rake said at the meeting cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
“A patient with cancer may be on immunotherapy and their risk for infection is quite low, but they may be at a huge risk for pneumonitis, which is treated completely differently,” she said. “So I think this should just raise alarms that close clinical monitoring for these conditions is really important.”
Dr. Cathcart-Rake disclosed that her institution receives research funding from Novartis. One study coinvestigator reported consulting or advisory roles with Trovagene, Genentech, Bristol-Myers Squibb, and Abbvie.
SOURCE: Cathcart-Rake EJ et al. 2018 Palliative and Supportive Care in Oncology Symposium. Abstract 184.
SAN DIEGO – Certain immune-related adverse events related to PD1/PD-L1 treatment of patients with non–small cell lung cancer (NSCLC) may be more common than reported in clinical trials, a recent analysis of administrative claims data suggests.
Pneumonitis was seen in 10.9% of patients up to 60 days after the last dose of immunotherapy, according to the analysis of data from a large, U.S. commercial insurance database, presented at the Palliative and Supportive Care in Oncology Symposium.
By comparison, pneumonitis was reported in just 5.8% of NSCLC patients during treatment with the PD-1 (programmed cell death-1) inhibitor pembrolizumab in KEYNOTE-024, a pivotal randomized phase 3 clinical trial, said Elizabeth Jane Cathcart-Rake, MD, senior study author and an oncology fellow at the Mayo Clinic, Rochester, Minn.
Rates of immune-related adverse events in this study were generally higher than in clinical trials, both for common side effects and more rare conditions such as hypophysitis, according to Dr. Cathcart-Rake.
These new claims-based data might be considered complementary to clinical trial data, the researcher said.
“Together, they may give us a better sense of the broader implications of these adverse events,” she said in an interview.
Joe Rotella, MD, a board member of the American Academy for Hospice and Palliative Care Medicine, said results of this insurance database study provide a perspective on the real-world incidence of adverse events associated with immune checkpoint inhibitors.
“We’ve only been using these therapies for a few years, so this new analysis gives us more information on the prevalence of these side effects in patients as the therapies gain wider use,” Dr. Rotella said in a news release.
In the study, Dr. Cathcart-Rake and coinvestigators queried the OptumLabs Data Warehouse to identify 3,164 patients with NSCLC who received PD-1 or PD-L1 (programmed death-ligand 1) inhibitors between 2015 and 2017. They looked at incidence of adverse events both at the time of the last immunotherapy dose and at 60 days after the last dose.
The incidence of pneumonitis, just 4.9% on the last date of immunotherapy, increased to 10.9% at 60 days after the last dose, Dr. Cathcart-Rake reported.
Beyond pneumonitis, the most common immunotherapy-related toxicities at 60 days were hypothyroidism in 7.0%, arrhythmia in 6.1%, and nephritis or acute kidney injury in 5.4%, according to the investigators.
Dr. Cathcart-Rake also highlighted the incidence of some less common immunotherapy-related toxicities such as hypophysitis or hypothalamic-pituitary-adrenal axis toxicity, seen in 2.8% of patients by 60 days.
“That’s a small number, but hypophysitis can be really profound, and frequently leads to hospitalization,” she said. “I think this just gives us enough of a signal that providers really need to be on top of looking for these adverse events and to counsel patients beforehand.”
These data could also be helpful for advising hospitalists, emergency room physicians, and other providers who may not be attuned to the potential risks of cancer immunotherapy as compared with traditional cytotoxic chemotherapy, Dr. Cathcart-Rake said at the meeting cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
“A patient with cancer may be on immunotherapy and their risk for infection is quite low, but they may be at a huge risk for pneumonitis, which is treated completely differently,” she said. “So I think this should just raise alarms that close clinical monitoring for these conditions is really important.”
Dr. Cathcart-Rake disclosed that her institution receives research funding from Novartis. One study coinvestigator reported consulting or advisory roles with Trovagene, Genentech, Bristol-Myers Squibb, and Abbvie.
SOURCE: Cathcart-Rake EJ et al. 2018 Palliative and Supportive Care in Oncology Symposium. Abstract 184.
REPORTING FROM PALLONC 2018
Key clinical point: In non–small cell lung cancer patients treated with PD-1/PD-L1 inhibitors, immune-related adverse events may occur more frequently than has been suggested by clinical trial data.
Major finding: Pneumonitis was seen in nearly 11% of patients up to 60 days after the last immunotherapy dose, which investigators said was higher than reported in a pivotal phase 3 study.
Study details: Analysis of administrative claims data for 3,164 NSCLC patients treated between 2015 and 2017.
Disclosures: Researchers reported institutional research funding from Novartis. One researcher reported consulting or advisory roles with Trovagene, Genentech, Bristol-Myers Squibb, and Abbvie.
Source: Cathcart-Rake EJ et al. Palliative and Supportive Care in Oncology Symposium. Abstract 184.
Early phase 2 data: Mocetinostat/durvalumab combo shows promise in mNSCLC
WASHINGTON, D.C. – (mNSCLC) – including patients who progressed on prior checkpoint inhibitor therapy (CIT), according to preliminary findings from a phase 2 trial.
Of 29 evaluable patients who progressed on prior checkpoint blockade, 12 had “some degree of tumor regression” and 5 achieved a confirmed partial response, Manish Patel, DO, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“Some of these responses were quite durable. The longest response ... was a little over 1 year,” said Dr. Patel, of the University of Minnesota Masonic Cancer Center, Minneapolis.
Several patients continue to show objective responses, and the initial estimate of response duration is a median of more than 5 months, he added.
Of note, no differences have been seen to date with respect to clinical benefit in patients who did and did not have prior clinical benefit on checkpoint blockade, Dr. Patel said.
Overall, the combination was very well tolerated. The most common adverse events were fatigue, nausea, and diarrhea, with more than 10% of patients experiencing grade 3 or higher fatigue.
“Otherwise the toxicities were relatively minor,” he said, noting, however, that 8% of patients had cardiac events during the study, including atrial fibrillation, pericardial effusion, and a few cases of pericardial tamponade.
Such effects have been described in prior mocetinostat monotherapy trials, and all patients in the current study underwent pretreatment echocardiograms and did not have evidence of pericardial effusion at the start.
“So I think this is likely to be related to mocetinostat,” Dr. Patel said.
Mocetinostat is a spectrum-selective class I and class IV histone deacetylase inhibitor with multiple potential immunomodulatory features.
For example, the agent induces major histocompatibility complex Class I and Class II expression on tumor cells, enhances the function of T effector cells, and decreases the function of immunosuppressive cell subsets, including regulatory T cells and myeloid derived suppressor cells, Dr. Patel noted.
“It was hypothesized that because of these pleiotropic immune-supportive effects, that the combination of mocetinostat and checkpoint blockade might be a successful strategy for patients with non–small cell lung cancer,” he said.
In phase 1, doses of 50 mg, 70 mg, or 90 mg given three times weekly in combination with 1,500 mg of durvalumab were studied in patients with advanced solid tumors. Based on the safety data from that phase of the study, the recommended phase 2 dose of mocetinostat was 70 mg three times weekly with 1,500 mg of durvalumab on day 1 of each 28-day cycle.
Study subjects were patients with mNSCLC who had received at least one platinum-based doublet and whose most recent treatment prior to enrollment was with a checkpoint inhibitor, or who were immunotherapy naive.
The findings show promising clinical efficacy and safety, and enrollment in the study, which began in June 2016, is currently ongoing in the United States, he said.
Dr. Patel is an advisory board member for Nektar Therapeutics and has received research funding from Merck.
SOURCE: Patel M et al. SITC 2018, Abstract 027.
WASHINGTON, D.C. – (mNSCLC) – including patients who progressed on prior checkpoint inhibitor therapy (CIT), according to preliminary findings from a phase 2 trial.
Of 29 evaluable patients who progressed on prior checkpoint blockade, 12 had “some degree of tumor regression” and 5 achieved a confirmed partial response, Manish Patel, DO, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“Some of these responses were quite durable. The longest response ... was a little over 1 year,” said Dr. Patel, of the University of Minnesota Masonic Cancer Center, Minneapolis.
Several patients continue to show objective responses, and the initial estimate of response duration is a median of more than 5 months, he added.
Of note, no differences have been seen to date with respect to clinical benefit in patients who did and did not have prior clinical benefit on checkpoint blockade, Dr. Patel said.
Overall, the combination was very well tolerated. The most common adverse events were fatigue, nausea, and diarrhea, with more than 10% of patients experiencing grade 3 or higher fatigue.
“Otherwise the toxicities were relatively minor,” he said, noting, however, that 8% of patients had cardiac events during the study, including atrial fibrillation, pericardial effusion, and a few cases of pericardial tamponade.
Such effects have been described in prior mocetinostat monotherapy trials, and all patients in the current study underwent pretreatment echocardiograms and did not have evidence of pericardial effusion at the start.
“So I think this is likely to be related to mocetinostat,” Dr. Patel said.
Mocetinostat is a spectrum-selective class I and class IV histone deacetylase inhibitor with multiple potential immunomodulatory features.
For example, the agent induces major histocompatibility complex Class I and Class II expression on tumor cells, enhances the function of T effector cells, and decreases the function of immunosuppressive cell subsets, including regulatory T cells and myeloid derived suppressor cells, Dr. Patel noted.
“It was hypothesized that because of these pleiotropic immune-supportive effects, that the combination of mocetinostat and checkpoint blockade might be a successful strategy for patients with non–small cell lung cancer,” he said.
In phase 1, doses of 50 mg, 70 mg, or 90 mg given three times weekly in combination with 1,500 mg of durvalumab were studied in patients with advanced solid tumors. Based on the safety data from that phase of the study, the recommended phase 2 dose of mocetinostat was 70 mg three times weekly with 1,500 mg of durvalumab on day 1 of each 28-day cycle.
Study subjects were patients with mNSCLC who had received at least one platinum-based doublet and whose most recent treatment prior to enrollment was with a checkpoint inhibitor, or who were immunotherapy naive.
The findings show promising clinical efficacy and safety, and enrollment in the study, which began in June 2016, is currently ongoing in the United States, he said.
Dr. Patel is an advisory board member for Nektar Therapeutics and has received research funding from Merck.
SOURCE: Patel M et al. SITC 2018, Abstract 027.
WASHINGTON, D.C. – (mNSCLC) – including patients who progressed on prior checkpoint inhibitor therapy (CIT), according to preliminary findings from a phase 2 trial.
Of 29 evaluable patients who progressed on prior checkpoint blockade, 12 had “some degree of tumor regression” and 5 achieved a confirmed partial response, Manish Patel, DO, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“Some of these responses were quite durable. The longest response ... was a little over 1 year,” said Dr. Patel, of the University of Minnesota Masonic Cancer Center, Minneapolis.
Several patients continue to show objective responses, and the initial estimate of response duration is a median of more than 5 months, he added.
Of note, no differences have been seen to date with respect to clinical benefit in patients who did and did not have prior clinical benefit on checkpoint blockade, Dr. Patel said.
Overall, the combination was very well tolerated. The most common adverse events were fatigue, nausea, and diarrhea, with more than 10% of patients experiencing grade 3 or higher fatigue.
“Otherwise the toxicities were relatively minor,” he said, noting, however, that 8% of patients had cardiac events during the study, including atrial fibrillation, pericardial effusion, and a few cases of pericardial tamponade.
Such effects have been described in prior mocetinostat monotherapy trials, and all patients in the current study underwent pretreatment echocardiograms and did not have evidence of pericardial effusion at the start.
“So I think this is likely to be related to mocetinostat,” Dr. Patel said.
Mocetinostat is a spectrum-selective class I and class IV histone deacetylase inhibitor with multiple potential immunomodulatory features.
For example, the agent induces major histocompatibility complex Class I and Class II expression on tumor cells, enhances the function of T effector cells, and decreases the function of immunosuppressive cell subsets, including regulatory T cells and myeloid derived suppressor cells, Dr. Patel noted.
“It was hypothesized that because of these pleiotropic immune-supportive effects, that the combination of mocetinostat and checkpoint blockade might be a successful strategy for patients with non–small cell lung cancer,” he said.
In phase 1, doses of 50 mg, 70 mg, or 90 mg given three times weekly in combination with 1,500 mg of durvalumab were studied in patients with advanced solid tumors. Based on the safety data from that phase of the study, the recommended phase 2 dose of mocetinostat was 70 mg three times weekly with 1,500 mg of durvalumab on day 1 of each 28-day cycle.
Study subjects were patients with mNSCLC who had received at least one platinum-based doublet and whose most recent treatment prior to enrollment was with a checkpoint inhibitor, or who were immunotherapy naive.
The findings show promising clinical efficacy and safety, and enrollment in the study, which began in June 2016, is currently ongoing in the United States, he said.
Dr. Patel is an advisory board member for Nektar Therapeutics and has received research funding from Merck.
SOURCE: Patel M et al. SITC 2018, Abstract 027.
REPORTING FROM SITC 2018
Key clinical point: Mocetinostat/durvalumab shows clinical activity and manageable side effects in metastatic NSCLC.
Major finding: Five patients achieved a confirmed partial response.
Study details: A phase 2 study including 29 NSCLC patients.
Disclosures: Dr. Patel is an advisory board member for Nektar Therapeutics and has received research funding from Merck.
Source: Patel M et al. SITC 2018, Abstract 027.
Capmatinib active against NSCLC with MET exon 14 mutations
MUNICH – The experimental agent capmatinib was associated with a high response rate when used in the first line for patients with advanced non–small cell lung cancers bearing MET exon 14–skipping mutations, said investigators in the Geometry MONO-1 trial.
Among a cohort of 25 patients with treatment-naive, MET exon 14–mutated non–small cell lung cancer (NSCLC), the primary endpoint of overall response rate (ORR) as determined by blinded, independent reviewers was 72%.
In contrast, the ORR among 69 patients who had received one or more prior lines of therapy was 39.1%, reported Juergen Wolf, MD, of University Hospital Cologne (Germany).
“The differential benefit observed between patients treated in the first line and relapsed [settings] highlights the need of early diagnosis of this aberration, and prompt targeted treatment of this challenging patient population,” he said at the European Society for Medical Oncology Congress.
MET exon14–skipping mutations occur in approximately 3%-4% of NSCLC cases. The mutation is thought to be an oncogenic driver and has been shown to be a poor prognostic factor for patients with advanced NSCLC. Patients with this mutation have poor responses to conventional therapy and immune checkpoint inhibitors, even when their tumors have high levels of programmed death–ligand 1 (PD-L1) and high mutational burden, Dr. Wolf said.
Capmatinib (INC280) is an oral, reversible inhibitor of the MET receptor tyrosine kinase and is highly selective for MET, with particular affinity for MET exon 14 mutations. It is also capable of crossing the blood-brain barrier and has shown activity in the brain in preliminary studies.
The Geometry MONO-1 trial is a phase 2 study of capmatinib in patients with stage IIIB/IV NSCLC with tumors that demonstrate MET amplification and/or carry the MET exon 14 mutation. Three study cohorts of patients with MET amplification were closed for futility. Dr. Wolf reported results from two cohorts of patients with MET exon 14–skipping mutations regardless of gene copy number: one with treatment-naive patients and the other with patients being treated in the second or third line.
As noted, the ORR in 25 patients in the treatment-naive cohort after a median follow-up of 5.6 months was 72%, including 18 partial responses and no complete responses. In addition, six patients (24%) had stable disease, for a disease control rate of 96%.
In the pretreated cohort, however, there were no complete responses among 69 patients, and 27 patients (39.1%) had partial responses. In this cohort, an additional 26 patients (37.7%) had stable disease, for an ORR of 39.1% and disease-control rate of 78.3%.
Dr. Wolf also highlighted preliminary evidence of capmatinib activity in the brain. He noted that one patient, an 80-year-old woman with multiple untreated brain metastases as well as lesions in dermal lymph nodes, liver, and pleura, had complete resolution of brain metastases at the first postbaseline CT scan, 42 days after starting capmatinib. The duration of response was 11.3 months, at which point the patient discontinued the drug because of extracranial progressive disease.
Among all patients in all study cohorts (302) the most common grade 3 or 4 adverse events were peripheral edema, dyspnea, fatigue, nausea, vomiting, and decreased appetite. Adverse drug-related events (grade 3 or 4) included peripheral edema, nausea, vomiting, fatigue, and decreased appetite. In all, 10.3% of patients discontinued for adverse events suspected to be related to capmatinib.
Invited discussant James Chih-Hsin Yang, MD, PhD, from the National Taiwan University Hospital in Taipei, said that the study shows that the MET exon 14–skipping mutation is an oncogenic driver and that capmatinib is an effective tyrosine kinase inhibitor (TKI) for patients with NSCLC harboring this mutation.
Questions that still need to be answered, he said, include whether patients with the mutation are heterogeneous and may have differing response to TKIs, how long the duration of response is, how long it will take for resistance to capmatinib to occur, how it compares with other MET inhibitors, and if there are additional biomarkers that could help select patients for treatment with the novel agent.
The study was funded by Novartis. Dr. Wolf reported advisory board participation, institutional research support, and lecture fees from Novartis and others. Dr. Yang reported honoraria from advisory board participation and/or speaking from Novartis and others. His institution participated in the Geometry MONO-1 study, but he was not personally involved.
MUNICH – The experimental agent capmatinib was associated with a high response rate when used in the first line for patients with advanced non–small cell lung cancers bearing MET exon 14–skipping mutations, said investigators in the Geometry MONO-1 trial.
Among a cohort of 25 patients with treatment-naive, MET exon 14–mutated non–small cell lung cancer (NSCLC), the primary endpoint of overall response rate (ORR) as determined by blinded, independent reviewers was 72%.
In contrast, the ORR among 69 patients who had received one or more prior lines of therapy was 39.1%, reported Juergen Wolf, MD, of University Hospital Cologne (Germany).
“The differential benefit observed between patients treated in the first line and relapsed [settings] highlights the need of early diagnosis of this aberration, and prompt targeted treatment of this challenging patient population,” he said at the European Society for Medical Oncology Congress.
MET exon14–skipping mutations occur in approximately 3%-4% of NSCLC cases. The mutation is thought to be an oncogenic driver and has been shown to be a poor prognostic factor for patients with advanced NSCLC. Patients with this mutation have poor responses to conventional therapy and immune checkpoint inhibitors, even when their tumors have high levels of programmed death–ligand 1 (PD-L1) and high mutational burden, Dr. Wolf said.
Capmatinib (INC280) is an oral, reversible inhibitor of the MET receptor tyrosine kinase and is highly selective for MET, with particular affinity for MET exon 14 mutations. It is also capable of crossing the blood-brain barrier and has shown activity in the brain in preliminary studies.
The Geometry MONO-1 trial is a phase 2 study of capmatinib in patients with stage IIIB/IV NSCLC with tumors that demonstrate MET amplification and/or carry the MET exon 14 mutation. Three study cohorts of patients with MET amplification were closed for futility. Dr. Wolf reported results from two cohorts of patients with MET exon 14–skipping mutations regardless of gene copy number: one with treatment-naive patients and the other with patients being treated in the second or third line.
As noted, the ORR in 25 patients in the treatment-naive cohort after a median follow-up of 5.6 months was 72%, including 18 partial responses and no complete responses. In addition, six patients (24%) had stable disease, for a disease control rate of 96%.
In the pretreated cohort, however, there were no complete responses among 69 patients, and 27 patients (39.1%) had partial responses. In this cohort, an additional 26 patients (37.7%) had stable disease, for an ORR of 39.1% and disease-control rate of 78.3%.
Dr. Wolf also highlighted preliminary evidence of capmatinib activity in the brain. He noted that one patient, an 80-year-old woman with multiple untreated brain metastases as well as lesions in dermal lymph nodes, liver, and pleura, had complete resolution of brain metastases at the first postbaseline CT scan, 42 days after starting capmatinib. The duration of response was 11.3 months, at which point the patient discontinued the drug because of extracranial progressive disease.
Among all patients in all study cohorts (302) the most common grade 3 or 4 adverse events were peripheral edema, dyspnea, fatigue, nausea, vomiting, and decreased appetite. Adverse drug-related events (grade 3 or 4) included peripheral edema, nausea, vomiting, fatigue, and decreased appetite. In all, 10.3% of patients discontinued for adverse events suspected to be related to capmatinib.
Invited discussant James Chih-Hsin Yang, MD, PhD, from the National Taiwan University Hospital in Taipei, said that the study shows that the MET exon 14–skipping mutation is an oncogenic driver and that capmatinib is an effective tyrosine kinase inhibitor (TKI) for patients with NSCLC harboring this mutation.
Questions that still need to be answered, he said, include whether patients with the mutation are heterogeneous and may have differing response to TKIs, how long the duration of response is, how long it will take for resistance to capmatinib to occur, how it compares with other MET inhibitors, and if there are additional biomarkers that could help select patients for treatment with the novel agent.
The study was funded by Novartis. Dr. Wolf reported advisory board participation, institutional research support, and lecture fees from Novartis and others. Dr. Yang reported honoraria from advisory board participation and/or speaking from Novartis and others. His institution participated in the Geometry MONO-1 study, but he was not personally involved.
MUNICH – The experimental agent capmatinib was associated with a high response rate when used in the first line for patients with advanced non–small cell lung cancers bearing MET exon 14–skipping mutations, said investigators in the Geometry MONO-1 trial.
Among a cohort of 25 patients with treatment-naive, MET exon 14–mutated non–small cell lung cancer (NSCLC), the primary endpoint of overall response rate (ORR) as determined by blinded, independent reviewers was 72%.
In contrast, the ORR among 69 patients who had received one or more prior lines of therapy was 39.1%, reported Juergen Wolf, MD, of University Hospital Cologne (Germany).
“The differential benefit observed between patients treated in the first line and relapsed [settings] highlights the need of early diagnosis of this aberration, and prompt targeted treatment of this challenging patient population,” he said at the European Society for Medical Oncology Congress.
MET exon14–skipping mutations occur in approximately 3%-4% of NSCLC cases. The mutation is thought to be an oncogenic driver and has been shown to be a poor prognostic factor for patients with advanced NSCLC. Patients with this mutation have poor responses to conventional therapy and immune checkpoint inhibitors, even when their tumors have high levels of programmed death–ligand 1 (PD-L1) and high mutational burden, Dr. Wolf said.
Capmatinib (INC280) is an oral, reversible inhibitor of the MET receptor tyrosine kinase and is highly selective for MET, with particular affinity for MET exon 14 mutations. It is also capable of crossing the blood-brain barrier and has shown activity in the brain in preliminary studies.
The Geometry MONO-1 trial is a phase 2 study of capmatinib in patients with stage IIIB/IV NSCLC with tumors that demonstrate MET amplification and/or carry the MET exon 14 mutation. Three study cohorts of patients with MET amplification were closed for futility. Dr. Wolf reported results from two cohorts of patients with MET exon 14–skipping mutations regardless of gene copy number: one with treatment-naive patients and the other with patients being treated in the second or third line.
As noted, the ORR in 25 patients in the treatment-naive cohort after a median follow-up of 5.6 months was 72%, including 18 partial responses and no complete responses. In addition, six patients (24%) had stable disease, for a disease control rate of 96%.
In the pretreated cohort, however, there were no complete responses among 69 patients, and 27 patients (39.1%) had partial responses. In this cohort, an additional 26 patients (37.7%) had stable disease, for an ORR of 39.1% and disease-control rate of 78.3%.
Dr. Wolf also highlighted preliminary evidence of capmatinib activity in the brain. He noted that one patient, an 80-year-old woman with multiple untreated brain metastases as well as lesions in dermal lymph nodes, liver, and pleura, had complete resolution of brain metastases at the first postbaseline CT scan, 42 days after starting capmatinib. The duration of response was 11.3 months, at which point the patient discontinued the drug because of extracranial progressive disease.
Among all patients in all study cohorts (302) the most common grade 3 or 4 adverse events were peripheral edema, dyspnea, fatigue, nausea, vomiting, and decreased appetite. Adverse drug-related events (grade 3 or 4) included peripheral edema, nausea, vomiting, fatigue, and decreased appetite. In all, 10.3% of patients discontinued for adverse events suspected to be related to capmatinib.
Invited discussant James Chih-Hsin Yang, MD, PhD, from the National Taiwan University Hospital in Taipei, said that the study shows that the MET exon 14–skipping mutation is an oncogenic driver and that capmatinib is an effective tyrosine kinase inhibitor (TKI) for patients with NSCLC harboring this mutation.
Questions that still need to be answered, he said, include whether patients with the mutation are heterogeneous and may have differing response to TKIs, how long the duration of response is, how long it will take for resistance to capmatinib to occur, how it compares with other MET inhibitors, and if there are additional biomarkers that could help select patients for treatment with the novel agent.
The study was funded by Novartis. Dr. Wolf reported advisory board participation, institutional research support, and lecture fees from Novartis and others. Dr. Yang reported honoraria from advisory board participation and/or speaking from Novartis and others. His institution participated in the Geometry MONO-1 study, but he was not personally involved.
REPORTING FROM ESMO 2018
Key clinical point: Patients with non–small cell lung cancer bearing a MET exon 14–skipping mutation had high overall response rates to the MET inhibitor capmatinib.
Major finding: The overall response rate in treatment-naive patients was 72%.
Study details: A phase 2 trial with previously treated and untreated patients with advanced non–small cell lung cancers bearing MET exon 14–skipping mutations.
Disclosures: The study was funded by Novartis. Dr. Wolf reported advisory board participation and lecture fees from Novartis and others and institutional research support from Novartis and others. Dr. Yang reported honoraria from advisory board participation and/or speaking from Novartis and others. His institution participated in the Geometry MONO-1 study, but he was not personally involved.
Cigarette smoking at lowest level ever
“This new all-time low in cigarette smoking among U.S. adults is a tremendous public health accomplishment, and it demonstrates the importance of continued proven strategies to reduce smoking,” CDC Director Robert Redfield said in a written statement.
In 2017, 19.3% of adults aged 18 years and older – approximately 47.4 million Americans – reported current use of some type of tobacco product, and current use of combustible tobacco was 16.7%, Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report. Current use was defined as use every day or some days, with an added requirement of at least 100 cigarettes in a lifetime added for cigarette smokers.
Data from the National Health Interview Survey showed that from 2016 to 2017, current use declined for any tobacco product, any combustible tobacco product, cigarettes, smokeless tobacco, and the combination of two or more tobacco products. The most common combination in 2017 was cigarettes and e-cigarettes, which was reported by 30.1% of the 9 million adults who used more than one product, Dr. Wang and her associates said.
Prevalence of current tobacco use was higher among men than women (24.8% vs. 14.2%), and adults aged 25-44 years (22.5%) had the highest level by age, followed by those aged 45-64 years (21.3%), 18-24 years (18.3%), and 65 years or older (11%). Use by race/ethnicity was highest among American Indian/Alaska Natives (29.8%), with the Midwest putting up the highest prevalence by region at 23.5%, they said.
“Although cigarette smoking among U.S. adults has declined considerably, tobacco products have evolved in recent years to include various combustible, noncombustible, and electronic products,” Dr. Wang and her associates wrote. “Implementation of evidence-based tobacco control interventions that address the diversity of tobacco products used by U.S. adults, in coordination with regulation of tobacco product manufacturing, marketing, and sales, can reduce tobacco-related disease and death in the United States.”
SOURCE: Wang TW et al. MMWR. 2018 Nov 9;67[44]:1225-32.

“This new all-time low in cigarette smoking among U.S. adults is a tremendous public health accomplishment, and it demonstrates the importance of continued proven strategies to reduce smoking,” CDC Director Robert Redfield said in a written statement.
In 2017, 19.3% of adults aged 18 years and older – approximately 47.4 million Americans – reported current use of some type of tobacco product, and current use of combustible tobacco was 16.7%, Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report. Current use was defined as use every day or some days, with an added requirement of at least 100 cigarettes in a lifetime added for cigarette smokers.
Data from the National Health Interview Survey showed that from 2016 to 2017, current use declined for any tobacco product, any combustible tobacco product, cigarettes, smokeless tobacco, and the combination of two or more tobacco products. The most common combination in 2017 was cigarettes and e-cigarettes, which was reported by 30.1% of the 9 million adults who used more than one product, Dr. Wang and her associates said.
Prevalence of current tobacco use was higher among men than women (24.8% vs. 14.2%), and adults aged 25-44 years (22.5%) had the highest level by age, followed by those aged 45-64 years (21.3%), 18-24 years (18.3%), and 65 years or older (11%). Use by race/ethnicity was highest among American Indian/Alaska Natives (29.8%), with the Midwest putting up the highest prevalence by region at 23.5%, they said.
“Although cigarette smoking among U.S. adults has declined considerably, tobacco products have evolved in recent years to include various combustible, noncombustible, and electronic products,” Dr. Wang and her associates wrote. “Implementation of evidence-based tobacco control interventions that address the diversity of tobacco products used by U.S. adults, in coordination with regulation of tobacco product manufacturing, marketing, and sales, can reduce tobacco-related disease and death in the United States.”
SOURCE: Wang TW et al. MMWR. 2018 Nov 9;67[44]:1225-32.

“This new all-time low in cigarette smoking among U.S. adults is a tremendous public health accomplishment, and it demonstrates the importance of continued proven strategies to reduce smoking,” CDC Director Robert Redfield said in a written statement.
In 2017, 19.3% of adults aged 18 years and older – approximately 47.4 million Americans – reported current use of some type of tobacco product, and current use of combustible tobacco was 16.7%, Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report. Current use was defined as use every day or some days, with an added requirement of at least 100 cigarettes in a lifetime added for cigarette smokers.
Data from the National Health Interview Survey showed that from 2016 to 2017, current use declined for any tobacco product, any combustible tobacco product, cigarettes, smokeless tobacco, and the combination of two or more tobacco products. The most common combination in 2017 was cigarettes and e-cigarettes, which was reported by 30.1% of the 9 million adults who used more than one product, Dr. Wang and her associates said.
Prevalence of current tobacco use was higher among men than women (24.8% vs. 14.2%), and adults aged 25-44 years (22.5%) had the highest level by age, followed by those aged 45-64 years (21.3%), 18-24 years (18.3%), and 65 years or older (11%). Use by race/ethnicity was highest among American Indian/Alaska Natives (29.8%), with the Midwest putting up the highest prevalence by region at 23.5%, they said.
“Although cigarette smoking among U.S. adults has declined considerably, tobacco products have evolved in recent years to include various combustible, noncombustible, and electronic products,” Dr. Wang and her associates wrote. “Implementation of evidence-based tobacco control interventions that address the diversity of tobacco products used by U.S. adults, in coordination with regulation of tobacco product manufacturing, marketing, and sales, can reduce tobacco-related disease and death in the United States.”
SOURCE: Wang TW et al. MMWR. 2018 Nov 9;67[44]:1225-32.
FROM MMWR
FDA approves lorlatinib as second line for ALK-positive advanced NSCLC
The Food and Drug Administration has granted accelerated approval to lorlatinib for patients with anaplastic lymphoma kinase (ALK)–positive metastatic non–small cell lung cancer (NSCLC) whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic disease or whose disease has progressed on alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease.
Approval of the next-generation ALK inhibitor was based on an overall response rate of 48% – with 4% complete and 44% partial – in a subgroup of 215 patients with ALK-positive metastatic NSCLC enrolled in a nonrandomized, phase 2 trial, the FDA said in a press announcement. All patients had been previously treated with one or more ALK kinase inhibitors.
The median response duration was 12.5 months (95% confidence interval, 8.4-23.7) and the intracranial overall response rate in 89 patients with measurable lesions in the CNS was 60% (95% CI, 49-70) with 21% complete and 38% partial responses.
Common adverse reactions in patients receiving lorlatinib were edema, peripheral neuropathy, cognitive effects, dyspnea, fatigue, weight gain, arthralgia, mood effects, and diarrhea. The most common laboratory abnormalities were hypercholesterolemia and hypertriglyceridemia, the FDA said.
The recommended dose of lorlatinib, to be marketed as Lorbrena by Pfizer, is 100 mg orally once daily.
The Food and Drug Administration has granted accelerated approval to lorlatinib for patients with anaplastic lymphoma kinase (ALK)–positive metastatic non–small cell lung cancer (NSCLC) whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic disease or whose disease has progressed on alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease.
Approval of the next-generation ALK inhibitor was based on an overall response rate of 48% – with 4% complete and 44% partial – in a subgroup of 215 patients with ALK-positive metastatic NSCLC enrolled in a nonrandomized, phase 2 trial, the FDA said in a press announcement. All patients had been previously treated with one or more ALK kinase inhibitors.
The median response duration was 12.5 months (95% confidence interval, 8.4-23.7) and the intracranial overall response rate in 89 patients with measurable lesions in the CNS was 60% (95% CI, 49-70) with 21% complete and 38% partial responses.
Common adverse reactions in patients receiving lorlatinib were edema, peripheral neuropathy, cognitive effects, dyspnea, fatigue, weight gain, arthralgia, mood effects, and diarrhea. The most common laboratory abnormalities were hypercholesterolemia and hypertriglyceridemia, the FDA said.
The recommended dose of lorlatinib, to be marketed as Lorbrena by Pfizer, is 100 mg orally once daily.
The Food and Drug Administration has granted accelerated approval to lorlatinib for patients with anaplastic lymphoma kinase (ALK)–positive metastatic non–small cell lung cancer (NSCLC) whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic disease or whose disease has progressed on alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease.
Approval of the next-generation ALK inhibitor was based on an overall response rate of 48% – with 4% complete and 44% partial – in a subgroup of 215 patients with ALK-positive metastatic NSCLC enrolled in a nonrandomized, phase 2 trial, the FDA said in a press announcement. All patients had been previously treated with one or more ALK kinase inhibitors.
The median response duration was 12.5 months (95% confidence interval, 8.4-23.7) and the intracranial overall response rate in 89 patients with measurable lesions in the CNS was 60% (95% CI, 49-70) with 21% complete and 38% partial responses.
Common adverse reactions in patients receiving lorlatinib were edema, peripheral neuropathy, cognitive effects, dyspnea, fatigue, weight gain, arthralgia, mood effects, and diarrhea. The most common laboratory abnormalities were hypercholesterolemia and hypertriglyceridemia, the FDA said.
The recommended dose of lorlatinib, to be marketed as Lorbrena by Pfizer, is 100 mg orally once daily.
FDA expands approval of pembrolizumab in NSCLC
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.