User login
Guidelines for proton therapy in mediastinal lymphomas
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but the treatment should only be used in patients expected to derive the most benefit, according to new guidelines from the International Lymphoma Radiation Oncology Group.
The guidelines note that proton therapy reduces the radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart.
However, the advantages of proton therapy are not always clear in other situations, such as when the target spans the right side of the heart or when the target is above the heart with no axillary involvement.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” said guideline author Bouthaina Dabaja, MD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues.
The group’s guidelines were published in Blood.
The guidelines note that proton therapy—like intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy—presents an opportunity for more conformal dose distribution and better sparing of organs at risk.
Proton therapy can greatly benefit certain patients with mediastinal disease, including:
- Young female patients in whom proton therapy would reduce the breast dose and decrease the risk of secondary breast cancer
- Patients at high risk of radiation-related toxicity due to previous treatment
- Patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines.
However, the consideration of proton therapy needs to factor in the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy is the least complex delivery technique, it is challenging because beams can conform only to one side of the target. In contrast, active mode pencil beam scanning proton therapy potentially provides better conformality and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said.
However, “motion management is of prime importance” with pencil beam scanning proton therapy, which is more sensitive to density changes in the beam path than is passive scattering proton therapy.
To that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
Dr. Dabaja and her coauthors reported no funding or conflicts of interest.
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but the treatment should only be used in patients expected to derive the most benefit, according to new guidelines from the International Lymphoma Radiation Oncology Group.
The guidelines note that proton therapy reduces the radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart.
However, the advantages of proton therapy are not always clear in other situations, such as when the target spans the right side of the heart or when the target is above the heart with no axillary involvement.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” said guideline author Bouthaina Dabaja, MD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues.
The group’s guidelines were published in Blood.
The guidelines note that proton therapy—like intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy—presents an opportunity for more conformal dose distribution and better sparing of organs at risk.
Proton therapy can greatly benefit certain patients with mediastinal disease, including:
- Young female patients in whom proton therapy would reduce the breast dose and decrease the risk of secondary breast cancer
- Patients at high risk of radiation-related toxicity due to previous treatment
- Patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines.
However, the consideration of proton therapy needs to factor in the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy is the least complex delivery technique, it is challenging because beams can conform only to one side of the target. In contrast, active mode pencil beam scanning proton therapy potentially provides better conformality and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said.
However, “motion management is of prime importance” with pencil beam scanning proton therapy, which is more sensitive to density changes in the beam path than is passive scattering proton therapy.
To that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
Dr. Dabaja and her coauthors reported no funding or conflicts of interest.
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but the treatment should only be used in patients expected to derive the most benefit, according to new guidelines from the International Lymphoma Radiation Oncology Group.
The guidelines note that proton therapy reduces the radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart.
However, the advantages of proton therapy are not always clear in other situations, such as when the target spans the right side of the heart or when the target is above the heart with no axillary involvement.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” said guideline author Bouthaina Dabaja, MD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues.
The group’s guidelines were published in Blood.
The guidelines note that proton therapy—like intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy—presents an opportunity for more conformal dose distribution and better sparing of organs at risk.
Proton therapy can greatly benefit certain patients with mediastinal disease, including:
- Young female patients in whom proton therapy would reduce the breast dose and decrease the risk of secondary breast cancer
- Patients at high risk of radiation-related toxicity due to previous treatment
- Patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines.
However, the consideration of proton therapy needs to factor in the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy is the least complex delivery technique, it is challenging because beams can conform only to one side of the target. In contrast, active mode pencil beam scanning proton therapy potentially provides better conformality and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said.
However, “motion management is of prime importance” with pencil beam scanning proton therapy, which is more sensitive to density changes in the beam path than is passive scattering proton therapy.
To that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
Dr. Dabaja and her coauthors reported no funding or conflicts of interest.
Risks of watchful waiting in follicular lymphoma
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
Researchers propose new acute leukemia subtypes
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
FROM NATURE
Key clinical point:
Major finding: In total, 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL.
Study details: Whole-genome, -exome, and RNA sequencing of 115 samples from pediatric patients with MPAL.
Disclosures: This research was supported by the National Cancer Institute and other organizations. The researchers reported having no competing interests.
Source: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
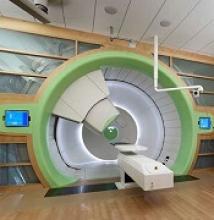
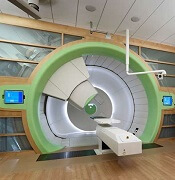
Best practices defined for proton therapy in mediastinal lymphomas
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but only when this modality offers a clear advantage over intensity-modulated radiotherapy, according to new guidelines from the International Lymphoma Radiation Oncology Group.
Proton therapy reduces radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart. The advantages are not always clear in other situations, such as when the target spans the right side of the heart, or when the target is above the heart with no axillary involvement, according to guideline authors Bouthaina Dabaja, MD, professor and section chief of hematology in the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” Dr. Dabaja and her coauthors said in the guidelines, which were published in the journal Blood (doi: 10.1182/blood-2018-03-837633).
Along with intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy, proton therapy presents “another opportunity” for more conformal dose distribution and better sparing of organs at risk, according to the consensus recommendations. Proton therapy can greatly benefit certain patients with mediastinal disease. These include young female patients to reduce breast dose and risk of a secondary breast cancer, patients at high risk of radiation-related toxicity due to previous treatment, and patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines. However, the consideration of proton therapy needs to factor the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy (PSPT) is the least complex delivery technique, it is challenging because beams can conform only to one side of the target; by contrast, the experts said, active mode pencil beam scanning proton therapy (PBSPT) potentially provides better conformity and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said in their report.
However, motion management is “of prime importance” with PBSPT, which is more sensitive to density changes in the beam path as compared to PSPT, they added. Toward that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
The guidelines list a total of 11 authors representing The University of Texas MD Anderson Cancer Center; University of Florida, Jacksonville; University of Pennsylvania, Philadelphia; University of Louisiana, Baton Rouge; Proton Therapy Center Czech, Prague; Motol University Hospital, Czech Republic; St. Thomas and Guy Hospital, London; Institut Curie, Paris; and Rigshospitalet, Copenhagen.
Dr. Dabaja and her guideline coauthors reported no funding or conflicts of interest.
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but only when this modality offers a clear advantage over intensity-modulated radiotherapy, according to new guidelines from the International Lymphoma Radiation Oncology Group.
Proton therapy reduces radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart. The advantages are not always clear in other situations, such as when the target spans the right side of the heart, or when the target is above the heart with no axillary involvement, according to guideline authors Bouthaina Dabaja, MD, professor and section chief of hematology in the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” Dr. Dabaja and her coauthors said in the guidelines, which were published in the journal Blood (doi: 10.1182/blood-2018-03-837633).
Along with intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy, proton therapy presents “another opportunity” for more conformal dose distribution and better sparing of organs at risk, according to the consensus recommendations. Proton therapy can greatly benefit certain patients with mediastinal disease. These include young female patients to reduce breast dose and risk of a secondary breast cancer, patients at high risk of radiation-related toxicity due to previous treatment, and patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines. However, the consideration of proton therapy needs to factor the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy (PSPT) is the least complex delivery technique, it is challenging because beams can conform only to one side of the target; by contrast, the experts said, active mode pencil beam scanning proton therapy (PBSPT) potentially provides better conformity and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said in their report.
However, motion management is “of prime importance” with PBSPT, which is more sensitive to density changes in the beam path as compared to PSPT, they added. Toward that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
The guidelines list a total of 11 authors representing The University of Texas MD Anderson Cancer Center; University of Florida, Jacksonville; University of Pennsylvania, Philadelphia; University of Louisiana, Baton Rouge; Proton Therapy Center Czech, Prague; Motol University Hospital, Czech Republic; St. Thomas and Guy Hospital, London; Institut Curie, Paris; and Rigshospitalet, Copenhagen.
Dr. Dabaja and her guideline coauthors reported no funding or conflicts of interest.
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but only when this modality offers a clear advantage over intensity-modulated radiotherapy, according to new guidelines from the International Lymphoma Radiation Oncology Group.
Proton therapy reduces radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart. The advantages are not always clear in other situations, such as when the target spans the right side of the heart, or when the target is above the heart with no axillary involvement, according to guideline authors Bouthaina Dabaja, MD, professor and section chief of hematology in the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” Dr. Dabaja and her coauthors said in the guidelines, which were published in the journal Blood (doi: 10.1182/blood-2018-03-837633).
Along with intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy, proton therapy presents “another opportunity” for more conformal dose distribution and better sparing of organs at risk, according to the consensus recommendations. Proton therapy can greatly benefit certain patients with mediastinal disease. These include young female patients to reduce breast dose and risk of a secondary breast cancer, patients at high risk of radiation-related toxicity due to previous treatment, and patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines. However, the consideration of proton therapy needs to factor the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy (PSPT) is the least complex delivery technique, it is challenging because beams can conform only to one side of the target; by contrast, the experts said, active mode pencil beam scanning proton therapy (PBSPT) potentially provides better conformity and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said in their report.
However, motion management is “of prime importance” with PBSPT, which is more sensitive to density changes in the beam path as compared to PSPT, they added. Toward that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
The guidelines list a total of 11 authors representing The University of Texas MD Anderson Cancer Center; University of Florida, Jacksonville; University of Pennsylvania, Philadelphia; University of Louisiana, Baton Rouge; Proton Therapy Center Czech, Prague; Motol University Hospital, Czech Republic; St. Thomas and Guy Hospital, London; Institut Curie, Paris; and Rigshospitalet, Copenhagen.
Dr. Dabaja and her guideline coauthors reported no funding or conflicts of interest.
FROM BLOOD
ASCO addresses financial barriers to cancer clinical trials
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
Implementation of Chemo and Anti-Neoplastic Extractor Cycle and Regimen Estimator (Cancer Care) in Follicular Lymphoma Patients Treated in VA
Rationale: Successfully extract, identify, and validate therapeutics lines used in the treatment of Veterans with follicular lymphoma.
Background: With the adoption of electronic health record (EHR) systems, leveraging this data is becoming increasingly important for clinical and observational research, especially in oncology, where precision oncology has become central to the Cancer Moonshot Initiative. One of the greatest challenges in using EHR data is extracting a cancer patient’s treatment history. The difficulty lies in identifying treatment “lines,” which may include one or more drugs, with each drug dispensation often recorded in an unstructured format within the EHR. Our objective was to conceptualize, develop, and validate an algorithm that reconstructs a cancer treatment line history using single-agent EHR pharmacy data in a cohort of follicular lymphoma patients treated in the Veterans Health Administration (VHA).
Methods: The CANCER CARE algorithm recreates and formalizes the heuristic a clinician uses to identify treatment lines dispensed using two inputs: (1) National Comprehensive Cancer Network treatment guidelines and the recommended chemotherapy lines and their comprising
antineoplastic agents that are used in the treatment of the cancer of interest; and (2) Single-agent dispensation information retrieved from the VA Corporate Data Warehouse. The algorithm uses rules to map concordant dispensation agents to a treatment line while taking into account common
practice variations such as omitted agents during the start or middle of a treatment line. It also identifies the initiation of a new line based on a change in agents received or time gaps between treatments. The algorithm was validated by comparing a set of 100 treatment lines that were independently annotated by a clinician in a cohort of patients with follicular lymphoma to the algorithm output. Accuracy, sensitivity, and precision were measured.
Results: CANCER CARE had an accuracy of 96%. Accuracy, sensitivity and precision for most prevalent lines were: 98%, 97% and 100% (rituximab), respectively; and 99%, 100%, and 95% (RCHOP), respectively. Accuracy, sensitivity, and precision for RCVP and BR were all 100%.
Conclusions: Cancer treatment line identification from EHR pharmacy dispensation data using a rule-based approach is feasible with high accuracy and can be used in real-world studies of cancer patient treatment practices and outcomes.
Rationale: Successfully extract, identify, and validate therapeutics lines used in the treatment of Veterans with follicular lymphoma.
Background: With the adoption of electronic health record (EHR) systems, leveraging this data is becoming increasingly important for clinical and observational research, especially in oncology, where precision oncology has become central to the Cancer Moonshot Initiative. One of the greatest challenges in using EHR data is extracting a cancer patient’s treatment history. The difficulty lies in identifying treatment “lines,” which may include one or more drugs, with each drug dispensation often recorded in an unstructured format within the EHR. Our objective was to conceptualize, develop, and validate an algorithm that reconstructs a cancer treatment line history using single-agent EHR pharmacy data in a cohort of follicular lymphoma patients treated in the Veterans Health Administration (VHA).
Methods: The CANCER CARE algorithm recreates and formalizes the heuristic a clinician uses to identify treatment lines dispensed using two inputs: (1) National Comprehensive Cancer Network treatment guidelines and the recommended chemotherapy lines and their comprising
antineoplastic agents that are used in the treatment of the cancer of interest; and (2) Single-agent dispensation information retrieved from the VA Corporate Data Warehouse. The algorithm uses rules to map concordant dispensation agents to a treatment line while taking into account common
practice variations such as omitted agents during the start or middle of a treatment line. It also identifies the initiation of a new line based on a change in agents received or time gaps between treatments. The algorithm was validated by comparing a set of 100 treatment lines that were independently annotated by a clinician in a cohort of patients with follicular lymphoma to the algorithm output. Accuracy, sensitivity, and precision were measured.
Results: CANCER CARE had an accuracy of 96%. Accuracy, sensitivity and precision for most prevalent lines were: 98%, 97% and 100% (rituximab), respectively; and 99%, 100%, and 95% (RCHOP), respectively. Accuracy, sensitivity, and precision for RCVP and BR were all 100%.
Conclusions: Cancer treatment line identification from EHR pharmacy dispensation data using a rule-based approach is feasible with high accuracy and can be used in real-world studies of cancer patient treatment practices and outcomes.
Rationale: Successfully extract, identify, and validate therapeutics lines used in the treatment of Veterans with follicular lymphoma.
Background: With the adoption of electronic health record (EHR) systems, leveraging this data is becoming increasingly important for clinical and observational research, especially in oncology, where precision oncology has become central to the Cancer Moonshot Initiative. One of the greatest challenges in using EHR data is extracting a cancer patient’s treatment history. The difficulty lies in identifying treatment “lines,” which may include one or more drugs, with each drug dispensation often recorded in an unstructured format within the EHR. Our objective was to conceptualize, develop, and validate an algorithm that reconstructs a cancer treatment line history using single-agent EHR pharmacy data in a cohort of follicular lymphoma patients treated in the Veterans Health Administration (VHA).
Methods: The CANCER CARE algorithm recreates and formalizes the heuristic a clinician uses to identify treatment lines dispensed using two inputs: (1) National Comprehensive Cancer Network treatment guidelines and the recommended chemotherapy lines and their comprising
antineoplastic agents that are used in the treatment of the cancer of interest; and (2) Single-agent dispensation information retrieved from the VA Corporate Data Warehouse. The algorithm uses rules to map concordant dispensation agents to a treatment line while taking into account common
practice variations such as omitted agents during the start or middle of a treatment line. It also identifies the initiation of a new line based on a change in agents received or time gaps between treatments. The algorithm was validated by comparing a set of 100 treatment lines that were independently annotated by a clinician in a cohort of patients with follicular lymphoma to the algorithm output. Accuracy, sensitivity, and precision were measured.
Results: CANCER CARE had an accuracy of 96%. Accuracy, sensitivity and precision for most prevalent lines were: 98%, 97% and 100% (rituximab), respectively; and 99%, 100%, and 95% (RCHOP), respectively. Accuracy, sensitivity, and precision for RCVP and BR were all 100%.
Conclusions: Cancer treatment line identification from EHR pharmacy dispensation data using a rule-based approach is feasible with high accuracy and can be used in real-world studies of cancer patient treatment practices and outcomes.
STORM trial shows response in penta-refractory myeloma
Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma, according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%. The median progression-free survival was 3.7 months and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Icahn School of Medicine at Mount Sinai, New York, presented these results at the annual meeting of the Society of Hematologic Oncology.
“The additional phase 2b clinical results… are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said in a statement. “Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM [Selinexor Treatment of Refractory Myeloma] study.”
STORM (NCT02336815) included 122 patients with penta-refractory multiple myeloma. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of seven prior treatment regimens. Their median age was 65 years, a little more than half were men, and more than half had high-risk cytogenetics. Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10–6 and one at 1 x 10–4.
Very good partial responses were seen in 4.9% of patients, 19.7% had partial responses, 13.1% had minimal responses (MRs), and 39.3% had stable disease. Progressive disease occurred in 13.1% of patients; 8.2% were not evaluable for response.
The overall response rate (partial response or better) was 26.2%, the clinical benefit rate (MR or better) was 39.3%, and the disease control rate (stable disease or better) was 78.7%.
The median duration of response was 4.4 months. The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had progressive disease or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. Overall survival was 15.6 months in patients with an MR or better and 1.7 months in patients who had progressive disease or were not evaluable (P less than .0001).
The “most important” grade 3/4 adverse events, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%). A total of 23 patients (19.5%) discontinued treatment because of a related adverse.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GlaxoSmithKline.
SOURCE: Jagannath S et al. SOHO 2018, Abstract MM-255
Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma, according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%. The median progression-free survival was 3.7 months and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Icahn School of Medicine at Mount Sinai, New York, presented these results at the annual meeting of the Society of Hematologic Oncology.
“The additional phase 2b clinical results… are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said in a statement. “Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM [Selinexor Treatment of Refractory Myeloma] study.”
STORM (NCT02336815) included 122 patients with penta-refractory multiple myeloma. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of seven prior treatment regimens. Their median age was 65 years, a little more than half were men, and more than half had high-risk cytogenetics. Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10–6 and one at 1 x 10–4.
Very good partial responses were seen in 4.9% of patients, 19.7% had partial responses, 13.1% had minimal responses (MRs), and 39.3% had stable disease. Progressive disease occurred in 13.1% of patients; 8.2% were not evaluable for response.
The overall response rate (partial response or better) was 26.2%, the clinical benefit rate (MR or better) was 39.3%, and the disease control rate (stable disease or better) was 78.7%.
The median duration of response was 4.4 months. The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had progressive disease or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. Overall survival was 15.6 months in patients with an MR or better and 1.7 months in patients who had progressive disease or were not evaluable (P less than .0001).
The “most important” grade 3/4 adverse events, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%). A total of 23 patients (19.5%) discontinued treatment because of a related adverse.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GlaxoSmithKline.
SOURCE: Jagannath S et al. SOHO 2018, Abstract MM-255
Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma, according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%. The median progression-free survival was 3.7 months and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Icahn School of Medicine at Mount Sinai, New York, presented these results at the annual meeting of the Society of Hematologic Oncology.
“The additional phase 2b clinical results… are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said in a statement. “Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM [Selinexor Treatment of Refractory Myeloma] study.”
STORM (NCT02336815) included 122 patients with penta-refractory multiple myeloma. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of seven prior treatment regimens. Their median age was 65 years, a little more than half were men, and more than half had high-risk cytogenetics. Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10–6 and one at 1 x 10–4.
Very good partial responses were seen in 4.9% of patients, 19.7% had partial responses, 13.1% had minimal responses (MRs), and 39.3% had stable disease. Progressive disease occurred in 13.1% of patients; 8.2% were not evaluable for response.
The overall response rate (partial response or better) was 26.2%, the clinical benefit rate (MR or better) was 39.3%, and the disease control rate (stable disease or better) was 78.7%.
The median duration of response was 4.4 months. The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had progressive disease or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. Overall survival was 15.6 months in patients with an MR or better and 1.7 months in patients who had progressive disease or were not evaluable (P less than .0001).
The “most important” grade 3/4 adverse events, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%). A total of 23 patients (19.5%) discontinued treatment because of a related adverse.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GlaxoSmithKline.
SOURCE: Jagannath S et al. SOHO 2018, Abstract MM-255
FROM SOHO 2018
Key clinical point:
Major finding: The overall response rate was 26.2% and the clinical benefit rate was 39.3%.
Study details: A phase 2 trial of 122 patients with penta-refractory multiple myeloma.
Disclosures: This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GlaxoSmithKline.
Source: Jagannath S et al. SOHO 2018, Abstract MM-255.
Be wary of watchful waiting in follicular lymphoma
A substantial proportion of patients with follicular lymphoma managed with watchful waiting develop organ dysfunction or transformation that may negatively impact survival outcomes, results of a retrospective study suggest.
About one-quarter of patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival that could not be predicted based on baseline characteristics, the study authors reported in Clinical Lymphoma, Myeloma & Leukemia.
The study confirmed certain benefits of watchful waiting, including a low risk of progression and an “excellent” rate of overall survival, the investigators said.
However, the substantial rate of organ dysfunction and transformation in a subset of patients is “clinically meaningful for informed decision making,” reported Gwynivere A. Davies, MD, of the University of Calgary (Alta.), and her coauthors.
“While consenting patients to initial [watchful waiting], patients need to be informed about the risk for these adverse events, as well as receiving education and the need for close monitoring regarding symptoms that may indicate serious progression events,” Dr. Davies and her coauthors wrote.
Alternatively, rituximab chemotherapy, with or without rituximab maintenance, might be warranted for watchful waiting patients with clear disease progression before organ dysfunction or transformation events, despite not meeting high-tumor burden therapy indications.
The retrospective study included data from the Alberta Lymphoma Database on patients with grade 1-3a follicular lymphoma aged 18-70 years who were diagnosed between 1994 and 2011. Investigators identified 238 patients initially managed with watchful waiting, with a median age of 54.1 years at diagnosis. More than 80% were advanced stage.
Only 71% of these patients progressed, with a median time to progression of about 30 months and a 10-year survival rate from diagnosis of 81.2%, investigators said. However, 58 patients (24.4%) had organ dysfunction or transformation at the time of progression.
Those adverse outcomes significantly affected overall survival. The 10-year overall survival was 65.4% for patients with transformation at progression versus 83.2% for those without (P = .0017). Likewise, 10-year overall survival was 71.5% and 82.7%, respectively, for those with organ dysfunction at progression and those without (P = .028).
Investigators also looked at a comparison group of 236 follicular lymphoma patients managed with immediate rituximab chemotherapy. They found survival outcomes in that group were similar to those in the subgroup of 56 watchful waiting patients who received primarily rituximab-containing regimens at the time of organ dysfunction or transformation.
Taken together, the findings suggest management changes may be warranted for follicular lymphoma patients managed according to a watchful waiting strategy, the investigators wrote. “Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events.”
Dr. Davies reported having no financial disclosures. Study coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
SOURCE: Davies GA et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 28. doi: 10.1016/j.clml.2018.08.015.
A substantial proportion of patients with follicular lymphoma managed with watchful waiting develop organ dysfunction or transformation that may negatively impact survival outcomes, results of a retrospective study suggest.
About one-quarter of patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival that could not be predicted based on baseline characteristics, the study authors reported in Clinical Lymphoma, Myeloma & Leukemia.
The study confirmed certain benefits of watchful waiting, including a low risk of progression and an “excellent” rate of overall survival, the investigators said.
However, the substantial rate of organ dysfunction and transformation in a subset of patients is “clinically meaningful for informed decision making,” reported Gwynivere A. Davies, MD, of the University of Calgary (Alta.), and her coauthors.
“While consenting patients to initial [watchful waiting], patients need to be informed about the risk for these adverse events, as well as receiving education and the need for close monitoring regarding symptoms that may indicate serious progression events,” Dr. Davies and her coauthors wrote.
Alternatively, rituximab chemotherapy, with or without rituximab maintenance, might be warranted for watchful waiting patients with clear disease progression before organ dysfunction or transformation events, despite not meeting high-tumor burden therapy indications.
The retrospective study included data from the Alberta Lymphoma Database on patients with grade 1-3a follicular lymphoma aged 18-70 years who were diagnosed between 1994 and 2011. Investigators identified 238 patients initially managed with watchful waiting, with a median age of 54.1 years at diagnosis. More than 80% were advanced stage.
Only 71% of these patients progressed, with a median time to progression of about 30 months and a 10-year survival rate from diagnosis of 81.2%, investigators said. However, 58 patients (24.4%) had organ dysfunction or transformation at the time of progression.
Those adverse outcomes significantly affected overall survival. The 10-year overall survival was 65.4% for patients with transformation at progression versus 83.2% for those without (P = .0017). Likewise, 10-year overall survival was 71.5% and 82.7%, respectively, for those with organ dysfunction at progression and those without (P = .028).
Investigators also looked at a comparison group of 236 follicular lymphoma patients managed with immediate rituximab chemotherapy. They found survival outcomes in that group were similar to those in the subgroup of 56 watchful waiting patients who received primarily rituximab-containing regimens at the time of organ dysfunction or transformation.
Taken together, the findings suggest management changes may be warranted for follicular lymphoma patients managed according to a watchful waiting strategy, the investigators wrote. “Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events.”
Dr. Davies reported having no financial disclosures. Study coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
SOURCE: Davies GA et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 28. doi: 10.1016/j.clml.2018.08.015.
A substantial proportion of patients with follicular lymphoma managed with watchful waiting develop organ dysfunction or transformation that may negatively impact survival outcomes, results of a retrospective study suggest.
About one-quarter of patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival that could not be predicted based on baseline characteristics, the study authors reported in Clinical Lymphoma, Myeloma & Leukemia.
The study confirmed certain benefits of watchful waiting, including a low risk of progression and an “excellent” rate of overall survival, the investigators said.
However, the substantial rate of organ dysfunction and transformation in a subset of patients is “clinically meaningful for informed decision making,” reported Gwynivere A. Davies, MD, of the University of Calgary (Alta.), and her coauthors.
“While consenting patients to initial [watchful waiting], patients need to be informed about the risk for these adverse events, as well as receiving education and the need for close monitoring regarding symptoms that may indicate serious progression events,” Dr. Davies and her coauthors wrote.
Alternatively, rituximab chemotherapy, with or without rituximab maintenance, might be warranted for watchful waiting patients with clear disease progression before organ dysfunction or transformation events, despite not meeting high-tumor burden therapy indications.
The retrospective study included data from the Alberta Lymphoma Database on patients with grade 1-3a follicular lymphoma aged 18-70 years who were diagnosed between 1994 and 2011. Investigators identified 238 patients initially managed with watchful waiting, with a median age of 54.1 years at diagnosis. More than 80% were advanced stage.
Only 71% of these patients progressed, with a median time to progression of about 30 months and a 10-year survival rate from diagnosis of 81.2%, investigators said. However, 58 patients (24.4%) had organ dysfunction or transformation at the time of progression.
Those adverse outcomes significantly affected overall survival. The 10-year overall survival was 65.4% for patients with transformation at progression versus 83.2% for those without (P = .0017). Likewise, 10-year overall survival was 71.5% and 82.7%, respectively, for those with organ dysfunction at progression and those without (P = .028).
Investigators also looked at a comparison group of 236 follicular lymphoma patients managed with immediate rituximab chemotherapy. They found survival outcomes in that group were similar to those in the subgroup of 56 watchful waiting patients who received primarily rituximab-containing regimens at the time of organ dysfunction or transformation.
Taken together, the findings suggest management changes may be warranted for follicular lymphoma patients managed according to a watchful waiting strategy, the investigators wrote. “Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events.”
Dr. Davies reported having no financial disclosures. Study coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
SOURCE: Davies GA et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 28. doi: 10.1016/j.clml.2018.08.015.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: A total of 58 patients (24.4%) had organ dysfunction or transformation at the time of progression and had worse survival outcomes, compared with patients who did not experience those events.
Study details: A retrospective study including data on 238 patients with grade 1-3a follicular lymphoma aged 18-70 years who were managed with watchful waiting.
Disclosures: Study authors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
Source: Davies GA et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 28. doi: 10.1016/j.clml.2018.08.015.
Pruritus linked to wide variety of cancers
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point:
Major finding: Blacks with pruritus had higher odds ratios for hematologic and soft tissue malignancies, while whites had higher ORs for skin and liver malignancies.
Study details: A retrospective study of 16,925 adults with itching or pruritus seen at a tertiary care center.
Disclosures: Dr. Kwatra reported serving as an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
Source: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
Evaluation of Acute Toxicity in Treating Pelvic Lymph Nodes With Prostate Boost With Hypofractionated Simultaneous Integrated Boost (SIB) Using Volumetric Arc Therapy (VMAT)
Purpose: It is conventional to treat pelvic lymph nodes, followed by prostate boost in a sequential manner, requiring 8-9 weeks to complete therapy. In the last several years there have been several randomized studies of implementing moderate hypofractionated radiotherapy in prostate cancer to shorten the treatment time, which has proven to be non-inferior to conventional treatment. The purpose of this retrospective study is to evaluate the acute toxicities of hypofractionated SIB radiotherapy in treating the lymph nodes with prostate boost.
Methods: Between 2015 and 2017, twenty five high risk prostate cancer patients received pelvic node radiotherapy with prostate boost in 25 fractions with SIB technique with neo-adjuvant and concurrent hormone therapy to 50 Gy/25 fractions at 2 Gy/fraction to pelvic nodes and prostate boost for a total of 67.5 - 75 Gy at 2.7 to 3.0 Gy/fraction. We followed QUNTAC dose-volume constraints for the rectum, bladder and bowel. All these patients received long-term hormone therapy.
Results: The median age was 66 years (range 57-81 years). There were 6 stage II C, 7 III A, 15 III C, and 1 stage IV A. All patients were restaged as per American Joint Committee on Cancer 8th edition. Gleason Score: 6 (1), 7 (4+3) (4), 8 (5), and 9-10 (15). The average PSA was 17.2 ng/mL with a range of 5.6 to 51.92 ng/mL, and average number of positive cores was 74%. These factors put the majority of patients into the very high risk group. The median follow up was 24 months. The majority of the patients tolerated treatment well. Grade 0 genitourinary (GU) toxicity occurred
in 8 (33%) patients, grade II 16 (67%) patients, one patient had a Foley catheter during treatment, a majority of patients were on alpha blockers either before, during or post radiotherapy. Grade 0 gastrointestinal (GI) toxicity occurred in 21 (84%) patients, grade 1 in one, and grade II in 3 (12%) patients. There were no grade 3 or 4 GU or GI toxicities.
Conclusions: Simultaneous integrated boost with VMAT is well tolerated in treating pelvic nodes and prostate boost, without any major acute toxicities. This technique is used in mostly for very high risk localized prostate cancer patients, reducing number of fractions from conventional sequential treatment.
Purpose: It is conventional to treat pelvic lymph nodes, followed by prostate boost in a sequential manner, requiring 8-9 weeks to complete therapy. In the last several years there have been several randomized studies of implementing moderate hypofractionated radiotherapy in prostate cancer to shorten the treatment time, which has proven to be non-inferior to conventional treatment. The purpose of this retrospective study is to evaluate the acute toxicities of hypofractionated SIB radiotherapy in treating the lymph nodes with prostate boost.
Methods: Between 2015 and 2017, twenty five high risk prostate cancer patients received pelvic node radiotherapy with prostate boost in 25 fractions with SIB technique with neo-adjuvant and concurrent hormone therapy to 50 Gy/25 fractions at 2 Gy/fraction to pelvic nodes and prostate boost for a total of 67.5 - 75 Gy at 2.7 to 3.0 Gy/fraction. We followed QUNTAC dose-volume constraints for the rectum, bladder and bowel. All these patients received long-term hormone therapy.
Results: The median age was 66 years (range 57-81 years). There were 6 stage II C, 7 III A, 15 III C, and 1 stage IV A. All patients were restaged as per American Joint Committee on Cancer 8th edition. Gleason Score: 6 (1), 7 (4+3) (4), 8 (5), and 9-10 (15). The average PSA was 17.2 ng/mL with a range of 5.6 to 51.92 ng/mL, and average number of positive cores was 74%. These factors put the majority of patients into the very high risk group. The median follow up was 24 months. The majority of the patients tolerated treatment well. Grade 0 genitourinary (GU) toxicity occurred
in 8 (33%) patients, grade II 16 (67%) patients, one patient had a Foley catheter during treatment, a majority of patients were on alpha blockers either before, during or post radiotherapy. Grade 0 gastrointestinal (GI) toxicity occurred in 21 (84%) patients, grade 1 in one, and grade II in 3 (12%) patients. There were no grade 3 or 4 GU or GI toxicities.
Conclusions: Simultaneous integrated boost with VMAT is well tolerated in treating pelvic nodes and prostate boost, without any major acute toxicities. This technique is used in mostly for very high risk localized prostate cancer patients, reducing number of fractions from conventional sequential treatment.
Purpose: It is conventional to treat pelvic lymph nodes, followed by prostate boost in a sequential manner, requiring 8-9 weeks to complete therapy. In the last several years there have been several randomized studies of implementing moderate hypofractionated radiotherapy in prostate cancer to shorten the treatment time, which has proven to be non-inferior to conventional treatment. The purpose of this retrospective study is to evaluate the acute toxicities of hypofractionated SIB radiotherapy in treating the lymph nodes with prostate boost.
Methods: Between 2015 and 2017, twenty five high risk prostate cancer patients received pelvic node radiotherapy with prostate boost in 25 fractions with SIB technique with neo-adjuvant and concurrent hormone therapy to 50 Gy/25 fractions at 2 Gy/fraction to pelvic nodes and prostate boost for a total of 67.5 - 75 Gy at 2.7 to 3.0 Gy/fraction. We followed QUNTAC dose-volume constraints for the rectum, bladder and bowel. All these patients received long-term hormone therapy.
Results: The median age was 66 years (range 57-81 years). There were 6 stage II C, 7 III A, 15 III C, and 1 stage IV A. All patients were restaged as per American Joint Committee on Cancer 8th edition. Gleason Score: 6 (1), 7 (4+3) (4), 8 (5), and 9-10 (15). The average PSA was 17.2 ng/mL with a range of 5.6 to 51.92 ng/mL, and average number of positive cores was 74%. These factors put the majority of patients into the very high risk group. The median follow up was 24 months. The majority of the patients tolerated treatment well. Grade 0 genitourinary (GU) toxicity occurred
in 8 (33%) patients, grade II 16 (67%) patients, one patient had a Foley catheter during treatment, a majority of patients were on alpha blockers either before, during or post radiotherapy. Grade 0 gastrointestinal (GI) toxicity occurred in 21 (84%) patients, grade 1 in one, and grade II in 3 (12%) patients. There were no grade 3 or 4 GU or GI toxicities.
Conclusions: Simultaneous integrated boost with VMAT is well tolerated in treating pelvic nodes and prostate boost, without any major acute toxicities. This technique is used in mostly for very high risk localized prostate cancer patients, reducing number of fractions from conventional sequential treatment.