User login
A Case of Systemic Mastocytosis With Associated Clonal Hematological Non-Mast Cell Lineage Disease at VA Pittsburgh Healthcare System
Introduction: Systemic mastocytosis (SM) is a rare myeloid neoplasm that is caused by accumulation of abnormal mast cells in the bone marrow, liver, spleen, and skin. The KIT D816V mutation encodes a constitutively activated receptor tyrosine kinase that drives disease pathogenesis. We present a case of systemic mastocytosis with associated clonal hematological non-mast cell disease (SM-AHNMD).
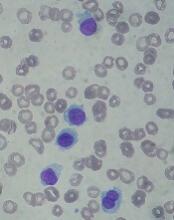
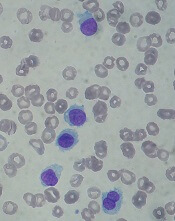
Background: A 71-year-old man presented with anemia, thrombocytopenia, absolute monocyte count of 2,000-4,000 and weight loss in August 2016. A CT showed splenomegaly and lymphadenopathy. Bone marrow biopsy revealed positive CD117 mast cells, CD34 myeloblasts and reticulin fibrosis consistent with SM. Immunohistochemistry confirmed the neoplastic cells were positive for CD25, but negative for CD2. PCR analysis revealed KIT D816V point mutation. Serum tryptase was 295 ug/L (normal 2.2-13.2). He was started on imatinib mesylate. However, his anemia, thrombocytopenia and splenomegaly worsened. He developed bilateral femoral neck fractures in April 2017. Imatinib was discontinued. He underwent bilateral hip hemiarthroplasty. Histology was consistent with SM (positive CD25 and CD117) with dysplastic megakaryocytes and increased monocytosis. By WHO classification he has SM-AHNMD with chronic myelomonocytic leukemia. He was started on cladribine for 4 cycles with good response in splenomegaly, anemia and thrombocytopenia, but he developed leukocytosis. Serum tryptase initially decreased to 141 but then rose to 243. Midostaurin 100 mg orally twice a day was initiated in December 2017. His cytopenia and splenomegaly improved. In March 2018 he was admitted for sigmoid colon obstruction due to inflammation or mass and underwent diverting loop ileostomy. Biopsy could not be performed. His serum tryptase decreased to 178 but increased to 275 in June 2018. He continues on midostaurin.
Discussion: SM-AHNMD constitutes approximately 40% of all SM with poor prognosis. SM is resistance to imatinib because of KIT D816V mutation. Cladribine has some activity. Midostaurin inhibits non-mutant and mutant KIT D816V with 58% response rate and median overall survival of 20 months. Our patient has a good response to both drugs.
Conclusions: Clinicians should be able to diagnose and treat SM. Cladribine and midostaurin are active drugs for SM.
Introduction: Systemic mastocytosis (SM) is a rare myeloid neoplasm that is caused by accumulation of abnormal mast cells in the bone marrow, liver, spleen, and skin. The KIT D816V mutation encodes a constitutively activated receptor tyrosine kinase that drives disease pathogenesis. We present a case of systemic mastocytosis with associated clonal hematological non-mast cell disease (SM-AHNMD).
Background: A 71-year-old man presented with anemia, thrombocytopenia, absolute monocyte count of 2,000-4,000 and weight loss in August 2016. A CT showed splenomegaly and lymphadenopathy. Bone marrow biopsy revealed positive CD117 mast cells, CD34 myeloblasts and reticulin fibrosis consistent with SM. Immunohistochemistry confirmed the neoplastic cells were positive for CD25, but negative for CD2. PCR analysis revealed KIT D816V point mutation. Serum tryptase was 295 ug/L (normal 2.2-13.2). He was started on imatinib mesylate. However, his anemia, thrombocytopenia and splenomegaly worsened. He developed bilateral femoral neck fractures in April 2017. Imatinib was discontinued. He underwent bilateral hip hemiarthroplasty. Histology was consistent with SM (positive CD25 and CD117) with dysplastic megakaryocytes and increased monocytosis. By WHO classification he has SM-AHNMD with chronic myelomonocytic leukemia. He was started on cladribine for 4 cycles with good response in splenomegaly, anemia and thrombocytopenia, but he developed leukocytosis. Serum tryptase initially decreased to 141 but then rose to 243. Midostaurin 100 mg orally twice a day was initiated in December 2017. His cytopenia and splenomegaly improved. In March 2018 he was admitted for sigmoid colon obstruction due to inflammation or mass and underwent diverting loop ileostomy. Biopsy could not be performed. His serum tryptase decreased to 178 but increased to 275 in June 2018. He continues on midostaurin.
Discussion: SM-AHNMD constitutes approximately 40% of all SM with poor prognosis. SM is resistance to imatinib because of KIT D816V mutation. Cladribine has some activity. Midostaurin inhibits non-mutant and mutant KIT D816V with 58% response rate and median overall survival of 20 months. Our patient has a good response to both drugs.
Conclusions: Clinicians should be able to diagnose and treat SM. Cladribine and midostaurin are active drugs for SM.
Introduction: Systemic mastocytosis (SM) is a rare myeloid neoplasm that is caused by accumulation of abnormal mast cells in the bone marrow, liver, spleen, and skin. The KIT D816V mutation encodes a constitutively activated receptor tyrosine kinase that drives disease pathogenesis. We present a case of systemic mastocytosis with associated clonal hematological non-mast cell disease (SM-AHNMD).
Background: A 71-year-old man presented with anemia, thrombocytopenia, absolute monocyte count of 2,000-4,000 and weight loss in August 2016. A CT showed splenomegaly and lymphadenopathy. Bone marrow biopsy revealed positive CD117 mast cells, CD34 myeloblasts and reticulin fibrosis consistent with SM. Immunohistochemistry confirmed the neoplastic cells were positive for CD25, but negative for CD2. PCR analysis revealed KIT D816V point mutation. Serum tryptase was 295 ug/L (normal 2.2-13.2). He was started on imatinib mesylate. However, his anemia, thrombocytopenia and splenomegaly worsened. He developed bilateral femoral neck fractures in April 2017. Imatinib was discontinued. He underwent bilateral hip hemiarthroplasty. Histology was consistent with SM (positive CD25 and CD117) with dysplastic megakaryocytes and increased monocytosis. By WHO classification he has SM-AHNMD with chronic myelomonocytic leukemia. He was started on cladribine for 4 cycles with good response in splenomegaly, anemia and thrombocytopenia, but he developed leukocytosis. Serum tryptase initially decreased to 141 but then rose to 243. Midostaurin 100 mg orally twice a day was initiated in December 2017. His cytopenia and splenomegaly improved. In March 2018 he was admitted for sigmoid colon obstruction due to inflammation or mass and underwent diverting loop ileostomy. Biopsy could not be performed. His serum tryptase decreased to 178 but increased to 275 in June 2018. He continues on midostaurin.
Discussion: SM-AHNMD constitutes approximately 40% of all SM with poor prognosis. SM is resistance to imatinib because of KIT D816V mutation. Cladribine has some activity. Midostaurin inhibits non-mutant and mutant KIT D816V with 58% response rate and median overall survival of 20 months. Our patient has a good response to both drugs.
Conclusions: Clinicians should be able to diagnose and treat SM. Cladribine and midostaurin are active drugs for SM.
Translocation T(11;14): Not Always Mantle Cell Lymphoma!
Background: The translocation t(11;14)(q13;q32) typically considered a hallmark of mantle cell lymphoma(MCL), has also been implicated in some cases of non-MCL lymphoproliferative disorders. Although uncommon, it has been reported in 2-5% of chronic lymphocytic leukemia (CLL) cases. Most of the cases identified have been observed mostly in relapsed CLL. This genetic aberration can be considered a significant prognostic indicator for CLL. t(11;14) positive CLL at the time of diagnosis has been rarely reported. We describe a case of a patient
diagnosed with CLL who was positive for this genetic abnormality.
Case Report: A 64-year-old white male presented with absolute lymphocytosis of 7 years. Lymphocyte immunophenotype detected a CD5(+) CD10(-) CD23(+) CD38(-) CD43(+) FMC7(partial dim) kappa-restricted B-cell population consistent with CLL. CT chest, abdomen, pelvis showed mildly prominent mediastinal and hilar lymph nodes only and was thus classified as Rai stage I. Peripheral FISH came back positive for t(11:14), cyclin D1-IgH translocation. His EPO, Jak2 and BCR-ABL mutation were all negative (done for mild erythrocytosis). Immunoglobulin and SPEP were negative. UPEP showed high Kappa/Lambda ratio. Although this tumor carries t(11;14) (q13;q32) translocation, immunostaining for BCL-1 was negative. It is possible that the gene is not expressed. Based on the staining and the clinical presentation, MCL was excluded. Per NCCN guidelines, patient is receiving clinical monitoring for stage I CLL.
Conclusions: Translocations involving the immunoglobulin genes are commonly identified. Uncommon genomic abnormalities in CLL should be recognized as significant independent predictors of disease progression and survival. It is important to recognize cases of CLL with t(11;14) translocation to achieve risk-adapted treatment strategies, which might be required to treat such patients.
Background: The translocation t(11;14)(q13;q32) typically considered a hallmark of mantle cell lymphoma(MCL), has also been implicated in some cases of non-MCL lymphoproliferative disorders. Although uncommon, it has been reported in 2-5% of chronic lymphocytic leukemia (CLL) cases. Most of the cases identified have been observed mostly in relapsed CLL. This genetic aberration can be considered a significant prognostic indicator for CLL. t(11;14) positive CLL at the time of diagnosis has been rarely reported. We describe a case of a patient
diagnosed with CLL who was positive for this genetic abnormality.
Case Report: A 64-year-old white male presented with absolute lymphocytosis of 7 years. Lymphocyte immunophenotype detected a CD5(+) CD10(-) CD23(+) CD38(-) CD43(+) FMC7(partial dim) kappa-restricted B-cell population consistent with CLL. CT chest, abdomen, pelvis showed mildly prominent mediastinal and hilar lymph nodes only and was thus classified as Rai stage I. Peripheral FISH came back positive for t(11:14), cyclin D1-IgH translocation. His EPO, Jak2 and BCR-ABL mutation were all negative (done for mild erythrocytosis). Immunoglobulin and SPEP were negative. UPEP showed high Kappa/Lambda ratio. Although this tumor carries t(11;14) (q13;q32) translocation, immunostaining for BCL-1 was negative. It is possible that the gene is not expressed. Based on the staining and the clinical presentation, MCL was excluded. Per NCCN guidelines, patient is receiving clinical monitoring for stage I CLL.
Conclusions: Translocations involving the immunoglobulin genes are commonly identified. Uncommon genomic abnormalities in CLL should be recognized as significant independent predictors of disease progression and survival. It is important to recognize cases of CLL with t(11;14) translocation to achieve risk-adapted treatment strategies, which might be required to treat such patients.
Background: The translocation t(11;14)(q13;q32) typically considered a hallmark of mantle cell lymphoma(MCL), has also been implicated in some cases of non-MCL lymphoproliferative disorders. Although uncommon, it has been reported in 2-5% of chronic lymphocytic leukemia (CLL) cases. Most of the cases identified have been observed mostly in relapsed CLL. This genetic aberration can be considered a significant prognostic indicator for CLL. t(11;14) positive CLL at the time of diagnosis has been rarely reported. We describe a case of a patient
diagnosed with CLL who was positive for this genetic abnormality.
Case Report: A 64-year-old white male presented with absolute lymphocytosis of 7 years. Lymphocyte immunophenotype detected a CD5(+) CD10(-) CD23(+) CD38(-) CD43(+) FMC7(partial dim) kappa-restricted B-cell population consistent with CLL. CT chest, abdomen, pelvis showed mildly prominent mediastinal and hilar lymph nodes only and was thus classified as Rai stage I. Peripheral FISH came back positive for t(11:14), cyclin D1-IgH translocation. His EPO, Jak2 and BCR-ABL mutation were all negative (done for mild erythrocytosis). Immunoglobulin and SPEP were negative. UPEP showed high Kappa/Lambda ratio. Although this tumor carries t(11;14) (q13;q32) translocation, immunostaining for BCL-1 was negative. It is possible that the gene is not expressed. Based on the staining and the clinical presentation, MCL was excluded. Per NCCN guidelines, patient is receiving clinical monitoring for stage I CLL.
Conclusions: Translocations involving the immunoglobulin genes are commonly identified. Uncommon genomic abnormalities in CLL should be recognized as significant independent predictors of disease progression and survival. It is important to recognize cases of CLL with t(11;14) translocation to achieve risk-adapted treatment strategies, which might be required to treat such patients.
FDA approves drug for hairy cell leukemia
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
FDA approves new hairy cell leukemia drug
The Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a boxed warning noting that the drug poses risks of capillary leak syndrome and hemolytic uremic syndrome. Other serious warnings include the risk of decreased renal function, infusion-related reactions, and electrolyte abnormalities.
The FDA granted the application for moxetumomab pasudotox fast track, priority review, and an orphan drug designation.
The agency approved AstraZeneca’s moxetumomab pasudotox based on results from a phase 3 trial (NCT01829711). Data from this study were presented at the 2018 annual meeting of the American Society of Clinical Oncology (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75%, the complete response (CR) rate was 41%, and the durable CR rate was 30%. Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most common treatment-related adverse events (AEs) were nausea, peripheral edema, headache, and pyrexia. Other treatment-related AEs included infections and neutropenia.
Treatment-related AEs that led to discontinuation included capillary leak syndrome, hemolytic uremic syndrome, and increased blood creatinine.
There were three deaths in this trial, but none of them were considered treatment related.
The Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a boxed warning noting that the drug poses risks of capillary leak syndrome and hemolytic uremic syndrome. Other serious warnings include the risk of decreased renal function, infusion-related reactions, and electrolyte abnormalities.
The FDA granted the application for moxetumomab pasudotox fast track, priority review, and an orphan drug designation.
The agency approved AstraZeneca’s moxetumomab pasudotox based on results from a phase 3 trial (NCT01829711). Data from this study were presented at the 2018 annual meeting of the American Society of Clinical Oncology (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75%, the complete response (CR) rate was 41%, and the durable CR rate was 30%. Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most common treatment-related adverse events (AEs) were nausea, peripheral edema, headache, and pyrexia. Other treatment-related AEs included infections and neutropenia.
Treatment-related AEs that led to discontinuation included capillary leak syndrome, hemolytic uremic syndrome, and increased blood creatinine.
There were three deaths in this trial, but none of them were considered treatment related.
The Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a boxed warning noting that the drug poses risks of capillary leak syndrome and hemolytic uremic syndrome. Other serious warnings include the risk of decreased renal function, infusion-related reactions, and electrolyte abnormalities.
The FDA granted the application for moxetumomab pasudotox fast track, priority review, and an orphan drug designation.
The agency approved AstraZeneca’s moxetumomab pasudotox based on results from a phase 3 trial (NCT01829711). Data from this study were presented at the 2018 annual meeting of the American Society of Clinical Oncology (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75%, the complete response (CR) rate was 41%, and the durable CR rate was 30%. Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most common treatment-related adverse events (AEs) were nausea, peripheral edema, headache, and pyrexia. Other treatment-related AEs included infections and neutropenia.
Treatment-related AEs that led to discontinuation included capillary leak syndrome, hemolytic uremic syndrome, and increased blood creatinine.
There were three deaths in this trial, but none of them were considered treatment related.
New U.S. cancer cases may exceed 2.3 million by 2035
The American Association for Cancer Research (AACR) has released its annual Cancer Progress Report, detailing recent advances in the fight against cancer and calling on elected officials to address the challenges that remain.
The AACR Cancer Progress Report 2018 lists the 22 new approvals for cancer treatments that have occurred during the last 12 months, including 12 therapies approved to treat hematologic malignancies.
However, the report also notes that cancer continues to pose immense public health challenges in the United States.
The estimated number of new cancer cases for 2018 is 1,735,350, and the estimated number of cancer deaths is 609,640.
The number of new cancer cases is predicted to increase to 2,387,304 in 2035. This is due, in large part, to the rising number of people age 65 and older, according to the report.
With this in mind, the AACR is calling on elected officials to:
Maintain “robust, sustained, and predictable growth” of the National Institutes of Health (NIH) budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
Make sure the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives—including the National Cancer Moonshot—“is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion—a $308 million increase above its FY 2018 level—to secure support for regulatory science and speed the development of medical products that are safe and effective.
Provide the Centers for Disease Control and Prevention’s Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
The American Association for Cancer Research (AACR) has released its annual Cancer Progress Report, detailing recent advances in the fight against cancer and calling on elected officials to address the challenges that remain.
The AACR Cancer Progress Report 2018 lists the 22 new approvals for cancer treatments that have occurred during the last 12 months, including 12 therapies approved to treat hematologic malignancies.
However, the report also notes that cancer continues to pose immense public health challenges in the United States.
The estimated number of new cancer cases for 2018 is 1,735,350, and the estimated number of cancer deaths is 609,640.
The number of new cancer cases is predicted to increase to 2,387,304 in 2035. This is due, in large part, to the rising number of people age 65 and older, according to the report.
With this in mind, the AACR is calling on elected officials to:
Maintain “robust, sustained, and predictable growth” of the National Institutes of Health (NIH) budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
Make sure the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives—including the National Cancer Moonshot—“is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion—a $308 million increase above its FY 2018 level—to secure support for regulatory science and speed the development of medical products that are safe and effective.
Provide the Centers for Disease Control and Prevention’s Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
The American Association for Cancer Research (AACR) has released its annual Cancer Progress Report, detailing recent advances in the fight against cancer and calling on elected officials to address the challenges that remain.
The AACR Cancer Progress Report 2018 lists the 22 new approvals for cancer treatments that have occurred during the last 12 months, including 12 therapies approved to treat hematologic malignancies.
However, the report also notes that cancer continues to pose immense public health challenges in the United States.
The estimated number of new cancer cases for 2018 is 1,735,350, and the estimated number of cancer deaths is 609,640.
The number of new cancer cases is predicted to increase to 2,387,304 in 2035. This is due, in large part, to the rising number of people age 65 and older, according to the report.
With this in mind, the AACR is calling on elected officials to:
Maintain “robust, sustained, and predictable growth” of the National Institutes of Health (NIH) budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
Make sure the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives—including the National Cancer Moonshot—“is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion—a $308 million increase above its FY 2018 level—to secure support for regulatory science and speed the development of medical products that are safe and effective.
Provide the Centers for Disease Control and Prevention’s Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
Novel AKT inhibitor active against MM cells
A novel inhibitor of AKT pathway signaling showed significant cytotoxic activity in mouse models and in human cells isolated from patients with primary or relapsed multiple myeloma (MM), investigators reported.
The experimental agent, labeled HS1793, is a derivative of the naturally occurring antioxidant compound resveratrol. In preclinical studies, HS1793 was shown to offer “great promise in eliminating MM cells and improving therapeutic responses in primary and relapsed/refractory MM patients,” according to Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues.
In a series of experiments, described in the journal Cancer Letters, the investigators demonstrated that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in multiple myeloma cells, and was cytotoxic and specific for myeloma cells in a mouse model of human metastatic myeloma, and in samples of human multiple myeloma cells.
When activated, AKT promotes oncogenesis by in turn activating other downstream pathways involved in proliferation or survival of malignant cells.
“AKT is frequently activated in MM cells and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They first screened 400 compounds, and narrowed in on resveratrol analogs, eventually choosing HS1793 as the most promising candidate.
This first experiment found evidence that suggested that the compound inhibits AKT activation by interfering with the interaction between AKT and its promoter HSP90.
They then showed in human MM cell lines that the antimyeloma action of HS1793 appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In a separate series of experiments, they found that the inhibition by HS1793 of AKT/HSP90 interaction results in cell death by suppressing nuclear factor kappa–B (NF-KB) pathway signaling. The investigators had previously reported that a different compound, an inhibitor of spindle protein kinesin, induced MM cell death via inhibition of NF-KB signaling.
Next, the investigators showed that HS1793-induced cell death was caused by the direct inhibition of AKT that in turn suppressed NF-KB activation.
Finally, they showed in a mouse model of multiple myeloma metastatic to bone that HS1793 “dramatically decreased” lytic skull and femur lesions in treated mice, compared with mice treated with a vehicle placebo, and increased survival of the mice that received the AKT inhibitor.
They also showed that HS1793 was cytotoxic to multiple myeloma cells but not to normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” they wrote.
The study was supported by grants from the Korean government. The researchers reported having no potential conflicts of interest.
SOURCE: Song IS et al. Cancer Lett. 2018;432:205-15.
A novel inhibitor of AKT pathway signaling showed significant cytotoxic activity in mouse models and in human cells isolated from patients with primary or relapsed multiple myeloma (MM), investigators reported.
The experimental agent, labeled HS1793, is a derivative of the naturally occurring antioxidant compound resveratrol. In preclinical studies, HS1793 was shown to offer “great promise in eliminating MM cells and improving therapeutic responses in primary and relapsed/refractory MM patients,” according to Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues.
In a series of experiments, described in the journal Cancer Letters, the investigators demonstrated that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in multiple myeloma cells, and was cytotoxic and specific for myeloma cells in a mouse model of human metastatic myeloma, and in samples of human multiple myeloma cells.
When activated, AKT promotes oncogenesis by in turn activating other downstream pathways involved in proliferation or survival of malignant cells.
“AKT is frequently activated in MM cells and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They first screened 400 compounds, and narrowed in on resveratrol analogs, eventually choosing HS1793 as the most promising candidate.
This first experiment found evidence that suggested that the compound inhibits AKT activation by interfering with the interaction between AKT and its promoter HSP90.
They then showed in human MM cell lines that the antimyeloma action of HS1793 appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In a separate series of experiments, they found that the inhibition by HS1793 of AKT/HSP90 interaction results in cell death by suppressing nuclear factor kappa–B (NF-KB) pathway signaling. The investigators had previously reported that a different compound, an inhibitor of spindle protein kinesin, induced MM cell death via inhibition of NF-KB signaling.
Next, the investigators showed that HS1793-induced cell death was caused by the direct inhibition of AKT that in turn suppressed NF-KB activation.
Finally, they showed in a mouse model of multiple myeloma metastatic to bone that HS1793 “dramatically decreased” lytic skull and femur lesions in treated mice, compared with mice treated with a vehicle placebo, and increased survival of the mice that received the AKT inhibitor.
They also showed that HS1793 was cytotoxic to multiple myeloma cells but not to normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” they wrote.
The study was supported by grants from the Korean government. The researchers reported having no potential conflicts of interest.
SOURCE: Song IS et al. Cancer Lett. 2018;432:205-15.
A novel inhibitor of AKT pathway signaling showed significant cytotoxic activity in mouse models and in human cells isolated from patients with primary or relapsed multiple myeloma (MM), investigators reported.
The experimental agent, labeled HS1793, is a derivative of the naturally occurring antioxidant compound resveratrol. In preclinical studies, HS1793 was shown to offer “great promise in eliminating MM cells and improving therapeutic responses in primary and relapsed/refractory MM patients,” according to Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues.
In a series of experiments, described in the journal Cancer Letters, the investigators demonstrated that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in multiple myeloma cells, and was cytotoxic and specific for myeloma cells in a mouse model of human metastatic myeloma, and in samples of human multiple myeloma cells.
When activated, AKT promotes oncogenesis by in turn activating other downstream pathways involved in proliferation or survival of malignant cells.
“AKT is frequently activated in MM cells and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They first screened 400 compounds, and narrowed in on resveratrol analogs, eventually choosing HS1793 as the most promising candidate.
This first experiment found evidence that suggested that the compound inhibits AKT activation by interfering with the interaction between AKT and its promoter HSP90.
They then showed in human MM cell lines that the antimyeloma action of HS1793 appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In a separate series of experiments, they found that the inhibition by HS1793 of AKT/HSP90 interaction results in cell death by suppressing nuclear factor kappa–B (NF-KB) pathway signaling. The investigators had previously reported that a different compound, an inhibitor of spindle protein kinesin, induced MM cell death via inhibition of NF-KB signaling.
Next, the investigators showed that HS1793-induced cell death was caused by the direct inhibition of AKT that in turn suppressed NF-KB activation.
Finally, they showed in a mouse model of multiple myeloma metastatic to bone that HS1793 “dramatically decreased” lytic skull and femur lesions in treated mice, compared with mice treated with a vehicle placebo, and increased survival of the mice that received the AKT inhibitor.
They also showed that HS1793 was cytotoxic to multiple myeloma cells but not to normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” they wrote.
The study was supported by grants from the Korean government. The researchers reported having no potential conflicts of interest.
SOURCE: Song IS et al. Cancer Lett. 2018;432:205-15.
FROM CANCER LETTERS
Key clinical point:
Major finding: HS1793 showed significant multiple myeloma cytotoxicity in mouse models and in human cells isolated from patients with primary or relapsed/refractory myeloma.
Study details: Preclinical investigations in cell lines, murine models, and isolated human multiple myeloma cells.
Disclosures: The study was supported by grants from the Korean government. The researchers reported having no potential conflicts of interest.
Source: Song IS et al. Cancer Lett. 2018:432:205-15.
Intravascular Lymphoma Presenting as Acute Abdomen With Intestinal Perforation
Background: We present a case of a 69-year-old male with a past medical history of RA, Afib, COPD, and DVT with pulmonary embolism. He presented to the emergency department with encephalopathy and severe abdominal pain. On exam the patient was septic with a diffusely tender abdominal exam with peritoneal signs. The CT scan showed pneumoperitoneum. The patient underwent emergent laparotomy which revealed fecal peritonitis from a cecal perforation. After washout, patient had bowel resection of the involved intestine with a primary anastomosis. Biopsy of his resected small bowel and cecum showed submucosal blood vessels with numerous lymphoid cells. Immunohistochemical staining showed aberrant expression for CD43 and CD30 with an increased proliferation index (Ki67 80-90%). Molecular studies of both the lymphoid aggregates
and the atypical intravascular cells were negative for Ig heavy chain, t(11:18) & t(14,19). A diagnosis of intravascular lymphoma was still made. Patient underwent four further abdominal washouts with reconstruction of anterior abdominal wall with Permacol™ biological mesh. The patient condition continued to deteriorate, and he was transitioned to palliative care. He died a month after. An autopsy was not performed.
Discussion: Intravascular lymphoma is a rare and very aggressive malignancy characterized by proliferation of atypical B cell confined mostly to the vascular lumen. Its presentation is protean depending on the organs involved. It has been referred to as “the oncologists great imitator.” In a series of 38 patients by Ferreri et al, the most common symptoms were fever, cutaneous symptoms, neurological symptoms followed by abdominal pain. Most patients present in an advanced state, one series of 96 patients by Murase et al, 91% of the patient presenting with clinical stage III or stage IV disease.
There remain no standard diagnostic criteria for intravascular lymphoma. First step is demonstration of lymphoma cells in small- and medium-sized blood vessels with characteristic sparing of surrounding tissue. B cell clones are most common, but T and NK cells have also been reported. Molecular, immune histochemical and flow cytometry techniques may aid in establishing diagnosis. Prognosis remains poor even with aggressive treatment, the largest series by Murase et al, with mean survival of just 13 months with treatment.
Background: We present a case of a 69-year-old male with a past medical history of RA, Afib, COPD, and DVT with pulmonary embolism. He presented to the emergency department with encephalopathy and severe abdominal pain. On exam the patient was septic with a diffusely tender abdominal exam with peritoneal signs. The CT scan showed pneumoperitoneum. The patient underwent emergent laparotomy which revealed fecal peritonitis from a cecal perforation. After washout, patient had bowel resection of the involved intestine with a primary anastomosis. Biopsy of his resected small bowel and cecum showed submucosal blood vessels with numerous lymphoid cells. Immunohistochemical staining showed aberrant expression for CD43 and CD30 with an increased proliferation index (Ki67 80-90%). Molecular studies of both the lymphoid aggregates
and the atypical intravascular cells were negative for Ig heavy chain, t(11:18) & t(14,19). A diagnosis of intravascular lymphoma was still made. Patient underwent four further abdominal washouts with reconstruction of anterior abdominal wall with Permacol™ biological mesh. The patient condition continued to deteriorate, and he was transitioned to palliative care. He died a month after. An autopsy was not performed.
Discussion: Intravascular lymphoma is a rare and very aggressive malignancy characterized by proliferation of atypical B cell confined mostly to the vascular lumen. Its presentation is protean depending on the organs involved. It has been referred to as “the oncologists great imitator.” In a series of 38 patients by Ferreri et al, the most common symptoms were fever, cutaneous symptoms, neurological symptoms followed by abdominal pain. Most patients present in an advanced state, one series of 96 patients by Murase et al, 91% of the patient presenting with clinical stage III or stage IV disease.
There remain no standard diagnostic criteria for intravascular lymphoma. First step is demonstration of lymphoma cells in small- and medium-sized blood vessels with characteristic sparing of surrounding tissue. B cell clones are most common, but T and NK cells have also been reported. Molecular, immune histochemical and flow cytometry techniques may aid in establishing diagnosis. Prognosis remains poor even with aggressive treatment, the largest series by Murase et al, with mean survival of just 13 months with treatment.
Background: We present a case of a 69-year-old male with a past medical history of RA, Afib, COPD, and DVT with pulmonary embolism. He presented to the emergency department with encephalopathy and severe abdominal pain. On exam the patient was septic with a diffusely tender abdominal exam with peritoneal signs. The CT scan showed pneumoperitoneum. The patient underwent emergent laparotomy which revealed fecal peritonitis from a cecal perforation. After washout, patient had bowel resection of the involved intestine with a primary anastomosis. Biopsy of his resected small bowel and cecum showed submucosal blood vessels with numerous lymphoid cells. Immunohistochemical staining showed aberrant expression for CD43 and CD30 with an increased proliferation index (Ki67 80-90%). Molecular studies of both the lymphoid aggregates
and the atypical intravascular cells were negative for Ig heavy chain, t(11:18) & t(14,19). A diagnosis of intravascular lymphoma was still made. Patient underwent four further abdominal washouts with reconstruction of anterior abdominal wall with Permacol™ biological mesh. The patient condition continued to deteriorate, and he was transitioned to palliative care. He died a month after. An autopsy was not performed.
Discussion: Intravascular lymphoma is a rare and very aggressive malignancy characterized by proliferation of atypical B cell confined mostly to the vascular lumen. Its presentation is protean depending on the organs involved. It has been referred to as “the oncologists great imitator.” In a series of 38 patients by Ferreri et al, the most common symptoms were fever, cutaneous symptoms, neurological symptoms followed by abdominal pain. Most patients present in an advanced state, one series of 96 patients by Murase et al, 91% of the patient presenting with clinical stage III or stage IV disease.
There remain no standard diagnostic criteria for intravascular lymphoma. First step is demonstration of lymphoma cells in small- and medium-sized blood vessels with characteristic sparing of surrounding tissue. B cell clones are most common, but T and NK cells have also been reported. Molecular, immune histochemical and flow cytometry techniques may aid in establishing diagnosis. Prognosis remains poor even with aggressive treatment, the largest series by Murase et al, with mean survival of just 13 months with treatment.
MRD data added to venetoclax label
The U.S. Food and Drug Administration (FDA) has expanded the label for venetoclax tablets (Venclexta®) to include data on minimal residual disease (MRD).
The drug’s prescribing information now includes details on MRD negativity in previously treated patients with chronic lymphocytic leukemia (CLL) who received venetoclax in combination with rituximab in the phase 3 MURANO trial.
The combination of venetoclax and rituximab was FDA approved in June for the treatment of patients with CLL or small lymphocytic lymphoma, with or without 17p deletion, who received at least one prior therapy.
The MURANO trial (NCT02005471), which supported the FDA approval, included 389 patients with relapsed or refractory CLL.
The patients were randomized to receive:
- Venetoclax at 400 mg daily for 24 months (after a 5-week ramp-up period) plus rituximab at 375 mg/m2 on day 1 for the first cycle and at 500 mg/m2 on day 1 for cycles 2 to 6 (n=194)
- Bendamustine at 70 mg/m2 on days 1 and 2 for 6 cycles plus rituximab at the same schedule as the venetoclax arm (n=195).
Researchers evaluated MRD in patients who achieved a partial response or better. MRD was assessed using allele-specific oligonucleotide polymerase chain reaction, and the definition of MRD negativity was less than one CLL cell per 10,000 lymphocytes.
The researchers assessed MRD in the peripheral blood 3 months after the last dose of rituximab. At that time, 53% (103/194) of patients in the venetoclax-rituximab arm were MRD negative, as were 12% (23/195) of patients in the bendamustine-rituximab arm.
The researchers also assessed MRD in the peripheral blood of patients with a complete response (CR) or CR with incomplete marrow recovery (CRi). MRD negativity was achieved by 3% (6/194) of these patients in the venetoclax-rituximab arm and 2% (3/195) in the bendamustine-rituximab arm.
Three percent (3/106) of patients in the venetoclax arm who achieved CR/CRi were MRD negative in both the peripheral blood and the bone marrow.
“The rates of MRD negativity seen with Venclexta plus rituximab are very encouraging,” said MURANO investigator John Seymour, MBBS, PhD, of the Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Additional results from the MURANO trial were published in The New England Journal of Medicine in March and are included in the prescribing information for venetoclax.
Venetoclax is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the U.S. and by AbbVie outside the U.S.
The U.S. Food and Drug Administration (FDA) has expanded the label for venetoclax tablets (Venclexta®) to include data on minimal residual disease (MRD).
The drug’s prescribing information now includes details on MRD negativity in previously treated patients with chronic lymphocytic leukemia (CLL) who received venetoclax in combination with rituximab in the phase 3 MURANO trial.
The combination of venetoclax and rituximab was FDA approved in June for the treatment of patients with CLL or small lymphocytic lymphoma, with or without 17p deletion, who received at least one prior therapy.
The MURANO trial (NCT02005471), which supported the FDA approval, included 389 patients with relapsed or refractory CLL.
The patients were randomized to receive:
- Venetoclax at 400 mg daily for 24 months (after a 5-week ramp-up period) plus rituximab at 375 mg/m2 on day 1 for the first cycle and at 500 mg/m2 on day 1 for cycles 2 to 6 (n=194)
- Bendamustine at 70 mg/m2 on days 1 and 2 for 6 cycles plus rituximab at the same schedule as the venetoclax arm (n=195).
Researchers evaluated MRD in patients who achieved a partial response or better. MRD was assessed using allele-specific oligonucleotide polymerase chain reaction, and the definition of MRD negativity was less than one CLL cell per 10,000 lymphocytes.
The researchers assessed MRD in the peripheral blood 3 months after the last dose of rituximab. At that time, 53% (103/194) of patients in the venetoclax-rituximab arm were MRD negative, as were 12% (23/195) of patients in the bendamustine-rituximab arm.
The researchers also assessed MRD in the peripheral blood of patients with a complete response (CR) or CR with incomplete marrow recovery (CRi). MRD negativity was achieved by 3% (6/194) of these patients in the venetoclax-rituximab arm and 2% (3/195) in the bendamustine-rituximab arm.
Three percent (3/106) of patients in the venetoclax arm who achieved CR/CRi were MRD negative in both the peripheral blood and the bone marrow.
“The rates of MRD negativity seen with Venclexta plus rituximab are very encouraging,” said MURANO investigator John Seymour, MBBS, PhD, of the Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Additional results from the MURANO trial were published in The New England Journal of Medicine in March and are included in the prescribing information for venetoclax.
Venetoclax is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the U.S. and by AbbVie outside the U.S.
The U.S. Food and Drug Administration (FDA) has expanded the label for venetoclax tablets (Venclexta®) to include data on minimal residual disease (MRD).
The drug’s prescribing information now includes details on MRD negativity in previously treated patients with chronic lymphocytic leukemia (CLL) who received venetoclax in combination with rituximab in the phase 3 MURANO trial.
The combination of venetoclax and rituximab was FDA approved in June for the treatment of patients with CLL or small lymphocytic lymphoma, with or without 17p deletion, who received at least one prior therapy.
The MURANO trial (NCT02005471), which supported the FDA approval, included 389 patients with relapsed or refractory CLL.
The patients were randomized to receive:
- Venetoclax at 400 mg daily for 24 months (after a 5-week ramp-up period) plus rituximab at 375 mg/m2 on day 1 for the first cycle and at 500 mg/m2 on day 1 for cycles 2 to 6 (n=194)
- Bendamustine at 70 mg/m2 on days 1 and 2 for 6 cycles plus rituximab at the same schedule as the venetoclax arm (n=195).
Researchers evaluated MRD in patients who achieved a partial response or better. MRD was assessed using allele-specific oligonucleotide polymerase chain reaction, and the definition of MRD negativity was less than one CLL cell per 10,000 lymphocytes.
The researchers assessed MRD in the peripheral blood 3 months after the last dose of rituximab. At that time, 53% (103/194) of patients in the venetoclax-rituximab arm were MRD negative, as were 12% (23/195) of patients in the bendamustine-rituximab arm.
The researchers also assessed MRD in the peripheral blood of patients with a complete response (CR) or CR with incomplete marrow recovery (CRi). MRD negativity was achieved by 3% (6/194) of these patients in the venetoclax-rituximab arm and 2% (3/195) in the bendamustine-rituximab arm.
Three percent (3/106) of patients in the venetoclax arm who achieved CR/CRi were MRD negative in both the peripheral blood and the bone marrow.
“The rates of MRD negativity seen with Venclexta plus rituximab are very encouraging,” said MURANO investigator John Seymour, MBBS, PhD, of the Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Additional results from the MURANO trial were published in The New England Journal of Medicine in March and are included in the prescribing information for venetoclax.
Venetoclax is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the U.S. and by AbbVie outside the U.S.
TP53 mutation plus complex karyotype equals poor prognosis
The presence of both TP53 gene mutation and complex karyotype may signal a dismal prognosis in patients with mantle cell lymphoma (MCL), according to results of a study.
All patients who had TP53 mutations and complex karyotype died within 1.2 years of diagnosis, while almost all patients with neither predictor were still alive at 2 years, according to study data reported in the journal Clinical Lymphoma, Myeloma & Leukemia.
By combining the markers, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment regimen, reported the study’s corresponding author Vít Procházka, MD, PhD, of the department of hemato-oncology faculty of medicine and dentistry at Palacký University, Olomouc, Czech Republic, and his coauthors.
“Our findings support a need to perform both tests, molecular cytogenetics, and next-generation sequencing simultaneously before initiation of treatment for MCL,” Dr. Procházka and his coauthors wrote.
In current clinical practice, TP53 mutational status and molecular cytogenetics are not routinely assessed prior to treatment initiation, they noted in their report.
The study, believed to be the first to evaluate the combined prognostic role of TP53 mutation and complex karyotype in an MCL cohort, included 74 consecutive adult patients newly diagnosed with MCL during 2000-2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or complex karyotype, was under observation without therapy.
Complex karyotype was found in 13 patients for whom fixed cells were available to perform cytogenetic analysis, the authors reported, while TP53 mutations were seen in 15 patients who were evaluated for that marker.
Altogether, 48 patients (64.9%) had biological material available to perform both analyses. Of those, 4 patients were found to have both TP53 mutation and complex karyotype, 12 had one of those two markers, and 32 had neither.
While patients with both markers all died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker; those differences were significant between groups (P less than .001), according to investigators.
Progression-free survival analyses showed similar results, with significant differences between the three groups, investigators reported.
Multivariate analysis showed that both TP53 mutation and complex karyotype were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index (MIPI).
While larger studies are needed to confirm the results, investigators suggested that novel treatment approaches might be warranted for patients in the highest-risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and [complex karyotype] might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” they said.
The study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
SOURCE: Obr A et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 23. doi: 10.1016/j.clml.2018.07.282.
The presence of both TP53 gene mutation and complex karyotype may signal a dismal prognosis in patients with mantle cell lymphoma (MCL), according to results of a study.
All patients who had TP53 mutations and complex karyotype died within 1.2 years of diagnosis, while almost all patients with neither predictor were still alive at 2 years, according to study data reported in the journal Clinical Lymphoma, Myeloma & Leukemia.
By combining the markers, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment regimen, reported the study’s corresponding author Vít Procházka, MD, PhD, of the department of hemato-oncology faculty of medicine and dentistry at Palacký University, Olomouc, Czech Republic, and his coauthors.
“Our findings support a need to perform both tests, molecular cytogenetics, and next-generation sequencing simultaneously before initiation of treatment for MCL,” Dr. Procházka and his coauthors wrote.
In current clinical practice, TP53 mutational status and molecular cytogenetics are not routinely assessed prior to treatment initiation, they noted in their report.
The study, believed to be the first to evaluate the combined prognostic role of TP53 mutation and complex karyotype in an MCL cohort, included 74 consecutive adult patients newly diagnosed with MCL during 2000-2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or complex karyotype, was under observation without therapy.
Complex karyotype was found in 13 patients for whom fixed cells were available to perform cytogenetic analysis, the authors reported, while TP53 mutations were seen in 15 patients who were evaluated for that marker.
Altogether, 48 patients (64.9%) had biological material available to perform both analyses. Of those, 4 patients were found to have both TP53 mutation and complex karyotype, 12 had one of those two markers, and 32 had neither.
While patients with both markers all died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker; those differences were significant between groups (P less than .001), according to investigators.
Progression-free survival analyses showed similar results, with significant differences between the three groups, investigators reported.
Multivariate analysis showed that both TP53 mutation and complex karyotype were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index (MIPI).
While larger studies are needed to confirm the results, investigators suggested that novel treatment approaches might be warranted for patients in the highest-risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and [complex karyotype] might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” they said.
The study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
SOURCE: Obr A et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 23. doi: 10.1016/j.clml.2018.07.282.
The presence of both TP53 gene mutation and complex karyotype may signal a dismal prognosis in patients with mantle cell lymphoma (MCL), according to results of a study.
All patients who had TP53 mutations and complex karyotype died within 1.2 years of diagnosis, while almost all patients with neither predictor were still alive at 2 years, according to study data reported in the journal Clinical Lymphoma, Myeloma & Leukemia.
By combining the markers, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment regimen, reported the study’s corresponding author Vít Procházka, MD, PhD, of the department of hemato-oncology faculty of medicine and dentistry at Palacký University, Olomouc, Czech Republic, and his coauthors.
“Our findings support a need to perform both tests, molecular cytogenetics, and next-generation sequencing simultaneously before initiation of treatment for MCL,” Dr. Procházka and his coauthors wrote.
In current clinical practice, TP53 mutational status and molecular cytogenetics are not routinely assessed prior to treatment initiation, they noted in their report.
The study, believed to be the first to evaluate the combined prognostic role of TP53 mutation and complex karyotype in an MCL cohort, included 74 consecutive adult patients newly diagnosed with MCL during 2000-2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or complex karyotype, was under observation without therapy.
Complex karyotype was found in 13 patients for whom fixed cells were available to perform cytogenetic analysis, the authors reported, while TP53 mutations were seen in 15 patients who were evaluated for that marker.
Altogether, 48 patients (64.9%) had biological material available to perform both analyses. Of those, 4 patients were found to have both TP53 mutation and complex karyotype, 12 had one of those two markers, and 32 had neither.
While patients with both markers all died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker; those differences were significant between groups (P less than .001), according to investigators.
Progression-free survival analyses showed similar results, with significant differences between the three groups, investigators reported.
Multivariate analysis showed that both TP53 mutation and complex karyotype were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index (MIPI).
While larger studies are needed to confirm the results, investigators suggested that novel treatment approaches might be warranted for patients in the highest-risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and [complex karyotype] might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” they said.
The study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
SOURCE: Obr A et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 23. doi: 10.1016/j.clml.2018.07.282.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Patients with both markers all died within 1.2 years, while 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker (P less than .001).
Study details: A study of 74 consecutive adult patients newly diagnosed with MCL during 2000-2014.
Disclosures: The study was supported by grants from the Czech Ministry of Health and Palacký University, Olomouc, Czech Republic. Study authors reported having no conflicts of interest.
Source: Obr A et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 23. doi: 10.1016/j.clml.2018.07.282.
New guidelines on antimicrobial prophylaxis
Experts have published updated guidelines on antimicrobial prophylaxis for adults with cancer-related immunosuppression.
The guidelines include antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions, such as hand hygiene, that may reduce infection risk.
The guidelines were developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA) and published in the Journal of Clinical Oncology.
For the most part, the expert panel that created these guidelines endorsed the previous ASCO recommendations, published in 2013.
However, the panel considered six new studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Recommendations
The ASCO/IDSA guidelines say health care providers should systematically assess the risk of febrile neutropenia, taking into account patient-, cancer-, and treatment-related factors.
Fluoroquinolone prophylaxis is recommended for patients at high risk of febrile neutropenia or profound, protracted neutropenia. This includes most patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) and patients undergoing hematopoietic stem cell transplant (HSCT) who are treated with myeloablative conditioning regimens.
Antifungal prophylaxis with an oral triazole or parenteral echinocandin is recommended for patients at risk of profound, protracted neutropenia, which includes HSCT recipients and most patients with AML/MDS.
However, neither antifungal nor antibiotic prophylaxis are routinely recommended for patients with solid tumors.
Prophylaxis with a nucleoside analog, such as acyclovir, is recommended in patients who are herpes simplex virus–seropositive and are undergoing allogeneic HSCT or leukemia induction.
Pneumocystis jirovecii prophylaxis, such as trimethoprim-sulfamethoxazole, is recommended for patients receiving chemotherapy regimens associated with a greater than 3.5% risk for pneumonia from P jirovecii.
Treatment with a nucleoside reverse transcription inhibitor, such as entecavir or tenofovir, is recommended for patients at high risk of hepatitis B virus reactivation.
Yearly influenza vaccination with an inactivated quadrivalent vaccine is recommended for all patients undergoing chemotherapy for malignancy as well as their family members, household contacts, and health care providers.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce the risk of pathogen transmission, according to the guidelines.
The guidelines also note that outpatients who develop neutropenia following cancer therapy should avoid prolonged contact with environments that have high concentrations of airborne fungal spores.
The guidelines do not recommend interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks. “Evidence of clinical benefit is lacking” for those interventions, the expert panel said.
Members of the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.
Experts have published updated guidelines on antimicrobial prophylaxis for adults with cancer-related immunosuppression.
The guidelines include antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions, such as hand hygiene, that may reduce infection risk.
The guidelines were developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA) and published in the Journal of Clinical Oncology.
For the most part, the expert panel that created these guidelines endorsed the previous ASCO recommendations, published in 2013.
However, the panel considered six new studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Recommendations
The ASCO/IDSA guidelines say health care providers should systematically assess the risk of febrile neutropenia, taking into account patient-, cancer-, and treatment-related factors.
Fluoroquinolone prophylaxis is recommended for patients at high risk of febrile neutropenia or profound, protracted neutropenia. This includes most patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) and patients undergoing hematopoietic stem cell transplant (HSCT) who are treated with myeloablative conditioning regimens.
Antifungal prophylaxis with an oral triazole or parenteral echinocandin is recommended for patients at risk of profound, protracted neutropenia, which includes HSCT recipients and most patients with AML/MDS.
However, neither antifungal nor antibiotic prophylaxis are routinely recommended for patients with solid tumors.
Prophylaxis with a nucleoside analog, such as acyclovir, is recommended in patients who are herpes simplex virus–seropositive and are undergoing allogeneic HSCT or leukemia induction.
Pneumocystis jirovecii prophylaxis, such as trimethoprim-sulfamethoxazole, is recommended for patients receiving chemotherapy regimens associated with a greater than 3.5% risk for pneumonia from P jirovecii.
Treatment with a nucleoside reverse transcription inhibitor, such as entecavir or tenofovir, is recommended for patients at high risk of hepatitis B virus reactivation.
Yearly influenza vaccination with an inactivated quadrivalent vaccine is recommended for all patients undergoing chemotherapy for malignancy as well as their family members, household contacts, and health care providers.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce the risk of pathogen transmission, according to the guidelines.
The guidelines also note that outpatients who develop neutropenia following cancer therapy should avoid prolonged contact with environments that have high concentrations of airborne fungal spores.
The guidelines do not recommend interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks. “Evidence of clinical benefit is lacking” for those interventions, the expert panel said.
Members of the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.
Experts have published updated guidelines on antimicrobial prophylaxis for adults with cancer-related immunosuppression.
The guidelines include antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions, such as hand hygiene, that may reduce infection risk.
The guidelines were developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA) and published in the Journal of Clinical Oncology.
For the most part, the expert panel that created these guidelines endorsed the previous ASCO recommendations, published in 2013.
However, the panel considered six new studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Recommendations
The ASCO/IDSA guidelines say health care providers should systematically assess the risk of febrile neutropenia, taking into account patient-, cancer-, and treatment-related factors.
Fluoroquinolone prophylaxis is recommended for patients at high risk of febrile neutropenia or profound, protracted neutropenia. This includes most patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) and patients undergoing hematopoietic stem cell transplant (HSCT) who are treated with myeloablative conditioning regimens.
Antifungal prophylaxis with an oral triazole or parenteral echinocandin is recommended for patients at risk of profound, protracted neutropenia, which includes HSCT recipients and most patients with AML/MDS.
However, neither antifungal nor antibiotic prophylaxis are routinely recommended for patients with solid tumors.
Prophylaxis with a nucleoside analog, such as acyclovir, is recommended in patients who are herpes simplex virus–seropositive and are undergoing allogeneic HSCT or leukemia induction.
Pneumocystis jirovecii prophylaxis, such as trimethoprim-sulfamethoxazole, is recommended for patients receiving chemotherapy regimens associated with a greater than 3.5% risk for pneumonia from P jirovecii.
Treatment with a nucleoside reverse transcription inhibitor, such as entecavir or tenofovir, is recommended for patients at high risk of hepatitis B virus reactivation.
Yearly influenza vaccination with an inactivated quadrivalent vaccine is recommended for all patients undergoing chemotherapy for malignancy as well as their family members, household contacts, and health care providers.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce the risk of pathogen transmission, according to the guidelines.
The guidelines also note that outpatients who develop neutropenia following cancer therapy should avoid prolonged contact with environments that have high concentrations of airborne fungal spores.
The guidelines do not recommend interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks. “Evidence of clinical benefit is lacking” for those interventions, the expert panel said.
Members of the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.