User login
Melanoma diagnosis does not deter pregnancy
Women in the United States do not appear to be delaying pregnancy after a diagnosis of melanoma, despite general recommendations to wait at least 2 years to attempt pregnancy because it might increase the risk of recurrence or exacerbate disease, investigators reported in the Journal of Surgical Research.
A review of records from a large national health care database showed that women aged 18-40 years with melanoma who were not pregnant on the index date had a significantly higher rate of pregnancy within 2 years, compared with matched controls, reported Julie A. DiSano, MD, from Penn State University, Hershey.
“These results suggest that a diagnosis of melanoma may serve as an impetus for some families to begin childbearing or have additional children sooner than they otherwise would have,” they wrote.
The investigators also found, reassuringly, that women who became pregnant after a melanoma diagnosis were not at increased risk for requiring additional therapy for the malignancy, at least in the short term.
Although earlier studies suggested that women who were pregnant at the time of a melanoma diagnosis had worse prognoses when compared with women who were not pregnant at the time of diagnosis, more recent studies have indicated women who are pregnant when diagnosed have similar outcomes as nonpregnant women with the same disease stage, the investigators noted.
“What is unclear and difficult to study is the relationship between melanoma and subsequent pregnancy rates, and pregnancy on melanoma outcomes. Very little data exist to guide women and physicians as to the safety of pregnancy after a diagnosis of melanoma. As a result, there are no formal guidelines for physicians who wish to counsel their patients regarding pregnancy after melanoma, and it is unknown whether women receive any counseling at all,” they wrote.
To get a clearer picture of the link between melanoma and subsequent pregnancy, the investigators scanned the Truven Health MarketScan database and identified 11,801 women from 18-40 years with melanoma who were not pregnant on the index date, determined by the earliest claim for melanoma diagnosis or therapy.
Each patient was matched on a 1:1 basis with women who did not have a melanoma claim at any time; cases were matched with controls on the basis of year of index date, age at index date, state of residence, and pregnancy status in the 90 days before the index date.
The authors found that the rate of pregnancy within 2 years of the index date was 15.8% for cases, compared with 13.6% for controls (P less than .001).
They also found, however, that women who required postsurgical therapy, suggesting more advanced disease stage or early recurrence, had a significantly lower probability of becoming pregnant within the first 9 months after the index date (hazard ratio, 0.26; P = .003).
There were no significant differences in the rate of postsurgical treatment by pregnancy status at either 3, 6, 9, or 12 months after surgery (P less than .05 for each), or in a Cox regression model for all time points (HR, 0.68, P = .23).
The authors offered several possible explanations for the higher pregnancy rates among women with melanoma, including the possibility that a cancer diagnosis could bring some couples closer together and “reorder” their priorities about starting a family.
“Another hypothesis is that families facing a melanoma diagnosis may decide to complete childbearing sooner in case the cancer recurs and subsequent treatment compromises fertility. Either way, the increased likelihood of pregnancy after melanoma diagnosis suggests an optimism about their future among families in the current childbearing generation in the United States,” they wrote.
The authors cautioned that the database does not include information about disease stage, and that “more detailed stage information is needed before revisiting recommendations.”
The study was supported by a Barsumian Trust grant; the authors reported having no conflicts of interest.
SOURCE: DiSano JA et al. J Surg Res. 2018 Jun 16. doi: 10.1016/j.jss.2018.05.026.
Women in the United States do not appear to be delaying pregnancy after a diagnosis of melanoma, despite general recommendations to wait at least 2 years to attempt pregnancy because it might increase the risk of recurrence or exacerbate disease, investigators reported in the Journal of Surgical Research.
A review of records from a large national health care database showed that women aged 18-40 years with melanoma who were not pregnant on the index date had a significantly higher rate of pregnancy within 2 years, compared with matched controls, reported Julie A. DiSano, MD, from Penn State University, Hershey.
“These results suggest that a diagnosis of melanoma may serve as an impetus for some families to begin childbearing or have additional children sooner than they otherwise would have,” they wrote.
The investigators also found, reassuringly, that women who became pregnant after a melanoma diagnosis were not at increased risk for requiring additional therapy for the malignancy, at least in the short term.
Although earlier studies suggested that women who were pregnant at the time of a melanoma diagnosis had worse prognoses when compared with women who were not pregnant at the time of diagnosis, more recent studies have indicated women who are pregnant when diagnosed have similar outcomes as nonpregnant women with the same disease stage, the investigators noted.
“What is unclear and difficult to study is the relationship between melanoma and subsequent pregnancy rates, and pregnancy on melanoma outcomes. Very little data exist to guide women and physicians as to the safety of pregnancy after a diagnosis of melanoma. As a result, there are no formal guidelines for physicians who wish to counsel their patients regarding pregnancy after melanoma, and it is unknown whether women receive any counseling at all,” they wrote.
To get a clearer picture of the link between melanoma and subsequent pregnancy, the investigators scanned the Truven Health MarketScan database and identified 11,801 women from 18-40 years with melanoma who were not pregnant on the index date, determined by the earliest claim for melanoma diagnosis or therapy.
Each patient was matched on a 1:1 basis with women who did not have a melanoma claim at any time; cases were matched with controls on the basis of year of index date, age at index date, state of residence, and pregnancy status in the 90 days before the index date.
The authors found that the rate of pregnancy within 2 years of the index date was 15.8% for cases, compared with 13.6% for controls (P less than .001).
They also found, however, that women who required postsurgical therapy, suggesting more advanced disease stage or early recurrence, had a significantly lower probability of becoming pregnant within the first 9 months after the index date (hazard ratio, 0.26; P = .003).
There were no significant differences in the rate of postsurgical treatment by pregnancy status at either 3, 6, 9, or 12 months after surgery (P less than .05 for each), or in a Cox regression model for all time points (HR, 0.68, P = .23).
The authors offered several possible explanations for the higher pregnancy rates among women with melanoma, including the possibility that a cancer diagnosis could bring some couples closer together and “reorder” their priorities about starting a family.
“Another hypothesis is that families facing a melanoma diagnosis may decide to complete childbearing sooner in case the cancer recurs and subsequent treatment compromises fertility. Either way, the increased likelihood of pregnancy after melanoma diagnosis suggests an optimism about their future among families in the current childbearing generation in the United States,” they wrote.
The authors cautioned that the database does not include information about disease stage, and that “more detailed stage information is needed before revisiting recommendations.”
The study was supported by a Barsumian Trust grant; the authors reported having no conflicts of interest.
SOURCE: DiSano JA et al. J Surg Res. 2018 Jun 16. doi: 10.1016/j.jss.2018.05.026.
Women in the United States do not appear to be delaying pregnancy after a diagnosis of melanoma, despite general recommendations to wait at least 2 years to attempt pregnancy because it might increase the risk of recurrence or exacerbate disease, investigators reported in the Journal of Surgical Research.
A review of records from a large national health care database showed that women aged 18-40 years with melanoma who were not pregnant on the index date had a significantly higher rate of pregnancy within 2 years, compared with matched controls, reported Julie A. DiSano, MD, from Penn State University, Hershey.
“These results suggest that a diagnosis of melanoma may serve as an impetus for some families to begin childbearing or have additional children sooner than they otherwise would have,” they wrote.
The investigators also found, reassuringly, that women who became pregnant after a melanoma diagnosis were not at increased risk for requiring additional therapy for the malignancy, at least in the short term.
Although earlier studies suggested that women who were pregnant at the time of a melanoma diagnosis had worse prognoses when compared with women who were not pregnant at the time of diagnosis, more recent studies have indicated women who are pregnant when diagnosed have similar outcomes as nonpregnant women with the same disease stage, the investigators noted.
“What is unclear and difficult to study is the relationship between melanoma and subsequent pregnancy rates, and pregnancy on melanoma outcomes. Very little data exist to guide women and physicians as to the safety of pregnancy after a diagnosis of melanoma. As a result, there are no formal guidelines for physicians who wish to counsel their patients regarding pregnancy after melanoma, and it is unknown whether women receive any counseling at all,” they wrote.
To get a clearer picture of the link between melanoma and subsequent pregnancy, the investigators scanned the Truven Health MarketScan database and identified 11,801 women from 18-40 years with melanoma who were not pregnant on the index date, determined by the earliest claim for melanoma diagnosis or therapy.
Each patient was matched on a 1:1 basis with women who did not have a melanoma claim at any time; cases were matched with controls on the basis of year of index date, age at index date, state of residence, and pregnancy status in the 90 days before the index date.
The authors found that the rate of pregnancy within 2 years of the index date was 15.8% for cases, compared with 13.6% for controls (P less than .001).
They also found, however, that women who required postsurgical therapy, suggesting more advanced disease stage or early recurrence, had a significantly lower probability of becoming pregnant within the first 9 months after the index date (hazard ratio, 0.26; P = .003).
There were no significant differences in the rate of postsurgical treatment by pregnancy status at either 3, 6, 9, or 12 months after surgery (P less than .05 for each), or in a Cox regression model for all time points (HR, 0.68, P = .23).
The authors offered several possible explanations for the higher pregnancy rates among women with melanoma, including the possibility that a cancer diagnosis could bring some couples closer together and “reorder” their priorities about starting a family.
“Another hypothesis is that families facing a melanoma diagnosis may decide to complete childbearing sooner in case the cancer recurs and subsequent treatment compromises fertility. Either way, the increased likelihood of pregnancy after melanoma diagnosis suggests an optimism about their future among families in the current childbearing generation in the United States,” they wrote.
The authors cautioned that the database does not include information about disease stage, and that “more detailed stage information is needed before revisiting recommendations.”
The study was supported by a Barsumian Trust grant; the authors reported having no conflicts of interest.
SOURCE: DiSano JA et al. J Surg Res. 2018 Jun 16. doi: 10.1016/j.jss.2018.05.026.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: Pregnancy after melanoma does not appear to increase risk for melanoma recurrence.
Major finding: The rate of pregnancy for women with melanoma was 15.8%, compared with 13.6% for controls (P less than .001).
Study details: A retrospective study of claims database records on 11,801 women with melanoma and an equal number of matched controls.
Disclosures: The study was supported by a Barsumian Trust grant; the authors reported having no conflicts of interest.
Source: DiSano JA et al. J Surg Res. 2018 Jun 16. doi: 10.1016/j.jss.2018.05.026.
Going Digital With Dermoscopy
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
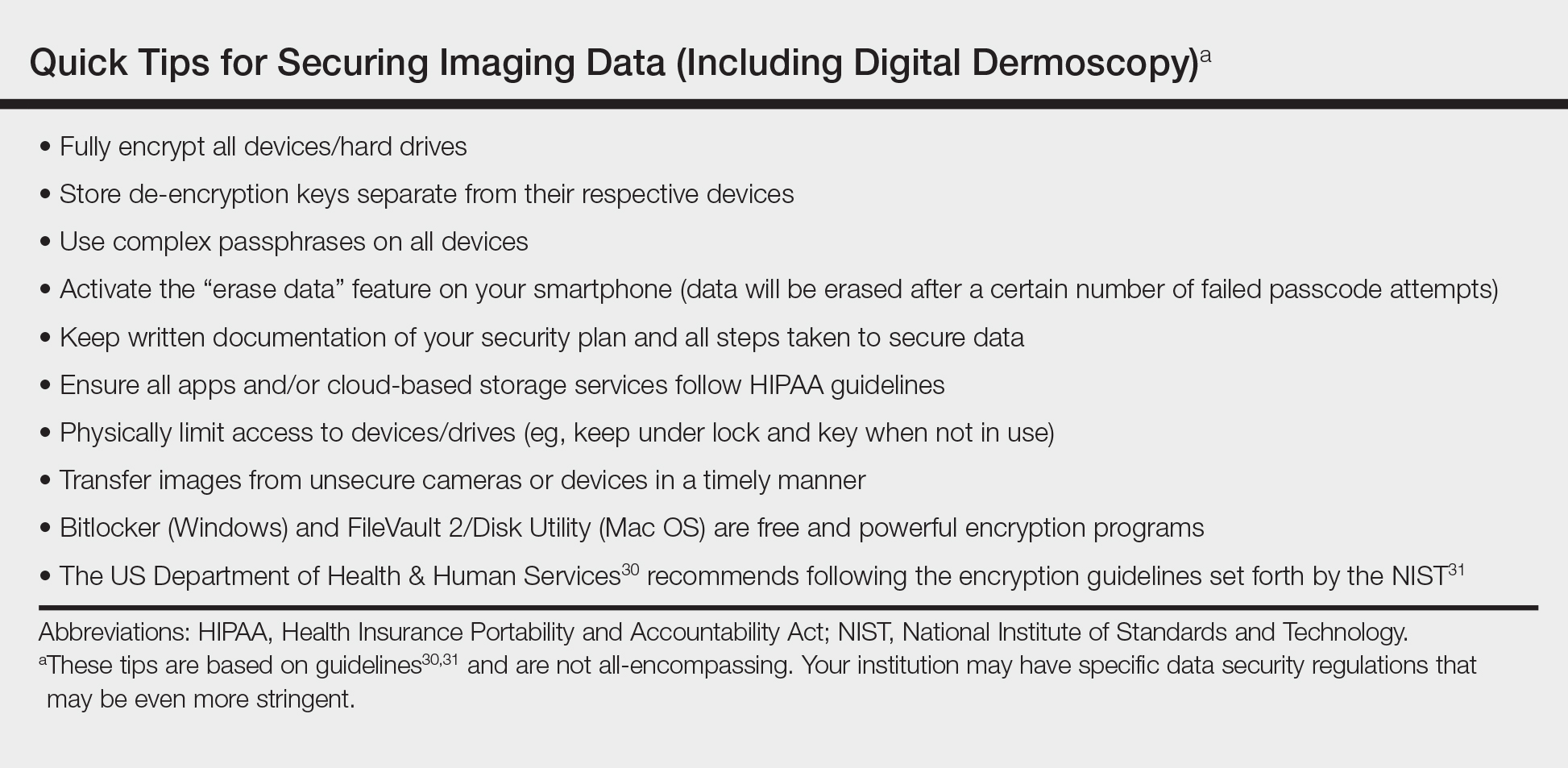
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
SPOTme addresses unmet need for skin cancer screening
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Free skin cancer screening programs help meet an unmet need for people at high risk for skin cancer.
Major finding: Of those who received a clinical diagnosis of melanoma during 1992-2010, 47% said they would not have seen a doctor for a skin exam if the free program had not been available.
Study details: The study analyzed data on almost 2 million people screened through the free SPOTme skin cancer screening program during 1986-2014.
Disclosures: The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
Source: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
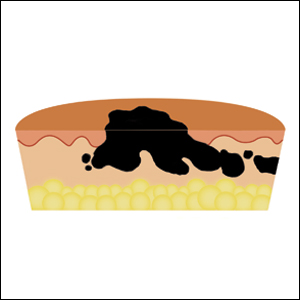
Melanoma survival shorter in those given high dose glucocorticoids for ipilimumab-induced hypophysitis
than did patients taking low-dose steroids for the adverse event, according to a new retrospective analysis in Cancer.
“Treatment with high-dose glucocorticoids does not appear to confer any obvious advantage to patients with IH (ipilimumab-induced hypophysitis) and may negatively affect tumor response to CPI (checkpoint-inhibitor therapy),” wrote Alexander Faje, MD, a neuroendocrinologist at Massachusetts General Hospital, Boston. “We recommend against the routine use of higher doses in these patients and that such treatment should be reserved for clinical indications like visual compromise or perhaps for intractable headache.”
Hypophysitis after treatment with a CTLA-4 inhibitor, such as ipilimumab, can approach 12%, though it is much less common with the checkpoint inhibitors that target PD-1 and PD-L1. Past studies examining the effects of glucocorticoids for immune-related adverse events have compared patients with severe events to those with minimal or no events. Since emergence of hypophysitis correlates with better overall survival, this is a flawed approach, the researchers said.
For their study, the researchers compared groups of patients with the same immune-related adverse events who received treatment with varying amounts of glucocorticoids.
They reviewed outcomes for 64 melanoma patients who had received ipilimumab monotherapy and were diagnosed with ipilimumab-induced hypophysitis treated in the Partners Healthcare system. Fourteen patients had received low-dose glucocorticoids, defined as a maximum average daily dose of 7.5 mg of prednisone or the equivalent. Fifty patients received high-dose glucocorticoids, defined as anything above that amount.
Overall survival and time to treatment failure were significantly higher in the low-dose group than the high-dose group (P = .002 for OS and P = .001 for TTF). Median overall survival was 23.3 months and time to treatment failure was 11.4 months in those given high-dose steroids. Median overall survival had not been reached in those given low-dose steroids.
While the findings are preliminary, the authors noted they may have implications for managing other immune-related adverse events. “Although the use of lower doses of immunosuppressive medications may be less of an option in many circumstances for other (immune-related adverse events), therapeutic parsimony would seem desirable with more tailored regimens as the biologic mechanisms underpinning these processes are further elucidated.”
SOURCE: Faje AT et al. Cancer. 2018 Jul 5.
The study results provide further evidence that hypophysitis appears to be an adverse effect of ipilimumab therapy that is linked with improved outcomes in melanoma. As hypophysitis tends to be self-limited, it can be treated safely with replacement therapy rather than high-dose steroid therapy, which was associated with reduced overall survival.
But the low number of patients on low-dose glucocorticoids – just 14 – is a limitation of the study and the group’s favorable outcomes could have been due to chance alone. Further, the 7.5-mg cut-off for high- vs. low-dose steroid therapy is somewhat arbitrary.
In support of the study’s overall conclusions, however, exploratory analyses have produced similar findings at somewhat higher cut-offs.
As the mechanism of hypophysitis is somewhat distinct compared with other immune-checkpoint toxicities, it might not be appropriate to generalize these findings to other toxic responses to these drugs.
The mechanisms of toxicities related to immune-checkpoint inhibitors are still not well defined. Unraveling those mechanisms may identify patients at high risk and hold the potential for aiding in the design of novel therapeutics that unleash antitumor immunity. Glucocorticoids are a fairly effective treatment for immune-related toxicities but remain a blunt, nonspecific way to suppress aberrant immunity. Designing inhibitors of culprit cellular populations or cytokines may combat toxicity without compromising the efficacy of immune therapy or promoting systemic immunosuppression.
Douglas B. Johnson, MD, is with Vanderbilt University Medical Center and Vanderbilt Ingram Cancer Center, Nashville, Tenn. He made his remarks in an editorial (Cancer 2018 Jul 5. doi: 10.1002/cncr.31627.
The study results provide further evidence that hypophysitis appears to be an adverse effect of ipilimumab therapy that is linked with improved outcomes in melanoma. As hypophysitis tends to be self-limited, it can be treated safely with replacement therapy rather than high-dose steroid therapy, which was associated with reduced overall survival.
But the low number of patients on low-dose glucocorticoids – just 14 – is a limitation of the study and the group’s favorable outcomes could have been due to chance alone. Further, the 7.5-mg cut-off for high- vs. low-dose steroid therapy is somewhat arbitrary.
In support of the study’s overall conclusions, however, exploratory analyses have produced similar findings at somewhat higher cut-offs.
As the mechanism of hypophysitis is somewhat distinct compared with other immune-checkpoint toxicities, it might not be appropriate to generalize these findings to other toxic responses to these drugs.
The mechanisms of toxicities related to immune-checkpoint inhibitors are still not well defined. Unraveling those mechanisms may identify patients at high risk and hold the potential for aiding in the design of novel therapeutics that unleash antitumor immunity. Glucocorticoids are a fairly effective treatment for immune-related toxicities but remain a blunt, nonspecific way to suppress aberrant immunity. Designing inhibitors of culprit cellular populations or cytokines may combat toxicity without compromising the efficacy of immune therapy or promoting systemic immunosuppression.
Douglas B. Johnson, MD, is with Vanderbilt University Medical Center and Vanderbilt Ingram Cancer Center, Nashville, Tenn. He made his remarks in an editorial (Cancer 2018 Jul 5. doi: 10.1002/cncr.31627.
The study results provide further evidence that hypophysitis appears to be an adverse effect of ipilimumab therapy that is linked with improved outcomes in melanoma. As hypophysitis tends to be self-limited, it can be treated safely with replacement therapy rather than high-dose steroid therapy, which was associated with reduced overall survival.
But the low number of patients on low-dose glucocorticoids – just 14 – is a limitation of the study and the group’s favorable outcomes could have been due to chance alone. Further, the 7.5-mg cut-off for high- vs. low-dose steroid therapy is somewhat arbitrary.
In support of the study’s overall conclusions, however, exploratory analyses have produced similar findings at somewhat higher cut-offs.
As the mechanism of hypophysitis is somewhat distinct compared with other immune-checkpoint toxicities, it might not be appropriate to generalize these findings to other toxic responses to these drugs.
The mechanisms of toxicities related to immune-checkpoint inhibitors are still not well defined. Unraveling those mechanisms may identify patients at high risk and hold the potential for aiding in the design of novel therapeutics that unleash antitumor immunity. Glucocorticoids are a fairly effective treatment for immune-related toxicities but remain a blunt, nonspecific way to suppress aberrant immunity. Designing inhibitors of culprit cellular populations or cytokines may combat toxicity without compromising the efficacy of immune therapy or promoting systemic immunosuppression.
Douglas B. Johnson, MD, is with Vanderbilt University Medical Center and Vanderbilt Ingram Cancer Center, Nashville, Tenn. He made his remarks in an editorial (Cancer 2018 Jul 5. doi: 10.1002/cncr.31627.
than did patients taking low-dose steroids for the adverse event, according to a new retrospective analysis in Cancer.
“Treatment with high-dose glucocorticoids does not appear to confer any obvious advantage to patients with IH (ipilimumab-induced hypophysitis) and may negatively affect tumor response to CPI (checkpoint-inhibitor therapy),” wrote Alexander Faje, MD, a neuroendocrinologist at Massachusetts General Hospital, Boston. “We recommend against the routine use of higher doses in these patients and that such treatment should be reserved for clinical indications like visual compromise or perhaps for intractable headache.”
Hypophysitis after treatment with a CTLA-4 inhibitor, such as ipilimumab, can approach 12%, though it is much less common with the checkpoint inhibitors that target PD-1 and PD-L1. Past studies examining the effects of glucocorticoids for immune-related adverse events have compared patients with severe events to those with minimal or no events. Since emergence of hypophysitis correlates with better overall survival, this is a flawed approach, the researchers said.
For their study, the researchers compared groups of patients with the same immune-related adverse events who received treatment with varying amounts of glucocorticoids.
They reviewed outcomes for 64 melanoma patients who had received ipilimumab monotherapy and were diagnosed with ipilimumab-induced hypophysitis treated in the Partners Healthcare system. Fourteen patients had received low-dose glucocorticoids, defined as a maximum average daily dose of 7.5 mg of prednisone or the equivalent. Fifty patients received high-dose glucocorticoids, defined as anything above that amount.
Overall survival and time to treatment failure were significantly higher in the low-dose group than the high-dose group (P = .002 for OS and P = .001 for TTF). Median overall survival was 23.3 months and time to treatment failure was 11.4 months in those given high-dose steroids. Median overall survival had not been reached in those given low-dose steroids.
While the findings are preliminary, the authors noted they may have implications for managing other immune-related adverse events. “Although the use of lower doses of immunosuppressive medications may be less of an option in many circumstances for other (immune-related adverse events), therapeutic parsimony would seem desirable with more tailored regimens as the biologic mechanisms underpinning these processes are further elucidated.”
SOURCE: Faje AT et al. Cancer. 2018 Jul 5.
than did patients taking low-dose steroids for the adverse event, according to a new retrospective analysis in Cancer.
“Treatment with high-dose glucocorticoids does not appear to confer any obvious advantage to patients with IH (ipilimumab-induced hypophysitis) and may negatively affect tumor response to CPI (checkpoint-inhibitor therapy),” wrote Alexander Faje, MD, a neuroendocrinologist at Massachusetts General Hospital, Boston. “We recommend against the routine use of higher doses in these patients and that such treatment should be reserved for clinical indications like visual compromise or perhaps for intractable headache.”
Hypophysitis after treatment with a CTLA-4 inhibitor, such as ipilimumab, can approach 12%, though it is much less common with the checkpoint inhibitors that target PD-1 and PD-L1. Past studies examining the effects of glucocorticoids for immune-related adverse events have compared patients with severe events to those with minimal or no events. Since emergence of hypophysitis correlates with better overall survival, this is a flawed approach, the researchers said.
For their study, the researchers compared groups of patients with the same immune-related adverse events who received treatment with varying amounts of glucocorticoids.
They reviewed outcomes for 64 melanoma patients who had received ipilimumab monotherapy and were diagnosed with ipilimumab-induced hypophysitis treated in the Partners Healthcare system. Fourteen patients had received low-dose glucocorticoids, defined as a maximum average daily dose of 7.5 mg of prednisone or the equivalent. Fifty patients received high-dose glucocorticoids, defined as anything above that amount.
Overall survival and time to treatment failure were significantly higher in the low-dose group than the high-dose group (P = .002 for OS and P = .001 for TTF). Median overall survival was 23.3 months and time to treatment failure was 11.4 months in those given high-dose steroids. Median overall survival had not been reached in those given low-dose steroids.
While the findings are preliminary, the authors noted they may have implications for managing other immune-related adverse events. “Although the use of lower doses of immunosuppressive medications may be less of an option in many circumstances for other (immune-related adverse events), therapeutic parsimony would seem desirable with more tailored regimens as the biologic mechanisms underpinning these processes are further elucidated.”
SOURCE: Faje AT et al. Cancer. 2018 Jul 5.
FROM CANCER
Key clinical point: Significantly lower overall survival and shorter time to treatment failure was seen in melanoma patients taking higher doses of glucocorticoids for ipilimumab-induced hypophysitis than in those taking lower doses.
Major finding: Median overall survival was 23.3 months and time to treatment failure was 11.4 months in those given high-dose steroids. Median overall survival had not been reached in those given low-dose steroids.
Study details: A retrospective review of 64 melanoma patients on single-agent ipilimumab therapy who were given glucocorticoids for ipilimumab-induced hypophysitis.
Disclosures: No funding source disclosed. The authors made no disclosures related to the submitted work.
Source: Faje AT et al. Cancer 2018 Jul 5.
Immunotherapies extend survival for melanoma patients with brain metastases
Since the Food and Drug Administration first approved checkpoint blockade immunotherapy (CBI) and BRAFV600-targeted therapy in 2011, survival times for patients with melanoma brain metastases (MBMs) have significantly improved, with a 91% increase in 4-year overall survival (OS) from 7.4% to 14.1%.
“The management of advanced melanoma has traditionally been tempered by limited responses to conventional therapies, resulting in a median overall survival (OS) of less than 1 year,” wrote J. Bryan Iorgulescu, MD, of Brigham and Women’s Hospital in Boston, and his colleagues. The report was published in Cancer Immunology Research. “The landscape of advanced melanoma treatment was revolutionized” by the approval of immunotherapy agents, beginning in 2011.
The current, retrospective study involved 2,753 patients with stage IV melanoma. Patient data were drawn from the National Cancer Database, with diagnoses made between 2010 and 2015. Patient management, overall survival, and disease characteristics were evaluated.
During initial review, the researchers found that 35.8% of patients with stage IV melanoma had brain involvement. These patients were further categorized by those with MBM only (39.7%) versus those with extracranial metastatic disease (60.3%), which included involvement of lung (82.9%), liver (8.1%), bone (6.0%), and lymph nodes or distant subcutaneous skin (3%). MBM-only disease was independently predicted by both younger age and geographic location.
Patients receiving first-line CBI therapy demonstrated improved 4-year OS (28.1% vs. 11.1%; P less than.001) and median OS (12.4 months vs. 5.2 months; P less than .001).
Improvements with CBI were most dramatic in patients with MBM-only disease. In these cases, 4-year OS improved from 16.9% to 51.5% (P less than .001), while median OS jumped from 7.7 months to 56.4 months (P less than .001).
Improved OS was also associated with fewer comorbidities, younger age, management at an academic cancer center, single-fraction stereotactic radiosurgery, and resection of the MBM.
“Our findings help bridge the gaps in early clinical trials of CBIs that largely excluded stage IV melanoma patients with MBMs, with checkpoint immunotherapy demonstrating a more than doubling of the median and 4-year OS of MBMs,” the authors concluded.
SOURCE: Iorgulescu et al. Cancer Immunol Res. 2018 July 12 doi: 10.1158/2326-6066.CIR-18-0067.
Since the Food and Drug Administration first approved checkpoint blockade immunotherapy (CBI) and BRAFV600-targeted therapy in 2011, survival times for patients with melanoma brain metastases (MBMs) have significantly improved, with a 91% increase in 4-year overall survival (OS) from 7.4% to 14.1%.
“The management of advanced melanoma has traditionally been tempered by limited responses to conventional therapies, resulting in a median overall survival (OS) of less than 1 year,” wrote J. Bryan Iorgulescu, MD, of Brigham and Women’s Hospital in Boston, and his colleagues. The report was published in Cancer Immunology Research. “The landscape of advanced melanoma treatment was revolutionized” by the approval of immunotherapy agents, beginning in 2011.
The current, retrospective study involved 2,753 patients with stage IV melanoma. Patient data were drawn from the National Cancer Database, with diagnoses made between 2010 and 2015. Patient management, overall survival, and disease characteristics were evaluated.
During initial review, the researchers found that 35.8% of patients with stage IV melanoma had brain involvement. These patients were further categorized by those with MBM only (39.7%) versus those with extracranial metastatic disease (60.3%), which included involvement of lung (82.9%), liver (8.1%), bone (6.0%), and lymph nodes or distant subcutaneous skin (3%). MBM-only disease was independently predicted by both younger age and geographic location.
Patients receiving first-line CBI therapy demonstrated improved 4-year OS (28.1% vs. 11.1%; P less than.001) and median OS (12.4 months vs. 5.2 months; P less than .001).
Improvements with CBI were most dramatic in patients with MBM-only disease. In these cases, 4-year OS improved from 16.9% to 51.5% (P less than .001), while median OS jumped from 7.7 months to 56.4 months (P less than .001).
Improved OS was also associated with fewer comorbidities, younger age, management at an academic cancer center, single-fraction stereotactic radiosurgery, and resection of the MBM.
“Our findings help bridge the gaps in early clinical trials of CBIs that largely excluded stage IV melanoma patients with MBMs, with checkpoint immunotherapy demonstrating a more than doubling of the median and 4-year OS of MBMs,” the authors concluded.
SOURCE: Iorgulescu et al. Cancer Immunol Res. 2018 July 12 doi: 10.1158/2326-6066.CIR-18-0067.
Since the Food and Drug Administration first approved checkpoint blockade immunotherapy (CBI) and BRAFV600-targeted therapy in 2011, survival times for patients with melanoma brain metastases (MBMs) have significantly improved, with a 91% increase in 4-year overall survival (OS) from 7.4% to 14.1%.
“The management of advanced melanoma has traditionally been tempered by limited responses to conventional therapies, resulting in a median overall survival (OS) of less than 1 year,” wrote J. Bryan Iorgulescu, MD, of Brigham and Women’s Hospital in Boston, and his colleagues. The report was published in Cancer Immunology Research. “The landscape of advanced melanoma treatment was revolutionized” by the approval of immunotherapy agents, beginning in 2011.
The current, retrospective study involved 2,753 patients with stage IV melanoma. Patient data were drawn from the National Cancer Database, with diagnoses made between 2010 and 2015. Patient management, overall survival, and disease characteristics were evaluated.
During initial review, the researchers found that 35.8% of patients with stage IV melanoma had brain involvement. These patients were further categorized by those with MBM only (39.7%) versus those with extracranial metastatic disease (60.3%), which included involvement of lung (82.9%), liver (8.1%), bone (6.0%), and lymph nodes or distant subcutaneous skin (3%). MBM-only disease was independently predicted by both younger age and geographic location.
Patients receiving first-line CBI therapy demonstrated improved 4-year OS (28.1% vs. 11.1%; P less than.001) and median OS (12.4 months vs. 5.2 months; P less than .001).
Improvements with CBI were most dramatic in patients with MBM-only disease. In these cases, 4-year OS improved from 16.9% to 51.5% (P less than .001), while median OS jumped from 7.7 months to 56.4 months (P less than .001).
Improved OS was also associated with fewer comorbidities, younger age, management at an academic cancer center, single-fraction stereotactic radiosurgery, and resection of the MBM.
“Our findings help bridge the gaps in early clinical trials of CBIs that largely excluded stage IV melanoma patients with MBMs, with checkpoint immunotherapy demonstrating a more than doubling of the median and 4-year OS of MBMs,” the authors concluded.
SOURCE: Iorgulescu et al. Cancer Immunol Res. 2018 July 12 doi: 10.1158/2326-6066.CIR-18-0067.
FROM CANCER IMMUNOLOGY RESEARCH
Key clinical point: Checkpoint blockade immunotherapy and BRAFV600-targeted therapy improve survival for patients with melanoma brain metastases.
Major finding: Patients with melanoma brain metastases receiving first-line checkpoint blockade immunotherapy had an improved 4-year overall survival (28.1% vs. 11.1%; P less than .001) and median overall survival (12.4 months vs. 5.2 months; P less than .001).
Study details: A retrospective study of 2,753 patients with stage IV melanoma and brain metastases, from the National Cancer Database, between 2010 and 2015.
Disclosures: The study was supported by the National Institute of Health, Abbvie, Bristol-Myers Squibb, Merck, and others. No conflicts of interest were reported.
Source: Iorgulescu et al. Cancer Immunol Res. 2018 July 12. doi: 10.1158/2326-6066.CIR-18-0067.
Sunscreen use in grade schoolers: Wide racial, ethnic disparities seen
and the figures were much lower for non-Hispanic black children.
Just 23% of fifth graders almost always used sunscreen, according to data drawn from the Healthy Passages study, which surveyed the parents or caregivers of 5,119 fifth graders. That figure was similar in the 1,802 Hispanic respondents, but fell to just 6% of the 1,748 non-Hispanic black respondents.
Some other factors that were associated with less chance of adherence to sunscreen use included being male and having lower socioeconomic status, wrote Christina M. Correnti, MD, and her study coauthors. The report was published in in Pediatric Dermatology. Perhaps surprisingly, they said, “School-based sun-safety education and involvement in team sports were not significant factors.”
Healthy Passages is a prospective multisite cohort study of child and adolescent health. Dr. Correnti, a dermatology resident at the University of Maryland, Baltimore, and her colleagues used baseline Healthy Passages data collected from the period of 2004-2006. Children enrolled in fifth grade at public schools in Birmingham, Ala., Houston, and Los Angeles, together with their caregivers, participated in the survey. Deidentified demographic data were collected, and participants were asked about four preventive health behaviors in addition to sunscreen use and flossing teeth: brushing teeth, helmet use, seatbelt use, and well-child examinations.
Dr. Correnti and her colleagues used multivariable analysis to calculate odds ratios for the association between the various demographic factors and other preventive behaviors and sunscreen use. They found that sunscreen adherence was correlated with all other preventive behaviors (P less than .001), but that the interrelationship with helmet use was confounded by racial and ethnic variables. Seatbelt use was not significantly correlated with sunscreen use for non-Hispanic black or Hispanic respondents.
“Children from more-educated and affluent households were more likely to use sun protection. Perhaps they had greater parental awareness and practice of sun safe habits,” wrote Dr. Correnti and her colleagues, noting that other work has shown that even low-income parents generally don’t see the cost of sunscreen as a barrier to use.
Although overall use of sunscreen among non-Hispanic black children was low, both non-Hispanic black and Hispanic children were more likely to use sunscreen if they had three or more sunburns within the prior 12 months. “Although darker skin tones may afford some sun protection, melanoma incidence is growing in Hispanic populations,” the researchers wrote.
To address these overall low rates of sunscreen use, the investigators discussed the utility of a variety of education options. The well-child visit affords an opportunity to reinforce the importance of preventive behaviors, but physicians may run into a time crunch and forgo thorough sun safety education, they said. Written materials can be a useful adjunct for clinicians in this setting.
“Health care practitioners may use absence of other preventive behaviors as potential markers for inadequate sunscreen use, prompting a point-of-care sun-safety intervention,” they suggested.
A school-based public health approach offers another route for education. “School sun-safety programs may alleviate the primary care burden,” wrote Dr. Correnti and her coinvestigators. The opportunity to deliver repeated, age-tailored messages as children progress through school may be effective in promoting healthy sun behaviors. Messaging that focuses on the negative effects of sun exposure on appearance such as age spots and wrinkles have been more effective than those warning of the risk of skin cancer for teens; investigating appearance-based content for this age group might be a good idea, the authors said.
The fact that the survey sites were in southern cities may mean that national rates of consistent sunscreen use for elementary schoolers may be even lower, said Dr. Correnti and her coauthors. Many other real-world factors, such as frequency and amount of sunscreen applied and the use of sun-protective clothing, couldn’t be captured by the survey, they acknowledged.
“Even in the most adherent group, non-Hispanic whites, only 44.8% always used sunscreen,” the researchers wrote. The study’s findings leave plenty of room for implementation of broad-based programs, especially in low-resource communities.
The National Institutes of Health funded the research. Dr. Correnti was supported by NIH awards.
SOURCE: Correnti CM et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13550.
and the figures were much lower for non-Hispanic black children.
Just 23% of fifth graders almost always used sunscreen, according to data drawn from the Healthy Passages study, which surveyed the parents or caregivers of 5,119 fifth graders. That figure was similar in the 1,802 Hispanic respondents, but fell to just 6% of the 1,748 non-Hispanic black respondents.
Some other factors that were associated with less chance of adherence to sunscreen use included being male and having lower socioeconomic status, wrote Christina M. Correnti, MD, and her study coauthors. The report was published in in Pediatric Dermatology. Perhaps surprisingly, they said, “School-based sun-safety education and involvement in team sports were not significant factors.”
Healthy Passages is a prospective multisite cohort study of child and adolescent health. Dr. Correnti, a dermatology resident at the University of Maryland, Baltimore, and her colleagues used baseline Healthy Passages data collected from the period of 2004-2006. Children enrolled in fifth grade at public schools in Birmingham, Ala., Houston, and Los Angeles, together with their caregivers, participated in the survey. Deidentified demographic data were collected, and participants were asked about four preventive health behaviors in addition to sunscreen use and flossing teeth: brushing teeth, helmet use, seatbelt use, and well-child examinations.
Dr. Correnti and her colleagues used multivariable analysis to calculate odds ratios for the association between the various demographic factors and other preventive behaviors and sunscreen use. They found that sunscreen adherence was correlated with all other preventive behaviors (P less than .001), but that the interrelationship with helmet use was confounded by racial and ethnic variables. Seatbelt use was not significantly correlated with sunscreen use for non-Hispanic black or Hispanic respondents.
“Children from more-educated and affluent households were more likely to use sun protection. Perhaps they had greater parental awareness and practice of sun safe habits,” wrote Dr. Correnti and her colleagues, noting that other work has shown that even low-income parents generally don’t see the cost of sunscreen as a barrier to use.
Although overall use of sunscreen among non-Hispanic black children was low, both non-Hispanic black and Hispanic children were more likely to use sunscreen if they had three or more sunburns within the prior 12 months. “Although darker skin tones may afford some sun protection, melanoma incidence is growing in Hispanic populations,” the researchers wrote.
To address these overall low rates of sunscreen use, the investigators discussed the utility of a variety of education options. The well-child visit affords an opportunity to reinforce the importance of preventive behaviors, but physicians may run into a time crunch and forgo thorough sun safety education, they said. Written materials can be a useful adjunct for clinicians in this setting.
“Health care practitioners may use absence of other preventive behaviors as potential markers for inadequate sunscreen use, prompting a point-of-care sun-safety intervention,” they suggested.
A school-based public health approach offers another route for education. “School sun-safety programs may alleviate the primary care burden,” wrote Dr. Correnti and her coinvestigators. The opportunity to deliver repeated, age-tailored messages as children progress through school may be effective in promoting healthy sun behaviors. Messaging that focuses on the negative effects of sun exposure on appearance such as age spots and wrinkles have been more effective than those warning of the risk of skin cancer for teens; investigating appearance-based content for this age group might be a good idea, the authors said.
The fact that the survey sites were in southern cities may mean that national rates of consistent sunscreen use for elementary schoolers may be even lower, said Dr. Correnti and her coauthors. Many other real-world factors, such as frequency and amount of sunscreen applied and the use of sun-protective clothing, couldn’t be captured by the survey, they acknowledged.
“Even in the most adherent group, non-Hispanic whites, only 44.8% always used sunscreen,” the researchers wrote. The study’s findings leave plenty of room for implementation of broad-based programs, especially in low-resource communities.
The National Institutes of Health funded the research. Dr. Correnti was supported by NIH awards.
SOURCE: Correnti CM et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13550.
and the figures were much lower for non-Hispanic black children.
Just 23% of fifth graders almost always used sunscreen, according to data drawn from the Healthy Passages study, which surveyed the parents or caregivers of 5,119 fifth graders. That figure was similar in the 1,802 Hispanic respondents, but fell to just 6% of the 1,748 non-Hispanic black respondents.
Some other factors that were associated with less chance of adherence to sunscreen use included being male and having lower socioeconomic status, wrote Christina M. Correnti, MD, and her study coauthors. The report was published in in Pediatric Dermatology. Perhaps surprisingly, they said, “School-based sun-safety education and involvement in team sports were not significant factors.”
Healthy Passages is a prospective multisite cohort study of child and adolescent health. Dr. Correnti, a dermatology resident at the University of Maryland, Baltimore, and her colleagues used baseline Healthy Passages data collected from the period of 2004-2006. Children enrolled in fifth grade at public schools in Birmingham, Ala., Houston, and Los Angeles, together with their caregivers, participated in the survey. Deidentified demographic data were collected, and participants were asked about four preventive health behaviors in addition to sunscreen use and flossing teeth: brushing teeth, helmet use, seatbelt use, and well-child examinations.
Dr. Correnti and her colleagues used multivariable analysis to calculate odds ratios for the association between the various demographic factors and other preventive behaviors and sunscreen use. They found that sunscreen adherence was correlated with all other preventive behaviors (P less than .001), but that the interrelationship with helmet use was confounded by racial and ethnic variables. Seatbelt use was not significantly correlated with sunscreen use for non-Hispanic black or Hispanic respondents.
“Children from more-educated and affluent households were more likely to use sun protection. Perhaps they had greater parental awareness and practice of sun safe habits,” wrote Dr. Correnti and her colleagues, noting that other work has shown that even low-income parents generally don’t see the cost of sunscreen as a barrier to use.
Although overall use of sunscreen among non-Hispanic black children was low, both non-Hispanic black and Hispanic children were more likely to use sunscreen if they had three or more sunburns within the prior 12 months. “Although darker skin tones may afford some sun protection, melanoma incidence is growing in Hispanic populations,” the researchers wrote.
To address these overall low rates of sunscreen use, the investigators discussed the utility of a variety of education options. The well-child visit affords an opportunity to reinforce the importance of preventive behaviors, but physicians may run into a time crunch and forgo thorough sun safety education, they said. Written materials can be a useful adjunct for clinicians in this setting.
“Health care practitioners may use absence of other preventive behaviors as potential markers for inadequate sunscreen use, prompting a point-of-care sun-safety intervention,” they suggested.
A school-based public health approach offers another route for education. “School sun-safety programs may alleviate the primary care burden,” wrote Dr. Correnti and her coinvestigators. The opportunity to deliver repeated, age-tailored messages as children progress through school may be effective in promoting healthy sun behaviors. Messaging that focuses on the negative effects of sun exposure on appearance such as age spots and wrinkles have been more effective than those warning of the risk of skin cancer for teens; investigating appearance-based content for this age group might be a good idea, the authors said.
The fact that the survey sites were in southern cities may mean that national rates of consistent sunscreen use for elementary schoolers may be even lower, said Dr. Correnti and her coauthors. Many other real-world factors, such as frequency and amount of sunscreen applied and the use of sun-protective clothing, couldn’t be captured by the survey, they acknowledged.
“Even in the most adherent group, non-Hispanic whites, only 44.8% always used sunscreen,” the researchers wrote. The study’s findings leave plenty of room for implementation of broad-based programs, especially in low-resource communities.
The National Institutes of Health funded the research. Dr. Correnti was supported by NIH awards.
SOURCE: Correnti CM et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13550.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: Most parents surveyed said their children didn’t use sunscreen consistently.
Major finding: Of non-Hispanic black children, 6% almost always used sunscreen.
Study details: Data drawn from Healthy Passages, a prospective cohort study of 5,119 fifth-graders and their parents or caregivers.
Disclosures: The National Institutes of Health funded the research. Dr. Correnti was supported by NIH awards.
Source: Correnti C et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13550.
Clinical Pearl: Mohs Cantaloupe Analogy for the Dermatology Resident
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).

In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.
Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
FDA approves encorafenib/binimetinib for advanced melanoma with BRAF mutations
The Food and Drug Administration has approved combination therapy of encorafenib (Braftovi) and binimetinib (Mektovi) for the treatment of unresectable or metastatic melanoma with BRAF V600E or BRAF V600K mutations; the FDA also has approved the THxID BRAF Kit as a companion diagnostic for this combination therapy.
The approval was based on results from the randomized, active-controlled, open-label, multicenter COLUMBUS trial, which included 517 patients. Progression-free survival, according to RECIST 1.1 criteria, was the major efficacy measure; the median progression-free survival was 14.9 months in the encorafenib/binimetinib combination arm versus 7.3 months in the vemurafenib (Zelboraf) monotherapy arm (hazard ratio, 0.54; 95% confidence interval, 0.41-0.71; P less than .0001).
Fatigue, nausea, diarrhea, vomiting, abdominal pain, and arthralgia were the most common adverse reactions. Discontinuation of therapy from adverse reactions occurred in 5% of patients receiving the combination, the FDA said in a press statement.
The full prescribing information for encorafenib and binimetinib can be found on the FDA website.
The Food and Drug Administration has approved combination therapy of encorafenib (Braftovi) and binimetinib (Mektovi) for the treatment of unresectable or metastatic melanoma with BRAF V600E or BRAF V600K mutations; the FDA also has approved the THxID BRAF Kit as a companion diagnostic for this combination therapy.
The approval was based on results from the randomized, active-controlled, open-label, multicenter COLUMBUS trial, which included 517 patients. Progression-free survival, according to RECIST 1.1 criteria, was the major efficacy measure; the median progression-free survival was 14.9 months in the encorafenib/binimetinib combination arm versus 7.3 months in the vemurafenib (Zelboraf) monotherapy arm (hazard ratio, 0.54; 95% confidence interval, 0.41-0.71; P less than .0001).
Fatigue, nausea, diarrhea, vomiting, abdominal pain, and arthralgia were the most common adverse reactions. Discontinuation of therapy from adverse reactions occurred in 5% of patients receiving the combination, the FDA said in a press statement.
The full prescribing information for encorafenib and binimetinib can be found on the FDA website.
The Food and Drug Administration has approved combination therapy of encorafenib (Braftovi) and binimetinib (Mektovi) for the treatment of unresectable or metastatic melanoma with BRAF V600E or BRAF V600K mutations; the FDA also has approved the THxID BRAF Kit as a companion diagnostic for this combination therapy.
The approval was based on results from the randomized, active-controlled, open-label, multicenter COLUMBUS trial, which included 517 patients. Progression-free survival, according to RECIST 1.1 criteria, was the major efficacy measure; the median progression-free survival was 14.9 months in the encorafenib/binimetinib combination arm versus 7.3 months in the vemurafenib (Zelboraf) monotherapy arm (hazard ratio, 0.54; 95% confidence interval, 0.41-0.71; P less than .0001).
Fatigue, nausea, diarrhea, vomiting, abdominal pain, and arthralgia were the most common adverse reactions. Discontinuation of therapy from adverse reactions occurred in 5% of patients receiving the combination, the FDA said in a press statement.
The full prescribing information for encorafenib and binimetinib can be found on the FDA website.
Registry data provide evidence that Mohs surgery remains underutilized
CHICAGO – Analysis of U.S. national cancer registry data shows that, contrary to expectation, the , Sean Condon, MD, reported at the annual meeting of the American College of Mohs Surgery.
Ditto for the use of Mohs in patients with the rare cutaneous malignancies for which published evidence clearly demonstrates Mohs outperforms wide local excision, which is employed seven times more frequently than Mohs in such situations.
“Mohs utilization did not increase after the Affordable Care Act [ACA], despite new health insurance coverage for 20 million previously uninsured adults,” Dr. Condon said. “Surprisingly, after the ACA we actually saw a decrease in Mohs use for melanoma in situ.”
Indeed, his retrospective study of more than 25,000 patients in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registries showed that the proportion of patients with melanoma in situ treated with Mohs declined from 13.9% during 2008-2009 – prior to ACA implementation – to 12.3% in 2011-2013, after the ACA took effect. That’s a statistically significant 13% drop, even though numerous published studies have shown outcomes in melanoma in situ are better with Mohs, said the dermatologist, who conducted the study while completing a Mohs surgery fellowship at the Cleveland Clinic. He is now in private practice in Thousand Oaks, Calif.
His analysis included 19,013 patients treated in 2008-2014 for melanoma in situ and 6,309 others treated for rare cutaneous malignancies deemed appropriate for Mohs according to the criteria formally developed jointly by the American Academy of Dermatology, the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery (J Am Acad Dermatol. 2012 Oct;67[4]:531-50). These rare malignancies include adnexal carcinoma, Merkel cell carcinoma, dermatofibrosarcoma, extramammary Paget disease, sebaceous adenocarcinoma, and leiomyosarcoma.
“These rare cutaneous malignancies were historically treated with wide local excision. However, numerous studies have lately shown that lower recurrence rates were found with Mohs compared with wide local excision,” Dr. Condon noted.
Nonetheless, the proportion of the rare cutaneous malignancies treated using Mohs was unaffected by implementation of the ACA. Nor was it influenced one way or the other by publication of the joint Mohs appropriate use criteria in 2012: The Mohs-treated proportion of such cases was 15.25% in 2010-2011 and 14.6% in 2013-2014.
Similarly, even though the appropriate use criteria identified melanoma in situ as Mohs appropriate, the proportion of those malignancies treated via Mohs was the same before and after the 2012 release of the criteria.
“It’s commonly thought that Mohs is overused. However, our study and our data clearly identify that Mohs is being underutilized for melanoma in situ and for rare cutaneous malignancies. This represents a knowledge gap for other specialties regarding best-practice therapy,” Dr. Condon said.
He and his coinvestigators searched for socioeconomic predictors of Mohs utilization by matching the nationally representative SEER data with U.S. census data. They examined the impact of three metrics: insurance status, income, and poverty. They found that low-income patients and those in the highest quartile of poverty were significantly less likely to have Mohs surgery for their melanoma in situ and rare cutaneous malignancies throughout the study years. Lack of health insurance had no impact on Mohs utilization for melanoma in situ but was independently associated with decreased likelihood of Mohs for the rare cutaneous malignancies. White patients were 2-fold to 2.4-fold more likely to have Mohs surgery for their rare cutaneous malignancies than were black patients.
“One can conclude that Mohs micrographic surgery may be skewed toward more affluent patients, and lower socioeconomic status areas have less Mohs access. So our data from this study support a role for targeted education and improved patient access to Mohs,” Dr. Condon said.
He noted that because the SEER registries don’t track squamous or basal cell carcinomas, it’s unknown whether Mohs is also underutilized for the higher-risk forms of these most common of all skin cancers.
Dr. Condon reported having no financial conflicts regarding his study, conducted free of commercial support.
CHICAGO – Analysis of U.S. national cancer registry data shows that, contrary to expectation, the , Sean Condon, MD, reported at the annual meeting of the American College of Mohs Surgery.
Ditto for the use of Mohs in patients with the rare cutaneous malignancies for which published evidence clearly demonstrates Mohs outperforms wide local excision, which is employed seven times more frequently than Mohs in such situations.
“Mohs utilization did not increase after the Affordable Care Act [ACA], despite new health insurance coverage for 20 million previously uninsured adults,” Dr. Condon said. “Surprisingly, after the ACA we actually saw a decrease in Mohs use for melanoma in situ.”
Indeed, his retrospective study of more than 25,000 patients in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registries showed that the proportion of patients with melanoma in situ treated with Mohs declined from 13.9% during 2008-2009 – prior to ACA implementation – to 12.3% in 2011-2013, after the ACA took effect. That’s a statistically significant 13% drop, even though numerous published studies have shown outcomes in melanoma in situ are better with Mohs, said the dermatologist, who conducted the study while completing a Mohs surgery fellowship at the Cleveland Clinic. He is now in private practice in Thousand Oaks, Calif.
His analysis included 19,013 patients treated in 2008-2014 for melanoma in situ and 6,309 others treated for rare cutaneous malignancies deemed appropriate for Mohs according to the criteria formally developed jointly by the American Academy of Dermatology, the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery (J Am Acad Dermatol. 2012 Oct;67[4]:531-50). These rare malignancies include adnexal carcinoma, Merkel cell carcinoma, dermatofibrosarcoma, extramammary Paget disease, sebaceous adenocarcinoma, and leiomyosarcoma.
“These rare cutaneous malignancies were historically treated with wide local excision. However, numerous studies have lately shown that lower recurrence rates were found with Mohs compared with wide local excision,” Dr. Condon noted.
Nonetheless, the proportion of the rare cutaneous malignancies treated using Mohs was unaffected by implementation of the ACA. Nor was it influenced one way or the other by publication of the joint Mohs appropriate use criteria in 2012: The Mohs-treated proportion of such cases was 15.25% in 2010-2011 and 14.6% in 2013-2014.
Similarly, even though the appropriate use criteria identified melanoma in situ as Mohs appropriate, the proportion of those malignancies treated via Mohs was the same before and after the 2012 release of the criteria.
“It’s commonly thought that Mohs is overused. However, our study and our data clearly identify that Mohs is being underutilized for melanoma in situ and for rare cutaneous malignancies. This represents a knowledge gap for other specialties regarding best-practice therapy,” Dr. Condon said.
He and his coinvestigators searched for socioeconomic predictors of Mohs utilization by matching the nationally representative SEER data with U.S. census data. They examined the impact of three metrics: insurance status, income, and poverty. They found that low-income patients and those in the highest quartile of poverty were significantly less likely to have Mohs surgery for their melanoma in situ and rare cutaneous malignancies throughout the study years. Lack of health insurance had no impact on Mohs utilization for melanoma in situ but was independently associated with decreased likelihood of Mohs for the rare cutaneous malignancies. White patients were 2-fold to 2.4-fold more likely to have Mohs surgery for their rare cutaneous malignancies than were black patients.
“One can conclude that Mohs micrographic surgery may be skewed toward more affluent patients, and lower socioeconomic status areas have less Mohs access. So our data from this study support a role for targeted education and improved patient access to Mohs,” Dr. Condon said.
He noted that because the SEER registries don’t track squamous or basal cell carcinomas, it’s unknown whether Mohs is also underutilized for the higher-risk forms of these most common of all skin cancers.
Dr. Condon reported having no financial conflicts regarding his study, conducted free of commercial support.
CHICAGO – Analysis of U.S. national cancer registry data shows that, contrary to expectation, the , Sean Condon, MD, reported at the annual meeting of the American College of Mohs Surgery.
Ditto for the use of Mohs in patients with the rare cutaneous malignancies for which published evidence clearly demonstrates Mohs outperforms wide local excision, which is employed seven times more frequently than Mohs in such situations.
“Mohs utilization did not increase after the Affordable Care Act [ACA], despite new health insurance coverage for 20 million previously uninsured adults,” Dr. Condon said. “Surprisingly, after the ACA we actually saw a decrease in Mohs use for melanoma in situ.”
Indeed, his retrospective study of more than 25,000 patients in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registries showed that the proportion of patients with melanoma in situ treated with Mohs declined from 13.9% during 2008-2009 – prior to ACA implementation – to 12.3% in 2011-2013, after the ACA took effect. That’s a statistically significant 13% drop, even though numerous published studies have shown outcomes in melanoma in situ are better with Mohs, said the dermatologist, who conducted the study while completing a Mohs surgery fellowship at the Cleveland Clinic. He is now in private practice in Thousand Oaks, Calif.
His analysis included 19,013 patients treated in 2008-2014 for melanoma in situ and 6,309 others treated for rare cutaneous malignancies deemed appropriate for Mohs according to the criteria formally developed jointly by the American Academy of Dermatology, the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery (J Am Acad Dermatol. 2012 Oct;67[4]:531-50). These rare malignancies include adnexal carcinoma, Merkel cell carcinoma, dermatofibrosarcoma, extramammary Paget disease, sebaceous adenocarcinoma, and leiomyosarcoma.
“These rare cutaneous malignancies were historically treated with wide local excision. However, numerous studies have lately shown that lower recurrence rates were found with Mohs compared with wide local excision,” Dr. Condon noted.
Nonetheless, the proportion of the rare cutaneous malignancies treated using Mohs was unaffected by implementation of the ACA. Nor was it influenced one way or the other by publication of the joint Mohs appropriate use criteria in 2012: The Mohs-treated proportion of such cases was 15.25% in 2010-2011 and 14.6% in 2013-2014.
Similarly, even though the appropriate use criteria identified melanoma in situ as Mohs appropriate, the proportion of those malignancies treated via Mohs was the same before and after the 2012 release of the criteria.
“It’s commonly thought that Mohs is overused. However, our study and our data clearly identify that Mohs is being underutilized for melanoma in situ and for rare cutaneous malignancies. This represents a knowledge gap for other specialties regarding best-practice therapy,” Dr. Condon said.
He and his coinvestigators searched for socioeconomic predictors of Mohs utilization by matching the nationally representative SEER data with U.S. census data. They examined the impact of three metrics: insurance status, income, and poverty. They found that low-income patients and those in the highest quartile of poverty were significantly less likely to have Mohs surgery for their melanoma in situ and rare cutaneous malignancies throughout the study years. Lack of health insurance had no impact on Mohs utilization for melanoma in situ but was independently associated with decreased likelihood of Mohs for the rare cutaneous malignancies. White patients were 2-fold to 2.4-fold more likely to have Mohs surgery for their rare cutaneous malignancies than were black patients.
“One can conclude that Mohs micrographic surgery may be skewed toward more affluent patients, and lower socioeconomic status areas have less Mohs access. So our data from this study support a role for targeted education and improved patient access to Mohs,” Dr. Condon said.
He noted that because the SEER registries don’t track squamous or basal cell carcinomas, it’s unknown whether Mohs is also underutilized for the higher-risk forms of these most common of all skin cancers.
Dr. Condon reported having no financial conflicts regarding his study, conducted free of commercial support.
REPORTING FROM THE ACMS 50TH ANNUAL MEETING
Key clinical point: Mohs micrographic surgery remains seriously underutilized for the skin cancers for which it is most advantageous.
Major finding: The use of Mohs micrographic surgery to treat melanoma in situ declined significantly after passage of the Affordable Care Act.
Study details: This was a retrospective study of national SEER data on more than 25,000 patients treated for melanoma in situ or rare cutaneous malignancies during 2008-2014.
Disclosures: The presenter reported having no financial conflicts regarding his study, conducted free of commercial support.