User login
Detecting Autoimmune Neural Antibodies Is Key to Preventing Misdiagnosis
LAS VEGAS—Advances in autoimmune neurology, a rapidly evolving field, have the potential to prevent misdiagnosis of many disorders, from multiple sclerosis to neurodegenerative diseases. The key: increased detection of autoimmune and paraneoplastic neural antibodies, said Sean J. Pittock, MD, at the American Academy of Neurology’s Fall 2016 Conference. The importance of autoimmune neurology “cannot be overstated because these are reversible, treatable conditions,” Dr. Pittock said. “If you miss them, you have done your patients a disservice.”
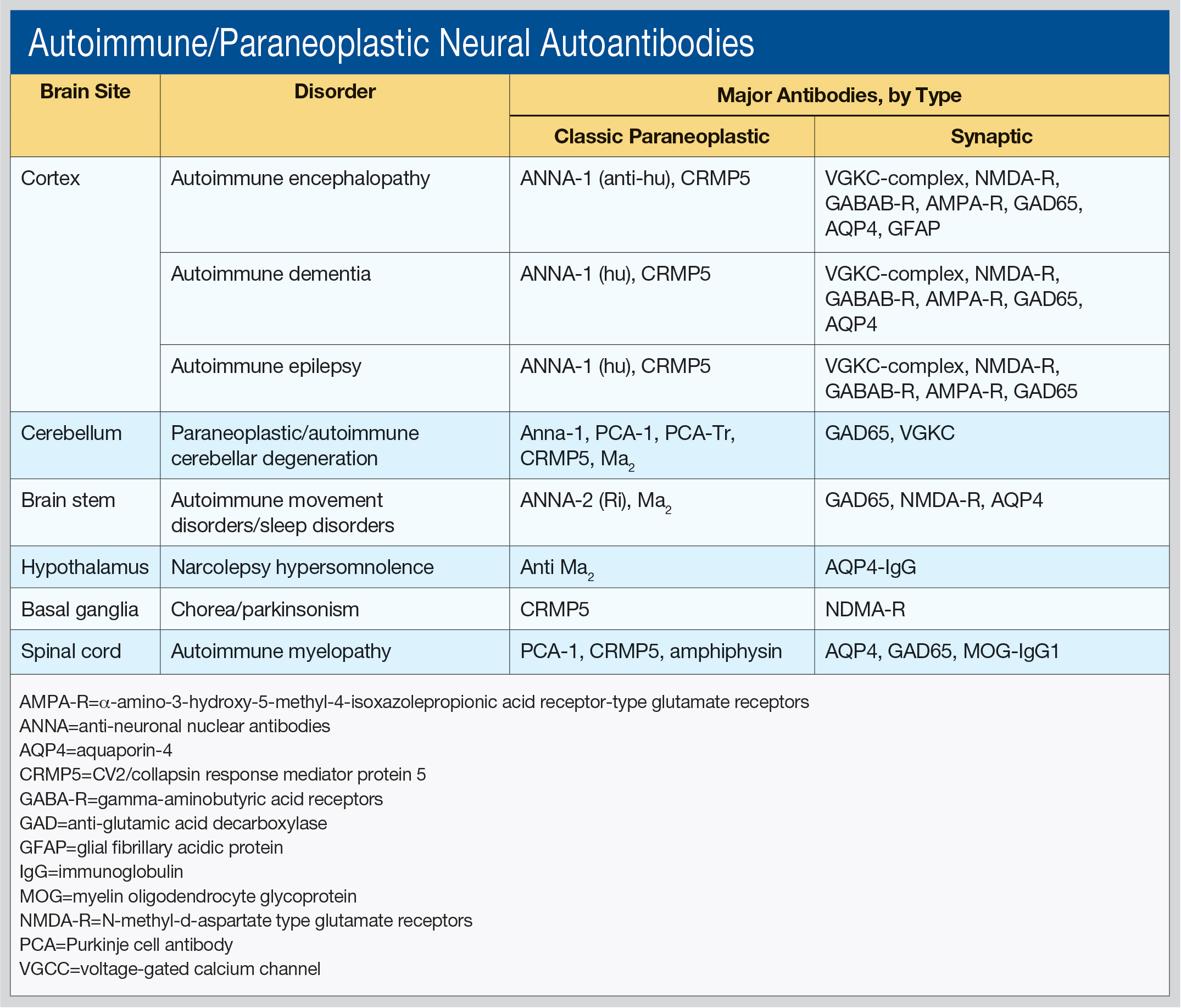
Dr. Pittock, Professor of Neurology, Director of the Neuroimmunology Laboratory, and Director of the Center for MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minnesota, said he works primarily “in the field of discovering novel antibodies that make sense to the clinician.” He outlined these antibodies by brain site (cortex, cerebellum, brainstem, hypothalamus, basal ganglia, and spinal cord), as well as associated disorders and type of antibodies (see table).
“Different antibodies tell you what cancers you should be looking for,” he said. The classic paraneoplastic antibodies have oncological associations with small cell and aerodigestive carcinomas, breast and gynecologic adenocarcinomas, Hodgkin lymphoma, and thymoma. The synaptic antibodies are associated with prostate, lung, thymic, and endometrial carcinomas, as well as with teratoma.
In his laboratory, Dr. Pittock uses a complex algorithm to test for 20 types of neural antibodies using diverse methodologies, from indirect immunofluorescence to cell-based and immunoprecipitation assays. Results are usually available within six to eight days. “We are moving towards a movement disorder evaluation, a CNS hyperexcitability evaluation, and a demyelinating disease evaluation,” he said.
“In paraneoplastic disorders, the tumors are often difficult to find,” Dr. Pittock said. “The immune system is having a dramatic impact on the tumor, and so these tumors are very small, so you may miss them.”
Dr. Pittock provided an overview of how clinicians might approach diagnosing autoimmune disorders. “For children, obviously, anaplastic diseases are rare, but if you have a patient with opsoclonus myoclonus, 50% of those children will have a cancer. For adolescents and young females, you always think about teratoma. If it is a young man, you really need to think about testicular tumors.”
The type of cancer defines what type of investigation is appropriate, he said. For example, due to low resolution, PET scans are not as useful for thymoma, teratoma, or testicular or gastrointestinal tumors. On the other hand, PET scans can help evaluate equivocal findings, such as pulmonary nodules or lymph nodes. With the exception of Medicare, however, PET scans are not generally covered by insurance. Medicare covers PET scanning under ICD-9 code V71.1, “observation for suspected malignant neoplasm,” and ICD-10, Z12, “encounter for screening for malignant neoplasm”; Z12.9, “site unspecified.”
At the Mayo Clinic, Dr. Pittock said they see five patients per week with NMDA-receptor encephalitis. To diagnose a possible autoimmune neurologic disorder, he asked that clinicians “please consider using the concept of immunotherapy as kind of a diagnostic test.” To do so, the patient should be treated acutely with IV methylprednisolone, IVIg, or plasma exchanges. If the patient improves, continue acute IV therapy and taper oral prednisone; other options include oral azathioprine or mycophenolate mofetil. If the patient does not improve, consider alternative acute therapy or no further therapy.
In one study that he and his colleagues conducted, 81% of patients who had failed antiepileptic drugs (AED) and were having daily seizures were treated with such a diagnostic test. Of these, 62% responded and 34% of patients became seizure-free. In contrast, Dr. Pittock said, patients treated with a third AED in a trial rarely experience meaningful improvement.
An autoimmune condition may also have a neuropsychiatric presentation. Dr. Pittock urged attendees to watch a recent film based on the 2013 book, “Brain on Fire: My Month of Madness,” by Susannah Cahalan, who went from psychosis to catatonia before a physician determined she had brain inflammation due to an autoimmune reaction.
“Many of these patients previously would have ended up in a psychiatric institution or in an ICU setting where, potentially, in the setting of intractable disease, they may have had their machine turned off, they may have died, when they have the potential to make a full recovery and, sometimes, spontaneously improve. The take-home message for this one is, if you want to investigate a patient with this, test spinal fluid.
“Autoimmune neurology is here to stay. It is a new field; it is very exciting; it has just been recognized by the American Academy of Neurology as having its own section,” Dr. Pittock said, and invited those interested to join.
Dr. Pittock has received research support from Alexion Pharmaceuticals, the Guthy-Jackson Charitable Foundation, and the National Institutes of Health.
—Debra Hughes
Suggested Reading
Linnoila J, Pittock SJ. Autoantibody-associated central nervous system neurologic disorders. Semin Neurol. 2016;36(4):382-396.
Toledano M, Britton JW, McKeon A, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578-1586.
LAS VEGAS—Advances in autoimmune neurology, a rapidly evolving field, have the potential to prevent misdiagnosis of many disorders, from multiple sclerosis to neurodegenerative diseases. The key: increased detection of autoimmune and paraneoplastic neural antibodies, said Sean J. Pittock, MD, at the American Academy of Neurology’s Fall 2016 Conference. The importance of autoimmune neurology “cannot be overstated because these are reversible, treatable conditions,” Dr. Pittock said. “If you miss them, you have done your patients a disservice.”
Dr. Pittock, Professor of Neurology, Director of the Neuroimmunology Laboratory, and Director of the Center for MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minnesota, said he works primarily “in the field of discovering novel antibodies that make sense to the clinician.” He outlined these antibodies by brain site (cortex, cerebellum, brainstem, hypothalamus, basal ganglia, and spinal cord), as well as associated disorders and type of antibodies (see table).
“Different antibodies tell you what cancers you should be looking for,” he said. The classic paraneoplastic antibodies have oncological associations with small cell and aerodigestive carcinomas, breast and gynecologic adenocarcinomas, Hodgkin lymphoma, and thymoma. The synaptic antibodies are associated with prostate, lung, thymic, and endometrial carcinomas, as well as with teratoma.
In his laboratory, Dr. Pittock uses a complex algorithm to test for 20 types of neural antibodies using diverse methodologies, from indirect immunofluorescence to cell-based and immunoprecipitation assays. Results are usually available within six to eight days. “We are moving towards a movement disorder evaluation, a CNS hyperexcitability evaluation, and a demyelinating disease evaluation,” he said.
“In paraneoplastic disorders, the tumors are often difficult to find,” Dr. Pittock said. “The immune system is having a dramatic impact on the tumor, and so these tumors are very small, so you may miss them.”
Dr. Pittock provided an overview of how clinicians might approach diagnosing autoimmune disorders. “For children, obviously, anaplastic diseases are rare, but if you have a patient with opsoclonus myoclonus, 50% of those children will have a cancer. For adolescents and young females, you always think about teratoma. If it is a young man, you really need to think about testicular tumors.”
The type of cancer defines what type of investigation is appropriate, he said. For example, due to low resolution, PET scans are not as useful for thymoma, teratoma, or testicular or gastrointestinal tumors. On the other hand, PET scans can help evaluate equivocal findings, such as pulmonary nodules or lymph nodes. With the exception of Medicare, however, PET scans are not generally covered by insurance. Medicare covers PET scanning under ICD-9 code V71.1, “observation for suspected malignant neoplasm,” and ICD-10, Z12, “encounter for screening for malignant neoplasm”; Z12.9, “site unspecified.”
At the Mayo Clinic, Dr. Pittock said they see five patients per week with NMDA-receptor encephalitis. To diagnose a possible autoimmune neurologic disorder, he asked that clinicians “please consider using the concept of immunotherapy as kind of a diagnostic test.” To do so, the patient should be treated acutely with IV methylprednisolone, IVIg, or plasma exchanges. If the patient improves, continue acute IV therapy and taper oral prednisone; other options include oral azathioprine or mycophenolate mofetil. If the patient does not improve, consider alternative acute therapy or no further therapy.
In one study that he and his colleagues conducted, 81% of patients who had failed antiepileptic drugs (AED) and were having daily seizures were treated with such a diagnostic test. Of these, 62% responded and 34% of patients became seizure-free. In contrast, Dr. Pittock said, patients treated with a third AED in a trial rarely experience meaningful improvement.
An autoimmune condition may also have a neuropsychiatric presentation. Dr. Pittock urged attendees to watch a recent film based on the 2013 book, “Brain on Fire: My Month of Madness,” by Susannah Cahalan, who went from psychosis to catatonia before a physician determined she had brain inflammation due to an autoimmune reaction.
“Many of these patients previously would have ended up in a psychiatric institution or in an ICU setting where, potentially, in the setting of intractable disease, they may have had their machine turned off, they may have died, when they have the potential to make a full recovery and, sometimes, spontaneously improve. The take-home message for this one is, if you want to investigate a patient with this, test spinal fluid.
“Autoimmune neurology is here to stay. It is a new field; it is very exciting; it has just been recognized by the American Academy of Neurology as having its own section,” Dr. Pittock said, and invited those interested to join.
Dr. Pittock has received research support from Alexion Pharmaceuticals, the Guthy-Jackson Charitable Foundation, and the National Institutes of Health.

—Debra Hughes
Suggested Reading
Linnoila J, Pittock SJ. Autoantibody-associated central nervous system neurologic disorders. Semin Neurol. 2016;36(4):382-396.
Toledano M, Britton JW, McKeon A, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578-1586.
LAS VEGAS—Advances in autoimmune neurology, a rapidly evolving field, have the potential to prevent misdiagnosis of many disorders, from multiple sclerosis to neurodegenerative diseases. The key: increased detection of autoimmune and paraneoplastic neural antibodies, said Sean J. Pittock, MD, at the American Academy of Neurology’s Fall 2016 Conference. The importance of autoimmune neurology “cannot be overstated because these are reversible, treatable conditions,” Dr. Pittock said. “If you miss them, you have done your patients a disservice.”
Dr. Pittock, Professor of Neurology, Director of the Neuroimmunology Laboratory, and Director of the Center for MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minnesota, said he works primarily “in the field of discovering novel antibodies that make sense to the clinician.” He outlined these antibodies by brain site (cortex, cerebellum, brainstem, hypothalamus, basal ganglia, and spinal cord), as well as associated disorders and type of antibodies (see table).
“Different antibodies tell you what cancers you should be looking for,” he said. The classic paraneoplastic antibodies have oncological associations with small cell and aerodigestive carcinomas, breast and gynecologic adenocarcinomas, Hodgkin lymphoma, and thymoma. The synaptic antibodies are associated with prostate, lung, thymic, and endometrial carcinomas, as well as with teratoma.
In his laboratory, Dr. Pittock uses a complex algorithm to test for 20 types of neural antibodies using diverse methodologies, from indirect immunofluorescence to cell-based and immunoprecipitation assays. Results are usually available within six to eight days. “We are moving towards a movement disorder evaluation, a CNS hyperexcitability evaluation, and a demyelinating disease evaluation,” he said.
“In paraneoplastic disorders, the tumors are often difficult to find,” Dr. Pittock said. “The immune system is having a dramatic impact on the tumor, and so these tumors are very small, so you may miss them.”
Dr. Pittock provided an overview of how clinicians might approach diagnosing autoimmune disorders. “For children, obviously, anaplastic diseases are rare, but if you have a patient with opsoclonus myoclonus, 50% of those children will have a cancer. For adolescents and young females, you always think about teratoma. If it is a young man, you really need to think about testicular tumors.”
The type of cancer defines what type of investigation is appropriate, he said. For example, due to low resolution, PET scans are not as useful for thymoma, teratoma, or testicular or gastrointestinal tumors. On the other hand, PET scans can help evaluate equivocal findings, such as pulmonary nodules or lymph nodes. With the exception of Medicare, however, PET scans are not generally covered by insurance. Medicare covers PET scanning under ICD-9 code V71.1, “observation for suspected malignant neoplasm,” and ICD-10, Z12, “encounter for screening for malignant neoplasm”; Z12.9, “site unspecified.”
At the Mayo Clinic, Dr. Pittock said they see five patients per week with NMDA-receptor encephalitis. To diagnose a possible autoimmune neurologic disorder, he asked that clinicians “please consider using the concept of immunotherapy as kind of a diagnostic test.” To do so, the patient should be treated acutely with IV methylprednisolone, IVIg, or plasma exchanges. If the patient improves, continue acute IV therapy and taper oral prednisone; other options include oral azathioprine or mycophenolate mofetil. If the patient does not improve, consider alternative acute therapy or no further therapy.
In one study that he and his colleagues conducted, 81% of patients who had failed antiepileptic drugs (AED) and were having daily seizures were treated with such a diagnostic test. Of these, 62% responded and 34% of patients became seizure-free. In contrast, Dr. Pittock said, patients treated with a third AED in a trial rarely experience meaningful improvement.
An autoimmune condition may also have a neuropsychiatric presentation. Dr. Pittock urged attendees to watch a recent film based on the 2013 book, “Brain on Fire: My Month of Madness,” by Susannah Cahalan, who went from psychosis to catatonia before a physician determined she had brain inflammation due to an autoimmune reaction.
“Many of these patients previously would have ended up in a psychiatric institution or in an ICU setting where, potentially, in the setting of intractable disease, they may have had their machine turned off, they may have died, when they have the potential to make a full recovery and, sometimes, spontaneously improve. The take-home message for this one is, if you want to investigate a patient with this, test spinal fluid.
“Autoimmune neurology is here to stay. It is a new field; it is very exciting; it has just been recognized by the American Academy of Neurology as having its own section,” Dr. Pittock said, and invited those interested to join.
Dr. Pittock has received research support from Alexion Pharmaceuticals, the Guthy-Jackson Charitable Foundation, and the National Institutes of Health.

—Debra Hughes
Suggested Reading
Linnoila J, Pittock SJ. Autoantibody-associated central nervous system neurologic disorders. Semin Neurol. 2016;36(4):382-396.
Toledano M, Britton JW, McKeon A, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578-1586.
Is physician-assisted suicide compatible with the Hippocratic Oath?
Do we stand by our Hippocratic Oath when providing physician-assisted suicide?
Physician-assisted suicide (PAS) is a form of euthanasia in which a physician provides the patient with the pertinent information, and, in certain cases, a prescription for the necessary lethal drugs, so that the individual can willingly and successfully terminate his or her own life. The justification for PAS is the compassionate relief of intractable human suffering. Euthanasia and PAS are accepted practices in European countries such as the Netherlands, Belgium, and Luxembourg.
Such patients are mentally incapable of making the difficult decision to end their life and therefore should require a psychiatric evaluation to include counseling prior to making a decision to engage in PAS. For patients with mental illness, PAS is even more problematic than for terminally ill patients, because patients may lack the capacity to make rational and responsible decisions. Indeed, there are sizable loopholes where our medical system lacks safeguards and similarly lacks the requirements for a thorough, pre-PAS mental health examination, family notifications, and consultations, and for the minimally necessary legal pressure required to ensure patient cooperation.
Critical role of mental health workers
Although PAS has been legalized in those five U.S. states, its support and cases have stalled in recent years, indicating serious ethical concerns, mostly because of multileveled challenges of combating and delineating cultural stereotypes, quantifying mental capacity, gauging quality of life, and deciding where to situate psychiatrists in the PAS decision.
The psychiatrist’s role is being debated. In the United States, opponents take issue with current PAS-legal state’s legislation regarding psychiatric evaluations. For example, Oregon stipulates a psychiatrist referral only in cases where a physician other than a psychiatrist believes the patient’s judgment is impaired. It’s agreed that psychiatrists have the best skill set to assess a patient’s perceptions. Other PAS-legal states require a psychiatrist or psychologist assessment before making the decision. Unfortunately, though, physicians have rarely referred these patients to psychiatrists before offering PAS as an option.
PAS opponents target standards by which concepts like “quality of life” or “contributing member of society” are judged – specifically, that “unbearable suffering” and its ramifications are ill defined – people whose lives are deemed “not worth living” (including the terminally ill) would be susceptible to “sympathetic death” via PAS that might result from PAS legalization. Opponents also argue that recognizing a suicide “right” contradicts that a significant number of suicide attempters have mental illness and need help. They say that legalizing PAS would enable mentally incapacitated people to commit the irreversible act based on their distorted perceptions without providing them the expected assistance from their profession.1
The use of euthanasia or PAS gradually is trending from physically terminally ill patients toward psychiatrically complex patients. There are cases in which euthanasia or PAS was requested by psychiatric patients who had chronic psychiatric, medical, and psychosocial histories rather than purely physical ailment histories. In one study, Scott Y. H. Kim, MD, PhD, and his associates reviewed cases in the Netherlands in which either euthanasia or PAS for psychiatric disorders was deployed. The granted PAS requests appeared to involve physician judgment without psychiatric input.
The study reviewed 66 cases: 55% of patients had chronic severe conditions with extensive histories of attempted suicides and psychiatric hospitalizations, demonstrating that the granted euthanasia and PAS requests had involved extensive evaluations. However, 11% of cases had no independent psychiatric input, and 24% involved disagreement among the physicians.2 PAS proponents and opponents support the involvement and expertise of psychiatrists in all of this.
Psychiatrists have long contended that suicide attempts are often a “cry for help,” not an earnest act to end one’s life. Legalizing PAS tells suicidal individuals that society does not care whether they live or die – a truly un-Hippocratic stance. The stereotypes tossed around in the PAS debate, which could mean life or death, need to be unpacked with specific criteria attached, rather than preconceptions.
Autonomy of patients and implications of PAS
In the PAS debate, there are serious life concerns exhibited by terminally ill/psychiatrically ill patients over losing their autonomy and becoming a burden to their families and caregivers. Stereotypes must be deconstructed, yes, but such patients generally have been considered to be rendered, by the sum effect of their illnesses, mentally incapable of making the decision to end their lives. Addressing this order of patient life concerns generally has been the realm of social workers, psychologists, and, in the most desirable cases, psychiatrists. Therefore, even in keeping with established practice, some form of competent psychiatric evaluation should be required before considering PAS as a viable option.
The call for this requirement is backed up by a study, which reported that 47% of patients who had considered or committed suicide previously were diagnosed with psychiatric disorders, and 15% had undiagnosed psychiatric disorders.3 Also, inventories of thwarted suicide attempters reveal that most lack the conviction to end their lives, and this obverse phenomenon is backed by a study that revealed that 75% of 96 suicidal patients were found to be ambivalent in their intent to end their lives. Suicide attempters simply may be trying to communicate with people in their lives in order to test their love and care.4 This indicates that those who attempt suicide are predominantly psychiatrically ill and prone to distorted perceptions, impaired judgment, frustration, escapism, and manipulative guilt.
Suicide is abhorrent to most human beings’ sensibilities, because humanity has an innate will to live.5 It is wrong to offer patients life-ending options when they might rejuvenate or ease into death naturally. With the debilitated or elderly, PAS could violate their human rights. If the attitude that they are a burden to society persists, then a certain segment of society might be tempted to avoid their intergenerational obligations to elders, particularly those elders presenting with concurrent mental illnesses. PAS would additionally fuel that problem. This would represent a profound injustice and gross violation of human dignity, while also serving as a denial of basic human rights, particularly the right to life and care.6
Conclusion
The complex physical, psychological, and social challenges associated with PAS and the difficulty in enforcing its laws necessitate more adept alternatives. Instead of conditionally legalizing suicide, we should ease patient suffering with compassion and calibrated treatment.6,7
Terminally ill patients often suffer from depression, affecting their rationality. Adequate counseling, medical care, and psychological care should be the response to terminally ill patients considering suicide. If society accepts that ending a life is a reasonable response to human suffering that could otherwise be treated or reversed, then those with the most serious psychiatric conditions are destined to become more vulnerable. Therefore, physicians and psychiatrists should instead work harder to help patients recover, rather than participating in their quest for death.
Simplistic legal and regulatory oversight is insufficient, because the questions evoked by PAS are complicated by life, death, and ethics. Further research is needed to look into the mechanisms and morality of how psychiatrists and other physicians will make judgments and recommendations that are vital elements in developing regulatory oversight on PAS. Finally, future studies should look at which practices have been successful and which might be deemed unethical.
References
1. Clin Med (Lond). 2010 Aug;109(4):323-5.
2. JAMA Psychiatry. 2016;73(4):362-8.
3. “The Final Months: A Study of the Lives of 134 Persons Who Committed Suicide,” New York: Oxford University Press, 1981.
4. “Why We Shouldn’t Legalize Assisting Suicide, Part I.” Department of Medical Ethics, National Right to Life Committee.
5. “Working with Suicidal Individuals: A Guide to Providing Understanding, Assessment and Support,” Philadelphia: Jessica Kingsley Publishers, 2010.
6. “Always Care, Never Kill: How Physician-Assisted Suicide Endangers the Weak, Corrupts Medicine, Compromises the Family, and Violates Human Dignity and Equality,” Washington: The Heritage Foundation, 2015.
7. Ann Intern Med. 2012 Jan 3;156(1 Pt 2):73-104.
Dr. Ahmed is a 2nd-year resident in the psychiatry & behavioral sciences department at the Nassau University Medical Center, New York. Over the last 3 years, he has published and presented papers at national and international forums. His interests include public social psychiatry, health care policy, health disparities, mental health stigma, and undiagnosed and overdiagnosed psychiatric illnesses in children. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
Do we stand by our Hippocratic Oath when providing physician-assisted suicide?
Physician-assisted suicide (PAS) is a form of euthanasia in which a physician provides the patient with the pertinent information, and, in certain cases, a prescription for the necessary lethal drugs, so that the individual can willingly and successfully terminate his or her own life. The justification for PAS is the compassionate relief of intractable human suffering. Euthanasia and PAS are accepted practices in European countries such as the Netherlands, Belgium, and Luxembourg.
Such patients are mentally incapable of making the difficult decision to end their life and therefore should require a psychiatric evaluation to include counseling prior to making a decision to engage in PAS. For patients with mental illness, PAS is even more problematic than for terminally ill patients, because patients may lack the capacity to make rational and responsible decisions. Indeed, there are sizable loopholes where our medical system lacks safeguards and similarly lacks the requirements for a thorough, pre-PAS mental health examination, family notifications, and consultations, and for the minimally necessary legal pressure required to ensure patient cooperation.
Critical role of mental health workers
Although PAS has been legalized in those five U.S. states, its support and cases have stalled in recent years, indicating serious ethical concerns, mostly because of multileveled challenges of combating and delineating cultural stereotypes, quantifying mental capacity, gauging quality of life, and deciding where to situate psychiatrists in the PAS decision.
The psychiatrist’s role is being debated. In the United States, opponents take issue with current PAS-legal state’s legislation regarding psychiatric evaluations. For example, Oregon stipulates a psychiatrist referral only in cases where a physician other than a psychiatrist believes the patient’s judgment is impaired. It’s agreed that psychiatrists have the best skill set to assess a patient’s perceptions. Other PAS-legal states require a psychiatrist or psychologist assessment before making the decision. Unfortunately, though, physicians have rarely referred these patients to psychiatrists before offering PAS as an option.
PAS opponents target standards by which concepts like “quality of life” or “contributing member of society” are judged – specifically, that “unbearable suffering” and its ramifications are ill defined – people whose lives are deemed “not worth living” (including the terminally ill) would be susceptible to “sympathetic death” via PAS that might result from PAS legalization. Opponents also argue that recognizing a suicide “right” contradicts that a significant number of suicide attempters have mental illness and need help. They say that legalizing PAS would enable mentally incapacitated people to commit the irreversible act based on their distorted perceptions without providing them the expected assistance from their profession.1
The use of euthanasia or PAS gradually is trending from physically terminally ill patients toward psychiatrically complex patients. There are cases in which euthanasia or PAS was requested by psychiatric patients who had chronic psychiatric, medical, and psychosocial histories rather than purely physical ailment histories. In one study, Scott Y. H. Kim, MD, PhD, and his associates reviewed cases in the Netherlands in which either euthanasia or PAS for psychiatric disorders was deployed. The granted PAS requests appeared to involve physician judgment without psychiatric input.
The study reviewed 66 cases: 55% of patients had chronic severe conditions with extensive histories of attempted suicides and psychiatric hospitalizations, demonstrating that the granted euthanasia and PAS requests had involved extensive evaluations. However, 11% of cases had no independent psychiatric input, and 24% involved disagreement among the physicians.2 PAS proponents and opponents support the involvement and expertise of psychiatrists in all of this.
Psychiatrists have long contended that suicide attempts are often a “cry for help,” not an earnest act to end one’s life. Legalizing PAS tells suicidal individuals that society does not care whether they live or die – a truly un-Hippocratic stance. The stereotypes tossed around in the PAS debate, which could mean life or death, need to be unpacked with specific criteria attached, rather than preconceptions.
Autonomy of patients and implications of PAS
In the PAS debate, there are serious life concerns exhibited by terminally ill/psychiatrically ill patients over losing their autonomy and becoming a burden to their families and caregivers. Stereotypes must be deconstructed, yes, but such patients generally have been considered to be rendered, by the sum effect of their illnesses, mentally incapable of making the decision to end their lives. Addressing this order of patient life concerns generally has been the realm of social workers, psychologists, and, in the most desirable cases, psychiatrists. Therefore, even in keeping with established practice, some form of competent psychiatric evaluation should be required before considering PAS as a viable option.
The call for this requirement is backed up by a study, which reported that 47% of patients who had considered or committed suicide previously were diagnosed with psychiatric disorders, and 15% had undiagnosed psychiatric disorders.3 Also, inventories of thwarted suicide attempters reveal that most lack the conviction to end their lives, and this obverse phenomenon is backed by a study that revealed that 75% of 96 suicidal patients were found to be ambivalent in their intent to end their lives. Suicide attempters simply may be trying to communicate with people in their lives in order to test their love and care.4 This indicates that those who attempt suicide are predominantly psychiatrically ill and prone to distorted perceptions, impaired judgment, frustration, escapism, and manipulative guilt.
Suicide is abhorrent to most human beings’ sensibilities, because humanity has an innate will to live.5 It is wrong to offer patients life-ending options when they might rejuvenate or ease into death naturally. With the debilitated or elderly, PAS could violate their human rights. If the attitude that they are a burden to society persists, then a certain segment of society might be tempted to avoid their intergenerational obligations to elders, particularly those elders presenting with concurrent mental illnesses. PAS would additionally fuel that problem. This would represent a profound injustice and gross violation of human dignity, while also serving as a denial of basic human rights, particularly the right to life and care.6
Conclusion
The complex physical, psychological, and social challenges associated with PAS and the difficulty in enforcing its laws necessitate more adept alternatives. Instead of conditionally legalizing suicide, we should ease patient suffering with compassion and calibrated treatment.6,7
Terminally ill patients often suffer from depression, affecting their rationality. Adequate counseling, medical care, and psychological care should be the response to terminally ill patients considering suicide. If society accepts that ending a life is a reasonable response to human suffering that could otherwise be treated or reversed, then those with the most serious psychiatric conditions are destined to become more vulnerable. Therefore, physicians and psychiatrists should instead work harder to help patients recover, rather than participating in their quest for death.
Simplistic legal and regulatory oversight is insufficient, because the questions evoked by PAS are complicated by life, death, and ethics. Further research is needed to look into the mechanisms and morality of how psychiatrists and other physicians will make judgments and recommendations that are vital elements in developing regulatory oversight on PAS. Finally, future studies should look at which practices have been successful and which might be deemed unethical.
References
1. Clin Med (Lond). 2010 Aug;109(4):323-5.
2. JAMA Psychiatry. 2016;73(4):362-8.
3. “The Final Months: A Study of the Lives of 134 Persons Who Committed Suicide,” New York: Oxford University Press, 1981.
4. “Why We Shouldn’t Legalize Assisting Suicide, Part I.” Department of Medical Ethics, National Right to Life Committee.
5. “Working with Suicidal Individuals: A Guide to Providing Understanding, Assessment and Support,” Philadelphia: Jessica Kingsley Publishers, 2010.
6. “Always Care, Never Kill: How Physician-Assisted Suicide Endangers the Weak, Corrupts Medicine, Compromises the Family, and Violates Human Dignity and Equality,” Washington: The Heritage Foundation, 2015.
7. Ann Intern Med. 2012 Jan 3;156(1 Pt 2):73-104.
Dr. Ahmed is a 2nd-year resident in the psychiatry & behavioral sciences department at the Nassau University Medical Center, New York. Over the last 3 years, he has published and presented papers at national and international forums. His interests include public social psychiatry, health care policy, health disparities, mental health stigma, and undiagnosed and overdiagnosed psychiatric illnesses in children. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
Do we stand by our Hippocratic Oath when providing physician-assisted suicide?
Physician-assisted suicide (PAS) is a form of euthanasia in which a physician provides the patient with the pertinent information, and, in certain cases, a prescription for the necessary lethal drugs, so that the individual can willingly and successfully terminate his or her own life. The justification for PAS is the compassionate relief of intractable human suffering. Euthanasia and PAS are accepted practices in European countries such as the Netherlands, Belgium, and Luxembourg.
Such patients are mentally incapable of making the difficult decision to end their life and therefore should require a psychiatric evaluation to include counseling prior to making a decision to engage in PAS. For patients with mental illness, PAS is even more problematic than for terminally ill patients, because patients may lack the capacity to make rational and responsible decisions. Indeed, there are sizable loopholes where our medical system lacks safeguards and similarly lacks the requirements for a thorough, pre-PAS mental health examination, family notifications, and consultations, and for the minimally necessary legal pressure required to ensure patient cooperation.
Critical role of mental health workers
Although PAS has been legalized in those five U.S. states, its support and cases have stalled in recent years, indicating serious ethical concerns, mostly because of multileveled challenges of combating and delineating cultural stereotypes, quantifying mental capacity, gauging quality of life, and deciding where to situate psychiatrists in the PAS decision.
The psychiatrist’s role is being debated. In the United States, opponents take issue with current PAS-legal state’s legislation regarding psychiatric evaluations. For example, Oregon stipulates a psychiatrist referral only in cases where a physician other than a psychiatrist believes the patient’s judgment is impaired. It’s agreed that psychiatrists have the best skill set to assess a patient’s perceptions. Other PAS-legal states require a psychiatrist or psychologist assessment before making the decision. Unfortunately, though, physicians have rarely referred these patients to psychiatrists before offering PAS as an option.
PAS opponents target standards by which concepts like “quality of life” or “contributing member of society” are judged – specifically, that “unbearable suffering” and its ramifications are ill defined – people whose lives are deemed “not worth living” (including the terminally ill) would be susceptible to “sympathetic death” via PAS that might result from PAS legalization. Opponents also argue that recognizing a suicide “right” contradicts that a significant number of suicide attempters have mental illness and need help. They say that legalizing PAS would enable mentally incapacitated people to commit the irreversible act based on their distorted perceptions without providing them the expected assistance from their profession.1
The use of euthanasia or PAS gradually is trending from physically terminally ill patients toward psychiatrically complex patients. There are cases in which euthanasia or PAS was requested by psychiatric patients who had chronic psychiatric, medical, and psychosocial histories rather than purely physical ailment histories. In one study, Scott Y. H. Kim, MD, PhD, and his associates reviewed cases in the Netherlands in which either euthanasia or PAS for psychiatric disorders was deployed. The granted PAS requests appeared to involve physician judgment without psychiatric input.
The study reviewed 66 cases: 55% of patients had chronic severe conditions with extensive histories of attempted suicides and psychiatric hospitalizations, demonstrating that the granted euthanasia and PAS requests had involved extensive evaluations. However, 11% of cases had no independent psychiatric input, and 24% involved disagreement among the physicians.2 PAS proponents and opponents support the involvement and expertise of psychiatrists in all of this.
Psychiatrists have long contended that suicide attempts are often a “cry for help,” not an earnest act to end one’s life. Legalizing PAS tells suicidal individuals that society does not care whether they live or die – a truly un-Hippocratic stance. The stereotypes tossed around in the PAS debate, which could mean life or death, need to be unpacked with specific criteria attached, rather than preconceptions.
Autonomy of patients and implications of PAS
In the PAS debate, there are serious life concerns exhibited by terminally ill/psychiatrically ill patients over losing their autonomy and becoming a burden to their families and caregivers. Stereotypes must be deconstructed, yes, but such patients generally have been considered to be rendered, by the sum effect of their illnesses, mentally incapable of making the decision to end their lives. Addressing this order of patient life concerns generally has been the realm of social workers, psychologists, and, in the most desirable cases, psychiatrists. Therefore, even in keeping with established practice, some form of competent psychiatric evaluation should be required before considering PAS as a viable option.
The call for this requirement is backed up by a study, which reported that 47% of patients who had considered or committed suicide previously were diagnosed with psychiatric disorders, and 15% had undiagnosed psychiatric disorders.3 Also, inventories of thwarted suicide attempters reveal that most lack the conviction to end their lives, and this obverse phenomenon is backed by a study that revealed that 75% of 96 suicidal patients were found to be ambivalent in their intent to end their lives. Suicide attempters simply may be trying to communicate with people in their lives in order to test their love and care.4 This indicates that those who attempt suicide are predominantly psychiatrically ill and prone to distorted perceptions, impaired judgment, frustration, escapism, and manipulative guilt.
Suicide is abhorrent to most human beings’ sensibilities, because humanity has an innate will to live.5 It is wrong to offer patients life-ending options when they might rejuvenate or ease into death naturally. With the debilitated or elderly, PAS could violate their human rights. If the attitude that they are a burden to society persists, then a certain segment of society might be tempted to avoid their intergenerational obligations to elders, particularly those elders presenting with concurrent mental illnesses. PAS would additionally fuel that problem. This would represent a profound injustice and gross violation of human dignity, while also serving as a denial of basic human rights, particularly the right to life and care.6
Conclusion
The complex physical, psychological, and social challenges associated with PAS and the difficulty in enforcing its laws necessitate more adept alternatives. Instead of conditionally legalizing suicide, we should ease patient suffering with compassion and calibrated treatment.6,7
Terminally ill patients often suffer from depression, affecting their rationality. Adequate counseling, medical care, and psychological care should be the response to terminally ill patients considering suicide. If society accepts that ending a life is a reasonable response to human suffering that could otherwise be treated or reversed, then those with the most serious psychiatric conditions are destined to become more vulnerable. Therefore, physicians and psychiatrists should instead work harder to help patients recover, rather than participating in their quest for death.
Simplistic legal and regulatory oversight is insufficient, because the questions evoked by PAS are complicated by life, death, and ethics. Further research is needed to look into the mechanisms and morality of how psychiatrists and other physicians will make judgments and recommendations that are vital elements in developing regulatory oversight on PAS. Finally, future studies should look at which practices have been successful and which might be deemed unethical.
References
1. Clin Med (Lond). 2010 Aug;109(4):323-5.
2. JAMA Psychiatry. 2016;73(4):362-8.
3. “The Final Months: A Study of the Lives of 134 Persons Who Committed Suicide,” New York: Oxford University Press, 1981.
4. “Why We Shouldn’t Legalize Assisting Suicide, Part I.” Department of Medical Ethics, National Right to Life Committee.
5. “Working with Suicidal Individuals: A Guide to Providing Understanding, Assessment and Support,” Philadelphia: Jessica Kingsley Publishers, 2010.
6. “Always Care, Never Kill: How Physician-Assisted Suicide Endangers the Weak, Corrupts Medicine, Compromises the Family, and Violates Human Dignity and Equality,” Washington: The Heritage Foundation, 2015.
7. Ann Intern Med. 2012 Jan 3;156(1 Pt 2):73-104.
Dr. Ahmed is a 2nd-year resident in the psychiatry & behavioral sciences department at the Nassau University Medical Center, New York. Over the last 3 years, he has published and presented papers at national and international forums. His interests include public social psychiatry, health care policy, health disparities, mental health stigma, and undiagnosed and overdiagnosed psychiatric illnesses in children. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
Less cognitive decline with stereotactic radiosurgery after brain metastases resection
BOSTON – For patients with resected brain metastases, stereotactic radiosurgery offers survival comparable with what’s seen with whole-brain radiotherapy, but with better quality of life and more effective preservation of cognitive function, investigators reported.
In the phase III N107C trial, there was no difference in overall survival between patients who were randomly assigned to undergo stereotactic radiosurgery (SRS) or whole-brain radiotherapy (WBRT), but patients who underwent WBRT had a twofold greater decline in cognitive function, compared with patients who underwent SRS, Paul D. Brown, MD, a radiation oncologist at the Mayo Clinic in Rochester, Minn., reported at the annual meeting of the American Society for Radiation Oncology.
In a similar prospective, randomized study, Anita Mahajan, MD, and colleagues from the University of Texas MD Anderson Cancer Center in Houston compared postoperative SRS after complete resection with observation alone in 128 patients, and found that although there was no difference in either distant brain metastases or overall survival, SRS was associated with significant improvements in local control.
“I think that going forward with the next patient I see with this scenario, I’m going to be a bit better informed and be able to inform my patient better of the trade-offs involved with regards to the decision of SRS vs. whole-brain radiotherapy,” commented George Rodrigues, MD, from the London (Ontario) Health Sciences Center in Canada. Dr. Rodrigues moderated a briefing during which Dr. Brown and Dr. Mahajan presented their data.
WBRT has been the standard of care for improving local control following surgical resection of brain metastases, but it does not offer a survival benefit and comes at a significant cost in side effects, including alopecia, fatigue, erythema, and, most distressing to patients, significant decline in cognitive function.
The precision of radiosurgery, on the other hand, allows the radiation dose to be concentrated on the surgical bed, limiting exposure of surrounding tissues and structures. For this reason, many centers have begun to adopt SRS for patients with resected brain metastases, but there is not level I evidence to back it up, Dr. Brown said.
WBRT vs. SRS
To rectify this situation, Dr. Brown and his colleagues at the Mayo Clinic and 47 other institutions conducted a clinical trial in 194 patients with one to four brain metastases.
Following surgical resection, the patients were stratified by age, duration of extracranial disease control, number of preoperative metastases, histology, maximum diameter of the resection cavity, and institution, and then randomly assigned to undergo either WBRT or SRS.
Patients were assessed for cognitive function (a coprimary endpoint with overall survival) at baseline and approximately every 3 months thereafter for up to 24 months. Other assessments included MRI scans, and FACT-Br (Functional Assessment of Cancer Therapy-Brain), a quality-of-life instrument.
After a median follow-up of 18.7 months, there was no difference in median overall survival, which was 11.5 months for WBRT and 11.8 months for SRS.
There was, however, a significant difference in cognitive deterioration–free survival, which was 2.8 months for WBRT vs. 3.3 months for SRS. The hazard ratio for WBRT was 2.05 (P = .0001). Cognitive deterioration–free survival rates at 6 months were 5.4% and 22.9%, respectively (P = .0012).
The declines in cognitive function were accounted for by significant differences in the Hopkins Verbal Learning Test (HVLT) domains of total and delayed recall and in the Trail Making Test (Part A).
Overall brain disease control was significantly better with WBRT than with SRS at 3 months (P = .003) and at 6 and 12 months (P less than .001 for each time point).
Surgical bed control was similar between the treatment groups at 6 and 12 months, but was significantly better with WBRT at 12 months, with surgical bed relapse occurring in 21.8% and 44.4% of patients, respectively.
Patients treated with SRS reported significantly better physical well being at 3 and 6 months (P = .002 and .014, respectively). There were 18 grade 3 or greater radiation-related adverse events among patients treated with WBRT, compared with 7 among patients treated with SRS.
SRS vs. observation
In the MD Anderson study, 45% of patients who underwent observation alone had local control of disease at 12 months, compared with 72% treated with SRS. The hazard ratio for SRS was 0.46 (P = .01). The median time to local recurrence was 7.6 months among patients on observation only, but no time point was reached for SRS-treated patients.
The evidence from the two trials suggests that “radiosurgery is a, but not the, standard of care following resection for brain metastasis,” said Vinai Gondi, MD, of the Northwestern Medicine Cancer Center in Warrenville, Ill., the invited discussant.
“While the MD Anderson trial clearly demonstrated that radiosurgery reduces the risk of surgical bed relapse, the N107C trial demonstrated a 44% risk of surgical bed relapse, a rate that is arguably too high in regards to the long survival of resected brain metastasis patients, and it also challenges and risks the resection of surgical bed relapse following radiosurgery,” he said.
The N107C trial was sponsored by the National Cancer Institute and the Alliance for Clinical Trials in Oncology. The MD Anderson trial was funded by a Cancer Center Grant. Dr. Brown, Dr. Mahajan, and Dr. Rodrigues reported no conflicts of interest.
BOSTON – For patients with resected brain metastases, stereotactic radiosurgery offers survival comparable with what’s seen with whole-brain radiotherapy, but with better quality of life and more effective preservation of cognitive function, investigators reported.
In the phase III N107C trial, there was no difference in overall survival between patients who were randomly assigned to undergo stereotactic radiosurgery (SRS) or whole-brain radiotherapy (WBRT), but patients who underwent WBRT had a twofold greater decline in cognitive function, compared with patients who underwent SRS, Paul D. Brown, MD, a radiation oncologist at the Mayo Clinic in Rochester, Minn., reported at the annual meeting of the American Society for Radiation Oncology.
In a similar prospective, randomized study, Anita Mahajan, MD, and colleagues from the University of Texas MD Anderson Cancer Center in Houston compared postoperative SRS after complete resection with observation alone in 128 patients, and found that although there was no difference in either distant brain metastases or overall survival, SRS was associated with significant improvements in local control.
“I think that going forward with the next patient I see with this scenario, I’m going to be a bit better informed and be able to inform my patient better of the trade-offs involved with regards to the decision of SRS vs. whole-brain radiotherapy,” commented George Rodrigues, MD, from the London (Ontario) Health Sciences Center in Canada. Dr. Rodrigues moderated a briefing during which Dr. Brown and Dr. Mahajan presented their data.
WBRT has been the standard of care for improving local control following surgical resection of brain metastases, but it does not offer a survival benefit and comes at a significant cost in side effects, including alopecia, fatigue, erythema, and, most distressing to patients, significant decline in cognitive function.
The precision of radiosurgery, on the other hand, allows the radiation dose to be concentrated on the surgical bed, limiting exposure of surrounding tissues and structures. For this reason, many centers have begun to adopt SRS for patients with resected brain metastases, but there is not level I evidence to back it up, Dr. Brown said.
WBRT vs. SRS
To rectify this situation, Dr. Brown and his colleagues at the Mayo Clinic and 47 other institutions conducted a clinical trial in 194 patients with one to four brain metastases.
Following surgical resection, the patients were stratified by age, duration of extracranial disease control, number of preoperative metastases, histology, maximum diameter of the resection cavity, and institution, and then randomly assigned to undergo either WBRT or SRS.
Patients were assessed for cognitive function (a coprimary endpoint with overall survival) at baseline and approximately every 3 months thereafter for up to 24 months. Other assessments included MRI scans, and FACT-Br (Functional Assessment of Cancer Therapy-Brain), a quality-of-life instrument.
After a median follow-up of 18.7 months, there was no difference in median overall survival, which was 11.5 months for WBRT and 11.8 months for SRS.
There was, however, a significant difference in cognitive deterioration–free survival, which was 2.8 months for WBRT vs. 3.3 months for SRS. The hazard ratio for WBRT was 2.05 (P = .0001). Cognitive deterioration–free survival rates at 6 months were 5.4% and 22.9%, respectively (P = .0012).
The declines in cognitive function were accounted for by significant differences in the Hopkins Verbal Learning Test (HVLT) domains of total and delayed recall and in the Trail Making Test (Part A).
Overall brain disease control was significantly better with WBRT than with SRS at 3 months (P = .003) and at 6 and 12 months (P less than .001 for each time point).
Surgical bed control was similar between the treatment groups at 6 and 12 months, but was significantly better with WBRT at 12 months, with surgical bed relapse occurring in 21.8% and 44.4% of patients, respectively.
Patients treated with SRS reported significantly better physical well being at 3 and 6 months (P = .002 and .014, respectively). There were 18 grade 3 or greater radiation-related adverse events among patients treated with WBRT, compared with 7 among patients treated with SRS.
SRS vs. observation
In the MD Anderson study, 45% of patients who underwent observation alone had local control of disease at 12 months, compared with 72% treated with SRS. The hazard ratio for SRS was 0.46 (P = .01). The median time to local recurrence was 7.6 months among patients on observation only, but no time point was reached for SRS-treated patients.
The evidence from the two trials suggests that “radiosurgery is a, but not the, standard of care following resection for brain metastasis,” said Vinai Gondi, MD, of the Northwestern Medicine Cancer Center in Warrenville, Ill., the invited discussant.
“While the MD Anderson trial clearly demonstrated that radiosurgery reduces the risk of surgical bed relapse, the N107C trial demonstrated a 44% risk of surgical bed relapse, a rate that is arguably too high in regards to the long survival of resected brain metastasis patients, and it also challenges and risks the resection of surgical bed relapse following radiosurgery,” he said.
The N107C trial was sponsored by the National Cancer Institute and the Alliance for Clinical Trials in Oncology. The MD Anderson trial was funded by a Cancer Center Grant. Dr. Brown, Dr. Mahajan, and Dr. Rodrigues reported no conflicts of interest.
BOSTON – For patients with resected brain metastases, stereotactic radiosurgery offers survival comparable with what’s seen with whole-brain radiotherapy, but with better quality of life and more effective preservation of cognitive function, investigators reported.
In the phase III N107C trial, there was no difference in overall survival between patients who were randomly assigned to undergo stereotactic radiosurgery (SRS) or whole-brain radiotherapy (WBRT), but patients who underwent WBRT had a twofold greater decline in cognitive function, compared with patients who underwent SRS, Paul D. Brown, MD, a radiation oncologist at the Mayo Clinic in Rochester, Minn., reported at the annual meeting of the American Society for Radiation Oncology.
In a similar prospective, randomized study, Anita Mahajan, MD, and colleagues from the University of Texas MD Anderson Cancer Center in Houston compared postoperative SRS after complete resection with observation alone in 128 patients, and found that although there was no difference in either distant brain metastases or overall survival, SRS was associated with significant improvements in local control.
“I think that going forward with the next patient I see with this scenario, I’m going to be a bit better informed and be able to inform my patient better of the trade-offs involved with regards to the decision of SRS vs. whole-brain radiotherapy,” commented George Rodrigues, MD, from the London (Ontario) Health Sciences Center in Canada. Dr. Rodrigues moderated a briefing during which Dr. Brown and Dr. Mahajan presented their data.
WBRT has been the standard of care for improving local control following surgical resection of brain metastases, but it does not offer a survival benefit and comes at a significant cost in side effects, including alopecia, fatigue, erythema, and, most distressing to patients, significant decline in cognitive function.
The precision of radiosurgery, on the other hand, allows the radiation dose to be concentrated on the surgical bed, limiting exposure of surrounding tissues and structures. For this reason, many centers have begun to adopt SRS for patients with resected brain metastases, but there is not level I evidence to back it up, Dr. Brown said.
WBRT vs. SRS
To rectify this situation, Dr. Brown and his colleagues at the Mayo Clinic and 47 other institutions conducted a clinical trial in 194 patients with one to four brain metastases.
Following surgical resection, the patients were stratified by age, duration of extracranial disease control, number of preoperative metastases, histology, maximum diameter of the resection cavity, and institution, and then randomly assigned to undergo either WBRT or SRS.
Patients were assessed for cognitive function (a coprimary endpoint with overall survival) at baseline and approximately every 3 months thereafter for up to 24 months. Other assessments included MRI scans, and FACT-Br (Functional Assessment of Cancer Therapy-Brain), a quality-of-life instrument.
After a median follow-up of 18.7 months, there was no difference in median overall survival, which was 11.5 months for WBRT and 11.8 months for SRS.
There was, however, a significant difference in cognitive deterioration–free survival, which was 2.8 months for WBRT vs. 3.3 months for SRS. The hazard ratio for WBRT was 2.05 (P = .0001). Cognitive deterioration–free survival rates at 6 months were 5.4% and 22.9%, respectively (P = .0012).
The declines in cognitive function were accounted for by significant differences in the Hopkins Verbal Learning Test (HVLT) domains of total and delayed recall and in the Trail Making Test (Part A).
Overall brain disease control was significantly better with WBRT than with SRS at 3 months (P = .003) and at 6 and 12 months (P less than .001 for each time point).
Surgical bed control was similar between the treatment groups at 6 and 12 months, but was significantly better with WBRT at 12 months, with surgical bed relapse occurring in 21.8% and 44.4% of patients, respectively.
Patients treated with SRS reported significantly better physical well being at 3 and 6 months (P = .002 and .014, respectively). There were 18 grade 3 or greater radiation-related adverse events among patients treated with WBRT, compared with 7 among patients treated with SRS.
SRS vs. observation
In the MD Anderson study, 45% of patients who underwent observation alone had local control of disease at 12 months, compared with 72% treated with SRS. The hazard ratio for SRS was 0.46 (P = .01). The median time to local recurrence was 7.6 months among patients on observation only, but no time point was reached for SRS-treated patients.
The evidence from the two trials suggests that “radiosurgery is a, but not the, standard of care following resection for brain metastasis,” said Vinai Gondi, MD, of the Northwestern Medicine Cancer Center in Warrenville, Ill., the invited discussant.
“While the MD Anderson trial clearly demonstrated that radiosurgery reduces the risk of surgical bed relapse, the N107C trial demonstrated a 44% risk of surgical bed relapse, a rate that is arguably too high in regards to the long survival of resected brain metastasis patients, and it also challenges and risks the resection of surgical bed relapse following radiosurgery,” he said.
The N107C trial was sponsored by the National Cancer Institute and the Alliance for Clinical Trials in Oncology. The MD Anderson trial was funded by a Cancer Center Grant. Dr. Brown, Dr. Mahajan, and Dr. Rodrigues reported no conflicts of interest.
AT ASTRO ANNUAL MEETING 2016
Key clinical point: Stereotactic radiosurgery following brain metastases resection was associated with similar survival but less toxicity than was whole-brain radiation therapy.
Major finding: WBRT was associated with a twofold greater risk for cognitive deterioration than SRS in one study, and SRS provided better local control than observation alone in another study.
Data source: A randomized, phase III trial in 194 patients from 48 centers in the United States and Canada, and a randomized trial in 128 patients from the MD Anderson Cancer Center.
Disclosures: The N107C trial was sponsored by the National Cancer Institute and the Alliance for Clinical Trials in Oncology. The MD Anderson trial was funded by a Cancer Center Grant. Dr. Brown, Dr. Mahajan, and Dr. Rodrigues reported no conflicts of interest.
Heat shock protein peptide vaccine appears safe, effective for glioblastoma patients
Chicago – A newly developed heat shock protein peptide vaccination appears to be safe and effective in treating patients with newly diagnosed glioblastoma (GBM), according to the results of a phase II single arm study.
In adding the vaccine to standard therapy for 46 patients with newly diagnosed GBM, median overall survival was 23.8 months, and there were no grade 3 or 4 adverse events associated with the vaccine, lead author Dr. Orin Bloch of Northwestern University, Chicago, reported at the annual meeting of the American Society of Clinical Oncology.
Standard therapy typically results in a median survival of 16 months, he said.
“There is a lot of information out there right now regarding CNS and other solid organ tumors particularly in the area of checkpoint modulation and its ability to stimulate an innate immune response against a tumor. I think in GBM we are facing a bit of a different scenario, however, because the tumor exists in a very privileged area behind the blood brain barrier and doesn’t regularly metastasize beyond the CNS,” Dr. Bloch said.
Therefore, only modulating checkpoints without stimulating and educating the immune system may not be the most effective approach. Adaptive immunity through vaccination or some other form of stimulation might be more successful, Dr. Bloch said.
“As a way of inducing immune stimulation and education using tumor-autologous peptides, one can capitalize on the native system of heat shock stimulation. Heat shock proteins are chaperone proteins that are ubiquitously expressed in cells and they’re bound to any number of intracellular peptides at any one time including, in tumor cells, neoantigens. If you extract these heat shock proteins with their bound antigens and deliver them in a naked form into the systemic circulation, their uptake into antigen-presenting cells through the CD91 receptor [will result in] the peptide [being] cleaved and presented on MHC class one and two for stimulation of CD8- and CD4-positive T cell response,” he said.
Heat shock proteins also interact with toll-like receptors and stimulate the secretion of pro-inflammatory cytokines, “acting as their own adjuvant,” Dr. Bloch further explained. Utilizing heat shock proteins activates both the innate and adaptive immune responses.
“This is an ideal platform for developing an immunotherapy for glioblastoma,” Dr. Bloch said.
In this phase II study, 46 adult patients with GBM underwent surgical resection of their tumors followed by chemoradiotherapy. At least four 25-microgram doses of vaccine were generated from tissue obtained during surgery. Within 5 weeks of completing radiotherapy, patients began receiving weekly vaccinations in combination with adjuvant temozolomide. Patients continued receiving vaccines until depletion or until tumor progression.
Median progression-free survival was 17.8 months (95% confidence interval, 11.3-21.6) and median overall survival was 23.8 months (95% CI, 19.8-30.2).
PD-L1 expression on circulating monocytes was also measured from peripheral blood samples obtained during surgery. Patients were classified as having either high PD-L1 expression (54.5% or more of monocytes) or low PD-L1 expression. Among patients classified as having high PD-L1 expression, the median overall survival was 18.0 months (95% CI, 10.0-23.3). Patients who had low PD-L1 expression had a significantly longer median overall survival time of 44.7 months with a confidence interval not calculable (hazard ratio, 3.35; 95% CI, 1.36-8.23; P = .003).
Finally, a multivariate proportional hazards model showed the MGMT methylation status and PD-L1 expression were the two greatest independent predictors of survival.
“Survival among patients who received the HSPPV-96 was greater than expected compared to historical controls... These results certainly, we feel, provide rationale for a phase III trial of vaccine plus standard of care versus standard of care alone,” Dr. Bloch said.
“PD-L1 expression on circulating myeloid cells is independently predictive of clinical response to vaccination, and it suggests that the low PD-L1 expressing population will most benefit from this anti-tumor vaccination scheme, but it also suggests that high PD-L1 expressing patients may benefit from combined checkpoint inhibition. Systemic immunosuppression driven by peripheral monocyte expression of PD-L1 is a previously unidentified factor that may mitigate vaccine efficacy,” Dr. Bloch further commented.
This study was funded by the National Cancer Institute, the National Institute of Neurological Disorders and Stroke, the National Brain Tumor Society, the American Brain Tumor Association, and Accelerated Brain Cancer Cure. Dr. Bloch reporting having no relevant disclosures.
On Twitter @jessnicolecraig
Chicago – A newly developed heat shock protein peptide vaccination appears to be safe and effective in treating patients with newly diagnosed glioblastoma (GBM), according to the results of a phase II single arm study.
In adding the vaccine to standard therapy for 46 patients with newly diagnosed GBM, median overall survival was 23.8 months, and there were no grade 3 or 4 adverse events associated with the vaccine, lead author Dr. Orin Bloch of Northwestern University, Chicago, reported at the annual meeting of the American Society of Clinical Oncology.
Standard therapy typically results in a median survival of 16 months, he said.
“There is a lot of information out there right now regarding CNS and other solid organ tumors particularly in the area of checkpoint modulation and its ability to stimulate an innate immune response against a tumor. I think in GBM we are facing a bit of a different scenario, however, because the tumor exists in a very privileged area behind the blood brain barrier and doesn’t regularly metastasize beyond the CNS,” Dr. Bloch said.
Therefore, only modulating checkpoints without stimulating and educating the immune system may not be the most effective approach. Adaptive immunity through vaccination or some other form of stimulation might be more successful, Dr. Bloch said.
“As a way of inducing immune stimulation and education using tumor-autologous peptides, one can capitalize on the native system of heat shock stimulation. Heat shock proteins are chaperone proteins that are ubiquitously expressed in cells and they’re bound to any number of intracellular peptides at any one time including, in tumor cells, neoantigens. If you extract these heat shock proteins with their bound antigens and deliver them in a naked form into the systemic circulation, their uptake into antigen-presenting cells through the CD91 receptor [will result in] the peptide [being] cleaved and presented on MHC class one and two for stimulation of CD8- and CD4-positive T cell response,” he said.
Heat shock proteins also interact with toll-like receptors and stimulate the secretion of pro-inflammatory cytokines, “acting as their own adjuvant,” Dr. Bloch further explained. Utilizing heat shock proteins activates both the innate and adaptive immune responses.
“This is an ideal platform for developing an immunotherapy for glioblastoma,” Dr. Bloch said.
In this phase II study, 46 adult patients with GBM underwent surgical resection of their tumors followed by chemoradiotherapy. At least four 25-microgram doses of vaccine were generated from tissue obtained during surgery. Within 5 weeks of completing radiotherapy, patients began receiving weekly vaccinations in combination with adjuvant temozolomide. Patients continued receiving vaccines until depletion or until tumor progression.
Median progression-free survival was 17.8 months (95% confidence interval, 11.3-21.6) and median overall survival was 23.8 months (95% CI, 19.8-30.2).
PD-L1 expression on circulating monocytes was also measured from peripheral blood samples obtained during surgery. Patients were classified as having either high PD-L1 expression (54.5% or more of monocytes) or low PD-L1 expression. Among patients classified as having high PD-L1 expression, the median overall survival was 18.0 months (95% CI, 10.0-23.3). Patients who had low PD-L1 expression had a significantly longer median overall survival time of 44.7 months with a confidence interval not calculable (hazard ratio, 3.35; 95% CI, 1.36-8.23; P = .003).
Finally, a multivariate proportional hazards model showed the MGMT methylation status and PD-L1 expression were the two greatest independent predictors of survival.
“Survival among patients who received the HSPPV-96 was greater than expected compared to historical controls... These results certainly, we feel, provide rationale for a phase III trial of vaccine plus standard of care versus standard of care alone,” Dr. Bloch said.
“PD-L1 expression on circulating myeloid cells is independently predictive of clinical response to vaccination, and it suggests that the low PD-L1 expressing population will most benefit from this anti-tumor vaccination scheme, but it also suggests that high PD-L1 expressing patients may benefit from combined checkpoint inhibition. Systemic immunosuppression driven by peripheral monocyte expression of PD-L1 is a previously unidentified factor that may mitigate vaccine efficacy,” Dr. Bloch further commented.
This study was funded by the National Cancer Institute, the National Institute of Neurological Disorders and Stroke, the National Brain Tumor Society, the American Brain Tumor Association, and Accelerated Brain Cancer Cure. Dr. Bloch reporting having no relevant disclosures.
On Twitter @jessnicolecraig
Chicago – A newly developed heat shock protein peptide vaccination appears to be safe and effective in treating patients with newly diagnosed glioblastoma (GBM), according to the results of a phase II single arm study.
In adding the vaccine to standard therapy for 46 patients with newly diagnosed GBM, median overall survival was 23.8 months, and there were no grade 3 or 4 adverse events associated with the vaccine, lead author Dr. Orin Bloch of Northwestern University, Chicago, reported at the annual meeting of the American Society of Clinical Oncology.
Standard therapy typically results in a median survival of 16 months, he said.
“There is a lot of information out there right now regarding CNS and other solid organ tumors particularly in the area of checkpoint modulation and its ability to stimulate an innate immune response against a tumor. I think in GBM we are facing a bit of a different scenario, however, because the tumor exists in a very privileged area behind the blood brain barrier and doesn’t regularly metastasize beyond the CNS,” Dr. Bloch said.
Therefore, only modulating checkpoints without stimulating and educating the immune system may not be the most effective approach. Adaptive immunity through vaccination or some other form of stimulation might be more successful, Dr. Bloch said.
“As a way of inducing immune stimulation and education using tumor-autologous peptides, one can capitalize on the native system of heat shock stimulation. Heat shock proteins are chaperone proteins that are ubiquitously expressed in cells and they’re bound to any number of intracellular peptides at any one time including, in tumor cells, neoantigens. If you extract these heat shock proteins with their bound antigens and deliver them in a naked form into the systemic circulation, their uptake into antigen-presenting cells through the CD91 receptor [will result in] the peptide [being] cleaved and presented on MHC class one and two for stimulation of CD8- and CD4-positive T cell response,” he said.
Heat shock proteins also interact with toll-like receptors and stimulate the secretion of pro-inflammatory cytokines, “acting as their own adjuvant,” Dr. Bloch further explained. Utilizing heat shock proteins activates both the innate and adaptive immune responses.
“This is an ideal platform for developing an immunotherapy for glioblastoma,” Dr. Bloch said.
In this phase II study, 46 adult patients with GBM underwent surgical resection of their tumors followed by chemoradiotherapy. At least four 25-microgram doses of vaccine were generated from tissue obtained during surgery. Within 5 weeks of completing radiotherapy, patients began receiving weekly vaccinations in combination with adjuvant temozolomide. Patients continued receiving vaccines until depletion or until tumor progression.
Median progression-free survival was 17.8 months (95% confidence interval, 11.3-21.6) and median overall survival was 23.8 months (95% CI, 19.8-30.2).
PD-L1 expression on circulating monocytes was also measured from peripheral blood samples obtained during surgery. Patients were classified as having either high PD-L1 expression (54.5% or more of monocytes) or low PD-L1 expression. Among patients classified as having high PD-L1 expression, the median overall survival was 18.0 months (95% CI, 10.0-23.3). Patients who had low PD-L1 expression had a significantly longer median overall survival time of 44.7 months with a confidence interval not calculable (hazard ratio, 3.35; 95% CI, 1.36-8.23; P = .003).
Finally, a multivariate proportional hazards model showed the MGMT methylation status and PD-L1 expression were the two greatest independent predictors of survival.
“Survival among patients who received the HSPPV-96 was greater than expected compared to historical controls... These results certainly, we feel, provide rationale for a phase III trial of vaccine plus standard of care versus standard of care alone,” Dr. Bloch said.
“PD-L1 expression on circulating myeloid cells is independently predictive of clinical response to vaccination, and it suggests that the low PD-L1 expressing population will most benefit from this anti-tumor vaccination scheme, but it also suggests that high PD-L1 expressing patients may benefit from combined checkpoint inhibition. Systemic immunosuppression driven by peripheral monocyte expression of PD-L1 is a previously unidentified factor that may mitigate vaccine efficacy,” Dr. Bloch further commented.
This study was funded by the National Cancer Institute, the National Institute of Neurological Disorders and Stroke, the National Brain Tumor Society, the American Brain Tumor Association, and Accelerated Brain Cancer Cure. Dr. Bloch reporting having no relevant disclosures.
On Twitter @jessnicolecraig
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: A heat shock protein peptide vaccine appears safe and effective for patients with glioblastoma in an early stage trial.
Major finding: Median progression-free survival was 17.8 months (95% CI, 11.3-21.6). Median overall survival was 23.8 months (95% CI, 19.8-30.2).
Data source: A phase II single arm study of 46 adult patients with glioblastoma.
Disclosures: This study was funded by the National Cancer Institute, the National Institute of Neurological Disorders and Stroke, the National Brain Tumor Society, the American Brain Tumor Association, and Accelerated Brain Cancer Cure. Dr. Bloch reporting having no relevant disclosures.
IL-2 adds only toxicity to neuroblastoma antibody tx
CHICAGO – Adding the cytokine IL-2 to front-line therapy with the anti-GD2 antibody ch14.18/CHO provided no additional survival benefit and only added to toxicity in the treatment of pediatric patients with high-risk neuroblastoma (NB), Dr. Ruth Ladenstein reported at the annual meeting of the American Society of Clinical Oncology.
A form of the antibody (dinutuximab) is approved for use in combination with granulocyte-macrophage colony stimulating factor, IL-2, and 13-cis-retinoic acid (RA) to treat high risk NB. A previous study (N Engl J Med. 2010;363:1324-34) showed that a combination of ch14.18 and the cytokines improved event free survival to 66% at 2 years, but the role of cytokines in this context remained unclear. Dr. Ladenstein and associates therefore performed a phase III trial that randomized patients to the antibody with or without subcutaneous (sc) IL-2.
High-risk NB was defined as patients with International Neuroblastoma Staging System stage 4 disease 1 year old or older, stage 4 less than 1 year old with MYCN amplification, or stage 2,3 patients up to age 21 years with MYCN amplification. Patients underwent a rapid induction therapy, followed by peripheral stem cell harvest, local control with complete tumor resection, myeloablative therapy with peripheral stem cell transplant, local control with radiotherapy, and then ch14.18 anti-GD2 monoclonal immunotherapy with RA, with or without sc IL-2.
Inclusion criteria were a complete response or partial response with three or fewer skeletal metastatic spots and no positive bone marrow biopsies on two aspirates. Randomization occurred between day 60 and 90 post stem cell infusion. RA was given on days 1-14 post randomization. For the arm receiving IL-2, it was given as 5 daily injections of 6 x 106 IU/m2 per day over 8 hours on days 15-19. IL-2 was repeated on days 22-26. Both groups also received the ch14.18 antibody on days 22-26. All patients received high-dose morphine for pain management.
For event free survival (EFS), the primary endpoint of the trial, “if we look at 3 years, we see with antibody alone it’s 57%. With IL-2, it’s 60%. It’s completely clear that there’s no superiority for the IL-2 arm,” said Dr. Ladenstein, professor of pediatrics at the Children’s Cancer Research Institute, Austria.
At 5 years, the EFS was no different for the two treatment arms, at 51% for antibody alone and 56% for antibody plus IL-2 (P = .561). There were 199/200 patients in the antibody-alone arm with follow-up after randomization and 203/206 in the antibody plus IL-2 arm. The same was true for the secondary endpoint of overall survival, with 66% survival with antibody-alone and 58% in the antibody plus IL-2 at 5 years.
The EFS for patients with a complete response prior to immunotherapy was 66% at 3 years and was 50% for patients with less than a complete response, a significant difference (P = .003) in favor of those with a complete response. IL-2 administration had no effect on the EFS of the patients with a complete response if it was given with the immunotherapy. Similarly, IL-2 made no difference for patients who had had a very good partial response or a partial response prior to immunotherapy. For complete, very good partial, or partial responses prior to immunotherapy, the overall response to immunotherapy was 51%.
“However, feasibility is a concern, particularly in the IL-2 arm. Only 61% of the cycles were completed whereas it was 85% in the antibody-only arm, and the interruptions are definitely related mainly to the IL-2 component,” Dr. Ladenstein said.
Toxicity was higher for those patients receiving IL-2 compared to those getting antibody alone: Lansky performance status of 30% or less was 41% vs. 17%, early termination of therapy was 39% vs. 15%, and Common Terminology Criteria grade 3/4 fever was 41% vs. 14%, respectively (all P less than .001). There were also significantly more grade 3/4 allergic reactions and incidences of capillary leak, as well as diarrhea, hypotension, central nervous toxicity, and pain with IL-2.
The outcomes were favorable with antibody immunotherapy alone, but the higher toxicity with IL-2 shows that “a less toxic treatment schedule therefore is needed for this late treatment phase,” Dr. Ladenstein said.
Commenting on the trial, Dr. Barbara Hero of University Children’s Hospital in Cologne, Germany, asked whether cytokines are a useful part of the regimen “because we know the cytokines add quite a lot of toxicity to the regimens.” Even if they are potentially useful, researchers still do not know which cytokines, route of administration, and at what doses and timing would be best. Also, it is not known if a different induction regimen or antibody treatment could make a difference in using cytokines.
Another question is whether cytokines may be of benefit in patients with a higher tumor burden, e.g., more than three skeletal spots, used as the eligibility cut-off in this trial, Dr. Hero said.
CHICAGO – Adding the cytokine IL-2 to front-line therapy with the anti-GD2 antibody ch14.18/CHO provided no additional survival benefit and only added to toxicity in the treatment of pediatric patients with high-risk neuroblastoma (NB), Dr. Ruth Ladenstein reported at the annual meeting of the American Society of Clinical Oncology.
A form of the antibody (dinutuximab) is approved for use in combination with granulocyte-macrophage colony stimulating factor, IL-2, and 13-cis-retinoic acid (RA) to treat high risk NB. A previous study (N Engl J Med. 2010;363:1324-34) showed that a combination of ch14.18 and the cytokines improved event free survival to 66% at 2 years, but the role of cytokines in this context remained unclear. Dr. Ladenstein and associates therefore performed a phase III trial that randomized patients to the antibody with or without subcutaneous (sc) IL-2.
High-risk NB was defined as patients with International Neuroblastoma Staging System stage 4 disease 1 year old or older, stage 4 less than 1 year old with MYCN amplification, or stage 2,3 patients up to age 21 years with MYCN amplification. Patients underwent a rapid induction therapy, followed by peripheral stem cell harvest, local control with complete tumor resection, myeloablative therapy with peripheral stem cell transplant, local control with radiotherapy, and then ch14.18 anti-GD2 monoclonal immunotherapy with RA, with or without sc IL-2.
Inclusion criteria were a complete response or partial response with three or fewer skeletal metastatic spots and no positive bone marrow biopsies on two aspirates. Randomization occurred between day 60 and 90 post stem cell infusion. RA was given on days 1-14 post randomization. For the arm receiving IL-2, it was given as 5 daily injections of 6 x 106 IU/m2 per day over 8 hours on days 15-19. IL-2 was repeated on days 22-26. Both groups also received the ch14.18 antibody on days 22-26. All patients received high-dose morphine for pain management.
For event free survival (EFS), the primary endpoint of the trial, “if we look at 3 years, we see with antibody alone it’s 57%. With IL-2, it’s 60%. It’s completely clear that there’s no superiority for the IL-2 arm,” said Dr. Ladenstein, professor of pediatrics at the Children’s Cancer Research Institute, Austria.
At 5 years, the EFS was no different for the two treatment arms, at 51% for antibody alone and 56% for antibody plus IL-2 (P = .561). There were 199/200 patients in the antibody-alone arm with follow-up after randomization and 203/206 in the antibody plus IL-2 arm. The same was true for the secondary endpoint of overall survival, with 66% survival with antibody-alone and 58% in the antibody plus IL-2 at 5 years.
The EFS for patients with a complete response prior to immunotherapy was 66% at 3 years and was 50% for patients with less than a complete response, a significant difference (P = .003) in favor of those with a complete response. IL-2 administration had no effect on the EFS of the patients with a complete response if it was given with the immunotherapy. Similarly, IL-2 made no difference for patients who had had a very good partial response or a partial response prior to immunotherapy. For complete, very good partial, or partial responses prior to immunotherapy, the overall response to immunotherapy was 51%.
“However, feasibility is a concern, particularly in the IL-2 arm. Only 61% of the cycles were completed whereas it was 85% in the antibody-only arm, and the interruptions are definitely related mainly to the IL-2 component,” Dr. Ladenstein said.
Toxicity was higher for those patients receiving IL-2 compared to those getting antibody alone: Lansky performance status of 30% or less was 41% vs. 17%, early termination of therapy was 39% vs. 15%, and Common Terminology Criteria grade 3/4 fever was 41% vs. 14%, respectively (all P less than .001). There were also significantly more grade 3/4 allergic reactions and incidences of capillary leak, as well as diarrhea, hypotension, central nervous toxicity, and pain with IL-2.
The outcomes were favorable with antibody immunotherapy alone, but the higher toxicity with IL-2 shows that “a less toxic treatment schedule therefore is needed for this late treatment phase,” Dr. Ladenstein said.
Commenting on the trial, Dr. Barbara Hero of University Children’s Hospital in Cologne, Germany, asked whether cytokines are a useful part of the regimen “because we know the cytokines add quite a lot of toxicity to the regimens.” Even if they are potentially useful, researchers still do not know which cytokines, route of administration, and at what doses and timing would be best. Also, it is not known if a different induction regimen or antibody treatment could make a difference in using cytokines.
Another question is whether cytokines may be of benefit in patients with a higher tumor burden, e.g., more than three skeletal spots, used as the eligibility cut-off in this trial, Dr. Hero said.
CHICAGO – Adding the cytokine IL-2 to front-line therapy with the anti-GD2 antibody ch14.18/CHO provided no additional survival benefit and only added to toxicity in the treatment of pediatric patients with high-risk neuroblastoma (NB), Dr. Ruth Ladenstein reported at the annual meeting of the American Society of Clinical Oncology.
A form of the antibody (dinutuximab) is approved for use in combination with granulocyte-macrophage colony stimulating factor, IL-2, and 13-cis-retinoic acid (RA) to treat high risk NB. A previous study (N Engl J Med. 2010;363:1324-34) showed that a combination of ch14.18 and the cytokines improved event free survival to 66% at 2 years, but the role of cytokines in this context remained unclear. Dr. Ladenstein and associates therefore performed a phase III trial that randomized patients to the antibody with or without subcutaneous (sc) IL-2.
High-risk NB was defined as patients with International Neuroblastoma Staging System stage 4 disease 1 year old or older, stage 4 less than 1 year old with MYCN amplification, or stage 2,3 patients up to age 21 years with MYCN amplification. Patients underwent a rapid induction therapy, followed by peripheral stem cell harvest, local control with complete tumor resection, myeloablative therapy with peripheral stem cell transplant, local control with radiotherapy, and then ch14.18 anti-GD2 monoclonal immunotherapy with RA, with or without sc IL-2.
Inclusion criteria were a complete response or partial response with three or fewer skeletal metastatic spots and no positive bone marrow biopsies on two aspirates. Randomization occurred between day 60 and 90 post stem cell infusion. RA was given on days 1-14 post randomization. For the arm receiving IL-2, it was given as 5 daily injections of 6 x 106 IU/m2 per day over 8 hours on days 15-19. IL-2 was repeated on days 22-26. Both groups also received the ch14.18 antibody on days 22-26. All patients received high-dose morphine for pain management.
For event free survival (EFS), the primary endpoint of the trial, “if we look at 3 years, we see with antibody alone it’s 57%. With IL-2, it’s 60%. It’s completely clear that there’s no superiority for the IL-2 arm,” said Dr. Ladenstein, professor of pediatrics at the Children’s Cancer Research Institute, Austria.
At 5 years, the EFS was no different for the two treatment arms, at 51% for antibody alone and 56% for antibody plus IL-2 (P = .561). There were 199/200 patients in the antibody-alone arm with follow-up after randomization and 203/206 in the antibody plus IL-2 arm. The same was true for the secondary endpoint of overall survival, with 66% survival with antibody-alone and 58% in the antibody plus IL-2 at 5 years.
The EFS for patients with a complete response prior to immunotherapy was 66% at 3 years and was 50% for patients with less than a complete response, a significant difference (P = .003) in favor of those with a complete response. IL-2 administration had no effect on the EFS of the patients with a complete response if it was given with the immunotherapy. Similarly, IL-2 made no difference for patients who had had a very good partial response or a partial response prior to immunotherapy. For complete, very good partial, or partial responses prior to immunotherapy, the overall response to immunotherapy was 51%.
“However, feasibility is a concern, particularly in the IL-2 arm. Only 61% of the cycles were completed whereas it was 85% in the antibody-only arm, and the interruptions are definitely related mainly to the IL-2 component,” Dr. Ladenstein said.
Toxicity was higher for those patients receiving IL-2 compared to those getting antibody alone: Lansky performance status of 30% or less was 41% vs. 17%, early termination of therapy was 39% vs. 15%, and Common Terminology Criteria grade 3/4 fever was 41% vs. 14%, respectively (all P less than .001). There were also significantly more grade 3/4 allergic reactions and incidences of capillary leak, as well as diarrhea, hypotension, central nervous toxicity, and pain with IL-2.
The outcomes were favorable with antibody immunotherapy alone, but the higher toxicity with IL-2 shows that “a less toxic treatment schedule therefore is needed for this late treatment phase,” Dr. Ladenstein said.
Commenting on the trial, Dr. Barbara Hero of University Children’s Hospital in Cologne, Germany, asked whether cytokines are a useful part of the regimen “because we know the cytokines add quite a lot of toxicity to the regimens.” Even if they are potentially useful, researchers still do not know which cytokines, route of administration, and at what doses and timing would be best. Also, it is not known if a different induction regimen or antibody treatment could make a difference in using cytokines.
Another question is whether cytokines may be of benefit in patients with a higher tumor burden, e.g., more than three skeletal spots, used as the eligibility cut-off in this trial, Dr. Hero said.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: IL-2 adds no benefit, only toxicity, to neuroblastoma antibody therapy.
Major finding: Only 61% of treatment cycles were completed with IL-2.
Data source: Phase III, randomized, two-arm study of 402 pediatric/adolescent neuroblastoma patients.
Disclosures: Dr. Ladenstein has received honoraria and has had a consulting or advisory role with Apeiron Biologics and Boehringer Ingelheim, and has research funding from, patents with, has provided expert testimony for, and has received travel expenses from Apeiron. Dr. Hero had no disclosures.
Early results positive for treating high-grade gliomas with virus-based therapy
An investigational virus-based therapy was safely given to patients with high-grade or recurrent gliomas in a phase I study, improving survival for some, investigators report.
In the phase I trial of Toca 511 (vocimagene amiretrorepvec) in combination with surgical resection, median overall survival was 13.6 months (95% confidence interval, 10.8-20.0) among all evaluable patients with high-grade glioblastoma (n = 43) and 14.4 months (95% CI, 11.3-32.3) for patients with first or second recurrence (n = 32), Dr. Timothy Cloughesy of the University of California, Los Angeles, and his associates reported (Sci Transl Med. 2016;8:1-11).
Investigators compared their data to those of external controls with glioblastoma at first and second recurrence treated with lomustine and saw an almost twofold improvement in overall survival (13.6 months vs. 7.1 months (hazard ratio, 0.45; P = .003).
Toca 511 dispatches a virus to rapidly dividing cancer cells, then delivers a gene encoding an enzyme that converts a nontoxic prodrug, Toca FC (extended-release 5-fluorocytosine), into its active form, 5-fluorouracil.
There were no treatment-related deaths, and there were fewer grade 3 adverse events, compared with the external lomustine control group.
“Recurrent HGG [high-grade glioblastoma] is associated with dismal clinical outcomes, and patients are in need of safe and more efficacious therapy. The nonlytic RRV [retroviral replicating vector] Toca 511 and an extended-release 5-FC [5-fluorocytosine] have the potential to fill this medical need,” the researchers said.
A randomized phase II/III trial in patients with recurrent glioblastoma and anaplastic astrocytoma is underway, they said.
This study was supported by the Accelerate Brain Cancer Cure Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musela Foundation, Voices Against Brain Cancer, and the National Institute of Neurological Disorders and Stroke. Thirteen investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies.
On Twitter @JessCraig_OP
An investigational virus-based therapy was safely given to patients with high-grade or recurrent gliomas in a phase I study, improving survival for some, investigators report.
In the phase I trial of Toca 511 (vocimagene amiretrorepvec) in combination with surgical resection, median overall survival was 13.6 months (95% confidence interval, 10.8-20.0) among all evaluable patients with high-grade glioblastoma (n = 43) and 14.4 months (95% CI, 11.3-32.3) for patients with first or second recurrence (n = 32), Dr. Timothy Cloughesy of the University of California, Los Angeles, and his associates reported (Sci Transl Med. 2016;8:1-11).
Investigators compared their data to those of external controls with glioblastoma at first and second recurrence treated with lomustine and saw an almost twofold improvement in overall survival (13.6 months vs. 7.1 months (hazard ratio, 0.45; P = .003).
Toca 511 dispatches a virus to rapidly dividing cancer cells, then delivers a gene encoding an enzyme that converts a nontoxic prodrug, Toca FC (extended-release 5-fluorocytosine), into its active form, 5-fluorouracil.
There were no treatment-related deaths, and there were fewer grade 3 adverse events, compared with the external lomustine control group.
“Recurrent HGG [high-grade glioblastoma] is associated with dismal clinical outcomes, and patients are in need of safe and more efficacious therapy. The nonlytic RRV [retroviral replicating vector] Toca 511 and an extended-release 5-FC [5-fluorocytosine] have the potential to fill this medical need,” the researchers said.
A randomized phase II/III trial in patients with recurrent glioblastoma and anaplastic astrocytoma is underway, they said.
This study was supported by the Accelerate Brain Cancer Cure Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musela Foundation, Voices Against Brain Cancer, and the National Institute of Neurological Disorders and Stroke. Thirteen investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies.
On Twitter @JessCraig_OP
An investigational virus-based therapy was safely given to patients with high-grade or recurrent gliomas in a phase I study, improving survival for some, investigators report.
In the phase I trial of Toca 511 (vocimagene amiretrorepvec) in combination with surgical resection, median overall survival was 13.6 months (95% confidence interval, 10.8-20.0) among all evaluable patients with high-grade glioblastoma (n = 43) and 14.4 months (95% CI, 11.3-32.3) for patients with first or second recurrence (n = 32), Dr. Timothy Cloughesy of the University of California, Los Angeles, and his associates reported (Sci Transl Med. 2016;8:1-11).
Investigators compared their data to those of external controls with glioblastoma at first and second recurrence treated with lomustine and saw an almost twofold improvement in overall survival (13.6 months vs. 7.1 months (hazard ratio, 0.45; P = .003).
Toca 511 dispatches a virus to rapidly dividing cancer cells, then delivers a gene encoding an enzyme that converts a nontoxic prodrug, Toca FC (extended-release 5-fluorocytosine), into its active form, 5-fluorouracil.
There were no treatment-related deaths, and there were fewer grade 3 adverse events, compared with the external lomustine control group.
“Recurrent HGG [high-grade glioblastoma] is associated with dismal clinical outcomes, and patients are in need of safe and more efficacious therapy. The nonlytic RRV [retroviral replicating vector] Toca 511 and an extended-release 5-FC [5-fluorocytosine] have the potential to fill this medical need,” the researchers said.
A randomized phase II/III trial in patients with recurrent glioblastoma and anaplastic astrocytoma is underway, they said.
This study was supported by the Accelerate Brain Cancer Cure Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musela Foundation, Voices Against Brain Cancer, and the National Institute of Neurological Disorders and Stroke. Thirteen investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies.
On Twitter @JessCraig_OP
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A phase I trial indicates that Toca 511 is safe and shows activity in treating patients with high-grade or recurrent glioblastoma.
Major finding: Median overall survival was 13.6 months (95% CI, 10.8-20.0) for patients with high-grade glioblastoma (n = 43), almost twice as long as that of similar patients from a separate trial treated with standard therapy (hazard ratio, 0.45; P = .003).
Data source: A phase I trial of 45 patients with glioblastoma, compared with an external control group.
Disclosures: This study was supported by the Accelerate Brain Cancer Cure Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musela Foundation, Voices Against Brain Cancer, and the National Institute of Neurological Disorders and Stroke. Thirteen investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies.
Proactive endocrine screening urged for pediatric brain tumor survivors
BOSTON – More than a third of 419 children treated for brain tumors at Cincinnati Children’s Hospital Medical Center later developed endocrine problems, according to a review presented at the Endocrine Society annual meeting.
Over 60% of the 96 suprasellar tumor patients developed endocrine dysfunction, which isn’t surprising considering the location of the tumor, but wide-ranging endocrine problems were also common in the 145 posterior fossa, 158 supratentorial, and 20 spinal cord cases, ranging from 14% in the spinal cord group to 42% in the posterior fossa group, after some combination of radiation, chemotherapy, and surgery based on tumor location and other factors.
“Even with tumors that aren’t supposed to be high risk, there was a high risk of endocrinopathies. We need yearly screening of these patients” for about 6 years, after which symptom-based screening may be sufficient. The clock should be restarted if there’s a recurrence. “Not everyone does this” at Cincinnati Children’s and probably most other institutions, said investigator and endocrinology fellow Dr. Vincent Horne.
The findings are “changing how our oncology department is thinking about [screening]; there’s a concentrated effort to increase proactive screening and follow these patients long term,” he said.
“Even within our specialized, multidisciplinary center,” endocrinopathy screening referrals were low, about 61% overall and only 80% in the suprasellar group. “Patients at highest risk” – those with craniopharyngioma – “are being seen early by us,” but others aren’t being referred. It’s possible that the extent of endocrine problems after pediatric brain tumor treatment is simply unrecognized, he said.
Endocrine abnormalities were found in 114 (45%) of the 254 patients evaluated, which translated to problems in more than a third of all patients.
More than half of the children had more than one problem, and most of the issues occurred within 6 years of treatment. Central hypothyroidism was found in 53% of the children, probably because Cincinnati Children’s already has thyroid screening in place.
About 40% were growth hormone deficient, and almost a third had precocious puberty. About 30% were gonadotropin-releasing hormone deficient, over 20% had primary hypothyroidism, and about the same had diabetes insipidus. Just over 6% were hyperprolactinemic.
Of the 151 patients who completed adrenocorticotropic hormone (ACTH) testing, 14.6% were deficient. ACTH deficient children were about evenly split between the suprasellar and supratentorial groups, with the remaining in the posterior fossa cohort.
“We are probably not thinking about” the risk of radiation “to locations like the posterior fossa. That group actually had the highest risk of primary hypothyroidism [20%] because of the spinal radiation. The supratentorial group is also receiving radiation; even though we think we are missing the hypothalamus, obviously that’s not necessarily the case,” Dr. Horne said.
His team looked into endocrine screening because previous studies “were limited and done years ago.” People are living longer now after treatment, “so we need to think about how to screen for endocrine disease. This is an attempt to clarify how we should do it,” he said.
Children were a median of 8 years old at diagnosis, and the median radiation dose was 54 Gy.
There was no industry funding for the work, and the investigators had no disclosures.
BOSTON – More than a third of 419 children treated for brain tumors at Cincinnati Children’s Hospital Medical Center later developed endocrine problems, according to a review presented at the Endocrine Society annual meeting.
Over 60% of the 96 suprasellar tumor patients developed endocrine dysfunction, which isn’t surprising considering the location of the tumor, but wide-ranging endocrine problems were also common in the 145 posterior fossa, 158 supratentorial, and 20 spinal cord cases, ranging from 14% in the spinal cord group to 42% in the posterior fossa group, after some combination of radiation, chemotherapy, and surgery based on tumor location and other factors.
“Even with tumors that aren’t supposed to be high risk, there was a high risk of endocrinopathies. We need yearly screening of these patients” for about 6 years, after which symptom-based screening may be sufficient. The clock should be restarted if there’s a recurrence. “Not everyone does this” at Cincinnati Children’s and probably most other institutions, said investigator and endocrinology fellow Dr. Vincent Horne.
The findings are “changing how our oncology department is thinking about [screening]; there’s a concentrated effort to increase proactive screening and follow these patients long term,” he said.
“Even within our specialized, multidisciplinary center,” endocrinopathy screening referrals were low, about 61% overall and only 80% in the suprasellar group. “Patients at highest risk” – those with craniopharyngioma – “are being seen early by us,” but others aren’t being referred. It’s possible that the extent of endocrine problems after pediatric brain tumor treatment is simply unrecognized, he said.
Endocrine abnormalities were found in 114 (45%) of the 254 patients evaluated, which translated to problems in more than a third of all patients.
More than half of the children had more than one problem, and most of the issues occurred within 6 years of treatment. Central hypothyroidism was found in 53% of the children, probably because Cincinnati Children’s already has thyroid screening in place.
About 40% were growth hormone deficient, and almost a third had precocious puberty. About 30% were gonadotropin-releasing hormone deficient, over 20% had primary hypothyroidism, and about the same had diabetes insipidus. Just over 6% were hyperprolactinemic.
Of the 151 patients who completed adrenocorticotropic hormone (ACTH) testing, 14.6% were deficient. ACTH deficient children were about evenly split between the suprasellar and supratentorial groups, with the remaining in the posterior fossa cohort.
“We are probably not thinking about” the risk of radiation “to locations like the posterior fossa. That group actually had the highest risk of primary hypothyroidism [20%] because of the spinal radiation. The supratentorial group is also receiving radiation; even though we think we are missing the hypothalamus, obviously that’s not necessarily the case,” Dr. Horne said.
His team looked into endocrine screening because previous studies “were limited and done years ago.” People are living longer now after treatment, “so we need to think about how to screen for endocrine disease. This is an attempt to clarify how we should do it,” he said.
Children were a median of 8 years old at diagnosis, and the median radiation dose was 54 Gy.
There was no industry funding for the work, and the investigators had no disclosures.
BOSTON – More than a third of 419 children treated for brain tumors at Cincinnati Children’s Hospital Medical Center later developed endocrine problems, according to a review presented at the Endocrine Society annual meeting.
Over 60% of the 96 suprasellar tumor patients developed endocrine dysfunction, which isn’t surprising considering the location of the tumor, but wide-ranging endocrine problems were also common in the 145 posterior fossa, 158 supratentorial, and 20 spinal cord cases, ranging from 14% in the spinal cord group to 42% in the posterior fossa group, after some combination of radiation, chemotherapy, and surgery based on tumor location and other factors.
“Even with tumors that aren’t supposed to be high risk, there was a high risk of endocrinopathies. We need yearly screening of these patients” for about 6 years, after which symptom-based screening may be sufficient. The clock should be restarted if there’s a recurrence. “Not everyone does this” at Cincinnati Children’s and probably most other institutions, said investigator and endocrinology fellow Dr. Vincent Horne.
The findings are “changing how our oncology department is thinking about [screening]; there’s a concentrated effort to increase proactive screening and follow these patients long term,” he said.
“Even within our specialized, multidisciplinary center,” endocrinopathy screening referrals were low, about 61% overall and only 80% in the suprasellar group. “Patients at highest risk” – those with craniopharyngioma – “are being seen early by us,” but others aren’t being referred. It’s possible that the extent of endocrine problems after pediatric brain tumor treatment is simply unrecognized, he said.
Endocrine abnormalities were found in 114 (45%) of the 254 patients evaluated, which translated to problems in more than a third of all patients.
More than half of the children had more than one problem, and most of the issues occurred within 6 years of treatment. Central hypothyroidism was found in 53% of the children, probably because Cincinnati Children’s already has thyroid screening in place.
About 40% were growth hormone deficient, and almost a third had precocious puberty. About 30% were gonadotropin-releasing hormone deficient, over 20% had primary hypothyroidism, and about the same had diabetes insipidus. Just over 6% were hyperprolactinemic.
Of the 151 patients who completed adrenocorticotropic hormone (ACTH) testing, 14.6% were deficient. ACTH deficient children were about evenly split between the suprasellar and supratentorial groups, with the remaining in the posterior fossa cohort.
“We are probably not thinking about” the risk of radiation “to locations like the posterior fossa. That group actually had the highest risk of primary hypothyroidism [20%] because of the spinal radiation. The supratentorial group is also receiving radiation; even though we think we are missing the hypothalamus, obviously that’s not necessarily the case,” Dr. Horne said.
His team looked into endocrine screening because previous studies “were limited and done years ago.” People are living longer now after treatment, “so we need to think about how to screen for endocrine disease. This is an attempt to clarify how we should do it,” he said.
Children were a median of 8 years old at diagnosis, and the median radiation dose was 54 Gy.
There was no industry funding for the work, and the investigators had no disclosures.
AT ENDO 2016
Key clinical point: Screen for endocrine dysfunction for 6 years after pediatric brain tumor treatment.
Major finding: Endocrine abnormalities were found in 114 of 254 patients (45%) evaluated, which translated to problems in more than a third of all patients.
Data source: Single-center review of 419 pediatric brain tumor cases.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
Adding chemo to radiation boosts survival from low-grade gliomas
Adding a chemotherapy combination to radiation therapy for initial treatment of low-grade gliomas significantly improved overall survival and progression-free survival, regardless of the tumor type, investigators report in the New England Journal of Medicine.
Grade 2 glioma patients who received radiation plus the combination of procarbazine, lomustine (also called CCNU), and vincristine (PCV) had a longer median overall survival than those who received radiation alone (13.3 vs. 7.8 years; hazard ratio for death, 0.59; P = .003). Of those who received radiation plus chemotherapy, the progression-free survival rate at 10 years was 51%, compared with 21% for the group who received radiation alone, Dr. Jan Buckner and his colleagues report (N Engl J Med. 2016;374:1344-55. doi: 10.1056/NEJMoa1500925).
“The magnitude of treatment benefit from combined chemotherapy plus radiation therapy is substantial, but the toxic effects are greater than those observed with radiation therapy alone,” wrote Dr. Buckner, professor of oncology at the Mayo Clinic, Rochester, Minn., and associates. Patients who received radiation plus PCV had more toxic side effects from their therapy, though most effects were grade 1 and 2.
For this study, 254 patients were randomized, with 128 assigned to radiotherapy alone, and 126 assigned to radiotherapy plus PCV. A total of 126 patients in the radiotherapy arm were included in the analysis, with one patient not receiving the intervention and 14 patients lost to follow-up at some point during the 10 years of the study. In the radiotherapy plus PCV arm, 125 patients were eligible for evaluation, and all of those patients were included in the analysis. Twenty-six patients in this arm were lost to follow-up, and 72 patients discontinued the intervention in this arm (this figure included four patients who died).
Tumor types included in the study were grade 2 astrocytoma, oligoastrocytoma, or oligodendroglioma.
Patients were included if they were between 18 and 39 years of age and had received a subtotal resection or biopsy of their tumor, or if they were 40 years of age or older and had any resection or biopsy of their tumor. Exclusion criteria included previous radiation to the head or neck, any previous chemotherapy, significant pulmonary disease, and a 5-year history of other cancers except cervical cancer in situ and non-melanoma skin cancer. Tumors could not have spread to noncontiguous leptomeninges, and patients could not have gliomatosis cerebri. Patients had to have a Karnofsky performance score of 60 or higher, and a neurological function score of 3 or less.
Dr. Buckner and his collaborators also assessed tumors for IDH1 mutational status by performing immunostaining with the mutation-specific monoclonal antibody IDH1 R132H; appropriate tissue was available for testing in slightly less than half of the patients in each study arm. The mutation was present in 35/57 patients (61%) in the radiotherapy-only arm, and 36/56 patients (64%) in the radiotherapy plus PCV arm. Patients with oligodendroglioma were most likely to have IDH1 R132H mutations. Sample sizes were too small to determine the effect of other IDH mutations or co-deletion of chromosome arms 1p and 19q.
In multivariable analysis, the presence of the IDH1 R132H mutation was identified as an independent prognostic factor for better overall survival (OS) and progression-free survival (PFS), regardless of the treatment administered. Those with the mutation still benefited significantly from receiving radiotherapy plus PCV rather than radiotherapy alone (P = .02 for OS, P less than .001 for PFS).
Exploratory analysis that broke down OS and PFS by cancer type showed that “the superiority of radiation therapy plus chemotherapy over radiation therapy alone was seen with all histologic diagnoses, although the difference did not reach significance among patients with astrocytoma,” wrote Dr. Buckner and his collaborators.
When all patients lost to follow-up in both groups were assessed as having died, the sensitivity analysis still showed benefit for radiotherapy plus PCV (HR for death, compared with radiotherapy alone, 0.72; P = .03).
The value of the long-term follow-up, wrote Dr. Buckner and his colleagues, was that “The separation of the progression-free survival curves of the two treatment groups did not begin until 2 to 3 years after randomization, although approximately 25% of the patients in each group had disease progression by then.”
Dr. Buckner and his collaborators emphasized that the patient-physician team should consider all factors in making treatment decisions, saying, “Patients and their physicians will have to weigh whether the longer survival justified the more toxic therapeutic approach.“
On Twitter @karioakes
Adding a chemotherapy combination to radiation therapy for initial treatment of low-grade gliomas significantly improved overall survival and progression-free survival, regardless of the tumor type, investigators report in the New England Journal of Medicine.
Grade 2 glioma patients who received radiation plus the combination of procarbazine, lomustine (also called CCNU), and vincristine (PCV) had a longer median overall survival than those who received radiation alone (13.3 vs. 7.8 years; hazard ratio for death, 0.59; P = .003). Of those who received radiation plus chemotherapy, the progression-free survival rate at 10 years was 51%, compared with 21% for the group who received radiation alone, Dr. Jan Buckner and his colleagues report (N Engl J Med. 2016;374:1344-55. doi: 10.1056/NEJMoa1500925).
“The magnitude of treatment benefit from combined chemotherapy plus radiation therapy is substantial, but the toxic effects are greater than those observed with radiation therapy alone,” wrote Dr. Buckner, professor of oncology at the Mayo Clinic, Rochester, Minn., and associates. Patients who received radiation plus PCV had more toxic side effects from their therapy, though most effects were grade 1 and 2.
For this study, 254 patients were randomized, with 128 assigned to radiotherapy alone, and 126 assigned to radiotherapy plus PCV. A total of 126 patients in the radiotherapy arm were included in the analysis, with one patient not receiving the intervention and 14 patients lost to follow-up at some point during the 10 years of the study. In the radiotherapy plus PCV arm, 125 patients were eligible for evaluation, and all of those patients were included in the analysis. Twenty-six patients in this arm were lost to follow-up, and 72 patients discontinued the intervention in this arm (this figure included four patients who died).
Tumor types included in the study were grade 2 astrocytoma, oligoastrocytoma, or oligodendroglioma.
Patients were included if they were between 18 and 39 years of age and had received a subtotal resection or biopsy of their tumor, or if they were 40 years of age or older and had any resection or biopsy of their tumor. Exclusion criteria included previous radiation to the head or neck, any previous chemotherapy, significant pulmonary disease, and a 5-year history of other cancers except cervical cancer in situ and non-melanoma skin cancer. Tumors could not have spread to noncontiguous leptomeninges, and patients could not have gliomatosis cerebri. Patients had to have a Karnofsky performance score of 60 or higher, and a neurological function score of 3 or less.
Dr. Buckner and his collaborators also assessed tumors for IDH1 mutational status by performing immunostaining with the mutation-specific monoclonal antibody IDH1 R132H; appropriate tissue was available for testing in slightly less than half of the patients in each study arm. The mutation was present in 35/57 patients (61%) in the radiotherapy-only arm, and 36/56 patients (64%) in the radiotherapy plus PCV arm. Patients with oligodendroglioma were most likely to have IDH1 R132H mutations. Sample sizes were too small to determine the effect of other IDH mutations or co-deletion of chromosome arms 1p and 19q.
In multivariable analysis, the presence of the IDH1 R132H mutation was identified as an independent prognostic factor for better overall survival (OS) and progression-free survival (PFS), regardless of the treatment administered. Those with the mutation still benefited significantly from receiving radiotherapy plus PCV rather than radiotherapy alone (P = .02 for OS, P less than .001 for PFS).
Exploratory analysis that broke down OS and PFS by cancer type showed that “the superiority of radiation therapy plus chemotherapy over radiation therapy alone was seen with all histologic diagnoses, although the difference did not reach significance among patients with astrocytoma,” wrote Dr. Buckner and his collaborators.
When all patients lost to follow-up in both groups were assessed as having died, the sensitivity analysis still showed benefit for radiotherapy plus PCV (HR for death, compared with radiotherapy alone, 0.72; P = .03).
The value of the long-term follow-up, wrote Dr. Buckner and his colleagues, was that “The separation of the progression-free survival curves of the two treatment groups did not begin until 2 to 3 years after randomization, although approximately 25% of the patients in each group had disease progression by then.”
Dr. Buckner and his collaborators emphasized that the patient-physician team should consider all factors in making treatment decisions, saying, “Patients and their physicians will have to weigh whether the longer survival justified the more toxic therapeutic approach.“
On Twitter @karioakes
Adding a chemotherapy combination to radiation therapy for initial treatment of low-grade gliomas significantly improved overall survival and progression-free survival, regardless of the tumor type, investigators report in the New England Journal of Medicine.
Grade 2 glioma patients who received radiation plus the combination of procarbazine, lomustine (also called CCNU), and vincristine (PCV) had a longer median overall survival than those who received radiation alone (13.3 vs. 7.8 years; hazard ratio for death, 0.59; P = .003). Of those who received radiation plus chemotherapy, the progression-free survival rate at 10 years was 51%, compared with 21% for the group who received radiation alone, Dr. Jan Buckner and his colleagues report (N Engl J Med. 2016;374:1344-55. doi: 10.1056/NEJMoa1500925).
“The magnitude of treatment benefit from combined chemotherapy plus radiation therapy is substantial, but the toxic effects are greater than those observed with radiation therapy alone,” wrote Dr. Buckner, professor of oncology at the Mayo Clinic, Rochester, Minn., and associates. Patients who received radiation plus PCV had more toxic side effects from their therapy, though most effects were grade 1 and 2.
For this study, 254 patients were randomized, with 128 assigned to radiotherapy alone, and 126 assigned to radiotherapy plus PCV. A total of 126 patients in the radiotherapy arm were included in the analysis, with one patient not receiving the intervention and 14 patients lost to follow-up at some point during the 10 years of the study. In the radiotherapy plus PCV arm, 125 patients were eligible for evaluation, and all of those patients were included in the analysis. Twenty-six patients in this arm were lost to follow-up, and 72 patients discontinued the intervention in this arm (this figure included four patients who died).
Tumor types included in the study were grade 2 astrocytoma, oligoastrocytoma, or oligodendroglioma.
Patients were included if they were between 18 and 39 years of age and had received a subtotal resection or biopsy of their tumor, or if they were 40 years of age or older and had any resection or biopsy of their tumor. Exclusion criteria included previous radiation to the head or neck, any previous chemotherapy, significant pulmonary disease, and a 5-year history of other cancers except cervical cancer in situ and non-melanoma skin cancer. Tumors could not have spread to noncontiguous leptomeninges, and patients could not have gliomatosis cerebri. Patients had to have a Karnofsky performance score of 60 or higher, and a neurological function score of 3 or less.
Dr. Buckner and his collaborators also assessed tumors for IDH1 mutational status by performing immunostaining with the mutation-specific monoclonal antibody IDH1 R132H; appropriate tissue was available for testing in slightly less than half of the patients in each study arm. The mutation was present in 35/57 patients (61%) in the radiotherapy-only arm, and 36/56 patients (64%) in the radiotherapy plus PCV arm. Patients with oligodendroglioma were most likely to have IDH1 R132H mutations. Sample sizes were too small to determine the effect of other IDH mutations or co-deletion of chromosome arms 1p and 19q.
In multivariable analysis, the presence of the IDH1 R132H mutation was identified as an independent prognostic factor for better overall survival (OS) and progression-free survival (PFS), regardless of the treatment administered. Those with the mutation still benefited significantly from receiving radiotherapy plus PCV rather than radiotherapy alone (P = .02 for OS, P less than .001 for PFS).
Exploratory analysis that broke down OS and PFS by cancer type showed that “the superiority of radiation therapy plus chemotherapy over radiation therapy alone was seen with all histologic diagnoses, although the difference did not reach significance among patients with astrocytoma,” wrote Dr. Buckner and his collaborators.
When all patients lost to follow-up in both groups were assessed as having died, the sensitivity analysis still showed benefit for radiotherapy plus PCV (HR for death, compared with radiotherapy alone, 0.72; P = .03).
The value of the long-term follow-up, wrote Dr. Buckner and his colleagues, was that “The separation of the progression-free survival curves of the two treatment groups did not begin until 2 to 3 years after randomization, although approximately 25% of the patients in each group had disease progression by then.”
Dr. Buckner and his collaborators emphasized that the patient-physician team should consider all factors in making treatment decisions, saying, “Patients and their physicians will have to weigh whether the longer survival justified the more toxic therapeutic approach.“
On Twitter @karioakes
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adding chemotherapy to radiotherapy improved progression-free survival and overall survival in patients with low-grade glioma.
Major finding: Median overall survival was 13.3 years for those receiving radiotherapy plus chemotherapy, compared with 7.8 years for radiotherapy alone (hazard ratio for death, 0.59; P = .003).
Data source: Longitudinal study of 254 patients with grade 2 gliomas receiving radiation therapy alone or radiation therapy plus procarbazine, lomustine, and vincristine.
Disclosures: The study was supported by the National Cancer Institute and did not receive funding from commercial sources. Dr. Bell and Dr. Chakravarti reported a planned patent application related to this work. Dr. Buckner, Dr. Gilbert, Dr. Mehta, and Dr. Suh reported support from pharmaceutical companies outside the scope of this study.
New CDC opioid guideline targets overprescribing for chronic pain
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Temsirolimus results in good but short-duration responses in primary CNS lymphoma
Single-agent therapy with temsirolimus was active in patients with relapsed/refractory primary central nervous system lymphoma, but most of the responses were short lived, results of a phase II trial show.
Among 37 patients with primary CNS lymphoma (PCNSL) for whom firstline therapy had failed, there were five complete responses (CR), three CR unconfirmed, and 12 partial responses (PR), for an overall response rate (ORR) of 54%, reported Dr. Agnieszka Korfel from Charité University Medicine Berlin (Germany) and colleagues.
The median progression-free survival (PFS), however, was just 2.1 months, although 1 patient had PFS of 15.8 months duration, and another had a response lasting for more than 44 months, the investigators noted in a study published online in the Journal of Clinical Oncology (doi: 10.1200/JCO.2015.64.9897).
The rationale for trying temsirolimus (Torisel), an inhibitor of the mammalian target of rapamycin (mTOR), came from studies showing the drug’s efficacy against relapsed/refractory mantle-cell lymphoma and against other, more aggressive forms of non-Hodgkin lymphoma. Patients with relapsed/refractory aggressive lymphomas tolerate temsirolimus relatively well, and the drug has the ability to penetrate brain tumor tissue, the authors noted.
They enrolled 37 patients with a median age of 70 years and a median time since their last treatment of 3.9 months into an open-label trial. The patients were all immuncompetent with histologically confirmed primary central nervous system lymphoma for whom high-dose methotrexate-based chemotherapy had failed and for whom high-dose chemotherapy with autologous stem cell transplant had either failed or was not an option.
The first six patients were treated with temsirolimus 25 mg intravenously once weekly, and the remaining 31 were treated with 75 mg IV once weekly until disease progression, intolerable toxicity, patient or physician decision to terminate, or death.
As noted before, ORR, the primary endpoint, was 54%. Median overall survival (OS), a secondary endpoint, was 3.7 months, and 1-year and 2-year OS were 19% and 16.2%, respectively.
The most frequently occurring toxicities include hyperglycemia, myelosuppression, pneumonias and other infections, and fatigue. A total of 28 severe adverse events occurred in 21 patients, including infectious episodes, hospitalizations because of disease progression, deep-vein thromboses, hyperglycemia, and one case each of seizures, grade 4 thrombocytopenia, drug fever, hyponatremia, renal insufficiency, and atrial fibrillation.
“Although most responses were short lived, some patients achieved long-term control. Thus, further evaluation in combination with other drugs seems reasonable. However, one has to be aware of the risk of hematotoxicity and infections necessitating primary antibiotic prophylaxis. Definition of biomarkers allowing identification of potential responders and those who are at particular risk for toxicity would be highly desirable,” the investigators concluded.
Single-agent therapy with temsirolimus was active in patients with relapsed/refractory primary central nervous system lymphoma, but most of the responses were short lived, results of a phase II trial show.
Among 37 patients with primary CNS lymphoma (PCNSL) for whom firstline therapy had failed, there were five complete responses (CR), three CR unconfirmed, and 12 partial responses (PR), for an overall response rate (ORR) of 54%, reported Dr. Agnieszka Korfel from Charité University Medicine Berlin (Germany) and colleagues.
The median progression-free survival (PFS), however, was just 2.1 months, although 1 patient had PFS of 15.8 months duration, and another had a response lasting for more than 44 months, the investigators noted in a study published online in the Journal of Clinical Oncology (doi: 10.1200/JCO.2015.64.9897).
The rationale for trying temsirolimus (Torisel), an inhibitor of the mammalian target of rapamycin (mTOR), came from studies showing the drug’s efficacy against relapsed/refractory mantle-cell lymphoma and against other, more aggressive forms of non-Hodgkin lymphoma. Patients with relapsed/refractory aggressive lymphomas tolerate temsirolimus relatively well, and the drug has the ability to penetrate brain tumor tissue, the authors noted.
They enrolled 37 patients with a median age of 70 years and a median time since their last treatment of 3.9 months into an open-label trial. The patients were all immuncompetent with histologically confirmed primary central nervous system lymphoma for whom high-dose methotrexate-based chemotherapy had failed and for whom high-dose chemotherapy with autologous stem cell transplant had either failed or was not an option.
The first six patients were treated with temsirolimus 25 mg intravenously once weekly, and the remaining 31 were treated with 75 mg IV once weekly until disease progression, intolerable toxicity, patient or physician decision to terminate, or death.
As noted before, ORR, the primary endpoint, was 54%. Median overall survival (OS), a secondary endpoint, was 3.7 months, and 1-year and 2-year OS were 19% and 16.2%, respectively.
The most frequently occurring toxicities include hyperglycemia, myelosuppression, pneumonias and other infections, and fatigue. A total of 28 severe adverse events occurred in 21 patients, including infectious episodes, hospitalizations because of disease progression, deep-vein thromboses, hyperglycemia, and one case each of seizures, grade 4 thrombocytopenia, drug fever, hyponatremia, renal insufficiency, and atrial fibrillation.
“Although most responses were short lived, some patients achieved long-term control. Thus, further evaluation in combination with other drugs seems reasonable. However, one has to be aware of the risk of hematotoxicity and infections necessitating primary antibiotic prophylaxis. Definition of biomarkers allowing identification of potential responders and those who are at particular risk for toxicity would be highly desirable,” the investigators concluded.
Single-agent therapy with temsirolimus was active in patients with relapsed/refractory primary central nervous system lymphoma, but most of the responses were short lived, results of a phase II trial show.
Among 37 patients with primary CNS lymphoma (PCNSL) for whom firstline therapy had failed, there were five complete responses (CR), three CR unconfirmed, and 12 partial responses (PR), for an overall response rate (ORR) of 54%, reported Dr. Agnieszka Korfel from Charité University Medicine Berlin (Germany) and colleagues.
The median progression-free survival (PFS), however, was just 2.1 months, although 1 patient had PFS of 15.8 months duration, and another had a response lasting for more than 44 months, the investigators noted in a study published online in the Journal of Clinical Oncology (doi: 10.1200/JCO.2015.64.9897).
The rationale for trying temsirolimus (Torisel), an inhibitor of the mammalian target of rapamycin (mTOR), came from studies showing the drug’s efficacy against relapsed/refractory mantle-cell lymphoma and against other, more aggressive forms of non-Hodgkin lymphoma. Patients with relapsed/refractory aggressive lymphomas tolerate temsirolimus relatively well, and the drug has the ability to penetrate brain tumor tissue, the authors noted.
They enrolled 37 patients with a median age of 70 years and a median time since their last treatment of 3.9 months into an open-label trial. The patients were all immuncompetent with histologically confirmed primary central nervous system lymphoma for whom high-dose methotrexate-based chemotherapy had failed and for whom high-dose chemotherapy with autologous stem cell transplant had either failed or was not an option.
The first six patients were treated with temsirolimus 25 mg intravenously once weekly, and the remaining 31 were treated with 75 mg IV once weekly until disease progression, intolerable toxicity, patient or physician decision to terminate, or death.
As noted before, ORR, the primary endpoint, was 54%. Median overall survival (OS), a secondary endpoint, was 3.7 months, and 1-year and 2-year OS were 19% and 16.2%, respectively.
The most frequently occurring toxicities include hyperglycemia, myelosuppression, pneumonias and other infections, and fatigue. A total of 28 severe adverse events occurred in 21 patients, including infectious episodes, hospitalizations because of disease progression, deep-vein thromboses, hyperglycemia, and one case each of seizures, grade 4 thrombocytopenia, drug fever, hyponatremia, renal insufficiency, and atrial fibrillation.
“Although most responses were short lived, some patients achieved long-term control. Thus, further evaluation in combination with other drugs seems reasonable. However, one has to be aware of the risk of hematotoxicity and infections necessitating primary antibiotic prophylaxis. Definition of biomarkers allowing identification of potential responders and those who are at particular risk for toxicity would be highly desirable,” the investigators concluded.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Relapsed/refractory primary CNS lymphoma has a poor prognosis and no standard treatment option.
Major finding: The overall response rate to once-weekly temsirolimus was 54%; most responses were short lived.
Data source: Open-label phase 2 study in 37 adults with relapsed/refractory primary CNS lymphoma.
Disclosures: The study was supported by Pfizer Germany. Dr. Korfel and several colleagues disclosed research support from or consulting/advising for the company.