User login
Microplastics Have Been Found in the Human Brain. Now What?
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six Updates on Stroke Management
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
Three Vascular Risk Factors May Up Severe Stroke Risk
TOPLINE:
, a global study shows.
METHODOLOGY:
- The INTERSTROKE case-control study included nearly 27,000 participants, half of whom had a first acute stroke (ischemic or hemorrhagic) and the other half acting as age- and sex-matched controls.
- Participants (mean age, 62 years; 40% women) were recruited across 142 centers in 32 countries between 2007 and 2015. Baseline demographics and lifestyle risk factors for stroke were gathered using standardized questionnaires
- Modified Rankin Scale (mRS) scores measured within 72 hours of hospital admission were used to classify stroke severity (0-3, nonsevere stroke; 4-6, severe stroke).
TAKEAWAY:
- Among the participants with acute stroke, 64% had nonsevere stroke and 36% had severe stroke, based on the mRS.
- Hypertension, atrial fibrillation, and smoking showed a significantly stronger association with severe stroke than with nonsevere stroke (odds ratios [ORs], 3.21 vs 2.87, 4.70 vs 3.61, and 1.87 vs 1.65, respectively; all P < .001).
- A high waist-to-hip ratio showed a stronger association with nonsevere stroke than with severe stroke (OR, 1.37 vs 1.11, respectively; P < .001).
- Diabetes, poor diet, physical inactivity, and stress were linked to increased odds of both severe and nonsevere stroke, whereas alcohol consumption and high apolipoprotein B levels were linked to higher odds of only nonsevere stroke. No significant differences in odds were observed between stroke severities in matched individuals.
IN PRACTICE:
“Our findings emphasize the importance of controlling high blood pressure, which is the most important modifiable risk factor for stroke globally,” lead author Catriona Reddin, MB BCh, BAO, MSc, School of Medicine, University of Galway, in Ireland, said in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
The study limitations included potential unmeasured confounders; reliance on the mRS score, which may have underestimated stroke severity; and challenges with recruiting patients with severe stroke in a case-control study. Smoking-related comorbidities and regional or sex-related variations in alcohol intake may also have influenced the results.
DISCLOSURES:
The study was funded by various organizations, including health research councils and foundations from Canada, Sweden, and Scotland, and pharmaceutical companies such as AstraZeneca, Boehringer Ingelheim, Pfizer, and MSD. One investigator reported receiving funding from the Irish Clinical Academic Training Programme, the Wellcome Trust and the Health Research Board, the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division in Northern Ireland. No other conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, a global study shows.
METHODOLOGY:
- The INTERSTROKE case-control study included nearly 27,000 participants, half of whom had a first acute stroke (ischemic or hemorrhagic) and the other half acting as age- and sex-matched controls.
- Participants (mean age, 62 years; 40% women) were recruited across 142 centers in 32 countries between 2007 and 2015. Baseline demographics and lifestyle risk factors for stroke were gathered using standardized questionnaires
- Modified Rankin Scale (mRS) scores measured within 72 hours of hospital admission were used to classify stroke severity (0-3, nonsevere stroke; 4-6, severe stroke).
TAKEAWAY:
- Among the participants with acute stroke, 64% had nonsevere stroke and 36% had severe stroke, based on the mRS.
- Hypertension, atrial fibrillation, and smoking showed a significantly stronger association with severe stroke than with nonsevere stroke (odds ratios [ORs], 3.21 vs 2.87, 4.70 vs 3.61, and 1.87 vs 1.65, respectively; all P < .001).
- A high waist-to-hip ratio showed a stronger association with nonsevere stroke than with severe stroke (OR, 1.37 vs 1.11, respectively; P < .001).
- Diabetes, poor diet, physical inactivity, and stress were linked to increased odds of both severe and nonsevere stroke, whereas alcohol consumption and high apolipoprotein B levels were linked to higher odds of only nonsevere stroke. No significant differences in odds were observed between stroke severities in matched individuals.
IN PRACTICE:
“Our findings emphasize the importance of controlling high blood pressure, which is the most important modifiable risk factor for stroke globally,” lead author Catriona Reddin, MB BCh, BAO, MSc, School of Medicine, University of Galway, in Ireland, said in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
The study limitations included potential unmeasured confounders; reliance on the mRS score, which may have underestimated stroke severity; and challenges with recruiting patients with severe stroke in a case-control study. Smoking-related comorbidities and regional or sex-related variations in alcohol intake may also have influenced the results.
DISCLOSURES:
The study was funded by various organizations, including health research councils and foundations from Canada, Sweden, and Scotland, and pharmaceutical companies such as AstraZeneca, Boehringer Ingelheim, Pfizer, and MSD. One investigator reported receiving funding from the Irish Clinical Academic Training Programme, the Wellcome Trust and the Health Research Board, the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division in Northern Ireland. No other conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, a global study shows.
METHODOLOGY:
- The INTERSTROKE case-control study included nearly 27,000 participants, half of whom had a first acute stroke (ischemic or hemorrhagic) and the other half acting as age- and sex-matched controls.
- Participants (mean age, 62 years; 40% women) were recruited across 142 centers in 32 countries between 2007 and 2015. Baseline demographics and lifestyle risk factors for stroke were gathered using standardized questionnaires
- Modified Rankin Scale (mRS) scores measured within 72 hours of hospital admission were used to classify stroke severity (0-3, nonsevere stroke; 4-6, severe stroke).
TAKEAWAY:
- Among the participants with acute stroke, 64% had nonsevere stroke and 36% had severe stroke, based on the mRS.
- Hypertension, atrial fibrillation, and smoking showed a significantly stronger association with severe stroke than with nonsevere stroke (odds ratios [ORs], 3.21 vs 2.87, 4.70 vs 3.61, and 1.87 vs 1.65, respectively; all P < .001).
- A high waist-to-hip ratio showed a stronger association with nonsevere stroke than with severe stroke (OR, 1.37 vs 1.11, respectively; P < .001).
- Diabetes, poor diet, physical inactivity, and stress were linked to increased odds of both severe and nonsevere stroke, whereas alcohol consumption and high apolipoprotein B levels were linked to higher odds of only nonsevere stroke. No significant differences in odds were observed between stroke severities in matched individuals.
IN PRACTICE:
“Our findings emphasize the importance of controlling high blood pressure, which is the most important modifiable risk factor for stroke globally,” lead author Catriona Reddin, MB BCh, BAO, MSc, School of Medicine, University of Galway, in Ireland, said in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
The study limitations included potential unmeasured confounders; reliance on the mRS score, which may have underestimated stroke severity; and challenges with recruiting patients with severe stroke in a case-control study. Smoking-related comorbidities and regional or sex-related variations in alcohol intake may also have influenced the results.
DISCLOSURES:
The study was funded by various organizations, including health research councils and foundations from Canada, Sweden, and Scotland, and pharmaceutical companies such as AstraZeneca, Boehringer Ingelheim, Pfizer, and MSD. One investigator reported receiving funding from the Irish Clinical Academic Training Programme, the Wellcome Trust and the Health Research Board, the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division in Northern Ireland. No other conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Food as Medicine: Diet’s Role in Parkinson’s Disease
For 15 years, John Duda, MD, national director of the VA Parkinson’s Disease Research, Education and Clinical Centers, has urged his patients to “keep waiting” for effective treatments to manage both motor and nonmotor symptoms of Parkinson’s disease.
However, Duda, who also serves as director of the Brain Wellness Clinic at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, recognized the persistent lack of effective drugs to address these symptoms. This prompted him to consider what other evidence-based strategies he could use to support his patients.
“I recognized that nutritional approaches within a broader program that includes medication review, stress management, social connections, adequate sleep, and physical exercise could make a real difference,” he said.
Observational studies have shown an inverse association between dietary patterns and Parkinson’s disease risk, age of onset, symptom severity, and mortality rates — particularly with the Mediterranean diet (MeDi) and the MIND diet, which combines elements of MeDi and the Dietary Approaches to Stop Hypertension (DASH) diet. Although randomized controlled trials are still limited, the epidemiologic evidence supporting dietary interventions is “compelling,” said Duda.
For example, a cross-sectional study comparing 167 participants with Parkinson’s disease vs 119 controls showed that later age of Parkinson’s disease onset correlated with adherence to the MIND diet in women, with a difference of up to 17.4 years (P < .001) between low and high dietary tertiles.
The MeDi was correlated with later onset in men, with differences of up to 8.4 years (P = .002). As previously reported, a healthy diet emphasizing vegetables, fruits, nuts, and grains was inversely associated with prodromal features of Parkinson’s disease, including constipation, excessive daytime sleepiness, and depression. In addition, lower rates of Parkinson’s disease have been shown in populations following vegetarian and vegan dietary patterns.
Does Parkinson’s disease Start in the Gut?
Parkinson’s disease is characterized by decreased short-chain fatty acid–producing bacteria and increased pro-inflammatory species linked to intestinal inflammation and alpha-synuclein aggregation. “There are reasons to believe that a-synuclein accumulation may start in the gut,” Duda noted.
Numerous studies implicate gut microbiome dysbiosis as a pathogenic mechanism in Parkinson’s disease, with gastrointestinal symptoms often predating motor symptoms. Dysbiosis might result in a pro-inflammatory state potentially linked to the recurrent gastrointestinal symptoms. Fecal microbiota transplant may restore a healthier gut environment and beneficially affect Parkinson’s disease symptoms, he said.
Some of the benefits conferred by the MeDi and other healthy diets may be mediated by improving the gut microbiome. Duda cited a study that showed that a 14-day ovo-lacto vegetarian diet intervention and a daily fecal enema for 8 days improved not only the microbiome but also Movement Disorder Society Unified Parkinson’s Disease Rating Scale—part III scores.
Duda also reviewed the role of dietary interventions in addressing common Parkinson’s disease symptoms, such as orthostatic hypotension. He recommended that Parkinson’s disease patients with this condition should avoid eating large meals, increase dietary salt intake, increase fluid intake, and decrease alcohol intake.
Malnutrition affects close to 25% of those with Parkinson’s disease, which is partially attributable to diminished olfaction. Because the experience of taste is largely driven by a sense of smell, patients may be less interested in eating. Duda recommended increasing herbs, spices, and other flavors in food. High caloric–density foods, including nuts, nut butters, and seeds, can boost weight, he said. However, he added, any patient with significant weight loss should consult a nutritionist.
Constipation is one of the most debilitating symptoms of Parkinson’s disease, affecting up to 66% of patients. Duda advised increasing fluid intake, exercise, and dietary fiber and use of stool softeners and laxatives. The MeDi may reduce symptoms of constipation and have a beneficial effect on gut microbiota.
Coffee may be helpful for sleepiness in Parkinson’s disease and may also confer neuroprotective, motor, and cognitive benefits. As an adjuvant treatment, caffeine may alter levodopa pharmacokinetics, reduce dyskinesia, improve gait in patients with freezing and may even reduce the risk of developing Parkinson’s disease, with a maximum benefit reached at approximately three cups of coffee daily.
Problematic Foods
There is also a growing body of evidence regarding the deleterious effects of ultraprocessed foods (UPFs), Duda said. He noted that a recent systematic review and meta-analysis of 28 studies showed that higher UPF intake was significantly associated with an enhanced risk for Parkinson’s disease (relative risk, 1.56; 95% CI, 1.21-2.02). As previously reported, UPFs have been tied to a host of adverse neurologic outcomes, including cognitive decline and stroke.
Although protein is a necessary nutrient, incorporating it into the diet of Parkinson’s disease patients taking levodopa is complicated. Levodopa, a large neutral amino acid (LNAA), competes with other LNAAs for transport to the brain from the small intestine, Duda explained.
“Some people notice that carbidopa-levodopa doesn’t work as well if taken with a high-protein meal.” He recommended taking carbidopa-levodopa 30 minutes before or 60 minutes after meals.
Rebecca Gilbert, MD, PhD, chief mission officer of the American Parkinson’s Disease Association, said that patients with Parkinson’s disease might want to avoid eating protein during the day, concentrating instead on carbohydrates and vegetables and saving the protein for the evening, which is closer to bedtime. Some evidence also supports the use of protein redistribution diets to enhance the clinical response to levodopa and reduce motor fluctuations.
What About Supplements?
It’s “hard to prove that one specific supplement can be protective against Parkinson’s disease because diet consists of many different components and the whole diet may be worth more than the sum of its parts,” Gilbert said. The evidence for individual supplements “isn’t robust enough to say they prevent or treat Parkinson’s disease.”
Research on the role of specific nutrients in Parkinson’s disease is conflicting, with no clear evidence supporting or refuting their benefits. For example, a study that followed participants for about 30 years showed no link between reduced Parkinson’s disease risk and vitamin B or folate intake.
On the other hand, there is research suggesting that certain vitamins may help reduce Parkinson’s disease risk, although these nutrients do not operate in isolation. For instance, one recent study showed a connection between vitamins C and E and reduced Parkinson’s disease risk, but factors such as body mass index and coffee consumption appeared to influence the strength of this association.
Consuming polyunsaturated fatty acids along with reducing saturated fatty acid intake has been tied to a reduced risk for Parkinson’s disease.
Additionally, certain foods may offer protective effects, including green and black tea, with consumption of three or more cups per day associated with a delay in motor symptom onset by 7.7 years. Foods high in nicotine content, such as those from the Solanaceae family — including peppers, tomatoes, tomato juice, and potatoes — have also been linked to potential protective benefits.
Diets rich in antioxidants, including carotenoids, lutein, and vitamins E and C, have been robustly linked to a reduced risk for parkinsonism and progression of parkinsonian symptoms in older adults.
Increasing the intake of dietary flavonoids, particularly tea, berry fruits, apples, red wine, and oranges or orange juice, can reduce Parkinson’s disease risk. One study showed that male participants in the highest quintile of total flavonoid consumption had a 40% lower Parkinson’s disease risk compared with those in the lowest quintile. Another study showed that flavonoid-rich foods were also associated with a lower risk for death in patients with Parkinson’s disease.
Food as Medicine
Although recent research shows that the drug development pipeline for Parkinson’s disease is robust, with a wide variety of approaches being developed and evaluated in phase 1 and 2, investigators note that only a limited number of disease-modifying treatments are transitioning to phase 3.
Duda noted that phytochemicals incorporated into the diet might target some of the same mechanisms that are targets of these drugs in development.
“Flavonoids have been shown to stabilize alpha-synuclein in vitro,” he said. “Caffeine, curcumin, resveratrol, and eliminating meat and dairy inhibit mTOR [mammalian target of rapamycin], and mTOR inhibition results in increased autophagy that may help clear alpha-synuclein. Genestein, an isoflavone in soybeans, protects dopaminergic neurons by inhibiting microglia activation. Flavonoids inhibit inflammation by inhibiting release of NO [nitric oxide] and pro-inflammatory cytokines,” he noted.
Ongoing studies of dietary interventions for Parkinson’s disease are exploring various areas, including the potential role of the ketogenic diet in protecting the gut microbiome, optimizing protein intake for muscle preservation and sleep, the effects of psyllium and wheat bran on weight and constipation, and the impact of a gluten-free diet.
Practical Tips for Healthy Eating
Gilbert emphasized that there are no medications or interventions currently available that can delay a Parkinson’s disease diagnosis by up to 17 years, as some dietary patterns have been shown to do, and she noted that it’s not possible to replicate the MeDi diet in a pill. However, she recommended a practical approach to eating that includes a diet low in ultraprocessed foods and high in beneficial nutrients. She encouraged people to shop for “real food” and enjoy a variety of colorful fruits and vegetables.
Duda acknowledged that motivating patients to follow a healthy diet can be difficult. As a result, the focus often shifts to making small adjustments and modifications. For example, he suggested that instead of pairing meat with French fries, people could opt for vegetables or add greens to their meals. Similarly, instead of having eggs and bacon for breakfast, they might choose oatmeal.
Preparing whole-food, plant-based meals may take more time than patients are accustomed to, so Duda suggests that, if possible, patients involve loved ones in both the meal preparation and the meal itself. He explained that a healthy meal can become an opportunity for bonding and that the key is educating them about new meal-related concepts.
Duda reported no relevant financial relationships with the pharmaceutical or food industries. He has received compensation from the Physicians Committee for Responsible Medicine for his lecture delivered at the conference and research grant support from the VA, the National Institutes of Health, the Michael J. Fox Foundation, and the Department of Defense unrelated to this topic. Gilbert reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
For 15 years, John Duda, MD, national director of the VA Parkinson’s Disease Research, Education and Clinical Centers, has urged his patients to “keep waiting” for effective treatments to manage both motor and nonmotor symptoms of Parkinson’s disease.
However, Duda, who also serves as director of the Brain Wellness Clinic at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, recognized the persistent lack of effective drugs to address these symptoms. This prompted him to consider what other evidence-based strategies he could use to support his patients.
“I recognized that nutritional approaches within a broader program that includes medication review, stress management, social connections, adequate sleep, and physical exercise could make a real difference,” he said.
Observational studies have shown an inverse association between dietary patterns and Parkinson’s disease risk, age of onset, symptom severity, and mortality rates — particularly with the Mediterranean diet (MeDi) and the MIND diet, which combines elements of MeDi and the Dietary Approaches to Stop Hypertension (DASH) diet. Although randomized controlled trials are still limited, the epidemiologic evidence supporting dietary interventions is “compelling,” said Duda.
For example, a cross-sectional study comparing 167 participants with Parkinson’s disease vs 119 controls showed that later age of Parkinson’s disease onset correlated with adherence to the MIND diet in women, with a difference of up to 17.4 years (P < .001) between low and high dietary tertiles.
The MeDi was correlated with later onset in men, with differences of up to 8.4 years (P = .002). As previously reported, a healthy diet emphasizing vegetables, fruits, nuts, and grains was inversely associated with prodromal features of Parkinson’s disease, including constipation, excessive daytime sleepiness, and depression. In addition, lower rates of Parkinson’s disease have been shown in populations following vegetarian and vegan dietary patterns.
Does Parkinson’s disease Start in the Gut?
Parkinson’s disease is characterized by decreased short-chain fatty acid–producing bacteria and increased pro-inflammatory species linked to intestinal inflammation and alpha-synuclein aggregation. “There are reasons to believe that a-synuclein accumulation may start in the gut,” Duda noted.
Numerous studies implicate gut microbiome dysbiosis as a pathogenic mechanism in Parkinson’s disease, with gastrointestinal symptoms often predating motor symptoms. Dysbiosis might result in a pro-inflammatory state potentially linked to the recurrent gastrointestinal symptoms. Fecal microbiota transplant may restore a healthier gut environment and beneficially affect Parkinson’s disease symptoms, he said.
Some of the benefits conferred by the MeDi and other healthy diets may be mediated by improving the gut microbiome. Duda cited a study that showed that a 14-day ovo-lacto vegetarian diet intervention and a daily fecal enema for 8 days improved not only the microbiome but also Movement Disorder Society Unified Parkinson’s Disease Rating Scale—part III scores.
Duda also reviewed the role of dietary interventions in addressing common Parkinson’s disease symptoms, such as orthostatic hypotension. He recommended that Parkinson’s disease patients with this condition should avoid eating large meals, increase dietary salt intake, increase fluid intake, and decrease alcohol intake.
Malnutrition affects close to 25% of those with Parkinson’s disease, which is partially attributable to diminished olfaction. Because the experience of taste is largely driven by a sense of smell, patients may be less interested in eating. Duda recommended increasing herbs, spices, and other flavors in food. High caloric–density foods, including nuts, nut butters, and seeds, can boost weight, he said. However, he added, any patient with significant weight loss should consult a nutritionist.
Constipation is one of the most debilitating symptoms of Parkinson’s disease, affecting up to 66% of patients. Duda advised increasing fluid intake, exercise, and dietary fiber and use of stool softeners and laxatives. The MeDi may reduce symptoms of constipation and have a beneficial effect on gut microbiota.
Coffee may be helpful for sleepiness in Parkinson’s disease and may also confer neuroprotective, motor, and cognitive benefits. As an adjuvant treatment, caffeine may alter levodopa pharmacokinetics, reduce dyskinesia, improve gait in patients with freezing and may even reduce the risk of developing Parkinson’s disease, with a maximum benefit reached at approximately three cups of coffee daily.
Problematic Foods
There is also a growing body of evidence regarding the deleterious effects of ultraprocessed foods (UPFs), Duda said. He noted that a recent systematic review and meta-analysis of 28 studies showed that higher UPF intake was significantly associated with an enhanced risk for Parkinson’s disease (relative risk, 1.56; 95% CI, 1.21-2.02). As previously reported, UPFs have been tied to a host of adverse neurologic outcomes, including cognitive decline and stroke.
Although protein is a necessary nutrient, incorporating it into the diet of Parkinson’s disease patients taking levodopa is complicated. Levodopa, a large neutral amino acid (LNAA), competes with other LNAAs for transport to the brain from the small intestine, Duda explained.
“Some people notice that carbidopa-levodopa doesn’t work as well if taken with a high-protein meal.” He recommended taking carbidopa-levodopa 30 minutes before or 60 minutes after meals.
Rebecca Gilbert, MD, PhD, chief mission officer of the American Parkinson’s Disease Association, said that patients with Parkinson’s disease might want to avoid eating protein during the day, concentrating instead on carbohydrates and vegetables and saving the protein for the evening, which is closer to bedtime. Some evidence also supports the use of protein redistribution diets to enhance the clinical response to levodopa and reduce motor fluctuations.
What About Supplements?
It’s “hard to prove that one specific supplement can be protective against Parkinson’s disease because diet consists of many different components and the whole diet may be worth more than the sum of its parts,” Gilbert said. The evidence for individual supplements “isn’t robust enough to say they prevent or treat Parkinson’s disease.”
Research on the role of specific nutrients in Parkinson’s disease is conflicting, with no clear evidence supporting or refuting their benefits. For example, a study that followed participants for about 30 years showed no link between reduced Parkinson’s disease risk and vitamin B or folate intake.
On the other hand, there is research suggesting that certain vitamins may help reduce Parkinson’s disease risk, although these nutrients do not operate in isolation. For instance, one recent study showed a connection between vitamins C and E and reduced Parkinson’s disease risk, but factors such as body mass index and coffee consumption appeared to influence the strength of this association.
Consuming polyunsaturated fatty acids along with reducing saturated fatty acid intake has been tied to a reduced risk for Parkinson’s disease.
Additionally, certain foods may offer protective effects, including green and black tea, with consumption of three or more cups per day associated with a delay in motor symptom onset by 7.7 years. Foods high in nicotine content, such as those from the Solanaceae family — including peppers, tomatoes, tomato juice, and potatoes — have also been linked to potential protective benefits.
Diets rich in antioxidants, including carotenoids, lutein, and vitamins E and C, have been robustly linked to a reduced risk for parkinsonism and progression of parkinsonian symptoms in older adults.
Increasing the intake of dietary flavonoids, particularly tea, berry fruits, apples, red wine, and oranges or orange juice, can reduce Parkinson’s disease risk. One study showed that male participants in the highest quintile of total flavonoid consumption had a 40% lower Parkinson’s disease risk compared with those in the lowest quintile. Another study showed that flavonoid-rich foods were also associated with a lower risk for death in patients with Parkinson’s disease.
Food as Medicine
Although recent research shows that the drug development pipeline for Parkinson’s disease is robust, with a wide variety of approaches being developed and evaluated in phase 1 and 2, investigators note that only a limited number of disease-modifying treatments are transitioning to phase 3.
Duda noted that phytochemicals incorporated into the diet might target some of the same mechanisms that are targets of these drugs in development.
“Flavonoids have been shown to stabilize alpha-synuclein in vitro,” he said. “Caffeine, curcumin, resveratrol, and eliminating meat and dairy inhibit mTOR [mammalian target of rapamycin], and mTOR inhibition results in increased autophagy that may help clear alpha-synuclein. Genestein, an isoflavone in soybeans, protects dopaminergic neurons by inhibiting microglia activation. Flavonoids inhibit inflammation by inhibiting release of NO [nitric oxide] and pro-inflammatory cytokines,” he noted.
Ongoing studies of dietary interventions for Parkinson’s disease are exploring various areas, including the potential role of the ketogenic diet in protecting the gut microbiome, optimizing protein intake for muscle preservation and sleep, the effects of psyllium and wheat bran on weight and constipation, and the impact of a gluten-free diet.
Practical Tips for Healthy Eating
Gilbert emphasized that there are no medications or interventions currently available that can delay a Parkinson’s disease diagnosis by up to 17 years, as some dietary patterns have been shown to do, and she noted that it’s not possible to replicate the MeDi diet in a pill. However, she recommended a practical approach to eating that includes a diet low in ultraprocessed foods and high in beneficial nutrients. She encouraged people to shop for “real food” and enjoy a variety of colorful fruits and vegetables.
Duda acknowledged that motivating patients to follow a healthy diet can be difficult. As a result, the focus often shifts to making small adjustments and modifications. For example, he suggested that instead of pairing meat with French fries, people could opt for vegetables or add greens to their meals. Similarly, instead of having eggs and bacon for breakfast, they might choose oatmeal.
Preparing whole-food, plant-based meals may take more time than patients are accustomed to, so Duda suggests that, if possible, patients involve loved ones in both the meal preparation and the meal itself. He explained that a healthy meal can become an opportunity for bonding and that the key is educating them about new meal-related concepts.
Duda reported no relevant financial relationships with the pharmaceutical or food industries. He has received compensation from the Physicians Committee for Responsible Medicine for his lecture delivered at the conference and research grant support from the VA, the National Institutes of Health, the Michael J. Fox Foundation, and the Department of Defense unrelated to this topic. Gilbert reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
For 15 years, John Duda, MD, national director of the VA Parkinson’s Disease Research, Education and Clinical Centers, has urged his patients to “keep waiting” for effective treatments to manage both motor and nonmotor symptoms of Parkinson’s disease.
However, Duda, who also serves as director of the Brain Wellness Clinic at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, recognized the persistent lack of effective drugs to address these symptoms. This prompted him to consider what other evidence-based strategies he could use to support his patients.
“I recognized that nutritional approaches within a broader program that includes medication review, stress management, social connections, adequate sleep, and physical exercise could make a real difference,” he said.
Observational studies have shown an inverse association between dietary patterns and Parkinson’s disease risk, age of onset, symptom severity, and mortality rates — particularly with the Mediterranean diet (MeDi) and the MIND diet, which combines elements of MeDi and the Dietary Approaches to Stop Hypertension (DASH) diet. Although randomized controlled trials are still limited, the epidemiologic evidence supporting dietary interventions is “compelling,” said Duda.
For example, a cross-sectional study comparing 167 participants with Parkinson’s disease vs 119 controls showed that later age of Parkinson’s disease onset correlated with adherence to the MIND diet in women, with a difference of up to 17.4 years (P < .001) between low and high dietary tertiles.
The MeDi was correlated with later onset in men, with differences of up to 8.4 years (P = .002). As previously reported, a healthy diet emphasizing vegetables, fruits, nuts, and grains was inversely associated with prodromal features of Parkinson’s disease, including constipation, excessive daytime sleepiness, and depression. In addition, lower rates of Parkinson’s disease have been shown in populations following vegetarian and vegan dietary patterns.
Does Parkinson’s disease Start in the Gut?
Parkinson’s disease is characterized by decreased short-chain fatty acid–producing bacteria and increased pro-inflammatory species linked to intestinal inflammation and alpha-synuclein aggregation. “There are reasons to believe that a-synuclein accumulation may start in the gut,” Duda noted.
Numerous studies implicate gut microbiome dysbiosis as a pathogenic mechanism in Parkinson’s disease, with gastrointestinal symptoms often predating motor symptoms. Dysbiosis might result in a pro-inflammatory state potentially linked to the recurrent gastrointestinal symptoms. Fecal microbiota transplant may restore a healthier gut environment and beneficially affect Parkinson’s disease symptoms, he said.
Some of the benefits conferred by the MeDi and other healthy diets may be mediated by improving the gut microbiome. Duda cited a study that showed that a 14-day ovo-lacto vegetarian diet intervention and a daily fecal enema for 8 days improved not only the microbiome but also Movement Disorder Society Unified Parkinson’s Disease Rating Scale—part III scores.
Duda also reviewed the role of dietary interventions in addressing common Parkinson’s disease symptoms, such as orthostatic hypotension. He recommended that Parkinson’s disease patients with this condition should avoid eating large meals, increase dietary salt intake, increase fluid intake, and decrease alcohol intake.
Malnutrition affects close to 25% of those with Parkinson’s disease, which is partially attributable to diminished olfaction. Because the experience of taste is largely driven by a sense of smell, patients may be less interested in eating. Duda recommended increasing herbs, spices, and other flavors in food. High caloric–density foods, including nuts, nut butters, and seeds, can boost weight, he said. However, he added, any patient with significant weight loss should consult a nutritionist.
Constipation is one of the most debilitating symptoms of Parkinson’s disease, affecting up to 66% of patients. Duda advised increasing fluid intake, exercise, and dietary fiber and use of stool softeners and laxatives. The MeDi may reduce symptoms of constipation and have a beneficial effect on gut microbiota.
Coffee may be helpful for sleepiness in Parkinson’s disease and may also confer neuroprotective, motor, and cognitive benefits. As an adjuvant treatment, caffeine may alter levodopa pharmacokinetics, reduce dyskinesia, improve gait in patients with freezing and may even reduce the risk of developing Parkinson’s disease, with a maximum benefit reached at approximately three cups of coffee daily.
Problematic Foods
There is also a growing body of evidence regarding the deleterious effects of ultraprocessed foods (UPFs), Duda said. He noted that a recent systematic review and meta-analysis of 28 studies showed that higher UPF intake was significantly associated with an enhanced risk for Parkinson’s disease (relative risk, 1.56; 95% CI, 1.21-2.02). As previously reported, UPFs have been tied to a host of adverse neurologic outcomes, including cognitive decline and stroke.
Although protein is a necessary nutrient, incorporating it into the diet of Parkinson’s disease patients taking levodopa is complicated. Levodopa, a large neutral amino acid (LNAA), competes with other LNAAs for transport to the brain from the small intestine, Duda explained.
“Some people notice that carbidopa-levodopa doesn’t work as well if taken with a high-protein meal.” He recommended taking carbidopa-levodopa 30 minutes before or 60 minutes after meals.
Rebecca Gilbert, MD, PhD, chief mission officer of the American Parkinson’s Disease Association, said that patients with Parkinson’s disease might want to avoid eating protein during the day, concentrating instead on carbohydrates and vegetables and saving the protein for the evening, which is closer to bedtime. Some evidence also supports the use of protein redistribution diets to enhance the clinical response to levodopa and reduce motor fluctuations.
What About Supplements?
It’s “hard to prove that one specific supplement can be protective against Parkinson’s disease because diet consists of many different components and the whole diet may be worth more than the sum of its parts,” Gilbert said. The evidence for individual supplements “isn’t robust enough to say they prevent or treat Parkinson’s disease.”
Research on the role of specific nutrients in Parkinson’s disease is conflicting, with no clear evidence supporting or refuting their benefits. For example, a study that followed participants for about 30 years showed no link between reduced Parkinson’s disease risk and vitamin B or folate intake.
On the other hand, there is research suggesting that certain vitamins may help reduce Parkinson’s disease risk, although these nutrients do not operate in isolation. For instance, one recent study showed a connection between vitamins C and E and reduced Parkinson’s disease risk, but factors such as body mass index and coffee consumption appeared to influence the strength of this association.
Consuming polyunsaturated fatty acids along with reducing saturated fatty acid intake has been tied to a reduced risk for Parkinson’s disease.
Additionally, certain foods may offer protective effects, including green and black tea, with consumption of three or more cups per day associated with a delay in motor symptom onset by 7.7 years. Foods high in nicotine content, such as those from the Solanaceae family — including peppers, tomatoes, tomato juice, and potatoes — have also been linked to potential protective benefits.
Diets rich in antioxidants, including carotenoids, lutein, and vitamins E and C, have been robustly linked to a reduced risk for parkinsonism and progression of parkinsonian symptoms in older adults.
Increasing the intake of dietary flavonoids, particularly tea, berry fruits, apples, red wine, and oranges or orange juice, can reduce Parkinson’s disease risk. One study showed that male participants in the highest quintile of total flavonoid consumption had a 40% lower Parkinson’s disease risk compared with those in the lowest quintile. Another study showed that flavonoid-rich foods were also associated with a lower risk for death in patients with Parkinson’s disease.
Food as Medicine
Although recent research shows that the drug development pipeline for Parkinson’s disease is robust, with a wide variety of approaches being developed and evaluated in phase 1 and 2, investigators note that only a limited number of disease-modifying treatments are transitioning to phase 3.
Duda noted that phytochemicals incorporated into the diet might target some of the same mechanisms that are targets of these drugs in development.
“Flavonoids have been shown to stabilize alpha-synuclein in vitro,” he said. “Caffeine, curcumin, resveratrol, and eliminating meat and dairy inhibit mTOR [mammalian target of rapamycin], and mTOR inhibition results in increased autophagy that may help clear alpha-synuclein. Genestein, an isoflavone in soybeans, protects dopaminergic neurons by inhibiting microglia activation. Flavonoids inhibit inflammation by inhibiting release of NO [nitric oxide] and pro-inflammatory cytokines,” he noted.
Ongoing studies of dietary interventions for Parkinson’s disease are exploring various areas, including the potential role of the ketogenic diet in protecting the gut microbiome, optimizing protein intake for muscle preservation and sleep, the effects of psyllium and wheat bran on weight and constipation, and the impact of a gluten-free diet.
Practical Tips for Healthy Eating
Gilbert emphasized that there are no medications or interventions currently available that can delay a Parkinson’s disease diagnosis by up to 17 years, as some dietary patterns have been shown to do, and she noted that it’s not possible to replicate the MeDi diet in a pill. However, she recommended a practical approach to eating that includes a diet low in ultraprocessed foods and high in beneficial nutrients. She encouraged people to shop for “real food” and enjoy a variety of colorful fruits and vegetables.
Duda acknowledged that motivating patients to follow a healthy diet can be difficult. As a result, the focus often shifts to making small adjustments and modifications. For example, he suggested that instead of pairing meat with French fries, people could opt for vegetables or add greens to their meals. Similarly, instead of having eggs and bacon for breakfast, they might choose oatmeal.
Preparing whole-food, plant-based meals may take more time than patients are accustomed to, so Duda suggests that, if possible, patients involve loved ones in both the meal preparation and the meal itself. He explained that a healthy meal can become an opportunity for bonding and that the key is educating them about new meal-related concepts.
Duda reported no relevant financial relationships with the pharmaceutical or food industries. He has received compensation from the Physicians Committee for Responsible Medicine for his lecture delivered at the conference and research grant support from the VA, the National Institutes of Health, the Michael J. Fox Foundation, and the Department of Defense unrelated to this topic. Gilbert reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
The Strange Untold Story of How Science Solved Narcolepsy
It was 1996, and Masashi Yanagisawa was on the brink of his next discovery.
The Japanese scientist had arrived at the University of Texas Southwestern in Dallas 5 years earlier, setting up his own lab at age 31. After earning his medical degree, he’d gained notoriety as a PhD student when he discovered endothelin, the body’s most potent vasoconstrictor.
Yanagisawa was about to prove this wasn’t a first-timer’s fluke.
His focus was G-protein–coupled receptors (GPCRs), cell surface receptors that respond to a range of molecules and a popular target for drug discovery. The Human Genome Project had just revealed a slew of newly discovered receptors, or “orphan” GPCRs, and identifying an activating molecule could yield a new drug. (That vasoconstrictor endothelin was one such success story, leading to four new drug approvals in the United States over the past quarter century.)
Yanagisawa and his team created 50 cell lines, each expressing one orphan receptor. They applied animal tissue to every line, along with a calcium-sensitive dye. If the cells glowed under the microscope, they had a hit.
“He was basically doing an elaborate fishing expedition,” said Jon Willie, MD, PhD, an associate professor of neurosurgery at Washington University School of Medicine in St. Louis, Missouri, who would later join Yanagisawa’s team.
It wasn’t long before the neon-green fluorescence signaled a match. After isolating the activating molecule, the scientists realized they were dealing with two neuropeptides.
No one had ever seen these proteins before. And no one knew their discovery would set off a decades-long journey that would finally solve a century-old medical mystery — and may even fix one of the biggest health crises of our time, as revealed by research published earlier in 2024. It’s a story of strange coincidences, serendipitous discoveries, and quirky details. Most of all, it’s a fascinating example of how basic science can revolutionize medicine — and how true breakthroughs happen over time and in real time.
But That’s Basic Science for You
Most basic science studies — the early, foundational research that provides the building blocks for science that follows — don’t lead to medical breakthroughs. But some do, often in surprising ways.
Also called curiosity-driven research, basic science aims to fill knowledge gaps to keep science moving, even if the trajectory isn’t always clear.
“The people working on the basic research that led to discoveries that transformed the modern world had no idea at the time,” said Isobel Ronai, PhD, a postdoctoral fellow in life sciences at Harvard University, Cambridge, Massachusetts. “Often, these stories can only be seen in hindsight,” sometimes decades later.
Case in point: For molecular biology techniques — things like DNA sequencing and gene targeting — the lag between basic science and breakthrough is, on average, 23 years. While many of the resulting techniques have received Nobel Prizes, few of the foundational discoveries have been awarded such accolades.
“The scientific glory is more often associated with the downstream applications,” said Ronai. “The importance of basic research can get lost. But it is the foundation for any future application, such as drug development.”
As funding is increasingly funneled toward applied research, basic science can require a certain persistence. What this under-appreciation can obscure is the pathway to discovery — which is often as compelling as the end result, full of unpredictable twists, turns, and even interpersonal intrigue.
And then there’s the fascinating — and definitely complicated — phenomenon of multiple independent discoveries.
As in: What happens when two independent teams discover the same thing at the same time?
Back to Yanagisawa’s Lab ...
... where he and his team learned a few things about those new neuropeptides. Rat brain studies pinpointed the lateral hypothalamus as the peptides’ area of activity — a region often called the brain’s feeding center.
“If you destroy that part of the brain, animals lose appetite,” said Yanagisawa. So these peptides must control feeding, the scientists thought.
Sure enough, injecting the proteins into rat brains led the rodents to start eating.
Satisfied, the team named them “orexin-A” and “orexin-B,” for the Greek word “orexis,” meaning appetite. The brain receptors became “orexin-1” and “orexin-2.” The team prepared to publish its findings in Cell.
But another group beat them to it.
Introducing the ‘Hypocretins’
In early January 1998, a team of Scripps Research Institute scientists, led by J. Gregor Sutcliffe, PhD, released a paper in the journal PNAS. They described a gene encoding for the precursor to two neuropeptides
As the peptides were in the hypothalamus and structurally like secretin (a gut hormone), they called them “hypocretins.” The hypocretin peptides excited neurons in the hypothalamus, and later that year, the scientists discovered that the neurons’ branches extended, tentacle-like, throughout the brain. “Many of the connected areas were involved in sleep-wake control,” said Thomas Kilduff, PhD, who joined the Sutcliffe lab just weeks before the hypocretin discovery. At the time, however, the significance of this finding was not yet clear.
Weeks later, in February 1998, Yanagisawa’s paper came out.
Somehow, two groups, over 1000 miles apart, had stumbled on the same neuropeptides at the same time.
“I first heard about [Yanagisawa’s] paper on NBC Nightly News,” recalls Kilduff. “I was skiing in the mountains, so I had to wait until Monday to get back to the lab to see what the paper was all about.”
He realized that Yanagisawa’s orexin was his lab’s hypocretin, although the study didn’t mention another team’s discovery.
“There may have been accusations. But as far as I know, it’s because [Yanagisawa] didn’t know [about the other paper],” said Willie. “This was not something he produced in 2 months. This was clearly years of work.”
‘Multiple Discovery’ Happens More Often Than You Think
In the mid-20th century, sociologist Robert Merton described the phenomenon of “multiple discovery,” where many scientific discoveries or inventions are made independently at roughly the same time.
“This happens much more frequently in scientific research than people suppose,” said David Pendlebury, head of research analysis at Clarivate’s Institute for Scientific Information, the analytics company’s research arm. (Last year, Pendlebury flagged the hypocretin/orexin discovery for Clarivate’s prestigious Citations Laureates award, an honor that aims to predict, often successfully, who will go on to win the Nobel Prize.)
“People have this idea of the lone researcher making a brilliant discovery,” Pendlebury said. “But more and more, teams find things at the same time.”
While this can — and does — lead to squabbling about who deserves credit, the desire to be first can also be highly motivating, said Mike Schneider, PhD, an assistant professor of philosophy at the University of Missouri, Columbia, who studies the social dynamics of science, potentially leading to faster scientific advancement.
The downside? If two groups produce the same or similar results, but one publishes first, scientific journals tend to reject the second, citing a lack of novelty.
Yet duplicating research is a key step in confirming the validity of a discovery.
That’s why, in 2018, the journal PLOS Biology created a provision for “scooped” scientists, allowing them to submit their paper within 6 months of the first as a complementary finding. Instead of viewing this as redundancy, the editors believe it adds robustness to the research.
‘What the Heck Is This Mouse Doing?’
Even though he’d been scooped, Yanagisawa forged on to the next challenge: Confirming whether orexin regulated feeding.
He began breeding mice missing the orexin gene. His team expected these “knockout” mice to eat less, resulting in a thinner body than other rodents. To the contrary, “they were on average fatter,” said Willie. “They were eating less but weighed more, indicating a slower metabolism.”
The researchers were befuddled. “We were really disappointed, almost desperate about what to do,” said Yanagisawa.
As nocturnal animals eat more at night, he decided they should study the mice after dark. One of his students, Richard Chemelli, MD, bought an infrared video camera from Radio Shack, filming the first 4 hours of the mice’s active period for several nights.
After watching the footage, “Rick called me and said, ‘Let’s get into the lab,’ ” said Willie. “It was four of us on a Saturday looking at these videos, saying, ‘What the heck is this mouse doing?’ ”
While exploring their habitat, the knockout mice would randomly fall over, pop back up after a minute or so, and resume normal activity. This happened over and over — and the scientists were unsure why.
They began monitoring the mice’s brains during these episodes — and made a startling discovery.
The mice weren’t having seizures. They were shifting directly into REM sleep, bypassing the non-REM stage, then quickly toggling back to wake mode.
“That’s when we knew these animals had something akin to narcolepsy,” said Willie.
The team recruited Thomas Scammell, MD, a Harvard neurologist, to investigate whether modafinil — an anti-narcoleptic drug without a clear mechanism — affected orexin neurons.
Two hours after injecting the mice with the medication, the scientists sacrificed them and stained their brains. Remarkably, the number of neurons showing orexin activity had increased ninefold. It seemed modafinil worked by activating the orexin system.
These findings had the potential to crack open the science of narcolepsy, one of the most mysterious sleep disorders.
Unless, of course, another team did it first.
The Mystery of Narcolepsy
Yet another multiple discovery, narcolepsy was first described by two scientists — one in Germany, the other in France — within a short span in the late 1800s.
It would be more than a hundred years before anyone understood the disorder’s cause, even though it affects about 1 in 2000 people.
“Patients were often labeled as lazy and malingerers,” said Kilduff, “since they were sleepy all the time and had this weird motor behavior called cataplexy” or the sudden loss of muscle tone.
In the early 1970s, William Dement, MD, PhD — “the father of sleep medicine” — was searching for a narcoleptic cat to study. He couldn’t find a feline, but several colleagues mentioned dogs with narcolepsy-like symptoms.
Dement, who died in 2020, had found his newest research subjects.
In 1973, he started a narcoleptic dog colony at Stanford University in Palo Alto, California. At first, he focused on poodles and beagles. After discovering their narcolepsy wasn’t genetic, he pivoted to dobermans and labradors. Their narcolepsy was inherited, so he could breed them to populate the colony.
Although human narcolepsy is rarely genetic, it’s otherwise a lot like the version in these dogs.
Both involve daytime sleepiness, “pathological” bouts of REM sleep, and the loss of muscle tone in response to emotions, often positive ones.
The researchers hoped the canines could unlock a treatment for human narcolepsy. They began laying out a path of dog kibble, then injecting the dogs with drugs such as selective serotonin reuptake inhibitors. They wanted to see what might help them stay awake as they excitedly chowed down.
Kilduff also started a molecular genetics program, trying to identify the genetic defect behind canine narcolepsy. But after a parvovirus outbreak, Kilduff resigned from the project, drained from the strain of seeing so many dogs die.
A decade after his departure from the dog colony, his work would dramatically intersect with that of his successor, Emmanuel Mignot, MD, PhD.
“I thought I had closed the narcolepsy chapter in my life forever,” said Kilduff. “Then in 1998, we described this novel neuropeptide, hypocretin, that turned out to be the key to understanding the disorder.”
Narcoleptic Dogs in California, Mutant Mice in Texas
It was modafinil — the same anti-narcoleptic drug Yanagisawa’s team studied — that brought Emmanuel Mignot to the United States. After training as a pharmacologist in France, his home country sent him to Stanford to study the drug, which was discovered by French scientists, as his required military service.
As Kilduff’s replacement at the dog colony, his goal was to figure out how modafinil worked, hoping to attract a US company to develop the drug.
The plan succeeded. Modafinil became Provigil, a billion-dollar narcolepsy drug, and Mignot became “completely fascinated” with the disorder.
“I realized quickly that there was no way we’d find the cause of narcolepsy by finding the mode of action of this drug,” Mignot said. “Most likely, the drug was acting downstream, not at the cause of the disorder.”
To discover the answer, he needed to become a geneticist. And so began his 11-year odyssey to find the cause of canine narcolepsy.
After mapping the dog genome, Mignot set out to find the smallest stretch of chromosome that the narcoleptic animals had in common. “For a very long time, we were stuck with a relatively large region [of DNA],” he recalls. “It was a no man’s land.”
Within that region was the gene for the hypocretin/orexin-2 receptor — the same receptor that Yanagisawa had identified in his first orexin paper. Mignot didn’t immediately pursue that gene as a possibility — even though his students suggested it. Why?
“The decision was simply: Should we lose time to test a possible candidate [gene] among many?” Mignot said.
As Mignot studied dog DNA in California, Yanagisawa was creating mutant mice in Texas. Unbeknownst to either scientist, their work was about to converge.
What Happened Next Is Somewhat Disputed
After diagnosing his mice with narcolepsy, Yanagisawa opted not to share this finding with Mignot, though he knew about Mignot’s interest in the condition. Instead, he asked a colleague to find out how far along Mignot was in his genetics research.
According to Yanagisawa, his colleague didn’t realize how quickly DNA sequencing could happen once a target gene was identified. At a sleep meeting, “he showed Emmanuel all of our raw data. Almost accidentally, he disclosed our findings,” he said. “It was a shock for me.”
Unsure whether he was part of the orexin group, Mignot decided not to reveal that he’d identified the hypocretin/orexin-2 receptor gene as the faulty one in his narcoleptic dogs.
Although he didn’t share this finding, Mignot said he did offer to speak with the lead researcher to see if their findings were the same. If they were, they could jointly submit their articles. But Mignot never heard back.
Meanwhile, back at his lab, Mignot buckled down. While he wasn’t convinced the mouse data proved anything, it did give him the motivation to move faster.
Within weeks, he submitted his findings to Cell, revealing a mutation in the hypocretin/orexin-2 receptor gene as the cause of canine narcolepsy. According to Yanagisawa, the journal’s editor invited him to peer-review the paper, tipping him off to its existence.
“I told him I had a conflict of interest,” said Yanagisawa. “And then we scrambled to finish our manuscript. We wrote up the paper within almost 5 days.”
For a moment, it seemed both papers would be published together in Cell. Instead, on August 6, 1999, Mignot’s study was splashed solo across the journal’s cover.
“At the time, our team was pissed off, but looking back, what else could Emmanuel have done?” said Willie, who was part of Yanagisawa’s team. “The grant he’d been working on for years was at risk. He had it within his power to do the final experiments. Of course he was going to finish.”
Two weeks later, Yanagisawa’s findings followed, also in Cell.
His paper proposed knockout mice as a model for human narcolepsy and orexin as a key regulator of the sleep/wake cycle. With orexin-activated neurons branching into other areas of the brain, the peptide seemed to promote wakefulness by synchronizing several arousal neurotransmitters, such as serotonin, norepinephrine, and histamine.
“If you don’t have orexin, each of those systems can still function, but they’re not as coordinated,” said Willie. “If you have narcolepsy, you’re capable of wakefulness, and you’re capable of sleep. What you can’t do is prevent inappropriately switching between states.”
Together, the two papers painted a clear picture: Narcolepsy was the result of a dysfunction in the hypocretin/orexin system.
After more than a century, the cause of narcolepsy was starting to come into focus.
“This was blockbuster,” said Willie.
By itself, either finding — one in dogs, one in mice — might have been met with skepticism. But in combination, they offered indisputable evidence about narcolepsy’s cause.
The Human Brains in Your Fridge Hold Secrets
Jerome Siegel had been searching for the cause of human narcolepsy for years. A PhD and professor at the University of California, Los Angeles, he had managed to acquire four human narcoleptic brains. As laughter is often the trigger for the sudden shift to REM sleep in humans, he focused on the amygdala, an area linked to emotion.
“I looked in the amygdala and didn’t see anything,” he said. “So the brains stayed in my refrigerator for probably 10 years.”
Then he was invited to review Yanagisawa’s study in Cell. The lightbulb clicked on: Maybe the hypothalamus — not the amygdala — was the area of abnormality. He and his team dug out the decade-old brains.
When they stained the brains, the massive loss of hypocretin-activated neurons was hard to miss: On average, the narcoleptic brains had only about 7000 of the cells versus 70,000 in the average human brain. The scientists also noticed scar tissue in the hypothalamus, indicating that the neurons had at some point died, rather than being absent from birth.
What Siegel didn’t know: Mignot had also acquired a handful of human narcoleptic brains.
Already, he had coauthored a study showing that hypocretin/orexin was undetectable in the cerebrospinal fluid of the majority of the people with narcolepsy his team tested. It seemed clear that the hypocretin/orexin system was flawed — or even broken — in people with the condition.
“It looked like the cause of narcolepsy in humans was indeed this lack of orexin in the brain,” he said. “That was the hypothesis immediately. To me, this is when we established that narcolepsy in humans was due to a lack of orexin. The next thing was to check that the cells were missing.”
Now he could do exactly that.
As expected, Mignot’s team observed a dramatic loss of hypocretin/orexin cells in the narcoleptic brains. They also noticed that a different cell type in the hypothalamus was unaffected. This implied the damage was specific to the hypocretin-activated cells and supported a hunch they already had: That the deficit was the result not of a genetic defect but of an autoimmune attack. (It’s a hypothesis Mignot has spent the last 15 years proving.)
It wasn’t until a gathering in Hawaii, in late August 2000, that the two realized the overlap of their work.
To celebrate his team’s finding, Mignot had invited a group of researchers to Big Island. With his paper scheduled for publication on September 1, he felt comfortable presenting his findings to his guests, which included Siegel.
Until then, “I didn’t know what he had found, and he didn’t know what I had found, which basically was the same thing,” said Siegel.
In yet another strange twist, the two papers were published just weeks apart, simultaneously revealing that human narcoleptics have a depleted supply of the neurons that bind to hypocretin/orexin. The cause of the disorder was at last a certainty.
“Even if I was first, what does it matter? In the end, you need confirmation,” said Mignot. “You need multiple people to make sure that it’s true. It’s good science when things like this happen.”
How All of This Changed Medicine
Since these groundbreaking discoveries, the diagnosis of narcolepsy has become much simpler. Lab tests can now easily measure hypocretin in cerebrospinal fluid, providing a definitive diagnosis.
But the development of narcolepsy treatments has lagged — even though hypocretin/orexin replacement therapy is the obvious answer.
“Almost 25 years have elapsed, and there’s no such therapeutic on the market,” said Kilduff, who now works for SRI International, a non-profit research and development institute.
That’s partly because agonists — drugs that bind to receptors in the brain — are challenging to create, as this requires mimicking the activating molecule’s structure, like copying the grooves of an intricate key.
Antagonists, by comparison, are easier to develop. These act as a gate, blocking access to the receptors. As a result, drugs that promote sleep by thwarting hypocretin/orexin have emerged more quickly, providing a flurry of new options for people with insomnia. The first, suvorexant, was launched in 2014. Two others followed in recent years.
Researchers are hopeful a hypocretin/orexin agonist is on the horizon.
“This is a very hot area of drug development,” said Kilduff. “It’s just a matter of who’s going to get the drug to market first.”
One More Hypocretin/Orexin Surprise — and It Could Be The Biggest
Several years ago, Siegel’s lab received what was supposed to be a healthy human brain — one they could use as a comparison for narcoleptic brains. But researcher Thomas Thannickal, PhD, lead author of the UCLA study linking hypocretin loss to human narcolepsy, noticed something strange: This brain had significantly more hypocretin neurons than average.
Was this due to a seizure? A traumatic death? Siegel called the brain bank to request the donor’s records. He was told they were missing.
Years later, Siegel happened to be visiting the brain bank for another project and found himself in a room adjacent to the medical records. “Nobody was there,” he said, “so I just opened a drawer.”
Shuffling through the brain bank’s files, Siegel found the medical records he’d been told were lost. In the file was a note from the donor, explaining that he was a former heroin addict.
“I almost fell out of my chair,” said Siegel. “I realized this guy’s heroin addiction likely had something to do with his very unusual brain.”
Obviously, opioids affected the orexin system. But how?
“It’s when people are happy that this peptide is released,” said Siegel. “The hypocretin system is not just related to alertness. It’s related to pleasure.”
As Yanagisawa observed early on, hypocretin/orexin does indeed play a role in eating — just not the one he initially thought. The peptides prompted pleasure seeking. So the rodents ate.
In 2018, after acquiring five more brains, Siegel’s group published a study in Translational Medicine showing 54% more detectable hypocretin neurons in the brains of heroin addicts than in those of control individuals.
In 2022, another breakthrough: His team showed that morphine significantly altered the pathways of hypocretin neurons in mice, sending their axons into brain regions associated with addiction. Then, when they removed the mice’s hypocretin neurons and discontinued their daily morphine dose, the rodents showed no symptoms of opioid withdrawal.
This fits the connection with narcolepsy: Among the standard treatments for the condition are amphetamines and other stimulants, which all have addictive potential. Yet, “narcoleptics never abuse these drugs,” Siegel said. “They seem to be uniquely resistant to addiction.”
This could powerfully change the way opioids are administered.
“If you prevent the hypocretin response to opioids, you may be able to prevent opioid addiction,” said Siegel. In other words, blocking the hypocretin system with a drug like those used to treat insomnia may allow patients to experience the pain-relieving benefits of opioids — without the risk for addiction.
His team is currently investigating treatments targeting the hypocretin/orexin system for opioid addiction.
In a study published in July, they found that mice who received suvorexant — the drug for insomnia — didn’t anticipate their daily dose of opioids the way other rodents did. This suggests the medication prevented addiction, without diminishing the pain-relieving effect of opioids.
If it translates to humans, this discovery could potentially save millions of lives.
“I think it’s just us working on this,” said Siegel.
But with hypocretin/orexin, you never know.
A version of this article appeared on Medscape.com.
It was 1996, and Masashi Yanagisawa was on the brink of his next discovery.
The Japanese scientist had arrived at the University of Texas Southwestern in Dallas 5 years earlier, setting up his own lab at age 31. After earning his medical degree, he’d gained notoriety as a PhD student when he discovered endothelin, the body’s most potent vasoconstrictor.
Yanagisawa was about to prove this wasn’t a first-timer’s fluke.
His focus was G-protein–coupled receptors (GPCRs), cell surface receptors that respond to a range of molecules and a popular target for drug discovery. The Human Genome Project had just revealed a slew of newly discovered receptors, or “orphan” GPCRs, and identifying an activating molecule could yield a new drug. (That vasoconstrictor endothelin was one such success story, leading to four new drug approvals in the United States over the past quarter century.)
Yanagisawa and his team created 50 cell lines, each expressing one orphan receptor. They applied animal tissue to every line, along with a calcium-sensitive dye. If the cells glowed under the microscope, they had a hit.
“He was basically doing an elaborate fishing expedition,” said Jon Willie, MD, PhD, an associate professor of neurosurgery at Washington University School of Medicine in St. Louis, Missouri, who would later join Yanagisawa’s team.
It wasn’t long before the neon-green fluorescence signaled a match. After isolating the activating molecule, the scientists realized they were dealing with two neuropeptides.
No one had ever seen these proteins before. And no one knew their discovery would set off a decades-long journey that would finally solve a century-old medical mystery — and may even fix one of the biggest health crises of our time, as revealed by research published earlier in 2024. It’s a story of strange coincidences, serendipitous discoveries, and quirky details. Most of all, it’s a fascinating example of how basic science can revolutionize medicine — and how true breakthroughs happen over time and in real time.
But That’s Basic Science for You
Most basic science studies — the early, foundational research that provides the building blocks for science that follows — don’t lead to medical breakthroughs. But some do, often in surprising ways.
Also called curiosity-driven research, basic science aims to fill knowledge gaps to keep science moving, even if the trajectory isn’t always clear.
“The people working on the basic research that led to discoveries that transformed the modern world had no idea at the time,” said Isobel Ronai, PhD, a postdoctoral fellow in life sciences at Harvard University, Cambridge, Massachusetts. “Often, these stories can only be seen in hindsight,” sometimes decades later.
Case in point: For molecular biology techniques — things like DNA sequencing and gene targeting — the lag between basic science and breakthrough is, on average, 23 years. While many of the resulting techniques have received Nobel Prizes, few of the foundational discoveries have been awarded such accolades.
“The scientific glory is more often associated with the downstream applications,” said Ronai. “The importance of basic research can get lost. But it is the foundation for any future application, such as drug development.”
As funding is increasingly funneled toward applied research, basic science can require a certain persistence. What this under-appreciation can obscure is the pathway to discovery — which is often as compelling as the end result, full of unpredictable twists, turns, and even interpersonal intrigue.
And then there’s the fascinating — and definitely complicated — phenomenon of multiple independent discoveries.
As in: What happens when two independent teams discover the same thing at the same time?
Back to Yanagisawa’s Lab ...
... where he and his team learned a few things about those new neuropeptides. Rat brain studies pinpointed the lateral hypothalamus as the peptides’ area of activity — a region often called the brain’s feeding center.
“If you destroy that part of the brain, animals lose appetite,” said Yanagisawa. So these peptides must control feeding, the scientists thought.
Sure enough, injecting the proteins into rat brains led the rodents to start eating.
Satisfied, the team named them “orexin-A” and “orexin-B,” for the Greek word “orexis,” meaning appetite. The brain receptors became “orexin-1” and “orexin-2.” The team prepared to publish its findings in Cell.
But another group beat them to it.
Introducing the ‘Hypocretins’
In early January 1998, a team of Scripps Research Institute scientists, led by J. Gregor Sutcliffe, PhD, released a paper in the journal PNAS. They described a gene encoding for the precursor to two neuropeptides
As the peptides were in the hypothalamus and structurally like secretin (a gut hormone), they called them “hypocretins.” The hypocretin peptides excited neurons in the hypothalamus, and later that year, the scientists discovered that the neurons’ branches extended, tentacle-like, throughout the brain. “Many of the connected areas were involved in sleep-wake control,” said Thomas Kilduff, PhD, who joined the Sutcliffe lab just weeks before the hypocretin discovery. At the time, however, the significance of this finding was not yet clear.
Weeks later, in February 1998, Yanagisawa’s paper came out.
Somehow, two groups, over 1000 miles apart, had stumbled on the same neuropeptides at the same time.
“I first heard about [Yanagisawa’s] paper on NBC Nightly News,” recalls Kilduff. “I was skiing in the mountains, so I had to wait until Monday to get back to the lab to see what the paper was all about.”
He realized that Yanagisawa’s orexin was his lab’s hypocretin, although the study didn’t mention another team’s discovery.
“There may have been accusations. But as far as I know, it’s because [Yanagisawa] didn’t know [about the other paper],” said Willie. “This was not something he produced in 2 months. This was clearly years of work.”
‘Multiple Discovery’ Happens More Often Than You Think
In the mid-20th century, sociologist Robert Merton described the phenomenon of “multiple discovery,” where many scientific discoveries or inventions are made independently at roughly the same time.
“This happens much more frequently in scientific research than people suppose,” said David Pendlebury, head of research analysis at Clarivate’s Institute for Scientific Information, the analytics company’s research arm. (Last year, Pendlebury flagged the hypocretin/orexin discovery for Clarivate’s prestigious Citations Laureates award, an honor that aims to predict, often successfully, who will go on to win the Nobel Prize.)
“People have this idea of the lone researcher making a brilliant discovery,” Pendlebury said. “But more and more, teams find things at the same time.”
While this can — and does — lead to squabbling about who deserves credit, the desire to be first can also be highly motivating, said Mike Schneider, PhD, an assistant professor of philosophy at the University of Missouri, Columbia, who studies the social dynamics of science, potentially leading to faster scientific advancement.
The downside? If two groups produce the same or similar results, but one publishes first, scientific journals tend to reject the second, citing a lack of novelty.
Yet duplicating research is a key step in confirming the validity of a discovery.
That’s why, in 2018, the journal PLOS Biology created a provision for “scooped” scientists, allowing them to submit their paper within 6 months of the first as a complementary finding. Instead of viewing this as redundancy, the editors believe it adds robustness to the research.
‘What the Heck Is This Mouse Doing?’
Even though he’d been scooped, Yanagisawa forged on to the next challenge: Confirming whether orexin regulated feeding.
He began breeding mice missing the orexin gene. His team expected these “knockout” mice to eat less, resulting in a thinner body than other rodents. To the contrary, “they were on average fatter,” said Willie. “They were eating less but weighed more, indicating a slower metabolism.”
The researchers were befuddled. “We were really disappointed, almost desperate about what to do,” said Yanagisawa.
As nocturnal animals eat more at night, he decided they should study the mice after dark. One of his students, Richard Chemelli, MD, bought an infrared video camera from Radio Shack, filming the first 4 hours of the mice’s active period for several nights.
After watching the footage, “Rick called me and said, ‘Let’s get into the lab,’ ” said Willie. “It was four of us on a Saturday looking at these videos, saying, ‘What the heck is this mouse doing?’ ”
While exploring their habitat, the knockout mice would randomly fall over, pop back up after a minute or so, and resume normal activity. This happened over and over — and the scientists were unsure why.
They began monitoring the mice’s brains during these episodes — and made a startling discovery.
The mice weren’t having seizures. They were shifting directly into REM sleep, bypassing the non-REM stage, then quickly toggling back to wake mode.
“That’s when we knew these animals had something akin to narcolepsy,” said Willie.
The team recruited Thomas Scammell, MD, a Harvard neurologist, to investigate whether modafinil — an anti-narcoleptic drug without a clear mechanism — affected orexin neurons.
Two hours after injecting the mice with the medication, the scientists sacrificed them and stained their brains. Remarkably, the number of neurons showing orexin activity had increased ninefold. It seemed modafinil worked by activating the orexin system.
These findings had the potential to crack open the science of narcolepsy, one of the most mysterious sleep disorders.
Unless, of course, another team did it first.
The Mystery of Narcolepsy
Yet another multiple discovery, narcolepsy was first described by two scientists — one in Germany, the other in France — within a short span in the late 1800s.
It would be more than a hundred years before anyone understood the disorder’s cause, even though it affects about 1 in 2000 people.
“Patients were often labeled as lazy and malingerers,” said Kilduff, “since they were sleepy all the time and had this weird motor behavior called cataplexy” or the sudden loss of muscle tone.
In the early 1970s, William Dement, MD, PhD — “the father of sleep medicine” — was searching for a narcoleptic cat to study. He couldn’t find a feline, but several colleagues mentioned dogs with narcolepsy-like symptoms.
Dement, who died in 2020, had found his newest research subjects.
In 1973, he started a narcoleptic dog colony at Stanford University in Palo Alto, California. At first, he focused on poodles and beagles. After discovering their narcolepsy wasn’t genetic, he pivoted to dobermans and labradors. Their narcolepsy was inherited, so he could breed them to populate the colony.
Although human narcolepsy is rarely genetic, it’s otherwise a lot like the version in these dogs.
Both involve daytime sleepiness, “pathological” bouts of REM sleep, and the loss of muscle tone in response to emotions, often positive ones.
The researchers hoped the canines could unlock a treatment for human narcolepsy. They began laying out a path of dog kibble, then injecting the dogs with drugs such as selective serotonin reuptake inhibitors. They wanted to see what might help them stay awake as they excitedly chowed down.
Kilduff also started a molecular genetics program, trying to identify the genetic defect behind canine narcolepsy. But after a parvovirus outbreak, Kilduff resigned from the project, drained from the strain of seeing so many dogs die.
A decade after his departure from the dog colony, his work would dramatically intersect with that of his successor, Emmanuel Mignot, MD, PhD.
“I thought I had closed the narcolepsy chapter in my life forever,” said Kilduff. “Then in 1998, we described this novel neuropeptide, hypocretin, that turned out to be the key to understanding the disorder.”
Narcoleptic Dogs in California, Mutant Mice in Texas
It was modafinil — the same anti-narcoleptic drug Yanagisawa’s team studied — that brought Emmanuel Mignot to the United States. After training as a pharmacologist in France, his home country sent him to Stanford to study the drug, which was discovered by French scientists, as his required military service.
As Kilduff’s replacement at the dog colony, his goal was to figure out how modafinil worked, hoping to attract a US company to develop the drug.
The plan succeeded. Modafinil became Provigil, a billion-dollar narcolepsy drug, and Mignot became “completely fascinated” with the disorder.
“I realized quickly that there was no way we’d find the cause of narcolepsy by finding the mode of action of this drug,” Mignot said. “Most likely, the drug was acting downstream, not at the cause of the disorder.”
To discover the answer, he needed to become a geneticist. And so began his 11-year odyssey to find the cause of canine narcolepsy.
After mapping the dog genome, Mignot set out to find the smallest stretch of chromosome that the narcoleptic animals had in common. “For a very long time, we were stuck with a relatively large region [of DNA],” he recalls. “It was a no man’s land.”
Within that region was the gene for the hypocretin/orexin-2 receptor — the same receptor that Yanagisawa had identified in his first orexin paper. Mignot didn’t immediately pursue that gene as a possibility — even though his students suggested it. Why?
“The decision was simply: Should we lose time to test a possible candidate [gene] among many?” Mignot said.
As Mignot studied dog DNA in California, Yanagisawa was creating mutant mice in Texas. Unbeknownst to either scientist, their work was about to converge.
What Happened Next Is Somewhat Disputed
After diagnosing his mice with narcolepsy, Yanagisawa opted not to share this finding with Mignot, though he knew about Mignot’s interest in the condition. Instead, he asked a colleague to find out how far along Mignot was in his genetics research.
According to Yanagisawa, his colleague didn’t realize how quickly DNA sequencing could happen once a target gene was identified. At a sleep meeting, “he showed Emmanuel all of our raw data. Almost accidentally, he disclosed our findings,” he said. “It was a shock for me.”
Unsure whether he was part of the orexin group, Mignot decided not to reveal that he’d identified the hypocretin/orexin-2 receptor gene as the faulty one in his narcoleptic dogs.
Although he didn’t share this finding, Mignot said he did offer to speak with the lead researcher to see if their findings were the same. If they were, they could jointly submit their articles. But Mignot never heard back.
Meanwhile, back at his lab, Mignot buckled down. While he wasn’t convinced the mouse data proved anything, it did give him the motivation to move faster.
Within weeks, he submitted his findings to Cell, revealing a mutation in the hypocretin/orexin-2 receptor gene as the cause of canine narcolepsy. According to Yanagisawa, the journal’s editor invited him to peer-review the paper, tipping him off to its existence.
“I told him I had a conflict of interest,” said Yanagisawa. “And then we scrambled to finish our manuscript. We wrote up the paper within almost 5 days.”
For a moment, it seemed both papers would be published together in Cell. Instead, on August 6, 1999, Mignot’s study was splashed solo across the journal’s cover.
“At the time, our team was pissed off, but looking back, what else could Emmanuel have done?” said Willie, who was part of Yanagisawa’s team. “The grant he’d been working on for years was at risk. He had it within his power to do the final experiments. Of course he was going to finish.”
Two weeks later, Yanagisawa’s findings followed, also in Cell.
His paper proposed knockout mice as a model for human narcolepsy and orexin as a key regulator of the sleep/wake cycle. With orexin-activated neurons branching into other areas of the brain, the peptide seemed to promote wakefulness by synchronizing several arousal neurotransmitters, such as serotonin, norepinephrine, and histamine.
“If you don’t have orexin, each of those systems can still function, but they’re not as coordinated,” said Willie. “If you have narcolepsy, you’re capable of wakefulness, and you’re capable of sleep. What you can’t do is prevent inappropriately switching between states.”
Together, the two papers painted a clear picture: Narcolepsy was the result of a dysfunction in the hypocretin/orexin system.
After more than a century, the cause of narcolepsy was starting to come into focus.
“This was blockbuster,” said Willie.
By itself, either finding — one in dogs, one in mice — might have been met with skepticism. But in combination, they offered indisputable evidence about narcolepsy’s cause.
The Human Brains in Your Fridge Hold Secrets
Jerome Siegel had been searching for the cause of human narcolepsy for years. A PhD and professor at the University of California, Los Angeles, he had managed to acquire four human narcoleptic brains. As laughter is often the trigger for the sudden shift to REM sleep in humans, he focused on the amygdala, an area linked to emotion.
“I looked in the amygdala and didn’t see anything,” he said. “So the brains stayed in my refrigerator for probably 10 years.”
Then he was invited to review Yanagisawa’s study in Cell. The lightbulb clicked on: Maybe the hypothalamus — not the amygdala — was the area of abnormality. He and his team dug out the decade-old brains.
When they stained the brains, the massive loss of hypocretin-activated neurons was hard to miss: On average, the narcoleptic brains had only about 7000 of the cells versus 70,000 in the average human brain. The scientists also noticed scar tissue in the hypothalamus, indicating that the neurons had at some point died, rather than being absent from birth.
What Siegel didn’t know: Mignot had also acquired a handful of human narcoleptic brains.
Already, he had coauthored a study showing that hypocretin/orexin was undetectable in the cerebrospinal fluid of the majority of the people with narcolepsy his team tested. It seemed clear that the hypocretin/orexin system was flawed — or even broken — in people with the condition.
“It looked like the cause of narcolepsy in humans was indeed this lack of orexin in the brain,” he said. “That was the hypothesis immediately. To me, this is when we established that narcolepsy in humans was due to a lack of orexin. The next thing was to check that the cells were missing.”
Now he could do exactly that.
As expected, Mignot’s team observed a dramatic loss of hypocretin/orexin cells in the narcoleptic brains. They also noticed that a different cell type in the hypothalamus was unaffected. This implied the damage was specific to the hypocretin-activated cells and supported a hunch they already had: That the deficit was the result not of a genetic defect but of an autoimmune attack. (It’s a hypothesis Mignot has spent the last 15 years proving.)
It wasn’t until a gathering in Hawaii, in late August 2000, that the two realized the overlap of their work.
To celebrate his team’s finding, Mignot had invited a group of researchers to Big Island. With his paper scheduled for publication on September 1, he felt comfortable presenting his findings to his guests, which included Siegel.
Until then, “I didn’t know what he had found, and he didn’t know what I had found, which basically was the same thing,” said Siegel.
In yet another strange twist, the two papers were published just weeks apart, simultaneously revealing that human narcoleptics have a depleted supply of the neurons that bind to hypocretin/orexin. The cause of the disorder was at last a certainty.
“Even if I was first, what does it matter? In the end, you need confirmation,” said Mignot. “You need multiple people to make sure that it’s true. It’s good science when things like this happen.”
How All of This Changed Medicine
Since these groundbreaking discoveries, the diagnosis of narcolepsy has become much simpler. Lab tests can now easily measure hypocretin in cerebrospinal fluid, providing a definitive diagnosis.
But the development of narcolepsy treatments has lagged — even though hypocretin/orexin replacement therapy is the obvious answer.
“Almost 25 years have elapsed, and there’s no such therapeutic on the market,” said Kilduff, who now works for SRI International, a non-profit research and development institute.
That’s partly because agonists — drugs that bind to receptors in the brain — are challenging to create, as this requires mimicking the activating molecule’s structure, like copying the grooves of an intricate key.
Antagonists, by comparison, are easier to develop. These act as a gate, blocking access to the receptors. As a result, drugs that promote sleep by thwarting hypocretin/orexin have emerged more quickly, providing a flurry of new options for people with insomnia. The first, suvorexant, was launched in 2014. Two others followed in recent years.
Researchers are hopeful a hypocretin/orexin agonist is on the horizon.
“This is a very hot area of drug development,” said Kilduff. “It’s just a matter of who’s going to get the drug to market first.”
One More Hypocretin/Orexin Surprise — and It Could Be The Biggest
Several years ago, Siegel’s lab received what was supposed to be a healthy human brain — one they could use as a comparison for narcoleptic brains. But researcher Thomas Thannickal, PhD, lead author of the UCLA study linking hypocretin loss to human narcolepsy, noticed something strange: This brain had significantly more hypocretin neurons than average.
Was this due to a seizure? A traumatic death? Siegel called the brain bank to request the donor’s records. He was told they were missing.
Years later, Siegel happened to be visiting the brain bank for another project and found himself in a room adjacent to the medical records. “Nobody was there,” he said, “so I just opened a drawer.”
Shuffling through the brain bank’s files, Siegel found the medical records he’d been told were lost. In the file was a note from the donor, explaining that he was a former heroin addict.
“I almost fell out of my chair,” said Siegel. “I realized this guy’s heroin addiction likely had something to do with his very unusual brain.”
Obviously, opioids affected the orexin system. But how?
“It’s when people are happy that this peptide is released,” said Siegel. “The hypocretin system is not just related to alertness. It’s related to pleasure.”
As Yanagisawa observed early on, hypocretin/orexin does indeed play a role in eating — just not the one he initially thought. The peptides prompted pleasure seeking. So the rodents ate.
In 2018, after acquiring five more brains, Siegel’s group published a study in Translational Medicine showing 54% more detectable hypocretin neurons in the brains of heroin addicts than in those of control individuals.
In 2022, another breakthrough: His team showed that morphine significantly altered the pathways of hypocretin neurons in mice, sending their axons into brain regions associated with addiction. Then, when they removed the mice’s hypocretin neurons and discontinued their daily morphine dose, the rodents showed no symptoms of opioid withdrawal.
This fits the connection with narcolepsy: Among the standard treatments for the condition are amphetamines and other stimulants, which all have addictive potential. Yet, “narcoleptics never abuse these drugs,” Siegel said. “They seem to be uniquely resistant to addiction.”
This could powerfully change the way opioids are administered.
“If you prevent the hypocretin response to opioids, you may be able to prevent opioid addiction,” said Siegel. In other words, blocking the hypocretin system with a drug like those used to treat insomnia may allow patients to experience the pain-relieving benefits of opioids — without the risk for addiction.
His team is currently investigating treatments targeting the hypocretin/orexin system for opioid addiction.
In a study published in July, they found that mice who received suvorexant — the drug for insomnia — didn’t anticipate their daily dose of opioids the way other rodents did. This suggests the medication prevented addiction, without diminishing the pain-relieving effect of opioids.
If it translates to humans, this discovery could potentially save millions of lives.
“I think it’s just us working on this,” said Siegel.
But with hypocretin/orexin, you never know.
A version of this article appeared on Medscape.com.
It was 1996, and Masashi Yanagisawa was on the brink of his next discovery.
The Japanese scientist had arrived at the University of Texas Southwestern in Dallas 5 years earlier, setting up his own lab at age 31. After earning his medical degree, he’d gained notoriety as a PhD student when he discovered endothelin, the body’s most potent vasoconstrictor.
Yanagisawa was about to prove this wasn’t a first-timer’s fluke.
His focus was G-protein–coupled receptors (GPCRs), cell surface receptors that respond to a range of molecules and a popular target for drug discovery. The Human Genome Project had just revealed a slew of newly discovered receptors, or “orphan” GPCRs, and identifying an activating molecule could yield a new drug. (That vasoconstrictor endothelin was one such success story, leading to four new drug approvals in the United States over the past quarter century.)
Yanagisawa and his team created 50 cell lines, each expressing one orphan receptor. They applied animal tissue to every line, along with a calcium-sensitive dye. If the cells glowed under the microscope, they had a hit.
“He was basically doing an elaborate fishing expedition,” said Jon Willie, MD, PhD, an associate professor of neurosurgery at Washington University School of Medicine in St. Louis, Missouri, who would later join Yanagisawa’s team.
It wasn’t long before the neon-green fluorescence signaled a match. After isolating the activating molecule, the scientists realized they were dealing with two neuropeptides.
No one had ever seen these proteins before. And no one knew their discovery would set off a decades-long journey that would finally solve a century-old medical mystery — and may even fix one of the biggest health crises of our time, as revealed by research published earlier in 2024. It’s a story of strange coincidences, serendipitous discoveries, and quirky details. Most of all, it’s a fascinating example of how basic science can revolutionize medicine — and how true breakthroughs happen over time and in real time.
But That’s Basic Science for You
Most basic science studies — the early, foundational research that provides the building blocks for science that follows — don’t lead to medical breakthroughs. But some do, often in surprising ways.
Also called curiosity-driven research, basic science aims to fill knowledge gaps to keep science moving, even if the trajectory isn’t always clear.
“The people working on the basic research that led to discoveries that transformed the modern world had no idea at the time,” said Isobel Ronai, PhD, a postdoctoral fellow in life sciences at Harvard University, Cambridge, Massachusetts. “Often, these stories can only be seen in hindsight,” sometimes decades later.
Case in point: For molecular biology techniques — things like DNA sequencing and gene targeting — the lag between basic science and breakthrough is, on average, 23 years. While many of the resulting techniques have received Nobel Prizes, few of the foundational discoveries have been awarded such accolades.
“The scientific glory is more often associated with the downstream applications,” said Ronai. “The importance of basic research can get lost. But it is the foundation for any future application, such as drug development.”
As funding is increasingly funneled toward applied research, basic science can require a certain persistence. What this under-appreciation can obscure is the pathway to discovery — which is often as compelling as the end result, full of unpredictable twists, turns, and even interpersonal intrigue.
And then there’s the fascinating — and definitely complicated — phenomenon of multiple independent discoveries.
As in: What happens when two independent teams discover the same thing at the same time?
Back to Yanagisawa’s Lab ...
... where he and his team learned a few things about those new neuropeptides. Rat brain studies pinpointed the lateral hypothalamus as the peptides’ area of activity — a region often called the brain’s feeding center.
“If you destroy that part of the brain, animals lose appetite,” said Yanagisawa. So these peptides must control feeding, the scientists thought.
Sure enough, injecting the proteins into rat brains led the rodents to start eating.
Satisfied, the team named them “orexin-A” and “orexin-B,” for the Greek word “orexis,” meaning appetite. The brain receptors became “orexin-1” and “orexin-2.” The team prepared to publish its findings in Cell.
But another group beat them to it.
Introducing the ‘Hypocretins’
In early January 1998, a team of Scripps Research Institute scientists, led by J. Gregor Sutcliffe, PhD, released a paper in the journal PNAS. They described a gene encoding for the precursor to two neuropeptides
As the peptides were in the hypothalamus and structurally like secretin (a gut hormone), they called them “hypocretins.” The hypocretin peptides excited neurons in the hypothalamus, and later that year, the scientists discovered that the neurons’ branches extended, tentacle-like, throughout the brain. “Many of the connected areas were involved in sleep-wake control,” said Thomas Kilduff, PhD, who joined the Sutcliffe lab just weeks before the hypocretin discovery. At the time, however, the significance of this finding was not yet clear.
Weeks later, in February 1998, Yanagisawa’s paper came out.
Somehow, two groups, over 1000 miles apart, had stumbled on the same neuropeptides at the same time.
“I first heard about [Yanagisawa’s] paper on NBC Nightly News,” recalls Kilduff. “I was skiing in the mountains, so I had to wait until Monday to get back to the lab to see what the paper was all about.”
He realized that Yanagisawa’s orexin was his lab’s hypocretin, although the study didn’t mention another team’s discovery.
“There may have been accusations. But as far as I know, it’s because [Yanagisawa] didn’t know [about the other paper],” said Willie. “This was not something he produced in 2 months. This was clearly years of work.”
‘Multiple Discovery’ Happens More Often Than You Think
In the mid-20th century, sociologist Robert Merton described the phenomenon of “multiple discovery,” where many scientific discoveries or inventions are made independently at roughly the same time.
“This happens much more frequently in scientific research than people suppose,” said David Pendlebury, head of research analysis at Clarivate’s Institute for Scientific Information, the analytics company’s research arm. (Last year, Pendlebury flagged the hypocretin/orexin discovery for Clarivate’s prestigious Citations Laureates award, an honor that aims to predict, often successfully, who will go on to win the Nobel Prize.)
“People have this idea of the lone researcher making a brilliant discovery,” Pendlebury said. “But more and more, teams find things at the same time.”
While this can — and does — lead to squabbling about who deserves credit, the desire to be first can also be highly motivating, said Mike Schneider, PhD, an assistant professor of philosophy at the University of Missouri, Columbia, who studies the social dynamics of science, potentially leading to faster scientific advancement.
The downside? If two groups produce the same or similar results, but one publishes first, scientific journals tend to reject the second, citing a lack of novelty.
Yet duplicating research is a key step in confirming the validity of a discovery.
That’s why, in 2018, the journal PLOS Biology created a provision for “scooped” scientists, allowing them to submit their paper within 6 months of the first as a complementary finding. Instead of viewing this as redundancy, the editors believe it adds robustness to the research.
‘What the Heck Is This Mouse Doing?’
Even though he’d been scooped, Yanagisawa forged on to the next challenge: Confirming whether orexin regulated feeding.
He began breeding mice missing the orexin gene. His team expected these “knockout” mice to eat less, resulting in a thinner body than other rodents. To the contrary, “they were on average fatter,” said Willie. “They were eating less but weighed more, indicating a slower metabolism.”
The researchers were befuddled. “We were really disappointed, almost desperate about what to do,” said Yanagisawa.
As nocturnal animals eat more at night, he decided they should study the mice after dark. One of his students, Richard Chemelli, MD, bought an infrared video camera from Radio Shack, filming the first 4 hours of the mice’s active period for several nights.
After watching the footage, “Rick called me and said, ‘Let’s get into the lab,’ ” said Willie. “It was four of us on a Saturday looking at these videos, saying, ‘What the heck is this mouse doing?’ ”
While exploring their habitat, the knockout mice would randomly fall over, pop back up after a minute or so, and resume normal activity. This happened over and over — and the scientists were unsure why.
They began monitoring the mice’s brains during these episodes — and made a startling discovery.
The mice weren’t having seizures. They were shifting directly into REM sleep, bypassing the non-REM stage, then quickly toggling back to wake mode.
“That’s when we knew these animals had something akin to narcolepsy,” said Willie.
The team recruited Thomas Scammell, MD, a Harvard neurologist, to investigate whether modafinil — an anti-narcoleptic drug without a clear mechanism — affected orexin neurons.
Two hours after injecting the mice with the medication, the scientists sacrificed them and stained their brains. Remarkably, the number of neurons showing orexin activity had increased ninefold. It seemed modafinil worked by activating the orexin system.
These findings had the potential to crack open the science of narcolepsy, one of the most mysterious sleep disorders.
Unless, of course, another team did it first.
The Mystery of Narcolepsy
Yet another multiple discovery, narcolepsy was first described by two scientists — one in Germany, the other in France — within a short span in the late 1800s.
It would be more than a hundred years before anyone understood the disorder’s cause, even though it affects about 1 in 2000 people.
“Patients were often labeled as lazy and malingerers,” said Kilduff, “since they were sleepy all the time and had this weird motor behavior called cataplexy” or the sudden loss of muscle tone.
In the early 1970s, William Dement, MD, PhD — “the father of sleep medicine” — was searching for a narcoleptic cat to study. He couldn’t find a feline, but several colleagues mentioned dogs with narcolepsy-like symptoms.
Dement, who died in 2020, had found his newest research subjects.
In 1973, he started a narcoleptic dog colony at Stanford University in Palo Alto, California. At first, he focused on poodles and beagles. After discovering their narcolepsy wasn’t genetic, he pivoted to dobermans and labradors. Their narcolepsy was inherited, so he could breed them to populate the colony.
Although human narcolepsy is rarely genetic, it’s otherwise a lot like the version in these dogs.
Both involve daytime sleepiness, “pathological” bouts of REM sleep, and the loss of muscle tone in response to emotions, often positive ones.
The researchers hoped the canines could unlock a treatment for human narcolepsy. They began laying out a path of dog kibble, then injecting the dogs with drugs such as selective serotonin reuptake inhibitors. They wanted to see what might help them stay awake as they excitedly chowed down.
Kilduff also started a molecular genetics program, trying to identify the genetic defect behind canine narcolepsy. But after a parvovirus outbreak, Kilduff resigned from the project, drained from the strain of seeing so many dogs die.
A decade after his departure from the dog colony, his work would dramatically intersect with that of his successor, Emmanuel Mignot, MD, PhD.
“I thought I had closed the narcolepsy chapter in my life forever,” said Kilduff. “Then in 1998, we described this novel neuropeptide, hypocretin, that turned out to be the key to understanding the disorder.”
Narcoleptic Dogs in California, Mutant Mice in Texas
It was modafinil — the same anti-narcoleptic drug Yanagisawa’s team studied — that brought Emmanuel Mignot to the United States. After training as a pharmacologist in France, his home country sent him to Stanford to study the drug, which was discovered by French scientists, as his required military service.
As Kilduff’s replacement at the dog colony, his goal was to figure out how modafinil worked, hoping to attract a US company to develop the drug.
The plan succeeded. Modafinil became Provigil, a billion-dollar narcolepsy drug, and Mignot became “completely fascinated” with the disorder.
“I realized quickly that there was no way we’d find the cause of narcolepsy by finding the mode of action of this drug,” Mignot said. “Most likely, the drug was acting downstream, not at the cause of the disorder.”
To discover the answer, he needed to become a geneticist. And so began his 11-year odyssey to find the cause of canine narcolepsy.
After mapping the dog genome, Mignot set out to find the smallest stretch of chromosome that the narcoleptic animals had in common. “For a very long time, we were stuck with a relatively large region [of DNA],” he recalls. “It was a no man’s land.”
Within that region was the gene for the hypocretin/orexin-2 receptor — the same receptor that Yanagisawa had identified in his first orexin paper. Mignot didn’t immediately pursue that gene as a possibility — even though his students suggested it. Why?
“The decision was simply: Should we lose time to test a possible candidate [gene] among many?” Mignot said.
As Mignot studied dog DNA in California, Yanagisawa was creating mutant mice in Texas. Unbeknownst to either scientist, their work was about to converge.
What Happened Next Is Somewhat Disputed
After diagnosing his mice with narcolepsy, Yanagisawa opted not to share this finding with Mignot, though he knew about Mignot’s interest in the condition. Instead, he asked a colleague to find out how far along Mignot was in his genetics research.
According to Yanagisawa, his colleague didn’t realize how quickly DNA sequencing could happen once a target gene was identified. At a sleep meeting, “he showed Emmanuel all of our raw data. Almost accidentally, he disclosed our findings,” he said. “It was a shock for me.”
Unsure whether he was part of the orexin group, Mignot decided not to reveal that he’d identified the hypocretin/orexin-2 receptor gene as the faulty one in his narcoleptic dogs.
Although he didn’t share this finding, Mignot said he did offer to speak with the lead researcher to see if their findings were the same. If they were, they could jointly submit their articles. But Mignot never heard back.
Meanwhile, back at his lab, Mignot buckled down. While he wasn’t convinced the mouse data proved anything, it did give him the motivation to move faster.
Within weeks, he submitted his findings to Cell, revealing a mutation in the hypocretin/orexin-2 receptor gene as the cause of canine narcolepsy. According to Yanagisawa, the journal’s editor invited him to peer-review the paper, tipping him off to its existence.
“I told him I had a conflict of interest,” said Yanagisawa. “And then we scrambled to finish our manuscript. We wrote up the paper within almost 5 days.”
For a moment, it seemed both papers would be published together in Cell. Instead, on August 6, 1999, Mignot’s study was splashed solo across the journal’s cover.
“At the time, our team was pissed off, but looking back, what else could Emmanuel have done?” said Willie, who was part of Yanagisawa’s team. “The grant he’d been working on for years was at risk. He had it within his power to do the final experiments. Of course he was going to finish.”
Two weeks later, Yanagisawa’s findings followed, also in Cell.
His paper proposed knockout mice as a model for human narcolepsy and orexin as a key regulator of the sleep/wake cycle. With orexin-activated neurons branching into other areas of the brain, the peptide seemed to promote wakefulness by synchronizing several arousal neurotransmitters, such as serotonin, norepinephrine, and histamine.
“If you don’t have orexin, each of those systems can still function, but they’re not as coordinated,” said Willie. “If you have narcolepsy, you’re capable of wakefulness, and you’re capable of sleep. What you can’t do is prevent inappropriately switching between states.”
Together, the two papers painted a clear picture: Narcolepsy was the result of a dysfunction in the hypocretin/orexin system.
After more than a century, the cause of narcolepsy was starting to come into focus.
“This was blockbuster,” said Willie.
By itself, either finding — one in dogs, one in mice — might have been met with skepticism. But in combination, they offered indisputable evidence about narcolepsy’s cause.
The Human Brains in Your Fridge Hold Secrets
Jerome Siegel had been searching for the cause of human narcolepsy for years. A PhD and professor at the University of California, Los Angeles, he had managed to acquire four human narcoleptic brains. As laughter is often the trigger for the sudden shift to REM sleep in humans, he focused on the amygdala, an area linked to emotion.
“I looked in the amygdala and didn’t see anything,” he said. “So the brains stayed in my refrigerator for probably 10 years.”
Then he was invited to review Yanagisawa’s study in Cell. The lightbulb clicked on: Maybe the hypothalamus — not the amygdala — was the area of abnormality. He and his team dug out the decade-old brains.
When they stained the brains, the massive loss of hypocretin-activated neurons was hard to miss: On average, the narcoleptic brains had only about 7000 of the cells versus 70,000 in the average human brain. The scientists also noticed scar tissue in the hypothalamus, indicating that the neurons had at some point died, rather than being absent from birth.
What Siegel didn’t know: Mignot had also acquired a handful of human narcoleptic brains.
Already, he had coauthored a study showing that hypocretin/orexin was undetectable in the cerebrospinal fluid of the majority of the people with narcolepsy his team tested. It seemed clear that the hypocretin/orexin system was flawed — or even broken — in people with the condition.
“It looked like the cause of narcolepsy in humans was indeed this lack of orexin in the brain,” he said. “That was the hypothesis immediately. To me, this is when we established that narcolepsy in humans was due to a lack of orexin. The next thing was to check that the cells were missing.”
Now he could do exactly that.
As expected, Mignot’s team observed a dramatic loss of hypocretin/orexin cells in the narcoleptic brains. They also noticed that a different cell type in the hypothalamus was unaffected. This implied the damage was specific to the hypocretin-activated cells and supported a hunch they already had: That the deficit was the result not of a genetic defect but of an autoimmune attack. (It’s a hypothesis Mignot has spent the last 15 years proving.)
It wasn’t until a gathering in Hawaii, in late August 2000, that the two realized the overlap of their work.
To celebrate his team’s finding, Mignot had invited a group of researchers to Big Island. With his paper scheduled for publication on September 1, he felt comfortable presenting his findings to his guests, which included Siegel.
Until then, “I didn’t know what he had found, and he didn’t know what I had found, which basically was the same thing,” said Siegel.
In yet another strange twist, the two papers were published just weeks apart, simultaneously revealing that human narcoleptics have a depleted supply of the neurons that bind to hypocretin/orexin. The cause of the disorder was at last a certainty.
“Even if I was first, what does it matter? In the end, you need confirmation,” said Mignot. “You need multiple people to make sure that it’s true. It’s good science when things like this happen.”
How All of This Changed Medicine
Since these groundbreaking discoveries, the diagnosis of narcolepsy has become much simpler. Lab tests can now easily measure hypocretin in cerebrospinal fluid, providing a definitive diagnosis.
But the development of narcolepsy treatments has lagged — even though hypocretin/orexin replacement therapy is the obvious answer.
“Almost 25 years have elapsed, and there’s no such therapeutic on the market,” said Kilduff, who now works for SRI International, a non-profit research and development institute.
That’s partly because agonists — drugs that bind to receptors in the brain — are challenging to create, as this requires mimicking the activating molecule’s structure, like copying the grooves of an intricate key.
Antagonists, by comparison, are easier to develop. These act as a gate, blocking access to the receptors. As a result, drugs that promote sleep by thwarting hypocretin/orexin have emerged more quickly, providing a flurry of new options for people with insomnia. The first, suvorexant, was launched in 2014. Two others followed in recent years.
Researchers are hopeful a hypocretin/orexin agonist is on the horizon.
“This is a very hot area of drug development,” said Kilduff. “It’s just a matter of who’s going to get the drug to market first.”
One More Hypocretin/Orexin Surprise — and It Could Be The Biggest
Several years ago, Siegel’s lab received what was supposed to be a healthy human brain — one they could use as a comparison for narcoleptic brains. But researcher Thomas Thannickal, PhD, lead author of the UCLA study linking hypocretin loss to human narcolepsy, noticed something strange: This brain had significantly more hypocretin neurons than average.
Was this due to a seizure? A traumatic death? Siegel called the brain bank to request the donor’s records. He was told they were missing.
Years later, Siegel happened to be visiting the brain bank for another project and found himself in a room adjacent to the medical records. “Nobody was there,” he said, “so I just opened a drawer.”
Shuffling through the brain bank’s files, Siegel found the medical records he’d been told were lost. In the file was a note from the donor, explaining that he was a former heroin addict.
“I almost fell out of my chair,” said Siegel. “I realized this guy’s heroin addiction likely had something to do with his very unusual brain.”
Obviously, opioids affected the orexin system. But how?
“It’s when people are happy that this peptide is released,” said Siegel. “The hypocretin system is not just related to alertness. It’s related to pleasure.”
As Yanagisawa observed early on, hypocretin/orexin does indeed play a role in eating — just not the one he initially thought. The peptides prompted pleasure seeking. So the rodents ate.
In 2018, after acquiring five more brains, Siegel’s group published a study in Translational Medicine showing 54% more detectable hypocretin neurons in the brains of heroin addicts than in those of control individuals.
In 2022, another breakthrough: His team showed that morphine significantly altered the pathways of hypocretin neurons in mice, sending their axons into brain regions associated with addiction. Then, when they removed the mice’s hypocretin neurons and discontinued their daily morphine dose, the rodents showed no symptoms of opioid withdrawal.
This fits the connection with narcolepsy: Among the standard treatments for the condition are amphetamines and other stimulants, which all have addictive potential. Yet, “narcoleptics never abuse these drugs,” Siegel said. “They seem to be uniquely resistant to addiction.”
This could powerfully change the way opioids are administered.
“If you prevent the hypocretin response to opioids, you may be able to prevent opioid addiction,” said Siegel. In other words, blocking the hypocretin system with a drug like those used to treat insomnia may allow patients to experience the pain-relieving benefits of opioids — without the risk for addiction.
His team is currently investigating treatments targeting the hypocretin/orexin system for opioid addiction.
In a study published in July, they found that mice who received suvorexant — the drug for insomnia — didn’t anticipate their daily dose of opioids the way other rodents did. This suggests the medication prevented addiction, without diminishing the pain-relieving effect of opioids.
If it translates to humans, this discovery could potentially save millions of lives.
“I think it’s just us working on this,” said Siegel.
But with hypocretin/orexin, you never know.
A version of this article appeared on Medscape.com.
Cancer Mortality Not Higher for Patients With Autoimmune Disease on Checkpoint Inhibitors
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Two Brain Stim Methods Better Than One for Depression?
TOPLINE:
a new study showed.
METHODOLOGY:
- Researchers conducted a double-blind, sham-controlled randomized clinical trial from 2021 to 2023 at three hospitals in China with 240 participants with MDD (mean age, 32.5 years; 58% women).
- Participants received active tDCS + active rTMS, sham tDCS + active rTMS, active tDCS + sham rTMS, or sham tDCS + sham rTMS with treatments administered five times per week for 2 weeks.
- tDCS was administered in 20-minute sessions using a 2-mA direct current stimulator, whereas rTMS involved 1600 pulses of 10-Hz stimulation targeting the left dorsolateral prefrontal cortex. Sham treatments used a pseudostimulation coil and only emitted sound.
- The primary outcome was change in the 24-item Hamilton Depression Rating Scale (HDRS-24) total score from baseline to week 2.
- Secondary outcomes included HDRS-24 total score change at week 4, remission rate (HDRS-24 total score ≤ 9), response rate (≥ 50% reduction in HDRS-24 total score), and adverse events.
TAKEAWAY:
- The active tDCS + active rTMS group demonstrated the greatest reduction in mean HDRS-24 score (18.33 ± 5.39) at week 2 compared with sham tDCS + active rTMS, active tDCS + sham rTMS, and sham tDCS + sham rTMS (P < .001).
- Response rates at week 2 were notably higher in the active tDCS + active rTMS group (85%) than in the active tDCS + sham rTMS (30%) and sham tDCS + sham rTMS groups (32%).
- The remission rate at week 4 reached 83% in the active tDCS + active rTMS group, which was significantly higher than the remission rates with the other interventions (P < .001).
- The treatments were well tolerated, with no serious adverse events, seizures, or manic symptoms reported across all intervention groups.
IN PRACTICE:
This trial “was the first to evaluate the safety, feasibility, and efficacy of combining tDCS and rTMS in treating depression. Future studies should focus on investigating the mechanism of this synergistic effect and improving the stimulation parameters to optimize the therapeutic effect,” the investigators wrote.
SOURCE:
This study was led by Dongsheng Zhou, MD, Ningbo Kangning Hospital, Ningbo, China. It was published online in JAMA Network Open.
LIMITATIONS:
The brief treatment duration involving 10 sessions may have been insufficient for tDCS and rTMS to demonstrate their full antidepressant potential. The inability to regulate participants’ antidepressant medications throughout the study period presented another limitation. Additionally, the lack of stratified randomization and adjustment for center effects may have introduced variability in the results.
DISCLOSURES:
This study received support from multiple grants, including from the Natural Science Foundation of Zhejiang Province, Basic Public Welfare Research Project of Zhejiang Province, Ningbo Medical and Health Brand Discipline, Ningbo Clinical Medical Research Centre for Mental Health, Ningbo Top Medical and Health Research Program, and the Zhejiang Medical and Health Science and Technology Plan Project. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
a new study showed.
METHODOLOGY:
- Researchers conducted a double-blind, sham-controlled randomized clinical trial from 2021 to 2023 at three hospitals in China with 240 participants with MDD (mean age, 32.5 years; 58% women).
- Participants received active tDCS + active rTMS, sham tDCS + active rTMS, active tDCS + sham rTMS, or sham tDCS + sham rTMS with treatments administered five times per week for 2 weeks.
- tDCS was administered in 20-minute sessions using a 2-mA direct current stimulator, whereas rTMS involved 1600 pulses of 10-Hz stimulation targeting the left dorsolateral prefrontal cortex. Sham treatments used a pseudostimulation coil and only emitted sound.
- The primary outcome was change in the 24-item Hamilton Depression Rating Scale (HDRS-24) total score from baseline to week 2.
- Secondary outcomes included HDRS-24 total score change at week 4, remission rate (HDRS-24 total score ≤ 9), response rate (≥ 50% reduction in HDRS-24 total score), and adverse events.
TAKEAWAY:
- The active tDCS + active rTMS group demonstrated the greatest reduction in mean HDRS-24 score (18.33 ± 5.39) at week 2 compared with sham tDCS + active rTMS, active tDCS + sham rTMS, and sham tDCS + sham rTMS (P < .001).
- Response rates at week 2 were notably higher in the active tDCS + active rTMS group (85%) than in the active tDCS + sham rTMS (30%) and sham tDCS + sham rTMS groups (32%).
- The remission rate at week 4 reached 83% in the active tDCS + active rTMS group, which was significantly higher than the remission rates with the other interventions (P < .001).
- The treatments were well tolerated, with no serious adverse events, seizures, or manic symptoms reported across all intervention groups.
IN PRACTICE:
This trial “was the first to evaluate the safety, feasibility, and efficacy of combining tDCS and rTMS in treating depression. Future studies should focus on investigating the mechanism of this synergistic effect and improving the stimulation parameters to optimize the therapeutic effect,” the investigators wrote.
SOURCE:
This study was led by Dongsheng Zhou, MD, Ningbo Kangning Hospital, Ningbo, China. It was published online in JAMA Network Open.
LIMITATIONS:
The brief treatment duration involving 10 sessions may have been insufficient for tDCS and rTMS to demonstrate their full antidepressant potential. The inability to regulate participants’ antidepressant medications throughout the study period presented another limitation. Additionally, the lack of stratified randomization and adjustment for center effects may have introduced variability in the results.
DISCLOSURES:
This study received support from multiple grants, including from the Natural Science Foundation of Zhejiang Province, Basic Public Welfare Research Project of Zhejiang Province, Ningbo Medical and Health Brand Discipline, Ningbo Clinical Medical Research Centre for Mental Health, Ningbo Top Medical and Health Research Program, and the Zhejiang Medical and Health Science and Technology Plan Project. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
a new study showed.
METHODOLOGY:
- Researchers conducted a double-blind, sham-controlled randomized clinical trial from 2021 to 2023 at three hospitals in China with 240 participants with MDD (mean age, 32.5 years; 58% women).
- Participants received active tDCS + active rTMS, sham tDCS + active rTMS, active tDCS + sham rTMS, or sham tDCS + sham rTMS with treatments administered five times per week for 2 weeks.
- tDCS was administered in 20-minute sessions using a 2-mA direct current stimulator, whereas rTMS involved 1600 pulses of 10-Hz stimulation targeting the left dorsolateral prefrontal cortex. Sham treatments used a pseudostimulation coil and only emitted sound.
- The primary outcome was change in the 24-item Hamilton Depression Rating Scale (HDRS-24) total score from baseline to week 2.
- Secondary outcomes included HDRS-24 total score change at week 4, remission rate (HDRS-24 total score ≤ 9), response rate (≥ 50% reduction in HDRS-24 total score), and adverse events.
TAKEAWAY:
- The active tDCS + active rTMS group demonstrated the greatest reduction in mean HDRS-24 score (18.33 ± 5.39) at week 2 compared with sham tDCS + active rTMS, active tDCS + sham rTMS, and sham tDCS + sham rTMS (P < .001).
- Response rates at week 2 were notably higher in the active tDCS + active rTMS group (85%) than in the active tDCS + sham rTMS (30%) and sham tDCS + sham rTMS groups (32%).
- The remission rate at week 4 reached 83% in the active tDCS + active rTMS group, which was significantly higher than the remission rates with the other interventions (P < .001).
- The treatments were well tolerated, with no serious adverse events, seizures, or manic symptoms reported across all intervention groups.
IN PRACTICE:
This trial “was the first to evaluate the safety, feasibility, and efficacy of combining tDCS and rTMS in treating depression. Future studies should focus on investigating the mechanism of this synergistic effect and improving the stimulation parameters to optimize the therapeutic effect,” the investigators wrote.
SOURCE:
This study was led by Dongsheng Zhou, MD, Ningbo Kangning Hospital, Ningbo, China. It was published online in JAMA Network Open.
LIMITATIONS:
The brief treatment duration involving 10 sessions may have been insufficient for tDCS and rTMS to demonstrate their full antidepressant potential. The inability to regulate participants’ antidepressant medications throughout the study period presented another limitation. Additionally, the lack of stratified randomization and adjustment for center effects may have introduced variability in the results.
DISCLOSURES:
This study received support from multiple grants, including from the Natural Science Foundation of Zhejiang Province, Basic Public Welfare Research Project of Zhejiang Province, Ningbo Medical and Health Brand Discipline, Ningbo Clinical Medical Research Centre for Mental Health, Ningbo Top Medical and Health Research Program, and the Zhejiang Medical and Health Science and Technology Plan Project. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Daytime Sleepiness May Flag Predementia Risk
TOPLINE:
a new study shows.
METHODOLOGY:
- Researchers included 445 older adults without dementia (mean age, 76 years; 57% women).
- Sleep components were assessed, and participants were classified as poor or good sleepers using the Pittsburgh Sleep Quality Index questionnaire.
- The primary outcome was incidence of MCR syndrome.
- The mean follow-up duration was 2.9 years.
TAKEAWAY:
- During the study period, 36 participants developed MCR syndrome.
- Poor sleepers had a higher risk for incident MCR syndrome, compared with good sleepers, after adjustment for age, sex, and educational level (adjusted hazard ratio [aHR], 2.6; 95% CI, 1.3-5.0; P < .05). However, this association was no longer significant after further adjustment for depressive symptoms.
- Sleep-related daytime dysfunction, defined as excessive sleepiness and lower enthusiasm for activities, was the only sleep component linked to a significant risk for MCR syndrome in fully adjusted models (aHR, 3.3; 95% CI, 1.5-7.4; P < .05).
- Prevalent MCR syndrome was not significantly associated with poor sleep quality (odds ratio, 1.1), suggesting that the relationship is unidirectional.
IN PRACTICE:
“Establishing the relationship between sleep dysfunction and MCR [syndrome] risk is important because early intervention may offer the best hope for preventing dementia,” the investigators wrote.
“Our findings emphasize the need for screening for sleep issues. There’s potential that people could get help with their sleep issues and prevent cognitive decline later in life,” lead author Victoire Leroy, MD, PhD, Albert Einstein College of Medicine, New York City, added in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
Study limitations included the lack of objective sleep measurements and potential recall bias in self-reported sleep complaints, particularly among participants with cognitive issues. In addition, the relatively short follow-up period may have resulted in a lower number of incident MCR syndrome cases. The sample population was also predominantly White (80%), which may have limited the generalizability of the findings to other populations.
DISCLOSURES:
The study was funded by the National Institute on Aging. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
a new study shows.
METHODOLOGY:
- Researchers included 445 older adults without dementia (mean age, 76 years; 57% women).
- Sleep components were assessed, and participants were classified as poor or good sleepers using the Pittsburgh Sleep Quality Index questionnaire.
- The primary outcome was incidence of MCR syndrome.
- The mean follow-up duration was 2.9 years.
TAKEAWAY:
- During the study period, 36 participants developed MCR syndrome.
- Poor sleepers had a higher risk for incident MCR syndrome, compared with good sleepers, after adjustment for age, sex, and educational level (adjusted hazard ratio [aHR], 2.6; 95% CI, 1.3-5.0; P < .05). However, this association was no longer significant after further adjustment for depressive symptoms.
- Sleep-related daytime dysfunction, defined as excessive sleepiness and lower enthusiasm for activities, was the only sleep component linked to a significant risk for MCR syndrome in fully adjusted models (aHR, 3.3; 95% CI, 1.5-7.4; P < .05).
- Prevalent MCR syndrome was not significantly associated with poor sleep quality (odds ratio, 1.1), suggesting that the relationship is unidirectional.
IN PRACTICE:
“Establishing the relationship between sleep dysfunction and MCR [syndrome] risk is important because early intervention may offer the best hope for preventing dementia,” the investigators wrote.
“Our findings emphasize the need for screening for sleep issues. There’s potential that people could get help with their sleep issues and prevent cognitive decline later in life,” lead author Victoire Leroy, MD, PhD, Albert Einstein College of Medicine, New York City, added in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
Study limitations included the lack of objective sleep measurements and potential recall bias in self-reported sleep complaints, particularly among participants with cognitive issues. In addition, the relatively short follow-up period may have resulted in a lower number of incident MCR syndrome cases. The sample population was also predominantly White (80%), which may have limited the generalizability of the findings to other populations.
DISCLOSURES:
The study was funded by the National Institute on Aging. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
a new study shows.
METHODOLOGY:
- Researchers included 445 older adults without dementia (mean age, 76 years; 57% women).
- Sleep components were assessed, and participants were classified as poor or good sleepers using the Pittsburgh Sleep Quality Index questionnaire.
- The primary outcome was incidence of MCR syndrome.
- The mean follow-up duration was 2.9 years.
TAKEAWAY:
- During the study period, 36 participants developed MCR syndrome.
- Poor sleepers had a higher risk for incident MCR syndrome, compared with good sleepers, after adjustment for age, sex, and educational level (adjusted hazard ratio [aHR], 2.6; 95% CI, 1.3-5.0; P < .05). However, this association was no longer significant after further adjustment for depressive symptoms.
- Sleep-related daytime dysfunction, defined as excessive sleepiness and lower enthusiasm for activities, was the only sleep component linked to a significant risk for MCR syndrome in fully adjusted models (aHR, 3.3; 95% CI, 1.5-7.4; P < .05).
- Prevalent MCR syndrome was not significantly associated with poor sleep quality (odds ratio, 1.1), suggesting that the relationship is unidirectional.
IN PRACTICE:
“Establishing the relationship between sleep dysfunction and MCR [syndrome] risk is important because early intervention may offer the best hope for preventing dementia,” the investigators wrote.
“Our findings emphasize the need for screening for sleep issues. There’s potential that people could get help with their sleep issues and prevent cognitive decline later in life,” lead author Victoire Leroy, MD, PhD, Albert Einstein College of Medicine, New York City, added in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
Study limitations included the lack of objective sleep measurements and potential recall bias in self-reported sleep complaints, particularly among participants with cognitive issues. In addition, the relatively short follow-up period may have resulted in a lower number of incident MCR syndrome cases. The sample population was also predominantly White (80%), which may have limited the generalizability of the findings to other populations.
DISCLOSURES:
The study was funded by the National Institute on Aging. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Impact and Recovery of VHA Epilepsy Care Services During the COVID-19 Pandemic
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.
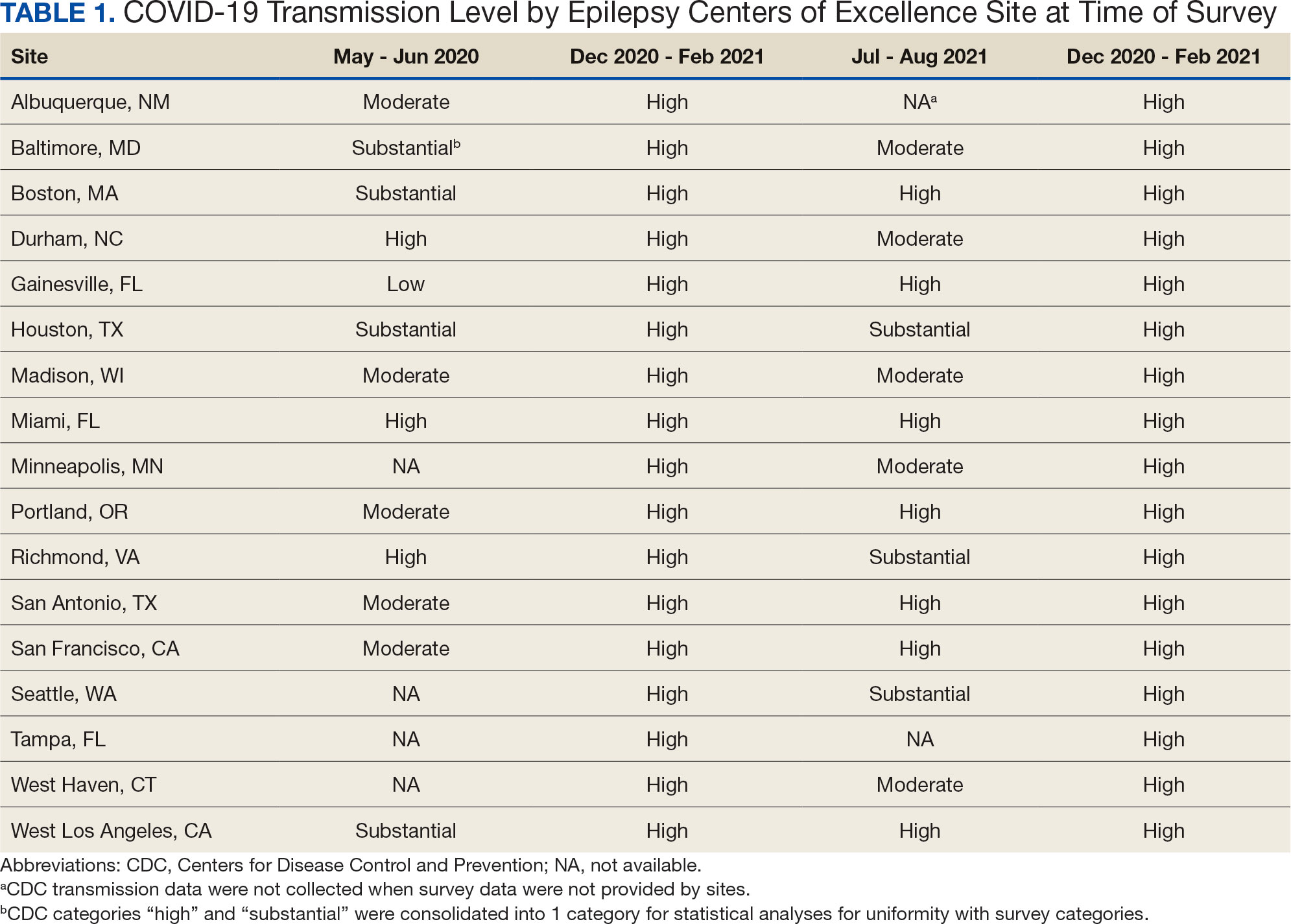
Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).
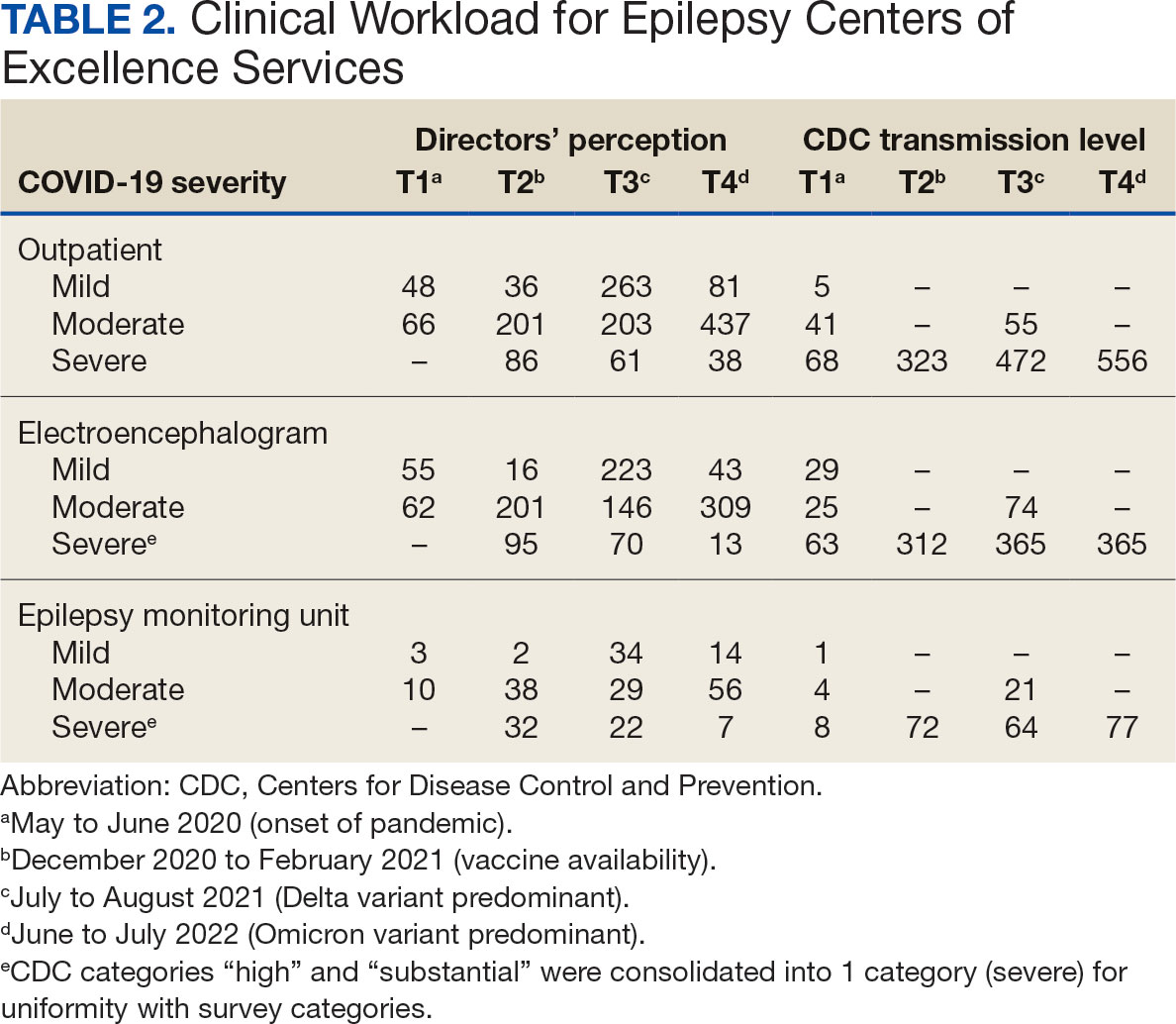
Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.

Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).

Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.

Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).

Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
Managing Diabetes and Dementia in Long-Term Care
VANCOUVER, BRITISH COLUMBIA — Conditions like diabetes and dementia are common in patients who are admitted to long-term care facilities, but aggressive management of these conditions in long-term care residents is not recommended, according to a presentation given at the Family Medicine Forum (FMF) 2024.
Hospitalizations for hypoglycemia are risky for patients with diabetes who are residents of long-term care facilities, particularly those aged 75 years or older, said Adam Gurau, MD, a family physician in Toronto. Gurau completed a fellowship in care of the elderly at the University of Toronto, in Ontario, Canada.
“A lot of studies have shown diabetes-related hospitalizations,” said Gurau. He cited a 2014 study that found that hypoglycemia hospitalization rates were twice as high in older patients (age, 75 years or older) as in younger patients (age, 65-74 years).
“It is important to keep in mind that our residents in long-term care are at increasing risk for hypoglycemia, and we really should try to reduce [this risk] and not use dangerous medications or potentially dangerous [means of] diabetes management,” said Gurau.
A Canadian study that examined the composite risk for emergency department visits, hospitalizations, or death within 30 days of reaching intensive glycemic control with high-risk agents (such as insulin or sulfonylureas) suggested little benefit and possible harm in using these agents in adults aged 75 years or older.
In addition, current guidelines on diabetes management encourage a different approach. “Looking at some of the more recent North American guidelines, many of them actually now recommend relaxing glycemic targets to reduce overtreatment and prevent hypoglycemia,” said Gurau.
Deprescribing Medications
Medication reviews present opportunities for taking a global view of a patient’s treatments and determining whether any drug can be removed from the list. “What we want to do is optimize medications,” said Gurau. “We’re not talking about adding medications. We’re talking about removing medications, which is, I think, what we should be doing.”
Some research suggests that patients are open to deprescribing. One survey examined older adults (mean age, 79.1 years) with three or more chronic conditions who had been prescribed at least five medications. The researchers found that most participants (77%) were willing to deprescribe one or more medicines if a doctor advised that it was possible. “General practitioners may be able to increase deprescribing by building trust with their patients and communicating evidence about the risks of medication use,” the researchers wrote.
About 62% of seniors living in a residential care home have a diagnosis of Alzheimer’s disease or another dementia, according to the Alzheimer Society of Canada. Evidence suggests that nonpharmacologic approaches, such as massage and touch therapy and music, can manage neuropsychiatric symptoms, such as aggression and agitation, that are associated with dementia in older adults, noted Gurau.
“We want to focus on nonpharmacologic approaches for many of these [long-term care] residents,” said Gurau. “We have to do as much as we can to exhaust all the nonpharmacologic approaches.”
Preventing Hospitalizations
Another challenge to tackle in long-term care is the unnecessary transfer of residents to hospital emergency departments, according to Gurau. “In many situations, it’s worth trying as hard as we can to treat them in the nursing home, as opposed to having them go to hospital.”
Researchers estimated that 25% of the transfers from long-term care facilities in Canada to hospital emergency departments in 2014 were potentially preventable.
Urinary tract infections accounted for 30% of hospital emergency department visits for potentially preventable conditions by older patients who are residents in long-term care, according to 2013-2014 data from the Canadian Institute for Health Information.
“There are lots of downsides to going to the hospital [from long-term care],” Gurau told this news organization. “There are risks for infections, risks for increasing delirium and agitation [in patients with dementia], and risks for other behavior that can really impact somebody’s life.”
Gurau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER, BRITISH COLUMBIA — Conditions like diabetes and dementia are common in patients who are admitted to long-term care facilities, but aggressive management of these conditions in long-term care residents is not recommended, according to a presentation given at the Family Medicine Forum (FMF) 2024.
Hospitalizations for hypoglycemia are risky for patients with diabetes who are residents of long-term care facilities, particularly those aged 75 years or older, said Adam Gurau, MD, a family physician in Toronto. Gurau completed a fellowship in care of the elderly at the University of Toronto, in Ontario, Canada.
“A lot of studies have shown diabetes-related hospitalizations,” said Gurau. He cited a 2014 study that found that hypoglycemia hospitalization rates were twice as high in older patients (age, 75 years or older) as in younger patients (age, 65-74 years).
“It is important to keep in mind that our residents in long-term care are at increasing risk for hypoglycemia, and we really should try to reduce [this risk] and not use dangerous medications or potentially dangerous [means of] diabetes management,” said Gurau.
A Canadian study that examined the composite risk for emergency department visits, hospitalizations, or death within 30 days of reaching intensive glycemic control with high-risk agents (such as insulin or sulfonylureas) suggested little benefit and possible harm in using these agents in adults aged 75 years or older.
In addition, current guidelines on diabetes management encourage a different approach. “Looking at some of the more recent North American guidelines, many of them actually now recommend relaxing glycemic targets to reduce overtreatment and prevent hypoglycemia,” said Gurau.
Deprescribing Medications
Medication reviews present opportunities for taking a global view of a patient’s treatments and determining whether any drug can be removed from the list. “What we want to do is optimize medications,” said Gurau. “We’re not talking about adding medications. We’re talking about removing medications, which is, I think, what we should be doing.”
Some research suggests that patients are open to deprescribing. One survey examined older adults (mean age, 79.1 years) with three or more chronic conditions who had been prescribed at least five medications. The researchers found that most participants (77%) were willing to deprescribe one or more medicines if a doctor advised that it was possible. “General practitioners may be able to increase deprescribing by building trust with their patients and communicating evidence about the risks of medication use,” the researchers wrote.
About 62% of seniors living in a residential care home have a diagnosis of Alzheimer’s disease or another dementia, according to the Alzheimer Society of Canada. Evidence suggests that nonpharmacologic approaches, such as massage and touch therapy and music, can manage neuropsychiatric symptoms, such as aggression and agitation, that are associated with dementia in older adults, noted Gurau.
“We want to focus on nonpharmacologic approaches for many of these [long-term care] residents,” said Gurau. “We have to do as much as we can to exhaust all the nonpharmacologic approaches.”
Preventing Hospitalizations
Another challenge to tackle in long-term care is the unnecessary transfer of residents to hospital emergency departments, according to Gurau. “In many situations, it’s worth trying as hard as we can to treat them in the nursing home, as opposed to having them go to hospital.”
Researchers estimated that 25% of the transfers from long-term care facilities in Canada to hospital emergency departments in 2014 were potentially preventable.
Urinary tract infections accounted for 30% of hospital emergency department visits for potentially preventable conditions by older patients who are residents in long-term care, according to 2013-2014 data from the Canadian Institute for Health Information.
“There are lots of downsides to going to the hospital [from long-term care],” Gurau told this news organization. “There are risks for infections, risks for increasing delirium and agitation [in patients with dementia], and risks for other behavior that can really impact somebody’s life.”
Gurau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER, BRITISH COLUMBIA — Conditions like diabetes and dementia are common in patients who are admitted to long-term care facilities, but aggressive management of these conditions in long-term care residents is not recommended, according to a presentation given at the Family Medicine Forum (FMF) 2024.
Hospitalizations for hypoglycemia are risky for patients with diabetes who are residents of long-term care facilities, particularly those aged 75 years or older, said Adam Gurau, MD, a family physician in Toronto. Gurau completed a fellowship in care of the elderly at the University of Toronto, in Ontario, Canada.
“A lot of studies have shown diabetes-related hospitalizations,” said Gurau. He cited a 2014 study that found that hypoglycemia hospitalization rates were twice as high in older patients (age, 75 years or older) as in younger patients (age, 65-74 years).
“It is important to keep in mind that our residents in long-term care are at increasing risk for hypoglycemia, and we really should try to reduce [this risk] and not use dangerous medications or potentially dangerous [means of] diabetes management,” said Gurau.
A Canadian study that examined the composite risk for emergency department visits, hospitalizations, or death within 30 days of reaching intensive glycemic control with high-risk agents (such as insulin or sulfonylureas) suggested little benefit and possible harm in using these agents in adults aged 75 years or older.
In addition, current guidelines on diabetes management encourage a different approach. “Looking at some of the more recent North American guidelines, many of them actually now recommend relaxing glycemic targets to reduce overtreatment and prevent hypoglycemia,” said Gurau.
Deprescribing Medications
Medication reviews present opportunities for taking a global view of a patient’s treatments and determining whether any drug can be removed from the list. “What we want to do is optimize medications,” said Gurau. “We’re not talking about adding medications. We’re talking about removing medications, which is, I think, what we should be doing.”
Some research suggests that patients are open to deprescribing. One survey examined older adults (mean age, 79.1 years) with three or more chronic conditions who had been prescribed at least five medications. The researchers found that most participants (77%) were willing to deprescribe one or more medicines if a doctor advised that it was possible. “General practitioners may be able to increase deprescribing by building trust with their patients and communicating evidence about the risks of medication use,” the researchers wrote.
About 62% of seniors living in a residential care home have a diagnosis of Alzheimer’s disease or another dementia, according to the Alzheimer Society of Canada. Evidence suggests that nonpharmacologic approaches, such as massage and touch therapy and music, can manage neuropsychiatric symptoms, such as aggression and agitation, that are associated with dementia in older adults, noted Gurau.
“We want to focus on nonpharmacologic approaches for many of these [long-term care] residents,” said Gurau. “We have to do as much as we can to exhaust all the nonpharmacologic approaches.”
Preventing Hospitalizations
Another challenge to tackle in long-term care is the unnecessary transfer of residents to hospital emergency departments, according to Gurau. “In many situations, it’s worth trying as hard as we can to treat them in the nursing home, as opposed to having them go to hospital.”
Researchers estimated that 25% of the transfers from long-term care facilities in Canada to hospital emergency departments in 2014 were potentially preventable.
Urinary tract infections accounted for 30% of hospital emergency department visits for potentially preventable conditions by older patients who are residents in long-term care, according to 2013-2014 data from the Canadian Institute for Health Information.
“There are lots of downsides to going to the hospital [from long-term care],” Gurau told this news organization. “There are risks for infections, risks for increasing delirium and agitation [in patients with dementia], and risks for other behavior that can really impact somebody’s life.”
Gurau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FMF 2024