User login
A (former) skeptic’s view of bariatric surgery
Because of the high prevalence of obesity and diabetes, bariatric surgery has become very popular. In the United States alone, there were an estimated 228,000 weight loss surgical procedures performed in 2017.1
But I must confess that for many years, I was skeptical about the value of surgery to treat obesity. Yes, everyone who had a bariatric procedure lost weight, but did the long-term benefits really outweigh the harms? I wondered if most people gradually gained back the weight they lost. And the harms can be significant, including dumping syndrome, hypoglycemia, and malabsorption—in addition to the potential for surgical complications and repeat surgery. And, I must confess that my views were likely affected by the death of a friend from complications of gastric bypass 25 years ago.
My skepticism, however, has changed to cautious optimism for carefully selected patients. I say this because we now have long-term follow-up studies demonstrating the value of bariatric procedures—especially for people with type 2 diabetes.
Most studies have been cohort studies that compare results to similar patients with obesity who did not have surgery, and the outcomes have been consistently better in patients who underwent surgery. Two recent meta-analyses summarized these results; one for all patients with obesity and the other for patients with type 2 diabetes.
Continue to: The first meta-analysis
The first meta-analysis included 11 randomized trials, 4 nonrandomized controlled trials, and 17 cohort studies and showed probable reductions in all-cause mortality and possible reductions in cancer and cardiovascular events.2 The second demonstrated significant improvements in microvascular and macrovascular disease and reduced mortality.3 The data were limited, however, because of the lack of large randomized trials with long-term follow-up.
The Stampede trial is one of a few bariatric surgery randomized trials focusing on patients with diabetes.4 The 5-year follow-up results are impressive. Nearly 30% of patients who had gastric bypass and 23% who had sleeve gastrectomy had an A1C ≤6 at 5 years compared to only 5% of those treated medically. Some patients discontinued all medications for diabetes, hypertension, and hyperlipidemia.
There is now adequate research to show that bariatric surgery provides significant benefits to properly selected patients who understand the risks. I no longer hesitate to refer patients for bariatric surgery who have been unsuccessful with weight loss—despite their best efforts.
Where do you stand?
1. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed September 18, 2018.
2. Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26:2590-2601.
3. Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27:2724-2732.
4. Schauer PR, Bhatt DL, Kirwan JP; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
Because of the high prevalence of obesity and diabetes, bariatric surgery has become very popular. In the United States alone, there were an estimated 228,000 weight loss surgical procedures performed in 2017.1
But I must confess that for many years, I was skeptical about the value of surgery to treat obesity. Yes, everyone who had a bariatric procedure lost weight, but did the long-term benefits really outweigh the harms? I wondered if most people gradually gained back the weight they lost. And the harms can be significant, including dumping syndrome, hypoglycemia, and malabsorption—in addition to the potential for surgical complications and repeat surgery. And, I must confess that my views were likely affected by the death of a friend from complications of gastric bypass 25 years ago.
My skepticism, however, has changed to cautious optimism for carefully selected patients. I say this because we now have long-term follow-up studies demonstrating the value of bariatric procedures—especially for people with type 2 diabetes.
Most studies have been cohort studies that compare results to similar patients with obesity who did not have surgery, and the outcomes have been consistently better in patients who underwent surgery. Two recent meta-analyses summarized these results; one for all patients with obesity and the other for patients with type 2 diabetes.
Continue to: The first meta-analysis
The first meta-analysis included 11 randomized trials, 4 nonrandomized controlled trials, and 17 cohort studies and showed probable reductions in all-cause mortality and possible reductions in cancer and cardiovascular events.2 The second demonstrated significant improvements in microvascular and macrovascular disease and reduced mortality.3 The data were limited, however, because of the lack of large randomized trials with long-term follow-up.
The Stampede trial is one of a few bariatric surgery randomized trials focusing on patients with diabetes.4 The 5-year follow-up results are impressive. Nearly 30% of patients who had gastric bypass and 23% who had sleeve gastrectomy had an A1C ≤6 at 5 years compared to only 5% of those treated medically. Some patients discontinued all medications for diabetes, hypertension, and hyperlipidemia.
There is now adequate research to show that bariatric surgery provides significant benefits to properly selected patients who understand the risks. I no longer hesitate to refer patients for bariatric surgery who have been unsuccessful with weight loss—despite their best efforts.
Where do you stand?
Because of the high prevalence of obesity and diabetes, bariatric surgery has become very popular. In the United States alone, there were an estimated 228,000 weight loss surgical procedures performed in 2017.1
But I must confess that for many years, I was skeptical about the value of surgery to treat obesity. Yes, everyone who had a bariatric procedure lost weight, but did the long-term benefits really outweigh the harms? I wondered if most people gradually gained back the weight they lost. And the harms can be significant, including dumping syndrome, hypoglycemia, and malabsorption—in addition to the potential for surgical complications and repeat surgery. And, I must confess that my views were likely affected by the death of a friend from complications of gastric bypass 25 years ago.
My skepticism, however, has changed to cautious optimism for carefully selected patients. I say this because we now have long-term follow-up studies demonstrating the value of bariatric procedures—especially for people with type 2 diabetes.
Most studies have been cohort studies that compare results to similar patients with obesity who did not have surgery, and the outcomes have been consistently better in patients who underwent surgery. Two recent meta-analyses summarized these results; one for all patients with obesity and the other for patients with type 2 diabetes.
Continue to: The first meta-analysis
The first meta-analysis included 11 randomized trials, 4 nonrandomized controlled trials, and 17 cohort studies and showed probable reductions in all-cause mortality and possible reductions in cancer and cardiovascular events.2 The second demonstrated significant improvements in microvascular and macrovascular disease and reduced mortality.3 The data were limited, however, because of the lack of large randomized trials with long-term follow-up.
The Stampede trial is one of a few bariatric surgery randomized trials focusing on patients with diabetes.4 The 5-year follow-up results are impressive. Nearly 30% of patients who had gastric bypass and 23% who had sleeve gastrectomy had an A1C ≤6 at 5 years compared to only 5% of those treated medically. Some patients discontinued all medications for diabetes, hypertension, and hyperlipidemia.
There is now adequate research to show that bariatric surgery provides significant benefits to properly selected patients who understand the risks. I no longer hesitate to refer patients for bariatric surgery who have been unsuccessful with weight loss—despite their best efforts.
Where do you stand?
1. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed September 18, 2018.
2. Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26:2590-2601.
3. Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27:2724-2732.
4. Schauer PR, Bhatt DL, Kirwan JP; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
1. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed September 18, 2018.
2. Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26:2590-2601.
3. Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27:2724-2732.
4. Schauer PR, Bhatt DL, Kirwan JP; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
Obesity: When to consider surgery
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5
For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
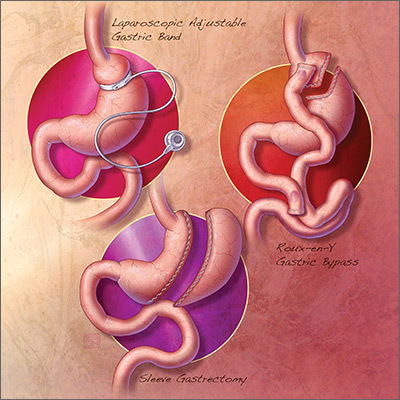
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; srb9023@nyp.org
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5
For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; srb9023@nyp.org
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5
For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; srb9023@nyp.org
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
PRACTICE RECOMMENDATIONS
Among adult patients with body mass index* ≥40, or ≥35 with obesity-related comorbid conditions:
› Consider bariatric surgery in those who are motivated to lose weight but who have not responded to lifestyle modification with or without pharmacotherapy in order to achieve sufficient and sustained weight loss. A
› Consider bariatric surgery to help patients achieve target health goals and reduce/improve obesity-related comorbidities. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
*Calculated as weight in kilograms divided by height in meters squared.
U.S. obesity continues to advance
The prevalence of adult obesity was at or above 35% for seven states in 2017, which is up from five states in 2016 and no states in 2012, according to the Centers for Disease Control and Prevention.
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.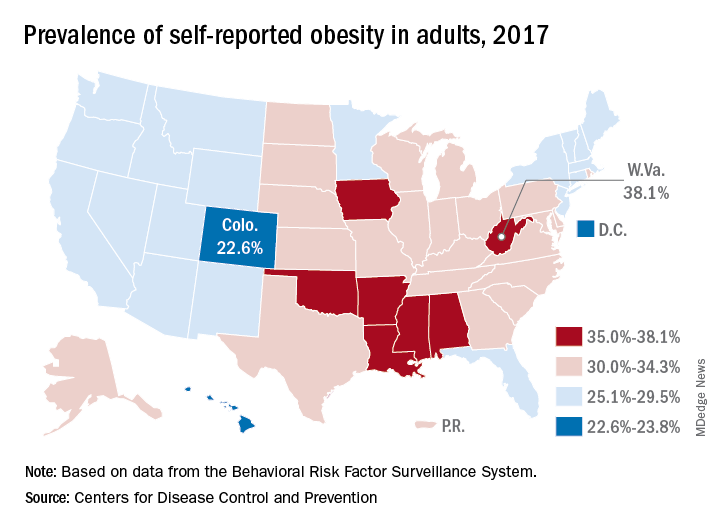
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at http://ow.ly/p1Fh30lOXYD
The prevalence of adult obesity was at or above 35% for seven states in 2017, which is up from five states in 2016 and no states in 2012, according to the Centers for Disease Control and Prevention.
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at http://ow.ly/p1Fh30lOXYD
The prevalence of adult obesity was at or above 35% for seven states in 2017, which is up from five states in 2016 and no states in 2012, according to the Centers for Disease Control and Prevention.
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at http://ow.ly/p1Fh30lOXYD
Online diabetes prevention programs as good as face-to-face programs
, researchers report.
Writing in background information to their paper, Tannaz Moin, MD, an endocrinologist at the VA Greater Los Angeles Healthcare System and the Veterans Affairs’ Health Services Research and Development Center for the Study of Healthcare Innovation, Implementation, and Policy, and her associates, said intensive lifestyle interventions such as diabetes prevention programs (DPP) could lower the risk of incident diabetes by 58%, but a lack of reach significantly attenuated their population impact in real-world settings.
“Building evidence for online DPP is important because of its potential for increasing reach because most U.S. adults (87%) use the Internet,” they wrote in their paper, published in the American Journal of Preventive Medicine.
They therefore set out to compare weight loss results from 114 veterans taking part in the Veterans Administration’s face-to-face standard-of-care weight management program MOVE! with an online program involving 268 obese or overweight veterans with prediabetes and 273 people taking part in an in-person program.
MOVE! included 8-12 face-to-face healthy-lifestyle sessions and monthly maintenance sessions but with no specified goals. The online program involved virtual groups of participants: live e-coaches who monitored group interactions and provided the participants with feedback via phone and private online messages; weekly educational modules on healthy eating and exercise; and wireless scales to record participant weights.
The in-person program consisted of 8-22 group-based face-to-face sessions focused on 7% weight loss and at least 150 minutes per session of moderate physical activity.
Weight loss, considered by the authors to be a significant predictor of diabetes risk reduction, was recorded at 6 months and then again at 12 months in all three interventions.
An analysis of 242 participants enrolled in the intensive, multifaceted online DPP intervention (26 were excluded because they did not have more than two available weights) revealed a significant weight change of –4.7 kg at 6 months and –4 kg at 12 months’ follow-up. On average, these participants lost 3.7% of their baseline weight at 12 months.
At both times weight change (kg and percentage) was not significantly different between the online intervention and those taking part in the in-person DPP (–4.8 and –4.1 kg for online vs –4 kg and –3.9 kg in-person for those completing more than one module/session). Both groups also had higher weight loss (percentage and kg) at 6 and 12 months compared with MOVE! participants (–1.1kg and 0.10 kg).
The research team noted that the online program had better participation than did the in-person program, with 87% of online participants completing eight or more sessions, compared with 59% for the in-person program and 55% for MOVE!
They suggested this was because the online program had several user-friendly features that increased the frequency of potential “touches” participants received over time.
“Future studies examining how inline DPP intervention components can work together to impact participation and engagement are key,” they said.
“This is one of the first studies to report weight outcomes irrespective of the level of engagement with an online DPP intervention and to examine outcomes compared with in person DPP. Overall, these findings may have important implications for national efforts to disseminate DPP,” they concluded.
The authors conceded that the generalizability of their study was limited as it included veterans receiving care in the VHA.
SOURCE: Am J Prev Med. 2018 Sep 24. doi: 10.1016/j.amepre.2018.06.028
, researchers report.
Writing in background information to their paper, Tannaz Moin, MD, an endocrinologist at the VA Greater Los Angeles Healthcare System and the Veterans Affairs’ Health Services Research and Development Center for the Study of Healthcare Innovation, Implementation, and Policy, and her associates, said intensive lifestyle interventions such as diabetes prevention programs (DPP) could lower the risk of incident diabetes by 58%, but a lack of reach significantly attenuated their population impact in real-world settings.
“Building evidence for online DPP is important because of its potential for increasing reach because most U.S. adults (87%) use the Internet,” they wrote in their paper, published in the American Journal of Preventive Medicine.
They therefore set out to compare weight loss results from 114 veterans taking part in the Veterans Administration’s face-to-face standard-of-care weight management program MOVE! with an online program involving 268 obese or overweight veterans with prediabetes and 273 people taking part in an in-person program.
MOVE! included 8-12 face-to-face healthy-lifestyle sessions and monthly maintenance sessions but with no specified goals. The online program involved virtual groups of participants: live e-coaches who monitored group interactions and provided the participants with feedback via phone and private online messages; weekly educational modules on healthy eating and exercise; and wireless scales to record participant weights.
The in-person program consisted of 8-22 group-based face-to-face sessions focused on 7% weight loss and at least 150 minutes per session of moderate physical activity.
Weight loss, considered by the authors to be a significant predictor of diabetes risk reduction, was recorded at 6 months and then again at 12 months in all three interventions.
An analysis of 242 participants enrolled in the intensive, multifaceted online DPP intervention (26 were excluded because they did not have more than two available weights) revealed a significant weight change of –4.7 kg at 6 months and –4 kg at 12 months’ follow-up. On average, these participants lost 3.7% of their baseline weight at 12 months.
At both times weight change (kg and percentage) was not significantly different between the online intervention and those taking part in the in-person DPP (–4.8 and –4.1 kg for online vs –4 kg and –3.9 kg in-person for those completing more than one module/session). Both groups also had higher weight loss (percentage and kg) at 6 and 12 months compared with MOVE! participants (–1.1kg and 0.10 kg).
The research team noted that the online program had better participation than did the in-person program, with 87% of online participants completing eight or more sessions, compared with 59% for the in-person program and 55% for MOVE!
They suggested this was because the online program had several user-friendly features that increased the frequency of potential “touches” participants received over time.
“Future studies examining how inline DPP intervention components can work together to impact participation and engagement are key,” they said.
“This is one of the first studies to report weight outcomes irrespective of the level of engagement with an online DPP intervention and to examine outcomes compared with in person DPP. Overall, these findings may have important implications for national efforts to disseminate DPP,” they concluded.
The authors conceded that the generalizability of their study was limited as it included veterans receiving care in the VHA.
SOURCE: Am J Prev Med. 2018 Sep 24. doi: 10.1016/j.amepre.2018.06.028
, researchers report.
Writing in background information to their paper, Tannaz Moin, MD, an endocrinologist at the VA Greater Los Angeles Healthcare System and the Veterans Affairs’ Health Services Research and Development Center for the Study of Healthcare Innovation, Implementation, and Policy, and her associates, said intensive lifestyle interventions such as diabetes prevention programs (DPP) could lower the risk of incident diabetes by 58%, but a lack of reach significantly attenuated their population impact in real-world settings.
“Building evidence for online DPP is important because of its potential for increasing reach because most U.S. adults (87%) use the Internet,” they wrote in their paper, published in the American Journal of Preventive Medicine.
They therefore set out to compare weight loss results from 114 veterans taking part in the Veterans Administration’s face-to-face standard-of-care weight management program MOVE! with an online program involving 268 obese or overweight veterans with prediabetes and 273 people taking part in an in-person program.
MOVE! included 8-12 face-to-face healthy-lifestyle sessions and monthly maintenance sessions but with no specified goals. The online program involved virtual groups of participants: live e-coaches who monitored group interactions and provided the participants with feedback via phone and private online messages; weekly educational modules on healthy eating and exercise; and wireless scales to record participant weights.
The in-person program consisted of 8-22 group-based face-to-face sessions focused on 7% weight loss and at least 150 minutes per session of moderate physical activity.
Weight loss, considered by the authors to be a significant predictor of diabetes risk reduction, was recorded at 6 months and then again at 12 months in all three interventions.
An analysis of 242 participants enrolled in the intensive, multifaceted online DPP intervention (26 were excluded because they did not have more than two available weights) revealed a significant weight change of –4.7 kg at 6 months and –4 kg at 12 months’ follow-up. On average, these participants lost 3.7% of their baseline weight at 12 months.
At both times weight change (kg and percentage) was not significantly different between the online intervention and those taking part in the in-person DPP (–4.8 and –4.1 kg for online vs –4 kg and –3.9 kg in-person for those completing more than one module/session). Both groups also had higher weight loss (percentage and kg) at 6 and 12 months compared with MOVE! participants (–1.1kg and 0.10 kg).
The research team noted that the online program had better participation than did the in-person program, with 87% of online participants completing eight or more sessions, compared with 59% for the in-person program and 55% for MOVE!
They suggested this was because the online program had several user-friendly features that increased the frequency of potential “touches” participants received over time.
“Future studies examining how inline DPP intervention components can work together to impact participation and engagement are key,” they said.
“This is one of the first studies to report weight outcomes irrespective of the level of engagement with an online DPP intervention and to examine outcomes compared with in person DPP. Overall, these findings may have important implications for national efforts to disseminate DPP,” they concluded.
The authors conceded that the generalizability of their study was limited as it included veterans receiving care in the VHA.
SOURCE: Am J Prev Med. 2018 Sep 24. doi: 10.1016/j.amepre.2018.06.028
FROM AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Online diabetes prevention programs (DPP) are as effective as in-person programs in terms of weight loss, and they have a wider reach.
Major finding: Participants enrolled in an intensive, multifaceted online DPP intervention had significant weight change of −4.7 kg at 6 months and −4.0 kg at 12-month follow-up, similar to that of participants enrolled in a face-to face program.
Study details: A large nonrandomized trial and a comparative analysis of individuals from a concurrent trial of two parallel in-person programs.
Disclosures: The Department of Veteran Affairs funded the study. One author reported co-owning shares in Amgen, and another reported receiving personal fees from two pharmaceutical companies.
Source: Am J Prev Med. 2018 Sep 24. doi: 10.1016/j.amepre.2018.06.028.
GBS in T2DM patients: Study highlights pros and cons, need for better patient selection
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
REPORTING FROM ADA 2018
Key clinical point: Bariatric surgery lowers mortality, CVD, and renal and other risks in obese T2DM patients but also has high complication rates.
Major finding: All-cause mortality, CVD, and renal disease risks were 49%, 34%, and 42% lower, respectively, in cases vs. controls.
Study details: A matched observational cohort study of 5,321 cases and 5,321 controls.
Disclosures: Dr. Liakopoulos reported having no disclosures.
Source: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
Task force advises behavioral intervention for obese adults
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
FROM JAMA
CDC: Obesity affects over 35% in 7 states
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
Obesity: Are shared medical appointments part of the answer?
Obesity is a major health problem in the United States. The facts are well known:
- Its prevalence has almost tripled since the early 1960s1
- More than 35% of US adults are obese (body mass index [BMI] ≥ 30 kg/m2)2
- It increases the risk of comorbid conditions including type 2 diabetes mellitus, heart disease, hypertension, obstructive sleep apnea, certain cancers, asthma, and osteoarthritis3,4
- It decreases life expectancy5
- Medical costs are up to 6 times higher per patient.6
Moreover, obesity is often not appropriately managed, owing to a variety of factors. In this article, we describe use of shared medical appointments as a strategy to improve the efficiency and effectiveness of treating patients with obesity.
Big benefits from small changes in weight
As little as 3% to 5% weight loss is associated with significant clinical benefits, such as improved glycemic control, reduced blood pressure, and reduced cholesterol levels.7,8 However, many patients are unable to reach this modest goal using current approaches to obesity management.
This failure is partially related to the complexity and chronic nature of obesity, which requires continued medical management from a multidisciplinary team. We believe this is an area of care that can be appropriately addressed through shared medical appointments.
CURRENT APPROACHES
Interventions for obesity have increased along with the prevalence of the disease. Hundreds of diets, exercise plans, natural products, and behavioral interventions are marketed, all claiming to be successful. More-intense treatment options include antiobesity medications, intra-abdominal weight loss devices, and bariatric surgery. Despite the availability of treatments, rates of obesity have not declined.
Counseling is important, but underused
Lifestyle modifications that encompass nutrition, physical activity, and behavioral interventions are the mainstay of obesity treatment.
Intensive interventions work better than less-intensive ones. In large clinical trials in overweight patients with diabetes, those who received intensive lifestyle interventions lost 3 to 5 kg more (3% to 8% of body weight) than those who received brief diet and nutrition counseling, as is often performed in a physician’s office.9–12 The US Preventive Services Task Force recommends that patients whose BMI is 30 kg/m2 or higher be offered intensive lifestyle intervention consisting of at least 12 sessions in 1 year.13
But fewer than half of primary care practitioners consistently provide specific guidance on diet, exercise, or weight control to patients with obesity, including those with a weight-related comorbidity.14 The rate has decreased since the 1990s despite the increase in obesity.15
One reason for the underuse is that many primary care practitioners do not have the training or time to deliver the recommended high-intensity obesity treatment.14 Plus, evidence does not clearly show a weight loss benefit from low-intensity interventions. Even when patients lose weight, most regain it, and only 20% are able to maintain their weight loss 1 year after treatment ends.16
Drugs and surgery also underused
Antiobesity medications and bariatric surgery are effective when added to lifestyle interventions, but they are also underused.
Bariatric surgery provides the greatest and most durable weight loss—15% to 30% of body weight—along with improvement in comorbidities such as type 2 diabetes, and its benefits are sustained for at least 10 years.17 However, fewer than 1% of eligible patients undergo bariatric surgery because of its limited availability, invasive nature, potential complications, limited insurance coverage, and high cost.17
The story is similar for antiobesity drugs. They are useful adjuncts to lifestyle interventions, providing an additional 3% to 7% weight loss,18 but fewer than 2% of eligible patients receive them.19 This may be attributed to their modest effectiveness, weight regain after discontinuation, potential adverse effects, and expense due to lack of insurance coverage.
ARE SHARED MEDICAL APPOINTMEMNTS AN ANSWER?
Although treatments have shown some effectiveness at producing weight loss, none has had a widespread impact on obesity. Lifestyle interventions, drugs, and bariatric surgery continue to be underused. Current treatment models are not providing patients with the intensive interventions needed.
Providers often find themselves offering repetitive advice to patients with obesity regarding nutrition and exercise, while simultaneously trying to manage obesity-related comorbidities, all in a 20-minute appointment. Too often, a patient returns home with prescriptions for hypertension or diabetes but no clear plan for weight management.
What can a shared medical appointment do?
A shared medical appointment is a group medical visit in which several patients with a similar clinical diagnosis, such as obesity, see a multidisciplinary team of healthcare providers. Typically, 5 to 10 patients have consultations with providers during a 60- to 90-minute appointment.20
Part of the session is dedicated to education on the patients’ common medical condition with the goal of improving their self-management, but most of the time is spent addressing individual patient concerns.
Each patient takes a turn consulting with a provider, as in a traditional medical appointment, but in a group setting. This allows others in the group to observe and learn from their peers’ experiences. During this consultation, the patient’s concerns are addressed, medications are managed, necessary tests are ordered, and a treatment plan is made.
Patients can continue to receive follow-up care through shared medical appointments at predetermined times, instead of traditional individual medical appointments.
BENEFITS OF SHARED APPOINTMENTS
Shared medical appointments could improve patient access, clinical outcomes, and patient and provider satisfaction and decrease costs.20,21 Since being introduced in the 1990s, their use has dramatically increased. For example, in the first 2 years of conducting shared medical appointments at Cleveland Clinic (2002–2004), there were just 385 shared medical appointments,21 but in 2017 there were approximately 12,300. They are used in a variety of medical and surgical specialties, and have been studied most for treating diabetes.22–24
Increased face time and access
Individual patient follow-up visits typically last 15 to 20 minutes, limiting the provider to seeing a maximum of 6 patients in 90 minutes. In that same time in the setting of a shared appointment, a multidisciplinary team can see up to 10 patients, and the patients receive up to 90 minutes of time with multiple providers.
Additionally, shared medical appointments can improve patient access to timely appointments. In a busy bariatric surgery practice, implementing shared medical appointments reduced patients’ wait time for an appointment by more than half.25 This is particularly important for patients with obesity, who usually require 12 to 26 appointments per year.
Improved patient outcomes
Use of shared medical appointments has improved clinical outcomes compared with traditional care. Patients with type 2 diabetes who attend shared medical appointments are more likely to reach target hemoglobin A1c and blood pressure levels.22−24 These benefits may be attributed to increased access to care, improved self-management skills, more frequent visits, peer support of the group, and the synergistic knowledge of multiple providers on the shared medical appointment team.
Although some trials reported patient retention rates of 75% to 90% in shared medical appointments, many trials did not report their rates. It is likely that some patients declined randomization to avoid shared medical appointments, which could have led to potential attrition and selection biases.23
Increased patient and provider satisfaction
Both patients and providers report high satisfaction with shared medical appointments.22,26 Although patients may initially hesitate to participate, their opinions significantly improve after attending 1 session.26 From 85% to 90% of patients who attend a shared medical appointment schedule their next follow-up appointment as a shared appointment as well.21,25
In comparative studies, patients who attended shared medical appointments had satisfaction rates equal to or higher than rates in patients who participated in usual care,22 noting better access to care and more sensitivity to their needs.27 Providers report greater satisfaction from working more directly with a team of providers, clearing up a backlogged schedule, and adding variety to their practice.21,24
Decreased costs
Data on the cost-effectiveness of shared medical appointments are mixed; however, some studies have shown that they are associated with a decrease in hospital admissions and emergency department visits.22 It seems reasonable to assume that, in an appropriate patient population, shared medical appointments can be cost-effective owing to increased provider productivity, but more research is needed to verify this.
CHALLENGES TO STARTING SHARED APPOINTMENTS FOR OBESITY
Despite their potential to provide comprehensive care to patients, shared medical appointments have limitations. These need to be addressed before implementing a shared medical appointment program.
Adequate resources and staff training
To be successful, a shared medical appointment program needs to have intensive physical and staffing resources. You need a space large enough to accommodate the group and access to the necessary equipment (eg, projector, whiteboard) for educational sessions. Larger or armless chairs may better accommodate patients with obesity. Facilitators need training in how to lead the group sessions, including time management and handling conflicts between patients. Schedulers and clinical intake staff need training in answering patient questions regarding these appointments.
Maintaining patient attendance
The benefits of provider efficiency rest on having an adequate number of patients attend the shared appointments.21 Patient cancellations and no-shows decrease both the efficiency and cost-effectiveness of this model, and they detract from the peer support and group learning that occurs in the group dynamic. To help minimize patient dropout, a discussion of patient expectations should take place prior to enrollment in shared medical appointments. This should include information on the concept of shared appointments, frequency and duration of appointments, and realistic weight loss goals.
Logistical challenges
A shared medical appointment requires a longer patient time slot and is usually less flexible than an individual appointment. Not all patients can take the time for a prescheduled 60- to 90-minute appointment. However, reduced waiting-room time and increased face time with a provider offset some of these challenges.
Recruiting patients
A shared medical appointment is a novel experience for some, and concerns about it may make it a challenge to recruit patients. Patients might worry that the presence of the group will compromise the patient-doctor relationship. Other concerns include potential irrelevance of other patients’ medical issues and reluctance to participate because of body image and the stigma of obesity.
One solution is to select patients from your existing practice so that the individual patient-provider relationship is established before introducing the concept of shared appointments. You will need to explain how shared appointments work, discuss their pros and cons, stress your expectations about attendance and confidentiality, and address any concerns of the patient. It is also important to emphasize that nearly all patients find shared medical appointments useful.
Once a group is established, it may be a challenge to keep a constant group membership to promote positive group dynamics. In practice, patients may drop out or be added, and facilitators need to be able to integrate new members into the group. It is important to emphasize to the group that obesity is chronic and that patients at all stages and levels of treatment can contribute to group learning.
Despite the advantages of shared medical appointments, some patients may not find them useful, even after attending several sessions. These patients should be offered individual follow-up visits. Also, shared appointments may not be suitable for patients who cannot speak English very well, are hearing-impaired, have significant cognitive impairment, or have acute medical issues.
Maintaining patient confidentiality
Maintaining confidentiality of personal and health information in a shared medical appointment is an important concern for patients but can be appropriately managed. In a survey of patients attending pulmonary hypertension shared medical appointments, 24% had concerns about confidentiality before participating, but after a few sessions, this rate was cut in half.28
Patients have reported initially withholding some information, but over time, they usually become more comfortable with the group and disclose more helpful information.29 Strategies to ensure confidentiality include having patients sign a confidentiality agreement at each appointment, providing specific instruction on what characterizes confidentiality breaches, and allowing patients the opportunity to schedule individual appointments as needed.
Ensuring insurance coverage
A shared medical appointment should be billed as an individual medical appointment for level of care, rather than time spent with the provider. This ensures that insurance coverage and copayments are the same as for individual medical appointments.
Lack of insurance coverage is a major barrier to obesity treatment in general. The US Centers for Medicare and Medicaid Services reimburses intensive behavioral obesity treatment delivered by a primary care practitioner, but limits it to 1 year of treatment and requires patients to meet weight loss goals. Some individual and employer-based healthcare plans do not cover dietitian visits, weight management programs, or antiobesity prescriptions.
EVIDENCE OF EFFECTIVENESS IN OBESITY
Few studies have investigated the use of shared medical appointments in obesity treatment. In the pediatric population, these programs significantly decreased BMI and some other anthropometric measurements,30–32 but they did not consistently involve a prescribing provider. This means they did not manage medications or comorbidities as would be expected in a shared medical appointment.
In adults, reported effects have been encouraging, although the studies are not particularly robust. In a 2-year observational study of a single physician conducting biweekly weight management shared medical appointments, participants lost 1% of their baseline weight, while those continuing with usual care gained 0.8%, a statistically significant difference.33 However, participation rates were low, with patients attending an average of only 3 shared medical appointments during the study.
In a meta-analysis of 13 randomized controlled trials of shared medical appointments for patients with type 2 diabetes, only 3 studies reported weight outcomes.23 These results indicated a trend toward weight loss among patients attending shared appointments, but they were not statistically significant.
Positive results also were reported by the Veterans Administration’s MOVE! (Managing Overweight/obesity for Veterans Everywhere) program.34 Participants in shared medical appointments reported that they felt empowered to make positive lifestyle changes, gained knowledge about obesity, were held accountable by their peers, and appreciated the individualized care they received from the multidisciplinary healthcare teams.
A systematic review involving 336 participants in group-based obesity interventions found group treatment produced more robust weight loss than individual treatment.35 However, shared medical appointments are different from weight loss groups in that they combine an educational session and a medical appointment in a peer-group setting, which requires a provider with prescribing privileges to be present. Thus, shared medical appointments can manage medications as well as weight-related comorbidities such as diabetes, hypertension, polycystic ovarian syndrome, and hyperlipidemia.
One more point is that continued attendance at shared medical appointments, even after successful weight loss, may help to maintain the weight loss, which has otherwise been found to be extremely challenging using traditional medical approaches.
WHO SHOULD BE ON THE TEAM?
Because obesity is multifactorial, it requires a comprehensive treatment approach that can be difficult to deliver given the limited time of an individual appointment. In a shared appointment, providers across multiple specialties can meet with patients at the same time to coordinate approaches to obesity treatment.
A multidisciplinary team for shared medical appointments for obesity needs a physician or a nurse practitioner—or ideally, both— who specializes in obesity to facilitate the session. Other key providers include a registered dietitian, an exercise physiologist, a behavioral health specialist, a sleep specialist, and a social worker to participate as needed in the educational component of the appointment or act as outside consultants.
WHAT ARE REALISTIC TARGETS?
- Nutrition
- Physical activity
- Appetite control
- Sleep
- Stress and mood disorders.
Nutrition
A calorie deficit of 500 to 750 calories per day is recommended for weight loss.7,8 Although there is no consensus on the best nutritional content of a diet, adherence to a diet is a significant predictor of weight loss.36 One reason diets fail to bring about weight loss is that patients tend to underestimate their caloric intake by almost 50%.37 Thus, they may benefit from a structured and supervised diet plan.
A dietitian can help patients develop an individualized diet plan that will promote adherence, which includes specific information on food choices, portion sizes, and timing of meals.
Physical activity
At least 150 minutes of physical activity per week is recommended for weight loss, and 200 to 300 minutes per week is recommended for long-term weight maintenance.7,8
An exercise physiologist can help patients design a personalized exercise plan to help achieve these goals. This plan should take into account the patient’s cardiac status, activity level, degree of mobility, and lifestyle.
Most patients are not able to achieve the recommended physical activity goals initially, and activity levels need to be gradually increased over a period of weeks to months. Patients who were previously inactive or have evidence of cardiovascular, renal, or metabolic disease may require a cardiopulmonary assessment, including an electrocardiogram and cardiac stress test, before starting an exercise program.
Appetite control
It is very difficult for patients to lose weight without appetite control. Weight loss that results from diet and exercise is often accompanied by a change in weight-regulating hormones (eg, leptin, ghrelin, peptide YY, and cholecystokinin) that promote weight regain.38 Thus, multiple compensatory mechanisms promote weight regain through increases in appetite and decreases in energy expenditure, resisting weight loss efforts.
Antiobesity drugs can help mitigate these adaptive weight-promoting responses through several mechanisms. They are indicated for use with lifestyle interventions for patients with a BMI of at least 30 mg/kg2 or a BMI of at least 27 kg/m2 with an obesity-related comorbidity.
These drugs promote an additional 3% to 7% weight loss when added to lifestyle interventions.18 But their effects are limited without appropriate lifestyle interventions.
Sleep
Adequate sleep is an often-overlooked component of obesity treatment. Inadequate sleep is associated with weight gain and an appetite-inducing hormone profile.39 Just 2 days of sleep deprivation in healthy normal-weight adult men was associated with a 70% increase in the ghrelin-to-leptin ratio, which showed a linear relationship with self-reported increased hunger.39 Sleep disorders, especially obstructive sleep apnea, are common in patients with obesity but are often underdiagnosed and undertreated.40
Healthy sleep habits and sleep quality should be addressed in shared medical appointments for obesity, as patients may be unaware of the impact that sleep may be having on their obesity treatment. The STOP-BANG questionnaire (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male sex) is a simple and reliable tool to screen for obstructive sleep apnea.41 Patients with symptoms of a sleep disorder should be referred to a sleep specialist for diagnosis and management.
Stress management and mood disorders
Stress and psychiatric disorders are underappreciated contributors to obesity. All patients receiving obesity treatment need to be screened for mood disorders and suicidal ideation.8
Chronic stress promotes weight gain through activation of the hypothalamic-pituitary-adrenocortical axis, whereby increased cortisol levels enhance appetite and accumulation of visceral fat.42 In addition, obesity is associated with a 25% increased risk of mood disorders, although the mechanism and direction of this association are unclear.43 Weight gain as a side effect of antidepressant or other psychiatric medications is another important consideration.
Management of stress and psychiatric disorders through goal-setting, self-monitoring, and patient education is vital to help patients fully participate in lifestyle changes and maximize weight loss. Patients participating in shared medical appointments usually benefit from consultations with psychiatrists or psychologists to manage psychiatric comorbidities and assist with adherence to behavior modification.
IN FAVOR OF SHARED MEDICAL APPOINTMENTS FOR OBESITY
Shared medical appointments can be an effective method of addressing the challenges of treating patients with obesity, using a multidisciplinary approach that combines nutrition, physical activity, appetite suppression, sleep improvement, and stress management. In addition, shared appointments allow practitioners to treat the primary problem of excess weight, rather than just its comorbidities, recognizing that obesity is a chronic disease that requires long-term, individualized treatment. Satisfaction rates are high for both patients and providers. Overall, education is essential to implementing and maintaining a successful shared medical appointment program.
- Ogden CL, Carroll MD. National Center for Health Statistics. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960-62 through 2007–2008. www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed August 8, 2018.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 315(21):2284–2291. doi:10.1001/jama.2016.6458
- Pantalone KM, Hobbs TM, Chagin KM, et al. Prevalence and recognition of obesity and its associated comorbidities: cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017; 7(11):e017583. doi:10.1136/bmjopen-2017-017583
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. doi:10.1186/1471-2458-9-88
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003; 289(2):187–193. pmid:12517229
- Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the United States: a quantitative systematic review. Int Assoc Study Obes Rev 2011; 12(1):50–61. doi:10.1111/j.1467-789X.2009.00708.x
- Jensen MD. Notice of duplicate publication of Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(25 suppl 2):S102–S138. doi:10.1161/01.cir.0000437739.71477.ee. J Am Coll Cardiol 2014; 63(25 Pt B):2985–3023. doi:10.1016/j.jacc.2013.11.004
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity: executive summary. Endocr Pract 2016; 22(7):842–884. doi:10.4158/EP161356.ESGL
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6):393–403. doi:10.1056/NEJMoa012512
- Eriksson J, Lindstrom J, Valle T, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: The Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999; 42(7):793–801. pmid:10440120
- Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30(6):1374–1383. doi:10.2337/dc07-0048
- Burguera B, Jesús Tur J, Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696. doi:10.1155/2015/194696
- Moyer VA; US Preventive Services Task Force. Screening for and management of obesity in adults: US Preventative Task Force Recommendation Statement. Ann Intern Med 2012; 157(5):373–378. doi:10.7326/0003-4819-157-5-201209040-00475
- Smith AW, Borowski LA, Liu B, et al. US primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med 2011; 41(1):33–42. doi:10.1016/j.amepre.2011.03.017
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51(2):186–192. doi:10.1097/MLR.0b013e3182726c33
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341. doi:10.1146/annurev.nutr.21.1.323
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017; 14(3):160–169. doi:10.1038/nrgastro.2016.170
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014; 311(1):74–86. doi:10.1001/jama.2013.281361
- Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015; 23(8):1721–1728. doi:10.1002/oby.21136
- Ramdas K, Darzi A. Adopting innovations in care delivery—the care of shared medical appointments. N Engl J Med 2017; 376(12):1105–1107. doi:10.1056/NEJMp1612803
- Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med 2004; 71(5):369–377. pmid:15195773
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ 2013; 185(13):E635–E644. doi:10.1503/cmaj.130053
- Housden LM, Wong ST. Using group medical visits with those who have diabetes: examining the evidence. Curr Diab Rep 2016; 16(12):134. doi:10.1007/s11892-016-0817-4
- Kaidar-Person O, Swartz EW, Lefkowitz M, et al. Shared medical appointments: new concept for high-volume follow-up for bariatric patients. Surg Obes Relat Dis 2006; 2(5):509–512. doi:10.1016/j.soard.2006.05.010
- Seager MJ, Egan RJ, Meredith HE, Bates SE, Norton SA, Morgan JD. Shared medical appointments for bariatric surgery follow-up: a patient satisfaction questionnaire. Obes Surg 2012; 22(4):641–645. doi:10.1007/s11695-012-0603-6
- Heyworth L, Rozenblum R, Burgess JF Jr, et al. Influence of shared medical appointments on patient satisfaction: a retrospective 3-year study. Ann Fam Med 2014; 12(4):324–330. doi:10.1370/afm.1660
- Rahaghi FF, Chastain VL, Benavides R, et al. Shared medical appointments in pulmonary hypertension. Pulm Circ 2014; 4(1):53–60. doi:10.1086/674883
- Wong ST, Lavoie JG, Browne AJ, Macleod ML, Chongo M. Patient confidentiality within the context of group medical visits: Is there cause for concern? Health Expect 2015; 18(5):727–739. doi:10.1111/hex.12156
- Geller JS, Dube ET, Cruz GA, Stevens J, Keating Bench K. Pediatric Obesity Empowerment Model Group Medical Visits (POEM-GMV) as treatment for pediatric obesity in an underserved community. Child Obes 2015; 11(5):638–646. doi:10.1089/chi.2014.0163
- Weigel C, Kokocinski K, Lederer P, Dötsch J, Rascher W, Knerr I. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav 2008; 40(6):369–373. doi:10.1016/j.jneb.2007.07.009
- Hinchman J, Beno L, Mims A. Kaiser Permanente Georgia’s experience with operation zero: a group medical appointment to address pediatric overweight. Perm J 2006; 10(3):66–71. pmid:21519478
- Palaniappan LP, Muzaffar AL, Wang EJ, Wong EC, Orchard TJ, Mbbch M. Shared medical appointments: promoting weight loss in a clinical setting. J Am Board Fam Med 2011; 24(3):326–328. doi:10.3122/jabfm.2011.03.100220
- Cohen S, Hartley S, Mavi J, Vest B, Wilson M. Veteran experiences related to participation in shared medical appointments. Mil Med 2012; 177(11):1287–1292. pmid:23198503
- Paul-Ebhohimhen V, Avenell A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes Facts 2009; 2(1):17–24. doi:10.1159/000186144
- Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360(9):859–873. doi:10.1056/NEJMoa0804748
- Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992; 327(27):1893–1898. doi:10.1056/NEJM199212313272701
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365(17):1597–1604. doi:10.1056/NEJMoa1105816
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141(11):846–850. pmid:15583226
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in US communities. Sleep Breath 2002; 6(2):49–54. doi:10.1007/s11325-002-0049-5
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67:259–284. doi:10.1146/annurev.physiol.67.040403.120816
- Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63(7):824-830. doi:10.1001/archpsyc.63.7.824
Obesity is a major health problem in the United States. The facts are well known:
- Its prevalence has almost tripled since the early 1960s1
- More than 35% of US adults are obese (body mass index [BMI] ≥ 30 kg/m2)2
- It increases the risk of comorbid conditions including type 2 diabetes mellitus, heart disease, hypertension, obstructive sleep apnea, certain cancers, asthma, and osteoarthritis3,4
- It decreases life expectancy5
- Medical costs are up to 6 times higher per patient.6
Moreover, obesity is often not appropriately managed, owing to a variety of factors. In this article, we describe use of shared medical appointments as a strategy to improve the efficiency and effectiveness of treating patients with obesity.
Big benefits from small changes in weight
As little as 3% to 5% weight loss is associated with significant clinical benefits, such as improved glycemic control, reduced blood pressure, and reduced cholesterol levels.7,8 However, many patients are unable to reach this modest goal using current approaches to obesity management.
This failure is partially related to the complexity and chronic nature of obesity, which requires continued medical management from a multidisciplinary team. We believe this is an area of care that can be appropriately addressed through shared medical appointments.
CURRENT APPROACHES
Interventions for obesity have increased along with the prevalence of the disease. Hundreds of diets, exercise plans, natural products, and behavioral interventions are marketed, all claiming to be successful. More-intense treatment options include antiobesity medications, intra-abdominal weight loss devices, and bariatric surgery. Despite the availability of treatments, rates of obesity have not declined.
Counseling is important, but underused
Lifestyle modifications that encompass nutrition, physical activity, and behavioral interventions are the mainstay of obesity treatment.
Intensive interventions work better than less-intensive ones. In large clinical trials in overweight patients with diabetes, those who received intensive lifestyle interventions lost 3 to 5 kg more (3% to 8% of body weight) than those who received brief diet and nutrition counseling, as is often performed in a physician’s office.9–12 The US Preventive Services Task Force recommends that patients whose BMI is 30 kg/m2 or higher be offered intensive lifestyle intervention consisting of at least 12 sessions in 1 year.13
But fewer than half of primary care practitioners consistently provide specific guidance on diet, exercise, or weight control to patients with obesity, including those with a weight-related comorbidity.14 The rate has decreased since the 1990s despite the increase in obesity.15
One reason for the underuse is that many primary care practitioners do not have the training or time to deliver the recommended high-intensity obesity treatment.14 Plus, evidence does not clearly show a weight loss benefit from low-intensity interventions. Even when patients lose weight, most regain it, and only 20% are able to maintain their weight loss 1 year after treatment ends.16
Drugs and surgery also underused
Antiobesity medications and bariatric surgery are effective when added to lifestyle interventions, but they are also underused.
Bariatric surgery provides the greatest and most durable weight loss—15% to 30% of body weight—along with improvement in comorbidities such as type 2 diabetes, and its benefits are sustained for at least 10 years.17 However, fewer than 1% of eligible patients undergo bariatric surgery because of its limited availability, invasive nature, potential complications, limited insurance coverage, and high cost.17
The story is similar for antiobesity drugs. They are useful adjuncts to lifestyle interventions, providing an additional 3% to 7% weight loss,18 but fewer than 2% of eligible patients receive them.19 This may be attributed to their modest effectiveness, weight regain after discontinuation, potential adverse effects, and expense due to lack of insurance coverage.
ARE SHARED MEDICAL APPOINTMEMNTS AN ANSWER?
Although treatments have shown some effectiveness at producing weight loss, none has had a widespread impact on obesity. Lifestyle interventions, drugs, and bariatric surgery continue to be underused. Current treatment models are not providing patients with the intensive interventions needed.
Providers often find themselves offering repetitive advice to patients with obesity regarding nutrition and exercise, while simultaneously trying to manage obesity-related comorbidities, all in a 20-minute appointment. Too often, a patient returns home with prescriptions for hypertension or diabetes but no clear plan for weight management.
What can a shared medical appointment do?
A shared medical appointment is a group medical visit in which several patients with a similar clinical diagnosis, such as obesity, see a multidisciplinary team of healthcare providers. Typically, 5 to 10 patients have consultations with providers during a 60- to 90-minute appointment.20
Part of the session is dedicated to education on the patients’ common medical condition with the goal of improving their self-management, but most of the time is spent addressing individual patient concerns.
Each patient takes a turn consulting with a provider, as in a traditional medical appointment, but in a group setting. This allows others in the group to observe and learn from their peers’ experiences. During this consultation, the patient’s concerns are addressed, medications are managed, necessary tests are ordered, and a treatment plan is made.
Patients can continue to receive follow-up care through shared medical appointments at predetermined times, instead of traditional individual medical appointments.
BENEFITS OF SHARED APPOINTMENTS
Shared medical appointments could improve patient access, clinical outcomes, and patient and provider satisfaction and decrease costs.20,21 Since being introduced in the 1990s, their use has dramatically increased. For example, in the first 2 years of conducting shared medical appointments at Cleveland Clinic (2002–2004), there were just 385 shared medical appointments,21 but in 2017 there were approximately 12,300. They are used in a variety of medical and surgical specialties, and have been studied most for treating diabetes.22–24
Increased face time and access
Individual patient follow-up visits typically last 15 to 20 minutes, limiting the provider to seeing a maximum of 6 patients in 90 minutes. In that same time in the setting of a shared appointment, a multidisciplinary team can see up to 10 patients, and the patients receive up to 90 minutes of time with multiple providers.
Additionally, shared medical appointments can improve patient access to timely appointments. In a busy bariatric surgery practice, implementing shared medical appointments reduced patients’ wait time for an appointment by more than half.25 This is particularly important for patients with obesity, who usually require 12 to 26 appointments per year.
Improved patient outcomes
Use of shared medical appointments has improved clinical outcomes compared with traditional care. Patients with type 2 diabetes who attend shared medical appointments are more likely to reach target hemoglobin A1c and blood pressure levels.22−24 These benefits may be attributed to increased access to care, improved self-management skills, more frequent visits, peer support of the group, and the synergistic knowledge of multiple providers on the shared medical appointment team.
Although some trials reported patient retention rates of 75% to 90% in shared medical appointments, many trials did not report their rates. It is likely that some patients declined randomization to avoid shared medical appointments, which could have led to potential attrition and selection biases.23
Increased patient and provider satisfaction
Both patients and providers report high satisfaction with shared medical appointments.22,26 Although patients may initially hesitate to participate, their opinions significantly improve after attending 1 session.26 From 85% to 90% of patients who attend a shared medical appointment schedule their next follow-up appointment as a shared appointment as well.21,25
In comparative studies, patients who attended shared medical appointments had satisfaction rates equal to or higher than rates in patients who participated in usual care,22 noting better access to care and more sensitivity to their needs.27 Providers report greater satisfaction from working more directly with a team of providers, clearing up a backlogged schedule, and adding variety to their practice.21,24
Decreased costs
Data on the cost-effectiveness of shared medical appointments are mixed; however, some studies have shown that they are associated with a decrease in hospital admissions and emergency department visits.22 It seems reasonable to assume that, in an appropriate patient population, shared medical appointments can be cost-effective owing to increased provider productivity, but more research is needed to verify this.
CHALLENGES TO STARTING SHARED APPOINTMENTS FOR OBESITY
Despite their potential to provide comprehensive care to patients, shared medical appointments have limitations. These need to be addressed before implementing a shared medical appointment program.
Adequate resources and staff training
To be successful, a shared medical appointment program needs to have intensive physical and staffing resources. You need a space large enough to accommodate the group and access to the necessary equipment (eg, projector, whiteboard) for educational sessions. Larger or armless chairs may better accommodate patients with obesity. Facilitators need training in how to lead the group sessions, including time management and handling conflicts between patients. Schedulers and clinical intake staff need training in answering patient questions regarding these appointments.
Maintaining patient attendance
The benefits of provider efficiency rest on having an adequate number of patients attend the shared appointments.21 Patient cancellations and no-shows decrease both the efficiency and cost-effectiveness of this model, and they detract from the peer support and group learning that occurs in the group dynamic. To help minimize patient dropout, a discussion of patient expectations should take place prior to enrollment in shared medical appointments. This should include information on the concept of shared appointments, frequency and duration of appointments, and realistic weight loss goals.
Logistical challenges
A shared medical appointment requires a longer patient time slot and is usually less flexible than an individual appointment. Not all patients can take the time for a prescheduled 60- to 90-minute appointment. However, reduced waiting-room time and increased face time with a provider offset some of these challenges.
Recruiting patients
A shared medical appointment is a novel experience for some, and concerns about it may make it a challenge to recruit patients. Patients might worry that the presence of the group will compromise the patient-doctor relationship. Other concerns include potential irrelevance of other patients’ medical issues and reluctance to participate because of body image and the stigma of obesity.
One solution is to select patients from your existing practice so that the individual patient-provider relationship is established before introducing the concept of shared appointments. You will need to explain how shared appointments work, discuss their pros and cons, stress your expectations about attendance and confidentiality, and address any concerns of the patient. It is also important to emphasize that nearly all patients find shared medical appointments useful.
Once a group is established, it may be a challenge to keep a constant group membership to promote positive group dynamics. In practice, patients may drop out or be added, and facilitators need to be able to integrate new members into the group. It is important to emphasize to the group that obesity is chronic and that patients at all stages and levels of treatment can contribute to group learning.
Despite the advantages of shared medical appointments, some patients may not find them useful, even after attending several sessions. These patients should be offered individual follow-up visits. Also, shared appointments may not be suitable for patients who cannot speak English very well, are hearing-impaired, have significant cognitive impairment, or have acute medical issues.
Maintaining patient confidentiality
Maintaining confidentiality of personal and health information in a shared medical appointment is an important concern for patients but can be appropriately managed. In a survey of patients attending pulmonary hypertension shared medical appointments, 24% had concerns about confidentiality before participating, but after a few sessions, this rate was cut in half.28
Patients have reported initially withholding some information, but over time, they usually become more comfortable with the group and disclose more helpful information.29 Strategies to ensure confidentiality include having patients sign a confidentiality agreement at each appointment, providing specific instruction on what characterizes confidentiality breaches, and allowing patients the opportunity to schedule individual appointments as needed.
Ensuring insurance coverage
A shared medical appointment should be billed as an individual medical appointment for level of care, rather than time spent with the provider. This ensures that insurance coverage and copayments are the same as for individual medical appointments.
Lack of insurance coverage is a major barrier to obesity treatment in general. The US Centers for Medicare and Medicaid Services reimburses intensive behavioral obesity treatment delivered by a primary care practitioner, but limits it to 1 year of treatment and requires patients to meet weight loss goals. Some individual and employer-based healthcare plans do not cover dietitian visits, weight management programs, or antiobesity prescriptions.
EVIDENCE OF EFFECTIVENESS IN OBESITY
Few studies have investigated the use of shared medical appointments in obesity treatment. In the pediatric population, these programs significantly decreased BMI and some other anthropometric measurements,30–32 but they did not consistently involve a prescribing provider. This means they did not manage medications or comorbidities as would be expected in a shared medical appointment.
In adults, reported effects have been encouraging, although the studies are not particularly robust. In a 2-year observational study of a single physician conducting biweekly weight management shared medical appointments, participants lost 1% of their baseline weight, while those continuing with usual care gained 0.8%, a statistically significant difference.33 However, participation rates were low, with patients attending an average of only 3 shared medical appointments during the study.
In a meta-analysis of 13 randomized controlled trials of shared medical appointments for patients with type 2 diabetes, only 3 studies reported weight outcomes.23 These results indicated a trend toward weight loss among patients attending shared appointments, but they were not statistically significant.
Positive results also were reported by the Veterans Administration’s MOVE! (Managing Overweight/obesity for Veterans Everywhere) program.34 Participants in shared medical appointments reported that they felt empowered to make positive lifestyle changes, gained knowledge about obesity, were held accountable by their peers, and appreciated the individualized care they received from the multidisciplinary healthcare teams.
A systematic review involving 336 participants in group-based obesity interventions found group treatment produced more robust weight loss than individual treatment.35 However, shared medical appointments are different from weight loss groups in that they combine an educational session and a medical appointment in a peer-group setting, which requires a provider with prescribing privileges to be present. Thus, shared medical appointments can manage medications as well as weight-related comorbidities such as diabetes, hypertension, polycystic ovarian syndrome, and hyperlipidemia.
One more point is that continued attendance at shared medical appointments, even after successful weight loss, may help to maintain the weight loss, which has otherwise been found to be extremely challenging using traditional medical approaches.
WHO SHOULD BE ON THE TEAM?
Because obesity is multifactorial, it requires a comprehensive treatment approach that can be difficult to deliver given the limited time of an individual appointment. In a shared appointment, providers across multiple specialties can meet with patients at the same time to coordinate approaches to obesity treatment.
A multidisciplinary team for shared medical appointments for obesity needs a physician or a nurse practitioner—or ideally, both— who specializes in obesity to facilitate the session. Other key providers include a registered dietitian, an exercise physiologist, a behavioral health specialist, a sleep specialist, and a social worker to participate as needed in the educational component of the appointment or act as outside consultants.
WHAT ARE REALISTIC TARGETS?
- Nutrition
- Physical activity
- Appetite control
- Sleep
- Stress and mood disorders.
Nutrition
A calorie deficit of 500 to 750 calories per day is recommended for weight loss.7,8 Although there is no consensus on the best nutritional content of a diet, adherence to a diet is a significant predictor of weight loss.36 One reason diets fail to bring about weight loss is that patients tend to underestimate their caloric intake by almost 50%.37 Thus, they may benefit from a structured and supervised diet plan.
A dietitian can help patients develop an individualized diet plan that will promote adherence, which includes specific information on food choices, portion sizes, and timing of meals.
Physical activity
At least 150 minutes of physical activity per week is recommended for weight loss, and 200 to 300 minutes per week is recommended for long-term weight maintenance.7,8
An exercise physiologist can help patients design a personalized exercise plan to help achieve these goals. This plan should take into account the patient’s cardiac status, activity level, degree of mobility, and lifestyle.
Most patients are not able to achieve the recommended physical activity goals initially, and activity levels need to be gradually increased over a period of weeks to months. Patients who were previously inactive or have evidence of cardiovascular, renal, or metabolic disease may require a cardiopulmonary assessment, including an electrocardiogram and cardiac stress test, before starting an exercise program.
Appetite control
It is very difficult for patients to lose weight without appetite control. Weight loss that results from diet and exercise is often accompanied by a change in weight-regulating hormones (eg, leptin, ghrelin, peptide YY, and cholecystokinin) that promote weight regain.38 Thus, multiple compensatory mechanisms promote weight regain through increases in appetite and decreases in energy expenditure, resisting weight loss efforts.
Antiobesity drugs can help mitigate these adaptive weight-promoting responses through several mechanisms. They are indicated for use with lifestyle interventions for patients with a BMI of at least 30 mg/kg2 or a BMI of at least 27 kg/m2 with an obesity-related comorbidity.
These drugs promote an additional 3% to 7% weight loss when added to lifestyle interventions.18 But their effects are limited without appropriate lifestyle interventions.
Sleep
Adequate sleep is an often-overlooked component of obesity treatment. Inadequate sleep is associated with weight gain and an appetite-inducing hormone profile.39 Just 2 days of sleep deprivation in healthy normal-weight adult men was associated with a 70% increase in the ghrelin-to-leptin ratio, which showed a linear relationship with self-reported increased hunger.39 Sleep disorders, especially obstructive sleep apnea, are common in patients with obesity but are often underdiagnosed and undertreated.40
Healthy sleep habits and sleep quality should be addressed in shared medical appointments for obesity, as patients may be unaware of the impact that sleep may be having on their obesity treatment. The STOP-BANG questionnaire (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male sex) is a simple and reliable tool to screen for obstructive sleep apnea.41 Patients with symptoms of a sleep disorder should be referred to a sleep specialist for diagnosis and management.
Stress management and mood disorders
Stress and psychiatric disorders are underappreciated contributors to obesity. All patients receiving obesity treatment need to be screened for mood disorders and suicidal ideation.8
Chronic stress promotes weight gain through activation of the hypothalamic-pituitary-adrenocortical axis, whereby increased cortisol levels enhance appetite and accumulation of visceral fat.42 In addition, obesity is associated with a 25% increased risk of mood disorders, although the mechanism and direction of this association are unclear.43 Weight gain as a side effect of antidepressant or other psychiatric medications is another important consideration.
Management of stress and psychiatric disorders through goal-setting, self-monitoring, and patient education is vital to help patients fully participate in lifestyle changes and maximize weight loss. Patients participating in shared medical appointments usually benefit from consultations with psychiatrists or psychologists to manage psychiatric comorbidities and assist with adherence to behavior modification.
IN FAVOR OF SHARED MEDICAL APPOINTMENTS FOR OBESITY
Shared medical appointments can be an effective method of addressing the challenges of treating patients with obesity, using a multidisciplinary approach that combines nutrition, physical activity, appetite suppression, sleep improvement, and stress management. In addition, shared appointments allow practitioners to treat the primary problem of excess weight, rather than just its comorbidities, recognizing that obesity is a chronic disease that requires long-term, individualized treatment. Satisfaction rates are high for both patients and providers. Overall, education is essential to implementing and maintaining a successful shared medical appointment program.
Obesity is a major health problem in the United States. The facts are well known:
- Its prevalence has almost tripled since the early 1960s1
- More than 35% of US adults are obese (body mass index [BMI] ≥ 30 kg/m2)2
- It increases the risk of comorbid conditions including type 2 diabetes mellitus, heart disease, hypertension, obstructive sleep apnea, certain cancers, asthma, and osteoarthritis3,4
- It decreases life expectancy5
- Medical costs are up to 6 times higher per patient.6
Moreover, obesity is often not appropriately managed, owing to a variety of factors. In this article, we describe use of shared medical appointments as a strategy to improve the efficiency and effectiveness of treating patients with obesity.
Big benefits from small changes in weight
As little as 3% to 5% weight loss is associated with significant clinical benefits, such as improved glycemic control, reduced blood pressure, and reduced cholesterol levels.7,8 However, many patients are unable to reach this modest goal using current approaches to obesity management.
This failure is partially related to the complexity and chronic nature of obesity, which requires continued medical management from a multidisciplinary team. We believe this is an area of care that can be appropriately addressed through shared medical appointments.
CURRENT APPROACHES
Interventions for obesity have increased along with the prevalence of the disease. Hundreds of diets, exercise plans, natural products, and behavioral interventions are marketed, all claiming to be successful. More-intense treatment options include antiobesity medications, intra-abdominal weight loss devices, and bariatric surgery. Despite the availability of treatments, rates of obesity have not declined.
Counseling is important, but underused
Lifestyle modifications that encompass nutrition, physical activity, and behavioral interventions are the mainstay of obesity treatment.
Intensive interventions work better than less-intensive ones. In large clinical trials in overweight patients with diabetes, those who received intensive lifestyle interventions lost 3 to 5 kg more (3% to 8% of body weight) than those who received brief diet and nutrition counseling, as is often performed in a physician’s office.9–12 The US Preventive Services Task Force recommends that patients whose BMI is 30 kg/m2 or higher be offered intensive lifestyle intervention consisting of at least 12 sessions in 1 year.13
But fewer than half of primary care practitioners consistently provide specific guidance on diet, exercise, or weight control to patients with obesity, including those with a weight-related comorbidity.14 The rate has decreased since the 1990s despite the increase in obesity.15
One reason for the underuse is that many primary care practitioners do not have the training or time to deliver the recommended high-intensity obesity treatment.14 Plus, evidence does not clearly show a weight loss benefit from low-intensity interventions. Even when patients lose weight, most regain it, and only 20% are able to maintain their weight loss 1 year after treatment ends.16
Drugs and surgery also underused
Antiobesity medications and bariatric surgery are effective when added to lifestyle interventions, but they are also underused.
Bariatric surgery provides the greatest and most durable weight loss—15% to 30% of body weight—along with improvement in comorbidities such as type 2 diabetes, and its benefits are sustained for at least 10 years.17 However, fewer than 1% of eligible patients undergo bariatric surgery because of its limited availability, invasive nature, potential complications, limited insurance coverage, and high cost.17
The story is similar for antiobesity drugs. They are useful adjuncts to lifestyle interventions, providing an additional 3% to 7% weight loss,18 but fewer than 2% of eligible patients receive them.19 This may be attributed to their modest effectiveness, weight regain after discontinuation, potential adverse effects, and expense due to lack of insurance coverage.
ARE SHARED MEDICAL APPOINTMEMNTS AN ANSWER?
Although treatments have shown some effectiveness at producing weight loss, none has had a widespread impact on obesity. Lifestyle interventions, drugs, and bariatric surgery continue to be underused. Current treatment models are not providing patients with the intensive interventions needed.
Providers often find themselves offering repetitive advice to patients with obesity regarding nutrition and exercise, while simultaneously trying to manage obesity-related comorbidities, all in a 20-minute appointment. Too often, a patient returns home with prescriptions for hypertension or diabetes but no clear plan for weight management.
What can a shared medical appointment do?
A shared medical appointment is a group medical visit in which several patients with a similar clinical diagnosis, such as obesity, see a multidisciplinary team of healthcare providers. Typically, 5 to 10 patients have consultations with providers during a 60- to 90-minute appointment.20
Part of the session is dedicated to education on the patients’ common medical condition with the goal of improving their self-management, but most of the time is spent addressing individual patient concerns.
Each patient takes a turn consulting with a provider, as in a traditional medical appointment, but in a group setting. This allows others in the group to observe and learn from their peers’ experiences. During this consultation, the patient’s concerns are addressed, medications are managed, necessary tests are ordered, and a treatment plan is made.
Patients can continue to receive follow-up care through shared medical appointments at predetermined times, instead of traditional individual medical appointments.
BENEFITS OF SHARED APPOINTMENTS
Shared medical appointments could improve patient access, clinical outcomes, and patient and provider satisfaction and decrease costs.20,21 Since being introduced in the 1990s, their use has dramatically increased. For example, in the first 2 years of conducting shared medical appointments at Cleveland Clinic (2002–2004), there were just 385 shared medical appointments,21 but in 2017 there were approximately 12,300. They are used in a variety of medical and surgical specialties, and have been studied most for treating diabetes.22–24
Increased face time and access
Individual patient follow-up visits typically last 15 to 20 minutes, limiting the provider to seeing a maximum of 6 patients in 90 minutes. In that same time in the setting of a shared appointment, a multidisciplinary team can see up to 10 patients, and the patients receive up to 90 minutes of time with multiple providers.
Additionally, shared medical appointments can improve patient access to timely appointments. In a busy bariatric surgery practice, implementing shared medical appointments reduced patients’ wait time for an appointment by more than half.25 This is particularly important for patients with obesity, who usually require 12 to 26 appointments per year.
Improved patient outcomes
Use of shared medical appointments has improved clinical outcomes compared with traditional care. Patients with type 2 diabetes who attend shared medical appointments are more likely to reach target hemoglobin A1c and blood pressure levels.22−24 These benefits may be attributed to increased access to care, improved self-management skills, more frequent visits, peer support of the group, and the synergistic knowledge of multiple providers on the shared medical appointment team.
Although some trials reported patient retention rates of 75% to 90% in shared medical appointments, many trials did not report their rates. It is likely that some patients declined randomization to avoid shared medical appointments, which could have led to potential attrition and selection biases.23
Increased patient and provider satisfaction
Both patients and providers report high satisfaction with shared medical appointments.22,26 Although patients may initially hesitate to participate, their opinions significantly improve after attending 1 session.26 From 85% to 90% of patients who attend a shared medical appointment schedule their next follow-up appointment as a shared appointment as well.21,25
In comparative studies, patients who attended shared medical appointments had satisfaction rates equal to or higher than rates in patients who participated in usual care,22 noting better access to care and more sensitivity to their needs.27 Providers report greater satisfaction from working more directly with a team of providers, clearing up a backlogged schedule, and adding variety to their practice.21,24
Decreased costs
Data on the cost-effectiveness of shared medical appointments are mixed; however, some studies have shown that they are associated with a decrease in hospital admissions and emergency department visits.22 It seems reasonable to assume that, in an appropriate patient population, shared medical appointments can be cost-effective owing to increased provider productivity, but more research is needed to verify this.
CHALLENGES TO STARTING SHARED APPOINTMENTS FOR OBESITY
Despite their potential to provide comprehensive care to patients, shared medical appointments have limitations. These need to be addressed before implementing a shared medical appointment program.
Adequate resources and staff training
To be successful, a shared medical appointment program needs to have intensive physical and staffing resources. You need a space large enough to accommodate the group and access to the necessary equipment (eg, projector, whiteboard) for educational sessions. Larger or armless chairs may better accommodate patients with obesity. Facilitators need training in how to lead the group sessions, including time management and handling conflicts between patients. Schedulers and clinical intake staff need training in answering patient questions regarding these appointments.
Maintaining patient attendance
The benefits of provider efficiency rest on having an adequate number of patients attend the shared appointments.21 Patient cancellations and no-shows decrease both the efficiency and cost-effectiveness of this model, and they detract from the peer support and group learning that occurs in the group dynamic. To help minimize patient dropout, a discussion of patient expectations should take place prior to enrollment in shared medical appointments. This should include information on the concept of shared appointments, frequency and duration of appointments, and realistic weight loss goals.
Logistical challenges
A shared medical appointment requires a longer patient time slot and is usually less flexible than an individual appointment. Not all patients can take the time for a prescheduled 60- to 90-minute appointment. However, reduced waiting-room time and increased face time with a provider offset some of these challenges.
Recruiting patients
A shared medical appointment is a novel experience for some, and concerns about it may make it a challenge to recruit patients. Patients might worry that the presence of the group will compromise the patient-doctor relationship. Other concerns include potential irrelevance of other patients’ medical issues and reluctance to participate because of body image and the stigma of obesity.
One solution is to select patients from your existing practice so that the individual patient-provider relationship is established before introducing the concept of shared appointments. You will need to explain how shared appointments work, discuss their pros and cons, stress your expectations about attendance and confidentiality, and address any concerns of the patient. It is also important to emphasize that nearly all patients find shared medical appointments useful.
Once a group is established, it may be a challenge to keep a constant group membership to promote positive group dynamics. In practice, patients may drop out or be added, and facilitators need to be able to integrate new members into the group. It is important to emphasize to the group that obesity is chronic and that patients at all stages and levels of treatment can contribute to group learning.
Despite the advantages of shared medical appointments, some patients may not find them useful, even after attending several sessions. These patients should be offered individual follow-up visits. Also, shared appointments may not be suitable for patients who cannot speak English very well, are hearing-impaired, have significant cognitive impairment, or have acute medical issues.
Maintaining patient confidentiality
Maintaining confidentiality of personal and health information in a shared medical appointment is an important concern for patients but can be appropriately managed. In a survey of patients attending pulmonary hypertension shared medical appointments, 24% had concerns about confidentiality before participating, but after a few sessions, this rate was cut in half.28
Patients have reported initially withholding some information, but over time, they usually become more comfortable with the group and disclose more helpful information.29 Strategies to ensure confidentiality include having patients sign a confidentiality agreement at each appointment, providing specific instruction on what characterizes confidentiality breaches, and allowing patients the opportunity to schedule individual appointments as needed.
Ensuring insurance coverage
A shared medical appointment should be billed as an individual medical appointment for level of care, rather than time spent with the provider. This ensures that insurance coverage and copayments are the same as for individual medical appointments.
Lack of insurance coverage is a major barrier to obesity treatment in general. The US Centers for Medicare and Medicaid Services reimburses intensive behavioral obesity treatment delivered by a primary care practitioner, but limits it to 1 year of treatment and requires patients to meet weight loss goals. Some individual and employer-based healthcare plans do not cover dietitian visits, weight management programs, or antiobesity prescriptions.
EVIDENCE OF EFFECTIVENESS IN OBESITY
Few studies have investigated the use of shared medical appointments in obesity treatment. In the pediatric population, these programs significantly decreased BMI and some other anthropometric measurements,30–32 but they did not consistently involve a prescribing provider. This means they did not manage medications or comorbidities as would be expected in a shared medical appointment.
In adults, reported effects have been encouraging, although the studies are not particularly robust. In a 2-year observational study of a single physician conducting biweekly weight management shared medical appointments, participants lost 1% of their baseline weight, while those continuing with usual care gained 0.8%, a statistically significant difference.33 However, participation rates were low, with patients attending an average of only 3 shared medical appointments during the study.
In a meta-analysis of 13 randomized controlled trials of shared medical appointments for patients with type 2 diabetes, only 3 studies reported weight outcomes.23 These results indicated a trend toward weight loss among patients attending shared appointments, but they were not statistically significant.
Positive results also were reported by the Veterans Administration’s MOVE! (Managing Overweight/obesity for Veterans Everywhere) program.34 Participants in shared medical appointments reported that they felt empowered to make positive lifestyle changes, gained knowledge about obesity, were held accountable by their peers, and appreciated the individualized care they received from the multidisciplinary healthcare teams.
A systematic review involving 336 participants in group-based obesity interventions found group treatment produced more robust weight loss than individual treatment.35 However, shared medical appointments are different from weight loss groups in that they combine an educational session and a medical appointment in a peer-group setting, which requires a provider with prescribing privileges to be present. Thus, shared medical appointments can manage medications as well as weight-related comorbidities such as diabetes, hypertension, polycystic ovarian syndrome, and hyperlipidemia.
One more point is that continued attendance at shared medical appointments, even after successful weight loss, may help to maintain the weight loss, which has otherwise been found to be extremely challenging using traditional medical approaches.
WHO SHOULD BE ON THE TEAM?
Because obesity is multifactorial, it requires a comprehensive treatment approach that can be difficult to deliver given the limited time of an individual appointment. In a shared appointment, providers across multiple specialties can meet with patients at the same time to coordinate approaches to obesity treatment.
A multidisciplinary team for shared medical appointments for obesity needs a physician or a nurse practitioner—or ideally, both— who specializes in obesity to facilitate the session. Other key providers include a registered dietitian, an exercise physiologist, a behavioral health specialist, a sleep specialist, and a social worker to participate as needed in the educational component of the appointment or act as outside consultants.
WHAT ARE REALISTIC TARGETS?
- Nutrition
- Physical activity
- Appetite control
- Sleep
- Stress and mood disorders.
Nutrition
A calorie deficit of 500 to 750 calories per day is recommended for weight loss.7,8 Although there is no consensus on the best nutritional content of a diet, adherence to a diet is a significant predictor of weight loss.36 One reason diets fail to bring about weight loss is that patients tend to underestimate their caloric intake by almost 50%.37 Thus, they may benefit from a structured and supervised diet plan.
A dietitian can help patients develop an individualized diet plan that will promote adherence, which includes specific information on food choices, portion sizes, and timing of meals.
Physical activity
At least 150 minutes of physical activity per week is recommended for weight loss, and 200 to 300 minutes per week is recommended for long-term weight maintenance.7,8
An exercise physiologist can help patients design a personalized exercise plan to help achieve these goals. This plan should take into account the patient’s cardiac status, activity level, degree of mobility, and lifestyle.
Most patients are not able to achieve the recommended physical activity goals initially, and activity levels need to be gradually increased over a period of weeks to months. Patients who were previously inactive or have evidence of cardiovascular, renal, or metabolic disease may require a cardiopulmonary assessment, including an electrocardiogram and cardiac stress test, before starting an exercise program.
Appetite control
It is very difficult for patients to lose weight without appetite control. Weight loss that results from diet and exercise is often accompanied by a change in weight-regulating hormones (eg, leptin, ghrelin, peptide YY, and cholecystokinin) that promote weight regain.38 Thus, multiple compensatory mechanisms promote weight regain through increases in appetite and decreases in energy expenditure, resisting weight loss efforts.
Antiobesity drugs can help mitigate these adaptive weight-promoting responses through several mechanisms. They are indicated for use with lifestyle interventions for patients with a BMI of at least 30 mg/kg2 or a BMI of at least 27 kg/m2 with an obesity-related comorbidity.
These drugs promote an additional 3% to 7% weight loss when added to lifestyle interventions.18 But their effects are limited without appropriate lifestyle interventions.
Sleep
Adequate sleep is an often-overlooked component of obesity treatment. Inadequate sleep is associated with weight gain and an appetite-inducing hormone profile.39 Just 2 days of sleep deprivation in healthy normal-weight adult men was associated with a 70% increase in the ghrelin-to-leptin ratio, which showed a linear relationship with self-reported increased hunger.39 Sleep disorders, especially obstructive sleep apnea, are common in patients with obesity but are often underdiagnosed and undertreated.40
Healthy sleep habits and sleep quality should be addressed in shared medical appointments for obesity, as patients may be unaware of the impact that sleep may be having on their obesity treatment. The STOP-BANG questionnaire (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male sex) is a simple and reliable tool to screen for obstructive sleep apnea.41 Patients with symptoms of a sleep disorder should be referred to a sleep specialist for diagnosis and management.
Stress management and mood disorders
Stress and psychiatric disorders are underappreciated contributors to obesity. All patients receiving obesity treatment need to be screened for mood disorders and suicidal ideation.8
Chronic stress promotes weight gain through activation of the hypothalamic-pituitary-adrenocortical axis, whereby increased cortisol levels enhance appetite and accumulation of visceral fat.42 In addition, obesity is associated with a 25% increased risk of mood disorders, although the mechanism and direction of this association are unclear.43 Weight gain as a side effect of antidepressant or other psychiatric medications is another important consideration.
Management of stress and psychiatric disorders through goal-setting, self-monitoring, and patient education is vital to help patients fully participate in lifestyle changes and maximize weight loss. Patients participating in shared medical appointments usually benefit from consultations with psychiatrists or psychologists to manage psychiatric comorbidities and assist with adherence to behavior modification.
IN FAVOR OF SHARED MEDICAL APPOINTMENTS FOR OBESITY
Shared medical appointments can be an effective method of addressing the challenges of treating patients with obesity, using a multidisciplinary approach that combines nutrition, physical activity, appetite suppression, sleep improvement, and stress management. In addition, shared appointments allow practitioners to treat the primary problem of excess weight, rather than just its comorbidities, recognizing that obesity is a chronic disease that requires long-term, individualized treatment. Satisfaction rates are high for both patients and providers. Overall, education is essential to implementing and maintaining a successful shared medical appointment program.
- Ogden CL, Carroll MD. National Center for Health Statistics. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960-62 through 2007–2008. www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed August 8, 2018.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 315(21):2284–2291. doi:10.1001/jama.2016.6458
- Pantalone KM, Hobbs TM, Chagin KM, et al. Prevalence and recognition of obesity and its associated comorbidities: cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017; 7(11):e017583. doi:10.1136/bmjopen-2017-017583
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. doi:10.1186/1471-2458-9-88
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003; 289(2):187–193. pmid:12517229
- Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the United States: a quantitative systematic review. Int Assoc Study Obes Rev 2011; 12(1):50–61. doi:10.1111/j.1467-789X.2009.00708.x
- Jensen MD. Notice of duplicate publication of Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(25 suppl 2):S102–S138. doi:10.1161/01.cir.0000437739.71477.ee. J Am Coll Cardiol 2014; 63(25 Pt B):2985–3023. doi:10.1016/j.jacc.2013.11.004
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity: executive summary. Endocr Pract 2016; 22(7):842–884. doi:10.4158/EP161356.ESGL
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6):393–403. doi:10.1056/NEJMoa012512
- Eriksson J, Lindstrom J, Valle T, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: The Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999; 42(7):793–801. pmid:10440120
- Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30(6):1374–1383. doi:10.2337/dc07-0048
- Burguera B, Jesús Tur J, Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696. doi:10.1155/2015/194696
- Moyer VA; US Preventive Services Task Force. Screening for and management of obesity in adults: US Preventative Task Force Recommendation Statement. Ann Intern Med 2012; 157(5):373–378. doi:10.7326/0003-4819-157-5-201209040-00475
- Smith AW, Borowski LA, Liu B, et al. US primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med 2011; 41(1):33–42. doi:10.1016/j.amepre.2011.03.017
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51(2):186–192. doi:10.1097/MLR.0b013e3182726c33
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341. doi:10.1146/annurev.nutr.21.1.323
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017; 14(3):160–169. doi:10.1038/nrgastro.2016.170
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014; 311(1):74–86. doi:10.1001/jama.2013.281361
- Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015; 23(8):1721–1728. doi:10.1002/oby.21136
- Ramdas K, Darzi A. Adopting innovations in care delivery—the care of shared medical appointments. N Engl J Med 2017; 376(12):1105–1107. doi:10.1056/NEJMp1612803
- Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med 2004; 71(5):369–377. pmid:15195773
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ 2013; 185(13):E635–E644. doi:10.1503/cmaj.130053
- Housden LM, Wong ST. Using group medical visits with those who have diabetes: examining the evidence. Curr Diab Rep 2016; 16(12):134. doi:10.1007/s11892-016-0817-4
- Kaidar-Person O, Swartz EW, Lefkowitz M, et al. Shared medical appointments: new concept for high-volume follow-up for bariatric patients. Surg Obes Relat Dis 2006; 2(5):509–512. doi:10.1016/j.soard.2006.05.010
- Seager MJ, Egan RJ, Meredith HE, Bates SE, Norton SA, Morgan JD. Shared medical appointments for bariatric surgery follow-up: a patient satisfaction questionnaire. Obes Surg 2012; 22(4):641–645. doi:10.1007/s11695-012-0603-6
- Heyworth L, Rozenblum R, Burgess JF Jr, et al. Influence of shared medical appointments on patient satisfaction: a retrospective 3-year study. Ann Fam Med 2014; 12(4):324–330. doi:10.1370/afm.1660
- Rahaghi FF, Chastain VL, Benavides R, et al. Shared medical appointments in pulmonary hypertension. Pulm Circ 2014; 4(1):53–60. doi:10.1086/674883
- Wong ST, Lavoie JG, Browne AJ, Macleod ML, Chongo M. Patient confidentiality within the context of group medical visits: Is there cause for concern? Health Expect 2015; 18(5):727–739. doi:10.1111/hex.12156
- Geller JS, Dube ET, Cruz GA, Stevens J, Keating Bench K. Pediatric Obesity Empowerment Model Group Medical Visits (POEM-GMV) as treatment for pediatric obesity in an underserved community. Child Obes 2015; 11(5):638–646. doi:10.1089/chi.2014.0163
- Weigel C, Kokocinski K, Lederer P, Dötsch J, Rascher W, Knerr I. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav 2008; 40(6):369–373. doi:10.1016/j.jneb.2007.07.009
- Hinchman J, Beno L, Mims A. Kaiser Permanente Georgia’s experience with operation zero: a group medical appointment to address pediatric overweight. Perm J 2006; 10(3):66–71. pmid:21519478
- Palaniappan LP, Muzaffar AL, Wang EJ, Wong EC, Orchard TJ, Mbbch M. Shared medical appointments: promoting weight loss in a clinical setting. J Am Board Fam Med 2011; 24(3):326–328. doi:10.3122/jabfm.2011.03.100220
- Cohen S, Hartley S, Mavi J, Vest B, Wilson M. Veteran experiences related to participation in shared medical appointments. Mil Med 2012; 177(11):1287–1292. pmid:23198503
- Paul-Ebhohimhen V, Avenell A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes Facts 2009; 2(1):17–24. doi:10.1159/000186144
- Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360(9):859–873. doi:10.1056/NEJMoa0804748
- Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992; 327(27):1893–1898. doi:10.1056/NEJM199212313272701
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365(17):1597–1604. doi:10.1056/NEJMoa1105816
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141(11):846–850. pmid:15583226
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in US communities. Sleep Breath 2002; 6(2):49–54. doi:10.1007/s11325-002-0049-5
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67:259–284. doi:10.1146/annurev.physiol.67.040403.120816
- Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63(7):824-830. doi:10.1001/archpsyc.63.7.824
- Ogden CL, Carroll MD. National Center for Health Statistics. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960-62 through 2007–2008. www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed August 8, 2018.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 315(21):2284–2291. doi:10.1001/jama.2016.6458
- Pantalone KM, Hobbs TM, Chagin KM, et al. Prevalence and recognition of obesity and its associated comorbidities: cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017; 7(11):e017583. doi:10.1136/bmjopen-2017-017583
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. doi:10.1186/1471-2458-9-88
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003; 289(2):187–193. pmid:12517229
- Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the United States: a quantitative systematic review. Int Assoc Study Obes Rev 2011; 12(1):50–61. doi:10.1111/j.1467-789X.2009.00708.x
- Jensen MD. Notice of duplicate publication of Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(25 suppl 2):S102–S138. doi:10.1161/01.cir.0000437739.71477.ee. J Am Coll Cardiol 2014; 63(25 Pt B):2985–3023. doi:10.1016/j.jacc.2013.11.004
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity: executive summary. Endocr Pract 2016; 22(7):842–884. doi:10.4158/EP161356.ESGL
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6):393–403. doi:10.1056/NEJMoa012512
- Eriksson J, Lindstrom J, Valle T, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: The Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999; 42(7):793–801. pmid:10440120
- Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30(6):1374–1383. doi:10.2337/dc07-0048
- Burguera B, Jesús Tur J, Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696. doi:10.1155/2015/194696
- Moyer VA; US Preventive Services Task Force. Screening for and management of obesity in adults: US Preventative Task Force Recommendation Statement. Ann Intern Med 2012; 157(5):373–378. doi:10.7326/0003-4819-157-5-201209040-00475
- Smith AW, Borowski LA, Liu B, et al. US primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med 2011; 41(1):33–42. doi:10.1016/j.amepre.2011.03.017
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51(2):186–192. doi:10.1097/MLR.0b013e3182726c33
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341. doi:10.1146/annurev.nutr.21.1.323
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017; 14(3):160–169. doi:10.1038/nrgastro.2016.170
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014; 311(1):74–86. doi:10.1001/jama.2013.281361
- Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015; 23(8):1721–1728. doi:10.1002/oby.21136
- Ramdas K, Darzi A. Adopting innovations in care delivery—the care of shared medical appointments. N Engl J Med 2017; 376(12):1105–1107. doi:10.1056/NEJMp1612803
- Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med 2004; 71(5):369–377. pmid:15195773
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ 2013; 185(13):E635–E644. doi:10.1503/cmaj.130053
- Housden LM, Wong ST. Using group medical visits with those who have diabetes: examining the evidence. Curr Diab Rep 2016; 16(12):134. doi:10.1007/s11892-016-0817-4
- Kaidar-Person O, Swartz EW, Lefkowitz M, et al. Shared medical appointments: new concept for high-volume follow-up for bariatric patients. Surg Obes Relat Dis 2006; 2(5):509–512. doi:10.1016/j.soard.2006.05.010
- Seager MJ, Egan RJ, Meredith HE, Bates SE, Norton SA, Morgan JD. Shared medical appointments for bariatric surgery follow-up: a patient satisfaction questionnaire. Obes Surg 2012; 22(4):641–645. doi:10.1007/s11695-012-0603-6
- Heyworth L, Rozenblum R, Burgess JF Jr, et al. Influence of shared medical appointments on patient satisfaction: a retrospective 3-year study. Ann Fam Med 2014; 12(4):324–330. doi:10.1370/afm.1660
- Rahaghi FF, Chastain VL, Benavides R, et al. Shared medical appointments in pulmonary hypertension. Pulm Circ 2014; 4(1):53–60. doi:10.1086/674883
- Wong ST, Lavoie JG, Browne AJ, Macleod ML, Chongo M. Patient confidentiality within the context of group medical visits: Is there cause for concern? Health Expect 2015; 18(5):727–739. doi:10.1111/hex.12156
- Geller JS, Dube ET, Cruz GA, Stevens J, Keating Bench K. Pediatric Obesity Empowerment Model Group Medical Visits (POEM-GMV) as treatment for pediatric obesity in an underserved community. Child Obes 2015; 11(5):638–646. doi:10.1089/chi.2014.0163
- Weigel C, Kokocinski K, Lederer P, Dötsch J, Rascher W, Knerr I. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav 2008; 40(6):369–373. doi:10.1016/j.jneb.2007.07.009
- Hinchman J, Beno L, Mims A. Kaiser Permanente Georgia’s experience with operation zero: a group medical appointment to address pediatric overweight. Perm J 2006; 10(3):66–71. pmid:21519478
- Palaniappan LP, Muzaffar AL, Wang EJ, Wong EC, Orchard TJ, Mbbch M. Shared medical appointments: promoting weight loss in a clinical setting. J Am Board Fam Med 2011; 24(3):326–328. doi:10.3122/jabfm.2011.03.100220
- Cohen S, Hartley S, Mavi J, Vest B, Wilson M. Veteran experiences related to participation in shared medical appointments. Mil Med 2012; 177(11):1287–1292. pmid:23198503
- Paul-Ebhohimhen V, Avenell A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes Facts 2009; 2(1):17–24. doi:10.1159/000186144
- Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360(9):859–873. doi:10.1056/NEJMoa0804748
- Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992; 327(27):1893–1898. doi:10.1056/NEJM199212313272701
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365(17):1597–1604. doi:10.1056/NEJMoa1105816
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141(11):846–850. pmid:15583226
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in US communities. Sleep Breath 2002; 6(2):49–54. doi:10.1007/s11325-002-0049-5
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67:259–284. doi:10.1146/annurev.physiol.67.040403.120816
- Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63(7):824-830. doi:10.1001/archpsyc.63.7.824
KEY POINTS
- Shared medical appointments have been shown to improve clinical outcomes and patient satisfaction compared with traditional care. However, they have not been well studied in patients with obesity.
- A shared medical appointment allows multiple patients to be medically managed by a multidisciplinary team, promoting more efficient delivery of care.
- Both patients and practitioners are satisfied with shared medical appointments and find them clinically useful.
Obesity Extends Viral Shedding of Flu
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Even modest alcohol use may worsen NAFLD
Patients with nonalcoholic fatty liver disease who consumed modest quantities of alcohol had significantly less improvement in steatosis and significantly lower odds of resolution of nonalcoholic steatohepatitis, compared with nondrinkers, according to the results of a longitudinal cohort study published in the Clinical Gastroenterology and Hepatology.
Modest drinkers also had significantly less improvement in their AST levels, compared with nondrinkers, said Veeral Ajmera, MD, of the University of California, San Diego, and his associates. “Importantly, our results suggest that cessation of alcohol use may mitigate these changes,” they wrote. Clinicians should consider the spectrum of nonalcoholic fatty liver disease (NAFLD), and especially nonalcoholic steatohepatitis (NASH), when making recommendations about alcohol use. “More advanced NAFLD severity may warrant counseling against [even] modest alcohol use.”
More than one in three adults in the United States has NAFLD and about two-thirds drink alcohol, almost always in moderation, the researchers noted. Modest alcohol use has been linked to decreased cardiovascular risk, which is particularly relevant because patients with NAFLD tend to have risk factors for cardiovascular disease. Results from at least two cross-sectional studies also suggest modest drinkers with NAFLD have less severe histology, including less NASH and fibrosis. However, modest drinkers tend to be more physically active, with lower body mass indices, higher physical activity levels, and less obesity, which are potential confounders. To better understand the effects of modest alcohol consumption on NAFLD, the researchers studied adults with NAFLD who participated in studies conducted by the multicenter NASH Clinical Research Network.
The 285 participants were typically aged in their late 40s, female, white, and obese, with an average body mass index of 34.7 kg/m2. In all, 168 participants (59%) reported consuming up to two drinks per day, while 41% abstained from alcohol use. During an average of 47 months between biopsies (standard deviation, 26 months), nondrinkers averaged a 0.49 reduction in steatosis grade, significantly more than that of modest drinkers (reduction, 0.30; P = .04). Nondrinkers also had a greater decrease in mean AST level (7 U/L), compared with drinkers (2 U/L; P = .04).
A total of 64% of patients were classified as having definite NASH, 19% had NAFLD without NASH, and 17% had borderline NASH. At baseline, 23% of patients did not have fibrosis, 32% had stage 1 fibrosis, 21% had stage 2, 21% had stage 3, and 3% had stage 4. Modest drinkers were more likely to be white and were less likely to be diagnosed with definitive NASH at baseline. After controlling for these potential confounders, modest drinkers had significantly lower odds of NASH resolution, compared with nondrinkers (adjusted odds ratio, 0.32; 95% confidence interval, 0.11-0.92; P = .04).
“[The] presence of NASH has consistently been shown to predict increased risk for fibrosis progression, and therefore, our finding of less NASH resolution among consistent modest drinkers is clinically relevant,” the investigators wrote. “Although we were unable to assess the association between modest alcohol consumption and cardiovascular risk, we did not see any significant changes in measured metabolic risk factors with known associations with cardiovascular disease including low-density lipoprotein and high-density lipoprotein cholesterol and insulin resistance.”
Funders of the study included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Advanced/Transplant Hepatology Fellowship, the American Association for the Study of Liver Diseases Foundation, and the Intramural Research Program of the National Institutes of Health.
SOURCE: Ajmera V et al. Clin Gastroenterol Hepatol. 2018 Mar 14. doi: 10.1016/j.cgh.2018.01.026.
Patients with nonalcoholic fatty liver disease who consumed modest quantities of alcohol had significantly less improvement in steatosis and significantly lower odds of resolution of nonalcoholic steatohepatitis, compared with nondrinkers, according to the results of a longitudinal cohort study published in the Clinical Gastroenterology and Hepatology.
Modest drinkers also had significantly less improvement in their AST levels, compared with nondrinkers, said Veeral Ajmera, MD, of the University of California, San Diego, and his associates. “Importantly, our results suggest that cessation of alcohol use may mitigate these changes,” they wrote. Clinicians should consider the spectrum of nonalcoholic fatty liver disease (NAFLD), and especially nonalcoholic steatohepatitis (NASH), when making recommendations about alcohol use. “More advanced NAFLD severity may warrant counseling against [even] modest alcohol use.”
More than one in three adults in the United States has NAFLD and about two-thirds drink alcohol, almost always in moderation, the researchers noted. Modest alcohol use has been linked to decreased cardiovascular risk, which is particularly relevant because patients with NAFLD tend to have risk factors for cardiovascular disease. Results from at least two cross-sectional studies also suggest modest drinkers with NAFLD have less severe histology, including less NASH and fibrosis. However, modest drinkers tend to be more physically active, with lower body mass indices, higher physical activity levels, and less obesity, which are potential confounders. To better understand the effects of modest alcohol consumption on NAFLD, the researchers studied adults with NAFLD who participated in studies conducted by the multicenter NASH Clinical Research Network.
The 285 participants were typically aged in their late 40s, female, white, and obese, with an average body mass index of 34.7 kg/m2. In all, 168 participants (59%) reported consuming up to two drinks per day, while 41% abstained from alcohol use. During an average of 47 months between biopsies (standard deviation, 26 months), nondrinkers averaged a 0.49 reduction in steatosis grade, significantly more than that of modest drinkers (reduction, 0.30; P = .04). Nondrinkers also had a greater decrease in mean AST level (7 U/L), compared with drinkers (2 U/L; P = .04).
A total of 64% of patients were classified as having definite NASH, 19% had NAFLD without NASH, and 17% had borderline NASH. At baseline, 23% of patients did not have fibrosis, 32% had stage 1 fibrosis, 21% had stage 2, 21% had stage 3, and 3% had stage 4. Modest drinkers were more likely to be white and were less likely to be diagnosed with definitive NASH at baseline. After controlling for these potential confounders, modest drinkers had significantly lower odds of NASH resolution, compared with nondrinkers (adjusted odds ratio, 0.32; 95% confidence interval, 0.11-0.92; P = .04).
“[The] presence of NASH has consistently been shown to predict increased risk for fibrosis progression, and therefore, our finding of less NASH resolution among consistent modest drinkers is clinically relevant,” the investigators wrote. “Although we were unable to assess the association between modest alcohol consumption and cardiovascular risk, we did not see any significant changes in measured metabolic risk factors with known associations with cardiovascular disease including low-density lipoprotein and high-density lipoprotein cholesterol and insulin resistance.”
Funders of the study included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Advanced/Transplant Hepatology Fellowship, the American Association for the Study of Liver Diseases Foundation, and the Intramural Research Program of the National Institutes of Health.
SOURCE: Ajmera V et al. Clin Gastroenterol Hepatol. 2018 Mar 14. doi: 10.1016/j.cgh.2018.01.026.
Patients with nonalcoholic fatty liver disease who consumed modest quantities of alcohol had significantly less improvement in steatosis and significantly lower odds of resolution of nonalcoholic steatohepatitis, compared with nondrinkers, according to the results of a longitudinal cohort study published in the Clinical Gastroenterology and Hepatology.
Modest drinkers also had significantly less improvement in their AST levels, compared with nondrinkers, said Veeral Ajmera, MD, of the University of California, San Diego, and his associates. “Importantly, our results suggest that cessation of alcohol use may mitigate these changes,” they wrote. Clinicians should consider the spectrum of nonalcoholic fatty liver disease (NAFLD), and especially nonalcoholic steatohepatitis (NASH), when making recommendations about alcohol use. “More advanced NAFLD severity may warrant counseling against [even] modest alcohol use.”
More than one in three adults in the United States has NAFLD and about two-thirds drink alcohol, almost always in moderation, the researchers noted. Modest alcohol use has been linked to decreased cardiovascular risk, which is particularly relevant because patients with NAFLD tend to have risk factors for cardiovascular disease. Results from at least two cross-sectional studies also suggest modest drinkers with NAFLD have less severe histology, including less NASH and fibrosis. However, modest drinkers tend to be more physically active, with lower body mass indices, higher physical activity levels, and less obesity, which are potential confounders. To better understand the effects of modest alcohol consumption on NAFLD, the researchers studied adults with NAFLD who participated in studies conducted by the multicenter NASH Clinical Research Network.
The 285 participants were typically aged in their late 40s, female, white, and obese, with an average body mass index of 34.7 kg/m2. In all, 168 participants (59%) reported consuming up to two drinks per day, while 41% abstained from alcohol use. During an average of 47 months between biopsies (standard deviation, 26 months), nondrinkers averaged a 0.49 reduction in steatosis grade, significantly more than that of modest drinkers (reduction, 0.30; P = .04). Nondrinkers also had a greater decrease in mean AST level (7 U/L), compared with drinkers (2 U/L; P = .04).
A total of 64% of patients were classified as having definite NASH, 19% had NAFLD without NASH, and 17% had borderline NASH. At baseline, 23% of patients did not have fibrosis, 32% had stage 1 fibrosis, 21% had stage 2, 21% had stage 3, and 3% had stage 4. Modest drinkers were more likely to be white and were less likely to be diagnosed with definitive NASH at baseline. After controlling for these potential confounders, modest drinkers had significantly lower odds of NASH resolution, compared with nondrinkers (adjusted odds ratio, 0.32; 95% confidence interval, 0.11-0.92; P = .04).
“[The] presence of NASH has consistently been shown to predict increased risk for fibrosis progression, and therefore, our finding of less NASH resolution among consistent modest drinkers is clinically relevant,” the investigators wrote. “Although we were unable to assess the association between modest alcohol consumption and cardiovascular risk, we did not see any significant changes in measured metabolic risk factors with known associations with cardiovascular disease including low-density lipoprotein and high-density lipoprotein cholesterol and insulin resistance.”
Funders of the study included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Advanced/Transplant Hepatology Fellowship, the American Association for the Study of Liver Diseases Foundation, and the Intramural Research Program of the National Institutes of Health.
SOURCE: Ajmera V et al. Clin Gastroenterol Hepatol. 2018 Mar 14. doi: 10.1016/j.cgh.2018.01.026.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Consider counseling patients with more advanced nonalcoholic fatty liver disease to avoid alcohol.
Major finding: Compared with nondrinkers, patients who reported modest alcohol use had significantly less improvement in steatosis and significantly lower odds of resolution of nonalcoholic steatohepatitis (P = .04 for both comparisons).
Study details: A longitudinal cohort study of 285 adults with nonalcoholic fatty liver disease with paired biopsy specimens obtained an average of 4 years apart.
Disclosures: Funders of the study included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Advanced/Transplant Hepatology Fellowship, the American Association for the Study of Liver Diseases Foundation, and the Intramural Research Program of the National Institutes of Health.
Source: Ajmera V et al. Clin Gastroenterol Hepatol. 2018 Mar 14. doi: 10.1016/j.cgh.2018.01.026.