User login
Preconception Health Care
CE/CME No: CR-1408
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define preconception and interconception health care and explain how these concepts relate to primary care.
• Describe three common clinical presentations in reproductive-age women that have implications for preconception and interconception health care.
• Explain the considerations, in terms of potential pregnancies, when prescribing pharmacologic treatment for reproductive-age women.
• Discuss examples of cultural factors and beliefs that may affect preconception health counseling provided to women of reproductive age.
FACULTY
Kathleen A. Ahonen is an Assistant Professor and Colleen Quinlan is an Associate Professor at the University of Toledo College of Nursing.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Because half of all pregnancies in the United States are unplanned, primary care constitutes preconception health care for women ages 15 to 44. Here are recommendations to incorporate into routine visits to improve outcomes of possible pregnancies.
Traditional interventions to improve pregnancy outcomes in the United States have focused on early, consistent prenatal care and on reducing the teenage pregnancy rate. Data indicate that early access to prenatal care has increased and that the teenage pregnancy rate has fallen.1,2
But while US rates of preterm births, low–birth-weight infants, and infant mortality have all declined since the mid-2000s,3 they remain higher than those in many other developed countries.4-6 Furthermore, there are significant differences in these rates by race and ethnicity.3
Many experts believe that waiting until a woman is pregnant to address the health needs and behaviors that adversely affect pregnancy outcomes is, at best, an incomplete solution to these problems. A more comprehensive view of women’s health care broadens this focus to stress the importance of preconception health care throughout the reproductive years. It is key to the achievement of optimal health, not only for women but also for their potential future children.7-9
The CDC defines preconception health simply as the health of women and men during their reproductive years. Preconception health care is defined as medical care during those years (for women, ages 15 to 44) that focuses on improving aspects of the patient’s health or health behaviors that can improve outcomes in the event of a pregnancy.10 The term interconception health care is also applied specifically to health care provided during the time between pregnancies.
This article highlights obesity, depression and other mood disorders, and nutritional deficiencies and reviews potential risk factors, particularly occurring very early in a pregnancy. Recommendations are offered for more effective health counseling at routine visits, with the goal of improving outcomes of potential pregnancies.
PRIMARY CARE IS PRECONCEPTION CARE
Researchers have reported that only about 50% of reproductive-age women receive counseling about health behaviors and pregnancy planning8 and that such counseling is even rarer for adolescent females.11,12 Because women of childbearing age commonly see both Ob-Gyn and family practice clinicians for health care, primary care providers are well positioned to deliver preconception health care by integrating health counseling, discussion of health behaviors, and primary and secondary prevention into patient care for reproductive-age women (see "Summary of CDC Recommendations to Improve Preconception Health and Health Care").6
On the next page: Obesity >>
OBESITY
Researchers in both the US and internationally have noted the connection between obesity and pregnancy complications such as gestational diabetes that increase the risk for poor pregnancy outcomes.13-15 Indeed, pregnancy outcomes can be altered for the better with weight loss in the preconception or interconception period.16
Weight loss is complex; it can be difficult to accomplish, and long-term maintenance also presents a challenge. Short-term weight loss is achievable, however, and can produce beneficial metabolic effects, such as a decrease in insulin resistance.17
Before weight loss attempts can be initiated, the overweight or obese patient’s weight must be acknowledged as a health problem. In one 2009 study, Callaway et al found that in their sample of 412 overweight and obese women, only 17% were advised by their health care providers to lose weight.18
Regular physical activity can facilitate the achievement and maintenance of a healthy weight. However, physical inactivity is common in nonpregnant women, especially those with higher BMIs. The CDC’s Pregnancy Risk Assessment Monitoring System (PRAMS; www.cdc.gov/prams), widely regarded as an important source of data about women’s health behaviors, reveals that nearly 40% of US women do not meet national recommendations for routine physical activity in the three months prior to pregnancy.19
Recent obesity guidelines recommend initial weight loss goals of 5% to 10% of baseline weight in six months before pregnancy.17 Some women may not achieve these goals because they are unaware of the potentially short time period between discontinuation of contraception and conception. Others, especially those older than 30, intentionally plan for short interconception intervals as part of their family planning strategy, which does not allow enough time for safe weight loss.20
Primary care clinicians should counsel these patients on the importance of a healthy weight and regular physical activity to the maintenance of optimal health. Particular emphasis should be placed on achieving weight loss slowly and safely and then maintaining it, at least for the short term. This strategy may be effective in helping a female patient achieve a lower BMI prior to pregnancy.
Bariatric surgery
Because the number of women undergoing bariatric surgery for morbid obesity is rapidly increasing,21 it is important to educate them about the specific effects of such procedures on reproductive health. A period of rapid weight loss—such as occurs after bariatric surgery—is not a time to consider pregnancy, despite the improvement in eventual pregnancy outcomes associated with a healthier BMI.
Many of these women may have been anovulatory when morbidly obese and unaware that fertility increases in the postoperative year, after a reduced BMI is achieved. If ovulation and menses have not yet normalized, a woman may become pregnant and not know it. This could result in the inadvertent exposure of the developing fetus to such teratogenic risks as alcohol, tobacco, and certain prescription drugs. While a pregnancy may be welcome, better outcomes are likely if pregnancy is avoided for at least 12 months after bariatric surgery.22 Effective contraception should be used until ovulation cycles stabilize.
Some surgical weight loss procedures, such as the Roux-en-Y gastric bypass, may alter the absorption of medication, including oral contraceptives (OCs), making the use of OCs after such procedures less than ideal. The more reliably absorbed injectable medroxyprogesterone is an option, but women who have undergone bariatric surgery often wish to avoid the associated risk for weight gain. Nonhormonal long-acting contraceptive methods not associated with weight gain, such as the copper intrauterine device, may be preferable for use in the first year postprocedure.21
On the next page: Depression and other mood disorders >>
DEPRESSION AND OTHER MOOD DISORDERS
Mood disorders include depression, bipolar disorder, and anxiety disorders. Selective serotonin reuptake inhibitors (SSRIs, such as paroxetine or sertraline) or serotonin and norepinephrine reuptake inhibitors (SNRIs, such as venlafaxine or duloxetine) are often prescribed for primary care management of depression and other mood disorders.
When SSRIs or SNRIs are not fully effective, clinicians may refer patients to mental health specialists for consultation and possible ongoing management. Women of reproductive age who receive specialty care for mood disorders are encouraged to continue their regular visits to primary care clinicians.
Medication: Risk for birth defects
Anticonvulsants, such as valproate, carbamazepine, and lamotrigine, are commonly used to treat bipolar disorder.23 When taken during the first trimester of pregnancy, these drugs pose well-documented risks to the rapidly developing fetus. Most evidence relates to the risk for neural tube defects, such as spina bifida, but other evidence suggests a risk for general cognitive impairment after prenatal valproate exposure. While the latter is based primarily on studies of women taking anti-epileptic drugs for seizure control—not psychiatric diagnoses—first-trimester risks appear to be independent of maternal seizures.23 Although folic acid supplementation decreases the incidence of neural tube defects (see discussion under “Nutritional Deficiencies"), it is unknown if such supplementation is effective in mitigating the additional risks to the fetus from exposure to anticonvulsants.
Female patients of childbearing age must be advised of the potential effects of these commonly prescribed mood-stabilizing drugs, not only as they relate to the diagnosis being treated but also regarding their possible effects on an early, undiagnosed pregnancy. Unfortunately, evidence indicates that insufficient attention is given to counseling reproductive-age women about the risks and benefits of these drugs as they relate to potential conception, at least in the context of specialty care.23 Therefore, the primary care clinician and the specialist should utilize a team approach, emphasizing careful reproductive planning to avoid pregnancy while under treatment with these drugs to ensure the best possible outcomes.
In the context of potential pregnancy, the need to manage depression and other mood disorders effectively is particularly important: Prepregnancy depressive mood has been significantly associated with preterm birth, and at least 14.5% of women experience a new episode of depression during pregnancy.24 Thus, effective treatment of mood disorders should be a priority, both as part of preconception care and during pregnancy.
Similarly, treatment strategies for postpartum depression—widely estimated to affect 10% to 20% of new mothers—must consider the potential risks of pharmacologic therapy to a fetus should the patient conceive during treatment.
On the next page: Nutritional deficiencies >>
NUTRITIONAL DEFICIENCIES
While there is widespread public awareness, at least on a basic level, of the importance of good nutrition during pregnancy, what that constitutes is not necessarily clearly understood. Even less well recognized is the importance of a woman’s nutritional status at the time of conception, at preimplantation, and during the early weeks of placental development, before pregnancy is known or confirmed. During this crucial time—three to seven weeks after the last menstrual period—an inadequate diet may result in low–birth-weight infants with lifelong health problems.25 These may include respiratory problems associated with barotrauma from ventilation at birth; neural tube defects; and orofacial clefts.25
Because of inadequate intake of fresh fruits and vegetables, many reproductive-age women in the US are deficient in vitamins A, C, B6, and E, as well as calcium, iron, zinc, magnesium, and folic acid. Although vitamin and mineral supplements are readily available, little clinical research—with the exception of folic acid—has been done on the efficacy of such supplementation.26
Until more is known, intake of these dietary components is best achieved as part of a well-balanced diet; however, this recommendation may need to be modified for African-American women. In a recent retrospective study of almost 2,500 white and African-American women who took a multivitamin supplement consistently during the month before conception, supplementation was associated with increased infant birth weight in the infants born to African-American women but not in those born to white women.26
Folic acid
In the specific case of folic acid, the crucial importance of preconception intake by reproductive-age women is hard to overstate. A well-established body of research supports supplementation to reduce the incidence of neural tube defects that may occur very early in development, before many women are aware of a pregnancy.25-27 However, it is estimated that only a minority of reproductive-age women take a regular folic acid supplement. This may be particularly true of women who are actively avoiding pregnancy and using regular contraception. Patients need to be educated that, as effective as current contraceptive methods are known to be, each method has a typical user failure rate, meaning that actual effectiveness is lower than theoretical effectiveness.
Considering that half of US pregnancies are unintended, with some occurring as a result of contraceptive method or user failures, the FDA Advisory Committee unanimously endorsed the concept of using OCs as a vehicle for folate supplementation.27 There are currently two FDA-approved OCs fortified with the equivalent of 0.4 mg of folic acid. Both contain drospirenone and therefore present a somewhat elevated risk for blood clots, especially in the first year of use.28,29 While this risk is small compared to the incidence of blood clots during pregnancy, a careful history should be taken to avoid prescribing these products to patients already at increased risk for blood clots (eg, obese women, smokers [even light smokers], those with a history of a blood clot after surgery or a motor vehicle accident). For women without risk factors, folic acid–supplemented OCs may be very beneficial should they become pregnant unintentionally or quickly after stopping contraception.
On the next page: Cultural considerations and conclusion >>
CULTURAL CONSIDERATIONS
Not all racial, ethnic, and socioeconomic groups consider health and pregnancy in the same cultural context, and nonmedical factors may affect health behaviors and sources of health counseling. Studies of women of different cultural backgrounds are illustrative.
In one study, increasing women’s evidence-based knowledge of preconception and interconception health behaviors, using group education and peer support, was shown to produce attitudinal and behavior changes in a sample of reproductive-age rural white women, especially with regard to nutrition and physical activity in the preconception period.30
Another study of primarily low-income Latina women with low levels of acculturation revealed that they had good understanding of the importance of attention to health once pregnancy is confirmed. However, they expressed much less belief in the ability of a woman to control her own preconception general health.31
A third study involving a sample of African-American women found that the women saw no clear role for preconception or interconception care through health care visits with primary care clinicians; rather, they looked to their social and cultural communities and families for such support.32
These diverse results suggest that both community-wide education and one-to-one health counseling are needed to effect improvements in health behaviors and knowledge. Subtle differences in cultural context must be recognized by health care providers who interact with a broad range of reproductive-age women.
CONCLUSION
Guidelines from the American Academy of Pediatrics, the American Congress of Obstetricians and Gynecologists, and the CDC all endorse the integration of preconception care into primary care encounters.33-36 The concept of preconception health care offers clinicians the opportunity to greatly influence the health of reproductive-age women in the primary care setting, with the potential to achieve small but clinically significant changes in health behaviors. Complications of pregnancy and poor pregnancy outcomes may be reduced when the overall health status of women of reproductive age is addressed, with awareness and mitigation of factors known to produce negative pregnancy outcomes.
There is a need, however, for ongoing research to develop effective, evidence-based strategies for use by primary care clinicians in the effort to improve women’s preconception health and, ultimately, pregnancy outcomes.
1. National Center for Health Statistics. Table 7: Prenatal care for live births, by detailed race and Hispanic origin of mother: United States, selected years 1970–2004. In: Health, United States, 2006. Report No: 2006-1232. www.ncbi.nlm.nih.gov/books/NBK21003. Accessed July 17, 2014.
2. Office of Adolescent Health, US Department of Health and Human Services. Trends in teen pregnancy and childbearing. www.hhs.gov/ash/oah/adolescent-health-topics/reproductive-health/teen-pregnancy/trends.html#_ftn6. Accessed July 17, 2014.
3. Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services. Child Health USA 2013. http://mchb.hrsa.gov/chusa13. Accessed July 17, 2014.
4. World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. www.who.int/pmnch/media/news/2012/201204_borntoosoon_countryranking.pdf. Accessed July 17, 2014.
5. Organization for Economic Cooperation and Development (OECD). Low birth weight infants, 2009 and change 1970-2009 (or nearest year). In: Health at a Glance 2011: OECD indicators. www.oecd.org/els/health-systems/49105858.pdf. Accessed July 17, 2014.
6. CDC. Infant mortality rates and international rankings. www.cdc.gov/nchs/data/hus/2013/016.pdf. Accessed July 17, 2014.
7. Livingood WC, Brady C, Pierce K, et al. Impact of pre-conception health care: evaluation of a social determinants focused intervention. Matern Child Health J. 2010;14:382-391.
8. Hillemeier MM, Weisman CS, Chase GA, et al. Women’s preconceptional health and use of health services: implications for preconception care. Health Serv Res. 2008;43(1):54-75.
9. Chuang CH, Weisman CS, Hillemeier MM, et al. Pregnancy intention and health behaviors: results from the Central Pennsylvania Women’s Health Study Cohort. Matern Child Health J. 2010;14:501-510.
10. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/overview.html. Accessed July 17, 2014.
11. Heavey E. Don’t miss preconception care opportunities for adolescents. Am J Matern Child Nurs. 2010;35(4):213-219.
12. Hoover KW, Tao G, Berman S, Kent CK. Utilization of health services in physician offices and outpatient clinics by adolescents and young women in the United States: implications for improving access to reproductive health services. J Adolesc Health. 2010;46:324-330.
13. Johnson K, Posner SF, Biermann J. Recommendations to improve preconception health and health care—United States. MMWR Recomm Rep. 2006;55:1-23.
14. Krishnamoorthy U, Schram CM, Hill SR. Maternal obesity in pregnancy: is it time for meaningful research to inform preventive and management strategies? Brit J Obstet Gynecol. 2006;113:1134-1140.
15. Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56-59.
16. Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502-1505.
17. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437739.71477.ee.full.pdf+html. Accessed July 17, 2014.
18. Callaway LK, O’Callaghan MJ, McIntyre HD. Barriers to addressing overweight and obesity before conception. Med J Aust. 2009;191(8):425-427.
19. Donahue SMA, Zimmerman FJ, Starr JR, Holt VL. Correlates of pre-pregnancy physical inactivity: results from the pregnancy risk assessment monitoring system. Matern Child Health J. 2010;14:235-244.
20. Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122(1):64-71.
21. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Obesity in pregnancy. Obstet Gynecol. 2013;121(1):213-217.
22. Ciangura C, Corigliano N, Basdevant A, et al. Etonogestrel concentrations in morbidly obese women following Roux-en-Y gastric bypass surgery: three case reports. Contraception. 2011;84:649-651.
23. Wieck A, Rao S, Sein K, Haddad PM. A survey of antiepileptic prescribing to women of childbearing potential in psychiatry. Arch Womens Ment Health. 2007;10:83-85.
24. Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA study. J Womens Health. 2009;18(6):803-811.
25. Gardiner PM, Nelson L, Shellhaas CS, et al. The clinical content of preconception care: nutrition and dietary supplements. Am J Obstet Gynecol. 2008;S345-S353.
26. Burris HH, Mitchell AA, Werler MM. Periconceptional multivitamin use and infant birth weight disparities. Ann Epidemiol. 2010;20(3):233-240.
27. Taylor TN, Farkouh RA, Graham JB, et al. Potential reduction in neural tube defects associated with use of Metafolin-fortified oral contraceptives in the United States. Am J Obstet Gynecol. 2011:e1-e8.
28. Beyaz [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
29. Safyral [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
30. Hillemeier MM, Downs DS, Feinberg ME, et al. Improving women’s preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania Women’s Health Study. Womens Health Issues. 2008;18(6 suppl):S87-S96.
31. Coonrod DV, Bruce NC, Malcolm TD, et al. Knowledge and attitudes regarding preconception care in a predominantly low-income Mexican American population. Am J Obstet Gynecol. 2009;686:e1-e7.
32. Canady RB, Tiedje LB, Lauber C. Preconception care and pregnancy planning: voices of African American women. Matern Child Nurs. 2008;33(2):90-97.
33. Bellanca HK, Hunter MS. ONE KEY QUESTION: preventive reproductive health is part of high quality primary care. Contraception. 2013;88(1):3-6.
34. American Academy of Pediatrics. Preconception health care. www.healthychildren.org/English/ages-stages/prenatal/Pages/Reduce-the-Risk-of-Birth-Defects.aspx. Accessed July 17, 2014.
35. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. The importance of preconception care in the continuum of women’s health care. www.acog.org/Resources_And_Publi cations/Committee_Opinions/Committee_on_Gynecologic_Practice/The_Importance_of_Preconception_Care_in_the_Continuum_of_Wom ens_Health_Care. Accessed July 17, 2014.
36. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/hcp/. Accessed July 17, 2014.
CE/CME No: CR-1408
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define preconception and interconception health care and explain how these concepts relate to primary care.
• Describe three common clinical presentations in reproductive-age women that have implications for preconception and interconception health care.
• Explain the considerations, in terms of potential pregnancies, when prescribing pharmacologic treatment for reproductive-age women.
• Discuss examples of cultural factors and beliefs that may affect preconception health counseling provided to women of reproductive age.
FACULTY
Kathleen A. Ahonen is an Assistant Professor and Colleen Quinlan is an Associate Professor at the University of Toledo College of Nursing.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Because half of all pregnancies in the United States are unplanned, primary care constitutes preconception health care for women ages 15 to 44. Here are recommendations to incorporate into routine visits to improve outcomes of possible pregnancies.
Traditional interventions to improve pregnancy outcomes in the United States have focused on early, consistent prenatal care and on reducing the teenage pregnancy rate. Data indicate that early access to prenatal care has increased and that the teenage pregnancy rate has fallen.1,2
But while US rates of preterm births, low–birth-weight infants, and infant mortality have all declined since the mid-2000s,3 they remain higher than those in many other developed countries.4-6 Furthermore, there are significant differences in these rates by race and ethnicity.3
Many experts believe that waiting until a woman is pregnant to address the health needs and behaviors that adversely affect pregnancy outcomes is, at best, an incomplete solution to these problems. A more comprehensive view of women’s health care broadens this focus to stress the importance of preconception health care throughout the reproductive years. It is key to the achievement of optimal health, not only for women but also for their potential future children.7-9
The CDC defines preconception health simply as the health of women and men during their reproductive years. Preconception health care is defined as medical care during those years (for women, ages 15 to 44) that focuses on improving aspects of the patient’s health or health behaviors that can improve outcomes in the event of a pregnancy.10 The term interconception health care is also applied specifically to health care provided during the time between pregnancies.
This article highlights obesity, depression and other mood disorders, and nutritional deficiencies and reviews potential risk factors, particularly occurring very early in a pregnancy. Recommendations are offered for more effective health counseling at routine visits, with the goal of improving outcomes of potential pregnancies.
PRIMARY CARE IS PRECONCEPTION CARE
Researchers have reported that only about 50% of reproductive-age women receive counseling about health behaviors and pregnancy planning8 and that such counseling is even rarer for adolescent females.11,12 Because women of childbearing age commonly see both Ob-Gyn and family practice clinicians for health care, primary care providers are well positioned to deliver preconception health care by integrating health counseling, discussion of health behaviors, and primary and secondary prevention into patient care for reproductive-age women (see "Summary of CDC Recommendations to Improve Preconception Health and Health Care").6
On the next page: Obesity >>
OBESITY
Researchers in both the US and internationally have noted the connection between obesity and pregnancy complications such as gestational diabetes that increase the risk for poor pregnancy outcomes.13-15 Indeed, pregnancy outcomes can be altered for the better with weight loss in the preconception or interconception period.16
Weight loss is complex; it can be difficult to accomplish, and long-term maintenance also presents a challenge. Short-term weight loss is achievable, however, and can produce beneficial metabolic effects, such as a decrease in insulin resistance.17
Before weight loss attempts can be initiated, the overweight or obese patient’s weight must be acknowledged as a health problem. In one 2009 study, Callaway et al found that in their sample of 412 overweight and obese women, only 17% were advised by their health care providers to lose weight.18
Regular physical activity can facilitate the achievement and maintenance of a healthy weight. However, physical inactivity is common in nonpregnant women, especially those with higher BMIs. The CDC’s Pregnancy Risk Assessment Monitoring System (PRAMS; www.cdc.gov/prams), widely regarded as an important source of data about women’s health behaviors, reveals that nearly 40% of US women do not meet national recommendations for routine physical activity in the three months prior to pregnancy.19
Recent obesity guidelines recommend initial weight loss goals of 5% to 10% of baseline weight in six months before pregnancy.17 Some women may not achieve these goals because they are unaware of the potentially short time period between discontinuation of contraception and conception. Others, especially those older than 30, intentionally plan for short interconception intervals as part of their family planning strategy, which does not allow enough time for safe weight loss.20
Primary care clinicians should counsel these patients on the importance of a healthy weight and regular physical activity to the maintenance of optimal health. Particular emphasis should be placed on achieving weight loss slowly and safely and then maintaining it, at least for the short term. This strategy may be effective in helping a female patient achieve a lower BMI prior to pregnancy.
Bariatric surgery
Because the number of women undergoing bariatric surgery for morbid obesity is rapidly increasing,21 it is important to educate them about the specific effects of such procedures on reproductive health. A period of rapid weight loss—such as occurs after bariatric surgery—is not a time to consider pregnancy, despite the improvement in eventual pregnancy outcomes associated with a healthier BMI.
Many of these women may have been anovulatory when morbidly obese and unaware that fertility increases in the postoperative year, after a reduced BMI is achieved. If ovulation and menses have not yet normalized, a woman may become pregnant and not know it. This could result in the inadvertent exposure of the developing fetus to such teratogenic risks as alcohol, tobacco, and certain prescription drugs. While a pregnancy may be welcome, better outcomes are likely if pregnancy is avoided for at least 12 months after bariatric surgery.22 Effective contraception should be used until ovulation cycles stabilize.
Some surgical weight loss procedures, such as the Roux-en-Y gastric bypass, may alter the absorption of medication, including oral contraceptives (OCs), making the use of OCs after such procedures less than ideal. The more reliably absorbed injectable medroxyprogesterone is an option, but women who have undergone bariatric surgery often wish to avoid the associated risk for weight gain. Nonhormonal long-acting contraceptive methods not associated with weight gain, such as the copper intrauterine device, may be preferable for use in the first year postprocedure.21
On the next page: Depression and other mood disorders >>
DEPRESSION AND OTHER MOOD DISORDERS
Mood disorders include depression, bipolar disorder, and anxiety disorders. Selective serotonin reuptake inhibitors (SSRIs, such as paroxetine or sertraline) or serotonin and norepinephrine reuptake inhibitors (SNRIs, such as venlafaxine or duloxetine) are often prescribed for primary care management of depression and other mood disorders.
When SSRIs or SNRIs are not fully effective, clinicians may refer patients to mental health specialists for consultation and possible ongoing management. Women of reproductive age who receive specialty care for mood disorders are encouraged to continue their regular visits to primary care clinicians.
Medication: Risk for birth defects
Anticonvulsants, such as valproate, carbamazepine, and lamotrigine, are commonly used to treat bipolar disorder.23 When taken during the first trimester of pregnancy, these drugs pose well-documented risks to the rapidly developing fetus. Most evidence relates to the risk for neural tube defects, such as spina bifida, but other evidence suggests a risk for general cognitive impairment after prenatal valproate exposure. While the latter is based primarily on studies of women taking anti-epileptic drugs for seizure control—not psychiatric diagnoses—first-trimester risks appear to be independent of maternal seizures.23 Although folic acid supplementation decreases the incidence of neural tube defects (see discussion under “Nutritional Deficiencies"), it is unknown if such supplementation is effective in mitigating the additional risks to the fetus from exposure to anticonvulsants.
Female patients of childbearing age must be advised of the potential effects of these commonly prescribed mood-stabilizing drugs, not only as they relate to the diagnosis being treated but also regarding their possible effects on an early, undiagnosed pregnancy. Unfortunately, evidence indicates that insufficient attention is given to counseling reproductive-age women about the risks and benefits of these drugs as they relate to potential conception, at least in the context of specialty care.23 Therefore, the primary care clinician and the specialist should utilize a team approach, emphasizing careful reproductive planning to avoid pregnancy while under treatment with these drugs to ensure the best possible outcomes.
In the context of potential pregnancy, the need to manage depression and other mood disorders effectively is particularly important: Prepregnancy depressive mood has been significantly associated with preterm birth, and at least 14.5% of women experience a new episode of depression during pregnancy.24 Thus, effective treatment of mood disorders should be a priority, both as part of preconception care and during pregnancy.
Similarly, treatment strategies for postpartum depression—widely estimated to affect 10% to 20% of new mothers—must consider the potential risks of pharmacologic therapy to a fetus should the patient conceive during treatment.
On the next page: Nutritional deficiencies >>
NUTRITIONAL DEFICIENCIES
While there is widespread public awareness, at least on a basic level, of the importance of good nutrition during pregnancy, what that constitutes is not necessarily clearly understood. Even less well recognized is the importance of a woman’s nutritional status at the time of conception, at preimplantation, and during the early weeks of placental development, before pregnancy is known or confirmed. During this crucial time—three to seven weeks after the last menstrual period—an inadequate diet may result in low–birth-weight infants with lifelong health problems.25 These may include respiratory problems associated with barotrauma from ventilation at birth; neural tube defects; and orofacial clefts.25
Because of inadequate intake of fresh fruits and vegetables, many reproductive-age women in the US are deficient in vitamins A, C, B6, and E, as well as calcium, iron, zinc, magnesium, and folic acid. Although vitamin and mineral supplements are readily available, little clinical research—with the exception of folic acid—has been done on the efficacy of such supplementation.26
Until more is known, intake of these dietary components is best achieved as part of a well-balanced diet; however, this recommendation may need to be modified for African-American women. In a recent retrospective study of almost 2,500 white and African-American women who took a multivitamin supplement consistently during the month before conception, supplementation was associated with increased infant birth weight in the infants born to African-American women but not in those born to white women.26
Folic acid
In the specific case of folic acid, the crucial importance of preconception intake by reproductive-age women is hard to overstate. A well-established body of research supports supplementation to reduce the incidence of neural tube defects that may occur very early in development, before many women are aware of a pregnancy.25-27 However, it is estimated that only a minority of reproductive-age women take a regular folic acid supplement. This may be particularly true of women who are actively avoiding pregnancy and using regular contraception. Patients need to be educated that, as effective as current contraceptive methods are known to be, each method has a typical user failure rate, meaning that actual effectiveness is lower than theoretical effectiveness.
Considering that half of US pregnancies are unintended, with some occurring as a result of contraceptive method or user failures, the FDA Advisory Committee unanimously endorsed the concept of using OCs as a vehicle for folate supplementation.27 There are currently two FDA-approved OCs fortified with the equivalent of 0.4 mg of folic acid. Both contain drospirenone and therefore present a somewhat elevated risk for blood clots, especially in the first year of use.28,29 While this risk is small compared to the incidence of blood clots during pregnancy, a careful history should be taken to avoid prescribing these products to patients already at increased risk for blood clots (eg, obese women, smokers [even light smokers], those with a history of a blood clot after surgery or a motor vehicle accident). For women without risk factors, folic acid–supplemented OCs may be very beneficial should they become pregnant unintentionally or quickly after stopping contraception.
On the next page: Cultural considerations and conclusion >>
CULTURAL CONSIDERATIONS
Not all racial, ethnic, and socioeconomic groups consider health and pregnancy in the same cultural context, and nonmedical factors may affect health behaviors and sources of health counseling. Studies of women of different cultural backgrounds are illustrative.
In one study, increasing women’s evidence-based knowledge of preconception and interconception health behaviors, using group education and peer support, was shown to produce attitudinal and behavior changes in a sample of reproductive-age rural white women, especially with regard to nutrition and physical activity in the preconception period.30
Another study of primarily low-income Latina women with low levels of acculturation revealed that they had good understanding of the importance of attention to health once pregnancy is confirmed. However, they expressed much less belief in the ability of a woman to control her own preconception general health.31
A third study involving a sample of African-American women found that the women saw no clear role for preconception or interconception care through health care visits with primary care clinicians; rather, they looked to their social and cultural communities and families for such support.32
These diverse results suggest that both community-wide education and one-to-one health counseling are needed to effect improvements in health behaviors and knowledge. Subtle differences in cultural context must be recognized by health care providers who interact with a broad range of reproductive-age women.
CONCLUSION
Guidelines from the American Academy of Pediatrics, the American Congress of Obstetricians and Gynecologists, and the CDC all endorse the integration of preconception care into primary care encounters.33-36 The concept of preconception health care offers clinicians the opportunity to greatly influence the health of reproductive-age women in the primary care setting, with the potential to achieve small but clinically significant changes in health behaviors. Complications of pregnancy and poor pregnancy outcomes may be reduced when the overall health status of women of reproductive age is addressed, with awareness and mitigation of factors known to produce negative pregnancy outcomes.
There is a need, however, for ongoing research to develop effective, evidence-based strategies for use by primary care clinicians in the effort to improve women’s preconception health and, ultimately, pregnancy outcomes.
CE/CME No: CR-1408
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define preconception and interconception health care and explain how these concepts relate to primary care.
• Describe three common clinical presentations in reproductive-age women that have implications for preconception and interconception health care.
• Explain the considerations, in terms of potential pregnancies, when prescribing pharmacologic treatment for reproductive-age women.
• Discuss examples of cultural factors and beliefs that may affect preconception health counseling provided to women of reproductive age.
FACULTY
Kathleen A. Ahonen is an Assistant Professor and Colleen Quinlan is an Associate Professor at the University of Toledo College of Nursing.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Because half of all pregnancies in the United States are unplanned, primary care constitutes preconception health care for women ages 15 to 44. Here are recommendations to incorporate into routine visits to improve outcomes of possible pregnancies.
Traditional interventions to improve pregnancy outcomes in the United States have focused on early, consistent prenatal care and on reducing the teenage pregnancy rate. Data indicate that early access to prenatal care has increased and that the teenage pregnancy rate has fallen.1,2
But while US rates of preterm births, low–birth-weight infants, and infant mortality have all declined since the mid-2000s,3 they remain higher than those in many other developed countries.4-6 Furthermore, there are significant differences in these rates by race and ethnicity.3
Many experts believe that waiting until a woman is pregnant to address the health needs and behaviors that adversely affect pregnancy outcomes is, at best, an incomplete solution to these problems. A more comprehensive view of women’s health care broadens this focus to stress the importance of preconception health care throughout the reproductive years. It is key to the achievement of optimal health, not only for women but also for their potential future children.7-9
The CDC defines preconception health simply as the health of women and men during their reproductive years. Preconception health care is defined as medical care during those years (for women, ages 15 to 44) that focuses on improving aspects of the patient’s health or health behaviors that can improve outcomes in the event of a pregnancy.10 The term interconception health care is also applied specifically to health care provided during the time between pregnancies.
This article highlights obesity, depression and other mood disorders, and nutritional deficiencies and reviews potential risk factors, particularly occurring very early in a pregnancy. Recommendations are offered for more effective health counseling at routine visits, with the goal of improving outcomes of potential pregnancies.
PRIMARY CARE IS PRECONCEPTION CARE
Researchers have reported that only about 50% of reproductive-age women receive counseling about health behaviors and pregnancy planning8 and that such counseling is even rarer for adolescent females.11,12 Because women of childbearing age commonly see both Ob-Gyn and family practice clinicians for health care, primary care providers are well positioned to deliver preconception health care by integrating health counseling, discussion of health behaviors, and primary and secondary prevention into patient care for reproductive-age women (see "Summary of CDC Recommendations to Improve Preconception Health and Health Care").6
On the next page: Obesity >>
OBESITY
Researchers in both the US and internationally have noted the connection between obesity and pregnancy complications such as gestational diabetes that increase the risk for poor pregnancy outcomes.13-15 Indeed, pregnancy outcomes can be altered for the better with weight loss in the preconception or interconception period.16
Weight loss is complex; it can be difficult to accomplish, and long-term maintenance also presents a challenge. Short-term weight loss is achievable, however, and can produce beneficial metabolic effects, such as a decrease in insulin resistance.17
Before weight loss attempts can be initiated, the overweight or obese patient’s weight must be acknowledged as a health problem. In one 2009 study, Callaway et al found that in their sample of 412 overweight and obese women, only 17% were advised by their health care providers to lose weight.18
Regular physical activity can facilitate the achievement and maintenance of a healthy weight. However, physical inactivity is common in nonpregnant women, especially those with higher BMIs. The CDC’s Pregnancy Risk Assessment Monitoring System (PRAMS; www.cdc.gov/prams), widely regarded as an important source of data about women’s health behaviors, reveals that nearly 40% of US women do not meet national recommendations for routine physical activity in the three months prior to pregnancy.19
Recent obesity guidelines recommend initial weight loss goals of 5% to 10% of baseline weight in six months before pregnancy.17 Some women may not achieve these goals because they are unaware of the potentially short time period between discontinuation of contraception and conception. Others, especially those older than 30, intentionally plan for short interconception intervals as part of their family planning strategy, which does not allow enough time for safe weight loss.20
Primary care clinicians should counsel these patients on the importance of a healthy weight and regular physical activity to the maintenance of optimal health. Particular emphasis should be placed on achieving weight loss slowly and safely and then maintaining it, at least for the short term. This strategy may be effective in helping a female patient achieve a lower BMI prior to pregnancy.
Bariatric surgery
Because the number of women undergoing bariatric surgery for morbid obesity is rapidly increasing,21 it is important to educate them about the specific effects of such procedures on reproductive health. A period of rapid weight loss—such as occurs after bariatric surgery—is not a time to consider pregnancy, despite the improvement in eventual pregnancy outcomes associated with a healthier BMI.
Many of these women may have been anovulatory when morbidly obese and unaware that fertility increases in the postoperative year, after a reduced BMI is achieved. If ovulation and menses have not yet normalized, a woman may become pregnant and not know it. This could result in the inadvertent exposure of the developing fetus to such teratogenic risks as alcohol, tobacco, and certain prescription drugs. While a pregnancy may be welcome, better outcomes are likely if pregnancy is avoided for at least 12 months after bariatric surgery.22 Effective contraception should be used until ovulation cycles stabilize.
Some surgical weight loss procedures, such as the Roux-en-Y gastric bypass, may alter the absorption of medication, including oral contraceptives (OCs), making the use of OCs after such procedures less than ideal. The more reliably absorbed injectable medroxyprogesterone is an option, but women who have undergone bariatric surgery often wish to avoid the associated risk for weight gain. Nonhormonal long-acting contraceptive methods not associated with weight gain, such as the copper intrauterine device, may be preferable for use in the first year postprocedure.21
On the next page: Depression and other mood disorders >>
DEPRESSION AND OTHER MOOD DISORDERS
Mood disorders include depression, bipolar disorder, and anxiety disorders. Selective serotonin reuptake inhibitors (SSRIs, such as paroxetine or sertraline) or serotonin and norepinephrine reuptake inhibitors (SNRIs, such as venlafaxine or duloxetine) are often prescribed for primary care management of depression and other mood disorders.
When SSRIs or SNRIs are not fully effective, clinicians may refer patients to mental health specialists for consultation and possible ongoing management. Women of reproductive age who receive specialty care for mood disorders are encouraged to continue their regular visits to primary care clinicians.
Medication: Risk for birth defects
Anticonvulsants, such as valproate, carbamazepine, and lamotrigine, are commonly used to treat bipolar disorder.23 When taken during the first trimester of pregnancy, these drugs pose well-documented risks to the rapidly developing fetus. Most evidence relates to the risk for neural tube defects, such as spina bifida, but other evidence suggests a risk for general cognitive impairment after prenatal valproate exposure. While the latter is based primarily on studies of women taking anti-epileptic drugs for seizure control—not psychiatric diagnoses—first-trimester risks appear to be independent of maternal seizures.23 Although folic acid supplementation decreases the incidence of neural tube defects (see discussion under “Nutritional Deficiencies"), it is unknown if such supplementation is effective in mitigating the additional risks to the fetus from exposure to anticonvulsants.
Female patients of childbearing age must be advised of the potential effects of these commonly prescribed mood-stabilizing drugs, not only as they relate to the diagnosis being treated but also regarding their possible effects on an early, undiagnosed pregnancy. Unfortunately, evidence indicates that insufficient attention is given to counseling reproductive-age women about the risks and benefits of these drugs as they relate to potential conception, at least in the context of specialty care.23 Therefore, the primary care clinician and the specialist should utilize a team approach, emphasizing careful reproductive planning to avoid pregnancy while under treatment with these drugs to ensure the best possible outcomes.
In the context of potential pregnancy, the need to manage depression and other mood disorders effectively is particularly important: Prepregnancy depressive mood has been significantly associated with preterm birth, and at least 14.5% of women experience a new episode of depression during pregnancy.24 Thus, effective treatment of mood disorders should be a priority, both as part of preconception care and during pregnancy.
Similarly, treatment strategies for postpartum depression—widely estimated to affect 10% to 20% of new mothers—must consider the potential risks of pharmacologic therapy to a fetus should the patient conceive during treatment.
On the next page: Nutritional deficiencies >>
NUTRITIONAL DEFICIENCIES
While there is widespread public awareness, at least on a basic level, of the importance of good nutrition during pregnancy, what that constitutes is not necessarily clearly understood. Even less well recognized is the importance of a woman’s nutritional status at the time of conception, at preimplantation, and during the early weeks of placental development, before pregnancy is known or confirmed. During this crucial time—three to seven weeks after the last menstrual period—an inadequate diet may result in low–birth-weight infants with lifelong health problems.25 These may include respiratory problems associated with barotrauma from ventilation at birth; neural tube defects; and orofacial clefts.25
Because of inadequate intake of fresh fruits and vegetables, many reproductive-age women in the US are deficient in vitamins A, C, B6, and E, as well as calcium, iron, zinc, magnesium, and folic acid. Although vitamin and mineral supplements are readily available, little clinical research—with the exception of folic acid—has been done on the efficacy of such supplementation.26
Until more is known, intake of these dietary components is best achieved as part of a well-balanced diet; however, this recommendation may need to be modified for African-American women. In a recent retrospective study of almost 2,500 white and African-American women who took a multivitamin supplement consistently during the month before conception, supplementation was associated with increased infant birth weight in the infants born to African-American women but not in those born to white women.26
Folic acid
In the specific case of folic acid, the crucial importance of preconception intake by reproductive-age women is hard to overstate. A well-established body of research supports supplementation to reduce the incidence of neural tube defects that may occur very early in development, before many women are aware of a pregnancy.25-27 However, it is estimated that only a minority of reproductive-age women take a regular folic acid supplement. This may be particularly true of women who are actively avoiding pregnancy and using regular contraception. Patients need to be educated that, as effective as current contraceptive methods are known to be, each method has a typical user failure rate, meaning that actual effectiveness is lower than theoretical effectiveness.
Considering that half of US pregnancies are unintended, with some occurring as a result of contraceptive method or user failures, the FDA Advisory Committee unanimously endorsed the concept of using OCs as a vehicle for folate supplementation.27 There are currently two FDA-approved OCs fortified with the equivalent of 0.4 mg of folic acid. Both contain drospirenone and therefore present a somewhat elevated risk for blood clots, especially in the first year of use.28,29 While this risk is small compared to the incidence of blood clots during pregnancy, a careful history should be taken to avoid prescribing these products to patients already at increased risk for blood clots (eg, obese women, smokers [even light smokers], those with a history of a blood clot after surgery or a motor vehicle accident). For women without risk factors, folic acid–supplemented OCs may be very beneficial should they become pregnant unintentionally or quickly after stopping contraception.
On the next page: Cultural considerations and conclusion >>
CULTURAL CONSIDERATIONS
Not all racial, ethnic, and socioeconomic groups consider health and pregnancy in the same cultural context, and nonmedical factors may affect health behaviors and sources of health counseling. Studies of women of different cultural backgrounds are illustrative.
In one study, increasing women’s evidence-based knowledge of preconception and interconception health behaviors, using group education and peer support, was shown to produce attitudinal and behavior changes in a sample of reproductive-age rural white women, especially with regard to nutrition and physical activity in the preconception period.30
Another study of primarily low-income Latina women with low levels of acculturation revealed that they had good understanding of the importance of attention to health once pregnancy is confirmed. However, they expressed much less belief in the ability of a woman to control her own preconception general health.31
A third study involving a sample of African-American women found that the women saw no clear role for preconception or interconception care through health care visits with primary care clinicians; rather, they looked to their social and cultural communities and families for such support.32
These diverse results suggest that both community-wide education and one-to-one health counseling are needed to effect improvements in health behaviors and knowledge. Subtle differences in cultural context must be recognized by health care providers who interact with a broad range of reproductive-age women.
CONCLUSION
Guidelines from the American Academy of Pediatrics, the American Congress of Obstetricians and Gynecologists, and the CDC all endorse the integration of preconception care into primary care encounters.33-36 The concept of preconception health care offers clinicians the opportunity to greatly influence the health of reproductive-age women in the primary care setting, with the potential to achieve small but clinically significant changes in health behaviors. Complications of pregnancy and poor pregnancy outcomes may be reduced when the overall health status of women of reproductive age is addressed, with awareness and mitigation of factors known to produce negative pregnancy outcomes.
There is a need, however, for ongoing research to develop effective, evidence-based strategies for use by primary care clinicians in the effort to improve women’s preconception health and, ultimately, pregnancy outcomes.
1. National Center for Health Statistics. Table 7: Prenatal care for live births, by detailed race and Hispanic origin of mother: United States, selected years 1970–2004. In: Health, United States, 2006. Report No: 2006-1232. www.ncbi.nlm.nih.gov/books/NBK21003. Accessed July 17, 2014.
2. Office of Adolescent Health, US Department of Health and Human Services. Trends in teen pregnancy and childbearing. www.hhs.gov/ash/oah/adolescent-health-topics/reproductive-health/teen-pregnancy/trends.html#_ftn6. Accessed July 17, 2014.
3. Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services. Child Health USA 2013. http://mchb.hrsa.gov/chusa13. Accessed July 17, 2014.
4. World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. www.who.int/pmnch/media/news/2012/201204_borntoosoon_countryranking.pdf. Accessed July 17, 2014.
5. Organization for Economic Cooperation and Development (OECD). Low birth weight infants, 2009 and change 1970-2009 (or nearest year). In: Health at a Glance 2011: OECD indicators. www.oecd.org/els/health-systems/49105858.pdf. Accessed July 17, 2014.
6. CDC. Infant mortality rates and international rankings. www.cdc.gov/nchs/data/hus/2013/016.pdf. Accessed July 17, 2014.
7. Livingood WC, Brady C, Pierce K, et al. Impact of pre-conception health care: evaluation of a social determinants focused intervention. Matern Child Health J. 2010;14:382-391.
8. Hillemeier MM, Weisman CS, Chase GA, et al. Women’s preconceptional health and use of health services: implications for preconception care. Health Serv Res. 2008;43(1):54-75.
9. Chuang CH, Weisman CS, Hillemeier MM, et al. Pregnancy intention and health behaviors: results from the Central Pennsylvania Women’s Health Study Cohort. Matern Child Health J. 2010;14:501-510.
10. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/overview.html. Accessed July 17, 2014.
11. Heavey E. Don’t miss preconception care opportunities for adolescents. Am J Matern Child Nurs. 2010;35(4):213-219.
12. Hoover KW, Tao G, Berman S, Kent CK. Utilization of health services in physician offices and outpatient clinics by adolescents and young women in the United States: implications for improving access to reproductive health services. J Adolesc Health. 2010;46:324-330.
13. Johnson K, Posner SF, Biermann J. Recommendations to improve preconception health and health care—United States. MMWR Recomm Rep. 2006;55:1-23.
14. Krishnamoorthy U, Schram CM, Hill SR. Maternal obesity in pregnancy: is it time for meaningful research to inform preventive and management strategies? Brit J Obstet Gynecol. 2006;113:1134-1140.
15. Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56-59.
16. Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502-1505.
17. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437739.71477.ee.full.pdf+html. Accessed July 17, 2014.
18. Callaway LK, O’Callaghan MJ, McIntyre HD. Barriers to addressing overweight and obesity before conception. Med J Aust. 2009;191(8):425-427.
19. Donahue SMA, Zimmerman FJ, Starr JR, Holt VL. Correlates of pre-pregnancy physical inactivity: results from the pregnancy risk assessment monitoring system. Matern Child Health J. 2010;14:235-244.
20. Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122(1):64-71.
21. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Obesity in pregnancy. Obstet Gynecol. 2013;121(1):213-217.
22. Ciangura C, Corigliano N, Basdevant A, et al. Etonogestrel concentrations in morbidly obese women following Roux-en-Y gastric bypass surgery: three case reports. Contraception. 2011;84:649-651.
23. Wieck A, Rao S, Sein K, Haddad PM. A survey of antiepileptic prescribing to women of childbearing potential in psychiatry. Arch Womens Ment Health. 2007;10:83-85.
24. Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA study. J Womens Health. 2009;18(6):803-811.
25. Gardiner PM, Nelson L, Shellhaas CS, et al. The clinical content of preconception care: nutrition and dietary supplements. Am J Obstet Gynecol. 2008;S345-S353.
26. Burris HH, Mitchell AA, Werler MM. Periconceptional multivitamin use and infant birth weight disparities. Ann Epidemiol. 2010;20(3):233-240.
27. Taylor TN, Farkouh RA, Graham JB, et al. Potential reduction in neural tube defects associated with use of Metafolin-fortified oral contraceptives in the United States. Am J Obstet Gynecol. 2011:e1-e8.
28. Beyaz [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
29. Safyral [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
30. Hillemeier MM, Downs DS, Feinberg ME, et al. Improving women’s preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania Women’s Health Study. Womens Health Issues. 2008;18(6 suppl):S87-S96.
31. Coonrod DV, Bruce NC, Malcolm TD, et al. Knowledge and attitudes regarding preconception care in a predominantly low-income Mexican American population. Am J Obstet Gynecol. 2009;686:e1-e7.
32. Canady RB, Tiedje LB, Lauber C. Preconception care and pregnancy planning: voices of African American women. Matern Child Nurs. 2008;33(2):90-97.
33. Bellanca HK, Hunter MS. ONE KEY QUESTION: preventive reproductive health is part of high quality primary care. Contraception. 2013;88(1):3-6.
34. American Academy of Pediatrics. Preconception health care. www.healthychildren.org/English/ages-stages/prenatal/Pages/Reduce-the-Risk-of-Birth-Defects.aspx. Accessed July 17, 2014.
35. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. The importance of preconception care in the continuum of women’s health care. www.acog.org/Resources_And_Publi cations/Committee_Opinions/Committee_on_Gynecologic_Practice/The_Importance_of_Preconception_Care_in_the_Continuum_of_Wom ens_Health_Care. Accessed July 17, 2014.
36. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/hcp/. Accessed July 17, 2014.
1. National Center for Health Statistics. Table 7: Prenatal care for live births, by detailed race and Hispanic origin of mother: United States, selected years 1970–2004. In: Health, United States, 2006. Report No: 2006-1232. www.ncbi.nlm.nih.gov/books/NBK21003. Accessed July 17, 2014.
2. Office of Adolescent Health, US Department of Health and Human Services. Trends in teen pregnancy and childbearing. www.hhs.gov/ash/oah/adolescent-health-topics/reproductive-health/teen-pregnancy/trends.html#_ftn6. Accessed July 17, 2014.
3. Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services. Child Health USA 2013. http://mchb.hrsa.gov/chusa13. Accessed July 17, 2014.
4. World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. www.who.int/pmnch/media/news/2012/201204_borntoosoon_countryranking.pdf. Accessed July 17, 2014.
5. Organization for Economic Cooperation and Development (OECD). Low birth weight infants, 2009 and change 1970-2009 (or nearest year). In: Health at a Glance 2011: OECD indicators. www.oecd.org/els/health-systems/49105858.pdf. Accessed July 17, 2014.
6. CDC. Infant mortality rates and international rankings. www.cdc.gov/nchs/data/hus/2013/016.pdf. Accessed July 17, 2014.
7. Livingood WC, Brady C, Pierce K, et al. Impact of pre-conception health care: evaluation of a social determinants focused intervention. Matern Child Health J. 2010;14:382-391.
8. Hillemeier MM, Weisman CS, Chase GA, et al. Women’s preconceptional health and use of health services: implications for preconception care. Health Serv Res. 2008;43(1):54-75.
9. Chuang CH, Weisman CS, Hillemeier MM, et al. Pregnancy intention and health behaviors: results from the Central Pennsylvania Women’s Health Study Cohort. Matern Child Health J. 2010;14:501-510.
10. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/overview.html. Accessed July 17, 2014.
11. Heavey E. Don’t miss preconception care opportunities for adolescents. Am J Matern Child Nurs. 2010;35(4):213-219.
12. Hoover KW, Tao G, Berman S, Kent CK. Utilization of health services in physician offices and outpatient clinics by adolescents and young women in the United States: implications for improving access to reproductive health services. J Adolesc Health. 2010;46:324-330.
13. Johnson K, Posner SF, Biermann J. Recommendations to improve preconception health and health care—United States. MMWR Recomm Rep. 2006;55:1-23.
14. Krishnamoorthy U, Schram CM, Hill SR. Maternal obesity in pregnancy: is it time for meaningful research to inform preventive and management strategies? Brit J Obstet Gynecol. 2006;113:1134-1140.
15. Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56-59.
16. Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502-1505.
17. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437739.71477.ee.full.pdf+html. Accessed July 17, 2014.
18. Callaway LK, O’Callaghan MJ, McIntyre HD. Barriers to addressing overweight and obesity before conception. Med J Aust. 2009;191(8):425-427.
19. Donahue SMA, Zimmerman FJ, Starr JR, Holt VL. Correlates of pre-pregnancy physical inactivity: results from the pregnancy risk assessment monitoring system. Matern Child Health J. 2010;14:235-244.
20. Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122(1):64-71.
21. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Obesity in pregnancy. Obstet Gynecol. 2013;121(1):213-217.
22. Ciangura C, Corigliano N, Basdevant A, et al. Etonogestrel concentrations in morbidly obese women following Roux-en-Y gastric bypass surgery: three case reports. Contraception. 2011;84:649-651.
23. Wieck A, Rao S, Sein K, Haddad PM. A survey of antiepileptic prescribing to women of childbearing potential in psychiatry. Arch Womens Ment Health. 2007;10:83-85.
24. Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA study. J Womens Health. 2009;18(6):803-811.
25. Gardiner PM, Nelson L, Shellhaas CS, et al. The clinical content of preconception care: nutrition and dietary supplements. Am J Obstet Gynecol. 2008;S345-S353.
26. Burris HH, Mitchell AA, Werler MM. Periconceptional multivitamin use and infant birth weight disparities. Ann Epidemiol. 2010;20(3):233-240.
27. Taylor TN, Farkouh RA, Graham JB, et al. Potential reduction in neural tube defects associated with use of Metafolin-fortified oral contraceptives in the United States. Am J Obstet Gynecol. 2011:e1-e8.
28. Beyaz [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
29. Safyral [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
30. Hillemeier MM, Downs DS, Feinberg ME, et al. Improving women’s preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania Women’s Health Study. Womens Health Issues. 2008;18(6 suppl):S87-S96.
31. Coonrod DV, Bruce NC, Malcolm TD, et al. Knowledge and attitudes regarding preconception care in a predominantly low-income Mexican American population. Am J Obstet Gynecol. 2009;686:e1-e7.
32. Canady RB, Tiedje LB, Lauber C. Preconception care and pregnancy planning: voices of African American women. Matern Child Nurs. 2008;33(2):90-97.
33. Bellanca HK, Hunter MS. ONE KEY QUESTION: preventive reproductive health is part of high quality primary care. Contraception. 2013;88(1):3-6.
34. American Academy of Pediatrics. Preconception health care. www.healthychildren.org/English/ages-stages/prenatal/Pages/Reduce-the-Risk-of-Birth-Defects.aspx. Accessed July 17, 2014.
35. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. The importance of preconception care in the continuum of women’s health care. www.acog.org/Resources_And_Publi cations/Committee_Opinions/Committee_on_Gynecologic_Practice/The_Importance_of_Preconception_Care_in_the_Continuum_of_Wom ens_Health_Care. Accessed July 17, 2014.
36. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/hcp/. Accessed July 17, 2014.
Transfer to the hospital for women planning a home birth: A high-risk obstetrics problem
CASE: Febrile laboring mother transferred to hospital
You are in the hospital managing the induction of labor for one of your nulliparous patients who is postterm. You are hoping for a quiet and uneventful shift.
At midnight the nursing administrator pages you and asks if you would please provide care to a pregnant woman attempting a home birth who is in labor and is being transferred to your hospital.
The woman is a 41-year-old G2P1 with one prior cesarean delivery who has attempted a trial of labor at home. According to the nursing administrator the patient has a temperature of 100.4°F and the most recent cervical examination shows her to be fully dilated at +3/5 station in an occiput posterior position. She has been fully dilated for 5 hours. The fetal heart rate, assessed by Doppler monitor, is reported to be reassuring.
What is your clinical plan?
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics recommend that pregnant women should deliver at certified birth centers or hospital-based obstetric units to optimize clinical outcomes for newborns and mothers.1,2 Both organizations also recognize a woman’s right to exercise her autonomy and choose a planned home birth.
In 2012, approximately 0.8% of pregnant women in the United States planned a home birth (31,500 home births and 3,999,386 total births).3 In 2009, three states had home birth rates above 1.9%, including Montana (2.6%), Oregon (1.96%), and Vermont (1.91%). Five states had home birth rates above 1.5%, including Idaho, Pennsylvania, Utah, Washington, and Wisconsin.4 Because planned home births may require transport to the hospital to complete the birth, all obstetric units should develop written plans for dealing with these high-risk patients.
Hospital transfer is common for women attempting a home birth
Many home birth experts regard the Netherlands as the country with the best organized and most successful home birth system that is fully integrated with hospital-based obstetric care. Approximately 23% of births in Holland occur at home supervised by a midwife. A key feature of the highly regulated Dutch system is that all pregnant women with a high-risk condition are required to give birth in a hospital and cannot have a home delivery. Consequently, only women with a low-risk pregnancy are permitted to attempt a home birth.
By contrast, in the United States, with a less well-regulated home birth system, women with high-risk conditions, such as one or more prior cesarean deliveries, may try to birth at home. In a Dutch study of 168,618 low-risk women attempting a home birth, 32% (N=53,809) were transferred to the hospital. Most of the transfers occurred during labor.5
In England about 2.8% of births occur at home. In a study of 16,840 planned home births in England, 21% (N=3,530) of the women were transferred to the hospital.6 Of the 3,530 transfers to the hospital, 70% were transferred before delivery and 30% after birth. In this study among 4,568 nulliparous women attempting home birth, 45% were transferred to the hospital. Among 12,272 multiparous women attempting home birth, 12% were transferred to the hospital.
Among the nulliparous women, but not among the multiparous women, there was a significantly increased risk of adverse newborn outcomes. Adverse newborn outcome was a composite measure that included perinatal death, stillbirth after the onset of labor, neonatal encephalopathy, meconium aspiration syndrome, brachial plexus injury, or fractured humerus or clavicle. The risk of an adverse newborn outcome among nulliparous women was 0.9% for those delivering at home and 0.5% for those delivering at the hospital.6
Your patient asks you, “Are home births safe for the baby?”
No large-scale randomized trials have compared home birth versus hospital birth.1 Consequently, the best evidence evaluating the risks and benefits of home birth is based on observational studies of large cohorts. Recent studies from the United States have reported that neonatal complications, including the risk of a low Apgar score and neonatal seizures, is significantly increased with planned home birth compared with birth at a hospital.2,3 In one study, the risk of a 5-minute Apgar score of zero was 1 in 615 home births and 1 in 6,493 hospital births.3 Studies from the Netherlands also have reported that planned home birth is associated with increased perinatal mortality and morbidity.4,5
Because planned home birth is associated with an increased risk of neonatal morbidity and mortality, some experts conclude that obstetricians have an ethical obligation to recommend against home birth and to respond to refusal of that recommendation with respectful persuasion.6
References
1. Olsen O, Clausen JA. Planned hospital birth versus planned home birth. Cochrane Database Syst Rev. 2012; (9):CD000352.
2. Grunebaum A, McCullough LB, Sapra KJ, et al. Apgar score of 0 at 5 minutes and neonatal seizures or serious neurologic dysfunction in relation to birth setting. Am J Obstet Gynecol. 2013;209(4):323.e1–e6.
3. Cheng YW, Snowden JM, King TL, Caughey AB. Selected perinatal outcomes associated with planned home births in the United States. Am J Obstet Gynecol. 2013;209(4).325.e1–e8.
4. Evers AC, Brouwers HA, Hukkelhoven CW, et al. Perinatal mortality and severe morbidity in low and high risk term pregnancies in the Netherlands: Prospective cohort study. BMJ. 2010;341:c5639.
5. Arabin B, Visser GHA. Comparison of obstetric care in Germany and in the Netherlands. J Health Med Informat. 2013;S11:014.
6. Chervenak FA, McCullough LB, Arabin B. Obstetric ethics: An essential dimension of planned home birth. Obstet Gynecol. 2011;117(5):1183–1187.
Interprofessional team care
For the woman planning a home birth, transfer from home to the hospital is a jarring experience. The woman may feel that she has not achieved a highly desired and important life goal. In a survey of women birthing in the Netherlands, transfer from home to the hospital was associated with a high rate of patient dissatisfaction with their birthing experience. Compared with women who were satisfied with their birth experience, women who were dissatisfied more often reported that the care providers at the hospital were rushed, insensitive, rude, inconsiderate, condescending, and unhelpful.7
Creating a positive birthing experience
Given that transfer to the hospital is associated with an increased rate of being dissatisfied with the birth experience, and that dissatisfied women may perceive their care providers negatively, it is important for the interprofessional hospital team to devote adequate time to listen the patient’s concerns, demonstrate a high degree of sensitivity, and be especially polite and helpful. It is probably best to avoid referring to the transfer as a “failed home birth.” Trust may be enhanced by asking open-ended questions about the patient’s expectations and expressing empathy for her situation. The hospital professional team might prioritize acknowledging the right of the woman to make informed choices and provide an overview of the standard procedures used at the hospital. The clinicians should explicitly state that the health of the mother and newborn are their top priority. The hospital team should also express confidence in the benefit of the standard practices they use to ensure a safe birth experience.
Successful negotiation: An art best achieved as a small group
When a laboring woman is transferred from home to the hospital, a negotiation begins with the hospital professionals about the best clinical path to a successful birth. The patient often arrives with a support team that includes her partner, a support person, and a midwife or trained birth attendant. These individuals often demonstrate strong group cohesion and may be skeptical of the benefits of hospital birthing practices including intravenous access, oxytocin administration, epidural anesthesia, and operative delivery. The goal for the patient and her support team and the hospital professionals is to achieve a safe birth for the baby and mother. Because the goal is aligned among all parties, the negotiation is focused on the clinical path that will best achieve the goal with minimal risks.
To enhance the likelihood of a successful negotiation, it is best if the team of hospital professionals, including an obstetrician, a senior nurse, and an obstetric anesthesiologist, jointly discuss hospital birthing practices with the patient and her support team. An obstetrician, negotiating independently, is in the difficult position of one professional trying to redirect the choices of a cohesive team of four individuals. Most experienced negotiators would not voluntarily enter a situation in which acting alone they needed to simultaneously negotiate with four people. A joint discussion between the interprofessional team and the patient reduces the opportunity for the patient and her team to generate disagreements among the hospital professionals.
An important issue is that the home midwife or trained birth attendant is not permitted to participate in the practice of medicine at the hospital. Only credentialed and licensed nurses, obstetricians, anesthesiologists, and pediatricians are permitted to participate in the practice of medicine at the hospital. It may be prudent to provide the home midwife a written statement from the hospital indicating that home midwives are not permitted to practice medicine at the institution.
Related article: Lay midwives and the ObGyn: Is collaboration risky? Lucia DiVenere, MA (Practice Management; May 2012)
Occasionally, negotiations between the hospital professional team and the patient and her support team are unsuccessful and the patient refuses the best advice of the hospital team. In these situations there should be a written plan of how the patient–clinician conflict will be communicated to other members of the hospital staff and hospital leadership. For example, another senior clinician may be asked to join in the planning process.
A high-risk patient population
In some cases of planned home birth, the patient and midwife have made management decisions that are inconsistent with standard obstetric protocols. Commonly encountered situations include 1) conservative home management of spontaneous rupture of the membranes at term, 2) prolonged conservative management of the arrest of the active phase of the first stage of labor, 3) prolonged second stage of labor, up to 24 hours in length, and 4) attempted home birth after multiple previous cesarean deliveries. I am also aware of multiple reports of attempted home birth of a fetus in the breech presentation.
On arrival to the hospital these patients and their newborns are at exceptionally high risk for adverse birth outcomes. If an adverse outcome were to occur, it would be unjust to assign sole or primary responsibility to the obstetrician for the adverse outcome. Hence, the hospital should have a written plan for helping to minimize the risk that the obstetrician, playing the role of Good Samaritan, will bear primary responsibility for an adverse outcome.
Related article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
CASE: Resolved
In the case presented above, the obstetrician, nurse, and obstetric anesthesiologist successfully negotiated with the patient. Intravenous access and an epidural anesthetic were established. Antibiotics were administered. Using ultrasound, the obstetrician confirmed that the fetus was in the occiput posterior position. The mother was exhausted from many hours of pushing and agreed to an operative delivery. Forceps were used to deliver a healthy baby and a perineal laceration was repaired.
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
2. American Academy of Pediatrics. Policy statement: Planned home birth Pediatrics. 2013;131(5):1016–1002.
3. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Matthews TH. Births: Final data for 2010. Natl Vital Stat Rep. 2012;61(1):1–72.
4. MacDorman MF, Mathews TJ, Declercq E. Home births in the United States, 1990-2009. NCHS Data Brief. 2012;(84):1–8.
5. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: A descriptive study. BJOG. 2008;115(5):570–578.
6. Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for health women with low risk pregnancies: The Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400.
7. Rijnders M, Baston H, Schonbeck Y, et al. Perinatal factors related to negative or positive recall of birth experience in women 3 years postpartum in the Netherlands. Birth. 2008;35:107–116.
CASE: Febrile laboring mother transferred to hospital
You are in the hospital managing the induction of labor for one of your nulliparous patients who is postterm. You are hoping for a quiet and uneventful shift.
At midnight the nursing administrator pages you and asks if you would please provide care to a pregnant woman attempting a home birth who is in labor and is being transferred to your hospital.
The woman is a 41-year-old G2P1 with one prior cesarean delivery who has attempted a trial of labor at home. According to the nursing administrator the patient has a temperature of 100.4°F and the most recent cervical examination shows her to be fully dilated at +3/5 station in an occiput posterior position. She has been fully dilated for 5 hours. The fetal heart rate, assessed by Doppler monitor, is reported to be reassuring.
What is your clinical plan?
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics recommend that pregnant women should deliver at certified birth centers or hospital-based obstetric units to optimize clinical outcomes for newborns and mothers.1,2 Both organizations also recognize a woman’s right to exercise her autonomy and choose a planned home birth.
In 2012, approximately 0.8% of pregnant women in the United States planned a home birth (31,500 home births and 3,999,386 total births).3 In 2009, three states had home birth rates above 1.9%, including Montana (2.6%), Oregon (1.96%), and Vermont (1.91%). Five states had home birth rates above 1.5%, including Idaho, Pennsylvania, Utah, Washington, and Wisconsin.4 Because planned home births may require transport to the hospital to complete the birth, all obstetric units should develop written plans for dealing with these high-risk patients.
Hospital transfer is common for women attempting a home birth
Many home birth experts regard the Netherlands as the country with the best organized and most successful home birth system that is fully integrated with hospital-based obstetric care. Approximately 23% of births in Holland occur at home supervised by a midwife. A key feature of the highly regulated Dutch system is that all pregnant women with a high-risk condition are required to give birth in a hospital and cannot have a home delivery. Consequently, only women with a low-risk pregnancy are permitted to attempt a home birth.
By contrast, in the United States, with a less well-regulated home birth system, women with high-risk conditions, such as one or more prior cesarean deliveries, may try to birth at home. In a Dutch study of 168,618 low-risk women attempting a home birth, 32% (N=53,809) were transferred to the hospital. Most of the transfers occurred during labor.5
In England about 2.8% of births occur at home. In a study of 16,840 planned home births in England, 21% (N=3,530) of the women were transferred to the hospital.6 Of the 3,530 transfers to the hospital, 70% were transferred before delivery and 30% after birth. In this study among 4,568 nulliparous women attempting home birth, 45% were transferred to the hospital. Among 12,272 multiparous women attempting home birth, 12% were transferred to the hospital.
Among the nulliparous women, but not among the multiparous women, there was a significantly increased risk of adverse newborn outcomes. Adverse newborn outcome was a composite measure that included perinatal death, stillbirth after the onset of labor, neonatal encephalopathy, meconium aspiration syndrome, brachial plexus injury, or fractured humerus or clavicle. The risk of an adverse newborn outcome among nulliparous women was 0.9% for those delivering at home and 0.5% for those delivering at the hospital.6
Your patient asks you, “Are home births safe for the baby?”
No large-scale randomized trials have compared home birth versus hospital birth.1 Consequently, the best evidence evaluating the risks and benefits of home birth is based on observational studies of large cohorts. Recent studies from the United States have reported that neonatal complications, including the risk of a low Apgar score and neonatal seizures, is significantly increased with planned home birth compared with birth at a hospital.2,3 In one study, the risk of a 5-minute Apgar score of zero was 1 in 615 home births and 1 in 6,493 hospital births.3 Studies from the Netherlands also have reported that planned home birth is associated with increased perinatal mortality and morbidity.4,5
Because planned home birth is associated with an increased risk of neonatal morbidity and mortality, some experts conclude that obstetricians have an ethical obligation to recommend against home birth and to respond to refusal of that recommendation with respectful persuasion.6
References
1. Olsen O, Clausen JA. Planned hospital birth versus planned home birth. Cochrane Database Syst Rev. 2012; (9):CD000352.
2. Grunebaum A, McCullough LB, Sapra KJ, et al. Apgar score of 0 at 5 minutes and neonatal seizures or serious neurologic dysfunction in relation to birth setting. Am J Obstet Gynecol. 2013;209(4):323.e1–e6.
3. Cheng YW, Snowden JM, King TL, Caughey AB. Selected perinatal outcomes associated with planned home births in the United States. Am J Obstet Gynecol. 2013;209(4).325.e1–e8.
4. Evers AC, Brouwers HA, Hukkelhoven CW, et al. Perinatal mortality and severe morbidity in low and high risk term pregnancies in the Netherlands: Prospective cohort study. BMJ. 2010;341:c5639.
5. Arabin B, Visser GHA. Comparison of obstetric care in Germany and in the Netherlands. J Health Med Informat. 2013;S11:014.
6. Chervenak FA, McCullough LB, Arabin B. Obstetric ethics: An essential dimension of planned home birth. Obstet Gynecol. 2011;117(5):1183–1187.
Interprofessional team care
For the woman planning a home birth, transfer from home to the hospital is a jarring experience. The woman may feel that she has not achieved a highly desired and important life goal. In a survey of women birthing in the Netherlands, transfer from home to the hospital was associated with a high rate of patient dissatisfaction with their birthing experience. Compared with women who were satisfied with their birth experience, women who were dissatisfied more often reported that the care providers at the hospital were rushed, insensitive, rude, inconsiderate, condescending, and unhelpful.7
Creating a positive birthing experience
Given that transfer to the hospital is associated with an increased rate of being dissatisfied with the birth experience, and that dissatisfied women may perceive their care providers negatively, it is important for the interprofessional hospital team to devote adequate time to listen the patient’s concerns, demonstrate a high degree of sensitivity, and be especially polite and helpful. It is probably best to avoid referring to the transfer as a “failed home birth.” Trust may be enhanced by asking open-ended questions about the patient’s expectations and expressing empathy for her situation. The hospital professional team might prioritize acknowledging the right of the woman to make informed choices and provide an overview of the standard procedures used at the hospital. The clinicians should explicitly state that the health of the mother and newborn are their top priority. The hospital team should also express confidence in the benefit of the standard practices they use to ensure a safe birth experience.
Successful negotiation: An art best achieved as a small group
When a laboring woman is transferred from home to the hospital, a negotiation begins with the hospital professionals about the best clinical path to a successful birth. The patient often arrives with a support team that includes her partner, a support person, and a midwife or trained birth attendant. These individuals often demonstrate strong group cohesion and may be skeptical of the benefits of hospital birthing practices including intravenous access, oxytocin administration, epidural anesthesia, and operative delivery. The goal for the patient and her support team and the hospital professionals is to achieve a safe birth for the baby and mother. Because the goal is aligned among all parties, the negotiation is focused on the clinical path that will best achieve the goal with minimal risks.
To enhance the likelihood of a successful negotiation, it is best if the team of hospital professionals, including an obstetrician, a senior nurse, and an obstetric anesthesiologist, jointly discuss hospital birthing practices with the patient and her support team. An obstetrician, negotiating independently, is in the difficult position of one professional trying to redirect the choices of a cohesive team of four individuals. Most experienced negotiators would not voluntarily enter a situation in which acting alone they needed to simultaneously negotiate with four people. A joint discussion between the interprofessional team and the patient reduces the opportunity for the patient and her team to generate disagreements among the hospital professionals.
An important issue is that the home midwife or trained birth attendant is not permitted to participate in the practice of medicine at the hospital. Only credentialed and licensed nurses, obstetricians, anesthesiologists, and pediatricians are permitted to participate in the practice of medicine at the hospital. It may be prudent to provide the home midwife a written statement from the hospital indicating that home midwives are not permitted to practice medicine at the institution.
Related article: Lay midwives and the ObGyn: Is collaboration risky? Lucia DiVenere, MA (Practice Management; May 2012)
Occasionally, negotiations between the hospital professional team and the patient and her support team are unsuccessful and the patient refuses the best advice of the hospital team. In these situations there should be a written plan of how the patient–clinician conflict will be communicated to other members of the hospital staff and hospital leadership. For example, another senior clinician may be asked to join in the planning process.
A high-risk patient population
In some cases of planned home birth, the patient and midwife have made management decisions that are inconsistent with standard obstetric protocols. Commonly encountered situations include 1) conservative home management of spontaneous rupture of the membranes at term, 2) prolonged conservative management of the arrest of the active phase of the first stage of labor, 3) prolonged second stage of labor, up to 24 hours in length, and 4) attempted home birth after multiple previous cesarean deliveries. I am also aware of multiple reports of attempted home birth of a fetus in the breech presentation.
On arrival to the hospital these patients and their newborns are at exceptionally high risk for adverse birth outcomes. If an adverse outcome were to occur, it would be unjust to assign sole or primary responsibility to the obstetrician for the adverse outcome. Hence, the hospital should have a written plan for helping to minimize the risk that the obstetrician, playing the role of Good Samaritan, will bear primary responsibility for an adverse outcome.
Related article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
CASE: Resolved
In the case presented above, the obstetrician, nurse, and obstetric anesthesiologist successfully negotiated with the patient. Intravenous access and an epidural anesthetic were established. Antibiotics were administered. Using ultrasound, the obstetrician confirmed that the fetus was in the occiput posterior position. The mother was exhausted from many hours of pushing and agreed to an operative delivery. Forceps were used to deliver a healthy baby and a perineal laceration was repaired.
CASE: Febrile laboring mother transferred to hospital
You are in the hospital managing the induction of labor for one of your nulliparous patients who is postterm. You are hoping for a quiet and uneventful shift.
At midnight the nursing administrator pages you and asks if you would please provide care to a pregnant woman attempting a home birth who is in labor and is being transferred to your hospital.
The woman is a 41-year-old G2P1 with one prior cesarean delivery who has attempted a trial of labor at home. According to the nursing administrator the patient has a temperature of 100.4°F and the most recent cervical examination shows her to be fully dilated at +3/5 station in an occiput posterior position. She has been fully dilated for 5 hours. The fetal heart rate, assessed by Doppler monitor, is reported to be reassuring.
What is your clinical plan?
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics recommend that pregnant women should deliver at certified birth centers or hospital-based obstetric units to optimize clinical outcomes for newborns and mothers.1,2 Both organizations also recognize a woman’s right to exercise her autonomy and choose a planned home birth.
In 2012, approximately 0.8% of pregnant women in the United States planned a home birth (31,500 home births and 3,999,386 total births).3 In 2009, three states had home birth rates above 1.9%, including Montana (2.6%), Oregon (1.96%), and Vermont (1.91%). Five states had home birth rates above 1.5%, including Idaho, Pennsylvania, Utah, Washington, and Wisconsin.4 Because planned home births may require transport to the hospital to complete the birth, all obstetric units should develop written plans for dealing with these high-risk patients.
Hospital transfer is common for women attempting a home birth
Many home birth experts regard the Netherlands as the country with the best organized and most successful home birth system that is fully integrated with hospital-based obstetric care. Approximately 23% of births in Holland occur at home supervised by a midwife. A key feature of the highly regulated Dutch system is that all pregnant women with a high-risk condition are required to give birth in a hospital and cannot have a home delivery. Consequently, only women with a low-risk pregnancy are permitted to attempt a home birth.
By contrast, in the United States, with a less well-regulated home birth system, women with high-risk conditions, such as one or more prior cesarean deliveries, may try to birth at home. In a Dutch study of 168,618 low-risk women attempting a home birth, 32% (N=53,809) were transferred to the hospital. Most of the transfers occurred during labor.5
In England about 2.8% of births occur at home. In a study of 16,840 planned home births in England, 21% (N=3,530) of the women were transferred to the hospital.6 Of the 3,530 transfers to the hospital, 70% were transferred before delivery and 30% after birth. In this study among 4,568 nulliparous women attempting home birth, 45% were transferred to the hospital. Among 12,272 multiparous women attempting home birth, 12% were transferred to the hospital.
Among the nulliparous women, but not among the multiparous women, there was a significantly increased risk of adverse newborn outcomes. Adverse newborn outcome was a composite measure that included perinatal death, stillbirth after the onset of labor, neonatal encephalopathy, meconium aspiration syndrome, brachial plexus injury, or fractured humerus or clavicle. The risk of an adverse newborn outcome among nulliparous women was 0.9% for those delivering at home and 0.5% for those delivering at the hospital.6
Your patient asks you, “Are home births safe for the baby?”
No large-scale randomized trials have compared home birth versus hospital birth.1 Consequently, the best evidence evaluating the risks and benefits of home birth is based on observational studies of large cohorts. Recent studies from the United States have reported that neonatal complications, including the risk of a low Apgar score and neonatal seizures, is significantly increased with planned home birth compared with birth at a hospital.2,3 In one study, the risk of a 5-minute Apgar score of zero was 1 in 615 home births and 1 in 6,493 hospital births.3 Studies from the Netherlands also have reported that planned home birth is associated with increased perinatal mortality and morbidity.4,5
Because planned home birth is associated with an increased risk of neonatal morbidity and mortality, some experts conclude that obstetricians have an ethical obligation to recommend against home birth and to respond to refusal of that recommendation with respectful persuasion.6
References
1. Olsen O, Clausen JA. Planned hospital birth versus planned home birth. Cochrane Database Syst Rev. 2012; (9):CD000352.
2. Grunebaum A, McCullough LB, Sapra KJ, et al. Apgar score of 0 at 5 minutes and neonatal seizures or serious neurologic dysfunction in relation to birth setting. Am J Obstet Gynecol. 2013;209(4):323.e1–e6.
3. Cheng YW, Snowden JM, King TL, Caughey AB. Selected perinatal outcomes associated with planned home births in the United States. Am J Obstet Gynecol. 2013;209(4).325.e1–e8.
4. Evers AC, Brouwers HA, Hukkelhoven CW, et al. Perinatal mortality and severe morbidity in low and high risk term pregnancies in the Netherlands: Prospective cohort study. BMJ. 2010;341:c5639.
5. Arabin B, Visser GHA. Comparison of obstetric care in Germany and in the Netherlands. J Health Med Informat. 2013;S11:014.
6. Chervenak FA, McCullough LB, Arabin B. Obstetric ethics: An essential dimension of planned home birth. Obstet Gynecol. 2011;117(5):1183–1187.
Interprofessional team care
For the woman planning a home birth, transfer from home to the hospital is a jarring experience. The woman may feel that she has not achieved a highly desired and important life goal. In a survey of women birthing in the Netherlands, transfer from home to the hospital was associated with a high rate of patient dissatisfaction with their birthing experience. Compared with women who were satisfied with their birth experience, women who were dissatisfied more often reported that the care providers at the hospital were rushed, insensitive, rude, inconsiderate, condescending, and unhelpful.7
Creating a positive birthing experience
Given that transfer to the hospital is associated with an increased rate of being dissatisfied with the birth experience, and that dissatisfied women may perceive their care providers negatively, it is important for the interprofessional hospital team to devote adequate time to listen the patient’s concerns, demonstrate a high degree of sensitivity, and be especially polite and helpful. It is probably best to avoid referring to the transfer as a “failed home birth.” Trust may be enhanced by asking open-ended questions about the patient’s expectations and expressing empathy for her situation. The hospital professional team might prioritize acknowledging the right of the woman to make informed choices and provide an overview of the standard procedures used at the hospital. The clinicians should explicitly state that the health of the mother and newborn are their top priority. The hospital team should also express confidence in the benefit of the standard practices they use to ensure a safe birth experience.
Successful negotiation: An art best achieved as a small group
When a laboring woman is transferred from home to the hospital, a negotiation begins with the hospital professionals about the best clinical path to a successful birth. The patient often arrives with a support team that includes her partner, a support person, and a midwife or trained birth attendant. These individuals often demonstrate strong group cohesion and may be skeptical of the benefits of hospital birthing practices including intravenous access, oxytocin administration, epidural anesthesia, and operative delivery. The goal for the patient and her support team and the hospital professionals is to achieve a safe birth for the baby and mother. Because the goal is aligned among all parties, the negotiation is focused on the clinical path that will best achieve the goal with minimal risks.
To enhance the likelihood of a successful negotiation, it is best if the team of hospital professionals, including an obstetrician, a senior nurse, and an obstetric anesthesiologist, jointly discuss hospital birthing practices with the patient and her support team. An obstetrician, negotiating independently, is in the difficult position of one professional trying to redirect the choices of a cohesive team of four individuals. Most experienced negotiators would not voluntarily enter a situation in which acting alone they needed to simultaneously negotiate with four people. A joint discussion between the interprofessional team and the patient reduces the opportunity for the patient and her team to generate disagreements among the hospital professionals.
An important issue is that the home midwife or trained birth attendant is not permitted to participate in the practice of medicine at the hospital. Only credentialed and licensed nurses, obstetricians, anesthesiologists, and pediatricians are permitted to participate in the practice of medicine at the hospital. It may be prudent to provide the home midwife a written statement from the hospital indicating that home midwives are not permitted to practice medicine at the institution.
Related article: Lay midwives and the ObGyn: Is collaboration risky? Lucia DiVenere, MA (Practice Management; May 2012)
Occasionally, negotiations between the hospital professional team and the patient and her support team are unsuccessful and the patient refuses the best advice of the hospital team. In these situations there should be a written plan of how the patient–clinician conflict will be communicated to other members of the hospital staff and hospital leadership. For example, another senior clinician may be asked to join in the planning process.
A high-risk patient population
In some cases of planned home birth, the patient and midwife have made management decisions that are inconsistent with standard obstetric protocols. Commonly encountered situations include 1) conservative home management of spontaneous rupture of the membranes at term, 2) prolonged conservative management of the arrest of the active phase of the first stage of labor, 3) prolonged second stage of labor, up to 24 hours in length, and 4) attempted home birth after multiple previous cesarean deliveries. I am also aware of multiple reports of attempted home birth of a fetus in the breech presentation.
On arrival to the hospital these patients and their newborns are at exceptionally high risk for adverse birth outcomes. If an adverse outcome were to occur, it would be unjust to assign sole or primary responsibility to the obstetrician for the adverse outcome. Hence, the hospital should have a written plan for helping to minimize the risk that the obstetrician, playing the role of Good Samaritan, will bear primary responsibility for an adverse outcome.
Related article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
CASE: Resolved
In the case presented above, the obstetrician, nurse, and obstetric anesthesiologist successfully negotiated with the patient. Intravenous access and an epidural anesthetic were established. Antibiotics were administered. Using ultrasound, the obstetrician confirmed that the fetus was in the occiput posterior position. The mother was exhausted from many hours of pushing and agreed to an operative delivery. Forceps were used to deliver a healthy baby and a perineal laceration was repaired.
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
2. American Academy of Pediatrics. Policy statement: Planned home birth Pediatrics. 2013;131(5):1016–1002.
3. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Matthews TH. Births: Final data for 2010. Natl Vital Stat Rep. 2012;61(1):1–72.
4. MacDorman MF, Mathews TJ, Declercq E. Home births in the United States, 1990-2009. NCHS Data Brief. 2012;(84):1–8.
5. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: A descriptive study. BJOG. 2008;115(5):570–578.
6. Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for health women with low risk pregnancies: The Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400.
7. Rijnders M, Baston H, Schonbeck Y, et al. Perinatal factors related to negative or positive recall of birth experience in women 3 years postpartum in the Netherlands. Birth. 2008;35:107–116.
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
2. American Academy of Pediatrics. Policy statement: Planned home birth Pediatrics. 2013;131(5):1016–1002.
3. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Matthews TH. Births: Final data for 2010. Natl Vital Stat Rep. 2012;61(1):1–72.
4. MacDorman MF, Mathews TJ, Declercq E. Home births in the United States, 1990-2009. NCHS Data Brief. 2012;(84):1–8.
5. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: A descriptive study. BJOG. 2008;115(5):570–578.
6. Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for health women with low risk pregnancies: The Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400.
7. Rijnders M, Baston H, Schonbeck Y, et al. Perinatal factors related to negative or positive recall of birth experience in women 3 years postpartum in the Netherlands. Birth. 2008;35:107–116.
Prenatal exposure to alcohol can result in persistent difficulties with math
BELLEVUE, WASH. – Many young adults who were exposed to alcohol prenatally have persistent difficulties with math, according to study findings reported at the annual meeting of the Teratology Society.
A team from Emory University in Atlanta performed long-term follow-up with repeated testing of a cohort of infants who were exposed to alcohol when their mothers drank during pregnancy. At the most recent assessment, they had a median age of 23 years; most were African American and of low socioeconomic status.
The study results showed that the young adults who had physical or cognitive effects from their prenatal alcohol exposure had summary math scores that were 10%-11% lower than those in unexposed peers and 3%-4% lower than those in peers who had had childhood disabilities requiring special education.
Detailed testing showed that these affected young adults had difficulty with even simple math skills, such as counting dots and identifying which of two numbers is bigger.
Alcohol-related math dysfunction, including deficits in math achievement as well as in the simple elements that support math functioning, "which one would expect an adult would be quite competent at," persists into adulthood, commented first author Claire D. Coles, Ph.D., professor of psychiatry and behavioral sciences and pediatrics and director of the maternal substance abuse and child development project at Emory.
"Even when compared with other individuals with similar demographic characteristics who have developmental deficits, adults who are alcohol affected demonstrate specific math deficits," she added.
"I believe that alcohol may target neuropsychological functions, like working memory and full-scale IQ, that support math achievement. But I believe there are some other factors involved as well," Dr. Coles said.
Session attendee Dr. Kenneth L. Jones of the University of California, San Diego, asked, "Is this specific enough that you think it could be used as a screen in 5- or 6-year-old children?"
"I think that there is a specific math deficit in kids, yes, and it’s persistent," Dr. Coles replied. "Obviously, there is not a math part of our brain. But I think that whatever it is that alcohol does affects the functions that underlie the ability to do mathematics so that you are going to pick it up regularly in people who are affected."
Session attendee Rajesh C. Miranda, Ph.D., of the Texas A&M Health Science Center in Bryan, asked, "Is it possible to overtrain a person affected by fetal alcohol syndrome to overcome the deficits?"
"We have actually done an intervention program called MILE, which is the Math Interactive Learning Experience, in which we worked with the family and child specifically on remediating these issues. And it seems to be quite effective," Dr. Coles replied. "I’m not saying it magically fixes everything, but it does help with developing some of these underlying factors and improving outcomes."
In the study, the investigators split the alcohol-exposed young adults into three groups: 48 who had physical effects from exposure, 37 who had only cognitive effects from exposure, and 38 who were clinically unaffected by their exposure.
They were compared with each other and with two control groups: 59 unexposed adults matched for socioeconomic status and 54 adults who had had disabilities as children requiring special education.
Scores on a test for math achievement, the Woodcock-Johnson, 3rd edition (WJ-III), showed a significant difference (P less than .01) across the five groups with respect to the broad math (summary) score and with respect to scores for the individual subtests (math calculation, math reasoning, math fluency, applied problems, and quantitative concepts), reported Dr. Coles.
There was a general pattern whereby scores were lower in the young adults prenatally exposed to alcohol who had physical or cognitive effects, compared with all of the other groups.
In a regression model attempting to sort out the factors explaining math performance, significant contributors (P less than .01) included study group, dysmorphic features, working memory, and full-scale IQ.
In addition, scores on the EC301 test of math skills, a standardized test for brain-damaged adults, showed that the group with physical effects of alcohol exposure performed more poorly than all other groups with respect to counting and precise number knowledge. And the groups with physical and cognitive effects performed more poorly than all of the other groups with respect to number comparisons, mental calculations, estimating the results of arithmetic operations, and other measures.
Dr. Coles disclosed no relevant financial conflicts.
BELLEVUE, WASH. – Many young adults who were exposed to alcohol prenatally have persistent difficulties with math, according to study findings reported at the annual meeting of the Teratology Society.
A team from Emory University in Atlanta performed long-term follow-up with repeated testing of a cohort of infants who were exposed to alcohol when their mothers drank during pregnancy. At the most recent assessment, they had a median age of 23 years; most were African American and of low socioeconomic status.
The study results showed that the young adults who had physical or cognitive effects from their prenatal alcohol exposure had summary math scores that were 10%-11% lower than those in unexposed peers and 3%-4% lower than those in peers who had had childhood disabilities requiring special education.
Detailed testing showed that these affected young adults had difficulty with even simple math skills, such as counting dots and identifying which of two numbers is bigger.
Alcohol-related math dysfunction, including deficits in math achievement as well as in the simple elements that support math functioning, "which one would expect an adult would be quite competent at," persists into adulthood, commented first author Claire D. Coles, Ph.D., professor of psychiatry and behavioral sciences and pediatrics and director of the maternal substance abuse and child development project at Emory.
"Even when compared with other individuals with similar demographic characteristics who have developmental deficits, adults who are alcohol affected demonstrate specific math deficits," she added.
"I believe that alcohol may target neuropsychological functions, like working memory and full-scale IQ, that support math achievement. But I believe there are some other factors involved as well," Dr. Coles said.
Session attendee Dr. Kenneth L. Jones of the University of California, San Diego, asked, "Is this specific enough that you think it could be used as a screen in 5- or 6-year-old children?"
"I think that there is a specific math deficit in kids, yes, and it’s persistent," Dr. Coles replied. "Obviously, there is not a math part of our brain. But I think that whatever it is that alcohol does affects the functions that underlie the ability to do mathematics so that you are going to pick it up regularly in people who are affected."
Session attendee Rajesh C. Miranda, Ph.D., of the Texas A&M Health Science Center in Bryan, asked, "Is it possible to overtrain a person affected by fetal alcohol syndrome to overcome the deficits?"
"We have actually done an intervention program called MILE, which is the Math Interactive Learning Experience, in which we worked with the family and child specifically on remediating these issues. And it seems to be quite effective," Dr. Coles replied. "I’m not saying it magically fixes everything, but it does help with developing some of these underlying factors and improving outcomes."
In the study, the investigators split the alcohol-exposed young adults into three groups: 48 who had physical effects from exposure, 37 who had only cognitive effects from exposure, and 38 who were clinically unaffected by their exposure.
They were compared with each other and with two control groups: 59 unexposed adults matched for socioeconomic status and 54 adults who had had disabilities as children requiring special education.
Scores on a test for math achievement, the Woodcock-Johnson, 3rd edition (WJ-III), showed a significant difference (P less than .01) across the five groups with respect to the broad math (summary) score and with respect to scores for the individual subtests (math calculation, math reasoning, math fluency, applied problems, and quantitative concepts), reported Dr. Coles.
There was a general pattern whereby scores were lower in the young adults prenatally exposed to alcohol who had physical or cognitive effects, compared with all of the other groups.
In a regression model attempting to sort out the factors explaining math performance, significant contributors (P less than .01) included study group, dysmorphic features, working memory, and full-scale IQ.
In addition, scores on the EC301 test of math skills, a standardized test for brain-damaged adults, showed that the group with physical effects of alcohol exposure performed more poorly than all other groups with respect to counting and precise number knowledge. And the groups with physical and cognitive effects performed more poorly than all of the other groups with respect to number comparisons, mental calculations, estimating the results of arithmetic operations, and other measures.
Dr. Coles disclosed no relevant financial conflicts.
BELLEVUE, WASH. – Many young adults who were exposed to alcohol prenatally have persistent difficulties with math, according to study findings reported at the annual meeting of the Teratology Society.
A team from Emory University in Atlanta performed long-term follow-up with repeated testing of a cohort of infants who were exposed to alcohol when their mothers drank during pregnancy. At the most recent assessment, they had a median age of 23 years; most were African American and of low socioeconomic status.
The study results showed that the young adults who had physical or cognitive effects from their prenatal alcohol exposure had summary math scores that were 10%-11% lower than those in unexposed peers and 3%-4% lower than those in peers who had had childhood disabilities requiring special education.
Detailed testing showed that these affected young adults had difficulty with even simple math skills, such as counting dots and identifying which of two numbers is bigger.
Alcohol-related math dysfunction, including deficits in math achievement as well as in the simple elements that support math functioning, "which one would expect an adult would be quite competent at," persists into adulthood, commented first author Claire D. Coles, Ph.D., professor of psychiatry and behavioral sciences and pediatrics and director of the maternal substance abuse and child development project at Emory.
"Even when compared with other individuals with similar demographic characteristics who have developmental deficits, adults who are alcohol affected demonstrate specific math deficits," she added.
"I believe that alcohol may target neuropsychological functions, like working memory and full-scale IQ, that support math achievement. But I believe there are some other factors involved as well," Dr. Coles said.
Session attendee Dr. Kenneth L. Jones of the University of California, San Diego, asked, "Is this specific enough that you think it could be used as a screen in 5- or 6-year-old children?"
"I think that there is a specific math deficit in kids, yes, and it’s persistent," Dr. Coles replied. "Obviously, there is not a math part of our brain. But I think that whatever it is that alcohol does affects the functions that underlie the ability to do mathematics so that you are going to pick it up regularly in people who are affected."
Session attendee Rajesh C. Miranda, Ph.D., of the Texas A&M Health Science Center in Bryan, asked, "Is it possible to overtrain a person affected by fetal alcohol syndrome to overcome the deficits?"
"We have actually done an intervention program called MILE, which is the Math Interactive Learning Experience, in which we worked with the family and child specifically on remediating these issues. And it seems to be quite effective," Dr. Coles replied. "I’m not saying it magically fixes everything, but it does help with developing some of these underlying factors and improving outcomes."
In the study, the investigators split the alcohol-exposed young adults into three groups: 48 who had physical effects from exposure, 37 who had only cognitive effects from exposure, and 38 who were clinically unaffected by their exposure.
They were compared with each other and with two control groups: 59 unexposed adults matched for socioeconomic status and 54 adults who had had disabilities as children requiring special education.
Scores on a test for math achievement, the Woodcock-Johnson, 3rd edition (WJ-III), showed a significant difference (P less than .01) across the five groups with respect to the broad math (summary) score and with respect to scores for the individual subtests (math calculation, math reasoning, math fluency, applied problems, and quantitative concepts), reported Dr. Coles.
There was a general pattern whereby scores were lower in the young adults prenatally exposed to alcohol who had physical or cognitive effects, compared with all of the other groups.
In a regression model attempting to sort out the factors explaining math performance, significant contributors (P less than .01) included study group, dysmorphic features, working memory, and full-scale IQ.
In addition, scores on the EC301 test of math skills, a standardized test for brain-damaged adults, showed that the group with physical effects of alcohol exposure performed more poorly than all other groups with respect to counting and precise number knowledge. And the groups with physical and cognitive effects performed more poorly than all of the other groups with respect to number comparisons, mental calculations, estimating the results of arithmetic operations, and other measures.
Dr. Coles disclosed no relevant financial conflicts.
AT TERATOLOGY SOCIETY 2014
Key clinical finding: Prenatal alcohol exposure can have lingering effects, even into young adulthood, in terms of math scores.
Major finding: Young adults who had had physical or cognitive effects from prenatal alcohol exposure had summary math scores that were 10%-11% lower than those in unexposed peers and 3%-4% lower than those in peers who had had childhood disabilities.
Data source: A cohort study of 123 adults prenatally exposed to alcohol, 59 unexposed adults, and 54 adults who had had childhood disabilities.
Disclosures: Dr. Coles disclosed no relevant conflicts of interest.
Calcium and Vitamin D Improve Metabolic Profile in Gestational Diabetes
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
Calcium and vitamin D can improve the metabolic profile of women with gestational diabetes mellitus, according to investigators in Iran who found significant reductions in fasting glucose, serum insulin levels, and low-density lipoprotein cholesterol associated with combined use of the supplements.
For their research, published June 23 in Diabetologia (doi:10.1007/s00125-014-3293-x), Zatollah Asemi, Ph.D., of Kashan (Iran) University and colleagues, randomized 56 women diagnosed with gestational diabetes mellitus (GDM) at between 24 and 28 weeks’ gestation to 1,000 mg calcium daily and to a 50,000 IU pearl of vitamin D3 at baseline and day 21 or placebo. Women and providers were blinded to treatment assignment.
At 6 weeks, compared with placebo, women assigned to the calcium-vitamin D group (n = 28) saw significantly lower fasting glucose (-0.89 ± 0.69 vs. +0.26 ± 0.92 mmol/L), serum insulin levels (-13.55 ± 35.25 vs. +9.17 ± 38.50 pmol/L) and homeostatic model assessment insulin resistance (-0.91 ± 1.18 vs + 0.63 ± 2.01).
They also saw a significant reduction in LDL cholesterol (-0.23 ± 0.79 vs. +0.26 ± 0.74 mmol/L). The treatment group saw increases in plasma glutathione, and supplementation was seen to prevent a rise in plasma malondialdehyde levels.
C-reactive protein and total antioxidant capacity were not affected by calcium and Vitamin D supplementation in this study, researchers wrote.
Dr. Asemi and colleagues, who have previously studied vitamin D supplementation in women with GDM and found it associated with improved insulin function and decreased total and LDL cholesterol (Am. J. Clin. Nutr. 2013;98:1425-32), hypothesized that calcium and vitamin D together would be more efficient in influencing metabolic profiles, possibly through their combined effects on cell cycle regulation, activation of antioxidant enzymes, and suppression of parathyroid hormone.
"GDM is associated with insulin resistance, increased inflammatory factors, and oxidative stress. Elevated circulating levels of inflammatory markers and impaired insulin metabolism in GDM can predict the progression to type 2 diabetes later in life and neonatal complications," investigators explained.
The researchers noted as weaknesses of their study that it did not capture pregnancy outcomes or certain biomarkers of inflammation and oxidative stress and that two subjects in the placebo group and three in the treatment group were lost to follow-up. The study was funded by Kashan University. None of the researchers disclosed conflicts of interest.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
Calcium and vitamin D can improve the metabolic profile of women with gestational diabetes mellitus, according to investigators in Iran who found significant reductions in fasting glucose, serum insulin levels, and low-density lipoprotein cholesterol associated with combined use of the supplements.
For their research, published June 23 in Diabetologia (doi:10.1007/s00125-014-3293-x), Zatollah Asemi, Ph.D., of Kashan (Iran) University and colleagues, randomized 56 women diagnosed with gestational diabetes mellitus (GDM) at between 24 and 28 weeks’ gestation to 1,000 mg calcium daily and to a 50,000 IU pearl of vitamin D3 at baseline and day 21 or placebo. Women and providers were blinded to treatment assignment.
At 6 weeks, compared with placebo, women assigned to the calcium-vitamin D group (n = 28) saw significantly lower fasting glucose (-0.89 ± 0.69 vs. +0.26 ± 0.92 mmol/L), serum insulin levels (-13.55 ± 35.25 vs. +9.17 ± 38.50 pmol/L) and homeostatic model assessment insulin resistance (-0.91 ± 1.18 vs + 0.63 ± 2.01).
They also saw a significant reduction in LDL cholesterol (-0.23 ± 0.79 vs. +0.26 ± 0.74 mmol/L). The treatment group saw increases in plasma glutathione, and supplementation was seen to prevent a rise in plasma malondialdehyde levels.
C-reactive protein and total antioxidant capacity were not affected by calcium and Vitamin D supplementation in this study, researchers wrote.
Dr. Asemi and colleagues, who have previously studied vitamin D supplementation in women with GDM and found it associated with improved insulin function and decreased total and LDL cholesterol (Am. J. Clin. Nutr. 2013;98:1425-32), hypothesized that calcium and vitamin D together would be more efficient in influencing metabolic profiles, possibly through their combined effects on cell cycle regulation, activation of antioxidant enzymes, and suppression of parathyroid hormone.
"GDM is associated with insulin resistance, increased inflammatory factors, and oxidative stress. Elevated circulating levels of inflammatory markers and impaired insulin metabolism in GDM can predict the progression to type 2 diabetes later in life and neonatal complications," investigators explained.
The researchers noted as weaknesses of their study that it did not capture pregnancy outcomes or certain biomarkers of inflammation and oxidative stress and that two subjects in the placebo group and three in the treatment group were lost to follow-up. The study was funded by Kashan University. None of the researchers disclosed conflicts of interest.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
Calcium and vitamin D can improve the metabolic profile of women with gestational diabetes mellitus, according to investigators in Iran who found significant reductions in fasting glucose, serum insulin levels, and low-density lipoprotein cholesterol associated with combined use of the supplements.
For their research, published June 23 in Diabetologia (doi:10.1007/s00125-014-3293-x), Zatollah Asemi, Ph.D., of Kashan (Iran) University and colleagues, randomized 56 women diagnosed with gestational diabetes mellitus (GDM) at between 24 and 28 weeks’ gestation to 1,000 mg calcium daily and to a 50,000 IU pearl of vitamin D3 at baseline and day 21 or placebo. Women and providers were blinded to treatment assignment.
At 6 weeks, compared with placebo, women assigned to the calcium-vitamin D group (n = 28) saw significantly lower fasting glucose (-0.89 ± 0.69 vs. +0.26 ± 0.92 mmol/L), serum insulin levels (-13.55 ± 35.25 vs. +9.17 ± 38.50 pmol/L) and homeostatic model assessment insulin resistance (-0.91 ± 1.18 vs + 0.63 ± 2.01).
They also saw a significant reduction in LDL cholesterol (-0.23 ± 0.79 vs. +0.26 ± 0.74 mmol/L). The treatment group saw increases in plasma glutathione, and supplementation was seen to prevent a rise in plasma malondialdehyde levels.
C-reactive protein and total antioxidant capacity were not affected by calcium and Vitamin D supplementation in this study, researchers wrote.
Dr. Asemi and colleagues, who have previously studied vitamin D supplementation in women with GDM and found it associated with improved insulin function and decreased total and LDL cholesterol (Am. J. Clin. Nutr. 2013;98:1425-32), hypothesized that calcium and vitamin D together would be more efficient in influencing metabolic profiles, possibly through their combined effects on cell cycle regulation, activation of antioxidant enzymes, and suppression of parathyroid hormone.
"GDM is associated with insulin resistance, increased inflammatory factors, and oxidative stress. Elevated circulating levels of inflammatory markers and impaired insulin metabolism in GDM can predict the progression to type 2 diabetes later in life and neonatal complications," investigators explained.
The researchers noted as weaknesses of their study that it did not capture pregnancy outcomes or certain biomarkers of inflammation and oxidative stress and that two subjects in the placebo group and three in the treatment group were lost to follow-up. The study was funded by Kashan University. None of the researchers disclosed conflicts of interest.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
FROM DIABETOLOGIA
Shoulder dystocia malpractice claims becoming more common
As ob.gyns. face a growing number of shoulder dystocia cases, also escalating are medical malpractice lawsuits that claim physicians are to blame for related injuries.
"Because there is an increasing incidence of obesity in the United States, as well as women entering pregnancy at high risk or already having diabetes, the risks of dystocia are rising," said Dr. Robert H. Debbs, director of the Pennsylvania Hospital Maternal Fetal Medicine, Philadelphia. "The trends appear to be suits now being filed not for failing to perform C [cesarean] sections, but for how the dystocia was handled; i.e., was it anticipated? Were the proper maneuvers done? Is there evidence of excessive force used? And was the ‘team’ responsive to the situation?"
Adding to legal risks is the difficulty of predicting shoulder dystocia, noted Dr. Debbs. A 2012 study in Archives of Gynecology and Obstetrics that analyzed 234 shoulder dystocia cases found only mode of delivery and birth weight were independent risk factors of the condition. The U.K. researchers concluded that exact birth weight and delivery mode is difficult to foretell, and therefore, occurrence of shoulder dystocia is highly unpredictable (Arch. Gynecol. Obstet. 2012;285:291-5).
"It’s clear that we are neither able to predict with accuracy, nor prevent dystocia accurately in that most cases occur in normal-weight babies, and only 25% of patients have risk factors," said Dr. Debbs, who recently spoke on the subject at the American Conference Institute obstetric claims forum in Philadelphia. "Performing elective cesarean sections for suspected large babies has not reduced significantly the number of dystocias, and we would have to perform close to 300 cesarean-sections to prevent one permanent nerve injury to a child, a number that places women at risk of future pregnancy complications in addition to morbidity and even mortality from surgery," according to Dr. Debbs.
Despite the literature, many plaintiffs’ attorneys argue the mere presence of a brachial plexus injury in a newborn indicates the physician pulled too hard or in the wrong direction, said Dr. Michael G. Ross, chair of the obstetrics and gynecology department for Harbor-UCLA Medical Center, Torrance, Calif.
"That’s simply not true," he said in an interview. "It’s the forces of the contraction, the forces of maternal pushing. It’s the baby’s movement down the birth canal," that causes the injuries.
To avoid litigation, ob.gyns. should be aware of American Congress of Obstetricians and Gynecologists guidelines pertaining to shoulder dystocia and ensure that the risks of dystocia and brachial plexus injuries are discussed with patients, Dr. Ross said.
Additionally, physicians and health care team members should attend a dystocia drill every 1-2 years and make sure their institution has designated appropriate staff to respond during dystocia events, Dr. Debbs said. Documentation of dystocia incidents and conversations with families after the fact also are crucial.
"Always sit down with patient and family and discuss what happened and how it was remedied in detail," he said. "Many lawsuits are filed after families feel no one spoke with them and explained things."
As ob.gyns. face a growing number of shoulder dystocia cases, also escalating are medical malpractice lawsuits that claim physicians are to blame for related injuries.
"Because there is an increasing incidence of obesity in the United States, as well as women entering pregnancy at high risk or already having diabetes, the risks of dystocia are rising," said Dr. Robert H. Debbs, director of the Pennsylvania Hospital Maternal Fetal Medicine, Philadelphia. "The trends appear to be suits now being filed not for failing to perform C [cesarean] sections, but for how the dystocia was handled; i.e., was it anticipated? Were the proper maneuvers done? Is there evidence of excessive force used? And was the ‘team’ responsive to the situation?"
Adding to legal risks is the difficulty of predicting shoulder dystocia, noted Dr. Debbs. A 2012 study in Archives of Gynecology and Obstetrics that analyzed 234 shoulder dystocia cases found only mode of delivery and birth weight were independent risk factors of the condition. The U.K. researchers concluded that exact birth weight and delivery mode is difficult to foretell, and therefore, occurrence of shoulder dystocia is highly unpredictable (Arch. Gynecol. Obstet. 2012;285:291-5).
"It’s clear that we are neither able to predict with accuracy, nor prevent dystocia accurately in that most cases occur in normal-weight babies, and only 25% of patients have risk factors," said Dr. Debbs, who recently spoke on the subject at the American Conference Institute obstetric claims forum in Philadelphia. "Performing elective cesarean sections for suspected large babies has not reduced significantly the number of dystocias, and we would have to perform close to 300 cesarean-sections to prevent one permanent nerve injury to a child, a number that places women at risk of future pregnancy complications in addition to morbidity and even mortality from surgery," according to Dr. Debbs.
Despite the literature, many plaintiffs’ attorneys argue the mere presence of a brachial plexus injury in a newborn indicates the physician pulled too hard or in the wrong direction, said Dr. Michael G. Ross, chair of the obstetrics and gynecology department for Harbor-UCLA Medical Center, Torrance, Calif.
"That’s simply not true," he said in an interview. "It’s the forces of the contraction, the forces of maternal pushing. It’s the baby’s movement down the birth canal," that causes the injuries.
To avoid litigation, ob.gyns. should be aware of American Congress of Obstetricians and Gynecologists guidelines pertaining to shoulder dystocia and ensure that the risks of dystocia and brachial plexus injuries are discussed with patients, Dr. Ross said.
Additionally, physicians and health care team members should attend a dystocia drill every 1-2 years and make sure their institution has designated appropriate staff to respond during dystocia events, Dr. Debbs said. Documentation of dystocia incidents and conversations with families after the fact also are crucial.
"Always sit down with patient and family and discuss what happened and how it was remedied in detail," he said. "Many lawsuits are filed after families feel no one spoke with them and explained things."
As ob.gyns. face a growing number of shoulder dystocia cases, also escalating are medical malpractice lawsuits that claim physicians are to blame for related injuries.
"Because there is an increasing incidence of obesity in the United States, as well as women entering pregnancy at high risk or already having diabetes, the risks of dystocia are rising," said Dr. Robert H. Debbs, director of the Pennsylvania Hospital Maternal Fetal Medicine, Philadelphia. "The trends appear to be suits now being filed not for failing to perform C [cesarean] sections, but for how the dystocia was handled; i.e., was it anticipated? Were the proper maneuvers done? Is there evidence of excessive force used? And was the ‘team’ responsive to the situation?"
Adding to legal risks is the difficulty of predicting shoulder dystocia, noted Dr. Debbs. A 2012 study in Archives of Gynecology and Obstetrics that analyzed 234 shoulder dystocia cases found only mode of delivery and birth weight were independent risk factors of the condition. The U.K. researchers concluded that exact birth weight and delivery mode is difficult to foretell, and therefore, occurrence of shoulder dystocia is highly unpredictable (Arch. Gynecol. Obstet. 2012;285:291-5).
"It’s clear that we are neither able to predict with accuracy, nor prevent dystocia accurately in that most cases occur in normal-weight babies, and only 25% of patients have risk factors," said Dr. Debbs, who recently spoke on the subject at the American Conference Institute obstetric claims forum in Philadelphia. "Performing elective cesarean sections for suspected large babies has not reduced significantly the number of dystocias, and we would have to perform close to 300 cesarean-sections to prevent one permanent nerve injury to a child, a number that places women at risk of future pregnancy complications in addition to morbidity and even mortality from surgery," according to Dr. Debbs.
Despite the literature, many plaintiffs’ attorneys argue the mere presence of a brachial plexus injury in a newborn indicates the physician pulled too hard or in the wrong direction, said Dr. Michael G. Ross, chair of the obstetrics and gynecology department for Harbor-UCLA Medical Center, Torrance, Calif.
"That’s simply not true," he said in an interview. "It’s the forces of the contraction, the forces of maternal pushing. It’s the baby’s movement down the birth canal," that causes the injuries.
To avoid litigation, ob.gyns. should be aware of American Congress of Obstetricians and Gynecologists guidelines pertaining to shoulder dystocia and ensure that the risks of dystocia and brachial plexus injuries are discussed with patients, Dr. Ross said.
Additionally, physicians and health care team members should attend a dystocia drill every 1-2 years and make sure their institution has designated appropriate staff to respond during dystocia events, Dr. Debbs said. Documentation of dystocia incidents and conversations with families after the fact also are crucial.
"Always sit down with patient and family and discuss what happened and how it was remedied in detail," he said. "Many lawsuits are filed after families feel no one spoke with them and explained things."
Black-white racial disparities seen in birth defects in Louisiana study
BELLEVUE, WASH. – Louisiana has marked black-white racial disparities when it comes to birth defects, suggests a cohort study of 113,696 live births in the state during 2006-2008.
Relative to peers born to black mothers, infants born to white mothers were 24%-177% more likely to have cardiac, gastrointestinal, and genitourinary defects, according to data reported at the annual meeting of the Teratology Society. But they were 35% less likely to have microcephalus.
"We can conclude from this study that most birth defects are more common in non-Hispanic white children, compared to non-Hispanic black children. We think this difference may be the result of genetic susceptibility or environmental factors that play a significant role," said Dr. Tri Tran, of the department of pediatrics at the Louisiana State University Health Sciences Center and an investigator with the Louisiana Department of Health and Hospitals Office of Public Health children and youth with special health needs program – both in New Orleans.
In the future, the investigators hope to conduct more in-depth study of genetic and environmental factors to determine reasons for the observed disparities, which could help inform effective prevention and intervention strategies, he added.
Session cochair Dr. James Mills, an investigator with the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md., commented, "I noticed that some of the defects that were more common in whites are things like ASD [atrial septal defect], VSD [ventricular septal defect], PDA [patent ductus arteriosus], and hypospadias, where there may be fairly trivial defects but really good diagnosis. Did you look at the data by socioeconomic class to see if there may be some bias in ascertainment?"
Analyses did not include that potential confounder, Dr. Tran replied.
For the study, the investigators used linked hospital records and data from the Louisiana Birth Defects Monitoring Network, a population-based surveillance system, to identify live births to non-Hispanic white women (57%) and non-Hispanic black women (43%) in which the birth weight was at least 350 g, and the gestational age was at least 20 weeks.
The investigators assessed birth defects captured in the first 3 years of life. Results were restricted to the 19 birth defects seen in at least 30 infants.
In adjusted analyses, infants born to white women were more likely to have ventricular septal defects (prevalence ratio, 1.59), atrial septal defects (1.24), atrioventricular septal defects (1.67), patent ductus arteriosus (1.31), cleft palate without cleft lip (2.20), pyloric stenosis (2.77), obstructive genitourinary defects (1.31), gastroschisis (2.71), Down syndrome (1.49), and hypospadias (1.50).
Only a single birth defect, microcephalus, was significantly less common in infants of white women, compared with infants of black women (prevalence ratio, 0.65), reported Dr. Tran.
Dr. Tran disclosed no relevant conflicts of interest.
BELLEVUE, WASH. – Louisiana has marked black-white racial disparities when it comes to birth defects, suggests a cohort study of 113,696 live births in the state during 2006-2008.
Relative to peers born to black mothers, infants born to white mothers were 24%-177% more likely to have cardiac, gastrointestinal, and genitourinary defects, according to data reported at the annual meeting of the Teratology Society. But they were 35% less likely to have microcephalus.
"We can conclude from this study that most birth defects are more common in non-Hispanic white children, compared to non-Hispanic black children. We think this difference may be the result of genetic susceptibility or environmental factors that play a significant role," said Dr. Tri Tran, of the department of pediatrics at the Louisiana State University Health Sciences Center and an investigator with the Louisiana Department of Health and Hospitals Office of Public Health children and youth with special health needs program – both in New Orleans.
In the future, the investigators hope to conduct more in-depth study of genetic and environmental factors to determine reasons for the observed disparities, which could help inform effective prevention and intervention strategies, he added.
Session cochair Dr. James Mills, an investigator with the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md., commented, "I noticed that some of the defects that were more common in whites are things like ASD [atrial septal defect], VSD [ventricular septal defect], PDA [patent ductus arteriosus], and hypospadias, where there may be fairly trivial defects but really good diagnosis. Did you look at the data by socioeconomic class to see if there may be some bias in ascertainment?"
Analyses did not include that potential confounder, Dr. Tran replied.
For the study, the investigators used linked hospital records and data from the Louisiana Birth Defects Monitoring Network, a population-based surveillance system, to identify live births to non-Hispanic white women (57%) and non-Hispanic black women (43%) in which the birth weight was at least 350 g, and the gestational age was at least 20 weeks.
The investigators assessed birth defects captured in the first 3 years of life. Results were restricted to the 19 birth defects seen in at least 30 infants.
In adjusted analyses, infants born to white women were more likely to have ventricular septal defects (prevalence ratio, 1.59), atrial septal defects (1.24), atrioventricular septal defects (1.67), patent ductus arteriosus (1.31), cleft palate without cleft lip (2.20), pyloric stenosis (2.77), obstructive genitourinary defects (1.31), gastroschisis (2.71), Down syndrome (1.49), and hypospadias (1.50).
Only a single birth defect, microcephalus, was significantly less common in infants of white women, compared with infants of black women (prevalence ratio, 0.65), reported Dr. Tran.
Dr. Tran disclosed no relevant conflicts of interest.
BELLEVUE, WASH. – Louisiana has marked black-white racial disparities when it comes to birth defects, suggests a cohort study of 113,696 live births in the state during 2006-2008.
Relative to peers born to black mothers, infants born to white mothers were 24%-177% more likely to have cardiac, gastrointestinal, and genitourinary defects, according to data reported at the annual meeting of the Teratology Society. But they were 35% less likely to have microcephalus.
"We can conclude from this study that most birth defects are more common in non-Hispanic white children, compared to non-Hispanic black children. We think this difference may be the result of genetic susceptibility or environmental factors that play a significant role," said Dr. Tri Tran, of the department of pediatrics at the Louisiana State University Health Sciences Center and an investigator with the Louisiana Department of Health and Hospitals Office of Public Health children and youth with special health needs program – both in New Orleans.
In the future, the investigators hope to conduct more in-depth study of genetic and environmental factors to determine reasons for the observed disparities, which could help inform effective prevention and intervention strategies, he added.
Session cochair Dr. James Mills, an investigator with the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md., commented, "I noticed that some of the defects that were more common in whites are things like ASD [atrial septal defect], VSD [ventricular septal defect], PDA [patent ductus arteriosus], and hypospadias, where there may be fairly trivial defects but really good diagnosis. Did you look at the data by socioeconomic class to see if there may be some bias in ascertainment?"
Analyses did not include that potential confounder, Dr. Tran replied.
For the study, the investigators used linked hospital records and data from the Louisiana Birth Defects Monitoring Network, a population-based surveillance system, to identify live births to non-Hispanic white women (57%) and non-Hispanic black women (43%) in which the birth weight was at least 350 g, and the gestational age was at least 20 weeks.
The investigators assessed birth defects captured in the first 3 years of life. Results were restricted to the 19 birth defects seen in at least 30 infants.
In adjusted analyses, infants born to white women were more likely to have ventricular septal defects (prevalence ratio, 1.59), atrial septal defects (1.24), atrioventricular septal defects (1.67), patent ductus arteriosus (1.31), cleft palate without cleft lip (2.20), pyloric stenosis (2.77), obstructive genitourinary defects (1.31), gastroschisis (2.71), Down syndrome (1.49), and hypospadias (1.50).
Only a single birth defect, microcephalus, was significantly less common in infants of white women, compared with infants of black women (prevalence ratio, 0.65), reported Dr. Tran.
Dr. Tran disclosed no relevant conflicts of interest.
AT TERATOLOGY SOCIETY 2014
Key clinical finding: Investigators concluded that most birth defects were more common in non-Hispanic white women than in non-Hispanic black women in this Louisiana study.
Major finding: Infants born to white women more often had cardiac, gastrointestinal, and genitourinary defects, whereas infants born to black women more often had microcephalus.
Data source: A cohort study of 113,696 live births in Louisiana between 2006 and 2008.
Disclosures: Dr. Tran disclosed no relevant conflicts of interest.
As severity of rheumatoid arthritis rises, so does risk of preterm delivery
BELLEVUE, WASH. – The severity of rheumatoid arthritis early in pregnancy is an independent risk factor for preterm delivery, according to data from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project.
The prospective cohort study of 447 pregnant women with rheumatoid arthritis who had a live-born infant from 2005-2013 found that the greater disease severity before 20 weeks of gestation, assessed by a variety of measures, the higher the adjusted risk of delivering preterm. But there was no significant impact on the adjusted risk of having an infant small for gestational age or a cesarean section.
"Disease severity in women with rheumatoid arthritis, measured early in pregnancy, is predictive of preterm delivery," noted Dr. Balambal Bharti, a researcher at the bioscience center, University of California, San Diego, in presenting the findings at the annual meeting of the Teratology Society.
The investigators are performing additional analyses to determine whether disease severity at different times – early versus late pregnancy – has a similar or differing impact, and to assess any effect of a change in severity during pregnancy.
"For future research, we’d like to investigate if better disease management early in pregnancy improves pregnancy outcome," she said.
Session cochair Suzan L. Carmichael, Ph.D., of the department of pediatrics (neonatology) at Stanford (Calif.) University asked, "Did you know if there were differences in disease severity or treatment based on whether women had planned their pregnancies, and whether that could have affected your results? I’m just wondering if there are certain women who had planned their pregnancy and had changed their treatment regimen in anticipation of that."
"We didn’t know whether women had planned their pregnancies," Dr. Bharti replied, although some data suggest that about half of pregnancies in the Organization of Teratology Information Specialists cohort are unplanned. The investigators also did not look at whether women changed their treatment before conceiving, she said.
Session attendee Dr. Jan M. Freidman of the University of British Columbia in Vancouver noted, "Two of the three outcome variables you looked at are actually continuous variables: birth weight (or birth weight for gestational age) and week of gestation at which you deliver. As a clinician, it would be useful to know what the size of the effect was in terms of those continuous variables: How much did [disease severity] reduce birth weight? How much was the reduction, or was there a reduction, in gestational age? Did you look at the analysis in that way, or just in the discrete way you presented here?"
"All of our outcomes were dichotomized," Dr. Bharti replied.
"There is more information there that you might want to look at," Dr. Friedman recommended.
The women studied were administered the 4-point Health Assessment Questionnaire Disability Index (HAQ-DI) at baseline, before 20 weeks of gestation. They also rated their pain and global health in the past week on 100-point scales.
Overall, 15% of the women had a preterm delivery (one occurring before 37 weeks of gestation), 9% gave birth to an infant who was small for gestational age, and 42% had a cesarean section.
In multivariate adjusted analyses, women’s risk of preterm birth rose with each 1% increase (worsening) in HAQ-DI score (relative risk, 1.55) and with each 20-point increase (worsening) in pain score (RR, 1.17) and score on the global scale of overall health (RR,1.22).
In contrast, none of the three measures of disease severity independently predicted small for gestational age or cesarean delivery.
Dr. Bharti said that, to the authors’ knowledge, only one other prospective study has looked at the impact of rheumatoid arthritis disease severity on pregnancy outcomes (Arthritis Rheum. 2009;60:3196-206). That study followed white Dutch-speaking women in a first pregnancy who were taking prednisone, sulfasalazine, or hydroxychloroquine.
"Our study adds to [that study] by having women of diverse ethnic background who were in a first or subsequent pregnancy, and they were either on no treatment for rheumatoid arthritis or on any kind of treatment," she commented.
The new findings are generally similar to those of that previous study but differ in that they show a positive association between disease severity and preterm delivery, according to Dr. Bharti.
She disclosed no conflicts of interest related to the research.
BELLEVUE, WASH. – The severity of rheumatoid arthritis early in pregnancy is an independent risk factor for preterm delivery, according to data from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project.
The prospective cohort study of 447 pregnant women with rheumatoid arthritis who had a live-born infant from 2005-2013 found that the greater disease severity before 20 weeks of gestation, assessed by a variety of measures, the higher the adjusted risk of delivering preterm. But there was no significant impact on the adjusted risk of having an infant small for gestational age or a cesarean section.
"Disease severity in women with rheumatoid arthritis, measured early in pregnancy, is predictive of preterm delivery," noted Dr. Balambal Bharti, a researcher at the bioscience center, University of California, San Diego, in presenting the findings at the annual meeting of the Teratology Society.
The investigators are performing additional analyses to determine whether disease severity at different times – early versus late pregnancy – has a similar or differing impact, and to assess any effect of a change in severity during pregnancy.
"For future research, we’d like to investigate if better disease management early in pregnancy improves pregnancy outcome," she said.
Session cochair Suzan L. Carmichael, Ph.D., of the department of pediatrics (neonatology) at Stanford (Calif.) University asked, "Did you know if there were differences in disease severity or treatment based on whether women had planned their pregnancies, and whether that could have affected your results? I’m just wondering if there are certain women who had planned their pregnancy and had changed their treatment regimen in anticipation of that."
"We didn’t know whether women had planned their pregnancies," Dr. Bharti replied, although some data suggest that about half of pregnancies in the Organization of Teratology Information Specialists cohort are unplanned. The investigators also did not look at whether women changed their treatment before conceiving, she said.
Session attendee Dr. Jan M. Freidman of the University of British Columbia in Vancouver noted, "Two of the three outcome variables you looked at are actually continuous variables: birth weight (or birth weight for gestational age) and week of gestation at which you deliver. As a clinician, it would be useful to know what the size of the effect was in terms of those continuous variables: How much did [disease severity] reduce birth weight? How much was the reduction, or was there a reduction, in gestational age? Did you look at the analysis in that way, or just in the discrete way you presented here?"
"All of our outcomes were dichotomized," Dr. Bharti replied.
"There is more information there that you might want to look at," Dr. Friedman recommended.
The women studied were administered the 4-point Health Assessment Questionnaire Disability Index (HAQ-DI) at baseline, before 20 weeks of gestation. They also rated their pain and global health in the past week on 100-point scales.
Overall, 15% of the women had a preterm delivery (one occurring before 37 weeks of gestation), 9% gave birth to an infant who was small for gestational age, and 42% had a cesarean section.
In multivariate adjusted analyses, women’s risk of preterm birth rose with each 1% increase (worsening) in HAQ-DI score (relative risk, 1.55) and with each 20-point increase (worsening) in pain score (RR, 1.17) and score on the global scale of overall health (RR,1.22).
In contrast, none of the three measures of disease severity independently predicted small for gestational age or cesarean delivery.
Dr. Bharti said that, to the authors’ knowledge, only one other prospective study has looked at the impact of rheumatoid arthritis disease severity on pregnancy outcomes (Arthritis Rheum. 2009;60:3196-206). That study followed white Dutch-speaking women in a first pregnancy who were taking prednisone, sulfasalazine, or hydroxychloroquine.
"Our study adds to [that study] by having women of diverse ethnic background who were in a first or subsequent pregnancy, and they were either on no treatment for rheumatoid arthritis or on any kind of treatment," she commented.
The new findings are generally similar to those of that previous study but differ in that they show a positive association between disease severity and preterm delivery, according to Dr. Bharti.
She disclosed no conflicts of interest related to the research.
BELLEVUE, WASH. – The severity of rheumatoid arthritis early in pregnancy is an independent risk factor for preterm delivery, according to data from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project.
The prospective cohort study of 447 pregnant women with rheumatoid arthritis who had a live-born infant from 2005-2013 found that the greater disease severity before 20 weeks of gestation, assessed by a variety of measures, the higher the adjusted risk of delivering preterm. But there was no significant impact on the adjusted risk of having an infant small for gestational age or a cesarean section.
"Disease severity in women with rheumatoid arthritis, measured early in pregnancy, is predictive of preterm delivery," noted Dr. Balambal Bharti, a researcher at the bioscience center, University of California, San Diego, in presenting the findings at the annual meeting of the Teratology Society.
The investigators are performing additional analyses to determine whether disease severity at different times – early versus late pregnancy – has a similar or differing impact, and to assess any effect of a change in severity during pregnancy.
"For future research, we’d like to investigate if better disease management early in pregnancy improves pregnancy outcome," she said.
Session cochair Suzan L. Carmichael, Ph.D., of the department of pediatrics (neonatology) at Stanford (Calif.) University asked, "Did you know if there were differences in disease severity or treatment based on whether women had planned their pregnancies, and whether that could have affected your results? I’m just wondering if there are certain women who had planned their pregnancy and had changed their treatment regimen in anticipation of that."
"We didn’t know whether women had planned their pregnancies," Dr. Bharti replied, although some data suggest that about half of pregnancies in the Organization of Teratology Information Specialists cohort are unplanned. The investigators also did not look at whether women changed their treatment before conceiving, she said.
Session attendee Dr. Jan M. Freidman of the University of British Columbia in Vancouver noted, "Two of the three outcome variables you looked at are actually continuous variables: birth weight (or birth weight for gestational age) and week of gestation at which you deliver. As a clinician, it would be useful to know what the size of the effect was in terms of those continuous variables: How much did [disease severity] reduce birth weight? How much was the reduction, or was there a reduction, in gestational age? Did you look at the analysis in that way, or just in the discrete way you presented here?"
"All of our outcomes were dichotomized," Dr. Bharti replied.
"There is more information there that you might want to look at," Dr. Friedman recommended.
The women studied were administered the 4-point Health Assessment Questionnaire Disability Index (HAQ-DI) at baseline, before 20 weeks of gestation. They also rated their pain and global health in the past week on 100-point scales.
Overall, 15% of the women had a preterm delivery (one occurring before 37 weeks of gestation), 9% gave birth to an infant who was small for gestational age, and 42% had a cesarean section.
In multivariate adjusted analyses, women’s risk of preterm birth rose with each 1% increase (worsening) in HAQ-DI score (relative risk, 1.55) and with each 20-point increase (worsening) in pain score (RR, 1.17) and score on the global scale of overall health (RR,1.22).
In contrast, none of the three measures of disease severity independently predicted small for gestational age or cesarean delivery.
Dr. Bharti said that, to the authors’ knowledge, only one other prospective study has looked at the impact of rheumatoid arthritis disease severity on pregnancy outcomes (Arthritis Rheum. 2009;60:3196-206). That study followed white Dutch-speaking women in a first pregnancy who were taking prednisone, sulfasalazine, or hydroxychloroquine.
"Our study adds to [that study] by having women of diverse ethnic background who were in a first or subsequent pregnancy, and they were either on no treatment for rheumatoid arthritis or on any kind of treatment," she commented.
The new findings are generally similar to those of that previous study but differ in that they show a positive association between disease severity and preterm delivery, according to Dr. Bharti.
She disclosed no conflicts of interest related to the research.
AT TERATOLOGY SOCIETY 2014
Key clinical point: Having RA under control before initiation of pregnancy may cut preterm birth risk.
Major finding: Women’s adjusted risk of preterm delivery increased with rheumatoid arthritis severity in early pregnancy as assessed by the HAQ-DI score (relative risk, 1.55), pain score (1.17), or patient global scale of overall health (1.22).
Data source: A prospective cohort study of 447 pregnant women with rheumatoid arthritis spanning 2005-2013.
Disclosures: Dr. Bharti disclosed no relevant conflicts of interest.
Birth registry data demonstrate familial cerebral palsy risk
Cerebral palsy appears to have a genetic component, with increased risk extending to third-degree relatives (first cousins), according to findings from a population-based cohort study.
These data "offer additional evidence that the underlying causes of cerebral palsy extend beyond the clinical management of delivery. However, the similar risks of cerebral palsy of co-twins of affected like-sex and unlike-sex twin pairs suggest that genetic influences are only part of a wide range of causes," the researchers concluded, noting that future studies should consider the possibility of genetic causes and genetic susceptibility to environmental causes.
Of over 2 million Norwegians born between 1967 and 2002 and included in the Medical Birth Registry of Norway, 3,649 were diagnosed with cerebral palsy, Dr. Mette C. Tollånes of the University of Bergen and her colleagues reported online July 15 in the British Journal of Medicine.
Individuals who had a twin with cerebral palsy had a 15.6-fold increase in the risk of cerebral palsy. Subsequent full or half siblings of a child with cerebral palsy had a 9.2-fold and 3.0-fold increase in the risk of cerebral palsy, respectively, and parents with cerebral palsy had a 6.5-fold increase in the risk of having an affected child, the investigators found (BMJ 2014 July 15 [doi:10.1136/bmj.g4294]).
Although the risk was lower than for first- and second-degree relatives, even those who had a first cousin with cerebral palsy were at a 1.5-fold increased risk of cerebral palsy, they said.
There was no evidence of a difference in the rate of transmission by affected mothers or fathers, and the risks seen in siblings and cousins were independent of the sex of the index case, Dr. Tollånes and her associates noted.
Prior studies have shown that, in addition to a number of risk factors in pregnancy and during the perinatal period – such as preterm delivery, multiple fetuses, birth asphyxia, and perinatal stroke – there also is a possible heritable component. In fact, a number of candidate genes and single nucleotide polymorphisms have been investigated to explain familial clustering of cerebral palsy, and some studies have extended the investigation to interactions with clinical factors and other genes.
Positive findings, however, have been difficult to replicate, the investigators said.
"We used data from a large population based cohort to ... shed light on patterns of inheritance," they wrote.
Although limited by a number of factors, including lack of information on cerebral palsy subtypes, an inability to identify cases with postneonatal causes, and a possible underestimation of recurrence risk because of the exclusion of those who didn’t survive past 3 years, the findings "suggest that cerebral palsy includes a genetic component, with a stronger recurrence among relatives with closer genetic relationship," Dr. Tollånes and her associates said.
This study was supported by grants from the University of Bergen; the Western Norway Regional Health Authority; and the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. The authors reported having no disclosures other than support from these funding sources.
Cerebral palsy appears to have a genetic component, with increased risk extending to third-degree relatives (first cousins), according to findings from a population-based cohort study.
These data "offer additional evidence that the underlying causes of cerebral palsy extend beyond the clinical management of delivery. However, the similar risks of cerebral palsy of co-twins of affected like-sex and unlike-sex twin pairs suggest that genetic influences are only part of a wide range of causes," the researchers concluded, noting that future studies should consider the possibility of genetic causes and genetic susceptibility to environmental causes.
Of over 2 million Norwegians born between 1967 and 2002 and included in the Medical Birth Registry of Norway, 3,649 were diagnosed with cerebral palsy, Dr. Mette C. Tollånes of the University of Bergen and her colleagues reported online July 15 in the British Journal of Medicine.
Individuals who had a twin with cerebral palsy had a 15.6-fold increase in the risk of cerebral palsy. Subsequent full or half siblings of a child with cerebral palsy had a 9.2-fold and 3.0-fold increase in the risk of cerebral palsy, respectively, and parents with cerebral palsy had a 6.5-fold increase in the risk of having an affected child, the investigators found (BMJ 2014 July 15 [doi:10.1136/bmj.g4294]).
Although the risk was lower than for first- and second-degree relatives, even those who had a first cousin with cerebral palsy were at a 1.5-fold increased risk of cerebral palsy, they said.
There was no evidence of a difference in the rate of transmission by affected mothers or fathers, and the risks seen in siblings and cousins were independent of the sex of the index case, Dr. Tollånes and her associates noted.
Prior studies have shown that, in addition to a number of risk factors in pregnancy and during the perinatal period – such as preterm delivery, multiple fetuses, birth asphyxia, and perinatal stroke – there also is a possible heritable component. In fact, a number of candidate genes and single nucleotide polymorphisms have been investigated to explain familial clustering of cerebral palsy, and some studies have extended the investigation to interactions with clinical factors and other genes.
Positive findings, however, have been difficult to replicate, the investigators said.
"We used data from a large population based cohort to ... shed light on patterns of inheritance," they wrote.
Although limited by a number of factors, including lack of information on cerebral palsy subtypes, an inability to identify cases with postneonatal causes, and a possible underestimation of recurrence risk because of the exclusion of those who didn’t survive past 3 years, the findings "suggest that cerebral palsy includes a genetic component, with a stronger recurrence among relatives with closer genetic relationship," Dr. Tollånes and her associates said.
This study was supported by grants from the University of Bergen; the Western Norway Regional Health Authority; and the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. The authors reported having no disclosures other than support from these funding sources.
Cerebral palsy appears to have a genetic component, with increased risk extending to third-degree relatives (first cousins), according to findings from a population-based cohort study.
These data "offer additional evidence that the underlying causes of cerebral palsy extend beyond the clinical management of delivery. However, the similar risks of cerebral palsy of co-twins of affected like-sex and unlike-sex twin pairs suggest that genetic influences are only part of a wide range of causes," the researchers concluded, noting that future studies should consider the possibility of genetic causes and genetic susceptibility to environmental causes.
Of over 2 million Norwegians born between 1967 and 2002 and included in the Medical Birth Registry of Norway, 3,649 were diagnosed with cerebral palsy, Dr. Mette C. Tollånes of the University of Bergen and her colleagues reported online July 15 in the British Journal of Medicine.
Individuals who had a twin with cerebral palsy had a 15.6-fold increase in the risk of cerebral palsy. Subsequent full or half siblings of a child with cerebral palsy had a 9.2-fold and 3.0-fold increase in the risk of cerebral palsy, respectively, and parents with cerebral palsy had a 6.5-fold increase in the risk of having an affected child, the investigators found (BMJ 2014 July 15 [doi:10.1136/bmj.g4294]).
Although the risk was lower than for first- and second-degree relatives, even those who had a first cousin with cerebral palsy were at a 1.5-fold increased risk of cerebral palsy, they said.
There was no evidence of a difference in the rate of transmission by affected mothers or fathers, and the risks seen in siblings and cousins were independent of the sex of the index case, Dr. Tollånes and her associates noted.
Prior studies have shown that, in addition to a number of risk factors in pregnancy and during the perinatal period – such as preterm delivery, multiple fetuses, birth asphyxia, and perinatal stroke – there also is a possible heritable component. In fact, a number of candidate genes and single nucleotide polymorphisms have been investigated to explain familial clustering of cerebral palsy, and some studies have extended the investigation to interactions with clinical factors and other genes.
Positive findings, however, have been difficult to replicate, the investigators said.
"We used data from a large population based cohort to ... shed light on patterns of inheritance," they wrote.
Although limited by a number of factors, including lack of information on cerebral palsy subtypes, an inability to identify cases with postneonatal causes, and a possible underestimation of recurrence risk because of the exclusion of those who didn’t survive past 3 years, the findings "suggest that cerebral palsy includes a genetic component, with a stronger recurrence among relatives with closer genetic relationship," Dr. Tollånes and her associates said.
This study was supported by grants from the University of Bergen; the Western Norway Regional Health Authority; and the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. The authors reported having no disclosures other than support from these funding sources.
FROM BMJ
Key clinical finding: It appears that cerebral palsy runs in families.
Major finding: The risk of cerebral palsy was increased 15.6-fold among twins, 6.5- to 9-fold among first-degree relatives, up to 3-fold among second-degree relatives, and 1.5-fold among third-degree relatives.
Data source: A population-based cohort study involving 2,036,741 Norwegians.
Disclosures: This study was supported by grants from the University of Bergen; the Western Norway Regional Health Authority; and the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. The authors reported having no disclosures other than support from these funding sources.
Fetal growth restriction may be underestimated in obese patients
Only 25% of babies who are born small for their gestational age are diagnosed prenatally, and this under-identification may be even higher in obese patients, according to researchers from The Penn State University College of Medicine. Because fetal growth restriction (FGR) is associated with poor perinatal outcomes, these researchers set out to retrospectively compare the accuracy of a customized growth curve with the standard growth curve (Hadlock), to identify FGR in obese and normal-weight patients.
A total of 300 nulliparous women were included in the single-institution, retrospective study (150 obese women with a body mass index [BMI] >30 mg/k2, and 150 women of normal weight with a BMI ≤25 mg/k2). These women were aged 18 to 50 years and gave birth between July 2008 and December 2012.
Obese women were twice as likely to have a fetus classified by third-trimester ultrasound as growth-restricted using the customized curve versus the Hadlock’s curve (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.4−3.2; P = .001). There was no difference in classification of growth restriction found in the women of normal weight (OR, 0.9; CI, 0.7−1.2; P = .41).
“Customized growth curves take into account certain maternal factors such as age, parity, BMI, and ethnicity,” said researcher Megha Gupta, MD. “The standard growth curves still used today were developed in the 1960s to 1980s in Colorado with primarily Caucasian women who did not have their BMI recorded. Those curves are outdated for today’s ethnically diverse population. With 30% of the US population obese, we need to move toward individualized medicine for the fetus.”
“Study limitations include our study’s retrospective nature and the fact that we could not exclude pathology, such as hypertension or smoking, which could have affected these results,” said Dr. Gupta. “We plan to follow this study up with a comparison between Lushenko and Fenton curves, which also are standardized curves for neonatal birth weight, and create customized growth charts. The ultimate goal is a prospective study to see if there are altered outcomes for babies that are detected to be growth-restricted, based on the customized growth chart.”
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Reference
Gupta M, Lauring J, Kunselman AR, Repke JT, Pauli JM. Fetal growth restriction may be underestimated in obese patients. Poster presented at: The American Congress of Obstetrics and Gynecology Annual Clinical Meeting, Chicago, IL; April 26, 2014.
Only 25% of babies who are born small for their gestational age are diagnosed prenatally, and this under-identification may be even higher in obese patients, according to researchers from The Penn State University College of Medicine. Because fetal growth restriction (FGR) is associated with poor perinatal outcomes, these researchers set out to retrospectively compare the accuracy of a customized growth curve with the standard growth curve (Hadlock), to identify FGR in obese and normal-weight patients.
A total of 300 nulliparous women were included in the single-institution, retrospective study (150 obese women with a body mass index [BMI] >30 mg/k2, and 150 women of normal weight with a BMI ≤25 mg/k2). These women were aged 18 to 50 years and gave birth between July 2008 and December 2012.
Obese women were twice as likely to have a fetus classified by third-trimester ultrasound as growth-restricted using the customized curve versus the Hadlock’s curve (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.4−3.2; P = .001). There was no difference in classification of growth restriction found in the women of normal weight (OR, 0.9; CI, 0.7−1.2; P = .41).
“Customized growth curves take into account certain maternal factors such as age, parity, BMI, and ethnicity,” said researcher Megha Gupta, MD. “The standard growth curves still used today were developed in the 1960s to 1980s in Colorado with primarily Caucasian women who did not have their BMI recorded. Those curves are outdated for today’s ethnically diverse population. With 30% of the US population obese, we need to move toward individualized medicine for the fetus.”
“Study limitations include our study’s retrospective nature and the fact that we could not exclude pathology, such as hypertension or smoking, which could have affected these results,” said Dr. Gupta. “We plan to follow this study up with a comparison between Lushenko and Fenton curves, which also are standardized curves for neonatal birth weight, and create customized growth charts. The ultimate goal is a prospective study to see if there are altered outcomes for babies that are detected to be growth-restricted, based on the customized growth chart.”
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Only 25% of babies who are born small for their gestational age are diagnosed prenatally, and this under-identification may be even higher in obese patients, according to researchers from The Penn State University College of Medicine. Because fetal growth restriction (FGR) is associated with poor perinatal outcomes, these researchers set out to retrospectively compare the accuracy of a customized growth curve with the standard growth curve (Hadlock), to identify FGR in obese and normal-weight patients.
A total of 300 nulliparous women were included in the single-institution, retrospective study (150 obese women with a body mass index [BMI] >30 mg/k2, and 150 women of normal weight with a BMI ≤25 mg/k2). These women were aged 18 to 50 years and gave birth between July 2008 and December 2012.
Obese women were twice as likely to have a fetus classified by third-trimester ultrasound as growth-restricted using the customized curve versus the Hadlock’s curve (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.4−3.2; P = .001). There was no difference in classification of growth restriction found in the women of normal weight (OR, 0.9; CI, 0.7−1.2; P = .41).
“Customized growth curves take into account certain maternal factors such as age, parity, BMI, and ethnicity,” said researcher Megha Gupta, MD. “The standard growth curves still used today were developed in the 1960s to 1980s in Colorado with primarily Caucasian women who did not have their BMI recorded. Those curves are outdated for today’s ethnically diverse population. With 30% of the US population obese, we need to move toward individualized medicine for the fetus.”
“Study limitations include our study’s retrospective nature and the fact that we could not exclude pathology, such as hypertension or smoking, which could have affected these results,” said Dr. Gupta. “We plan to follow this study up with a comparison between Lushenko and Fenton curves, which also are standardized curves for neonatal birth weight, and create customized growth charts. The ultimate goal is a prospective study to see if there are altered outcomes for babies that are detected to be growth-restricted, based on the customized growth chart.”
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Reference
Gupta M, Lauring J, Kunselman AR, Repke JT, Pauli JM. Fetal growth restriction may be underestimated in obese patients. Poster presented at: The American Congress of Obstetrics and Gynecology Annual Clinical Meeting, Chicago, IL; April 26, 2014.
Reference
Gupta M, Lauring J, Kunselman AR, Repke JT, Pauli JM. Fetal growth restriction may be underestimated in obese patients. Poster presented at: The American Congress of Obstetrics and Gynecology Annual Clinical Meeting, Chicago, IL; April 26, 2014.
Sleep apnea raises cardiomyopathy risk ninefold in pregnancy
Pregnant women with obstructive sleep apnea are more likely to experience adverse clinical conditions than are pregnant women who do not have sleep apnea, according to an analysis of over 55 million pregnancy-related hospital discharges from 1998 to 2009.
Women with obstructive sleep apnea (OSA) were nine times more likely to have cardiomyopathy and 8.9 times more likely to have congestive heart failure during pregnancy than were women without OSA, after adjustment for numerous factors, including maternal age and obesity, race/ethnicity, household income, heart disease, hyperlipidemia, and prepregnancy diabetes, reported Dr. Judette M. Louis of the University of South Florida, Tampa, and her associates.
Women with OSA also were more likely to have pulmonary edema (adjusted odds ratio, 7.5), in-hospital mortality (AOR, 5.3), pulmonary embolism and infarction (AOR, 4.5), stroke (AOR, 2.9), and acute renal failure (AOR, 2.7), Dr. Louis and her associates said (Sleep 2014;37:843-9).
The investigators analyzed data on 55,781,965 maternal hospital discharges from 1998 to 2009 in the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample. The institution of one investigator received a grant from ResMed Inc. and equipment from ResMed and Philips Respironics for use in clinical trials.
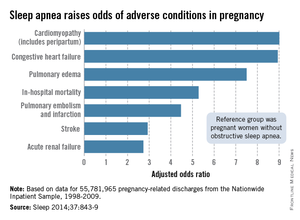
Pregnant women with obstructive sleep apnea are more likely to experience adverse clinical conditions than are pregnant women who do not have sleep apnea, according to an analysis of over 55 million pregnancy-related hospital discharges from 1998 to 2009.
Women with obstructive sleep apnea (OSA) were nine times more likely to have cardiomyopathy and 8.9 times more likely to have congestive heart failure during pregnancy than were women without OSA, after adjustment for numerous factors, including maternal age and obesity, race/ethnicity, household income, heart disease, hyperlipidemia, and prepregnancy diabetes, reported Dr. Judette M. Louis of the University of South Florida, Tampa, and her associates.
Women with OSA also were more likely to have pulmonary edema (adjusted odds ratio, 7.5), in-hospital mortality (AOR, 5.3), pulmonary embolism and infarction (AOR, 4.5), stroke (AOR, 2.9), and acute renal failure (AOR, 2.7), Dr. Louis and her associates said (Sleep 2014;37:843-9).
The investigators analyzed data on 55,781,965 maternal hospital discharges from 1998 to 2009 in the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample. The institution of one investigator received a grant from ResMed Inc. and equipment from ResMed and Philips Respironics for use in clinical trials.

Pregnant women with obstructive sleep apnea are more likely to experience adverse clinical conditions than are pregnant women who do not have sleep apnea, according to an analysis of over 55 million pregnancy-related hospital discharges from 1998 to 2009.
Women with obstructive sleep apnea (OSA) were nine times more likely to have cardiomyopathy and 8.9 times more likely to have congestive heart failure during pregnancy than were women without OSA, after adjustment for numerous factors, including maternal age and obesity, race/ethnicity, household income, heart disease, hyperlipidemia, and prepregnancy diabetes, reported Dr. Judette M. Louis of the University of South Florida, Tampa, and her associates.
Women with OSA also were more likely to have pulmonary edema (adjusted odds ratio, 7.5), in-hospital mortality (AOR, 5.3), pulmonary embolism and infarction (AOR, 4.5), stroke (AOR, 2.9), and acute renal failure (AOR, 2.7), Dr. Louis and her associates said (Sleep 2014;37:843-9).
The investigators analyzed data on 55,781,965 maternal hospital discharges from 1998 to 2009 in the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample. The institution of one investigator received a grant from ResMed Inc. and equipment from ResMed and Philips Respironics for use in clinical trials.

FROM SLEEP