User login
Infant deaths from birth defects decline, but some disparities widen
according to the Centers for Disease Control and Prevention.
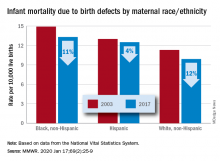
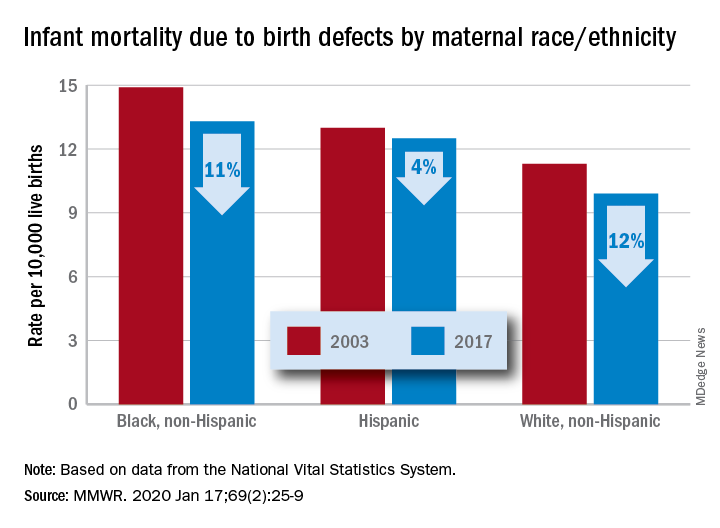
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
according to the Centers for Disease Control and Prevention.
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
according to the Centers for Disease Control and Prevention.
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
FROM MMWR
Cognitive problems after extremely preterm birth persist
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
FROM PEDIATRICS
2020 Update on obstetrics
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
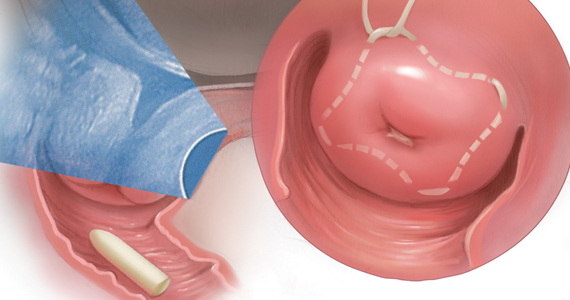
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
US rates of preterm birth
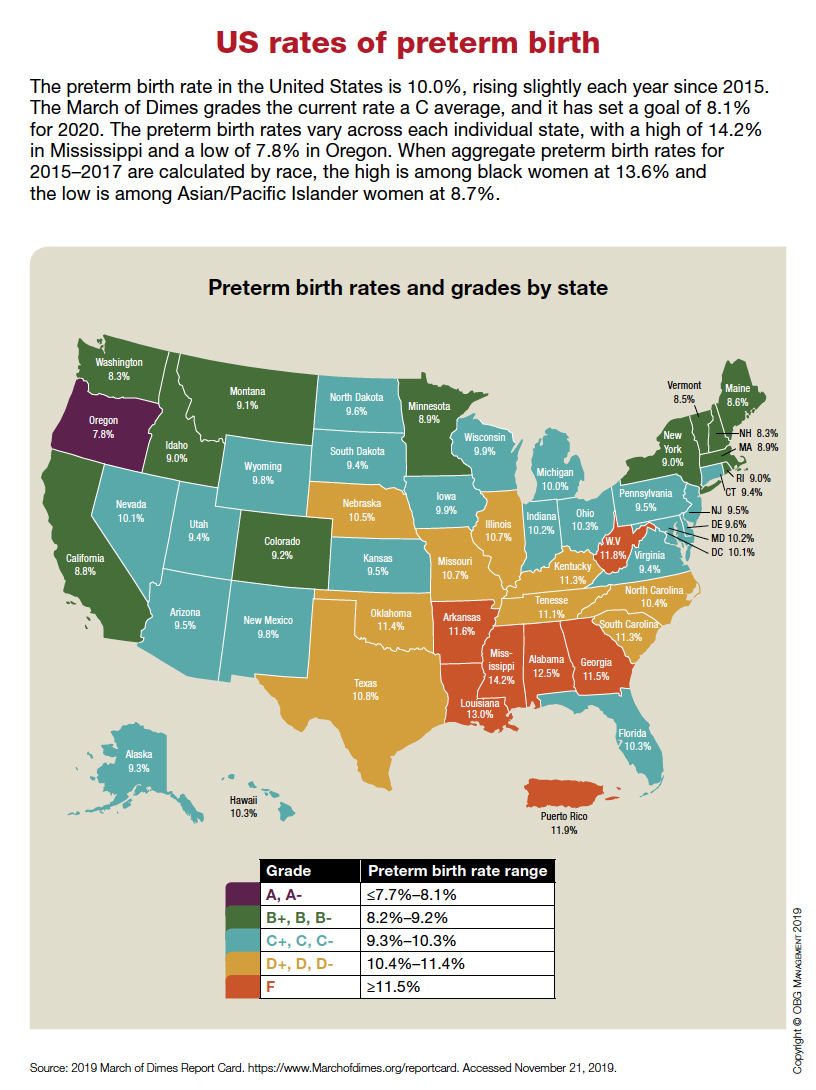


Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
Does planned early delivery make sense in women with preterm preeclampsia?
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
Data build on cardiovascular disease risk after GDM, HDP
WASHINGTON – Cardiovascular risk factors may be elevated “as soon as the first postpartum year” in women who have gestational diabetes or hypertensive disorders of pregnancy, recent findings have affirmed, Deborah B. Ehrenthal, MD, MPH, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
Dr. Ehrenthal was one of several researchers who urged innovative strategies and improved care coordination to boost women’s follow-up after gestational diabetes mellitus (GDM) and other adverse pregnancy outcomes and complications. “The metabolic stress of pregnancy can uncover underlying susceptibilities,” she said. “And ”
Evidence that adverse pregnancy outcomes – including GDM and hypertensive disorders of pregnancy (HDP) – can elevate cardiovascular risk comes most recently from the Nulliparous Pregnancy Outcomes Study – Monitoring Mothers to be Heart Health Study (nuMoM2b–HHS study), a prospective observational cohort that followed 4,484 women 2-7 years after their first pregnancy. Women had a follow-up exam, with blood pressure and anthropometric measurements and clinical/biological testing, an average of 3 years post partum.
An analysis published in October 2019 in the Journal of the American Heart Association shows that women with HDP (including preeclampsia and gestational hypertension) had a relative risk of hypertension of 2.5 at follow-up, compared with women without HDP. Women who had preeclampsia specifically were 2.3 times as likely as were women who did not have preeclampsia to have incident hypertension at follow-up, said Dr. Ehrenthal, a coinvestigator of the study.
The analysis focused on incident hypertension as the primary outcome, and adjusted for age, body mass index, and other important cardiovascular disease risk factors, she noted. Researchers utilized the diagnostic threshold for hypertension extant at the time of study design: A systolic blood pressure of 140 mm Hg or greater, or a diastolic BP of 90 mm Hg or greater (J Am Heart Assoc. 2019;8:e013092).
HDP was the most common adverse pregnancy outcome in the nuMoM2b–HHS study (14%). Among all participants, 4% had GDM. Approximately 82% had neither HDP nor GDM. Other adverse pregnancy outcomes included in the analysis were preterm birth, small-for-gestational-age birth, and stillbirth.
Additional preliminary estimates presented by Dr. Ehrenthal show that, based on the new (2017) lower threshold for hypertension – 130 mg Hg systolic or 80 mm Hg diastolic – the disorder afflicted 37% of women who had experienced HDP (relative risk 2.1), and 32% of women who had GDM (RR 1.8). Prediabetes/diabetes (using a fasting blood glucose threshold of 100 mg/dL) at follow-up affected an estimated 21% of women who had HDP (RR 1.4) and 38% of women who had GDM (RR 2.5).
Notably, across the entire study cohort, 20% had hypertension at follow-up, “which is extraordinary” considering the short time frame from pregnancy and the young age of the study population – a mean maternal age of 27 years, said Dr. Ehrenthal, associate professor of population health sciences and obstetrics & gynecology at the University of Wisconsin, Madison.
Also across the cohort, 15% had prediabetes/diabetes at follow-up. “We need to think about women more generally,” she cautioned. “While we recognize the significant elevated risk of HDP and GDM [for the development of subsequent hypertension and cardiovascular risk], we will miss a lot of women [if we focus only on the history of HDP and GDM.]”
The majority of women found to have hypertension or prediabetes/diabetes at follow-up had experienced neither HDP nor GDM, but a good many of them (47% of those who had hypertension and 47% of those found to have prediabetes/diabetes) had a BMI of 30 or above, Dr. Ehrenthal said at the DPSG-NA meeting.
Nurses Health Study, hyperglycemia and adverse pregnancy outcome follow-up data
The new findings from the nuMoM2b–HHS study add to a robust and growing body of evidence that pregnancy is an important window to future health, and that follow up and screening after GDM and HDP are crucial.
Regarding GDM specifically, “there’s quite a bit of literature by now demonstrating that GDM history is a risk factor for hypertension, even 1-2 years post partum, and that the risk is elevated as well for dyslipidemia and vascular dysfunction,” Deirdre K. Tobias, D.Sc., an epidemiologist at Brigham and Women’s Hospital and assistant professor of nutrition at Harvard TH Chan School of Public Health, Boston, said at the DPSG meeting.
An analysis of the Nurses Health Study II (NHS II) cohort published in 2017 found a 40% higher relative risk of cardiovascular disease events (largely myocardial infarction) in women who had GDM, compared with women who did not have GDM over a median follow-up of 26 years. This was after adjustments were made for age, time since pregnancy, menopausal status, family history of MI or stroke, hypertension in pregnancy, white race/ethnicity, prepregnancy BMI, and other factors (JAMA Intern Med. 2017;177[12]:1735-42).
The NHS data also have shown, however, that the elevated risk for cardiovascular disease after a GDM pregnancy “can be mitigated by adopting a healthy lifestyle,” said Dr. Tobias, lead author of the 2017 NHS II analysis. Adjustments for postpregnancy weight gain and lifestyle factors attenuated the relative risk of cardiovascular disease events after a GDM pregnancy to a 30% increased risk.
Dr. Tobias and colleagues currently are looking within the NHS cohort for “metabolomic signatures” or signals – various amino acid and lipid metabolites – to identify the progression of GDM to type 2 diabetes. Metabolomics “may help further refine our understanding of the long-term links between GDM and prevention of type 2 diabetes and of cardiovascular disease in mothers,” she said.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Follow-Up Study, in the meantime, is documenting associations of maternal glucose levels during pregnancy not only with prediabetes or type 2 diabetes 10-14 years later, but also with measures of cardiovascular risk in mothers 10-14 years later.
Just as perinatal outcomes were strongly associated with glucose as a continuous variable in the original HAPO study, “it’s clear there’s a progressive increase in the risk of [later] disorders of glucose metabolism as [fasting blood glucose levels and 1- and-2-hour glucose values] in pregnancy are higher,” said Boyd E. Metzger, MD, the Tom D. Spies emeritus professor of metabolism and nutrition at Northwestern University, Chicago, and principal investigator of the original HAPO study and its follow up.
“Another message is that the more normal you are in pregnancy, the more normal you will be many years later. Good values [during pregnancy] produce good outcomes.”
Currently unpublished data from the HAPO Follow-Up Study are being analyzed, but it appears thus far that GDM is not associated with hypertension (per the old diagnostic threshold) in this cohort after adjustment for maternal age, BMI, smoking, and family history of hypertension. GDM appears to be a significant risk factor for dyslipidemia, however. HDL cholesterol at follow-up was significantly lower for mothers who had GDM compared with those without, whereas LDL cholesterol and triglycerides at follow-up were significantly higher for mothers with GDM, Dr. Metzger said.
Racial/ethnic disparities, postpartum care
Neither long-term study – the NHS II or the HAPO Follow-Up Study – has looked at racial and ethnic differences. The HAPO cohort is racially-ethnically diverse but the NHS II cohort is predominantly white women.
Research suggests that GDM is a heterogeneous condition with some unique phenotypes in subgroups that vary by race and ethnicity. And just as there appear to be racial-ethnic differences in the pathophysiology of GDM, there appear to be racial-ethnic differences in the progression to type 2 diabetes – a known risk factor for cardiovascular disease, said Monique Henderson, PhD, a research scientist at Kaiser Permanente Northern California (KPNC).
On the broadest level, while Asian Americans have the highest prevalence of GDM, African Americans have the highest rates of progressing to type 2 diabetes, Dr. Henderson said. Disparities “may [stem from] metabolic differences in terms of insulin resistance and secretion that are different between pregnancy and the postpartum period, and that might vary [across racial-ethnic subgroups],” she said. Lifestyle differences and variation in postpartum screening rates also may play a role.
At KPNC, where women with GDM receive calls and letters reminding them of the need for postpartum screening, only 48% overall completed an oral glucose tolerance test at 4-12 weeks post partum, as recommended by both the American Diabetes Association and the American College of Obstetricians and Gynecologists. Both before and after adjustment for education, attendance at a postpartum visit, and other variables, Chinese women were most likely to have screening, and black women were least likely, said Dr. Henderson, referring to ongoing research.
A study Dr. Ehrenthal led of women with GDM or HDP recruited from the postpartum service of a large community-based, academic obstetrical hospital in Delaware showed that while nearly all women attended a 6-week postpartum visit with their ob.gyns., 59% of women with GDM had not yet completed diabetes screening when they were interviewed 3 months post partum. Most women with HDP indicated they had follow-up blood pressure testing, and just over half of women with either diagnosis recalled having ever had lipid testing (J Women’s Health 2014;23[9]:760-4).
Women least likely to complete screening tests were those who had no college education, those who had less than a high school level of health literacy, and those who were not privately insured, Dr. Ehrenthal said.
A large national study of privately insured women also found low rates of follow-up testing, however. While the majority of women with GDM had a postpartum visit with an obstetrician or primary care physician within a year after delivery, only a minority of women had a glycemic screening test completed (Obstet Gynecol. 2016;128[1]:159-67).
“We can’t place the blame on women,” Dr. Ehrenthal said. “We need increased attention to screening,” including screening for cardiovascular disease risk factors, and a “deliberate hand-off to primary care.”
For follow-up cardiovascular disease risk factor assessment after HDP, ACOG recommends periodic (perhaps annually) assessment and referral for treatment as needed, and the cardiology professional organizations recommend that pregnancy history be considered when assessing risk in order to decide on lipid treatment, she noted.
Each of the speakers reported that they have no financial or other interests that pose a conflict of interest. The HAPO Follow-Up Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the nuMoM2b–HHS study has been funded by several National Institutes of Health institutes and other programs and initiatives.
WASHINGTON – Cardiovascular risk factors may be elevated “as soon as the first postpartum year” in women who have gestational diabetes or hypertensive disorders of pregnancy, recent findings have affirmed, Deborah B. Ehrenthal, MD, MPH, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
Dr. Ehrenthal was one of several researchers who urged innovative strategies and improved care coordination to boost women’s follow-up after gestational diabetes mellitus (GDM) and other adverse pregnancy outcomes and complications. “The metabolic stress of pregnancy can uncover underlying susceptibilities,” she said. “And ”
Evidence that adverse pregnancy outcomes – including GDM and hypertensive disorders of pregnancy (HDP) – can elevate cardiovascular risk comes most recently from the Nulliparous Pregnancy Outcomes Study – Monitoring Mothers to be Heart Health Study (nuMoM2b–HHS study), a prospective observational cohort that followed 4,484 women 2-7 years after their first pregnancy. Women had a follow-up exam, with blood pressure and anthropometric measurements and clinical/biological testing, an average of 3 years post partum.
An analysis published in October 2019 in the Journal of the American Heart Association shows that women with HDP (including preeclampsia and gestational hypertension) had a relative risk of hypertension of 2.5 at follow-up, compared with women without HDP. Women who had preeclampsia specifically were 2.3 times as likely as were women who did not have preeclampsia to have incident hypertension at follow-up, said Dr. Ehrenthal, a coinvestigator of the study.
The analysis focused on incident hypertension as the primary outcome, and adjusted for age, body mass index, and other important cardiovascular disease risk factors, she noted. Researchers utilized the diagnostic threshold for hypertension extant at the time of study design: A systolic blood pressure of 140 mm Hg or greater, or a diastolic BP of 90 mm Hg or greater (J Am Heart Assoc. 2019;8:e013092).
HDP was the most common adverse pregnancy outcome in the nuMoM2b–HHS study (14%). Among all participants, 4% had GDM. Approximately 82% had neither HDP nor GDM. Other adverse pregnancy outcomes included in the analysis were preterm birth, small-for-gestational-age birth, and stillbirth.
Additional preliminary estimates presented by Dr. Ehrenthal show that, based on the new (2017) lower threshold for hypertension – 130 mg Hg systolic or 80 mm Hg diastolic – the disorder afflicted 37% of women who had experienced HDP (relative risk 2.1), and 32% of women who had GDM (RR 1.8). Prediabetes/diabetes (using a fasting blood glucose threshold of 100 mg/dL) at follow-up affected an estimated 21% of women who had HDP (RR 1.4) and 38% of women who had GDM (RR 2.5).
Notably, across the entire study cohort, 20% had hypertension at follow-up, “which is extraordinary” considering the short time frame from pregnancy and the young age of the study population – a mean maternal age of 27 years, said Dr. Ehrenthal, associate professor of population health sciences and obstetrics & gynecology at the University of Wisconsin, Madison.
Also across the cohort, 15% had prediabetes/diabetes at follow-up. “We need to think about women more generally,” she cautioned. “While we recognize the significant elevated risk of HDP and GDM [for the development of subsequent hypertension and cardiovascular risk], we will miss a lot of women [if we focus only on the history of HDP and GDM.]”
The majority of women found to have hypertension or prediabetes/diabetes at follow-up had experienced neither HDP nor GDM, but a good many of them (47% of those who had hypertension and 47% of those found to have prediabetes/diabetes) had a BMI of 30 or above, Dr. Ehrenthal said at the DPSG-NA meeting.
Nurses Health Study, hyperglycemia and adverse pregnancy outcome follow-up data
The new findings from the nuMoM2b–HHS study add to a robust and growing body of evidence that pregnancy is an important window to future health, and that follow up and screening after GDM and HDP are crucial.
Regarding GDM specifically, “there’s quite a bit of literature by now demonstrating that GDM history is a risk factor for hypertension, even 1-2 years post partum, and that the risk is elevated as well for dyslipidemia and vascular dysfunction,” Deirdre K. Tobias, D.Sc., an epidemiologist at Brigham and Women’s Hospital and assistant professor of nutrition at Harvard TH Chan School of Public Health, Boston, said at the DPSG meeting.
An analysis of the Nurses Health Study II (NHS II) cohort published in 2017 found a 40% higher relative risk of cardiovascular disease events (largely myocardial infarction) in women who had GDM, compared with women who did not have GDM over a median follow-up of 26 years. This was after adjustments were made for age, time since pregnancy, menopausal status, family history of MI or stroke, hypertension in pregnancy, white race/ethnicity, prepregnancy BMI, and other factors (JAMA Intern Med. 2017;177[12]:1735-42).
The NHS data also have shown, however, that the elevated risk for cardiovascular disease after a GDM pregnancy “can be mitigated by adopting a healthy lifestyle,” said Dr. Tobias, lead author of the 2017 NHS II analysis. Adjustments for postpregnancy weight gain and lifestyle factors attenuated the relative risk of cardiovascular disease events after a GDM pregnancy to a 30% increased risk.
Dr. Tobias and colleagues currently are looking within the NHS cohort for “metabolomic signatures” or signals – various amino acid and lipid metabolites – to identify the progression of GDM to type 2 diabetes. Metabolomics “may help further refine our understanding of the long-term links between GDM and prevention of type 2 diabetes and of cardiovascular disease in mothers,” she said.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Follow-Up Study, in the meantime, is documenting associations of maternal glucose levels during pregnancy not only with prediabetes or type 2 diabetes 10-14 years later, but also with measures of cardiovascular risk in mothers 10-14 years later.
Just as perinatal outcomes were strongly associated with glucose as a continuous variable in the original HAPO study, “it’s clear there’s a progressive increase in the risk of [later] disorders of glucose metabolism as [fasting blood glucose levels and 1- and-2-hour glucose values] in pregnancy are higher,” said Boyd E. Metzger, MD, the Tom D. Spies emeritus professor of metabolism and nutrition at Northwestern University, Chicago, and principal investigator of the original HAPO study and its follow up.
“Another message is that the more normal you are in pregnancy, the more normal you will be many years later. Good values [during pregnancy] produce good outcomes.”
Currently unpublished data from the HAPO Follow-Up Study are being analyzed, but it appears thus far that GDM is not associated with hypertension (per the old diagnostic threshold) in this cohort after adjustment for maternal age, BMI, smoking, and family history of hypertension. GDM appears to be a significant risk factor for dyslipidemia, however. HDL cholesterol at follow-up was significantly lower for mothers who had GDM compared with those without, whereas LDL cholesterol and triglycerides at follow-up were significantly higher for mothers with GDM, Dr. Metzger said.
Racial/ethnic disparities, postpartum care
Neither long-term study – the NHS II or the HAPO Follow-Up Study – has looked at racial and ethnic differences. The HAPO cohort is racially-ethnically diverse but the NHS II cohort is predominantly white women.
Research suggests that GDM is a heterogeneous condition with some unique phenotypes in subgroups that vary by race and ethnicity. And just as there appear to be racial-ethnic differences in the pathophysiology of GDM, there appear to be racial-ethnic differences in the progression to type 2 diabetes – a known risk factor for cardiovascular disease, said Monique Henderson, PhD, a research scientist at Kaiser Permanente Northern California (KPNC).
On the broadest level, while Asian Americans have the highest prevalence of GDM, African Americans have the highest rates of progressing to type 2 diabetes, Dr. Henderson said. Disparities “may [stem from] metabolic differences in terms of insulin resistance and secretion that are different between pregnancy and the postpartum period, and that might vary [across racial-ethnic subgroups],” she said. Lifestyle differences and variation in postpartum screening rates also may play a role.
At KPNC, where women with GDM receive calls and letters reminding them of the need for postpartum screening, only 48% overall completed an oral glucose tolerance test at 4-12 weeks post partum, as recommended by both the American Diabetes Association and the American College of Obstetricians and Gynecologists. Both before and after adjustment for education, attendance at a postpartum visit, and other variables, Chinese women were most likely to have screening, and black women were least likely, said Dr. Henderson, referring to ongoing research.
A study Dr. Ehrenthal led of women with GDM or HDP recruited from the postpartum service of a large community-based, academic obstetrical hospital in Delaware showed that while nearly all women attended a 6-week postpartum visit with their ob.gyns., 59% of women with GDM had not yet completed diabetes screening when they were interviewed 3 months post partum. Most women with HDP indicated they had follow-up blood pressure testing, and just over half of women with either diagnosis recalled having ever had lipid testing (J Women’s Health 2014;23[9]:760-4).
Women least likely to complete screening tests were those who had no college education, those who had less than a high school level of health literacy, and those who were not privately insured, Dr. Ehrenthal said.
A large national study of privately insured women also found low rates of follow-up testing, however. While the majority of women with GDM had a postpartum visit with an obstetrician or primary care physician within a year after delivery, only a minority of women had a glycemic screening test completed (Obstet Gynecol. 2016;128[1]:159-67).
“We can’t place the blame on women,” Dr. Ehrenthal said. “We need increased attention to screening,” including screening for cardiovascular disease risk factors, and a “deliberate hand-off to primary care.”
For follow-up cardiovascular disease risk factor assessment after HDP, ACOG recommends periodic (perhaps annually) assessment and referral for treatment as needed, and the cardiology professional organizations recommend that pregnancy history be considered when assessing risk in order to decide on lipid treatment, she noted.
Each of the speakers reported that they have no financial or other interests that pose a conflict of interest. The HAPO Follow-Up Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the nuMoM2b–HHS study has been funded by several National Institutes of Health institutes and other programs and initiatives.
WASHINGTON – Cardiovascular risk factors may be elevated “as soon as the first postpartum year” in women who have gestational diabetes or hypertensive disorders of pregnancy, recent findings have affirmed, Deborah B. Ehrenthal, MD, MPH, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
Dr. Ehrenthal was one of several researchers who urged innovative strategies and improved care coordination to boost women’s follow-up after gestational diabetes mellitus (GDM) and other adverse pregnancy outcomes and complications. “The metabolic stress of pregnancy can uncover underlying susceptibilities,” she said. “And ”
Evidence that adverse pregnancy outcomes – including GDM and hypertensive disorders of pregnancy (HDP) – can elevate cardiovascular risk comes most recently from the Nulliparous Pregnancy Outcomes Study – Monitoring Mothers to be Heart Health Study (nuMoM2b–HHS study), a prospective observational cohort that followed 4,484 women 2-7 years after their first pregnancy. Women had a follow-up exam, with blood pressure and anthropometric measurements and clinical/biological testing, an average of 3 years post partum.
An analysis published in October 2019 in the Journal of the American Heart Association shows that women with HDP (including preeclampsia and gestational hypertension) had a relative risk of hypertension of 2.5 at follow-up, compared with women without HDP. Women who had preeclampsia specifically were 2.3 times as likely as were women who did not have preeclampsia to have incident hypertension at follow-up, said Dr. Ehrenthal, a coinvestigator of the study.
The analysis focused on incident hypertension as the primary outcome, and adjusted for age, body mass index, and other important cardiovascular disease risk factors, she noted. Researchers utilized the diagnostic threshold for hypertension extant at the time of study design: A systolic blood pressure of 140 mm Hg or greater, or a diastolic BP of 90 mm Hg or greater (J Am Heart Assoc. 2019;8:e013092).
HDP was the most common adverse pregnancy outcome in the nuMoM2b–HHS study (14%). Among all participants, 4% had GDM. Approximately 82% had neither HDP nor GDM. Other adverse pregnancy outcomes included in the analysis were preterm birth, small-for-gestational-age birth, and stillbirth.
Additional preliminary estimates presented by Dr. Ehrenthal show that, based on the new (2017) lower threshold for hypertension – 130 mg Hg systolic or 80 mm Hg diastolic – the disorder afflicted 37% of women who had experienced HDP (relative risk 2.1), and 32% of women who had GDM (RR 1.8). Prediabetes/diabetes (using a fasting blood glucose threshold of 100 mg/dL) at follow-up affected an estimated 21% of women who had HDP (RR 1.4) and 38% of women who had GDM (RR 2.5).
Notably, across the entire study cohort, 20% had hypertension at follow-up, “which is extraordinary” considering the short time frame from pregnancy and the young age of the study population – a mean maternal age of 27 years, said Dr. Ehrenthal, associate professor of population health sciences and obstetrics & gynecology at the University of Wisconsin, Madison.
Also across the cohort, 15% had prediabetes/diabetes at follow-up. “We need to think about women more generally,” she cautioned. “While we recognize the significant elevated risk of HDP and GDM [for the development of subsequent hypertension and cardiovascular risk], we will miss a lot of women [if we focus only on the history of HDP and GDM.]”
The majority of women found to have hypertension or prediabetes/diabetes at follow-up had experienced neither HDP nor GDM, but a good many of them (47% of those who had hypertension and 47% of those found to have prediabetes/diabetes) had a BMI of 30 or above, Dr. Ehrenthal said at the DPSG-NA meeting.
Nurses Health Study, hyperglycemia and adverse pregnancy outcome follow-up data
The new findings from the nuMoM2b–HHS study add to a robust and growing body of evidence that pregnancy is an important window to future health, and that follow up and screening after GDM and HDP are crucial.
Regarding GDM specifically, “there’s quite a bit of literature by now demonstrating that GDM history is a risk factor for hypertension, even 1-2 years post partum, and that the risk is elevated as well for dyslipidemia and vascular dysfunction,” Deirdre K. Tobias, D.Sc., an epidemiologist at Brigham and Women’s Hospital and assistant professor of nutrition at Harvard TH Chan School of Public Health, Boston, said at the DPSG meeting.
An analysis of the Nurses Health Study II (NHS II) cohort published in 2017 found a 40% higher relative risk of cardiovascular disease events (largely myocardial infarction) in women who had GDM, compared with women who did not have GDM over a median follow-up of 26 years. This was after adjustments were made for age, time since pregnancy, menopausal status, family history of MI or stroke, hypertension in pregnancy, white race/ethnicity, prepregnancy BMI, and other factors (JAMA Intern Med. 2017;177[12]:1735-42).
The NHS data also have shown, however, that the elevated risk for cardiovascular disease after a GDM pregnancy “can be mitigated by adopting a healthy lifestyle,” said Dr. Tobias, lead author of the 2017 NHS II analysis. Adjustments for postpregnancy weight gain and lifestyle factors attenuated the relative risk of cardiovascular disease events after a GDM pregnancy to a 30% increased risk.
Dr. Tobias and colleagues currently are looking within the NHS cohort for “metabolomic signatures” or signals – various amino acid and lipid metabolites – to identify the progression of GDM to type 2 diabetes. Metabolomics “may help further refine our understanding of the long-term links between GDM and prevention of type 2 diabetes and of cardiovascular disease in mothers,” she said.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Follow-Up Study, in the meantime, is documenting associations of maternal glucose levels during pregnancy not only with prediabetes or type 2 diabetes 10-14 years later, but also with measures of cardiovascular risk in mothers 10-14 years later.
Just as perinatal outcomes were strongly associated with glucose as a continuous variable in the original HAPO study, “it’s clear there’s a progressive increase in the risk of [later] disorders of glucose metabolism as [fasting blood glucose levels and 1- and-2-hour glucose values] in pregnancy are higher,” said Boyd E. Metzger, MD, the Tom D. Spies emeritus professor of metabolism and nutrition at Northwestern University, Chicago, and principal investigator of the original HAPO study and its follow up.
“Another message is that the more normal you are in pregnancy, the more normal you will be many years later. Good values [during pregnancy] produce good outcomes.”
Currently unpublished data from the HAPO Follow-Up Study are being analyzed, but it appears thus far that GDM is not associated with hypertension (per the old diagnostic threshold) in this cohort after adjustment for maternal age, BMI, smoking, and family history of hypertension. GDM appears to be a significant risk factor for dyslipidemia, however. HDL cholesterol at follow-up was significantly lower for mothers who had GDM compared with those without, whereas LDL cholesterol and triglycerides at follow-up were significantly higher for mothers with GDM, Dr. Metzger said.
Racial/ethnic disparities, postpartum care
Neither long-term study – the NHS II or the HAPO Follow-Up Study – has looked at racial and ethnic differences. The HAPO cohort is racially-ethnically diverse but the NHS II cohort is predominantly white women.
Research suggests that GDM is a heterogeneous condition with some unique phenotypes in subgroups that vary by race and ethnicity. And just as there appear to be racial-ethnic differences in the pathophysiology of GDM, there appear to be racial-ethnic differences in the progression to type 2 diabetes – a known risk factor for cardiovascular disease, said Monique Henderson, PhD, a research scientist at Kaiser Permanente Northern California (KPNC).
On the broadest level, while Asian Americans have the highest prevalence of GDM, African Americans have the highest rates of progressing to type 2 diabetes, Dr. Henderson said. Disparities “may [stem from] metabolic differences in terms of insulin resistance and secretion that are different between pregnancy and the postpartum period, and that might vary [across racial-ethnic subgroups],” she said. Lifestyle differences and variation in postpartum screening rates also may play a role.
At KPNC, where women with GDM receive calls and letters reminding them of the need for postpartum screening, only 48% overall completed an oral glucose tolerance test at 4-12 weeks post partum, as recommended by both the American Diabetes Association and the American College of Obstetricians and Gynecologists. Both before and after adjustment for education, attendance at a postpartum visit, and other variables, Chinese women were most likely to have screening, and black women were least likely, said Dr. Henderson, referring to ongoing research.
A study Dr. Ehrenthal led of women with GDM or HDP recruited from the postpartum service of a large community-based, academic obstetrical hospital in Delaware showed that while nearly all women attended a 6-week postpartum visit with their ob.gyns., 59% of women with GDM had not yet completed diabetes screening when they were interviewed 3 months post partum. Most women with HDP indicated they had follow-up blood pressure testing, and just over half of women with either diagnosis recalled having ever had lipid testing (J Women’s Health 2014;23[9]:760-4).
Women least likely to complete screening tests were those who had no college education, those who had less than a high school level of health literacy, and those who were not privately insured, Dr. Ehrenthal said.
A large national study of privately insured women also found low rates of follow-up testing, however. While the majority of women with GDM had a postpartum visit with an obstetrician or primary care physician within a year after delivery, only a minority of women had a glycemic screening test completed (Obstet Gynecol. 2016;128[1]:159-67).
“We can’t place the blame on women,” Dr. Ehrenthal said. “We need increased attention to screening,” including screening for cardiovascular disease risk factors, and a “deliberate hand-off to primary care.”
For follow-up cardiovascular disease risk factor assessment after HDP, ACOG recommends periodic (perhaps annually) assessment and referral for treatment as needed, and the cardiology professional organizations recommend that pregnancy history be considered when assessing risk in order to decide on lipid treatment, she noted.
Each of the speakers reported that they have no financial or other interests that pose a conflict of interest. The HAPO Follow-Up Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the nuMoM2b–HHS study has been funded by several National Institutes of Health institutes and other programs and initiatives.
REPORTING FROM THE DPSG-NA 2019
Subclinical hypothyroidism and pregnancy: Public health problem or lab finding with minimal clinical significance?
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.
Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
U.S. infant mortality continued slow decline in 2017
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.
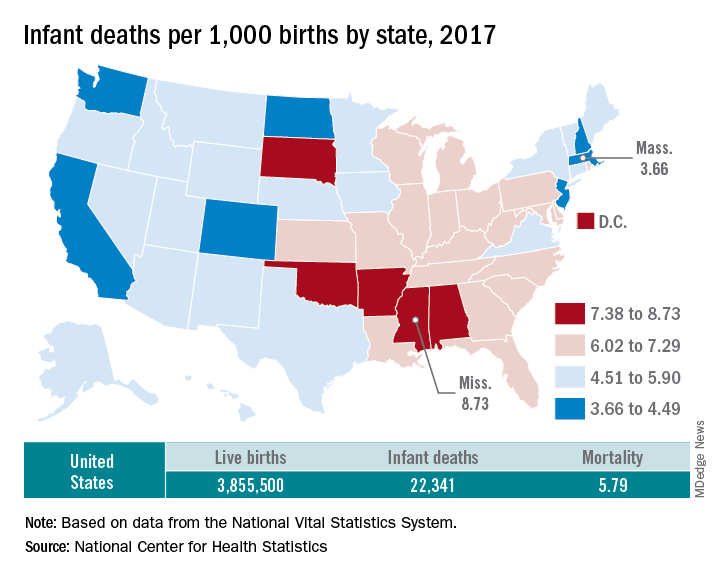
The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.

The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.

The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
U.S. fertility rate, teen births are on the decline
The general fertility rate in the United States decreased 2% between 2017 and 2018, according to a report from the Centers for Disease Control and Prevention.
Fertility rates, defined as births per 1,000 women aged 15-44 years, declined for all racial/ethnic groups studied.
Teen birth rates, or births among girls aged 15-19 years, declined from 2017 to 2018 as well.
These data come from the National Vital Statistics System’s Natality Data File, which includes information from birth certificates for all births in the United States.
The data show a decline in the general fertility rate from 60.3 per 1,000 women in 2017 to 59.1 per 1,000 women in 2018, a significant decrease (P less than .05).
Fertility rates declined across the three largest racial/ethnic groups studied, decreasing:
- 3% in Hispanic women, from 67.6 to 65.9 per 1,000.
- 2% in non-Hispanic black women, from 63.1 to 62.0 per 1,000.
- 2% in non-Hispanic white women, from 57.2 to 56.3 per 1,000.
Similarly, teen birth rates declined 7% from 2017 to 2018, decreasing from 18.8 to 17.4 births per 1,000 girls aged 15-19 years (P less than .05). Rates decreased:
- 8% in Hispanic teens, from 28.9 to 26.7 per 1,000.
- 4% in non-Hispanic black teens, from 27.5 to 26.3 per 1,000.
- 8% in non-Hispanic white teens, from 13.2 to 12.1 per 1,000.
The data also show an increase in the rate of vaginal births after previous cesarean (VBAC) delivery. The percentage of VBAC deliveries increased from 12.8% in 2017 to 13.3% in 2018 (P less than .05).
VBAC delivery rates increased across all racial/ethnic groups studied, although the increase among non-Hispanic back women was not significant.
Finally, the report shows an increase in preterm and early term births from 2017 to 2018. Preterm deliveries (less than 37 weeks of gestation) increased from 9.93% to 10.02%, and early term deliveries (37-38 weeks) increased from 26.00% to 26.53% (P less than .05).
At the same time, full-term births (39-40 weeks) decreased from 57.49% to 57.24%, and late- and post-term births (41 weeks or more) decreased from 6.58 % to 6.20% (P less than .05). These findings were consistent across the racial/ethnic groups studied.
SOURCE: Martin JA et al. NCHS Data Brief. 2019 July; no 346.
The general fertility rate in the United States decreased 2% between 2017 and 2018, according to a report from the Centers for Disease Control and Prevention.
Fertility rates, defined as births per 1,000 women aged 15-44 years, declined for all racial/ethnic groups studied.
Teen birth rates, or births among girls aged 15-19 years, declined from 2017 to 2018 as well.
These data come from the National Vital Statistics System’s Natality Data File, which includes information from birth certificates for all births in the United States.
The data show a decline in the general fertility rate from 60.3 per 1,000 women in 2017 to 59.1 per 1,000 women in 2018, a significant decrease (P less than .05).
Fertility rates declined across the three largest racial/ethnic groups studied, decreasing:
- 3% in Hispanic women, from 67.6 to 65.9 per 1,000.
- 2% in non-Hispanic black women, from 63.1 to 62.0 per 1,000.
- 2% in non-Hispanic white women, from 57.2 to 56.3 per 1,000.
Similarly, teen birth rates declined 7% from 2017 to 2018, decreasing from 18.8 to 17.4 births per 1,000 girls aged 15-19 years (P less than .05). Rates decreased:
- 8% in Hispanic teens, from 28.9 to 26.7 per 1,000.
- 4% in non-Hispanic black teens, from 27.5 to 26.3 per 1,000.
- 8% in non-Hispanic white teens, from 13.2 to 12.1 per 1,000.
The data also show an increase in the rate of vaginal births after previous cesarean (VBAC) delivery. The percentage of VBAC deliveries increased from 12.8% in 2017 to 13.3% in 2018 (P less than .05).
VBAC delivery rates increased across all racial/ethnic groups studied, although the increase among non-Hispanic back women was not significant.
Finally, the report shows an increase in preterm and early term births from 2017 to 2018. Preterm deliveries (less than 37 weeks of gestation) increased from 9.93% to 10.02%, and early term deliveries (37-38 weeks) increased from 26.00% to 26.53% (P less than .05).
At the same time, full-term births (39-40 weeks) decreased from 57.49% to 57.24%, and late- and post-term births (41 weeks or more) decreased from 6.58 % to 6.20% (P less than .05). These findings were consistent across the racial/ethnic groups studied.
SOURCE: Martin JA et al. NCHS Data Brief. 2019 July; no 346.
The general fertility rate in the United States decreased 2% between 2017 and 2018, according to a report from the Centers for Disease Control and Prevention.
Fertility rates, defined as births per 1,000 women aged 15-44 years, declined for all racial/ethnic groups studied.
Teen birth rates, or births among girls aged 15-19 years, declined from 2017 to 2018 as well.
These data come from the National Vital Statistics System’s Natality Data File, which includes information from birth certificates for all births in the United States.
The data show a decline in the general fertility rate from 60.3 per 1,000 women in 2017 to 59.1 per 1,000 women in 2018, a significant decrease (P less than .05).
Fertility rates declined across the three largest racial/ethnic groups studied, decreasing:
- 3% in Hispanic women, from 67.6 to 65.9 per 1,000.
- 2% in non-Hispanic black women, from 63.1 to 62.0 per 1,000.
- 2% in non-Hispanic white women, from 57.2 to 56.3 per 1,000.
Similarly, teen birth rates declined 7% from 2017 to 2018, decreasing from 18.8 to 17.4 births per 1,000 girls aged 15-19 years (P less than .05). Rates decreased:
- 8% in Hispanic teens, from 28.9 to 26.7 per 1,000.
- 4% in non-Hispanic black teens, from 27.5 to 26.3 per 1,000.
- 8% in non-Hispanic white teens, from 13.2 to 12.1 per 1,000.
The data also show an increase in the rate of vaginal births after previous cesarean (VBAC) delivery. The percentage of VBAC deliveries increased from 12.8% in 2017 to 13.3% in 2018 (P less than .05).
VBAC delivery rates increased across all racial/ethnic groups studied, although the increase among non-Hispanic back women was not significant.
Finally, the report shows an increase in preterm and early term births from 2017 to 2018. Preterm deliveries (less than 37 weeks of gestation) increased from 9.93% to 10.02%, and early term deliveries (37-38 weeks) increased from 26.00% to 26.53% (P less than .05).
At the same time, full-term births (39-40 weeks) decreased from 57.49% to 57.24%, and late- and post-term births (41 weeks or more) decreased from 6.58 % to 6.20% (P less than .05). These findings were consistent across the racial/ethnic groups studied.
SOURCE: Martin JA et al. NCHS Data Brief. 2019 July; no 346.