User login
LARC prolongs interpregnancy intervals but doesn’t cut preterm birth risk
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
REPORTING FROM ACOG 2019
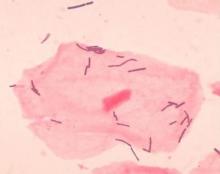
Diverse vaginal microbiome may signal risk for preterm birth
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
FROM NATURE MEDICINE
In women with late preterm mild hypertensive disorders, does immediate delivery versus expectant management differ in terms of neonatal neurodevelopmental outcomes?
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
Zwertbroek EF, Franssen MT, Broekhuijsen K, et al; HYPITAT-II Study Group. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring of mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2019.03.024.
EXPERT COMMENTARY
In women with mild hypertensive disorders in the preterm period, the maternal benefits of delivery should be weighed against the consequences of preterm birth for the neonate. In a recent study, Zwertbroek and colleagues sought to evaluate the long-term neurodevelopmental effects of this decision on the offspring.
Details of the study
The authors conducted a follow-up study of the randomized, controlled Hypertension and Preeclampsia Intervention Trial At Term II (HYPITAT-II), in which 704 women diagnosed with late preterm (34–37 weeks) hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension, or mild preeclampsia) were randomly assigned to immediate delivery or expectant management.
Expectant management consisted of close monitoring until 37 weeks or until an indication for delivery occurred, whichever came first. Children born to those mothers were eligible for this study (women enrolled during 2011–2015) when they reached 2 years of age; 342 children were included in this analysis. Of note, children from the expectant management group had been delivered at a more advanced gestational age (median, 37.0 vs 36.1 weeks; P<.001) than those in the immediate-delivery group.
Survey tools. Parents completed 2 response surveys, the Ages and Stages Questionnaire (ASQ) and the Child Behavior Checklist (CBCL), between 23 and 26 months’ corrected age. The ASQ is designed to detect developmental delay, while the CBCL assesses behavioral and emotional problems. The primary outcome was an abnormal result on either screen.
Results. Based on 330 returned questionnaires, the authors found more abnormal ASQ scores (45 of 162 [28%] vs 27 of 148 [18%] children; P = .045) in the immediate-delivery group versus the expectant management group, most pronounced in the fine motor domain. They found no difference in the CBCL scores. The authors concluded that immediate delivery for women with late preterm mild hypertensive disorders in pregnancy increases the risk of developmental delay in the children.
Study strengths and limitations
This study is unique as a planned follow-up to a randomized, controlled trial, allowing for 2-year outcomes to be assessed on children of enrolled women with mild hypertensive disorders in the late preterm period. The authors used validated surveys that are known to predict long-term neurodevelopmental outcomes.
Continue to: This work has several limitations...
This work has several limitations, however. Randomization was not truly maintained given the less than 50% response rate of original participants. Additionally, parents completed the surveys and provider confirmation of developmental concerns or diagnoses was not obtained. Further, assessments at 2 years of age may be too early to detect subtle differences, with evaluations at 5 years more predictive of long-term outcomes; the authors stated that these data already are being collected.
Finally, while these data importantly reinforce the conclusions of the parent HYPITAT-II trial, which support expectant management for mild hypertensive disorders in the late preterm period,1 clinicians must always take care to individualize decisions in the face of worsening maternal disease.
This follow-up study of the HYPITAT-II randomized, controlled trial demonstrates poorer neurodevelopmental outcomes in offspring of late preterm mild hypertensives who undergo immediate delivery. These data support current practice recommendations to expectantly manage women with late preterm mild hypertensive disease until 37 weeks or signs of clinical worsening, whichever comes first.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
- Broekjuijsen K, van Baaren GJ, van Pampus MG, et al; HYPITAT-II Study Group. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385:2492-2501.
Marijuana during prenatal OUD treatment increases premature birth
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
REPORTING FROM PAS 2019
Key clinical point: Warn pregnant women being treated for opioid use disorder to stay away from marijuana.
Major finding: Marijuana use more than doubled the risk of prematurity and low birth weight.
Study details: Review of 2,375 opioid-exposed pregnancies at six hospitals
Disclosures: There was no industry funding for the work, and the lead investigator had no disclosures.
Antenatal steroids for preterm birth is cost effective
Administering antenatal corticosteroids to pregnant women at high risk for preterm birth was a cost-effective intervention that improved infant respiratory outcomes, according to a new study.
“This intervention has a potential cost saving in the United States of approximately $100 million dollars annually from the benefit in the immediate neonatal outcome alone,” Cynthia Gyamfi-Bannerman, MD, of Columbia University, New York, and her associates reported in JAMA Pediatrics. “Because late preterm birth comprises a large proportion of all preterm births, our findings have the potential for a large influence on public health.”
The researchers conducted a retrospective secondary analysis of the randomized Antenatal Late Preterm Steroids (ALPS) clinical trial October 2010 to February 2015. The trial enrolled randomly assigned antenatal administration of betamethasone or placebo to women pregnant with a singleton and at high risk for preterm birth while between 34 weeks, 6 days, and 36 weeks, 0 days, of gestation.
Antenatal corticosteroid administration was regarded as effective if a newborn did not require treatment in the first 72 hours for respiratory distress or illness. Treatment could include “continuous positive airway pressure or high-flow nasal cannula for 2 hours or more, supplemental oxygen with a fraction of inspired oxygen of 30% or more for 4 hours or more, and extracorporeal membrane oxygenation or mechanical ventilation,” Dr. Gyamfi-Bannerman and her associates wrote.
To tally the costs, the researchers used Medicaid rates to estimate the total in 2015 U.S. dollars for betamethasone, outpatient visits or inpatient stays to administer it, and all direct newborn care costs, including neonatal ICU daily costs stratified by respiratory illness severity. Betamethasone administration included an initial 12-mg intramuscular dose followed by another after 24 hours if the infant had not been delivered.
“Because therapy often persists for longer than this 72-hour duration, we measured costs through hospital discharge,” the authors wrote. “The analysis took the perspective of a third-party payer in which we included direct medical costs and associated overhead accruing to hospitals and medical payers for the care of enrolled patients and their infants.”
Among 2,821 mothers not lost to follow-up during the secondary analysis, 1,426 received betamethasone and 1,395 received placebo. For mothers who received betamethasone antenatally, the total mean cost was $4,681 per mother-infant pair. Total mean cost for those in the placebo group was $5,379 per pair, resulting in a significant mean $698 savings (P = .02). Respiratory morbidity was 2.9% lower in infants whose mothers received antenatal corticosteroid treatment.
“Thus, because the treated group had lower costs and this strategy was more effective, administration of betamethasone to women at risk for late preterm birth was judged to be a dominant strategy, which is defined as one in which costs are lower and effectiveness is higher than a comparator (incremental cost-effectiveness ratio [ICER], −23 986),” Dr. Gyamfi-Bannerman and her associates reported. ICER is defined as the difference in mean total cost per patient in the betamethasone and placebo arms divided by the difference in the effectiveness.
Study limitations were an inability to estimate costs according to quality-adjusted life years or to include families’/caregivers’ costs.
SOURCE: Gyamfi-Bannerman C. JAMA Pediatr. 2019 Mar 11. doi: 10.1001/jamapediatrics.2019.0032.
Administering antenatal corticosteroids to pregnant women at high risk for preterm birth was a cost-effective intervention that improved infant respiratory outcomes, according to a new study.
“This intervention has a potential cost saving in the United States of approximately $100 million dollars annually from the benefit in the immediate neonatal outcome alone,” Cynthia Gyamfi-Bannerman, MD, of Columbia University, New York, and her associates reported in JAMA Pediatrics. “Because late preterm birth comprises a large proportion of all preterm births, our findings have the potential for a large influence on public health.”
The researchers conducted a retrospective secondary analysis of the randomized Antenatal Late Preterm Steroids (ALPS) clinical trial October 2010 to February 2015. The trial enrolled randomly assigned antenatal administration of betamethasone or placebo to women pregnant with a singleton and at high risk for preterm birth while between 34 weeks, 6 days, and 36 weeks, 0 days, of gestation.
Antenatal corticosteroid administration was regarded as effective if a newborn did not require treatment in the first 72 hours for respiratory distress or illness. Treatment could include “continuous positive airway pressure or high-flow nasal cannula for 2 hours or more, supplemental oxygen with a fraction of inspired oxygen of 30% or more for 4 hours or more, and extracorporeal membrane oxygenation or mechanical ventilation,” Dr. Gyamfi-Bannerman and her associates wrote.
To tally the costs, the researchers used Medicaid rates to estimate the total in 2015 U.S. dollars for betamethasone, outpatient visits or inpatient stays to administer it, and all direct newborn care costs, including neonatal ICU daily costs stratified by respiratory illness severity. Betamethasone administration included an initial 12-mg intramuscular dose followed by another after 24 hours if the infant had not been delivered.
“Because therapy often persists for longer than this 72-hour duration, we measured costs through hospital discharge,” the authors wrote. “The analysis took the perspective of a third-party payer in which we included direct medical costs and associated overhead accruing to hospitals and medical payers for the care of enrolled patients and their infants.”
Among 2,821 mothers not lost to follow-up during the secondary analysis, 1,426 received betamethasone and 1,395 received placebo. For mothers who received betamethasone antenatally, the total mean cost was $4,681 per mother-infant pair. Total mean cost for those in the placebo group was $5,379 per pair, resulting in a significant mean $698 savings (P = .02). Respiratory morbidity was 2.9% lower in infants whose mothers received antenatal corticosteroid treatment.
“Thus, because the treated group had lower costs and this strategy was more effective, administration of betamethasone to women at risk for late preterm birth was judged to be a dominant strategy, which is defined as one in which costs are lower and effectiveness is higher than a comparator (incremental cost-effectiveness ratio [ICER], −23 986),” Dr. Gyamfi-Bannerman and her associates reported. ICER is defined as the difference in mean total cost per patient in the betamethasone and placebo arms divided by the difference in the effectiveness.
Study limitations were an inability to estimate costs according to quality-adjusted life years or to include families’/caregivers’ costs.
SOURCE: Gyamfi-Bannerman C. JAMA Pediatr. 2019 Mar 11. doi: 10.1001/jamapediatrics.2019.0032.
Administering antenatal corticosteroids to pregnant women at high risk for preterm birth was a cost-effective intervention that improved infant respiratory outcomes, according to a new study.
“This intervention has a potential cost saving in the United States of approximately $100 million dollars annually from the benefit in the immediate neonatal outcome alone,” Cynthia Gyamfi-Bannerman, MD, of Columbia University, New York, and her associates reported in JAMA Pediatrics. “Because late preterm birth comprises a large proportion of all preterm births, our findings have the potential for a large influence on public health.”
The researchers conducted a retrospective secondary analysis of the randomized Antenatal Late Preterm Steroids (ALPS) clinical trial October 2010 to February 2015. The trial enrolled randomly assigned antenatal administration of betamethasone or placebo to women pregnant with a singleton and at high risk for preterm birth while between 34 weeks, 6 days, and 36 weeks, 0 days, of gestation.
Antenatal corticosteroid administration was regarded as effective if a newborn did not require treatment in the first 72 hours for respiratory distress or illness. Treatment could include “continuous positive airway pressure or high-flow nasal cannula for 2 hours or more, supplemental oxygen with a fraction of inspired oxygen of 30% or more for 4 hours or more, and extracorporeal membrane oxygenation or mechanical ventilation,” Dr. Gyamfi-Bannerman and her associates wrote.
To tally the costs, the researchers used Medicaid rates to estimate the total in 2015 U.S. dollars for betamethasone, outpatient visits or inpatient stays to administer it, and all direct newborn care costs, including neonatal ICU daily costs stratified by respiratory illness severity. Betamethasone administration included an initial 12-mg intramuscular dose followed by another after 24 hours if the infant had not been delivered.
“Because therapy often persists for longer than this 72-hour duration, we measured costs through hospital discharge,” the authors wrote. “The analysis took the perspective of a third-party payer in which we included direct medical costs and associated overhead accruing to hospitals and medical payers for the care of enrolled patients and their infants.”
Among 2,821 mothers not lost to follow-up during the secondary analysis, 1,426 received betamethasone and 1,395 received placebo. For mothers who received betamethasone antenatally, the total mean cost was $4,681 per mother-infant pair. Total mean cost for those in the placebo group was $5,379 per pair, resulting in a significant mean $698 savings (P = .02). Respiratory morbidity was 2.9% lower in infants whose mothers received antenatal corticosteroid treatment.
“Thus, because the treated group had lower costs and this strategy was more effective, administration of betamethasone to women at risk for late preterm birth was judged to be a dominant strategy, which is defined as one in which costs are lower and effectiveness is higher than a comparator (incremental cost-effectiveness ratio [ICER], −23 986),” Dr. Gyamfi-Bannerman and her associates reported. ICER is defined as the difference in mean total cost per patient in the betamethasone and placebo arms divided by the difference in the effectiveness.
Study limitations were an inability to estimate costs according to quality-adjusted life years or to include families’/caregivers’ costs.
SOURCE: Gyamfi-Bannerman C. JAMA Pediatr. 2019 Mar 11. doi: 10.1001/jamapediatrics.2019.0032.
FROM JAMA PEDIATRICS
Omega-3 fatty acid supplementation reduces risk of preterm birth
Taking omega-3 long-chain polyunsaturated fatty acids during pregnancy was associated with reduced risk of preterm birth, and also may reduce the risk of babies born at a low birth weight and risk of requiring neonatal intensive care, according to a Cochrane review of 70 randomized controlled trials.
“There are not many options for preventing premature birth, so these new findings are very important for pregnant women, babies, and the health professionals who care for them,” Philippa Middleton, MPH, PhD, of Cochrane Pregnancy and Childbirth Group and the South Australian Health and Medical Research Institute, in Adelaide, stated in a press release. “We don’t yet fully understand the causes of premature labor, so predicting and preventing early birth has always been a challenge. This is one of the reasons omega-3 supplementation in pregnancy is of such great interest to researchers around the world.”
Dr. Middleton and her colleagues performed a search of the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform and identified 70 randomized controlled trials (RCTs) where 19,927 women at varying levels of risk for preterm birth received omega-3 long-chain polyunsaturated fatty acids (LCPUFA), placebo, or no omega-3.
“Many pregnant women in the UK are already taking omega-3 supplements by personal choice rather than as a result of advice from health professionals,” Dr. Middleton said in the release. “It’s worth noting though that many supplements currently on the market don’t contain the optimal dose or type of omega-3 for preventing premature birth. Our review found the optimum dose was a daily supplement containing between 500 and 1,000 milligrams of long-chain omega-3 fats (containing at least 500 mg of DHA [docosahexaenoic acid]) starting at 12 weeks of pregnancy.”
In 26 RCTs (10,304 women), the risk of preterm birth under 37 weeks was 11% lower for women who took omega-3 LCPUFA compared with women who did not take omega-3 (relative risk, 0.89; 95% confidence interval, 0.81-0.97), while the risk for preterm birth under 34 weeks in 9 RCTs (5,204 women) was 42% lower for women compared with women who did not take omega-3 (RR, 0.58; 95% CI, 0.44-0.77).
With regard to infant health, use of omega-3 LCPUFA during pregnancy was associated in 10 RCTs (7,416 women) with a potential reduced risk of perinatal mortality (RR, 0.75; 95% CI, 0.54-1.03) and, in 9 RCTs (6,920 women), a reduced risk of neonatal intensive care admission (RR, 0.92; 95% CI, 0.83-1.03). The researchers noted that omega-3 use in 15 trials (8,449 women) was potentially associated with a reduced number of babies with low birth weight (RR, 0.90; 95% CI, 0.82-0.99), but an increase in babies who were large for their gestational age in 3,722 women from 6 RCTs (RR, 1.15; 95% CI, 0.97-1.36). There was no significant difference among groups with regard to babies who were born small for their gestational age or in uterine growth restriction, they said.
While maternal outcomes were examined, Dr. Middleton and her colleagues found no significant differences between groups in factors such as postterm induction, serious adverse events, admission to intensive care, and postnatal depression.
“Ultimately, we hope this review will make a real contribution to the evidence base we need to reduce premature births, which continue to be one of the most pressing and intractable maternal and child health problems in every country around the world,” Dr. Middleton said.
The National Institutes of Health funded the review. The authors reported no conflicts of interest.
SOURCE: Middleton P et al. Cochrane Database Syst Rev. 2018; doi: 10.1002/14651858.CD003402.pub3.
Taking omega-3 long-chain polyunsaturated fatty acids during pregnancy was associated with reduced risk of preterm birth, and also may reduce the risk of babies born at a low birth weight and risk of requiring neonatal intensive care, according to a Cochrane review of 70 randomized controlled trials.
“There are not many options for preventing premature birth, so these new findings are very important for pregnant women, babies, and the health professionals who care for them,” Philippa Middleton, MPH, PhD, of Cochrane Pregnancy and Childbirth Group and the South Australian Health and Medical Research Institute, in Adelaide, stated in a press release. “We don’t yet fully understand the causes of premature labor, so predicting and preventing early birth has always been a challenge. This is one of the reasons omega-3 supplementation in pregnancy is of such great interest to researchers around the world.”
Dr. Middleton and her colleagues performed a search of the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform and identified 70 randomized controlled trials (RCTs) where 19,927 women at varying levels of risk for preterm birth received omega-3 long-chain polyunsaturated fatty acids (LCPUFA), placebo, or no omega-3.
“Many pregnant women in the UK are already taking omega-3 supplements by personal choice rather than as a result of advice from health professionals,” Dr. Middleton said in the release. “It’s worth noting though that many supplements currently on the market don’t contain the optimal dose or type of omega-3 for preventing premature birth. Our review found the optimum dose was a daily supplement containing between 500 and 1,000 milligrams of long-chain omega-3 fats (containing at least 500 mg of DHA [docosahexaenoic acid]) starting at 12 weeks of pregnancy.”
In 26 RCTs (10,304 women), the risk of preterm birth under 37 weeks was 11% lower for women who took omega-3 LCPUFA compared with women who did not take omega-3 (relative risk, 0.89; 95% confidence interval, 0.81-0.97), while the risk for preterm birth under 34 weeks in 9 RCTs (5,204 women) was 42% lower for women compared with women who did not take omega-3 (RR, 0.58; 95% CI, 0.44-0.77).
With regard to infant health, use of omega-3 LCPUFA during pregnancy was associated in 10 RCTs (7,416 women) with a potential reduced risk of perinatal mortality (RR, 0.75; 95% CI, 0.54-1.03) and, in 9 RCTs (6,920 women), a reduced risk of neonatal intensive care admission (RR, 0.92; 95% CI, 0.83-1.03). The researchers noted that omega-3 use in 15 trials (8,449 women) was potentially associated with a reduced number of babies with low birth weight (RR, 0.90; 95% CI, 0.82-0.99), but an increase in babies who were large for their gestational age in 3,722 women from 6 RCTs (RR, 1.15; 95% CI, 0.97-1.36). There was no significant difference among groups with regard to babies who were born small for their gestational age or in uterine growth restriction, they said.
While maternal outcomes were examined, Dr. Middleton and her colleagues found no significant differences between groups in factors such as postterm induction, serious adverse events, admission to intensive care, and postnatal depression.
“Ultimately, we hope this review will make a real contribution to the evidence base we need to reduce premature births, which continue to be one of the most pressing and intractable maternal and child health problems in every country around the world,” Dr. Middleton said.
The National Institutes of Health funded the review. The authors reported no conflicts of interest.
SOURCE: Middleton P et al. Cochrane Database Syst Rev. 2018; doi: 10.1002/14651858.CD003402.pub3.
Taking omega-3 long-chain polyunsaturated fatty acids during pregnancy was associated with reduced risk of preterm birth, and also may reduce the risk of babies born at a low birth weight and risk of requiring neonatal intensive care, according to a Cochrane review of 70 randomized controlled trials.
“There are not many options for preventing premature birth, so these new findings are very important for pregnant women, babies, and the health professionals who care for them,” Philippa Middleton, MPH, PhD, of Cochrane Pregnancy and Childbirth Group and the South Australian Health and Medical Research Institute, in Adelaide, stated in a press release. “We don’t yet fully understand the causes of premature labor, so predicting and preventing early birth has always been a challenge. This is one of the reasons omega-3 supplementation in pregnancy is of such great interest to researchers around the world.”
Dr. Middleton and her colleagues performed a search of the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform and identified 70 randomized controlled trials (RCTs) where 19,927 women at varying levels of risk for preterm birth received omega-3 long-chain polyunsaturated fatty acids (LCPUFA), placebo, or no omega-3.
“Many pregnant women in the UK are already taking omega-3 supplements by personal choice rather than as a result of advice from health professionals,” Dr. Middleton said in the release. “It’s worth noting though that many supplements currently on the market don’t contain the optimal dose or type of omega-3 for preventing premature birth. Our review found the optimum dose was a daily supplement containing between 500 and 1,000 milligrams of long-chain omega-3 fats (containing at least 500 mg of DHA [docosahexaenoic acid]) starting at 12 weeks of pregnancy.”
In 26 RCTs (10,304 women), the risk of preterm birth under 37 weeks was 11% lower for women who took omega-3 LCPUFA compared with women who did not take omega-3 (relative risk, 0.89; 95% confidence interval, 0.81-0.97), while the risk for preterm birth under 34 weeks in 9 RCTs (5,204 women) was 42% lower for women compared with women who did not take omega-3 (RR, 0.58; 95% CI, 0.44-0.77).
With regard to infant health, use of omega-3 LCPUFA during pregnancy was associated in 10 RCTs (7,416 women) with a potential reduced risk of perinatal mortality (RR, 0.75; 95% CI, 0.54-1.03) and, in 9 RCTs (6,920 women), a reduced risk of neonatal intensive care admission (RR, 0.92; 95% CI, 0.83-1.03). The researchers noted that omega-3 use in 15 trials (8,449 women) was potentially associated with a reduced number of babies with low birth weight (RR, 0.90; 95% CI, 0.82-0.99), but an increase in babies who were large for their gestational age in 3,722 women from 6 RCTs (RR, 1.15; 95% CI, 0.97-1.36). There was no significant difference among groups with regard to babies who were born small for their gestational age or in uterine growth restriction, they said.
While maternal outcomes were examined, Dr. Middleton and her colleagues found no significant differences between groups in factors such as postterm induction, serious adverse events, admission to intensive care, and postnatal depression.
“Ultimately, we hope this review will make a real contribution to the evidence base we need to reduce premature births, which continue to be one of the most pressing and intractable maternal and child health problems in every country around the world,” Dr. Middleton said.
The National Institutes of Health funded the review. The authors reported no conflicts of interest.
SOURCE: Middleton P et al. Cochrane Database Syst Rev. 2018; doi: 10.1002/14651858.CD003402.pub3.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point:
Major finding: In 26 randomized controlled trials, the risk of preterm birth at 37 weeks (10,304 women) was 11% lower and the risk of preterm birth at 34 weeks (5,204 women) in 9 RCTs was 42% lower for women taking omega-3, compared with women not taking omega-3.
Study details: A Cochrane review of 70 RCTs with a total of 19,927 women at varying levels of risk for preterm birth who received omega-3 long-chain polyunsaturated fatty acids, placebo, or no omega-3.
Disclosures: The National Institutes of Health funded the review. The authors reported no conflicts of interest.
Source: Middleton P et al. Cochrane Database Syst Rev. 2018. doi: 10.1002/14651858.CD003402.pub3.
Platelet transfusion threshold matters for preterm infants
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The odds of new major bleeding or death were 57% higher in preterm infants who received a platelet transfusion at a higher threshold of 50,000 per mm3 than at a lower threshold of 25,000 mm3 (P = .02).Study details: Randomized study in 660 preterm infants with severe thrombocytopenia.
Disclosures: The study was supported by the National Health Service Blood and Transplant Research and Development Committee, and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
Source: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
Does the preterm birth racial disparity persist among black and white IVF users?
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1
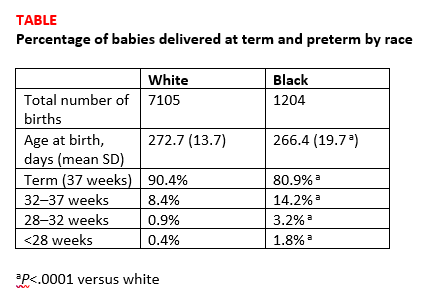
Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1

Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1

Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
Diagnosing placenta accreta spectrum with prenatal ultrasound
Placenta accreta spectrum (PAS) describes abnormal invasion of placental tissue into or through the myometrium, comprising 3 distinct conditions: placenta accreta, placenta increta, and placenta percreta. This complication is relatively new to obstetrics, first described in 1937.1
The overall incidence of PAS has been increasing over several decades, in parallel to an increasing rate of cesarean delivery (CD), with an incidence from 1982 through 2002 of 1 in 533 pregnancies, representing a 5-fold increase since the 1980s.2 PAS is associated with significant morbidity and mortality, including fetal growth restriction, preterm delivery, placental abruption antenatally, and hemorrhage during delivery or postpartum.
Prenatal diagnosis of PAS and planned delivery at an experienced center are associated with significant reduction in maternal and fetal morbidity.3 In an era of advanced imaging modalities, prenatal detection of PAS regrettably remains variable and largely subjective: As many as 20% to 50% of cases of PAS escape prenatal diagnosis.3,4
In this article, we review the sonographic markers of PAS, including diagnostic accuracy, and propose a standardized approach to prenatal diagnosis. Throughout our discussion, we describe protocols for detection of PAS practiced at our Maternal-Fetal Medicine Program in the Department of Obstetrics and Gynecology, Eastern Virginia Medical School (also see “US evaluation of PAS risk: The authors’ recommended approach”).
Numerous risk factors
There are many risk factors for PAS, including prior uterine surgery or instrumentation, such as CD, uterine curettage, myomectomy, pelvic radiation, and endometrial ablation. Other risk factors include smoking, in vitro fertilization, advanced maternal age, multiparity, and a brief interval between prior CD and subsequent pregnancy.5 Of major significance is the increased risk of PAS in the presence of placenta previa with prior CD.6 Knowledge of clinical risk factors by the interpreting physician appears to be associated with improved detection of PAS on ultrasonography (US).4
Ultrasonographic markers of PAS
First-trimester markers
Sonographic markers of PAS in the first trimester include:
- a gestational sac implanted in the lower uterine segment or in a CD scar
- multiple hypoechoic spaces within the placenta (lacunae).7
Lower uterine-segment implantation has been defined by Ballas and colleagues as 1) a gestational sac implanted in the lower one-third of the uterus between 8 and 10 weeks’ gestation or 2) a gestational sac occupying primarily the lower uterine segment from 10 weeks’ gestation onward (FIGURE 1).8 Our experience is that it is difficult to accurately assess lower uterine-segment implantation beyond 13 weeks of gestation because the sac typically expands to fill the upper uterine cavity.
Continue to: Color Doppler US...
Color Doppler US can help differentiate lower uterine-segment implantation from a gestational sac of a failed pregnancy in the process of expulsion by demonstrating loss of circumferential blood flow in the failed pregnancy. Furthermore, applying pressure to the anterior surface of the uterus will result in downward movement of the gestational sac of a failed pregnancy.9
Not all gestational sacs that implant in the lower uterine segment lead to PAS: Subsequent normal pregnancies have been reported in this circumstance. In such cases, a normal thick myometrium is noted anterior to the gestational sac.7 A patient with lower uterine-segment implantation without evidence of anterior myometrial thinning remains at risk for third-trimester placenta previa.7
Cesarean scar pregnancy carries significant risk of PAS. In these cases, the gestational sac is typically implanted within the scar, resulting in a thin anterior myometrium and significantly increased vascularity of the placental–myometrial and bladder–uterine wall interfaces (FIGURE 2).9 Differentiating cesarean scar pregnancy from a lower uterine-segment implantation is easier to perform before the eighth week of gestation but becomes more difficult as pregnancy advances. Although it might be useful to distinguish between true cesarean scar pregnancy and lower uterine-segment implantation adjacent to or involving the scar, both carry considerable risk of PAS and excessive hemorrhage, and the approach to treating both conditions is quite similar.
Lacunae, with or without documented blood flow on color Doppler US, are the third marker of PAS in the first trimester.8 Although some retrospective series and case reports describe the finding of lacunae in the first trimester of patients with diagnosed PAS, more recent literature suggests that these spaces are seen infrequently and at a similar frequency in women with and without PAS at delivery.7
Second- and third-trimester markers
Multiple diagnostic sonographic markers of PAS have been described in the second and third trimesters.
Placental location is a significant risk factor for PAS. Placenta previa in the setting of prior CD carries the highest risk of PAS—as high as 61% in women with both placenta previa and a history of 3 CDs.10 An anterior placenta appears to be a stronger risk factor for PAS than a posterior placenta in women with prior CD; the location of the placenta should therefore be evaluated in all women in the second trimester.
Continue to: Lacunae
Lacunae. The finding of multiple hypoechoic vascular spaces within the placental parenchyma has been associated with PAS (FIGURES 3 and 4). The pathogenesis of this finding is probably related to alterations in placental tissue resulting from long-term exposure to pulsatile blood flow.11
Finberg and colleagues introduced a grading system for placental lacunae in 1992 that is still used:
- Grade 0: no lacunae seen
- Grade 1: 1 to 3 lacunae seen
- Grade 2: 4 to 6 lacunae seen
- Grade 3: multiple lacunae seen throughout the placenta.12
The sensitivity and specificity of lacunae as an independent marker for PAS have been reported to be 77% and 95%, respectively.13 Despite these findings, several studies report a range of sensitivity (73% to 100%) and negative predictive value (88% to 100%).14 Even in Finberg’s original work, 27% of cases of confirmed PAS had Grade 0 or Grade 1 placental lacunae and 11% of cases of placenta previa, without PAS, demonstrated Grade 2 lacunae.12 There is agreement, however, that, the more lacunae, the higher the risk of PAS.
Continue to: Other US markers for PAS
Other US markers of PAS
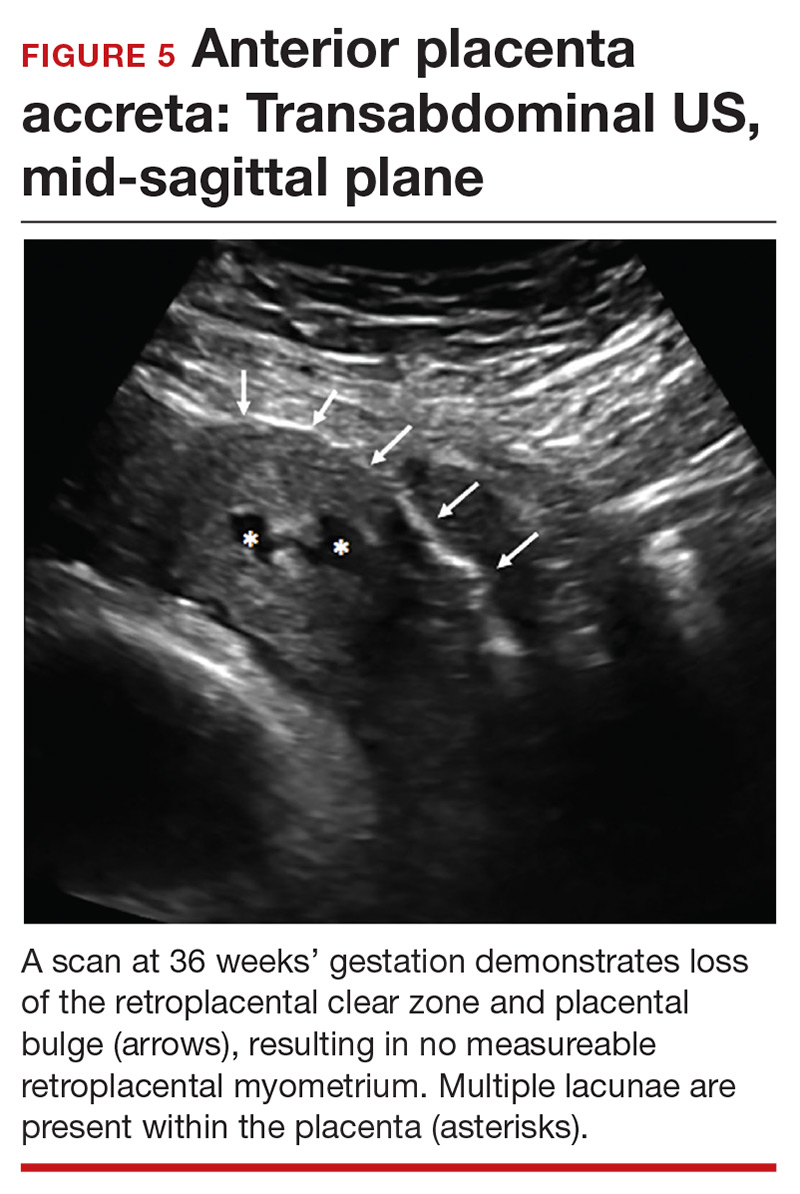
Retroplacental–myometrial interface

Loss of the normal hypoechoic (clear) retroplacental zone, also referred to as loss of the clear space between placenta and uterus, is another marker of PAS (FIGURE 5). This finding corresponds to pathologic loss of the decidua basalis as trophoblastic tissue invades directly through the myometrium.15 This sonographic finding has been reported to have a detection rate of approximately 93%, with sensitivity of 52% and specificity of 57%, for PAS; the false-positive rate, however, has been in the range of 21% or higher. This marker should not be used alone because it is angle-dependent and can be found (as an absent clear zone) in normal anterior placentas.16
The strength of this US marker is in its negative predictive value, which ranges from 96% to 100%. The presence of a hypoechoic retroplacental clear space that extends the length of the placenta makes PAS unlikely.17 Of note, the clear zone may appear falsely absent as a result of increased pressure from the US probe.
Retroplacental myometrial thickness
Retroplacental myometrial thickness is difficult to assess because the lower uterine-segment myometrium thins in normal pregnancy as term approaches. This measurement also can be influenced by direct pressure of the US probe and fullness of the maternal bladder.18 In patients who have had a CD but who do not have PAS, the median myometrial thickness of the lower uterine segment in the third trimester is 2.4 mm.19
Thinning of the myometrium in the upper uterine segment always should be of concern. Studies of this marker have reported sensitivity of US ranging from 22% to 100% and specificity from 72% to 100%.9,20 Given such variability, it is important to standardize the gestational age and sonographic approach for this marker.
Continue to: Uterovesical interface
Uterovesical interface
Studies also have reported that abnormalities of the uterovesical interface are predictive of PAS. The uterovesical interface is best evaluated in a sagittal plane containing the lower uterine segment and a partially full bladder in gray-scale and color Doppler US.15 The normal uterovesical interface appears as a smooth line, without irregularities or increased vascularity on sagittal imaging.
Abnormalities include focal interruption of the hyperechoic bladder wall, bulging of the bladder wall, and increased vascularity, such as varicosities (FIGURES 5, 6, and 7).15 These findings may be seen as early as the first trimester but are more commonly noted in the second and third trimesters.7 The authors of a recent meta-analysis concluded that irregularity of the uterovesical interface is the most specific marker for invasive placentation (99.75% confidence interval; range, 99.5% to 99.9%).13
Other US markers and modalities
Three-dimensional US. Studies have evaluated the role of 3-dimensional (3D) US for predicting PAS. Application of 3D US in vascular mode has shown promise because it allows for semiquantitative assessment of placental vasculature.22 Using 3D US to screen for PAS presents drawbacks, however: The technology is not well-standardized and requires significant operator expertise for volume acquisition and manipulation. Prospective studies are needed before 3D US can be applied routinely to screen for and diagnose PAS.
Color Doppler US. As an adjunct to gray-scale US, color Doppler US can be used for making a diagnosis of PAS. Color Doppler US helps differentiate a normal subplacental venous complex with nonpulsatile, low-velocity venous blood flow waveforms from markedly dilated peripheral subplacental vascular channels with pulsatile venous-type flow, which suggests PAS. These vascular channels are often located directly over the cervix. In addition, the observation of bridging vessels linking the placenta and bladder with high diastolic arterial blood flow also suggests invasion.21 In a meta-analysis, overall sensitivity of color Doppler US for the diagnosis of PAS was 91%, with specificity of 87%.13
The value of utilizing multiple markers
The accuracy of US diagnosis of PAS is likely improved by using more than 1 sonographic marker. Pilloni and colleagues,20 in a prospective analysis, found that 81% of cases of confirmed PAS had ≥2 markers and 51% of cases had ≥3 markers.
Several scoring systems have been proposed for making the diagnosis of PAS using combinations of sonographic markers, placental location, and clinical history.19,24,25 In 2016, Tovbin and colleagues,25 in a prospective study, evaluated a scoring system that included:
- number of previous CDs
- number of, maximum dimension of, and presence of blood flow in lacunae
- loss of uteroplacental clear zone
- placental location
- hypervascularity of the uterovesical or uteroplacental interface.
Tovbin assigned 1 or 2 points to each criterion. Each sonographic marker was found to be significantly associated with PAS when compared to a high-risk control group. A score of ≥8 was considered “at high risk” and predicted 69% of PAS cases.
Regrettably, no combination of US markers reliably predicts the depth of invasion of the placenta.26
Continue to: A standardized approach is needed
A standardized approach is needed
To decrease variability and improve the US diagnosis of PAS, it is important to define and standardize the diagnosis of each sonographic marker for PAS.4 In 2016, the European Working Group on Abnormally Invasive Placenta (EW-AIP) proposed a set of US markers that always should be reported when performing an US examination for suspected abnormal placentation (TABLE).23 Despite this effort by the EW-AIP, ambiguity remains over sonographic definitions of several PAS markers. For example, what determines a placental lacuna on US? And what constitutes an abnormal uterovesical interface? There is a need for a more objective definition of US markers of PAS and a standardized approach to the US examination in at-risk pregnancies.
The Society for Maternal-Fetal Medicine is coordinating a multi-society task force to address the need to define and standardize the US diagnosis of PAS.
Observations on other PAS diagnostic modalities
Magnetic resonance imaging
Adjunctive role. Magnetic resonance imaging (MRI) is often used as an adjunctive diagnostic modality in cases of suspected PAS. Several markers for PAS have been described on MRI, including15:
- intraplacental T2-weighted dark bands
- abnormal intraplacental vascularity
- heterogeneous intraplacental signal intensity
- focal interruption of the myometrium by the placenta
- uterine bulging.
- Assess a priori risk for the patient before initiating the US exam
- In the presence of a placenta previa, or low-lying placenta, we strongly recommend a transvaginal, in addition to transabdominal, US to further assess for the presence of placenta accreta spectrum (PAS) markers
- Until prospective studies clearly define the diagnostic accuracy of PAS sonographic markers and their performance in high-risk and low-risk pregnancies, we recommend that US findings be reported as a risk profile—that is, high, moderate, and low risk of PAS
- Be especially cautious with patients who are at substantially increased risk for PAS, such as those with placenta previa and prior multiple CDs. In this setting, a low-risk report for PAS only should be provided when none of the PAS markers are seen on transabdominal and transvaginal US examinations
- While awaiting national guidelines that 1) standardize the approach to the US examination and 2) define PAS US markers, we encourage US laboratories to develop local protocols to standardize the sonographic evaluation of the placenta and ensure uniform and complete placental assessment
Based on a recent meta-analysis, overall sensitivity of MRI for detecting PAS is 86% to 95%, with specificity of 80% to 95%. Although this is comparable to the sensitivity and specificity of US,27 studies of MRI in PAS are smaller and more prone to bias than in studies of US, because MRI typically is used only in patients at highest risk for PAS. Few studies comparing US to MRI for PAS have been performed; all are small and lack statistical power.
Complementary role. MRI can be complementary to US in cases in which the placenta is posterior or located laterally28 but, importantly, rarely changes decisions about surgical management when used in conjunction with US to assess patients for the diagnosis of PAS. (An exception might lie in the ability of MRI to assess the degree or depth of invasion of the placenta and discerning placenta percreta from placenta accreta.15)
Enhancement with contrast. Addition of gadolinium-based contrast might improve the ability of MRI to make a diagnosis of PAS, but gadolinium crosses the placenta barrier. Although fetal effects of gadolinium have not been observed, American College of Radiology guidelines recommend avoiding this contrast agent during pregnancy unless absolutely essential.29
Specific indications. MRI without contrast should be considered 1) when US is inconclusive and 2) to further evaluate a posterior placenta suspicious for invasion, to define the precise topography of extrauterine placental invasion. The additional information offered by MRI might alter surgical planning.15
Overall, based on current literature, gray-scale US appears to be an excellent tool for prenatal diagnosis of PAS in women at risk: Sensitivity has been reported in the range of 80% to 90%; specificity, 91% to 98%; positive predictive value, 65% to 93%; and negative predictive value, 98%.5,6
However, these values might overestimate the true ability of prenatal US to predict PAS. Why? Early studies that assessed the accuracy of US prediction of PAS might have been biased by inclusion of single-expert observations, high suspicion of placenta accreta, and prior knowledge of patients’ risk factors. In addition, small sample size, retrospective design, and wide variability in the definition of PAS and inclusion criteria led to inconsistency in performance and skewed sensitivity.7
In fact, when experienced providers, reviewing the same US images, were blinded to patients’ clinical history, the accuracy of US diagnosis of PAS decreased in regard to sensitivity (to 54%), specificity (88%), positive (82%) and negative (65%) predictive value, and accuracy (65%).4 Investigators also found wide inter-observer variability in the interpretation of markers of PAS.4 Furthermore, there is evidence that several PAS US markers are commonly seen in low-risk normal pregnancy.
Although studies have yielded variable findings of the precise sensitivity and positive predictive value of US in the diagnosis of PAS, there is a general agreement that US should be the primary imaging modality for this purpose, and can be used exclusively in most cases.
References
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171-181.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2013;42:509-517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153-2158.
Biomarkers
Multiple serum biomarkers have been proposed to predict PAS in high-risk women. PAS might be associated with increased levels of first-trimester pregnancy-associated plasma protein A, second-trimester maternal serum alpha fetoprotein, and human chorionic gonadotropin, but studies of the utility of these biomarkers have yielded contradictory results.30,31 Biomarkers are of interest and have significant clinical applicability, but none of the ones identified to date have high sensitivity or specificity for predicting PAS prenatally. Research is ongoing to identify markers of PAS that have sufficient predictive power.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Irving FC, Hertig AT. A study of placenta accreta. Surg Gynec Obstet. 1937:64:178–200.
- Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458–1461.
- Hall T, Wax JR, Lucas FL, et al. Prenatal sonographic diagnosis of placenta accreta—impact on maternal and neonatal outcomes. J Clin Ultrasound. 2014;42:449–455.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153–2158.
- Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126:654–668.
- Silver RM, Landon MB, Rouse DJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–1232.
- Rac MW, Moschos E, Wells CE, et al. Sonographic findings of morbidly adherent placenta in the first trimester. J Ultrasound Med. 2016;35:263–269.
- Ballas J, Pretorius D, Hull AD, et al. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31:1835–1841.
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171–181.
- Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am J Obstet Gynecol. 2011;205:262.e1–e8.
- Baughman WC, Corteville JE, Shah RR. Placenta accreta: spectrum of US and MR imaging findings. Radiographics. 2008;28:1905–1916.
- Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333–343.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:509–517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135–1140.
- D’Antonio F, Palacios-Jaraquemada J, Lim PS, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol. 2016;47:290–301.
- Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509–517.
- Wong HS, Cheung YK, Zuccollo J, et al. Evaluation of sonographic diagnostic criteria for placenta accreta. J Clin Ultrasound. 2008;36:551–559.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75–87.
- Rac MW, Dashe JS, Wells CE, et al. Ultrasound predictors of placental invasion: the Placenta Accreta Index. Am J Obstet Gynecol. 2015;212:343.e1–e7.
- Pilloni E, Alemanno MG, Gaglioti P, et al. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet Gynecol. 2016;47:302–307.
- Comstock CH. Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol. 2005;26:89–96.
- Collins SL, Stevenson GN, Al-Khan A, et al. Three-dimensional power Doppler ultrasonography for diagnosing abnormally invasive placenta and quantifying the risk. Obstet Gynecol. 2015;126:645–653.
- Collins SL, Ashcroft A, Braun T, et al; European Working Group on Abnormally Invasive Placenta (EW-AIP). Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271–275.
- Gilboa Y, Spira M, Mazaki-Tovi S, et al. A novel sonographic scoring system for antenatal risk assessment of obstetric complications in suspected morbidly adherent placenta. J Ultrasound Med. 2015;34:561–567.
- Tovbin J, Melcer Y, Shor S, et al. Prediction of morbidly adherent placenta using a scoring system. Ultrasound Obstet Gynecol. 2016;48:504–510.
- Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016:215:712–721.
- Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:507–520.
- Rezk MA, Shawky M. Grey-scale and colour Doppler ultrasound versus magnetic resonance imaging for the prenatal diagnosis of placenta accreta. J Matern Fetal Neonatal Med. 2016;29:218–223.
- Expert Panel on MR Safety; Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530.
- Pekar-Zlotin M, Melcer Y, Maymon R, Jauniaux E. Secondtrimester levels of fetoplacental hormones among women with placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140:377–378.
- Lyell DJ, Faucett AM, Baer RJ, et al. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J Perinatol. 2015;35:570–574.
Placenta accreta spectrum (PAS) describes abnormal invasion of placental tissue into or through the myometrium, comprising 3 distinct conditions: placenta accreta, placenta increta, and placenta percreta. This complication is relatively new to obstetrics, first described in 1937.1
The overall incidence of PAS has been increasing over several decades, in parallel to an increasing rate of cesarean delivery (CD), with an incidence from 1982 through 2002 of 1 in 533 pregnancies, representing a 5-fold increase since the 1980s.2 PAS is associated with significant morbidity and mortality, including fetal growth restriction, preterm delivery, placental abruption antenatally, and hemorrhage during delivery or postpartum.
Prenatal diagnosis of PAS and planned delivery at an experienced center are associated with significant reduction in maternal and fetal morbidity.3 In an era of advanced imaging modalities, prenatal detection of PAS regrettably remains variable and largely subjective: As many as 20% to 50% of cases of PAS escape prenatal diagnosis.3,4
In this article, we review the sonographic markers of PAS, including diagnostic accuracy, and propose a standardized approach to prenatal diagnosis. Throughout our discussion, we describe protocols for detection of PAS practiced at our Maternal-Fetal Medicine Program in the Department of Obstetrics and Gynecology, Eastern Virginia Medical School (also see “US evaluation of PAS risk: The authors’ recommended approach”).
Numerous risk factors
There are many risk factors for PAS, including prior uterine surgery or instrumentation, such as CD, uterine curettage, myomectomy, pelvic radiation, and endometrial ablation. Other risk factors include smoking, in vitro fertilization, advanced maternal age, multiparity, and a brief interval between prior CD and subsequent pregnancy.5 Of major significance is the increased risk of PAS in the presence of placenta previa with prior CD.6 Knowledge of clinical risk factors by the interpreting physician appears to be associated with improved detection of PAS on ultrasonography (US).4
Ultrasonographic markers of PAS
First-trimester markers
Sonographic markers of PAS in the first trimester include:
- a gestational sac implanted in the lower uterine segment or in a CD scar
- multiple hypoechoic spaces within the placenta (lacunae).7
Lower uterine-segment implantation has been defined by Ballas and colleagues as 1) a gestational sac implanted in the lower one-third of the uterus between 8 and 10 weeks’ gestation or 2) a gestational sac occupying primarily the lower uterine segment from 10 weeks’ gestation onward (FIGURE 1).8 Our experience is that it is difficult to accurately assess lower uterine-segment implantation beyond 13 weeks of gestation because the sac typically expands to fill the upper uterine cavity.
Continue to: Color Doppler US...
Color Doppler US can help differentiate lower uterine-segment implantation from a gestational sac of a failed pregnancy in the process of expulsion by demonstrating loss of circumferential blood flow in the failed pregnancy. Furthermore, applying pressure to the anterior surface of the uterus will result in downward movement of the gestational sac of a failed pregnancy.9
Not all gestational sacs that implant in the lower uterine segment lead to PAS: Subsequent normal pregnancies have been reported in this circumstance. In such cases, a normal thick myometrium is noted anterior to the gestational sac.7 A patient with lower uterine-segment implantation without evidence of anterior myometrial thinning remains at risk for third-trimester placenta previa.7
Cesarean scar pregnancy carries significant risk of PAS. In these cases, the gestational sac is typically implanted within the scar, resulting in a thin anterior myometrium and significantly increased vascularity of the placental–myometrial and bladder–uterine wall interfaces (FIGURE 2).9 Differentiating cesarean scar pregnancy from a lower uterine-segment implantation is easier to perform before the eighth week of gestation but becomes more difficult as pregnancy advances. Although it might be useful to distinguish between true cesarean scar pregnancy and lower uterine-segment implantation adjacent to or involving the scar, both carry considerable risk of PAS and excessive hemorrhage, and the approach to treating both conditions is quite similar.
Lacunae, with or without documented blood flow on color Doppler US, are the third marker of PAS in the first trimester.8 Although some retrospective series and case reports describe the finding of lacunae in the first trimester of patients with diagnosed PAS, more recent literature suggests that these spaces are seen infrequently and at a similar frequency in women with and without PAS at delivery.7
Second- and third-trimester markers
Multiple diagnostic sonographic markers of PAS have been described in the second and third trimesters.
Placental location is a significant risk factor for PAS. Placenta previa in the setting of prior CD carries the highest risk of PAS—as high as 61% in women with both placenta previa and a history of 3 CDs.10 An anterior placenta appears to be a stronger risk factor for PAS than a posterior placenta in women with prior CD; the location of the placenta should therefore be evaluated in all women in the second trimester.
Continue to: Lacunae
Lacunae. The finding of multiple hypoechoic vascular spaces within the placental parenchyma has been associated with PAS (FIGURES 3 and 4). The pathogenesis of this finding is probably related to alterations in placental tissue resulting from long-term exposure to pulsatile blood flow.11
Finberg and colleagues introduced a grading system for placental lacunae in 1992 that is still used:
- Grade 0: no lacunae seen
- Grade 1: 1 to 3 lacunae seen
- Grade 2: 4 to 6 lacunae seen
- Grade 3: multiple lacunae seen throughout the placenta.12
The sensitivity and specificity of lacunae as an independent marker for PAS have been reported to be 77% and 95%, respectively.13 Despite these findings, several studies report a range of sensitivity (73% to 100%) and negative predictive value (88% to 100%).14 Even in Finberg’s original work, 27% of cases of confirmed PAS had Grade 0 or Grade 1 placental lacunae and 11% of cases of placenta previa, without PAS, demonstrated Grade 2 lacunae.12 There is agreement, however, that, the more lacunae, the higher the risk of PAS.
Continue to: Other US markers for PAS
Other US markers of PAS

Retroplacental–myometrial interface

Loss of the normal hypoechoic (clear) retroplacental zone, also referred to as loss of the clear space between placenta and uterus, is another marker of PAS (FIGURE 5). This finding corresponds to pathologic loss of the decidua basalis as trophoblastic tissue invades directly through the myometrium.15 This sonographic finding has been reported to have a detection rate of approximately 93%, with sensitivity of 52% and specificity of 57%, for PAS; the false-positive rate, however, has been in the range of 21% or higher. This marker should not be used alone because it is angle-dependent and can be found (as an absent clear zone) in normal anterior placentas.16
The strength of this US marker is in its negative predictive value, which ranges from 96% to 100%. The presence of a hypoechoic retroplacental clear space that extends the length of the placenta makes PAS unlikely.17 Of note, the clear zone may appear falsely absent as a result of increased pressure from the US probe.
Retroplacental myometrial thickness
Retroplacental myometrial thickness is difficult to assess because the lower uterine-segment myometrium thins in normal pregnancy as term approaches. This measurement also can be influenced by direct pressure of the US probe and fullness of the maternal bladder.18 In patients who have had a CD but who do not have PAS, the median myometrial thickness of the lower uterine segment in the third trimester is 2.4 mm.19
Thinning of the myometrium in the upper uterine segment always should be of concern. Studies of this marker have reported sensitivity of US ranging from 22% to 100% and specificity from 72% to 100%.9,20 Given such variability, it is important to standardize the gestational age and sonographic approach for this marker.
Continue to: Uterovesical interface
Uterovesical interface
Studies also have reported that abnormalities of the uterovesical interface are predictive of PAS. The uterovesical interface is best evaluated in a sagittal plane containing the lower uterine segment and a partially full bladder in gray-scale and color Doppler US.15 The normal uterovesical interface appears as a smooth line, without irregularities or increased vascularity on sagittal imaging.
Abnormalities include focal interruption of the hyperechoic bladder wall, bulging of the bladder wall, and increased vascularity, such as varicosities (FIGURES 5, 6, and 7).15 These findings may be seen as early as the first trimester but are more commonly noted in the second and third trimesters.7 The authors of a recent meta-analysis concluded that irregularity of the uterovesical interface is the most specific marker for invasive placentation (99.75% confidence interval; range, 99.5% to 99.9%).13
Other US markers and modalities
Three-dimensional US. Studies have evaluated the role of 3-dimensional (3D) US for predicting PAS. Application of 3D US in vascular mode has shown promise because it allows for semiquantitative assessment of placental vasculature.22 Using 3D US to screen for PAS presents drawbacks, however: The technology is not well-standardized and requires significant operator expertise for volume acquisition and manipulation. Prospective studies are needed before 3D US can be applied routinely to screen for and diagnose PAS.
Color Doppler US. As an adjunct to gray-scale US, color Doppler US can be used for making a diagnosis of PAS. Color Doppler US helps differentiate a normal subplacental venous complex with nonpulsatile, low-velocity venous blood flow waveforms from markedly dilated peripheral subplacental vascular channels with pulsatile venous-type flow, which suggests PAS. These vascular channels are often located directly over the cervix. In addition, the observation of bridging vessels linking the placenta and bladder with high diastolic arterial blood flow also suggests invasion.21 In a meta-analysis, overall sensitivity of color Doppler US for the diagnosis of PAS was 91%, with specificity of 87%.13
The value of utilizing multiple markers
The accuracy of US diagnosis of PAS is likely improved by using more than 1 sonographic marker. Pilloni and colleagues,20 in a prospective analysis, found that 81% of cases of confirmed PAS had ≥2 markers and 51% of cases had ≥3 markers.
Several scoring systems have been proposed for making the diagnosis of PAS using combinations of sonographic markers, placental location, and clinical history.19,24,25 In 2016, Tovbin and colleagues,25 in a prospective study, evaluated a scoring system that included:
- number of previous CDs
- number of, maximum dimension of, and presence of blood flow in lacunae
- loss of uteroplacental clear zone
- placental location
- hypervascularity of the uterovesical or uteroplacental interface.
Tovbin assigned 1 or 2 points to each criterion. Each sonographic marker was found to be significantly associated with PAS when compared to a high-risk control group. A score of ≥8 was considered “at high risk” and predicted 69% of PAS cases.
Regrettably, no combination of US markers reliably predicts the depth of invasion of the placenta.26
Continue to: A standardized approach is needed
A standardized approach is needed
To decrease variability and improve the US diagnosis of PAS, it is important to define and standardize the diagnosis of each sonographic marker for PAS.4 In 2016, the European Working Group on Abnormally Invasive Placenta (EW-AIP) proposed a set of US markers that always should be reported when performing an US examination for suspected abnormal placentation (TABLE).23 Despite this effort by the EW-AIP, ambiguity remains over sonographic definitions of several PAS markers. For example, what determines a placental lacuna on US? And what constitutes an abnormal uterovesical interface? There is a need for a more objective definition of US markers of PAS and a standardized approach to the US examination in at-risk pregnancies.
The Society for Maternal-Fetal Medicine is coordinating a multi-society task force to address the need to define and standardize the US diagnosis of PAS.
Observations on other PAS diagnostic modalities
Magnetic resonance imaging
Adjunctive role. Magnetic resonance imaging (MRI) is often used as an adjunctive diagnostic modality in cases of suspected PAS. Several markers for PAS have been described on MRI, including15:
- intraplacental T2-weighted dark bands
- abnormal intraplacental vascularity
- heterogeneous intraplacental signal intensity
- focal interruption of the myometrium by the placenta
- uterine bulging.
- Assess a priori risk for the patient before initiating the US exam
- In the presence of a placenta previa, or low-lying placenta, we strongly recommend a transvaginal, in addition to transabdominal, US to further assess for the presence of placenta accreta spectrum (PAS) markers
- Until prospective studies clearly define the diagnostic accuracy of PAS sonographic markers and their performance in high-risk and low-risk pregnancies, we recommend that US findings be reported as a risk profile—that is, high, moderate, and low risk of PAS
- Be especially cautious with patients who are at substantially increased risk for PAS, such as those with placenta previa and prior multiple CDs. In this setting, a low-risk report for PAS only should be provided when none of the PAS markers are seen on transabdominal and transvaginal US examinations
- While awaiting national guidelines that 1) standardize the approach to the US examination and 2) define PAS US markers, we encourage US laboratories to develop local protocols to standardize the sonographic evaluation of the placenta and ensure uniform and complete placental assessment
Based on a recent meta-analysis, overall sensitivity of MRI for detecting PAS is 86% to 95%, with specificity of 80% to 95%. Although this is comparable to the sensitivity and specificity of US,27 studies of MRI in PAS are smaller and more prone to bias than in studies of US, because MRI typically is used only in patients at highest risk for PAS. Few studies comparing US to MRI for PAS have been performed; all are small and lack statistical power.
Complementary role. MRI can be complementary to US in cases in which the placenta is posterior or located laterally28 but, importantly, rarely changes decisions about surgical management when used in conjunction with US to assess patients for the diagnosis of PAS. (An exception might lie in the ability of MRI to assess the degree or depth of invasion of the placenta and discerning placenta percreta from placenta accreta.15)
Enhancement with contrast. Addition of gadolinium-based contrast might improve the ability of MRI to make a diagnosis of PAS, but gadolinium crosses the placenta barrier. Although fetal effects of gadolinium have not been observed, American College of Radiology guidelines recommend avoiding this contrast agent during pregnancy unless absolutely essential.29
Specific indications. MRI without contrast should be considered 1) when US is inconclusive and 2) to further evaluate a posterior placenta suspicious for invasion, to define the precise topography of extrauterine placental invasion. The additional information offered by MRI might alter surgical planning.15
Overall, based on current literature, gray-scale US appears to be an excellent tool for prenatal diagnosis of PAS in women at risk: Sensitivity has been reported in the range of 80% to 90%; specificity, 91% to 98%; positive predictive value, 65% to 93%; and negative predictive value, 98%.5,6
However, these values might overestimate the true ability of prenatal US to predict PAS. Why? Early studies that assessed the accuracy of US prediction of PAS might have been biased by inclusion of single-expert observations, high suspicion of placenta accreta, and prior knowledge of patients’ risk factors. In addition, small sample size, retrospective design, and wide variability in the definition of PAS and inclusion criteria led to inconsistency in performance and skewed sensitivity.7
In fact, when experienced providers, reviewing the same US images, were blinded to patients’ clinical history, the accuracy of US diagnosis of PAS decreased in regard to sensitivity (to 54%), specificity (88%), positive (82%) and negative (65%) predictive value, and accuracy (65%).4 Investigators also found wide inter-observer variability in the interpretation of markers of PAS.4 Furthermore, there is evidence that several PAS US markers are commonly seen in low-risk normal pregnancy.
Although studies have yielded variable findings of the precise sensitivity and positive predictive value of US in the diagnosis of PAS, there is a general agreement that US should be the primary imaging modality for this purpose, and can be used exclusively in most cases.
References
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171-181.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2013;42:509-517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153-2158.
Biomarkers
Multiple serum biomarkers have been proposed to predict PAS in high-risk women. PAS might be associated with increased levels of first-trimester pregnancy-associated plasma protein A, second-trimester maternal serum alpha fetoprotein, and human chorionic gonadotropin, but studies of the utility of these biomarkers have yielded contradictory results.30,31 Biomarkers are of interest and have significant clinical applicability, but none of the ones identified to date have high sensitivity or specificity for predicting PAS prenatally. Research is ongoing to identify markers of PAS that have sufficient predictive power.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Placenta accreta spectrum (PAS) describes abnormal invasion of placental tissue into or through the myometrium, comprising 3 distinct conditions: placenta accreta, placenta increta, and placenta percreta. This complication is relatively new to obstetrics, first described in 1937.1
The overall incidence of PAS has been increasing over several decades, in parallel to an increasing rate of cesarean delivery (CD), with an incidence from 1982 through 2002 of 1 in 533 pregnancies, representing a 5-fold increase since the 1980s.2 PAS is associated with significant morbidity and mortality, including fetal growth restriction, preterm delivery, placental abruption antenatally, and hemorrhage during delivery or postpartum.
Prenatal diagnosis of PAS and planned delivery at an experienced center are associated with significant reduction in maternal and fetal morbidity.3 In an era of advanced imaging modalities, prenatal detection of PAS regrettably remains variable and largely subjective: As many as 20% to 50% of cases of PAS escape prenatal diagnosis.3,4
In this article, we review the sonographic markers of PAS, including diagnostic accuracy, and propose a standardized approach to prenatal diagnosis. Throughout our discussion, we describe protocols for detection of PAS practiced at our Maternal-Fetal Medicine Program in the Department of Obstetrics and Gynecology, Eastern Virginia Medical School (also see “US evaluation of PAS risk: The authors’ recommended approach”).
Numerous risk factors
There are many risk factors for PAS, including prior uterine surgery or instrumentation, such as CD, uterine curettage, myomectomy, pelvic radiation, and endometrial ablation. Other risk factors include smoking, in vitro fertilization, advanced maternal age, multiparity, and a brief interval between prior CD and subsequent pregnancy.5 Of major significance is the increased risk of PAS in the presence of placenta previa with prior CD.6 Knowledge of clinical risk factors by the interpreting physician appears to be associated with improved detection of PAS on ultrasonography (US).4
Ultrasonographic markers of PAS
First-trimester markers
Sonographic markers of PAS in the first trimester include:
- a gestational sac implanted in the lower uterine segment or in a CD scar
- multiple hypoechoic spaces within the placenta (lacunae).7
Lower uterine-segment implantation has been defined by Ballas and colleagues as 1) a gestational sac implanted in the lower one-third of the uterus between 8 and 10 weeks’ gestation or 2) a gestational sac occupying primarily the lower uterine segment from 10 weeks’ gestation onward (FIGURE 1).8 Our experience is that it is difficult to accurately assess lower uterine-segment implantation beyond 13 weeks of gestation because the sac typically expands to fill the upper uterine cavity.
Continue to: Color Doppler US...
Color Doppler US can help differentiate lower uterine-segment implantation from a gestational sac of a failed pregnancy in the process of expulsion by demonstrating loss of circumferential blood flow in the failed pregnancy. Furthermore, applying pressure to the anterior surface of the uterus will result in downward movement of the gestational sac of a failed pregnancy.9
Not all gestational sacs that implant in the lower uterine segment lead to PAS: Subsequent normal pregnancies have been reported in this circumstance. In such cases, a normal thick myometrium is noted anterior to the gestational sac.7 A patient with lower uterine-segment implantation without evidence of anterior myometrial thinning remains at risk for third-trimester placenta previa.7
Cesarean scar pregnancy carries significant risk of PAS. In these cases, the gestational sac is typically implanted within the scar, resulting in a thin anterior myometrium and significantly increased vascularity of the placental–myometrial and bladder–uterine wall interfaces (FIGURE 2).9 Differentiating cesarean scar pregnancy from a lower uterine-segment implantation is easier to perform before the eighth week of gestation but becomes more difficult as pregnancy advances. Although it might be useful to distinguish between true cesarean scar pregnancy and lower uterine-segment implantation adjacent to or involving the scar, both carry considerable risk of PAS and excessive hemorrhage, and the approach to treating both conditions is quite similar.
Lacunae, with or without documented blood flow on color Doppler US, are the third marker of PAS in the first trimester.8 Although some retrospective series and case reports describe the finding of lacunae in the first trimester of patients with diagnosed PAS, more recent literature suggests that these spaces are seen infrequently and at a similar frequency in women with and without PAS at delivery.7
Second- and third-trimester markers
Multiple diagnostic sonographic markers of PAS have been described in the second and third trimesters.
Placental location is a significant risk factor for PAS. Placenta previa in the setting of prior CD carries the highest risk of PAS—as high as 61% in women with both placenta previa and a history of 3 CDs.10 An anterior placenta appears to be a stronger risk factor for PAS than a posterior placenta in women with prior CD; the location of the placenta should therefore be evaluated in all women in the second trimester.
Continue to: Lacunae
Lacunae. The finding of multiple hypoechoic vascular spaces within the placental parenchyma has been associated with PAS (FIGURES 3 and 4). The pathogenesis of this finding is probably related to alterations in placental tissue resulting from long-term exposure to pulsatile blood flow.11
Finberg and colleagues introduced a grading system for placental lacunae in 1992 that is still used:
- Grade 0: no lacunae seen
- Grade 1: 1 to 3 lacunae seen
- Grade 2: 4 to 6 lacunae seen
- Grade 3: multiple lacunae seen throughout the placenta.12
The sensitivity and specificity of lacunae as an independent marker for PAS have been reported to be 77% and 95%, respectively.13 Despite these findings, several studies report a range of sensitivity (73% to 100%) and negative predictive value (88% to 100%).14 Even in Finberg’s original work, 27% of cases of confirmed PAS had Grade 0 or Grade 1 placental lacunae and 11% of cases of placenta previa, without PAS, demonstrated Grade 2 lacunae.12 There is agreement, however, that, the more lacunae, the higher the risk of PAS.
Continue to: Other US markers for PAS
Other US markers of PAS

Retroplacental–myometrial interface

Loss of the normal hypoechoic (clear) retroplacental zone, also referred to as loss of the clear space between placenta and uterus, is another marker of PAS (FIGURE 5). This finding corresponds to pathologic loss of the decidua basalis as trophoblastic tissue invades directly through the myometrium.15 This sonographic finding has been reported to have a detection rate of approximately 93%, with sensitivity of 52% and specificity of 57%, for PAS; the false-positive rate, however, has been in the range of 21% or higher. This marker should not be used alone because it is angle-dependent and can be found (as an absent clear zone) in normal anterior placentas.16
The strength of this US marker is in its negative predictive value, which ranges from 96% to 100%. The presence of a hypoechoic retroplacental clear space that extends the length of the placenta makes PAS unlikely.17 Of note, the clear zone may appear falsely absent as a result of increased pressure from the US probe.
Retroplacental myometrial thickness
Retroplacental myometrial thickness is difficult to assess because the lower uterine-segment myometrium thins in normal pregnancy as term approaches. This measurement also can be influenced by direct pressure of the US probe and fullness of the maternal bladder.18 In patients who have had a CD but who do not have PAS, the median myometrial thickness of the lower uterine segment in the third trimester is 2.4 mm.19
Thinning of the myometrium in the upper uterine segment always should be of concern. Studies of this marker have reported sensitivity of US ranging from 22% to 100% and specificity from 72% to 100%.9,20 Given such variability, it is important to standardize the gestational age and sonographic approach for this marker.
Continue to: Uterovesical interface
Uterovesical interface
Studies also have reported that abnormalities of the uterovesical interface are predictive of PAS. The uterovesical interface is best evaluated in a sagittal plane containing the lower uterine segment and a partially full bladder in gray-scale and color Doppler US.15 The normal uterovesical interface appears as a smooth line, without irregularities or increased vascularity on sagittal imaging.
Abnormalities include focal interruption of the hyperechoic bladder wall, bulging of the bladder wall, and increased vascularity, such as varicosities (FIGURES 5, 6, and 7).15 These findings may be seen as early as the first trimester but are more commonly noted in the second and third trimesters.7 The authors of a recent meta-analysis concluded that irregularity of the uterovesical interface is the most specific marker for invasive placentation (99.75% confidence interval; range, 99.5% to 99.9%).13
Other US markers and modalities
Three-dimensional US. Studies have evaluated the role of 3-dimensional (3D) US for predicting PAS. Application of 3D US in vascular mode has shown promise because it allows for semiquantitative assessment of placental vasculature.22 Using 3D US to screen for PAS presents drawbacks, however: The technology is not well-standardized and requires significant operator expertise for volume acquisition and manipulation. Prospective studies are needed before 3D US can be applied routinely to screen for and diagnose PAS.
Color Doppler US. As an adjunct to gray-scale US, color Doppler US can be used for making a diagnosis of PAS. Color Doppler US helps differentiate a normal subplacental venous complex with nonpulsatile, low-velocity venous blood flow waveforms from markedly dilated peripheral subplacental vascular channels with pulsatile venous-type flow, which suggests PAS. These vascular channels are often located directly over the cervix. In addition, the observation of bridging vessels linking the placenta and bladder with high diastolic arterial blood flow also suggests invasion.21 In a meta-analysis, overall sensitivity of color Doppler US for the diagnosis of PAS was 91%, with specificity of 87%.13
The value of utilizing multiple markers
The accuracy of US diagnosis of PAS is likely improved by using more than 1 sonographic marker. Pilloni and colleagues,20 in a prospective analysis, found that 81% of cases of confirmed PAS had ≥2 markers and 51% of cases had ≥3 markers.
Several scoring systems have been proposed for making the diagnosis of PAS using combinations of sonographic markers, placental location, and clinical history.19,24,25 In 2016, Tovbin and colleagues,25 in a prospective study, evaluated a scoring system that included:
- number of previous CDs
- number of, maximum dimension of, and presence of blood flow in lacunae
- loss of uteroplacental clear zone
- placental location
- hypervascularity of the uterovesical or uteroplacental interface.
Tovbin assigned 1 or 2 points to each criterion. Each sonographic marker was found to be significantly associated with PAS when compared to a high-risk control group. A score of ≥8 was considered “at high risk” and predicted 69% of PAS cases.
Regrettably, no combination of US markers reliably predicts the depth of invasion of the placenta.26
Continue to: A standardized approach is needed
A standardized approach is needed
To decrease variability and improve the US diagnosis of PAS, it is important to define and standardize the diagnosis of each sonographic marker for PAS.4 In 2016, the European Working Group on Abnormally Invasive Placenta (EW-AIP) proposed a set of US markers that always should be reported when performing an US examination for suspected abnormal placentation (TABLE).23 Despite this effort by the EW-AIP, ambiguity remains over sonographic definitions of several PAS markers. For example, what determines a placental lacuna on US? And what constitutes an abnormal uterovesical interface? There is a need for a more objective definition of US markers of PAS and a standardized approach to the US examination in at-risk pregnancies.
The Society for Maternal-Fetal Medicine is coordinating a multi-society task force to address the need to define and standardize the US diagnosis of PAS.
Observations on other PAS diagnostic modalities
Magnetic resonance imaging
Adjunctive role. Magnetic resonance imaging (MRI) is often used as an adjunctive diagnostic modality in cases of suspected PAS. Several markers for PAS have been described on MRI, including15:
- intraplacental T2-weighted dark bands
- abnormal intraplacental vascularity
- heterogeneous intraplacental signal intensity
- focal interruption of the myometrium by the placenta
- uterine bulging.
- Assess a priori risk for the patient before initiating the US exam
- In the presence of a placenta previa, or low-lying placenta, we strongly recommend a transvaginal, in addition to transabdominal, US to further assess for the presence of placenta accreta spectrum (PAS) markers
- Until prospective studies clearly define the diagnostic accuracy of PAS sonographic markers and their performance in high-risk and low-risk pregnancies, we recommend that US findings be reported as a risk profile—that is, high, moderate, and low risk of PAS
- Be especially cautious with patients who are at substantially increased risk for PAS, such as those with placenta previa and prior multiple CDs. In this setting, a low-risk report for PAS only should be provided when none of the PAS markers are seen on transabdominal and transvaginal US examinations
- While awaiting national guidelines that 1) standardize the approach to the US examination and 2) define PAS US markers, we encourage US laboratories to develop local protocols to standardize the sonographic evaluation of the placenta and ensure uniform and complete placental assessment
Based on a recent meta-analysis, overall sensitivity of MRI for detecting PAS is 86% to 95%, with specificity of 80% to 95%. Although this is comparable to the sensitivity and specificity of US,27 studies of MRI in PAS are smaller and more prone to bias than in studies of US, because MRI typically is used only in patients at highest risk for PAS. Few studies comparing US to MRI for PAS have been performed; all are small and lack statistical power.
Complementary role. MRI can be complementary to US in cases in which the placenta is posterior or located laterally28 but, importantly, rarely changes decisions about surgical management when used in conjunction with US to assess patients for the diagnosis of PAS. (An exception might lie in the ability of MRI to assess the degree or depth of invasion of the placenta and discerning placenta percreta from placenta accreta.15)
Enhancement with contrast. Addition of gadolinium-based contrast might improve the ability of MRI to make a diagnosis of PAS, but gadolinium crosses the placenta barrier. Although fetal effects of gadolinium have not been observed, American College of Radiology guidelines recommend avoiding this contrast agent during pregnancy unless absolutely essential.29
Specific indications. MRI without contrast should be considered 1) when US is inconclusive and 2) to further evaluate a posterior placenta suspicious for invasion, to define the precise topography of extrauterine placental invasion. The additional information offered by MRI might alter surgical planning.15
Overall, based on current literature, gray-scale US appears to be an excellent tool for prenatal diagnosis of PAS in women at risk: Sensitivity has been reported in the range of 80% to 90%; specificity, 91% to 98%; positive predictive value, 65% to 93%; and negative predictive value, 98%.5,6
However, these values might overestimate the true ability of prenatal US to predict PAS. Why? Early studies that assessed the accuracy of US prediction of PAS might have been biased by inclusion of single-expert observations, high suspicion of placenta accreta, and prior knowledge of patients’ risk factors. In addition, small sample size, retrospective design, and wide variability in the definition of PAS and inclusion criteria led to inconsistency in performance and skewed sensitivity.7
In fact, when experienced providers, reviewing the same US images, were blinded to patients’ clinical history, the accuracy of US diagnosis of PAS decreased in regard to sensitivity (to 54%), specificity (88%), positive (82%) and negative (65%) predictive value, and accuracy (65%).4 Investigators also found wide inter-observer variability in the interpretation of markers of PAS.4 Furthermore, there is evidence that several PAS US markers are commonly seen in low-risk normal pregnancy.
Although studies have yielded variable findings of the precise sensitivity and positive predictive value of US in the diagnosis of PAS, there is a general agreement that US should be the primary imaging modality for this purpose, and can be used exclusively in most cases.
References
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171-181.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2013;42:509-517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153-2158.
Biomarkers
Multiple serum biomarkers have been proposed to predict PAS in high-risk women. PAS might be associated with increased levels of first-trimester pregnancy-associated plasma protein A, second-trimester maternal serum alpha fetoprotein, and human chorionic gonadotropin, but studies of the utility of these biomarkers have yielded contradictory results.30,31 Biomarkers are of interest and have significant clinical applicability, but none of the ones identified to date have high sensitivity or specificity for predicting PAS prenatally. Research is ongoing to identify markers of PAS that have sufficient predictive power.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Irving FC, Hertig AT. A study of placenta accreta. Surg Gynec Obstet. 1937:64:178–200.
- Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458–1461.
- Hall T, Wax JR, Lucas FL, et al. Prenatal sonographic diagnosis of placenta accreta—impact on maternal and neonatal outcomes. J Clin Ultrasound. 2014;42:449–455.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153–2158.
- Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126:654–668.
- Silver RM, Landon MB, Rouse DJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–1232.
- Rac MW, Moschos E, Wells CE, et al. Sonographic findings of morbidly adherent placenta in the first trimester. J Ultrasound Med. 2016;35:263–269.
- Ballas J, Pretorius D, Hull AD, et al. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31:1835–1841.
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171–181.
- Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am J Obstet Gynecol. 2011;205:262.e1–e8.
- Baughman WC, Corteville JE, Shah RR. Placenta accreta: spectrum of US and MR imaging findings. Radiographics. 2008;28:1905–1916.
- Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333–343.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:509–517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135–1140.
- D’Antonio F, Palacios-Jaraquemada J, Lim PS, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol. 2016;47:290–301.
- Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509–517.
- Wong HS, Cheung YK, Zuccollo J, et al. Evaluation of sonographic diagnostic criteria for placenta accreta. J Clin Ultrasound. 2008;36:551–559.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75–87.
- Rac MW, Dashe JS, Wells CE, et al. Ultrasound predictors of placental invasion: the Placenta Accreta Index. Am J Obstet Gynecol. 2015;212:343.e1–e7.
- Pilloni E, Alemanno MG, Gaglioti P, et al. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet Gynecol. 2016;47:302–307.
- Comstock CH. Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol. 2005;26:89–96.
- Collins SL, Stevenson GN, Al-Khan A, et al. Three-dimensional power Doppler ultrasonography for diagnosing abnormally invasive placenta and quantifying the risk. Obstet Gynecol. 2015;126:645–653.
- Collins SL, Ashcroft A, Braun T, et al; European Working Group on Abnormally Invasive Placenta (EW-AIP). Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271–275.
- Gilboa Y, Spira M, Mazaki-Tovi S, et al. A novel sonographic scoring system for antenatal risk assessment of obstetric complications in suspected morbidly adherent placenta. J Ultrasound Med. 2015;34:561–567.
- Tovbin J, Melcer Y, Shor S, et al. Prediction of morbidly adherent placenta using a scoring system. Ultrasound Obstet Gynecol. 2016;48:504–510.
- Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016:215:712–721.
- Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:507–520.
- Rezk MA, Shawky M. Grey-scale and colour Doppler ultrasound versus magnetic resonance imaging for the prenatal diagnosis of placenta accreta. J Matern Fetal Neonatal Med. 2016;29:218–223.
- Expert Panel on MR Safety; Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530.
- Pekar-Zlotin M, Melcer Y, Maymon R, Jauniaux E. Secondtrimester levels of fetoplacental hormones among women with placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140:377–378.
- Lyell DJ, Faucett AM, Baer RJ, et al. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J Perinatol. 2015;35:570–574.
- Irving FC, Hertig AT. A study of placenta accreta. Surg Gynec Obstet. 1937:64:178–200.
- Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458–1461.
- Hall T, Wax JR, Lucas FL, et al. Prenatal sonographic diagnosis of placenta accreta—impact on maternal and neonatal outcomes. J Clin Ultrasound. 2014;42:449–455.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153–2158.
- Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126:654–668.
- Silver RM, Landon MB, Rouse DJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–1232.
- Rac MW, Moschos E, Wells CE, et al. Sonographic findings of morbidly adherent placenta in the first trimester. J Ultrasound Med. 2016;35:263–269.
- Ballas J, Pretorius D, Hull AD, et al. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31:1835–1841.
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171–181.
- Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am J Obstet Gynecol. 2011;205:262.e1–e8.
- Baughman WC, Corteville JE, Shah RR. Placenta accreta: spectrum of US and MR imaging findings. Radiographics. 2008;28:1905–1916.
- Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333–343.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:509–517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135–1140.
- D’Antonio F, Palacios-Jaraquemada J, Lim PS, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol. 2016;47:290–301.
- Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509–517.
- Wong HS, Cheung YK, Zuccollo J, et al. Evaluation of sonographic diagnostic criteria for placenta accreta. J Clin Ultrasound. 2008;36:551–559.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75–87.
- Rac MW, Dashe JS, Wells CE, et al. Ultrasound predictors of placental invasion: the Placenta Accreta Index. Am J Obstet Gynecol. 2015;212:343.e1–e7.
- Pilloni E, Alemanno MG, Gaglioti P, et al. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet Gynecol. 2016;47:302–307.
- Comstock CH. Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol. 2005;26:89–96.
- Collins SL, Stevenson GN, Al-Khan A, et al. Three-dimensional power Doppler ultrasonography for diagnosing abnormally invasive placenta and quantifying the risk. Obstet Gynecol. 2015;126:645–653.
- Collins SL, Ashcroft A, Braun T, et al; European Working Group on Abnormally Invasive Placenta (EW-AIP). Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271–275.
- Gilboa Y, Spira M, Mazaki-Tovi S, et al. A novel sonographic scoring system for antenatal risk assessment of obstetric complications in suspected morbidly adherent placenta. J Ultrasound Med. 2015;34:561–567.
- Tovbin J, Melcer Y, Shor S, et al. Prediction of morbidly adherent placenta using a scoring system. Ultrasound Obstet Gynecol. 2016;48:504–510.
- Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016:215:712–721.
- Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:507–520.
- Rezk MA, Shawky M. Grey-scale and colour Doppler ultrasound versus magnetic resonance imaging for the prenatal diagnosis of placenta accreta. J Matern Fetal Neonatal Med. 2016;29:218–223.
- Expert Panel on MR Safety; Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530.
- Pekar-Zlotin M, Melcer Y, Maymon R, Jauniaux E. Secondtrimester levels of fetoplacental hormones among women with placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140:377–378.
- Lyell DJ, Faucett AM, Baer RJ, et al. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J Perinatol. 2015;35:570–574.
Recommending HPV vaccination: How would you grade yourself?
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers
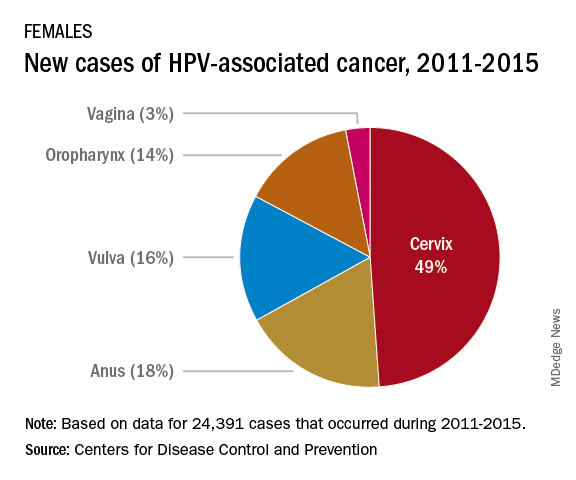
The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.
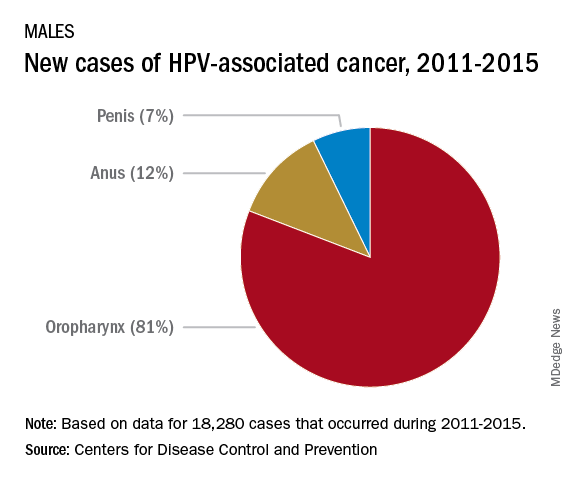
Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.