User login
'Near Remission' May Predict 3-Year Outcome in Early RA
BERLIN – "Near remission," which is based on joint counts and acute phase reactants, predicts disease status at 3 years in patients with early inflammatory arthritis as well as the definition of remission that was proposed by the American College of Rheumatology/European League Against Rheumatism in 2011. The ACR/EULAR definition includes patient global assessment of status, according to Dr. Laure Gossec, who presented findings from the ESPOIR trial at the annual European Congress of Rheumatology.
"Near-remission is much more frequent than ACR/EULAR remission in early arthritis. It appears from this analysis that near-remission (not taking into account patient global) predicts radiographic progression over 3 years in early arthritis, as well as ACR/EULAR remission, or as the definition of remission that takes into account patient-reported fatigue. Near-remission may be a valid predictive outcome in early arthritis," said Dr. Gossec, associate professor of rheumatology at Descartes University, Paris, who currently is a visiting scholar at Manchester (England) University Arthritis Epidemiology Unit.
The 2011 ACR/EULAR proposed definition of Boolean remission of early rheumatoid arthritis comprises one or no tender joints; one or no swollen joints; C-reactive protein (CRP) level equal to or less than 1 mg/dL; and a patient global assessment score of no higher than 1 (Ann. Rheum. Dis. 2011;70:404-13). However, last year at the 2011 Annual European Congress of Rheumatology, some authors noted that patient global assessment was often a limiting factor to reach this remission, and proposed near-remission as an alternative outcome (Arthritis Rheum. 2011, ACR Congress, abstract 2459). "The question is would we lose predictive information, by not taking into account the patient’s point of view? And reversely, should we be assessing fatigue; would that add to predictive information?" Dr. Gossec noted in an interview.
Dr. Gossec and her associates undertook the ESPOIR observational study to assess if patient reported outcomes, particularly patient global and fatigue, predict radiologic joint destruction at 3 years, in patients already in near remission as judged by their joint counts and levels of acute phase reactants. Specifically, the investigators assessed the predictive value of both the ACR/EULAR proposed definition of remission at 6 and 12 months after diagnosis as well as the definition of "near remission," which included three of the four proposed ACR/EULAR end points but did not include patient global assessment of status, and "fatigue-remission" in which patient’s self-report of fatigue substitutes for the patient global.
Dr. Gossec and her associates followed 776 patients with early arthritis. The patients underwent swollen and tender joint counts and CRP measurements at 6 and 12 months after diagnosis. In addition, they completed a patient global assessment of their status at those times and a fatigue visual analog scale assessment. The outcome was change in the total Sharp-van der Heijde score between baseline and at 3 years.
Of the 776 patients, 57 patients (7.4%) met the proposed ACR/EULAR definition of remission both at 6 and 12 months, whereas 145 patients (18.7%) reached near-remission, and only 24 patients (3.1%) reached fatigue-remission. Agreement between ACR/EULAR remission and the other definitions was moderate: kappa, 0.51 (95% confidence interval, 0.43-0.60) and 0.39 (95% CI, 0.26-0.53), respectively. Prediction of radiographic progression was similar no matter which definition of remission was used.
However, the relation between radiographic progression and remission was strongest for the definition of near remission and the proposed ACR/EULAR remission. In stepwise selection only the variables in near-remission were predictive. Thus, it appears that the strongest drivers of radiographic progression are joint counts and acute phase reactants, and that for patients already in remission for those criteria, patient-reported outcomes add little to the prediction of radiographic progression. Dr. Gossec concluded that "assessing patient-reported outcomes is important to understand the patient’s perspective, but have only some added value to predict radiographic outcomes if objective criteria are already well controlled."
Dr. Gossec reported that she has no conflicts of interest that are relevant to this project.
BERLIN – "Near remission," which is based on joint counts and acute phase reactants, predicts disease status at 3 years in patients with early inflammatory arthritis as well as the definition of remission that was proposed by the American College of Rheumatology/European League Against Rheumatism in 2011. The ACR/EULAR definition includes patient global assessment of status, according to Dr. Laure Gossec, who presented findings from the ESPOIR trial at the annual European Congress of Rheumatology.
"Near-remission is much more frequent than ACR/EULAR remission in early arthritis. It appears from this analysis that near-remission (not taking into account patient global) predicts radiographic progression over 3 years in early arthritis, as well as ACR/EULAR remission, or as the definition of remission that takes into account patient-reported fatigue. Near-remission may be a valid predictive outcome in early arthritis," said Dr. Gossec, associate professor of rheumatology at Descartes University, Paris, who currently is a visiting scholar at Manchester (England) University Arthritis Epidemiology Unit.
The 2011 ACR/EULAR proposed definition of Boolean remission of early rheumatoid arthritis comprises one or no tender joints; one or no swollen joints; C-reactive protein (CRP) level equal to or less than 1 mg/dL; and a patient global assessment score of no higher than 1 (Ann. Rheum. Dis. 2011;70:404-13). However, last year at the 2011 Annual European Congress of Rheumatology, some authors noted that patient global assessment was often a limiting factor to reach this remission, and proposed near-remission as an alternative outcome (Arthritis Rheum. 2011, ACR Congress, abstract 2459). "The question is would we lose predictive information, by not taking into account the patient’s point of view? And reversely, should we be assessing fatigue; would that add to predictive information?" Dr. Gossec noted in an interview.
Dr. Gossec and her associates undertook the ESPOIR observational study to assess if patient reported outcomes, particularly patient global and fatigue, predict radiologic joint destruction at 3 years, in patients already in near remission as judged by their joint counts and levels of acute phase reactants. Specifically, the investigators assessed the predictive value of both the ACR/EULAR proposed definition of remission at 6 and 12 months after diagnosis as well as the definition of "near remission," which included three of the four proposed ACR/EULAR end points but did not include patient global assessment of status, and "fatigue-remission" in which patient’s self-report of fatigue substitutes for the patient global.
Dr. Gossec and her associates followed 776 patients with early arthritis. The patients underwent swollen and tender joint counts and CRP measurements at 6 and 12 months after diagnosis. In addition, they completed a patient global assessment of their status at those times and a fatigue visual analog scale assessment. The outcome was change in the total Sharp-van der Heijde score between baseline and at 3 years.
Of the 776 patients, 57 patients (7.4%) met the proposed ACR/EULAR definition of remission both at 6 and 12 months, whereas 145 patients (18.7%) reached near-remission, and only 24 patients (3.1%) reached fatigue-remission. Agreement between ACR/EULAR remission and the other definitions was moderate: kappa, 0.51 (95% confidence interval, 0.43-0.60) and 0.39 (95% CI, 0.26-0.53), respectively. Prediction of radiographic progression was similar no matter which definition of remission was used.
However, the relation between radiographic progression and remission was strongest for the definition of near remission and the proposed ACR/EULAR remission. In stepwise selection only the variables in near-remission were predictive. Thus, it appears that the strongest drivers of radiographic progression are joint counts and acute phase reactants, and that for patients already in remission for those criteria, patient-reported outcomes add little to the prediction of radiographic progression. Dr. Gossec concluded that "assessing patient-reported outcomes is important to understand the patient’s perspective, but have only some added value to predict radiographic outcomes if objective criteria are already well controlled."
Dr. Gossec reported that she has no conflicts of interest that are relevant to this project.
BERLIN – "Near remission," which is based on joint counts and acute phase reactants, predicts disease status at 3 years in patients with early inflammatory arthritis as well as the definition of remission that was proposed by the American College of Rheumatology/European League Against Rheumatism in 2011. The ACR/EULAR definition includes patient global assessment of status, according to Dr. Laure Gossec, who presented findings from the ESPOIR trial at the annual European Congress of Rheumatology.
"Near-remission is much more frequent than ACR/EULAR remission in early arthritis. It appears from this analysis that near-remission (not taking into account patient global) predicts radiographic progression over 3 years in early arthritis, as well as ACR/EULAR remission, or as the definition of remission that takes into account patient-reported fatigue. Near-remission may be a valid predictive outcome in early arthritis," said Dr. Gossec, associate professor of rheumatology at Descartes University, Paris, who currently is a visiting scholar at Manchester (England) University Arthritis Epidemiology Unit.
The 2011 ACR/EULAR proposed definition of Boolean remission of early rheumatoid arthritis comprises one or no tender joints; one or no swollen joints; C-reactive protein (CRP) level equal to or less than 1 mg/dL; and a patient global assessment score of no higher than 1 (Ann. Rheum. Dis. 2011;70:404-13). However, last year at the 2011 Annual European Congress of Rheumatology, some authors noted that patient global assessment was often a limiting factor to reach this remission, and proposed near-remission as an alternative outcome (Arthritis Rheum. 2011, ACR Congress, abstract 2459). "The question is would we lose predictive information, by not taking into account the patient’s point of view? And reversely, should we be assessing fatigue; would that add to predictive information?" Dr. Gossec noted in an interview.
Dr. Gossec and her associates undertook the ESPOIR observational study to assess if patient reported outcomes, particularly patient global and fatigue, predict radiologic joint destruction at 3 years, in patients already in near remission as judged by their joint counts and levels of acute phase reactants. Specifically, the investigators assessed the predictive value of both the ACR/EULAR proposed definition of remission at 6 and 12 months after diagnosis as well as the definition of "near remission," which included three of the four proposed ACR/EULAR end points but did not include patient global assessment of status, and "fatigue-remission" in which patient’s self-report of fatigue substitutes for the patient global.
Dr. Gossec and her associates followed 776 patients with early arthritis. The patients underwent swollen and tender joint counts and CRP measurements at 6 and 12 months after diagnosis. In addition, they completed a patient global assessment of their status at those times and a fatigue visual analog scale assessment. The outcome was change in the total Sharp-van der Heijde score between baseline and at 3 years.
Of the 776 patients, 57 patients (7.4%) met the proposed ACR/EULAR definition of remission both at 6 and 12 months, whereas 145 patients (18.7%) reached near-remission, and only 24 patients (3.1%) reached fatigue-remission. Agreement between ACR/EULAR remission and the other definitions was moderate: kappa, 0.51 (95% confidence interval, 0.43-0.60) and 0.39 (95% CI, 0.26-0.53), respectively. Prediction of radiographic progression was similar no matter which definition of remission was used.
However, the relation between radiographic progression and remission was strongest for the definition of near remission and the proposed ACR/EULAR remission. In stepwise selection only the variables in near-remission were predictive. Thus, it appears that the strongest drivers of radiographic progression are joint counts and acute phase reactants, and that for patients already in remission for those criteria, patient-reported outcomes add little to the prediction of radiographic progression. Dr. Gossec concluded that "assessing patient-reported outcomes is important to understand the patient’s perspective, but have only some added value to predict radiographic outcomes if objective criteria are already well controlled."
Dr. Gossec reported that she has no conflicts of interest that are relevant to this project.
FROM THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Anti-TNF Treatment Linked to Reduced CV Risk
BERLIN – Treatment with a drug that blocked the action of tumor necrosis factor led to a significant reduction of cardiovascular events in patients with rheumatoid arthritis, compared with treatment with methotrexate or other nonbiologic disease modifying antirheumatic drugs, in a review of more than 100,000 patients treated during routine practice, according to Dr. Michael T. Nurmohamed.
Although this finding adds to the accumulating circumstantial evidence that treating patients with rheumatoid arthritis (RA) with a drug that blocks tumor necrosis factor (TNF) reduces their cardiovascular disease risk better than does treatment with disease-modifying antirheumatic drugs (DMARDs), current evidence does not yet clearly support an early start to anti-TNF treatment to maximize cardiovascular risk reduction, Dr. Nurmohamed said in an interview at the annual European Congress of Rheumatology.
"However, the literature does indicate that effective inflammatory suppression is essential [for patients with RA], and that if this is not achieved within a reasonable time frame with high-dose methotrexate or another DMARD then treatment with a biological, anti-TNF drug should be considered," he noted.
"The rheumatologic aim of treatment should be minimal RA disease activity and preferably remission. An additional aim of treatment might be cardiovascular risk reduction. If anti-TNF treatment stops when an RA patient achieves disease remission, we need to know whether the arterial inflammatory process also stays in remission. Investigations of this are now underway, and these studies may reveal new biomarkers of cardiovascular risk in RA patients. Until these results are available, cardiovascular risk management in patients with RA should follow EULAR recommendations (Ann. Rheum. Dis. 2010;69:325-31)," said Dr. Nurmohamed, a rheumatologist at VU University Medical Centre in Amsterdam.
"It appears that in patients with RA, atherosclerotic plaques rupture earlier," he said in an interview. "We know that inflammation is very important in that process. It might be that when you treat patients with an anti-TNF drug or other anti-inflammatory, you reduce the inflammatory content of the plaque. Therefore the plaque ruptures later. That may be the way that anti-TNF drugs lower myocardial infarctions," according to Dr. Nurmohamed.
The new results he reported came from a review of 109,462 United States patients diagnosed with RA who had filled at least one prescription for an anti-TNF drug, methotrexate, or another DMARD, and were included in a database maintained by Thomson Reuters MarketScan The researchers reviewed the patient records following the date when they filled their index prescription until they developed a cardiovascular event, ended their enrollment in their health plan, or until 6 months after they stopped taking their index drug, whichever came first. The review included 48,210 patient-years of follow-up while on treatment with an anti-TNF drug, 13,540 patient-years of treatment with methotrexate, and 7,320 patient-years of treatment with another nonbiologic DMARD. The database included more than 75,000 patient-years of follow-up when patients received monotherapy with one of these three treatments, according to Dr. Nurmohamed.
During follow-up, 1,743 patients (1.6%) had a cardiovascular event, a myocardial infarction (MI), unstable angina, heart failure, or stroke. Events primarily occurred as MI, unstable angina, or heart failure. Dr. Nurmohamed said that he and his associates then ran a series of Cox proportional hazard models that assessed the outcomes from cumulative exposure to the various treatments and controlled for patient demographics, use of cardiovascular medications, smoking, comorbidities, hypertension, diabetes, history of cardiovascular events, and medical-resource use.
The analyses showed that for every 6 months of treatment with an anti-TNF drug, the rate of all cardiovascular events fell by 13%, compared with patients not treated with an anti-TNF drug. Six months of anti-TNF-drug treatment cut the rate of MIs specifically by 20%, compared with nonuse of an anti-TNF drug. Both effects were statistically significant.
The analyses also showed that the cardiovascular-event effect of anti-TNF drugs was cumulative. A year of use cut the cardiovascular event rate by 24%, 2 years cut the rate by 42%, and 3 years cut the rate by 56%, compared with patients who did not receive an anti-TNF drug, Dr. Nurmohamed reported. This cumulative effect was not surprising because "prolonged inflammatory suppression will lead to more pronounced risk reduction," he said.
The analyses also showed that the cardiovascular-risk reduction of treatment with an anti-TNF drug, compared with patients who did not get this drug class, was a statistically significant 14% in patients who were at least 50 years old, and a significant 15% in patients with no prior exposure to methotrexate. The analyses failed to find a significant impact on cardiovascular disease from treatment with methotrexate or other nonbiologic DMARDs.
Dr. Nurmohamed said that he had no relevant disclosures.
BERLIN – Treatment with a drug that blocked the action of tumor necrosis factor led to a significant reduction of cardiovascular events in patients with rheumatoid arthritis, compared with treatment with methotrexate or other nonbiologic disease modifying antirheumatic drugs, in a review of more than 100,000 patients treated during routine practice, according to Dr. Michael T. Nurmohamed.
Although this finding adds to the accumulating circumstantial evidence that treating patients with rheumatoid arthritis (RA) with a drug that blocks tumor necrosis factor (TNF) reduces their cardiovascular disease risk better than does treatment with disease-modifying antirheumatic drugs (DMARDs), current evidence does not yet clearly support an early start to anti-TNF treatment to maximize cardiovascular risk reduction, Dr. Nurmohamed said in an interview at the annual European Congress of Rheumatology.
"However, the literature does indicate that effective inflammatory suppression is essential [for patients with RA], and that if this is not achieved within a reasonable time frame with high-dose methotrexate or another DMARD then treatment with a biological, anti-TNF drug should be considered," he noted.
"The rheumatologic aim of treatment should be minimal RA disease activity and preferably remission. An additional aim of treatment might be cardiovascular risk reduction. If anti-TNF treatment stops when an RA patient achieves disease remission, we need to know whether the arterial inflammatory process also stays in remission. Investigations of this are now underway, and these studies may reveal new biomarkers of cardiovascular risk in RA patients. Until these results are available, cardiovascular risk management in patients with RA should follow EULAR recommendations (Ann. Rheum. Dis. 2010;69:325-31)," said Dr. Nurmohamed, a rheumatologist at VU University Medical Centre in Amsterdam.
"It appears that in patients with RA, atherosclerotic plaques rupture earlier," he said in an interview. "We know that inflammation is very important in that process. It might be that when you treat patients with an anti-TNF drug or other anti-inflammatory, you reduce the inflammatory content of the plaque. Therefore the plaque ruptures later. That may be the way that anti-TNF drugs lower myocardial infarctions," according to Dr. Nurmohamed.
The new results he reported came from a review of 109,462 United States patients diagnosed with RA who had filled at least one prescription for an anti-TNF drug, methotrexate, or another DMARD, and were included in a database maintained by Thomson Reuters MarketScan The researchers reviewed the patient records following the date when they filled their index prescription until they developed a cardiovascular event, ended their enrollment in their health plan, or until 6 months after they stopped taking their index drug, whichever came first. The review included 48,210 patient-years of follow-up while on treatment with an anti-TNF drug, 13,540 patient-years of treatment with methotrexate, and 7,320 patient-years of treatment with another nonbiologic DMARD. The database included more than 75,000 patient-years of follow-up when patients received monotherapy with one of these three treatments, according to Dr. Nurmohamed.
During follow-up, 1,743 patients (1.6%) had a cardiovascular event, a myocardial infarction (MI), unstable angina, heart failure, or stroke. Events primarily occurred as MI, unstable angina, or heart failure. Dr. Nurmohamed said that he and his associates then ran a series of Cox proportional hazard models that assessed the outcomes from cumulative exposure to the various treatments and controlled for patient demographics, use of cardiovascular medications, smoking, comorbidities, hypertension, diabetes, history of cardiovascular events, and medical-resource use.
The analyses showed that for every 6 months of treatment with an anti-TNF drug, the rate of all cardiovascular events fell by 13%, compared with patients not treated with an anti-TNF drug. Six months of anti-TNF-drug treatment cut the rate of MIs specifically by 20%, compared with nonuse of an anti-TNF drug. Both effects were statistically significant.
The analyses also showed that the cardiovascular-event effect of anti-TNF drugs was cumulative. A year of use cut the cardiovascular event rate by 24%, 2 years cut the rate by 42%, and 3 years cut the rate by 56%, compared with patients who did not receive an anti-TNF drug, Dr. Nurmohamed reported. This cumulative effect was not surprising because "prolonged inflammatory suppression will lead to more pronounced risk reduction," he said.
The analyses also showed that the cardiovascular-risk reduction of treatment with an anti-TNF drug, compared with patients who did not get this drug class, was a statistically significant 14% in patients who were at least 50 years old, and a significant 15% in patients with no prior exposure to methotrexate. The analyses failed to find a significant impact on cardiovascular disease from treatment with methotrexate or other nonbiologic DMARDs.
Dr. Nurmohamed said that he had no relevant disclosures.
BERLIN – Treatment with a drug that blocked the action of tumor necrosis factor led to a significant reduction of cardiovascular events in patients with rheumatoid arthritis, compared with treatment with methotrexate or other nonbiologic disease modifying antirheumatic drugs, in a review of more than 100,000 patients treated during routine practice, according to Dr. Michael T. Nurmohamed.
Although this finding adds to the accumulating circumstantial evidence that treating patients with rheumatoid arthritis (RA) with a drug that blocks tumor necrosis factor (TNF) reduces their cardiovascular disease risk better than does treatment with disease-modifying antirheumatic drugs (DMARDs), current evidence does not yet clearly support an early start to anti-TNF treatment to maximize cardiovascular risk reduction, Dr. Nurmohamed said in an interview at the annual European Congress of Rheumatology.
"However, the literature does indicate that effective inflammatory suppression is essential [for patients with RA], and that if this is not achieved within a reasonable time frame with high-dose methotrexate or another DMARD then treatment with a biological, anti-TNF drug should be considered," he noted.
"The rheumatologic aim of treatment should be minimal RA disease activity and preferably remission. An additional aim of treatment might be cardiovascular risk reduction. If anti-TNF treatment stops when an RA patient achieves disease remission, we need to know whether the arterial inflammatory process also stays in remission. Investigations of this are now underway, and these studies may reveal new biomarkers of cardiovascular risk in RA patients. Until these results are available, cardiovascular risk management in patients with RA should follow EULAR recommendations (Ann. Rheum. Dis. 2010;69:325-31)," said Dr. Nurmohamed, a rheumatologist at VU University Medical Centre in Amsterdam.
"It appears that in patients with RA, atherosclerotic plaques rupture earlier," he said in an interview. "We know that inflammation is very important in that process. It might be that when you treat patients with an anti-TNF drug or other anti-inflammatory, you reduce the inflammatory content of the plaque. Therefore the plaque ruptures later. That may be the way that anti-TNF drugs lower myocardial infarctions," according to Dr. Nurmohamed.
The new results he reported came from a review of 109,462 United States patients diagnosed with RA who had filled at least one prescription for an anti-TNF drug, methotrexate, or another DMARD, and were included in a database maintained by Thomson Reuters MarketScan The researchers reviewed the patient records following the date when they filled their index prescription until they developed a cardiovascular event, ended their enrollment in their health plan, or until 6 months after they stopped taking their index drug, whichever came first. The review included 48,210 patient-years of follow-up while on treatment with an anti-TNF drug, 13,540 patient-years of treatment with methotrexate, and 7,320 patient-years of treatment with another nonbiologic DMARD. The database included more than 75,000 patient-years of follow-up when patients received monotherapy with one of these three treatments, according to Dr. Nurmohamed.
During follow-up, 1,743 patients (1.6%) had a cardiovascular event, a myocardial infarction (MI), unstable angina, heart failure, or stroke. Events primarily occurred as MI, unstable angina, or heart failure. Dr. Nurmohamed said that he and his associates then ran a series of Cox proportional hazard models that assessed the outcomes from cumulative exposure to the various treatments and controlled for patient demographics, use of cardiovascular medications, smoking, comorbidities, hypertension, diabetes, history of cardiovascular events, and medical-resource use.
The analyses showed that for every 6 months of treatment with an anti-TNF drug, the rate of all cardiovascular events fell by 13%, compared with patients not treated with an anti-TNF drug. Six months of anti-TNF-drug treatment cut the rate of MIs specifically by 20%, compared with nonuse of an anti-TNF drug. Both effects were statistically significant.
The analyses also showed that the cardiovascular-event effect of anti-TNF drugs was cumulative. A year of use cut the cardiovascular event rate by 24%, 2 years cut the rate by 42%, and 3 years cut the rate by 56%, compared with patients who did not receive an anti-TNF drug, Dr. Nurmohamed reported. This cumulative effect was not surprising because "prolonged inflammatory suppression will lead to more pronounced risk reduction," he said.
The analyses also showed that the cardiovascular-risk reduction of treatment with an anti-TNF drug, compared with patients who did not get this drug class, was a statistically significant 14% in patients who were at least 50 years old, and a significant 15% in patients with no prior exposure to methotrexate. The analyses failed to find a significant impact on cardiovascular disease from treatment with methotrexate or other nonbiologic DMARDs.
Dr. Nurmohamed said that he had no relevant disclosures.
FROM THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Major Finding: Six months of anti-TNF treatment cut cardiovascular events in RA patients by 13%, compared with patients not on an anti-TNF drug.
Data Source: Data came from a review of 109,462 U.S. patients with rheumatoid arthritis in a database maintained by Thomson Reuters MarketScan.
Disclosures: Dr. Nurmohamed said that he had no relevant disclosures.
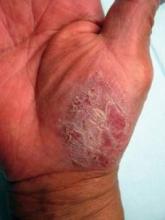
High-Dose Ustekinumab Stomps Out Palmoplantar Psoriasis
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: The primary endpoint of a palm-sole PGA score of 0 or 1 was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose
Data Source: An open-label, 24-week study of 20 patients treated with ustekinumab for moderate-to-severe palmoplantar psoriasis refractory to topical corticosteroids.
Disclosures: This investigator-initiated study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator, Dr. Alice B. Gottlieb, reported having numerous industry relationships.
Could a Urine Test Predict Response to Biologics?
GLASGOW, SCOTLAND – Urine analysis might help to predict if patients with rheumatoid arthritis are likely to respond to biologic treatments, the results of a small metabolomics study suggest.
Pretreatment levels of histamine, glutamine, xanthurenic acid, and ethanolamine were consistently correlated to response to anti–tumor necrosis factor–alpha (anti-TNF-alpha) therapy at 12 weeks in the 36-patient evaluation.
Further research is needed, of course, before any practical application of the research can be confirmed, but the finding does raise the possibly that a simple urine-based dipstick test could one day be available in the physician’s office.
"TNF has huge effects on metabolism," said Dr. Sabrina Kapoor at the annual meeting of the British Society for Rheumatology. These include effects on rheumatoid cachexia, angiogenesis, the acute phase response, and the recruitment of leukocytes to sites of inflammation.
"Metabolomics assess many metabolites together in biological samples, such as urine or blood," explained Dr. Kapoor, an Arthritis U.K. clinical research fellow in the Rheumatology Research Group at the University of Birmingham (England). The questions were, could such metabolites be found in the urine of patients with arthritic disease, and if they were present, did the metabolites change in response to treatment?
Dr. Kapoor and her associates obtained frozen urine samples from 16 patients with RA and 20 with psoriatic arthritis (PsA).
The samples had been taken during a randomized, three-center clinical study investigating patient responses to treatment with infliximab or etanercept (Rheumatology 2012;51[suppl.3]:abstract O15).
Nuclear magnetic resonance (NMR) spectroscopy was used to analyze the metabolomic profiles of the urine samples that were taken before and 12 weeks after anti-TNF treatment.
All patients in the study received methotrexate and had a disease duration of more than 6 months. RA patients were rheumatoid factor (RF) positive, anti–cyclic citrullinated protein (CCP) antibody positive, or both, and had a DAS28 (Disease Activity Score based on a 28-joint count) greater than 4. PsA patients were negative for RF and anti-CCP antibodies, with three or more swollen or tender joints.
Only the samples from the patients with RA could be linked to treatment effect. All the patients with PsA had a good response to treatment according to EULAR criteria (defined as improvement in two or more of the following: tender joint score, swollen joint score, patient global score, and physician global score).
Changes in the DAS28 were used to identify patients with RA who did (n = 7) or did not (n = 9) respond to anti-TNF therapy at 12 weeks. Good responders were those who achieved a DAS28 lower than 3.2, or an improvement in score greater than 1.2.
The baseline clinical characteristics of "good responders" and "not good responders" were similar: The mean age was about 50 years, all patients were women, and all had a similar history of steroid or nonsteroidal anti-inflammatory drug use.
RA patients who responded well to anti-TNF therapy had a distinct metabolomic profile compared with those who did not exhibit a good response to treatment.
"There was a significant [P = .04] correlation between baseline metabolomic profiles in the urine samples and the extent of change in DAS28," Dr. Kapoor said.
Baseline metabolomic analysis of the urine in RA patients had 85.9% sensitivity and 85.7%, specificity to detect treatment response.
"Urine is actually a cleaner biofluid than other biofluids, such as serum," Dr. Kapoor noted in the discussion that followed her presentation, noting that there are fewer proteins involved that could interfere with the NMR.
The samples tested were randomly obtained, but there is no evidence that any special collection protocols (such as early-morning collection or dietary restrictions) would be needed, she said. There is also no suggestion that urine would need handling in a particular way, although perhaps antibacterial treatment might be needed to keep specimens fresh.
These data, of course, need to be confirmed in a much larger, independent cohort of patients before any subsequent investigation of specific collection or storage requirements.
Dr. Kapoor had no financial disclosures. Merck sponsored the original study, but the company did not sponsor the metabolomics analysis reported here.
GLASGOW, SCOTLAND – Urine analysis might help to predict if patients with rheumatoid arthritis are likely to respond to biologic treatments, the results of a small metabolomics study suggest.
Pretreatment levels of histamine, glutamine, xanthurenic acid, and ethanolamine were consistently correlated to response to anti–tumor necrosis factor–alpha (anti-TNF-alpha) therapy at 12 weeks in the 36-patient evaluation.
Further research is needed, of course, before any practical application of the research can be confirmed, but the finding does raise the possibly that a simple urine-based dipstick test could one day be available in the physician’s office.
"TNF has huge effects on metabolism," said Dr. Sabrina Kapoor at the annual meeting of the British Society for Rheumatology. These include effects on rheumatoid cachexia, angiogenesis, the acute phase response, and the recruitment of leukocytes to sites of inflammation.
"Metabolomics assess many metabolites together in biological samples, such as urine or blood," explained Dr. Kapoor, an Arthritis U.K. clinical research fellow in the Rheumatology Research Group at the University of Birmingham (England). The questions were, could such metabolites be found in the urine of patients with arthritic disease, and if they were present, did the metabolites change in response to treatment?
Dr. Kapoor and her associates obtained frozen urine samples from 16 patients with RA and 20 with psoriatic arthritis (PsA).
The samples had been taken during a randomized, three-center clinical study investigating patient responses to treatment with infliximab or etanercept (Rheumatology 2012;51[suppl.3]:abstract O15).
Nuclear magnetic resonance (NMR) spectroscopy was used to analyze the metabolomic profiles of the urine samples that were taken before and 12 weeks after anti-TNF treatment.
All patients in the study received methotrexate and had a disease duration of more than 6 months. RA patients were rheumatoid factor (RF) positive, anti–cyclic citrullinated protein (CCP) antibody positive, or both, and had a DAS28 (Disease Activity Score based on a 28-joint count) greater than 4. PsA patients were negative for RF and anti-CCP antibodies, with three or more swollen or tender joints.
Only the samples from the patients with RA could be linked to treatment effect. All the patients with PsA had a good response to treatment according to EULAR criteria (defined as improvement in two or more of the following: tender joint score, swollen joint score, patient global score, and physician global score).
Changes in the DAS28 were used to identify patients with RA who did (n = 7) or did not (n = 9) respond to anti-TNF therapy at 12 weeks. Good responders were those who achieved a DAS28 lower than 3.2, or an improvement in score greater than 1.2.
The baseline clinical characteristics of "good responders" and "not good responders" were similar: The mean age was about 50 years, all patients were women, and all had a similar history of steroid or nonsteroidal anti-inflammatory drug use.
RA patients who responded well to anti-TNF therapy had a distinct metabolomic profile compared with those who did not exhibit a good response to treatment.
"There was a significant [P = .04] correlation between baseline metabolomic profiles in the urine samples and the extent of change in DAS28," Dr. Kapoor said.
Baseline metabolomic analysis of the urine in RA patients had 85.9% sensitivity and 85.7%, specificity to detect treatment response.
"Urine is actually a cleaner biofluid than other biofluids, such as serum," Dr. Kapoor noted in the discussion that followed her presentation, noting that there are fewer proteins involved that could interfere with the NMR.
The samples tested were randomly obtained, but there is no evidence that any special collection protocols (such as early-morning collection or dietary restrictions) would be needed, she said. There is also no suggestion that urine would need handling in a particular way, although perhaps antibacterial treatment might be needed to keep specimens fresh.
These data, of course, need to be confirmed in a much larger, independent cohort of patients before any subsequent investigation of specific collection or storage requirements.
Dr. Kapoor had no financial disclosures. Merck sponsored the original study, but the company did not sponsor the metabolomics analysis reported here.
GLASGOW, SCOTLAND – Urine analysis might help to predict if patients with rheumatoid arthritis are likely to respond to biologic treatments, the results of a small metabolomics study suggest.
Pretreatment levels of histamine, glutamine, xanthurenic acid, and ethanolamine were consistently correlated to response to anti–tumor necrosis factor–alpha (anti-TNF-alpha) therapy at 12 weeks in the 36-patient evaluation.
Further research is needed, of course, before any practical application of the research can be confirmed, but the finding does raise the possibly that a simple urine-based dipstick test could one day be available in the physician’s office.
"TNF has huge effects on metabolism," said Dr. Sabrina Kapoor at the annual meeting of the British Society for Rheumatology. These include effects on rheumatoid cachexia, angiogenesis, the acute phase response, and the recruitment of leukocytes to sites of inflammation.
"Metabolomics assess many metabolites together in biological samples, such as urine or blood," explained Dr. Kapoor, an Arthritis U.K. clinical research fellow in the Rheumatology Research Group at the University of Birmingham (England). The questions were, could such metabolites be found in the urine of patients with arthritic disease, and if they were present, did the metabolites change in response to treatment?
Dr. Kapoor and her associates obtained frozen urine samples from 16 patients with RA and 20 with psoriatic arthritis (PsA).
The samples had been taken during a randomized, three-center clinical study investigating patient responses to treatment with infliximab or etanercept (Rheumatology 2012;51[suppl.3]:abstract O15).
Nuclear magnetic resonance (NMR) spectroscopy was used to analyze the metabolomic profiles of the urine samples that were taken before and 12 weeks after anti-TNF treatment.
All patients in the study received methotrexate and had a disease duration of more than 6 months. RA patients were rheumatoid factor (RF) positive, anti–cyclic citrullinated protein (CCP) antibody positive, or both, and had a DAS28 (Disease Activity Score based on a 28-joint count) greater than 4. PsA patients were negative for RF and anti-CCP antibodies, with three or more swollen or tender joints.
Only the samples from the patients with RA could be linked to treatment effect. All the patients with PsA had a good response to treatment according to EULAR criteria (defined as improvement in two or more of the following: tender joint score, swollen joint score, patient global score, and physician global score).
Changes in the DAS28 were used to identify patients with RA who did (n = 7) or did not (n = 9) respond to anti-TNF therapy at 12 weeks. Good responders were those who achieved a DAS28 lower than 3.2, or an improvement in score greater than 1.2.
The baseline clinical characteristics of "good responders" and "not good responders" were similar: The mean age was about 50 years, all patients were women, and all had a similar history of steroid or nonsteroidal anti-inflammatory drug use.
RA patients who responded well to anti-TNF therapy had a distinct metabolomic profile compared with those who did not exhibit a good response to treatment.
"There was a significant [P = .04] correlation between baseline metabolomic profiles in the urine samples and the extent of change in DAS28," Dr. Kapoor said.
Baseline metabolomic analysis of the urine in RA patients had 85.9% sensitivity and 85.7%, specificity to detect treatment response.
"Urine is actually a cleaner biofluid than other biofluids, such as serum," Dr. Kapoor noted in the discussion that followed her presentation, noting that there are fewer proteins involved that could interfere with the NMR.
The samples tested were randomly obtained, but there is no evidence that any special collection protocols (such as early-morning collection or dietary restrictions) would be needed, she said. There is also no suggestion that urine would need handling in a particular way, although perhaps antibacterial treatment might be needed to keep specimens fresh.
These data, of course, need to be confirmed in a much larger, independent cohort of patients before any subsequent investigation of specific collection or storage requirements.
Dr. Kapoor had no financial disclosures. Merck sponsored the original study, but the company did not sponsor the metabolomics analysis reported here.
FROM THE ANNUAL MEETING OF THE BRITISH SOCIETY FOR RHEUMATOLOGY
Major Finding: Baseline metabolomic analysis of pretreatment levels of histamine, glutamine, xanthurenic acid, and ethanolamine in the urine of RA patients had 85.9% sensitivity and 85.7% specificity to detect treatment response to anti-TNF therapy.
Data Source: Data came from an examination of frozen urine samples from 16 patients with RA and 20 with psoriatic arthritis who participated in a randomized clinical study comparing responses to infliximab and etanercept treatment.
Disclosures: Dr. Kapoor had no financial disclosures. Merck sponsored the original study but the company did not sponsor the metabolomics analysis reported here.
Smokers Less Likely to Respond to Biologic Treatment for RA
GLASGOW, SCOTLAND – Patients who smoke are substantially less likely to respond to biologic treatment for rheumatoid arthritis than are those who have never smoked.
The percentage of current smokers who responded to treatment with anti–tumor necrosis factor-alpha (anti-TNF-alpha) drugs at 6 months was just 27%, compared with 90% of never smokers and 63% of ex-smokers in a retrospective study of 359 patients.
Similarly poor response to rituximab was seen in patients who were current smokers, with respective response rates for current, ex-, and never smokers of 20%, 68%, and 98%.
A response was defined as a mean change in 28-joint disease activity score (DAS28) of 1.2 or greater, according to the U.K. National Clinical for Health and Clinical of Excellence (NICE) definition.
This begs the controversial question of whether current smokers should be given treatment with biologic agents unless they quit smoking, said Dr. Abdul Khan, a rheumatology specialist trainee at St. George’s Hospital in London, speaking at the annual meeting of the British Society for Rheumatology.
Working with Dr. David L. Scott, Dr. Khan assessed whether two simple pretreatment biomarkers – rheumatoid factor (RF) and smoking status – could help predict the response to biologic therapy for RA (Rheumatology 2012;51:iii41-2, abstract O40). They studied 209 patients treated with anti-TNFs and 150 treated with rituximab. The mean age of patients was 56 years for the anti-TNF treated patients and 61 years for the rituximab group. The mean disease duration was 8 years and 13 years, respectively, and 61% and 53% were RF positive.
Primary outcome assessments included the 6-month change in DAS28 and calculation of NICE responders (DAS28 change greater than or equal to 1.2). Smoking status was assessed as being current, previous, or never. Dr. Khan observed that a more detailed evaluation of smoking history might be warranted in future investigations, such as the calculation of pack years. RF status was determined, and anticitrullinated protein autoantibody (ACPA) positivity was determined for patients receiving rituximab therapy.
The mean change in DAS28 scores after 6 months’ anti-TNF therapy for never smokers was 2.6. For current smokers, the mean change was just 0.9 and for ex-smokers, it was 1.39. Corresponding figures for rituximab were 2.92, 0.63, and 1.49.
RF status predicted responses to rituximab but not to anti-TNFs, with a mean change of 2.14 for RF-positive patients and 0.98 for RF-negative patients treated with rituximab after 6 months.
Combining RF and ACPA status showed significant effects with regards to response to rituximab – 80% of never but only 22% of current smokers responded to treatment at 6 month if they were positive for both RF and ACPA
"Smoking and rheumatoid factor/ACPA status had an additive effect on DAS28 responses," Dr. Khan reported. Of 55 never smokers, 46 were RF/ACPA positive and nine were negative and almost all (98%) were NICE responders, with the mean fall in DAS28 score of 2.77.
Strikingly different results were seen for current smokers, however, with 50% of RF-positive patients responding, compared with 3% of RF-negative patients. Previous smokers showed intermediate response rates between those of current and never smokers.
Aside from the other well-documented health risks of smoking – including an increased risk of cardiovascular and pulmonary complications such as chronic obstructive pulmonary disease and lung cancer – these data suggest that patients with RA would do well to give up smoking if they currently smoke.
Indeed, in a press statement, Dr. Scott, professor of rheumatology at King’s College in London, suggested that "these findings show what a dramatic effect modifying your lifestyle, such as giving up smoking, can have on treatment outcomes."
Dr. Khan noted that they also raise an ethical dilemma for clinicians. "The balance of evidence suggests that biologics are unlikely to be cost effective in RA patients who continue to smoke.
"This observation creates a complex ethical dilemma which needs to be addressed."
Dr. Khan and Dr. Scott had no financial disclosures.
GLASGOW, SCOTLAND – Patients who smoke are substantially less likely to respond to biologic treatment for rheumatoid arthritis than are those who have never smoked.
The percentage of current smokers who responded to treatment with anti–tumor necrosis factor-alpha (anti-TNF-alpha) drugs at 6 months was just 27%, compared with 90% of never smokers and 63% of ex-smokers in a retrospective study of 359 patients.
Similarly poor response to rituximab was seen in patients who were current smokers, with respective response rates for current, ex-, and never smokers of 20%, 68%, and 98%.
A response was defined as a mean change in 28-joint disease activity score (DAS28) of 1.2 or greater, according to the U.K. National Clinical for Health and Clinical of Excellence (NICE) definition.
This begs the controversial question of whether current smokers should be given treatment with biologic agents unless they quit smoking, said Dr. Abdul Khan, a rheumatology specialist trainee at St. George’s Hospital in London, speaking at the annual meeting of the British Society for Rheumatology.
Working with Dr. David L. Scott, Dr. Khan assessed whether two simple pretreatment biomarkers – rheumatoid factor (RF) and smoking status – could help predict the response to biologic therapy for RA (Rheumatology 2012;51:iii41-2, abstract O40). They studied 209 patients treated with anti-TNFs and 150 treated with rituximab. The mean age of patients was 56 years for the anti-TNF treated patients and 61 years for the rituximab group. The mean disease duration was 8 years and 13 years, respectively, and 61% and 53% were RF positive.
Primary outcome assessments included the 6-month change in DAS28 and calculation of NICE responders (DAS28 change greater than or equal to 1.2). Smoking status was assessed as being current, previous, or never. Dr. Khan observed that a more detailed evaluation of smoking history might be warranted in future investigations, such as the calculation of pack years. RF status was determined, and anticitrullinated protein autoantibody (ACPA) positivity was determined for patients receiving rituximab therapy.
The mean change in DAS28 scores after 6 months’ anti-TNF therapy for never smokers was 2.6. For current smokers, the mean change was just 0.9 and for ex-smokers, it was 1.39. Corresponding figures for rituximab were 2.92, 0.63, and 1.49.
RF status predicted responses to rituximab but not to anti-TNFs, with a mean change of 2.14 for RF-positive patients and 0.98 for RF-negative patients treated with rituximab after 6 months.
Combining RF and ACPA status showed significant effects with regards to response to rituximab – 80% of never but only 22% of current smokers responded to treatment at 6 month if they were positive for both RF and ACPA
"Smoking and rheumatoid factor/ACPA status had an additive effect on DAS28 responses," Dr. Khan reported. Of 55 never smokers, 46 were RF/ACPA positive and nine were negative and almost all (98%) were NICE responders, with the mean fall in DAS28 score of 2.77.
Strikingly different results were seen for current smokers, however, with 50% of RF-positive patients responding, compared with 3% of RF-negative patients. Previous smokers showed intermediate response rates between those of current and never smokers.
Aside from the other well-documented health risks of smoking – including an increased risk of cardiovascular and pulmonary complications such as chronic obstructive pulmonary disease and lung cancer – these data suggest that patients with RA would do well to give up smoking if they currently smoke.
Indeed, in a press statement, Dr. Scott, professor of rheumatology at King’s College in London, suggested that "these findings show what a dramatic effect modifying your lifestyle, such as giving up smoking, can have on treatment outcomes."
Dr. Khan noted that they also raise an ethical dilemma for clinicians. "The balance of evidence suggests that biologics are unlikely to be cost effective in RA patients who continue to smoke.
"This observation creates a complex ethical dilemma which needs to be addressed."
Dr. Khan and Dr. Scott had no financial disclosures.
GLASGOW, SCOTLAND – Patients who smoke are substantially less likely to respond to biologic treatment for rheumatoid arthritis than are those who have never smoked.
The percentage of current smokers who responded to treatment with anti–tumor necrosis factor-alpha (anti-TNF-alpha) drugs at 6 months was just 27%, compared with 90% of never smokers and 63% of ex-smokers in a retrospective study of 359 patients.
Similarly poor response to rituximab was seen in patients who were current smokers, with respective response rates for current, ex-, and never smokers of 20%, 68%, and 98%.
A response was defined as a mean change in 28-joint disease activity score (DAS28) of 1.2 or greater, according to the U.K. National Clinical for Health and Clinical of Excellence (NICE) definition.
This begs the controversial question of whether current smokers should be given treatment with biologic agents unless they quit smoking, said Dr. Abdul Khan, a rheumatology specialist trainee at St. George’s Hospital in London, speaking at the annual meeting of the British Society for Rheumatology.
Working with Dr. David L. Scott, Dr. Khan assessed whether two simple pretreatment biomarkers – rheumatoid factor (RF) and smoking status – could help predict the response to biologic therapy for RA (Rheumatology 2012;51:iii41-2, abstract O40). They studied 209 patients treated with anti-TNFs and 150 treated with rituximab. The mean age of patients was 56 years for the anti-TNF treated patients and 61 years for the rituximab group. The mean disease duration was 8 years and 13 years, respectively, and 61% and 53% were RF positive.
Primary outcome assessments included the 6-month change in DAS28 and calculation of NICE responders (DAS28 change greater than or equal to 1.2). Smoking status was assessed as being current, previous, or never. Dr. Khan observed that a more detailed evaluation of smoking history might be warranted in future investigations, such as the calculation of pack years. RF status was determined, and anticitrullinated protein autoantibody (ACPA) positivity was determined for patients receiving rituximab therapy.
The mean change in DAS28 scores after 6 months’ anti-TNF therapy for never smokers was 2.6. For current smokers, the mean change was just 0.9 and for ex-smokers, it was 1.39. Corresponding figures for rituximab were 2.92, 0.63, and 1.49.
RF status predicted responses to rituximab but not to anti-TNFs, with a mean change of 2.14 for RF-positive patients and 0.98 for RF-negative patients treated with rituximab after 6 months.
Combining RF and ACPA status showed significant effects with regards to response to rituximab – 80% of never but only 22% of current smokers responded to treatment at 6 month if they were positive for both RF and ACPA
"Smoking and rheumatoid factor/ACPA status had an additive effect on DAS28 responses," Dr. Khan reported. Of 55 never smokers, 46 were RF/ACPA positive and nine were negative and almost all (98%) were NICE responders, with the mean fall in DAS28 score of 2.77.
Strikingly different results were seen for current smokers, however, with 50% of RF-positive patients responding, compared with 3% of RF-negative patients. Previous smokers showed intermediate response rates between those of current and never smokers.
Aside from the other well-documented health risks of smoking – including an increased risk of cardiovascular and pulmonary complications such as chronic obstructive pulmonary disease and lung cancer – these data suggest that patients with RA would do well to give up smoking if they currently smoke.
Indeed, in a press statement, Dr. Scott, professor of rheumatology at King’s College in London, suggested that "these findings show what a dramatic effect modifying your lifestyle, such as giving up smoking, can have on treatment outcomes."
Dr. Khan noted that they also raise an ethical dilemma for clinicians. "The balance of evidence suggests that biologics are unlikely to be cost effective in RA patients who continue to smoke.
"This observation creates a complex ethical dilemma which needs to be addressed."
Dr. Khan and Dr. Scott had no financial disclosures.
FROM THE ANNUAL MEETING OF THE BRITISH SOCIETY FOR RHEUMATOLOGY
Major Finding: Response rates to 6 months of anti-TNF therapy were 27% for current, 63% for previous, and 90% for never smokers. Corresponding data for rituximab were 20%, 68%, and 98%.
Data Source: This was a retrospective study of 359 RA patients treated with anti-TNF agents or rituximab.
Disclosures: Dr. Khan and Dr. Scott had no financial disclosures.
Avoid Certain Vaccine-Biologic Combos
NEW YORK – Handy mnemonic devices can help predict vaccine response in patients taking biologics for rheumatoid arthritis, according to Dr. Daniel E. Furst.
Vaccinating patients taking biologic agents for rheumatoid arthritis is tricky. The effects of both the vaccine and biologic can be T-cell dependent or independent, or sometimes both, and when the mechanisms match, the vaccine’s response will likely be attenuated, he said at a rheumatology meeting sponsored by New York University.
Think ‘PITH’ – (Human) Papillomavirus, Influenza A/B, Tetanus, and Hepatitis B – to remember which vaccines have protein-based antigens and are T-cell dependent, said Dr. Furst, a rheumatology professor at the University of California in Los Angeles.
For vaccines that have carbohydrate-based antigens, which are largely T-cell independent (B-cell dependent), remember ‘PHIM’: Pneumococcus, H. influenzae B, Influenza A/B, and Meningococcus.
Biologic agents that affect T-cells include methotrexate, the TNF inhibitors, abatacept, and tocilizumab. As a general rule, biologics that affect T-cells decrease one’s response to T-cell dependent vaccines. For example, etanercept has been shown to decrease by as much as half, as well as delay the response to, the hepatitis B vaccine in both normal controls and patients with RA.
In normal volunteers abatacept blunted the response to tetanus vaccine given 2 weeks later by about 50% and given 8 weeks later by about 20%, compared to normal controls (Arthritis Res. Ther. 2007;9:R38).
Likewise, tocilizumab has been shown to reduce the response to the influenza vaccine by about 15%, according to a study presented at EULAR 2007 in Barcelona.
B-cell dependent vaccine responses are diminished by B-cell directed therapy. For example, the SIERRA study of pneumococcal vaccination in rituximab-treated patients showed that, compared to methotrexate alone, a combination of rituximab plus methotrexate decreased the response to the vaccination at 4 weeks by 30% to 60% (Arthritis Rheum. 2010;62:64-74). "That’s just what we would expect," Dr. Furst noted.
It is important to note that biologics that affect T-cell independent mechanisms (B-cell directed therapy), such as rituximab, will not usually affect T-cell mediated vaccine responses. For example, rituximab does not change the response to tetanus toxoid, said Dr. Furst. Similarly, T-cell dependent biologics will not affect T-cell independent vaccinations, citing as an example the lack of effect of adalimumab on the response to the pneumococcal vaccine, according to a paper presented at EULAR in 2006.
The influenza A/B vaccine is an exception to the rule, because it first requires an early B-cell response followed by a T-cell response.
Dr. Furst recommends avoiding live attenuated virus vaccines in patients on biologics or immunosuppressive agents. Vaccines that are likely to be affected include those for varicella/zoster, intranasal influenza/H1N1, measles/mumps/rubella (MMR), yellow fever, oral polio, typhoid (Ty21a oral), vaccinia (smallpox), the BCG vaccine for tuberculosis, and rotavirus. Live viruses can result in disseminated disease in such patients, he said. Ideally patients should be immunized with these vaccines as needed before starting a biologic or any other immunosuppressive therapy.
Dr. Furst is the recipient of research funding/consultant/advisory board member of Abbott Laboratories, Actelion Pharmaceuticals, Amgen, Biogen Idec, Bristol-Myers Squibb, Centocor Ortho Biotech, CORRONA, Gilead Sciences, GlaxoSmithKline, Novartis Pharmaceuticals, Pfizer, Roche Pharmaceuticals/Genentech, and UCB.
Vaccinating patients, biologic agents, Think ‘PITH’, (Human) Papillomavirus, Influenza A/B, Tetanus, and Hepatitis B, vaccines have protein-based antigens, are T-cell dependent, T-cell independent, B-cell dependent, PHIM, Pneumococcus, H. influenzae B, Influenza A/B, and Meningococcus, methotrexate, the TNF inhibitors, abatacept, and tocilizumab.
NEW YORK – Handy mnemonic devices can help predict vaccine response in patients taking biologics for rheumatoid arthritis, according to Dr. Daniel E. Furst.
Vaccinating patients taking biologic agents for rheumatoid arthritis is tricky. The effects of both the vaccine and biologic can be T-cell dependent or independent, or sometimes both, and when the mechanisms match, the vaccine’s response will likely be attenuated, he said at a rheumatology meeting sponsored by New York University.
Think ‘PITH’ – (Human) Papillomavirus, Influenza A/B, Tetanus, and Hepatitis B – to remember which vaccines have protein-based antigens and are T-cell dependent, said Dr. Furst, a rheumatology professor at the University of California in Los Angeles.
For vaccines that have carbohydrate-based antigens, which are largely T-cell independent (B-cell dependent), remember ‘PHIM’: Pneumococcus, H. influenzae B, Influenza A/B, and Meningococcus.
Biologic agents that affect T-cells include methotrexate, the TNF inhibitors, abatacept, and tocilizumab. As a general rule, biologics that affect T-cells decrease one’s response to T-cell dependent vaccines. For example, etanercept has been shown to decrease by as much as half, as well as delay the response to, the hepatitis B vaccine in both normal controls and patients with RA.
In normal volunteers abatacept blunted the response to tetanus vaccine given 2 weeks later by about 50% and given 8 weeks later by about 20%, compared to normal controls (Arthritis Res. Ther. 2007;9:R38).
Likewise, tocilizumab has been shown to reduce the response to the influenza vaccine by about 15%, according to a study presented at EULAR 2007 in Barcelona.
B-cell dependent vaccine responses are diminished by B-cell directed therapy. For example, the SIERRA study of pneumococcal vaccination in rituximab-treated patients showed that, compared to methotrexate alone, a combination of rituximab plus methotrexate decreased the response to the vaccination at 4 weeks by 30% to 60% (Arthritis Rheum. 2010;62:64-74). "That’s just what we would expect," Dr. Furst noted.
It is important to note that biologics that affect T-cell independent mechanisms (B-cell directed therapy), such as rituximab, will not usually affect T-cell mediated vaccine responses. For example, rituximab does not change the response to tetanus toxoid, said Dr. Furst. Similarly, T-cell dependent biologics will not affect T-cell independent vaccinations, citing as an example the lack of effect of adalimumab on the response to the pneumococcal vaccine, according to a paper presented at EULAR in 2006.
The influenza A/B vaccine is an exception to the rule, because it first requires an early B-cell response followed by a T-cell response.
Dr. Furst recommends avoiding live attenuated virus vaccines in patients on biologics or immunosuppressive agents. Vaccines that are likely to be affected include those for varicella/zoster, intranasal influenza/H1N1, measles/mumps/rubella (MMR), yellow fever, oral polio, typhoid (Ty21a oral), vaccinia (smallpox), the BCG vaccine for tuberculosis, and rotavirus. Live viruses can result in disseminated disease in such patients, he said. Ideally patients should be immunized with these vaccines as needed before starting a biologic or any other immunosuppressive therapy.
Dr. Furst is the recipient of research funding/consultant/advisory board member of Abbott Laboratories, Actelion Pharmaceuticals, Amgen, Biogen Idec, Bristol-Myers Squibb, Centocor Ortho Biotech, CORRONA, Gilead Sciences, GlaxoSmithKline, Novartis Pharmaceuticals, Pfizer, Roche Pharmaceuticals/Genentech, and UCB.
NEW YORK – Handy mnemonic devices can help predict vaccine response in patients taking biologics for rheumatoid arthritis, according to Dr. Daniel E. Furst.
Vaccinating patients taking biologic agents for rheumatoid arthritis is tricky. The effects of both the vaccine and biologic can be T-cell dependent or independent, or sometimes both, and when the mechanisms match, the vaccine’s response will likely be attenuated, he said at a rheumatology meeting sponsored by New York University.
Think ‘PITH’ – (Human) Papillomavirus, Influenza A/B, Tetanus, and Hepatitis B – to remember which vaccines have protein-based antigens and are T-cell dependent, said Dr. Furst, a rheumatology professor at the University of California in Los Angeles.
For vaccines that have carbohydrate-based antigens, which are largely T-cell independent (B-cell dependent), remember ‘PHIM’: Pneumococcus, H. influenzae B, Influenza A/B, and Meningococcus.
Biologic agents that affect T-cells include methotrexate, the TNF inhibitors, abatacept, and tocilizumab. As a general rule, biologics that affect T-cells decrease one’s response to T-cell dependent vaccines. For example, etanercept has been shown to decrease by as much as half, as well as delay the response to, the hepatitis B vaccine in both normal controls and patients with RA.
In normal volunteers abatacept blunted the response to tetanus vaccine given 2 weeks later by about 50% and given 8 weeks later by about 20%, compared to normal controls (Arthritis Res. Ther. 2007;9:R38).
Likewise, tocilizumab has been shown to reduce the response to the influenza vaccine by about 15%, according to a study presented at EULAR 2007 in Barcelona.
B-cell dependent vaccine responses are diminished by B-cell directed therapy. For example, the SIERRA study of pneumococcal vaccination in rituximab-treated patients showed that, compared to methotrexate alone, a combination of rituximab plus methotrexate decreased the response to the vaccination at 4 weeks by 30% to 60% (Arthritis Rheum. 2010;62:64-74). "That’s just what we would expect," Dr. Furst noted.
It is important to note that biologics that affect T-cell independent mechanisms (B-cell directed therapy), such as rituximab, will not usually affect T-cell mediated vaccine responses. For example, rituximab does not change the response to tetanus toxoid, said Dr. Furst. Similarly, T-cell dependent biologics will not affect T-cell independent vaccinations, citing as an example the lack of effect of adalimumab on the response to the pneumococcal vaccine, according to a paper presented at EULAR in 2006.
The influenza A/B vaccine is an exception to the rule, because it first requires an early B-cell response followed by a T-cell response.
Dr. Furst recommends avoiding live attenuated virus vaccines in patients on biologics or immunosuppressive agents. Vaccines that are likely to be affected include those for varicella/zoster, intranasal influenza/H1N1, measles/mumps/rubella (MMR), yellow fever, oral polio, typhoid (Ty21a oral), vaccinia (smallpox), the BCG vaccine for tuberculosis, and rotavirus. Live viruses can result in disseminated disease in such patients, he said. Ideally patients should be immunized with these vaccines as needed before starting a biologic or any other immunosuppressive therapy.
Dr. Furst is the recipient of research funding/consultant/advisory board member of Abbott Laboratories, Actelion Pharmaceuticals, Amgen, Biogen Idec, Bristol-Myers Squibb, Centocor Ortho Biotech, CORRONA, Gilead Sciences, GlaxoSmithKline, Novartis Pharmaceuticals, Pfizer, Roche Pharmaceuticals/Genentech, and UCB.
Vaccinating patients, biologic agents, Think ‘PITH’, (Human) Papillomavirus, Influenza A/B, Tetanus, and Hepatitis B, vaccines have protein-based antigens, are T-cell dependent, T-cell independent, B-cell dependent, PHIM, Pneumococcus, H. influenzae B, Influenza A/B, and Meningococcus, methotrexate, the TNF inhibitors, abatacept, and tocilizumab.
Vaccinating patients, biologic agents, Think ‘PITH’, (Human) Papillomavirus, Influenza A/B, Tetanus, and Hepatitis B, vaccines have protein-based antigens, are T-cell dependent, T-cell independent, B-cell dependent, PHIM, Pneumococcus, H. influenzae B, Influenza A/B, and Meningococcus, methotrexate, the TNF inhibitors, abatacept, and tocilizumab.
EXPERT ANALYSIS FROM A RHEUMATOLOGY MEETING SPONSORED BY NEW YORK UNIVERSITY
Rheumatoid Vasculitis 5-Year Mortality Is 60%
GLASGOW, SCOTLAND – Five-year mortality following the diagnosis of systemic rheumatoid vasculitis hovers around 60%, according to an analysis of 34 cases identified in a British-based patient registry.
Although the annual incidence of SRV has reportedly been decreasing since the 1990s, these data suggest it remains a problem despite the introduction of modern immunosuppressive therapies and the use of earlier and more aggressive treatment for rheumatoid arthritis, Dr. Eleana Ntatsaki, a rheumatology specialist trainee at Norfolk and Norwich (England) University Hospital, said at the annual meeting of the British Society for Rheumatology.
The Norfolk Vasculitis Register (NORVASC) is a prospective patient registry established in 1988 to log cases of vasculitis that occur in the county of Norfolk, England. This is a region particularly well suited to epidemiological study, as it is an isolated geographical area with a stable and well-defined population. This is also one of the reasons the Norfolk Arthritis Register (NOAR) is situated in this part of the country.
Between Jan. 1, 2001, and Dec. 31, 2010, a total of 34 cases were reviewed in detail, with 18 patients (10 men) confirmed as having SRV according to previously published criteria (Am. J. Med. 1984;76:377-84), chart review, and independent physician assessment.
The median age of confirmed cases at diagnosis was 72 years and the average disease duration before SRV diagnosis was nearly 16 years. All patients were rheumatoid factor positive, 13 had documented erosive disease, and three had rheumatoid nodules.
All patients had been treated with steroids, a median of two prior DMARDs had been used (methotrexate in 65% of patients), and two patients had received biologic agents. SRV had been treated with intravenous cyclophosphamide (median six cycles) in all but one patient.
The average annual incidence of SRV was calculated to be 3.9 per million (95% confidence interval [CI], 2.3-6.2). This was substantially lower than the 9.1 per million (95% CI, 6.8-12.0) seen in a reference population of 47 patients who developed the complication between 1998 and 2000.
The average annual incidence rates in 2001-2010 were 4.5 (95% CI, 2.2-8.3) for men and 3.4 (95% CI, 1.4-6.6) for women. In 1998-2000, the rates were 8.9 (95% CI, 5.7-13.1) for men and 8.7 (95% CI, 5.6-12.8) for women.
No statistically significant differences were found in the number of patients experiencing systemic, cutaneous, neurologic, pulmonary, renal, ophthalmic, or gastrointestinal manifestations between the two time periods.
Mortality rates at 1 year were also similar between the cohorts, at 12% for 2001-2010 and 14% for 1998-2000. Five-year mortality rates were 60% and 51%, respectively.
"Modern immunosuppression therapy seems to be associated with a decrease in the incidence" of systemic rheumatoid vasculitis, said Dr. Ntatsaki, "but has had no significant influence on clinical features, or outcome."
Whether the decrease in incidence can truly be attributed to methotrexate or improvements in RA treatment in general is questionable, suggested Dr. Deborah Symmons, professor of rheumatology and musculoskeletal epidemiology at the University of Manchester (England).
This decline in the incidence of rheumatoid vasculitis is not necessarily because rheumatoid arthritis treatments have improved, she said. "Perhaps it is just the natural history of rheumatoid arthritis that vasculitis is becoming rare. Clearly when people do get it they do just as badly as they ever did, and perhaps all this about methotrexate is completely coincidental."
Dr. Ntatsaki reported having no financial disclosures.
GLASGOW, SCOTLAND – Five-year mortality following the diagnosis of systemic rheumatoid vasculitis hovers around 60%, according to an analysis of 34 cases identified in a British-based patient registry.
Although the annual incidence of SRV has reportedly been decreasing since the 1990s, these data suggest it remains a problem despite the introduction of modern immunosuppressive therapies and the use of earlier and more aggressive treatment for rheumatoid arthritis, Dr. Eleana Ntatsaki, a rheumatology specialist trainee at Norfolk and Norwich (England) University Hospital, said at the annual meeting of the British Society for Rheumatology.
The Norfolk Vasculitis Register (NORVASC) is a prospective patient registry established in 1988 to log cases of vasculitis that occur in the county of Norfolk, England. This is a region particularly well suited to epidemiological study, as it is an isolated geographical area with a stable and well-defined population. This is also one of the reasons the Norfolk Arthritis Register (NOAR) is situated in this part of the country.
Between Jan. 1, 2001, and Dec. 31, 2010, a total of 34 cases were reviewed in detail, with 18 patients (10 men) confirmed as having SRV according to previously published criteria (Am. J. Med. 1984;76:377-84), chart review, and independent physician assessment.
The median age of confirmed cases at diagnosis was 72 years and the average disease duration before SRV diagnosis was nearly 16 years. All patients were rheumatoid factor positive, 13 had documented erosive disease, and three had rheumatoid nodules.
All patients had been treated with steroids, a median of two prior DMARDs had been used (methotrexate in 65% of patients), and two patients had received biologic agents. SRV had been treated with intravenous cyclophosphamide (median six cycles) in all but one patient.
The average annual incidence of SRV was calculated to be 3.9 per million (95% confidence interval [CI], 2.3-6.2). This was substantially lower than the 9.1 per million (95% CI, 6.8-12.0) seen in a reference population of 47 patients who developed the complication between 1998 and 2000.
The average annual incidence rates in 2001-2010 were 4.5 (95% CI, 2.2-8.3) for men and 3.4 (95% CI, 1.4-6.6) for women. In 1998-2000, the rates were 8.9 (95% CI, 5.7-13.1) for men and 8.7 (95% CI, 5.6-12.8) for women.
No statistically significant differences were found in the number of patients experiencing systemic, cutaneous, neurologic, pulmonary, renal, ophthalmic, or gastrointestinal manifestations between the two time periods.
Mortality rates at 1 year were also similar between the cohorts, at 12% for 2001-2010 and 14% for 1998-2000. Five-year mortality rates were 60% and 51%, respectively.
"Modern immunosuppression therapy seems to be associated with a decrease in the incidence" of systemic rheumatoid vasculitis, said Dr. Ntatsaki, "but has had no significant influence on clinical features, or outcome."
Whether the decrease in incidence can truly be attributed to methotrexate or improvements in RA treatment in general is questionable, suggested Dr. Deborah Symmons, professor of rheumatology and musculoskeletal epidemiology at the University of Manchester (England).
This decline in the incidence of rheumatoid vasculitis is not necessarily because rheumatoid arthritis treatments have improved, she said. "Perhaps it is just the natural history of rheumatoid arthritis that vasculitis is becoming rare. Clearly when people do get it they do just as badly as they ever did, and perhaps all this about methotrexate is completely coincidental."
Dr. Ntatsaki reported having no financial disclosures.
GLASGOW, SCOTLAND – Five-year mortality following the diagnosis of systemic rheumatoid vasculitis hovers around 60%, according to an analysis of 34 cases identified in a British-based patient registry.
Although the annual incidence of SRV has reportedly been decreasing since the 1990s, these data suggest it remains a problem despite the introduction of modern immunosuppressive therapies and the use of earlier and more aggressive treatment for rheumatoid arthritis, Dr. Eleana Ntatsaki, a rheumatology specialist trainee at Norfolk and Norwich (England) University Hospital, said at the annual meeting of the British Society for Rheumatology.
The Norfolk Vasculitis Register (NORVASC) is a prospective patient registry established in 1988 to log cases of vasculitis that occur in the county of Norfolk, England. This is a region particularly well suited to epidemiological study, as it is an isolated geographical area with a stable and well-defined population. This is also one of the reasons the Norfolk Arthritis Register (NOAR) is situated in this part of the country.
Between Jan. 1, 2001, and Dec. 31, 2010, a total of 34 cases were reviewed in detail, with 18 patients (10 men) confirmed as having SRV according to previously published criteria (Am. J. Med. 1984;76:377-84), chart review, and independent physician assessment.
The median age of confirmed cases at diagnosis was 72 years and the average disease duration before SRV diagnosis was nearly 16 years. All patients were rheumatoid factor positive, 13 had documented erosive disease, and three had rheumatoid nodules.
All patients had been treated with steroids, a median of two prior DMARDs had been used (methotrexate in 65% of patients), and two patients had received biologic agents. SRV had been treated with intravenous cyclophosphamide (median six cycles) in all but one patient.
The average annual incidence of SRV was calculated to be 3.9 per million (95% confidence interval [CI], 2.3-6.2). This was substantially lower than the 9.1 per million (95% CI, 6.8-12.0) seen in a reference population of 47 patients who developed the complication between 1998 and 2000.
The average annual incidence rates in 2001-2010 were 4.5 (95% CI, 2.2-8.3) for men and 3.4 (95% CI, 1.4-6.6) for women. In 1998-2000, the rates were 8.9 (95% CI, 5.7-13.1) for men and 8.7 (95% CI, 5.6-12.8) for women.
No statistically significant differences were found in the number of patients experiencing systemic, cutaneous, neurologic, pulmonary, renal, ophthalmic, or gastrointestinal manifestations between the two time periods.
Mortality rates at 1 year were also similar between the cohorts, at 12% for 2001-2010 and 14% for 1998-2000. Five-year mortality rates were 60% and 51%, respectively.
"Modern immunosuppression therapy seems to be associated with a decrease in the incidence" of systemic rheumatoid vasculitis, said Dr. Ntatsaki, "but has had no significant influence on clinical features, or outcome."
Whether the decrease in incidence can truly be attributed to methotrexate or improvements in RA treatment in general is questionable, suggested Dr. Deborah Symmons, professor of rheumatology and musculoskeletal epidemiology at the University of Manchester (England).
This decline in the incidence of rheumatoid vasculitis is not necessarily because rheumatoid arthritis treatments have improved, she said. "Perhaps it is just the natural history of rheumatoid arthritis that vasculitis is becoming rare. Clearly when people do get it they do just as badly as they ever did, and perhaps all this about methotrexate is completely coincidental."
Dr. Ntatsaki reported having no financial disclosures.
FROM THE ANNUAL MEETING OF THE BRITISH SOCIETY FOR RHEUMATOLOGY
Major Finding: Five-year mortality from systemic rheumatoid vasculitis was 60% during 2001-2010 and 51% during 1988-2000.
Data Source: The findings are based on an analysis of data collated between 2001 and 2010 for the prospective Norfolk Vasculitis (NORVASC) register.
Disclosures: Dr. Ntatsaki reported having no financial disclosures.
Biologics for RA Do Not Increase Solid Cancer Risk
GLASGOW – Biologic treatment for rheumatoid arthritis does not increase the overall risk of solid cancers beyond that of more traditional, nonbiologic drugs, according to long-awaited data from the British Society for Rheumatology Biologics Register.
At a median follow-up of almost 5 years, the adjusted hazard ratio for all solid cancers was 0.88 (95% confidence interval, 0.65-1.17) in a comparison between anti–tumor necrosis factor (anti-TNF)-alpha therapy and nonbiologic disease-modifying antirheumatic drugs (nbDMARDs, n = 3,543) in patients without a prior history of cancer.
There was no significant difference in cancer risk between the anti-TNF drugs included in the analysis, namely etanercept (adjusted hazard ratio, 0.94; 95% CI, 0.68-1.29), infliximab (aHR, 0.87; 95% CI, 0.61-1.25), and adalimumab (aHR, 0.81; 95% CI, 0.57-1.14) versus nbDMARDs. Neither duration of follow-up nor the overall site where tumors occurred had any effect on the occurrence of solid cancers.
These data lend further support to the general safety of TNF inhibitors, and add to findings reported from the British Society for Rheumatology Biologics Register (BSRBR) and other European registries, such as the Danish Biologics Registry (DANBIO), with regard to malignancy (Rheumatology News, June 2011, p. 22).
"We do still need further follow-up to address site-specific [cancer] risk and to allow for longer latency," said Dr. Louise Mercer, May 3, at the British Society for Rheumatology annual conference (Rheumatology 2012;51:iii40, abstract O37).
Dr. Mercer, a clinical research fellow at the Arthritis Research UK Epidemiology Unit, University of Manchester (England), noted that that cancer risk is already higher in patients with RA than in the general population (Rheumatology News, April 2011, p. 31). However, these latest findings from the BSRBR highlight that the use of biologic therapy doesn’t appear to add to this risk over that seen with nbDMARDs.
Data on 11,719 patients without a history of cancer who were enrolled in the BSRBR between 2001 and 2009 were used for the current analysis. Data after 2009 were not considered in order to allow for the lag time in cancer reporting from the U.K.’s National Cancer Registry into the Biologics Register. A group of 3,543 patients treated with nbDMARDs was used as the reference population.
Data adjustment took account of patients’ age at baseline, gender, duration of disease, use of nonsteroidal anti-inflammatory drugs, comorbidities, and age at time of enrollment in the U.K.-based register.
A total of 295 cancers were reported in patients treated with anti-TNF agents during a mean follow up of 4.6 years, giving a crude cancer rate of 63 per 10,000 patient-years. A total of 91 cancers were reported in patients treated with nbDMARDs over a median follow-up of 3.4 years, representing a crude cancer rate of 84 per 10,000 patient years.
With regard to the site of cancer, anti-TNFs appeared to be associated with a lower risk of lung cancer (aHR, 0.89; 95% CI, 0.46-1.74) and breast cancer (aHR, 0.99; 95% CI, 0.51-1.92) than were nbDMARDs. There was an increase in the risk of colorectal cancer (HR, 1.21; 95% CI, 0.54-2.70), but "the confidence intervals have become wider," Dr. Mercer observed.
Data on patients with a prior history of cancer have already been published by the team (Arthritis Care Res. 2010;62:755-63), but new data on patients with cervical carcinoma in situ (CIS) were reported during a poster presentation at the meeting (Rheumatology 2012;51:iii77, abstract 70).
Considering data collated through March 2011, 238 women had a prior history of cervical CIS – 48 of 2,654 (1.8%) women treated with nbDMARDs and 190 of 9,084 (2.1%) treated with anti-TNFs. Two women subsequently developed genitourinary cancer – both were treated with nbDMARDs. The rate of incident cancer was 13 per 1,000 person-years (95% CI, 2-45).
Dr. Mercer and her associates noted that there was a low level of reporting cervical CIS, which could reflect rheumatologists not being aware of abnormal cervical changes or choosing not to report in situ cancers or these prior cancers at baseline.
They concluded that "there were no new or recurrent female genital cancers among women with preexisting cervical CIS selected for treatment with anti-TNF," which should prove reassuring to women who may have a history of cervical abnormalities and who are considering anti-TNF therapy.
The BSRBR is funded by a grant from the British Society for Rheumatology. The BSR receives funding from Abbott Laboratories, Swedish Orphan Biovitrum (SOBI), Merck, Pfizer, Roche Products, and UCB Pharma. This income finances a separate contract between the BSR and the University of Manchester, which provides and runs the BSRBR. All decisions concerning data analysis, interpretation, and publications are made autonomously of any industrial contribution. Dr. Mercer reported having no personal conflicts of interest.
GLASGOW – Biologic treatment for rheumatoid arthritis does not increase the overall risk of solid cancers beyond that of more traditional, nonbiologic drugs, according to long-awaited data from the British Society for Rheumatology Biologics Register.
At a median follow-up of almost 5 years, the adjusted hazard ratio for all solid cancers was 0.88 (95% confidence interval, 0.65-1.17) in a comparison between anti–tumor necrosis factor (anti-TNF)-alpha therapy and nonbiologic disease-modifying antirheumatic drugs (nbDMARDs, n = 3,543) in patients without a prior history of cancer.
There was no significant difference in cancer risk between the anti-TNF drugs included in the analysis, namely etanercept (adjusted hazard ratio, 0.94; 95% CI, 0.68-1.29), infliximab (aHR, 0.87; 95% CI, 0.61-1.25), and adalimumab (aHR, 0.81; 95% CI, 0.57-1.14) versus nbDMARDs. Neither duration of follow-up nor the overall site where tumors occurred had any effect on the occurrence of solid cancers.
These data lend further support to the general safety of TNF inhibitors, and add to findings reported from the British Society for Rheumatology Biologics Register (BSRBR) and other European registries, such as the Danish Biologics Registry (DANBIO), with regard to malignancy (Rheumatology News, June 2011, p. 22).
"We do still need further follow-up to address site-specific [cancer] risk and to allow for longer latency," said Dr. Louise Mercer, May 3, at the British Society for Rheumatology annual conference (Rheumatology 2012;51:iii40, abstract O37).
Dr. Mercer, a clinical research fellow at the Arthritis Research UK Epidemiology Unit, University of Manchester (England), noted that that cancer risk is already higher in patients with RA than in the general population (Rheumatology News, April 2011, p. 31). However, these latest findings from the BSRBR highlight that the use of biologic therapy doesn’t appear to add to this risk over that seen with nbDMARDs.
Data on 11,719 patients without a history of cancer who were enrolled in the BSRBR between 2001 and 2009 were used for the current analysis. Data after 2009 were not considered in order to allow for the lag time in cancer reporting from the U.K.’s National Cancer Registry into the Biologics Register. A group of 3,543 patients treated with nbDMARDs was used as the reference population.
Data adjustment took account of patients’ age at baseline, gender, duration of disease, use of nonsteroidal anti-inflammatory drugs, comorbidities, and age at time of enrollment in the U.K.-based register.
A total of 295 cancers were reported in patients treated with anti-TNF agents during a mean follow up of 4.6 years, giving a crude cancer rate of 63 per 10,000 patient-years. A total of 91 cancers were reported in patients treated with nbDMARDs over a median follow-up of 3.4 years, representing a crude cancer rate of 84 per 10,000 patient years.
With regard to the site of cancer, anti-TNFs appeared to be associated with a lower risk of lung cancer (aHR, 0.89; 95% CI, 0.46-1.74) and breast cancer (aHR, 0.99; 95% CI, 0.51-1.92) than were nbDMARDs. There was an increase in the risk of colorectal cancer (HR, 1.21; 95% CI, 0.54-2.70), but "the confidence intervals have become wider," Dr. Mercer observed.
Data on patients with a prior history of cancer have already been published by the team (Arthritis Care Res. 2010;62:755-63), but new data on patients with cervical carcinoma in situ (CIS) were reported during a poster presentation at the meeting (Rheumatology 2012;51:iii77, abstract 70).
Considering data collated through March 2011, 238 women had a prior history of cervical CIS – 48 of 2,654 (1.8%) women treated with nbDMARDs and 190 of 9,084 (2.1%) treated with anti-TNFs. Two women subsequently developed genitourinary cancer – both were treated with nbDMARDs. The rate of incident cancer was 13 per 1,000 person-years (95% CI, 2-45).
Dr. Mercer and her associates noted that there was a low level of reporting cervical CIS, which could reflect rheumatologists not being aware of abnormal cervical changes or choosing not to report in situ cancers or these prior cancers at baseline.
They concluded that "there were no new or recurrent female genital cancers among women with preexisting cervical CIS selected for treatment with anti-TNF," which should prove reassuring to women who may have a history of cervical abnormalities and who are considering anti-TNF therapy.
The BSRBR is funded by a grant from the British Society for Rheumatology. The BSR receives funding from Abbott Laboratories, Swedish Orphan Biovitrum (SOBI), Merck, Pfizer, Roche Products, and UCB Pharma. This income finances a separate contract between the BSR and the University of Manchester, which provides and runs the BSRBR. All decisions concerning data analysis, interpretation, and publications are made autonomously of any industrial contribution. Dr. Mercer reported having no personal conflicts of interest.
GLASGOW – Biologic treatment for rheumatoid arthritis does not increase the overall risk of solid cancers beyond that of more traditional, nonbiologic drugs, according to long-awaited data from the British Society for Rheumatology Biologics Register.
At a median follow-up of almost 5 years, the adjusted hazard ratio for all solid cancers was 0.88 (95% confidence interval, 0.65-1.17) in a comparison between anti–tumor necrosis factor (anti-TNF)-alpha therapy and nonbiologic disease-modifying antirheumatic drugs (nbDMARDs, n = 3,543) in patients without a prior history of cancer.
There was no significant difference in cancer risk between the anti-TNF drugs included in the analysis, namely etanercept (adjusted hazard ratio, 0.94; 95% CI, 0.68-1.29), infliximab (aHR, 0.87; 95% CI, 0.61-1.25), and adalimumab (aHR, 0.81; 95% CI, 0.57-1.14) versus nbDMARDs. Neither duration of follow-up nor the overall site where tumors occurred had any effect on the occurrence of solid cancers.
These data lend further support to the general safety of TNF inhibitors, and add to findings reported from the British Society for Rheumatology Biologics Register (BSRBR) and other European registries, such as the Danish Biologics Registry (DANBIO), with regard to malignancy (Rheumatology News, June 2011, p. 22).
"We do still need further follow-up to address site-specific [cancer] risk and to allow for longer latency," said Dr. Louise Mercer, May 3, at the British Society for Rheumatology annual conference (Rheumatology 2012;51:iii40, abstract O37).
Dr. Mercer, a clinical research fellow at the Arthritis Research UK Epidemiology Unit, University of Manchester (England), noted that that cancer risk is already higher in patients with RA than in the general population (Rheumatology News, April 2011, p. 31). However, these latest findings from the BSRBR highlight that the use of biologic therapy doesn’t appear to add to this risk over that seen with nbDMARDs.
Data on 11,719 patients without a history of cancer who were enrolled in the BSRBR between 2001 and 2009 were used for the current analysis. Data after 2009 were not considered in order to allow for the lag time in cancer reporting from the U.K.’s National Cancer Registry into the Biologics Register. A group of 3,543 patients treated with nbDMARDs was used as the reference population.
Data adjustment took account of patients’ age at baseline, gender, duration of disease, use of nonsteroidal anti-inflammatory drugs, comorbidities, and age at time of enrollment in the U.K.-based register.
A total of 295 cancers were reported in patients treated with anti-TNF agents during a mean follow up of 4.6 years, giving a crude cancer rate of 63 per 10,000 patient-years. A total of 91 cancers were reported in patients treated with nbDMARDs over a median follow-up of 3.4 years, representing a crude cancer rate of 84 per 10,000 patient years.
With regard to the site of cancer, anti-TNFs appeared to be associated with a lower risk of lung cancer (aHR, 0.89; 95% CI, 0.46-1.74) and breast cancer (aHR, 0.99; 95% CI, 0.51-1.92) than were nbDMARDs. There was an increase in the risk of colorectal cancer (HR, 1.21; 95% CI, 0.54-2.70), but "the confidence intervals have become wider," Dr. Mercer observed.
Data on patients with a prior history of cancer have already been published by the team (Arthritis Care Res. 2010;62:755-63), but new data on patients with cervical carcinoma in situ (CIS) were reported during a poster presentation at the meeting (Rheumatology 2012;51:iii77, abstract 70).
Considering data collated through March 2011, 238 women had a prior history of cervical CIS – 48 of 2,654 (1.8%) women treated with nbDMARDs and 190 of 9,084 (2.1%) treated with anti-TNFs. Two women subsequently developed genitourinary cancer – both were treated with nbDMARDs. The rate of incident cancer was 13 per 1,000 person-years (95% CI, 2-45).
Dr. Mercer and her associates noted that there was a low level of reporting cervical CIS, which could reflect rheumatologists not being aware of abnormal cervical changes or choosing not to report in situ cancers or these prior cancers at baseline.
They concluded that "there were no new or recurrent female genital cancers among women with preexisting cervical CIS selected for treatment with anti-TNF," which should prove reassuring to women who may have a history of cervical abnormalities and who are considering anti-TNF therapy.
The BSRBR is funded by a grant from the British Society for Rheumatology. The BSR receives funding from Abbott Laboratories, Swedish Orphan Biovitrum (SOBI), Merck, Pfizer, Roche Products, and UCB Pharma. This income finances a separate contract between the BSR and the University of Manchester, which provides and runs the BSRBR. All decisions concerning data analysis, interpretation, and publications are made autonomously of any industrial contribution. Dr. Mercer reported having no personal conflicts of interest.
FROM THE ANNUAL MEETING OF THE BRITISH SOCIETY FOR RHEUMATOLOGY
Exercise Eases Upper-Limb Dysfunction in Early RA
GLASGOW, SCOTLAND – Upper-limb dysfunction in early rheumatoid arthritis patients can be significantly improved by a combined exercise and education strategy compared with usual care, based on preliminary findings of an assessor-blinded, randomized controlled trial.
Disability, function, hand grip strength, and self-efficacy assessed using the patient-rated Arthritis Self-Efficacy Scale (Arthritis Rheum. 1989;32:37-44) were all significantly (P less than .05) improved at 3 months in the EXTRA (Education and Exercise Upper-Limb Training in Early Rheumatoid Arthritis) study
"Whilst self-efficacy improved throughout the study, the [effects of the] other outcome measures did diminish, [and] efficacy was not sustained at 9 months," according to chief investigator for the trial Dr. Lindsay Bearne.
"However, the program is safe in people with early RA with moderate to high disease activity," Dr. Bearne, a lecturer in physiotherapy at King’s College London, added at the annual meeting of the British Society for Rheumatology.
The management approach being tested in the EXTRA study differs from other trials of exercise in RA in that it specifically addresses upper-limb dysfunction. The few previous trials that have been done focused on the effects of whole-body or lower-limb exercise or assessed only disability in the hand and wrist.
The aim of the EXTRA study, therefore, was to specifically look at the combined 12-week efficacy of a home-based exercise regimen supplemented with four group discussion and exercise sessions, versus usual care, for upper-limb rehabilitation in patients with early RA.
The primary hypothesis was that greater upper-limb function and less disability would be achieved with the exercise and education program than with usual care.
A total of 108 adults (82 women) with an average age of 55 years participated. All had early RA, with an average disease duration of 20 months. Patients who had received steroid injections in the previous 4 weeks or who had upper-limb surgery or physiotherapy in the past 6 months had been excluded.
The primary efficacy measure was improvement in upper-limb dysfunction assessed via the Disability of Arm, Shoulder, and Hand (DASH) outcome questionnaire. This is a 30-item disability/symptom scale with which patients rate their responses on a 5-point scale. An overall score from 0 (no disability) to 100 is obtained (BMC Musculoskeletal Disord. 2003;4:11).
Secondary assessments included hand-grip strength, self-efficacy, and a grip ability test for function. The 28-joint disease activity score (DAS28) was also used, and pain was assessed using a visual analog scale.
The daily home exercise program used in the study involved six simple exercises selected to suit the patient from an overall list of 16 exercises. The main "menu" of exercises selected to improve upper-limb strength and function was based on expert opinion and the published literature, Dr. Bearne explained, and included arm curls and squeezing a ball of putty.
DAS28 and pain scores at 3 months were both significantly lower (P less than .05) in the patients who had been randomized to the EXTRA program versus the usual care group. DAS28 and pain scores at baseline and at 9 months’ follow-up were not significantly different between the groups.
These data highlight that personalized, well-described global upper-limb exercises and a self-management program can help improve upper-limb dysfunction, if only temporarily.
"The challenge of sustaining long-term exercise remains," said Dr. Bearne.
"We appear to be able to motivate people to exercise, and to initiate exercise; [the difficulty is] to take that initial burst of enthusiasm and convert it to a longer-term habit."
Further data, including the results of a health economic evaluation, are expected from the EXTRA study.
Dr. Bearne reported no relevant financial disclosures. EXTRA is funded by the Chartered Society of Physiotherapy/Physiotherapy Research Foundation and sponsored by King’s College London.
GLASGOW, SCOTLAND – Upper-limb dysfunction in early rheumatoid arthritis patients can be significantly improved by a combined exercise and education strategy compared with usual care, based on preliminary findings of an assessor-blinded, randomized controlled trial.
Disability, function, hand grip strength, and self-efficacy assessed using the patient-rated Arthritis Self-Efficacy Scale (Arthritis Rheum. 1989;32:37-44) were all significantly (P less than .05) improved at 3 months in the EXTRA (Education and Exercise Upper-Limb Training in Early Rheumatoid Arthritis) study
"Whilst self-efficacy improved throughout the study, the [effects of the] other outcome measures did diminish, [and] efficacy was not sustained at 9 months," according to chief investigator for the trial Dr. Lindsay Bearne.
"However, the program is safe in people with early RA with moderate to high disease activity," Dr. Bearne, a lecturer in physiotherapy at King’s College London, added at the annual meeting of the British Society for Rheumatology.
The management approach being tested in the EXTRA study differs from other trials of exercise in RA in that it specifically addresses upper-limb dysfunction. The few previous trials that have been done focused on the effects of whole-body or lower-limb exercise or assessed only disability in the hand and wrist.
The aim of the EXTRA study, therefore, was to specifically look at the combined 12-week efficacy of a home-based exercise regimen supplemented with four group discussion and exercise sessions, versus usual care, for upper-limb rehabilitation in patients with early RA.
The primary hypothesis was that greater upper-limb function and less disability would be achieved with the exercise and education program than with usual care.
A total of 108 adults (82 women) with an average age of 55 years participated. All had early RA, with an average disease duration of 20 months. Patients who had received steroid injections in the previous 4 weeks or who had upper-limb surgery or physiotherapy in the past 6 months had been excluded.
The primary efficacy measure was improvement in upper-limb dysfunction assessed via the Disability of Arm, Shoulder, and Hand (DASH) outcome questionnaire. This is a 30-item disability/symptom scale with which patients rate their responses on a 5-point scale. An overall score from 0 (no disability) to 100 is obtained (BMC Musculoskeletal Disord. 2003;4:11).
Secondary assessments included hand-grip strength, self-efficacy, and a grip ability test for function. The 28-joint disease activity score (DAS28) was also used, and pain was assessed using a visual analog scale.
The daily home exercise program used in the study involved six simple exercises selected to suit the patient from an overall list of 16 exercises. The main "menu" of exercises selected to improve upper-limb strength and function was based on expert opinion and the published literature, Dr. Bearne explained, and included arm curls and squeezing a ball of putty.
DAS28 and pain scores at 3 months were both significantly lower (P less than .05) in the patients who had been randomized to the EXTRA program versus the usual care group. DAS28 and pain scores at baseline and at 9 months’ follow-up were not significantly different between the groups.
These data highlight that personalized, well-described global upper-limb exercises and a self-management program can help improve upper-limb dysfunction, if only temporarily.
"The challenge of sustaining long-term exercise remains," said Dr. Bearne.
"We appear to be able to motivate people to exercise, and to initiate exercise; [the difficulty is] to take that initial burst of enthusiasm and convert it to a longer-term habit."
Further data, including the results of a health economic evaluation, are expected from the EXTRA study.
Dr. Bearne reported no relevant financial disclosures. EXTRA is funded by the Chartered Society of Physiotherapy/Physiotherapy Research Foundation and sponsored by King’s College London.
GLASGOW, SCOTLAND – Upper-limb dysfunction in early rheumatoid arthritis patients can be significantly improved by a combined exercise and education strategy compared with usual care, based on preliminary findings of an assessor-blinded, randomized controlled trial.
Disability, function, hand grip strength, and self-efficacy assessed using the patient-rated Arthritis Self-Efficacy Scale (Arthritis Rheum. 1989;32:37-44) were all significantly (P less than .05) improved at 3 months in the EXTRA (Education and Exercise Upper-Limb Training in Early Rheumatoid Arthritis) study
"Whilst self-efficacy improved throughout the study, the [effects of the] other outcome measures did diminish, [and] efficacy was not sustained at 9 months," according to chief investigator for the trial Dr. Lindsay Bearne.
"However, the program is safe in people with early RA with moderate to high disease activity," Dr. Bearne, a lecturer in physiotherapy at King’s College London, added at the annual meeting of the British Society for Rheumatology.
The management approach being tested in the EXTRA study differs from other trials of exercise in RA in that it specifically addresses upper-limb dysfunction. The few previous trials that have been done focused on the effects of whole-body or lower-limb exercise or assessed only disability in the hand and wrist.
The aim of the EXTRA study, therefore, was to specifically look at the combined 12-week efficacy of a home-based exercise regimen supplemented with four group discussion and exercise sessions, versus usual care, for upper-limb rehabilitation in patients with early RA.
The primary hypothesis was that greater upper-limb function and less disability would be achieved with the exercise and education program than with usual care.
A total of 108 adults (82 women) with an average age of 55 years participated. All had early RA, with an average disease duration of 20 months. Patients who had received steroid injections in the previous 4 weeks or who had upper-limb surgery or physiotherapy in the past 6 months had been excluded.
The primary efficacy measure was improvement in upper-limb dysfunction assessed via the Disability of Arm, Shoulder, and Hand (DASH) outcome questionnaire. This is a 30-item disability/symptom scale with which patients rate their responses on a 5-point scale. An overall score from 0 (no disability) to 100 is obtained (BMC Musculoskeletal Disord. 2003;4:11).
Secondary assessments included hand-grip strength, self-efficacy, and a grip ability test for function. The 28-joint disease activity score (DAS28) was also used, and pain was assessed using a visual analog scale.
The daily home exercise program used in the study involved six simple exercises selected to suit the patient from an overall list of 16 exercises. The main "menu" of exercises selected to improve upper-limb strength and function was based on expert opinion and the published literature, Dr. Bearne explained, and included arm curls and squeezing a ball of putty.
DAS28 and pain scores at 3 months were both significantly lower (P less than .05) in the patients who had been randomized to the EXTRA program versus the usual care group. DAS28 and pain scores at baseline and at 9 months’ follow-up were not significantly different between the groups.
These data highlight that personalized, well-described global upper-limb exercises and a self-management program can help improve upper-limb dysfunction, if only temporarily.
"The challenge of sustaining long-term exercise remains," said Dr. Bearne.
"We appear to be able to motivate people to exercise, and to initiate exercise; [the difficulty is] to take that initial burst of enthusiasm and convert it to a longer-term habit."
Further data, including the results of a health economic evaluation, are expected from the EXTRA study.
Dr. Bearne reported no relevant financial disclosures. EXTRA is funded by the Chartered Society of Physiotherapy/Physiotherapy Research Foundation and sponsored by King’s College London.
FROM THE ANNUAL MEETING OF THE BRITISH SOCIETY FOR RHEUMATOLOGY
Major Finding: Disability, function, hand grip strength, self-efficacy, and DAS28 and pain scores were all significantly (P less than .05) improved in patients randomized to the EXTRA program versus usual care.
Data Source: The data came from a study of 108 patients with RA and upper-limb dysfunction who were randomized to a combined home exercise, group exercise, and education program versus usual care.
Disclosures: Dr. Bearne reported no relevant financial disclosures. EXTRA is funded by the Chartered Society of Physiotherapy/Physiotherapy Research Foundation and sponsored by King’s College London.
Obese RA Patients Less Likely to Respond to DMARDs at 1 Year
GLASGOW, SCOTLAND – Early arthritis patients who are obese are less likely to achieve a good response to disease-modifying antirheumatic drugs in their first year of treatment than their lighter counterparts, judging from the results of a 212-patient study.
The median 28-joint count disease activity score (DAS28) was higher (3.1 vs. 2.6) and fewer patients achieved DAS28 remission (40.3% vs. 52.3%) comparing obese with normal-weight or underweight patients.
A good EULAR response was achieved by 40% of patient who were classified as obese vs. 58.5% of those classified as being of normal weight or underweight.
"There was not much difference in initial treatment across the whole cohort of patients, but at the endpoint of 1 year we found that the obese patients just were not responding as well," said Dr. Stephanie Ling in an interview at the annual meeting of the British Society for Rheumatology.
"This study has highlighted the alarming levels of obesity and poor fitness in a typical well-controlled RA population."
"There are a number of reasons that could be behind this. It could be we are not adjusting the dose to weight, or it could be because of our hypothesis that inflammatory markers are higher in the obese patients anyway."
Dr. Ling of the University of Liverpool (England) and coworkers have previously assessed how obesity affects disease activity in rheumatoid arthritis (RA), finding that the higher the body mass index (BMI), the greater the DAS28 and inflammatory disease activity (Rheumatology News, May 2011, p. 34).
"When we broke down the disease activity score components, there was not much difference between obese and non-obese patients in the tender and swollen joint counts," she said.
"When you got down to the levels of the inflammatory markers in the blood, however, those were statistically significantly raised in the very obese patients." This could be influencing the response to treatment, and possibly explain the impaired response to disease-modifying antirheumatic drugs (DMARDs) that was seen in the present study (Rheumatology 2012;51:iii162–3, abstract P295).
The current investigation assessed the effects of obesity on disease activity in the first year of DMARD therapy in an inception cohort of RA patients who had a symptom duration of less than 1 year.
The mean age of patients was 57.7 years, 60.1% were female, and 71.2% were positive for anticitrullinated protein antibodies (ACPA). The median body mass index (BMI) was 27.5 kg/m2, with a third (34%) of the cohort classified as being obese (BMI greater than 30).
At 1 year, the median DAS28 score for the entire cohort was 2.6, and 51% were in DAS28 remission; 58% had achieved a good EULAR response. When outcomes were split according to BMI, however, the obese patients did significantly worse.
The median DAS28 score in overweight (BMI 25-29.9) patients at 1 year was 2.4; 45% achieved DAS28 remission, and 66.7% achieved a good EULAR response.
A trend for association between obesity and high baseline DAS28 (greater than 5.1) was found (odds ratio, 1.7; 95% confidence interval, 0.9-3.1), which grew stronger when the analysis was limited to patients who were ACPA-positive (OR, 2.0; 95% CI, 1.0-4.0). However, ACPA-positivity by itself was not associated with treatment response at 1 year.
Inverse correlations between baseline obesity and DAS28 remission and EULAR response at 1 year were found to be more prominent in female than male patients.
"I think we definitely need to think about dosing according to weight instead of giving everyone the same dose," Dr. Ling suggested. Dosing by weight is done in pediatric but not adult practice.
Losing weight may also be of benefit but requires further investigation to see if this in itself could help improve the response to therapy. Anecdotally, Dr. Ling noted the case of a woman who had been on high-dose treatment and lost more than 238 pounds and subsequently went into remission, without further need for drug therapy.
Other work presented by Dr. Corrinne Ellis showed that obesity is linked to greater functional disability (Rheumatology 2012;51:iii163, abstract P296). Analysis of data on 803 patients (21% obese) with inflammatory polyarthritis in the Norfolk Arthritis Register (NOAR) revealed that baseline obesity was associated with higher disability, as determined by the Health Assessment Questionnaire (HAQ) at 1 year.
Perhaps the reason HAQ scores are higher in obese patients is because they are just too unfit or obese to exercise. Indeed, other research presented at this meeting showed that cardiorespiratory fitness assessed using a simple step test was low in patients with RA (Rheumatology 2012;51:iii162–3, abstract P80).
Cardiorespiratory fitness is an independent risk factor for heart disease and was found to be decreased in the study of 100 patients with RA regardless of whether traditional cardiovascular risk factors were also present. Cardiorespiratory fitness was linked to obesity and the metabolic syndrome.
"This study has highlighted the alarming levels of obesity and poor fitness in a typical well-controlled RA population," according to Ms. Jennifer Cooney of the University of Bangor (Wales) and associates.
"Thus more attentions and understanding is required on addressing these factors than the traditional risk factors alone."
Dr. Ling reported no financial disclosures.
GLASGOW, SCOTLAND – Early arthritis patients who are obese are less likely to achieve a good response to disease-modifying antirheumatic drugs in their first year of treatment than their lighter counterparts, judging from the results of a 212-patient study.
The median 28-joint count disease activity score (DAS28) was higher (3.1 vs. 2.6) and fewer patients achieved DAS28 remission (40.3% vs. 52.3%) comparing obese with normal-weight or underweight patients.
A good EULAR response was achieved by 40% of patient who were classified as obese vs. 58.5% of those classified as being of normal weight or underweight.
"There was not much difference in initial treatment across the whole cohort of patients, but at the endpoint of 1 year we found that the obese patients just were not responding as well," said Dr. Stephanie Ling in an interview at the annual meeting of the British Society for Rheumatology.
"This study has highlighted the alarming levels of obesity and poor fitness in a typical well-controlled RA population."
"There are a number of reasons that could be behind this. It could be we are not adjusting the dose to weight, or it could be because of our hypothesis that inflammatory markers are higher in the obese patients anyway."
Dr. Ling of the University of Liverpool (England) and coworkers have previously assessed how obesity affects disease activity in rheumatoid arthritis (RA), finding that the higher the body mass index (BMI), the greater the DAS28 and inflammatory disease activity (Rheumatology News, May 2011, p. 34).
"When we broke down the disease activity score components, there was not much difference between obese and non-obese patients in the tender and swollen joint counts," she said.
"When you got down to the levels of the inflammatory markers in the blood, however, those were statistically significantly raised in the very obese patients." This could be influencing the response to treatment, and possibly explain the impaired response to disease-modifying antirheumatic drugs (DMARDs) that was seen in the present study (Rheumatology 2012;51:iii162–3, abstract P295).
The current investigation assessed the effects of obesity on disease activity in the first year of DMARD therapy in an inception cohort of RA patients who had a symptom duration of less than 1 year.
The mean age of patients was 57.7 years, 60.1% were female, and 71.2% were positive for anticitrullinated protein antibodies (ACPA). The median body mass index (BMI) was 27.5 kg/m2, with a third (34%) of the cohort classified as being obese (BMI greater than 30).
At 1 year, the median DAS28 score for the entire cohort was 2.6, and 51% were in DAS28 remission; 58% had achieved a good EULAR response. When outcomes were split according to BMI, however, the obese patients did significantly worse.
The median DAS28 score in overweight (BMI 25-29.9) patients at 1 year was 2.4; 45% achieved DAS28 remission, and 66.7% achieved a good EULAR response.
A trend for association between obesity and high baseline DAS28 (greater than 5.1) was found (odds ratio, 1.7; 95% confidence interval, 0.9-3.1), which grew stronger when the analysis was limited to patients who were ACPA-positive (OR, 2.0; 95% CI, 1.0-4.0). However, ACPA-positivity by itself was not associated with treatment response at 1 year.
Inverse correlations between baseline obesity and DAS28 remission and EULAR response at 1 year were found to be more prominent in female than male patients.
"I think we definitely need to think about dosing according to weight instead of giving everyone the same dose," Dr. Ling suggested. Dosing by weight is done in pediatric but not adult practice.
Losing weight may also be of benefit but requires further investigation to see if this in itself could help improve the response to therapy. Anecdotally, Dr. Ling noted the case of a woman who had been on high-dose treatment and lost more than 238 pounds and subsequently went into remission, without further need for drug therapy.
Other work presented by Dr. Corrinne Ellis showed that obesity is linked to greater functional disability (Rheumatology 2012;51:iii163, abstract P296). Analysis of data on 803 patients (21% obese) with inflammatory polyarthritis in the Norfolk Arthritis Register (NOAR) revealed that baseline obesity was associated with higher disability, as determined by the Health Assessment Questionnaire (HAQ) at 1 year.
Perhaps the reason HAQ scores are higher in obese patients is because they are just too unfit or obese to exercise. Indeed, other research presented at this meeting showed that cardiorespiratory fitness assessed using a simple step test was low in patients with RA (Rheumatology 2012;51:iii162–3, abstract P80).
Cardiorespiratory fitness is an independent risk factor for heart disease and was found to be decreased in the study of 100 patients with RA regardless of whether traditional cardiovascular risk factors were also present. Cardiorespiratory fitness was linked to obesity and the metabolic syndrome.
"This study has highlighted the alarming levels of obesity and poor fitness in a typical well-controlled RA population," according to Ms. Jennifer Cooney of the University of Bangor (Wales) and associates.
"Thus more attentions and understanding is required on addressing these factors than the traditional risk factors alone."
Dr. Ling reported no financial disclosures.
GLASGOW, SCOTLAND – Early arthritis patients who are obese are less likely to achieve a good response to disease-modifying antirheumatic drugs in their first year of treatment than their lighter counterparts, judging from the results of a 212-patient study.
The median 28-joint count disease activity score (DAS28) was higher (3.1 vs. 2.6) and fewer patients achieved DAS28 remission (40.3% vs. 52.3%) comparing obese with normal-weight or underweight patients.
A good EULAR response was achieved by 40% of patient who were classified as obese vs. 58.5% of those classified as being of normal weight or underweight.
"There was not much difference in initial treatment across the whole cohort of patients, but at the endpoint of 1 year we found that the obese patients just were not responding as well," said Dr. Stephanie Ling in an interview at the annual meeting of the British Society for Rheumatology.
"This study has highlighted the alarming levels of obesity and poor fitness in a typical well-controlled RA population."
"There are a number of reasons that could be behind this. It could be we are not adjusting the dose to weight, or it could be because of our hypothesis that inflammatory markers are higher in the obese patients anyway."
Dr. Ling of the University of Liverpool (England) and coworkers have previously assessed how obesity affects disease activity in rheumatoid arthritis (RA), finding that the higher the body mass index (BMI), the greater the DAS28 and inflammatory disease activity (Rheumatology News, May 2011, p. 34).
"When we broke down the disease activity score components, there was not much difference between obese and non-obese patients in the tender and swollen joint counts," she said.
"When you got down to the levels of the inflammatory markers in the blood, however, those were statistically significantly raised in the very obese patients." This could be influencing the response to treatment, and possibly explain the impaired response to disease-modifying antirheumatic drugs (DMARDs) that was seen in the present study (Rheumatology 2012;51:iii162–3, abstract P295).
The current investigation assessed the effects of obesity on disease activity in the first year of DMARD therapy in an inception cohort of RA patients who had a symptom duration of less than 1 year.
The mean age of patients was 57.7 years, 60.1% were female, and 71.2% were positive for anticitrullinated protein antibodies (ACPA). The median body mass index (BMI) was 27.5 kg/m2, with a third (34%) of the cohort classified as being obese (BMI greater than 30).
At 1 year, the median DAS28 score for the entire cohort was 2.6, and 51% were in DAS28 remission; 58% had achieved a good EULAR response. When outcomes were split according to BMI, however, the obese patients did significantly worse.
The median DAS28 score in overweight (BMI 25-29.9) patients at 1 year was 2.4; 45% achieved DAS28 remission, and 66.7% achieved a good EULAR response.
A trend for association between obesity and high baseline DAS28 (greater than 5.1) was found (odds ratio, 1.7; 95% confidence interval, 0.9-3.1), which grew stronger when the analysis was limited to patients who were ACPA-positive (OR, 2.0; 95% CI, 1.0-4.0). However, ACPA-positivity by itself was not associated with treatment response at 1 year.
Inverse correlations between baseline obesity and DAS28 remission and EULAR response at 1 year were found to be more prominent in female than male patients.
"I think we definitely need to think about dosing according to weight instead of giving everyone the same dose," Dr. Ling suggested. Dosing by weight is done in pediatric but not adult practice.
Losing weight may also be of benefit but requires further investigation to see if this in itself could help improve the response to therapy. Anecdotally, Dr. Ling noted the case of a woman who had been on high-dose treatment and lost more than 238 pounds and subsequently went into remission, without further need for drug therapy.
Other work presented by Dr. Corrinne Ellis showed that obesity is linked to greater functional disability (Rheumatology 2012;51:iii163, abstract P296). Analysis of data on 803 patients (21% obese) with inflammatory polyarthritis in the Norfolk Arthritis Register (NOAR) revealed that baseline obesity was associated with higher disability, as determined by the Health Assessment Questionnaire (HAQ) at 1 year.
Perhaps the reason HAQ scores are higher in obese patients is because they are just too unfit or obese to exercise. Indeed, other research presented at this meeting showed that cardiorespiratory fitness assessed using a simple step test was low in patients with RA (Rheumatology 2012;51:iii162–3, abstract P80).
Cardiorespiratory fitness is an independent risk factor for heart disease and was found to be decreased in the study of 100 patients with RA regardless of whether traditional cardiovascular risk factors were also present. Cardiorespiratory fitness was linked to obesity and the metabolic syndrome.
"This study has highlighted the alarming levels of obesity and poor fitness in a typical well-controlled RA population," according to Ms. Jennifer Cooney of the University of Bangor (Wales) and associates.
"Thus more attentions and understanding is required on addressing these factors than the traditional risk factors alone."
Dr. Ling reported no financial disclosures.
FROM THE ANNUAL MEETING OF THE BRITISH SOCIETY FOR RHEUMATOLOGY
Major Finding: A good EULAR response was achieved by 40% of obese patients vs. 58.5% of those categorized as normal weight or underweight.
Data Source: The findings were based on data from an inception cohort of 212 patients with early rheumatoid arthritis).
Disclosures: Dr. Ling reported no financial disclosures.