User login
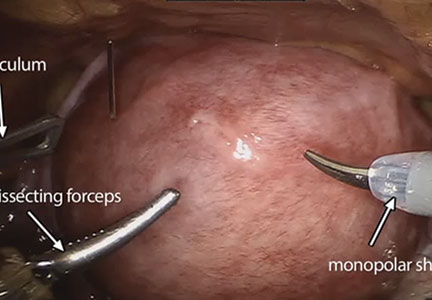
FDA allows marketing of morcellation containment system
The Food and Drug Administration has cleared for marketing a novel tissue containment system for use with certain laparoscopic power morcellators.
The PneumoLiner system is intended to be used to contain morcellated uterine tissue in a limited population of patients, including women without uterine fibroids undergoing hysterectomy and some premenopausal women with fibroids who want to maintain their fertility, according to the FDA. The agency is requiring the manufacturer to warn patients and physicians that the device has not been proven to reduce the risk of spreading cancer during surgery.
This approval – through the FDA’s de novo classification process for novel, low- and moderate-risk medical devices – comes about 2 years after the FDA first warned physicians and patients about the risk of spreading unsuspected uterine sarcomas during laparoscopic power morcellation in hysterectomy or myomectomy.
“This new device does not change our position on the risks associated with power morcellation,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in an April 7 statement. “We are continuing to warn against the use of power morcellators for the vast majority of women undergoing removal of the uterus or uterine fibroids.”
The device, which is manufactured by Advanced Surgical Concepts of Bray, Ireland, consists of a containment bag and a tube-like plunger to deliver the device into the abdominal cavity. The tissue being removed is placed in the bag and the bag is sealed and inflated. The inflation is intended to create working space around the tissue and better visualization during morcellation to prevent the morcellator tip or other surgical instruments from puncturing the bag. During laboratory testing, the containment bag was found to be impermeable to substances similar in molecular size to tissues, cells, and body fluids, according to the FDA.
Risks associated with the device include dissemination of morcellated tissue, injury to surrounding tissues or organs, infections, and a potentially longer surgical time. The FDA is requiring that the device’s label state that use of the PneumoLiner system is limited to physicians who have successfully completed the manufacturer’s validated training program.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The Food and Drug Administration has cleared for marketing a novel tissue containment system for use with certain laparoscopic power morcellators.
The PneumoLiner system is intended to be used to contain morcellated uterine tissue in a limited population of patients, including women without uterine fibroids undergoing hysterectomy and some premenopausal women with fibroids who want to maintain their fertility, according to the FDA. The agency is requiring the manufacturer to warn patients and physicians that the device has not been proven to reduce the risk of spreading cancer during surgery.
This approval – through the FDA’s de novo classification process for novel, low- and moderate-risk medical devices – comes about 2 years after the FDA first warned physicians and patients about the risk of spreading unsuspected uterine sarcomas during laparoscopic power morcellation in hysterectomy or myomectomy.
“This new device does not change our position on the risks associated with power morcellation,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in an April 7 statement. “We are continuing to warn against the use of power morcellators for the vast majority of women undergoing removal of the uterus or uterine fibroids.”
The device, which is manufactured by Advanced Surgical Concepts of Bray, Ireland, consists of a containment bag and a tube-like plunger to deliver the device into the abdominal cavity. The tissue being removed is placed in the bag and the bag is sealed and inflated. The inflation is intended to create working space around the tissue and better visualization during morcellation to prevent the morcellator tip or other surgical instruments from puncturing the bag. During laboratory testing, the containment bag was found to be impermeable to substances similar in molecular size to tissues, cells, and body fluids, according to the FDA.
Risks associated with the device include dissemination of morcellated tissue, injury to surrounding tissues or organs, infections, and a potentially longer surgical time. The FDA is requiring that the device’s label state that use of the PneumoLiner system is limited to physicians who have successfully completed the manufacturer’s validated training program.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The Food and Drug Administration has cleared for marketing a novel tissue containment system for use with certain laparoscopic power morcellators.
The PneumoLiner system is intended to be used to contain morcellated uterine tissue in a limited population of patients, including women without uterine fibroids undergoing hysterectomy and some premenopausal women with fibroids who want to maintain their fertility, according to the FDA. The agency is requiring the manufacturer to warn patients and physicians that the device has not been proven to reduce the risk of spreading cancer during surgery.
This approval – through the FDA’s de novo classification process for novel, low- and moderate-risk medical devices – comes about 2 years after the FDA first warned physicians and patients about the risk of spreading unsuspected uterine sarcomas during laparoscopic power morcellation in hysterectomy or myomectomy.
“This new device does not change our position on the risks associated with power morcellation,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in an April 7 statement. “We are continuing to warn against the use of power morcellators for the vast majority of women undergoing removal of the uterus or uterine fibroids.”
The device, which is manufactured by Advanced Surgical Concepts of Bray, Ireland, consists of a containment bag and a tube-like plunger to deliver the device into the abdominal cavity. The tissue being removed is placed in the bag and the bag is sealed and inflated. The inflation is intended to create working space around the tissue and better visualization during morcellation to prevent the morcellator tip or other surgical instruments from puncturing the bag. During laboratory testing, the containment bag was found to be impermeable to substances similar in molecular size to tissues, cells, and body fluids, according to the FDA.
Risks associated with the device include dissemination of morcellated tissue, injury to surrounding tissues or organs, infections, and a potentially longer surgical time. The FDA is requiring that the device’s label state that use of the PneumoLiner system is limited to physicians who have successfully completed the manufacturer’s validated training program.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
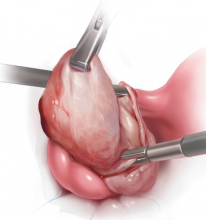
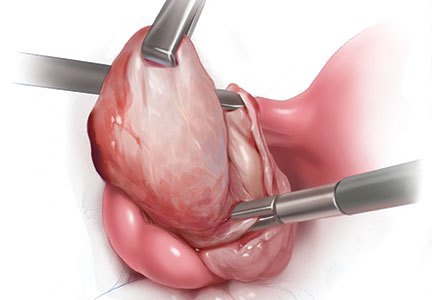
Robot-assisted laparoscopic myomectomy
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
New Findings Show: Factors Contributing to the Prevalence in readmission for Bariatric Surgery Patients
NEW YORK (Reuters Health) - About one in 20 bariatric surgery patients are readmitted to the hospital within 30 days of having the procedure, according to new findings.
Readmissions are increasingly being used as a quality metric for surgical procedures, Dr. John Morton of Stanford University in California and colleagues note in their report, published online March 19 in the American Journal of Surgery.
"While (the Centers for Medicare and Medicaid Services) has not addressed bariatric surgery readmissions to date, other payors have made readmissions a priority," they add. "Data regarding bariatric surgery readmissions are critical to help better understand and drive quality improvement in this area.
"To investigate the prevalence, causes and risk factors for readmission following bariatric surgery, the researchers looked at data from the 2012 American College of Surgeons National Surgical Quality Improvement Program Public Use File dataset on nearly 18,300 bariatric patients, of whom 55% had laparoscopic Roux-en-Y gastric bypass (LRYGB), 10% had laparoscopic adjustable gastric banding (LAGB), and 35% had laparoscopic sleeve gastrectomy (LSG).
There were 955 readmissions (5.22%), most commonly for gastrointestinal causes (45%), dietary reasons (34%) and bleeding (7%). Readmission rates were nearly 7% for LRYGB; just under 2% for LAGB; and 4% for LSG.
The patients who were readmitted had a significantly longer average operating time (132 vs. 115 minutes) and length of stay (2.76 days vs. 2.23). Forty percent had a complication, versus 4% of patients who were not readmitted. Patients who were readmitted were also more likely to have a body mass index above 50, preoperative diabetes, chronic obstructive pulmonary disease, and hypertension.
Factors independently associated with readmission included African-American race (odds ratio, 1.53), complication (OR, 11.3) and resident involvement (OR, 0.53).
"Other studies have also demonstrated similar predictors of readmission and have also demonstrated that length of stay may also play a role in readmission rates," Dr. Morton and his team state. "This study helps demonstrate that bariatric surgery readmissions are prevalent and potentially preventable."
NEW YORK (Reuters Health) - About one in 20 bariatric surgery patients are readmitted to the hospital within 30 days of having the procedure, according to new findings.
Readmissions are increasingly being used as a quality metric for surgical procedures, Dr. John Morton of Stanford University in California and colleagues note in their report, published online March 19 in the American Journal of Surgery.
"While (the Centers for Medicare and Medicaid Services) has not addressed bariatric surgery readmissions to date, other payors have made readmissions a priority," they add. "Data regarding bariatric surgery readmissions are critical to help better understand and drive quality improvement in this area.
"To investigate the prevalence, causes and risk factors for readmission following bariatric surgery, the researchers looked at data from the 2012 American College of Surgeons National Surgical Quality Improvement Program Public Use File dataset on nearly 18,300 bariatric patients, of whom 55% had laparoscopic Roux-en-Y gastric bypass (LRYGB), 10% had laparoscopic adjustable gastric banding (LAGB), and 35% had laparoscopic sleeve gastrectomy (LSG).
There were 955 readmissions (5.22%), most commonly for gastrointestinal causes (45%), dietary reasons (34%) and bleeding (7%). Readmission rates were nearly 7% for LRYGB; just under 2% for LAGB; and 4% for LSG.
The patients who were readmitted had a significantly longer average operating time (132 vs. 115 minutes) and length of stay (2.76 days vs. 2.23). Forty percent had a complication, versus 4% of patients who were not readmitted. Patients who were readmitted were also more likely to have a body mass index above 50, preoperative diabetes, chronic obstructive pulmonary disease, and hypertension.
Factors independently associated with readmission included African-American race (odds ratio, 1.53), complication (OR, 11.3) and resident involvement (OR, 0.53).
"Other studies have also demonstrated similar predictors of readmission and have also demonstrated that length of stay may also play a role in readmission rates," Dr. Morton and his team state. "This study helps demonstrate that bariatric surgery readmissions are prevalent and potentially preventable."
NEW YORK (Reuters Health) - About one in 20 bariatric surgery patients are readmitted to the hospital within 30 days of having the procedure, according to new findings.
Readmissions are increasingly being used as a quality metric for surgical procedures, Dr. John Morton of Stanford University in California and colleagues note in their report, published online March 19 in the American Journal of Surgery.
"While (the Centers for Medicare and Medicaid Services) has not addressed bariatric surgery readmissions to date, other payors have made readmissions a priority," they add. "Data regarding bariatric surgery readmissions are critical to help better understand and drive quality improvement in this area.
"To investigate the prevalence, causes and risk factors for readmission following bariatric surgery, the researchers looked at data from the 2012 American College of Surgeons National Surgical Quality Improvement Program Public Use File dataset on nearly 18,300 bariatric patients, of whom 55% had laparoscopic Roux-en-Y gastric bypass (LRYGB), 10% had laparoscopic adjustable gastric banding (LAGB), and 35% had laparoscopic sleeve gastrectomy (LSG).
There were 955 readmissions (5.22%), most commonly for gastrointestinal causes (45%), dietary reasons (34%) and bleeding (7%). Readmission rates were nearly 7% for LRYGB; just under 2% for LAGB; and 4% for LSG.
The patients who were readmitted had a significantly longer average operating time (132 vs. 115 minutes) and length of stay (2.76 days vs. 2.23). Forty percent had a complication, versus 4% of patients who were not readmitted. Patients who were readmitted were also more likely to have a body mass index above 50, preoperative diabetes, chronic obstructive pulmonary disease, and hypertension.
Factors independently associated with readmission included African-American race (odds ratio, 1.53), complication (OR, 11.3) and resident involvement (OR, 0.53).
"Other studies have also demonstrated similar predictors of readmission and have also demonstrated that length of stay may also play a role in readmission rates," Dr. Morton and his team state. "This study helps demonstrate that bariatric surgery readmissions are prevalent and potentially preventable."
2016 Update on minimally invasive gynecologic surgery
Rightly so, the topics of mechanical tissue extraction and hysterectomy approach have dominated the field of obstetrics and gynecology over the past 12 months and more. A profusion of literature has been published on these subjects. However, there are 2 important topics within the field of minimally invasive gynecologic surgery that deserve our attention as well, and I have chosen to focus on these for this Update.
First, laparoscopic treatment of ovarian endometriomas is one of the most commonly performed gynecologic procedures worldwide. Many women undergoing such surgery are of childbearing age and have the desire for future pregnancy. What are best practices for preserving ovarian function in these women? Two studies recently published in the Journal of Minimally Invasive Gynecology addressed this question.
Second, until recently, the rate of bowel injury at laparoscopic gynecologic surgery has not been well established.1 Moreover, mechanical bowel preparation is commonly employed in case intestinal injury does occur, despite the lack of evidence that outcomes of these possible injuries can be improved.2 Understanding the rate of bowel injury can shed light on the overall value of the perceived benefits of bowel preparation. Therefore, I examine 2 recent systematic reviews that analyze the incidence of bowel injury and the value of bowel prep in gynecologic laparoscopic surgery.
bipolar coagulation inferior to suturing or hemostatic sealant for preserving ovarian function
Song T, Kim WY, Lee KW, Kim KH. Effect on ovarian reserve of hemostasis by bipolar coagulation versus suture during laparoendoscopic single-site cystectomy for ovarian endometriomas. J Minim Invasive Gynecol. 2015;22(3):415−420.
Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar dessication. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2015;22(3):363−372.
The customary surgical approach for laparoscopic cystectomy is by mechanical stripping of the cyst wall (FIGURE) and the use of bipolar desiccation for hemostasis. Stripping inevitably leads to removal of healthy ovarian cortex,3 especially in inexperienced hands,4 and ovarian follicles inevitably are destroyed during electrosurgical desiccation. When compared with the use of suturing or a hemostatic agent to control bleeding in the ovarian defect, the use of bipolar electrosurgery may harm more of the ovarian cortex, resulting in a comparatively diminished follicular cohort.
Possible deleterious effects on the ovarian reserve can be determined with a blood test to measure anti-Müllerian hormone (AMH) levels postoperatively. Produced by the granulosa cells of the ovary, this hormone directly reflects the remaining ovarian egg supply. Lower levels of AMH have been shown to significantly decrease the success rate of in vitro fertilization (IVF), especially in women older than age 35.5 Moreover, AMH levels in the late reproductive years can be used as a predictive marker of menopause, with lower levels predicting significantly earlier onset.6
Data from 2 recent studies, a quasi-randomized trial by Song and colleagues and a systematic review and meta-analysis by Ata and colleagues emphasize that bipolar desiccation for hemostasis may not be best practice for protecting ovarian reserve during laparoscopic ovarian cystectomy for an endometrioma.
AMH levels decline more significantly for women undergoing bipolar desiccation
Song and colleagues conducted a prospective quasi-randomized study of 125 women whose endometriomas were laparoscopically removed via a single-site approach and managed for hemostasis with either bipolar desiccation or suturing of the ovarian defect with a 2-0 barbed suture. All surgeries were conducted by a single surgeon.
At 3 months postsurgery, mean AMH levels had declined from baseline by 42.2% (interquartile range [IR], 16.5−53.0 ng/mL) in the desiccation group and by 24.6% (IR, 11.6−37.0 ng/mL) in the suture group (P = .001). Multivariate analysis showed that the method used for hemostasis was the only determinant for reduced ovarian reserve.
In their systematic review and meta-analysis, Ata and colleagues included 10 studies--6 qualitative and 4 quantitative. All studies examined the rate of change of serum AMH levels 3 months after laparoscopic removal of an endometrioma.
In their qualitative analysis, 5 of the 6 studies reported a significantly greater decrease in ovarian reserve after bipolar desiccation (varying from 13% to 44%) or a strong trend in the same direction. In the sixth study, the desiccation group had a lower decline in absolute AMH level than in the other 5 studies. The authors note that this 2.7% decline was much lower than the values reported for the bipolar desiccation group of any other study. (Those declines ranged between 19% and 58%.)
Although not significant, in all 3 of the included randomized controlled trials (RCTs), the desiccation groups had a greater loss in AMH level than the hemostatic sealant groups, and in 2 of these RCTs, bipolar desiccation groups had a greater loss than the suturing groups.
Among the 213 study participants in the 3 RCTs and the prospective cohort study included in the quantitative meta-analysis, alternative methods to bipolar desiccation were associated with a 6.95% lower decrease in AMH-level decline (95% confidence interval [CI], −13.0% to −0.9%; P = .02).
What this EVIDENCE means for practice
Compared with the use of bipolar electrosurgery to attain hemostasis, the use of a topical biosurgical agent or suturing could be significantly better for protection of the ovarian follicles during laparoscopic ovarian cystectomy for endometrioma. These alternative methods especially could benefit those women desiring future pregnancy who are demonstrated preoperatively to have a low ovarian reserve. As needed, electrosurgery should be sparingly employed for ovarian hemostasis.
Large Study identifies incidence of bowel injury during gynecologic laproscopy
Llarena NC, Shah AB, Milad MP. Bowel injury in gynecologic laparoscopy: a systematic review. Am J Obstet Gynecol. 2015;125(6):1407−1417.
In no aspect of laparoscopic surgery are preventive strategies more cautiously employed than during peritoneal access. Regardless of the applied technique, there is an irreducible risk of injury to the underlying viscera by either adhesions between the underlying bowel and abdominal wall or during the course of pilot error. Moreover, in the best of hands, bowel injury can occur whenever normal anatomic relationships need to be restored using intra-abdominal adhesiolysis. Given the ubiquity, these risks are never out of the surgeon's mind. Gynecologists are obliged to discuss these risks during the informed consent process.
Until recently, the rate of bowel injury has not been well established. Llarena and colleagues recently have conducted the largest systematic review of the medical literature to date for incidence, presentation, mortality, cause, and location of bowel injury associated with laparoscopic surgery while not necessarily distinguishing for the type of bowel injury. Sixty retrospective and 27 prospective studies met inclusion criteria.
The risk of bowel injury overall and defined
Among 474,063 laparoscopic surgeries conducted between 1972 and 2014, 604 bowel injuries were found, for an incidence of 1 in 769, or 0.13% (95% CI, 0.12−0.14%).
The rate of bowel injury varied by procedure, year, study type, and definition of bowel injury. The incidence of injury according to:
- definition, was 1 in 416 (0.24%) for studies that clearly included serosal injuries and enterotomies versus 1 in 833 (0.12%) for studies not clearly defining the type of bowel injury (relative risk [RR], 0.47; 95% CI, 0.38−0.59; P<.001)
- study type, was 1 in 666 (0.15%) for prospective studies versus 1 in 909 (0.11%) for retrospective studies (RR, 0.78; 95% CI, 0.63−0.96; P = .02)
- procedure, was 1 in 3,333 (0.03%; 95% CI, 0.01−0.03%) for sterilization and 1 in 256 (0.39%; 95% CI, 0.35−0.45%) for hysterectomy
- year, for laparoscopic hysterectomy only, was 1 in 222 (0.45%) before the year 2000 and 1 in 294 (0.34%) after 2000 (RR, 0.75; 95% CI, 0.57−0.98; P = .03).
How were injuries caused, found, and managed?
Thirty studies described the laparoscopic instrument used during 366 reported bowel injuries. The majority of injuries (55%) occurred during initial peritoneal access, with the Veress needle or trocar causing the damage. This was followed by electrosurgery (29%), dissection (11%), and forceps or scissors (4.1%).
According to 40 studies describing 307 injuries, bowel injuries most often were managed by converting to laparotomy (80%); only 8% of injuries were managed with laparoscopy and 2% expectantly.
Surgery to repair the bowel injury was delayed in 154 (41%) of 375 cases. The median time to injury discovery was 3 days (range, 1−13 days).
In only 19 cases were the presenting signs and symptoms of bowel injury recorded. Those reported from most to least often were: peritonitis, abdominal pain, fever, abdominal distention, leukocytosis, leukopenia, and septic shock.
Mortality
Mortality as an outcome was only reported in 29 of the total 90 studies; therefore, mortality may be underreported. Overall, however, death occurred in 1 (0.8%) of 125 bowel injuries.
The overall mortality rate from bowel injury--calculated from the only 42 studies that explicitly mentioned mortality as an outcome--was 1 in 125, or 0.8% (95% CI, 0.36%-1.9%). All 5 reported deaths occurred as a result of delayed recognition of bowel injury, which made the mortality rate for unrecognized bowel injury 1 in 31, or 3.2% (95% CI, 1%-7%). No deaths occurred when the bowel injury was noted intraoperatively.
What this EVIDENCE means for practice
In this review of 474,063 laparoscopic procedures, bowel injury occurred in 1 in 769, or 0.13% of procedures. Bowel injury is more apt to occur during more complicated laparoscopic procedures (compared with laparoscopic sterilization procedures, the risk during hysterectomy was greater than 10-fold).
Most of the injuries were managed by laparotomic surgery despite the potential to repair bowel injury by laparoscopy. Validating that peritoneal access is a high risk part of laparoscopic surgery, the majority of the injuries occurred during insufflation with a Veress needle or during abdominal access by trocar insertion. Nearly one-third of the injuries were from the use of electrosurgery, which are typically associated with a delay in presentation.
In this study, 41% of the injuries were unrecognized at the time of surgery. All 5 of the reported deaths were associated with a delay in diagnosis, with an overall mortality rate of 1 of 125, or 0.8%. Since all of these deaths were associated with a delay in diagnosis, the rate of mortality in unrecognized bowel injury was 5 of 154, or 3.2%. Among women who experienced delayed diagnosis, only 19 of 154 experienced signs or symptoms diagnostic for an underlying bowel injury, particularly when the small bowel was injured.
Can mechanical bowel prep positively affect outcomes in gynecologic laparoscopy, or should it be discarded?
Arnold A, Aitchison LP, Abbott J. Preoperative mechanical bowel preparation for abdominal, laparoscopic, and vaginal surgery: a systematic review. J Minim Invasive Gynecol. 2015;22(5):737−752.
Popularized for more than 4 decades, the practice of presurgical bowel preparation is predicated on the notion that the presence of less, versus more, feces can minimize bacterial count and thereby reduce peritoneal contamination. Logically then, surgical site infections (SSIs) should be reduced with bowel preparation. Moreover, the surgical view and bowel handling during laparoscopic surgery should be improved, with surgical times consequently reduced.
Surgeons must weigh the putative benefits of mechanical bowel preparation against the unpleasant experience it causes for patients, as well as the risks of dehydration or electrolyte disturbance it may cause. To this day, a considerable percentage of gynecologists and colorectal surgeons routinely prep the bowel after weighing all of these factors, despite the paucity of evidence for the practice's efficacy to reduce SSI and improve surgical outcomes.7
The results of this recent systematic review critically question the usefulness of preoperative bowel preparation for abdominal, laparoscopic, and vaginal surgery.
Details of the analysis
The authors evaluated high-quality studies on mechanical bowel preparation to assess evidence for:
- surgeon outcomes, including the surgical field and bowel handling
- operative outcomes, including intraoperative complications and operative times
- patient outcomes, including postoperative complications, overall morbidity, and length of stay.
The authors identified RCTs and prospective or retrospective cohort studies in various surgical specialties comparing preoperative bowel preparation to no such prep. Forty-three studies met inclusion criteria: 38 compared prep to no prep, and 5 compared prep to a single rectal enema. Five high-grade studies in gynecology were included (n = 795), with 4 of them RCTs of gynecologic laparoscopy (n = 645).
Operative field and duration
Of the studies comparing bowel prep with no prep, only the 5 gynecologic ones assessed operative field. Surgical view was perceived as improved in only 1 study. In another, surgeons only could guess allocation half the time.
Sixteen studies evaluated impact of mechanical bowel preparation on duration of surgery: 1 high-quality study found a significant reduction in OR time with bowel prep, and 1 moderate-quality study found longer operative time with bowel prep.
Patient outcomes
Of all studies assessing patient outcomes, 3 high-quality studies of colorectal patients (n = 490) found increased complications from prep versus no prep, including anastomotic dehiscence (P = .05), abdominal complications (P = .028), and infectious complications (P = .05).
Length of stay was assessed in 26 studies, with 4 reporting longer hospital stay with bowel prep and the remaining finding no difference between prep and no prep.
Across all specialties, only 2 studies reported improved outcomes with mechanical bowel preparation. One was a high-quality study reporting reduced 30-day morbidity (P = .018) and infectious complication rates (P = .018), and the other was a moderate-quality study that found reduced SSI (P = .0001) and organ space infection (P = .024) in patients undergoing bowel prep.
Mechanical bowel preparation vs enema
Bowel prep was compared with a single rectal enema in 5 studies. In 2 of these, patient outcomes were worse with enema. One high-quality study of 294 patients reported increased intra-abdominal fecal soiling (P = .008) in the enema group. (The surgeons believed that bowel preparation was more likely to be inadequate in this group, 25% compared with 6%, P<.05.) Whereas there was no statistical difference in the incidence of anastomotic leak between these groups, there was higher reoperation rate in the enema-only group where leakage was diagnosed (6 [4.1%] vs 0, respectively; P = .013).
Bowel prep and preoperative and postoperative symptoms
Six high-quality studies reported on the impact of mechanical bowel preparation on patient symptoms, such as nausea, weakness, abdominal distention, and satisfaction before and after surgery. In all but 1 study patients had significantly greater discomfort with bowel preparation. In 2 of the 6 studies, patients had more diarrhea (P = .0003), a delay in the first bowel movement (P = .001), and a slower return to normal diet (P = .004).
What this EVIDENCE means for practice
The theory behind mechanical bowel preparation is not supported by the evidence. Despite the fact that the bowel is not customarily entered, up to 50% of gynecologic surgeons employ bowel preparation, with the hope of improving visualization and decreasing risk of an anastomotic leak. The colorectal studies in this review demonstrate no evidence for decreased anastomotic leak or infectious complications. By extrapolation, there is no evidence that using preoperative bowel prep bestows any benefit if bowel injury occurs inadvertently and if resection or reanastomosis is then required.
Among the 7 studies examining bowel prep in laparoscopy (4 gynecology, 3 urology, and 1 colorectal), only data from 1 demonstrated an improved surgical field (and in this case only by 1 out of 10 on a Likert scale). The impact of mechanical bowel preparation on the visual field is the same for diagnostic or complex laparoscopic surgeries. One high-quality study with deep endometriosis resection demonstrated no change in the operative field as reflected by no practical differences in OR time or complications.
Preparing the bowel for surgery is an intrusive process that reduces patient satisfaction by inducing weakness, abdominal distention, nausea, vomiting, hunger, and thirst. Whereas this systematic analysis failed to confirm any benefit of the process, it provides evidence for the potential for harm. Mechanical bowel preparation should be discarded as a routine preoperative treatment for patients undergoing minimally invasive gynecologic surgery.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Rightly so, the topics of mechanical tissue extraction and hysterectomy approach have dominated the field of obstetrics and gynecology over the past 12 months and more. A profusion of literature has been published on these subjects. However, there are 2 important topics within the field of minimally invasive gynecologic surgery that deserve our attention as well, and I have chosen to focus on these for this Update.
First, laparoscopic treatment of ovarian endometriomas is one of the most commonly performed gynecologic procedures worldwide. Many women undergoing such surgery are of childbearing age and have the desire for future pregnancy. What are best practices for preserving ovarian function in these women? Two studies recently published in the Journal of Minimally Invasive Gynecology addressed this question.
Second, until recently, the rate of bowel injury at laparoscopic gynecologic surgery has not been well established.1 Moreover, mechanical bowel preparation is commonly employed in case intestinal injury does occur, despite the lack of evidence that outcomes of these possible injuries can be improved.2 Understanding the rate of bowel injury can shed light on the overall value of the perceived benefits of bowel preparation. Therefore, I examine 2 recent systematic reviews that analyze the incidence of bowel injury and the value of bowel prep in gynecologic laparoscopic surgery.
bipolar coagulation inferior to suturing or hemostatic sealant for preserving ovarian function
Song T, Kim WY, Lee KW, Kim KH. Effect on ovarian reserve of hemostasis by bipolar coagulation versus suture during laparoendoscopic single-site cystectomy for ovarian endometriomas. J Minim Invasive Gynecol. 2015;22(3):415−420.
Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar dessication. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2015;22(3):363−372.
The customary surgical approach for laparoscopic cystectomy is by mechanical stripping of the cyst wall (FIGURE) and the use of bipolar desiccation for hemostasis. Stripping inevitably leads to removal of healthy ovarian cortex,3 especially in inexperienced hands,4 and ovarian follicles inevitably are destroyed during electrosurgical desiccation. When compared with the use of suturing or a hemostatic agent to control bleeding in the ovarian defect, the use of bipolar electrosurgery may harm more of the ovarian cortex, resulting in a comparatively diminished follicular cohort.
Possible deleterious effects on the ovarian reserve can be determined with a blood test to measure anti-Müllerian hormone (AMH) levels postoperatively. Produced by the granulosa cells of the ovary, this hormone directly reflects the remaining ovarian egg supply. Lower levels of AMH have been shown to significantly decrease the success rate of in vitro fertilization (IVF), especially in women older than age 35.5 Moreover, AMH levels in the late reproductive years can be used as a predictive marker of menopause, with lower levels predicting significantly earlier onset.6
Data from 2 recent studies, a quasi-randomized trial by Song and colleagues and a systematic review and meta-analysis by Ata and colleagues emphasize that bipolar desiccation for hemostasis may not be best practice for protecting ovarian reserve during laparoscopic ovarian cystectomy for an endometrioma.
AMH levels decline more significantly for women undergoing bipolar desiccation
Song and colleagues conducted a prospective quasi-randomized study of 125 women whose endometriomas were laparoscopically removed via a single-site approach and managed for hemostasis with either bipolar desiccation or suturing of the ovarian defect with a 2-0 barbed suture. All surgeries were conducted by a single surgeon.
At 3 months postsurgery, mean AMH levels had declined from baseline by 42.2% (interquartile range [IR], 16.5−53.0 ng/mL) in the desiccation group and by 24.6% (IR, 11.6−37.0 ng/mL) in the suture group (P = .001). Multivariate analysis showed that the method used for hemostasis was the only determinant for reduced ovarian reserve.
In their systematic review and meta-analysis, Ata and colleagues included 10 studies--6 qualitative and 4 quantitative. All studies examined the rate of change of serum AMH levels 3 months after laparoscopic removal of an endometrioma.
In their qualitative analysis, 5 of the 6 studies reported a significantly greater decrease in ovarian reserve after bipolar desiccation (varying from 13% to 44%) or a strong trend in the same direction. In the sixth study, the desiccation group had a lower decline in absolute AMH level than in the other 5 studies. The authors note that this 2.7% decline was much lower than the values reported for the bipolar desiccation group of any other study. (Those declines ranged between 19% and 58%.)
Although not significant, in all 3 of the included randomized controlled trials (RCTs), the desiccation groups had a greater loss in AMH level than the hemostatic sealant groups, and in 2 of these RCTs, bipolar desiccation groups had a greater loss than the suturing groups.
Among the 213 study participants in the 3 RCTs and the prospective cohort study included in the quantitative meta-analysis, alternative methods to bipolar desiccation were associated with a 6.95% lower decrease in AMH-level decline (95% confidence interval [CI], −13.0% to −0.9%; P = .02).
What this EVIDENCE means for practice
Compared with the use of bipolar electrosurgery to attain hemostasis, the use of a topical biosurgical agent or suturing could be significantly better for protection of the ovarian follicles during laparoscopic ovarian cystectomy for endometrioma. These alternative methods especially could benefit those women desiring future pregnancy who are demonstrated preoperatively to have a low ovarian reserve. As needed, electrosurgery should be sparingly employed for ovarian hemostasis.
Large Study identifies incidence of bowel injury during gynecologic laproscopy
Llarena NC, Shah AB, Milad MP. Bowel injury in gynecologic laparoscopy: a systematic review. Am J Obstet Gynecol. 2015;125(6):1407−1417.
In no aspect of laparoscopic surgery are preventive strategies more cautiously employed than during peritoneal access. Regardless of the applied technique, there is an irreducible risk of injury to the underlying viscera by either adhesions between the underlying bowel and abdominal wall or during the course of pilot error. Moreover, in the best of hands, bowel injury can occur whenever normal anatomic relationships need to be restored using intra-abdominal adhesiolysis. Given the ubiquity, these risks are never out of the surgeon's mind. Gynecologists are obliged to discuss these risks during the informed consent process.
Until recently, the rate of bowel injury has not been well established. Llarena and colleagues recently have conducted the largest systematic review of the medical literature to date for incidence, presentation, mortality, cause, and location of bowel injury associated with laparoscopic surgery while not necessarily distinguishing for the type of bowel injury. Sixty retrospective and 27 prospective studies met inclusion criteria.
The risk of bowel injury overall and defined
Among 474,063 laparoscopic surgeries conducted between 1972 and 2014, 604 bowel injuries were found, for an incidence of 1 in 769, or 0.13% (95% CI, 0.12−0.14%).
The rate of bowel injury varied by procedure, year, study type, and definition of bowel injury. The incidence of injury according to:
- definition, was 1 in 416 (0.24%) for studies that clearly included serosal injuries and enterotomies versus 1 in 833 (0.12%) for studies not clearly defining the type of bowel injury (relative risk [RR], 0.47; 95% CI, 0.38−0.59; P<.001)
- study type, was 1 in 666 (0.15%) for prospective studies versus 1 in 909 (0.11%) for retrospective studies (RR, 0.78; 95% CI, 0.63−0.96; P = .02)
- procedure, was 1 in 3,333 (0.03%; 95% CI, 0.01−0.03%) for sterilization and 1 in 256 (0.39%; 95% CI, 0.35−0.45%) for hysterectomy
- year, for laparoscopic hysterectomy only, was 1 in 222 (0.45%) before the year 2000 and 1 in 294 (0.34%) after 2000 (RR, 0.75; 95% CI, 0.57−0.98; P = .03).
How were injuries caused, found, and managed?
Thirty studies described the laparoscopic instrument used during 366 reported bowel injuries. The majority of injuries (55%) occurred during initial peritoneal access, with the Veress needle or trocar causing the damage. This was followed by electrosurgery (29%), dissection (11%), and forceps or scissors (4.1%).
According to 40 studies describing 307 injuries, bowel injuries most often were managed by converting to laparotomy (80%); only 8% of injuries were managed with laparoscopy and 2% expectantly.
Surgery to repair the bowel injury was delayed in 154 (41%) of 375 cases. The median time to injury discovery was 3 days (range, 1−13 days).
In only 19 cases were the presenting signs and symptoms of bowel injury recorded. Those reported from most to least often were: peritonitis, abdominal pain, fever, abdominal distention, leukocytosis, leukopenia, and septic shock.
Mortality
Mortality as an outcome was only reported in 29 of the total 90 studies; therefore, mortality may be underreported. Overall, however, death occurred in 1 (0.8%) of 125 bowel injuries.
The overall mortality rate from bowel injury--calculated from the only 42 studies that explicitly mentioned mortality as an outcome--was 1 in 125, or 0.8% (95% CI, 0.36%-1.9%). All 5 reported deaths occurred as a result of delayed recognition of bowel injury, which made the mortality rate for unrecognized bowel injury 1 in 31, or 3.2% (95% CI, 1%-7%). No deaths occurred when the bowel injury was noted intraoperatively.
What this EVIDENCE means for practice
In this review of 474,063 laparoscopic procedures, bowel injury occurred in 1 in 769, or 0.13% of procedures. Bowel injury is more apt to occur during more complicated laparoscopic procedures (compared with laparoscopic sterilization procedures, the risk during hysterectomy was greater than 10-fold).
Most of the injuries were managed by laparotomic surgery despite the potential to repair bowel injury by laparoscopy. Validating that peritoneal access is a high risk part of laparoscopic surgery, the majority of the injuries occurred during insufflation with a Veress needle or during abdominal access by trocar insertion. Nearly one-third of the injuries were from the use of electrosurgery, which are typically associated with a delay in presentation.
In this study, 41% of the injuries were unrecognized at the time of surgery. All 5 of the reported deaths were associated with a delay in diagnosis, with an overall mortality rate of 1 of 125, or 0.8%. Since all of these deaths were associated with a delay in diagnosis, the rate of mortality in unrecognized bowel injury was 5 of 154, or 3.2%. Among women who experienced delayed diagnosis, only 19 of 154 experienced signs or symptoms diagnostic for an underlying bowel injury, particularly when the small bowel was injured.
Can mechanical bowel prep positively affect outcomes in gynecologic laparoscopy, or should it be discarded?
Arnold A, Aitchison LP, Abbott J. Preoperative mechanical bowel preparation for abdominal, laparoscopic, and vaginal surgery: a systematic review. J Minim Invasive Gynecol. 2015;22(5):737−752.
Popularized for more than 4 decades, the practice of presurgical bowel preparation is predicated on the notion that the presence of less, versus more, feces can minimize bacterial count and thereby reduce peritoneal contamination. Logically then, surgical site infections (SSIs) should be reduced with bowel preparation. Moreover, the surgical view and bowel handling during laparoscopic surgery should be improved, with surgical times consequently reduced.
Surgeons must weigh the putative benefits of mechanical bowel preparation against the unpleasant experience it causes for patients, as well as the risks of dehydration or electrolyte disturbance it may cause. To this day, a considerable percentage of gynecologists and colorectal surgeons routinely prep the bowel after weighing all of these factors, despite the paucity of evidence for the practice's efficacy to reduce SSI and improve surgical outcomes.7
The results of this recent systematic review critically question the usefulness of preoperative bowel preparation for abdominal, laparoscopic, and vaginal surgery.
Details of the analysis
The authors evaluated high-quality studies on mechanical bowel preparation to assess evidence for:
- surgeon outcomes, including the surgical field and bowel handling
- operative outcomes, including intraoperative complications and operative times
- patient outcomes, including postoperative complications, overall morbidity, and length of stay.
The authors identified RCTs and prospective or retrospective cohort studies in various surgical specialties comparing preoperative bowel preparation to no such prep. Forty-three studies met inclusion criteria: 38 compared prep to no prep, and 5 compared prep to a single rectal enema. Five high-grade studies in gynecology were included (n = 795), with 4 of them RCTs of gynecologic laparoscopy (n = 645).
Operative field and duration
Of the studies comparing bowel prep with no prep, only the 5 gynecologic ones assessed operative field. Surgical view was perceived as improved in only 1 study. In another, surgeons only could guess allocation half the time.
Sixteen studies evaluated impact of mechanical bowel preparation on duration of surgery: 1 high-quality study found a significant reduction in OR time with bowel prep, and 1 moderate-quality study found longer operative time with bowel prep.
Patient outcomes
Of all studies assessing patient outcomes, 3 high-quality studies of colorectal patients (n = 490) found increased complications from prep versus no prep, including anastomotic dehiscence (P = .05), abdominal complications (P = .028), and infectious complications (P = .05).
Length of stay was assessed in 26 studies, with 4 reporting longer hospital stay with bowel prep and the remaining finding no difference between prep and no prep.
Across all specialties, only 2 studies reported improved outcomes with mechanical bowel preparation. One was a high-quality study reporting reduced 30-day morbidity (P = .018) and infectious complication rates (P = .018), and the other was a moderate-quality study that found reduced SSI (P = .0001) and organ space infection (P = .024) in patients undergoing bowel prep.
Mechanical bowel preparation vs enema
Bowel prep was compared with a single rectal enema in 5 studies. In 2 of these, patient outcomes were worse with enema. One high-quality study of 294 patients reported increased intra-abdominal fecal soiling (P = .008) in the enema group. (The surgeons believed that bowel preparation was more likely to be inadequate in this group, 25% compared with 6%, P<.05.) Whereas there was no statistical difference in the incidence of anastomotic leak between these groups, there was higher reoperation rate in the enema-only group where leakage was diagnosed (6 [4.1%] vs 0, respectively; P = .013).
Bowel prep and preoperative and postoperative symptoms
Six high-quality studies reported on the impact of mechanical bowel preparation on patient symptoms, such as nausea, weakness, abdominal distention, and satisfaction before and after surgery. In all but 1 study patients had significantly greater discomfort with bowel preparation. In 2 of the 6 studies, patients had more diarrhea (P = .0003), a delay in the first bowel movement (P = .001), and a slower return to normal diet (P = .004).
What this EVIDENCE means for practice
The theory behind mechanical bowel preparation is not supported by the evidence. Despite the fact that the bowel is not customarily entered, up to 50% of gynecologic surgeons employ bowel preparation, with the hope of improving visualization and decreasing risk of an anastomotic leak. The colorectal studies in this review demonstrate no evidence for decreased anastomotic leak or infectious complications. By extrapolation, there is no evidence that using preoperative bowel prep bestows any benefit if bowel injury occurs inadvertently and if resection or reanastomosis is then required.
Among the 7 studies examining bowel prep in laparoscopy (4 gynecology, 3 urology, and 1 colorectal), only data from 1 demonstrated an improved surgical field (and in this case only by 1 out of 10 on a Likert scale). The impact of mechanical bowel preparation on the visual field is the same for diagnostic or complex laparoscopic surgeries. One high-quality study with deep endometriosis resection demonstrated no change in the operative field as reflected by no practical differences in OR time or complications.
Preparing the bowel for surgery is an intrusive process that reduces patient satisfaction by inducing weakness, abdominal distention, nausea, vomiting, hunger, and thirst. Whereas this systematic analysis failed to confirm any benefit of the process, it provides evidence for the potential for harm. Mechanical bowel preparation should be discarded as a routine preoperative treatment for patients undergoing minimally invasive gynecologic surgery.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Rightly so, the topics of mechanical tissue extraction and hysterectomy approach have dominated the field of obstetrics and gynecology over the past 12 months and more. A profusion of literature has been published on these subjects. However, there are 2 important topics within the field of minimally invasive gynecologic surgery that deserve our attention as well, and I have chosen to focus on these for this Update.
First, laparoscopic treatment of ovarian endometriomas is one of the most commonly performed gynecologic procedures worldwide. Many women undergoing such surgery are of childbearing age and have the desire for future pregnancy. What are best practices for preserving ovarian function in these women? Two studies recently published in the Journal of Minimally Invasive Gynecology addressed this question.
Second, until recently, the rate of bowel injury at laparoscopic gynecologic surgery has not been well established.1 Moreover, mechanical bowel preparation is commonly employed in case intestinal injury does occur, despite the lack of evidence that outcomes of these possible injuries can be improved.2 Understanding the rate of bowel injury can shed light on the overall value of the perceived benefits of bowel preparation. Therefore, I examine 2 recent systematic reviews that analyze the incidence of bowel injury and the value of bowel prep in gynecologic laparoscopic surgery.
bipolar coagulation inferior to suturing or hemostatic sealant for preserving ovarian function
Song T, Kim WY, Lee KW, Kim KH. Effect on ovarian reserve of hemostasis by bipolar coagulation versus suture during laparoendoscopic single-site cystectomy for ovarian endometriomas. J Minim Invasive Gynecol. 2015;22(3):415−420.
Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar dessication. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2015;22(3):363−372.
The customary surgical approach for laparoscopic cystectomy is by mechanical stripping of the cyst wall (FIGURE) and the use of bipolar desiccation for hemostasis. Stripping inevitably leads to removal of healthy ovarian cortex,3 especially in inexperienced hands,4 and ovarian follicles inevitably are destroyed during electrosurgical desiccation. When compared with the use of suturing or a hemostatic agent to control bleeding in the ovarian defect, the use of bipolar electrosurgery may harm more of the ovarian cortex, resulting in a comparatively diminished follicular cohort.
Possible deleterious effects on the ovarian reserve can be determined with a blood test to measure anti-Müllerian hormone (AMH) levels postoperatively. Produced by the granulosa cells of the ovary, this hormone directly reflects the remaining ovarian egg supply. Lower levels of AMH have been shown to significantly decrease the success rate of in vitro fertilization (IVF), especially in women older than age 35.5 Moreover, AMH levels in the late reproductive years can be used as a predictive marker of menopause, with lower levels predicting significantly earlier onset.6
Data from 2 recent studies, a quasi-randomized trial by Song and colleagues and a systematic review and meta-analysis by Ata and colleagues emphasize that bipolar desiccation for hemostasis may not be best practice for protecting ovarian reserve during laparoscopic ovarian cystectomy for an endometrioma.
AMH levels decline more significantly for women undergoing bipolar desiccation
Song and colleagues conducted a prospective quasi-randomized study of 125 women whose endometriomas were laparoscopically removed via a single-site approach and managed for hemostasis with either bipolar desiccation or suturing of the ovarian defect with a 2-0 barbed suture. All surgeries were conducted by a single surgeon.
At 3 months postsurgery, mean AMH levels had declined from baseline by 42.2% (interquartile range [IR], 16.5−53.0 ng/mL) in the desiccation group and by 24.6% (IR, 11.6−37.0 ng/mL) in the suture group (P = .001). Multivariate analysis showed that the method used for hemostasis was the only determinant for reduced ovarian reserve.
In their systematic review and meta-analysis, Ata and colleagues included 10 studies--6 qualitative and 4 quantitative. All studies examined the rate of change of serum AMH levels 3 months after laparoscopic removal of an endometrioma.
In their qualitative analysis, 5 of the 6 studies reported a significantly greater decrease in ovarian reserve after bipolar desiccation (varying from 13% to 44%) or a strong trend in the same direction. In the sixth study, the desiccation group had a lower decline in absolute AMH level than in the other 5 studies. The authors note that this 2.7% decline was much lower than the values reported for the bipolar desiccation group of any other study. (Those declines ranged between 19% and 58%.)
Although not significant, in all 3 of the included randomized controlled trials (RCTs), the desiccation groups had a greater loss in AMH level than the hemostatic sealant groups, and in 2 of these RCTs, bipolar desiccation groups had a greater loss than the suturing groups.
Among the 213 study participants in the 3 RCTs and the prospective cohort study included in the quantitative meta-analysis, alternative methods to bipolar desiccation were associated with a 6.95% lower decrease in AMH-level decline (95% confidence interval [CI], −13.0% to −0.9%; P = .02).
What this EVIDENCE means for practice
Compared with the use of bipolar electrosurgery to attain hemostasis, the use of a topical biosurgical agent or suturing could be significantly better for protection of the ovarian follicles during laparoscopic ovarian cystectomy for endometrioma. These alternative methods especially could benefit those women desiring future pregnancy who are demonstrated preoperatively to have a low ovarian reserve. As needed, electrosurgery should be sparingly employed for ovarian hemostasis.
Large Study identifies incidence of bowel injury during gynecologic laproscopy
Llarena NC, Shah AB, Milad MP. Bowel injury in gynecologic laparoscopy: a systematic review. Am J Obstet Gynecol. 2015;125(6):1407−1417.
In no aspect of laparoscopic surgery are preventive strategies more cautiously employed than during peritoneal access. Regardless of the applied technique, there is an irreducible risk of injury to the underlying viscera by either adhesions between the underlying bowel and abdominal wall or during the course of pilot error. Moreover, in the best of hands, bowel injury can occur whenever normal anatomic relationships need to be restored using intra-abdominal adhesiolysis. Given the ubiquity, these risks are never out of the surgeon's mind. Gynecologists are obliged to discuss these risks during the informed consent process.
Until recently, the rate of bowel injury has not been well established. Llarena and colleagues recently have conducted the largest systematic review of the medical literature to date for incidence, presentation, mortality, cause, and location of bowel injury associated with laparoscopic surgery while not necessarily distinguishing for the type of bowel injury. Sixty retrospective and 27 prospective studies met inclusion criteria.
The risk of bowel injury overall and defined
Among 474,063 laparoscopic surgeries conducted between 1972 and 2014, 604 bowel injuries were found, for an incidence of 1 in 769, or 0.13% (95% CI, 0.12−0.14%).
The rate of bowel injury varied by procedure, year, study type, and definition of bowel injury. The incidence of injury according to:
- definition, was 1 in 416 (0.24%) for studies that clearly included serosal injuries and enterotomies versus 1 in 833 (0.12%) for studies not clearly defining the type of bowel injury (relative risk [RR], 0.47; 95% CI, 0.38−0.59; P<.001)
- study type, was 1 in 666 (0.15%) for prospective studies versus 1 in 909 (0.11%) for retrospective studies (RR, 0.78; 95% CI, 0.63−0.96; P = .02)
- procedure, was 1 in 3,333 (0.03%; 95% CI, 0.01−0.03%) for sterilization and 1 in 256 (0.39%; 95% CI, 0.35−0.45%) for hysterectomy
- year, for laparoscopic hysterectomy only, was 1 in 222 (0.45%) before the year 2000 and 1 in 294 (0.34%) after 2000 (RR, 0.75; 95% CI, 0.57−0.98; P = .03).
How were injuries caused, found, and managed?
Thirty studies described the laparoscopic instrument used during 366 reported bowel injuries. The majority of injuries (55%) occurred during initial peritoneal access, with the Veress needle or trocar causing the damage. This was followed by electrosurgery (29%), dissection (11%), and forceps or scissors (4.1%).
According to 40 studies describing 307 injuries, bowel injuries most often were managed by converting to laparotomy (80%); only 8% of injuries were managed with laparoscopy and 2% expectantly.
Surgery to repair the bowel injury was delayed in 154 (41%) of 375 cases. The median time to injury discovery was 3 days (range, 1−13 days).
In only 19 cases were the presenting signs and symptoms of bowel injury recorded. Those reported from most to least often were: peritonitis, abdominal pain, fever, abdominal distention, leukocytosis, leukopenia, and septic shock.
Mortality
Mortality as an outcome was only reported in 29 of the total 90 studies; therefore, mortality may be underreported. Overall, however, death occurred in 1 (0.8%) of 125 bowel injuries.
The overall mortality rate from bowel injury--calculated from the only 42 studies that explicitly mentioned mortality as an outcome--was 1 in 125, or 0.8% (95% CI, 0.36%-1.9%). All 5 reported deaths occurred as a result of delayed recognition of bowel injury, which made the mortality rate for unrecognized bowel injury 1 in 31, or 3.2% (95% CI, 1%-7%). No deaths occurred when the bowel injury was noted intraoperatively.
What this EVIDENCE means for practice
In this review of 474,063 laparoscopic procedures, bowel injury occurred in 1 in 769, or 0.13% of procedures. Bowel injury is more apt to occur during more complicated laparoscopic procedures (compared with laparoscopic sterilization procedures, the risk during hysterectomy was greater than 10-fold).
Most of the injuries were managed by laparotomic surgery despite the potential to repair bowel injury by laparoscopy. Validating that peritoneal access is a high risk part of laparoscopic surgery, the majority of the injuries occurred during insufflation with a Veress needle or during abdominal access by trocar insertion. Nearly one-third of the injuries were from the use of electrosurgery, which are typically associated with a delay in presentation.
In this study, 41% of the injuries were unrecognized at the time of surgery. All 5 of the reported deaths were associated with a delay in diagnosis, with an overall mortality rate of 1 of 125, or 0.8%. Since all of these deaths were associated with a delay in diagnosis, the rate of mortality in unrecognized bowel injury was 5 of 154, or 3.2%. Among women who experienced delayed diagnosis, only 19 of 154 experienced signs or symptoms diagnostic for an underlying bowel injury, particularly when the small bowel was injured.
Can mechanical bowel prep positively affect outcomes in gynecologic laparoscopy, or should it be discarded?
Arnold A, Aitchison LP, Abbott J. Preoperative mechanical bowel preparation for abdominal, laparoscopic, and vaginal surgery: a systematic review. J Minim Invasive Gynecol. 2015;22(5):737−752.
Popularized for more than 4 decades, the practice of presurgical bowel preparation is predicated on the notion that the presence of less, versus more, feces can minimize bacterial count and thereby reduce peritoneal contamination. Logically then, surgical site infections (SSIs) should be reduced with bowel preparation. Moreover, the surgical view and bowel handling during laparoscopic surgery should be improved, with surgical times consequently reduced.
Surgeons must weigh the putative benefits of mechanical bowel preparation against the unpleasant experience it causes for patients, as well as the risks of dehydration or electrolyte disturbance it may cause. To this day, a considerable percentage of gynecologists and colorectal surgeons routinely prep the bowel after weighing all of these factors, despite the paucity of evidence for the practice's efficacy to reduce SSI and improve surgical outcomes.7
The results of this recent systematic review critically question the usefulness of preoperative bowel preparation for abdominal, laparoscopic, and vaginal surgery.
Details of the analysis
The authors evaluated high-quality studies on mechanical bowel preparation to assess evidence for:
- surgeon outcomes, including the surgical field and bowel handling
- operative outcomes, including intraoperative complications and operative times
- patient outcomes, including postoperative complications, overall morbidity, and length of stay.
The authors identified RCTs and prospective or retrospective cohort studies in various surgical specialties comparing preoperative bowel preparation to no such prep. Forty-three studies met inclusion criteria: 38 compared prep to no prep, and 5 compared prep to a single rectal enema. Five high-grade studies in gynecology were included (n = 795), with 4 of them RCTs of gynecologic laparoscopy (n = 645).
Operative field and duration
Of the studies comparing bowel prep with no prep, only the 5 gynecologic ones assessed operative field. Surgical view was perceived as improved in only 1 study. In another, surgeons only could guess allocation half the time.
Sixteen studies evaluated impact of mechanical bowel preparation on duration of surgery: 1 high-quality study found a significant reduction in OR time with bowel prep, and 1 moderate-quality study found longer operative time with bowel prep.
Patient outcomes
Of all studies assessing patient outcomes, 3 high-quality studies of colorectal patients (n = 490) found increased complications from prep versus no prep, including anastomotic dehiscence (P = .05), abdominal complications (P = .028), and infectious complications (P = .05).
Length of stay was assessed in 26 studies, with 4 reporting longer hospital stay with bowel prep and the remaining finding no difference between prep and no prep.
Across all specialties, only 2 studies reported improved outcomes with mechanical bowel preparation. One was a high-quality study reporting reduced 30-day morbidity (P = .018) and infectious complication rates (P = .018), and the other was a moderate-quality study that found reduced SSI (P = .0001) and organ space infection (P = .024) in patients undergoing bowel prep.
Mechanical bowel preparation vs enema
Bowel prep was compared with a single rectal enema in 5 studies. In 2 of these, patient outcomes were worse with enema. One high-quality study of 294 patients reported increased intra-abdominal fecal soiling (P = .008) in the enema group. (The surgeons believed that bowel preparation was more likely to be inadequate in this group, 25% compared with 6%, P<.05.) Whereas there was no statistical difference in the incidence of anastomotic leak between these groups, there was higher reoperation rate in the enema-only group where leakage was diagnosed (6 [4.1%] vs 0, respectively; P = .013).
Bowel prep and preoperative and postoperative symptoms
Six high-quality studies reported on the impact of mechanical bowel preparation on patient symptoms, such as nausea, weakness, abdominal distention, and satisfaction before and after surgery. In all but 1 study patients had significantly greater discomfort with bowel preparation. In 2 of the 6 studies, patients had more diarrhea (P = .0003), a delay in the first bowel movement (P = .001), and a slower return to normal diet (P = .004).
What this EVIDENCE means for practice
The theory behind mechanical bowel preparation is not supported by the evidence. Despite the fact that the bowel is not customarily entered, up to 50% of gynecologic surgeons employ bowel preparation, with the hope of improving visualization and decreasing risk of an anastomotic leak. The colorectal studies in this review demonstrate no evidence for decreased anastomotic leak or infectious complications. By extrapolation, there is no evidence that using preoperative bowel prep bestows any benefit if bowel injury occurs inadvertently and if resection or reanastomosis is then required.
Among the 7 studies examining bowel prep in laparoscopy (4 gynecology, 3 urology, and 1 colorectal), only data from 1 demonstrated an improved surgical field (and in this case only by 1 out of 10 on a Likert scale). The impact of mechanical bowel preparation on the visual field is the same for diagnostic or complex laparoscopic surgeries. One high-quality study with deep endometriosis resection demonstrated no change in the operative field as reflected by no practical differences in OR time or complications.
Preparing the bowel for surgery is an intrusive process that reduces patient satisfaction by inducing weakness, abdominal distention, nausea, vomiting, hunger, and thirst. Whereas this systematic analysis failed to confirm any benefit of the process, it provides evidence for the potential for harm. Mechanical bowel preparation should be discarded as a routine preoperative treatment for patients undergoing minimally invasive gynecologic surgery.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In this article
- Preserving ovarian function at laparoscopic cystectomy
- Incidence of bowel injury during gyn surgery
- Usefulness and safety of mechanical bowel preparation
Cesarean scar defect: What is it and how should it be treated?
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
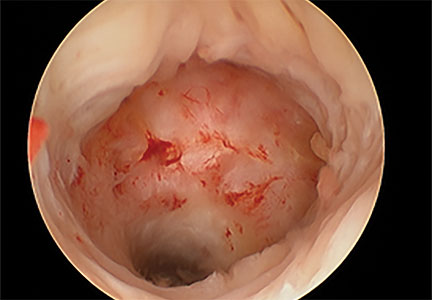
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).
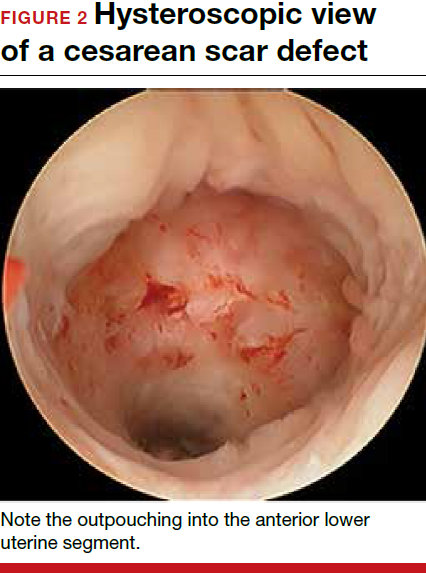
Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
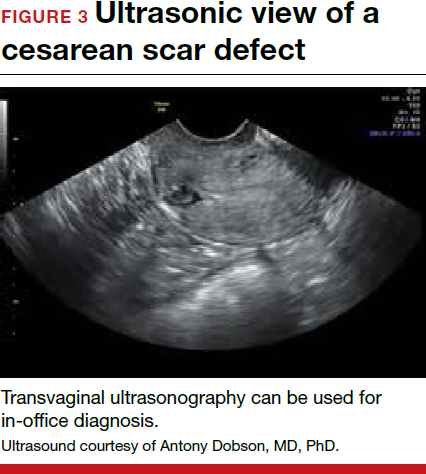
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.
Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9
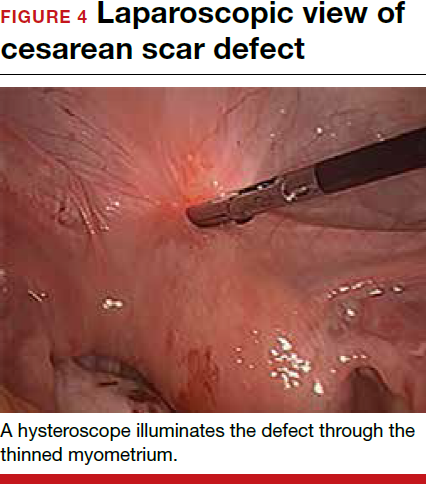
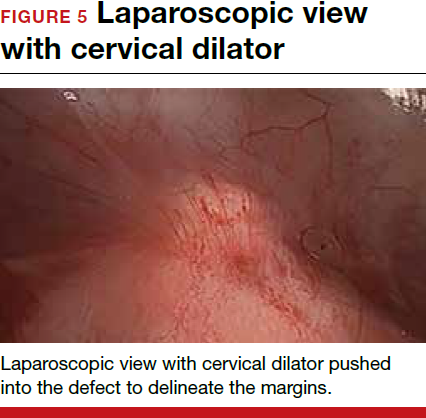
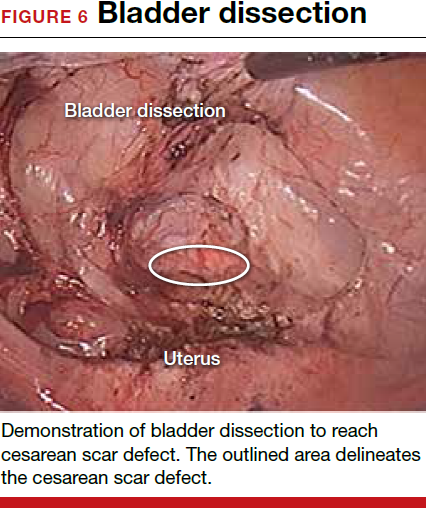
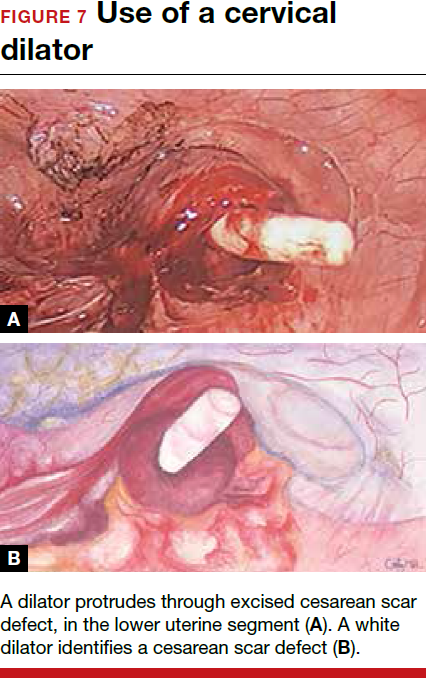
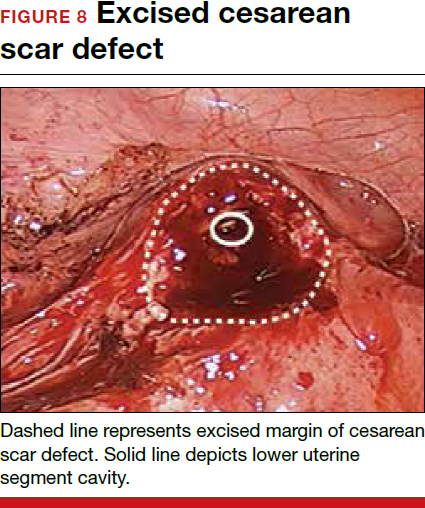
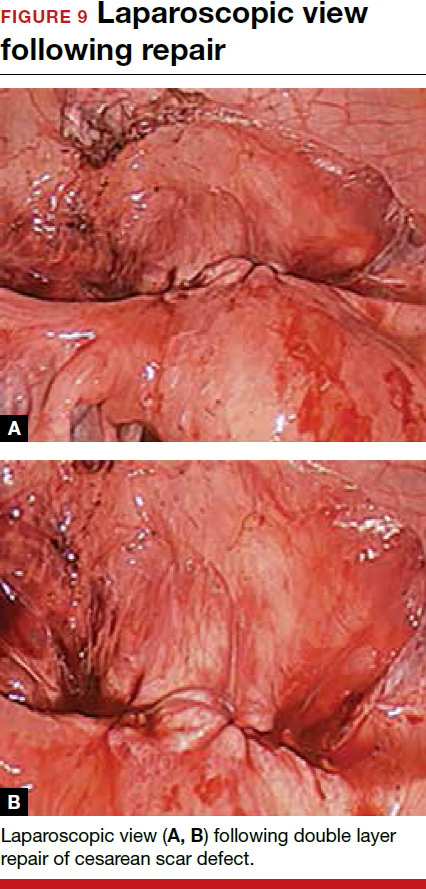
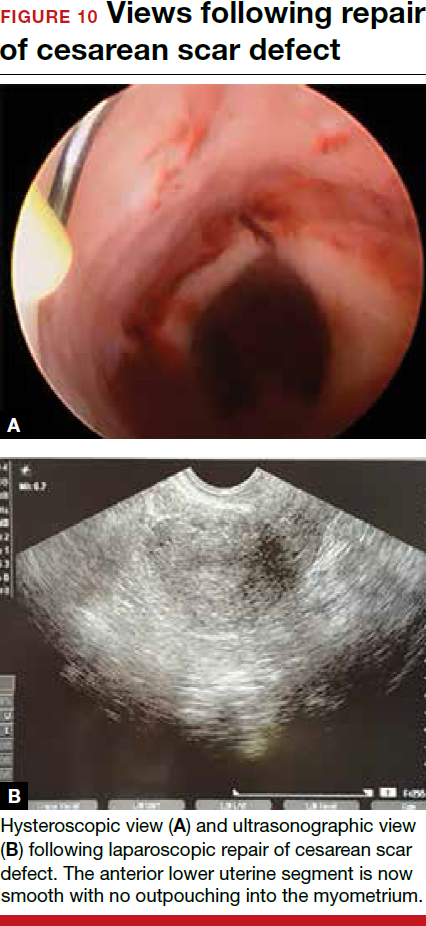
Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).

Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.

Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9







Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).

Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.

Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9







Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In this Article
FDA investigates Boston Scientific’s surgical mesh
The Food and Drug Administration is investigating allegations that Boston Scientific is using counterfeit raw materials in its urogynecologic surgical mesh.
For women who already have the Boston Scientific mesh implanted, the FDA is not recommending removal. The available data do not suggest any decreased benefit with the device and the risk of mesh removal outweighs any potential risk from mesh manufactured from alleged counterfeit raw materials, according to the FDA.
The investigation, announced by the FDA on April 1, 2016, comes after the agency received a citizen’s petition alleging that Boston Scientific was using a resin manufactured in China rather than the authentic Marlex HGX-030-01 resin.
Teresa Stevens, who is also involved in a federal class action lawsuit against Boston Scientific, submitted the citizen’s petition asking the FDA to begin an immediate Class I recall of all Boston Scientific mesh products made with the alleged counterfeit resin.
Boston Scientific is denying the allegations made in the petition.
“Boston Scientific does not use ‘counterfeit’ or ‘adulterated’ materials in our medical devices,” company officials wrote in an April 1 statement. “We have the highest confidence in the safety of our mesh devices. We have shared our test data with the Food and Drug Administration and are fully cooperating with the agency’s requests for information as part of our ongoing discussions. Additionally, we have offered to conduct further biocompatibility and chemical characterization testing to complement the results from existing tests.”
Company officials said they located a new supplier of Marlex resin in 2011 and put samples of the materials through a battery of tests to demonstrate equivalency.
The FDA acknowledged that it is not uncommon for companies to change the source of their raw materials after a device has been cleared for marketing and that the change often does not require premarket review by the agency. But due to the allegations in the citizen’s petition, the FDA will evaluate the results of new testing conducted by the company, including chemical characterization and toxicologic risk assessment of the raw material alleged to be counterfeit, as well as chemical characterization and biocompatibility of the final finished urogynecologic surgical mesh.
“The additional testing should be sufficient for the FDA to determine whether or not the urogynecologic surgical mesh manufactured from the alleged counterfeit raw material are equivalent to the urogynecologic surgical mesh manufactured from the original raw material supplier,” FDA officials wrote. “We expect that this testing will take several months to complete.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The Food and Drug Administration is investigating allegations that Boston Scientific is using counterfeit raw materials in its urogynecologic surgical mesh.
For women who already have the Boston Scientific mesh implanted, the FDA is not recommending removal. The available data do not suggest any decreased benefit with the device and the risk of mesh removal outweighs any potential risk from mesh manufactured from alleged counterfeit raw materials, according to the FDA.
The investigation, announced by the FDA on April 1, 2016, comes after the agency received a citizen’s petition alleging that Boston Scientific was using a resin manufactured in China rather than the authentic Marlex HGX-030-01 resin.
Teresa Stevens, who is also involved in a federal class action lawsuit against Boston Scientific, submitted the citizen’s petition asking the FDA to begin an immediate Class I recall of all Boston Scientific mesh products made with the alleged counterfeit resin.
Boston Scientific is denying the allegations made in the petition.
“Boston Scientific does not use ‘counterfeit’ or ‘adulterated’ materials in our medical devices,” company officials wrote in an April 1 statement. “We have the highest confidence in the safety of our mesh devices. We have shared our test data with the Food and Drug Administration and are fully cooperating with the agency’s requests for information as part of our ongoing discussions. Additionally, we have offered to conduct further biocompatibility and chemical characterization testing to complement the results from existing tests.”
Company officials said they located a new supplier of Marlex resin in 2011 and put samples of the materials through a battery of tests to demonstrate equivalency.
The FDA acknowledged that it is not uncommon for companies to change the source of their raw materials after a device has been cleared for marketing and that the change often does not require premarket review by the agency. But due to the allegations in the citizen’s petition, the FDA will evaluate the results of new testing conducted by the company, including chemical characterization and toxicologic risk assessment of the raw material alleged to be counterfeit, as well as chemical characterization and biocompatibility of the final finished urogynecologic surgical mesh.
“The additional testing should be sufficient for the FDA to determine whether or not the urogynecologic surgical mesh manufactured from the alleged counterfeit raw material are equivalent to the urogynecologic surgical mesh manufactured from the original raw material supplier,” FDA officials wrote. “We expect that this testing will take several months to complete.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The Food and Drug Administration is investigating allegations that Boston Scientific is using counterfeit raw materials in its urogynecologic surgical mesh.
For women who already have the Boston Scientific mesh implanted, the FDA is not recommending removal. The available data do not suggest any decreased benefit with the device and the risk of mesh removal outweighs any potential risk from mesh manufactured from alleged counterfeit raw materials, according to the FDA.
The investigation, announced by the FDA on April 1, 2016, comes after the agency received a citizen’s petition alleging that Boston Scientific was using a resin manufactured in China rather than the authentic Marlex HGX-030-01 resin.
Teresa Stevens, who is also involved in a federal class action lawsuit against Boston Scientific, submitted the citizen’s petition asking the FDA to begin an immediate Class I recall of all Boston Scientific mesh products made with the alleged counterfeit resin.
Boston Scientific is denying the allegations made in the petition.
“Boston Scientific does not use ‘counterfeit’ or ‘adulterated’ materials in our medical devices,” company officials wrote in an April 1 statement. “We have the highest confidence in the safety of our mesh devices. We have shared our test data with the Food and Drug Administration and are fully cooperating with the agency’s requests for information as part of our ongoing discussions. Additionally, we have offered to conduct further biocompatibility and chemical characterization testing to complement the results from existing tests.”
Company officials said they located a new supplier of Marlex resin in 2011 and put samples of the materials through a battery of tests to demonstrate equivalency.
The FDA acknowledged that it is not uncommon for companies to change the source of their raw materials after a device has been cleared for marketing and that the change often does not require premarket review by the agency. But due to the allegations in the citizen’s petition, the FDA will evaluate the results of new testing conducted by the company, including chemical characterization and toxicologic risk assessment of the raw material alleged to be counterfeit, as well as chemical characterization and biocompatibility of the final finished urogynecologic surgical mesh.
“The additional testing should be sufficient for the FDA to determine whether or not the urogynecologic surgical mesh manufactured from the alleged counterfeit raw material are equivalent to the urogynecologic surgical mesh manufactured from the original raw material supplier,” FDA officials wrote. “We expect that this testing will take several months to complete.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Reducing Surgical Site Infections in a Children’s Hospital: The Fuzzy Elements of Change
From the Hospital for Sick Children, Toronto, ON.
Abstract
- Objective: To describe the iterative and adaptive process used in implementing strategies to reduce surgical site infections (SSI) in a pediatric academic health science center.
- Methods: A multidisciplinary group was tasked with implementing strategies to reduce SSI with a focus on evaluating the use of a guideline for the use of prophylactic antibiotics and determining the rate of SSI.
- Results: The task force initially addressed surgical preparation solution, hair removal, oxygenation, and normothermia. The task force subsequently revised a guideline for the use of prophylactic antibiotics and implemented the guideline iteratively with multiple strategies including audit and feedback, communication and dissemination, and computerised order entry. The appropriate use of the guideline was associated with a 30% reduction in the rate of SSI.
- Conclusion: Using iterative and adaptive strategies over many years, the SSI rate was reduced by 30%.
Improving quality of care is a prime concern for clinicians, patients, families, and health systems [1]. Quality improvement methods are used widely in medicine for studying and addressing problems with care and have successfully addressed gaps in quality. The challenges include defining quality, obtaining complete and accurate data about quality, developing meaningful and cost-effective interventions to improve quality, and to successfully change clinician’s behaviour with commensurate improvement in quality of care.
Quality improvement in health care involves effecting and assessing change in a setting of complexity and uncertainty. Whereas the randomized trial may be used to measure the effectiveness of a particular treatment, quality improvement implementation involves an iterative and adaptive process in response to local events as the implementation proceeds [2]. These context-specific iterative changes to the implementation process are the fuzzy elements of change. This article describes a quality improvement initative to to reduce surgical site infections at an academic health science center with a focus on the fuzziness inherent in the process and our iterative responses to local events.
Setting
The Hospital for Sick Children (Sickkids) is a childrens’ academic health science center in Toronto, Ontario, Canada. The largest children’s hospital in Canada, with 8000 health care professionals, scientists, trainees, administrative and support staff, it has approximately 300 beds, 15,000 inpatient admissions, 12,000 surgical procedures, 70,000 emergency visits, and 300,000 outpatient visits annually. The hospital is a Level 1 trauma unit and performs the full spectrum of pediatric surgical care including transplant and cardiac procedures. The hospital and physician staffs are affiliated with the University of Toronto. The hospital has 16 theatre operating rooms, with 11 perioperative divisions and departments.
The departmental and divisional structure of the hospital, which emulates the university organizational structure, does not represent the size and level of clinical activity of the groups. For example, the department of otolaryngology, head and neck surgery has 5 surgeons whereas the division of orthopedics (as one of 6 divisions in the department of surgery) has 9 orthopedic surgeons. Furthermore, a divisional and departmental structure arguably does not match the institutional operational aims related to patient care delivery. Thus, in 2007 the 3 departments of surgery, the departments of critical care, anaesthesia and pain medicine, and dentistry were clustered together as “perioperative services,” reporting to a chief of perioperative services who in turn reported directly to the CEO. The chief of perioperative services, responsible for all operational issues, was concurrently the surgeon-in-chief.
Physicians at Sickkids are not paid fee-for-service. Each division/department receives compensation according to their specific speciality on a full-time equivalent (FTE) basis. While clinical and academic productivity is measured, physicians do not receive activity-based compensation. The perioperative service chiefs have primary responsibility for the clinical operations and academic activity. A perioperative care unit (POCU) executive has primarily responsibility for policy and financial oversight of the operating rooms.
As this was primarily a quality improvement initiative, we obtained institutional approval through that process.
Defining the Target for Quality improvement
To determine shared objectives for quality improvement, the surgeon-in-chief organized a daylong retreat in 2005 of all physicians (of the 11 divisions and departments that was later called perioperative services), nurses, and other disciplines involved in delivering surgical care. All scheduled clinics and OR activity were cancelled. The start and end of the retreat day matched the nursing day shift with a voluntary social event at the end. In the morning after meeting together, the 3 disciplines of nursing, surgery and anaesthesia met to discuss speciality-specific issues. In the afternoon, the 3 disciplines reconvened in small multidisciplinary groups of 8 to 10 individuals to discuss the objectives for improvement using the Institute of Medicine framework [1]. Outcomes of the small group discussions were presented to, and discussed by, the entire group, and those initiatives that achieved general endorsement were approved. A report summarising all recommendations arising from the day was widely circulated for comment. Recommendations were grouped, where appropriate, and assigned to task forces. Task forces were multidisciplinary groups co-led by 2 disciplines, with specific objectives arising from the retreat recommendations with measurable goals and a timeline of 12 to 18 months for completion of the recommendations.
The retreat of the perioperative services group recognized that many aspects of high quality care were hampered by variable diagnoses, comorbidities, and multiple and complex interventions with a critical lack of easily measured and cogent outcomes. The 4 areas that were relevant to all disciplines, most amenable to evaluation, and where significant quality gains were perceived to be necessary and possible were safety, perioperative pain, access to surgery, and surgical site infection (SSI). This paper reports on the SSI QI program.
Initial Task Force Work
An SSI task force initially addressed surgical preparation solution, hair clipping, oxygenation and normothermia. All razors were physically removed from the ORs and replaced by electric clippers. Multi-use proviodine preparation solution was replaced by single-use 70% isopropyl alcohol with 2% chlorhexidene (except for open wounds and neonates). Pilot studies of patients arriving in the POCU revealed that hypoxia was not an issue and normothermia was seldom an issue. Thereafter the prime focus shifted to the use of prophylactic antibiotics to reduce SSI.
Compliance with Antibiotic Prophylaxis Guideline
Guideline Update Process
A guideline for the use of prophylactic antibiotics to prevent SSI had been in place at Sickkids for many years. However, a chart review revealed only 40% of patients were receiving the correct drug, dose, duration, and time of administration relative to the incision, and few patients were receiving appropriate intraoperative top-ups [3]. In addition, the existing guideline was incomplete for all specialities and procedures, did not consider the issue of beta-lactam antibiotic allergy, and had no specific dosing for neonates. Therefore, the guideline needed to be updated and be more comprehensive before any attempts to increase compliance with the guideline was initiated. The infection control specialist and pharmacist reviewed evidence-based guidelines from the literature on adults to create a guideline comprehensive for speciality and procedure with specific dosing for neonates and alternative antibiotics for patients allergic to penicillin [3]. Updating the guidelines took almost a year.
The next step was to seek endorsement of all the surgical subspecialities. The guidelines were circulated to all specialities for comments. While a few specialists provided minor comments, as discussed further below, this step did not result in substantive feedback and again took almost a year.
The final guidelines were discussed at multiple meetings of the members of perioperative services and approved by the hospital drug and therapeutics committee. A date was set to introduce the new guideline and announced at departmental meetings, in emails, and on banners in the OR.
The revised guidelines replaced the old guidelines on the e-formulary. Hard copies were attached to the anaesthetic machine in each OR and the need for antibiotics was made part of the “time-out” before commencement of the procedure.
Early Monitoring of Guideline Use
To monitor the use of the guidelines, the use of an antibiotic and the timing related to the surgical incision became part of charting by nurses. Nurses charted many aspects of the surgical procedure through a surgical information management system (SIS, Alpharetta, GA). While documentation of the specific drug and dose was considered important information, the additional charting burden for nurses was considered to be too great. Thus the compromise was to chart if a drug was given and the time of administration to allow determination if the drug was given within an hour of the surgical incision.
Early results from monitoring of antibiotic administration revealed that drugs often were given well in advance of the 1-hour target. To address this issue, first, antibiotics given “on call to OR” was eliminated (because the duration from the call to go to the OR and until the surgical incision was never less than 1 hour) and thereafter all antibiotics were given in the OR. Second, due to prolonged anesthetic times prior to surgical start for complex cases, anesthetists changed their practise to give antibiotics as one of the final steps prior to start of surgery.
The next step was to monitor the use and timing of antibiotics by surgical division/department automatically using data from SIS. Concurrent with the efforts to improve the use of prophylactic antibiotic, a score card had been created to monitor quality and efficiency activities within perioperative services. The use and timing of prophylactic antibiotics became part of that monthly report. While the appropriate use of antibiotics improved over 6 months, a repeat audit revealed that compliance with the guideline for patients to receive, or not receive, antibiotics was only moderately improved [5]. Furthermore, whereas the guideline stated that antibiotics were needed only intra-operatively for the majority of procedures, antibiotics were extended postoperatively for periods ranging from 24 to 72 hours.
Addressing Compliance Issues
First, semi-annual mandatory lectures were presented to residents and fellows delineating the importance of the guidelines, with a specific focus on correct duration of antibiotics. Furthermore a “stop warning” was added to the computerized physician order entry system (orders are completed almost exclusively by house staff). In addition, we introduced an individual audit and feedback mechanism (see below).
Automated Audit and Feedback Process and Results
Each surgeon and anesthetist received an automated email the morning after the procedure detailing whether antibiotics had been indicated and whether they had been given or held appropriately. To accomplish this required that all surgical procedures (entered on SIS by the nurses) were matched to the guidelines. With the assistance of each division and department, each SIS procedural code was matched to the guideline as to whether antibiotics were indicated or not. In the case of multiple procedures, if any of the procedures warranted antibiotics then antibiotics were indicated for that patient. The automatic email sent to the staff acknowledged potential errors due to incorrect matching of the surgical procedure to guideline, incorrect charting by nurses, and incorrect indication of the guideline to receive (or not receive) antibiotics.
The response to this email had several impacts. First, the response identified many errors related to matching of SIS procedure to guidelines. Second, the email served as impetus to improve nurse charting. Third, through the automated emails we determined that some patients were on antibiotics for a pre-existing infection. Thus a separate notation in the SIS charting by the nursing staff was added to indicate a pre-existing infection (to prevent an automated email). Fourth, while circulation of the guidelines to all divisions and departments had provided little feedback to the final draft of guideline, responses to the emails resulted in refinement of ambiguities in guideline related to procedure description, and in some cases changes to the guideline based on the use of antibiotics. Fifth, the emails improved compliance with the guideline [3].
While audit and feedback resulted in a substantial rise in the appropriate use and timing of antibiotics, the nurses were often harassed about their charting, placing them in the uncomfortable position of seen to be enforcing the guideline. Also, some surgeons vehemently disliked the emails, pointing to occasional inaccuracies of the emails. Finally, the audit and feedback provided feedback after the surgical event, and while increasing attention on the guideline, did nothing for the individual patient. An alternative proposed strategy was that at the time of SIS charting of the procedure that SIS could serve as a decision tool and indicate whether antibiotics were indicated, and indicate the correct antibiotic. However SIS is proprietary software and we were unable to make the necessary programming changes.
Measuring SSI Rate
Concurrently with focusing on the process measures of the appropriate use of antibiotics, we also developed a mechanism to measure SSI [4]. Prior to this quality improvement initiative, the existing mechanism to measure institutional SSI was based on daily visits to surgical wards by infection control practitioners (ICPs) supplemented by identification of patients by positive wound cultures in microbiology. Due to the expense of active monitoring across all surgical disciplines, this program had been restricted to neurosurgery, cardiac surgery, and spine surgery (areas of high risk for SSI identified in the past). Because the hospital did not have the resources to expand ICP monitoring to all surgical areas, an alternative strategy of using health record coders was explored as a means to provide comprehensive rates of SSI for all disciplines.
The first step in using health records as a means to identify SSI was to perform a review of all SSIs identified by health records in the 3 priority areas monitored by the ICPs. All health records identified “SSI” were reviewed by a surgeon to determine which were and were not SSI, according to the Centers for Disease Control criteria [5]. The review identified that the International Classification of Disease (ICD−10) coding for SSI included, in addition to SSI, multiple types of infections such as sepsis and central line infections. The review also identified that the health record coders had no specific criteria and therefore were variable in how they coded “SSI.” The review identified that the ICPs missed some true infections that were identified by health record coders.
To address the ambiguity of ICD coding, extension codes to the ICD codes were added to code specifically for SSI. To address the lack of criteria for SSI, the health record coders were trained by ICPs to use Centers for Disease Control criteria for SSI [5]. While both of these steps improved the identification of SSI by health record coders, a subsequent chart audit identified false positive and false negative recording of SSI by both ICPs and health record coders. The task force accepted that no method was completely accurate and that health record coding for SSI was financially feasible and provided SSI rates for all surgical disciplines. The task force concluded that health record coding would serve the purpose of monitoring trends in SSIs.
Impact of Guideline Compliance
The final step in the quality improvement initiative of reducing SSI was to evaluate trends in use of prophylactic antibiotics and the relationship with SSI. Through the multiple iterative strategies described above, the administration of an antibiotic within an hour of the incision increased to over 80% of patients. To evaluate the impact of guideline compliance, approximately 9000 procedures were reviewed over a 21-month period [4]. In the approximately 4500 patients who had a guideline-based indication to receive antibiotics, the 80% who received correct administration of an antibiotic within 1 hour of the incision had a reduction in the rate of SSI by one third compared with the 20% who didn’t receive antibiotics. Of the approximately 4500 patients who did not have an indication for antibiotics, 80% did not receive antibiotics (20% did receive despite no indication) and had a (statistically insignificant) lower rate of SSI compared to the 20% who received antibiotics inappropriately. In summary, only 50% of children having surgery had an indication for antibiotics, and not receiving antibiotics saved money, reduced antibiotic exposure, and did not increase the rate of SSI. In the 50% of patients who received antibiotics according to the guidelines the rate of SSI was reduced by 30% [6].
Discussion
Duration of Project
The total duration of the Sickkids effort to measure and reduce the rate of SSI and thereby improve the quality of surgical care took almost 8 years. The duration, which ideally should have been about one quarter of that time, was due to multiple issues. First, there were many simultaneous competing demands to improve quality in other IOM domains such as safety and efficiency. Second, no one on the task force had protected time and thus meetings could be no more than monthly because people could not complete tasks in a shorter time frame. Third, many of the steps relied on wider physician involvement such as reviewing the revised guidelines. The physicians were slow to respond and only after all 9 surgical disciplines had signed off on the guidelines could implementation proceed. Finally, many of the important issues came up only after implementation of a specific step. For example, the recognition of the need for an individual audit and feedback mechanism created the need of mapping the procedures to guidelines to SIS procedures, a process that took more than a year to complete. Also the responses to the emails created the need for revisions to the guideline with subsequent delays for re-approval with hospital and IT support for eformulary changes.
Success Factors and Impediments
The factors that in retrospect seem critical to effecting positive change started with a general endorsement of the perioperative services group for improving quality and specifically SSI. The retreat and an open forum involving multiple disciplines was critical in creating a mandate for change. Second, the task force not only had multiple and key discipline representation for each aspect of the change management strategy, but the task force members were passionate about the importance of reducing SSI. Third, the multiple strategies used for change needed to be adaptive and iterative to new findings as they arose. While the task force attempted to anticipate barriers to change, only once the quality initiative started did the task force truly understand the barriers and respond in turn. Finally, the need for relentless energy by the leaders and task force was critical to seeing the project to completion.
While the appropriate use of antibiotics increased with a reduction in SSI, several aspects of this initiative were not successful. First, despite the surgeon-in-chief’s semi-annual lectures, this initiative did not successfully engage the majority of the house staff manifested by their continued habit of prescribing postoperative antibiotics for hours to days despite the guideline advice. Second, because nurses were tasked with asking about and recording the use of antibiotics, an unintended consequence was that they took the brunt of disgruntled physicians. Despite all our attempts, many nurses felt this initiative brought negative responses of physicians toward their charting duties. Third, while audit and feedback was an important strategy to improve guideline compliance, many physicians saw the daily emails in response to noncompliance with the guidelines as intrusive and irritating. Also we could not program SIS to make it a decision support in real time rather than documenting an event after the fact and, thereby, not enhancing care for that individual patient. Finally, we adopted a strategy of health record coding for SSI due to the prohibitive expenses of a comprehensive active monitoring strategies by ICPs.
Exportability
The strategies used in this quality improvement project to reduce SSI may be exportable to other hospitals with similar results. However, the emphasis on which element of change management strategy is most important would likely vary by context [2,6]. The elements most essential for success were a mechanism to develop group buy-in, a dedicated multidisciplinary task force with leader(s) with relentless commitment to achieving meaningful change, and a mechanism to evaluate both the process measures and the final outcome. The elements of change would vary by site and including consideration of the mechanism for physician compensation, commitment of physicians to institutional initiatives to enhance quality, and institutional resources to support quality initiatives.
None of the observed changes in this quality improvement initiative can be confidently attributed to any of the specific interventions. The interventions were completed in stages, but most importantly were constantly changed, emphasized and de-emphasized according to the responses. This is the fuzzy nature of change whereby leaders take reasonable steps to effect change but have to constantly adapt to barriers to change. While a specific change strategy generalizable to all contexts would be ideal, in the end at an institutional level, positive change is the ultimate aim rather than determining which interventions are effective. This response to events as they arise as illustrated in our quality improvement journey, is the fuzzy side of change management.
Conclusion
In conclusion, through a long period with a multitude of strategies, use of a guideline for prophylactic antibiotics increased and was associated with a reduction in SSI. Future directions need to consider cost-effective strategies to actively monitor SSI and testing of other strategies to reduce SSI. Institutions embarking on change need to consider that initiatives will likely need to adapt to specific contextual responses.
Corresponding author: James G. Wright, MD, PMH, FRCS, Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, University of Oxford Botnar Research Centre, Windmill Road, Oxford, OX3 7LD, UK, james.wright@ndorms.ox.ac.uk.
Funding/support: RB Salter Chair in Paediatric Surgical Research.
1. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
2. Grol R, Wensing M, Eccles M, Davis D, editors. Improving patient care: the implementation of change in health care. 2nd ed. Wiley Blackwell; 2013.
3. So JP, Aleem IS, Tsang DS, et al. Increasing compliance with an antibiotic prophylaxis guideline to prevent pediatric surgical site infection: before and after study. Ann Surg 2015;262:403–8.
4. Khoshbin A, So JP, Aleem IS, et al. Antibiotic prophylaxis to prevent surgical site infections in children: a prospective cohort study. Ann Surg 2015;262:397–402.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132.
6. Curran JA, Grimshaw JM, Hayden JA, Campbell B. Knowledge translation research: the science of moving research into policy and practice. J Contin Educ Health Prof 2011;31:174–80.
From the Hospital for Sick Children, Toronto, ON.
Abstract
- Objective: To describe the iterative and adaptive process used in implementing strategies to reduce surgical site infections (SSI) in a pediatric academic health science center.
- Methods: A multidisciplinary group was tasked with implementing strategies to reduce SSI with a focus on evaluating the use of a guideline for the use of prophylactic antibiotics and determining the rate of SSI.
- Results: The task force initially addressed surgical preparation solution, hair removal, oxygenation, and normothermia. The task force subsequently revised a guideline for the use of prophylactic antibiotics and implemented the guideline iteratively with multiple strategies including audit and feedback, communication and dissemination, and computerised order entry. The appropriate use of the guideline was associated with a 30% reduction in the rate of SSI.
- Conclusion: Using iterative and adaptive strategies over many years, the SSI rate was reduced by 30%.
Improving quality of care is a prime concern for clinicians, patients, families, and health systems [1]. Quality improvement methods are used widely in medicine for studying and addressing problems with care and have successfully addressed gaps in quality. The challenges include defining quality, obtaining complete and accurate data about quality, developing meaningful and cost-effective interventions to improve quality, and to successfully change clinician’s behaviour with commensurate improvement in quality of care.
Quality improvement in health care involves effecting and assessing change in a setting of complexity and uncertainty. Whereas the randomized trial may be used to measure the effectiveness of a particular treatment, quality improvement implementation involves an iterative and adaptive process in response to local events as the implementation proceeds [2]. These context-specific iterative changes to the implementation process are the fuzzy elements of change. This article describes a quality improvement initative to to reduce surgical site infections at an academic health science center with a focus on the fuzziness inherent in the process and our iterative responses to local events.
Setting
The Hospital for Sick Children (Sickkids) is a childrens’ academic health science center in Toronto, Ontario, Canada. The largest children’s hospital in Canada, with 8000 health care professionals, scientists, trainees, administrative and support staff, it has approximately 300 beds, 15,000 inpatient admissions, 12,000 surgical procedures, 70,000 emergency visits, and 300,000 outpatient visits annually. The hospital is a Level 1 trauma unit and performs the full spectrum of pediatric surgical care including transplant and cardiac procedures. The hospital and physician staffs are affiliated with the University of Toronto. The hospital has 16 theatre operating rooms, with 11 perioperative divisions and departments.
The departmental and divisional structure of the hospital, which emulates the university organizational structure, does not represent the size and level of clinical activity of the groups. For example, the department of otolaryngology, head and neck surgery has 5 surgeons whereas the division of orthopedics (as one of 6 divisions in the department of surgery) has 9 orthopedic surgeons. Furthermore, a divisional and departmental structure arguably does not match the institutional operational aims related to patient care delivery. Thus, in 2007 the 3 departments of surgery, the departments of critical care, anaesthesia and pain medicine, and dentistry were clustered together as “perioperative services,” reporting to a chief of perioperative services who in turn reported directly to the CEO. The chief of perioperative services, responsible for all operational issues, was concurrently the surgeon-in-chief.
Physicians at Sickkids are not paid fee-for-service. Each division/department receives compensation according to their specific speciality on a full-time equivalent (FTE) basis. While clinical and academic productivity is measured, physicians do not receive activity-based compensation. The perioperative service chiefs have primary responsibility for the clinical operations and academic activity. A perioperative care unit (POCU) executive has primarily responsibility for policy and financial oversight of the operating rooms.
As this was primarily a quality improvement initiative, we obtained institutional approval through that process.
Defining the Target for Quality improvement
To determine shared objectives for quality improvement, the surgeon-in-chief organized a daylong retreat in 2005 of all physicians (of the 11 divisions and departments that was later called perioperative services), nurses, and other disciplines involved in delivering surgical care. All scheduled clinics and OR activity were cancelled. The start and end of the retreat day matched the nursing day shift with a voluntary social event at the end. In the morning after meeting together, the 3 disciplines of nursing, surgery and anaesthesia met to discuss speciality-specific issues. In the afternoon, the 3 disciplines reconvened in small multidisciplinary groups of 8 to 10 individuals to discuss the objectives for improvement using the Institute of Medicine framework [1]. Outcomes of the small group discussions were presented to, and discussed by, the entire group, and those initiatives that achieved general endorsement were approved. A report summarising all recommendations arising from the day was widely circulated for comment. Recommendations were grouped, where appropriate, and assigned to task forces. Task forces were multidisciplinary groups co-led by 2 disciplines, with specific objectives arising from the retreat recommendations with measurable goals and a timeline of 12 to 18 months for completion of the recommendations.
The retreat of the perioperative services group recognized that many aspects of high quality care were hampered by variable diagnoses, comorbidities, and multiple and complex interventions with a critical lack of easily measured and cogent outcomes. The 4 areas that were relevant to all disciplines, most amenable to evaluation, and where significant quality gains were perceived to be necessary and possible were safety, perioperative pain, access to surgery, and surgical site infection (SSI). This paper reports on the SSI QI program.
Initial Task Force Work
An SSI task force initially addressed surgical preparation solution, hair clipping, oxygenation and normothermia. All razors were physically removed from the ORs and replaced by electric clippers. Multi-use proviodine preparation solution was replaced by single-use 70% isopropyl alcohol with 2% chlorhexidene (except for open wounds and neonates). Pilot studies of patients arriving in the POCU revealed that hypoxia was not an issue and normothermia was seldom an issue. Thereafter the prime focus shifted to the use of prophylactic antibiotics to reduce SSI.
Compliance with Antibiotic Prophylaxis Guideline
Guideline Update Process
A guideline for the use of prophylactic antibiotics to prevent SSI had been in place at Sickkids for many years. However, a chart review revealed only 40% of patients were receiving the correct drug, dose, duration, and time of administration relative to the incision, and few patients were receiving appropriate intraoperative top-ups [3]. In addition, the existing guideline was incomplete for all specialities and procedures, did not consider the issue of beta-lactam antibiotic allergy, and had no specific dosing for neonates. Therefore, the guideline needed to be updated and be more comprehensive before any attempts to increase compliance with the guideline was initiated. The infection control specialist and pharmacist reviewed evidence-based guidelines from the literature on adults to create a guideline comprehensive for speciality and procedure with specific dosing for neonates and alternative antibiotics for patients allergic to penicillin [3]. Updating the guidelines took almost a year.
The next step was to seek endorsement of all the surgical subspecialities. The guidelines were circulated to all specialities for comments. While a few specialists provided minor comments, as discussed further below, this step did not result in substantive feedback and again took almost a year.
The final guidelines were discussed at multiple meetings of the members of perioperative services and approved by the hospital drug and therapeutics committee. A date was set to introduce the new guideline and announced at departmental meetings, in emails, and on banners in the OR.
The revised guidelines replaced the old guidelines on the e-formulary. Hard copies were attached to the anaesthetic machine in each OR and the need for antibiotics was made part of the “time-out” before commencement of the procedure.
Early Monitoring of Guideline Use
To monitor the use of the guidelines, the use of an antibiotic and the timing related to the surgical incision became part of charting by nurses. Nurses charted many aspects of the surgical procedure through a surgical information management system (SIS, Alpharetta, GA). While documentation of the specific drug and dose was considered important information, the additional charting burden for nurses was considered to be too great. Thus the compromise was to chart if a drug was given and the time of administration to allow determination if the drug was given within an hour of the surgical incision.
Early results from monitoring of antibiotic administration revealed that drugs often were given well in advance of the 1-hour target. To address this issue, first, antibiotics given “on call to OR” was eliminated (because the duration from the call to go to the OR and until the surgical incision was never less than 1 hour) and thereafter all antibiotics were given in the OR. Second, due to prolonged anesthetic times prior to surgical start for complex cases, anesthetists changed their practise to give antibiotics as one of the final steps prior to start of surgery.
The next step was to monitor the use and timing of antibiotics by surgical division/department automatically using data from SIS. Concurrent with the efforts to improve the use of prophylactic antibiotic, a score card had been created to monitor quality and efficiency activities within perioperative services. The use and timing of prophylactic antibiotics became part of that monthly report. While the appropriate use of antibiotics improved over 6 months, a repeat audit revealed that compliance with the guideline for patients to receive, or not receive, antibiotics was only moderately improved [5]. Furthermore, whereas the guideline stated that antibiotics were needed only intra-operatively for the majority of procedures, antibiotics were extended postoperatively for periods ranging from 24 to 72 hours.
Addressing Compliance Issues
First, semi-annual mandatory lectures were presented to residents and fellows delineating the importance of the guidelines, with a specific focus on correct duration of antibiotics. Furthermore a “stop warning” was added to the computerized physician order entry system (orders are completed almost exclusively by house staff). In addition, we introduced an individual audit and feedback mechanism (see below).
Automated Audit and Feedback Process and Results
Each surgeon and anesthetist received an automated email the morning after the procedure detailing whether antibiotics had been indicated and whether they had been given or held appropriately. To accomplish this required that all surgical procedures (entered on SIS by the nurses) were matched to the guidelines. With the assistance of each division and department, each SIS procedural code was matched to the guideline as to whether antibiotics were indicated or not. In the case of multiple procedures, if any of the procedures warranted antibiotics then antibiotics were indicated for that patient. The automatic email sent to the staff acknowledged potential errors due to incorrect matching of the surgical procedure to guideline, incorrect charting by nurses, and incorrect indication of the guideline to receive (or not receive) antibiotics.
The response to this email had several impacts. First, the response identified many errors related to matching of SIS procedure to guidelines. Second, the email served as impetus to improve nurse charting. Third, through the automated emails we determined that some patients were on antibiotics for a pre-existing infection. Thus a separate notation in the SIS charting by the nursing staff was added to indicate a pre-existing infection (to prevent an automated email). Fourth, while circulation of the guidelines to all divisions and departments had provided little feedback to the final draft of guideline, responses to the emails resulted in refinement of ambiguities in guideline related to procedure description, and in some cases changes to the guideline based on the use of antibiotics. Fifth, the emails improved compliance with the guideline [3].
While audit and feedback resulted in a substantial rise in the appropriate use and timing of antibiotics, the nurses were often harassed about their charting, placing them in the uncomfortable position of seen to be enforcing the guideline. Also, some surgeons vehemently disliked the emails, pointing to occasional inaccuracies of the emails. Finally, the audit and feedback provided feedback after the surgical event, and while increasing attention on the guideline, did nothing for the individual patient. An alternative proposed strategy was that at the time of SIS charting of the procedure that SIS could serve as a decision tool and indicate whether antibiotics were indicated, and indicate the correct antibiotic. However SIS is proprietary software and we were unable to make the necessary programming changes.
Measuring SSI Rate
Concurrently with focusing on the process measures of the appropriate use of antibiotics, we also developed a mechanism to measure SSI [4]. Prior to this quality improvement initiative, the existing mechanism to measure institutional SSI was based on daily visits to surgical wards by infection control practitioners (ICPs) supplemented by identification of patients by positive wound cultures in microbiology. Due to the expense of active monitoring across all surgical disciplines, this program had been restricted to neurosurgery, cardiac surgery, and spine surgery (areas of high risk for SSI identified in the past). Because the hospital did not have the resources to expand ICP monitoring to all surgical areas, an alternative strategy of using health record coders was explored as a means to provide comprehensive rates of SSI for all disciplines.
The first step in using health records as a means to identify SSI was to perform a review of all SSIs identified by health records in the 3 priority areas monitored by the ICPs. All health records identified “SSI” were reviewed by a surgeon to determine which were and were not SSI, according to the Centers for Disease Control criteria [5]. The review identified that the International Classification of Disease (ICD−10) coding for SSI included, in addition to SSI, multiple types of infections such as sepsis and central line infections. The review also identified that the health record coders had no specific criteria and therefore were variable in how they coded “SSI.” The review identified that the ICPs missed some true infections that were identified by health record coders.
To address the ambiguity of ICD coding, extension codes to the ICD codes were added to code specifically for SSI. To address the lack of criteria for SSI, the health record coders were trained by ICPs to use Centers for Disease Control criteria for SSI [5]. While both of these steps improved the identification of SSI by health record coders, a subsequent chart audit identified false positive and false negative recording of SSI by both ICPs and health record coders. The task force accepted that no method was completely accurate and that health record coding for SSI was financially feasible and provided SSI rates for all surgical disciplines. The task force concluded that health record coding would serve the purpose of monitoring trends in SSIs.
Impact of Guideline Compliance
The final step in the quality improvement initiative of reducing SSI was to evaluate trends in use of prophylactic antibiotics and the relationship with SSI. Through the multiple iterative strategies described above, the administration of an antibiotic within an hour of the incision increased to over 80% of patients. To evaluate the impact of guideline compliance, approximately 9000 procedures were reviewed over a 21-month period [4]. In the approximately 4500 patients who had a guideline-based indication to receive antibiotics, the 80% who received correct administration of an antibiotic within 1 hour of the incision had a reduction in the rate of SSI by one third compared with the 20% who didn’t receive antibiotics. Of the approximately 4500 patients who did not have an indication for antibiotics, 80% did not receive antibiotics (20% did receive despite no indication) and had a (statistically insignificant) lower rate of SSI compared to the 20% who received antibiotics inappropriately. In summary, only 50% of children having surgery had an indication for antibiotics, and not receiving antibiotics saved money, reduced antibiotic exposure, and did not increase the rate of SSI. In the 50% of patients who received antibiotics according to the guidelines the rate of SSI was reduced by 30% [6].
Discussion
Duration of Project
The total duration of the Sickkids effort to measure and reduce the rate of SSI and thereby improve the quality of surgical care took almost 8 years. The duration, which ideally should have been about one quarter of that time, was due to multiple issues. First, there were many simultaneous competing demands to improve quality in other IOM domains such as safety and efficiency. Second, no one on the task force had protected time and thus meetings could be no more than monthly because people could not complete tasks in a shorter time frame. Third, many of the steps relied on wider physician involvement such as reviewing the revised guidelines. The physicians were slow to respond and only after all 9 surgical disciplines had signed off on the guidelines could implementation proceed. Finally, many of the important issues came up only after implementation of a specific step. For example, the recognition of the need for an individual audit and feedback mechanism created the need of mapping the procedures to guidelines to SIS procedures, a process that took more than a year to complete. Also the responses to the emails created the need for revisions to the guideline with subsequent delays for re-approval with hospital and IT support for eformulary changes.
Success Factors and Impediments
The factors that in retrospect seem critical to effecting positive change started with a general endorsement of the perioperative services group for improving quality and specifically SSI. The retreat and an open forum involving multiple disciplines was critical in creating a mandate for change. Second, the task force not only had multiple and key discipline representation for each aspect of the change management strategy, but the task force members were passionate about the importance of reducing SSI. Third, the multiple strategies used for change needed to be adaptive and iterative to new findings as they arose. While the task force attempted to anticipate barriers to change, only once the quality initiative started did the task force truly understand the barriers and respond in turn. Finally, the need for relentless energy by the leaders and task force was critical to seeing the project to completion.
While the appropriate use of antibiotics increased with a reduction in SSI, several aspects of this initiative were not successful. First, despite the surgeon-in-chief’s semi-annual lectures, this initiative did not successfully engage the majority of the house staff manifested by their continued habit of prescribing postoperative antibiotics for hours to days despite the guideline advice. Second, because nurses were tasked with asking about and recording the use of antibiotics, an unintended consequence was that they took the brunt of disgruntled physicians. Despite all our attempts, many nurses felt this initiative brought negative responses of physicians toward their charting duties. Third, while audit and feedback was an important strategy to improve guideline compliance, many physicians saw the daily emails in response to noncompliance with the guidelines as intrusive and irritating. Also we could not program SIS to make it a decision support in real time rather than documenting an event after the fact and, thereby, not enhancing care for that individual patient. Finally, we adopted a strategy of health record coding for SSI due to the prohibitive expenses of a comprehensive active monitoring strategies by ICPs.
Exportability
The strategies used in this quality improvement project to reduce SSI may be exportable to other hospitals with similar results. However, the emphasis on which element of change management strategy is most important would likely vary by context [2,6]. The elements most essential for success were a mechanism to develop group buy-in, a dedicated multidisciplinary task force with leader(s) with relentless commitment to achieving meaningful change, and a mechanism to evaluate both the process measures and the final outcome. The elements of change would vary by site and including consideration of the mechanism for physician compensation, commitment of physicians to institutional initiatives to enhance quality, and institutional resources to support quality initiatives.
None of the observed changes in this quality improvement initiative can be confidently attributed to any of the specific interventions. The interventions were completed in stages, but most importantly were constantly changed, emphasized and de-emphasized according to the responses. This is the fuzzy nature of change whereby leaders take reasonable steps to effect change but have to constantly adapt to barriers to change. While a specific change strategy generalizable to all contexts would be ideal, in the end at an institutional level, positive change is the ultimate aim rather than determining which interventions are effective. This response to events as they arise as illustrated in our quality improvement journey, is the fuzzy side of change management.
Conclusion
In conclusion, through a long period with a multitude of strategies, use of a guideline for prophylactic antibiotics increased and was associated with a reduction in SSI. Future directions need to consider cost-effective strategies to actively monitor SSI and testing of other strategies to reduce SSI. Institutions embarking on change need to consider that initiatives will likely need to adapt to specific contextual responses.
Corresponding author: James G. Wright, MD, PMH, FRCS, Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, University of Oxford Botnar Research Centre, Windmill Road, Oxford, OX3 7LD, UK, james.wright@ndorms.ox.ac.uk.
Funding/support: RB Salter Chair in Paediatric Surgical Research.
From the Hospital for Sick Children, Toronto, ON.
Abstract
- Objective: To describe the iterative and adaptive process used in implementing strategies to reduce surgical site infections (SSI) in a pediatric academic health science center.
- Methods: A multidisciplinary group was tasked with implementing strategies to reduce SSI with a focus on evaluating the use of a guideline for the use of prophylactic antibiotics and determining the rate of SSI.
- Results: The task force initially addressed surgical preparation solution, hair removal, oxygenation, and normothermia. The task force subsequently revised a guideline for the use of prophylactic antibiotics and implemented the guideline iteratively with multiple strategies including audit and feedback, communication and dissemination, and computerised order entry. The appropriate use of the guideline was associated with a 30% reduction in the rate of SSI.
- Conclusion: Using iterative and adaptive strategies over many years, the SSI rate was reduced by 30%.
Improving quality of care is a prime concern for clinicians, patients, families, and health systems [1]. Quality improvement methods are used widely in medicine for studying and addressing problems with care and have successfully addressed gaps in quality. The challenges include defining quality, obtaining complete and accurate data about quality, developing meaningful and cost-effective interventions to improve quality, and to successfully change clinician’s behaviour with commensurate improvement in quality of care.
Quality improvement in health care involves effecting and assessing change in a setting of complexity and uncertainty. Whereas the randomized trial may be used to measure the effectiveness of a particular treatment, quality improvement implementation involves an iterative and adaptive process in response to local events as the implementation proceeds [2]. These context-specific iterative changes to the implementation process are the fuzzy elements of change. This article describes a quality improvement initative to to reduce surgical site infections at an academic health science center with a focus on the fuzziness inherent in the process and our iterative responses to local events.
Setting
The Hospital for Sick Children (Sickkids) is a childrens’ academic health science center in Toronto, Ontario, Canada. The largest children’s hospital in Canada, with 8000 health care professionals, scientists, trainees, administrative and support staff, it has approximately 300 beds, 15,000 inpatient admissions, 12,000 surgical procedures, 70,000 emergency visits, and 300,000 outpatient visits annually. The hospital is a Level 1 trauma unit and performs the full spectrum of pediatric surgical care including transplant and cardiac procedures. The hospital and physician staffs are affiliated with the University of Toronto. The hospital has 16 theatre operating rooms, with 11 perioperative divisions and departments.
The departmental and divisional structure of the hospital, which emulates the university organizational structure, does not represent the size and level of clinical activity of the groups. For example, the department of otolaryngology, head and neck surgery has 5 surgeons whereas the division of orthopedics (as one of 6 divisions in the department of surgery) has 9 orthopedic surgeons. Furthermore, a divisional and departmental structure arguably does not match the institutional operational aims related to patient care delivery. Thus, in 2007 the 3 departments of surgery, the departments of critical care, anaesthesia and pain medicine, and dentistry were clustered together as “perioperative services,” reporting to a chief of perioperative services who in turn reported directly to the CEO. The chief of perioperative services, responsible for all operational issues, was concurrently the surgeon-in-chief.
Physicians at Sickkids are not paid fee-for-service. Each division/department receives compensation according to their specific speciality on a full-time equivalent (FTE) basis. While clinical and academic productivity is measured, physicians do not receive activity-based compensation. The perioperative service chiefs have primary responsibility for the clinical operations and academic activity. A perioperative care unit (POCU) executive has primarily responsibility for policy and financial oversight of the operating rooms.
As this was primarily a quality improvement initiative, we obtained institutional approval through that process.
Defining the Target for Quality improvement
To determine shared objectives for quality improvement, the surgeon-in-chief organized a daylong retreat in 2005 of all physicians (of the 11 divisions and departments that was later called perioperative services), nurses, and other disciplines involved in delivering surgical care. All scheduled clinics and OR activity were cancelled. The start and end of the retreat day matched the nursing day shift with a voluntary social event at the end. In the morning after meeting together, the 3 disciplines of nursing, surgery and anaesthesia met to discuss speciality-specific issues. In the afternoon, the 3 disciplines reconvened in small multidisciplinary groups of 8 to 10 individuals to discuss the objectives for improvement using the Institute of Medicine framework [1]. Outcomes of the small group discussions were presented to, and discussed by, the entire group, and those initiatives that achieved general endorsement were approved. A report summarising all recommendations arising from the day was widely circulated for comment. Recommendations were grouped, where appropriate, and assigned to task forces. Task forces were multidisciplinary groups co-led by 2 disciplines, with specific objectives arising from the retreat recommendations with measurable goals and a timeline of 12 to 18 months for completion of the recommendations.
The retreat of the perioperative services group recognized that many aspects of high quality care were hampered by variable diagnoses, comorbidities, and multiple and complex interventions with a critical lack of easily measured and cogent outcomes. The 4 areas that were relevant to all disciplines, most amenable to evaluation, and where significant quality gains were perceived to be necessary and possible were safety, perioperative pain, access to surgery, and surgical site infection (SSI). This paper reports on the SSI QI program.
Initial Task Force Work
An SSI task force initially addressed surgical preparation solution, hair clipping, oxygenation and normothermia. All razors were physically removed from the ORs and replaced by electric clippers. Multi-use proviodine preparation solution was replaced by single-use 70% isopropyl alcohol with 2% chlorhexidene (except for open wounds and neonates). Pilot studies of patients arriving in the POCU revealed that hypoxia was not an issue and normothermia was seldom an issue. Thereafter the prime focus shifted to the use of prophylactic antibiotics to reduce SSI.
Compliance with Antibiotic Prophylaxis Guideline
Guideline Update Process
A guideline for the use of prophylactic antibiotics to prevent SSI had been in place at Sickkids for many years. However, a chart review revealed only 40% of patients were receiving the correct drug, dose, duration, and time of administration relative to the incision, and few patients were receiving appropriate intraoperative top-ups [3]. In addition, the existing guideline was incomplete for all specialities and procedures, did not consider the issue of beta-lactam antibiotic allergy, and had no specific dosing for neonates. Therefore, the guideline needed to be updated and be more comprehensive before any attempts to increase compliance with the guideline was initiated. The infection control specialist and pharmacist reviewed evidence-based guidelines from the literature on adults to create a guideline comprehensive for speciality and procedure with specific dosing for neonates and alternative antibiotics for patients allergic to penicillin [3]. Updating the guidelines took almost a year.
The next step was to seek endorsement of all the surgical subspecialities. The guidelines were circulated to all specialities for comments. While a few specialists provided minor comments, as discussed further below, this step did not result in substantive feedback and again took almost a year.
The final guidelines were discussed at multiple meetings of the members of perioperative services and approved by the hospital drug and therapeutics committee. A date was set to introduce the new guideline and announced at departmental meetings, in emails, and on banners in the OR.
The revised guidelines replaced the old guidelines on the e-formulary. Hard copies were attached to the anaesthetic machine in each OR and the need for antibiotics was made part of the “time-out” before commencement of the procedure.
Early Monitoring of Guideline Use
To monitor the use of the guidelines, the use of an antibiotic and the timing related to the surgical incision became part of charting by nurses. Nurses charted many aspects of the surgical procedure through a surgical information management system (SIS, Alpharetta, GA). While documentation of the specific drug and dose was considered important information, the additional charting burden for nurses was considered to be too great. Thus the compromise was to chart if a drug was given and the time of administration to allow determination if the drug was given within an hour of the surgical incision.
Early results from monitoring of antibiotic administration revealed that drugs often were given well in advance of the 1-hour target. To address this issue, first, antibiotics given “on call to OR” was eliminated (because the duration from the call to go to the OR and until the surgical incision was never less than 1 hour) and thereafter all antibiotics were given in the OR. Second, due to prolonged anesthetic times prior to surgical start for complex cases, anesthetists changed their practise to give antibiotics as one of the final steps prior to start of surgery.
The next step was to monitor the use and timing of antibiotics by surgical division/department automatically using data from SIS. Concurrent with the efforts to improve the use of prophylactic antibiotic, a score card had been created to monitor quality and efficiency activities within perioperative services. The use and timing of prophylactic antibiotics became part of that monthly report. While the appropriate use of antibiotics improved over 6 months, a repeat audit revealed that compliance with the guideline for patients to receive, or not receive, antibiotics was only moderately improved [5]. Furthermore, whereas the guideline stated that antibiotics were needed only intra-operatively for the majority of procedures, antibiotics were extended postoperatively for periods ranging from 24 to 72 hours.
Addressing Compliance Issues
First, semi-annual mandatory lectures were presented to residents and fellows delineating the importance of the guidelines, with a specific focus on correct duration of antibiotics. Furthermore a “stop warning” was added to the computerized physician order entry system (orders are completed almost exclusively by house staff). In addition, we introduced an individual audit and feedback mechanism (see below).
Automated Audit and Feedback Process and Results
Each surgeon and anesthetist received an automated email the morning after the procedure detailing whether antibiotics had been indicated and whether they had been given or held appropriately. To accomplish this required that all surgical procedures (entered on SIS by the nurses) were matched to the guidelines. With the assistance of each division and department, each SIS procedural code was matched to the guideline as to whether antibiotics were indicated or not. In the case of multiple procedures, if any of the procedures warranted antibiotics then antibiotics were indicated for that patient. The automatic email sent to the staff acknowledged potential errors due to incorrect matching of the surgical procedure to guideline, incorrect charting by nurses, and incorrect indication of the guideline to receive (or not receive) antibiotics.
The response to this email had several impacts. First, the response identified many errors related to matching of SIS procedure to guidelines. Second, the email served as impetus to improve nurse charting. Third, through the automated emails we determined that some patients were on antibiotics for a pre-existing infection. Thus a separate notation in the SIS charting by the nursing staff was added to indicate a pre-existing infection (to prevent an automated email). Fourth, while circulation of the guidelines to all divisions and departments had provided little feedback to the final draft of guideline, responses to the emails resulted in refinement of ambiguities in guideline related to procedure description, and in some cases changes to the guideline based on the use of antibiotics. Fifth, the emails improved compliance with the guideline [3].
While audit and feedback resulted in a substantial rise in the appropriate use and timing of antibiotics, the nurses were often harassed about their charting, placing them in the uncomfortable position of seen to be enforcing the guideline. Also, some surgeons vehemently disliked the emails, pointing to occasional inaccuracies of the emails. Finally, the audit and feedback provided feedback after the surgical event, and while increasing attention on the guideline, did nothing for the individual patient. An alternative proposed strategy was that at the time of SIS charting of the procedure that SIS could serve as a decision tool and indicate whether antibiotics were indicated, and indicate the correct antibiotic. However SIS is proprietary software and we were unable to make the necessary programming changes.
Measuring SSI Rate
Concurrently with focusing on the process measures of the appropriate use of antibiotics, we also developed a mechanism to measure SSI [4]. Prior to this quality improvement initiative, the existing mechanism to measure institutional SSI was based on daily visits to surgical wards by infection control practitioners (ICPs) supplemented by identification of patients by positive wound cultures in microbiology. Due to the expense of active monitoring across all surgical disciplines, this program had been restricted to neurosurgery, cardiac surgery, and spine surgery (areas of high risk for SSI identified in the past). Because the hospital did not have the resources to expand ICP monitoring to all surgical areas, an alternative strategy of using health record coders was explored as a means to provide comprehensive rates of SSI for all disciplines.
The first step in using health records as a means to identify SSI was to perform a review of all SSIs identified by health records in the 3 priority areas monitored by the ICPs. All health records identified “SSI” were reviewed by a surgeon to determine which were and were not SSI, according to the Centers for Disease Control criteria [5]. The review identified that the International Classification of Disease (ICD−10) coding for SSI included, in addition to SSI, multiple types of infections such as sepsis and central line infections. The review also identified that the health record coders had no specific criteria and therefore were variable in how they coded “SSI.” The review identified that the ICPs missed some true infections that were identified by health record coders.
To address the ambiguity of ICD coding, extension codes to the ICD codes were added to code specifically for SSI. To address the lack of criteria for SSI, the health record coders were trained by ICPs to use Centers for Disease Control criteria for SSI [5]. While both of these steps improved the identification of SSI by health record coders, a subsequent chart audit identified false positive and false negative recording of SSI by both ICPs and health record coders. The task force accepted that no method was completely accurate and that health record coding for SSI was financially feasible and provided SSI rates for all surgical disciplines. The task force concluded that health record coding would serve the purpose of monitoring trends in SSIs.
Impact of Guideline Compliance
The final step in the quality improvement initiative of reducing SSI was to evaluate trends in use of prophylactic antibiotics and the relationship with SSI. Through the multiple iterative strategies described above, the administration of an antibiotic within an hour of the incision increased to over 80% of patients. To evaluate the impact of guideline compliance, approximately 9000 procedures were reviewed over a 21-month period [4]. In the approximately 4500 patients who had a guideline-based indication to receive antibiotics, the 80% who received correct administration of an antibiotic within 1 hour of the incision had a reduction in the rate of SSI by one third compared with the 20% who didn’t receive antibiotics. Of the approximately 4500 patients who did not have an indication for antibiotics, 80% did not receive antibiotics (20% did receive despite no indication) and had a (statistically insignificant) lower rate of SSI compared to the 20% who received antibiotics inappropriately. In summary, only 50% of children having surgery had an indication for antibiotics, and not receiving antibiotics saved money, reduced antibiotic exposure, and did not increase the rate of SSI. In the 50% of patients who received antibiotics according to the guidelines the rate of SSI was reduced by 30% [6].
Discussion
Duration of Project
The total duration of the Sickkids effort to measure and reduce the rate of SSI and thereby improve the quality of surgical care took almost 8 years. The duration, which ideally should have been about one quarter of that time, was due to multiple issues. First, there were many simultaneous competing demands to improve quality in other IOM domains such as safety and efficiency. Second, no one on the task force had protected time and thus meetings could be no more than monthly because people could not complete tasks in a shorter time frame. Third, many of the steps relied on wider physician involvement such as reviewing the revised guidelines. The physicians were slow to respond and only after all 9 surgical disciplines had signed off on the guidelines could implementation proceed. Finally, many of the important issues came up only after implementation of a specific step. For example, the recognition of the need for an individual audit and feedback mechanism created the need of mapping the procedures to guidelines to SIS procedures, a process that took more than a year to complete. Also the responses to the emails created the need for revisions to the guideline with subsequent delays for re-approval with hospital and IT support for eformulary changes.
Success Factors and Impediments
The factors that in retrospect seem critical to effecting positive change started with a general endorsement of the perioperative services group for improving quality and specifically SSI. The retreat and an open forum involving multiple disciplines was critical in creating a mandate for change. Second, the task force not only had multiple and key discipline representation for each aspect of the change management strategy, but the task force members were passionate about the importance of reducing SSI. Third, the multiple strategies used for change needed to be adaptive and iterative to new findings as they arose. While the task force attempted to anticipate barriers to change, only once the quality initiative started did the task force truly understand the barriers and respond in turn. Finally, the need for relentless energy by the leaders and task force was critical to seeing the project to completion.
While the appropriate use of antibiotics increased with a reduction in SSI, several aspects of this initiative were not successful. First, despite the surgeon-in-chief’s semi-annual lectures, this initiative did not successfully engage the majority of the house staff manifested by their continued habit of prescribing postoperative antibiotics for hours to days despite the guideline advice. Second, because nurses were tasked with asking about and recording the use of antibiotics, an unintended consequence was that they took the brunt of disgruntled physicians. Despite all our attempts, many nurses felt this initiative brought negative responses of physicians toward their charting duties. Third, while audit and feedback was an important strategy to improve guideline compliance, many physicians saw the daily emails in response to noncompliance with the guidelines as intrusive and irritating. Also we could not program SIS to make it a decision support in real time rather than documenting an event after the fact and, thereby, not enhancing care for that individual patient. Finally, we adopted a strategy of health record coding for SSI due to the prohibitive expenses of a comprehensive active monitoring strategies by ICPs.
Exportability
The strategies used in this quality improvement project to reduce SSI may be exportable to other hospitals with similar results. However, the emphasis on which element of change management strategy is most important would likely vary by context [2,6]. The elements most essential for success were a mechanism to develop group buy-in, a dedicated multidisciplinary task force with leader(s) with relentless commitment to achieving meaningful change, and a mechanism to evaluate both the process measures and the final outcome. The elements of change would vary by site and including consideration of the mechanism for physician compensation, commitment of physicians to institutional initiatives to enhance quality, and institutional resources to support quality initiatives.
None of the observed changes in this quality improvement initiative can be confidently attributed to any of the specific interventions. The interventions were completed in stages, but most importantly were constantly changed, emphasized and de-emphasized according to the responses. This is the fuzzy nature of change whereby leaders take reasonable steps to effect change but have to constantly adapt to barriers to change. While a specific change strategy generalizable to all contexts would be ideal, in the end at an institutional level, positive change is the ultimate aim rather than determining which interventions are effective. This response to events as they arise as illustrated in our quality improvement journey, is the fuzzy side of change management.
Conclusion
In conclusion, through a long period with a multitude of strategies, use of a guideline for prophylactic antibiotics increased and was associated with a reduction in SSI. Future directions need to consider cost-effective strategies to actively monitor SSI and testing of other strategies to reduce SSI. Institutions embarking on change need to consider that initiatives will likely need to adapt to specific contextual responses.
Corresponding author: James G. Wright, MD, PMH, FRCS, Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, University of Oxford Botnar Research Centre, Windmill Road, Oxford, OX3 7LD, UK, james.wright@ndorms.ox.ac.uk.
Funding/support: RB Salter Chair in Paediatric Surgical Research.
1. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
2. Grol R, Wensing M, Eccles M, Davis D, editors. Improving patient care: the implementation of change in health care. 2nd ed. Wiley Blackwell; 2013.
3. So JP, Aleem IS, Tsang DS, et al. Increasing compliance with an antibiotic prophylaxis guideline to prevent pediatric surgical site infection: before and after study. Ann Surg 2015;262:403–8.
4. Khoshbin A, So JP, Aleem IS, et al. Antibiotic prophylaxis to prevent surgical site infections in children: a prospective cohort study. Ann Surg 2015;262:397–402.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132.
6. Curran JA, Grimshaw JM, Hayden JA, Campbell B. Knowledge translation research: the science of moving research into policy and practice. J Contin Educ Health Prof 2011;31:174–80.
1. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
2. Grol R, Wensing M, Eccles M, Davis D, editors. Improving patient care: the implementation of change in health care. 2nd ed. Wiley Blackwell; 2013.
3. So JP, Aleem IS, Tsang DS, et al. Increasing compliance with an antibiotic prophylaxis guideline to prevent pediatric surgical site infection: before and after study. Ann Surg 2015;262:403–8.
4. Khoshbin A, So JP, Aleem IS, et al. Antibiotic prophylaxis to prevent surgical site infections in children: a prospective cohort study. Ann Surg 2015;262:397–402.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132.
6. Curran JA, Grimshaw JM, Hayden JA, Campbell B. Knowledge translation research: the science of moving research into policy and practice. J Contin Educ Health Prof 2011;31:174–80.
The crushing of innovation for treating female pelvic floor disorders: A story of “lead or be led”
With the decision by Astora Women’s Health to discontinue operations as of March 31, 2016, we have lost midurethral slings and pelvic organ prolapse repair mesh, technologies and kits that have been among the most widely used and studied (Steve Blum, Senior Vice President and General Manager, Astora Women’s Health, and Kathie J. Lenzen, Senior Vice President and General Manager, Endo Device Operations, e-mail communication to physician customers, February 29, 2016). US Food and Drug Administration (FDA)−mandated 522 postmarket surveillance studies on these products have stopped enrolling patients, and we will therefore never glean the full science from fully enrolled and completed studies. This is a horrible precedent. How did this happen, and what do we need to do now to prevent further loss of helpful innovative technologies that benefit our patients with pelvic floor disorders?
Liability challenges precipitated shut downEndo Pharmaceuticals, the parent company of Astora (previously American Medical Systems Women’s Health division), last year offered $1.5 billion to settle a majority of its pending mesh litigation cases. I was told that the company wanted to put all of the negative noise from the relentless plaintiff attorney public media campaign behind it and refocus its attention on helping women with pelvic floor disorders.
Over the past year, 4 interested and capable buyers have been in discussions with the company to purchase and continue its product line. The company’s recent decision to not sell its product line and discontinue all operations was based on “the current legal environment and the ongoing challenges associated with vaginal mesh product liability” (Astora Women’s Health, e-mail communication to physician customers, February 29, 2016). If it had chosen to sell its product line, the company always would have remained a potential deep-pocketed codefendant in any future litigation against the company that purchased its products, technologies, and intellectual properties.
This is a frightening scenario that threatens existing companies that want to remain in the prolapse and incontinence product space. This is a threat to all future innovation for pelvic floor disorder therapies, and it discourages anyone or any company to invest in innovative products that may help our patients. In addition, it is a threat to our mission as physicians and surgeons to provide the very best therapies to our patients who deserve and expect us to do so.
Let me be crystal clear: Currently available midurethral slings are also in the crosshairs of plaintiff attorneys, and we are at risk of losing them as well if we do not act quickly, decisively, and as a unified force. More than 60% of the mesh lawsuits have been against midurethral slings, not the prolapse mesh kits focused on in the FDA Public Health notice of July 2011.1 In their class action lawsuits, plaintiff attorneys lumped together any procedure involving mesh in the pelvis to increase the number of their patient clients involved, which can drive up settlement awards, and they succeeded. In 2014, 128,030 sling procedures for incontinence were performed. Does anyone truly believe that the scientific literature supports that these patients would have been best served by 128,030 Burch procedures?
Some believe that Endo Pharmaceuticals’ placement of $1.5 billion in settlement funds was an error, “threw blood in the water,” and led to what has happened. Some believe that companies should fight every lawsuit to win and not settle. By the companies winning cases, the plaintiff attorneys lose their incentives to advertise and file more cases, as they only receive money if they win (or get a settlement) and are out of pocket for their costs and time if they lose.
Plaintiff attorneys have a responsibility to zealously advocate for their patient clients. Defense attorneys have a responsibility to zealously defend their corporate clients. We surgeons must realize that we have a responsibility to zealously advocate for our patients and do whatever is needed to best serve them and to protect the use (and development) of innovative products and therapies that give them value and a better quality of life.
Proactive steps surgeons can take 
How do we do this? I suggest the following:
Implement expert oversight for litigation. Some of the large plaintiff awards were assisted by expert testimony based on a highly questionable scientific foundation. Judges give expert witnesses great latitude in their testimony, relying on the jury to discern the truth. I recommend that professional societies, such as the American College of Obstetricians and Gynecologists (ACOG), AmericanUrological Association (AUA), American Urogynecologic Society (AUGS), Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and Society of Gynecologic Surgeons (SGS), establish a panel to review and carefully evaluate plaintiff expert testimony that has a questionable scientific foundation. If such a panel finds the scientific basis of testimony to be biased, untruthful, or unethical, the societies must publicly reprimand and sanction these experts. Only then would these experts no longer be used by the plaintiff attorneys.
Such an expert panel also could serve to educate the judges in federal and state courts on real science and not manufactured opinions.
We need juries that can understand the science so they truly can decide on cases involving complex technologies.
Support professional leadership efforts. I am encouraged that AUGS is working to establish guidelines for the management of mesh complications. I have seen cases in which a small amount of mesh exposure, best treated by limited local excision of the exposed mesh, instead has been treated by complete excision of every polypropylene fiber placed, resulting in an unnecessarily morbid surgery that leaves a scarred and small vagina. Notably, some of the surgeons who excise every polypropylene fiber are also working as plaintiff experts, who may then testify that the scarred, small vagina was caused by the mesh and the implanting surgeon.
Our professional society leadership and volunteer committees, especially from AUGS, have done a tremendous amount of work in assisting with the FDA-required 522 postmarket surveillance study research design; establishing a Pelvic Floor Disorders Registry (http://www.pfdr.org/) and a sling registry; and developing credentialing guidelines for sacrocolpopexy, transvaginal mesh, and slings. They deserve our gratitude and our participation in the registries. It would be a tragedy if all of this work does not lead to fully enrolled and completed 522 studies so that we can scientifically make decisions on products before any more treatment options are removed from the market.
Use video to scrutinize surgical outcomes data. The surgical literature shows extreme variance in outcomes and complications for vaginal mesh surgery, including exposure rates from 1% to 20% with the same mesh products. This only can be explained by depth of surgical dissection and implanting technique. Surgical outcomes have been shown to be related directly to surgical volumes and experience.2 I propose that going forward, any authors who publish their study outcomes and complication data on a surgical procedure must submit a surgical video that demonstrates exactly how the surgery was done.
Best serve the patient. We all need to rigorously follow our own surgery results, improve our techniques, and keep within our surgical skill sets. We need to share our outcome and complication data with our patients during the informed consent process, since we, and not the surgical literature, are performing their surgeries.
We need to be transparent and respectful of our colleagues with different skill sets, putting what is best for patients ahead of everything else. We must be mindful of our inherent biases toward surgeries we are personally very good at and comfortable with. We must respect that other surgeons may achieve better clinical outcomes than us with the same surgery. We need to teach each other the best reproducible surgical techniques to maximize outcomes and minimize complications.
We must humbly accept that not every surgeon can do every surgery (and should not try). If a patient would be best served with a surgery we are not skilled in, we must refer that patient to a colleague who is.
Encourage industry’s part in training. As new technologies are developed, we must be brutally honest with ourselves about whether or not we have the skill sets to use them. Industry must gauge the complexity of the surgical skill set necessary to use their products and limit attendance at their teaching labs to surgeons who have the skills required to obtain good outcomes and minimize complications.
We have reached the tipping pointWe have seen the enemy, and it is us. We now need to advocate zealously for our patients. We will succeed only if we keep what is best for our patients at the forefront of everything we do. We must today decide to lead or be led. If we do not lead, we will be led by others—to places that may not best serve our patients. Make no mistake, this is a tipping point. The future of midurethral slings and potential future innovations lie in our hands right now.
Notably, just days prior to Astora’s letter to its physician customers announcing the decision to discontinue all of its operations, the transobturator postanal sling system (TOPAS) for fecal incontinence, a product in the pipeline at Astora, received 3 unanimous 8-0 votes from an FDA device advisory panel on safety, efficacy, and benefit outweighing risk.3 The future of this technology is now uncertain as well.
I ask Endo Pharmaceuticals to reconsider abandoning all of its products and intellectual properties. I ask it to entertain discussions with large companies that want its technologies and intellectual properties and can indemnify it from future litigation. While there never is a guarantee of complete indemnification and the company does have a fiduciary responsibility to its shareholders, industry also has a responsibility to patients and surgeons to allow helpful technologies to persist.
According to Astora’s letter to its physician customers, “Patient health has always been our number one priority. As such, the business closure has been expedited so that you and your patients have the opportunity to assess alternative treatment options as soon as possible.”
That letter was dated February 29. I do not feel that 31 days’ notice is enough time for surgeons to assess—let alone learn and master—new treatment options. It would have been helpful if Endo Pharmaceuticals had given more notice and would at least have allowed other interested companies the option to purchase useful technologies and intellectual property to mitigate its rapid departure from the space. The company remains in the health care arena with its pharmaceutical products, and how it behaves leaving the surgical space will be noted and impact its brand and reputation.
Lessons from the morcellation situationHow quickly the power morcellator disappeared is a lesson to note very carefully, and it has important parallels to what we now face. I highly recommend that you read and study Lisa Rosenbaum’s article in New England Journal of Medicine, “N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation.”4 She eloquently discusses how decisions to terminate technologies based on passionate anecdotal stories and media campaigns, and not scientific study, does not serve the greater good. She explores lessons learned from the silicone breast implant saga as well, stating “the tendency to focus on eliminating an immediate harm while failing to consider potentially greater harms caused by that reaction is heightened by the power of tragic stories.”4
We need a calmer, less emotional, and balanced scientific approach to evaluate technologies. We need to consider what harm is done by not allowing new technologies to be adequately studied, improved, and implemented. Dr. Rosenbaum discusses what Cass Sunstein and Timur Kuran call the “availability cascade,” “a phenomenon whereby stories inform public perceptions and anyone challenging those perceptions is vilified.”4,5
No technology will ever be risk free, and there always will be some risks and complications that could be significant and chilling. However, patient autonomy requires a full discussion of a risk/benefit ratio that is based on science, and these scientific data must be allowed to be collected and learned. There even can be more significant and chilling complications from not using a technology as well.
It is challenging to speak science to emotion that is driven by tragic outcomes, but we can remain compassionate as we seek the science that will serve the greater good. Condemning proponents of carefully studied and properly implemented technologies as immoral is neither helpful nor constructive. Crushing the ability to thoroughly and scientifically study new technologies is not in the best interest of our patients with pelvic floor disorders.
It is time to reawaken the better angels of our natureWill we do the necessary work now no matter how uncomfortable it may make us feel? Or will we be intimidated and remain silent and disjointed? Will we participate in the registries and follow best clinical practice and credentialing guidelines? Will we hold ourselves and our colleagues accountable? It is time to remember why we became surgeons, and to start acting on our convictions.
To that end, we must ask ourselves, will we:
- honor the Hippocratic Oath that we took in medical school and “respect the hard-won scientific gains of those physicians in whose steps I walk, and gladly share such knowledge as is mine with those who are to follow”6
- “not be ashamed to say ‘I know not,’ nor will I fail to call in my colleagues when the skills of another are needed for a patient’s recovery”6
- zealously advocate for our patients to ensure we can offer them the very best therapies
- honor and respect the sacred trust patients place in us when we take them to the operating room
- lead or be led?
This is personal for me. My mother struggled with pelvic floor disorders. I always felt it grossly unfair that women who chose to give us life could suffer for the rest of theirs for that decision. These women deserve our very best. The 40 million women with pelvic floor disorders deserve—and expect—that we lead. Will we?
I am hopeful that we will. I believe we will rise to today’s challenges and protect and fight for our patients. I believe that years from now we will look back and be proud that we did the right thing, and in so doing protected and encouraged innovations that significantly enhanced the quality of our patients’ lives. I believe patients will recognize our genuine efforts and in so doing give our profession the respect and trust that I feel has been diminished.
I believe we will draw the needed courage and resolve from the oath we recited in medical school and remember that, “If I do not violate this oath, may I enjoy life and art, respected while I live and remembered with affection thereafter. May I always act so as to preserve the finest traditions of my calling and may I long experience the joy of healing those who seek my help.”6
I do believe we will.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Published July 2011. Accessed March 21, 2016.
- Meyer CP, Trinh QD. Complications after surgery for stress urinary incontinence: untangling a mesh of uncertainties. JAMA Surg. 2015;150(12):1175-1176.
- Food and Drug Administration Center for Devices and Radiological Health. Brief summary of the Gastroenterology and Urology Devices Panel of the Medical Devices Advisory Committee Meeting--February 25, 2016. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM488397.pdf. Accessed March 21, 2016.
- Rosenbaum L. N-of-1--tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986-990.
- Kuran T, Sunstein C. Availability cascades and risk regulation. Stanford Law Rev. 1999;51:683-768.
- Hippocratic oath, modern version. Adapted by Louis Lasagna. 1964. Johns Hopkins Sheridan Libraries and University Museums website. http://guides.library.jhu.edu/c.php?g=202502&p=1335759. Updated December 8, 2015. Accessed March 22, 2016.
With the decision by Astora Women’s Health to discontinue operations as of March 31, 2016, we have lost midurethral slings and pelvic organ prolapse repair mesh, technologies and kits that have been among the most widely used and studied (Steve Blum, Senior Vice President and General Manager, Astora Women’s Health, and Kathie J. Lenzen, Senior Vice President and General Manager, Endo Device Operations, e-mail communication to physician customers, February 29, 2016). US Food and Drug Administration (FDA)−mandated 522 postmarket surveillance studies on these products have stopped enrolling patients, and we will therefore never glean the full science from fully enrolled and completed studies. This is a horrible precedent. How did this happen, and what do we need to do now to prevent further loss of helpful innovative technologies that benefit our patients with pelvic floor disorders?
Liability challenges precipitated shut downEndo Pharmaceuticals, the parent company of Astora (previously American Medical Systems Women’s Health division), last year offered $1.5 billion to settle a majority of its pending mesh litigation cases. I was told that the company wanted to put all of the negative noise from the relentless plaintiff attorney public media campaign behind it and refocus its attention on helping women with pelvic floor disorders.
Over the past year, 4 interested and capable buyers have been in discussions with the company to purchase and continue its product line. The company’s recent decision to not sell its product line and discontinue all operations was based on “the current legal environment and the ongoing challenges associated with vaginal mesh product liability” (Astora Women’s Health, e-mail communication to physician customers, February 29, 2016). If it had chosen to sell its product line, the company always would have remained a potential deep-pocketed codefendant in any future litigation against the company that purchased its products, technologies, and intellectual properties.
This is a frightening scenario that threatens existing companies that want to remain in the prolapse and incontinence product space. This is a threat to all future innovation for pelvic floor disorder therapies, and it discourages anyone or any company to invest in innovative products that may help our patients. In addition, it is a threat to our mission as physicians and surgeons to provide the very best therapies to our patients who deserve and expect us to do so.
Let me be crystal clear: Currently available midurethral slings are also in the crosshairs of plaintiff attorneys, and we are at risk of losing them as well if we do not act quickly, decisively, and as a unified force. More than 60% of the mesh lawsuits have been against midurethral slings, not the prolapse mesh kits focused on in the FDA Public Health notice of July 2011.1 In their class action lawsuits, plaintiff attorneys lumped together any procedure involving mesh in the pelvis to increase the number of their patient clients involved, which can drive up settlement awards, and they succeeded. In 2014, 128,030 sling procedures for incontinence were performed. Does anyone truly believe that the scientific literature supports that these patients would have been best served by 128,030 Burch procedures?
Some believe that Endo Pharmaceuticals’ placement of $1.5 billion in settlement funds was an error, “threw blood in the water,” and led to what has happened. Some believe that companies should fight every lawsuit to win and not settle. By the companies winning cases, the plaintiff attorneys lose their incentives to advertise and file more cases, as they only receive money if they win (or get a settlement) and are out of pocket for their costs and time if they lose.
Plaintiff attorneys have a responsibility to zealously advocate for their patient clients. Defense attorneys have a responsibility to zealously defend their corporate clients. We surgeons must realize that we have a responsibility to zealously advocate for our patients and do whatever is needed to best serve them and to protect the use (and development) of innovative products and therapies that give them value and a better quality of life.
Proactive steps surgeons can take 
How do we do this? I suggest the following:
Implement expert oversight for litigation. Some of the large plaintiff awards were assisted by expert testimony based on a highly questionable scientific foundation. Judges give expert witnesses great latitude in their testimony, relying on the jury to discern the truth. I recommend that professional societies, such as the American College of Obstetricians and Gynecologists (ACOG), AmericanUrological Association (AUA), American Urogynecologic Society (AUGS), Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and Society of Gynecologic Surgeons (SGS), establish a panel to review and carefully evaluate plaintiff expert testimony that has a questionable scientific foundation. If such a panel finds the scientific basis of testimony to be biased, untruthful, or unethical, the societies must publicly reprimand and sanction these experts. Only then would these experts no longer be used by the plaintiff attorneys.
Such an expert panel also could serve to educate the judges in federal and state courts on real science and not manufactured opinions.
We need juries that can understand the science so they truly can decide on cases involving complex technologies.
Support professional leadership efforts. I am encouraged that AUGS is working to establish guidelines for the management of mesh complications. I have seen cases in which a small amount of mesh exposure, best treated by limited local excision of the exposed mesh, instead has been treated by complete excision of every polypropylene fiber placed, resulting in an unnecessarily morbid surgery that leaves a scarred and small vagina. Notably, some of the surgeons who excise every polypropylene fiber are also working as plaintiff experts, who may then testify that the scarred, small vagina was caused by the mesh and the implanting surgeon.
Our professional society leadership and volunteer committees, especially from AUGS, have done a tremendous amount of work in assisting with the FDA-required 522 postmarket surveillance study research design; establishing a Pelvic Floor Disorders Registry (http://www.pfdr.org/) and a sling registry; and developing credentialing guidelines for sacrocolpopexy, transvaginal mesh, and slings. They deserve our gratitude and our participation in the registries. It would be a tragedy if all of this work does not lead to fully enrolled and completed 522 studies so that we can scientifically make decisions on products before any more treatment options are removed from the market.
Use video to scrutinize surgical outcomes data. The surgical literature shows extreme variance in outcomes and complications for vaginal mesh surgery, including exposure rates from 1% to 20% with the same mesh products. This only can be explained by depth of surgical dissection and implanting technique. Surgical outcomes have been shown to be related directly to surgical volumes and experience.2 I propose that going forward, any authors who publish their study outcomes and complication data on a surgical procedure must submit a surgical video that demonstrates exactly how the surgery was done.
Best serve the patient. We all need to rigorously follow our own surgery results, improve our techniques, and keep within our surgical skill sets. We need to share our outcome and complication data with our patients during the informed consent process, since we, and not the surgical literature, are performing their surgeries.
We need to be transparent and respectful of our colleagues with different skill sets, putting what is best for patients ahead of everything else. We must be mindful of our inherent biases toward surgeries we are personally very good at and comfortable with. We must respect that other surgeons may achieve better clinical outcomes than us with the same surgery. We need to teach each other the best reproducible surgical techniques to maximize outcomes and minimize complications.
We must humbly accept that not every surgeon can do every surgery (and should not try). If a patient would be best served with a surgery we are not skilled in, we must refer that patient to a colleague who is.
Encourage industry’s part in training. As new technologies are developed, we must be brutally honest with ourselves about whether or not we have the skill sets to use them. Industry must gauge the complexity of the surgical skill set necessary to use their products and limit attendance at their teaching labs to surgeons who have the skills required to obtain good outcomes and minimize complications.
We have reached the tipping pointWe have seen the enemy, and it is us. We now need to advocate zealously for our patients. We will succeed only if we keep what is best for our patients at the forefront of everything we do. We must today decide to lead or be led. If we do not lead, we will be led by others—to places that may not best serve our patients. Make no mistake, this is a tipping point. The future of midurethral slings and potential future innovations lie in our hands right now.
Notably, just days prior to Astora’s letter to its physician customers announcing the decision to discontinue all of its operations, the transobturator postanal sling system (TOPAS) for fecal incontinence, a product in the pipeline at Astora, received 3 unanimous 8-0 votes from an FDA device advisory panel on safety, efficacy, and benefit outweighing risk.3 The future of this technology is now uncertain as well.
I ask Endo Pharmaceuticals to reconsider abandoning all of its products and intellectual properties. I ask it to entertain discussions with large companies that want its technologies and intellectual properties and can indemnify it from future litigation. While there never is a guarantee of complete indemnification and the company does have a fiduciary responsibility to its shareholders, industry also has a responsibility to patients and surgeons to allow helpful technologies to persist.
According to Astora’s letter to its physician customers, “Patient health has always been our number one priority. As such, the business closure has been expedited so that you and your patients have the opportunity to assess alternative treatment options as soon as possible.”
That letter was dated February 29. I do not feel that 31 days’ notice is enough time for surgeons to assess—let alone learn and master—new treatment options. It would have been helpful if Endo Pharmaceuticals had given more notice and would at least have allowed other interested companies the option to purchase useful technologies and intellectual property to mitigate its rapid departure from the space. The company remains in the health care arena with its pharmaceutical products, and how it behaves leaving the surgical space will be noted and impact its brand and reputation.
Lessons from the morcellation situationHow quickly the power morcellator disappeared is a lesson to note very carefully, and it has important parallels to what we now face. I highly recommend that you read and study Lisa Rosenbaum’s article in New England Journal of Medicine, “N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation.”4 She eloquently discusses how decisions to terminate technologies based on passionate anecdotal stories and media campaigns, and not scientific study, does not serve the greater good. She explores lessons learned from the silicone breast implant saga as well, stating “the tendency to focus on eliminating an immediate harm while failing to consider potentially greater harms caused by that reaction is heightened by the power of tragic stories.”4
We need a calmer, less emotional, and balanced scientific approach to evaluate technologies. We need to consider what harm is done by not allowing new technologies to be adequately studied, improved, and implemented. Dr. Rosenbaum discusses what Cass Sunstein and Timur Kuran call the “availability cascade,” “a phenomenon whereby stories inform public perceptions and anyone challenging those perceptions is vilified.”4,5
No technology will ever be risk free, and there always will be some risks and complications that could be significant and chilling. However, patient autonomy requires a full discussion of a risk/benefit ratio that is based on science, and these scientific data must be allowed to be collected and learned. There even can be more significant and chilling complications from not using a technology as well.
It is challenging to speak science to emotion that is driven by tragic outcomes, but we can remain compassionate as we seek the science that will serve the greater good. Condemning proponents of carefully studied and properly implemented technologies as immoral is neither helpful nor constructive. Crushing the ability to thoroughly and scientifically study new technologies is not in the best interest of our patients with pelvic floor disorders.
It is time to reawaken the better angels of our natureWill we do the necessary work now no matter how uncomfortable it may make us feel? Or will we be intimidated and remain silent and disjointed? Will we participate in the registries and follow best clinical practice and credentialing guidelines? Will we hold ourselves and our colleagues accountable? It is time to remember why we became surgeons, and to start acting on our convictions.
To that end, we must ask ourselves, will we:
- honor the Hippocratic Oath that we took in medical school and “respect the hard-won scientific gains of those physicians in whose steps I walk, and gladly share such knowledge as is mine with those who are to follow”6
- “not be ashamed to say ‘I know not,’ nor will I fail to call in my colleagues when the skills of another are needed for a patient’s recovery”6
- zealously advocate for our patients to ensure we can offer them the very best therapies
- honor and respect the sacred trust patients place in us when we take them to the operating room
- lead or be led?
This is personal for me. My mother struggled with pelvic floor disorders. I always felt it grossly unfair that women who chose to give us life could suffer for the rest of theirs for that decision. These women deserve our very best. The 40 million women with pelvic floor disorders deserve—and expect—that we lead. Will we?
I am hopeful that we will. I believe we will rise to today’s challenges and protect and fight for our patients. I believe that years from now we will look back and be proud that we did the right thing, and in so doing protected and encouraged innovations that significantly enhanced the quality of our patients’ lives. I believe patients will recognize our genuine efforts and in so doing give our profession the respect and trust that I feel has been diminished.
I believe we will draw the needed courage and resolve from the oath we recited in medical school and remember that, “If I do not violate this oath, may I enjoy life and art, respected while I live and remembered with affection thereafter. May I always act so as to preserve the finest traditions of my calling and may I long experience the joy of healing those who seek my help.”6
I do believe we will.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
With the decision by Astora Women’s Health to discontinue operations as of March 31, 2016, we have lost midurethral slings and pelvic organ prolapse repair mesh, technologies and kits that have been among the most widely used and studied (Steve Blum, Senior Vice President and General Manager, Astora Women’s Health, and Kathie J. Lenzen, Senior Vice President and General Manager, Endo Device Operations, e-mail communication to physician customers, February 29, 2016). US Food and Drug Administration (FDA)−mandated 522 postmarket surveillance studies on these products have stopped enrolling patients, and we will therefore never glean the full science from fully enrolled and completed studies. This is a horrible precedent. How did this happen, and what do we need to do now to prevent further loss of helpful innovative technologies that benefit our patients with pelvic floor disorders?
Liability challenges precipitated shut downEndo Pharmaceuticals, the parent company of Astora (previously American Medical Systems Women’s Health division), last year offered $1.5 billion to settle a majority of its pending mesh litigation cases. I was told that the company wanted to put all of the negative noise from the relentless plaintiff attorney public media campaign behind it and refocus its attention on helping women with pelvic floor disorders.
Over the past year, 4 interested and capable buyers have been in discussions with the company to purchase and continue its product line. The company’s recent decision to not sell its product line and discontinue all operations was based on “the current legal environment and the ongoing challenges associated with vaginal mesh product liability” (Astora Women’s Health, e-mail communication to physician customers, February 29, 2016). If it had chosen to sell its product line, the company always would have remained a potential deep-pocketed codefendant in any future litigation against the company that purchased its products, technologies, and intellectual properties.
This is a frightening scenario that threatens existing companies that want to remain in the prolapse and incontinence product space. This is a threat to all future innovation for pelvic floor disorder therapies, and it discourages anyone or any company to invest in innovative products that may help our patients. In addition, it is a threat to our mission as physicians and surgeons to provide the very best therapies to our patients who deserve and expect us to do so.
Let me be crystal clear: Currently available midurethral slings are also in the crosshairs of plaintiff attorneys, and we are at risk of losing them as well if we do not act quickly, decisively, and as a unified force. More than 60% of the mesh lawsuits have been against midurethral slings, not the prolapse mesh kits focused on in the FDA Public Health notice of July 2011.1 In their class action lawsuits, plaintiff attorneys lumped together any procedure involving mesh in the pelvis to increase the number of their patient clients involved, which can drive up settlement awards, and they succeeded. In 2014, 128,030 sling procedures for incontinence were performed. Does anyone truly believe that the scientific literature supports that these patients would have been best served by 128,030 Burch procedures?
Some believe that Endo Pharmaceuticals’ placement of $1.5 billion in settlement funds was an error, “threw blood in the water,” and led to what has happened. Some believe that companies should fight every lawsuit to win and not settle. By the companies winning cases, the plaintiff attorneys lose their incentives to advertise and file more cases, as they only receive money if they win (or get a settlement) and are out of pocket for their costs and time if they lose.
Plaintiff attorneys have a responsibility to zealously advocate for their patient clients. Defense attorneys have a responsibility to zealously defend their corporate clients. We surgeons must realize that we have a responsibility to zealously advocate for our patients and do whatever is needed to best serve them and to protect the use (and development) of innovative products and therapies that give them value and a better quality of life.
Proactive steps surgeons can take 
How do we do this? I suggest the following:
Implement expert oversight for litigation. Some of the large plaintiff awards were assisted by expert testimony based on a highly questionable scientific foundation. Judges give expert witnesses great latitude in their testimony, relying on the jury to discern the truth. I recommend that professional societies, such as the American College of Obstetricians and Gynecologists (ACOG), AmericanUrological Association (AUA), American Urogynecologic Society (AUGS), Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and Society of Gynecologic Surgeons (SGS), establish a panel to review and carefully evaluate plaintiff expert testimony that has a questionable scientific foundation. If such a panel finds the scientific basis of testimony to be biased, untruthful, or unethical, the societies must publicly reprimand and sanction these experts. Only then would these experts no longer be used by the plaintiff attorneys.
Such an expert panel also could serve to educate the judges in federal and state courts on real science and not manufactured opinions.
We need juries that can understand the science so they truly can decide on cases involving complex technologies.
Support professional leadership efforts. I am encouraged that AUGS is working to establish guidelines for the management of mesh complications. I have seen cases in which a small amount of mesh exposure, best treated by limited local excision of the exposed mesh, instead has been treated by complete excision of every polypropylene fiber placed, resulting in an unnecessarily morbid surgery that leaves a scarred and small vagina. Notably, some of the surgeons who excise every polypropylene fiber are also working as plaintiff experts, who may then testify that the scarred, small vagina was caused by the mesh and the implanting surgeon.
Our professional society leadership and volunteer committees, especially from AUGS, have done a tremendous amount of work in assisting with the FDA-required 522 postmarket surveillance study research design; establishing a Pelvic Floor Disorders Registry (http://www.pfdr.org/) and a sling registry; and developing credentialing guidelines for sacrocolpopexy, transvaginal mesh, and slings. They deserve our gratitude and our participation in the registries. It would be a tragedy if all of this work does not lead to fully enrolled and completed 522 studies so that we can scientifically make decisions on products before any more treatment options are removed from the market.
Use video to scrutinize surgical outcomes data. The surgical literature shows extreme variance in outcomes and complications for vaginal mesh surgery, including exposure rates from 1% to 20% with the same mesh products. This only can be explained by depth of surgical dissection and implanting technique. Surgical outcomes have been shown to be related directly to surgical volumes and experience.2 I propose that going forward, any authors who publish their study outcomes and complication data on a surgical procedure must submit a surgical video that demonstrates exactly how the surgery was done.
Best serve the patient. We all need to rigorously follow our own surgery results, improve our techniques, and keep within our surgical skill sets. We need to share our outcome and complication data with our patients during the informed consent process, since we, and not the surgical literature, are performing their surgeries.
We need to be transparent and respectful of our colleagues with different skill sets, putting what is best for patients ahead of everything else. We must be mindful of our inherent biases toward surgeries we are personally very good at and comfortable with. We must respect that other surgeons may achieve better clinical outcomes than us with the same surgery. We need to teach each other the best reproducible surgical techniques to maximize outcomes and minimize complications.
We must humbly accept that not every surgeon can do every surgery (and should not try). If a patient would be best served with a surgery we are not skilled in, we must refer that patient to a colleague who is.
Encourage industry’s part in training. As new technologies are developed, we must be brutally honest with ourselves about whether or not we have the skill sets to use them. Industry must gauge the complexity of the surgical skill set necessary to use their products and limit attendance at their teaching labs to surgeons who have the skills required to obtain good outcomes and minimize complications.
We have reached the tipping pointWe have seen the enemy, and it is us. We now need to advocate zealously for our patients. We will succeed only if we keep what is best for our patients at the forefront of everything we do. We must today decide to lead or be led. If we do not lead, we will be led by others—to places that may not best serve our patients. Make no mistake, this is a tipping point. The future of midurethral slings and potential future innovations lie in our hands right now.
Notably, just days prior to Astora’s letter to its physician customers announcing the decision to discontinue all of its operations, the transobturator postanal sling system (TOPAS) for fecal incontinence, a product in the pipeline at Astora, received 3 unanimous 8-0 votes from an FDA device advisory panel on safety, efficacy, and benefit outweighing risk.3 The future of this technology is now uncertain as well.
I ask Endo Pharmaceuticals to reconsider abandoning all of its products and intellectual properties. I ask it to entertain discussions with large companies that want its technologies and intellectual properties and can indemnify it from future litigation. While there never is a guarantee of complete indemnification and the company does have a fiduciary responsibility to its shareholders, industry also has a responsibility to patients and surgeons to allow helpful technologies to persist.
According to Astora’s letter to its physician customers, “Patient health has always been our number one priority. As such, the business closure has been expedited so that you and your patients have the opportunity to assess alternative treatment options as soon as possible.”
That letter was dated February 29. I do not feel that 31 days’ notice is enough time for surgeons to assess—let alone learn and master—new treatment options. It would have been helpful if Endo Pharmaceuticals had given more notice and would at least have allowed other interested companies the option to purchase useful technologies and intellectual property to mitigate its rapid departure from the space. The company remains in the health care arena with its pharmaceutical products, and how it behaves leaving the surgical space will be noted and impact its brand and reputation.
Lessons from the morcellation situationHow quickly the power morcellator disappeared is a lesson to note very carefully, and it has important parallels to what we now face. I highly recommend that you read and study Lisa Rosenbaum’s article in New England Journal of Medicine, “N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation.”4 She eloquently discusses how decisions to terminate technologies based on passionate anecdotal stories and media campaigns, and not scientific study, does not serve the greater good. She explores lessons learned from the silicone breast implant saga as well, stating “the tendency to focus on eliminating an immediate harm while failing to consider potentially greater harms caused by that reaction is heightened by the power of tragic stories.”4
We need a calmer, less emotional, and balanced scientific approach to evaluate technologies. We need to consider what harm is done by not allowing new technologies to be adequately studied, improved, and implemented. Dr. Rosenbaum discusses what Cass Sunstein and Timur Kuran call the “availability cascade,” “a phenomenon whereby stories inform public perceptions and anyone challenging those perceptions is vilified.”4,5
No technology will ever be risk free, and there always will be some risks and complications that could be significant and chilling. However, patient autonomy requires a full discussion of a risk/benefit ratio that is based on science, and these scientific data must be allowed to be collected and learned. There even can be more significant and chilling complications from not using a technology as well.
It is challenging to speak science to emotion that is driven by tragic outcomes, but we can remain compassionate as we seek the science that will serve the greater good. Condemning proponents of carefully studied and properly implemented technologies as immoral is neither helpful nor constructive. Crushing the ability to thoroughly and scientifically study new technologies is not in the best interest of our patients with pelvic floor disorders.
It is time to reawaken the better angels of our natureWill we do the necessary work now no matter how uncomfortable it may make us feel? Or will we be intimidated and remain silent and disjointed? Will we participate in the registries and follow best clinical practice and credentialing guidelines? Will we hold ourselves and our colleagues accountable? It is time to remember why we became surgeons, and to start acting on our convictions.
To that end, we must ask ourselves, will we:
- honor the Hippocratic Oath that we took in medical school and “respect the hard-won scientific gains of those physicians in whose steps I walk, and gladly share such knowledge as is mine with those who are to follow”6
- “not be ashamed to say ‘I know not,’ nor will I fail to call in my colleagues when the skills of another are needed for a patient’s recovery”6
- zealously advocate for our patients to ensure we can offer them the very best therapies
- honor and respect the sacred trust patients place in us when we take them to the operating room
- lead or be led?
This is personal for me. My mother struggled with pelvic floor disorders. I always felt it grossly unfair that women who chose to give us life could suffer for the rest of theirs for that decision. These women deserve our very best. The 40 million women with pelvic floor disorders deserve—and expect—that we lead. Will we?
I am hopeful that we will. I believe we will rise to today’s challenges and protect and fight for our patients. I believe that years from now we will look back and be proud that we did the right thing, and in so doing protected and encouraged innovations that significantly enhanced the quality of our patients’ lives. I believe patients will recognize our genuine efforts and in so doing give our profession the respect and trust that I feel has been diminished.
I believe we will draw the needed courage and resolve from the oath we recited in medical school and remember that, “If I do not violate this oath, may I enjoy life and art, respected while I live and remembered with affection thereafter. May I always act so as to preserve the finest traditions of my calling and may I long experience the joy of healing those who seek my help.”6
I do believe we will.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Published July 2011. Accessed March 21, 2016.
- Meyer CP, Trinh QD. Complications after surgery for stress urinary incontinence: untangling a mesh of uncertainties. JAMA Surg. 2015;150(12):1175-1176.
- Food and Drug Administration Center for Devices and Radiological Health. Brief summary of the Gastroenterology and Urology Devices Panel of the Medical Devices Advisory Committee Meeting--February 25, 2016. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM488397.pdf. Accessed March 21, 2016.
- Rosenbaum L. N-of-1--tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986-990.
- Kuran T, Sunstein C. Availability cascades and risk regulation. Stanford Law Rev. 1999;51:683-768.
- Hippocratic oath, modern version. Adapted by Louis Lasagna. 1964. Johns Hopkins Sheridan Libraries and University Museums website. http://guides.library.jhu.edu/c.php?g=202502&p=1335759. Updated December 8, 2015. Accessed March 22, 2016.
- Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Published July 2011. Accessed March 21, 2016.
- Meyer CP, Trinh QD. Complications after surgery for stress urinary incontinence: untangling a mesh of uncertainties. JAMA Surg. 2015;150(12):1175-1176.
- Food and Drug Administration Center for Devices and Radiological Health. Brief summary of the Gastroenterology and Urology Devices Panel of the Medical Devices Advisory Committee Meeting--February 25, 2016. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM488397.pdf. Accessed March 21, 2016.
- Rosenbaum L. N-of-1--tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986-990.
- Kuran T, Sunstein C. Availability cascades and risk regulation. Stanford Law Rev. 1999;51:683-768.
- Hippocratic oath, modern version. Adapted by Louis Lasagna. 1964. Johns Hopkins Sheridan Libraries and University Museums website. http://guides.library.jhu.edu/c.php?g=202502&p=1335759. Updated December 8, 2015. Accessed March 22, 2016.
Gynecologic oncologists often missing from pediatric pelvic evaluations
SAN DIEGO – Young women and girls with gynecologic malignancies more often present with pain and masses greater than 5 cm in size, compared with their counterparts who have benign disease. Additionally, gynecologic oncologists are inconsistently involved in the management of this patient population.
Those are key findings from a study that set out to compare the clinical presentation and surgical outcomes of women and girls younger than 21 years old who had a pelvic mass.
“If something is suspicious, it’s not a bad idea to get your colleagues who specialize in gynecologic cancer involved sooner rather than later,” Dr. Teuta Shemshedini, the lead study author, said in an interview at the annual meeting of the Society of Gynecologic Oncology. Clinicians who specialize in gynecologic oncology “were often talked to either intraoperatively or postoperatively, so we were kind of working backwards when we could have sat with patients and the families before the surgery and worked forward.”
Dr. Shemshedini, who is a fourth-year resident in the department of obstetrics and gynecology at Westchester Medical Center, Valhalla, N.Y., and her associates reviewed medical records of all women and girls younger than 21 years old who underwent primary surgery for a pelvic mass at the medical center from 2010 to 2015.
Of the 138 patients evaluated, 77 were included in the final analysis: 57 who had benign disease and 20 who had malignant disease. The mean age of the patients was 13.5 years and the mean adnexal mass size was 9.8 cm in the benign group, compared with 15.5 cm in the malignant group (P = .005). The most common presentation was pain, which occurred in 75% of all cases.
Gynecologic oncologists were consulted on 10 cases (13%), with six of the 10 consults (60%) requested by pediatric gynecologists. However, only two of eight (25%) were preoperative consults in malignant cases.
The researchers also observed that tumors greater than 10 cm in size were found in 75% of malignancies, and all tumors 5 cm or smaller were benign (14%). Clinicians did not use tumor markers in 29% of the entire study group, even though tumor markers were elevated in 70% of the malignant cases.
Laparoscopic surgery was performed in 35 patients (45%), with a majority of cases being benign. The most common benign tumors were mature teratomas (70%). The most common malignant tumors were borderline ovarian tumors (35%), followed by immature teratomas (20%), and mixed germ cell tumors (20%). More than half of malignant tumors (55%) were stage I.
“The most surprising part was that we weren’t getting gynecologic oncology involved soon enough,” Dr. Shemshedini said. “I think most people are very surprised when a mass comes back as cancer in kids, especially ovarian cancer. In adults we see epithelial cancer most commonly, while in kids it’s more of the germ cell tumors. Those are rare.”
Dr. Shemshedini reported having no financial disclosures.
SAN DIEGO – Young women and girls with gynecologic malignancies more often present with pain and masses greater than 5 cm in size, compared with their counterparts who have benign disease. Additionally, gynecologic oncologists are inconsistently involved in the management of this patient population.
Those are key findings from a study that set out to compare the clinical presentation and surgical outcomes of women and girls younger than 21 years old who had a pelvic mass.
“If something is suspicious, it’s not a bad idea to get your colleagues who specialize in gynecologic cancer involved sooner rather than later,” Dr. Teuta Shemshedini, the lead study author, said in an interview at the annual meeting of the Society of Gynecologic Oncology. Clinicians who specialize in gynecologic oncology “were often talked to either intraoperatively or postoperatively, so we were kind of working backwards when we could have sat with patients and the families before the surgery and worked forward.”
Dr. Shemshedini, who is a fourth-year resident in the department of obstetrics and gynecology at Westchester Medical Center, Valhalla, N.Y., and her associates reviewed medical records of all women and girls younger than 21 years old who underwent primary surgery for a pelvic mass at the medical center from 2010 to 2015.
Of the 138 patients evaluated, 77 were included in the final analysis: 57 who had benign disease and 20 who had malignant disease. The mean age of the patients was 13.5 years and the mean adnexal mass size was 9.8 cm in the benign group, compared with 15.5 cm in the malignant group (P = .005). The most common presentation was pain, which occurred in 75% of all cases.
Gynecologic oncologists were consulted on 10 cases (13%), with six of the 10 consults (60%) requested by pediatric gynecologists. However, only two of eight (25%) were preoperative consults in malignant cases.
The researchers also observed that tumors greater than 10 cm in size were found in 75% of malignancies, and all tumors 5 cm or smaller were benign (14%). Clinicians did not use tumor markers in 29% of the entire study group, even though tumor markers were elevated in 70% of the malignant cases.
Laparoscopic surgery was performed in 35 patients (45%), with a majority of cases being benign. The most common benign tumors were mature teratomas (70%). The most common malignant tumors were borderline ovarian tumors (35%), followed by immature teratomas (20%), and mixed germ cell tumors (20%). More than half of malignant tumors (55%) were stage I.
“The most surprising part was that we weren’t getting gynecologic oncology involved soon enough,” Dr. Shemshedini said. “I think most people are very surprised when a mass comes back as cancer in kids, especially ovarian cancer. In adults we see epithelial cancer most commonly, while in kids it’s more of the germ cell tumors. Those are rare.”
Dr. Shemshedini reported having no financial disclosures.
SAN DIEGO – Young women and girls with gynecologic malignancies more often present with pain and masses greater than 5 cm in size, compared with their counterparts who have benign disease. Additionally, gynecologic oncologists are inconsistently involved in the management of this patient population.
Those are key findings from a study that set out to compare the clinical presentation and surgical outcomes of women and girls younger than 21 years old who had a pelvic mass.
“If something is suspicious, it’s not a bad idea to get your colleagues who specialize in gynecologic cancer involved sooner rather than later,” Dr. Teuta Shemshedini, the lead study author, said in an interview at the annual meeting of the Society of Gynecologic Oncology. Clinicians who specialize in gynecologic oncology “were often talked to either intraoperatively or postoperatively, so we were kind of working backwards when we could have sat with patients and the families before the surgery and worked forward.”
Dr. Shemshedini, who is a fourth-year resident in the department of obstetrics and gynecology at Westchester Medical Center, Valhalla, N.Y., and her associates reviewed medical records of all women and girls younger than 21 years old who underwent primary surgery for a pelvic mass at the medical center from 2010 to 2015.
Of the 138 patients evaluated, 77 were included in the final analysis: 57 who had benign disease and 20 who had malignant disease. The mean age of the patients was 13.5 years and the mean adnexal mass size was 9.8 cm in the benign group, compared with 15.5 cm in the malignant group (P = .005). The most common presentation was pain, which occurred in 75% of all cases.
Gynecologic oncologists were consulted on 10 cases (13%), with six of the 10 consults (60%) requested by pediatric gynecologists. However, only two of eight (25%) were preoperative consults in malignant cases.
The researchers also observed that tumors greater than 10 cm in size were found in 75% of malignancies, and all tumors 5 cm or smaller were benign (14%). Clinicians did not use tumor markers in 29% of the entire study group, even though tumor markers were elevated in 70% of the malignant cases.
Laparoscopic surgery was performed in 35 patients (45%), with a majority of cases being benign. The most common benign tumors were mature teratomas (70%). The most common malignant tumors were borderline ovarian tumors (35%), followed by immature teratomas (20%), and mixed germ cell tumors (20%). More than half of malignant tumors (55%) were stage I.
“The most surprising part was that we weren’t getting gynecologic oncology involved soon enough,” Dr. Shemshedini said. “I think most people are very surprised when a mass comes back as cancer in kids, especially ovarian cancer. In adults we see epithelial cancer most commonly, while in kids it’s more of the germ cell tumors. Those are rare.”
Dr. Shemshedini reported having no financial disclosures.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Among young women and girls with pelvic malignancies, the mass is often greater than 5 cm in size.
Major finding: The mean adnexal mass size was 9.8 cm in the benign group, compared with 15.5 cm in the malignant group (P =.005).
Data source: A review of medical records from 77 women and girls younger than 21 years old who underwent primary surgery for a pelvic mass from 2010 to 2015.
Disclosures: Dr. Shemshedini reported having no financial disclosures.
ADHD Medications Are Linked to Diminished Bone Density in Young Patients
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.