User login
Advancements help guide achalasia management, experts say
at a pace that has left the line-tracing technology considered to have debatable merit just 15 years ago “now as obsolete as a typewriter,” experts said recently in a review in Gastro Hep Advances.
“We have come to conceptualize esophageal motility disorders by specific aspects of physiological dysfunction,” wrote a trio of experts – Peter Kahrilas, MD, professor of medicine; Dustin Carlson, MD, MS, assistant professor of medicine, and John Pandolfino, MD, chief of gastroenterology and hepatology, all at Northwestern University, Chicago. “A major implication of this approach is a shift in management strategy toward rendering treatment in a phenotype-specific manner.”
High-resolution manometry (HRM) was trail-blazing, they said, as it replaced line-tracing manometry in evaluating the motility of the esophagus. HRM led to the subtyping of achalasia based on the three patterns of pressurization in the esophagus that are associated with obstruction at the esophagogastric junction. But the field has continued to advance.
“It has since become clear that obstructive physiology also occurs in syndromes besides achalasia involving the esophagogastric junction and/or distal esophagus,” Dr. Kahrilas, Dr. Carlson, and Dr. Pandolfino said. “In fact, obstructive physiology is increasingly recognized as the fundamental abnormality leading to the perception of dysphagia with esophageal motility disorders. This concept of obstructive physiology as the fundamental abnormality has substantially morphed the clinical management of esophageal motility disorders.”
HRM, has many limitations, but in cases of an uncertain achalasia diagnosis, functional luminal imaging probe (FLIP) technology can help, they said. FLIP can also help surgeons tailor myotomy procedures.
In FLIP, a probe is carefully filled with fluid, causing distension of the esophagus. In the test, the distensibility of the esophagogastric junction is measured. The procedure allows a more refined assessment of the movement of the esophagus, and the subtypes of achalasia.
Identifying the achalasia subtype is crucial to choosing the right treatment, data suggests. There have been no randomized controlled trials on achalasia management that prospectively consider achalasia subtype, but retrospective analysis of RCT data “suggests that achalasia subtypes are of great relevance in forecasting treatment effectiveness,” they said.
In one trial, pneumatic dilation was effective in 100% of type II achalasia, which involves panesophageal pressurization, significantly better than laparoscopic Heller myotomy (LHM). But it was much less effective than LHM in type III achalasia, the spastic form, although a significance couldn’t be established because of the number of cases. Data from a meta-analysis showed that peroral endoscopic myotomy, which allows for a longer myotomy if needed, was better than LHM for classic achalasia and spastic achalasia and was most efficacious overall.
The writers said that the diagnostic classifications for achalasia are likely to continue to evolve, pointing to the dynamic nature of the Chicago Classification for the disorder.
“The fact that it has now gone through four iterations since 2008 emphasizes that this is a work in progress and that no classification scheme of esophageal motility disorders based on a single test will ever be perfect,” they said. “After all, there are no biomarkers of esophageal motility disorders and, in the absence of a biomarker, there can be no ‘gold standard’ for diagnosis.”
Dr. Pandolfino, Dr. Kahrilas, and Northwestern University hold shared intellectual property rights and ownership surrounding FLIP Panometry systems, methods, and apparatus with Medtronic. Dr. Kahrilas reported consulting with Ironwood, Reckitt, and Phathom. Dr. Carlson reported conflicts of interest with Medtronic and Phathom Pharmaceuticals. Dr. Pandolfino reported conflicts of interest with Sandhill Scientific/Diversatek, Takeda, AstraZeneca, Medtronic, Torax, and Ironwood.
16% of the U.S. population experience dysphagia, only half of whom seek medical care and the others manage their symptoms by modifying diet.
X-ray barium swallow and endoscopy with biopsy to exclude eosinophilic esophagitis are the initial tests for dysphagia diagnosis. If the above are normal, a high-resolution esophageal manometry impedance (HRMZ) is recommended to diagnose primary and secondary esophageal motility disorder.
However, only in a minority of patients is it likely to cause dysphagia because uncontrolled studies show that therapeutic strategies to address EGJOO (botox, dilation, and myotomy) relieve dysphagia symptoms in a minority of patients. Hence, in significant number of patients the cause of dysphagia symptoms remains obscure. It might be that our testing is inadequate, or possibly, patients have functional dysphagia (sensory dysfunction of the esophagus). My opinion is that it is the former.
The esophagus has only one simple function, that is, to transfer the pharyngeal pump driven, that is, swallowed contents to the stomach, for which its luminal cross-sectional area must be larger than that of the swallowed bolus and contraction (measured by manometry) behind the bolus must be of adequate strength. The latter is likely less relevant because humans eat in the upright position and gravity provides propulsion for the bolus. Stated simply, as long as esophagus can distend well and there is no resistance to the outflow at the EGJ, esophagus can achieve its goal. However, until recently, there was no single test to determine the distension and contraction, the two essential elements of primary esophageal peristalsis.
Endoscopy and x-ray barium swallow are tests to determine the luminal diameter but have limitations. Endoflip measures the opening function of the EGJ and is useful when the HRM is normal. However, pressures that are currently being used to measure the EGJ distensibility by Endoflip are not physiological. Furthermore, esophageal body motor function assessed by a bag that distends a long segment of the esophagus under high pressure is unphysiological. The distension-contraction plots, which determines the luminal CSA and contraction simultaneously during primary peristalsis is ideally suited to study the pathophysiology of esophageal motility disorders. Several studies from my laboratory show that in patients with nutcracker esophagus, EGJOO and normal HRM, the esophagus distends significantly less than that of normal subjects during primary peristalsis. I suspect that an esophageal contraction pushing bolus through a narrow lumen esophagus is the cause of dysphagia sensation in many patients that have been labeled as functional dysphagia.
The last 2 decades have seen significant progress in the diagnosis of esophageal motility disorders using HRM, Endoflip, and distension-contraction plots of peristalsis. Furthermore, endoscopic treatment of achalasia and “achalasia-like syndromes” is revolutionary. What is desperately needed is an understanding of the pathogenesis of esophageal motor disorders, pharmacotherapy of esophageal symptoms, such as chest pain, proton pump inhibitor–resistant heartburn, and others because dysfunctional esophagus is a huge burden on health care expenditures worldwide.
Ravinder K. Mittal, MD, is a professor of medicine and gastroenterologist with UC San Diego Health. He has patent application pending on the computer software Dplots.
16% of the U.S. population experience dysphagia, only half of whom seek medical care and the others manage their symptoms by modifying diet.
X-ray barium swallow and endoscopy with biopsy to exclude eosinophilic esophagitis are the initial tests for dysphagia diagnosis. If the above are normal, a high-resolution esophageal manometry impedance (HRMZ) is recommended to diagnose primary and secondary esophageal motility disorder.
However, only in a minority of patients is it likely to cause dysphagia because uncontrolled studies show that therapeutic strategies to address EGJOO (botox, dilation, and myotomy) relieve dysphagia symptoms in a minority of patients. Hence, in significant number of patients the cause of dysphagia symptoms remains obscure. It might be that our testing is inadequate, or possibly, patients have functional dysphagia (sensory dysfunction of the esophagus). My opinion is that it is the former.
The esophagus has only one simple function, that is, to transfer the pharyngeal pump driven, that is, swallowed contents to the stomach, for which its luminal cross-sectional area must be larger than that of the swallowed bolus and contraction (measured by manometry) behind the bolus must be of adequate strength. The latter is likely less relevant because humans eat in the upright position and gravity provides propulsion for the bolus. Stated simply, as long as esophagus can distend well and there is no resistance to the outflow at the EGJ, esophagus can achieve its goal. However, until recently, there was no single test to determine the distension and contraction, the two essential elements of primary esophageal peristalsis.
Endoscopy and x-ray barium swallow are tests to determine the luminal diameter but have limitations. Endoflip measures the opening function of the EGJ and is useful when the HRM is normal. However, pressures that are currently being used to measure the EGJ distensibility by Endoflip are not physiological. Furthermore, esophageal body motor function assessed by a bag that distends a long segment of the esophagus under high pressure is unphysiological. The distension-contraction plots, which determines the luminal CSA and contraction simultaneously during primary peristalsis is ideally suited to study the pathophysiology of esophageal motility disorders. Several studies from my laboratory show that in patients with nutcracker esophagus, EGJOO and normal HRM, the esophagus distends significantly less than that of normal subjects during primary peristalsis. I suspect that an esophageal contraction pushing bolus through a narrow lumen esophagus is the cause of dysphagia sensation in many patients that have been labeled as functional dysphagia.
The last 2 decades have seen significant progress in the diagnosis of esophageal motility disorders using HRM, Endoflip, and distension-contraction plots of peristalsis. Furthermore, endoscopic treatment of achalasia and “achalasia-like syndromes” is revolutionary. What is desperately needed is an understanding of the pathogenesis of esophageal motor disorders, pharmacotherapy of esophageal symptoms, such as chest pain, proton pump inhibitor–resistant heartburn, and others because dysfunctional esophagus is a huge burden on health care expenditures worldwide.
Ravinder K. Mittal, MD, is a professor of medicine and gastroenterologist with UC San Diego Health. He has patent application pending on the computer software Dplots.
16% of the U.S. population experience dysphagia, only half of whom seek medical care and the others manage their symptoms by modifying diet.
X-ray barium swallow and endoscopy with biopsy to exclude eosinophilic esophagitis are the initial tests for dysphagia diagnosis. If the above are normal, a high-resolution esophageal manometry impedance (HRMZ) is recommended to diagnose primary and secondary esophageal motility disorder.
However, only in a minority of patients is it likely to cause dysphagia because uncontrolled studies show that therapeutic strategies to address EGJOO (botox, dilation, and myotomy) relieve dysphagia symptoms in a minority of patients. Hence, in significant number of patients the cause of dysphagia symptoms remains obscure. It might be that our testing is inadequate, or possibly, patients have functional dysphagia (sensory dysfunction of the esophagus). My opinion is that it is the former.
The esophagus has only one simple function, that is, to transfer the pharyngeal pump driven, that is, swallowed contents to the stomach, for which its luminal cross-sectional area must be larger than that of the swallowed bolus and contraction (measured by manometry) behind the bolus must be of adequate strength. The latter is likely less relevant because humans eat in the upright position and gravity provides propulsion for the bolus. Stated simply, as long as esophagus can distend well and there is no resistance to the outflow at the EGJ, esophagus can achieve its goal. However, until recently, there was no single test to determine the distension and contraction, the two essential elements of primary esophageal peristalsis.
Endoscopy and x-ray barium swallow are tests to determine the luminal diameter but have limitations. Endoflip measures the opening function of the EGJ and is useful when the HRM is normal. However, pressures that are currently being used to measure the EGJ distensibility by Endoflip are not physiological. Furthermore, esophageal body motor function assessed by a bag that distends a long segment of the esophagus under high pressure is unphysiological. The distension-contraction plots, which determines the luminal CSA and contraction simultaneously during primary peristalsis is ideally suited to study the pathophysiology of esophageal motility disorders. Several studies from my laboratory show that in patients with nutcracker esophagus, EGJOO and normal HRM, the esophagus distends significantly less than that of normal subjects during primary peristalsis. I suspect that an esophageal contraction pushing bolus through a narrow lumen esophagus is the cause of dysphagia sensation in many patients that have been labeled as functional dysphagia.
The last 2 decades have seen significant progress in the diagnosis of esophageal motility disorders using HRM, Endoflip, and distension-contraction plots of peristalsis. Furthermore, endoscopic treatment of achalasia and “achalasia-like syndromes” is revolutionary. What is desperately needed is an understanding of the pathogenesis of esophageal motor disorders, pharmacotherapy of esophageal symptoms, such as chest pain, proton pump inhibitor–resistant heartburn, and others because dysfunctional esophagus is a huge burden on health care expenditures worldwide.
Ravinder K. Mittal, MD, is a professor of medicine and gastroenterologist with UC San Diego Health. He has patent application pending on the computer software Dplots.
at a pace that has left the line-tracing technology considered to have debatable merit just 15 years ago “now as obsolete as a typewriter,” experts said recently in a review in Gastro Hep Advances.
“We have come to conceptualize esophageal motility disorders by specific aspects of physiological dysfunction,” wrote a trio of experts – Peter Kahrilas, MD, professor of medicine; Dustin Carlson, MD, MS, assistant professor of medicine, and John Pandolfino, MD, chief of gastroenterology and hepatology, all at Northwestern University, Chicago. “A major implication of this approach is a shift in management strategy toward rendering treatment in a phenotype-specific manner.”
High-resolution manometry (HRM) was trail-blazing, they said, as it replaced line-tracing manometry in evaluating the motility of the esophagus. HRM led to the subtyping of achalasia based on the three patterns of pressurization in the esophagus that are associated with obstruction at the esophagogastric junction. But the field has continued to advance.
“It has since become clear that obstructive physiology also occurs in syndromes besides achalasia involving the esophagogastric junction and/or distal esophagus,” Dr. Kahrilas, Dr. Carlson, and Dr. Pandolfino said. “In fact, obstructive physiology is increasingly recognized as the fundamental abnormality leading to the perception of dysphagia with esophageal motility disorders. This concept of obstructive physiology as the fundamental abnormality has substantially morphed the clinical management of esophageal motility disorders.”
HRM, has many limitations, but in cases of an uncertain achalasia diagnosis, functional luminal imaging probe (FLIP) technology can help, they said. FLIP can also help surgeons tailor myotomy procedures.
In FLIP, a probe is carefully filled with fluid, causing distension of the esophagus. In the test, the distensibility of the esophagogastric junction is measured. The procedure allows a more refined assessment of the movement of the esophagus, and the subtypes of achalasia.
Identifying the achalasia subtype is crucial to choosing the right treatment, data suggests. There have been no randomized controlled trials on achalasia management that prospectively consider achalasia subtype, but retrospective analysis of RCT data “suggests that achalasia subtypes are of great relevance in forecasting treatment effectiveness,” they said.
In one trial, pneumatic dilation was effective in 100% of type II achalasia, which involves panesophageal pressurization, significantly better than laparoscopic Heller myotomy (LHM). But it was much less effective than LHM in type III achalasia, the spastic form, although a significance couldn’t be established because of the number of cases. Data from a meta-analysis showed that peroral endoscopic myotomy, which allows for a longer myotomy if needed, was better than LHM for classic achalasia and spastic achalasia and was most efficacious overall.
The writers said that the diagnostic classifications for achalasia are likely to continue to evolve, pointing to the dynamic nature of the Chicago Classification for the disorder.
“The fact that it has now gone through four iterations since 2008 emphasizes that this is a work in progress and that no classification scheme of esophageal motility disorders based on a single test will ever be perfect,” they said. “After all, there are no biomarkers of esophageal motility disorders and, in the absence of a biomarker, there can be no ‘gold standard’ for diagnosis.”
Dr. Pandolfino, Dr. Kahrilas, and Northwestern University hold shared intellectual property rights and ownership surrounding FLIP Panometry systems, methods, and apparatus with Medtronic. Dr. Kahrilas reported consulting with Ironwood, Reckitt, and Phathom. Dr. Carlson reported conflicts of interest with Medtronic and Phathom Pharmaceuticals. Dr. Pandolfino reported conflicts of interest with Sandhill Scientific/Diversatek, Takeda, AstraZeneca, Medtronic, Torax, and Ironwood.
at a pace that has left the line-tracing technology considered to have debatable merit just 15 years ago “now as obsolete as a typewriter,” experts said recently in a review in Gastro Hep Advances.
“We have come to conceptualize esophageal motility disorders by specific aspects of physiological dysfunction,” wrote a trio of experts – Peter Kahrilas, MD, professor of medicine; Dustin Carlson, MD, MS, assistant professor of medicine, and John Pandolfino, MD, chief of gastroenterology and hepatology, all at Northwestern University, Chicago. “A major implication of this approach is a shift in management strategy toward rendering treatment in a phenotype-specific manner.”
High-resolution manometry (HRM) was trail-blazing, they said, as it replaced line-tracing manometry in evaluating the motility of the esophagus. HRM led to the subtyping of achalasia based on the three patterns of pressurization in the esophagus that are associated with obstruction at the esophagogastric junction. But the field has continued to advance.
“It has since become clear that obstructive physiology also occurs in syndromes besides achalasia involving the esophagogastric junction and/or distal esophagus,” Dr. Kahrilas, Dr. Carlson, and Dr. Pandolfino said. “In fact, obstructive physiology is increasingly recognized as the fundamental abnormality leading to the perception of dysphagia with esophageal motility disorders. This concept of obstructive physiology as the fundamental abnormality has substantially morphed the clinical management of esophageal motility disorders.”
HRM, has many limitations, but in cases of an uncertain achalasia diagnosis, functional luminal imaging probe (FLIP) technology can help, they said. FLIP can also help surgeons tailor myotomy procedures.
In FLIP, a probe is carefully filled with fluid, causing distension of the esophagus. In the test, the distensibility of the esophagogastric junction is measured. The procedure allows a more refined assessment of the movement of the esophagus, and the subtypes of achalasia.
Identifying the achalasia subtype is crucial to choosing the right treatment, data suggests. There have been no randomized controlled trials on achalasia management that prospectively consider achalasia subtype, but retrospective analysis of RCT data “suggests that achalasia subtypes are of great relevance in forecasting treatment effectiveness,” they said.
In one trial, pneumatic dilation was effective in 100% of type II achalasia, which involves panesophageal pressurization, significantly better than laparoscopic Heller myotomy (LHM). But it was much less effective than LHM in type III achalasia, the spastic form, although a significance couldn’t be established because of the number of cases. Data from a meta-analysis showed that peroral endoscopic myotomy, which allows for a longer myotomy if needed, was better than LHM for classic achalasia and spastic achalasia and was most efficacious overall.
The writers said that the diagnostic classifications for achalasia are likely to continue to evolve, pointing to the dynamic nature of the Chicago Classification for the disorder.
“The fact that it has now gone through four iterations since 2008 emphasizes that this is a work in progress and that no classification scheme of esophageal motility disorders based on a single test will ever be perfect,” they said. “After all, there are no biomarkers of esophageal motility disorders and, in the absence of a biomarker, there can be no ‘gold standard’ for diagnosis.”
Dr. Pandolfino, Dr. Kahrilas, and Northwestern University hold shared intellectual property rights and ownership surrounding FLIP Panometry systems, methods, and apparatus with Medtronic. Dr. Kahrilas reported consulting with Ironwood, Reckitt, and Phathom. Dr. Carlson reported conflicts of interest with Medtronic and Phathom Pharmaceuticals. Dr. Pandolfino reported conflicts of interest with Sandhill Scientific/Diversatek, Takeda, AstraZeneca, Medtronic, Torax, and Ironwood.
FROM GASTRO HEP ADVANCES
Can ChatGPT help clinicians manage GERD?
managing gastroesophageal reflux disease (GERD), investigators have found.
The researchers say the tool’s conversational format could improve clinical efficiency and reduce the volume of patient messages and calls, potentially diminishing clinician burnout.
However, inconsistencies and content errors observed require a certain level of clinical oversight, caution the researchers, led by Jacqueline Henson, MD, with the division of gastroenterology, Duke University, Durham, N.C.
The study was published online in the American Journal of Gastroenterology.
Putting ChatGPT to the GERD test
Affecting nearly 30% of U.S. adults, GERD is a common and increasingly complex condition to manage. AI technologies like ChatGPT (Open AI/Microsoft) have demonstrated an increasing role in medicine, although the ability of ChatGPT to provide guidance for GERD management is uncertain.
Dr. Henson and colleagues assessed ChatGPT’s ability to provide accurate and specific responses to questions regarding GERD care.
They generated 23 GERD management prompts based on published clinical guidelines and expert consensus recommendations. Five questions were about diagnosis, eleven on treatment, and seven on both diagnosis and treatment.
Each prompt was submitted to ChatGPT 3.5 (version 3/14/2023) three times on separate occasions without feedback to assess the consistency of the answer. Responses were rated by three board-certified gastroenterologists for appropriateness and specificity.
ChatGPT returned appropriate responses to 63 of 69 (91.3%) queries, with 29% considered completely appropriate and 62.3% mostly appropriate.
However, responses to the same prompt were often inconsistent, with 16 of 23 (70%) prompts yielding varying appropriateness, including three (13%) with both inappropriate and appropriate responses.
Prompts regarding treatment received the highest proportion of completely appropriate responses (39.4%), while prompts for diagnosis and management had the highest proportion of mostly inappropriate responses (14.3%).
For example, the chatbot failed to recommend consideration of Roux-en-Y gastric bypass for ongoing GERD symptoms with pathologic acid exposure in the setting of obesity, and some potential risks associated with proton pump inhibitor therapy were stated as fact.
However, the majority (78.3%) of responses contained at least some specific guidance, especially for prompts assessing diagnosis (93.3%). In all responses, ChatGPT suggested contacting a health care professional for further advice.
Eight patients from a range of educational backgrounds who provided feedback on the responses generally felt that the ChatGPT responses were both understandable and useful.
Overall, ChatGPT “provided largely appropriate and at least some specific guidance for GERD management, highlighting the potential for this technology to serve as a source of information for patients, as well as an aid for clinicians,” Dr. Henson and colleagues write.
However, “the presence of inappropriate responses with inconsistencies to the same prompt largely preclude its application within health care in its present state, at least for GERD,” they add.
The study had no commercial funding. Dr. Henson has served as a consultant for Medtronic.
A version of this article first appeared on Medscape.com.
managing gastroesophageal reflux disease (GERD), investigators have found.
The researchers say the tool’s conversational format could improve clinical efficiency and reduce the volume of patient messages and calls, potentially diminishing clinician burnout.
However, inconsistencies and content errors observed require a certain level of clinical oversight, caution the researchers, led by Jacqueline Henson, MD, with the division of gastroenterology, Duke University, Durham, N.C.
The study was published online in the American Journal of Gastroenterology.
Putting ChatGPT to the GERD test
Affecting nearly 30% of U.S. adults, GERD is a common and increasingly complex condition to manage. AI technologies like ChatGPT (Open AI/Microsoft) have demonstrated an increasing role in medicine, although the ability of ChatGPT to provide guidance for GERD management is uncertain.
Dr. Henson and colleagues assessed ChatGPT’s ability to provide accurate and specific responses to questions regarding GERD care.
They generated 23 GERD management prompts based on published clinical guidelines and expert consensus recommendations. Five questions were about diagnosis, eleven on treatment, and seven on both diagnosis and treatment.
Each prompt was submitted to ChatGPT 3.5 (version 3/14/2023) three times on separate occasions without feedback to assess the consistency of the answer. Responses were rated by three board-certified gastroenterologists for appropriateness and specificity.
ChatGPT returned appropriate responses to 63 of 69 (91.3%) queries, with 29% considered completely appropriate and 62.3% mostly appropriate.
However, responses to the same prompt were often inconsistent, with 16 of 23 (70%) prompts yielding varying appropriateness, including three (13%) with both inappropriate and appropriate responses.
Prompts regarding treatment received the highest proportion of completely appropriate responses (39.4%), while prompts for diagnosis and management had the highest proportion of mostly inappropriate responses (14.3%).
For example, the chatbot failed to recommend consideration of Roux-en-Y gastric bypass for ongoing GERD symptoms with pathologic acid exposure in the setting of obesity, and some potential risks associated with proton pump inhibitor therapy were stated as fact.
However, the majority (78.3%) of responses contained at least some specific guidance, especially for prompts assessing diagnosis (93.3%). In all responses, ChatGPT suggested contacting a health care professional for further advice.
Eight patients from a range of educational backgrounds who provided feedback on the responses generally felt that the ChatGPT responses were both understandable and useful.
Overall, ChatGPT “provided largely appropriate and at least some specific guidance for GERD management, highlighting the potential for this technology to serve as a source of information for patients, as well as an aid for clinicians,” Dr. Henson and colleagues write.
However, “the presence of inappropriate responses with inconsistencies to the same prompt largely preclude its application within health care in its present state, at least for GERD,” they add.
The study had no commercial funding. Dr. Henson has served as a consultant for Medtronic.
A version of this article first appeared on Medscape.com.
managing gastroesophageal reflux disease (GERD), investigators have found.
The researchers say the tool’s conversational format could improve clinical efficiency and reduce the volume of patient messages and calls, potentially diminishing clinician burnout.
However, inconsistencies and content errors observed require a certain level of clinical oversight, caution the researchers, led by Jacqueline Henson, MD, with the division of gastroenterology, Duke University, Durham, N.C.
The study was published online in the American Journal of Gastroenterology.
Putting ChatGPT to the GERD test
Affecting nearly 30% of U.S. adults, GERD is a common and increasingly complex condition to manage. AI technologies like ChatGPT (Open AI/Microsoft) have demonstrated an increasing role in medicine, although the ability of ChatGPT to provide guidance for GERD management is uncertain.
Dr. Henson and colleagues assessed ChatGPT’s ability to provide accurate and specific responses to questions regarding GERD care.
They generated 23 GERD management prompts based on published clinical guidelines and expert consensus recommendations. Five questions were about diagnosis, eleven on treatment, and seven on both diagnosis and treatment.
Each prompt was submitted to ChatGPT 3.5 (version 3/14/2023) three times on separate occasions without feedback to assess the consistency of the answer. Responses were rated by three board-certified gastroenterologists for appropriateness and specificity.
ChatGPT returned appropriate responses to 63 of 69 (91.3%) queries, with 29% considered completely appropriate and 62.3% mostly appropriate.
However, responses to the same prompt were often inconsistent, with 16 of 23 (70%) prompts yielding varying appropriateness, including three (13%) with both inappropriate and appropriate responses.
Prompts regarding treatment received the highest proportion of completely appropriate responses (39.4%), while prompts for diagnosis and management had the highest proportion of mostly inappropriate responses (14.3%).
For example, the chatbot failed to recommend consideration of Roux-en-Y gastric bypass for ongoing GERD symptoms with pathologic acid exposure in the setting of obesity, and some potential risks associated with proton pump inhibitor therapy were stated as fact.
However, the majority (78.3%) of responses contained at least some specific guidance, especially for prompts assessing diagnosis (93.3%). In all responses, ChatGPT suggested contacting a health care professional for further advice.
Eight patients from a range of educational backgrounds who provided feedback on the responses generally felt that the ChatGPT responses were both understandable and useful.
Overall, ChatGPT “provided largely appropriate and at least some specific guidance for GERD management, highlighting the potential for this technology to serve as a source of information for patients, as well as an aid for clinicians,” Dr. Henson and colleagues write.
However, “the presence of inappropriate responses with inconsistencies to the same prompt largely preclude its application within health care in its present state, at least for GERD,” they add.
The study had no commercial funding. Dr. Henson has served as a consultant for Medtronic.
A version of this article first appeared on Medscape.com.
Eosinophilic esophagitis: A year in review
At the AGA postgraduate course in May, we highlighted recent noteworthy randomized controlled trials (RCT) using eosinophil-targeting biologic therapy, esophageal-optimized corticosteroid preparations, and dietary elimination in EoE.
Dupilumab, a monoclonal antibody that blocks interleukin-4 and IL-13 signaling, was tested in a phase 3 trial for adults and adolescents with EoE.1 In this double-blind, randomized, placebo-controlled trial, the efficacy of subcutaneous dupilumab 300 mg weekly or every other week was compared against placebo. Stringent histologic remission (≤ 6 eosinophils/high power field) occurred in approximately 60% who received dupilumab (either dose) versus 5% in placebo. However, significant symptom improvement was seen only with 300 g weekly dupilumab.
On the topical corticosteroid front, the results of two RCTs using fluticasone orally disintegrating tablet (APT-1011) and budesonide oral suspension (BOS) were published. In the APT-1011 phase 2b trial, patients were randomized to receive 1.5 mg or 3 mg daily or b.i.d. versus placebo for 12 weeks.2 High histologic response rates and improvement in dysphagia frequency were seen with all ≥ 3-mg daily-dose APT-1011, compared with placebo. However, adverse events (that is, candidiasis) were highest among those on 3 mg b.i.d. Thus, 3 mg daily APT-1011 was thought to offer the most favorable risk-benefit profile. In the BOS phase 3 trial, patients were randomized 2:1 to received BOS 2 mg b.i.d. or placebo for 12 weeks.3 BOS was superior to placebo in histologic, symptomatic, and endoscopic outcomes.
Diet remains the only therapy targeting the cause of EoE and offers a potential drug-free remission. In the randomized, open label trial of 1- versus 6-food elimination diet, adult patients were allocated 1:1 to 1FED (animal milk) or 6FED (animal milk, wheat, egg, soy, fish/shellfish, and peanuts/tree nuts) for 6 weeks.4 No significant difference in partial or stringent remission was found between the two groups. Step-up therapy resulted in an additional 43% histologic response in those who underwent 6FED after failing 1FED and 82% histologic response in those who received swallowed fluticasone 880 mcg b.i.d after failing 6FED. Hence, eliminating animal milk alone in a step-up treatment approach is reasonable.
We have witnessed major progress to expand EoE treatment options in the last year. Long-term efficacy and side-effect data, as well as studies comparing between therapies are needed to improve shared decision-making and strategies to implement tailored care in EoE.
Dr. Chen is with the division of gastroenterology and hepatology, department of internal medicine at the University of Michigan, Ann Arbor. She disclosed consultancy work with Phathom Pharmaceuticals.
References
1. Dellon ES et al. N Engl J Med. 2022;387(25):2317-30.
2. Dellon ES et al. Clin Gastroenterol Hepatol. 2022;20(11):2485-94e15.
3. Hirano I et al. Budesonide. Clin Gastroenterol Hepatol. 2022;20(3):525-34e10.
4. Kliewer KL et al. Lancet Gastroenterol Hepatol. 2023;8(5):408-21.
At the AGA postgraduate course in May, we highlighted recent noteworthy randomized controlled trials (RCT) using eosinophil-targeting biologic therapy, esophageal-optimized corticosteroid preparations, and dietary elimination in EoE.
Dupilumab, a monoclonal antibody that blocks interleukin-4 and IL-13 signaling, was tested in a phase 3 trial for adults and adolescents with EoE.1 In this double-blind, randomized, placebo-controlled trial, the efficacy of subcutaneous dupilumab 300 mg weekly or every other week was compared against placebo. Stringent histologic remission (≤ 6 eosinophils/high power field) occurred in approximately 60% who received dupilumab (either dose) versus 5% in placebo. However, significant symptom improvement was seen only with 300 g weekly dupilumab.
On the topical corticosteroid front, the results of two RCTs using fluticasone orally disintegrating tablet (APT-1011) and budesonide oral suspension (BOS) were published. In the APT-1011 phase 2b trial, patients were randomized to receive 1.5 mg or 3 mg daily or b.i.d. versus placebo for 12 weeks.2 High histologic response rates and improvement in dysphagia frequency were seen with all ≥ 3-mg daily-dose APT-1011, compared with placebo. However, adverse events (that is, candidiasis) were highest among those on 3 mg b.i.d. Thus, 3 mg daily APT-1011 was thought to offer the most favorable risk-benefit profile. In the BOS phase 3 trial, patients were randomized 2:1 to received BOS 2 mg b.i.d. or placebo for 12 weeks.3 BOS was superior to placebo in histologic, symptomatic, and endoscopic outcomes.
Diet remains the only therapy targeting the cause of EoE and offers a potential drug-free remission. In the randomized, open label trial of 1- versus 6-food elimination diet, adult patients were allocated 1:1 to 1FED (animal milk) or 6FED (animal milk, wheat, egg, soy, fish/shellfish, and peanuts/tree nuts) for 6 weeks.4 No significant difference in partial or stringent remission was found between the two groups. Step-up therapy resulted in an additional 43% histologic response in those who underwent 6FED after failing 1FED and 82% histologic response in those who received swallowed fluticasone 880 mcg b.i.d after failing 6FED. Hence, eliminating animal milk alone in a step-up treatment approach is reasonable.
We have witnessed major progress to expand EoE treatment options in the last year. Long-term efficacy and side-effect data, as well as studies comparing between therapies are needed to improve shared decision-making and strategies to implement tailored care in EoE.
Dr. Chen is with the division of gastroenterology and hepatology, department of internal medicine at the University of Michigan, Ann Arbor. She disclosed consultancy work with Phathom Pharmaceuticals.
References
1. Dellon ES et al. N Engl J Med. 2022;387(25):2317-30.
2. Dellon ES et al. Clin Gastroenterol Hepatol. 2022;20(11):2485-94e15.
3. Hirano I et al. Budesonide. Clin Gastroenterol Hepatol. 2022;20(3):525-34e10.
4. Kliewer KL et al. Lancet Gastroenterol Hepatol. 2023;8(5):408-21.
At the AGA postgraduate course in May, we highlighted recent noteworthy randomized controlled trials (RCT) using eosinophil-targeting biologic therapy, esophageal-optimized corticosteroid preparations, and dietary elimination in EoE.
Dupilumab, a monoclonal antibody that blocks interleukin-4 and IL-13 signaling, was tested in a phase 3 trial for adults and adolescents with EoE.1 In this double-blind, randomized, placebo-controlled trial, the efficacy of subcutaneous dupilumab 300 mg weekly or every other week was compared against placebo. Stringent histologic remission (≤ 6 eosinophils/high power field) occurred in approximately 60% who received dupilumab (either dose) versus 5% in placebo. However, significant symptom improvement was seen only with 300 g weekly dupilumab.
On the topical corticosteroid front, the results of two RCTs using fluticasone orally disintegrating tablet (APT-1011) and budesonide oral suspension (BOS) were published. In the APT-1011 phase 2b trial, patients were randomized to receive 1.5 mg or 3 mg daily or b.i.d. versus placebo for 12 weeks.2 High histologic response rates and improvement in dysphagia frequency were seen with all ≥ 3-mg daily-dose APT-1011, compared with placebo. However, adverse events (that is, candidiasis) were highest among those on 3 mg b.i.d. Thus, 3 mg daily APT-1011 was thought to offer the most favorable risk-benefit profile. In the BOS phase 3 trial, patients were randomized 2:1 to received BOS 2 mg b.i.d. or placebo for 12 weeks.3 BOS was superior to placebo in histologic, symptomatic, and endoscopic outcomes.
Diet remains the only therapy targeting the cause of EoE and offers a potential drug-free remission. In the randomized, open label trial of 1- versus 6-food elimination diet, adult patients were allocated 1:1 to 1FED (animal milk) or 6FED (animal milk, wheat, egg, soy, fish/shellfish, and peanuts/tree nuts) for 6 weeks.4 No significant difference in partial or stringent remission was found between the two groups. Step-up therapy resulted in an additional 43% histologic response in those who underwent 6FED after failing 1FED and 82% histologic response in those who received swallowed fluticasone 880 mcg b.i.d after failing 6FED. Hence, eliminating animal milk alone in a step-up treatment approach is reasonable.
We have witnessed major progress to expand EoE treatment options in the last year. Long-term efficacy and side-effect data, as well as studies comparing between therapies are needed to improve shared decision-making and strategies to implement tailored care in EoE.
Dr. Chen is with the division of gastroenterology and hepatology, department of internal medicine at the University of Michigan, Ann Arbor. She disclosed consultancy work with Phathom Pharmaceuticals.
References
1. Dellon ES et al. N Engl J Med. 2022;387(25):2317-30.
2. Dellon ES et al. Clin Gastroenterol Hepatol. 2022;20(11):2485-94e15.
3. Hirano I et al. Budesonide. Clin Gastroenterol Hepatol. 2022;20(3):525-34e10.
4. Kliewer KL et al. Lancet Gastroenterol Hepatol. 2023;8(5):408-21.
Esophageal diseases: Key new concepts
CHICAGO – These include novel care approaches for esophageal diseases that were published in recent AGA best practice updates on gastroesophageal reflux disease (GERD), extraesophageal reflux, and Barrett’s esophagus, as well as randomized clinical trial data examining therapeutic approaches for erosive esophagitis and eosinophilic esophagitis.
Here are a few highlights: Complications of chronic gastroesophageal reflux include erosive esophagitis for which healing and maintenance of healing is crucial to reduce further erosive sequelae. Healing is typically achieved with pump inhibitor (PPI) therapy. Potassium competitive acid blockers are active prodrugs that bind to the H+/K+ ATPase and have been demonstrated to have a more potent and faster onset in suppressing gastric acid secretion, compared with PPIs.
In a recent phase 3 randomized trial of more than 1,000 adults with erosive esophagitis, the potassium competitive acid blocker vonoprazan was found to be noninferior to lansoprazole in inducing and maintaining healing of erosive esophagitis. Overall, the proportions of subjects that achieved healing by week 8 and maintained healing up to 24 weeks were higher with vonoprazan, when compared with lansoprazole, with a greater treatment effect seen in subjects with severe erosive esophagitis (Los Angeles grade C or D) (Laine L et al. Gastroenterology. Jan 2023;164[1]:61-71).
Screening patients at risk of Barrett’s esophagus (BE), another erosive sequelae of chronic GERD, is critical for early detection and prevention of esophageal cancer. Upper GI endoscopy is standard for Barrett’s screening; however, screening rates of at-risk populations are suboptimal.
In a recent retrospective analysis of a multipractice health care network, only 39% of a screen-eligible population were noted to have undergone upper GI endoscopy. These findings highlight the critical need to improve screening for Barrett’s, including potential of the newer nonendoscopic screening modalities such as swallowable capsule devices combined with a biomarker or cell-collection devices, as well as the need for risk stratification/prediction tools and collaboration with primary care physicians (Eluri S et al. Am J Gastroenterol. Nov 2022;117[11]:1764-71).
Therapeutic options for eosinophilic esophagitis (EoE) have expanded over the past year. Randomized trials demonstrate the efficacy of varied therapeutic approaches including the monoclonal antibody dupilumab as well as topical corticosteroids such as fluticasone propionate orally disintegrated tablet and budesonide oral suspension.
In terms of food elimination diets, a recent multicenter randomized open-label trial identified comparable rates of partial histologic remission with both a traditional six-food elimination diet and a one-food animal milk elimination diet in patients with EoE, though those treated with a six-food elimination were more likely to achieve complete remission (< 1 eosinophil/high power field). Results suggest elimination of animal milk alone is an acceptable initial dietary therapy for EoE, with potential to convert to six-food elimination or alternative therapy when histologic response is not achieved (Kliewer K. Lancet Gastroenterol Hepatol. [published online Feb 2023]).
Dr. Yadlapati is an associate professor in gastroenterology at the University of California, San Diego. She disclosed relationships with Medtronic (Institutional), Ironwood Pharmaceuticals (Institutional), Phathom Pharmaceuticals, and Ironwood Pharmaceuticals. She serves on the advisory board with stock options for RJS Mediagnostix.
These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – These include novel care approaches for esophageal diseases that were published in recent AGA best practice updates on gastroesophageal reflux disease (GERD), extraesophageal reflux, and Barrett’s esophagus, as well as randomized clinical trial data examining therapeutic approaches for erosive esophagitis and eosinophilic esophagitis.
Here are a few highlights: Complications of chronic gastroesophageal reflux include erosive esophagitis for which healing and maintenance of healing is crucial to reduce further erosive sequelae. Healing is typically achieved with pump inhibitor (PPI) therapy. Potassium competitive acid blockers are active prodrugs that bind to the H+/K+ ATPase and have been demonstrated to have a more potent and faster onset in suppressing gastric acid secretion, compared with PPIs.
In a recent phase 3 randomized trial of more than 1,000 adults with erosive esophagitis, the potassium competitive acid blocker vonoprazan was found to be noninferior to lansoprazole in inducing and maintaining healing of erosive esophagitis. Overall, the proportions of subjects that achieved healing by week 8 and maintained healing up to 24 weeks were higher with vonoprazan, when compared with lansoprazole, with a greater treatment effect seen in subjects with severe erosive esophagitis (Los Angeles grade C or D) (Laine L et al. Gastroenterology. Jan 2023;164[1]:61-71).
Screening patients at risk of Barrett’s esophagus (BE), another erosive sequelae of chronic GERD, is critical for early detection and prevention of esophageal cancer. Upper GI endoscopy is standard for Barrett’s screening; however, screening rates of at-risk populations are suboptimal.
In a recent retrospective analysis of a multipractice health care network, only 39% of a screen-eligible population were noted to have undergone upper GI endoscopy. These findings highlight the critical need to improve screening for Barrett’s, including potential of the newer nonendoscopic screening modalities such as swallowable capsule devices combined with a biomarker or cell-collection devices, as well as the need for risk stratification/prediction tools and collaboration with primary care physicians (Eluri S et al. Am J Gastroenterol. Nov 2022;117[11]:1764-71).
Therapeutic options for eosinophilic esophagitis (EoE) have expanded over the past year. Randomized trials demonstrate the efficacy of varied therapeutic approaches including the monoclonal antibody dupilumab as well as topical corticosteroids such as fluticasone propionate orally disintegrated tablet and budesonide oral suspension.
In terms of food elimination diets, a recent multicenter randomized open-label trial identified comparable rates of partial histologic remission with both a traditional six-food elimination diet and a one-food animal milk elimination diet in patients with EoE, though those treated with a six-food elimination were more likely to achieve complete remission (< 1 eosinophil/high power field). Results suggest elimination of animal milk alone is an acceptable initial dietary therapy for EoE, with potential to convert to six-food elimination or alternative therapy when histologic response is not achieved (Kliewer K. Lancet Gastroenterol Hepatol. [published online Feb 2023]).
Dr. Yadlapati is an associate professor in gastroenterology at the University of California, San Diego. She disclosed relationships with Medtronic (Institutional), Ironwood Pharmaceuticals (Institutional), Phathom Pharmaceuticals, and Ironwood Pharmaceuticals. She serves on the advisory board with stock options for RJS Mediagnostix.
These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – These include novel care approaches for esophageal diseases that were published in recent AGA best practice updates on gastroesophageal reflux disease (GERD), extraesophageal reflux, and Barrett’s esophagus, as well as randomized clinical trial data examining therapeutic approaches for erosive esophagitis and eosinophilic esophagitis.
Here are a few highlights: Complications of chronic gastroesophageal reflux include erosive esophagitis for which healing and maintenance of healing is crucial to reduce further erosive sequelae. Healing is typically achieved with pump inhibitor (PPI) therapy. Potassium competitive acid blockers are active prodrugs that bind to the H+/K+ ATPase and have been demonstrated to have a more potent and faster onset in suppressing gastric acid secretion, compared with PPIs.
In a recent phase 3 randomized trial of more than 1,000 adults with erosive esophagitis, the potassium competitive acid blocker vonoprazan was found to be noninferior to lansoprazole in inducing and maintaining healing of erosive esophagitis. Overall, the proportions of subjects that achieved healing by week 8 and maintained healing up to 24 weeks were higher with vonoprazan, when compared with lansoprazole, with a greater treatment effect seen in subjects with severe erosive esophagitis (Los Angeles grade C or D) (Laine L et al. Gastroenterology. Jan 2023;164[1]:61-71).
Screening patients at risk of Barrett’s esophagus (BE), another erosive sequelae of chronic GERD, is critical for early detection and prevention of esophageal cancer. Upper GI endoscopy is standard for Barrett’s screening; however, screening rates of at-risk populations are suboptimal.
In a recent retrospective analysis of a multipractice health care network, only 39% of a screen-eligible population were noted to have undergone upper GI endoscopy. These findings highlight the critical need to improve screening for Barrett’s, including potential of the newer nonendoscopic screening modalities such as swallowable capsule devices combined with a biomarker or cell-collection devices, as well as the need for risk stratification/prediction tools and collaboration with primary care physicians (Eluri S et al. Am J Gastroenterol. Nov 2022;117[11]:1764-71).
Therapeutic options for eosinophilic esophagitis (EoE) have expanded over the past year. Randomized trials demonstrate the efficacy of varied therapeutic approaches including the monoclonal antibody dupilumab as well as topical corticosteroids such as fluticasone propionate orally disintegrated tablet and budesonide oral suspension.
In terms of food elimination diets, a recent multicenter randomized open-label trial identified comparable rates of partial histologic remission with both a traditional six-food elimination diet and a one-food animal milk elimination diet in patients with EoE, though those treated with a six-food elimination were more likely to achieve complete remission (< 1 eosinophil/high power field). Results suggest elimination of animal milk alone is an acceptable initial dietary therapy for EoE, with potential to convert to six-food elimination or alternative therapy when histologic response is not achieved (Kliewer K. Lancet Gastroenterol Hepatol. [published online Feb 2023]).
Dr. Yadlapati is an associate professor in gastroenterology at the University of California, San Diego. She disclosed relationships with Medtronic (Institutional), Ironwood Pharmaceuticals (Institutional), Phathom Pharmaceuticals, and Ironwood Pharmaceuticals. She serves on the advisory board with stock options for RJS Mediagnostix.
These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
AT DDW 2023
AGA clinical practice update: Extraesophageal gastroesophageal reflux disease
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.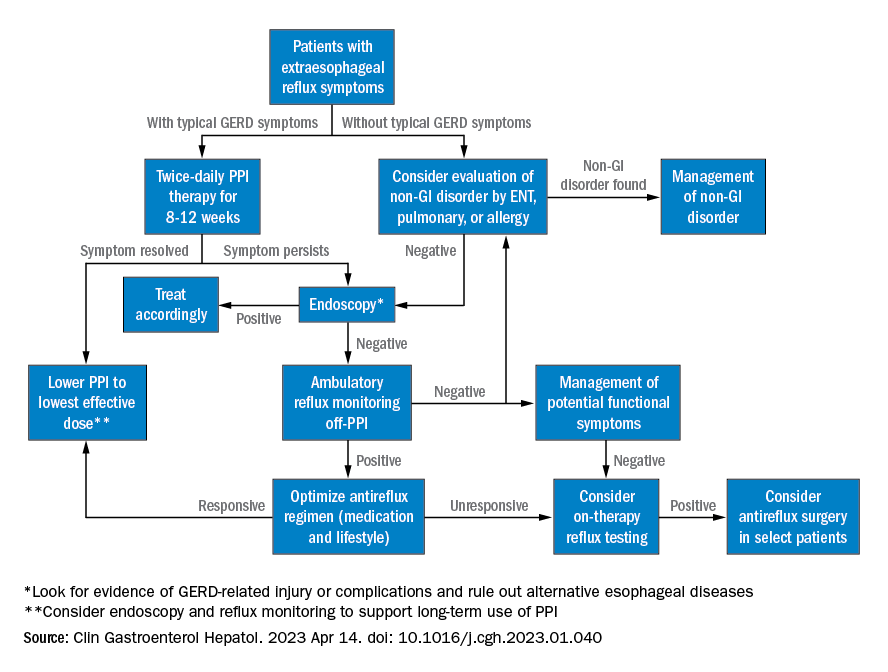
Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.
Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.
Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Approach to dysphagia
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).
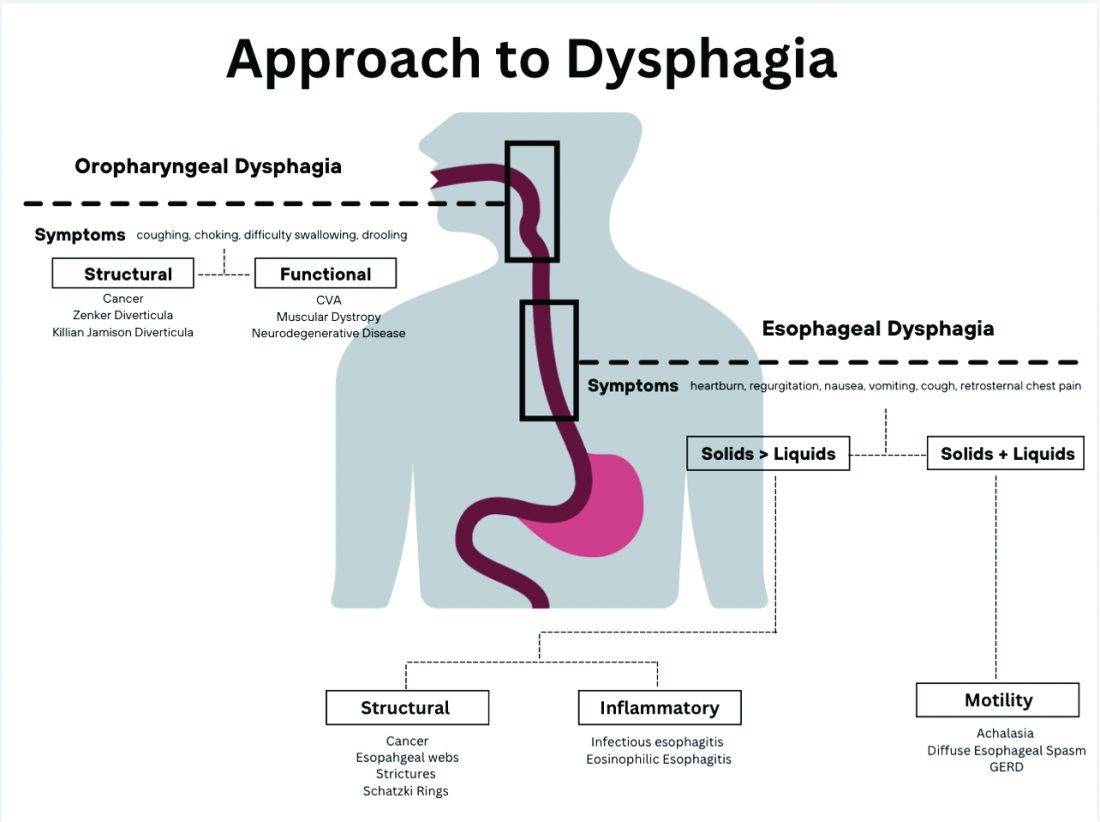
Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).

Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).

Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
COVID raises risk for long-term GI complications
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
EoE: One-food elimination works as well as six-food elimination
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
FROM THE LANCET GASTROENTEROLOGY AND HEPATOLOGY
FDA puts hold on esophagitis, H. pylori drug, owing to impurity concerns
The Food and Drug Administration
The FDA approved the company’s two vonoprazan-based treatments for H. pylori infection, Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), last May.
The target action date of the company’s NDA for vonoprazan for the treatment of erosive esophagitis was Jan. 11, 2023.
In a news release, Phathom said it has received complete response letters from the FDA related to its erosive esophagitis NDA and H. pylori postapproval supplement.
Both letters address specifications and controls for a nitrosamine drug substance–related impurity, N-nitroso-vonoprazan (NVP), which was detected in the initial commercial launch materials of both products.
The FDA letters ask Phathom to provide “additional stability data to demonstrate that levels of the impurity previously found in vonoprazan drug product will remain at or below the daily acceptable intake throughout the proposed shelf life of the product,” Phathom said.
Phathom said it has conducted “extensive root cause investigations regarding the trace levels of the impurity since it was detected and has implemented mitigation measures to control the levels of NVP below the acceptable intake.”
The company expects to meet with the FDA before the end of March to discuss a resubmission plan and a timeline for the vonoprazan products.
Owing to the regulatory delays, Phathom said it no longer expects product launches for H. pylori or erosive esophagitis in the first quarter of 2023.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration
The FDA approved the company’s two vonoprazan-based treatments for H. pylori infection, Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), last May.
The target action date of the company’s NDA for vonoprazan for the treatment of erosive esophagitis was Jan. 11, 2023.
In a news release, Phathom said it has received complete response letters from the FDA related to its erosive esophagitis NDA and H. pylori postapproval supplement.
Both letters address specifications and controls for a nitrosamine drug substance–related impurity, N-nitroso-vonoprazan (NVP), which was detected in the initial commercial launch materials of both products.
The FDA letters ask Phathom to provide “additional stability data to demonstrate that levels of the impurity previously found in vonoprazan drug product will remain at or below the daily acceptable intake throughout the proposed shelf life of the product,” Phathom said.
Phathom said it has conducted “extensive root cause investigations regarding the trace levels of the impurity since it was detected and has implemented mitigation measures to control the levels of NVP below the acceptable intake.”
The company expects to meet with the FDA before the end of March to discuss a resubmission plan and a timeline for the vonoprazan products.
Owing to the regulatory delays, Phathom said it no longer expects product launches for H. pylori or erosive esophagitis in the first quarter of 2023.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration
The FDA approved the company’s two vonoprazan-based treatments for H. pylori infection, Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), last May.
The target action date of the company’s NDA for vonoprazan for the treatment of erosive esophagitis was Jan. 11, 2023.
In a news release, Phathom said it has received complete response letters from the FDA related to its erosive esophagitis NDA and H. pylori postapproval supplement.
Both letters address specifications and controls for a nitrosamine drug substance–related impurity, N-nitroso-vonoprazan (NVP), which was detected in the initial commercial launch materials of both products.
The FDA letters ask Phathom to provide “additional stability data to demonstrate that levels of the impurity previously found in vonoprazan drug product will remain at or below the daily acceptable intake throughout the proposed shelf life of the product,” Phathom said.
Phathom said it has conducted “extensive root cause investigations regarding the trace levels of the impurity since it was detected and has implemented mitigation measures to control the levels of NVP below the acceptable intake.”
The company expects to meet with the FDA before the end of March to discuss a resubmission plan and a timeline for the vonoprazan products.
Owing to the regulatory delays, Phathom said it no longer expects product launches for H. pylori or erosive esophagitis in the first quarter of 2023.
A version of this article first appeared on Medscape.com.
Room for improvement in Barrett esophagus care
, an analysis of U.S. registry data shows.
“As the GI Quality Improvement Consortium (GIQuIC) registry represents the ‘best-case scenario’ for adherence, since sites and endoscopists enrolled in this quality registry are aware that their practices are being monitored, these results indicate that there is still room for improvement and better consistency,” the researchers write.
The study was published online in The American Journal of Gastroenterology.
Quality care in BE, which is a precursor to esophageal adenocarcinoma (EAC), includes adherence to the Seattle biopsy protocol for sampling the BE segment (four-quadrant biopsies every 2 cm) and to a surveillance interval of 3-5 years for patients with nondysplastic BE (NDBE).
Previous studies have found poor adherence to these two QIs, but those studies only provided overall estimates, and individual endoscopists or different sites were not taken into consideration.
Jennifer Kolb, MD, with the University of California, Los Angeles, and colleagues say their study is the first to highlight variation in adherence to these measures at the center and endoscopist levels.
The study is also the first U.S. population–based study to report the dysplasia detection rate (DDR), which is a proposed quality indicator. The findings on this metric also demonstrate marked variability across endoscopists and sites.
Study details
Using the nationwide GIQuIC registry, the researchers evaluated endoscopist and site-based adherence to the Seattle protocol and surveillance interval advice from January 2018 to May 2021.
Among 255 practices with 1,195 endoscopists who performed 20,155 upper endoscopies for suspected or established BE, overall adherence to the Seattle protocol was 86%, which is considerably higher than the 51% reported in a study conducted from 2002 to 2007, Dr. Kolb and colleagues note.
When researchers looked specifically at 572 endoscopists for whom there were at least 10 endoscopy records in the registry, they found high variability in adherence to the Seattle protocol (median, 93.8%; interquartile range, 18.9%).
Adherence to the Seattle protocol was also variable among 153 practices for which there were at least 20 endoscopy records (median, 90%; IQR, 20.1%).
Of the 12,100 upper endoscopies with documented NDBE, 8,517 (70.4%) had a guideline-concordant–recommended surveillance interval of 3-5 years, with variability at both the endoscopist (median, 82.4%; IQR, 36.3%) and site level (median, 77.2%; IQR, 28.9%).
Endoscopist and site adherence to the Seattle protocol and surveillance guidance generally rose along with volume of upper endoscopies performed.
The overall DDR was 3.1%; it varied among endoscopists and sites (mean, 3.3% for both).
The investigators note that the 95% confidence intervals for each provider for DDR were “highly variable” and ranged from –20% to 119.3%. Notably, increasing upper endoscopy volume had an inconsistent effect on adherence rates and DDR by endoscopists and sites.
The investigators saw no correlation between overall DDR and Seattle protocol adherence among sites and only weak but statistically significant negative correlation between DDR and Seattle protocol adherence among individual endoscopists.
Practical approaches to improvement
The researchers say their observations from the GIQuIC database “most accurately represent the real-world experience in Barrett’s endoscopy.”
The results can serve as a “benchmark for quality initiatives and intervention trials aimed at improving outcomes for patients with BE,” they say.
Improving adherence to key QI measures and ensuring more consistent clinical behavior across practice groups and endoscopists are “critical first steps” to ensure high-quality BE care, Dr. Kolb and colleagues say.
To that end, they encourage professional societies to emphasize these metrics to their members and to streamline the reporting systems for QIs within the electronic health records used across various practice settings.
Avenues to improve examination quality may include educational interventions, such as online learning platforms that teach dysplasia detection or that highlight best practices, they add. These educational tools should be easy to use and should emphasize quality improvement measures.
“Future efforts are warranted to identify and extinguish predictors of this variability and to determine whether these interventions can improve DDR and adherence rates to QIs among endoscopists doing these examinations with the goal to improve EAC outcomes,” they conclude.
The study had no financial support. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, an analysis of U.S. registry data shows.
“As the GI Quality Improvement Consortium (GIQuIC) registry represents the ‘best-case scenario’ for adherence, since sites and endoscopists enrolled in this quality registry are aware that their practices are being monitored, these results indicate that there is still room for improvement and better consistency,” the researchers write.
The study was published online in The American Journal of Gastroenterology.
Quality care in BE, which is a precursor to esophageal adenocarcinoma (EAC), includes adherence to the Seattle biopsy protocol for sampling the BE segment (four-quadrant biopsies every 2 cm) and to a surveillance interval of 3-5 years for patients with nondysplastic BE (NDBE).
Previous studies have found poor adherence to these two QIs, but those studies only provided overall estimates, and individual endoscopists or different sites were not taken into consideration.
Jennifer Kolb, MD, with the University of California, Los Angeles, and colleagues say their study is the first to highlight variation in adherence to these measures at the center and endoscopist levels.
The study is also the first U.S. population–based study to report the dysplasia detection rate (DDR), which is a proposed quality indicator. The findings on this metric also demonstrate marked variability across endoscopists and sites.
Study details
Using the nationwide GIQuIC registry, the researchers evaluated endoscopist and site-based adherence to the Seattle protocol and surveillance interval advice from January 2018 to May 2021.
Among 255 practices with 1,195 endoscopists who performed 20,155 upper endoscopies for suspected or established BE, overall adherence to the Seattle protocol was 86%, which is considerably higher than the 51% reported in a study conducted from 2002 to 2007, Dr. Kolb and colleagues note.
When researchers looked specifically at 572 endoscopists for whom there were at least 10 endoscopy records in the registry, they found high variability in adherence to the Seattle protocol (median, 93.8%; interquartile range, 18.9%).
Adherence to the Seattle protocol was also variable among 153 practices for which there were at least 20 endoscopy records (median, 90%; IQR, 20.1%).
Of the 12,100 upper endoscopies with documented NDBE, 8,517 (70.4%) had a guideline-concordant–recommended surveillance interval of 3-5 years, with variability at both the endoscopist (median, 82.4%; IQR, 36.3%) and site level (median, 77.2%; IQR, 28.9%).
Endoscopist and site adherence to the Seattle protocol and surveillance guidance generally rose along with volume of upper endoscopies performed.
The overall DDR was 3.1%; it varied among endoscopists and sites (mean, 3.3% for both).
The investigators note that the 95% confidence intervals for each provider for DDR were “highly variable” and ranged from –20% to 119.3%. Notably, increasing upper endoscopy volume had an inconsistent effect on adherence rates and DDR by endoscopists and sites.
The investigators saw no correlation between overall DDR and Seattle protocol adherence among sites and only weak but statistically significant negative correlation between DDR and Seattle protocol adherence among individual endoscopists.
Practical approaches to improvement
The researchers say their observations from the GIQuIC database “most accurately represent the real-world experience in Barrett’s endoscopy.”
The results can serve as a “benchmark for quality initiatives and intervention trials aimed at improving outcomes for patients with BE,” they say.
Improving adherence to key QI measures and ensuring more consistent clinical behavior across practice groups and endoscopists are “critical first steps” to ensure high-quality BE care, Dr. Kolb and colleagues say.
To that end, they encourage professional societies to emphasize these metrics to their members and to streamline the reporting systems for QIs within the electronic health records used across various practice settings.
Avenues to improve examination quality may include educational interventions, such as online learning platforms that teach dysplasia detection or that highlight best practices, they add. These educational tools should be easy to use and should emphasize quality improvement measures.
“Future efforts are warranted to identify and extinguish predictors of this variability and to determine whether these interventions can improve DDR and adherence rates to QIs among endoscopists doing these examinations with the goal to improve EAC outcomes,” they conclude.
The study had no financial support. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, an analysis of U.S. registry data shows.
“As the GI Quality Improvement Consortium (GIQuIC) registry represents the ‘best-case scenario’ for adherence, since sites and endoscopists enrolled in this quality registry are aware that their practices are being monitored, these results indicate that there is still room for improvement and better consistency,” the researchers write.
The study was published online in The American Journal of Gastroenterology.
Quality care in BE, which is a precursor to esophageal adenocarcinoma (EAC), includes adherence to the Seattle biopsy protocol for sampling the BE segment (four-quadrant biopsies every 2 cm) and to a surveillance interval of 3-5 years for patients with nondysplastic BE (NDBE).
Previous studies have found poor adherence to these two QIs, but those studies only provided overall estimates, and individual endoscopists or different sites were not taken into consideration.
Jennifer Kolb, MD, with the University of California, Los Angeles, and colleagues say their study is the first to highlight variation in adherence to these measures at the center and endoscopist levels.
The study is also the first U.S. population–based study to report the dysplasia detection rate (DDR), which is a proposed quality indicator. The findings on this metric also demonstrate marked variability across endoscopists and sites.
Study details
Using the nationwide GIQuIC registry, the researchers evaluated endoscopist and site-based adherence to the Seattle protocol and surveillance interval advice from January 2018 to May 2021.
Among 255 practices with 1,195 endoscopists who performed 20,155 upper endoscopies for suspected or established BE, overall adherence to the Seattle protocol was 86%, which is considerably higher than the 51% reported in a study conducted from 2002 to 2007, Dr. Kolb and colleagues note.
When researchers looked specifically at 572 endoscopists for whom there were at least 10 endoscopy records in the registry, they found high variability in adherence to the Seattle protocol (median, 93.8%; interquartile range, 18.9%).
Adherence to the Seattle protocol was also variable among 153 practices for which there were at least 20 endoscopy records (median, 90%; IQR, 20.1%).
Of the 12,100 upper endoscopies with documented NDBE, 8,517 (70.4%) had a guideline-concordant–recommended surveillance interval of 3-5 years, with variability at both the endoscopist (median, 82.4%; IQR, 36.3%) and site level (median, 77.2%; IQR, 28.9%).
Endoscopist and site adherence to the Seattle protocol and surveillance guidance generally rose along with volume of upper endoscopies performed.
The overall DDR was 3.1%; it varied among endoscopists and sites (mean, 3.3% for both).
The investigators note that the 95% confidence intervals for each provider for DDR were “highly variable” and ranged from –20% to 119.3%. Notably, increasing upper endoscopy volume had an inconsistent effect on adherence rates and DDR by endoscopists and sites.
The investigators saw no correlation between overall DDR and Seattle protocol adherence among sites and only weak but statistically significant negative correlation between DDR and Seattle protocol adherence among individual endoscopists.
Practical approaches to improvement
The researchers say their observations from the GIQuIC database “most accurately represent the real-world experience in Barrett’s endoscopy.”
The results can serve as a “benchmark for quality initiatives and intervention trials aimed at improving outcomes for patients with BE,” they say.
Improving adherence to key QI measures and ensuring more consistent clinical behavior across practice groups and endoscopists are “critical first steps” to ensure high-quality BE care, Dr. Kolb and colleagues say.
To that end, they encourage professional societies to emphasize these metrics to their members and to streamline the reporting systems for QIs within the electronic health records used across various practice settings.
Avenues to improve examination quality may include educational interventions, such as online learning platforms that teach dysplasia detection or that highlight best practices, they add. These educational tools should be easy to use and should emphasize quality improvement measures.
“Future efforts are warranted to identify and extinguish predictors of this variability and to determine whether these interventions can improve DDR and adherence rates to QIs among endoscopists doing these examinations with the goal to improve EAC outcomes,” they conclude.
The study had no financial support. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY











