User login
ACIP approves changes to HPV, Tdap, DTaP, MenB vaccination guidance
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices approved a series of minor changes to the current guidance for meningococcal, Tdap, DTaP, and human papillomavirus vaccination schedules.
Regarding meningococcal vaccinations, the committee voted to change the recommendations to state that individuals who are at an increased risk of contracting the disease should receive a three-dose regimen of Trumenba at 0 months, 1-2 months, and 6 months. The same regimen also should apply during any outbreaks of serogroup B meningococcal virus. In addition, a two-dose regimen at 0 and 6 months should be given to adolescents who are not considered high risk, and if the second dose is given fewer than 6 months following the first, then a third dose must be given within 6 months of the initial dose.
“This new recommendation enables flexible vaccination dosing intervals depending on one’s risk of exposure to meningococcal group B disease, also known as MenB, which makes it easier for health care providers to help protect adolescents and young adults from this uncommon but life-threatening disease,” the CDC announced in a statement.
For Tdap and DTaP vaccines, changes to the language of the recommendations were approved unanimously by the committee. These changes will contain the routine recommendations for DTaP, Tdap, and TB, which previously were published as separate statements, along with Tdap recommendations made after the 2005 recommendations and published in Morbidity and Mortality Weekly Report policy notes.
“This statement also contains updates, such as DTaP vaccines that became available after the 1997 DTaP statement, and updates to the label indications on various DTaP and Tdap products,” Jennifer Liang, DVM, of the CDC’s National Center for Immunization and Respiratory Diseases, explained at the ACIP meeting. “Also included in the statement are the following updates: mention of the discontinuation of monovalent tetanus toxoid vaccine, the contraindications and precautions for DTaP are now consistent with the [American Academy of Pediatrics’] Red Book, and for persons aged 7-10 years who received a dose of Tdap as part of the catchup series, an adolescent Tdap dose may be given at age 11-12 years.”
Dr. Liang added that these updated changes would bring the guidance in line with the recommendations for children who are administered Tdap inadvertently.
With one recusal, changes to the HPV vaccination guidance also were unanimously approved. No changes were proposed to the routine and catch-up age groups for HPV vaccination, and for contraindications and precautions. Major additions were made, however, to the sections on dosing schedules, and people with prior vaccination. Clarifying language was added for the sections on interrupted schedules, special populations, and medical conditions.
For individuals initiating vaccination before the 15th birthday, the recommended immunization schedule is two doses of HPV vaccine. The second dose should be administered 6-12 months after the first dose (0 months, 6-12 months schedule). For people initiating vaccination on or after the 15th birthday, the recommendations remain the same as before: three doses of HPV vaccine, with the second dose administered 1-2 months after the first dose, and the third dose administered within 6 months of the first dose.
Those with prior vaccinations who initiated with 9-valent HPV, 4-valent HPV, or 2-valent HPV before their 15th birthday and received either two or three doses at the recommended dosing schedule should be considered adequately vaccinated. Those who initiated any of those three HPV vaccinations on or after their 15th birthday and received three doses at the currently recommended dosing schedule should be considered adequately vaccinated, too.
With regard to the minimum intervals, the proposed change was to add a footnote defining minimum intervals: in a two-dose series of HPV vaccines, the minimum interval is 5 months between the first and second dose, and in a three-dose series, 5 months between the first and third dose. All other language remains as is. Special population language also was changed to “gay, bisexual, and other” men rather than simply men who have sex with men, to broaden the scope of the language. Language also will be amended to include transgender patients.
Finally, for those with other medical conditions, ACIP still recommends that all immunocompromised males and females aged 9-26 years get a three-dose HPV vaccination at 0, 1-2, and 6 months, but now the language change will read that “Persons who should receive three doses are those with primary or secondary immunocompromising conditions that might reduce cell-mediated or humoral immunity, such as B lymphocyte antibody deficiencies, T lymphocyte complete or partial defects, HIV infection, malignant neoplasm, transplantation, autoimmune disease, or immunosuppressive therapy, since response to vaccination may be attenuated.”
In addition, there will be a footnote stating that these recommendations for a three-dose schedule do not apply to children under the age of 15 years with asplenia, asthma, chronic granulomatous disease, chronic heart/liver/lung/renal disease, central nervous system anatomic barrier defects, complement deficiency, diabetes, or sickle cell disease.”
The recommendations agreed upon will be submitted for approval to CDC Director Tom Frieden, MD. If approved, the recommendations will be published by Jan. 1, 2017, at which point, they will go into effect.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices approved a series of minor changes to the current guidance for meningococcal, Tdap, DTaP, and human papillomavirus vaccination schedules.
Regarding meningococcal vaccinations, the committee voted to change the recommendations to state that individuals who are at an increased risk of contracting the disease should receive a three-dose regimen of Trumenba at 0 months, 1-2 months, and 6 months. The same regimen also should apply during any outbreaks of serogroup B meningococcal virus. In addition, a two-dose regimen at 0 and 6 months should be given to adolescents who are not considered high risk, and if the second dose is given fewer than 6 months following the first, then a third dose must be given within 6 months of the initial dose.
“This new recommendation enables flexible vaccination dosing intervals depending on one’s risk of exposure to meningococcal group B disease, also known as MenB, which makes it easier for health care providers to help protect adolescents and young adults from this uncommon but life-threatening disease,” the CDC announced in a statement.
For Tdap and DTaP vaccines, changes to the language of the recommendations were approved unanimously by the committee. These changes will contain the routine recommendations for DTaP, Tdap, and TB, which previously were published as separate statements, along with Tdap recommendations made after the 2005 recommendations and published in Morbidity and Mortality Weekly Report policy notes.
“This statement also contains updates, such as DTaP vaccines that became available after the 1997 DTaP statement, and updates to the label indications on various DTaP and Tdap products,” Jennifer Liang, DVM, of the CDC’s National Center for Immunization and Respiratory Diseases, explained at the ACIP meeting. “Also included in the statement are the following updates: mention of the discontinuation of monovalent tetanus toxoid vaccine, the contraindications and precautions for DTaP are now consistent with the [American Academy of Pediatrics’] Red Book, and for persons aged 7-10 years who received a dose of Tdap as part of the catchup series, an adolescent Tdap dose may be given at age 11-12 years.”
Dr. Liang added that these updated changes would bring the guidance in line with the recommendations for children who are administered Tdap inadvertently.
With one recusal, changes to the HPV vaccination guidance also were unanimously approved. No changes were proposed to the routine and catch-up age groups for HPV vaccination, and for contraindications and precautions. Major additions were made, however, to the sections on dosing schedules, and people with prior vaccination. Clarifying language was added for the sections on interrupted schedules, special populations, and medical conditions.
For individuals initiating vaccination before the 15th birthday, the recommended immunization schedule is two doses of HPV vaccine. The second dose should be administered 6-12 months after the first dose (0 months, 6-12 months schedule). For people initiating vaccination on or after the 15th birthday, the recommendations remain the same as before: three doses of HPV vaccine, with the second dose administered 1-2 months after the first dose, and the third dose administered within 6 months of the first dose.
Those with prior vaccinations who initiated with 9-valent HPV, 4-valent HPV, or 2-valent HPV before their 15th birthday and received either two or three doses at the recommended dosing schedule should be considered adequately vaccinated. Those who initiated any of those three HPV vaccinations on or after their 15th birthday and received three doses at the currently recommended dosing schedule should be considered adequately vaccinated, too.
With regard to the minimum intervals, the proposed change was to add a footnote defining minimum intervals: in a two-dose series of HPV vaccines, the minimum interval is 5 months between the first and second dose, and in a three-dose series, 5 months between the first and third dose. All other language remains as is. Special population language also was changed to “gay, bisexual, and other” men rather than simply men who have sex with men, to broaden the scope of the language. Language also will be amended to include transgender patients.
Finally, for those with other medical conditions, ACIP still recommends that all immunocompromised males and females aged 9-26 years get a three-dose HPV vaccination at 0, 1-2, and 6 months, but now the language change will read that “Persons who should receive three doses are those with primary or secondary immunocompromising conditions that might reduce cell-mediated or humoral immunity, such as B lymphocyte antibody deficiencies, T lymphocyte complete or partial defects, HIV infection, malignant neoplasm, transplantation, autoimmune disease, or immunosuppressive therapy, since response to vaccination may be attenuated.”
In addition, there will be a footnote stating that these recommendations for a three-dose schedule do not apply to children under the age of 15 years with asplenia, asthma, chronic granulomatous disease, chronic heart/liver/lung/renal disease, central nervous system anatomic barrier defects, complement deficiency, diabetes, or sickle cell disease.”
The recommendations agreed upon will be submitted for approval to CDC Director Tom Frieden, MD. If approved, the recommendations will be published by Jan. 1, 2017, at which point, they will go into effect.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices approved a series of minor changes to the current guidance for meningococcal, Tdap, DTaP, and human papillomavirus vaccination schedules.
Regarding meningococcal vaccinations, the committee voted to change the recommendations to state that individuals who are at an increased risk of contracting the disease should receive a three-dose regimen of Trumenba at 0 months, 1-2 months, and 6 months. The same regimen also should apply during any outbreaks of serogroup B meningococcal virus. In addition, a two-dose regimen at 0 and 6 months should be given to adolescents who are not considered high risk, and if the second dose is given fewer than 6 months following the first, then a third dose must be given within 6 months of the initial dose.
“This new recommendation enables flexible vaccination dosing intervals depending on one’s risk of exposure to meningococcal group B disease, also known as MenB, which makes it easier for health care providers to help protect adolescents and young adults from this uncommon but life-threatening disease,” the CDC announced in a statement.
For Tdap and DTaP vaccines, changes to the language of the recommendations were approved unanimously by the committee. These changes will contain the routine recommendations for DTaP, Tdap, and TB, which previously were published as separate statements, along with Tdap recommendations made after the 2005 recommendations and published in Morbidity and Mortality Weekly Report policy notes.
“This statement also contains updates, such as DTaP vaccines that became available after the 1997 DTaP statement, and updates to the label indications on various DTaP and Tdap products,” Jennifer Liang, DVM, of the CDC’s National Center for Immunization and Respiratory Diseases, explained at the ACIP meeting. “Also included in the statement are the following updates: mention of the discontinuation of monovalent tetanus toxoid vaccine, the contraindications and precautions for DTaP are now consistent with the [American Academy of Pediatrics’] Red Book, and for persons aged 7-10 years who received a dose of Tdap as part of the catchup series, an adolescent Tdap dose may be given at age 11-12 years.”
Dr. Liang added that these updated changes would bring the guidance in line with the recommendations for children who are administered Tdap inadvertently.
With one recusal, changes to the HPV vaccination guidance also were unanimously approved. No changes were proposed to the routine and catch-up age groups for HPV vaccination, and for contraindications and precautions. Major additions were made, however, to the sections on dosing schedules, and people with prior vaccination. Clarifying language was added for the sections on interrupted schedules, special populations, and medical conditions.
For individuals initiating vaccination before the 15th birthday, the recommended immunization schedule is two doses of HPV vaccine. The second dose should be administered 6-12 months after the first dose (0 months, 6-12 months schedule). For people initiating vaccination on or after the 15th birthday, the recommendations remain the same as before: three doses of HPV vaccine, with the second dose administered 1-2 months after the first dose, and the third dose administered within 6 months of the first dose.
Those with prior vaccinations who initiated with 9-valent HPV, 4-valent HPV, or 2-valent HPV before their 15th birthday and received either two or three doses at the recommended dosing schedule should be considered adequately vaccinated. Those who initiated any of those three HPV vaccinations on or after their 15th birthday and received three doses at the currently recommended dosing schedule should be considered adequately vaccinated, too.
With regard to the minimum intervals, the proposed change was to add a footnote defining minimum intervals: in a two-dose series of HPV vaccines, the minimum interval is 5 months between the first and second dose, and in a three-dose series, 5 months between the first and third dose. All other language remains as is. Special population language also was changed to “gay, bisexual, and other” men rather than simply men who have sex with men, to broaden the scope of the language. Language also will be amended to include transgender patients.
Finally, for those with other medical conditions, ACIP still recommends that all immunocompromised males and females aged 9-26 years get a three-dose HPV vaccination at 0, 1-2, and 6 months, but now the language change will read that “Persons who should receive three doses are those with primary or secondary immunocompromising conditions that might reduce cell-mediated or humoral immunity, such as B lymphocyte antibody deficiencies, T lymphocyte complete or partial defects, HIV infection, malignant neoplasm, transplantation, autoimmune disease, or immunosuppressive therapy, since response to vaccination may be attenuated.”
In addition, there will be a footnote stating that these recommendations for a three-dose schedule do not apply to children under the age of 15 years with asplenia, asthma, chronic granulomatous disease, chronic heart/liver/lung/renal disease, central nervous system anatomic barrier defects, complement deficiency, diabetes, or sickle cell disease.”
The recommendations agreed upon will be submitted for approval to CDC Director Tom Frieden, MD. If approved, the recommendations will be published by Jan. 1, 2017, at which point, they will go into effect.
FROM AN ACIP MEETING
Use of 2D bar coding with vaccines may be the future in pediatric practice
ATLANTA – Since the first bar coded consumer product, a pack of gum, was scanned in June of 1974, the soon widespread use of bar codes changed little until 2D bar codes arrived toward the end of last century. Today, the increasing use of 2D bar code technology with vaccines offers practices the potential for greater accuracy and efficiency with vaccine administration and data entry – if they have the resources to take the plunge.
An overview of 2D bar code use with vaccines, presented at a conference sponsored by the Centers for Disease Control and Prevention, provided a glimpse into both the types of changes practices might see with adoption of the technology and the way some clinics have made the transition.
Ken Gerlach, MPH, of the Immunization Services Division at the CDC in Atlanta, outlined the history of bar code use in immunizations, starting with a November 1999 Institute of Medicine report that identified the contribution of human error to disease and led the Food and Drug Administration to begin requiring linear bar codes on pharmaceutical unit-of-use products to reduce errors.
Then, a meeting organized by the American Academy of Pediatrics in January 2009 with the FDA, CDC, vaccine manufacturers, and other stakeholders led to a bar code rule change by the FDA in August 2011 that allowed alternatives to the traditional linear bar codes on vaccine vials and syringes.
“They essentially indicated to the pharmaceutical companies that it’s okay to add 2D bar codes, and this is essentially the point where things began to take off,” Mr. Gerlach explained. Until then, there had been no 2D bar codes on vaccines, but today the majority of vaccine products have them, as do all Vaccine Information Statements. In addition to the standard information included on traditional bar codes – Global Trade Item Number (GTIN), lot and serial numbers, and the expiration date – 2D bar codes also can include most relevant patient information that would go into the EMR except the injection site and immunization route. But a practice cannot simply jump over to scanning the 2D bar codes without ensuring that its EMR system is configured to accept the scanning.
Mr. Gerlach described a three-part project by the CDC, from 2011 through 2017, that assesses the impact of 2D coding on vaccination data quality and work flow, facilitates the adoption of 2D bar code scanning in health care practices, and then assesses the potential for expanding 2D bar code use in a large health care system. The first part of the project, which ran from 2011 to 2014, involved two vaccine manufacturers and 217 health care practices with more than 1.4 million de-identified vaccination records, 18.1% of which had been 2D bar coded.
Analysis of data quality from that pilot revealed an 8% increase in the correctness of lot numbers and 11% increase for expiration dates, with a time savings of 3.4 seconds per vaccine administration. Among the 116 staff users who completed surveys, 86% agreed that 2D bar coding improves accuracy and completeness, and 60% agreed it was easy to integrate the bar coding into their usual data recording process.
The pilot revealed challenges as well, however: not all individuals units of vaccines were 2D bar coded, users did not always consistently scan the bar codes, and some bar codes were difficult to read, such as one that was brown and wouldn’t scan. Another obstacle was having different lot numbers on the unit of use versus the unit of sale with 10% of the vaccines. Further, because inventory management typically involves unit of sale, it does not always match well with scanning unit of use.
Clinicians’ beliefs and attitudes toward 2D bar coding
As more practices consider adopting the technology, buy-in will important. At the conference, Sharon Humiston, MD, and Jill Hernandez, MPH, of Children’s Mercy Hospital in Kansas City, Mo., shared the findings of an online questionnaire about 2D bar coding and practices’ current systems for vaccine inventory and recording patient immunization information. The researchers distributed the questionnaire link to various AAP sections and committees in listservs and emails. Those eligible to complete the 15-minute survey were primary care personnel who used EMRs but not 2D bar code scanning for vaccines. They also needed to be key decision makers in the process of purchasing technology for the practice, and their practice needed to be enrolled in the Vaccines for Children program.
Among the 77 respondents who met all the inclusion criteria (61% of all who started the survey), 1 in 5 were private practices with one or two physicians, just over a third (36%) were private practices with more than two physicians, and a quarter were multispecialty group practices. Overall, respondents administered an average 116 doses of DTaP and 50 doses of Tdap each month.
Protocols for immunization management varied considerably across the respondents. For recording vaccine information, 49% reported that an administrator pre-entered it into an EMR, but 43% reported that staff manually enter it into an EMR. About 55% of practices entered the information before vaccine administration, and 42% entered it afterward. Although 57% of respondents’ practices upload the vaccination information directly from the EMR to their state’s Immunization Information System (IIS), 30% must enter it both into the EMR and into the state IIS separately, and 11% don’t enter it into a state IIS.
More than half (56%) of the respondents were extremely interested in having a bar code scanner system, and 31% were moderately to strongly interested, rating a 6 to 9 on a scale of 1 to 10. If provided evidence that 2D bar codes reduced errors in vaccine documentation, 56% of respondents said it would greatly increase their interest, and 32% said it would somewhat increase it. Only 23% said their interest would greatly increase if the bar code technology allowed the vaccine information statement to be scanned into EMRs.
Nearly all the respondents agreed that 2D bar code scanning technology would improve efficiency and accuracy of entering vaccine information into medical records and tracking vaccine inventory. Further, 81% believed it would reduce medical malpractice liability, and 85% believed it would reduce risk of harm to patients. However, 23% thought bar code technology would disrupt office work flow, and a quarter believed the technology’s costs would exceeds its benefits.
Despite the strong interest overall, respondents reported a number of barriers to adopting 2D bar code technology. The greatest barrier, reported by more than 70%, was the upfront cost of purchasing software for the EMR interface, followed by the cost of the bar code scanners. Other barriers, reported by 25%-45% of respondents, were the need for staff training, the need to service and maintain electronics for the technology, and the purchase of additional computers for scanner sites. If a bar code system cost less than $5,000, then 80% of the respondents would definitely or probably adopt such a system. Few would adopt it if the system cost $10,000 or more, but 42% probably would if it cost between $5,000 and $9,999. Even this small survey of self-selected volunteers, however, suggested strong interest in using 2D bar code technology for vaccines – although initial costs for a system presented a significant barrier to most practices.
One influenza vaccine clinic’s experience
Interest based on hypothetical questions is one thing. The process of actually implementing a 2D bar code scanning system into a health care center is another. In a separate presentation, Jane Glaser, MSN, RN, executive director of Campbell County Public Health in Gillette, Wyo., reviewed how such a system was implemented for mass influenza vaccination.
Campbell County, in the northeast corner of Wyoming, covers more than 4,800 square miles, has a population base of nearly 50,000 people, and also serves individuals from Montana, South Dakota, and North Dakota. Although the community as a whole works 24/7 in the county because of the oil, mining, and farming industries, the mass flu clinic is open 7 a.m. to 7 p.m., during which it provides an estimated 700 to 1,500 flu vaccines daily. Personnel comprises 13 public health nurses, 5 administrative assistants, and 3-4 community volunteers.
After 20 years of using an IIS, the clinic’s leadership decided to begin using 2D bar code scanners in October 2011 after observing it at a state immunization conference. Their goals in changing systems were to increase clinic flow, decrease registration time, and decrease overtime due to data entry. The new work flow went as follows: Those with Wyoming driver licenses or state ID cards have the linear bar code on their ID scanned in the immunization registry, which automatically populates the patient’s record. Then the staff member enters the vaccine information directly into the IIS registry in real time after the client receives the vaccine.
Ms. Glaser describes a number of improvements that resulted from use of the bar code scanning system, starting with reduced time for clinic registration and improved clinic flow. They also found that using bar code scanning reduced manual entry errors and improved the efficiency of assessing vaccination status and needed vaccines. Entering data in real time at point of care reduced time spent on data entry later on, thereby leading to a decrease in overtime and subsequent cost savings.
For providers and practices interested in learning more about 2D bar coding, the CDC offers a current list of 2D bar coded vaccines, data from the pilot program, training materials, and AAP guidance about 2D bar code use.
None of three presentations noted external funding, and all the researchers reported no financial relationships with companies that profit from bar code scanning technology. Deloitte Consulting, was involved in the three-part project conducted by the CDC.
ATLANTA – Since the first bar coded consumer product, a pack of gum, was scanned in June of 1974, the soon widespread use of bar codes changed little until 2D bar codes arrived toward the end of last century. Today, the increasing use of 2D bar code technology with vaccines offers practices the potential for greater accuracy and efficiency with vaccine administration and data entry – if they have the resources to take the plunge.
An overview of 2D bar code use with vaccines, presented at a conference sponsored by the Centers for Disease Control and Prevention, provided a glimpse into both the types of changes practices might see with adoption of the technology and the way some clinics have made the transition.
Ken Gerlach, MPH, of the Immunization Services Division at the CDC in Atlanta, outlined the history of bar code use in immunizations, starting with a November 1999 Institute of Medicine report that identified the contribution of human error to disease and led the Food and Drug Administration to begin requiring linear bar codes on pharmaceutical unit-of-use products to reduce errors.
Then, a meeting organized by the American Academy of Pediatrics in January 2009 with the FDA, CDC, vaccine manufacturers, and other stakeholders led to a bar code rule change by the FDA in August 2011 that allowed alternatives to the traditional linear bar codes on vaccine vials and syringes.
“They essentially indicated to the pharmaceutical companies that it’s okay to add 2D bar codes, and this is essentially the point where things began to take off,” Mr. Gerlach explained. Until then, there had been no 2D bar codes on vaccines, but today the majority of vaccine products have them, as do all Vaccine Information Statements. In addition to the standard information included on traditional bar codes – Global Trade Item Number (GTIN), lot and serial numbers, and the expiration date – 2D bar codes also can include most relevant patient information that would go into the EMR except the injection site and immunization route. But a practice cannot simply jump over to scanning the 2D bar codes without ensuring that its EMR system is configured to accept the scanning.
Mr. Gerlach described a three-part project by the CDC, from 2011 through 2017, that assesses the impact of 2D coding on vaccination data quality and work flow, facilitates the adoption of 2D bar code scanning in health care practices, and then assesses the potential for expanding 2D bar code use in a large health care system. The first part of the project, which ran from 2011 to 2014, involved two vaccine manufacturers and 217 health care practices with more than 1.4 million de-identified vaccination records, 18.1% of which had been 2D bar coded.
Analysis of data quality from that pilot revealed an 8% increase in the correctness of lot numbers and 11% increase for expiration dates, with a time savings of 3.4 seconds per vaccine administration. Among the 116 staff users who completed surveys, 86% agreed that 2D bar coding improves accuracy and completeness, and 60% agreed it was easy to integrate the bar coding into their usual data recording process.
The pilot revealed challenges as well, however: not all individuals units of vaccines were 2D bar coded, users did not always consistently scan the bar codes, and some bar codes were difficult to read, such as one that was brown and wouldn’t scan. Another obstacle was having different lot numbers on the unit of use versus the unit of sale with 10% of the vaccines. Further, because inventory management typically involves unit of sale, it does not always match well with scanning unit of use.
Clinicians’ beliefs and attitudes toward 2D bar coding
As more practices consider adopting the technology, buy-in will important. At the conference, Sharon Humiston, MD, and Jill Hernandez, MPH, of Children’s Mercy Hospital in Kansas City, Mo., shared the findings of an online questionnaire about 2D bar coding and practices’ current systems for vaccine inventory and recording patient immunization information. The researchers distributed the questionnaire link to various AAP sections and committees in listservs and emails. Those eligible to complete the 15-minute survey were primary care personnel who used EMRs but not 2D bar code scanning for vaccines. They also needed to be key decision makers in the process of purchasing technology for the practice, and their practice needed to be enrolled in the Vaccines for Children program.
Among the 77 respondents who met all the inclusion criteria (61% of all who started the survey), 1 in 5 were private practices with one or two physicians, just over a third (36%) were private practices with more than two physicians, and a quarter were multispecialty group practices. Overall, respondents administered an average 116 doses of DTaP and 50 doses of Tdap each month.
Protocols for immunization management varied considerably across the respondents. For recording vaccine information, 49% reported that an administrator pre-entered it into an EMR, but 43% reported that staff manually enter it into an EMR. About 55% of practices entered the information before vaccine administration, and 42% entered it afterward. Although 57% of respondents’ practices upload the vaccination information directly from the EMR to their state’s Immunization Information System (IIS), 30% must enter it both into the EMR and into the state IIS separately, and 11% don’t enter it into a state IIS.
More than half (56%) of the respondents were extremely interested in having a bar code scanner system, and 31% were moderately to strongly interested, rating a 6 to 9 on a scale of 1 to 10. If provided evidence that 2D bar codes reduced errors in vaccine documentation, 56% of respondents said it would greatly increase their interest, and 32% said it would somewhat increase it. Only 23% said their interest would greatly increase if the bar code technology allowed the vaccine information statement to be scanned into EMRs.
Nearly all the respondents agreed that 2D bar code scanning technology would improve efficiency and accuracy of entering vaccine information into medical records and tracking vaccine inventory. Further, 81% believed it would reduce medical malpractice liability, and 85% believed it would reduce risk of harm to patients. However, 23% thought bar code technology would disrupt office work flow, and a quarter believed the technology’s costs would exceeds its benefits.
Despite the strong interest overall, respondents reported a number of barriers to adopting 2D bar code technology. The greatest barrier, reported by more than 70%, was the upfront cost of purchasing software for the EMR interface, followed by the cost of the bar code scanners. Other barriers, reported by 25%-45% of respondents, were the need for staff training, the need to service and maintain electronics for the technology, and the purchase of additional computers for scanner sites. If a bar code system cost less than $5,000, then 80% of the respondents would definitely or probably adopt such a system. Few would adopt it if the system cost $10,000 or more, but 42% probably would if it cost between $5,000 and $9,999. Even this small survey of self-selected volunteers, however, suggested strong interest in using 2D bar code technology for vaccines – although initial costs for a system presented a significant barrier to most practices.
One influenza vaccine clinic’s experience
Interest based on hypothetical questions is one thing. The process of actually implementing a 2D bar code scanning system into a health care center is another. In a separate presentation, Jane Glaser, MSN, RN, executive director of Campbell County Public Health in Gillette, Wyo., reviewed how such a system was implemented for mass influenza vaccination.
Campbell County, in the northeast corner of Wyoming, covers more than 4,800 square miles, has a population base of nearly 50,000 people, and also serves individuals from Montana, South Dakota, and North Dakota. Although the community as a whole works 24/7 in the county because of the oil, mining, and farming industries, the mass flu clinic is open 7 a.m. to 7 p.m., during which it provides an estimated 700 to 1,500 flu vaccines daily. Personnel comprises 13 public health nurses, 5 administrative assistants, and 3-4 community volunteers.
After 20 years of using an IIS, the clinic’s leadership decided to begin using 2D bar code scanners in October 2011 after observing it at a state immunization conference. Their goals in changing systems were to increase clinic flow, decrease registration time, and decrease overtime due to data entry. The new work flow went as follows: Those with Wyoming driver licenses or state ID cards have the linear bar code on their ID scanned in the immunization registry, which automatically populates the patient’s record. Then the staff member enters the vaccine information directly into the IIS registry in real time after the client receives the vaccine.
Ms. Glaser describes a number of improvements that resulted from use of the bar code scanning system, starting with reduced time for clinic registration and improved clinic flow. They also found that using bar code scanning reduced manual entry errors and improved the efficiency of assessing vaccination status and needed vaccines. Entering data in real time at point of care reduced time spent on data entry later on, thereby leading to a decrease in overtime and subsequent cost savings.
For providers and practices interested in learning more about 2D bar coding, the CDC offers a current list of 2D bar coded vaccines, data from the pilot program, training materials, and AAP guidance about 2D bar code use.
None of three presentations noted external funding, and all the researchers reported no financial relationships with companies that profit from bar code scanning technology. Deloitte Consulting, was involved in the three-part project conducted by the CDC.
ATLANTA – Since the first bar coded consumer product, a pack of gum, was scanned in June of 1974, the soon widespread use of bar codes changed little until 2D bar codes arrived toward the end of last century. Today, the increasing use of 2D bar code technology with vaccines offers practices the potential for greater accuracy and efficiency with vaccine administration and data entry – if they have the resources to take the plunge.
An overview of 2D bar code use with vaccines, presented at a conference sponsored by the Centers for Disease Control and Prevention, provided a glimpse into both the types of changes practices might see with adoption of the technology and the way some clinics have made the transition.
Ken Gerlach, MPH, of the Immunization Services Division at the CDC in Atlanta, outlined the history of bar code use in immunizations, starting with a November 1999 Institute of Medicine report that identified the contribution of human error to disease and led the Food and Drug Administration to begin requiring linear bar codes on pharmaceutical unit-of-use products to reduce errors.
Then, a meeting organized by the American Academy of Pediatrics in January 2009 with the FDA, CDC, vaccine manufacturers, and other stakeholders led to a bar code rule change by the FDA in August 2011 that allowed alternatives to the traditional linear bar codes on vaccine vials and syringes.
“They essentially indicated to the pharmaceutical companies that it’s okay to add 2D bar codes, and this is essentially the point where things began to take off,” Mr. Gerlach explained. Until then, there had been no 2D bar codes on vaccines, but today the majority of vaccine products have them, as do all Vaccine Information Statements. In addition to the standard information included on traditional bar codes – Global Trade Item Number (GTIN), lot and serial numbers, and the expiration date – 2D bar codes also can include most relevant patient information that would go into the EMR except the injection site and immunization route. But a practice cannot simply jump over to scanning the 2D bar codes without ensuring that its EMR system is configured to accept the scanning.
Mr. Gerlach described a three-part project by the CDC, from 2011 through 2017, that assesses the impact of 2D coding on vaccination data quality and work flow, facilitates the adoption of 2D bar code scanning in health care practices, and then assesses the potential for expanding 2D bar code use in a large health care system. The first part of the project, which ran from 2011 to 2014, involved two vaccine manufacturers and 217 health care practices with more than 1.4 million de-identified vaccination records, 18.1% of which had been 2D bar coded.
Analysis of data quality from that pilot revealed an 8% increase in the correctness of lot numbers and 11% increase for expiration dates, with a time savings of 3.4 seconds per vaccine administration. Among the 116 staff users who completed surveys, 86% agreed that 2D bar coding improves accuracy and completeness, and 60% agreed it was easy to integrate the bar coding into their usual data recording process.
The pilot revealed challenges as well, however: not all individuals units of vaccines were 2D bar coded, users did not always consistently scan the bar codes, and some bar codes were difficult to read, such as one that was brown and wouldn’t scan. Another obstacle was having different lot numbers on the unit of use versus the unit of sale with 10% of the vaccines. Further, because inventory management typically involves unit of sale, it does not always match well with scanning unit of use.
Clinicians’ beliefs and attitudes toward 2D bar coding
As more practices consider adopting the technology, buy-in will important. At the conference, Sharon Humiston, MD, and Jill Hernandez, MPH, of Children’s Mercy Hospital in Kansas City, Mo., shared the findings of an online questionnaire about 2D bar coding and practices’ current systems for vaccine inventory and recording patient immunization information. The researchers distributed the questionnaire link to various AAP sections and committees in listservs and emails. Those eligible to complete the 15-minute survey were primary care personnel who used EMRs but not 2D bar code scanning for vaccines. They also needed to be key decision makers in the process of purchasing technology for the practice, and their practice needed to be enrolled in the Vaccines for Children program.
Among the 77 respondents who met all the inclusion criteria (61% of all who started the survey), 1 in 5 were private practices with one or two physicians, just over a third (36%) were private practices with more than two physicians, and a quarter were multispecialty group practices. Overall, respondents administered an average 116 doses of DTaP and 50 doses of Tdap each month.
Protocols for immunization management varied considerably across the respondents. For recording vaccine information, 49% reported that an administrator pre-entered it into an EMR, but 43% reported that staff manually enter it into an EMR. About 55% of practices entered the information before vaccine administration, and 42% entered it afterward. Although 57% of respondents’ practices upload the vaccination information directly from the EMR to their state’s Immunization Information System (IIS), 30% must enter it both into the EMR and into the state IIS separately, and 11% don’t enter it into a state IIS.
More than half (56%) of the respondents were extremely interested in having a bar code scanner system, and 31% were moderately to strongly interested, rating a 6 to 9 on a scale of 1 to 10. If provided evidence that 2D bar codes reduced errors in vaccine documentation, 56% of respondents said it would greatly increase their interest, and 32% said it would somewhat increase it. Only 23% said their interest would greatly increase if the bar code technology allowed the vaccine information statement to be scanned into EMRs.
Nearly all the respondents agreed that 2D bar code scanning technology would improve efficiency and accuracy of entering vaccine information into medical records and tracking vaccine inventory. Further, 81% believed it would reduce medical malpractice liability, and 85% believed it would reduce risk of harm to patients. However, 23% thought bar code technology would disrupt office work flow, and a quarter believed the technology’s costs would exceeds its benefits.
Despite the strong interest overall, respondents reported a number of barriers to adopting 2D bar code technology. The greatest barrier, reported by more than 70%, was the upfront cost of purchasing software for the EMR interface, followed by the cost of the bar code scanners. Other barriers, reported by 25%-45% of respondents, were the need for staff training, the need to service and maintain electronics for the technology, and the purchase of additional computers for scanner sites. If a bar code system cost less than $5,000, then 80% of the respondents would definitely or probably adopt such a system. Few would adopt it if the system cost $10,000 or more, but 42% probably would if it cost between $5,000 and $9,999. Even this small survey of self-selected volunteers, however, suggested strong interest in using 2D bar code technology for vaccines – although initial costs for a system presented a significant barrier to most practices.
One influenza vaccine clinic’s experience
Interest based on hypothetical questions is one thing. The process of actually implementing a 2D bar code scanning system into a health care center is another. In a separate presentation, Jane Glaser, MSN, RN, executive director of Campbell County Public Health in Gillette, Wyo., reviewed how such a system was implemented for mass influenza vaccination.
Campbell County, in the northeast corner of Wyoming, covers more than 4,800 square miles, has a population base of nearly 50,000 people, and also serves individuals from Montana, South Dakota, and North Dakota. Although the community as a whole works 24/7 in the county because of the oil, mining, and farming industries, the mass flu clinic is open 7 a.m. to 7 p.m., during which it provides an estimated 700 to 1,500 flu vaccines daily. Personnel comprises 13 public health nurses, 5 administrative assistants, and 3-4 community volunteers.
After 20 years of using an IIS, the clinic’s leadership decided to begin using 2D bar code scanners in October 2011 after observing it at a state immunization conference. Their goals in changing systems were to increase clinic flow, decrease registration time, and decrease overtime due to data entry. The new work flow went as follows: Those with Wyoming driver licenses or state ID cards have the linear bar code on their ID scanned in the immunization registry, which automatically populates the patient’s record. Then the staff member enters the vaccine information directly into the IIS registry in real time after the client receives the vaccine.
Ms. Glaser describes a number of improvements that resulted from use of the bar code scanning system, starting with reduced time for clinic registration and improved clinic flow. They also found that using bar code scanning reduced manual entry errors and improved the efficiency of assessing vaccination status and needed vaccines. Entering data in real time at point of care reduced time spent on data entry later on, thereby leading to a decrease in overtime and subsequent cost savings.
For providers and practices interested in learning more about 2D bar coding, the CDC offers a current list of 2D bar coded vaccines, data from the pilot program, training materials, and AAP guidance about 2D bar code use.
None of three presentations noted external funding, and all the researchers reported no financial relationships with companies that profit from bar code scanning technology. Deloitte Consulting, was involved in the three-part project conducted by the CDC.
EXPERT ANALYSIS FROM AAP 16
Key clinical point: 2D bar coding with vaccines offers benefits and challenges.
Major finding:
Data source: A CDC study, an online questionnaire, and experience in a Wyoming flu clinic.
Disclosures: None of three presentations noted external funding, and all researchers reported no financial relationships with companies that profit from bar code scanning technology. Deloitte Consulting was involved in the three-part project conducted by the CDC.
ACIP approves change to hepatitis B vaccination guidelines
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted in favor of changes to the current recommendations that would remove the permissive language for the hepatitis B birth dose to be administered after discharge from the hospital.
The revised language will state that for all medically stable infants weighing greater than or equal to 2,000 g at birth and born to hepatitis B surface antigen–negative mothers, the first dose of vaccine should be administered within 24 hours of birth. Originally, the recommendations were to state that vaccination should take place before hospital discharge, but this language was changed via a unanimously approved amendment to broaden the scope of the guidelines. Furthermore, the recommendations state that only single-antigen hepatitis B vaccine should be used for the birth dose.
“The language regarding vaccination within 24 hours of birth is consistent with what the [World Health Organization] says,” she explained.
The proposed changes came after the hepatitis B workgroup held five teleconference meetings between February and September of this year to discuss and determine what changes, if any, need to be made. A draft was prepared and presented at the ACIP meeting, at which the changes were unanimously approved by the 14-member advisory committee.
The recommendations agreed upon will be submitted for approval to CDC Director Tom Frieden, MD, and, if approved, will be published by Jan. 1, 2017, at which point they will go into effect.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted in favor of changes to the current recommendations that would remove the permissive language for the hepatitis B birth dose to be administered after discharge from the hospital.
The revised language will state that for all medically stable infants weighing greater than or equal to 2,000 g at birth and born to hepatitis B surface antigen–negative mothers, the first dose of vaccine should be administered within 24 hours of birth. Originally, the recommendations were to state that vaccination should take place before hospital discharge, but this language was changed via a unanimously approved amendment to broaden the scope of the guidelines. Furthermore, the recommendations state that only single-antigen hepatitis B vaccine should be used for the birth dose.
“The language regarding vaccination within 24 hours of birth is consistent with what the [World Health Organization] says,” she explained.
The proposed changes came after the hepatitis B workgroup held five teleconference meetings between February and September of this year to discuss and determine what changes, if any, need to be made. A draft was prepared and presented at the ACIP meeting, at which the changes were unanimously approved by the 14-member advisory committee.
The recommendations agreed upon will be submitted for approval to CDC Director Tom Frieden, MD, and, if approved, will be published by Jan. 1, 2017, at which point they will go into effect.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted in favor of changes to the current recommendations that would remove the permissive language for the hepatitis B birth dose to be administered after discharge from the hospital.
The revised language will state that for all medically stable infants weighing greater than or equal to 2,000 g at birth and born to hepatitis B surface antigen–negative mothers, the first dose of vaccine should be administered within 24 hours of birth. Originally, the recommendations were to state that vaccination should take place before hospital discharge, but this language was changed via a unanimously approved amendment to broaden the scope of the guidelines. Furthermore, the recommendations state that only single-antigen hepatitis B vaccine should be used for the birth dose.
“The language regarding vaccination within 24 hours of birth is consistent with what the [World Health Organization] says,” she explained.
The proposed changes came after the hepatitis B workgroup held five teleconference meetings between February and September of this year to discuss and determine what changes, if any, need to be made. A draft was prepared and presented at the ACIP meeting, at which the changes were unanimously approved by the 14-member advisory committee.
The recommendations agreed upon will be submitted for approval to CDC Director Tom Frieden, MD, and, if approved, will be published by Jan. 1, 2017, at which point they will go into effect.
Unvaccinated patients rack up billions in preventable costs
Adult patients who avoid vaccines cost the health care system $7 billion in preventable illness in 2015, according to a meta-analysis.
Sachiko Ozawa, PhD., of the University of North Carolina at Chapel Hill and her colleagues estimated the annual economic burden of diseases associated with 10 adult vaccines recommended by the Centers for Disease Control and Prevention that protect against 14 pathogens by looking at studies with U.S. cost data for adult age groups and using cost-of-illness modeling (Health Affairs 2016 Oct. doi:10.1377/hlthaff.2016.0462).
The cost of outpatient care ranged between $108 and $457 per patient, while the cost of medication ranged from $0 per patient for diseases that do not have curative drug treatments to $605 per patients treated for tetanus, investigators found. When it came to inpatient care, costs ranged from $5,770 per patient for those hospitalized for influenza to $15,600 for those hospitalized for invasive meningococcal disease.
Outpatient productivity loss per patient ranged from $29 for patients requiring a single outpatient visit to $154 for patients diagnosed with HPV-related cancers. Inpatient productivity loss per person ranged from $122 for patients with mumps to $580 for patients with tetanus.
The results underscore the need for improved uptake of vaccines among adults and the need for patients to better appreciate the value of vaccines, Dr. Ozawa said in an interview.
“If these individuals were to be vaccinated, than $7 billion in costs would be eliminated every year from the U.S. economy,” she said. “That’s pretty big. That’s the high-level takeaway.”
Dr. Ozawa said that she hopes the study will spur some creative policy solutions to increase vaccine usage, while preserving the autonomy of patients to make more informed choices.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Adult patients who avoid vaccines cost the health care system $7 billion in preventable illness in 2015, according to a meta-analysis.
Sachiko Ozawa, PhD., of the University of North Carolina at Chapel Hill and her colleagues estimated the annual economic burden of diseases associated with 10 adult vaccines recommended by the Centers for Disease Control and Prevention that protect against 14 pathogens by looking at studies with U.S. cost data for adult age groups and using cost-of-illness modeling (Health Affairs 2016 Oct. doi:10.1377/hlthaff.2016.0462).
The cost of outpatient care ranged between $108 and $457 per patient, while the cost of medication ranged from $0 per patient for diseases that do not have curative drug treatments to $605 per patients treated for tetanus, investigators found. When it came to inpatient care, costs ranged from $5,770 per patient for those hospitalized for influenza to $15,600 for those hospitalized for invasive meningococcal disease.
Outpatient productivity loss per patient ranged from $29 for patients requiring a single outpatient visit to $154 for patients diagnosed with HPV-related cancers. Inpatient productivity loss per person ranged from $122 for patients with mumps to $580 for patients with tetanus.
The results underscore the need for improved uptake of vaccines among adults and the need for patients to better appreciate the value of vaccines, Dr. Ozawa said in an interview.
“If these individuals were to be vaccinated, than $7 billion in costs would be eliminated every year from the U.S. economy,” she said. “That’s pretty big. That’s the high-level takeaway.”
Dr. Ozawa said that she hopes the study will spur some creative policy solutions to increase vaccine usage, while preserving the autonomy of patients to make more informed choices.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Adult patients who avoid vaccines cost the health care system $7 billion in preventable illness in 2015, according to a meta-analysis.
Sachiko Ozawa, PhD., of the University of North Carolina at Chapel Hill and her colleagues estimated the annual economic burden of diseases associated with 10 adult vaccines recommended by the Centers for Disease Control and Prevention that protect against 14 pathogens by looking at studies with U.S. cost data for adult age groups and using cost-of-illness modeling (Health Affairs 2016 Oct. doi:10.1377/hlthaff.2016.0462).
The cost of outpatient care ranged between $108 and $457 per patient, while the cost of medication ranged from $0 per patient for diseases that do not have curative drug treatments to $605 per patients treated for tetanus, investigators found. When it came to inpatient care, costs ranged from $5,770 per patient for those hospitalized for influenza to $15,600 for those hospitalized for invasive meningococcal disease.
Outpatient productivity loss per patient ranged from $29 for patients requiring a single outpatient visit to $154 for patients diagnosed with HPV-related cancers. Inpatient productivity loss per person ranged from $122 for patients with mumps to $580 for patients with tetanus.
The results underscore the need for improved uptake of vaccines among adults and the need for patients to better appreciate the value of vaccines, Dr. Ozawa said in an interview.
“If these individuals were to be vaccinated, than $7 billion in costs would be eliminated every year from the U.S. economy,” she said. “That’s pretty big. That’s the high-level takeaway.”
Dr. Ozawa said that she hopes the study will spur some creative policy solutions to increase vaccine usage, while preserving the autonomy of patients to make more informed choices.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Dengue vaccine beneficial only in moderate to high transmission settings
Pediatric patients with previous natural exposure to dengue virus benefit from the dengue virus vaccine, while vaccination of seronegative patients leads to an increased risk for hospitalization because of dengue, according to the results of a mathematical model simulation.
Because of the first approved dengue vaccine’s highly variable efficacy rates among pediatric patients, the vaccine should only be used in moderate to high transmission settings, the investigators who designed the model concluded in a paper published in Science.
Dengvaxia, developed by Sanofi-Pasteur, is a recombinant chimeric live attenuated dengue virus vaccine based on a yellow fever vaccine backbone. The vaccine’s development was “considerably more challenging than for other Flavivirus infections because of the immunological interactions between the four dengue virus serotypes and the risk of immune-mediated enhancement of disease” which causes secondary infections to lead to more severe disease, Neil Ferguson, PhD, of the Imperial College of London and his associates wrote (Science. 2016 Sep 2;353:1033-6. doi: 10.1126/science.aaf9590).
Despite the complexity of the virus and vaccine, Dengvaxia was recently approved for use in six countries, and two large multicenter phase III clinical trials recently concluded. Investigators for the trials, which involved over 30,000 children in Southeast Asia and Latin America, reported an overall vaccine efficacy of about 60% in cases of symptomatic dengue disease. However, the vaccine’s efficacy varied by severity of dengue infection and by age and serotype of the patient at time of vaccination. Investigators for both trials reported higher efficacy in patients with severe infection and in patients who were seropositive for dengue virus (indicating previous exposure to the virus) at the time of vaccination. In addition, investigators for both trials reported lower vaccine efficacies in younger patients, a pattern “consistent with reduced efficacy in individuals who have not lived long enough to experience a natural infection,” the authors noted.
In an effort to provide guidance for future clinical trials and to predict the impact of wide-scale use of Dengvaxia, investigators developed a mathematical model of dengue transmission based on data from the two trials.
The model confirmed that secondary infections were nearly twice as likely to cause symptomatic infection, compared with primary and postsecondary infections.
In a highly important result, the model simulation showed that seropositive recipients always gained a substantial benefit – more than a 90% reduction in the risk of hospitalization because of dengue – from vaccination. However, among seronegative recipients, the vaccine initially induced near-perfect protection, but this protection rapidly decayed (mean duration, 7 months). Moreover, the model showed that seronegative recipients who received the vaccine were at an increased risk for hospitalization with dengue.
“This is true both in the short term and in the long term and raises fundamental issues about individual versus population benefits of vaccination,” investigators wrote. “Individual serological testing, if feasible, might radically improve the benefit-risk trade-off.”
The model also demonstrated that the optimal age for vaccination depends on the transmission intensity rate in a region where a child lives. In high-transmission settings, the optimal age to target for vaccination can be 9 years or younger, and as intensity of transmission decreases, optimal age of vaccination should increase, according to investigators.
The study was funded by the UK Medical Research Council, the UK National Institute of Health Research, the National Institutes of Health, and the Bill and Melinda Gates Foundation. Authors did not report any relevant disclosures.
jcraig@frontlinemedcom.com
On Twitter @jessnicolecraig
Pediatric patients with previous natural exposure to dengue virus benefit from the dengue virus vaccine, while vaccination of seronegative patients leads to an increased risk for hospitalization because of dengue, according to the results of a mathematical model simulation.
Because of the first approved dengue vaccine’s highly variable efficacy rates among pediatric patients, the vaccine should only be used in moderate to high transmission settings, the investigators who designed the model concluded in a paper published in Science.
Dengvaxia, developed by Sanofi-Pasteur, is a recombinant chimeric live attenuated dengue virus vaccine based on a yellow fever vaccine backbone. The vaccine’s development was “considerably more challenging than for other Flavivirus infections because of the immunological interactions between the four dengue virus serotypes and the risk of immune-mediated enhancement of disease” which causes secondary infections to lead to more severe disease, Neil Ferguson, PhD, of the Imperial College of London and his associates wrote (Science. 2016 Sep 2;353:1033-6. doi: 10.1126/science.aaf9590).
Despite the complexity of the virus and vaccine, Dengvaxia was recently approved for use in six countries, and two large multicenter phase III clinical trials recently concluded. Investigators for the trials, which involved over 30,000 children in Southeast Asia and Latin America, reported an overall vaccine efficacy of about 60% in cases of symptomatic dengue disease. However, the vaccine’s efficacy varied by severity of dengue infection and by age and serotype of the patient at time of vaccination. Investigators for both trials reported higher efficacy in patients with severe infection and in patients who were seropositive for dengue virus (indicating previous exposure to the virus) at the time of vaccination. In addition, investigators for both trials reported lower vaccine efficacies in younger patients, a pattern “consistent with reduced efficacy in individuals who have not lived long enough to experience a natural infection,” the authors noted.
In an effort to provide guidance for future clinical trials and to predict the impact of wide-scale use of Dengvaxia, investigators developed a mathematical model of dengue transmission based on data from the two trials.
The model confirmed that secondary infections were nearly twice as likely to cause symptomatic infection, compared with primary and postsecondary infections.
In a highly important result, the model simulation showed that seropositive recipients always gained a substantial benefit – more than a 90% reduction in the risk of hospitalization because of dengue – from vaccination. However, among seronegative recipients, the vaccine initially induced near-perfect protection, but this protection rapidly decayed (mean duration, 7 months). Moreover, the model showed that seronegative recipients who received the vaccine were at an increased risk for hospitalization with dengue.
“This is true both in the short term and in the long term and raises fundamental issues about individual versus population benefits of vaccination,” investigators wrote. “Individual serological testing, if feasible, might radically improve the benefit-risk trade-off.”
The model also demonstrated that the optimal age for vaccination depends on the transmission intensity rate in a region where a child lives. In high-transmission settings, the optimal age to target for vaccination can be 9 years or younger, and as intensity of transmission decreases, optimal age of vaccination should increase, according to investigators.
The study was funded by the UK Medical Research Council, the UK National Institute of Health Research, the National Institutes of Health, and the Bill and Melinda Gates Foundation. Authors did not report any relevant disclosures.
jcraig@frontlinemedcom.com
On Twitter @jessnicolecraig
Pediatric patients with previous natural exposure to dengue virus benefit from the dengue virus vaccine, while vaccination of seronegative patients leads to an increased risk for hospitalization because of dengue, according to the results of a mathematical model simulation.
Because of the first approved dengue vaccine’s highly variable efficacy rates among pediatric patients, the vaccine should only be used in moderate to high transmission settings, the investigators who designed the model concluded in a paper published in Science.
Dengvaxia, developed by Sanofi-Pasteur, is a recombinant chimeric live attenuated dengue virus vaccine based on a yellow fever vaccine backbone. The vaccine’s development was “considerably more challenging than for other Flavivirus infections because of the immunological interactions between the four dengue virus serotypes and the risk of immune-mediated enhancement of disease” which causes secondary infections to lead to more severe disease, Neil Ferguson, PhD, of the Imperial College of London and his associates wrote (Science. 2016 Sep 2;353:1033-6. doi: 10.1126/science.aaf9590).
Despite the complexity of the virus and vaccine, Dengvaxia was recently approved for use in six countries, and two large multicenter phase III clinical trials recently concluded. Investigators for the trials, which involved over 30,000 children in Southeast Asia and Latin America, reported an overall vaccine efficacy of about 60% in cases of symptomatic dengue disease. However, the vaccine’s efficacy varied by severity of dengue infection and by age and serotype of the patient at time of vaccination. Investigators for both trials reported higher efficacy in patients with severe infection and in patients who were seropositive for dengue virus (indicating previous exposure to the virus) at the time of vaccination. In addition, investigators for both trials reported lower vaccine efficacies in younger patients, a pattern “consistent with reduced efficacy in individuals who have not lived long enough to experience a natural infection,” the authors noted.
In an effort to provide guidance for future clinical trials and to predict the impact of wide-scale use of Dengvaxia, investigators developed a mathematical model of dengue transmission based on data from the two trials.
The model confirmed that secondary infections were nearly twice as likely to cause symptomatic infection, compared with primary and postsecondary infections.
In a highly important result, the model simulation showed that seropositive recipients always gained a substantial benefit – more than a 90% reduction in the risk of hospitalization because of dengue – from vaccination. However, among seronegative recipients, the vaccine initially induced near-perfect protection, but this protection rapidly decayed (mean duration, 7 months). Moreover, the model showed that seronegative recipients who received the vaccine were at an increased risk for hospitalization with dengue.
“This is true both in the short term and in the long term and raises fundamental issues about individual versus population benefits of vaccination,” investigators wrote. “Individual serological testing, if feasible, might radically improve the benefit-risk trade-off.”
The model also demonstrated that the optimal age for vaccination depends on the transmission intensity rate in a region where a child lives. In high-transmission settings, the optimal age to target for vaccination can be 9 years or younger, and as intensity of transmission decreases, optimal age of vaccination should increase, according to investigators.
The study was funded by the UK Medical Research Council, the UK National Institute of Health Research, the National Institutes of Health, and the Bill and Melinda Gates Foundation. Authors did not report any relevant disclosures.
jcraig@frontlinemedcom.com
On Twitter @jessnicolecraig
FROM SCIENCE
Key clinical point:
Major finding: Vaccine should only be used in moderate to high transmission settings. In high-transmission settings, the optimal age to target for vaccination is 9 years or younger.
Data source: Mathematical model simulation based on two large, multicenter, phase III clinical trials.
Disclosures: This study was funded by the UK Medical Research Council, the UK National Institute of Health Research, the National Institutes of Health, and the Bill and Melinda Gates Foundation. Authors did not report any relevant disclosures.
Missed Adult Vaccines Cost U.S. Nearly $9 Billion in 2015
Just how important are adult vaccines? According to a new study from Health Affairs missed vaccines cost the U.S. economy $8.95 billion in 2015. “This review not only estimated the direct costs and productivity losses due to inpatient and outpatient visits associated with vaccine-preventable diseases…but [it also] examined this across all vaccines recommended for US adults,” authors Sachiko Ozawa, Allison Portnoy, Hiwote Getaneh, Samantha Clark, Maria Knoll, David Bishai, H. Keri Yang, and Pallavi D. Patwardhan explained.
“Low rates of vaccine uptake lead to costs to individuals and society in terms of deaths and disabilities, which are avoidable, and they create economic losses from doctor visits, hospitalizations, and lost income,” the authors argued. “To identify the magnitude of this problem, we calculated the current economic burden that is attributable to vaccine-preventable diseases among US adults.
Not surprisingly, preventable influenza exacted the highest cost, estimated to be $5.8 billion. Pneumococcal disease was second, with an estimated cost of $1.9 billion, followed by herpes zoster ($782 million), human papillomavirus, and hepatitis B ($173 million).
Researchers were from University of North Carolina at Chapel Hill, Harvard T. H. Chan School of Public Health, in Boston, Massachusetts, MRDC in New York City, Johns Hopkins Bloomberg School of Public Health, in Baltimore, Maryland, and Merck. This study was funded by Merck. The study “highlights the need for US adults to better appreciate the value of vaccines to prevent economic burden,” they said.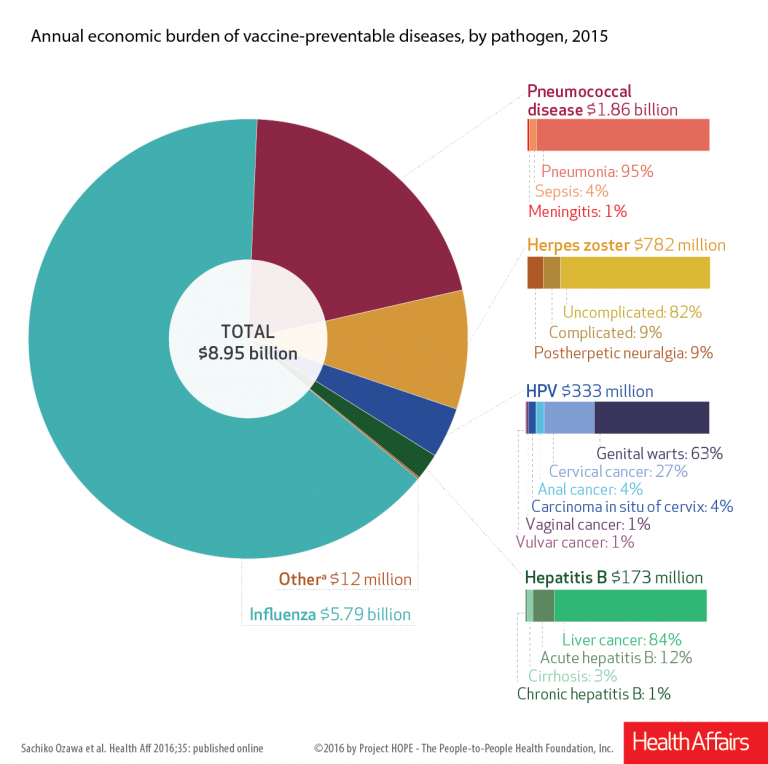
Just how important are adult vaccines? According to a new study from Health Affairs missed vaccines cost the U.S. economy $8.95 billion in 2015. “This review not only estimated the direct costs and productivity losses due to inpatient and outpatient visits associated with vaccine-preventable diseases…but [it also] examined this across all vaccines recommended for US adults,” authors Sachiko Ozawa, Allison Portnoy, Hiwote Getaneh, Samantha Clark, Maria Knoll, David Bishai, H. Keri Yang, and Pallavi D. Patwardhan explained.
“Low rates of vaccine uptake lead to costs to individuals and society in terms of deaths and disabilities, which are avoidable, and they create economic losses from doctor visits, hospitalizations, and lost income,” the authors argued. “To identify the magnitude of this problem, we calculated the current economic burden that is attributable to vaccine-preventable diseases among US adults.
Not surprisingly, preventable influenza exacted the highest cost, estimated to be $5.8 billion. Pneumococcal disease was second, with an estimated cost of $1.9 billion, followed by herpes zoster ($782 million), human papillomavirus, and hepatitis B ($173 million).
Researchers were from University of North Carolina at Chapel Hill, Harvard T. H. Chan School of Public Health, in Boston, Massachusetts, MRDC in New York City, Johns Hopkins Bloomberg School of Public Health, in Baltimore, Maryland, and Merck. This study was funded by Merck. The study “highlights the need for US adults to better appreciate the value of vaccines to prevent economic burden,” they said.
Just how important are adult vaccines? According to a new study from Health Affairs missed vaccines cost the U.S. economy $8.95 billion in 2015. “This review not only estimated the direct costs and productivity losses due to inpatient and outpatient visits associated with vaccine-preventable diseases…but [it also] examined this across all vaccines recommended for US adults,” authors Sachiko Ozawa, Allison Portnoy, Hiwote Getaneh, Samantha Clark, Maria Knoll, David Bishai, H. Keri Yang, and Pallavi D. Patwardhan explained.
“Low rates of vaccine uptake lead to costs to individuals and society in terms of deaths and disabilities, which are avoidable, and they create economic losses from doctor visits, hospitalizations, and lost income,” the authors argued. “To identify the magnitude of this problem, we calculated the current economic burden that is attributable to vaccine-preventable diseases among US adults.
Not surprisingly, preventable influenza exacted the highest cost, estimated to be $5.8 billion. Pneumococcal disease was second, with an estimated cost of $1.9 billion, followed by herpes zoster ($782 million), human papillomavirus, and hepatitis B ($173 million).
Researchers were from University of North Carolina at Chapel Hill, Harvard T. H. Chan School of Public Health, in Boston, Massachusetts, MRDC in New York City, Johns Hopkins Bloomberg School of Public Health, in Baltimore, Maryland, and Merck. This study was funded by Merck. The study “highlights the need for US adults to better appreciate the value of vaccines to prevent economic burden,” they said.
Novel Messenger RNA Vaccine in Development for Zika
A novel anti-Zika vaccine based on messenger RNA (mRNA) technology received financial backing from HHS. Moderna Therapeutics, Cambridge, Massachusetts, will get $8.2 million to accelerate the development of the vaccine.
Messenger RNA carries specific genetic codes to parts of the cell. The vaccine uses mRNA containing the genetic sequence of the Zika virus to generate an immune response.
This technology produces vaccine faster than other methods, which require the growth and purification of an attenuated or inactivated virus, HHS says. Moderna also is designing the vaccine to be easy to administer by not requiring any specialized delivery devices.
Under the initial 4-year agreement, HHS’ Biomedical Advanced Research and Development Authority (BARDA) will support a phase 1 clinical trial, toxicology studies, vaccine formulation, and manufacturing. If additional funding is identified the agreement could be extended to 5 years, with a total of $125.5 million to cover phase 2 and 3 clinical trials and large-scale manufacturing.
The funding is part of an obligated $85 million BARDA “reprogrammed” for Zika work. The funds are also being used to develop other Zika vaccines, blood screening tests, and pathogen reduction technologies.
A novel anti-Zika vaccine based on messenger RNA (mRNA) technology received financial backing from HHS. Moderna Therapeutics, Cambridge, Massachusetts, will get $8.2 million to accelerate the development of the vaccine.
Messenger RNA carries specific genetic codes to parts of the cell. The vaccine uses mRNA containing the genetic sequence of the Zika virus to generate an immune response.
This technology produces vaccine faster than other methods, which require the growth and purification of an attenuated or inactivated virus, HHS says. Moderna also is designing the vaccine to be easy to administer by not requiring any specialized delivery devices.
Under the initial 4-year agreement, HHS’ Biomedical Advanced Research and Development Authority (BARDA) will support a phase 1 clinical trial, toxicology studies, vaccine formulation, and manufacturing. If additional funding is identified the agreement could be extended to 5 years, with a total of $125.5 million to cover phase 2 and 3 clinical trials and large-scale manufacturing.
The funding is part of an obligated $85 million BARDA “reprogrammed” for Zika work. The funds are also being used to develop other Zika vaccines, blood screening tests, and pathogen reduction technologies.
A novel anti-Zika vaccine based on messenger RNA (mRNA) technology received financial backing from HHS. Moderna Therapeutics, Cambridge, Massachusetts, will get $8.2 million to accelerate the development of the vaccine.
Messenger RNA carries specific genetic codes to parts of the cell. The vaccine uses mRNA containing the genetic sequence of the Zika virus to generate an immune response.
This technology produces vaccine faster than other methods, which require the growth and purification of an attenuated or inactivated virus, HHS says. Moderna also is designing the vaccine to be easy to administer by not requiring any specialized delivery devices.
Under the initial 4-year agreement, HHS’ Biomedical Advanced Research and Development Authority (BARDA) will support a phase 1 clinical trial, toxicology studies, vaccine formulation, and manufacturing. If additional funding is identified the agreement could be extended to 5 years, with a total of $125.5 million to cover phase 2 and 3 clinical trials and large-scale manufacturing.
The funding is part of an obligated $85 million BARDA “reprogrammed” for Zika work. The funds are also being used to develop other Zika vaccines, blood screening tests, and pathogen reduction technologies.
Vaccination rates up in U.S. kindergartners in 2015, steady in 19- to 35-month-olds
Vaccination coverage for MMR and DTaP increased for children in kindergarten during the 2015-2016 school year, but remained steady for children aged 19-35 months in 2015, according to reports from the Centers for Disease Control and Prevention.
The median MMR vaccination rate for kindergartners in 2015 was 94.6%, up significantly from 92.6% in 2014. DTaP coverage also increased, rising from 92.4% to 94.2%. A total of 32 states saw an increase in MMR coverage in 2015, with 22 states reporting greater than 95% coverage. Only 3 states and the District of Columbia reported less than 90% coverage, down from 7 states and D.C. in 2014.
While the median vaccination rates were increased in 2015, the median exemption rate also increased by nearly 11% to 1.9% overall. This was caused in part by the addition of reports from Texas and Wyoming, neither of which reported the number of exemptions in 2014, the CDC investigators remarked.
In a second CDC report from Dr. Holly Hill and her associates based on data collected from the National Immunization Survey, the vaccination rate for children aged 19-35 months in 2015 did not increase significantly from the previous year (MMWR. 2016 Oct 6. doi: 10.15585/mmwr.mm639a4). The rate of children who received four or more doses of DTaP and at least one dose of MMR increased by 0.4 percentage points each from 84.2% to 84.6% and from 91.5% to 91.9%, respectively. The largest increase was seen in hepatitis A vaccine, where the rate of vaccination increased from 57.5% to 59.6%.
Healthy People 2020 goals for greater than 90% coverage for children aged 19-35 months were met for four vaccines in 2015: three or more doses of poliovirus vaccine, one or more doses of MMR, three or more doses of hepatitis B vaccine, and one or more doses of varicella vaccine. Vaccine coverage was lower in almost all cases for children living below the poverty level. The largest discrepancies were seen in rotavirus and varicella vaccines. The combined seven-vaccine series rate for children at or above the poverty line was 74.7%, and was 68.7% for children below the poverty line.
“Continued surveillance is needed to monitor coverage, locate pockets of susceptibility, and evaluate the impact of interventions designed to ensure that all children remain adequately protected against vaccine-preventable diseases,” Dr. Hill and her associates noted.
The CDC investigators had no relevant financial disclosures to report.
Vaccination coverage for MMR and DTaP increased for children in kindergarten during the 2015-2016 school year, but remained steady for children aged 19-35 months in 2015, according to reports from the Centers for Disease Control and Prevention.
The median MMR vaccination rate for kindergartners in 2015 was 94.6%, up significantly from 92.6% in 2014. DTaP coverage also increased, rising from 92.4% to 94.2%. A total of 32 states saw an increase in MMR coverage in 2015, with 22 states reporting greater than 95% coverage. Only 3 states and the District of Columbia reported less than 90% coverage, down from 7 states and D.C. in 2014.
While the median vaccination rates were increased in 2015, the median exemption rate also increased by nearly 11% to 1.9% overall. This was caused in part by the addition of reports from Texas and Wyoming, neither of which reported the number of exemptions in 2014, the CDC investigators remarked.
In a second CDC report from Dr. Holly Hill and her associates based on data collected from the National Immunization Survey, the vaccination rate for children aged 19-35 months in 2015 did not increase significantly from the previous year (MMWR. 2016 Oct 6. doi: 10.15585/mmwr.mm639a4). The rate of children who received four or more doses of DTaP and at least one dose of MMR increased by 0.4 percentage points each from 84.2% to 84.6% and from 91.5% to 91.9%, respectively. The largest increase was seen in hepatitis A vaccine, where the rate of vaccination increased from 57.5% to 59.6%.
Healthy People 2020 goals for greater than 90% coverage for children aged 19-35 months were met for four vaccines in 2015: three or more doses of poliovirus vaccine, one or more doses of MMR, three or more doses of hepatitis B vaccine, and one or more doses of varicella vaccine. Vaccine coverage was lower in almost all cases for children living below the poverty level. The largest discrepancies were seen in rotavirus and varicella vaccines. The combined seven-vaccine series rate for children at or above the poverty line was 74.7%, and was 68.7% for children below the poverty line.
“Continued surveillance is needed to monitor coverage, locate pockets of susceptibility, and evaluate the impact of interventions designed to ensure that all children remain adequately protected against vaccine-preventable diseases,” Dr. Hill and her associates noted.
The CDC investigators had no relevant financial disclosures to report.
Vaccination coverage for MMR and DTaP increased for children in kindergarten during the 2015-2016 school year, but remained steady for children aged 19-35 months in 2015, according to reports from the Centers for Disease Control and Prevention.
The median MMR vaccination rate for kindergartners in 2015 was 94.6%, up significantly from 92.6% in 2014. DTaP coverage also increased, rising from 92.4% to 94.2%. A total of 32 states saw an increase in MMR coverage in 2015, with 22 states reporting greater than 95% coverage. Only 3 states and the District of Columbia reported less than 90% coverage, down from 7 states and D.C. in 2014.
While the median vaccination rates were increased in 2015, the median exemption rate also increased by nearly 11% to 1.9% overall. This was caused in part by the addition of reports from Texas and Wyoming, neither of which reported the number of exemptions in 2014, the CDC investigators remarked.
In a second CDC report from Dr. Holly Hill and her associates based on data collected from the National Immunization Survey, the vaccination rate for children aged 19-35 months in 2015 did not increase significantly from the previous year (MMWR. 2016 Oct 6. doi: 10.15585/mmwr.mm639a4). The rate of children who received four or more doses of DTaP and at least one dose of MMR increased by 0.4 percentage points each from 84.2% to 84.6% and from 91.5% to 91.9%, respectively. The largest increase was seen in hepatitis A vaccine, where the rate of vaccination increased from 57.5% to 59.6%.
Healthy People 2020 goals for greater than 90% coverage for children aged 19-35 months were met for four vaccines in 2015: three or more doses of poliovirus vaccine, one or more doses of MMR, three or more doses of hepatitis B vaccine, and one or more doses of varicella vaccine. Vaccine coverage was lower in almost all cases for children living below the poverty level. The largest discrepancies were seen in rotavirus and varicella vaccines. The combined seven-vaccine series rate for children at or above the poverty line was 74.7%, and was 68.7% for children below the poverty line.
“Continued surveillance is needed to monitor coverage, locate pockets of susceptibility, and evaluate the impact of interventions designed to ensure that all children remain adequately protected against vaccine-preventable diseases,” Dr. Hill and her associates noted.
The CDC investigators had no relevant financial disclosures to report.
Promise of effective RSV vaccines on horizon
ATLANTA – A new vaccine for respiratory syncytial virus may truly be on the horizon, given recent advances in basic science and a marked increase in interest in the pharmaceutical industry.
That’s the conclusion of Larry Anderson, MD, professor of infectious disease in the Emory University department of pediatrics, who presented the most updated research and progress on a respiratory syncytial virus (RSV) vaccine during a conference sponsored by the Centers for Disease Control and Prevention.
The high hospitalization rates of infants with RSV, also associated with later development of reactive airway disease and asthma, highlight the challenge of developing a vaccine, Dr. Anderson said.
“The infant has an immature immune system less able to respond vigorously to a vaccine,” he said. “Also, it is highly susceptible to the disease of RSV, and therefore safety becomes an issue at least in terms of the live virus vaccine.” Furthermore, RSV causes multiple repeat infections throughout life, “which underlines the difficulty in inducing a protective immune response,” he added.
But Dr. Anderson said he believes there is light at the end of the tunnel when it comes to a vaccine for the virus.
“I think in terms of [the] potential of having an RSV vaccine in the near future, now is the most promising time, recognizing that work on an RSV vaccine has been going on for over 50 years without success to date,” he said. Significant advances in basic biology, immunology, and vaccinology have led to a better understanding of the virus, and new tools such as reverse genetics make “it possible to make any live virus you want as long as you know what you want,” he added.
Dr. Anderson provided an overview of published and preliminary data on the progress of more than five dozen groups working on an RSV vaccine. About 70% of these candidates remain in preclinical research, primarily in animal models. Of the dozen in phase I, several look promising, he said. Another six vaccines are in phase II or phase III testing, and MedImmune’s Synagis is market approved. But not all target infants.
“The first and highest priority is the young infant, particularly the under 2- to 4-month-old,” he said. In infants aged 4-6 months, it’s likely easier to induce an immune response, and there’s less susceptibility to disease with replication of the virus, he said. The elderly, also at high risk for RSV, would be another target population.
Potentially “the lowest apple on the tree for immunization,” Dr. Anderson said, would be pregnant women because a vaccine could prevent infection, disease, and transmission to their infant before he might be able to be vaccinated.
“There, the primary purpose is to increase the kind of antibody that is transferred across the placenta to the fetus to protect from RSV disease” in the infant after birth, he said. Data suggest it’s possible to increase titer antibodies in infants up to 4 months from maternal immunization, possibly longer, depending on how much the vaccine can induce antibodies in the woman.
For young children, he noted that five live attenuated RSV vaccines are in phase I testing, and four others are in phase I that use a virus vector to deliver the F protein – three using adenovirus and one with a modified vaccinia Ankara virus. A handful of subunit vaccines have reached phase II, and Novavax is furthest along in phase III, but these target older children and adults, including pregnant women.
“There’s going to be a lot of data in the coming year on completed clinical trials, and that’s going to tell us a lot about where we are,” Dr. Anderson said. “The young infant is the most challenging for a vaccine.” But, he added, “new information on protective immunity and disease pathogenesis should help achieve or improve vaccines in the future.”
Dr. Anderson has consulted on RSV vaccines for MedImmune, Novartis, Crucell Holland, and AVC, and has served on a Moderna Therapeutics scientific advisory board. His lab also has received grant funding from Trellis RSV Holdings, and he coinvented several RSV-related vaccine and treatment patents held by the CDC.
ATLANTA – A new vaccine for respiratory syncytial virus may truly be on the horizon, given recent advances in basic science and a marked increase in interest in the pharmaceutical industry.
That’s the conclusion of Larry Anderson, MD, professor of infectious disease in the Emory University department of pediatrics, who presented the most updated research and progress on a respiratory syncytial virus (RSV) vaccine during a conference sponsored by the Centers for Disease Control and Prevention.
The high hospitalization rates of infants with RSV, also associated with later development of reactive airway disease and asthma, highlight the challenge of developing a vaccine, Dr. Anderson said.
“The infant has an immature immune system less able to respond vigorously to a vaccine,” he said. “Also, it is highly susceptible to the disease of RSV, and therefore safety becomes an issue at least in terms of the live virus vaccine.” Furthermore, RSV causes multiple repeat infections throughout life, “which underlines the difficulty in inducing a protective immune response,” he added.
But Dr. Anderson said he believes there is light at the end of the tunnel when it comes to a vaccine for the virus.
“I think in terms of [the] potential of having an RSV vaccine in the near future, now is the most promising time, recognizing that work on an RSV vaccine has been going on for over 50 years without success to date,” he said. Significant advances in basic biology, immunology, and vaccinology have led to a better understanding of the virus, and new tools such as reverse genetics make “it possible to make any live virus you want as long as you know what you want,” he added.
Dr. Anderson provided an overview of published and preliminary data on the progress of more than five dozen groups working on an RSV vaccine. About 70% of these candidates remain in preclinical research, primarily in animal models. Of the dozen in phase I, several look promising, he said. Another six vaccines are in phase II or phase III testing, and MedImmune’s Synagis is market approved. But not all target infants.
“The first and highest priority is the young infant, particularly the under 2- to 4-month-old,” he said. In infants aged 4-6 months, it’s likely easier to induce an immune response, and there’s less susceptibility to disease with replication of the virus, he said. The elderly, also at high risk for RSV, would be another target population.
Potentially “the lowest apple on the tree for immunization,” Dr. Anderson said, would be pregnant women because a vaccine could prevent infection, disease, and transmission to their infant before he might be able to be vaccinated.
“There, the primary purpose is to increase the kind of antibody that is transferred across the placenta to the fetus to protect from RSV disease” in the infant after birth, he said. Data suggest it’s possible to increase titer antibodies in infants up to 4 months from maternal immunization, possibly longer, depending on how much the vaccine can induce antibodies in the woman.
For young children, he noted that five live attenuated RSV vaccines are in phase I testing, and four others are in phase I that use a virus vector to deliver the F protein – three using adenovirus and one with a modified vaccinia Ankara virus. A handful of subunit vaccines have reached phase II, and Novavax is furthest along in phase III, but these target older children and adults, including pregnant women.
“There’s going to be a lot of data in the coming year on completed clinical trials, and that’s going to tell us a lot about where we are,” Dr. Anderson said. “The young infant is the most challenging for a vaccine.” But, he added, “new information on protective immunity and disease pathogenesis should help achieve or improve vaccines in the future.”
Dr. Anderson has consulted on RSV vaccines for MedImmune, Novartis, Crucell Holland, and AVC, and has served on a Moderna Therapeutics scientific advisory board. His lab also has received grant funding from Trellis RSV Holdings, and he coinvented several RSV-related vaccine and treatment patents held by the CDC.
ATLANTA – A new vaccine for respiratory syncytial virus may truly be on the horizon, given recent advances in basic science and a marked increase in interest in the pharmaceutical industry.
That’s the conclusion of Larry Anderson, MD, professor of infectious disease in the Emory University department of pediatrics, who presented the most updated research and progress on a respiratory syncytial virus (RSV) vaccine during a conference sponsored by the Centers for Disease Control and Prevention.
The high hospitalization rates of infants with RSV, also associated with later development of reactive airway disease and asthma, highlight the challenge of developing a vaccine, Dr. Anderson said.
“The infant has an immature immune system less able to respond vigorously to a vaccine,” he said. “Also, it is highly susceptible to the disease of RSV, and therefore safety becomes an issue at least in terms of the live virus vaccine.” Furthermore, RSV causes multiple repeat infections throughout life, “which underlines the difficulty in inducing a protective immune response,” he added.
But Dr. Anderson said he believes there is light at the end of the tunnel when it comes to a vaccine for the virus.
“I think in terms of [the] potential of having an RSV vaccine in the near future, now is the most promising time, recognizing that work on an RSV vaccine has been going on for over 50 years without success to date,” he said. Significant advances in basic biology, immunology, and vaccinology have led to a better understanding of the virus, and new tools such as reverse genetics make “it possible to make any live virus you want as long as you know what you want,” he added.
Dr. Anderson provided an overview of published and preliminary data on the progress of more than five dozen groups working on an RSV vaccine. About 70% of these candidates remain in preclinical research, primarily in animal models. Of the dozen in phase I, several look promising, he said. Another six vaccines are in phase II or phase III testing, and MedImmune’s Synagis is market approved. But not all target infants.
“The first and highest priority is the young infant, particularly the under 2- to 4-month-old,” he said. In infants aged 4-6 months, it’s likely easier to induce an immune response, and there’s less susceptibility to disease with replication of the virus, he said. The elderly, also at high risk for RSV, would be another target population.
Potentially “the lowest apple on the tree for immunization,” Dr. Anderson said, would be pregnant women because a vaccine could prevent infection, disease, and transmission to their infant before he might be able to be vaccinated.
“There, the primary purpose is to increase the kind of antibody that is transferred across the placenta to the fetus to protect from RSV disease” in the infant after birth, he said. Data suggest it’s possible to increase titer antibodies in infants up to 4 months from maternal immunization, possibly longer, depending on how much the vaccine can induce antibodies in the woman.
For young children, he noted that five live attenuated RSV vaccines are in phase I testing, and four others are in phase I that use a virus vector to deliver the F protein – three using adenovirus and one with a modified vaccinia Ankara virus. A handful of subunit vaccines have reached phase II, and Novavax is furthest along in phase III, but these target older children and adults, including pregnant women.
“There’s going to be a lot of data in the coming year on completed clinical trials, and that’s going to tell us a lot about where we are,” Dr. Anderson said. “The young infant is the most challenging for a vaccine.” But, he added, “new information on protective immunity and disease pathogenesis should help achieve or improve vaccines in the future.”
Dr. Anderson has consulted on RSV vaccines for MedImmune, Novartis, Crucell Holland, and AVC, and has served on a Moderna Therapeutics scientific advisory board. His lab also has received grant funding from Trellis RSV Holdings, and he coinvented several RSV-related vaccine and treatment patents held by the CDC.
EXPERT ANALYSIS FROM THE NATIONAL IMMUNIZATION CONFERENCE
Key clinical point: A respiratory syncytial virus vaccine is closer to reality now than at any other time.
Major finding: 62 RSV vaccines are in development, with approximately 70% in preclinical studies.
Data source: Based on a review of the current state of research into an RSV vaccine and the burden of RSV disease.
Disclosures: Dr. Anderson has consulted on RSV vaccines for MedImmune, Novartis, Crucell Holland, and AVC, and has served on a Moderna Therapeutics scientific advisory board. His lab also has received grant funding from Trellis RSV Holdings, and he coinvented several RSV-related vaccine and treatment patents held by the CDC.
Strongly recommend HPV vaccine to increase uptake
To convince parents to get their kids vaccinated against human papillomavirus (HPV), tell them “I strongly believe in the importance of this cancer-preventing vaccine for [their child’s name],” researchers concluded after an online survey of 1,504 parents of children aged 11-17 years.
Sixty-five percent of parents agreed it was a persuasive argument, the highest percentage among 15 statements in favor of vaccination; 69% of 776 surveyed physicians said they’d use it in practice, also the highest physician endorsement for the various arguments (Cancer Epidemiol Biomarkers Prev. 2016 Sep 30. doi: 10.1158/1055-9965.EPI-16-0224).
In general, parents disinclined to vaccinate were most receptive to messages about HPV infection being common, cancers caused by HPV, and HPV vaccine effectiveness.
Other persuasive arguments included “[child’s name] can get anal/cervical cancer as an adult, but you can stop that right now;” and “there will be many things in [child’s name]’s life that you can’t control. But you can control whether [he/she] gets some dangerous kinds of HPV.”
Both “placed the onus of protection on the parent and emphasized the control they possess over their child’s health. This finding is aligned with the tenets of various theories of fear appeals, which posit that fear messages inspire action if the receiver believes he or she has some control over the situation. Parents without prior intentions to vaccinate their child may be particularly receptive to messages that arouse fear while fostering a sense of efficacy,” said Teri Malo, PhD, of the University of North Carolina, Chapel Hill, and associates.
Although almost 60% of parents said they’d pay attention if told they could control if their child gets HPV cancer, only 37% of physicians said they’d use the argument. It’s not clear why, but the team said the finding was “concerning ... given that it was one of the top messages that would persuade parents without prior intentions to vaccinate their child.”
At present, 40% of girls and 22% of boys aged 13-17 years receive all three HPV shots. The goal of the study was to help doctors increase those numbers with persuasive arguments. “Physician communication about human papillomavirus vaccine is a key determinant of uptake,” but not all physicians strongly recommend the vaccine, especially if they anticipate parent resistance. “We sought to identify messages that would motivate HPV vaccination,” the researchers said.
Argument by analogy was the weakest approach. Only 5% of parents found “would you wait until [child’s name] is in a car accident before you tell [him/her] to wear a seatbelt?” persuasive, and only 9% of physicians said they’d use it, which made it the least endorsed statement in the study.
The same arguments seemed to work regardless of parents’ race, education, or income, or their child’s age or sex, which led the team to suggest the messages will work across demographic subgroups.
Slightly more moms than dads filled out the survey, and most were white, with about 9% of parents black and 14% Hispanic. Most parents lived in metropolitan areas, nearly two-thirds had at least some college, and almost half reported an annual household income of at least $75,000. For simplicity, parents limited their responses to their child with the most recent birthday. About half of children were boys, and just over half hadn’t been vaccinated against HPV.
About two-thirds of the physicians were men. Over half had practiced for at least 20 years, just over half were pediatricians, and the rest were family practitioners.
The work was funded by an unrestricted educational grant from Pfizer to senior investigator Noel Brewer, PhD. Dr. Brewer receives commercial research grants from Merck, maker of the HPV vaccine Gardasil, and Pfizer. He is also a Merck consultant and advisory board member. Coauthor Melissa B. Gilkey, PhD, and Dr. Malo were supported by other grants. The report noted that “the costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with” U.S. law.
To convince parents to get their kids vaccinated against human papillomavirus (HPV), tell them “I strongly believe in the importance of this cancer-preventing vaccine for [their child’s name],” researchers concluded after an online survey of 1,504 parents of children aged 11-17 years.
Sixty-five percent of parents agreed it was a persuasive argument, the highest percentage among 15 statements in favor of vaccination; 69% of 776 surveyed physicians said they’d use it in practice, also the highest physician endorsement for the various arguments (Cancer Epidemiol Biomarkers Prev. 2016 Sep 30. doi: 10.1158/1055-9965.EPI-16-0224).
In general, parents disinclined to vaccinate were most receptive to messages about HPV infection being common, cancers caused by HPV, and HPV vaccine effectiveness.
Other persuasive arguments included “[child’s name] can get anal/cervical cancer as an adult, but you can stop that right now;” and “there will be many things in [child’s name]’s life that you can’t control. But you can control whether [he/she] gets some dangerous kinds of HPV.”
Both “placed the onus of protection on the parent and emphasized the control they possess over their child’s health. This finding is aligned with the tenets of various theories of fear appeals, which posit that fear messages inspire action if the receiver believes he or she has some control over the situation. Parents without prior intentions to vaccinate their child may be particularly receptive to messages that arouse fear while fostering a sense of efficacy,” said Teri Malo, PhD, of the University of North Carolina, Chapel Hill, and associates.
Although almost 60% of parents said they’d pay attention if told they could control if their child gets HPV cancer, only 37% of physicians said they’d use the argument. It’s not clear why, but the team said the finding was “concerning ... given that it was one of the top messages that would persuade parents without prior intentions to vaccinate their child.”
At present, 40% of girls and 22% of boys aged 13-17 years receive all three HPV shots. The goal of the study was to help doctors increase those numbers with persuasive arguments. “Physician communication about human papillomavirus vaccine is a key determinant of uptake,” but not all physicians strongly recommend the vaccine, especially if they anticipate parent resistance. “We sought to identify messages that would motivate HPV vaccination,” the researchers said.
Argument by analogy was the weakest approach. Only 5% of parents found “would you wait until [child’s name] is in a car accident before you tell [him/her] to wear a seatbelt?” persuasive, and only 9% of physicians said they’d use it, which made it the least endorsed statement in the study.
The same arguments seemed to work regardless of parents’ race, education, or income, or their child’s age or sex, which led the team to suggest the messages will work across demographic subgroups.
Slightly more moms than dads filled out the survey, and most were white, with about 9% of parents black and 14% Hispanic. Most parents lived in metropolitan areas, nearly two-thirds had at least some college, and almost half reported an annual household income of at least $75,000. For simplicity, parents limited their responses to their child with the most recent birthday. About half of children were boys, and just over half hadn’t been vaccinated against HPV.
About two-thirds of the physicians were men. Over half had practiced for at least 20 years, just over half were pediatricians, and the rest were family practitioners.
The work was funded by an unrestricted educational grant from Pfizer to senior investigator Noel Brewer, PhD. Dr. Brewer receives commercial research grants from Merck, maker of the HPV vaccine Gardasil, and Pfizer. He is also a Merck consultant and advisory board member. Coauthor Melissa B. Gilkey, PhD, and Dr. Malo were supported by other grants. The report noted that “the costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with” U.S. law.
To convince parents to get their kids vaccinated against human papillomavirus (HPV), tell them “I strongly believe in the importance of this cancer-preventing vaccine for [their child’s name],” researchers concluded after an online survey of 1,504 parents of children aged 11-17 years.
Sixty-five percent of parents agreed it was a persuasive argument, the highest percentage among 15 statements in favor of vaccination; 69% of 776 surveyed physicians said they’d use it in practice, also the highest physician endorsement for the various arguments (Cancer Epidemiol Biomarkers Prev. 2016 Sep 30. doi: 10.1158/1055-9965.EPI-16-0224).
In general, parents disinclined to vaccinate were most receptive to messages about HPV infection being common, cancers caused by HPV, and HPV vaccine effectiveness.
Other persuasive arguments included “[child’s name] can get anal/cervical cancer as an adult, but you can stop that right now;” and “there will be many things in [child’s name]’s life that you can’t control. But you can control whether [he/she] gets some dangerous kinds of HPV.”
Both “placed the onus of protection on the parent and emphasized the control they possess over their child’s health. This finding is aligned with the tenets of various theories of fear appeals, which posit that fear messages inspire action if the receiver believes he or she has some control over the situation. Parents without prior intentions to vaccinate their child may be particularly receptive to messages that arouse fear while fostering a sense of efficacy,” said Teri Malo, PhD, of the University of North Carolina, Chapel Hill, and associates.
Although almost 60% of parents said they’d pay attention if told they could control if their child gets HPV cancer, only 37% of physicians said they’d use the argument. It’s not clear why, but the team said the finding was “concerning ... given that it was one of the top messages that would persuade parents without prior intentions to vaccinate their child.”
At present, 40% of girls and 22% of boys aged 13-17 years receive all three HPV shots. The goal of the study was to help doctors increase those numbers with persuasive arguments. “Physician communication about human papillomavirus vaccine is a key determinant of uptake,” but not all physicians strongly recommend the vaccine, especially if they anticipate parent resistance. “We sought to identify messages that would motivate HPV vaccination,” the researchers said.
Argument by analogy was the weakest approach. Only 5% of parents found “would you wait until [child’s name] is in a car accident before you tell [him/her] to wear a seatbelt?” persuasive, and only 9% of physicians said they’d use it, which made it the least endorsed statement in the study.
The same arguments seemed to work regardless of parents’ race, education, or income, or their child’s age or sex, which led the team to suggest the messages will work across demographic subgroups.
Slightly more moms than dads filled out the survey, and most were white, with about 9% of parents black and 14% Hispanic. Most parents lived in metropolitan areas, nearly two-thirds had at least some college, and almost half reported an annual household income of at least $75,000. For simplicity, parents limited their responses to their child with the most recent birthday. About half of children were boys, and just over half hadn’t been vaccinated against HPV.
About two-thirds of the physicians were men. Over half had practiced for at least 20 years, just over half were pediatricians, and the rest were family practitioners.
The work was funded by an unrestricted educational grant from Pfizer to senior investigator Noel Brewer, PhD. Dr. Brewer receives commercial research grants from Merck, maker of the HPV vaccine Gardasil, and Pfizer. He is also a Merck consultant and advisory board member. Coauthor Melissa B. Gilkey, PhD, and Dr. Malo were supported by other grants. The report noted that “the costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with” U.S. law.
Key clinical point:
Major finding: Sixty-five percent of parents agreed it was a persuasive argument, the highest percentage among 15 statements in favor of vaccination; 69% of 776 surveyed physicians said they’d use it in practice.
Data source: Online survey of 1,504 parents of children aged 11-17 years old, and 776 physicians.
Disclosures: The work was funded by an unrestricted educational grant from Pfizer to senior investigator Noel Brewer, PhD. Dr. Brewer receives commercial research grants from Merck, maker of the HPV vaccine Gardasil, and Pfizer. He is also a Merck consultant and advisory board member. Coauthor Melissa B. Gilkey, PhD, and Dr. Malo were supported by other grants. The report noted that “the costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with” U.S. law.