User login
Abaloparatide shows no effect on cardiovascular risk in postmenopausal women
Osteoporosis treatment with abaloparatide in postmenopausal women does not lead to increased cardiovascular risk, according to a post hoc analysis of the pivotal ACTIVE and ACTIVExtend trials.
“Neither treatment with abaloparatide or teriparatide was associated with an increase in serious cardiac [adverse events],” wrote Felicia Cosman, MD, of Columbia University, New York, and coauthors. The study was published in the Journal of Clinical Endocrinology.
To assess the cardiovascular safety profile of abaloparatide, a synthetic analogue of parathyroid hormone–related peptide, the researchers analyzed data on heart rate, blood pressure and cardiovascular-related adverse events (AEs) from patients taking part in the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE) trial and its ACTIVExtend extension study.
The 2,460 participants in the ACTIVE trial were postmenopausal women between the ages of 49 and 86 years with osteoporosis; they were given 80 mcg of daily subcutaneous abaloparatide, 20 mcg of open-label daily subcutaneous teriparatide, or placebo in roughly equal numbers for 18 months. After a 1-month treatment-free period, 1,133 eligible participants from either the abaloparatide or placebo groups were enrolled in ACTIVExtend and given 70 mg of open-label alendronate once a week for 24 months. Because heart rate was only assessed pre- and post dose in the ACTIVE trial, an additional pharmacology study of abaloparatide involving 55 healthy volunteers (32 men and 23 women) was undertaken. After a dose of either abaloparatide or placebo, heart rate was measured at 15, 30, and 45 minutes and 1, 1.5, 2, 2.5, 4, 6, 8, and 12 hours.
Overall, treatment-emergent AEs were higher in the abaloparatide (165, 20.1%) and teriparatide (106, 13%) groups, compared with placebo (74, 9%), as were AEs that led to discontinuation of the study and were potentially associated with changes in heart rate or BP (27 in abaloparatide, 11 in teriparatide, and 5 in placebo). However, the percentage of patients with serious cardiac AEs was similar across groups (1%, 1%, and 0.9%, respectively).
During the ACTIVE trial, major cardiac adverse events plus heart failure were more common in the placebo group (1.7%) than the abaloparatide (0.5%) or teriparatide (0.6%) groups. During ACTIVExtend, major cardiac adverse plus heart failure were similarly common in the abaloparatide/alendronate (1.6%) and the placebo/alendronate (1.6%) groups.
On day 1 of treatment during ACTIVE, the mean change in heart rate from pretreatment to an hour post treatment was 7.9 bpm, 5.3 bpm, and 1.2 bpm for abaloparatide, teriparatide, and placebo, respectively (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .05 for abaloparatide vs. teriparatide).
Subsequent visits saw similar changes. The mean maximum heart rate at 1 hour post dose was 80.7 bpm for abaloparatide, 79.0 bpm for teriparatide, and 73.7 bpm for placebo (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .01 for abaloparatide vs. teriparatide). In the study of healthy volunteers, HR peaked at 15 minutes after dosing and then declined, resolving within 2.5-4 hours.
From predose to 1 hour post dose, small but significant decreases were observed in mean supine systolic and diastolic BP across groups (–2.7/–3.6 mm Hg with abaloparatide, –2.0/–3.6 with teriparatide, –1.5/–2.3 with placebo). During the first year of ACTIVE, the mean maximal decrease in BP from predose to 1 hour post dose was slightly higher (1-2 mm Hg) in the abaloparatide and teriparatide groups, compared with the placebo group (P < .05).
The authors acknowledged their study’s limitations, including the analysis of major cardiac adverse plus heart failure in ACTIVE being limited because of a low number of events and the trial not being designed in that regard.
Abaloparatide was approved by the Food and Drug Administration in 2017 on the basis of results from the ACTIVE and ACTIVExtend trials showing significant reductions in new vertebral and nonvertebral fractures, compared with placebo.
The analysis was partially funded by Radius Health. Its authors acknowledged numerous potential conflicts of interest, including receiving grants and research support from various organizations and pharmaceutical companies.
SOURCE: Cosman F et al. J Clin Endocrinol Metab. 2020 Jul 13. doi: 10.1210/clinem/dgaa450.
Osteoporosis treatment with abaloparatide in postmenopausal women does not lead to increased cardiovascular risk, according to a post hoc analysis of the pivotal ACTIVE and ACTIVExtend trials.
“Neither treatment with abaloparatide or teriparatide was associated with an increase in serious cardiac [adverse events],” wrote Felicia Cosman, MD, of Columbia University, New York, and coauthors. The study was published in the Journal of Clinical Endocrinology.
To assess the cardiovascular safety profile of abaloparatide, a synthetic analogue of parathyroid hormone–related peptide, the researchers analyzed data on heart rate, blood pressure and cardiovascular-related adverse events (AEs) from patients taking part in the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE) trial and its ACTIVExtend extension study.
The 2,460 participants in the ACTIVE trial were postmenopausal women between the ages of 49 and 86 years with osteoporosis; they were given 80 mcg of daily subcutaneous abaloparatide, 20 mcg of open-label daily subcutaneous teriparatide, or placebo in roughly equal numbers for 18 months. After a 1-month treatment-free period, 1,133 eligible participants from either the abaloparatide or placebo groups were enrolled in ACTIVExtend and given 70 mg of open-label alendronate once a week for 24 months. Because heart rate was only assessed pre- and post dose in the ACTIVE trial, an additional pharmacology study of abaloparatide involving 55 healthy volunteers (32 men and 23 women) was undertaken. After a dose of either abaloparatide or placebo, heart rate was measured at 15, 30, and 45 minutes and 1, 1.5, 2, 2.5, 4, 6, 8, and 12 hours.
Overall, treatment-emergent AEs were higher in the abaloparatide (165, 20.1%) and teriparatide (106, 13%) groups, compared with placebo (74, 9%), as were AEs that led to discontinuation of the study and were potentially associated with changes in heart rate or BP (27 in abaloparatide, 11 in teriparatide, and 5 in placebo). However, the percentage of patients with serious cardiac AEs was similar across groups (1%, 1%, and 0.9%, respectively).
During the ACTIVE trial, major cardiac adverse events plus heart failure were more common in the placebo group (1.7%) than the abaloparatide (0.5%) or teriparatide (0.6%) groups. During ACTIVExtend, major cardiac adverse plus heart failure were similarly common in the abaloparatide/alendronate (1.6%) and the placebo/alendronate (1.6%) groups.
On day 1 of treatment during ACTIVE, the mean change in heart rate from pretreatment to an hour post treatment was 7.9 bpm, 5.3 bpm, and 1.2 bpm for abaloparatide, teriparatide, and placebo, respectively (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .05 for abaloparatide vs. teriparatide).
Subsequent visits saw similar changes. The mean maximum heart rate at 1 hour post dose was 80.7 bpm for abaloparatide, 79.0 bpm for teriparatide, and 73.7 bpm for placebo (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .01 for abaloparatide vs. teriparatide). In the study of healthy volunteers, HR peaked at 15 minutes after dosing and then declined, resolving within 2.5-4 hours.
From predose to 1 hour post dose, small but significant decreases were observed in mean supine systolic and diastolic BP across groups (–2.7/–3.6 mm Hg with abaloparatide, –2.0/–3.6 with teriparatide, –1.5/–2.3 with placebo). During the first year of ACTIVE, the mean maximal decrease in BP from predose to 1 hour post dose was slightly higher (1-2 mm Hg) in the abaloparatide and teriparatide groups, compared with the placebo group (P < .05).
The authors acknowledged their study’s limitations, including the analysis of major cardiac adverse plus heart failure in ACTIVE being limited because of a low number of events and the trial not being designed in that regard.
Abaloparatide was approved by the Food and Drug Administration in 2017 on the basis of results from the ACTIVE and ACTIVExtend trials showing significant reductions in new vertebral and nonvertebral fractures, compared with placebo.
The analysis was partially funded by Radius Health. Its authors acknowledged numerous potential conflicts of interest, including receiving grants and research support from various organizations and pharmaceutical companies.
SOURCE: Cosman F et al. J Clin Endocrinol Metab. 2020 Jul 13. doi: 10.1210/clinem/dgaa450.
Osteoporosis treatment with abaloparatide in postmenopausal women does not lead to increased cardiovascular risk, according to a post hoc analysis of the pivotal ACTIVE and ACTIVExtend trials.
“Neither treatment with abaloparatide or teriparatide was associated with an increase in serious cardiac [adverse events],” wrote Felicia Cosman, MD, of Columbia University, New York, and coauthors. The study was published in the Journal of Clinical Endocrinology.
To assess the cardiovascular safety profile of abaloparatide, a synthetic analogue of parathyroid hormone–related peptide, the researchers analyzed data on heart rate, blood pressure and cardiovascular-related adverse events (AEs) from patients taking part in the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE) trial and its ACTIVExtend extension study.
The 2,460 participants in the ACTIVE trial were postmenopausal women between the ages of 49 and 86 years with osteoporosis; they were given 80 mcg of daily subcutaneous abaloparatide, 20 mcg of open-label daily subcutaneous teriparatide, or placebo in roughly equal numbers for 18 months. After a 1-month treatment-free period, 1,133 eligible participants from either the abaloparatide or placebo groups were enrolled in ACTIVExtend and given 70 mg of open-label alendronate once a week for 24 months. Because heart rate was only assessed pre- and post dose in the ACTIVE trial, an additional pharmacology study of abaloparatide involving 55 healthy volunteers (32 men and 23 women) was undertaken. After a dose of either abaloparatide or placebo, heart rate was measured at 15, 30, and 45 minutes and 1, 1.5, 2, 2.5, 4, 6, 8, and 12 hours.
Overall, treatment-emergent AEs were higher in the abaloparatide (165, 20.1%) and teriparatide (106, 13%) groups, compared with placebo (74, 9%), as were AEs that led to discontinuation of the study and were potentially associated with changes in heart rate or BP (27 in abaloparatide, 11 in teriparatide, and 5 in placebo). However, the percentage of patients with serious cardiac AEs was similar across groups (1%, 1%, and 0.9%, respectively).
During the ACTIVE trial, major cardiac adverse events plus heart failure were more common in the placebo group (1.7%) than the abaloparatide (0.5%) or teriparatide (0.6%) groups. During ACTIVExtend, major cardiac adverse plus heart failure were similarly common in the abaloparatide/alendronate (1.6%) and the placebo/alendronate (1.6%) groups.
On day 1 of treatment during ACTIVE, the mean change in heart rate from pretreatment to an hour post treatment was 7.9 bpm, 5.3 bpm, and 1.2 bpm for abaloparatide, teriparatide, and placebo, respectively (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .05 for abaloparatide vs. teriparatide).
Subsequent visits saw similar changes. The mean maximum heart rate at 1 hour post dose was 80.7 bpm for abaloparatide, 79.0 bpm for teriparatide, and 73.7 bpm for placebo (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .01 for abaloparatide vs. teriparatide). In the study of healthy volunteers, HR peaked at 15 minutes after dosing and then declined, resolving within 2.5-4 hours.
From predose to 1 hour post dose, small but significant decreases were observed in mean supine systolic and diastolic BP across groups (–2.7/–3.6 mm Hg with abaloparatide, –2.0/–3.6 with teriparatide, –1.5/–2.3 with placebo). During the first year of ACTIVE, the mean maximal decrease in BP from predose to 1 hour post dose was slightly higher (1-2 mm Hg) in the abaloparatide and teriparatide groups, compared with the placebo group (P < .05).
The authors acknowledged their study’s limitations, including the analysis of major cardiac adverse plus heart failure in ACTIVE being limited because of a low number of events and the trial not being designed in that regard.
Abaloparatide was approved by the Food and Drug Administration in 2017 on the basis of results from the ACTIVE and ACTIVExtend trials showing significant reductions in new vertebral and nonvertebral fractures, compared with placebo.
The analysis was partially funded by Radius Health. Its authors acknowledged numerous potential conflicts of interest, including receiving grants and research support from various organizations and pharmaceutical companies.
SOURCE: Cosman F et al. J Clin Endocrinol Metab. 2020 Jul 13. doi: 10.1210/clinem/dgaa450.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
USPSTF expands options for cervical cancer screening
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
PRACTICE CHANGER
Offer women ages 30 to 65 years the option of being screened for cervical cancer using a high-risk human papillomavirus assay every 5 years.1,2
STRENGTH OF RECOMMENDATION
A: Based on a US Preventive Services Task Force recommendation statement.
Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
Early screening may halve breast cancer mortality in childhood cancer survivors
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
FROM ANNALS OF INTERNAL MEDICINE
Do-it-yourself cervical cancer screening?
ILLUSTRATIVE CASE
A 40-year-old woman presents to your office to establish care. During your interview you realize that she has never been screened for cervical cancer. In fact, she has not had a pelvic exam because she is fearful of the procedure. She would like to know if alternatives exist for cervical cancer screening. What can you suggest?
Although deaths from cervical cancer decreased in the United States from 1975 to 2017, demographic and social disparities in the burden of the disease remain.2,3 Data from 2016 reveal that cervical cancer incidence per 100,000 women is lowest among white (7.5), Asian-Pacific Islander (5.8), and American Indian/Alaska native (5.6) women, and highest among Hispanic (9.8) and black (8.7) women, which could be explained by lower screening rates in these populations.4,5 The National Cancer Institute’s publication on reducing cancer health disparities states that the most effective way to reduce cervical cancer incidence and mortality is by increasing screening rates among women who have not been screened or who have not been screened regularly.6
The US Food and Drug Administration (FDA) approved the first human papillomavirus (HPV) screening test in 2003.7 Evidence now suggests that high-risk HPV screening provides greater protection against cervical cancer than screening with cytology alone.8 The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) have changed their recommendations to include primary HPV testing as an alternative method to Pap smears for cervical cancer screening.9
An advantage of primary HPV screening is that it can be performed on a specimen collected by the patient, which could potentially increase rates of screening and help to decrease demographic and social disparities. A randomized trial of almost 2000 women ages 21 to 65 years that evaluated the acceptability of this method to patients revealed that more than half of women prefer the idea of a self-collected specimen to one that is collected by a clinician because it is more convenient and obviates the need for a pelvic exam.10
A meta-analysis of 36 studies and more than 150,000 women concluded that when self-collected samples were used with signal-based assays, the tests were not as sensitive or specific as when clinician-collected samples were used.11 However, the meta-analysis also found that some polymerase chain reaction (PCR)-based HPV tests were similarly sensitive for both self- and clinician-collected samples.
STUDY SUMMARY
PCR vs signal amplification HPV tests with collection by patients vs clinicians
This meta-analysis compared the accuracy of high-risk HPV self-screening with clinician collection of samples (56 diagnostic accuracy trials; total N not provided) in identifying cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) with signal amplification and PCR tests evaluated separately.1 In addition, this review evaluated strategies to screen women who are underscreened or not screened, which was defined as women who were irregularly or never screened, or did not respond to reminder letters about cervical cancer screening (25 randomized controlled trials [RCTs]; total N not provided).
In the diagnostic accuracy studies, patients collected a vaginal sample themselves and then had a sample taken by a clinician. CIN 2+ or 3+ was confirmed by either colposcopy and biopsy performed on all patients or by a positive high-risk HPV test result. Studies were further divided into those using assays based on signal amplification or PCR.
Continue to: In signal amplification assays...
In signal amplification assays, the pooled sensitivity for CIN 2+ was lower in the group with the self-collected samples than in the clinician-collected sample group (77%; 95% confidence interval [CI], 69%-82% vs 93%; 95% CI, 89%-96%). The pooled specificity to exclude CIN 2+ was also lower in the group with the self-collected samples (84%; 95% CI, 77%-88% vs 86%; 95% CI, 81%-90%). In high-risk HPV assays based on PCR, there was no difference in sensitivity (96%) or specificity (79%) between the specimen groups.
With regard to the pooled relative sensitivity and specificity of signal amplification assays, those using self-swab samples were less sensitive and less specific for CIN 2+ (sensitivity ratio = 0.85; 95% CI, 0.80-0.89; specificity ratio = 0.96; 95% CI, 0.93-0.98) and CIN 3+ (sensitivity ratio = 0.86; 95% CI, 0.76-0.98; specificity ratio = 0.97; 95% CI, 0.95-0.99). Using PCR assays, there was no difference between groups in relative sensitivity for the diagnosis of CIN 2+ (sensitivity ratio = 0.99; 95% CI, 0.97-1.02) and CIN 3+ (sensitivity ratio = 0.99; 95% CI, 0.96-1.02). Relative specificity was slightly lower in the self-swab group for CIN 2+ (specificity ratio = 0.98; 95% CI, 0.97-0.99) and CIN 3+ (specificity ratio = 0.98; 95% CI, 0.97-0.99).
The second analysis to evaluate which outreach strategies are effective methods for screening underscreened/unscreened women found that delivering self-sample kits to patients was more effective than the control method, which was sending reminders to women to undergo conventional screening (95% vs 53%; mean difference [MD], 41%; 95% CI, 3%-78%). Similarly, mailing kits to patients compared favorably to the control method (25% vs 12%; MD, 13%; 95% CI, 10%-15%).
WHAT’S NEW
Self-collected specimens can beas reliable as clinician-collected ones
This is the first study to provide robust evidence that high-risk HPV PCR-based assays using patient self-collected specimens are as sensitive at diagnosing CIN 2+ or 3+ as using clinician-collected samples.
CAVEATS
Balancing lower specificity with reaching underscreened populations
Patients with a positive HPV test result require additional testing. The success rates for this follow-up are not known and could be a barrier to accurate diagnoses because of accessibility and patient willingness to follow up with a pelvic exam. In addition, self-collection may be less specific than cytology and could increase colposcopy referrals that lead to negative findings and overtreatment.12 However, the increased acceptance of this screening method could make it an effective strategy to reach underscreened or reluctant patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Availability of PCR-based HPV assays may be an issue
HPV PCR assays may not be available at all laboratories, but signal amplification HPV tests have been shown to be inferior to PCR assays. Physicians will have to confirm with their laboratories whether PCR-based HPV assays are available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Arbyn M, Smith SB, Temin S, et al; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: cervical cancer. www.seer.cancer.gov/statfacts/html/cervix.html. Accessed June 29, 2020.
3. Singh GK, Azuine RE, Siahpush M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. Int J MCH AIDS. 2012;1:17‐30.
4. US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999-2016): US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. June 2019. www.cdc.gov/cancer/dataviz. Accessed June 29, 2020.
5. MacLaughlin KL, Jacobson RM, Breitkopf CR, et al. Trends over time in Pap and Pap-HPV cotesting for cervical cancer screening. J Womens Health. 2019;28:244-249.
6. Freeman HP, Wingrove BK. Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities. NIH Pub. No. 05–5282. Rockville, MD: National Cancer Institute, Center to Reduce Cancer Health Disparities, May 2005. www.cancer.gov/about-nci/organization/crchd/about-health-disparities/resources/excess-cervical-cancer-mortality.pdf. Accessed June 29, 2020.
7. FDA approves expanded use of HPV test. Infection Control Today. March 31, 2003. https://www.infectioncontroltoday.com/view/fda-approves-expanded-use-hpv-test. Accessed June 29, 2020.
8. Ronco G, Dillner J, Elfström K, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
9. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed June 29, 2020.
10. Mao C, Kulasingam S, Whitham H, et al. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health. 2017;26:609-615.
11. Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172-183.
12. Lazcano-Ponce E, Lorincz A, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868-1873.
ILLUSTRATIVE CASE
A 40-year-old woman presents to your office to establish care. During your interview you realize that she has never been screened for cervical cancer. In fact, she has not had a pelvic exam because she is fearful of the procedure. She would like to know if alternatives exist for cervical cancer screening. What can you suggest?
Although deaths from cervical cancer decreased in the United States from 1975 to 2017, demographic and social disparities in the burden of the disease remain.2,3 Data from 2016 reveal that cervical cancer incidence per 100,000 women is lowest among white (7.5), Asian-Pacific Islander (5.8), and American Indian/Alaska native (5.6) women, and highest among Hispanic (9.8) and black (8.7) women, which could be explained by lower screening rates in these populations.4,5 The National Cancer Institute’s publication on reducing cancer health disparities states that the most effective way to reduce cervical cancer incidence and mortality is by increasing screening rates among women who have not been screened or who have not been screened regularly.6
The US Food and Drug Administration (FDA) approved the first human papillomavirus (HPV) screening test in 2003.7 Evidence now suggests that high-risk HPV screening provides greater protection against cervical cancer than screening with cytology alone.8 The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) have changed their recommendations to include primary HPV testing as an alternative method to Pap smears for cervical cancer screening.9
An advantage of primary HPV screening is that it can be performed on a specimen collected by the patient, which could potentially increase rates of screening and help to decrease demographic and social disparities. A randomized trial of almost 2000 women ages 21 to 65 years that evaluated the acceptability of this method to patients revealed that more than half of women prefer the idea of a self-collected specimen to one that is collected by a clinician because it is more convenient and obviates the need for a pelvic exam.10
A meta-analysis of 36 studies and more than 150,000 women concluded that when self-collected samples were used with signal-based assays, the tests were not as sensitive or specific as when clinician-collected samples were used.11 However, the meta-analysis also found that some polymerase chain reaction (PCR)-based HPV tests were similarly sensitive for both self- and clinician-collected samples.
STUDY SUMMARY
PCR vs signal amplification HPV tests with collection by patients vs clinicians
This meta-analysis compared the accuracy of high-risk HPV self-screening with clinician collection of samples (56 diagnostic accuracy trials; total N not provided) in identifying cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) with signal amplification and PCR tests evaluated separately.1 In addition, this review evaluated strategies to screen women who are underscreened or not screened, which was defined as women who were irregularly or never screened, or did not respond to reminder letters about cervical cancer screening (25 randomized controlled trials [RCTs]; total N not provided).
In the diagnostic accuracy studies, patients collected a vaginal sample themselves and then had a sample taken by a clinician. CIN 2+ or 3+ was confirmed by either colposcopy and biopsy performed on all patients or by a positive high-risk HPV test result. Studies were further divided into those using assays based on signal amplification or PCR.
Continue to: In signal amplification assays...
In signal amplification assays, the pooled sensitivity for CIN 2+ was lower in the group with the self-collected samples than in the clinician-collected sample group (77%; 95% confidence interval [CI], 69%-82% vs 93%; 95% CI, 89%-96%). The pooled specificity to exclude CIN 2+ was also lower in the group with the self-collected samples (84%; 95% CI, 77%-88% vs 86%; 95% CI, 81%-90%). In high-risk HPV assays based on PCR, there was no difference in sensitivity (96%) or specificity (79%) between the specimen groups.
With regard to the pooled relative sensitivity and specificity of signal amplification assays, those using self-swab samples were less sensitive and less specific for CIN 2+ (sensitivity ratio = 0.85; 95% CI, 0.80-0.89; specificity ratio = 0.96; 95% CI, 0.93-0.98) and CIN 3+ (sensitivity ratio = 0.86; 95% CI, 0.76-0.98; specificity ratio = 0.97; 95% CI, 0.95-0.99). Using PCR assays, there was no difference between groups in relative sensitivity for the diagnosis of CIN 2+ (sensitivity ratio = 0.99; 95% CI, 0.97-1.02) and CIN 3+ (sensitivity ratio = 0.99; 95% CI, 0.96-1.02). Relative specificity was slightly lower in the self-swab group for CIN 2+ (specificity ratio = 0.98; 95% CI, 0.97-0.99) and CIN 3+ (specificity ratio = 0.98; 95% CI, 0.97-0.99).
The second analysis to evaluate which outreach strategies are effective methods for screening underscreened/unscreened women found that delivering self-sample kits to patients was more effective than the control method, which was sending reminders to women to undergo conventional screening (95% vs 53%; mean difference [MD], 41%; 95% CI, 3%-78%). Similarly, mailing kits to patients compared favorably to the control method (25% vs 12%; MD, 13%; 95% CI, 10%-15%).
WHAT’S NEW
Self-collected specimens can beas reliable as clinician-collected ones
This is the first study to provide robust evidence that high-risk HPV PCR-based assays using patient self-collected specimens are as sensitive at diagnosing CIN 2+ or 3+ as using clinician-collected samples.
CAVEATS
Balancing lower specificity with reaching underscreened populations
Patients with a positive HPV test result require additional testing. The success rates for this follow-up are not known and could be a barrier to accurate diagnoses because of accessibility and patient willingness to follow up with a pelvic exam. In addition, self-collection may be less specific than cytology and could increase colposcopy referrals that lead to negative findings and overtreatment.12 However, the increased acceptance of this screening method could make it an effective strategy to reach underscreened or reluctant patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Availability of PCR-based HPV assays may be an issue
HPV PCR assays may not be available at all laboratories, but signal amplification HPV tests have been shown to be inferior to PCR assays. Physicians will have to confirm with their laboratories whether PCR-based HPV assays are available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 40-year-old woman presents to your office to establish care. During your interview you realize that she has never been screened for cervical cancer. In fact, she has not had a pelvic exam because she is fearful of the procedure. She would like to know if alternatives exist for cervical cancer screening. What can you suggest?
Although deaths from cervical cancer decreased in the United States from 1975 to 2017, demographic and social disparities in the burden of the disease remain.2,3 Data from 2016 reveal that cervical cancer incidence per 100,000 women is lowest among white (7.5), Asian-Pacific Islander (5.8), and American Indian/Alaska native (5.6) women, and highest among Hispanic (9.8) and black (8.7) women, which could be explained by lower screening rates in these populations.4,5 The National Cancer Institute’s publication on reducing cancer health disparities states that the most effective way to reduce cervical cancer incidence and mortality is by increasing screening rates among women who have not been screened or who have not been screened regularly.6
The US Food and Drug Administration (FDA) approved the first human papillomavirus (HPV) screening test in 2003.7 Evidence now suggests that high-risk HPV screening provides greater protection against cervical cancer than screening with cytology alone.8 The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) have changed their recommendations to include primary HPV testing as an alternative method to Pap smears for cervical cancer screening.9
An advantage of primary HPV screening is that it can be performed on a specimen collected by the patient, which could potentially increase rates of screening and help to decrease demographic and social disparities. A randomized trial of almost 2000 women ages 21 to 65 years that evaluated the acceptability of this method to patients revealed that more than half of women prefer the idea of a self-collected specimen to one that is collected by a clinician because it is more convenient and obviates the need for a pelvic exam.10
A meta-analysis of 36 studies and more than 150,000 women concluded that when self-collected samples were used with signal-based assays, the tests were not as sensitive or specific as when clinician-collected samples were used.11 However, the meta-analysis also found that some polymerase chain reaction (PCR)-based HPV tests were similarly sensitive for both self- and clinician-collected samples.
STUDY SUMMARY
PCR vs signal amplification HPV tests with collection by patients vs clinicians
This meta-analysis compared the accuracy of high-risk HPV self-screening with clinician collection of samples (56 diagnostic accuracy trials; total N not provided) in identifying cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) with signal amplification and PCR tests evaluated separately.1 In addition, this review evaluated strategies to screen women who are underscreened or not screened, which was defined as women who were irregularly or never screened, or did not respond to reminder letters about cervical cancer screening (25 randomized controlled trials [RCTs]; total N not provided).
In the diagnostic accuracy studies, patients collected a vaginal sample themselves and then had a sample taken by a clinician. CIN 2+ or 3+ was confirmed by either colposcopy and biopsy performed on all patients or by a positive high-risk HPV test result. Studies were further divided into those using assays based on signal amplification or PCR.
Continue to: In signal amplification assays...
In signal amplification assays, the pooled sensitivity for CIN 2+ was lower in the group with the self-collected samples than in the clinician-collected sample group (77%; 95% confidence interval [CI], 69%-82% vs 93%; 95% CI, 89%-96%). The pooled specificity to exclude CIN 2+ was also lower in the group with the self-collected samples (84%; 95% CI, 77%-88% vs 86%; 95% CI, 81%-90%). In high-risk HPV assays based on PCR, there was no difference in sensitivity (96%) or specificity (79%) between the specimen groups.
With regard to the pooled relative sensitivity and specificity of signal amplification assays, those using self-swab samples were less sensitive and less specific for CIN 2+ (sensitivity ratio = 0.85; 95% CI, 0.80-0.89; specificity ratio = 0.96; 95% CI, 0.93-0.98) and CIN 3+ (sensitivity ratio = 0.86; 95% CI, 0.76-0.98; specificity ratio = 0.97; 95% CI, 0.95-0.99). Using PCR assays, there was no difference between groups in relative sensitivity for the diagnosis of CIN 2+ (sensitivity ratio = 0.99; 95% CI, 0.97-1.02) and CIN 3+ (sensitivity ratio = 0.99; 95% CI, 0.96-1.02). Relative specificity was slightly lower in the self-swab group for CIN 2+ (specificity ratio = 0.98; 95% CI, 0.97-0.99) and CIN 3+ (specificity ratio = 0.98; 95% CI, 0.97-0.99).
The second analysis to evaluate which outreach strategies are effective methods for screening underscreened/unscreened women found that delivering self-sample kits to patients was more effective than the control method, which was sending reminders to women to undergo conventional screening (95% vs 53%; mean difference [MD], 41%; 95% CI, 3%-78%). Similarly, mailing kits to patients compared favorably to the control method (25% vs 12%; MD, 13%; 95% CI, 10%-15%).
WHAT’S NEW
Self-collected specimens can beas reliable as clinician-collected ones
This is the first study to provide robust evidence that high-risk HPV PCR-based assays using patient self-collected specimens are as sensitive at diagnosing CIN 2+ or 3+ as using clinician-collected samples.
CAVEATS
Balancing lower specificity with reaching underscreened populations
Patients with a positive HPV test result require additional testing. The success rates for this follow-up are not known and could be a barrier to accurate diagnoses because of accessibility and patient willingness to follow up with a pelvic exam. In addition, self-collection may be less specific than cytology and could increase colposcopy referrals that lead to negative findings and overtreatment.12 However, the increased acceptance of this screening method could make it an effective strategy to reach underscreened or reluctant patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Availability of PCR-based HPV assays may be an issue
HPV PCR assays may not be available at all laboratories, but signal amplification HPV tests have been shown to be inferior to PCR assays. Physicians will have to confirm with their laboratories whether PCR-based HPV assays are available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Arbyn M, Smith SB, Temin S, et al; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: cervical cancer. www.seer.cancer.gov/statfacts/html/cervix.html. Accessed June 29, 2020.
3. Singh GK, Azuine RE, Siahpush M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. Int J MCH AIDS. 2012;1:17‐30.
4. US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999-2016): US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. June 2019. www.cdc.gov/cancer/dataviz. Accessed June 29, 2020.
5. MacLaughlin KL, Jacobson RM, Breitkopf CR, et al. Trends over time in Pap and Pap-HPV cotesting for cervical cancer screening. J Womens Health. 2019;28:244-249.
6. Freeman HP, Wingrove BK. Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities. NIH Pub. No. 05–5282. Rockville, MD: National Cancer Institute, Center to Reduce Cancer Health Disparities, May 2005. www.cancer.gov/about-nci/organization/crchd/about-health-disparities/resources/excess-cervical-cancer-mortality.pdf. Accessed June 29, 2020.
7. FDA approves expanded use of HPV test. Infection Control Today. March 31, 2003. https://www.infectioncontroltoday.com/view/fda-approves-expanded-use-hpv-test. Accessed June 29, 2020.
8. Ronco G, Dillner J, Elfström K, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
9. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed June 29, 2020.
10. Mao C, Kulasingam S, Whitham H, et al. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health. 2017;26:609-615.
11. Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172-183.
12. Lazcano-Ponce E, Lorincz A, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868-1873.
1. Arbyn M, Smith SB, Temin S, et al; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: cervical cancer. www.seer.cancer.gov/statfacts/html/cervix.html. Accessed June 29, 2020.
3. Singh GK, Azuine RE, Siahpush M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. Int J MCH AIDS. 2012;1:17‐30.
4. US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999-2016): US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. June 2019. www.cdc.gov/cancer/dataviz. Accessed June 29, 2020.
5. MacLaughlin KL, Jacobson RM, Breitkopf CR, et al. Trends over time in Pap and Pap-HPV cotesting for cervical cancer screening. J Womens Health. 2019;28:244-249.
6. Freeman HP, Wingrove BK. Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities. NIH Pub. No. 05–5282. Rockville, MD: National Cancer Institute, Center to Reduce Cancer Health Disparities, May 2005. www.cancer.gov/about-nci/organization/crchd/about-health-disparities/resources/excess-cervical-cancer-mortality.pdf. Accessed June 29, 2020.
7. FDA approves expanded use of HPV test. Infection Control Today. March 31, 2003. https://www.infectioncontroltoday.com/view/fda-approves-expanded-use-hpv-test. Accessed June 29, 2020.
8. Ronco G, Dillner J, Elfström K, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
9. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed June 29, 2020.
10. Mao C, Kulasingam S, Whitham H, et al. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health. 2017;26:609-615.
11. Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172-183.
12. Lazcano-Ponce E, Lorincz A, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868-1873.
PRACTICE CHANGER
Have patients who decline a pelvic examination self-collect a specimen for human papillomavirus polymerase chain reaction testing as an alternative to a clinician-collected one.
STRENGTH OF RECOMMENDATION
B: Meta-analysis of observational trials.1
Arbyn M, Smith SB, Temin S, et al; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching under-screened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823.
Some women use prescription opioids during pregnancy
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.
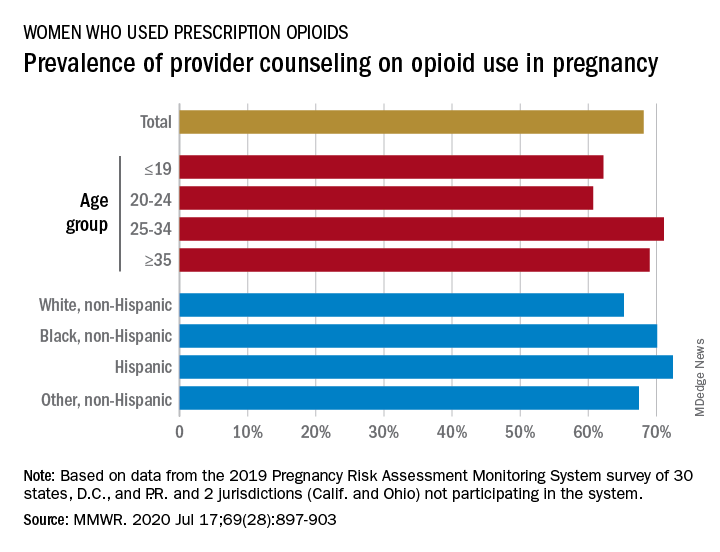
Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
FROM MMWR
Limit customized compounded hormones to special circumstances
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
obnews@mdedge.com
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
obnews@mdedge.com
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
obnews@mdedge.com
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
Cerclage in twin pregnancies reduces perinatal mortality in randomized trial
according to a randomized controlled trial. The trial, which was published in the American Journal of Obstetrics and Gynecology, included 30 patients at 8 centers. The investigators stopped the trial early because perinatal mortality occurred more often in the group that did not receive the intervention.
The research suggests that a combination of physical exam–indicated cerclage, indomethacin, and antibiotics decreased the incidence of spontaneous preterm birth and prolonged the period from diagnosis to delivery by an average of 5.6 weeks, compared with no cerclage.
“We’ve already incorporated this cerclage into our practice and have been able to offer this to pregnant mothers with twins with great success,” senior author Vincenzo Berghella, MD, said in a news release.
“These results have the potential to change practice and help many more women have healthy twin babies,” said Dr. Berghella, director of the division of maternal fetal medicine at Thomas Jefferson University in Philadelphia.
A shift in perspective
More research is needed to establish a standardized approach, but the trial should “open physicians’ perspectives to think about how, in selected cases and with the proper approach, cerclage can work well,” said Ozhan M. Turan, MD, PhD, director of the division of maternal and fetal medicine and director of fetal therapy and complex obstetric surgery at University of Maryland in Baltimore.
Although many physicians use cerclage for twin pregnancies in select situations, the practice is not well established. “If you look at the guidelines or books, mostly everyone thinks that doing a cerclage in twins is not a good idea,” Dr. Turan said in an interview.
In the present trial, the researchers controlled for many factors and carefully selected patients with no signs of preterm labor or infection. It is not simply a matter of saying, “Do the stitch,” he said. “But it is proven: if you select patients well and use the appropriate approach, then you could improve the outcome.”
The study is the first randomized controlled trial of physical exam–indicated cerclage focused on twins, according to its authors. It enrolled patients between July 2015 and July 2019. In the end, the researchers analyzed data from 30 pregnancies, rather than the originally intended 52. They stopped the trial after a data and safety monitoring board considered it “unethical to continue the study due to the considerable perinatal mortality in one of the arms ... and requested to unmask the arms of the study,” the researchers said.
Perinatal mortality occurred in 18% of neonates in the cerclage group (6 of 34), compared with 77% in the group without cerclage (20 of 26). All perinatal mortality cases were associated with delivery before 24 weeks.
“The small number of participants reflects how rare this condition is among all pregnancies,” first author Amanda Roman, MD, of the division of maternal fetal medicine at Thomas Jefferson University, Philadelphia, said in the news release. “But because women were randomized to treatment and nontreatment groups, the results are strong, as confirmed by the independent data and safety monitoring board.”
The researchers enrolled women with twin pregnancies and asymptomatic cervical dilation from 1 to 4 cm before 24 weeks. Exclusion criteria included monochorionic-monoamniotic pregnancy, selective fetal growth restriction, twin-twin transfusion syndrome, major fetal malformation, known genetic anomaly, placenta previa, signs of labor, or clinical chorioamnionitis.
In all, 17 women were randomized to cerclage and 13 to the no-cerclage group. Both groups had similar patient characteristics. About 93% of the twin gestations were diamniotic-dichorionic. Assisted reproductive technology was used by about 36% of the participants, and 20% had a history of singleton preterm birth. Four women assigned to cerclage did not undergo the procedure but were included in the intention-to-treat analysis. Two of the four patients had contraindications that occurred soon after randomization (rupture of amniotic membranes and vaginal bleeding), one had a friable cervix, and one declined cerclage after being randomized.
Spontaneous preterm birth before 34 weeks of gestation, the primary outcome, occurred in 12 of 17 women in the cerclage group and in all 13 women in the no-cerclage group (70% vs. 100%).
Trial to assess ultrasound indicated cerclage
“Expectant management with no cerclage is the current standard of care for these women,” Dr. Roman and coauthors wrote. “Despite small sample size, we were able to show a significant benefit to physical exam–indicated cerclage.”
Inability to place the cerclage in one patient due to friable cervix was the only intraoperative complication. “Larger cohorts in singleton pregnancies have informed a 10%-20% risk of intraoperative rupture of the membranes, cervical laceration, and bleeding during the procedure,” the researchers noted.
All women who received cerclage also received indomethacin and antibiotics, although these elements of management were not prespecified. Given the relatively small sample size, it is unclear what role factors such as indomethacin, which was administered to 82% of the cerclage group versus 31% of the no-cerclage group, and antibiotics may have played, said Dr. Turan.
Prospective studies may help clarify how the degree of cervical dilation, gestational age, use of progesterone, or surgical techniques may influence outcomes. In addition, the researchers are enrolling patients in another trial. That study aims to assess whether cerclage reduces the incidence of spontaneous preterm birth in asymptomatic women with twin gestations and cervical length of 15 mm or less diagnosed by transvaginal ultrasound between 16 and 24 weeks of gestation.
The study had no external financial support. The authors had no conflicts of interest. Dr. Turan said he had no relevant financial disclosures.
SOURCE: Roman A et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.047.
according to a randomized controlled trial. The trial, which was published in the American Journal of Obstetrics and Gynecology, included 30 patients at 8 centers. The investigators stopped the trial early because perinatal mortality occurred more often in the group that did not receive the intervention.
The research suggests that a combination of physical exam–indicated cerclage, indomethacin, and antibiotics decreased the incidence of spontaneous preterm birth and prolonged the period from diagnosis to delivery by an average of 5.6 weeks, compared with no cerclage.
“We’ve already incorporated this cerclage into our practice and have been able to offer this to pregnant mothers with twins with great success,” senior author Vincenzo Berghella, MD, said in a news release.
“These results have the potential to change practice and help many more women have healthy twin babies,” said Dr. Berghella, director of the division of maternal fetal medicine at Thomas Jefferson University in Philadelphia.
A shift in perspective
More research is needed to establish a standardized approach, but the trial should “open physicians’ perspectives to think about how, in selected cases and with the proper approach, cerclage can work well,” said Ozhan M. Turan, MD, PhD, director of the division of maternal and fetal medicine and director of fetal therapy and complex obstetric surgery at University of Maryland in Baltimore.
Although many physicians use cerclage for twin pregnancies in select situations, the practice is not well established. “If you look at the guidelines or books, mostly everyone thinks that doing a cerclage in twins is not a good idea,” Dr. Turan said in an interview.
In the present trial, the researchers controlled for many factors and carefully selected patients with no signs of preterm labor or infection. It is not simply a matter of saying, “Do the stitch,” he said. “But it is proven: if you select patients well and use the appropriate approach, then you could improve the outcome.”
The study is the first randomized controlled trial of physical exam–indicated cerclage focused on twins, according to its authors. It enrolled patients between July 2015 and July 2019. In the end, the researchers analyzed data from 30 pregnancies, rather than the originally intended 52. They stopped the trial after a data and safety monitoring board considered it “unethical to continue the study due to the considerable perinatal mortality in one of the arms ... and requested to unmask the arms of the study,” the researchers said.
Perinatal mortality occurred in 18% of neonates in the cerclage group (6 of 34), compared with 77% in the group without cerclage (20 of 26). All perinatal mortality cases were associated with delivery before 24 weeks.
“The small number of participants reflects how rare this condition is among all pregnancies,” first author Amanda Roman, MD, of the division of maternal fetal medicine at Thomas Jefferson University, Philadelphia, said in the news release. “But because women were randomized to treatment and nontreatment groups, the results are strong, as confirmed by the independent data and safety monitoring board.”
The researchers enrolled women with twin pregnancies and asymptomatic cervical dilation from 1 to 4 cm before 24 weeks. Exclusion criteria included monochorionic-monoamniotic pregnancy, selective fetal growth restriction, twin-twin transfusion syndrome, major fetal malformation, known genetic anomaly, placenta previa, signs of labor, or clinical chorioamnionitis.
In all, 17 women were randomized to cerclage and 13 to the no-cerclage group. Both groups had similar patient characteristics. About 93% of the twin gestations were diamniotic-dichorionic. Assisted reproductive technology was used by about 36% of the participants, and 20% had a history of singleton preterm birth. Four women assigned to cerclage did not undergo the procedure but were included in the intention-to-treat analysis. Two of the four patients had contraindications that occurred soon after randomization (rupture of amniotic membranes and vaginal bleeding), one had a friable cervix, and one declined cerclage after being randomized.
Spontaneous preterm birth before 34 weeks of gestation, the primary outcome, occurred in 12 of 17 women in the cerclage group and in all 13 women in the no-cerclage group (70% vs. 100%).
Trial to assess ultrasound indicated cerclage
“Expectant management with no cerclage is the current standard of care for these women,” Dr. Roman and coauthors wrote. “Despite small sample size, we were able to show a significant benefit to physical exam–indicated cerclage.”
Inability to place the cerclage in one patient due to friable cervix was the only intraoperative complication. “Larger cohorts in singleton pregnancies have informed a 10%-20% risk of intraoperative rupture of the membranes, cervical laceration, and bleeding during the procedure,” the researchers noted.
All women who received cerclage also received indomethacin and antibiotics, although these elements of management were not prespecified. Given the relatively small sample size, it is unclear what role factors such as indomethacin, which was administered to 82% of the cerclage group versus 31% of the no-cerclage group, and antibiotics may have played, said Dr. Turan.
Prospective studies may help clarify how the degree of cervical dilation, gestational age, use of progesterone, or surgical techniques may influence outcomes. In addition, the researchers are enrolling patients in another trial. That study aims to assess whether cerclage reduces the incidence of spontaneous preterm birth in asymptomatic women with twin gestations and cervical length of 15 mm or less diagnosed by transvaginal ultrasound between 16 and 24 weeks of gestation.
The study had no external financial support. The authors had no conflicts of interest. Dr. Turan said he had no relevant financial disclosures.
SOURCE: Roman A et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.047.
according to a randomized controlled trial. The trial, which was published in the American Journal of Obstetrics and Gynecology, included 30 patients at 8 centers. The investigators stopped the trial early because perinatal mortality occurred more often in the group that did not receive the intervention.
The research suggests that a combination of physical exam–indicated cerclage, indomethacin, and antibiotics decreased the incidence of spontaneous preterm birth and prolonged the period from diagnosis to delivery by an average of 5.6 weeks, compared with no cerclage.
“We’ve already incorporated this cerclage into our practice and have been able to offer this to pregnant mothers with twins with great success,” senior author Vincenzo Berghella, MD, said in a news release.
“These results have the potential to change practice and help many more women have healthy twin babies,” said Dr. Berghella, director of the division of maternal fetal medicine at Thomas Jefferson University in Philadelphia.
A shift in perspective
More research is needed to establish a standardized approach, but the trial should “open physicians’ perspectives to think about how, in selected cases and with the proper approach, cerclage can work well,” said Ozhan M. Turan, MD, PhD, director of the division of maternal and fetal medicine and director of fetal therapy and complex obstetric surgery at University of Maryland in Baltimore.
Although many physicians use cerclage for twin pregnancies in select situations, the practice is not well established. “If you look at the guidelines or books, mostly everyone thinks that doing a cerclage in twins is not a good idea,” Dr. Turan said in an interview.
In the present trial, the researchers controlled for many factors and carefully selected patients with no signs of preterm labor or infection. It is not simply a matter of saying, “Do the stitch,” he said. “But it is proven: if you select patients well and use the appropriate approach, then you could improve the outcome.”
The study is the first randomized controlled trial of physical exam–indicated cerclage focused on twins, according to its authors. It enrolled patients between July 2015 and July 2019. In the end, the researchers analyzed data from 30 pregnancies, rather than the originally intended 52. They stopped the trial after a data and safety monitoring board considered it “unethical to continue the study due to the considerable perinatal mortality in one of the arms ... and requested to unmask the arms of the study,” the researchers said.
Perinatal mortality occurred in 18% of neonates in the cerclage group (6 of 34), compared with 77% in the group without cerclage (20 of 26). All perinatal mortality cases were associated with delivery before 24 weeks.
“The small number of participants reflects how rare this condition is among all pregnancies,” first author Amanda Roman, MD, of the division of maternal fetal medicine at Thomas Jefferson University, Philadelphia, said in the news release. “But because women were randomized to treatment and nontreatment groups, the results are strong, as confirmed by the independent data and safety monitoring board.”
The researchers enrolled women with twin pregnancies and asymptomatic cervical dilation from 1 to 4 cm before 24 weeks. Exclusion criteria included monochorionic-monoamniotic pregnancy, selective fetal growth restriction, twin-twin transfusion syndrome, major fetal malformation, known genetic anomaly, placenta previa, signs of labor, or clinical chorioamnionitis.
In all, 17 women were randomized to cerclage and 13 to the no-cerclage group. Both groups had similar patient characteristics. About 93% of the twin gestations were diamniotic-dichorionic. Assisted reproductive technology was used by about 36% of the participants, and 20% had a history of singleton preterm birth. Four women assigned to cerclage did not undergo the procedure but were included in the intention-to-treat analysis. Two of the four patients had contraindications that occurred soon after randomization (rupture of amniotic membranes and vaginal bleeding), one had a friable cervix, and one declined cerclage after being randomized.
Spontaneous preterm birth before 34 weeks of gestation, the primary outcome, occurred in 12 of 17 women in the cerclage group and in all 13 women in the no-cerclage group (70% vs. 100%).
Trial to assess ultrasound indicated cerclage
“Expectant management with no cerclage is the current standard of care for these women,” Dr. Roman and coauthors wrote. “Despite small sample size, we were able to show a significant benefit to physical exam–indicated cerclage.”
Inability to place the cerclage in one patient due to friable cervix was the only intraoperative complication. “Larger cohorts in singleton pregnancies have informed a 10%-20% risk of intraoperative rupture of the membranes, cervical laceration, and bleeding during the procedure,” the researchers noted.
All women who received cerclage also received indomethacin and antibiotics, although these elements of management were not prespecified. Given the relatively small sample size, it is unclear what role factors such as indomethacin, which was administered to 82% of the cerclage group versus 31% of the no-cerclage group, and antibiotics may have played, said Dr. Turan.
Prospective studies may help clarify how the degree of cervical dilation, gestational age, use of progesterone, or surgical techniques may influence outcomes. In addition, the researchers are enrolling patients in another trial. That study aims to assess whether cerclage reduces the incidence of spontaneous preterm birth in asymptomatic women with twin gestations and cervical length of 15 mm or less diagnosed by transvaginal ultrasound between 16 and 24 weeks of gestation.
The study had no external financial support. The authors had no conflicts of interest. Dr. Turan said he had no relevant financial disclosures.
SOURCE: Roman A et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.047.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
One-week postsurgical interval for voiding trial increases pass rate
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
FROM AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Prior beta-blockers predict extra burden of heart failure in women with ACS
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Novel program cuts weight retention after gestational diabetes
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ADA 2020