User login
Do you plan to incorporate dynamic ultrasonography (use of the vaginal probe to help examine a patient, for pelvic pain for instance) into your practice?
[polldaddy:10873738]
[polldaddy:10873738]
[polldaddy:10873738]
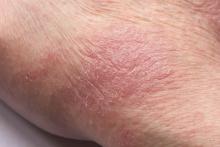
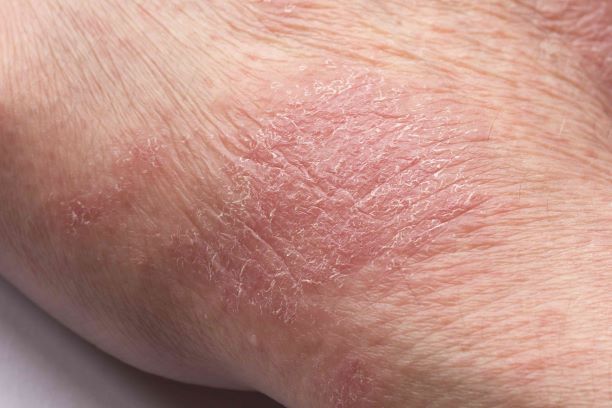
Man presents with diffuse pruritus
This patient has atopic dermatitis (AD), but on the basis of the image and description above, by no means would this be an intuitive diagnosis; the findings are not characteristic of those in younger patients with AD. Further, clinicians might find it difficult to diagnose AD in an older patient because older patients generally tend to have more comorbidities and medication side effects, including chronic pruritus of unknown origin and xerosis, which could confound the diagnosis.
Finally, specific guidelines are lacking for clinicians to distinguish AD from other pruritic skin conditions in the older patient. Currently, according to one report, older patients are diagnosed with AD after at least 6 months of symptom assessment and exclusion of other conditions, including cutaneous T-cell lymphoma, allergic contact dermatitis, psoriasis, drug reactions, and chronic idiopathic or secondary erythroderma.
AD arising de novo in older persons is a discrete form of the disease that characteristically involves the face, neck, trunk, and hands, while sparing the flexural areas, which are prominently involved in younger patients. The eczema can become erythrodermic. Older men are affected threefold more often than older women.
Skin manifestations in older patients with AD generally match those of adolescents and young adults with AD, but the reverse sign of lichenified eczema around unaffected folds of the elbows and knees is more common than the classic sign of localized lichenified eczema at those folds.
Factors rendering older people susceptible to AD include innate physiologic changes of aging, notably a decline in skin barrier function, dysregulation of innate immune cells, and skewing of adaptive immunity to a Th2 response.
Much about how to best treat AD in older patients remains unclear. It is a challenge to treat older patients according to standardized guidelines for general AD treatment because dermatologists and others need to consider comorbidities and the medications that these patients might already be taking. Some examples: Dermatologists might limit cyclosporine use in patients with hypertension and reduced kidney function, or limit systemic steroid use in patients with osteoporosis. Older patients have a greater propensity for infection, which might cause dermatologists to limit systemic immunosuppressant drugs. And skin thinning and diffuse photoaging might cause doctors to limit even topical steroid treatment in these patients.
As in other age groups, regular application of moisturizers in combination with calcineurin inhibitors, adjunctive administration of oral antihistamines and avoidance of exacerbating factors comprise basic treatments for AD in older patients.
Although antihistamines such as hydroxyzine can work for itching in some individuals, they are generally lacking in efficacy in most patients with AD.
Brian S. Kim, MD, is Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
This patient has atopic dermatitis (AD), but on the basis of the image and description above, by no means would this be an intuitive diagnosis; the findings are not characteristic of those in younger patients with AD. Further, clinicians might find it difficult to diagnose AD in an older patient because older patients generally tend to have more comorbidities and medication side effects, including chronic pruritus of unknown origin and xerosis, which could confound the diagnosis.
Finally, specific guidelines are lacking for clinicians to distinguish AD from other pruritic skin conditions in the older patient. Currently, according to one report, older patients are diagnosed with AD after at least 6 months of symptom assessment and exclusion of other conditions, including cutaneous T-cell lymphoma, allergic contact dermatitis, psoriasis, drug reactions, and chronic idiopathic or secondary erythroderma.
AD arising de novo in older persons is a discrete form of the disease that characteristically involves the face, neck, trunk, and hands, while sparing the flexural areas, which are prominently involved in younger patients. The eczema can become erythrodermic. Older men are affected threefold more often than older women.
Skin manifestations in older patients with AD generally match those of adolescents and young adults with AD, but the reverse sign of lichenified eczema around unaffected folds of the elbows and knees is more common than the classic sign of localized lichenified eczema at those folds.
Factors rendering older people susceptible to AD include innate physiologic changes of aging, notably a decline in skin barrier function, dysregulation of innate immune cells, and skewing of adaptive immunity to a Th2 response.
Much about how to best treat AD in older patients remains unclear. It is a challenge to treat older patients according to standardized guidelines for general AD treatment because dermatologists and others need to consider comorbidities and the medications that these patients might already be taking. Some examples: Dermatologists might limit cyclosporine use in patients with hypertension and reduced kidney function, or limit systemic steroid use in patients with osteoporosis. Older patients have a greater propensity for infection, which might cause dermatologists to limit systemic immunosuppressant drugs. And skin thinning and diffuse photoaging might cause doctors to limit even topical steroid treatment in these patients.
As in other age groups, regular application of moisturizers in combination with calcineurin inhibitors, adjunctive administration of oral antihistamines and avoidance of exacerbating factors comprise basic treatments for AD in older patients.
Although antihistamines such as hydroxyzine can work for itching in some individuals, they are generally lacking in efficacy in most patients with AD.
Brian S. Kim, MD, is Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
This patient has atopic dermatitis (AD), but on the basis of the image and description above, by no means would this be an intuitive diagnosis; the findings are not characteristic of those in younger patients with AD. Further, clinicians might find it difficult to diagnose AD in an older patient because older patients generally tend to have more comorbidities and medication side effects, including chronic pruritus of unknown origin and xerosis, which could confound the diagnosis.
Finally, specific guidelines are lacking for clinicians to distinguish AD from other pruritic skin conditions in the older patient. Currently, according to one report, older patients are diagnosed with AD after at least 6 months of symptom assessment and exclusion of other conditions, including cutaneous T-cell lymphoma, allergic contact dermatitis, psoriasis, drug reactions, and chronic idiopathic or secondary erythroderma.
AD arising de novo in older persons is a discrete form of the disease that characteristically involves the face, neck, trunk, and hands, while sparing the flexural areas, which are prominently involved in younger patients. The eczema can become erythrodermic. Older men are affected threefold more often than older women.
Skin manifestations in older patients with AD generally match those of adolescents and young adults with AD, but the reverse sign of lichenified eczema around unaffected folds of the elbows and knees is more common than the classic sign of localized lichenified eczema at those folds.
Factors rendering older people susceptible to AD include innate physiologic changes of aging, notably a decline in skin barrier function, dysregulation of innate immune cells, and skewing of adaptive immunity to a Th2 response.
Much about how to best treat AD in older patients remains unclear. It is a challenge to treat older patients according to standardized guidelines for general AD treatment because dermatologists and others need to consider comorbidities and the medications that these patients might already be taking. Some examples: Dermatologists might limit cyclosporine use in patients with hypertension and reduced kidney function, or limit systemic steroid use in patients with osteoporosis. Older patients have a greater propensity for infection, which might cause dermatologists to limit systemic immunosuppressant drugs. And skin thinning and diffuse photoaging might cause doctors to limit even topical steroid treatment in these patients.
As in other age groups, regular application of moisturizers in combination with calcineurin inhibitors, adjunctive administration of oral antihistamines and avoidance of exacerbating factors comprise basic treatments for AD in older patients.
Although antihistamines such as hydroxyzine can work for itching in some individuals, they are generally lacking in efficacy in most patients with AD.
Brian S. Kim, MD, is Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
A 59-year-old man presents with diffuse pruritus that first appeared 6 months earlier. He developed worsening eczematous, erythematous papules on his hands and face which waxed and waned. The areas of eczema on his hands and face were erythrodermic. The pruritus was intermittently controlled with the antihistamine hydroxyzine, a potent topical corticosteroid, and over-the-counter skin lotions. The patient said he had no history of allergies or asthma.
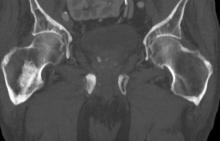
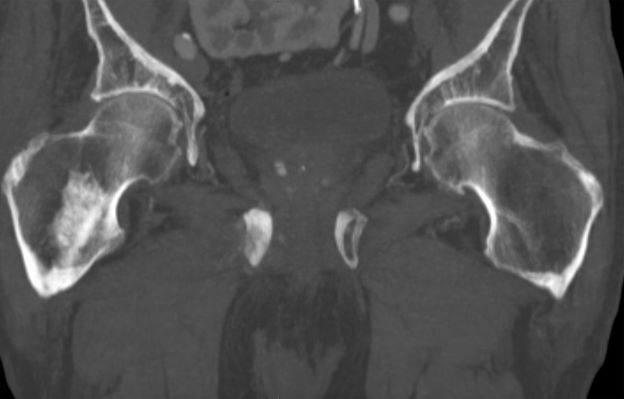
Sudden onset of severe pain in left thigh
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
A 74-year-old man presents to the emergency department with sudden onset of severe pain in his left thigh, a 3-month history of unexplained weight loss and general weakness, and progressive difficulty in walking for the past 3 weeks. The patient states that he has always been in excellent health and he has not seen a physician in at least 10 years. Cachexia is noted on physical examination. Laboratory findings include hemoglobin, 12.4 g/dL; white blood cells, 8.12 cells/µL; platelets, 310,000 cells/µL; creatinine, 1.4 mg/dL; sodium, 137 mmol/L; potassium, 4.4 mmol/L; calcium, 10.1 mg/dL; prostate-specific antigen, 31 ng/mL; aspartate aminotransferase, 37 IU/L; and gamma-glutamyltransferase, 16 IU/L. Proteinuria and hematuria are detected by urinalysis. CT reveals multiple diffuse osteoblastic lesions in the right proximal femur.
Intermittent fever and gradually progressive low back pain
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
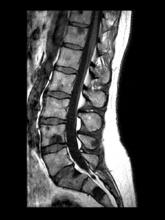
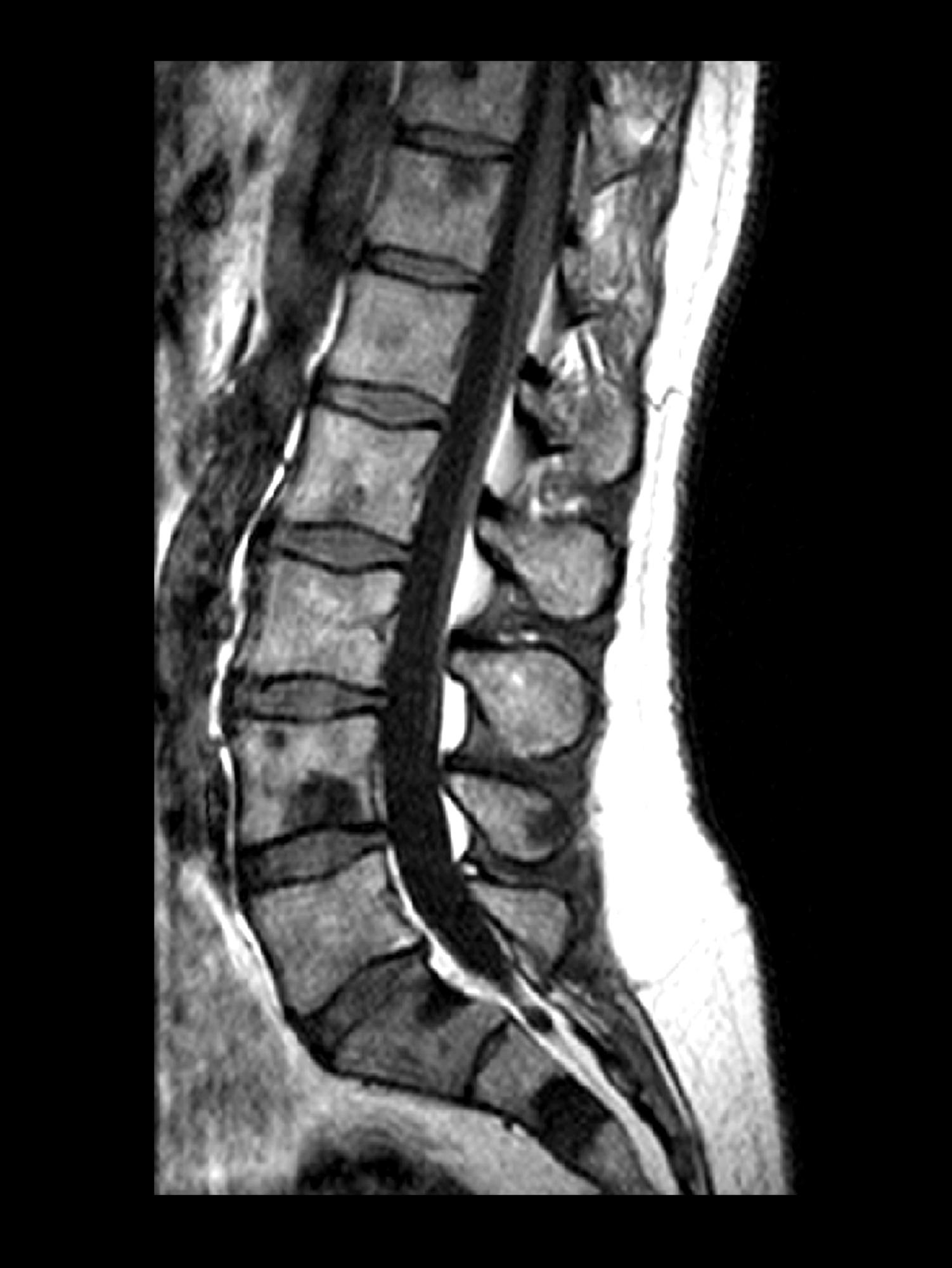
A 71-year-old homeless man presents to the emergency department (ED) with intermittent fever, gradually progressive low back pain restricting physical activities and movement, fatigue, exertional dyspnea, and poor appetite. The patient has been seen in the same ED sporadically over the years for various problems, and his medical history is notable for chronic obstructive pulmonary disease, tobacco use, alcoholism, and foot infections. Physical examination findings include tenderness to percussion over the thoracic and lumbar spine and a mildly enlarged prostate that appears to be smooth, normal in texture, and lacking nodules on digital rectal exam. Complete blood cell count and chemistry panel are normal. Both alkaline phosphatase and prostate-specific antigen (PSA) levels are elevated, at 240 U/L and 115 ng/mL, respectively. Urinalysis shows hematuria. CT shows osteolytic lesions in the patient's lumbar spine and femur.
DEMO: Dyspnea and intercostal retractions
The patient is probably having a delayed allergenic response to an indoor mold allergen. Early reactions can occur immediately following allergen inhalation but can also manifest 4-10 hours after exposure to wet and damp areas, such as bathrooms and basements.
Allergic asthma represents the most common asthma phenotype. The average age of onset is younger than that of nonallergic asthma, with allergens triggering exacerbations in 60%-90% of children compared with 50% of adults. Triggers can include mold spores, seasonal pollen, dust mites, and animal allergens. There is also an increased incidence of co-occurring allergic rhinoconjunctivitis and atopic dermatitis in patients with allergic asthma.
During an acute asthma exacerbation, lung examination findings may include wheezing, rhonchi, hyperinflation, or a prolonged expiratory phase. In children, supraclavicular and intercostal retractions and nasal flaring, as well as abdominal breathing, are particularly telling. Total serum immunoglobulin E levels are usually higher in allergic vs nonallergic asthma (> 100 IU), although this marker is not specific to asthma.
The allergic asthma phenotype usually responds to inhaled corticosteroid therapy. Although evidence suggests that allergic vs nonallergic asthma is less severe in many cases, compelling data point to a causal relationship between mold allergy and asthma severity in a subgroup of younger asthma patients; specifically, mold sensitization may be associated with asthma attacks requiring hospital admission in this population.
Although spirometry assessments should be obtained as the primary test to establish the asthma diagnosis, to test for allergic sensitivity to specific allergens in the environment, allergy skin tests and blood radioallergosorbent tests may be used. Up to 85% of patients with asthma demonstrate positive skin test results, and in children, this sensitization is reflective of disease activity. Such testing for allergen-specific immunoglobulin E is critical in prescribing allergen avoidance techniques and allergen immunotherapy regimens, although the method shows a relatively high false positive rate and should not be performed during an exacerbation.
The patient is probably having a delayed allergenic response to an indoor mold allergen. Early reactions can occur immediately following allergen inhalation but can also manifest 4-10 hours after exposure to wet and damp areas, such as bathrooms and basements.
Allergic asthma represents the most common asthma phenotype. The average age of onset is younger than that of nonallergic asthma, with allergens triggering exacerbations in 60%-90% of children compared with 50% of adults. Triggers can include mold spores, seasonal pollen, dust mites, and animal allergens. There is also an increased incidence of co-occurring allergic rhinoconjunctivitis and atopic dermatitis in patients with allergic asthma.
During an acute asthma exacerbation, lung examination findings may include wheezing, rhonchi, hyperinflation, or a prolonged expiratory phase. In children, supraclavicular and intercostal retractions and nasal flaring, as well as abdominal breathing, are particularly telling. Total serum immunoglobulin E levels are usually higher in allergic vs nonallergic asthma (> 100 IU), although this marker is not specific to asthma.
The allergic asthma phenotype usually responds to inhaled corticosteroid therapy. Although evidence suggests that allergic vs nonallergic asthma is less severe in many cases, compelling data point to a causal relationship between mold allergy and asthma severity in a subgroup of younger asthma patients; specifically, mold sensitization may be associated with asthma attacks requiring hospital admission in this population.
Although spirometry assessments should be obtained as the primary test to establish the asthma diagnosis, to test for allergic sensitivity to specific allergens in the environment, allergy skin tests and blood radioallergosorbent tests may be used. Up to 85% of patients with asthma demonstrate positive skin test results, and in children, this sensitization is reflective of disease activity. Such testing for allergen-specific immunoglobulin E is critical in prescribing allergen avoidance techniques and allergen immunotherapy regimens, although the method shows a relatively high false positive rate and should not be performed during an exacerbation.
The patient is probably having a delayed allergenic response to an indoor mold allergen. Early reactions can occur immediately following allergen inhalation but can also manifest 4-10 hours after exposure to wet and damp areas, such as bathrooms and basements.
Allergic asthma represents the most common asthma phenotype. The average age of onset is younger than that of nonallergic asthma, with allergens triggering exacerbations in 60%-90% of children compared with 50% of adults. Triggers can include mold spores, seasonal pollen, dust mites, and animal allergens. There is also an increased incidence of co-occurring allergic rhinoconjunctivitis and atopic dermatitis in patients with allergic asthma.
During an acute asthma exacerbation, lung examination findings may include wheezing, rhonchi, hyperinflation, or a prolonged expiratory phase. In children, supraclavicular and intercostal retractions and nasal flaring, as well as abdominal breathing, are particularly telling. Total serum immunoglobulin E levels are usually higher in allergic vs nonallergic asthma (> 100 IU), although this marker is not specific to asthma.
The allergic asthma phenotype usually responds to inhaled corticosteroid therapy. Although evidence suggests that allergic vs nonallergic asthma is less severe in many cases, compelling data point to a causal relationship between mold allergy and asthma severity in a subgroup of younger asthma patients; specifically, mold sensitization may be associated with asthma attacks requiring hospital admission in this population.
Although spirometry assessments should be obtained as the primary test to establish the asthma diagnosis, to test for allergic sensitivity to specific allergens in the environment, allergy skin tests and blood radioallergosorbent tests may be used. Up to 85% of patients with asthma demonstrate positive skin test results, and in children, this sensitization is reflective of disease activity. Such testing for allergen-specific immunoglobulin E is critical in prescribing allergen avoidance techniques and allergen immunotherapy regimens, although the method shows a relatively high false positive rate and should not be performed during an exacerbation.
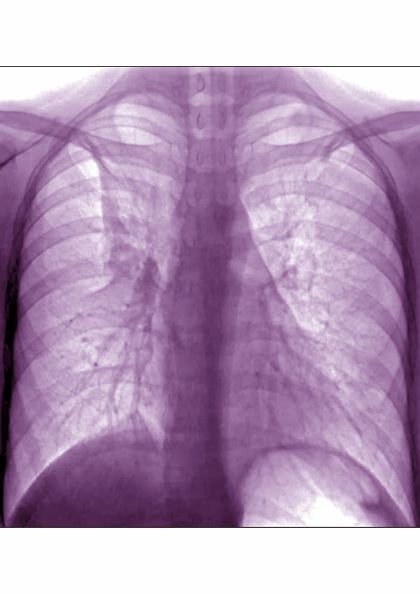
A 17-year-old boy came to the hospital with shortness of breath and loud wheezing. His mother explained that earlier that day, he visited a friend’s house where they played video games together in the basement. When he returned home, he noticed that he was becoming breathless while talking. His chest radiograph findings were normal, but intercostal retractions were observed. His heart rate is 120 beats/minute.
DEMO: Persistent cough and dyspnea
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
A 6-year-old girl came to the hospital with a persistent cough, episodes of dyspnea, and a slight fever. Her mother reported that she had been diagnosed with asthma when she was 4 years old. Despite strict adherence to anti-inflammatory medication, she experiences frequent symptoms, especially at night, and she had been hospitalized for an asthma exacerbation 8 months ago. Posterior-anterior chest radiograph revealed blurring of the right border of the heart.
What's your diagnosis?
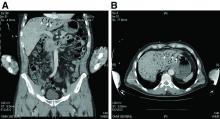
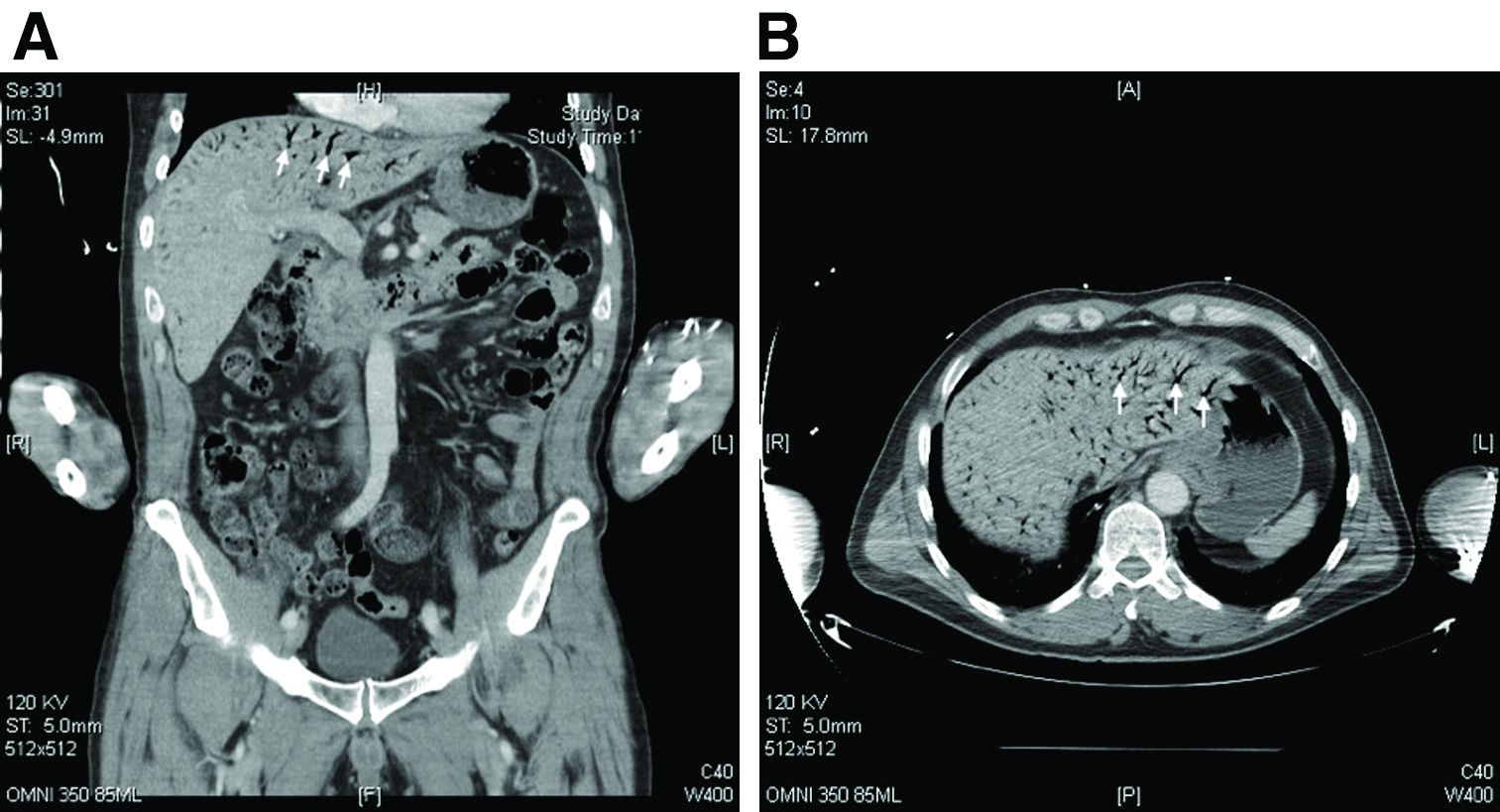
Hepatic portal venous gas
The CT scan of the abdomen and pelvis depicts portal venous gas throughout the liver (Figure A, B, white arrows). Hepatic portal venous gas is traditionally regarded as an ominous radiologic sign and appears as a branching area of low attenuation on CT scanning extending to within 2 cm of the liver capsule.1 It is commonly associated with numerous underlying abdominal diseases, ranging from benign processes to potentially lethal etiologies requiring immediate surgical intervention. The mechanism of hepatic portal venous gas can involve mechanical injury to the bowel lumen or gas-producing bacteria in the intestine.2 In the specific case of caustic ingestion of H2O2, the presence of bubbles in the portal vein could result from the oxygen generated by the caustic after passage through damaged gastric mucosa or from generation of oxygen in the blood after absorption of the caustic.3
Despite numerous reports of satisfactory outcomes with conservative management, the discovery of portal venous gas should not be dismissed quickly. Ultimately, management should be tailored to the underlying etiology and may include urgent surgical intervention. When appropriate, conservative management may include intravenous fluids and proton pump inhibitors.2,3 However, in cases involving caustic ingestion and massive gas embolization, providers should maintain a high index of clinical suspicion for neurologic as well as cardiac complications, because these complications may benefit from hyperbaric oxygen therapy.2
In this case, the patient had severe symptoms. Therefore, a decision was made to treat him with intravenous fluids, proton pump inhibitors, and two rounds of hyperbaric oxygen therapy. The patient ultimately had an uneventful recovery.
The quiz authors disclose no conflicts.
References
1. Sebastia C et al. Radiographics. 2000 Sep-Oct;20(5):1213-24.
2. Abboud B et al. World J Gastroenterol. 2009 Aug 7;15(29):3585-90.
3. Lewin M et al. Eur Radiol. 2002 Dec;12(Suppl 3):S59-61.
Hepatic portal venous gas
The CT scan of the abdomen and pelvis depicts portal venous gas throughout the liver (Figure A, B, white arrows). Hepatic portal venous gas is traditionally regarded as an ominous radiologic sign and appears as a branching area of low attenuation on CT scanning extending to within 2 cm of the liver capsule.1 It is commonly associated with numerous underlying abdominal diseases, ranging from benign processes to potentially lethal etiologies requiring immediate surgical intervention. The mechanism of hepatic portal venous gas can involve mechanical injury to the bowel lumen or gas-producing bacteria in the intestine.2 In the specific case of caustic ingestion of H2O2, the presence of bubbles in the portal vein could result from the oxygen generated by the caustic after passage through damaged gastric mucosa or from generation of oxygen in the blood after absorption of the caustic.3
Despite numerous reports of satisfactory outcomes with conservative management, the discovery of portal venous gas should not be dismissed quickly. Ultimately, management should be tailored to the underlying etiology and may include urgent surgical intervention. When appropriate, conservative management may include intravenous fluids and proton pump inhibitors.2,3 However, in cases involving caustic ingestion and massive gas embolization, providers should maintain a high index of clinical suspicion for neurologic as well as cardiac complications, because these complications may benefit from hyperbaric oxygen therapy.2
In this case, the patient had severe symptoms. Therefore, a decision was made to treat him with intravenous fluids, proton pump inhibitors, and two rounds of hyperbaric oxygen therapy. The patient ultimately had an uneventful recovery.
The quiz authors disclose no conflicts.
References
1. Sebastia C et al. Radiographics. 2000 Sep-Oct;20(5):1213-24.
2. Abboud B et al. World J Gastroenterol. 2009 Aug 7;15(29):3585-90.
3. Lewin M et al. Eur Radiol. 2002 Dec;12(Suppl 3):S59-61.
Hepatic portal venous gas
The CT scan of the abdomen and pelvis depicts portal venous gas throughout the liver (Figure A, B, white arrows). Hepatic portal venous gas is traditionally regarded as an ominous radiologic sign and appears as a branching area of low attenuation on CT scanning extending to within 2 cm of the liver capsule.1 It is commonly associated with numerous underlying abdominal diseases, ranging from benign processes to potentially lethal etiologies requiring immediate surgical intervention. The mechanism of hepatic portal venous gas can involve mechanical injury to the bowel lumen or gas-producing bacteria in the intestine.2 In the specific case of caustic ingestion of H2O2, the presence of bubbles in the portal vein could result from the oxygen generated by the caustic after passage through damaged gastric mucosa or from generation of oxygen in the blood after absorption of the caustic.3
Despite numerous reports of satisfactory outcomes with conservative management, the discovery of portal venous gas should not be dismissed quickly. Ultimately, management should be tailored to the underlying etiology and may include urgent surgical intervention. When appropriate, conservative management may include intravenous fluids and proton pump inhibitors.2,3 However, in cases involving caustic ingestion and massive gas embolization, providers should maintain a high index of clinical suspicion for neurologic as well as cardiac complications, because these complications may benefit from hyperbaric oxygen therapy.2
In this case, the patient had severe symptoms. Therefore, a decision was made to treat him with intravenous fluids, proton pump inhibitors, and two rounds of hyperbaric oxygen therapy. The patient ultimately had an uneventful recovery.
The quiz authors disclose no conflicts.
References
1. Sebastia C et al. Radiographics. 2000 Sep-Oct;20(5):1213-24.
2. Abboud B et al. World J Gastroenterol. 2009 Aug 7;15(29):3585-90.
3. Lewin M et al. Eur Radiol. 2002 Dec;12(Suppl 3):S59-61.
How should this condition be managed?
Sudden eruption of pruritus and pain
The patient is empirically diagnosed with AD and eczema herpeticum. A Tzanck smear is done and is positive for multinucleate giant cells with nuclear molding and ballooning degeneration of the keratinocytes.
Eczema herpeticum, or Kaposi varicelliform eruption, is a rare but potentially life-threatening complication of AD. It is an extensive cutaneous vesicular eruption that arises from pre-existing skin disease — in most cases, AD — and is caused by disseminated cutaneous viral infection, usually with the herpes simplex virus (HSV). The virus can infect the epidermis because of the skin barrier defects that are inherently associated with AD. Patients with AD and filaggrin gene null mutations may have an increased risk for eczema herpeticum.
Eczema herpeticum is associated with significant morbidity and is considered a medical emergency. Early diagnosis and treatment is essential to prevent or minimize complications. Complications of eczema herpeticum can include keratoconjunctivitis, which may lead to vision loss, as well as multiorgan involvement with meningoencephalitis and/or septic shock.
Eczema herpeticum occurs on areas of preexisting AD (or other skin disease) and presents as small, dome-shaped, grouped papule-vesicles on an erythematous base. When these vesicles rupture, punched-out ulcers are formed. It is seen most often on the face, neck, and upper trunk. Fever, malaise, and lymphadenopathies may also be present.
Among patients with severe or poorly controlled AD, even the classic presentation of eczema herpeticum can be challenging to recognize. Clinicians should maintain a high index of suspicion for this complication in any patient with AD who presents with an acute pruritic and painful rash, including pediatric patients, who have the highest risk for the development of eczema herpeticum.
Viral PCR on vesicle fluid can confirm the diagnosis of eczema herpeticum and determine the type of HSV with high sensitivity and specificity. If unavailable, a Tzanck smear of an opened vesicle or erosion can confirm an HSV infection and provide rapid diagnosis, but its sensitivity and specificity can vary. Direct fluorescence antigen testing is a fast and inexpensive method for detecting an HSV antigen and distinguishing between HSV-1 and HSV-2 infections. Bacterial cultures should be performed when there is a concern for impetiginization. A skin biopsy may be indicated in cases involving atypical presentation.
Immediate treatment with oral acyclovir should be initiated for any patient with eczema herpeticum; patients with severe disease or who are immunocompromised may require hospitalization for administration of systemic antivirals. Because secondary bacterial infections are common, prophylactic antibiotics (eg, cephalexin, clindamycin, doxycycline, or trimethoprim-sulfamethoxazole) may also be indicated. Finally, consultation with an ophthalmologist is indicated when herpes keratitis is suspected.
Richard Vinson, MD, President, Mountain View Dermatology, El Paso, Texas.
Richard Vinson, MD, has disclosed no relevant financial relationships.
The patient is empirically diagnosed with AD and eczema herpeticum. A Tzanck smear is done and is positive for multinucleate giant cells with nuclear molding and ballooning degeneration of the keratinocytes.
Eczema herpeticum, or Kaposi varicelliform eruption, is a rare but potentially life-threatening complication of AD. It is an extensive cutaneous vesicular eruption that arises from pre-existing skin disease — in most cases, AD — and is caused by disseminated cutaneous viral infection, usually with the herpes simplex virus (HSV). The virus can infect the epidermis because of the skin barrier defects that are inherently associated with AD. Patients with AD and filaggrin gene null mutations may have an increased risk for eczema herpeticum.
Eczema herpeticum is associated with significant morbidity and is considered a medical emergency. Early diagnosis and treatment is essential to prevent or minimize complications. Complications of eczema herpeticum can include keratoconjunctivitis, which may lead to vision loss, as well as multiorgan involvement with meningoencephalitis and/or septic shock.
Eczema herpeticum occurs on areas of preexisting AD (or other skin disease) and presents as small, dome-shaped, grouped papule-vesicles on an erythematous base. When these vesicles rupture, punched-out ulcers are formed. It is seen most often on the face, neck, and upper trunk. Fever, malaise, and lymphadenopathies may also be present.
Among patients with severe or poorly controlled AD, even the classic presentation of eczema herpeticum can be challenging to recognize. Clinicians should maintain a high index of suspicion for this complication in any patient with AD who presents with an acute pruritic and painful rash, including pediatric patients, who have the highest risk for the development of eczema herpeticum.
Viral PCR on vesicle fluid can confirm the diagnosis of eczema herpeticum and determine the type of HSV with high sensitivity and specificity. If unavailable, a Tzanck smear of an opened vesicle or erosion can confirm an HSV infection and provide rapid diagnosis, but its sensitivity and specificity can vary. Direct fluorescence antigen testing is a fast and inexpensive method for detecting an HSV antigen and distinguishing between HSV-1 and HSV-2 infections. Bacterial cultures should be performed when there is a concern for impetiginization. A skin biopsy may be indicated in cases involving atypical presentation.
Immediate treatment with oral acyclovir should be initiated for any patient with eczema herpeticum; patients with severe disease or who are immunocompromised may require hospitalization for administration of systemic antivirals. Because secondary bacterial infections are common, prophylactic antibiotics (eg, cephalexin, clindamycin, doxycycline, or trimethoprim-sulfamethoxazole) may also be indicated. Finally, consultation with an ophthalmologist is indicated when herpes keratitis is suspected.
Richard Vinson, MD, President, Mountain View Dermatology, El Paso, Texas.
Richard Vinson, MD, has disclosed no relevant financial relationships.
The patient is empirically diagnosed with AD and eczema herpeticum. A Tzanck smear is done and is positive for multinucleate giant cells with nuclear molding and ballooning degeneration of the keratinocytes.
Eczema herpeticum, or Kaposi varicelliform eruption, is a rare but potentially life-threatening complication of AD. It is an extensive cutaneous vesicular eruption that arises from pre-existing skin disease — in most cases, AD — and is caused by disseminated cutaneous viral infection, usually with the herpes simplex virus (HSV). The virus can infect the epidermis because of the skin barrier defects that are inherently associated with AD. Patients with AD and filaggrin gene null mutations may have an increased risk for eczema herpeticum.
Eczema herpeticum is associated with significant morbidity and is considered a medical emergency. Early diagnosis and treatment is essential to prevent or minimize complications. Complications of eczema herpeticum can include keratoconjunctivitis, which may lead to vision loss, as well as multiorgan involvement with meningoencephalitis and/or septic shock.
Eczema herpeticum occurs on areas of preexisting AD (or other skin disease) and presents as small, dome-shaped, grouped papule-vesicles on an erythematous base. When these vesicles rupture, punched-out ulcers are formed. It is seen most often on the face, neck, and upper trunk. Fever, malaise, and lymphadenopathies may also be present.
Among patients with severe or poorly controlled AD, even the classic presentation of eczema herpeticum can be challenging to recognize. Clinicians should maintain a high index of suspicion for this complication in any patient with AD who presents with an acute pruritic and painful rash, including pediatric patients, who have the highest risk for the development of eczema herpeticum.
Viral PCR on vesicle fluid can confirm the diagnosis of eczema herpeticum and determine the type of HSV with high sensitivity and specificity. If unavailable, a Tzanck smear of an opened vesicle or erosion can confirm an HSV infection and provide rapid diagnosis, but its sensitivity and specificity can vary. Direct fluorescence antigen testing is a fast and inexpensive method for detecting an HSV antigen and distinguishing between HSV-1 and HSV-2 infections. Bacterial cultures should be performed when there is a concern for impetiginization. A skin biopsy may be indicated in cases involving atypical presentation.
Immediate treatment with oral acyclovir should be initiated for any patient with eczema herpeticum; patients with severe disease or who are immunocompromised may require hospitalization for administration of systemic antivirals. Because secondary bacterial infections are common, prophylactic antibiotics (eg, cephalexin, clindamycin, doxycycline, or trimethoprim-sulfamethoxazole) may also be indicated. Finally, consultation with an ophthalmologist is indicated when herpes keratitis is suspected.
Richard Vinson, MD, President, Mountain View Dermatology, El Paso, Texas.
Richard Vinson, MD, has disclosed no relevant financial relationships.
A 24-year-old woman with a history of moderate atopic dermatitis (AD) and mild intermittent asthma presents with a sudden eruption of widespread pruritus and pain affecting her torso. Physical examination reveals small, dome-shaped, grouped papule-vesicles and some punched-out erosions on an erythematous base. The patient was last treated with topical triamcinolone for AD 6 weeks before. Current medications include an inhaled short-acting, beta-2 agonist used as needed for asthma symptoms and levonorgestrel-ethinyl estradiol tablets for oral contraception.
Widespread pruritic lesions on torso
The patient is diagnosed with atopic dermatitis (AD) on the basis of clinical findings and historical features, morphology and distribution of skin lesions, and associated clinical signs.
AD continues to be a clinical diagnosis. Pruritus and eczema (acute, subacute, or chronic) are essential features for the diagnosis of AD. The eczema should follow characteristic morphology and age-specific patterns (eg, facial/neck/extensor involvement in children, flexural involvement in any age group, sparing of the groin and axillary regions). A personal or family history of atopy is an important historical feature that supports the diagnosis of AD, as are xerosis and early age of onset.
The majority of patients with AD (60%) develop symptoms during the first year of life and 90% experience an eruption by 5 years of age, but disease onset can occur at any age. Some studies have found an association between late-onset AD and persistence of disease into adulthood.
At present, there are no reliable biomarkers that can differentiate AD from other entities. An elevated total and/or allergen-specific serum IgE level is the most commonly associated laboratory feature, but it is not seen in about 20% of individuals with AD. Additionally, elevated allergen-specific IgE levels are found in 55% of the general population in the United States, making it nonspecific for AD. Moreover, while total IgE level usually varies in accordance with disease severity, it is not a reliable marker of disease severity, and it is possible for individuals with severe disease to have normal levels. Nonatopic conditions (eg, parasitic infection, some cancers, and autoimmune disease) may also lead to elevations in IgE levels.
Consistent prognostic markers are also lacking, although elevated total serum IgE levels and filaggrin gene null mutations do tend to be predictive of a more severe and prolonged disease course.
Topical agents including nonpharmacologic moisturizers and topical corticosteroids are the mainstay of treatment for AD; immunomodulatory agents and targeted biologic therapies are also available. For patients with more severe or recalcitrant disease, systemic therapy or phototherapy may be used, often in conjunction with topical therapies.
Richard Vinson, MD, President, Mountain View Dermatology, El Paso, Texas.
Richard Vinson, MD, has disclosed no relevant financial relationships.
The patient is diagnosed with atopic dermatitis (AD) on the basis of clinical findings and historical features, morphology and distribution of skin lesions, and associated clinical signs.
AD continues to be a clinical diagnosis. Pruritus and eczema (acute, subacute, or chronic) are essential features for the diagnosis of AD. The eczema should follow characteristic morphology and age-specific patterns (eg, facial/neck/extensor involvement in children, flexural involvement in any age group, sparing of the groin and axillary regions). A personal or family history of atopy is an important historical feature that supports the diagnosis of AD, as are xerosis and early age of onset.
The majority of patients with AD (60%) develop symptoms during the first year of life and 90% experience an eruption by 5 years of age, but disease onset can occur at any age. Some studies have found an association between late-onset AD and persistence of disease into adulthood.
At present, there are no reliable biomarkers that can differentiate AD from other entities. An elevated total and/or allergen-specific serum IgE level is the most commonly associated laboratory feature, but it is not seen in about 20% of individuals with AD. Additionally, elevated allergen-specific IgE levels are found in 55% of the general population in the United States, making it nonspecific for AD. Moreover, while total IgE level usually varies in accordance with disease severity, it is not a reliable marker of disease severity, and it is possible for individuals with severe disease to have normal levels. Nonatopic conditions (eg, parasitic infection, some cancers, and autoimmune disease) may also lead to elevations in IgE levels.
Consistent prognostic markers are also lacking, although elevated total serum IgE levels and filaggrin gene null mutations do tend to be predictive of a more severe and prolonged disease course.
Topical agents including nonpharmacologic moisturizers and topical corticosteroids are the mainstay of treatment for AD; immunomodulatory agents and targeted biologic therapies are also available. For patients with more severe or recalcitrant disease, systemic therapy or phototherapy may be used, often in conjunction with topical therapies.
Richard Vinson, MD, President, Mountain View Dermatology, El Paso, Texas.
Richard Vinson, MD, has disclosed no relevant financial relationships.
The patient is diagnosed with atopic dermatitis (AD) on the basis of clinical findings and historical features, morphology and distribution of skin lesions, and associated clinical signs.
AD continues to be a clinical diagnosis. Pruritus and eczema (acute, subacute, or chronic) are essential features for the diagnosis of AD. The eczema should follow characteristic morphology and age-specific patterns (eg, facial/neck/extensor involvement in children, flexural involvement in any age group, sparing of the groin and axillary regions). A personal or family history of atopy is an important historical feature that supports the diagnosis of AD, as are xerosis and early age of onset.
The majority of patients with AD (60%) develop symptoms during the first year of life and 90% experience an eruption by 5 years of age, but disease onset can occur at any age. Some studies have found an association between late-onset AD and persistence of disease into adulthood.
At present, there are no reliable biomarkers that can differentiate AD from other entities. An elevated total and/or allergen-specific serum IgE level is the most commonly associated laboratory feature, but it is not seen in about 20% of individuals with AD. Additionally, elevated allergen-specific IgE levels are found in 55% of the general population in the United States, making it nonspecific for AD. Moreover, while total IgE level usually varies in accordance with disease severity, it is not a reliable marker of disease severity, and it is possible for individuals with severe disease to have normal levels. Nonatopic conditions (eg, parasitic infection, some cancers, and autoimmune disease) may also lead to elevations in IgE levels.
Consistent prognostic markers are also lacking, although elevated total serum IgE levels and filaggrin gene null mutations do tend to be predictive of a more severe and prolonged disease course.
Topical agents including nonpharmacologic moisturizers and topical corticosteroids are the mainstay of treatment for AD; immunomodulatory agents and targeted biologic therapies are also available. For patients with more severe or recalcitrant disease, systemic therapy or phototherapy may be used, often in conjunction with topical therapies.
Richard Vinson, MD, President, Mountain View Dermatology, El Paso, Texas.
Richard Vinson, MD, has disclosed no relevant financial relationships.
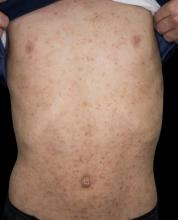
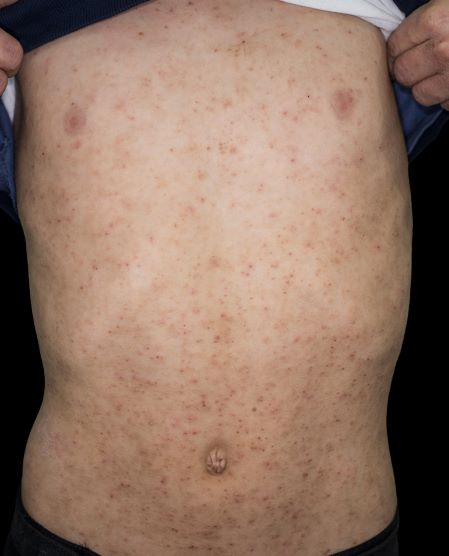
A 15-year-old boy presents with widespread pruritic lesions on his torso. His mother explains that he had been seen by his pediatrician 2 weeks before and was diagnosed with scabies. The patient was treated with a topical scabicidal agent (permethrin 5% lotion) on diagnosis, with a repeat application 7 days later. Despite treatment, no improvement in the patient's symptoms have been noted. Physical examination reveals poorly defined, erythematous, scaly, and crusted patches and plaques. No other family members are experiencing symptoms. There is a positive family history for atopy.
Persistent cough and dyspnea
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
A 6-year-old girl came to the hospital with a persistent cough, episodes of dyspnea, and a slight fever. Her mother reported that she had been diagnosed with asthma when she was 4 years old. Despite strict adherence to anti-inflammatory medication, she experiences frequent symptoms, especially at night, and she had been hospitalized for an asthma exacerbation 8 months ago. Posterior-anterior chest radiograph revealed blurring of the right border of the heart.