User login
For MD-IQ only
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
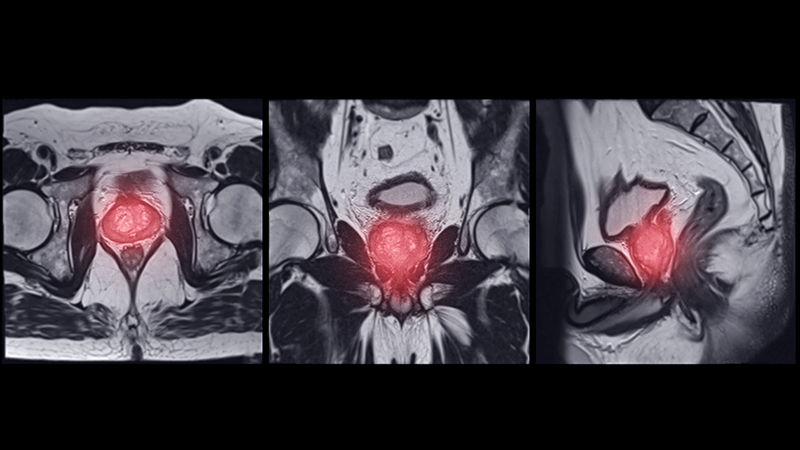
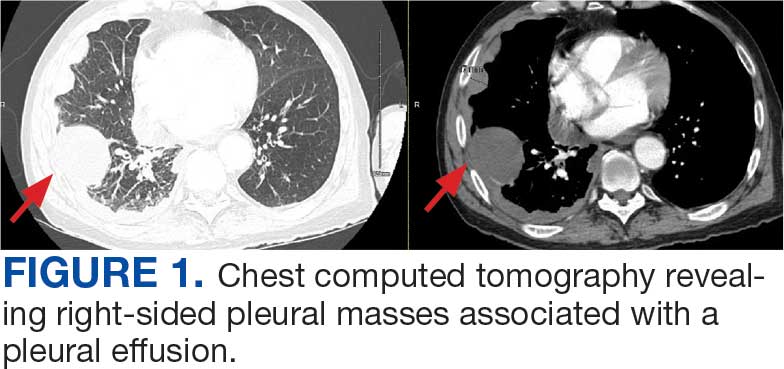
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.
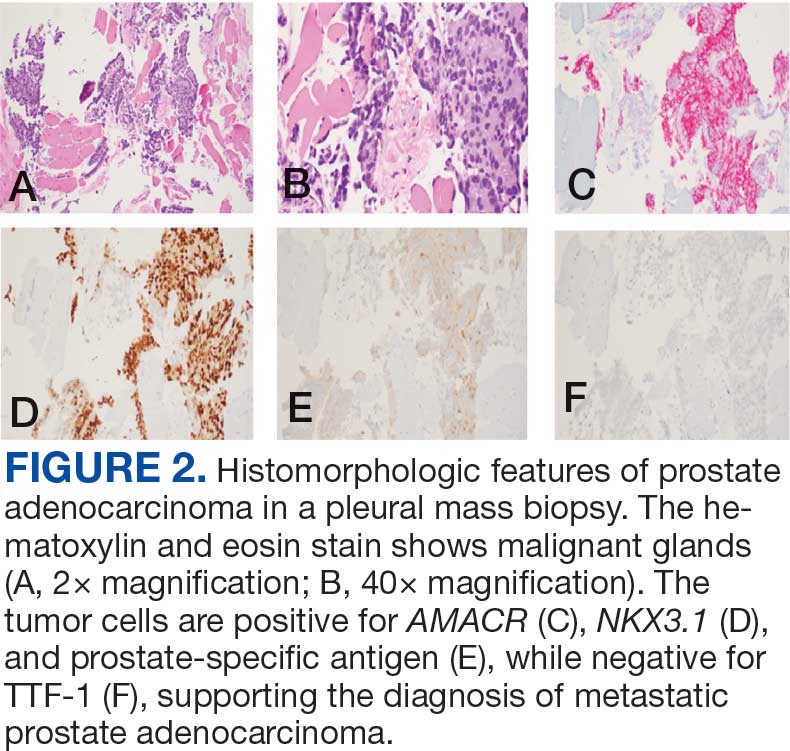
Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).
Case 2
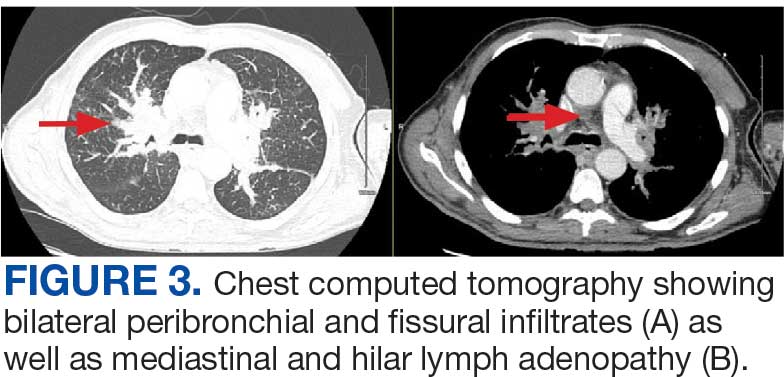
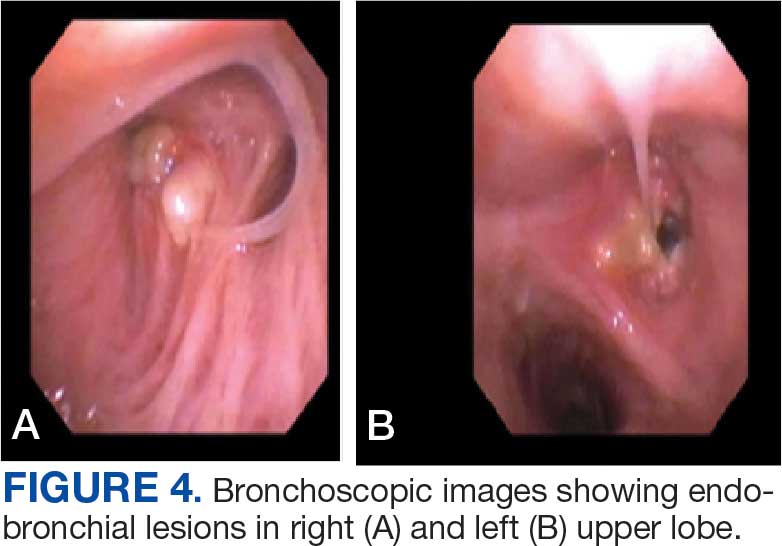
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).
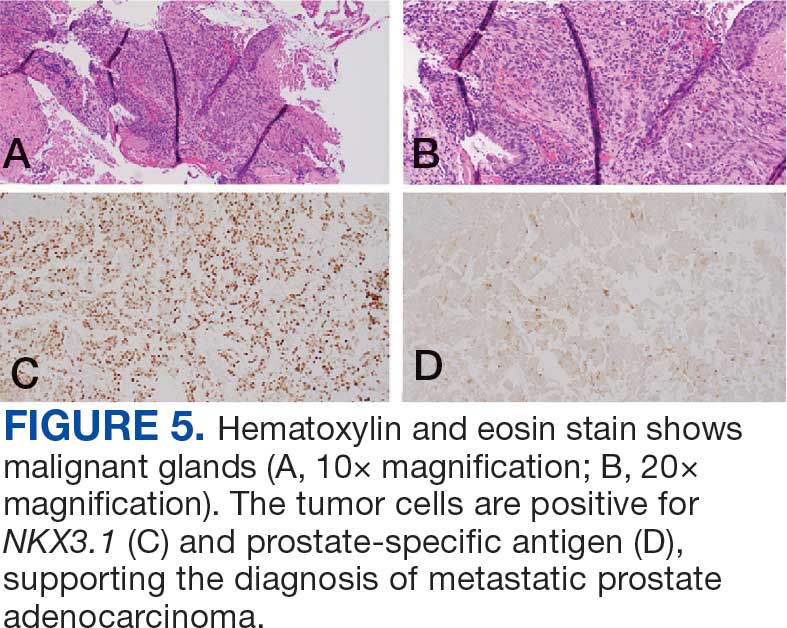
The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.
Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
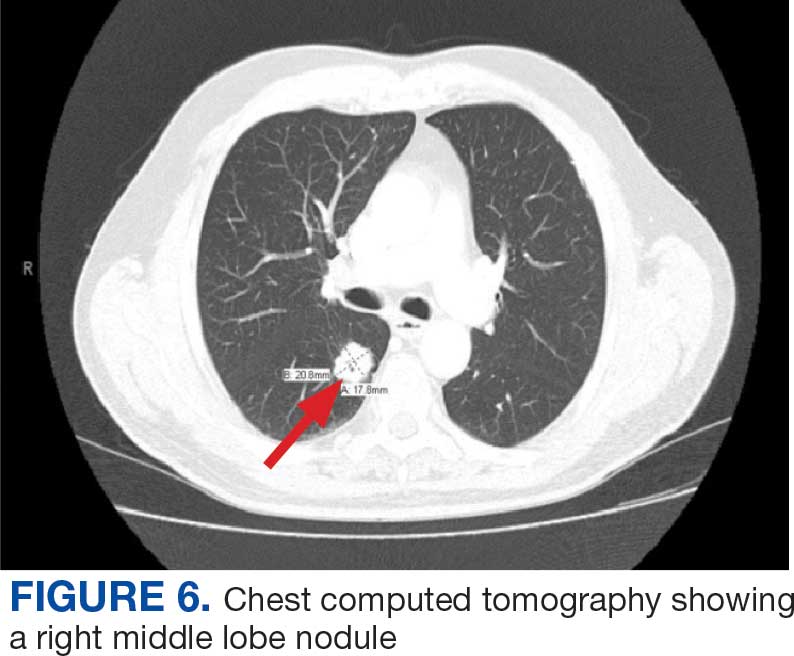
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.
The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.

Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).

Case 2
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).


The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.

Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.

The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.

Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).

Case 2
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).


The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.

Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.

The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Cancer Data Trends 2025
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
Million Veteran Program Drives Prostate Cancer Research
About 15,000 veterans are annually diagnosed with prostate cancer. Fortunately, those veterans enrolled in the US Department of Veterans Affairs (VA) Million Veteran Program (MVP) provide researchers with a deep pool of genetic data that can help identify causes, aid diagnosis, and guide targeted treatments.
More than 1,000,000 veterans have enrolled in MVP and donated their anonymized DNA to foster research. It is also one of the most genetically diverse health-related databases: 20% of participants identify as Black, 8% as Hispanic, 2% as Asian American, and 1% as Native American.
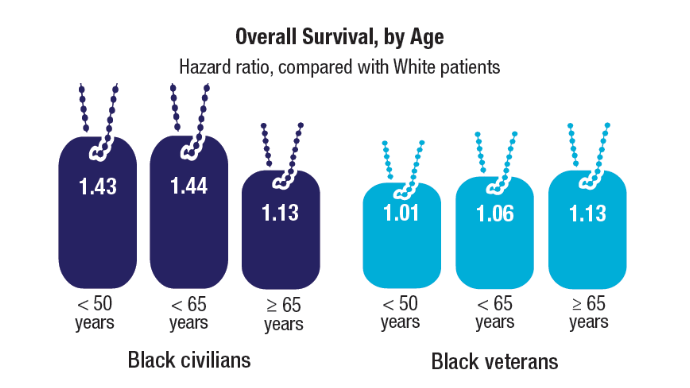
Ethnically and racially diverse data are particularly important for advancing the treatment of underserved groups. In a 2020 review, researchers found a number of areas where Black veterans differed from White veterans, including prostate-specific antigen (PSA) levels, incidence (almost 60% higher), clinical course, and mortality rate (2 to 3 times greater). To facilitate research, the MVP developed the “DNA chip,” a custom-designed tool that tests for > 750,000 genetic variants, including > 300,000 that are more common in minority populations.
“The whole thing about understanding genetics and diversity is like a circular feedback loop,” Director of MVP Dr. Sumitra Muralidhar said in a VA news article. “The more people you have represented from different racial and ethnic backgrounds, the more we’ll be able to discover genetic variants that contribute to their health. The more we discover, the more we can help that group. It’s a complete circular feedback loop.”
In addition to veterans’ blood samples and 600,000-plus baseline surveys on lifestyle, military service, and health, the MVP has collected upwards of 825,000 germline DNA samples, which have helped inform research into prostate cancer, the most commonly diagnosed solid tumor among veterans. By mining these data, researchers have built more evidence of how genes add to risk and disease progression.
In one study preprint that has not been peer reviewed, VA researchers investigated the significance of high polygenic hazard scores. The scores are strongly associated with age at diagnosis of any prostate cancer, as well as lifetime risk of metastatic and fatal prostate cancer. However, because they’re associated with any prostate cancer, the researchers say, there is concern that screening men with high polygenic risk could increase overdiagnosis of indolent cancers.
The researchers analyzed genetic and phenotypic data from 69,901 men in the MVP who have been diagnosed with prostate cancer (6413 metastatic). They found their hypothesis to be correct: Among men eventually diagnosed with prostate cancer, those with higher polygenic risk were more likely to develop metastatic disease.
Genetic risk scores like PHS601, a 601-variant polygenic score, can be performed on a saliva sample at any time during a person’s life, the researchers note. Thus, the scores provide the earliest information about age-specific risk of developing aggressive prostate cancer. These scores might be useful, they suggest, to support clinical decisions not only about whom to screen but also at what age.
Another study led by Stanford University researchers and published in Nature Genetics aimed to make screening more targeted, in this case prostate specific antigen screening. Estimates about PSA heritability vary from 40% to 45%, with genome-wide evaluations putting it at 25% to 30%, suggesting that incorporating genetic factors could improve screening.
This study involved 296,754 men (211,342 with European ancestry, 58,236 with African ancestry, 23,546 with Hispanic/Latino ancestry, and 3630 with Asian ancestry; 96.5% of participants were from MVP)—a sample size more than triple that in previous work.
The researchers detected 448 genome-wide significant variants, including 295 that were novel (to the best of their knowledge). The variance explained by genome-wide polygenic risk scores ranged from 11.6% to 16.6% for European ancestry, 5.5% to 9.5% for African ancestry, 13.5% to 18.2% for Hispanic/Latino ancestry, and 8.6% to 15.3% for Asian ancestry, and decreased with increasing age. Midlife genetically adjusted PSA levels were more strongly associated with overall and aggressive prostate cancer than unadjusted PSA levels.
The researchers say their study highlights how including higher proportions of participants from underrepresented populations can improve genetic prediction of PSA levels, offering the potential to personalize prostate cancer screening. Adjusting PSA for individuals’ predispositions in the absence of prostate cancer could improve the specificity (to reduce overdiagnosis) and sensitivity (to prevent more deaths) of screening.
Their findings, the researchers suggest, also explain additional variation in PSA, especially among men of African heritage, who experience the highest prostate cancer morbidity and mortality. They note that this work “moved us closer to leveraging genetic information to personalize PSA and substantially improved our understanding of PSA across diverse ancestries.”
A third study from a team at the VA Tennessee Valley Healthcare System also investigated the risk of inheriting a predisposition to prostate cancer. These researchers explored pathogenic variants using both genome-wide single-allele and identity-by-descent analytic approaches. They then tested their candidate variants for replication across independent biobanks, including MVP.
The researchers discovered the gene WNT9B E152K more than doubled the risk of familial prostate cancer. Meta-analysis, collectively encompassing 500,000 patients, confirmed the genome-wide significance. The researchers say WNT9B shares an “unexpected commonality” with the previously established prostate cancer risk genes HOXB13 and HNF1B: Each are required for embryonic prostate development. Based on that finding, the researchers also evaluated 2 additional genes, KMT2D and DHCR7, which are known to cause Mendelian genitourinary developmental defects. They, too, were nominally associated with prostate cancer under meta-analyses.
Tens of thousands of participants in MVP have had prostate cancer. The genetic research they participate in advances detection, prediction, and treatment for themselves and others, and science in general. The research is not only about finding causes, but what to do if the cancer develops. An “acting on MVP prostate cancer findings” study at VA Puget Sound Health Care System is testing how communicating with veterans about MVP prostate cancer results will affect their care. Those with prostate cancer will be screened to determine genetic contributions to their cancers. Those found to have a gene-based cancer diagnosis will be offered genetic counseling. Their immediate family will also be offered screening to test for inherited prostate cancer risk.
In 2016, the VA partnered with the Prostate Cancer Foundation to establish the Precision Oncology Program for Cancer of the Prostate (POPCaP). In collaboration with MVP and the Genomic Medicine Service, the program uses genetic information to individualize treatments for veterans with advanced prostate cancer.
US Army Veteran James Perry is one of the beneficiaries of the program. First diagnosed with prostate cancer in 2001, he was initially treated with radiation therapy, but the cancer recurred and spread to his lung. The John J. Cochran Veterans Hospital in St. Louis sent a sample of Perry's lung tumor to the laboratory for genetic testing, where they discovered he had a BRCA1 gene mutation.
His oncologist, Dr. Martin Schoen, recommended Perry enroll in AMPLITUDE, a clinical trial testing the effectiveness of poly-ADP ribose polymerase inhibitors, a new class of drugs to treat hormone-sensitive prostate cancer. One year later, Perry’s lung tumor could barely be seen on computed tomography, and his PSA levels were undetectable.
"I would highly recommend enrolling in a trial," Perry told VA Research Currents. “If a veteran has that opportunity, I would encourage it—anything that is going to give you a few more days is worth it.” In the interview, Perry said he enjoyed being part of the trial because he knows he is getting the most advanced care possible and is proud to help others like himself.
"We are honored to support VA's work to improve the lives of veterans who are living with advanced prostate cancer," Vice President and National Director of the PCF Veterans Health Initiative Rebecca Levine said. "Clinical trials play a vital role in bringing new treatments to patients who need them most. Mr. Perry's experience illustrates VA's commitment to provide state-of-the-art cancer care to all veterans who need it."
About 15,000 veterans are annually diagnosed with prostate cancer. Fortunately, those veterans enrolled in the US Department of Veterans Affairs (VA) Million Veteran Program (MVP) provide researchers with a deep pool of genetic data that can help identify causes, aid diagnosis, and guide targeted treatments.
More than 1,000,000 veterans have enrolled in MVP and donated their anonymized DNA to foster research. It is also one of the most genetically diverse health-related databases: 20% of participants identify as Black, 8% as Hispanic, 2% as Asian American, and 1% as Native American.
Ethnically and racially diverse data are particularly important for advancing the treatment of underserved groups. In a 2020 review, researchers found a number of areas where Black veterans differed from White veterans, including prostate-specific antigen (PSA) levels, incidence (almost 60% higher), clinical course, and mortality rate (2 to 3 times greater). To facilitate research, the MVP developed the “DNA chip,” a custom-designed tool that tests for > 750,000 genetic variants, including > 300,000 that are more common in minority populations.
“The whole thing about understanding genetics and diversity is like a circular feedback loop,” Director of MVP Dr. Sumitra Muralidhar said in a VA news article. “The more people you have represented from different racial and ethnic backgrounds, the more we’ll be able to discover genetic variants that contribute to their health. The more we discover, the more we can help that group. It’s a complete circular feedback loop.”
In addition to veterans’ blood samples and 600,000-plus baseline surveys on lifestyle, military service, and health, the MVP has collected upwards of 825,000 germline DNA samples, which have helped inform research into prostate cancer, the most commonly diagnosed solid tumor among veterans. By mining these data, researchers have built more evidence of how genes add to risk and disease progression.
In one study preprint that has not been peer reviewed, VA researchers investigated the significance of high polygenic hazard scores. The scores are strongly associated with age at diagnosis of any prostate cancer, as well as lifetime risk of metastatic and fatal prostate cancer. However, because they’re associated with any prostate cancer, the researchers say, there is concern that screening men with high polygenic risk could increase overdiagnosis of indolent cancers.
The researchers analyzed genetic and phenotypic data from 69,901 men in the MVP who have been diagnosed with prostate cancer (6413 metastatic). They found their hypothesis to be correct: Among men eventually diagnosed with prostate cancer, those with higher polygenic risk were more likely to develop metastatic disease.
Genetic risk scores like PHS601, a 601-variant polygenic score, can be performed on a saliva sample at any time during a person’s life, the researchers note. Thus, the scores provide the earliest information about age-specific risk of developing aggressive prostate cancer. These scores might be useful, they suggest, to support clinical decisions not only about whom to screen but also at what age.
Another study led by Stanford University researchers and published in Nature Genetics aimed to make screening more targeted, in this case prostate specific antigen screening. Estimates about PSA heritability vary from 40% to 45%, with genome-wide evaluations putting it at 25% to 30%, suggesting that incorporating genetic factors could improve screening.
This study involved 296,754 men (211,342 with European ancestry, 58,236 with African ancestry, 23,546 with Hispanic/Latino ancestry, and 3630 with Asian ancestry; 96.5% of participants were from MVP)—a sample size more than triple that in previous work.
The researchers detected 448 genome-wide significant variants, including 295 that were novel (to the best of their knowledge). The variance explained by genome-wide polygenic risk scores ranged from 11.6% to 16.6% for European ancestry, 5.5% to 9.5% for African ancestry, 13.5% to 18.2% for Hispanic/Latino ancestry, and 8.6% to 15.3% for Asian ancestry, and decreased with increasing age. Midlife genetically adjusted PSA levels were more strongly associated with overall and aggressive prostate cancer than unadjusted PSA levels.
The researchers say their study highlights how including higher proportions of participants from underrepresented populations can improve genetic prediction of PSA levels, offering the potential to personalize prostate cancer screening. Adjusting PSA for individuals’ predispositions in the absence of prostate cancer could improve the specificity (to reduce overdiagnosis) and sensitivity (to prevent more deaths) of screening.
Their findings, the researchers suggest, also explain additional variation in PSA, especially among men of African heritage, who experience the highest prostate cancer morbidity and mortality. They note that this work “moved us closer to leveraging genetic information to personalize PSA and substantially improved our understanding of PSA across diverse ancestries.”
A third study from a team at the VA Tennessee Valley Healthcare System also investigated the risk of inheriting a predisposition to prostate cancer. These researchers explored pathogenic variants using both genome-wide single-allele and identity-by-descent analytic approaches. They then tested their candidate variants for replication across independent biobanks, including MVP.
The researchers discovered the gene WNT9B E152K more than doubled the risk of familial prostate cancer. Meta-analysis, collectively encompassing 500,000 patients, confirmed the genome-wide significance. The researchers say WNT9B shares an “unexpected commonality” with the previously established prostate cancer risk genes HOXB13 and HNF1B: Each are required for embryonic prostate development. Based on that finding, the researchers also evaluated 2 additional genes, KMT2D and DHCR7, which are known to cause Mendelian genitourinary developmental defects. They, too, were nominally associated with prostate cancer under meta-analyses.
Tens of thousands of participants in MVP have had prostate cancer. The genetic research they participate in advances detection, prediction, and treatment for themselves and others, and science in general. The research is not only about finding causes, but what to do if the cancer develops. An “acting on MVP prostate cancer findings” study at VA Puget Sound Health Care System is testing how communicating with veterans about MVP prostate cancer results will affect their care. Those with prostate cancer will be screened to determine genetic contributions to their cancers. Those found to have a gene-based cancer diagnosis will be offered genetic counseling. Their immediate family will also be offered screening to test for inherited prostate cancer risk.
In 2016, the VA partnered with the Prostate Cancer Foundation to establish the Precision Oncology Program for Cancer of the Prostate (POPCaP). In collaboration with MVP and the Genomic Medicine Service, the program uses genetic information to individualize treatments for veterans with advanced prostate cancer.
US Army Veteran James Perry is one of the beneficiaries of the program. First diagnosed with prostate cancer in 2001, he was initially treated with radiation therapy, but the cancer recurred and spread to his lung. The John J. Cochran Veterans Hospital in St. Louis sent a sample of Perry's lung tumor to the laboratory for genetic testing, where they discovered he had a BRCA1 gene mutation.
His oncologist, Dr. Martin Schoen, recommended Perry enroll in AMPLITUDE, a clinical trial testing the effectiveness of poly-ADP ribose polymerase inhibitors, a new class of drugs to treat hormone-sensitive prostate cancer. One year later, Perry’s lung tumor could barely be seen on computed tomography, and his PSA levels were undetectable.
"I would highly recommend enrolling in a trial," Perry told VA Research Currents. “If a veteran has that opportunity, I would encourage it—anything that is going to give you a few more days is worth it.” In the interview, Perry said he enjoyed being part of the trial because he knows he is getting the most advanced care possible and is proud to help others like himself.
"We are honored to support VA's work to improve the lives of veterans who are living with advanced prostate cancer," Vice President and National Director of the PCF Veterans Health Initiative Rebecca Levine said. "Clinical trials play a vital role in bringing new treatments to patients who need them most. Mr. Perry's experience illustrates VA's commitment to provide state-of-the-art cancer care to all veterans who need it."
About 15,000 veterans are annually diagnosed with prostate cancer. Fortunately, those veterans enrolled in the US Department of Veterans Affairs (VA) Million Veteran Program (MVP) provide researchers with a deep pool of genetic data that can help identify causes, aid diagnosis, and guide targeted treatments.
More than 1,000,000 veterans have enrolled in MVP and donated their anonymized DNA to foster research. It is also one of the most genetically diverse health-related databases: 20% of participants identify as Black, 8% as Hispanic, 2% as Asian American, and 1% as Native American.
Ethnically and racially diverse data are particularly important for advancing the treatment of underserved groups. In a 2020 review, researchers found a number of areas where Black veterans differed from White veterans, including prostate-specific antigen (PSA) levels, incidence (almost 60% higher), clinical course, and mortality rate (2 to 3 times greater). To facilitate research, the MVP developed the “DNA chip,” a custom-designed tool that tests for > 750,000 genetic variants, including > 300,000 that are more common in minority populations.
“The whole thing about understanding genetics and diversity is like a circular feedback loop,” Director of MVP Dr. Sumitra Muralidhar said in a VA news article. “The more people you have represented from different racial and ethnic backgrounds, the more we’ll be able to discover genetic variants that contribute to their health. The more we discover, the more we can help that group. It’s a complete circular feedback loop.”
In addition to veterans’ blood samples and 600,000-plus baseline surveys on lifestyle, military service, and health, the MVP has collected upwards of 825,000 germline DNA samples, which have helped inform research into prostate cancer, the most commonly diagnosed solid tumor among veterans. By mining these data, researchers have built more evidence of how genes add to risk and disease progression.
In one study preprint that has not been peer reviewed, VA researchers investigated the significance of high polygenic hazard scores. The scores are strongly associated with age at diagnosis of any prostate cancer, as well as lifetime risk of metastatic and fatal prostate cancer. However, because they’re associated with any prostate cancer, the researchers say, there is concern that screening men with high polygenic risk could increase overdiagnosis of indolent cancers.
The researchers analyzed genetic and phenotypic data from 69,901 men in the MVP who have been diagnosed with prostate cancer (6413 metastatic). They found their hypothesis to be correct: Among men eventually diagnosed with prostate cancer, those with higher polygenic risk were more likely to develop metastatic disease.
Genetic risk scores like PHS601, a 601-variant polygenic score, can be performed on a saliva sample at any time during a person’s life, the researchers note. Thus, the scores provide the earliest information about age-specific risk of developing aggressive prostate cancer. These scores might be useful, they suggest, to support clinical decisions not only about whom to screen but also at what age.
Another study led by Stanford University researchers and published in Nature Genetics aimed to make screening more targeted, in this case prostate specific antigen screening. Estimates about PSA heritability vary from 40% to 45%, with genome-wide evaluations putting it at 25% to 30%, suggesting that incorporating genetic factors could improve screening.
This study involved 296,754 men (211,342 with European ancestry, 58,236 with African ancestry, 23,546 with Hispanic/Latino ancestry, and 3630 with Asian ancestry; 96.5% of participants were from MVP)—a sample size more than triple that in previous work.
The researchers detected 448 genome-wide significant variants, including 295 that were novel (to the best of their knowledge). The variance explained by genome-wide polygenic risk scores ranged from 11.6% to 16.6% for European ancestry, 5.5% to 9.5% for African ancestry, 13.5% to 18.2% for Hispanic/Latino ancestry, and 8.6% to 15.3% for Asian ancestry, and decreased with increasing age. Midlife genetically adjusted PSA levels were more strongly associated with overall and aggressive prostate cancer than unadjusted PSA levels.
The researchers say their study highlights how including higher proportions of participants from underrepresented populations can improve genetic prediction of PSA levels, offering the potential to personalize prostate cancer screening. Adjusting PSA for individuals’ predispositions in the absence of prostate cancer could improve the specificity (to reduce overdiagnosis) and sensitivity (to prevent more deaths) of screening.
Their findings, the researchers suggest, also explain additional variation in PSA, especially among men of African heritage, who experience the highest prostate cancer morbidity and mortality. They note that this work “moved us closer to leveraging genetic information to personalize PSA and substantially improved our understanding of PSA across diverse ancestries.”
A third study from a team at the VA Tennessee Valley Healthcare System also investigated the risk of inheriting a predisposition to prostate cancer. These researchers explored pathogenic variants using both genome-wide single-allele and identity-by-descent analytic approaches. They then tested their candidate variants for replication across independent biobanks, including MVP.
The researchers discovered the gene WNT9B E152K more than doubled the risk of familial prostate cancer. Meta-analysis, collectively encompassing 500,000 patients, confirmed the genome-wide significance. The researchers say WNT9B shares an “unexpected commonality” with the previously established prostate cancer risk genes HOXB13 and HNF1B: Each are required for embryonic prostate development. Based on that finding, the researchers also evaluated 2 additional genes, KMT2D and DHCR7, which are known to cause Mendelian genitourinary developmental defects. They, too, were nominally associated with prostate cancer under meta-analyses.
Tens of thousands of participants in MVP have had prostate cancer. The genetic research they participate in advances detection, prediction, and treatment for themselves and others, and science in general. The research is not only about finding causes, but what to do if the cancer develops. An “acting on MVP prostate cancer findings” study at VA Puget Sound Health Care System is testing how communicating with veterans about MVP prostate cancer results will affect their care. Those with prostate cancer will be screened to determine genetic contributions to their cancers. Those found to have a gene-based cancer diagnosis will be offered genetic counseling. Their immediate family will also be offered screening to test for inherited prostate cancer risk.
In 2016, the VA partnered with the Prostate Cancer Foundation to establish the Precision Oncology Program for Cancer of the Prostate (POPCaP). In collaboration with MVP and the Genomic Medicine Service, the program uses genetic information to individualize treatments for veterans with advanced prostate cancer.
US Army Veteran James Perry is one of the beneficiaries of the program. First diagnosed with prostate cancer in 2001, he was initially treated with radiation therapy, but the cancer recurred and spread to his lung. The John J. Cochran Veterans Hospital in St. Louis sent a sample of Perry's lung tumor to the laboratory for genetic testing, where they discovered he had a BRCA1 gene mutation.
His oncologist, Dr. Martin Schoen, recommended Perry enroll in AMPLITUDE, a clinical trial testing the effectiveness of poly-ADP ribose polymerase inhibitors, a new class of drugs to treat hormone-sensitive prostate cancer. One year later, Perry’s lung tumor could barely be seen on computed tomography, and his PSA levels were undetectable.
"I would highly recommend enrolling in a trial," Perry told VA Research Currents. “If a veteran has that opportunity, I would encourage it—anything that is going to give you a few more days is worth it.” In the interview, Perry said he enjoyed being part of the trial because he knows he is getting the most advanced care possible and is proud to help others like himself.
"We are honored to support VA's work to improve the lives of veterans who are living with advanced prostate cancer," Vice President and National Director of the PCF Veterans Health Initiative Rebecca Levine said. "Clinical trials play a vital role in bringing new treatments to patients who need them most. Mr. Perry's experience illustrates VA's commitment to provide state-of-the-art cancer care to all veterans who need it."
Service Connection Expanded to Additional Cancers
The US Department of Veterans Affairs (VA) is "lowering the burden of proof" for thousands, making acute and chronic leukemias, multiple myelomas, myelodysplastic syndromes, myelofibrosis, urinary bladder, ureter, and related genitourinary cancers presumptive for service connection.
The Jan. 8 decision included Gulf War veterans, those who served in Somalia or the Southwest Asia theater of operations during the Persian Gulf War on or after Aug. 2, 1990; and post-9/11 veterans, those who served in Afghanistan, Iraq, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, or Uzbekistan and the airspace above these locations during the Gulf War on or after Sept. 11, 2001. It also includes veterans who served at the Karshi-Khanabad (K2) base in Uzbekistan after Sept. 11, 2001.
Veterans no longer must prove their service caused their condition to receive benefits. This landmark decision allows them access to free health care for that condition.
According to the VA, these steps are also part of a comprehensive effort to ensure that K2 veterans—and their survivors—receive the care and benefits they deserve. K2 veterans have higher claim and approval rates than any other cohort of veterans: 13,002 are enrolled in VA health care, and the average K2 veteran is service connected for 14.6 conditions.
The 2022 PACT Act was the largest expansion of veteran benefits in generations. The VA then made millions of veterans eligible for health care and benefits years earlier than called for by the law. It also launched the largest outreach campaign in the history of the VA to encourage veterans to apply.
Nearly 890,000 veterans have signed up for VA health care since the bill was signed into law, a nearly 40% increase over the previous equivalent period, and veterans have submitted > 4.8 million applications for VA benefits (a 42% increase over the previous equivalent period and an all-time record). The VA has delivered > $600 billion in earned benefits directly to veterans, their families, and survivors during that time.
The VA encourages all eligible veterans—including those with previously denied claims—to apply for benefits. To apply for benefits, veterans and survivors may visit VA.gov or call 1-800-MYVA411.
The US Department of Veterans Affairs (VA) is "lowering the burden of proof" for thousands, making acute and chronic leukemias, multiple myelomas, myelodysplastic syndromes, myelofibrosis, urinary bladder, ureter, and related genitourinary cancers presumptive for service connection.
The Jan. 8 decision included Gulf War veterans, those who served in Somalia or the Southwest Asia theater of operations during the Persian Gulf War on or after Aug. 2, 1990; and post-9/11 veterans, those who served in Afghanistan, Iraq, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, or Uzbekistan and the airspace above these locations during the Gulf War on or after Sept. 11, 2001. It also includes veterans who served at the Karshi-Khanabad (K2) base in Uzbekistan after Sept. 11, 2001.
Veterans no longer must prove their service caused their condition to receive benefits. This landmark decision allows them access to free health care for that condition.
According to the VA, these steps are also part of a comprehensive effort to ensure that K2 veterans—and their survivors—receive the care and benefits they deserve. K2 veterans have higher claim and approval rates than any other cohort of veterans: 13,002 are enrolled in VA health care, and the average K2 veteran is service connected for 14.6 conditions.
The 2022 PACT Act was the largest expansion of veteran benefits in generations. The VA then made millions of veterans eligible for health care and benefits years earlier than called for by the law. It also launched the largest outreach campaign in the history of the VA to encourage veterans to apply.
Nearly 890,000 veterans have signed up for VA health care since the bill was signed into law, a nearly 40% increase over the previous equivalent period, and veterans have submitted > 4.8 million applications for VA benefits (a 42% increase over the previous equivalent period and an all-time record). The VA has delivered > $600 billion in earned benefits directly to veterans, their families, and survivors during that time.
The VA encourages all eligible veterans—including those with previously denied claims—to apply for benefits. To apply for benefits, veterans and survivors may visit VA.gov or call 1-800-MYVA411.
The US Department of Veterans Affairs (VA) is "lowering the burden of proof" for thousands, making acute and chronic leukemias, multiple myelomas, myelodysplastic syndromes, myelofibrosis, urinary bladder, ureter, and related genitourinary cancers presumptive for service connection.
The Jan. 8 decision included Gulf War veterans, those who served in Somalia or the Southwest Asia theater of operations during the Persian Gulf War on or after Aug. 2, 1990; and post-9/11 veterans, those who served in Afghanistan, Iraq, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, or Uzbekistan and the airspace above these locations during the Gulf War on or after Sept. 11, 2001. It also includes veterans who served at the Karshi-Khanabad (K2) base in Uzbekistan after Sept. 11, 2001.
Veterans no longer must prove their service caused their condition to receive benefits. This landmark decision allows them access to free health care for that condition.
According to the VA, these steps are also part of a comprehensive effort to ensure that K2 veterans—and their survivors—receive the care and benefits they deserve. K2 veterans have higher claim and approval rates than any other cohort of veterans: 13,002 are enrolled in VA health care, and the average K2 veteran is service connected for 14.6 conditions.
The 2022 PACT Act was the largest expansion of veteran benefits in generations. The VA then made millions of veterans eligible for health care and benefits years earlier than called for by the law. It also launched the largest outreach campaign in the history of the VA to encourage veterans to apply.
Nearly 890,000 veterans have signed up for VA health care since the bill was signed into law, a nearly 40% increase over the previous equivalent period, and veterans have submitted > 4.8 million applications for VA benefits (a 42% increase over the previous equivalent period and an all-time record). The VA has delivered > $600 billion in earned benefits directly to veterans, their families, and survivors during that time.
The VA encourages all eligible veterans—including those with previously denied claims—to apply for benefits. To apply for benefits, veterans and survivors may visit VA.gov or call 1-800-MYVA411.
FDA OKs Blood-Based Test to Help Diagnose Prostate Cancer
FDA OKs Blood-Based Test to Help Diagnose Prostate Cancer
The FDA has granted Cleveland Diagnostics' IsoPSA test premarket approval (PMA) to help detect prostate cancer in men aged ≥ 50 years with elevated PSA levels.
IsoPSA is a blood assay that detects variations of the PSA protein that signal a higher likelihood of high-grade tumors. It is one of several biomarker tests included in the National Comprehensive Cancer Network's guidelines on early detection of prostate cancer.
Cleveland Diagnostics noted that 75% of prostate biopsies are negative for high-grade disease. IsoPSA and similar tests aim to help identify men who need a biopsy while allowing others avoid an unnecessary procedure.
IsoPSA has been available since 2020 under the FDA's Laboratory-Developed Test rubric, meaning that blood samples had to be shipped for analysis to Cleveland Diagnostics' lab. With the PMA, testing can now be done at CLIA-certified labs across the country.
The company expects the approval should increase access to IsoPSA and reduce turnaround time. "We remain focused on executing our commercial strategy and expanding access to IsoPSA," company President and CEO Arnon Chait, PhD, said in a press release.
The approval was based, in part, on a prospective validation study of 888 men scheduled for prostate biopsy. IsoPSA demonstrated an AUC of 0.783 for high-grade tumors, with a sensitivity of 90.2% and a specificity of 45.5%. In a real-world clinical utility study with 900 patients, IsoPSA testing led to a 55% decrease in biopsy recommendations.
The test is covered by Medicare and a growing number of commercial payers, Cleveland Diagnostics said.
M. Alexander Otto is a physician assistant with a master's degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape Medical News. Alex is also an MIT Knight Science Journalism Fellow. Email: aotto@mdedge.com.
A version of this article first appeared on Medscape.com.
The FDA has granted Cleveland Diagnostics' IsoPSA test premarket approval (PMA) to help detect prostate cancer in men aged ≥ 50 years with elevated PSA levels.
IsoPSA is a blood assay that detects variations of the PSA protein that signal a higher likelihood of high-grade tumors. It is one of several biomarker tests included in the National Comprehensive Cancer Network's guidelines on early detection of prostate cancer.
Cleveland Diagnostics noted that 75% of prostate biopsies are negative for high-grade disease. IsoPSA and similar tests aim to help identify men who need a biopsy while allowing others avoid an unnecessary procedure.
IsoPSA has been available since 2020 under the FDA's Laboratory-Developed Test rubric, meaning that blood samples had to be shipped for analysis to Cleveland Diagnostics' lab. With the PMA, testing can now be done at CLIA-certified labs across the country.
The company expects the approval should increase access to IsoPSA and reduce turnaround time. "We remain focused on executing our commercial strategy and expanding access to IsoPSA," company President and CEO Arnon Chait, PhD, said in a press release.
The approval was based, in part, on a prospective validation study of 888 men scheduled for prostate biopsy. IsoPSA demonstrated an AUC of 0.783 for high-grade tumors, with a sensitivity of 90.2% and a specificity of 45.5%. In a real-world clinical utility study with 900 patients, IsoPSA testing led to a 55% decrease in biopsy recommendations.
The test is covered by Medicare and a growing number of commercial payers, Cleveland Diagnostics said.
M. Alexander Otto is a physician assistant with a master's degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape Medical News. Alex is also an MIT Knight Science Journalism Fellow. Email: aotto@mdedge.com.
A version of this article first appeared on Medscape.com.
The FDA has granted Cleveland Diagnostics' IsoPSA test premarket approval (PMA) to help detect prostate cancer in men aged ≥ 50 years with elevated PSA levels.
IsoPSA is a blood assay that detects variations of the PSA protein that signal a higher likelihood of high-grade tumors. It is one of several biomarker tests included in the National Comprehensive Cancer Network's guidelines on early detection of prostate cancer.
Cleveland Diagnostics noted that 75% of prostate biopsies are negative for high-grade disease. IsoPSA and similar tests aim to help identify men who need a biopsy while allowing others avoid an unnecessary procedure.
IsoPSA has been available since 2020 under the FDA's Laboratory-Developed Test rubric, meaning that blood samples had to be shipped for analysis to Cleveland Diagnostics' lab. With the PMA, testing can now be done at CLIA-certified labs across the country.
The company expects the approval should increase access to IsoPSA and reduce turnaround time. "We remain focused on executing our commercial strategy and expanding access to IsoPSA," company President and CEO Arnon Chait, PhD, said in a press release.
The approval was based, in part, on a prospective validation study of 888 men scheduled for prostate biopsy. IsoPSA demonstrated an AUC of 0.783 for high-grade tumors, with a sensitivity of 90.2% and a specificity of 45.5%. In a real-world clinical utility study with 900 patients, IsoPSA testing led to a 55% decrease in biopsy recommendations.
The test is covered by Medicare and a growing number of commercial payers, Cleveland Diagnostics said.
M. Alexander Otto is a physician assistant with a master's degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape Medical News. Alex is also an MIT Knight Science Journalism Fellow. Email: aotto@mdedge.com.
A version of this article first appeared on Medscape.com.
FDA OKs Blood-Based Test to Help Diagnose Prostate Cancer
FDA OKs Blood-Based Test to Help Diagnose Prostate Cancer
Identical Survival for Abiraterone and Enzalutamide in Vets With Metastatic Hormone-Sensitive Prostate Cancer
Abiraterone and enzalutamide showed identical survival outcomes when used as first-line treatment for metastatic hormone-sensitive prostate cancer (mHSPC), according to a new study using US Department of Veterans Affairs (VA) data. The report represents the first head-to-head clinical analysis of these commonly used androgen receptor inhibitors.
Among 1258 veterans treated with abiraterone and 311 treated with enzalutamide, median overall survival was 36.2 months for both drugs. Patients were followed for a mean of 28.7 months (abiraterone) and 30.8 months (enzalutamide), reported by Martin W. Schoen, MD, MPH, from Saint Louis University School of Medicine and the St. Louis VA Medical Center, in JAMA Network Open.
Notably, there was no significant difference in outcomes among Black veterans, who often have poorer outcomes in prostate cancer, and in patients with cardiovascular disease.
“This is the first direct comparison of abiraterone and enzalutamide for mHSPC in a clinical practice setting,” Schoen told Federal Practitioner. “At the population level, there are no differences based on initial treatment choice.”
Abiraterone Is Preferred in the VA Due to Cost
According to Schoen, abiraterone and enzalutamide are the most commonly used androgen receptor inhibitors to treat mHSPC within the VA. A 2025 study by Schoen and colleagues found that 53.7% of veterans with mHSPC in 2022 received androgen receptor inhibitor therapy, up from 16.9% in 2017.
“In the VA, the preference for most patients is abiraterone since it is the least expensive agent,” he said. A generic version has been available for several years.
Additionally, abiraterone “has been on the market for the longest, and therefore clinicians are familiar with its use,” Schoen said. However, “clinicians have little idea of the comparative efficacy between these 2 agents,” he added.
The authors suggest that the cost and toxicities of the medications should guide clinician decisions, Schoen said. “There is data that abiraterone may worsen diabetes, since it is given with prednisone and could increase the risk of cardiovascular events,” he said.
He added that 2 newer drugs, apalutamide and darolutamide, are also “viable options.” Chemotherapies and certain targeted drugs are also available, “but they are only used in a select group of patients.”
Outside Specialist: Diverse Study Population Is a Plus
Hematologist-oncologist Natalie Reizine, MD, of the University of Illinois College of Medicine, Chicago, who was not involved in the study, told Federal Practitioner that the real-world data are valuable given the limitations of clinical trial populations.
“It’s difficult to compare clinical trials because they enroll different groups of patients,” she said. And, she said, they often exclude patients with significant comorbidities. “If they have bad cardiovascular disease, for instance, or poorly controlled diabetes, they're excluded from the clinical trial. But in real life, many of our patients have other medical problems that we have to manage.”
Reizine also emphasized the significance of the study’s diverse patient population. “Black men are very underrepresented in clinical trials. Many clinical trials that lead to drug approval will have only few or no Black men at all, yet these drugs go on to be widely prescribed to all men with prostate cancer.”
Results Are ‘Reassuring’
Reizine described the overall study findings as “reassuring,” especially in light of “studies that show that abiraterone and prednisone may be associated with worse cardiovascular outcomes. This study showed that in this VA population, even for patients who had cardiovascular disease, there was not a difference in how they did.”
As for choosing between agents, she recommended considering comorbidities and potential drug-drug interactions. “One of the big reasons that you may not be able to safely prescribe enzalutamide, for instance, is if a patient is on an anticoagulant, which is incredibly common in cancer patients. Enzalutamide has more drug-drug interactions than abiraterone and prednisone.”
Study Demographics and Findings
The study included all patients with mHSPC who initiated abiraterone or enzalutamide between July 2017 and April 2023.
Median ages were 73 (abiraterone) and 74 years (enzalutamide, P = .29). Racial distribution was similar between groups: abiraterone (68.1% White, 25.0% Black, 6.9% other/unknown) and enzalutamide (66.6% White, 27.0% Black, 6.4% other/unknown; P = .74). Ethnicity was 89.2% non-Hispanic, 4.4% Hispanic, and 6.4% unknown in the abiraterone group vs 88.4% non-Hispanic, 3.5% Hispanic, and 8.0% unknown in the enzalutamide group (P = .50).
The groups had similar rates of the most common comorbidities: diabetes (40.5% vs 46.3%, respectively, P = .07), peripheral vascular disease (40.2% vs 37.6%, respectively, P = .44), and chronic pulmonary disease (37.0% vs 40.5%, P = .29).
In an inverse probability weighting analysis with abiraterone as reference, weighted median overall survival was comparable across the entire cohort (36.2 months, P = .32), Black veterans (39.7 months, P = .90), and those with cardiovascular disease (31.5 months, P = .30).
The authors noted limitations such as the observational cohort design and data constraints.
The study was supported by the American Society of Clinical Oncology Conquer Cancer Foundation, the Prostate Cancer Foundation, and the Blavatnik Family Foundation.
Schoen discloses relationships with the Prostate Cancer Foundation, Astellas, and US Department of Defense. Other authors disclose relationships with the American Society of Clinical Oncology, Pfizer, Exelixis, Eli Lilly, Sanofi, Merck, Seagen, Bellicum, and BMS.
Outside the submitted work. Reizine discloses relationships with the US Department of Defense, Sanofi, Exelexis, Janssen, AstraZeneca, EMD Serono, Janssen, Merck, and Tempus.
Abiraterone and enzalutamide showed identical survival outcomes when used as first-line treatment for metastatic hormone-sensitive prostate cancer (mHSPC), according to a new study using US Department of Veterans Affairs (VA) data. The report represents the first head-to-head clinical analysis of these commonly used androgen receptor inhibitors.
Among 1258 veterans treated with abiraterone and 311 treated with enzalutamide, median overall survival was 36.2 months for both drugs. Patients were followed for a mean of 28.7 months (abiraterone) and 30.8 months (enzalutamide), reported by Martin W. Schoen, MD, MPH, from Saint Louis University School of Medicine and the St. Louis VA Medical Center, in JAMA Network Open.
Notably, there was no significant difference in outcomes among Black veterans, who often have poorer outcomes in prostate cancer, and in patients with cardiovascular disease.
“This is the first direct comparison of abiraterone and enzalutamide for mHSPC in a clinical practice setting,” Schoen told Federal Practitioner. “At the population level, there are no differences based on initial treatment choice.”
Abiraterone Is Preferred in the VA Due to Cost
According to Schoen, abiraterone and enzalutamide are the most commonly used androgen receptor inhibitors to treat mHSPC within the VA. A 2025 study by Schoen and colleagues found that 53.7% of veterans with mHSPC in 2022 received androgen receptor inhibitor therapy, up from 16.9% in 2017.
“In the VA, the preference for most patients is abiraterone since it is the least expensive agent,” he said. A generic version has been available for several years.
Additionally, abiraterone “has been on the market for the longest, and therefore clinicians are familiar with its use,” Schoen said. However, “clinicians have little idea of the comparative efficacy between these 2 agents,” he added.
The authors suggest that the cost and toxicities of the medications should guide clinician decisions, Schoen said. “There is data that abiraterone may worsen diabetes, since it is given with prednisone and could increase the risk of cardiovascular events,” he said.
He added that 2 newer drugs, apalutamide and darolutamide, are also “viable options.” Chemotherapies and certain targeted drugs are also available, “but they are only used in a select group of patients.”
Outside Specialist: Diverse Study Population Is a Plus
Hematologist-oncologist Natalie Reizine, MD, of the University of Illinois College of Medicine, Chicago, who was not involved in the study, told Federal Practitioner that the real-world data are valuable given the limitations of clinical trial populations.
“It’s difficult to compare clinical trials because they enroll different groups of patients,” she said. And, she said, they often exclude patients with significant comorbidities. “If they have bad cardiovascular disease, for instance, or poorly controlled diabetes, they're excluded from the clinical trial. But in real life, many of our patients have other medical problems that we have to manage.”
Reizine also emphasized the significance of the study’s diverse patient population. “Black men are very underrepresented in clinical trials. Many clinical trials that lead to drug approval will have only few or no Black men at all, yet these drugs go on to be widely prescribed to all men with prostate cancer.”
Results Are ‘Reassuring’
Reizine described the overall study findings as “reassuring,” especially in light of “studies that show that abiraterone and prednisone may be associated with worse cardiovascular outcomes. This study showed that in this VA population, even for patients who had cardiovascular disease, there was not a difference in how they did.”
As for choosing between agents, she recommended considering comorbidities and potential drug-drug interactions. “One of the big reasons that you may not be able to safely prescribe enzalutamide, for instance, is if a patient is on an anticoagulant, which is incredibly common in cancer patients. Enzalutamide has more drug-drug interactions than abiraterone and prednisone.”
Study Demographics and Findings
The study included all patients with mHSPC who initiated abiraterone or enzalutamide between July 2017 and April 2023.
Median ages were 73 (abiraterone) and 74 years (enzalutamide, P = .29). Racial distribution was similar between groups: abiraterone (68.1% White, 25.0% Black, 6.9% other/unknown) and enzalutamide (66.6% White, 27.0% Black, 6.4% other/unknown; P = .74). Ethnicity was 89.2% non-Hispanic, 4.4% Hispanic, and 6.4% unknown in the abiraterone group vs 88.4% non-Hispanic, 3.5% Hispanic, and 8.0% unknown in the enzalutamide group (P = .50).
The groups had similar rates of the most common comorbidities: diabetes (40.5% vs 46.3%, respectively, P = .07), peripheral vascular disease (40.2% vs 37.6%, respectively, P = .44), and chronic pulmonary disease (37.0% vs 40.5%, P = .29).
In an inverse probability weighting analysis with abiraterone as reference, weighted median overall survival was comparable across the entire cohort (36.2 months, P = .32), Black veterans (39.7 months, P = .90), and those with cardiovascular disease (31.5 months, P = .30).
The authors noted limitations such as the observational cohort design and data constraints.
The study was supported by the American Society of Clinical Oncology Conquer Cancer Foundation, the Prostate Cancer Foundation, and the Blavatnik Family Foundation.
Schoen discloses relationships with the Prostate Cancer Foundation, Astellas, and US Department of Defense. Other authors disclose relationships with the American Society of Clinical Oncology, Pfizer, Exelixis, Eli Lilly, Sanofi, Merck, Seagen, Bellicum, and BMS.
Outside the submitted work. Reizine discloses relationships with the US Department of Defense, Sanofi, Exelexis, Janssen, AstraZeneca, EMD Serono, Janssen, Merck, and Tempus.
Abiraterone and enzalutamide showed identical survival outcomes when used as first-line treatment for metastatic hormone-sensitive prostate cancer (mHSPC), according to a new study using US Department of Veterans Affairs (VA) data. The report represents the first head-to-head clinical analysis of these commonly used androgen receptor inhibitors.
Among 1258 veterans treated with abiraterone and 311 treated with enzalutamide, median overall survival was 36.2 months for both drugs. Patients were followed for a mean of 28.7 months (abiraterone) and 30.8 months (enzalutamide), reported by Martin W. Schoen, MD, MPH, from Saint Louis University School of Medicine and the St. Louis VA Medical Center, in JAMA Network Open.
Notably, there was no significant difference in outcomes among Black veterans, who often have poorer outcomes in prostate cancer, and in patients with cardiovascular disease.
“This is the first direct comparison of abiraterone and enzalutamide for mHSPC in a clinical practice setting,” Schoen told Federal Practitioner. “At the population level, there are no differences based on initial treatment choice.”
Abiraterone Is Preferred in the VA Due to Cost
According to Schoen, abiraterone and enzalutamide are the most commonly used androgen receptor inhibitors to treat mHSPC within the VA. A 2025 study by Schoen and colleagues found that 53.7% of veterans with mHSPC in 2022 received androgen receptor inhibitor therapy, up from 16.9% in 2017.
“In the VA, the preference for most patients is abiraterone since it is the least expensive agent,” he said. A generic version has been available for several years.
Additionally, abiraterone “has been on the market for the longest, and therefore clinicians are familiar with its use,” Schoen said. However, “clinicians have little idea of the comparative efficacy between these 2 agents,” he added.
The authors suggest that the cost and toxicities of the medications should guide clinician decisions, Schoen said. “There is data that abiraterone may worsen diabetes, since it is given with prednisone and could increase the risk of cardiovascular events,” he said.
He added that 2 newer drugs, apalutamide and darolutamide, are also “viable options.” Chemotherapies and certain targeted drugs are also available, “but they are only used in a select group of patients.”
Outside Specialist: Diverse Study Population Is a Plus
Hematologist-oncologist Natalie Reizine, MD, of the University of Illinois College of Medicine, Chicago, who was not involved in the study, told Federal Practitioner that the real-world data are valuable given the limitations of clinical trial populations.
“It’s difficult to compare clinical trials because they enroll different groups of patients,” she said. And, she said, they often exclude patients with significant comorbidities. “If they have bad cardiovascular disease, for instance, or poorly controlled diabetes, they're excluded from the clinical trial. But in real life, many of our patients have other medical problems that we have to manage.”
Reizine also emphasized the significance of the study’s diverse patient population. “Black men are very underrepresented in clinical trials. Many clinical trials that lead to drug approval will have only few or no Black men at all, yet these drugs go on to be widely prescribed to all men with prostate cancer.”
Results Are ‘Reassuring’
Reizine described the overall study findings as “reassuring,” especially in light of “studies that show that abiraterone and prednisone may be associated with worse cardiovascular outcomes. This study showed that in this VA population, even for patients who had cardiovascular disease, there was not a difference in how they did.”
As for choosing between agents, she recommended considering comorbidities and potential drug-drug interactions. “One of the big reasons that you may not be able to safely prescribe enzalutamide, for instance, is if a patient is on an anticoagulant, which is incredibly common in cancer patients. Enzalutamide has more drug-drug interactions than abiraterone and prednisone.”
Study Demographics and Findings
The study included all patients with mHSPC who initiated abiraterone or enzalutamide between July 2017 and April 2023.
Median ages were 73 (abiraterone) and 74 years (enzalutamide, P = .29). Racial distribution was similar between groups: abiraterone (68.1% White, 25.0% Black, 6.9% other/unknown) and enzalutamide (66.6% White, 27.0% Black, 6.4% other/unknown; P = .74). Ethnicity was 89.2% non-Hispanic, 4.4% Hispanic, and 6.4% unknown in the abiraterone group vs 88.4% non-Hispanic, 3.5% Hispanic, and 8.0% unknown in the enzalutamide group (P = .50).
The groups had similar rates of the most common comorbidities: diabetes (40.5% vs 46.3%, respectively, P = .07), peripheral vascular disease (40.2% vs 37.6%, respectively, P = .44), and chronic pulmonary disease (37.0% vs 40.5%, P = .29).
In an inverse probability weighting analysis with abiraterone as reference, weighted median overall survival was comparable across the entire cohort (36.2 months, P = .32), Black veterans (39.7 months, P = .90), and those with cardiovascular disease (31.5 months, P = .30).
The authors noted limitations such as the observational cohort design and data constraints.
The study was supported by the American Society of Clinical Oncology Conquer Cancer Foundation, the Prostate Cancer Foundation, and the Blavatnik Family Foundation.
Schoen discloses relationships with the Prostate Cancer Foundation, Astellas, and US Department of Defense. Other authors disclose relationships with the American Society of Clinical Oncology, Pfizer, Exelixis, Eli Lilly, Sanofi, Merck, Seagen, Bellicum, and BMS.
Outside the submitted work. Reizine discloses relationships with the US Department of Defense, Sanofi, Exelexis, Janssen, AstraZeneca, EMD Serono, Janssen, Merck, and Tempus.
NICE Endorses Oral Alternative to Chemo in Prostate Cancer
A faster, oral alternative to docetaxel is set to reach NHS clinics after the National Institute for Health and Care Excellence (NICE) recommended darolutamide (Nubeqa, Bayer) in combination with androgen deprivation therapy (ADT) for men with metastatic hormone-sensitive prostate cancer who are unable to receive or tolerate chemotherapy.
Detailed in NICE’s final draft guidance, the decision will make darolutamide available through the NHS in England and Wales to approximately 6000 patients, offering a new oral therapy for those who with limited alternatives to docetaxel or other androgen-receptor inhibitors.
New Option for Chemo-Ineligible Patients
Darolutamide functions by blocking hormones that fuel cancer growth, specifically depriving prostate cancer cells of testosterone required for multiplication and spread. Patients take two tablets twice daily alongside standard ADT.
Peter Johnson, national clinical director for cancer at NHS England, welcomed the decision and expects this approval to give clinicians and their patients “more flexibility to choose the approach best suited to individual circumstances and clinical needs.”
The guidance was finalised 5 weeks ahead of the standard review timeline, underscoring NICE’s commitment to accelerating access to effective prostate cancer treatments.
Clinical Trial Evidence
The NICE’s decision was supported by evidence from the phase 3 ARASENS trial (N = 1306).
The results showed that adding darolutamide to ADT and docetaxel significantly improved overall survival in metastatic hormone-sensitive prostate cancer, reducing the risk for death by 32% compared with ADT and docetaxel alone. Progression-free outcomes, measured by time to castration-resistant disease or death, also favoured darolutamide.
A NICE network meta-analysis of the TITAN, ARCHES, LATITUDE, and STAMPEDE trials suggested that combining ADT with androgen-receptor pathway inhibitors such as apalutamide, enzalutamide, and abiraterone provides comparable survival benefits in this disease setting.
Cost and Implementation
NICE determined that darolutamide plus ADT delivers similar or lower overall costs to the NHS compared with apalutamide plus ADT. The list price is £4040.00 for a 28-day supply (112 × 300-mg tablets), though Bayer has agreed to a confidential commercial discount.
The guidance requires healthcare providers to use the least expensive suitable treatment option, considering administration costs, dosages, price per dose, and commercial arrangements when choosing between darolutamide plus ADT and apalutamide plus ADT.
NHS England and integrated care boards must provide funding within 30 days of final publication, with routine commissioning beginning after this interim period.
A version of this article first appeared on Medscape.com.
A faster, oral alternative to docetaxel is set to reach NHS clinics after the National Institute for Health and Care Excellence (NICE) recommended darolutamide (Nubeqa, Bayer) in combination with androgen deprivation therapy (ADT) for men with metastatic hormone-sensitive prostate cancer who are unable to receive or tolerate chemotherapy.
Detailed in NICE’s final draft guidance, the decision will make darolutamide available through the NHS in England and Wales to approximately 6000 patients, offering a new oral therapy for those who with limited alternatives to docetaxel or other androgen-receptor inhibitors.
New Option for Chemo-Ineligible Patients
Darolutamide functions by blocking hormones that fuel cancer growth, specifically depriving prostate cancer cells of testosterone required for multiplication and spread. Patients take two tablets twice daily alongside standard ADT.
Peter Johnson, national clinical director for cancer at NHS England, welcomed the decision and expects this approval to give clinicians and their patients “more flexibility to choose the approach best suited to individual circumstances and clinical needs.”
The guidance was finalised 5 weeks ahead of the standard review timeline, underscoring NICE’s commitment to accelerating access to effective prostate cancer treatments.
Clinical Trial Evidence
The NICE’s decision was supported by evidence from the phase 3 ARASENS trial (N = 1306).
The results showed that adding darolutamide to ADT and docetaxel significantly improved overall survival in metastatic hormone-sensitive prostate cancer, reducing the risk for death by 32% compared with ADT and docetaxel alone. Progression-free outcomes, measured by time to castration-resistant disease or death, also favoured darolutamide.
A NICE network meta-analysis of the TITAN, ARCHES, LATITUDE, and STAMPEDE trials suggested that combining ADT with androgen-receptor pathway inhibitors such as apalutamide, enzalutamide, and abiraterone provides comparable survival benefits in this disease setting.
Cost and Implementation
NICE determined that darolutamide plus ADT delivers similar or lower overall costs to the NHS compared with apalutamide plus ADT. The list price is £4040.00 for a 28-day supply (112 × 300-mg tablets), though Bayer has agreed to a confidential commercial discount.
The guidance requires healthcare providers to use the least expensive suitable treatment option, considering administration costs, dosages, price per dose, and commercial arrangements when choosing between darolutamide plus ADT and apalutamide plus ADT.
NHS England and integrated care boards must provide funding within 30 days of final publication, with routine commissioning beginning after this interim period.
A version of this article first appeared on Medscape.com.
A faster, oral alternative to docetaxel is set to reach NHS clinics after the National Institute for Health and Care Excellence (NICE) recommended darolutamide (Nubeqa, Bayer) in combination with androgen deprivation therapy (ADT) for men with metastatic hormone-sensitive prostate cancer who are unable to receive or tolerate chemotherapy.
Detailed in NICE’s final draft guidance, the decision will make darolutamide available through the NHS in England and Wales to approximately 6000 patients, offering a new oral therapy for those who with limited alternatives to docetaxel or other androgen-receptor inhibitors.
New Option for Chemo-Ineligible Patients
Darolutamide functions by blocking hormones that fuel cancer growth, specifically depriving prostate cancer cells of testosterone required for multiplication and spread. Patients take two tablets twice daily alongside standard ADT.
Peter Johnson, national clinical director for cancer at NHS England, welcomed the decision and expects this approval to give clinicians and their patients “more flexibility to choose the approach best suited to individual circumstances and clinical needs.”
The guidance was finalised 5 weeks ahead of the standard review timeline, underscoring NICE’s commitment to accelerating access to effective prostate cancer treatments.
Clinical Trial Evidence
The NICE’s decision was supported by evidence from the phase 3 ARASENS trial (N = 1306).
The results showed that adding darolutamide to ADT and docetaxel significantly improved overall survival in metastatic hormone-sensitive prostate cancer, reducing the risk for death by 32% compared with ADT and docetaxel alone. Progression-free outcomes, measured by time to castration-resistant disease or death, also favoured darolutamide.
A NICE network meta-analysis of the TITAN, ARCHES, LATITUDE, and STAMPEDE trials suggested that combining ADT with androgen-receptor pathway inhibitors such as apalutamide, enzalutamide, and abiraterone provides comparable survival benefits in this disease setting.
Cost and Implementation
NICE determined that darolutamide plus ADT delivers similar or lower overall costs to the NHS compared with apalutamide plus ADT. The list price is £4040.00 for a 28-day supply (112 × 300-mg tablets), though Bayer has agreed to a confidential commercial discount.
The guidance requires healthcare providers to use the least expensive suitable treatment option, considering administration costs, dosages, price per dose, and commercial arrangements when choosing between darolutamide plus ADT and apalutamide plus ADT.
NHS England and integrated care boards must provide funding within 30 days of final publication, with routine commissioning beginning after this interim period.
A version of this article first appeared on Medscape.com.
Findings from (ImPaCT): Improving Patients With Prostate Cancer’s Access to Germline Testing
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Enhancing Molecular Testing Documentation in Prostate Cancer
Background
Prostate cancer is the most common non-cutaneous malignancy at the Veterans Health Administration (VHA) and every year approximately 15,000 Veterans are diagnosed and treated. Many advanced prostate cancer cases harbor genetic mutations that significantly impact prognosis, treatment decisions, and familial screening. In February 2021, the Prostate Cancer Molecular Testing Pathway (PCMTP) flow map was developed to increase appropriate genetic testing.
Methods
VHA initiated the Oncology Clinical Pathways (OCP) program to standardize cancer care for Veterans. The PCMTP was developed by a multidisciplinary team that created interactive templates within the Computerized Patient Record System (CPRS), to facilitate identification of eligible Veterans for germline and comprehensive genomic profiling (CGP). Clinical decision-making for these tests is documented as Health Factors (HF), in CPRS, allowing for assessment of pathway adherence and overall uptake.
Results
The PCMTP has achieved success, as there is over 90% compliance to molecular testing among participating Veterans which exceeds the pathway benchmark of 80%. PCMTP has been utilized at 88 VA sites, by over 700 distinct VA providers, with over 7,000 Veterans participating. This implementation has yielded over 19,200 Health Factors within CPRS.
Conclusions
The PCMTP has markedly improved the documentation and application of germline and CGP testing among Veterans diagnosed with prostate cancer. By facilitating genomic testing in appropriate patients, the PCMTP aims to enhance patient outcomes and optimize the quality of care. Prior to PCMTP establishment, assessing the prevalence of germline and CGP testing in eligible Veterans posed significant challenges. Future work will concentrate on increasing PCMTP utilization, evaluating downstream outcomes from genomic testing, including the identification of pathogenic variants, utilization of genetic counseling services, referrals to clinical trials, and the genomic impact on treatment strategies.
Background
Prostate cancer is the most common non-cutaneous malignancy at the Veterans Health Administration (VHA) and every year approximately 15,000 Veterans are diagnosed and treated. Many advanced prostate cancer cases harbor genetic mutations that significantly impact prognosis, treatment decisions, and familial screening. In February 2021, the Prostate Cancer Molecular Testing Pathway (PCMTP) flow map was developed to increase appropriate genetic testing.
Methods
VHA initiated the Oncology Clinical Pathways (OCP) program to standardize cancer care for Veterans. The PCMTP was developed by a multidisciplinary team that created interactive templates within the Computerized Patient Record System (CPRS), to facilitate identification of eligible Veterans for germline and comprehensive genomic profiling (CGP). Clinical decision-making for these tests is documented as Health Factors (HF), in CPRS, allowing for assessment of pathway adherence and overall uptake.
Results
The PCMTP has achieved success, as there is over 90% compliance to molecular testing among participating Veterans which exceeds the pathway benchmark of 80%. PCMTP has been utilized at 88 VA sites, by over 700 distinct VA providers, with over 7,000 Veterans participating. This implementation has yielded over 19,200 Health Factors within CPRS.
Conclusions
The PCMTP has markedly improved the documentation and application of germline and CGP testing among Veterans diagnosed with prostate cancer. By facilitating genomic testing in appropriate patients, the PCMTP aims to enhance patient outcomes and optimize the quality of care. Prior to PCMTP establishment, assessing the prevalence of germline and CGP testing in eligible Veterans posed significant challenges. Future work will concentrate on increasing PCMTP utilization, evaluating downstream outcomes from genomic testing, including the identification of pathogenic variants, utilization of genetic counseling services, referrals to clinical trials, and the genomic impact on treatment strategies.
Background
Prostate cancer is the most common non-cutaneous malignancy at the Veterans Health Administration (VHA) and every year approximately 15,000 Veterans are diagnosed and treated. Many advanced prostate cancer cases harbor genetic mutations that significantly impact prognosis, treatment decisions, and familial screening. In February 2021, the Prostate Cancer Molecular Testing Pathway (PCMTP) flow map was developed to increase appropriate genetic testing.
Methods
VHA initiated the Oncology Clinical Pathways (OCP) program to standardize cancer care for Veterans. The PCMTP was developed by a multidisciplinary team that created interactive templates within the Computerized Patient Record System (CPRS), to facilitate identification of eligible Veterans for germline and comprehensive genomic profiling (CGP). Clinical decision-making for these tests is documented as Health Factors (HF), in CPRS, allowing for assessment of pathway adherence and overall uptake.
Results
The PCMTP has achieved success, as there is over 90% compliance to molecular testing among participating Veterans which exceeds the pathway benchmark of 80%. PCMTP has been utilized at 88 VA sites, by over 700 distinct VA providers, with over 7,000 Veterans participating. This implementation has yielded over 19,200 Health Factors within CPRS.
Conclusions
The PCMTP has markedly improved the documentation and application of germline and CGP testing among Veterans diagnosed with prostate cancer. By facilitating genomic testing in appropriate patients, the PCMTP aims to enhance patient outcomes and optimize the quality of care. Prior to PCMTP establishment, assessing the prevalence of germline and CGP testing in eligible Veterans posed significant challenges. Future work will concentrate on increasing PCMTP utilization, evaluating downstream outcomes from genomic testing, including the identification of pathogenic variants, utilization of genetic counseling services, referrals to clinical trials, and the genomic impact on treatment strategies.
The Urology Prostate Cancer Note, One Tool to Increase Prostate Cancer Clinical Pathway Utilization
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Methods
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator.
Results
The UPCN functions as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. Since the UPCN national deployment on 9/6/24, documentation of high risk prostate cancer pathway utilization has increased 75% from 226 unique Veterans prior to launch to 395 unique Veterans after launch.
Conclusions
This collaborative effort did improve pathway utilization and documentation however other tools will need to be developed to improve provider PCCP documentation.
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Methods
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator.
Results
The UPCN functions as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. Since the UPCN national deployment on 9/6/24, documentation of high risk prostate cancer pathway utilization has increased 75% from 226 unique Veterans prior to launch to 395 unique Veterans after launch.
Conclusions
This collaborative effort did improve pathway utilization and documentation however other tools will need to be developed to improve provider PCCP documentation.
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Methods
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator.
Results
The UPCN functions as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. Since the UPCN national deployment on 9/6/24, documentation of high risk prostate cancer pathway utilization has increased 75% from 226 unique Veterans prior to launch to 395 unique Veterans after launch.
Conclusions
This collaborative effort did improve pathway utilization and documentation however other tools will need to be developed to improve provider PCCP documentation.