User login
VIDEO: More aggressive treatment with docetaxel boosts survival in prostate cancer
CHICAGO – Adding docetaxel chemotherapy to standard management improved survival in men with high-risk, localized, hormone-sensitive prostate cancer, results from the phase III RTOG 0521 study show.
At 4 years, overall survival was 89% without docetaxel and 93% with the addition of docetaxel (Toxotere) and prednisone after completing radiotherapy and long-term hormonal suppression (Hazard ratio, 0.70; P = .04).
“For the first time, improvement in overall survival was observed with adjuvant chemotherapy for localized, high-risk, hormone-sensitive prostate cancer,” Dr. Howard Sandler said in a press conference in advance of the formal presentation of the late-breaking study at the annual meeting of the American Society of Clinical Oncology.
The implications of the study are clinically relevant and wide-ranging, particularly in patients with high-risk disease, press briefing moderator Dr. Don Dizon, commented.
Dr. Sandler, chair of radiation oncology at Cedars-Sinai Medical Center, Los Angeles, gives details of this practice-changing study in a video interview.
The study was funded by the National Institutes of Health. Dr. Sandler reported financial relationships with several firms including Sanofi Aventis, maker of docetaxel.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
CHICAGO – Adding docetaxel chemotherapy to standard management improved survival in men with high-risk, localized, hormone-sensitive prostate cancer, results from the phase III RTOG 0521 study show.
At 4 years, overall survival was 89% without docetaxel and 93% with the addition of docetaxel (Toxotere) and prednisone after completing radiotherapy and long-term hormonal suppression (Hazard ratio, 0.70; P = .04).
“For the first time, improvement in overall survival was observed with adjuvant chemotherapy for localized, high-risk, hormone-sensitive prostate cancer,” Dr. Howard Sandler said in a press conference in advance of the formal presentation of the late-breaking study at the annual meeting of the American Society of Clinical Oncology.
The implications of the study are clinically relevant and wide-ranging, particularly in patients with high-risk disease, press briefing moderator Dr. Don Dizon, commented.
Dr. Sandler, chair of radiation oncology at Cedars-Sinai Medical Center, Los Angeles, gives details of this practice-changing study in a video interview.
The study was funded by the National Institutes of Health. Dr. Sandler reported financial relationships with several firms including Sanofi Aventis, maker of docetaxel.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
CHICAGO – Adding docetaxel chemotherapy to standard management improved survival in men with high-risk, localized, hormone-sensitive prostate cancer, results from the phase III RTOG 0521 study show.
At 4 years, overall survival was 89% without docetaxel and 93% with the addition of docetaxel (Toxotere) and prednisone after completing radiotherapy and long-term hormonal suppression (Hazard ratio, 0.70; P = .04).
“For the first time, improvement in overall survival was observed with adjuvant chemotherapy for localized, high-risk, hormone-sensitive prostate cancer,” Dr. Howard Sandler said in a press conference in advance of the formal presentation of the late-breaking study at the annual meeting of the American Society of Clinical Oncology.
The implications of the study are clinically relevant and wide-ranging, particularly in patients with high-risk disease, press briefing moderator Dr. Don Dizon, commented.
Dr. Sandler, chair of radiation oncology at Cedars-Sinai Medical Center, Los Angeles, gives details of this practice-changing study in a video interview.
The study was funded by the National Institutes of Health. Dr. Sandler reported financial relationships with several firms including Sanofi Aventis, maker of docetaxel.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
AT THE ASCO ANNUAL MEETING 2015
VIDEO: Nivolumab puts brakes on advanced liver cancer
CHICAGO – Immune checkpoint inhibition with nivolumab every 2 weeks provides a better response than does standard treatment for advanced liver cancer, phase I/II results suggest.
The overall response rate was 19%, with 8 of the 42 evaluable patients experiencing at least 30% tumor shrinkage.
Moreover, responses to nivolumab (Opdivo) have been durable, with 62% of patients still alive at 12 months, Dr. Anthony El-Khoueiry reported in a press briefing in advance of the formal presentation of the study at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In contrast, just 2% of patients have at least 30% tumor shrinkage with sorafenib (Nexavar) and the average overall survival is about 10-11 months. Sorafenib, a multitargeted tyrosine kinase inhibitor, is currently the only Food and Drug Administration–approved systemic treatment for advanced liver disease, he noted.
Nivolumab, a programmed death-1 (PD-1) inhibitor, is approved for previously treated melanoma and gained a second indication in March for use in previously treated squamous non–small cell lung cancer.
“This is the first study to show antitumor activity of a PD-1 immune checkpoint inhibitor in patients with liver cancer,” said Dr. El-Khoueiry of the University of Southern California Norris Comprehensive Cancer Center, Los Angeles.The durability of those responses was particularly impressive, Dr. Peter Paul Yu, ASCO president, said in an interview.
“This is a small study, but the signal is unusually robust in comparison to what the standard of care would be, which is why this is so promising,” he added.
Press briefing moderator Dr. Lynn Schuchter, chief of hematology-oncology at University of Pennsylvania in Philadelphia, said in a statement, “The fact that this drug might stop advanced liver cancer in its tracks for months, even a year, is great news for patients. To understand the full impact of this approach, however, larger trials are needed.”
Hear more about the promising results from the late-breaking abstract in our interview with Dr. El-Khoueiry.
The study was funded by Bristol-Myers Squibb. Dr. El-Khoueiry reported financial relationships with Bristol-Myers Squibb, GlaxoSmithKline, Genentech/Roche, Amgen, Exelixis, AstraZeneca, and Astex Pharmaceuticals. Several coauthors also had ties to BMS including employment and/or stock ownership. Dr. Schuchter reported institutional research funding from BMS, Genentech, GSK, Merck, and Roche.
On Twitter @pwendl
CHICAGO – Immune checkpoint inhibition with nivolumab every 2 weeks provides a better response than does standard treatment for advanced liver cancer, phase I/II results suggest.
The overall response rate was 19%, with 8 of the 42 evaluable patients experiencing at least 30% tumor shrinkage.
Moreover, responses to nivolumab (Opdivo) have been durable, with 62% of patients still alive at 12 months, Dr. Anthony El-Khoueiry reported in a press briefing in advance of the formal presentation of the study at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In contrast, just 2% of patients have at least 30% tumor shrinkage with sorafenib (Nexavar) and the average overall survival is about 10-11 months. Sorafenib, a multitargeted tyrosine kinase inhibitor, is currently the only Food and Drug Administration–approved systemic treatment for advanced liver disease, he noted.
Nivolumab, a programmed death-1 (PD-1) inhibitor, is approved for previously treated melanoma and gained a second indication in March for use in previously treated squamous non–small cell lung cancer.
“This is the first study to show antitumor activity of a PD-1 immune checkpoint inhibitor in patients with liver cancer,” said Dr. El-Khoueiry of the University of Southern California Norris Comprehensive Cancer Center, Los Angeles.The durability of those responses was particularly impressive, Dr. Peter Paul Yu, ASCO president, said in an interview.
“This is a small study, but the signal is unusually robust in comparison to what the standard of care would be, which is why this is so promising,” he added.
Press briefing moderator Dr. Lynn Schuchter, chief of hematology-oncology at University of Pennsylvania in Philadelphia, said in a statement, “The fact that this drug might stop advanced liver cancer in its tracks for months, even a year, is great news for patients. To understand the full impact of this approach, however, larger trials are needed.”
Hear more about the promising results from the late-breaking abstract in our interview with Dr. El-Khoueiry.
The study was funded by Bristol-Myers Squibb. Dr. El-Khoueiry reported financial relationships with Bristol-Myers Squibb, GlaxoSmithKline, Genentech/Roche, Amgen, Exelixis, AstraZeneca, and Astex Pharmaceuticals. Several coauthors also had ties to BMS including employment and/or stock ownership. Dr. Schuchter reported institutional research funding from BMS, Genentech, GSK, Merck, and Roche.
On Twitter @pwendl
CHICAGO – Immune checkpoint inhibition with nivolumab every 2 weeks provides a better response than does standard treatment for advanced liver cancer, phase I/II results suggest.
The overall response rate was 19%, with 8 of the 42 evaluable patients experiencing at least 30% tumor shrinkage.
Moreover, responses to nivolumab (Opdivo) have been durable, with 62% of patients still alive at 12 months, Dr. Anthony El-Khoueiry reported in a press briefing in advance of the formal presentation of the study at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In contrast, just 2% of patients have at least 30% tumor shrinkage with sorafenib (Nexavar) and the average overall survival is about 10-11 months. Sorafenib, a multitargeted tyrosine kinase inhibitor, is currently the only Food and Drug Administration–approved systemic treatment for advanced liver disease, he noted.
Nivolumab, a programmed death-1 (PD-1) inhibitor, is approved for previously treated melanoma and gained a second indication in March for use in previously treated squamous non–small cell lung cancer.
“This is the first study to show antitumor activity of a PD-1 immune checkpoint inhibitor in patients with liver cancer,” said Dr. El-Khoueiry of the University of Southern California Norris Comprehensive Cancer Center, Los Angeles.The durability of those responses was particularly impressive, Dr. Peter Paul Yu, ASCO president, said in an interview.
“This is a small study, but the signal is unusually robust in comparison to what the standard of care would be, which is why this is so promising,” he added.
Press briefing moderator Dr. Lynn Schuchter, chief of hematology-oncology at University of Pennsylvania in Philadelphia, said in a statement, “The fact that this drug might stop advanced liver cancer in its tracks for months, even a year, is great news for patients. To understand the full impact of this approach, however, larger trials are needed.”
Hear more about the promising results from the late-breaking abstract in our interview with Dr. El-Khoueiry.
The study was funded by Bristol-Myers Squibb. Dr. El-Khoueiry reported financial relationships with Bristol-Myers Squibb, GlaxoSmithKline, Genentech/Roche, Amgen, Exelixis, AstraZeneca, and Astex Pharmaceuticals. Several coauthors also had ties to BMS including employment and/or stock ownership. Dr. Schuchter reported institutional research funding from BMS, Genentech, GSK, Merck, and Roche.
On Twitter @pwendl
AT THE ASCO ANNUAL MEETING 2015
VIDEO: Genomic biomarker predicts pembrolizumab response
CHICAGO – Tumors that carry a genetic signature known as mismatch repair deficiency are highly responsive to immune checkpoint blockade with pembrolizumab, according to results from a small proof-of-concept study.
“This is the first study to use genetics in a prospective manner to guide immunotherapy,” study author Dr. Dung T. Le said in a press conference at the annual meeting of the American Society of Clinical Oncology.
The phase II study, also published online May 30 in the New England Journal of Medicine, enrolled three cohorts, all of which had previously treated metastatic cancer. Pembrolizumab (Keytruda) 10 mg/kg was given intravenously every two weeks.
An objective response was observed in 62% of 25 patients with mismatch repair-deficient colorectal cancer versus none of the 25 patients with mismatch repair-proficient colorectal cancer, she said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Disease control rates, which also included stable disease for at least 12 weeks, were 92% vs. 16%, respectively.
In a third cohort of 21 patients with a variety of mismatch repair-deficient cancers including pancreatic/bile duct, uterine, small bowel, stomach, and prostate, the response rate was 60% and disease control rate 70%.
Median overall survival has not been reached in the mismatch repair-deficient cohorts, with some patients continuing to respond to the immunotherapy drug for more than a year Dr. Le, with the Johns Hopkins Kimmel Cancer Center in Baltimore, said. Median overall survival was about 7.6 months in the mismatch repair-proficient colorectal cohort.
“These data suggest that genomics is more influential than histology for mismatch repair-deficient tumors when treated with anti-PD-1,” she said.
Defects in mismatch repair genes disable cells’ ability to repair errors in the DNA replication process. As a result, tumors deficient in mismatch repair proteins produce hundreds to thousands of mutations. Immunotherapy may work best in patients with more mutations in their tumors because multiple mutations trigger production of more abnormal proteins in cancer cells, and in turn prompt the immune system to mount a bigger response.
Indeed, higher somatic mutation loads were associated with better response and prolonged progression free-survival. On average, mismatch repair-deficient tumors had about 1,700 mutations vs. about 70 mutations in mismatch repair-proficient tumors (P = .007), Dr. Le said.
“Seventeen-hundred mutations: that’s like you put a red flag on the cancer cell and say to the immune system, ‘Here I am,’ ” press conference moderator Dr. Lynn Schuchter, chief of hematology-oncology at University of Pennsylvania in Philadelphia, remarked to reporters.
The study was funded by Swim Across America, The Commonwealth Fund, The Ludwig Center at Johns Hopkins, and the National Institutes of Health. Merck provided the study drug. Personal Genome Diagnostics performed the sequencing analysis. Dr. Le reported having no financial disclosures. Dr. Schuchter reported institutional research funding from Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche.
CHICAGO – Tumors that carry a genetic signature known as mismatch repair deficiency are highly responsive to immune checkpoint blockade with pembrolizumab, according to results from a small proof-of-concept study.
“This is the first study to use genetics in a prospective manner to guide immunotherapy,” study author Dr. Dung T. Le said in a press conference at the annual meeting of the American Society of Clinical Oncology.
The phase II study, also published online May 30 in the New England Journal of Medicine, enrolled three cohorts, all of which had previously treated metastatic cancer. Pembrolizumab (Keytruda) 10 mg/kg was given intravenously every two weeks.
An objective response was observed in 62% of 25 patients with mismatch repair-deficient colorectal cancer versus none of the 25 patients with mismatch repair-proficient colorectal cancer, she said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Disease control rates, which also included stable disease for at least 12 weeks, were 92% vs. 16%, respectively.
In a third cohort of 21 patients with a variety of mismatch repair-deficient cancers including pancreatic/bile duct, uterine, small bowel, stomach, and prostate, the response rate was 60% and disease control rate 70%.
Median overall survival has not been reached in the mismatch repair-deficient cohorts, with some patients continuing to respond to the immunotherapy drug for more than a year Dr. Le, with the Johns Hopkins Kimmel Cancer Center in Baltimore, said. Median overall survival was about 7.6 months in the mismatch repair-proficient colorectal cohort.
“These data suggest that genomics is more influential than histology for mismatch repair-deficient tumors when treated with anti-PD-1,” she said.
Defects in mismatch repair genes disable cells’ ability to repair errors in the DNA replication process. As a result, tumors deficient in mismatch repair proteins produce hundreds to thousands of mutations. Immunotherapy may work best in patients with more mutations in their tumors because multiple mutations trigger production of more abnormal proteins in cancer cells, and in turn prompt the immune system to mount a bigger response.
Indeed, higher somatic mutation loads were associated with better response and prolonged progression free-survival. On average, mismatch repair-deficient tumors had about 1,700 mutations vs. about 70 mutations in mismatch repair-proficient tumors (P = .007), Dr. Le said.
“Seventeen-hundred mutations: that’s like you put a red flag on the cancer cell and say to the immune system, ‘Here I am,’ ” press conference moderator Dr. Lynn Schuchter, chief of hematology-oncology at University of Pennsylvania in Philadelphia, remarked to reporters.
The study was funded by Swim Across America, The Commonwealth Fund, The Ludwig Center at Johns Hopkins, and the National Institutes of Health. Merck provided the study drug. Personal Genome Diagnostics performed the sequencing analysis. Dr. Le reported having no financial disclosures. Dr. Schuchter reported institutional research funding from Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche.
CHICAGO – Tumors that carry a genetic signature known as mismatch repair deficiency are highly responsive to immune checkpoint blockade with pembrolizumab, according to results from a small proof-of-concept study.
“This is the first study to use genetics in a prospective manner to guide immunotherapy,” study author Dr. Dung T. Le said in a press conference at the annual meeting of the American Society of Clinical Oncology.
The phase II study, also published online May 30 in the New England Journal of Medicine, enrolled three cohorts, all of which had previously treated metastatic cancer. Pembrolizumab (Keytruda) 10 mg/kg was given intravenously every two weeks.
An objective response was observed in 62% of 25 patients with mismatch repair-deficient colorectal cancer versus none of the 25 patients with mismatch repair-proficient colorectal cancer, she said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Disease control rates, which also included stable disease for at least 12 weeks, were 92% vs. 16%, respectively.
In a third cohort of 21 patients with a variety of mismatch repair-deficient cancers including pancreatic/bile duct, uterine, small bowel, stomach, and prostate, the response rate was 60% and disease control rate 70%.
Median overall survival has not been reached in the mismatch repair-deficient cohorts, with some patients continuing to respond to the immunotherapy drug for more than a year Dr. Le, with the Johns Hopkins Kimmel Cancer Center in Baltimore, said. Median overall survival was about 7.6 months in the mismatch repair-proficient colorectal cohort.
“These data suggest that genomics is more influential than histology for mismatch repair-deficient tumors when treated with anti-PD-1,” she said.
Defects in mismatch repair genes disable cells’ ability to repair errors in the DNA replication process. As a result, tumors deficient in mismatch repair proteins produce hundreds to thousands of mutations. Immunotherapy may work best in patients with more mutations in their tumors because multiple mutations trigger production of more abnormal proteins in cancer cells, and in turn prompt the immune system to mount a bigger response.
Indeed, higher somatic mutation loads were associated with better response and prolonged progression free-survival. On average, mismatch repair-deficient tumors had about 1,700 mutations vs. about 70 mutations in mismatch repair-proficient tumors (P = .007), Dr. Le said.
“Seventeen-hundred mutations: that’s like you put a red flag on the cancer cell and say to the immune system, ‘Here I am,’ ” press conference moderator Dr. Lynn Schuchter, chief of hematology-oncology at University of Pennsylvania in Philadelphia, remarked to reporters.
The study was funded by Swim Across America, The Commonwealth Fund, The Ludwig Center at Johns Hopkins, and the National Institutes of Health. Merck provided the study drug. Personal Genome Diagnostics performed the sequencing analysis. Dr. Le reported having no financial disclosures. Dr. Schuchter reported institutional research funding from Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche.
AT THE ASCO ANNUAL MEETING 2015
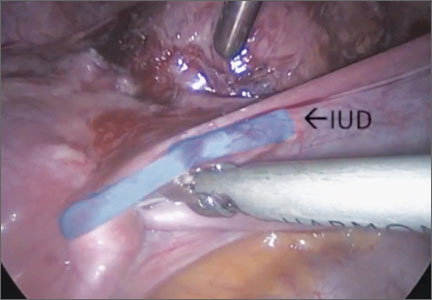
Surgical removal of malpositioned IUDs
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
VIDEO: Cannabis further compromises cognitive function in some MS patients
INDIANAPOLIS – Although an estimated 10%-20% of multiple sclerosis patients currently smoke cannabis for pain control or to alleviate symptoms, new research from Canada suggests that smoking the drug can worsen cognitive function in some patients with the disease.
“We already know that 40%-70% of people with MS already have cognitive problems,” Dr. Anthony Feinstein, professor of psychiatrist at the University of Toronto, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. “Some of the research that I’ve done shows that, in the presence of cannabis, these rates go up.”
Even so, he emphasized that patients should weigh such side effects against the benefits of use.
“I think it’s going to be an individual decision,” he said. “If it’s helping with pain, that’s good. Our data needs to be replicated by other groups.”
Dr. Feinstein disclosed that his work on MS and cannabis is funded by the Multiple Sclerosis Society of Canada.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
INDIANAPOLIS – Although an estimated 10%-20% of multiple sclerosis patients currently smoke cannabis for pain control or to alleviate symptoms, new research from Canada suggests that smoking the drug can worsen cognitive function in some patients with the disease.
“We already know that 40%-70% of people with MS already have cognitive problems,” Dr. Anthony Feinstein, professor of psychiatrist at the University of Toronto, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. “Some of the research that I’ve done shows that, in the presence of cannabis, these rates go up.”
Even so, he emphasized that patients should weigh such side effects against the benefits of use.
“I think it’s going to be an individual decision,” he said. “If it’s helping with pain, that’s good. Our data needs to be replicated by other groups.”
Dr. Feinstein disclosed that his work on MS and cannabis is funded by the Multiple Sclerosis Society of Canada.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
INDIANAPOLIS – Although an estimated 10%-20% of multiple sclerosis patients currently smoke cannabis for pain control or to alleviate symptoms, new research from Canada suggests that smoking the drug can worsen cognitive function in some patients with the disease.
“We already know that 40%-70% of people with MS already have cognitive problems,” Dr. Anthony Feinstein, professor of psychiatrist at the University of Toronto, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. “Some of the research that I’ve done shows that, in the presence of cannabis, these rates go up.”
Even so, he emphasized that patients should weigh such side effects against the benefits of use.
“I think it’s going to be an individual decision,” he said. “If it’s helping with pain, that’s good. Our data needs to be replicated by other groups.”
Dr. Feinstein disclosed that his work on MS and cannabis is funded by the Multiple Sclerosis Society of Canada.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
AT THE CMSC ANNUAL MEETING
Rx: Preventive care
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Riley Bove, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Gerard Gioia, PhD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Is it IBS? Blood test may offer conclusive answer
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2015
VIDEO: Value-based care will help improve mental health care delivery
TORONTO – The federal mental health parity law, combined with the new focus on value-based payment, will accelerate the revolution in mental health assessment and treatment in the United States and will reduce the financial risk physicians will be exposed to in their practices.
In this interview at the annual meeting of the American Psychiatric Association, former U.S. Rep. Patrick J. Kennedy (D-R.I.) – who, while in Congress, sponsored* the Mental Health Parity and Addiction Equity Act of 2008 – shared his thoughts on how mental health care is about to change.
*Correction, 5/19/2015: An earlier version of this story misstated former Rep. Kennedy's connection to the parity legislation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
TORONTO – The federal mental health parity law, combined with the new focus on value-based payment, will accelerate the revolution in mental health assessment and treatment in the United States and will reduce the financial risk physicians will be exposed to in their practices.
In this interview at the annual meeting of the American Psychiatric Association, former U.S. Rep. Patrick J. Kennedy (D-R.I.) – who, while in Congress, sponsored* the Mental Health Parity and Addiction Equity Act of 2008 – shared his thoughts on how mental health care is about to change.
*Correction, 5/19/2015: An earlier version of this story misstated former Rep. Kennedy's connection to the parity legislation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
TORONTO – The federal mental health parity law, combined with the new focus on value-based payment, will accelerate the revolution in mental health assessment and treatment in the United States and will reduce the financial risk physicians will be exposed to in their practices.
In this interview at the annual meeting of the American Psychiatric Association, former U.S. Rep. Patrick J. Kennedy (D-R.I.) – who, while in Congress, sponsored* the Mental Health Parity and Addiction Equity Act of 2008 – shared his thoughts on how mental health care is about to change.
*Correction, 5/19/2015: An earlier version of this story misstated former Rep. Kennedy's connection to the parity legislation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
AT THE APA ANNUAL MEETING