User login
VIDEO: Efficacy of DMTs decreases with age
San Diego – , and high-efficacy drugs do a better job of inhibiting MS disability compared with low-efficacy drugs only in patients younger than 40.5 years.
Those are the key conclusions from a meta-analysis of the age-dependent efficacy of MS treatments that was published in the November 2017 issue of Frontiers in Neurology. In a video interview, Ann Marie Weideman, lead study author, discussed highlights from the meta-analysis at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The meta-analysis drew from more than 28,000 individuals with MS participating in 38 trials of 13 categories of immunomodulatory drugs.
Ms. Weideman is an IRTA Fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md. She reported that study coauthor Bibiana Bielekova, MD, is coinventor of several patents related to daclizumab.
San Diego – , and high-efficacy drugs do a better job of inhibiting MS disability compared with low-efficacy drugs only in patients younger than 40.5 years.
Those are the key conclusions from a meta-analysis of the age-dependent efficacy of MS treatments that was published in the November 2017 issue of Frontiers in Neurology. In a video interview, Ann Marie Weideman, lead study author, discussed highlights from the meta-analysis at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The meta-analysis drew from more than 28,000 individuals with MS participating in 38 trials of 13 categories of immunomodulatory drugs.
Ms. Weideman is an IRTA Fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md. She reported that study coauthor Bibiana Bielekova, MD, is coinventor of several patents related to daclizumab.
San Diego – , and high-efficacy drugs do a better job of inhibiting MS disability compared with low-efficacy drugs only in patients younger than 40.5 years.
Those are the key conclusions from a meta-analysis of the age-dependent efficacy of MS treatments that was published in the November 2017 issue of Frontiers in Neurology. In a video interview, Ann Marie Weideman, lead study author, discussed highlights from the meta-analysis at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The meta-analysis drew from more than 28,000 individuals with MS participating in 38 trials of 13 categories of immunomodulatory drugs.
Ms. Weideman is an IRTA Fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md. She reported that study coauthor Bibiana Bielekova, MD, is coinventor of several patents related to daclizumab.
REPORTING FROM ACTRIMS FORUM 2018
VIDEO: New MS ambulatory measure could fill clinical gap
REPORTING FROM ACTRIMS FORUM 2018
SAN DIEGO – Although clinical tools to assess ambulatory function among people with multiple sclerosis exist, some measure it as part of a comprehensive assessment while others require the patient to answer many questions and then clinicians to calculate a score.
To devise a more targeted, simpler instrument, Emily Evans, MD, and her colleagues developed the PDAS or Patient Derived Ambulation Scale. They evaluated the correlation of this single-item scale to assess ambulation – an important measure of patient function – and evaluated how the results correlated with existing tools such as the Patient Determined Disease Steps and 12-item MS Walking Scale. Dr. Evans presented preliminary findings at the ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“We feel this is a quick test that can be readily implemented into clinical practice,” Dr. Evans, a neurologist at the John L. Trotter MS Center at Washington University in St. Louis, said in a video interview.
REPORTING FROM ACTRIMS FORUM 2018
SAN DIEGO – Although clinical tools to assess ambulatory function among people with multiple sclerosis exist, some measure it as part of a comprehensive assessment while others require the patient to answer many questions and then clinicians to calculate a score.
To devise a more targeted, simpler instrument, Emily Evans, MD, and her colleagues developed the PDAS or Patient Derived Ambulation Scale. They evaluated the correlation of this single-item scale to assess ambulation – an important measure of patient function – and evaluated how the results correlated with existing tools such as the Patient Determined Disease Steps and 12-item MS Walking Scale. Dr. Evans presented preliminary findings at the ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“We feel this is a quick test that can be readily implemented into clinical practice,” Dr. Evans, a neurologist at the John L. Trotter MS Center at Washington University in St. Louis, said in a video interview.
REPORTING FROM ACTRIMS FORUM 2018
SAN DIEGO – Although clinical tools to assess ambulatory function among people with multiple sclerosis exist, some measure it as part of a comprehensive assessment while others require the patient to answer many questions and then clinicians to calculate a score.
To devise a more targeted, simpler instrument, Emily Evans, MD, and her colleagues developed the PDAS or Patient Derived Ambulation Scale. They evaluated the correlation of this single-item scale to assess ambulation – an important measure of patient function – and evaluated how the results correlated with existing tools such as the Patient Determined Disease Steps and 12-item MS Walking Scale. Dr. Evans presented preliminary findings at the ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“We feel this is a quick test that can be readily implemented into clinical practice,” Dr. Evans, a neurologist at the John L. Trotter MS Center at Washington University in St. Louis, said in a video interview.
VIDEO: Alemtuzumab associated with long-term MS control in TOPAZ study
SAN DIEGO – A majority of patients with active relapsing-remitting multiple sclerosis and inadequate response to previous therapy achieved a durable response after treatment with alemtuzumab in the TOPAZ trial, a 5-year extension to the CARE-MS II study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Almost half of the 317 participants in TOPAZ received no further therapy beyond their initial two courses of alemtuzumab infusion therapy that they received as part of the CARE-MS II study.
“If you follow patients over time ... you’re seeing a significant group of patients who have improvement. It’s very unexpected, especially when you look at the patients who entered the clinical trial who had a fair amount of active disease,” said Barry A. Singer, MD, director of The MS Center for Innovations in Care at Missouri Baptist Medical Center in St. Louis.
At the 7-year evaluation of patients in TOPAZ, the annualized relapse rate was 0.14. In addition, 87% of patients remained relapse-free in year 7. Dr. Singer and his colleagues also reported that 73% of TOPAZ participants were stable or improved based on their Expanded Disability Status Scale (EDSS) scores.
“As we follow the data out and follow these patients out, we’re seeing how the clinical course for these patients is dramatically improving for the majority of patients,” Dr. Singer said in a video interview at ACTRIMS Forum 2018, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The TOPAZ study also revealed that 69% of patients were free of clinical disease worsening and 44% experienced clinical disease improvement in the 6 months before year 7. The majority also had no evidence of disease activity, Dr. Singer reported.
“One of the attributes that makes alemtuzumab so attractive as a clinician and for patients is you can go through a couple of series of medication [treatments] ... and really alter your disease course – that is the exciting thing,” he said.
The Food and Drug Administration approved alemtuzumab (Lemtrada) in November 2014 for the treatment of patients with relapsing forms of multiple sclerosis. Use of alemtuzumab is generally reserved for patients who have had an inadequate response to two or more previous drugs indicated for the treatment of multiple sclerosis.
In CARE-MS II, participants received two annual courses of alemtuzumab: intravenous infusion of 12 mg/day for 5 days at baseline and again for 3 days at 12 months. Additional treatment in TOPAZ for relapse or MRI evidence of disease was at the discretion of the investigator and could include alemtuzumab retreatment 12 mg/day on 3 consecutive days 12 months or more after a previous course, or another disease-modifying therapy at any time. Annual follow-up exams included an MRI scan.
A durable treatment effect was achieved by a majority of patients, even though 47% received no further treatment with alemtuzumab or another disease-modifying therapy after the initial two alemtuzumab courses.
The incidence of most adverse events, including infusion-associated reactions and infections, decreased over the course of the TOPAZ study and were lower than the incidence reported in the 2-year CARE-MS II trial. Of note, the incidence of thyroid-related adverse events peaked in the third year of the follow-up and continued to decline out to 7 years, Dr. Singer said. “We’re not seeing any new safety issues.”
Dr. Singer and his coinvestigators plan to continue the research, monitoring and scoring patients over time.
The TOPAZ trial was funded by Sanofi Genzyme, which markets alemtuzumab. Dr. Singer disclosed that he receives clinical research support and is a speaker for Sanofi Genzyme.
SOURCE: Singer B et al. ACTRIMS Forum 2018, abstract P026.
SAN DIEGO – A majority of patients with active relapsing-remitting multiple sclerosis and inadequate response to previous therapy achieved a durable response after treatment with alemtuzumab in the TOPAZ trial, a 5-year extension to the CARE-MS II study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Almost half of the 317 participants in TOPAZ received no further therapy beyond their initial two courses of alemtuzumab infusion therapy that they received as part of the CARE-MS II study.
“If you follow patients over time ... you’re seeing a significant group of patients who have improvement. It’s very unexpected, especially when you look at the patients who entered the clinical trial who had a fair amount of active disease,” said Barry A. Singer, MD, director of The MS Center for Innovations in Care at Missouri Baptist Medical Center in St. Louis.
At the 7-year evaluation of patients in TOPAZ, the annualized relapse rate was 0.14. In addition, 87% of patients remained relapse-free in year 7. Dr. Singer and his colleagues also reported that 73% of TOPAZ participants were stable or improved based on their Expanded Disability Status Scale (EDSS) scores.
“As we follow the data out and follow these patients out, we’re seeing how the clinical course for these patients is dramatically improving for the majority of patients,” Dr. Singer said in a video interview at ACTRIMS Forum 2018, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The TOPAZ study also revealed that 69% of patients were free of clinical disease worsening and 44% experienced clinical disease improvement in the 6 months before year 7. The majority also had no evidence of disease activity, Dr. Singer reported.
“One of the attributes that makes alemtuzumab so attractive as a clinician and for patients is you can go through a couple of series of medication [treatments] ... and really alter your disease course – that is the exciting thing,” he said.
The Food and Drug Administration approved alemtuzumab (Lemtrada) in November 2014 for the treatment of patients with relapsing forms of multiple sclerosis. Use of alemtuzumab is generally reserved for patients who have had an inadequate response to two or more previous drugs indicated for the treatment of multiple sclerosis.
In CARE-MS II, participants received two annual courses of alemtuzumab: intravenous infusion of 12 mg/day for 5 days at baseline and again for 3 days at 12 months. Additional treatment in TOPAZ for relapse or MRI evidence of disease was at the discretion of the investigator and could include alemtuzumab retreatment 12 mg/day on 3 consecutive days 12 months or more after a previous course, or another disease-modifying therapy at any time. Annual follow-up exams included an MRI scan.
A durable treatment effect was achieved by a majority of patients, even though 47% received no further treatment with alemtuzumab or another disease-modifying therapy after the initial two alemtuzumab courses.
The incidence of most adverse events, including infusion-associated reactions and infections, decreased over the course of the TOPAZ study and were lower than the incidence reported in the 2-year CARE-MS II trial. Of note, the incidence of thyroid-related adverse events peaked in the third year of the follow-up and continued to decline out to 7 years, Dr. Singer said. “We’re not seeing any new safety issues.”
Dr. Singer and his coinvestigators plan to continue the research, monitoring and scoring patients over time.
The TOPAZ trial was funded by Sanofi Genzyme, which markets alemtuzumab. Dr. Singer disclosed that he receives clinical research support and is a speaker for Sanofi Genzyme.
SOURCE: Singer B et al. ACTRIMS Forum 2018, abstract P026.
SAN DIEGO – A majority of patients with active relapsing-remitting multiple sclerosis and inadequate response to previous therapy achieved a durable response after treatment with alemtuzumab in the TOPAZ trial, a 5-year extension to the CARE-MS II study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Almost half of the 317 participants in TOPAZ received no further therapy beyond their initial two courses of alemtuzumab infusion therapy that they received as part of the CARE-MS II study.
“If you follow patients over time ... you’re seeing a significant group of patients who have improvement. It’s very unexpected, especially when you look at the patients who entered the clinical trial who had a fair amount of active disease,” said Barry A. Singer, MD, director of The MS Center for Innovations in Care at Missouri Baptist Medical Center in St. Louis.
At the 7-year evaluation of patients in TOPAZ, the annualized relapse rate was 0.14. In addition, 87% of patients remained relapse-free in year 7. Dr. Singer and his colleagues also reported that 73% of TOPAZ participants were stable or improved based on their Expanded Disability Status Scale (EDSS) scores.
“As we follow the data out and follow these patients out, we’re seeing how the clinical course for these patients is dramatically improving for the majority of patients,” Dr. Singer said in a video interview at ACTRIMS Forum 2018, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The TOPAZ study also revealed that 69% of patients were free of clinical disease worsening and 44% experienced clinical disease improvement in the 6 months before year 7. The majority also had no evidence of disease activity, Dr. Singer reported.
“One of the attributes that makes alemtuzumab so attractive as a clinician and for patients is you can go through a couple of series of medication [treatments] ... and really alter your disease course – that is the exciting thing,” he said.
The Food and Drug Administration approved alemtuzumab (Lemtrada) in November 2014 for the treatment of patients with relapsing forms of multiple sclerosis. Use of alemtuzumab is generally reserved for patients who have had an inadequate response to two or more previous drugs indicated for the treatment of multiple sclerosis.
In CARE-MS II, participants received two annual courses of alemtuzumab: intravenous infusion of 12 mg/day for 5 days at baseline and again for 3 days at 12 months. Additional treatment in TOPAZ for relapse or MRI evidence of disease was at the discretion of the investigator and could include alemtuzumab retreatment 12 mg/day on 3 consecutive days 12 months or more after a previous course, or another disease-modifying therapy at any time. Annual follow-up exams included an MRI scan.
A durable treatment effect was achieved by a majority of patients, even though 47% received no further treatment with alemtuzumab or another disease-modifying therapy after the initial two alemtuzumab courses.
The incidence of most adverse events, including infusion-associated reactions and infections, decreased over the course of the TOPAZ study and were lower than the incidence reported in the 2-year CARE-MS II trial. Of note, the incidence of thyroid-related adverse events peaked in the third year of the follow-up and continued to decline out to 7 years, Dr. Singer said. “We’re not seeing any new safety issues.”
Dr. Singer and his coinvestigators plan to continue the research, monitoring and scoring patients over time.
The TOPAZ trial was funded by Sanofi Genzyme, which markets alemtuzumab. Dr. Singer disclosed that he receives clinical research support and is a speaker for Sanofi Genzyme.
SOURCE: Singer B et al. ACTRIMS Forum 2018, abstract P026.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: The annualized relapse rate was 0.14 at year 7 among the 87% of participants who remained in the TOPAZ study.
Study details: A 5-year extension study of 317 participants from the initial CARE-MS II trial.
Disclosures: The TOPAZ trial was funded by Sanofi Genzyme, which markets alemtuzumab. Dr. Singer disclosed that he receives clinical research support and is a speaker for Sanofi Genzyme.
Source: Singer B et al. ACTRIMS Forum 2018, abstract P026.
VIDEO: Could targeting gut dysbiosis in MS prevent disease?
SAN DIEGO – Compelling findings in a genetically engineered mouse model of multiple sclerosis identify mechanisms of how adolescence and gut dysbiosis contribute to the risk of MS. In addition, disparities in gut microbiome species could explain why some people are at higher risk for developing multiple sclerosis, while others seem to enjoy a protective effect against development of this and other autoimmune diseases.
The hope is that these findings could pave the way for clinicians to potentially prevent development of multiple sclerosis in people at higher risk, perhaps through altering the gut flora and probiotic therapy, Suhayl Dhib-Jalbut, MD, said in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Dhib-Jalbut and his team discovered these findings using humanized transgenic mice – in other words, mice containing risk genes for triggering disease transferred from a patient with multiple sclerosis. The mice were more likely to develop MS-like disease at certain ages and in the presence of an altered gut microbiome or gut dysbiosis (Proc Natl Acad Sci U S A. 2017 Oct 31;114[44]:E9318-27).
Dr. Dhib-Jalbut is past president of ACTRIMS and is professor and chairman of the departments of neurology at Rutgers–Robert Wood Johnson Medical School, New Brunswick, N.J., and New Jersey Medical School, Newark. He has received research grants from Biogen and Teva, and is a consultant for Genzyme, Teva, Celgene, and, Mallinckrodt.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Compelling findings in a genetically engineered mouse model of multiple sclerosis identify mechanisms of how adolescence and gut dysbiosis contribute to the risk of MS. In addition, disparities in gut microbiome species could explain why some people are at higher risk for developing multiple sclerosis, while others seem to enjoy a protective effect against development of this and other autoimmune diseases.
The hope is that these findings could pave the way for clinicians to potentially prevent development of multiple sclerosis in people at higher risk, perhaps through altering the gut flora and probiotic therapy, Suhayl Dhib-Jalbut, MD, said in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Dhib-Jalbut and his team discovered these findings using humanized transgenic mice – in other words, mice containing risk genes for triggering disease transferred from a patient with multiple sclerosis. The mice were more likely to develop MS-like disease at certain ages and in the presence of an altered gut microbiome or gut dysbiosis (Proc Natl Acad Sci U S A. 2017 Oct 31;114[44]:E9318-27).
Dr. Dhib-Jalbut is past president of ACTRIMS and is professor and chairman of the departments of neurology at Rutgers–Robert Wood Johnson Medical School, New Brunswick, N.J., and New Jersey Medical School, Newark. He has received research grants from Biogen and Teva, and is a consultant for Genzyme, Teva, Celgene, and, Mallinckrodt.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Compelling findings in a genetically engineered mouse model of multiple sclerosis identify mechanisms of how adolescence and gut dysbiosis contribute to the risk of MS. In addition, disparities in gut microbiome species could explain why some people are at higher risk for developing multiple sclerosis, while others seem to enjoy a protective effect against development of this and other autoimmune diseases.
The hope is that these findings could pave the way for clinicians to potentially prevent development of multiple sclerosis in people at higher risk, perhaps through altering the gut flora and probiotic therapy, Suhayl Dhib-Jalbut, MD, said in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Dhib-Jalbut and his team discovered these findings using humanized transgenic mice – in other words, mice containing risk genes for triggering disease transferred from a patient with multiple sclerosis. The mice were more likely to develop MS-like disease at certain ages and in the presence of an altered gut microbiome or gut dysbiosis (Proc Natl Acad Sci U S A. 2017 Oct 31;114[44]:E9318-27).
Dr. Dhib-Jalbut is past president of ACTRIMS and is professor and chairman of the departments of neurology at Rutgers–Robert Wood Johnson Medical School, New Brunswick, N.J., and New Jersey Medical School, Newark. He has received research grants from Biogen and Teva, and is a consultant for Genzyme, Teva, Celgene, and, Mallinckrodt.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM ACTRIMS FORUM 2018
VIDEO: Cystic fibrosis patients need earlier, more frequent colorectal cancer screening
Adults with cystic fibrosis (CF) should undergo screening colonoscopy for colorectal cancer every 5 years beginning at age 40 years, unless they have had a solid organ transplant – in which case, screening should begin at age 30 years. For both groups, screening intervals should be shortened to 3 years if any adenomatous polyps are recovered.
The new screening recommendation is 1 of 10 set forth by the Cystic Fibrosis Foundation, in conjunction with the American Gastroenterological Association. The document reflects the significantly increased risk of colorectal cancer among adults with the chronic lung disorder, Denis Hadjiliadis, MD, and his colleagues wrote in the February issue of Gastroenterology. ; the risk approaches a 30-fold increase among CF patients who have undergone a lung transplant.
SOURCE: American Gastroenterological Association
In addition to making recommendations on screening intervals and protocols, the document asks clinicians to reframe their thinking of CF as a respiratory-only disease.
“Physicians should recognize that CF is a colon cancer syndrome,” wrote Dr. Hadjiliadis, director of the Adult Cystic Fibrosis Program at the University of Pennsylvania, Philadelphia, and his coauthors.
The increased colorectal cancer risk has become increasingly evident as CF patients live longer, Dr. Hadjiliadis and the panel wrote.
“The current median predicted survival is 41 years, and persons born in 2015 have an estimated average life expectancy of 45 years. The increasing longevity of adults with CF puts them at risk for other diseases, such as gastrointestinal cancer.”
In addition to the normal age-related risk, however, CF patients seem to have an elevated risk profile unique to the disease. The underlying causes have not been fully elucidated but may have to do with mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which are responsible for the excess thickened mucosal secretions that characterize CF. CFTR also is a tumor-suppressor gene in the intestinal tract of mice, and is important in gastrointestinal epithelial homeostasis. “Absence of CFTR is associated with dysregulation of the immune response, intestinal stem cells, and growth signaling regulators,” the authors noted.
In response to this observed increased risk of colorectal cancers among CF patients, the Cystic Fibrosis Foundation convened an 18-member task force to review the extant literature and compile colorectal cancer screening recommendations for CF patients who show no signs of such malignancies. The team reviewed 1,159 articles and based its findings on the 50 most relevant. The papers comprised observational studies, case-control studies, and case reports; there are no randomized clinical trials of screening for this population.
The American Gastroenterological Association reviewed and approved all of the recommendations:
- Screening decisions should be a collaborative process between the CF patient and clinician, taking into account comorbidities, safety, and quality of life. This should include a discussion of expected lifespan; patients with limited lifespan won’t benefit from screening for a slow-growing cancer. Patients should also consider that the colonoscopy prep for CF patients is somewhat more complex than for non-CF patients. “Given these complexities, the task force agreed that individuals with CF and their providers should … carefully assess the risks and benefits of CRC screening and its impact on the health and quality of life for the adult with CF.”
- The decision team should include an endoscopist. An endoscopist with CF training is preferred, but the panel noted these specialists are rare.
- Colonoscopy is the preferred method of screening for CF patients, since it can both detect and remove polyps. “This is one of the main reasons why colonoscopy is the screening procedure of choice for other high-risk groups,” the panel noted.
- There is insufficient evidence to recommend alternate screening methods in CF patients, including CT scanning, colonography, stool-based tests, or flexible sigmoidoscopy.
- In CF patients without signs of CRC, screening should commence at age 40 years and be repeated every 5 years as long as the results are negative.
- Any CF patient who has had adenomatous polyps on a screening colonoscopy should have a repeat colonoscopy within 3 years, unless clinical findings support more frequent screening.
- For any adult CF patient older than age 30 years who has undergone a solid organ transplant, screening colonoscopy should commence within 2 years of transplantation. “Although the absolute risk of CRC in individuals with CF is extremely low for patients younger than 30 years, the risk … greatly increases after lung transplantation,” to 25-30 times the age-adjusted baseline, the panel wrote. “Increased posttransplantation survival means that many transplant patients will enter older age groups where there is an increased risk of cancer.” Screening should be performed after recovery and within 2 years, unless there was a negative colonoscopy in the 5 years before transplant.
- Thereafter, patients who have had a solid organ transplant should undergo colonoscopy every 5 years, based on their life expectancy. “In cases where the expected survival time is limited (less than 10 years), screening should not be performed. For adults appropriately selected, lung transplantation usually increases survival probability. Therefore, a lung transplantation candidate with a short life expectancy is likely to become a screening candidate before and after transplantation at the appropriate ages described here, because the potential survival increases to approximately 10 years.”
- Colonoscopy should be repeated every 3 years on CF patients with transplants with a history of adenomatous polyps. This interval may be as short as 1 year for patients with high-risk, large, or multiple polyps.
- CF patients should undergo more intense bowel prep for colonoscopy, with three-four washes of a minimum of one liter of purgative per wash; the last wash should occur 4-6 hours before the procedure. Split-prep regimens (several smaller-volume washes) are better than a single larger-volume wash. The panel suggested a sample CF-specific regimen available from the Minnesota Cystic Fibrosis Center.
The new document reflects expert consensus on the currently available data, the panel said. As more data emerge, the recommendations might change.
“It is possible that different subpopulations will need more or less frequent schedules for rescreening and surveillance. Our recommendations are making an effort to balance the risk of missing advanced colorectal cancer and minimizing the burden and risk of too frequent examinations.”
None of the panel members had any financial disclosures.
SOURCE: Hadjiliadis D et al. Gastroenterology. 2017 Dec 28. doi. org/10.1053/j.gastro.2017.12.012
According to the Cystic Fibrosis Foundation Patient Registry, more than 30,000 people are living with cystic fibrosis (CF ) in the United States. More than half of the CF population is over 18 years of age! It is extremely important to talk to patients about preventative medicine which was not a topic of conversation CF healthcare providers were adding to their management plan in the past.
According to the Cystic Fibrosis Foundation Patient Registry, more than 30,000 people are living with cystic fibrosis (CF ) in the United States. More than half of the CF population is over 18 years of age! It is extremely important to talk to patients about preventative medicine which was not a topic of conversation CF healthcare providers were adding to their management plan in the past.
According to the Cystic Fibrosis Foundation Patient Registry, more than 30,000 people are living with cystic fibrosis (CF ) in the United States. More than half of the CF population is over 18 years of age! It is extremely important to talk to patients about preventative medicine which was not a topic of conversation CF healthcare providers were adding to their management plan in the past.
Adults with cystic fibrosis (CF) should undergo screening colonoscopy for colorectal cancer every 5 years beginning at age 40 years, unless they have had a solid organ transplant – in which case, screening should begin at age 30 years. For both groups, screening intervals should be shortened to 3 years if any adenomatous polyps are recovered.
The new screening recommendation is 1 of 10 set forth by the Cystic Fibrosis Foundation, in conjunction with the American Gastroenterological Association. The document reflects the significantly increased risk of colorectal cancer among adults with the chronic lung disorder, Denis Hadjiliadis, MD, and his colleagues wrote in the February issue of Gastroenterology. ; the risk approaches a 30-fold increase among CF patients who have undergone a lung transplant.
SOURCE: American Gastroenterological Association
In addition to making recommendations on screening intervals and protocols, the document asks clinicians to reframe their thinking of CF as a respiratory-only disease.
“Physicians should recognize that CF is a colon cancer syndrome,” wrote Dr. Hadjiliadis, director of the Adult Cystic Fibrosis Program at the University of Pennsylvania, Philadelphia, and his coauthors.
The increased colorectal cancer risk has become increasingly evident as CF patients live longer, Dr. Hadjiliadis and the panel wrote.
“The current median predicted survival is 41 years, and persons born in 2015 have an estimated average life expectancy of 45 years. The increasing longevity of adults with CF puts them at risk for other diseases, such as gastrointestinal cancer.”
In addition to the normal age-related risk, however, CF patients seem to have an elevated risk profile unique to the disease. The underlying causes have not been fully elucidated but may have to do with mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which are responsible for the excess thickened mucosal secretions that characterize CF. CFTR also is a tumor-suppressor gene in the intestinal tract of mice, and is important in gastrointestinal epithelial homeostasis. “Absence of CFTR is associated with dysregulation of the immune response, intestinal stem cells, and growth signaling regulators,” the authors noted.
In response to this observed increased risk of colorectal cancers among CF patients, the Cystic Fibrosis Foundation convened an 18-member task force to review the extant literature and compile colorectal cancer screening recommendations for CF patients who show no signs of such malignancies. The team reviewed 1,159 articles and based its findings on the 50 most relevant. The papers comprised observational studies, case-control studies, and case reports; there are no randomized clinical trials of screening for this population.
The American Gastroenterological Association reviewed and approved all of the recommendations:
- Screening decisions should be a collaborative process between the CF patient and clinician, taking into account comorbidities, safety, and quality of life. This should include a discussion of expected lifespan; patients with limited lifespan won’t benefit from screening for a slow-growing cancer. Patients should also consider that the colonoscopy prep for CF patients is somewhat more complex than for non-CF patients. “Given these complexities, the task force agreed that individuals with CF and their providers should … carefully assess the risks and benefits of CRC screening and its impact on the health and quality of life for the adult with CF.”
- The decision team should include an endoscopist. An endoscopist with CF training is preferred, but the panel noted these specialists are rare.
- Colonoscopy is the preferred method of screening for CF patients, since it can both detect and remove polyps. “This is one of the main reasons why colonoscopy is the screening procedure of choice for other high-risk groups,” the panel noted.
- There is insufficient evidence to recommend alternate screening methods in CF patients, including CT scanning, colonography, stool-based tests, or flexible sigmoidoscopy.
- In CF patients without signs of CRC, screening should commence at age 40 years and be repeated every 5 years as long as the results are negative.
- Any CF patient who has had adenomatous polyps on a screening colonoscopy should have a repeat colonoscopy within 3 years, unless clinical findings support more frequent screening.
- For any adult CF patient older than age 30 years who has undergone a solid organ transplant, screening colonoscopy should commence within 2 years of transplantation. “Although the absolute risk of CRC in individuals with CF is extremely low for patients younger than 30 years, the risk … greatly increases after lung transplantation,” to 25-30 times the age-adjusted baseline, the panel wrote. “Increased posttransplantation survival means that many transplant patients will enter older age groups where there is an increased risk of cancer.” Screening should be performed after recovery and within 2 years, unless there was a negative colonoscopy in the 5 years before transplant.
- Thereafter, patients who have had a solid organ transplant should undergo colonoscopy every 5 years, based on their life expectancy. “In cases where the expected survival time is limited (less than 10 years), screening should not be performed. For adults appropriately selected, lung transplantation usually increases survival probability. Therefore, a lung transplantation candidate with a short life expectancy is likely to become a screening candidate before and after transplantation at the appropriate ages described here, because the potential survival increases to approximately 10 years.”
- Colonoscopy should be repeated every 3 years on CF patients with transplants with a history of adenomatous polyps. This interval may be as short as 1 year for patients with high-risk, large, or multiple polyps.
- CF patients should undergo more intense bowel prep for colonoscopy, with three-four washes of a minimum of one liter of purgative per wash; the last wash should occur 4-6 hours before the procedure. Split-prep regimens (several smaller-volume washes) are better than a single larger-volume wash. The panel suggested a sample CF-specific regimen available from the Minnesota Cystic Fibrosis Center.
The new document reflects expert consensus on the currently available data, the panel said. As more data emerge, the recommendations might change.
“It is possible that different subpopulations will need more or less frequent schedules for rescreening and surveillance. Our recommendations are making an effort to balance the risk of missing advanced colorectal cancer and minimizing the burden and risk of too frequent examinations.”
None of the panel members had any financial disclosures.
SOURCE: Hadjiliadis D et al. Gastroenterology. 2017 Dec 28. doi. org/10.1053/j.gastro.2017.12.012
Adults with cystic fibrosis (CF) should undergo screening colonoscopy for colorectal cancer every 5 years beginning at age 40 years, unless they have had a solid organ transplant – in which case, screening should begin at age 30 years. For both groups, screening intervals should be shortened to 3 years if any adenomatous polyps are recovered.
The new screening recommendation is 1 of 10 set forth by the Cystic Fibrosis Foundation, in conjunction with the American Gastroenterological Association. The document reflects the significantly increased risk of colorectal cancer among adults with the chronic lung disorder, Denis Hadjiliadis, MD, and his colleagues wrote in the February issue of Gastroenterology. ; the risk approaches a 30-fold increase among CF patients who have undergone a lung transplant.
SOURCE: American Gastroenterological Association
In addition to making recommendations on screening intervals and protocols, the document asks clinicians to reframe their thinking of CF as a respiratory-only disease.
“Physicians should recognize that CF is a colon cancer syndrome,” wrote Dr. Hadjiliadis, director of the Adult Cystic Fibrosis Program at the University of Pennsylvania, Philadelphia, and his coauthors.
The increased colorectal cancer risk has become increasingly evident as CF patients live longer, Dr. Hadjiliadis and the panel wrote.
“The current median predicted survival is 41 years, and persons born in 2015 have an estimated average life expectancy of 45 years. The increasing longevity of adults with CF puts them at risk for other diseases, such as gastrointestinal cancer.”
In addition to the normal age-related risk, however, CF patients seem to have an elevated risk profile unique to the disease. The underlying causes have not been fully elucidated but may have to do with mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which are responsible for the excess thickened mucosal secretions that characterize CF. CFTR also is a tumor-suppressor gene in the intestinal tract of mice, and is important in gastrointestinal epithelial homeostasis. “Absence of CFTR is associated with dysregulation of the immune response, intestinal stem cells, and growth signaling regulators,” the authors noted.
In response to this observed increased risk of colorectal cancers among CF patients, the Cystic Fibrosis Foundation convened an 18-member task force to review the extant literature and compile colorectal cancer screening recommendations for CF patients who show no signs of such malignancies. The team reviewed 1,159 articles and based its findings on the 50 most relevant. The papers comprised observational studies, case-control studies, and case reports; there are no randomized clinical trials of screening for this population.
The American Gastroenterological Association reviewed and approved all of the recommendations:
- Screening decisions should be a collaborative process between the CF patient and clinician, taking into account comorbidities, safety, and quality of life. This should include a discussion of expected lifespan; patients with limited lifespan won’t benefit from screening for a slow-growing cancer. Patients should also consider that the colonoscopy prep for CF patients is somewhat more complex than for non-CF patients. “Given these complexities, the task force agreed that individuals with CF and their providers should … carefully assess the risks and benefits of CRC screening and its impact on the health and quality of life for the adult with CF.”
- The decision team should include an endoscopist. An endoscopist with CF training is preferred, but the panel noted these specialists are rare.
- Colonoscopy is the preferred method of screening for CF patients, since it can both detect and remove polyps. “This is one of the main reasons why colonoscopy is the screening procedure of choice for other high-risk groups,” the panel noted.
- There is insufficient evidence to recommend alternate screening methods in CF patients, including CT scanning, colonography, stool-based tests, or flexible sigmoidoscopy.
- In CF patients without signs of CRC, screening should commence at age 40 years and be repeated every 5 years as long as the results are negative.
- Any CF patient who has had adenomatous polyps on a screening colonoscopy should have a repeat colonoscopy within 3 years, unless clinical findings support more frequent screening.
- For any adult CF patient older than age 30 years who has undergone a solid organ transplant, screening colonoscopy should commence within 2 years of transplantation. “Although the absolute risk of CRC in individuals with CF is extremely low for patients younger than 30 years, the risk … greatly increases after lung transplantation,” to 25-30 times the age-adjusted baseline, the panel wrote. “Increased posttransplantation survival means that many transplant patients will enter older age groups where there is an increased risk of cancer.” Screening should be performed after recovery and within 2 years, unless there was a negative colonoscopy in the 5 years before transplant.
- Thereafter, patients who have had a solid organ transplant should undergo colonoscopy every 5 years, based on their life expectancy. “In cases where the expected survival time is limited (less than 10 years), screening should not be performed. For adults appropriately selected, lung transplantation usually increases survival probability. Therefore, a lung transplantation candidate with a short life expectancy is likely to become a screening candidate before and after transplantation at the appropriate ages described here, because the potential survival increases to approximately 10 years.”
- Colonoscopy should be repeated every 3 years on CF patients with transplants with a history of adenomatous polyps. This interval may be as short as 1 year for patients with high-risk, large, or multiple polyps.
- CF patients should undergo more intense bowel prep for colonoscopy, with three-four washes of a minimum of one liter of purgative per wash; the last wash should occur 4-6 hours before the procedure. Split-prep regimens (several smaller-volume washes) are better than a single larger-volume wash. The panel suggested a sample CF-specific regimen available from the Minnesota Cystic Fibrosis Center.
The new document reflects expert consensus on the currently available data, the panel said. As more data emerge, the recommendations might change.
“It is possible that different subpopulations will need more or less frequent schedules for rescreening and surveillance. Our recommendations are making an effort to balance the risk of missing advanced colorectal cancer and minimizing the burden and risk of too frequent examinations.”
None of the panel members had any financial disclosures.
SOURCE: Hadjiliadis D et al. Gastroenterology. 2017 Dec 28. doi. org/10.1053/j.gastro.2017.12.012
FROM GASTROENTEROLOGY
VIDEO: Gluten-free diet tied to heavy metal bioaccumulation
A gluten-free diet was associated with significantly increased blood levels of mercury, lead, and cadmium and with significantly increased urinary levels of arsenic in a large cross-sectional population-based survey study.
Source: American Gastroenterological Association
After researchers controlled for demographic characteristics, “levels of all heavy metals remained significantly higher in persons following a gluten-free diet, compared with those not following a gluten-free diet,” Stephanie L. Raehsler, MPH, of Mayo Clinic in Rochester, Minn., wrote with her associates in an article published in the February issue of Clinical Gastroenterology and Hepatology.
The purported (unproven) benefits of a gluten-free diet (GFD) have propelled them into the mainstream outside the settings of celiac disease, dermatitis herpetiformis, and wheat allergy. However, GFDs have been linked to nutritional deficits of iron, ferritin, zinc, and fiber, to increased consumption of sugar, fats, and salt, and to excessive bioaccumulation of mercury, the investigators noted.
High intake of rice, a staple of many GFDs, also has been associated with elevated urinary excretion of arsenic (PLoS One. 2014 Sep 8;9[9]:e104768. doi: 10.1371/journal.pone.0104768). To further characterize these relationships, the researchers analyzed data for 2009 through 2012 from 11,354 participants in the National Health and Nutrition Examination Survey (NHANES). Blood levels of lead, mercury, and cadmium were available from 115 participants who reported following a GFD, and data on urinary arsenic levels were available from 32 such individuals.
In the overall study group, blood mercury levels averaged 1.37 mcg/L (95% confidence interval, 1.02-1.85 mcg/L) among persons on a GFD and 0.93 mcg/L (95% CI, 0.86-1.0 mcg/L) in persons not on a GFD (P = .008). Individuals on a GFD also had significantly higher total blood levels of lead (1.42 vs. 1.13 mcg/L; P = .007 ) and cadmium (0.42 vs. 0.34; P = .03), and they had significantly higher urinary levels of total arsenic (15.2 vs. 8.4 mcg/L; P = .003). These significant differences persisted after researchers controlled for age, sex, race, and smoking status.
Additionally, among 101 individuals on GFDs who had no laboratory or clinical indication of celiac disease, blood levels of total mercury were significantly elevated, compared with individuals not on a GFD (1.40 vs. 0.93 mcg/L; P = .02), as were blood lead concentrations (1.44 vs. 1.13 mcg/L; P = .01) and urinary arsenic levels (14.7 vs. 8.3 mcg/L; P = .01). Blood cadmium levels also were increased (0.42 vs. 0.34 mcg/L), but this difference did not reach statistical significance (P = .06).
Individuals who reported eating fish or shellfish in the past month had higher blood mercury levels than those who did not, regardless of whether they were on a GFD. However, only two individuals in the study exceeded the toxicity threshold for mercury and neither was on a GFD, the researchers said. For most individuals on a GFD, levels of all heavy metals except urinary arsenic stayed under the recognized limits for toxicity, they noted.
The number of respondents following a GFD was small, but the investigators followed NHANES recommendations on sampling weights and sample design variables. Also, although the NHANES included only one question on GFDs, trained interviewers were used to help minimize bias. “Studies are needed to determine the long-term effects of accumulation of these elements in persons on a GFD,” the researchers concluded.
The Centers for Disease Control and Prevention provided partial funding. The researchers reported having no conflicts of interest.
SOURCE: Raehsler S et al. Clin Gastro Hepatol. 2018;(in press).
A gluten-free diet was associated with significantly increased blood levels of mercury, lead, and cadmium and with significantly increased urinary levels of arsenic in a large cross-sectional population-based survey study.
Source: American Gastroenterological Association
After researchers controlled for demographic characteristics, “levels of all heavy metals remained significantly higher in persons following a gluten-free diet, compared with those not following a gluten-free diet,” Stephanie L. Raehsler, MPH, of Mayo Clinic in Rochester, Minn., wrote with her associates in an article published in the February issue of Clinical Gastroenterology and Hepatology.
The purported (unproven) benefits of a gluten-free diet (GFD) have propelled them into the mainstream outside the settings of celiac disease, dermatitis herpetiformis, and wheat allergy. However, GFDs have been linked to nutritional deficits of iron, ferritin, zinc, and fiber, to increased consumption of sugar, fats, and salt, and to excessive bioaccumulation of mercury, the investigators noted.
High intake of rice, a staple of many GFDs, also has been associated with elevated urinary excretion of arsenic (PLoS One. 2014 Sep 8;9[9]:e104768. doi: 10.1371/journal.pone.0104768). To further characterize these relationships, the researchers analyzed data for 2009 through 2012 from 11,354 participants in the National Health and Nutrition Examination Survey (NHANES). Blood levels of lead, mercury, and cadmium were available from 115 participants who reported following a GFD, and data on urinary arsenic levels were available from 32 such individuals.
In the overall study group, blood mercury levels averaged 1.37 mcg/L (95% confidence interval, 1.02-1.85 mcg/L) among persons on a GFD and 0.93 mcg/L (95% CI, 0.86-1.0 mcg/L) in persons not on a GFD (P = .008). Individuals on a GFD also had significantly higher total blood levels of lead (1.42 vs. 1.13 mcg/L; P = .007 ) and cadmium (0.42 vs. 0.34; P = .03), and they had significantly higher urinary levels of total arsenic (15.2 vs. 8.4 mcg/L; P = .003). These significant differences persisted after researchers controlled for age, sex, race, and smoking status.
Additionally, among 101 individuals on GFDs who had no laboratory or clinical indication of celiac disease, blood levels of total mercury were significantly elevated, compared with individuals not on a GFD (1.40 vs. 0.93 mcg/L; P = .02), as were blood lead concentrations (1.44 vs. 1.13 mcg/L; P = .01) and urinary arsenic levels (14.7 vs. 8.3 mcg/L; P = .01). Blood cadmium levels also were increased (0.42 vs. 0.34 mcg/L), but this difference did not reach statistical significance (P = .06).
Individuals who reported eating fish or shellfish in the past month had higher blood mercury levels than those who did not, regardless of whether they were on a GFD. However, only two individuals in the study exceeded the toxicity threshold for mercury and neither was on a GFD, the researchers said. For most individuals on a GFD, levels of all heavy metals except urinary arsenic stayed under the recognized limits for toxicity, they noted.
The number of respondents following a GFD was small, but the investigators followed NHANES recommendations on sampling weights and sample design variables. Also, although the NHANES included only one question on GFDs, trained interviewers were used to help minimize bias. “Studies are needed to determine the long-term effects of accumulation of these elements in persons on a GFD,” the researchers concluded.
The Centers for Disease Control and Prevention provided partial funding. The researchers reported having no conflicts of interest.
SOURCE: Raehsler S et al. Clin Gastro Hepatol. 2018;(in press).
A gluten-free diet was associated with significantly increased blood levels of mercury, lead, and cadmium and with significantly increased urinary levels of arsenic in a large cross-sectional population-based survey study.
Source: American Gastroenterological Association
After researchers controlled for demographic characteristics, “levels of all heavy metals remained significantly higher in persons following a gluten-free diet, compared with those not following a gluten-free diet,” Stephanie L. Raehsler, MPH, of Mayo Clinic in Rochester, Minn., wrote with her associates in an article published in the February issue of Clinical Gastroenterology and Hepatology.
The purported (unproven) benefits of a gluten-free diet (GFD) have propelled them into the mainstream outside the settings of celiac disease, dermatitis herpetiformis, and wheat allergy. However, GFDs have been linked to nutritional deficits of iron, ferritin, zinc, and fiber, to increased consumption of sugar, fats, and salt, and to excessive bioaccumulation of mercury, the investigators noted.
High intake of rice, a staple of many GFDs, also has been associated with elevated urinary excretion of arsenic (PLoS One. 2014 Sep 8;9[9]:e104768. doi: 10.1371/journal.pone.0104768). To further characterize these relationships, the researchers analyzed data for 2009 through 2012 from 11,354 participants in the National Health and Nutrition Examination Survey (NHANES). Blood levels of lead, mercury, and cadmium were available from 115 participants who reported following a GFD, and data on urinary arsenic levels were available from 32 such individuals.
In the overall study group, blood mercury levels averaged 1.37 mcg/L (95% confidence interval, 1.02-1.85 mcg/L) among persons on a GFD and 0.93 mcg/L (95% CI, 0.86-1.0 mcg/L) in persons not on a GFD (P = .008). Individuals on a GFD also had significantly higher total blood levels of lead (1.42 vs. 1.13 mcg/L; P = .007 ) and cadmium (0.42 vs. 0.34; P = .03), and they had significantly higher urinary levels of total arsenic (15.2 vs. 8.4 mcg/L; P = .003). These significant differences persisted after researchers controlled for age, sex, race, and smoking status.
Additionally, among 101 individuals on GFDs who had no laboratory or clinical indication of celiac disease, blood levels of total mercury were significantly elevated, compared with individuals not on a GFD (1.40 vs. 0.93 mcg/L; P = .02), as were blood lead concentrations (1.44 vs. 1.13 mcg/L; P = .01) and urinary arsenic levels (14.7 vs. 8.3 mcg/L; P = .01). Blood cadmium levels also were increased (0.42 vs. 0.34 mcg/L), but this difference did not reach statistical significance (P = .06).
Individuals who reported eating fish or shellfish in the past month had higher blood mercury levels than those who did not, regardless of whether they were on a GFD. However, only two individuals in the study exceeded the toxicity threshold for mercury and neither was on a GFD, the researchers said. For most individuals on a GFD, levels of all heavy metals except urinary arsenic stayed under the recognized limits for toxicity, they noted.
The number of respondents following a GFD was small, but the investigators followed NHANES recommendations on sampling weights and sample design variables. Also, although the NHANES included only one question on GFDs, trained interviewers were used to help minimize bias. “Studies are needed to determine the long-term effects of accumulation of these elements in persons on a GFD,” the researchers concluded.
The Centers for Disease Control and Prevention provided partial funding. The researchers reported having no conflicts of interest.
SOURCE: Raehsler S et al. Clin Gastro Hepatol. 2018;(in press).
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: A gluten-free diet was associated with significantly increased bioaccumulation of several heavy metals.
Major finding: After accounting for demographic factors, blood or urinary levels of lead, cadmium, arsenic, and mercury were significantly higher in persons following a gluten-free diet, compared with those who did not follow a gluten-free diet.
Data source: A population-based, cross-sectional study of 11,354 respondents to NHANES 2009-2012, including 115 persons on a gluten-free diet.
Disclosures: The Centers for Disease Control and Prevention provided partial funding. The researchers reported having no conflicts of interest.
Source: Raehsler S et al. Clin Gastro Hepatol. 2018 (in press).
Neurodegenerative aspects of psychiatric disorders
Complex regional pain syndrome: Steps that FPs can take
How to Investigate Thyroid Nodules Like A Pro
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
Chronic constipation: Practical approaches and novel therapies
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance
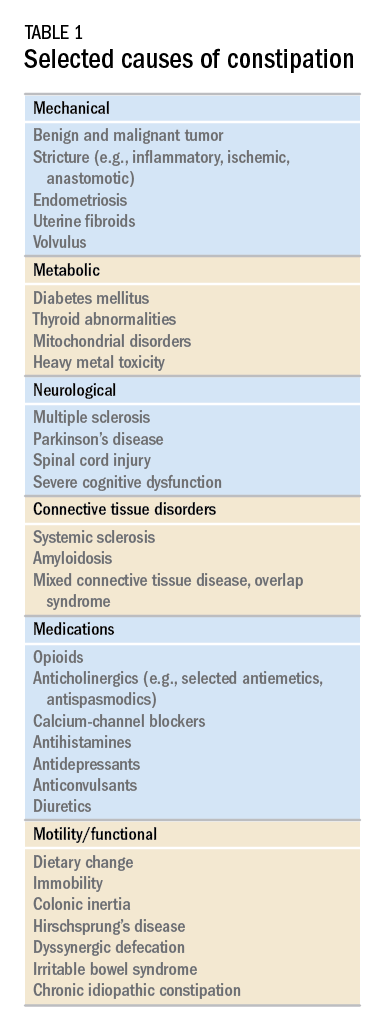
While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance

While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance

While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.