User login
Uveitis prevalence, risk eyeballed in spondyloarthritis patients
DENVER – Anterior uveitis occurred in 13% of military veterans with spondyloarthritis followed longitudinally in the PULSAR registry, Dr. Maureen Dubreuil reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
One factor proved to be independently associated with the risk of developing uveitis in a multivariate logistic regression analysis: being positive for HLA-B27. This conferred an 11.3-fold increased risk of developing the serious ophthalmologic complication, according to Dr. Dubreuil of Boston University.
PULSAR (the Program to Understand Long-Term Outcomes in Spondyloarthritis) is an eight-site longitudinal registry funded by the Department of Veterans Affairs since 2007.
Thirty-five of 268 men (13.1%) with spondyloarthritis (SpA) were diagnosed with anterior uveitis a mean of 10.3 years following diagnosis of SpA. The diagnosis of uveitis was made by a participating PULSAR rheumatologist and confirmed by an ophthalmologist.
Sixty-two percent of subjects with uveitis experienced a mean of 5.7 recurrences, while the remainder had encountered a solitary episode at the time of the study. Two-thirds of the time, uveitis episodes occurred concurrently with periods of SpA activity. The uveitis occurred in an alternating unilateral pattern in nearly half of cases.
One-quarter of subjects with uveitis experienced complications, including cataracts, glaucoma, and blindness in two patients.
Uveitis is recognized as one of the most common extra-articular manifestations of radiographic axial SpA; however, the picture in patients with non-axial SpA has been far less clear. In the PULSAR analysis, the prevalence of uveitis was 24% among the 95 subjects with axial SpA. In a univariate analysis, this translated to a 2.45-fold increased likelihood of uveitis in individuals with axial SpA, compared with patients with non-axial SpA. Nonetheless, in a multivariate logistic regression analysis, axial SpA wasn’t associated with a significantly increased risk of uveitis. Neither were demographic variables, smoking status, SpA duration, or inflammatory markers, Dr. Dubreuil said.
She reported having no financial conflicts regarding the study, which was funded by the Department of Veterans Affairs.
DENVER – Anterior uveitis occurred in 13% of military veterans with spondyloarthritis followed longitudinally in the PULSAR registry, Dr. Maureen Dubreuil reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
One factor proved to be independently associated with the risk of developing uveitis in a multivariate logistic regression analysis: being positive for HLA-B27. This conferred an 11.3-fold increased risk of developing the serious ophthalmologic complication, according to Dr. Dubreuil of Boston University.
PULSAR (the Program to Understand Long-Term Outcomes in Spondyloarthritis) is an eight-site longitudinal registry funded by the Department of Veterans Affairs since 2007.
Thirty-five of 268 men (13.1%) with spondyloarthritis (SpA) were diagnosed with anterior uveitis a mean of 10.3 years following diagnosis of SpA. The diagnosis of uveitis was made by a participating PULSAR rheumatologist and confirmed by an ophthalmologist.
Sixty-two percent of subjects with uveitis experienced a mean of 5.7 recurrences, while the remainder had encountered a solitary episode at the time of the study. Two-thirds of the time, uveitis episodes occurred concurrently with periods of SpA activity. The uveitis occurred in an alternating unilateral pattern in nearly half of cases.
One-quarter of subjects with uveitis experienced complications, including cataracts, glaucoma, and blindness in two patients.
Uveitis is recognized as one of the most common extra-articular manifestations of radiographic axial SpA; however, the picture in patients with non-axial SpA has been far less clear. In the PULSAR analysis, the prevalence of uveitis was 24% among the 95 subjects with axial SpA. In a univariate analysis, this translated to a 2.45-fold increased likelihood of uveitis in individuals with axial SpA, compared with patients with non-axial SpA. Nonetheless, in a multivariate logistic regression analysis, axial SpA wasn’t associated with a significantly increased risk of uveitis. Neither were demographic variables, smoking status, SpA duration, or inflammatory markers, Dr. Dubreuil said.
She reported having no financial conflicts regarding the study, which was funded by the Department of Veterans Affairs.
DENVER – Anterior uveitis occurred in 13% of military veterans with spondyloarthritis followed longitudinally in the PULSAR registry, Dr. Maureen Dubreuil reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
One factor proved to be independently associated with the risk of developing uveitis in a multivariate logistic regression analysis: being positive for HLA-B27. This conferred an 11.3-fold increased risk of developing the serious ophthalmologic complication, according to Dr. Dubreuil of Boston University.
PULSAR (the Program to Understand Long-Term Outcomes in Spondyloarthritis) is an eight-site longitudinal registry funded by the Department of Veterans Affairs since 2007.
Thirty-five of 268 men (13.1%) with spondyloarthritis (SpA) were diagnosed with anterior uveitis a mean of 10.3 years following diagnosis of SpA. The diagnosis of uveitis was made by a participating PULSAR rheumatologist and confirmed by an ophthalmologist.
Sixty-two percent of subjects with uveitis experienced a mean of 5.7 recurrences, while the remainder had encountered a solitary episode at the time of the study. Two-thirds of the time, uveitis episodes occurred concurrently with periods of SpA activity. The uveitis occurred in an alternating unilateral pattern in nearly half of cases.
One-quarter of subjects with uveitis experienced complications, including cataracts, glaucoma, and blindness in two patients.
Uveitis is recognized as one of the most common extra-articular manifestations of radiographic axial SpA; however, the picture in patients with non-axial SpA has been far less clear. In the PULSAR analysis, the prevalence of uveitis was 24% among the 95 subjects with axial SpA. In a univariate analysis, this translated to a 2.45-fold increased likelihood of uveitis in individuals with axial SpA, compared with patients with non-axial SpA. Nonetheless, in a multivariate logistic regression analysis, axial SpA wasn’t associated with a significantly increased risk of uveitis. Neither were demographic variables, smoking status, SpA duration, or inflammatory markers, Dr. Dubreuil said.
She reported having no financial conflicts regarding the study, which was funded by the Department of Veterans Affairs.
AT THE 2015 SPARTAN ANNUAL MEETING
Key clinical point: The risk of anterior uveitis in patients with spondyloarthritis was 11.3-fold greater among those who were HLA-B27-positive.
Major finding: Thirteen percent of men with spondyloarthritis developed anterior uveitis a mean of 10.3 years following diagnosis of their rheumatologic disease.
Data source: An analysis of 268 male military veterans with spondyloarthritis in the Program to Understand Long-Term Outcomes in Spondyloarthritis (PULSAR) registry.
Disclosures: PULSAR is funded by the Department of Veterans Affairs. The presenter reported having no financial conflicts of interest.
Study yields insight into nonradiographic axial spondyloarthritis progression
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
AT THE 2015 SPARTAN ANNUAL MEETING
Key clinical point: Radiographic evidence of sacroiliitis is s-l-o-w to develop in patients with nonradiographic axial spondyloarthritis.
Major finding: Fifteen years after patients were identified as having nonradiographic axial spondyloarthritis based upon MRI or clinical findings, 26% of them had progressed to ankylosing spondylitis as defined by sacroiliitis on x-ray.
Data source: The Rochester (Minn.) Epidemiology Project, a unique medical records database incorporating all residents of Olmsted County, Minn.
Disclosures: The National Institutes of Health funded the study. The presenter reported having no relevant financial disclosures.
IHC: Botox for migraine also improves depression
VALENCIA, SPAIN – OnabotulinumtoxinA injections given for treatment of chronic migraine provide a major side benefit: clinically meaningful improvement in comorbid moderate to severe depression, Dr. Stewart J. Tepper reported at the International Headache Congress.
He presented an uncontrolled, retrospective study with prospectively collected patient-reported outcomes data in 429 chronic migraine patients who underwent two or more sessions of onabotulinumtoxinA (Botox) injections, a treatment approved by the Food and Drug Administration for chronic migraine since 2010.
Since the safety and efficacy of the treatment for chronic migraine are already well established, the goal of this study was to examine change in depression, a common comorbid condition in this population. The primary outcome measure was change in Patient Health Questionnaire-9 (PHQ-9) scores from baseline to follow-up at 6-12 months.
In the overall study population, PHQ-9 scores improved significantly, from a mean of 14.4 at baseline to 11.3 post Botox. But the results became considerably more intriguing when broken by baseline level of depression, according to Dr. Tepper, professor of medicine (neurology) at Case Western Reserve University, Cleveland, and director of research at the Neurological Center for Pain at the Cleveland Clinic.
Seventy percent of the chronic migraine patients had at least mild depression as reflected in a baseline PHQ-9 score of 5 or more. Among the 145 patients with mild depression as evidenced by a PHQ-9 of 5-9, Botox therapy wasn’t associated with any impact on depression scores: a mean of 6.8 at baseline and 6.8 at follow-up.
In contrast, among the 75 patients with moderate depression as defined by a PHQ-9 score of 10-14, depression scores improved significantly from 11.8 to 9.6. Moreover, in the 30 patients with baseline moderate/severe depression as evidenced by a PHQ-9 of 15-19, scores improved from 17 at pretreatment to 13.4 at follow-up. The largest absolute improvement in depression scores was seen in those who were most severely depressed: in the 16 patients with baseline severe depression as defined by a PHQ-9 score of 20-27, mean scores improved from 21.8 at baseline to 15.2, Dr. Tepper reported at the meeting sponsored by the International Headache Society and the American Headache Society.
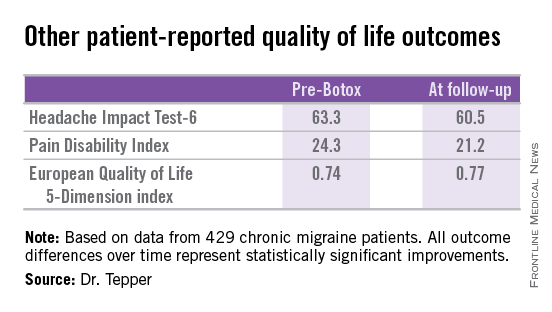
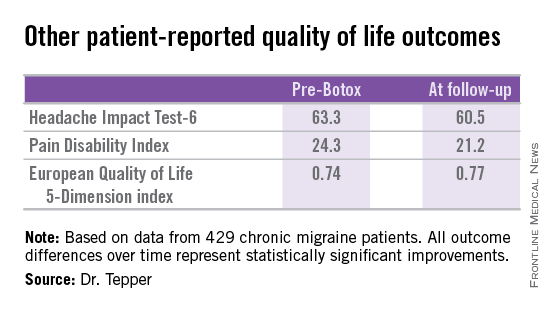
Other patient-reported outcomes also improved significantly following two or more onabotulinumtoxinA treatment sessions.
Dr. Tepper reported no financial conflicts regarding this study, which was conducted exclusively with institutional funds.
VALENCIA, SPAIN – OnabotulinumtoxinA injections given for treatment of chronic migraine provide a major side benefit: clinically meaningful improvement in comorbid moderate to severe depression, Dr. Stewart J. Tepper reported at the International Headache Congress.
He presented an uncontrolled, retrospective study with prospectively collected patient-reported outcomes data in 429 chronic migraine patients who underwent two or more sessions of onabotulinumtoxinA (Botox) injections, a treatment approved by the Food and Drug Administration for chronic migraine since 2010.

Since the safety and efficacy of the treatment for chronic migraine are already well established, the goal of this study was to examine change in depression, a common comorbid condition in this population. The primary outcome measure was change in Patient Health Questionnaire-9 (PHQ-9) scores from baseline to follow-up at 6-12 months.
In the overall study population, PHQ-9 scores improved significantly, from a mean of 14.4 at baseline to 11.3 post Botox. But the results became considerably more intriguing when broken by baseline level of depression, according to Dr. Tepper, professor of medicine (neurology) at Case Western Reserve University, Cleveland, and director of research at the Neurological Center for Pain at the Cleveland Clinic.
Seventy percent of the chronic migraine patients had at least mild depression as reflected in a baseline PHQ-9 score of 5 or more. Among the 145 patients with mild depression as evidenced by a PHQ-9 of 5-9, Botox therapy wasn’t associated with any impact on depression scores: a mean of 6.8 at baseline and 6.8 at follow-up.
In contrast, among the 75 patients with moderate depression as defined by a PHQ-9 score of 10-14, depression scores improved significantly from 11.8 to 9.6. Moreover, in the 30 patients with baseline moderate/severe depression as evidenced by a PHQ-9 of 15-19, scores improved from 17 at pretreatment to 13.4 at follow-up. The largest absolute improvement in depression scores was seen in those who were most severely depressed: in the 16 patients with baseline severe depression as defined by a PHQ-9 score of 20-27, mean scores improved from 21.8 at baseline to 15.2, Dr. Tepper reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Other patient-reported outcomes also improved significantly following two or more onabotulinumtoxinA treatment sessions.
Dr. Tepper reported no financial conflicts regarding this study, which was conducted exclusively with institutional funds.
VALENCIA, SPAIN – OnabotulinumtoxinA injections given for treatment of chronic migraine provide a major side benefit: clinically meaningful improvement in comorbid moderate to severe depression, Dr. Stewart J. Tepper reported at the International Headache Congress.
He presented an uncontrolled, retrospective study with prospectively collected patient-reported outcomes data in 429 chronic migraine patients who underwent two or more sessions of onabotulinumtoxinA (Botox) injections, a treatment approved by the Food and Drug Administration for chronic migraine since 2010.

Since the safety and efficacy of the treatment for chronic migraine are already well established, the goal of this study was to examine change in depression, a common comorbid condition in this population. The primary outcome measure was change in Patient Health Questionnaire-9 (PHQ-9) scores from baseline to follow-up at 6-12 months.
In the overall study population, PHQ-9 scores improved significantly, from a mean of 14.4 at baseline to 11.3 post Botox. But the results became considerably more intriguing when broken by baseline level of depression, according to Dr. Tepper, professor of medicine (neurology) at Case Western Reserve University, Cleveland, and director of research at the Neurological Center for Pain at the Cleveland Clinic.
Seventy percent of the chronic migraine patients had at least mild depression as reflected in a baseline PHQ-9 score of 5 or more. Among the 145 patients with mild depression as evidenced by a PHQ-9 of 5-9, Botox therapy wasn’t associated with any impact on depression scores: a mean of 6.8 at baseline and 6.8 at follow-up.
In contrast, among the 75 patients with moderate depression as defined by a PHQ-9 score of 10-14, depression scores improved significantly from 11.8 to 9.6. Moreover, in the 30 patients with baseline moderate/severe depression as evidenced by a PHQ-9 of 15-19, scores improved from 17 at pretreatment to 13.4 at follow-up. The largest absolute improvement in depression scores was seen in those who were most severely depressed: in the 16 patients with baseline severe depression as defined by a PHQ-9 score of 20-27, mean scores improved from 21.8 at baseline to 15.2, Dr. Tepper reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Other patient-reported outcomes also improved significantly following two or more onabotulinumtoxinA treatment sessions.
Dr. Tepper reported no financial conflicts regarding this study, which was conducted exclusively with institutional funds.
AT IHC 2015
Key clinical point: OnabotulinumtoxinA injections for chronic migraine significantly improved depression scores in patients with moderate or severe depression at baseline.
Major finding: Mean depression scores on the Patient Health Questionnaire-9 in chronic migraine patients with baseline severe depression improved from 21.8 out of a possible 27 at baseline to 15.2 at 6-12 months’ follow-up after two or more onabotulinumtoxinA treatment sessions.
Data source: This was an uncontrolled, retrospective study of 429 patients who underwent two or more sessions of onabotulinumtoxinA injections for chronic migraine and were assessed for change in depression scores.
Disclosures: This study was conducted entirely with institutional funds. The presenter reported having no financial conflicts.
In melanoma, sentinel node results will drive targeted therapies
VANCOUVER, B.C. – Why should dermatologists care about the role of sentinel lymph node biopsy in melanoma patients? Because SNLB results will inform the use of an explosion of immunotherapies and targeted therapies emerging for melanoma and – because even after melanoma patients have been referred to a comprehensive cancer center – patients will continue to view dermatologists as the primary care providers with respect to the cancer, Dr. Timothy M. Johnson said in a plenary address at the World Congress of Dermatology.
“I guarantee you of that” scenario, predicted Dr. Johnson, professor of dermatology and clinical director of the multidisciplinary melanoma program at the University of Michigan, Ann Arbor, where more than 2,000 melanoma patients are seen each year.
New melanoma therapies are “coming in waves. They seem to be coming weekly,” he said. In June, the National Cancer Institute listed 218 therapeutic clinical trials for advanced melanoma in the United States alone. And some of these new treatments for stage IV melanoma are already starting to make their way into the adjuvant setting for stage III melanoma.
“The first round of adjuvant therapy trials has been done, and the data are being analyzed now. A new round is coming like gangbusters,” Dr. Johnson said. “This is one of the most exciting times ever in melanoma.”
Should some of these novel agents prove safe and effective as adjuvant therapy, SLNB results will determine the need for such therapy based upon individualized patient prognosis. And the SLNB results will determine the type of adjuvant therapy that’s most appropriate based upon the tumor molecular profile.
“Once we have effective adjuvant therapy, it will eradicate the need for total lymph node dissection in patients with a positive node on SLNB. Those with a positive node will likely undergo systemic therapy based on a personalized approach,” he predicted.
For the present, in Dr. Johnson’s view, much of the best guidance on when and how to employ SLNB in melanoma patients comes from the landmark National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial (MSLT-1).
The MSLT-1 results (N Engl J Med. 2014 Feb 13;370:599-609) are deemed controversial by some, Dr. Johnson noted. But they provide a strong case for widespread application of SLNB for accurate staging and regional control of the nodal basin based upon the findings in 1,270 participants with intermediate-thickness melanomas of 1.2-3.5 mm and 290 others with melanomas greater than 3.5 mm thick, he said. Both groups showed significant benefit in terms of 10-year melanoma-specific survival if randomized to SLNB with immediate total lymph node dissection if SLNB positive, as opposed to watchful waiting for an occult nodal metastasis to become clinically evident.
An important finding was that roughly 27% of patients in the observation arms who experienced a clinically evident nodal metastasis during follow-up and then underwent completion total lymph node dissection had four or more positive nodes at that time, compared to 1.6% of those with a positive SLNB who underwent immediate completion dissection.
“If an occult metastasis is present in the lymph node it’s going to grow to the point of becoming clinically evident in an average of 2-3 years. You can deal with it now or you can deal with it later, but you’re likely going to have to deal with it. Failure to detect and treat that disease early will result in increased tumor burden upon completion lymph node dissection, increased morbidity and side effects in order to remove those nodes, and a small but increased likelihood of dying from that disease. That’s a very powerful conversation to have with patients and families to help them make the best informed decision for themselves,” Dr. Johnson said.
At the University of Michigan melanoma program – the nation’s largest – patients are counseled to seriously consider SLNB if they have a clinically localized melanoma 1 mm or more in thickness provided their functional status is favorable. In contrast, there is essentially no evidence to support SLNB in patients with a melanoma less than 0.75 mm in thickness.
In patients with a melanoma thickness of 0.75-0.99 mm, however, Dr. Johnson and his colleagues may recommend SLNB in the presence of certain predictors of increased likelihood of a positive biopsy: ulceration, angiolymphatic invasion, younger age, a positive deep margin on a shave biopsy, extensive dermal regression in excess of 1 mm, and/or a mitotic rate of at least 1 mm2. Age and mitotic rate are continuous variables: The younger the patient and the higher the mitotic rate, the greater the likelihood of a positive SLNB in the setting of a thin melanoma.
At present, the decision regarding whether to recommend SLNB in a patient with a thin melanoma is based upon a general gestalt, said Dr. Johnson. He and his colleagues have received a grant to study more than 2,000 cases of thin melanoma in an effort to develop a system for weighting the individual risk factors.
“As dermatologists – you, me, we – should be one of the lead dogs with respect to melanoma advancement, knowledge, management, and guidance. To do that most effectively, you must learn from and work closely with other specialists – collegially, collaboratively, and humbly,” he concluded.
Dr. Johnson reported having no financial conflicts of interest regarding his talk.
VANCOUVER, B.C. – Why should dermatologists care about the role of sentinel lymph node biopsy in melanoma patients? Because SNLB results will inform the use of an explosion of immunotherapies and targeted therapies emerging for melanoma and – because even after melanoma patients have been referred to a comprehensive cancer center – patients will continue to view dermatologists as the primary care providers with respect to the cancer, Dr. Timothy M. Johnson said in a plenary address at the World Congress of Dermatology.
“I guarantee you of that” scenario, predicted Dr. Johnson, professor of dermatology and clinical director of the multidisciplinary melanoma program at the University of Michigan, Ann Arbor, where more than 2,000 melanoma patients are seen each year.
New melanoma therapies are “coming in waves. They seem to be coming weekly,” he said. In June, the National Cancer Institute listed 218 therapeutic clinical trials for advanced melanoma in the United States alone. And some of these new treatments for stage IV melanoma are already starting to make their way into the adjuvant setting for stage III melanoma.
“The first round of adjuvant therapy trials has been done, and the data are being analyzed now. A new round is coming like gangbusters,” Dr. Johnson said. “This is one of the most exciting times ever in melanoma.”
Should some of these novel agents prove safe and effective as adjuvant therapy, SLNB results will determine the need for such therapy based upon individualized patient prognosis. And the SLNB results will determine the type of adjuvant therapy that’s most appropriate based upon the tumor molecular profile.
“Once we have effective adjuvant therapy, it will eradicate the need for total lymph node dissection in patients with a positive node on SLNB. Those with a positive node will likely undergo systemic therapy based on a personalized approach,” he predicted.
For the present, in Dr. Johnson’s view, much of the best guidance on when and how to employ SLNB in melanoma patients comes from the landmark National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial (MSLT-1).
The MSLT-1 results (N Engl J Med. 2014 Feb 13;370:599-609) are deemed controversial by some, Dr. Johnson noted. But they provide a strong case for widespread application of SLNB for accurate staging and regional control of the nodal basin based upon the findings in 1,270 participants with intermediate-thickness melanomas of 1.2-3.5 mm and 290 others with melanomas greater than 3.5 mm thick, he said. Both groups showed significant benefit in terms of 10-year melanoma-specific survival if randomized to SLNB with immediate total lymph node dissection if SLNB positive, as opposed to watchful waiting for an occult nodal metastasis to become clinically evident.
An important finding was that roughly 27% of patients in the observation arms who experienced a clinically evident nodal metastasis during follow-up and then underwent completion total lymph node dissection had four or more positive nodes at that time, compared to 1.6% of those with a positive SLNB who underwent immediate completion dissection.
“If an occult metastasis is present in the lymph node it’s going to grow to the point of becoming clinically evident in an average of 2-3 years. You can deal with it now or you can deal with it later, but you’re likely going to have to deal with it. Failure to detect and treat that disease early will result in increased tumor burden upon completion lymph node dissection, increased morbidity and side effects in order to remove those nodes, and a small but increased likelihood of dying from that disease. That’s a very powerful conversation to have with patients and families to help them make the best informed decision for themselves,” Dr. Johnson said.
At the University of Michigan melanoma program – the nation’s largest – patients are counseled to seriously consider SLNB if they have a clinically localized melanoma 1 mm or more in thickness provided their functional status is favorable. In contrast, there is essentially no evidence to support SLNB in patients with a melanoma less than 0.75 mm in thickness.
In patients with a melanoma thickness of 0.75-0.99 mm, however, Dr. Johnson and his colleagues may recommend SLNB in the presence of certain predictors of increased likelihood of a positive biopsy: ulceration, angiolymphatic invasion, younger age, a positive deep margin on a shave biopsy, extensive dermal regression in excess of 1 mm, and/or a mitotic rate of at least 1 mm2. Age and mitotic rate are continuous variables: The younger the patient and the higher the mitotic rate, the greater the likelihood of a positive SLNB in the setting of a thin melanoma.
At present, the decision regarding whether to recommend SLNB in a patient with a thin melanoma is based upon a general gestalt, said Dr. Johnson. He and his colleagues have received a grant to study more than 2,000 cases of thin melanoma in an effort to develop a system for weighting the individual risk factors.
“As dermatologists – you, me, we – should be one of the lead dogs with respect to melanoma advancement, knowledge, management, and guidance. To do that most effectively, you must learn from and work closely with other specialists – collegially, collaboratively, and humbly,” he concluded.
Dr. Johnson reported having no financial conflicts of interest regarding his talk.
VANCOUVER, B.C. – Why should dermatologists care about the role of sentinel lymph node biopsy in melanoma patients? Because SNLB results will inform the use of an explosion of immunotherapies and targeted therapies emerging for melanoma and – because even after melanoma patients have been referred to a comprehensive cancer center – patients will continue to view dermatologists as the primary care providers with respect to the cancer, Dr. Timothy M. Johnson said in a plenary address at the World Congress of Dermatology.
“I guarantee you of that” scenario, predicted Dr. Johnson, professor of dermatology and clinical director of the multidisciplinary melanoma program at the University of Michigan, Ann Arbor, where more than 2,000 melanoma patients are seen each year.
New melanoma therapies are “coming in waves. They seem to be coming weekly,” he said. In June, the National Cancer Institute listed 218 therapeutic clinical trials for advanced melanoma in the United States alone. And some of these new treatments for stage IV melanoma are already starting to make their way into the adjuvant setting for stage III melanoma.
“The first round of adjuvant therapy trials has been done, and the data are being analyzed now. A new round is coming like gangbusters,” Dr. Johnson said. “This is one of the most exciting times ever in melanoma.”
Should some of these novel agents prove safe and effective as adjuvant therapy, SLNB results will determine the need for such therapy based upon individualized patient prognosis. And the SLNB results will determine the type of adjuvant therapy that’s most appropriate based upon the tumor molecular profile.
“Once we have effective adjuvant therapy, it will eradicate the need for total lymph node dissection in patients with a positive node on SLNB. Those with a positive node will likely undergo systemic therapy based on a personalized approach,” he predicted.
For the present, in Dr. Johnson’s view, much of the best guidance on when and how to employ SLNB in melanoma patients comes from the landmark National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial (MSLT-1).
The MSLT-1 results (N Engl J Med. 2014 Feb 13;370:599-609) are deemed controversial by some, Dr. Johnson noted. But they provide a strong case for widespread application of SLNB for accurate staging and regional control of the nodal basin based upon the findings in 1,270 participants with intermediate-thickness melanomas of 1.2-3.5 mm and 290 others with melanomas greater than 3.5 mm thick, he said. Both groups showed significant benefit in terms of 10-year melanoma-specific survival if randomized to SLNB with immediate total lymph node dissection if SLNB positive, as opposed to watchful waiting for an occult nodal metastasis to become clinically evident.
An important finding was that roughly 27% of patients in the observation arms who experienced a clinically evident nodal metastasis during follow-up and then underwent completion total lymph node dissection had four or more positive nodes at that time, compared to 1.6% of those with a positive SLNB who underwent immediate completion dissection.
“If an occult metastasis is present in the lymph node it’s going to grow to the point of becoming clinically evident in an average of 2-3 years. You can deal with it now or you can deal with it later, but you’re likely going to have to deal with it. Failure to detect and treat that disease early will result in increased tumor burden upon completion lymph node dissection, increased morbidity and side effects in order to remove those nodes, and a small but increased likelihood of dying from that disease. That’s a very powerful conversation to have with patients and families to help them make the best informed decision for themselves,” Dr. Johnson said.
At the University of Michigan melanoma program – the nation’s largest – patients are counseled to seriously consider SLNB if they have a clinically localized melanoma 1 mm or more in thickness provided their functional status is favorable. In contrast, there is essentially no evidence to support SLNB in patients with a melanoma less than 0.75 mm in thickness.
In patients with a melanoma thickness of 0.75-0.99 mm, however, Dr. Johnson and his colleagues may recommend SLNB in the presence of certain predictors of increased likelihood of a positive biopsy: ulceration, angiolymphatic invasion, younger age, a positive deep margin on a shave biopsy, extensive dermal regression in excess of 1 mm, and/or a mitotic rate of at least 1 mm2. Age and mitotic rate are continuous variables: The younger the patient and the higher the mitotic rate, the greater the likelihood of a positive SLNB in the setting of a thin melanoma.
At present, the decision regarding whether to recommend SLNB in a patient with a thin melanoma is based upon a general gestalt, said Dr. Johnson. He and his colleagues have received a grant to study more than 2,000 cases of thin melanoma in an effort to develop a system for weighting the individual risk factors.
“As dermatologists – you, me, we – should be one of the lead dogs with respect to melanoma advancement, knowledge, management, and guidance. To do that most effectively, you must learn from and work closely with other specialists – collegially, collaboratively, and humbly,” he concluded.
Dr. Johnson reported having no financial conflicts of interest regarding his talk.
EXPERT ANALYSIS FROM WCD 2015
IHC: Infantile colic portends adolescent migraine
VALENCIA, SPAIN – Infantile colic appears to be a potent predictor of subsequent adolescent migraine without aura, according to a large, prospective, population-based Finnish study.
This is the latest of several studies to document a relationship between infantile colic and later migraine. It’s particularly persuasive by virtue of its 18 years of prospective follow-up, Dr. Kenneth J. Mack commented at the International Headache Congress.
Indeed, he hailed the Finnish study (Cephalalgia. 2015 Mar 9. pii: 0333102415576225.) led by investigators at the University of Turku as one of the top developments in the field of pediatric headache within the past year.
The Finnish investigators followed 1,267 infants, all the first-born in their families, and 13% of whom were diagnosed with colic by age 3 months. Of the 787 subjects captured at follow-up at age 18 years, 129 had been diagnosed with migraine, and 96 of the 787 had a history of infantile colic.
Migraine was present in 23% of the 18-year-olds with a history of infantile colic but in only 11% of those without such a history, noted Dr. Mack, professor of neurology and pediatrics at the Mayo Clinic, Rochester, Minn.
Fourteen of 22 adolescent migraineurs with a history of infantile colic had migraine without aura. The remaining eight had migraine with aura. Thus, infantile colic was associated with a highly significant 2.8-fold increased risk for adolescent migraine without aura but no increased risk for developing migraine with aura, Dr. Mack continued at the meeting sponsored by the International Headache Society and the American Headache Society.
This study solidifies the link between infantile colic and subsequent migraine, but Dr. Mack said he has long suspected the existence of such an association based upon personal experience: “In my own family, two out of three colicky boys developed migraine.”
As the Finnish investigators noted, their study leaves unanswered the question of whether infantile colic is through some as-yet unknown mechanism a risk factor for the subsequent development of migraine or, alternatively, infantile colic might actually be an expression of infantile migraine.
The study was sponsored by the University of Turku, Finland. Dr. Mack reported having no financial conflicts regarding the Finnish study.
VALENCIA, SPAIN – Infantile colic appears to be a potent predictor of subsequent adolescent migraine without aura, according to a large, prospective, population-based Finnish study.
This is the latest of several studies to document a relationship between infantile colic and later migraine. It’s particularly persuasive by virtue of its 18 years of prospective follow-up, Dr. Kenneth J. Mack commented at the International Headache Congress.
Indeed, he hailed the Finnish study (Cephalalgia. 2015 Mar 9. pii: 0333102415576225.) led by investigators at the University of Turku as one of the top developments in the field of pediatric headache within the past year.
The Finnish investigators followed 1,267 infants, all the first-born in their families, and 13% of whom were diagnosed with colic by age 3 months. Of the 787 subjects captured at follow-up at age 18 years, 129 had been diagnosed with migraine, and 96 of the 787 had a history of infantile colic.
Migraine was present in 23% of the 18-year-olds with a history of infantile colic but in only 11% of those without such a history, noted Dr. Mack, professor of neurology and pediatrics at the Mayo Clinic, Rochester, Minn.
Fourteen of 22 adolescent migraineurs with a history of infantile colic had migraine without aura. The remaining eight had migraine with aura. Thus, infantile colic was associated with a highly significant 2.8-fold increased risk for adolescent migraine without aura but no increased risk for developing migraine with aura, Dr. Mack continued at the meeting sponsored by the International Headache Society and the American Headache Society.
This study solidifies the link between infantile colic and subsequent migraine, but Dr. Mack said he has long suspected the existence of such an association based upon personal experience: “In my own family, two out of three colicky boys developed migraine.”
As the Finnish investigators noted, their study leaves unanswered the question of whether infantile colic is through some as-yet unknown mechanism a risk factor for the subsequent development of migraine or, alternatively, infantile colic might actually be an expression of infantile migraine.
The study was sponsored by the University of Turku, Finland. Dr. Mack reported having no financial conflicts regarding the Finnish study.
VALENCIA, SPAIN – Infantile colic appears to be a potent predictor of subsequent adolescent migraine without aura, according to a large, prospective, population-based Finnish study.
This is the latest of several studies to document a relationship between infantile colic and later migraine. It’s particularly persuasive by virtue of its 18 years of prospective follow-up, Dr. Kenneth J. Mack commented at the International Headache Congress.
Indeed, he hailed the Finnish study (Cephalalgia. 2015 Mar 9. pii: 0333102415576225.) led by investigators at the University of Turku as one of the top developments in the field of pediatric headache within the past year.
The Finnish investigators followed 1,267 infants, all the first-born in their families, and 13% of whom were diagnosed with colic by age 3 months. Of the 787 subjects captured at follow-up at age 18 years, 129 had been diagnosed with migraine, and 96 of the 787 had a history of infantile colic.
Migraine was present in 23% of the 18-year-olds with a history of infantile colic but in only 11% of those without such a history, noted Dr. Mack, professor of neurology and pediatrics at the Mayo Clinic, Rochester, Minn.
Fourteen of 22 adolescent migraineurs with a history of infantile colic had migraine without aura. The remaining eight had migraine with aura. Thus, infantile colic was associated with a highly significant 2.8-fold increased risk for adolescent migraine without aura but no increased risk for developing migraine with aura, Dr. Mack continued at the meeting sponsored by the International Headache Society and the American Headache Society.
This study solidifies the link between infantile colic and subsequent migraine, but Dr. Mack said he has long suspected the existence of such an association based upon personal experience: “In my own family, two out of three colicky boys developed migraine.”
As the Finnish investigators noted, their study leaves unanswered the question of whether infantile colic is through some as-yet unknown mechanism a risk factor for the subsequent development of migraine or, alternatively, infantile colic might actually be an expression of infantile migraine.
The study was sponsored by the University of Turku, Finland. Dr. Mack reported having no financial conflicts regarding the Finnish study.
AT IHC 2015
Key clinical point: Infantile colic is associated with a nearly threefold increased risk of having migraine by age 18 years.
Major finding: Migraine was present at age 18 in 23% of a group of Finnish youths with a history of infantile colic but in only 11% of subjects without such a history.
Data source: This was a prospective, population-based cohort study involving 18 years of follow-up of 1,267 Finnish infants.
Disclosures: The study was sponsored by the University of Turku, Finland. The presenter reported having no financial conflicts.
WCD: Watch for These Emerging Infections
VANCOUVER – Two serious emerging skin and soft tissue infections whose progress physicians will want to chart are melioidosis and Acinetobacter baumannii infection, Dr. Dirk M. Elston advised at the World Congress of Dermatology.
Both Burkholderia pseudomallei – the cause of melioidosis – and Acinetobacter baumannii are gram-negative organisms that laboratory staff sometimes mistakenly dismiss as culture contaminants. But melioidosis has a case fatality rate of up to 40%, and A. baumannii is an increasingly multidrug-resistant cause of community-acquired cellulitis, according to Dr. Elston, chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina, Charleston.
Dr. Elston, also managing director of the Ackerman Academy of Dermatopathology in New York, offered his views on these two emerging infections.
Melioidosis
“We know melioidosis from the rice paddies of Vietnam as a plaguelike ulceroglandular syndrome. It has reemerged in the Caribbean,” Dr. Elston reported.
Indeed, investigators at the Centers for Disease Control and Prevention reported earlier this year that melioidosis is now endemic in Puerto Rico based upon its findings of high seropositivity rates among patient contacts plus isolation of the causative organism from soil samples (Clin Infect Dis. 2015 Jan 15;60(2):243-250). The infection, which is believed to be underdiagnosed, also has been reported at numerous other sites in the Caribbean basin and in Latin America and Africa, as well as in Southeast Asia.
Although skin and soft tissue abscesses are common manifestations of this acute febrile illness, the most common clinical presentation of melioidosis is acute pneumonia with or without septicemia, which can be fulminant.
According to the CDC investigators, up to 80% of patients with melioidosis have diabetes, chronic lung disease, and/or excessive alcohol use as risk factors for the infection. In the Puerto Rican study, a history of injection drug use was for the first time identified as another risk factor. When in endemic areas such as Puerto Rico, individuals with diabetes or other risk factors should protect themselves from direct exposure to soil and water to reduce their risk of what is believed to be a transcutaneously acquired infection. The investigators advised that individuals with skin wounds or sores do the same.
The recommended treatment for melioidosis is intravenous ceftazidime, imipenem, or meropenem (N Engl J Med. 2012 Sep 13;367(11):1035-1044).
A. baumannii infection
In a recent report, Dr. Adam J. Friedman and his colleagues at the Albert Einstein College of Medicine in New York said that A. baumannii’s pattern of evolution to date is strikingly similar to that of methicillin-resistant Staphylococcus aureus. A. baumannii has displayed increasing pathogenicity and antibiotic resistance. The investigators warned that there is a real danger that, like MRSA, extensively drug-resistant A. baumannii will become a common community-acquired infection arising in previously healthy patients (JAMA Dermatol. 2014 Aug;150(8):905-906).
“There are some strains of gonococcus and some strains of Acinetobacter baumannii that appear to be resistant to all known antibiotics,” Dr. Elston said.
He reported having no relevant financial conflicts of interest.
VANCOUVER – Two serious emerging skin and soft tissue infections whose progress physicians will want to chart are melioidosis and Acinetobacter baumannii infection, Dr. Dirk M. Elston advised at the World Congress of Dermatology.
Both Burkholderia pseudomallei – the cause of melioidosis – and Acinetobacter baumannii are gram-negative organisms that laboratory staff sometimes mistakenly dismiss as culture contaminants. But melioidosis has a case fatality rate of up to 40%, and A. baumannii is an increasingly multidrug-resistant cause of community-acquired cellulitis, according to Dr. Elston, chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina, Charleston.
Dr. Elston, also managing director of the Ackerman Academy of Dermatopathology in New York, offered his views on these two emerging infections.
Melioidosis
“We know melioidosis from the rice paddies of Vietnam as a plaguelike ulceroglandular syndrome. It has reemerged in the Caribbean,” Dr. Elston reported.
Indeed, investigators at the Centers for Disease Control and Prevention reported earlier this year that melioidosis is now endemic in Puerto Rico based upon its findings of high seropositivity rates among patient contacts plus isolation of the causative organism from soil samples (Clin Infect Dis. 2015 Jan 15;60(2):243-250). The infection, which is believed to be underdiagnosed, also has been reported at numerous other sites in the Caribbean basin and in Latin America and Africa, as well as in Southeast Asia.
Although skin and soft tissue abscesses are common manifestations of this acute febrile illness, the most common clinical presentation of melioidosis is acute pneumonia with or without septicemia, which can be fulminant.
According to the CDC investigators, up to 80% of patients with melioidosis have diabetes, chronic lung disease, and/or excessive alcohol use as risk factors for the infection. In the Puerto Rican study, a history of injection drug use was for the first time identified as another risk factor. When in endemic areas such as Puerto Rico, individuals with diabetes or other risk factors should protect themselves from direct exposure to soil and water to reduce their risk of what is believed to be a transcutaneously acquired infection. The investigators advised that individuals with skin wounds or sores do the same.
The recommended treatment for melioidosis is intravenous ceftazidime, imipenem, or meropenem (N Engl J Med. 2012 Sep 13;367(11):1035-1044).
A. baumannii infection
In a recent report, Dr. Adam J. Friedman and his colleagues at the Albert Einstein College of Medicine in New York said that A. baumannii’s pattern of evolution to date is strikingly similar to that of methicillin-resistant Staphylococcus aureus. A. baumannii has displayed increasing pathogenicity and antibiotic resistance. The investigators warned that there is a real danger that, like MRSA, extensively drug-resistant A. baumannii will become a common community-acquired infection arising in previously healthy patients (JAMA Dermatol. 2014 Aug;150(8):905-906).
“There are some strains of gonococcus and some strains of Acinetobacter baumannii that appear to be resistant to all known antibiotics,” Dr. Elston said.
He reported having no relevant financial conflicts of interest.
VANCOUVER – Two serious emerging skin and soft tissue infections whose progress physicians will want to chart are melioidosis and Acinetobacter baumannii infection, Dr. Dirk M. Elston advised at the World Congress of Dermatology.
Both Burkholderia pseudomallei – the cause of melioidosis – and Acinetobacter baumannii are gram-negative organisms that laboratory staff sometimes mistakenly dismiss as culture contaminants. But melioidosis has a case fatality rate of up to 40%, and A. baumannii is an increasingly multidrug-resistant cause of community-acquired cellulitis, according to Dr. Elston, chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina, Charleston.
Dr. Elston, also managing director of the Ackerman Academy of Dermatopathology in New York, offered his views on these two emerging infections.
Melioidosis
“We know melioidosis from the rice paddies of Vietnam as a plaguelike ulceroglandular syndrome. It has reemerged in the Caribbean,” Dr. Elston reported.
Indeed, investigators at the Centers for Disease Control and Prevention reported earlier this year that melioidosis is now endemic in Puerto Rico based upon its findings of high seropositivity rates among patient contacts plus isolation of the causative organism from soil samples (Clin Infect Dis. 2015 Jan 15;60(2):243-250). The infection, which is believed to be underdiagnosed, also has been reported at numerous other sites in the Caribbean basin and in Latin America and Africa, as well as in Southeast Asia.
Although skin and soft tissue abscesses are common manifestations of this acute febrile illness, the most common clinical presentation of melioidosis is acute pneumonia with or without septicemia, which can be fulminant.
According to the CDC investigators, up to 80% of patients with melioidosis have diabetes, chronic lung disease, and/or excessive alcohol use as risk factors for the infection. In the Puerto Rican study, a history of injection drug use was for the first time identified as another risk factor. When in endemic areas such as Puerto Rico, individuals with diabetes or other risk factors should protect themselves from direct exposure to soil and water to reduce their risk of what is believed to be a transcutaneously acquired infection. The investigators advised that individuals with skin wounds or sores do the same.
The recommended treatment for melioidosis is intravenous ceftazidime, imipenem, or meropenem (N Engl J Med. 2012 Sep 13;367(11):1035-1044).
A. baumannii infection
In a recent report, Dr. Adam J. Friedman and his colleagues at the Albert Einstein College of Medicine in New York said that A. baumannii’s pattern of evolution to date is strikingly similar to that of methicillin-resistant Staphylococcus aureus. A. baumannii has displayed increasing pathogenicity and antibiotic resistance. The investigators warned that there is a real danger that, like MRSA, extensively drug-resistant A. baumannii will become a common community-acquired infection arising in previously healthy patients (JAMA Dermatol. 2014 Aug;150(8):905-906).
“There are some strains of gonococcus and some strains of Acinetobacter baumannii that appear to be resistant to all known antibiotics,” Dr. Elston said.
He reported having no relevant financial conflicts of interest.
EXPERT ANALYSIS FROM WCD 2015
WCD: Watch for these emerging infections
VANCOUVER – Two serious emerging skin and soft tissue infections whose progress physicians will want to chart are melioidosis and Acinetobacter baumannii infection, Dr. Dirk M. Elston advised at the World Congress of Dermatology.
Both Burkholderia pseudomallei – the cause of melioidosis – and Acinetobacter baumannii are gram-negative organisms that laboratory staff sometimes mistakenly dismiss as culture contaminants. But melioidosis has a case fatality rate of up to 40%, and A. baumannii is an increasingly multidrug-resistant cause of community-acquired cellulitis, according to Dr. Elston, chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina, Charleston.
Dr. Elston, also managing director of the Ackerman Academy of Dermatopathology in New York, offered his views on these two emerging infections.
Melioidosis
“We know melioidosis from the rice paddies of Vietnam as a plaguelike ulceroglandular syndrome. It has reemerged in the Caribbean,” Dr. Elston reported.
Indeed, investigators at the Centers for Disease Control and Prevention reported earlier this year that melioidosis is now endemic in Puerto Rico based upon its findings of high seropositivity rates among patient contacts plus isolation of the causative organism from soil samples (Clin Infect Dis. 2015 Jan 15;60(2):243-250). The infection, which is believed to be underdiagnosed, also has been reported at numerous other sites in the Caribbean basin and in Latin America and Africa, as well as in Southeast Asia.
Although skin and soft tissue abscesses are common manifestations of this acute febrile illness, the most common clinical presentation of melioidosis is acute pneumonia with or without septicemia, which can be fulminant.
According to the CDC investigators, up to 80% of patients with melioidosis have diabetes, chronic lung disease, and/or excessive alcohol use as risk factors for the infection. In the Puerto Rican study, a history of injection drug use was for the first time identified as another risk factor. When in endemic areas such as Puerto Rico, individuals with diabetes or other risk factors should protect themselves from direct exposure to soil and water to reduce their risk of what is believed to be a transcutaneously acquired infection. The investigators advised that individuals with skin wounds or sores do the same.
The recommended treatment for melioidosis is intravenous ceftazidime, imipenem, or meropenem (N Engl J Med. 2012 Sep 13;367(11):1035-1044).
A. baumannii infection
In a recent report, Dr. Adam J. Friedman and his colleagues at the Albert Einstein College of Medicine in New York said that A. baumannii’s pattern of evolution to date is strikingly similar to that of methicillin-resistant Staphylococcus aureus. A. baumannii has displayed increasing pathogenicity and antibiotic resistance. The investigators warned that there is a real danger that, like MRSA, extensively drug-resistant A. baumannii will become a common community-acquired infection arising in previously healthy patients (JAMA Dermatol. 2014 Aug;150(8):905-906).
“There are some strains of gonococcus and some strains of Acinetobacter baumannii that appear to be resistant to all known antibiotics,” Dr. Elston said.
He reported having no relevant financial conflicts of interest.
VANCOUVER – Two serious emerging skin and soft tissue infections whose progress physicians will want to chart are melioidosis and Acinetobacter baumannii infection, Dr. Dirk M. Elston advised at the World Congress of Dermatology.
Both Burkholderia pseudomallei – the cause of melioidosis – and Acinetobacter baumannii are gram-negative organisms that laboratory staff sometimes mistakenly dismiss as culture contaminants. But melioidosis has a case fatality rate of up to 40%, and A. baumannii is an increasingly multidrug-resistant cause of community-acquired cellulitis, according to Dr. Elston, chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina, Charleston.
Dr. Elston, also managing director of the Ackerman Academy of Dermatopathology in New York, offered his views on these two emerging infections.
Melioidosis
“We know melioidosis from the rice paddies of Vietnam as a plaguelike ulceroglandular syndrome. It has reemerged in the Caribbean,” Dr. Elston reported.
Indeed, investigators at the Centers for Disease Control and Prevention reported earlier this year that melioidosis is now endemic in Puerto Rico based upon its findings of high seropositivity rates among patient contacts plus isolation of the causative organism from soil samples (Clin Infect Dis. 2015 Jan 15;60(2):243-250). The infection, which is believed to be underdiagnosed, also has been reported at numerous other sites in the Caribbean basin and in Latin America and Africa, as well as in Southeast Asia.
Although skin and soft tissue abscesses are common manifestations of this acute febrile illness, the most common clinical presentation of melioidosis is acute pneumonia with or without septicemia, which can be fulminant.
According to the CDC investigators, up to 80% of patients with melioidosis have diabetes, chronic lung disease, and/or excessive alcohol use as risk factors for the infection. In the Puerto Rican study, a history of injection drug use was for the first time identified as another risk factor. When in endemic areas such as Puerto Rico, individuals with diabetes or other risk factors should protect themselves from direct exposure to soil and water to reduce their risk of what is believed to be a transcutaneously acquired infection. The investigators advised that individuals with skin wounds or sores do the same.
The recommended treatment for melioidosis is intravenous ceftazidime, imipenem, or meropenem (N Engl J Med. 2012 Sep 13;367(11):1035-1044).
A. baumannii infection
In a recent report, Dr. Adam J. Friedman and his colleagues at the Albert Einstein College of Medicine in New York said that A. baumannii’s pattern of evolution to date is strikingly similar to that of methicillin-resistant Staphylococcus aureus. A. baumannii has displayed increasing pathogenicity and antibiotic resistance. The investigators warned that there is a real danger that, like MRSA, extensively drug-resistant A. baumannii will become a common community-acquired infection arising in previously healthy patients (JAMA Dermatol. 2014 Aug;150(8):905-906).
“There are some strains of gonococcus and some strains of Acinetobacter baumannii that appear to be resistant to all known antibiotics,” Dr. Elston said.
He reported having no relevant financial conflicts of interest.
VANCOUVER – Two serious emerging skin and soft tissue infections whose progress physicians will want to chart are melioidosis and Acinetobacter baumannii infection, Dr. Dirk M. Elston advised at the World Congress of Dermatology.
Both Burkholderia pseudomallei – the cause of melioidosis – and Acinetobacter baumannii are gram-negative organisms that laboratory staff sometimes mistakenly dismiss as culture contaminants. But melioidosis has a case fatality rate of up to 40%, and A. baumannii is an increasingly multidrug-resistant cause of community-acquired cellulitis, according to Dr. Elston, chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina, Charleston.
Dr. Elston, also managing director of the Ackerman Academy of Dermatopathology in New York, offered his views on these two emerging infections.
Melioidosis
“We know melioidosis from the rice paddies of Vietnam as a plaguelike ulceroglandular syndrome. It has reemerged in the Caribbean,” Dr. Elston reported.
Indeed, investigators at the Centers for Disease Control and Prevention reported earlier this year that melioidosis is now endemic in Puerto Rico based upon its findings of high seropositivity rates among patient contacts plus isolation of the causative organism from soil samples (Clin Infect Dis. 2015 Jan 15;60(2):243-250). The infection, which is believed to be underdiagnosed, also has been reported at numerous other sites in the Caribbean basin and in Latin America and Africa, as well as in Southeast Asia.
Although skin and soft tissue abscesses are common manifestations of this acute febrile illness, the most common clinical presentation of melioidosis is acute pneumonia with or without septicemia, which can be fulminant.
According to the CDC investigators, up to 80% of patients with melioidosis have diabetes, chronic lung disease, and/or excessive alcohol use as risk factors for the infection. In the Puerto Rican study, a history of injection drug use was for the first time identified as another risk factor. When in endemic areas such as Puerto Rico, individuals with diabetes or other risk factors should protect themselves from direct exposure to soil and water to reduce their risk of what is believed to be a transcutaneously acquired infection. The investigators advised that individuals with skin wounds or sores do the same.
The recommended treatment for melioidosis is intravenous ceftazidime, imipenem, or meropenem (N Engl J Med. 2012 Sep 13;367(11):1035-1044).
A. baumannii infection
In a recent report, Dr. Adam J. Friedman and his colleagues at the Albert Einstein College of Medicine in New York said that A. baumannii’s pattern of evolution to date is strikingly similar to that of methicillin-resistant Staphylococcus aureus. A. baumannii has displayed increasing pathogenicity and antibiotic resistance. The investigators warned that there is a real danger that, like MRSA, extensively drug-resistant A. baumannii will become a common community-acquired infection arising in previously healthy patients (JAMA Dermatol. 2014 Aug;150(8):905-906).
“There are some strains of gonococcus and some strains of Acinetobacter baumannii that appear to be resistant to all known antibiotics,” Dr. Elston said.
He reported having no relevant financial conflicts of interest.
EXPERT ANALYSIS FROM WCD 2015
IHC: Sucked clonazepam douses burning mouth syndrome
VALENCIA, SPAIN – Sucking on a clonazepam tablet for 3 minutes after every meal effectively reduced pain, paresthesia, dry mouth, and altered sense of taste in patients with burning mouth syndrome in a retrospective study.
Seventy-two patients who met International Headache Society criteria for burning mouth syndrome were instructed to place a 1-mg tablet of clonazepam in their mouths after each meal and suck on it for 3 minutes, holding their saliva in the painful areas of the mouth all the while without swallowing. After the 3 minutes were up, the dregs of the tablet and the saliva were to be spit out, Dr. Maialen Mendizabal reported at the International Headache Congress.
Controlled trials previously have shown orally ingested clonazepam to be effective in patients with burning mouth syndrome. Sucked clonazepam provides the advantages of a topical therapy, added Dr. Mendizabal of the University of the Basque Country, Bilbao, Spain.
Pain was assessed via the validated Brief Pain Inventory at baseline, 2 months, and 6 months, as were the sensory disturbances and other symptoms that are often featured prominently in patients with burning mouth syndrome.
At 2 months, 31% of patients reported a greater than 50% reduction from baseline in pain scores, and an additional 10% had a 30%-50% reduction in pain. At 6 months, 39% of patients reported a greater than 50% reduction in pain, and 8% had a 30%-50% decrease.
Fifteen patients (20%) were completely asymptomatic at 6 months. An additional three patients were pain free but continued to experience dry mouth or other symptoms associated with burning mouth syndrome, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dry mouth and altered taste showed a particularly favorable response to topical clonazepam. Two-thirds of subjects had an altered sense of taste at baseline, one-third after 2 months of clonazepam sucking, and none at 6 months. Likewise, two-thirds of patients reported dry mouth at baseline, 47% at 2 months, and just 13% at 6 months.
Although this retrospective, uncontrolled study certainly can’t be considered the final word, the results are sufficiently encouraging to warrant a randomized trial, Dr. Mendizabal said.
The International Headache Society diagnostic criteria for burning mouth syndrome require the presence of pain in the mouth on a daily basis and persisting for most of the day, normal appearing oral mucosa, and exclusion of other local and systemic diseases.
Burning mouth syndrome is mainly a disorder of postmenopausal women. In this study, the mean age of the population was 63 years, and 82% were female.
Dr. Mendizabal reported having no financial conflicts regarding this study, which was conducted free of commercial support.
VALENCIA, SPAIN – Sucking on a clonazepam tablet for 3 minutes after every meal effectively reduced pain, paresthesia, dry mouth, and altered sense of taste in patients with burning mouth syndrome in a retrospective study.
Seventy-two patients who met International Headache Society criteria for burning mouth syndrome were instructed to place a 1-mg tablet of clonazepam in their mouths after each meal and suck on it for 3 minutes, holding their saliva in the painful areas of the mouth all the while without swallowing. After the 3 minutes were up, the dregs of the tablet and the saliva were to be spit out, Dr. Maialen Mendizabal reported at the International Headache Congress.
Controlled trials previously have shown orally ingested clonazepam to be effective in patients with burning mouth syndrome. Sucked clonazepam provides the advantages of a topical therapy, added Dr. Mendizabal of the University of the Basque Country, Bilbao, Spain.
Pain was assessed via the validated Brief Pain Inventory at baseline, 2 months, and 6 months, as were the sensory disturbances and other symptoms that are often featured prominently in patients with burning mouth syndrome.
At 2 months, 31% of patients reported a greater than 50% reduction from baseline in pain scores, and an additional 10% had a 30%-50% reduction in pain. At 6 months, 39% of patients reported a greater than 50% reduction in pain, and 8% had a 30%-50% decrease.
Fifteen patients (20%) were completely asymptomatic at 6 months. An additional three patients were pain free but continued to experience dry mouth or other symptoms associated with burning mouth syndrome, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dry mouth and altered taste showed a particularly favorable response to topical clonazepam. Two-thirds of subjects had an altered sense of taste at baseline, one-third after 2 months of clonazepam sucking, and none at 6 months. Likewise, two-thirds of patients reported dry mouth at baseline, 47% at 2 months, and just 13% at 6 months.
Although this retrospective, uncontrolled study certainly can’t be considered the final word, the results are sufficiently encouraging to warrant a randomized trial, Dr. Mendizabal said.
The International Headache Society diagnostic criteria for burning mouth syndrome require the presence of pain in the mouth on a daily basis and persisting for most of the day, normal appearing oral mucosa, and exclusion of other local and systemic diseases.
Burning mouth syndrome is mainly a disorder of postmenopausal women. In this study, the mean age of the population was 63 years, and 82% were female.
Dr. Mendizabal reported having no financial conflicts regarding this study, which was conducted free of commercial support.
VALENCIA, SPAIN – Sucking on a clonazepam tablet for 3 minutes after every meal effectively reduced pain, paresthesia, dry mouth, and altered sense of taste in patients with burning mouth syndrome in a retrospective study.
Seventy-two patients who met International Headache Society criteria for burning mouth syndrome were instructed to place a 1-mg tablet of clonazepam in their mouths after each meal and suck on it for 3 minutes, holding their saliva in the painful areas of the mouth all the while without swallowing. After the 3 minutes were up, the dregs of the tablet and the saliva were to be spit out, Dr. Maialen Mendizabal reported at the International Headache Congress.
Controlled trials previously have shown orally ingested clonazepam to be effective in patients with burning mouth syndrome. Sucked clonazepam provides the advantages of a topical therapy, added Dr. Mendizabal of the University of the Basque Country, Bilbao, Spain.
Pain was assessed via the validated Brief Pain Inventory at baseline, 2 months, and 6 months, as were the sensory disturbances and other symptoms that are often featured prominently in patients with burning mouth syndrome.
At 2 months, 31% of patients reported a greater than 50% reduction from baseline in pain scores, and an additional 10% had a 30%-50% reduction in pain. At 6 months, 39% of patients reported a greater than 50% reduction in pain, and 8% had a 30%-50% decrease.
Fifteen patients (20%) were completely asymptomatic at 6 months. An additional three patients were pain free but continued to experience dry mouth or other symptoms associated with burning mouth syndrome, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dry mouth and altered taste showed a particularly favorable response to topical clonazepam. Two-thirds of subjects had an altered sense of taste at baseline, one-third after 2 months of clonazepam sucking, and none at 6 months. Likewise, two-thirds of patients reported dry mouth at baseline, 47% at 2 months, and just 13% at 6 months.
Although this retrospective, uncontrolled study certainly can’t be considered the final word, the results are sufficiently encouraging to warrant a randomized trial, Dr. Mendizabal said.
The International Headache Society diagnostic criteria for burning mouth syndrome require the presence of pain in the mouth on a daily basis and persisting for most of the day, normal appearing oral mucosa, and exclusion of other local and systemic diseases.
Burning mouth syndrome is mainly a disorder of postmenopausal women. In this study, the mean age of the population was 63 years, and 82% were female.
Dr. Mendizabal reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT IHC 2015
Key clinical point: Sucking on clonazepam tablets reduces the pain and other symptoms of burning mouth syndrome.
Major finding: After 6 months of sucking on a clonazepam tablet for 3 minutes after every meal, 28 of 72 patients with burning mouth syndrome reported a greater than 50% reduction in pain compared with baseline, including 15 patients with no pain at all.
Data source: This was a retrospective uncontrolled study of 72 patients with burning mouth syndrome treated with topical clonazepam and followed for 6 months.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AAS: CAMS protects against suicidality during, after hospitalization
ATLANTA – The suicide-specific intervention known as CAMS, or Collaborative Assessment and Management of Suicidality, provides protection against suicidal events during psychiatric hospitalization and in the first weeks post discharge, according to a study from the Menninger Clinic.
In this study, 52 patients who received CAMS in addition to the Menninger Clinic’s usual very intensive treatment regimen showed significantly greater improvement on measures of depression, suicidal ideation, hopelessness, and psychological flexibility at discharge, compared with 52 propensity-matched controls who receive the same regimen minus the CAMS. And, in the first 6 months after discharge, the CAMS group made half as many suicide attempts as did the controls, Thomas E. Ellis, Psy.D., reported at the annual conference of the American Association of Suicidology.
“We’re thinking that CAMS has some kind of buffering or protective effect in the weeks following discharge, which is the highest-risk period,” said Dr. Ellis, who is director of psychology at the Menninger Clinic and professor of psychiatry at the Baylor College of Medicine, both in Houston.
A total of 4%-7% of all suicides occurring in the United States each year happen in inpatient psychiatric settings. That’s a surprising statistic given that psychiatric hospitalization aims to provide a safe harbor for individuals bent on self-harm. Investigators have identified two sharp peaks of elevated risk of suicide in connection with psychiatric hospitalization: one in the first week after admission, the other in the first week after discharge. CAMS appears to flatten out these peaks, according to the psychologist.
CAMS is an intervention developed by David A. Jobes, Ph.D., professor of psychology at Catholic University in Washington. The intervention often is described as a theoretical framework in which suicide is seen as the primary focus rather than as a symptom. CAMS is nondenominational in terms of its psychotherapeutic orientation: It is being used today by therapists whose approach runs the gamut from psychodynamic to cognitive-behavioral therapy. The emphasis is on helping the patient problem-solve to find alternatives to suicide as a coping response and providing psychotherapy to address underlying vulnerabilities. A key element of CAMS is early assessment via the CAMS Suicide Status Form, which is used for problem identification and treatment planning, Dr. Ellis explained.
In recent years, he and Dr. Jobes have worked to adapt the CAMS approach to the unique setting of the Menninger Clinic. The clinic is a 100-bed private psychiatric hospital that often is seen as “a last chance” for patients who previously have been hospitalized elsewhere for multiple treatment-resistant conditions, often including substance abuse and personality disorders as well as refractory major depression. The average length of stay is 6-7 weeks – “that’s almost unheard of,” he noted – in contrast to the 3- to 7-day hospitalizations that are typical elsewhere.
The 104 study participants had a mean of 1.7 prior suicide attempts. The 52 controls were selected from a pool of 310 suicidal patients on the basis of propensity score matching by age, sex, treatment unit, severity of suicidal ideation, and number of previous suicide attempts. All subjects got the usual Menninger treatment package, which includes medications, group therapy, psychosocial groups, milieu therapy, nursing care, family counseling, vocational counseling, and individual psychotherapy. The only difference was that the individual psychotherapy included CAMS in 52 patients, while the other 52 received their individual psychotherapy from practitioners who were not involved in CAMS.
Both groups showed significant improvement during hospitalization, but the CAMS group showed significantly greater gains on measures of depression, suicidal ideation, and suicidal cognition.
However, when patients were reassessed at 3 and 6 months post discharge, the picture became more complex. The controls showed late improvement in these measures such that by 3 months’ follow-up, there were no longer significant differences between the two groups on measures of suicide ideation intention on the Columbia Suicide Severity Rating Scale, functional impairment on the World Health Organization Disability Assessment Scale, and thoughts of self-harm on the Patient Health Questionnaire-9 (PHQ-9). It’s not that the CAMS group was backsliding, they were in fact remaining stable on these measures post hospitalization while the controls were getting better. For example, depression scores on the PHQ-9 in the CAMS group were 8.8 at discharge, 9.2 at 3 months, and 9.5 at 6 months, in contrast to 13.7, 10.4, and 10.5 in controls.
Three suicide attempts occurred in the CAMS group post discharge at a mean of 53 and median of 57 days, compared with six attempts in controls at a mean of 38 and median 19 days. But while suicide attempts were fewer in number and occurred later in the CAMS group, these favorable outcome trends did not achieve statistical significance, as the study was not powered to look at that relatively infrequent endpoint, Dr. Ellis said.
To his dismay, rehospitalization rates were higher in the CAMS group: 0 versus 3.8% in controls during the first 2 weeks post discharge, but 9.8%, compared with 5.8% at 3 months and 15.4% vs. 7.6% in controls at 6 months.
Those differences are not statistically significant, but “the raw numbers are of concern,” Dr. Ellis said. He added that the rehospitalization data were compiled only quite recently, and he and his coinvestigators are still trying to figure out the explanation. That will be key in achieving their goal, which is to sustain the gains achieved via CAMS during hospitalization and the first few weeks afterward out to 6 months and beyond.
The study was funded by the Menninger Foundation and other nonprofit organizations. Dr. Ellis reported having no financial conflicts.
ATLANTA – The suicide-specific intervention known as CAMS, or Collaborative Assessment and Management of Suicidality, provides protection against suicidal events during psychiatric hospitalization and in the first weeks post discharge, according to a study from the Menninger Clinic.
In this study, 52 patients who received CAMS in addition to the Menninger Clinic’s usual very intensive treatment regimen showed significantly greater improvement on measures of depression, suicidal ideation, hopelessness, and psychological flexibility at discharge, compared with 52 propensity-matched controls who receive the same regimen minus the CAMS. And, in the first 6 months after discharge, the CAMS group made half as many suicide attempts as did the controls, Thomas E. Ellis, Psy.D., reported at the annual conference of the American Association of Suicidology.
“We’re thinking that CAMS has some kind of buffering or protective effect in the weeks following discharge, which is the highest-risk period,” said Dr. Ellis, who is director of psychology at the Menninger Clinic and professor of psychiatry at the Baylor College of Medicine, both in Houston.
A total of 4%-7% of all suicides occurring in the United States each year happen in inpatient psychiatric settings. That’s a surprising statistic given that psychiatric hospitalization aims to provide a safe harbor for individuals bent on self-harm. Investigators have identified two sharp peaks of elevated risk of suicide in connection with psychiatric hospitalization: one in the first week after admission, the other in the first week after discharge. CAMS appears to flatten out these peaks, according to the psychologist.
CAMS is an intervention developed by David A. Jobes, Ph.D., professor of psychology at Catholic University in Washington. The intervention often is described as a theoretical framework in which suicide is seen as the primary focus rather than as a symptom. CAMS is nondenominational in terms of its psychotherapeutic orientation: It is being used today by therapists whose approach runs the gamut from psychodynamic to cognitive-behavioral therapy. The emphasis is on helping the patient problem-solve to find alternatives to suicide as a coping response and providing psychotherapy to address underlying vulnerabilities. A key element of CAMS is early assessment via the CAMS Suicide Status Form, which is used for problem identification and treatment planning, Dr. Ellis explained.
In recent years, he and Dr. Jobes have worked to adapt the CAMS approach to the unique setting of the Menninger Clinic. The clinic is a 100-bed private psychiatric hospital that often is seen as “a last chance” for patients who previously have been hospitalized elsewhere for multiple treatment-resistant conditions, often including substance abuse and personality disorders as well as refractory major depression. The average length of stay is 6-7 weeks – “that’s almost unheard of,” he noted – in contrast to the 3- to 7-day hospitalizations that are typical elsewhere.
The 104 study participants had a mean of 1.7 prior suicide attempts. The 52 controls were selected from a pool of 310 suicidal patients on the basis of propensity score matching by age, sex, treatment unit, severity of suicidal ideation, and number of previous suicide attempts. All subjects got the usual Menninger treatment package, which includes medications, group therapy, psychosocial groups, milieu therapy, nursing care, family counseling, vocational counseling, and individual psychotherapy. The only difference was that the individual psychotherapy included CAMS in 52 patients, while the other 52 received their individual psychotherapy from practitioners who were not involved in CAMS.
Both groups showed significant improvement during hospitalization, but the CAMS group showed significantly greater gains on measures of depression, suicidal ideation, and suicidal cognition.
However, when patients were reassessed at 3 and 6 months post discharge, the picture became more complex. The controls showed late improvement in these measures such that by 3 months’ follow-up, there were no longer significant differences between the two groups on measures of suicide ideation intention on the Columbia Suicide Severity Rating Scale, functional impairment on the World Health Organization Disability Assessment Scale, and thoughts of self-harm on the Patient Health Questionnaire-9 (PHQ-9). It’s not that the CAMS group was backsliding, they were in fact remaining stable on these measures post hospitalization while the controls were getting better. For example, depression scores on the PHQ-9 in the CAMS group were 8.8 at discharge, 9.2 at 3 months, and 9.5 at 6 months, in contrast to 13.7, 10.4, and 10.5 in controls.
Three suicide attempts occurred in the CAMS group post discharge at a mean of 53 and median of 57 days, compared with six attempts in controls at a mean of 38 and median 19 days. But while suicide attempts were fewer in number and occurred later in the CAMS group, these favorable outcome trends did not achieve statistical significance, as the study was not powered to look at that relatively infrequent endpoint, Dr. Ellis said.
To his dismay, rehospitalization rates were higher in the CAMS group: 0 versus 3.8% in controls during the first 2 weeks post discharge, but 9.8%, compared with 5.8% at 3 months and 15.4% vs. 7.6% in controls at 6 months.
Those differences are not statistically significant, but “the raw numbers are of concern,” Dr. Ellis said. He added that the rehospitalization data were compiled only quite recently, and he and his coinvestigators are still trying to figure out the explanation. That will be key in achieving their goal, which is to sustain the gains achieved via CAMS during hospitalization and the first few weeks afterward out to 6 months and beyond.
The study was funded by the Menninger Foundation and other nonprofit organizations. Dr. Ellis reported having no financial conflicts.
ATLANTA – The suicide-specific intervention known as CAMS, or Collaborative Assessment and Management of Suicidality, provides protection against suicidal events during psychiatric hospitalization and in the first weeks post discharge, according to a study from the Menninger Clinic.
In this study, 52 patients who received CAMS in addition to the Menninger Clinic’s usual very intensive treatment regimen showed significantly greater improvement on measures of depression, suicidal ideation, hopelessness, and psychological flexibility at discharge, compared with 52 propensity-matched controls who receive the same regimen minus the CAMS. And, in the first 6 months after discharge, the CAMS group made half as many suicide attempts as did the controls, Thomas E. Ellis, Psy.D., reported at the annual conference of the American Association of Suicidology.
“We’re thinking that CAMS has some kind of buffering or protective effect in the weeks following discharge, which is the highest-risk period,” said Dr. Ellis, who is director of psychology at the Menninger Clinic and professor of psychiatry at the Baylor College of Medicine, both in Houston.
A total of 4%-7% of all suicides occurring in the United States each year happen in inpatient psychiatric settings. That’s a surprising statistic given that psychiatric hospitalization aims to provide a safe harbor for individuals bent on self-harm. Investigators have identified two sharp peaks of elevated risk of suicide in connection with psychiatric hospitalization: one in the first week after admission, the other in the first week after discharge. CAMS appears to flatten out these peaks, according to the psychologist.
CAMS is an intervention developed by David A. Jobes, Ph.D., professor of psychology at Catholic University in Washington. The intervention often is described as a theoretical framework in which suicide is seen as the primary focus rather than as a symptom. CAMS is nondenominational in terms of its psychotherapeutic orientation: It is being used today by therapists whose approach runs the gamut from psychodynamic to cognitive-behavioral therapy. The emphasis is on helping the patient problem-solve to find alternatives to suicide as a coping response and providing psychotherapy to address underlying vulnerabilities. A key element of CAMS is early assessment via the CAMS Suicide Status Form, which is used for problem identification and treatment planning, Dr. Ellis explained.
In recent years, he and Dr. Jobes have worked to adapt the CAMS approach to the unique setting of the Menninger Clinic. The clinic is a 100-bed private psychiatric hospital that often is seen as “a last chance” for patients who previously have been hospitalized elsewhere for multiple treatment-resistant conditions, often including substance abuse and personality disorders as well as refractory major depression. The average length of stay is 6-7 weeks – “that’s almost unheard of,” he noted – in contrast to the 3- to 7-day hospitalizations that are typical elsewhere.
The 104 study participants had a mean of 1.7 prior suicide attempts. The 52 controls were selected from a pool of 310 suicidal patients on the basis of propensity score matching by age, sex, treatment unit, severity of suicidal ideation, and number of previous suicide attempts. All subjects got the usual Menninger treatment package, which includes medications, group therapy, psychosocial groups, milieu therapy, nursing care, family counseling, vocational counseling, and individual psychotherapy. The only difference was that the individual psychotherapy included CAMS in 52 patients, while the other 52 received their individual psychotherapy from practitioners who were not involved in CAMS.
Both groups showed significant improvement during hospitalization, but the CAMS group showed significantly greater gains on measures of depression, suicidal ideation, and suicidal cognition.
However, when patients were reassessed at 3 and 6 months post discharge, the picture became more complex. The controls showed late improvement in these measures such that by 3 months’ follow-up, there were no longer significant differences between the two groups on measures of suicide ideation intention on the Columbia Suicide Severity Rating Scale, functional impairment on the World Health Organization Disability Assessment Scale, and thoughts of self-harm on the Patient Health Questionnaire-9 (PHQ-9). It’s not that the CAMS group was backsliding, they were in fact remaining stable on these measures post hospitalization while the controls were getting better. For example, depression scores on the PHQ-9 in the CAMS group were 8.8 at discharge, 9.2 at 3 months, and 9.5 at 6 months, in contrast to 13.7, 10.4, and 10.5 in controls.
Three suicide attempts occurred in the CAMS group post discharge at a mean of 53 and median of 57 days, compared with six attempts in controls at a mean of 38 and median 19 days. But while suicide attempts were fewer in number and occurred later in the CAMS group, these favorable outcome trends did not achieve statistical significance, as the study was not powered to look at that relatively infrequent endpoint, Dr. Ellis said.
To his dismay, rehospitalization rates were higher in the CAMS group: 0 versus 3.8% in controls during the first 2 weeks post discharge, but 9.8%, compared with 5.8% at 3 months and 15.4% vs. 7.6% in controls at 6 months.
Those differences are not statistically significant, but “the raw numbers are of concern,” Dr. Ellis said. He added that the rehospitalization data were compiled only quite recently, and he and his coinvestigators are still trying to figure out the explanation. That will be key in achieving their goal, which is to sustain the gains achieved via CAMS during hospitalization and the first few weeks afterward out to 6 months and beyond.
The study was funded by the Menninger Foundation and other nonprofit organizations. Dr. Ellis reported having no financial conflicts.
AT THE ANNUAL AAS CONFERENCE
Key clinical point: CAMS therapy for suicidal inpatients markedly improves suicidal ideation and cognition at discharge.
Major finding: Mean scores on the Suicide Cognitions Scale improved markedly from 53.6 at admission to 23.3 at discharge in psychiatric inpatients who received the Collaborative Assessment and Management of Suicidality (CAMS), compared with a change from 59.9 to 50.8 in controls who received the same intensive therapy program minus CAMS.
Data source: Fifty-two suicidal inpatients at the Menninger Clinic who received CAMS were propensity score-matched with 52 controls who did not; all were followed for 6 months post discharge.
Disclosures: The study was funded by the Menninger Foundation and other nonprofit organizations. The presenter reported having no financial disclosures.
Growing buzz surrounds CGRP inhibitors in migraine
VALENCIA, SPAIN – In the lecture halls and corridors at the International Headache Congress, far and away the dominant topic of conversation was the latest highly promising data for the CGRP (calcitonin gene–related peptide)-inhibiting monoclonal antibodies being developed for migraine prophylaxis.
“The question of efficacy, to me, has been resolved: They’re spectacularly effective. The whole reason for the phase III studies is to establish safety,” Dr. Marcelo E. Bigal, a vice president at Teva Pharmaceuticals, said in the opening plenary session.
“This is the second revolution in the history of migraine therapy,” declared another opening plenary speaker, Dr. Messoud Ashina, professor of neurology at the University of Copenhagen.
“It is a really exciting emerging field. It kind of reminds me of the triptan story,” observed Richard J. Hargreaves, Ph.D., vice president for discovery science at Biogen in Cambridge, Mass.
With a long track record of success in new drug development and having conducted research on CGRP for more than 2 decades, it fell to Dr. Hargreaves to provide an introductory overview of the CGRP inhibitors. Each of the monoclonal antibodies has a different mechanism of action. However, they share several key characteristics: They are highly specific in their mechanisms of action, they have long circulating plasma half-lives, they are largely peripherally restricted rather than acting at the level of the central nervous system, and they typically have a low toxicity profile. Indeed, in phase I and -II studies the type and frequency of adverse events was essentially indistinguishable from placebo.
Now advancing through the developmental pipeline are three CGRP ligand-neutralizing antibodies and one CGRP receptor antibody.
“The race is on. It’s estimated that 40% of migraine patients are candidates for prophylaxis. That’s 14 million U.S. patients. And preventive therapy represents a significant unmet medical need,” Dr. Hargreaves said at the meeting sponsored by the International Headache Society and the American Headache Society.
“Clearly the CGRP monoclonal antibodies aren’t going to be used for acute migraine therapy. That’s going to be the province for oral small-molecule CGRP inhibitors because of the need for rapid activity. But if the antibodies prevent well, then hopefully the need for acute medications will go down,” he continued.
A particularly impressive feature of the investigational agents is that a substantial proportion of treated patients are hyperresponders – that is, individuals who experience at least a 75% and in some cases a 100% reduction in migraine days per month.
“Understanding these hyperresponders in the antibody trials is a real goal for the field. What’s the biomarker that predicts you can cure a patient of migraine headaches? How can you match an individual’s phenotype to the pharmacology of the medicine you’re giving them and get a better outcome?” he asked.
Dr. Hargreaves left his audience of headache specialists with another question to ponder: “If triptans inhibit CGRP release, and CGRP modulators, such as the monoclonal antibodies block CGRP’s action, then why aren’t triptans useful preventive agents? It’s something for the field to think about. I’ll leave you with the thought that maybe CGRP is the volume control for trigeminovascular sensory transmission.”
New data on three CGRP inhibitors was presented at the congress:
ALD403: A single 1,000-mg IV dose of this humanized IgG1 CGRP antibody produced lasting efficacy for 6 months in a phase II, randomized, double-blind, placebo-controlled study.
The study, conducted at 28 U.S. sites, included 163 patients with high-frequency episodic migraine, defined as an average of 5-14 migraine days per month.
One month post infusion, 51% of ALD403-treated patients and 24% of controls had a 75% reduction in monthly migraine days; in addition, 26% of the ALD403 group and 5% of controls had a 100% response, meaning they had no migraines.
At 12 weeks, 33% of the ALD403 group and 9% of controls had a 75% response, while 16% of ALD403-treated patients and zero controls had a 100% response.
At 24 weeks – again, after just a single dose – 26% of the ALD403 group and 7% of controls had a 75% response, while 11% of the active treatment group and no controls had a 100% response.
“There is relatively little difference between the 3-month and 24-week data. So in initial responders, the antibody is still working at 6 months. Some patients simply never had a migraine from the end of the needle to the end of the study,” said Dr. Jeffrey T.L. Smith, senior vice president at Alden BioPharmaceuticals in Bothell, Wash.
One audience member asked how the antibody can reduce the frequency of migraines so swiftly – starting within the first several weeks – when conventional prophylactic medications take months and months to work.
“An antibody having this high an affinity will shut down the biology of CGRP very quickly. The same is true for the very high–affinity anti-TNF and anti-interleukin-6 antibodies used in rheumatology. ALD403 will not only bind to free CGRP, it will actually pull CGRP off the receptor. Everything goes towards the antibody; it acts as a sink,” Dr. Smith replied.
This study was too small to identify predictors of hyperresponsiveness. Investigators hope to hunt down useful biomarkers in the large upcoming phase III studies, he added.
TEV-48125: Dr. Bigal presented the first-ever clinical trial of any CGRP-inhibiting antibody in patients with chronic migraine, defined as 15 or more headache days per month. All other studies to date have been conducted in patients with episodic migraine, who typically have far less cardiovascular disease and other comorbidities. Another unique feature of this trial was that current users of migraine preventive medications weren’t excluded; indeed, roughly half of participants were current users.
In this 263-patient, multicenter, double-blind, placebo-controlled, 3-month, phase II study, once-monthly subcutaneous TEV-48125 at 900 mg resulted in a 6.3-day reduction in the monthly number of moderate to severe headache days, while a regimen consisting of a 675-mg loading dose followed by 225 mg achieved a 6-day reduction, compared with baseline. Both of these outcomes were significantly better than the 4-day reduction seen with placebo.
Moreover, efficacy was evident just 1 week into the study. At that point, patients on either the high or low dose of TEV-48125 already showed a significantly greater reduction in the number of headache hours recorded in an electronic headache diary than did controls.
The monoclonal antibody–treated patients also resorted to acute medications significantly less frequently than did controls by a margin of roughly 2 fewer days per month.
The benefit of both TEV-48125 dosing regimens was equally robust regardless of whether they were on conventional prophylactic medications.
A 75% or greater reduction in monthly headache days was achieved in 32% of patients on the 900-mg dose, in nearly 30% of those on 675/225 mg, and in 16% of controls.
“These patients had suffered with frequent migraine for an average of 18 years, and now they come in and say, ‘I’m basically free of headaches for the first time in my life,’” Dr. Bigal said.
AMG 334: This fully human monoclonal antibody to the CGRP receptor was the focus of a multicenter, phase IIb, double-blind, dose-ranging study in 483 patients with 4-14 migraine days per month at baseline. Patients with comorbid depression and/or anxiety disorders were eligible to participate.
The most effective dose, and the one being carried forward into phase III, was 70 mg given subcutaneously once per month. The mean number of monthly migraine days was reduced by 3.4 days in patients on that regimen from a baseline of 8.7 days, significantly better than the 2.28-day reduction with placebo. In a prespecified secondary analysis, there was no difference in efficacy between patients with more than 8 monthly migraine days at baseline and those with fewer than 8 days, reported Dr. Robert Lenz of Amgen in Thousand Oaks, Calif.
The safety data showed no signal of any adverse event. Fewer than 3% of patients of the AMG 334 group discontinued treatment for any reason.
VALENCIA, SPAIN – In the lecture halls and corridors at the International Headache Congress, far and away the dominant topic of conversation was the latest highly promising data for the CGRP (calcitonin gene–related peptide)-inhibiting monoclonal antibodies being developed for migraine prophylaxis.
“The question of efficacy, to me, has been resolved: They’re spectacularly effective. The whole reason for the phase III studies is to establish safety,” Dr. Marcelo E. Bigal, a vice president at Teva Pharmaceuticals, said in the opening plenary session.
“This is the second revolution in the history of migraine therapy,” declared another opening plenary speaker, Dr. Messoud Ashina, professor of neurology at the University of Copenhagen.
“It is a really exciting emerging field. It kind of reminds me of the triptan story,” observed Richard J. Hargreaves, Ph.D., vice president for discovery science at Biogen in Cambridge, Mass.
With a long track record of success in new drug development and having conducted research on CGRP for more than 2 decades, it fell to Dr. Hargreaves to provide an introductory overview of the CGRP inhibitors. Each of the monoclonal antibodies has a different mechanism of action. However, they share several key characteristics: They are highly specific in their mechanisms of action, they have long circulating plasma half-lives, they are largely peripherally restricted rather than acting at the level of the central nervous system, and they typically have a low toxicity profile. Indeed, in phase I and -II studies the type and frequency of adverse events was essentially indistinguishable from placebo.
Now advancing through the developmental pipeline are three CGRP ligand-neutralizing antibodies and one CGRP receptor antibody.
“The race is on. It’s estimated that 40% of migraine patients are candidates for prophylaxis. That’s 14 million U.S. patients. And preventive therapy represents a significant unmet medical need,” Dr. Hargreaves said at the meeting sponsored by the International Headache Society and the American Headache Society.
“Clearly the CGRP monoclonal antibodies aren’t going to be used for acute migraine therapy. That’s going to be the province for oral small-molecule CGRP inhibitors because of the need for rapid activity. But if the antibodies prevent well, then hopefully the need for acute medications will go down,” he continued.
A particularly impressive feature of the investigational agents is that a substantial proportion of treated patients are hyperresponders – that is, individuals who experience at least a 75% and in some cases a 100% reduction in migraine days per month.
“Understanding these hyperresponders in the antibody trials is a real goal for the field. What’s the biomarker that predicts you can cure a patient of migraine headaches? How can you match an individual’s phenotype to the pharmacology of the medicine you’re giving them and get a better outcome?” he asked.
Dr. Hargreaves left his audience of headache specialists with another question to ponder: “If triptans inhibit CGRP release, and CGRP modulators, such as the monoclonal antibodies block CGRP’s action, then why aren’t triptans useful preventive agents? It’s something for the field to think about. I’ll leave you with the thought that maybe CGRP is the volume control for trigeminovascular sensory transmission.”
New data on three CGRP inhibitors was presented at the congress:
ALD403: A single 1,000-mg IV dose of this humanized IgG1 CGRP antibody produced lasting efficacy for 6 months in a phase II, randomized, double-blind, placebo-controlled study.
The study, conducted at 28 U.S. sites, included 163 patients with high-frequency episodic migraine, defined as an average of 5-14 migraine days per month.
One month post infusion, 51% of ALD403-treated patients and 24% of controls had a 75% reduction in monthly migraine days; in addition, 26% of the ALD403 group and 5% of controls had a 100% response, meaning they had no migraines.
At 12 weeks, 33% of the ALD403 group and 9% of controls had a 75% response, while 16% of ALD403-treated patients and zero controls had a 100% response.
At 24 weeks – again, after just a single dose – 26% of the ALD403 group and 7% of controls had a 75% response, while 11% of the active treatment group and no controls had a 100% response.
“There is relatively little difference between the 3-month and 24-week data. So in initial responders, the antibody is still working at 6 months. Some patients simply never had a migraine from the end of the needle to the end of the study,” said Dr. Jeffrey T.L. Smith, senior vice president at Alden BioPharmaceuticals in Bothell, Wash.
One audience member asked how the antibody can reduce the frequency of migraines so swiftly – starting within the first several weeks – when conventional prophylactic medications take months and months to work.
“An antibody having this high an affinity will shut down the biology of CGRP very quickly. The same is true for the very high–affinity anti-TNF and anti-interleukin-6 antibodies used in rheumatology. ALD403 will not only bind to free CGRP, it will actually pull CGRP off the receptor. Everything goes towards the antibody; it acts as a sink,” Dr. Smith replied.
This study was too small to identify predictors of hyperresponsiveness. Investigators hope to hunt down useful biomarkers in the large upcoming phase III studies, he added.
TEV-48125: Dr. Bigal presented the first-ever clinical trial of any CGRP-inhibiting antibody in patients with chronic migraine, defined as 15 or more headache days per month. All other studies to date have been conducted in patients with episodic migraine, who typically have far less cardiovascular disease and other comorbidities. Another unique feature of this trial was that current users of migraine preventive medications weren’t excluded; indeed, roughly half of participants were current users.
In this 263-patient, multicenter, double-blind, placebo-controlled, 3-month, phase II study, once-monthly subcutaneous TEV-48125 at 900 mg resulted in a 6.3-day reduction in the monthly number of moderate to severe headache days, while a regimen consisting of a 675-mg loading dose followed by 225 mg achieved a 6-day reduction, compared with baseline. Both of these outcomes were significantly better than the 4-day reduction seen with placebo.
Moreover, efficacy was evident just 1 week into the study. At that point, patients on either the high or low dose of TEV-48125 already showed a significantly greater reduction in the number of headache hours recorded in an electronic headache diary than did controls.
The monoclonal antibody–treated patients also resorted to acute medications significantly less frequently than did controls by a margin of roughly 2 fewer days per month.
The benefit of both TEV-48125 dosing regimens was equally robust regardless of whether they were on conventional prophylactic medications.
A 75% or greater reduction in monthly headache days was achieved in 32% of patients on the 900-mg dose, in nearly 30% of those on 675/225 mg, and in 16% of controls.
“These patients had suffered with frequent migraine for an average of 18 years, and now they come in and say, ‘I’m basically free of headaches for the first time in my life,’” Dr. Bigal said.
AMG 334: This fully human monoclonal antibody to the CGRP receptor was the focus of a multicenter, phase IIb, double-blind, dose-ranging study in 483 patients with 4-14 migraine days per month at baseline. Patients with comorbid depression and/or anxiety disorders were eligible to participate.
The most effective dose, and the one being carried forward into phase III, was 70 mg given subcutaneously once per month. The mean number of monthly migraine days was reduced by 3.4 days in patients on that regimen from a baseline of 8.7 days, significantly better than the 2.28-day reduction with placebo. In a prespecified secondary analysis, there was no difference in efficacy between patients with more than 8 monthly migraine days at baseline and those with fewer than 8 days, reported Dr. Robert Lenz of Amgen in Thousand Oaks, Calif.
The safety data showed no signal of any adverse event. Fewer than 3% of patients of the AMG 334 group discontinued treatment for any reason.
VALENCIA, SPAIN – In the lecture halls and corridors at the International Headache Congress, far and away the dominant topic of conversation was the latest highly promising data for the CGRP (calcitonin gene–related peptide)-inhibiting monoclonal antibodies being developed for migraine prophylaxis.
“The question of efficacy, to me, has been resolved: They’re spectacularly effective. The whole reason for the phase III studies is to establish safety,” Dr. Marcelo E. Bigal, a vice president at Teva Pharmaceuticals, said in the opening plenary session.
“This is the second revolution in the history of migraine therapy,” declared another opening plenary speaker, Dr. Messoud Ashina, professor of neurology at the University of Copenhagen.
“It is a really exciting emerging field. It kind of reminds me of the triptan story,” observed Richard J. Hargreaves, Ph.D., vice president for discovery science at Biogen in Cambridge, Mass.
With a long track record of success in new drug development and having conducted research on CGRP for more than 2 decades, it fell to Dr. Hargreaves to provide an introductory overview of the CGRP inhibitors. Each of the monoclonal antibodies has a different mechanism of action. However, they share several key characteristics: They are highly specific in their mechanisms of action, they have long circulating plasma half-lives, they are largely peripherally restricted rather than acting at the level of the central nervous system, and they typically have a low toxicity profile. Indeed, in phase I and -II studies the type and frequency of adverse events was essentially indistinguishable from placebo.
Now advancing through the developmental pipeline are three CGRP ligand-neutralizing antibodies and one CGRP receptor antibody.
“The race is on. It’s estimated that 40% of migraine patients are candidates for prophylaxis. That’s 14 million U.S. patients. And preventive therapy represents a significant unmet medical need,” Dr. Hargreaves said at the meeting sponsored by the International Headache Society and the American Headache Society.
“Clearly the CGRP monoclonal antibodies aren’t going to be used for acute migraine therapy. That’s going to be the province for oral small-molecule CGRP inhibitors because of the need for rapid activity. But if the antibodies prevent well, then hopefully the need for acute medications will go down,” he continued.
A particularly impressive feature of the investigational agents is that a substantial proportion of treated patients are hyperresponders – that is, individuals who experience at least a 75% and in some cases a 100% reduction in migraine days per month.
“Understanding these hyperresponders in the antibody trials is a real goal for the field. What’s the biomarker that predicts you can cure a patient of migraine headaches? How can you match an individual’s phenotype to the pharmacology of the medicine you’re giving them and get a better outcome?” he asked.
Dr. Hargreaves left his audience of headache specialists with another question to ponder: “If triptans inhibit CGRP release, and CGRP modulators, such as the monoclonal antibodies block CGRP’s action, then why aren’t triptans useful preventive agents? It’s something for the field to think about. I’ll leave you with the thought that maybe CGRP is the volume control for trigeminovascular sensory transmission.”
New data on three CGRP inhibitors was presented at the congress:
ALD403: A single 1,000-mg IV dose of this humanized IgG1 CGRP antibody produced lasting efficacy for 6 months in a phase II, randomized, double-blind, placebo-controlled study.
The study, conducted at 28 U.S. sites, included 163 patients with high-frequency episodic migraine, defined as an average of 5-14 migraine days per month.
One month post infusion, 51% of ALD403-treated patients and 24% of controls had a 75% reduction in monthly migraine days; in addition, 26% of the ALD403 group and 5% of controls had a 100% response, meaning they had no migraines.
At 12 weeks, 33% of the ALD403 group and 9% of controls had a 75% response, while 16% of ALD403-treated patients and zero controls had a 100% response.
At 24 weeks – again, after just a single dose – 26% of the ALD403 group and 7% of controls had a 75% response, while 11% of the active treatment group and no controls had a 100% response.
“There is relatively little difference between the 3-month and 24-week data. So in initial responders, the antibody is still working at 6 months. Some patients simply never had a migraine from the end of the needle to the end of the study,” said Dr. Jeffrey T.L. Smith, senior vice president at Alden BioPharmaceuticals in Bothell, Wash.
One audience member asked how the antibody can reduce the frequency of migraines so swiftly – starting within the first several weeks – when conventional prophylactic medications take months and months to work.
“An antibody having this high an affinity will shut down the biology of CGRP very quickly. The same is true for the very high–affinity anti-TNF and anti-interleukin-6 antibodies used in rheumatology. ALD403 will not only bind to free CGRP, it will actually pull CGRP off the receptor. Everything goes towards the antibody; it acts as a sink,” Dr. Smith replied.
This study was too small to identify predictors of hyperresponsiveness. Investigators hope to hunt down useful biomarkers in the large upcoming phase III studies, he added.
TEV-48125: Dr. Bigal presented the first-ever clinical trial of any CGRP-inhibiting antibody in patients with chronic migraine, defined as 15 or more headache days per month. All other studies to date have been conducted in patients with episodic migraine, who typically have far less cardiovascular disease and other comorbidities. Another unique feature of this trial was that current users of migraine preventive medications weren’t excluded; indeed, roughly half of participants were current users.
In this 263-patient, multicenter, double-blind, placebo-controlled, 3-month, phase II study, once-monthly subcutaneous TEV-48125 at 900 mg resulted in a 6.3-day reduction in the monthly number of moderate to severe headache days, while a regimen consisting of a 675-mg loading dose followed by 225 mg achieved a 6-day reduction, compared with baseline. Both of these outcomes were significantly better than the 4-day reduction seen with placebo.
Moreover, efficacy was evident just 1 week into the study. At that point, patients on either the high or low dose of TEV-48125 already showed a significantly greater reduction in the number of headache hours recorded in an electronic headache diary than did controls.
The monoclonal antibody–treated patients also resorted to acute medications significantly less frequently than did controls by a margin of roughly 2 fewer days per month.
The benefit of both TEV-48125 dosing regimens was equally robust regardless of whether they were on conventional prophylactic medications.
A 75% or greater reduction in monthly headache days was achieved in 32% of patients on the 900-mg dose, in nearly 30% of those on 675/225 mg, and in 16% of controls.
“These patients had suffered with frequent migraine for an average of 18 years, and now they come in and say, ‘I’m basically free of headaches for the first time in my life,’” Dr. Bigal said.
AMG 334: This fully human monoclonal antibody to the CGRP receptor was the focus of a multicenter, phase IIb, double-blind, dose-ranging study in 483 patients with 4-14 migraine days per month at baseline. Patients with comorbid depression and/or anxiety disorders were eligible to participate.
The most effective dose, and the one being carried forward into phase III, was 70 mg given subcutaneously once per month. The mean number of monthly migraine days was reduced by 3.4 days in patients on that regimen from a baseline of 8.7 days, significantly better than the 2.28-day reduction with placebo. In a prespecified secondary analysis, there was no difference in efficacy between patients with more than 8 monthly migraine days at baseline and those with fewer than 8 days, reported Dr. Robert Lenz of Amgen in Thousand Oaks, Calif.
The safety data showed no signal of any adverse event. Fewer than 3% of patients of the AMG 334 group discontinued treatment for any reason.
AT IHC 2015