User login
PAS: Early adverse childhood experiences linked to poor literacy
SAN DIEGO – Exposure to various forms of abuse or other early adverse childhood experiences are associated with below-average literacy and attention problems in kindergartners, which in turn are strong predictors of educational trajectory, according to Dr. Manuel Jimenez.
“These findings emphasize the importance of strategies that address the developmental needs of vulnerable children, including strengthening families and early literacy promotion,” said Dr. Jimenez of the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
It’s well established that adverse childhood experiences (ACEs) are associated with adult disability, social problems, and engagement in health-risky behaviors. Much less is known about the impact of early ACEs on the kindergarten experience. This was the impetus for Dr. Jimenez’s analysis of data from the Fragile Families and Child Wellbeing Study. He focused on an urban cohort of 1,008 children evaluated by teacher survey and mother report at age 5 years. Forty-six percent of the children were African American, and 57% of their mothers had a high school diploma or less.
The mother-reported adverse childhood events were defined as in an earlier Centers for Disease Control and Prevention study. There were eight under consideration: exposure to maternal depression, incarceration of a household member, household substance use, the mother having been treated violently, neglect, or physical, sexual, or psychological abuse.
The prevalence of the individual ACEs was in the 10%-16% range with the exception of sexual abuse, which was reported in just 0.6% of the cohort. Forty-eight percent of the children had experienced at least one ACE and 22% two or more.
In teacher ratings conducted in the final month of kindergarten, the presence of any two or more ACEs was associated with a 1.7-fold increased likelihood of below-average literacy skills, a 1.6-fold greater likelihood of below-average math skills, a 1.8-fold increase in child disability, and a 3-fold increase in the likelihood of being in the top 10% of the class in attention problems.
Taking a more in-depth look at the teacher ratings of emergent literary skills, children with two or more ACEs were 1.9-fold more likely to be rated as “not yet” able to easily and quickly name all uppercase and lowercase letters or as “beginning to” do so; 1.8-fold more likely to not yet be able to understand or interpret a story or other text read to them; and 1.7-fold more likely not to demonstrate an understanding of some of the conventions of print.
“It’s reasonable to assume that children at the end of their kindergarten year would have mastered, or be proficient in, each of these skills,” Dr. Jimenez said at the annual meeting of the Pediatric Academic Societies.
Children with two or more ACEs were also 4.4-fold more likely to score in the top 10% for attention problems as rated by parents using the Child Behavior Checklist attention subscale.
All of these significantly increased odds ratios were adjusted for potential confounders in multivariate logistic regression analysis, including race, sex, ethnicity, maternal education at childbirth, and household income, he noted.
One audience member asked whether Dr. Jimenez thinks it’s useful to screen for ACEs in clinical practice.
“We’ve actually thought a lot about this,” he replied. “With problems as big as these, it’s often possible to feel very small. But as pediatricians we really can make an incredible difference in children’s lives because we get to see kids so often. The Bright Futures project (brightfutures.aap.org) is filled with tools we can use to screen for and find ACEs as well as the adverse outcomes that are associated with them. And there is an opportunity to work with families and connect them with community resources that can help build resilience and strength. In my own line of work, I’ve been fortunate to be able to partner with community groups in New Brunswick. We’re actively trying to link pediatricians in the community – the medical home – to the resources that are out there.”
The Fragile Families and Child Wellbeing Study is supported by the National Institute of Child Health and Human Development. Dr. Jimenez reported having no relevant financial disclosures.
SAN DIEGO – Exposure to various forms of abuse or other early adverse childhood experiences are associated with below-average literacy and attention problems in kindergartners, which in turn are strong predictors of educational trajectory, according to Dr. Manuel Jimenez.
“These findings emphasize the importance of strategies that address the developmental needs of vulnerable children, including strengthening families and early literacy promotion,” said Dr. Jimenez of the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
It’s well established that adverse childhood experiences (ACEs) are associated with adult disability, social problems, and engagement in health-risky behaviors. Much less is known about the impact of early ACEs on the kindergarten experience. This was the impetus for Dr. Jimenez’s analysis of data from the Fragile Families and Child Wellbeing Study. He focused on an urban cohort of 1,008 children evaluated by teacher survey and mother report at age 5 years. Forty-six percent of the children were African American, and 57% of their mothers had a high school diploma or less.
The mother-reported adverse childhood events were defined as in an earlier Centers for Disease Control and Prevention study. There were eight under consideration: exposure to maternal depression, incarceration of a household member, household substance use, the mother having been treated violently, neglect, or physical, sexual, or psychological abuse.
The prevalence of the individual ACEs was in the 10%-16% range with the exception of sexual abuse, which was reported in just 0.6% of the cohort. Forty-eight percent of the children had experienced at least one ACE and 22% two or more.
In teacher ratings conducted in the final month of kindergarten, the presence of any two or more ACEs was associated with a 1.7-fold increased likelihood of below-average literacy skills, a 1.6-fold greater likelihood of below-average math skills, a 1.8-fold increase in child disability, and a 3-fold increase in the likelihood of being in the top 10% of the class in attention problems.
Taking a more in-depth look at the teacher ratings of emergent literary skills, children with two or more ACEs were 1.9-fold more likely to be rated as “not yet” able to easily and quickly name all uppercase and lowercase letters or as “beginning to” do so; 1.8-fold more likely to not yet be able to understand or interpret a story or other text read to them; and 1.7-fold more likely not to demonstrate an understanding of some of the conventions of print.
“It’s reasonable to assume that children at the end of their kindergarten year would have mastered, or be proficient in, each of these skills,” Dr. Jimenez said at the annual meeting of the Pediatric Academic Societies.
Children with two or more ACEs were also 4.4-fold more likely to score in the top 10% for attention problems as rated by parents using the Child Behavior Checklist attention subscale.
All of these significantly increased odds ratios were adjusted for potential confounders in multivariate logistic regression analysis, including race, sex, ethnicity, maternal education at childbirth, and household income, he noted.
One audience member asked whether Dr. Jimenez thinks it’s useful to screen for ACEs in clinical practice.
“We’ve actually thought a lot about this,” he replied. “With problems as big as these, it’s often possible to feel very small. But as pediatricians we really can make an incredible difference in children’s lives because we get to see kids so often. The Bright Futures project (brightfutures.aap.org) is filled with tools we can use to screen for and find ACEs as well as the adverse outcomes that are associated with them. And there is an opportunity to work with families and connect them with community resources that can help build resilience and strength. In my own line of work, I’ve been fortunate to be able to partner with community groups in New Brunswick. We’re actively trying to link pediatricians in the community – the medical home – to the resources that are out there.”
The Fragile Families and Child Wellbeing Study is supported by the National Institute of Child Health and Human Development. Dr. Jimenez reported having no relevant financial disclosures.
SAN DIEGO – Exposure to various forms of abuse or other early adverse childhood experiences are associated with below-average literacy and attention problems in kindergartners, which in turn are strong predictors of educational trajectory, according to Dr. Manuel Jimenez.
“These findings emphasize the importance of strategies that address the developmental needs of vulnerable children, including strengthening families and early literacy promotion,” said Dr. Jimenez of the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
It’s well established that adverse childhood experiences (ACEs) are associated with adult disability, social problems, and engagement in health-risky behaviors. Much less is known about the impact of early ACEs on the kindergarten experience. This was the impetus for Dr. Jimenez’s analysis of data from the Fragile Families and Child Wellbeing Study. He focused on an urban cohort of 1,008 children evaluated by teacher survey and mother report at age 5 years. Forty-six percent of the children were African American, and 57% of their mothers had a high school diploma or less.
The mother-reported adverse childhood events were defined as in an earlier Centers for Disease Control and Prevention study. There were eight under consideration: exposure to maternal depression, incarceration of a household member, household substance use, the mother having been treated violently, neglect, or physical, sexual, or psychological abuse.
The prevalence of the individual ACEs was in the 10%-16% range with the exception of sexual abuse, which was reported in just 0.6% of the cohort. Forty-eight percent of the children had experienced at least one ACE and 22% two or more.
In teacher ratings conducted in the final month of kindergarten, the presence of any two or more ACEs was associated with a 1.7-fold increased likelihood of below-average literacy skills, a 1.6-fold greater likelihood of below-average math skills, a 1.8-fold increase in child disability, and a 3-fold increase in the likelihood of being in the top 10% of the class in attention problems.
Taking a more in-depth look at the teacher ratings of emergent literary skills, children with two or more ACEs were 1.9-fold more likely to be rated as “not yet” able to easily and quickly name all uppercase and lowercase letters or as “beginning to” do so; 1.8-fold more likely to not yet be able to understand or interpret a story or other text read to them; and 1.7-fold more likely not to demonstrate an understanding of some of the conventions of print.
“It’s reasonable to assume that children at the end of their kindergarten year would have mastered, or be proficient in, each of these skills,” Dr. Jimenez said at the annual meeting of the Pediatric Academic Societies.
Children with two or more ACEs were also 4.4-fold more likely to score in the top 10% for attention problems as rated by parents using the Child Behavior Checklist attention subscale.
All of these significantly increased odds ratios were adjusted for potential confounders in multivariate logistic regression analysis, including race, sex, ethnicity, maternal education at childbirth, and household income, he noted.
One audience member asked whether Dr. Jimenez thinks it’s useful to screen for ACEs in clinical practice.
“We’ve actually thought a lot about this,” he replied. “With problems as big as these, it’s often possible to feel very small. But as pediatricians we really can make an incredible difference in children’s lives because we get to see kids so often. The Bright Futures project (brightfutures.aap.org) is filled with tools we can use to screen for and find ACEs as well as the adverse outcomes that are associated with them. And there is an opportunity to work with families and connect them with community resources that can help build resilience and strength. In my own line of work, I’ve been fortunate to be able to partner with community groups in New Brunswick. We’re actively trying to link pediatricians in the community – the medical home – to the resources that are out there.”
The Fragile Families and Child Wellbeing Study is supported by the National Institute of Child Health and Human Development. Dr. Jimenez reported having no relevant financial disclosures.
AT THE PAS ANNUAL MEETING
Key clinical point: Kindergartners with a history of two or more of a possible eight early adverse childhood experiences had below-average teacher ratings for literacy and high ratings for attention problems.
Major finding: Twenty-two percent of the kindergartners had two or more early adverse childhood experiences by parental report.
Data source: A cross-sectional analysis of teacher and parent ratings of 1,008 urban 5-year-olds.
Disclosures: The Fragile Families and Child Wellbeing Study is supported by the National Institute of Child Health and Human Development. Dr. Jimenez reported having no relevant financial disclosures.
PAS: For pediatric mental conditions, PCPs do the heavy lifting
SAN DIEGO – The bulk of outpatient pediatric mental health care is provided solely by primary care physicians, not psychiatrists, according to a national study.
The national data also showed that primary care physicians (PCPs) are more likely than psychiatrists to prescribe psychotropic medications for children and adolescents with mental health conditions, L. Elizabeth Anderson reported at the annual meeting of the Pediatric Academic Societies.
She presented an analysis of the Agency for Healthcare Quality and Research’s Medical Expenditure Panel Survey(MEPS), which contains data on a nationally representative cohort’s types of outpatient visits, the specialties of the physicians seen, conditions diagnosed, prescribed medications, and sociodemographic factors.
The MEPS data highlight just how commonly children and adolescents seek professional help for anxiety, depression, conduct disorders, and behavioral disorders. Extrapolating from this analysis of 1,778 youths aged 2-21 years with a mental disorder, an estimated 5.2% of U.S. children and adolescents have had one or more outpatient mental health visits within the past year, according to Ms. Anderson, a medical student at the University of Tennessee, Memphis.
Attention-deficit/hyperactivity disorder accounted for 65% of these outpatient visits, mood disorders 24%, anxiety 19%, pervasive developmental disorder 7%, and psychoses 1.3%. Patients could have more than one diagnosis.
Fully 35% of the youths received their outpatient care for mental health conditions solely from a PCP. Another 26% saw a psychiatrist only, and 15% saw a psychologist and/or social worker only. Roughly one-quarter of subjects saw multiple providers of mental health services, but patient comanagement by a PCP and psychiatrist occurred in only 6.7% of cases.
Primary care physicians appeared to be more comfortable serving as sole care providers for youths with ADHD than those with anxiety and/or mood disorders: 41% of youths with ADHD had a PCP as their sole provider, compared with 17% of those with an anxiety and/or mood disorder.
Sixty-nine percent of patients with a mental health disorder that was managed solely by a PCP received a prescription for psychotropic medication, as did 62% of those seen by a psychiatrist only. The difference in prescribing patterns was more marked in patients with ADHD: 73% of those treated by a PCP received a prescription, compared with 58% of those treated by a psychiatrist.
Thus, the likelihood that a patient with ADHD who was seen by a PCP would receive a prescription for a stimulant or atomoxetine was 65% greater than if seen by a psychiatrist, Ms. Anderson continued.
No consistent associations were seen between provider type or medication prescribing and sociodemographic factors including ethnicity, household income, or geographic locale.
With only 8.6 child and adolescent psychiatrists available per 100,000 U.S. pediatric patients, these study findings showing that PCPs now provide a substantial amount of pediatric mental health care provide a strong argument for programs aimed at improving quality of care via point-of-care advice to PCPs from mental health specialists, she said.
Ms. Anderson’s study was supported by a grant from the American Pediatric Society/Society for Pediatric Research student research training program. She reported having no financial conflicts.
SAN DIEGO – The bulk of outpatient pediatric mental health care is provided solely by primary care physicians, not psychiatrists, according to a national study.
The national data also showed that primary care physicians (PCPs) are more likely than psychiatrists to prescribe psychotropic medications for children and adolescents with mental health conditions, L. Elizabeth Anderson reported at the annual meeting of the Pediatric Academic Societies.
She presented an analysis of the Agency for Healthcare Quality and Research’s Medical Expenditure Panel Survey(MEPS), which contains data on a nationally representative cohort’s types of outpatient visits, the specialties of the physicians seen, conditions diagnosed, prescribed medications, and sociodemographic factors.
The MEPS data highlight just how commonly children and adolescents seek professional help for anxiety, depression, conduct disorders, and behavioral disorders. Extrapolating from this analysis of 1,778 youths aged 2-21 years with a mental disorder, an estimated 5.2% of U.S. children and adolescents have had one or more outpatient mental health visits within the past year, according to Ms. Anderson, a medical student at the University of Tennessee, Memphis.
Attention-deficit/hyperactivity disorder accounted for 65% of these outpatient visits, mood disorders 24%, anxiety 19%, pervasive developmental disorder 7%, and psychoses 1.3%. Patients could have more than one diagnosis.
Fully 35% of the youths received their outpatient care for mental health conditions solely from a PCP. Another 26% saw a psychiatrist only, and 15% saw a psychologist and/or social worker only. Roughly one-quarter of subjects saw multiple providers of mental health services, but patient comanagement by a PCP and psychiatrist occurred in only 6.7% of cases.
Primary care physicians appeared to be more comfortable serving as sole care providers for youths with ADHD than those with anxiety and/or mood disorders: 41% of youths with ADHD had a PCP as their sole provider, compared with 17% of those with an anxiety and/or mood disorder.
Sixty-nine percent of patients with a mental health disorder that was managed solely by a PCP received a prescription for psychotropic medication, as did 62% of those seen by a psychiatrist only. The difference in prescribing patterns was more marked in patients with ADHD: 73% of those treated by a PCP received a prescription, compared with 58% of those treated by a psychiatrist.
Thus, the likelihood that a patient with ADHD who was seen by a PCP would receive a prescription for a stimulant or atomoxetine was 65% greater than if seen by a psychiatrist, Ms. Anderson continued.
No consistent associations were seen between provider type or medication prescribing and sociodemographic factors including ethnicity, household income, or geographic locale.
With only 8.6 child and adolescent psychiatrists available per 100,000 U.S. pediatric patients, these study findings showing that PCPs now provide a substantial amount of pediatric mental health care provide a strong argument for programs aimed at improving quality of care via point-of-care advice to PCPs from mental health specialists, she said.
Ms. Anderson’s study was supported by a grant from the American Pediatric Society/Society for Pediatric Research student research training program. She reported having no financial conflicts.
SAN DIEGO – The bulk of outpatient pediatric mental health care is provided solely by primary care physicians, not psychiatrists, according to a national study.
The national data also showed that primary care physicians (PCPs) are more likely than psychiatrists to prescribe psychotropic medications for children and adolescents with mental health conditions, L. Elizabeth Anderson reported at the annual meeting of the Pediatric Academic Societies.
She presented an analysis of the Agency for Healthcare Quality and Research’s Medical Expenditure Panel Survey(MEPS), which contains data on a nationally representative cohort’s types of outpatient visits, the specialties of the physicians seen, conditions diagnosed, prescribed medications, and sociodemographic factors.
The MEPS data highlight just how commonly children and adolescents seek professional help for anxiety, depression, conduct disorders, and behavioral disorders. Extrapolating from this analysis of 1,778 youths aged 2-21 years with a mental disorder, an estimated 5.2% of U.S. children and adolescents have had one or more outpatient mental health visits within the past year, according to Ms. Anderson, a medical student at the University of Tennessee, Memphis.
Attention-deficit/hyperactivity disorder accounted for 65% of these outpatient visits, mood disorders 24%, anxiety 19%, pervasive developmental disorder 7%, and psychoses 1.3%. Patients could have more than one diagnosis.
Fully 35% of the youths received their outpatient care for mental health conditions solely from a PCP. Another 26% saw a psychiatrist only, and 15% saw a psychologist and/or social worker only. Roughly one-quarter of subjects saw multiple providers of mental health services, but patient comanagement by a PCP and psychiatrist occurred in only 6.7% of cases.
Primary care physicians appeared to be more comfortable serving as sole care providers for youths with ADHD than those with anxiety and/or mood disorders: 41% of youths with ADHD had a PCP as their sole provider, compared with 17% of those with an anxiety and/or mood disorder.
Sixty-nine percent of patients with a mental health disorder that was managed solely by a PCP received a prescription for psychotropic medication, as did 62% of those seen by a psychiatrist only. The difference in prescribing patterns was more marked in patients with ADHD: 73% of those treated by a PCP received a prescription, compared with 58% of those treated by a psychiatrist.
Thus, the likelihood that a patient with ADHD who was seen by a PCP would receive a prescription for a stimulant or atomoxetine was 65% greater than if seen by a psychiatrist, Ms. Anderson continued.
No consistent associations were seen between provider type or medication prescribing and sociodemographic factors including ethnicity, household income, or geographic locale.
With only 8.6 child and adolescent psychiatrists available per 100,000 U.S. pediatric patients, these study findings showing that PCPs now provide a substantial amount of pediatric mental health care provide a strong argument for programs aimed at improving quality of care via point-of-care advice to PCPs from mental health specialists, she said.
Ms. Anderson’s study was supported by a grant from the American Pediatric Society/Society for Pediatric Research student research training program. She reported having no financial conflicts.
AT THE PAS ANNUAL MEETING
Key clinical point: Primary care physicians provide a substantial amount of pediatric outpatient care for mental health conditions.
Major finding: 35% of U.S. youths receiving outpatient care for a mental health condition get it solely from a primary care physician, while 26% see a psychiatrist only.
Data source: This study analyzed national data from the Agency for Healthcare Quality and Research’s Medical Expenditure Panel Survey.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was funded free of commercial support.
PAS: Brief neonatal antibiotic exposure disrupts gut microbiota long term
SAN DIEGO – As little as 2-3 days of neonatal antibiotic exposure are sufficient to disrupt the development of the gut microbiota at 1 and 6 months of age, according to a case-control study.
The study design enabled investigators to conclude that the aberrant gut colonization was due to the brief course of antibiotics rather than the supposed infection for which the drugs were prescribed, Dr. Samuli Rautava said at the annual meeting of the Pediatric Academic Societies.
“I’m surprised that all of the effects on the microbiota we see are more prominent at 6 months of age than at 1 month. We do not have direct evidence that these relatively long-term perturbations of the gut microbiota lead to increased risk of disease, but if you go through the literature, I think it’s worrisome,” said Dr. Rautava, a pediatrician at the University of Turku (Finland).
He noted that prior studies have linked early antibiotic exposure to increased risks of developing necrotizing enterocolitis, asthma, inflammatory bowel disease, and most recently obesity (Pediatrics 2015;135:617-26).
Dr. Rautava reported on a prospective comparison of gut microbiota development in 36 term or late-preterm infants. The study population consisted of 6 infants with a documented infection for which they received a 7-day course of gentamicin or penicillin G beginning within the first 3 days after birth, 6 infants who received the same drugs on an empiric basis for up to 2-3 days before treatment was discontinued because infection had been ruled out, and 24 non–antibiotic exposed controls matched for duration of breastfeeding, mode of birth, and maternal use of probiotics.
The purpose of the study was to tease out the relative impact on gut microbiota development of early antibiotic exposure versus the infections for which the drugs are given. Gut microbiota composition was analyzed from fecal samples assessed at 1 and 6 months of age by quantitative polymerase chain reaction, 16S rRNA pyrosequencing, and denaturing gradient gel electrophoresis.
Among the key findings: Both groups of antibiotic-exposed neonates had markedly low levels of Bifidobacterium species, including B. bifidum, at 1 and 6 months. These are “good” gut microbes, and low levels early in life have been associated with obesity and allergies.
Bacteroides species were virtually absent at 1 month of age in both groups with early antibiotic exposure. Again, low levels early in life have been linked to obesity and allergies.
“The situation is a little better at age 6 months, but there is still some scarcity of Bacteroides species in these infants at that time,” according to the pediatrician.
In contrast, Clostridium species are more abundant in the gut microbiota of antibiotic-exposed children at both 1 and 6 months than in controls. This makes sense, since gentamicin and penicillin G are not effective against Clostridium species, which have been linked to disease.
Dr. Rautava said these study findings have significant implications for clinical practice: “We cannot not give antibiotics to infants who might have sepsis, but we need to develop means of finding the bacterially infected neonates who truly need the antibiotics so we can avoid unnecessary antibiotic exposure during this vulnerable period.”
“I told my post-doc when we did this study that the scientist in me wants to see huge changes in the gut microbiota, but the clinician in me wants to see no changes at all. So I’m torn: I’m happy to have something to report, but I’m devastated at what we’re actually doing with our early antibiotic therapy,” Dr. Rautava said.
Dr. Rautava’s study was funded by the University of Turku and other noncommercial sources. He reported having no financial conflicts.
SAN DIEGO – As little as 2-3 days of neonatal antibiotic exposure are sufficient to disrupt the development of the gut microbiota at 1 and 6 months of age, according to a case-control study.
The study design enabled investigators to conclude that the aberrant gut colonization was due to the brief course of antibiotics rather than the supposed infection for which the drugs were prescribed, Dr. Samuli Rautava said at the annual meeting of the Pediatric Academic Societies.
“I’m surprised that all of the effects on the microbiota we see are more prominent at 6 months of age than at 1 month. We do not have direct evidence that these relatively long-term perturbations of the gut microbiota lead to increased risk of disease, but if you go through the literature, I think it’s worrisome,” said Dr. Rautava, a pediatrician at the University of Turku (Finland).
He noted that prior studies have linked early antibiotic exposure to increased risks of developing necrotizing enterocolitis, asthma, inflammatory bowel disease, and most recently obesity (Pediatrics 2015;135:617-26).
Dr. Rautava reported on a prospective comparison of gut microbiota development in 36 term or late-preterm infants. The study population consisted of 6 infants with a documented infection for which they received a 7-day course of gentamicin or penicillin G beginning within the first 3 days after birth, 6 infants who received the same drugs on an empiric basis for up to 2-3 days before treatment was discontinued because infection had been ruled out, and 24 non–antibiotic exposed controls matched for duration of breastfeeding, mode of birth, and maternal use of probiotics.
The purpose of the study was to tease out the relative impact on gut microbiota development of early antibiotic exposure versus the infections for which the drugs are given. Gut microbiota composition was analyzed from fecal samples assessed at 1 and 6 months of age by quantitative polymerase chain reaction, 16S rRNA pyrosequencing, and denaturing gradient gel electrophoresis.
Among the key findings: Both groups of antibiotic-exposed neonates had markedly low levels of Bifidobacterium species, including B. bifidum, at 1 and 6 months. These are “good” gut microbes, and low levels early in life have been associated with obesity and allergies.
Bacteroides species were virtually absent at 1 month of age in both groups with early antibiotic exposure. Again, low levels early in life have been linked to obesity and allergies.
“The situation is a little better at age 6 months, but there is still some scarcity of Bacteroides species in these infants at that time,” according to the pediatrician.
In contrast, Clostridium species are more abundant in the gut microbiota of antibiotic-exposed children at both 1 and 6 months than in controls. This makes sense, since gentamicin and penicillin G are not effective against Clostridium species, which have been linked to disease.
Dr. Rautava said these study findings have significant implications for clinical practice: “We cannot not give antibiotics to infants who might have sepsis, but we need to develop means of finding the bacterially infected neonates who truly need the antibiotics so we can avoid unnecessary antibiotic exposure during this vulnerable period.”
“I told my post-doc when we did this study that the scientist in me wants to see huge changes in the gut microbiota, but the clinician in me wants to see no changes at all. So I’m torn: I’m happy to have something to report, but I’m devastated at what we’re actually doing with our early antibiotic therapy,” Dr. Rautava said.
Dr. Rautava’s study was funded by the University of Turku and other noncommercial sources. He reported having no financial conflicts.
SAN DIEGO – As little as 2-3 days of neonatal antibiotic exposure are sufficient to disrupt the development of the gut microbiota at 1 and 6 months of age, according to a case-control study.
The study design enabled investigators to conclude that the aberrant gut colonization was due to the brief course of antibiotics rather than the supposed infection for which the drugs were prescribed, Dr. Samuli Rautava said at the annual meeting of the Pediatric Academic Societies.
“I’m surprised that all of the effects on the microbiota we see are more prominent at 6 months of age than at 1 month. We do not have direct evidence that these relatively long-term perturbations of the gut microbiota lead to increased risk of disease, but if you go through the literature, I think it’s worrisome,” said Dr. Rautava, a pediatrician at the University of Turku (Finland).
He noted that prior studies have linked early antibiotic exposure to increased risks of developing necrotizing enterocolitis, asthma, inflammatory bowel disease, and most recently obesity (Pediatrics 2015;135:617-26).
Dr. Rautava reported on a prospective comparison of gut microbiota development in 36 term or late-preterm infants. The study population consisted of 6 infants with a documented infection for which they received a 7-day course of gentamicin or penicillin G beginning within the first 3 days after birth, 6 infants who received the same drugs on an empiric basis for up to 2-3 days before treatment was discontinued because infection had been ruled out, and 24 non–antibiotic exposed controls matched for duration of breastfeeding, mode of birth, and maternal use of probiotics.
The purpose of the study was to tease out the relative impact on gut microbiota development of early antibiotic exposure versus the infections for which the drugs are given. Gut microbiota composition was analyzed from fecal samples assessed at 1 and 6 months of age by quantitative polymerase chain reaction, 16S rRNA pyrosequencing, and denaturing gradient gel electrophoresis.
Among the key findings: Both groups of antibiotic-exposed neonates had markedly low levels of Bifidobacterium species, including B. bifidum, at 1 and 6 months. These are “good” gut microbes, and low levels early in life have been associated with obesity and allergies.
Bacteroides species were virtually absent at 1 month of age in both groups with early antibiotic exposure. Again, low levels early in life have been linked to obesity and allergies.
“The situation is a little better at age 6 months, but there is still some scarcity of Bacteroides species in these infants at that time,” according to the pediatrician.
In contrast, Clostridium species are more abundant in the gut microbiota of antibiotic-exposed children at both 1 and 6 months than in controls. This makes sense, since gentamicin and penicillin G are not effective against Clostridium species, which have been linked to disease.
Dr. Rautava said these study findings have significant implications for clinical practice: “We cannot not give antibiotics to infants who might have sepsis, but we need to develop means of finding the bacterially infected neonates who truly need the antibiotics so we can avoid unnecessary antibiotic exposure during this vulnerable period.”
“I told my post-doc when we did this study that the scientist in me wants to see huge changes in the gut microbiota, but the clinician in me wants to see no changes at all. So I’m torn: I’m happy to have something to report, but I’m devastated at what we’re actually doing with our early antibiotic therapy,” Dr. Rautava said.
Dr. Rautava’s study was funded by the University of Turku and other noncommercial sources. He reported having no financial conflicts.
AT THE PAS ANNUAL MEETING
Key clinical point: Even a couple of days of exposure to antibiotics in the early neonatal period has a long-term adverse impact upon development of the gut microbiota.
Major finding: Particularly striking was the increase in Clostridium species along with low levels of Bifidobacterium and Bacteroides species at 6 months of age in the infants exposed to antibiotics as neonates.
Data source: This was a prospective case-control study involving 6 infants given a course of antibiotics for documented infections within the first 3 days of life, another 6 infants who were briefly on empiric therapy with the same antibiotics until infection was ruled out, and 24 matched controls.
Disclosures: The study was funded by the University of Turku and other noncommercial sources. The presenter reported having no financial conflicts.
PAS: Even 1 hour TV daily linked to kindergartner obesity
SAN DIEGO – Watching at least 1 hour of television daily was associated with increased likelihood of overweight and obesity in kindergartners and first graders in a large national longitudinal study.
The American Academy of Pediatrics recommends that children watch less than 2 hours of TV daily, but this new analysis indicates that watching at least 1 hour but less than 2 hours is associated with unhealthy weight gain, Dr. Mark D. DeBoer said at the annual meeting of the Pediatric Academic Societies.
“The AAP may wish to consider reducing the recommended limits of TV viewing, with an emphasis on replacing this time with physical activities and learning opportunities,” declared Dr. DeBoer, a pediatrician at the University of Virginia, Charlottesville.
He and his coinvestigators analyzed data on 12,389 young children participating in the nationally representative Early Childhood Longitudinal Study-Kindergarten Cohort 2011. The study collected parentally reported hours and minutes of weekly daytime TV watching and determined body mass index z scores when the children were in kindergarten and again a year later as first graders.
As kindergartners, participants averaged 2.0 hours of TV per day, with 33% of the children exceeding the AAP-recommended 2-hour limit. As first graders, they averaged 1.6 hours of TV daily, and 15.7% of them exceeded 2 hours; however, when the first graders’ computer screen time was added in, 36.7% of them exceeded the AAP recommendation.
In a logistic regression analysis adjusted for race/ethnicity, gender, socioeconomic status, and amount of computer use, kindergartners who watched at least 1 hour and less than 2 hours of TV daily were 43% more likely to be overweight and 47% more likely to be obese than their peers who watched less than 1 hour. Moreover, kindergartners who watched 2 or more hours of TV per day were 58% and 75% more likely to be overweight and obese, respectively, than those who watched less than 1 hour.
A similar cross-sectional analysis conducted during first grade showed much the same: Children who watched at least 1 but less than 2 hours of TV daily were 40% more likely to be overweight and 48% more likely to be obese than those watching less than 1 hour per day. Children who watched at least 2 hours of TV daily were 53% and 61% more likely to be overweight and obese, respectively.
Dr. DeBoer and coworkers also conducted a longitudinal analysis examining the relationship between TV time and change in BMI z scores over the course of 1 year of follow-up from kindergarten and first grade. The group watching less than 1 hour of TV per day showed a slight decrease in BMI z score during the year, while those watching 1 to less than 2 hours had a mean 0.16-point increase in z score, which translates into roughly a 0.3-kg weight gain for an average-height child in this age group.
Computer use and video game time had no association with weight status.
The mechanism for the relationship between TV viewing and weight gain in children is believed to involve the sedentary nature of the activity along with exposure to advertisements for snack foods.
Several audience members rose to take issue with Dr. DeBoer’s call for the AAP to lower its recommended maximum amount of TV viewing.
“You’ve described an association,” one pediatrician said. “It isn’t necessarily the case that if you reduce the TV time, you reduce obesity in these individuals.”
Dr. DeBoer agreed that the observed association “may or may not be causal,” but he noted further, “There have been some studies that targeted reducing TV viewing that have shown favorable effects on BMI over time, so that is supportive.”
The Early Childhood Longitudinal Study-Kindergarten Cohort 2011 is funded by the U.S. Department of Education’s National Center for Education Statistics. The TV time/body weight analysis was funded by the Doris Duke Foundation. Dr. DeBoer reported having no financial conflicts.
SAN DIEGO – Watching at least 1 hour of television daily was associated with increased likelihood of overweight and obesity in kindergartners and first graders in a large national longitudinal study.
The American Academy of Pediatrics recommends that children watch less than 2 hours of TV daily, but this new analysis indicates that watching at least 1 hour but less than 2 hours is associated with unhealthy weight gain, Dr. Mark D. DeBoer said at the annual meeting of the Pediatric Academic Societies.
“The AAP may wish to consider reducing the recommended limits of TV viewing, with an emphasis on replacing this time with physical activities and learning opportunities,” declared Dr. DeBoer, a pediatrician at the University of Virginia, Charlottesville.
He and his coinvestigators analyzed data on 12,389 young children participating in the nationally representative Early Childhood Longitudinal Study-Kindergarten Cohort 2011. The study collected parentally reported hours and minutes of weekly daytime TV watching and determined body mass index z scores when the children were in kindergarten and again a year later as first graders.
As kindergartners, participants averaged 2.0 hours of TV per day, with 33% of the children exceeding the AAP-recommended 2-hour limit. As first graders, they averaged 1.6 hours of TV daily, and 15.7% of them exceeded 2 hours; however, when the first graders’ computer screen time was added in, 36.7% of them exceeded the AAP recommendation.
In a logistic regression analysis adjusted for race/ethnicity, gender, socioeconomic status, and amount of computer use, kindergartners who watched at least 1 hour and less than 2 hours of TV daily were 43% more likely to be overweight and 47% more likely to be obese than their peers who watched less than 1 hour. Moreover, kindergartners who watched 2 or more hours of TV per day were 58% and 75% more likely to be overweight and obese, respectively, than those who watched less than 1 hour.
A similar cross-sectional analysis conducted during first grade showed much the same: Children who watched at least 1 but less than 2 hours of TV daily were 40% more likely to be overweight and 48% more likely to be obese than those watching less than 1 hour per day. Children who watched at least 2 hours of TV daily were 53% and 61% more likely to be overweight and obese, respectively.
Dr. DeBoer and coworkers also conducted a longitudinal analysis examining the relationship between TV time and change in BMI z scores over the course of 1 year of follow-up from kindergarten and first grade. The group watching less than 1 hour of TV per day showed a slight decrease in BMI z score during the year, while those watching 1 to less than 2 hours had a mean 0.16-point increase in z score, which translates into roughly a 0.3-kg weight gain for an average-height child in this age group.
Computer use and video game time had no association with weight status.
The mechanism for the relationship between TV viewing and weight gain in children is believed to involve the sedentary nature of the activity along with exposure to advertisements for snack foods.
Several audience members rose to take issue with Dr. DeBoer’s call for the AAP to lower its recommended maximum amount of TV viewing.
“You’ve described an association,” one pediatrician said. “It isn’t necessarily the case that if you reduce the TV time, you reduce obesity in these individuals.”
Dr. DeBoer agreed that the observed association “may or may not be causal,” but he noted further, “There have been some studies that targeted reducing TV viewing that have shown favorable effects on BMI over time, so that is supportive.”
The Early Childhood Longitudinal Study-Kindergarten Cohort 2011 is funded by the U.S. Department of Education’s National Center for Education Statistics. The TV time/body weight analysis was funded by the Doris Duke Foundation. Dr. DeBoer reported having no financial conflicts.
SAN DIEGO – Watching at least 1 hour of television daily was associated with increased likelihood of overweight and obesity in kindergartners and first graders in a large national longitudinal study.
The American Academy of Pediatrics recommends that children watch less than 2 hours of TV daily, but this new analysis indicates that watching at least 1 hour but less than 2 hours is associated with unhealthy weight gain, Dr. Mark D. DeBoer said at the annual meeting of the Pediatric Academic Societies.
“The AAP may wish to consider reducing the recommended limits of TV viewing, with an emphasis on replacing this time with physical activities and learning opportunities,” declared Dr. DeBoer, a pediatrician at the University of Virginia, Charlottesville.
He and his coinvestigators analyzed data on 12,389 young children participating in the nationally representative Early Childhood Longitudinal Study-Kindergarten Cohort 2011. The study collected parentally reported hours and minutes of weekly daytime TV watching and determined body mass index z scores when the children were in kindergarten and again a year later as first graders.
As kindergartners, participants averaged 2.0 hours of TV per day, with 33% of the children exceeding the AAP-recommended 2-hour limit. As first graders, they averaged 1.6 hours of TV daily, and 15.7% of them exceeded 2 hours; however, when the first graders’ computer screen time was added in, 36.7% of them exceeded the AAP recommendation.
In a logistic regression analysis adjusted for race/ethnicity, gender, socioeconomic status, and amount of computer use, kindergartners who watched at least 1 hour and less than 2 hours of TV daily were 43% more likely to be overweight and 47% more likely to be obese than their peers who watched less than 1 hour. Moreover, kindergartners who watched 2 or more hours of TV per day were 58% and 75% more likely to be overweight and obese, respectively, than those who watched less than 1 hour.
A similar cross-sectional analysis conducted during first grade showed much the same: Children who watched at least 1 but less than 2 hours of TV daily were 40% more likely to be overweight and 48% more likely to be obese than those watching less than 1 hour per day. Children who watched at least 2 hours of TV daily were 53% and 61% more likely to be overweight and obese, respectively.
Dr. DeBoer and coworkers also conducted a longitudinal analysis examining the relationship between TV time and change in BMI z scores over the course of 1 year of follow-up from kindergarten and first grade. The group watching less than 1 hour of TV per day showed a slight decrease in BMI z score during the year, while those watching 1 to less than 2 hours had a mean 0.16-point increase in z score, which translates into roughly a 0.3-kg weight gain for an average-height child in this age group.
Computer use and video game time had no association with weight status.
The mechanism for the relationship between TV viewing and weight gain in children is believed to involve the sedentary nature of the activity along with exposure to advertisements for snack foods.
Several audience members rose to take issue with Dr. DeBoer’s call for the AAP to lower its recommended maximum amount of TV viewing.
“You’ve described an association,” one pediatrician said. “It isn’t necessarily the case that if you reduce the TV time, you reduce obesity in these individuals.”
Dr. DeBoer agreed that the observed association “may or may not be causal,” but he noted further, “There have been some studies that targeted reducing TV viewing that have shown favorable effects on BMI over time, so that is supportive.”
The Early Childhood Longitudinal Study-Kindergarten Cohort 2011 is funded by the U.S. Department of Education’s National Center for Education Statistics. The TV time/body weight analysis was funded by the Doris Duke Foundation. Dr. DeBoer reported having no financial conflicts.
AT THE PAS ANNUAL MEETING
Key clinical point: The American Academy of Pediatrics’ recommended maximum of less than 2 hours of television viewing per day may be set too high.
Major finding: Kindergartners and first graders watching at least 1 hour of television per day are 40%-76% more likely to be overweight or obese than their peers watching less.
Data source: This analysis included more than 12,000 U.S. children participating in the Early Childhood Longitudinal Study.
Disclosures: The presenter reported having no relevant financial conflicts.
PAS: Psychotropic polypharmacy widespread in pediatric primary care
SAN DIEGO – Of children and teens on psychotropic medication prescribed by their primary care pediatrician or family physician, 21% simultaneously receive two or more different classes of the drugs for at least 3 months, according to a massive study.
“Given that the safety of pediatric monotherapy for some of these psychotropic medications is unexplored, these patterns of polypharmacy prescribing are alarming and warrant further investigation,” Dr. Robert Grundmeier said in presenting the study results at the annual meeting of the Pediatric Academic Societies.
He presented a study of 891,717 patients up to 18 years of age who received health care at 1 of 222 primary care pediatric or family medicine sites participating in the Comparative Effectiveness Research Through Collaborative Electronic Reporting (CER2) Consortium. He described CER2 as a “supernetwork” comprised of multiple electronic health record–based networks and led by the American Academy of Pediatrics. The consortium was designed to carry out very large pharmacoepidemiologic and comparative effectiveness studies.
The initial study population consisted of more than 1.2 million patients up to age 18 years, a figure that investigators whittled down to just under 900,000 by excluding those with a seizure disorder or less than 12 months of follow-up data.
Among the 891,717 subjects, 8.3% of boys and 5.3% of girls were treated with psychotropic medication by their primary care physician at some point. Of these 60,430 patients, 21% simultaneously received psychotropic drugs from two or more different classes for at least 3 months. Another 5,813 patients – 0.9% of all boys and 0.4% of all girls in the study – got psychotropic medications from three or more classes simultaneously, reported Dr. Grundmeier of Children’s Hospital of Philadelphia.
The most frequently prescribed category of medication was stimulants/
atomoxetine, a treatment mainstay in attention-deficit/hyperactivity disorder. Antidepressants were No. 2 and were prescribed in 2.1% of all boys and 2.8% of girls. Atypical antipsychotics were prescribed for 1.4% of boys and 0.8% of girls.
The most widely prescribed two-class combination among patients receiving psychotropic medication was a stimulant and an alpha-agonist, given to 10.7% of boys and 5.6% of girls. The second most popular two-drug combination was a stimulant and an antidepressant, which was prescribed for 7.3% of boys and 7.1% of girls who took psychotropic medication.
The use of combination therapy incorporating an atypical antipsychotic was of particular interest because health policy makers have identified this as an issue of concern. The drugs aren’t approved by the Food and Drug Administration for patients younger than 5 years old, and yet the Agency for Healthcare Research and Quality has reported that the use of these medications in very young children with behavior problems doubled between 1999-2001 and 2007. Moreover, more than three-quarters of youths on Medicaid are taking an atypical antipsychotic for non–FDA-approved indications, including ADHD and aggressive behavior, according to Centers for Medicare & Medicaid Services data.
Dr. Grundmeier reported that a stimulant and an atypical antipsychotic were prescribed together for 7.5% of boys and 4.4% of girls on psychotropic medication, and an antidepressant and an atypical antipsychotic were given to 4.9% of boys and 6.4% of girls.
These CER2 data probably underestimate the true rate of psychotropic drug prescribing for children and adolescents, since prescriptions written by a psychiatrist wouldn’t necessarily be included in the primary care electronic medical record, Dr. Grundmeier said.
Audience member Dr. James M. Perrin, professor of pediatrics at Harvard Medical School and director of general pediatrics at Massachusetts General Hospital, both in Boston, commented, “One of the interesting things about these polypharmacy kids is they come in in acute crisis and someone adds a third or fourth drug, but when the kid gets out of the crisis, no one thinks about taking the drug away; he’s always on it.”
Dr. Grundmeier agreed that’s the classic pattern. Accordingly, a likely next step in this research project will be to scrutinize the CER2 Consortium data set in search of patterns of subtracting psychotropic drugs and simplifying management on the part of some primary care physicians. The ultimate goal is to gain an understanding of the effectiveness and potential adverse effects of psychotropic polypharmacy in order to develop evidence-based tools, such as clinical pathways to help support primary care physicians who are treating these young patients.
Another audience member, a pediatric critical care specialist at an urban public hospital, commented that the high rates of off-label psychotropic polypharmacy by primary care physicians documented in the CER2 study constitute an indictment of widespread inadequate psychiatric services for young Medicaid patients.
“There’s not a night on call when I’m not admitting a teenager trying to kill himself. So when I see your data, I split it into the ADHD group and the antidepressant group. And the patients admitted for suicidality have to leave the hospital after a maximum of 3 days, generally without psychiatric or social support services to supplement these medications,” she said.
This study was funded by the Health Resources and Services Administration. Dr. Grundmeier reported having no relevant financial conflicts.
SAN DIEGO – Of children and teens on psychotropic medication prescribed by their primary care pediatrician or family physician, 21% simultaneously receive two or more different classes of the drugs for at least 3 months, according to a massive study.
“Given that the safety of pediatric monotherapy for some of these psychotropic medications is unexplored, these patterns of polypharmacy prescribing are alarming and warrant further investigation,” Dr. Robert Grundmeier said in presenting the study results at the annual meeting of the Pediatric Academic Societies.
He presented a study of 891,717 patients up to 18 years of age who received health care at 1 of 222 primary care pediatric or family medicine sites participating in the Comparative Effectiveness Research Through Collaborative Electronic Reporting (CER2) Consortium. He described CER2 as a “supernetwork” comprised of multiple electronic health record–based networks and led by the American Academy of Pediatrics. The consortium was designed to carry out very large pharmacoepidemiologic and comparative effectiveness studies.
The initial study population consisted of more than 1.2 million patients up to age 18 years, a figure that investigators whittled down to just under 900,000 by excluding those with a seizure disorder or less than 12 months of follow-up data.
Among the 891,717 subjects, 8.3% of boys and 5.3% of girls were treated with psychotropic medication by their primary care physician at some point. Of these 60,430 patients, 21% simultaneously received psychotropic drugs from two or more different classes for at least 3 months. Another 5,813 patients – 0.9% of all boys and 0.4% of all girls in the study – got psychotropic medications from three or more classes simultaneously, reported Dr. Grundmeier of Children’s Hospital of Philadelphia.
The most frequently prescribed category of medication was stimulants/
atomoxetine, a treatment mainstay in attention-deficit/hyperactivity disorder. Antidepressants were No. 2 and were prescribed in 2.1% of all boys and 2.8% of girls. Atypical antipsychotics were prescribed for 1.4% of boys and 0.8% of girls.
The most widely prescribed two-class combination among patients receiving psychotropic medication was a stimulant and an alpha-agonist, given to 10.7% of boys and 5.6% of girls. The second most popular two-drug combination was a stimulant and an antidepressant, which was prescribed for 7.3% of boys and 7.1% of girls who took psychotropic medication.
The use of combination therapy incorporating an atypical antipsychotic was of particular interest because health policy makers have identified this as an issue of concern. The drugs aren’t approved by the Food and Drug Administration for patients younger than 5 years old, and yet the Agency for Healthcare Research and Quality has reported that the use of these medications in very young children with behavior problems doubled between 1999-2001 and 2007. Moreover, more than three-quarters of youths on Medicaid are taking an atypical antipsychotic for non–FDA-approved indications, including ADHD and aggressive behavior, according to Centers for Medicare & Medicaid Services data.
Dr. Grundmeier reported that a stimulant and an atypical antipsychotic were prescribed together for 7.5% of boys and 4.4% of girls on psychotropic medication, and an antidepressant and an atypical antipsychotic were given to 4.9% of boys and 6.4% of girls.
These CER2 data probably underestimate the true rate of psychotropic drug prescribing for children and adolescents, since prescriptions written by a psychiatrist wouldn’t necessarily be included in the primary care electronic medical record, Dr. Grundmeier said.
Audience member Dr. James M. Perrin, professor of pediatrics at Harvard Medical School and director of general pediatrics at Massachusetts General Hospital, both in Boston, commented, “One of the interesting things about these polypharmacy kids is they come in in acute crisis and someone adds a third or fourth drug, but when the kid gets out of the crisis, no one thinks about taking the drug away; he’s always on it.”
Dr. Grundmeier agreed that’s the classic pattern. Accordingly, a likely next step in this research project will be to scrutinize the CER2 Consortium data set in search of patterns of subtracting psychotropic drugs and simplifying management on the part of some primary care physicians. The ultimate goal is to gain an understanding of the effectiveness and potential adverse effects of psychotropic polypharmacy in order to develop evidence-based tools, such as clinical pathways to help support primary care physicians who are treating these young patients.
Another audience member, a pediatric critical care specialist at an urban public hospital, commented that the high rates of off-label psychotropic polypharmacy by primary care physicians documented in the CER2 study constitute an indictment of widespread inadequate psychiatric services for young Medicaid patients.
“There’s not a night on call when I’m not admitting a teenager trying to kill himself. So when I see your data, I split it into the ADHD group and the antidepressant group. And the patients admitted for suicidality have to leave the hospital after a maximum of 3 days, generally without psychiatric or social support services to supplement these medications,” she said.
This study was funded by the Health Resources and Services Administration. Dr. Grundmeier reported having no relevant financial conflicts.
SAN DIEGO – Of children and teens on psychotropic medication prescribed by their primary care pediatrician or family physician, 21% simultaneously receive two or more different classes of the drugs for at least 3 months, according to a massive study.
“Given that the safety of pediatric monotherapy for some of these psychotropic medications is unexplored, these patterns of polypharmacy prescribing are alarming and warrant further investigation,” Dr. Robert Grundmeier said in presenting the study results at the annual meeting of the Pediatric Academic Societies.
He presented a study of 891,717 patients up to 18 years of age who received health care at 1 of 222 primary care pediatric or family medicine sites participating in the Comparative Effectiveness Research Through Collaborative Electronic Reporting (CER2) Consortium. He described CER2 as a “supernetwork” comprised of multiple electronic health record–based networks and led by the American Academy of Pediatrics. The consortium was designed to carry out very large pharmacoepidemiologic and comparative effectiveness studies.
The initial study population consisted of more than 1.2 million patients up to age 18 years, a figure that investigators whittled down to just under 900,000 by excluding those with a seizure disorder or less than 12 months of follow-up data.
Among the 891,717 subjects, 8.3% of boys and 5.3% of girls were treated with psychotropic medication by their primary care physician at some point. Of these 60,430 patients, 21% simultaneously received psychotropic drugs from two or more different classes for at least 3 months. Another 5,813 patients – 0.9% of all boys and 0.4% of all girls in the study – got psychotropic medications from three or more classes simultaneously, reported Dr. Grundmeier of Children’s Hospital of Philadelphia.
The most frequently prescribed category of medication was stimulants/
atomoxetine, a treatment mainstay in attention-deficit/hyperactivity disorder. Antidepressants were No. 2 and were prescribed in 2.1% of all boys and 2.8% of girls. Atypical antipsychotics were prescribed for 1.4% of boys and 0.8% of girls.
The most widely prescribed two-class combination among patients receiving psychotropic medication was a stimulant and an alpha-agonist, given to 10.7% of boys and 5.6% of girls. The second most popular two-drug combination was a stimulant and an antidepressant, which was prescribed for 7.3% of boys and 7.1% of girls who took psychotropic medication.
The use of combination therapy incorporating an atypical antipsychotic was of particular interest because health policy makers have identified this as an issue of concern. The drugs aren’t approved by the Food and Drug Administration for patients younger than 5 years old, and yet the Agency for Healthcare Research and Quality has reported that the use of these medications in very young children with behavior problems doubled between 1999-2001 and 2007. Moreover, more than three-quarters of youths on Medicaid are taking an atypical antipsychotic for non–FDA-approved indications, including ADHD and aggressive behavior, according to Centers for Medicare & Medicaid Services data.
Dr. Grundmeier reported that a stimulant and an atypical antipsychotic were prescribed together for 7.5% of boys and 4.4% of girls on psychotropic medication, and an antidepressant and an atypical antipsychotic were given to 4.9% of boys and 6.4% of girls.
These CER2 data probably underestimate the true rate of psychotropic drug prescribing for children and adolescents, since prescriptions written by a psychiatrist wouldn’t necessarily be included in the primary care electronic medical record, Dr. Grundmeier said.
Audience member Dr. James M. Perrin, professor of pediatrics at Harvard Medical School and director of general pediatrics at Massachusetts General Hospital, both in Boston, commented, “One of the interesting things about these polypharmacy kids is they come in in acute crisis and someone adds a third or fourth drug, but when the kid gets out of the crisis, no one thinks about taking the drug away; he’s always on it.”
Dr. Grundmeier agreed that’s the classic pattern. Accordingly, a likely next step in this research project will be to scrutinize the CER2 Consortium data set in search of patterns of subtracting psychotropic drugs and simplifying management on the part of some primary care physicians. The ultimate goal is to gain an understanding of the effectiveness and potential adverse effects of psychotropic polypharmacy in order to develop evidence-based tools, such as clinical pathways to help support primary care physicians who are treating these young patients.
Another audience member, a pediatric critical care specialist at an urban public hospital, commented that the high rates of off-label psychotropic polypharmacy by primary care physicians documented in the CER2 study constitute an indictment of widespread inadequate psychiatric services for young Medicaid patients.
“There’s not a night on call when I’m not admitting a teenager trying to kill himself. So when I see your data, I split it into the ADHD group and the antidepressant group. And the patients admitted for suicidality have to leave the hospital after a maximum of 3 days, generally without psychiatric or social support services to supplement these medications,” she said.
This study was funded by the Health Resources and Services Administration. Dr. Grundmeier reported having no relevant financial conflicts.
AT THE PAS ANNUAL MEETING
Key clinical point: Psychotropic polypharmacy has reached what investigators have deemed “alarming” levels in pediatric primary care.
Major finding: Of 60,430 pediatric patients who were prescribed psychotropic medication by their primary care physician, 21% simultaneously received two or more different classes of the drugs.
Data source: A retrospective study of electronic health record data for 891,717 patients up to age 18 years in the CER2 data set.
Disclosures: The study was funded by the Health Resources and Services Administration. Dr. Grundmeier reported having no relevant financial conflicts.
AAS: Experts say suicide research needs a reboot
ATLANTA– Progress has stalled in understanding the predictors and prevention of suicide, and it’s time for researchers to step up their game, experts agreed at the annual conference of the American Association of Suicidology.
“In the past couple of decades we’ve learned a fair amount about suicidal behavior. However, I think progress has been fairly slow – some might even say a little stagnant – in our pushing things forward and improving our understanding,” Matthew K. Nock, Ph.D., said in the meeting’s opening plenary talk.
He cited a soon-to-be-published meta-analysis led by his post-doctoral fellow Joseph C. Franklin, Ph.D., which evaluated all of the studies of predictors of suicide attempts and completed suicides published during the last 5 decades. The eye opening finding: The predictive odds ratios for the standard risk factors have remained essentially the same – namely, weak – for the past 50 years.
“In general, we’re not getting better in our ability to predict suicidal behavior – and that’s a serious problem for us. We still have enormous gaps in our understanding and in our ability to predict and prevent these outcomes,” declared Dr. Nock, professor of psychology at Harvard University, Boston.
The necessity for a fresh approach to suicide and suicide risk also was emphasized by E. David Klonsky, Ph.D., in his Edwin Shneidman Award Lecture.
“Despite what seems like a very large body of knowledge, suicide rates in the U.S. have increased for numerous consecutive years, and the same is true worldwide,” observed Dr. Klonsky, a psychologist at the University of British Columbia, Vancouver.
“What’s really hard to wrap our heads around is that we’re still only at a 1960s level in our ability to predict suicide. And the main reason for that is our risk factors don’t tell us what we think they do,” he continued.
This was first demonstrated in a 1999 study by Dr. Ronald C. Kessler of Harvard Medical School and coworkers (Arch. Gen. Psychiatry 1999;56:617-26).
They showed that the widely accepted suicide risk factors -- including any mood or anxiety disorder or substance disorders -- are strong predictors of suicidal ideation, but not significant predictors of who will transition from ideation to suicidal action. This finding has subsequently been confirmed by Dr. Nock and others in both adults and adolescents in a massive World Health Organization-sponsored project. Yet to date the concept hasn’t really sunk in broadly in the mental health and medical fields, according to Dr. Klonsky.
In his plenary talk, Dr. Nock focused on four key gaps in the current understanding of how to predict and prevent suicide and outlined how he and others are addressing these needs:
The need for objective markers of suicidal risk: Historically, nearly all patient assessments have relied upon self-report and cross-sectional surveys. That has an obvious limitation, since people are often motivated to conceal their thoughts of suicide. For example, one study found that 78% of patients who died by suicide while in a psychiatric hospital denied suicidal thoughts or intent in their last assessment.
The emerging emphasis is on creating brief computerized tests of memory and reaction time to gain a window into people’s implicit cognitions. Dr. Nock and colleagues have developed one such test, the Implicit Association Test. They had patients who presented to a psychiatric emergency department take the 5-minute word association test and demonstrated that those who scored high for implicit associations between death and suicide were six-fold more likely to make a suicide attempt in the next 6 months (Psychol. Sci. 2010;21:511-7). These findings have since been confirmed by a Canadian group (Psychol. Assess. 2013;25:714-21). The test is available online (www.ImplicitMentalHealth.com) with expert feedback provided as a public education tool and as a means for Dr. Nock and coinvestigators to gather large quantities of data.
Other objective tests for suicide risk that measure physiologic and neural responses to suicide-related stimuli include the Suicide Stroop and Affect Misattribution Procedure.
The need for better predictors of the transition from ideation to attempt: There are a few early leads on such predictors from the WHO dataset and other large studies. These include disorders characterized by aggression, agitation, and/or anxiety, such as conduct disorder, bipolar disorder, and a history of physical or sexual abuse. In a large study in the U.S. Army, the number-one predictor is intermittent explosive disorder.
The need for methods of combining risk factor data: Nearly all studies of suicide risk factors have utilized bivariate analysis -- that is, they examine risk based upon the presence or absence of an individual risk factor, such as a personal history of a mental disorder. But in a study led by Guilherme Borges, Sc.D., of the National Institute of Psychiatry in Mexico City, a group including Dr. Nock showed using National Comorbidity Survey Replication data that by simply together individual risk factors to create a 0-11 scale it became possible to identify a high-risk subgroup consisting of 13.7% of survey participants. This subgroup accounted for 67% of all suicide attempts within the next 12 months (Psychol. Med. 2006;36:1747-57).
The investigators have gone on to validate this approach in more than 108,000 subjects in 21 countries participating in the World Health Organization mental health project (J. Clin. Psychiatry 2010;71:1617-28).
Simple addition of suicidality risk factors, while a big step forward in risk assessment, is still a relatively crude predictive tool. More recently, Dr. Kessler, collaborating with Dr. Nock and others, has developed a much more sophisticated actuarial risk algorithm and applied it to more than 54,000 U.S. Army soldiers hospitalized for psychiatric disorders. They found that subjects who scored in the top 5% in terms of predicted suicide risk accounted for 53% of all suicides that occurred within the next 12 months. The suicide rate in this highest-risk group was massive: 3,624 per 100,000 per year as compared to a background rate of 18.5/100,000/year in the Army overall.
Moreover, nearly one-half of soldiers with a risk score in the top 5% had a 12-month composite adverse outcome, defined as another suicide attempt, death by suicide, accidental death, or psychiatric rehospitalization (JAMA Psychiatry 2015;72:49-57).
The need for data on imminent risk: Dr. Nock called this the biggest unmet need in suicidology; it’s what clinicians and family members desperately want but don’t have. At present there is “approximately zero data” on how to predict suicidal behavior in the hours, days, or weeks before it occurs, Dr. Nock said. Indeed, Dr. Franklin’s meta-analysis showed that in the past 50 years more than three-quarters of studies examining suicide risk have looked at risk a year or more in the future. Only 2% of studies have looked at risk during the window of the next month or so.
Numerous groups are now looking at real-time patient monitoring using cell phones and smart watches as a means of developing short-term risk predictors. These tools enable investigators to monitor changes in mood, thoughts, behavior, and physiology in large populations in order to see what leads up to a suicide attempt. Dr. Nock’s group is collaborating with information scientists at Massachusetts Intitute of Technology on such projects.
This technology also shows promise for therapeutic intervention. Dr. Franklin and coworkers have developed a brief, game-like mobile app to administer what he calls Therapeutic Evaluative Conditioning. In three soon-to-be-published randomized controlled trials, he has shown that this simple intervention – essentially, playing a game on a cell phone – resulted in reductions of 42%-49% in self-cutting and other nonsuicidal self-injury, 21%-64% reductions in suicidal planning, and 20%-57% decreases in suicidal behaviors, according to Dr. Nock.
Dr. Nock’s research is funded chiefly by the National Institute of Mental Health, the World Health Organization, and the Department of Defense; he reported having no financial conflicts. Dr. Klonsky’s research is largely supported by the American Foundation for Suicide Prevention.
ATLANTA– Progress has stalled in understanding the predictors and prevention of suicide, and it’s time for researchers to step up their game, experts agreed at the annual conference of the American Association of Suicidology.
“In the past couple of decades we’ve learned a fair amount about suicidal behavior. However, I think progress has been fairly slow – some might even say a little stagnant – in our pushing things forward and improving our understanding,” Matthew K. Nock, Ph.D., said in the meeting’s opening plenary talk.
He cited a soon-to-be-published meta-analysis led by his post-doctoral fellow Joseph C. Franklin, Ph.D., which evaluated all of the studies of predictors of suicide attempts and completed suicides published during the last 5 decades. The eye opening finding: The predictive odds ratios for the standard risk factors have remained essentially the same – namely, weak – for the past 50 years.
“In general, we’re not getting better in our ability to predict suicidal behavior – and that’s a serious problem for us. We still have enormous gaps in our understanding and in our ability to predict and prevent these outcomes,” declared Dr. Nock, professor of psychology at Harvard University, Boston.
The necessity for a fresh approach to suicide and suicide risk also was emphasized by E. David Klonsky, Ph.D., in his Edwin Shneidman Award Lecture.
“Despite what seems like a very large body of knowledge, suicide rates in the U.S. have increased for numerous consecutive years, and the same is true worldwide,” observed Dr. Klonsky, a psychologist at the University of British Columbia, Vancouver.
“What’s really hard to wrap our heads around is that we’re still only at a 1960s level in our ability to predict suicide. And the main reason for that is our risk factors don’t tell us what we think they do,” he continued.
This was first demonstrated in a 1999 study by Dr. Ronald C. Kessler of Harvard Medical School and coworkers (Arch. Gen. Psychiatry 1999;56:617-26).
They showed that the widely accepted suicide risk factors -- including any mood or anxiety disorder or substance disorders -- are strong predictors of suicidal ideation, but not significant predictors of who will transition from ideation to suicidal action. This finding has subsequently been confirmed by Dr. Nock and others in both adults and adolescents in a massive World Health Organization-sponsored project. Yet to date the concept hasn’t really sunk in broadly in the mental health and medical fields, according to Dr. Klonsky.
In his plenary talk, Dr. Nock focused on four key gaps in the current understanding of how to predict and prevent suicide and outlined how he and others are addressing these needs:
The need for objective markers of suicidal risk: Historically, nearly all patient assessments have relied upon self-report and cross-sectional surveys. That has an obvious limitation, since people are often motivated to conceal their thoughts of suicide. For example, one study found that 78% of patients who died by suicide while in a psychiatric hospital denied suicidal thoughts or intent in their last assessment.
The emerging emphasis is on creating brief computerized tests of memory and reaction time to gain a window into people’s implicit cognitions. Dr. Nock and colleagues have developed one such test, the Implicit Association Test. They had patients who presented to a psychiatric emergency department take the 5-minute word association test and demonstrated that those who scored high for implicit associations between death and suicide were six-fold more likely to make a suicide attempt in the next 6 months (Psychol. Sci. 2010;21:511-7). These findings have since been confirmed by a Canadian group (Psychol. Assess. 2013;25:714-21). The test is available online (www.ImplicitMentalHealth.com) with expert feedback provided as a public education tool and as a means for Dr. Nock and coinvestigators to gather large quantities of data.
Other objective tests for suicide risk that measure physiologic and neural responses to suicide-related stimuli include the Suicide Stroop and Affect Misattribution Procedure.
The need for better predictors of the transition from ideation to attempt: There are a few early leads on such predictors from the WHO dataset and other large studies. These include disorders characterized by aggression, agitation, and/or anxiety, such as conduct disorder, bipolar disorder, and a history of physical or sexual abuse. In a large study in the U.S. Army, the number-one predictor is intermittent explosive disorder.
The need for methods of combining risk factor data: Nearly all studies of suicide risk factors have utilized bivariate analysis -- that is, they examine risk based upon the presence or absence of an individual risk factor, such as a personal history of a mental disorder. But in a study led by Guilherme Borges, Sc.D., of the National Institute of Psychiatry in Mexico City, a group including Dr. Nock showed using National Comorbidity Survey Replication data that by simply together individual risk factors to create a 0-11 scale it became possible to identify a high-risk subgroup consisting of 13.7% of survey participants. This subgroup accounted for 67% of all suicide attempts within the next 12 months (Psychol. Med. 2006;36:1747-57).
The investigators have gone on to validate this approach in more than 108,000 subjects in 21 countries participating in the World Health Organization mental health project (J. Clin. Psychiatry 2010;71:1617-28).
Simple addition of suicidality risk factors, while a big step forward in risk assessment, is still a relatively crude predictive tool. More recently, Dr. Kessler, collaborating with Dr. Nock and others, has developed a much more sophisticated actuarial risk algorithm and applied it to more than 54,000 U.S. Army soldiers hospitalized for psychiatric disorders. They found that subjects who scored in the top 5% in terms of predicted suicide risk accounted for 53% of all suicides that occurred within the next 12 months. The suicide rate in this highest-risk group was massive: 3,624 per 100,000 per year as compared to a background rate of 18.5/100,000/year in the Army overall.
Moreover, nearly one-half of soldiers with a risk score in the top 5% had a 12-month composite adverse outcome, defined as another suicide attempt, death by suicide, accidental death, or psychiatric rehospitalization (JAMA Psychiatry 2015;72:49-57).
The need for data on imminent risk: Dr. Nock called this the biggest unmet need in suicidology; it’s what clinicians and family members desperately want but don’t have. At present there is “approximately zero data” on how to predict suicidal behavior in the hours, days, or weeks before it occurs, Dr. Nock said. Indeed, Dr. Franklin’s meta-analysis showed that in the past 50 years more than three-quarters of studies examining suicide risk have looked at risk a year or more in the future. Only 2% of studies have looked at risk during the window of the next month or so.
Numerous groups are now looking at real-time patient monitoring using cell phones and smart watches as a means of developing short-term risk predictors. These tools enable investigators to monitor changes in mood, thoughts, behavior, and physiology in large populations in order to see what leads up to a suicide attempt. Dr. Nock’s group is collaborating with information scientists at Massachusetts Intitute of Technology on such projects.
This technology also shows promise for therapeutic intervention. Dr. Franklin and coworkers have developed a brief, game-like mobile app to administer what he calls Therapeutic Evaluative Conditioning. In three soon-to-be-published randomized controlled trials, he has shown that this simple intervention – essentially, playing a game on a cell phone – resulted in reductions of 42%-49% in self-cutting and other nonsuicidal self-injury, 21%-64% reductions in suicidal planning, and 20%-57% decreases in suicidal behaviors, according to Dr. Nock.
Dr. Nock’s research is funded chiefly by the National Institute of Mental Health, the World Health Organization, and the Department of Defense; he reported having no financial conflicts. Dr. Klonsky’s research is largely supported by the American Foundation for Suicide Prevention.
ATLANTA– Progress has stalled in understanding the predictors and prevention of suicide, and it’s time for researchers to step up their game, experts agreed at the annual conference of the American Association of Suicidology.
“In the past couple of decades we’ve learned a fair amount about suicidal behavior. However, I think progress has been fairly slow – some might even say a little stagnant – in our pushing things forward and improving our understanding,” Matthew K. Nock, Ph.D., said in the meeting’s opening plenary talk.
He cited a soon-to-be-published meta-analysis led by his post-doctoral fellow Joseph C. Franklin, Ph.D., which evaluated all of the studies of predictors of suicide attempts and completed suicides published during the last 5 decades. The eye opening finding: The predictive odds ratios for the standard risk factors have remained essentially the same – namely, weak – for the past 50 years.
“In general, we’re not getting better in our ability to predict suicidal behavior – and that’s a serious problem for us. We still have enormous gaps in our understanding and in our ability to predict and prevent these outcomes,” declared Dr. Nock, professor of psychology at Harvard University, Boston.
The necessity for a fresh approach to suicide and suicide risk also was emphasized by E. David Klonsky, Ph.D., in his Edwin Shneidman Award Lecture.
“Despite what seems like a very large body of knowledge, suicide rates in the U.S. have increased for numerous consecutive years, and the same is true worldwide,” observed Dr. Klonsky, a psychologist at the University of British Columbia, Vancouver.
“What’s really hard to wrap our heads around is that we’re still only at a 1960s level in our ability to predict suicide. And the main reason for that is our risk factors don’t tell us what we think they do,” he continued.
This was first demonstrated in a 1999 study by Dr. Ronald C. Kessler of Harvard Medical School and coworkers (Arch. Gen. Psychiatry 1999;56:617-26).
They showed that the widely accepted suicide risk factors -- including any mood or anxiety disorder or substance disorders -- are strong predictors of suicidal ideation, but not significant predictors of who will transition from ideation to suicidal action. This finding has subsequently been confirmed by Dr. Nock and others in both adults and adolescents in a massive World Health Organization-sponsored project. Yet to date the concept hasn’t really sunk in broadly in the mental health and medical fields, according to Dr. Klonsky.
In his plenary talk, Dr. Nock focused on four key gaps in the current understanding of how to predict and prevent suicide and outlined how he and others are addressing these needs:
The need for objective markers of suicidal risk: Historically, nearly all patient assessments have relied upon self-report and cross-sectional surveys. That has an obvious limitation, since people are often motivated to conceal their thoughts of suicide. For example, one study found that 78% of patients who died by suicide while in a psychiatric hospital denied suicidal thoughts or intent in their last assessment.
The emerging emphasis is on creating brief computerized tests of memory and reaction time to gain a window into people’s implicit cognitions. Dr. Nock and colleagues have developed one such test, the Implicit Association Test. They had patients who presented to a psychiatric emergency department take the 5-minute word association test and demonstrated that those who scored high for implicit associations between death and suicide were six-fold more likely to make a suicide attempt in the next 6 months (Psychol. Sci. 2010;21:511-7). These findings have since been confirmed by a Canadian group (Psychol. Assess. 2013;25:714-21). The test is available online (www.ImplicitMentalHealth.com) with expert feedback provided as a public education tool and as a means for Dr. Nock and coinvestigators to gather large quantities of data.
Other objective tests for suicide risk that measure physiologic and neural responses to suicide-related stimuli include the Suicide Stroop and Affect Misattribution Procedure.
The need for better predictors of the transition from ideation to attempt: There are a few early leads on such predictors from the WHO dataset and other large studies. These include disorders characterized by aggression, agitation, and/or anxiety, such as conduct disorder, bipolar disorder, and a history of physical or sexual abuse. In a large study in the U.S. Army, the number-one predictor is intermittent explosive disorder.
The need for methods of combining risk factor data: Nearly all studies of suicide risk factors have utilized bivariate analysis -- that is, they examine risk based upon the presence or absence of an individual risk factor, such as a personal history of a mental disorder. But in a study led by Guilherme Borges, Sc.D., of the National Institute of Psychiatry in Mexico City, a group including Dr. Nock showed using National Comorbidity Survey Replication data that by simply together individual risk factors to create a 0-11 scale it became possible to identify a high-risk subgroup consisting of 13.7% of survey participants. This subgroup accounted for 67% of all suicide attempts within the next 12 months (Psychol. Med. 2006;36:1747-57).
The investigators have gone on to validate this approach in more than 108,000 subjects in 21 countries participating in the World Health Organization mental health project (J. Clin. Psychiatry 2010;71:1617-28).
Simple addition of suicidality risk factors, while a big step forward in risk assessment, is still a relatively crude predictive tool. More recently, Dr. Kessler, collaborating with Dr. Nock and others, has developed a much more sophisticated actuarial risk algorithm and applied it to more than 54,000 U.S. Army soldiers hospitalized for psychiatric disorders. They found that subjects who scored in the top 5% in terms of predicted suicide risk accounted for 53% of all suicides that occurred within the next 12 months. The suicide rate in this highest-risk group was massive: 3,624 per 100,000 per year as compared to a background rate of 18.5/100,000/year in the Army overall.
Moreover, nearly one-half of soldiers with a risk score in the top 5% had a 12-month composite adverse outcome, defined as another suicide attempt, death by suicide, accidental death, or psychiatric rehospitalization (JAMA Psychiatry 2015;72:49-57).
The need for data on imminent risk: Dr. Nock called this the biggest unmet need in suicidology; it’s what clinicians and family members desperately want but don’t have. At present there is “approximately zero data” on how to predict suicidal behavior in the hours, days, or weeks before it occurs, Dr. Nock said. Indeed, Dr. Franklin’s meta-analysis showed that in the past 50 years more than three-quarters of studies examining suicide risk have looked at risk a year or more in the future. Only 2% of studies have looked at risk during the window of the next month or so.
Numerous groups are now looking at real-time patient monitoring using cell phones and smart watches as a means of developing short-term risk predictors. These tools enable investigators to monitor changes in mood, thoughts, behavior, and physiology in large populations in order to see what leads up to a suicide attempt. Dr. Nock’s group is collaborating with information scientists at Massachusetts Intitute of Technology on such projects.
This technology also shows promise for therapeutic intervention. Dr. Franklin and coworkers have developed a brief, game-like mobile app to administer what he calls Therapeutic Evaluative Conditioning. In three soon-to-be-published randomized controlled trials, he has shown that this simple intervention – essentially, playing a game on a cell phone – resulted in reductions of 42%-49% in self-cutting and other nonsuicidal self-injury, 21%-64% reductions in suicidal planning, and 20%-57% decreases in suicidal behaviors, according to Dr. Nock.
Dr. Nock’s research is funded chiefly by the National Institute of Mental Health, the World Health Organization, and the Department of Defense; he reported having no financial conflicts. Dr. Klonsky’s research is largely supported by the American Foundation for Suicide Prevention.
EXPERT ANALYSIS FROM THE ANNUAL AAS CONFERENCE
Applications expand for vorapaxar in cardiovascular prevention
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
AT ACC 15
Key clinical point: Vorapaxar reduced the composite outcome of cardiovascular death, MI, or stroke by 29% compared with placebo in patients with a history of MI and/or peripheral arterial disease and prior coronary artery bypass surgery.
Major finding: The novel antiplatelet agent also reduced acute limb ischemia events by 42% in patients with symptomatic peripheral arterial disease.
Data source: These were findings from post hoc, exploratory analyses of a 26,449-patient, randomized, double-blind pivotal trial of vorapaxar for secondary cardiovascular prevention.
Disclosures: The TRA 2°P TIMI-50 trial was sponsored by Merck. The presenters reported having no financial conflicts.
PAS: Low-dose hydrocortisone improves outcomes in extreme preemies
SAN DIEGO – Early prophylactic very-low-dose hydrocortisone improved survival free of bronchopulmonary dysplasia in extremely preterm neonates in a large French randomized trial.
The intervention also brought positive results on multiple secondary endpoints, including rates of extubation by day 10 and patent ductus arteriosus closure without resort to ligation, Dr. Olivier Baud reported at the annual meeting of the Pediatric Academic Societies.
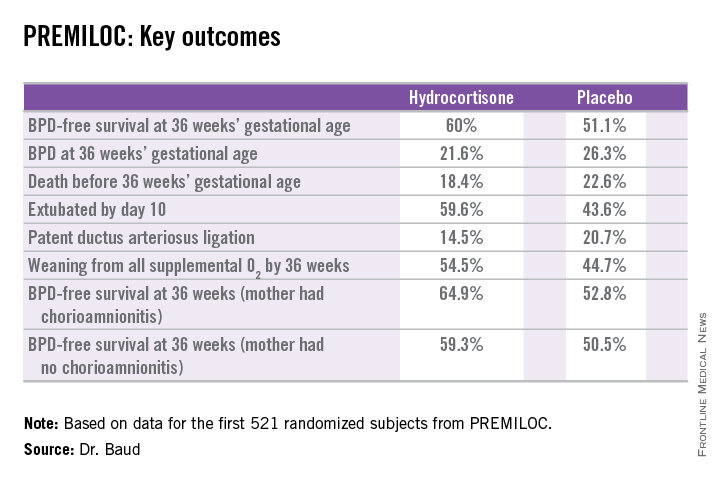
He presented the findings of PREMILOC, a double-blind, randomized, placebo-controlled, 21-center French study. The study was halted by the data safety monitoring board for ethical reasons, based upon compelling evidence of superiority after scrutinizing results in the first 521 randomized subjects.
The primary outcome – survival free of bronchopulmonary dysplasia (BPD) at 36 weeks of gestational age – occurred in 60% of the hydrocortisone group, compared with 51% of placebo-treated controls, for an adjusted 48% increased likelihood of favorable outcome. The number-needed-to-treat was 11 patients, said Dr. Baud, professor of pediatrics and chief of the neonatal medicine unit at Robert Debré Hospital in Paris and Paris Diderot University.
The intervention involved administration of intravenous hydrocortisone sodium succinate for the first 10 postnatal days. The dose was 0.5 mg/kg/12 hours for 7 days followed by 0.5 mg/kg/24 hours for 3 days. Treatment began as soon as possible after birth and always within 24 hours. The total cumulative dose of hydrocortisone was 8.5 mg/kg. That’s 15-30 times less corticosteroid than employed in earlier studies of higher-dose dexamethasone, where gastrointestinal bleeding, intestinal perforation, and other serious adverse events were a major problem.
“This is the lowest hydrocortisone dose ever studied in a randomized controlled trial,” according to the neonatologist.
All study participants were born at 24-27 weeks’ gestational age. In order to minimize the risk of treatment-related serious adverse events, especially GI perforation – patients with intrauterine growth retardation or who were on nonsteroidal anti-inflammatory agents were ineligible for the trial.
The rationale for the study, Dr. Baud explained, comes from the landmark work of Dr. Kristi L. Watterberg, professor of pediatrics at the University of New Mexico, Albuquerque, who has argued that early adrenal insufficiency in extremely small-for-gestational-age infants is linked to BPD, patent ductus arteriosus, persistent lung inflammation, and poor enteral nutrition. She introduced the concept that administration of very-low-dose corticosteroids at a dose comparable to normal endogenous steroid production could serve as a global therapy addressing these multiple health problems.
Two prior studies of low-dose hydrocortisone in extremely preterm babies were halted due to an increase in GI perforation, Dr. Baud noted, which is why the French investigators excluded neonates at increased risk for this complication.
In a prespecified PREMILOC subgroup analysis stratified by gestational age, the rate of survival free of BPD at 36 weeks among neonates born at 24-25 weeks was 33.7% with hydrocortisone, compared with 23.3% with placebo, for an adjusted 67% increased likelihood of a positive outcome with active treatment. In babies born at 26-27 weeks, the rates were 72.7% and 65.3%, respectively. On the other hand, hydrocortisone was associated with a highly significant 55% relative risk reduction for neonatal mortality in infants born at 26-27 weeks.
Post hoc analysis spotlighted several factors which were unexpectedly associated with differential impacts on outcome. For example, hydrocortisone achieved a significant benefit in terms of survival free of BPD at 36 weeks only in females, where the rates were 69.4% versus 53%, for an adjusted 2.25-fold increased rate. In males, the rate was 51.1% with hydrocortisone, compared with 49.7% in controls.
There were no differences between the hydrocortisone and placebo groups in rates of any serious adverse events, including GI perforation, necrotizing enterocolitis, air leaks, severe sepsis, or persistent pulmonary hypertension.
Dr. Baud said the next stage of the PREMILOC study will be to report 2-year outcomes; a greater than 90% follow-up rate is anticipated. Also, he and his coinvestigators are planning ancillary studies examining placental findings, the impact of pretreatment serum cortisol levels, thyroid function, and other issues.
“Our goal is to identify a targeted population that could strongly benefit from prophylactic hydrocortisone,” the neonatologist said.
He reported having no financial conflicts regarding the study, supported by INSERM and other French national research organizations.
SAN DIEGO – Early prophylactic very-low-dose hydrocortisone improved survival free of bronchopulmonary dysplasia in extremely preterm neonates in a large French randomized trial.
The intervention also brought positive results on multiple secondary endpoints, including rates of extubation by day 10 and patent ductus arteriosus closure without resort to ligation, Dr. Olivier Baud reported at the annual meeting of the Pediatric Academic Societies.

He presented the findings of PREMILOC, a double-blind, randomized, placebo-controlled, 21-center French study. The study was halted by the data safety monitoring board for ethical reasons, based upon compelling evidence of superiority after scrutinizing results in the first 521 randomized subjects.
The primary outcome – survival free of bronchopulmonary dysplasia (BPD) at 36 weeks of gestational age – occurred in 60% of the hydrocortisone group, compared with 51% of placebo-treated controls, for an adjusted 48% increased likelihood of favorable outcome. The number-needed-to-treat was 11 patients, said Dr. Baud, professor of pediatrics and chief of the neonatal medicine unit at Robert Debré Hospital in Paris and Paris Diderot University.
The intervention involved administration of intravenous hydrocortisone sodium succinate for the first 10 postnatal days. The dose was 0.5 mg/kg/12 hours for 7 days followed by 0.5 mg/kg/24 hours for 3 days. Treatment began as soon as possible after birth and always within 24 hours. The total cumulative dose of hydrocortisone was 8.5 mg/kg. That’s 15-30 times less corticosteroid than employed in earlier studies of higher-dose dexamethasone, where gastrointestinal bleeding, intestinal perforation, and other serious adverse events were a major problem.
“This is the lowest hydrocortisone dose ever studied in a randomized controlled trial,” according to the neonatologist.
All study participants were born at 24-27 weeks’ gestational age. In order to minimize the risk of treatment-related serious adverse events, especially GI perforation – patients with intrauterine growth retardation or who were on nonsteroidal anti-inflammatory agents were ineligible for the trial.
The rationale for the study, Dr. Baud explained, comes from the landmark work of Dr. Kristi L. Watterberg, professor of pediatrics at the University of New Mexico, Albuquerque, who has argued that early adrenal insufficiency in extremely small-for-gestational-age infants is linked to BPD, patent ductus arteriosus, persistent lung inflammation, and poor enteral nutrition. She introduced the concept that administration of very-low-dose corticosteroids at a dose comparable to normal endogenous steroid production could serve as a global therapy addressing these multiple health problems.
Two prior studies of low-dose hydrocortisone in extremely preterm babies were halted due to an increase in GI perforation, Dr. Baud noted, which is why the French investigators excluded neonates at increased risk for this complication.
In a prespecified PREMILOC subgroup analysis stratified by gestational age, the rate of survival free of BPD at 36 weeks among neonates born at 24-25 weeks was 33.7% with hydrocortisone, compared with 23.3% with placebo, for an adjusted 67% increased likelihood of a positive outcome with active treatment. In babies born at 26-27 weeks, the rates were 72.7% and 65.3%, respectively. On the other hand, hydrocortisone was associated with a highly significant 55% relative risk reduction for neonatal mortality in infants born at 26-27 weeks.
Post hoc analysis spotlighted several factors which were unexpectedly associated with differential impacts on outcome. For example, hydrocortisone achieved a significant benefit in terms of survival free of BPD at 36 weeks only in females, where the rates were 69.4% versus 53%, for an adjusted 2.25-fold increased rate. In males, the rate was 51.1% with hydrocortisone, compared with 49.7% in controls.
There were no differences between the hydrocortisone and placebo groups in rates of any serious adverse events, including GI perforation, necrotizing enterocolitis, air leaks, severe sepsis, or persistent pulmonary hypertension.
Dr. Baud said the next stage of the PREMILOC study will be to report 2-year outcomes; a greater than 90% follow-up rate is anticipated. Also, he and his coinvestigators are planning ancillary studies examining placental findings, the impact of pretreatment serum cortisol levels, thyroid function, and other issues.
“Our goal is to identify a targeted population that could strongly benefit from prophylactic hydrocortisone,” the neonatologist said.
He reported having no financial conflicts regarding the study, supported by INSERM and other French national research organizations.
SAN DIEGO – Early prophylactic very-low-dose hydrocortisone improved survival free of bronchopulmonary dysplasia in extremely preterm neonates in a large French randomized trial.
The intervention also brought positive results on multiple secondary endpoints, including rates of extubation by day 10 and patent ductus arteriosus closure without resort to ligation, Dr. Olivier Baud reported at the annual meeting of the Pediatric Academic Societies.

He presented the findings of PREMILOC, a double-blind, randomized, placebo-controlled, 21-center French study. The study was halted by the data safety monitoring board for ethical reasons, based upon compelling evidence of superiority after scrutinizing results in the first 521 randomized subjects.
The primary outcome – survival free of bronchopulmonary dysplasia (BPD) at 36 weeks of gestational age – occurred in 60% of the hydrocortisone group, compared with 51% of placebo-treated controls, for an adjusted 48% increased likelihood of favorable outcome. The number-needed-to-treat was 11 patients, said Dr. Baud, professor of pediatrics and chief of the neonatal medicine unit at Robert Debré Hospital in Paris and Paris Diderot University.
The intervention involved administration of intravenous hydrocortisone sodium succinate for the first 10 postnatal days. The dose was 0.5 mg/kg/12 hours for 7 days followed by 0.5 mg/kg/24 hours for 3 days. Treatment began as soon as possible after birth and always within 24 hours. The total cumulative dose of hydrocortisone was 8.5 mg/kg. That’s 15-30 times less corticosteroid than employed in earlier studies of higher-dose dexamethasone, where gastrointestinal bleeding, intestinal perforation, and other serious adverse events were a major problem.
“This is the lowest hydrocortisone dose ever studied in a randomized controlled trial,” according to the neonatologist.
All study participants were born at 24-27 weeks’ gestational age. In order to minimize the risk of treatment-related serious adverse events, especially GI perforation – patients with intrauterine growth retardation or who were on nonsteroidal anti-inflammatory agents were ineligible for the trial.
The rationale for the study, Dr. Baud explained, comes from the landmark work of Dr. Kristi L. Watterberg, professor of pediatrics at the University of New Mexico, Albuquerque, who has argued that early adrenal insufficiency in extremely small-for-gestational-age infants is linked to BPD, patent ductus arteriosus, persistent lung inflammation, and poor enteral nutrition. She introduced the concept that administration of very-low-dose corticosteroids at a dose comparable to normal endogenous steroid production could serve as a global therapy addressing these multiple health problems.
Two prior studies of low-dose hydrocortisone in extremely preterm babies were halted due to an increase in GI perforation, Dr. Baud noted, which is why the French investigators excluded neonates at increased risk for this complication.
In a prespecified PREMILOC subgroup analysis stratified by gestational age, the rate of survival free of BPD at 36 weeks among neonates born at 24-25 weeks was 33.7% with hydrocortisone, compared with 23.3% with placebo, for an adjusted 67% increased likelihood of a positive outcome with active treatment. In babies born at 26-27 weeks, the rates were 72.7% and 65.3%, respectively. On the other hand, hydrocortisone was associated with a highly significant 55% relative risk reduction for neonatal mortality in infants born at 26-27 weeks.
Post hoc analysis spotlighted several factors which were unexpectedly associated with differential impacts on outcome. For example, hydrocortisone achieved a significant benefit in terms of survival free of BPD at 36 weeks only in females, where the rates were 69.4% versus 53%, for an adjusted 2.25-fold increased rate. In males, the rate was 51.1% with hydrocortisone, compared with 49.7% in controls.
There were no differences between the hydrocortisone and placebo groups in rates of any serious adverse events, including GI perforation, necrotizing enterocolitis, air leaks, severe sepsis, or persistent pulmonary hypertension.
Dr. Baud said the next stage of the PREMILOC study will be to report 2-year outcomes; a greater than 90% follow-up rate is anticipated. Also, he and his coinvestigators are planning ancillary studies examining placental findings, the impact of pretreatment serum cortisol levels, thyroid function, and other issues.
“Our goal is to identify a targeted population that could strongly benefit from prophylactic hydrocortisone,” the neonatologist said.
He reported having no financial conflicts regarding the study, supported by INSERM and other French national research organizations.
AT THE PAS ANNUAL MEETING
Key clinical point: In extremely premature neonates, prophylactic very-low-dose hydrocortisone improved the likelihood of survival free of bronchopulmonary dysplasia at 36 weeks’ gestational age by roughly 50%.
Major finding: The number needed to treat to achieve one additional survival without bronchopulmonary dysplasia at 36 weeks’ gestational age was 11.
Data source: This was a randomized, double-blind, placebo-controlled, multicenter French study involving 521 patients born at 24-27 weeks’ gestational age.
Disclosures: The PREMILOC study was supported by INSERM and other French scientific research organizations. The presenter reported having no financial conflicts.
PAS: Screen for postpartum depression during infant hospitalization
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
bjancin@frontlinemedcom.com
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
bjancin@frontlinemedcom.com
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
bjancin@frontlinemedcom.com
AT THE PAS ANNUAL MEETING
Key clinical point: Screening for maternal postpartum depression during an infant’s hospitalization captures large numbers of previously unscreened women.
Major finding: Twenty-eight percent of women screened during their infant’s hospital admission were deemed at risk for postpartum depression.
Data source: The study included 310 mothers, only 18% of whom had been screened for postpartum depression during their infant’s routine office visits as guidelines recommend.
Disclosures: This study was conducted free of commercial support. The presenter reported having no financial conflicts.
PAS: Screen for postpartum depression during infant hospitalization
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT THE PAS ANNUAL MEETING
Key clinical point: Screening for maternal postpartum depression during an infant’s hospitalization captures large numbers of previously unscreened women.
Major finding: Twenty-eight percent of women screened during their infant’s hospital admission were deemed at risk for postpartum depression.
Data source: The study included 310 mothers, only 18% of whom had been screened for postpartum depression during their infant’s routine office visits as guidelines recommend.
Disclosures: This study was conducted free of commercial support. The presenter reported having no financial conflicts.