User login
Annual recurrence rate of anaphylaxis in kids is nearly 30%
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: The risk of recurrent anaphylaxis in children who’ve experienced a first episode may be higher than previously recognized.
Major finding: The annual anaphylaxis recurrence rate was 29% in a group of prospectively followed Montreal children.
Data source: A prospective study of 96 children.
Disclosures: The study was supported by research grants from AllerGen, Health Canada, and Sanofi. The presenter reported having no financial conflicts of interest.
Reslizumab aces pivotal trials in asthma with eosinophilia
HOUSTON – Reslizumab, a next-generation molecular-based asthma therapy, achieved its primary and secondary endpoints and demonstrated favorable safety in patients with moderate to severe asthma and eosinophilia in two pivotal clinical trials.
“We believe that reslizumab is an effective therapy for controlling asthma in patients with elevated blood eosinophils who are inadequately controlled on medium to high dose inhaled corticosteroid–based regimens,” Dr. Mario Castro concluded in presenting the two phase III results at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The frequency of clinical asthma exacerbations was reduced by more than half in reslizumab-treated patients, compared with controls in the two year-long studies. In addition, the reslizumab group experienced an early improvement in lung function as expressed in forced expiratory volume in 1 second (FEV1) that was sustained throughout the year-long trials, as well as improvements in other measures of asthma control, including quality of life, reported Dr. Castro, professor of pulmonary and critical care medicine and pediatrics at Washington University in St. Louis.
Elevated blood and sputum levels of eosinophils define an asthma phenotype at increased risk for serious asthma exacerbations. Reslizumab is a humanized monoclonal antibody that binds circulating interleukin-5 and prevents binding to the IL-5 receptor, thereby disrupting eosinophil production and function.
Dr. Castro presented two identically designed phase III, double-blind, placebo-controlled, 12-month studies totaling 953 adolescents and adults. They were randomized to intravenous reslizumab at 3 mg/kg or placebo every 4 weeks for a year.
The primary endpoint was frequency of clinical asthma exacerbations (CAEs), an independently adjudicated composite outcome which required an episode featuring an increase in corticosteroids, an asthma-related ER visit or unscheduled office visit, evidence of asthma worsening in the form of at least a 20% drop from baseline in FEV1 or a 30% reduction in peak expiratory flow rate on 2 consecutive days, and worsening clinical symptoms.
Participants averaged two CAEs during the year prior to enrollment. The placebo-treated controls maintained that event rate during the two year-long studies, while the reslizumab-treated patients experienced 50% and 59% reductions relative to controls (P < .0001).
Reslizumab also increased the time to first CAE. In the two trials, 61% and 73% of reslizumab-treated patients didn’t develop a single CAE during 52 weeks, compared with 44% and 52% of controls.
The more CAEs a patient had in the year prior to enrollment, the greater the magnitude of benefit with reslizumab. While the relative risk reduction was 54%, compared with placebo in the combined studies, it climbed to 64% in patients with four or more CAEs in the previous year.
FEV1 improved after the first dose of reslizumab. The benefit – placebo-subtracted gains of 0.126 L in one trial and 0.09 L in the other – was sustained throughout the 52 weeks.
The reslizumab group also outperformed controls in terms of Asthma Control Questionnaire scores and Asthma Quality of Life Questionnaire scores. For example, 74% and 73% of reslizumab-treated patients in the two studies experienced at least a 0.5-point improvement in the AQLQ, which is considered the minimal clinically important difference, compared with 65% and 62% of controls, Dr. Castro continued.
Study discontinuation due to adverse events occurred in 2% of patients on reslizumab, with worsening asthma the No. 1 reason. Two patients on reslizumab experienced anaphylactoid reactions, neither requiring epinephrine. Three percent of reslizumab-treated patients developed low-titer, generally transient antidrug antibodies that didn’t affect eosinophil levels, which plunged with the first dose of reslizumab and stayed low throughout.
Reslizumab is one of a cluster of novel agents in development for severe or treatment-resistant asthma. These are targeted therapies directed at specific patient phenotypes. Biomarkers such as eosinophilia provide guidance as to the specific asthmatic inflammatory pathways involved.
Recent European Respiratory Society/American Thoracic Society guidelines stress the major unmet need for new treatments for severe asthma (Eur. Respir. J. 2014;43:343-73).
In an interview, Dr. James E. Gern, who wasn’t involved in the reslizumab studies, said the various severe asthma phenotypes account for a relatively small proportion of the total asthma population, but a tremendously disproportionate amount of health care utilization.
“These new medications that are coming out are probably going to be indicated for a relatively small number of people. But for those people, it’ll make a huge difference because of the huge burden that severe asthma has on quality of life,” said Dr. Gern, professor of pediatrics at the University of Wisconsin, Madison.
The two pivotal reslizumab studies were sponsored by Teva. Dr. Castro is on the company’s speakers’ bureau and receives research grants from more than a dozen pharmaceutical and device companies as well as from the National Institutes of Health and Centers for Disease Control and Prevention. Simultaneously with Dr. Castro’s presentation at AAAAI, the study results were published online (Lancet Respir. Med. 2015 [doi.org/10.1016/S2213-2600(15)00042-9]).
HOUSTON – Reslizumab, a next-generation molecular-based asthma therapy, achieved its primary and secondary endpoints and demonstrated favorable safety in patients with moderate to severe asthma and eosinophilia in two pivotal clinical trials.
“We believe that reslizumab is an effective therapy for controlling asthma in patients with elevated blood eosinophils who are inadequately controlled on medium to high dose inhaled corticosteroid–based regimens,” Dr. Mario Castro concluded in presenting the two phase III results at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The frequency of clinical asthma exacerbations was reduced by more than half in reslizumab-treated patients, compared with controls in the two year-long studies. In addition, the reslizumab group experienced an early improvement in lung function as expressed in forced expiratory volume in 1 second (FEV1) that was sustained throughout the year-long trials, as well as improvements in other measures of asthma control, including quality of life, reported Dr. Castro, professor of pulmonary and critical care medicine and pediatrics at Washington University in St. Louis.
Elevated blood and sputum levels of eosinophils define an asthma phenotype at increased risk for serious asthma exacerbations. Reslizumab is a humanized monoclonal antibody that binds circulating interleukin-5 and prevents binding to the IL-5 receptor, thereby disrupting eosinophil production and function.
Dr. Castro presented two identically designed phase III, double-blind, placebo-controlled, 12-month studies totaling 953 adolescents and adults. They were randomized to intravenous reslizumab at 3 mg/kg or placebo every 4 weeks for a year.
The primary endpoint was frequency of clinical asthma exacerbations (CAEs), an independently adjudicated composite outcome which required an episode featuring an increase in corticosteroids, an asthma-related ER visit or unscheduled office visit, evidence of asthma worsening in the form of at least a 20% drop from baseline in FEV1 or a 30% reduction in peak expiratory flow rate on 2 consecutive days, and worsening clinical symptoms.
Participants averaged two CAEs during the year prior to enrollment. The placebo-treated controls maintained that event rate during the two year-long studies, while the reslizumab-treated patients experienced 50% and 59% reductions relative to controls (P < .0001).
Reslizumab also increased the time to first CAE. In the two trials, 61% and 73% of reslizumab-treated patients didn’t develop a single CAE during 52 weeks, compared with 44% and 52% of controls.
The more CAEs a patient had in the year prior to enrollment, the greater the magnitude of benefit with reslizumab. While the relative risk reduction was 54%, compared with placebo in the combined studies, it climbed to 64% in patients with four or more CAEs in the previous year.
FEV1 improved after the first dose of reslizumab. The benefit – placebo-subtracted gains of 0.126 L in one trial and 0.09 L in the other – was sustained throughout the 52 weeks.
The reslizumab group also outperformed controls in terms of Asthma Control Questionnaire scores and Asthma Quality of Life Questionnaire scores. For example, 74% and 73% of reslizumab-treated patients in the two studies experienced at least a 0.5-point improvement in the AQLQ, which is considered the minimal clinically important difference, compared with 65% and 62% of controls, Dr. Castro continued.
Study discontinuation due to adverse events occurred in 2% of patients on reslizumab, with worsening asthma the No. 1 reason. Two patients on reslizumab experienced anaphylactoid reactions, neither requiring epinephrine. Three percent of reslizumab-treated patients developed low-titer, generally transient antidrug antibodies that didn’t affect eosinophil levels, which plunged with the first dose of reslizumab and stayed low throughout.
Reslizumab is one of a cluster of novel agents in development for severe or treatment-resistant asthma. These are targeted therapies directed at specific patient phenotypes. Biomarkers such as eosinophilia provide guidance as to the specific asthmatic inflammatory pathways involved.
Recent European Respiratory Society/American Thoracic Society guidelines stress the major unmet need for new treatments for severe asthma (Eur. Respir. J. 2014;43:343-73).
In an interview, Dr. James E. Gern, who wasn’t involved in the reslizumab studies, said the various severe asthma phenotypes account for a relatively small proportion of the total asthma population, but a tremendously disproportionate amount of health care utilization.
“These new medications that are coming out are probably going to be indicated for a relatively small number of people. But for those people, it’ll make a huge difference because of the huge burden that severe asthma has on quality of life,” said Dr. Gern, professor of pediatrics at the University of Wisconsin, Madison.
The two pivotal reslizumab studies were sponsored by Teva. Dr. Castro is on the company’s speakers’ bureau and receives research grants from more than a dozen pharmaceutical and device companies as well as from the National Institutes of Health and Centers for Disease Control and Prevention. Simultaneously with Dr. Castro’s presentation at AAAAI, the study results were published online (Lancet Respir. Med. 2015 [doi.org/10.1016/S2213-2600(15)00042-9]).
HOUSTON – Reslizumab, a next-generation molecular-based asthma therapy, achieved its primary and secondary endpoints and demonstrated favorable safety in patients with moderate to severe asthma and eosinophilia in two pivotal clinical trials.
“We believe that reslizumab is an effective therapy for controlling asthma in patients with elevated blood eosinophils who are inadequately controlled on medium to high dose inhaled corticosteroid–based regimens,” Dr. Mario Castro concluded in presenting the two phase III results at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The frequency of clinical asthma exacerbations was reduced by more than half in reslizumab-treated patients, compared with controls in the two year-long studies. In addition, the reslizumab group experienced an early improvement in lung function as expressed in forced expiratory volume in 1 second (FEV1) that was sustained throughout the year-long trials, as well as improvements in other measures of asthma control, including quality of life, reported Dr. Castro, professor of pulmonary and critical care medicine and pediatrics at Washington University in St. Louis.
Elevated blood and sputum levels of eosinophils define an asthma phenotype at increased risk for serious asthma exacerbations. Reslizumab is a humanized monoclonal antibody that binds circulating interleukin-5 and prevents binding to the IL-5 receptor, thereby disrupting eosinophil production and function.
Dr. Castro presented two identically designed phase III, double-blind, placebo-controlled, 12-month studies totaling 953 adolescents and adults. They were randomized to intravenous reslizumab at 3 mg/kg or placebo every 4 weeks for a year.
The primary endpoint was frequency of clinical asthma exacerbations (CAEs), an independently adjudicated composite outcome which required an episode featuring an increase in corticosteroids, an asthma-related ER visit or unscheduled office visit, evidence of asthma worsening in the form of at least a 20% drop from baseline in FEV1 or a 30% reduction in peak expiratory flow rate on 2 consecutive days, and worsening clinical symptoms.
Participants averaged two CAEs during the year prior to enrollment. The placebo-treated controls maintained that event rate during the two year-long studies, while the reslizumab-treated patients experienced 50% and 59% reductions relative to controls (P < .0001).
Reslizumab also increased the time to first CAE. In the two trials, 61% and 73% of reslizumab-treated patients didn’t develop a single CAE during 52 weeks, compared with 44% and 52% of controls.
The more CAEs a patient had in the year prior to enrollment, the greater the magnitude of benefit with reslizumab. While the relative risk reduction was 54%, compared with placebo in the combined studies, it climbed to 64% in patients with four or more CAEs in the previous year.
FEV1 improved after the first dose of reslizumab. The benefit – placebo-subtracted gains of 0.126 L in one trial and 0.09 L in the other – was sustained throughout the 52 weeks.
The reslizumab group also outperformed controls in terms of Asthma Control Questionnaire scores and Asthma Quality of Life Questionnaire scores. For example, 74% and 73% of reslizumab-treated patients in the two studies experienced at least a 0.5-point improvement in the AQLQ, which is considered the minimal clinically important difference, compared with 65% and 62% of controls, Dr. Castro continued.
Study discontinuation due to adverse events occurred in 2% of patients on reslizumab, with worsening asthma the No. 1 reason. Two patients on reslizumab experienced anaphylactoid reactions, neither requiring epinephrine. Three percent of reslizumab-treated patients developed low-titer, generally transient antidrug antibodies that didn’t affect eosinophil levels, which plunged with the first dose of reslizumab and stayed low throughout.
Reslizumab is one of a cluster of novel agents in development for severe or treatment-resistant asthma. These are targeted therapies directed at specific patient phenotypes. Biomarkers such as eosinophilia provide guidance as to the specific asthmatic inflammatory pathways involved.
Recent European Respiratory Society/American Thoracic Society guidelines stress the major unmet need for new treatments for severe asthma (Eur. Respir. J. 2014;43:343-73).
In an interview, Dr. James E. Gern, who wasn’t involved in the reslizumab studies, said the various severe asthma phenotypes account for a relatively small proportion of the total asthma population, but a tremendously disproportionate amount of health care utilization.
“These new medications that are coming out are probably going to be indicated for a relatively small number of people. But for those people, it’ll make a huge difference because of the huge burden that severe asthma has on quality of life,” said Dr. Gern, professor of pediatrics at the University of Wisconsin, Madison.
The two pivotal reslizumab studies were sponsored by Teva. Dr. Castro is on the company’s speakers’ bureau and receives research grants from more than a dozen pharmaceutical and device companies as well as from the National Institutes of Health and Centers for Disease Control and Prevention. Simultaneously with Dr. Castro’s presentation at AAAAI, the study results were published online (Lancet Respir. Med. 2015 [doi.org/10.1016/S2213-2600(15)00042-9]).
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: A monoclonal antibody against interleukin-5 significantly reduced the frequency of clinical asthma exacerbations in an important subset of asthma patients.
Major finding: Intravenous reslizumab given every 4 weeks reduced the annual rate of clinical asthma exacerbations by 50% and 59%, compared with placebo in two phase III trials.
Data source: The two randomized, double-blind, placebo-controlled, 1-year pivotal trials totaled 953 patients with moderate to severe asthma with elevated blood eosinophils inadequately controlled using inhaled corticosteroid–based regimens.
Disclosures: The studies were sponsored by Teva. The presenter is on the company’s speakers’ bureau and receives research grants from more than a dozen pharmaceutical and device companies.
Respiratory harm reversal seen in asthmatic smokers on e-cigarettes
HOUSTON – Asthmatic smokers who switched to electronic cigarettes showed evidence suggestive of respiratory harm reversal in a retrospective pilot study.
“Electronic cigarette use improves respiratory physiology and subjective asthma outcomes in asthmatic smokers. E-cigarettes are a safer alternative to conventional cigarettes in this vulnerable population,” Dr. Cristina Russo declared at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
She said that her small retrospective study is the first to examine the respiratory health impact of a switch to e-cigarettes by asthmatic smokers.
Every one of the objective and subjective measures of asthma status evaluated in the study showed statistically significant improvement 1 year after patients adopted e-cigarettes, and the e-cigarette users’ consumption of conventional cigarettes dropped precipitously, reported Dr. Russo of the University of Catania (Italy).
She and her colleagues in the university asthma clinic have taken to suggesting the use of battery-powered e-cigarettes to their asthmatic smokers who haven’t benefited from or aren’t interested in trying the more conventional approaches to smoking cessation or reduction, including medications. While abstinence from cigarette smoking is best, the available evidence indicates e-cigarettes are at least 95% less harmful than conventional cigarettes in the general population, she said.
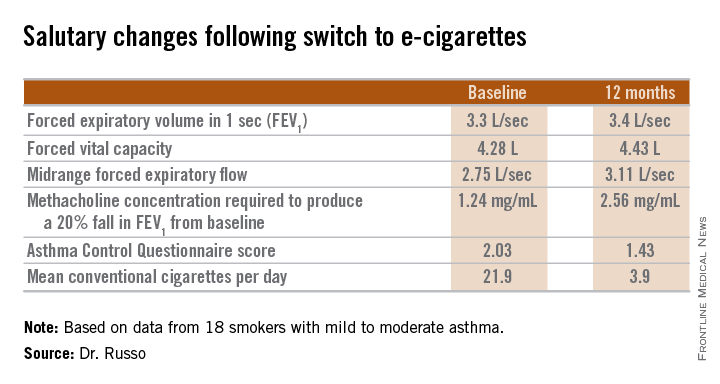
The study included 18 smokers with mild to moderate asthma who switched to e-cigarettes and underwent spirometry and other testing at baseline and 6 and 12 months of follow-up. Ten patients switched over to e-cigarettes exclusively, while the other 8 used both conventional and e-cigarettes.
Among the highlights: The mid-range forced expiratory flow (25%-75%) showed a major, clinically important improvement, increasing from 2.75 L/sec to 3.11 L/sec. And patients’ mean self-reported conventional cigarette consumption dropped from 21.9 per day at baseline to 5 at 6 months and 3.9 per day at 12 months.
Among the group at large, no significant change was seen in the frequency of asthma exacerbations resulting in hospitalization. However, among the frequent exacerbators – the six patients with two or more exacerbations during the 6 months prior to baseline – exacerbation frequency was cut in half both 6 and 12 months following the switch to e-cigarettes.
Dr. Russo’s presentation sparked vigorous audience discussion. Several physicians cited a Centers for Disease Control and Prevention warning about the unknowns regarding e-cigarette safety, and one allergist declared he didn’t think physicians should ever encourage patients to smoke anything. But others defended the “lesser of two evils” approach adopted by Dr. Russo and coworkers.
Dr. Russo noted that the prevalence of smoking among asthma patients is similar to that of the general population. She called smoking and asthma “a dangerous liaison.” Smoking accelerates asthma patients’ decline in lung function, worsens persistent airways obstruction, and increases insensitivity to corticosteroids.
Her study was supported by a university grant and the Italian League Against Smoking. She reported having no financial conflicts.
HOUSTON – Asthmatic smokers who switched to electronic cigarettes showed evidence suggestive of respiratory harm reversal in a retrospective pilot study.
“Electronic cigarette use improves respiratory physiology and subjective asthma outcomes in asthmatic smokers. E-cigarettes are a safer alternative to conventional cigarettes in this vulnerable population,” Dr. Cristina Russo declared at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.

She said that her small retrospective study is the first to examine the respiratory health impact of a switch to e-cigarettes by asthmatic smokers.
Every one of the objective and subjective measures of asthma status evaluated in the study showed statistically significant improvement 1 year after patients adopted e-cigarettes, and the e-cigarette users’ consumption of conventional cigarettes dropped precipitously, reported Dr. Russo of the University of Catania (Italy).
She and her colleagues in the university asthma clinic have taken to suggesting the use of battery-powered e-cigarettes to their asthmatic smokers who haven’t benefited from or aren’t interested in trying the more conventional approaches to smoking cessation or reduction, including medications. While abstinence from cigarette smoking is best, the available evidence indicates e-cigarettes are at least 95% less harmful than conventional cigarettes in the general population, she said.
The study included 18 smokers with mild to moderate asthma who switched to e-cigarettes and underwent spirometry and other testing at baseline and 6 and 12 months of follow-up. Ten patients switched over to e-cigarettes exclusively, while the other 8 used both conventional and e-cigarettes.
Among the highlights: The mid-range forced expiratory flow (25%-75%) showed a major, clinically important improvement, increasing from 2.75 L/sec to 3.11 L/sec. And patients’ mean self-reported conventional cigarette consumption dropped from 21.9 per day at baseline to 5 at 6 months and 3.9 per day at 12 months.
Among the group at large, no significant change was seen in the frequency of asthma exacerbations resulting in hospitalization. However, among the frequent exacerbators – the six patients with two or more exacerbations during the 6 months prior to baseline – exacerbation frequency was cut in half both 6 and 12 months following the switch to e-cigarettes.
Dr. Russo’s presentation sparked vigorous audience discussion. Several physicians cited a Centers for Disease Control and Prevention warning about the unknowns regarding e-cigarette safety, and one allergist declared he didn’t think physicians should ever encourage patients to smoke anything. But others defended the “lesser of two evils” approach adopted by Dr. Russo and coworkers.
Dr. Russo noted that the prevalence of smoking among asthma patients is similar to that of the general population. She called smoking and asthma “a dangerous liaison.” Smoking accelerates asthma patients’ decline in lung function, worsens persistent airways obstruction, and increases insensitivity to corticosteroids.
Her study was supported by a university grant and the Italian League Against Smoking. She reported having no financial conflicts.
HOUSTON – Asthmatic smokers who switched to electronic cigarettes showed evidence suggestive of respiratory harm reversal in a retrospective pilot study.
“Electronic cigarette use improves respiratory physiology and subjective asthma outcomes in asthmatic smokers. E-cigarettes are a safer alternative to conventional cigarettes in this vulnerable population,” Dr. Cristina Russo declared at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.

She said that her small retrospective study is the first to examine the respiratory health impact of a switch to e-cigarettes by asthmatic smokers.
Every one of the objective and subjective measures of asthma status evaluated in the study showed statistically significant improvement 1 year after patients adopted e-cigarettes, and the e-cigarette users’ consumption of conventional cigarettes dropped precipitously, reported Dr. Russo of the University of Catania (Italy).
She and her colleagues in the university asthma clinic have taken to suggesting the use of battery-powered e-cigarettes to their asthmatic smokers who haven’t benefited from or aren’t interested in trying the more conventional approaches to smoking cessation or reduction, including medications. While abstinence from cigarette smoking is best, the available evidence indicates e-cigarettes are at least 95% less harmful than conventional cigarettes in the general population, she said.
The study included 18 smokers with mild to moderate asthma who switched to e-cigarettes and underwent spirometry and other testing at baseline and 6 and 12 months of follow-up. Ten patients switched over to e-cigarettes exclusively, while the other 8 used both conventional and e-cigarettes.
Among the highlights: The mid-range forced expiratory flow (25%-75%) showed a major, clinically important improvement, increasing from 2.75 L/sec to 3.11 L/sec. And patients’ mean self-reported conventional cigarette consumption dropped from 21.9 per day at baseline to 5 at 6 months and 3.9 per day at 12 months.
Among the group at large, no significant change was seen in the frequency of asthma exacerbations resulting in hospitalization. However, among the frequent exacerbators – the six patients with two or more exacerbations during the 6 months prior to baseline – exacerbation frequency was cut in half both 6 and 12 months following the switch to e-cigarettes.
Dr. Russo’s presentation sparked vigorous audience discussion. Several physicians cited a Centers for Disease Control and Prevention warning about the unknowns regarding e-cigarette safety, and one allergist declared he didn’t think physicians should ever encourage patients to smoke anything. But others defended the “lesser of two evils” approach adopted by Dr. Russo and coworkers.
Dr. Russo noted that the prevalence of smoking among asthma patients is similar to that of the general population. She called smoking and asthma “a dangerous liaison.” Smoking accelerates asthma patients’ decline in lung function, worsens persistent airways obstruction, and increases insensitivity to corticosteroids.
Her study was supported by a university grant and the Italian League Against Smoking. She reported having no financial conflicts.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Asthmatic smokers who adopted physician advice to switch to electronic cigarettes showed significant 1-year improvements in lung function, methacholine-provoked airway hyperresponsiveness, and asthma-related quality of life.
Major finding: Self-reported daily consumption of conventional cigarettes by asthmatic smokers who switched to e-cigarettes fell from a mean of 21.9 per day at baseline to 5.0 at 6 months and 3.9 at 12 months of follow-up.
Data source: This retrospective pilot study included 18 asthmatic smokers who switched to e-cigarettes.
Disclosures: The study was supported by a university grant and the Italian League Against Smoking. The presenter reported having no financial conflicts.
What’s new with methotrexate for rheumatoid arthritis
SNOWMASS, COLO. – Can an old rheumatoid arthritis drug – like an old dog – learn new tricks?
When that drug is methotrexate, the answer is definitely yes, according to Dr. Michael E. Weinblatt, professor of medicine at Harvard Medical School, Boston.
Clinically important advances and refinements continue to be made in the use of this venerable drug, a treatment mainstay for RA since the mid-1980s.
“I had the pleasure of participating in a symposium on methotrexate organized by Dr. Joel Kremer at last year’s annual ACR meeting in Boston. This is the second one we’ve done, and I must tell you, the number of people in attendance was extraordinary. We probably filled one of the largest conference rooms for the second time in 2 years. And the questions went on forever. So even though methotrexate is an old drug, there still remains great interest in it,” he observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Here’s what’s new:
Subcutaneous methotrexate for RA is finally legit.
Multiple studies over the past 15 years have established that at methotrexate doses above 15-20 mg/week, subcutaneous therapy achieves superior efficacy, compared with oral therapy. For this reason, rheumatologists have long used once-weekly subcutaneous injections of methotrexate in RA patients with an inadequate clinical response to oral therapy. However, as payers have often informed them, this has been off-label usage. Not any longer. Rheumatoid arthritis is now an FDA-approved indication for subcutaneous methotrexate. Moreover, two easy-to-use, single-dose, once-weekly, autoinjector formulations are now on the market, known as Otrexup and Rasuvo.
In a randomized, crossover study of various doses of oral methotrexate versus Otrexup in 49 RA patients, the systemic bioavailability of oral methotrexate plateaued at doses of 15 mg/week or above. In contrast, the systemic exposure of subcutaneous methotrexate continued to increase linearly out to 25 mg/week, the highest dose studied. And this greater bioavailability wasn’t associated with an increase in adverse events (Ann. Rheum. Dis. 2014;73:1549-51).
Split-dose oral therapy improves bioavailability.
When methotrexate was first prescribed for RA in the mid-1980s, it was given in a pulsed-dose regimen: 8 a.m., 8 p.m., and again at 8 a.m. the next morning. Three doses over a 24-hour period, once weekly.
“Over time, most of us shifted to a once-a-week regimen for convenience, ease-of-administration, and because of some concerns about liver exposure. We’ve come to appreciate, however, that if you dose-escalate methotrexate, a significant percentage of patients do not absorb the drug at doses above 15-20 mg once weekly. But pharmacokinetic studies have demonstrated you get higher drug concentrations with a split dose,” according to the rheumatologist.
For example, a crossover study involving 10 patients on stable doses of oral methotrexate at 25-35 mg/week showed that splitting a dose into two halves given 8 hours apart resulted in 28% greater bioavailability, compared with the standard single dose. Indeed, the bioavailability with the oral split dose was comparable to that achieved with subcutaneous administration (J. Rheumatol. 2006;33:481-5).
“Many of us are now splitting the dose of methotrexate at doses above 17.5-20 mg/week. I would like to caution, however, that this does not mean you should give the drug on Mondays and Thursdays. This was studied 40 years ago at the [National Institutes of Health]. It caused more acute toxicity, including bone marrow suppression, and more chronic toxicity, such as liver disease. So the drug should only be administered over one 24-hour period per week,” Dr. Weinblatt advised.
Methotrexate: the next statin?
Four cohort studies over the past 10 years have reported that methotrexate appears to reduce cardiovascular mortality in RA patients. Registry data are also supportive. The evidence is sufficiently strong that a definitive randomized clinical trial is now well underway. This major study, known as the Cardiovascular Inflammation Reduction Trial (CIRT), is funded by the National Heart, Lung, and Blood Institute and led by renowned cardiologist Dr. Paul Ridker, the Eugene Braunwald Professor of Medicine at Harvard Medical School. It will enroll 7,000 subjects – none with RA – at roughly 350 U.S. and Canadian sites.
Participants are high–cardiovascular risk patients with a history of acute MI within the previous 5 years plus type 2 diabetes or metabolic syndrome. They are being randomized to guideline-directed medical care plus either oral methotrexate at 10-20 mg/week or placebo for 2-4 years. The primary endpoint is cardiovascular death, nonfatal MI, or stroke.
The proposed mechanism of benefit is methotrexate’s anti-inflammatory effect. If the results of CIRT are positive, this low-cost generic drug with a thoroughly understood safety profile could transform preventive cardiology; lowering systemic inflammation would potentially join LDL lowering via statins as a cornerstone of disease prevention.
“This is one of the most exciting areas involving methotrexate,” commented Dr. Weinblatt, a participating investigator in CIRT.
Methotrexate boosts efficacy of selected biologics.
Concomitant methotrexate is known to increase the clinical efficacy of anti–tumor necrosis factor biologics and rituximab. Studies in patients on infliximab or adalimumab have shown that background methotrexate raises serum concentrations of the biologics by 20%-25%, which is presumably the explanation for the increased efficacy. Whether methotrexate enhances or attenuates the efficacy of the oral small-molecule JAK inhibitors is now under study.
One intriguing cohort study has shown that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion; the higher the dose of methotrexate, the less ability patients had to develop blocking antibodies against adalimumab (Ann. Rheum. Dis. 2012;71:1914-5).
More recently, the randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients extended this work. Participants were randomized to open-label adalimumab plus weekly blinded methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and other measures were significantly better in patients on 10 or 20 mg/week of methotrexate. Serum adalimumab levels were higher in patients on those doses of methotrexate than in those on 2.5 or 5 mg/week as well (Ann. Rheum. Dis. 2014 Feb. 18 [doi:10.1136/annrheumdis-2013-204769]).
There’s an important lesson here for patient management: “If you’re going to dose-reduce your methotrexate in patients with low disease activity or in remission, there is a threshold at which you should probably stop reducing the methotrexate, at least when your patient is on this biologic,” according to Dr. Weinblatt.
In contrast, no methotrexate dose-threshold effect occurs with etanercept, he added.
Methotrexate and the liver.
A national observational cohort study of 659 military veterans over age 65 when they started methotrexate for RA or other rheumatic diseases found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. The investigators identified a series of independent risk factors for liver function test abnormalities. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, abnormal liver function tests premethotrexate was associated with a 3.2-fold increased risk, and lack of folic acid supplementation brought a 2.2-fold increase in risk (Arthritis Care Res. 2014;66:1159-66).
“This study confirms what a lot of us have been worried about,” Dr. Weinblatt commented. That is, the risk of methotrexate-related liver toxicity is particularly high in older patients with characteristics consistent with the metabolic syndrome, a condition that has reached epidemic proportions.
A strong take-home message from this study, he added, is that patients should not start on methotrexate if they have abnormal liver function tests. That guidance was included in the original guidelines for methotrexate in RA published back in the mid-1990s.
“I think rheumatologists have kind of lost sight of that. You probably should find out why those tests are elevated. In many cases you’ll find it may be related to NASH [nonalcoholic steatohepatitis],” he said. “I actually think obesity is one of the greatest risk factors for methotrexate toxicity.”
Dr. Weinblatt reported receiving consulting fees or other remuneration from more than two dozen pharmaceutical companies.
SNOWMASS, COLO. – Can an old rheumatoid arthritis drug – like an old dog – learn new tricks?
When that drug is methotrexate, the answer is definitely yes, according to Dr. Michael E. Weinblatt, professor of medicine at Harvard Medical School, Boston.
Clinically important advances and refinements continue to be made in the use of this venerable drug, a treatment mainstay for RA since the mid-1980s.
“I had the pleasure of participating in a symposium on methotrexate organized by Dr. Joel Kremer at last year’s annual ACR meeting in Boston. This is the second one we’ve done, and I must tell you, the number of people in attendance was extraordinary. We probably filled one of the largest conference rooms for the second time in 2 years. And the questions went on forever. So even though methotrexate is an old drug, there still remains great interest in it,” he observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Here’s what’s new:
Subcutaneous methotrexate for RA is finally legit.
Multiple studies over the past 15 years have established that at methotrexate doses above 15-20 mg/week, subcutaneous therapy achieves superior efficacy, compared with oral therapy. For this reason, rheumatologists have long used once-weekly subcutaneous injections of methotrexate in RA patients with an inadequate clinical response to oral therapy. However, as payers have often informed them, this has been off-label usage. Not any longer. Rheumatoid arthritis is now an FDA-approved indication for subcutaneous methotrexate. Moreover, two easy-to-use, single-dose, once-weekly, autoinjector formulations are now on the market, known as Otrexup and Rasuvo.
In a randomized, crossover study of various doses of oral methotrexate versus Otrexup in 49 RA patients, the systemic bioavailability of oral methotrexate plateaued at doses of 15 mg/week or above. In contrast, the systemic exposure of subcutaneous methotrexate continued to increase linearly out to 25 mg/week, the highest dose studied. And this greater bioavailability wasn’t associated with an increase in adverse events (Ann. Rheum. Dis. 2014;73:1549-51).
Split-dose oral therapy improves bioavailability.
When methotrexate was first prescribed for RA in the mid-1980s, it was given in a pulsed-dose regimen: 8 a.m., 8 p.m., and again at 8 a.m. the next morning. Three doses over a 24-hour period, once weekly.
“Over time, most of us shifted to a once-a-week regimen for convenience, ease-of-administration, and because of some concerns about liver exposure. We’ve come to appreciate, however, that if you dose-escalate methotrexate, a significant percentage of patients do not absorb the drug at doses above 15-20 mg once weekly. But pharmacokinetic studies have demonstrated you get higher drug concentrations with a split dose,” according to the rheumatologist.
For example, a crossover study involving 10 patients on stable doses of oral methotrexate at 25-35 mg/week showed that splitting a dose into two halves given 8 hours apart resulted in 28% greater bioavailability, compared with the standard single dose. Indeed, the bioavailability with the oral split dose was comparable to that achieved with subcutaneous administration (J. Rheumatol. 2006;33:481-5).
“Many of us are now splitting the dose of methotrexate at doses above 17.5-20 mg/week. I would like to caution, however, that this does not mean you should give the drug on Mondays and Thursdays. This was studied 40 years ago at the [National Institutes of Health]. It caused more acute toxicity, including bone marrow suppression, and more chronic toxicity, such as liver disease. So the drug should only be administered over one 24-hour period per week,” Dr. Weinblatt advised.
Methotrexate: the next statin?
Four cohort studies over the past 10 years have reported that methotrexate appears to reduce cardiovascular mortality in RA patients. Registry data are also supportive. The evidence is sufficiently strong that a definitive randomized clinical trial is now well underway. This major study, known as the Cardiovascular Inflammation Reduction Trial (CIRT), is funded by the National Heart, Lung, and Blood Institute and led by renowned cardiologist Dr. Paul Ridker, the Eugene Braunwald Professor of Medicine at Harvard Medical School. It will enroll 7,000 subjects – none with RA – at roughly 350 U.S. and Canadian sites.
Participants are high–cardiovascular risk patients with a history of acute MI within the previous 5 years plus type 2 diabetes or metabolic syndrome. They are being randomized to guideline-directed medical care plus either oral methotrexate at 10-20 mg/week or placebo for 2-4 years. The primary endpoint is cardiovascular death, nonfatal MI, or stroke.
The proposed mechanism of benefit is methotrexate’s anti-inflammatory effect. If the results of CIRT are positive, this low-cost generic drug with a thoroughly understood safety profile could transform preventive cardiology; lowering systemic inflammation would potentially join LDL lowering via statins as a cornerstone of disease prevention.
“This is one of the most exciting areas involving methotrexate,” commented Dr. Weinblatt, a participating investigator in CIRT.
Methotrexate boosts efficacy of selected biologics.
Concomitant methotrexate is known to increase the clinical efficacy of anti–tumor necrosis factor biologics and rituximab. Studies in patients on infliximab or adalimumab have shown that background methotrexate raises serum concentrations of the biologics by 20%-25%, which is presumably the explanation for the increased efficacy. Whether methotrexate enhances or attenuates the efficacy of the oral small-molecule JAK inhibitors is now under study.
One intriguing cohort study has shown that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion; the higher the dose of methotrexate, the less ability patients had to develop blocking antibodies against adalimumab (Ann. Rheum. Dis. 2012;71:1914-5).
More recently, the randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients extended this work. Participants were randomized to open-label adalimumab plus weekly blinded methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and other measures were significantly better in patients on 10 or 20 mg/week of methotrexate. Serum adalimumab levels were higher in patients on those doses of methotrexate than in those on 2.5 or 5 mg/week as well (Ann. Rheum. Dis. 2014 Feb. 18 [doi:10.1136/annrheumdis-2013-204769]).
There’s an important lesson here for patient management: “If you’re going to dose-reduce your methotrexate in patients with low disease activity or in remission, there is a threshold at which you should probably stop reducing the methotrexate, at least when your patient is on this biologic,” according to Dr. Weinblatt.
In contrast, no methotrexate dose-threshold effect occurs with etanercept, he added.
Methotrexate and the liver.
A national observational cohort study of 659 military veterans over age 65 when they started methotrexate for RA or other rheumatic diseases found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. The investigators identified a series of independent risk factors for liver function test abnormalities. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, abnormal liver function tests premethotrexate was associated with a 3.2-fold increased risk, and lack of folic acid supplementation brought a 2.2-fold increase in risk (Arthritis Care Res. 2014;66:1159-66).
“This study confirms what a lot of us have been worried about,” Dr. Weinblatt commented. That is, the risk of methotrexate-related liver toxicity is particularly high in older patients with characteristics consistent with the metabolic syndrome, a condition that has reached epidemic proportions.
A strong take-home message from this study, he added, is that patients should not start on methotrexate if they have abnormal liver function tests. That guidance was included in the original guidelines for methotrexate in RA published back in the mid-1990s.
“I think rheumatologists have kind of lost sight of that. You probably should find out why those tests are elevated. In many cases you’ll find it may be related to NASH [nonalcoholic steatohepatitis],” he said. “I actually think obesity is one of the greatest risk factors for methotrexate toxicity.”
Dr. Weinblatt reported receiving consulting fees or other remuneration from more than two dozen pharmaceutical companies.
SNOWMASS, COLO. – Can an old rheumatoid arthritis drug – like an old dog – learn new tricks?
When that drug is methotrexate, the answer is definitely yes, according to Dr. Michael E. Weinblatt, professor of medicine at Harvard Medical School, Boston.
Clinically important advances and refinements continue to be made in the use of this venerable drug, a treatment mainstay for RA since the mid-1980s.
“I had the pleasure of participating in a symposium on methotrexate organized by Dr. Joel Kremer at last year’s annual ACR meeting in Boston. This is the second one we’ve done, and I must tell you, the number of people in attendance was extraordinary. We probably filled one of the largest conference rooms for the second time in 2 years. And the questions went on forever. So even though methotrexate is an old drug, there still remains great interest in it,” he observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Here’s what’s new:
Subcutaneous methotrexate for RA is finally legit.
Multiple studies over the past 15 years have established that at methotrexate doses above 15-20 mg/week, subcutaneous therapy achieves superior efficacy, compared with oral therapy. For this reason, rheumatologists have long used once-weekly subcutaneous injections of methotrexate in RA patients with an inadequate clinical response to oral therapy. However, as payers have often informed them, this has been off-label usage. Not any longer. Rheumatoid arthritis is now an FDA-approved indication for subcutaneous methotrexate. Moreover, two easy-to-use, single-dose, once-weekly, autoinjector formulations are now on the market, known as Otrexup and Rasuvo.
In a randomized, crossover study of various doses of oral methotrexate versus Otrexup in 49 RA patients, the systemic bioavailability of oral methotrexate plateaued at doses of 15 mg/week or above. In contrast, the systemic exposure of subcutaneous methotrexate continued to increase linearly out to 25 mg/week, the highest dose studied. And this greater bioavailability wasn’t associated with an increase in adverse events (Ann. Rheum. Dis. 2014;73:1549-51).
Split-dose oral therapy improves bioavailability.
When methotrexate was first prescribed for RA in the mid-1980s, it was given in a pulsed-dose regimen: 8 a.m., 8 p.m., and again at 8 a.m. the next morning. Three doses over a 24-hour period, once weekly.
“Over time, most of us shifted to a once-a-week regimen for convenience, ease-of-administration, and because of some concerns about liver exposure. We’ve come to appreciate, however, that if you dose-escalate methotrexate, a significant percentage of patients do not absorb the drug at doses above 15-20 mg once weekly. But pharmacokinetic studies have demonstrated you get higher drug concentrations with a split dose,” according to the rheumatologist.
For example, a crossover study involving 10 patients on stable doses of oral methotrexate at 25-35 mg/week showed that splitting a dose into two halves given 8 hours apart resulted in 28% greater bioavailability, compared with the standard single dose. Indeed, the bioavailability with the oral split dose was comparable to that achieved with subcutaneous administration (J. Rheumatol. 2006;33:481-5).
“Many of us are now splitting the dose of methotrexate at doses above 17.5-20 mg/week. I would like to caution, however, that this does not mean you should give the drug on Mondays and Thursdays. This was studied 40 years ago at the [National Institutes of Health]. It caused more acute toxicity, including bone marrow suppression, and more chronic toxicity, such as liver disease. So the drug should only be administered over one 24-hour period per week,” Dr. Weinblatt advised.
Methotrexate: the next statin?
Four cohort studies over the past 10 years have reported that methotrexate appears to reduce cardiovascular mortality in RA patients. Registry data are also supportive. The evidence is sufficiently strong that a definitive randomized clinical trial is now well underway. This major study, known as the Cardiovascular Inflammation Reduction Trial (CIRT), is funded by the National Heart, Lung, and Blood Institute and led by renowned cardiologist Dr. Paul Ridker, the Eugene Braunwald Professor of Medicine at Harvard Medical School. It will enroll 7,000 subjects – none with RA – at roughly 350 U.S. and Canadian sites.
Participants are high–cardiovascular risk patients with a history of acute MI within the previous 5 years plus type 2 diabetes or metabolic syndrome. They are being randomized to guideline-directed medical care plus either oral methotrexate at 10-20 mg/week or placebo for 2-4 years. The primary endpoint is cardiovascular death, nonfatal MI, or stroke.
The proposed mechanism of benefit is methotrexate’s anti-inflammatory effect. If the results of CIRT are positive, this low-cost generic drug with a thoroughly understood safety profile could transform preventive cardiology; lowering systemic inflammation would potentially join LDL lowering via statins as a cornerstone of disease prevention.
“This is one of the most exciting areas involving methotrexate,” commented Dr. Weinblatt, a participating investigator in CIRT.
Methotrexate boosts efficacy of selected biologics.
Concomitant methotrexate is known to increase the clinical efficacy of anti–tumor necrosis factor biologics and rituximab. Studies in patients on infliximab or adalimumab have shown that background methotrexate raises serum concentrations of the biologics by 20%-25%, which is presumably the explanation for the increased efficacy. Whether methotrexate enhances or attenuates the efficacy of the oral small-molecule JAK inhibitors is now under study.
One intriguing cohort study has shown that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion; the higher the dose of methotrexate, the less ability patients had to develop blocking antibodies against adalimumab (Ann. Rheum. Dis. 2012;71:1914-5).
More recently, the randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients extended this work. Participants were randomized to open-label adalimumab plus weekly blinded methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and other measures were significantly better in patients on 10 or 20 mg/week of methotrexate. Serum adalimumab levels were higher in patients on those doses of methotrexate than in those on 2.5 or 5 mg/week as well (Ann. Rheum. Dis. 2014 Feb. 18 [doi:10.1136/annrheumdis-2013-204769]).
There’s an important lesson here for patient management: “If you’re going to dose-reduce your methotrexate in patients with low disease activity or in remission, there is a threshold at which you should probably stop reducing the methotrexate, at least when your patient is on this biologic,” according to Dr. Weinblatt.
In contrast, no methotrexate dose-threshold effect occurs with etanercept, he added.
Methotrexate and the liver.
A national observational cohort study of 659 military veterans over age 65 when they started methotrexate for RA or other rheumatic diseases found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. The investigators identified a series of independent risk factors for liver function test abnormalities. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, abnormal liver function tests premethotrexate was associated with a 3.2-fold increased risk, and lack of folic acid supplementation brought a 2.2-fold increase in risk (Arthritis Care Res. 2014;66:1159-66).
“This study confirms what a lot of us have been worried about,” Dr. Weinblatt commented. That is, the risk of methotrexate-related liver toxicity is particularly high in older patients with characteristics consistent with the metabolic syndrome, a condition that has reached epidemic proportions.
A strong take-home message from this study, he added, is that patients should not start on methotrexate if they have abnormal liver function tests. That guidance was included in the original guidelines for methotrexate in RA published back in the mid-1990s.
“I think rheumatologists have kind of lost sight of that. You probably should find out why those tests are elevated. In many cases you’ll find it may be related to NASH [nonalcoholic steatohepatitis],” he said. “I actually think obesity is one of the greatest risk factors for methotrexate toxicity.”
Dr. Weinblatt reported receiving consulting fees or other remuneration from more than two dozen pharmaceutical companies.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Rituximab for RA: Think like a European
SNOWMASS, COLO. – When it comes to prescribing rituximab in patients with rheumatoid arthritis, maybe it’s time to think like a European.
In response to a persuasive French study, rheumatologists across Europe have embraced a lower-dose rituximab regimen: Patients who achieve a moderate or good EULAR response to the standard initial induction dosing of two 1,000-mg doses given intravenously 2 weeks apart receive thereafter a single 1,000-mg dose every 6 months rather than the licensed maintenance dosing of two 1,000-mg IV injections every 6 months.
“They showed that one dose every 6 months is as good as two doses every 6 months. This is a strategy that our European colleagues have actively adopted. It’s a cost-effective strategy, and I think it’s actually an appropriate strategy to use and one we’re using routinely now in our rheumatoid patients after we’ve induced a response with the initial induction dosing,” Dr. Michael E. Weinblatt, professor of medicine at Harvard Medical School, Boston, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
The French study was an open-label, prospective, multicenter, noninferiority study involving 225 rheumatoid arthritis patients with an inadequate response to anti–tumor necrosis factor (anti-TNF) agents and a 6-month moderate or good EULAR response to rituximab (Rituxan) induction therapy coupled with methotrexate. They were randomized to rituximab retreatment at 6-month intervals at either a single 1,000-mg dose or the two 1,000-mg doses as described in the product labeling. At 104 weeks, disease activity as measured by the mean disease activity in 28 joints plus C-reactive protein level area under the curve was similar in the two treatment arms. So was the safety profile (Ann. Rheum. Dis. 2014;73:1508-14).
Dr. Weinblatt also provided an update on rituximab and infections. Two studies presented at last fall’s annual meeting of the American College of Rheumatology in Boston shed new light on this perennially hot topic.
In one, Dr. Kenneth G. Saag of the University of Alabama, Birmingham, and coinvestigators provided an update from the ongoing prospective, observational SUNSTONE cohort study, whose goal is to evaluate in a real-world setting the safety of rituximab in rheumatoid arthritis patients refractory to anti-TNF therapies. At a mean 4-year duration of follow-up in 938 patients who received a mean of four courses of rituximab, 17% of patients had experienced significant infections as defined either by the Food and Drug Administration serious adverse event criteria or need for intravenous antibiotics. The key finding from this intermediate analysis: The serious infection incidence rate did not increase with time and multiple courses of rituximab.
That’s reassuring, Dr. Weinblatt observed, and so is the finding that, in the 338 SUNSTONE participants who switched from rituximab to another biologic agent, there wasn’t any associated increased risk of serious infection.
All of the biologics carry an increased risk of infection. The one rituximab-associated infection that gives rheumatologists particular pause – and that has blunted the use of the anti-B-cell agent in the United States – is progressive multifocal leukoencephalopathy (PML). Indeed, rituximab is the only biologic agent or small molecule prescribed by rheumatologists that carries a warning label about PML, and the only one whose use is restricted to patients who’ve failed a first biologic.
“That means you have to – and should – discuss this with your patients,” Dr. Weinblatt noted.
At last November’s annual ACR meeting, Dr. Leonard H. Calabrese of the Cleveland Clinic and Dr. Eamonn Molloy of St. Vincent University Hospital, Dublin, provided an analysis of data released in response to their Freedom of Information Act request that the FDA provide information on all cases of PML recorded in the agency’s Adverse Event Reporting System through August 2012. In Dr. Weinblatt’s view, these data are reassuring.
“The rates are actually quite low. It’s a rare event. It’s clearly associated with rituximab, though,” he said.
Indeed, 30 confirmed cases of PML have been associated with biologic therapies for autoimmune rheumatic diseases, including 11 in patients with RA, 11 with systemic lupus erythematosus, and 5 with dermato/polymyositis. The median age was 53 years, and 25 of the 30 patients were women. Twenty-six cases occurred in patients who had most recently been on rituximab, the other four in patients who were on anti-TNF therapies at the time. Abatacept, belimumab, tocilizumab, and anakinra were not linked to PML.
PML developed after a median of two courses of rituximab. The median time interval was 15 months from the first rituximab infusion and 5 months from the last, but many confounders were present, making it difficult to draw definitive conclusions about causality. For example, four patients with PML had been on concomitant rituximab and cyclophosphamide, and another five had received cyclophosphamide prior to going on rituximab. Eighteen of 26 rituximab-treated patients were on one or more additional immunosuppressive agents at the time they were diagnosed with PML. Two patients had previously received cancer chemotherapy. Another five had significant lymphopenia.
Dr. Weinblatt reported receiving consulting fees or other remuneration from more than two dozen pharmaceutical companies, including Genentech, which markets rituximab.
SNOWMASS, COLO. – When it comes to prescribing rituximab in patients with rheumatoid arthritis, maybe it’s time to think like a European.
In response to a persuasive French study, rheumatologists across Europe have embraced a lower-dose rituximab regimen: Patients who achieve a moderate or good EULAR response to the standard initial induction dosing of two 1,000-mg doses given intravenously 2 weeks apart receive thereafter a single 1,000-mg dose every 6 months rather than the licensed maintenance dosing of two 1,000-mg IV injections every 6 months.
“They showed that one dose every 6 months is as good as two doses every 6 months. This is a strategy that our European colleagues have actively adopted. It’s a cost-effective strategy, and I think it’s actually an appropriate strategy to use and one we’re using routinely now in our rheumatoid patients after we’ve induced a response with the initial induction dosing,” Dr. Michael E. Weinblatt, professor of medicine at Harvard Medical School, Boston, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
The French study was an open-label, prospective, multicenter, noninferiority study involving 225 rheumatoid arthritis patients with an inadequate response to anti–tumor necrosis factor (anti-TNF) agents and a 6-month moderate or good EULAR response to rituximab (Rituxan) induction therapy coupled with methotrexate. They were randomized to rituximab retreatment at 6-month intervals at either a single 1,000-mg dose or the two 1,000-mg doses as described in the product labeling. At 104 weeks, disease activity as measured by the mean disease activity in 28 joints plus C-reactive protein level area under the curve was similar in the two treatment arms. So was the safety profile (Ann. Rheum. Dis. 2014;73:1508-14).
Dr. Weinblatt also provided an update on rituximab and infections. Two studies presented at last fall’s annual meeting of the American College of Rheumatology in Boston shed new light on this perennially hot topic.
In one, Dr. Kenneth G. Saag of the University of Alabama, Birmingham, and coinvestigators provided an update from the ongoing prospective, observational SUNSTONE cohort study, whose goal is to evaluate in a real-world setting the safety of rituximab in rheumatoid arthritis patients refractory to anti-TNF therapies. At a mean 4-year duration of follow-up in 938 patients who received a mean of four courses of rituximab, 17% of patients had experienced significant infections as defined either by the Food and Drug Administration serious adverse event criteria or need for intravenous antibiotics. The key finding from this intermediate analysis: The serious infection incidence rate did not increase with time and multiple courses of rituximab.
That’s reassuring, Dr. Weinblatt observed, and so is the finding that, in the 338 SUNSTONE participants who switched from rituximab to another biologic agent, there wasn’t any associated increased risk of serious infection.
All of the biologics carry an increased risk of infection. The one rituximab-associated infection that gives rheumatologists particular pause – and that has blunted the use of the anti-B-cell agent in the United States – is progressive multifocal leukoencephalopathy (PML). Indeed, rituximab is the only biologic agent or small molecule prescribed by rheumatologists that carries a warning label about PML, and the only one whose use is restricted to patients who’ve failed a first biologic.
“That means you have to – and should – discuss this with your patients,” Dr. Weinblatt noted.
At last November’s annual ACR meeting, Dr. Leonard H. Calabrese of the Cleveland Clinic and Dr. Eamonn Molloy of St. Vincent University Hospital, Dublin, provided an analysis of data released in response to their Freedom of Information Act request that the FDA provide information on all cases of PML recorded in the agency’s Adverse Event Reporting System through August 2012. In Dr. Weinblatt’s view, these data are reassuring.
“The rates are actually quite low. It’s a rare event. It’s clearly associated with rituximab, though,” he said.
Indeed, 30 confirmed cases of PML have been associated with biologic therapies for autoimmune rheumatic diseases, including 11 in patients with RA, 11 with systemic lupus erythematosus, and 5 with dermato/polymyositis. The median age was 53 years, and 25 of the 30 patients were women. Twenty-six cases occurred in patients who had most recently been on rituximab, the other four in patients who were on anti-TNF therapies at the time. Abatacept, belimumab, tocilizumab, and anakinra were not linked to PML.
PML developed after a median of two courses of rituximab. The median time interval was 15 months from the first rituximab infusion and 5 months from the last, but many confounders were present, making it difficult to draw definitive conclusions about causality. For example, four patients with PML had been on concomitant rituximab and cyclophosphamide, and another five had received cyclophosphamide prior to going on rituximab. Eighteen of 26 rituximab-treated patients were on one or more additional immunosuppressive agents at the time they were diagnosed with PML. Two patients had previously received cancer chemotherapy. Another five had significant lymphopenia.
Dr. Weinblatt reported receiving consulting fees or other remuneration from more than two dozen pharmaceutical companies, including Genentech, which markets rituximab.
SNOWMASS, COLO. – When it comes to prescribing rituximab in patients with rheumatoid arthritis, maybe it’s time to think like a European.
In response to a persuasive French study, rheumatologists across Europe have embraced a lower-dose rituximab regimen: Patients who achieve a moderate or good EULAR response to the standard initial induction dosing of two 1,000-mg doses given intravenously 2 weeks apart receive thereafter a single 1,000-mg dose every 6 months rather than the licensed maintenance dosing of two 1,000-mg IV injections every 6 months.
“They showed that one dose every 6 months is as good as two doses every 6 months. This is a strategy that our European colleagues have actively adopted. It’s a cost-effective strategy, and I think it’s actually an appropriate strategy to use and one we’re using routinely now in our rheumatoid patients after we’ve induced a response with the initial induction dosing,” Dr. Michael E. Weinblatt, professor of medicine at Harvard Medical School, Boston, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
The French study was an open-label, prospective, multicenter, noninferiority study involving 225 rheumatoid arthritis patients with an inadequate response to anti–tumor necrosis factor (anti-TNF) agents and a 6-month moderate or good EULAR response to rituximab (Rituxan) induction therapy coupled with methotrexate. They were randomized to rituximab retreatment at 6-month intervals at either a single 1,000-mg dose or the two 1,000-mg doses as described in the product labeling. At 104 weeks, disease activity as measured by the mean disease activity in 28 joints plus C-reactive protein level area under the curve was similar in the two treatment arms. So was the safety profile (Ann. Rheum. Dis. 2014;73:1508-14).
Dr. Weinblatt also provided an update on rituximab and infections. Two studies presented at last fall’s annual meeting of the American College of Rheumatology in Boston shed new light on this perennially hot topic.
In one, Dr. Kenneth G. Saag of the University of Alabama, Birmingham, and coinvestigators provided an update from the ongoing prospective, observational SUNSTONE cohort study, whose goal is to evaluate in a real-world setting the safety of rituximab in rheumatoid arthritis patients refractory to anti-TNF therapies. At a mean 4-year duration of follow-up in 938 patients who received a mean of four courses of rituximab, 17% of patients had experienced significant infections as defined either by the Food and Drug Administration serious adverse event criteria or need for intravenous antibiotics. The key finding from this intermediate analysis: The serious infection incidence rate did not increase with time and multiple courses of rituximab.
That’s reassuring, Dr. Weinblatt observed, and so is the finding that, in the 338 SUNSTONE participants who switched from rituximab to another biologic agent, there wasn’t any associated increased risk of serious infection.
All of the biologics carry an increased risk of infection. The one rituximab-associated infection that gives rheumatologists particular pause – and that has blunted the use of the anti-B-cell agent in the United States – is progressive multifocal leukoencephalopathy (PML). Indeed, rituximab is the only biologic agent or small molecule prescribed by rheumatologists that carries a warning label about PML, and the only one whose use is restricted to patients who’ve failed a first biologic.
“That means you have to – and should – discuss this with your patients,” Dr. Weinblatt noted.
At last November’s annual ACR meeting, Dr. Leonard H. Calabrese of the Cleveland Clinic and Dr. Eamonn Molloy of St. Vincent University Hospital, Dublin, provided an analysis of data released in response to their Freedom of Information Act request that the FDA provide information on all cases of PML recorded in the agency’s Adverse Event Reporting System through August 2012. In Dr. Weinblatt’s view, these data are reassuring.
“The rates are actually quite low. It’s a rare event. It’s clearly associated with rituximab, though,” he said.
Indeed, 30 confirmed cases of PML have been associated with biologic therapies for autoimmune rheumatic diseases, including 11 in patients with RA, 11 with systemic lupus erythematosus, and 5 with dermato/polymyositis. The median age was 53 years, and 25 of the 30 patients were women. Twenty-six cases occurred in patients who had most recently been on rituximab, the other four in patients who were on anti-TNF therapies at the time. Abatacept, belimumab, tocilizumab, and anakinra were not linked to PML.
PML developed after a median of two courses of rituximab. The median time interval was 15 months from the first rituximab infusion and 5 months from the last, but many confounders were present, making it difficult to draw definitive conclusions about causality. For example, four patients with PML had been on concomitant rituximab and cyclophosphamide, and another five had received cyclophosphamide prior to going on rituximab. Eighteen of 26 rituximab-treated patients were on one or more additional immunosuppressive agents at the time they were diagnosed with PML. Two patients had previously received cancer chemotherapy. Another five had significant lymphopenia.
Dr. Weinblatt reported receiving consulting fees or other remuneration from more than two dozen pharmaceutical companies, including Genentech, which markets rituximab.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Skin patch therapy for peanut allergy wins plaudits
HOUSTON – A peanut protein–bearing skin patch showed considerable promise in the largest randomized trial to date of any potential treatment for peanut allergy.
One year of treatment with the investigational proprietary Viaskin Peanut skin patch raised the threshold of exposure to peanut protein required to elicit an allergic reaction to a level that patients and families would consider life changing, Dr. Hugh A. Sampson said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“One of the biggest problems for the family of a patient with peanut allergy is the fear of getting contamination. If they could rest assured that they could tolerate something like a gram’s worth of contamination with peanut protein, much of that concern would go away. They could go to restaurants and birthday parties. They still need to be vigilant and tell people they’re peanut allergic, but the chance of these low-level contaminations that lead to most of the reactions we see would largely be taken care of,” said Dr. Sampson, professor of pediatrics, allergy, and immunology and dean for translational biomedical research at Icahn School of Medicine at Mount Sinai, New York.
He presented the findings of a phase IIb randomized, double-blind, multinational, placebo-controlled trial of what is being called epicutaneous immunotherapy. The trial involved 221 subjects with documented peanut allergy. Roughly half were aged 6-11 years; the rest were adolescents and adults up to age 55. They were randomized to one of four study arms: the skin patch at a dose of 50, 100, or 250 mcg or a placebo patch. As a requirement for study participation, all subjects had to have a peanut protein reaction–eliciting dose of 300 mg or less on baseline formal oral peanut challenge testing; half of the children reacted to 30 mg or less.
The primary endpoint was the ability to tolerate on a repeat oral challenge at 1 year either at least 1 g of peanut protein or 10 times the amount they tolerated on baseline testing. In the overall study population, the 250-mcg patch was the top performer. It was the only patch dose significantly better than placebo: 50% of all patients on the 250-mcg patch met the primary endpoint, compared with 25% of placebo-treated controls.
Peanut allergy typically starts early in life, so it’s noteworthy that the children in the study had a markedly more robust response to epicutaneous immunotherapy than the older patients. Indeed, among the 6- to 11-year-olds, all three patch doses outperformed placebo. The mean cumulative dose of peanut protein required to elicit a reaction on structured oral challenge testing was 1,121 mg greater at 1 year than at baseline among children on the 250-mcg patch, 570 mg greater with the 100-mcg patch, and 471 mg greater than at baseline among those using the 50-mcg patch.
Similarly, a robust dose-dependent increase was seen in protective peanut-specific IgG4 levels in response to 1 year of epicutaneous immunotherapy. The median level of this biomarker of therapeutic benefit climbed 19-fold over baseline in children on the 250-mcg patch, 7-fold with the 100-mcg patch, and 5.5-fold with the 50-mcg patch.
The compliance rate with patch therapy was greater than 96%. Only 2 of the 221 participants dropped out of the study due to adverse events, both because of a flare of atopic dermatitis around the patch site. There were no serious adverse events in the study.
The trial is being extended for a second year of immunotherapy. “My anticipation based on what we see with other forms of immunotherapy is that we might very well see even more protection,” according to Dr. Sampson.
In an interview, he said he’d like to see the skin patch studied at doses above 250 mcg in adolescents and adults.
“I would also love to see epicutaneous immunotherapy looked at in very young children,” he added. “You get a much better response, and it may be longer lasting because we think that the immune system in an infant is much more plastic than it is once they get older. Trying to reverse a set response is much more difficult.”
The skin patch is about the size of a small, round Band-Aid. It is placed over the back or inner upper arm and changed daily. The concept is that as humidity develops under the patch, a small amount of peanut protein permeates the outer skin. Langerhans cells then pick it up and transport it to regional lymph nodes, where T cells can activate desensitization.
“I think this concept of using a low amount of protein in a convenient, safe way could change the way we do immunotherapy,” Dr. Sampson predicted.
Asked to comment, peanut allergy researcher Dr. Brian P. Vickery of the University of North Carolina, Chapel Hill, agreed with Dr. Sampson’s assessment that patch therapy could be a game changer, especially given that there is no FDA-approved treatment for peanut allergy.
“Right now the standard of care is avoidance. So anything that improves upon that to allow a margin of safety that lets a patient get along in the world with the reassurance that a contamination event up to, say, a gram would be well tolerated would be transformative,” he said.
HOUSTON – A peanut protein–bearing skin patch showed considerable promise in the largest randomized trial to date of any potential treatment for peanut allergy.
One year of treatment with the investigational proprietary Viaskin Peanut skin patch raised the threshold of exposure to peanut protein required to elicit an allergic reaction to a level that patients and families would consider life changing, Dr. Hugh A. Sampson said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“One of the biggest problems for the family of a patient with peanut allergy is the fear of getting contamination. If they could rest assured that they could tolerate something like a gram’s worth of contamination with peanut protein, much of that concern would go away. They could go to restaurants and birthday parties. They still need to be vigilant and tell people they’re peanut allergic, but the chance of these low-level contaminations that lead to most of the reactions we see would largely be taken care of,” said Dr. Sampson, professor of pediatrics, allergy, and immunology and dean for translational biomedical research at Icahn School of Medicine at Mount Sinai, New York.
He presented the findings of a phase IIb randomized, double-blind, multinational, placebo-controlled trial of what is being called epicutaneous immunotherapy. The trial involved 221 subjects with documented peanut allergy. Roughly half were aged 6-11 years; the rest were adolescents and adults up to age 55. They were randomized to one of four study arms: the skin patch at a dose of 50, 100, or 250 mcg or a placebo patch. As a requirement for study participation, all subjects had to have a peanut protein reaction–eliciting dose of 300 mg or less on baseline formal oral peanut challenge testing; half of the children reacted to 30 mg or less.
The primary endpoint was the ability to tolerate on a repeat oral challenge at 1 year either at least 1 g of peanut protein or 10 times the amount they tolerated on baseline testing. In the overall study population, the 250-mcg patch was the top performer. It was the only patch dose significantly better than placebo: 50% of all patients on the 250-mcg patch met the primary endpoint, compared with 25% of placebo-treated controls.
Peanut allergy typically starts early in life, so it’s noteworthy that the children in the study had a markedly more robust response to epicutaneous immunotherapy than the older patients. Indeed, among the 6- to 11-year-olds, all three patch doses outperformed placebo. The mean cumulative dose of peanut protein required to elicit a reaction on structured oral challenge testing was 1,121 mg greater at 1 year than at baseline among children on the 250-mcg patch, 570 mg greater with the 100-mcg patch, and 471 mg greater than at baseline among those using the 50-mcg patch.
Similarly, a robust dose-dependent increase was seen in protective peanut-specific IgG4 levels in response to 1 year of epicutaneous immunotherapy. The median level of this biomarker of therapeutic benefit climbed 19-fold over baseline in children on the 250-mcg patch, 7-fold with the 100-mcg patch, and 5.5-fold with the 50-mcg patch.
The compliance rate with patch therapy was greater than 96%. Only 2 of the 221 participants dropped out of the study due to adverse events, both because of a flare of atopic dermatitis around the patch site. There were no serious adverse events in the study.
The trial is being extended for a second year of immunotherapy. “My anticipation based on what we see with other forms of immunotherapy is that we might very well see even more protection,” according to Dr. Sampson.
In an interview, he said he’d like to see the skin patch studied at doses above 250 mcg in adolescents and adults.
“I would also love to see epicutaneous immunotherapy looked at in very young children,” he added. “You get a much better response, and it may be longer lasting because we think that the immune system in an infant is much more plastic than it is once they get older. Trying to reverse a set response is much more difficult.”
The skin patch is about the size of a small, round Band-Aid. It is placed over the back or inner upper arm and changed daily. The concept is that as humidity develops under the patch, a small amount of peanut protein permeates the outer skin. Langerhans cells then pick it up and transport it to regional lymph nodes, where T cells can activate desensitization.
“I think this concept of using a low amount of protein in a convenient, safe way could change the way we do immunotherapy,” Dr. Sampson predicted.
Asked to comment, peanut allergy researcher Dr. Brian P. Vickery of the University of North Carolina, Chapel Hill, agreed with Dr. Sampson’s assessment that patch therapy could be a game changer, especially given that there is no FDA-approved treatment for peanut allergy.
“Right now the standard of care is avoidance. So anything that improves upon that to allow a margin of safety that lets a patient get along in the world with the reassurance that a contamination event up to, say, a gram would be well tolerated would be transformative,” he said.
HOUSTON – A peanut protein–bearing skin patch showed considerable promise in the largest randomized trial to date of any potential treatment for peanut allergy.
One year of treatment with the investigational proprietary Viaskin Peanut skin patch raised the threshold of exposure to peanut protein required to elicit an allergic reaction to a level that patients and families would consider life changing, Dr. Hugh A. Sampson said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“One of the biggest problems for the family of a patient with peanut allergy is the fear of getting contamination. If they could rest assured that they could tolerate something like a gram’s worth of contamination with peanut protein, much of that concern would go away. They could go to restaurants and birthday parties. They still need to be vigilant and tell people they’re peanut allergic, but the chance of these low-level contaminations that lead to most of the reactions we see would largely be taken care of,” said Dr. Sampson, professor of pediatrics, allergy, and immunology and dean for translational biomedical research at Icahn School of Medicine at Mount Sinai, New York.
He presented the findings of a phase IIb randomized, double-blind, multinational, placebo-controlled trial of what is being called epicutaneous immunotherapy. The trial involved 221 subjects with documented peanut allergy. Roughly half were aged 6-11 years; the rest were adolescents and adults up to age 55. They were randomized to one of four study arms: the skin patch at a dose of 50, 100, or 250 mcg or a placebo patch. As a requirement for study participation, all subjects had to have a peanut protein reaction–eliciting dose of 300 mg or less on baseline formal oral peanut challenge testing; half of the children reacted to 30 mg or less.
The primary endpoint was the ability to tolerate on a repeat oral challenge at 1 year either at least 1 g of peanut protein or 10 times the amount they tolerated on baseline testing. In the overall study population, the 250-mcg patch was the top performer. It was the only patch dose significantly better than placebo: 50% of all patients on the 250-mcg patch met the primary endpoint, compared with 25% of placebo-treated controls.
Peanut allergy typically starts early in life, so it’s noteworthy that the children in the study had a markedly more robust response to epicutaneous immunotherapy than the older patients. Indeed, among the 6- to 11-year-olds, all three patch doses outperformed placebo. The mean cumulative dose of peanut protein required to elicit a reaction on structured oral challenge testing was 1,121 mg greater at 1 year than at baseline among children on the 250-mcg patch, 570 mg greater with the 100-mcg patch, and 471 mg greater than at baseline among those using the 50-mcg patch.
Similarly, a robust dose-dependent increase was seen in protective peanut-specific IgG4 levels in response to 1 year of epicutaneous immunotherapy. The median level of this biomarker of therapeutic benefit climbed 19-fold over baseline in children on the 250-mcg patch, 7-fold with the 100-mcg patch, and 5.5-fold with the 50-mcg patch.
The compliance rate with patch therapy was greater than 96%. Only 2 of the 221 participants dropped out of the study due to adverse events, both because of a flare of atopic dermatitis around the patch site. There were no serious adverse events in the study.
The trial is being extended for a second year of immunotherapy. “My anticipation based on what we see with other forms of immunotherapy is that we might very well see even more protection,” according to Dr. Sampson.
In an interview, he said he’d like to see the skin patch studied at doses above 250 mcg in adolescents and adults.
“I would also love to see epicutaneous immunotherapy looked at in very young children,” he added. “You get a much better response, and it may be longer lasting because we think that the immune system in an infant is much more plastic than it is once they get older. Trying to reverse a set response is much more difficult.”
The skin patch is about the size of a small, round Band-Aid. It is placed over the back or inner upper arm and changed daily. The concept is that as humidity develops under the patch, a small amount of peanut protein permeates the outer skin. Langerhans cells then pick it up and transport it to regional lymph nodes, where T cells can activate desensitization.
“I think this concept of using a low amount of protein in a convenient, safe way could change the way we do immunotherapy,” Dr. Sampson predicted.
Asked to comment, peanut allergy researcher Dr. Brian P. Vickery of the University of North Carolina, Chapel Hill, agreed with Dr. Sampson’s assessment that patch therapy could be a game changer, especially given that there is no FDA-approved treatment for peanut allergy.
“Right now the standard of care is avoidance. So anything that improves upon that to allow a margin of safety that lets a patient get along in the world with the reassurance that a contamination event up to, say, a gram would be well tolerated would be transformative,” he said.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Epicutaneous immunotherapy in patients with peanut allergy is safe, well-tolerated, and shows dose- and age-dependent efficacy.
Major finding: Half of peanut-allergic patients who wore a proprietary skin patch containing 250 mcg of peanut protein experienced a clinically meaningful increase in the threshold of exposure required to elicit a reaction, compared with 25% of controls using a placebo patch. The results were more dramatic in children than older subjects.
Data source: This was a 12-month, randomized, double-blind, placebo-controlled, multinational phase IIb study involving 221 peanut-allergic patients aged 6-55 years.
Disclosures: The study was funded by DBV Technologies. The presenter reported serving as an unpaid member of the company’s scientific advisory board.
How to halt ‘a heart attack of the finger’
SNOWMASS, COLO. – Persistent, widespread, intense ischemic pain in the vicinity of a digital ulcer in a patient with secondary Raynaud’s phenomenon signals impending tissue infarction and gangrene, Dr. Fredrick M. Wigley warned at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“When you see this you’ve got a heart attack of the finger occurring. It’s a medical emergency,” emphasized Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
The pain associated with this condition is severe enough that opiates are often required. Therefore, one of Dr. Wigley’s first moves in the office is often to administer a digital block for immediate pain relief. He sticks the needle into the web of the finger and infiltrates 2% lidocaine. It’s an easy procedure to do, and it often breaks the acute event.
If the digital block doesn’t succeed in terminating the ischemic event, however, then his go-to therapy is prostacyclin infusion.
“We’re very keen at our center, and at other centers around the country as well, to go right to a prostacyclin analogue. You can do that for prevention or during an acute event. Set up a peripheral line, run in prostacyclin for 3-5 days, and it has a magical effect that lasts for 10-12 weeks. It can really break an ischemic event quickly and may also have some preventive benefit. What we have available in the U.S. is epoprostenol. In can be administered on an inpatient basis, but we set it up as outpatient therapy. Patients come in for an 8-hour period for 3-5 days in a row,” the rheumatologist explained.
This is off-label therapy. Epoprostenol’s approved indication is in treating pulmonary arterial hypertension. But there is persuasive evidence of the effectiveness of epoprostenol (Flolan) and the prostacyclins available in other countries in aborting acute digital ischemic events in patients with Raynaud’s, he said.
One prostacyclin, treprostinil, is now available as Orenitram in an oral formulation approved for treatment of pulmonary arterial hypertension. Other oral prostacyclins are in the developmental pipeline. Despite their promise of much greater patient convenience, however, at this point their use in patients with acute digital critical ischemia isn’t supported by evidence.
During those multiple, daylong outpatient treatment sessions, Dr. Wigley emphasizes the importance of staying warm, resting, and avoiding stress. He also makes sure the patient is on an optimal vasodilatory medication program, the linchpin of which is a long-acting calcium channel blocker titrated to the maximum tolerated dose.
He also does special testing in search of a potential obstruction in a larger upstream vessel that might be amenable to a corrective procedure. These tests include Doppler ultrasound, the Allen’s test, magnetic resonance angiography, and/or digital subtraction x-ray.
This multiday period of prostacyclin therapy is an excellent opportunity to initiate the use of vasculoprotective medications. The ones he’s found most helpful include statins, low-dose aspirin or another antiplatelet agent, antioxidants, and in the case of a suspected early thrombotic or embolic event, 24-72 hours of low-molecular-weight or unfractionated heparin.
There is clinical trial evidence that bosentan (Tracleer) reduces the frequency of recurrent digital ulcers. Dr. Wigley rarely uses it, however, because it’s expensive and has lots of toxicity.
Surgical sympathectomy can be finger saving.
“Don’t dillydally,” urged Dr. Wigley. “If your patient is not responding to medical therapy, don’t say, ‘Come back in a few weeks, and we’ll see how you’re doing.’ You’ll lose the finger.”
Sympathectomy has several salutary effects: the procedure rips sympathetic nerve fibers from the over-sensitive blood vessels and also gets rid of fibrous material entrapping the vessels.
In a meta-analysis of studies that included 511 proximal sympathectomies in 449 patients with secondary Raynaud’s, 89% reported sustained improvement (J. Vasc. Surg. 2011;54:273-7). The available data on the less morbid alternative digital sympathectomy procedure are less extensive, but the rates of complete healing and/or decrease in digital ulcers are impressive.
Remember: Avoid tunnel vision, Dr. Wigley cautioned. Always consider the likely possibility of macrovascular disease in the setting of lower-extremity digital ischemia. Measurement of the ankle-brachial pressure index is considered a mandatory part of the clinical work-up in these patients. An ankle-brachial index of less than 0.9 has 95% sensitivity for the presence of angiographically evident cardiovascular disease.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from Kinemed, MedImmune, and CSL Behring.
SNOWMASS, COLO. – Persistent, widespread, intense ischemic pain in the vicinity of a digital ulcer in a patient with secondary Raynaud’s phenomenon signals impending tissue infarction and gangrene, Dr. Fredrick M. Wigley warned at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“When you see this you’ve got a heart attack of the finger occurring. It’s a medical emergency,” emphasized Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
The pain associated with this condition is severe enough that opiates are often required. Therefore, one of Dr. Wigley’s first moves in the office is often to administer a digital block for immediate pain relief. He sticks the needle into the web of the finger and infiltrates 2% lidocaine. It’s an easy procedure to do, and it often breaks the acute event.
If the digital block doesn’t succeed in terminating the ischemic event, however, then his go-to therapy is prostacyclin infusion.
“We’re very keen at our center, and at other centers around the country as well, to go right to a prostacyclin analogue. You can do that for prevention or during an acute event. Set up a peripheral line, run in prostacyclin for 3-5 days, and it has a magical effect that lasts for 10-12 weeks. It can really break an ischemic event quickly and may also have some preventive benefit. What we have available in the U.S. is epoprostenol. In can be administered on an inpatient basis, but we set it up as outpatient therapy. Patients come in for an 8-hour period for 3-5 days in a row,” the rheumatologist explained.
This is off-label therapy. Epoprostenol’s approved indication is in treating pulmonary arterial hypertension. But there is persuasive evidence of the effectiveness of epoprostenol (Flolan) and the prostacyclins available in other countries in aborting acute digital ischemic events in patients with Raynaud’s, he said.
One prostacyclin, treprostinil, is now available as Orenitram in an oral formulation approved for treatment of pulmonary arterial hypertension. Other oral prostacyclins are in the developmental pipeline. Despite their promise of much greater patient convenience, however, at this point their use in patients with acute digital critical ischemia isn’t supported by evidence.
During those multiple, daylong outpatient treatment sessions, Dr. Wigley emphasizes the importance of staying warm, resting, and avoiding stress. He also makes sure the patient is on an optimal vasodilatory medication program, the linchpin of which is a long-acting calcium channel blocker titrated to the maximum tolerated dose.
He also does special testing in search of a potential obstruction in a larger upstream vessel that might be amenable to a corrective procedure. These tests include Doppler ultrasound, the Allen’s test, magnetic resonance angiography, and/or digital subtraction x-ray.
This multiday period of prostacyclin therapy is an excellent opportunity to initiate the use of vasculoprotective medications. The ones he’s found most helpful include statins, low-dose aspirin or another antiplatelet agent, antioxidants, and in the case of a suspected early thrombotic or embolic event, 24-72 hours of low-molecular-weight or unfractionated heparin.
There is clinical trial evidence that bosentan (Tracleer) reduces the frequency of recurrent digital ulcers. Dr. Wigley rarely uses it, however, because it’s expensive and has lots of toxicity.
Surgical sympathectomy can be finger saving.
“Don’t dillydally,” urged Dr. Wigley. “If your patient is not responding to medical therapy, don’t say, ‘Come back in a few weeks, and we’ll see how you’re doing.’ You’ll lose the finger.”
Sympathectomy has several salutary effects: the procedure rips sympathetic nerve fibers from the over-sensitive blood vessels and also gets rid of fibrous material entrapping the vessels.
In a meta-analysis of studies that included 511 proximal sympathectomies in 449 patients with secondary Raynaud’s, 89% reported sustained improvement (J. Vasc. Surg. 2011;54:273-7). The available data on the less morbid alternative digital sympathectomy procedure are less extensive, but the rates of complete healing and/or decrease in digital ulcers are impressive.
Remember: Avoid tunnel vision, Dr. Wigley cautioned. Always consider the likely possibility of macrovascular disease in the setting of lower-extremity digital ischemia. Measurement of the ankle-brachial pressure index is considered a mandatory part of the clinical work-up in these patients. An ankle-brachial index of less than 0.9 has 95% sensitivity for the presence of angiographically evident cardiovascular disease.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from Kinemed, MedImmune, and CSL Behring.
SNOWMASS, COLO. – Persistent, widespread, intense ischemic pain in the vicinity of a digital ulcer in a patient with secondary Raynaud’s phenomenon signals impending tissue infarction and gangrene, Dr. Fredrick M. Wigley warned at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“When you see this you’ve got a heart attack of the finger occurring. It’s a medical emergency,” emphasized Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
The pain associated with this condition is severe enough that opiates are often required. Therefore, one of Dr. Wigley’s first moves in the office is often to administer a digital block for immediate pain relief. He sticks the needle into the web of the finger and infiltrates 2% lidocaine. It’s an easy procedure to do, and it often breaks the acute event.
If the digital block doesn’t succeed in terminating the ischemic event, however, then his go-to therapy is prostacyclin infusion.
“We’re very keen at our center, and at other centers around the country as well, to go right to a prostacyclin analogue. You can do that for prevention or during an acute event. Set up a peripheral line, run in prostacyclin for 3-5 days, and it has a magical effect that lasts for 10-12 weeks. It can really break an ischemic event quickly and may also have some preventive benefit. What we have available in the U.S. is epoprostenol. In can be administered on an inpatient basis, but we set it up as outpatient therapy. Patients come in for an 8-hour period for 3-5 days in a row,” the rheumatologist explained.
This is off-label therapy. Epoprostenol’s approved indication is in treating pulmonary arterial hypertension. But there is persuasive evidence of the effectiveness of epoprostenol (Flolan) and the prostacyclins available in other countries in aborting acute digital ischemic events in patients with Raynaud’s, he said.
One prostacyclin, treprostinil, is now available as Orenitram in an oral formulation approved for treatment of pulmonary arterial hypertension. Other oral prostacyclins are in the developmental pipeline. Despite their promise of much greater patient convenience, however, at this point their use in patients with acute digital critical ischemia isn’t supported by evidence.
During those multiple, daylong outpatient treatment sessions, Dr. Wigley emphasizes the importance of staying warm, resting, and avoiding stress. He also makes sure the patient is on an optimal vasodilatory medication program, the linchpin of which is a long-acting calcium channel blocker titrated to the maximum tolerated dose.
He also does special testing in search of a potential obstruction in a larger upstream vessel that might be amenable to a corrective procedure. These tests include Doppler ultrasound, the Allen’s test, magnetic resonance angiography, and/or digital subtraction x-ray.
This multiday period of prostacyclin therapy is an excellent opportunity to initiate the use of vasculoprotective medications. The ones he’s found most helpful include statins, low-dose aspirin or another antiplatelet agent, antioxidants, and in the case of a suspected early thrombotic or embolic event, 24-72 hours of low-molecular-weight or unfractionated heparin.
There is clinical trial evidence that bosentan (Tracleer) reduces the frequency of recurrent digital ulcers. Dr. Wigley rarely uses it, however, because it’s expensive and has lots of toxicity.
Surgical sympathectomy can be finger saving.
“Don’t dillydally,” urged Dr. Wigley. “If your patient is not responding to medical therapy, don’t say, ‘Come back in a few weeks, and we’ll see how you’re doing.’ You’ll lose the finger.”
Sympathectomy has several salutary effects: the procedure rips sympathetic nerve fibers from the over-sensitive blood vessels and also gets rid of fibrous material entrapping the vessels.
In a meta-analysis of studies that included 511 proximal sympathectomies in 449 patients with secondary Raynaud’s, 89% reported sustained improvement (J. Vasc. Surg. 2011;54:273-7). The available data on the less morbid alternative digital sympathectomy procedure are less extensive, but the rates of complete healing and/or decrease in digital ulcers are impressive.
Remember: Avoid tunnel vision, Dr. Wigley cautioned. Always consider the likely possibility of macrovascular disease in the setting of lower-extremity digital ischemia. Measurement of the ankle-brachial pressure index is considered a mandatory part of the clinical work-up in these patients. An ankle-brachial index of less than 0.9 has 95% sensitivity for the presence of angiographically evident cardiovascular disease.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from Kinemed, MedImmune, and CSL Behring.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Differentiating between primary and secondary Raynaud’s
SNOWMASS, COLO.– Physical examination of the hands yields strong clues that a patient presenting with sharply demarcated color changes of the digits has primary Raynaud’s phenomenon due to reversible small-vessel vasospasm rather than secondary Raynaud’s involving vasospasm plus structural disease in the microcirculation, Dr. Fredrick M. Wigley said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Primary Raynaud’s phenomenon is precipitated by cold temperatures or emotional stress. The attacks are symmetric and involve all the fingers on both hands. The pallor doesn’t extend beyond the metacarpophalangeal joints into the palms; if it does, this is not just a digital arteriolar vasospastic event, but rather one involving compromise of larger vessels, as occurs in Raynaud’s from a secondary cause such as scleroderma, said Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
Examination of the nailfold capillaries under magnification using immersion oil is informative, he added. Normal nailfold capillaries are consistent with primary Raynaud’s phenomenon. But abnormal nailfolds in a patient with no history or physical findings suggestive of a secondary cause of Raynaud’s are associated with a 20% chance of developing a rheumatic disease – most often scleroderma – within the next 2 years. And if the nailfold capillaries are abnormal and the patient also has anticentromere or other autoantibodies, the 2-year likelihood of a rheumatic disease diagnosis jumps to 80% (Rheum. Dis. Clin. North Am. 2008;34:89-114).
Patients with primary Raynaud’s phenomenon often show a livedo reticularis pattern of mottled, purplish, lacelike vessels on their skin, especially on the legs. The thermoregulatory shunts present in the blood vessels of patients with primary Raynaud’s phenomenon are abnormally sensitive to sympathetic tone, and the livedo reticularis is believed to be another consequence of these upregulated circulatory shunts.
A major clinical distinction between primary and secondary Raynaud’s is that patients with primary Raynaud’s phenomenon do not get digital ulcers, gangrene, or signs of tissue injury.
Only about one-third of scleroderma patients with severe secondary Raynaud’s develop ischemic digital ulcers. That’s surprising because scleroderma patients with Raynaud’s have intimal proliferation and luminal narrowing of their digital vessels, making them prone to occlusion, along with reduced levels of endogenous vasodilators and elevated levels of endothelin-1 and other vasoconstrictors that render their vascular endothelial layer easily provoked into spasm.
“If you do an angiogram – and I generally don’t – you’ll be frightened because there’s an incredible amount of disease present despite the fact that many patients don’t get ischemic ulcers,” Dr. Wigley said.
In the two-thirds of patients with scleroderma and severe Raynaud’s phenomenon attacks who don’t get ulcers, it’s “certainly reasonable” to manage their digital disease as if they had primary Raynaud’s, according to the rheumatologist.
One quick way to predict whether a scleroderma patient with severe, dramatically cyanotic Raynaud’s phenomenon is likely to develop worrisome digital ulcers is simply to press on the cold fingers, then release them.
“If you see a good, rapid flush you’ve got good blood flow and you’re probably not going to get into trouble with an ischemic event,” Dr. Wigley said.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from KineMed, MedImmune, and CSL Behring.
SNOWMASS, COLO.– Physical examination of the hands yields strong clues that a patient presenting with sharply demarcated color changes of the digits has primary Raynaud’s phenomenon due to reversible small-vessel vasospasm rather than secondary Raynaud’s involving vasospasm plus structural disease in the microcirculation, Dr. Fredrick M. Wigley said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Primary Raynaud’s phenomenon is precipitated by cold temperatures or emotional stress. The attacks are symmetric and involve all the fingers on both hands. The pallor doesn’t extend beyond the metacarpophalangeal joints into the palms; if it does, this is not just a digital arteriolar vasospastic event, but rather one involving compromise of larger vessels, as occurs in Raynaud’s from a secondary cause such as scleroderma, said Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
Examination of the nailfold capillaries under magnification using immersion oil is informative, he added. Normal nailfold capillaries are consistent with primary Raynaud’s phenomenon. But abnormal nailfolds in a patient with no history or physical findings suggestive of a secondary cause of Raynaud’s are associated with a 20% chance of developing a rheumatic disease – most often scleroderma – within the next 2 years. And if the nailfold capillaries are abnormal and the patient also has anticentromere or other autoantibodies, the 2-year likelihood of a rheumatic disease diagnosis jumps to 80% (Rheum. Dis. Clin. North Am. 2008;34:89-114).
Patients with primary Raynaud’s phenomenon often show a livedo reticularis pattern of mottled, purplish, lacelike vessels on their skin, especially on the legs. The thermoregulatory shunts present in the blood vessels of patients with primary Raynaud’s phenomenon are abnormally sensitive to sympathetic tone, and the livedo reticularis is believed to be another consequence of these upregulated circulatory shunts.
A major clinical distinction between primary and secondary Raynaud’s is that patients with primary Raynaud’s phenomenon do not get digital ulcers, gangrene, or signs of tissue injury.
Only about one-third of scleroderma patients with severe secondary Raynaud’s develop ischemic digital ulcers. That’s surprising because scleroderma patients with Raynaud’s have intimal proliferation and luminal narrowing of their digital vessels, making them prone to occlusion, along with reduced levels of endogenous vasodilators and elevated levels of endothelin-1 and other vasoconstrictors that render their vascular endothelial layer easily provoked into spasm.
“If you do an angiogram – and I generally don’t – you’ll be frightened because there’s an incredible amount of disease present despite the fact that many patients don’t get ischemic ulcers,” Dr. Wigley said.
In the two-thirds of patients with scleroderma and severe Raynaud’s phenomenon attacks who don’t get ulcers, it’s “certainly reasonable” to manage their digital disease as if they had primary Raynaud’s, according to the rheumatologist.
One quick way to predict whether a scleroderma patient with severe, dramatically cyanotic Raynaud’s phenomenon is likely to develop worrisome digital ulcers is simply to press on the cold fingers, then release them.
“If you see a good, rapid flush you’ve got good blood flow and you’re probably not going to get into trouble with an ischemic event,” Dr. Wigley said.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from KineMed, MedImmune, and CSL Behring.
SNOWMASS, COLO.– Physical examination of the hands yields strong clues that a patient presenting with sharply demarcated color changes of the digits has primary Raynaud’s phenomenon due to reversible small-vessel vasospasm rather than secondary Raynaud’s involving vasospasm plus structural disease in the microcirculation, Dr. Fredrick M. Wigley said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Primary Raynaud’s phenomenon is precipitated by cold temperatures or emotional stress. The attacks are symmetric and involve all the fingers on both hands. The pallor doesn’t extend beyond the metacarpophalangeal joints into the palms; if it does, this is not just a digital arteriolar vasospastic event, but rather one involving compromise of larger vessels, as occurs in Raynaud’s from a secondary cause such as scleroderma, said Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
Examination of the nailfold capillaries under magnification using immersion oil is informative, he added. Normal nailfold capillaries are consistent with primary Raynaud’s phenomenon. But abnormal nailfolds in a patient with no history or physical findings suggestive of a secondary cause of Raynaud’s are associated with a 20% chance of developing a rheumatic disease – most often scleroderma – within the next 2 years. And if the nailfold capillaries are abnormal and the patient also has anticentromere or other autoantibodies, the 2-year likelihood of a rheumatic disease diagnosis jumps to 80% (Rheum. Dis. Clin. North Am. 2008;34:89-114).
Patients with primary Raynaud’s phenomenon often show a livedo reticularis pattern of mottled, purplish, lacelike vessels on their skin, especially on the legs. The thermoregulatory shunts present in the blood vessels of patients with primary Raynaud’s phenomenon are abnormally sensitive to sympathetic tone, and the livedo reticularis is believed to be another consequence of these upregulated circulatory shunts.
A major clinical distinction between primary and secondary Raynaud’s is that patients with primary Raynaud’s phenomenon do not get digital ulcers, gangrene, or signs of tissue injury.
Only about one-third of scleroderma patients with severe secondary Raynaud’s develop ischemic digital ulcers. That’s surprising because scleroderma patients with Raynaud’s have intimal proliferation and luminal narrowing of their digital vessels, making them prone to occlusion, along with reduced levels of endogenous vasodilators and elevated levels of endothelin-1 and other vasoconstrictors that render their vascular endothelial layer easily provoked into spasm.
“If you do an angiogram – and I generally don’t – you’ll be frightened because there’s an incredible amount of disease present despite the fact that many patients don’t get ischemic ulcers,” Dr. Wigley said.
In the two-thirds of patients with scleroderma and severe Raynaud’s phenomenon attacks who don’t get ulcers, it’s “certainly reasonable” to manage their digital disease as if they had primary Raynaud’s, according to the rheumatologist.
One quick way to predict whether a scleroderma patient with severe, dramatically cyanotic Raynaud’s phenomenon is likely to develop worrisome digital ulcers is simply to press on the cold fingers, then release them.
“If you see a good, rapid flush you’ve got good blood flow and you’re probably not going to get into trouble with an ischemic event,” Dr. Wigley said.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from KineMed, MedImmune, and CSL Behring.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
An expert on primary Raynaud’s phenomenon describes treatment approach
SNOWMASS, COLO. – Plenty of medication options exist for treatment of primary Raynaud’s phenomenon caused by reversible cutaneous arteriolar vasospasm. But actually, for most affected patients, no drug therapy is required, according to Dr. Fredrick M. Wigley.
“The environment – physical and emotional – has a huge impact on the severity of Raynaud’s phenomenon,” he emphasized at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Whatever I tell you in terms of drugs, there’s nothing more potent than getting the patient out of a stressful, cold environment. So I tell patients who have digital ischemia to stop work, go home, get in bed, put the blanket up to your neck, keep warm, and often that’s more effective than the drug therapy that we try,” said Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
Primary Raynaud’s phenomenon is typically reversible within 15-20 minutes after rewarming, he added.
Aggravators: Stress, trauma, smoking, certain drugs
Besides cold temperatures, another important aggravating factor in primary Raynaud’s phenomenon is emotional stress. Anxiety, like cold, increases sympathetic tone, triggering cutaneous vasoconstriction and rapid shunting of blood into the body core in vulnerable patients. Other major triggers of Raynaud’s are physical trauma – especially vibration – and smoking.
“I can tell you that in patients who have complicated Raynaud’s with digital lesions, if they continue to smoke, it doesn’t matter what drug you give them; you’re not going to get good control or fast healing of those digital lesions,” the rheumatologist continued.
Drugs known to aggravate Raynaud’s phenomenon include the serotonin agonists prescribed for migraine headaches, interferons, opiates, some cancer chemotherapy drugs, and medications used in treating attention-deficit/hyperactivity disorder.
“Thirty to forty percent of ADHD kids develop Raynaud’s phenomenon. Methylphenidate, dextroamphetamine, atomoxetine – those are definitely potent sympathomimetic drugs. And the vascular shunts in the skin are very sensitive to sympathomimetic agonists,” according to Dr. Wigley.
Poor evidence for behavioral therapies
The notion of biofeedback, acupuncture, herbals, autogenic training, and other nondrug treatments appeals to many, but in his view, there is no good supporting evidence. What clinical trials have been done have been negative.
“Those therapies have all sort of gone belly up. I wouldn’t be recommending behavioral therapies,” he continued.
When to use vasodilators
In the minority of patients with severe primary Raynaud’s phenomenon, which affects their quality of life and doesn’t sufficiently respond to strategies aimed at avoiding environmental triggers, calcium channel blocker monotherapy is king.
“Amlodipine is my favorite because it has less cardiac effects. I use the long-acting form rather than short-acting agents. I titrate the calcium channel blocker to the maximum tolerated dose before considering combining it with a second agent. It’s a rare patient that I don’t have good control with just a calcium channel blocker,” he said.
“Usually it’s at 15-20 mg/day of amlodipine when patients start getting edema and intolerance to the drug, so you’ve got a lot of room. You usually see about a 40% reduction in the severity of Raynaud’s with 5-10 mg/day. And understand that you’re not going to get rid of Raynaud’s phenomenon. But bringing it down to where you get improved quality of life, along with all the nondrug things that you do, can be all you need to do,” Dr. Wigley said.
When he needs to resort to a second vasodilator, it’s typically a phosphodiesterase-5 inhibitor.
“I use them,” the rheumatologist said. “The best benefit I’ve gotten is when I add it to a calcium channel blocker. If someone tolerates, say, 5 mg/day of amlodipine but not 10 mg, then I might add 10 mg of sildenafil t.i.d. [three times daily] to the calcium channel blocker.”
He was quick to add, however, that the supporting evidence base for the phosphodiesterase-5 inhibitors is weak. All of the studies to date have been small – too small, in fact, to persuade insurance companies to routinely authorize this use of the medications without a fight. For example, a recent double-blind, randomized, crossover trial showed udenafil and amlodipine had equal efficacy in reducing the frequency of Raynaud’s attacks and the phosphodiesterase-5 inhibitor achieved greater blood flow in the digital arteries (Rheumatology 2014;53:658-64); however, there were only 29 subjects, and the drug is not approved in the United States.
“I’m just talking about my own experience. The studies aren’t there,” Dr. Wigley observed. “The fact of the matter is we need a large, well-controlled, double-blind study to exactly define the role of the phosphodiesterase inhibitors.”
Botox in need of supporting evidence
OnabotulinumtoxinA (Botox) has become very popular. On the plus side, it has no adverse impact upon blood pressure, and in case series, 75%-100% of treated patients report significant improvement. The negatives? It’s costly, the benefit doesn’t kick in until after about 48 hours, and the supporting evidence to date is weak, although Dr. Wigley and coinvestigators have an ongoing placebo-controlled trial.
“I have to say, in my own experience in patients with garden-variety primary Raynaud’s, [onabotulinumtoxinA] has not been very exciting, but in patients with critical ischemia, where they’re losing a finger, I’ve been very impressed. So I do think that it works. We need evidence to prove it,” he said.
Alternative vasodilator options with some demonstrated benefit in mild disease include the selective serotonin reuptake inhibitor fluoxetine, the angiotensin receptor blocker losartan, cilostazol, pentoxifylline, and topical nitrates, although the benefits of nitrates tend to wane over time and headaches are often a problem.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from KineMed, MedImmune, and CSL Behring.
SNOWMASS, COLO. – Plenty of medication options exist for treatment of primary Raynaud’s phenomenon caused by reversible cutaneous arteriolar vasospasm. But actually, for most affected patients, no drug therapy is required, according to Dr. Fredrick M. Wigley.
“The environment – physical and emotional – has a huge impact on the severity of Raynaud’s phenomenon,” he emphasized at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Whatever I tell you in terms of drugs, there’s nothing more potent than getting the patient out of a stressful, cold environment. So I tell patients who have digital ischemia to stop work, go home, get in bed, put the blanket up to your neck, keep warm, and often that’s more effective than the drug therapy that we try,” said Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
Primary Raynaud’s phenomenon is typically reversible within 15-20 minutes after rewarming, he added.
Aggravators: Stress, trauma, smoking, certain drugs
Besides cold temperatures, another important aggravating factor in primary Raynaud’s phenomenon is emotional stress. Anxiety, like cold, increases sympathetic tone, triggering cutaneous vasoconstriction and rapid shunting of blood into the body core in vulnerable patients. Other major triggers of Raynaud’s are physical trauma – especially vibration – and smoking.
“I can tell you that in patients who have complicated Raynaud’s with digital lesions, if they continue to smoke, it doesn’t matter what drug you give them; you’re not going to get good control or fast healing of those digital lesions,” the rheumatologist continued.
Drugs known to aggravate Raynaud’s phenomenon include the serotonin agonists prescribed for migraine headaches, interferons, opiates, some cancer chemotherapy drugs, and medications used in treating attention-deficit/hyperactivity disorder.
“Thirty to forty percent of ADHD kids develop Raynaud’s phenomenon. Methylphenidate, dextroamphetamine, atomoxetine – those are definitely potent sympathomimetic drugs. And the vascular shunts in the skin are very sensitive to sympathomimetic agonists,” according to Dr. Wigley.
Poor evidence for behavioral therapies
The notion of biofeedback, acupuncture, herbals, autogenic training, and other nondrug treatments appeals to many, but in his view, there is no good supporting evidence. What clinical trials have been done have been negative.
“Those therapies have all sort of gone belly up. I wouldn’t be recommending behavioral therapies,” he continued.
When to use vasodilators
In the minority of patients with severe primary Raynaud’s phenomenon, which affects their quality of life and doesn’t sufficiently respond to strategies aimed at avoiding environmental triggers, calcium channel blocker monotherapy is king.
“Amlodipine is my favorite because it has less cardiac effects. I use the long-acting form rather than short-acting agents. I titrate the calcium channel blocker to the maximum tolerated dose before considering combining it with a second agent. It’s a rare patient that I don’t have good control with just a calcium channel blocker,” he said.
“Usually it’s at 15-20 mg/day of amlodipine when patients start getting edema and intolerance to the drug, so you’ve got a lot of room. You usually see about a 40% reduction in the severity of Raynaud’s with 5-10 mg/day. And understand that you’re not going to get rid of Raynaud’s phenomenon. But bringing it down to where you get improved quality of life, along with all the nondrug things that you do, can be all you need to do,” Dr. Wigley said.
When he needs to resort to a second vasodilator, it’s typically a phosphodiesterase-5 inhibitor.
“I use them,” the rheumatologist said. “The best benefit I’ve gotten is when I add it to a calcium channel blocker. If someone tolerates, say, 5 mg/day of amlodipine but not 10 mg, then I might add 10 mg of sildenafil t.i.d. [three times daily] to the calcium channel blocker.”
He was quick to add, however, that the supporting evidence base for the phosphodiesterase-5 inhibitors is weak. All of the studies to date have been small – too small, in fact, to persuade insurance companies to routinely authorize this use of the medications without a fight. For example, a recent double-blind, randomized, crossover trial showed udenafil and amlodipine had equal efficacy in reducing the frequency of Raynaud’s attacks and the phosphodiesterase-5 inhibitor achieved greater blood flow in the digital arteries (Rheumatology 2014;53:658-64); however, there were only 29 subjects, and the drug is not approved in the United States.
“I’m just talking about my own experience. The studies aren’t there,” Dr. Wigley observed. “The fact of the matter is we need a large, well-controlled, double-blind study to exactly define the role of the phosphodiesterase inhibitors.”
Botox in need of supporting evidence
OnabotulinumtoxinA (Botox) has become very popular. On the plus side, it has no adverse impact upon blood pressure, and in case series, 75%-100% of treated patients report significant improvement. The negatives? It’s costly, the benefit doesn’t kick in until after about 48 hours, and the supporting evidence to date is weak, although Dr. Wigley and coinvestigators have an ongoing placebo-controlled trial.
“I have to say, in my own experience in patients with garden-variety primary Raynaud’s, [onabotulinumtoxinA] has not been very exciting, but in patients with critical ischemia, where they’re losing a finger, I’ve been very impressed. So I do think that it works. We need evidence to prove it,” he said.
Alternative vasodilator options with some demonstrated benefit in mild disease include the selective serotonin reuptake inhibitor fluoxetine, the angiotensin receptor blocker losartan, cilostazol, pentoxifylline, and topical nitrates, although the benefits of nitrates tend to wane over time and headaches are often a problem.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from KineMed, MedImmune, and CSL Behring.
SNOWMASS, COLO. – Plenty of medication options exist for treatment of primary Raynaud’s phenomenon caused by reversible cutaneous arteriolar vasospasm. But actually, for most affected patients, no drug therapy is required, according to Dr. Fredrick M. Wigley.
“The environment – physical and emotional – has a huge impact on the severity of Raynaud’s phenomenon,” he emphasized at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Whatever I tell you in terms of drugs, there’s nothing more potent than getting the patient out of a stressful, cold environment. So I tell patients who have digital ischemia to stop work, go home, get in bed, put the blanket up to your neck, keep warm, and often that’s more effective than the drug therapy that we try,” said Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
Primary Raynaud’s phenomenon is typically reversible within 15-20 minutes after rewarming, he added.
Aggravators: Stress, trauma, smoking, certain drugs
Besides cold temperatures, another important aggravating factor in primary Raynaud’s phenomenon is emotional stress. Anxiety, like cold, increases sympathetic tone, triggering cutaneous vasoconstriction and rapid shunting of blood into the body core in vulnerable patients. Other major triggers of Raynaud’s are physical trauma – especially vibration – and smoking.
“I can tell you that in patients who have complicated Raynaud’s with digital lesions, if they continue to smoke, it doesn’t matter what drug you give them; you’re not going to get good control or fast healing of those digital lesions,” the rheumatologist continued.
Drugs known to aggravate Raynaud’s phenomenon include the serotonin agonists prescribed for migraine headaches, interferons, opiates, some cancer chemotherapy drugs, and medications used in treating attention-deficit/hyperactivity disorder.
“Thirty to forty percent of ADHD kids develop Raynaud’s phenomenon. Methylphenidate, dextroamphetamine, atomoxetine – those are definitely potent sympathomimetic drugs. And the vascular shunts in the skin are very sensitive to sympathomimetic agonists,” according to Dr. Wigley.
Poor evidence for behavioral therapies
The notion of biofeedback, acupuncture, herbals, autogenic training, and other nondrug treatments appeals to many, but in his view, there is no good supporting evidence. What clinical trials have been done have been negative.
“Those therapies have all sort of gone belly up. I wouldn’t be recommending behavioral therapies,” he continued.
When to use vasodilators
In the minority of patients with severe primary Raynaud’s phenomenon, which affects their quality of life and doesn’t sufficiently respond to strategies aimed at avoiding environmental triggers, calcium channel blocker monotherapy is king.
“Amlodipine is my favorite because it has less cardiac effects. I use the long-acting form rather than short-acting agents. I titrate the calcium channel blocker to the maximum tolerated dose before considering combining it with a second agent. It’s a rare patient that I don’t have good control with just a calcium channel blocker,” he said.
“Usually it’s at 15-20 mg/day of amlodipine when patients start getting edema and intolerance to the drug, so you’ve got a lot of room. You usually see about a 40% reduction in the severity of Raynaud’s with 5-10 mg/day. And understand that you’re not going to get rid of Raynaud’s phenomenon. But bringing it down to where you get improved quality of life, along with all the nondrug things that you do, can be all you need to do,” Dr. Wigley said.
When he needs to resort to a second vasodilator, it’s typically a phosphodiesterase-5 inhibitor.
“I use them,” the rheumatologist said. “The best benefit I’ve gotten is when I add it to a calcium channel blocker. If someone tolerates, say, 5 mg/day of amlodipine but not 10 mg, then I might add 10 mg of sildenafil t.i.d. [three times daily] to the calcium channel blocker.”
He was quick to add, however, that the supporting evidence base for the phosphodiesterase-5 inhibitors is weak. All of the studies to date have been small – too small, in fact, to persuade insurance companies to routinely authorize this use of the medications without a fight. For example, a recent double-blind, randomized, crossover trial showed udenafil and amlodipine had equal efficacy in reducing the frequency of Raynaud’s attacks and the phosphodiesterase-5 inhibitor achieved greater blood flow in the digital arteries (Rheumatology 2014;53:658-64); however, there were only 29 subjects, and the drug is not approved in the United States.
“I’m just talking about my own experience. The studies aren’t there,” Dr. Wigley observed. “The fact of the matter is we need a large, well-controlled, double-blind study to exactly define the role of the phosphodiesterase inhibitors.”
Botox in need of supporting evidence
OnabotulinumtoxinA (Botox) has become very popular. On the plus side, it has no adverse impact upon blood pressure, and in case series, 75%-100% of treated patients report significant improvement. The negatives? It’s costly, the benefit doesn’t kick in until after about 48 hours, and the supporting evidence to date is weak, although Dr. Wigley and coinvestigators have an ongoing placebo-controlled trial.
“I have to say, in my own experience in patients with garden-variety primary Raynaud’s, [onabotulinumtoxinA] has not been very exciting, but in patients with critical ischemia, where they’re losing a finger, I’ve been very impressed. So I do think that it works. We need evidence to prove it,” he said.
Alternative vasodilator options with some demonstrated benefit in mild disease include the selective serotonin reuptake inhibitor fluoxetine, the angiotensin receptor blocker losartan, cilostazol, pentoxifylline, and topical nitrates, although the benefits of nitrates tend to wane over time and headaches are often a problem.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from KineMed, MedImmune, and CSL Behring.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
ACL repair: ‘We have to do better’
SNOWMASS, COLO. – A novel approach to repairing anterior cruciate ligament injuries – and perhaps thereby avoiding a downstream tidal wave of knee osteoarthritis – is creating major buzz in sports medicine circles.
“You’ll probably hear much more about this bioenhanced repair, with the expectation of achieving strength equal to that of ACL reconstruction and perhaps preventing the development of osteoarthritis 15 years down the road,” Dr. M. Timothy Hresko predicted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He cited research led by his colleague Dr. Martha M. Murray of Boston Children’s Hospital, which has resulted in development of a surgical technique combining a tissue-engineered composite scaffold with a suture repair of the torn ACL in what Dr. Murray has termed a bioenhanced repair.
Her work, to date preclinical, has garnered major awards from both the American Orthopaedic Society for Sports Medicine and the American Academy of Orthopaedic Surgeons. The Food and Drug Administration recently granted approval for the first clinical safety studies, to begin this year.
There is a major unmet need for better methods of repairing ACL injuries. They’re common, with an estimated 550,000 cases per year. The peak incidence occurs in 15- to 19-year-old female athletes. And the current gold standard therapy consisting of ACL reconstruction using an allograft or hamstring graft has a disturbingly high failure rate, both early and late. The graft failure rate is up to 20% in the first 2 years, climbing to 50% at 10 years.
“We just have to do better,” conceded Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“One of the interesting and unfortunate facts,” he continued, “is that roughly 80% of people who have an ACL injury, with or without reconstruction, are still going to have osteoarthritis 14 years after the injury. So, if this is your 15-year-old daughter who plays basketball, she’ll only be 30 and will already have degenerative arthritis of the knee at what should still be a very active period of life.”
The bioenhanced repair now under study uses an extracellular matrix-based scaffold, which is loaded with a few milliliters of the patient’s own platelet-enriched plasma. The scaffold is applied between the torn ligament ends in order to stimulate collagen production and promote ligament healing. The suture repair of the ligament entails much less trauma than does standard reconstructive surgery.
In large-animal studies, the bioenhanced repair resulted in the same yield load, stiffness, and other desirable biomechanical properties at 1 year as with major reconstructive surgery. However, while premature posttraumatic osteoarthritis occurred in 80% of the knees treated with standard ACL reconstruction, there was no evidence of such damage 1 year following bioenhanced repair. Nor have adverse reactions to the scaffold been noted in the porcine model.
Dr. Hresko reported serving as a consultant to Depuy Spine.
SNOWMASS, COLO. – A novel approach to repairing anterior cruciate ligament injuries – and perhaps thereby avoiding a downstream tidal wave of knee osteoarthritis – is creating major buzz in sports medicine circles.
“You’ll probably hear much more about this bioenhanced repair, with the expectation of achieving strength equal to that of ACL reconstruction and perhaps preventing the development of osteoarthritis 15 years down the road,” Dr. M. Timothy Hresko predicted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He cited research led by his colleague Dr. Martha M. Murray of Boston Children’s Hospital, which has resulted in development of a surgical technique combining a tissue-engineered composite scaffold with a suture repair of the torn ACL in what Dr. Murray has termed a bioenhanced repair.
Her work, to date preclinical, has garnered major awards from both the American Orthopaedic Society for Sports Medicine and the American Academy of Orthopaedic Surgeons. The Food and Drug Administration recently granted approval for the first clinical safety studies, to begin this year.
There is a major unmet need for better methods of repairing ACL injuries. They’re common, with an estimated 550,000 cases per year. The peak incidence occurs in 15- to 19-year-old female athletes. And the current gold standard therapy consisting of ACL reconstruction using an allograft or hamstring graft has a disturbingly high failure rate, both early and late. The graft failure rate is up to 20% in the first 2 years, climbing to 50% at 10 years.
“We just have to do better,” conceded Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“One of the interesting and unfortunate facts,” he continued, “is that roughly 80% of people who have an ACL injury, with or without reconstruction, are still going to have osteoarthritis 14 years after the injury. So, if this is your 15-year-old daughter who plays basketball, she’ll only be 30 and will already have degenerative arthritis of the knee at what should still be a very active period of life.”
The bioenhanced repair now under study uses an extracellular matrix-based scaffold, which is loaded with a few milliliters of the patient’s own platelet-enriched plasma. The scaffold is applied between the torn ligament ends in order to stimulate collagen production and promote ligament healing. The suture repair of the ligament entails much less trauma than does standard reconstructive surgery.
In large-animal studies, the bioenhanced repair resulted in the same yield load, stiffness, and other desirable biomechanical properties at 1 year as with major reconstructive surgery. However, while premature posttraumatic osteoarthritis occurred in 80% of the knees treated with standard ACL reconstruction, there was no evidence of such damage 1 year following bioenhanced repair. Nor have adverse reactions to the scaffold been noted in the porcine model.
Dr. Hresko reported serving as a consultant to Depuy Spine.
SNOWMASS, COLO. – A novel approach to repairing anterior cruciate ligament injuries – and perhaps thereby avoiding a downstream tidal wave of knee osteoarthritis – is creating major buzz in sports medicine circles.
“You’ll probably hear much more about this bioenhanced repair, with the expectation of achieving strength equal to that of ACL reconstruction and perhaps preventing the development of osteoarthritis 15 years down the road,” Dr. M. Timothy Hresko predicted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He cited research led by his colleague Dr. Martha M. Murray of Boston Children’s Hospital, which has resulted in development of a surgical technique combining a tissue-engineered composite scaffold with a suture repair of the torn ACL in what Dr. Murray has termed a bioenhanced repair.
Her work, to date preclinical, has garnered major awards from both the American Orthopaedic Society for Sports Medicine and the American Academy of Orthopaedic Surgeons. The Food and Drug Administration recently granted approval for the first clinical safety studies, to begin this year.
There is a major unmet need for better methods of repairing ACL injuries. They’re common, with an estimated 550,000 cases per year. The peak incidence occurs in 15- to 19-year-old female athletes. And the current gold standard therapy consisting of ACL reconstruction using an allograft or hamstring graft has a disturbingly high failure rate, both early and late. The graft failure rate is up to 20% in the first 2 years, climbing to 50% at 10 years.
“We just have to do better,” conceded Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“One of the interesting and unfortunate facts,” he continued, “is that roughly 80% of people who have an ACL injury, with or without reconstruction, are still going to have osteoarthritis 14 years after the injury. So, if this is your 15-year-old daughter who plays basketball, she’ll only be 30 and will already have degenerative arthritis of the knee at what should still be a very active period of life.”
The bioenhanced repair now under study uses an extracellular matrix-based scaffold, which is loaded with a few milliliters of the patient’s own platelet-enriched plasma. The scaffold is applied between the torn ligament ends in order to stimulate collagen production and promote ligament healing. The suture repair of the ligament entails much less trauma than does standard reconstructive surgery.
In large-animal studies, the bioenhanced repair resulted in the same yield load, stiffness, and other desirable biomechanical properties at 1 year as with major reconstructive surgery. However, while premature posttraumatic osteoarthritis occurred in 80% of the knees treated with standard ACL reconstruction, there was no evidence of such damage 1 year following bioenhanced repair. Nor have adverse reactions to the scaffold been noted in the porcine model.
Dr. Hresko reported serving as a consultant to Depuy Spine.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM