User login
Psoriatic disease inflammation linked to heart failure
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
Clinicians address psoriatic disease risk in the era of COVID-19
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
FROM THE GRAPPA 2020 VIRTUAL ANNUAL MEETING
PASDAS beats DAS28 in measuring psoriatic arthritis treat-to-target success
Measuring success with a treat-to-target strategy in psoriatic arthritis patients proved to be more comprehensive with the Psoriatic Arthritis Disease Activity Score (PASDAS) than it was with the Disease Activity Score in 28 joints (DAS28), according to findings from a prospective cohort study.
Fewer patients had a low disease activity score according to DAS28, and a higher percentage of patients deemed adequately treated according to DAS28 were found to have residual disease activity, compared with the number of patients so categorized according to PASDAS, researcher Michelle Mulder reported in her presentation of the study at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
“PASDAS implementation in a tightly monitored PsA [psoriatic arthritis] cohort suggests relevant residual disease burden, even though DAS28 was measured at every visit previously,” said Ms. Mulder, an MD/PhD student at Sint Maartenskliniek in Nijmegen, The Netherlands.
The presentation was convincing to Philip Helliwell, MD, PhD, who is a professor of clinical rheumatology at Leeds (England) University, and was also one of the developers of PASDAS. “We know it can be used in clinical practice with a certain amount of organization and clinical staff to help you,” he said during another presentation at GRAPPA.
Treat to target is a widely accepted therapeutic strategy. It’s particularly common in rheumatoid arthritis, but increasing evidence suggests that it improves patient outcomes in psoriatic arthritis. DAS28 is frequently used in treat-to-target approaches in rheumatoid arthritis, and often gets applied to psoriatic arthritis since rheumatologists are already comfortable with it, according to Ms. Mulder. “However, DAS28 has shown some limitations when used in psoriatic arthritis. For example, its joint count is limited to only 28 joints, and it does not take all PsA domains into account,” she said.
DAS28 was previously used at Sint Maartenskliniek in combination with psoriatic arthritis–specific assessment recommendations, but the institution opted in 2019 to switch to PASDAS, which was developed by GRAPPA and the European League Against Rheumatism. “To better adhere to international PsA guidelines, we chose to implement PASDAS in our cohort with the assumption that it might improve patient care,” Ms. Mulder said.
With DAS28, clinicians measured the C-reactive protein (CRP) and Patient Global Visual Analog Scale (VAS) domains and were advised to examine 28 joints for tender and swollen joint count domains. Under the PASDAS guidance, clinicians examined 68 joints for tenderness, 66 joints for swelling, CRP, Patient Global VAS, Physician Global VAS, Leeds Enthesitis Index, dactylitis, and the 12-item Short Form Physical Composite Scale. They also examined the skin, nails, and axial disease.
To examine the effects of the switch from DAS28 to PASDAS, the researchers compared outcomes in 855 patients before and after the change during March to December 2019. The mean age of patients was 55 years, and 46% were female. The mean disease duration was 10 years, and the mean PASDAS score was 3.1. A total of 96% of participants were negative for anti-cyclic citrullinated peptide. Overall, 30% had arthritis, 9% had axial disease, 3% had dactylitis, 21% had enthesitis, 51% had skin disease, and 42% had nail disease.
About three-quarters (77.4%) of patients reached the threshold of low disease activity (LDA) according to the DAS28 measure, while 53.1% did so using the PASDAS. High disease activity occurred in 7.8% of patients according to DAS28, compared with 2.7% as measured by PASDAS. Patients who reached only the DAS28 LDA target but not the PASDAS target, compared with patients who reached the LDA target in both measures, had significantly worse counts for swelling in 66 joints (0.7 vs. 0.2; P < .001) and tenderness in 68 joints (2.1 vs. 0.7; P < .001), as well as worse scores for enthesitis (0.5 vs. 0.1; P < .001), dactylitis (4% vs. 1%; P = .005), patient global VAS (44.0 vs. 14.4; P < .001), Health Assessment Questionnaire (0.8 vs. 0.4; P < .001) and Patient Acceptable Symptom State (unacceptable score in 17% vs. 3%; P < .001).
Ms. Mulder acknowledged that PASDAS imposes a significant burden on clinicians, and noted that Sint Maartenskliniek created patient infrastructure to handle the load. “It’s very important that you set up your clinic in a specific way. When the patient comes in, we draw blood immediately and we ask them to fill in the questionnaires, and then they go to a specialized nurse who measures all the different components of the PASDAS. It took a lot of time to train the specialized nurses and to implement the PASDAS score in our electronic health records. After we did those things, it was quite easy because we have this whole setup. It takes time and it is difficult, but it is definitely possible to do it,” Ms. Mulder said during a live Q&A following her prerecorded presentation.
The study received no funding. Ms. Mulder had no relevant financial disclosures. Dr. Helliwell has financial ties to AbbVie, Amgen, Celgen, Galapagos, Janssen, Novartis, Pfizer, and UCB.
SOURCE: Mulder M et al. GRAPPA 2020 Virtual Annual Meeting.
Measuring success with a treat-to-target strategy in psoriatic arthritis patients proved to be more comprehensive with the Psoriatic Arthritis Disease Activity Score (PASDAS) than it was with the Disease Activity Score in 28 joints (DAS28), according to findings from a prospective cohort study.
Fewer patients had a low disease activity score according to DAS28, and a higher percentage of patients deemed adequately treated according to DAS28 were found to have residual disease activity, compared with the number of patients so categorized according to PASDAS, researcher Michelle Mulder reported in her presentation of the study at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
“PASDAS implementation in a tightly monitored PsA [psoriatic arthritis] cohort suggests relevant residual disease burden, even though DAS28 was measured at every visit previously,” said Ms. Mulder, an MD/PhD student at Sint Maartenskliniek in Nijmegen, The Netherlands.
The presentation was convincing to Philip Helliwell, MD, PhD, who is a professor of clinical rheumatology at Leeds (England) University, and was also one of the developers of PASDAS. “We know it can be used in clinical practice with a certain amount of organization and clinical staff to help you,” he said during another presentation at GRAPPA.
Treat to target is a widely accepted therapeutic strategy. It’s particularly common in rheumatoid arthritis, but increasing evidence suggests that it improves patient outcomes in psoriatic arthritis. DAS28 is frequently used in treat-to-target approaches in rheumatoid arthritis, and often gets applied to psoriatic arthritis since rheumatologists are already comfortable with it, according to Ms. Mulder. “However, DAS28 has shown some limitations when used in psoriatic arthritis. For example, its joint count is limited to only 28 joints, and it does not take all PsA domains into account,” she said.
DAS28 was previously used at Sint Maartenskliniek in combination with psoriatic arthritis–specific assessment recommendations, but the institution opted in 2019 to switch to PASDAS, which was developed by GRAPPA and the European League Against Rheumatism. “To better adhere to international PsA guidelines, we chose to implement PASDAS in our cohort with the assumption that it might improve patient care,” Ms. Mulder said.
With DAS28, clinicians measured the C-reactive protein (CRP) and Patient Global Visual Analog Scale (VAS) domains and were advised to examine 28 joints for tender and swollen joint count domains. Under the PASDAS guidance, clinicians examined 68 joints for tenderness, 66 joints for swelling, CRP, Patient Global VAS, Physician Global VAS, Leeds Enthesitis Index, dactylitis, and the 12-item Short Form Physical Composite Scale. They also examined the skin, nails, and axial disease.
To examine the effects of the switch from DAS28 to PASDAS, the researchers compared outcomes in 855 patients before and after the change during March to December 2019. The mean age of patients was 55 years, and 46% were female. The mean disease duration was 10 years, and the mean PASDAS score was 3.1. A total of 96% of participants were negative for anti-cyclic citrullinated peptide. Overall, 30% had arthritis, 9% had axial disease, 3% had dactylitis, 21% had enthesitis, 51% had skin disease, and 42% had nail disease.
About three-quarters (77.4%) of patients reached the threshold of low disease activity (LDA) according to the DAS28 measure, while 53.1% did so using the PASDAS. High disease activity occurred in 7.8% of patients according to DAS28, compared with 2.7% as measured by PASDAS. Patients who reached only the DAS28 LDA target but not the PASDAS target, compared with patients who reached the LDA target in both measures, had significantly worse counts for swelling in 66 joints (0.7 vs. 0.2; P < .001) and tenderness in 68 joints (2.1 vs. 0.7; P < .001), as well as worse scores for enthesitis (0.5 vs. 0.1; P < .001), dactylitis (4% vs. 1%; P = .005), patient global VAS (44.0 vs. 14.4; P < .001), Health Assessment Questionnaire (0.8 vs. 0.4; P < .001) and Patient Acceptable Symptom State (unacceptable score in 17% vs. 3%; P < .001).
Ms. Mulder acknowledged that PASDAS imposes a significant burden on clinicians, and noted that Sint Maartenskliniek created patient infrastructure to handle the load. “It’s very important that you set up your clinic in a specific way. When the patient comes in, we draw blood immediately and we ask them to fill in the questionnaires, and then they go to a specialized nurse who measures all the different components of the PASDAS. It took a lot of time to train the specialized nurses and to implement the PASDAS score in our electronic health records. After we did those things, it was quite easy because we have this whole setup. It takes time and it is difficult, but it is definitely possible to do it,” Ms. Mulder said during a live Q&A following her prerecorded presentation.
The study received no funding. Ms. Mulder had no relevant financial disclosures. Dr. Helliwell has financial ties to AbbVie, Amgen, Celgen, Galapagos, Janssen, Novartis, Pfizer, and UCB.
SOURCE: Mulder M et al. GRAPPA 2020 Virtual Annual Meeting.
Measuring success with a treat-to-target strategy in psoriatic arthritis patients proved to be more comprehensive with the Psoriatic Arthritis Disease Activity Score (PASDAS) than it was with the Disease Activity Score in 28 joints (DAS28), according to findings from a prospective cohort study.
Fewer patients had a low disease activity score according to DAS28, and a higher percentage of patients deemed adequately treated according to DAS28 were found to have residual disease activity, compared with the number of patients so categorized according to PASDAS, researcher Michelle Mulder reported in her presentation of the study at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
“PASDAS implementation in a tightly monitored PsA [psoriatic arthritis] cohort suggests relevant residual disease burden, even though DAS28 was measured at every visit previously,” said Ms. Mulder, an MD/PhD student at Sint Maartenskliniek in Nijmegen, The Netherlands.
The presentation was convincing to Philip Helliwell, MD, PhD, who is a professor of clinical rheumatology at Leeds (England) University, and was also one of the developers of PASDAS. “We know it can be used in clinical practice with a certain amount of organization and clinical staff to help you,” he said during another presentation at GRAPPA.
Treat to target is a widely accepted therapeutic strategy. It’s particularly common in rheumatoid arthritis, but increasing evidence suggests that it improves patient outcomes in psoriatic arthritis. DAS28 is frequently used in treat-to-target approaches in rheumatoid arthritis, and often gets applied to psoriatic arthritis since rheumatologists are already comfortable with it, according to Ms. Mulder. “However, DAS28 has shown some limitations when used in psoriatic arthritis. For example, its joint count is limited to only 28 joints, and it does not take all PsA domains into account,” she said.
DAS28 was previously used at Sint Maartenskliniek in combination with psoriatic arthritis–specific assessment recommendations, but the institution opted in 2019 to switch to PASDAS, which was developed by GRAPPA and the European League Against Rheumatism. “To better adhere to international PsA guidelines, we chose to implement PASDAS in our cohort with the assumption that it might improve patient care,” Ms. Mulder said.
With DAS28, clinicians measured the C-reactive protein (CRP) and Patient Global Visual Analog Scale (VAS) domains and were advised to examine 28 joints for tender and swollen joint count domains. Under the PASDAS guidance, clinicians examined 68 joints for tenderness, 66 joints for swelling, CRP, Patient Global VAS, Physician Global VAS, Leeds Enthesitis Index, dactylitis, and the 12-item Short Form Physical Composite Scale. They also examined the skin, nails, and axial disease.
To examine the effects of the switch from DAS28 to PASDAS, the researchers compared outcomes in 855 patients before and after the change during March to December 2019. The mean age of patients was 55 years, and 46% were female. The mean disease duration was 10 years, and the mean PASDAS score was 3.1. A total of 96% of participants were negative for anti-cyclic citrullinated peptide. Overall, 30% had arthritis, 9% had axial disease, 3% had dactylitis, 21% had enthesitis, 51% had skin disease, and 42% had nail disease.
About three-quarters (77.4%) of patients reached the threshold of low disease activity (LDA) according to the DAS28 measure, while 53.1% did so using the PASDAS. High disease activity occurred in 7.8% of patients according to DAS28, compared with 2.7% as measured by PASDAS. Patients who reached only the DAS28 LDA target but not the PASDAS target, compared with patients who reached the LDA target in both measures, had significantly worse counts for swelling in 66 joints (0.7 vs. 0.2; P < .001) and tenderness in 68 joints (2.1 vs. 0.7; P < .001), as well as worse scores for enthesitis (0.5 vs. 0.1; P < .001), dactylitis (4% vs. 1%; P = .005), patient global VAS (44.0 vs. 14.4; P < .001), Health Assessment Questionnaire (0.8 vs. 0.4; P < .001) and Patient Acceptable Symptom State (unacceptable score in 17% vs. 3%; P < .001).
Ms. Mulder acknowledged that PASDAS imposes a significant burden on clinicians, and noted that Sint Maartenskliniek created patient infrastructure to handle the load. “It’s very important that you set up your clinic in a specific way. When the patient comes in, we draw blood immediately and we ask them to fill in the questionnaires, and then they go to a specialized nurse who measures all the different components of the PASDAS. It took a lot of time to train the specialized nurses and to implement the PASDAS score in our electronic health records. After we did those things, it was quite easy because we have this whole setup. It takes time and it is difficult, but it is definitely possible to do it,” Ms. Mulder said during a live Q&A following her prerecorded presentation.
The study received no funding. Ms. Mulder had no relevant financial disclosures. Dr. Helliwell has financial ties to AbbVie, Amgen, Celgen, Galapagos, Janssen, Novartis, Pfizer, and UCB.
SOURCE: Mulder M et al. GRAPPA 2020 Virtual Annual Meeting.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
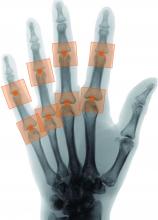
Automated RA image scoring could be coming
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
FROM THE EULAR 2020 E-CONGRESS
Visualization tool aids migraine management
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.
A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
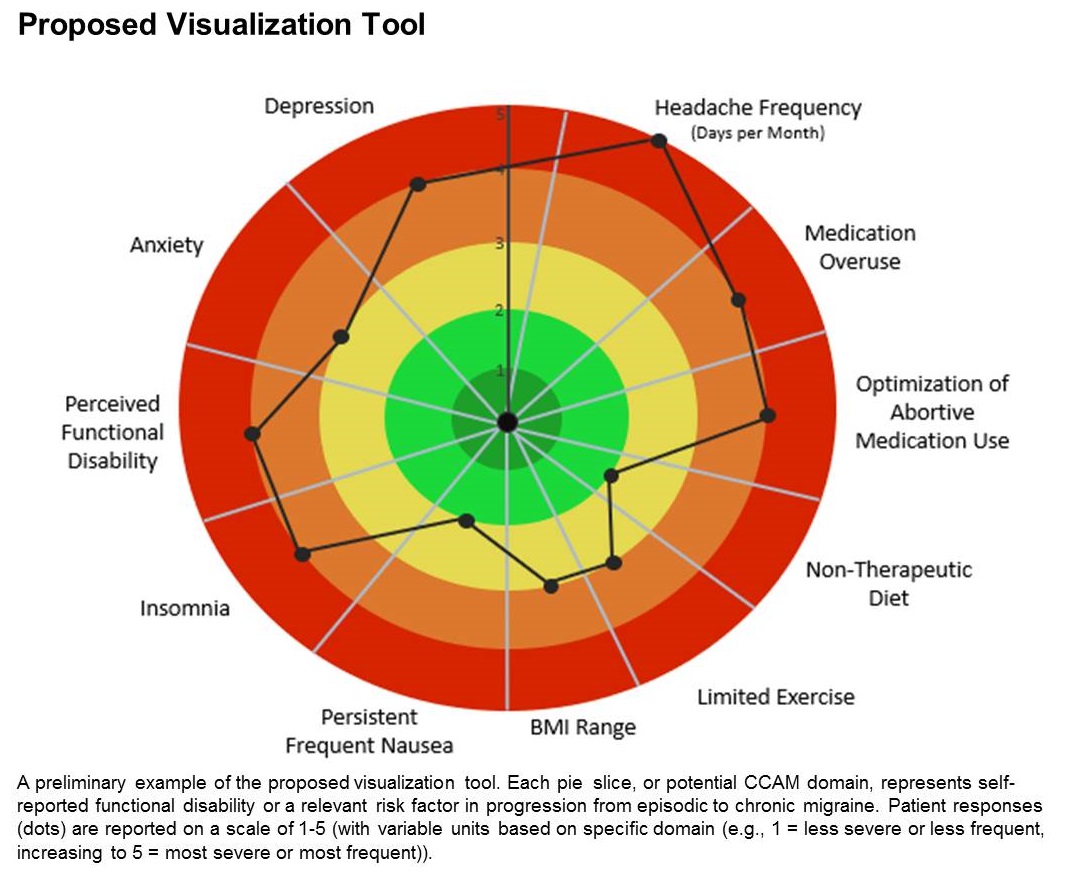
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.

A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.

A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
FROM AHS 2020
Early hypertensive disorders in pregnancy linked to obesity
Rising classes of obesity are linked with progressively increased risk of early-onset hypertensive disorders in pregnant women, as has been established for late-onset hypertensive disorders, according to a U.S.-based retrospective cohort study.
Between 4% and 8% of pregnancies are impacted by hypertensive disorders, and preeclampsia is associated with a doubling of adverse neonatal events and causes 16% of maternal deaths in developed countries, previous studies have found. This study showed a clear risk of early-onset hypertensive disorders (less than 34 weeks’ gestation), which may be more deadly than late-onset disease: Compared with later-developing disorders, early hypertensive disorders are linked to a 400% increased risk of perinatal death and a 100%-300% increased risk of severe cardiovascular, renal, or hepatic maternal morbidity, according to previous studies.
The new research, led by Matthew Bicocca, MD, of the University of Texas Health Science Center, Houston, was published in Obstetrics & Gynecology. The researchers analyzed data from U.S. Vital Statistics, including over 14 million singleton births. The sample excluded women with chronic hypertension and a body mass index (BMI) below 18.5 kg/m2.
Previous studies demonstrated that obesity is a risk factor for late-onset hypertensive disorders, but studies of early-onset hypertensive disorders have yielded conflicting results. That could be because early-onset disorders are rare, representing just 5%-10% of hypertensive disorders during pregnancy, making it difficult to obtain a sufficient sample size to show a relationship.
“We know that obese pregnant women are at increased risk for adverse pregnancy outcomes, and this is of particular importance with the increasing prevalence of obesity in the United States. As this is a nationwide cohort with a large sample size, it allowed for evaluation of the rare outcome of early-onset hypertensive disorders of pregnancy,” said Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, who was asked to comment on the study.
The researchers classified the women in the study as nonobese (BMI, 18.5-29.9 kg/m2; 46%), class I obese (BMI, 30.0-34.9; 29%), class II obese (BMI, 35.0-39.9; 15%), or class III obese (BMI, 40 or higher; 10%). About 6% of the participants developed hypertensive disorders during pregnancy (0.3% early onset), and the associated risk was greater with increasing class of obesity. Compared with nonobese women, class III obesity was associated with the highest adjusted risk ratio (2.18; 95% confidence interval, 2.12-2.24) for early-onset hypertensive disorders, followed by class II obesity (aRR, 1.57; 95% CI, 1.53-1.62) and class I (aRR, 1.13; 95% CI, 1.10-1.16). A similar pattern was observed with late-onset hypertensive disorders, with the highest risk associated with class III obese (aRR, 3.93; 95% CI, 3.91-3.96), followed by class II (aRR, 2.60; 95% CI, 2.58-2.62) and class I (aRR, 1.71; 95% CI, 1.70-1.73).
The mechanism underlying any potential link between obesity and risk of hypertensive orders of pregnancy isn’t completely understood, especially because the early-onset and late-onset hypertensive disorders have differing pathophysiology. “Early onset is the result from abnormal placentation [leading to] chronic placental insufficiency, and late onset likely [results from] placental insufficiency paired with oxidative stress from conditions such as obesity and insulin resistance,” Dr. Krishna said.
The new research reinforces the need for obese women to receive early prenatal care and counseling on nutrition and exercise “to mitigate weight gain during pregnancy in hopes of reducing their risk for adverse pregnancy outcomes, such as hypertensive disorders of pregnancy,” she concluded.
No source of funding was disclosed. The authors reported having no potential conflicts of interest.
SOURCE: Bicocca M et al. Obstet Gynecol. 2020 Jun 11. doi: 10.1097/AOG.0000000000003901.
Rising classes of obesity are linked with progressively increased risk of early-onset hypertensive disorders in pregnant women, as has been established for late-onset hypertensive disorders, according to a U.S.-based retrospective cohort study.
Between 4% and 8% of pregnancies are impacted by hypertensive disorders, and preeclampsia is associated with a doubling of adverse neonatal events and causes 16% of maternal deaths in developed countries, previous studies have found. This study showed a clear risk of early-onset hypertensive disorders (less than 34 weeks’ gestation), which may be more deadly than late-onset disease: Compared with later-developing disorders, early hypertensive disorders are linked to a 400% increased risk of perinatal death and a 100%-300% increased risk of severe cardiovascular, renal, or hepatic maternal morbidity, according to previous studies.
The new research, led by Matthew Bicocca, MD, of the University of Texas Health Science Center, Houston, was published in Obstetrics & Gynecology. The researchers analyzed data from U.S. Vital Statistics, including over 14 million singleton births. The sample excluded women with chronic hypertension and a body mass index (BMI) below 18.5 kg/m2.
Previous studies demonstrated that obesity is a risk factor for late-onset hypertensive disorders, but studies of early-onset hypertensive disorders have yielded conflicting results. That could be because early-onset disorders are rare, representing just 5%-10% of hypertensive disorders during pregnancy, making it difficult to obtain a sufficient sample size to show a relationship.
“We know that obese pregnant women are at increased risk for adverse pregnancy outcomes, and this is of particular importance with the increasing prevalence of obesity in the United States. As this is a nationwide cohort with a large sample size, it allowed for evaluation of the rare outcome of early-onset hypertensive disorders of pregnancy,” said Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, who was asked to comment on the study.
The researchers classified the women in the study as nonobese (BMI, 18.5-29.9 kg/m2; 46%), class I obese (BMI, 30.0-34.9; 29%), class II obese (BMI, 35.0-39.9; 15%), or class III obese (BMI, 40 or higher; 10%). About 6% of the participants developed hypertensive disorders during pregnancy (0.3% early onset), and the associated risk was greater with increasing class of obesity. Compared with nonobese women, class III obesity was associated with the highest adjusted risk ratio (2.18; 95% confidence interval, 2.12-2.24) for early-onset hypertensive disorders, followed by class II obesity (aRR, 1.57; 95% CI, 1.53-1.62) and class I (aRR, 1.13; 95% CI, 1.10-1.16). A similar pattern was observed with late-onset hypertensive disorders, with the highest risk associated with class III obese (aRR, 3.93; 95% CI, 3.91-3.96), followed by class II (aRR, 2.60; 95% CI, 2.58-2.62) and class I (aRR, 1.71; 95% CI, 1.70-1.73).
The mechanism underlying any potential link between obesity and risk of hypertensive orders of pregnancy isn’t completely understood, especially because the early-onset and late-onset hypertensive disorders have differing pathophysiology. “Early onset is the result from abnormal placentation [leading to] chronic placental insufficiency, and late onset likely [results from] placental insufficiency paired with oxidative stress from conditions such as obesity and insulin resistance,” Dr. Krishna said.
The new research reinforces the need for obese women to receive early prenatal care and counseling on nutrition and exercise “to mitigate weight gain during pregnancy in hopes of reducing their risk for adverse pregnancy outcomes, such as hypertensive disorders of pregnancy,” she concluded.
No source of funding was disclosed. The authors reported having no potential conflicts of interest.
SOURCE: Bicocca M et al. Obstet Gynecol. 2020 Jun 11. doi: 10.1097/AOG.0000000000003901.
Rising classes of obesity are linked with progressively increased risk of early-onset hypertensive disorders in pregnant women, as has been established for late-onset hypertensive disorders, according to a U.S.-based retrospective cohort study.
Between 4% and 8% of pregnancies are impacted by hypertensive disorders, and preeclampsia is associated with a doubling of adverse neonatal events and causes 16% of maternal deaths in developed countries, previous studies have found. This study showed a clear risk of early-onset hypertensive disorders (less than 34 weeks’ gestation), which may be more deadly than late-onset disease: Compared with later-developing disorders, early hypertensive disorders are linked to a 400% increased risk of perinatal death and a 100%-300% increased risk of severe cardiovascular, renal, or hepatic maternal morbidity, according to previous studies.
The new research, led by Matthew Bicocca, MD, of the University of Texas Health Science Center, Houston, was published in Obstetrics & Gynecology. The researchers analyzed data from U.S. Vital Statistics, including over 14 million singleton births. The sample excluded women with chronic hypertension and a body mass index (BMI) below 18.5 kg/m2.
Previous studies demonstrated that obesity is a risk factor for late-onset hypertensive disorders, but studies of early-onset hypertensive disorders have yielded conflicting results. That could be because early-onset disorders are rare, representing just 5%-10% of hypertensive disorders during pregnancy, making it difficult to obtain a sufficient sample size to show a relationship.
“We know that obese pregnant women are at increased risk for adverse pregnancy outcomes, and this is of particular importance with the increasing prevalence of obesity in the United States. As this is a nationwide cohort with a large sample size, it allowed for evaluation of the rare outcome of early-onset hypertensive disorders of pregnancy,” said Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, who was asked to comment on the study.
The researchers classified the women in the study as nonobese (BMI, 18.5-29.9 kg/m2; 46%), class I obese (BMI, 30.0-34.9; 29%), class II obese (BMI, 35.0-39.9; 15%), or class III obese (BMI, 40 or higher; 10%). About 6% of the participants developed hypertensive disorders during pregnancy (0.3% early onset), and the associated risk was greater with increasing class of obesity. Compared with nonobese women, class III obesity was associated with the highest adjusted risk ratio (2.18; 95% confidence interval, 2.12-2.24) for early-onset hypertensive disorders, followed by class II obesity (aRR, 1.57; 95% CI, 1.53-1.62) and class I (aRR, 1.13; 95% CI, 1.10-1.16). A similar pattern was observed with late-onset hypertensive disorders, with the highest risk associated with class III obese (aRR, 3.93; 95% CI, 3.91-3.96), followed by class II (aRR, 2.60; 95% CI, 2.58-2.62) and class I (aRR, 1.71; 95% CI, 1.70-1.73).
The mechanism underlying any potential link between obesity and risk of hypertensive orders of pregnancy isn’t completely understood, especially because the early-onset and late-onset hypertensive disorders have differing pathophysiology. “Early onset is the result from abnormal placentation [leading to] chronic placental insufficiency, and late onset likely [results from] placental insufficiency paired with oxidative stress from conditions such as obesity and insulin resistance,” Dr. Krishna said.
The new research reinforces the need for obese women to receive early prenatal care and counseling on nutrition and exercise “to mitigate weight gain during pregnancy in hopes of reducing their risk for adverse pregnancy outcomes, such as hypertensive disorders of pregnancy,” she concluded.
No source of funding was disclosed. The authors reported having no potential conflicts of interest.
SOURCE: Bicocca M et al. Obstet Gynecol. 2020 Jun 11. doi: 10.1097/AOG.0000000000003901.
FROM OBSTETRICS & GYNECOLOGY
Marijuana for migraine? Some tentative evidence
according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
Many patients ask about medical marijuana, but there is relatively little data on its effects on headache. Studies are generally retrospective, and often focus on marijuana use for general pain, with subset analyses looking at headache, according to coauthor Claire Ceriani, MD, who is a headache fellow at Jefferson. “A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Dr. Ceriani in an interview.
Although the research is far from a final word on the subject, it did have some take-home messages, said Dr. Ceriani. “Most people seem to find it effective as an abortive medication that might be able to take the place of some of the prescription medications that they were previously using,” she said.
The study was part of the virtual annual meeting of the American Headache Society.
An effective abortive therapy?
The study began shortly after the Jefferson Headache Center became certified to offer medical marijuana around the beginning of 2019. “We wanted to start keeping track of these patients from the get-go so we’d be able to learn as much as possible from them and help guide the recommendations we give to patients in the future,” said Dr. Ceriani.
The study included 48 patients with migraine or other types of chronic headache who received medical marijuana treatment between January and September 2019. After collecting baseline information from medical records and questionnaires filled out at marijuana treatment initiation, the researchers followed up periodically with telephone questionnaires to assess treatment response and side effects. About half of the participants (56.3%) reported daily headache. 14.6% had posttraumatic headache, 10.4% new daily persistent headache, and 4.2% tension-type headache. Additional symptoms were common, including anxiety (72.9%) and insomnia (62.5%).
A total of 28 subjects completed a follow-up questionnaire over the phone. Out of the 28 participants , 3 had stopped using marijuana. Of 25 subjects who continued use, 71.4% used it two or more times per week, and 25.0% used it every day. Among participants, 50% used a THC-dominant strain of marijuana. Overall, 71.4% used an inhaled form.
Side effects included dry mouth/throat (46.4%), dry/red eyes (35.7%), fatigue/lethargy (35.7%), and increased appetite (35.7%).
Before starting on marijuana, 46.4% of the subjects used abortive medications at least 10 days per month. After starting marijuana treatment, the rate dropped to 25.0%. Marijuana use was associated with improvements in anxiety: 57.1% who had anxiety reported improvement with marijuana use, as did 78.6% with insomnia. On a scale of 10, the average rating of marijuana’s usefulness was 5.9, and 17.9% rated it as 10.
Several concerns
The study has numerous limitations. It has a small sample size, it is from a single center, and the patient population had relatively severe symptoms. Such studies are “fraught with possible bias,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, when asked to comment.
He pointed out that one key concern for marijuana is concerns over worsening of the condition or refractoriness caused by medication overuse. The cannabinoid receptors it acts on bear some similarity to opioid receptors, and opioid overuse headache is well known. The recent changes in marijuana laws makes it an important issue, one that patients often asked about. But prospective clinical trials face a range of roadblocks: Marijuana remains a controlled substance, it would be difficult to create a placebo control, and no large companies are likely to sponsor such a trial.
“But I think it’s important to keep talking about and developing evidence as much as we can and addressing not just the benefits but also being keenly aware of the possible adverse effects, especially medication overuse,” said Dr. Charles.
The authors also acknowledged the study’s limitations, “but I think there is value, because there are definitely specific patterns we were able to find in terms of what’s helpful for patients, and we also found that a lot of patients also have other disorders in addition to headache, like anxiety and insomnia. And we found that those patients in particular seemed to have more benefit than most with medical marijuana,” said coauthor Angela Hou, MD, who is also a headache fellow at Jefferson.
Dr. Hou and Dr. Ceriani cautioned against use of marijuana in any patient with a substance use disorder, as well as the inhaled form in patients with chronic lung conditions.
The study received no funding. Dr. Ceriani and Dr. Hou had no relevant financial disclosures. Dr. Charles has consulted for Amgen, Biohaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Marmura MJ et al. AHS 2020, Abstract 842679.
according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
Many patients ask about medical marijuana, but there is relatively little data on its effects on headache. Studies are generally retrospective, and often focus on marijuana use for general pain, with subset analyses looking at headache, according to coauthor Claire Ceriani, MD, who is a headache fellow at Jefferson. “A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Dr. Ceriani in an interview.
Although the research is far from a final word on the subject, it did have some take-home messages, said Dr. Ceriani. “Most people seem to find it effective as an abortive medication that might be able to take the place of some of the prescription medications that they were previously using,” she said.
The study was part of the virtual annual meeting of the American Headache Society.
An effective abortive therapy?
The study began shortly after the Jefferson Headache Center became certified to offer medical marijuana around the beginning of 2019. “We wanted to start keeping track of these patients from the get-go so we’d be able to learn as much as possible from them and help guide the recommendations we give to patients in the future,” said Dr. Ceriani.
The study included 48 patients with migraine or other types of chronic headache who received medical marijuana treatment between January and September 2019. After collecting baseline information from medical records and questionnaires filled out at marijuana treatment initiation, the researchers followed up periodically with telephone questionnaires to assess treatment response and side effects. About half of the participants (56.3%) reported daily headache. 14.6% had posttraumatic headache, 10.4% new daily persistent headache, and 4.2% tension-type headache. Additional symptoms were common, including anxiety (72.9%) and insomnia (62.5%).
A total of 28 subjects completed a follow-up questionnaire over the phone. Out of the 28 participants , 3 had stopped using marijuana. Of 25 subjects who continued use, 71.4% used it two or more times per week, and 25.0% used it every day. Among participants, 50% used a THC-dominant strain of marijuana. Overall, 71.4% used an inhaled form.
Side effects included dry mouth/throat (46.4%), dry/red eyes (35.7%), fatigue/lethargy (35.7%), and increased appetite (35.7%).
Before starting on marijuana, 46.4% of the subjects used abortive medications at least 10 days per month. After starting marijuana treatment, the rate dropped to 25.0%. Marijuana use was associated with improvements in anxiety: 57.1% who had anxiety reported improvement with marijuana use, as did 78.6% with insomnia. On a scale of 10, the average rating of marijuana’s usefulness was 5.9, and 17.9% rated it as 10.
Several concerns
The study has numerous limitations. It has a small sample size, it is from a single center, and the patient population had relatively severe symptoms. Such studies are “fraught with possible bias,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, when asked to comment.
He pointed out that one key concern for marijuana is concerns over worsening of the condition or refractoriness caused by medication overuse. The cannabinoid receptors it acts on bear some similarity to opioid receptors, and opioid overuse headache is well known. The recent changes in marijuana laws makes it an important issue, one that patients often asked about. But prospective clinical trials face a range of roadblocks: Marijuana remains a controlled substance, it would be difficult to create a placebo control, and no large companies are likely to sponsor such a trial.
“But I think it’s important to keep talking about and developing evidence as much as we can and addressing not just the benefits but also being keenly aware of the possible adverse effects, especially medication overuse,” said Dr. Charles.
The authors also acknowledged the study’s limitations, “but I think there is value, because there are definitely specific patterns we were able to find in terms of what’s helpful for patients, and we also found that a lot of patients also have other disorders in addition to headache, like anxiety and insomnia. And we found that those patients in particular seemed to have more benefit than most with medical marijuana,” said coauthor Angela Hou, MD, who is also a headache fellow at Jefferson.
Dr. Hou and Dr. Ceriani cautioned against use of marijuana in any patient with a substance use disorder, as well as the inhaled form in patients with chronic lung conditions.
The study received no funding. Dr. Ceriani and Dr. Hou had no relevant financial disclosures. Dr. Charles has consulted for Amgen, Biohaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Marmura MJ et al. AHS 2020, Abstract 842679.
according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
Many patients ask about medical marijuana, but there is relatively little data on its effects on headache. Studies are generally retrospective, and often focus on marijuana use for general pain, with subset analyses looking at headache, according to coauthor Claire Ceriani, MD, who is a headache fellow at Jefferson. “A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Dr. Ceriani in an interview.
Although the research is far from a final word on the subject, it did have some take-home messages, said Dr. Ceriani. “Most people seem to find it effective as an abortive medication that might be able to take the place of some of the prescription medications that they were previously using,” she said.
The study was part of the virtual annual meeting of the American Headache Society.
An effective abortive therapy?
The study began shortly after the Jefferson Headache Center became certified to offer medical marijuana around the beginning of 2019. “We wanted to start keeping track of these patients from the get-go so we’d be able to learn as much as possible from them and help guide the recommendations we give to patients in the future,” said Dr. Ceriani.
The study included 48 patients with migraine or other types of chronic headache who received medical marijuana treatment between January and September 2019. After collecting baseline information from medical records and questionnaires filled out at marijuana treatment initiation, the researchers followed up periodically with telephone questionnaires to assess treatment response and side effects. About half of the participants (56.3%) reported daily headache. 14.6% had posttraumatic headache, 10.4% new daily persistent headache, and 4.2% tension-type headache. Additional symptoms were common, including anxiety (72.9%) and insomnia (62.5%).
A total of 28 subjects completed a follow-up questionnaire over the phone. Out of the 28 participants , 3 had stopped using marijuana. Of 25 subjects who continued use, 71.4% used it two or more times per week, and 25.0% used it every day. Among participants, 50% used a THC-dominant strain of marijuana. Overall, 71.4% used an inhaled form.
Side effects included dry mouth/throat (46.4%), dry/red eyes (35.7%), fatigue/lethargy (35.7%), and increased appetite (35.7%).
Before starting on marijuana, 46.4% of the subjects used abortive medications at least 10 days per month. After starting marijuana treatment, the rate dropped to 25.0%. Marijuana use was associated with improvements in anxiety: 57.1% who had anxiety reported improvement with marijuana use, as did 78.6% with insomnia. On a scale of 10, the average rating of marijuana’s usefulness was 5.9, and 17.9% rated it as 10.
Several concerns
The study has numerous limitations. It has a small sample size, it is from a single center, and the patient population had relatively severe symptoms. Such studies are “fraught with possible bias,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, when asked to comment.
He pointed out that one key concern for marijuana is concerns over worsening of the condition or refractoriness caused by medication overuse. The cannabinoid receptors it acts on bear some similarity to opioid receptors, and opioid overuse headache is well known. The recent changes in marijuana laws makes it an important issue, one that patients often asked about. But prospective clinical trials face a range of roadblocks: Marijuana remains a controlled substance, it would be difficult to create a placebo control, and no large companies are likely to sponsor such a trial.
“But I think it’s important to keep talking about and developing evidence as much as we can and addressing not just the benefits but also being keenly aware of the possible adverse effects, especially medication overuse,” said Dr. Charles.
The authors also acknowledged the study’s limitations, “but I think there is value, because there are definitely specific patterns we were able to find in terms of what’s helpful for patients, and we also found that a lot of patients also have other disorders in addition to headache, like anxiety and insomnia. And we found that those patients in particular seemed to have more benefit than most with medical marijuana,” said coauthor Angela Hou, MD, who is also a headache fellow at Jefferson.
Dr. Hou and Dr. Ceriani cautioned against use of marijuana in any patient with a substance use disorder, as well as the inhaled form in patients with chronic lung conditions.
The study received no funding. Dr. Ceriani and Dr. Hou had no relevant financial disclosures. Dr. Charles has consulted for Amgen, Biohaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Marmura MJ et al. AHS 2020, Abstract 842679.
FROM AHS 2020
NSAID/triptan combination improves treatment-resistant migraine
The combination (AXS-07), in development by Axsome Therapeutics, was also safe and well tolerated, according to Cedric O’Gorman, MD, Axsome senior vice president for clinical development and medical affairs. It was tested in subjects who had inadequately responded to previous treatment and who had an average of 2-8 migraines per month.
The therapy combines 10-mg rizatriptan with 20-mg meloxicam delivered by the company’s MoSEIC technology. “Treatment with AXS-07 resulted in rapid, sustained, substantial, and statistically significant effect as compared with rizatriptan and placebo. The enhanced effect of AXS-07 may be especially relevant for patients with more difficult-to-treat migraine,” said Dr. O’Gorman during a presentation of the study at the virtual annual meeting of the American Headache Society.
Matthew Robbins, MD, said in an interview, “This combination may be particularly useful for patients who want to take an oral medication but still need rapid and sustained pain freedom.” Dr. Robbins is the neurology residency program director at New York Presbyterian Hospital and an associate professor of neurology at Weill Cornell Medicine, New York. He was not involved in the research.
The study randomized 1,594 patients 2:2:2:1 to AXS-07, rizatriptan alone, MoSEIC meloxicam alone, or placebo, which could be administered immediately after a migraine event. Between 35% and 40% of participants across the groups had previously used triptans. The mean migraine treatment optimization questionnaire (mTOQ4) score was 3.6, indicating that the population was made up of people with poor responses to medication. Among patients in the study group, 37%-43% had severe pain intensity, 41%-47% were obese, and 35%-37% had morning migraine.
At 2 hours, more patients in the AXS-07 group than in the placebo group were pain free (19.9% vs. 6.7%; P < 0.001). They were also more likely to experience freedom from the most bothersome symptom at 2 hours (36.9% vs. 24.4%; P = 0.002). Secondary outcome measures favored the AXS-07 group when compared with the rizatriptan-only group, including 1-hour pain relief (44% vs. 37%; P = 0.04), 2- to 24-hour sustained pain relief (53% vs. 44%; P = 0.006), 2- to 48-hour sustained pain relief (47% vs. 37%; P = 0.003), 2- to 24-hour sustained pain freedom (16% vs. 11%; P = 0.038), 2- to 48-hour sustained pain freedom (15% vs. 8.8%; P = 0.003), rescue medication use (23% vs. 35%; P < 0.001), a rating of much or very much improved on the Patient Global Impression of Change (PGI-C) scale (47% vs. 39%; P = 0.022), and functional improvement at 24 hours (64% vs. 56%; P = 0.027).
“The percentage of patients achieving pain relief with AXS-07 was numerically greater than with rizatriptan at every time point measure, starting at 15 minutes, and was statistically significant by 60 minutes. This is significant because rizatriptan is widely recognized as the fastest-acting and one of the most effective oral triptans,” said Dr. O’Gorman.
The frequency of adverse events was 11.0% in the AXS-07, 15.4% in the rizatriptan group, 11.5% in the meloxicam group, and 6.0% in the placebo group.
“The added benefit of this study was the demonstration of efficacy in patients who have previously failed other acute treatments. We know that ineffective acute treatments are a likely risk factor for the progression of episodic migraine to chronic migraine, and the more options that we have for our patients, the better,” Dr. Robbins commented.
He remains concerned about cost and access, however. A limited number of tablets per month for acute treatments prompt clinicians to prescribe the medications individually and advise patients to take them in combination. “Rizatriptan is generally available in 12 monthly tablets by many coverage plans, and I would hope that, if ultimately FDA approved, a similar allotment is made affordable and accessible,” he said.
The study was funded by Axsome Therapeutics. Dr. O’Gorman is an employee of Axsome. Dr. Robbins has no relevant financial disclosures.
SOURCE: O’Gorman C et al. AHS 2020, Abstract 840673.
The combination (AXS-07), in development by Axsome Therapeutics, was also safe and well tolerated, according to Cedric O’Gorman, MD, Axsome senior vice president for clinical development and medical affairs. It was tested in subjects who had inadequately responded to previous treatment and who had an average of 2-8 migraines per month.
The therapy combines 10-mg rizatriptan with 20-mg meloxicam delivered by the company’s MoSEIC technology. “Treatment with AXS-07 resulted in rapid, sustained, substantial, and statistically significant effect as compared with rizatriptan and placebo. The enhanced effect of AXS-07 may be especially relevant for patients with more difficult-to-treat migraine,” said Dr. O’Gorman during a presentation of the study at the virtual annual meeting of the American Headache Society.
Matthew Robbins, MD, said in an interview, “This combination may be particularly useful for patients who want to take an oral medication but still need rapid and sustained pain freedom.” Dr. Robbins is the neurology residency program director at New York Presbyterian Hospital and an associate professor of neurology at Weill Cornell Medicine, New York. He was not involved in the research.
The study randomized 1,594 patients 2:2:2:1 to AXS-07, rizatriptan alone, MoSEIC meloxicam alone, or placebo, which could be administered immediately after a migraine event. Between 35% and 40% of participants across the groups had previously used triptans. The mean migraine treatment optimization questionnaire (mTOQ4) score was 3.6, indicating that the population was made up of people with poor responses to medication. Among patients in the study group, 37%-43% had severe pain intensity, 41%-47% were obese, and 35%-37% had morning migraine.
At 2 hours, more patients in the AXS-07 group than in the placebo group were pain free (19.9% vs. 6.7%; P < 0.001). They were also more likely to experience freedom from the most bothersome symptom at 2 hours (36.9% vs. 24.4%; P = 0.002). Secondary outcome measures favored the AXS-07 group when compared with the rizatriptan-only group, including 1-hour pain relief (44% vs. 37%; P = 0.04), 2- to 24-hour sustained pain relief (53% vs. 44%; P = 0.006), 2- to 48-hour sustained pain relief (47% vs. 37%; P = 0.003), 2- to 24-hour sustained pain freedom (16% vs. 11%; P = 0.038), 2- to 48-hour sustained pain freedom (15% vs. 8.8%; P = 0.003), rescue medication use (23% vs. 35%; P < 0.001), a rating of much or very much improved on the Patient Global Impression of Change (PGI-C) scale (47% vs. 39%; P = 0.022), and functional improvement at 24 hours (64% vs. 56%; P = 0.027).
“The percentage of patients achieving pain relief with AXS-07 was numerically greater than with rizatriptan at every time point measure, starting at 15 minutes, and was statistically significant by 60 minutes. This is significant because rizatriptan is widely recognized as the fastest-acting and one of the most effective oral triptans,” said Dr. O’Gorman.
The frequency of adverse events was 11.0% in the AXS-07, 15.4% in the rizatriptan group, 11.5% in the meloxicam group, and 6.0% in the placebo group.
“The added benefit of this study was the demonstration of efficacy in patients who have previously failed other acute treatments. We know that ineffective acute treatments are a likely risk factor for the progression of episodic migraine to chronic migraine, and the more options that we have for our patients, the better,” Dr. Robbins commented.
He remains concerned about cost and access, however. A limited number of tablets per month for acute treatments prompt clinicians to prescribe the medications individually and advise patients to take them in combination. “Rizatriptan is generally available in 12 monthly tablets by many coverage plans, and I would hope that, if ultimately FDA approved, a similar allotment is made affordable and accessible,” he said.
The study was funded by Axsome Therapeutics. Dr. O’Gorman is an employee of Axsome. Dr. Robbins has no relevant financial disclosures.
SOURCE: O’Gorman C et al. AHS 2020, Abstract 840673.
The combination (AXS-07), in development by Axsome Therapeutics, was also safe and well tolerated, according to Cedric O’Gorman, MD, Axsome senior vice president for clinical development and medical affairs. It was tested in subjects who had inadequately responded to previous treatment and who had an average of 2-8 migraines per month.
The therapy combines 10-mg rizatriptan with 20-mg meloxicam delivered by the company’s MoSEIC technology. “Treatment with AXS-07 resulted in rapid, sustained, substantial, and statistically significant effect as compared with rizatriptan and placebo. The enhanced effect of AXS-07 may be especially relevant for patients with more difficult-to-treat migraine,” said Dr. O’Gorman during a presentation of the study at the virtual annual meeting of the American Headache Society.
Matthew Robbins, MD, said in an interview, “This combination may be particularly useful for patients who want to take an oral medication but still need rapid and sustained pain freedom.” Dr. Robbins is the neurology residency program director at New York Presbyterian Hospital and an associate professor of neurology at Weill Cornell Medicine, New York. He was not involved in the research.
The study randomized 1,594 patients 2:2:2:1 to AXS-07, rizatriptan alone, MoSEIC meloxicam alone, or placebo, which could be administered immediately after a migraine event. Between 35% and 40% of participants across the groups had previously used triptans. The mean migraine treatment optimization questionnaire (mTOQ4) score was 3.6, indicating that the population was made up of people with poor responses to medication. Among patients in the study group, 37%-43% had severe pain intensity, 41%-47% were obese, and 35%-37% had morning migraine.
At 2 hours, more patients in the AXS-07 group than in the placebo group were pain free (19.9% vs. 6.7%; P < 0.001). They were also more likely to experience freedom from the most bothersome symptom at 2 hours (36.9% vs. 24.4%; P = 0.002). Secondary outcome measures favored the AXS-07 group when compared with the rizatriptan-only group, including 1-hour pain relief (44% vs. 37%; P = 0.04), 2- to 24-hour sustained pain relief (53% vs. 44%; P = 0.006), 2- to 48-hour sustained pain relief (47% vs. 37%; P = 0.003), 2- to 24-hour sustained pain freedom (16% vs. 11%; P = 0.038), 2- to 48-hour sustained pain freedom (15% vs. 8.8%; P = 0.003), rescue medication use (23% vs. 35%; P < 0.001), a rating of much or very much improved on the Patient Global Impression of Change (PGI-C) scale (47% vs. 39%; P = 0.022), and functional improvement at 24 hours (64% vs. 56%; P = 0.027).
“The percentage of patients achieving pain relief with AXS-07 was numerically greater than with rizatriptan at every time point measure, starting at 15 minutes, and was statistically significant by 60 minutes. This is significant because rizatriptan is widely recognized as the fastest-acting and one of the most effective oral triptans,” said Dr. O’Gorman.
The frequency of adverse events was 11.0% in the AXS-07, 15.4% in the rizatriptan group, 11.5% in the meloxicam group, and 6.0% in the placebo group.
“The added benefit of this study was the demonstration of efficacy in patients who have previously failed other acute treatments. We know that ineffective acute treatments are a likely risk factor for the progression of episodic migraine to chronic migraine, and the more options that we have for our patients, the better,” Dr. Robbins commented.
He remains concerned about cost and access, however. A limited number of tablets per month for acute treatments prompt clinicians to prescribe the medications individually and advise patients to take them in combination. “Rizatriptan is generally available in 12 monthly tablets by many coverage plans, and I would hope that, if ultimately FDA approved, a similar allotment is made affordable and accessible,” he said.
The study was funded by Axsome Therapeutics. Dr. O’Gorman is an employee of Axsome. Dr. Robbins has no relevant financial disclosures.
SOURCE: O’Gorman C et al. AHS 2020, Abstract 840673.
FROM AHS 2020
Intranasal DHE shows promise in migraine
, according to results from a phase 3 clinical trial. In development by Impel NeuroPharma, the new formulation could offer patients an at-home alternative to intramuscular infusions or intravenous injections currently used to deliver DHE.
“Our analysis of the data suggests that nothing new or untoward seemed to be happening as a result of delivering DHE to the upper nasal space,” Stephen Shrewsbury, MD, chief medical officer of Impel NeuroPharma, said in an interview. The company released key results from its phase 3 clinical trial, while a poster examining patient satisfaction was presented by Dr. Shrewsbury at the virtual annual meeting of the American Headache Society.
An improved intranasal formulation
The product isn’t the first effort to develop an inhaled form of DHE. An inhaled version called Migranal, marketed by Bausch Health, delivers DHE to the front part of the nose, where it may be lost to the upper lip or down the throat, according to Dr. Shrewsbury. Impel’s formulation (INP104) delivers the drug to the upper nasal space, where an earlier phase 1 trial demonstrated it could achieve higher serum concentrations compared with Migranal.
In 2018, MAP Pharmaceuticals came close to a product, but it was ultimately rejected by the Food and Drug Administration because DHE was not stable in the propellant used in the formulation. This time is different, said Dr. Shrewsbury, who was chief medical officer at MAP before joining Impel. The new device holds DHE and the propellant in separate compartments until they are combined right before use, which should circumvent stability problems.
Dr. Shrewsbury believes that patients will welcome an inhaled version of DHE. “People with migraines don’t want to have to go into hospital or even an infusion center if they can help it,” he said.
The study was one of a number of presentations at the AHS meeting that focused on novel delivery methods for established drugs. “The idea of taking things that we know work and improving upon them, both in terms of formulation and then delivery, that’s a common theme. My impression is that this will be an interesting arrow to have in our sling,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, who was not involved in the study.
Open-label trial results
The STOP 301 phase 3 open-label safety and tolerability trial treated over 5,650 migraine attacks in 354 patients who self-administered INP104 for up to 52 weeks. They were provided up to three doses per week (1.45 mg in a dose of two puffs, one per nostril). Maximum doses included two per day and three per week.
There were no new safety signals or concern trends in nasal safety findings. 15.0% of patients experienced nasal congestion, 6.8% nausea, 5.1% nasal discomfort, and 5.1% unpleasant taste.
A total of 66.3% of participants reported pain relief by 2 hours (severe or moderate pain reduced to mild or none, or mild pain reduced to none) following a dose, and 38% had freedom from pain. 16.3% reported pain relief onset at 15 minutes, with continued improvement over time. During weeks 21-24 of the study, 98.4% and 95% of patients reporting no recurrence of their migraine or use of rescue medications during the 24- and 48-hour periods after using INP104. “Once they got rid of the pain, it didn’t come back, and that’s been one of the shortcomings of many of the available oral therapies – although some of them can be quite effective, that effect can wear off and people can find their migraine comes back within a 24- or 48-hour period,” said Dr. Shrewsbury.
The drug was also rated as convenient, with 83.6% of participants strongly agreeing (50%) or agreeing (33.6%) that it is easy to use.
“It certainly looks like compliance will be good. The possibility is that this will be quite useful,” said Dr. Charles, who is also enthusiastic about some of the other drug formulations announced at the meeting. “It really is just fun times for us as clinicians to be able to have so many different options for patients,” he said.
Dr. Shrewsbury is an employee of Impel NeuroPharma, which funded the study.* Dr. Charles consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Shrewsbury S, et al. AHS 2020. Abstract 832509.
*Correction, 6/19/20: An earlier version of this article misstated the name of Impel NeuroPharma.
, according to results from a phase 3 clinical trial. In development by Impel NeuroPharma, the new formulation could offer patients an at-home alternative to intramuscular infusions or intravenous injections currently used to deliver DHE.
“Our analysis of the data suggests that nothing new or untoward seemed to be happening as a result of delivering DHE to the upper nasal space,” Stephen Shrewsbury, MD, chief medical officer of Impel NeuroPharma, said in an interview. The company released key results from its phase 3 clinical trial, while a poster examining patient satisfaction was presented by Dr. Shrewsbury at the virtual annual meeting of the American Headache Society.
An improved intranasal formulation
The product isn’t the first effort to develop an inhaled form of DHE. An inhaled version called Migranal, marketed by Bausch Health, delivers DHE to the front part of the nose, where it may be lost to the upper lip or down the throat, according to Dr. Shrewsbury. Impel’s formulation (INP104) delivers the drug to the upper nasal space, where an earlier phase 1 trial demonstrated it could achieve higher serum concentrations compared with Migranal.
In 2018, MAP Pharmaceuticals came close to a product, but it was ultimately rejected by the Food and Drug Administration because DHE was not stable in the propellant used in the formulation. This time is different, said Dr. Shrewsbury, who was chief medical officer at MAP before joining Impel. The new device holds DHE and the propellant in separate compartments until they are combined right before use, which should circumvent stability problems.
Dr. Shrewsbury believes that patients will welcome an inhaled version of DHE. “People with migraines don’t want to have to go into hospital or even an infusion center if they can help it,” he said.
The study was one of a number of presentations at the AHS meeting that focused on novel delivery methods for established drugs. “The idea of taking things that we know work and improving upon them, both in terms of formulation and then delivery, that’s a common theme. My impression is that this will be an interesting arrow to have in our sling,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, who was not involved in the study.
Open-label trial results
The STOP 301 phase 3 open-label safety and tolerability trial treated over 5,650 migraine attacks in 354 patients who self-administered INP104 for up to 52 weeks. They were provided up to three doses per week (1.45 mg in a dose of two puffs, one per nostril). Maximum doses included two per day and three per week.
There were no new safety signals or concern trends in nasal safety findings. 15.0% of patients experienced nasal congestion, 6.8% nausea, 5.1% nasal discomfort, and 5.1% unpleasant taste.
A total of 66.3% of participants reported pain relief by 2 hours (severe or moderate pain reduced to mild or none, or mild pain reduced to none) following a dose, and 38% had freedom from pain. 16.3% reported pain relief onset at 15 minutes, with continued improvement over time. During weeks 21-24 of the study, 98.4% and 95% of patients reporting no recurrence of their migraine or use of rescue medications during the 24- and 48-hour periods after using INP104. “Once they got rid of the pain, it didn’t come back, and that’s been one of the shortcomings of many of the available oral therapies – although some of them can be quite effective, that effect can wear off and people can find their migraine comes back within a 24- or 48-hour period,” said Dr. Shrewsbury.
The drug was also rated as convenient, with 83.6% of participants strongly agreeing (50%) or agreeing (33.6%) that it is easy to use.
“It certainly looks like compliance will be good. The possibility is that this will be quite useful,” said Dr. Charles, who is also enthusiastic about some of the other drug formulations announced at the meeting. “It really is just fun times for us as clinicians to be able to have so many different options for patients,” he said.
Dr. Shrewsbury is an employee of Impel NeuroPharma, which funded the study.* Dr. Charles consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Shrewsbury S, et al. AHS 2020. Abstract 832509.
*Correction, 6/19/20: An earlier version of this article misstated the name of Impel NeuroPharma.
, according to results from a phase 3 clinical trial. In development by Impel NeuroPharma, the new formulation could offer patients an at-home alternative to intramuscular infusions or intravenous injections currently used to deliver DHE.
“Our analysis of the data suggests that nothing new or untoward seemed to be happening as a result of delivering DHE to the upper nasal space,” Stephen Shrewsbury, MD, chief medical officer of Impel NeuroPharma, said in an interview. The company released key results from its phase 3 clinical trial, while a poster examining patient satisfaction was presented by Dr. Shrewsbury at the virtual annual meeting of the American Headache Society.
An improved intranasal formulation
The product isn’t the first effort to develop an inhaled form of DHE. An inhaled version called Migranal, marketed by Bausch Health, delivers DHE to the front part of the nose, where it may be lost to the upper lip or down the throat, according to Dr. Shrewsbury. Impel’s formulation (INP104) delivers the drug to the upper nasal space, where an earlier phase 1 trial demonstrated it could achieve higher serum concentrations compared with Migranal.
In 2018, MAP Pharmaceuticals came close to a product, but it was ultimately rejected by the Food and Drug Administration because DHE was not stable in the propellant used in the formulation. This time is different, said Dr. Shrewsbury, who was chief medical officer at MAP before joining Impel. The new device holds DHE and the propellant in separate compartments until they are combined right before use, which should circumvent stability problems.
Dr. Shrewsbury believes that patients will welcome an inhaled version of DHE. “People with migraines don’t want to have to go into hospital or even an infusion center if they can help it,” he said.
The study was one of a number of presentations at the AHS meeting that focused on novel delivery methods for established drugs. “The idea of taking things that we know work and improving upon them, both in terms of formulation and then delivery, that’s a common theme. My impression is that this will be an interesting arrow to have in our sling,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, who was not involved in the study.
Open-label trial results
The STOP 301 phase 3 open-label safety and tolerability trial treated over 5,650 migraine attacks in 354 patients who self-administered INP104 for up to 52 weeks. They were provided up to three doses per week (1.45 mg in a dose of two puffs, one per nostril). Maximum doses included two per day and three per week.
There were no new safety signals or concern trends in nasal safety findings. 15.0% of patients experienced nasal congestion, 6.8% nausea, 5.1% nasal discomfort, and 5.1% unpleasant taste.
A total of 66.3% of participants reported pain relief by 2 hours (severe or moderate pain reduced to mild or none, or mild pain reduced to none) following a dose, and 38% had freedom from pain. 16.3% reported pain relief onset at 15 minutes, with continued improvement over time. During weeks 21-24 of the study, 98.4% and 95% of patients reporting no recurrence of their migraine or use of rescue medications during the 24- and 48-hour periods after using INP104. “Once they got rid of the pain, it didn’t come back, and that’s been one of the shortcomings of many of the available oral therapies – although some of them can be quite effective, that effect can wear off and people can find their migraine comes back within a 24- or 48-hour period,” said Dr. Shrewsbury.
The drug was also rated as convenient, with 83.6% of participants strongly agreeing (50%) or agreeing (33.6%) that it is easy to use.
“It certainly looks like compliance will be good. The possibility is that this will be quite useful,” said Dr. Charles, who is also enthusiastic about some of the other drug formulations announced at the meeting. “It really is just fun times for us as clinicians to be able to have so many different options for patients,” he said.
Dr. Shrewsbury is an employee of Impel NeuroPharma, which funded the study.* Dr. Charles consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Shrewsbury S, et al. AHS 2020. Abstract 832509.
*Correction, 6/19/20: An earlier version of this article misstated the name of Impel NeuroPharma.
FROM AHS 2020
Persistent posttraumatic headache risk factors confirmed
It also revealed a surprisingly high frequency of misdiagnosis. The original sample included 500 patients drawn from the Stanford Research Repository Cohort Discovery Tool, but a review found 200 records that were misdiagnosed and had to be excluded.
“It’s very easy to label someone who suffered a head injury and say this is the reason why they have this (headache),” said lead author Tommy Chan, MBBS, a headache fellow in the department of neurology at Stanford (Calif.) University, in an interview. Such patients are often seen by ED or primary care physicians who do not have a lot of experience with posttraumatic headache, and that can lead to negative consequences if a low-pressure headache is mistaken as stemming from a skull fracture. “It’s a very different treatment plan for one versus the other,” said Dr. Chan in an interview.
He noted that it can help to take a patient history that includes the preaccident headache frequency and determine if there was a change in frequency post injury.
Dr. Chan presented the results at the virtual annual meeting of the American Headache Society.
“The results are what one might expect, although we haven’t studied it enough to really know. We haven’t systematically characterized these risk factors for chronic posttraumatic headache very well, [so] it’s useful to have this information,” said Andrew Charles, MD, professor neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program, who was not involved in the study. However, Dr. Charles emphasized the need to confirm the results prospectively.
Defining risk factors
The analysis found that a history of migraines, medication overuse, psychological disorders, and new posttraumatic headache–associated comorbidities were all associated with a greater risk for persistent posttraumatic headache. None of those came as a surprise, “but we live in a world where medicine is practiced based on evidence, and providers want to see data to support that. I think that this will help with resource allocation. It’s important to address [a patient’s] overuse of medications, or if they’re having psychological symptoms,” said Dr. Chan.
A total of 150 patients in the analysis had acute posttraumatic headache (mean duration, 0.7 months) while 150 had persistent posttraumatic headache (mean duration, 24 months; P < .00001). Clinical factors associated with risk of persistent headache included a history migraine (relative risk, 2.4; P < .0001), a previous head injury (odds ratio, 5.8; P < .0001), medication overuse (RR, 2.6; P < .0001), preexisting psychological history (OR, 5; P < .0001), and new posttraumatic headache–associated comorbidities, such as vertigo or posttraumatic stress disorder (RR, 9.8; P < .0001).
Identifying patient subgroups
The researchers also identified four subcategories of patients with persistent posttraumatic headache, each with differing risk factors and clinical characteristics. It’s too soon to use these identifiers to make clinical recommendations, but Dr. Chan hopes that further study of these groups will be informative. “It might point us toward (the idea) that each patient population is actually different, even within the chronic persistent posttraumatic headache population, we can’t group them all under the same umbrella term. If we can tease out that a patient has truly had a head injury, but no history of migraine, no overuse of medication, no psychological history, and no other associated symptoms, this would be a very interesting population to study because they would help us understand the pathophysiology [of persistent posttraumatic headache].”
Although the study was conducted by defining persistent posttraumatic headache as lasting at least 3 months, Dr. Chan took issue with that commonly held definition. That choice is arbitrary, with no pathophysiological basis or data to support it, and is based more on clinical trials testing preventative treatments. But when it is used in clinical practice, it can muddy communication with patients. “When this timeline is told to a patient, and when it’s not achieved, they might become disappointed. We should not put too much emphasis on time. Everybody is different,” he said.
The study did not receive any funding. Dr. Chan had no relevant financial disclosures. Dr. Charles consults for consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
It also revealed a surprisingly high frequency of misdiagnosis. The original sample included 500 patients drawn from the Stanford Research Repository Cohort Discovery Tool, but a review found 200 records that were misdiagnosed and had to be excluded.
“It’s very easy to label someone who suffered a head injury and say this is the reason why they have this (headache),” said lead author Tommy Chan, MBBS, a headache fellow in the department of neurology at Stanford (Calif.) University, in an interview. Such patients are often seen by ED or primary care physicians who do not have a lot of experience with posttraumatic headache, and that can lead to negative consequences if a low-pressure headache is mistaken as stemming from a skull fracture. “It’s a very different treatment plan for one versus the other,” said Dr. Chan in an interview.
He noted that it can help to take a patient history that includes the preaccident headache frequency and determine if there was a change in frequency post injury.
Dr. Chan presented the results at the virtual annual meeting of the American Headache Society.
“The results are what one might expect, although we haven’t studied it enough to really know. We haven’t systematically characterized these risk factors for chronic posttraumatic headache very well, [so] it’s useful to have this information,” said Andrew Charles, MD, professor neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program, who was not involved in the study. However, Dr. Charles emphasized the need to confirm the results prospectively.
Defining risk factors
The analysis found that a history of migraines, medication overuse, psychological disorders, and new posttraumatic headache–associated comorbidities were all associated with a greater risk for persistent posttraumatic headache. None of those came as a surprise, “but we live in a world where medicine is practiced based on evidence, and providers want to see data to support that. I think that this will help with resource allocation. It’s important to address [a patient’s] overuse of medications, or if they’re having psychological symptoms,” said Dr. Chan.
A total of 150 patients in the analysis had acute posttraumatic headache (mean duration, 0.7 months) while 150 had persistent posttraumatic headache (mean duration, 24 months; P < .00001). Clinical factors associated with risk of persistent headache included a history migraine (relative risk, 2.4; P < .0001), a previous head injury (odds ratio, 5.8; P < .0001), medication overuse (RR, 2.6; P < .0001), preexisting psychological history (OR, 5; P < .0001), and new posttraumatic headache–associated comorbidities, such as vertigo or posttraumatic stress disorder (RR, 9.8; P < .0001).
Identifying patient subgroups
The researchers also identified four subcategories of patients with persistent posttraumatic headache, each with differing risk factors and clinical characteristics. It’s too soon to use these identifiers to make clinical recommendations, but Dr. Chan hopes that further study of these groups will be informative. “It might point us toward (the idea) that each patient population is actually different, even within the chronic persistent posttraumatic headache population, we can’t group them all under the same umbrella term. If we can tease out that a patient has truly had a head injury, but no history of migraine, no overuse of medication, no psychological history, and no other associated symptoms, this would be a very interesting population to study because they would help us understand the pathophysiology [of persistent posttraumatic headache].”
Although the study was conducted by defining persistent posttraumatic headache as lasting at least 3 months, Dr. Chan took issue with that commonly held definition. That choice is arbitrary, with no pathophysiological basis or data to support it, and is based more on clinical trials testing preventative treatments. But when it is used in clinical practice, it can muddy communication with patients. “When this timeline is told to a patient, and when it’s not achieved, they might become disappointed. We should not put too much emphasis on time. Everybody is different,” he said.
The study did not receive any funding. Dr. Chan had no relevant financial disclosures. Dr. Charles consults for consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
It also revealed a surprisingly high frequency of misdiagnosis. The original sample included 500 patients drawn from the Stanford Research Repository Cohort Discovery Tool, but a review found 200 records that were misdiagnosed and had to be excluded.
“It’s very easy to label someone who suffered a head injury and say this is the reason why they have this (headache),” said lead author Tommy Chan, MBBS, a headache fellow in the department of neurology at Stanford (Calif.) University, in an interview. Such patients are often seen by ED or primary care physicians who do not have a lot of experience with posttraumatic headache, and that can lead to negative consequences if a low-pressure headache is mistaken as stemming from a skull fracture. “It’s a very different treatment plan for one versus the other,” said Dr. Chan in an interview.
He noted that it can help to take a patient history that includes the preaccident headache frequency and determine if there was a change in frequency post injury.
Dr. Chan presented the results at the virtual annual meeting of the American Headache Society.
“The results are what one might expect, although we haven’t studied it enough to really know. We haven’t systematically characterized these risk factors for chronic posttraumatic headache very well, [so] it’s useful to have this information,” said Andrew Charles, MD, professor neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program, who was not involved in the study. However, Dr. Charles emphasized the need to confirm the results prospectively.
Defining risk factors
The analysis found that a history of migraines, medication overuse, psychological disorders, and new posttraumatic headache–associated comorbidities were all associated with a greater risk for persistent posttraumatic headache. None of those came as a surprise, “but we live in a world where medicine is practiced based on evidence, and providers want to see data to support that. I think that this will help with resource allocation. It’s important to address [a patient’s] overuse of medications, or if they’re having psychological symptoms,” said Dr. Chan.
A total of 150 patients in the analysis had acute posttraumatic headache (mean duration, 0.7 months) while 150 had persistent posttraumatic headache (mean duration, 24 months; P < .00001). Clinical factors associated with risk of persistent headache included a history migraine (relative risk, 2.4; P < .0001), a previous head injury (odds ratio, 5.8; P < .0001), medication overuse (RR, 2.6; P < .0001), preexisting psychological history (OR, 5; P < .0001), and new posttraumatic headache–associated comorbidities, such as vertigo or posttraumatic stress disorder (RR, 9.8; P < .0001).
Identifying patient subgroups
The researchers also identified four subcategories of patients with persistent posttraumatic headache, each with differing risk factors and clinical characteristics. It’s too soon to use these identifiers to make clinical recommendations, but Dr. Chan hopes that further study of these groups will be informative. “It might point us toward (the idea) that each patient population is actually different, even within the chronic persistent posttraumatic headache population, we can’t group them all under the same umbrella term. If we can tease out that a patient has truly had a head injury, but no history of migraine, no overuse of medication, no psychological history, and no other associated symptoms, this would be a very interesting population to study because they would help us understand the pathophysiology [of persistent posttraumatic headache].”
Although the study was conducted by defining persistent posttraumatic headache as lasting at least 3 months, Dr. Chan took issue with that commonly held definition. That choice is arbitrary, with no pathophysiological basis or data to support it, and is based more on clinical trials testing preventative treatments. But when it is used in clinical practice, it can muddy communication with patients. “When this timeline is told to a patient, and when it’s not achieved, they might become disappointed. We should not put too much emphasis on time. Everybody is different,” he said.
The study did not receive any funding. Dr. Chan had no relevant financial disclosures. Dr. Charles consults for consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
FROM AHS 2020