User login
Dabigatran raises major bleeding risk
Dabigatran significantly raises the risk of major bleeding and gastrointestinal bleeding across all subgroups of patients with atrial fibrillation, and particularly in African Americans and patients with chronic kidney disease, according to a report published online Nov. 3 in JAMA Internal Medicine.
Physicians should only prescribe dabigatran with caution, and should fully explain to patients who do take the drug how to identify abnormal bleeding so that it can be detected and controlled as early as possible, said Inmaculada Hernandez, Pharm.D., of the department of health policy and management, University of Pittsburgh, and her associates.
The FDA approved dabigatran in 2010 via an accelerated pathway after only 6 months of review, based largely on findings from a single clinical study, the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial, which did not adjust for patient characteristics (N. Engl. J. Med. 2009;361:1139-51). That study reported lower bleeding risks with dabigatran than with warfarin. Several months later, the agency’s Adverse Event Reporting System received “a large number” of reports of severe bleeding associated with dabigatran; and the relative bleeding risk associated with the two drugs is still unclear.
Dr. Hernandez and her colleagues examined the issue using data from a nationally representative random sample of 9,404 Medicare beneficiaries newly diagnosed as having nonvalvular atrial fibrillation during a 1-year period and treated in real-world practice. A total of 1,302 patients were given dabigatran and 8,102 were given warfarin to prevent stroke and systemic embolism. They were followed for a median of about 200 days, until discontinuing or switching their anticoagulant, dying, or reaching the study’s cutoff date. Nine categories of bleeding were assessed, and the data were adjusted to account for numerous demographic and clinical characteristics known to affect bleeding risk.
Compared with warfarin, dabigatran was associated with a significantly higher risk of major bleeding (9.0% vs 5.9%), with a hazard ratio of 1.58. Dabigatran also was associated with a significantly higher risk of GI bleeding (HR, 1.85), hematuria (HR, 1.41), vaginal bleeding (HR, 2.27), hemarthrosis (HR, 2.78), and hemoptysis (HR, 1.49). In contrast, dabigatran was associated with a slightly lower (0.6%) rate of intracranial bleeding, and also with lower rates of epistaxis and nonspecified bleeding, the investigators reported (JAMA Intern. Med. 2014 Nov. 3 [doi: 10.1001/jamainternmed.2014.5398]).
These differences were consistent across numerous subgroups of patients assessed, and were especially strong among African Americans and patients with chronic kidney disease.
This study was supported by the Commonwealth Foundation and the U.S. Agency for Healthcare Research and Quality. Dr. Hernandez and her associates reported having no financial conflicts of interest.
The bleeding risk for dabigatran appears to be higher than that for warfarin and significantly greater than it initially seemed at the time of FDA approval.
Hernandez et al. noted that the study on which the FDA based its approval failed to adjust for important differences in patient characteristics, which likely biased the results. They remind us that postmarketing data are crucial for us to advise our patients accurately.
Dr. Rita F. Redberg is the editor of JAMA Internal Medicine and director of women’s cardiovascular services at the Philip R. Lee Institute for Health Policy Studies at the University of California, San Francisco, Medical Center. She reported no financial conflicts of interest. Dr. Redberg made these remarks in an Editor’s Note accompanying Dr. Hernandez’s report (JAMA Intern. Med. 2014 Nov. 3).
The bleeding risk for dabigatran appears to be higher than that for warfarin and significantly greater than it initially seemed at the time of FDA approval.
Hernandez et al. noted that the study on which the FDA based its approval failed to adjust for important differences in patient characteristics, which likely biased the results. They remind us that postmarketing data are crucial for us to advise our patients accurately.
Dr. Rita F. Redberg is the editor of JAMA Internal Medicine and director of women’s cardiovascular services at the Philip R. Lee Institute for Health Policy Studies at the University of California, San Francisco, Medical Center. She reported no financial conflicts of interest. Dr. Redberg made these remarks in an Editor’s Note accompanying Dr. Hernandez’s report (JAMA Intern. Med. 2014 Nov. 3).
The bleeding risk for dabigatran appears to be higher than that for warfarin and significantly greater than it initially seemed at the time of FDA approval.
Hernandez et al. noted that the study on which the FDA based its approval failed to adjust for important differences in patient characteristics, which likely biased the results. They remind us that postmarketing data are crucial for us to advise our patients accurately.
Dr. Rita F. Redberg is the editor of JAMA Internal Medicine and director of women’s cardiovascular services at the Philip R. Lee Institute for Health Policy Studies at the University of California, San Francisco, Medical Center. She reported no financial conflicts of interest. Dr. Redberg made these remarks in an Editor’s Note accompanying Dr. Hernandez’s report (JAMA Intern. Med. 2014 Nov. 3).
Dabigatran significantly raises the risk of major bleeding and gastrointestinal bleeding across all subgroups of patients with atrial fibrillation, and particularly in African Americans and patients with chronic kidney disease, according to a report published online Nov. 3 in JAMA Internal Medicine.
Physicians should only prescribe dabigatran with caution, and should fully explain to patients who do take the drug how to identify abnormal bleeding so that it can be detected and controlled as early as possible, said Inmaculada Hernandez, Pharm.D., of the department of health policy and management, University of Pittsburgh, and her associates.
The FDA approved dabigatran in 2010 via an accelerated pathway after only 6 months of review, based largely on findings from a single clinical study, the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial, which did not adjust for patient characteristics (N. Engl. J. Med. 2009;361:1139-51). That study reported lower bleeding risks with dabigatran than with warfarin. Several months later, the agency’s Adverse Event Reporting System received “a large number” of reports of severe bleeding associated with dabigatran; and the relative bleeding risk associated with the two drugs is still unclear.
Dr. Hernandez and her colleagues examined the issue using data from a nationally representative random sample of 9,404 Medicare beneficiaries newly diagnosed as having nonvalvular atrial fibrillation during a 1-year period and treated in real-world practice. A total of 1,302 patients were given dabigatran and 8,102 were given warfarin to prevent stroke and systemic embolism. They were followed for a median of about 200 days, until discontinuing or switching their anticoagulant, dying, or reaching the study’s cutoff date. Nine categories of bleeding were assessed, and the data were adjusted to account for numerous demographic and clinical characteristics known to affect bleeding risk.
Compared with warfarin, dabigatran was associated with a significantly higher risk of major bleeding (9.0% vs 5.9%), with a hazard ratio of 1.58. Dabigatran also was associated with a significantly higher risk of GI bleeding (HR, 1.85), hematuria (HR, 1.41), vaginal bleeding (HR, 2.27), hemarthrosis (HR, 2.78), and hemoptysis (HR, 1.49). In contrast, dabigatran was associated with a slightly lower (0.6%) rate of intracranial bleeding, and also with lower rates of epistaxis and nonspecified bleeding, the investigators reported (JAMA Intern. Med. 2014 Nov. 3 [doi: 10.1001/jamainternmed.2014.5398]).
These differences were consistent across numerous subgroups of patients assessed, and were especially strong among African Americans and patients with chronic kidney disease.
This study was supported by the Commonwealth Foundation and the U.S. Agency for Healthcare Research and Quality. Dr. Hernandez and her associates reported having no financial conflicts of interest.
Dabigatran significantly raises the risk of major bleeding and gastrointestinal bleeding across all subgroups of patients with atrial fibrillation, and particularly in African Americans and patients with chronic kidney disease, according to a report published online Nov. 3 in JAMA Internal Medicine.
Physicians should only prescribe dabigatran with caution, and should fully explain to patients who do take the drug how to identify abnormal bleeding so that it can be detected and controlled as early as possible, said Inmaculada Hernandez, Pharm.D., of the department of health policy and management, University of Pittsburgh, and her associates.
The FDA approved dabigatran in 2010 via an accelerated pathway after only 6 months of review, based largely on findings from a single clinical study, the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial, which did not adjust for patient characteristics (N. Engl. J. Med. 2009;361:1139-51). That study reported lower bleeding risks with dabigatran than with warfarin. Several months later, the agency’s Adverse Event Reporting System received “a large number” of reports of severe bleeding associated with dabigatran; and the relative bleeding risk associated with the two drugs is still unclear.
Dr. Hernandez and her colleagues examined the issue using data from a nationally representative random sample of 9,404 Medicare beneficiaries newly diagnosed as having nonvalvular atrial fibrillation during a 1-year period and treated in real-world practice. A total of 1,302 patients were given dabigatran and 8,102 were given warfarin to prevent stroke and systemic embolism. They were followed for a median of about 200 days, until discontinuing or switching their anticoagulant, dying, or reaching the study’s cutoff date. Nine categories of bleeding were assessed, and the data were adjusted to account for numerous demographic and clinical characteristics known to affect bleeding risk.
Compared with warfarin, dabigatran was associated with a significantly higher risk of major bleeding (9.0% vs 5.9%), with a hazard ratio of 1.58. Dabigatran also was associated with a significantly higher risk of GI bleeding (HR, 1.85), hematuria (HR, 1.41), vaginal bleeding (HR, 2.27), hemarthrosis (HR, 2.78), and hemoptysis (HR, 1.49). In contrast, dabigatran was associated with a slightly lower (0.6%) rate of intracranial bleeding, and also with lower rates of epistaxis and nonspecified bleeding, the investigators reported (JAMA Intern. Med. 2014 Nov. 3 [doi: 10.1001/jamainternmed.2014.5398]).
These differences were consistent across numerous subgroups of patients assessed, and were especially strong among African Americans and patients with chronic kidney disease.
This study was supported by the Commonwealth Foundation and the U.S. Agency for Healthcare Research and Quality. Dr. Hernandez and her associates reported having no financial conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Dabigatran raises the risk of major bleeding, contrary to initial reports that fast-tracked FDA approval.
Major finding: Compared with warfarin, dabigatran was associated with a significantly higher risk of major bleeding (9.0% vs. 5.9%), with a hazard ratio of 1.58.
Data source: A retrospective cohort study of bleeding risks in 1,302 dabigatran users and 8,102 warfarin users who had newly diagnosed nonvalvular atrial fibrillation.
Disclosures: This study was supported by the Commonwealth Foundation and the U.S. Agency for Healthcare Research and Quality. Dr. Hernandez and her associates reported having no financial conflicts of interest.
ROR score aids prognosis after 5 years on tamoxifen
The risk of recurrence (ROR) score provides clinically meaningful prognostic information after postmenopausal women with early-stage breast cancer complete 5 years of tamoxifen therapy, according to a report published online Oct. 27 in Journal of Clinical Oncology.
The ROR score is derived from a quantitative assessment of a tumor’s expression of 46 genes related to breast cancer plus a measure of the tumor’s size. In this study, it was added to the Clinical Treatment Score, a standard prognostic tool that takes into account nodal status, tumor size and grade, patient age, and treatment modalities, said Ivana Sestak, Ph.D., of the Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Queen Mary University, London, and her associates.
To examine the usefulness of the ROR score in assessing risk for late recurrence of breast cancer, researchers analyzed combined data from two large clinical trials involving 2,137 postmenopausal women with early-stage hormone receptor–positive breast cancer who received 5 years of endocrine therapy but no chemotherapy and were followed for a further 5 years. There were 148 distant recurrences during that time.
Women classified by ROR score as high risk were 6.9 times more likely to develop a late recurrence than were those classified as low risk, and women classified by ROR score as intermediate risk were 3.3 times more likely to develop a late recurrence. The addition of ROR score to the Clinical Treatment Score reclassified 32 women as high risk, and those women did go on to develop a late recurrence. Similarly, adding to ROR score to the Clinical Treatment Score reclassified three women as low risk, and those women did not go on to develop a late recurrence, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.55.6894]).
In particular, the ROR score was helpful in assigning a “low-risk” designation to women who had node-negative and node-negative/HER2-negative disease, even though some of them had large tumors. These women could safely be spared from further endocrine therapy. Likewise, the ROR score was helpful in identifying women at high risk of recurrence who may wish to extend endocrine therapy beyond 5 years, Dr. Sestak and her associates said.
The risk of recurrence (ROR) score provides clinically meaningful prognostic information after postmenopausal women with early-stage breast cancer complete 5 years of tamoxifen therapy, according to a report published online Oct. 27 in Journal of Clinical Oncology.
The ROR score is derived from a quantitative assessment of a tumor’s expression of 46 genes related to breast cancer plus a measure of the tumor’s size. In this study, it was added to the Clinical Treatment Score, a standard prognostic tool that takes into account nodal status, tumor size and grade, patient age, and treatment modalities, said Ivana Sestak, Ph.D., of the Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Queen Mary University, London, and her associates.
To examine the usefulness of the ROR score in assessing risk for late recurrence of breast cancer, researchers analyzed combined data from two large clinical trials involving 2,137 postmenopausal women with early-stage hormone receptor–positive breast cancer who received 5 years of endocrine therapy but no chemotherapy and were followed for a further 5 years. There were 148 distant recurrences during that time.
Women classified by ROR score as high risk were 6.9 times more likely to develop a late recurrence than were those classified as low risk, and women classified by ROR score as intermediate risk were 3.3 times more likely to develop a late recurrence. The addition of ROR score to the Clinical Treatment Score reclassified 32 women as high risk, and those women did go on to develop a late recurrence. Similarly, adding to ROR score to the Clinical Treatment Score reclassified three women as low risk, and those women did not go on to develop a late recurrence, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.55.6894]).
In particular, the ROR score was helpful in assigning a “low-risk” designation to women who had node-negative and node-negative/HER2-negative disease, even though some of them had large tumors. These women could safely be spared from further endocrine therapy. Likewise, the ROR score was helpful in identifying women at high risk of recurrence who may wish to extend endocrine therapy beyond 5 years, Dr. Sestak and her associates said.
The risk of recurrence (ROR) score provides clinically meaningful prognostic information after postmenopausal women with early-stage breast cancer complete 5 years of tamoxifen therapy, according to a report published online Oct. 27 in Journal of Clinical Oncology.
The ROR score is derived from a quantitative assessment of a tumor’s expression of 46 genes related to breast cancer plus a measure of the tumor’s size. In this study, it was added to the Clinical Treatment Score, a standard prognostic tool that takes into account nodal status, tumor size and grade, patient age, and treatment modalities, said Ivana Sestak, Ph.D., of the Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Queen Mary University, London, and her associates.
To examine the usefulness of the ROR score in assessing risk for late recurrence of breast cancer, researchers analyzed combined data from two large clinical trials involving 2,137 postmenopausal women with early-stage hormone receptor–positive breast cancer who received 5 years of endocrine therapy but no chemotherapy and were followed for a further 5 years. There were 148 distant recurrences during that time.
Women classified by ROR score as high risk were 6.9 times more likely to develop a late recurrence than were those classified as low risk, and women classified by ROR score as intermediate risk were 3.3 times more likely to develop a late recurrence. The addition of ROR score to the Clinical Treatment Score reclassified 32 women as high risk, and those women did go on to develop a late recurrence. Similarly, adding to ROR score to the Clinical Treatment Score reclassified three women as low risk, and those women did not go on to develop a late recurrence, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.55.6894]).
In particular, the ROR score was helpful in assigning a “low-risk” designation to women who had node-negative and node-negative/HER2-negative disease, even though some of them had large tumors. These women could safely be spared from further endocrine therapy. Likewise, the ROR score was helpful in identifying women at high risk of recurrence who may wish to extend endocrine therapy beyond 5 years, Dr. Sestak and her associates said.
Key clinical point: The ROR score adds meaningful prognostic information 5-10 years after patients complete a 5-year course of tamoxifen for breast cancer.
Major finding: Women classified by ROR score as high risk were 6.9 times more likely to develop a late recurrence, and women classified by ROR score as intermediate risk were 3.3 times more likely to develop a late recurrence, compared with those classified as low risk.
Data source: A secondary, combined analysis of data from two large breast cancer trials involving 2,137 women followed for an additional 5 years after completing a 5-year course of tamoxifen.
Disclosures: This study was supported by Breakthrough Breast Cancer, the National Institute for Health Research Biomedical Research Centre at Royal Marsden Hospital, and Cancer Research U.K. Dr. Sestak reported having no financial conflicts of interest; her associates reported ties to numerous industry sources.
None of 19 potential biomarkers predicted pertuzumab response
None of the 19 potential biomarkers assessed in an exploratory study were found to help select which patients with HER2-positive metastatic breast cancer would benefit most from treatment with the monoclonal anti-HER2 antibody pertuzumab – other than the HER2 biomarker already in use for that purpose, according to a report published online Oct. 27 in Journal of Clinical Oncology.
Even though anti-HER2 therapies for metastatic breast cancer have been in use for more than 15 years, HER2 status itself remains the only marker of sensitivity to those agents, and it is an imperfect one. “We therefore aimed to explore within the HER2-positive patient population whether subgroups that derive differential progression-free survival or overall survival benefit from HER2-targeted treatment can be identified based on biomarker profiles,” said Dr. José Baselga, physician in chief and chief medical officer at Memorial Sloan Kettering Cancer Center, New York, and his associates.
They analyzed tumor, serum, and whole blood samples from 808 participants in a phase III randomized clinical trial assessing response to pertuzumab and trastuzumab therapy. These patients had locally recurrent nonresectable or metastatic breast cancer and had not received any chemotherapy. Each tissue sample was tested using a panel of 19 potentially useful biomarkers thought to be involved in either resistance to HER2-targeted agents or regulation of HER2-signaling pathways, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2013.54.5384]).
None of the biomarkers were found to correlate with treatment response, as measured by the patients’ progression-free and overall survival. One biomarker, a mutation in the PIK3CA gene, appeared to identify tumors that would not respond well to pertuzumab even though they expressed HER2, but further study of this possible association is needed, Dr. Baselga and his associates said.
None of the 19 potential biomarkers assessed in an exploratory study were found to help select which patients with HER2-positive metastatic breast cancer would benefit most from treatment with the monoclonal anti-HER2 antibody pertuzumab – other than the HER2 biomarker already in use for that purpose, according to a report published online Oct. 27 in Journal of Clinical Oncology.
Even though anti-HER2 therapies for metastatic breast cancer have been in use for more than 15 years, HER2 status itself remains the only marker of sensitivity to those agents, and it is an imperfect one. “We therefore aimed to explore within the HER2-positive patient population whether subgroups that derive differential progression-free survival or overall survival benefit from HER2-targeted treatment can be identified based on biomarker profiles,” said Dr. José Baselga, physician in chief and chief medical officer at Memorial Sloan Kettering Cancer Center, New York, and his associates.
They analyzed tumor, serum, and whole blood samples from 808 participants in a phase III randomized clinical trial assessing response to pertuzumab and trastuzumab therapy. These patients had locally recurrent nonresectable or metastatic breast cancer and had not received any chemotherapy. Each tissue sample was tested using a panel of 19 potentially useful biomarkers thought to be involved in either resistance to HER2-targeted agents or regulation of HER2-signaling pathways, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2013.54.5384]).
None of the biomarkers were found to correlate with treatment response, as measured by the patients’ progression-free and overall survival. One biomarker, a mutation in the PIK3CA gene, appeared to identify tumors that would not respond well to pertuzumab even though they expressed HER2, but further study of this possible association is needed, Dr. Baselga and his associates said.
None of the 19 potential biomarkers assessed in an exploratory study were found to help select which patients with HER2-positive metastatic breast cancer would benefit most from treatment with the monoclonal anti-HER2 antibody pertuzumab – other than the HER2 biomarker already in use for that purpose, according to a report published online Oct. 27 in Journal of Clinical Oncology.
Even though anti-HER2 therapies for metastatic breast cancer have been in use for more than 15 years, HER2 status itself remains the only marker of sensitivity to those agents, and it is an imperfect one. “We therefore aimed to explore within the HER2-positive patient population whether subgroups that derive differential progression-free survival or overall survival benefit from HER2-targeted treatment can be identified based on biomarker profiles,” said Dr. José Baselga, physician in chief and chief medical officer at Memorial Sloan Kettering Cancer Center, New York, and his associates.
They analyzed tumor, serum, and whole blood samples from 808 participants in a phase III randomized clinical trial assessing response to pertuzumab and trastuzumab therapy. These patients had locally recurrent nonresectable or metastatic breast cancer and had not received any chemotherapy. Each tissue sample was tested using a panel of 19 potentially useful biomarkers thought to be involved in either resistance to HER2-targeted agents or regulation of HER2-signaling pathways, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2013.54.5384]).
None of the biomarkers were found to correlate with treatment response, as measured by the patients’ progression-free and overall survival. One biomarker, a mutation in the PIK3CA gene, appeared to identify tumors that would not respond well to pertuzumab even though they expressed HER2, but further study of this possible association is needed, Dr. Baselga and his associates said.
Key clinical point: None of the 19 biomarkers assessed in an exploratory genetic study were found to aid in patient selection for pertuzumab-based treatment of metastatic breast cancer,
Major finding: None of the biomarkers were found to correlate with treatment response, as measured by the patients’ progression-free and overall survival.
Data source: A prospective laboratory assessment of 19 potential biomarkers from 808 tumor and serum samples taken from participants in a clinical trial assessing treatment response to pertuzumab.
Disclosures: The clinical trial on which this study was based was sponsored by Hoffmann-LaRoche, which also participated in the study design, data interpretation, and the writing and publication of the report. Dr. Baselga reported serving as a consultant for Genentech and Novartis; his associates reported ties to numerous industry sources.
Nine weeks of biochemotherapy effective for high-risk melanoma
A 9-week course of multiagent biochemotherapy markedly improved relapse-free survival in patients with high-risk melanoma, compared with the 1-year course of high-dose interferon that has been the unchallenged standard of care for this disease for decades, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
“This [phase III] randomized trial is the first to compare a multiagent regimen against high-dose interferon … and the first to demonstrate a statistically significant relapse-free survival benefit for any treatment regimen over high-dose interferon,” said Dr. Lawrence E. Flaherty of Wayne State University and the Karmanos Cancer Institute, Detroit, and his associates.
Moreover, the median relapse-free survival of 4 years achieved with biochemotherapy “represents a value not previously observed in any adjuvant therapy trial for patients with high-risk melanoma,” the investigators said. Unfortunately, this benefit did not translate into increased overall survival in this study, which was 56% at 5 years for both treatment groups.
The investigators enrolled patients aged 10-74 years (median age, 47 years) who had undergone wide excision of a cutaneous primary melanoma (stage IIIA-N2a and above) with pathologically negative margins and a complete regional lymphadenectomy. These study participants had no clinical, radiologic, or pathologic evidence of residual or metastatic melanoma, and had never undergone radiotherapy, chemotherapy, or immunotherapy for any type of cancer. They were randomly assigned to receive either high-dose intravenous interferon for 52 weeks (203 patients), or cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon every 21 days for a total of three cycles (199 patients).
After a median of 7.2 years of follow-up, the median relapse-free survival was 4.0 years for biochemotherapy, compared with only 1.9 years for high-dose interferon alone. The 5-year relapse-free survival rate was 48% for biochemotherapy, compared with only 39% for high-dose interferon alone. However, there was no corresponding improvement in overall survival: The median overall survival was 9.9 years for biochemotherapy, compared with 6.7 years for high-dose interferon alone, and 5-year overall survival was 56% for both study groups, Dr. Flaherty and his associates reported (J. Clin. Oncol. October 2014 [doi:10.1200/JCO.2013.53.1590]).
“Toxicities for biochemotherapy and high-dose interferon are different but comparable in magnitude, particularly when discontinuation for toxicity is considered,” they wrote. Biochemotherapy was associated with a higher rate of grade 4 toxicity (40% vs 7%), but rates of grade 3 and 4 toxicity were similar (76% vs 64%), and most toxicity related to biochemotherapy was hematologic and of short duration. The rate of discontinuation of treatment was 15% for biochemotherapy and 19% for high-dose interferon alone, a nonsignificant difference.
These findings indicate that biochemotherapy can be considered an alternative adjuvant treatment for high-risk melanoma “in appropriately selected patients by physicians at centers experienced in the use of this regimen,” Dr. Flaherty and his associates said.
A 9-week course of multiagent biochemotherapy markedly improved relapse-free survival in patients with high-risk melanoma, compared with the 1-year course of high-dose interferon that has been the unchallenged standard of care for this disease for decades, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
“This [phase III] randomized trial is the first to compare a multiagent regimen against high-dose interferon … and the first to demonstrate a statistically significant relapse-free survival benefit for any treatment regimen over high-dose interferon,” said Dr. Lawrence E. Flaherty of Wayne State University and the Karmanos Cancer Institute, Detroit, and his associates.
Moreover, the median relapse-free survival of 4 years achieved with biochemotherapy “represents a value not previously observed in any adjuvant therapy trial for patients with high-risk melanoma,” the investigators said. Unfortunately, this benefit did not translate into increased overall survival in this study, which was 56% at 5 years for both treatment groups.
The investigators enrolled patients aged 10-74 years (median age, 47 years) who had undergone wide excision of a cutaneous primary melanoma (stage IIIA-N2a and above) with pathologically negative margins and a complete regional lymphadenectomy. These study participants had no clinical, radiologic, or pathologic evidence of residual or metastatic melanoma, and had never undergone radiotherapy, chemotherapy, or immunotherapy for any type of cancer. They were randomly assigned to receive either high-dose intravenous interferon for 52 weeks (203 patients), or cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon every 21 days for a total of three cycles (199 patients).
After a median of 7.2 years of follow-up, the median relapse-free survival was 4.0 years for biochemotherapy, compared with only 1.9 years for high-dose interferon alone. The 5-year relapse-free survival rate was 48% for biochemotherapy, compared with only 39% for high-dose interferon alone. However, there was no corresponding improvement in overall survival: The median overall survival was 9.9 years for biochemotherapy, compared with 6.7 years for high-dose interferon alone, and 5-year overall survival was 56% for both study groups, Dr. Flaherty and his associates reported (J. Clin. Oncol. October 2014 [doi:10.1200/JCO.2013.53.1590]).
“Toxicities for biochemotherapy and high-dose interferon are different but comparable in magnitude, particularly when discontinuation for toxicity is considered,” they wrote. Biochemotherapy was associated with a higher rate of grade 4 toxicity (40% vs 7%), but rates of grade 3 and 4 toxicity were similar (76% vs 64%), and most toxicity related to biochemotherapy was hematologic and of short duration. The rate of discontinuation of treatment was 15% for biochemotherapy and 19% for high-dose interferon alone, a nonsignificant difference.
These findings indicate that biochemotherapy can be considered an alternative adjuvant treatment for high-risk melanoma “in appropriately selected patients by physicians at centers experienced in the use of this regimen,” Dr. Flaherty and his associates said.
A 9-week course of multiagent biochemotherapy markedly improved relapse-free survival in patients with high-risk melanoma, compared with the 1-year course of high-dose interferon that has been the unchallenged standard of care for this disease for decades, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
“This [phase III] randomized trial is the first to compare a multiagent regimen against high-dose interferon … and the first to demonstrate a statistically significant relapse-free survival benefit for any treatment regimen over high-dose interferon,” said Dr. Lawrence E. Flaherty of Wayne State University and the Karmanos Cancer Institute, Detroit, and his associates.
Moreover, the median relapse-free survival of 4 years achieved with biochemotherapy “represents a value not previously observed in any adjuvant therapy trial for patients with high-risk melanoma,” the investigators said. Unfortunately, this benefit did not translate into increased overall survival in this study, which was 56% at 5 years for both treatment groups.
The investigators enrolled patients aged 10-74 years (median age, 47 years) who had undergone wide excision of a cutaneous primary melanoma (stage IIIA-N2a and above) with pathologically negative margins and a complete regional lymphadenectomy. These study participants had no clinical, radiologic, or pathologic evidence of residual or metastatic melanoma, and had never undergone radiotherapy, chemotherapy, or immunotherapy for any type of cancer. They were randomly assigned to receive either high-dose intravenous interferon for 52 weeks (203 patients), or cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon every 21 days for a total of three cycles (199 patients).
After a median of 7.2 years of follow-up, the median relapse-free survival was 4.0 years for biochemotherapy, compared with only 1.9 years for high-dose interferon alone. The 5-year relapse-free survival rate was 48% for biochemotherapy, compared with only 39% for high-dose interferon alone. However, there was no corresponding improvement in overall survival: The median overall survival was 9.9 years for biochemotherapy, compared with 6.7 years for high-dose interferon alone, and 5-year overall survival was 56% for both study groups, Dr. Flaherty and his associates reported (J. Clin. Oncol. October 2014 [doi:10.1200/JCO.2013.53.1590]).
“Toxicities for biochemotherapy and high-dose interferon are different but comparable in magnitude, particularly when discontinuation for toxicity is considered,” they wrote. Biochemotherapy was associated with a higher rate of grade 4 toxicity (40% vs 7%), but rates of grade 3 and 4 toxicity were similar (76% vs 64%), and most toxicity related to biochemotherapy was hematologic and of short duration. The rate of discontinuation of treatment was 15% for biochemotherapy and 19% for high-dose interferon alone, a nonsignificant difference.
These findings indicate that biochemotherapy can be considered an alternative adjuvant treatment for high-risk melanoma “in appropriately selected patients by physicians at centers experienced in the use of this regimen,” Dr. Flaherty and his associates said.
Key clinical point: A 9-week course of biochemotherapy markedly improved relapse-free survival in high-risk melanoma, compared with standard-of-care 1-year treatment.
Major finding: The median relapse-free survival was 4.0 years for multiagent biochemotherapy, compared with only 1.9 years for high-dose interferon alone.
Data source: A phase III randomized clinical trial involving 402 patients aged 10-74 years who had high-risk melanoma and were followed for a median of 7.2 years.
Disclosures: This study was supported in part by numerous Public Health Service grants from the National Cancer Institute and by Novartis. Dr. Flaherty reported ties to Novartis and Merck, and his associates reported ties to numerous industry sources.
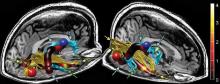
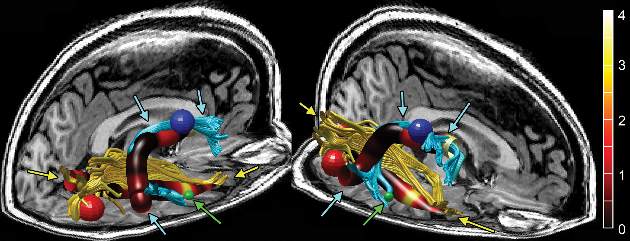
Brain changes identified in chronic fatigue syndrome
Imaging to assess brain microstructure identified increased fractional anisotropy in the anterior right arcuate fasciculus of patients with chronic fatigue syndrome, which was not present in age- and sex-matched control subjects.
The right-sided abnormality was particularly marked in right-handed chronic fatigue syndrome (CFS) patients, and the degree of increase correlated with the severity of their disorder. A finding of increased fractional anisotropy in this area of the brain may eventually serve as a biomarker for CFS, said Dr. Michael M. Zeineh of the department of radiology at Stanford (Calif.) University, and his associates (Radiology 2014 Oct. 29 [doi:10.1148/radiol.14141079]).
In what they described as the first study to examine brain microstructure in CFS, the investigators used volumetric MRI with diffusion-tensor imaging (DTI) to detect microstructural abnormalities in the patients – who had been evaluated at the university’s CFS Clinic during the preceding 5 years – and in healthy volunteers. The researchers also performed gray- and white-matter volumetric studies to identify differences between the two study groups in gross brain structure, as well as pseudocontinuous arterial spin labeling to assess global alterations in brain perfusion.
The mean age of the study participants was 46 years, and the mean duration of CFS symptoms was 12 years.
In the DTI analysis, the only significant difference between the two study groups was that the anterior right arcuate fasciculus – identified in 13 of 15 CFS patients and in all 14 control subjects in the study – showed significantly higher fractional anisotropy in CFS patients than in control subjects. This was confirmed in a second dataset from the same study subjects. The difference between CFS patients and control subjects was most pronounced in the subgroup of right-handed participants, “suggesting that hemispheric differences with handedness and language are an additional source of variance,” Dr. Zeineh and his associates said.
In addition, “two cortical regions connected via the arcuate fasciculus exhibited increased thickness: the right middle temporal and precentral gyri,” they noted.
The volumetric studies showed significantly lower total supratentorial white-matter volume in CFS, even after the data were adjusted to account for subject age, total intracranial volume, and handedness. However, total cortical gray-matter volume was equivalent between the two study groups, as were prefrontal cortex volume and global cortical thickness.
There were no differences between patients and controls in any perfusion measure, including perfusion to the cortex, supratentorial white matter, basal ganglia, thalami, or hippocampi. This finding is consistent with the results of a previous perfusion study involving monozygotic twins. Taken together, the findings suggest that brain perfusion is not affected in CFS.
Given the small number of subjects in this study, the findings must be verified in larger studies. It also would be valuable to assess possible changes in brain microstructure over time in a longitudinal study, and to determine whether treatment interventions exert any beneficial effects, the investigators added.
Imaging to assess brain microstructure identified increased fractional anisotropy in the anterior right arcuate fasciculus of patients with chronic fatigue syndrome, which was not present in age- and sex-matched control subjects.
The right-sided abnormality was particularly marked in right-handed chronic fatigue syndrome (CFS) patients, and the degree of increase correlated with the severity of their disorder. A finding of increased fractional anisotropy in this area of the brain may eventually serve as a biomarker for CFS, said Dr. Michael M. Zeineh of the department of radiology at Stanford (Calif.) University, and his associates (Radiology 2014 Oct. 29 [doi:10.1148/radiol.14141079]).
In what they described as the first study to examine brain microstructure in CFS, the investigators used volumetric MRI with diffusion-tensor imaging (DTI) to detect microstructural abnormalities in the patients – who had been evaluated at the university’s CFS Clinic during the preceding 5 years – and in healthy volunteers. The researchers also performed gray- and white-matter volumetric studies to identify differences between the two study groups in gross brain structure, as well as pseudocontinuous arterial spin labeling to assess global alterations in brain perfusion.
The mean age of the study participants was 46 years, and the mean duration of CFS symptoms was 12 years.
In the DTI analysis, the only significant difference between the two study groups was that the anterior right arcuate fasciculus – identified in 13 of 15 CFS patients and in all 14 control subjects in the study – showed significantly higher fractional anisotropy in CFS patients than in control subjects. This was confirmed in a second dataset from the same study subjects. The difference between CFS patients and control subjects was most pronounced in the subgroup of right-handed participants, “suggesting that hemispheric differences with handedness and language are an additional source of variance,” Dr. Zeineh and his associates said.
In addition, “two cortical regions connected via the arcuate fasciculus exhibited increased thickness: the right middle temporal and precentral gyri,” they noted.
The volumetric studies showed significantly lower total supratentorial white-matter volume in CFS, even after the data were adjusted to account for subject age, total intracranial volume, and handedness. However, total cortical gray-matter volume was equivalent between the two study groups, as were prefrontal cortex volume and global cortical thickness.
There were no differences between patients and controls in any perfusion measure, including perfusion to the cortex, supratentorial white matter, basal ganglia, thalami, or hippocampi. This finding is consistent with the results of a previous perfusion study involving monozygotic twins. Taken together, the findings suggest that brain perfusion is not affected in CFS.
Given the small number of subjects in this study, the findings must be verified in larger studies. It also would be valuable to assess possible changes in brain microstructure over time in a longitudinal study, and to determine whether treatment interventions exert any beneficial effects, the investigators added.
Imaging to assess brain microstructure identified increased fractional anisotropy in the anterior right arcuate fasciculus of patients with chronic fatigue syndrome, which was not present in age- and sex-matched control subjects.
The right-sided abnormality was particularly marked in right-handed chronic fatigue syndrome (CFS) patients, and the degree of increase correlated with the severity of their disorder. A finding of increased fractional anisotropy in this area of the brain may eventually serve as a biomarker for CFS, said Dr. Michael M. Zeineh of the department of radiology at Stanford (Calif.) University, and his associates (Radiology 2014 Oct. 29 [doi:10.1148/radiol.14141079]).
In what they described as the first study to examine brain microstructure in CFS, the investigators used volumetric MRI with diffusion-tensor imaging (DTI) to detect microstructural abnormalities in the patients – who had been evaluated at the university’s CFS Clinic during the preceding 5 years – and in healthy volunteers. The researchers also performed gray- and white-matter volumetric studies to identify differences between the two study groups in gross brain structure, as well as pseudocontinuous arterial spin labeling to assess global alterations in brain perfusion.
The mean age of the study participants was 46 years, and the mean duration of CFS symptoms was 12 years.
In the DTI analysis, the only significant difference between the two study groups was that the anterior right arcuate fasciculus – identified in 13 of 15 CFS patients and in all 14 control subjects in the study – showed significantly higher fractional anisotropy in CFS patients than in control subjects. This was confirmed in a second dataset from the same study subjects. The difference between CFS patients and control subjects was most pronounced in the subgroup of right-handed participants, “suggesting that hemispheric differences with handedness and language are an additional source of variance,” Dr. Zeineh and his associates said.
In addition, “two cortical regions connected via the arcuate fasciculus exhibited increased thickness: the right middle temporal and precentral gyri,” they noted.
The volumetric studies showed significantly lower total supratentorial white-matter volume in CFS, even after the data were adjusted to account for subject age, total intracranial volume, and handedness. However, total cortical gray-matter volume was equivalent between the two study groups, as were prefrontal cortex volume and global cortical thickness.
There were no differences between patients and controls in any perfusion measure, including perfusion to the cortex, supratentorial white matter, basal ganglia, thalami, or hippocampi. This finding is consistent with the results of a previous perfusion study involving monozygotic twins. Taken together, the findings suggest that brain perfusion is not affected in CFS.
Given the small number of subjects in this study, the findings must be verified in larger studies. It also would be valuable to assess possible changes in brain microstructure over time in a longitudinal study, and to determine whether treatment interventions exert any beneficial effects, the investigators added.
FROM RADIOLOGY
Key clinical point: Increased fractional anisotropy in the anterior right arcuate fasciculus may eventually serve as a biomarker for CFS if larger studies confirm the finding.
Major finding: The anterior right arcuate fasciculus showed significantly higher fractional anisotropy in CFS patients than in control subjects
Data source: A case-control study in which brain imaging was compared between 15 patients with chronic fatigue syndrome and 14 age- and sex-matched control subjects.
Disclosures: This study was supported by Stanford’s division of infectious disease CFS Fund. Dr. Zeineh reported receiving support from GE Healthcare unrelated to this study. He and his associates reported having no other financial disclosures.
Adjuvant ovarian suppression impairs QOL in women with breast cancer
For premenopausal women who have undergone definitive surgery for node-negative invasive breast cancer, adjuvant therapy with tamoxifen plus ovarian function suppression significantly impairs quality of life, compared with tamoxifen alone, according to a report published online October 27 in Journal of Clinical Oncology.
The role of ovarian function suppression in this setting remains uncertain because most studies examining the issue have been relatively short (2 years or less) and have included patients undergoing cytotoxic chemotherapy, which makes it difficult to tease out which drugs produce which outcomes of interest. In contrast, this open-label, phase III, randomized trial by the Eastern Cooperative Oncology Group had a mean follow-up of 10 years, and it included only women with hormone receptor–positive tumors measuring 3 cm or less who received no adjuvant chemotherapy, said Dr. Amye J. Tevaawerk of the University of Wisconsin, Madison, and her associates.
The 345 study participants were randomly assigned to receive either tamoxifen plus ovarian function suppression via goserelin, leuprolide, surgical ablation, or radiation ablation (174 women) or tamoxifen alone (171 women). The median age at baseline was 45 years (range, 26-55 years). The trial was designed to compare multiple outcomes, including disease-free survival, overall survival, and quality of life, but it was terminated early because of slow accrual and ultimately was only powered to detect differences in quality of life (QOL).
Throughout follow-up, women who received tamoxifen plus ovarian suppression reported inferior health-related QOL as measured by the Functional Assessment of Cancer Therapy – General (FACT-G) and the Functional Assessment of Cancer Therapy – Breast (FACT-B). They had more menopausal symptoms and lower levels of sexual function than women who received tamoxifen alone, regardless of patient age or race, tumor hormone-receptor status, tumor size, or type of surgical procedure. The differences between the two study groups were both statistically significant and clinically meaningful, Dr. Tevaawerk and her associates said (J. Clin. Oncol. 2014 October 27 [doi:10.1200/JCO.2014.55.6993]).
The negative impact of ovarian suppression gradually diminished after 3 years, most likely because increasing numbers of women receiving tamoxifen alone reached menopause. “Our results suggest that [studies with] durations of less than 3 years fail to capture the adverse effect peak and the health-related QOL nadir from ovarian function suppression,” the investigators said. “Given these data and in the absence of definitive data for improvement in disease-free survival or overall survival with ovarian function suppression, the American Society of Clinical Oncology guidelines are appropriately cautious about adding ovarian function suppression to tamoxifen,” they noted.
For premenopausal women who have undergone definitive surgery for node-negative invasive breast cancer, adjuvant therapy with tamoxifen plus ovarian function suppression significantly impairs quality of life, compared with tamoxifen alone, according to a report published online October 27 in Journal of Clinical Oncology.
The role of ovarian function suppression in this setting remains uncertain because most studies examining the issue have been relatively short (2 years or less) and have included patients undergoing cytotoxic chemotherapy, which makes it difficult to tease out which drugs produce which outcomes of interest. In contrast, this open-label, phase III, randomized trial by the Eastern Cooperative Oncology Group had a mean follow-up of 10 years, and it included only women with hormone receptor–positive tumors measuring 3 cm or less who received no adjuvant chemotherapy, said Dr. Amye J. Tevaawerk of the University of Wisconsin, Madison, and her associates.
The 345 study participants were randomly assigned to receive either tamoxifen plus ovarian function suppression via goserelin, leuprolide, surgical ablation, or radiation ablation (174 women) or tamoxifen alone (171 women). The median age at baseline was 45 years (range, 26-55 years). The trial was designed to compare multiple outcomes, including disease-free survival, overall survival, and quality of life, but it was terminated early because of slow accrual and ultimately was only powered to detect differences in quality of life (QOL).
Throughout follow-up, women who received tamoxifen plus ovarian suppression reported inferior health-related QOL as measured by the Functional Assessment of Cancer Therapy – General (FACT-G) and the Functional Assessment of Cancer Therapy – Breast (FACT-B). They had more menopausal symptoms and lower levels of sexual function than women who received tamoxifen alone, regardless of patient age or race, tumor hormone-receptor status, tumor size, or type of surgical procedure. The differences between the two study groups were both statistically significant and clinically meaningful, Dr. Tevaawerk and her associates said (J. Clin. Oncol. 2014 October 27 [doi:10.1200/JCO.2014.55.6993]).
The negative impact of ovarian suppression gradually diminished after 3 years, most likely because increasing numbers of women receiving tamoxifen alone reached menopause. “Our results suggest that [studies with] durations of less than 3 years fail to capture the adverse effect peak and the health-related QOL nadir from ovarian function suppression,” the investigators said. “Given these data and in the absence of definitive data for improvement in disease-free survival or overall survival with ovarian function suppression, the American Society of Clinical Oncology guidelines are appropriately cautious about adding ovarian function suppression to tamoxifen,” they noted.
For premenopausal women who have undergone definitive surgery for node-negative invasive breast cancer, adjuvant therapy with tamoxifen plus ovarian function suppression significantly impairs quality of life, compared with tamoxifen alone, according to a report published online October 27 in Journal of Clinical Oncology.
The role of ovarian function suppression in this setting remains uncertain because most studies examining the issue have been relatively short (2 years or less) and have included patients undergoing cytotoxic chemotherapy, which makes it difficult to tease out which drugs produce which outcomes of interest. In contrast, this open-label, phase III, randomized trial by the Eastern Cooperative Oncology Group had a mean follow-up of 10 years, and it included only women with hormone receptor–positive tumors measuring 3 cm or less who received no adjuvant chemotherapy, said Dr. Amye J. Tevaawerk of the University of Wisconsin, Madison, and her associates.
The 345 study participants were randomly assigned to receive either tamoxifen plus ovarian function suppression via goserelin, leuprolide, surgical ablation, or radiation ablation (174 women) or tamoxifen alone (171 women). The median age at baseline was 45 years (range, 26-55 years). The trial was designed to compare multiple outcomes, including disease-free survival, overall survival, and quality of life, but it was terminated early because of slow accrual and ultimately was only powered to detect differences in quality of life (QOL).
Throughout follow-up, women who received tamoxifen plus ovarian suppression reported inferior health-related QOL as measured by the Functional Assessment of Cancer Therapy – General (FACT-G) and the Functional Assessment of Cancer Therapy – Breast (FACT-B). They had more menopausal symptoms and lower levels of sexual function than women who received tamoxifen alone, regardless of patient age or race, tumor hormone-receptor status, tumor size, or type of surgical procedure. The differences between the two study groups were both statistically significant and clinically meaningful, Dr. Tevaawerk and her associates said (J. Clin. Oncol. 2014 October 27 [doi:10.1200/JCO.2014.55.6993]).
The negative impact of ovarian suppression gradually diminished after 3 years, most likely because increasing numbers of women receiving tamoxifen alone reached menopause. “Our results suggest that [studies with] durations of less than 3 years fail to capture the adverse effect peak and the health-related QOL nadir from ovarian function suppression,” the investigators said. “Given these data and in the absence of definitive data for improvement in disease-free survival or overall survival with ovarian function suppression, the American Society of Clinical Oncology guidelines are appropriately cautious about adding ovarian function suppression to tamoxifen,” they noted.
Key clinical point: Adding ovarian function suppression to standard tamoxifen in premenopausal women with early breast cancer definitely impairs QOL.
Major finding: Women who received tamoxifen plus ovarian suppression reported inferior health-related QOL, with more menopausal symptoms and lower levels of sexual function, than women who received tamoxifen alone, regardless of patient age or race, tumor hormone receptor status, tumor size, or type of surgical procedure.
Data source: An open-label, phase III, randomized, controlled trial comparing adjuvant tamoxifen alone against tamoxifen plus ovarian function suppression in 345 premenopausal women with early-stage breast cancer.
Disclosures: This Eastern Cooperative Oncology Group study was supported in part by Public Health Service grants from the National Cancer Institute and by the National Institutes of Health National Center for Advancing Translational Sciences. Dr. Tevaawerk and her associates reported having no financial conflicts of interest.
Aggressive radiotherapy dramatically improves limited metastatic NSCLC
Stereotactic body radiation therapy dramatically prolonged progression-free survival and overall survival in a phase II study of 24 patients who had limited but progressive metastatic non–small-cell lung cancer.
The findings were published online Oct. 27 in Journal of Clinical Oncology.
When added to erlotinib, the aggressive local radiation technique, which delivers high doses in a few treatment sessions to extracranial malignant deposits, more than doubled progression-free and overall survival, compared with that in historical control subjects. These findings “encourage consideration of a new treatment paradigm with the inclusion of aggressive noninvasive local therapy in the form of stereotactic body radiation therapy,” said Dr. Puneeth Iyengar, a radiation oncologist at the University of Texas Southwestern Medical Center, Dallas, and his associates.
The study participants were adults with stage IV NSCLC who had undergone at least one systemic chemotherapy regimen and still had up to six metastases, most often in the lung parenchyma, mediastinal or hilar nodes, adrenal glands, liver, and spine. The contours of 52 metastatic deposits were mapped and treated with stereotactic body radiation therapy (SBRT). All the patients also received erlotinib, beginning 1 week before radiotherapy began and continuing until disease progressed or patients opted to stop the drug.
The primary efficacy outcome, median 6-month progression-free survival, was 69%. After a mean follow-up of 17 months (range, 3-60 months), median progression-free survival was 14.7 months and median overall survival was 20.4 months. In comparison, progression-free survival was only 2-4 months and overall survival only 6-9 months in historical cohorts of patients with stage IV NSCLC who progressed through multiple rounds of cytotoxic chemotherapy but did not receive SBRT.
At 3-month follow-up, 10 of 47 evaluable lesions were no longer visible and 24 others showed a reduction in volume of at least 30%. None of the metastases treated with SBRT showed any progression for at least 9 months.
“Our approach dramatically changed the pattern of relapse, with a shift in failure from existing sites (i.e., local) to new sites (i.e., distant). One-third of patients were able to receive additional cytotoxic chemotherapy after progression on our trial, with SBRT potentially allowing this subset to tolerate subsequent agents by delaying continued use,” Dr. Iyengar and his associates wrote (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.56.7412]).
It is important to note that all single-arm studies like this one by Iyengar and colleagues are by their nature nonrandomized and subject to selection bias.
Patients eligible for this study had to have tolerated one or more rounds of chemotherapy and had to have been considered good candidates for radiotherapy, meaning that they likely had no negative prognostic factors such as pleural effusion, active brain metastases, or large tumors. Selection of such patients could itself improve survival measures. And tumor sensitivity to chemotherapy may likewise select for more favorable disease biology.
Dr. Salma K. Jabbour is a radiation oncologist at Rutgers Cancer Institute of New Jersey in New Brunswick. She reported being on the speakers bureau for Abbott Laboratories. Dr. Jabbour made these remarks in an editorial accompanying Dr. Iyengar’s report (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.58.5539]).
It is important to note that all single-arm studies like this one by Iyengar and colleagues are by their nature nonrandomized and subject to selection bias.
Patients eligible for this study had to have tolerated one or more rounds of chemotherapy and had to have been considered good candidates for radiotherapy, meaning that they likely had no negative prognostic factors such as pleural effusion, active brain metastases, or large tumors. Selection of such patients could itself improve survival measures. And tumor sensitivity to chemotherapy may likewise select for more favorable disease biology.
Dr. Salma K. Jabbour is a radiation oncologist at Rutgers Cancer Institute of New Jersey in New Brunswick. She reported being on the speakers bureau for Abbott Laboratories. Dr. Jabbour made these remarks in an editorial accompanying Dr. Iyengar’s report (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.58.5539]).
It is important to note that all single-arm studies like this one by Iyengar and colleagues are by their nature nonrandomized and subject to selection bias.
Patients eligible for this study had to have tolerated one or more rounds of chemotherapy and had to have been considered good candidates for radiotherapy, meaning that they likely had no negative prognostic factors such as pleural effusion, active brain metastases, or large tumors. Selection of such patients could itself improve survival measures. And tumor sensitivity to chemotherapy may likewise select for more favorable disease biology.
Dr. Salma K. Jabbour is a radiation oncologist at Rutgers Cancer Institute of New Jersey in New Brunswick. She reported being on the speakers bureau for Abbott Laboratories. Dr. Jabbour made these remarks in an editorial accompanying Dr. Iyengar’s report (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.58.5539]).
Stereotactic body radiation therapy dramatically prolonged progression-free survival and overall survival in a phase II study of 24 patients who had limited but progressive metastatic non–small-cell lung cancer.
The findings were published online Oct. 27 in Journal of Clinical Oncology.
When added to erlotinib, the aggressive local radiation technique, which delivers high doses in a few treatment sessions to extracranial malignant deposits, more than doubled progression-free and overall survival, compared with that in historical control subjects. These findings “encourage consideration of a new treatment paradigm with the inclusion of aggressive noninvasive local therapy in the form of stereotactic body radiation therapy,” said Dr. Puneeth Iyengar, a radiation oncologist at the University of Texas Southwestern Medical Center, Dallas, and his associates.
The study participants were adults with stage IV NSCLC who had undergone at least one systemic chemotherapy regimen and still had up to six metastases, most often in the lung parenchyma, mediastinal or hilar nodes, adrenal glands, liver, and spine. The contours of 52 metastatic deposits were mapped and treated with stereotactic body radiation therapy (SBRT). All the patients also received erlotinib, beginning 1 week before radiotherapy began and continuing until disease progressed or patients opted to stop the drug.
The primary efficacy outcome, median 6-month progression-free survival, was 69%. After a mean follow-up of 17 months (range, 3-60 months), median progression-free survival was 14.7 months and median overall survival was 20.4 months. In comparison, progression-free survival was only 2-4 months and overall survival only 6-9 months in historical cohorts of patients with stage IV NSCLC who progressed through multiple rounds of cytotoxic chemotherapy but did not receive SBRT.
At 3-month follow-up, 10 of 47 evaluable lesions were no longer visible and 24 others showed a reduction in volume of at least 30%. None of the metastases treated with SBRT showed any progression for at least 9 months.
“Our approach dramatically changed the pattern of relapse, with a shift in failure from existing sites (i.e., local) to new sites (i.e., distant). One-third of patients were able to receive additional cytotoxic chemotherapy after progression on our trial, with SBRT potentially allowing this subset to tolerate subsequent agents by delaying continued use,” Dr. Iyengar and his associates wrote (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.56.7412]).
Stereotactic body radiation therapy dramatically prolonged progression-free survival and overall survival in a phase II study of 24 patients who had limited but progressive metastatic non–small-cell lung cancer.
The findings were published online Oct. 27 in Journal of Clinical Oncology.
When added to erlotinib, the aggressive local radiation technique, which delivers high doses in a few treatment sessions to extracranial malignant deposits, more than doubled progression-free and overall survival, compared with that in historical control subjects. These findings “encourage consideration of a new treatment paradigm with the inclusion of aggressive noninvasive local therapy in the form of stereotactic body radiation therapy,” said Dr. Puneeth Iyengar, a radiation oncologist at the University of Texas Southwestern Medical Center, Dallas, and his associates.
The study participants were adults with stage IV NSCLC who had undergone at least one systemic chemotherapy regimen and still had up to six metastases, most often in the lung parenchyma, mediastinal or hilar nodes, adrenal glands, liver, and spine. The contours of 52 metastatic deposits were mapped and treated with stereotactic body radiation therapy (SBRT). All the patients also received erlotinib, beginning 1 week before radiotherapy began and continuing until disease progressed or patients opted to stop the drug.
The primary efficacy outcome, median 6-month progression-free survival, was 69%. After a mean follow-up of 17 months (range, 3-60 months), median progression-free survival was 14.7 months and median overall survival was 20.4 months. In comparison, progression-free survival was only 2-4 months and overall survival only 6-9 months in historical cohorts of patients with stage IV NSCLC who progressed through multiple rounds of cytotoxic chemotherapy but did not receive SBRT.
At 3-month follow-up, 10 of 47 evaluable lesions were no longer visible and 24 others showed a reduction in volume of at least 30%. None of the metastases treated with SBRT showed any progression for at least 9 months.
“Our approach dramatically changed the pattern of relapse, with a shift in failure from existing sites (i.e., local) to new sites (i.e., distant). One-third of patients were able to receive additional cytotoxic chemotherapy after progression on our trial, with SBRT potentially allowing this subset to tolerate subsequent agents by delaying continued use,” Dr. Iyengar and his associates wrote (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.56.7412]).
Key clinical point: Stereotactic body radiation therapy plus erlotinib dramatically improves survival in limited but progressive metastatic NSCLC.
Major finding: Median progression-free survival was 14.7 months and median overall survival was 20.4 months, compared with 2-4 months and 6-9 months, respectively, in historical cohorts of similar patients.
Data source: A prospective phase II clinical trial assessing survival after stereotactic body radiation therapy plus erlotinib in 24 patients who had six or fewer metastases of NSCLC.
Disclosures: Dr. Iyengar reported having no financial disclosures; his associates reported ties to numerous industry sources.
Hippocampus-sparing brain radiotherapy preserves memory, QOL
For cancer patients who have multiple brain metastases, tailoring whole-brain radiotherapy so that it avoids the hippocampus preserves memory and quality of life for at least 6 months, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
Injury to the compartment of neural stem cells located in the subgranular zone of the hippocampal dentate gyrus is thought to suppress the formation of new memory and to impair recall, and injury to this region by relatively low doses of radiotherapy is thought to account for radiation-induced early cognitive decline. Researchers performed a multicenter phase II trial to determine whether sparing this region would prevent such cognitive decline. They assessed 100 patients who had brain metastases of nonhematopoietic malignancies and underwent irradiation of the whole-brain parenchyma minus the “hippocampal avoidance regions” that had been designated using advanced imaging techniques, said Dr. Vinai Gondi of the Cadence Brain Tumor Center and CDH Proton Center, Warrenville, Ill., and his associates.
The study participants underwent cognitive assessment at baseline and at regular intervals following radiotherapy, as well as assessment of health-related quality of life. Their results were compared with those of 208 historical control subjects who had received standard whole-brain radiotherapy without hippocampal avoidance in an unrelated clinical trial. The radiation-sparing technique, which reduced the mean dose to the neural stem compartment by an estimated 80%, produced significant memory preservation that persisted for up to 6 months of follow-up. The mean probability of cognitive deterioration at 4 months was only 7%, compared with 30% in the historical control group.
The hippocampal-sparing technique also preserved physical, social/family, emotional, and functional well-being, as assessed by the patient and his or her family, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.57.2909]).
The risk that metastases would develop in the nonradiated hippocampus was considered low, as only three patients (4.5%) developed such metastases. Previously, investigators have predicted that the risk would be closer to 10%, but they appear to have overestimated the actual risk, Dr. Gondi and his associates said.
These promising results require further validation in phase III trials. Studies are now underway to assess whether further reducing the radiation dose to the hippocampal area may improve outcomes even more, and future studies also are being planned to assess whether hippocampal avoidance prevents longer-term cognitive decline, beyond the 6-month mark established in this study, the investigators added.
This study was supported by the National Cancer Institute’s Radiation Therapy Oncology Group and Community Clinical Oncology Program. Dr. Gondi reported having no financial disclosures; his associates reported numerous ties to industry sources.
The findings of this phase II study need to be replicated and expanded upon before this innovative treatment approach can be offered to patients.
To justify the increased cost, time, and effort involved in hippocampal-avoidance whole-brain radiotherapy, it may be necessary to prove that the technique improves survival, not just QOL. And the influence of factors such as the number and size of brain metastases, extracranial disease status, and prognostic assessment scores should be addressed in future studies, to narrow down which patients will benefit most from the therapy.
Dr. John H. Suh is in the department of radiation oncology at the Cleveland Clinic. He reported receiving honoraria and serving as a consultant or adviser to Varian Medical Systems. Dr. Suh made these remarks in an editorial accompanying Dr. Gondi’s report (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.58.4367]).
The findings of this phase II study need to be replicated and expanded upon before this innovative treatment approach can be offered to patients.
To justify the increased cost, time, and effort involved in hippocampal-avoidance whole-brain radiotherapy, it may be necessary to prove that the technique improves survival, not just QOL. And the influence of factors such as the number and size of brain metastases, extracranial disease status, and prognostic assessment scores should be addressed in future studies, to narrow down which patients will benefit most from the therapy.
Dr. John H. Suh is in the department of radiation oncology at the Cleveland Clinic. He reported receiving honoraria and serving as a consultant or adviser to Varian Medical Systems. Dr. Suh made these remarks in an editorial accompanying Dr. Gondi’s report (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.58.4367]).
The findings of this phase II study need to be replicated and expanded upon before this innovative treatment approach can be offered to patients.
To justify the increased cost, time, and effort involved in hippocampal-avoidance whole-brain radiotherapy, it may be necessary to prove that the technique improves survival, not just QOL. And the influence of factors such as the number and size of brain metastases, extracranial disease status, and prognostic assessment scores should be addressed in future studies, to narrow down which patients will benefit most from the therapy.
Dr. John H. Suh is in the department of radiation oncology at the Cleveland Clinic. He reported receiving honoraria and serving as a consultant or adviser to Varian Medical Systems. Dr. Suh made these remarks in an editorial accompanying Dr. Gondi’s report (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.58.4367]).
For cancer patients who have multiple brain metastases, tailoring whole-brain radiotherapy so that it avoids the hippocampus preserves memory and quality of life for at least 6 months, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
Injury to the compartment of neural stem cells located in the subgranular zone of the hippocampal dentate gyrus is thought to suppress the formation of new memory and to impair recall, and injury to this region by relatively low doses of radiotherapy is thought to account for radiation-induced early cognitive decline. Researchers performed a multicenter phase II trial to determine whether sparing this region would prevent such cognitive decline. They assessed 100 patients who had brain metastases of nonhematopoietic malignancies and underwent irradiation of the whole-brain parenchyma minus the “hippocampal avoidance regions” that had been designated using advanced imaging techniques, said Dr. Vinai Gondi of the Cadence Brain Tumor Center and CDH Proton Center, Warrenville, Ill., and his associates.
The study participants underwent cognitive assessment at baseline and at regular intervals following radiotherapy, as well as assessment of health-related quality of life. Their results were compared with those of 208 historical control subjects who had received standard whole-brain radiotherapy without hippocampal avoidance in an unrelated clinical trial. The radiation-sparing technique, which reduced the mean dose to the neural stem compartment by an estimated 80%, produced significant memory preservation that persisted for up to 6 months of follow-up. The mean probability of cognitive deterioration at 4 months was only 7%, compared with 30% in the historical control group.
The hippocampal-sparing technique also preserved physical, social/family, emotional, and functional well-being, as assessed by the patient and his or her family, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.57.2909]).
The risk that metastases would develop in the nonradiated hippocampus was considered low, as only three patients (4.5%) developed such metastases. Previously, investigators have predicted that the risk would be closer to 10%, but they appear to have overestimated the actual risk, Dr. Gondi and his associates said.
These promising results require further validation in phase III trials. Studies are now underway to assess whether further reducing the radiation dose to the hippocampal area may improve outcomes even more, and future studies also are being planned to assess whether hippocampal avoidance prevents longer-term cognitive decline, beyond the 6-month mark established in this study, the investigators added.
This study was supported by the National Cancer Institute’s Radiation Therapy Oncology Group and Community Clinical Oncology Program. Dr. Gondi reported having no financial disclosures; his associates reported numerous ties to industry sources.
For cancer patients who have multiple brain metastases, tailoring whole-brain radiotherapy so that it avoids the hippocampus preserves memory and quality of life for at least 6 months, according to a report published online Oct. 27 in the Journal of Clinical Oncology.
Injury to the compartment of neural stem cells located in the subgranular zone of the hippocampal dentate gyrus is thought to suppress the formation of new memory and to impair recall, and injury to this region by relatively low doses of radiotherapy is thought to account for radiation-induced early cognitive decline. Researchers performed a multicenter phase II trial to determine whether sparing this region would prevent such cognitive decline. They assessed 100 patients who had brain metastases of nonhematopoietic malignancies and underwent irradiation of the whole-brain parenchyma minus the “hippocampal avoidance regions” that had been designated using advanced imaging techniques, said Dr. Vinai Gondi of the Cadence Brain Tumor Center and CDH Proton Center, Warrenville, Ill., and his associates.
The study participants underwent cognitive assessment at baseline and at regular intervals following radiotherapy, as well as assessment of health-related quality of life. Their results were compared with those of 208 historical control subjects who had received standard whole-brain radiotherapy without hippocampal avoidance in an unrelated clinical trial. The radiation-sparing technique, which reduced the mean dose to the neural stem compartment by an estimated 80%, produced significant memory preservation that persisted for up to 6 months of follow-up. The mean probability of cognitive deterioration at 4 months was only 7%, compared with 30% in the historical control group.
The hippocampal-sparing technique also preserved physical, social/family, emotional, and functional well-being, as assessed by the patient and his or her family, the investigators said (J. Clin. Oncol. 2014 Oct. 27 [doi:10.1200/JCO.2014.57.2909]).
The risk that metastases would develop in the nonradiated hippocampus was considered low, as only three patients (4.5%) developed such metastases. Previously, investigators have predicted that the risk would be closer to 10%, but they appear to have overestimated the actual risk, Dr. Gondi and his associates said.
These promising results require further validation in phase III trials. Studies are now underway to assess whether further reducing the radiation dose to the hippocampal area may improve outcomes even more, and future studies also are being planned to assess whether hippocampal avoidance prevents longer-term cognitive decline, beyond the 6-month mark established in this study, the investigators added.
This study was supported by the National Cancer Institute’s Radiation Therapy Oncology Group and Community Clinical Oncology Program. Dr. Gondi reported having no financial disclosures; his associates reported numerous ties to industry sources.
Key clinical point: Whole-brain radiotherapy that spares the hippocampal neural stem cell compartment preserves memory and QOL in cancer patients with brain metastases.
Major finding: The mean probability of cognitive deterioration at 4 months was only 7% in patients who underwent hippocampus-sparing brain radiotherapy, compared with 30% in a historical control group.
Data source: A multicenter phase II study comparing cognitive function and QOL between 100 patients with brain metastases who had hippocampus-sparing radiotherapy and 208 historical control subjects who had standard whole-brain radiotherapy.
Disclosures: This study was supported by the National Cancer Institute’s Radiation Therapy Oncology Group and Community Clinical Oncology Program. Dr. Gondi reported having no financial disclosures; his associates reported numerous ties to industry sources.
Short-course TB therapy fails in three international trials
Three different short-course treatment regimens for tuberculosis failed to show noninferiority to the standard 6-month course in separate phase III randomized clinical trials, even though the experimental regimens produced a more rapid decline in bacterial load, as expected, according to reports published online Oct. 23 in the New England Journal of Medicine.
The main reason that the short-course (4-month) approaches failed to measure up to standard treatment was that, despite their greater bactericidal activity, the rate of relapse was excessive after treatment was completed.
Researchers undertook these large international trials because the data from several phase II and murine studies had been so promising: replacing either the isoniazid or the ethambutol in the standard anti-TB regimen with a fluoroquinolone appeared to permit shortening of the treatment period without sacrificing efficacy. But the consistently negative results from these phase III studies clearly demonstrate that this approach is not effective.
The first trial involved 1,931 adults with newly diagnosed and untreated Mycobacterium tuberculosis infection who were treated in South Africa, India, Tanzania, Kenya, Thailand, Malaysia, Zambia, China, and Mexico. These patients were randomly assigned to receive the standard regimen of isoniazid, rifampin, pyrazinamide, and ethambutol for 8 weeks, followed by 18 weeks of isoniazid plus rifampin (control group, 640 participants); or an experimental regimen in which ethambutol was replaced by moxifloxacin for 17 weeks, followed by 9 weeks of placebo (655 participants); or an experimental regimen in which isoniazid was replaced by moxifloxacin in the same way (636 participants), said Dr. Stephen H. Gillespie of the University of St. Andrews (England) and University College London and his associates.
Patients in the two experimental groups converted to culture-negative status more rapidly than did those in the control group. However, in a per-protocol analysis, 92% of the control group achieved a favorable final outcome, compared with only 85% and 80% of the experimental groups, respectively. Results of a modified intention-to-treat analysis and of more than 20 sensitivity analyses showed the same pattern. At the end of active treatment, only 12 patients in the control group had a relapse of TB infection, compared with 64 patients and 46 patients, respectively, in the experimental groups, Dr. Gillespie and his colleagues said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1407426]).
The second trial was an open-label noninferiority study involving 1,836 patients in Benin, Guinea, Kenya, Senegal, and South Africa. They were randomly assigned to standard 6-month treatment (919 control subjects) or an experimental treatment in which gatifloxacin was substituted for ethambutol and the course of therapy was shortened to 4 months (917 patients), said Dr. Corinne S. Merle of the London School of Hygiene and Tropical Medicine and her associates.
The primary efficacy endpoint, the percentage of patients with an unfavorable outcome after 24 months, was 17.2% in the control group and 21.0% in the experimental group, a significant difference. In particular, more than twice as many patients in the experimental group (14.6%) had a relapse than in the control group (7.1%). As with Dr. Gillespie’s study, Dr. Merle’s study failed to show that the short-course regimen was noninferior to the standard regimen. Again, “the expectations raised by [phase I and II trials] were not borne out in this phase III trial,” they said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1315817]).
The third trial, involving 827 patients in South Africa, Zimbabwe, Botswana, and Zambia, compared the standard control regimen against a 4-month regimen in which isoniazid was replaced by moxifloxacin and a 6-month regimen in which isoniazid was replaced by moxifloxacin, said Dr. Amina Jindani of St. George’s University of London and University College London and her associates.
In the per-protocol analysis, unfavorable outcomes occurred in 4.9% of the control group and 3.2% of the 6-month experimental group, compared with 18.2% of the short-course experimental group. The corresponding figures for the intention-to-treat analysis were 14.4%, 13.7%, and 26.9%, respectively.
Again, this discrepancy was attributed primarily to the significantly higher relapse rate in the short-course, compared with the control treatments – 26 cases with 4 months of treatment vs. 5 cases each with 6 months, Dr. Jindani and her associates said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1314210]).
Although the studies described here have established the capacity for large, multicenter trials across disease-endemic countries, the design and selection of future experimental regimens will need to incorporate a triage process that can mitigate risks while enabling the accelerated development of much-needed treatment-shortening therapies. The disconnect between the phase II data that motivated these trials and the phase III results reinforces the idea that small sample sizes limit the utility of short trials in predicting the success of treatment-shortening regimens.
As these three trials have confirmed, our understanding of the science underlying positive clinical outcomes remains rudimentary. It’s time to go back to basics.
Digby F. Warner, Ph.D., and Valerie Mizrahi, Ph.D., are in the molecular mycobacteriology research unit at the Institute of Infectious Disease and Molecular Medicine, Cape Town and in the department of clinical laboratory sciences at the University of Cape Town, both in South Africa. Dr. Warner reported receiving funding from the South African Medical Research Council, Medical Research Foundation South Africa, and Wellcome Trust. Dr. Mizrahi reported funding from the European & Developing Countries Clinical Trials Partnership, the Bill and Melinda Gates Foundation, the Wellcome Trust, and USAID. Dr. Mizrahi also serves on the scientific advisory committee of the Global Alliance for TB Drug Development, which supported Dr. Gillespie’s study. This comment is excerpted from an editorial by Dr. Warner and Dr. Mizrahi that accompanied the three reports (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMe1410977]).
Although the studies described here have established the capacity for large, multicenter trials across disease-endemic countries, the design and selection of future experimental regimens will need to incorporate a triage process that can mitigate risks while enabling the accelerated development of much-needed treatment-shortening therapies. The disconnect between the phase II data that motivated these trials and the phase III results reinforces the idea that small sample sizes limit the utility of short trials in predicting the success of treatment-shortening regimens.
As these three trials have confirmed, our understanding of the science underlying positive clinical outcomes remains rudimentary. It’s time to go back to basics.
Digby F. Warner, Ph.D., and Valerie Mizrahi, Ph.D., are in the molecular mycobacteriology research unit at the Institute of Infectious Disease and Molecular Medicine, Cape Town and in the department of clinical laboratory sciences at the University of Cape Town, both in South Africa. Dr. Warner reported receiving funding from the South African Medical Research Council, Medical Research Foundation South Africa, and Wellcome Trust. Dr. Mizrahi reported funding from the European & Developing Countries Clinical Trials Partnership, the Bill and Melinda Gates Foundation, the Wellcome Trust, and USAID. Dr. Mizrahi also serves on the scientific advisory committee of the Global Alliance for TB Drug Development, which supported Dr. Gillespie’s study. This comment is excerpted from an editorial by Dr. Warner and Dr. Mizrahi that accompanied the three reports (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMe1410977]).
Although the studies described here have established the capacity for large, multicenter trials across disease-endemic countries, the design and selection of future experimental regimens will need to incorporate a triage process that can mitigate risks while enabling the accelerated development of much-needed treatment-shortening therapies. The disconnect between the phase II data that motivated these trials and the phase III results reinforces the idea that small sample sizes limit the utility of short trials in predicting the success of treatment-shortening regimens.
As these three trials have confirmed, our understanding of the science underlying positive clinical outcomes remains rudimentary. It’s time to go back to basics.
Digby F. Warner, Ph.D., and Valerie Mizrahi, Ph.D., are in the molecular mycobacteriology research unit at the Institute of Infectious Disease and Molecular Medicine, Cape Town and in the department of clinical laboratory sciences at the University of Cape Town, both in South Africa. Dr. Warner reported receiving funding from the South African Medical Research Council, Medical Research Foundation South Africa, and Wellcome Trust. Dr. Mizrahi reported funding from the European & Developing Countries Clinical Trials Partnership, the Bill and Melinda Gates Foundation, the Wellcome Trust, and USAID. Dr. Mizrahi also serves on the scientific advisory committee of the Global Alliance for TB Drug Development, which supported Dr. Gillespie’s study. This comment is excerpted from an editorial by Dr. Warner and Dr. Mizrahi that accompanied the three reports (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMe1410977]).
Three different short-course treatment regimens for tuberculosis failed to show noninferiority to the standard 6-month course in separate phase III randomized clinical trials, even though the experimental regimens produced a more rapid decline in bacterial load, as expected, according to reports published online Oct. 23 in the New England Journal of Medicine.
The main reason that the short-course (4-month) approaches failed to measure up to standard treatment was that, despite their greater bactericidal activity, the rate of relapse was excessive after treatment was completed.
Researchers undertook these large international trials because the data from several phase II and murine studies had been so promising: replacing either the isoniazid or the ethambutol in the standard anti-TB regimen with a fluoroquinolone appeared to permit shortening of the treatment period without sacrificing efficacy. But the consistently negative results from these phase III studies clearly demonstrate that this approach is not effective.
The first trial involved 1,931 adults with newly diagnosed and untreated Mycobacterium tuberculosis infection who were treated in South Africa, India, Tanzania, Kenya, Thailand, Malaysia, Zambia, China, and Mexico. These patients were randomly assigned to receive the standard regimen of isoniazid, rifampin, pyrazinamide, and ethambutol for 8 weeks, followed by 18 weeks of isoniazid plus rifampin (control group, 640 participants); or an experimental regimen in which ethambutol was replaced by moxifloxacin for 17 weeks, followed by 9 weeks of placebo (655 participants); or an experimental regimen in which isoniazid was replaced by moxifloxacin in the same way (636 participants), said Dr. Stephen H. Gillespie of the University of St. Andrews (England) and University College London and his associates.
Patients in the two experimental groups converted to culture-negative status more rapidly than did those in the control group. However, in a per-protocol analysis, 92% of the control group achieved a favorable final outcome, compared with only 85% and 80% of the experimental groups, respectively. Results of a modified intention-to-treat analysis and of more than 20 sensitivity analyses showed the same pattern. At the end of active treatment, only 12 patients in the control group had a relapse of TB infection, compared with 64 patients and 46 patients, respectively, in the experimental groups, Dr. Gillespie and his colleagues said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1407426]).
The second trial was an open-label noninferiority study involving 1,836 patients in Benin, Guinea, Kenya, Senegal, and South Africa. They were randomly assigned to standard 6-month treatment (919 control subjects) or an experimental treatment in which gatifloxacin was substituted for ethambutol and the course of therapy was shortened to 4 months (917 patients), said Dr. Corinne S. Merle of the London School of Hygiene and Tropical Medicine and her associates.
The primary efficacy endpoint, the percentage of patients with an unfavorable outcome after 24 months, was 17.2% in the control group and 21.0% in the experimental group, a significant difference. In particular, more than twice as many patients in the experimental group (14.6%) had a relapse than in the control group (7.1%). As with Dr. Gillespie’s study, Dr. Merle’s study failed to show that the short-course regimen was noninferior to the standard regimen. Again, “the expectations raised by [phase I and II trials] were not borne out in this phase III trial,” they said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1315817]).
The third trial, involving 827 patients in South Africa, Zimbabwe, Botswana, and Zambia, compared the standard control regimen against a 4-month regimen in which isoniazid was replaced by moxifloxacin and a 6-month regimen in which isoniazid was replaced by moxifloxacin, said Dr. Amina Jindani of St. George’s University of London and University College London and her associates.
In the per-protocol analysis, unfavorable outcomes occurred in 4.9% of the control group and 3.2% of the 6-month experimental group, compared with 18.2% of the short-course experimental group. The corresponding figures for the intention-to-treat analysis were 14.4%, 13.7%, and 26.9%, respectively.
Again, this discrepancy was attributed primarily to the significantly higher relapse rate in the short-course, compared with the control treatments – 26 cases with 4 months of treatment vs. 5 cases each with 6 months, Dr. Jindani and her associates said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1314210]).
Three different short-course treatment regimens for tuberculosis failed to show noninferiority to the standard 6-month course in separate phase III randomized clinical trials, even though the experimental regimens produced a more rapid decline in bacterial load, as expected, according to reports published online Oct. 23 in the New England Journal of Medicine.
The main reason that the short-course (4-month) approaches failed to measure up to standard treatment was that, despite their greater bactericidal activity, the rate of relapse was excessive after treatment was completed.
Researchers undertook these large international trials because the data from several phase II and murine studies had been so promising: replacing either the isoniazid or the ethambutol in the standard anti-TB regimen with a fluoroquinolone appeared to permit shortening of the treatment period without sacrificing efficacy. But the consistently negative results from these phase III studies clearly demonstrate that this approach is not effective.
The first trial involved 1,931 adults with newly diagnosed and untreated Mycobacterium tuberculosis infection who were treated in South Africa, India, Tanzania, Kenya, Thailand, Malaysia, Zambia, China, and Mexico. These patients were randomly assigned to receive the standard regimen of isoniazid, rifampin, pyrazinamide, and ethambutol for 8 weeks, followed by 18 weeks of isoniazid plus rifampin (control group, 640 participants); or an experimental regimen in which ethambutol was replaced by moxifloxacin for 17 weeks, followed by 9 weeks of placebo (655 participants); or an experimental regimen in which isoniazid was replaced by moxifloxacin in the same way (636 participants), said Dr. Stephen H. Gillespie of the University of St. Andrews (England) and University College London and his associates.
Patients in the two experimental groups converted to culture-negative status more rapidly than did those in the control group. However, in a per-protocol analysis, 92% of the control group achieved a favorable final outcome, compared with only 85% and 80% of the experimental groups, respectively. Results of a modified intention-to-treat analysis and of more than 20 sensitivity analyses showed the same pattern. At the end of active treatment, only 12 patients in the control group had a relapse of TB infection, compared with 64 patients and 46 patients, respectively, in the experimental groups, Dr. Gillespie and his colleagues said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1407426]).
The second trial was an open-label noninferiority study involving 1,836 patients in Benin, Guinea, Kenya, Senegal, and South Africa. They were randomly assigned to standard 6-month treatment (919 control subjects) or an experimental treatment in which gatifloxacin was substituted for ethambutol and the course of therapy was shortened to 4 months (917 patients), said Dr. Corinne S. Merle of the London School of Hygiene and Tropical Medicine and her associates.
The primary efficacy endpoint, the percentage of patients with an unfavorable outcome after 24 months, was 17.2% in the control group and 21.0% in the experimental group, a significant difference. In particular, more than twice as many patients in the experimental group (14.6%) had a relapse than in the control group (7.1%). As with Dr. Gillespie’s study, Dr. Merle’s study failed to show that the short-course regimen was noninferior to the standard regimen. Again, “the expectations raised by [phase I and II trials] were not borne out in this phase III trial,” they said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1315817]).
The third trial, involving 827 patients in South Africa, Zimbabwe, Botswana, and Zambia, compared the standard control regimen against a 4-month regimen in which isoniazid was replaced by moxifloxacin and a 6-month regimen in which isoniazid was replaced by moxifloxacin, said Dr. Amina Jindani of St. George’s University of London and University College London and her associates.
In the per-protocol analysis, unfavorable outcomes occurred in 4.9% of the control group and 3.2% of the 6-month experimental group, compared with 18.2% of the short-course experimental group. The corresponding figures for the intention-to-treat analysis were 14.4%, 13.7%, and 26.9%, respectively.
Again, this discrepancy was attributed primarily to the significantly higher relapse rate in the short-course, compared with the control treatments – 26 cases with 4 months of treatment vs. 5 cases each with 6 months, Dr. Jindani and her associates said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1314210]).
Key clinical point: Shortening tuberculosis treatment to 4 months is not proven to be effective in three phase III studies.
Major finding: Relapse was seen in more than twice as many patients (14.6%) receiving treatment in which gatifloxacin was substituted for ethambutol and the course of therapy was shortened to 4 months in lieu of the standard 6-month treatment (7.1%).
Data source: An open-label noninferiority study involving 1,836 patients.
Disclosures: The researchers reported no relevant financial conflicts.
FOLFOXIRI + bevacizumab for metastatic colorectal cancer
Initial treatment with FOLFOXIRI plus bevacizumab, rather than FOLFIRI plus bevacizumab, improved progression-free survival in adults with inoperable metastatic colorectal cancer participating in a phase III randomized clinical trial, according to a report published online Oct. 23 in the New England Journal of Medicine.
However, patients who received FOLFOXIRI developed more grade 3 or 4 adverse events such as neutropenia, diarrhea, stomatitis, and peripheral neuropathy. The trial assessed treatment efficacy but not quality of life, so the impact of these toxicities was not addressed, said Dr. Fotios Loupakis of the University of Pisa and his associates in the Triplet plus Bevacizumab (TRIBE) trial.
In the open-label study, patients with unresectable metastatic colorectal cancer who had not received chemotherapy or biologic therapy for their metastatic disease were treated at 34 medical centers across Italy. They were randomly assigned to receive up to 12 cycles of either the experimental treatment FOLFOXIRI (fluorouracil plus leucovorin and irinotecan and oxaliplatin) plus bevacizumab (252 patients) or the control treatment FOLFIRI (fluorouracil plus leucovorin and irinotecan) plus bevacizumab (256 patients). Both study groups then received maintenance therapy with fluorouracil plus bevacizumab until the cancer progressed or they withdrew from the study.
After a median follow-up of 32 months (range, 24.7-40.6 months), the primary outcome measure, progression-free survival, was 12.1 months with the experimental therapy, which was significantly longer than the 9.7 months in the control group (hazard ratio, 0.75) was. In addition, the treatment response rate was 12% higher and median overall survival was 5 months longer in the experimental group, the investigators said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1403108]).
The percentage of bevacizumab-related adverse events was not significantly different between the two study groups, which indicates that intensifying the chemotherapy doesn’t affect the safety profile of the antiangiogenic agent. The rate of adverse events was significantly higher in the experimental-therapy group, but the rate of serious adverse events was similar (20.4% with FOLFOXIRI and 19.7% with FOLFIRI), as was the number of patients who died from adverse events (6 and 4, respectively).
Initial treatment with FOLFOXIRI plus bevacizumab, rather than FOLFIRI plus bevacizumab, improved progression-free survival in adults with inoperable metastatic colorectal cancer participating in a phase III randomized clinical trial, according to a report published online Oct. 23 in the New England Journal of Medicine.
However, patients who received FOLFOXIRI developed more grade 3 or 4 adverse events such as neutropenia, diarrhea, stomatitis, and peripheral neuropathy. The trial assessed treatment efficacy but not quality of life, so the impact of these toxicities was not addressed, said Dr. Fotios Loupakis of the University of Pisa and his associates in the Triplet plus Bevacizumab (TRIBE) trial.
In the open-label study, patients with unresectable metastatic colorectal cancer who had not received chemotherapy or biologic therapy for their metastatic disease were treated at 34 medical centers across Italy. They were randomly assigned to receive up to 12 cycles of either the experimental treatment FOLFOXIRI (fluorouracil plus leucovorin and irinotecan and oxaliplatin) plus bevacizumab (252 patients) or the control treatment FOLFIRI (fluorouracil plus leucovorin and irinotecan) plus bevacizumab (256 patients). Both study groups then received maintenance therapy with fluorouracil plus bevacizumab until the cancer progressed or they withdrew from the study.
After a median follow-up of 32 months (range, 24.7-40.6 months), the primary outcome measure, progression-free survival, was 12.1 months with the experimental therapy, which was significantly longer than the 9.7 months in the control group (hazard ratio, 0.75) was. In addition, the treatment response rate was 12% higher and median overall survival was 5 months longer in the experimental group, the investigators said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1403108]).
The percentage of bevacizumab-related adverse events was not significantly different between the two study groups, which indicates that intensifying the chemotherapy doesn’t affect the safety profile of the antiangiogenic agent. The rate of adverse events was significantly higher in the experimental-therapy group, but the rate of serious adverse events was similar (20.4% with FOLFOXIRI and 19.7% with FOLFIRI), as was the number of patients who died from adverse events (6 and 4, respectively).
Initial treatment with FOLFOXIRI plus bevacizumab, rather than FOLFIRI plus bevacizumab, improved progression-free survival in adults with inoperable metastatic colorectal cancer participating in a phase III randomized clinical trial, according to a report published online Oct. 23 in the New England Journal of Medicine.
However, patients who received FOLFOXIRI developed more grade 3 or 4 adverse events such as neutropenia, diarrhea, stomatitis, and peripheral neuropathy. The trial assessed treatment efficacy but not quality of life, so the impact of these toxicities was not addressed, said Dr. Fotios Loupakis of the University of Pisa and his associates in the Triplet plus Bevacizumab (TRIBE) trial.
In the open-label study, patients with unresectable metastatic colorectal cancer who had not received chemotherapy or biologic therapy for their metastatic disease were treated at 34 medical centers across Italy. They were randomly assigned to receive up to 12 cycles of either the experimental treatment FOLFOXIRI (fluorouracil plus leucovorin and irinotecan and oxaliplatin) plus bevacizumab (252 patients) or the control treatment FOLFIRI (fluorouracil plus leucovorin and irinotecan) plus bevacizumab (256 patients). Both study groups then received maintenance therapy with fluorouracil plus bevacizumab until the cancer progressed or they withdrew from the study.
After a median follow-up of 32 months (range, 24.7-40.6 months), the primary outcome measure, progression-free survival, was 12.1 months with the experimental therapy, which was significantly longer than the 9.7 months in the control group (hazard ratio, 0.75) was. In addition, the treatment response rate was 12% higher and median overall survival was 5 months longer in the experimental group, the investigators said (N. Engl. J. Med. 2014 Oct. 23 [doi:10.1056/NEJMoa1403108]).
The percentage of bevacizumab-related adverse events was not significantly different between the two study groups, which indicates that intensifying the chemotherapy doesn’t affect the safety profile of the antiangiogenic agent. The rate of adverse events was significantly higher in the experimental-therapy group, but the rate of serious adverse events was similar (20.4% with FOLFOXIRI and 19.7% with FOLFIRI), as was the number of patients who died from adverse events (6 and 4, respectively).
Key clinical point: Initial therapy with FOLFOXIRI + bevacizumab improved progression-free survival in metastatic colorectal cancer.
Major finding: After a median follow-up of 32 months, the primary outcome measure, progression-free survival, was 12.1 months with the experimental therapy and 9.7 months in the control group (HR, 0.75).
Data source: A phase III randomized open-label multicenter clinical trial in Italy, involving 508 adults with metastatic colorectal cancer who were followed for a median of 32 months.
Disclosures: This trial was supported by the Gruppo Oncologico Nord Ovest, the ARCO Foundation, and Hoffmann-La Roche. Dr. Loupakis reported ties to Roche, Amgen, Sanofi-Aventis, Bayer, and Merck Serono, and his associates reported ties to numerous industry sources.