User login
Pediatric Dermatology Consult - August 2017
BY AYAN KUSARI AND CATALINA MATIZ, MD
The patient was diagnosed with eruptive vellus hair cysts (EVHC). Treatment with a keratolytic, such as 12% lactic acid cream, was recommended. Hydrocortisone 2.5% once daily as needed also was recommended to treat the patient’s itch.
EVHC are benign middermal cysts characterized by epidermoid keratinization of the cyst wall, as well as lamellar keratin and vellus hairs within the cyst.1 The term “eruptive vellus hair cysts” was first used to describe a longstanding hyperpigmented, monomorphous papular eruption in two children by Esterly, Fretzin, and Pinkus in 1977.2 Clinically, EVHC present as 1- to 3-mm follicular, dome-shaped papules that are often skin-colored but also have been described as being brown, gray, green or black colored.3,4 They appear suddenly and sometimes are associated with mild tenderness and pruritus.1,5 EVHC most commonly present on the anterior chest but also can present on the upper and lower extremities, face, neck, axillae, and buttocks.4
Furthermore, although spontaneous resolution is possible through transepidermal elimination of cyst products, cases may persist for years in the absence of treatment.1
Accurate diagnosis of eruptive vellus hair cysts is important to guide therapy.
Keratosis pilaris consists of follicular-based papules with variable erythema.4 It may be widespread – including over the anterior chest – but is most commonly seen on the cheeks, extensor surfaces of proximal upper extremities, and the anterior thighs.4 It is related to excessive keratinization, which leads to formation of horny plugs within hair-follicle orifices.1
Steatocystoma multiplex is typically characterized by firm, yellow-to-flesh–colored dermal cysts ranging from a few millimeters to 1 cm in size.1 They are sometimes clinically hard to distinguish from EVHC, and both are associated with keratin 17 gene mutations and type 2 pachyonychia congenita.1 Nonetheless, this patient’s lesions did not have any features – such as size or drainage – that would point toward steatocystoma multiplex or other skin findings suggestive of pachyonychia congenita.
Superficial folliculitis, also known as Bockhart’s impetigo, is an infection of the follicular ostium and typically presents with perifollicular pustules on an erythematous base that may be painful or pruritic and can occur throughout the corpus, including the anterior trunk.1
Acne vulgaris is a very common disease that involves the pilosebaceous unit and occurs most frequently on the face, back, upper arms, and chest. However, it is characterized by open and closed comedones, papules, and pustules and, in severe cases, nodules and cysts that may leave postinflammatory hyperpigmentation and scarring. Tiny, hyperpigmented, dome-shaped macules occurring exclusively on the chest would not be characteristic.
Patients with hypohidrotic ectodermal dysplasia, also known as Christ-Siemens-Touraine syndrome, can present with EVHC. This condition is characterized by a triad of fair, sparse short hair; hyperthermia related to decreased sweating; and missing teeth.4 Although EVHC have been reported in association with hypohidrotic ectodermal dysplasia, this patient does not have any of the dysmorphic features associated with this syndrome.11
Patients with pachyonychia congenita (type 2) also may have EVHC as part of their presentation, but this patient does not have nail dystrophy, focal palmoplantar keratoderma, follicular keratoses, or multiple steatocysts which also are features of this condition.4
Treatment may be offered to patients who are distressed by the lesions or seek cosmesis. A 2012 review of 220 cases of EVHC found that topical retinoic acid, incision/excision, CO2 laser, erbium:yttrium-aluminum-garnet laser, needle evacuation, dermabrasion, and 10% urea cream were each associated with successful treatment in multiple cases.3
Forty years have passed since EVHC was identified as a distinct disease entity. Despite this, eruptive vellus hair cysts remains somewhat understudied, and further research is needed to determine an ideal treatment algorithm for patients with this condition. Our approach was to attempt noninvasive keratolytic therapy before considering retinoids or surgical options; we also recommended steroid treatment for symptom relief. Providers should keep EVHC in the differential for eruptions consisting of tiny papules so that appropriate treatment may be offered.
Dr. Matiz is a pediatric dermatologist at Rady Children’s Hospital, San Diego, and an assistant clinical professor in the department of pediatric and adolescent dermatology at the University of California, San Diego. Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures.
Email them at pdnews@frontlinemedcom.com.
References
1. “Dermatology.” 3rd ed. (Philadelphia: Saunders, 2012).
2. Arch Dermatol. 1977 Apr;113(4):500-3.
3. Am J Clin Dermatol. 2012 Feb 1;13(1):19-28.
5. Indian Dermatol Online J. 2013 Jul;4(3):213-5.
6. J Am Acad Dermatol. 1980 Oct;3(4):425-9.
7. Am J Dermatopathol. 1997 Jun;19(3):250-3.
8. Hum Mol Genet. 1998 Jul;7(7):1143-8.
9. Dermatology. 1998;196(4):392-6.
BY AYAN KUSARI AND CATALINA MATIZ, MD
The patient was diagnosed with eruptive vellus hair cysts (EVHC). Treatment with a keratolytic, such as 12% lactic acid cream, was recommended. Hydrocortisone 2.5% once daily as needed also was recommended to treat the patient’s itch.
EVHC are benign middermal cysts characterized by epidermoid keratinization of the cyst wall, as well as lamellar keratin and vellus hairs within the cyst.1 The term “eruptive vellus hair cysts” was first used to describe a longstanding hyperpigmented, monomorphous papular eruption in two children by Esterly, Fretzin, and Pinkus in 1977.2 Clinically, EVHC present as 1- to 3-mm follicular, dome-shaped papules that are often skin-colored but also have been described as being brown, gray, green or black colored.3,4 They appear suddenly and sometimes are associated with mild tenderness and pruritus.1,5 EVHC most commonly present on the anterior chest but also can present on the upper and lower extremities, face, neck, axillae, and buttocks.4
Furthermore, although spontaneous resolution is possible through transepidermal elimination of cyst products, cases may persist for years in the absence of treatment.1
Accurate diagnosis of eruptive vellus hair cysts is important to guide therapy.
Keratosis pilaris consists of follicular-based papules with variable erythema.4 It may be widespread – including over the anterior chest – but is most commonly seen on the cheeks, extensor surfaces of proximal upper extremities, and the anterior thighs.4 It is related to excessive keratinization, which leads to formation of horny plugs within hair-follicle orifices.1
Steatocystoma multiplex is typically characterized by firm, yellow-to-flesh–colored dermal cysts ranging from a few millimeters to 1 cm in size.1 They are sometimes clinically hard to distinguish from EVHC, and both are associated with keratin 17 gene mutations and type 2 pachyonychia congenita.1 Nonetheless, this patient’s lesions did not have any features – such as size or drainage – that would point toward steatocystoma multiplex or other skin findings suggestive of pachyonychia congenita.
Superficial folliculitis, also known as Bockhart’s impetigo, is an infection of the follicular ostium and typically presents with perifollicular pustules on an erythematous base that may be painful or pruritic and can occur throughout the corpus, including the anterior trunk.1
Acne vulgaris is a very common disease that involves the pilosebaceous unit and occurs most frequently on the face, back, upper arms, and chest. However, it is characterized by open and closed comedones, papules, and pustules and, in severe cases, nodules and cysts that may leave postinflammatory hyperpigmentation and scarring. Tiny, hyperpigmented, dome-shaped macules occurring exclusively on the chest would not be characteristic.
Patients with hypohidrotic ectodermal dysplasia, also known as Christ-Siemens-Touraine syndrome, can present with EVHC. This condition is characterized by a triad of fair, sparse short hair; hyperthermia related to decreased sweating; and missing teeth.4 Although EVHC have been reported in association with hypohidrotic ectodermal dysplasia, this patient does not have any of the dysmorphic features associated with this syndrome.11
Patients with pachyonychia congenita (type 2) also may have EVHC as part of their presentation, but this patient does not have nail dystrophy, focal palmoplantar keratoderma, follicular keratoses, or multiple steatocysts which also are features of this condition.4
Treatment may be offered to patients who are distressed by the lesions or seek cosmesis. A 2012 review of 220 cases of EVHC found that topical retinoic acid, incision/excision, CO2 laser, erbium:yttrium-aluminum-garnet laser, needle evacuation, dermabrasion, and 10% urea cream were each associated with successful treatment in multiple cases.3
Forty years have passed since EVHC was identified as a distinct disease entity. Despite this, eruptive vellus hair cysts remains somewhat understudied, and further research is needed to determine an ideal treatment algorithm for patients with this condition. Our approach was to attempt noninvasive keratolytic therapy before considering retinoids or surgical options; we also recommended steroid treatment for symptom relief. Providers should keep EVHC in the differential for eruptions consisting of tiny papules so that appropriate treatment may be offered.
Dr. Matiz is a pediatric dermatologist at Rady Children’s Hospital, San Diego, and an assistant clinical professor in the department of pediatric and adolescent dermatology at the University of California, San Diego. Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures.
Email them at pdnews@frontlinemedcom.com.
References
1. “Dermatology.” 3rd ed. (Philadelphia: Saunders, 2012).
2. Arch Dermatol. 1977 Apr;113(4):500-3.
3. Am J Clin Dermatol. 2012 Feb 1;13(1):19-28.
5. Indian Dermatol Online J. 2013 Jul;4(3):213-5.
6. J Am Acad Dermatol. 1980 Oct;3(4):425-9.
7. Am J Dermatopathol. 1997 Jun;19(3):250-3.
8. Hum Mol Genet. 1998 Jul;7(7):1143-8.
9. Dermatology. 1998;196(4):392-6.
BY AYAN KUSARI AND CATALINA MATIZ, MD
The patient was diagnosed with eruptive vellus hair cysts (EVHC). Treatment with a keratolytic, such as 12% lactic acid cream, was recommended. Hydrocortisone 2.5% once daily as needed also was recommended to treat the patient’s itch.
EVHC are benign middermal cysts characterized by epidermoid keratinization of the cyst wall, as well as lamellar keratin and vellus hairs within the cyst.1 The term “eruptive vellus hair cysts” was first used to describe a longstanding hyperpigmented, monomorphous papular eruption in two children by Esterly, Fretzin, and Pinkus in 1977.2 Clinically, EVHC present as 1- to 3-mm follicular, dome-shaped papules that are often skin-colored but also have been described as being brown, gray, green or black colored.3,4 They appear suddenly and sometimes are associated with mild tenderness and pruritus.1,5 EVHC most commonly present on the anterior chest but also can present on the upper and lower extremities, face, neck, axillae, and buttocks.4
Furthermore, although spontaneous resolution is possible through transepidermal elimination of cyst products, cases may persist for years in the absence of treatment.1
Accurate diagnosis of eruptive vellus hair cysts is important to guide therapy.
Keratosis pilaris consists of follicular-based papules with variable erythema.4 It may be widespread – including over the anterior chest – but is most commonly seen on the cheeks, extensor surfaces of proximal upper extremities, and the anterior thighs.4 It is related to excessive keratinization, which leads to formation of horny plugs within hair-follicle orifices.1
Steatocystoma multiplex is typically characterized by firm, yellow-to-flesh–colored dermal cysts ranging from a few millimeters to 1 cm in size.1 They are sometimes clinically hard to distinguish from EVHC, and both are associated with keratin 17 gene mutations and type 2 pachyonychia congenita.1 Nonetheless, this patient’s lesions did not have any features – such as size or drainage – that would point toward steatocystoma multiplex or other skin findings suggestive of pachyonychia congenita.
Superficial folliculitis, also known as Bockhart’s impetigo, is an infection of the follicular ostium and typically presents with perifollicular pustules on an erythematous base that may be painful or pruritic and can occur throughout the corpus, including the anterior trunk.1
Acne vulgaris is a very common disease that involves the pilosebaceous unit and occurs most frequently on the face, back, upper arms, and chest. However, it is characterized by open and closed comedones, papules, and pustules and, in severe cases, nodules and cysts that may leave postinflammatory hyperpigmentation and scarring. Tiny, hyperpigmented, dome-shaped macules occurring exclusively on the chest would not be characteristic.
Patients with hypohidrotic ectodermal dysplasia, also known as Christ-Siemens-Touraine syndrome, can present with EVHC. This condition is characterized by a triad of fair, sparse short hair; hyperthermia related to decreased sweating; and missing teeth.4 Although EVHC have been reported in association with hypohidrotic ectodermal dysplasia, this patient does not have any of the dysmorphic features associated with this syndrome.11
Patients with pachyonychia congenita (type 2) also may have EVHC as part of their presentation, but this patient does not have nail dystrophy, focal palmoplantar keratoderma, follicular keratoses, or multiple steatocysts which also are features of this condition.4
Treatment may be offered to patients who are distressed by the lesions or seek cosmesis. A 2012 review of 220 cases of EVHC found that topical retinoic acid, incision/excision, CO2 laser, erbium:yttrium-aluminum-garnet laser, needle evacuation, dermabrasion, and 10% urea cream were each associated with successful treatment in multiple cases.3
Forty years have passed since EVHC was identified as a distinct disease entity. Despite this, eruptive vellus hair cysts remains somewhat understudied, and further research is needed to determine an ideal treatment algorithm for patients with this condition. Our approach was to attempt noninvasive keratolytic therapy before considering retinoids or surgical options; we also recommended steroid treatment for symptom relief. Providers should keep EVHC in the differential for eruptions consisting of tiny papules so that appropriate treatment may be offered.
Dr. Matiz is a pediatric dermatologist at Rady Children’s Hospital, San Diego, and an assistant clinical professor in the department of pediatric and adolescent dermatology at the University of California, San Diego. Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures.
Email them at pdnews@frontlinemedcom.com.
References
1. “Dermatology.” 3rd ed. (Philadelphia: Saunders, 2012).
2. Arch Dermatol. 1977 Apr;113(4):500-3.
3. Am J Clin Dermatol. 2012 Feb 1;13(1):19-28.
5. Indian Dermatol Online J. 2013 Jul;4(3):213-5.
6. J Am Acad Dermatol. 1980 Oct;3(4):425-9.
7. Am J Dermatopathol. 1997 Jun;19(3):250-3.
8. Hum Mol Genet. 1998 Jul;7(7):1143-8.
9. Dermatology. 1998;196(4):392-6.
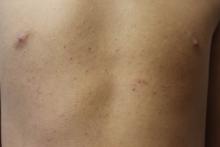
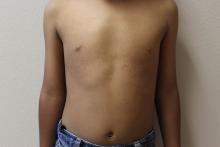
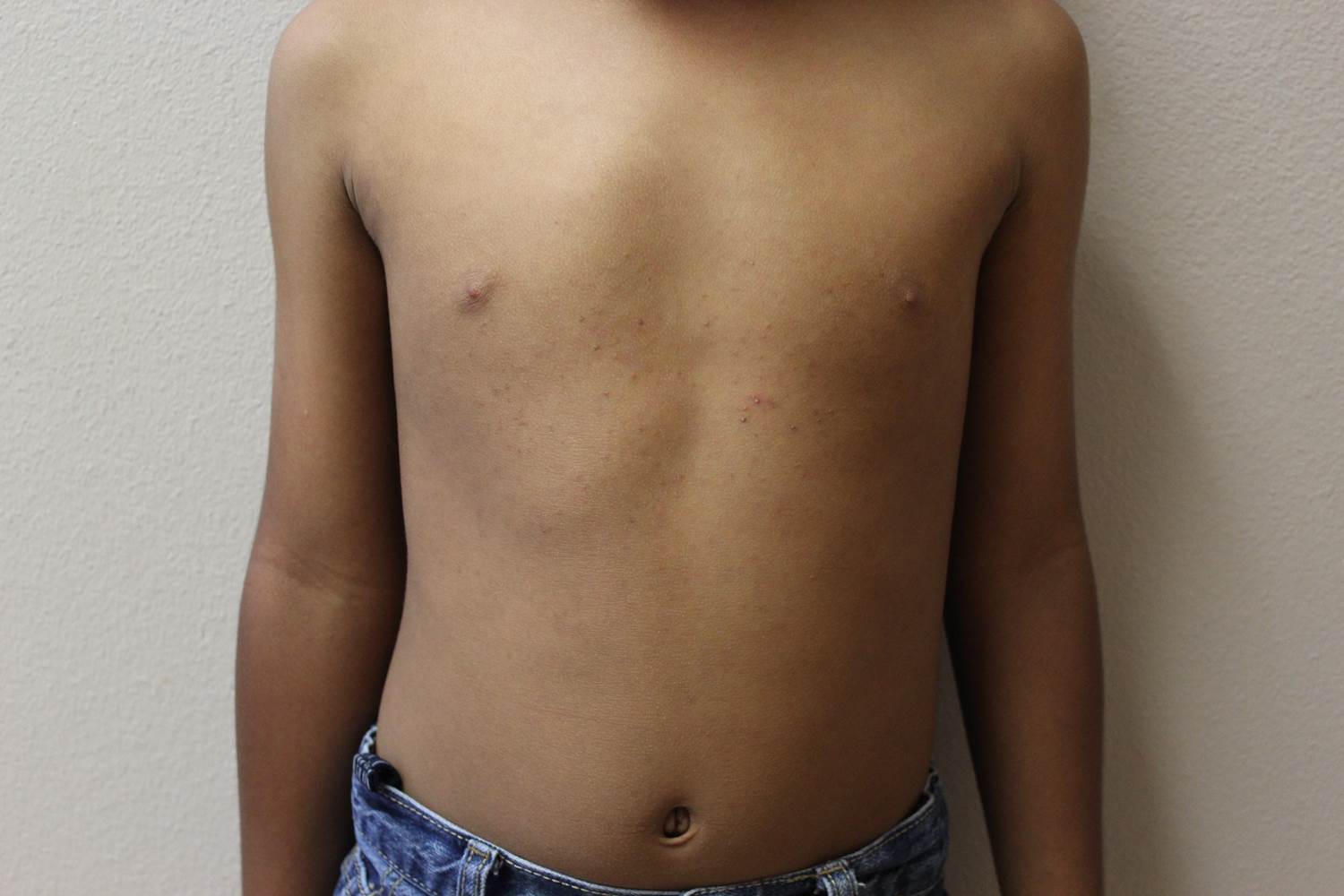
A 6-year-old boy presents with bumps on his chest and lower abdomen that have been present for 6 months. The patient’s mother states that the bumps are occasionally pruritic but not painful. She reports that the bumps first appeared on the chest and subsequently spread downward to involve the upper abdomen.
The patient is otherwise healthy. No similar lesions are present beyond the trunk. The patient’s past medical history and developmental history are unremarkable aside from bilateral amblyopia and high myopia. The patient’s mother denies any other family members with similar lesions. There is no history of teeth or nail abnormalities.
On exam, you find symmetrically distributed, firm, nontender, tiny 1- to 2-mm hyperpigmented dome-shaped papules on the anterior chest with no similar lesions elsewhere on the body. The remainder of the physical exam discloses no abnormalities.
Florence A. Blanchfield: A Lifetime of Nursing Leadership
The U.S. Army hospital at Fort Campbell, Kentucky, was named for army nurse, Colonel Florence A. Blanchfield—making it the only current army hospital named for a nurse.
Florence Aby Blanchfield was born into a large family in Shepherdstown, West Virginia, in 1882. Her mother was a nurse, and her father was a mason and stonecutter. She grew up in Oranda, Virginia, and attended both public and private schools. Following in her mother’s footsteps to become a nurse, she attended Southside Hospital Training School in Pittsburgh, Pennsylvania, and graduated in 1906. She moved to Baltimore after graduation and worked with Howard Atwood Kelly, one of the “Big Four” along with William Osler, William Henry Welch, and William Stewart Halsted who were known as the founding physicians of the Johns Hopkins Hospital.
After what must have been a remarkable experience with the innovative Kelly (inventor of many groundbreaking medical instruments and procedures, including the Kelly clamp), Blanchfield returned to Pittsburgh. She held positions of increasing responsibility over several years, including operating room supervisor at Southside Hospital and Montefiore Hospital and superintendent of the training school at Suburban General Hospital. Looking for adventure as well as service, she gave up her positions of leadership and headed to Panama in 1913 to become an operating room nurse and an anesthetist at Ancon Hospital in the U.S. Canal Zone.
As America prepared for its probable entry into World War I, Blanchfield joined the Army Nurse Corps (ANC) at age 35 to serve with the Medical School Unit of the University of Pittsburgh’s Base Hospital 27. She arrived in France in October 1917 and became acting chief nurse of Base Hospital 27 in Angers, Maine et Loire department. She also served as acting chief nurse of Camp Hospital 15 at Coëtquidan, Ille et Vil department.
Blanchfield returned to civilian life following World War I for a short period but returned to active duty in 1920. Over the next 15 years, she had several assignments within the continental U.S. and overseas in the Philippines and in Tianjin, China (formally known in English as Tientsin). In 1935, Blanchfield joined the Office of the Army Surgeon General in Washington, DC, where she was assigned to work on personnel matters in the office of the superintendent of the ANC. She became assistant superintendent in 1939, acting superintendent in 1942, and served as superintendent from June 1943 until September 1947. During World War II, she presided over the growth of the ANC from about 7,000 nurses on the day Pearl Harbor was attacked to more than 50,000 by the end of the war. She was awarded the Distinguished Service Medal for her contributions and accomplishments during World War II.
Blanchfield, a long-time senior leader in the ANC, was instrumental in many of the significant changes that took place during and after World War II, including nurses gaining full rank and benefits. This was an incremental process that culminated with passage of the Army and Navy Nurse Corps Act of April 1947, with nurses being granted full commissioned status. As a result of this act, she became a lieutenant colonel and the first woman to receive a commission in the regular army.
Blanchfield remained active in national nursing affairs after her retirement from the U.S. Army. At a time when many believed that nurses did not need specialty training, she promoted the establishment of specialized courses of study. In 1951, she received the Florence Nightingale Medal of the International Red Cross.
Blanchfield died on May 12, 1971, and was buried in the nurse’s section of Arlington National Cemetery with full military honors. In 1978, ANC leadership began a drive to memorialize Blanchfield by naming the new hospital at Fort Campbell, Kentucky, in her honor. A successful letter writing campaign by army nurses inundated the senior commander at Fort Campbell. The Colonel Florence A. Blanchfield Army Community Hospital, which was dedicated in her memory on September 17, 1982.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual your facility was named for or to offer a topic suggestion, contact us at fedprac@frontlinemedcom.com or on Facebook.
The U.S. Army hospital at Fort Campbell, Kentucky, was named for army nurse, Colonel Florence A. Blanchfield—making it the only current army hospital named for a nurse.
Florence Aby Blanchfield was born into a large family in Shepherdstown, West Virginia, in 1882. Her mother was a nurse, and her father was a mason and stonecutter. She grew up in Oranda, Virginia, and attended both public and private schools. Following in her mother’s footsteps to become a nurse, she attended Southside Hospital Training School in Pittsburgh, Pennsylvania, and graduated in 1906. She moved to Baltimore after graduation and worked with Howard Atwood Kelly, one of the “Big Four” along with William Osler, William Henry Welch, and William Stewart Halsted who were known as the founding physicians of the Johns Hopkins Hospital.
After what must have been a remarkable experience with the innovative Kelly (inventor of many groundbreaking medical instruments and procedures, including the Kelly clamp), Blanchfield returned to Pittsburgh. She held positions of increasing responsibility over several years, including operating room supervisor at Southside Hospital and Montefiore Hospital and superintendent of the training school at Suburban General Hospital. Looking for adventure as well as service, she gave up her positions of leadership and headed to Panama in 1913 to become an operating room nurse and an anesthetist at Ancon Hospital in the U.S. Canal Zone.
As America prepared for its probable entry into World War I, Blanchfield joined the Army Nurse Corps (ANC) at age 35 to serve with the Medical School Unit of the University of Pittsburgh’s Base Hospital 27. She arrived in France in October 1917 and became acting chief nurse of Base Hospital 27 in Angers, Maine et Loire department. She also served as acting chief nurse of Camp Hospital 15 at Coëtquidan, Ille et Vil department.
Blanchfield returned to civilian life following World War I for a short period but returned to active duty in 1920. Over the next 15 years, she had several assignments within the continental U.S. and overseas in the Philippines and in Tianjin, China (formally known in English as Tientsin). In 1935, Blanchfield joined the Office of the Army Surgeon General in Washington, DC, where she was assigned to work on personnel matters in the office of the superintendent of the ANC. She became assistant superintendent in 1939, acting superintendent in 1942, and served as superintendent from June 1943 until September 1947. During World War II, she presided over the growth of the ANC from about 7,000 nurses on the day Pearl Harbor was attacked to more than 50,000 by the end of the war. She was awarded the Distinguished Service Medal for her contributions and accomplishments during World War II.
Blanchfield, a long-time senior leader in the ANC, was instrumental in many of the significant changes that took place during and after World War II, including nurses gaining full rank and benefits. This was an incremental process that culminated with passage of the Army and Navy Nurse Corps Act of April 1947, with nurses being granted full commissioned status. As a result of this act, she became a lieutenant colonel and the first woman to receive a commission in the regular army.
Blanchfield remained active in national nursing affairs after her retirement from the U.S. Army. At a time when many believed that nurses did not need specialty training, she promoted the establishment of specialized courses of study. In 1951, she received the Florence Nightingale Medal of the International Red Cross.
Blanchfield died on May 12, 1971, and was buried in the nurse’s section of Arlington National Cemetery with full military honors. In 1978, ANC leadership began a drive to memorialize Blanchfield by naming the new hospital at Fort Campbell, Kentucky, in her honor. A successful letter writing campaign by army nurses inundated the senior commander at Fort Campbell. The Colonel Florence A. Blanchfield Army Community Hospital, which was dedicated in her memory on September 17, 1982.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual your facility was named for or to offer a topic suggestion, contact us at fedprac@frontlinemedcom.com or on Facebook.
The U.S. Army hospital at Fort Campbell, Kentucky, was named for army nurse, Colonel Florence A. Blanchfield—making it the only current army hospital named for a nurse.
Florence Aby Blanchfield was born into a large family in Shepherdstown, West Virginia, in 1882. Her mother was a nurse, and her father was a mason and stonecutter. She grew up in Oranda, Virginia, and attended both public and private schools. Following in her mother’s footsteps to become a nurse, she attended Southside Hospital Training School in Pittsburgh, Pennsylvania, and graduated in 1906. She moved to Baltimore after graduation and worked with Howard Atwood Kelly, one of the “Big Four” along with William Osler, William Henry Welch, and William Stewart Halsted who were known as the founding physicians of the Johns Hopkins Hospital.
After what must have been a remarkable experience with the innovative Kelly (inventor of many groundbreaking medical instruments and procedures, including the Kelly clamp), Blanchfield returned to Pittsburgh. She held positions of increasing responsibility over several years, including operating room supervisor at Southside Hospital and Montefiore Hospital and superintendent of the training school at Suburban General Hospital. Looking for adventure as well as service, she gave up her positions of leadership and headed to Panama in 1913 to become an operating room nurse and an anesthetist at Ancon Hospital in the U.S. Canal Zone.
As America prepared for its probable entry into World War I, Blanchfield joined the Army Nurse Corps (ANC) at age 35 to serve with the Medical School Unit of the University of Pittsburgh’s Base Hospital 27. She arrived in France in October 1917 and became acting chief nurse of Base Hospital 27 in Angers, Maine et Loire department. She also served as acting chief nurse of Camp Hospital 15 at Coëtquidan, Ille et Vil department.
Blanchfield returned to civilian life following World War I for a short period but returned to active duty in 1920. Over the next 15 years, she had several assignments within the continental U.S. and overseas in the Philippines and in Tianjin, China (formally known in English as Tientsin). In 1935, Blanchfield joined the Office of the Army Surgeon General in Washington, DC, where she was assigned to work on personnel matters in the office of the superintendent of the ANC. She became assistant superintendent in 1939, acting superintendent in 1942, and served as superintendent from June 1943 until September 1947. During World War II, she presided over the growth of the ANC from about 7,000 nurses on the day Pearl Harbor was attacked to more than 50,000 by the end of the war. She was awarded the Distinguished Service Medal for her contributions and accomplishments during World War II.
Blanchfield, a long-time senior leader in the ANC, was instrumental in many of the significant changes that took place during and after World War II, including nurses gaining full rank and benefits. This was an incremental process that culminated with passage of the Army and Navy Nurse Corps Act of April 1947, with nurses being granted full commissioned status. As a result of this act, she became a lieutenant colonel and the first woman to receive a commission in the regular army.
Blanchfield remained active in national nursing affairs after her retirement from the U.S. Army. At a time when many believed that nurses did not need specialty training, she promoted the establishment of specialized courses of study. In 1951, she received the Florence Nightingale Medal of the International Red Cross.
Blanchfield died on May 12, 1971, and was buried in the nurse’s section of Arlington National Cemetery with full military honors. In 1978, ANC leadership began a drive to memorialize Blanchfield by naming the new hospital at Fort Campbell, Kentucky, in her honor. A successful letter writing campaign by army nurses inundated the senior commander at Fort Campbell. The Colonel Florence A. Blanchfield Army Community Hospital, which was dedicated in her memory on September 17, 1982.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual your facility was named for or to offer a topic suggestion, contact us at fedprac@frontlinemedcom.com or on Facebook.
An ASCO 2017 recap: significant advances continue
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
Cartilage Restoration in the Patellofemoral Joint
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
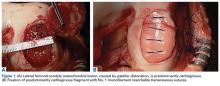
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
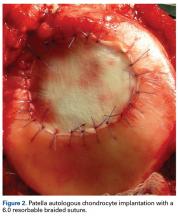
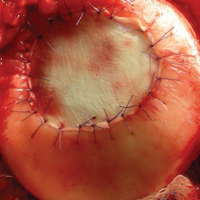
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
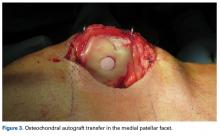
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
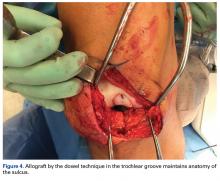
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
Ribociclib: another CDK inhibitor hits the mark in breast cancer
This spring, the US Food and Drug Administration approved a second cyclin-dependent kinase (CDK) inhibitor for the treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced/metastatic breast cancer in combination with aromatase inhibitors (AIs).1 The drug, ribociclib, joins palbociclib as the second drug in this class, which targets key regulators of the mammalian cell cycle and can help to overcome resistance to endocrine therapy–like AIs, a standard front-line treatment option in this group of patients. Palbociclib (Ibrance) was approved last year in combination with the AI letrozole, which was recently expanded to include its use in combination with all AIs, the same indication for which ribociclib received approval.
The ribociclib approval was based on the results of a phase 3, randomized, double-blind, placebo-controlled, international clinical trial called MONALEESA-2.2 The trial, conducted in 29 countries, compared the effects of ribociclib plus letrozole with letrozole plus placebo in 668 postmenopausal women with locally confirmed, HR-positive, HER2-negative, recurrent or metastatic breast cancer.
Patients had not received previous systemic therapy for advanced disease, had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (range, 1-5; 0, fully active and 5, dead), and had adequate bone marrow and organ function.
Patients were excluded if they had received previous CDK4/6 therapy, any previous systemic chemotherapy, endocrine therapy for advanced disease, previous neoadjuvant or adjuvant therapy with any nonsteroidal AI (unless they had been disease free for more than 12 months), and had inflammatory breast cancer, central nervous system metastases, history of cardiac disease or dysfunction, or impaired gastrointestinal function that alters drug absorption.
Patients were treated with ribociclib at a dose of 600 mg daily on a 3-weeks-on, 1-week-off schedule in 28-day cycles or placebo, which were combined with letrozole at a dose of 2.5 mg a day on a continuous schedule. Randomization was stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression, unacceptable toxicity, death or discontinuation of treatment. Dose reductions of ribociclib were allowed, to manage adverse events (AEs), but treatment crossover was not permitted.
Tumor assessments were performed at screening, every 8 weeks during the first 18 months, every 12 weeks thereafter until disease progression, and at the end of treatment, and were assessed by an independent review committee. The baseline characteristics of the patient population were well balanced; patients had a median age of 62 years, all were HR positive except 1 patient who was HER2 positive.
The trial was ended prematurely after an initial interim analysis demonstrated a significant benefit in favor of ribociclib in the primary endpoint, progression-free survival (PFS). Over a median duration of follow-up of 15.3 months, the median PFS was not yet reached in the ribociclib arm, compared with 14.7 months in the placebo arm (hazard ratio, 0.556; P < .0001). In a subsequent analysis with 11 months of additional follow-up, the median PFS was 25.3 months in the combination arm, compared with 16 months in the placebo arm, which translated into a 44% reduction in the risk of disease progression or death. The PFS benefit with ribociclib was observed across all preplanned subgroup analyses. The objective response rates were 52.7% in the ribociclib arm, compared with 37.1% in the placebo arm, but overall survival data were immature.
The frequency and severity of AEs were increased in the combination arm; most common were neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, and back pain. The most common grade 3 or 4 AEs experienced with ribociclib were neutropenia, leukopenia, abnormal liver function tests, lymphopenia, and vomiting.
Ribociclib is accompanied by warnings and precautions about QT interval prolongation, hepatobiliary toxicity, and neutropenia. Clinicians are advised to monitor electrocardiograms and electrolytes before the start of ribociclib therapy and to begin treatment only in patients with QTcF values <450 ms and in whom electrolyte abnormalities have been corrected. ECG should be repeated at around day 14 of the first cycle, the beginning of the second cycle, and as deemed clinically necessary.
Liver function tests should be performed before starting treatment, every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, and as clinically indicated. For aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels greater than 3-5 times the upper limit of normal (ULN, grade 2), ribociclib should be interrupted until recovery to baseline or lower. For levels >5-20 times the ULN (grade 3) or recurring grade 2 increases, treatment should be interrupted until recovery to baseline or lower and then resumed at the next lowest dose level. Treatment with ribociclib should be discontinued in the event of recurring grade 3 elevations or for AST/ALT elevations >3 times ULN in combination with total bilirubin >2 times ULN.
Complete blood counts should be performed before starting treatment and monitored every 2 weeks for the first 2 cycles, at the beginning of each of the 4 subsequent cycles, and as clinically needed. If absolute neutrophil counts are 500-1,000 mm3 (grade 3), treatment should be discontinued until recovery to grade 2 or lower. If grade 3 neutropenia recurs or for grade 3 febrile neutropenia or grade 4 neutropenia, treatment should resume at a lower dose level upon recovery to grade 2 or lower.
Pregnant women and those of reproductive age should be warned of the risk of fetal harm and the need for effective contraception during treatment and for at least 3 weeks after the last dose. Ribociclib is marketed as Kisqali by Novartis.
1. Ribociclib (Kisqali). US Food and Drug Administration website. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm546438.htm. Last updated March 14, 2017. Accessed April 3, 2017.
2. Kisqali (ribociclib) tables, for oral use. Prescribing information. Novartis Pharmaceuticals Corp. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf. March 2017. Accessed April 3, 2017.
3. Horobagyi GN, Stemmer SN, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-1748.
This spring, the US Food and Drug Administration approved a second cyclin-dependent kinase (CDK) inhibitor for the treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced/metastatic breast cancer in combination with aromatase inhibitors (AIs).1 The drug, ribociclib, joins palbociclib as the second drug in this class, which targets key regulators of the mammalian cell cycle and can help to overcome resistance to endocrine therapy–like AIs, a standard front-line treatment option in this group of patients. Palbociclib (Ibrance) was approved last year in combination with the AI letrozole, which was recently expanded to include its use in combination with all AIs, the same indication for which ribociclib received approval.
The ribociclib approval was based on the results of a phase 3, randomized, double-blind, placebo-controlled, international clinical trial called MONALEESA-2.2 The trial, conducted in 29 countries, compared the effects of ribociclib plus letrozole with letrozole plus placebo in 668 postmenopausal women with locally confirmed, HR-positive, HER2-negative, recurrent or metastatic breast cancer.
Patients had not received previous systemic therapy for advanced disease, had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (range, 1-5; 0, fully active and 5, dead), and had adequate bone marrow and organ function.
Patients were excluded if they had received previous CDK4/6 therapy, any previous systemic chemotherapy, endocrine therapy for advanced disease, previous neoadjuvant or adjuvant therapy with any nonsteroidal AI (unless they had been disease free for more than 12 months), and had inflammatory breast cancer, central nervous system metastases, history of cardiac disease or dysfunction, or impaired gastrointestinal function that alters drug absorption.
Patients were treated with ribociclib at a dose of 600 mg daily on a 3-weeks-on, 1-week-off schedule in 28-day cycles or placebo, which were combined with letrozole at a dose of 2.5 mg a day on a continuous schedule. Randomization was stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression, unacceptable toxicity, death or discontinuation of treatment. Dose reductions of ribociclib were allowed, to manage adverse events (AEs), but treatment crossover was not permitted.
Tumor assessments were performed at screening, every 8 weeks during the first 18 months, every 12 weeks thereafter until disease progression, and at the end of treatment, and were assessed by an independent review committee. The baseline characteristics of the patient population were well balanced; patients had a median age of 62 years, all were HR positive except 1 patient who was HER2 positive.
The trial was ended prematurely after an initial interim analysis demonstrated a significant benefit in favor of ribociclib in the primary endpoint, progression-free survival (PFS). Over a median duration of follow-up of 15.3 months, the median PFS was not yet reached in the ribociclib arm, compared with 14.7 months in the placebo arm (hazard ratio, 0.556; P < .0001). In a subsequent analysis with 11 months of additional follow-up, the median PFS was 25.3 months in the combination arm, compared with 16 months in the placebo arm, which translated into a 44% reduction in the risk of disease progression or death. The PFS benefit with ribociclib was observed across all preplanned subgroup analyses. The objective response rates were 52.7% in the ribociclib arm, compared with 37.1% in the placebo arm, but overall survival data were immature.
The frequency and severity of AEs were increased in the combination arm; most common were neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, and back pain. The most common grade 3 or 4 AEs experienced with ribociclib were neutropenia, leukopenia, abnormal liver function tests, lymphopenia, and vomiting.
Ribociclib is accompanied by warnings and precautions about QT interval prolongation, hepatobiliary toxicity, and neutropenia. Clinicians are advised to monitor electrocardiograms and electrolytes before the start of ribociclib therapy and to begin treatment only in patients with QTcF values <450 ms and in whom electrolyte abnormalities have been corrected. ECG should be repeated at around day 14 of the first cycle, the beginning of the second cycle, and as deemed clinically necessary.
Liver function tests should be performed before starting treatment, every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, and as clinically indicated. For aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels greater than 3-5 times the upper limit of normal (ULN, grade 2), ribociclib should be interrupted until recovery to baseline or lower. For levels >5-20 times the ULN (grade 3) or recurring grade 2 increases, treatment should be interrupted until recovery to baseline or lower and then resumed at the next lowest dose level. Treatment with ribociclib should be discontinued in the event of recurring grade 3 elevations or for AST/ALT elevations >3 times ULN in combination with total bilirubin >2 times ULN.
Complete blood counts should be performed before starting treatment and monitored every 2 weeks for the first 2 cycles, at the beginning of each of the 4 subsequent cycles, and as clinically needed. If absolute neutrophil counts are 500-1,000 mm3 (grade 3), treatment should be discontinued until recovery to grade 2 or lower. If grade 3 neutropenia recurs or for grade 3 febrile neutropenia or grade 4 neutropenia, treatment should resume at a lower dose level upon recovery to grade 2 or lower.
Pregnant women and those of reproductive age should be warned of the risk of fetal harm and the need for effective contraception during treatment and for at least 3 weeks after the last dose. Ribociclib is marketed as Kisqali by Novartis.
This spring, the US Food and Drug Administration approved a second cyclin-dependent kinase (CDK) inhibitor for the treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced/metastatic breast cancer in combination with aromatase inhibitors (AIs).1 The drug, ribociclib, joins palbociclib as the second drug in this class, which targets key regulators of the mammalian cell cycle and can help to overcome resistance to endocrine therapy–like AIs, a standard front-line treatment option in this group of patients. Palbociclib (Ibrance) was approved last year in combination with the AI letrozole, which was recently expanded to include its use in combination with all AIs, the same indication for which ribociclib received approval.
The ribociclib approval was based on the results of a phase 3, randomized, double-blind, placebo-controlled, international clinical trial called MONALEESA-2.2 The trial, conducted in 29 countries, compared the effects of ribociclib plus letrozole with letrozole plus placebo in 668 postmenopausal women with locally confirmed, HR-positive, HER2-negative, recurrent or metastatic breast cancer.
Patients had not received previous systemic therapy for advanced disease, had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (range, 1-5; 0, fully active and 5, dead), and had adequate bone marrow and organ function.
Patients were excluded if they had received previous CDK4/6 therapy, any previous systemic chemotherapy, endocrine therapy for advanced disease, previous neoadjuvant or adjuvant therapy with any nonsteroidal AI (unless they had been disease free for more than 12 months), and had inflammatory breast cancer, central nervous system metastases, history of cardiac disease or dysfunction, or impaired gastrointestinal function that alters drug absorption.
Patients were treated with ribociclib at a dose of 600 mg daily on a 3-weeks-on, 1-week-off schedule in 28-day cycles or placebo, which were combined with letrozole at a dose of 2.5 mg a day on a continuous schedule. Randomization was stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression, unacceptable toxicity, death or discontinuation of treatment. Dose reductions of ribociclib were allowed, to manage adverse events (AEs), but treatment crossover was not permitted.
Tumor assessments were performed at screening, every 8 weeks during the first 18 months, every 12 weeks thereafter until disease progression, and at the end of treatment, and were assessed by an independent review committee. The baseline characteristics of the patient population were well balanced; patients had a median age of 62 years, all were HR positive except 1 patient who was HER2 positive.
The trial was ended prematurely after an initial interim analysis demonstrated a significant benefit in favor of ribociclib in the primary endpoint, progression-free survival (PFS). Over a median duration of follow-up of 15.3 months, the median PFS was not yet reached in the ribociclib arm, compared with 14.7 months in the placebo arm (hazard ratio, 0.556; P < .0001). In a subsequent analysis with 11 months of additional follow-up, the median PFS was 25.3 months in the combination arm, compared with 16 months in the placebo arm, which translated into a 44% reduction in the risk of disease progression or death. The PFS benefit with ribociclib was observed across all preplanned subgroup analyses. The objective response rates were 52.7% in the ribociclib arm, compared with 37.1% in the placebo arm, but overall survival data were immature.
The frequency and severity of AEs were increased in the combination arm; most common were neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, and back pain. The most common grade 3 or 4 AEs experienced with ribociclib were neutropenia, leukopenia, abnormal liver function tests, lymphopenia, and vomiting.
Ribociclib is accompanied by warnings and precautions about QT interval prolongation, hepatobiliary toxicity, and neutropenia. Clinicians are advised to monitor electrocardiograms and electrolytes before the start of ribociclib therapy and to begin treatment only in patients with QTcF values <450 ms and in whom electrolyte abnormalities have been corrected. ECG should be repeated at around day 14 of the first cycle, the beginning of the second cycle, and as deemed clinically necessary.
Liver function tests should be performed before starting treatment, every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, and as clinically indicated. For aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels greater than 3-5 times the upper limit of normal (ULN, grade 2), ribociclib should be interrupted until recovery to baseline or lower. For levels >5-20 times the ULN (grade 3) or recurring grade 2 increases, treatment should be interrupted until recovery to baseline or lower and then resumed at the next lowest dose level. Treatment with ribociclib should be discontinued in the event of recurring grade 3 elevations or for AST/ALT elevations >3 times ULN in combination with total bilirubin >2 times ULN.
Complete blood counts should be performed before starting treatment and monitored every 2 weeks for the first 2 cycles, at the beginning of each of the 4 subsequent cycles, and as clinically needed. If absolute neutrophil counts are 500-1,000 mm3 (grade 3), treatment should be discontinued until recovery to grade 2 or lower. If grade 3 neutropenia recurs or for grade 3 febrile neutropenia or grade 4 neutropenia, treatment should resume at a lower dose level upon recovery to grade 2 or lower.
Pregnant women and those of reproductive age should be warned of the risk of fetal harm and the need for effective contraception during treatment and for at least 3 weeks after the last dose. Ribociclib is marketed as Kisqali by Novartis.
1. Ribociclib (Kisqali). US Food and Drug Administration website. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm546438.htm. Last updated March 14, 2017. Accessed April 3, 2017.
2. Kisqali (ribociclib) tables, for oral use. Prescribing information. Novartis Pharmaceuticals Corp. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf. March 2017. Accessed April 3, 2017.
3. Horobagyi GN, Stemmer SN, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-1748.
1. Ribociclib (Kisqali). US Food and Drug Administration website. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm546438.htm. Last updated March 14, 2017. Accessed April 3, 2017.
2. Kisqali (ribociclib) tables, for oral use. Prescribing information. Novartis Pharmaceuticals Corp. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf. March 2017. Accessed April 3, 2017.
3. Horobagyi GN, Stemmer SN, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-1748.
Approval makes olaratumab the first first-line treatment option for soft tissue sarcoma in more than 40 years
When the US Food and Drug Administration approved olaratumab as a first-line treatment for patients with soft tissue sarcoma (STS) in the fall of 2016, it marked the first approval since the chemotherapy drug doxorubicin became standard of care more than 40 years ago.1 Though rare, STS, which comprises a host of different histologic subtypes, has proven difficult to treat. Like pazopanib, which was approved in 2012 for the treatment of STS in the second-line setting, olaratumab targets the platelet-derived growth factor receptor alpha (PDGFRα), a tyrosine kinase receptor involved in cell signaling pathways that promotes key hallmark abilities in both cancer cells and the cells of the tumor microenvironment. Olaratumab, however, is a much more specific inhibitor of PDGFRα compared with pazopanib.
Accelerated approval was granted for the treatment of patients with STS that is not amenable to curative treatment with radiotherapy or surgery and with a subtype that cannot be treated effectively with an anthracycline-containing regimen. The approval was based on the phase 2 JGDG study, a randomized, active-controlled clinical trial in which 133 patients were randomized 1:1 to receive olaratumab plus doxorubicin, or doxorubicin alone.2
Eligible patients included those aged 18 years and over, with histologically confirmed diagnosis of locally advanced or metastatic STS not previously treated with an anthracycline, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 (range, 1-5; 0, fully active and 5, dead), and with available tumor tissue for determination of PDGFRα expression by immunohistochemistry. Patients were enrolled at 16 clinical sites in 16 cities and 15 states in the United States from October 2010 to January 2013.
Patients were excluded if they had histologically or cytologically confirmed Kaposi sarcoma; untreated central nervous system metastases; received prior treatment with doxorubicin or other anthracyclines and anthracenediones, or any drug targeting PDGF or the PDGFRs; received concurrent treatment with other anticancer therapy within 4 weeks before study entry; unstable angina pectoris, angioplasty, cardiac stenting, or myocardial infarction within 6 months before study entry; HIV infection; or if they were pregnant or lactating.
Olaratumab was administered at 15 mg/kg as an intravenous infusion on days 1 and 8 of each 21-day cycle, and doxorubicin at 75 mg/m2 as an intravenous infusion on day 1 of each cycle, for a maximum of 8 cycles. Patients were permitted to receive dexarozoxane on cycles 5-8 and crossover was permitted. Tumor response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) every 6 weeks, and survival assessed every 2 months, until study completion. PDGFR expression was assessed by immunohistochemistry at a central academic laboratory before randomization.
The primary endpoint of the study was progression-free survival (PFS) and the combination of olaratumab–doxorubicin significantly extended PFS in this patient population: median PFS was 6.6 months in the combination arm, compared with 4.1 months in the doxorubicin-alone arm (hazard ratio [HR], 0.672; P = .0615). The objective response rate (ORR) and median overall survival (OS), which were secondary endpoints in the trial, were also significantly improved with combination therapy compared with doxorubicin alone (ORR, 18.2% vs 11.9%, respectively; median OS, 26.5 months vs 14.7 months). The benefits of combination therapy were observed across prespecified subgroups, including histological tumor type, number of previous treatments, and PDGFRα expression level.
The most common adverse events (AEs) in the patients taking olaratumab were nausea, fatigue, neutropenia, musculoskeletal pain, mucositis, alopecia, vomiting, diarrhea, decreased appetite, abdominal pain, neuropathy, and headache. Grade 3/4 AEs were also higher for the combination than for doxorubicin alone. The most common AE leading to discontinuation of olaratumab was infusion-related reactions, which occurred in 13% of patients.
According to the prescribing information, the recommended dose for olaratumab is 15 mg/kg as an intravenous infusion over 60 minutes on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity, in combination with doxorubicin for the first 8 cycles. Patients should be premedicated with dexamethasone and diphenhydramine, to help protect against infusion-related reactions.
Olaratumab, marketed as Lartruvo by Lilly Oncology, has warnings and precautions relating to infusion-related reactions and embryofetal toxicity. Patients should be monitored for signs and symptoms of the former during and after infusion and olaratumab should be administered in a setting with available resuscitation equipment. Olaratumab should be permanently discontinued in the event of grade 3/4 infusion-related reactions. Olaratumab can cause fetal harm and female patients should be advised of the potential risk to a fetus and the need for effective contraception during treatment and for 3 months after the last dose.
1. FDA grants accelerated approval to new treatment for advanced soft tissue sarcoma. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm525878.htm. Last updated October 19, 2016. Accessed March 6, 2017.
2. Tap WD, Jones RL, Van Tine BA, et al. Olaratumumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488-497.
3. Lartruvo (olaratumumab) injection, for intravenous use. Prescribing information. Eli Lilly and Co. http://pi.lilly.com/us/lartruvo-uspi.pdf. Last update October 2016. Accessed March 6, 2017.
When the US Food and Drug Administration approved olaratumab as a first-line treatment for patients with soft tissue sarcoma (STS) in the fall of 2016, it marked the first approval since the chemotherapy drug doxorubicin became standard of care more than 40 years ago.1 Though rare, STS, which comprises a host of different histologic subtypes, has proven difficult to treat. Like pazopanib, which was approved in 2012 for the treatment of STS in the second-line setting, olaratumab targets the platelet-derived growth factor receptor alpha (PDGFRα), a tyrosine kinase receptor involved in cell signaling pathways that promotes key hallmark abilities in both cancer cells and the cells of the tumor microenvironment. Olaratumab, however, is a much more specific inhibitor of PDGFRα compared with pazopanib.
Accelerated approval was granted for the treatment of patients with STS that is not amenable to curative treatment with radiotherapy or surgery and with a subtype that cannot be treated effectively with an anthracycline-containing regimen. The approval was based on the phase 2 JGDG study, a randomized, active-controlled clinical trial in which 133 patients were randomized 1:1 to receive olaratumab plus doxorubicin, or doxorubicin alone.2
Eligible patients included those aged 18 years and over, with histologically confirmed diagnosis of locally advanced or metastatic STS not previously treated with an anthracycline, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 (range, 1-5; 0, fully active and 5, dead), and with available tumor tissue for determination of PDGFRα expression by immunohistochemistry. Patients were enrolled at 16 clinical sites in 16 cities and 15 states in the United States from October 2010 to January 2013.
Patients were excluded if they had histologically or cytologically confirmed Kaposi sarcoma; untreated central nervous system metastases; received prior treatment with doxorubicin or other anthracyclines and anthracenediones, or any drug targeting PDGF or the PDGFRs; received concurrent treatment with other anticancer therapy within 4 weeks before study entry; unstable angina pectoris, angioplasty, cardiac stenting, or myocardial infarction within 6 months before study entry; HIV infection; or if they were pregnant or lactating.
Olaratumab was administered at 15 mg/kg as an intravenous infusion on days 1 and 8 of each 21-day cycle, and doxorubicin at 75 mg/m2 as an intravenous infusion on day 1 of each cycle, for a maximum of 8 cycles. Patients were permitted to receive dexarozoxane on cycles 5-8 and crossover was permitted. Tumor response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) every 6 weeks, and survival assessed every 2 months, until study completion. PDGFR expression was assessed by immunohistochemistry at a central academic laboratory before randomization.
The primary endpoint of the study was progression-free survival (PFS) and the combination of olaratumab–doxorubicin significantly extended PFS in this patient population: median PFS was 6.6 months in the combination arm, compared with 4.1 months in the doxorubicin-alone arm (hazard ratio [HR], 0.672; P = .0615). The objective response rate (ORR) and median overall survival (OS), which were secondary endpoints in the trial, were also significantly improved with combination therapy compared with doxorubicin alone (ORR, 18.2% vs 11.9%, respectively; median OS, 26.5 months vs 14.7 months). The benefits of combination therapy were observed across prespecified subgroups, including histological tumor type, number of previous treatments, and PDGFRα expression level.
The most common adverse events (AEs) in the patients taking olaratumab were nausea, fatigue, neutropenia, musculoskeletal pain, mucositis, alopecia, vomiting, diarrhea, decreased appetite, abdominal pain, neuropathy, and headache. Grade 3/4 AEs were also higher for the combination than for doxorubicin alone. The most common AE leading to discontinuation of olaratumab was infusion-related reactions, which occurred in 13% of patients.
According to the prescribing information, the recommended dose for olaratumab is 15 mg/kg as an intravenous infusion over 60 minutes on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity, in combination with doxorubicin for the first 8 cycles. Patients should be premedicated with dexamethasone and diphenhydramine, to help protect against infusion-related reactions.
Olaratumab, marketed as Lartruvo by Lilly Oncology, has warnings and precautions relating to infusion-related reactions and embryofetal toxicity. Patients should be monitored for signs and symptoms of the former during and after infusion and olaratumab should be administered in a setting with available resuscitation equipment. Olaratumab should be permanently discontinued in the event of grade 3/4 infusion-related reactions. Olaratumab can cause fetal harm and female patients should be advised of the potential risk to a fetus and the need for effective contraception during treatment and for 3 months after the last dose.
When the US Food and Drug Administration approved olaratumab as a first-line treatment for patients with soft tissue sarcoma (STS) in the fall of 2016, it marked the first approval since the chemotherapy drug doxorubicin became standard of care more than 40 years ago.1 Though rare, STS, which comprises a host of different histologic subtypes, has proven difficult to treat. Like pazopanib, which was approved in 2012 for the treatment of STS in the second-line setting, olaratumab targets the platelet-derived growth factor receptor alpha (PDGFRα), a tyrosine kinase receptor involved in cell signaling pathways that promotes key hallmark abilities in both cancer cells and the cells of the tumor microenvironment. Olaratumab, however, is a much more specific inhibitor of PDGFRα compared with pazopanib.
Accelerated approval was granted for the treatment of patients with STS that is not amenable to curative treatment with radiotherapy or surgery and with a subtype that cannot be treated effectively with an anthracycline-containing regimen. The approval was based on the phase 2 JGDG study, a randomized, active-controlled clinical trial in which 133 patients were randomized 1:1 to receive olaratumab plus doxorubicin, or doxorubicin alone.2
Eligible patients included those aged 18 years and over, with histologically confirmed diagnosis of locally advanced or metastatic STS not previously treated with an anthracycline, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 (range, 1-5; 0, fully active and 5, dead), and with available tumor tissue for determination of PDGFRα expression by immunohistochemistry. Patients were enrolled at 16 clinical sites in 16 cities and 15 states in the United States from October 2010 to January 2013.
Patients were excluded if they had histologically or cytologically confirmed Kaposi sarcoma; untreated central nervous system metastases; received prior treatment with doxorubicin or other anthracyclines and anthracenediones, or any drug targeting PDGF or the PDGFRs; received concurrent treatment with other anticancer therapy within 4 weeks before study entry; unstable angina pectoris, angioplasty, cardiac stenting, or myocardial infarction within 6 months before study entry; HIV infection; or if they were pregnant or lactating.
Olaratumab was administered at 15 mg/kg as an intravenous infusion on days 1 and 8 of each 21-day cycle, and doxorubicin at 75 mg/m2 as an intravenous infusion on day 1 of each cycle, for a maximum of 8 cycles. Patients were permitted to receive dexarozoxane on cycles 5-8 and crossover was permitted. Tumor response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) every 6 weeks, and survival assessed every 2 months, until study completion. PDGFR expression was assessed by immunohistochemistry at a central academic laboratory before randomization.
The primary endpoint of the study was progression-free survival (PFS) and the combination of olaratumab–doxorubicin significantly extended PFS in this patient population: median PFS was 6.6 months in the combination arm, compared with 4.1 months in the doxorubicin-alone arm (hazard ratio [HR], 0.672; P = .0615). The objective response rate (ORR) and median overall survival (OS), which were secondary endpoints in the trial, were also significantly improved with combination therapy compared with doxorubicin alone (ORR, 18.2% vs 11.9%, respectively; median OS, 26.5 months vs 14.7 months). The benefits of combination therapy were observed across prespecified subgroups, including histological tumor type, number of previous treatments, and PDGFRα expression level.
The most common adverse events (AEs) in the patients taking olaratumab were nausea, fatigue, neutropenia, musculoskeletal pain, mucositis, alopecia, vomiting, diarrhea, decreased appetite, abdominal pain, neuropathy, and headache. Grade 3/4 AEs were also higher for the combination than for doxorubicin alone. The most common AE leading to discontinuation of olaratumab was infusion-related reactions, which occurred in 13% of patients.
According to the prescribing information, the recommended dose for olaratumab is 15 mg/kg as an intravenous infusion over 60 minutes on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity, in combination with doxorubicin for the first 8 cycles. Patients should be premedicated with dexamethasone and diphenhydramine, to help protect against infusion-related reactions.
Olaratumab, marketed as Lartruvo by Lilly Oncology, has warnings and precautions relating to infusion-related reactions and embryofetal toxicity. Patients should be monitored for signs and symptoms of the former during and after infusion and olaratumab should be administered in a setting with available resuscitation equipment. Olaratumab should be permanently discontinued in the event of grade 3/4 infusion-related reactions. Olaratumab can cause fetal harm and female patients should be advised of the potential risk to a fetus and the need for effective contraception during treatment and for 3 months after the last dose.
1. FDA grants accelerated approval to new treatment for advanced soft tissue sarcoma. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm525878.htm. Last updated October 19, 2016. Accessed March 6, 2017.
2. Tap WD, Jones RL, Van Tine BA, et al. Olaratumumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488-497.
3. Lartruvo (olaratumumab) injection, for intravenous use. Prescribing information. Eli Lilly and Co. http://pi.lilly.com/us/lartruvo-uspi.pdf. Last update October 2016. Accessed March 6, 2017.
1. FDA grants accelerated approval to new treatment for advanced soft tissue sarcoma. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm525878.htm. Last updated October 19, 2016. Accessed March 6, 2017.
2. Tap WD, Jones RL, Van Tine BA, et al. Olaratumumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488-497.
3. Lartruvo (olaratumumab) injection, for intravenous use. Prescribing information. Eli Lilly and Co. http://pi.lilly.com/us/lartruvo-uspi.pdf. Last update October 2016. Accessed March 6, 2017.
Multiple Myeloma: Updates on Diagnosis and Management
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level. There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy,
hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly
myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the
bone is destroyed, an MRI offers a more sensitive image.
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic
SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma.For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of
lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these
criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall
survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics,
which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require
therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to 3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients
with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS
and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either
be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared
with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that
could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib,
doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is
often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials
have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and
safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and
OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide
plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are
integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered
to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit
not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective
and well tolerated. There has been a general shift to using these agents upfront in transplant-ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered
in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay
before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as
neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refreactory/Relapsed Disease Treatments
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined
significance (MGUS) consistently precedes multiple myeloma: a prospective
study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed
multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal
Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney
injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop
Consensus Panel 3. Consensus recommendations for standard investigative workup:
report of the International Myeloma Workshop Consensus Panel 3. Blood.
2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working
Group consensus statement for the management, treatment, and supportive care
of patients with myeloma not eligible for standard autologous stem-cell transplantation.
J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working
Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol.
2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more
entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation
of measured myeloma cell mass with presenting clinical features, response to
treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple
myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival
in multiple myeloma: changes in early mortality and outcomes in older patients.
Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification,
and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis
in monoclonal gammopathy of undetermined significance. N Engl J Med.
2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering
(asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering
multiple myeloma: biological insights and early treatment strategies. Hematology
Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone
for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple
myeloma limited to high-risk subgroup identified by gene expression profiling.
Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes.
Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in
oncology: multiple myeloma. National Comprehensive Cancer Network Website.
http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated
March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study
(EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide,
and lenalidomide in previously untreated multiple myeloma. Blood.
2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus
reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment
before autologous stem cell transplantation in newly diagnosed multiple
myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous
administration of bortezomib in patients with relapsed multiple myeloma: a randomized,
phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in
multiple myeloma linked to bortezomib in total therapy 3: comparison with total
therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem
cell mobilization and engraftment post-peripheral blood stem cell transplantation
in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group.
Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell
collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining
regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis
for patients with newly diagnosed multiple myeloma patients treated with
lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in
combination with lenalidomide and low dose dexamethasone as a frontline treatment
for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of
carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended
dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed
multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide
plus dexamethasone in newly diagnosed multiple myeloma: a comparative
analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous
bone marrow transplantation and chemotherapy in multiple myeloma.
Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance
therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous
stem cell transplantation in multiple myeloma: up-front or rescue treatment?
Results of a multicenter sequential randomized clinical trial. Blood.
1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second
on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol.
2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the
FIRST (frontline investigation of lenalidomide + dexamethasone versus standard
thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma
(NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood.
2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan
and prednisone for initial treatment of multiple myeloma. N Engl J Med.
2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan
and prednisone plus thalidomide versus melphalan and prednisone
alone or reduced-intensity autologous stem cell transplantation in
elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet.
2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide
in patients older than 75 years with newly diagnosed multiple myeloma.
IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stemcell
transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome
(IFM). Maintenance therapy with thalidomide improves survival in patients with
multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose
thalidomide and prednisolone prolongs the survival of multiple myeloma patients
undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol.
2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and
maintenance treatment in patients with newly diagnosed multiple myeloma:
results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol.
2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome
Inhibition for Extending Remissions (APEX) Investigators. Bortezomib
or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med.
2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated
liposomal doxorubicin plus bortezomib compared with bortezomib alone
in relapsed or refractory multiple myeloma: combination therapy improves time
to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group.
Risk of progression and survival in multiple myeloma relapsing after therapy
with IMiDs and bortezomib: a multicenter international myeloma working group
study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus lowdose
dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol.
2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib
(PX-171-003-A1) in patients with relapsed and refractory multiple myeloma.
Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib
and dexamethasone versus placebo plus bortezomib and dexamethasone
in patients with relapsed or relapsed and refractory multiple
myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol.
2014;15(11):1195-1206.
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level. There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy,
hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly
myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the
bone is destroyed, an MRI offers a more sensitive image.
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic
SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma.For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of
lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these
criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall
survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics,
which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require
therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to 3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients
with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS
and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either
be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared
with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that
could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib,
doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is
often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials
have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and
safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and
OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide
plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are
integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered
to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit
not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective
and well tolerated. There has been a general shift to using these agents upfront in transplant-ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered
in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay
before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as
neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refreactory/Relapsed Disease Treatments
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level. There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy,
hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly
myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the
bone is destroyed, an MRI offers a more sensitive image.
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic
SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma.For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of
lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these
criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall
survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics,
which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require
therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to 3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients
with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS
and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either
be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared
with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that
could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib,
doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is
often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials
have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and
safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and
OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide
plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are
integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered
to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit
not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective
and well tolerated. There has been a general shift to using these agents upfront in transplant-ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered
in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay
before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as
neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refreactory/Relapsed Disease Treatments
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined
significance (MGUS) consistently precedes multiple myeloma: a prospective
study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed
multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal
Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney
injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop
Consensus Panel 3. Consensus recommendations for standard investigative workup:
report of the International Myeloma Workshop Consensus Panel 3. Blood.
2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working
Group consensus statement for the management, treatment, and supportive care
of patients with myeloma not eligible for standard autologous stem-cell transplantation.
J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working
Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol.
2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more
entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation
of measured myeloma cell mass with presenting clinical features, response to
treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple
myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival
in multiple myeloma: changes in early mortality and outcomes in older patients.
Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification,
and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis
in monoclonal gammopathy of undetermined significance. N Engl J Med.
2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering
(asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering
multiple myeloma: biological insights and early treatment strategies. Hematology
Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone
for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple
myeloma limited to high-risk subgroup identified by gene expression profiling.
Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes.
Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in
oncology: multiple myeloma. National Comprehensive Cancer Network Website.
http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated
March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study
(EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide,
and lenalidomide in previously untreated multiple myeloma. Blood.
2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus
reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment
before autologous stem cell transplantation in newly diagnosed multiple
myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous
administration of bortezomib in patients with relapsed multiple myeloma: a randomized,
phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in
multiple myeloma linked to bortezomib in total therapy 3: comparison with total
therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem
cell mobilization and engraftment post-peripheral blood stem cell transplantation
in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group.
Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell
collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining
regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis
for patients with newly diagnosed multiple myeloma patients treated with
lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in
combination with lenalidomide and low dose dexamethasone as a frontline treatment
for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of
carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended
dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed
multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide
plus dexamethasone in newly diagnosed multiple myeloma: a comparative
analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous
bone marrow transplantation and chemotherapy in multiple myeloma.
Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance
therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous
stem cell transplantation in multiple myeloma: up-front or rescue treatment?
Results of a multicenter sequential randomized clinical trial. Blood.
1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second
on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol.
2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the
FIRST (frontline investigation of lenalidomide + dexamethasone versus standard
thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma
(NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood.
2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan
and prednisone for initial treatment of multiple myeloma. N Engl J Med.
2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan
and prednisone plus thalidomide versus melphalan and prednisone
alone or reduced-intensity autologous stem cell transplantation in
elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet.
2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide
in patients older than 75 years with newly diagnosed multiple myeloma.
IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stemcell
transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome
(IFM). Maintenance therapy with thalidomide improves survival in patients with
multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose
thalidomide and prednisolone prolongs the survival of multiple myeloma patients
undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol.
2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and
maintenance treatment in patients with newly diagnosed multiple myeloma:
results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol.
2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome
Inhibition for Extending Remissions (APEX) Investigators. Bortezomib
or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med.
2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated
liposomal doxorubicin plus bortezomib compared with bortezomib alone
in relapsed or refractory multiple myeloma: combination therapy improves time
to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group.
Risk of progression and survival in multiple myeloma relapsing after therapy
with IMiDs and bortezomib: a multicenter international myeloma working group
study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus lowdose
dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol.
2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib
(PX-171-003-A1) in patients with relapsed and refractory multiple myeloma.
Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib
and dexamethasone versus placebo plus bortezomib and dexamethasone
in patients with relapsed or relapsed and refractory multiple
myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol.
2014;15(11):1195-1206.
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined
significance (MGUS) consistently precedes multiple myeloma: a prospective
study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed
multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal
Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney
injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop
Consensus Panel 3. Consensus recommendations for standard investigative workup:
report of the International Myeloma Workshop Consensus Panel 3. Blood.
2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working
Group consensus statement for the management, treatment, and supportive care
of patients with myeloma not eligible for standard autologous stem-cell transplantation.
J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working
Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol.
2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more
entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation
of measured myeloma cell mass with presenting clinical features, response to
treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple
myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival
in multiple myeloma: changes in early mortality and outcomes in older patients.
Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification,
and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis
in monoclonal gammopathy of undetermined significance. N Engl J Med.
2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering
(asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering
multiple myeloma: biological insights and early treatment strategies. Hematology
Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone
for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple
myeloma limited to high-risk subgroup identified by gene expression profiling.
Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes.
Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in
oncology: multiple myeloma. National Comprehensive Cancer Network Website.
http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated
March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study
(EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide,
and lenalidomide in previously untreated multiple myeloma. Blood.
2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus
reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment
before autologous stem cell transplantation in newly diagnosed multiple
myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous
administration of bortezomib in patients with relapsed multiple myeloma: a randomized,
phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in
multiple myeloma linked to bortezomib in total therapy 3: comparison with total
therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem
cell mobilization and engraftment post-peripheral blood stem cell transplantation
in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group.
Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell
collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining
regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis
for patients with newly diagnosed multiple myeloma patients treated with
lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in
combination with lenalidomide and low dose dexamethasone as a frontline treatment
for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of
carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended
dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed
multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide
plus dexamethasone in newly diagnosed multiple myeloma: a comparative
analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous
bone marrow transplantation and chemotherapy in multiple myeloma.
Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance
therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous
stem cell transplantation in multiple myeloma: up-front or rescue treatment?
Results of a multicenter sequential randomized clinical trial. Blood.
1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second
on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol.
2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the
FIRST (frontline investigation of lenalidomide + dexamethasone versus standard
thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma
(NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood.
2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan
and prednisone for initial treatment of multiple myeloma. N Engl J Med.
2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan
and prednisone plus thalidomide versus melphalan and prednisone
alone or reduced-intensity autologous stem cell transplantation in
elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet.
2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide
in patients older than 75 years with newly diagnosed multiple myeloma.
IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stemcell
transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome
(IFM). Maintenance therapy with thalidomide improves survival in patients with
multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose
thalidomide and prednisolone prolongs the survival of multiple myeloma patients
undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol.
2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and
maintenance treatment in patients with newly diagnosed multiple myeloma:
results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol.
2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome
Inhibition for Extending Remissions (APEX) Investigators. Bortezomib
or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med.
2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated
liposomal doxorubicin plus bortezomib compared with bortezomib alone
in relapsed or refractory multiple myeloma: combination therapy improves time
to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group.
Risk of progression and survival in multiple myeloma relapsing after therapy
with IMiDs and bortezomib: a multicenter international myeloma working group
study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus lowdose
dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol.
2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib
(PX-171-003-A1) in patients with relapsed and refractory multiple myeloma.
Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib
and dexamethasone versus placebo plus bortezomib and dexamethasone
in patients with relapsed or relapsed and refractory multiple
myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol.
2014;15(11):1195-1206.
Implementation of a Communication Training Program Is Associated with Reduction of Antipsychotic Medication Use in Nursing Homes
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
Fixed-Dose Combination Pills Enhance Adherence and Persistence to Antihypertensive Medications
Study Overview
Objective. To evaluate long-term adherence to antihypertensive therapy among patients on fixed-dose combination medication as well as antihypertensive monotherapy; and to identify demographic and clinical risk factors associated with selection of and adherence and persistence to antihypertensive medication therapy.
Design. Retrospective cohort study using claims data from a large nationwide insurer.
Setting and participants. The study population included patients older than age 18 who initiated antihypertensive medication between 1 January 2009 and 31 December 2012 and who were continually enrolled at least 180 days before and 365 days after the index date, defined as the date of initiation of antihypertensive therapy. Patients were excluded from the study if they had previously filled any antihypertensive medication at any time prior to the index date. Patients were categorized based on the number and type of antihypertensive medications (fixed-dose combination, defined as a single pill containing multiple medications; multi-pill combination, defined as 2 or more distinct antihypertensive tablets or capsules; or single therapy, defined as only 1 medication) using National Drug Codes (NDC). Study authors also measured patient baseline characteristics, such as age, region, gender, diagnoses as defined by ICD-9 codes, patient utilization characteristics (both outpatient visits and hospitalizations) and characteristics of the initiated medication, including patient copayment and number of days of medication supplied.
Main outcome measures. The primary outcome of inte-rest was persistence, defined as having supply for any antihypertensive medication that overlapped with the 365th day after initiation (index date), whether the initiated medication or other antihypertensive. Additional outcomes included adherence to at least 1 antihypertensive in the 12 months after initiation and refilling at least 1 antihypertensive medication. To determine adherence, the study authors calculated the proportion of days the patient had any antihypertensive available to them (proportion of days covered; PDC). PDC > 80% to at least 1 antihypertensive in the 12 months after initiation was defined as “fully adherent.”
Statistical analysis utilized modified multivariable Poisson regression models and sensitivity analyses were performed. The main study comparisons focused on patients initiating fixed-dose combination therapy and monotherapy because these groups were more comparable in terms of baseline characteristics and medications initiated than the multi-pill combination group.
Main results. The study sample consisted of 484,493 patients who initiated an oral antihypertensive, including 78,958 patient initiating fixed-dose combinations, 380,269 filled a single therapy, and 22,266 who initiated multi-pill combinations. The most frequently initiated fixed-dose combination was lisinopril-hydrochlorothiazide. Lisinopril, hydrochlorothiazide, and amlodipine with the most frequently initiated monotherapy. The mean age of the study population was 47.2 years and 51.8% were women. Patients initiating multiple pill combinations were older (mean age 52.5) and tended to be sicker with more comorbidities than fixed-dose combinations or monotherapy. Patients initiating fixed-dose combination had higher prescription copayments than patients using single medication (prescription copay $14.4 versus $9.6). Patients initiating fixed-dose combinations were 9% more likely to be persistent (relative risk [RR] 1.09, 95% CI 1.08–1.10) and 13% more likely to be adherent (RR 1.13, 95% CI 1.11–1.14) than those who started on a monotherapy. Refill rates were also slightly higher among fixed-dose combination initiators (RR 1.06, 95% CI 1.05-1.07).
Conclusion. Compared with monotherapy, fixed-dose combination therapy appears to improve adherence and persistence to antihypertensive medications.
Commentary
Approximately half of US of individuals with diagnosed hypertension obtain control of their condition based on currently defined targets [1]. The most effective approach to blood pressure management has been controversial. The JNC8 [2] guidelines liberalized blood pressure targets, while recent results from the SPRINT (systolic blood pressure intervention trial) [3] indicates that lower blood pressure targets are able to prevent hypertension-related complications without significant additional risk. Given these conflicts, there is clearly ambiguity in the most effective approach to initiating antihypertensive treatment. Prior studies have shown that fewer than 50% of patients continue to take their medications just 12 months after initiation [4,5].
Fixed-dose combination therapy for blood pressure management has been cited as better for adherence and is now making its way into clinical guidelines [6–8]. However, it should be noted that fixed-dose combination therapy for blood pressure management limits dosing flexibility. Dose titration may be needed, potentially leading to additional prescriptions, thus potentially complicating the drug regimen and adding additional cost. Complicating matters further, quality metrics and reporting requirements for hypertension require primary care providers to achieve blood pressure control while also ensuring patient adherence and concomitantly avoiding side effects related to medication therapy.
This study was conducted using claims data for commercially insured patients or those with Medicare Advan-tage and is unlikely to be representative of the entire population. Additionally, the study authors did not have detailed clinical information about patients, limiting the ability to understand the true clinical implications. Further, patients may have initiated medications for indications other than hypertension. In addition, causality cannot be established given the retrospective observational cohort nature of this study.
Applications for Clinical Practice
Primary care physicians face substantial challenges in the treatment of hypertension, including with respect to selection of initial medication therapy. Results from this study add to the evidence base that fixed-dose combination therapy is more effective in obtaining blood pressure control than monotherapy or multiple-pill therapy. Medication adherence in primary care practice is challenging. Strategies such as fixed-dose combination therapy are reasonable to employ to improve medication adherence; however, costs must be considered.
—Ajay Dharod, MD, Wake Forest School of Medicine, Winston-Salem, NC
1. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension. Circulation 2012;126:2105–14.
2. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
3. Group TSR. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16.
4. Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15:728–40.
5. Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation 2010;121:1455–8.
6. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120:713–9.
7. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents. Hypertension 2010;55:399–407.
8. Pan F, Chernew ME, Fendrick AM. Impact of fixed-dose combination drugs on adherence to prescription medications. J Gen Intern Med 2008;23:611–4.
Study Overview
Objective. To evaluate long-term adherence to antihypertensive therapy among patients on fixed-dose combination medication as well as antihypertensive monotherapy; and to identify demographic and clinical risk factors associated with selection of and adherence and persistence to antihypertensive medication therapy.
Design. Retrospective cohort study using claims data from a large nationwide insurer.
Setting and participants. The study population included patients older than age 18 who initiated antihypertensive medication between 1 January 2009 and 31 December 2012 and who were continually enrolled at least 180 days before and 365 days after the index date, defined as the date of initiation of antihypertensive therapy. Patients were excluded from the study if they had previously filled any antihypertensive medication at any time prior to the index date. Patients were categorized based on the number and type of antihypertensive medications (fixed-dose combination, defined as a single pill containing multiple medications; multi-pill combination, defined as 2 or more distinct antihypertensive tablets or capsules; or single therapy, defined as only 1 medication) using National Drug Codes (NDC). Study authors also measured patient baseline characteristics, such as age, region, gender, diagnoses as defined by ICD-9 codes, patient utilization characteristics (both outpatient visits and hospitalizations) and characteristics of the initiated medication, including patient copayment and number of days of medication supplied.
Main outcome measures. The primary outcome of inte-rest was persistence, defined as having supply for any antihypertensive medication that overlapped with the 365th day after initiation (index date), whether the initiated medication or other antihypertensive. Additional outcomes included adherence to at least 1 antihypertensive in the 12 months after initiation and refilling at least 1 antihypertensive medication. To determine adherence, the study authors calculated the proportion of days the patient had any antihypertensive available to them (proportion of days covered; PDC). PDC > 80% to at least 1 antihypertensive in the 12 months after initiation was defined as “fully adherent.”
Statistical analysis utilized modified multivariable Poisson regression models and sensitivity analyses were performed. The main study comparisons focused on patients initiating fixed-dose combination therapy and monotherapy because these groups were more comparable in terms of baseline characteristics and medications initiated than the multi-pill combination group.
Main results. The study sample consisted of 484,493 patients who initiated an oral antihypertensive, including 78,958 patient initiating fixed-dose combinations, 380,269 filled a single therapy, and 22,266 who initiated multi-pill combinations. The most frequently initiated fixed-dose combination was lisinopril-hydrochlorothiazide. Lisinopril, hydrochlorothiazide, and amlodipine with the most frequently initiated monotherapy. The mean age of the study population was 47.2 years and 51.8% were women. Patients initiating multiple pill combinations were older (mean age 52.5) and tended to be sicker with more comorbidities than fixed-dose combinations or monotherapy. Patients initiating fixed-dose combination had higher prescription copayments than patients using single medication (prescription copay $14.4 versus $9.6). Patients initiating fixed-dose combinations were 9% more likely to be persistent (relative risk [RR] 1.09, 95% CI 1.08–1.10) and 13% more likely to be adherent (RR 1.13, 95% CI 1.11–1.14) than those who started on a monotherapy. Refill rates were also slightly higher among fixed-dose combination initiators (RR 1.06, 95% CI 1.05-1.07).
Conclusion. Compared with monotherapy, fixed-dose combination therapy appears to improve adherence and persistence to antihypertensive medications.
Commentary
Approximately half of US of individuals with diagnosed hypertension obtain control of their condition based on currently defined targets [1]. The most effective approach to blood pressure management has been controversial. The JNC8 [2] guidelines liberalized blood pressure targets, while recent results from the SPRINT (systolic blood pressure intervention trial) [3] indicates that lower blood pressure targets are able to prevent hypertension-related complications without significant additional risk. Given these conflicts, there is clearly ambiguity in the most effective approach to initiating antihypertensive treatment. Prior studies have shown that fewer than 50% of patients continue to take their medications just 12 months after initiation [4,5].
Fixed-dose combination therapy for blood pressure management has been cited as better for adherence and is now making its way into clinical guidelines [6–8]. However, it should be noted that fixed-dose combination therapy for blood pressure management limits dosing flexibility. Dose titration may be needed, potentially leading to additional prescriptions, thus potentially complicating the drug regimen and adding additional cost. Complicating matters further, quality metrics and reporting requirements for hypertension require primary care providers to achieve blood pressure control while also ensuring patient adherence and concomitantly avoiding side effects related to medication therapy.
This study was conducted using claims data for commercially insured patients or those with Medicare Advan-tage and is unlikely to be representative of the entire population. Additionally, the study authors did not have detailed clinical information about patients, limiting the ability to understand the true clinical implications. Further, patients may have initiated medications for indications other than hypertension. In addition, causality cannot be established given the retrospective observational cohort nature of this study.
Applications for Clinical Practice
Primary care physicians face substantial challenges in the treatment of hypertension, including with respect to selection of initial medication therapy. Results from this study add to the evidence base that fixed-dose combination therapy is more effective in obtaining blood pressure control than monotherapy or multiple-pill therapy. Medication adherence in primary care practice is challenging. Strategies such as fixed-dose combination therapy are reasonable to employ to improve medication adherence; however, costs must be considered.
—Ajay Dharod, MD, Wake Forest School of Medicine, Winston-Salem, NC
Study Overview
Objective. To evaluate long-term adherence to antihypertensive therapy among patients on fixed-dose combination medication as well as antihypertensive monotherapy; and to identify demographic and clinical risk factors associated with selection of and adherence and persistence to antihypertensive medication therapy.
Design. Retrospective cohort study using claims data from a large nationwide insurer.
Setting and participants. The study population included patients older than age 18 who initiated antihypertensive medication between 1 January 2009 and 31 December 2012 and who were continually enrolled at least 180 days before and 365 days after the index date, defined as the date of initiation of antihypertensive therapy. Patients were excluded from the study if they had previously filled any antihypertensive medication at any time prior to the index date. Patients were categorized based on the number and type of antihypertensive medications (fixed-dose combination, defined as a single pill containing multiple medications; multi-pill combination, defined as 2 or more distinct antihypertensive tablets or capsules; or single therapy, defined as only 1 medication) using National Drug Codes (NDC). Study authors also measured patient baseline characteristics, such as age, region, gender, diagnoses as defined by ICD-9 codes, patient utilization characteristics (both outpatient visits and hospitalizations) and characteristics of the initiated medication, including patient copayment and number of days of medication supplied.
Main outcome measures. The primary outcome of inte-rest was persistence, defined as having supply for any antihypertensive medication that overlapped with the 365th day after initiation (index date), whether the initiated medication or other antihypertensive. Additional outcomes included adherence to at least 1 antihypertensive in the 12 months after initiation and refilling at least 1 antihypertensive medication. To determine adherence, the study authors calculated the proportion of days the patient had any antihypertensive available to them (proportion of days covered; PDC). PDC > 80% to at least 1 antihypertensive in the 12 months after initiation was defined as “fully adherent.”
Statistical analysis utilized modified multivariable Poisson regression models and sensitivity analyses were performed. The main study comparisons focused on patients initiating fixed-dose combination therapy and monotherapy because these groups were more comparable in terms of baseline characteristics and medications initiated than the multi-pill combination group.
Main results. The study sample consisted of 484,493 patients who initiated an oral antihypertensive, including 78,958 patient initiating fixed-dose combinations, 380,269 filled a single therapy, and 22,266 who initiated multi-pill combinations. The most frequently initiated fixed-dose combination was lisinopril-hydrochlorothiazide. Lisinopril, hydrochlorothiazide, and amlodipine with the most frequently initiated monotherapy. The mean age of the study population was 47.2 years and 51.8% were women. Patients initiating multiple pill combinations were older (mean age 52.5) and tended to be sicker with more comorbidities than fixed-dose combinations or monotherapy. Patients initiating fixed-dose combination had higher prescription copayments than patients using single medication (prescription copay $14.4 versus $9.6). Patients initiating fixed-dose combinations were 9% more likely to be persistent (relative risk [RR] 1.09, 95% CI 1.08–1.10) and 13% more likely to be adherent (RR 1.13, 95% CI 1.11–1.14) than those who started on a monotherapy. Refill rates were also slightly higher among fixed-dose combination initiators (RR 1.06, 95% CI 1.05-1.07).
Conclusion. Compared with monotherapy, fixed-dose combination therapy appears to improve adherence and persistence to antihypertensive medications.
Commentary
Approximately half of US of individuals with diagnosed hypertension obtain control of their condition based on currently defined targets [1]. The most effective approach to blood pressure management has been controversial. The JNC8 [2] guidelines liberalized blood pressure targets, while recent results from the SPRINT (systolic blood pressure intervention trial) [3] indicates that lower blood pressure targets are able to prevent hypertension-related complications without significant additional risk. Given these conflicts, there is clearly ambiguity in the most effective approach to initiating antihypertensive treatment. Prior studies have shown that fewer than 50% of patients continue to take their medications just 12 months after initiation [4,5].
Fixed-dose combination therapy for blood pressure management has been cited as better for adherence and is now making its way into clinical guidelines [6–8]. However, it should be noted that fixed-dose combination therapy for blood pressure management limits dosing flexibility. Dose titration may be needed, potentially leading to additional prescriptions, thus potentially complicating the drug regimen and adding additional cost. Complicating matters further, quality metrics and reporting requirements for hypertension require primary care providers to achieve blood pressure control while also ensuring patient adherence and concomitantly avoiding side effects related to medication therapy.
This study was conducted using claims data for commercially insured patients or those with Medicare Advan-tage and is unlikely to be representative of the entire population. Additionally, the study authors did not have detailed clinical information about patients, limiting the ability to understand the true clinical implications. Further, patients may have initiated medications for indications other than hypertension. In addition, causality cannot be established given the retrospective observational cohort nature of this study.
Applications for Clinical Practice
Primary care physicians face substantial challenges in the treatment of hypertension, including with respect to selection of initial medication therapy. Results from this study add to the evidence base that fixed-dose combination therapy is more effective in obtaining blood pressure control than monotherapy or multiple-pill therapy. Medication adherence in primary care practice is challenging. Strategies such as fixed-dose combination therapy are reasonable to employ to improve medication adherence; however, costs must be considered.
—Ajay Dharod, MD, Wake Forest School of Medicine, Winston-Salem, NC
1. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension. Circulation 2012;126:2105–14.
2. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
3. Group TSR. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16.
4. Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15:728–40.
5. Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation 2010;121:1455–8.
6. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120:713–9.
7. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents. Hypertension 2010;55:399–407.
8. Pan F, Chernew ME, Fendrick AM. Impact of fixed-dose combination drugs on adherence to prescription medications. J Gen Intern Med 2008;23:611–4.
1. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension. Circulation 2012;126:2105–14.
2. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
3. Group TSR. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16.
4. Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15:728–40.
5. Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation 2010;121:1455–8.
6. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120:713–9.
7. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents. Hypertension 2010;55:399–407.
8. Pan F, Chernew ME, Fendrick AM. Impact of fixed-dose combination drugs on adherence to prescription medications. J Gen Intern Med 2008;23:611–4.
Leonard Wood: Advocate of Military Preparedness
Unless you have been assigned to the post or the hospital, you have probably never heard of Leonard Wood. Leonard Wood arguably had the most distinguished military-government career of someone who did not become president. Wood was a Harvard-educated physician, pursued the Apache Chief Geronimo, received the Medal of Honor, was physician to 2 U.S. presidents, served as U.S. army chief of staff, was a successful military governor, ran for president, was a colleague of Walter Reed, and was commander-inarms for President Theodore Roosevelt.
Wood was born in 1860 to an established New England family; his father was a Union Army physician during the Civil War and was practicing on Cape Cod when he died unexpectedly in 1880. The family was left destitute, but Wood was able to continue his education when a wealthy family friend agreed to pay for him to attend Harvard Medical School, which at the time did not require any prior college. He graduated in 1883 and was selected for a prized internship at Boston City Hospital; however, he was dismissed for a rule violation that the program director later admitted was a mistake.
Unable to support himself in practice in Boston, Wood turned to the U.S. Army, a decision that would change his life. Assigned to Fort Huachuca in Arizona, Wood participated in the yearlong pursuit and final surrender of Geronimo; for his role he was awarded the Medal of Honor in 1898. His experiences in the wild and rugged terrain of the west triggered a legendary and lifelong pursuit of hard and stressful physical activity. Transferred to California, Wood met Louise Condit-Smith, ward of an associate justice of the U.S. Supreme Court. When they married in November 1890 in Washington, DC, the ceremony was attended by all of the Supreme Court justices.
In 1893 while assigned to Fort McPherson outside Atlanta, Wood, whose duties were not demanding, needed a physical outlet for his unbounded energies. He enrolled at Georgia Tech at age 33 to play football. He was eligible to play because he had not previously attended college. He scored 5 touchdowns, winning the game against rival University of Georgia.
Later, Wood was assigned to Washington, where he quickly became known and sought after as a physician. He served many of the political and military elite, including presidents Grover Cleveland and William McKinley. In 1897, he met Theodore Roosevelt, the 38-year-old assistant secretary of the U.S. Navy who shared his love of outdoor adventure and the military. They became fast friends/companions/competitors; Roosevelt wrote to a friend that he had found a “playmate.”
When the U.S.S. Maine was sunk in Havana Harbor in 1898 and war was declared on Spain, Wood and Roosevelt schemed on how to go to war together. Wood the career soldier and Roosevelt the career politician had excellent connections and became commander and deputy commander of the First Volunteer Calvary, later famously known as the Rough Riders. When a more senior general became ill, Wood was promoted to brigadier general, and Roosevelt became the regiment colonel.
After the war, Wood became military governor of Cuba and major general of volunteers. During the U.S. occupation, Walter Reed was sent to investigate infectious diseases, including yellow fever. Wood provided $10,000 to fund the second phase of Reed’s research and approved the use of human volunteers. When the U.S. occupation ended in 1902, Wood was to revert to captain, medical corps.
Wood’s success in Cuba was obvious and wel l known; President McKinley promoted him to U.S. Army brigadier general. At that time, as a brigadier general, Wood was essentially guaranteed a second star and a rotation through the chief of staff position. He served as chief of staff from 1910 to 1914, the only physician ever to do so. As chief of staff he eliminated the antiquated bureau system, developed the maneuver unit concept, and laid the groundwork for the Reserve Officers’ Training Corps.
Wood stayed on active duty and rotated through other senior-level positions. Because of Wood’s political activity promoting universal service and improving readiness, President Woodrow Wilson passed over him, instead selecting John J. Pershing to command the American Expeditionary Force in World War I. Wood stayed politically active and ran for the Republican presidential nomination in 1920, losing to Warren G. Harding at the convention. Wood was appointed governor general of the Philippines, a position he held until his death in 1927.
While in Cuba, Wood was severely injured by striking his head on a chandelier, most likely resulting in an undiagnosed skull fracture. Over time he developed neurologic symptoms and was seen by neurosurgeon Harvey Cushing, MD, at Johns Hopkins, who removed a meningioma in February 1910. Wood made a dramatic recovery. Over a decade later while in the Philippines, his symptoms returned, and after significant delay he went home to see Cushing who was then at Harvard Medical School. When Wood died after surgery, Cushing admitted that he should not have tackled such a difficult case so quickly after returning from a trip to Europe.
Fort Leonard Wood in Missouri and the on-base General Leonard Wood U.S. Army Community Hospital are named in Wood’s honor.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual
your facility was named for or to offer a topic suggestion, contact us at fedprac@frontlinemedcom.com or on Facebook.
Unless you have been assigned to the post or the hospital, you have probably never heard of Leonard Wood. Leonard Wood arguably had the most distinguished military-government career of someone who did not become president. Wood was a Harvard-educated physician, pursued the Apache Chief Geronimo, received the Medal of Honor, was physician to 2 U.S. presidents, served as U.S. army chief of staff, was a successful military governor, ran for president, was a colleague of Walter Reed, and was commander-inarms for President Theodore Roosevelt.
Wood was born in 1860 to an established New England family; his father was a Union Army physician during the Civil War and was practicing on Cape Cod when he died unexpectedly in 1880. The family was left destitute, but Wood was able to continue his education when a wealthy family friend agreed to pay for him to attend Harvard Medical School, which at the time did not require any prior college. He graduated in 1883 and was selected for a prized internship at Boston City Hospital; however, he was dismissed for a rule violation that the program director later admitted was a mistake.
Unable to support himself in practice in Boston, Wood turned to the U.S. Army, a decision that would change his life. Assigned to Fort Huachuca in Arizona, Wood participated in the yearlong pursuit and final surrender of Geronimo; for his role he was awarded the Medal of Honor in 1898. His experiences in the wild and rugged terrain of the west triggered a legendary and lifelong pursuit of hard and stressful physical activity. Transferred to California, Wood met Louise Condit-Smith, ward of an associate justice of the U.S. Supreme Court. When they married in November 1890 in Washington, DC, the ceremony was attended by all of the Supreme Court justices.
In 1893 while assigned to Fort McPherson outside Atlanta, Wood, whose duties were not demanding, needed a physical outlet for his unbounded energies. He enrolled at Georgia Tech at age 33 to play football. He was eligible to play because he had not previously attended college. He scored 5 touchdowns, winning the game against rival University of Georgia.
Later, Wood was assigned to Washington, where he quickly became known and sought after as a physician. He served many of the political and military elite, including presidents Grover Cleveland and William McKinley. In 1897, he met Theodore Roosevelt, the 38-year-old assistant secretary of the U.S. Navy who shared his love of outdoor adventure and the military. They became fast friends/companions/competitors; Roosevelt wrote to a friend that he had found a “playmate.”
When the U.S.S. Maine was sunk in Havana Harbor in 1898 and war was declared on Spain, Wood and Roosevelt schemed on how to go to war together. Wood the career soldier and Roosevelt the career politician had excellent connections and became commander and deputy commander of the First Volunteer Calvary, later famously known as the Rough Riders. When a more senior general became ill, Wood was promoted to brigadier general, and Roosevelt became the regiment colonel.
After the war, Wood became military governor of Cuba and major general of volunteers. During the U.S. occupation, Walter Reed was sent to investigate infectious diseases, including yellow fever. Wood provided $10,000 to fund the second phase of Reed’s research and approved the use of human volunteers. When the U.S. occupation ended in 1902, Wood was to revert to captain, medical corps.
Wood’s success in Cuba was obvious and wel l known; President McKinley promoted him to U.S. Army brigadier general. At that time, as a brigadier general, Wood was essentially guaranteed a second star and a rotation through the chief of staff position. He served as chief of staff from 1910 to 1914, the only physician ever to do so. As chief of staff he eliminated the antiquated bureau system, developed the maneuver unit concept, and laid the groundwork for the Reserve Officers’ Training Corps.
Wood stayed on active duty and rotated through other senior-level positions. Because of Wood’s political activity promoting universal service and improving readiness, President Woodrow Wilson passed over him, instead selecting John J. Pershing to command the American Expeditionary Force in World War I. Wood stayed politically active and ran for the Republican presidential nomination in 1920, losing to Warren G. Harding at the convention. Wood was appointed governor general of the Philippines, a position he held until his death in 1927.
While in Cuba, Wood was severely injured by striking his head on a chandelier, most likely resulting in an undiagnosed skull fracture. Over time he developed neurologic symptoms and was seen by neurosurgeon Harvey Cushing, MD, at Johns Hopkins, who removed a meningioma in February 1910. Wood made a dramatic recovery. Over a decade later while in the Philippines, his symptoms returned, and after significant delay he went home to see Cushing who was then at Harvard Medical School. When Wood died after surgery, Cushing admitted that he should not have tackled such a difficult case so quickly after returning from a trip to Europe.
Fort Leonard Wood in Missouri and the on-base General Leonard Wood U.S. Army Community Hospital are named in Wood’s honor.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual
your facility was named for or to offer a topic suggestion, contact us at fedprac@frontlinemedcom.com or on Facebook.
Unless you have been assigned to the post or the hospital, you have probably never heard of Leonard Wood. Leonard Wood arguably had the most distinguished military-government career of someone who did not become president. Wood was a Harvard-educated physician, pursued the Apache Chief Geronimo, received the Medal of Honor, was physician to 2 U.S. presidents, served as U.S. army chief of staff, was a successful military governor, ran for president, was a colleague of Walter Reed, and was commander-inarms for President Theodore Roosevelt.
Wood was born in 1860 to an established New England family; his father was a Union Army physician during the Civil War and was practicing on Cape Cod when he died unexpectedly in 1880. The family was left destitute, but Wood was able to continue his education when a wealthy family friend agreed to pay for him to attend Harvard Medical School, which at the time did not require any prior college. He graduated in 1883 and was selected for a prized internship at Boston City Hospital; however, he was dismissed for a rule violation that the program director later admitted was a mistake.
Unable to support himself in practice in Boston, Wood turned to the U.S. Army, a decision that would change his life. Assigned to Fort Huachuca in Arizona, Wood participated in the yearlong pursuit and final surrender of Geronimo; for his role he was awarded the Medal of Honor in 1898. His experiences in the wild and rugged terrain of the west triggered a legendary and lifelong pursuit of hard and stressful physical activity. Transferred to California, Wood met Louise Condit-Smith, ward of an associate justice of the U.S. Supreme Court. When they married in November 1890 in Washington, DC, the ceremony was attended by all of the Supreme Court justices.
In 1893 while assigned to Fort McPherson outside Atlanta, Wood, whose duties were not demanding, needed a physical outlet for his unbounded energies. He enrolled at Georgia Tech at age 33 to play football. He was eligible to play because he had not previously attended college. He scored 5 touchdowns, winning the game against rival University of Georgia.
Later, Wood was assigned to Washington, where he quickly became known and sought after as a physician. He served many of the political and military elite, including presidents Grover Cleveland and William McKinley. In 1897, he met Theodore Roosevelt, the 38-year-old assistant secretary of the U.S. Navy who shared his love of outdoor adventure and the military. They became fast friends/companions/competitors; Roosevelt wrote to a friend that he had found a “playmate.”
When the U.S.S. Maine was sunk in Havana Harbor in 1898 and war was declared on Spain, Wood and Roosevelt schemed on how to go to war together. Wood the career soldier and Roosevelt the career politician had excellent connections and became commander and deputy commander of the First Volunteer Calvary, later famously known as the Rough Riders. When a more senior general became ill, Wood was promoted to brigadier general, and Roosevelt became the regiment colonel.
After the war, Wood became military governor of Cuba and major general of volunteers. During the U.S. occupation, Walter Reed was sent to investigate infectious diseases, including yellow fever. Wood provided $10,000 to fund the second phase of Reed’s research and approved the use of human volunteers. When the U.S. occupation ended in 1902, Wood was to revert to captain, medical corps.
Wood’s success in Cuba was obvious and wel l known; President McKinley promoted him to U.S. Army brigadier general. At that time, as a brigadier general, Wood was essentially guaranteed a second star and a rotation through the chief of staff position. He served as chief of staff from 1910 to 1914, the only physician ever to do so. As chief of staff he eliminated the antiquated bureau system, developed the maneuver unit concept, and laid the groundwork for the Reserve Officers’ Training Corps.
Wood stayed on active duty and rotated through other senior-level positions. Because of Wood’s political activity promoting universal service and improving readiness, President Woodrow Wilson passed over him, instead selecting John J. Pershing to command the American Expeditionary Force in World War I. Wood stayed politically active and ran for the Republican presidential nomination in 1920, losing to Warren G. Harding at the convention. Wood was appointed governor general of the Philippines, a position he held until his death in 1927.
While in Cuba, Wood was severely injured by striking his head on a chandelier, most likely resulting in an undiagnosed skull fracture. Over time he developed neurologic symptoms and was seen by neurosurgeon Harvey Cushing, MD, at Johns Hopkins, who removed a meningioma in February 1910. Wood made a dramatic recovery. Over a decade later while in the Philippines, his symptoms returned, and after significant delay he went home to see Cushing who was then at Harvard Medical School. When Wood died after surgery, Cushing admitted that he should not have tackled such a difficult case so quickly after returning from a trip to Europe.
Fort Leonard Wood in Missouri and the on-base General Leonard Wood U.S. Army Community Hospital are named in Wood’s honor.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual
your facility was named for or to offer a topic suggestion, contact us at fedprac@frontlinemedcom.com or on Facebook.