User login
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
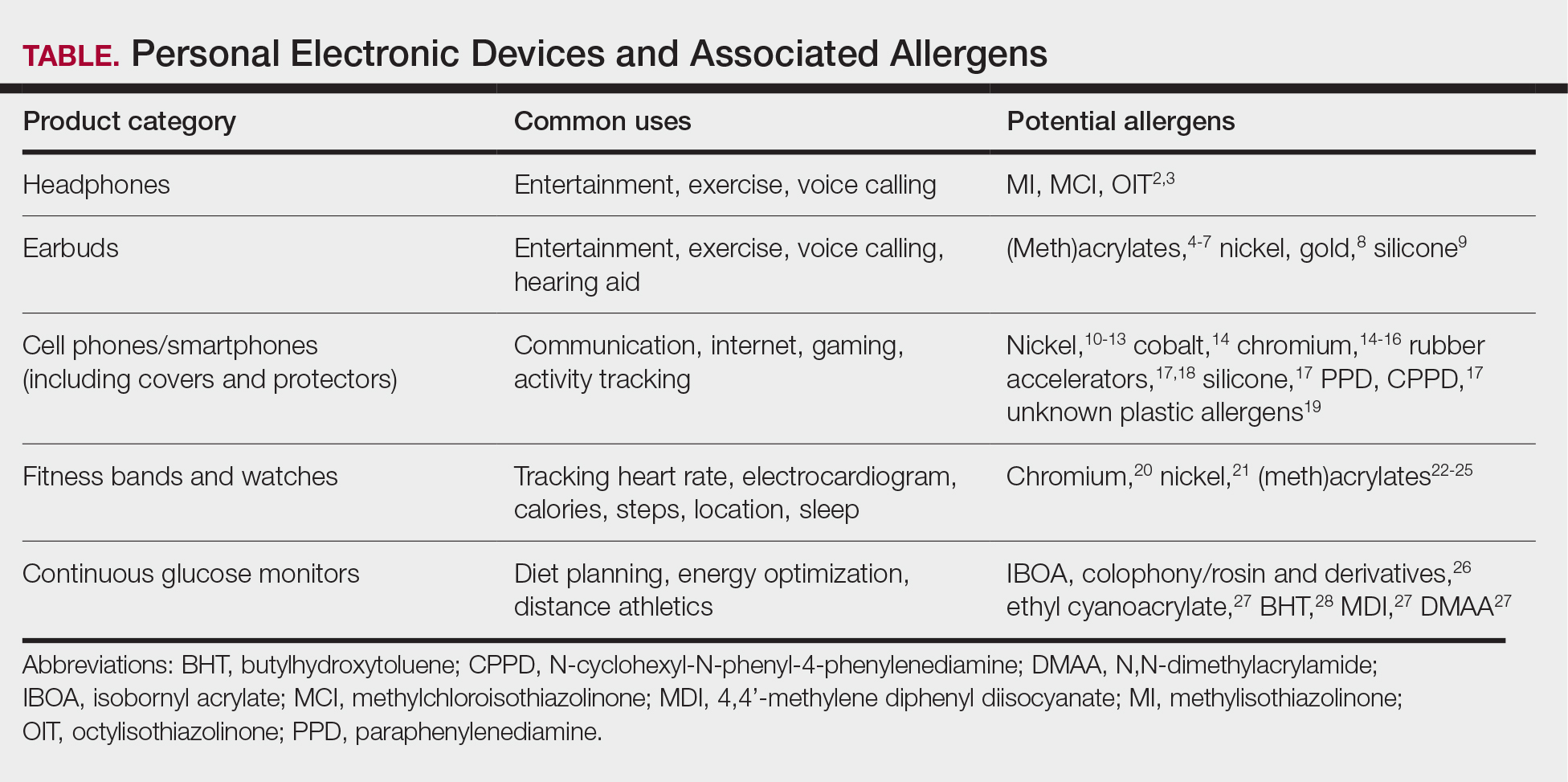
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).
Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
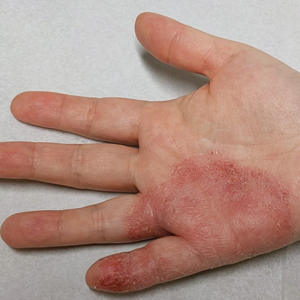
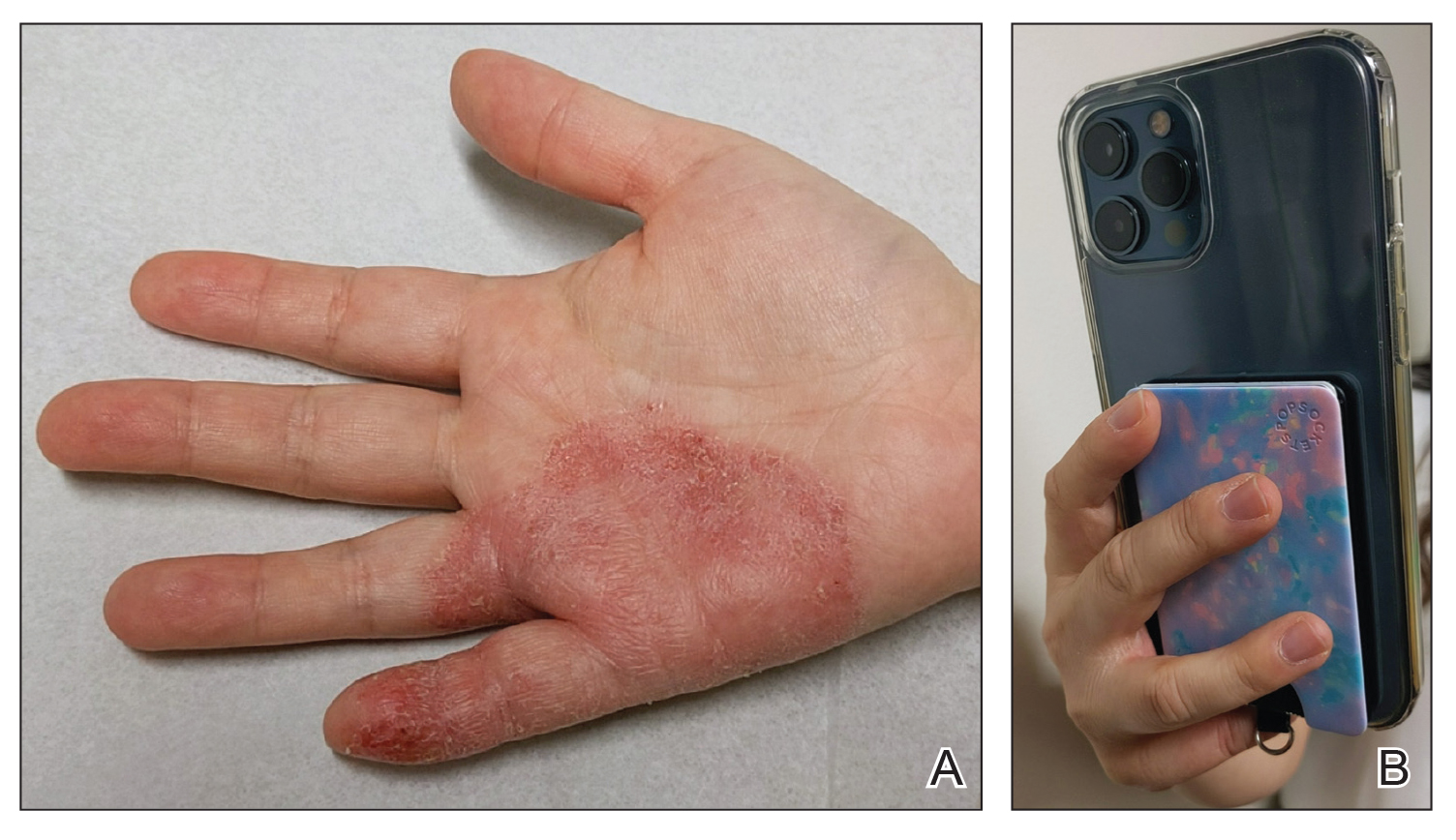
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.
Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).

Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.

Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).

Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.

Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
PRACTICE POINTS
- Personal electronic devices including smart phones, headphones, watches, and continuous glucose monitors represent an emerging source of allergic contact dermatitis.
- Reactions often are localized to areas of skin contact including the face, ears, wrists, and hands.
- Reported allergens in personal electronic devices include (meth)acrylates, metals, and rubber compounds.
- Patch testing is key in detecting and avoiding culprit allergens, but a major challenge is lack of transparency regarding device composition and ingredients.
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15
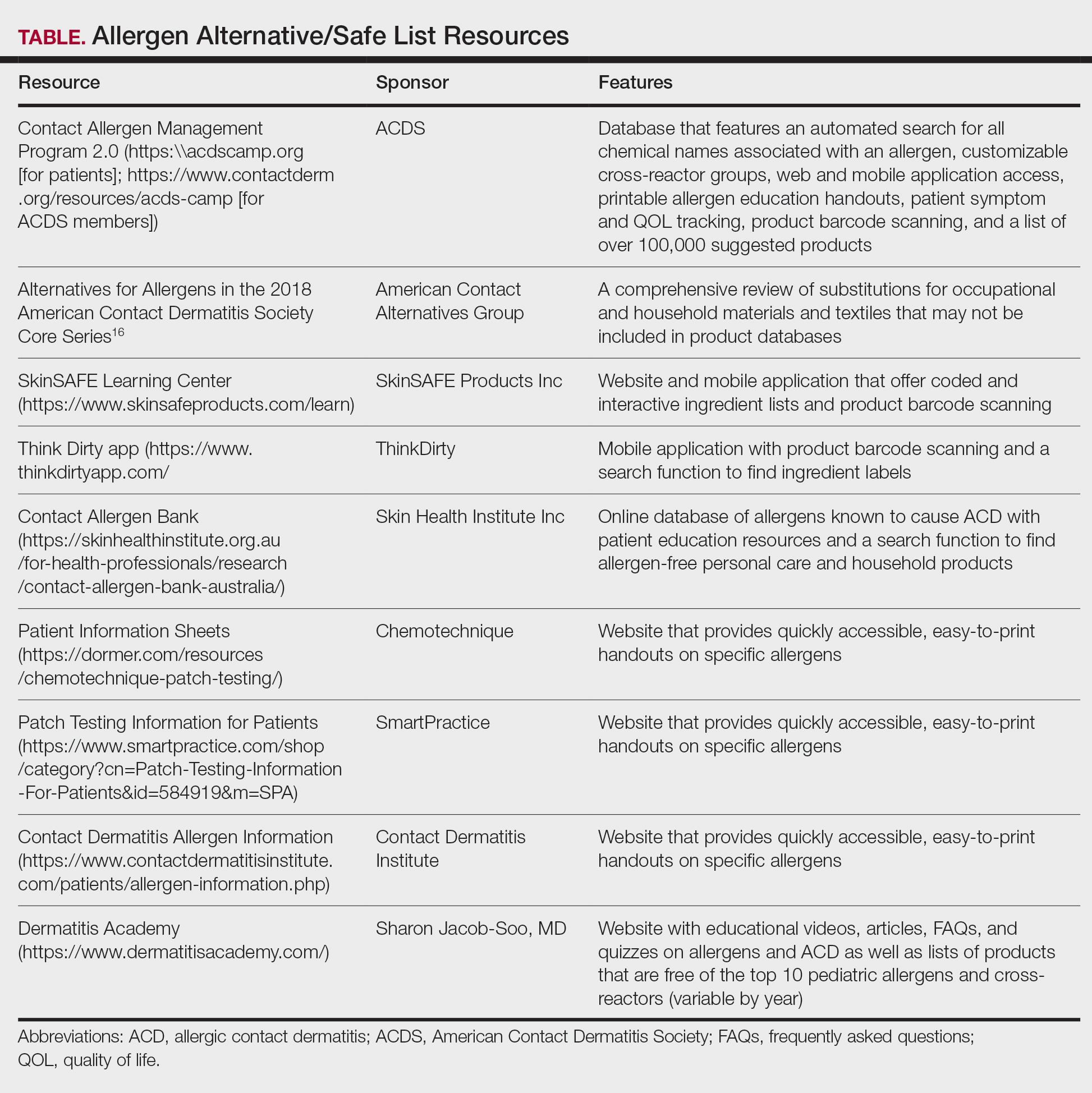
Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.
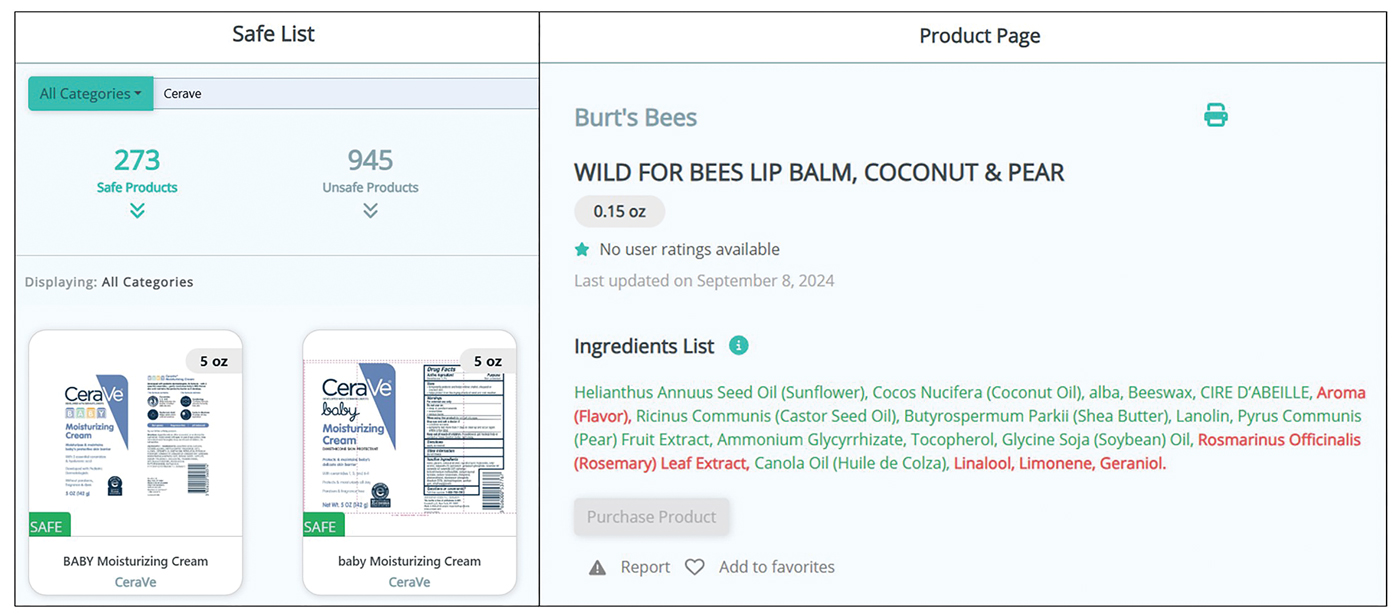
Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
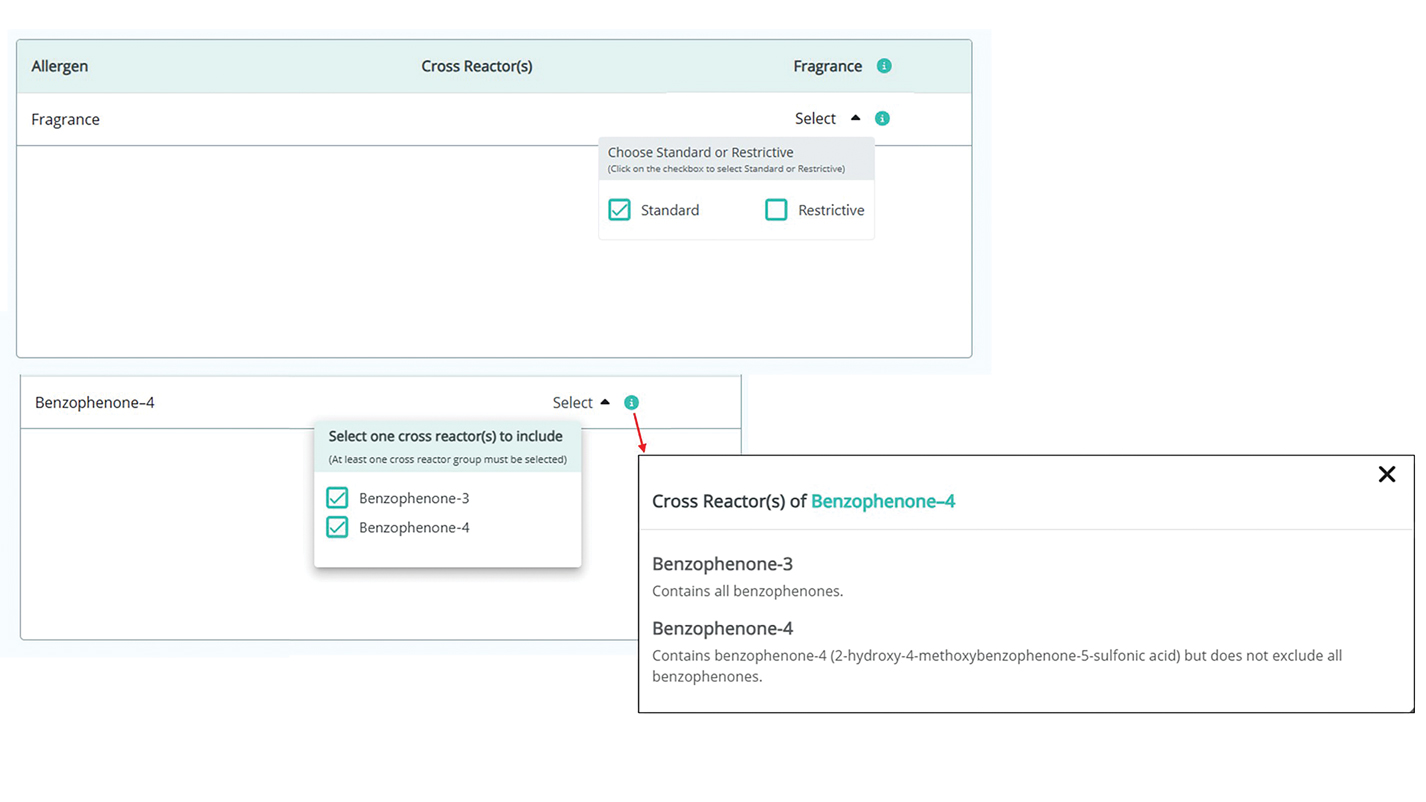
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.
Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
PRACTICE POINTS
- Comprehensive patient education is critical for appropriate allergen avoidance after patch testing, and allergen databases and product safe lists are invaluable tools to complement clinical guidance.
- The updated Contact Allergen Management Program 2.0 offers an updated approach to patient guidance, including a database of more than 100,000 products and an easy-to-use platform to find safe, allergen-free products.
- Interactive learning resources, product pages, and quality-of-life tracking tools can help equip patients with information to encourage further autonomy in allergen avoidance.