User login
Top 50 Authors in Dermatology by Publication Rate (2017-2022)
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
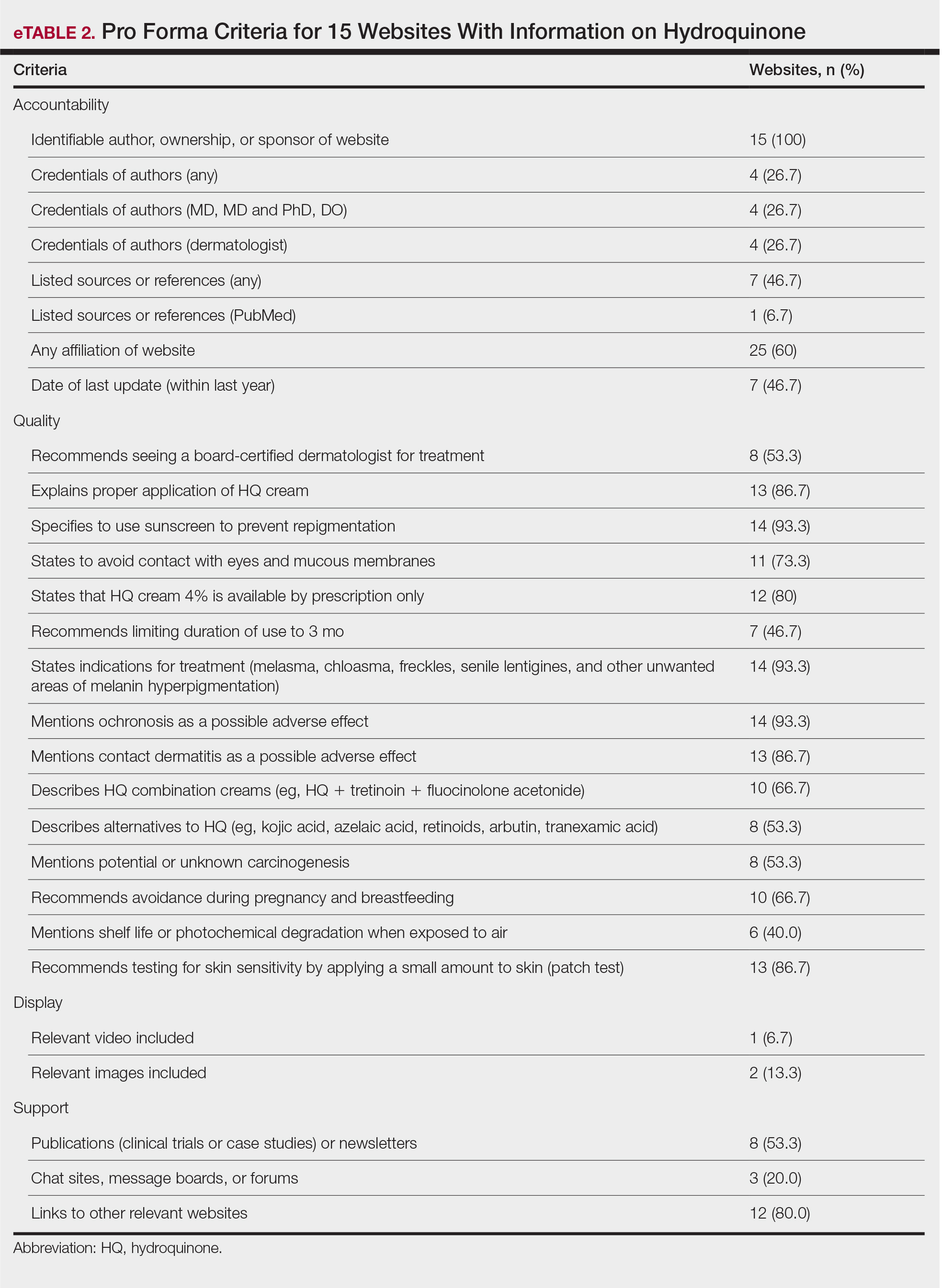
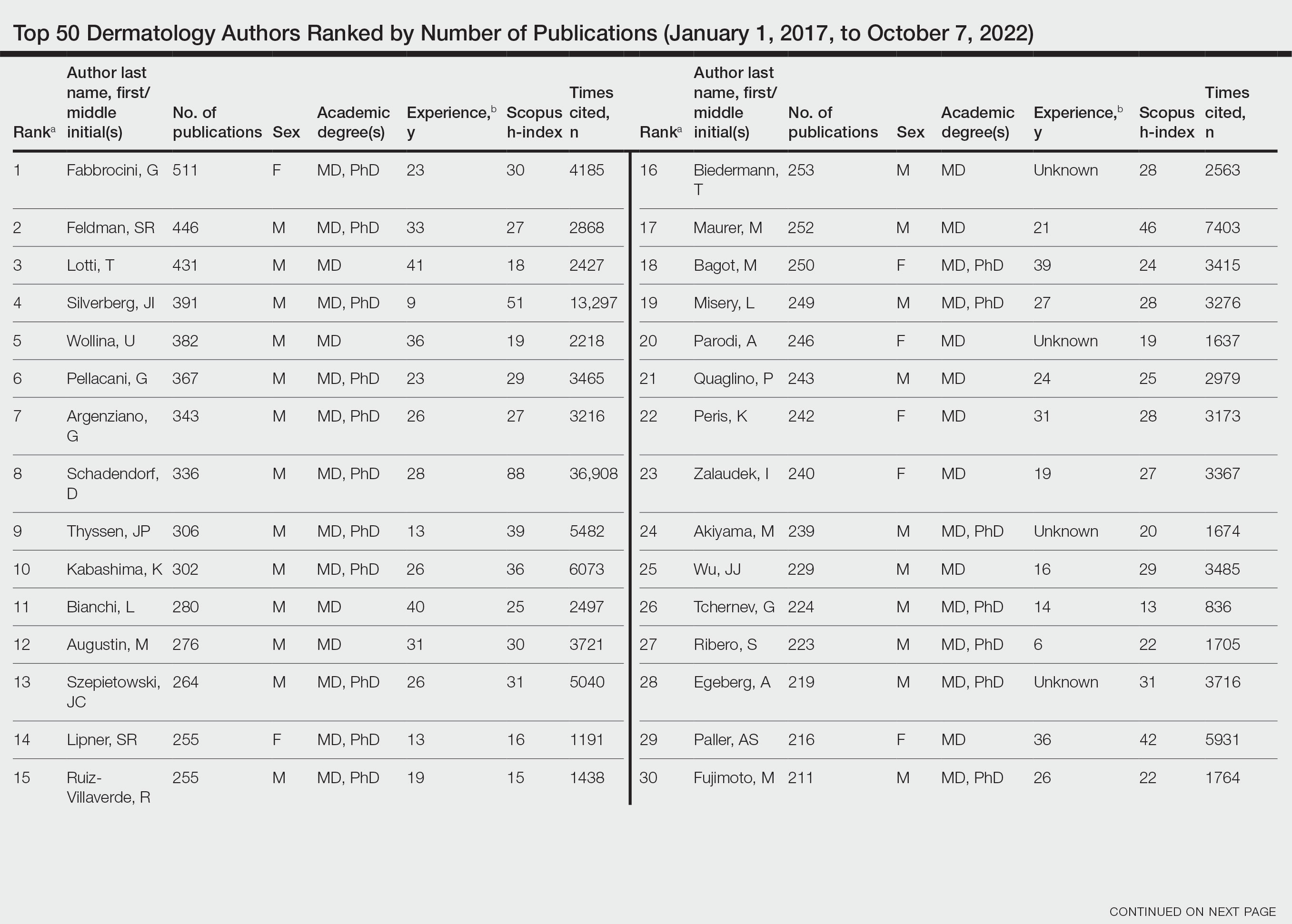
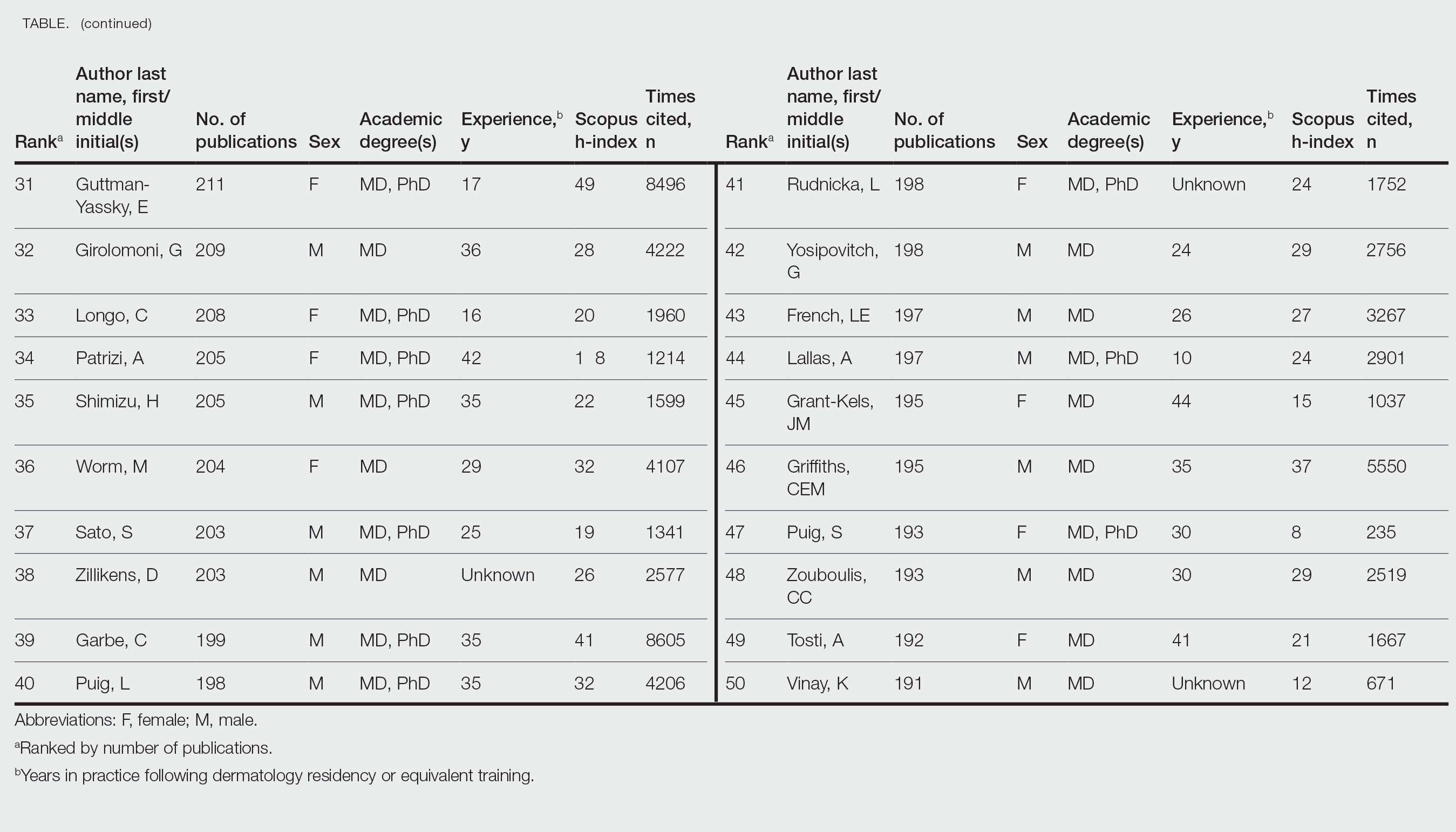
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).
Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).


Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).


Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
Practice Points
- Academic scholarship often is measured by number of citations and h-index. Using these measures, female dermatologists are infrequently represented on top author lists.
- Using the Scopus database to search for the 50 most published dermatology authors from January 1, 2017, to October 7, 2022, 30% were female.
- Higher proportions of female dermatology trainees as well as efforts to increase mentorship and research support for female dermatologists may improve equality in top lists of dermatology citations and h-index values.
Dermatology Author Gender Trends During the COVID-19 Pandemic
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.
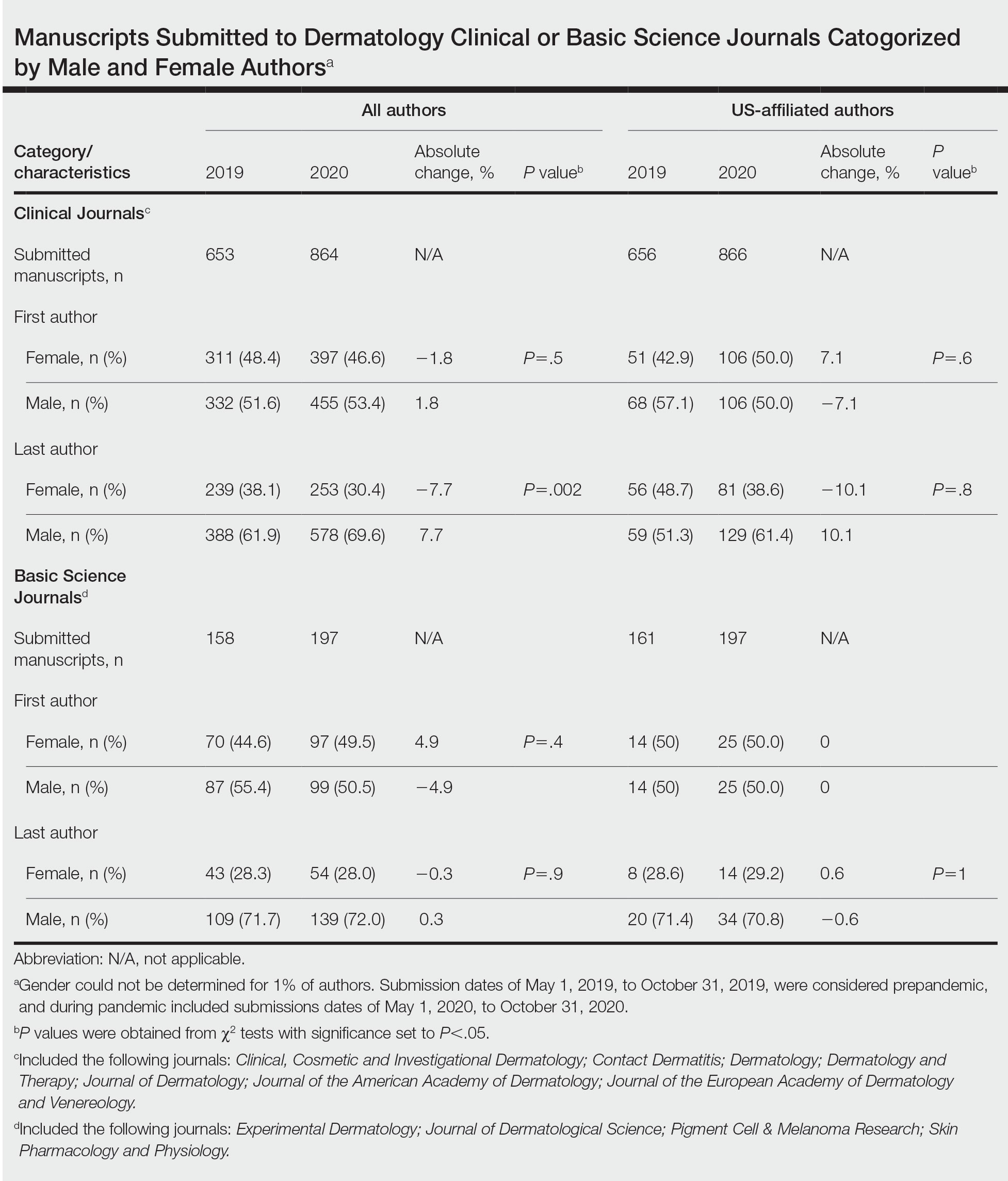
Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.

Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.

Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
Practice Points
- The academic productivity of female dermatologists as last authors in dermatology clinical journals has potentially been impacted by the COVID-19 pandemic.
- To potentially aid in the resurgence of female dermatologist authors impacted by the pandemic, academic institutions and funding agencies may consider implementing strategies such as extending grant end dates, providing dedicated funding opportunities, and prioritizing female-authored submissions in dermatology research.
Cross-sectional Analysis of Matched Dermatology Residency Applicants Without US Home Programs
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.
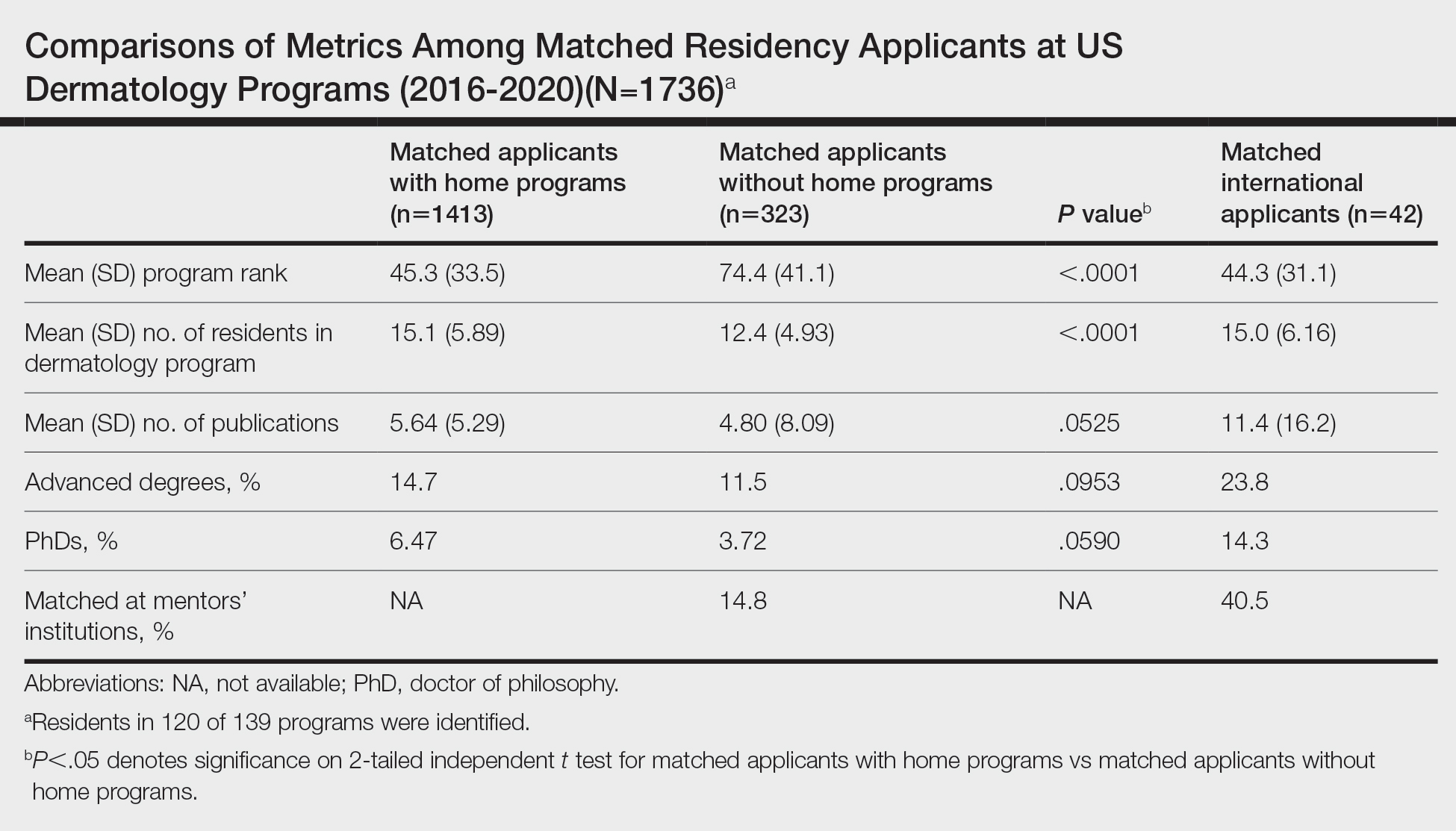
Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.

Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.

Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
Practice Points
- Our study suggests that matched dermatology applicants with and without home programs (HPs) had similar achievements, on average, for number of publications and holding advanced degrees.
- Because of the potential benefits of having program connections for matching in dermatology, applicants without HPs should seek dermatology research mentors.
Polyurethane Tubing to Minimize Pain During Nail Injections
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
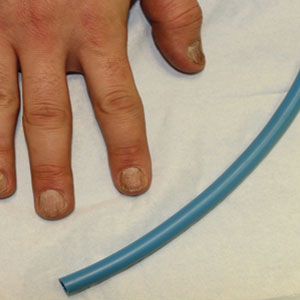
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.
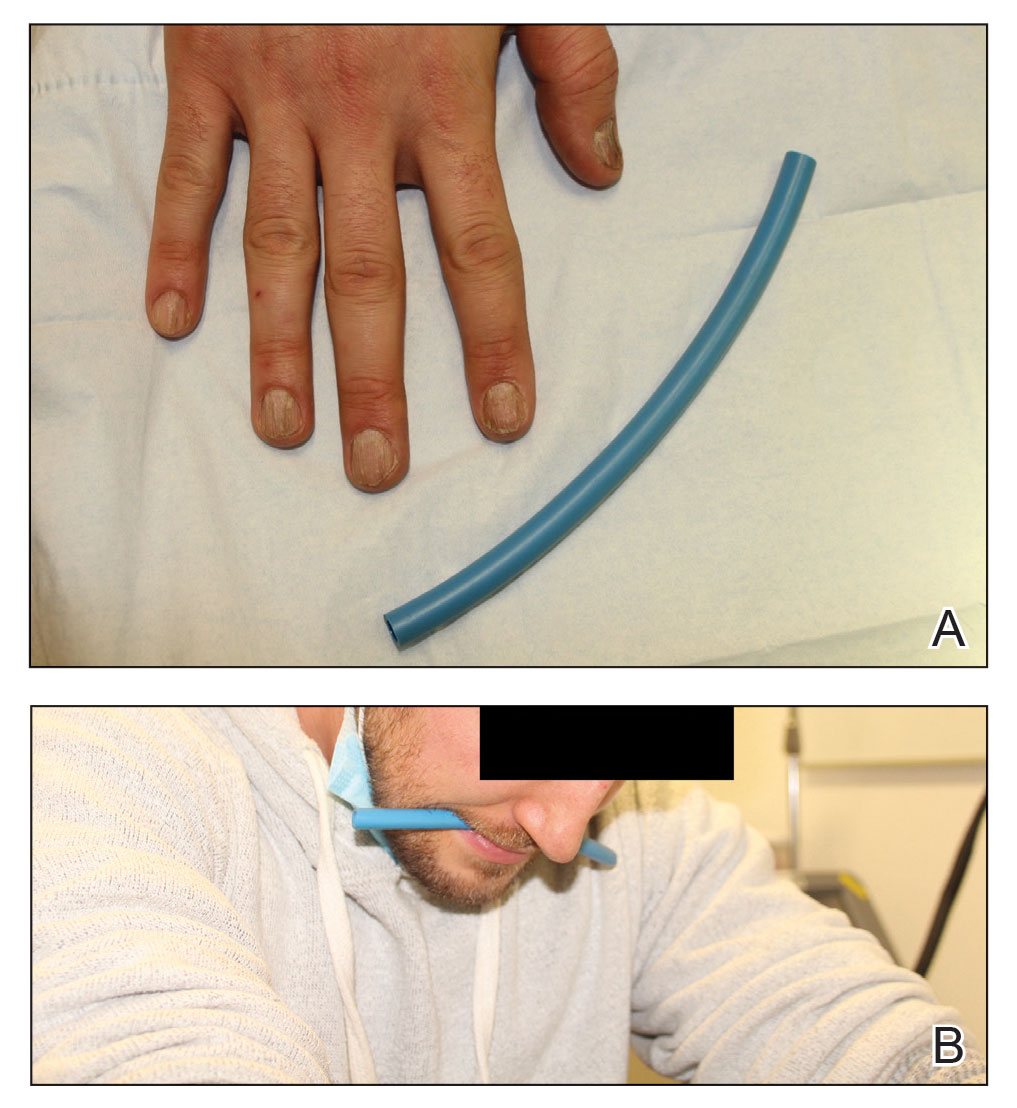
What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.

What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.

What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
A “Solution” for Patients Unable to Swallow a Pill: Crushed Terbinafine Mixed With Syrup
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
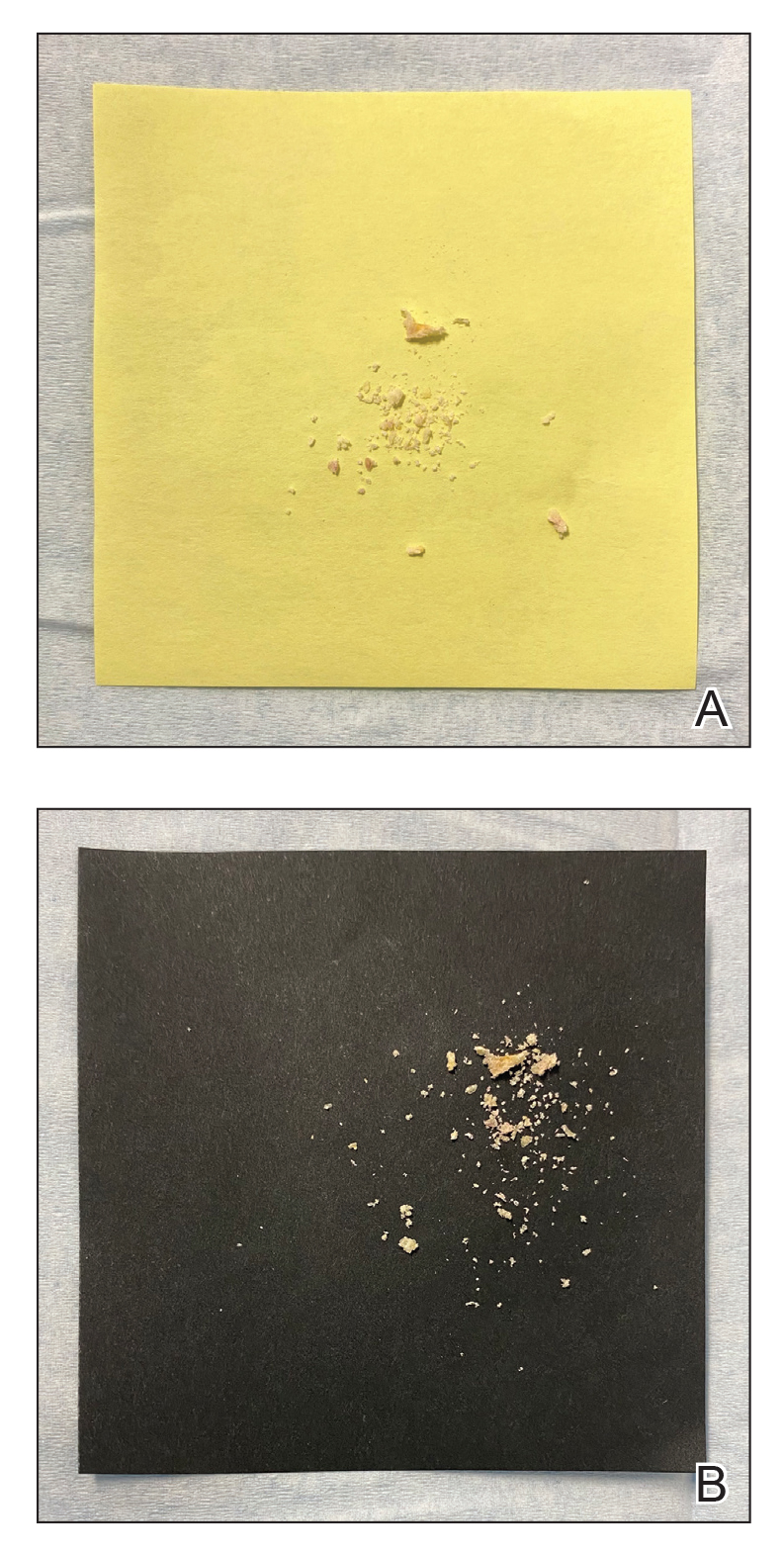
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
Dermatology Articles in Preprint Servers: A Cross-sectional Study
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).
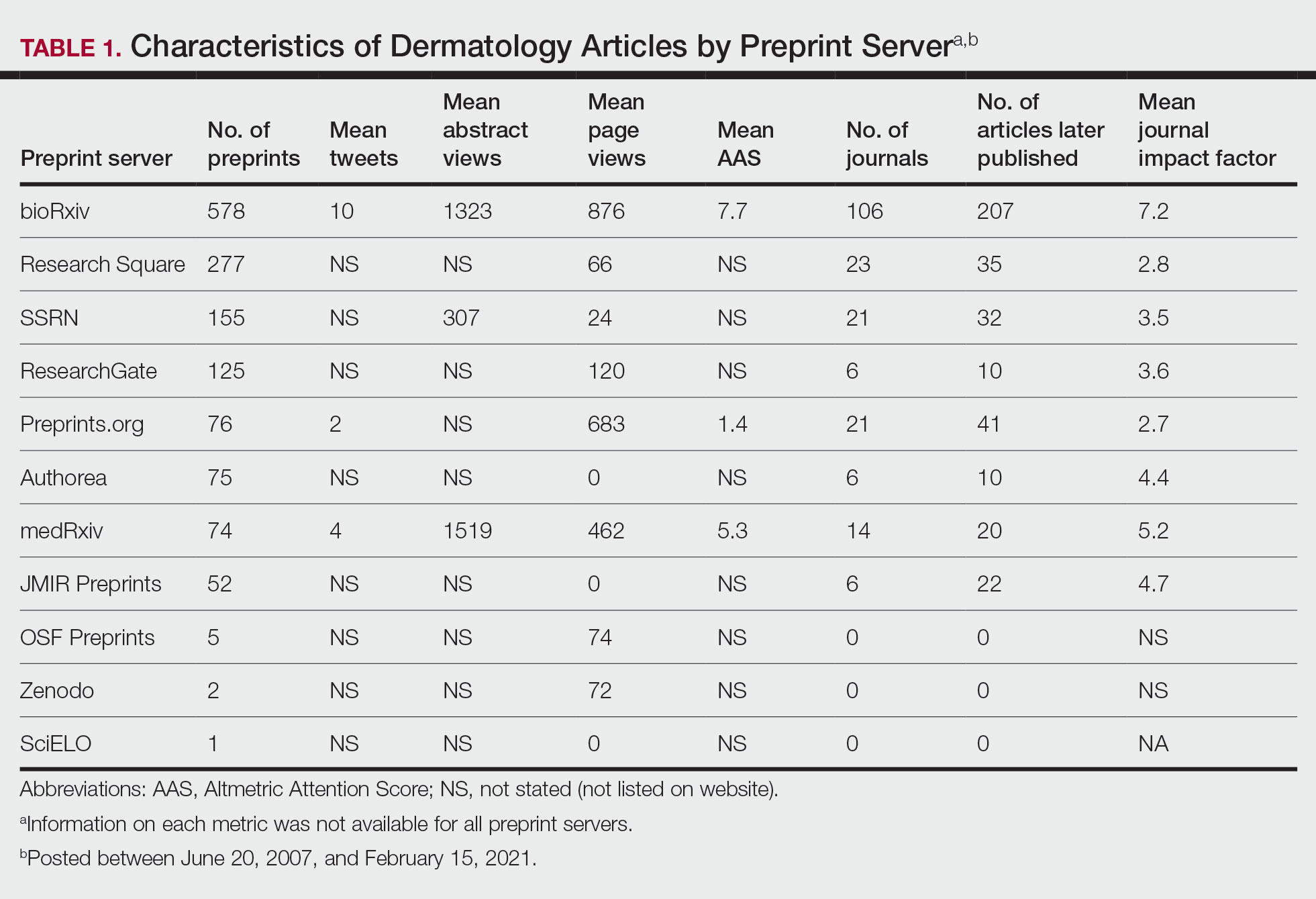
A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.
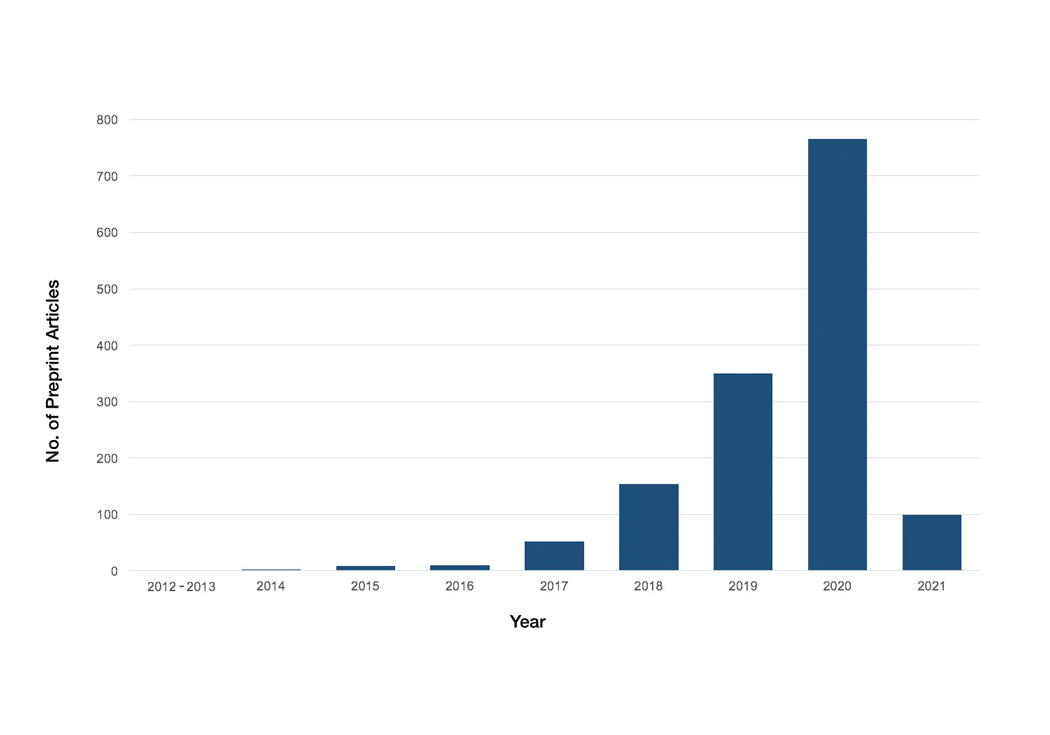
Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.
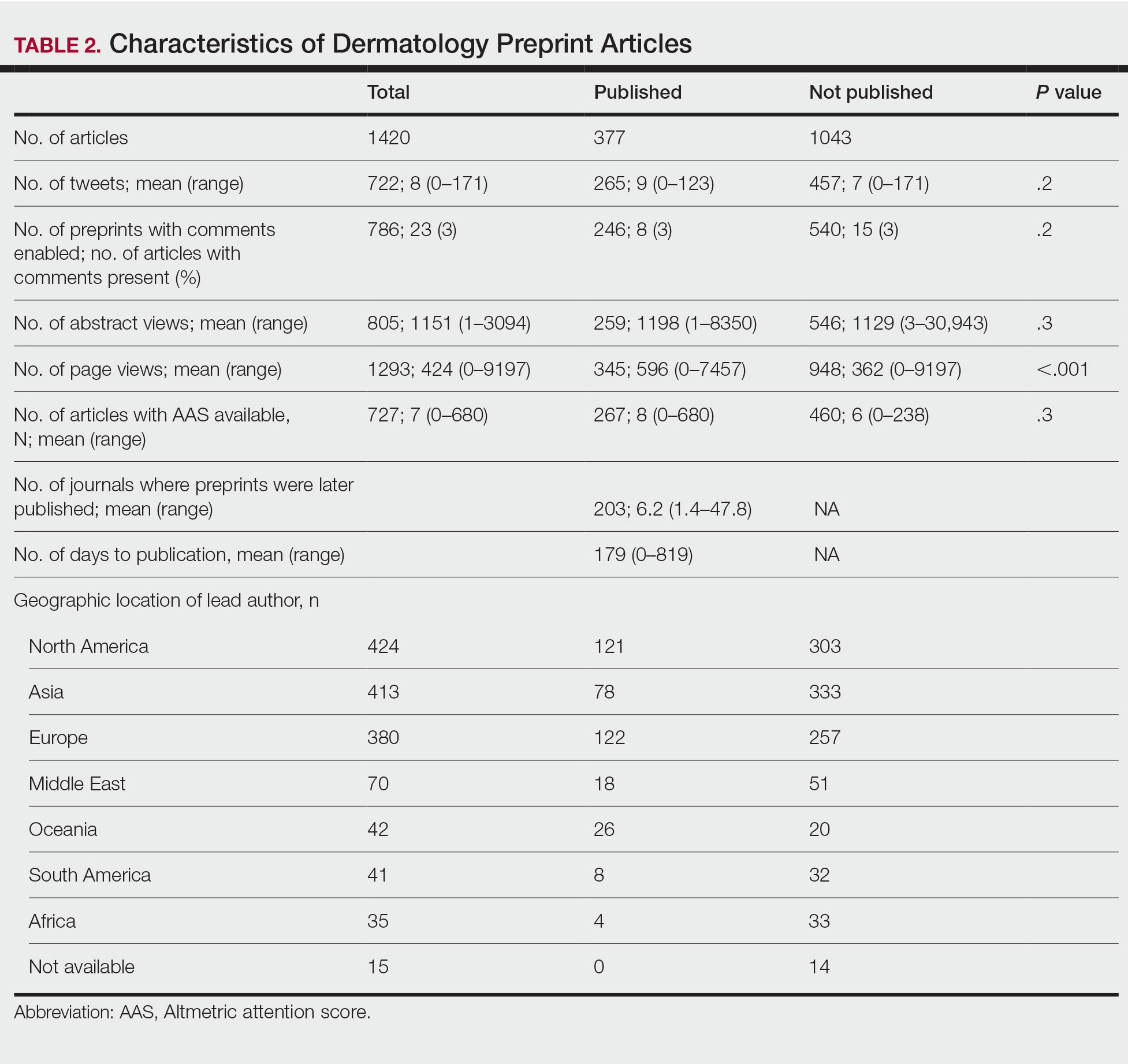
Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
PRACTICE POINTS
- Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals.
- The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission; therefore, dermatologists may use these servers to disseminate research quickly and freely but may not receive constructive criticism.
- Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice.
Nail Changes Associated With Thyroid Disease
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.
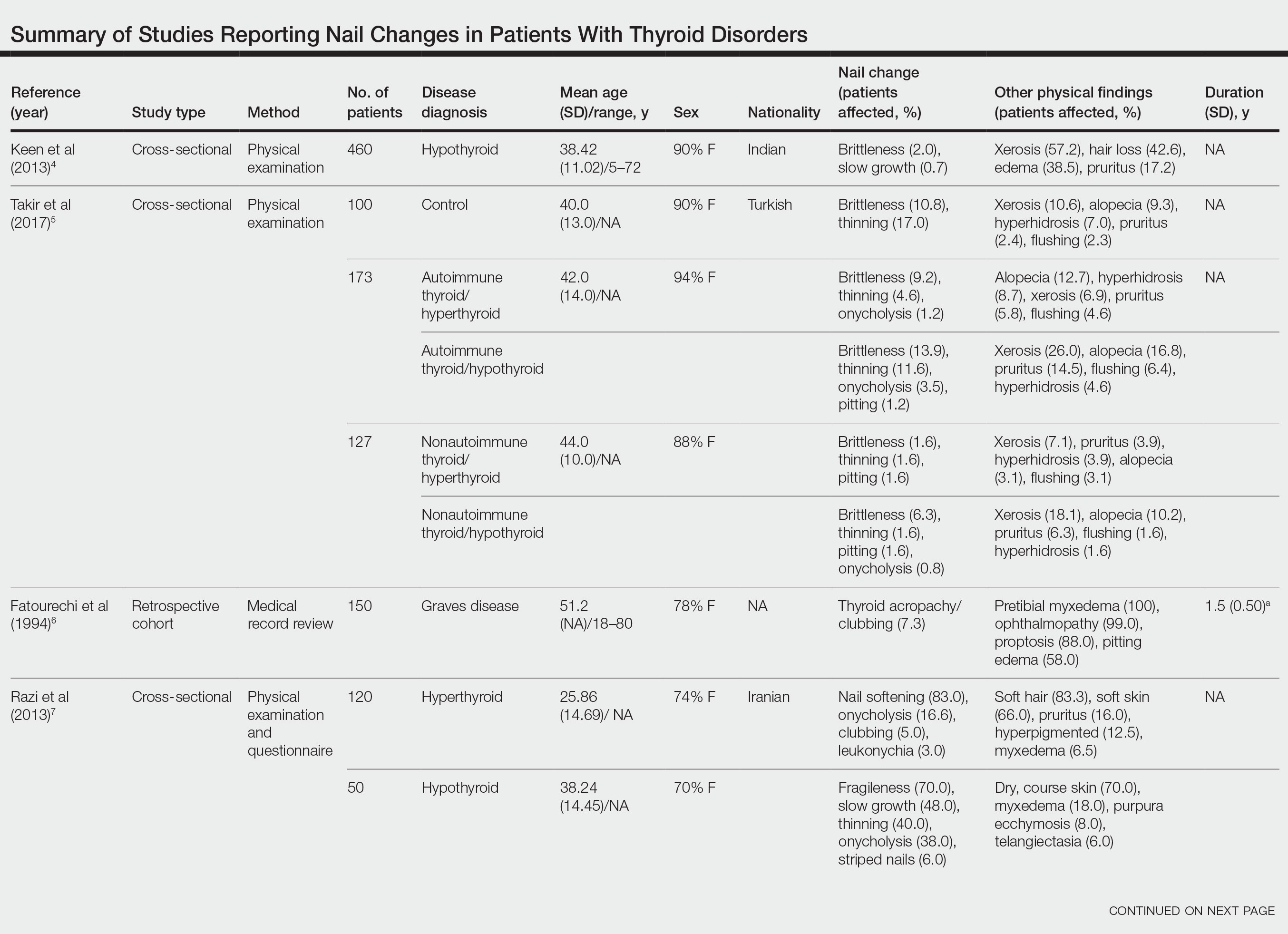
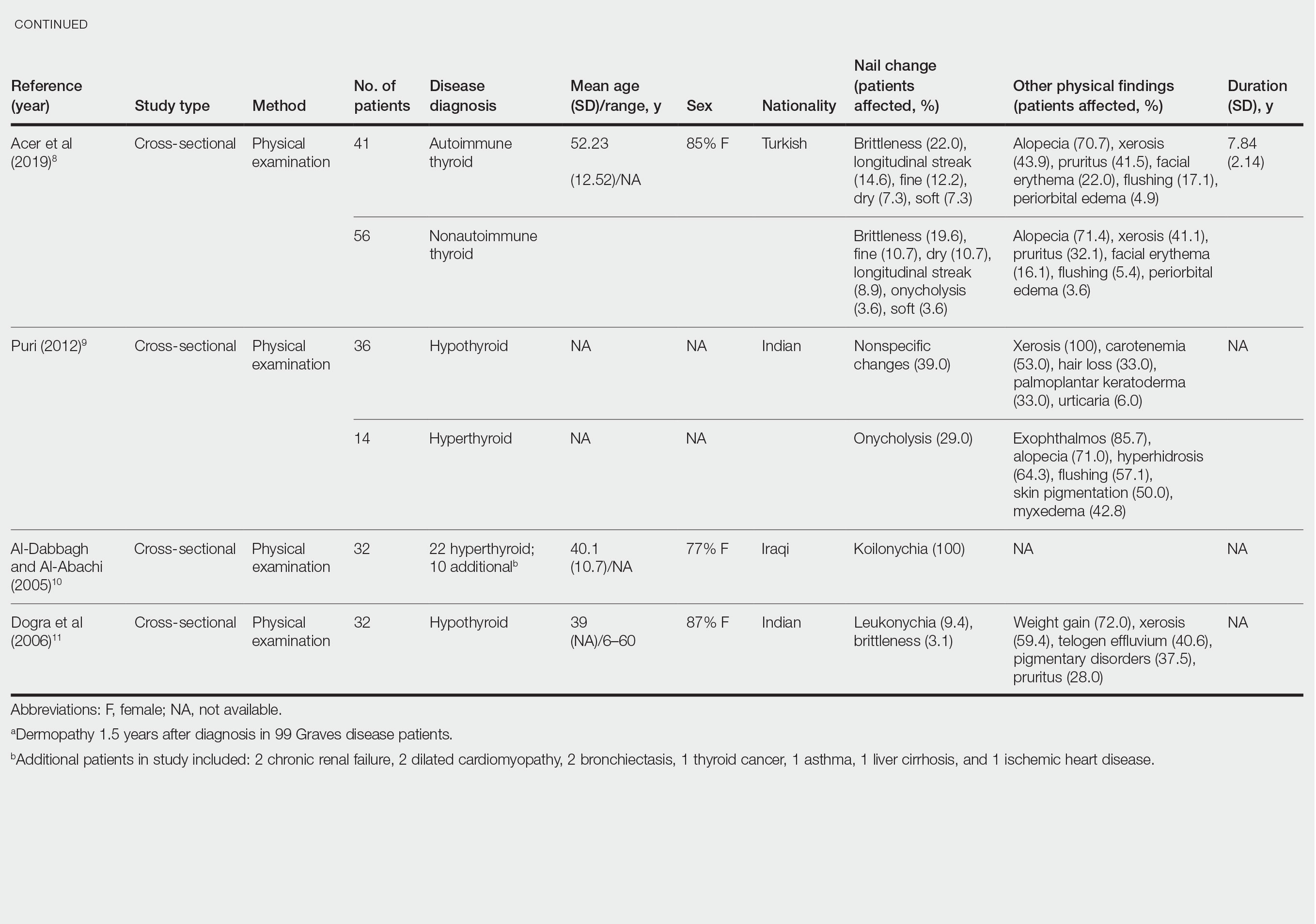
Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
Practice Points
- Koilonychia is associated with hyperthyroidism.
- Clubbing is a manifestation of thyroid acropachy in Graves disease and also affects other patients with hyperthyroidism.
- Onycholysis improves in patients with hypothyroidism treated with thyroid hormone replacement therapy.
Online Information About Hydroquinone: An Assessment of Accuracy and Readability
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3
Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3
Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3
Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
Practice Points
- Hydroquinone (HQ) 4% is US Food and Drug Administration (FDA) approved for skin hyperpigmentation including melasma.
- In September 2020, the FDA enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform, announcing that HQ is not classified as Category II/not generally recognized as safe and effective, thus prohibiting the distribution of OTC HQ products.
- Exogenous ochronosis is a potential side effect associated with HQ.
- There is a need for dermatologists to develop online patient education materials on HQ that are characterized by high-quality and up-to-date medical information.
A Contrasting Dark Background for Nail Sampling
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
The Top 100 Most-Cited Articles on Nail Psoriasis: A Bibliometric Analysis
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072