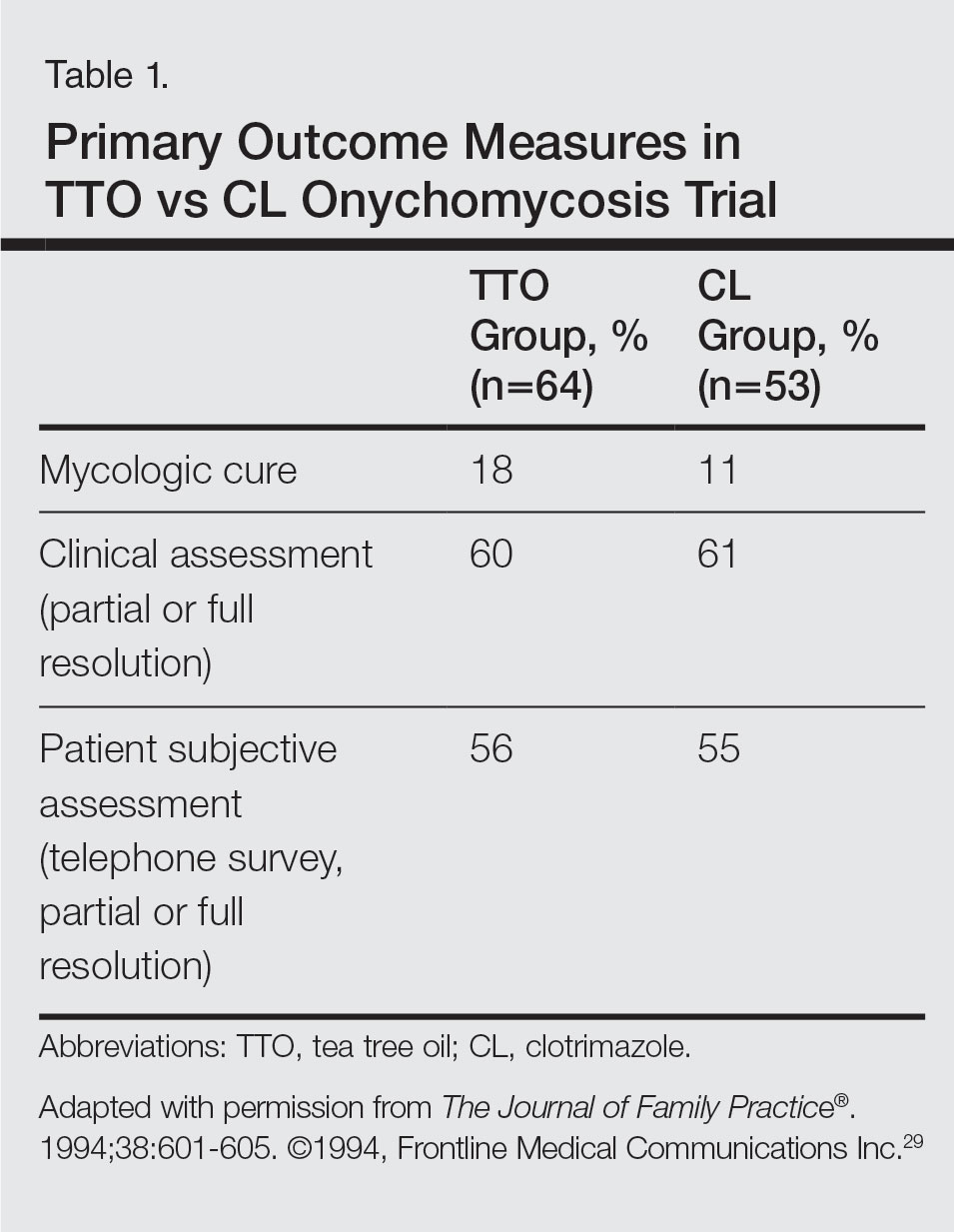
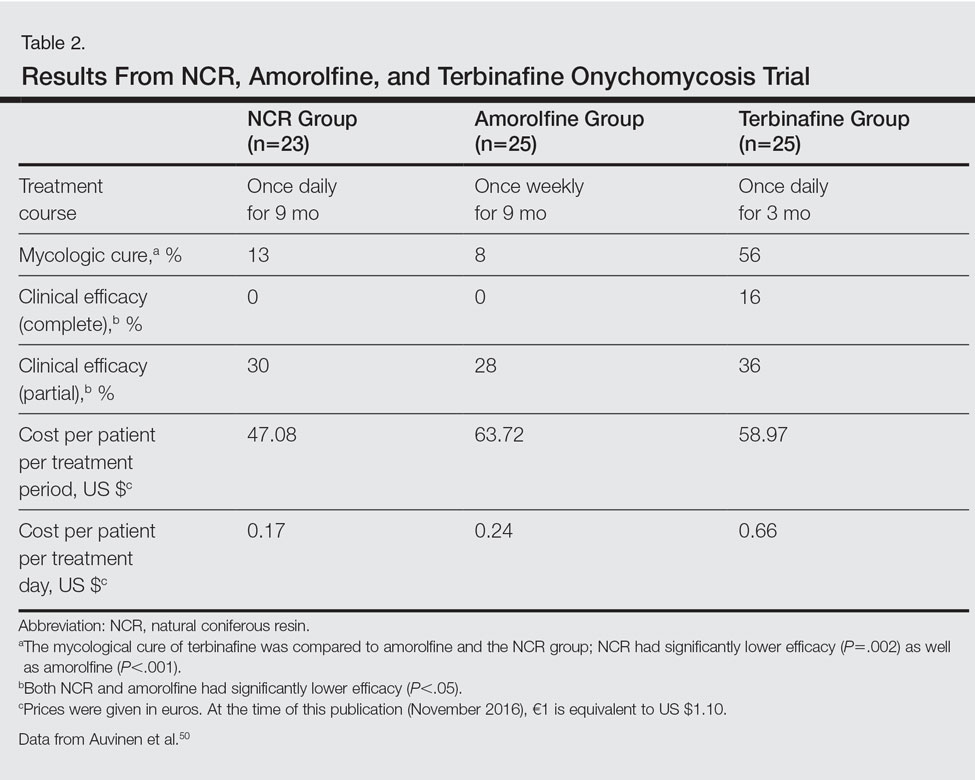
User login
Enlarging Nodule on the Nipple
The Diagnosis: Nipple Adenoma (Florid Papillomatosis of the Nipple)
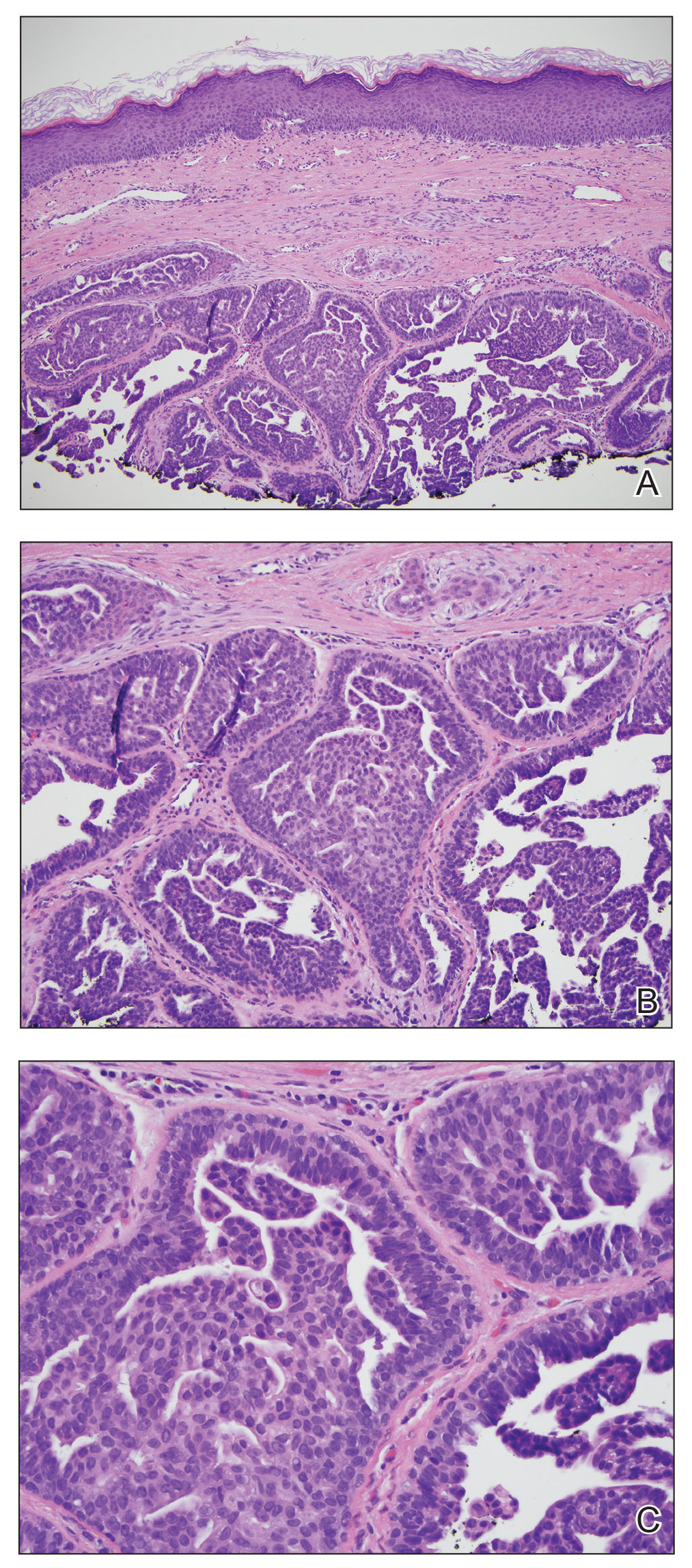
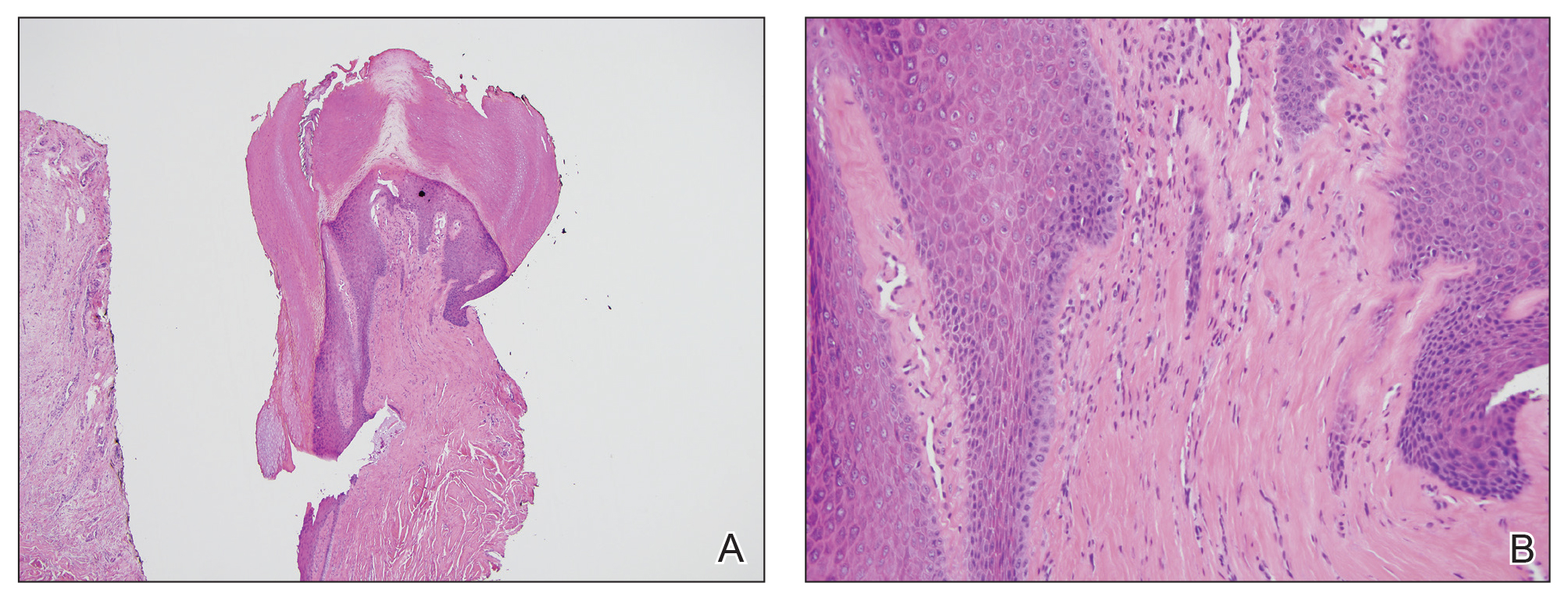
Biopsy of the nodule showed florid papillary hyperplasia of the ductal epithelium within the dermis that was sharply demarcated from the background stroma (Figure, A and B). Neither cytological nor architectural atypia were evident. There was no notable necrosis (Figure C). There was a background of fibrosis whereby the glandular ductal structures assumed a somewhat irregular growth pattern within the dermis with attendant hemorrhage. The patient underwent complete excision of the lesion. No evidence of carcinoma was seen on the final pathology, and the final margins were negative.
First described in 1923 and fully characterized in 1955, nipple adenoma (also known as florid papillomatosis of the nipple) is a benign proliferative neoplasm that originates in the lactiferous ducts of the nipple.1,2 It most commonly affects women aged 40 to 50 years (range, 0-89 years); less than 5% of cases are reported in men.3,4 It predominantly is unilateral, with only rare cases of bilateral papillomatosis reported. Patients often present with serous or serosanguineous discharge and an itching or burning sensation. Symptoms may worsen with the menstrual cycle.4 On physical examination, it presents as an ill-defined red nodule on the nipple with crusting, erosion, or erythema of the nipple surface. Although imaging generally is not used to confirm the diagnosis, mammography should be performed prior to biopsy to rule out underlying breast pathology. Dermoscopy may show linear cherry red structures or red serpiginous and annular structures.5,6 The differential diagnosis of nipple adenoma includes Paget disease of the breast, adenomyoepithelioma, subareolar subsclerosing duct hyperplasia, syringomatous adenoma, adenosis tumor, low-grade adenosquamous carcinoma, low-grade ductal carcinoma in situ, tubular carcinoma, and sweat gland tumors.3
Microscopic features of nipple adenoma have been categorized into 4 subtypes: sclerosing papillomatosis, papillomatosis, adenosis, and a mixed pattern.3,7 The tumors may have keratin cysts and focal necrosis but no atypia, and the myoepithelial cell layer is retained. Nipple adenomas show a glandular proliferation in the dermis that is relatively well circumscribed with glands that vary in appearance between a simple adenosislike pattern of growth to a papillary hyperplasia and/or usual ductal hyperplasia growth pattern. A pseudoinfiltrative pattern can occur when the glandular epithelium is entrapped within stromal fibrosis; however, the myoepithelial layer is retained. Occasionally, the glandular epithelium can grow in continuity with the surface squamous epithelium of the nipple, clinically simulating Paget disease of the breast.8 Immunohistochemical stains, specifically p63, p40, calponin 1, h-caldesmon, cytokeratin 5/6, CD10, and α; smooth muscle actin, highlight the myoepithelial cells, while cytokeratin 7 identifies the ductal epithelium, supporting the diagnosis.6 In addition to biopsy and microscopic tissue examination, touch preparation cytology, curettage cytology, and fine needle aspiration techniques have been used to perform cytologic examination of the lesions, aiding in identification of the benign or malignant nature of the neoplasm.6 Nipple adenoma also is referred to as florid papillomatosis of the nipple, papillary adenoma, erosive adenomatosis, and subareolar duct papillomatosis.7
Although nipple adenoma is a benign tumor, up to 16.5% of affected patients had an ipsilateral or contralateral mammary carcinoma.9 The majority arose coincidentally but separately in the same breast, and carcinoma arose directly from the nipple adenoma in 8 cases; 3 cases were carcinomas that arose in men.10 A definitive association or causal relationship between nipple adenoma and subsequent development of breast cancer has not been identified, and the incidence of nipple adenoma in patients with a positive family history of breast cancer has not been examined. Therefore, although various treatments including cryosurgery, nipple splitting enucleation, and Mohs micrographic surgery have been proposed, complete excision remains the gold standard of therapy. Regular breast examinations and digital mammography are necessary to screen for local recurrences.
- Miller E, Lewis D. The significance of serohemorrhagic or hemorrhagic discharge from the nipple. JAMA. 1923;81:1651-1657.
- Jones DB. Florid papillomatosis of the nipple ducts. Cancer. 1955;8:315-319.
- Rosen PP. Rosen's Breast Pathology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:120-128.
- Brownstein MH, Phelps RG, Maqnin PH. Papillary adenoma of the nipple: analysis of fifteen new cases. J Am Acad Dermatol. 1985;12:707-715.
- Takashima S, Fujita Y, Miyauchi T, et al. Dermoscopic observation in adenoma of the nipple. J Dermatol. 2015;42:341-342.
- Spohn G, Trotter S, Tozbikian G, et al. Nipple adenoma in a female patient presenting with persistent erythema of the right nipple skin: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin. BMC Dermatol. 2016;16:4.
- Shin SJ. Nipple adenoma (florid papillomatosis of the nipple). In: Dabbs DJ, ed. Breast Pathology. Philadelphia, PA: Elsevier Saunders; 2012:286-292.
- Schnitt SJ, Collins LC. Biopsy Interpretation of the Breast. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
- Salemis NS. Florid papillomatosis of the nipple: a rare presentation and review of the literature. Breast Dis. 2015;35:153-156.
- Di Bonito M, Cantile M, Collina F, et al. Adenoma of the nipple: a clinicopathological report of 13 cases. Oncol Lett. 2014;7:1839-1842.
The Diagnosis: Nipple Adenoma (Florid Papillomatosis of the Nipple)
Biopsy of the nodule showed florid papillary hyperplasia of the ductal epithelium within the dermis that was sharply demarcated from the background stroma (Figure, A and B). Neither cytological nor architectural atypia were evident. There was no notable necrosis (Figure C). There was a background of fibrosis whereby the glandular ductal structures assumed a somewhat irregular growth pattern within the dermis with attendant hemorrhage. The patient underwent complete excision of the lesion. No evidence of carcinoma was seen on the final pathology, and the final margins were negative.
First described in 1923 and fully characterized in 1955, nipple adenoma (also known as florid papillomatosis of the nipple) is a benign proliferative neoplasm that originates in the lactiferous ducts of the nipple.1,2 It most commonly affects women aged 40 to 50 years (range, 0-89 years); less than 5% of cases are reported in men.3,4 It predominantly is unilateral, with only rare cases of bilateral papillomatosis reported. Patients often present with serous or serosanguineous discharge and an itching or burning sensation. Symptoms may worsen with the menstrual cycle.4 On physical examination, it presents as an ill-defined red nodule on the nipple with crusting, erosion, or erythema of the nipple surface. Although imaging generally is not used to confirm the diagnosis, mammography should be performed prior to biopsy to rule out underlying breast pathology. Dermoscopy may show linear cherry red structures or red serpiginous and annular structures.5,6 The differential diagnosis of nipple adenoma includes Paget disease of the breast, adenomyoepithelioma, subareolar subsclerosing duct hyperplasia, syringomatous adenoma, adenosis tumor, low-grade adenosquamous carcinoma, low-grade ductal carcinoma in situ, tubular carcinoma, and sweat gland tumors.3
Microscopic features of nipple adenoma have been categorized into 4 subtypes: sclerosing papillomatosis, papillomatosis, adenosis, and a mixed pattern.3,7 The tumors may have keratin cysts and focal necrosis but no atypia, and the myoepithelial cell layer is retained. Nipple adenomas show a glandular proliferation in the dermis that is relatively well circumscribed with glands that vary in appearance between a simple adenosislike pattern of growth to a papillary hyperplasia and/or usual ductal hyperplasia growth pattern. A pseudoinfiltrative pattern can occur when the glandular epithelium is entrapped within stromal fibrosis; however, the myoepithelial layer is retained. Occasionally, the glandular epithelium can grow in continuity with the surface squamous epithelium of the nipple, clinically simulating Paget disease of the breast.8 Immunohistochemical stains, specifically p63, p40, calponin 1, h-caldesmon, cytokeratin 5/6, CD10, and α; smooth muscle actin, highlight the myoepithelial cells, while cytokeratin 7 identifies the ductal epithelium, supporting the diagnosis.6 In addition to biopsy and microscopic tissue examination, touch preparation cytology, curettage cytology, and fine needle aspiration techniques have been used to perform cytologic examination of the lesions, aiding in identification of the benign or malignant nature of the neoplasm.6 Nipple adenoma also is referred to as florid papillomatosis of the nipple, papillary adenoma, erosive adenomatosis, and subareolar duct papillomatosis.7
Although nipple adenoma is a benign tumor, up to 16.5% of affected patients had an ipsilateral or contralateral mammary carcinoma.9 The majority arose coincidentally but separately in the same breast, and carcinoma arose directly from the nipple adenoma in 8 cases; 3 cases were carcinomas that arose in men.10 A definitive association or causal relationship between nipple adenoma and subsequent development of breast cancer has not been identified, and the incidence of nipple adenoma in patients with a positive family history of breast cancer has not been examined. Therefore, although various treatments including cryosurgery, nipple splitting enucleation, and Mohs micrographic surgery have been proposed, complete excision remains the gold standard of therapy. Regular breast examinations and digital mammography are necessary to screen for local recurrences.
The Diagnosis: Nipple Adenoma (Florid Papillomatosis of the Nipple)
Biopsy of the nodule showed florid papillary hyperplasia of the ductal epithelium within the dermis that was sharply demarcated from the background stroma (Figure, A and B). Neither cytological nor architectural atypia were evident. There was no notable necrosis (Figure C). There was a background of fibrosis whereby the glandular ductal structures assumed a somewhat irregular growth pattern within the dermis with attendant hemorrhage. The patient underwent complete excision of the lesion. No evidence of carcinoma was seen on the final pathology, and the final margins were negative.
First described in 1923 and fully characterized in 1955, nipple adenoma (also known as florid papillomatosis of the nipple) is a benign proliferative neoplasm that originates in the lactiferous ducts of the nipple.1,2 It most commonly affects women aged 40 to 50 years (range, 0-89 years); less than 5% of cases are reported in men.3,4 It predominantly is unilateral, with only rare cases of bilateral papillomatosis reported. Patients often present with serous or serosanguineous discharge and an itching or burning sensation. Symptoms may worsen with the menstrual cycle.4 On physical examination, it presents as an ill-defined red nodule on the nipple with crusting, erosion, or erythema of the nipple surface. Although imaging generally is not used to confirm the diagnosis, mammography should be performed prior to biopsy to rule out underlying breast pathology. Dermoscopy may show linear cherry red structures or red serpiginous and annular structures.5,6 The differential diagnosis of nipple adenoma includes Paget disease of the breast, adenomyoepithelioma, subareolar subsclerosing duct hyperplasia, syringomatous adenoma, adenosis tumor, low-grade adenosquamous carcinoma, low-grade ductal carcinoma in situ, tubular carcinoma, and sweat gland tumors.3
Microscopic features of nipple adenoma have been categorized into 4 subtypes: sclerosing papillomatosis, papillomatosis, adenosis, and a mixed pattern.3,7 The tumors may have keratin cysts and focal necrosis but no atypia, and the myoepithelial cell layer is retained. Nipple adenomas show a glandular proliferation in the dermis that is relatively well circumscribed with glands that vary in appearance between a simple adenosislike pattern of growth to a papillary hyperplasia and/or usual ductal hyperplasia growth pattern. A pseudoinfiltrative pattern can occur when the glandular epithelium is entrapped within stromal fibrosis; however, the myoepithelial layer is retained. Occasionally, the glandular epithelium can grow in continuity with the surface squamous epithelium of the nipple, clinically simulating Paget disease of the breast.8 Immunohistochemical stains, specifically p63, p40, calponin 1, h-caldesmon, cytokeratin 5/6, CD10, and α; smooth muscle actin, highlight the myoepithelial cells, while cytokeratin 7 identifies the ductal epithelium, supporting the diagnosis.6 In addition to biopsy and microscopic tissue examination, touch preparation cytology, curettage cytology, and fine needle aspiration techniques have been used to perform cytologic examination of the lesions, aiding in identification of the benign or malignant nature of the neoplasm.6 Nipple adenoma also is referred to as florid papillomatosis of the nipple, papillary adenoma, erosive adenomatosis, and subareolar duct papillomatosis.7
Although nipple adenoma is a benign tumor, up to 16.5% of affected patients had an ipsilateral or contralateral mammary carcinoma.9 The majority arose coincidentally but separately in the same breast, and carcinoma arose directly from the nipple adenoma in 8 cases; 3 cases were carcinomas that arose in men.10 A definitive association or causal relationship between nipple adenoma and subsequent development of breast cancer has not been identified, and the incidence of nipple adenoma in patients with a positive family history of breast cancer has not been examined. Therefore, although various treatments including cryosurgery, nipple splitting enucleation, and Mohs micrographic surgery have been proposed, complete excision remains the gold standard of therapy. Regular breast examinations and digital mammography are necessary to screen for local recurrences.
- Miller E, Lewis D. The significance of serohemorrhagic or hemorrhagic discharge from the nipple. JAMA. 1923;81:1651-1657.
- Jones DB. Florid papillomatosis of the nipple ducts. Cancer. 1955;8:315-319.
- Rosen PP. Rosen's Breast Pathology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:120-128.
- Brownstein MH, Phelps RG, Maqnin PH. Papillary adenoma of the nipple: analysis of fifteen new cases. J Am Acad Dermatol. 1985;12:707-715.
- Takashima S, Fujita Y, Miyauchi T, et al. Dermoscopic observation in adenoma of the nipple. J Dermatol. 2015;42:341-342.
- Spohn G, Trotter S, Tozbikian G, et al. Nipple adenoma in a female patient presenting with persistent erythema of the right nipple skin: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin. BMC Dermatol. 2016;16:4.
- Shin SJ. Nipple adenoma (florid papillomatosis of the nipple). In: Dabbs DJ, ed. Breast Pathology. Philadelphia, PA: Elsevier Saunders; 2012:286-292.
- Schnitt SJ, Collins LC. Biopsy Interpretation of the Breast. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
- Salemis NS. Florid papillomatosis of the nipple: a rare presentation and review of the literature. Breast Dis. 2015;35:153-156.
- Di Bonito M, Cantile M, Collina F, et al. Adenoma of the nipple: a clinicopathological report of 13 cases. Oncol Lett. 2014;7:1839-1842.
- Miller E, Lewis D. The significance of serohemorrhagic or hemorrhagic discharge from the nipple. JAMA. 1923;81:1651-1657.
- Jones DB. Florid papillomatosis of the nipple ducts. Cancer. 1955;8:315-319.
- Rosen PP. Rosen's Breast Pathology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:120-128.
- Brownstein MH, Phelps RG, Maqnin PH. Papillary adenoma of the nipple: analysis of fifteen new cases. J Am Acad Dermatol. 1985;12:707-715.
- Takashima S, Fujita Y, Miyauchi T, et al. Dermoscopic observation in adenoma of the nipple. J Dermatol. 2015;42:341-342.
- Spohn G, Trotter S, Tozbikian G, et al. Nipple adenoma in a female patient presenting with persistent erythema of the right nipple skin: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin. BMC Dermatol. 2016;16:4.
- Shin SJ. Nipple adenoma (florid papillomatosis of the nipple). In: Dabbs DJ, ed. Breast Pathology. Philadelphia, PA: Elsevier Saunders; 2012:286-292.
- Schnitt SJ, Collins LC. Biopsy Interpretation of the Breast. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
- Salemis NS. Florid papillomatosis of the nipple: a rare presentation and review of the literature. Breast Dis. 2015;35:153-156.
- Di Bonito M, Cantile M, Collina F, et al. Adenoma of the nipple: a clinicopathological report of 13 cases. Oncol Lett. 2014;7:1839-1842.
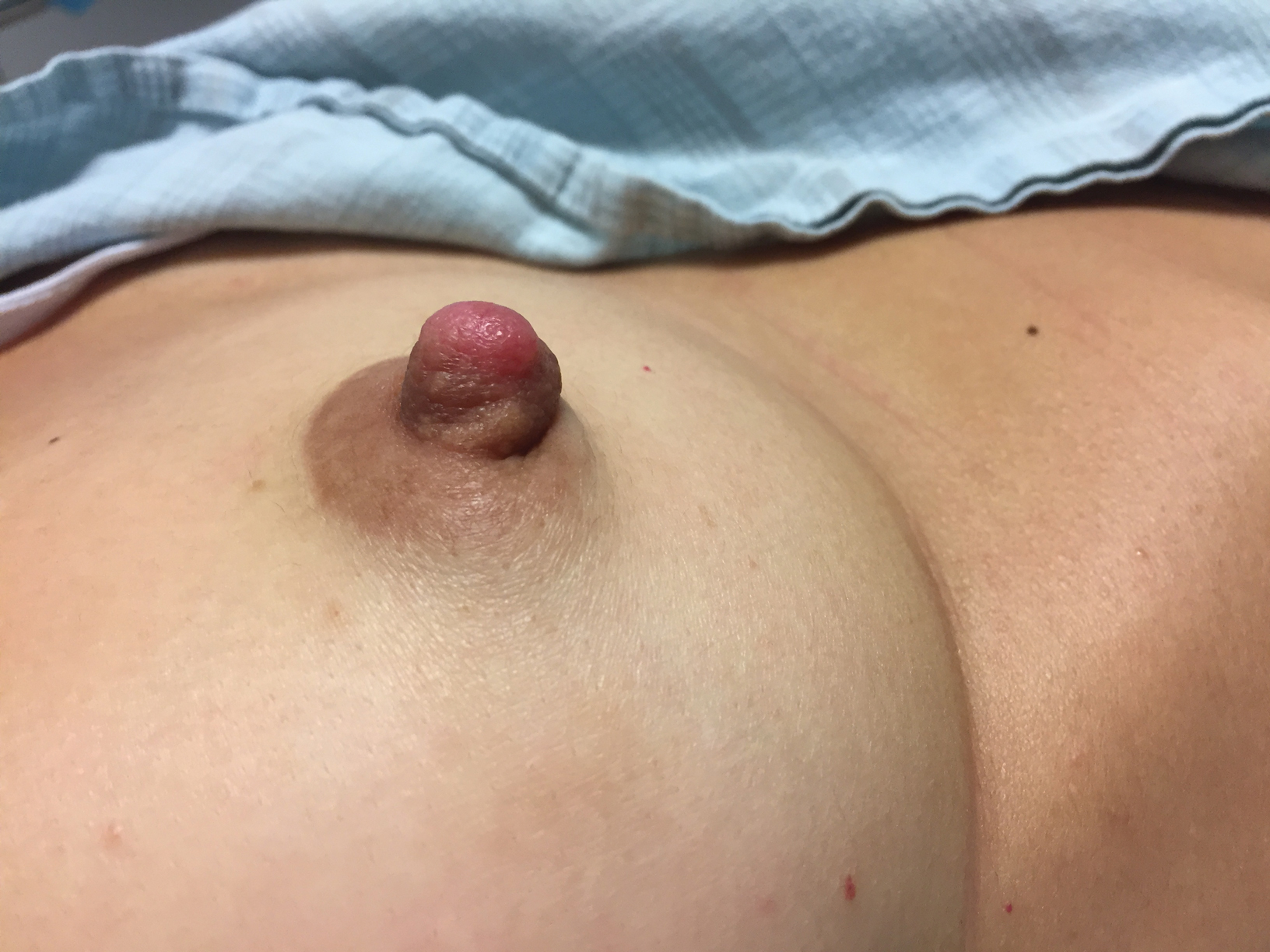
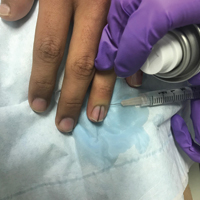
A healthy 48-year-old woman presented with a growth on the right nipple that had been slowly enlarging over the last few months. She initially noticed mild swelling in the area that persisted and formed a soft lump. She described mild pain with intermittent drainage but no bleeding. Her medical history was unremarkable, including a negative personal and family history of breast and skin cancer. She was taking no medications prior to development of the mass. She had no recent history of pregnancy or breastfeeding. A mammogram and breast ultrasound were not concerning for carcinoma. Physical examination showed a soft, exophytic, mildly tender, pink nodule on the right nipple that measured 12.2×7 mm; no drainage, bleeding, or ulceration was present. The surrounding skin of the areola and breast demonstrated no clinical changes. The contralateral breast, areola, and nipple were unaffected. The patient had no appreciable axillary or cervical lymphadenopathy. A deep shave biopsy of the nodule was performed and sent for histopathologic examination.
Clinical Pearl: Benzethonium Chloride for Habit-Tic Nail Deformity
Practice Gap
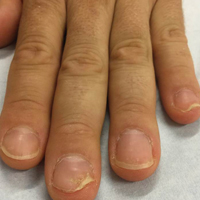
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
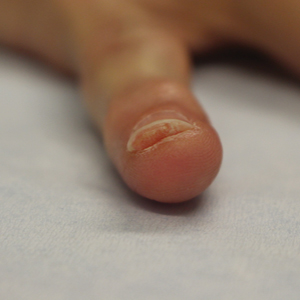
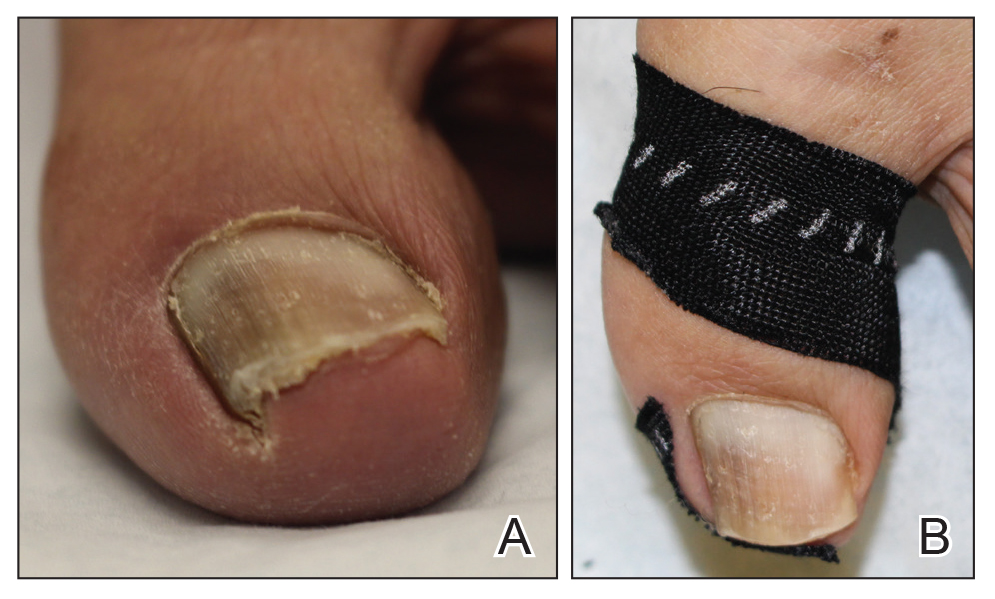
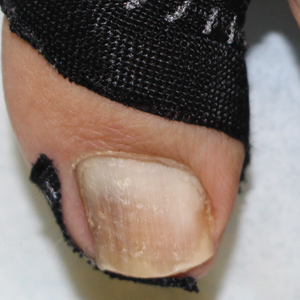
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).
Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).
Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).
Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
Acquired Digital Fibrokeratoma Presenting as a Painless Nodule on the Right Fifth Fingernail
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
Practice Points
- Acquired digital fibrokeratoma is a benign tumor of the nail bed and periungual area.
- Histopathology shows epidermal acanthosis and hyperkeratosis, and collagen bundles are arranged in a vertical orientation to the long axis of the epidermis.
- Acquired digital fibrokeratoma should be considered in the differential diagnosis of flesh-colored papules on the nail unit associated with longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.
Analysis of Nail-Related Content in the Basic Dermatology Curriculum
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
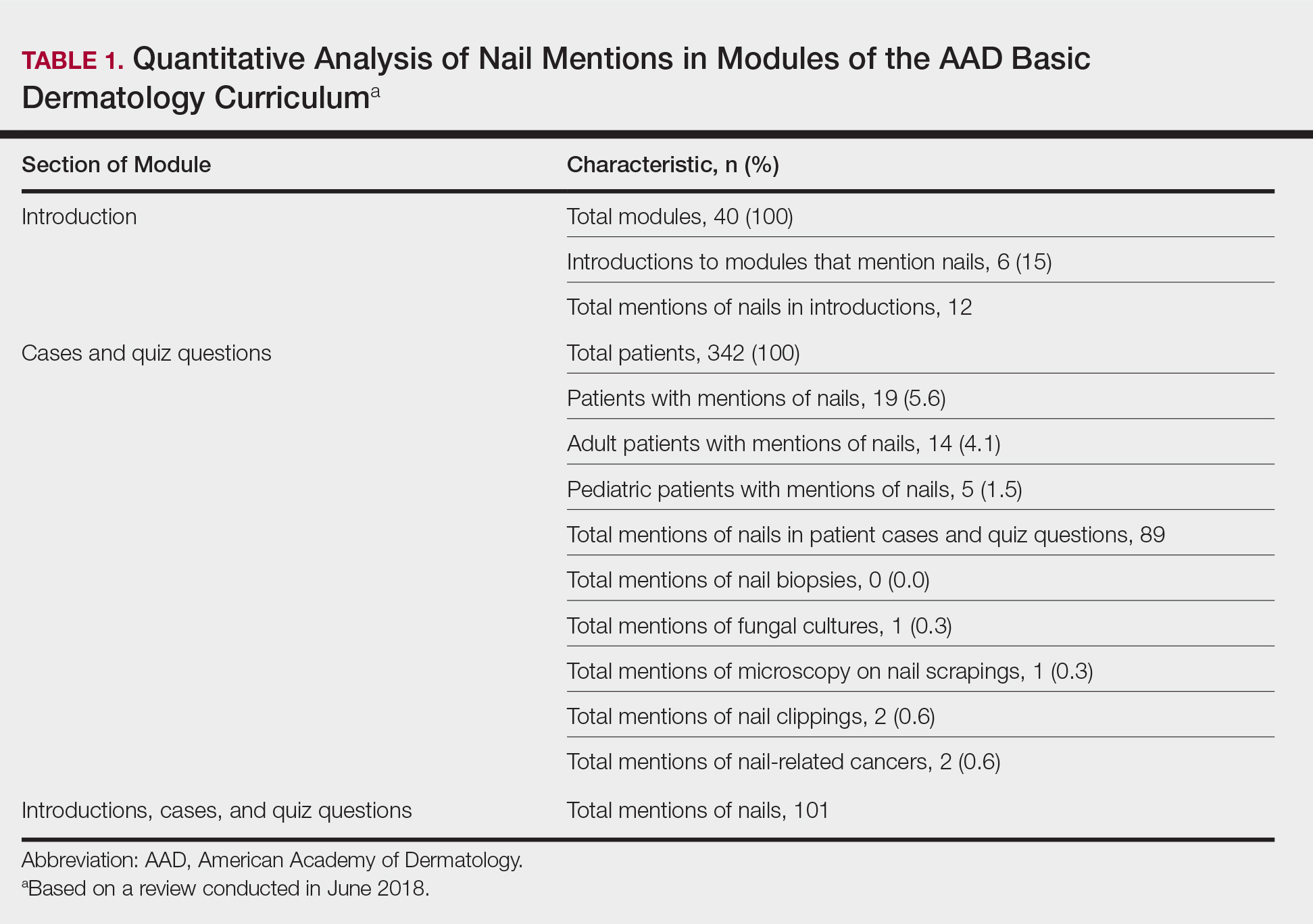
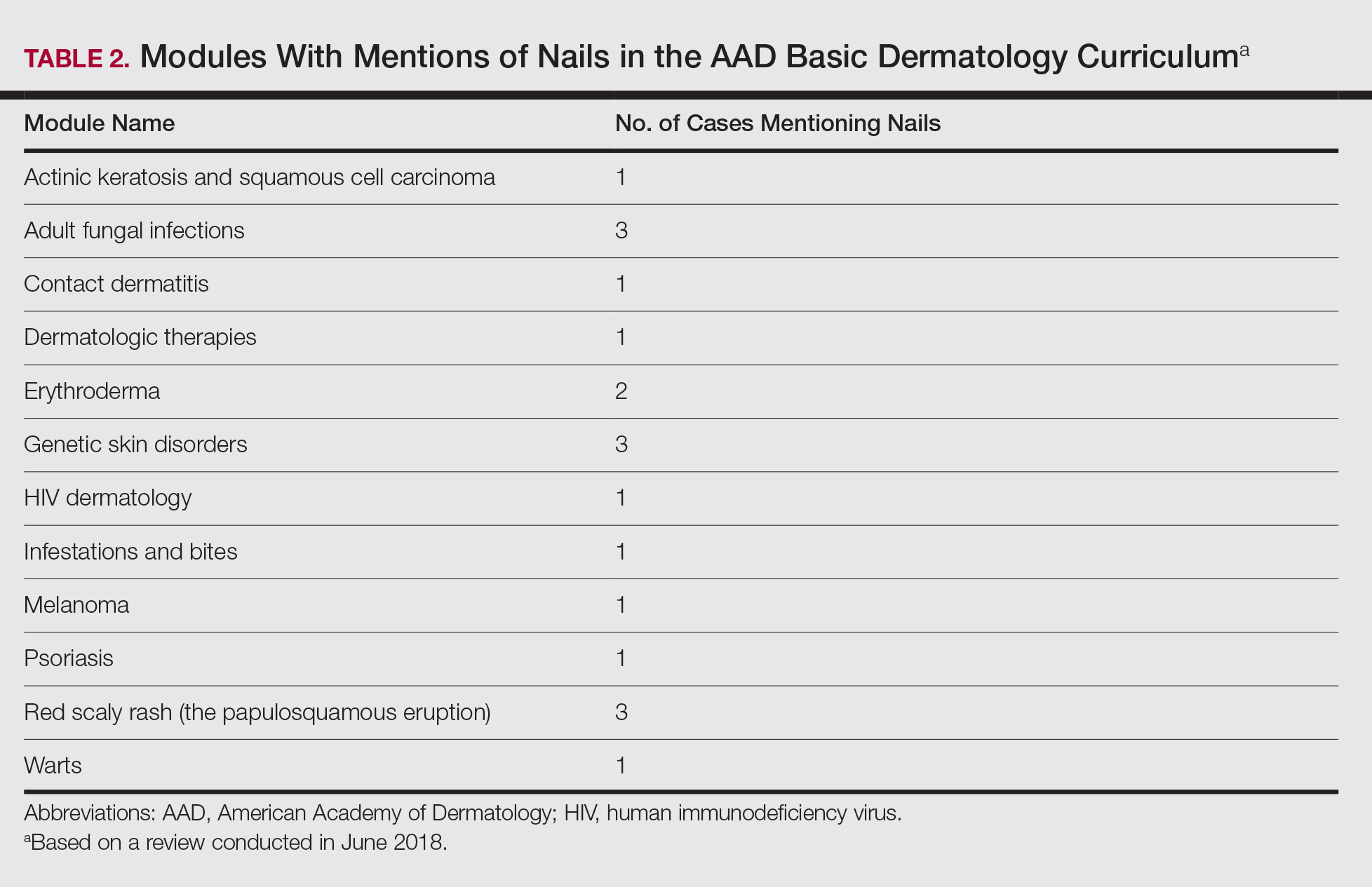
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3
Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3

Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3

Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
Practice Points
- Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving.
- Education on diagnosis and management of nail conditions is deficient in the American Academy of Dermatology (AAD) Basic Dermatology Curriculum.
- Increased efforts are needed to incorporate relevant nail education materials into the AAD Basic Dermatology Curriculum.
Clinical Pearl: Kinesiology Tape for Onychocryptosis
Practice Gap
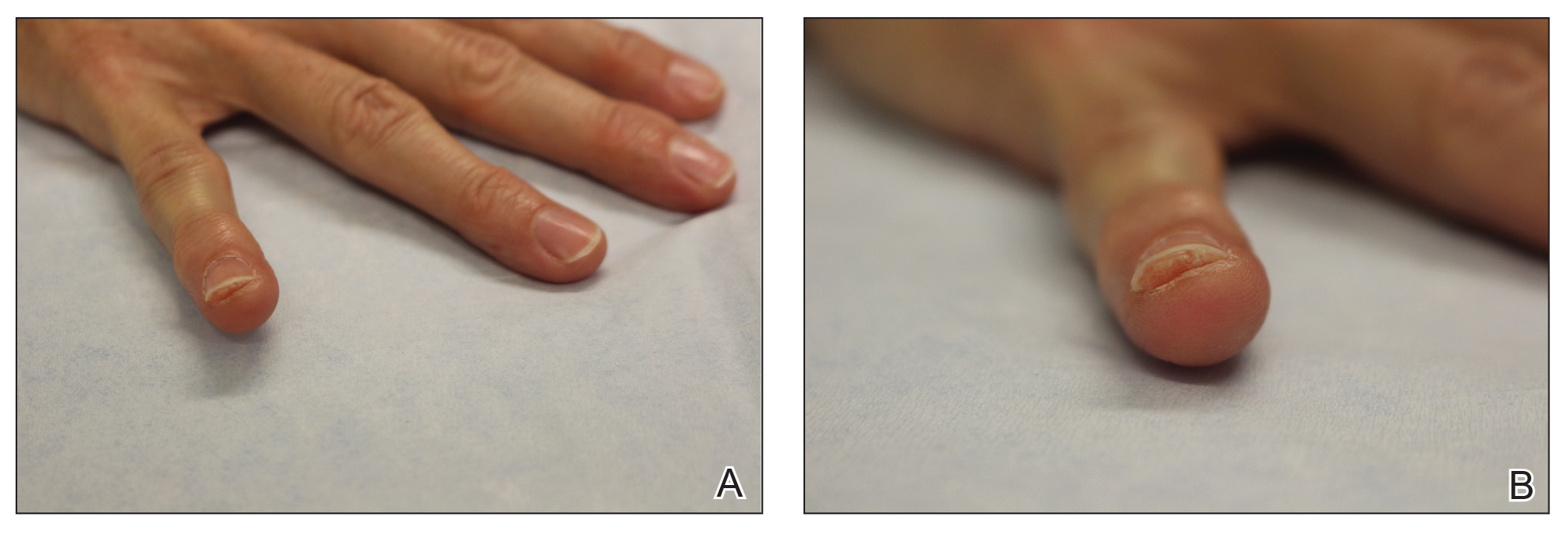
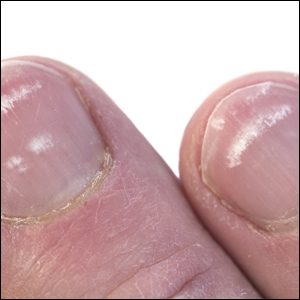
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
Practice Gap
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
Practice Gap
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
Optimizing Topical Therapy for Onychomycosis: The Importance of Patient Education
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
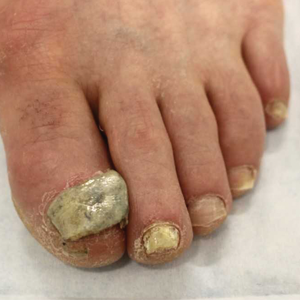
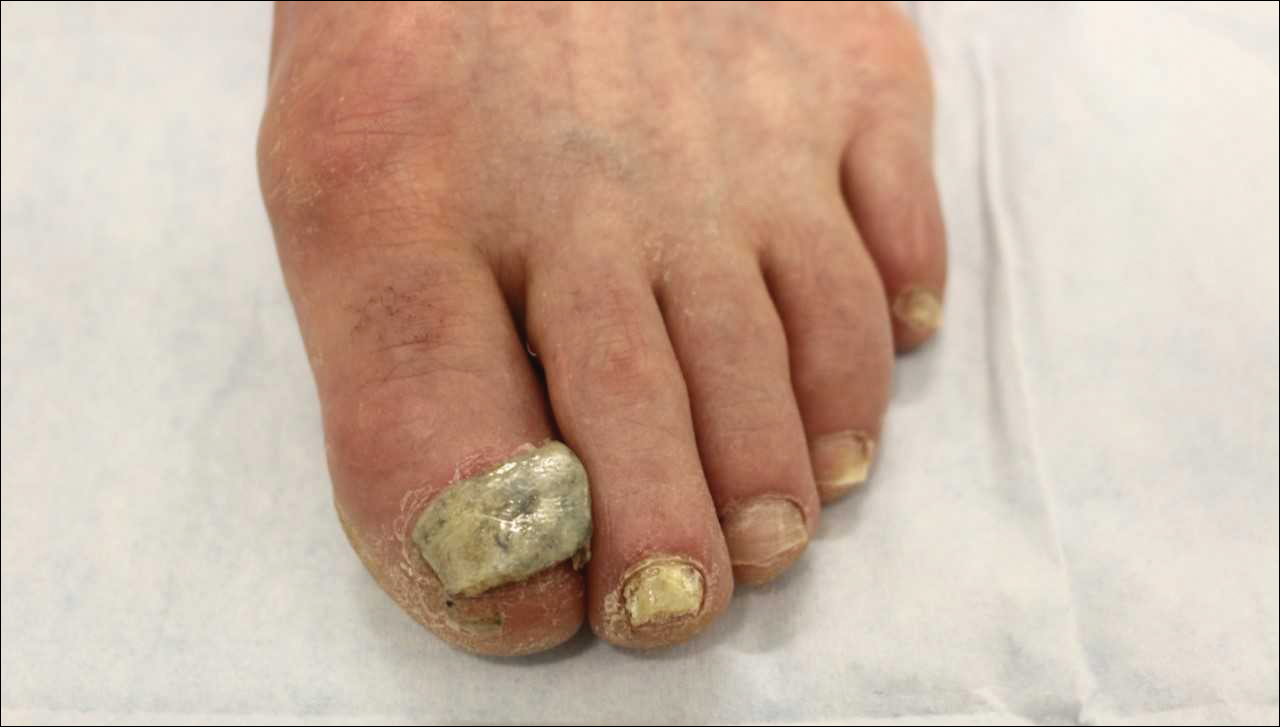
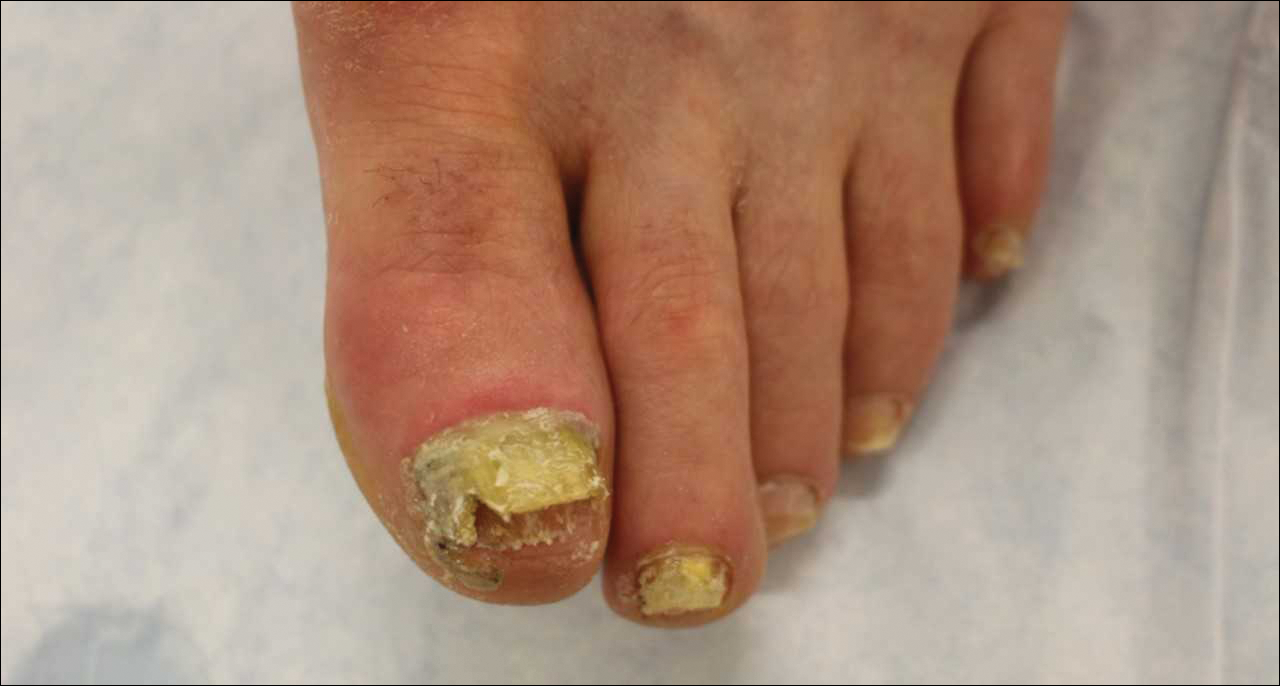
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15
Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15


Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15


Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
Onychomycosis Diagnosis and Long-term Treatment
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
Pain-Minimizing Strategies for Nail Surgery
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11
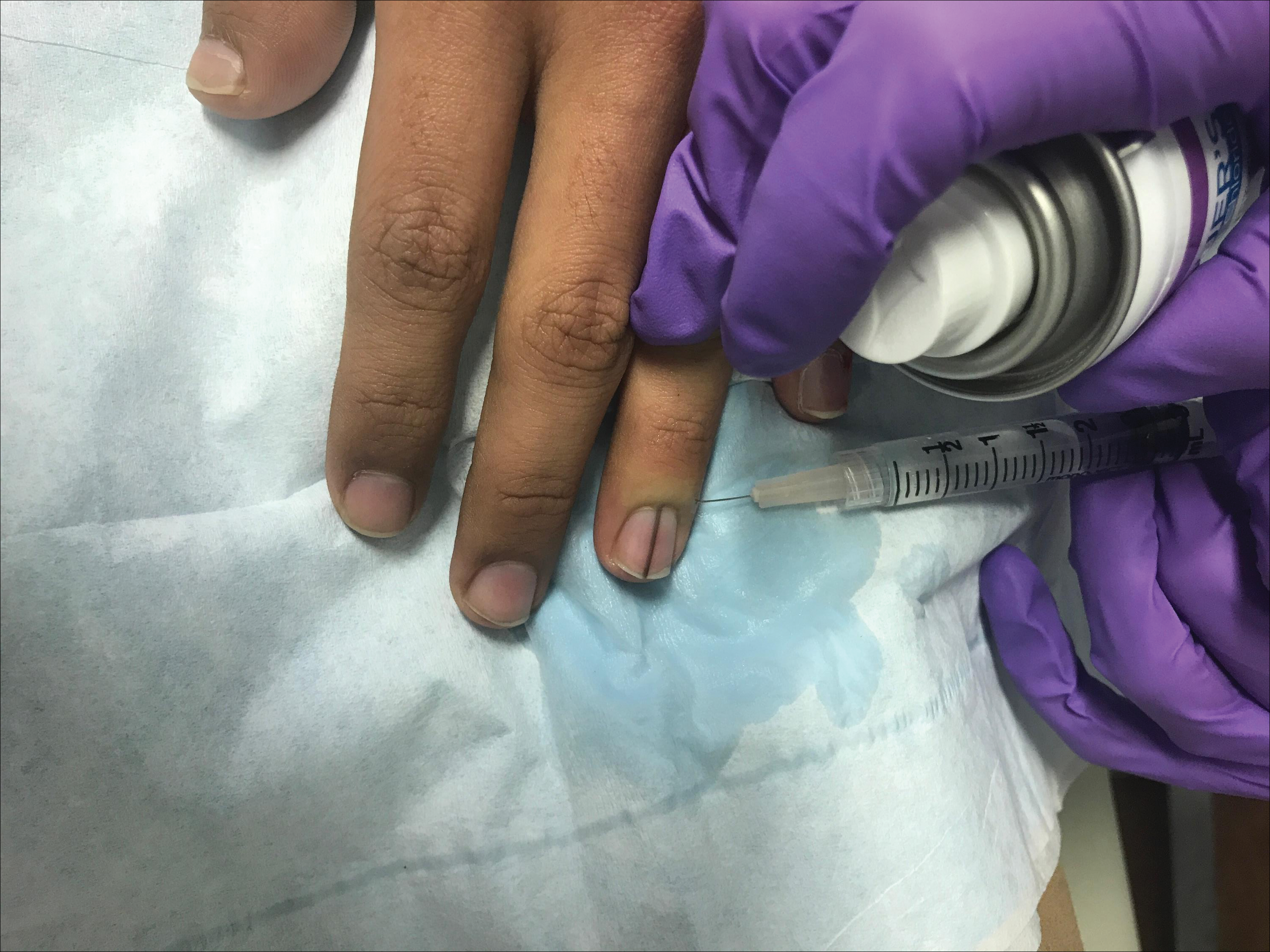
Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
Clinical Pearl: Early Diagnosis of Nail Psoriasis and Psoriatic Arthritis
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).
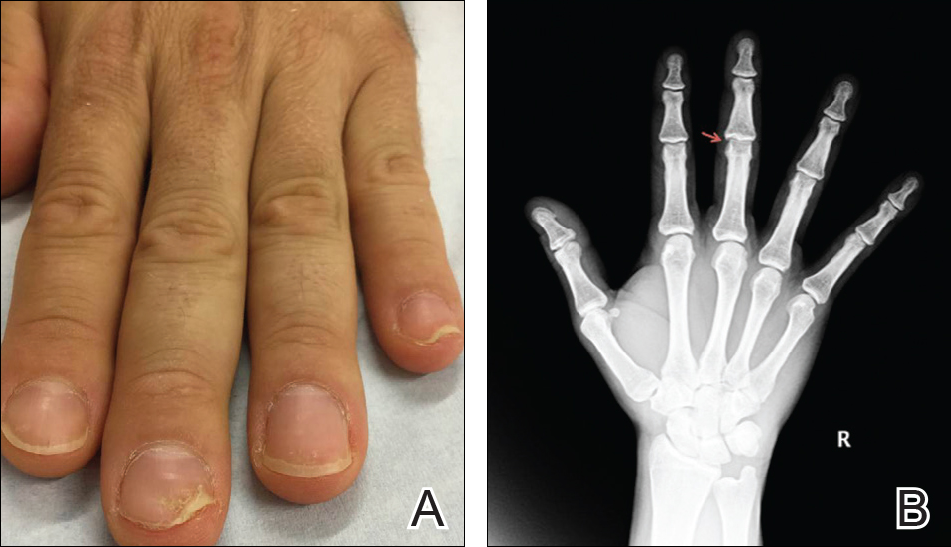
Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).

Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).

Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
Over-the-counter and Natural Remedies for Onychomycosis: Do They Really Work?
Onychomycosis is a fungal infection of the nail unit by dermatophytes, yeasts, and nondermatophyte molds. It is characterized by a white or yellow discoloration of the nail plate; hyperkeratosis of the nail bed; distal detachment of the nail plate from its bed (onycholysis); and nail plate dystrophy, including thickening, crumbling, and ridging. Onychomycosis is an important problem, representing 30% of all superficial fungal infections and an estimated 50% of all nail diseases.1 Reported prevalence rates of onychomycosis in the United States and worldwide are varied, but the mean prevalence based on population-based studies in Europe and North America is estimated to be 4.3%.2 It is more common in older individuals, with an incidence rate of 20% in those older than 60 years and 50% in those older than 70 years.3 Onychomycosis is more common in patients with diabetes and 1.9 to 2.8 times higher than the general population.4 Dermatophytes are responsible for the majority of cases of onychomycosis, particularly Trichophyton rubrum and Trichophyton mentagrophytes.5
Onychomycosis is divided into different subtypes based on clinical presentation, which in turn are characterized by varying infecting organisms and prognoses. The subtypes of onychomycosis are distal and lateral subungual (DLSO), proximal subungual, superficial, endonyx, mixed pattern, total dystrophic, and secondary. Distal and lateral subungual onychomycosis are by far the most common presentation and begins when the infecting organism invades the hyponychium and distal or lateral nail bed. Trichophyton rubrum is the most common organism and T mentagrophytes is second, but Candida parapsilosis and Candida albicans also are possibilities. Proximal subungual onychomycosis is far less frequent than DLSO and is usually caused by T rubrum. The fungus invades the proximal nail folds and penetrates the newly growing nail plate.6 This pattern is more common in immunosuppressed patients and should prompt testing for human immunodeficiency virus.7 Total dystrophic onychomycosis is the end stage of fungal nail plate invasion, may follow DLSO or proximal subungual onychomycosis, and is difficult to treat.6
Onychomycosis causes pain, paresthesia, and difficulty with ambulation.8 In patients with peripheral neuropathy and vascular problems, including diabetes, onychomycosis can increase the risk for foot ulcers, with amputation in severe cases.9 Patients also may present with aesthetic concerns that may impact their quality of life.10
Given the effect on quality of life along with medical risks associated with onychomycosis, a safe and successful treatment modality with a low risk of recurrence is desirable. Unfortunately, treatment of nail fungus is quite challenging for a number of reasons. First, the thickness of the nail and/or the fungal mass may be a barrier to the delivery of topical and systemic drugs at the source of the infection. In addition, the nail plate does not have intrinsic immunity. Also, recurrence after treatment is common due to residual hyphae or spores that were not previously eliminated.11 Finally, many topical medications require long treatment courses, which may limit patient compliance, especially in patients who want to use nail polish for cosmesis or camouflage.
Currently Approved Therapies for Onychomycosis
Several definitions are needed to better interpret the results of onychomycosis clinical trials. Complete cure is defined as a negative potassium hydroxide preparation and negative fungal culture with a completely normal appearance of the nail. Mycological cure is defined as potassium hydroxide microscopy and fungal culture negative. Clinical cure is stated as 0% nail plate involvement but at times is reported as less than 5% and less than 10% involvement.
Terbinafine and itraconazole are the only US Food and Drug Administration (FDA)–approved systemic therapies, and ciclopirox, efinaconazole, and tavaborole are the only FDA-approved topicals. Advantages of systemic agents generally are higher cure rates and shorter treatment courses, thus better compliance. Disadvantages include greater incidence of systemic side effects and drug-drug interactions as well as the need for laboratory monitoring. Pros of topical therapies are low potential for adverse effects, no drug-drug interactions, and no monitoring of blood work. Cons include lower efficacy, long treatment courses, and poor patient compliance.
Terbinafine, an allylamine, taken orally once daily (250 mg) for 12 weeks for toenails and 6 weeks for fingernails currently is the preferred systemic treatment of onychomycosis, with complete cure rates of 38% and 59% and mycological cure rates of 70% and 79% for toenails and fingernails, respectively.12 Itraconazole, an azole, is dosed orally at 200 mg daily for 3 months for toenails, with a complete cure rate of 14% and mycological cure rate of 54%.13 For fingernail onychomycosis only, itraconazole is dosed at 200 mg twice daily for 1 week, followed by a treatment-free period of 3 weeks, and then another 1-week course at thesame dose. The complete cure rate is 47% and the mycological cure is 61% for this pulse regimen.13
Ciclopirox is a hydroxypyridone and the 8% nail lacquer formulation was approved in 1999, making it the first topical medication to gain FDA approval for the treatment of toenail onychomycosis. Based on 2 clinical trials, complete cure rates for toenails are 5.5% and 8.5% and mycological cure rates are 29% and 36% at 48 weeks with removal of residual lacquer and debridement.14Efinaconazole is an azole and the 10% solution was FDA approved for the treatment of toenail onychomycosis in 2014.15 In 2 clinical trials, complete cure rates were 17.8% and 15.2% and mycological cure rates were 55.2% and 53.4% with once daily toenail application for 48 weeks.16 Tavaborole is a benzoxaborole and the 5% solution also was approved for the treatment of toenail onychomycosis in 2014.17 Two clinical trials reported complete cure rates of 6.5% and 9.1% and mycological cure rates of 31.1% and 35.9% with once daily toenail application for 48 weeks.18
Given the poor efficacy, systemic side effects, potential for drug-drug interactions, long-term treatment courses, and cost associated with current systemic and/or topical treatments, there has been a renewed interest in natural remedies and over-the-counter (OTC) therapies for onychomycosis. This review summarizes the in vitro and in vivo data, mechanisms of action, and clinical efficacy of various natural and OTC agents for the treatment of onychomycosis. Specifically, we summarize the data on tea tree oil (TTO), a popular topical cough suppressant (TCS), natural coniferous resin (NCR) lacquer, Ageratina pichinchensis (AP) extract, and ozonized sunflower oil.
Tea Tree Oil
Background
Tea tree oil is a volatile oil whose medicinal use dates back to the early 20th century when the Bundjabung aborigines of North and New South Wales extracted TTO from the dried leaves of the Melaleuca alternifolia plant and used it to treat superficial wounds.19 Tea tree oil has been shown to be an effective treatment of tinea pedis,20 and it is widely used in Australia as well as in Europe and North America.21 Tea tree oil also has been investigated as an antifungal agent for the treatment of onychomycosis, both in vitro22-28 and in clinical trials.29,30
In Vitro Data
Because TTO is composed of more than 100 active components,23 the antifungal activity of these individual components was investigated against 14 fungal isolates, including C albicans, T mentagrophytes, and Aspergillus species. The minimum inhibitory concentration (MIC) for α-pinene was less than 0.004% for T mentagrophytes and the components with the greatest MIC and minimum fungicidal concentration for the fungi tested were terpinen-4-ol and α-terpineol, respectively.22 The antifungal activity of TTO also was tested using disk diffusion assay experiments with 58 clinical isolates of fungi including C albicans, T rubrum, T mentagrophytes, and Aspergillus niger.24 Tea tree oil was most effective at inhibiting T rubrum followed by T mentagrophytes,24 which are the 2 most common etiologies of onychomycosis.5 In another report, the authors determined the MIC of TTO utilizing 4 different experiments with T rubrum as the infecting organism. Because TTO inhibited the growth of T rubrum at all concentrations greater than 0.1%, they found that the MIC was 0.1%.25 Given the lack of adequate nail penetration of most topical therapies, TTO in nanocapsules (TTO-NC), TTO nanoemulsions, and normal emulsions were tested in vitro for their ability to inhibit the growth of T rubrum inoculated into nail shavings. Colony growth decreased significantly within the first week of treatment, with TTO-NC showing maximum efficacy (P<.001). This study showed that TTO, particularly TTO-NC, was effective in inhibiting the growth of T rubrum in vitro and that using nanocapsule technology may increase nail penetration and bioavailability.31
Much of what we know about TTO’s antifungal mechanism of action comes from experiments involving C albicans. To date, it has not been studied in T rubrum or T mentagrophytes, the 2 most common etiologies of onychomycosis.5 In C albicans, TTO causes altered permeability of plasma membranes,32 dose-dependent alteration of respiration,33 decreased glucose-induced acidification of media surrounding fungi,32 and reversible inhibition of germ tube formation.19,34
Clinical Trials
A randomized, double-blind, multicenter trial was performed on 117 patients with culture-proven DLSO who were randomized to receive TTO 100% or clotrimazole solution 1% applied twice daily to affected toenails for 6 months.29 Primary outcome measures were mycologic cure, clinical assessment, and patient subjective assessment (Table 1). There were no statistical differences between the 2 treatment groups. Erythema and irritation were the most common adverse reactions occurring in 7.8% (5/64) of the TTO group.29
Another study was a double-blind, placebo-controlled trial involving 60 patients with clinical and mycologic evidence of DLSO who were randomized to treatment with a cream containing butenafine hydrochloride 2% and TTO 5% (n=40) or a control cream containing only TTO (n=20), with active treatment for 8 weeks and final follow-up at 36 weeks.30 Patients were instructed to apply the cream 3 times daily under occlusion for 8 weeks and the nail was debrided between weeks 4 and 6 if feasible. If the nail could not be debrided after 8 weeks, it was considered resistant to treatment. At the end of the study, the complete cure rate was 80% in the active group compared to 0% in the placebo group (P<.0001), and the mean time to complete healing with progressive nail growth was 29 weeks. There were no adverse effects in the placebo group, but 4 patients in the active group had mild skin inflammation.30
Topical Cough Suppressant
Background
Topical cough suppressants, which are made up of several natural ingredients, are OTC ointments for adults and children 2 years and older that are indicated as cough suppressants when applied to the chest and throat and as relief of mild muscle and joint pains.35 The active ingredients are camphor 4.8%, eucalyptus oil 1.2%, and menthol 2.6%, while the inactive ingredients are cedarleaf oil, nutmeg oil, petrolatum, thymol, and turpentine oil.35 Some of the active and inactive ingredients in TCSs have shown efficacy against dermatophytes in vitro,36-38 and although they are not specifically indicated for onychomycosis, they have been popularized as home remedies for fungal nail infections.36,39 A TCS has been evaluated for its efficacy for the treatment of onychomycosis in one clinical trial.40
In Vitro Data
An in vitro study was performed to evaluate the antifungal activity of the individual and combined components of TCS on 16 different dermatophytes, nondermatophytes, and molds. The zones of inhibition against these organisms were greatest for camphor, menthol, thymol, and eucalyptus oil. Interestingly, there were large zones of inhibition and a synergistic effect when a mixture of components was used against T rubrum and T mentagrophytes.36 The in vitro activity of thymol, a component of TCS, was tested against Candida species.37 The essential oil subtypes Thymus vulgaris and Thymus zygis (subspecies zygis) showed similar antifungal activity, which was superior to Thymus mastichina, and all 3 compounds had similar MIC and minimal lethal concentration values. The authors showed that the antifungal mechanism was due to cell membrane damage and inhibition of germ tube formation.37 It should be noted that Candida species are less common causes of onychomycosis, and it is not known whether this data is applicable to T rubrum. In another study, the authors investigated the antifungal activity of Thymus pulegioides and found that MIC ranged from 0.16 to 0.32 μL/mL for dermatophytes and Aspergillus strains and 0.32 to 0.64 μL/mL for Candida species. When an essential oil concentration of 0.08 μL/mL was used against T rubrum, ergosterol content decreased by 70 %, indicating that T pulegioides inhibits ergosterol biosynthesis in T rubrum.38
Clinical Observations and Clinical Trial
There is one report documenting the clinical observations on a group of patients with a clinical diagnosis of onychomycosis who were instructed to apply TCS to affected nail(s) once daily.36 Eighty-five charts were reviewed (mean age, 77 years), and although follow-up was not complete or standardized, the following data were reported: 32 (38%) cleared their fungal infection, 21 (25%) had no record of change but also no record of compliance, 19 (22%) had only 1 documented follow-up visit, 9 (11%) reported they did not use the treatment, and 4 (5%) did not return for a follow-up visit. Of the 32 patients whose nails were cured, 3 (9%) had clearance within 5 months, 8 (25%) within 7 months, 11 (34%) within 9 months, 4 (13%) within 11 months, and 6 (19%) within 16 months.36
A small pilot study was performed to evaluate the efficacy of daily application of TCS in the treatment of onychomycosis in patients 18 years and older with at least 1 great toenail affected.40 The primary end points were mycologic cure at 48 weeks and clinical cure at the end of the study graded as complete, partial, or no change. The secondary end point was patient satisfaction with the appearance of the affected nail at 48 weeks. Eighteen participants completed the study; 55% (10/18) were male, with an average age of 51 years (age range, 30–85 years). The mean initial amount of affected nail was 62% (range, 16%–100%), and cultures included dermatophytes, nondermatophytes, and molds. With TCS treatment, 27.8% (5/18) showed mycologic cure of which 4 (22.2%) had a complete clinical cure. Ten participants (55.6%) had partial clinical cure and 3 (16.7%) had no clinical improvement. Interestingly, the 4 participants who had complete clinical cure had baseline cultures positive for either T mentagrophytes or C parapsilosis. Most patients were content with the treatment, as 9 participants stated that they were very satisfied and 9 stated that they were satisfied. The average ratio of affected to total nail area declined from 63% at screening to 41% at the end of the study (P<.001). No adverse effects were reported with study drug.40
NCR Lacquer
Background
Resins are natural products derived from coniferous trees and are believed to protect trees against insects and microbial pathogens.41 Natural coniferous resin derived from the Norway spruce tree (Picea abies) mixed with boiled animal fat or butter has been used topically for centuries in Finland and Sweden to treat infections and wounds.42-44 The activity of NCR has been studied against a wide range of microbes, demonstrating broad-spectrum antimicrobial activity against both gram-positive bacteria and fungi.45-48 There are 2 published clinical trials evaluating NCR in the treatment of onychomycosis.49,50
In Vitro Data
Natural coniferous resin has shown antifungal activity against T mentagrophytes, Trichophyton tonsurans, and T rubrum in vitro, which was demonstrated using medicated disks of resin on petri dishes inoculated with these organisms.46 In another study, the authors evaluated the antifungal activity of NCR against human pathogenic fungi and yeasts using agar plate diffusion tests and showed that the resin had antifungal activity against Trichophyton species but not against Fusarium and most Candida species. Electron microscopy of T mentagrophytes exposed to NCR showed that all cells were dead inside the inhibition zone, with striking changes seen in the hyphal cell walls, while fungal cells outside the inhibition zone were morphologically normal.47 In another report, utilizing the European Pharmacopoeia challenge test, NCR was highly effective against gram-positive and gram-negative bacteria as well as C albicans.42
Clinical Trials
In one preliminary observational and prospective clinical trial, 15 participants with clinical and mycologic evidence of onychomycosis were instructed to apply NCR lacquer once daily for 9 months with a 4-week washout period, with the primary outcome measures being clinical and mycologic cure.49 Thirteen (87%) enrolled participants were male and the average age was 65 years (age range, 37–80 years). The DLSO subtype was present in 9 (60%) participants. The mycologic cure rate at the end of the study was 65% (95% CI, 42%-87%), and none achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail.49
The second trial was a prospective, controlled, investigator-blinded study of 73 patients with clinical and mycologic evidence of toenail onychomycosis who were randomized to receive NCR 30%, amorolfine lacquer 5%, or 250 mg oral terbinafine.50 The primary end point was mycologic cure at 10 months, and secondary end points were clinical efficacy, cost-effectiveness, and patient compliance. Clinical efficacy was based on the proximal linear growth of healthy nail and was classified as unchanged, partial, or complete. Partial responses were described as substantial decreases in onycholysis, subungual hyperkeratosis, and streaks. A complete response was defined as a fully normal appearance of the toenail. Most patients were male in the NCR (91% [21/23]), amorolfine (80% [20/25]), and terbinafine (68% [17/25]) groups; the average ages were 64, 63, and 64 years, respectively. Trichophyton rubrum was cultured most often in all 3 groups: NCR, 87% (20/23); amorolfine, 96% (24/25); and terbinafine, 84% (21/25). The remaining cases were from T mentagrophytes. A summary of the results is shown in Table 2. Patient compliance was 100% in all except 1 patient in the amorolfine treatment group with moderate compliance. There were no adverse events, except for 2 in the terbinafine group: diarrhea and rash.50
AP Extract
Background
Ageratina pichinchensis, a member of the Asteraceae family, has been used historically in Mexico for fungal infections of the skin.51,52 Fresh or dried leaves were extracted with alcohol and the product was administered topically onto damaged skin without considerable skin irritation.53 Multiple studies have demonstrated that AP extract has in vitro antifungal activity along with other members of the Asteraceae family.54-56 There also is evidence from clinical trials that AP extract is effective against superficial dermatophyte infections such as tinea pedis.57 Given the positive antifungal in vitro data, the potential use of this agent was investigated for onychomycosis treatment.53,58
In Vitro Data
The antifungal properties of the Asteraceae family have been tested in several in vitro experiments. Eupatorium aschenbornianum, described as synonymous with A pichinchensis,59 was found to be most active against the dermatophytes T rubrum and T mentagrophytes with MICs of 0.3 and 0.03 mg/mL, respectively.54 It is thought that the primary antimycotic activity is due to encecalin, an acetylchromene compound that was identified in other plants from the Asteraceae family and has activity against dermatophytes.55 In another study, Ageratum houstanianum Mill, a comparable member of the Asteraceae family, had fungitoxic activity against T rubrum and C albicans isolated from nail infections.56
Clinical Trials
A double-blind controlled trial was performed on 110 patients with clinical and mycologic evidence of mild to moderate toenail onychomycosis randomized to treatment with AP lacquer or ciclopirox lacquer 8% (control).58 Primary end points were clinical effectiveness (completely normal nails) and mycologic cure. Patients were instructed to apply the lacquer once every third day during the first month, twice a week for the second month, and once a week for 16 weeks, with removal of the lacquer weekly. Demographics were similar between the AP lacquer and control groups, with mean ages of 44.6 and 46.5 years, respectively; women made up 74.5% and 67.2%, respectively, of each treatment group, with most patients having a 2- to 5-year history of disease (41.8% and 40.1%, respectively).58 A summary of the data is shown in Table 3. No severe side effects were documented, but minimal nail fold skin pain was reported in 3 patients in the control group in the first week, resolving later in the trial.58
A follow-up study was performed to determine the optimal concentration of AP lacquer for the treatment of onychomycosis.53 One hundred twenty-two patients aged 19 to 65 years with clinical and mycologic evidence of mild to moderate DLSO were randomized to receive 12.6% or 16.8% AP lacquer applied once daily to the affected nails for 6 months. The nails were graded as healthy, mild, or moderately affected before and after treatment. There were no significant differences in demographics between the 2 treatment groups, and 77% of patients were women with a median age of 47 years. There were no significant side effects from either concentration of AP lacquer.53
Ozonized Sunflower Oil
Background
Ozonized sunflower oil is derived by reacting ozone (O3) with sunflower plant (Helianthus annuus) oil to form a petroleum jelly–like material.60 It was originally shown to have antibacterial properties in vitro,61 and further studies have confirmed these findings and demonstrated anti-inflammatory, wound healing, and antifungal properties.62-64 A formulation of ozonized sunflower oil used in Cuba is clinically indicated for the treatment of tinea pedis and impetigo.65 The clinical efficacy of this product has been evaluated in a clinical trial for the treatment of onychomycosis.65
In Vitro Data
A compound made up of 30% ozonized sunflower oil with 0.5% of α-lipoic acid was found to have antifungal activity against C albicans using the disk diffusion method, in addition to other bacterial organisms. The MIC values ranged from 2.0 to 3.5 mg/mL.62 Another study was designed to evaluate the in vitro antifungal activity of this formulation on samples cultured from patients with onychomycosis using the disk diffusion method. They found inhibition of growth of C albicans, C parapsilosis, and Candida tropicalis, which was inferior to amphotericin B, ketoconazole, fluconazole, and itraconazole.64
Clinical Trial
A single-blind, controlled, phase 3 study was performed on 400 patients with clinical and mycologic evidence of onychomycosis. Patients were randomized to treatment with an ozonized sunflower oil solution or ketoconazole cream 2% applied to affected nails twice daily for 3 months, with filing and massage of the affected nails upon application of treatment.65 Cured was defined as mycologic cure in addition to a healthy appearing nail, improved as an increase in healthy appearing nail in addition to a decrease in symptoms (ie, paresthesia, pain, itching) but positive mycological testing, same as no clinical change in appearance with positive mycological findings, and worse as increasing diseased nail involvement in the presence of positive mycological findings. Demographics were similar between groups with a mean age of 35 years. Men accounted for 80% of the study population, and 65% of the study population was white. The mean duration of disease was 30 months. They also reported on a 1-year follow-up, with 2.8% of patients in the ozonized sunflower oil solution group and 37.0% of patients in the ketoconazole group describing relapses. Trichophyton rubrum and C albicans were cultured from these patients.65
Comment
Due to the poor efficacy, long-term treatment courses, inability to use nail polish, and high cost associated with many FDA-approved topical treatments, along with the systemic side effects, potential for drug-drug interactions, and cost associated with many oral therapies approved for onychomycosis, there has been a renewed interest in natural remedies and OTC treatments. Overall, TTO, TCS, NCR, AP extract, and ozonized sunflower oil have shown efficacy in vitro against some dermatophytes, nondermatophytes, and molds responsible for onychomycosis. One or more clinical trials were performed with each of these agents for the treatment of onychomycosis. They were mostly small pilot studies, and due to differences in trial design, the results cannot be compared with each other or with currently FDA-approved treatments. We can conclude that because adverse events were rare with all of these therapies—most commonly skin irritation or mild skin pain—they exhibit good safety.
For TTO, there was no statistical difference between the clotrimazole and TTO treatment groups in mycologic cure, clinical assessment, or patient subjective assessment of the nails.29 Although there was an 80% complete cure in the butenafine and TTO group, it was 0% in the TTO group at week 36.30 Trial design, longer treatment periods, incorporation into nanocapsules, or combination treatment with other antifungal agents may influence our future use of TTO for onychomycosis, but based on the present data we cannot recommend this treatment in clinical practice.
With TCS, 27.8% of participants had a mycologic cure and 22.2% had complete clinical cure.40 Although it is difficult to draw firm conclusions from this small pilot study, there may be some benefit to treating toenail onychomycosis due to T mentagrophytes or C parapsilosis with TCS but no benefit in treating onychomycosis due to T rubrum, the more common cause of onychomycosis. Limitations of this study were lack of a placebo group, small sample size, wide variety of represented pathogens that may not be representative of the true population, and lack of stratification by baseline severity or involvement of nail. A larger randomized controlled clinical trial would be necessary to confirm the results of this small study and make formal recommendations.
In one clinical trial with NCR, mycologic cure was 65% at the end of the study.49 No participants achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail. Because this study was small (N=15), it is difficult to draw firm conclusions.49 In another study with NCR, mycologic cure rates with NCR, amorolfine, and terbinafine were 13%, 8%, and 56%, respectively. Based on these results, NCR has similar antifungal efficacy to amorolfine but was inferior to oral terbinafine.50 A larger randomized controlled clinical trial with more homogenous and less severely affected patients and longer treatment periods would be necessary to confirm the results of these small studies and make formal recommendations.
Because there were no significant differences in clinical effectiveness of mycologic cure rates between AP lacquer 10% and ciclopirox lacquer 8% in one clinical trial,58 AP does not seem to be more effective than at least one of the current FDA-approved topical treatments; however, because AP lacquer 16.8% was shown to be more effective than AP lacquer 12.6% in one onychomycosis clinical trial, using higher concentrations of AP may yield better results in future trials.53
One trial comparing ozonized sunflower oil to ketoconazole cream 2% showed 90.5% and 13.5% cure rates, respectively.65 Although there is good in vitro antifungal activity and a clinical trial showing efficacy using ozonized sunflower oil for the treatment of onychomycosis, confirmatory studies are necessary before we can recommend this OTC treatment to our patients. Specifically, we will get the most data from large randomized controlled trials with strict inclusion/exclusion and efficacy criteria.
Conclusion
Over-the-counter and natural remedies may be an emerging area of research in the treatment of onychomycosis. This review summarizes the laboratory data and clinical trials on several of these agents and, when available, compares their clinical and mycologic efficacy with FDA-approved therapies. Shortcomings of some of these studies include a small study population, lack of adequate controls, nonstandardized mycologic testing, and abbreviated posttreatment evaluation times. It may be concluded that these products have varying degrees of efficacy and appear to be safe in the studies cited; however, at present, we cannot recommend any of them to our patients until there are larger randomized clinical trials with appropriate controls demonstrating their efficacy.
- Scher RK, Daniel CR. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005.
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480-1491.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Mayser P, Freund V, Budihardja D. Toenail onychomycosis in diabetic patients: issues and management. Am J Clin Dermatol. 2009;10:211-220.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification J Am Acad Dermatol. 2011;65:1219-1227.
- Elewski B. Clinical pearl: proximal white subungual onychomycosis in AIDS. J Am Acad Dermatol. 1993;29:631-632.
- Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):15.
- Boyko EJ, Ahroni JH, Cohen V, et al. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006;29:1202-1207.
- Szepietowski JC, Reich A, Pacan P, et al. Evaluation of quality of life in patients with toenail onychomycosis by Polish version of an international onychomycosis-specific questionnaire. J Eur Acad Dermatol Venereol. 2007;21:491-496.
- Scher RK, Baron R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Sporanox [package insert]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001
- Penlac [package insert]. Bridgewater, NJ: Dermik Laboratories; 2006.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; 2014.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies [published online May 5, 2015]. J Am Acad Dermatol. 2015;73:62-69.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: a randomized, placebo-controlled, blinded study. Australas J Dermatol. 2002;43:175-178.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853-860.
- Brophy JJ, Davies NW, Southwell IA, et al. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem. 1989;37:1330-1335.
- Concha JM, Moore LS, Holloway WJ. 1998 William J. Stickel Bronze Award. Antifungal activity of Melaleuca alternifolia (tea-tree) oil against various pathogenic organisms. J Am Podiatr Med Assoc. 1998;88:489-492.
- Benger S, Townsend P, Ashford RL, et al. An in vitro study to determine the minimum inhibitory concentration of Melaleuca alternifolia against the dermatophyte Trichophyton rubrum. Foot. 2004;14:86-91.
- Hammer KA, Carson CF, Riley TV. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother. 1998;42:591-595.
- Altman P. Australian tea tree oil. Aust J Pharm. 1998;69:276-278.
- Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147-157.
- Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract. 1994;38:601-605.
- Syed TA, Qureshi ZA, Ali SM, et al. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Med Int Health. 1999;4:284-287.
- Flores FC, de Lima JA, Ribeiro RF, et al. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of Trichophyton rubrum. Mycopathologia. 2013;175:281-286.
- Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081-1085.
- Cox SD, Mann CM, Markham JL, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88:170-175.
- Hammer KA, Carson CF, Riley TV. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med Mycol. 2000;38:355-362.
- Vicks VapoRub [package insert]. Gross-Gerau, Germany: Proctor & Gamble; 2010.
- Ramsewak RS, Nair MG, Stommel M, et al. In vitro antagonistic activity of monoterpenes and their mixtures against ‘toe nail fungus’ pathogens. Phytother Res. 2003;17:376-379.
- Pina-Vaz C, Gonçalves Rodrigues A, Pinto E, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73-78.
- Pinto E, Pina-Vaz C, Salgueiro L, et al. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol. 2006;55:1367-1373.
- Vicks VapoRub might help fight toenail fungus. Consumer Reports. 2006;71:49.
- Derby R, Rohal P, Jackson C, et al. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med. 2011;24:69-74.
- Trapp S, Croteau R. Defensive resin biosynthesis in conifers. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:689-724.
- Sipponen A, Laitinen K. Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS. 2011;119:720-724.
- Sipponen A, Lohi J. Lappish gum care “new” treatment of pressure ulcers? People’s improvement at it’s best. Eng Med J. 2003;58:2775-2776.
- Benedictus O. Een Nyttigh Läkare. Malmö: Kroon; 1938.
- Rautio M, Sipponen A, Peltola R, et al. Antibacterial effects of home-made resin salve from Norway spruce (Picea abies). APMIS. 2007;115:335-340.
- Laitinen K, Sipponen A, Jokinen JJ, et al. Resin salve from Norway spruce is antifungal against dermatophytes causing nail infections. EWMA. 2009;56:289-296.
- Rautio M, Sipponen A, Lohi J, et al. In vitro fungistatic effects of natural coniferous resin from Norway spruce (Picea abies). Eur J Clin Microbiol Infect Dis. 2012;31:1783-1789.
- Sipponen A, Peltola R, Jokinen JJ, et al. Effects of Norway spruce (Picea abies) resin on cell wall and cell membrane of Staphylococcus aureus. Ultrastruct Pathol. 2009;33:128-135.
- Sipponen P, Sipponen A, Lohi J, et al. Natural coniferous resin lacquer in treatment of toenail onychomycosis: an observational study. Mycoses. 2013;56:289-296.
- Auvinen T, Tiihonen R, Soini M, et al. Efficacy of topical resin lacquer, amorolfine, and oral terbinafine for treating toenail onychomycosis: a prospective, randomized, controlled, investigator-blinded, parallel-group clinical trial. Br J Dermatol. 2015;173:940-948.
- Argueta A, Cano L, Rodarte M. Atlas de las Plantas de la Medicina Tradicional Mexicana. Vol 3. Mexico City, Mexico: Instituto Nacional Indigenista; 1994:72-680.
- Avilés M, Suárez G. Catálogo de Plantas Medicinales del Jardín Etnobotánico. Peru: Instituto Nacional de Antropología e Historia; 1994.
- Romero-Cerecero O, Roman-Ramos R, Zamilpa A, et al. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J Ethnopharmacol. 2009;126:74-78.
- Navarro Garcia VM, Gonzalez A, Fuentes M, et al. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003;87:85-88.
- Castañeda P, Gómez L, Mata R, et al. Phytogrowth-inhibitory and antifungal constituents of Helianthella quinquenervis. J Nat Prod. 1996;59:323-326.
- Kumar N. Inhibition of nail infecting fungi of peoples of North Eastern UP causing Tinea unguium through leaf essential oil of Ageratum houstonianum Mill. IOSR J Pharm. June 2014;4:36-42.
- Romero-Cerecero O, Rojas G, Navarro V, et al. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: an explorative pilot study controlled with ketoconazole. Planta Med. 2006;72:1257-1261.
- Romero-Cerecero O, Zamilpa A, Jimenez-Ferrer JE, et al. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. a comparative study with ciclopirox. Planta Med. 2008;74:1430-1435.
- Rzedowski J, De Rzedowski GC. Flora Fanerogámica del Valle de México. Mexico City, Mexico: Instituto de Ecología Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional; 1985.
- Bocci V. Biological and clinical effects of ozone. has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56:270-279.
- Sechi LA, Lezcano I, Nunez N, et al. Antibacterial activity of ozonized sunflower oil (Oleozon). J Appl Microbiol. 2001;90:279-284.
- Rodrigues KL, Cardoso CC, Caputo LR, et al. Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds. Inflammopharmacology. 2004;12:261-270.
- Daud FV, Ueda SMY, Navarini A, et al. The use of ozonized oil in the treatment of dermatophitosis caused by Microsporum canis in rabbits. Braz J Microbiol. 2011;42:274-281.
- Guerrer LV, Cunha KC, Nogueira MC, et al. “In vitro” antifungal activity of ozonized sunflower oil on yeasts from onychomycosis. Braz J Microbiol. 2012;43:1315-1318.
- Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON in patients suffering from onychomycosis. Mycoses. 2011;54:E272-E277.
Onychomycosis is a fungal infection of the nail unit by dermatophytes, yeasts, and nondermatophyte molds. It is characterized by a white or yellow discoloration of the nail plate; hyperkeratosis of the nail bed; distal detachment of the nail plate from its bed (onycholysis); and nail plate dystrophy, including thickening, crumbling, and ridging. Onychomycosis is an important problem, representing 30% of all superficial fungal infections and an estimated 50% of all nail diseases.1 Reported prevalence rates of onychomycosis in the United States and worldwide are varied, but the mean prevalence based on population-based studies in Europe and North America is estimated to be 4.3%.2 It is more common in older individuals, with an incidence rate of 20% in those older than 60 years and 50% in those older than 70 years.3 Onychomycosis is more common in patients with diabetes and 1.9 to 2.8 times higher than the general population.4 Dermatophytes are responsible for the majority of cases of onychomycosis, particularly Trichophyton rubrum and Trichophyton mentagrophytes.5
Onychomycosis is divided into different subtypes based on clinical presentation, which in turn are characterized by varying infecting organisms and prognoses. The subtypes of onychomycosis are distal and lateral subungual (DLSO), proximal subungual, superficial, endonyx, mixed pattern, total dystrophic, and secondary. Distal and lateral subungual onychomycosis are by far the most common presentation and begins when the infecting organism invades the hyponychium and distal or lateral nail bed. Trichophyton rubrum is the most common organism and T mentagrophytes is second, but Candida parapsilosis and Candida albicans also are possibilities. Proximal subungual onychomycosis is far less frequent than DLSO and is usually caused by T rubrum. The fungus invades the proximal nail folds and penetrates the newly growing nail plate.6 This pattern is more common in immunosuppressed patients and should prompt testing for human immunodeficiency virus.7 Total dystrophic onychomycosis is the end stage of fungal nail plate invasion, may follow DLSO or proximal subungual onychomycosis, and is difficult to treat.6
Onychomycosis causes pain, paresthesia, and difficulty with ambulation.8 In patients with peripheral neuropathy and vascular problems, including diabetes, onychomycosis can increase the risk for foot ulcers, with amputation in severe cases.9 Patients also may present with aesthetic concerns that may impact their quality of life.10
Given the effect on quality of life along with medical risks associated with onychomycosis, a safe and successful treatment modality with a low risk of recurrence is desirable. Unfortunately, treatment of nail fungus is quite challenging for a number of reasons. First, the thickness of the nail and/or the fungal mass may be a barrier to the delivery of topical and systemic drugs at the source of the infection. In addition, the nail plate does not have intrinsic immunity. Also, recurrence after treatment is common due to residual hyphae or spores that were not previously eliminated.11 Finally, many topical medications require long treatment courses, which may limit patient compliance, especially in patients who want to use nail polish for cosmesis or camouflage.
Currently Approved Therapies for Onychomycosis
Several definitions are needed to better interpret the results of onychomycosis clinical trials. Complete cure is defined as a negative potassium hydroxide preparation and negative fungal culture with a completely normal appearance of the nail. Mycological cure is defined as potassium hydroxide microscopy and fungal culture negative. Clinical cure is stated as 0% nail plate involvement but at times is reported as less than 5% and less than 10% involvement.
Terbinafine and itraconazole are the only US Food and Drug Administration (FDA)–approved systemic therapies, and ciclopirox, efinaconazole, and tavaborole are the only FDA-approved topicals. Advantages of systemic agents generally are higher cure rates and shorter treatment courses, thus better compliance. Disadvantages include greater incidence of systemic side effects and drug-drug interactions as well as the need for laboratory monitoring. Pros of topical therapies are low potential for adverse effects, no drug-drug interactions, and no monitoring of blood work. Cons include lower efficacy, long treatment courses, and poor patient compliance.
Terbinafine, an allylamine, taken orally once daily (250 mg) for 12 weeks for toenails and 6 weeks for fingernails currently is the preferred systemic treatment of onychomycosis, with complete cure rates of 38% and 59% and mycological cure rates of 70% and 79% for toenails and fingernails, respectively.12 Itraconazole, an azole, is dosed orally at 200 mg daily for 3 months for toenails, with a complete cure rate of 14% and mycological cure rate of 54%.13 For fingernail onychomycosis only, itraconazole is dosed at 200 mg twice daily for 1 week, followed by a treatment-free period of 3 weeks, and then another 1-week course at thesame dose. The complete cure rate is 47% and the mycological cure is 61% for this pulse regimen.13
Ciclopirox is a hydroxypyridone and the 8% nail lacquer formulation was approved in 1999, making it the first topical medication to gain FDA approval for the treatment of toenail onychomycosis. Based on 2 clinical trials, complete cure rates for toenails are 5.5% and 8.5% and mycological cure rates are 29% and 36% at 48 weeks with removal of residual lacquer and debridement.14Efinaconazole is an azole and the 10% solution was FDA approved for the treatment of toenail onychomycosis in 2014.15 In 2 clinical trials, complete cure rates were 17.8% and 15.2% and mycological cure rates were 55.2% and 53.4% with once daily toenail application for 48 weeks.16 Tavaborole is a benzoxaborole and the 5% solution also was approved for the treatment of toenail onychomycosis in 2014.17 Two clinical trials reported complete cure rates of 6.5% and 9.1% and mycological cure rates of 31.1% and 35.9% with once daily toenail application for 48 weeks.18
Given the poor efficacy, systemic side effects, potential for drug-drug interactions, long-term treatment courses, and cost associated with current systemic and/or topical treatments, there has been a renewed interest in natural remedies and over-the-counter (OTC) therapies for onychomycosis. This review summarizes the in vitro and in vivo data, mechanisms of action, and clinical efficacy of various natural and OTC agents for the treatment of onychomycosis. Specifically, we summarize the data on tea tree oil (TTO), a popular topical cough suppressant (TCS), natural coniferous resin (NCR) lacquer, Ageratina pichinchensis (AP) extract, and ozonized sunflower oil.
Tea Tree Oil
Background
Tea tree oil is a volatile oil whose medicinal use dates back to the early 20th century when the Bundjabung aborigines of North and New South Wales extracted TTO from the dried leaves of the Melaleuca alternifolia plant and used it to treat superficial wounds.19 Tea tree oil has been shown to be an effective treatment of tinea pedis,20 and it is widely used in Australia as well as in Europe and North America.21 Tea tree oil also has been investigated as an antifungal agent for the treatment of onychomycosis, both in vitro22-28 and in clinical trials.29,30
In Vitro Data
Because TTO is composed of more than 100 active components,23 the antifungal activity of these individual components was investigated against 14 fungal isolates, including C albicans, T mentagrophytes, and Aspergillus species. The minimum inhibitory concentration (MIC) for α-pinene was less than 0.004% for T mentagrophytes and the components with the greatest MIC and minimum fungicidal concentration for the fungi tested were terpinen-4-ol and α-terpineol, respectively.22 The antifungal activity of TTO also was tested using disk diffusion assay experiments with 58 clinical isolates of fungi including C albicans, T rubrum, T mentagrophytes, and Aspergillus niger.24 Tea tree oil was most effective at inhibiting T rubrum followed by T mentagrophytes,24 which are the 2 most common etiologies of onychomycosis.5 In another report, the authors determined the MIC of TTO utilizing 4 different experiments with T rubrum as the infecting organism. Because TTO inhibited the growth of T rubrum at all concentrations greater than 0.1%, they found that the MIC was 0.1%.25 Given the lack of adequate nail penetration of most topical therapies, TTO in nanocapsules (TTO-NC), TTO nanoemulsions, and normal emulsions were tested in vitro for their ability to inhibit the growth of T rubrum inoculated into nail shavings. Colony growth decreased significantly within the first week of treatment, with TTO-NC showing maximum efficacy (P<.001). This study showed that TTO, particularly TTO-NC, was effective in inhibiting the growth of T rubrum in vitro and that using nanocapsule technology may increase nail penetration and bioavailability.31
Much of what we know about TTO’s antifungal mechanism of action comes from experiments involving C albicans. To date, it has not been studied in T rubrum or T mentagrophytes, the 2 most common etiologies of onychomycosis.5 In C albicans, TTO causes altered permeability of plasma membranes,32 dose-dependent alteration of respiration,33 decreased glucose-induced acidification of media surrounding fungi,32 and reversible inhibition of germ tube formation.19,34
Clinical Trials
A randomized, double-blind, multicenter trial was performed on 117 patients with culture-proven DLSO who were randomized to receive TTO 100% or clotrimazole solution 1% applied twice daily to affected toenails for 6 months.29 Primary outcome measures were mycologic cure, clinical assessment, and patient subjective assessment (Table 1). There were no statistical differences between the 2 treatment groups. Erythema and irritation were the most common adverse reactions occurring in 7.8% (5/64) of the TTO group.29
Another study was a double-blind, placebo-controlled trial involving 60 patients with clinical and mycologic evidence of DLSO who were randomized to treatment with a cream containing butenafine hydrochloride 2% and TTO 5% (n=40) or a control cream containing only TTO (n=20), with active treatment for 8 weeks and final follow-up at 36 weeks.30 Patients were instructed to apply the cream 3 times daily under occlusion for 8 weeks and the nail was debrided between weeks 4 and 6 if feasible. If the nail could not be debrided after 8 weeks, it was considered resistant to treatment. At the end of the study, the complete cure rate was 80% in the active group compared to 0% in the placebo group (P<.0001), and the mean time to complete healing with progressive nail growth was 29 weeks. There were no adverse effects in the placebo group, but 4 patients in the active group had mild skin inflammation.30
Topical Cough Suppressant
Background
Topical cough suppressants, which are made up of several natural ingredients, are OTC ointments for adults and children 2 years and older that are indicated as cough suppressants when applied to the chest and throat and as relief of mild muscle and joint pains.35 The active ingredients are camphor 4.8%, eucalyptus oil 1.2%, and menthol 2.6%, while the inactive ingredients are cedarleaf oil, nutmeg oil, petrolatum, thymol, and turpentine oil.35 Some of the active and inactive ingredients in TCSs have shown efficacy against dermatophytes in vitro,36-38 and although they are not specifically indicated for onychomycosis, they have been popularized as home remedies for fungal nail infections.36,39 A TCS has been evaluated for its efficacy for the treatment of onychomycosis in one clinical trial.40
In Vitro Data
An in vitro study was performed to evaluate the antifungal activity of the individual and combined components of TCS on 16 different dermatophytes, nondermatophytes, and molds. The zones of inhibition against these organisms were greatest for camphor, menthol, thymol, and eucalyptus oil. Interestingly, there were large zones of inhibition and a synergistic effect when a mixture of components was used against T rubrum and T mentagrophytes.36 The in vitro activity of thymol, a component of TCS, was tested against Candida species.37 The essential oil subtypes Thymus vulgaris and Thymus zygis (subspecies zygis) showed similar antifungal activity, which was superior to Thymus mastichina, and all 3 compounds had similar MIC and minimal lethal concentration values. The authors showed that the antifungal mechanism was due to cell membrane damage and inhibition of germ tube formation.37 It should be noted that Candida species are less common causes of onychomycosis, and it is not known whether this data is applicable to T rubrum. In another study, the authors investigated the antifungal activity of Thymus pulegioides and found that MIC ranged from 0.16 to 0.32 μL/mL for dermatophytes and Aspergillus strains and 0.32 to 0.64 μL/mL for Candida species. When an essential oil concentration of 0.08 μL/mL was used against T rubrum, ergosterol content decreased by 70 %, indicating that T pulegioides inhibits ergosterol biosynthesis in T rubrum.38
Clinical Observations and Clinical Trial
There is one report documenting the clinical observations on a group of patients with a clinical diagnosis of onychomycosis who were instructed to apply TCS to affected nail(s) once daily.36 Eighty-five charts were reviewed (mean age, 77 years), and although follow-up was not complete or standardized, the following data were reported: 32 (38%) cleared their fungal infection, 21 (25%) had no record of change but also no record of compliance, 19 (22%) had only 1 documented follow-up visit, 9 (11%) reported they did not use the treatment, and 4 (5%) did not return for a follow-up visit. Of the 32 patients whose nails were cured, 3 (9%) had clearance within 5 months, 8 (25%) within 7 months, 11 (34%) within 9 months, 4 (13%) within 11 months, and 6 (19%) within 16 months.36
A small pilot study was performed to evaluate the efficacy of daily application of TCS in the treatment of onychomycosis in patients 18 years and older with at least 1 great toenail affected.40 The primary end points were mycologic cure at 48 weeks and clinical cure at the end of the study graded as complete, partial, or no change. The secondary end point was patient satisfaction with the appearance of the affected nail at 48 weeks. Eighteen participants completed the study; 55% (10/18) were male, with an average age of 51 years (age range, 30–85 years). The mean initial amount of affected nail was 62% (range, 16%–100%), and cultures included dermatophytes, nondermatophytes, and molds. With TCS treatment, 27.8% (5/18) showed mycologic cure of which 4 (22.2%) had a complete clinical cure. Ten participants (55.6%) had partial clinical cure and 3 (16.7%) had no clinical improvement. Interestingly, the 4 participants who had complete clinical cure had baseline cultures positive for either T mentagrophytes or C parapsilosis. Most patients were content with the treatment, as 9 participants stated that they were very satisfied and 9 stated that they were satisfied. The average ratio of affected to total nail area declined from 63% at screening to 41% at the end of the study (P<.001). No adverse effects were reported with study drug.40
NCR Lacquer
Background
Resins are natural products derived from coniferous trees and are believed to protect trees against insects and microbial pathogens.41 Natural coniferous resin derived from the Norway spruce tree (Picea abies) mixed with boiled animal fat or butter has been used topically for centuries in Finland and Sweden to treat infections and wounds.42-44 The activity of NCR has been studied against a wide range of microbes, demonstrating broad-spectrum antimicrobial activity against both gram-positive bacteria and fungi.45-48 There are 2 published clinical trials evaluating NCR in the treatment of onychomycosis.49,50
In Vitro Data
Natural coniferous resin has shown antifungal activity against T mentagrophytes, Trichophyton tonsurans, and T rubrum in vitro, which was demonstrated using medicated disks of resin on petri dishes inoculated with these organisms.46 In another study, the authors evaluated the antifungal activity of NCR against human pathogenic fungi and yeasts using agar plate diffusion tests and showed that the resin had antifungal activity against Trichophyton species but not against Fusarium and most Candida species. Electron microscopy of T mentagrophytes exposed to NCR showed that all cells were dead inside the inhibition zone, with striking changes seen in the hyphal cell walls, while fungal cells outside the inhibition zone were morphologically normal.47 In another report, utilizing the European Pharmacopoeia challenge test, NCR was highly effective against gram-positive and gram-negative bacteria as well as C albicans.42
Clinical Trials
In one preliminary observational and prospective clinical trial, 15 participants with clinical and mycologic evidence of onychomycosis were instructed to apply NCR lacquer once daily for 9 months with a 4-week washout period, with the primary outcome measures being clinical and mycologic cure.49 Thirteen (87%) enrolled participants were male and the average age was 65 years (age range, 37–80 years). The DLSO subtype was present in 9 (60%) participants. The mycologic cure rate at the end of the study was 65% (95% CI, 42%-87%), and none achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail.49
The second trial was a prospective, controlled, investigator-blinded study of 73 patients with clinical and mycologic evidence of toenail onychomycosis who were randomized to receive NCR 30%, amorolfine lacquer 5%, or 250 mg oral terbinafine.50 The primary end point was mycologic cure at 10 months, and secondary end points were clinical efficacy, cost-effectiveness, and patient compliance. Clinical efficacy was based on the proximal linear growth of healthy nail and was classified as unchanged, partial, or complete. Partial responses were described as substantial decreases in onycholysis, subungual hyperkeratosis, and streaks. A complete response was defined as a fully normal appearance of the toenail. Most patients were male in the NCR (91% [21/23]), amorolfine (80% [20/25]), and terbinafine (68% [17/25]) groups; the average ages were 64, 63, and 64 years, respectively. Trichophyton rubrum was cultured most often in all 3 groups: NCR, 87% (20/23); amorolfine, 96% (24/25); and terbinafine, 84% (21/25). The remaining cases were from T mentagrophytes. A summary of the results is shown in Table 2. Patient compliance was 100% in all except 1 patient in the amorolfine treatment group with moderate compliance. There were no adverse events, except for 2 in the terbinafine group: diarrhea and rash.50
AP Extract
Background
Ageratina pichinchensis, a member of the Asteraceae family, has been used historically in Mexico for fungal infections of the skin.51,52 Fresh or dried leaves were extracted with alcohol and the product was administered topically onto damaged skin without considerable skin irritation.53 Multiple studies have demonstrated that AP extract has in vitro antifungal activity along with other members of the Asteraceae family.54-56 There also is evidence from clinical trials that AP extract is effective against superficial dermatophyte infections such as tinea pedis.57 Given the positive antifungal in vitro data, the potential use of this agent was investigated for onychomycosis treatment.53,58
In Vitro Data
The antifungal properties of the Asteraceae family have been tested in several in vitro experiments. Eupatorium aschenbornianum, described as synonymous with A pichinchensis,59 was found to be most active against the dermatophytes T rubrum and T mentagrophytes with MICs of 0.3 and 0.03 mg/mL, respectively.54 It is thought that the primary antimycotic activity is due to encecalin, an acetylchromene compound that was identified in other plants from the Asteraceae family and has activity against dermatophytes.55 In another study, Ageratum houstanianum Mill, a comparable member of the Asteraceae family, had fungitoxic activity against T rubrum and C albicans isolated from nail infections.56
Clinical Trials
A double-blind controlled trial was performed on 110 patients with clinical and mycologic evidence of mild to moderate toenail onychomycosis randomized to treatment with AP lacquer or ciclopirox lacquer 8% (control).58 Primary end points were clinical effectiveness (completely normal nails) and mycologic cure. Patients were instructed to apply the lacquer once every third day during the first month, twice a week for the second month, and once a week for 16 weeks, with removal of the lacquer weekly. Demographics were similar between the AP lacquer and control groups, with mean ages of 44.6 and 46.5 years, respectively; women made up 74.5% and 67.2%, respectively, of each treatment group, with most patients having a 2- to 5-year history of disease (41.8% and 40.1%, respectively).58 A summary of the data is shown in Table 3. No severe side effects were documented, but minimal nail fold skin pain was reported in 3 patients in the control group in the first week, resolving later in the trial.58
A follow-up study was performed to determine the optimal concentration of AP lacquer for the treatment of onychomycosis.53 One hundred twenty-two patients aged 19 to 65 years with clinical and mycologic evidence of mild to moderate DLSO were randomized to receive 12.6% or 16.8% AP lacquer applied once daily to the affected nails for 6 months. The nails were graded as healthy, mild, or moderately affected before and after treatment. There were no significant differences in demographics between the 2 treatment groups, and 77% of patients were women with a median age of 47 years. There were no significant side effects from either concentration of AP lacquer.53
Ozonized Sunflower Oil
Background
Ozonized sunflower oil is derived by reacting ozone (O3) with sunflower plant (Helianthus annuus) oil to form a petroleum jelly–like material.60 It was originally shown to have antibacterial properties in vitro,61 and further studies have confirmed these findings and demonstrated anti-inflammatory, wound healing, and antifungal properties.62-64 A formulation of ozonized sunflower oil used in Cuba is clinically indicated for the treatment of tinea pedis and impetigo.65 The clinical efficacy of this product has been evaluated in a clinical trial for the treatment of onychomycosis.65
In Vitro Data
A compound made up of 30% ozonized sunflower oil with 0.5% of α-lipoic acid was found to have antifungal activity against C albicans using the disk diffusion method, in addition to other bacterial organisms. The MIC values ranged from 2.0 to 3.5 mg/mL.62 Another study was designed to evaluate the in vitro antifungal activity of this formulation on samples cultured from patients with onychomycosis using the disk diffusion method. They found inhibition of growth of C albicans, C parapsilosis, and Candida tropicalis, which was inferior to amphotericin B, ketoconazole, fluconazole, and itraconazole.64
Clinical Trial
A single-blind, controlled, phase 3 study was performed on 400 patients with clinical and mycologic evidence of onychomycosis. Patients were randomized to treatment with an ozonized sunflower oil solution or ketoconazole cream 2% applied to affected nails twice daily for 3 months, with filing and massage of the affected nails upon application of treatment.65 Cured was defined as mycologic cure in addition to a healthy appearing nail, improved as an increase in healthy appearing nail in addition to a decrease in symptoms (ie, paresthesia, pain, itching) but positive mycological testing, same as no clinical change in appearance with positive mycological findings, and worse as increasing diseased nail involvement in the presence of positive mycological findings. Demographics were similar between groups with a mean age of 35 years. Men accounted for 80% of the study population, and 65% of the study population was white. The mean duration of disease was 30 months. They also reported on a 1-year follow-up, with 2.8% of patients in the ozonized sunflower oil solution group and 37.0% of patients in the ketoconazole group describing relapses. Trichophyton rubrum and C albicans were cultured from these patients.65
Comment
Due to the poor efficacy, long-term treatment courses, inability to use nail polish, and high cost associated with many FDA-approved topical treatments, along with the systemic side effects, potential for drug-drug interactions, and cost associated with many oral therapies approved for onychomycosis, there has been a renewed interest in natural remedies and OTC treatments. Overall, TTO, TCS, NCR, AP extract, and ozonized sunflower oil have shown efficacy in vitro against some dermatophytes, nondermatophytes, and molds responsible for onychomycosis. One or more clinical trials were performed with each of these agents for the treatment of onychomycosis. They were mostly small pilot studies, and due to differences in trial design, the results cannot be compared with each other or with currently FDA-approved treatments. We can conclude that because adverse events were rare with all of these therapies—most commonly skin irritation or mild skin pain—they exhibit good safety.
For TTO, there was no statistical difference between the clotrimazole and TTO treatment groups in mycologic cure, clinical assessment, or patient subjective assessment of the nails.29 Although there was an 80% complete cure in the butenafine and TTO group, it was 0% in the TTO group at week 36.30 Trial design, longer treatment periods, incorporation into nanocapsules, or combination treatment with other antifungal agents may influence our future use of TTO for onychomycosis, but based on the present data we cannot recommend this treatment in clinical practice.
With TCS, 27.8% of participants had a mycologic cure and 22.2% had complete clinical cure.40 Although it is difficult to draw firm conclusions from this small pilot study, there may be some benefit to treating toenail onychomycosis due to T mentagrophytes or C parapsilosis with TCS but no benefit in treating onychomycosis due to T rubrum, the more common cause of onychomycosis. Limitations of this study were lack of a placebo group, small sample size, wide variety of represented pathogens that may not be representative of the true population, and lack of stratification by baseline severity or involvement of nail. A larger randomized controlled clinical trial would be necessary to confirm the results of this small study and make formal recommendations.
In one clinical trial with NCR, mycologic cure was 65% at the end of the study.49 No participants achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail. Because this study was small (N=15), it is difficult to draw firm conclusions.49 In another study with NCR, mycologic cure rates with NCR, amorolfine, and terbinafine were 13%, 8%, and 56%, respectively. Based on these results, NCR has similar antifungal efficacy to amorolfine but was inferior to oral terbinafine.50 A larger randomized controlled clinical trial with more homogenous and less severely affected patients and longer treatment periods would be necessary to confirm the results of these small studies and make formal recommendations.
Because there were no significant differences in clinical effectiveness of mycologic cure rates between AP lacquer 10% and ciclopirox lacquer 8% in one clinical trial,58 AP does not seem to be more effective than at least one of the current FDA-approved topical treatments; however, because AP lacquer 16.8% was shown to be more effective than AP lacquer 12.6% in one onychomycosis clinical trial, using higher concentrations of AP may yield better results in future trials.53
One trial comparing ozonized sunflower oil to ketoconazole cream 2% showed 90.5% and 13.5% cure rates, respectively.65 Although there is good in vitro antifungal activity and a clinical trial showing efficacy using ozonized sunflower oil for the treatment of onychomycosis, confirmatory studies are necessary before we can recommend this OTC treatment to our patients. Specifically, we will get the most data from large randomized controlled trials with strict inclusion/exclusion and efficacy criteria.
Conclusion
Over-the-counter and natural remedies may be an emerging area of research in the treatment of onychomycosis. This review summarizes the laboratory data and clinical trials on several of these agents and, when available, compares their clinical and mycologic efficacy with FDA-approved therapies. Shortcomings of some of these studies include a small study population, lack of adequate controls, nonstandardized mycologic testing, and abbreviated posttreatment evaluation times. It may be concluded that these products have varying degrees of efficacy and appear to be safe in the studies cited; however, at present, we cannot recommend any of them to our patients until there are larger randomized clinical trials with appropriate controls demonstrating their efficacy.
Onychomycosis is a fungal infection of the nail unit by dermatophytes, yeasts, and nondermatophyte molds. It is characterized by a white or yellow discoloration of the nail plate; hyperkeratosis of the nail bed; distal detachment of the nail plate from its bed (onycholysis); and nail plate dystrophy, including thickening, crumbling, and ridging. Onychomycosis is an important problem, representing 30% of all superficial fungal infections and an estimated 50% of all nail diseases.1 Reported prevalence rates of onychomycosis in the United States and worldwide are varied, but the mean prevalence based on population-based studies in Europe and North America is estimated to be 4.3%.2 It is more common in older individuals, with an incidence rate of 20% in those older than 60 years and 50% in those older than 70 years.3 Onychomycosis is more common in patients with diabetes and 1.9 to 2.8 times higher than the general population.4 Dermatophytes are responsible for the majority of cases of onychomycosis, particularly Trichophyton rubrum and Trichophyton mentagrophytes.5
Onychomycosis is divided into different subtypes based on clinical presentation, which in turn are characterized by varying infecting organisms and prognoses. The subtypes of onychomycosis are distal and lateral subungual (DLSO), proximal subungual, superficial, endonyx, mixed pattern, total dystrophic, and secondary. Distal and lateral subungual onychomycosis are by far the most common presentation and begins when the infecting organism invades the hyponychium and distal or lateral nail bed. Trichophyton rubrum is the most common organism and T mentagrophytes is second, but Candida parapsilosis and Candida albicans also are possibilities. Proximal subungual onychomycosis is far less frequent than DLSO and is usually caused by T rubrum. The fungus invades the proximal nail folds and penetrates the newly growing nail plate.6 This pattern is more common in immunosuppressed patients and should prompt testing for human immunodeficiency virus.7 Total dystrophic onychomycosis is the end stage of fungal nail plate invasion, may follow DLSO or proximal subungual onychomycosis, and is difficult to treat.6
Onychomycosis causes pain, paresthesia, and difficulty with ambulation.8 In patients with peripheral neuropathy and vascular problems, including diabetes, onychomycosis can increase the risk for foot ulcers, with amputation in severe cases.9 Patients also may present with aesthetic concerns that may impact their quality of life.10
Given the effect on quality of life along with medical risks associated with onychomycosis, a safe and successful treatment modality with a low risk of recurrence is desirable. Unfortunately, treatment of nail fungus is quite challenging for a number of reasons. First, the thickness of the nail and/or the fungal mass may be a barrier to the delivery of topical and systemic drugs at the source of the infection. In addition, the nail plate does not have intrinsic immunity. Also, recurrence after treatment is common due to residual hyphae or spores that were not previously eliminated.11 Finally, many topical medications require long treatment courses, which may limit patient compliance, especially in patients who want to use nail polish for cosmesis or camouflage.
Currently Approved Therapies for Onychomycosis
Several definitions are needed to better interpret the results of onychomycosis clinical trials. Complete cure is defined as a negative potassium hydroxide preparation and negative fungal culture with a completely normal appearance of the nail. Mycological cure is defined as potassium hydroxide microscopy and fungal culture negative. Clinical cure is stated as 0% nail plate involvement but at times is reported as less than 5% and less than 10% involvement.
Terbinafine and itraconazole are the only US Food and Drug Administration (FDA)–approved systemic therapies, and ciclopirox, efinaconazole, and tavaborole are the only FDA-approved topicals. Advantages of systemic agents generally are higher cure rates and shorter treatment courses, thus better compliance. Disadvantages include greater incidence of systemic side effects and drug-drug interactions as well as the need for laboratory monitoring. Pros of topical therapies are low potential for adverse effects, no drug-drug interactions, and no monitoring of blood work. Cons include lower efficacy, long treatment courses, and poor patient compliance.
Terbinafine, an allylamine, taken orally once daily (250 mg) for 12 weeks for toenails and 6 weeks for fingernails currently is the preferred systemic treatment of onychomycosis, with complete cure rates of 38% and 59% and mycological cure rates of 70% and 79% for toenails and fingernails, respectively.12 Itraconazole, an azole, is dosed orally at 200 mg daily for 3 months for toenails, with a complete cure rate of 14% and mycological cure rate of 54%.13 For fingernail onychomycosis only, itraconazole is dosed at 200 mg twice daily for 1 week, followed by a treatment-free period of 3 weeks, and then another 1-week course at thesame dose. The complete cure rate is 47% and the mycological cure is 61% for this pulse regimen.13
Ciclopirox is a hydroxypyridone and the 8% nail lacquer formulation was approved in 1999, making it the first topical medication to gain FDA approval for the treatment of toenail onychomycosis. Based on 2 clinical trials, complete cure rates for toenails are 5.5% and 8.5% and mycological cure rates are 29% and 36% at 48 weeks with removal of residual lacquer and debridement.14Efinaconazole is an azole and the 10% solution was FDA approved for the treatment of toenail onychomycosis in 2014.15 In 2 clinical trials, complete cure rates were 17.8% and 15.2% and mycological cure rates were 55.2% and 53.4% with once daily toenail application for 48 weeks.16 Tavaborole is a benzoxaborole and the 5% solution also was approved for the treatment of toenail onychomycosis in 2014.17 Two clinical trials reported complete cure rates of 6.5% and 9.1% and mycological cure rates of 31.1% and 35.9% with once daily toenail application for 48 weeks.18
Given the poor efficacy, systemic side effects, potential for drug-drug interactions, long-term treatment courses, and cost associated with current systemic and/or topical treatments, there has been a renewed interest in natural remedies and over-the-counter (OTC) therapies for onychomycosis. This review summarizes the in vitro and in vivo data, mechanisms of action, and clinical efficacy of various natural and OTC agents for the treatment of onychomycosis. Specifically, we summarize the data on tea tree oil (TTO), a popular topical cough suppressant (TCS), natural coniferous resin (NCR) lacquer, Ageratina pichinchensis (AP) extract, and ozonized sunflower oil.
Tea Tree Oil
Background
Tea tree oil is a volatile oil whose medicinal use dates back to the early 20th century when the Bundjabung aborigines of North and New South Wales extracted TTO from the dried leaves of the Melaleuca alternifolia plant and used it to treat superficial wounds.19 Tea tree oil has been shown to be an effective treatment of tinea pedis,20 and it is widely used in Australia as well as in Europe and North America.21 Tea tree oil also has been investigated as an antifungal agent for the treatment of onychomycosis, both in vitro22-28 and in clinical trials.29,30
In Vitro Data
Because TTO is composed of more than 100 active components,23 the antifungal activity of these individual components was investigated against 14 fungal isolates, including C albicans, T mentagrophytes, and Aspergillus species. The minimum inhibitory concentration (MIC) for α-pinene was less than 0.004% for T mentagrophytes and the components with the greatest MIC and minimum fungicidal concentration for the fungi tested were terpinen-4-ol and α-terpineol, respectively.22 The antifungal activity of TTO also was tested using disk diffusion assay experiments with 58 clinical isolates of fungi including C albicans, T rubrum, T mentagrophytes, and Aspergillus niger.24 Tea tree oil was most effective at inhibiting T rubrum followed by T mentagrophytes,24 which are the 2 most common etiologies of onychomycosis.5 In another report, the authors determined the MIC of TTO utilizing 4 different experiments with T rubrum as the infecting organism. Because TTO inhibited the growth of T rubrum at all concentrations greater than 0.1%, they found that the MIC was 0.1%.25 Given the lack of adequate nail penetration of most topical therapies, TTO in nanocapsules (TTO-NC), TTO nanoemulsions, and normal emulsions were tested in vitro for their ability to inhibit the growth of T rubrum inoculated into nail shavings. Colony growth decreased significantly within the first week of treatment, with TTO-NC showing maximum efficacy (P<.001). This study showed that TTO, particularly TTO-NC, was effective in inhibiting the growth of T rubrum in vitro and that using nanocapsule technology may increase nail penetration and bioavailability.31
Much of what we know about TTO’s antifungal mechanism of action comes from experiments involving C albicans. To date, it has not been studied in T rubrum or T mentagrophytes, the 2 most common etiologies of onychomycosis.5 In C albicans, TTO causes altered permeability of plasma membranes,32 dose-dependent alteration of respiration,33 decreased glucose-induced acidification of media surrounding fungi,32 and reversible inhibition of germ tube formation.19,34
Clinical Trials
A randomized, double-blind, multicenter trial was performed on 117 patients with culture-proven DLSO who were randomized to receive TTO 100% or clotrimazole solution 1% applied twice daily to affected toenails for 6 months.29 Primary outcome measures were mycologic cure, clinical assessment, and patient subjective assessment (Table 1). There were no statistical differences between the 2 treatment groups. Erythema and irritation were the most common adverse reactions occurring in 7.8% (5/64) of the TTO group.29
Another study was a double-blind, placebo-controlled trial involving 60 patients with clinical and mycologic evidence of DLSO who were randomized to treatment with a cream containing butenafine hydrochloride 2% and TTO 5% (n=40) or a control cream containing only TTO (n=20), with active treatment for 8 weeks and final follow-up at 36 weeks.30 Patients were instructed to apply the cream 3 times daily under occlusion for 8 weeks and the nail was debrided between weeks 4 and 6 if feasible. If the nail could not be debrided after 8 weeks, it was considered resistant to treatment. At the end of the study, the complete cure rate was 80% in the active group compared to 0% in the placebo group (P<.0001), and the mean time to complete healing with progressive nail growth was 29 weeks. There were no adverse effects in the placebo group, but 4 patients in the active group had mild skin inflammation.30
Topical Cough Suppressant
Background
Topical cough suppressants, which are made up of several natural ingredients, are OTC ointments for adults and children 2 years and older that are indicated as cough suppressants when applied to the chest and throat and as relief of mild muscle and joint pains.35 The active ingredients are camphor 4.8%, eucalyptus oil 1.2%, and menthol 2.6%, while the inactive ingredients are cedarleaf oil, nutmeg oil, petrolatum, thymol, and turpentine oil.35 Some of the active and inactive ingredients in TCSs have shown efficacy against dermatophytes in vitro,36-38 and although they are not specifically indicated for onychomycosis, they have been popularized as home remedies for fungal nail infections.36,39 A TCS has been evaluated for its efficacy for the treatment of onychomycosis in one clinical trial.40
In Vitro Data
An in vitro study was performed to evaluate the antifungal activity of the individual and combined components of TCS on 16 different dermatophytes, nondermatophytes, and molds. The zones of inhibition against these organisms were greatest for camphor, menthol, thymol, and eucalyptus oil. Interestingly, there were large zones of inhibition and a synergistic effect when a mixture of components was used against T rubrum and T mentagrophytes.36 The in vitro activity of thymol, a component of TCS, was tested against Candida species.37 The essential oil subtypes Thymus vulgaris and Thymus zygis (subspecies zygis) showed similar antifungal activity, which was superior to Thymus mastichina, and all 3 compounds had similar MIC and minimal lethal concentration values. The authors showed that the antifungal mechanism was due to cell membrane damage and inhibition of germ tube formation.37 It should be noted that Candida species are less common causes of onychomycosis, and it is not known whether this data is applicable to T rubrum. In another study, the authors investigated the antifungal activity of Thymus pulegioides and found that MIC ranged from 0.16 to 0.32 μL/mL for dermatophytes and Aspergillus strains and 0.32 to 0.64 μL/mL for Candida species. When an essential oil concentration of 0.08 μL/mL was used against T rubrum, ergosterol content decreased by 70 %, indicating that T pulegioides inhibits ergosterol biosynthesis in T rubrum.38
Clinical Observations and Clinical Trial
There is one report documenting the clinical observations on a group of patients with a clinical diagnosis of onychomycosis who were instructed to apply TCS to affected nail(s) once daily.36 Eighty-five charts were reviewed (mean age, 77 years), and although follow-up was not complete or standardized, the following data were reported: 32 (38%) cleared their fungal infection, 21 (25%) had no record of change but also no record of compliance, 19 (22%) had only 1 documented follow-up visit, 9 (11%) reported they did not use the treatment, and 4 (5%) did not return for a follow-up visit. Of the 32 patients whose nails were cured, 3 (9%) had clearance within 5 months, 8 (25%) within 7 months, 11 (34%) within 9 months, 4 (13%) within 11 months, and 6 (19%) within 16 months.36
A small pilot study was performed to evaluate the efficacy of daily application of TCS in the treatment of onychomycosis in patients 18 years and older with at least 1 great toenail affected.40 The primary end points were mycologic cure at 48 weeks and clinical cure at the end of the study graded as complete, partial, or no change. The secondary end point was patient satisfaction with the appearance of the affected nail at 48 weeks. Eighteen participants completed the study; 55% (10/18) were male, with an average age of 51 years (age range, 30–85 years). The mean initial amount of affected nail was 62% (range, 16%–100%), and cultures included dermatophytes, nondermatophytes, and molds. With TCS treatment, 27.8% (5/18) showed mycologic cure of which 4 (22.2%) had a complete clinical cure. Ten participants (55.6%) had partial clinical cure and 3 (16.7%) had no clinical improvement. Interestingly, the 4 participants who had complete clinical cure had baseline cultures positive for either T mentagrophytes or C parapsilosis. Most patients were content with the treatment, as 9 participants stated that they were very satisfied and 9 stated that they were satisfied. The average ratio of affected to total nail area declined from 63% at screening to 41% at the end of the study (P<.001). No adverse effects were reported with study drug.40
NCR Lacquer
Background
Resins are natural products derived from coniferous trees and are believed to protect trees against insects and microbial pathogens.41 Natural coniferous resin derived from the Norway spruce tree (Picea abies) mixed with boiled animal fat or butter has been used topically for centuries in Finland and Sweden to treat infections and wounds.42-44 The activity of NCR has been studied against a wide range of microbes, demonstrating broad-spectrum antimicrobial activity against both gram-positive bacteria and fungi.45-48 There are 2 published clinical trials evaluating NCR in the treatment of onychomycosis.49,50
In Vitro Data
Natural coniferous resin has shown antifungal activity against T mentagrophytes, Trichophyton tonsurans, and T rubrum in vitro, which was demonstrated using medicated disks of resin on petri dishes inoculated with these organisms.46 In another study, the authors evaluated the antifungal activity of NCR against human pathogenic fungi and yeasts using agar plate diffusion tests and showed that the resin had antifungal activity against Trichophyton species but not against Fusarium and most Candida species. Electron microscopy of T mentagrophytes exposed to NCR showed that all cells were dead inside the inhibition zone, with striking changes seen in the hyphal cell walls, while fungal cells outside the inhibition zone were morphologically normal.47 In another report, utilizing the European Pharmacopoeia challenge test, NCR was highly effective against gram-positive and gram-negative bacteria as well as C albicans.42
Clinical Trials
In one preliminary observational and prospective clinical trial, 15 participants with clinical and mycologic evidence of onychomycosis were instructed to apply NCR lacquer once daily for 9 months with a 4-week washout period, with the primary outcome measures being clinical and mycologic cure.49 Thirteen (87%) enrolled participants were male and the average age was 65 years (age range, 37–80 years). The DLSO subtype was present in 9 (60%) participants. The mycologic cure rate at the end of the study was 65% (95% CI, 42%-87%), and none achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail.49
The second trial was a prospective, controlled, investigator-blinded study of 73 patients with clinical and mycologic evidence of toenail onychomycosis who were randomized to receive NCR 30%, amorolfine lacquer 5%, or 250 mg oral terbinafine.50 The primary end point was mycologic cure at 10 months, and secondary end points were clinical efficacy, cost-effectiveness, and patient compliance. Clinical efficacy was based on the proximal linear growth of healthy nail and was classified as unchanged, partial, or complete. Partial responses were described as substantial decreases in onycholysis, subungual hyperkeratosis, and streaks. A complete response was defined as a fully normal appearance of the toenail. Most patients were male in the NCR (91% [21/23]), amorolfine (80% [20/25]), and terbinafine (68% [17/25]) groups; the average ages were 64, 63, and 64 years, respectively. Trichophyton rubrum was cultured most often in all 3 groups: NCR, 87% (20/23); amorolfine, 96% (24/25); and terbinafine, 84% (21/25). The remaining cases were from T mentagrophytes. A summary of the results is shown in Table 2. Patient compliance was 100% in all except 1 patient in the amorolfine treatment group with moderate compliance. There were no adverse events, except for 2 in the terbinafine group: diarrhea and rash.50
AP Extract
Background
Ageratina pichinchensis, a member of the Asteraceae family, has been used historically in Mexico for fungal infections of the skin.51,52 Fresh or dried leaves were extracted with alcohol and the product was administered topically onto damaged skin without considerable skin irritation.53 Multiple studies have demonstrated that AP extract has in vitro antifungal activity along with other members of the Asteraceae family.54-56 There also is evidence from clinical trials that AP extract is effective against superficial dermatophyte infections such as tinea pedis.57 Given the positive antifungal in vitro data, the potential use of this agent was investigated for onychomycosis treatment.53,58
In Vitro Data
The antifungal properties of the Asteraceae family have been tested in several in vitro experiments. Eupatorium aschenbornianum, described as synonymous with A pichinchensis,59 was found to be most active against the dermatophytes T rubrum and T mentagrophytes with MICs of 0.3 and 0.03 mg/mL, respectively.54 It is thought that the primary antimycotic activity is due to encecalin, an acetylchromene compound that was identified in other plants from the Asteraceae family and has activity against dermatophytes.55 In another study, Ageratum houstanianum Mill, a comparable member of the Asteraceae family, had fungitoxic activity against T rubrum and C albicans isolated from nail infections.56
Clinical Trials
A double-blind controlled trial was performed on 110 patients with clinical and mycologic evidence of mild to moderate toenail onychomycosis randomized to treatment with AP lacquer or ciclopirox lacquer 8% (control).58 Primary end points were clinical effectiveness (completely normal nails) and mycologic cure. Patients were instructed to apply the lacquer once every third day during the first month, twice a week for the second month, and once a week for 16 weeks, with removal of the lacquer weekly. Demographics were similar between the AP lacquer and control groups, with mean ages of 44.6 and 46.5 years, respectively; women made up 74.5% and 67.2%, respectively, of each treatment group, with most patients having a 2- to 5-year history of disease (41.8% and 40.1%, respectively).58 A summary of the data is shown in Table 3. No severe side effects were documented, but minimal nail fold skin pain was reported in 3 patients in the control group in the first week, resolving later in the trial.58
A follow-up study was performed to determine the optimal concentration of AP lacquer for the treatment of onychomycosis.53 One hundred twenty-two patients aged 19 to 65 years with clinical and mycologic evidence of mild to moderate DLSO were randomized to receive 12.6% or 16.8% AP lacquer applied once daily to the affected nails for 6 months. The nails were graded as healthy, mild, or moderately affected before and after treatment. There were no significant differences in demographics between the 2 treatment groups, and 77% of patients were women with a median age of 47 years. There were no significant side effects from either concentration of AP lacquer.53
Ozonized Sunflower Oil
Background
Ozonized sunflower oil is derived by reacting ozone (O3) with sunflower plant (Helianthus annuus) oil to form a petroleum jelly–like material.60 It was originally shown to have antibacterial properties in vitro,61 and further studies have confirmed these findings and demonstrated anti-inflammatory, wound healing, and antifungal properties.62-64 A formulation of ozonized sunflower oil used in Cuba is clinically indicated for the treatment of tinea pedis and impetigo.65 The clinical efficacy of this product has been evaluated in a clinical trial for the treatment of onychomycosis.65
In Vitro Data
A compound made up of 30% ozonized sunflower oil with 0.5% of α-lipoic acid was found to have antifungal activity against C albicans using the disk diffusion method, in addition to other bacterial organisms. The MIC values ranged from 2.0 to 3.5 mg/mL.62 Another study was designed to evaluate the in vitro antifungal activity of this formulation on samples cultured from patients with onychomycosis using the disk diffusion method. They found inhibition of growth of C albicans, C parapsilosis, and Candida tropicalis, which was inferior to amphotericin B, ketoconazole, fluconazole, and itraconazole.64
Clinical Trial
A single-blind, controlled, phase 3 study was performed on 400 patients with clinical and mycologic evidence of onychomycosis. Patients were randomized to treatment with an ozonized sunflower oil solution or ketoconazole cream 2% applied to affected nails twice daily for 3 months, with filing and massage of the affected nails upon application of treatment.65 Cured was defined as mycologic cure in addition to a healthy appearing nail, improved as an increase in healthy appearing nail in addition to a decrease in symptoms (ie, paresthesia, pain, itching) but positive mycological testing, same as no clinical change in appearance with positive mycological findings, and worse as increasing diseased nail involvement in the presence of positive mycological findings. Demographics were similar between groups with a mean age of 35 years. Men accounted for 80% of the study population, and 65% of the study population was white. The mean duration of disease was 30 months. They also reported on a 1-year follow-up, with 2.8% of patients in the ozonized sunflower oil solution group and 37.0% of patients in the ketoconazole group describing relapses. Trichophyton rubrum and C albicans were cultured from these patients.65
Comment
Due to the poor efficacy, long-term treatment courses, inability to use nail polish, and high cost associated with many FDA-approved topical treatments, along with the systemic side effects, potential for drug-drug interactions, and cost associated with many oral therapies approved for onychomycosis, there has been a renewed interest in natural remedies and OTC treatments. Overall, TTO, TCS, NCR, AP extract, and ozonized sunflower oil have shown efficacy in vitro against some dermatophytes, nondermatophytes, and molds responsible for onychomycosis. One or more clinical trials were performed with each of these agents for the treatment of onychomycosis. They were mostly small pilot studies, and due to differences in trial design, the results cannot be compared with each other or with currently FDA-approved treatments. We can conclude that because adverse events were rare with all of these therapies—most commonly skin irritation or mild skin pain—they exhibit good safety.
For TTO, there was no statistical difference between the clotrimazole and TTO treatment groups in mycologic cure, clinical assessment, or patient subjective assessment of the nails.29 Although there was an 80% complete cure in the butenafine and TTO group, it was 0% in the TTO group at week 36.30 Trial design, longer treatment periods, incorporation into nanocapsules, or combination treatment with other antifungal agents may influence our future use of TTO for onychomycosis, but based on the present data we cannot recommend this treatment in clinical practice.
With TCS, 27.8% of participants had a mycologic cure and 22.2% had complete clinical cure.40 Although it is difficult to draw firm conclusions from this small pilot study, there may be some benefit to treating toenail onychomycosis due to T mentagrophytes or C parapsilosis with TCS but no benefit in treating onychomycosis due to T rubrum, the more common cause of onychomycosis. Limitations of this study were lack of a placebo group, small sample size, wide variety of represented pathogens that may not be representative of the true population, and lack of stratification by baseline severity or involvement of nail. A larger randomized controlled clinical trial would be necessary to confirm the results of this small study and make formal recommendations.
In one clinical trial with NCR, mycologic cure was 65% at the end of the study.49 No participants achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail. Because this study was small (N=15), it is difficult to draw firm conclusions.49 In another study with NCR, mycologic cure rates with NCR, amorolfine, and terbinafine were 13%, 8%, and 56%, respectively. Based on these results, NCR has similar antifungal efficacy to amorolfine but was inferior to oral terbinafine.50 A larger randomized controlled clinical trial with more homogenous and less severely affected patients and longer treatment periods would be necessary to confirm the results of these small studies and make formal recommendations.
Because there were no significant differences in clinical effectiveness of mycologic cure rates between AP lacquer 10% and ciclopirox lacquer 8% in one clinical trial,58 AP does not seem to be more effective than at least one of the current FDA-approved topical treatments; however, because AP lacquer 16.8% was shown to be more effective than AP lacquer 12.6% in one onychomycosis clinical trial, using higher concentrations of AP may yield better results in future trials.53
One trial comparing ozonized sunflower oil to ketoconazole cream 2% showed 90.5% and 13.5% cure rates, respectively.65 Although there is good in vitro antifungal activity and a clinical trial showing efficacy using ozonized sunflower oil for the treatment of onychomycosis, confirmatory studies are necessary before we can recommend this OTC treatment to our patients. Specifically, we will get the most data from large randomized controlled trials with strict inclusion/exclusion and efficacy criteria.
Conclusion
Over-the-counter and natural remedies may be an emerging area of research in the treatment of onychomycosis. This review summarizes the laboratory data and clinical trials on several of these agents and, when available, compares their clinical and mycologic efficacy with FDA-approved therapies. Shortcomings of some of these studies include a small study population, lack of adequate controls, nonstandardized mycologic testing, and abbreviated posttreatment evaluation times. It may be concluded that these products have varying degrees of efficacy and appear to be safe in the studies cited; however, at present, we cannot recommend any of them to our patients until there are larger randomized clinical trials with appropriate controls demonstrating their efficacy.
- Scher RK, Daniel CR. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005.
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480-1491.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Mayser P, Freund V, Budihardja D. Toenail onychomycosis in diabetic patients: issues and management. Am J Clin Dermatol. 2009;10:211-220.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification J Am Acad Dermatol. 2011;65:1219-1227.
- Elewski B. Clinical pearl: proximal white subungual onychomycosis in AIDS. J Am Acad Dermatol. 1993;29:631-632.
- Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):15.
- Boyko EJ, Ahroni JH, Cohen V, et al. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006;29:1202-1207.
- Szepietowski JC, Reich A, Pacan P, et al. Evaluation of quality of life in patients with toenail onychomycosis by Polish version of an international onychomycosis-specific questionnaire. J Eur Acad Dermatol Venereol. 2007;21:491-496.
- Scher RK, Baron R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Sporanox [package insert]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001
- Penlac [package insert]. Bridgewater, NJ: Dermik Laboratories; 2006.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; 2014.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies [published online May 5, 2015]. J Am Acad Dermatol. 2015;73:62-69.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: a randomized, placebo-controlled, blinded study. Australas J Dermatol. 2002;43:175-178.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853-860.
- Brophy JJ, Davies NW, Southwell IA, et al. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem. 1989;37:1330-1335.
- Concha JM, Moore LS, Holloway WJ. 1998 William J. Stickel Bronze Award. Antifungal activity of Melaleuca alternifolia (tea-tree) oil against various pathogenic organisms. J Am Podiatr Med Assoc. 1998;88:489-492.
- Benger S, Townsend P, Ashford RL, et al. An in vitro study to determine the minimum inhibitory concentration of Melaleuca alternifolia against the dermatophyte Trichophyton rubrum. Foot. 2004;14:86-91.
- Hammer KA, Carson CF, Riley TV. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother. 1998;42:591-595.
- Altman P. Australian tea tree oil. Aust J Pharm. 1998;69:276-278.
- Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147-157.
- Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract. 1994;38:601-605.
- Syed TA, Qureshi ZA, Ali SM, et al. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Med Int Health. 1999;4:284-287.
- Flores FC, de Lima JA, Ribeiro RF, et al. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of Trichophyton rubrum. Mycopathologia. 2013;175:281-286.
- Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081-1085.
- Cox SD, Mann CM, Markham JL, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88:170-175.
- Hammer KA, Carson CF, Riley TV. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med Mycol. 2000;38:355-362.
- Vicks VapoRub [package insert]. Gross-Gerau, Germany: Proctor & Gamble; 2010.
- Ramsewak RS, Nair MG, Stommel M, et al. In vitro antagonistic activity of monoterpenes and their mixtures against ‘toe nail fungus’ pathogens. Phytother Res. 2003;17:376-379.
- Pina-Vaz C, Gonçalves Rodrigues A, Pinto E, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73-78.
- Pinto E, Pina-Vaz C, Salgueiro L, et al. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol. 2006;55:1367-1373.
- Vicks VapoRub might help fight toenail fungus. Consumer Reports. 2006;71:49.
- Derby R, Rohal P, Jackson C, et al. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med. 2011;24:69-74.
- Trapp S, Croteau R. Defensive resin biosynthesis in conifers. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:689-724.
- Sipponen A, Laitinen K. Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS. 2011;119:720-724.
- Sipponen A, Lohi J. Lappish gum care “new” treatment of pressure ulcers? People’s improvement at it’s best. Eng Med J. 2003;58:2775-2776.
- Benedictus O. Een Nyttigh Läkare. Malmö: Kroon; 1938.
- Rautio M, Sipponen A, Peltola R, et al. Antibacterial effects of home-made resin salve from Norway spruce (Picea abies). APMIS. 2007;115:335-340.
- Laitinen K, Sipponen A, Jokinen JJ, et al. Resin salve from Norway spruce is antifungal against dermatophytes causing nail infections. EWMA. 2009;56:289-296.
- Rautio M, Sipponen A, Lohi J, et al. In vitro fungistatic effects of natural coniferous resin from Norway spruce (Picea abies). Eur J Clin Microbiol Infect Dis. 2012;31:1783-1789.
- Sipponen A, Peltola R, Jokinen JJ, et al. Effects of Norway spruce (Picea abies) resin on cell wall and cell membrane of Staphylococcus aureus. Ultrastruct Pathol. 2009;33:128-135.
- Sipponen P, Sipponen A, Lohi J, et al. Natural coniferous resin lacquer in treatment of toenail onychomycosis: an observational study. Mycoses. 2013;56:289-296.
- Auvinen T, Tiihonen R, Soini M, et al. Efficacy of topical resin lacquer, amorolfine, and oral terbinafine for treating toenail onychomycosis: a prospective, randomized, controlled, investigator-blinded, parallel-group clinical trial. Br J Dermatol. 2015;173:940-948.
- Argueta A, Cano L, Rodarte M. Atlas de las Plantas de la Medicina Tradicional Mexicana. Vol 3. Mexico City, Mexico: Instituto Nacional Indigenista; 1994:72-680.
- Avilés M, Suárez G. Catálogo de Plantas Medicinales del Jardín Etnobotánico. Peru: Instituto Nacional de Antropología e Historia; 1994.
- Romero-Cerecero O, Roman-Ramos R, Zamilpa A, et al. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J Ethnopharmacol. 2009;126:74-78.
- Navarro Garcia VM, Gonzalez A, Fuentes M, et al. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003;87:85-88.
- Castañeda P, Gómez L, Mata R, et al. Phytogrowth-inhibitory and antifungal constituents of Helianthella quinquenervis. J Nat Prod. 1996;59:323-326.
- Kumar N. Inhibition of nail infecting fungi of peoples of North Eastern UP causing Tinea unguium through leaf essential oil of Ageratum houstonianum Mill. IOSR J Pharm. June 2014;4:36-42.
- Romero-Cerecero O, Rojas G, Navarro V, et al. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: an explorative pilot study controlled with ketoconazole. Planta Med. 2006;72:1257-1261.
- Romero-Cerecero O, Zamilpa A, Jimenez-Ferrer JE, et al. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. a comparative study with ciclopirox. Planta Med. 2008;74:1430-1435.
- Rzedowski J, De Rzedowski GC. Flora Fanerogámica del Valle de México. Mexico City, Mexico: Instituto de Ecología Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional; 1985.
- Bocci V. Biological and clinical effects of ozone. has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56:270-279.
- Sechi LA, Lezcano I, Nunez N, et al. Antibacterial activity of ozonized sunflower oil (Oleozon). J Appl Microbiol. 2001;90:279-284.
- Rodrigues KL, Cardoso CC, Caputo LR, et al. Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds. Inflammopharmacology. 2004;12:261-270.
- Daud FV, Ueda SMY, Navarini A, et al. The use of ozonized oil in the treatment of dermatophitosis caused by Microsporum canis in rabbits. Braz J Microbiol. 2011;42:274-281.
- Guerrer LV, Cunha KC, Nogueira MC, et al. “In vitro” antifungal activity of ozonized sunflower oil on yeasts from onychomycosis. Braz J Microbiol. 2012;43:1315-1318.
- Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON in patients suffering from onychomycosis. Mycoses. 2011;54:E272-E277.
- Scher RK, Daniel CR. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005.
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480-1491.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Mayser P, Freund V, Budihardja D. Toenail onychomycosis in diabetic patients: issues and management. Am J Clin Dermatol. 2009;10:211-220.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification J Am Acad Dermatol. 2011;65:1219-1227.
- Elewski B. Clinical pearl: proximal white subungual onychomycosis in AIDS. J Am Acad Dermatol. 1993;29:631-632.
- Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):15.
- Boyko EJ, Ahroni JH, Cohen V, et al. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006;29:1202-1207.
- Szepietowski JC, Reich A, Pacan P, et al. Evaluation of quality of life in patients with toenail onychomycosis by Polish version of an international onychomycosis-specific questionnaire. J Eur Acad Dermatol Venereol. 2007;21:491-496.
- Scher RK, Baron R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Sporanox [package insert]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001
- Penlac [package insert]. Bridgewater, NJ: Dermik Laboratories; 2006.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; 2014.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies [published online May 5, 2015]. J Am Acad Dermatol. 2015;73:62-69.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: a randomized, placebo-controlled, blinded study. Australas J Dermatol. 2002;43:175-178.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853-860.
- Brophy JJ, Davies NW, Southwell IA, et al. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem. 1989;37:1330-1335.
- Concha JM, Moore LS, Holloway WJ. 1998 William J. Stickel Bronze Award. Antifungal activity of Melaleuca alternifolia (tea-tree) oil against various pathogenic organisms. J Am Podiatr Med Assoc. 1998;88:489-492.
- Benger S, Townsend P, Ashford RL, et al. An in vitro study to determine the minimum inhibitory concentration of Melaleuca alternifolia against the dermatophyte Trichophyton rubrum. Foot. 2004;14:86-91.
- Hammer KA, Carson CF, Riley TV. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother. 1998;42:591-595.
- Altman P. Australian tea tree oil. Aust J Pharm. 1998;69:276-278.
- Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147-157.
- Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract. 1994;38:601-605.
- Syed TA, Qureshi ZA, Ali SM, et al. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Med Int Health. 1999;4:284-287.
- Flores FC, de Lima JA, Ribeiro RF, et al. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of Trichophyton rubrum. Mycopathologia. 2013;175:281-286.
- Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081-1085.
- Cox SD, Mann CM, Markham JL, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88:170-175.
- Hammer KA, Carson CF, Riley TV. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med Mycol. 2000;38:355-362.
- Vicks VapoRub [package insert]. Gross-Gerau, Germany: Proctor & Gamble; 2010.
- Ramsewak RS, Nair MG, Stommel M, et al. In vitro antagonistic activity of monoterpenes and their mixtures against ‘toe nail fungus’ pathogens. Phytother Res. 2003;17:376-379.
- Pina-Vaz C, Gonçalves Rodrigues A, Pinto E, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73-78.
- Pinto E, Pina-Vaz C, Salgueiro L, et al. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol. 2006;55:1367-1373.
- Vicks VapoRub might help fight toenail fungus. Consumer Reports. 2006;71:49.
- Derby R, Rohal P, Jackson C, et al. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med. 2011;24:69-74.
- Trapp S, Croteau R. Defensive resin biosynthesis in conifers. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:689-724.
- Sipponen A, Laitinen K. Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS. 2011;119:720-724.
- Sipponen A, Lohi J. Lappish gum care “new” treatment of pressure ulcers? People’s improvement at it’s best. Eng Med J. 2003;58:2775-2776.
- Benedictus O. Een Nyttigh Läkare. Malmö: Kroon; 1938.
- Rautio M, Sipponen A, Peltola R, et al. Antibacterial effects of home-made resin salve from Norway spruce (Picea abies). APMIS. 2007;115:335-340.
- Laitinen K, Sipponen A, Jokinen JJ, et al. Resin salve from Norway spruce is antifungal against dermatophytes causing nail infections. EWMA. 2009;56:289-296.
- Rautio M, Sipponen A, Lohi J, et al. In vitro fungistatic effects of natural coniferous resin from Norway spruce (Picea abies). Eur J Clin Microbiol Infect Dis. 2012;31:1783-1789.
- Sipponen A, Peltola R, Jokinen JJ, et al. Effects of Norway spruce (Picea abies) resin on cell wall and cell membrane of Staphylococcus aureus. Ultrastruct Pathol. 2009;33:128-135.
- Sipponen P, Sipponen A, Lohi J, et al. Natural coniferous resin lacquer in treatment of toenail onychomycosis: an observational study. Mycoses. 2013;56:289-296.
- Auvinen T, Tiihonen R, Soini M, et al. Efficacy of topical resin lacquer, amorolfine, and oral terbinafine for treating toenail onychomycosis: a prospective, randomized, controlled, investigator-blinded, parallel-group clinical trial. Br J Dermatol. 2015;173:940-948.
- Argueta A, Cano L, Rodarte M. Atlas de las Plantas de la Medicina Tradicional Mexicana. Vol 3. Mexico City, Mexico: Instituto Nacional Indigenista; 1994:72-680.
- Avilés M, Suárez G. Catálogo de Plantas Medicinales del Jardín Etnobotánico. Peru: Instituto Nacional de Antropología e Historia; 1994.
- Romero-Cerecero O, Roman-Ramos R, Zamilpa A, et al. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J Ethnopharmacol. 2009;126:74-78.
- Navarro Garcia VM, Gonzalez A, Fuentes M, et al. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003;87:85-88.
- Castañeda P, Gómez L, Mata R, et al. Phytogrowth-inhibitory and antifungal constituents of Helianthella quinquenervis. J Nat Prod. 1996;59:323-326.
- Kumar N. Inhibition of nail infecting fungi of peoples of North Eastern UP causing Tinea unguium through leaf essential oil of Ageratum houstonianum Mill. IOSR J Pharm. June 2014;4:36-42.
- Romero-Cerecero O, Rojas G, Navarro V, et al. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: an explorative pilot study controlled with ketoconazole. Planta Med. 2006;72:1257-1261.
- Romero-Cerecero O, Zamilpa A, Jimenez-Ferrer JE, et al. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. a comparative study with ciclopirox. Planta Med. 2008;74:1430-1435.
- Rzedowski J, De Rzedowski GC. Flora Fanerogámica del Valle de México. Mexico City, Mexico: Instituto de Ecología Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional; 1985.
- Bocci V. Biological and clinical effects of ozone. has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56:270-279.
- Sechi LA, Lezcano I, Nunez N, et al. Antibacterial activity of ozonized sunflower oil (Oleozon). J Appl Microbiol. 2001;90:279-284.
- Rodrigues KL, Cardoso CC, Caputo LR, et al. Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds. Inflammopharmacology. 2004;12:261-270.
- Daud FV, Ueda SMY, Navarini A, et al. The use of ozonized oil in the treatment of dermatophitosis caused by Microsporum canis in rabbits. Braz J Microbiol. 2011;42:274-281.
- Guerrer LV, Cunha KC, Nogueira MC, et al. “In vitro” antifungal activity of ozonized sunflower oil on yeasts from onychomycosis. Braz J Microbiol. 2012;43:1315-1318.
- Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON in patients suffering from onychomycosis. Mycoses. 2011;54:E272-E277.
Practice Points
- Natural remedies, including tea tree oil, natural topical cough suppressants, natural coniferous resin lacquer, Ageratina pichinchensis extract, and ozonized sunflower oil, have shown antifungal activities in in vitro studies.
- Some of these products have efficacy and appear to be safe in clinical studies.
- Larger randomized clinical trials demonstrating efficacy are required before we can recommend these products to our patients.