User login
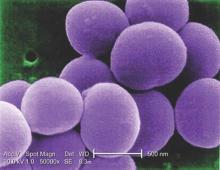
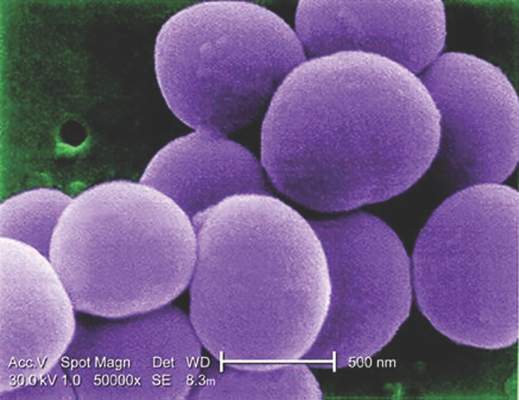
MRSA incidence decreased in children as clindamycin resistance increased
The incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections has decreased in children in recent years, but resistance to clindamycin has increased over the same period, a study showed.
“The epidemic of skin and soft tissue infections and invasive MRSA led to modifications of antimicrobial prescribing practices for suspected S. aureus infections,” reported Dr. Deena E. Sutter of the San Antonio Military Medical Center in Fort Sam Houston, Tex., and her associates. “Over the study period, erythromycin susceptibility among methicillin-susceptible S. aureus (MSSA) remained stable, suggesting that declining clindamycin susceptibility is a result of an increase in inducible resistance.”
The steady decline in clindamycin susceptibility “may lead to some concern about the continued reliance on clindamycin for the empirical treatment of presumptive S. aureus infections, although it is probably premature to abandon this effective antibiotic choice,” they wrote (Pediatrics. 2016 Mar. 1. doi: 10.1542/peds.2015-3099). “It is crucial that clinicians remain knowledgeable about local susceptibility rates as it would be prudent to consider [alternative] antimicrobial agents for empirical use when the local clindamycin susceptibility rate drops below 85%.”
The researchers retrospectively analyzed lab results from 39,209 patients under age 18 who were treated for S. aureus infections at one of the 266 U.S. facilities of the Military Health System from 2005 to 2014. The data included 41,745 S. aureus isolates, classified as MRSA if found resistant to cefoxitin, methicillin, or oxacillin and as methicillin susceptible (MSSA) if susceptible to those antimicrobials. The isolates had also been tested for susceptibility to ciprofloxacin, clindamycin, erythromycin, gentamicin, oxacillin, penicillin, rifampin, tetracycline, and trimethoprim/sulfamethoxazole (TMP/SMX).
During that decade, overall S. aureus susceptibility to clindamycin, ciprofloxacin, and TMP/SMX decreased – although susceptibility to TMP/SMX in 2014 stayed high at 98% – while overall susceptibility to erythromycin, gentamicin, and oxacillin increased. Specifically, 59% of S. aureus isolates were susceptible to oxacillin in 2005, which dropped briefly to 54% in 2007 before climbing to the 2014 rate of 68%.
Meanwhile, overall susceptibility to clindamycin dropped from 91% in 2005 to 86% in 2014, and MSSA susceptibility to clindamycin dropped from 91% in 2005 to 84% in 2014. “Erythromycin susceptibility remained stable among MSSA isolates throughout the study period at 63.5%, whereas MRSA susceptibility to erythromycin increased from 12.1% to 20.5%,” Dr. Sutter and her associates reported. “Ciprofloxacin susceptibility significantly decreased overall, although an initial decrease of 10.6% over the first 7 years of the study was subsequently followed by an increase of 6% between 2011 and 2014.”
Most of the isolates came from patients with skin and soft tissue infections, which were less likely to be susceptible to oxacillin than were other infections. Infections in children aged 1-5 years also were less likely to be susceptible to oxacillin, compared with infections in children of other age groups.
If the local clindamycin susceptibility rate falls below 85%, “beta-lactams, TMP/SMX, or tetracyclines may be used for less severe infections with intravenous vancomycin employed in severe cases,” the investigators said. “If overall MRSA rates continue to decline and clindamycin resistance among MSSA continues to increase, we may see a return to antistaphylococcal beta-lactam antimicrobial agents such as oxacillin or first-generation cephalosporins as preferred empirical therapy for presumed S. aureus infections.”
The research did not use external funding, and the authors reported no relevant financial disclosures.
Staphylococcus aureus is one of the most common organisms isolated from children with health care–associated infections, regardless of whether these infections had their onset in the community or were acquired in the hospital. Thus, the initial empiric treatment of a skin or soft tissue infection or invasive infection in a child almost always includes an antibiotic effective against S. aureus.
However, over the years, clindamycin susceptibility among S. aureus isolates has declined, likely related to the increased use of this agent for empiric as well as definitive treatment of community-acquired (CA) MRSA infections, encouraging the transmission of the genes associated with clindamycin resistance.
What are the implications of the findings from the report by Sutter et al. with respect to the selection of empiric antibiotics for children with suspected S. aureus infections? Currently, considering the still substantial MRSA resistance rates that exceed the 10%-15% level suggested by many experts as the threshold above which agents effective against CA-MRSA isolates should be administered for empiric treatment, changes in the selection of empiric antibiotics are not warranted. If rates of MRSA among S. aureus isolates from otherwise normal children are documented to drop below the 10%-15% threshold in different communities, a modification of current recommendations should be considered. It would also be important to understand why methicillin resistance is declining among S. aureus isolates from CA infections; this information may provide clues for preventing CA-MRSA infections with the use of vaccines or other means. The epidemiology of S. aureus infections in children has been changing over the past 2 decades, which is why it is critical to keep a very close eye on this common pathogen.
These comments were excerpted from an accompanying commentary by Dr. Sheldon L. Kaplan of the infectious disease service at Texas Children’s Hospital in Houston (Pediatrics. 2016 Mar 1. doi: 10.1542/peds.2016-0101). Dr. Kaplan has received research funds from Pfizer, Forest Laboratories, and Cubist.
Staphylococcus aureus is one of the most common organisms isolated from children with health care–associated infections, regardless of whether these infections had their onset in the community or were acquired in the hospital. Thus, the initial empiric treatment of a skin or soft tissue infection or invasive infection in a child almost always includes an antibiotic effective against S. aureus.
However, over the years, clindamycin susceptibility among S. aureus isolates has declined, likely related to the increased use of this agent for empiric as well as definitive treatment of community-acquired (CA) MRSA infections, encouraging the transmission of the genes associated with clindamycin resistance.
What are the implications of the findings from the report by Sutter et al. with respect to the selection of empiric antibiotics for children with suspected S. aureus infections? Currently, considering the still substantial MRSA resistance rates that exceed the 10%-15% level suggested by many experts as the threshold above which agents effective against CA-MRSA isolates should be administered for empiric treatment, changes in the selection of empiric antibiotics are not warranted. If rates of MRSA among S. aureus isolates from otherwise normal children are documented to drop below the 10%-15% threshold in different communities, a modification of current recommendations should be considered. It would also be important to understand why methicillin resistance is declining among S. aureus isolates from CA infections; this information may provide clues for preventing CA-MRSA infections with the use of vaccines or other means. The epidemiology of S. aureus infections in children has been changing over the past 2 decades, which is why it is critical to keep a very close eye on this common pathogen.
These comments were excerpted from an accompanying commentary by Dr. Sheldon L. Kaplan of the infectious disease service at Texas Children’s Hospital in Houston (Pediatrics. 2016 Mar 1. doi: 10.1542/peds.2016-0101). Dr. Kaplan has received research funds from Pfizer, Forest Laboratories, and Cubist.
Staphylococcus aureus is one of the most common organisms isolated from children with health care–associated infections, regardless of whether these infections had their onset in the community or were acquired in the hospital. Thus, the initial empiric treatment of a skin or soft tissue infection or invasive infection in a child almost always includes an antibiotic effective against S. aureus.
However, over the years, clindamycin susceptibility among S. aureus isolates has declined, likely related to the increased use of this agent for empiric as well as definitive treatment of community-acquired (CA) MRSA infections, encouraging the transmission of the genes associated with clindamycin resistance.
What are the implications of the findings from the report by Sutter et al. with respect to the selection of empiric antibiotics for children with suspected S. aureus infections? Currently, considering the still substantial MRSA resistance rates that exceed the 10%-15% level suggested by many experts as the threshold above which agents effective against CA-MRSA isolates should be administered for empiric treatment, changes in the selection of empiric antibiotics are not warranted. If rates of MRSA among S. aureus isolates from otherwise normal children are documented to drop below the 10%-15% threshold in different communities, a modification of current recommendations should be considered. It would also be important to understand why methicillin resistance is declining among S. aureus isolates from CA infections; this information may provide clues for preventing CA-MRSA infections with the use of vaccines or other means. The epidemiology of S. aureus infections in children has been changing over the past 2 decades, which is why it is critical to keep a very close eye on this common pathogen.
These comments were excerpted from an accompanying commentary by Dr. Sheldon L. Kaplan of the infectious disease service at Texas Children’s Hospital in Houston (Pediatrics. 2016 Mar 1. doi: 10.1542/peds.2016-0101). Dr. Kaplan has received research funds from Pfizer, Forest Laboratories, and Cubist.
The incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections has decreased in children in recent years, but resistance to clindamycin has increased over the same period, a study showed.
“The epidemic of skin and soft tissue infections and invasive MRSA led to modifications of antimicrobial prescribing practices for suspected S. aureus infections,” reported Dr. Deena E. Sutter of the San Antonio Military Medical Center in Fort Sam Houston, Tex., and her associates. “Over the study period, erythromycin susceptibility among methicillin-susceptible S. aureus (MSSA) remained stable, suggesting that declining clindamycin susceptibility is a result of an increase in inducible resistance.”
The steady decline in clindamycin susceptibility “may lead to some concern about the continued reliance on clindamycin for the empirical treatment of presumptive S. aureus infections, although it is probably premature to abandon this effective antibiotic choice,” they wrote (Pediatrics. 2016 Mar. 1. doi: 10.1542/peds.2015-3099). “It is crucial that clinicians remain knowledgeable about local susceptibility rates as it would be prudent to consider [alternative] antimicrobial agents for empirical use when the local clindamycin susceptibility rate drops below 85%.”
The researchers retrospectively analyzed lab results from 39,209 patients under age 18 who were treated for S. aureus infections at one of the 266 U.S. facilities of the Military Health System from 2005 to 2014. The data included 41,745 S. aureus isolates, classified as MRSA if found resistant to cefoxitin, methicillin, or oxacillin and as methicillin susceptible (MSSA) if susceptible to those antimicrobials. The isolates had also been tested for susceptibility to ciprofloxacin, clindamycin, erythromycin, gentamicin, oxacillin, penicillin, rifampin, tetracycline, and trimethoprim/sulfamethoxazole (TMP/SMX).
During that decade, overall S. aureus susceptibility to clindamycin, ciprofloxacin, and TMP/SMX decreased – although susceptibility to TMP/SMX in 2014 stayed high at 98% – while overall susceptibility to erythromycin, gentamicin, and oxacillin increased. Specifically, 59% of S. aureus isolates were susceptible to oxacillin in 2005, which dropped briefly to 54% in 2007 before climbing to the 2014 rate of 68%.
Meanwhile, overall susceptibility to clindamycin dropped from 91% in 2005 to 86% in 2014, and MSSA susceptibility to clindamycin dropped from 91% in 2005 to 84% in 2014. “Erythromycin susceptibility remained stable among MSSA isolates throughout the study period at 63.5%, whereas MRSA susceptibility to erythromycin increased from 12.1% to 20.5%,” Dr. Sutter and her associates reported. “Ciprofloxacin susceptibility significantly decreased overall, although an initial decrease of 10.6% over the first 7 years of the study was subsequently followed by an increase of 6% between 2011 and 2014.”
Most of the isolates came from patients with skin and soft tissue infections, which were less likely to be susceptible to oxacillin than were other infections. Infections in children aged 1-5 years also were less likely to be susceptible to oxacillin, compared with infections in children of other age groups.
If the local clindamycin susceptibility rate falls below 85%, “beta-lactams, TMP/SMX, or tetracyclines may be used for less severe infections with intravenous vancomycin employed in severe cases,” the investigators said. “If overall MRSA rates continue to decline and clindamycin resistance among MSSA continues to increase, we may see a return to antistaphylococcal beta-lactam antimicrobial agents such as oxacillin or first-generation cephalosporins as preferred empirical therapy for presumed S. aureus infections.”
The research did not use external funding, and the authors reported no relevant financial disclosures.
The incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections has decreased in children in recent years, but resistance to clindamycin has increased over the same period, a study showed.
“The epidemic of skin and soft tissue infections and invasive MRSA led to modifications of antimicrobial prescribing practices for suspected S. aureus infections,” reported Dr. Deena E. Sutter of the San Antonio Military Medical Center in Fort Sam Houston, Tex., and her associates. “Over the study period, erythromycin susceptibility among methicillin-susceptible S. aureus (MSSA) remained stable, suggesting that declining clindamycin susceptibility is a result of an increase in inducible resistance.”
The steady decline in clindamycin susceptibility “may lead to some concern about the continued reliance on clindamycin for the empirical treatment of presumptive S. aureus infections, although it is probably premature to abandon this effective antibiotic choice,” they wrote (Pediatrics. 2016 Mar. 1. doi: 10.1542/peds.2015-3099). “It is crucial that clinicians remain knowledgeable about local susceptibility rates as it would be prudent to consider [alternative] antimicrobial agents for empirical use when the local clindamycin susceptibility rate drops below 85%.”
The researchers retrospectively analyzed lab results from 39,209 patients under age 18 who were treated for S. aureus infections at one of the 266 U.S. facilities of the Military Health System from 2005 to 2014. The data included 41,745 S. aureus isolates, classified as MRSA if found resistant to cefoxitin, methicillin, or oxacillin and as methicillin susceptible (MSSA) if susceptible to those antimicrobials. The isolates had also been tested for susceptibility to ciprofloxacin, clindamycin, erythromycin, gentamicin, oxacillin, penicillin, rifampin, tetracycline, and trimethoprim/sulfamethoxazole (TMP/SMX).
During that decade, overall S. aureus susceptibility to clindamycin, ciprofloxacin, and TMP/SMX decreased – although susceptibility to TMP/SMX in 2014 stayed high at 98% – while overall susceptibility to erythromycin, gentamicin, and oxacillin increased. Specifically, 59% of S. aureus isolates were susceptible to oxacillin in 2005, which dropped briefly to 54% in 2007 before climbing to the 2014 rate of 68%.
Meanwhile, overall susceptibility to clindamycin dropped from 91% in 2005 to 86% in 2014, and MSSA susceptibility to clindamycin dropped from 91% in 2005 to 84% in 2014. “Erythromycin susceptibility remained stable among MSSA isolates throughout the study period at 63.5%, whereas MRSA susceptibility to erythromycin increased from 12.1% to 20.5%,” Dr. Sutter and her associates reported. “Ciprofloxacin susceptibility significantly decreased overall, although an initial decrease of 10.6% over the first 7 years of the study was subsequently followed by an increase of 6% between 2011 and 2014.”
Most of the isolates came from patients with skin and soft tissue infections, which were less likely to be susceptible to oxacillin than were other infections. Infections in children aged 1-5 years also were less likely to be susceptible to oxacillin, compared with infections in children of other age groups.
If the local clindamycin susceptibility rate falls below 85%, “beta-lactams, TMP/SMX, or tetracyclines may be used for less severe infections with intravenous vancomycin employed in severe cases,” the investigators said. “If overall MRSA rates continue to decline and clindamycin resistance among MSSA continues to increase, we may see a return to antistaphylococcal beta-lactam antimicrobial agents such as oxacillin or first-generation cephalosporins as preferred empirical therapy for presumed S. aureus infections.”
The research did not use external funding, and the authors reported no relevant financial disclosures.
FROM PEDIATRICS
Key clinical point: The incidence of methicillin-resistant Staphylococcus aureus infections has decreased in children in recent years while resistance to clindamycin has increased.
Major finding: MRSA susceptibility to oxacillin increased to 68.4% in 2014, and susceptibility dropped to 86% for clindamycin.
Data source: A retrospective analysis of 41,745 S. aureus isolates from 39,209 patients under age 18 years in the U.S. Military Health System between 2005 and 2014.
Disclosures: The research did not use external funding, and the authors reported no relevant financial disclosures.
Risperidone, aripiprazole treat irritability in children with ASD
Risperidone and aripiprazole, followed by N-acetylcysteine, appear most effective in treating irritability and aggression in youth with autism spectrum disorders (ASD), according to a systematic review and meta-analysis published in the February issue of Pediatrics.
“Although risperidone and aripiprazole have the strongest evidence for reducing ABC-I [Aberrant Behavioral Checklist–Irritability] in youth with autism spectrum disorders, they also have evidence for significant adverse events for a subset of patients,” reported Dr. Lawrence K. Fung of Stanford (Calif) University, and his associates (Pediatrics. 2016 Feb.;137[Suppl 2]:S124-35).
More than half of autistic individuals show significant emotion dysregulation, and about 20% have irritability or aggression at moderate to severe levels.
The authors combed Medline, PsychINFO, Embase, and review articles to identify randomized placebo-controlled trials evaluating medications to treat irritability and aggression in ASD youth, aged 2-17 years. The researchers used Aberrant Behavioral Checklist–Irritability scores as the primary endpoint in assessing effect size for improvement of emotional and behavioral symptoms in individuals with ASD.
Of 35 randomized controlled trials involving 1,673 participants included in the systematic review, 25 trials used the ABC-I, with 11 targeting irritability as the primary outcome and the other 14 targeting a different primary outcome. Five of the 11 trials targeting irritability tested the effectiveness of risperidone, while 2 tested aripiprazole, 2 tested valproate, 1 tested N-acetylcysteine, and 1 tested amantadine. Risperidone, aripiprazole, and N-acetylcysteine showed improvement in ABC-I scores with a moderate to large effect size. Valproate showed significant improvement to a lesser extent, but amantadine did not.
Among medications tested in the other 14 trials using the ABC-I, clonidine, methylphenidate, and tianeptine showed improvement with moderate effect sizes ,while venlafaxine and naltrexone showed improvement with a small effect size. Other medications tested included atomoxetine, citalopram, dextromethorphan, levetiracetam, mecamylamine, omega-3 fatty acid, and secretin. Hyperactivity and impulsivity improved in trials testing atomoxetine and dextromethorphan.
In trials using assessments other than ABC-I, risperidone and haloperidol showed effectiveness, compared with placebos, although risperidone beat haloperidol in a head-to-head trial. Some improvement in irritability also occurred with clomipramine, naltrexone, and a supplement with 19 vitamins and 9 minerals, compared with placebo.
The researchers calculated a number needed to treat (NNT) of two for risperidone at a dose between 1.2 and 1.9 mg, but the number needed to harm (NNH) for sedation from risperidone was also two. Risperidone’s NNH for extrapyramidal symptoms was six to seven. Flexible dosing with aripiprazole yielded an NNT of three, but fixed dosing led to an NNT of five to seven. The NNH for sedation with aripiprazole was 16, and the NNH for extrapyramidal symptoms was 20.
Sedation also occurred with haloperidol (NNH = 1) and amantadine (NNH = 10), and haloperidol caused extrapyramidal symptoms (NNH = 10). Weight gain occurred with risperidone, aripiprazole, and valproate.
“The reason for more compounds failing to show efficacy is not clear, but some of the negative studies had small sample sizes,” the authors noted. “Therefore, it is possible that some of these negative studies may represent false negatives,” and some of the studies used baseline ABC-I scores below the typical cutoff of at least 18 for inclusion.
The research was conducted through the Autism Speaks Autism Treatment Network and was supported by the U.S. Department of Health and Human Services, and the maternal and child health research program at Massachusetts General Hospital. Dr. Daniel Coury has received research funding from Autism Speaks, SynapDx, and Neuren Pharmaceuticals; has consulted for Cognoa; and is a speaker for Abbott/Innovative Biopharma. Dr. Jeremy Veenstra-VanderWeele has received research funding from Seaside Therapeutics, Roche Pharmaceuticals, Novartis, Forest, Sunovion, and SynapDx and has consulted for or advised Roche Pharmaceuticals, SynapDx, and Novartis.
Risperidone and aripiprazole, followed by N-acetylcysteine, appear most effective in treating irritability and aggression in youth with autism spectrum disorders (ASD), according to a systematic review and meta-analysis published in the February issue of Pediatrics.
“Although risperidone and aripiprazole have the strongest evidence for reducing ABC-I [Aberrant Behavioral Checklist–Irritability] in youth with autism spectrum disorders, they also have evidence for significant adverse events for a subset of patients,” reported Dr. Lawrence K. Fung of Stanford (Calif) University, and his associates (Pediatrics. 2016 Feb.;137[Suppl 2]:S124-35).
More than half of autistic individuals show significant emotion dysregulation, and about 20% have irritability or aggression at moderate to severe levels.
The authors combed Medline, PsychINFO, Embase, and review articles to identify randomized placebo-controlled trials evaluating medications to treat irritability and aggression in ASD youth, aged 2-17 years. The researchers used Aberrant Behavioral Checklist–Irritability scores as the primary endpoint in assessing effect size for improvement of emotional and behavioral symptoms in individuals with ASD.
Of 35 randomized controlled trials involving 1,673 participants included in the systematic review, 25 trials used the ABC-I, with 11 targeting irritability as the primary outcome and the other 14 targeting a different primary outcome. Five of the 11 trials targeting irritability tested the effectiveness of risperidone, while 2 tested aripiprazole, 2 tested valproate, 1 tested N-acetylcysteine, and 1 tested amantadine. Risperidone, aripiprazole, and N-acetylcysteine showed improvement in ABC-I scores with a moderate to large effect size. Valproate showed significant improvement to a lesser extent, but amantadine did not.
Among medications tested in the other 14 trials using the ABC-I, clonidine, methylphenidate, and tianeptine showed improvement with moderate effect sizes ,while venlafaxine and naltrexone showed improvement with a small effect size. Other medications tested included atomoxetine, citalopram, dextromethorphan, levetiracetam, mecamylamine, omega-3 fatty acid, and secretin. Hyperactivity and impulsivity improved in trials testing atomoxetine and dextromethorphan.
In trials using assessments other than ABC-I, risperidone and haloperidol showed effectiveness, compared with placebos, although risperidone beat haloperidol in a head-to-head trial. Some improvement in irritability also occurred with clomipramine, naltrexone, and a supplement with 19 vitamins and 9 minerals, compared with placebo.
The researchers calculated a number needed to treat (NNT) of two for risperidone at a dose between 1.2 and 1.9 mg, but the number needed to harm (NNH) for sedation from risperidone was also two. Risperidone’s NNH for extrapyramidal symptoms was six to seven. Flexible dosing with aripiprazole yielded an NNT of three, but fixed dosing led to an NNT of five to seven. The NNH for sedation with aripiprazole was 16, and the NNH for extrapyramidal symptoms was 20.
Sedation also occurred with haloperidol (NNH = 1) and amantadine (NNH = 10), and haloperidol caused extrapyramidal symptoms (NNH = 10). Weight gain occurred with risperidone, aripiprazole, and valproate.
“The reason for more compounds failing to show efficacy is not clear, but some of the negative studies had small sample sizes,” the authors noted. “Therefore, it is possible that some of these negative studies may represent false negatives,” and some of the studies used baseline ABC-I scores below the typical cutoff of at least 18 for inclusion.
The research was conducted through the Autism Speaks Autism Treatment Network and was supported by the U.S. Department of Health and Human Services, and the maternal and child health research program at Massachusetts General Hospital. Dr. Daniel Coury has received research funding from Autism Speaks, SynapDx, and Neuren Pharmaceuticals; has consulted for Cognoa; and is a speaker for Abbott/Innovative Biopharma. Dr. Jeremy Veenstra-VanderWeele has received research funding from Seaside Therapeutics, Roche Pharmaceuticals, Novartis, Forest, Sunovion, and SynapDx and has consulted for or advised Roche Pharmaceuticals, SynapDx, and Novartis.
Risperidone and aripiprazole, followed by N-acetylcysteine, appear most effective in treating irritability and aggression in youth with autism spectrum disorders (ASD), according to a systematic review and meta-analysis published in the February issue of Pediatrics.
“Although risperidone and aripiprazole have the strongest evidence for reducing ABC-I [Aberrant Behavioral Checklist–Irritability] in youth with autism spectrum disorders, they also have evidence for significant adverse events for a subset of patients,” reported Dr. Lawrence K. Fung of Stanford (Calif) University, and his associates (Pediatrics. 2016 Feb.;137[Suppl 2]:S124-35).
More than half of autistic individuals show significant emotion dysregulation, and about 20% have irritability or aggression at moderate to severe levels.
The authors combed Medline, PsychINFO, Embase, and review articles to identify randomized placebo-controlled trials evaluating medications to treat irritability and aggression in ASD youth, aged 2-17 years. The researchers used Aberrant Behavioral Checklist–Irritability scores as the primary endpoint in assessing effect size for improvement of emotional and behavioral symptoms in individuals with ASD.
Of 35 randomized controlled trials involving 1,673 participants included in the systematic review, 25 trials used the ABC-I, with 11 targeting irritability as the primary outcome and the other 14 targeting a different primary outcome. Five of the 11 trials targeting irritability tested the effectiveness of risperidone, while 2 tested aripiprazole, 2 tested valproate, 1 tested N-acetylcysteine, and 1 tested amantadine. Risperidone, aripiprazole, and N-acetylcysteine showed improvement in ABC-I scores with a moderate to large effect size. Valproate showed significant improvement to a lesser extent, but amantadine did not.
Among medications tested in the other 14 trials using the ABC-I, clonidine, methylphenidate, and tianeptine showed improvement with moderate effect sizes ,while venlafaxine and naltrexone showed improvement with a small effect size. Other medications tested included atomoxetine, citalopram, dextromethorphan, levetiracetam, mecamylamine, omega-3 fatty acid, and secretin. Hyperactivity and impulsivity improved in trials testing atomoxetine and dextromethorphan.
In trials using assessments other than ABC-I, risperidone and haloperidol showed effectiveness, compared with placebos, although risperidone beat haloperidol in a head-to-head trial. Some improvement in irritability also occurred with clomipramine, naltrexone, and a supplement with 19 vitamins and 9 minerals, compared with placebo.
The researchers calculated a number needed to treat (NNT) of two for risperidone at a dose between 1.2 and 1.9 mg, but the number needed to harm (NNH) for sedation from risperidone was also two. Risperidone’s NNH for extrapyramidal symptoms was six to seven. Flexible dosing with aripiprazole yielded an NNT of three, but fixed dosing led to an NNT of five to seven. The NNH for sedation with aripiprazole was 16, and the NNH for extrapyramidal symptoms was 20.
Sedation also occurred with haloperidol (NNH = 1) and amantadine (NNH = 10), and haloperidol caused extrapyramidal symptoms (NNH = 10). Weight gain occurred with risperidone, aripiprazole, and valproate.
“The reason for more compounds failing to show efficacy is not clear, but some of the negative studies had small sample sizes,” the authors noted. “Therefore, it is possible that some of these negative studies may represent false negatives,” and some of the studies used baseline ABC-I scores below the typical cutoff of at least 18 for inclusion.
The research was conducted through the Autism Speaks Autism Treatment Network and was supported by the U.S. Department of Health and Human Services, and the maternal and child health research program at Massachusetts General Hospital. Dr. Daniel Coury has received research funding from Autism Speaks, SynapDx, and Neuren Pharmaceuticals; has consulted for Cognoa; and is a speaker for Abbott/Innovative Biopharma. Dr. Jeremy Veenstra-VanderWeele has received research funding from Seaside Therapeutics, Roche Pharmaceuticals, Novartis, Forest, Sunovion, and SynapDx and has consulted for or advised Roche Pharmaceuticals, SynapDx, and Novartis.
FROM PEDIATRICS
Key clinical point: Risperidone and aripiprazole most effectively treat irritability and aggression in youth with autism spectrum disorders.
Major finding: The number needed to treat was two for risperidone and three with flexible dosing of aripiprazole, with varying numbers needed to harm depending on adverse events.
Data source: A systematic review and meta-analysis of 35 randomized controlled studies assessing the effectiveness of medications in the treatment of irritability and aggression in youth with ASD.
Disclosures: The research was conducted through the Autism Speaks Autism Treatment Network and was supported by the U.S. Department of Health and Human Services, and the maternal and child health research program at Massachusetts General Hospital. Dr. Daniel Coury has received research funding from Autism Speaks, SynapDx, and Neuren Pharmaceuticals; has consulted for Cognoa; and is a speaker for Abbott/Innovative Biopharma. Dr. Jeremy Veenstra-VanderWeele has received research funding from Seaside Therapeutics, Roche Pharmaceuticals, Novartis, Forest, Sunovion, and SynapDx and has consulted for or advised Roche Pharmaceuticals, SynapDx, and Novartis.
Sleep problems common among youth with ASD
The majority of children with autism spectrum disorders have sleeping problems and many take medications for sleep, but those taking medications have worse daytime behaviors and poorer quality of life, according to a study published in the February issue of Pediatrics.
“These findings underscore the need for both longitudinal and interventional studies to determine whether improvement of sleep disturbance with medications also improves daytime behaviors and quality of life,” reported Dr. Beth A. Malow of Vanderbilt University in Nashville, and her associates (Pediatrics. 2016;137[S2]:e20152851H).
The researchers analyzed data from parent questionnaires and clinical forms filled out between April 2009 and December 2013 for 1,518 children, aged 4-10 years, in the Autism Speaks Autism Treatment Network Registry. Most of the children were white boys; only 16% were girls and 20% were nonwhite.
Although only 30% of children (P less than .0001) had sleep diagnoses in clinical reports, parents reported that 71% of the children had significant sleep problems, indicated by a score of at least 41 on the Children’s Sleep Habits Questionnaire. The most common sleep diagnosis was sleep disturbance not otherwise specified, followed by inadequate sleep hygiene, behavioral insomnia of childhood, other sleep disorder, and organic insomnia unspecified.
One reason for the discrepancy between diagnoses and parent-reported difficulties, the researchers suggested, was that “sleep concerns may be eclipsed by other needs [of children with ASD], especially in the limited time available at a clinician visit.” At the same time, however, they note that 41 may be too low a scale cutoff for autistic children.
Among those with a sleep diagnosis, 46% were taking any medication for sleep. Just over a third (36%) of those with a sleep diagnosis took melatonin, and 14% took alpha-agonists. Only 2% of those without a sleep diagnosis took alpha-agonists, but 13% took melatonin and 15% took any medication. Other medications children took for sleep included antidepressants, antihistamines, atypical antipsychotics, benzodiazepines, beta-blockers, sedatives, iron supplements, and vitamins/dietary supplements.
The children taking medications for sleep had more insomnia, significantly lower scores for quality of life, and significantly higher scores for irritability and for internalizing and externalizing behaviors.
“It is possible that sleep disturbance itself is driving this relationship,” Dr. Malow and her associates said. “It is also possible that a clinician would be more likely to use a medication for sleep in a child with more difficult daytime behaviors” or that sleep medications influence behaviors and quality of life.
The research was supported by the Autism Speaks Autism Treatment Network, the U.S. Department of Health & Human Services, and the Maternal and Child Health Research Program to the Massachusetts General Hospital. Dr. Malow has received grant funding from Neurim Pharmaceuticals for a study of prolonged release melatonin (Circadin), and Dr. Reynolds has received grant funding from Mead Johnson.
The majority of children with autism spectrum disorders have sleeping problems and many take medications for sleep, but those taking medications have worse daytime behaviors and poorer quality of life, according to a study published in the February issue of Pediatrics.
“These findings underscore the need for both longitudinal and interventional studies to determine whether improvement of sleep disturbance with medications also improves daytime behaviors and quality of life,” reported Dr. Beth A. Malow of Vanderbilt University in Nashville, and her associates (Pediatrics. 2016;137[S2]:e20152851H).
The researchers analyzed data from parent questionnaires and clinical forms filled out between April 2009 and December 2013 for 1,518 children, aged 4-10 years, in the Autism Speaks Autism Treatment Network Registry. Most of the children were white boys; only 16% were girls and 20% were nonwhite.
Although only 30% of children (P less than .0001) had sleep diagnoses in clinical reports, parents reported that 71% of the children had significant sleep problems, indicated by a score of at least 41 on the Children’s Sleep Habits Questionnaire. The most common sleep diagnosis was sleep disturbance not otherwise specified, followed by inadequate sleep hygiene, behavioral insomnia of childhood, other sleep disorder, and organic insomnia unspecified.
One reason for the discrepancy between diagnoses and parent-reported difficulties, the researchers suggested, was that “sleep concerns may be eclipsed by other needs [of children with ASD], especially in the limited time available at a clinician visit.” At the same time, however, they note that 41 may be too low a scale cutoff for autistic children.
Among those with a sleep diagnosis, 46% were taking any medication for sleep. Just over a third (36%) of those with a sleep diagnosis took melatonin, and 14% took alpha-agonists. Only 2% of those without a sleep diagnosis took alpha-agonists, but 13% took melatonin and 15% took any medication. Other medications children took for sleep included antidepressants, antihistamines, atypical antipsychotics, benzodiazepines, beta-blockers, sedatives, iron supplements, and vitamins/dietary supplements.
The children taking medications for sleep had more insomnia, significantly lower scores for quality of life, and significantly higher scores for irritability and for internalizing and externalizing behaviors.
“It is possible that sleep disturbance itself is driving this relationship,” Dr. Malow and her associates said. “It is also possible that a clinician would be more likely to use a medication for sleep in a child with more difficult daytime behaviors” or that sleep medications influence behaviors and quality of life.
The research was supported by the Autism Speaks Autism Treatment Network, the U.S. Department of Health & Human Services, and the Maternal and Child Health Research Program to the Massachusetts General Hospital. Dr. Malow has received grant funding from Neurim Pharmaceuticals for a study of prolonged release melatonin (Circadin), and Dr. Reynolds has received grant funding from Mead Johnson.
The majority of children with autism spectrum disorders have sleeping problems and many take medications for sleep, but those taking medications have worse daytime behaviors and poorer quality of life, according to a study published in the February issue of Pediatrics.
“These findings underscore the need for both longitudinal and interventional studies to determine whether improvement of sleep disturbance with medications also improves daytime behaviors and quality of life,” reported Dr. Beth A. Malow of Vanderbilt University in Nashville, and her associates (Pediatrics. 2016;137[S2]:e20152851H).
The researchers analyzed data from parent questionnaires and clinical forms filled out between April 2009 and December 2013 for 1,518 children, aged 4-10 years, in the Autism Speaks Autism Treatment Network Registry. Most of the children were white boys; only 16% were girls and 20% were nonwhite.
Although only 30% of children (P less than .0001) had sleep diagnoses in clinical reports, parents reported that 71% of the children had significant sleep problems, indicated by a score of at least 41 on the Children’s Sleep Habits Questionnaire. The most common sleep diagnosis was sleep disturbance not otherwise specified, followed by inadequate sleep hygiene, behavioral insomnia of childhood, other sleep disorder, and organic insomnia unspecified.
One reason for the discrepancy between diagnoses and parent-reported difficulties, the researchers suggested, was that “sleep concerns may be eclipsed by other needs [of children with ASD], especially in the limited time available at a clinician visit.” At the same time, however, they note that 41 may be too low a scale cutoff for autistic children.
Among those with a sleep diagnosis, 46% were taking any medication for sleep. Just over a third (36%) of those with a sleep diagnosis took melatonin, and 14% took alpha-agonists. Only 2% of those without a sleep diagnosis took alpha-agonists, but 13% took melatonin and 15% took any medication. Other medications children took for sleep included antidepressants, antihistamines, atypical antipsychotics, benzodiazepines, beta-blockers, sedatives, iron supplements, and vitamins/dietary supplements.
The children taking medications for sleep had more insomnia, significantly lower scores for quality of life, and significantly higher scores for irritability and for internalizing and externalizing behaviors.
“It is possible that sleep disturbance itself is driving this relationship,” Dr. Malow and her associates said. “It is also possible that a clinician would be more likely to use a medication for sleep in a child with more difficult daytime behaviors” or that sleep medications influence behaviors and quality of life.
The research was supported by the Autism Speaks Autism Treatment Network, the U.S. Department of Health & Human Services, and the Maternal and Child Health Research Program to the Massachusetts General Hospital. Dr. Malow has received grant funding from Neurim Pharmaceuticals for a study of prolonged release melatonin (Circadin), and Dr. Reynolds has received grant funding from Mead Johnson.
FROM PEDIATRICS
Key clinical point: Many autistic children have sleeping problems, and many take medications for sleep.
Major finding: 71% of children with autism spectrum disorders have sleeping difficulties, and 30% have a sleep diagnosis, of whom 46% take medications for sleep.
Data source: The findings are based on analysis of questionnaires and clinical reports for 1,518 children, aged 4-10 years, in the Autism Speaks Autism Treatment Network Registry seen between April 2009 and December 2013.
Disclosures: The research was conducted through the Autism Speaks Autism Treatment Network and was supported by the U.S. Department of Health and Human Services, and Massachusetts General Hospital. Dr. Malow has received grant funding from Neurim Pharmaceuticals for a study of prolonged release melatonin (Circadin), and Dr. Reynolds has received grant funding from Mead Johnson.
More teens complete HPV vaccine series when parents choose reminder methods
Asking parents how they want to be reminded of the need for their child’s second dose of the human papillomavirus (HPV) vaccine appears to increase the likelihood of their child completing the three-dose series, a recent study found.
“For the promise of the HPV vaccine to be realized, rates of vaccine initiation and series completion must be markedly increased,” wrote Dr. Allison Kempe of the University of Colorado, Aurora, and her associates. “Results of this study demonstrate that preference-based recall could have a major impact on increasing HPV series completion rates and in increasing the timeliness of full vaccination” (Pediatrics. 2016 Feb 26. doi: 10.1542/peds.2015-2857). “The intervention was most effective for younger adolescents, and reminding the adolescent, in addition to the parent, did not increase effectiveness.”
The researchers randomly assigned three pediatric practices from Kaiser Permanente Colorado to offer usual care and four practices to the intervention, during January to June 2013. They limited the practices to those with a similar proportion of African American and Hispanic patients, a similar number of patients aged 11-17 years, and a similar proportion of Medicaid-covered patients. At the start of the study, the intervention sites had an 18% rate for the first dose of the HPV vaccine and a 6% series completion rate, compared with 20% and 7%, respectively, at the control sites.
When parents brought their children in for the first dose of the HPV vaccine, staff at the intervention practices asked the parents if they wanted to receive a reminder about the next dose. If they did, they chose whether they wanted a text message, an email, or an automated phone message (they could choose up to two) and whether they wanted their child contacted as well. Parents of 43% of the 867 eligible adolescents participated.
The researchers compared HPV vaccines series completion rates between those 374 adolescents and the 555 eligible adolescents at the control practices. At the intervention practices, 83% of the teens received the second dose, and 63% completed the vaccine series. At the control practice, 71% of the teens received the second dose, and 38% completed the series – similar to the 33% completion rate of unenrolled teens at the intervention practices. Overall, 46% of all the teens – enrolled and unenrolled – at the intervention practices completed the series, compared with 38% at the control practices – for an adjusted risk ratio of 1.22 (P less than .01)
The most popular recall method was text messaging alone, requested by 39% of parents and particularly preferred by parents of Hispanic adolescents. Both text and email were requested by 19% of parents, while 18% of parents requested email only, 9% requested text and phone, and 9% requested phone only. Only 6% requested phone and email, yet this was the only recall method associated with higher series completion rates that significantly differed from the other methods. Nearly one in five parents (19%) requested the practice to remind their child too, but these reminders had no apparent impact on completion rates.
“Whether this method [of preference-based recall] could also increase initiation of the series also should be examined, as barriers to initiation and to completion have been shown to differ,” the authors wrote.
The Centers for Disease Control and Prevention funded the study. The authors reported no disclosures.
Asking parents how they want to be reminded of the need for their child’s second dose of the human papillomavirus (HPV) vaccine appears to increase the likelihood of their child completing the three-dose series, a recent study found.
“For the promise of the HPV vaccine to be realized, rates of vaccine initiation and series completion must be markedly increased,” wrote Dr. Allison Kempe of the University of Colorado, Aurora, and her associates. “Results of this study demonstrate that preference-based recall could have a major impact on increasing HPV series completion rates and in increasing the timeliness of full vaccination” (Pediatrics. 2016 Feb 26. doi: 10.1542/peds.2015-2857). “The intervention was most effective for younger adolescents, and reminding the adolescent, in addition to the parent, did not increase effectiveness.”
The researchers randomly assigned three pediatric practices from Kaiser Permanente Colorado to offer usual care and four practices to the intervention, during January to June 2013. They limited the practices to those with a similar proportion of African American and Hispanic patients, a similar number of patients aged 11-17 years, and a similar proportion of Medicaid-covered patients. At the start of the study, the intervention sites had an 18% rate for the first dose of the HPV vaccine and a 6% series completion rate, compared with 20% and 7%, respectively, at the control sites.
When parents brought their children in for the first dose of the HPV vaccine, staff at the intervention practices asked the parents if they wanted to receive a reminder about the next dose. If they did, they chose whether they wanted a text message, an email, or an automated phone message (they could choose up to two) and whether they wanted their child contacted as well. Parents of 43% of the 867 eligible adolescents participated.
The researchers compared HPV vaccines series completion rates between those 374 adolescents and the 555 eligible adolescents at the control practices. At the intervention practices, 83% of the teens received the second dose, and 63% completed the vaccine series. At the control practice, 71% of the teens received the second dose, and 38% completed the series – similar to the 33% completion rate of unenrolled teens at the intervention practices. Overall, 46% of all the teens – enrolled and unenrolled – at the intervention practices completed the series, compared with 38% at the control practices – for an adjusted risk ratio of 1.22 (P less than .01)
The most popular recall method was text messaging alone, requested by 39% of parents and particularly preferred by parents of Hispanic adolescents. Both text and email were requested by 19% of parents, while 18% of parents requested email only, 9% requested text and phone, and 9% requested phone only. Only 6% requested phone and email, yet this was the only recall method associated with higher series completion rates that significantly differed from the other methods. Nearly one in five parents (19%) requested the practice to remind their child too, but these reminders had no apparent impact on completion rates.
“Whether this method [of preference-based recall] could also increase initiation of the series also should be examined, as barriers to initiation and to completion have been shown to differ,” the authors wrote.
The Centers for Disease Control and Prevention funded the study. The authors reported no disclosures.
Asking parents how they want to be reminded of the need for their child’s second dose of the human papillomavirus (HPV) vaccine appears to increase the likelihood of their child completing the three-dose series, a recent study found.
“For the promise of the HPV vaccine to be realized, rates of vaccine initiation and series completion must be markedly increased,” wrote Dr. Allison Kempe of the University of Colorado, Aurora, and her associates. “Results of this study demonstrate that preference-based recall could have a major impact on increasing HPV series completion rates and in increasing the timeliness of full vaccination” (Pediatrics. 2016 Feb 26. doi: 10.1542/peds.2015-2857). “The intervention was most effective for younger adolescents, and reminding the adolescent, in addition to the parent, did not increase effectiveness.”
The researchers randomly assigned three pediatric practices from Kaiser Permanente Colorado to offer usual care and four practices to the intervention, during January to June 2013. They limited the practices to those with a similar proportion of African American and Hispanic patients, a similar number of patients aged 11-17 years, and a similar proportion of Medicaid-covered patients. At the start of the study, the intervention sites had an 18% rate for the first dose of the HPV vaccine and a 6% series completion rate, compared with 20% and 7%, respectively, at the control sites.
When parents brought their children in for the first dose of the HPV vaccine, staff at the intervention practices asked the parents if they wanted to receive a reminder about the next dose. If they did, they chose whether they wanted a text message, an email, or an automated phone message (they could choose up to two) and whether they wanted their child contacted as well. Parents of 43% of the 867 eligible adolescents participated.
The researchers compared HPV vaccines series completion rates between those 374 adolescents and the 555 eligible adolescents at the control practices. At the intervention practices, 83% of the teens received the second dose, and 63% completed the vaccine series. At the control practice, 71% of the teens received the second dose, and 38% completed the series – similar to the 33% completion rate of unenrolled teens at the intervention practices. Overall, 46% of all the teens – enrolled and unenrolled – at the intervention practices completed the series, compared with 38% at the control practices – for an adjusted risk ratio of 1.22 (P less than .01)
The most popular recall method was text messaging alone, requested by 39% of parents and particularly preferred by parents of Hispanic adolescents. Both text and email were requested by 19% of parents, while 18% of parents requested email only, 9% requested text and phone, and 9% requested phone only. Only 6% requested phone and email, yet this was the only recall method associated with higher series completion rates that significantly differed from the other methods. Nearly one in five parents (19%) requested the practice to remind their child too, but these reminders had no apparent impact on completion rates.
“Whether this method [of preference-based recall] could also increase initiation of the series also should be examined, as barriers to initiation and to completion have been shown to differ,” the authors wrote.
The Centers for Disease Control and Prevention funded the study. The authors reported no disclosures.
FROM PEDIATRICS
Key clinical point: Preference-based reminders increased HPV vaccine series completion rates.
Major finding: 46% of adolescents at intervention practices completed the series, compared with 38% at practices providing usual care.
Data source: The findings are based on a cluster randomized trial of seven pediatric practices in the Kaiser Permanent Colorado system involving 1,422 patients aged 11-17 years.
Disclosures: The Centers for Disease Control and Prevention funded the study. The authors reported no disclosures.
Pediatric BMI increases linked to rises in blood pressure, hypertension risk
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
FROM PEDIATRICS
Key clinical point: The risk of blood pressure increases and even hypertension rises with increasing BMI among youth aged 3-17 years.
Major finding: Incident hypertension risk doubled for children and adolescents with obesity (HR, 2.02 and HR, 2.20, respectively) and quadrupled for those with severe obesity (HR, 4.42 and HR, 4.46, respectively).
Data source: The findings are based on a retrospective cohort study of 100,606 individuals, aged 3-17 years, from one of three U.S. health systems who were tracked over a median 3.1 years.
Disclosures: The study was funded by the National Heart, Lung, and Blood Institute. Dr. Lo has received previous research funding from Sanofi.
Tdap effectiveness wanes rapidly in teens
The Tdap vaccine’s effectiveness against pertussis wanes so rapidly in the year after administration in teens that it provides too little protection to prevent outbreaks, according to results of a new study.
The Tdap is the booster vaccine against tetanus, diphtheria, and pertussis that adults and children ages 10 and older can receive. The corresponding acellular pertussis vaccine for children ages 6 and younger is the DTaP.
“Widespread Tdap vaccination, although associated with a transient decrease in pertussis incidence, did not prevent outbreaks among this population of teenagers who have only ever received acellular pertussis vaccines,” reported Dr. Nicola P. Klein and her associates at the Kaiser Permanente Vaccine Study Center in Oakland, Calif. “This study demonstrates that despite high rates of Tdap vaccination, the growing cohort of adolescents who have only received acellular pertussis vaccines continue to be at high risk of contracting pertussis and sustaining epidemics,” they wrote online (Pediatrics. 2016 Feb 5. [doi: 10.1542/peds.2015-3326]).
The researchers tracked 279,493 members of Kaiser Permanente Northern California who had received only DTaP vaccines, as opposed to the whole-cell DTwP formulation previously used, from age 10 onward. These included all members who were born from 1999 onward or were born from 1996-1998 and received three infant doses of DTaP, excluding children who had previously received Tdap or had pertussis. Among these, 175,094 children received the Tdap.
For the purposes of tracking pertussis cases in unvaccinated versus vaccinated adolescents, individuals were considered unvaccinated in the analysis until they received their first Tdap, after which they were vaccinated and time since vaccination was a continuous variable. Overall, 96.5% of the children were vaccinated by their 14th birthday.
Across 792,418 person-years between January 2006 and March 2015, including 418,595 vaccinated person-years for the children who received the Tdap, 1,207 cases of pertussis occurred. The vast majority of these – 85% – occurred among those ages 10-13 years. Teens aged 14-16 years comprised 15% of the cases, and only 0.5% of cases occurred among older teens. During each year of outbreaks, incidence dropped off precipitously among the age groups composed of children who would have received the whole-cell pertussis vaccine.
For example, “pertussis incidence in the 2010 outbreak sharply declined after this peak [of 10- to 11-year-olds] and stayed low at older ages, a decline that we have previously demonstrated to be associated with the receipt of whole cell instead of acellular pertussis vaccines in infancy and childhood as well as with Tdap receipt,” the authors wrote.
The researchers estimated the Tdap’s effectiveness at 69% throughout the first year after vaccination. This dropped to 57% in the second year after vaccination and then to 25% in the third year. By 4 years or later after Tdap receipt, the vaccine’s effectiveness sat at just 9%.
For each year after Tdap vaccination, children’s risk of pertussis increased 35% (hazard ratio, 1.35), and cases were mild or moderate regardless of vaccination status. Nearly all (98%) of the children with pertussis had visited the doctor within 5 days on either side of their positive polymerase chain reaction test, 86% received a diagnosis of pertussis, and 96% received a prescription for azithromycin, except for 1 for erythromycin. In addition, 4% (50) of the cases visited the emergency department. No differences in rate of ED visits or prescriptions existed between vaccinated and unvaccinated children.
“The strategy of routinely vaccinating adolescents to prevent future disease did not prevent the 2014 epidemic, arguably because the protection afforded by a dose of Tdap was too short-lived,” the authors noted. They also pointed out that Tdap waning estimates likely also include ongoing DTaP waning.“This study was unable to disentangle the waning of Tdap effectiveness from the ongoing waning of previous doses of DTaP because the years since vaccination for Tdap and the fifth DTaP dose are closely correlated,” they wrote.
Most of the Tdap vaccines administered were Adacel (Sanofi Pasteur), but Boostrix (GlaxoSmithKline) was used as well. Waning occurred in both brands, and although not directly compared, no major differences in waning seemed to exist.
“We expect future pertussis epidemics to be larger as the cohort that has only received acellular pertussis vaccines ages,” the authors concluded. “The results in this study raise serious questions regarding the benefits of routinely administering a single dose of Tdap to every adolescent aged 11 or 12 years.”
The research was funded by Kaiser Permanente. Dr. Klein has received research funding from GlaxoSmithKline for a separate pertussis vaccine effectiveness study, and Dr. Klein and one coauthor also have received research funding from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Protein Science, Nuron Biotech, and MedImmune. The remaining coauthors said they had no relevant financial disclosures. GlaxoSmithKline and Sanofi Pasteur manufacture the pertussis vaccines purchased by Kaiser Permanente for this study.
When adolescents contract whooping cough, a.k.a. pertussis, they usually don’t whoop but they cough, sometimes for 3 months. The illness is impactful for them and they are contagious to others, so the infection spreads to classmates and within families. Waning immunity after experiencing pertussis infection, after receiving the old DTwP vaccine that was discontinued in the United States years ago due to safety issues, and after receiving the newer DTaP and Tdap vaccines, has been known to occur for many years. What is new in this article is the rapidity of waning immunity in the study population.
 |
Dr. Michael E. Pichichero |
First, it is important to note that the study population is from California, a state where pertussis has been circulating much more than in most other states. The exact reasons for a higher prevalence of pertussis in California are not fully understood, but a high rate of vaccine refusers may be a significant factor. Secondly, the study uses a mathematical model, so it is an estimate of waning immunity. Nevertheless, the observations alert health care providers and the community that pertussis can occur even in vaccinated persons, especially as time passes after vaccination.
The solutions are few at this time. Public health care officials are unlikely to recommend boosters more frequently than already advocated, although that is an option. Alternative formulations of Tdap to include other or additional ingredients could be a path forward, but the vaccine industry is tackling so many new diseases with vaccine development programs that a push for a better DTaP or Tdap is unlikely in the near term.
Dr. Michael E. Pichichero, specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures.
When adolescents contract whooping cough, a.k.a. pertussis, they usually don’t whoop but they cough, sometimes for 3 months. The illness is impactful for them and they are contagious to others, so the infection spreads to classmates and within families. Waning immunity after experiencing pertussis infection, after receiving the old DTwP vaccine that was discontinued in the United States years ago due to safety issues, and after receiving the newer DTaP and Tdap vaccines, has been known to occur for many years. What is new in this article is the rapidity of waning immunity in the study population.
 |
Dr. Michael E. Pichichero |
First, it is important to note that the study population is from California, a state where pertussis has been circulating much more than in most other states. The exact reasons for a higher prevalence of pertussis in California are not fully understood, but a high rate of vaccine refusers may be a significant factor. Secondly, the study uses a mathematical model, so it is an estimate of waning immunity. Nevertheless, the observations alert health care providers and the community that pertussis can occur even in vaccinated persons, especially as time passes after vaccination.
The solutions are few at this time. Public health care officials are unlikely to recommend boosters more frequently than already advocated, although that is an option. Alternative formulations of Tdap to include other or additional ingredients could be a path forward, but the vaccine industry is tackling so many new diseases with vaccine development programs that a push for a better DTaP or Tdap is unlikely in the near term.
Dr. Michael E. Pichichero, specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures.
When adolescents contract whooping cough, a.k.a. pertussis, they usually don’t whoop but they cough, sometimes for 3 months. The illness is impactful for them and they are contagious to others, so the infection spreads to classmates and within families. Waning immunity after experiencing pertussis infection, after receiving the old DTwP vaccine that was discontinued in the United States years ago due to safety issues, and after receiving the newer DTaP and Tdap vaccines, has been known to occur for many years. What is new in this article is the rapidity of waning immunity in the study population.
 |
Dr. Michael E. Pichichero |
First, it is important to note that the study population is from California, a state where pertussis has been circulating much more than in most other states. The exact reasons for a higher prevalence of pertussis in California are not fully understood, but a high rate of vaccine refusers may be a significant factor. Secondly, the study uses a mathematical model, so it is an estimate of waning immunity. Nevertheless, the observations alert health care providers and the community that pertussis can occur even in vaccinated persons, especially as time passes after vaccination.
The solutions are few at this time. Public health care officials are unlikely to recommend boosters more frequently than already advocated, although that is an option. Alternative formulations of Tdap to include other or additional ingredients could be a path forward, but the vaccine industry is tackling so many new diseases with vaccine development programs that a push for a better DTaP or Tdap is unlikely in the near term.
Dr. Michael E. Pichichero, specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures.
The Tdap vaccine’s effectiveness against pertussis wanes so rapidly in the year after administration in teens that it provides too little protection to prevent outbreaks, according to results of a new study.
The Tdap is the booster vaccine against tetanus, diphtheria, and pertussis that adults and children ages 10 and older can receive. The corresponding acellular pertussis vaccine for children ages 6 and younger is the DTaP.
“Widespread Tdap vaccination, although associated with a transient decrease in pertussis incidence, did not prevent outbreaks among this population of teenagers who have only ever received acellular pertussis vaccines,” reported Dr. Nicola P. Klein and her associates at the Kaiser Permanente Vaccine Study Center in Oakland, Calif. “This study demonstrates that despite high rates of Tdap vaccination, the growing cohort of adolescents who have only received acellular pertussis vaccines continue to be at high risk of contracting pertussis and sustaining epidemics,” they wrote online (Pediatrics. 2016 Feb 5. [doi: 10.1542/peds.2015-3326]).
The researchers tracked 279,493 members of Kaiser Permanente Northern California who had received only DTaP vaccines, as opposed to the whole-cell DTwP formulation previously used, from age 10 onward. These included all members who were born from 1999 onward or were born from 1996-1998 and received three infant doses of DTaP, excluding children who had previously received Tdap or had pertussis. Among these, 175,094 children received the Tdap.
For the purposes of tracking pertussis cases in unvaccinated versus vaccinated adolescents, individuals were considered unvaccinated in the analysis until they received their first Tdap, after which they were vaccinated and time since vaccination was a continuous variable. Overall, 96.5% of the children were vaccinated by their 14th birthday.
Across 792,418 person-years between January 2006 and March 2015, including 418,595 vaccinated person-years for the children who received the Tdap, 1,207 cases of pertussis occurred. The vast majority of these – 85% – occurred among those ages 10-13 years. Teens aged 14-16 years comprised 15% of the cases, and only 0.5% of cases occurred among older teens. During each year of outbreaks, incidence dropped off precipitously among the age groups composed of children who would have received the whole-cell pertussis vaccine.
For example, “pertussis incidence in the 2010 outbreak sharply declined after this peak [of 10- to 11-year-olds] and stayed low at older ages, a decline that we have previously demonstrated to be associated with the receipt of whole cell instead of acellular pertussis vaccines in infancy and childhood as well as with Tdap receipt,” the authors wrote.
The researchers estimated the Tdap’s effectiveness at 69% throughout the first year after vaccination. This dropped to 57% in the second year after vaccination and then to 25% in the third year. By 4 years or later after Tdap receipt, the vaccine’s effectiveness sat at just 9%.
For each year after Tdap vaccination, children’s risk of pertussis increased 35% (hazard ratio, 1.35), and cases were mild or moderate regardless of vaccination status. Nearly all (98%) of the children with pertussis had visited the doctor within 5 days on either side of their positive polymerase chain reaction test, 86% received a diagnosis of pertussis, and 96% received a prescription for azithromycin, except for 1 for erythromycin. In addition, 4% (50) of the cases visited the emergency department. No differences in rate of ED visits or prescriptions existed between vaccinated and unvaccinated children.
“The strategy of routinely vaccinating adolescents to prevent future disease did not prevent the 2014 epidemic, arguably because the protection afforded by a dose of Tdap was too short-lived,” the authors noted. They also pointed out that Tdap waning estimates likely also include ongoing DTaP waning.“This study was unable to disentangle the waning of Tdap effectiveness from the ongoing waning of previous doses of DTaP because the years since vaccination for Tdap and the fifth DTaP dose are closely correlated,” they wrote.
Most of the Tdap vaccines administered were Adacel (Sanofi Pasteur), but Boostrix (GlaxoSmithKline) was used as well. Waning occurred in both brands, and although not directly compared, no major differences in waning seemed to exist.
“We expect future pertussis epidemics to be larger as the cohort that has only received acellular pertussis vaccines ages,” the authors concluded. “The results in this study raise serious questions regarding the benefits of routinely administering a single dose of Tdap to every adolescent aged 11 or 12 years.”
The research was funded by Kaiser Permanente. Dr. Klein has received research funding from GlaxoSmithKline for a separate pertussis vaccine effectiveness study, and Dr. Klein and one coauthor also have received research funding from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Protein Science, Nuron Biotech, and MedImmune. The remaining coauthors said they had no relevant financial disclosures. GlaxoSmithKline and Sanofi Pasteur manufacture the pertussis vaccines purchased by Kaiser Permanente for this study.
The Tdap vaccine’s effectiveness against pertussis wanes so rapidly in the year after administration in teens that it provides too little protection to prevent outbreaks, according to results of a new study.
The Tdap is the booster vaccine against tetanus, diphtheria, and pertussis that adults and children ages 10 and older can receive. The corresponding acellular pertussis vaccine for children ages 6 and younger is the DTaP.
“Widespread Tdap vaccination, although associated with a transient decrease in pertussis incidence, did not prevent outbreaks among this population of teenagers who have only ever received acellular pertussis vaccines,” reported Dr. Nicola P. Klein and her associates at the Kaiser Permanente Vaccine Study Center in Oakland, Calif. “This study demonstrates that despite high rates of Tdap vaccination, the growing cohort of adolescents who have only received acellular pertussis vaccines continue to be at high risk of contracting pertussis and sustaining epidemics,” they wrote online (Pediatrics. 2016 Feb 5. [doi: 10.1542/peds.2015-3326]).
The researchers tracked 279,493 members of Kaiser Permanente Northern California who had received only DTaP vaccines, as opposed to the whole-cell DTwP formulation previously used, from age 10 onward. These included all members who were born from 1999 onward or were born from 1996-1998 and received three infant doses of DTaP, excluding children who had previously received Tdap or had pertussis. Among these, 175,094 children received the Tdap.
For the purposes of tracking pertussis cases in unvaccinated versus vaccinated adolescents, individuals were considered unvaccinated in the analysis until they received their first Tdap, after which they were vaccinated and time since vaccination was a continuous variable. Overall, 96.5% of the children were vaccinated by their 14th birthday.
Across 792,418 person-years between January 2006 and March 2015, including 418,595 vaccinated person-years for the children who received the Tdap, 1,207 cases of pertussis occurred. The vast majority of these – 85% – occurred among those ages 10-13 years. Teens aged 14-16 years comprised 15% of the cases, and only 0.5% of cases occurred among older teens. During each year of outbreaks, incidence dropped off precipitously among the age groups composed of children who would have received the whole-cell pertussis vaccine.
For example, “pertussis incidence in the 2010 outbreak sharply declined after this peak [of 10- to 11-year-olds] and stayed low at older ages, a decline that we have previously demonstrated to be associated with the receipt of whole cell instead of acellular pertussis vaccines in infancy and childhood as well as with Tdap receipt,” the authors wrote.
The researchers estimated the Tdap’s effectiveness at 69% throughout the first year after vaccination. This dropped to 57% in the second year after vaccination and then to 25% in the third year. By 4 years or later after Tdap receipt, the vaccine’s effectiveness sat at just 9%.
For each year after Tdap vaccination, children’s risk of pertussis increased 35% (hazard ratio, 1.35), and cases were mild or moderate regardless of vaccination status. Nearly all (98%) of the children with pertussis had visited the doctor within 5 days on either side of their positive polymerase chain reaction test, 86% received a diagnosis of pertussis, and 96% received a prescription for azithromycin, except for 1 for erythromycin. In addition, 4% (50) of the cases visited the emergency department. No differences in rate of ED visits or prescriptions existed between vaccinated and unvaccinated children.
“The strategy of routinely vaccinating adolescents to prevent future disease did not prevent the 2014 epidemic, arguably because the protection afforded by a dose of Tdap was too short-lived,” the authors noted. They also pointed out that Tdap waning estimates likely also include ongoing DTaP waning.“This study was unable to disentangle the waning of Tdap effectiveness from the ongoing waning of previous doses of DTaP because the years since vaccination for Tdap and the fifth DTaP dose are closely correlated,” they wrote.
Most of the Tdap vaccines administered were Adacel (Sanofi Pasteur), but Boostrix (GlaxoSmithKline) was used as well. Waning occurred in both brands, and although not directly compared, no major differences in waning seemed to exist.
“We expect future pertussis epidemics to be larger as the cohort that has only received acellular pertussis vaccines ages,” the authors concluded. “The results in this study raise serious questions regarding the benefits of routinely administering a single dose of Tdap to every adolescent aged 11 or 12 years.”
The research was funded by Kaiser Permanente. Dr. Klein has received research funding from GlaxoSmithKline for a separate pertussis vaccine effectiveness study, and Dr. Klein and one coauthor also have received research funding from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Protein Science, Nuron Biotech, and MedImmune. The remaining coauthors said they had no relevant financial disclosures. GlaxoSmithKline and Sanofi Pasteur manufacture the pertussis vaccines purchased by Kaiser Permanente for this study.
FROM PEDIATRICS
Key clinical point: Tdap protection against pertussis in adolescents wanes rapidly after the first year.
Major finding: Tdap effectiveness against pertussis dropped from 68.6% effective in the first year after vaccination to 8.9% by 4 years later; each year after Tdap increased the risk of pertussis in vaccinated teens by 35% (hazard ratio per year: 1.35).
Data source: The findings are based on analysis of 1,207 cases of pertussis among 279,493 California teens who were unvaccinated or who received the Tdap booster after recommended childhood doses of DTaP.
Disclosures: The research was funded by Kaiser Permanente. Dr. Klein has received research funding from GlaxoSmithKline for a separate pertussis vaccine effectiveness study, and Dr. Klein and one coauthor also have received research funding from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Protein Science, Nuron Biotech and MedImmune. The remaining coauthors said they had no relevant financial disclosures. GlaxoSmithKline and Sanofi Pasteur manufacture the pertussis vaccines purchased by Kaiser Permanente for this study.
Mixed rotavirus vaccine schedules safe and effective
Mixing the RotaTeq and Rotarix rotavirus vaccines schedules resulted in noninferior immunogenicity compared with using only one vaccine schedule, found a new multicenter, open-label study. Safety profiles were also similar among mixed-vaccine and single-vaccine groups, according to Dr. Romina Libster of Vanderbilt University, Nashville, Tenn., and her associates.
“These encouraging data are supported by an earlier study involving precursors of both vaccines and natural rotavirus infections,” wrote Dr. Libster and her colleagues online (Pediatrics. 2016 Jan 28. doi: 10.1542/peds.2015-2603).
The researchers randomly assigned 1,393 healthy infants who were between 6 and 14 weeks old to receive one of five different rotavirus vaccine schedules. The children, enrolled from March 2011 through September 2013, either received all doses from one vaccine or a mixture of the two different rotavirus vaccines, concurrently with other routine immunizations, accordingly:
• 244 children received three doses of RotaTeq (RV5), a live, oral vaccine containing a combination of five human-bovine reassortant rotaviruses.
• 330 children received two doses of Rotarix (RV1), a live-attenuated human rotavirus vaccine from a single human strain.
• 250 children received one dose of RotaTeq, followed by two doses of Rotarix.
• 240 children received two consecutive doses of RotaTeq followed by a dose of Rotarix.
• 329 children received one dose of Rotarix, followed by two consecutive doses of RotaTeq.
Among the 1,236 infants remaining in the study at follow-up, 77%-96% of them across the study groups attained seropositivity defined as IgA greater than or equal to 20 U/mL against at least one vaccine antigen 1 month after the last vaccine dose. Each of the mixed schedules was noninferior to each of the single-vaccine–type schedules.
In fact, a significantly greater proportion of infants receiving one Rotarix vaccine dose followed by two RotaTeq doses achieved seropositivity against both WC3 and 89-12, compared with those receiving two Rotarix doses. Similarly, infants receiving two doses of RotaTeq and one dose of Rotarix (in either order) had higher average titers against WC3 and 89-12 than infants in either of the single-vaccine groups.
“As this study was not aimed to evaluate vaccine efficacy and there is a gap of knowledge regarding a precise correlate of protection for rotavirus vaccine, the clinical relevance of the differences in immunogenicity is unclear,” the authors wrote.
No significant differences in rates of fever, diarrhea, or vomiting occurred when comparing the RV5-RV1-RV1 and RV5-RV5-RV1 groups to the single RotaTeq (RV4) group. The RV1-RV5-RV5 group, however, showed significantly more fever and vomiting than did the single Rotarix group.
“However, when the associations between group and presence of solicited symptoms were stratified by vaccine dose, there were no statistically significant differences between the two groups for the first or second doses of rotavirus vaccine,” the authors wrote.
The most commonly reported adverse event was irritability. Of 70 infants hospitalized during the study, only one case was linked to the vaccine: a 2-month-old girl receiving two doses of Rotarix and diagnosed after the first dose with gastroenteritis concurrent with an Escherichia coli urinary tract infection. Among 33 infants with bloody stools across the groups, 14 cases were determined to have been related to the vaccine. One case of intussusception occurring 91 days after the last dose was classified as unrelated to the vaccine.
The research was funded by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, and the US Department of Health and Human Services. Dr. Libster and her colleagues all reported some kind of relationship with a variety of companies including Merck, Novartis, bioCSL, and others. Dr. Turley also holds publicly traded Abbott and Pfizer stock, and Ms. McNeal has laboratory service agreements with Merck and GSK.
Expert opinion has led the American Academy of Pediatrics’ Committee on Infectious Diseases to advise providers that timely completion of immunization with any available licensed product is better than delaying immunization for a specific product. The article by Libster et al. in this issue of Pediatrics supports this principle and reassures us that for rotavirus vaccines, immunization with a mixed series of vaccines is safe and results in an immune response that is noninferior to that generated by immunization with any single product.
Importantly, the study met the enrollment goals for statistical power. The proportion of children seropositive for at least one vaccine antigen was high and was similar for all vaccine combinations. All combinations of vaccine were well tolerated, and serious adverse events were rare with any of the vaccine combinations and, in the majority of cases, unrelated to vaccine administration.
 |
Dr. Yvonne Maldonado |
The findings of Libster et al, in addition to demonstrating the efficacy of multiple vaccine combinations for rotavirus, also serve as a reminder of the importance of the infrastructures of the National Institutes of Health–supported Vaccine and Treatment Evaluation Units and the Clinical and Translational Science Awards in supporting the health of children. Without these federally funded resources, it is unlikely that the evaluation of multiple rotavirus vaccine combinations would have been conducted. Individual vaccine manufacturers have little or no incentive to test their licensed products in combination with those of other manufacturers. As child health advocates, we regularly encourage the use of vaccines. We must remember that our advocacy should also include working to ensure that the research infrastructure of the nation is strong and that adequate resources are devoted to investigations that will benefit the health of children.
These comments have been edited from a commentary accompanying the study (Pediatrics. 2016 Jan 28. doi: 10.1542/peds.2015-3618). Dr. Carrie L. Byington is with the University of Utah, Salt Lake City, department of pediatrics and Dr. Yvonne Maldonado is with the Stanford (Calif.) University department of pediatrics. Dr. Byington is supported by an H.A. and Edna Benning Presidential Endowment and the National Center for Advancing Translational Sciences. Dr. Maldonado is supported by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Byington has intellectual property in and receives royalties from BioFire Diagnostics and Dr. Maldonado is a member of a data safety monitoring board for Pfizer.
Expert opinion has led the American Academy of Pediatrics’ Committee on Infectious Diseases to advise providers that timely completion of immunization with any available licensed product is better than delaying immunization for a specific product. The article by Libster et al. in this issue of Pediatrics supports this principle and reassures us that for rotavirus vaccines, immunization with a mixed series of vaccines is safe and results in an immune response that is noninferior to that generated by immunization with any single product.
Importantly, the study met the enrollment goals for statistical power. The proportion of children seropositive for at least one vaccine antigen was high and was similar for all vaccine combinations. All combinations of vaccine were well tolerated, and serious adverse events were rare with any of the vaccine combinations and, in the majority of cases, unrelated to vaccine administration.
 |
Dr. Yvonne Maldonado |
The findings of Libster et al, in addition to demonstrating the efficacy of multiple vaccine combinations for rotavirus, also serve as a reminder of the importance of the infrastructures of the National Institutes of Health–supported Vaccine and Treatment Evaluation Units and the Clinical and Translational Science Awards in supporting the health of children. Without these federally funded resources, it is unlikely that the evaluation of multiple rotavirus vaccine combinations would have been conducted. Individual vaccine manufacturers have little or no incentive to test their licensed products in combination with those of other manufacturers. As child health advocates, we regularly encourage the use of vaccines. We must remember that our advocacy should also include working to ensure that the research infrastructure of the nation is strong and that adequate resources are devoted to investigations that will benefit the health of children.
These comments have been edited from a commentary accompanying the study (Pediatrics. 2016 Jan 28. doi: 10.1542/peds.2015-3618). Dr. Carrie L. Byington is with the University of Utah, Salt Lake City, department of pediatrics and Dr. Yvonne Maldonado is with the Stanford (Calif.) University department of pediatrics. Dr. Byington is supported by an H.A. and Edna Benning Presidential Endowment and the National Center for Advancing Translational Sciences. Dr. Maldonado is supported by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Byington has intellectual property in and receives royalties from BioFire Diagnostics and Dr. Maldonado is a member of a data safety monitoring board for Pfizer.
Expert opinion has led the American Academy of Pediatrics’ Committee on Infectious Diseases to advise providers that timely completion of immunization with any available licensed product is better than delaying immunization for a specific product. The article by Libster et al. in this issue of Pediatrics supports this principle and reassures us that for rotavirus vaccines, immunization with a mixed series of vaccines is safe and results in an immune response that is noninferior to that generated by immunization with any single product.
Importantly, the study met the enrollment goals for statistical power. The proportion of children seropositive for at least one vaccine antigen was high and was similar for all vaccine combinations. All combinations of vaccine were well tolerated, and serious adverse events were rare with any of the vaccine combinations and, in the majority of cases, unrelated to vaccine administration.
 |
Dr. Yvonne Maldonado |
The findings of Libster et al, in addition to demonstrating the efficacy of multiple vaccine combinations for rotavirus, also serve as a reminder of the importance of the infrastructures of the National Institutes of Health–supported Vaccine and Treatment Evaluation Units and the Clinical and Translational Science Awards in supporting the health of children. Without these federally funded resources, it is unlikely that the evaluation of multiple rotavirus vaccine combinations would have been conducted. Individual vaccine manufacturers have little or no incentive to test their licensed products in combination with those of other manufacturers. As child health advocates, we regularly encourage the use of vaccines. We must remember that our advocacy should also include working to ensure that the research infrastructure of the nation is strong and that adequate resources are devoted to investigations that will benefit the health of children.
These comments have been edited from a commentary accompanying the study (Pediatrics. 2016 Jan 28. doi: 10.1542/peds.2015-3618). Dr. Carrie L. Byington is with the University of Utah, Salt Lake City, department of pediatrics and Dr. Yvonne Maldonado is with the Stanford (Calif.) University department of pediatrics. Dr. Byington is supported by an H.A. and Edna Benning Presidential Endowment and the National Center for Advancing Translational Sciences. Dr. Maldonado is supported by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Byington has intellectual property in and receives royalties from BioFire Diagnostics and Dr. Maldonado is a member of a data safety monitoring board for Pfizer.
Mixing the RotaTeq and Rotarix rotavirus vaccines schedules resulted in noninferior immunogenicity compared with using only one vaccine schedule, found a new multicenter, open-label study. Safety profiles were also similar among mixed-vaccine and single-vaccine groups, according to Dr. Romina Libster of Vanderbilt University, Nashville, Tenn., and her associates.
“These encouraging data are supported by an earlier study involving precursors of both vaccines and natural rotavirus infections,” wrote Dr. Libster and her colleagues online (Pediatrics. 2016 Jan 28. doi: 10.1542/peds.2015-2603).
The researchers randomly assigned 1,393 healthy infants who were between 6 and 14 weeks old to receive one of five different rotavirus vaccine schedules. The children, enrolled from March 2011 through September 2013, either received all doses from one vaccine or a mixture of the two different rotavirus vaccines, concurrently with other routine immunizations, accordingly:
• 244 children received three doses of RotaTeq (RV5), a live, oral vaccine containing a combination of five human-bovine reassortant rotaviruses.
• 330 children received two doses of Rotarix (RV1), a live-attenuated human rotavirus vaccine from a single human strain.
• 250 children received one dose of RotaTeq, followed by two doses of Rotarix.
• 240 children received two consecutive doses of RotaTeq followed by a dose of Rotarix.
• 329 children received one dose of Rotarix, followed by two consecutive doses of RotaTeq.
Among the 1,236 infants remaining in the study at follow-up, 77%-96% of them across the study groups attained seropositivity defined as IgA greater than or equal to 20 U/mL against at least one vaccine antigen 1 month after the last vaccine dose. Each of the mixed schedules was noninferior to each of the single-vaccine–type schedules.
In fact, a significantly greater proportion of infants receiving one Rotarix vaccine dose followed by two RotaTeq doses achieved seropositivity against both WC3 and 89-12, compared with those receiving two Rotarix doses. Similarly, infants receiving two doses of RotaTeq and one dose of Rotarix (in either order) had higher average titers against WC3 and 89-12 than infants in either of the single-vaccine groups.
“As this study was not aimed to evaluate vaccine efficacy and there is a gap of knowledge regarding a precise correlate of protection for rotavirus vaccine, the clinical relevance of the differences in immunogenicity is unclear,” the authors wrote.
No significant differences in rates of fever, diarrhea, or vomiting occurred when comparing the RV5-RV1-RV1 and RV5-RV5-RV1 groups to the single RotaTeq (RV4) group. The RV1-RV5-RV5 group, however, showed significantly more fever and vomiting than did the single Rotarix group.
“However, when the associations between group and presence of solicited symptoms were stratified by vaccine dose, there were no statistically significant differences between the two groups for the first or second doses of rotavirus vaccine,” the authors wrote.
The most commonly reported adverse event was irritability. Of 70 infants hospitalized during the study, only one case was linked to the vaccine: a 2-month-old girl receiving two doses of Rotarix and diagnosed after the first dose with gastroenteritis concurrent with an Escherichia coli urinary tract infection. Among 33 infants with bloody stools across the groups, 14 cases were determined to have been related to the vaccine. One case of intussusception occurring 91 days after the last dose was classified as unrelated to the vaccine.
The research was funded by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, and the US Department of Health and Human Services. Dr. Libster and her colleagues all reported some kind of relationship with a variety of companies including Merck, Novartis, bioCSL, and others. Dr. Turley also holds publicly traded Abbott and Pfizer stock, and Ms. McNeal has laboratory service agreements with Merck and GSK.
Mixing the RotaTeq and Rotarix rotavirus vaccines schedules resulted in noninferior immunogenicity compared with using only one vaccine schedule, found a new multicenter, open-label study. Safety profiles were also similar among mixed-vaccine and single-vaccine groups, according to Dr. Romina Libster of Vanderbilt University, Nashville, Tenn., and her associates.
“These encouraging data are supported by an earlier study involving precursors of both vaccines and natural rotavirus infections,” wrote Dr. Libster and her colleagues online (Pediatrics. 2016 Jan 28. doi: 10.1542/peds.2015-2603).
The researchers randomly assigned 1,393 healthy infants who were between 6 and 14 weeks old to receive one of five different rotavirus vaccine schedules. The children, enrolled from March 2011 through September 2013, either received all doses from one vaccine or a mixture of the two different rotavirus vaccines, concurrently with other routine immunizations, accordingly:
• 244 children received three doses of RotaTeq (RV5), a live, oral vaccine containing a combination of five human-bovine reassortant rotaviruses.
• 330 children received two doses of Rotarix (RV1), a live-attenuated human rotavirus vaccine from a single human strain.
• 250 children received one dose of RotaTeq, followed by two doses of Rotarix.
• 240 children received two consecutive doses of RotaTeq followed by a dose of Rotarix.
• 329 children received one dose of Rotarix, followed by two consecutive doses of RotaTeq.
Among the 1,236 infants remaining in the study at follow-up, 77%-96% of them across the study groups attained seropositivity defined as IgA greater than or equal to 20 U/mL against at least one vaccine antigen 1 month after the last vaccine dose. Each of the mixed schedules was noninferior to each of the single-vaccine–type schedules.
In fact, a significantly greater proportion of infants receiving one Rotarix vaccine dose followed by two RotaTeq doses achieved seropositivity against both WC3 and 89-12, compared with those receiving two Rotarix doses. Similarly, infants receiving two doses of RotaTeq and one dose of Rotarix (in either order) had higher average titers against WC3 and 89-12 than infants in either of the single-vaccine groups.
“As this study was not aimed to evaluate vaccine efficacy and there is a gap of knowledge regarding a precise correlate of protection for rotavirus vaccine, the clinical relevance of the differences in immunogenicity is unclear,” the authors wrote.
No significant differences in rates of fever, diarrhea, or vomiting occurred when comparing the RV5-RV1-RV1 and RV5-RV5-RV1 groups to the single RotaTeq (RV4) group. The RV1-RV5-RV5 group, however, showed significantly more fever and vomiting than did the single Rotarix group.
“However, when the associations between group and presence of solicited symptoms were stratified by vaccine dose, there were no statistically significant differences between the two groups for the first or second doses of rotavirus vaccine,” the authors wrote.
The most commonly reported adverse event was irritability. Of 70 infants hospitalized during the study, only one case was linked to the vaccine: a 2-month-old girl receiving two doses of Rotarix and diagnosed after the first dose with gastroenteritis concurrent with an Escherichia coli urinary tract infection. Among 33 infants with bloody stools across the groups, 14 cases were determined to have been related to the vaccine. One case of intussusception occurring 91 days after the last dose was classified as unrelated to the vaccine.
The research was funded by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, and the US Department of Health and Human Services. Dr. Libster and her colleagues all reported some kind of relationship with a variety of companies including Merck, Novartis, bioCSL, and others. Dr. Turley also holds publicly traded Abbott and Pfizer stock, and Ms. McNeal has laboratory service agreements with Merck and GSK.
FROM PEDIATRICS
Key clinical point: Mixing rotavirus vaccine schedules is safe and comparable to single vaccine schedules.
Major finding: 77%-96% of infants receiving single vaccine schedules or a mixed schedule achieved seropositivity.
Data source: The findings are based on a randomized, multicenter, open-label study involving 1,393 children between 6 and 14 weeks old, enrolled from March 2011 through September 2013 and randomized to receive one of five different rotavirus vaccine schedules.
Disclosures: The research was funded by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, and the U.S. Department of Health & Human Services. Dr. Libster and her colleagues all reported some kind of relationship with a variety of companies including Merck, Novartis, bioCSL, and others. Dr. Turley also holds publicly traded Abbott and Pfizer stock, and Ms. McNeal has laboratory service agreements with Merck and GSK.
AAP says newborn pain management plans essential for all providers
Repeated exposure to physical pain can have both short-term and long-term adverse effects in newborns, yet providers too inconsistently assess and adequately manage pain in neonates, the American Academy of Pediatrics said in an updated policy statement on preventing and managing procedural pain in these infants.
“Neonates are frequently subjected to painful procedures, with the most immature infants receiving the highest number of painful events,” wrote the AAP Committee on Fetus and Newborn and the AAP Section on Anesthesiology and Pain Medicine. Premature infants also are at the highest risk for long-term sequelae, the committees noted. “These sequelae include physiologic instability; altered brain development; and abnormal neurodevelopment, somatosensory, and stress response systems, which can persist into childhood” (Pediatrics. 2016 Jan 25. doi: 10.1542/peds.2015-4271).
The statement nicely summarizes current evidence on the evaluation and management of pain in neonates, as well as potential short-term and long-term negative effects, said Dr. Clay T. Jones, a neonatal hospitalist at Newton-Wellesley Hospital in Newton, Mass., who was not involved in drafting the statement.
“It is a nice testament to how far we’ve come since the days of performing major surgeries in newborns without the use of analgesic medications, but there is still considerable room for improvement,” Dr. Jones said in an interview. “Although we’ve learned a lot about pain in this population over the past few decades, the authors go to great lengths to make it clear that the optimal way to treat pain hasn’t been established yet, and that the evaluation of risk versus benefit in regards to management using pharmaceutical agents is ongoing.”
The statement described the challenges of effective pain management, including foremost the newborn’s inability to communicate and the dearth of information about effective pain assessments and management in this population.
“Contextual factors such as gestational age and behavioral state may play a significant role in pain assessment and are beginning to be included in some assessment tools,” the committees wrote. “New and emerging technologies to measure pain responses, such as near-infrared spectroscopy, amplitude-integrated electroencephalography, functional MRI, skin conductance, and heart rate variability assessment, are being investigated.”
In the meantime, however, all facilities caring for newborns should implement a pain prevention program that takes advantage of what pain management strategies do exist. In addition to reviewing these strategies, the statement recommended all providers implement and use pain assessment and management plans that include both pharmacologic and nonpharmacologic therapies for major procedures and routine minor interventions that might still cause pain.
“Where this report might have the largest impact is in the outpatient offices of pediatric medical providers and in newborn nurseries,” Dr. Jones said. “It is not uncommon for young infants to undergo painful medical procedures, such as heel sticks and circumcisions, without a systematic approach to pain evaluation and inconsistent efforts to prevent or reduce pain.”
Yet there is no excuse for this lack of a consistent approach, he said.
“Nonpharmaceutical interventions are safe, easy to implement, [and] inexpensive, and there is good evidence that they reduce physiologic surrogate markers for pain such as blood pressure, heart rate, and respiratory rate,” Dr. Jones said.
The statement’s first recommendation is that all institutions have written guidelines for a stepwise plan of pain prevention and treatment in newborns. This plan also should include use of currently available and validated neonatal assessment tools “before, during, and after painful procedures to monitor the effectiveness of pain relief interventions.”
Another recommendation urged pediatricians and other neonatal health care providers and family members to receive ongoing education about recognizing and assessing pain in newborns and then managing it as much as current knowledge and evidence allow. The committees called for more research into pain assessment tools and pain prevention and amelioration strategies, including pharmacologic options.
The nonpharmacologic strategies shown to be effective for reducing pain during short-term mild or moderately painful procedures include facilitated tucking, sensorial stimulation, nonnutritive sucking, and breastfeeding or providing expressed human milk.
“Anything we can do to ease pain will improve a baby’s quality of life,” said Dr. Nathan Boonstra of Blank Children’s Hospital pediatric clinic in Des Moines, Iowa, who was not involved in writing the statement.
“Pediatricians should always be judicious when deciding to draw blood, but when we need to, we should reflexively think about what can keep the procedure’s pain at a minimum,” he said in an interview. “Many of my patients’ mothers instinctively want to breastfeed to help their newborns through something painful, and their instinct serves them well.”
The policy statement mentioned oral sucrose and/or glucose solutions as effective analgesic options for mild to moderately painful procedures, but recommended these be prescribed and tracked as medication.
This strategy carries risks and other drawbacks, however, Dr. Thomas M Seman, a pediatrician in group practice in Danvers, Mass., said in an interview
“Having sucrose or glucose solutions in the office can be dangerous because of the risk of overuse and hyperglycemia as well as the cost of these items,” said Dr. Seman, who was not involved with drafting the AAP statement. His office policy primarily focuses on the parents holding their children and talking, singing, or humming to them during procedures, followed by feeding and/or acetaminophen, he said.
“The other medications used are prohibitive for a number of reasons,” said Dr. Seman, although he added that most of the procedures described in the statement are performed on premature infants in a NICU with only a few done in private practices.
For example, opioids such as fentanyl and morphine are most frequently used for persistent pain, yet the data on appropriate dosing and long-term effects in the newborn period are “woefully lacking and/or conflicting,” the statement noted. The evidence for benzodiazepines, often used for sedation in the neonatal intensive care, shows little additional analgesic benefit, but these agents can potentiate the risk of respiratory depression and hypotension associated with opioid use. Caution should particularly be exercised before using methadone, ketamine, propofol, and dexmedetomidine because so little is known about safe and effective dosing of these medications in neonates. They also carry various potential risks ranging from neurotoxicity to bradycardia, desaturations, and prolonged hypotension.
While NSAIDs are not recommended at this young age, oral or intravenous acetaminophen has sufficient preliminary safety and efficacy to be considered for postoperative pain relief, according to the statement.
The AAP did not report disclosures for committee members. Dr. Boonstra, Dr. Jones, and Dr. Seman had no relevant financial disclosures.
Repeated exposure to physical pain can have both short-term and long-term adverse effects in newborns, yet providers too inconsistently assess and adequately manage pain in neonates, the American Academy of Pediatrics said in an updated policy statement on preventing and managing procedural pain in these infants.
“Neonates are frequently subjected to painful procedures, with the most immature infants receiving the highest number of painful events,” wrote the AAP Committee on Fetus and Newborn and the AAP Section on Anesthesiology and Pain Medicine. Premature infants also are at the highest risk for long-term sequelae, the committees noted. “These sequelae include physiologic instability; altered brain development; and abnormal neurodevelopment, somatosensory, and stress response systems, which can persist into childhood” (Pediatrics. 2016 Jan 25. doi: 10.1542/peds.2015-4271).
The statement nicely summarizes current evidence on the evaluation and management of pain in neonates, as well as potential short-term and long-term negative effects, said Dr. Clay T. Jones, a neonatal hospitalist at Newton-Wellesley Hospital in Newton, Mass., who was not involved in drafting the statement.
“It is a nice testament to how far we’ve come since the days of performing major surgeries in newborns without the use of analgesic medications, but there is still considerable room for improvement,” Dr. Jones said in an interview. “Although we’ve learned a lot about pain in this population over the past few decades, the authors go to great lengths to make it clear that the optimal way to treat pain hasn’t been established yet, and that the evaluation of risk versus benefit in regards to management using pharmaceutical agents is ongoing.”
The statement described the challenges of effective pain management, including foremost the newborn’s inability to communicate and the dearth of information about effective pain assessments and management in this population.
“Contextual factors such as gestational age and behavioral state may play a significant role in pain assessment and are beginning to be included in some assessment tools,” the committees wrote. “New and emerging technologies to measure pain responses, such as near-infrared spectroscopy, amplitude-integrated electroencephalography, functional MRI, skin conductance, and heart rate variability assessment, are being investigated.”
In the meantime, however, all facilities caring for newborns should implement a pain prevention program that takes advantage of what pain management strategies do exist. In addition to reviewing these strategies, the statement recommended all providers implement and use pain assessment and management plans that include both pharmacologic and nonpharmacologic therapies for major procedures and routine minor interventions that might still cause pain.
“Where this report might have the largest impact is in the outpatient offices of pediatric medical providers and in newborn nurseries,” Dr. Jones said. “It is not uncommon for young infants to undergo painful medical procedures, such as heel sticks and circumcisions, without a systematic approach to pain evaluation and inconsistent efforts to prevent or reduce pain.”
Yet there is no excuse for this lack of a consistent approach, he said.
“Nonpharmaceutical interventions are safe, easy to implement, [and] inexpensive, and there is good evidence that they reduce physiologic surrogate markers for pain such as blood pressure, heart rate, and respiratory rate,” Dr. Jones said.
The statement’s first recommendation is that all institutions have written guidelines for a stepwise plan of pain prevention and treatment in newborns. This plan also should include use of currently available and validated neonatal assessment tools “before, during, and after painful procedures to monitor the effectiveness of pain relief interventions.”
Another recommendation urged pediatricians and other neonatal health care providers and family members to receive ongoing education about recognizing and assessing pain in newborns and then managing it as much as current knowledge and evidence allow. The committees called for more research into pain assessment tools and pain prevention and amelioration strategies, including pharmacologic options.
The nonpharmacologic strategies shown to be effective for reducing pain during short-term mild or moderately painful procedures include facilitated tucking, sensorial stimulation, nonnutritive sucking, and breastfeeding or providing expressed human milk.
“Anything we can do to ease pain will improve a baby’s quality of life,” said Dr. Nathan Boonstra of Blank Children’s Hospital pediatric clinic in Des Moines, Iowa, who was not involved in writing the statement.
“Pediatricians should always be judicious when deciding to draw blood, but when we need to, we should reflexively think about what can keep the procedure’s pain at a minimum,” he said in an interview. “Many of my patients’ mothers instinctively want to breastfeed to help their newborns through something painful, and their instinct serves them well.”
The policy statement mentioned oral sucrose and/or glucose solutions as effective analgesic options for mild to moderately painful procedures, but recommended these be prescribed and tracked as medication.
This strategy carries risks and other drawbacks, however, Dr. Thomas M Seman, a pediatrician in group practice in Danvers, Mass., said in an interview
“Having sucrose or glucose solutions in the office can be dangerous because of the risk of overuse and hyperglycemia as well as the cost of these items,” said Dr. Seman, who was not involved with drafting the AAP statement. His office policy primarily focuses on the parents holding their children and talking, singing, or humming to them during procedures, followed by feeding and/or acetaminophen, he said.
“The other medications used are prohibitive for a number of reasons,” said Dr. Seman, although he added that most of the procedures described in the statement are performed on premature infants in a NICU with only a few done in private practices.
For example, opioids such as fentanyl and morphine are most frequently used for persistent pain, yet the data on appropriate dosing and long-term effects in the newborn period are “woefully lacking and/or conflicting,” the statement noted. The evidence for benzodiazepines, often used for sedation in the neonatal intensive care, shows little additional analgesic benefit, but these agents can potentiate the risk of respiratory depression and hypotension associated with opioid use. Caution should particularly be exercised before using methadone, ketamine, propofol, and dexmedetomidine because so little is known about safe and effective dosing of these medications in neonates. They also carry various potential risks ranging from neurotoxicity to bradycardia, desaturations, and prolonged hypotension.
While NSAIDs are not recommended at this young age, oral or intravenous acetaminophen has sufficient preliminary safety and efficacy to be considered for postoperative pain relief, according to the statement.
The AAP did not report disclosures for committee members. Dr. Boonstra, Dr. Jones, and Dr. Seman had no relevant financial disclosures.
Repeated exposure to physical pain can have both short-term and long-term adverse effects in newborns, yet providers too inconsistently assess and adequately manage pain in neonates, the American Academy of Pediatrics said in an updated policy statement on preventing and managing procedural pain in these infants.
“Neonates are frequently subjected to painful procedures, with the most immature infants receiving the highest number of painful events,” wrote the AAP Committee on Fetus and Newborn and the AAP Section on Anesthesiology and Pain Medicine. Premature infants also are at the highest risk for long-term sequelae, the committees noted. “These sequelae include physiologic instability; altered brain development; and abnormal neurodevelopment, somatosensory, and stress response systems, which can persist into childhood” (Pediatrics. 2016 Jan 25. doi: 10.1542/peds.2015-4271).
The statement nicely summarizes current evidence on the evaluation and management of pain in neonates, as well as potential short-term and long-term negative effects, said Dr. Clay T. Jones, a neonatal hospitalist at Newton-Wellesley Hospital in Newton, Mass., who was not involved in drafting the statement.
“It is a nice testament to how far we’ve come since the days of performing major surgeries in newborns without the use of analgesic medications, but there is still considerable room for improvement,” Dr. Jones said in an interview. “Although we’ve learned a lot about pain in this population over the past few decades, the authors go to great lengths to make it clear that the optimal way to treat pain hasn’t been established yet, and that the evaluation of risk versus benefit in regards to management using pharmaceutical agents is ongoing.”
The statement described the challenges of effective pain management, including foremost the newborn’s inability to communicate and the dearth of information about effective pain assessments and management in this population.
“Contextual factors such as gestational age and behavioral state may play a significant role in pain assessment and are beginning to be included in some assessment tools,” the committees wrote. “New and emerging technologies to measure pain responses, such as near-infrared spectroscopy, amplitude-integrated electroencephalography, functional MRI, skin conductance, and heart rate variability assessment, are being investigated.”
In the meantime, however, all facilities caring for newborns should implement a pain prevention program that takes advantage of what pain management strategies do exist. In addition to reviewing these strategies, the statement recommended all providers implement and use pain assessment and management plans that include both pharmacologic and nonpharmacologic therapies for major procedures and routine minor interventions that might still cause pain.
“Where this report might have the largest impact is in the outpatient offices of pediatric medical providers and in newborn nurseries,” Dr. Jones said. “It is not uncommon for young infants to undergo painful medical procedures, such as heel sticks and circumcisions, without a systematic approach to pain evaluation and inconsistent efforts to prevent or reduce pain.”
Yet there is no excuse for this lack of a consistent approach, he said.
“Nonpharmaceutical interventions are safe, easy to implement, [and] inexpensive, and there is good evidence that they reduce physiologic surrogate markers for pain such as blood pressure, heart rate, and respiratory rate,” Dr. Jones said.
The statement’s first recommendation is that all institutions have written guidelines for a stepwise plan of pain prevention and treatment in newborns. This plan also should include use of currently available and validated neonatal assessment tools “before, during, and after painful procedures to monitor the effectiveness of pain relief interventions.”
Another recommendation urged pediatricians and other neonatal health care providers and family members to receive ongoing education about recognizing and assessing pain in newborns and then managing it as much as current knowledge and evidence allow. The committees called for more research into pain assessment tools and pain prevention and amelioration strategies, including pharmacologic options.
The nonpharmacologic strategies shown to be effective for reducing pain during short-term mild or moderately painful procedures include facilitated tucking, sensorial stimulation, nonnutritive sucking, and breastfeeding or providing expressed human milk.
“Anything we can do to ease pain will improve a baby’s quality of life,” said Dr. Nathan Boonstra of Blank Children’s Hospital pediatric clinic in Des Moines, Iowa, who was not involved in writing the statement.
“Pediatricians should always be judicious when deciding to draw blood, but when we need to, we should reflexively think about what can keep the procedure’s pain at a minimum,” he said in an interview. “Many of my patients’ mothers instinctively want to breastfeed to help their newborns through something painful, and their instinct serves them well.”
The policy statement mentioned oral sucrose and/or glucose solutions as effective analgesic options for mild to moderately painful procedures, but recommended these be prescribed and tracked as medication.
This strategy carries risks and other drawbacks, however, Dr. Thomas M Seman, a pediatrician in group practice in Danvers, Mass., said in an interview
“Having sucrose or glucose solutions in the office can be dangerous because of the risk of overuse and hyperglycemia as well as the cost of these items,” said Dr. Seman, who was not involved with drafting the AAP statement. His office policy primarily focuses on the parents holding their children and talking, singing, or humming to them during procedures, followed by feeding and/or acetaminophen, he said.
“The other medications used are prohibitive for a number of reasons,” said Dr. Seman, although he added that most of the procedures described in the statement are performed on premature infants in a NICU with only a few done in private practices.
For example, opioids such as fentanyl and morphine are most frequently used for persistent pain, yet the data on appropriate dosing and long-term effects in the newborn period are “woefully lacking and/or conflicting,” the statement noted. The evidence for benzodiazepines, often used for sedation in the neonatal intensive care, shows little additional analgesic benefit, but these agents can potentiate the risk of respiratory depression and hypotension associated with opioid use. Caution should particularly be exercised before using methadone, ketamine, propofol, and dexmedetomidine because so little is known about safe and effective dosing of these medications in neonates. They also carry various potential risks ranging from neurotoxicity to bradycardia, desaturations, and prolonged hypotension.
While NSAIDs are not recommended at this young age, oral or intravenous acetaminophen has sufficient preliminary safety and efficacy to be considered for postoperative pain relief, according to the statement.
The AAP did not report disclosures for committee members. Dr. Boonstra, Dr. Jones, and Dr. Seman had no relevant financial disclosures.
FROM PEDIATRICS
Antibiotic course durations for uncomplicated SSTIs reduced by hospital initiative
Using targeted quality improvement methods, a hospital more than tripled the proportion of short courses of antibiotics prescribed for children discharged from hospitalization for uncomplicated skin and soft tissue infections (SSTIs), according to a recent study.
“Short courses of antibiotics may be beneficial to help prevent development of resistant bacteria, lessen cost, and reduce the chances of unintended effects of antimicrobial therapy,” explained Dr. Christine L. Schuler and her associates at the Cincinnati Children’s Hospital Medical Center (Pediatrics. 2016 Jan 18. doi: 10.1542/peds.2015-1223). They aimed specifically to increase the percentage of patients receiving antibiotic prescriptions for 7 days or fewer from their baseline of 23% to 80% over 6 months.
For nonpurulent cellulitis, 5-day courses of antibiotics are recommended as sufficiently effective by the Infectious Diseases Society of America, which recommends an extension only if the patient hasn’t improved in that time. Awareness of these guidelines and relevant evidence through two 15-minute education sessions comprised one of the four key components for the initiative’s implementation. The three other components involved “changing the culture of physician prescribing to include discussion of duration of therapy at admission, buy in from prescribers, and effective monitoring of prescribing,” Dr. Schuler and her associates wrote.
Following the sessions, a second intervention provided clinicians with reminder lanyard cards containing information about recommended treatment regimes. Next, electronic order defaults for antibiotics for SSTIs were changed from 14-day courses of antibiotics to 7-day courses, which then automatically subtracted the days on antibiotics during hospitalization, a median 1.2 days.
Last, the hospital started reviewing medical records daily to identify all patients admitted with SSTIs. An intern or senior resident then contacted the patient’s medical team and reminded them that the patient may be appropriate for a short course of antibiotics, that the lanyard card provided therapy recommendations, and that the medical team could contact the project team with questions.
Of 641 patients – aged 3 months to 18 years – admitted for SSTIs between January 2012 and November 2013, one-third occurred before the intervention and two-thirds after. Clindamycin was prescribed in 88% of cases, trimethoprim-sulfamethoxazole in 8%, and cephalexin in 4%.
The proportion of short courses of antibiotics prescribed increased from a baseline of 23% to 74% by February 2013, where it remained for the next 6 months. From March to November 2013, the median duration of antibiotic courses prescribed was 7 days. A total of 2% of the patients were readmitted for recurrence, and 2% were admitted for treatment failure, but these numbers were similar before and after the intervention.
“We suspect that our sustained improvement in prescriptions for short courses of antibiotics over multiple months was in large part due to modification of our electronic order set used to generate outpatient prescriptions,” the authors wrote.
Using targeted quality improvement methods, a hospital more than tripled the proportion of short courses of antibiotics prescribed for children discharged from hospitalization for uncomplicated skin and soft tissue infections (SSTIs), according to a recent study.
“Short courses of antibiotics may be beneficial to help prevent development of resistant bacteria, lessen cost, and reduce the chances of unintended effects of antimicrobial therapy,” explained Dr. Christine L. Schuler and her associates at the Cincinnati Children’s Hospital Medical Center (Pediatrics. 2016 Jan 18. doi: 10.1542/peds.2015-1223). They aimed specifically to increase the percentage of patients receiving antibiotic prescriptions for 7 days or fewer from their baseline of 23% to 80% over 6 months.
For nonpurulent cellulitis, 5-day courses of antibiotics are recommended as sufficiently effective by the Infectious Diseases Society of America, which recommends an extension only if the patient hasn’t improved in that time. Awareness of these guidelines and relevant evidence through two 15-minute education sessions comprised one of the four key components for the initiative’s implementation. The three other components involved “changing the culture of physician prescribing to include discussion of duration of therapy at admission, buy in from prescribers, and effective monitoring of prescribing,” Dr. Schuler and her associates wrote.
Following the sessions, a second intervention provided clinicians with reminder lanyard cards containing information about recommended treatment regimes. Next, electronic order defaults for antibiotics for SSTIs were changed from 14-day courses of antibiotics to 7-day courses, which then automatically subtracted the days on antibiotics during hospitalization, a median 1.2 days.
Last, the hospital started reviewing medical records daily to identify all patients admitted with SSTIs. An intern or senior resident then contacted the patient’s medical team and reminded them that the patient may be appropriate for a short course of antibiotics, that the lanyard card provided therapy recommendations, and that the medical team could contact the project team with questions.
Of 641 patients – aged 3 months to 18 years – admitted for SSTIs between January 2012 and November 2013, one-third occurred before the intervention and two-thirds after. Clindamycin was prescribed in 88% of cases, trimethoprim-sulfamethoxazole in 8%, and cephalexin in 4%.
The proportion of short courses of antibiotics prescribed increased from a baseline of 23% to 74% by February 2013, where it remained for the next 6 months. From March to November 2013, the median duration of antibiotic courses prescribed was 7 days. A total of 2% of the patients were readmitted for recurrence, and 2% were admitted for treatment failure, but these numbers were similar before and after the intervention.
“We suspect that our sustained improvement in prescriptions for short courses of antibiotics over multiple months was in large part due to modification of our electronic order set used to generate outpatient prescriptions,” the authors wrote.
Using targeted quality improvement methods, a hospital more than tripled the proportion of short courses of antibiotics prescribed for children discharged from hospitalization for uncomplicated skin and soft tissue infections (SSTIs), according to a recent study.
“Short courses of antibiotics may be beneficial to help prevent development of resistant bacteria, lessen cost, and reduce the chances of unintended effects of antimicrobial therapy,” explained Dr. Christine L. Schuler and her associates at the Cincinnati Children’s Hospital Medical Center (Pediatrics. 2016 Jan 18. doi: 10.1542/peds.2015-1223). They aimed specifically to increase the percentage of patients receiving antibiotic prescriptions for 7 days or fewer from their baseline of 23% to 80% over 6 months.
For nonpurulent cellulitis, 5-day courses of antibiotics are recommended as sufficiently effective by the Infectious Diseases Society of America, which recommends an extension only if the patient hasn’t improved in that time. Awareness of these guidelines and relevant evidence through two 15-minute education sessions comprised one of the four key components for the initiative’s implementation. The three other components involved “changing the culture of physician prescribing to include discussion of duration of therapy at admission, buy in from prescribers, and effective monitoring of prescribing,” Dr. Schuler and her associates wrote.
Following the sessions, a second intervention provided clinicians with reminder lanyard cards containing information about recommended treatment regimes. Next, electronic order defaults for antibiotics for SSTIs were changed from 14-day courses of antibiotics to 7-day courses, which then automatically subtracted the days on antibiotics during hospitalization, a median 1.2 days.
Last, the hospital started reviewing medical records daily to identify all patients admitted with SSTIs. An intern or senior resident then contacted the patient’s medical team and reminded them that the patient may be appropriate for a short course of antibiotics, that the lanyard card provided therapy recommendations, and that the medical team could contact the project team with questions.
Of 641 patients – aged 3 months to 18 years – admitted for SSTIs between January 2012 and November 2013, one-third occurred before the intervention and two-thirds after. Clindamycin was prescribed in 88% of cases, trimethoprim-sulfamethoxazole in 8%, and cephalexin in 4%.
The proportion of short courses of antibiotics prescribed increased from a baseline of 23% to 74% by February 2013, where it remained for the next 6 months. From March to November 2013, the median duration of antibiotic courses prescribed was 7 days. A total of 2% of the patients were readmitted for recurrence, and 2% were admitted for treatment failure, but these numbers were similar before and after the intervention.
“We suspect that our sustained improvement in prescriptions for short courses of antibiotics over multiple months was in large part due to modification of our electronic order set used to generate outpatient prescriptions,” the authors wrote.
FROM PEDIATRICS
Key clinical point: Quality improvement methods effectively decreased duration of antibiotic courses prescribed for uncomplicated skin and soft tissue infections.
Major finding: A hospital increased its 5-day course antibiotic prescriptions at discharge from 23% to 74% over 13 months.
Data source: The findings are based on one hospital’s data on short courses of antibiotics prescribed between January 2012 and November 2013.
Disclosures: The research did not use external funding, and the authors reported no disclosures.
Therapy, medication together best to treat pediatric mood disorders
Effective management of depression and other mood disorders in children may involve both pharmacotherapy with behavioral recommendations and psychotherapy, according to Stephen P. Whiteside, Ph.D., and Dr. John Huxsahl of the Mayo Clinic in Rochester Minn.
In a presentation at the American Academy of Pediatrics annual meeting in Washington, they reviewed the differences between various mood disorders because accurate diagnosis is a key component to management.
A major depressive episode is among the more common mood disorders among children, but others include disruptive mood dysregulation disorder, persistent depressive disorder, and bipolar I disorder. Because most pediatricians have just 15 minutes or so with patients, Dr. Whiteside and Dr. Huxsahl recommended using screening questionnaires to identify children at risk for a mood disorder. Trying to determine why a child is experiencing these symptoms or feelings can help clinicians determine whether this is a chronic issue or a situational one. Asking about other concerns, such as anxiety or use of substances, also can help with diagnosis.
Depressive episodes
Occurring among approximately 2% of children and 4%-8% of adolescents, a major depressive episode lasts a median of 8 months, but has a very high rate of recurrence: 20%-60% at 1 or 2 years after remission and 70% at 5 years after remission. In a significant proportion of children – about 20%-40% – a major depressive episode forecasts bipolar disorder.
In fact, having a parent with a mood disorder doubles to quadruples a child’s risk of major depressive episodes, and the disorder frequently occurs with anxiety, estimated in approximately 61%-65% of children with a major depressive episode. An episode generally appears to result from a combination of genetic factors and environmental ones, including abuse, neglect, family conflict, childhood adversity, losses, and comorbid disorders.
More heterogeneous than in adults, major depressive episodes in children look different based on a child’s age, according to Dr. Whiteside and Dr. Huxsahl:
• Birth to age 2 years. Symptoms include whining, decreased growth, lack of responsiveness, disrupted sleep, and excessive fears.
• Ages 3-5 years. Symptoms include anxiety, somatic symptoms, tantrums, sadness, weight gain, tiredness/sleepiness, suicidal ideation, anger or irritability, apathy, illness, and social withdrawal
• Ages 6-12 years. Symptoms include sadness, an inability to experience pleasure, decreased energy, low self-esteem, irritability, and suicidal ideation.
• Ages 12-18 years. Symptoms include a volatile mood, rage, acting out, self-consciousness, withdrawal, suicidal ideation, and overeating and/or oversleeping.
Other mood disorders
Disruptive mood dysregulation disorder (DMDD) involves much more acting out at a younger age than major depressive episodes. In children with DMDD, outbursts greatly exceed what would be expected in response to a situation, whether in terms of how long the tantrums last or how intense they are. These outbursts also are unexpected developmentally, with an onset before age 10 years, although the disorder should not be diagnosed earlier than age 6 years. Diagnostic criteria also require that the outbursts occur at least three times weekly, on average, for at least 12 months in at least two settings with a persistently angry or irritable mood between outbursts.
One way to support a diagnosis of DMDD is to rule out what it’s not. DMDD can exist with comorbidities of major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), conduct disorder, and substance use disorders, and it’s most likely to grow into depression or anxiety. It cannot coexist with oppositional defiant disorder, intermittent explosive disorder, or bipolar disorder, and it does not develop into bipolar disorder.
Persistent depressive disorder resembles MDD, with either a depressed mood lasting at least 2 years or an irritable mood lasting at least 1 year plus at least two of the following symptoms: poor appetite or overeating; insomnia or hypersomnia; low energy or fatigue; poor concretion or difficulty making decisions; feelings of hopelessness; and low self-esteem.
Bipolar disorder
“The most important part of managing bipolar I is episodes, episodes, episodes,” Dr. Whiteside said. Alternating with either hypomanic or major depressive episodes are manic episodes, in which a child experiences an abnormal “high,” a period of at least a week of extremely high energy nearly all day, every day, with a “persistently elevated, expansive, or irritable mood.”
For an episode to be considered manic, however, at least three of the following seven other symptoms must be present, and deviate from the patient’s otherwise normal behavior: grandiosity or an especially inflated sense of self-esteem; a decreased need for sleep (not just that the patient doesn’t sleep but doesn’t actually seem to need to); extreme talkativeness or use of pressured speech; a feeling of having racing thoughts or ideas flying about; a tendency to be easily distracted or unfocused; an increase in activities aimed at accomplishing certain goals (in any sphere, for example, school, work, social, or sexual); and risky behaviors with potentially severe, long-term consequences, such as more frequent sexual behavior or shopping sprees.
Dr. Whiteside compared bipolar disorder to ADHD to help clinicians appreciate how to best understand it. Key differences with ADHD are an earlier age of onset, a more consistent presence of symptoms without episodes, a need for sleep despite possible insomnia, and only situational depression. In a 1998 study, a comparison of bipolar I and ADHD revealed that 89% of bipolar children had an elevated mood, compared with just 14% of those with ADHD. A similar gulf existed with grandiose thoughts, occurring in 86% of children with bipolar disorder and 5% of those with ADHD. Likewise, a much greater proportion of children with bipolar disorder experienced “flights of ideas,” decreased sleep, or hypersexuality, compared with these symptoms in children with ADHD.
Treating depression: Behavioral interventions
Psychopharmacology can be beneficial, but it works best when combined with behavioral interventions and simply explaining the disorder, said Dr. Whiteside. “There’s a lot of evidence that exercise can be a powerful treatment with depression,” he said. “Education is especially important since one of the four features of depression is a sense of hopelessness and belief that nothing is going to [get] better, so for them to know there are steps they can take is a very powerful intervention itself.”
The most effective intervention, however, is cognitive-behavioral therapy, with an 81% success rate 1 year out and a 98% success rate 2 years out. After the initial intervention, however, the numbers are more modest, with 67% of children no longer meeting major depressive disorder criteria when undergoing cognitive-behavioral therapy, compared with 48% in the waiting-list control group.
An important aspect to education starts with developing an alliance with a family so that the clinician can explain the condition and its treatment, build trust, and instill hope. In doing so, both parents’ and children’s expectations can be adjusted so that children feel less guilty and parents respond less negatively to their children.
Treatment with medication
Although only fluoxetine and escitalopram have Food and Drug Administration approval for MDD, Dr. Huxsahl recommended starting kids with mixed anxiety and depression on fluoxetine, fluvoxamine, or sertraline, and suggested clinicians not shy away from the target doses recommended for each drug. After 2-4 months of acute treatment, clinicians also should help families understand that continuing the treatment for at least 4-9 months and then potentially taking a maintenance dose for up to 3 years may be recommended.
“I would encourage you to educate the parents, and especially the teenagers, that they need to take the medication beyond the time that they are depressed,” he explained. Initially, however, start with a low dose and then increase it within 4 weeks if no response occurs. After 8 weeks, switch agents if no response has occurred, a process that requires monthly visits for the first 3-6 months of treatment.
Another question families and clinicians face is when to taper off medication when moving to a maintenance dose or working toward no longer taking the medication.
“In at least one study where they randomized kids to tapering during the school year or in the summer, the findings showed that the kids actually did better in the summer,” said Dr. Huxsahl. But follow-up is important during cessation because during the first 2-3 months is when there is the greatest risk for relapse or recurrence.
Dr. Whiteside and Dr. Huxsahl said they had no relevant financial disclosures.
Effective management of depression and other mood disorders in children may involve both pharmacotherapy with behavioral recommendations and psychotherapy, according to Stephen P. Whiteside, Ph.D., and Dr. John Huxsahl of the Mayo Clinic in Rochester Minn.
In a presentation at the American Academy of Pediatrics annual meeting in Washington, they reviewed the differences between various mood disorders because accurate diagnosis is a key component to management.
A major depressive episode is among the more common mood disorders among children, but others include disruptive mood dysregulation disorder, persistent depressive disorder, and bipolar I disorder. Because most pediatricians have just 15 minutes or so with patients, Dr. Whiteside and Dr. Huxsahl recommended using screening questionnaires to identify children at risk for a mood disorder. Trying to determine why a child is experiencing these symptoms or feelings can help clinicians determine whether this is a chronic issue or a situational one. Asking about other concerns, such as anxiety or use of substances, also can help with diagnosis.
Depressive episodes
Occurring among approximately 2% of children and 4%-8% of adolescents, a major depressive episode lasts a median of 8 months, but has a very high rate of recurrence: 20%-60% at 1 or 2 years after remission and 70% at 5 years after remission. In a significant proportion of children – about 20%-40% – a major depressive episode forecasts bipolar disorder.
In fact, having a parent with a mood disorder doubles to quadruples a child’s risk of major depressive episodes, and the disorder frequently occurs with anxiety, estimated in approximately 61%-65% of children with a major depressive episode. An episode generally appears to result from a combination of genetic factors and environmental ones, including abuse, neglect, family conflict, childhood adversity, losses, and comorbid disorders.
More heterogeneous than in adults, major depressive episodes in children look different based on a child’s age, according to Dr. Whiteside and Dr. Huxsahl:
• Birth to age 2 years. Symptoms include whining, decreased growth, lack of responsiveness, disrupted sleep, and excessive fears.
• Ages 3-5 years. Symptoms include anxiety, somatic symptoms, tantrums, sadness, weight gain, tiredness/sleepiness, suicidal ideation, anger or irritability, apathy, illness, and social withdrawal
• Ages 6-12 years. Symptoms include sadness, an inability to experience pleasure, decreased energy, low self-esteem, irritability, and suicidal ideation.
• Ages 12-18 years. Symptoms include a volatile mood, rage, acting out, self-consciousness, withdrawal, suicidal ideation, and overeating and/or oversleeping.
Other mood disorders
Disruptive mood dysregulation disorder (DMDD) involves much more acting out at a younger age than major depressive episodes. In children with DMDD, outbursts greatly exceed what would be expected in response to a situation, whether in terms of how long the tantrums last or how intense they are. These outbursts also are unexpected developmentally, with an onset before age 10 years, although the disorder should not be diagnosed earlier than age 6 years. Diagnostic criteria also require that the outbursts occur at least three times weekly, on average, for at least 12 months in at least two settings with a persistently angry or irritable mood between outbursts.
One way to support a diagnosis of DMDD is to rule out what it’s not. DMDD can exist with comorbidities of major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), conduct disorder, and substance use disorders, and it’s most likely to grow into depression or anxiety. It cannot coexist with oppositional defiant disorder, intermittent explosive disorder, or bipolar disorder, and it does not develop into bipolar disorder.
Persistent depressive disorder resembles MDD, with either a depressed mood lasting at least 2 years or an irritable mood lasting at least 1 year plus at least two of the following symptoms: poor appetite or overeating; insomnia or hypersomnia; low energy or fatigue; poor concretion or difficulty making decisions; feelings of hopelessness; and low self-esteem.
Bipolar disorder
“The most important part of managing bipolar I is episodes, episodes, episodes,” Dr. Whiteside said. Alternating with either hypomanic or major depressive episodes are manic episodes, in which a child experiences an abnormal “high,” a period of at least a week of extremely high energy nearly all day, every day, with a “persistently elevated, expansive, or irritable mood.”
For an episode to be considered manic, however, at least three of the following seven other symptoms must be present, and deviate from the patient’s otherwise normal behavior: grandiosity or an especially inflated sense of self-esteem; a decreased need for sleep (not just that the patient doesn’t sleep but doesn’t actually seem to need to); extreme talkativeness or use of pressured speech; a feeling of having racing thoughts or ideas flying about; a tendency to be easily distracted or unfocused; an increase in activities aimed at accomplishing certain goals (in any sphere, for example, school, work, social, or sexual); and risky behaviors with potentially severe, long-term consequences, such as more frequent sexual behavior or shopping sprees.
Dr. Whiteside compared bipolar disorder to ADHD to help clinicians appreciate how to best understand it. Key differences with ADHD are an earlier age of onset, a more consistent presence of symptoms without episodes, a need for sleep despite possible insomnia, and only situational depression. In a 1998 study, a comparison of bipolar I and ADHD revealed that 89% of bipolar children had an elevated mood, compared with just 14% of those with ADHD. A similar gulf existed with grandiose thoughts, occurring in 86% of children with bipolar disorder and 5% of those with ADHD. Likewise, a much greater proportion of children with bipolar disorder experienced “flights of ideas,” decreased sleep, or hypersexuality, compared with these symptoms in children with ADHD.
Treating depression: Behavioral interventions
Psychopharmacology can be beneficial, but it works best when combined with behavioral interventions and simply explaining the disorder, said Dr. Whiteside. “There’s a lot of evidence that exercise can be a powerful treatment with depression,” he said. “Education is especially important since one of the four features of depression is a sense of hopelessness and belief that nothing is going to [get] better, so for them to know there are steps they can take is a very powerful intervention itself.”
The most effective intervention, however, is cognitive-behavioral therapy, with an 81% success rate 1 year out and a 98% success rate 2 years out. After the initial intervention, however, the numbers are more modest, with 67% of children no longer meeting major depressive disorder criteria when undergoing cognitive-behavioral therapy, compared with 48% in the waiting-list control group.
An important aspect to education starts with developing an alliance with a family so that the clinician can explain the condition and its treatment, build trust, and instill hope. In doing so, both parents’ and children’s expectations can be adjusted so that children feel less guilty and parents respond less negatively to their children.
Treatment with medication
Although only fluoxetine and escitalopram have Food and Drug Administration approval for MDD, Dr. Huxsahl recommended starting kids with mixed anxiety and depression on fluoxetine, fluvoxamine, or sertraline, and suggested clinicians not shy away from the target doses recommended for each drug. After 2-4 months of acute treatment, clinicians also should help families understand that continuing the treatment for at least 4-9 months and then potentially taking a maintenance dose for up to 3 years may be recommended.
“I would encourage you to educate the parents, and especially the teenagers, that they need to take the medication beyond the time that they are depressed,” he explained. Initially, however, start with a low dose and then increase it within 4 weeks if no response occurs. After 8 weeks, switch agents if no response has occurred, a process that requires monthly visits for the first 3-6 months of treatment.
Another question families and clinicians face is when to taper off medication when moving to a maintenance dose or working toward no longer taking the medication.
“In at least one study where they randomized kids to tapering during the school year or in the summer, the findings showed that the kids actually did better in the summer,” said Dr. Huxsahl. But follow-up is important during cessation because during the first 2-3 months is when there is the greatest risk for relapse or recurrence.
Dr. Whiteside and Dr. Huxsahl said they had no relevant financial disclosures.
Effective management of depression and other mood disorders in children may involve both pharmacotherapy with behavioral recommendations and psychotherapy, according to Stephen P. Whiteside, Ph.D., and Dr. John Huxsahl of the Mayo Clinic in Rochester Minn.
In a presentation at the American Academy of Pediatrics annual meeting in Washington, they reviewed the differences between various mood disorders because accurate diagnosis is a key component to management.
A major depressive episode is among the more common mood disorders among children, but others include disruptive mood dysregulation disorder, persistent depressive disorder, and bipolar I disorder. Because most pediatricians have just 15 minutes or so with patients, Dr. Whiteside and Dr. Huxsahl recommended using screening questionnaires to identify children at risk for a mood disorder. Trying to determine why a child is experiencing these symptoms or feelings can help clinicians determine whether this is a chronic issue or a situational one. Asking about other concerns, such as anxiety or use of substances, also can help with diagnosis.
Depressive episodes
Occurring among approximately 2% of children and 4%-8% of adolescents, a major depressive episode lasts a median of 8 months, but has a very high rate of recurrence: 20%-60% at 1 or 2 years after remission and 70% at 5 years after remission. In a significant proportion of children – about 20%-40% – a major depressive episode forecasts bipolar disorder.
In fact, having a parent with a mood disorder doubles to quadruples a child’s risk of major depressive episodes, and the disorder frequently occurs with anxiety, estimated in approximately 61%-65% of children with a major depressive episode. An episode generally appears to result from a combination of genetic factors and environmental ones, including abuse, neglect, family conflict, childhood adversity, losses, and comorbid disorders.
More heterogeneous than in adults, major depressive episodes in children look different based on a child’s age, according to Dr. Whiteside and Dr. Huxsahl:
• Birth to age 2 years. Symptoms include whining, decreased growth, lack of responsiveness, disrupted sleep, and excessive fears.
• Ages 3-5 years. Symptoms include anxiety, somatic symptoms, tantrums, sadness, weight gain, tiredness/sleepiness, suicidal ideation, anger or irritability, apathy, illness, and social withdrawal
• Ages 6-12 years. Symptoms include sadness, an inability to experience pleasure, decreased energy, low self-esteem, irritability, and suicidal ideation.
• Ages 12-18 years. Symptoms include a volatile mood, rage, acting out, self-consciousness, withdrawal, suicidal ideation, and overeating and/or oversleeping.
Other mood disorders
Disruptive mood dysregulation disorder (DMDD) involves much more acting out at a younger age than major depressive episodes. In children with DMDD, outbursts greatly exceed what would be expected in response to a situation, whether in terms of how long the tantrums last or how intense they are. These outbursts also are unexpected developmentally, with an onset before age 10 years, although the disorder should not be diagnosed earlier than age 6 years. Diagnostic criteria also require that the outbursts occur at least three times weekly, on average, for at least 12 months in at least two settings with a persistently angry or irritable mood between outbursts.
One way to support a diagnosis of DMDD is to rule out what it’s not. DMDD can exist with comorbidities of major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), conduct disorder, and substance use disorders, and it’s most likely to grow into depression or anxiety. It cannot coexist with oppositional defiant disorder, intermittent explosive disorder, or bipolar disorder, and it does not develop into bipolar disorder.
Persistent depressive disorder resembles MDD, with either a depressed mood lasting at least 2 years or an irritable mood lasting at least 1 year plus at least two of the following symptoms: poor appetite or overeating; insomnia or hypersomnia; low energy or fatigue; poor concretion or difficulty making decisions; feelings of hopelessness; and low self-esteem.
Bipolar disorder
“The most important part of managing bipolar I is episodes, episodes, episodes,” Dr. Whiteside said. Alternating with either hypomanic or major depressive episodes are manic episodes, in which a child experiences an abnormal “high,” a period of at least a week of extremely high energy nearly all day, every day, with a “persistently elevated, expansive, or irritable mood.”
For an episode to be considered manic, however, at least three of the following seven other symptoms must be present, and deviate from the patient’s otherwise normal behavior: grandiosity or an especially inflated sense of self-esteem; a decreased need for sleep (not just that the patient doesn’t sleep but doesn’t actually seem to need to); extreme talkativeness or use of pressured speech; a feeling of having racing thoughts or ideas flying about; a tendency to be easily distracted or unfocused; an increase in activities aimed at accomplishing certain goals (in any sphere, for example, school, work, social, or sexual); and risky behaviors with potentially severe, long-term consequences, such as more frequent sexual behavior or shopping sprees.
Dr. Whiteside compared bipolar disorder to ADHD to help clinicians appreciate how to best understand it. Key differences with ADHD are an earlier age of onset, a more consistent presence of symptoms without episodes, a need for sleep despite possible insomnia, and only situational depression. In a 1998 study, a comparison of bipolar I and ADHD revealed that 89% of bipolar children had an elevated mood, compared with just 14% of those with ADHD. A similar gulf existed with grandiose thoughts, occurring in 86% of children with bipolar disorder and 5% of those with ADHD. Likewise, a much greater proportion of children with bipolar disorder experienced “flights of ideas,” decreased sleep, or hypersexuality, compared with these symptoms in children with ADHD.
Treating depression: Behavioral interventions
Psychopharmacology can be beneficial, but it works best when combined with behavioral interventions and simply explaining the disorder, said Dr. Whiteside. “There’s a lot of evidence that exercise can be a powerful treatment with depression,” he said. “Education is especially important since one of the four features of depression is a sense of hopelessness and belief that nothing is going to [get] better, so for them to know there are steps they can take is a very powerful intervention itself.”
The most effective intervention, however, is cognitive-behavioral therapy, with an 81% success rate 1 year out and a 98% success rate 2 years out. After the initial intervention, however, the numbers are more modest, with 67% of children no longer meeting major depressive disorder criteria when undergoing cognitive-behavioral therapy, compared with 48% in the waiting-list control group.
An important aspect to education starts with developing an alliance with a family so that the clinician can explain the condition and its treatment, build trust, and instill hope. In doing so, both parents’ and children’s expectations can be adjusted so that children feel less guilty and parents respond less negatively to their children.
Treatment with medication
Although only fluoxetine and escitalopram have Food and Drug Administration approval for MDD, Dr. Huxsahl recommended starting kids with mixed anxiety and depression on fluoxetine, fluvoxamine, or sertraline, and suggested clinicians not shy away from the target doses recommended for each drug. After 2-4 months of acute treatment, clinicians also should help families understand that continuing the treatment for at least 4-9 months and then potentially taking a maintenance dose for up to 3 years may be recommended.
“I would encourage you to educate the parents, and especially the teenagers, that they need to take the medication beyond the time that they are depressed,” he explained. Initially, however, start with a low dose and then increase it within 4 weeks if no response occurs. After 8 weeks, switch agents if no response has occurred, a process that requires monthly visits for the first 3-6 months of treatment.
Another question families and clinicians face is when to taper off medication when moving to a maintenance dose or working toward no longer taking the medication.
“In at least one study where they randomized kids to tapering during the school year or in the summer, the findings showed that the kids actually did better in the summer,” said Dr. Huxsahl. But follow-up is important during cessation because during the first 2-3 months is when there is the greatest risk for relapse or recurrence.
Dr. Whiteside and Dr. Huxsahl said they had no relevant financial disclosures.