User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Lifestyle Medicine: Not Just for the Wealthy
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Time-Restricted Eating Is Not a Metabolic Magic Bullet
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.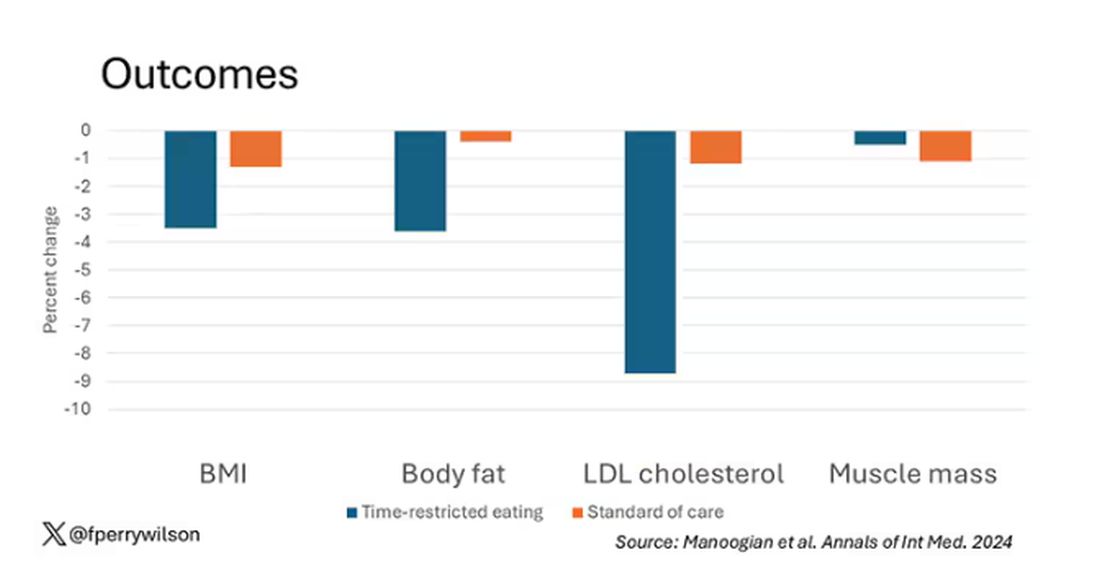
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.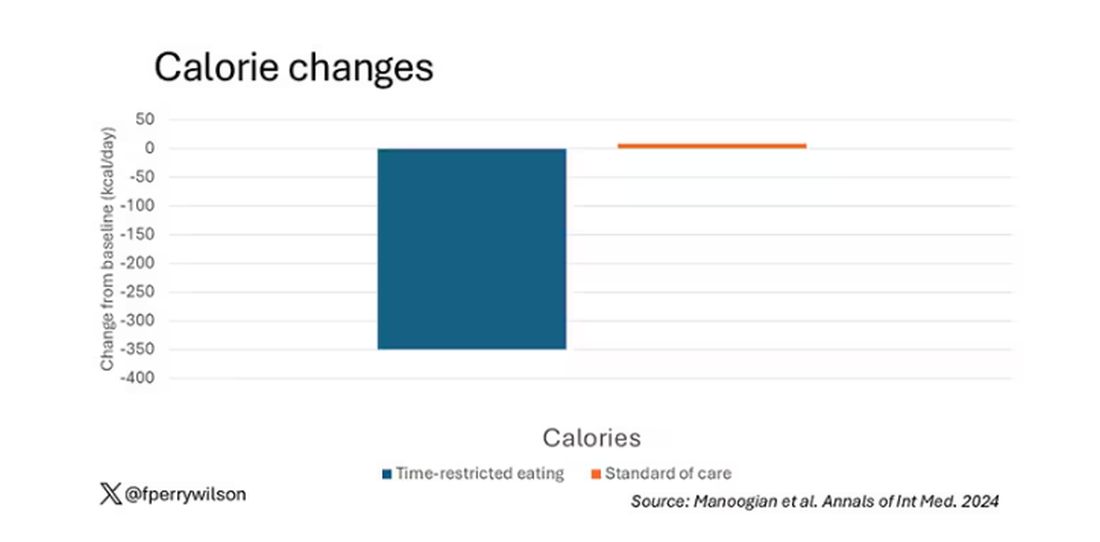
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 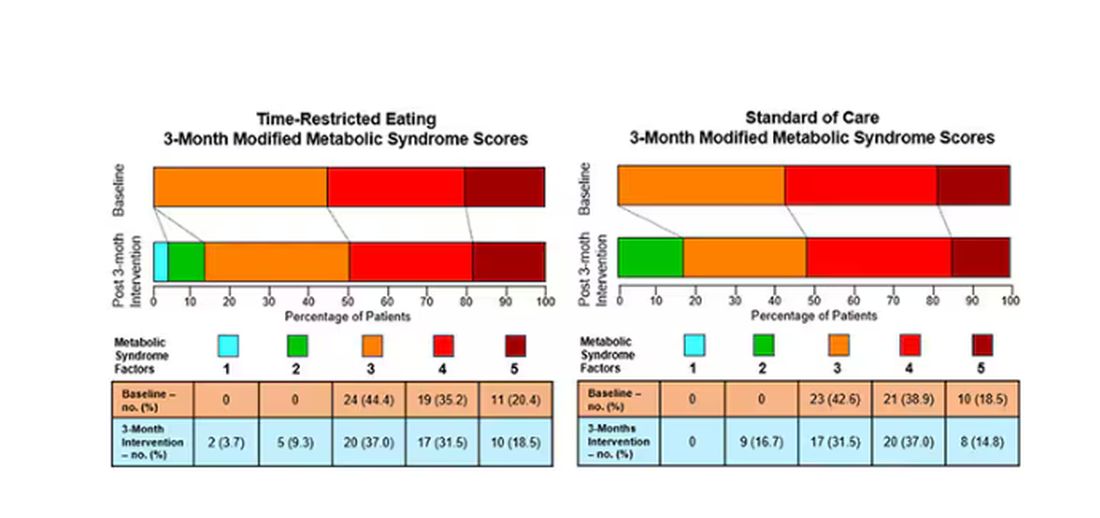
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Detecting Type 2 Diabetes Through Voice: How Does It Work?
An international study, Colive Voice, presented at the European Association for the Study of Diabetes (EASD) 2024 conference, shows that These results “open up possibilities for developing a first-line, noninvasive, and rapid screening tool for T2D, feasible with just a few seconds of voice recording on a smartphone or during consultations,” explained the study’s principal investigator Guy Fagherazzi, PhD, a diabetes epidemiologist at the Luxembourg Institute of Health, in an interview with this news organization.
How did the idea of detecting diabetes through voice come about?
During the COVID-19 pandemic, we began analyzing voice recordings from patients with chronic diseases. We wanted to find solutions to assess people’s health remotely, without physical contact. We quickly realized that this approach could be extended to other diseases. Because my main research focus has always been diabetes, I looked into how voice characteristics might correlate with diabetes. Previous studies had indicated that patients with diabetes have distinct voices compared with the general population, and this insight formed the starting point.
What mechanism could explain why patients with T2D have different voice characteristics?
It’s challenging to pinpoint a single factor that would explain why patients with T2D have different voices from those without diabetes. Several factors are involved.
Some biological mechanisms, especially those affecting the vascular system, influence symptoms in people with metabolic diseases such as diabetes. For example, people with T2D have more frequent cardiorespiratory fatigue. Obesity and overweight are also key factors, as these conditions can slightly alter vocal parameters compared with people of normal weight. Hypertension, common in patients with T2D, adds to the complexity.
Neurologic complications can affect the nerves and muscles involved in voice production, particularly the vocal cords.
Therefore, respiratory fatigue, neuropathies, and other conditions such as dehydration and gastric acid reflux, which are more common in patients with diabetes, can contribute to differences in voice.
These differences might not be noticeable to the human ear. That’s why we often don’t notice the link between voice and diabetes. However, technological advancements in signal processing and artificial intelligence allow us to extract a large amount of information from these subtle variations. By analyzing these small differences, we can detect diabetes with a reasonable degree of accuracy.
In your study, you mention that voice tone can indicate diabetic status. Could you elaborate?
Yes, voice tone can be affected, though it’s a complex, multidimensional phenomenon.
Patients who have had diabetes for 5-10 years, or longer, tend to have a rougher voice than those without diabetes of the same age and gender. In our study, we were able to extract many voice characteristics from the raw audio signal, which is why it’s difficult to isolate one specific factor that stands out.
Is there a difference in voice changes between patients with well-managed diabetes and those whose disease is uncontrolled?
The roughness of the voice tends to increase with the duration of diabetes. It’s more noticeable in people with poorly controlled diabetes. Our hypothesis, based on the results we presented at the EASD conference, is that fluctuations in blood sugar levels, both hypo- and hyperglycemia, may cause short-term changes in the voice. There are also many subtle, rapid changes that could potentially be detected, though we haven’t confirmed this yet. We’re currently conducting additional studies to explore this.
Why did you ask participants to read a passage from the Universal Declaration of Human Rights?
We used a highly standardized approach. Participants completed several recordings, including holding the sound “Aaaaaa” for as long as possible in one breath. They also read a passage, which helps us better distinguish between patients with and those without diabetes. This method works slightly better than other sounds typically used for analyzing diseases. We chose this particular text in the participant’s native language because it’s neutral and doesn’t trigger emotional fluctuations. Because Colive Voice is an international, multilingual study, we use official translations in various languages.
Your research focuses on T2D. Do you plan to study type 1 diabetes (T1D) as well?
We believe that individuals with T1D also exhibit voice changes over time. However, our current focus is on T2D because our goal is to develop large-scale screening methods. T1D, typically diagnosed in childhood, requires different screening approaches. For now, our research mainly involves adults.
Were there any gender differences in the accuracy of your voice analysis?
Yes, voice studies generally show that women have different vocal signatures from men, partly owing to hormonal fluctuations that affect pitch and tone. Detecting differences between healthy individuals and those with diabetes can sometimes be more challenging in women, depending on the condition. In our study, we achieved about 70% accuracy for women compared with 75% for men.
The EASD results focused on a US-based population. When can we expect data from France?
We started with the US because we could quickly gather a large number of patients. Now, we’re expanding to global and language-specific analyses. French data are certainly a priority, and we’re working on it. We encourage people to participate — it takes only 20 minutes and contributes to innovative research on noninvasive diabetes detection. Participants can sign up at www.colivevoice.org
Dr. Fagherazzi heads the Deep Digital Phenotyping laboratory and the Department of Precision Health at the Luxembourg Institute of Health. His research focuses on integrating new technologies and digital data into diabetes research. He has declared no relevant financial relationships.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
An international study, Colive Voice, presented at the European Association for the Study of Diabetes (EASD) 2024 conference, shows that These results “open up possibilities for developing a first-line, noninvasive, and rapid screening tool for T2D, feasible with just a few seconds of voice recording on a smartphone or during consultations,” explained the study’s principal investigator Guy Fagherazzi, PhD, a diabetes epidemiologist at the Luxembourg Institute of Health, in an interview with this news organization.
How did the idea of detecting diabetes through voice come about?
During the COVID-19 pandemic, we began analyzing voice recordings from patients with chronic diseases. We wanted to find solutions to assess people’s health remotely, without physical contact. We quickly realized that this approach could be extended to other diseases. Because my main research focus has always been diabetes, I looked into how voice characteristics might correlate with diabetes. Previous studies had indicated that patients with diabetes have distinct voices compared with the general population, and this insight formed the starting point.
What mechanism could explain why patients with T2D have different voice characteristics?
It’s challenging to pinpoint a single factor that would explain why patients with T2D have different voices from those without diabetes. Several factors are involved.
Some biological mechanisms, especially those affecting the vascular system, influence symptoms in people with metabolic diseases such as diabetes. For example, people with T2D have more frequent cardiorespiratory fatigue. Obesity and overweight are also key factors, as these conditions can slightly alter vocal parameters compared with people of normal weight. Hypertension, common in patients with T2D, adds to the complexity.
Neurologic complications can affect the nerves and muscles involved in voice production, particularly the vocal cords.
Therefore, respiratory fatigue, neuropathies, and other conditions such as dehydration and gastric acid reflux, which are more common in patients with diabetes, can contribute to differences in voice.
These differences might not be noticeable to the human ear. That’s why we often don’t notice the link between voice and diabetes. However, technological advancements in signal processing and artificial intelligence allow us to extract a large amount of information from these subtle variations. By analyzing these small differences, we can detect diabetes with a reasonable degree of accuracy.
In your study, you mention that voice tone can indicate diabetic status. Could you elaborate?
Yes, voice tone can be affected, though it’s a complex, multidimensional phenomenon.
Patients who have had diabetes for 5-10 years, or longer, tend to have a rougher voice than those without diabetes of the same age and gender. In our study, we were able to extract many voice characteristics from the raw audio signal, which is why it’s difficult to isolate one specific factor that stands out.
Is there a difference in voice changes between patients with well-managed diabetes and those whose disease is uncontrolled?
The roughness of the voice tends to increase with the duration of diabetes. It’s more noticeable in people with poorly controlled diabetes. Our hypothesis, based on the results we presented at the EASD conference, is that fluctuations in blood sugar levels, both hypo- and hyperglycemia, may cause short-term changes in the voice. There are also many subtle, rapid changes that could potentially be detected, though we haven’t confirmed this yet. We’re currently conducting additional studies to explore this.
Why did you ask participants to read a passage from the Universal Declaration of Human Rights?
We used a highly standardized approach. Participants completed several recordings, including holding the sound “Aaaaaa” for as long as possible in one breath. They also read a passage, which helps us better distinguish between patients with and those without diabetes. This method works slightly better than other sounds typically used for analyzing diseases. We chose this particular text in the participant’s native language because it’s neutral and doesn’t trigger emotional fluctuations. Because Colive Voice is an international, multilingual study, we use official translations in various languages.
Your research focuses on T2D. Do you plan to study type 1 diabetes (T1D) as well?
We believe that individuals with T1D also exhibit voice changes over time. However, our current focus is on T2D because our goal is to develop large-scale screening methods. T1D, typically diagnosed in childhood, requires different screening approaches. For now, our research mainly involves adults.
Were there any gender differences in the accuracy of your voice analysis?
Yes, voice studies generally show that women have different vocal signatures from men, partly owing to hormonal fluctuations that affect pitch and tone. Detecting differences between healthy individuals and those with diabetes can sometimes be more challenging in women, depending on the condition. In our study, we achieved about 70% accuracy for women compared with 75% for men.
The EASD results focused on a US-based population. When can we expect data from France?
We started with the US because we could quickly gather a large number of patients. Now, we’re expanding to global and language-specific analyses. French data are certainly a priority, and we’re working on it. We encourage people to participate — it takes only 20 minutes and contributes to innovative research on noninvasive diabetes detection. Participants can sign up at www.colivevoice.org
Dr. Fagherazzi heads the Deep Digital Phenotyping laboratory and the Department of Precision Health at the Luxembourg Institute of Health. His research focuses on integrating new technologies and digital data into diabetes research. He has declared no relevant financial relationships.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
An international study, Colive Voice, presented at the European Association for the Study of Diabetes (EASD) 2024 conference, shows that These results “open up possibilities for developing a first-line, noninvasive, and rapid screening tool for T2D, feasible with just a few seconds of voice recording on a smartphone or during consultations,” explained the study’s principal investigator Guy Fagherazzi, PhD, a diabetes epidemiologist at the Luxembourg Institute of Health, in an interview with this news organization.
How did the idea of detecting diabetes through voice come about?
During the COVID-19 pandemic, we began analyzing voice recordings from patients with chronic diseases. We wanted to find solutions to assess people’s health remotely, without physical contact. We quickly realized that this approach could be extended to other diseases. Because my main research focus has always been diabetes, I looked into how voice characteristics might correlate with diabetes. Previous studies had indicated that patients with diabetes have distinct voices compared with the general population, and this insight formed the starting point.
What mechanism could explain why patients with T2D have different voice characteristics?
It’s challenging to pinpoint a single factor that would explain why patients with T2D have different voices from those without diabetes. Several factors are involved.
Some biological mechanisms, especially those affecting the vascular system, influence symptoms in people with metabolic diseases such as diabetes. For example, people with T2D have more frequent cardiorespiratory fatigue. Obesity and overweight are also key factors, as these conditions can slightly alter vocal parameters compared with people of normal weight. Hypertension, common in patients with T2D, adds to the complexity.
Neurologic complications can affect the nerves and muscles involved in voice production, particularly the vocal cords.
Therefore, respiratory fatigue, neuropathies, and other conditions such as dehydration and gastric acid reflux, which are more common in patients with diabetes, can contribute to differences in voice.
These differences might not be noticeable to the human ear. That’s why we often don’t notice the link between voice and diabetes. However, technological advancements in signal processing and artificial intelligence allow us to extract a large amount of information from these subtle variations. By analyzing these small differences, we can detect diabetes with a reasonable degree of accuracy.
In your study, you mention that voice tone can indicate diabetic status. Could you elaborate?
Yes, voice tone can be affected, though it’s a complex, multidimensional phenomenon.
Patients who have had diabetes for 5-10 years, or longer, tend to have a rougher voice than those without diabetes of the same age and gender. In our study, we were able to extract many voice characteristics from the raw audio signal, which is why it’s difficult to isolate one specific factor that stands out.
Is there a difference in voice changes between patients with well-managed diabetes and those whose disease is uncontrolled?
The roughness of the voice tends to increase with the duration of diabetes. It’s more noticeable in people with poorly controlled diabetes. Our hypothesis, based on the results we presented at the EASD conference, is that fluctuations in blood sugar levels, both hypo- and hyperglycemia, may cause short-term changes in the voice. There are also many subtle, rapid changes that could potentially be detected, though we haven’t confirmed this yet. We’re currently conducting additional studies to explore this.
Why did you ask participants to read a passage from the Universal Declaration of Human Rights?
We used a highly standardized approach. Participants completed several recordings, including holding the sound “Aaaaaa” for as long as possible in one breath. They also read a passage, which helps us better distinguish between patients with and those without diabetes. This method works slightly better than other sounds typically used for analyzing diseases. We chose this particular text in the participant’s native language because it’s neutral and doesn’t trigger emotional fluctuations. Because Colive Voice is an international, multilingual study, we use official translations in various languages.
Your research focuses on T2D. Do you plan to study type 1 diabetes (T1D) as well?
We believe that individuals with T1D also exhibit voice changes over time. However, our current focus is on T2D because our goal is to develop large-scale screening methods. T1D, typically diagnosed in childhood, requires different screening approaches. For now, our research mainly involves adults.
Were there any gender differences in the accuracy of your voice analysis?
Yes, voice studies generally show that women have different vocal signatures from men, partly owing to hormonal fluctuations that affect pitch and tone. Detecting differences between healthy individuals and those with diabetes can sometimes be more challenging in women, depending on the condition. In our study, we achieved about 70% accuracy for women compared with 75% for men.
The EASD results focused on a US-based population. When can we expect data from France?
We started with the US because we could quickly gather a large number of patients. Now, we’re expanding to global and language-specific analyses. French data are certainly a priority, and we’re working on it. We encourage people to participate — it takes only 20 minutes and contributes to innovative research on noninvasive diabetes detection. Participants can sign up at www.colivevoice.org
Dr. Fagherazzi heads the Deep Digital Phenotyping laboratory and the Department of Precision Health at the Luxembourg Institute of Health. His research focuses on integrating new technologies and digital data into diabetes research. He has declared no relevant financial relationships.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM EASD 2024
Cannabis Use Rising in Diabetes: What Do Endos Need to Know?
A recent US prevalence study estimated that 9% adults with diabetes used cannabis in the last month, a 33.7% increase between 2021 and 2022. Nearly half (48.9%) of users were younger than 50 years.
Cannabis use is also increasing sharply among those aged 65 years or older, many of whom have diabetes and other chronic conditions. In this demographic, the perceived risk surrounding regular cannabis use has dropped significantly, even as the data tell another story — that they are particularly at risk from emergency department visits for cannabis poisoning.
As legalization continues and cannabis products proliferate, endocrinologists will likely face more patients of all ages seeking advice about its use. Yet with few evidence-based resources to turn to, endocrinologists advising patients in this area are mostly left fending for themselves.
Evidence ‘Limited’
“The evidence on cannabis is limited mainly because of its scheduling in the United States,” Jay Shubrook, DO, a professor and diabetologist at College of Osteopathic Medicine, Touro University California, in Vallejo, California, told this news organization.
“It was declared to be a schedule I drug in the 1970s, which meant it was ‘dangerous’ and ‘had no medical benefit.’ This made it hard to access and study in human trials.”
That will likely change soon. On May 16, 2024, the US Department of Justice submitted a proposal to move marijuana from a schedule I to a schedule III drug under the Controlled Substances Act, emphasizing its accepted medical use. If approved, the door will open to more investigators seeking to study the effects of cannabis.
Yet, even in Canada, where recreational use has been legal since 2018 and cannabis is sold widely with government support, there are little hard data to guide practice. In 2019, Diabetes Canada issued a position statement on recreational cannabis use in people with type 1 diabetes (T1D) and type 2 diabetes (T2D). It sought to evaluate the effects of cannabis on metabolic factors and diabetes complications, as well as self-management behaviors in those aged 13 years or older.
The authors noted that five of the six studies upon which the statement was based did not consider or report the routes of cannabis administration, which have differing risks. In addition, their recommendations were based on grade D evidence and consensus.
What Patients Are Taking
Cannabis — also known as marijuana, weed, pot, or bud — refers to the dried flowers, leaves, stems, and seeds of the cannabis plant. The plant contains more than 100 compounds, including tetrahydrocannabinol (THC), which is responsible for the euphoric “high,” and other active compounds, including cannabidiol (CBD), which by itself is not mind-altering.
Cannabis can be ingested in several ways. It can be smoked (ie, joints, blunts, pipes, and water pipes), ingested in edible form (mixed or infused into foods), and inhaled using electronic vaporizing devices (ie, e-cigarettes or vape pens).
Compounds in cannabis can also be extracted to make oils and concentrates that can be vaped or inhaled. Smoking oils, concentrates, and extracts from the cannabis plant, known as “dabbing,” are on the rise in the United States.
There are no validated or standard dosage recommendations for cannabis strains and formulations, THC/CBD ratios, or modes of administration. Therefore, the Canadian Pharmacists Association prepared a guide for finding a safe and effective dose for medical purposes. GoodRx, a website with information on prescription drug prices, says that larger doses of THC pose greater risks, noting that the potency of cannabis has increased from 4% in 1995 to about 14% in 2019.
Potential Risks and Benefits: Canadian and US Perspectives
Health and safety risks vary with each of the different ways of using cannabis for individuals with and without diabetes, depending on a host of patient- and product-specific factors.
In a recent article proposing a “THC unit” for Canada’s legal cannabis market, researchers reported that consumers lack familiarity with THC levels, don’t know what constitutes a “low” or “high” THC amount, have trouble dosing, overconsume, and commonly experience adverse health events from cannabis use.
A recent study suggested that most clinicians are similarly uninformed, with “a lack of knowledge of beneficial effects, adverse effects, and of how to advise patients,” even for medical cannabis.
Diabetes Canada takes a stab at summarizing what’s known with respect to cannabis and diabetes, stating that:
“Research on recreational cannabis use suggests it may negatively impact diabetes metabolic factors and self-management behaviors. The safety of recreational cannabis use has not been demonstrated, whereas regular cannabis use is associated with worsening glycemic control, more diabetes-related complications, and poorer self-care behaviors, such as adequate glucose monitoring, adherence to medications, and compliance with dietary and physical activity recommendations for people living with both type 1 and type 2 diabetes.”
The American Diabetes Association’s information on cannabis consists of a patient-oriented article on CBD oil. The article stated:
“There’s a lot of hype surrounding CBD oil and diabetes. There is no noticeable effect on blood glucose (blood sugar) or insulin levels in people with type 2 diabetes. Researchers continue to study the effects of CBD on diabetes in animal studies.”
It concludes that:
“Although many claims continue to be made about CBD oil, there is little evidence of any benefit. It’s certainly not an alternative to traditional diabetes management. The safety of CBD is also unknown — it may have dangerous side effects that we won’t know about unless further research is done.”
A Roundup of Recent Studies
A smattering of recent studies have touched on various aspects of cannabis consumption and diabetes.
Angela Bryan, PhD, professor and co-director of CUChange at the University of Colorado Boulder, has been evaluating cannabis use in young adults (ages 21-40 years) in the SONIC study. Dr. Bryan reported at the American Diabetes Association (ADA) 84th Scientific Sessions that cannabis users were more likely to have a lower body mass index and less likely to develop T2D. Furthermore, chronic cannabis users were less likely to have measures of inflammation and no loss of insulin sensitivity.
Another study by Dr. Bryan’s group found that CBD-dominant forms of cannabis were associated with acute tension reduction, which might lead to longer-term reductions in anxiety. Bryan said the findings could be relevant in the context of diabetes distress.
Similarly positive results were found in a 15-week, double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD spray for neuropathic pain among treatment-resistant patients. The investigators reported that “clinically important improvements” were seen in pain, sleep quality, and subjective impressions of pain. Another small study of inhaled cannabis in treatment-refractory patients found a dose-dependent reduction in diabetic peripheral neuropathy pain.
Findings from a 9-year longitudinal study of approximately 18,000 Swedish men and women suggested no association between cannabis and subsequent T2D development after controlling for age, although these authors also called for longer follow-up and more detailed information about cannabis use to make “more robust” conclusions.
On the other side of the spectrum, a “rapid” review of recreational cannabis use in people with T1D and T2D found that recreational cannabis use may negatively impact diabetes metabolic factors and self-management behaviors and may increase risks for peripheral arterial occlusion, myocardial infarction, and renal disease. However, the authors cautioned that more robust research is needed to confirm the potential impact of cannabis on diabetes.
How to Advise Patients
When Dr. Shubrook was working with patients with diabetes in his family medicine practice in Ohio, cannabis wasn’t legal.
“’Don’t ask, don’t tell’ was the way we handled it then,” he said.
By contrast, in California, where he’s currently located, “it’s pretty well accepted and legal, and patients volunteer information about use, even if it’s recreational,” he said. “Realizing this was something we could talk about was really eye-opening to me.”
Talking to patients about cannabis use is a “20-minute conversation that details what they’re doing,” he said. He proceeds by asking questions: Are you using for recreational or medicinal purposes? What do you take? What do you take it for? Does it work?
“People will tell you,” Dr. Shubrook said. “They know exactly what it works or doesn’t work for and how it affects their glucose control, which in most cases is only minimally.”
He tells patients he would prefer they don’t inhale cannabis, given the risks posed to the lungs.
“Edibles may have a slower onset of effect, but depending on what they’re adding it to, glucose might be affected,” he noted. “And I have seen that chronic use can lead to hyperemesis syndrome.”
Overall, he said, “Take the time to talk to your patients about cannabis — it will allow them to be honest with you, and you can improve the specificity and safety of its use. If cannabis is legal in your state, encourage people to go to legal dispensaries, which will reduce the risk of it being laced with another drug that could increase the danger of use.”
A recent US prevalence study found that people with diabetes who use cannabis likely engage in other substance and psychoactive substance use, including tobacco use, binge drinking, and misuse of opioids and stimulants.
“Use of these additional substances could further exacerbate the health risks associated with diabetes and also emphasizes the importance of addressing polysubstance use among adults with diabetes,” the study’s author Benjamin H. Han, MD, Division of Geriatrics, Gerontology and Palliative Care, Department of Medicine, US San Diego School of Medicine in La Jolla, California, told this news organization.
“We were surprised at how strong the associations were, especially with use of substances that can increase cardiovascular risk,” Dr. Han added. “And given the strong association we found between cannabis use and use of other psychoactive substances in diabetes, clinicians must screen all their patients for psychoactive substance use.”
Diabetes Canada’s position paper states that despite the limited evidence, “there were sufficient data to begin developing recommendations for type 1 and type 2 diabetes about education, counseling, and management related to recreational cannabis use.”
Their recommendations include the following:
- Healthcare professionals should engage their patients in discussions about substance use on a regular basis, with a nonjudgmental approach.
- The use of recreational cannabis is not recommended for adolescents and adults with diabetes.
- People with T1D should avoid recreational cannabis use because of the increased risk for diabetic ketoacidosis.
- For adults with T1D or T2D who intend to use cannabis recreationally, individualized assessment and counseling should be offered to inform them of the general risks of cannabis, with a focus on harm reduction and reduction of the risk for potential adverse effects on diabetes management and complications.
- People with T1D or T2D should be offered education on and encouraged to read public information available through resources from various Canadian health authorities about the general risks of cannabis use to reduce the risk for nondiabetes-related adverse effects of cannabis consumption.
Of note, in 2018, the Canadian government produced an exhaustive compendium of information on cannabis for healthcare professionals that includes information relevant to managing patients with diabetes.
Dr. Shubrook and Dr. Han reported no competing interests.
A version of this article appeared on Medscape.com.
A recent US prevalence study estimated that 9% adults with diabetes used cannabis in the last month, a 33.7% increase between 2021 and 2022. Nearly half (48.9%) of users were younger than 50 years.
Cannabis use is also increasing sharply among those aged 65 years or older, many of whom have diabetes and other chronic conditions. In this demographic, the perceived risk surrounding regular cannabis use has dropped significantly, even as the data tell another story — that they are particularly at risk from emergency department visits for cannabis poisoning.
As legalization continues and cannabis products proliferate, endocrinologists will likely face more patients of all ages seeking advice about its use. Yet with few evidence-based resources to turn to, endocrinologists advising patients in this area are mostly left fending for themselves.
Evidence ‘Limited’
“The evidence on cannabis is limited mainly because of its scheduling in the United States,” Jay Shubrook, DO, a professor and diabetologist at College of Osteopathic Medicine, Touro University California, in Vallejo, California, told this news organization.
“It was declared to be a schedule I drug in the 1970s, which meant it was ‘dangerous’ and ‘had no medical benefit.’ This made it hard to access and study in human trials.”
That will likely change soon. On May 16, 2024, the US Department of Justice submitted a proposal to move marijuana from a schedule I to a schedule III drug under the Controlled Substances Act, emphasizing its accepted medical use. If approved, the door will open to more investigators seeking to study the effects of cannabis.
Yet, even in Canada, where recreational use has been legal since 2018 and cannabis is sold widely with government support, there are little hard data to guide practice. In 2019, Diabetes Canada issued a position statement on recreational cannabis use in people with type 1 diabetes (T1D) and type 2 diabetes (T2D). It sought to evaluate the effects of cannabis on metabolic factors and diabetes complications, as well as self-management behaviors in those aged 13 years or older.
The authors noted that five of the six studies upon which the statement was based did not consider or report the routes of cannabis administration, which have differing risks. In addition, their recommendations were based on grade D evidence and consensus.
What Patients Are Taking
Cannabis — also known as marijuana, weed, pot, or bud — refers to the dried flowers, leaves, stems, and seeds of the cannabis plant. The plant contains more than 100 compounds, including tetrahydrocannabinol (THC), which is responsible for the euphoric “high,” and other active compounds, including cannabidiol (CBD), which by itself is not mind-altering.
Cannabis can be ingested in several ways. It can be smoked (ie, joints, blunts, pipes, and water pipes), ingested in edible form (mixed or infused into foods), and inhaled using electronic vaporizing devices (ie, e-cigarettes or vape pens).
Compounds in cannabis can also be extracted to make oils and concentrates that can be vaped or inhaled. Smoking oils, concentrates, and extracts from the cannabis plant, known as “dabbing,” are on the rise in the United States.
There are no validated or standard dosage recommendations for cannabis strains and formulations, THC/CBD ratios, or modes of administration. Therefore, the Canadian Pharmacists Association prepared a guide for finding a safe and effective dose for medical purposes. GoodRx, a website with information on prescription drug prices, says that larger doses of THC pose greater risks, noting that the potency of cannabis has increased from 4% in 1995 to about 14% in 2019.
Potential Risks and Benefits: Canadian and US Perspectives
Health and safety risks vary with each of the different ways of using cannabis for individuals with and without diabetes, depending on a host of patient- and product-specific factors.
In a recent article proposing a “THC unit” for Canada’s legal cannabis market, researchers reported that consumers lack familiarity with THC levels, don’t know what constitutes a “low” or “high” THC amount, have trouble dosing, overconsume, and commonly experience adverse health events from cannabis use.
A recent study suggested that most clinicians are similarly uninformed, with “a lack of knowledge of beneficial effects, adverse effects, and of how to advise patients,” even for medical cannabis.
Diabetes Canada takes a stab at summarizing what’s known with respect to cannabis and diabetes, stating that:
“Research on recreational cannabis use suggests it may negatively impact diabetes metabolic factors and self-management behaviors. The safety of recreational cannabis use has not been demonstrated, whereas regular cannabis use is associated with worsening glycemic control, more diabetes-related complications, and poorer self-care behaviors, such as adequate glucose monitoring, adherence to medications, and compliance with dietary and physical activity recommendations for people living with both type 1 and type 2 diabetes.”
The American Diabetes Association’s information on cannabis consists of a patient-oriented article on CBD oil. The article stated:
“There’s a lot of hype surrounding CBD oil and diabetes. There is no noticeable effect on blood glucose (blood sugar) or insulin levels in people with type 2 diabetes. Researchers continue to study the effects of CBD on diabetes in animal studies.”
It concludes that:
“Although many claims continue to be made about CBD oil, there is little evidence of any benefit. It’s certainly not an alternative to traditional diabetes management. The safety of CBD is also unknown — it may have dangerous side effects that we won’t know about unless further research is done.”
A Roundup of Recent Studies
A smattering of recent studies have touched on various aspects of cannabis consumption and diabetes.
Angela Bryan, PhD, professor and co-director of CUChange at the University of Colorado Boulder, has been evaluating cannabis use in young adults (ages 21-40 years) in the SONIC study. Dr. Bryan reported at the American Diabetes Association (ADA) 84th Scientific Sessions that cannabis users were more likely to have a lower body mass index and less likely to develop T2D. Furthermore, chronic cannabis users were less likely to have measures of inflammation and no loss of insulin sensitivity.
Another study by Dr. Bryan’s group found that CBD-dominant forms of cannabis were associated with acute tension reduction, which might lead to longer-term reductions in anxiety. Bryan said the findings could be relevant in the context of diabetes distress.
Similarly positive results were found in a 15-week, double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD spray for neuropathic pain among treatment-resistant patients. The investigators reported that “clinically important improvements” were seen in pain, sleep quality, and subjective impressions of pain. Another small study of inhaled cannabis in treatment-refractory patients found a dose-dependent reduction in diabetic peripheral neuropathy pain.
Findings from a 9-year longitudinal study of approximately 18,000 Swedish men and women suggested no association between cannabis and subsequent T2D development after controlling for age, although these authors also called for longer follow-up and more detailed information about cannabis use to make “more robust” conclusions.
On the other side of the spectrum, a “rapid” review of recreational cannabis use in people with T1D and T2D found that recreational cannabis use may negatively impact diabetes metabolic factors and self-management behaviors and may increase risks for peripheral arterial occlusion, myocardial infarction, and renal disease. However, the authors cautioned that more robust research is needed to confirm the potential impact of cannabis on diabetes.
How to Advise Patients
When Dr. Shubrook was working with patients with diabetes in his family medicine practice in Ohio, cannabis wasn’t legal.
“’Don’t ask, don’t tell’ was the way we handled it then,” he said.
By contrast, in California, where he’s currently located, “it’s pretty well accepted and legal, and patients volunteer information about use, even if it’s recreational,” he said. “Realizing this was something we could talk about was really eye-opening to me.”
Talking to patients about cannabis use is a “20-minute conversation that details what they’re doing,” he said. He proceeds by asking questions: Are you using for recreational or medicinal purposes? What do you take? What do you take it for? Does it work?
“People will tell you,” Dr. Shubrook said. “They know exactly what it works or doesn’t work for and how it affects their glucose control, which in most cases is only minimally.”
He tells patients he would prefer they don’t inhale cannabis, given the risks posed to the lungs.
“Edibles may have a slower onset of effect, but depending on what they’re adding it to, glucose might be affected,” he noted. “And I have seen that chronic use can lead to hyperemesis syndrome.”
Overall, he said, “Take the time to talk to your patients about cannabis — it will allow them to be honest with you, and you can improve the specificity and safety of its use. If cannabis is legal in your state, encourage people to go to legal dispensaries, which will reduce the risk of it being laced with another drug that could increase the danger of use.”
A recent US prevalence study found that people with diabetes who use cannabis likely engage in other substance and psychoactive substance use, including tobacco use, binge drinking, and misuse of opioids and stimulants.
“Use of these additional substances could further exacerbate the health risks associated with diabetes and also emphasizes the importance of addressing polysubstance use among adults with diabetes,” the study’s author Benjamin H. Han, MD, Division of Geriatrics, Gerontology and Palliative Care, Department of Medicine, US San Diego School of Medicine in La Jolla, California, told this news organization.
“We were surprised at how strong the associations were, especially with use of substances that can increase cardiovascular risk,” Dr. Han added. “And given the strong association we found between cannabis use and use of other psychoactive substances in diabetes, clinicians must screen all their patients for psychoactive substance use.”
Diabetes Canada’s position paper states that despite the limited evidence, “there were sufficient data to begin developing recommendations for type 1 and type 2 diabetes about education, counseling, and management related to recreational cannabis use.”
Their recommendations include the following:
- Healthcare professionals should engage their patients in discussions about substance use on a regular basis, with a nonjudgmental approach.
- The use of recreational cannabis is not recommended for adolescents and adults with diabetes.
- People with T1D should avoid recreational cannabis use because of the increased risk for diabetic ketoacidosis.
- For adults with T1D or T2D who intend to use cannabis recreationally, individualized assessment and counseling should be offered to inform them of the general risks of cannabis, with a focus on harm reduction and reduction of the risk for potential adverse effects on diabetes management and complications.
- People with T1D or T2D should be offered education on and encouraged to read public information available through resources from various Canadian health authorities about the general risks of cannabis use to reduce the risk for nondiabetes-related adverse effects of cannabis consumption.
Of note, in 2018, the Canadian government produced an exhaustive compendium of information on cannabis for healthcare professionals that includes information relevant to managing patients with diabetes.
Dr. Shubrook and Dr. Han reported no competing interests.
A version of this article appeared on Medscape.com.
A recent US prevalence study estimated that 9% adults with diabetes used cannabis in the last month, a 33.7% increase between 2021 and 2022. Nearly half (48.9%) of users were younger than 50 years.
Cannabis use is also increasing sharply among those aged 65 years or older, many of whom have diabetes and other chronic conditions. In this demographic, the perceived risk surrounding regular cannabis use has dropped significantly, even as the data tell another story — that they are particularly at risk from emergency department visits for cannabis poisoning.
As legalization continues and cannabis products proliferate, endocrinologists will likely face more patients of all ages seeking advice about its use. Yet with few evidence-based resources to turn to, endocrinologists advising patients in this area are mostly left fending for themselves.
Evidence ‘Limited’
“The evidence on cannabis is limited mainly because of its scheduling in the United States,” Jay Shubrook, DO, a professor and diabetologist at College of Osteopathic Medicine, Touro University California, in Vallejo, California, told this news organization.
“It was declared to be a schedule I drug in the 1970s, which meant it was ‘dangerous’ and ‘had no medical benefit.’ This made it hard to access and study in human trials.”
That will likely change soon. On May 16, 2024, the US Department of Justice submitted a proposal to move marijuana from a schedule I to a schedule III drug under the Controlled Substances Act, emphasizing its accepted medical use. If approved, the door will open to more investigators seeking to study the effects of cannabis.
Yet, even in Canada, where recreational use has been legal since 2018 and cannabis is sold widely with government support, there are little hard data to guide practice. In 2019, Diabetes Canada issued a position statement on recreational cannabis use in people with type 1 diabetes (T1D) and type 2 diabetes (T2D). It sought to evaluate the effects of cannabis on metabolic factors and diabetes complications, as well as self-management behaviors in those aged 13 years or older.
The authors noted that five of the six studies upon which the statement was based did not consider or report the routes of cannabis administration, which have differing risks. In addition, their recommendations were based on grade D evidence and consensus.
What Patients Are Taking
Cannabis — also known as marijuana, weed, pot, or bud — refers to the dried flowers, leaves, stems, and seeds of the cannabis plant. The plant contains more than 100 compounds, including tetrahydrocannabinol (THC), which is responsible for the euphoric “high,” and other active compounds, including cannabidiol (CBD), which by itself is not mind-altering.
Cannabis can be ingested in several ways. It can be smoked (ie, joints, blunts, pipes, and water pipes), ingested in edible form (mixed or infused into foods), and inhaled using electronic vaporizing devices (ie, e-cigarettes or vape pens).
Compounds in cannabis can also be extracted to make oils and concentrates that can be vaped or inhaled. Smoking oils, concentrates, and extracts from the cannabis plant, known as “dabbing,” are on the rise in the United States.
There are no validated or standard dosage recommendations for cannabis strains and formulations, THC/CBD ratios, or modes of administration. Therefore, the Canadian Pharmacists Association prepared a guide for finding a safe and effective dose for medical purposes. GoodRx, a website with information on prescription drug prices, says that larger doses of THC pose greater risks, noting that the potency of cannabis has increased from 4% in 1995 to about 14% in 2019.
Potential Risks and Benefits: Canadian and US Perspectives
Health and safety risks vary with each of the different ways of using cannabis for individuals with and without diabetes, depending on a host of patient- and product-specific factors.
In a recent article proposing a “THC unit” for Canada’s legal cannabis market, researchers reported that consumers lack familiarity with THC levels, don’t know what constitutes a “low” or “high” THC amount, have trouble dosing, overconsume, and commonly experience adverse health events from cannabis use.
A recent study suggested that most clinicians are similarly uninformed, with “a lack of knowledge of beneficial effects, adverse effects, and of how to advise patients,” even for medical cannabis.
Diabetes Canada takes a stab at summarizing what’s known with respect to cannabis and diabetes, stating that:
“Research on recreational cannabis use suggests it may negatively impact diabetes metabolic factors and self-management behaviors. The safety of recreational cannabis use has not been demonstrated, whereas regular cannabis use is associated with worsening glycemic control, more diabetes-related complications, and poorer self-care behaviors, such as adequate glucose monitoring, adherence to medications, and compliance with dietary and physical activity recommendations for people living with both type 1 and type 2 diabetes.”
The American Diabetes Association’s information on cannabis consists of a patient-oriented article on CBD oil. The article stated:
“There’s a lot of hype surrounding CBD oil and diabetes. There is no noticeable effect on blood glucose (blood sugar) or insulin levels in people with type 2 diabetes. Researchers continue to study the effects of CBD on diabetes in animal studies.”
It concludes that:
“Although many claims continue to be made about CBD oil, there is little evidence of any benefit. It’s certainly not an alternative to traditional diabetes management. The safety of CBD is also unknown — it may have dangerous side effects that we won’t know about unless further research is done.”
A Roundup of Recent Studies
A smattering of recent studies have touched on various aspects of cannabis consumption and diabetes.
Angela Bryan, PhD, professor and co-director of CUChange at the University of Colorado Boulder, has been evaluating cannabis use in young adults (ages 21-40 years) in the SONIC study. Dr. Bryan reported at the American Diabetes Association (ADA) 84th Scientific Sessions that cannabis users were more likely to have a lower body mass index and less likely to develop T2D. Furthermore, chronic cannabis users were less likely to have measures of inflammation and no loss of insulin sensitivity.
Another study by Dr. Bryan’s group found that CBD-dominant forms of cannabis were associated with acute tension reduction, which might lead to longer-term reductions in anxiety. Bryan said the findings could be relevant in the context of diabetes distress.
Similarly positive results were found in a 15-week, double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD spray for neuropathic pain among treatment-resistant patients. The investigators reported that “clinically important improvements” were seen in pain, sleep quality, and subjective impressions of pain. Another small study of inhaled cannabis in treatment-refractory patients found a dose-dependent reduction in diabetic peripheral neuropathy pain.
Findings from a 9-year longitudinal study of approximately 18,000 Swedish men and women suggested no association between cannabis and subsequent T2D development after controlling for age, although these authors also called for longer follow-up and more detailed information about cannabis use to make “more robust” conclusions.
On the other side of the spectrum, a “rapid” review of recreational cannabis use in people with T1D and T2D found that recreational cannabis use may negatively impact diabetes metabolic factors and self-management behaviors and may increase risks for peripheral arterial occlusion, myocardial infarction, and renal disease. However, the authors cautioned that more robust research is needed to confirm the potential impact of cannabis on diabetes.
How to Advise Patients
When Dr. Shubrook was working with patients with diabetes in his family medicine practice in Ohio, cannabis wasn’t legal.
“’Don’t ask, don’t tell’ was the way we handled it then,” he said.
By contrast, in California, where he’s currently located, “it’s pretty well accepted and legal, and patients volunteer information about use, even if it’s recreational,” he said. “Realizing this was something we could talk about was really eye-opening to me.”
Talking to patients about cannabis use is a “20-minute conversation that details what they’re doing,” he said. He proceeds by asking questions: Are you using for recreational or medicinal purposes? What do you take? What do you take it for? Does it work?
“People will tell you,” Dr. Shubrook said. “They know exactly what it works or doesn’t work for and how it affects their glucose control, which in most cases is only minimally.”
He tells patients he would prefer they don’t inhale cannabis, given the risks posed to the lungs.
“Edibles may have a slower onset of effect, but depending on what they’re adding it to, glucose might be affected,” he noted. “And I have seen that chronic use can lead to hyperemesis syndrome.”
Overall, he said, “Take the time to talk to your patients about cannabis — it will allow them to be honest with you, and you can improve the specificity and safety of its use. If cannabis is legal in your state, encourage people to go to legal dispensaries, which will reduce the risk of it being laced with another drug that could increase the danger of use.”
A recent US prevalence study found that people with diabetes who use cannabis likely engage in other substance and psychoactive substance use, including tobacco use, binge drinking, and misuse of opioids and stimulants.
“Use of these additional substances could further exacerbate the health risks associated with diabetes and also emphasizes the importance of addressing polysubstance use among adults with diabetes,” the study’s author Benjamin H. Han, MD, Division of Geriatrics, Gerontology and Palliative Care, Department of Medicine, US San Diego School of Medicine in La Jolla, California, told this news organization.
“We were surprised at how strong the associations were, especially with use of substances that can increase cardiovascular risk,” Dr. Han added. “And given the strong association we found between cannabis use and use of other psychoactive substances in diabetes, clinicians must screen all their patients for psychoactive substance use.”
Diabetes Canada’s position paper states that despite the limited evidence, “there were sufficient data to begin developing recommendations for type 1 and type 2 diabetes about education, counseling, and management related to recreational cannabis use.”
Their recommendations include the following:
- Healthcare professionals should engage their patients in discussions about substance use on a regular basis, with a nonjudgmental approach.
- The use of recreational cannabis is not recommended for adolescents and adults with diabetes.
- People with T1D should avoid recreational cannabis use because of the increased risk for diabetic ketoacidosis.
- For adults with T1D or T2D who intend to use cannabis recreationally, individualized assessment and counseling should be offered to inform them of the general risks of cannabis, with a focus on harm reduction and reduction of the risk for potential adverse effects on diabetes management and complications.
- People with T1D or T2D should be offered education on and encouraged to read public information available through resources from various Canadian health authorities about the general risks of cannabis use to reduce the risk for nondiabetes-related adverse effects of cannabis consumption.
Of note, in 2018, the Canadian government produced an exhaustive compendium of information on cannabis for healthcare professionals that includes information relevant to managing patients with diabetes.
Dr. Shubrook and Dr. Han reported no competing interests.
A version of this article appeared on Medscape.com.
Hypothyroidism Treatment Does Not Affect Cognitive Decline in Menopausal Women
TOPLINE:
Women with hypothyroidism treated with levothyroxine show no significant cognitive decline across the menopausal transition compared with those without thyroid disease.
METHODOLOGY:
- Levothyroxine, the primary treatment for hypothyroidism, has been linked to perceived cognitive deficits, yet it is unclear whether this is due to the underlying condition being inadequately treated or other factors.
- Using data collected from the Study of Women’s Health Across the Nation, which encompasses five ethnic/racial groups from seven centers across the United States, researchers compared cognitive function over time between women with hypothyroidism treated with levothyroxine and those without thyroid disease.
- Participants underwent cognitive testing across three domains — processing speed, working memory, and episodic memory — which were assessed over a mean follow-up of 13 years.
- Further analyses assessed the impact of abnormal levels of thyroid-stimulating hormone on cognitive outcomes.
TAKEAWAY:
- Of 2033 women included, 227 (mean age, 49.8 years) had levothyroxine-treated hypothyroidism and 1806 (mean age, 50.0 years) did not have thyroid disease; the proportion of women with premenopausal or early perimenopausal status at baseline was higher in the hypothyroidism group (54.2% vs 49.8%; P = .010).
- At baseline, levothyroxine-treated women had higher scores for processing speed (mean score, 56.5 vs 54.4; P = .006) and working memory (mean score, 6.8 vs 6.4; P = .018) than those without thyroid disease; however, no difference in episodic memory was observed between the groups.
- Over the study period, there was no significant difference in cognitive decline between the groups.
- There was no significant effect of levothyroxine-treated hypothyroidism on working memory or episodic memory, although an annual decline in processing speed was observed (P < .001).
- Sensitivity analyses determined that abnormal levels of thyroid-stimulating hormone did not affect cognitive outcomes in women with hypothyroidism.
IN PRACTICE:
When cognitive decline is observed in these patients, the authors advised that “clinicians should resist anchoring on inadequate treatment of hypothyroidism as the cause of these symptoms and may investigate other disease processes (eg, iron deficiency, B12 deficiency, sleep apnea, celiac disease).”
SOURCE:
The study, led by Matthew D. Ettleson, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, was published online in Thyroid.
LIMITATIONS:
The cognitive assessments in the study were not designed to provide a thorough evaluation of all aspects of cognitive function. The study may not have been adequately powered to detect small effects of levothyroxine-treated hypothyroidism on cognitive outcomes. The higher levels of education attained by the study population may have acted as a protective factor against cognitive decline, potentially biasing the results.
DISCLOSURES:
The Study of Women’s Health Across the Nation was supported by grants from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with hypothyroidism treated with levothyroxine show no significant cognitive decline across the menopausal transition compared with those without thyroid disease.
METHODOLOGY:
- Levothyroxine, the primary treatment for hypothyroidism, has been linked to perceived cognitive deficits, yet it is unclear whether this is due to the underlying condition being inadequately treated or other factors.
- Using data collected from the Study of Women’s Health Across the Nation, which encompasses five ethnic/racial groups from seven centers across the United States, researchers compared cognitive function over time between women with hypothyroidism treated with levothyroxine and those without thyroid disease.
- Participants underwent cognitive testing across three domains — processing speed, working memory, and episodic memory — which were assessed over a mean follow-up of 13 years.
- Further analyses assessed the impact of abnormal levels of thyroid-stimulating hormone on cognitive outcomes.
TAKEAWAY:
- Of 2033 women included, 227 (mean age, 49.8 years) had levothyroxine-treated hypothyroidism and 1806 (mean age, 50.0 years) did not have thyroid disease; the proportion of women with premenopausal or early perimenopausal status at baseline was higher in the hypothyroidism group (54.2% vs 49.8%; P = .010).
- At baseline, levothyroxine-treated women had higher scores for processing speed (mean score, 56.5 vs 54.4; P = .006) and working memory (mean score, 6.8 vs 6.4; P = .018) than those without thyroid disease; however, no difference in episodic memory was observed between the groups.
- Over the study period, there was no significant difference in cognitive decline between the groups.
- There was no significant effect of levothyroxine-treated hypothyroidism on working memory or episodic memory, although an annual decline in processing speed was observed (P < .001).
- Sensitivity analyses determined that abnormal levels of thyroid-stimulating hormone did not affect cognitive outcomes in women with hypothyroidism.
IN PRACTICE:
When cognitive decline is observed in these patients, the authors advised that “clinicians should resist anchoring on inadequate treatment of hypothyroidism as the cause of these symptoms and may investigate other disease processes (eg, iron deficiency, B12 deficiency, sleep apnea, celiac disease).”
SOURCE:
The study, led by Matthew D. Ettleson, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, was published online in Thyroid.
LIMITATIONS:
The cognitive assessments in the study were not designed to provide a thorough evaluation of all aspects of cognitive function. The study may not have been adequately powered to detect small effects of levothyroxine-treated hypothyroidism on cognitive outcomes. The higher levels of education attained by the study population may have acted as a protective factor against cognitive decline, potentially biasing the results.
DISCLOSURES:
The Study of Women’s Health Across the Nation was supported by grants from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with hypothyroidism treated with levothyroxine show no significant cognitive decline across the menopausal transition compared with those without thyroid disease.
METHODOLOGY:
- Levothyroxine, the primary treatment for hypothyroidism, has been linked to perceived cognitive deficits, yet it is unclear whether this is due to the underlying condition being inadequately treated or other factors.
- Using data collected from the Study of Women’s Health Across the Nation, which encompasses five ethnic/racial groups from seven centers across the United States, researchers compared cognitive function over time between women with hypothyroidism treated with levothyroxine and those without thyroid disease.
- Participants underwent cognitive testing across three domains — processing speed, working memory, and episodic memory — which were assessed over a mean follow-up of 13 years.
- Further analyses assessed the impact of abnormal levels of thyroid-stimulating hormone on cognitive outcomes.
TAKEAWAY:
- Of 2033 women included, 227 (mean age, 49.8 years) had levothyroxine-treated hypothyroidism and 1806 (mean age, 50.0 years) did not have thyroid disease; the proportion of women with premenopausal or early perimenopausal status at baseline was higher in the hypothyroidism group (54.2% vs 49.8%; P = .010).
- At baseline, levothyroxine-treated women had higher scores for processing speed (mean score, 56.5 vs 54.4; P = .006) and working memory (mean score, 6.8 vs 6.4; P = .018) than those without thyroid disease; however, no difference in episodic memory was observed between the groups.
- Over the study period, there was no significant difference in cognitive decline between the groups.
- There was no significant effect of levothyroxine-treated hypothyroidism on working memory or episodic memory, although an annual decline in processing speed was observed (P < .001).
- Sensitivity analyses determined that abnormal levels of thyroid-stimulating hormone did not affect cognitive outcomes in women with hypothyroidism.
IN PRACTICE:
When cognitive decline is observed in these patients, the authors advised that “clinicians should resist anchoring on inadequate treatment of hypothyroidism as the cause of these symptoms and may investigate other disease processes (eg, iron deficiency, B12 deficiency, sleep apnea, celiac disease).”
SOURCE:
The study, led by Matthew D. Ettleson, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, was published online in Thyroid.
LIMITATIONS:
The cognitive assessments in the study were not designed to provide a thorough evaluation of all aspects of cognitive function. The study may not have been adequately powered to detect small effects of levothyroxine-treated hypothyroidism on cognitive outcomes. The higher levels of education attained by the study population may have acted as a protective factor against cognitive decline, potentially biasing the results.
DISCLOSURES:
The Study of Women’s Health Across the Nation was supported by grants from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
First Patient-Derived Stem Cell Transplant a Success in T1D
The chemically induced pluripotent stem cell–derived islets came from the somatic cells of the patient, a 25-year-old woman who had lived for 11 years with unstable T1D with less than 50% time-in-target glucose range despite intensive insulin therapy. By 1 year following the transplantation of the cells into her abdomen, her glucose levels were nearly 100% in range, and her hemoglobin A1c had come down from 7.4%-8.0% to nondiabetic range (~5%).
Of note, she was already under immunosuppression for a prior liver transplant and remained on it throughout. There were no major safety concerns.
“We are very encouraged by the positive clinical findings seen in this first patient using this combination of technologies. These findings set a strong foundation for further development of stem cell–derived islet transplantation as a feasible treatment modality for diabetes,” study authors Soon Yi Liew, PhD, and Hongkui Deng, PhD, both of Peking University Health Science Center, Beijing, China, told this news organization in an email. Dr. Deng, the lead author, is the director of the university’s Institute of Stem Cell Research.
The findings were published in Cell.
What’s New With This Approach?
The use of the patient’s own cells is one of several ways in which this approach differs from other ongoing efforts in treating T1D with pluripotent stem cell–derived islets, such as those of the companies Vertex and Sernova, Dr. Liew and Dr. Deng explained.
Another difference is that “the patient-specific stem cell–derived islets used in this study were produced from induced pluripotent stem cells generated using chemical reprogramming, which is a nontransgenic approach to inducing pluripotent stem cells from somatic cells that uses only small molecules, different from the conventional method of viral transduction of transcription factors. ... Employing small molecules as reprogramming factors provides a greater degree of control — small molecules have defined structures easily manufactured and standardized, are not genome integrating, and are cost effective,” Dr. Liew and Dr. Deng said.
A third difference, they noted, is the placement of the stem cell–derived islets underneath the abdominal anterior rectus sheath of the patient, as opposed to the more commonly used hepatic portal vein. In addition to better ease of visualization, prior evidence suggested that this approach could lead to an improved engraftment and graft function and could circumvent graft loss from blood-mediated inflammatory responses associated with the liver site.
Moreover, they added, “to our knowledge, the rapidity with which insulin-independence was achieved post transplantation of stem cell–derived islets, 75 days post-transplantation, is also a first.”
Immunosuppression Remains a Challenge
Asked to comment, David M. Harlan, MD, the William and Doris Krupp professor of medicine and codirector of the Diabetes Center of Excellence at the University of Massachusetts Chan Medical School, Worcester, told this news organization, “on the one hand, it seems like a great breakthrough that you could take each individual cells and use those to make islets, but ... that process takes a long time, is very, very expensive, and then the T1D recipient still needs to be immunosuppressed. From a business point of view, I just don’t see it as getting any legs.”
Dr. Harlan, who had been involved in the islet transplantation field for several decades, pointed out that the additional autoimmunity of T1D poses a challenge beyond that of the body’s immune reaction to foreign tissue. “Because transplants have been around since the 1950s, we know a lot about how to prevent allogeneic rejection, from one person to another, but we know very little about how to prevent autoimmunity, so that’s still a very difficult nut to crack. I actually think the major effort should be focused on making the beta cells more hardy [via genetic modification] as opposed to focusing on the immune system. And there’s a lot of data to support that now, and that’s what we’re working on.”
Indeed, Dr. Liew and Dr. Deng said, “New immunomodulatory strategies to address graft longevity without immunosuppression remain to be established and tested. With reports of therapeutic efficacy of stem cell–derived islet transplantation such as with our study, stem cell–derived therapy without need for immunosuppression would be a meaningful next step in the treatment of this disease.”
The team has now performed the same procedure in two more patients and will report their data “in due course.”
Dr. Liew had no disclosures. Dr. Deng is a scientific adviser at Hangzhou Reprogenix Bioscience. Two coauthors are employees of Hangzhou Reprogenix Bioscience. Another is a former employee of Hangzhou Reprogenix Bioscience and is now affiliated with the Hangzhou Institute of Medicine, Chinese Academy of Sciences. Four coauthors have patent applications related to this work. Dr. Harlan is chief scientific officer and cofounder of Stability Health. He had no other disclosures.
A version of this article first appeared on Medscape.com.
The chemically induced pluripotent stem cell–derived islets came from the somatic cells of the patient, a 25-year-old woman who had lived for 11 years with unstable T1D with less than 50% time-in-target glucose range despite intensive insulin therapy. By 1 year following the transplantation of the cells into her abdomen, her glucose levels were nearly 100% in range, and her hemoglobin A1c had come down from 7.4%-8.0% to nondiabetic range (~5%).
Of note, she was already under immunosuppression for a prior liver transplant and remained on it throughout. There were no major safety concerns.
“We are very encouraged by the positive clinical findings seen in this first patient using this combination of technologies. These findings set a strong foundation for further development of stem cell–derived islet transplantation as a feasible treatment modality for diabetes,” study authors Soon Yi Liew, PhD, and Hongkui Deng, PhD, both of Peking University Health Science Center, Beijing, China, told this news organization in an email. Dr. Deng, the lead author, is the director of the university’s Institute of Stem Cell Research.
The findings were published in Cell.
What’s New With This Approach?
The use of the patient’s own cells is one of several ways in which this approach differs from other ongoing efforts in treating T1D with pluripotent stem cell–derived islets, such as those of the companies Vertex and Sernova, Dr. Liew and Dr. Deng explained.
Another difference is that “the patient-specific stem cell–derived islets used in this study were produced from induced pluripotent stem cells generated using chemical reprogramming, which is a nontransgenic approach to inducing pluripotent stem cells from somatic cells that uses only small molecules, different from the conventional method of viral transduction of transcription factors. ... Employing small molecules as reprogramming factors provides a greater degree of control — small molecules have defined structures easily manufactured and standardized, are not genome integrating, and are cost effective,” Dr. Liew and Dr. Deng said.
A third difference, they noted, is the placement of the stem cell–derived islets underneath the abdominal anterior rectus sheath of the patient, as opposed to the more commonly used hepatic portal vein. In addition to better ease of visualization, prior evidence suggested that this approach could lead to an improved engraftment and graft function and could circumvent graft loss from blood-mediated inflammatory responses associated with the liver site.
Moreover, they added, “to our knowledge, the rapidity with which insulin-independence was achieved post transplantation of stem cell–derived islets, 75 days post-transplantation, is also a first.”
Immunosuppression Remains a Challenge
Asked to comment, David M. Harlan, MD, the William and Doris Krupp professor of medicine and codirector of the Diabetes Center of Excellence at the University of Massachusetts Chan Medical School, Worcester, told this news organization, “on the one hand, it seems like a great breakthrough that you could take each individual cells and use those to make islets, but ... that process takes a long time, is very, very expensive, and then the T1D recipient still needs to be immunosuppressed. From a business point of view, I just don’t see it as getting any legs.”
Dr. Harlan, who had been involved in the islet transplantation field for several decades, pointed out that the additional autoimmunity of T1D poses a challenge beyond that of the body’s immune reaction to foreign tissue. “Because transplants have been around since the 1950s, we know a lot about how to prevent allogeneic rejection, from one person to another, but we know very little about how to prevent autoimmunity, so that’s still a very difficult nut to crack. I actually think the major effort should be focused on making the beta cells more hardy [via genetic modification] as opposed to focusing on the immune system. And there’s a lot of data to support that now, and that’s what we’re working on.”
Indeed, Dr. Liew and Dr. Deng said, “New immunomodulatory strategies to address graft longevity without immunosuppression remain to be established and tested. With reports of therapeutic efficacy of stem cell–derived islet transplantation such as with our study, stem cell–derived therapy without need for immunosuppression would be a meaningful next step in the treatment of this disease.”
The team has now performed the same procedure in two more patients and will report their data “in due course.”
Dr. Liew had no disclosures. Dr. Deng is a scientific adviser at Hangzhou Reprogenix Bioscience. Two coauthors are employees of Hangzhou Reprogenix Bioscience. Another is a former employee of Hangzhou Reprogenix Bioscience and is now affiliated with the Hangzhou Institute of Medicine, Chinese Academy of Sciences. Four coauthors have patent applications related to this work. Dr. Harlan is chief scientific officer and cofounder of Stability Health. He had no other disclosures.
A version of this article first appeared on Medscape.com.
The chemically induced pluripotent stem cell–derived islets came from the somatic cells of the patient, a 25-year-old woman who had lived for 11 years with unstable T1D with less than 50% time-in-target glucose range despite intensive insulin therapy. By 1 year following the transplantation of the cells into her abdomen, her glucose levels were nearly 100% in range, and her hemoglobin A1c had come down from 7.4%-8.0% to nondiabetic range (~5%).
Of note, she was already under immunosuppression for a prior liver transplant and remained on it throughout. There were no major safety concerns.
“We are very encouraged by the positive clinical findings seen in this first patient using this combination of technologies. These findings set a strong foundation for further development of stem cell–derived islet transplantation as a feasible treatment modality for diabetes,” study authors Soon Yi Liew, PhD, and Hongkui Deng, PhD, both of Peking University Health Science Center, Beijing, China, told this news organization in an email. Dr. Deng, the lead author, is the director of the university’s Institute of Stem Cell Research.
The findings were published in Cell.
What’s New With This Approach?
The use of the patient’s own cells is one of several ways in which this approach differs from other ongoing efforts in treating T1D with pluripotent stem cell–derived islets, such as those of the companies Vertex and Sernova, Dr. Liew and Dr. Deng explained.
Another difference is that “the patient-specific stem cell–derived islets used in this study were produced from induced pluripotent stem cells generated using chemical reprogramming, which is a nontransgenic approach to inducing pluripotent stem cells from somatic cells that uses only small molecules, different from the conventional method of viral transduction of transcription factors. ... Employing small molecules as reprogramming factors provides a greater degree of control — small molecules have defined structures easily manufactured and standardized, are not genome integrating, and are cost effective,” Dr. Liew and Dr. Deng said.
A third difference, they noted, is the placement of the stem cell–derived islets underneath the abdominal anterior rectus sheath of the patient, as opposed to the more commonly used hepatic portal vein. In addition to better ease of visualization, prior evidence suggested that this approach could lead to an improved engraftment and graft function and could circumvent graft loss from blood-mediated inflammatory responses associated with the liver site.
Moreover, they added, “to our knowledge, the rapidity with which insulin-independence was achieved post transplantation of stem cell–derived islets, 75 days post-transplantation, is also a first.”
Immunosuppression Remains a Challenge
Asked to comment, David M. Harlan, MD, the William and Doris Krupp professor of medicine and codirector of the Diabetes Center of Excellence at the University of Massachusetts Chan Medical School, Worcester, told this news organization, “on the one hand, it seems like a great breakthrough that you could take each individual cells and use those to make islets, but ... that process takes a long time, is very, very expensive, and then the T1D recipient still needs to be immunosuppressed. From a business point of view, I just don’t see it as getting any legs.”
Dr. Harlan, who had been involved in the islet transplantation field for several decades, pointed out that the additional autoimmunity of T1D poses a challenge beyond that of the body’s immune reaction to foreign tissue. “Because transplants have been around since the 1950s, we know a lot about how to prevent allogeneic rejection, from one person to another, but we know very little about how to prevent autoimmunity, so that’s still a very difficult nut to crack. I actually think the major effort should be focused on making the beta cells more hardy [via genetic modification] as opposed to focusing on the immune system. And there’s a lot of data to support that now, and that’s what we’re working on.”
Indeed, Dr. Liew and Dr. Deng said, “New immunomodulatory strategies to address graft longevity without immunosuppression remain to be established and tested. With reports of therapeutic efficacy of stem cell–derived islet transplantation such as with our study, stem cell–derived therapy without need for immunosuppression would be a meaningful next step in the treatment of this disease.”
The team has now performed the same procedure in two more patients and will report their data “in due course.”
Dr. Liew had no disclosures. Dr. Deng is a scientific adviser at Hangzhou Reprogenix Bioscience. Two coauthors are employees of Hangzhou Reprogenix Bioscience. Another is a former employee of Hangzhou Reprogenix Bioscience and is now affiliated with the Hangzhou Institute of Medicine, Chinese Academy of Sciences. Four coauthors have patent applications related to this work. Dr. Harlan is chief scientific officer and cofounder of Stability Health. He had no other disclosures.
A version of this article first appeared on Medscape.com.
FROM CELL
Millennial Clinicians Face Pay Disparities by Specialty, Other Factors
Salaries for millennial physicians are slightly increasing, but clinicians still face pay disparities across location, practice type, and gender.
Medscape Medical News reviewed survey data from more than 1200 practicing doctors under age 40 across 29 specialties over a 4-month period starting in October 2023.
The average annual total compensation (including any bonuses) for young clinicians rose from $326,000 to $338,000, about 4%, between 2022 and 2023. Among millennials, primary care physicians saw a 5% increase. But a large pay gap exists between fields: Specialists under age 40 earned an average of $357,000 in 2023, compared with the average primary care clinician salary of $271,000.
“Procedures are reimbursed too high, while very little value is placed on primary care,” one survey respondent complained.
The type of practice plays a major part in compensation. Millennial doctors in office-based, single-specialty group practices earned an average of $358,000 per year, followed by those in office-based multispecialty group practices at 355,000 per year. Those in outpatient clinics earned $278,000 per year.
“I believe the practice situation is a huge portion of compensation,” said Tiffany Di Pietro, DO, a cardiologist and internal medicine physician in Fort Lauderdale, Florida. “Owning your own private practice is generally more lucrative (if you have good business sense), but it is also quite a bit more time-consuming, whereas employed physicians usually make less but have fewer concerns with staffing and overhead.”
Like in previous years, a gender pay gap equated to men outearning women. Female physicians under age 40 of any kind earned about $302,000 per year, 24% less than their male counterparts, on average.
Millennial doctors in the Midwest brought home the biggest earnings, with an average salary of $343,000 vs $332,000 on the West Coast.
Millennial physicians also reported higher levels of dissatisfaction. In the 2022 report, 46% said they were not paid fairly. That figure rose to 49%. Just 68% of millennial doctors would choose medicine again if they could do things over, down from 76% in the 2021 report.
“Doctors go through multiple years of school and then have to act like we are working at Dunkin’ Donuts — like we’re on an assembly line,” one survey respondent said. “We should not have to be paid per patient seen but valued for 8-9 years of training.”
Despite these complaints, close to 7 out of 10 millennial respondents said pay was not a major factor in what area of medicine they chose, with 29% saying it played no role at all in their decision.
Psychiatrists and anesthesiologists were the happiest with their earnings, with 61% of both specialties reporting that they felt fairly paid. They were followed by dermatologists and emergency medicine doctors, both of whom 60% reported fair earnings.
Many millennial doctors are finding ways to make money outside of their practice, with 18% securing other medical-related work, 15% doing medical moonlighting, and 5% taking on non–medical-related work.
A version of this article first appeared on Medscape.com.
Salaries for millennial physicians are slightly increasing, but clinicians still face pay disparities across location, practice type, and gender.
Medscape Medical News reviewed survey data from more than 1200 practicing doctors under age 40 across 29 specialties over a 4-month period starting in October 2023.
The average annual total compensation (including any bonuses) for young clinicians rose from $326,000 to $338,000, about 4%, between 2022 and 2023. Among millennials, primary care physicians saw a 5% increase. But a large pay gap exists between fields: Specialists under age 40 earned an average of $357,000 in 2023, compared with the average primary care clinician salary of $271,000.
“Procedures are reimbursed too high, while very little value is placed on primary care,” one survey respondent complained.
The type of practice plays a major part in compensation. Millennial doctors in office-based, single-specialty group practices earned an average of $358,000 per year, followed by those in office-based multispecialty group practices at 355,000 per year. Those in outpatient clinics earned $278,000 per year.
“I believe the practice situation is a huge portion of compensation,” said Tiffany Di Pietro, DO, a cardiologist and internal medicine physician in Fort Lauderdale, Florida. “Owning your own private practice is generally more lucrative (if you have good business sense), but it is also quite a bit more time-consuming, whereas employed physicians usually make less but have fewer concerns with staffing and overhead.”
Like in previous years, a gender pay gap equated to men outearning women. Female physicians under age 40 of any kind earned about $302,000 per year, 24% less than their male counterparts, on average.
Millennial doctors in the Midwest brought home the biggest earnings, with an average salary of $343,000 vs $332,000 on the West Coast.
Millennial physicians also reported higher levels of dissatisfaction. In the 2022 report, 46% said they were not paid fairly. That figure rose to 49%. Just 68% of millennial doctors would choose medicine again if they could do things over, down from 76% in the 2021 report.
“Doctors go through multiple years of school and then have to act like we are working at Dunkin’ Donuts — like we’re on an assembly line,” one survey respondent said. “We should not have to be paid per patient seen but valued for 8-9 years of training.”
Despite these complaints, close to 7 out of 10 millennial respondents said pay was not a major factor in what area of medicine they chose, with 29% saying it played no role at all in their decision.
Psychiatrists and anesthesiologists were the happiest with their earnings, with 61% of both specialties reporting that they felt fairly paid. They were followed by dermatologists and emergency medicine doctors, both of whom 60% reported fair earnings.
Many millennial doctors are finding ways to make money outside of their practice, with 18% securing other medical-related work, 15% doing medical moonlighting, and 5% taking on non–medical-related work.
A version of this article first appeared on Medscape.com.
Salaries for millennial physicians are slightly increasing, but clinicians still face pay disparities across location, practice type, and gender.
Medscape Medical News reviewed survey data from more than 1200 practicing doctors under age 40 across 29 specialties over a 4-month period starting in October 2023.
The average annual total compensation (including any bonuses) for young clinicians rose from $326,000 to $338,000, about 4%, between 2022 and 2023. Among millennials, primary care physicians saw a 5% increase. But a large pay gap exists between fields: Specialists under age 40 earned an average of $357,000 in 2023, compared with the average primary care clinician salary of $271,000.
“Procedures are reimbursed too high, while very little value is placed on primary care,” one survey respondent complained.
The type of practice plays a major part in compensation. Millennial doctors in office-based, single-specialty group practices earned an average of $358,000 per year, followed by those in office-based multispecialty group practices at 355,000 per year. Those in outpatient clinics earned $278,000 per year.
“I believe the practice situation is a huge portion of compensation,” said Tiffany Di Pietro, DO, a cardiologist and internal medicine physician in Fort Lauderdale, Florida. “Owning your own private practice is generally more lucrative (if you have good business sense), but it is also quite a bit more time-consuming, whereas employed physicians usually make less but have fewer concerns with staffing and overhead.”
Like in previous years, a gender pay gap equated to men outearning women. Female physicians under age 40 of any kind earned about $302,000 per year, 24% less than their male counterparts, on average.
Millennial doctors in the Midwest brought home the biggest earnings, with an average salary of $343,000 vs $332,000 on the West Coast.
Millennial physicians also reported higher levels of dissatisfaction. In the 2022 report, 46% said they were not paid fairly. That figure rose to 49%. Just 68% of millennial doctors would choose medicine again if they could do things over, down from 76% in the 2021 report.
“Doctors go through multiple years of school and then have to act like we are working at Dunkin’ Donuts — like we’re on an assembly line,” one survey respondent said. “We should not have to be paid per patient seen but valued for 8-9 years of training.”
Despite these complaints, close to 7 out of 10 millennial respondents said pay was not a major factor in what area of medicine they chose, with 29% saying it played no role at all in their decision.
Psychiatrists and anesthesiologists were the happiest with their earnings, with 61% of both specialties reporting that they felt fairly paid. They were followed by dermatologists and emergency medicine doctors, both of whom 60% reported fair earnings.
Many millennial doctors are finding ways to make money outside of their practice, with 18% securing other medical-related work, 15% doing medical moonlighting, and 5% taking on non–medical-related work.
A version of this article first appeared on Medscape.com.
Severe Autoimmune Diseases Linked to Premature Ovarian Insufficiency
TOPLINE:
Women with premature ovarian insufficiency (POI) have a 2.6 times higher prevalence of severe autoimmune diseases before diagnosis and a 2- to 3-fold increased risk for these diseases after diagnosis.
METHODOLOGY:
- Researchers conducted a population-based registry study including 3972 women diagnosed with spontaneous POI between 1988 and 2017.
- A total of 15,708 female population controls matched by age and municipality of residence were included for comparison.
- Autoimmune disease diagnoses were evaluated from childhood until the end of 2017 using the Hospital Discharge Registry.
- Women with a history of cancer or bilateral oophorectomy were excluded from the study.
TAKEAWAY:
- Women with POI had a 2.6 times higher prevalence of severe autoimmune diseases before diagnosis compared to controls (odds ratio [OR], 2.6; 95% CI, 2.2-3.1).
- The prevalence of specific autoimmune diseases such as polyglandular autoimmune diseases (OR, 25.8; 95% CI, 9.0-74.1) and Addison disease (OR, 22.9; 95% CI, 7.9-66.1) was significantly higher in women with POI.
- The standardized incidence ratios for being diagnosed with a severe autoimmune disease after POI diagnosis was 2.8 (95% CI, 2.3-3.4) during the first 3 years, decreasing to 1.3 (95% CI, 1.1-1.6) after 12 years.
- No significant difference was found in the prevalence of diabetes type 1 and ankylosing spondylitis between women with POI and the reference cohort.
IN PRACTICE:
“The study results strengthen the hypothesis that autoimmune mechanisms play an important role in the pathogenesis of POI. Future studies should focus on the immunological mechanism of POI from preventative and curative perspectives,” wrote the authors of the study.
SOURCE:
The study was led by Susanna M. Savukoski, Oulu University Hospital in Finland. It was published online in Human Reproduction.
LIMITATIONS:
The study included only autoimmune disorders diagnosed in specialized health care, which may underestimate the overall prevalence of autoimmune disorders in women with POI. Additionally, the study did not account for confounders such as body mass index and smoking, which are associated with the risk for autoimmune disease and POI.
DISCLOSURES:
Ms. Savukoski received grants from the Finnish Menopause Society, the Finnish Medical Foundation, and the Juho Vainio Foundation. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Women with premature ovarian insufficiency (POI) have a 2.6 times higher prevalence of severe autoimmune diseases before diagnosis and a 2- to 3-fold increased risk for these diseases after diagnosis.
METHODOLOGY:
- Researchers conducted a population-based registry study including 3972 women diagnosed with spontaneous POI between 1988 and 2017.
- A total of 15,708 female population controls matched by age and municipality of residence were included for comparison.
- Autoimmune disease diagnoses were evaluated from childhood until the end of 2017 using the Hospital Discharge Registry.
- Women with a history of cancer or bilateral oophorectomy were excluded from the study.
TAKEAWAY:
- Women with POI had a 2.6 times higher prevalence of severe autoimmune diseases before diagnosis compared to controls (odds ratio [OR], 2.6; 95% CI, 2.2-3.1).
- The prevalence of specific autoimmune diseases such as polyglandular autoimmune diseases (OR, 25.8; 95% CI, 9.0-74.1) and Addison disease (OR, 22.9; 95% CI, 7.9-66.1) was significantly higher in women with POI.
- The standardized incidence ratios for being diagnosed with a severe autoimmune disease after POI diagnosis was 2.8 (95% CI, 2.3-3.4) during the first 3 years, decreasing to 1.3 (95% CI, 1.1-1.6) after 12 years.
- No significant difference was found in the prevalence of diabetes type 1 and ankylosing spondylitis between women with POI and the reference cohort.
IN PRACTICE:
“The study results strengthen the hypothesis that autoimmune mechanisms play an important role in the pathogenesis of POI. Future studies should focus on the immunological mechanism of POI from preventative and curative perspectives,” wrote the authors of the study.
SOURCE:
The study was led by Susanna M. Savukoski, Oulu University Hospital in Finland. It was published online in Human Reproduction.
LIMITATIONS:
The study included only autoimmune disorders diagnosed in specialized health care, which may underestimate the overall prevalence of autoimmune disorders in women with POI. Additionally, the study did not account for confounders such as body mass index and smoking, which are associated with the risk for autoimmune disease and POI.
DISCLOSURES:
Ms. Savukoski received grants from the Finnish Menopause Society, the Finnish Medical Foundation, and the Juho Vainio Foundation. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Women with premature ovarian insufficiency (POI) have a 2.6 times higher prevalence of severe autoimmune diseases before diagnosis and a 2- to 3-fold increased risk for these diseases after diagnosis.
METHODOLOGY:
- Researchers conducted a population-based registry study including 3972 women diagnosed with spontaneous POI between 1988 and 2017.
- A total of 15,708 female population controls matched by age and municipality of residence were included for comparison.
- Autoimmune disease diagnoses were evaluated from childhood until the end of 2017 using the Hospital Discharge Registry.
- Women with a history of cancer or bilateral oophorectomy were excluded from the study.
TAKEAWAY:
- Women with POI had a 2.6 times higher prevalence of severe autoimmune diseases before diagnosis compared to controls (odds ratio [OR], 2.6; 95% CI, 2.2-3.1).
- The prevalence of specific autoimmune diseases such as polyglandular autoimmune diseases (OR, 25.8; 95% CI, 9.0-74.1) and Addison disease (OR, 22.9; 95% CI, 7.9-66.1) was significantly higher in women with POI.
- The standardized incidence ratios for being diagnosed with a severe autoimmune disease after POI diagnosis was 2.8 (95% CI, 2.3-3.4) during the first 3 years, decreasing to 1.3 (95% CI, 1.1-1.6) after 12 years.
- No significant difference was found in the prevalence of diabetes type 1 and ankylosing spondylitis between women with POI and the reference cohort.
IN PRACTICE:
“The study results strengthen the hypothesis that autoimmune mechanisms play an important role in the pathogenesis of POI. Future studies should focus on the immunological mechanism of POI from preventative and curative perspectives,” wrote the authors of the study.
SOURCE:
The study was led by Susanna M. Savukoski, Oulu University Hospital in Finland. It was published online in Human Reproduction.
LIMITATIONS:
The study included only autoimmune disorders diagnosed in specialized health care, which may underestimate the overall prevalence of autoimmune disorders in women with POI. Additionally, the study did not account for confounders such as body mass index and smoking, which are associated with the risk for autoimmune disease and POI.
DISCLOSURES:
Ms. Savukoski received grants from the Finnish Menopause Society, the Finnish Medical Foundation, and the Juho Vainio Foundation. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New Guidelines Emphasize Liver Care in T2D, Obesity
MADRID — Individuals with type 2 diabetes and/or obesity plus one or more metabolic risk factors are at a higher risk for metabolic dysfunction–associated steatotic liver disease (MASLD) with fibrosis and progression to more severe liver disease, stated new European guidelines that provide recommendations for diagnosis and management.
“The availability of improved treatment options underlines the need to identify at-risk individuals with MASLD early, as we now possess the tools to positively influence the course of the diseases, which is expected to prevent relevant clinical events,” stated the clinical practice guidelines, updated for the first time since 2016.
“Now we have guidelines that tell clinicians how to monitor the liver,” said Amalia Gastaldelli, PhD, research director at the Institute of Clinical Physiology of the National Research Council in Pisa, Italy, and a member of the panel that developed the guidelines.
Dr. Gastaldelli moderated a session focused on the guidelines at the annual meeting of the European Association for the Study of Diabetes (EASD). In an interview after the session, Dr. Gastaldelli, who leads a cardiometabolic risk research group, stressed the importance of the liver’s role in the body and the need for diabetes specialists to start paying more attention to this vital organ.
“It’s an important organ for monitoring because liver disease is silent, and the patient doesn’t feel unwell until disease is severe,” she said. “Diabetologists already monitor the eye, the heart, the kidney, and so on, but the liver is often neglected,” she said. A 2024 study found that the global pooled prevalence of MASLD among patients with type 2 diabetes was 65.33%.
Dr. Gastaldelli noted the importance of liver status in diabetes care. The liver makes triglycerides and very-low-density lipoprotein cholesterol, which are all major risk factors for atherosclerosis and cardiovascular disease (CVD), she said, as well as producing glucose, which in excess can lead to hyperglycemia.
The guidelines were jointly written by EASD, the European Association for the Study of the Liver, and the European Association for the Study of Obesity, and published in Diabetologia, The Journal of Hepatology, and Obesity Facts.
A Metabolic Condition
In the EASD meeting session, Dr. Gastaldelli discussed the reasons for, and implications of, shifting the name from nonalcoholic fatty liver disease (NAFLD) to MASLD.
“The name change focuses on the fact that this is a metabolic disease, while NAFLD had no mention of this and was considered stigmatizing by patients, especially in relation to the words ‘fatty’ and ‘nonalcoholic,’” she said.
According to the guidelines, MASLD is defined as liver steatosis in the presence of one or more cardiometabolic risk factor(s) and the absence of excess alcohol intake.
MASLD has become the most common chronic liver disease and includes isolated steatosis, metabolic dysfunction-associated steatohepatitis (MASH, previously NASH), MASH-related fibrosis, and cirrhosis.
In the overarching group of steatotic liver disease, a totally new intermediate category has been added: MASLD with moderate (increased) alcohol intake (MetALD), which represents MASLD in people who consume greater amounts of alcohol per week (140-350 g/week and 210-420 g/week for women and men, respectively).
The change in the nomenclature has been incremental and regional, Dr. Gastaldelli said. “The definition first changed from NAFLD to MAFLD, which recognizes the importance of metabolism in the pathophysiology of this disease but does not take into account alcohol intake. MAFLD is still used in Asia, Australasia, and North Africa, while Europe and the Americas have endorsed MASLD.”
Case-Finding and Diagnosis
Identifying MASLD cases in people at risk remains incidental, largely because it is a silent disease and is symptom-free until it becomes severe, said Dr. Gastaldelli.
The guideline recognizes that individuals with type 2 diabetes or obesity with additional metabolic risk factor(s) are at a higher risk for MASLD with fibrosis and progression to MASH.
Assessment strategies for severe liver fibrosis in MASLD include the use of noninvasive tests in people who have cardiometabolic risk factors, abnormal liver enzymes, and/or radiological signs of hepatic steatosis, particularly in the presence of type 2 diabetes or obesity or in the presence of one or more metabolic risk factors.
Dr. Gastaldelli noted that type 2 diabetes, metabolic syndrome, and obesity, including abdominal obesity identified by large waist circumference, are the major risk factors and should be warning signs.
“We need to consider abdominal obesity too — we’ve published data in relatively lean people, body mass index < 25, with MASH but without diabetes. Most of the patients accumulated fat viscerally and in the liver and had hypertriglyceridemia and hypercholesterolemia,” she said.
“The guidelines reflect this because the definition of MASLD includes steatosis plus at least one metabolic factor — waist circumference, for example, which is related to visceral fat, hyperlipidemia, or hyperglycemia. Of note, in both pharmacological and diet-induced weight loss, the decrease in liver fat was associated with the decrease in visceral fat.”
The noninvasive biomarker test, Fibrosis-4 (FIB-4) may be used to assess the risk for liver fibrosis. The FIB-4 index is calculated using a patient’s age and results of three blood tests — aspartate aminotransferase, alanine aminotransferase, and platelet count.
Advanced fibrosis (grade F3-F4) “is a major risk factor for severe outcomes,” said Dr. Gastaldelli. A FIB-4 test result below 1.3 indicates low risk for advanced liver fibrosis, 1.30-2.67 indicates intermediate risk, and above 2.67 indicates high risk.
“When fibrosis increases, then liver enzymes increase and the platelets decrease,” said Dr. Gastaldelli. “It is not a perfect tool, and we need to add in age because at a young age, it is prone to false negatives and when very old — false positives. It’s important to take a global view, especially if the patient has persistent high liver enzymes, but FIB-4 is low.”
“And if they have more than one metabolic risk factor, proceed with more tests, for example, transient elastography,” she advised. Imaging techniques such as transient elastography may rule out or rule in advanced fibrosis, which is predictive of liver-related outcomes.
“However, imaging techniques only diagnose steatosis and fibrosis, and right now, MASH can only be diagnosed with liver biopsy because we do not have any markers of liver inflammation and ballooning. In the future, noninvasive tests based on imaging and blood tests will be used to identify patients with MASH,” she added.
Management of MASLD — Lifestyle and Treatment
“Pharmacological treatments are designed for [patients] with MASH and fibrosis grade F2 or F3, but not MASLD,” Dr. Gastaldelli said. As such, lifestyle interventions are the mainstay of management — including weight loss, dietary changes, physical exercise, and low to no alcohol consumption. “Eating good-quality food and reducing calories are both important because the metabolism responds differently to different nutrients,” Dr. Gastaldelli said.
“In particular, the guidelines advise dietary management because some foods carry liver toxicity, for example, sugary foods with sucrose/fructose especially,” she said, adding that, “complex carbohydrates are less harmful than refined carbohydrates. Processed foods should be avoided if possible because they contain sugars, [as well as] saturated fats and hydrogenated fat, which is particularly bad for the liver. Olive oil is better than butter or margarine, which are rich in saturated fat, and fish and white meat are preferable.”
She added that a diet to help manage type 2 diabetes was not so dissimilar because sugar again needs to be reduced.
If a patient has severe obesity (and MASLD), data show that bariatric surgery is beneficial. “It not only helps weight loss, but it improves liver histology and has been shown to improve or resolve type 2 diabetes and reduce CVD risk. Importantly, regarding fibrosis, nutritional management after the bariatric surgery is the most important thing,” said Dr. Gastaldelli.
Optimal management of comorbidities — including the use of incretin-based therapies such as semaglutide or tirzepatide for type 2 diabetes or obesity, if indicated — is advised, according to the guidelines.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been shown to have a beneficial effect on MASH, said Dr. Gastaldelli. “They have not shown effectiveness in the resolution of fibrosis, but this might take longer to manifest. However, if the medication is started early enough, it may prevent severe fibrosis. Significant weight loss, both with lifestyle and pharmacological treatment, should lead to an improvement in the liver too.”
There are currently no drugs available in Europe for the treatment of noncirrhotic MASH and severe fibrosis (stage ≥ 2). Resmetirom is the first approved MASH-targeted treatment in noncirrhotic MASH and significant liver fibrosis, with histological effectiveness on steatohepatitis and fibrosis, together with an acceptable safety and tolerability profile, but, for the moment, this agent is only available in United States.
Finally, turning to MASH-related cirrhosis, the guidelines advise adaptations of metabolic drugs, nutritional counseling, and surveillance for portal hypertension and hepatocellular carcinoma, as well as liver transplantation in decompensated cirrhosis.
After the session, this news organization spoke to Tushy Kailayanathan, MBBS BSc, medical director of the liver MRI company, Perspectum, who reviewed the limitations of the FIB-4 test. The FIB-4 test identifies those with advanced fibrosis in the liver, for example, patients with hepatitis C, she noted; however, “it performs worse in type 2 diabetic patients and in the elderly. There is little clinical guidance on the adjustment of FIB-4 thresholds needed for these high cardiometabolic risk groups. The priority patients are missed by FIB-4 because those individuals with early and active disease may not yet have progressed to advanced disease detected by FIB-4.”
These individuals are exactly those amenable to primary care prevention strategies, said Dr. Kailayanathan. Because of the nature of early and active liver disease in patients with high cardiometabolic risk, it would make sense to shift some diagnostic protocols into primary care.
“These individuals are exactly those amenable to primary care prevention strategies at annual diabetic review because they are likely to have modifiable cardiometabolic risk factors such as metabolic syndrome and would benefit from lifestyle and therapeutic intervention, including GLP-1 RAs and SGLT2is [sodium-glucose cotransporter-2 inhibitors],” she said. “Case-finding and detection of early-stage MASLD is a priority in diabetics, and there is an unmet need for accurate biomarkers to measure liver fat and inflammation early.”
Dr. Gastaldelli has been on the advisory board or consulting for Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Fractyl, Pfizer, Merck-MSD, MetaDeq and a speaker for Eli Lilly, Novo Nordisk, and Pfizer. Dr. Kailayanathan is medical director at Perspectum, a UK-based company involved in liver imaging technology.
A version of this article first appeared on Medscape.com.
MADRID — Individuals with type 2 diabetes and/or obesity plus one or more metabolic risk factors are at a higher risk for metabolic dysfunction–associated steatotic liver disease (MASLD) with fibrosis and progression to more severe liver disease, stated new European guidelines that provide recommendations for diagnosis and management.
“The availability of improved treatment options underlines the need to identify at-risk individuals with MASLD early, as we now possess the tools to positively influence the course of the diseases, which is expected to prevent relevant clinical events,” stated the clinical practice guidelines, updated for the first time since 2016.
“Now we have guidelines that tell clinicians how to monitor the liver,” said Amalia Gastaldelli, PhD, research director at the Institute of Clinical Physiology of the National Research Council in Pisa, Italy, and a member of the panel that developed the guidelines.
Dr. Gastaldelli moderated a session focused on the guidelines at the annual meeting of the European Association for the Study of Diabetes (EASD). In an interview after the session, Dr. Gastaldelli, who leads a cardiometabolic risk research group, stressed the importance of the liver’s role in the body and the need for diabetes specialists to start paying more attention to this vital organ.
“It’s an important organ for monitoring because liver disease is silent, and the patient doesn’t feel unwell until disease is severe,” she said. “Diabetologists already monitor the eye, the heart, the kidney, and so on, but the liver is often neglected,” she said. A 2024 study found that the global pooled prevalence of MASLD among patients with type 2 diabetes was 65.33%.
Dr. Gastaldelli noted the importance of liver status in diabetes care. The liver makes triglycerides and very-low-density lipoprotein cholesterol, which are all major risk factors for atherosclerosis and cardiovascular disease (CVD), she said, as well as producing glucose, which in excess can lead to hyperglycemia.
The guidelines were jointly written by EASD, the European Association for the Study of the Liver, and the European Association for the Study of Obesity, and published in Diabetologia, The Journal of Hepatology, and Obesity Facts.
A Metabolic Condition
In the EASD meeting session, Dr. Gastaldelli discussed the reasons for, and implications of, shifting the name from nonalcoholic fatty liver disease (NAFLD) to MASLD.
“The name change focuses on the fact that this is a metabolic disease, while NAFLD had no mention of this and was considered stigmatizing by patients, especially in relation to the words ‘fatty’ and ‘nonalcoholic,’” she said.
According to the guidelines, MASLD is defined as liver steatosis in the presence of one or more cardiometabolic risk factor(s) and the absence of excess alcohol intake.
MASLD has become the most common chronic liver disease and includes isolated steatosis, metabolic dysfunction-associated steatohepatitis (MASH, previously NASH), MASH-related fibrosis, and cirrhosis.
In the overarching group of steatotic liver disease, a totally new intermediate category has been added: MASLD with moderate (increased) alcohol intake (MetALD), which represents MASLD in people who consume greater amounts of alcohol per week (140-350 g/week and 210-420 g/week for women and men, respectively).
The change in the nomenclature has been incremental and regional, Dr. Gastaldelli said. “The definition first changed from NAFLD to MAFLD, which recognizes the importance of metabolism in the pathophysiology of this disease but does not take into account alcohol intake. MAFLD is still used in Asia, Australasia, and North Africa, while Europe and the Americas have endorsed MASLD.”
Case-Finding and Diagnosis
Identifying MASLD cases in people at risk remains incidental, largely because it is a silent disease and is symptom-free until it becomes severe, said Dr. Gastaldelli.
The guideline recognizes that individuals with type 2 diabetes or obesity with additional metabolic risk factor(s) are at a higher risk for MASLD with fibrosis and progression to MASH.
Assessment strategies for severe liver fibrosis in MASLD include the use of noninvasive tests in people who have cardiometabolic risk factors, abnormal liver enzymes, and/or radiological signs of hepatic steatosis, particularly in the presence of type 2 diabetes or obesity or in the presence of one or more metabolic risk factors.
Dr. Gastaldelli noted that type 2 diabetes, metabolic syndrome, and obesity, including abdominal obesity identified by large waist circumference, are the major risk factors and should be warning signs.
“We need to consider abdominal obesity too — we’ve published data in relatively lean people, body mass index < 25, with MASH but without diabetes. Most of the patients accumulated fat viscerally and in the liver and had hypertriglyceridemia and hypercholesterolemia,” she said.
“The guidelines reflect this because the definition of MASLD includes steatosis plus at least one metabolic factor — waist circumference, for example, which is related to visceral fat, hyperlipidemia, or hyperglycemia. Of note, in both pharmacological and diet-induced weight loss, the decrease in liver fat was associated with the decrease in visceral fat.”
The noninvasive biomarker test, Fibrosis-4 (FIB-4) may be used to assess the risk for liver fibrosis. The FIB-4 index is calculated using a patient’s age and results of three blood tests — aspartate aminotransferase, alanine aminotransferase, and platelet count.
Advanced fibrosis (grade F3-F4) “is a major risk factor for severe outcomes,” said Dr. Gastaldelli. A FIB-4 test result below 1.3 indicates low risk for advanced liver fibrosis, 1.30-2.67 indicates intermediate risk, and above 2.67 indicates high risk.
“When fibrosis increases, then liver enzymes increase and the platelets decrease,” said Dr. Gastaldelli. “It is not a perfect tool, and we need to add in age because at a young age, it is prone to false negatives and when very old — false positives. It’s important to take a global view, especially if the patient has persistent high liver enzymes, but FIB-4 is low.”
“And if they have more than one metabolic risk factor, proceed with more tests, for example, transient elastography,” she advised. Imaging techniques such as transient elastography may rule out or rule in advanced fibrosis, which is predictive of liver-related outcomes.
“However, imaging techniques only diagnose steatosis and fibrosis, and right now, MASH can only be diagnosed with liver biopsy because we do not have any markers of liver inflammation and ballooning. In the future, noninvasive tests based on imaging and blood tests will be used to identify patients with MASH,” she added.
Management of MASLD — Lifestyle and Treatment
“Pharmacological treatments are designed for [patients] with MASH and fibrosis grade F2 or F3, but not MASLD,” Dr. Gastaldelli said. As such, lifestyle interventions are the mainstay of management — including weight loss, dietary changes, physical exercise, and low to no alcohol consumption. “Eating good-quality food and reducing calories are both important because the metabolism responds differently to different nutrients,” Dr. Gastaldelli said.
“In particular, the guidelines advise dietary management because some foods carry liver toxicity, for example, sugary foods with sucrose/fructose especially,” she said, adding that, “complex carbohydrates are less harmful than refined carbohydrates. Processed foods should be avoided if possible because they contain sugars, [as well as] saturated fats and hydrogenated fat, which is particularly bad for the liver. Olive oil is better than butter or margarine, which are rich in saturated fat, and fish and white meat are preferable.”
She added that a diet to help manage type 2 diabetes was not so dissimilar because sugar again needs to be reduced.
If a patient has severe obesity (and MASLD), data show that bariatric surgery is beneficial. “It not only helps weight loss, but it improves liver histology and has been shown to improve or resolve type 2 diabetes and reduce CVD risk. Importantly, regarding fibrosis, nutritional management after the bariatric surgery is the most important thing,” said Dr. Gastaldelli.
Optimal management of comorbidities — including the use of incretin-based therapies such as semaglutide or tirzepatide for type 2 diabetes or obesity, if indicated — is advised, according to the guidelines.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been shown to have a beneficial effect on MASH, said Dr. Gastaldelli. “They have not shown effectiveness in the resolution of fibrosis, but this might take longer to manifest. However, if the medication is started early enough, it may prevent severe fibrosis. Significant weight loss, both with lifestyle and pharmacological treatment, should lead to an improvement in the liver too.”
There are currently no drugs available in Europe for the treatment of noncirrhotic MASH and severe fibrosis (stage ≥ 2). Resmetirom is the first approved MASH-targeted treatment in noncirrhotic MASH and significant liver fibrosis, with histological effectiveness on steatohepatitis and fibrosis, together with an acceptable safety and tolerability profile, but, for the moment, this agent is only available in United States.
Finally, turning to MASH-related cirrhosis, the guidelines advise adaptations of metabolic drugs, nutritional counseling, and surveillance for portal hypertension and hepatocellular carcinoma, as well as liver transplantation in decompensated cirrhosis.
After the session, this news organization spoke to Tushy Kailayanathan, MBBS BSc, medical director of the liver MRI company, Perspectum, who reviewed the limitations of the FIB-4 test. The FIB-4 test identifies those with advanced fibrosis in the liver, for example, patients with hepatitis C, she noted; however, “it performs worse in type 2 diabetic patients and in the elderly. There is little clinical guidance on the adjustment of FIB-4 thresholds needed for these high cardiometabolic risk groups. The priority patients are missed by FIB-4 because those individuals with early and active disease may not yet have progressed to advanced disease detected by FIB-4.”
These individuals are exactly those amenable to primary care prevention strategies, said Dr. Kailayanathan. Because of the nature of early and active liver disease in patients with high cardiometabolic risk, it would make sense to shift some diagnostic protocols into primary care.
“These individuals are exactly those amenable to primary care prevention strategies at annual diabetic review because they are likely to have modifiable cardiometabolic risk factors such as metabolic syndrome and would benefit from lifestyle and therapeutic intervention, including GLP-1 RAs and SGLT2is [sodium-glucose cotransporter-2 inhibitors],” she said. “Case-finding and detection of early-stage MASLD is a priority in diabetics, and there is an unmet need for accurate biomarkers to measure liver fat and inflammation early.”
Dr. Gastaldelli has been on the advisory board or consulting for Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Fractyl, Pfizer, Merck-MSD, MetaDeq and a speaker for Eli Lilly, Novo Nordisk, and Pfizer. Dr. Kailayanathan is medical director at Perspectum, a UK-based company involved in liver imaging technology.
A version of this article first appeared on Medscape.com.
MADRID — Individuals with type 2 diabetes and/or obesity plus one or more metabolic risk factors are at a higher risk for metabolic dysfunction–associated steatotic liver disease (MASLD) with fibrosis and progression to more severe liver disease, stated new European guidelines that provide recommendations for diagnosis and management.
“The availability of improved treatment options underlines the need to identify at-risk individuals with MASLD early, as we now possess the tools to positively influence the course of the diseases, which is expected to prevent relevant clinical events,” stated the clinical practice guidelines, updated for the first time since 2016.
“Now we have guidelines that tell clinicians how to monitor the liver,” said Amalia Gastaldelli, PhD, research director at the Institute of Clinical Physiology of the National Research Council in Pisa, Italy, and a member of the panel that developed the guidelines.
Dr. Gastaldelli moderated a session focused on the guidelines at the annual meeting of the European Association for the Study of Diabetes (EASD). In an interview after the session, Dr. Gastaldelli, who leads a cardiometabolic risk research group, stressed the importance of the liver’s role in the body and the need for diabetes specialists to start paying more attention to this vital organ.
“It’s an important organ for monitoring because liver disease is silent, and the patient doesn’t feel unwell until disease is severe,” she said. “Diabetologists already monitor the eye, the heart, the kidney, and so on, but the liver is often neglected,” she said. A 2024 study found that the global pooled prevalence of MASLD among patients with type 2 diabetes was 65.33%.
Dr. Gastaldelli noted the importance of liver status in diabetes care. The liver makes triglycerides and very-low-density lipoprotein cholesterol, which are all major risk factors for atherosclerosis and cardiovascular disease (CVD), she said, as well as producing glucose, which in excess can lead to hyperglycemia.
The guidelines were jointly written by EASD, the European Association for the Study of the Liver, and the European Association for the Study of Obesity, and published in Diabetologia, The Journal of Hepatology, and Obesity Facts.
A Metabolic Condition
In the EASD meeting session, Dr. Gastaldelli discussed the reasons for, and implications of, shifting the name from nonalcoholic fatty liver disease (NAFLD) to MASLD.
“The name change focuses on the fact that this is a metabolic disease, while NAFLD had no mention of this and was considered stigmatizing by patients, especially in relation to the words ‘fatty’ and ‘nonalcoholic,’” she said.
According to the guidelines, MASLD is defined as liver steatosis in the presence of one or more cardiometabolic risk factor(s) and the absence of excess alcohol intake.
MASLD has become the most common chronic liver disease and includes isolated steatosis, metabolic dysfunction-associated steatohepatitis (MASH, previously NASH), MASH-related fibrosis, and cirrhosis.
In the overarching group of steatotic liver disease, a totally new intermediate category has been added: MASLD with moderate (increased) alcohol intake (MetALD), which represents MASLD in people who consume greater amounts of alcohol per week (140-350 g/week and 210-420 g/week for women and men, respectively).
The change in the nomenclature has been incremental and regional, Dr. Gastaldelli said. “The definition first changed from NAFLD to MAFLD, which recognizes the importance of metabolism in the pathophysiology of this disease but does not take into account alcohol intake. MAFLD is still used in Asia, Australasia, and North Africa, while Europe and the Americas have endorsed MASLD.”
Case-Finding and Diagnosis
Identifying MASLD cases in people at risk remains incidental, largely because it is a silent disease and is symptom-free until it becomes severe, said Dr. Gastaldelli.
The guideline recognizes that individuals with type 2 diabetes or obesity with additional metabolic risk factor(s) are at a higher risk for MASLD with fibrosis and progression to MASH.
Assessment strategies for severe liver fibrosis in MASLD include the use of noninvasive tests in people who have cardiometabolic risk factors, abnormal liver enzymes, and/or radiological signs of hepatic steatosis, particularly in the presence of type 2 diabetes or obesity or in the presence of one or more metabolic risk factors.
Dr. Gastaldelli noted that type 2 diabetes, metabolic syndrome, and obesity, including abdominal obesity identified by large waist circumference, are the major risk factors and should be warning signs.
“We need to consider abdominal obesity too — we’ve published data in relatively lean people, body mass index < 25, with MASH but without diabetes. Most of the patients accumulated fat viscerally and in the liver and had hypertriglyceridemia and hypercholesterolemia,” she said.
“The guidelines reflect this because the definition of MASLD includes steatosis plus at least one metabolic factor — waist circumference, for example, which is related to visceral fat, hyperlipidemia, or hyperglycemia. Of note, in both pharmacological and diet-induced weight loss, the decrease in liver fat was associated with the decrease in visceral fat.”
The noninvasive biomarker test, Fibrosis-4 (FIB-4) may be used to assess the risk for liver fibrosis. The FIB-4 index is calculated using a patient’s age and results of three blood tests — aspartate aminotransferase, alanine aminotransferase, and platelet count.
Advanced fibrosis (grade F3-F4) “is a major risk factor for severe outcomes,” said Dr. Gastaldelli. A FIB-4 test result below 1.3 indicates low risk for advanced liver fibrosis, 1.30-2.67 indicates intermediate risk, and above 2.67 indicates high risk.
“When fibrosis increases, then liver enzymes increase and the platelets decrease,” said Dr. Gastaldelli. “It is not a perfect tool, and we need to add in age because at a young age, it is prone to false negatives and when very old — false positives. It’s important to take a global view, especially if the patient has persistent high liver enzymes, but FIB-4 is low.”
“And if they have more than one metabolic risk factor, proceed with more tests, for example, transient elastography,” she advised. Imaging techniques such as transient elastography may rule out or rule in advanced fibrosis, which is predictive of liver-related outcomes.
“However, imaging techniques only diagnose steatosis and fibrosis, and right now, MASH can only be diagnosed with liver biopsy because we do not have any markers of liver inflammation and ballooning. In the future, noninvasive tests based on imaging and blood tests will be used to identify patients with MASH,” she added.
Management of MASLD — Lifestyle and Treatment
“Pharmacological treatments are designed for [patients] with MASH and fibrosis grade F2 or F3, but not MASLD,” Dr. Gastaldelli said. As such, lifestyle interventions are the mainstay of management — including weight loss, dietary changes, physical exercise, and low to no alcohol consumption. “Eating good-quality food and reducing calories are both important because the metabolism responds differently to different nutrients,” Dr. Gastaldelli said.
“In particular, the guidelines advise dietary management because some foods carry liver toxicity, for example, sugary foods with sucrose/fructose especially,” she said, adding that, “complex carbohydrates are less harmful than refined carbohydrates. Processed foods should be avoided if possible because they contain sugars, [as well as] saturated fats and hydrogenated fat, which is particularly bad for the liver. Olive oil is better than butter or margarine, which are rich in saturated fat, and fish and white meat are preferable.”
She added that a diet to help manage type 2 diabetes was not so dissimilar because sugar again needs to be reduced.
If a patient has severe obesity (and MASLD), data show that bariatric surgery is beneficial. “It not only helps weight loss, but it improves liver histology and has been shown to improve or resolve type 2 diabetes and reduce CVD risk. Importantly, regarding fibrosis, nutritional management after the bariatric surgery is the most important thing,” said Dr. Gastaldelli.
Optimal management of comorbidities — including the use of incretin-based therapies such as semaglutide or tirzepatide for type 2 diabetes or obesity, if indicated — is advised, according to the guidelines.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been shown to have a beneficial effect on MASH, said Dr. Gastaldelli. “They have not shown effectiveness in the resolution of fibrosis, but this might take longer to manifest. However, if the medication is started early enough, it may prevent severe fibrosis. Significant weight loss, both with lifestyle and pharmacological treatment, should lead to an improvement in the liver too.”
There are currently no drugs available in Europe for the treatment of noncirrhotic MASH and severe fibrosis (stage ≥ 2). Resmetirom is the first approved MASH-targeted treatment in noncirrhotic MASH and significant liver fibrosis, with histological effectiveness on steatohepatitis and fibrosis, together with an acceptable safety and tolerability profile, but, for the moment, this agent is only available in United States.
Finally, turning to MASH-related cirrhosis, the guidelines advise adaptations of metabolic drugs, nutritional counseling, and surveillance for portal hypertension and hepatocellular carcinoma, as well as liver transplantation in decompensated cirrhosis.
After the session, this news organization spoke to Tushy Kailayanathan, MBBS BSc, medical director of the liver MRI company, Perspectum, who reviewed the limitations of the FIB-4 test. The FIB-4 test identifies those with advanced fibrosis in the liver, for example, patients with hepatitis C, she noted; however, “it performs worse in type 2 diabetic patients and in the elderly. There is little clinical guidance on the adjustment of FIB-4 thresholds needed for these high cardiometabolic risk groups. The priority patients are missed by FIB-4 because those individuals with early and active disease may not yet have progressed to advanced disease detected by FIB-4.”
These individuals are exactly those amenable to primary care prevention strategies, said Dr. Kailayanathan. Because of the nature of early and active liver disease in patients with high cardiometabolic risk, it would make sense to shift some diagnostic protocols into primary care.
“These individuals are exactly those amenable to primary care prevention strategies at annual diabetic review because they are likely to have modifiable cardiometabolic risk factors such as metabolic syndrome and would benefit from lifestyle and therapeutic intervention, including GLP-1 RAs and SGLT2is [sodium-glucose cotransporter-2 inhibitors],” she said. “Case-finding and detection of early-stage MASLD is a priority in diabetics, and there is an unmet need for accurate biomarkers to measure liver fat and inflammation early.”
Dr. Gastaldelli has been on the advisory board or consulting for Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Fractyl, Pfizer, Merck-MSD, MetaDeq and a speaker for Eli Lilly, Novo Nordisk, and Pfizer. Dr. Kailayanathan is medical director at Perspectum, a UK-based company involved in liver imaging technology.
A version of this article first appeared on Medscape.com.
FROM EASD 2024
Hyperandrogenic PCOS Linked to Lower Pregnancy and Live Birth Rates
TOPLINE:
Women with hyperandrogenic polycystic ovary syndrome (PCOS) have lower pregnancy (29.9%) and live birth rates (20.1%) than those with nonhyperandrogenic PCOS (40.2% and 33.1%, respectively).
METHODOLOGY:
- Researchers conducted a retrospective cohort study of 1376 participants from the PPCOS I and II trials, all meeting National Institutes of Health diagnostic criteria for PCOS.
- Participants were categorized into hyperandrogenic (A and B) and nonhyperandrogenic (D) PCOS phenotypes on the basis of medical interviews, demographics, physical examinations, and laboratory data.
- Outcomes of interest included clinical pregnancy, pregnancy loss, live birth, obstetric complications, and neonatal outcomes.
- Fasting blood samples were analyzed for hormonal assays, and Homeostatic Model Assessment for Insulin Resistance scores were calculated using fasting glucose and insulin values.
TAKEAWAY:
- Participants with hyperandrogenic PCOS had higher body mass index (35.5 ± 8.9 vs 31.9 ± 9.3; P < .001) and fasting insulin levels (21.6 ± 27.7 vs 14.7 ± 15.0 μIU/mL; P < .001) than those with nonhyperandrogenic PCOS.
- Participants with hyperandrogenic PCOS had lower odds of achieving pregnancy (odds ratio [OR], 0.63; 95% CI, 0.44-0.92) and live birth (OR, 0.51; 95% CI, 0.34-0.76) than those with nonhyperandrogenic PCOS.
- No significant differences were found in pregnancy loss rates (23.9% vs 32.3%, P = .06) or neonatal outcomes between the two groups.
- The study lacked the power to detect differences in neonatal outcomes because of the low prevalence of these outcomes.
IN PRACTICE:
“Patients with nonhyperandrogenic PCOS may represent a different disease process with unique morbidities and outcomes and could be counseled differently than hyperandrogenic PCOS,” wrote the authors of the study.
SOURCE:
The study was led by Jessica L. Chan, MD, MSCE, Cedars-Sinai Medical Center in Los Angeles, California. It was published online in Obstetrics & Gynecology.
LIMITATIONS:
The primary limitation of this study was that it is a secondary analysis of previously collected randomized controlled trial data, which may affect the availability of certain information. Additionally, the lower number of participants in the nonhyperandrogenic PCOS group could affect the power of the results. The study was underpowered to detect statistically significant differences in neonatal outcomes because of their low prevalence.
DISCLOSURES:
The study was supported by a grant from the ASRM/NICHD/Duke Clinical Research/Reproductive Scientist Training Program. One coauthor disclosed receiving payments from Celmatix, Ferring Pharmaceuticals, Exeltis, Organon, and Monsanto; another disclosed receiving payments from Ferring Pharmaceuticals. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with hyperandrogenic polycystic ovary syndrome (PCOS) have lower pregnancy (29.9%) and live birth rates (20.1%) than those with nonhyperandrogenic PCOS (40.2% and 33.1%, respectively).
METHODOLOGY:
- Researchers conducted a retrospective cohort study of 1376 participants from the PPCOS I and II trials, all meeting National Institutes of Health diagnostic criteria for PCOS.
- Participants were categorized into hyperandrogenic (A and B) and nonhyperandrogenic (D) PCOS phenotypes on the basis of medical interviews, demographics, physical examinations, and laboratory data.
- Outcomes of interest included clinical pregnancy, pregnancy loss, live birth, obstetric complications, and neonatal outcomes.
- Fasting blood samples were analyzed for hormonal assays, and Homeostatic Model Assessment for Insulin Resistance scores were calculated using fasting glucose and insulin values.
TAKEAWAY:
- Participants with hyperandrogenic PCOS had higher body mass index (35.5 ± 8.9 vs 31.9 ± 9.3; P < .001) and fasting insulin levels (21.6 ± 27.7 vs 14.7 ± 15.0 μIU/mL; P < .001) than those with nonhyperandrogenic PCOS.
- Participants with hyperandrogenic PCOS had lower odds of achieving pregnancy (odds ratio [OR], 0.63; 95% CI, 0.44-0.92) and live birth (OR, 0.51; 95% CI, 0.34-0.76) than those with nonhyperandrogenic PCOS.
- No significant differences were found in pregnancy loss rates (23.9% vs 32.3%, P = .06) or neonatal outcomes between the two groups.
- The study lacked the power to detect differences in neonatal outcomes because of the low prevalence of these outcomes.
IN PRACTICE:
“Patients with nonhyperandrogenic PCOS may represent a different disease process with unique morbidities and outcomes and could be counseled differently than hyperandrogenic PCOS,” wrote the authors of the study.
SOURCE:
The study was led by Jessica L. Chan, MD, MSCE, Cedars-Sinai Medical Center in Los Angeles, California. It was published online in Obstetrics & Gynecology.
LIMITATIONS:
The primary limitation of this study was that it is a secondary analysis of previously collected randomized controlled trial data, which may affect the availability of certain information. Additionally, the lower number of participants in the nonhyperandrogenic PCOS group could affect the power of the results. The study was underpowered to detect statistically significant differences in neonatal outcomes because of their low prevalence.
DISCLOSURES:
The study was supported by a grant from the ASRM/NICHD/Duke Clinical Research/Reproductive Scientist Training Program. One coauthor disclosed receiving payments from Celmatix, Ferring Pharmaceuticals, Exeltis, Organon, and Monsanto; another disclosed receiving payments from Ferring Pharmaceuticals. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with hyperandrogenic polycystic ovary syndrome (PCOS) have lower pregnancy (29.9%) and live birth rates (20.1%) than those with nonhyperandrogenic PCOS (40.2% and 33.1%, respectively).
METHODOLOGY:
- Researchers conducted a retrospective cohort study of 1376 participants from the PPCOS I and II trials, all meeting National Institutes of Health diagnostic criteria for PCOS.
- Participants were categorized into hyperandrogenic (A and B) and nonhyperandrogenic (D) PCOS phenotypes on the basis of medical interviews, demographics, physical examinations, and laboratory data.
- Outcomes of interest included clinical pregnancy, pregnancy loss, live birth, obstetric complications, and neonatal outcomes.
- Fasting blood samples were analyzed for hormonal assays, and Homeostatic Model Assessment for Insulin Resistance scores were calculated using fasting glucose and insulin values.
TAKEAWAY:
- Participants with hyperandrogenic PCOS had higher body mass index (35.5 ± 8.9 vs 31.9 ± 9.3; P < .001) and fasting insulin levels (21.6 ± 27.7 vs 14.7 ± 15.0 μIU/mL; P < .001) than those with nonhyperandrogenic PCOS.
- Participants with hyperandrogenic PCOS had lower odds of achieving pregnancy (odds ratio [OR], 0.63; 95% CI, 0.44-0.92) and live birth (OR, 0.51; 95% CI, 0.34-0.76) than those with nonhyperandrogenic PCOS.
- No significant differences were found in pregnancy loss rates (23.9% vs 32.3%, P = .06) or neonatal outcomes between the two groups.
- The study lacked the power to detect differences in neonatal outcomes because of the low prevalence of these outcomes.
IN PRACTICE:
“Patients with nonhyperandrogenic PCOS may represent a different disease process with unique morbidities and outcomes and could be counseled differently than hyperandrogenic PCOS,” wrote the authors of the study.
SOURCE:
The study was led by Jessica L. Chan, MD, MSCE, Cedars-Sinai Medical Center in Los Angeles, California. It was published online in Obstetrics & Gynecology.
LIMITATIONS:
The primary limitation of this study was that it is a secondary analysis of previously collected randomized controlled trial data, which may affect the availability of certain information. Additionally, the lower number of participants in the nonhyperandrogenic PCOS group could affect the power of the results. The study was underpowered to detect statistically significant differences in neonatal outcomes because of their low prevalence.
DISCLOSURES:
The study was supported by a grant from the ASRM/NICHD/Duke Clinical Research/Reproductive Scientist Training Program. One coauthor disclosed receiving payments from Celmatix, Ferring Pharmaceuticals, Exeltis, Organon, and Monsanto; another disclosed receiving payments from Ferring Pharmaceuticals. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.

