User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Artificial Intelligence for Clinical Decision Support
There is abundant research being conducted on the use of artificial intelligence (AI) to improve diagnosis in dermatology. Recently, convolutional neural networks trained using large image libraries have achieved parity with dermatologists in discriminating between benign and malignant lesions.1 There are expectations that these systems, as they improve and are implemented in mobile electronic devices, will revolutionize diagnosis. Substantially less attention has been given to the use of AI to guide management options following a diagnosis. There are several reasons this area lends itself to the application of AI.
In 2015, the National Library of Medicine indexed more than 800,000 articles.2 Medical literature is growing at an overwhelming pace that makes it challenging for health care professionals to read, retain, and appropriately implement the latest research into their care. One survey found that physicians spend no more than 4 hours per week reading medical journals, and for the majority of articles, only the abstracts are read.3 Conversely, AI networks today are able to interpret millions of pages of data within seconds. It is worth investigating how AI can be used to improve treatment and management decisions made by physicians.
Cognitive computing is a modern approach to AI that incorporates natural language processing, machine learning, and other techniques to answer questions. One cognitive computing system developed by IBM research in 2007, Watson, can interpret a user’s query using natural language processing and then generate hypotheses. It searches data sources extensively to find and score evidence for each candidate hypothesis.4 This information is synthesized to provide a simple output: ranked answers with associated confidence scores. Machine learning is used to improve the answers with feedback, training, and repetition.4,5
Watson Oncology, an ongoing collaboration between IBM and Memorial Sloan Kettering Cancer Center, is an application of cognitive computing to medicine. At Memorial Sloan Kettering, Watson has been trained by expert clinicians to provide an individualized, evidence-based list of therapeutic options for oncologists and patients to discuss. Furthermore, Watson is capable of taking patient preferences into consideration.4
In the near future, there also may be a role that cognitive computing could play in aiding dermatologists. Dermatologists manage a multitude of conditions requiring systemic therapies such as chemotherapeutics, biologics, and immunosuppressant medications. Frequently, the patient population has a complicated medical history with multiple comorbidities. Although current electronic health record (EHR) systems are able to assist physicians with structured numerical data such as vitals and laboratory results, cognitive computing systems could interpret the natural language of journal articles, textbooks, and published guidelines, as well as the narrative components of EHR notes. Outcomes from similar patients also could be used as inputs. With enough data, cognitive computing systems may be able to identify associations and epidemiologic trends that would not otherwise be noticed. As described by Miotto et al,6 one system, “deep patient,” was able to accurately predict the development of schizophrenia, diabetes mellitus, and various cancers based on EHR data. Patient genetic information also could one day be used to generate new insights into pharmacogenomics.
The benefit of a cognitive computing decision support system is that ineffective treatments and adverse reactions could be minimized, which may improve outcomes and reduce costs. Artificial intelligence also could help to decrease work burden so that physicians can spend more time with their patients, resulting in improved patient satisfaction and overall increased access to the specialty.
As with other clinical decision support systems, a number of challenges exist with the integration of cognitive computing into real care. One obstacle unique to machine learning algorithms is the black box problem. For instance, the skin lesion–identifying neural network cannot be questioned to determine which factors it used to arrive at its diagnosis. This shortcoming can lead to dangerous situations, such as the one reported by Caruana et al.7 A predictive model classified patients with pneumonia and a history of asthma as having a lower mortality risk than those with pneumonia alone because the model was unable to recognize the confounder that asthmatic patients were preemptively admitted to the intensive care unit and treated more aggressively, which is another reason that AI recommendations must always be evaluated by a physician.7 Physician and patient input also will be integral to incorporate contextual and qualitative information that may not be accessible to computers.8
As cognitive computing decision support systems are primarily used in oncology, they will need to be adjusted to optimize them for dermatologic conditions. It also will be up to health care providers to benchmark the performance of these systems.
Current clinical decision support systems that do not use AI have struggled to improve major patient outcomes such as mortality. These systems have been hobbled by poor usability and human-computer integration. Clinicians find their alerts and warnings to be a nuisance. The adoption of cognitive computing systems has the potential to give clinicians an intelligent partner. Their natural language processing, ability to comprehend questions, and easily understandable output give them an inherent ease of use that simplifies interactions with clinicians. Rather than replacing physicians, these systems will free clinicians to spend more of their time on the components of care that only a human can provide.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The National Library of Medicine fact sheet. U.S. National Library of Medicine website https://www.nlm.nih.gov/pubs/factsheets/nlm.html. Updated October 20, 2016. Accessed June 18, 2018.
- Saint S, Christakis DA, Saha S, et al. Journal reading habits of internists. J Gen Intern Med. 2000;15:881-884.
- Kelly JE III, Hamm S. Smart Machines: IBM’s Watson and the Era of Cognitive Computing. New York, NY: Columbia University Press; 2013.
- Ferrucci D, Levas A, Bagchi S, et al. Watson: beyond Jeopardy! Artificial Intelligence. 2013;199:93-105.
- Miotto R, Li L, Kidd BA, et al. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094.
- Caruana R, Lou Y, Gehrke J, et al. Intelligible models for healthcare: predicting pneumonia risk and hospital 30-day readmission. Paper presented at: 21st ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 2015; August 10-13, 2015; Sydney, Australia.
- Verghese A, Shah NH, Harrington RA. What this computer needs is a physician: humanism and artificial intelligence. JAMA. 2018;319:19-20.
There is abundant research being conducted on the use of artificial intelligence (AI) to improve diagnosis in dermatology. Recently, convolutional neural networks trained using large image libraries have achieved parity with dermatologists in discriminating between benign and malignant lesions.1 There are expectations that these systems, as they improve and are implemented in mobile electronic devices, will revolutionize diagnosis. Substantially less attention has been given to the use of AI to guide management options following a diagnosis. There are several reasons this area lends itself to the application of AI.
In 2015, the National Library of Medicine indexed more than 800,000 articles.2 Medical literature is growing at an overwhelming pace that makes it challenging for health care professionals to read, retain, and appropriately implement the latest research into their care. One survey found that physicians spend no more than 4 hours per week reading medical journals, and for the majority of articles, only the abstracts are read.3 Conversely, AI networks today are able to interpret millions of pages of data within seconds. It is worth investigating how AI can be used to improve treatment and management decisions made by physicians.
Cognitive computing is a modern approach to AI that incorporates natural language processing, machine learning, and other techniques to answer questions. One cognitive computing system developed by IBM research in 2007, Watson, can interpret a user’s query using natural language processing and then generate hypotheses. It searches data sources extensively to find and score evidence for each candidate hypothesis.4 This information is synthesized to provide a simple output: ranked answers with associated confidence scores. Machine learning is used to improve the answers with feedback, training, and repetition.4,5
Watson Oncology, an ongoing collaboration between IBM and Memorial Sloan Kettering Cancer Center, is an application of cognitive computing to medicine. At Memorial Sloan Kettering, Watson has been trained by expert clinicians to provide an individualized, evidence-based list of therapeutic options for oncologists and patients to discuss. Furthermore, Watson is capable of taking patient preferences into consideration.4
In the near future, there also may be a role that cognitive computing could play in aiding dermatologists. Dermatologists manage a multitude of conditions requiring systemic therapies such as chemotherapeutics, biologics, and immunosuppressant medications. Frequently, the patient population has a complicated medical history with multiple comorbidities. Although current electronic health record (EHR) systems are able to assist physicians with structured numerical data such as vitals and laboratory results, cognitive computing systems could interpret the natural language of journal articles, textbooks, and published guidelines, as well as the narrative components of EHR notes. Outcomes from similar patients also could be used as inputs. With enough data, cognitive computing systems may be able to identify associations and epidemiologic trends that would not otherwise be noticed. As described by Miotto et al,6 one system, “deep patient,” was able to accurately predict the development of schizophrenia, diabetes mellitus, and various cancers based on EHR data. Patient genetic information also could one day be used to generate new insights into pharmacogenomics.
The benefit of a cognitive computing decision support system is that ineffective treatments and adverse reactions could be minimized, which may improve outcomes and reduce costs. Artificial intelligence also could help to decrease work burden so that physicians can spend more time with their patients, resulting in improved patient satisfaction and overall increased access to the specialty.
As with other clinical decision support systems, a number of challenges exist with the integration of cognitive computing into real care. One obstacle unique to machine learning algorithms is the black box problem. For instance, the skin lesion–identifying neural network cannot be questioned to determine which factors it used to arrive at its diagnosis. This shortcoming can lead to dangerous situations, such as the one reported by Caruana et al.7 A predictive model classified patients with pneumonia and a history of asthma as having a lower mortality risk than those with pneumonia alone because the model was unable to recognize the confounder that asthmatic patients were preemptively admitted to the intensive care unit and treated more aggressively, which is another reason that AI recommendations must always be evaluated by a physician.7 Physician and patient input also will be integral to incorporate contextual and qualitative information that may not be accessible to computers.8
As cognitive computing decision support systems are primarily used in oncology, they will need to be adjusted to optimize them for dermatologic conditions. It also will be up to health care providers to benchmark the performance of these systems.
Current clinical decision support systems that do not use AI have struggled to improve major patient outcomes such as mortality. These systems have been hobbled by poor usability and human-computer integration. Clinicians find their alerts and warnings to be a nuisance. The adoption of cognitive computing systems has the potential to give clinicians an intelligent partner. Their natural language processing, ability to comprehend questions, and easily understandable output give them an inherent ease of use that simplifies interactions with clinicians. Rather than replacing physicians, these systems will free clinicians to spend more of their time on the components of care that only a human can provide.
There is abundant research being conducted on the use of artificial intelligence (AI) to improve diagnosis in dermatology. Recently, convolutional neural networks trained using large image libraries have achieved parity with dermatologists in discriminating between benign and malignant lesions.1 There are expectations that these systems, as they improve and are implemented in mobile electronic devices, will revolutionize diagnosis. Substantially less attention has been given to the use of AI to guide management options following a diagnosis. There are several reasons this area lends itself to the application of AI.
In 2015, the National Library of Medicine indexed more than 800,000 articles.2 Medical literature is growing at an overwhelming pace that makes it challenging for health care professionals to read, retain, and appropriately implement the latest research into their care. One survey found that physicians spend no more than 4 hours per week reading medical journals, and for the majority of articles, only the abstracts are read.3 Conversely, AI networks today are able to interpret millions of pages of data within seconds. It is worth investigating how AI can be used to improve treatment and management decisions made by physicians.
Cognitive computing is a modern approach to AI that incorporates natural language processing, machine learning, and other techniques to answer questions. One cognitive computing system developed by IBM research in 2007, Watson, can interpret a user’s query using natural language processing and then generate hypotheses. It searches data sources extensively to find and score evidence for each candidate hypothesis.4 This information is synthesized to provide a simple output: ranked answers with associated confidence scores. Machine learning is used to improve the answers with feedback, training, and repetition.4,5
Watson Oncology, an ongoing collaboration between IBM and Memorial Sloan Kettering Cancer Center, is an application of cognitive computing to medicine. At Memorial Sloan Kettering, Watson has been trained by expert clinicians to provide an individualized, evidence-based list of therapeutic options for oncologists and patients to discuss. Furthermore, Watson is capable of taking patient preferences into consideration.4
In the near future, there also may be a role that cognitive computing could play in aiding dermatologists. Dermatologists manage a multitude of conditions requiring systemic therapies such as chemotherapeutics, biologics, and immunosuppressant medications. Frequently, the patient population has a complicated medical history with multiple comorbidities. Although current electronic health record (EHR) systems are able to assist physicians with structured numerical data such as vitals and laboratory results, cognitive computing systems could interpret the natural language of journal articles, textbooks, and published guidelines, as well as the narrative components of EHR notes. Outcomes from similar patients also could be used as inputs. With enough data, cognitive computing systems may be able to identify associations and epidemiologic trends that would not otherwise be noticed. As described by Miotto et al,6 one system, “deep patient,” was able to accurately predict the development of schizophrenia, diabetes mellitus, and various cancers based on EHR data. Patient genetic information also could one day be used to generate new insights into pharmacogenomics.
The benefit of a cognitive computing decision support system is that ineffective treatments and adverse reactions could be minimized, which may improve outcomes and reduce costs. Artificial intelligence also could help to decrease work burden so that physicians can spend more time with their patients, resulting in improved patient satisfaction and overall increased access to the specialty.
As with other clinical decision support systems, a number of challenges exist with the integration of cognitive computing into real care. One obstacle unique to machine learning algorithms is the black box problem. For instance, the skin lesion–identifying neural network cannot be questioned to determine which factors it used to arrive at its diagnosis. This shortcoming can lead to dangerous situations, such as the one reported by Caruana et al.7 A predictive model classified patients with pneumonia and a history of asthma as having a lower mortality risk than those with pneumonia alone because the model was unable to recognize the confounder that asthmatic patients were preemptively admitted to the intensive care unit and treated more aggressively, which is another reason that AI recommendations must always be evaluated by a physician.7 Physician and patient input also will be integral to incorporate contextual and qualitative information that may not be accessible to computers.8
As cognitive computing decision support systems are primarily used in oncology, they will need to be adjusted to optimize them for dermatologic conditions. It also will be up to health care providers to benchmark the performance of these systems.
Current clinical decision support systems that do not use AI have struggled to improve major patient outcomes such as mortality. These systems have been hobbled by poor usability and human-computer integration. Clinicians find their alerts and warnings to be a nuisance. The adoption of cognitive computing systems has the potential to give clinicians an intelligent partner. Their natural language processing, ability to comprehend questions, and easily understandable output give them an inherent ease of use that simplifies interactions with clinicians. Rather than replacing physicians, these systems will free clinicians to spend more of their time on the components of care that only a human can provide.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The National Library of Medicine fact sheet. U.S. National Library of Medicine website https://www.nlm.nih.gov/pubs/factsheets/nlm.html. Updated October 20, 2016. Accessed June 18, 2018.
- Saint S, Christakis DA, Saha S, et al. Journal reading habits of internists. J Gen Intern Med. 2000;15:881-884.
- Kelly JE III, Hamm S. Smart Machines: IBM’s Watson and the Era of Cognitive Computing. New York, NY: Columbia University Press; 2013.
- Ferrucci D, Levas A, Bagchi S, et al. Watson: beyond Jeopardy! Artificial Intelligence. 2013;199:93-105.
- Miotto R, Li L, Kidd BA, et al. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094.
- Caruana R, Lou Y, Gehrke J, et al. Intelligible models for healthcare: predicting pneumonia risk and hospital 30-day readmission. Paper presented at: 21st ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 2015; August 10-13, 2015; Sydney, Australia.
- Verghese A, Shah NH, Harrington RA. What this computer needs is a physician: humanism and artificial intelligence. JAMA. 2018;319:19-20.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The National Library of Medicine fact sheet. U.S. National Library of Medicine website https://www.nlm.nih.gov/pubs/factsheets/nlm.html. Updated October 20, 2016. Accessed June 18, 2018.
- Saint S, Christakis DA, Saha S, et al. Journal reading habits of internists. J Gen Intern Med. 2000;15:881-884.
- Kelly JE III, Hamm S. Smart Machines: IBM’s Watson and the Era of Cognitive Computing. New York, NY: Columbia University Press; 2013.
- Ferrucci D, Levas A, Bagchi S, et al. Watson: beyond Jeopardy! Artificial Intelligence. 2013;199:93-105.
- Miotto R, Li L, Kidd BA, et al. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094.
- Caruana R, Lou Y, Gehrke J, et al. Intelligible models for healthcare: predicting pneumonia risk and hospital 30-day readmission. Paper presented at: 21st ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 2015; August 10-13, 2015; Sydney, Australia.
- Verghese A, Shah NH, Harrington RA. What this computer needs is a physician: humanism and artificial intelligence. JAMA. 2018;319:19-20.
Topical Corticosteroids for Treatment-Resistant Atopic Dermatitis
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
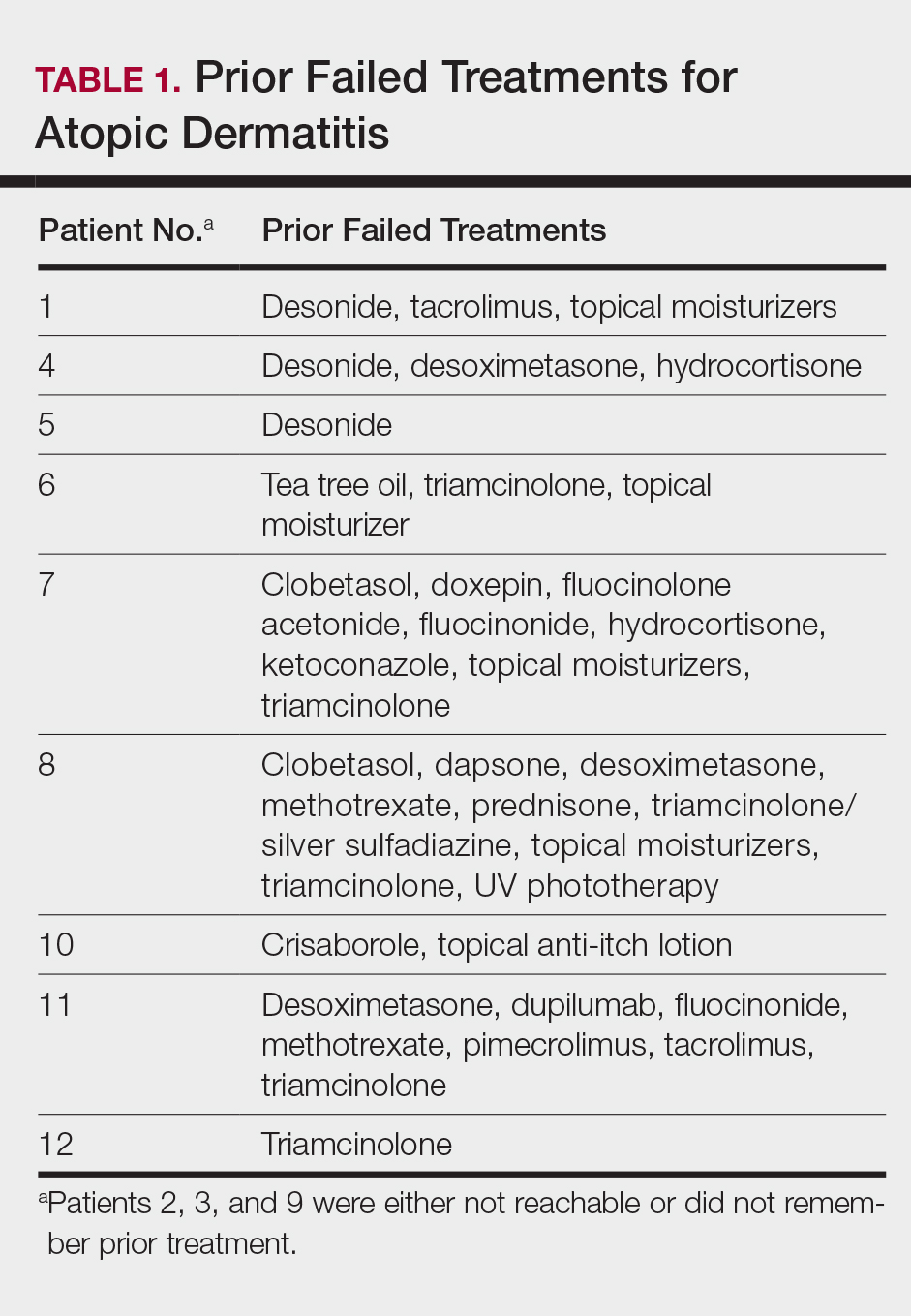
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
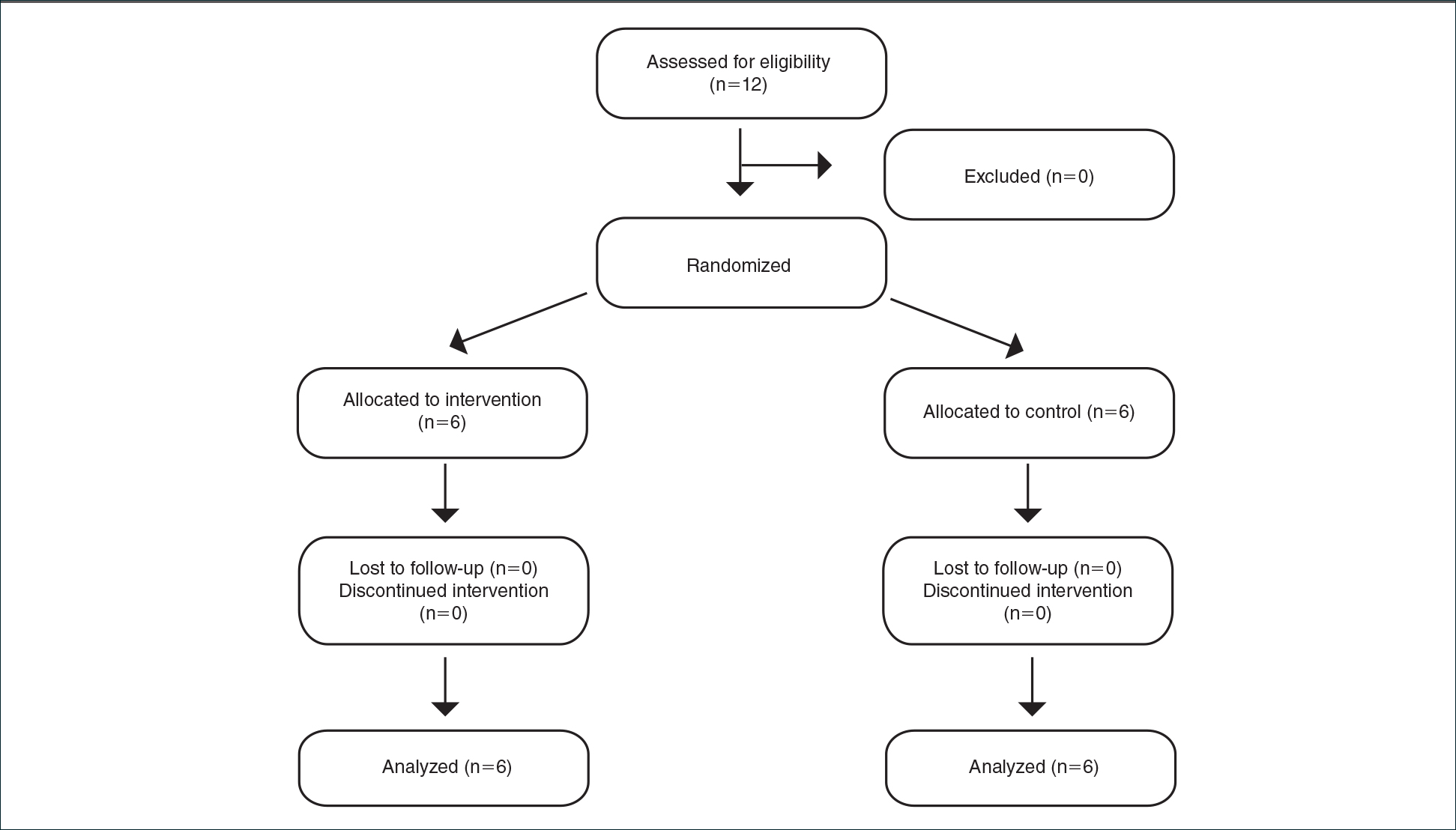
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
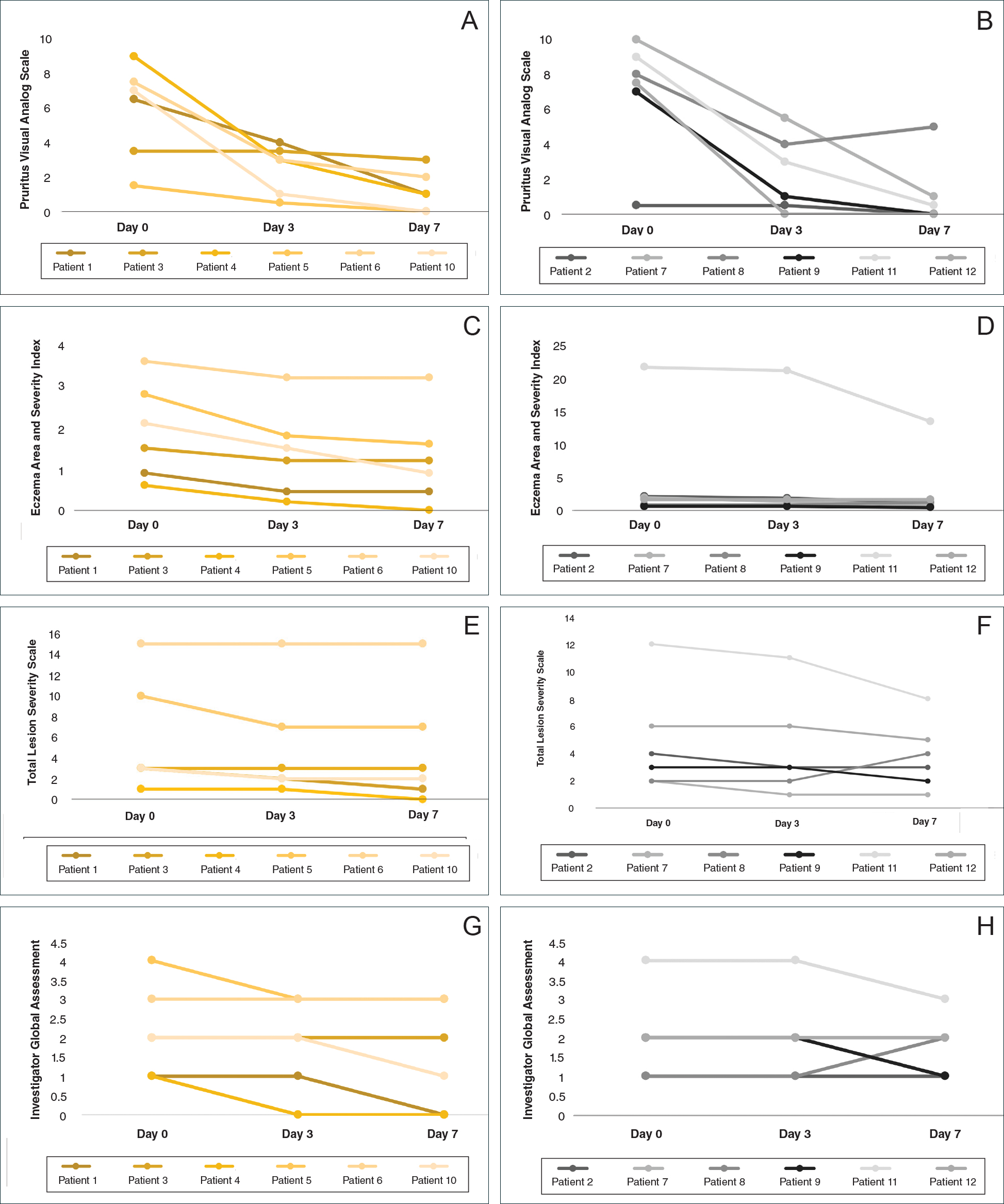
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
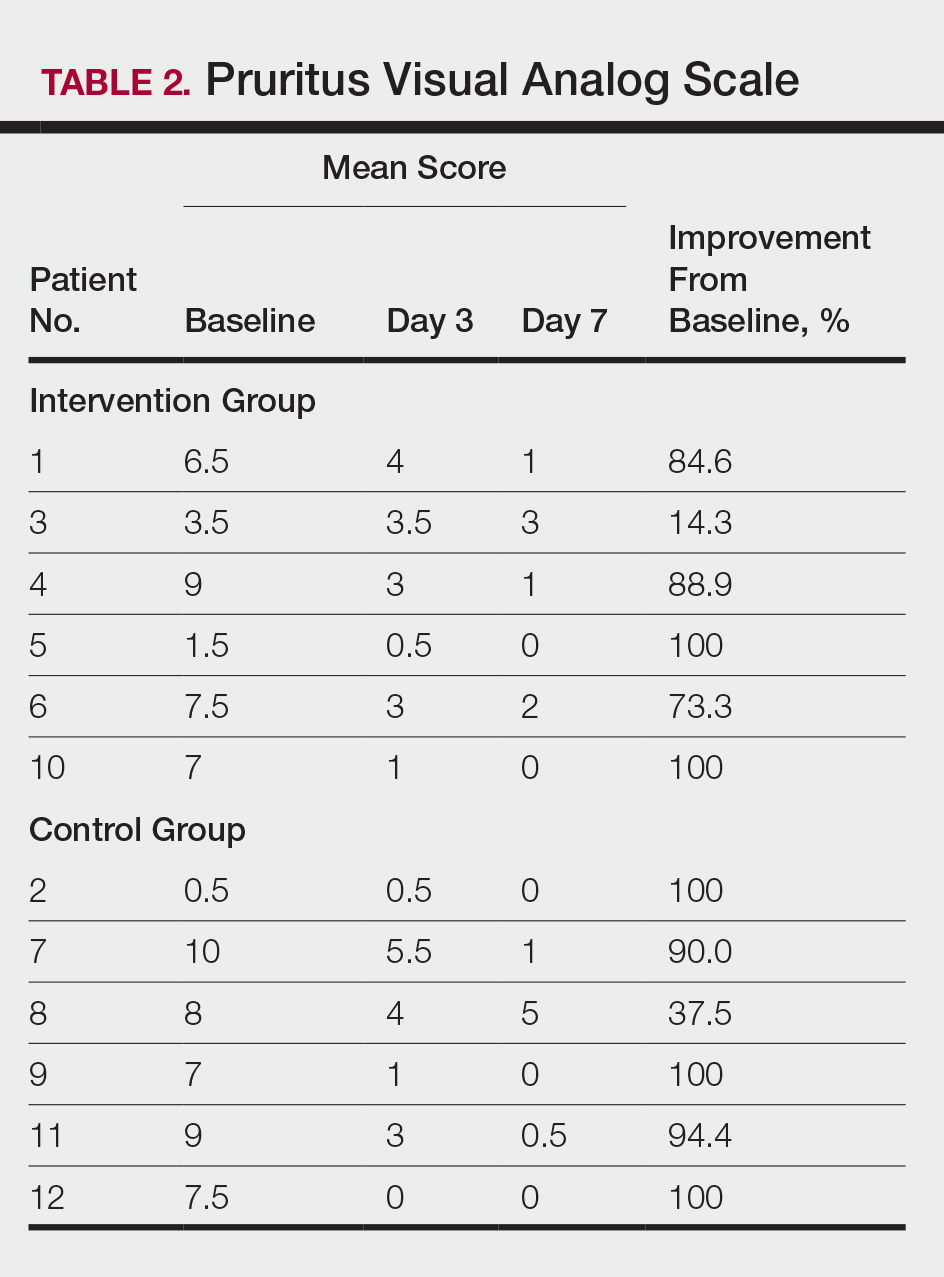
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.
Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.

Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.

Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
Practice Points
- Mid-potency corticosteroids are the first-line treatment of atopic dermatitis (AD).
- Atopic dermatitis may fail to respond to topical corticosteroids initially or lose response over time, a phenomenon known as tachyphylaxis.
- Nonadherence to medication is the most likely cause of treatment resistance in patients with AD.
Dupilumab for Off-Label Treatment of Moderate to Severe Childhood Atopic Dermatitis
Case Report
A 7-year-old boy with a history of shellfish anaphylaxis, pollen allergy, asthma, rhinoconjunctivitis, frequent headaches and ear infections, sinusitis, bronchitis, vitiligo, warts, and cold sores presented to our dermatology clinic for evaluation of a widespread crusting, cracking, red rash that had been present since 6 months of age. The patient’s mother reported that he had many sleepless nights from uncontrolled itching. His medications included albuterol solution for nebulization, loratadine, and montelukast. Prior to the current presentation he had been treated with triamcinolone and betamethasone creams by the pediatrician. Despite compliance with topical therapy, his mother stated the itching persisted and lesions lingered with minimal improvement. He also was treated with oral corticosteroids for episodic sinusitis and bronchitis, which was beneficial to the skin lesions for only a short duration. The patient was adopted and therefore his family history was unavailable.
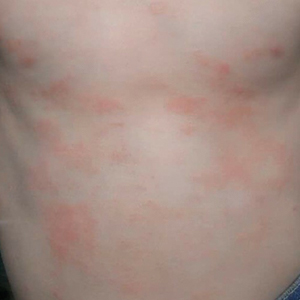
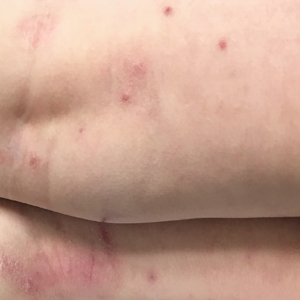
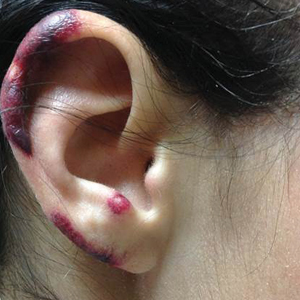
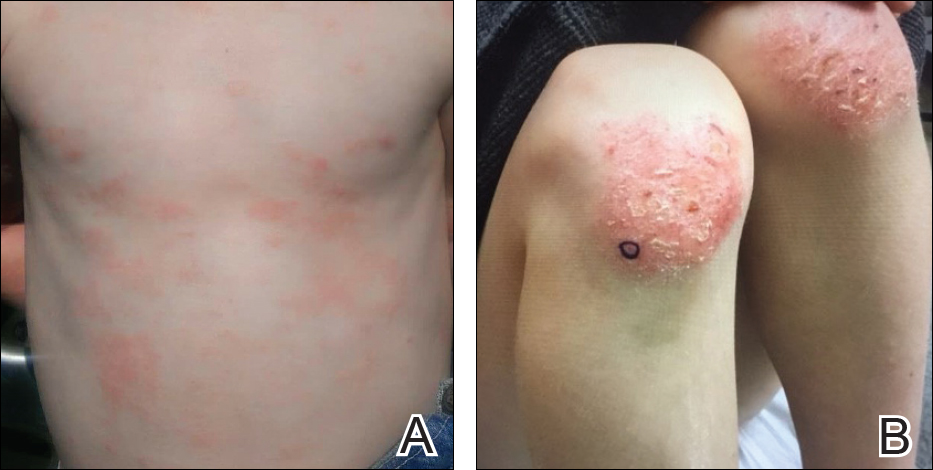
During physical examination, the patient was in the fetal position on the examination table and appeared uncomfortable, scratching himself. The patient admitted to severe widespread itching and burning. On skin examination, multiple thick, lichenified, highly pruritic plaques coalesced on the knees, ankles, arms, and wrists, and very discreet scaly patches were present on the scalp. Annular patches covered 50% of the patient’s body, with highly inflamed lesions concentrated in skin folds (Figure 1), leading to diagnosis of atopic dermatitis (AD).
Over the course of several months, a number of topical therapies were prescribed. The calcineurin inhibitor pimecrolimus cream 1% proffered minimal relief, and the patient experienced burning with crisaborole despite attempts to combine it with emollients and topical corticosteroids. The patient and his mother favored intermittent use of topical corticosteroids alone; however, he experienced frequent disease flares. Stabilized hypochlorous acid spray and mupirocin 2% antibiotic ointment were included in the treatment regimen as adjunctive topical therapies. Additionally, the patient underwent bleach and vinegar bath therapy without success.
Although UVA and UVB phototherapy has shown to be safe and effective in children, our patient had limited treatment options due to insurance restrictions. The patient had been taking oral corticosteroids on and off for years prior to presentation to our dermatology clinic.
Our patient weighed approximately 40 lb and was prescribed methotrexate 5 mg once weekly for 2 weeks along with oral folic acid 1 mg once daily, except when taking the methotrexate. Laboratory workup was ordered at 2- and then 4-week intervals. After 2 weeks of treatment, methotrexate was increased to 10 mg once weekly. His asthma was carefully monitored by the allergist, and his mother was instructed to stop the medication if he had worsening shortness of breath or exacerbation of asthma symptoms. He tolerated methotrexate at 10 mg once weekly well without clinical side effects for 6 months. His mother observed less frequent ear and sinus infections during methotrexate therapy; however, he developed anemia over time and the methotrexate was discontinued. Understanding the nature of off-label use in administering dupilumab, the patient’s mother consented to a scheduled dosage of 300 mg subcutaneous (SQ) injection every month in the absence of a loading dose with the assumption of future modifications pending his response to therapy.
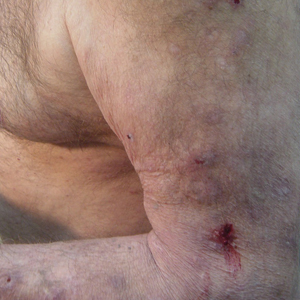
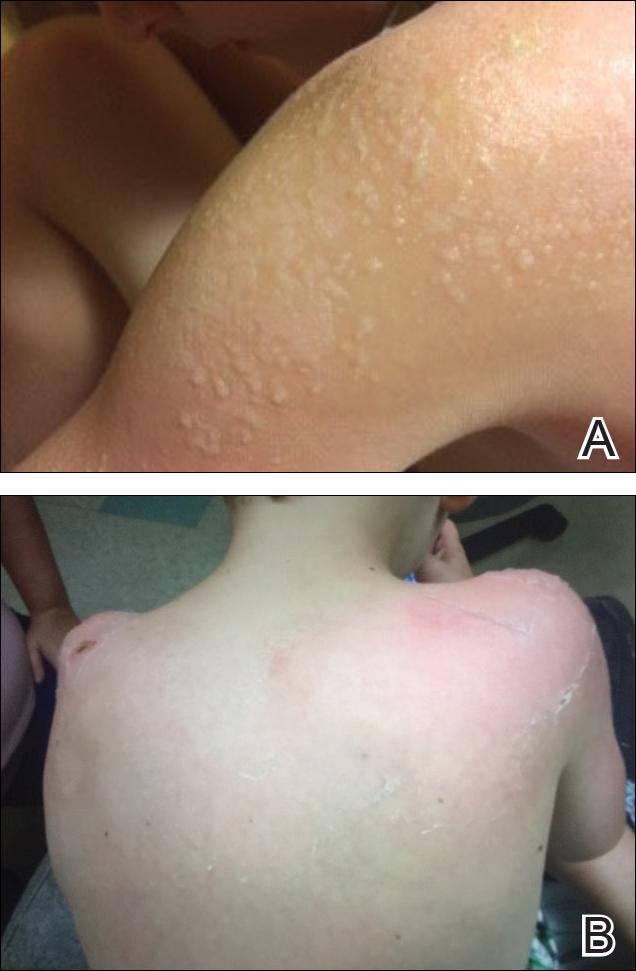
Five days after treatment with a 300-mg SQ dupilumab injection, the patient returned to clinic for evaluation of a vesicular rash with subsequent peeling confined to the shoulders (Figure 2). He and his mother denied any UV exposure, citing he had been completely out of the sun. He denied constitutional symptoms including fever, malaise, swelling, joint pain, headache, muscle pain, nausea, vomiting, diarrhea, enlarged lymph glands, difficulty urinating, breathing, or neurological disturbance. Upon physical examination, the rash was not considered to be a drug eruption. Had a mild drug reaction been suspected, a careful rechallenge, weighing the risks and benefits, would have been considered and was discussed with the mother and patient. New-onset or worsening eye symptoms should be reported; therefore, a referral to ophthalmology was prompted due to our patient’s history of rhinoconjunctivitis and persistent conjunctival injection observed early after initiating dupilumab therapy. Nothing remarkable was found.
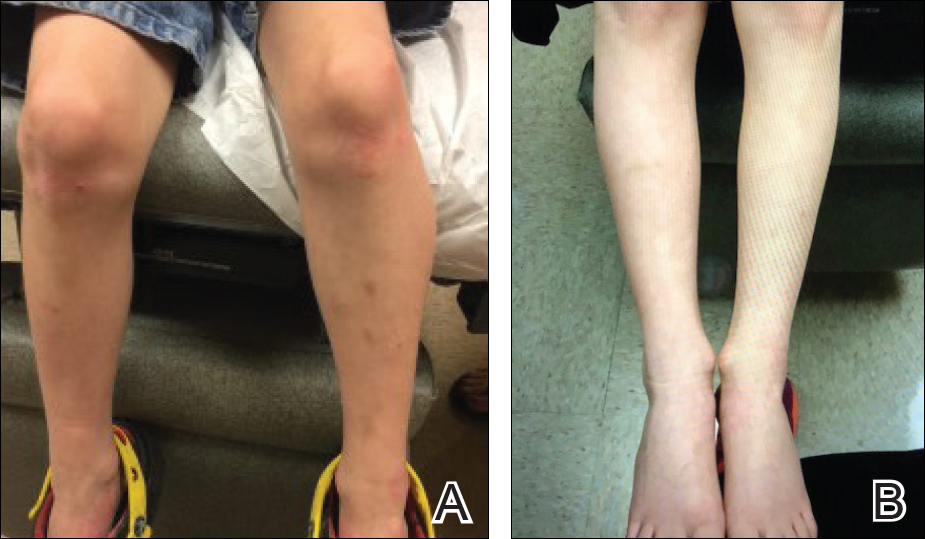
The patient was eager to continue dupilumab therapy due to considerable reduction of itching and elimination of lesions. His mother reported that the greatest benefit 1 month after starting dupilumab was almost no itching (Figure 3A). Additionally, he denied headache or nasopharyngitis at his 1-month office visit. After 2 months of dupilumab therapy, the patient reported persistent lesions on the feet and ankles despite concomitant treatment with topical corticosteroids. The decision to increase the dupilumab dose to 300-mg SQ injection once every 3 weeks for a total of 3 doses was made, which resulted in resolution of all lesions (Figure 3B).
Comment
Prevalence and Pathogenesis
Atopic dermatitis affects 31.6 million individuals in the United States, with 17.8 million experiencing moderate to severe lesions.1 The current prevalence of AD in the pediatric population ranges from 10% to 30% compared to 2% to 10% in adults. Fortunately, up to 70% of young children enter remission or improve by 12 years of age. Atopic diatheses may simultaneously occur, which includes asthma and rhinoconjunctivitis.2
Complications from AD include bacterial and viral infections and ocular disease. Furthermore, impaired growth in stature has been correlated with individuals who have extensive disease.2 Of interest, our 7-year-old patient gained 7 lb and grew almost 3 in within 6 months of being on immunosuppressant therapy. Children with AD have poorer sleep efficiency in contrast to children without AD.3 Eczema is associated with more frequent headaches in childhood, especially in those with sleep disturbances,4 as our patient had experienced prior to systemic therapy.
The pathogenesis of AD is complex, and one must take into consideration the multiple cellular activities including inflammatory mechanics in the absence of IgE-mediated sensitization, epidermal barrier changes, epicutaneous sensitization, dendritic cell roles, T-cell responses and cytokine orchestrations, actions of microbial colonization, and involvement of autoimmunity.5 Select patients with AD have IgE antibodies focused against self-proteins. Disease severity correlates with ubiquity of these antibodies. Moreover, certain autoallergens induce helper T cell (TH1) responses.5 Circulating TH2 cytokines and chemokines IL-4, IL4ra, and IL-13 also have been linked to AD pathogenesis. Additionally, nonlesional skin abnormalities have been observed.6 Most recently, researchers have identified a caspase recruitment domain family member 11 (CARD11) gene mutation possibly leading to AD.7 Clinically, our patient responded favorably to dupilumab, which inhibits TH2 cytokines IL-4 and IL13. He experienced a considerable decrease in itching and inflammation and reduced lesion count after 1 month of treatment with dupilumab. No skin lesions were identified on visual examination at week 17 and inevitably the patient discontinued messy topicals.
Treatment Options
Because AD is characterized by episodes of remission and relapse, management generally is comprised of trigger avoidance, including known allergens and irritants; a skin care regimen that promotes healthy epidermal barrier function; anti-inflammatory therapies to control both flares and subclinical inflammation; and adjunctive therapy for additional symptomatic control (eg, phototherapy, stabilized hypochlorous acid, topical antibiotic treatment) when needed. Avoidance of excessive washing or irritants, food provocation, and emotional stress, as well as toleration of body temperature fluctuations and humidity, is recommended to amend exacerbations.5
Current topical therapies include emollients; corticosteroids; calcineurin inhibitors; and crisaborole, a newer phosphodiesterase 4 inhibitor. There are a number of emollients and moisturizers available, and one over-the-counter preparation showed tolerability and improved skin hydration in AD patients and demonstrated less transepidermal water loss than the control group.8 Ointments such as petrolatum usually do not include ingredients such as preservatives, gelling agents, or humectants that can promote stinging or burning.9 Topical corticosteroids, which ameliorate inflammation by subduing proinflammatory cytokine expression, have been the mainstay of treatment for more than 60 years; however, caution should be used due to the potential for side effects, mainly but not limited to systemic absorption in children, development of striae, and skin atrophy. Calcineurin inhibitors prohibit T-cell activity, modify mast cell response, and decrease dendritic cells in the epidermis. Since 2000, calcineurin inhibitors have been utilized as steroid-sparing agents10; however, prior authorization is still necessary with some insurance providers. Crisaborole ointment 2%, the newest topical agent for AD treatment in the market, has shown improvement of erythema, exudation, and pruritus. Approved for patients aged 2 years and older, twice-daily application of topical crisaborole as a steroid-sparing agent has rendered AD symptom relief.11 It has been reported that 4% of patients encounter stinging or burning with topical crisaborole application, whereas up to 50% of calcineurin inhibitors induce these adverse effects.12 Stabilized hypochlorous acid spray or gel acts as an antipruritic and antimicrobial agent, relieving pain associated with skin irritations. Topical antimicrobial preparations such as mupirocin 2% antibiotic ointment can reduce Staphylococcus colonization when applied in the nasal passage as well as to affected skin lesions.2
In children, UVA and UVB phototherapy has proven safe and effective and can be utilized in AD when suitable.13 When patients inadequately respond to topical therapies and phototherapy, systemic immunomodulatory agents have been recommended as treatment options.A child’s developing immune system indeed may be sensitive to systemic therapies as the innate immune system fully matures in adolescence and his/her adaptive immune system is undergoing vigorous definition.14 Systemic immunomodulatory agents such as cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate have been used off label for years and pose certain challenges in being identified as durable therapies due to potential side effects. Cyclosporine is effective for the treatment of AD; however, long-term administration should be dosed up to a 12-month period and then stopped to decrease cumulative exposure to the drug. Therefore, further treatment options must be considered. For children, cyclosporine should be administered in a dose of 3 to 6 mg/kg daily. Fluctuations in blood pressure and renal function should be monitored. The recommended pediatric dose for azathioprine is 1 to 4 mg/kg daily with laboratory monitoring, particularly of liver enzymes and complete blood cell count. Obtaining the patient’s thiopurine methyltransferase level may aid in dosing. Gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea are common. Phototherapy is not advised in conjunction with azathioprine due to an increased risk of photocarcinogenicity.13 The literature supporting mycophenolate mofetil in children with AD is limited.
Biologic therapies targeting IgE, B-lymphocyte antigen CD20, IL-5, thymic stromal lymphopoietin, TH17 cells, IL-12, IL-23, interferon gamma, IL-6 receptors, tumor necrosis factor, phosphodiesterase 4, Janus kinase, chymase, and nuclear receptors expressed on adipocytes and immune cells have undergone investigation for treatment of AD.17 Additionally, biologic agents targeting IL-31, IL-13, and IL-22 also have been evaluated.1 Currently, there are no US Food and Drug Administration–approved biologic agents for moderate to severe childhood AD.
Dupilumab, an IL-4Rα and IL-13Rα antagonist, recently has been approved for treatment of moderate to severe AD in adults but not yet for children. Potential side effects include nasopharyngitis, headache, hypersensitivity reactions, and ocular symptoms,11 namely keratitis and conjunctivitis.18 Less than 1% of patients experienced keratitis in clinical trials, while conjunctivitis was reported in 4% of patients taking dupilumab with topical corticosteroids at 52 weeks.18 However, possible ocular findings on slit-lamp examination in AD patients include atopic keratoconjunctivitis, blepharitis, palpebral conjunctival scarring, papillary conjunctival reaction, Horner-Trantas dots, keratoconus, and atopic cataracts. Spontaneous retinal detachment is seen more commonly in individuals with AD than in the general population.19
In clinical trials, hypersensitivity reactions included urticaria and serum sickness or serum sickness–like reactions in less than 1% of patients taking dupilumab.18
Conclusion
Childhood AD can be debilitating, and affected individuals often lead a poorer quality of life if left untreated. Embarrassment and isolation are commonly experienced. Increased responsibility and work in tending for a child with eczema may result in parental exhaustion.21 As with psoriasis, AD can impair activity and productivity.22 Currently, dupilumab has proven to positively impact health-related quality of life for adults.23 Pending the outcome of ongoing pediatric clinical trials, dupilumab may become a benchmark therapy for children younger than 18 years.
- Samalonis L. What’s new in eczema and atopic dermatitis research. The Dermatologist. November 19, 2015. http://www.the-dermatologist.com/content/whats-new-eczema-and-atopic-dermatitis-research. Accessed July 19, 2018.
- Habif T. Atopic dermatitis. In: Bonnet C, Pinczewski A, Cook L, eds. Clinical Dermatology. 5th ed. Edinburgh, Scotland: Mosby Elsevier; 2010:160-180.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case control study [published online October 28, 2017]. J Am Acad Dermatol. 2018;78:336-341.
- Silverberg J. Association between childhood eczema and headaches: an analysis of 19 US population-based studies [published online August 29, 2015]. J Allergy Clin Immunol. 2016;137:492-499.e5.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Saunders Elsevier; 2012:203-216.
- Suarez-Farinas M, Tintle S, Shemer A, et al. Non-lesional atopic dermatitis (AD) skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954-964.
- Hilton L. AD gene mutation identified: discovery may lead to new therapeutic option for patients. Dermatol Times. 2017;38:30.
- Zeichner JA, Dryer L. Effect of CeraVe Healing Ointment on skin hydration and barrier function on normal and barrier-impaired skin. Poster presented at: Orlando Dermatology Aesthetic & Clinical Conference; January 15-16, 2016; Orlando, FL.
- Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Delivery. 2015;22:969-987.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Koutnik-Fotopoulous E. Update on the latest eczema treatments. The Dermatologist. February 17, 2016. http://www.the-dermatologist.com/content/update-latest-eczema-treatments. Accessed August 16, 2018.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Sidbury R, Davis D, Cohen D, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents . J Am Acad Dermatol. 2014;71:327-349.
- van der Merwe R, Gianella-Borradori A. Industry perspective on the clinical development of systemic products for the treatment of atopic dermatitis in pediatric patients with inadequate response to topical prescription therapy. Presented at: FDA Dermatologic and Ophthalmic Drugs Advisory Committee Meeting; March 9, 2015; Silver Spring, MD.
- Heller M, Shin HT, Orlow SJ, et al. Mycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patients. Br J Dermatol. 2007;157:127-132.
- Callen JP, Kulp-Shorten CL. Methotrexate. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Saunders Elsevier; 2013:169-181.
- Guttman-Yassky E, Dhingra N, Leung DY. New era of biological therapeutics in atopic dermatitis [published online January 16, 2013]. Expert Opin Biol Ther. 2013;13:549-561.
- Dupixent [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
- Lowery RS. Ophthalmologic manifestations of atopic dermatitis clinical presentation. Medscape website. emedicine.medscape.com/article/1197636-clinical#b4. Updated September 7, 2016. Accessed July 19, 2018.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601-609.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984-992.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the Unites States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol, 2017;77:274-279.
- Tsianakas A, Luger TA, Radin A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a randomized, placebo-controlled clinical trial [published online January 11, 2018]. Br J Dermatol. 2018;178:406-414.
Case Report
A 7-year-old boy with a history of shellfish anaphylaxis, pollen allergy, asthma, rhinoconjunctivitis, frequent headaches and ear infections, sinusitis, bronchitis, vitiligo, warts, and cold sores presented to our dermatology clinic for evaluation of a widespread crusting, cracking, red rash that had been present since 6 months of age. The patient’s mother reported that he had many sleepless nights from uncontrolled itching. His medications included albuterol solution for nebulization, loratadine, and montelukast. Prior to the current presentation he had been treated with triamcinolone and betamethasone creams by the pediatrician. Despite compliance with topical therapy, his mother stated the itching persisted and lesions lingered with minimal improvement. He also was treated with oral corticosteroids for episodic sinusitis and bronchitis, which was beneficial to the skin lesions for only a short duration. The patient was adopted and therefore his family history was unavailable.
During physical examination, the patient was in the fetal position on the examination table and appeared uncomfortable, scratching himself. The patient admitted to severe widespread itching and burning. On skin examination, multiple thick, lichenified, highly pruritic plaques coalesced on the knees, ankles, arms, and wrists, and very discreet scaly patches were present on the scalp. Annular patches covered 50% of the patient’s body, with highly inflamed lesions concentrated in skin folds (Figure 1), leading to diagnosis of atopic dermatitis (AD).

Over the course of several months, a number of topical therapies were prescribed. The calcineurin inhibitor pimecrolimus cream 1% proffered minimal relief, and the patient experienced burning with crisaborole despite attempts to combine it with emollients and topical corticosteroids. The patient and his mother favored intermittent use of topical corticosteroids alone; however, he experienced frequent disease flares. Stabilized hypochlorous acid spray and mupirocin 2% antibiotic ointment were included in the treatment regimen as adjunctive topical therapies. Additionally, the patient underwent bleach and vinegar bath therapy without success.
Although UVA and UVB phototherapy has shown to be safe and effective in children, our patient had limited treatment options due to insurance restrictions. The patient had been taking oral corticosteroids on and off for years prior to presentation to our dermatology clinic.
Our patient weighed approximately 40 lb and was prescribed methotrexate 5 mg once weekly for 2 weeks along with oral folic acid 1 mg once daily, except when taking the methotrexate. Laboratory workup was ordered at 2- and then 4-week intervals. After 2 weeks of treatment, methotrexate was increased to 10 mg once weekly. His asthma was carefully monitored by the allergist, and his mother was instructed to stop the medication if he had worsening shortness of breath or exacerbation of asthma symptoms. He tolerated methotrexate at 10 mg once weekly well without clinical side effects for 6 months. His mother observed less frequent ear and sinus infections during methotrexate therapy; however, he developed anemia over time and the methotrexate was discontinued. Understanding the nature of off-label use in administering dupilumab, the patient’s mother consented to a scheduled dosage of 300 mg subcutaneous (SQ) injection every month in the absence of a loading dose with the assumption of future modifications pending his response to therapy.
Five days after treatment with a 300-mg SQ dupilumab injection, the patient returned to clinic for evaluation of a vesicular rash with subsequent peeling confined to the shoulders (Figure 2). He and his mother denied any UV exposure, citing he had been completely out of the sun. He denied constitutional symptoms including fever, malaise, swelling, joint pain, headache, muscle pain, nausea, vomiting, diarrhea, enlarged lymph glands, difficulty urinating, breathing, or neurological disturbance. Upon physical examination, the rash was not considered to be a drug eruption. Had a mild drug reaction been suspected, a careful rechallenge, weighing the risks and benefits, would have been considered and was discussed with the mother and patient. New-onset or worsening eye symptoms should be reported; therefore, a referral to ophthalmology was prompted due to our patient’s history of rhinoconjunctivitis and persistent conjunctival injection observed early after initiating dupilumab therapy. Nothing remarkable was found.

The patient was eager to continue dupilumab therapy due to considerable reduction of itching and elimination of lesions. His mother reported that the greatest benefit 1 month after starting dupilumab was almost no itching (Figure 3A). Additionally, he denied headache or nasopharyngitis at his 1-month office visit. After 2 months of dupilumab therapy, the patient reported persistent lesions on the feet and ankles despite concomitant treatment with topical corticosteroids. The decision to increase the dupilumab dose to 300-mg SQ injection once every 3 weeks for a total of 3 doses was made, which resulted in resolution of all lesions (Figure 3B).

Comment
Prevalence and Pathogenesis
Atopic dermatitis affects 31.6 million individuals in the United States, with 17.8 million experiencing moderate to severe lesions.1 The current prevalence of AD in the pediatric population ranges from 10% to 30% compared to 2% to 10% in adults. Fortunately, up to 70% of young children enter remission or improve by 12 years of age. Atopic diatheses may simultaneously occur, which includes asthma and rhinoconjunctivitis.2
Complications from AD include bacterial and viral infections and ocular disease. Furthermore, impaired growth in stature has been correlated with individuals who have extensive disease.2 Of interest, our 7-year-old patient gained 7 lb and grew almost 3 in within 6 months of being on immunosuppressant therapy. Children with AD have poorer sleep efficiency in contrast to children without AD.3 Eczema is associated with more frequent headaches in childhood, especially in those with sleep disturbances,4 as our patient had experienced prior to systemic therapy.
The pathogenesis of AD is complex, and one must take into consideration the multiple cellular activities including inflammatory mechanics in the absence of IgE-mediated sensitization, epidermal barrier changes, epicutaneous sensitization, dendritic cell roles, T-cell responses and cytokine orchestrations, actions of microbial colonization, and involvement of autoimmunity.5 Select patients with AD have IgE antibodies focused against self-proteins. Disease severity correlates with ubiquity of these antibodies. Moreover, certain autoallergens induce helper T cell (TH1) responses.5 Circulating TH2 cytokines and chemokines IL-4, IL4ra, and IL-13 also have been linked to AD pathogenesis. Additionally, nonlesional skin abnormalities have been observed.6 Most recently, researchers have identified a caspase recruitment domain family member 11 (CARD11) gene mutation possibly leading to AD.7 Clinically, our patient responded favorably to dupilumab, which inhibits TH2 cytokines IL-4 and IL13. He experienced a considerable decrease in itching and inflammation and reduced lesion count after 1 month of treatment with dupilumab. No skin lesions were identified on visual examination at week 17 and inevitably the patient discontinued messy topicals.
Treatment Options
Because AD is characterized by episodes of remission and relapse, management generally is comprised of trigger avoidance, including known allergens and irritants; a skin care regimen that promotes healthy epidermal barrier function; anti-inflammatory therapies to control both flares and subclinical inflammation; and adjunctive therapy for additional symptomatic control (eg, phototherapy, stabilized hypochlorous acid, topical antibiotic treatment) when needed. Avoidance of excessive washing or irritants, food provocation, and emotional stress, as well as toleration of body temperature fluctuations and humidity, is recommended to amend exacerbations.5
Current topical therapies include emollients; corticosteroids; calcineurin inhibitors; and crisaborole, a newer phosphodiesterase 4 inhibitor. There are a number of emollients and moisturizers available, and one over-the-counter preparation showed tolerability and improved skin hydration in AD patients and demonstrated less transepidermal water loss than the control group.8 Ointments such as petrolatum usually do not include ingredients such as preservatives, gelling agents, or humectants that can promote stinging or burning.9 Topical corticosteroids, which ameliorate inflammation by subduing proinflammatory cytokine expression, have been the mainstay of treatment for more than 60 years; however, caution should be used due to the potential for side effects, mainly but not limited to systemic absorption in children, development of striae, and skin atrophy. Calcineurin inhibitors prohibit T-cell activity, modify mast cell response, and decrease dendritic cells in the epidermis. Since 2000, calcineurin inhibitors have been utilized as steroid-sparing agents10; however, prior authorization is still necessary with some insurance providers. Crisaborole ointment 2%, the newest topical agent for AD treatment in the market, has shown improvement of erythema, exudation, and pruritus. Approved for patients aged 2 years and older, twice-daily application of topical crisaborole as a steroid-sparing agent has rendered AD symptom relief.11 It has been reported that 4% of patients encounter stinging or burning with topical crisaborole application, whereas up to 50% of calcineurin inhibitors induce these adverse effects.12 Stabilized hypochlorous acid spray or gel acts as an antipruritic and antimicrobial agent, relieving pain associated with skin irritations. Topical antimicrobial preparations such as mupirocin 2% antibiotic ointment can reduce Staphylococcus colonization when applied in the nasal passage as well as to affected skin lesions.2
In children, UVA and UVB phototherapy has proven safe and effective and can be utilized in AD when suitable.13 When patients inadequately respond to topical therapies and phototherapy, systemic immunomodulatory agents have been recommended as treatment options.A child’s developing immune system indeed may be sensitive to systemic therapies as the innate immune system fully matures in adolescence and his/her adaptive immune system is undergoing vigorous definition.14 Systemic immunomodulatory agents such as cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate have been used off label for years and pose certain challenges in being identified as durable therapies due to potential side effects. Cyclosporine is effective for the treatment of AD; however, long-term administration should be dosed up to a 12-month period and then stopped to decrease cumulative exposure to the drug. Therefore, further treatment options must be considered. For children, cyclosporine should be administered in a dose of 3 to 6 mg/kg daily. Fluctuations in blood pressure and renal function should be monitored. The recommended pediatric dose for azathioprine is 1 to 4 mg/kg daily with laboratory monitoring, particularly of liver enzymes and complete blood cell count. Obtaining the patient’s thiopurine methyltransferase level may aid in dosing. Gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea are common. Phototherapy is not advised in conjunction with azathioprine due to an increased risk of photocarcinogenicity.13 The literature supporting mycophenolate mofetil in children with AD is limited.
Biologic therapies targeting IgE, B-lymphocyte antigen CD20, IL-5, thymic stromal lymphopoietin, TH17 cells, IL-12, IL-23, interferon gamma, IL-6 receptors, tumor necrosis factor, phosphodiesterase 4, Janus kinase, chymase, and nuclear receptors expressed on adipocytes and immune cells have undergone investigation for treatment of AD.17 Additionally, biologic agents targeting IL-31, IL-13, and IL-22 also have been evaluated.1 Currently, there are no US Food and Drug Administration–approved biologic agents for moderate to severe childhood AD.
Dupilumab, an IL-4Rα and IL-13Rα antagonist, recently has been approved for treatment of moderate to severe AD in adults but not yet for children. Potential side effects include nasopharyngitis, headache, hypersensitivity reactions, and ocular symptoms,11 namely keratitis and conjunctivitis.18 Less than 1% of patients experienced keratitis in clinical trials, while conjunctivitis was reported in 4% of patients taking dupilumab with topical corticosteroids at 52 weeks.18 However, possible ocular findings on slit-lamp examination in AD patients include atopic keratoconjunctivitis, blepharitis, palpebral conjunctival scarring, papillary conjunctival reaction, Horner-Trantas dots, keratoconus, and atopic cataracts. Spontaneous retinal detachment is seen more commonly in individuals with AD than in the general population.19
In clinical trials, hypersensitivity reactions included urticaria and serum sickness or serum sickness–like reactions in less than 1% of patients taking dupilumab.18
Conclusion
Childhood AD can be debilitating, and affected individuals often lead a poorer quality of life if left untreated. Embarrassment and isolation are commonly experienced. Increased responsibility and work in tending for a child with eczema may result in parental exhaustion.21 As with psoriasis, AD can impair activity and productivity.22 Currently, dupilumab has proven to positively impact health-related quality of life for adults.23 Pending the outcome of ongoing pediatric clinical trials, dupilumab may become a benchmark therapy for children younger than 18 years.
Case Report
A 7-year-old boy with a history of shellfish anaphylaxis, pollen allergy, asthma, rhinoconjunctivitis, frequent headaches and ear infections, sinusitis, bronchitis, vitiligo, warts, and cold sores presented to our dermatology clinic for evaluation of a widespread crusting, cracking, red rash that had been present since 6 months of age. The patient’s mother reported that he had many sleepless nights from uncontrolled itching. His medications included albuterol solution for nebulization, loratadine, and montelukast. Prior to the current presentation he had been treated with triamcinolone and betamethasone creams by the pediatrician. Despite compliance with topical therapy, his mother stated the itching persisted and lesions lingered with minimal improvement. He also was treated with oral corticosteroids for episodic sinusitis and bronchitis, which was beneficial to the skin lesions for only a short duration. The patient was adopted and therefore his family history was unavailable.
During physical examination, the patient was in the fetal position on the examination table and appeared uncomfortable, scratching himself. The patient admitted to severe widespread itching and burning. On skin examination, multiple thick, lichenified, highly pruritic plaques coalesced on the knees, ankles, arms, and wrists, and very discreet scaly patches were present on the scalp. Annular patches covered 50% of the patient’s body, with highly inflamed lesions concentrated in skin folds (Figure 1), leading to diagnosis of atopic dermatitis (AD).

Over the course of several months, a number of topical therapies were prescribed. The calcineurin inhibitor pimecrolimus cream 1% proffered minimal relief, and the patient experienced burning with crisaborole despite attempts to combine it with emollients and topical corticosteroids. The patient and his mother favored intermittent use of topical corticosteroids alone; however, he experienced frequent disease flares. Stabilized hypochlorous acid spray and mupirocin 2% antibiotic ointment were included in the treatment regimen as adjunctive topical therapies. Additionally, the patient underwent bleach and vinegar bath therapy without success.
Although UVA and UVB phototherapy has shown to be safe and effective in children, our patient had limited treatment options due to insurance restrictions. The patient had been taking oral corticosteroids on and off for years prior to presentation to our dermatology clinic.
Our patient weighed approximately 40 lb and was prescribed methotrexate 5 mg once weekly for 2 weeks along with oral folic acid 1 mg once daily, except when taking the methotrexate. Laboratory workup was ordered at 2- and then 4-week intervals. After 2 weeks of treatment, methotrexate was increased to 10 mg once weekly. His asthma was carefully monitored by the allergist, and his mother was instructed to stop the medication if he had worsening shortness of breath or exacerbation of asthma symptoms. He tolerated methotrexate at 10 mg once weekly well without clinical side effects for 6 months. His mother observed less frequent ear and sinus infections during methotrexate therapy; however, he developed anemia over time and the methotrexate was discontinued. Understanding the nature of off-label use in administering dupilumab, the patient’s mother consented to a scheduled dosage of 300 mg subcutaneous (SQ) injection every month in the absence of a loading dose with the assumption of future modifications pending his response to therapy.
Five days after treatment with a 300-mg SQ dupilumab injection, the patient returned to clinic for evaluation of a vesicular rash with subsequent peeling confined to the shoulders (Figure 2). He and his mother denied any UV exposure, citing he had been completely out of the sun. He denied constitutional symptoms including fever, malaise, swelling, joint pain, headache, muscle pain, nausea, vomiting, diarrhea, enlarged lymph glands, difficulty urinating, breathing, or neurological disturbance. Upon physical examination, the rash was not considered to be a drug eruption. Had a mild drug reaction been suspected, a careful rechallenge, weighing the risks and benefits, would have been considered and was discussed with the mother and patient. New-onset or worsening eye symptoms should be reported; therefore, a referral to ophthalmology was prompted due to our patient’s history of rhinoconjunctivitis and persistent conjunctival injection observed early after initiating dupilumab therapy. Nothing remarkable was found.

The patient was eager to continue dupilumab therapy due to considerable reduction of itching and elimination of lesions. His mother reported that the greatest benefit 1 month after starting dupilumab was almost no itching (Figure 3A). Additionally, he denied headache or nasopharyngitis at his 1-month office visit. After 2 months of dupilumab therapy, the patient reported persistent lesions on the feet and ankles despite concomitant treatment with topical corticosteroids. The decision to increase the dupilumab dose to 300-mg SQ injection once every 3 weeks for a total of 3 doses was made, which resulted in resolution of all lesions (Figure 3B).

Comment
Prevalence and Pathogenesis
Atopic dermatitis affects 31.6 million individuals in the United States, with 17.8 million experiencing moderate to severe lesions.1 The current prevalence of AD in the pediatric population ranges from 10% to 30% compared to 2% to 10% in adults. Fortunately, up to 70% of young children enter remission or improve by 12 years of age. Atopic diatheses may simultaneously occur, which includes asthma and rhinoconjunctivitis.2
Complications from AD include bacterial and viral infections and ocular disease. Furthermore, impaired growth in stature has been correlated with individuals who have extensive disease.2 Of interest, our 7-year-old patient gained 7 lb and grew almost 3 in within 6 months of being on immunosuppressant therapy. Children with AD have poorer sleep efficiency in contrast to children without AD.3 Eczema is associated with more frequent headaches in childhood, especially in those with sleep disturbances,4 as our patient had experienced prior to systemic therapy.
The pathogenesis of AD is complex, and one must take into consideration the multiple cellular activities including inflammatory mechanics in the absence of IgE-mediated sensitization, epidermal barrier changes, epicutaneous sensitization, dendritic cell roles, T-cell responses and cytokine orchestrations, actions of microbial colonization, and involvement of autoimmunity.5 Select patients with AD have IgE antibodies focused against self-proteins. Disease severity correlates with ubiquity of these antibodies. Moreover, certain autoallergens induce helper T cell (TH1) responses.5 Circulating TH2 cytokines and chemokines IL-4, IL4ra, and IL-13 also have been linked to AD pathogenesis. Additionally, nonlesional skin abnormalities have been observed.6 Most recently, researchers have identified a caspase recruitment domain family member 11 (CARD11) gene mutation possibly leading to AD.7 Clinically, our patient responded favorably to dupilumab, which inhibits TH2 cytokines IL-4 and IL13. He experienced a considerable decrease in itching and inflammation and reduced lesion count after 1 month of treatment with dupilumab. No skin lesions were identified on visual examination at week 17 and inevitably the patient discontinued messy topicals.
Treatment Options
Because AD is characterized by episodes of remission and relapse, management generally is comprised of trigger avoidance, including known allergens and irritants; a skin care regimen that promotes healthy epidermal barrier function; anti-inflammatory therapies to control both flares and subclinical inflammation; and adjunctive therapy for additional symptomatic control (eg, phototherapy, stabilized hypochlorous acid, topical antibiotic treatment) when needed. Avoidance of excessive washing or irritants, food provocation, and emotional stress, as well as toleration of body temperature fluctuations and humidity, is recommended to amend exacerbations.5
Current topical therapies include emollients; corticosteroids; calcineurin inhibitors; and crisaborole, a newer phosphodiesterase 4 inhibitor. There are a number of emollients and moisturizers available, and one over-the-counter preparation showed tolerability and improved skin hydration in AD patients and demonstrated less transepidermal water loss than the control group.8 Ointments such as petrolatum usually do not include ingredients such as preservatives, gelling agents, or humectants that can promote stinging or burning.9 Topical corticosteroids, which ameliorate inflammation by subduing proinflammatory cytokine expression, have been the mainstay of treatment for more than 60 years; however, caution should be used due to the potential for side effects, mainly but not limited to systemic absorption in children, development of striae, and skin atrophy. Calcineurin inhibitors prohibit T-cell activity, modify mast cell response, and decrease dendritic cells in the epidermis. Since 2000, calcineurin inhibitors have been utilized as steroid-sparing agents10; however, prior authorization is still necessary with some insurance providers. Crisaborole ointment 2%, the newest topical agent for AD treatment in the market, has shown improvement of erythema, exudation, and pruritus. Approved for patients aged 2 years and older, twice-daily application of topical crisaborole as a steroid-sparing agent has rendered AD symptom relief.11 It has been reported that 4% of patients encounter stinging or burning with topical crisaborole application, whereas up to 50% of calcineurin inhibitors induce these adverse effects.12 Stabilized hypochlorous acid spray or gel acts as an antipruritic and antimicrobial agent, relieving pain associated with skin irritations. Topical antimicrobial preparations such as mupirocin 2% antibiotic ointment can reduce Staphylococcus colonization when applied in the nasal passage as well as to affected skin lesions.2
In children, UVA and UVB phototherapy has proven safe and effective and can be utilized in AD when suitable.13 When patients inadequately respond to topical therapies and phototherapy, systemic immunomodulatory agents have been recommended as treatment options.A child’s developing immune system indeed may be sensitive to systemic therapies as the innate immune system fully matures in adolescence and his/her adaptive immune system is undergoing vigorous definition.14 Systemic immunomodulatory agents such as cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate have been used off label for years and pose certain challenges in being identified as durable therapies due to potential side effects. Cyclosporine is effective for the treatment of AD; however, long-term administration should be dosed up to a 12-month period and then stopped to decrease cumulative exposure to the drug. Therefore, further treatment options must be considered. For children, cyclosporine should be administered in a dose of 3 to 6 mg/kg daily. Fluctuations in blood pressure and renal function should be monitored. The recommended pediatric dose for azathioprine is 1 to 4 mg/kg daily with laboratory monitoring, particularly of liver enzymes and complete blood cell count. Obtaining the patient’s thiopurine methyltransferase level may aid in dosing. Gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea are common. Phototherapy is not advised in conjunction with azathioprine due to an increased risk of photocarcinogenicity.13 The literature supporting mycophenolate mofetil in children with AD is limited.
Biologic therapies targeting IgE, B-lymphocyte antigen CD20, IL-5, thymic stromal lymphopoietin, TH17 cells, IL-12, IL-23, interferon gamma, IL-6 receptors, tumor necrosis factor, phosphodiesterase 4, Janus kinase, chymase, and nuclear receptors expressed on adipocytes and immune cells have undergone investigation for treatment of AD.17 Additionally, biologic agents targeting IL-31, IL-13, and IL-22 also have been evaluated.1 Currently, there are no US Food and Drug Administration–approved biologic agents for moderate to severe childhood AD.
Dupilumab, an IL-4Rα and IL-13Rα antagonist, recently has been approved for treatment of moderate to severe AD in adults but not yet for children. Potential side effects include nasopharyngitis, headache, hypersensitivity reactions, and ocular symptoms,11 namely keratitis and conjunctivitis.18 Less than 1% of patients experienced keratitis in clinical trials, while conjunctivitis was reported in 4% of patients taking dupilumab with topical corticosteroids at 52 weeks.18 However, possible ocular findings on slit-lamp examination in AD patients include atopic keratoconjunctivitis, blepharitis, palpebral conjunctival scarring, papillary conjunctival reaction, Horner-Trantas dots, keratoconus, and atopic cataracts. Spontaneous retinal detachment is seen more commonly in individuals with AD than in the general population.19
In clinical trials, hypersensitivity reactions included urticaria and serum sickness or serum sickness–like reactions in less than 1% of patients taking dupilumab.18
Conclusion
Childhood AD can be debilitating, and affected individuals often lead a poorer quality of life if left untreated. Embarrassment and isolation are commonly experienced. Increased responsibility and work in tending for a child with eczema may result in parental exhaustion.21 As with psoriasis, AD can impair activity and productivity.22 Currently, dupilumab has proven to positively impact health-related quality of life for adults.23 Pending the outcome of ongoing pediatric clinical trials, dupilumab may become a benchmark therapy for children younger than 18 years.
- Samalonis L. What’s new in eczema and atopic dermatitis research. The Dermatologist. November 19, 2015. http://www.the-dermatologist.com/content/whats-new-eczema-and-atopic-dermatitis-research. Accessed July 19, 2018.
- Habif T. Atopic dermatitis. In: Bonnet C, Pinczewski A, Cook L, eds. Clinical Dermatology. 5th ed. Edinburgh, Scotland: Mosby Elsevier; 2010:160-180.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case control study [published online October 28, 2017]. J Am Acad Dermatol. 2018;78:336-341.
- Silverberg J. Association between childhood eczema and headaches: an analysis of 19 US population-based studies [published online August 29, 2015]. J Allergy Clin Immunol. 2016;137:492-499.e5.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Saunders Elsevier; 2012:203-216.
- Suarez-Farinas M, Tintle S, Shemer A, et al. Non-lesional atopic dermatitis (AD) skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954-964.
- Hilton L. AD gene mutation identified: discovery may lead to new therapeutic option for patients. Dermatol Times. 2017;38:30.
- Zeichner JA, Dryer L. Effect of CeraVe Healing Ointment on skin hydration and barrier function on normal and barrier-impaired skin. Poster presented at: Orlando Dermatology Aesthetic & Clinical Conference; January 15-16, 2016; Orlando, FL.
- Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Delivery. 2015;22:969-987.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Koutnik-Fotopoulous E. Update on the latest eczema treatments. The Dermatologist. February 17, 2016. http://www.the-dermatologist.com/content/update-latest-eczema-treatments. Accessed August 16, 2018.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Sidbury R, Davis D, Cohen D, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents . J Am Acad Dermatol. 2014;71:327-349.
- van der Merwe R, Gianella-Borradori A. Industry perspective on the clinical development of systemic products for the treatment of atopic dermatitis in pediatric patients with inadequate response to topical prescription therapy. Presented at: FDA Dermatologic and Ophthalmic Drugs Advisory Committee Meeting; March 9, 2015; Silver Spring, MD.
- Heller M, Shin HT, Orlow SJ, et al. Mycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patients. Br J Dermatol. 2007;157:127-132.
- Callen JP, Kulp-Shorten CL. Methotrexate. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Saunders Elsevier; 2013:169-181.
- Guttman-Yassky E, Dhingra N, Leung DY. New era of biological therapeutics in atopic dermatitis [published online January 16, 2013]. Expert Opin Biol Ther. 2013;13:549-561.
- Dupixent [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
- Lowery RS. Ophthalmologic manifestations of atopic dermatitis clinical presentation. Medscape website. emedicine.medscape.com/article/1197636-clinical#b4. Updated September 7, 2016. Accessed July 19, 2018.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601-609.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984-992.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the Unites States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol, 2017;77:274-279.
- Tsianakas A, Luger TA, Radin A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a randomized, placebo-controlled clinical trial [published online January 11, 2018]. Br J Dermatol. 2018;178:406-414.
- Samalonis L. What’s new in eczema and atopic dermatitis research. The Dermatologist. November 19, 2015. http://www.the-dermatologist.com/content/whats-new-eczema-and-atopic-dermatitis-research. Accessed July 19, 2018.
- Habif T. Atopic dermatitis. In: Bonnet C, Pinczewski A, Cook L, eds. Clinical Dermatology. 5th ed. Edinburgh, Scotland: Mosby Elsevier; 2010:160-180.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case control study [published online October 28, 2017]. J Am Acad Dermatol. 2018;78:336-341.
- Silverberg J. Association between childhood eczema and headaches: an analysis of 19 US population-based studies [published online August 29, 2015]. J Allergy Clin Immunol. 2016;137:492-499.e5.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Saunders Elsevier; 2012:203-216.
- Suarez-Farinas M, Tintle S, Shemer A, et al. Non-lesional atopic dermatitis (AD) skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954-964.
- Hilton L. AD gene mutation identified: discovery may lead to new therapeutic option for patients. Dermatol Times. 2017;38:30.
- Zeichner JA, Dryer L. Effect of CeraVe Healing Ointment on skin hydration and barrier function on normal and barrier-impaired skin. Poster presented at: Orlando Dermatology Aesthetic & Clinical Conference; January 15-16, 2016; Orlando, FL.
- Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Delivery. 2015;22:969-987.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Koutnik-Fotopoulous E. Update on the latest eczema treatments. The Dermatologist. February 17, 2016. http://www.the-dermatologist.com/content/update-latest-eczema-treatments. Accessed August 16, 2018.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Sidbury R, Davis D, Cohen D, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents . J Am Acad Dermatol. 2014;71:327-349.
- van der Merwe R, Gianella-Borradori A. Industry perspective on the clinical development of systemic products for the treatment of atopic dermatitis in pediatric patients with inadequate response to topical prescription therapy. Presented at: FDA Dermatologic and Ophthalmic Drugs Advisory Committee Meeting; March 9, 2015; Silver Spring, MD.
- Heller M, Shin HT, Orlow SJ, et al. Mycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patients. Br J Dermatol. 2007;157:127-132.
- Callen JP, Kulp-Shorten CL. Methotrexate. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Saunders Elsevier; 2013:169-181.
- Guttman-Yassky E, Dhingra N, Leung DY. New era of biological therapeutics in atopic dermatitis [published online January 16, 2013]. Expert Opin Biol Ther. 2013;13:549-561.
- Dupixent [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
- Lowery RS. Ophthalmologic manifestations of atopic dermatitis clinical presentation. Medscape website. emedicine.medscape.com/article/1197636-clinical#b4. Updated September 7, 2016. Accessed July 19, 2018.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601-609.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984-992.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the Unites States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol, 2017;77:274-279.
- Tsianakas A, Luger TA, Radin A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a randomized, placebo-controlled clinical trial [published online January 11, 2018]. Br J Dermatol. 2018;178:406-414.
Practice Points
- Childhood atopic dermatitis can be debilitating, and affected individuals often experience poorer quality of life if left untreated.
- Dupilumab may become a benchmark therapy for children younger than 18 years.
Product News: 09 2018
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Molluscum Contagiosum Virus Infection Can Trigger Atopic Dermatitis Disease Onset or Flare
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).
The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.
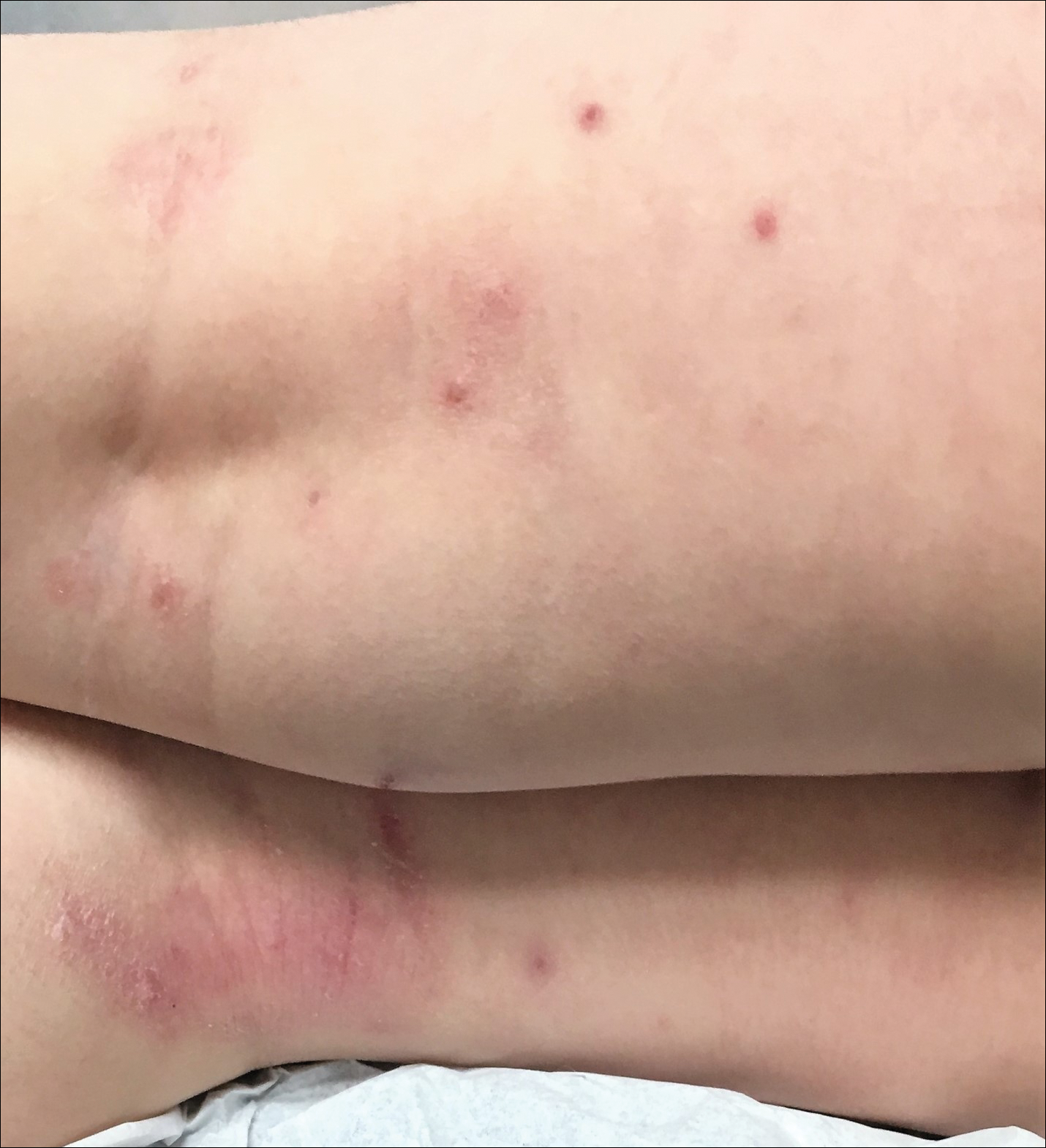
Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).
The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.

Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).
The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.

Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
Practice Points
- Molluscum contagiosum virus (MCV) infection appears to aggravate atopic dermatitis (AD) symptoms in a subset of pediatric patients.
- In susceptible children, the first onset of AD symptoms can occur during the course of MCV infection.
Concurrent Notalgia Paresthetica and Brachioradial Pruritus Associated With Cervical Degenerative Disc Disease
Case Report
A 74-year-old man presented with persistent episodes of severe pruritus with exacerbations on the bilateral forearms, arms, and left side of the mid back of 4 years’ duration. He had a refractory and debilitating disease that had failed extensive therapies including topical antipruritics, antihistamines, oral hydroxyzine, capsaicin, potent topical steroids (ie, clobetasol, fluocinonide, triamcinolone), phototherapy with narrowband UVB, and various dietary modifications including a gluten-free trial. The patient reported he had exhausted all medical evaluation through care with more than 7 physicians and multiple dermatologists, including a university-based dermatology department for repeated consultations; he was seen by our dermatology center for an eighth opinion.
Initial dermatologic examination revealed multiple secondarily excoriated, hemorrhagic, hyperpigmented plaques and nodules on the right side of the mid upper back indicative of notalgia paresthetica (NP) with secondary chronic skin changes (Figure 1). Additional examination of the left arm and forearm revealed several open erosions, raised nodules, and lichenified skin plaques indicative of brachioradial pruritus (BP) with secondary skin changes (Figure 2). In addition, multiple lichenified plaques of the left side of the mid back were associated with decreased sensory alternations to light touch and pin prick. Of note, the localized pruritus pattern, particularly of the unilateral infrascapular back region, heralded the possibility of a neuropathic pruritus condition originating from the cervical spine. Examination confirmed decreased range of motion in the neck with associated marked palpable bilateral cervical muscle spasm and tenderness. Laboratory testing confirmed Staphylococcus aureus secondary skin infection that was treated empirically with chlorhexidine wash. General pruritus serology and imaging workup was ordered with contributory results. The patient’s medical history was notable for noninsulin-dependent diabetes mellitus, obesity, deep venous thrombosis, asthma, vein surgery, cardiovascular disease, atrial fibrillation, atopy, allergies, asthma, and keratosis pilaris, as well as drug intolerances of warfarin sodium, sitagliptin, and clopidogrel. His medications on presentation included glyburide, digoxin, prednisone, aspirin, cetirizine, cimetidine, and hydroxyzine. Based on the relatively classic localized pruritus symptoms and the anatomical distribution of skin findings, a clinical diagnosis of concurrent NP and BRP was made, and radiologic studies of the cervical spine were ordered.

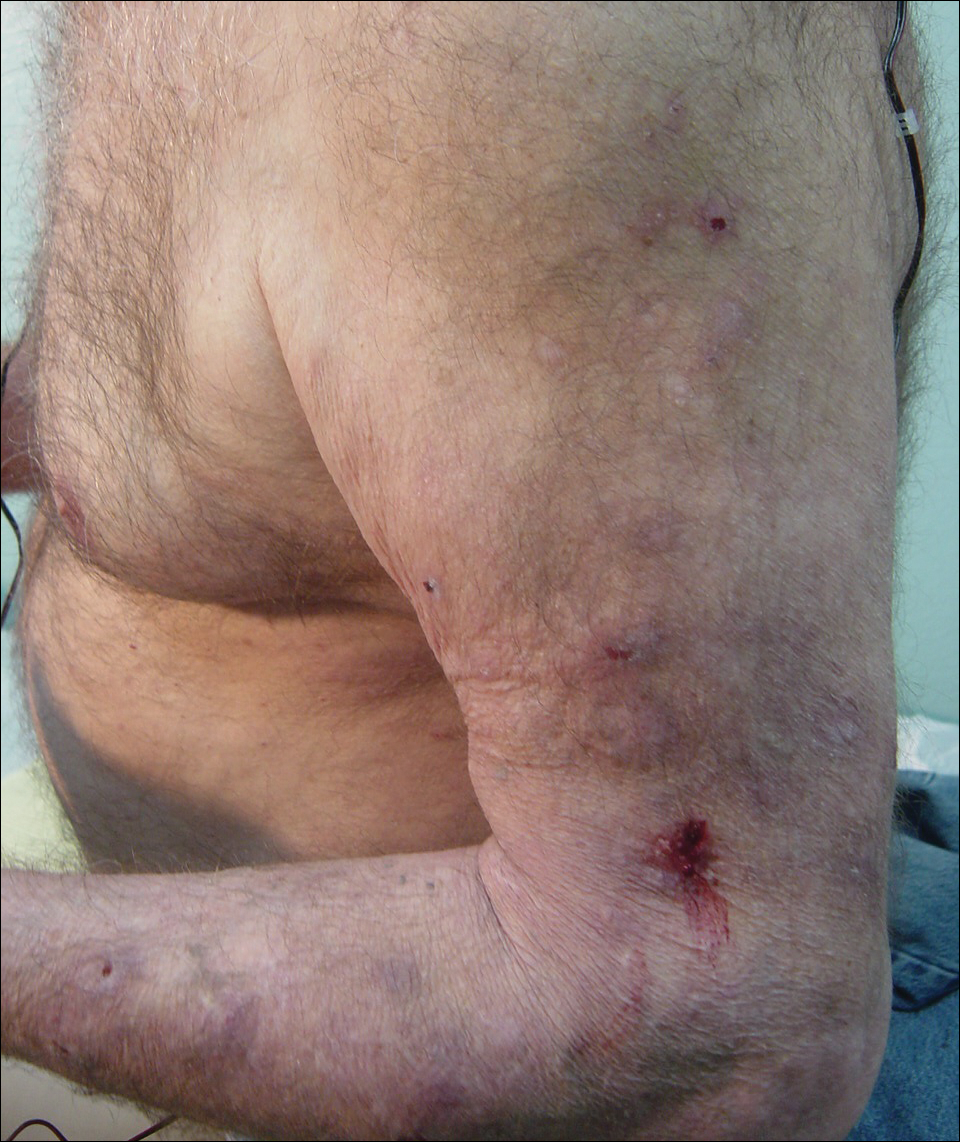
Magnetic resonance imaging (MRI) of the cervical spine showed severe central canal stenosis at C3-C4 secondary to disc disease slight asymmetric toward the right side, severe central canal stenosis at C4-C5 slightly more prominent in the midline, severe central stenosis at C5-C6 more prominent in the midline, and mild changes at other levels as described. Laboratory workup revealed an abnormal complete blood cell count with mildly elevated white blood cell count (11,800/µL [reference range, 4000–10,500/µL]), elevated neutrophils (8600/µL [reference range, 1800–7800/µL]), elevated eosinophils (600/µL [reference range, 0–450/µL ]), and elevated IgE (160 IU/mL [reference range, 0–100 IU/mL]). Further testing revealed negative results for Helicobacter pylori IgG and IgM, human immunodeficiency virus, and hepatitis B and C screening panels; antinuclear antibody negative; normal thyroid-stimulating hormone; and normal thyroid peroxidase antibody. Chest radiograph and computed tomography of the chest, abdomen, and pelvis were negative.
We referred the patient for a neurosurgical consultation that uncovered newly diagnosed severe cervical stenosis with mild to moderate canal compromise at C3, C4, C5, and C6. His motor examination revealed full strength in the upper extremities (5/5). Sensory examination showed patchy sensory alteration on the mid back. He declined oral antibiotics as advised for the skin staphylococcal infection and neurosurgical treatment for the cervical disease.
During the 4 years prior to presentation at our center, the patient reported failure to improve with a dermatologically prescribed gluten-free diet as well as all topical and oral steroid treatments. He was presented at a university grand rounds where a suggestion for UVB light treatment was made; the patient reported possible worsening of symptoms with narrowband UVB phototherapy.
At the patient’s first visit at our center, for immediate symptom relief he underwent therapy with transcutaneous electronic nerve stimulation (TENS) with acupuncture of the cervicothoracic spine (Figure 3). He agreed to discontinue oral prednisone and begin chlorhexidine cleansing body wash, low-dose hydroxyzine 10-mg tablets up to 60 mg every 6 hours as required for pruritus, and mupirocin intranasal ointment. At 1-week follow-up, he reported at least 50% improvement in his symptoms with decreased pruritus, improved sleep, and enhanced quality of life. Within 2 weeks of initial assessment, there was a notable 70% clinical improvement of both the NP and BRP, with a notable decrease in cutaneous erosions and flattening of the pruritic skin nodules. He reported adequate control of symptoms with continued TENS for at-home use 3 times daily for 5- to 10-minute intervals.
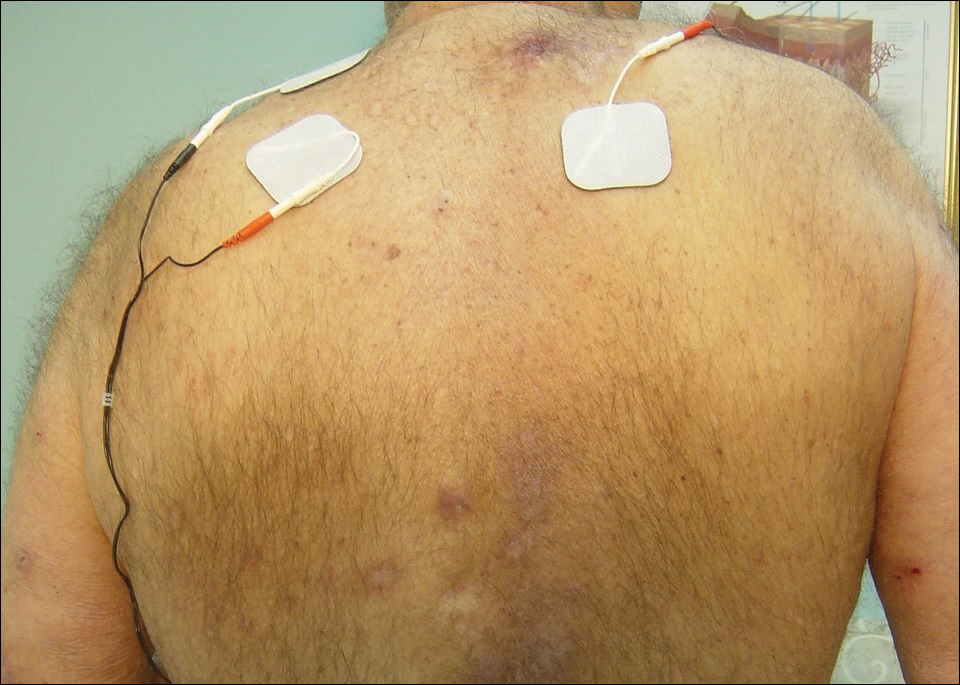
Comment
NP Presentation
Notalgia paresthetica is a common, albeit heavily unrecognized and underdiagnosed, sensory neuropathic syndrome of the back, classically of the unilateral infrascapular region. Notalg
The dermatologic condition may consist of other symptoms that include but are not limited to localized burning, pain, tenderness, hyperalgesia, or dysesthesia. Notalgia paresthetica typically is associated with a poorly confined tan or hyperpigmented patch in the symptomatic area, though the skin may have no visible findings in many early cases. Notalgia paresthetica tends to be a chronic condition with periodic remissions and exacerbations. It is generally not associated with other comorbidities and is not life threatening; however, it does frequently decrease quality of life, causing much discomfort and annoyance to the affected patients.
Treatment
Topical therapies for NP have generally failed and are considered difficult because of the out-of-reach affected location. There is no uniformly effective treatment of NP.
Pathogenesis
The etiologies for NP and BRP are evolving and remain to be fully elucidated. Although the exact etiology remains uncertain, there are several possible mechanisms that have been proposed for NP: (1) neuropathy from degenerative cervicothoracic disc disease or direct nerve impingement,1 and (2) localized increased sensory innervations of the affected skin areas.2
Differential and Workup
The differential diagnosis in NP may include allergic or irritant contact dermatitis, fixed drug eruption, dermatophytosis, neoplasm, lichen amyloidosis, arthropod reaction, lichenified skin reactions including lichen simplex chronicus, neurodermatitis, infection, and other hypersensitivity reaction.
It is important during the initial assessment of patients with NP and/or BRP to obtain a thorough history of osteoarthritis, neck trauma, motor vehicle accident(s), vertebral fracture, cervical neoplasm or malignancy, family history of NP or BRP, or cervical disc disease. Radiographs or MRIs of the cervical spine may aid in diagnosis and treatment, and perhaps more so if there is an absence of contributory medical history. Radiographic imaging also may be indicated if there is a positive family history of osteoarthritis or vertebral disc disease.
A full laboratory workup including complete blood cell count, chemistry panel including renal and liver functions, and other laboratory tests (eg, IgE levels) may be warranted if pruritus is generalized and persistent to exclude other causes. Proper management of NP and BRP may involve a multispecialty effort of dermatology with radiology; orthopedic surgery; neurosurgery; neurology; and adjunctive fields including acupuncture, massage, chiropractic, and physical therapy.
NP and BRB Overlap
Because NP and BRP often originate from varying degrees of cervical disease, particularly at the C5-C6 level, traditional topical therapies aimed at treating the affected skin of the mid back and forearms may be ineffectual or partially effective as basic emollients. Due to NP and BRP’s periodic spontaneous remissions and exacerbations, it may be reasonably difficult to accurately measure direct response to various therapies. Some topical therapies aimed at the mid back skin or forearms may appear partially effective from a placebo-type perspective.
Currently, uniformly effective treatment of NP and BRP include the following: (1) therapies aimed at the cervical spine at C5-C6, including TENS, cervical massage, physical therapy, and acupuncture; and (2) therapies targeting the underlying lowered pruritus threshold such as oral antihistamines and narrowband UVB. Although uniformly effective treatments in this space had been previously lacking, traditional therapeutic options for NP and BRP included capsaicin cream, eutectic mixture of local anesthetic cream, topical steroids, pramoxine cream, topical cooling, oral steroids, menthol creams, various commercially available topical mixtures of menthol and methyl salicylate, cordran tape, intralesional corticosteroid injections, botulinum toxin injections,3 oral antihistamines, hydroxyzine, doxepin, topiramate, carbamazepine, antidepressants, gabapentin, oxcarbazepine, topiramate, thalidomide,4 paravertebral local anesthetic block, cervical epidural injection, surgical resection of the rib, and many others. Some of the tried systemic therapies exert their effect through the spinal nerves and central nervous system, thereby supporting the neuropathic etiology of NP.
Cervical Disc Disease
Alai et al1 reported a 37-year-old with documented NP on the right side of the back with MRI findings of disc disease at C5-C6 and mild nerve impingement that strongly suggest the association of cervical degenerative disc disease and NP.
Savk and Savk5 reported that 7 of 10 patients with NP demonstrated normal neurological examination and standard electrodiagnostic results. All had skin histopathology compatible with postinflammatory hyperpigmentation. There were no amyloid deposits or other described pathology on pathologic examination of the skin. Seven of 10 cases confirmed radiographic changes in the vertebra corresponding to the dermatome of the cutaneous lesion.5
An earlier study by Springall et al6 evaluating the mechanism of NP studied whether the cutaneous symptoms were caused by alternations on the cutaneous innervation of the involved infrascapular area. They p
Histologic studies have shown cutaneous changes in a few cases including lichen amyloidosis that may be secondary to the localized chronic scratching and rubbing.7 Clinical observations in orthopedics have established a clear relationship between the upper thoracic and interscapular region and the lower cervical spine. Frequently, cervical disc disease presents as referred pain in the upper thoracic and interscapular area. Similarly, some tumors of the cervical medulla also have presented as interscapular pain.8
Some have speculated direct involvement and actual entrapment of the posterior rami of T2-T6 spinal nerves.9 However, there are referred symptoms from the cervical area directly to the interscapular back. Degenerative vertebral and disc changes corresponding to the affected dermatome may be observed in some cases. Radiographic imaging of cervical and thoracic spine will help to exclude disc disease and possible nerve compromise.8
With advances in radiography and availability of MRI, earlier detection and intervention of cervical disc disease is possible. Early recognition may promote timely intervention and treatment to prevent cervical spine disease progression. In addition to degenerative cervical discs, osteoarthritis, and cervical spine strain and muscle spasm, there may be neoplasms or other pathology of the cervical spine contributing to NP and BRP.
There is some thought that there may be a relationship between NP and BRP. The described association of many cases of BRP and cervical spine disease6 and description of these diseases as likely neuropathic/neurogenic pruritic conditions also support a probable association of these two conditions. In contrast, NP has classically been described as unilateral in distribution, while BRP may involve unilateral or bilateral dorsolateral forearms. Most recently, as seen in our case, there are increasing incidences of nonclassic presentations of both of these diseases that may involve additional skin areas and be a basis for diagnostic challenges to the clinician.
First-line therapies for NP and BRP with associated cervical spinal disease are currently evolving and may include nondermatologic and noninvasive treatments such as spinal manipulation, physical therapy, acupuncture, cervical soft collars, massage, cervical traction, cervical muscle strengthening and increased range on motion, oral nonsteroidal anti-inflammatory medications (eg, ibuprofen, celecoxib, ketorolac), and oral muscle relaxants (eg, carisoprodol, cyclobenzaprine, methocarbamol, metaxalone). Curren
Conclusion
Notalgia paresthetica and BRP may not be solely skin diseases but rather cutaneous signs of an underlying cervical spine disease. The striking association of NP with BRP we present as well as the degenerative and/or traumatic cervicothoracic spine disease suggests that early spinal nerve impingement or cervical muscle spasm may contribute to the pathogenesis of these skin symptoms. Additional studies are needed to further assess the relationship of NP and BRP as well as the association of each disease entity independently with cervical spine disease, as it is unknown if these are causal or coincidental findings. Although topical therapies may seemingly help decrease the localized symptoms in NP and BRP in some cases, systemic or broader-scope cervical spinal evaluation may be warranted to fully evaluate refractory cases. Cervical spinal imaging and treatment, particularly at C5-C6 levels, may be appropriate as primary or first-line therapy in many cases of NP and BRP. The paradigm shift in thinking will more likely than not be to treat the cervical spine and the skin will follow.
- Alai NN, Skinner HB, Nabili S, et al. Notalgia paresthetica associated with cervical spinal stenosis and cervicothoracic disk disease at C4 through C7. Cutis. 2010;85:77-81.
- Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170.
- Goodless DR, Eaglstein WH. Brachioradial pruritus treatment with topical capsaicin. J Am Acad Dermatol. 1993;29(5, pt 1):783-784.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
- Springall DR, Karanth SS, Kirkham N, et al. Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97:555-561.
- Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
- Misery L. What is notalgia paresthetica? Dermatology. 2002;204:86-87.
- Pleet AB, Massey EW. Notalgia paresthetica. Neurology. 1978;28:1310-1312.
- Findlay C, Ayis S, Demetriades AK. Total disc replacement versus anterior cervical discectomy and fusion. Bone Joint J. 2018;100-B:991-1001.
Case Report
A 74-year-old man presented with persistent episodes of severe pruritus with exacerbations on the bilateral forearms, arms, and left side of the mid back of 4 years’ duration. He had a refractory and debilitating disease that had failed extensive therapies including topical antipruritics, antihistamines, oral hydroxyzine, capsaicin, potent topical steroids (ie, clobetasol, fluocinonide, triamcinolone), phototherapy with narrowband UVB, and various dietary modifications including a gluten-free trial. The patient reported he had exhausted all medical evaluation through care with more than 7 physicians and multiple dermatologists, including a university-based dermatology department for repeated consultations; he was seen by our dermatology center for an eighth opinion.
Initial dermatologic examination revealed multiple secondarily excoriated, hemorrhagic, hyperpigmented plaques and nodules on the right side of the mid upper back indicative of notalgia paresthetica (NP) with secondary chronic skin changes (Figure 1). Additional examination of the left arm and forearm revealed several open erosions, raised nodules, and lichenified skin plaques indicative of brachioradial pruritus (BP) with secondary skin changes (Figure 2). In addition, multiple lichenified plaques of the left side of the mid back were associated with decreased sensory alternations to light touch and pin prick. Of note, the localized pruritus pattern, particularly of the unilateral infrascapular back region, heralded the possibility of a neuropathic pruritus condition originating from the cervical spine. Examination confirmed decreased range of motion in the neck with associated marked palpable bilateral cervical muscle spasm and tenderness. Laboratory testing confirmed Staphylococcus aureus secondary skin infection that was treated empirically with chlorhexidine wash. General pruritus serology and imaging workup was ordered with contributory results. The patient’s medical history was notable for noninsulin-dependent diabetes mellitus, obesity, deep venous thrombosis, asthma, vein surgery, cardiovascular disease, atrial fibrillation, atopy, allergies, asthma, and keratosis pilaris, as well as drug intolerances of warfarin sodium, sitagliptin, and clopidogrel. His medications on presentation included glyburide, digoxin, prednisone, aspirin, cetirizine, cimetidine, and hydroxyzine. Based on the relatively classic localized pruritus symptoms and the anatomical distribution of skin findings, a clinical diagnosis of concurrent NP and BRP was made, and radiologic studies of the cervical spine were ordered.


Magnetic resonance imaging (MRI) of the cervical spine showed severe central canal stenosis at C3-C4 secondary to disc disease slight asymmetric toward the right side, severe central canal stenosis at C4-C5 slightly more prominent in the midline, severe central stenosis at C5-C6 more prominent in the midline, and mild changes at other levels as described. Laboratory workup revealed an abnormal complete blood cell count with mildly elevated white blood cell count (11,800/µL [reference range, 4000–10,500/µL]), elevated neutrophils (8600/µL [reference range, 1800–7800/µL]), elevated eosinophils (600/µL [reference range, 0–450/µL ]), and elevated IgE (160 IU/mL [reference range, 0–100 IU/mL]). Further testing revealed negative results for Helicobacter pylori IgG and IgM, human immunodeficiency virus, and hepatitis B and C screening panels; antinuclear antibody negative; normal thyroid-stimulating hormone; and normal thyroid peroxidase antibody. Chest radiograph and computed tomography of the chest, abdomen, and pelvis were negative.
We referred the patient for a neurosurgical consultation that uncovered newly diagnosed severe cervical stenosis with mild to moderate canal compromise at C3, C4, C5, and C6. His motor examination revealed full strength in the upper extremities (5/5). Sensory examination showed patchy sensory alteration on the mid back. He declined oral antibiotics as advised for the skin staphylococcal infection and neurosurgical treatment for the cervical disease.
During the 4 years prior to presentation at our center, the patient reported failure to improve with a dermatologically prescribed gluten-free diet as well as all topical and oral steroid treatments. He was presented at a university grand rounds where a suggestion for UVB light treatment was made; the patient reported possible worsening of symptoms with narrowband UVB phototherapy.
At the patient’s first visit at our center, for immediate symptom relief he underwent therapy with transcutaneous electronic nerve stimulation (TENS) with acupuncture of the cervicothoracic spine (Figure 3). He agreed to discontinue oral prednisone and begin chlorhexidine cleansing body wash, low-dose hydroxyzine 10-mg tablets up to 60 mg every 6 hours as required for pruritus, and mupirocin intranasal ointment. At 1-week follow-up, he reported at least 50% improvement in his symptoms with decreased pruritus, improved sleep, and enhanced quality of life. Within 2 weeks of initial assessment, there was a notable 70% clinical improvement of both the NP and BRP, with a notable decrease in cutaneous erosions and flattening of the pruritic skin nodules. He reported adequate control of symptoms with continued TENS for at-home use 3 times daily for 5- to 10-minute intervals.

Comment
NP Presentation
Notalgia paresthetica is a common, albeit heavily unrecognized and underdiagnosed, sensory neuropathic syndrome of the back, classically of the unilateral infrascapular region. Notalg
The dermatologic condition may consist of other symptoms that include but are not limited to localized burning, pain, tenderness, hyperalgesia, or dysesthesia. Notalgia paresthetica typically is associated with a poorly confined tan or hyperpigmented patch in the symptomatic area, though the skin may have no visible findings in many early cases. Notalgia paresthetica tends to be a chronic condition with periodic remissions and exacerbations. It is generally not associated with other comorbidities and is not life threatening; however, it does frequently decrease quality of life, causing much discomfort and annoyance to the affected patients.
Treatment
Topical therapies for NP have generally failed and are considered difficult because of the out-of-reach affected location. There is no uniformly effective treatment of NP.
Pathogenesis
The etiologies for NP and BRP are evolving and remain to be fully elucidated. Although the exact etiology remains uncertain, there are several possible mechanisms that have been proposed for NP: (1) neuropathy from degenerative cervicothoracic disc disease or direct nerve impingement,1 and (2) localized increased sensory innervations of the affected skin areas.2
Differential and Workup
The differential diagnosis in NP may include allergic or irritant contact dermatitis, fixed drug eruption, dermatophytosis, neoplasm, lichen amyloidosis, arthropod reaction, lichenified skin reactions including lichen simplex chronicus, neurodermatitis, infection, and other hypersensitivity reaction.
It is important during the initial assessment of patients with NP and/or BRP to obtain a thorough history of osteoarthritis, neck trauma, motor vehicle accident(s), vertebral fracture, cervical neoplasm or malignancy, family history of NP or BRP, or cervical disc disease. Radiographs or MRIs of the cervical spine may aid in diagnosis and treatment, and perhaps more so if there is an absence of contributory medical history. Radiographic imaging also may be indicated if there is a positive family history of osteoarthritis or vertebral disc disease.
A full laboratory workup including complete blood cell count, chemistry panel including renal and liver functions, and other laboratory tests (eg, IgE levels) may be warranted if pruritus is generalized and persistent to exclude other causes. Proper management of NP and BRP may involve a multispecialty effort of dermatology with radiology; orthopedic surgery; neurosurgery; neurology; and adjunctive fields including acupuncture, massage, chiropractic, and physical therapy.
NP and BRB Overlap
Because NP and BRP often originate from varying degrees of cervical disease, particularly at the C5-C6 level, traditional topical therapies aimed at treating the affected skin of the mid back and forearms may be ineffectual or partially effective as basic emollients. Due to NP and BRP’s periodic spontaneous remissions and exacerbations, it may be reasonably difficult to accurately measure direct response to various therapies. Some topical therapies aimed at the mid back skin or forearms may appear partially effective from a placebo-type perspective.
Currently, uniformly effective treatment of NP and BRP include the following: (1) therapies aimed at the cervical spine at C5-C6, including TENS, cervical massage, physical therapy, and acupuncture; and (2) therapies targeting the underlying lowered pruritus threshold such as oral antihistamines and narrowband UVB. Although uniformly effective treatments in this space had been previously lacking, traditional therapeutic options for NP and BRP included capsaicin cream, eutectic mixture of local anesthetic cream, topical steroids, pramoxine cream, topical cooling, oral steroids, menthol creams, various commercially available topical mixtures of menthol and methyl salicylate, cordran tape, intralesional corticosteroid injections, botulinum toxin injections,3 oral antihistamines, hydroxyzine, doxepin, topiramate, carbamazepine, antidepressants, gabapentin, oxcarbazepine, topiramate, thalidomide,4 paravertebral local anesthetic block, cervical epidural injection, surgical resection of the rib, and many others. Some of the tried systemic therapies exert their effect through the spinal nerves and central nervous system, thereby supporting the neuropathic etiology of NP.
Cervical Disc Disease
Alai et al1 reported a 37-year-old with documented NP on the right side of the back with MRI findings of disc disease at C5-C6 and mild nerve impingement that strongly suggest the association of cervical degenerative disc disease and NP.
Savk and Savk5 reported that 7 of 10 patients with NP demonstrated normal neurological examination and standard electrodiagnostic results. All had skin histopathology compatible with postinflammatory hyperpigmentation. There were no amyloid deposits or other described pathology on pathologic examination of the skin. Seven of 10 cases confirmed radiographic changes in the vertebra corresponding to the dermatome of the cutaneous lesion.5
An earlier study by Springall et al6 evaluating the mechanism of NP studied whether the cutaneous symptoms were caused by alternations on the cutaneous innervation of the involved infrascapular area. They p
Histologic studies have shown cutaneous changes in a few cases including lichen amyloidosis that may be secondary to the localized chronic scratching and rubbing.7 Clinical observations in orthopedics have established a clear relationship between the upper thoracic and interscapular region and the lower cervical spine. Frequently, cervical disc disease presents as referred pain in the upper thoracic and interscapular area. Similarly, some tumors of the cervical medulla also have presented as interscapular pain.8
Some have speculated direct involvement and actual entrapment of the posterior rami of T2-T6 spinal nerves.9 However, there are referred symptoms from the cervical area directly to the interscapular back. Degenerative vertebral and disc changes corresponding to the affected dermatome may be observed in some cases. Radiographic imaging of cervical and thoracic spine will help to exclude disc disease and possible nerve compromise.8
With advances in radiography and availability of MRI, earlier detection and intervention of cervical disc disease is possible. Early recognition may promote timely intervention and treatment to prevent cervical spine disease progression. In addition to degenerative cervical discs, osteoarthritis, and cervical spine strain and muscle spasm, there may be neoplasms or other pathology of the cervical spine contributing to NP and BRP.
There is some thought that there may be a relationship between NP and BRP. The described association of many cases of BRP and cervical spine disease6 and description of these diseases as likely neuropathic/neurogenic pruritic conditions also support a probable association of these two conditions. In contrast, NP has classically been described as unilateral in distribution, while BRP may involve unilateral or bilateral dorsolateral forearms. Most recently, as seen in our case, there are increasing incidences of nonclassic presentations of both of these diseases that may involve additional skin areas and be a basis for diagnostic challenges to the clinician.
First-line therapies for NP and BRP with associated cervical spinal disease are currently evolving and may include nondermatologic and noninvasive treatments such as spinal manipulation, physical therapy, acupuncture, cervical soft collars, massage, cervical traction, cervical muscle strengthening and increased range on motion, oral nonsteroidal anti-inflammatory medications (eg, ibuprofen, celecoxib, ketorolac), and oral muscle relaxants (eg, carisoprodol, cyclobenzaprine, methocarbamol, metaxalone). Curren
Conclusion
Notalgia paresthetica and BRP may not be solely skin diseases but rather cutaneous signs of an underlying cervical spine disease. The striking association of NP with BRP we present as well as the degenerative and/or traumatic cervicothoracic spine disease suggests that early spinal nerve impingement or cervical muscle spasm may contribute to the pathogenesis of these skin symptoms. Additional studies are needed to further assess the relationship of NP and BRP as well as the association of each disease entity independently with cervical spine disease, as it is unknown if these are causal or coincidental findings. Although topical therapies may seemingly help decrease the localized symptoms in NP and BRP in some cases, systemic or broader-scope cervical spinal evaluation may be warranted to fully evaluate refractory cases. Cervical spinal imaging and treatment, particularly at C5-C6 levels, may be appropriate as primary or first-line therapy in many cases of NP and BRP. The paradigm shift in thinking will more likely than not be to treat the cervical spine and the skin will follow.
Case Report
A 74-year-old man presented with persistent episodes of severe pruritus with exacerbations on the bilateral forearms, arms, and left side of the mid back of 4 years’ duration. He had a refractory and debilitating disease that had failed extensive therapies including topical antipruritics, antihistamines, oral hydroxyzine, capsaicin, potent topical steroids (ie, clobetasol, fluocinonide, triamcinolone), phototherapy with narrowband UVB, and various dietary modifications including a gluten-free trial. The patient reported he had exhausted all medical evaluation through care with more than 7 physicians and multiple dermatologists, including a university-based dermatology department for repeated consultations; he was seen by our dermatology center for an eighth opinion.
Initial dermatologic examination revealed multiple secondarily excoriated, hemorrhagic, hyperpigmented plaques and nodules on the right side of the mid upper back indicative of notalgia paresthetica (NP) with secondary chronic skin changes (Figure 1). Additional examination of the left arm and forearm revealed several open erosions, raised nodules, and lichenified skin plaques indicative of brachioradial pruritus (BP) with secondary skin changes (Figure 2). In addition, multiple lichenified plaques of the left side of the mid back were associated with decreased sensory alternations to light touch and pin prick. Of note, the localized pruritus pattern, particularly of the unilateral infrascapular back region, heralded the possibility of a neuropathic pruritus condition originating from the cervical spine. Examination confirmed decreased range of motion in the neck with associated marked palpable bilateral cervical muscle spasm and tenderness. Laboratory testing confirmed Staphylococcus aureus secondary skin infection that was treated empirically with chlorhexidine wash. General pruritus serology and imaging workup was ordered with contributory results. The patient’s medical history was notable for noninsulin-dependent diabetes mellitus, obesity, deep venous thrombosis, asthma, vein surgery, cardiovascular disease, atrial fibrillation, atopy, allergies, asthma, and keratosis pilaris, as well as drug intolerances of warfarin sodium, sitagliptin, and clopidogrel. His medications on presentation included glyburide, digoxin, prednisone, aspirin, cetirizine, cimetidine, and hydroxyzine. Based on the relatively classic localized pruritus symptoms and the anatomical distribution of skin findings, a clinical diagnosis of concurrent NP and BRP was made, and radiologic studies of the cervical spine were ordered.


Magnetic resonance imaging (MRI) of the cervical spine showed severe central canal stenosis at C3-C4 secondary to disc disease slight asymmetric toward the right side, severe central canal stenosis at C4-C5 slightly more prominent in the midline, severe central stenosis at C5-C6 more prominent in the midline, and mild changes at other levels as described. Laboratory workup revealed an abnormal complete blood cell count with mildly elevated white blood cell count (11,800/µL [reference range, 4000–10,500/µL]), elevated neutrophils (8600/µL [reference range, 1800–7800/µL]), elevated eosinophils (600/µL [reference range, 0–450/µL ]), and elevated IgE (160 IU/mL [reference range, 0–100 IU/mL]). Further testing revealed negative results for Helicobacter pylori IgG and IgM, human immunodeficiency virus, and hepatitis B and C screening panels; antinuclear antibody negative; normal thyroid-stimulating hormone; and normal thyroid peroxidase antibody. Chest radiograph and computed tomography of the chest, abdomen, and pelvis were negative.
We referred the patient for a neurosurgical consultation that uncovered newly diagnosed severe cervical stenosis with mild to moderate canal compromise at C3, C4, C5, and C6. His motor examination revealed full strength in the upper extremities (5/5). Sensory examination showed patchy sensory alteration on the mid back. He declined oral antibiotics as advised for the skin staphylococcal infection and neurosurgical treatment for the cervical disease.
During the 4 years prior to presentation at our center, the patient reported failure to improve with a dermatologically prescribed gluten-free diet as well as all topical and oral steroid treatments. He was presented at a university grand rounds where a suggestion for UVB light treatment was made; the patient reported possible worsening of symptoms with narrowband UVB phototherapy.
At the patient’s first visit at our center, for immediate symptom relief he underwent therapy with transcutaneous electronic nerve stimulation (TENS) with acupuncture of the cervicothoracic spine (Figure 3). He agreed to discontinue oral prednisone and begin chlorhexidine cleansing body wash, low-dose hydroxyzine 10-mg tablets up to 60 mg every 6 hours as required for pruritus, and mupirocin intranasal ointment. At 1-week follow-up, he reported at least 50% improvement in his symptoms with decreased pruritus, improved sleep, and enhanced quality of life. Within 2 weeks of initial assessment, there was a notable 70% clinical improvement of both the NP and BRP, with a notable decrease in cutaneous erosions and flattening of the pruritic skin nodules. He reported adequate control of symptoms with continued TENS for at-home use 3 times daily for 5- to 10-minute intervals.

Comment
NP Presentation
Notalgia paresthetica is a common, albeit heavily unrecognized and underdiagnosed, sensory neuropathic syndrome of the back, classically of the unilateral infrascapular region. Notalg
The dermatologic condition may consist of other symptoms that include but are not limited to localized burning, pain, tenderness, hyperalgesia, or dysesthesia. Notalgia paresthetica typically is associated with a poorly confined tan or hyperpigmented patch in the symptomatic area, though the skin may have no visible findings in many early cases. Notalgia paresthetica tends to be a chronic condition with periodic remissions and exacerbations. It is generally not associated with other comorbidities and is not life threatening; however, it does frequently decrease quality of life, causing much discomfort and annoyance to the affected patients.
Treatment
Topical therapies for NP have generally failed and are considered difficult because of the out-of-reach affected location. There is no uniformly effective treatment of NP.
Pathogenesis
The etiologies for NP and BRP are evolving and remain to be fully elucidated. Although the exact etiology remains uncertain, there are several possible mechanisms that have been proposed for NP: (1) neuropathy from degenerative cervicothoracic disc disease or direct nerve impingement,1 and (2) localized increased sensory innervations of the affected skin areas.2
Differential and Workup
The differential diagnosis in NP may include allergic or irritant contact dermatitis, fixed drug eruption, dermatophytosis, neoplasm, lichen amyloidosis, arthropod reaction, lichenified skin reactions including lichen simplex chronicus, neurodermatitis, infection, and other hypersensitivity reaction.
It is important during the initial assessment of patients with NP and/or BRP to obtain a thorough history of osteoarthritis, neck trauma, motor vehicle accident(s), vertebral fracture, cervical neoplasm or malignancy, family history of NP or BRP, or cervical disc disease. Radiographs or MRIs of the cervical spine may aid in diagnosis and treatment, and perhaps more so if there is an absence of contributory medical history. Radiographic imaging also may be indicated if there is a positive family history of osteoarthritis or vertebral disc disease.
A full laboratory workup including complete blood cell count, chemistry panel including renal and liver functions, and other laboratory tests (eg, IgE levels) may be warranted if pruritus is generalized and persistent to exclude other causes. Proper management of NP and BRP may involve a multispecialty effort of dermatology with radiology; orthopedic surgery; neurosurgery; neurology; and adjunctive fields including acupuncture, massage, chiropractic, and physical therapy.
NP and BRB Overlap
Because NP and BRP often originate from varying degrees of cervical disease, particularly at the C5-C6 level, traditional topical therapies aimed at treating the affected skin of the mid back and forearms may be ineffectual or partially effective as basic emollients. Due to NP and BRP’s periodic spontaneous remissions and exacerbations, it may be reasonably difficult to accurately measure direct response to various therapies. Some topical therapies aimed at the mid back skin or forearms may appear partially effective from a placebo-type perspective.
Currently, uniformly effective treatment of NP and BRP include the following: (1) therapies aimed at the cervical spine at C5-C6, including TENS, cervical massage, physical therapy, and acupuncture; and (2) therapies targeting the underlying lowered pruritus threshold such as oral antihistamines and narrowband UVB. Although uniformly effective treatments in this space had been previously lacking, traditional therapeutic options for NP and BRP included capsaicin cream, eutectic mixture of local anesthetic cream, topical steroids, pramoxine cream, topical cooling, oral steroids, menthol creams, various commercially available topical mixtures of menthol and methyl salicylate, cordran tape, intralesional corticosteroid injections, botulinum toxin injections,3 oral antihistamines, hydroxyzine, doxepin, topiramate, carbamazepine, antidepressants, gabapentin, oxcarbazepine, topiramate, thalidomide,4 paravertebral local anesthetic block, cervical epidural injection, surgical resection of the rib, and many others. Some of the tried systemic therapies exert their effect through the spinal nerves and central nervous system, thereby supporting the neuropathic etiology of NP.
Cervical Disc Disease
Alai et al1 reported a 37-year-old with documented NP on the right side of the back with MRI findings of disc disease at C5-C6 and mild nerve impingement that strongly suggest the association of cervical degenerative disc disease and NP.
Savk and Savk5 reported that 7 of 10 patients with NP demonstrated normal neurological examination and standard electrodiagnostic results. All had skin histopathology compatible with postinflammatory hyperpigmentation. There were no amyloid deposits or other described pathology on pathologic examination of the skin. Seven of 10 cases confirmed radiographic changes in the vertebra corresponding to the dermatome of the cutaneous lesion.5
An earlier study by Springall et al6 evaluating the mechanism of NP studied whether the cutaneous symptoms were caused by alternations on the cutaneous innervation of the involved infrascapular area. They p
Histologic studies have shown cutaneous changes in a few cases including lichen amyloidosis that may be secondary to the localized chronic scratching and rubbing.7 Clinical observations in orthopedics have established a clear relationship between the upper thoracic and interscapular region and the lower cervical spine. Frequently, cervical disc disease presents as referred pain in the upper thoracic and interscapular area. Similarly, some tumors of the cervical medulla also have presented as interscapular pain.8
Some have speculated direct involvement and actual entrapment of the posterior rami of T2-T6 spinal nerves.9 However, there are referred symptoms from the cervical area directly to the interscapular back. Degenerative vertebral and disc changes corresponding to the affected dermatome may be observed in some cases. Radiographic imaging of cervical and thoracic spine will help to exclude disc disease and possible nerve compromise.8
With advances in radiography and availability of MRI, earlier detection and intervention of cervical disc disease is possible. Early recognition may promote timely intervention and treatment to prevent cervical spine disease progression. In addition to degenerative cervical discs, osteoarthritis, and cervical spine strain and muscle spasm, there may be neoplasms or other pathology of the cervical spine contributing to NP and BRP.
There is some thought that there may be a relationship between NP and BRP. The described association of many cases of BRP and cervical spine disease6 and description of these diseases as likely neuropathic/neurogenic pruritic conditions also support a probable association of these two conditions. In contrast, NP has classically been described as unilateral in distribution, while BRP may involve unilateral or bilateral dorsolateral forearms. Most recently, as seen in our case, there are increasing incidences of nonclassic presentations of both of these diseases that may involve additional skin areas and be a basis for diagnostic challenges to the clinician.
First-line therapies for NP and BRP with associated cervical spinal disease are currently evolving and may include nondermatologic and noninvasive treatments such as spinal manipulation, physical therapy, acupuncture, cervical soft collars, massage, cervical traction, cervical muscle strengthening and increased range on motion, oral nonsteroidal anti-inflammatory medications (eg, ibuprofen, celecoxib, ketorolac), and oral muscle relaxants (eg, carisoprodol, cyclobenzaprine, methocarbamol, metaxalone). Curren
Conclusion
Notalgia paresthetica and BRP may not be solely skin diseases but rather cutaneous signs of an underlying cervical spine disease. The striking association of NP with BRP we present as well as the degenerative and/or traumatic cervicothoracic spine disease suggests that early spinal nerve impingement or cervical muscle spasm may contribute to the pathogenesis of these skin symptoms. Additional studies are needed to further assess the relationship of NP and BRP as well as the association of each disease entity independently with cervical spine disease, as it is unknown if these are causal or coincidental findings. Although topical therapies may seemingly help decrease the localized symptoms in NP and BRP in some cases, systemic or broader-scope cervical spinal evaluation may be warranted to fully evaluate refractory cases. Cervical spinal imaging and treatment, particularly at C5-C6 levels, may be appropriate as primary or first-line therapy in many cases of NP and BRP. The paradigm shift in thinking will more likely than not be to treat the cervical spine and the skin will follow.
- Alai NN, Skinner HB, Nabili S, et al. Notalgia paresthetica associated with cervical spinal stenosis and cervicothoracic disk disease at C4 through C7. Cutis. 2010;85:77-81.
- Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170.
- Goodless DR, Eaglstein WH. Brachioradial pruritus treatment with topical capsaicin. J Am Acad Dermatol. 1993;29(5, pt 1):783-784.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
- Springall DR, Karanth SS, Kirkham N, et al. Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97:555-561.
- Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
- Misery L. What is notalgia paresthetica? Dermatology. 2002;204:86-87.
- Pleet AB, Massey EW. Notalgia paresthetica. Neurology. 1978;28:1310-1312.
- Findlay C, Ayis S, Demetriades AK. Total disc replacement versus anterior cervical discectomy and fusion. Bone Joint J. 2018;100-B:991-1001.
- Alai NN, Skinner HB, Nabili S, et al. Notalgia paresthetica associated with cervical spinal stenosis and cervicothoracic disk disease at C4 through C7. Cutis. 2010;85:77-81.
- Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170.
- Goodless DR, Eaglstein WH. Brachioradial pruritus treatment with topical capsaicin. J Am Acad Dermatol. 1993;29(5, pt 1):783-784.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
- Springall DR, Karanth SS, Kirkham N, et al. Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97:555-561.
- Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
- Misery L. What is notalgia paresthetica? Dermatology. 2002;204:86-87.
- Pleet AB, Massey EW. Notalgia paresthetica. Neurology. 1978;28:1310-1312.
- Findlay C, Ayis S, Demetriades AK. Total disc replacement versus anterior cervical discectomy and fusion. Bone Joint J. 2018;100-B:991-1001.
Practice Points
- Assess the spine in patients with notalgia paresthetica (NP) and brachioradial pruritus (BRP). Cervical spinal disease, especially at the C5-C6 level, may be the root cause of BRP and/or NP.
- Consider a multimodality approach to treatment, including physical therapy, acupuncture, massage, and transcutaneous electronic nerve stimulation.
- Treat the neck (underlying cause) and expect the skin (cutaneous symptoms) to follow.
Angioimmunoblastic T-Cell Lymphoma Mimicking Diffuse Large B-Cell Lymphoma
Angioimmunoblastic T-cell lymphoma (AITL) is a rare, often aggressive type of peripheral T-cell lymphoma. It comprises 18% of peripheral T-cell lymphomas and 1% to 2% of all non-Hodgkin lymphomas.1 The incidence of AITL in the United States is estimated to be 0.05 cases per 100,000 person-years,2 and there is a slight male predominance.1,3,4 It typically presents in the seventh decade of life; however, cases have been reported in adults ranging from 20 to 91 years of age.3
Angioimmunoblastic T-cell lymphoma presents with lymphadenopathy, hepatosplenomegaly, and systemic B symptoms (eg, fever, night sweats, weight loss, generalized pruritus).4-6 There are cutaneous manifestations in up to 50% of cases4,5,7 and frequently signs of autoimmune disorder.4,5 The diagnosis often is made by excisional lymph node biopsy. Lymph node specimens characteristically have a mixed inflammatory infiltrate that includes numerous B cells often infected with Epstein-Barr virus (EBV) and a relatively small population of atypical T lymphocytes.8 Identification of this neoplastic population of CD4+CD8− T lymphocytes expressing normal follicular helper T-cell markers CD10, chemokine CXCL13, programmed cell death protein 1 (PD-1), and B-cell lymphoma 6 (BCL-6) confirms the diagnosis of AITL.9,10 These malignant cells can be identified in skin specimens in cases of cutaneous metastatic disease.11,12 We present a case originally misdiagnosed as diffuse large B-cell lymphoma that was later identified as AITL on skin biopsy.
Case Report
A 72-year-old woman presented with a pruritic erythematous eruption around the neck of 3 weeks’ duration (Figure 1). Her medical history was notable for diffuse large B-cell non-Hodgkin lymphoma diagnosed 3 months prior based on results from a right cervical lymph node biopsy. She was treated with bendamustine and rituximab. On physical examination there were erythematous edematous papules coalescing into indurated plaques around the neck. The differential diagnosis included drug hypersensitivity reaction, herpes zoster, urticaria, and cutaneous metastasis. Two punch biopsies were taken for hematoxylin and eosin and tissue culture.
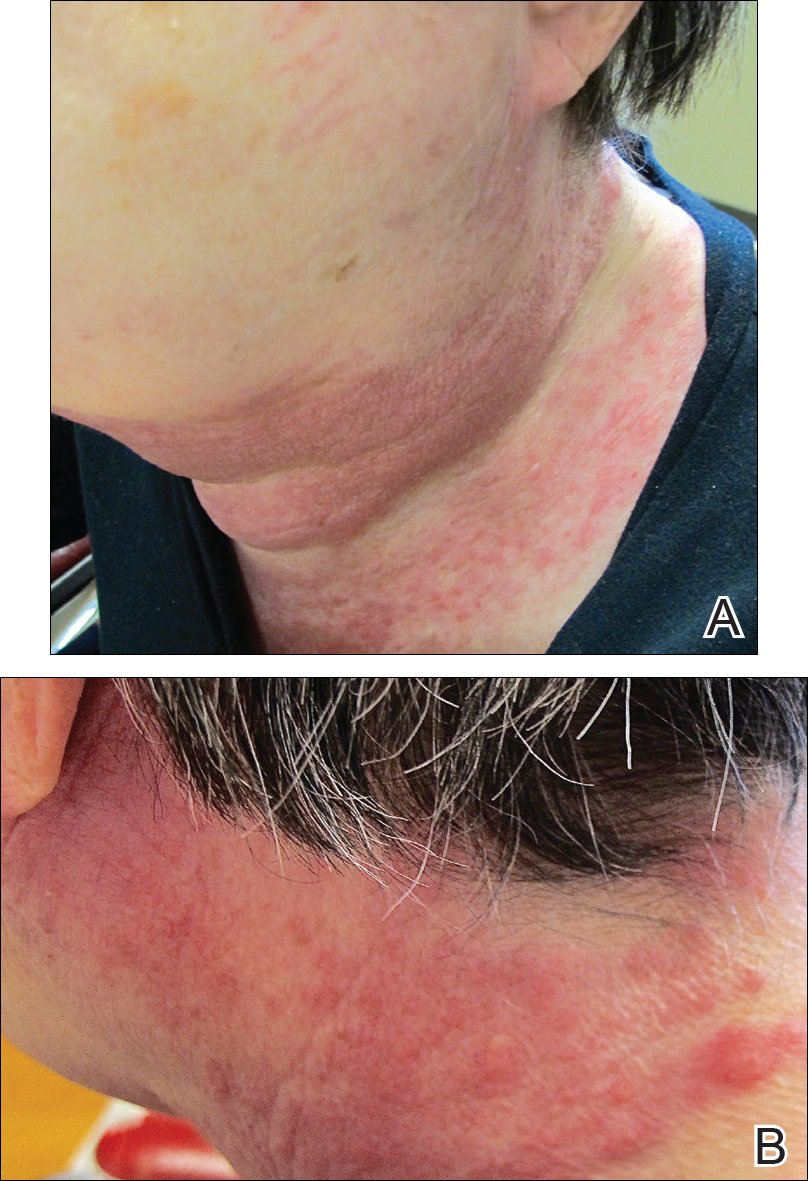
Tissue cultures and viral polymerase chain reaction were negative. Histopathologic examination revealed a scant atypical lymphoid infiltrate focally involving the deep dermis. The cells were medium to large in size and contained hyperchromatic pleomorphic nuclei (Figure 2). They were positive for CD3 and CD4, which was concerning for T-cell lymphoma. The histologic report of the excisional lymph node biopsy done 3 months prior described an atypical lymphoid neoplasm with extensive necrosis and extranodal spread that stained positively for CD20 (Figure 3).
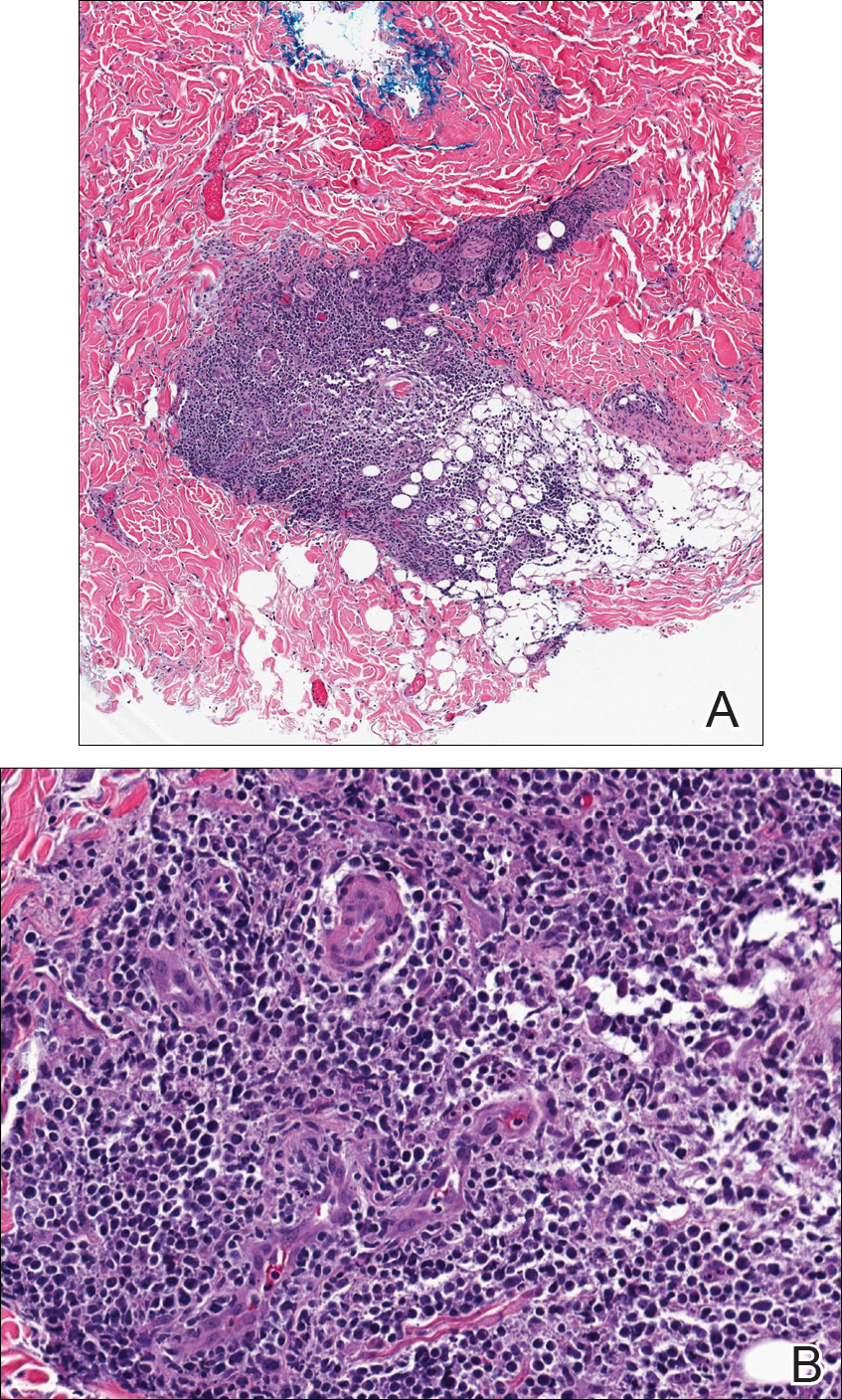
Further staining of this cervical lymph node specimen revealed large atypical lymphoid cells positive for CD3, CD10, B-cell lymphoma 2 (BCL-2), BCL-6, and PD-1. There were intermixed mature B lymphocytes positive for CD20 and BCL-2. Chromogenic in situ hybridization with probes for EBV showed numerous positive cells throughout the infiltrate. Polymerase chain reaction demonstrated a T-cell population with clonally rearranged T-cell receptor genes. Primers for immunoglobulin heavy and light chains showed no evidence of a clonal B-cell population.
Additional staining of the atypical cutaneous lymphocytes revealed positivity for CD3, CD10, and PD-1. The morphologic and immunophenotypic findings of both specimens supported the diagnosis of AITL.
The patient declined further treatment and chose hospice care.
Comment
Etiology
Angioimmunoblastic T-cell lymphoma was originally named angioimmunoblastic lymphadenopathy with dysproteinemia. It was initially thought to be a benign hyperreactive immune process driven by B cells, and patients often died of infectious complications not long after the diagnosis was made.13 As more cases were reported with clonal rearrangements and signs of progressive lymphoma, AITL was recognized as a malignancy.
Presentation
Patients with AITL often present with advanced stage III or IV disease with extranodal and bone marrow involvement.3-6 Cutaneous disease occurs in up to half of patients and portends a poor prognosis.7 The rash often is a nonspecific erythematous macular and papular eruption mimicking a morbilliform viral exanthem or drug reaction. Urticarial, nodular, petechial, purpuric, eczematous, erythrodermic, and vesiculobullous presentations have been described.4,11,12 In up to one-third of cases, the eruption occurs in association with a new medication, often leading to an initial misdiagnosis of drug hypersensitivity reaction.4,11 In a review conducted by Balaraman et al,14 84% of patients with AITL reported having pruritus.
There is an association of autoimmune phenomena in patients with AITL, which is likely a result of immune dysregulation associated with poorly functioning follicular helper T cells. Patients may present with arthralgia, hemolytic anemia, or thrombocytopenic purpura. Hypergammaglobulinemia has been reported in 30% to 50% of AITL patients.4,6 Other pertinent immunologic findings include positive Coombs test, cold agglutinins, cryoglobulinemia, hypocomplementemia, and positive antinuclear antibodies.4-7
Gene Analysis
Affected lymph nodes have a characteristically effaced architecture with proliferative arborizing venules; a hyperplastic population of follicular dendritic cells; and a mixed inflammatory infiltrate that is comprised of atypical lymphocytes and variable numbers of reactive lymphocytes, histiocytes, eosinophils, and plasma cells. The malignant T lymphocytes often account for only a small portion of the infiltrate.8 T-cell gene rearrangement studies identify clonal cells with β and γ rearrangements in the majority of cases.4 These cells are predominantly CD4+CD8− and express normal follicular helper T-cell markers CD10, CXCL13, BCL-6,5,9 and PD-1.10 Numerous B cells are seen intermixed with follicular dendritic cells. They are frequently infected with EBV and can have an atypical Reed-Sternberg cell–like appearance.4,5,15 In the evaluation of AITL, polymerase chain reaction studies with primers for immunoglobulin heavy and light chain should be performed to look for clonal B-cell populations and rule out a possible secondary B-cell lymphoma.
Histology
Five histologic patterns have been described with cutaneous AITL: (1) superficial perivascular infiltrate of eosinophils and lymphocytes that lack atypia, (2) sparse perivascular infiltrate with atypical lymphocytes, (3) dense dermal infiltrate of pleomorphic lymphocytes, (4) leukocytoclastic vasculitis without atypical lymphocytes,11 and (5) necrotizing vasculitis.12 The finding of vascular hyperplasia, perivascular infiltrate, or vasculitis has been reported in 91% of cases in the literature. Although these findings are nonspecific, an analysis of cutaneous cases reported in the literature found that 87% demonstrated T-cell receptor gene rearrangements.14 Lymphoid cells are positive for CD10 and PD-1, as was demonstrated in our case, and are CXCL13 positive in the majority of cases.12 Atypical and EBV-infected B cells also can be found in the skin.11,12
Differential Diagnosis
Angioimmunoblastic T-cell lymphoma can mimic infectious, autoimmune, or allergic etiologies, and misdiagnosis of another type of lymphoma is not uncommon, as occurred in our case. Patients who have a delay in the correct diagnosis have similar outcomes to those correctly diagnosed at first presentation.4
Treatment
There are no effective therapies for AITL. Poor prognostic factors include age (>60 years), stages III to IV disease, male gender, elevated serum lactate dehydrogenase level,3,5,10 and cutaneous involvement.7 Corticosteroids, anthracycline-based chemotherapy, and autologous stem cell transplant are currently the mainstays of therapy. Initial response to chemotherapy is promising, but duration of response is poor overall and there is no increased survival.5,15 A large population-based study of 1207 cases by Xu and Liu3 showed the overall survival rate at 2 and 10 years was 46.8% and 21.9%, respectively. Ten-year disease-specific survival was 35.9%, and there was no demonstrable improvement in survival over the last 2 decades.3 Case reports have demonstrated that thalidomide,16 lenalidomide,17 and cyclosporine plus dexamethasone18 have been successfully used to achieve remission for up to 3 years.
Conclusion
Angioimmunoblastic T-cell lymphoma is difficult to diagnose due to nonspecific clinical and histologic findings. Cutaneous manifestations are seen in AITL in up to half of cases that may occur early or in advanced disease. Similar to all cutaneous metastases, the appearance of the lesions can greatly vary. Our case demonstrates that dermatologists and dermatopathologists can make this diagnosis in the appropriate clinicopathologic context utilizing appropriate immunohistochemical staining and gene rearrangement studies.
- Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkins Lymphoma Classification Project. Ann Oncol. 2002;13:140-149.
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-276.
- Xu B, Liu P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PLoS One. 2014;9:e92585.
- Lachenal F, Berger F, Ghesquieres H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore). 2007;86:282-292.
- Mourad N, Mounier N, Briére J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470.
- Frederico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the International Peripheral T-cell Lymphoma Project. J Clin Oncol. 2013;31:240-246.
- Siegert W, Nerl C, Agthe A, et al. Angioimmunoblastic lym-phadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol. 1995;6:659-664.
- Attygalle AD, Chuang SS, Diss TC, et al. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype, and molecular genetics. Histopathology. 2007;50:498-508.
- Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper cells. Am J Surg Pathol. 2006;30:490-494.
- Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293-1296.
- Martel P, Laroche L, Courville P, et al. Cutaneous involvementin patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000;136:881-886.
- Ortonne N, Dupuis J, Plonquet A, et al. Characterization of CXCL13+ neoplastic t cells in cutaneous lesions of angioimmunoblastic T-cell lymphoma (AITL). Am J Surg Pathol. 2007;31:1068-1076.
- Frizzera G, Moran E, Rappaport H. Angioimmunoblastic lymphadenopathy with dysproteinemia. Lancet. 1974;1:1070-1073.
- Balaraman B, Conley JA, Sheinbein DM. Evaluation of cutaneous angioimmunoblastic T-cell lymphoma [published online May 6, 2011]. J Am Acad Dermatol. 2011;65:855-862.
- Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood. 2012;119:2837-2843.
- Dogan A, Ngu LSP, Ng SH, et al. Pathology and clinical features of angioimmunoblastic T-cell lymphoma after successful treatment with thalidomide. Leukemia. 2005;19:873-875.
- Fabbri A, Cencini E, Pietrini A, et al. Impressive activity of lenalidomide monotherapy in refractory angioimmunoblastic T-cell lymphoma: report of a case with long-term follow-up. Hematol Oncol. 2013;31:213-217.
- Kobayashi T, Kuroda J, Uchiyama H, et al. Successful treatment of chemotherapy-refractory angioimmunoblastic T cell lymphoma with cyclosporin A. Acta Haematol. 2012;127:10-15.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare, often aggressive type of peripheral T-cell lymphoma. It comprises 18% of peripheral T-cell lymphomas and 1% to 2% of all non-Hodgkin lymphomas.1 The incidence of AITL in the United States is estimated to be 0.05 cases per 100,000 person-years,2 and there is a slight male predominance.1,3,4 It typically presents in the seventh decade of life; however, cases have been reported in adults ranging from 20 to 91 years of age.3
Angioimmunoblastic T-cell lymphoma presents with lymphadenopathy, hepatosplenomegaly, and systemic B symptoms (eg, fever, night sweats, weight loss, generalized pruritus).4-6 There are cutaneous manifestations in up to 50% of cases4,5,7 and frequently signs of autoimmune disorder.4,5 The diagnosis often is made by excisional lymph node biopsy. Lymph node specimens characteristically have a mixed inflammatory infiltrate that includes numerous B cells often infected with Epstein-Barr virus (EBV) and a relatively small population of atypical T lymphocytes.8 Identification of this neoplastic population of CD4+CD8− T lymphocytes expressing normal follicular helper T-cell markers CD10, chemokine CXCL13, programmed cell death protein 1 (PD-1), and B-cell lymphoma 6 (BCL-6) confirms the diagnosis of AITL.9,10 These malignant cells can be identified in skin specimens in cases of cutaneous metastatic disease.11,12 We present a case originally misdiagnosed as diffuse large B-cell lymphoma that was later identified as AITL on skin biopsy.
Case Report
A 72-year-old woman presented with a pruritic erythematous eruption around the neck of 3 weeks’ duration (Figure 1). Her medical history was notable for diffuse large B-cell non-Hodgkin lymphoma diagnosed 3 months prior based on results from a right cervical lymph node biopsy. She was treated with bendamustine and rituximab. On physical examination there were erythematous edematous papules coalescing into indurated plaques around the neck. The differential diagnosis included drug hypersensitivity reaction, herpes zoster, urticaria, and cutaneous metastasis. Two punch biopsies were taken for hematoxylin and eosin and tissue culture.

Tissue cultures and viral polymerase chain reaction were negative. Histopathologic examination revealed a scant atypical lymphoid infiltrate focally involving the deep dermis. The cells were medium to large in size and contained hyperchromatic pleomorphic nuclei (Figure 2). They were positive for CD3 and CD4, which was concerning for T-cell lymphoma. The histologic report of the excisional lymph node biopsy done 3 months prior described an atypical lymphoid neoplasm with extensive necrosis and extranodal spread that stained positively for CD20 (Figure 3).

Further staining of this cervical lymph node specimen revealed large atypical lymphoid cells positive for CD3, CD10, B-cell lymphoma 2 (BCL-2), BCL-6, and PD-1. There were intermixed mature B lymphocytes positive for CD20 and BCL-2. Chromogenic in situ hybridization with probes for EBV showed numerous positive cells throughout the infiltrate. Polymerase chain reaction demonstrated a T-cell population with clonally rearranged T-cell receptor genes. Primers for immunoglobulin heavy and light chains showed no evidence of a clonal B-cell population.
Additional staining of the atypical cutaneous lymphocytes revealed positivity for CD3, CD10, and PD-1. The morphologic and immunophenotypic findings of both specimens supported the diagnosis of AITL.
The patient declined further treatment and chose hospice care.
Comment
Etiology
Angioimmunoblastic T-cell lymphoma was originally named angioimmunoblastic lymphadenopathy with dysproteinemia. It was initially thought to be a benign hyperreactive immune process driven by B cells, and patients often died of infectious complications not long after the diagnosis was made.13 As more cases were reported with clonal rearrangements and signs of progressive lymphoma, AITL was recognized as a malignancy.
Presentation
Patients with AITL often present with advanced stage III or IV disease with extranodal and bone marrow involvement.3-6 Cutaneous disease occurs in up to half of patients and portends a poor prognosis.7 The rash often is a nonspecific erythematous macular and papular eruption mimicking a morbilliform viral exanthem or drug reaction. Urticarial, nodular, petechial, purpuric, eczematous, erythrodermic, and vesiculobullous presentations have been described.4,11,12 In up to one-third of cases, the eruption occurs in association with a new medication, often leading to an initial misdiagnosis of drug hypersensitivity reaction.4,11 In a review conducted by Balaraman et al,14 84% of patients with AITL reported having pruritus.
There is an association of autoimmune phenomena in patients with AITL, which is likely a result of immune dysregulation associated with poorly functioning follicular helper T cells. Patients may present with arthralgia, hemolytic anemia, or thrombocytopenic purpura. Hypergammaglobulinemia has been reported in 30% to 50% of AITL patients.4,6 Other pertinent immunologic findings include positive Coombs test, cold agglutinins, cryoglobulinemia, hypocomplementemia, and positive antinuclear antibodies.4-7
Gene Analysis
Affected lymph nodes have a characteristically effaced architecture with proliferative arborizing venules; a hyperplastic population of follicular dendritic cells; and a mixed inflammatory infiltrate that is comprised of atypical lymphocytes and variable numbers of reactive lymphocytes, histiocytes, eosinophils, and plasma cells. The malignant T lymphocytes often account for only a small portion of the infiltrate.8 T-cell gene rearrangement studies identify clonal cells with β and γ rearrangements in the majority of cases.4 These cells are predominantly CD4+CD8− and express normal follicular helper T-cell markers CD10, CXCL13, BCL-6,5,9 and PD-1.10 Numerous B cells are seen intermixed with follicular dendritic cells. They are frequently infected with EBV and can have an atypical Reed-Sternberg cell–like appearance.4,5,15 In the evaluation of AITL, polymerase chain reaction studies with primers for immunoglobulin heavy and light chain should be performed to look for clonal B-cell populations and rule out a possible secondary B-cell lymphoma.
Histology
Five histologic patterns have been described with cutaneous AITL: (1) superficial perivascular infiltrate of eosinophils and lymphocytes that lack atypia, (2) sparse perivascular infiltrate with atypical lymphocytes, (3) dense dermal infiltrate of pleomorphic lymphocytes, (4) leukocytoclastic vasculitis without atypical lymphocytes,11 and (5) necrotizing vasculitis.12 The finding of vascular hyperplasia, perivascular infiltrate, or vasculitis has been reported in 91% of cases in the literature. Although these findings are nonspecific, an analysis of cutaneous cases reported in the literature found that 87% demonstrated T-cell receptor gene rearrangements.14 Lymphoid cells are positive for CD10 and PD-1, as was demonstrated in our case, and are CXCL13 positive in the majority of cases.12 Atypical and EBV-infected B cells also can be found in the skin.11,12
Differential Diagnosis
Angioimmunoblastic T-cell lymphoma can mimic infectious, autoimmune, or allergic etiologies, and misdiagnosis of another type of lymphoma is not uncommon, as occurred in our case. Patients who have a delay in the correct diagnosis have similar outcomes to those correctly diagnosed at first presentation.4
Treatment
There are no effective therapies for AITL. Poor prognostic factors include age (>60 years), stages III to IV disease, male gender, elevated serum lactate dehydrogenase level,3,5,10 and cutaneous involvement.7 Corticosteroids, anthracycline-based chemotherapy, and autologous stem cell transplant are currently the mainstays of therapy. Initial response to chemotherapy is promising, but duration of response is poor overall and there is no increased survival.5,15 A large population-based study of 1207 cases by Xu and Liu3 showed the overall survival rate at 2 and 10 years was 46.8% and 21.9%, respectively. Ten-year disease-specific survival was 35.9%, and there was no demonstrable improvement in survival over the last 2 decades.3 Case reports have demonstrated that thalidomide,16 lenalidomide,17 and cyclosporine plus dexamethasone18 have been successfully used to achieve remission for up to 3 years.
Conclusion
Angioimmunoblastic T-cell lymphoma is difficult to diagnose due to nonspecific clinical and histologic findings. Cutaneous manifestations are seen in AITL in up to half of cases that may occur early or in advanced disease. Similar to all cutaneous metastases, the appearance of the lesions can greatly vary. Our case demonstrates that dermatologists and dermatopathologists can make this diagnosis in the appropriate clinicopathologic context utilizing appropriate immunohistochemical staining and gene rearrangement studies.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare, often aggressive type of peripheral T-cell lymphoma. It comprises 18% of peripheral T-cell lymphomas and 1% to 2% of all non-Hodgkin lymphomas.1 The incidence of AITL in the United States is estimated to be 0.05 cases per 100,000 person-years,2 and there is a slight male predominance.1,3,4 It typically presents in the seventh decade of life; however, cases have been reported in adults ranging from 20 to 91 years of age.3
Angioimmunoblastic T-cell lymphoma presents with lymphadenopathy, hepatosplenomegaly, and systemic B symptoms (eg, fever, night sweats, weight loss, generalized pruritus).4-6 There are cutaneous manifestations in up to 50% of cases4,5,7 and frequently signs of autoimmune disorder.4,5 The diagnosis often is made by excisional lymph node biopsy. Lymph node specimens characteristically have a mixed inflammatory infiltrate that includes numerous B cells often infected with Epstein-Barr virus (EBV) and a relatively small population of atypical T lymphocytes.8 Identification of this neoplastic population of CD4+CD8− T lymphocytes expressing normal follicular helper T-cell markers CD10, chemokine CXCL13, programmed cell death protein 1 (PD-1), and B-cell lymphoma 6 (BCL-6) confirms the diagnosis of AITL.9,10 These malignant cells can be identified in skin specimens in cases of cutaneous metastatic disease.11,12 We present a case originally misdiagnosed as diffuse large B-cell lymphoma that was later identified as AITL on skin biopsy.
Case Report
A 72-year-old woman presented with a pruritic erythematous eruption around the neck of 3 weeks’ duration (Figure 1). Her medical history was notable for diffuse large B-cell non-Hodgkin lymphoma diagnosed 3 months prior based on results from a right cervical lymph node biopsy. She was treated with bendamustine and rituximab. On physical examination there were erythematous edematous papules coalescing into indurated plaques around the neck. The differential diagnosis included drug hypersensitivity reaction, herpes zoster, urticaria, and cutaneous metastasis. Two punch biopsies were taken for hematoxylin and eosin and tissue culture.

Tissue cultures and viral polymerase chain reaction were negative. Histopathologic examination revealed a scant atypical lymphoid infiltrate focally involving the deep dermis. The cells were medium to large in size and contained hyperchromatic pleomorphic nuclei (Figure 2). They were positive for CD3 and CD4, which was concerning for T-cell lymphoma. The histologic report of the excisional lymph node biopsy done 3 months prior described an atypical lymphoid neoplasm with extensive necrosis and extranodal spread that stained positively for CD20 (Figure 3).

Further staining of this cervical lymph node specimen revealed large atypical lymphoid cells positive for CD3, CD10, B-cell lymphoma 2 (BCL-2), BCL-6, and PD-1. There were intermixed mature B lymphocytes positive for CD20 and BCL-2. Chromogenic in situ hybridization with probes for EBV showed numerous positive cells throughout the infiltrate. Polymerase chain reaction demonstrated a T-cell population with clonally rearranged T-cell receptor genes. Primers for immunoglobulin heavy and light chains showed no evidence of a clonal B-cell population.
Additional staining of the atypical cutaneous lymphocytes revealed positivity for CD3, CD10, and PD-1. The morphologic and immunophenotypic findings of both specimens supported the diagnosis of AITL.
The patient declined further treatment and chose hospice care.
Comment
Etiology
Angioimmunoblastic T-cell lymphoma was originally named angioimmunoblastic lymphadenopathy with dysproteinemia. It was initially thought to be a benign hyperreactive immune process driven by B cells, and patients often died of infectious complications not long after the diagnosis was made.13 As more cases were reported with clonal rearrangements and signs of progressive lymphoma, AITL was recognized as a malignancy.
Presentation
Patients with AITL often present with advanced stage III or IV disease with extranodal and bone marrow involvement.3-6 Cutaneous disease occurs in up to half of patients and portends a poor prognosis.7 The rash often is a nonspecific erythematous macular and papular eruption mimicking a morbilliform viral exanthem or drug reaction. Urticarial, nodular, petechial, purpuric, eczematous, erythrodermic, and vesiculobullous presentations have been described.4,11,12 In up to one-third of cases, the eruption occurs in association with a new medication, often leading to an initial misdiagnosis of drug hypersensitivity reaction.4,11 In a review conducted by Balaraman et al,14 84% of patients with AITL reported having pruritus.
There is an association of autoimmune phenomena in patients with AITL, which is likely a result of immune dysregulation associated with poorly functioning follicular helper T cells. Patients may present with arthralgia, hemolytic anemia, or thrombocytopenic purpura. Hypergammaglobulinemia has been reported in 30% to 50% of AITL patients.4,6 Other pertinent immunologic findings include positive Coombs test, cold agglutinins, cryoglobulinemia, hypocomplementemia, and positive antinuclear antibodies.4-7
Gene Analysis
Affected lymph nodes have a characteristically effaced architecture with proliferative arborizing venules; a hyperplastic population of follicular dendritic cells; and a mixed inflammatory infiltrate that is comprised of atypical lymphocytes and variable numbers of reactive lymphocytes, histiocytes, eosinophils, and plasma cells. The malignant T lymphocytes often account for only a small portion of the infiltrate.8 T-cell gene rearrangement studies identify clonal cells with β and γ rearrangements in the majority of cases.4 These cells are predominantly CD4+CD8− and express normal follicular helper T-cell markers CD10, CXCL13, BCL-6,5,9 and PD-1.10 Numerous B cells are seen intermixed with follicular dendritic cells. They are frequently infected with EBV and can have an atypical Reed-Sternberg cell–like appearance.4,5,15 In the evaluation of AITL, polymerase chain reaction studies with primers for immunoglobulin heavy and light chain should be performed to look for clonal B-cell populations and rule out a possible secondary B-cell lymphoma.
Histology
Five histologic patterns have been described with cutaneous AITL: (1) superficial perivascular infiltrate of eosinophils and lymphocytes that lack atypia, (2) sparse perivascular infiltrate with atypical lymphocytes, (3) dense dermal infiltrate of pleomorphic lymphocytes, (4) leukocytoclastic vasculitis without atypical lymphocytes,11 and (5) necrotizing vasculitis.12 The finding of vascular hyperplasia, perivascular infiltrate, or vasculitis has been reported in 91% of cases in the literature. Although these findings are nonspecific, an analysis of cutaneous cases reported in the literature found that 87% demonstrated T-cell receptor gene rearrangements.14 Lymphoid cells are positive for CD10 and PD-1, as was demonstrated in our case, and are CXCL13 positive in the majority of cases.12 Atypical and EBV-infected B cells also can be found in the skin.11,12
Differential Diagnosis
Angioimmunoblastic T-cell lymphoma can mimic infectious, autoimmune, or allergic etiologies, and misdiagnosis of another type of lymphoma is not uncommon, as occurred in our case. Patients who have a delay in the correct diagnosis have similar outcomes to those correctly diagnosed at first presentation.4
Treatment
There are no effective therapies for AITL. Poor prognostic factors include age (>60 years), stages III to IV disease, male gender, elevated serum lactate dehydrogenase level,3,5,10 and cutaneous involvement.7 Corticosteroids, anthracycline-based chemotherapy, and autologous stem cell transplant are currently the mainstays of therapy. Initial response to chemotherapy is promising, but duration of response is poor overall and there is no increased survival.5,15 A large population-based study of 1207 cases by Xu and Liu3 showed the overall survival rate at 2 and 10 years was 46.8% and 21.9%, respectively. Ten-year disease-specific survival was 35.9%, and there was no demonstrable improvement in survival over the last 2 decades.3 Case reports have demonstrated that thalidomide,16 lenalidomide,17 and cyclosporine plus dexamethasone18 have been successfully used to achieve remission for up to 3 years.
Conclusion
Angioimmunoblastic T-cell lymphoma is difficult to diagnose due to nonspecific clinical and histologic findings. Cutaneous manifestations are seen in AITL in up to half of cases that may occur early or in advanced disease. Similar to all cutaneous metastases, the appearance of the lesions can greatly vary. Our case demonstrates that dermatologists and dermatopathologists can make this diagnosis in the appropriate clinicopathologic context utilizing appropriate immunohistochemical staining and gene rearrangement studies.
- Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkins Lymphoma Classification Project. Ann Oncol. 2002;13:140-149.
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-276.
- Xu B, Liu P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PLoS One. 2014;9:e92585.
- Lachenal F, Berger F, Ghesquieres H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore). 2007;86:282-292.
- Mourad N, Mounier N, Briére J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470.
- Frederico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the International Peripheral T-cell Lymphoma Project. J Clin Oncol. 2013;31:240-246.
- Siegert W, Nerl C, Agthe A, et al. Angioimmunoblastic lym-phadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol. 1995;6:659-664.
- Attygalle AD, Chuang SS, Diss TC, et al. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype, and molecular genetics. Histopathology. 2007;50:498-508.
- Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper cells. Am J Surg Pathol. 2006;30:490-494.
- Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293-1296.
- Martel P, Laroche L, Courville P, et al. Cutaneous involvementin patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000;136:881-886.
- Ortonne N, Dupuis J, Plonquet A, et al. Characterization of CXCL13+ neoplastic t cells in cutaneous lesions of angioimmunoblastic T-cell lymphoma (AITL). Am J Surg Pathol. 2007;31:1068-1076.
- Frizzera G, Moran E, Rappaport H. Angioimmunoblastic lymphadenopathy with dysproteinemia. Lancet. 1974;1:1070-1073.
- Balaraman B, Conley JA, Sheinbein DM. Evaluation of cutaneous angioimmunoblastic T-cell lymphoma [published online May 6, 2011]. J Am Acad Dermatol. 2011;65:855-862.
- Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood. 2012;119:2837-2843.
- Dogan A, Ngu LSP, Ng SH, et al. Pathology and clinical features of angioimmunoblastic T-cell lymphoma after successful treatment with thalidomide. Leukemia. 2005;19:873-875.
- Fabbri A, Cencini E, Pietrini A, et al. Impressive activity of lenalidomide monotherapy in refractory angioimmunoblastic T-cell lymphoma: report of a case with long-term follow-up. Hematol Oncol. 2013;31:213-217.
- Kobayashi T, Kuroda J, Uchiyama H, et al. Successful treatment of chemotherapy-refractory angioimmunoblastic T cell lymphoma with cyclosporin A. Acta Haematol. 2012;127:10-15.
- Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkins Lymphoma Classification Project. Ann Oncol. 2002;13:140-149.
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-276.
- Xu B, Liu P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PLoS One. 2014;9:e92585.
- Lachenal F, Berger F, Ghesquieres H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore). 2007;86:282-292.
- Mourad N, Mounier N, Briére J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470.
- Frederico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the International Peripheral T-cell Lymphoma Project. J Clin Oncol. 2013;31:240-246.
- Siegert W, Nerl C, Agthe A, et al. Angioimmunoblastic lym-phadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol. 1995;6:659-664.
- Attygalle AD, Chuang SS, Diss TC, et al. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype, and molecular genetics. Histopathology. 2007;50:498-508.
- Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper cells. Am J Surg Pathol. 2006;30:490-494.
- Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293-1296.
- Martel P, Laroche L, Courville P, et al. Cutaneous involvementin patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000;136:881-886.
- Ortonne N, Dupuis J, Plonquet A, et al. Characterization of CXCL13+ neoplastic t cells in cutaneous lesions of angioimmunoblastic T-cell lymphoma (AITL). Am J Surg Pathol. 2007;31:1068-1076.
- Frizzera G, Moran E, Rappaport H. Angioimmunoblastic lymphadenopathy with dysproteinemia. Lancet. 1974;1:1070-1073.
- Balaraman B, Conley JA, Sheinbein DM. Evaluation of cutaneous angioimmunoblastic T-cell lymphoma [published online May 6, 2011]. J Am Acad Dermatol. 2011;65:855-862.
- Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood. 2012;119:2837-2843.
- Dogan A, Ngu LSP, Ng SH, et al. Pathology and clinical features of angioimmunoblastic T-cell lymphoma after successful treatment with thalidomide. Leukemia. 2005;19:873-875.
- Fabbri A, Cencini E, Pietrini A, et al. Impressive activity of lenalidomide monotherapy in refractory angioimmunoblastic T-cell lymphoma: report of a case with long-term follow-up. Hematol Oncol. 2013;31:213-217.
- Kobayashi T, Kuroda J, Uchiyama H, et al. Successful treatment of chemotherapy-refractory angioimmunoblastic T cell lymphoma with cyclosporin A. Acta Haematol. 2012;127:10-15.
Practice Points
- Angioimmunoblastic T-cell lymphoma (AITL) is a rare, often aggressive type of peripheral T-cell lymphoma.
- Cutaneous manifestations have been seen in up to 50% of cases.
- Immunohistochemical markers for normal follicular helper T cells—CD-10, chemokine CXCL-13, and programmed cell death protein 1 (PD-1)—can be used to differentiate AITL from other types of lymphoma.
- The prognosis of AITL is poor.
Levamisole-Induced Vasculopathy With Gastric Involvement in a Cocaine User
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.
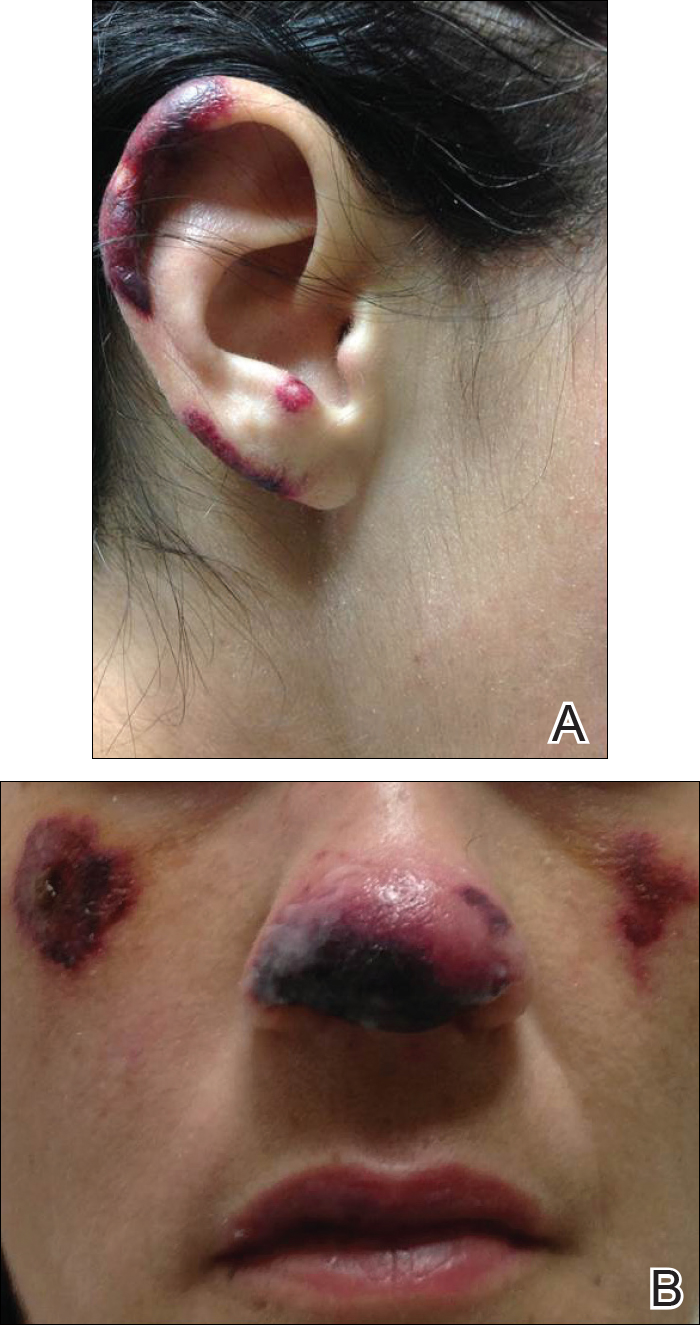
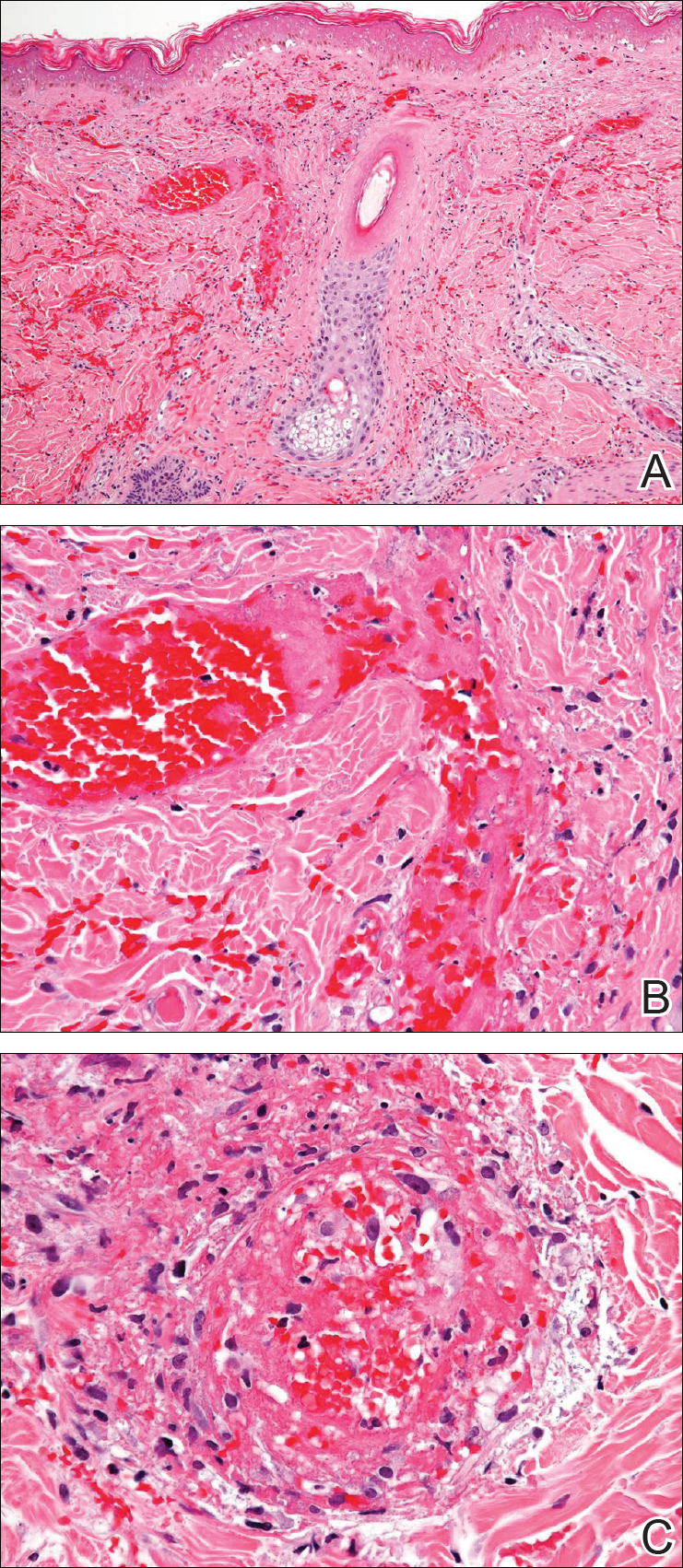
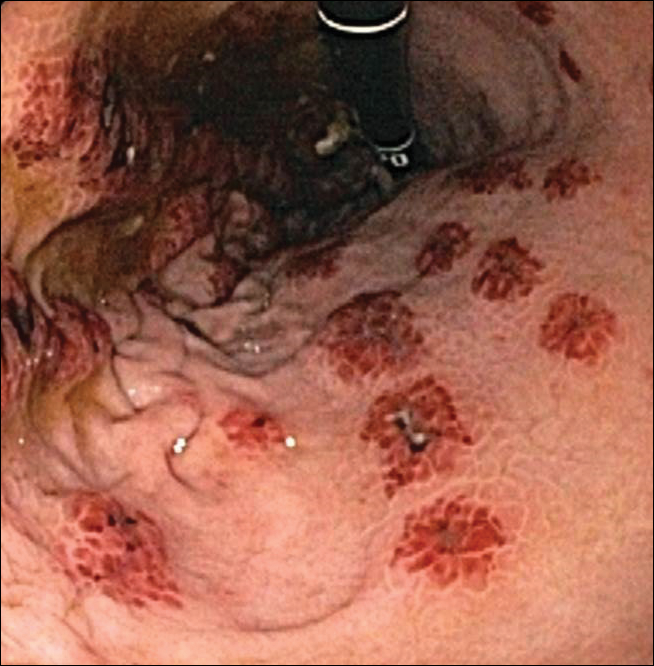
Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.



Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.



Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
Practice Points
- More than half of the cocaine illicitly consumed in the United States is contaminated with levamisole, a veterinary drug that can incite a vasculitic/vasculopathic response in the skin as well as in other organ systems.
- Because dermatologists often are the specialists to make the diagnosis of levamisole-induced vasculopathy, clinicians should be made aware that consumption of levamisole-contaminated cocaine may affect more than the skin alone.
Tetrad Bodies in Skin
The Diagnosis: Bacterial Infection
The tetrad arrangement of organisms seen in this case was classic for Micrococcus and Sarcina species. Both are gram-positive cocci that occur in tetrads, but Micrococcus is aerobic and catalase positive, whereas Sarcina species are anaerobic, catalase negative, acidophilic, and form spores in alkaline pH.1 Although difficult to definitively differentiate on light microscopy, micrococci are smaller in size, ranging from 0.5 to 2.0 μm, and occur in tight clusters, as seen in this case (quiz images), in contrast to Sarcina species, which are relatively larger (1.8-3.0 μm).2 Sarcinae typically are found in soil and air, are considered pathogenic, and are associated with gastric symptoms (Sarcina ventriculi).1Sarcina species also are reported to colonize the skin of patients with diabetes mellitus, but no pathogenic activity is known in the skin.3Micrococcus species, with the majority being Micrococcus luteus, are part of the normal flora of the human skin as well as the oral and nasal cavities. Occasional reports of pneumonia, endocarditis, meningitis, arthritis, endophthalmitis, and sepsis have been reported in immunocompromised individuals.4 In the skin, Micrococcus is a commensal organism; however, Micrococcus sedentarius has been associated with pitted keratolysis, and reports of Micrococcus folliculitis in human immunodeficiency virus patients also are described in the literature.5,6 Micrococci are considered opportunistic bacteria and may worsen and prolong a localized cutaneous infection caused by other organisms under favorable conditions.7Micrococcus luteus is one of the most common bacteria cultured from skin and soft tissue infections caused by fungal organisms.8 Depending on the immune status of an individual, use of broad-spectrum antibiotic and/or elimination of favorable milieu (ie, primary pathogen, breaks in skin) usually treats the infection.
Because of the rarity of infections caused and being part of the normal flora, the clinical implications of subtyping and sensitivity studies via culture or molecular studies may not be important; however, incidental presence of these organisms with unfamiliar morphology may cause confusion for the dermatopathologist. An extremely small size (0.5-2.0 μm) compared to red blood cells (7-8 μm) and white blood cells (10-12 μm) in a tight tetrad arrangement should raise the suspicion for Micrococcus.1 The refractive nature of these organisms from a thick extracellular layer can mimic fungus or plant matter; a negative Grocott-Gomori methenamine-silver stain in this case helped in not only differentiating but also ruling out secondary fungal infection. Finally, a Gram stain with violet staining of these organisms reaffirmed the diagnosis of gram-positive bacterial organisms, most consistent with Micrococcus species (Figure 1). Culture studies were not performed because of contamination of the tissue specimen and resolution of the patient's symptoms.
The presence of foreign material in the skin may be traumatic, occupational, cosmetic, iatrogenic, or self-inflicted, including a wide variety of substances that appear in different morphological forms on hematoxylin and eosin (H&E)-stained sections, depending on their structure and physiochemical properties.9 Although not all foreign bodies may polarize, examining the sample under polarized light is considered an important step to narrow down the differential diagnosis. The tissue reaction is primarily dependent on the nature of the substance and duration, consisting of histiocytes, macrophages, plasma cells, lymphocytes, and fibrosis.9 Activated histiocytes, multinucleated giant cells, and granulomas are classic findings that generally are seen surrounding and engulfing the foreign material (Figure 2). In addition to foreign material, substances such as calcium salts, urate crystals, extruded keratin, ruptured cysts, and hair follicles may act as foreign materials and can incite a tissue response.9 Absence of histiocytic response, granuloma formation, and fibrosis in a lesion of 1 month's duration made the tetrad bodies unlikely to be foreign material.
Demodex mites are superficial inhabitants of human skin that are acquired shortly after birth, live in or near pilosebaceous units, and obtain nourishment from skin cells and sebum.10,11 The mites can be recovered on 10% of skin biopsies, most commonly on the face due to high sebum production.10 Adult mites range from 0.1 to 0.4 mm in length and are round to oval in shape. Females lay eggs inside the hair follicle or sebaceous glands.11 They usually are asymptomatic, but their infestation may become pathogenic, especially in immunocompromised individuals.10 The clinical picture may resemble bacterial folliculitis, rosacea, and perioral dermatitis, while histology typically is characterized by spongiosis, lymphohistiocytic inflammation around infested follicles, and mite(s) in follicular infundibula (Figure 3). Sometimes the protrusion of mites and keratin from the follicles is seen as follicular spines on histology and referred to as pityriasis folliculorum.
Deposits of urate crystals in skin occur from the elevated serum uric acid levels in gout. The cutaneous deposits are mainly in the dermis and subcutaneous tissue and are extremely painful.12 Urate crystals get dissolved during formalin fixation and leave needlelike clefts in a homogenous, lightly basophilic material on H&E slide (Figure 4). For the same reason, polarized microscopy also is not helpful despite the birefringent nature of urate crystals.12
Fungal yeast forms appear round to oval under light microscopy, ranging from 2 to 100 μm in size.13 The common superficial forms involving the epidermis or hair follicles similar to the current case of bacterial infection include Malassezia and dermatophyte infections. Malassezia is part of the normal flora of sebum-rich areas of skin and is associated with superficial infections such as folliculitis, atopic dermatitis, psoriasis, seborrheic dermatitis, and dandruff.14Malassezia appear as clusters of yeast cells that are pleomorphic and round to oval in shape, ranging from 2 to 6 μm in size. It forms hyphae in its pathogenic form and gives rise to the classic spaghetti and meatball-like appearance that can be highlighted by periodic acid-Schiff (Figure 5) and Grocott-Gomori methenamine-silver special stains. Dermatophytes include 3 genera--Trichophyton, Microsporum, and Epidermophyton--with at least 40 species that causes skin infections in humans.14 Fungal spores and hyphae forms are restricted to the stratum corneum. The hyphae forms may not be apparent on H&E stain, and periodic acid-Schiff staining is helpful in visualizing the fungal elements. The presence of neutrophils in the corneal layer, basket weave hyperkeratosis, and presence of fungal hyphae within the corneal layer fissures (sandwich sign) are clues to the dermatophyte infection.15 Other smaller fungi such as Histoplasma capsulatum (2-4 μm), Candida (3-5 μm), and Pneumocystis (2-5 μm) species can be found in skin in disseminated infections, usually affecting immunocompromised individuals.13Histoplasma is a basophilic yeast that exhibits narrow-based budding and appears clustered within or outside of macrophages. Candida species generally are dimorphic, and yeasts are found intermingled with filamentous forms. Pneumocystis infection in skin is extremely rare, and the fungi appear as spherical or crescent-shaped bodies in a foamy amorphous material.16

- Al Rasheed MR, Senseng CG. Sarcina ventriculi: review of the literature. Arch Pathol Lab Med. 2016;140:1441-1445.
- Lam-Himlin D, Tsiatis AC, Montgomery E, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:1700-1705.
- Somerville DA, Lancaster-Smith M. The aerobic cutaneous microflora of diabetic subjects. Br J Dermatol. 1973;89:395-400.
- Hetem DJ, Rooijakkers S, Ekkelenkamp MB. Staphylococci and Micrococci. In: Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 4th ed. Vol 2. New York, NY: Elsevier; 2017:1509-1522.
- Nordstrom KM, McGinley KJ, Cappiello L, et al. Pitted keratolysis. the role of Micrococcus sedentarius. Arch Dermatol. 1987;123:1320-1325.
- Smith KJ, Neafie R, Yeager J, et al. Micrococcus folliculitis in HIV-1 disease. Br J Dermatol. 1999;141:558-561.
- van Rensburg JJ, Lin H, Gao X, et al. The human skin microbiome associates with the outcome of and is influenced by bacterial infection. mBio. 2015;6:E01315-15. doi:10.1128/mBio.01315-15.
- Chuku A, Nwankiti OO. Association of bacteria with fungal infection of skin and soft tissue lesions in plateau state, Nigeria. Br Microbiol Res J. 2013;3:470-477.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Rather PA, Hassan I. Human Demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014;59:60-66.
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- White TC, Findley K, Dawson TL Jr, et al. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med. 2014;4. pii:a019802. doi:10.1101/cshperspect.a019802.
- Gottlieb GJ, Ackerman AB. The "sandwich sign" of dermatophytosis. Am J Dermatopathol. 1986;8:347.
- Hennessey NP, Parro EL, Cockerell CJ. Cutaneous Pneumocystis carinii infection in patients with acquired immunodeficiency syndrome. Arch Dermatol. 1991;127:1699-1701.
The Diagnosis: Bacterial Infection
The tetrad arrangement of organisms seen in this case was classic for Micrococcus and Sarcina species. Both are gram-positive cocci that occur in tetrads, but Micrococcus is aerobic and catalase positive, whereas Sarcina species are anaerobic, catalase negative, acidophilic, and form spores in alkaline pH.1 Although difficult to definitively differentiate on light microscopy, micrococci are smaller in size, ranging from 0.5 to 2.0 μm, and occur in tight clusters, as seen in this case (quiz images), in contrast to Sarcina species, which are relatively larger (1.8-3.0 μm).2 Sarcinae typically are found in soil and air, are considered pathogenic, and are associated with gastric symptoms (Sarcina ventriculi).1Sarcina species also are reported to colonize the skin of patients with diabetes mellitus, but no pathogenic activity is known in the skin.3Micrococcus species, with the majority being Micrococcus luteus, are part of the normal flora of the human skin as well as the oral and nasal cavities. Occasional reports of pneumonia, endocarditis, meningitis, arthritis, endophthalmitis, and sepsis have been reported in immunocompromised individuals.4 In the skin, Micrococcus is a commensal organism; however, Micrococcus sedentarius has been associated with pitted keratolysis, and reports of Micrococcus folliculitis in human immunodeficiency virus patients also are described in the literature.5,6 Micrococci are considered opportunistic bacteria and may worsen and prolong a localized cutaneous infection caused by other organisms under favorable conditions.7Micrococcus luteus is one of the most common bacteria cultured from skin and soft tissue infections caused by fungal organisms.8 Depending on the immune status of an individual, use of broad-spectrum antibiotic and/or elimination of favorable milieu (ie, primary pathogen, breaks in skin) usually treats the infection.
Because of the rarity of infections caused and being part of the normal flora, the clinical implications of subtyping and sensitivity studies via culture or molecular studies may not be important; however, incidental presence of these organisms with unfamiliar morphology may cause confusion for the dermatopathologist. An extremely small size (0.5-2.0 μm) compared to red blood cells (7-8 μm) and white blood cells (10-12 μm) in a tight tetrad arrangement should raise the suspicion for Micrococcus.1 The refractive nature of these organisms from a thick extracellular layer can mimic fungus or plant matter; a negative Grocott-Gomori methenamine-silver stain in this case helped in not only differentiating but also ruling out secondary fungal infection. Finally, a Gram stain with violet staining of these organisms reaffirmed the diagnosis of gram-positive bacterial organisms, most consistent with Micrococcus species (Figure 1). Culture studies were not performed because of contamination of the tissue specimen and resolution of the patient's symptoms.

The presence of foreign material in the skin may be traumatic, occupational, cosmetic, iatrogenic, or self-inflicted, including a wide variety of substances that appear in different morphological forms on hematoxylin and eosin (H&E)-stained sections, depending on their structure and physiochemical properties.9 Although not all foreign bodies may polarize, examining the sample under polarized light is considered an important step to narrow down the differential diagnosis. The tissue reaction is primarily dependent on the nature of the substance and duration, consisting of histiocytes, macrophages, plasma cells, lymphocytes, and fibrosis.9 Activated histiocytes, multinucleated giant cells, and granulomas are classic findings that generally are seen surrounding and engulfing the foreign material (Figure 2). In addition to foreign material, substances such as calcium salts, urate crystals, extruded keratin, ruptured cysts, and hair follicles may act as foreign materials and can incite a tissue response.9 Absence of histiocytic response, granuloma formation, and fibrosis in a lesion of 1 month's duration made the tetrad bodies unlikely to be foreign material.

Demodex mites are superficial inhabitants of human skin that are acquired shortly after birth, live in or near pilosebaceous units, and obtain nourishment from skin cells and sebum.10,11 The mites can be recovered on 10% of skin biopsies, most commonly on the face due to high sebum production.10 Adult mites range from 0.1 to 0.4 mm in length and are round to oval in shape. Females lay eggs inside the hair follicle or sebaceous glands.11 They usually are asymptomatic, but their infestation may become pathogenic, especially in immunocompromised individuals.10 The clinical picture may resemble bacterial folliculitis, rosacea, and perioral dermatitis, while histology typically is characterized by spongiosis, lymphohistiocytic inflammation around infested follicles, and mite(s) in follicular infundibula (Figure 3). Sometimes the protrusion of mites and keratin from the follicles is seen as follicular spines on histology and referred to as pityriasis folliculorum.

Deposits of urate crystals in skin occur from the elevated serum uric acid levels in gout. The cutaneous deposits are mainly in the dermis and subcutaneous tissue and are extremely painful.12 Urate crystals get dissolved during formalin fixation and leave needlelike clefts in a homogenous, lightly basophilic material on H&E slide (Figure 4). For the same reason, polarized microscopy also is not helpful despite the birefringent nature of urate crystals.12

Fungal yeast forms appear round to oval under light microscopy, ranging from 2 to 100 μm in size.13 The common superficial forms involving the epidermis or hair follicles similar to the current case of bacterial infection include Malassezia and dermatophyte infections. Malassezia is part of the normal flora of sebum-rich areas of skin and is associated with superficial infections such as folliculitis, atopic dermatitis, psoriasis, seborrheic dermatitis, and dandruff.14Malassezia appear as clusters of yeast cells that are pleomorphic and round to oval in shape, ranging from 2 to 6 μm in size. It forms hyphae in its pathogenic form and gives rise to the classic spaghetti and meatball-like appearance that can be highlighted by periodic acid-Schiff (Figure 5) and Grocott-Gomori methenamine-silver special stains. Dermatophytes include 3 genera--Trichophyton, Microsporum, and Epidermophyton--with at least 40 species that causes skin infections in humans.14 Fungal spores and hyphae forms are restricted to the stratum corneum. The hyphae forms may not be apparent on H&E stain, and periodic acid-Schiff staining is helpful in visualizing the fungal elements. The presence of neutrophils in the corneal layer, basket weave hyperkeratosis, and presence of fungal hyphae within the corneal layer fissures (sandwich sign) are clues to the dermatophyte infection.15 Other smaller fungi such as Histoplasma capsulatum (2-4 μm), Candida (3-5 μm), and Pneumocystis (2-5 μm) species can be found in skin in disseminated infections, usually affecting immunocompromised individuals.13Histoplasma is a basophilic yeast that exhibits narrow-based budding and appears clustered within or outside of macrophages. Candida species generally are dimorphic, and yeasts are found intermingled with filamentous forms. Pneumocystis infection in skin is extremely rare, and the fungi appear as spherical or crescent-shaped bodies in a foamy amorphous material.16

The Diagnosis: Bacterial Infection
The tetrad arrangement of organisms seen in this case was classic for Micrococcus and Sarcina species. Both are gram-positive cocci that occur in tetrads, but Micrococcus is aerobic and catalase positive, whereas Sarcina species are anaerobic, catalase negative, acidophilic, and form spores in alkaline pH.1 Although difficult to definitively differentiate on light microscopy, micrococci are smaller in size, ranging from 0.5 to 2.0 μm, and occur in tight clusters, as seen in this case (quiz images), in contrast to Sarcina species, which are relatively larger (1.8-3.0 μm).2 Sarcinae typically are found in soil and air, are considered pathogenic, and are associated with gastric symptoms (Sarcina ventriculi).1Sarcina species also are reported to colonize the skin of patients with diabetes mellitus, but no pathogenic activity is known in the skin.3Micrococcus species, with the majority being Micrococcus luteus, are part of the normal flora of the human skin as well as the oral and nasal cavities. Occasional reports of pneumonia, endocarditis, meningitis, arthritis, endophthalmitis, and sepsis have been reported in immunocompromised individuals.4 In the skin, Micrococcus is a commensal organism; however, Micrococcus sedentarius has been associated with pitted keratolysis, and reports of Micrococcus folliculitis in human immunodeficiency virus patients also are described in the literature.5,6 Micrococci are considered opportunistic bacteria and may worsen and prolong a localized cutaneous infection caused by other organisms under favorable conditions.7Micrococcus luteus is one of the most common bacteria cultured from skin and soft tissue infections caused by fungal organisms.8 Depending on the immune status of an individual, use of broad-spectrum antibiotic and/or elimination of favorable milieu (ie, primary pathogen, breaks in skin) usually treats the infection.
Because of the rarity of infections caused and being part of the normal flora, the clinical implications of subtyping and sensitivity studies via culture or molecular studies may not be important; however, incidental presence of these organisms with unfamiliar morphology may cause confusion for the dermatopathologist. An extremely small size (0.5-2.0 μm) compared to red blood cells (7-8 μm) and white blood cells (10-12 μm) in a tight tetrad arrangement should raise the suspicion for Micrococcus.1 The refractive nature of these organisms from a thick extracellular layer can mimic fungus or plant matter; a negative Grocott-Gomori methenamine-silver stain in this case helped in not only differentiating but also ruling out secondary fungal infection. Finally, a Gram stain with violet staining of these organisms reaffirmed the diagnosis of gram-positive bacterial organisms, most consistent with Micrococcus species (Figure 1). Culture studies were not performed because of contamination of the tissue specimen and resolution of the patient's symptoms.

The presence of foreign material in the skin may be traumatic, occupational, cosmetic, iatrogenic, or self-inflicted, including a wide variety of substances that appear in different morphological forms on hematoxylin and eosin (H&E)-stained sections, depending on their structure and physiochemical properties.9 Although not all foreign bodies may polarize, examining the sample under polarized light is considered an important step to narrow down the differential diagnosis. The tissue reaction is primarily dependent on the nature of the substance and duration, consisting of histiocytes, macrophages, plasma cells, lymphocytes, and fibrosis.9 Activated histiocytes, multinucleated giant cells, and granulomas are classic findings that generally are seen surrounding and engulfing the foreign material (Figure 2). In addition to foreign material, substances such as calcium salts, urate crystals, extruded keratin, ruptured cysts, and hair follicles may act as foreign materials and can incite a tissue response.9 Absence of histiocytic response, granuloma formation, and fibrosis in a lesion of 1 month's duration made the tetrad bodies unlikely to be foreign material.

Demodex mites are superficial inhabitants of human skin that are acquired shortly after birth, live in or near pilosebaceous units, and obtain nourishment from skin cells and sebum.10,11 The mites can be recovered on 10% of skin biopsies, most commonly on the face due to high sebum production.10 Adult mites range from 0.1 to 0.4 mm in length and are round to oval in shape. Females lay eggs inside the hair follicle or sebaceous glands.11 They usually are asymptomatic, but their infestation may become pathogenic, especially in immunocompromised individuals.10 The clinical picture may resemble bacterial folliculitis, rosacea, and perioral dermatitis, while histology typically is characterized by spongiosis, lymphohistiocytic inflammation around infested follicles, and mite(s) in follicular infundibula (Figure 3). Sometimes the protrusion of mites and keratin from the follicles is seen as follicular spines on histology and referred to as pityriasis folliculorum.

Deposits of urate crystals in skin occur from the elevated serum uric acid levels in gout. The cutaneous deposits are mainly in the dermis and subcutaneous tissue and are extremely painful.12 Urate crystals get dissolved during formalin fixation and leave needlelike clefts in a homogenous, lightly basophilic material on H&E slide (Figure 4). For the same reason, polarized microscopy also is not helpful despite the birefringent nature of urate crystals.12

Fungal yeast forms appear round to oval under light microscopy, ranging from 2 to 100 μm in size.13 The common superficial forms involving the epidermis or hair follicles similar to the current case of bacterial infection include Malassezia and dermatophyte infections. Malassezia is part of the normal flora of sebum-rich areas of skin and is associated with superficial infections such as folliculitis, atopic dermatitis, psoriasis, seborrheic dermatitis, and dandruff.14Malassezia appear as clusters of yeast cells that are pleomorphic and round to oval in shape, ranging from 2 to 6 μm in size. It forms hyphae in its pathogenic form and gives rise to the classic spaghetti and meatball-like appearance that can be highlighted by periodic acid-Schiff (Figure 5) and Grocott-Gomori methenamine-silver special stains. Dermatophytes include 3 genera--Trichophyton, Microsporum, and Epidermophyton--with at least 40 species that causes skin infections in humans.14 Fungal spores and hyphae forms are restricted to the stratum corneum. The hyphae forms may not be apparent on H&E stain, and periodic acid-Schiff staining is helpful in visualizing the fungal elements. The presence of neutrophils in the corneal layer, basket weave hyperkeratosis, and presence of fungal hyphae within the corneal layer fissures (sandwich sign) are clues to the dermatophyte infection.15 Other smaller fungi such as Histoplasma capsulatum (2-4 μm), Candida (3-5 μm), and Pneumocystis (2-5 μm) species can be found in skin in disseminated infections, usually affecting immunocompromised individuals.13Histoplasma is a basophilic yeast that exhibits narrow-based budding and appears clustered within or outside of macrophages. Candida species generally are dimorphic, and yeasts are found intermingled with filamentous forms. Pneumocystis infection in skin is extremely rare, and the fungi appear as spherical or crescent-shaped bodies in a foamy amorphous material.16

- Al Rasheed MR, Senseng CG. Sarcina ventriculi: review of the literature. Arch Pathol Lab Med. 2016;140:1441-1445.
- Lam-Himlin D, Tsiatis AC, Montgomery E, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:1700-1705.
- Somerville DA, Lancaster-Smith M. The aerobic cutaneous microflora of diabetic subjects. Br J Dermatol. 1973;89:395-400.
- Hetem DJ, Rooijakkers S, Ekkelenkamp MB. Staphylococci and Micrococci. In: Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 4th ed. Vol 2. New York, NY: Elsevier; 2017:1509-1522.
- Nordstrom KM, McGinley KJ, Cappiello L, et al. Pitted keratolysis. the role of Micrococcus sedentarius. Arch Dermatol. 1987;123:1320-1325.
- Smith KJ, Neafie R, Yeager J, et al. Micrococcus folliculitis in HIV-1 disease. Br J Dermatol. 1999;141:558-561.
- van Rensburg JJ, Lin H, Gao X, et al. The human skin microbiome associates with the outcome of and is influenced by bacterial infection. mBio. 2015;6:E01315-15. doi:10.1128/mBio.01315-15.
- Chuku A, Nwankiti OO. Association of bacteria with fungal infection of skin and soft tissue lesions in plateau state, Nigeria. Br Microbiol Res J. 2013;3:470-477.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Rather PA, Hassan I. Human Demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014;59:60-66.
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- White TC, Findley K, Dawson TL Jr, et al. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med. 2014;4. pii:a019802. doi:10.1101/cshperspect.a019802.
- Gottlieb GJ, Ackerman AB. The "sandwich sign" of dermatophytosis. Am J Dermatopathol. 1986;8:347.
- Hennessey NP, Parro EL, Cockerell CJ. Cutaneous Pneumocystis carinii infection in patients with acquired immunodeficiency syndrome. Arch Dermatol. 1991;127:1699-1701.
- Al Rasheed MR, Senseng CG. Sarcina ventriculi: review of the literature. Arch Pathol Lab Med. 2016;140:1441-1445.
- Lam-Himlin D, Tsiatis AC, Montgomery E, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:1700-1705.
- Somerville DA, Lancaster-Smith M. The aerobic cutaneous microflora of diabetic subjects. Br J Dermatol. 1973;89:395-400.
- Hetem DJ, Rooijakkers S, Ekkelenkamp MB. Staphylococci and Micrococci. In: Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 4th ed. Vol 2. New York, NY: Elsevier; 2017:1509-1522.
- Nordstrom KM, McGinley KJ, Cappiello L, et al. Pitted keratolysis. the role of Micrococcus sedentarius. Arch Dermatol. 1987;123:1320-1325.
- Smith KJ, Neafie R, Yeager J, et al. Micrococcus folliculitis in HIV-1 disease. Br J Dermatol. 1999;141:558-561.
- van Rensburg JJ, Lin H, Gao X, et al. The human skin microbiome associates with the outcome of and is influenced by bacterial infection. mBio. 2015;6:E01315-15. doi:10.1128/mBio.01315-15.
- Chuku A, Nwankiti OO. Association of bacteria with fungal infection of skin and soft tissue lesions in plateau state, Nigeria. Br Microbiol Res J. 2013;3:470-477.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Rather PA, Hassan I. Human Demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014;59:60-66.
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- White TC, Findley K, Dawson TL Jr, et al. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med. 2014;4. pii:a019802. doi:10.1101/cshperspect.a019802.
- Gottlieb GJ, Ackerman AB. The "sandwich sign" of dermatophytosis. Am J Dermatopathol. 1986;8:347.
- Hennessey NP, Parro EL, Cockerell CJ. Cutaneous Pneumocystis carinii infection in patients with acquired immunodeficiency syndrome. Arch Dermatol. 1991;127:1699-1701.
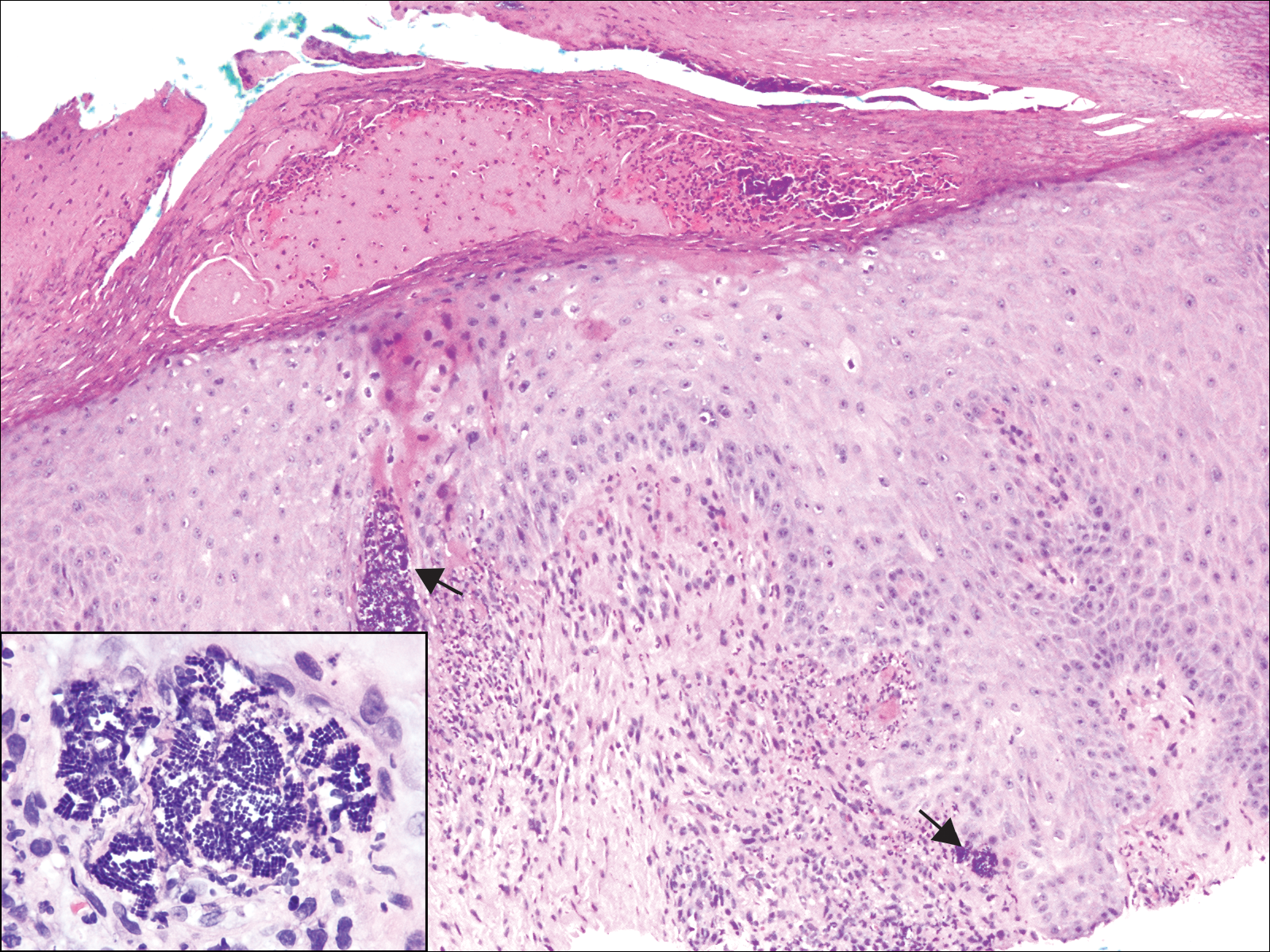
A 72-year-old woman with a medical history notable for multiple sclerosis and intravenous drug abuse presented to the dermatology clinic with a 0.6×0.5-cm, pruritic, wartlike, inflamed, keratotic papule on the palmar aspect of the right finger of more than 1 month's duration. A shave biopsy was performed that showed excoriation with serum crust, parakeratosis, and neutrophilic infiltrate in the papillary dermis. Within the serum crust and at the dermoepidermal junction, clusters of refractive basophilic bodies (arrows) in tetrad arrangement also were noted (inset). The papule resolved after the biopsy without any additional treatment.
Outcomes Associated With Shorter Wait Times at a County Hospital Outpatient Dermatology Clinic
Maximizing productivity is prudent for outpatient subspecialty clinics to improve access to care. The outpatient dermatology clinic at Parkland Health and Hospital System in Dallas, Texas, which is a safety-net hospital in Dallas County, decreased wait times for new patients (from 377 to 48 days) and follow-up patients (from 95 to 34 days) from May 2012 to September 2015.1 Changes in clinic productivity measures that occur with decreased wait times are not well characterized; therefore, we sought to address this knowledge gap. We propose that decreased wait times are associated with improvement in additional clinic productivity measures, specifically decreases in nonattendance and cycle times (defined as time between patient check-in and discharge) as well as increases in referrals.
In our retrospective cohort study of patients seen in the Parkland outpatient dermatology clinic between fiscal year (FY) 2012 and FY 2015 (between October 2011 and September 2015), we collected data on patient nonattendance rates, cycle times, and referral volumes. Categorical variables were compared using χ2 tests, and changes in cycle times were analyzed using 2-way analysis of variance. P<.05 was considered statistically significant.
There were 52,775 scheduled clinic visits from FY 2012 to FY 2015. The overall proportion of patient nonattendance rates decreased from 34.6% (4202/12,141) to 31.4% (4429/14,119)(P<.001)(Figure), despite an increase in completed patient visits during the study period (7939 vs 9690). New patient nonattendance rates decreased from 42.9% (1831/4269) to 30.2% (1474/4874)(P<.001). The number of completed visits for new patients increased from 2438 in FY 2012 to 3400 in FY 2015. Follow-up nonattendance rates increased from 30.1% (2371/7872) to 32.0% (2955/9245)(P<.001). Follow-up completed visits increased from 5501 in FY 2012 to 6290 in FY 2015. Overall, average cycle time showed a trend to decrease from 159 to 123 minutes (22.6%)(Figure). Average cycle times were reduced from 159 to 128 minutes (19.5%) for new patients and from 161 to 115 minutes (28.6%) for follow-up patients (P=.02). Overall, referrals increased by 14.1% (816/5799)(P<.001), which was largely due to the increase in volume of referrals observed between FY 2014 (n=5770) and FY 2015 (n=6615).
We have demonstrated that decreased wait times can be associated with improvements in clinic productivity measures, namely decreased nonattendance rates and cycle times and increased referrals. Patient nonattendance is a burden on clinic resources and has been described in the dermatology clinic setting.2-6 Increased likelihood of nonattendance has been associated with prolonged wait times.3,7 We propose that decreased wait times can lead to diminished nonattendance rates, as patients are more likely to keep their appointments rather than seek other providers for dermatologic care. The difference in trends between new patient and follow-up nonattendance rates may be attributed to the larger relative increase in completed new patient visits compared to follow-ups during the study period.
Furthermore, the decrease in average cycle time reflected our clinic’s ability to see a larger number of patients per clinic, with subsequently shorter wait times. The greater reduction in cycle times for follow-up patients may be attributed to the increased continuity of providers who had previously seen these patients. Although the cycle times may seem high in our clinic compared to other practice settings, we believe that this marker of productivity is widely applicable to various clinic settings, including private practices and other outpatient specialty clinics. Increased clinic referrals can be a downstream effect of decreased wait times due to improvements in access to care, as shown in other specialty clinics.8 Effects of confounding variables on referral volumes, including nationwide health insurance changes during our study period, could not be ruled out.
Limitations of this study include unavailable data on patient and provider satisfaction and changes in patients’ health insurance. This study provides evidence of changes in clinical productivity measures associated with decreased wait times that can demonstrate widespread benefits to the health system.
Acknowledgments
The authors would like to thank Michael Estabrooks, RN, and Trung Vu for providing aggregate data, as well as Linda Hynan, PhD, for statistical advice (all Dallas, Texas).
- O’Brien JC, Chong BF. Reducing outpatient dermatology clinic wait times in a safety net health system in Dallas, Texas. J Am Acad Dermatol. 2016;75:631-632.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
- Cohen AD, Dreiher J, Vardy DA, et al. Nonattendance in a dermatology clinic—a large sample analysis. J Eur Acad Dermatol Venereol. 2008;22:1178-1183.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Tsang MW, Resneck JS Jr. Even patients with changing moles face long dermatology appointment wait-times: a study of simulated patient calls to dermatologists. J Am Acad Dermatol. 2006;55:54-58.
- Rosenbach M, Kagan S, Leventhal S. Dermatology urgent care clinic: a survey of referring physician satisfaction. J Am Acad Dermatol. 2013;69:1067-1069.e1.
- Dickey W, Morrow JI. Can outpatient non-attendance be predicted from the referral letter? an audit of default at neurology clinics. J R Soc Med. 1991;8:662-663.
- Bungard TJ, Smigorowsky MJ, Lalonde LD, et al. Cardiac EASE (Ensuring Access and Speedy Evaluation)—the impact of a single-point-of-entry multidisciplinary outpatient cardiology consultation program on wait times in Canada. Can J Cardiol. 2009;25:697-702.
Maximizing productivity is prudent for outpatient subspecialty clinics to improve access to care. The outpatient dermatology clinic at Parkland Health and Hospital System in Dallas, Texas, which is a safety-net hospital in Dallas County, decreased wait times for new patients (from 377 to 48 days) and follow-up patients (from 95 to 34 days) from May 2012 to September 2015.1 Changes in clinic productivity measures that occur with decreased wait times are not well characterized; therefore, we sought to address this knowledge gap. We propose that decreased wait times are associated with improvement in additional clinic productivity measures, specifically decreases in nonattendance and cycle times (defined as time between patient check-in and discharge) as well as increases in referrals.
In our retrospective cohort study of patients seen in the Parkland outpatient dermatology clinic between fiscal year (FY) 2012 and FY 2015 (between October 2011 and September 2015), we collected data on patient nonattendance rates, cycle times, and referral volumes. Categorical variables were compared using χ2 tests, and changes in cycle times were analyzed using 2-way analysis of variance. P<.05 was considered statistically significant.
There were 52,775 scheduled clinic visits from FY 2012 to FY 2015. The overall proportion of patient nonattendance rates decreased from 34.6% (4202/12,141) to 31.4% (4429/14,119)(P<.001)(Figure), despite an increase in completed patient visits during the study period (7939 vs 9690). New patient nonattendance rates decreased from 42.9% (1831/4269) to 30.2% (1474/4874)(P<.001). The number of completed visits for new patients increased from 2438 in FY 2012 to 3400 in FY 2015. Follow-up nonattendance rates increased from 30.1% (2371/7872) to 32.0% (2955/9245)(P<.001). Follow-up completed visits increased from 5501 in FY 2012 to 6290 in FY 2015. Overall, average cycle time showed a trend to decrease from 159 to 123 minutes (22.6%)(Figure). Average cycle times were reduced from 159 to 128 minutes (19.5%) for new patients and from 161 to 115 minutes (28.6%) for follow-up patients (P=.02). Overall, referrals increased by 14.1% (816/5799)(P<.001), which was largely due to the increase in volume of referrals observed between FY 2014 (n=5770) and FY 2015 (n=6615).
We have demonstrated that decreased wait times can be associated with improvements in clinic productivity measures, namely decreased nonattendance rates and cycle times and increased referrals. Patient nonattendance is a burden on clinic resources and has been described in the dermatology clinic setting.2-6 Increased likelihood of nonattendance has been associated with prolonged wait times.3,7 We propose that decreased wait times can lead to diminished nonattendance rates, as patients are more likely to keep their appointments rather than seek other providers for dermatologic care. The difference in trends between new patient and follow-up nonattendance rates may be attributed to the larger relative increase in completed new patient visits compared to follow-ups during the study period.
Furthermore, the decrease in average cycle time reflected our clinic’s ability to see a larger number of patients per clinic, with subsequently shorter wait times. The greater reduction in cycle times for follow-up patients may be attributed to the increased continuity of providers who had previously seen these patients. Although the cycle times may seem high in our clinic compared to other practice settings, we believe that this marker of productivity is widely applicable to various clinic settings, including private practices and other outpatient specialty clinics. Increased clinic referrals can be a downstream effect of decreased wait times due to improvements in access to care, as shown in other specialty clinics.8 Effects of confounding variables on referral volumes, including nationwide health insurance changes during our study period, could not be ruled out.
Limitations of this study include unavailable data on patient and provider satisfaction and changes in patients’ health insurance. This study provides evidence of changes in clinical productivity measures associated with decreased wait times that can demonstrate widespread benefits to the health system.
Acknowledgments
The authors would like to thank Michael Estabrooks, RN, and Trung Vu for providing aggregate data, as well as Linda Hynan, PhD, for statistical advice (all Dallas, Texas).
Maximizing productivity is prudent for outpatient subspecialty clinics to improve access to care. The outpatient dermatology clinic at Parkland Health and Hospital System in Dallas, Texas, which is a safety-net hospital in Dallas County, decreased wait times for new patients (from 377 to 48 days) and follow-up patients (from 95 to 34 days) from May 2012 to September 2015.1 Changes in clinic productivity measures that occur with decreased wait times are not well characterized; therefore, we sought to address this knowledge gap. We propose that decreased wait times are associated with improvement in additional clinic productivity measures, specifically decreases in nonattendance and cycle times (defined as time between patient check-in and discharge) as well as increases in referrals.
In our retrospective cohort study of patients seen in the Parkland outpatient dermatology clinic between fiscal year (FY) 2012 and FY 2015 (between October 2011 and September 2015), we collected data on patient nonattendance rates, cycle times, and referral volumes. Categorical variables were compared using χ2 tests, and changes in cycle times were analyzed using 2-way analysis of variance. P<.05 was considered statistically significant.
There were 52,775 scheduled clinic visits from FY 2012 to FY 2015. The overall proportion of patient nonattendance rates decreased from 34.6% (4202/12,141) to 31.4% (4429/14,119)(P<.001)(Figure), despite an increase in completed patient visits during the study period (7939 vs 9690). New patient nonattendance rates decreased from 42.9% (1831/4269) to 30.2% (1474/4874)(P<.001). The number of completed visits for new patients increased from 2438 in FY 2012 to 3400 in FY 2015. Follow-up nonattendance rates increased from 30.1% (2371/7872) to 32.0% (2955/9245)(P<.001). Follow-up completed visits increased from 5501 in FY 2012 to 6290 in FY 2015. Overall, average cycle time showed a trend to decrease from 159 to 123 minutes (22.6%)(Figure). Average cycle times were reduced from 159 to 128 minutes (19.5%) for new patients and from 161 to 115 minutes (28.6%) for follow-up patients (P=.02). Overall, referrals increased by 14.1% (816/5799)(P<.001), which was largely due to the increase in volume of referrals observed between FY 2014 (n=5770) and FY 2015 (n=6615).
We have demonstrated that decreased wait times can be associated with improvements in clinic productivity measures, namely decreased nonattendance rates and cycle times and increased referrals. Patient nonattendance is a burden on clinic resources and has been described in the dermatology clinic setting.2-6 Increased likelihood of nonattendance has been associated with prolonged wait times.3,7 We propose that decreased wait times can lead to diminished nonattendance rates, as patients are more likely to keep their appointments rather than seek other providers for dermatologic care. The difference in trends between new patient and follow-up nonattendance rates may be attributed to the larger relative increase in completed new patient visits compared to follow-ups during the study period.
Furthermore, the decrease in average cycle time reflected our clinic’s ability to see a larger number of patients per clinic, with subsequently shorter wait times. The greater reduction in cycle times for follow-up patients may be attributed to the increased continuity of providers who had previously seen these patients. Although the cycle times may seem high in our clinic compared to other practice settings, we believe that this marker of productivity is widely applicable to various clinic settings, including private practices and other outpatient specialty clinics. Increased clinic referrals can be a downstream effect of decreased wait times due to improvements in access to care, as shown in other specialty clinics.8 Effects of confounding variables on referral volumes, including nationwide health insurance changes during our study period, could not be ruled out.
Limitations of this study include unavailable data on patient and provider satisfaction and changes in patients’ health insurance. This study provides evidence of changes in clinical productivity measures associated with decreased wait times that can demonstrate widespread benefits to the health system.
Acknowledgments
The authors would like to thank Michael Estabrooks, RN, and Trung Vu for providing aggregate data, as well as Linda Hynan, PhD, for statistical advice (all Dallas, Texas).
- O’Brien JC, Chong BF. Reducing outpatient dermatology clinic wait times in a safety net health system in Dallas, Texas. J Am Acad Dermatol. 2016;75:631-632.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
- Cohen AD, Dreiher J, Vardy DA, et al. Nonattendance in a dermatology clinic—a large sample analysis. J Eur Acad Dermatol Venereol. 2008;22:1178-1183.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Tsang MW, Resneck JS Jr. Even patients with changing moles face long dermatology appointment wait-times: a study of simulated patient calls to dermatologists. J Am Acad Dermatol. 2006;55:54-58.
- Rosenbach M, Kagan S, Leventhal S. Dermatology urgent care clinic: a survey of referring physician satisfaction. J Am Acad Dermatol. 2013;69:1067-1069.e1.
- Dickey W, Morrow JI. Can outpatient non-attendance be predicted from the referral letter? an audit of default at neurology clinics. J R Soc Med. 1991;8:662-663.
- Bungard TJ, Smigorowsky MJ, Lalonde LD, et al. Cardiac EASE (Ensuring Access and Speedy Evaluation)—the impact of a single-point-of-entry multidisciplinary outpatient cardiology consultation program on wait times in Canada. Can J Cardiol. 2009;25:697-702.
- O’Brien JC, Chong BF. Reducing outpatient dermatology clinic wait times in a safety net health system in Dallas, Texas. J Am Acad Dermatol. 2016;75:631-632.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
- Cohen AD, Dreiher J, Vardy DA, et al. Nonattendance in a dermatology clinic—a large sample analysis. J Eur Acad Dermatol Venereol. 2008;22:1178-1183.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Tsang MW, Resneck JS Jr. Even patients with changing moles face long dermatology appointment wait-times: a study of simulated patient calls to dermatologists. J Am Acad Dermatol. 2006;55:54-58.
- Rosenbach M, Kagan S, Leventhal S. Dermatology urgent care clinic: a survey of referring physician satisfaction. J Am Acad Dermatol. 2013;69:1067-1069.e1.
- Dickey W, Morrow JI. Can outpatient non-attendance be predicted from the referral letter? an audit of default at neurology clinics. J R Soc Med. 1991;8:662-663.
- Bungard TJ, Smigorowsky MJ, Lalonde LD, et al. Cardiac EASE (Ensuring Access and Speedy Evaluation)—the impact of a single-point-of-entry multidisciplinary outpatient cardiology consultation program on wait times in Canada. Can J Cardiol. 2009;25:697-702.