User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Perceptions of Tanning Risk Among Melanoma Patients With a History of Indoor Tanning
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
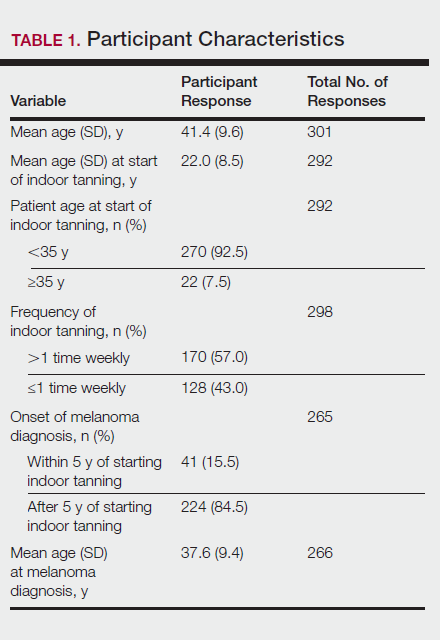
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).
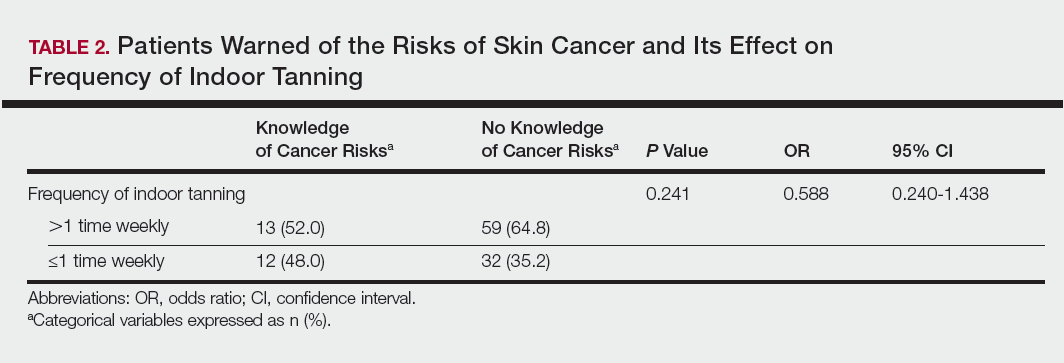
Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).
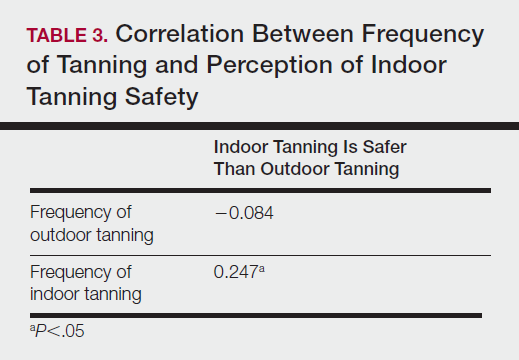
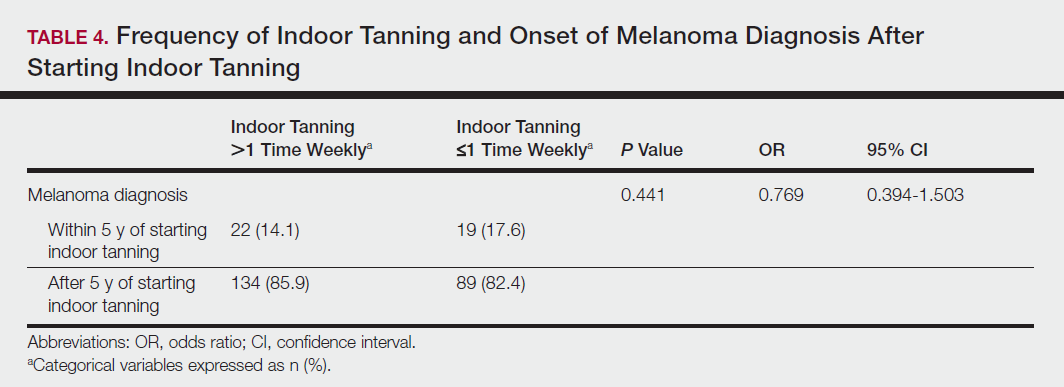
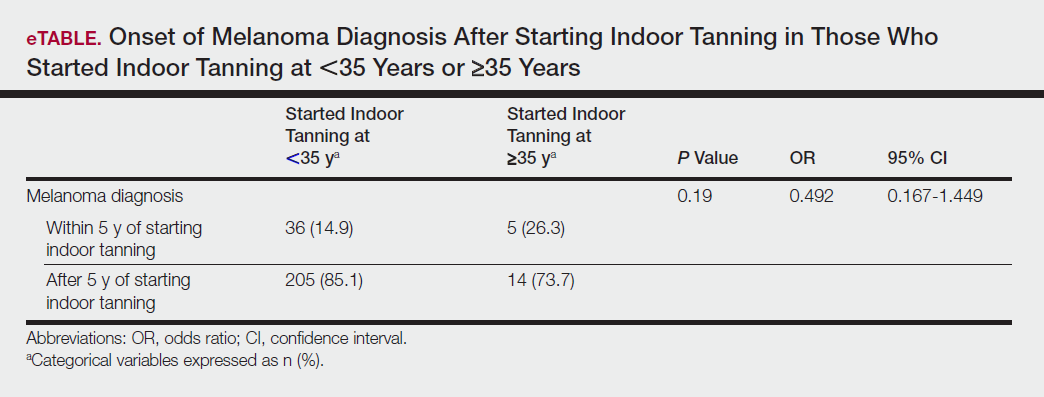
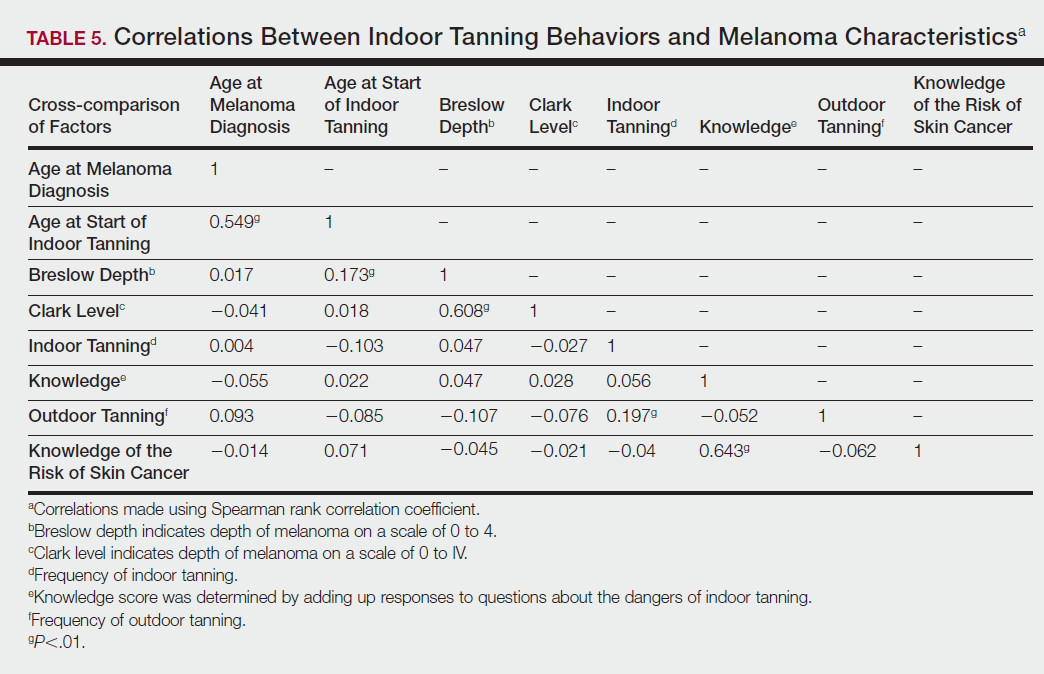
Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).
Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).


Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).



Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).

Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).


Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).



Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).

Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
Practice Points
- Despite US Food and Drug Administration reclassification and publicity of the risks of skin cancer, many patients continue to use sunbeds.
- It is important to assess how patients are obtaining information regarding sunbed safety, as indoor tanning companies are promoting sunbeds as “safe” tans.
- The increased combination of sunbed use and outdoor tanning is putting people at greater risk for the development of melanoma and nonmelanoma skin cancer.
Richner-Hanhart Syndrome (Tyrosinemia Type II)
To the Editor:
Richner-Hanhart syndrome, also known as tyrosinemia type II or oculocutaneous tyrosinemia, is a rare autosomal-recessive, childhood-onset, metabolic hereditary disease.1 A deficiency of tyrosine aminotransferase leads to an accumulation of tyrosine amino acid. It is characterized by the association of palmoplantar hyperkeratosis, bilateral keratitis, and neurological disorders.
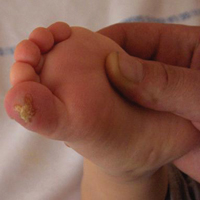
An 18-month-old girl with recurrent warts of 6 months' duration was admitted to the dermatology department. She had been treated repeatedly with acyclovir for recurrent bilateral herpetic keratitis with major photophobia since 9 months of age with no response. Clinical presentation included punctate hyperkeratosis of the fingers and toes (Figure, A), severe photophobia with decreased visual acuity, and speech delay.
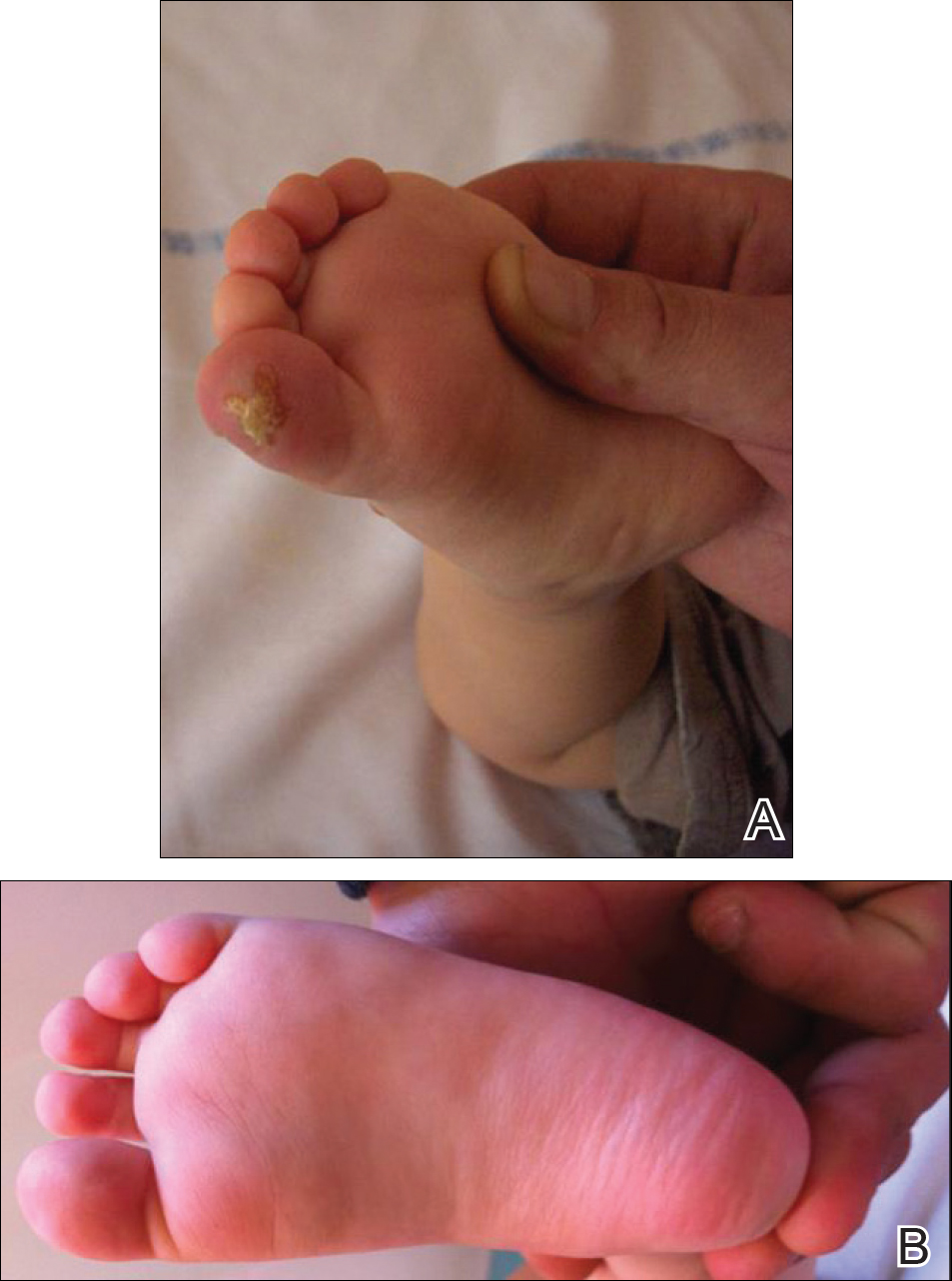
Her medical record showed a break of the growth curve with a weight of 9.25 kg (3rd percentile), a height of 80 cm (50th percentile), and a head circumference of 45 cm (50th percentile). Her parents were nonconsanguineous. The association of bilateral dendritic keratitis with punctate palmoplantar keratosis suggested a diagnosis of Richner-Hanhart syndrome. Diagnosis was confirmed by an elevated plasma level of tyrosine (1580 µmol/L; reference range, 40-80 µmol/L).
A low tyrosine and low phenylalanine diet (no animal proteins) was immediately introduced, with supplementation of amino acids, vitamins, and trace elements. After 8 days, the plasma level of tyrosinemia decreased by a factor of 4 (392 µmol/L). After 1 month, the cutaneous and ocular lesions completely resolved (Figure, B). Discrete psychomotor slowing still persisted for 1 year and then reached complete normalization. Genetic analysis showed a composite heterozygous mutation of the tyrosine aminotransferase gene, TAT, on chromosome 16. The mutation detected in the patient's mother was an A to V substitution at codon 147 (A147V). The second mutation was detected in the father; it was an 8 nucleotides duplication and then a substitution leading to a premature stop codon at codon 37 (R37X).
Richner-Hanhart syndrome is a rare autosomal-recessive disorder that is more common in Italy and in areas where inbreeding is prevalent1,2; however, no data are available on disease prevalence. It is caused by a homozygous mutation in the TAT gene located on chromosome 16q22.3 Tyrosine aminotransferase is an important enzyme involved in the tyrosine and phenylalanine metabolic degradation pathway located in the hepatic cytosol. Symptoms are due to the accumulation of tyrosine and its metabolite. Diagnosis is confirmed by an elevated plasma level of tyrosine (>500 µmol/L). This oculocutaneous syndrome is characterized by bilateral pseudodendritic keratitis, palmoplantar hyperkeratosis, and a variable degree of mental retardation.1 In contrast to tyrosinemia type II, types I and III do not affect the skin.
Intrafamilial and interfamilial phenotypic variability is reported. A large spectrum of mutations within the TAT gene have been reported.4-7 These mutations lead to a reduction or an absence in the activity of hepatic tyrosine aminotransferase. The degradation pathway of tyrosine involving TAT occurs mainly in the liver. This process also is present in the mitochondria where the enzyme is called aspartate aminotransferase.1,2 The mechanism by which Richner-Hanhart syndrome causes painful palmoplantar keratosis and keratitis remains unknown. It has been suggested that intracellular L-tyrosine crystals initiate an inflammation process resulting in the typical skin lesions and keratitis.8 There is some evidence that patients with higher values of tyrosine in early life are more likely to develop neurological problems.1 In addition, phenotype variability has been observed, even among individuals sharing the same pathogenic mutation.4
Tyrosinemia type II typically demonstrates ocular symptoms (75% of cases) that usually occur in the first year of life.8 They are characterized by photophobia, redness, and increase of lacrimation. Examination reveals a superficial and bilateral punctate keratosis with corneal dystrophy, often misdiagnosed as herpetic keratosis, as in our case, which may delay the diagnosis.9,10 Bilateral ocular lesions are suggestive, even if they are asymmetric.8,11 Furthermore, negative fluorescein staining, negative culture, and resistance to antiviral treatment exclude the diagnosis of herpetic keratosis.9,10
Skin lesions (85% of cases) typically appear in the first year of life. They are characterized by painful, irregular, limited, punctate hyperkeratosis on the palms and soles.1 They are more frequent in weight-bearing areas and tend to improve during summer, possibly due to a seasonal change in dietary behavior.4,12 Hyperkeratotic papules in a linear pattern also have been described on the flexor aspects of the fingers or toes.13 In our case, the lesions were misdiagnosed as warts for 6 months.
Retarded development affects 60% of patients with tyrosinemia type II. Expression of neurological symptoms is variable and could include mental retardation, nystagmus, tremors, ataxia, and convulsion.4 Lifetime follow-up of these patients is recommended.
Early initiation of a tyrosine-phenylalanine-restricted diet in infancy is the most effective therapy for Richner-Hanhart syndrome.13 The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into amino acid tyrosine. Thus, dietary treatment of Richner-Hanhart syndrome requires restricting or eliminating foods high in phenylalanine and tyrosine with protein "medical food" substitute. The dietary treatment allows resolution of both eye and skin symptoms after a few days or weeks and also may prevent mental retardation. It is effective in lowering the plasma level to less than 400 µmol/L. The diet must be introduced as soon as Richner-Hanhart syndrome is suspected. Supplementation with essential amino acids, vitamins, and trace elements is needed. Early screening of siblings in families with Richner-Hanhart syndrome history is recommended, even in the absence of clinical findings. Careful dietary control of maternal plasma tyrosine level must be considered during future pregnancy for women.4,14,15
Richner-Hanhart syndrome should be suspected in patients demonstrating cutaneous lesions, especially palmoplantar keratosis associated with bilateral pseudodendritic corneal lesions unresponsive to antiviral therapy.
- Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin Med Genet. 2006;142C:121-126.
- Meissner T, Betz RC, Pasternack SM, et al. Richner-Hanhart syndrome detected by expanded newborn screening. Pediatr Dermatol. 2008;25:378-380.
- Natt E, Kida K, Odievre M, et al. Point mutations in the tyrosine aminotransferase gene in tyrosinemia type II. Proc Natl Acad Sci USA. 1992;89:9297-9301.
- Charfeddine C, Monastiri K, Mokni M, et al. Clinical and mutational investigations of tyrosinemia type II in Northern Tunisia: identification and structural characterization of two novel TAT mutations. Mol Genet Metab. 2006;88:184-191.
- Legarda M, Wlodarczyk K, Lage S, et al. A large TAT deletion in a tyrosinaemia type II patient. Mol Genet Metab. 2011;104:407-409.
- Culic V, Betz RC, Refke M, et al. Tyrosinemia type II (Richner-Hanhart syndrome): a new mutation in the TAT gene. Eur J Med Genet. 2011;54:205-208.
- Pasternack SM, Betz RC, Brandrup F, et al. Identification of two new mutations in the TAT gene in a Danish family with tyrosinaemia type II. Br J Dermatol. 2009;160:704-706.
- Macsai MS, Schwartz TL, Hinkle D, et al. Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol. 2001;132:522-527.
- Kymionis GD, Kankariya VP, Kontadakis GA, et al. Isolated corneal pseudodendrites as the initial manifestation of tyrosinemia type II in monozygotic twins. J Pediatr Ophthalmol Strabismus.2012;49:E33-E36.
- Iskeleli G, Bilgeç MD, Arici C, et al. Richner-Hanhart syndrome (tyrosinemia type II): a case report of delayed diagnosis with pseudodendritic corneal lesion. Turk J Pediatr. 2011;53:692-694.
- Rehák A, Selim MM, Yadav G. Richner-Hanhart syndrome (tyrosinaemia-II)(report of four cases without ocular involvement). Br J Dermatol. 1981;104:469-475.
- Viglizzo GM, Occella C, Bleidl D, et al. Richner-Hanhart syndrome (tyrosinemia II): early diagnosis of an incomplete presentation with unusual findings. Pediatr Dermatol. 2006;23:259-261.
- Machino H, Miki Y, Kawatsu T, et al. Successful dietary control of tyrosinemia II. J Am Acad Dermatol. 1983;9:533-539.
- el-Badramany MH, Fawzy AR, Farag TI. Familial Richner-Hanhart syndrome in Kuwait: twelve-year clinical reassessment by a multidisciplinary approach. Am J Med Genet. 1995;60:353-355.
- Cerone R, Fantasia AR, Castellano E, et al. Pregnancy and tyrosinaemia type II. J Inherit Metab Dis. 2002;25:317-318.
To the Editor:
Richner-Hanhart syndrome, also known as tyrosinemia type II or oculocutaneous tyrosinemia, is a rare autosomal-recessive, childhood-onset, metabolic hereditary disease.1 A deficiency of tyrosine aminotransferase leads to an accumulation of tyrosine amino acid. It is characterized by the association of palmoplantar hyperkeratosis, bilateral keratitis, and neurological disorders.
An 18-month-old girl with recurrent warts of 6 months' duration was admitted to the dermatology department. She had been treated repeatedly with acyclovir for recurrent bilateral herpetic keratitis with major photophobia since 9 months of age with no response. Clinical presentation included punctate hyperkeratosis of the fingers and toes (Figure, A), severe photophobia with decreased visual acuity, and speech delay.

Her medical record showed a break of the growth curve with a weight of 9.25 kg (3rd percentile), a height of 80 cm (50th percentile), and a head circumference of 45 cm (50th percentile). Her parents were nonconsanguineous. The association of bilateral dendritic keratitis with punctate palmoplantar keratosis suggested a diagnosis of Richner-Hanhart syndrome. Diagnosis was confirmed by an elevated plasma level of tyrosine (1580 µmol/L; reference range, 40-80 µmol/L).
A low tyrosine and low phenylalanine diet (no animal proteins) was immediately introduced, with supplementation of amino acids, vitamins, and trace elements. After 8 days, the plasma level of tyrosinemia decreased by a factor of 4 (392 µmol/L). After 1 month, the cutaneous and ocular lesions completely resolved (Figure, B). Discrete psychomotor slowing still persisted for 1 year and then reached complete normalization. Genetic analysis showed a composite heterozygous mutation of the tyrosine aminotransferase gene, TAT, on chromosome 16. The mutation detected in the patient's mother was an A to V substitution at codon 147 (A147V). The second mutation was detected in the father; it was an 8 nucleotides duplication and then a substitution leading to a premature stop codon at codon 37 (R37X).
Richner-Hanhart syndrome is a rare autosomal-recessive disorder that is more common in Italy and in areas where inbreeding is prevalent1,2; however, no data are available on disease prevalence. It is caused by a homozygous mutation in the TAT gene located on chromosome 16q22.3 Tyrosine aminotransferase is an important enzyme involved in the tyrosine and phenylalanine metabolic degradation pathway located in the hepatic cytosol. Symptoms are due to the accumulation of tyrosine and its metabolite. Diagnosis is confirmed by an elevated plasma level of tyrosine (>500 µmol/L). This oculocutaneous syndrome is characterized by bilateral pseudodendritic keratitis, palmoplantar hyperkeratosis, and a variable degree of mental retardation.1 In contrast to tyrosinemia type II, types I and III do not affect the skin.
Intrafamilial and interfamilial phenotypic variability is reported. A large spectrum of mutations within the TAT gene have been reported.4-7 These mutations lead to a reduction or an absence in the activity of hepatic tyrosine aminotransferase. The degradation pathway of tyrosine involving TAT occurs mainly in the liver. This process also is present in the mitochondria where the enzyme is called aspartate aminotransferase.1,2 The mechanism by which Richner-Hanhart syndrome causes painful palmoplantar keratosis and keratitis remains unknown. It has been suggested that intracellular L-tyrosine crystals initiate an inflammation process resulting in the typical skin lesions and keratitis.8 There is some evidence that patients with higher values of tyrosine in early life are more likely to develop neurological problems.1 In addition, phenotype variability has been observed, even among individuals sharing the same pathogenic mutation.4
Tyrosinemia type II typically demonstrates ocular symptoms (75% of cases) that usually occur in the first year of life.8 They are characterized by photophobia, redness, and increase of lacrimation. Examination reveals a superficial and bilateral punctate keratosis with corneal dystrophy, often misdiagnosed as herpetic keratosis, as in our case, which may delay the diagnosis.9,10 Bilateral ocular lesions are suggestive, even if they are asymmetric.8,11 Furthermore, negative fluorescein staining, negative culture, and resistance to antiviral treatment exclude the diagnosis of herpetic keratosis.9,10
Skin lesions (85% of cases) typically appear in the first year of life. They are characterized by painful, irregular, limited, punctate hyperkeratosis on the palms and soles.1 They are more frequent in weight-bearing areas and tend to improve during summer, possibly due to a seasonal change in dietary behavior.4,12 Hyperkeratotic papules in a linear pattern also have been described on the flexor aspects of the fingers or toes.13 In our case, the lesions were misdiagnosed as warts for 6 months.
Retarded development affects 60% of patients with tyrosinemia type II. Expression of neurological symptoms is variable and could include mental retardation, nystagmus, tremors, ataxia, and convulsion.4 Lifetime follow-up of these patients is recommended.
Early initiation of a tyrosine-phenylalanine-restricted diet in infancy is the most effective therapy for Richner-Hanhart syndrome.13 The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into amino acid tyrosine. Thus, dietary treatment of Richner-Hanhart syndrome requires restricting or eliminating foods high in phenylalanine and tyrosine with protein "medical food" substitute. The dietary treatment allows resolution of both eye and skin symptoms after a few days or weeks and also may prevent mental retardation. It is effective in lowering the plasma level to less than 400 µmol/L. The diet must be introduced as soon as Richner-Hanhart syndrome is suspected. Supplementation with essential amino acids, vitamins, and trace elements is needed. Early screening of siblings in families with Richner-Hanhart syndrome history is recommended, even in the absence of clinical findings. Careful dietary control of maternal plasma tyrosine level must be considered during future pregnancy for women.4,14,15
Richner-Hanhart syndrome should be suspected in patients demonstrating cutaneous lesions, especially palmoplantar keratosis associated with bilateral pseudodendritic corneal lesions unresponsive to antiviral therapy.
To the Editor:
Richner-Hanhart syndrome, also known as tyrosinemia type II or oculocutaneous tyrosinemia, is a rare autosomal-recessive, childhood-onset, metabolic hereditary disease.1 A deficiency of tyrosine aminotransferase leads to an accumulation of tyrosine amino acid. It is characterized by the association of palmoplantar hyperkeratosis, bilateral keratitis, and neurological disorders.
An 18-month-old girl with recurrent warts of 6 months' duration was admitted to the dermatology department. She had been treated repeatedly with acyclovir for recurrent bilateral herpetic keratitis with major photophobia since 9 months of age with no response. Clinical presentation included punctate hyperkeratosis of the fingers and toes (Figure, A), severe photophobia with decreased visual acuity, and speech delay.

Her medical record showed a break of the growth curve with a weight of 9.25 kg (3rd percentile), a height of 80 cm (50th percentile), and a head circumference of 45 cm (50th percentile). Her parents were nonconsanguineous. The association of bilateral dendritic keratitis with punctate palmoplantar keratosis suggested a diagnosis of Richner-Hanhart syndrome. Diagnosis was confirmed by an elevated plasma level of tyrosine (1580 µmol/L; reference range, 40-80 µmol/L).
A low tyrosine and low phenylalanine diet (no animal proteins) was immediately introduced, with supplementation of amino acids, vitamins, and trace elements. After 8 days, the plasma level of tyrosinemia decreased by a factor of 4 (392 µmol/L). After 1 month, the cutaneous and ocular lesions completely resolved (Figure, B). Discrete psychomotor slowing still persisted for 1 year and then reached complete normalization. Genetic analysis showed a composite heterozygous mutation of the tyrosine aminotransferase gene, TAT, on chromosome 16. The mutation detected in the patient's mother was an A to V substitution at codon 147 (A147V). The second mutation was detected in the father; it was an 8 nucleotides duplication and then a substitution leading to a premature stop codon at codon 37 (R37X).
Richner-Hanhart syndrome is a rare autosomal-recessive disorder that is more common in Italy and in areas where inbreeding is prevalent1,2; however, no data are available on disease prevalence. It is caused by a homozygous mutation in the TAT gene located on chromosome 16q22.3 Tyrosine aminotransferase is an important enzyme involved in the tyrosine and phenylalanine metabolic degradation pathway located in the hepatic cytosol. Symptoms are due to the accumulation of tyrosine and its metabolite. Diagnosis is confirmed by an elevated plasma level of tyrosine (>500 µmol/L). This oculocutaneous syndrome is characterized by bilateral pseudodendritic keratitis, palmoplantar hyperkeratosis, and a variable degree of mental retardation.1 In contrast to tyrosinemia type II, types I and III do not affect the skin.
Intrafamilial and interfamilial phenotypic variability is reported. A large spectrum of mutations within the TAT gene have been reported.4-7 These mutations lead to a reduction or an absence in the activity of hepatic tyrosine aminotransferase. The degradation pathway of tyrosine involving TAT occurs mainly in the liver. This process also is present in the mitochondria where the enzyme is called aspartate aminotransferase.1,2 The mechanism by which Richner-Hanhart syndrome causes painful palmoplantar keratosis and keratitis remains unknown. It has been suggested that intracellular L-tyrosine crystals initiate an inflammation process resulting in the typical skin lesions and keratitis.8 There is some evidence that patients with higher values of tyrosine in early life are more likely to develop neurological problems.1 In addition, phenotype variability has been observed, even among individuals sharing the same pathogenic mutation.4
Tyrosinemia type II typically demonstrates ocular symptoms (75% of cases) that usually occur in the first year of life.8 They are characterized by photophobia, redness, and increase of lacrimation. Examination reveals a superficial and bilateral punctate keratosis with corneal dystrophy, often misdiagnosed as herpetic keratosis, as in our case, which may delay the diagnosis.9,10 Bilateral ocular lesions are suggestive, even if they are asymmetric.8,11 Furthermore, negative fluorescein staining, negative culture, and resistance to antiviral treatment exclude the diagnosis of herpetic keratosis.9,10
Skin lesions (85% of cases) typically appear in the first year of life. They are characterized by painful, irregular, limited, punctate hyperkeratosis on the palms and soles.1 They are more frequent in weight-bearing areas and tend to improve during summer, possibly due to a seasonal change in dietary behavior.4,12 Hyperkeratotic papules in a linear pattern also have been described on the flexor aspects of the fingers or toes.13 In our case, the lesions were misdiagnosed as warts for 6 months.
Retarded development affects 60% of patients with tyrosinemia type II. Expression of neurological symptoms is variable and could include mental retardation, nystagmus, tremors, ataxia, and convulsion.4 Lifetime follow-up of these patients is recommended.
Early initiation of a tyrosine-phenylalanine-restricted diet in infancy is the most effective therapy for Richner-Hanhart syndrome.13 The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into amino acid tyrosine. Thus, dietary treatment of Richner-Hanhart syndrome requires restricting or eliminating foods high in phenylalanine and tyrosine with protein "medical food" substitute. The dietary treatment allows resolution of both eye and skin symptoms after a few days or weeks and also may prevent mental retardation. It is effective in lowering the plasma level to less than 400 µmol/L. The diet must be introduced as soon as Richner-Hanhart syndrome is suspected. Supplementation with essential amino acids, vitamins, and trace elements is needed. Early screening of siblings in families with Richner-Hanhart syndrome history is recommended, even in the absence of clinical findings. Careful dietary control of maternal plasma tyrosine level must be considered during future pregnancy for women.4,14,15
Richner-Hanhart syndrome should be suspected in patients demonstrating cutaneous lesions, especially palmoplantar keratosis associated with bilateral pseudodendritic corneal lesions unresponsive to antiviral therapy.
- Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin Med Genet. 2006;142C:121-126.
- Meissner T, Betz RC, Pasternack SM, et al. Richner-Hanhart syndrome detected by expanded newborn screening. Pediatr Dermatol. 2008;25:378-380.
- Natt E, Kida K, Odievre M, et al. Point mutations in the tyrosine aminotransferase gene in tyrosinemia type II. Proc Natl Acad Sci USA. 1992;89:9297-9301.
- Charfeddine C, Monastiri K, Mokni M, et al. Clinical and mutational investigations of tyrosinemia type II in Northern Tunisia: identification and structural characterization of two novel TAT mutations. Mol Genet Metab. 2006;88:184-191.
- Legarda M, Wlodarczyk K, Lage S, et al. A large TAT deletion in a tyrosinaemia type II patient. Mol Genet Metab. 2011;104:407-409.
- Culic V, Betz RC, Refke M, et al. Tyrosinemia type II (Richner-Hanhart syndrome): a new mutation in the TAT gene. Eur J Med Genet. 2011;54:205-208.
- Pasternack SM, Betz RC, Brandrup F, et al. Identification of two new mutations in the TAT gene in a Danish family with tyrosinaemia type II. Br J Dermatol. 2009;160:704-706.
- Macsai MS, Schwartz TL, Hinkle D, et al. Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol. 2001;132:522-527.
- Kymionis GD, Kankariya VP, Kontadakis GA, et al. Isolated corneal pseudodendrites as the initial manifestation of tyrosinemia type II in monozygotic twins. J Pediatr Ophthalmol Strabismus.2012;49:E33-E36.
- Iskeleli G, Bilgeç MD, Arici C, et al. Richner-Hanhart syndrome (tyrosinemia type II): a case report of delayed diagnosis with pseudodendritic corneal lesion. Turk J Pediatr. 2011;53:692-694.
- Rehák A, Selim MM, Yadav G. Richner-Hanhart syndrome (tyrosinaemia-II)(report of four cases without ocular involvement). Br J Dermatol. 1981;104:469-475.
- Viglizzo GM, Occella C, Bleidl D, et al. Richner-Hanhart syndrome (tyrosinemia II): early diagnosis of an incomplete presentation with unusual findings. Pediatr Dermatol. 2006;23:259-261.
- Machino H, Miki Y, Kawatsu T, et al. Successful dietary control of tyrosinemia II. J Am Acad Dermatol. 1983;9:533-539.
- el-Badramany MH, Fawzy AR, Farag TI. Familial Richner-Hanhart syndrome in Kuwait: twelve-year clinical reassessment by a multidisciplinary approach. Am J Med Genet. 1995;60:353-355.
- Cerone R, Fantasia AR, Castellano E, et al. Pregnancy and tyrosinaemia type II. J Inherit Metab Dis. 2002;25:317-318.
- Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin Med Genet. 2006;142C:121-126.
- Meissner T, Betz RC, Pasternack SM, et al. Richner-Hanhart syndrome detected by expanded newborn screening. Pediatr Dermatol. 2008;25:378-380.
- Natt E, Kida K, Odievre M, et al. Point mutations in the tyrosine aminotransferase gene in tyrosinemia type II. Proc Natl Acad Sci USA. 1992;89:9297-9301.
- Charfeddine C, Monastiri K, Mokni M, et al. Clinical and mutational investigations of tyrosinemia type II in Northern Tunisia: identification and structural characterization of two novel TAT mutations. Mol Genet Metab. 2006;88:184-191.
- Legarda M, Wlodarczyk K, Lage S, et al. A large TAT deletion in a tyrosinaemia type II patient. Mol Genet Metab. 2011;104:407-409.
- Culic V, Betz RC, Refke M, et al. Tyrosinemia type II (Richner-Hanhart syndrome): a new mutation in the TAT gene. Eur J Med Genet. 2011;54:205-208.
- Pasternack SM, Betz RC, Brandrup F, et al. Identification of two new mutations in the TAT gene in a Danish family with tyrosinaemia type II. Br J Dermatol. 2009;160:704-706.
- Macsai MS, Schwartz TL, Hinkle D, et al. Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol. 2001;132:522-527.
- Kymionis GD, Kankariya VP, Kontadakis GA, et al. Isolated corneal pseudodendrites as the initial manifestation of tyrosinemia type II in monozygotic twins. J Pediatr Ophthalmol Strabismus.2012;49:E33-E36.
- Iskeleli G, Bilgeç MD, Arici C, et al. Richner-Hanhart syndrome (tyrosinemia type II): a case report of delayed diagnosis with pseudodendritic corneal lesion. Turk J Pediatr. 2011;53:692-694.
- Rehák A, Selim MM, Yadav G. Richner-Hanhart syndrome (tyrosinaemia-II)(report of four cases without ocular involvement). Br J Dermatol. 1981;104:469-475.
- Viglizzo GM, Occella C, Bleidl D, et al. Richner-Hanhart syndrome (tyrosinemia II): early diagnosis of an incomplete presentation with unusual findings. Pediatr Dermatol. 2006;23:259-261.
- Machino H, Miki Y, Kawatsu T, et al. Successful dietary control of tyrosinemia II. J Am Acad Dermatol. 1983;9:533-539.
- el-Badramany MH, Fawzy AR, Farag TI. Familial Richner-Hanhart syndrome in Kuwait: twelve-year clinical reassessment by a multidisciplinary approach. Am J Med Genet. 1995;60:353-355.
- Cerone R, Fantasia AR, Castellano E, et al. Pregnancy and tyrosinaemia type II. J Inherit Metab Dis. 2002;25:317-318.
Practice Points
- Richner-Hanhart syndrome (tyrosinemia type II) should be suspected in patients demonstrating cutaneous lesions, especially palmoplantar keratosis associated with bilateral pseudodendritic corneal lesions unresponsive to antiviral therapy.
- Early diagnosis and initiation of a tyrosinephenylalanine–restricted diet in infancy is the most effective therapy to prevent mental retardation.
Cosmetic Corner: Dermatologists Weigh in on Pigment Correctors
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Debunking Psoriasis Myths: How to Help Patients Who Are Afraid of Injections
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.
Pediatric Leg Ulcers: Going Out on a Limb for the Diagnosis
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.
Pseudomyogenic Hemangioendothelioma
Pseudomyogenic hemangioendothelioma (PMHE), also referred to as epithelioid sarcoma–like hemangioendothelioma,1 is a rare soft tissue tumor that was described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. It predominantly affects males between the second and fifth decades of life and most commonly presents as multiple nodules that may involve either the superficial or deep soft tissues of the legs and less often the arms. It also can arise on the trunk. We present a case of PMHE occurring in a young man and briefly review the literature on clinical presentation and histologic differentiation of this unique tumor, comparing these findings to its mimickers.
Case Report
A 20-year-old man presented with skin lesions on the left leg that had been present for 1 year. The patient described the lesions as tender pimples that would drain yellow discharge on occasion but had now transformed into large brown plaques. Physical examination showed 4 verrucous plaques ranging in size from 1 to 3 cm with hyperpigmentation and a central crust (Figure 1). Initially, the patient thought the lesions appeared due to shaving his legs for sports. He presented to the emergency department multiple times over the past year; pain control was provided and local skin care was recommended. Culture of the discharge had been performed 6 months prior to biopsy with negative results. No biopsy was performed on initial presentation and the lesions were diagnosed in the emergency department clinically as boils.
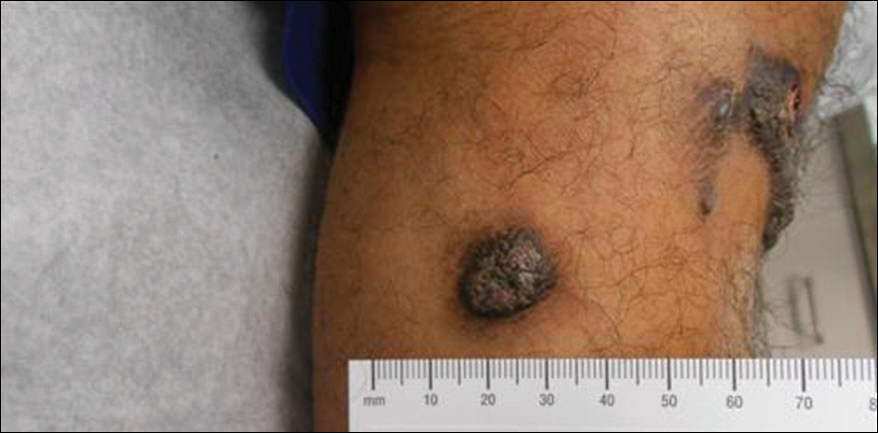
After failing to improve, the patient was seen by an outside dermatologist and the clinical differential diagnosis included deep fungal infection, atypical mycobacterial infection, and keloids. A 4-mm punch biopsy was taken from the periphery of one of the lesions and demonstrated hyperkeratosis, papillomatosis, and acanthosis (Figure 2). Within the superficial and deep dermis and focally extending into the subcutaneous tissue, there were sheets of spindled to epithelioid-appearing cells with moderate cytologic atypia (Figure 3). The tumor showed infiltrative margins. There was moderate cellularity. The individual cells had a rhabdoid appearance with large eccentric vesicular nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (Figure 4). No definitive evidence of glandular, squamous, or vascular differentiation was present. There was an associated moderate inflammatory host response composed of neutrophils and lymphocytes. Occasional extravasated red blood cells were present. Immunohistochemistry staining was performed and the atypical cells demonstrated diffuse positive staining for friend leukemia integration 1 transcription factor (FLI-1), erythroblastosis virus E26 transforming sequence-related gene (ERG)(Figure 5), CD31, and CD68. There was patchy positive staining for cytokeratin AE1/AE3, CD10, and factor VIII. There was no remarkable staining for human herpesvirus 8, epithelial membrane antigen, S-100, CD34, cytokeratin 903, and desmin. Overall, the histologic features in conjunction with the immunohistochemistry staining were consistent with a diagnosis of PMHE.

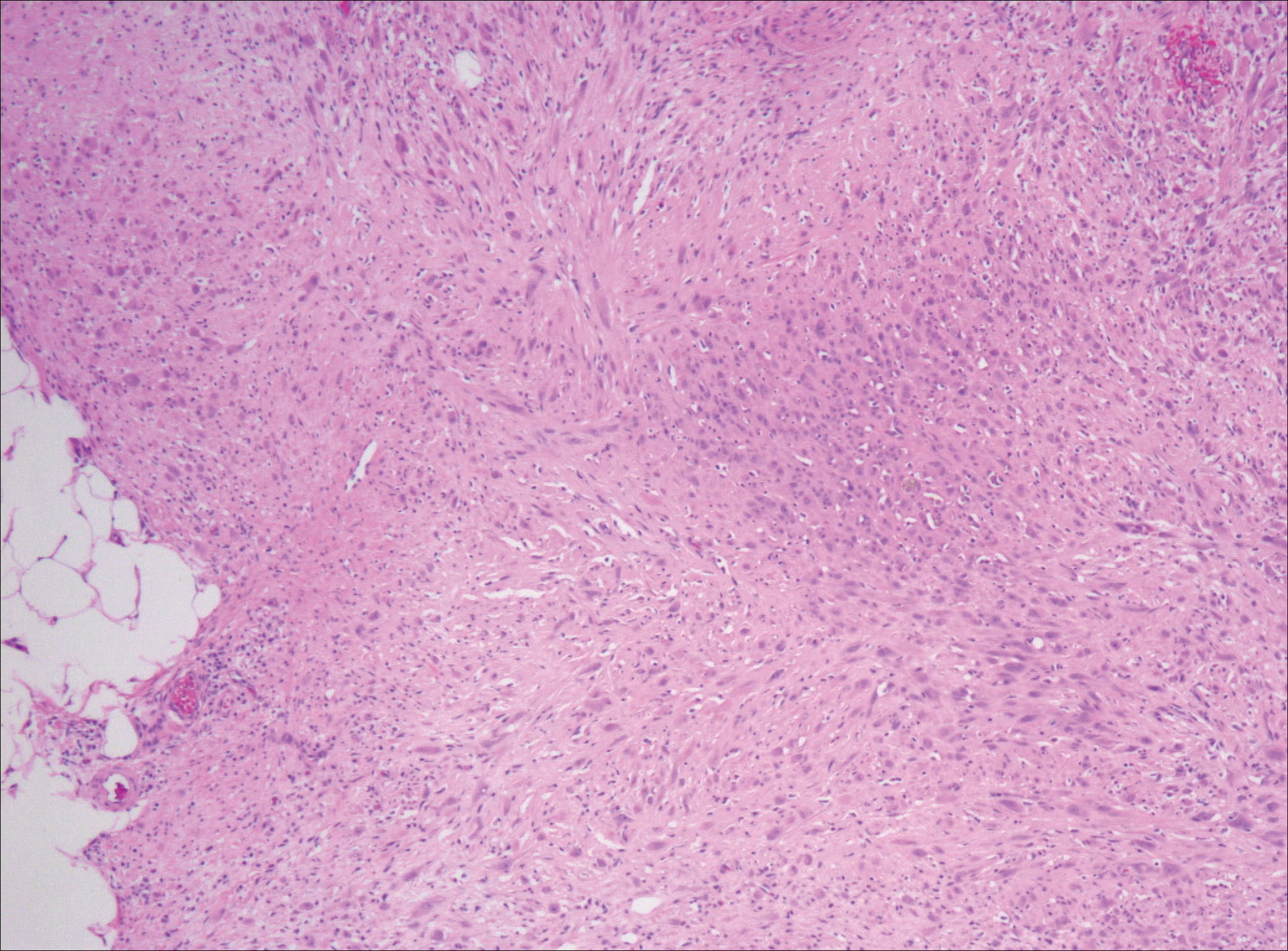
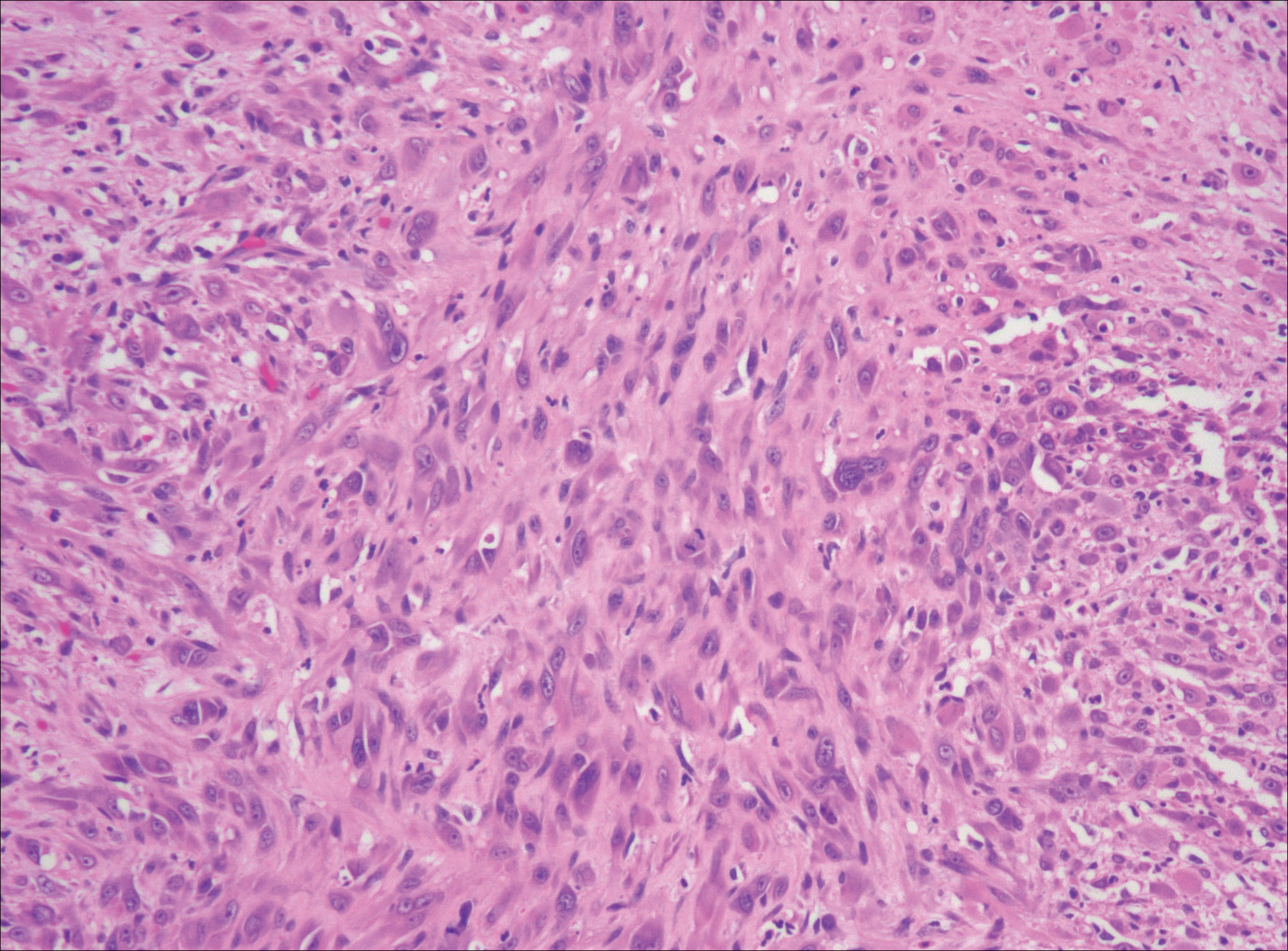
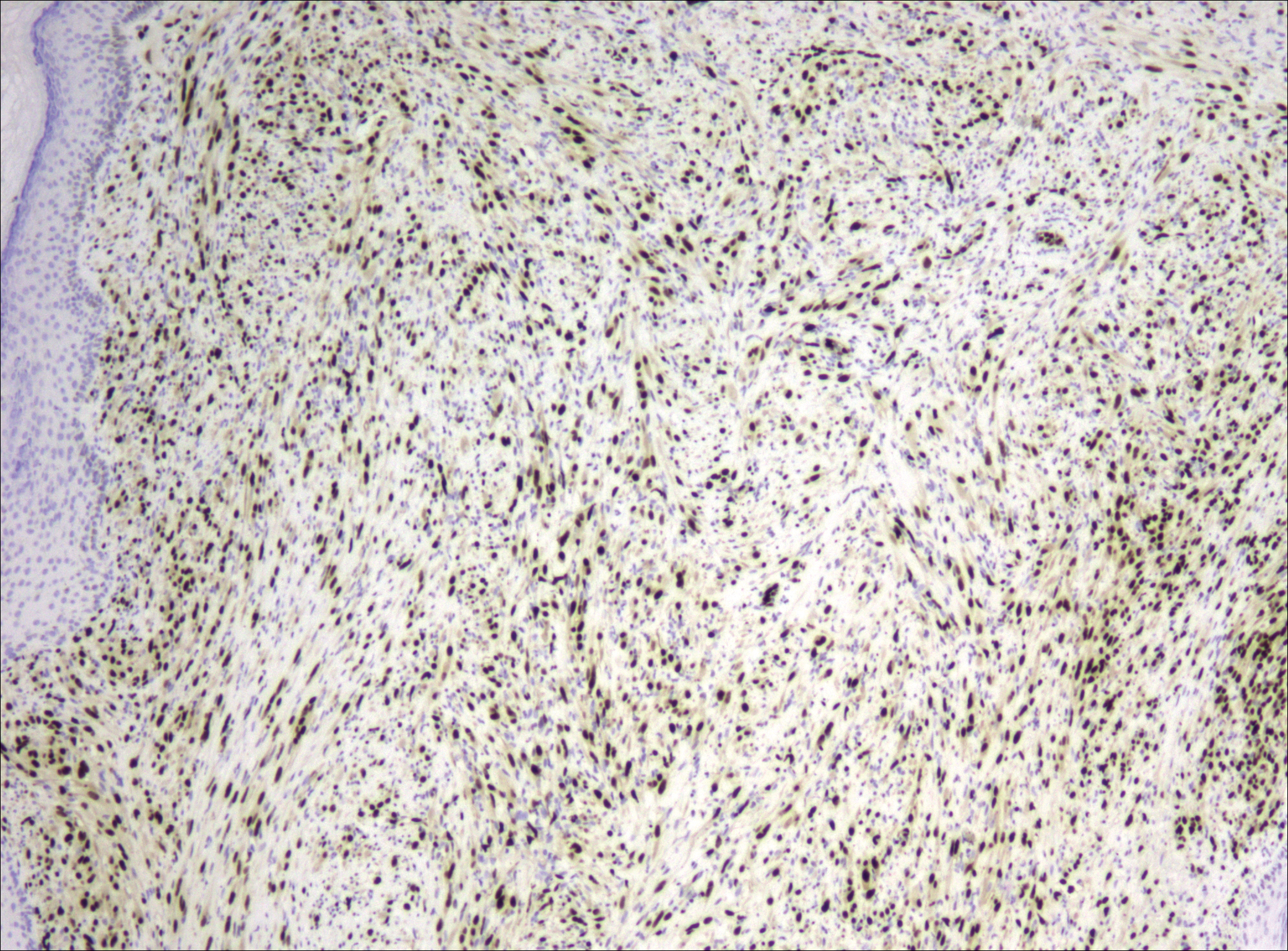
Magnetic resonance imaging was then performed to evaluate the depth and extent of the lesions for surgical excision planning (Figure 6), which showed 5 nodular lesions within the dermis and subcutis adjacent to the proximal aspect of the left tibia and medial aspect of the left knee. An additional lesion was noted between the sartorius and semimembranosus muscles, which was thought to represent either a lymph node or an additional neoplastic lesion. Chest computed tomography also displayed indeterminate lesions in the lungs.
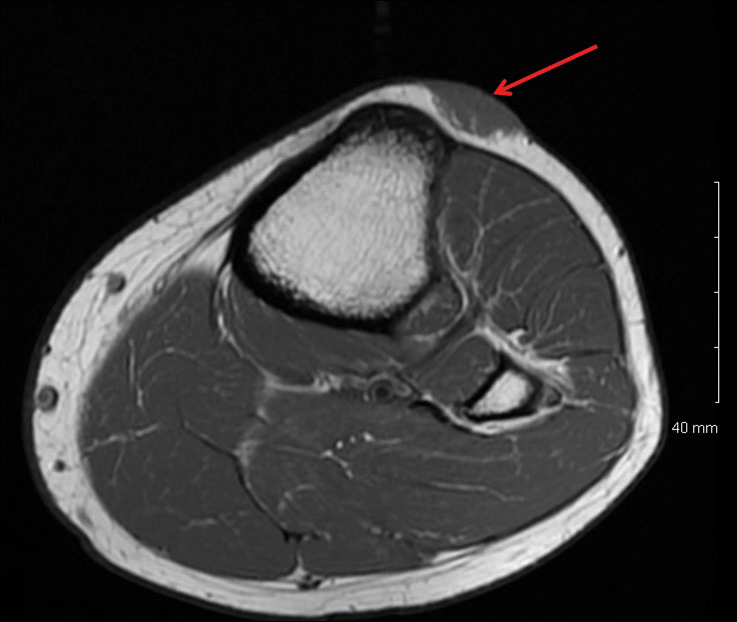
Excision of the superficial lesions was performed. All of the lesions demonstrated similar histologic changes to the previously described biopsy specimen. The tumor was limited to the dermis and subcutaneous tissue. The patient was lost to follow-up and the etiology of the lung lesions was unknown.
Comment
Nomenclature
Pseudomyogenic hemangioendothelioma is a relatively new type of vascular tumor that has been included in the updated 2013 edition of the World Health Organization classification as an intermediate malignant tumor that rarely metastasizes.3 It typically involves multiple tissue planes, most notably the dermis and subcutaneous layers but also muscle and bone.4 The term pseudomyogenic refers to the histologic resemblance of some of the cells to rhabdomyoblasts; however, these tumors are negative for all immunohistochemical muscle markers, most notably myogenin, desmin, and α-smooth muscle actin.5
Clinical Presentation
Gross features of PMHE typically include multiple firm nodules with ill-defined margins. The tumor was initially described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. In 2003, a series of 7 cases of PMHE was reported by Billings et al6 under the term epithelioid sarcomalike hemangioendothelioma. Other than the predominance of an epithelioid morphology, the cases reported as epithelioid sarcomalike hemangioendothelioma had similar clinical features and immunophenotype to what has been reported as PMHE.
Based on a PubMed search of articles indexed for MEDLINE using the term pseudomyogenic hemangioendothelioma, the 2 largest case series were reported by Pradhan et al7 (N=8) in 2017 and Hornick and Fletcher4 (N=50) in 2011. Hornick and Fletcher4 reported a male (41/50 [82.0%]) to female (9/50 [18.0%]) ratio of 4.6 to 1, and an average age at presentation of 31 years with 82% (41/50) of patients 40 years or younger. Pradhan et al7 also reported a male predominance (7/8 [87.5%]) with a similar average age at presentation of 29 years (age range, 9–62 years). The size of individual tumors ranged from 0.3 to 5.5 cm (mean size, 1.9 cm) in the series by Hornick and Fletcher4 and 0.3 to 6.0 com in the series by Pradhan et al.7 Hornick and Fletcher4 reported the most common site of involvement was the leg (27/50 [54.0%]), followed by the arm (12/50 [24.0%]), trunk (9/50 [18.0%]), and head and neck (2/50 [4.0%]). The leg (6/8 [75.0%]) also was the most common site of involvement in the series by Pradhan et al,7 with 2 cases occurring on the arm. In the series by Hornick and Fletcher,4 the tumors typically involved the dermis and subcutaneous tissue (26/50 [52%]) with a smaller number involving skeletal muscle (17/50 [34%]) and bone (7/50 [14%]). They reported 66% of their patients (33/50) had multifocal disease at presentation.4 Pradhan et al7 also reported 2 (25.0%) cases being limited to the superficial soft tissue, 2 (25.0%) being limited to the deep soft tissue, and 4 (50.0%) involving the bone; 5 (62.5%) patients had multifocal disease at presentation. The presentation of our patient in regards to gender, age, and tumor characteristics is consistent with other published cases.5-10
Histopathology
Microscopic features of PMHE include sheets of spindled to epithelioid-appearing cells with mild to moderate nuclear atypia and eosinophilic cytoplasm. The tumor has an infiltrative growth pattern. Some of the cells may resemble rhabdomyoblastlike cells, hence the moniker pseudomyogenic. There is no recapitulation of vascular structures or remarkable cytoplasmic vacuolization. Mitotic rate is low and there is no tumor necrosis.4 The tumor cells do not appear to arise from a vessel or display an angiocentric growth pattern. Many cases report the presence of an inflammatory infiltrate containing neutrophils interspersed within the tumor.4,5,7 The overlying epidermis will commonly show hyperkeratosis, epidermal hyperplasia, and acanthosis.4,11
Differential Diagnosis
The histopathologic differential diagnosis would include epithelioid sarcoma, epithelioid hemangioendothelioma, and to a lesser extent dermatofibrosarcoma protuberans (DFSP) and rhabdomyosarcoma. Dermatofibrosarcoma protuberans is the most commonly encountered of these tumors. Histologically, DFSP is characterized by a cellular proliferation of small spindle cells with plump nuclei arranged in a storiform or cartwheel pattern. Dermatofibrosarcoma protuberans tends to be limited to the dermis and subcutaneous tissue and only rarely involves underlying skeletal muscle. The presence of the storiform growth pattern in conjunction with the lack of rhabdoid changes would favor a diagnosis of DFSP. Another characteristic histologic finding typically only associated with DFSP is the interdigitating growth pattern of the spindle cells within the lobules of the subcutaneous tissue, creating a lacelike or honeycomb appearance.
Immunohistochemistry staining is necessary to help differentiate PMHE from other tumors in the differential diagnosis. Pseudomyogenic hemangioendothelioma stains positive for cytokeratin AE1/AE3; integrase interactor 1; and vascular markers FLI-1, CD31, and ERG, and negative for CD34.4,6,12-15 In contrast to epithelioid hemangioendothelioma, DFSP, and to a lesser extent epithelioid sarcoma, all of which are positive for CD34, epithelioid sarcoma is negative for both CD31 and integrase interactor 1. Dermatofibrosarcoma protuberans is negative for cytokeratin AE1/AE3. Rhabdomyosarcomas are positive for myogenic markers such as MyoD1 and myogenin, unlike any of the other tumors mentioned. Histologically, epithelioid sarcomas will tend to have a granulomalike growth pattern with central necrosis, unlike PMHE.12 Epithelioid hemangioendothelioma often will have a cordlike growth pattern in a myxochondroid background. Unlike PMHE, these tumors often will appear to be arising from vessels, and intracytoplasmic vacuoles are common. Three cases of PMHE have been reported to have a t(7;19)(q22;q13) chromosomal anomaly, which is not consistent with every case.16
Treatment Options
Standard treatment typically includes wide excision of the lesions, as was done in our case. Because of the substantial risk of local recurrence, which was up to 58% in the series by Hornick and Fletcher,4 adjuvant therapy may be considered if positive margins are found on excision. Metastasis to lymph nodes and the lungs has been reported but is rare.2,4 Most cases have been shown to have a favorable prognosis; however, local recurrence seems to be common. Rarely, amputation of the limb may be required.5 In contrast, epithelioid sarcomas have been found to spread to lymph nodes and the lungs in up to 50% of cases with a 5-year survival rate of 10% to 30%.13
Conclusion
In summary, we describe a case of PMHE involving the lower leg in a 20-year-old man. These tumors often are multinodular and multiplanar, with the dermis and subcutaneous tissues being the most common areas affected. It has a high rate of local recurrence but rarely has distant metastasis. Pseudomyogenic hemangioendothelioma, similar to other soft tissue tumors, can be difficult to diagnose on shave biopsy or superficial punch biopsy not extending into subcutaneous tissue. Deep incisional or punch biopsies are required to more definitively diagnose these types of tumors. The diagnosis of PMHE versus other soft tissue tumors requires correlation of histology and immunohistochemistry staining with clinical information and radiographic findings.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma (pseudomyogenic hemangioendothelioma). Am J Surg Pathol. 2011;35:1088; author reply 1088-1089.
- Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104.
- Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190-201.
- Sheng W, Pan Y, Wang J. Pseudomyogenic hemangioendothelioma: report of an additional case with aggressive clinical course. Am J Dermatopathol. 2013;35:597-600.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27:48-57.
- Pradhan D, Schoedel K, McGough RL, et al. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue—a clinicopathological, immunohistochemical and fluorescence in situ hybridization study [published online November 2, 2017]. Hum Pathol. 2017. doi:0.1016/j.humpath.2017.10.023.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- McGinity M, Bartanusz V, Dengler B, et al. Pseudomyogenic hemangioendothelioma (epithelioid sarcoma-like hemangioendothelioma, fibroma-like variant of epithelioid sarcoma) of the thoracic spine. Eur Spine J. 2013;22(suppl 3):S506-S511.
- Stuart LN, Gardner JM, Lauer SR, et al. Epithelioid sarcoma-like (pseudomyogenic) hemangioendothelioma, clinically mimicking dermatofibroma, diagnosed by skin biopsy in a 30-year-old man. J Cutan Pathol. 2013;40:909-913.
- Amary MF, O’Donnell P, Berisha F, et al. Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma: characterization of five cases. Skeletal Radiol. 2013;42:947-957.
- Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542-550.
- Chbani L, Guillou L, Terrier P, et al. Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French Sarcoma Group. Am J Clin Pathol. 2009;131:222-227.
- Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- Trombetta D, Magnusson L, von Steyern FV, et al. Translocation t(7;19)(q22;q13)—a recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer Genet. 2011;204:211-215.
Pseudomyogenic hemangioendothelioma (PMHE), also referred to as epithelioid sarcoma–like hemangioendothelioma,1 is a rare soft tissue tumor that was described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. It predominantly affects males between the second and fifth decades of life and most commonly presents as multiple nodules that may involve either the superficial or deep soft tissues of the legs and less often the arms. It also can arise on the trunk. We present a case of PMHE occurring in a young man and briefly review the literature on clinical presentation and histologic differentiation of this unique tumor, comparing these findings to its mimickers.
Case Report
A 20-year-old man presented with skin lesions on the left leg that had been present for 1 year. The patient described the lesions as tender pimples that would drain yellow discharge on occasion but had now transformed into large brown plaques. Physical examination showed 4 verrucous plaques ranging in size from 1 to 3 cm with hyperpigmentation and a central crust (Figure 1). Initially, the patient thought the lesions appeared due to shaving his legs for sports. He presented to the emergency department multiple times over the past year; pain control was provided and local skin care was recommended. Culture of the discharge had been performed 6 months prior to biopsy with negative results. No biopsy was performed on initial presentation and the lesions were diagnosed in the emergency department clinically as boils.

After failing to improve, the patient was seen by an outside dermatologist and the clinical differential diagnosis included deep fungal infection, atypical mycobacterial infection, and keloids. A 4-mm punch biopsy was taken from the periphery of one of the lesions and demonstrated hyperkeratosis, papillomatosis, and acanthosis (Figure 2). Within the superficial and deep dermis and focally extending into the subcutaneous tissue, there were sheets of spindled to epithelioid-appearing cells with moderate cytologic atypia (Figure 3). The tumor showed infiltrative margins. There was moderate cellularity. The individual cells had a rhabdoid appearance with large eccentric vesicular nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (Figure 4). No definitive evidence of glandular, squamous, or vascular differentiation was present. There was an associated moderate inflammatory host response composed of neutrophils and lymphocytes. Occasional extravasated red blood cells were present. Immunohistochemistry staining was performed and the atypical cells demonstrated diffuse positive staining for friend leukemia integration 1 transcription factor (FLI-1), erythroblastosis virus E26 transforming sequence-related gene (ERG)(Figure 5), CD31, and CD68. There was patchy positive staining for cytokeratin AE1/AE3, CD10, and factor VIII. There was no remarkable staining for human herpesvirus 8, epithelial membrane antigen, S-100, CD34, cytokeratin 903, and desmin. Overall, the histologic features in conjunction with the immunohistochemistry staining were consistent with a diagnosis of PMHE.




Magnetic resonance imaging was then performed to evaluate the depth and extent of the lesions for surgical excision planning (Figure 6), which showed 5 nodular lesions within the dermis and subcutis adjacent to the proximal aspect of the left tibia and medial aspect of the left knee. An additional lesion was noted between the sartorius and semimembranosus muscles, which was thought to represent either a lymph node or an additional neoplastic lesion. Chest computed tomography also displayed indeterminate lesions in the lungs.

Excision of the superficial lesions was performed. All of the lesions demonstrated similar histologic changes to the previously described biopsy specimen. The tumor was limited to the dermis and subcutaneous tissue. The patient was lost to follow-up and the etiology of the lung lesions was unknown.
Comment
Nomenclature
Pseudomyogenic hemangioendothelioma is a relatively new type of vascular tumor that has been included in the updated 2013 edition of the World Health Organization classification as an intermediate malignant tumor that rarely metastasizes.3 It typically involves multiple tissue planes, most notably the dermis and subcutaneous layers but also muscle and bone.4 The term pseudomyogenic refers to the histologic resemblance of some of the cells to rhabdomyoblasts; however, these tumors are negative for all immunohistochemical muscle markers, most notably myogenin, desmin, and α-smooth muscle actin.5
Clinical Presentation
Gross features of PMHE typically include multiple firm nodules with ill-defined margins. The tumor was initially described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. In 2003, a series of 7 cases of PMHE was reported by Billings et al6 under the term epithelioid sarcomalike hemangioendothelioma. Other than the predominance of an epithelioid morphology, the cases reported as epithelioid sarcomalike hemangioendothelioma had similar clinical features and immunophenotype to what has been reported as PMHE.
Based on a PubMed search of articles indexed for MEDLINE using the term pseudomyogenic hemangioendothelioma, the 2 largest case series were reported by Pradhan et al7 (N=8) in 2017 and Hornick and Fletcher4 (N=50) in 2011. Hornick and Fletcher4 reported a male (41/50 [82.0%]) to female (9/50 [18.0%]) ratio of 4.6 to 1, and an average age at presentation of 31 years with 82% (41/50) of patients 40 years or younger. Pradhan et al7 also reported a male predominance (7/8 [87.5%]) with a similar average age at presentation of 29 years (age range, 9–62 years). The size of individual tumors ranged from 0.3 to 5.5 cm (mean size, 1.9 cm) in the series by Hornick and Fletcher4 and 0.3 to 6.0 com in the series by Pradhan et al.7 Hornick and Fletcher4 reported the most common site of involvement was the leg (27/50 [54.0%]), followed by the arm (12/50 [24.0%]), trunk (9/50 [18.0%]), and head and neck (2/50 [4.0%]). The leg (6/8 [75.0%]) also was the most common site of involvement in the series by Pradhan et al,7 with 2 cases occurring on the arm. In the series by Hornick and Fletcher,4 the tumors typically involved the dermis and subcutaneous tissue (26/50 [52%]) with a smaller number involving skeletal muscle (17/50 [34%]) and bone (7/50 [14%]). They reported 66% of their patients (33/50) had multifocal disease at presentation.4 Pradhan et al7 also reported 2 (25.0%) cases being limited to the superficial soft tissue, 2 (25.0%) being limited to the deep soft tissue, and 4 (50.0%) involving the bone; 5 (62.5%) patients had multifocal disease at presentation. The presentation of our patient in regards to gender, age, and tumor characteristics is consistent with other published cases.5-10
Histopathology
Microscopic features of PMHE include sheets of spindled to epithelioid-appearing cells with mild to moderate nuclear atypia and eosinophilic cytoplasm. The tumor has an infiltrative growth pattern. Some of the cells may resemble rhabdomyoblastlike cells, hence the moniker pseudomyogenic. There is no recapitulation of vascular structures or remarkable cytoplasmic vacuolization. Mitotic rate is low and there is no tumor necrosis.4 The tumor cells do not appear to arise from a vessel or display an angiocentric growth pattern. Many cases report the presence of an inflammatory infiltrate containing neutrophils interspersed within the tumor.4,5,7 The overlying epidermis will commonly show hyperkeratosis, epidermal hyperplasia, and acanthosis.4,11
Differential Diagnosis
The histopathologic differential diagnosis would include epithelioid sarcoma, epithelioid hemangioendothelioma, and to a lesser extent dermatofibrosarcoma protuberans (DFSP) and rhabdomyosarcoma. Dermatofibrosarcoma protuberans is the most commonly encountered of these tumors. Histologically, DFSP is characterized by a cellular proliferation of small spindle cells with plump nuclei arranged in a storiform or cartwheel pattern. Dermatofibrosarcoma protuberans tends to be limited to the dermis and subcutaneous tissue and only rarely involves underlying skeletal muscle. The presence of the storiform growth pattern in conjunction with the lack of rhabdoid changes would favor a diagnosis of DFSP. Another characteristic histologic finding typically only associated with DFSP is the interdigitating growth pattern of the spindle cells within the lobules of the subcutaneous tissue, creating a lacelike or honeycomb appearance.
Immunohistochemistry staining is necessary to help differentiate PMHE from other tumors in the differential diagnosis. Pseudomyogenic hemangioendothelioma stains positive for cytokeratin AE1/AE3; integrase interactor 1; and vascular markers FLI-1, CD31, and ERG, and negative for CD34.4,6,12-15 In contrast to epithelioid hemangioendothelioma, DFSP, and to a lesser extent epithelioid sarcoma, all of which are positive for CD34, epithelioid sarcoma is negative for both CD31 and integrase interactor 1. Dermatofibrosarcoma protuberans is negative for cytokeratin AE1/AE3. Rhabdomyosarcomas are positive for myogenic markers such as MyoD1 and myogenin, unlike any of the other tumors mentioned. Histologically, epithelioid sarcomas will tend to have a granulomalike growth pattern with central necrosis, unlike PMHE.12 Epithelioid hemangioendothelioma often will have a cordlike growth pattern in a myxochondroid background. Unlike PMHE, these tumors often will appear to be arising from vessels, and intracytoplasmic vacuoles are common. Three cases of PMHE have been reported to have a t(7;19)(q22;q13) chromosomal anomaly, which is not consistent with every case.16
Treatment Options
Standard treatment typically includes wide excision of the lesions, as was done in our case. Because of the substantial risk of local recurrence, which was up to 58% in the series by Hornick and Fletcher,4 adjuvant therapy may be considered if positive margins are found on excision. Metastasis to lymph nodes and the lungs has been reported but is rare.2,4 Most cases have been shown to have a favorable prognosis; however, local recurrence seems to be common. Rarely, amputation of the limb may be required.5 In contrast, epithelioid sarcomas have been found to spread to lymph nodes and the lungs in up to 50% of cases with a 5-year survival rate of 10% to 30%.13
Conclusion
In summary, we describe a case of PMHE involving the lower leg in a 20-year-old man. These tumors often are multinodular and multiplanar, with the dermis and subcutaneous tissues being the most common areas affected. It has a high rate of local recurrence but rarely has distant metastasis. Pseudomyogenic hemangioendothelioma, similar to other soft tissue tumors, can be difficult to diagnose on shave biopsy or superficial punch biopsy not extending into subcutaneous tissue. Deep incisional or punch biopsies are required to more definitively diagnose these types of tumors. The diagnosis of PMHE versus other soft tissue tumors requires correlation of histology and immunohistochemistry staining with clinical information and radiographic findings.
Pseudomyogenic hemangioendothelioma (PMHE), also referred to as epithelioid sarcoma–like hemangioendothelioma,1 is a rare soft tissue tumor that was described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. It predominantly affects males between the second and fifth decades of life and most commonly presents as multiple nodules that may involve either the superficial or deep soft tissues of the legs and less often the arms. It also can arise on the trunk. We present a case of PMHE occurring in a young man and briefly review the literature on clinical presentation and histologic differentiation of this unique tumor, comparing these findings to its mimickers.
Case Report
A 20-year-old man presented with skin lesions on the left leg that had been present for 1 year. The patient described the lesions as tender pimples that would drain yellow discharge on occasion but had now transformed into large brown plaques. Physical examination showed 4 verrucous plaques ranging in size from 1 to 3 cm with hyperpigmentation and a central crust (Figure 1). Initially, the patient thought the lesions appeared due to shaving his legs for sports. He presented to the emergency department multiple times over the past year; pain control was provided and local skin care was recommended. Culture of the discharge had been performed 6 months prior to biopsy with negative results. No biopsy was performed on initial presentation and the lesions were diagnosed in the emergency department clinically as boils.

After failing to improve, the patient was seen by an outside dermatologist and the clinical differential diagnosis included deep fungal infection, atypical mycobacterial infection, and keloids. A 4-mm punch biopsy was taken from the periphery of one of the lesions and demonstrated hyperkeratosis, papillomatosis, and acanthosis (Figure 2). Within the superficial and deep dermis and focally extending into the subcutaneous tissue, there were sheets of spindled to epithelioid-appearing cells with moderate cytologic atypia (Figure 3). The tumor showed infiltrative margins. There was moderate cellularity. The individual cells had a rhabdoid appearance with large eccentric vesicular nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (Figure 4). No definitive evidence of glandular, squamous, or vascular differentiation was present. There was an associated moderate inflammatory host response composed of neutrophils and lymphocytes. Occasional extravasated red blood cells were present. Immunohistochemistry staining was performed and the atypical cells demonstrated diffuse positive staining for friend leukemia integration 1 transcription factor (FLI-1), erythroblastosis virus E26 transforming sequence-related gene (ERG)(Figure 5), CD31, and CD68. There was patchy positive staining for cytokeratin AE1/AE3, CD10, and factor VIII. There was no remarkable staining for human herpesvirus 8, epithelial membrane antigen, S-100, CD34, cytokeratin 903, and desmin. Overall, the histologic features in conjunction with the immunohistochemistry staining were consistent with a diagnosis of PMHE.




Magnetic resonance imaging was then performed to evaluate the depth and extent of the lesions for surgical excision planning (Figure 6), which showed 5 nodular lesions within the dermis and subcutis adjacent to the proximal aspect of the left tibia and medial aspect of the left knee. An additional lesion was noted between the sartorius and semimembranosus muscles, which was thought to represent either a lymph node or an additional neoplastic lesion. Chest computed tomography also displayed indeterminate lesions in the lungs.

Excision of the superficial lesions was performed. All of the lesions demonstrated similar histologic changes to the previously described biopsy specimen. The tumor was limited to the dermis and subcutaneous tissue. The patient was lost to follow-up and the etiology of the lung lesions was unknown.
Comment
Nomenclature
Pseudomyogenic hemangioendothelioma is a relatively new type of vascular tumor that has been included in the updated 2013 edition of the World Health Organization classification as an intermediate malignant tumor that rarely metastasizes.3 It typically involves multiple tissue planes, most notably the dermis and subcutaneous layers but also muscle and bone.4 The term pseudomyogenic refers to the histologic resemblance of some of the cells to rhabdomyoblasts; however, these tumors are negative for all immunohistochemical muscle markers, most notably myogenin, desmin, and α-smooth muscle actin.5
Clinical Presentation
Gross features of PMHE typically include multiple firm nodules with ill-defined margins. The tumor was initially described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. In 2003, a series of 7 cases of PMHE was reported by Billings et al6 under the term epithelioid sarcomalike hemangioendothelioma. Other than the predominance of an epithelioid morphology, the cases reported as epithelioid sarcomalike hemangioendothelioma had similar clinical features and immunophenotype to what has been reported as PMHE.
Based on a PubMed search of articles indexed for MEDLINE using the term pseudomyogenic hemangioendothelioma, the 2 largest case series were reported by Pradhan et al7 (N=8) in 2017 and Hornick and Fletcher4 (N=50) in 2011. Hornick and Fletcher4 reported a male (41/50 [82.0%]) to female (9/50 [18.0%]) ratio of 4.6 to 1, and an average age at presentation of 31 years with 82% (41/50) of patients 40 years or younger. Pradhan et al7 also reported a male predominance (7/8 [87.5%]) with a similar average age at presentation of 29 years (age range, 9–62 years). The size of individual tumors ranged from 0.3 to 5.5 cm (mean size, 1.9 cm) in the series by Hornick and Fletcher4 and 0.3 to 6.0 com in the series by Pradhan et al.7 Hornick and Fletcher4 reported the most common site of involvement was the leg (27/50 [54.0%]), followed by the arm (12/50 [24.0%]), trunk (9/50 [18.0%]), and head and neck (2/50 [4.0%]). The leg (6/8 [75.0%]) also was the most common site of involvement in the series by Pradhan et al,7 with 2 cases occurring on the arm. In the series by Hornick and Fletcher,4 the tumors typically involved the dermis and subcutaneous tissue (26/50 [52%]) with a smaller number involving skeletal muscle (17/50 [34%]) and bone (7/50 [14%]). They reported 66% of their patients (33/50) had multifocal disease at presentation.4 Pradhan et al7 also reported 2 (25.0%) cases being limited to the superficial soft tissue, 2 (25.0%) being limited to the deep soft tissue, and 4 (50.0%) involving the bone; 5 (62.5%) patients had multifocal disease at presentation. The presentation of our patient in regards to gender, age, and tumor characteristics is consistent with other published cases.5-10
Histopathology
Microscopic features of PMHE include sheets of spindled to epithelioid-appearing cells with mild to moderate nuclear atypia and eosinophilic cytoplasm. The tumor has an infiltrative growth pattern. Some of the cells may resemble rhabdomyoblastlike cells, hence the moniker pseudomyogenic. There is no recapitulation of vascular structures or remarkable cytoplasmic vacuolization. Mitotic rate is low and there is no tumor necrosis.4 The tumor cells do not appear to arise from a vessel or display an angiocentric growth pattern. Many cases report the presence of an inflammatory infiltrate containing neutrophils interspersed within the tumor.4,5,7 The overlying epidermis will commonly show hyperkeratosis, epidermal hyperplasia, and acanthosis.4,11
Differential Diagnosis
The histopathologic differential diagnosis would include epithelioid sarcoma, epithelioid hemangioendothelioma, and to a lesser extent dermatofibrosarcoma protuberans (DFSP) and rhabdomyosarcoma. Dermatofibrosarcoma protuberans is the most commonly encountered of these tumors. Histologically, DFSP is characterized by a cellular proliferation of small spindle cells with plump nuclei arranged in a storiform or cartwheel pattern. Dermatofibrosarcoma protuberans tends to be limited to the dermis and subcutaneous tissue and only rarely involves underlying skeletal muscle. The presence of the storiform growth pattern in conjunction with the lack of rhabdoid changes would favor a diagnosis of DFSP. Another characteristic histologic finding typically only associated with DFSP is the interdigitating growth pattern of the spindle cells within the lobules of the subcutaneous tissue, creating a lacelike or honeycomb appearance.
Immunohistochemistry staining is necessary to help differentiate PMHE from other tumors in the differential diagnosis. Pseudomyogenic hemangioendothelioma stains positive for cytokeratin AE1/AE3; integrase interactor 1; and vascular markers FLI-1, CD31, and ERG, and negative for CD34.4,6,12-15 In contrast to epithelioid hemangioendothelioma, DFSP, and to a lesser extent epithelioid sarcoma, all of which are positive for CD34, epithelioid sarcoma is negative for both CD31 and integrase interactor 1. Dermatofibrosarcoma protuberans is negative for cytokeratin AE1/AE3. Rhabdomyosarcomas are positive for myogenic markers such as MyoD1 and myogenin, unlike any of the other tumors mentioned. Histologically, epithelioid sarcomas will tend to have a granulomalike growth pattern with central necrosis, unlike PMHE.12 Epithelioid hemangioendothelioma often will have a cordlike growth pattern in a myxochondroid background. Unlike PMHE, these tumors often will appear to be arising from vessels, and intracytoplasmic vacuoles are common. Three cases of PMHE have been reported to have a t(7;19)(q22;q13) chromosomal anomaly, which is not consistent with every case.16
Treatment Options
Standard treatment typically includes wide excision of the lesions, as was done in our case. Because of the substantial risk of local recurrence, which was up to 58% in the series by Hornick and Fletcher,4 adjuvant therapy may be considered if positive margins are found on excision. Metastasis to lymph nodes and the lungs has been reported but is rare.2,4 Most cases have been shown to have a favorable prognosis; however, local recurrence seems to be common. Rarely, amputation of the limb may be required.5 In contrast, epithelioid sarcomas have been found to spread to lymph nodes and the lungs in up to 50% of cases with a 5-year survival rate of 10% to 30%.13
Conclusion
In summary, we describe a case of PMHE involving the lower leg in a 20-year-old man. These tumors often are multinodular and multiplanar, with the dermis and subcutaneous tissues being the most common areas affected. It has a high rate of local recurrence but rarely has distant metastasis. Pseudomyogenic hemangioendothelioma, similar to other soft tissue tumors, can be difficult to diagnose on shave biopsy or superficial punch biopsy not extending into subcutaneous tissue. Deep incisional or punch biopsies are required to more definitively diagnose these types of tumors. The diagnosis of PMHE versus other soft tissue tumors requires correlation of histology and immunohistochemistry staining with clinical information and radiographic findings.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma (pseudomyogenic hemangioendothelioma). Am J Surg Pathol. 2011;35:1088; author reply 1088-1089.
- Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104.
- Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190-201.
- Sheng W, Pan Y, Wang J. Pseudomyogenic hemangioendothelioma: report of an additional case with aggressive clinical course. Am J Dermatopathol. 2013;35:597-600.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27:48-57.
- Pradhan D, Schoedel K, McGough RL, et al. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue—a clinicopathological, immunohistochemical and fluorescence in situ hybridization study [published online November 2, 2017]. Hum Pathol. 2017. doi:0.1016/j.humpath.2017.10.023.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- McGinity M, Bartanusz V, Dengler B, et al. Pseudomyogenic hemangioendothelioma (epithelioid sarcoma-like hemangioendothelioma, fibroma-like variant of epithelioid sarcoma) of the thoracic spine. Eur Spine J. 2013;22(suppl 3):S506-S511.
- Stuart LN, Gardner JM, Lauer SR, et al. Epithelioid sarcoma-like (pseudomyogenic) hemangioendothelioma, clinically mimicking dermatofibroma, diagnosed by skin biopsy in a 30-year-old man. J Cutan Pathol. 2013;40:909-913.
- Amary MF, O’Donnell P, Berisha F, et al. Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma: characterization of five cases. Skeletal Radiol. 2013;42:947-957.
- Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542-550.
- Chbani L, Guillou L, Terrier P, et al. Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French Sarcoma Group. Am J Clin Pathol. 2009;131:222-227.
- Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- Trombetta D, Magnusson L, von Steyern FV, et al. Translocation t(7;19)(q22;q13)—a recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer Genet. 2011;204:211-215.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma (pseudomyogenic hemangioendothelioma). Am J Surg Pathol. 2011;35:1088; author reply 1088-1089.
- Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104.
- Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190-201.
- Sheng W, Pan Y, Wang J. Pseudomyogenic hemangioendothelioma: report of an additional case with aggressive clinical course. Am J Dermatopathol. 2013;35:597-600.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27:48-57.
- Pradhan D, Schoedel K, McGough RL, et al. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue—a clinicopathological, immunohistochemical and fluorescence in situ hybridization study [published online November 2, 2017]. Hum Pathol. 2017. doi:0.1016/j.humpath.2017.10.023.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- McGinity M, Bartanusz V, Dengler B, et al. Pseudomyogenic hemangioendothelioma (epithelioid sarcoma-like hemangioendothelioma, fibroma-like variant of epithelioid sarcoma) of the thoracic spine. Eur Spine J. 2013;22(suppl 3):S506-S511.
- Stuart LN, Gardner JM, Lauer SR, et al. Epithelioid sarcoma-like (pseudomyogenic) hemangioendothelioma, clinically mimicking dermatofibroma, diagnosed by skin biopsy in a 30-year-old man. J Cutan Pathol. 2013;40:909-913.
- Amary MF, O’Donnell P, Berisha F, et al. Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma: characterization of five cases. Skeletal Radiol. 2013;42:947-957.
- Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542-550.
- Chbani L, Guillou L, Terrier P, et al. Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French Sarcoma Group. Am J Clin Pathol. 2009;131:222-227.
- Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- Trombetta D, Magnusson L, von Steyern FV, et al. Translocation t(7;19)(q22;q13)—a recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer Genet. 2011;204:211-215.
Practice Points
- Pseudomyogenic hemangioendothelioma (PMHE) is an uncommon vascular tumor that most often presents as multiple distinct nodules on the legs in young men.
- Pseudomyogenic hemangioendothelioma has an unusual immunohistochemistry staining pattern, with positive staining for cytokeratin AE1/AE3, CD31, and ERG but negative for CD34.
- Although local reoccurrence is common, PMHE metastasis is very uncommon.
Red Patches on a Newborn
The Diagnosis: Congenital Unilateral Nevoid Telangiectasia
Two weeks later the patches were noticeably lighter (Figures 1A and 1B). She continued to be in good health, but gynecomastia was notably present on examination (Figure 1C). At 3 months of age, all patches on the right arm, superior aspect of the chest, and superior aspect of the back had resolved, along with the gynecomastia (Figure 2).
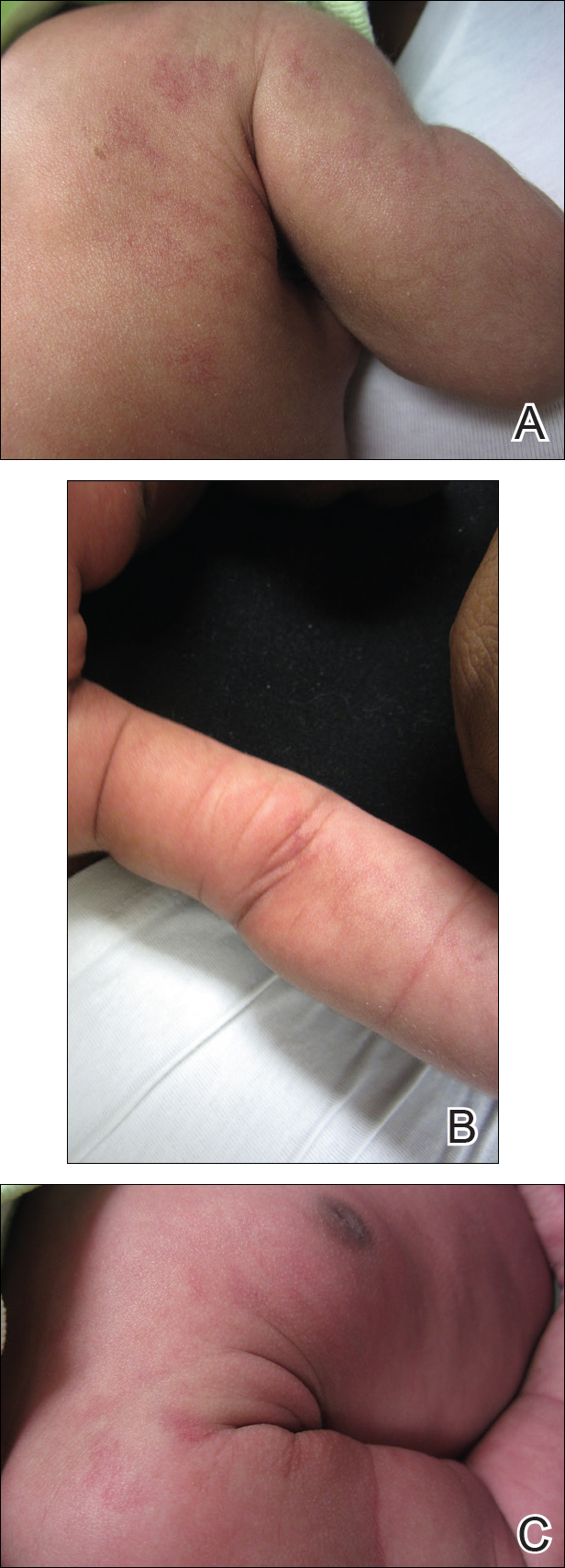
This case describes the rare condition of congenital unilateral nevoid telangiectasia (UNT). Unilateral nevoid telangiectasia is a rare cutaneous vascular condition first described by Blaschko1 in 1899. It is characterized by the presence of unilateral superficial telangiectases occurring most often in the cervical and upper thoracic dermatomes in a linear pattern.2 Females are more often affected than males (2:1 ratio), and cases of UNT are either congenital or acquired.3 Although most UNT cases are acquired and often found in females, approximately 15% of cases are congenital and are comprised largely by males. Acquired cases have been hypothesized to occur in association with hyperestrogenemic states such as pregnancy, puberty, oral contraceptive use and hormonal therapy, alcoholism, and liver disease including hepatitis B and C infections.4,5 There is conflicting evidence as to whether there is an absolute increase in the presence of estrogen and progesterone receptors in the skin, as many case reports show no increase. Instead, others hypothesize that the condition is actually a result of somatic mosaicism and that the cutaneous lesions are genetically predisposed to becoming visibly evident under conditions of elevated estrogen.2
In our case, we hypothesize that the cause was elevated maternal estrogen levels present at higher than normal levels in the fetal circulation. The presence of gynecomastia seen in our patient supports the hypothesis that increased circulating estrogen may be present in infants with UNT.
- Blaschko A. Teleangiektasien. versammlungen. Berliner Dermatologische Gesellschaft. Monatschr prakt Dermat. 1899;28:451.
- Karakas¸ M, Durdu M, Sönmezoğlu S, et al. Unilateral nevoidtelangiectasia. J Dermatol. 2004;31:109-112.
- Wenson SF, Farhana J, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Hynes LR, Shenefelt PD. Unilateral nevoid telangiectasia: occurrence in two patients with hepatitis C. J Am Acad Dermatol. 1997;36(5 pt 2):819-822.
- Guedes R, Leite L. Unilateral nevoid telangiectasia: a rare disease? Indian J Dermatol. 2012;57:138-140.
The Diagnosis: Congenital Unilateral Nevoid Telangiectasia
Two weeks later the patches were noticeably lighter (Figures 1A and 1B). She continued to be in good health, but gynecomastia was notably present on examination (Figure 1C). At 3 months of age, all patches on the right arm, superior aspect of the chest, and superior aspect of the back had resolved, along with the gynecomastia (Figure 2).


This case describes the rare condition of congenital unilateral nevoid telangiectasia (UNT). Unilateral nevoid telangiectasia is a rare cutaneous vascular condition first described by Blaschko1 in 1899. It is characterized by the presence of unilateral superficial telangiectases occurring most often in the cervical and upper thoracic dermatomes in a linear pattern.2 Females are more often affected than males (2:1 ratio), and cases of UNT are either congenital or acquired.3 Although most UNT cases are acquired and often found in females, approximately 15% of cases are congenital and are comprised largely by males. Acquired cases have been hypothesized to occur in association with hyperestrogenemic states such as pregnancy, puberty, oral contraceptive use and hormonal therapy, alcoholism, and liver disease including hepatitis B and C infections.4,5 There is conflicting evidence as to whether there is an absolute increase in the presence of estrogen and progesterone receptors in the skin, as many case reports show no increase. Instead, others hypothesize that the condition is actually a result of somatic mosaicism and that the cutaneous lesions are genetically predisposed to becoming visibly evident under conditions of elevated estrogen.2
In our case, we hypothesize that the cause was elevated maternal estrogen levels present at higher than normal levels in the fetal circulation. The presence of gynecomastia seen in our patient supports the hypothesis that increased circulating estrogen may be present in infants with UNT.
The Diagnosis: Congenital Unilateral Nevoid Telangiectasia
Two weeks later the patches were noticeably lighter (Figures 1A and 1B). She continued to be in good health, but gynecomastia was notably present on examination (Figure 1C). At 3 months of age, all patches on the right arm, superior aspect of the chest, and superior aspect of the back had resolved, along with the gynecomastia (Figure 2).


This case describes the rare condition of congenital unilateral nevoid telangiectasia (UNT). Unilateral nevoid telangiectasia is a rare cutaneous vascular condition first described by Blaschko1 in 1899. It is characterized by the presence of unilateral superficial telangiectases occurring most often in the cervical and upper thoracic dermatomes in a linear pattern.2 Females are more often affected than males (2:1 ratio), and cases of UNT are either congenital or acquired.3 Although most UNT cases are acquired and often found in females, approximately 15% of cases are congenital and are comprised largely by males. Acquired cases have been hypothesized to occur in association with hyperestrogenemic states such as pregnancy, puberty, oral contraceptive use and hormonal therapy, alcoholism, and liver disease including hepatitis B and C infections.4,5 There is conflicting evidence as to whether there is an absolute increase in the presence of estrogen and progesterone receptors in the skin, as many case reports show no increase. Instead, others hypothesize that the condition is actually a result of somatic mosaicism and that the cutaneous lesions are genetically predisposed to becoming visibly evident under conditions of elevated estrogen.2
In our case, we hypothesize that the cause was elevated maternal estrogen levels present at higher than normal levels in the fetal circulation. The presence of gynecomastia seen in our patient supports the hypothesis that increased circulating estrogen may be present in infants with UNT.
- Blaschko A. Teleangiektasien. versammlungen. Berliner Dermatologische Gesellschaft. Monatschr prakt Dermat. 1899;28:451.
- Karakas¸ M, Durdu M, Sönmezoğlu S, et al. Unilateral nevoidtelangiectasia. J Dermatol. 2004;31:109-112.
- Wenson SF, Farhana J, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Hynes LR, Shenefelt PD. Unilateral nevoid telangiectasia: occurrence in two patients with hepatitis C. J Am Acad Dermatol. 1997;36(5 pt 2):819-822.
- Guedes R, Leite L. Unilateral nevoid telangiectasia: a rare disease? Indian J Dermatol. 2012;57:138-140.
- Blaschko A. Teleangiektasien. versammlungen. Berliner Dermatologische Gesellschaft. Monatschr prakt Dermat. 1899;28:451.
- Karakas¸ M, Durdu M, Sönmezoğlu S, et al. Unilateral nevoidtelangiectasia. J Dermatol. 2004;31:109-112.
- Wenson SF, Farhana J, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Hynes LR, Shenefelt PD. Unilateral nevoid telangiectasia: occurrence in two patients with hepatitis C. J Am Acad Dermatol. 1997;36(5 pt 2):819-822.
- Guedes R, Leite L. Unilateral nevoid telangiectasia: a rare disease? Indian J Dermatol. 2012;57:138-140.
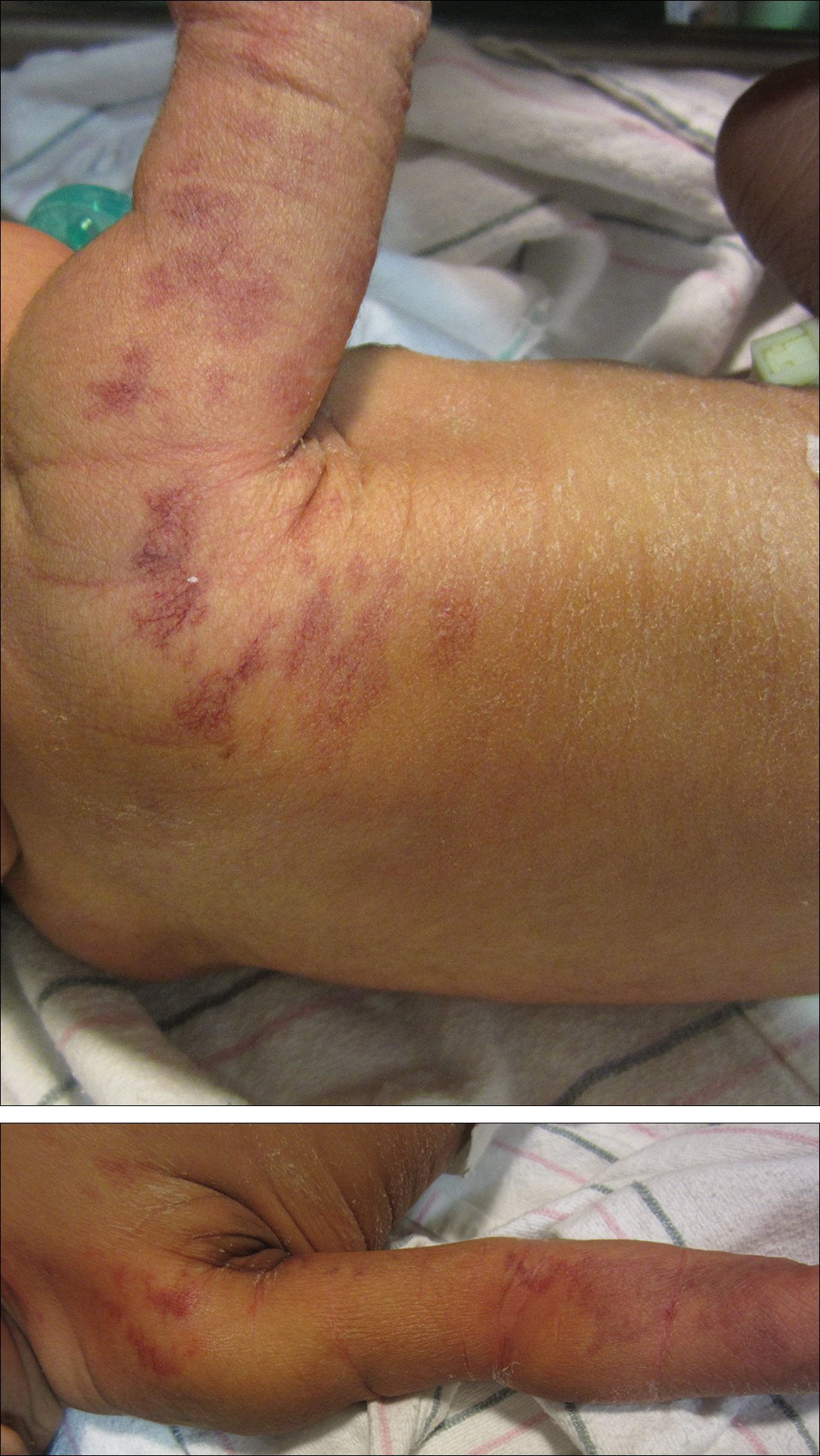
A 1-day-old female infant presented with red patches on the right arm that had been present since delivery. The patient was born to a healthy mother by spontaneous vaginal delivery without complications and with a good Apgar score. The newborn moved both arms and legs well and blood work was unremarkable. Her mother noted being healthy during pregnancy, and she had not taken any additional medications aside from prenatal vitamins. Examination of the infant revealed red blanchable reticulate patches in a dermatomal distribution extending from the posterior aspect of the right shoulder (top) down to the flexural aspect of the arm (bottom). There also were a few coalescing reticulate patches on the superior aspect of the right side of the chest and superior aspect of the right side of the back that resolved by 3 months of age.
Juvéderm Voluma for Cheek Rejuvenation



Preview of Best of Acne: 2017
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Social Media Creates Anxiety for Teenagers With Acne
Adolescents with acne experience anxiety over using social media, according to a recent online survey of teenagers in the United States. The results of the survey, conducted by Harris Poll on behalf of Cutanea Life Sciences, Inc, demonstrate the negative impact of acne on body image and self-esteem.
Of 1010 teens surveyed (age range, 15–19 years), 86% said they have had acne, and a majority of respondents said that acne has a negative effect on their body image and attractiveness (71%) as well as their self-esteem (67%). Fifty-one percent of respondents who use social media said it makes having acne harder and 72% agreed most teenagers with acne are self-conscious about showing their acne on social media. As a result, 68% reported that most of their peers with acne edit or alter their photographs on social media, and 58% have offered to take a photograph to avoid being in a picture. Half of the respondents have taken at least 1 of the following actions to avoid displaying their acne on social media:
- Chose not to include a photograph on social media
- Deleted or untagged a photograph that showed their acne
- Asked someone else to take down a photograph because it showed their acne
- Altered, edited, retouched, or cropped a photograph to try and hide their acne
- Avoided having their picture taken with someone who had clearer skin
- Stayed off social media so they would not have to post or see photographs of themselves
Dermatologists should be aware of the psychosocial impact of acne in teenagers to provide effective management strategies. Although the majority of teens (61%) said they were doing everything possible to manage their acne, 1 in 3 respondents admitted to having difficulty managing their condition. To effectively treat acne, more than three-quarters said that it was at least very important to use a therapy that worked quickly (83%) and was affordable (80%) and easy to use (78%). Be sure to address the psychosocial impact of acne with your teenaged patients, especially pertaining to social media.
Adolescents with acne experience anxiety over using social media, according to a recent online survey of teenagers in the United States. The results of the survey, conducted by Harris Poll on behalf of Cutanea Life Sciences, Inc, demonstrate the negative impact of acne on body image and self-esteem.
Of 1010 teens surveyed (age range, 15–19 years), 86% said they have had acne, and a majority of respondents said that acne has a negative effect on their body image and attractiveness (71%) as well as their self-esteem (67%). Fifty-one percent of respondents who use social media said it makes having acne harder and 72% agreed most teenagers with acne are self-conscious about showing their acne on social media. As a result, 68% reported that most of their peers with acne edit or alter their photographs on social media, and 58% have offered to take a photograph to avoid being in a picture. Half of the respondents have taken at least 1 of the following actions to avoid displaying their acne on social media:
- Chose not to include a photograph on social media
- Deleted or untagged a photograph that showed their acne
- Asked someone else to take down a photograph because it showed their acne
- Altered, edited, retouched, or cropped a photograph to try and hide their acne
- Avoided having their picture taken with someone who had clearer skin
- Stayed off social media so they would not have to post or see photographs of themselves
Dermatologists should be aware of the psychosocial impact of acne in teenagers to provide effective management strategies. Although the majority of teens (61%) said they were doing everything possible to manage their acne, 1 in 3 respondents admitted to having difficulty managing their condition. To effectively treat acne, more than three-quarters said that it was at least very important to use a therapy that worked quickly (83%) and was affordable (80%) and easy to use (78%). Be sure to address the psychosocial impact of acne with your teenaged patients, especially pertaining to social media.
Adolescents with acne experience anxiety over using social media, according to a recent online survey of teenagers in the United States. The results of the survey, conducted by Harris Poll on behalf of Cutanea Life Sciences, Inc, demonstrate the negative impact of acne on body image and self-esteem.
Of 1010 teens surveyed (age range, 15–19 years), 86% said they have had acne, and a majority of respondents said that acne has a negative effect on their body image and attractiveness (71%) as well as their self-esteem (67%). Fifty-one percent of respondents who use social media said it makes having acne harder and 72% agreed most teenagers with acne are self-conscious about showing their acne on social media. As a result, 68% reported that most of their peers with acne edit or alter their photographs on social media, and 58% have offered to take a photograph to avoid being in a picture. Half of the respondents have taken at least 1 of the following actions to avoid displaying their acne on social media:
- Chose not to include a photograph on social media
- Deleted or untagged a photograph that showed their acne
- Asked someone else to take down a photograph because it showed their acne
- Altered, edited, retouched, or cropped a photograph to try and hide their acne
- Avoided having their picture taken with someone who had clearer skin
- Stayed off social media so they would not have to post or see photographs of themselves
Dermatologists should be aware of the psychosocial impact of acne in teenagers to provide effective management strategies. Although the majority of teens (61%) said they were doing everything possible to manage their acne, 1 in 3 respondents admitted to having difficulty managing their condition. To effectively treat acne, more than three-quarters said that it was at least very important to use a therapy that worked quickly (83%) and was affordable (80%) and easy to use (78%). Be sure to address the psychosocial impact of acne with your teenaged patients, especially pertaining to social media.