User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Best of Acne: 2017
The Best of Acne collection consists of our top-accessed content online this year on acne in one convenient file.
Topics include:
- Hormonal therapies such as oral contraceptives and spironolactone
- Oral therapies
- Alternative therapies for acne scarring
- Patient management in populations such as children
Gary Goldenberg, MD, Digital Editor, Cutis, provides commentary on the top 10 articles.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage acne patients.
The Best of Acne collection consists of our top-accessed content online this year on acne in one convenient file.
Topics include:
- Hormonal therapies such as oral contraceptives and spironolactone
- Oral therapies
- Alternative therapies for acne scarring
- Patient management in populations such as children
Gary Goldenberg, MD, Digital Editor, Cutis, provides commentary on the top 10 articles.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage acne patients.
The Best of Acne collection consists of our top-accessed content online this year on acne in one convenient file.
Topics include:
- Hormonal therapies such as oral contraceptives and spironolactone
- Oral therapies
- Alternative therapies for acne scarring
- Patient management in populations such as children
Gary Goldenberg, MD, Digital Editor, Cutis, provides commentary on the top 10 articles.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage acne patients.

Lichen Planus and Lichenoid Drug Eruption After Vaccination
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
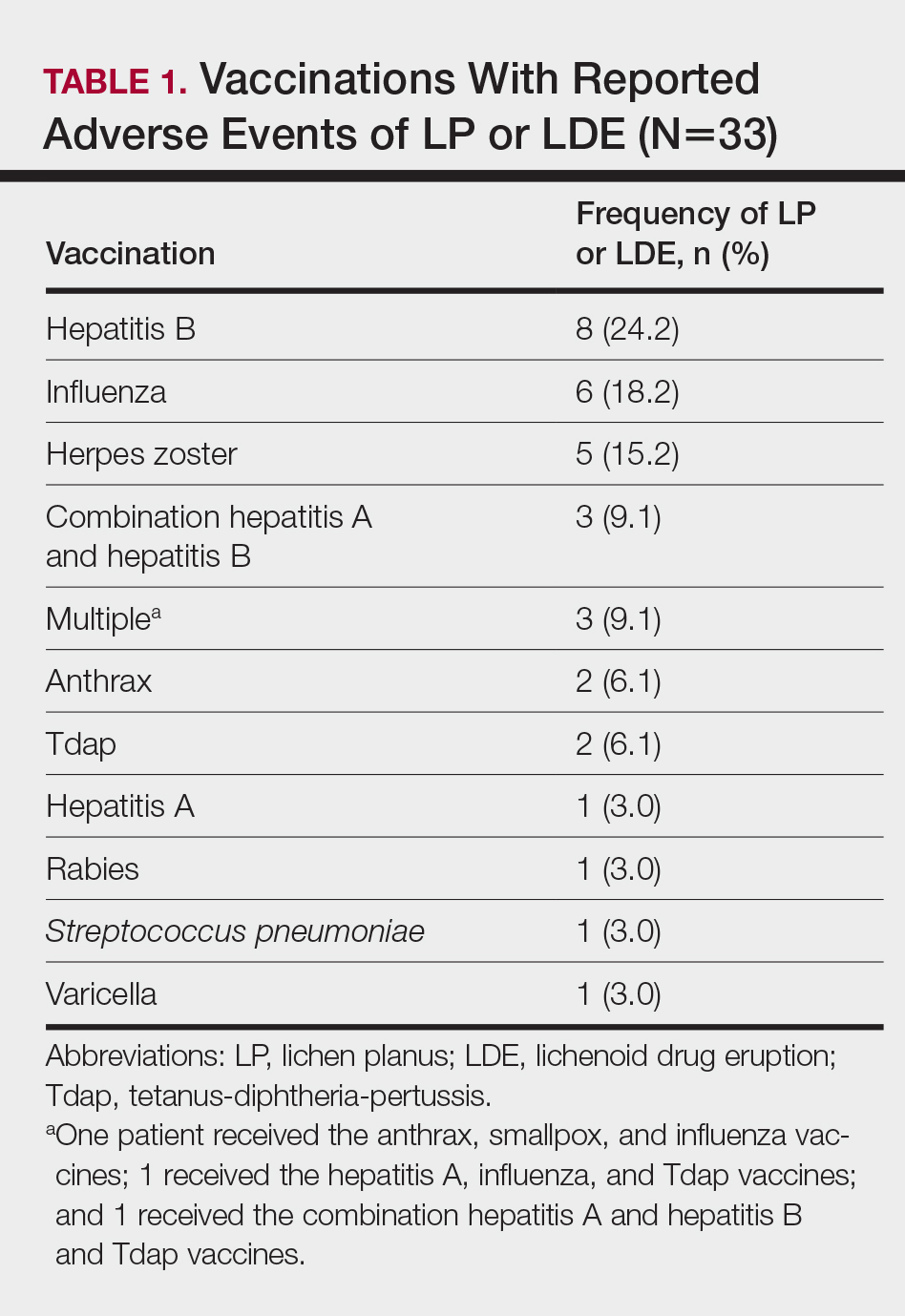
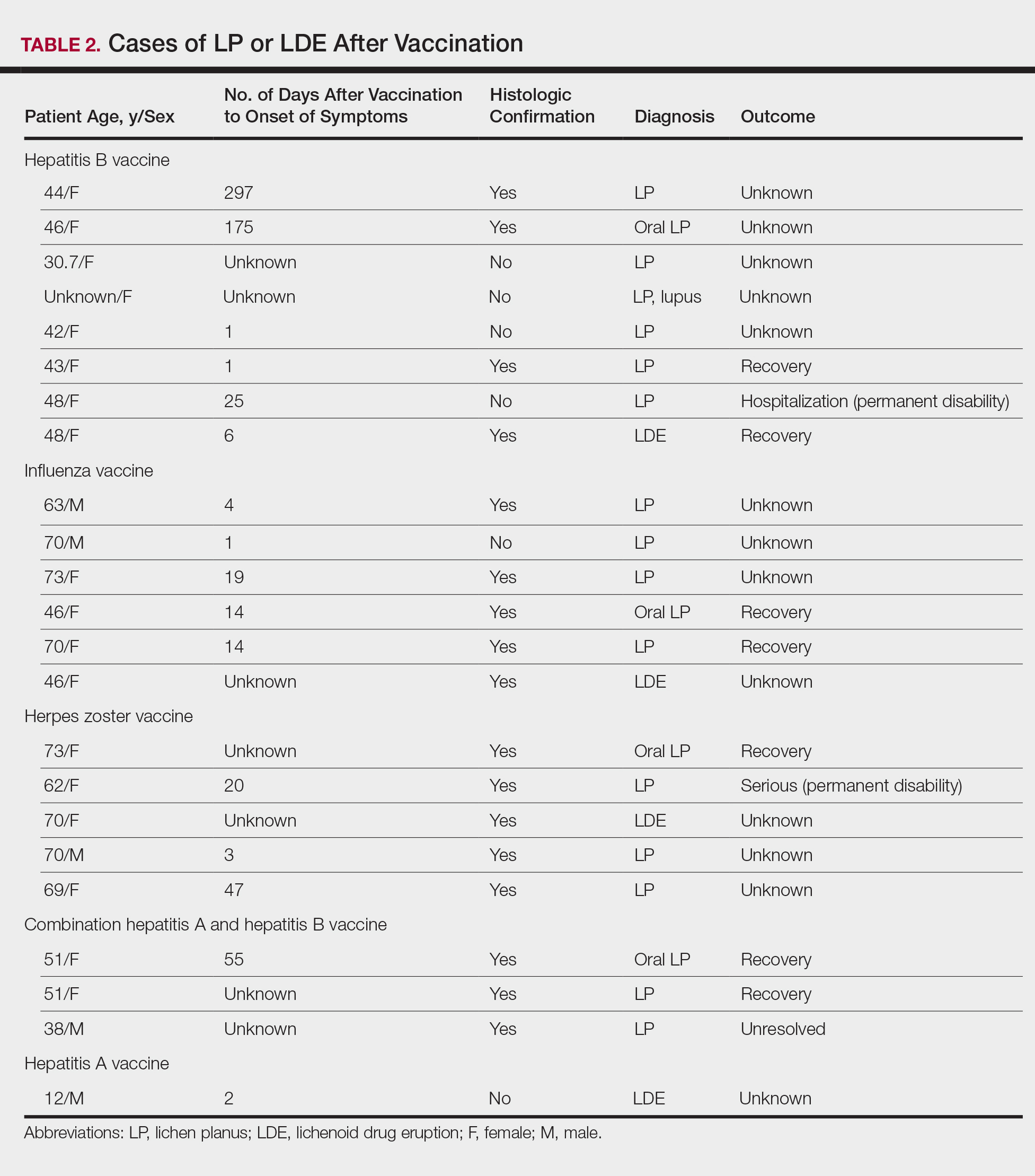
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.
Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
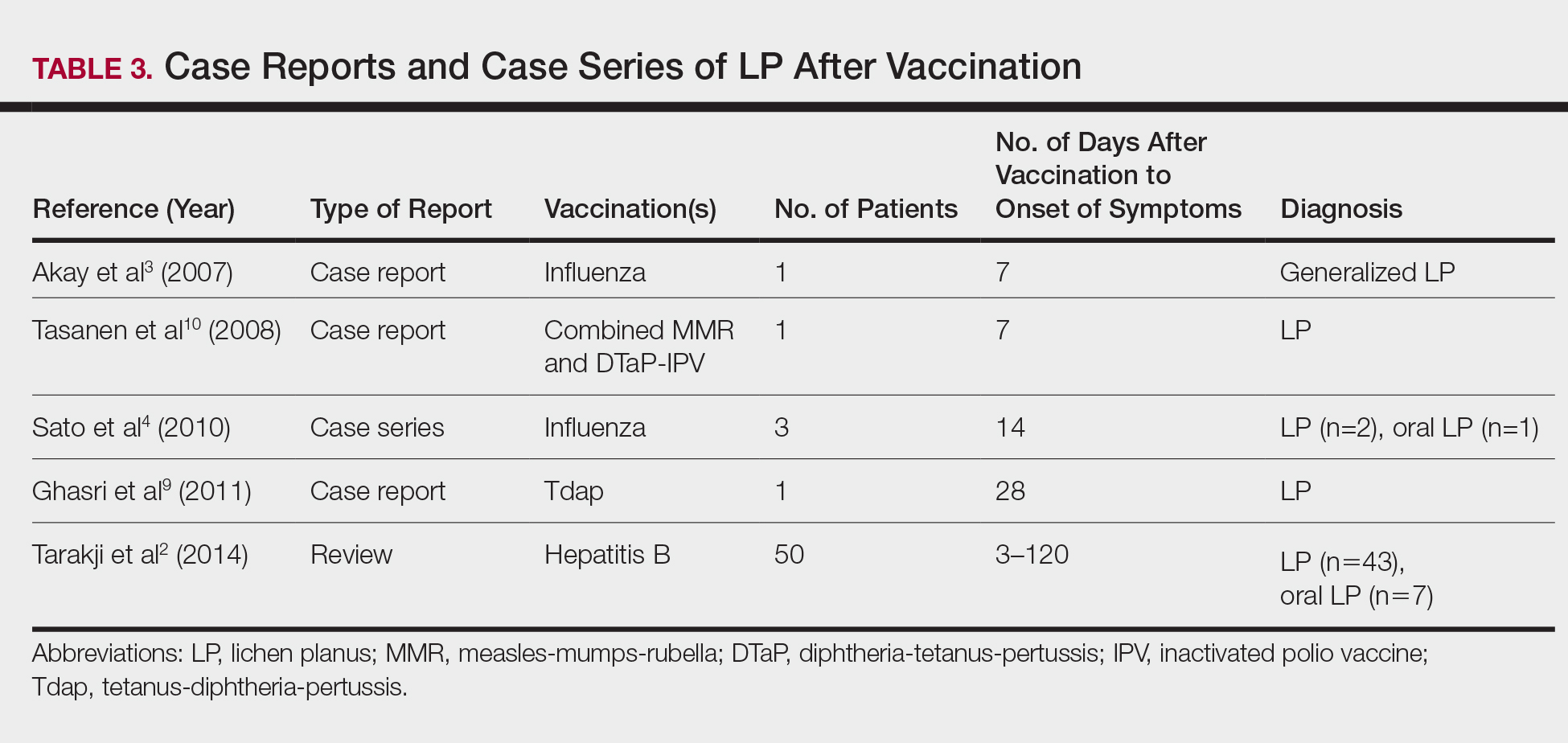
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.
The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.
Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.
The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.
Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.
The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
Practice Points
- Lichen planus (LP) and lichenoid drug eruptions (LDEs) can uncommonly occur after vaccination.
- Common vaccines associated with LP and LDEs include hepatitis B and influenza vaccinations.
- It is important to be cognizant of such reactions, especially in patients who have recently received these common vaccines.
A Peek at Our December 2017 Issue
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NORD Welcomes Five New Member Organizations
As the leading advocacy organization for the 30 million Americans with rare diseases, NORD works closely with its more than 260 member organizations representing specific rare disease communities. The NORD board has approved five additional organizations for NORD membership.
They are the Advocacy & Awareness for Immune Disorders Association; Amniotic Fluid Embolism (AFE) Foundation; Atypical HUS Foundation; HCU (Homocystinuria) Network America; and Lung Transplant Foundation.
As the leading advocacy organization for the 30 million Americans with rare diseases, NORD works closely with its more than 260 member organizations representing specific rare disease communities. The NORD board has approved five additional organizations for NORD membership.
They are the Advocacy & Awareness for Immune Disorders Association; Amniotic Fluid Embolism (AFE) Foundation; Atypical HUS Foundation; HCU (Homocystinuria) Network America; and Lung Transplant Foundation.
As the leading advocacy organization for the 30 million Americans with rare diseases, NORD works closely with its more than 260 member organizations representing specific rare disease communities. The NORD board has approved five additional organizations for NORD membership.
They are the Advocacy & Awareness for Immune Disorders Association; Amniotic Fluid Embolism (AFE) Foundation; Atypical HUS Foundation; HCU (Homocystinuria) Network America; and Lung Transplant Foundation.
Beta Version of ClinicalTrials.gov Website Available for Public Testing
In an effort to make the primary national website for clinical trial postings more user-friendly, a beta version of an updated and enhanced site is now available for public testing. Comments, questions, and suggestions on this new version are welcomed. ClinicalTrials.gov is hosted by the National Library of Medicine of the National Institutes of Health.
In an effort to make the primary national website for clinical trial postings more user-friendly, a beta version of an updated and enhanced site is now available for public testing. Comments, questions, and suggestions on this new version are welcomed. ClinicalTrials.gov is hosted by the National Library of Medicine of the National Institutes of Health.
In an effort to make the primary national website for clinical trial postings more user-friendly, a beta version of an updated and enhanced site is now available for public testing. Comments, questions, and suggestions on this new version are welcomed. ClinicalTrials.gov is hosted by the National Library of Medicine of the National Institutes of Health.
An Atypical Syphilis Presentation
Syphilis is a chronic systemic infection that has been allotted the epithet “the great imitator” for its gross and histologic similarity to numerous other skin pathologies. Well-characterized for centuries, syphilis features diverse clinical manifestations including a number of cutaneous symptoms.1
RELATED AUDIOCAST: The Syphilis Epidemic: Dermatologists on the Frontline of Treatment and Diagnosis
The primary stage of infection is classically defined by an asymptomatic chancre at the inoculation site. The secondary stage results from the systemic dissemination of the infection and typically is characterized by cutaneous eruptions, regional lymphadenopathy, and flulike symptoms. This stage gained its notoriety as the great imitator owing to its ability to present with a variety of papulosquamous eruptions. The secondary stage is followed by an asymptomatic latent period that may last months to years, followed by the tertiary stage, which is characterized by the neurologic, cardiovascular, and/or gummatous manifestations that represent the major sources of morbidity and mortality associated with syphilis. It is during the primary, secondary, and early latent stages that the infection is communicable.1
Case Report
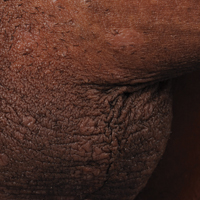
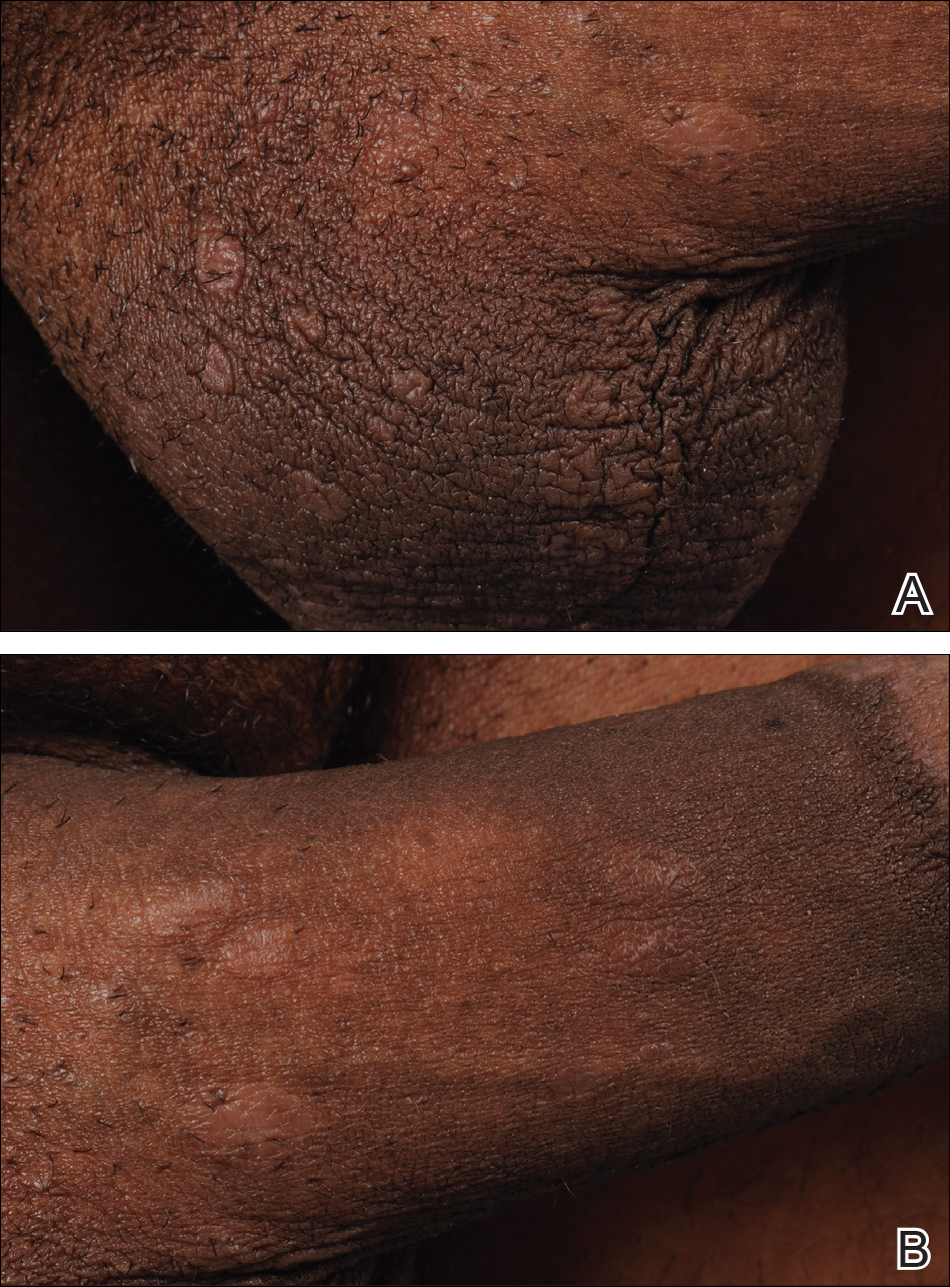
A 40-year-old man presented with multiple intensely pruritic, scattered, erythematous and slightly violaceous, flat-topped papules on the scrotum (Figure 1A) and penile shaft (Figure 1B) of 1 week’s duration. Some of these lesions were annular in appearance. The patient denied any other dermatologic concerns and showed no other skin lesions. A shave biopsy of the right side of the penile shaft was performed, revealing minimal papillary dermis and superficial perivascular dermatitis with substantial perivascular plasmalymphocytic infiltration. The epidermal layer was mildly acanthotic with parakeratosis. A tentative diagnosis of secondary syphilis of unknown latency was made and confirmatory laboratory studies were ordered.
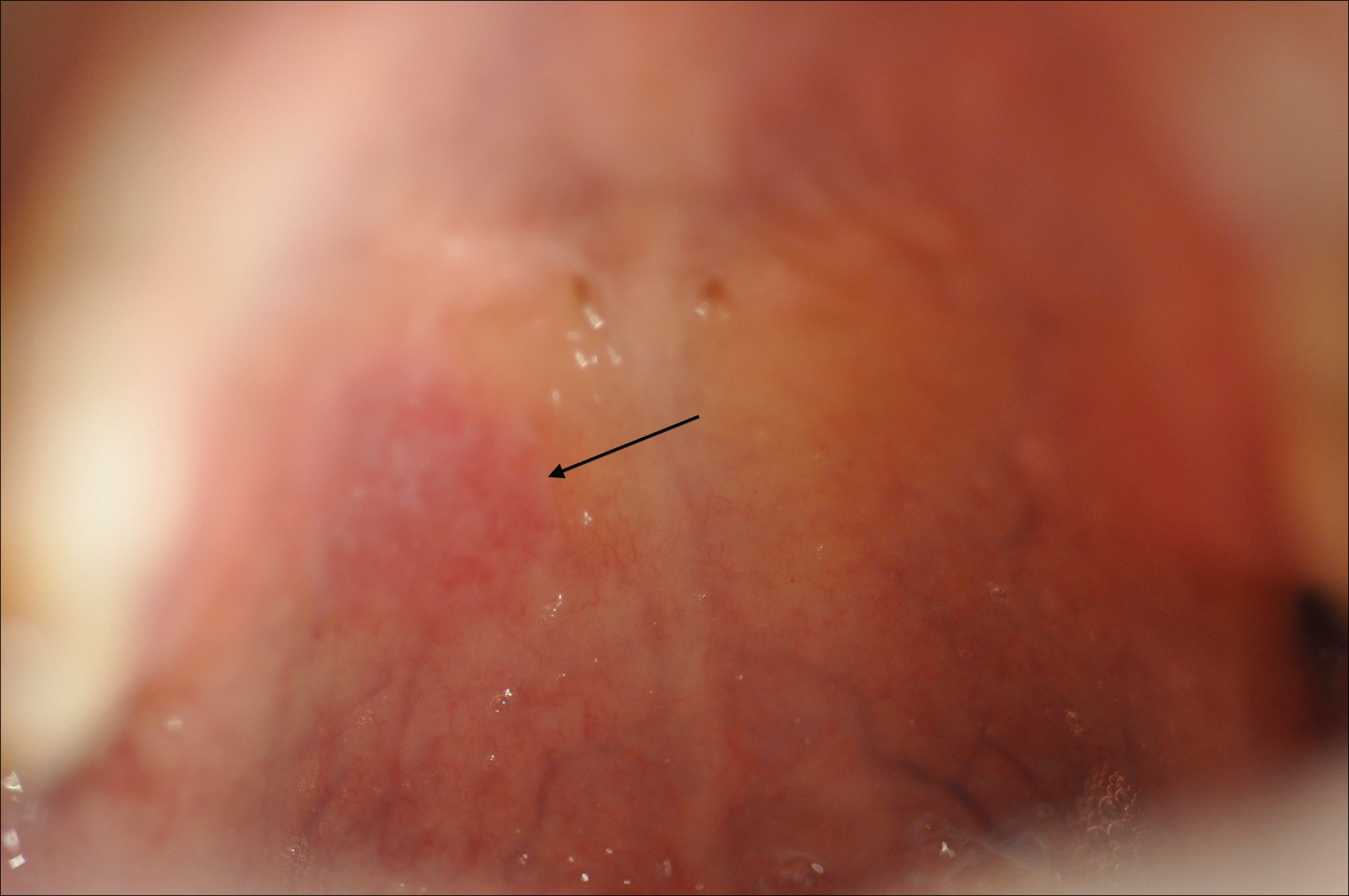
Within weeks, the patient developed a painful 7-mm white patch on the right lower mucosal lip followed several days later by the appearance of a painful lesion on the hard palate (Figure 2 [arrow indicates palatal lesion]) and odynophagia. He presented to the emergency department roughly 3 weeks from the time of index presentation and was started empirically on amoxicillin 500 mg 3 times daily for 10 days for suspicion of strep throat. At a scheduled follow-up with his dermatologist 1 week later, physical examination showed complete resolution of the mucosal lip patch and genital lesions. A round erythematous patch on the right hard palate consistent with a resolving mucosal patch also was noted. A diagnosis of secondary syphilitic infection was made with a rapid plasma reagin (RPR) titer of 1:32 (reference range, <1:1) and positive Treponema antibodies. The patient was treated with a single dose of intramuscular benzathine penicillin G 2.4 million U to prevent the development of tertiary syphilis.
Comment
Incidence
Syphilis has been well characterized since the early 15th century, though its geographic origin remains a topic of controversy.2 Although acquired syphilis infections represented a major source of morbidity and mortality in the early 20th century, the prevalence of syphilis in the United States declined substantially thereafter due to improved public health management.2 Syphilis was relatively rare in the United States by the year 1956, with fewer than 7000 cases of primary and secondary disease reported annually.3 The incidence of primary and secondary syphilis infections in the United States increased gradually until 1990 before declining precipitously and reaching an unprecedented low of 2.2 cases per 100,000 individuals in 2000.4 These shifts ultimately have resulted in decreased clinical familiarity with the disease presentation of syphilis among many health care providers. Since 2000, the incidence of syphilis infection has increased in the United States, with the greatest increases seen in men who have sex with men, intravenous drug users, and human immunodeficiency virus–infected individuals.5-7
RELATED ARTICLE: Syphilis and the Dermatologist
Pathogenesis and Transmission
The causative agent in syphilis infection is the bacterium Treponema pallidum, a member of the family Spirochaetaceae, which is distinguished by its thin, regularly coiled form and distinctive corkscrew motility.8 Syphilis is communicated primarily by sexual contact or in utero exposure during the primary and secondary stages of maternal infection.9 At the time of presentation, our patient denied having any new sexual partners or practices. He reported a monogamous heterosexual relationship within the months preceding presentation, suggesting historical inaccuracy on the part of the patient or probable infidelity in the reported relationship as an alternative means of infection transmission. Untreated individuals may be contagious for longer than 1 year,9 making transmission patterns difficult to track clinically.
Presentation
The clinical presentation of infection with T pallidum results from dual humoral and cell-mediated inflammatory responses in the host. The primary stage is classically defined by a single chancre, which develops at the inoculation site(s) 9 to 90 days following exposure. The chancre typically begins as a small papule that rapidly develops into a painless ulcer characterized by an indurated border, red base, bordering edema, and a diameter of 2 cm or less. Indolent regional lymphadenopathy often is observed in conjunction with the primary chancre.10 Our case is notable for the absence of a primary syphilitic lesion and lack of adenopathy. The primary chancre of syphilis typically resolves within 3 to 6 weeks of onset regardless of whether the patient is treated,4 thus suggesting the rare possibility that our patient developed a painless primary chancre without realizing it.
The secondary stage of syphilis infection arises weeks to months after resolution of the primary chancre and is triggered by hematogenous and lymphatic dissemination of the bacteria. The symptoms of secondary syphilis are primarily flulike and may include headache, malaise, fatigue, sore throat, arthralgia, and low-grade fever.9 Nontender regional lymphadenopathy and splenomegaly also have been reported.11 Our patient denied any systemic concerns throughout the duration of his illness, with the exception of odynophagia in association with ulceration of the oral mucosa. Abnormal laboratory findings in secondary syphilis are nonspecific and may include an elevated erythrocyte sedimentation rate and/or an increased white blood cell count with absolute lymphocytosis.12 Laboratory studies drawn at the time of presentation showed no such abnormalities in our patient.
The cutaneous signs of secondary syphilis arise concurrently with systemic manifestations and are a common finding, with lesions of the skin or oral mucosa present in up to 80% of patients,13 as in our case. Oral lesions classically involve ulcerations at the tip and sides of the tongue,12 which is distinct from our patient who developed oral lesions of the mucosal lip and hard palate.
Secondary syphilis classically features a copper-colored maculopapular rash with sharply delineated margins typically present on the palmar and plantar surfaces.14 Verrucous lesions appearing as moist exophytic plaques on the genitals, intertriginous areas, and/or perineum also have been described and are referred to as condyloma lata in the setting of secondary syphilis.15 In contrast to these classic findings, our patient demonstrated lichenoid lesions on the genitalia and white mucosal patches on the oral mucosa. Our case also was highly unusual because of the intense pruritus associated with the genital lesions, which starkly contrasts most secondary-stage cutaneous lesions that are classically asymptomatic.14 Additionally, our case was distinctive due to the lack of palmar or plantar involvement, which is considered a characteristic feature of secondary cutaneous syphilis.1 Finally, our case was notable for the presence of multiple annular cutaneous lesions, which indicated a late secondary-stage infection during which involution of the lesions produced endarteritis as deeper vessels became involved. A 20-year retrospective study by Abell et al11 demonstrated that 40% of syphilitic rashes are macular, 40% are maculopapular, 10% are papular (as in our case), and the remaining 10% are not easily grouped within these categories.
Differential Diagnosis
It has been estimated that approximately 8% of cutaneous syphilitic lesions demonstrate morphology and distributions suggestive of other dermatologic conditions, including atopic dermatitis, pityriasis rosea, psoriasis, drug-induced eruptions, erythema multiforme, mycosis fungoides, and far more uncommonly lichenoid lesions,16,17 as in our case.
Histopathology
It has been demonstrated that the gross appearance of the secondary syphilitic lesion depends both on the degree of inflammatory infiltrate and the extent of vascular involvement producing ischemia of the skin.1 Our case presented with small, flat-topped, papular lesions that grossly resembled lichen planus and were ultimately shown to be the product of dense lymphomononuclear infiltration extending perivascularly and throughout the superficial and deep dermis.
Biopsy of a lesion is one means of diagnosis, though the histologic appearance of secondary syphilis can mimic many other diseases. In primary and secondary syphilis, skin biopsy characteristically shows central thinning or ulceration of the epidermal layer with heavy dermal lymphocyte infiltration, lymphovascular proliferation with endarteritis, small-vessel thrombosis, and dermal necrosis. Lichen planus–type dermatitis is histologically characterized by hyperkeratosis, irregular epidermal hyperplasia, and a dermoepidermal junction that may be obscured by a dense lymphomononuclear infiltrate.9 The specimen taken from our patient showed minimal infiltrate in the papillary dermis, suggesting a diagnosis of secondary syphilis with lichenoid features. Despite a gross appearance consistent with lichen planus, the biopsy lacked the hydropic degeneration of the basal layer and keratinocyte necrosis that typically characterize this condition.
Diagnosis
Serologic testing for syphilis infection is comprised of nontreponemal and treponemal studies. Nontreponemal testing, which includes the RPR and VDRL test, detects antibodies to cardiolipin-lecithin antigen, a lipid component of the cell membranes of T pallidum. Because the specificity of these tests is fairly low, they typically are used only for screening and monitoring of disease progression and/or response to treatment. Approximately 25% of cases in the United States of primary syphilis are not detected by nontreponemal testing, whereas a nonreactive test nearly always excludes a diagnosis of secondary or latent-stage syphilitic infection.9 Indeed, nontreponemal studies show the highest antibody titers during the late secondary and early latent stages of infection with declining titers thereafter, even in the absence of antibiotic treatment. In our case, diagnosis was made by biopsy and RPR was used for staging; RPR was reactive at a dilution of 1:32, indicative of secondary or early latent infection.
Treponemal testing, which includes the fluorescent treponemal antibody absorption test, and multiplex flow immunoassay detects antibodies that are specific to syphilis infection. Treponemal antibodies are detectable earlier in the course of infection than nontreponemal antibodies and remain permanently detectable even following treatment. Because of its high specificity, treponemal testing often is used to confirm diagnosis after positive screening with nontreponemal tests.4 Positive fluorescent treponemal antibody absorption testing and positive multiplex flow immunoassay may be used to confirm the diagnosis of T pallidum infection.
The tertiary stage of syphilis infection can occur years after conclusion of the secondary stage and is comprised of one or more of the following: gummas, aortic dilatation or dissection, and neurosyphilitic manifestations such as tabes dorsalis or general paresis.1 It is of vital importance to identify syphilis infection prior to the onset of the tertiary stage to prevent substantial morbidity and mortality.
Treatment
Our patient’s symptoms abated after empiric treatment with amoxicillin for presumed streptococcal throat infection after he presented to the emergency department with odynophagia, which is not surprising given the moderate-spectrum coverage of this β-lactam antibiotic as well as the near-complete susceptibility of Treponema spirochetes to amoxicillin in primary and secondary syphilis with notably lower efficacy in latent or tertiary disease. It was essential to treat the patient with a single dose of intramuscular benzathine penicillin G 2.4 million U, which has been shown to reliably prevent recurrence of infection or progression to tertiary syphilis.18
Conclusion
We present a rare case of lichenoid secondary syphilis in the absence of lesions on the palmar and plantar surfaces. The patient lacked any other cutaneous or systemic manifestations, except for odynophagia in association with oral mucosal lesions. He denied any new sexual partners and did not recall having a primary chancre. Also strikingly unusual in this case was the intense pruritus associated with the genital eruption, which is unlike the classic lack of symptoms experienced in the great majority of eruptions due to secondary syphilis. A clinical appreciation of the many cutaneous manifestations of syphilis infection remains critical to early identification of the disease prior to progression to the tertiary stage and its devastating sequelae.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Kilmarx PH, St Louis ME. The evolving epidemiology of syphilis. Am J Public Health. 1995;85(8, pt 1):1053-1054.
- Patton ME, Su JR, Nelson R, et al. Primary and secondary syphilis—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:402-406.
- Coffin LS, Newberry A, Hagan H, et al. Syphilis in drug users in low and middle income countries. Int J Drug Policy. 2010;21:20-27.
- Gao L, Zhang L, Jin Q. Meta-analysis: prevalence of HIV infection and syphilis among MSM in China. Sex Transm Infect. 2009;85:354-358.
- Karp G, Schlaeffer F, Jotkowitz A, et al. Syphilis and HIV co-infection. Eur J Int Med. 2009;20:9-13.
- Hol EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121:4584-4592.
- Schnirring-Judge M, Gustaferro C, Terol C. Vesiculobullous syphilis: a case involving an unusual cutaneous manifestation of secondary syphilis. J Foot Ankle Surg. 2011;50:96-101.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Abell E, Marks R, Jones W. Secondary syphilis: a clinicopathological review. Br J Dermatol. 1975;93:53-61.
- Fiumara N. The treponematoses. Int Dermatol. 1992;1:953-974.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Morton RS. The treponematoses. In: Champion RH, Bourton JL, Burns DA, et al. Rook’s Textbook of Dermatology. 6th ed. London, United Kingdom: Blackwell Science; 1998:1237-1275.
- Rosen T, Hwong H. Pedal interdigital condylomata lata: a rare sign of secondary syphilis. Sex Transm Dis. 2001;28:184-186.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Tang MBY, Yosipovitch G, Tan SH. Secondary syphilis presenting as a lichen planus-like rash. J Eur Acad Dermatol Venereol. 2004;18:185-187.
- Onoda Y. Clinical evaluation of amoxicillin in the treatment of syphilis. J Int Med. 1979;7:539-545.
Syphilis is a chronic systemic infection that has been allotted the epithet “the great imitator” for its gross and histologic similarity to numerous other skin pathologies. Well-characterized for centuries, syphilis features diverse clinical manifestations including a number of cutaneous symptoms.1
RELATED AUDIOCAST: The Syphilis Epidemic: Dermatologists on the Frontline of Treatment and Diagnosis
The primary stage of infection is classically defined by an asymptomatic chancre at the inoculation site. The secondary stage results from the systemic dissemination of the infection and typically is characterized by cutaneous eruptions, regional lymphadenopathy, and flulike symptoms. This stage gained its notoriety as the great imitator owing to its ability to present with a variety of papulosquamous eruptions. The secondary stage is followed by an asymptomatic latent period that may last months to years, followed by the tertiary stage, which is characterized by the neurologic, cardiovascular, and/or gummatous manifestations that represent the major sources of morbidity and mortality associated with syphilis. It is during the primary, secondary, and early latent stages that the infection is communicable.1
Case Report
A 40-year-old man presented with multiple intensely pruritic, scattered, erythematous and slightly violaceous, flat-topped papules on the scrotum (Figure 1A) and penile shaft (Figure 1B) of 1 week’s duration. Some of these lesions were annular in appearance. The patient denied any other dermatologic concerns and showed no other skin lesions. A shave biopsy of the right side of the penile shaft was performed, revealing minimal papillary dermis and superficial perivascular dermatitis with substantial perivascular plasmalymphocytic infiltration. The epidermal layer was mildly acanthotic with parakeratosis. A tentative diagnosis of secondary syphilis of unknown latency was made and confirmatory laboratory studies were ordered.

Within weeks, the patient developed a painful 7-mm white patch on the right lower mucosal lip followed several days later by the appearance of a painful lesion on the hard palate (Figure 2 [arrow indicates palatal lesion]) and odynophagia. He presented to the emergency department roughly 3 weeks from the time of index presentation and was started empirically on amoxicillin 500 mg 3 times daily for 10 days for suspicion of strep throat. At a scheduled follow-up with his dermatologist 1 week later, physical examination showed complete resolution of the mucosal lip patch and genital lesions. A round erythematous patch on the right hard palate consistent with a resolving mucosal patch also was noted. A diagnosis of secondary syphilitic infection was made with a rapid plasma reagin (RPR) titer of 1:32 (reference range, <1:1) and positive Treponema antibodies. The patient was treated with a single dose of intramuscular benzathine penicillin G 2.4 million U to prevent the development of tertiary syphilis.

Comment
Incidence
Syphilis has been well characterized since the early 15th century, though its geographic origin remains a topic of controversy.2 Although acquired syphilis infections represented a major source of morbidity and mortality in the early 20th century, the prevalence of syphilis in the United States declined substantially thereafter due to improved public health management.2 Syphilis was relatively rare in the United States by the year 1956, with fewer than 7000 cases of primary and secondary disease reported annually.3 The incidence of primary and secondary syphilis infections in the United States increased gradually until 1990 before declining precipitously and reaching an unprecedented low of 2.2 cases per 100,000 individuals in 2000.4 These shifts ultimately have resulted in decreased clinical familiarity with the disease presentation of syphilis among many health care providers. Since 2000, the incidence of syphilis infection has increased in the United States, with the greatest increases seen in men who have sex with men, intravenous drug users, and human immunodeficiency virus–infected individuals.5-7
RELATED ARTICLE: Syphilis and the Dermatologist
Pathogenesis and Transmission
The causative agent in syphilis infection is the bacterium Treponema pallidum, a member of the family Spirochaetaceae, which is distinguished by its thin, regularly coiled form and distinctive corkscrew motility.8 Syphilis is communicated primarily by sexual contact or in utero exposure during the primary and secondary stages of maternal infection.9 At the time of presentation, our patient denied having any new sexual partners or practices. He reported a monogamous heterosexual relationship within the months preceding presentation, suggesting historical inaccuracy on the part of the patient or probable infidelity in the reported relationship as an alternative means of infection transmission. Untreated individuals may be contagious for longer than 1 year,9 making transmission patterns difficult to track clinically.
Presentation
The clinical presentation of infection with T pallidum results from dual humoral and cell-mediated inflammatory responses in the host. The primary stage is classically defined by a single chancre, which develops at the inoculation site(s) 9 to 90 days following exposure. The chancre typically begins as a small papule that rapidly develops into a painless ulcer characterized by an indurated border, red base, bordering edema, and a diameter of 2 cm or less. Indolent regional lymphadenopathy often is observed in conjunction with the primary chancre.10 Our case is notable for the absence of a primary syphilitic lesion and lack of adenopathy. The primary chancre of syphilis typically resolves within 3 to 6 weeks of onset regardless of whether the patient is treated,4 thus suggesting the rare possibility that our patient developed a painless primary chancre without realizing it.
The secondary stage of syphilis infection arises weeks to months after resolution of the primary chancre and is triggered by hematogenous and lymphatic dissemination of the bacteria. The symptoms of secondary syphilis are primarily flulike and may include headache, malaise, fatigue, sore throat, arthralgia, and low-grade fever.9 Nontender regional lymphadenopathy and splenomegaly also have been reported.11 Our patient denied any systemic concerns throughout the duration of his illness, with the exception of odynophagia in association with ulceration of the oral mucosa. Abnormal laboratory findings in secondary syphilis are nonspecific and may include an elevated erythrocyte sedimentation rate and/or an increased white blood cell count with absolute lymphocytosis.12 Laboratory studies drawn at the time of presentation showed no such abnormalities in our patient.
The cutaneous signs of secondary syphilis arise concurrently with systemic manifestations and are a common finding, with lesions of the skin or oral mucosa present in up to 80% of patients,13 as in our case. Oral lesions classically involve ulcerations at the tip and sides of the tongue,12 which is distinct from our patient who developed oral lesions of the mucosal lip and hard palate.
Secondary syphilis classically features a copper-colored maculopapular rash with sharply delineated margins typically present on the palmar and plantar surfaces.14 Verrucous lesions appearing as moist exophytic plaques on the genitals, intertriginous areas, and/or perineum also have been described and are referred to as condyloma lata in the setting of secondary syphilis.15 In contrast to these classic findings, our patient demonstrated lichenoid lesions on the genitalia and white mucosal patches on the oral mucosa. Our case also was highly unusual because of the intense pruritus associated with the genital lesions, which starkly contrasts most secondary-stage cutaneous lesions that are classically asymptomatic.14 Additionally, our case was distinctive due to the lack of palmar or plantar involvement, which is considered a characteristic feature of secondary cutaneous syphilis.1 Finally, our case was notable for the presence of multiple annular cutaneous lesions, which indicated a late secondary-stage infection during which involution of the lesions produced endarteritis as deeper vessels became involved. A 20-year retrospective study by Abell et al11 demonstrated that 40% of syphilitic rashes are macular, 40% are maculopapular, 10% are papular (as in our case), and the remaining 10% are not easily grouped within these categories.
Differential Diagnosis
It has been estimated that approximately 8% of cutaneous syphilitic lesions demonstrate morphology and distributions suggestive of other dermatologic conditions, including atopic dermatitis, pityriasis rosea, psoriasis, drug-induced eruptions, erythema multiforme, mycosis fungoides, and far more uncommonly lichenoid lesions,16,17 as in our case.
Histopathology
It has been demonstrated that the gross appearance of the secondary syphilitic lesion depends both on the degree of inflammatory infiltrate and the extent of vascular involvement producing ischemia of the skin.1 Our case presented with small, flat-topped, papular lesions that grossly resembled lichen planus and were ultimately shown to be the product of dense lymphomononuclear infiltration extending perivascularly and throughout the superficial and deep dermis.
Biopsy of a lesion is one means of diagnosis, though the histologic appearance of secondary syphilis can mimic many other diseases. In primary and secondary syphilis, skin biopsy characteristically shows central thinning or ulceration of the epidermal layer with heavy dermal lymphocyte infiltration, lymphovascular proliferation with endarteritis, small-vessel thrombosis, and dermal necrosis. Lichen planus–type dermatitis is histologically characterized by hyperkeratosis, irregular epidermal hyperplasia, and a dermoepidermal junction that may be obscured by a dense lymphomononuclear infiltrate.9 The specimen taken from our patient showed minimal infiltrate in the papillary dermis, suggesting a diagnosis of secondary syphilis with lichenoid features. Despite a gross appearance consistent with lichen planus, the biopsy lacked the hydropic degeneration of the basal layer and keratinocyte necrosis that typically characterize this condition.
Diagnosis
Serologic testing for syphilis infection is comprised of nontreponemal and treponemal studies. Nontreponemal testing, which includes the RPR and VDRL test, detects antibodies to cardiolipin-lecithin antigen, a lipid component of the cell membranes of T pallidum. Because the specificity of these tests is fairly low, they typically are used only for screening and monitoring of disease progression and/or response to treatment. Approximately 25% of cases in the United States of primary syphilis are not detected by nontreponemal testing, whereas a nonreactive test nearly always excludes a diagnosis of secondary or latent-stage syphilitic infection.9 Indeed, nontreponemal studies show the highest antibody titers during the late secondary and early latent stages of infection with declining titers thereafter, even in the absence of antibiotic treatment. In our case, diagnosis was made by biopsy and RPR was used for staging; RPR was reactive at a dilution of 1:32, indicative of secondary or early latent infection.
Treponemal testing, which includes the fluorescent treponemal antibody absorption test, and multiplex flow immunoassay detects antibodies that are specific to syphilis infection. Treponemal antibodies are detectable earlier in the course of infection than nontreponemal antibodies and remain permanently detectable even following treatment. Because of its high specificity, treponemal testing often is used to confirm diagnosis after positive screening with nontreponemal tests.4 Positive fluorescent treponemal antibody absorption testing and positive multiplex flow immunoassay may be used to confirm the diagnosis of T pallidum infection.
The tertiary stage of syphilis infection can occur years after conclusion of the secondary stage and is comprised of one or more of the following: gummas, aortic dilatation or dissection, and neurosyphilitic manifestations such as tabes dorsalis or general paresis.1 It is of vital importance to identify syphilis infection prior to the onset of the tertiary stage to prevent substantial morbidity and mortality.
Treatment
Our patient’s symptoms abated after empiric treatment with amoxicillin for presumed streptococcal throat infection after he presented to the emergency department with odynophagia, which is not surprising given the moderate-spectrum coverage of this β-lactam antibiotic as well as the near-complete susceptibility of Treponema spirochetes to amoxicillin in primary and secondary syphilis with notably lower efficacy in latent or tertiary disease. It was essential to treat the patient with a single dose of intramuscular benzathine penicillin G 2.4 million U, which has been shown to reliably prevent recurrence of infection or progression to tertiary syphilis.18
Conclusion
We present a rare case of lichenoid secondary syphilis in the absence of lesions on the palmar and plantar surfaces. The patient lacked any other cutaneous or systemic manifestations, except for odynophagia in association with oral mucosal lesions. He denied any new sexual partners and did not recall having a primary chancre. Also strikingly unusual in this case was the intense pruritus associated with the genital eruption, which is unlike the classic lack of symptoms experienced in the great majority of eruptions due to secondary syphilis. A clinical appreciation of the many cutaneous manifestations of syphilis infection remains critical to early identification of the disease prior to progression to the tertiary stage and its devastating sequelae.
Syphilis is a chronic systemic infection that has been allotted the epithet “the great imitator” for its gross and histologic similarity to numerous other skin pathologies. Well-characterized for centuries, syphilis features diverse clinical manifestations including a number of cutaneous symptoms.1
RELATED AUDIOCAST: The Syphilis Epidemic: Dermatologists on the Frontline of Treatment and Diagnosis
The primary stage of infection is classically defined by an asymptomatic chancre at the inoculation site. The secondary stage results from the systemic dissemination of the infection and typically is characterized by cutaneous eruptions, regional lymphadenopathy, and flulike symptoms. This stage gained its notoriety as the great imitator owing to its ability to present with a variety of papulosquamous eruptions. The secondary stage is followed by an asymptomatic latent period that may last months to years, followed by the tertiary stage, which is characterized by the neurologic, cardiovascular, and/or gummatous manifestations that represent the major sources of morbidity and mortality associated with syphilis. It is during the primary, secondary, and early latent stages that the infection is communicable.1
Case Report
A 40-year-old man presented with multiple intensely pruritic, scattered, erythematous and slightly violaceous, flat-topped papules on the scrotum (Figure 1A) and penile shaft (Figure 1B) of 1 week’s duration. Some of these lesions were annular in appearance. The patient denied any other dermatologic concerns and showed no other skin lesions. A shave biopsy of the right side of the penile shaft was performed, revealing minimal papillary dermis and superficial perivascular dermatitis with substantial perivascular plasmalymphocytic infiltration. The epidermal layer was mildly acanthotic with parakeratosis. A tentative diagnosis of secondary syphilis of unknown latency was made and confirmatory laboratory studies were ordered.

Within weeks, the patient developed a painful 7-mm white patch on the right lower mucosal lip followed several days later by the appearance of a painful lesion on the hard palate (Figure 2 [arrow indicates palatal lesion]) and odynophagia. He presented to the emergency department roughly 3 weeks from the time of index presentation and was started empirically on amoxicillin 500 mg 3 times daily for 10 days for suspicion of strep throat. At a scheduled follow-up with his dermatologist 1 week later, physical examination showed complete resolution of the mucosal lip patch and genital lesions. A round erythematous patch on the right hard palate consistent with a resolving mucosal patch also was noted. A diagnosis of secondary syphilitic infection was made with a rapid plasma reagin (RPR) titer of 1:32 (reference range, <1:1) and positive Treponema antibodies. The patient was treated with a single dose of intramuscular benzathine penicillin G 2.4 million U to prevent the development of tertiary syphilis.

Comment
Incidence
Syphilis has been well characterized since the early 15th century, though its geographic origin remains a topic of controversy.2 Although acquired syphilis infections represented a major source of morbidity and mortality in the early 20th century, the prevalence of syphilis in the United States declined substantially thereafter due to improved public health management.2 Syphilis was relatively rare in the United States by the year 1956, with fewer than 7000 cases of primary and secondary disease reported annually.3 The incidence of primary and secondary syphilis infections in the United States increased gradually until 1990 before declining precipitously and reaching an unprecedented low of 2.2 cases per 100,000 individuals in 2000.4 These shifts ultimately have resulted in decreased clinical familiarity with the disease presentation of syphilis among many health care providers. Since 2000, the incidence of syphilis infection has increased in the United States, with the greatest increases seen in men who have sex with men, intravenous drug users, and human immunodeficiency virus–infected individuals.5-7
RELATED ARTICLE: Syphilis and the Dermatologist
Pathogenesis and Transmission
The causative agent in syphilis infection is the bacterium Treponema pallidum, a member of the family Spirochaetaceae, which is distinguished by its thin, regularly coiled form and distinctive corkscrew motility.8 Syphilis is communicated primarily by sexual contact or in utero exposure during the primary and secondary stages of maternal infection.9 At the time of presentation, our patient denied having any new sexual partners or practices. He reported a monogamous heterosexual relationship within the months preceding presentation, suggesting historical inaccuracy on the part of the patient or probable infidelity in the reported relationship as an alternative means of infection transmission. Untreated individuals may be contagious for longer than 1 year,9 making transmission patterns difficult to track clinically.
Presentation
The clinical presentation of infection with T pallidum results from dual humoral and cell-mediated inflammatory responses in the host. The primary stage is classically defined by a single chancre, which develops at the inoculation site(s) 9 to 90 days following exposure. The chancre typically begins as a small papule that rapidly develops into a painless ulcer characterized by an indurated border, red base, bordering edema, and a diameter of 2 cm or less. Indolent regional lymphadenopathy often is observed in conjunction with the primary chancre.10 Our case is notable for the absence of a primary syphilitic lesion and lack of adenopathy. The primary chancre of syphilis typically resolves within 3 to 6 weeks of onset regardless of whether the patient is treated,4 thus suggesting the rare possibility that our patient developed a painless primary chancre without realizing it.
The secondary stage of syphilis infection arises weeks to months after resolution of the primary chancre and is triggered by hematogenous and lymphatic dissemination of the bacteria. The symptoms of secondary syphilis are primarily flulike and may include headache, malaise, fatigue, sore throat, arthralgia, and low-grade fever.9 Nontender regional lymphadenopathy and splenomegaly also have been reported.11 Our patient denied any systemic concerns throughout the duration of his illness, with the exception of odynophagia in association with ulceration of the oral mucosa. Abnormal laboratory findings in secondary syphilis are nonspecific and may include an elevated erythrocyte sedimentation rate and/or an increased white blood cell count with absolute lymphocytosis.12 Laboratory studies drawn at the time of presentation showed no such abnormalities in our patient.
The cutaneous signs of secondary syphilis arise concurrently with systemic manifestations and are a common finding, with lesions of the skin or oral mucosa present in up to 80% of patients,13 as in our case. Oral lesions classically involve ulcerations at the tip and sides of the tongue,12 which is distinct from our patient who developed oral lesions of the mucosal lip and hard palate.
Secondary syphilis classically features a copper-colored maculopapular rash with sharply delineated margins typically present on the palmar and plantar surfaces.14 Verrucous lesions appearing as moist exophytic plaques on the genitals, intertriginous areas, and/or perineum also have been described and are referred to as condyloma lata in the setting of secondary syphilis.15 In contrast to these classic findings, our patient demonstrated lichenoid lesions on the genitalia and white mucosal patches on the oral mucosa. Our case also was highly unusual because of the intense pruritus associated with the genital lesions, which starkly contrasts most secondary-stage cutaneous lesions that are classically asymptomatic.14 Additionally, our case was distinctive due to the lack of palmar or plantar involvement, which is considered a characteristic feature of secondary cutaneous syphilis.1 Finally, our case was notable for the presence of multiple annular cutaneous lesions, which indicated a late secondary-stage infection during which involution of the lesions produced endarteritis as deeper vessels became involved. A 20-year retrospective study by Abell et al11 demonstrated that 40% of syphilitic rashes are macular, 40% are maculopapular, 10% are papular (as in our case), and the remaining 10% are not easily grouped within these categories.
Differential Diagnosis
It has been estimated that approximately 8% of cutaneous syphilitic lesions demonstrate morphology and distributions suggestive of other dermatologic conditions, including atopic dermatitis, pityriasis rosea, psoriasis, drug-induced eruptions, erythema multiforme, mycosis fungoides, and far more uncommonly lichenoid lesions,16,17 as in our case.
Histopathology
It has been demonstrated that the gross appearance of the secondary syphilitic lesion depends both on the degree of inflammatory infiltrate and the extent of vascular involvement producing ischemia of the skin.1 Our case presented with small, flat-topped, papular lesions that grossly resembled lichen planus and were ultimately shown to be the product of dense lymphomononuclear infiltration extending perivascularly and throughout the superficial and deep dermis.
Biopsy of a lesion is one means of diagnosis, though the histologic appearance of secondary syphilis can mimic many other diseases. In primary and secondary syphilis, skin biopsy characteristically shows central thinning or ulceration of the epidermal layer with heavy dermal lymphocyte infiltration, lymphovascular proliferation with endarteritis, small-vessel thrombosis, and dermal necrosis. Lichen planus–type dermatitis is histologically characterized by hyperkeratosis, irregular epidermal hyperplasia, and a dermoepidermal junction that may be obscured by a dense lymphomononuclear infiltrate.9 The specimen taken from our patient showed minimal infiltrate in the papillary dermis, suggesting a diagnosis of secondary syphilis with lichenoid features. Despite a gross appearance consistent with lichen planus, the biopsy lacked the hydropic degeneration of the basal layer and keratinocyte necrosis that typically characterize this condition.
Diagnosis
Serologic testing for syphilis infection is comprised of nontreponemal and treponemal studies. Nontreponemal testing, which includes the RPR and VDRL test, detects antibodies to cardiolipin-lecithin antigen, a lipid component of the cell membranes of T pallidum. Because the specificity of these tests is fairly low, they typically are used only for screening and monitoring of disease progression and/or response to treatment. Approximately 25% of cases in the United States of primary syphilis are not detected by nontreponemal testing, whereas a nonreactive test nearly always excludes a diagnosis of secondary or latent-stage syphilitic infection.9 Indeed, nontreponemal studies show the highest antibody titers during the late secondary and early latent stages of infection with declining titers thereafter, even in the absence of antibiotic treatment. In our case, diagnosis was made by biopsy and RPR was used for staging; RPR was reactive at a dilution of 1:32, indicative of secondary or early latent infection.
Treponemal testing, which includes the fluorescent treponemal antibody absorption test, and multiplex flow immunoassay detects antibodies that are specific to syphilis infection. Treponemal antibodies are detectable earlier in the course of infection than nontreponemal antibodies and remain permanently detectable even following treatment. Because of its high specificity, treponemal testing often is used to confirm diagnosis after positive screening with nontreponemal tests.4 Positive fluorescent treponemal antibody absorption testing and positive multiplex flow immunoassay may be used to confirm the diagnosis of T pallidum infection.
The tertiary stage of syphilis infection can occur years after conclusion of the secondary stage and is comprised of one or more of the following: gummas, aortic dilatation or dissection, and neurosyphilitic manifestations such as tabes dorsalis or general paresis.1 It is of vital importance to identify syphilis infection prior to the onset of the tertiary stage to prevent substantial morbidity and mortality.
Treatment
Our patient’s symptoms abated after empiric treatment with amoxicillin for presumed streptococcal throat infection after he presented to the emergency department with odynophagia, which is not surprising given the moderate-spectrum coverage of this β-lactam antibiotic as well as the near-complete susceptibility of Treponema spirochetes to amoxicillin in primary and secondary syphilis with notably lower efficacy in latent or tertiary disease. It was essential to treat the patient with a single dose of intramuscular benzathine penicillin G 2.4 million U, which has been shown to reliably prevent recurrence of infection or progression to tertiary syphilis.18
Conclusion
We present a rare case of lichenoid secondary syphilis in the absence of lesions on the palmar and plantar surfaces. The patient lacked any other cutaneous or systemic manifestations, except for odynophagia in association with oral mucosal lesions. He denied any new sexual partners and did not recall having a primary chancre. Also strikingly unusual in this case was the intense pruritus associated with the genital eruption, which is unlike the classic lack of symptoms experienced in the great majority of eruptions due to secondary syphilis. A clinical appreciation of the many cutaneous manifestations of syphilis infection remains critical to early identification of the disease prior to progression to the tertiary stage and its devastating sequelae.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Kilmarx PH, St Louis ME. The evolving epidemiology of syphilis. Am J Public Health. 1995;85(8, pt 1):1053-1054.
- Patton ME, Su JR, Nelson R, et al. Primary and secondary syphilis—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:402-406.
- Coffin LS, Newberry A, Hagan H, et al. Syphilis in drug users in low and middle income countries. Int J Drug Policy. 2010;21:20-27.
- Gao L, Zhang L, Jin Q. Meta-analysis: prevalence of HIV infection and syphilis among MSM in China. Sex Transm Infect. 2009;85:354-358.
- Karp G, Schlaeffer F, Jotkowitz A, et al. Syphilis and HIV co-infection. Eur J Int Med. 2009;20:9-13.
- Hol EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121:4584-4592.
- Schnirring-Judge M, Gustaferro C, Terol C. Vesiculobullous syphilis: a case involving an unusual cutaneous manifestation of secondary syphilis. J Foot Ankle Surg. 2011;50:96-101.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Abell E, Marks R, Jones W. Secondary syphilis: a clinicopathological review. Br J Dermatol. 1975;93:53-61.
- Fiumara N. The treponematoses. Int Dermatol. 1992;1:953-974.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Morton RS. The treponematoses. In: Champion RH, Bourton JL, Burns DA, et al. Rook’s Textbook of Dermatology. 6th ed. London, United Kingdom: Blackwell Science; 1998:1237-1275.
- Rosen T, Hwong H. Pedal interdigital condylomata lata: a rare sign of secondary syphilis. Sex Transm Dis. 2001;28:184-186.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Tang MBY, Yosipovitch G, Tan SH. Secondary syphilis presenting as a lichen planus-like rash. J Eur Acad Dermatol Venereol. 2004;18:185-187.
- Onoda Y. Clinical evaluation of amoxicillin in the treatment of syphilis. J Int Med. 1979;7:539-545.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Kilmarx PH, St Louis ME. The evolving epidemiology of syphilis. Am J Public Health. 1995;85(8, pt 1):1053-1054.
- Patton ME, Su JR, Nelson R, et al. Primary and secondary syphilis—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:402-406.
- Coffin LS, Newberry A, Hagan H, et al. Syphilis in drug users in low and middle income countries. Int J Drug Policy. 2010;21:20-27.
- Gao L, Zhang L, Jin Q. Meta-analysis: prevalence of HIV infection and syphilis among MSM in China. Sex Transm Infect. 2009;85:354-358.
- Karp G, Schlaeffer F, Jotkowitz A, et al. Syphilis and HIV co-infection. Eur J Int Med. 2009;20:9-13.
- Hol EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121:4584-4592.
- Schnirring-Judge M, Gustaferro C, Terol C. Vesiculobullous syphilis: a case involving an unusual cutaneous manifestation of secondary syphilis. J Foot Ankle Surg. 2011;50:96-101.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Abell E, Marks R, Jones W. Secondary syphilis: a clinicopathological review. Br J Dermatol. 1975;93:53-61.
- Fiumara N. The treponematoses. Int Dermatol. 1992;1:953-974.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Morton RS. The treponematoses. In: Champion RH, Bourton JL, Burns DA, et al. Rook’s Textbook of Dermatology. 6th ed. London, United Kingdom: Blackwell Science; 1998:1237-1275.
- Rosen T, Hwong H. Pedal interdigital condylomata lata: a rare sign of secondary syphilis. Sex Transm Dis. 2001;28:184-186.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Tang MBY, Yosipovitch G, Tan SH. Secondary syphilis presenting as a lichen planus-like rash. J Eur Acad Dermatol Venereol. 2004;18:185-187.
- Onoda Y. Clinical evaluation of amoxicillin in the treatment of syphilis. J Int Med. 1979;7:539-545.
Practice Points
- Syphilis retains its reputation as “the great imitator” due to its wide variability in clinical presentation and propensity for misdiagnosis.
- Lichenoid syphilis is a well-described cutaneous presentation of secondary syphilis, though the characteristics of these lesions remain highly variable and require a high degree of clinical suspicion.
- Treponema pallidum is partially susceptible to most β-lactam antibiotics in primary and early secondary stages of infection; thus, use of these medications can obscure symptoms without adequately treating the infection.
Poster Abstracts Due January 31 for European Conference on Rare Diseases and Orphan Products
Hosted by EURORDIS, the European Conference on Rare Diseases and Orphan Products is the largest conference in Europe for the rare disease community. More than 800 stakeholders gather to discuss research, development of new treatments, health care, public health policies, and other topics. The conference will take place May 10-12, 2018. Poster abstracts are due Jan. 31.
Hosted by EURORDIS, the European Conference on Rare Diseases and Orphan Products is the largest conference in Europe for the rare disease community. More than 800 stakeholders gather to discuss research, development of new treatments, health care, public health policies, and other topics. The conference will take place May 10-12, 2018. Poster abstracts are due Jan. 31.
Hosted by EURORDIS, the European Conference on Rare Diseases and Orphan Products is the largest conference in Europe for the rare disease community. More than 800 stakeholders gather to discuss research, development of new treatments, health care, public health policies, and other topics. The conference will take place May 10-12, 2018. Poster abstracts are due Jan. 31.
NORD Reopens Call for Abstracts for Research Grants
NORD has reopened the call to submit abstracts and letters of intent for the following research grants. The deadline is ongoing and all US and international researchers interested in studying these diseases are encouraged to consider applying. RFPs are posted on the NORD website.
- Cat Eye Syndrome
- Malonic Aciduria
- Post-Orgasmic Illness Syndrome
NORD has reopened the call to submit abstracts and letters of intent for the following research grants. The deadline is ongoing and all US and international researchers interested in studying these diseases are encouraged to consider applying. RFPs are posted on the NORD website.
- Cat Eye Syndrome
- Malonic Aciduria
- Post-Orgasmic Illness Syndrome
NORD has reopened the call to submit abstracts and letters of intent for the following research grants. The deadline is ongoing and all US and international researchers interested in studying these diseases are encouraged to consider applying. RFPs are posted on the NORD website.
- Cat Eye Syndrome
- Malonic Aciduria
- Post-Orgasmic Illness Syndrome
Nominate a Colleague or Patient for a NORD Rare Impact Award
Nominations are now open for the 2018 NORD Rare Impact Awards. These awards honor individuals who have made a positive impact on the rare disease community through research, patient care, advocacy, or other areas of involvement.
Awards are presented in May each year at NORD’s annual Rare Impact Celebration. Nominations may be submitted online. The deadline is January 12.
Honorees from previous years have included members of Congress, staff and senior officials from NIH and FDA, medical researchers and clinicians, patient organization leaders, and individual patients and caregivers. The awards honor those who have helped to improve the lives of those affected by rare diseases.
Nominations are now open for the 2018 NORD Rare Impact Awards. These awards honor individuals who have made a positive impact on the rare disease community through research, patient care, advocacy, or other areas of involvement.
Awards are presented in May each year at NORD’s annual Rare Impact Celebration. Nominations may be submitted online. The deadline is January 12.
Honorees from previous years have included members of Congress, staff and senior officials from NIH and FDA, medical researchers and clinicians, patient organization leaders, and individual patients and caregivers. The awards honor those who have helped to improve the lives of those affected by rare diseases.
Nominations are now open for the 2018 NORD Rare Impact Awards. These awards honor individuals who have made a positive impact on the rare disease community through research, patient care, advocacy, or other areas of involvement.
Awards are presented in May each year at NORD’s annual Rare Impact Celebration. Nominations may be submitted online. The deadline is January 12.
Honorees from previous years have included members of Congress, staff and senior officials from NIH and FDA, medical researchers and clinicians, patient organization leaders, and individual patients and caregivers. The awards honor those who have helped to improve the lives of those affected by rare diseases.
Webinar Recording on Open Enrollment Available
With the December 15th deadline for selecting an Affordable Care Act health plan fast approaching, NORD hosted and recorded a free public webinar that now is available online. The webinar helps patients with rare disease and caregivers navigate what can be a confusing process.
It addresses how to enroll in an insurance plan, the difference between federal and state exchanges, key dates to keep in mind, and more. Access the recording.
With the December 15th deadline for selecting an Affordable Care Act health plan fast approaching, NORD hosted and recorded a free public webinar that now is available online. The webinar helps patients with rare disease and caregivers navigate what can be a confusing process.
It addresses how to enroll in an insurance plan, the difference between federal and state exchanges, key dates to keep in mind, and more. Access the recording.
With the December 15th deadline for selecting an Affordable Care Act health plan fast approaching, NORD hosted and recorded a free public webinar that now is available online. The webinar helps patients with rare disease and caregivers navigate what can be a confusing process.
It addresses how to enroll in an insurance plan, the difference between federal and state exchanges, key dates to keep in mind, and more. Access the recording.