User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Petrolatum Is Effective as a Moisturizer, But There Are More Uses for It
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
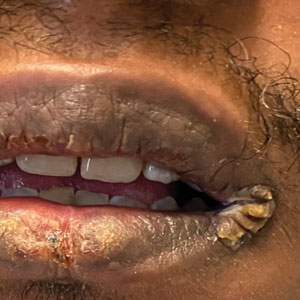
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Learning Experiences in LGBT Health During Dermatology Residency
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
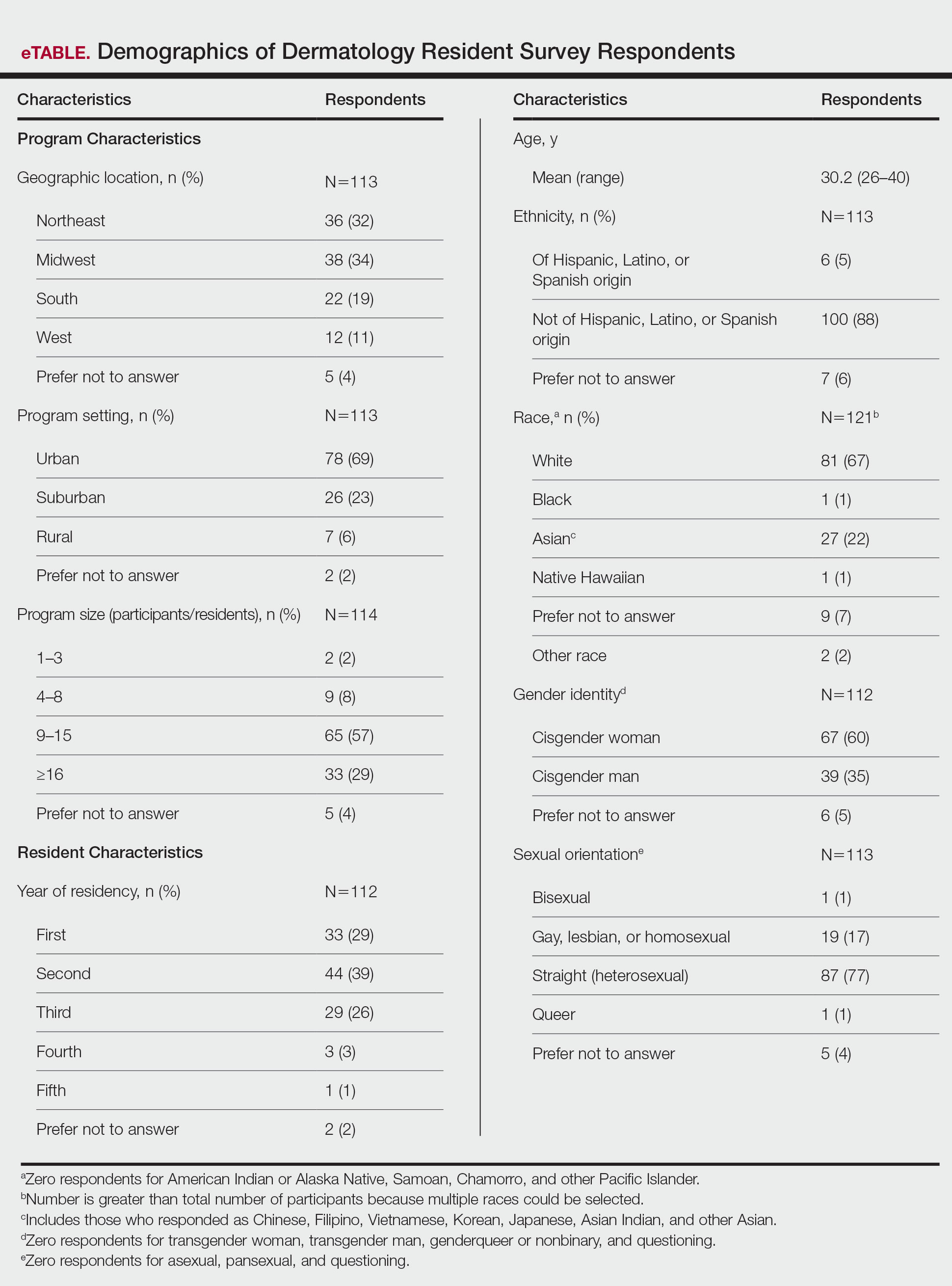
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.
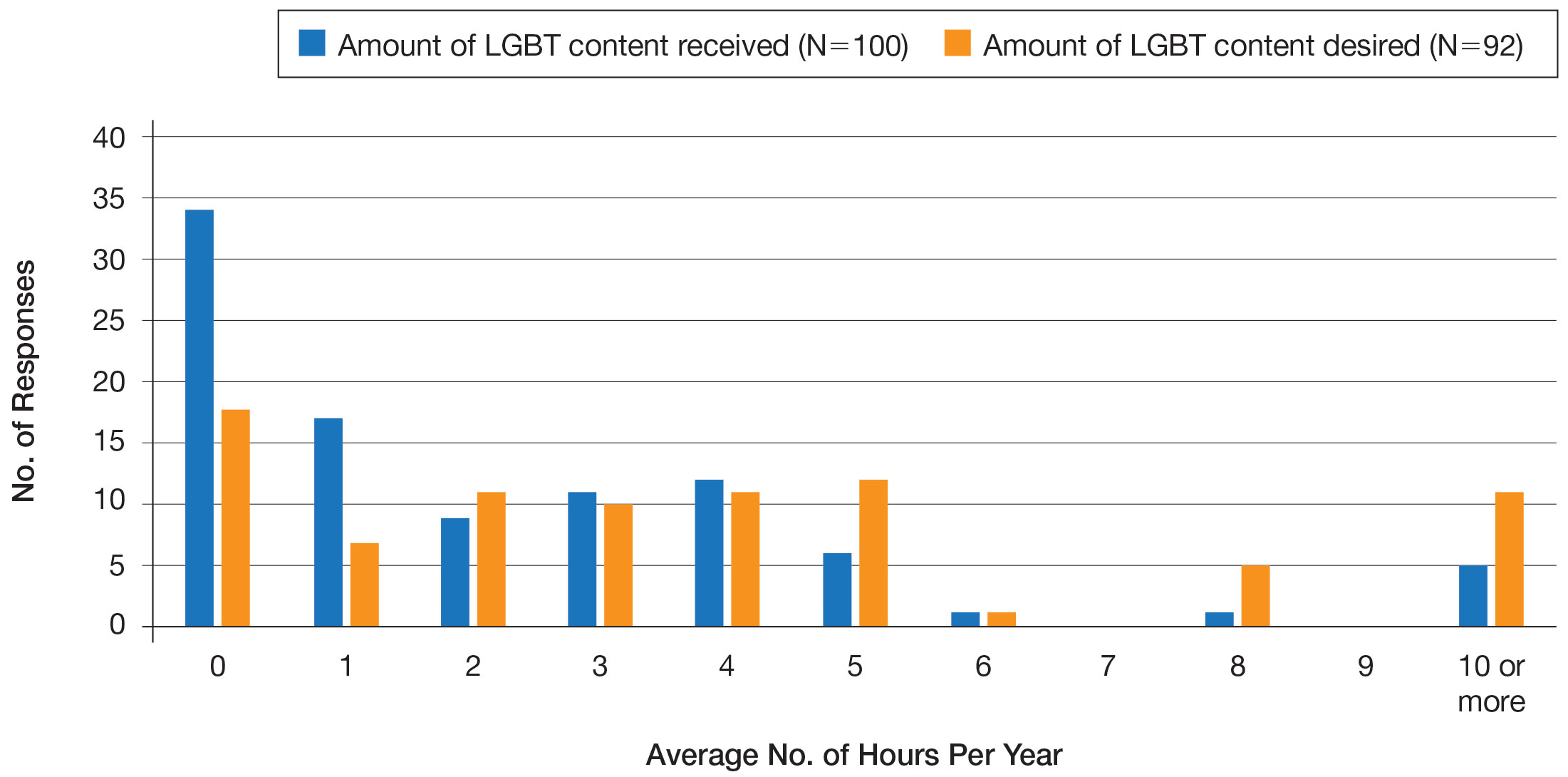
LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.
Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.
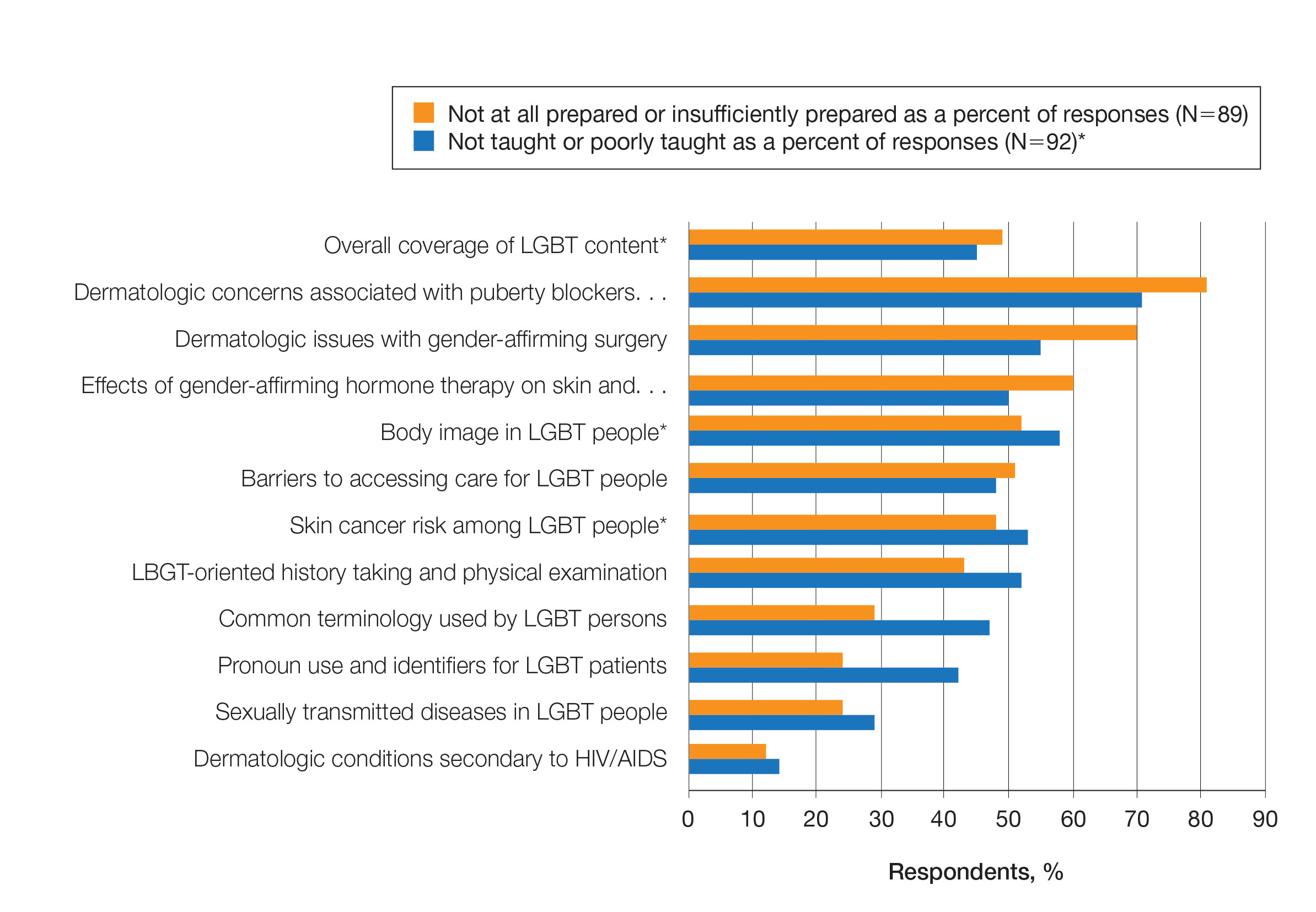
Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.

LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.

Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.

Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.

LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.

Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.

Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
Practice Points
- Dermatologists have the potential to greatly impact lesbian, gay, bisexual, transgender (LGBT) health since many health concerns in this population are cutaneous.
- Improving LGBT health education and training in dermatology residency likely will increase dermatology residents' comfort level in treating this population.
Cutaneous Eruption in an Immunocompromised Patient
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.
Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
A 29-year-old Black man with long-standing untreated HIV presented with mildly pruritic, scaly plaques on the palms and soles of 2 weeks’ duration. His medical history was notable for primary syphilis treated approximately 1 year prior. A review of symptoms was positive for blurry vision and floaters but negative for constitutional symptoms. Physical examination revealed well-defined scaly plaques over the palms, soles, and elbows with subungual hyperkeratosis. Patches of nonscarring alopecia over the scalp and split papules at the oral commissures also were noted. There were no palpable lymph nodes or genital involvement. Eye examination showed conjunctival injection and 20 cells per field in the vitreous humor. Laboratory evaluation revealed an HIV viral load of 31,623 copies/mL and a CD4 count of 47 cells/μL (reference range, 362–1531 cells/μL). A shave biopsy of the left elbow was performed for histopathologic evaluation.
Dual-Physician Marriages: Understanding the Challenges and Rewards
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
Resident Pearl
- As more physicians marry other physicians, there is an increasing need to understand the challenges and rewards of these relationships.
HIV Pre-exposure Prophylaxis (PrEP): A Survey of Dermatologists’ Knowledge and Practice Patterns
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.
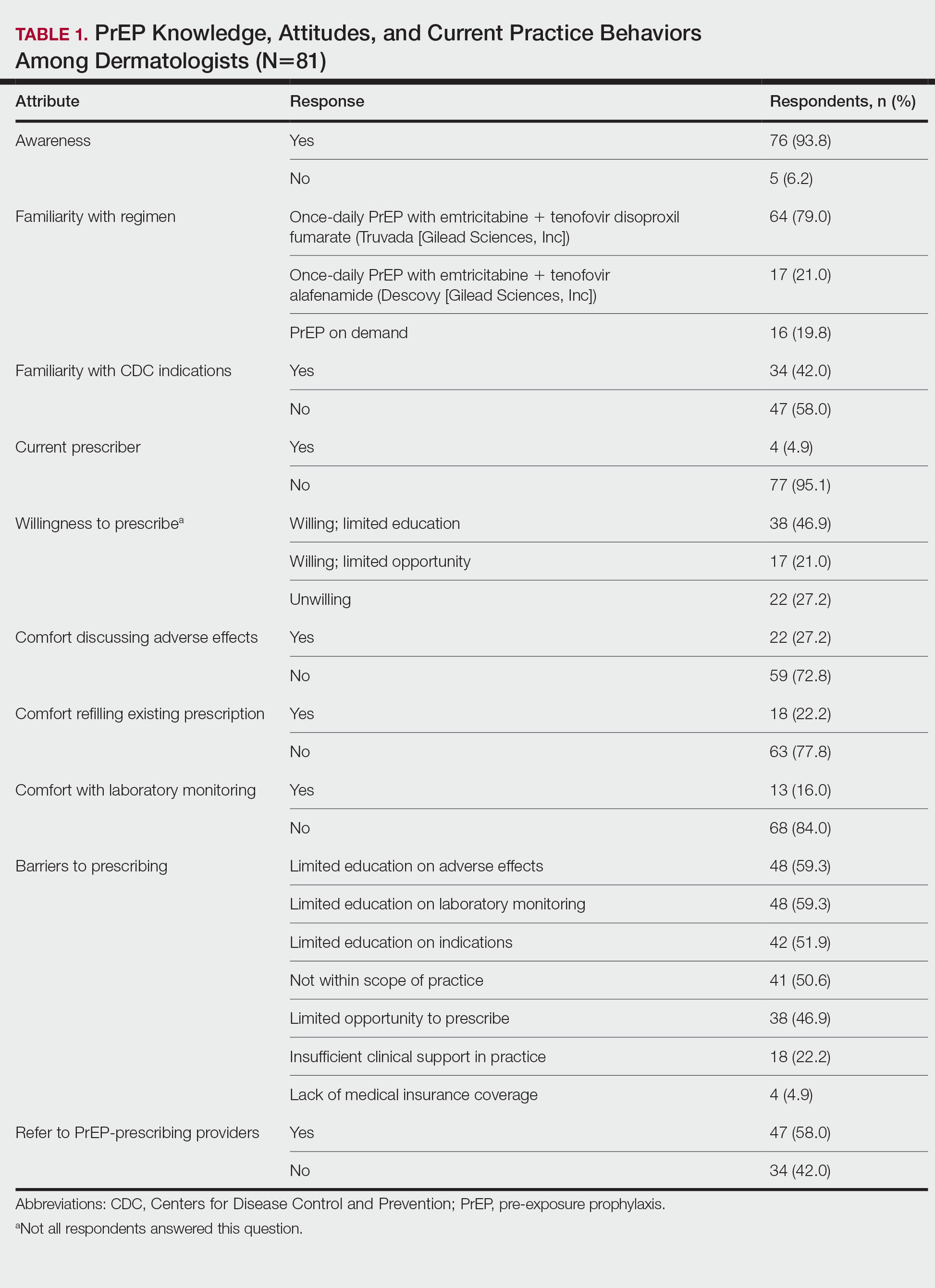
Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).
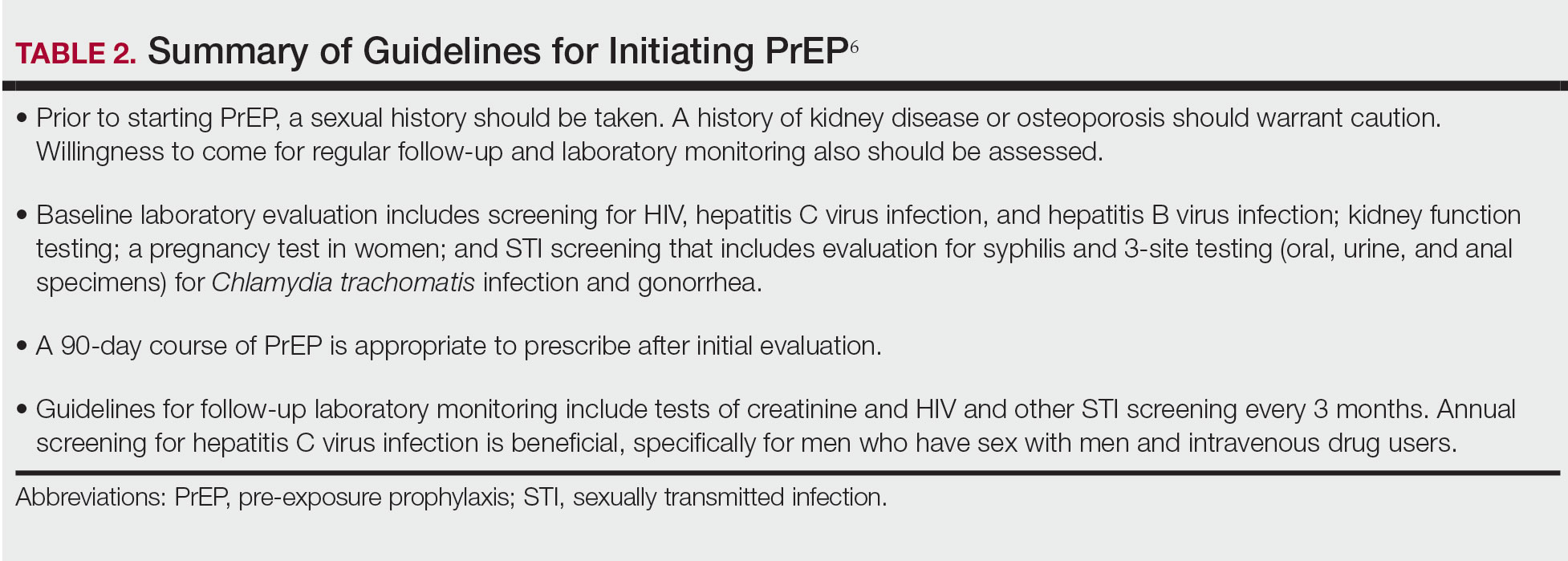
In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
Practice Points
- Sexually transmitted infections (STIs) often have skin manifestations, with patients presenting to dermatologists.
- Pre-exposure prophylaxis (PrEP) uses antiretrovirals taken prophylactically to prevent transmission of and infection with HIV. Dermatologists are aware of PrEP, but several barriers prevent them from being prescribers.
- Patients with a history of an STI should be considered for PrEP.
Tender Nonhealing Lesion on the Leg
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
A 50-year-old woman presented to our dermatology clinic with an exquisitely tender, nonhealing lesion on the left leg of 2 weeks’ duration that began as a small red-purplish spot. She applied a triple antibiotic ointment and wrapped the area with gauze daily but reported that it continued to enlarge and darken in color before forming a “scab.” She noted occasional seropurulent discharge and denied any trauma or new exposures to the area. She was seen at a local emergency department 3 days prior to presentation and was prescribed oral clindamycin for suspected cellulitis, but she denied any improvement with the initiation of antibiotics. Her medical history was notable for obesity, depression, hypothyroidism, and liver disease secondary to alcohol use disorder. She reported that she drank a pint of vodka daily. Her medications included pantoprazole, spironolactone, bumetanide, citalopram, levothyroxine, naltrexone, tramadol, and a multivitamin. Physical examination revealed violaceous mottling with areas of superficial erythema and ulceration with necrotic eschars on the proximal left thigh that were extremely painful. A biopsy was obtained for confirmation of diagnosis, but the patient died before the results were returned.
Open Clinical Trials for Psoriasis
The psoriasis clinical trials listed below are all phase 3 and recruiting participants as of July 19, 2022. For additional information on the study design, eligibility criteria, and contacts/locations, visit ClinicalTrials.gov.
GENERALIZED PUSTULAR PSORIASIS
Long-Term Safety and Efficacy of Imsidolimab (ANB019) in Subjects With Generalized Pustular Psoriasis (GEMINI2)
ClinicalTrials.gov Identifier: NCT05366855
An Expanded Access Trial in Japan to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have no Other Treatment Options
ClinicalTrials.gov Identifier: NCT05200247
An Expanded Access Program in China to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have No Other Treatment Options
ClinicalTrials.gov Identifier: NCT05239039
Study to Evaluate the Efficacy and Safety of Imsidolimab (ANB019) in the Treatment of Subjects With GPP (GEMINI1)
ClinicalTrials.gov Identifier: NCT05352893
NAIL PSORIASIS
Efficacy and Safety Study of Tildrakizumab in the Treatment of Nail Psoriasis
ClinicalTrials.gov Identifier: NCT03897075
PALMOPLANTAR PUSTULOSIS
Phase 3, Randomized Study of Apremilast in Japanese Participants With Palmoplantar Pustulosis (PPP)
ClinicalTrials.gov Identifier: NCT05174065
PLAQUE PSORIASIS
A Long-term Extension Study of Apremilast (CC-10004) in Pediatric Subjects From 6 Through 17 Years of Age With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04175613
A Phase III Efficacy and Safety Study of Hemay005 in Subjects With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04839328
A Study of Subcutaneous Risankizumab Injection for Pediatric Participants With Moderate to Severe Plaque Psoriasis to Assess Change in Disease Symptoms
ClinicalTrials.gov Identifier: NCT04435600
A Study to Evaluate the Drug Levels, Efficacy and Safety of Deucravacitinib in Adolescent Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04772079
Investigator Initiated Trial to Study Duobrii® Lotion in the Treatment of Mild Plaque Psoriasis in Adults
ClinicalTrials.gov Identifier: NCT05203315
Comparative Study of BAT2206 With Stelara® in Patients With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04728360
A Study to Evaluate the Efficacy and Safety of Bimekizumab in Adult Korean Study Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT05020249
Comparing Efficacy and Safety of Bmab 1200 and Stelara in Patients With Moderate to Severe Chronic Plaque Psoriasis (STELLAR-2)
ClinicalTrials.gov Identifier: NCT05335356
A Study to Evaluate the Efficacy, Safety, and Drug Concentration of Certolizumab Pegol (CZP) in Children and Adolescent Study Participants With Moderate to Severe Chronic Plaque Psoriasis (PSO)(CIMcare)
ClinicalTrials.gov Identifier: NCT04123795
A Study of Tildrakizumab in Pediatric Subjects With Chronic Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT03997786
Tapinarof for the Treatment of Plaque Psoriasis in Pediatric Subjects
ClinicalTrials.gov Identifier: NCT05172726
A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Subcutaneously Administered Guselkumab for the Treatment of Chronic Plaque Psoriasis in Pediatric Participants (PROTOSTAR)
ClinicalTrials.gov Identifier: NCT03451851
PSORIATIC ARTHRITIS
Efficacy of Secukinumab Compared to Ustekinumab in Adults With Active Psoriatic Arthritis and Failure of TNFα-Inhibitor Treatment (AgAIN)
ClinicalTrials.gov Identifier: NCT04632927
A Long-term Extension Study of Ustekinumab in Pediatric Participants (UNITED)
ClinicalTrials.gov Identifier: NCT05092269
A Study of Ustekinumab or Guselkumab in Pediatric Participants With Active Juvenile Psoriatic Arthritis (PSUMMIT-Jr)
ClinicalTrials.gov Identifier: NCT05083182
Comparative Study of BAT2506 With Simponi® in Participants With Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT05046431
Long Term Evaluation of Safety and Efficacy of Tildrakizumab in Patients With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04991116
To Evaluate the Efficacy and Safety of SHR0302 Tablet in Subjects of Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04957550
PSORIATIC ARTHRITIS (continued)
Guselkumab in Active Psoriatic Arthritis Participants With Inadequate Response/Intolerance to One Prior Anti-TNF Alpha Agent (SOLSTICE)
ClinicalTrials.gov Identifier: NCT04936308
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease-modifying Anti-rheumatic Drugs
ClinicalTrials.gov Identifier: NCT04908202
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease Modifying Anti-rheumatic Drugs or Had Previously Received TNFα Inhibitor Treatment
ClinicalTrials.gov Identifier: NCT04908189
A Study of Guselkumab in Participants With Active Psoriatic Arthritis (APEX) ClinicalTrials.gov Identifier: NCT04882098 Apremilast Pediatric Study in Children With Active Juvenile Psoriatic Arthritis (PEAPOD)
ClinicalTrials.gov Identifier: NCT04804553
Impact of Tapering Immunosuppressants on Maintaining Minimal Disease Activity in Adult Subjects With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04610476
A Study of Ixekizumab (LY2439821) in Children With Juvenile Idiopathic Arthritis Categories of Enthesitis-related Arthritis (Including Juvenile Onset Ankylosing Spondylitis) and Juvenile Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04527380
Efficacy and Safety of Tildrakizumab Compared to Placebo in Subjects With Active Psoriatic Arthritis I (INSPIRE 1)
ClinicalTrials.gov Identifier: NCT04314544
Efficacy and Safety of Tildrakizumab Compared to Placebo in Anti- TNF naïve Subjects With Active Psoriatic Arthritis II (INSPIRE 2)
ClinicalTrials.gov Identifier: NCT04314531
The psoriasis clinical trials listed below are all phase 3 and recruiting participants as of July 19, 2022. For additional information on the study design, eligibility criteria, and contacts/locations, visit ClinicalTrials.gov.
GENERALIZED PUSTULAR PSORIASIS
Long-Term Safety and Efficacy of Imsidolimab (ANB019) in Subjects With Generalized Pustular Psoriasis (GEMINI2)
ClinicalTrials.gov Identifier: NCT05366855
An Expanded Access Trial in Japan to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have no Other Treatment Options
ClinicalTrials.gov Identifier: NCT05200247
An Expanded Access Program in China to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have No Other Treatment Options
ClinicalTrials.gov Identifier: NCT05239039
Study to Evaluate the Efficacy and Safety of Imsidolimab (ANB019) in the Treatment of Subjects With GPP (GEMINI1)
ClinicalTrials.gov Identifier: NCT05352893
NAIL PSORIASIS
Efficacy and Safety Study of Tildrakizumab in the Treatment of Nail Psoriasis
ClinicalTrials.gov Identifier: NCT03897075
PALMOPLANTAR PUSTULOSIS
Phase 3, Randomized Study of Apremilast in Japanese Participants With Palmoplantar Pustulosis (PPP)
ClinicalTrials.gov Identifier: NCT05174065
PLAQUE PSORIASIS
A Long-term Extension Study of Apremilast (CC-10004) in Pediatric Subjects From 6 Through 17 Years of Age With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04175613
A Phase III Efficacy and Safety Study of Hemay005 in Subjects With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04839328
A Study of Subcutaneous Risankizumab Injection for Pediatric Participants With Moderate to Severe Plaque Psoriasis to Assess Change in Disease Symptoms
ClinicalTrials.gov Identifier: NCT04435600
A Study to Evaluate the Drug Levels, Efficacy and Safety of Deucravacitinib in Adolescent Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04772079
Investigator Initiated Trial to Study Duobrii® Lotion in the Treatment of Mild Plaque Psoriasis in Adults
ClinicalTrials.gov Identifier: NCT05203315
Comparative Study of BAT2206 With Stelara® in Patients With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04728360
A Study to Evaluate the Efficacy and Safety of Bimekizumab in Adult Korean Study Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT05020249
Comparing Efficacy and Safety of Bmab 1200 and Stelara in Patients With Moderate to Severe Chronic Plaque Psoriasis (STELLAR-2)
ClinicalTrials.gov Identifier: NCT05335356
A Study to Evaluate the Efficacy, Safety, and Drug Concentration of Certolizumab Pegol (CZP) in Children and Adolescent Study Participants With Moderate to Severe Chronic Plaque Psoriasis (PSO)(CIMcare)
ClinicalTrials.gov Identifier: NCT04123795
A Study of Tildrakizumab in Pediatric Subjects With Chronic Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT03997786
Tapinarof for the Treatment of Plaque Psoriasis in Pediatric Subjects
ClinicalTrials.gov Identifier: NCT05172726
A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Subcutaneously Administered Guselkumab for the Treatment of Chronic Plaque Psoriasis in Pediatric Participants (PROTOSTAR)
ClinicalTrials.gov Identifier: NCT03451851
PSORIATIC ARTHRITIS
Efficacy of Secukinumab Compared to Ustekinumab in Adults With Active Psoriatic Arthritis and Failure of TNFα-Inhibitor Treatment (AgAIN)
ClinicalTrials.gov Identifier: NCT04632927
A Long-term Extension Study of Ustekinumab in Pediatric Participants (UNITED)
ClinicalTrials.gov Identifier: NCT05092269
A Study of Ustekinumab or Guselkumab in Pediatric Participants With Active Juvenile Psoriatic Arthritis (PSUMMIT-Jr)
ClinicalTrials.gov Identifier: NCT05083182
Comparative Study of BAT2506 With Simponi® in Participants With Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT05046431
Long Term Evaluation of Safety and Efficacy of Tildrakizumab in Patients With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04991116
To Evaluate the Efficacy and Safety of SHR0302 Tablet in Subjects of Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04957550
PSORIATIC ARTHRITIS (continued)
Guselkumab in Active Psoriatic Arthritis Participants With Inadequate Response/Intolerance to One Prior Anti-TNF Alpha Agent (SOLSTICE)
ClinicalTrials.gov Identifier: NCT04936308
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease-modifying Anti-rheumatic Drugs
ClinicalTrials.gov Identifier: NCT04908202
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease Modifying Anti-rheumatic Drugs or Had Previously Received TNFα Inhibitor Treatment
ClinicalTrials.gov Identifier: NCT04908189
A Study of Guselkumab in Participants With Active Psoriatic Arthritis (APEX) ClinicalTrials.gov Identifier: NCT04882098 Apremilast Pediatric Study in Children With Active Juvenile Psoriatic Arthritis (PEAPOD)
ClinicalTrials.gov Identifier: NCT04804553
Impact of Tapering Immunosuppressants on Maintaining Minimal Disease Activity in Adult Subjects With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04610476
A Study of Ixekizumab (LY2439821) in Children With Juvenile Idiopathic Arthritis Categories of Enthesitis-related Arthritis (Including Juvenile Onset Ankylosing Spondylitis) and Juvenile Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04527380
Efficacy and Safety of Tildrakizumab Compared to Placebo in Subjects With Active Psoriatic Arthritis I (INSPIRE 1)
ClinicalTrials.gov Identifier: NCT04314544
Efficacy and Safety of Tildrakizumab Compared to Placebo in Anti- TNF naïve Subjects With Active Psoriatic Arthritis II (INSPIRE 2)
ClinicalTrials.gov Identifier: NCT04314531
The psoriasis clinical trials listed below are all phase 3 and recruiting participants as of July 19, 2022. For additional information on the study design, eligibility criteria, and contacts/locations, visit ClinicalTrials.gov.
GENERALIZED PUSTULAR PSORIASIS
Long-Term Safety and Efficacy of Imsidolimab (ANB019) in Subjects With Generalized Pustular Psoriasis (GEMINI2)
ClinicalTrials.gov Identifier: NCT05366855
An Expanded Access Trial in Japan to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have no Other Treatment Options
ClinicalTrials.gov Identifier: NCT05200247
An Expanded Access Program in China to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have No Other Treatment Options
ClinicalTrials.gov Identifier: NCT05239039
Study to Evaluate the Efficacy and Safety of Imsidolimab (ANB019) in the Treatment of Subjects With GPP (GEMINI1)
ClinicalTrials.gov Identifier: NCT05352893
NAIL PSORIASIS
Efficacy and Safety Study of Tildrakizumab in the Treatment of Nail Psoriasis
ClinicalTrials.gov Identifier: NCT03897075
PALMOPLANTAR PUSTULOSIS
Phase 3, Randomized Study of Apremilast in Japanese Participants With Palmoplantar Pustulosis (PPP)
ClinicalTrials.gov Identifier: NCT05174065
PLAQUE PSORIASIS
A Long-term Extension Study of Apremilast (CC-10004) in Pediatric Subjects From 6 Through 17 Years of Age With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04175613
A Phase III Efficacy and Safety Study of Hemay005 in Subjects With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04839328
A Study of Subcutaneous Risankizumab Injection for Pediatric Participants With Moderate to Severe Plaque Psoriasis to Assess Change in Disease Symptoms
ClinicalTrials.gov Identifier: NCT04435600
A Study to Evaluate the Drug Levels, Efficacy and Safety of Deucravacitinib in Adolescent Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04772079
Investigator Initiated Trial to Study Duobrii® Lotion in the Treatment of Mild Plaque Psoriasis in Adults
ClinicalTrials.gov Identifier: NCT05203315
Comparative Study of BAT2206 With Stelara® in Patients With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04728360
A Study to Evaluate the Efficacy and Safety of Bimekizumab in Adult Korean Study Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT05020249
Comparing Efficacy and Safety of Bmab 1200 and Stelara in Patients With Moderate to Severe Chronic Plaque Psoriasis (STELLAR-2)
ClinicalTrials.gov Identifier: NCT05335356
A Study to Evaluate the Efficacy, Safety, and Drug Concentration of Certolizumab Pegol (CZP) in Children and Adolescent Study Participants With Moderate to Severe Chronic Plaque Psoriasis (PSO)(CIMcare)
ClinicalTrials.gov Identifier: NCT04123795
A Study of Tildrakizumab in Pediatric Subjects With Chronic Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT03997786
Tapinarof for the Treatment of Plaque Psoriasis in Pediatric Subjects
ClinicalTrials.gov Identifier: NCT05172726
A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Subcutaneously Administered Guselkumab for the Treatment of Chronic Plaque Psoriasis in Pediatric Participants (PROTOSTAR)
ClinicalTrials.gov Identifier: NCT03451851
PSORIATIC ARTHRITIS
Efficacy of Secukinumab Compared to Ustekinumab in Adults With Active Psoriatic Arthritis and Failure of TNFα-Inhibitor Treatment (AgAIN)
ClinicalTrials.gov Identifier: NCT04632927
A Long-term Extension Study of Ustekinumab in Pediatric Participants (UNITED)
ClinicalTrials.gov Identifier: NCT05092269
A Study of Ustekinumab or Guselkumab in Pediatric Participants With Active Juvenile Psoriatic Arthritis (PSUMMIT-Jr)
ClinicalTrials.gov Identifier: NCT05083182
Comparative Study of BAT2506 With Simponi® in Participants With Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT05046431
Long Term Evaluation of Safety and Efficacy of Tildrakizumab in Patients With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04991116
To Evaluate the Efficacy and Safety of SHR0302 Tablet in Subjects of Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04957550
PSORIATIC ARTHRITIS (continued)
Guselkumab in Active Psoriatic Arthritis Participants With Inadequate Response/Intolerance to One Prior Anti-TNF Alpha Agent (SOLSTICE)
ClinicalTrials.gov Identifier: NCT04936308
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease-modifying Anti-rheumatic Drugs
ClinicalTrials.gov Identifier: NCT04908202
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease Modifying Anti-rheumatic Drugs or Had Previously Received TNFα Inhibitor Treatment
ClinicalTrials.gov Identifier: NCT04908189
A Study of Guselkumab in Participants With Active Psoriatic Arthritis (APEX) ClinicalTrials.gov Identifier: NCT04882098 Apremilast Pediatric Study in Children With Active Juvenile Psoriatic Arthritis (PEAPOD)
ClinicalTrials.gov Identifier: NCT04804553
Impact of Tapering Immunosuppressants on Maintaining Minimal Disease Activity in Adult Subjects With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04610476
A Study of Ixekizumab (LY2439821) in Children With Juvenile Idiopathic Arthritis Categories of Enthesitis-related Arthritis (Including Juvenile Onset Ankylosing Spondylitis) and Juvenile Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04527380
Efficacy and Safety of Tildrakizumab Compared to Placebo in Subjects With Active Psoriatic Arthritis I (INSPIRE 1)
ClinicalTrials.gov Identifier: NCT04314544
Efficacy and Safety of Tildrakizumab Compared to Placebo in Anti- TNF naïve Subjects With Active Psoriatic Arthritis II (INSPIRE 2)
ClinicalTrials.gov Identifier: NCT04314531
Optimizing Narrowband UVB Phototherapy: Is It More Challenging for Your Older Patients?
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—
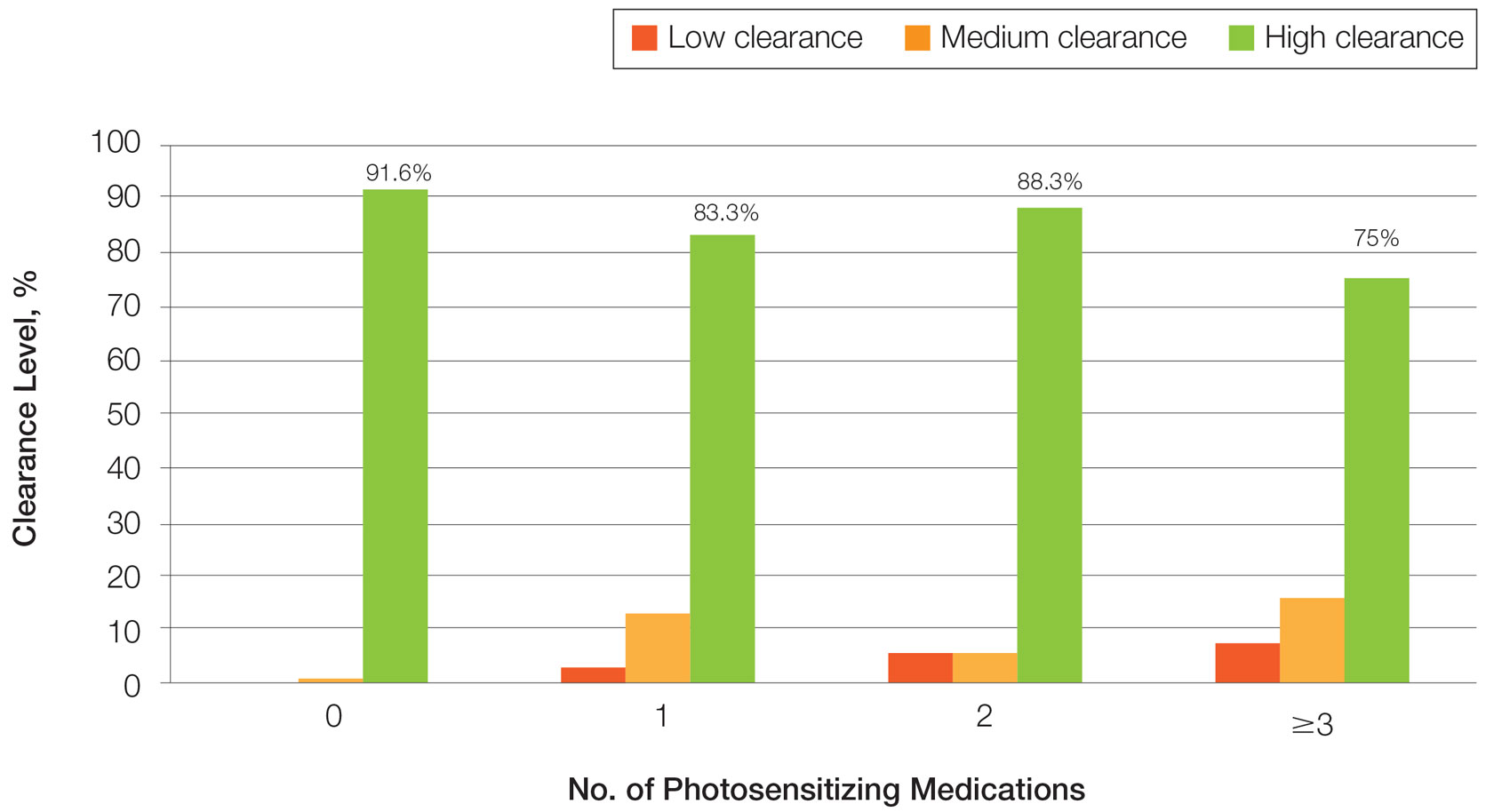
Photosensitizing Medications, Clearance Levels, and Clearance Rates—
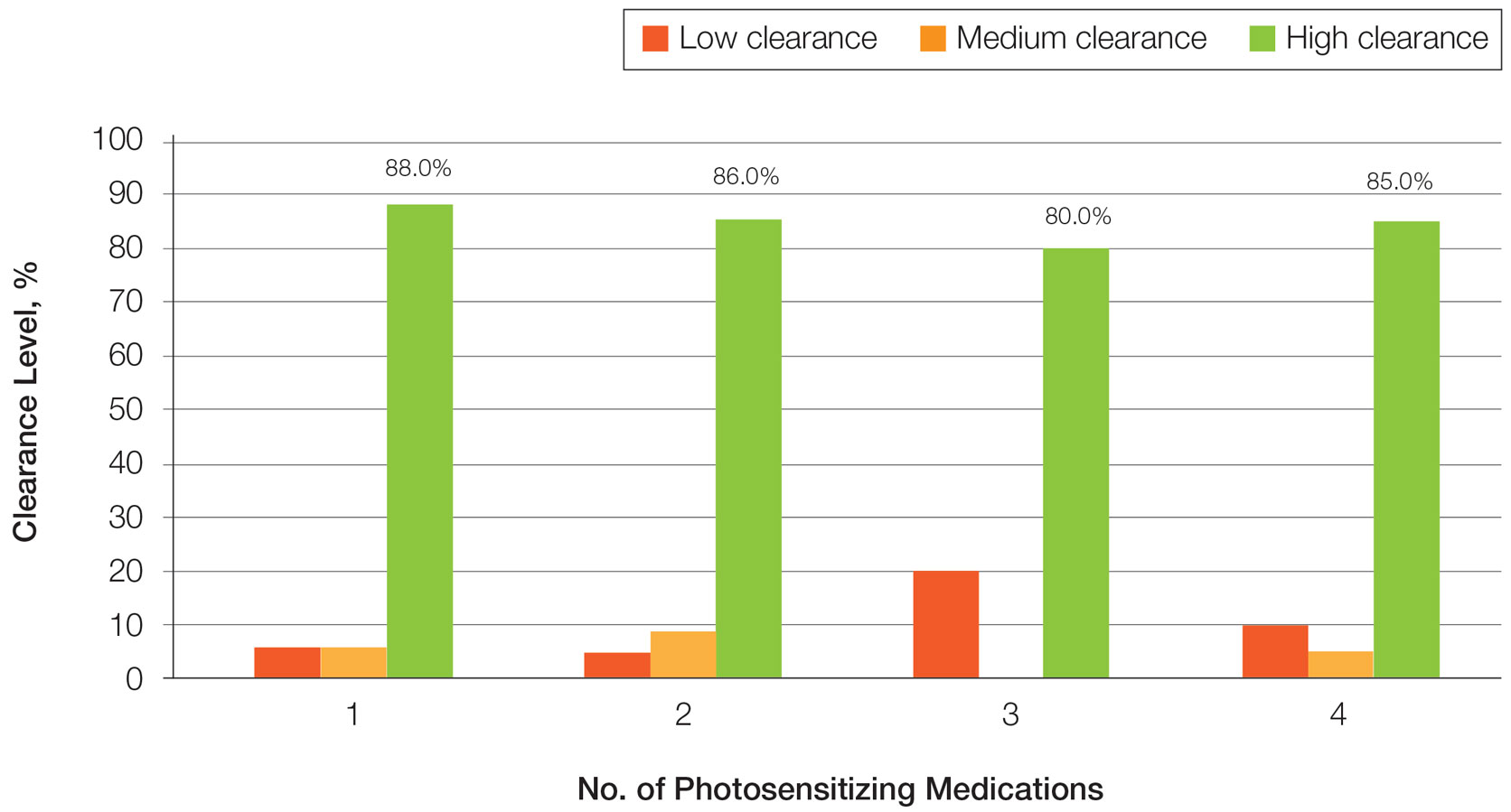
Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.
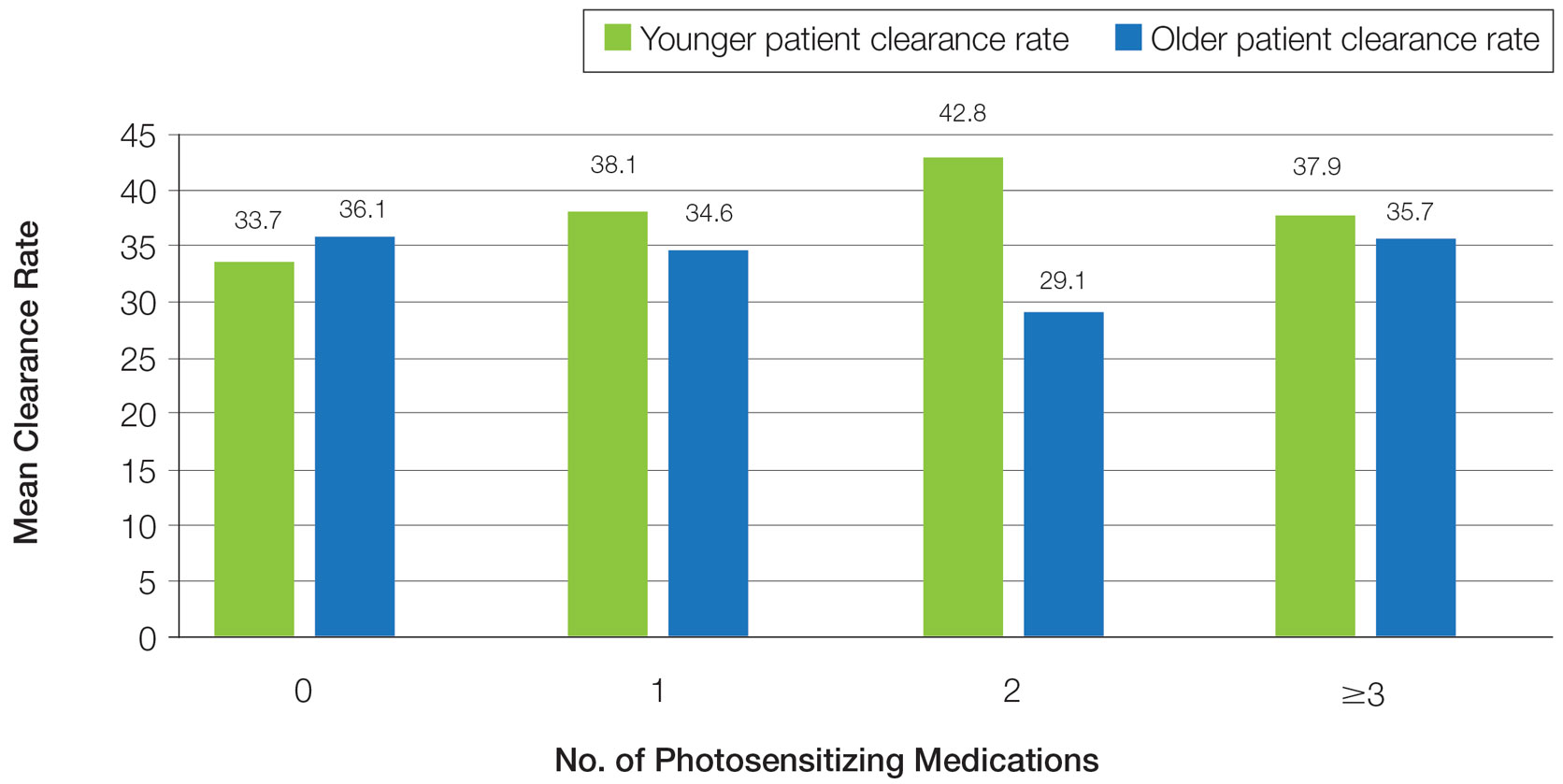
Photosensitizing Medications and Erythema Rates—
Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).
Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Practice Points
- Narrowband UVB (NB-UVB) phototherapy remains a safe and efficacious nonpharmacologic treatment for dermatologic conditions in older and younger adults.
- Compared to younger adults, older adults using the same protocols need similar or even fewer treatments to achieve high levels of clearance.
- Individuals taking 3 or more photosensitizing medications, regardless of age, may be at higher risk for substantial erythema with NB-UVB phototherapy.
- Phototherapy program monitoring is important to ensure quality care and investigate opportunities for care optimization.