User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
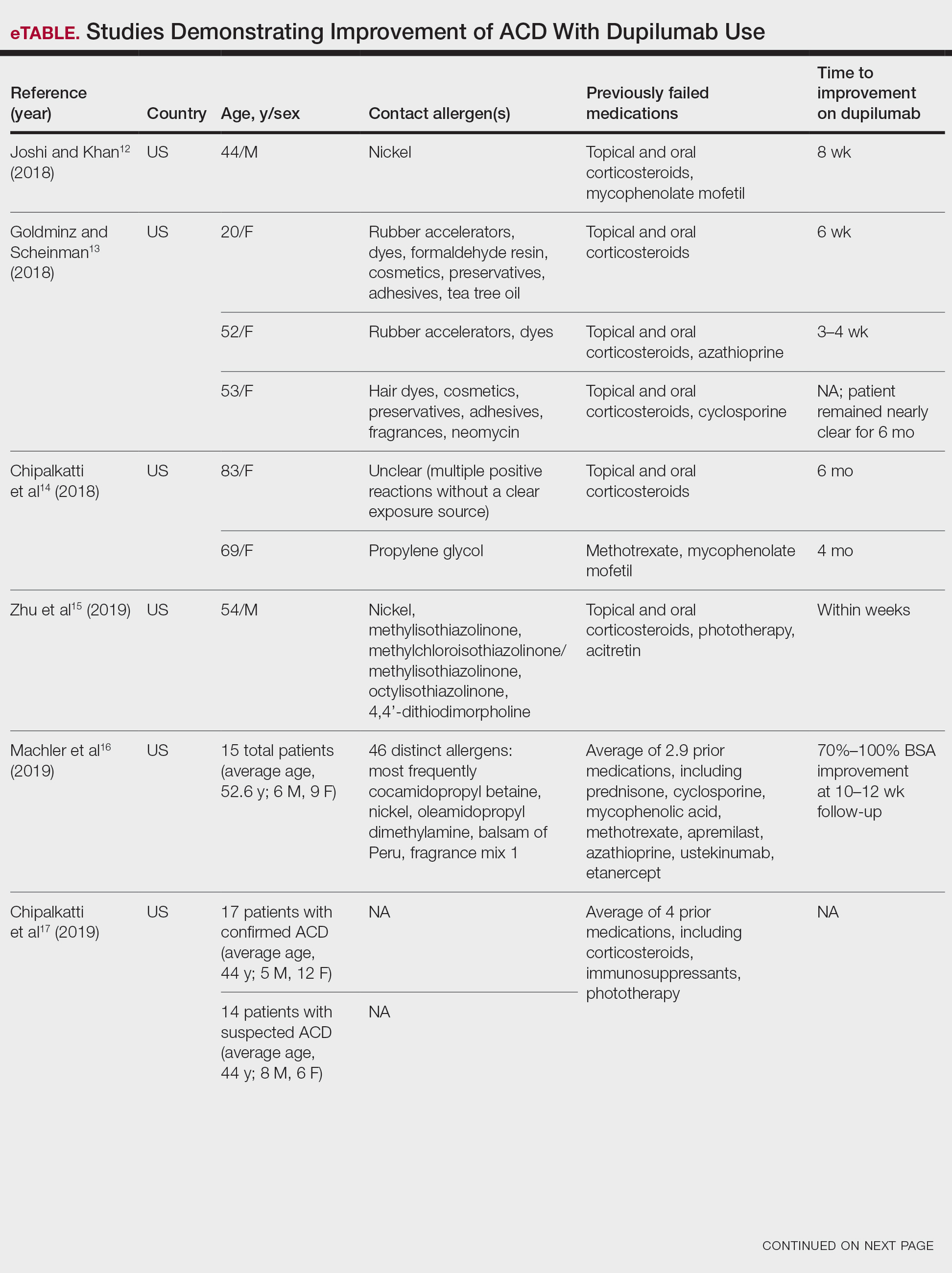
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Calcified Urachal Remnant in a Young Adult: An Unusual Case
To the Editor:
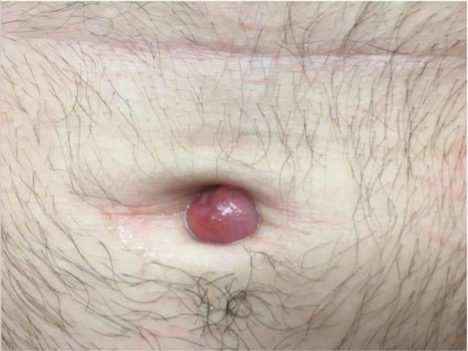
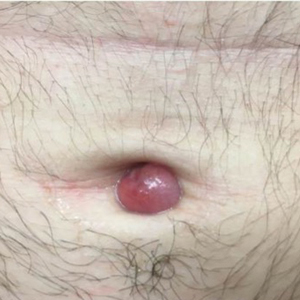
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.
Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.
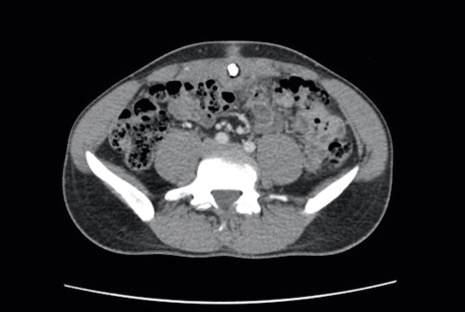
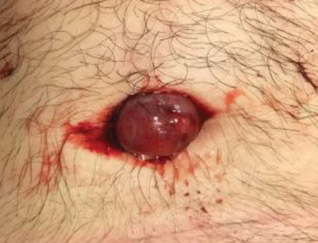
The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.
We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
To the Editor:
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.
Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.

The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.
We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
To the Editor:
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.
Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.

The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.
We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
Practice Points
- Visible umbilical nodules occur infrequently; the differential diagnosis is broad and consists of various benign and malignant pathologies.
- Disruption of the involution of the urachus during development can lead to various rare anomalies.
- Urachal anomalies are important to diagnose given the potential for secondary infection or malignancy.
Postirradiation Pseudosclerodermatous Panniculitis: A Rare Complication of Megavoltage External Beam Radiotherapy
To the Editor:
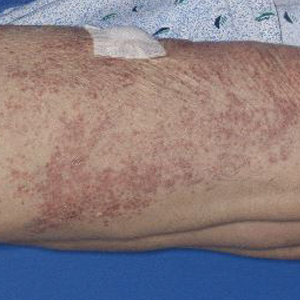
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
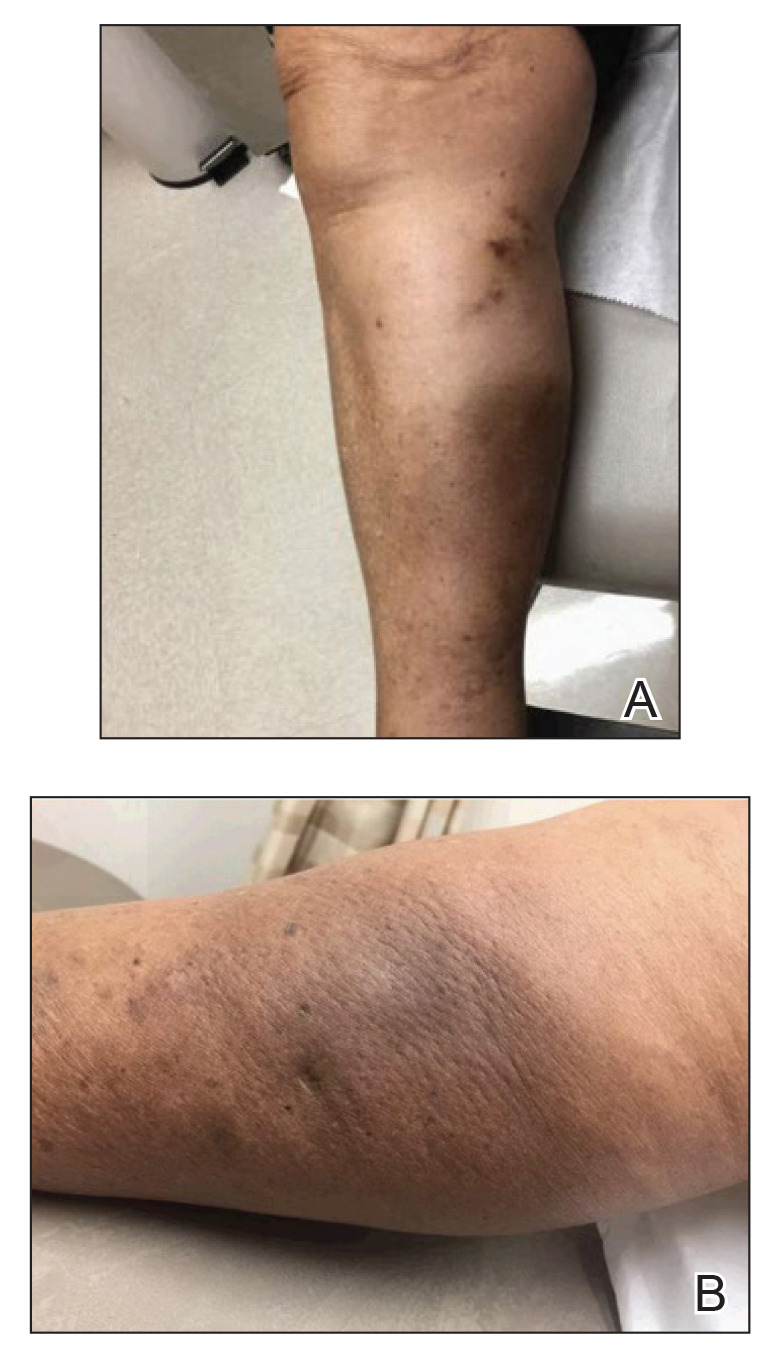
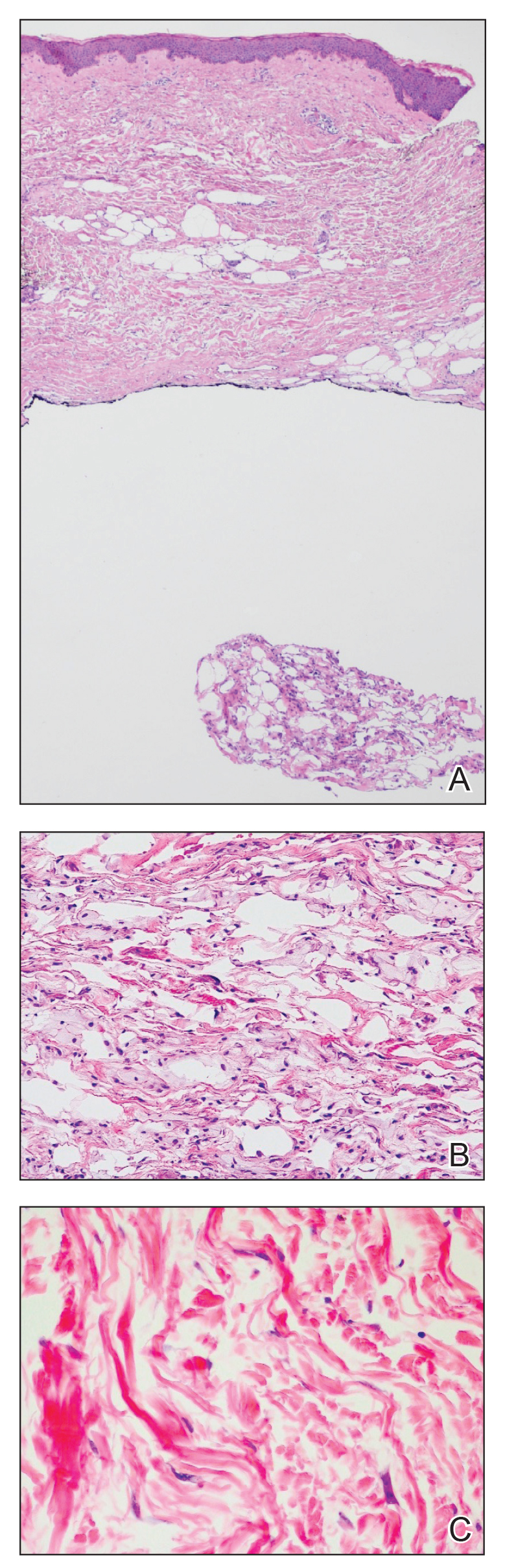
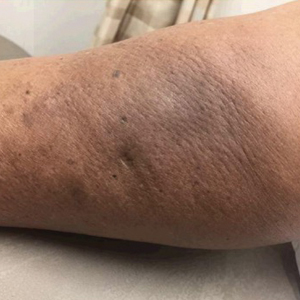
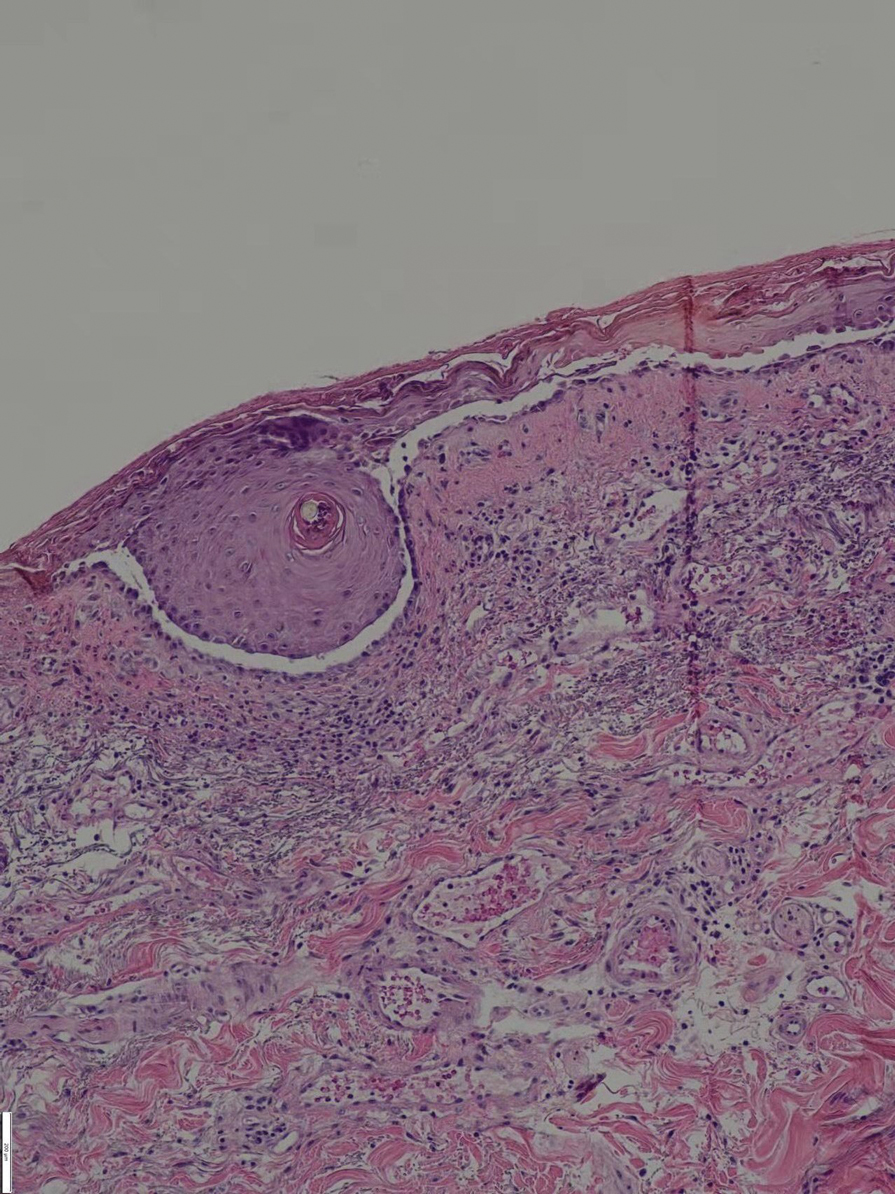
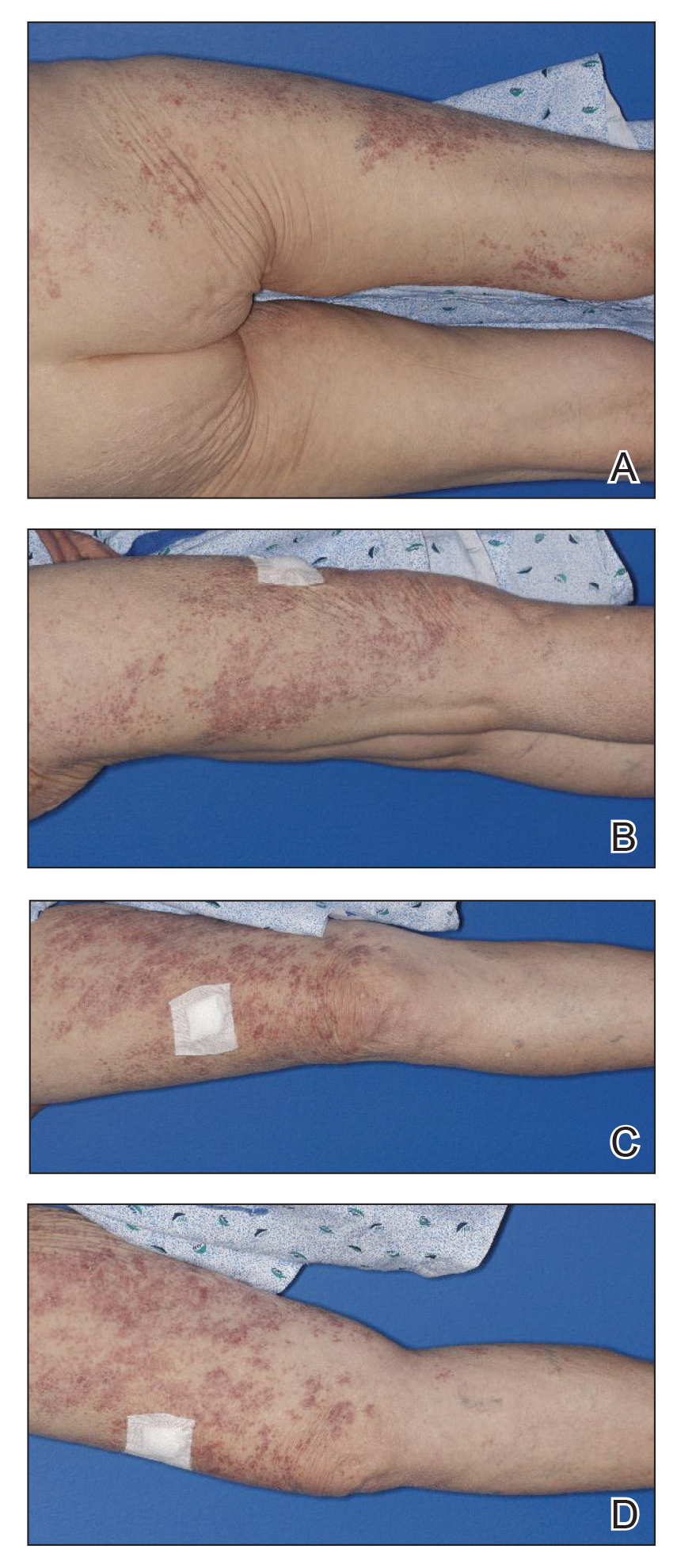
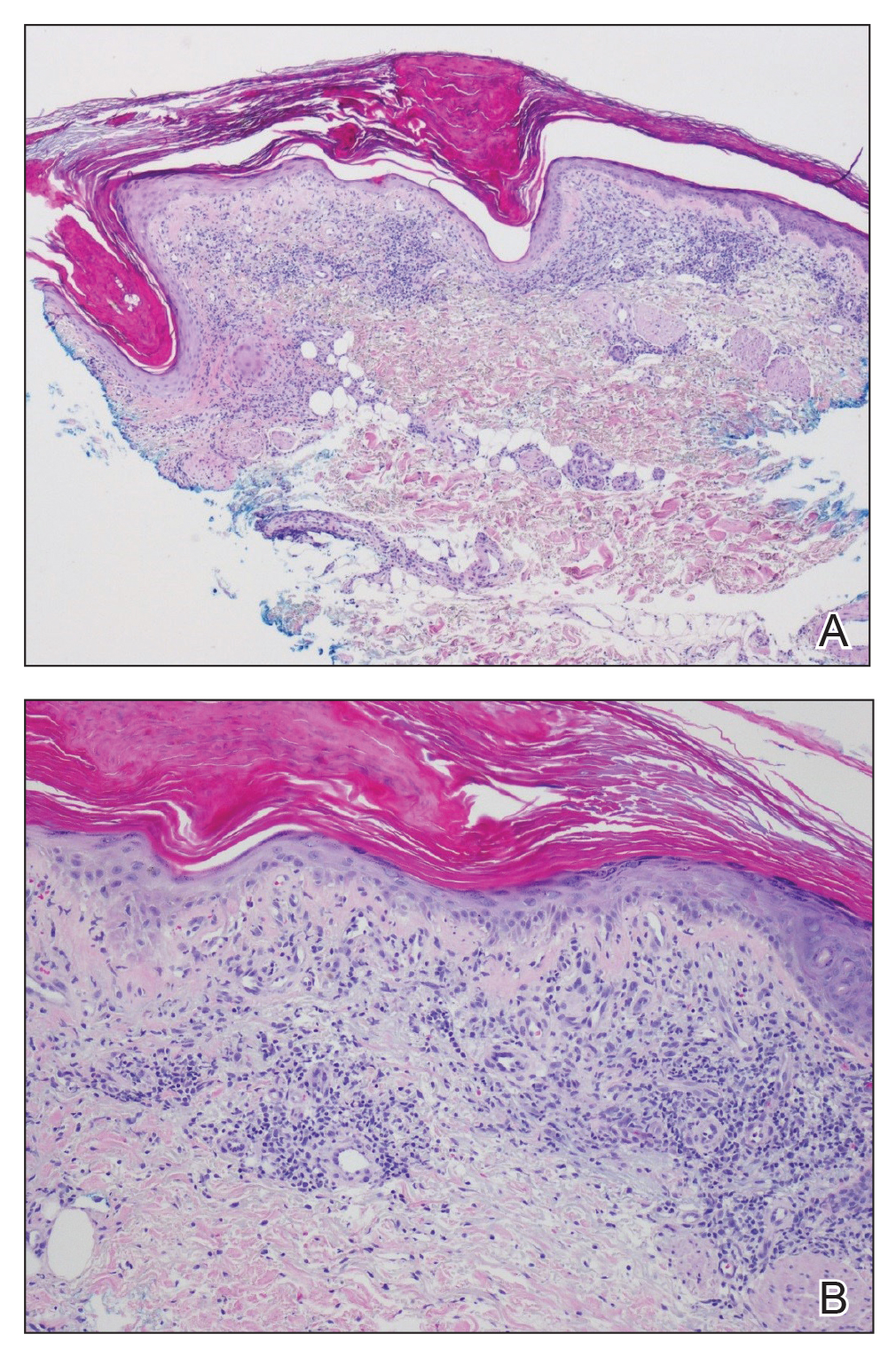
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.
The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.
Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.
The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.
Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.
The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.
Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
Practice Points
- Postirradiation pseudosclerodermatous panniculitis presents as an erythematous or indurated plaque at a site of prior radiotherapy.
- This rare entity may be underreported and requires biopsy for accurate diagnosis.
Vascular Plaque in a Pregnant Patient With a History of Breast Cancer
The Diagnosis: Tufted Angioma
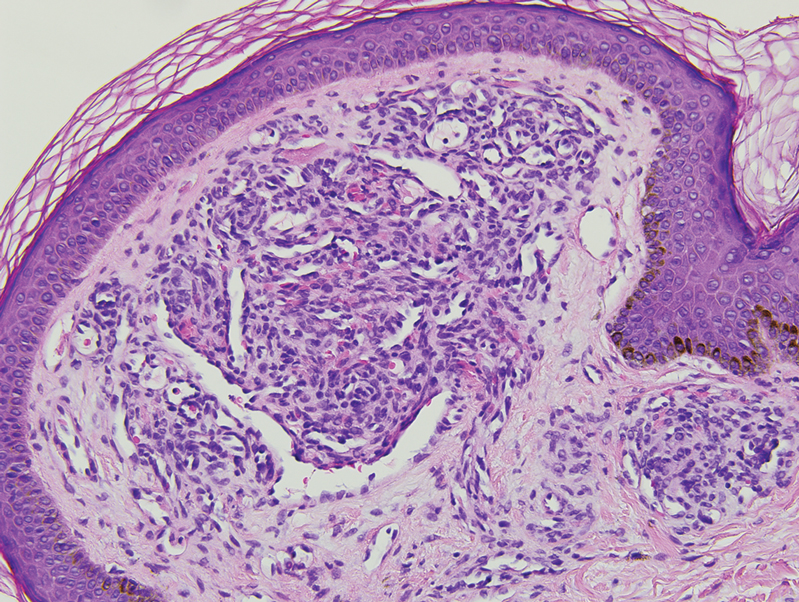
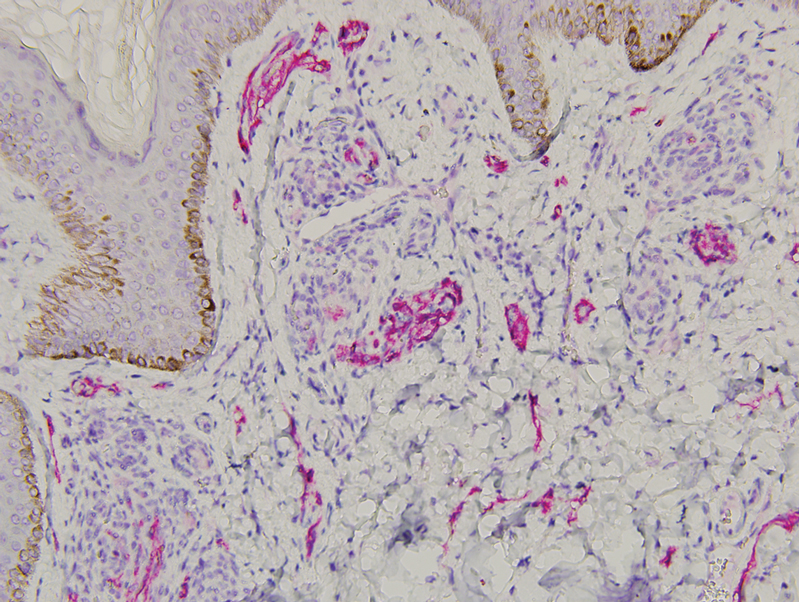
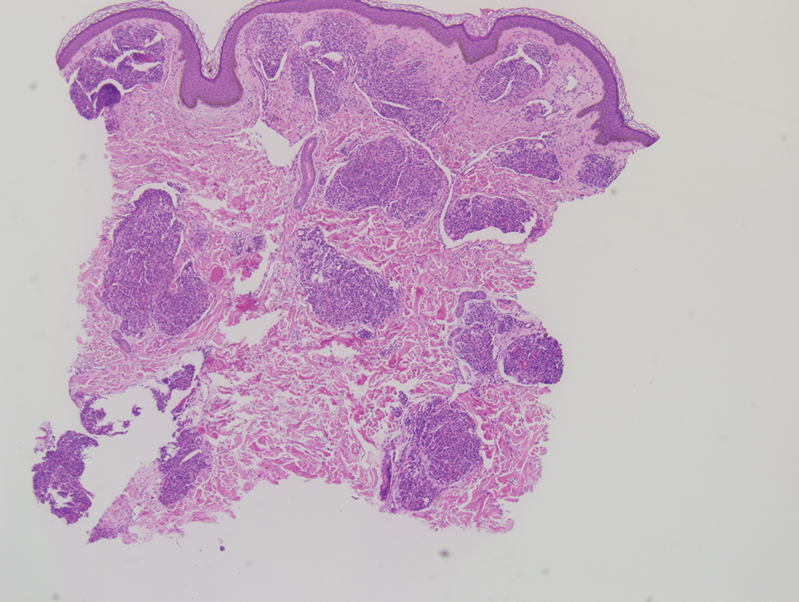
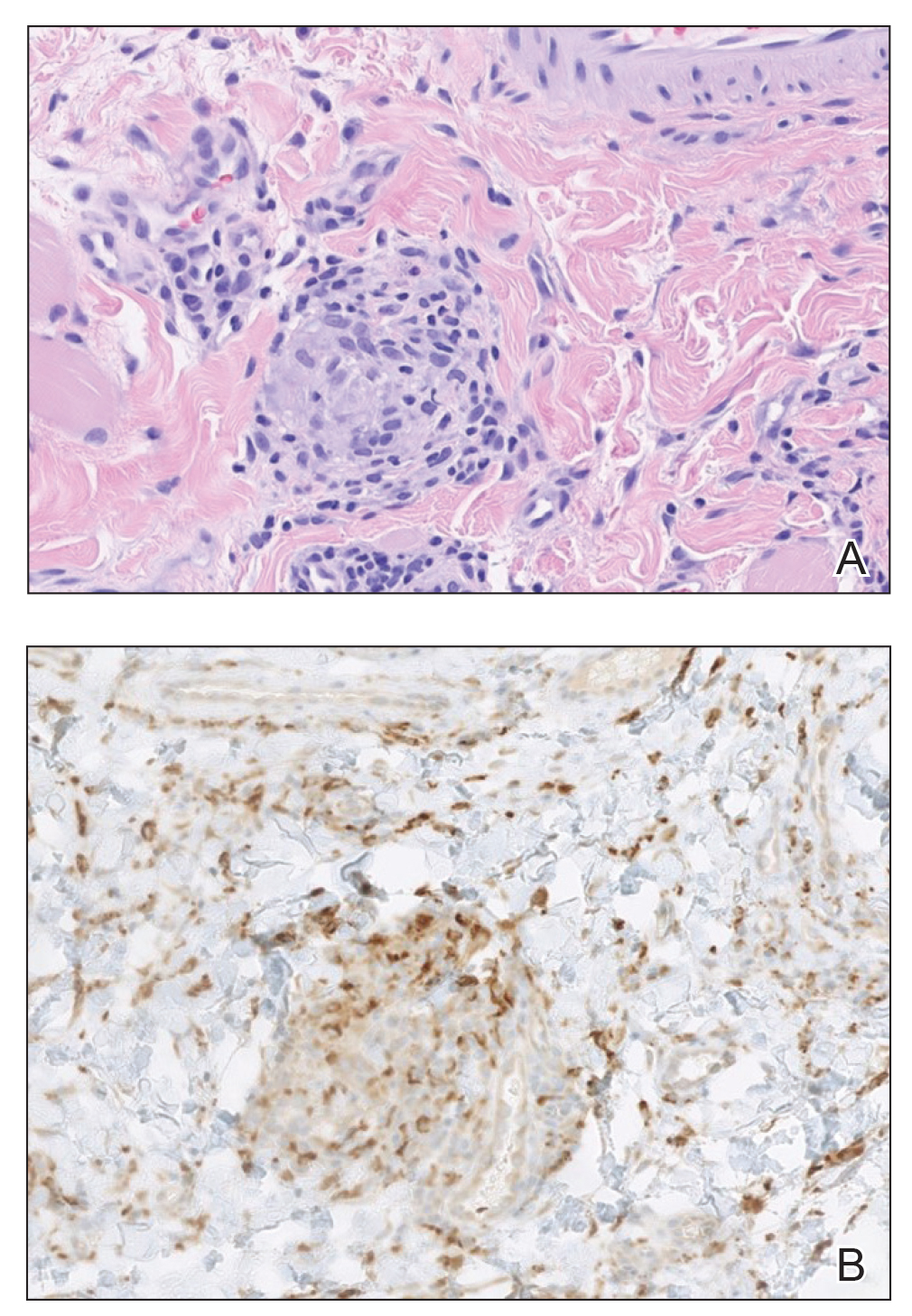
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.
Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1
Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4
Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
The Diagnosis: Tufted Angioma
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.
Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1
Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4
Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
The Diagnosis: Tufted Angioma
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.
Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1
Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4
Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
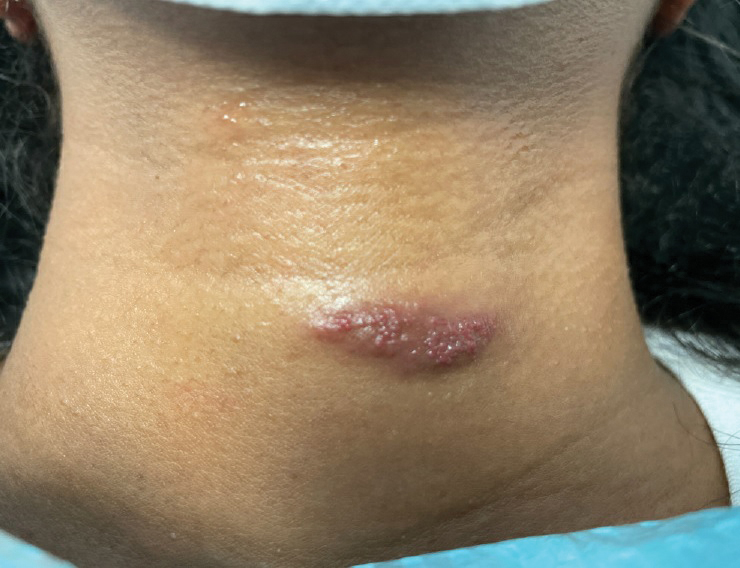
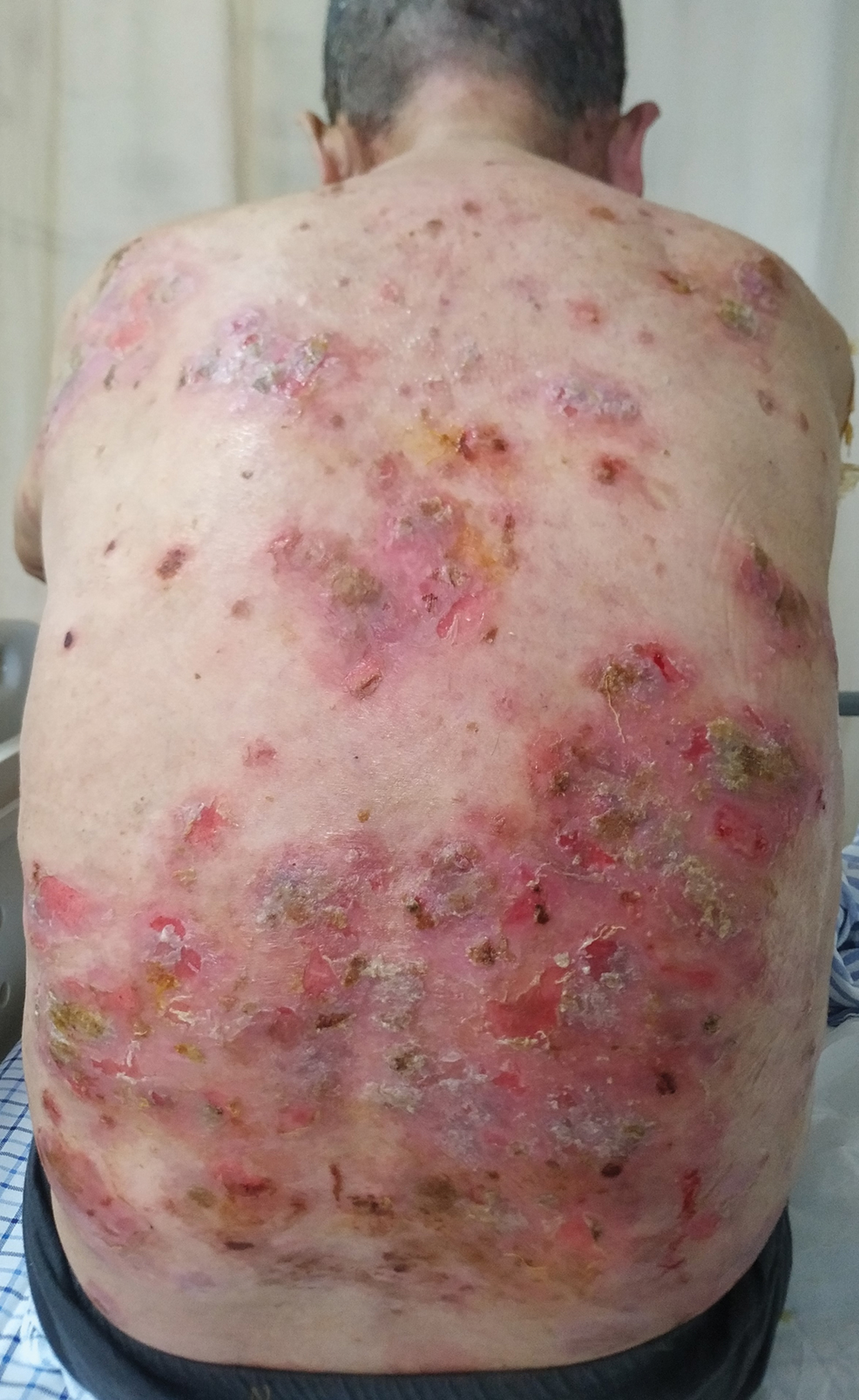
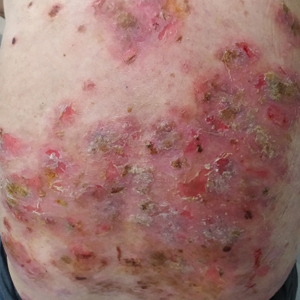
A 31-year-old woman at 34 weeks’ gestation presented with skin discoloration of the anterior neck of 7 months’ duration. Her pregnancy had been complicated by a diagnosis of invasive papillary carcinoma of the breast with unilateral complete mastectomy and negative sentinel lymph node biopsy in the first trimester. The lesion was tender, darkening, and rapidly enlarging. Physical examination demonstrated a linear, violaceous, vascular, and indurated plaque with microvesiculation that was 3.5 cm in width. She had no history of blistering sunburns, frequent UV exposure, or skin cancer.
Pemphigus Vulgaris Aggravated: Rifampicin Found at the Scene of the Crime
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
Practice Points
- Long-term use of immunosuppressants requires constant attention for infections, especially latent infections in the body.
- Clinicians should carefully inquire with patients about concomitant diseases and medications used, and be vigilant about drug interactions.
Forceps for Milia Extraction
To the Editor:
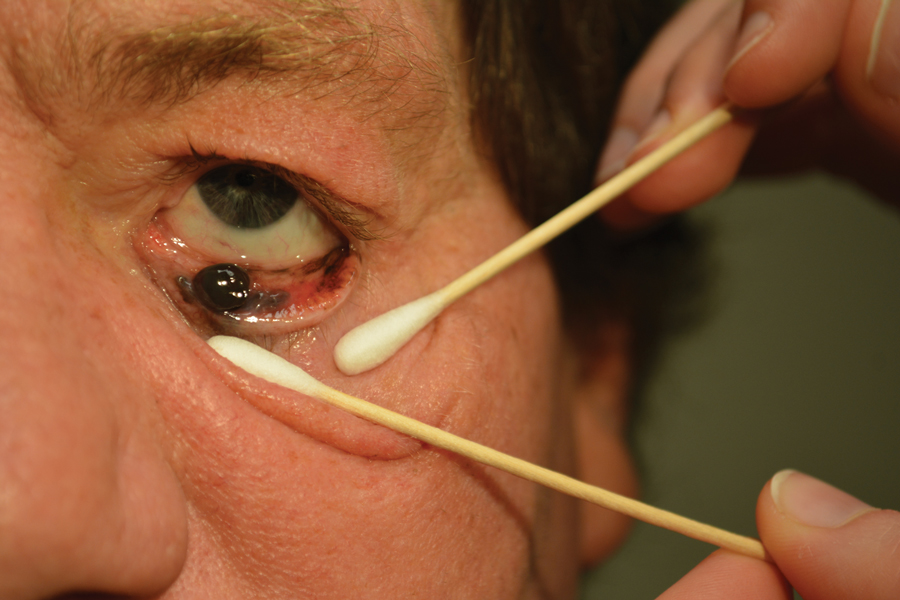
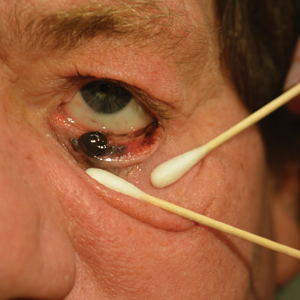
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
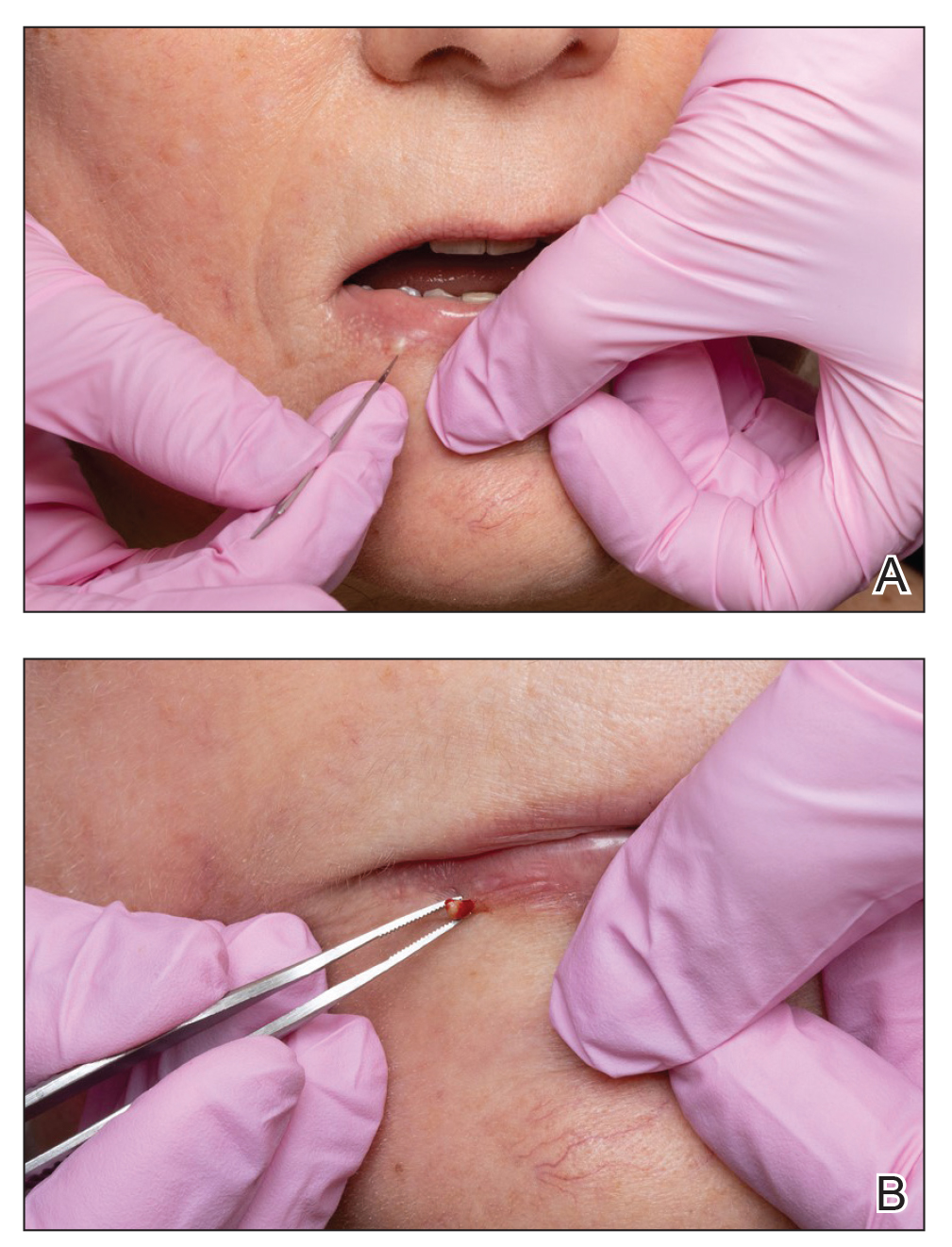
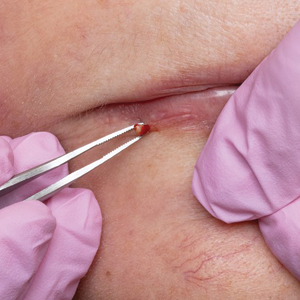
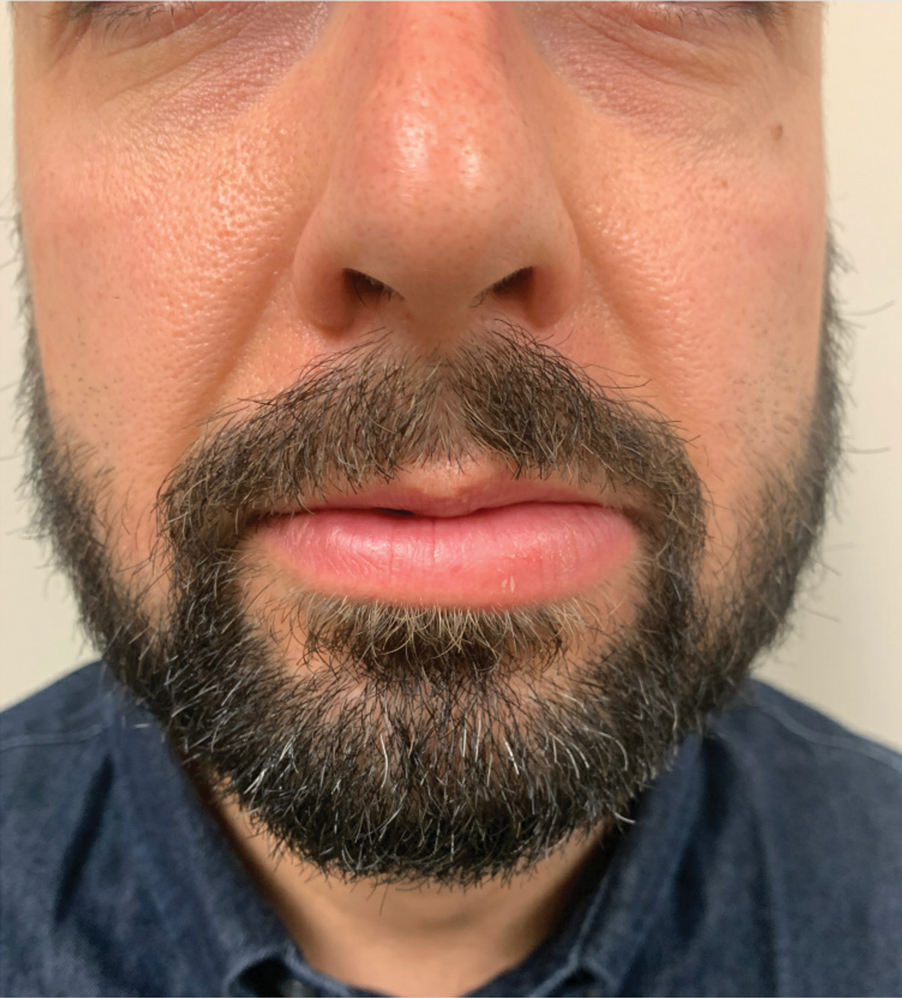
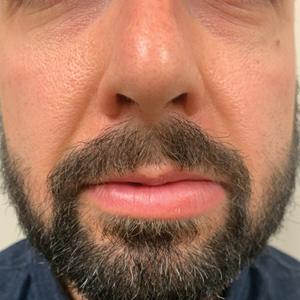
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.
Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.
Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.
Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
Practice Points
- Milia are common benign lesions that are cosmetically undesirable to some patients.
- Although some methods of milia removal can be painful, removal with forceps is quick and effective.
Navigating Motherhood and Dermatology Residency
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
Resident Pearl
- Female dermatology residents seeking motherhood during training have many obstacles to navigate, including the timing of pregnancy, maternity leave scheduling, planning for breastfeeding while working, and arranging for childcare. With supportive policies and resources, motherhood and dermatology training can be rewarding complements to one another.
Conjunctival Melanoma of the Left Lower Eyelid
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.
A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.
A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.
A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
Practice Points
- Ophthalmologists should carefully examine palpebral and bulbar conjunctiva at each annual visit paying careful attention to pigmented nevi.
- Conjunctival abnormalities should be thoroughly documented via color photography to accurately follow for suspicious change.
Cutaneous Lupus Erythematosus–like Isotopic Response to Herpes Zoster Infection
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.
Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.
Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.
Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.
Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.
Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.
Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
Practice Points
- Wolf isotopic response describes the occurrence of a new skin condition at the site of a previously healed and unrelated skin disorder; a granulomatous reaction is a commonly reported isotopic response.
- Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses.
Persistent Lip Swelling
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.
Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.
Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.
Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
A 36-year-old man with allergic rhinitis presented with lower lip swelling of several months’ duration. The swelling was persistent and predominantly on the left side of the lower lip but occasionally spread to the entire lower lip. The episodes of increased swelling would last for several days and were not associated with any apparent triggers. He denied any pain, pruritus, or dryness. He noted more drooling from the affected side but denied any associated breathing difficulty or throat discomfort. Treatment with an oral antihistamine provided no relief. He denied any recent nonsteroidal anti-inflammatory drug or angiotensinconverting enzyme inhibitor use. His family history was notable for lupus in his maternal grandmother and maternal aunt. He denied any personal or family history of inflammatory bowel disease or recent gastrointestinal tract symptoms. Physical examination revealed nontender edema in the left side of the lower lip with no surface changes. No warmth or erythema were noted. The tongue and the rest of the oral cavity were unremarkable.
Dupilumab for Allergic Contact Dermatitis: An Overview of Its Use and Impact on Patch Testing
Dupilumab is a humanized monoclonal antibody approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe atopic dermatitis. Through inhibition of the IL-4R α subunit, it prevents activation of the IL-4/IL-13 signaling cascade. This dampens the T H 2 inflammatory response, thereby improving the symptoms associated with atopic dermatitis. 1,2 Recent literature suggests that dupilumab may be useful in the treatment of other chronic dermatologic conditions, including allergic contact dermatitis (ACD) refractory to allergen avoidance and other treatments. Herein, we provide an overview of ACD, the role that dupilumab may play in its management, and its impact on patch testing results.
Pathogenesis of ACD
Allergic contact dermatitis is a cell-mediated type IV hypersensitivity reaction that develops through 2 distinct stages. In the sensitization phase, an allergen penetrates the skin and subsequently is engulfed by a cutaneous antigen-presenting cell. The allergen is then combined with a peptide to form a complex that is presented to naïve T lymphocytes in regional lymph nodes. The result is clonal expansion of a T-cell population that recognizes the allergen. In the elicitation phase, repeat exposure to the allergen leads to the recruitment of primed T cells to the skin, followed by cytokine release, inflammation, and resultant dermatitis.3
Historically, ACD was thought to be primarily driven by the TH1 inflammatory response; however, it is now known that TH2, TH9, TH17, and TH22 also may play a role in its pathogenesis.4,5 Another key finding is that the immune response in ACD appears to be at least partially allergen specific. Molecular profiling has revealed that nickel primarily induces a TH1/TH17 response, while allergens such as fragrance and rubber primarily induce a TH2 response.4
Management of ACD
Allergen avoidance is the mainstay of ACD treatment; however, in some patients, this approach does not always improve symptoms. In addition, eliminating the source of the allergen may not be possible in those with certain occupational, environmental, or medical exposures.
There are no FDA-approved treatments for ACD. When allergen avoidance alone is insufficient, first-line pharmacologic therapy typically includes topical or oral corticosteroids, the choice of which depends on the extent and severity of the dermatitis; however, a steroid-sparing agent often is preferred to avoid the unfavorable effects of long-term steroid use. Other systemic treatments for ACD include methotrexate, cyclosporine, mycophenolate mofetil, and azathioprine.6 These agents are used for severe ACD and typically are chosen as a last resort due to their immunosuppressive activity.
Phototherapy is another option, often as an adjunct to other therapies. Narrowband UVB and psoralen plus UVA have both been used. Psoralen plus UVA tends to have more side effects; therefore, narrowband UVB often is preferred.7,8
Use of Dupilumab in ACD
Biologics are unique, as they can target a single step in the immune response to improve a wide variety of symptoms. Research investigating their role as a treatment modality for ACD is still evolving alongside our increasing knowledge of its pathophysiology.9 Of note, studies examining the anti–IL-17 biologic secukinumab revealed it to be ineffective against ACD,10,11 which suggests that targeting specific immune components may not always result in improvement of ACD symptoms, likely because its pathophysiology involves several pathways.
There have been multiple reports demonstrating the effectiveness of dupilumab in the treatment of ACD (eTable).12-20 The findings from these studies show that dupilumab can improve recalcitrant dermatitis caused by a broad range of contact allergens, including nickel. This highlights its ability to improve ACD caused by allergens with a TH1 bias, despite its primarily TH2-dampening effects. Notably, several studies have reported successful use of dupilumab for systemic ACD.12,18 In addition, dupilumab may be able to improve symptoms of ACD in as little as 1 to 4 weeks. Unlike some systemic therapies for ACD, dupilumab also benefits from its lack of notable immunosuppressive effects.9 A phase 4 clinical trial at Brigham and Women’s Hospital (Boston, Massachusetts) is recruiting participants, with a primary goal of investigating dupilumab’s impact on ACD in patients who have not improved despite allergen avoidance (ClinicalTrials.gov identifier NCT03935971).
There are a few potential disadvantages to dupilumab. Because it is not yet FDA approved for the treatment of ACD, insurance companies may deny coverage, making it likely to be unaffordable for most patients. Furthermore, the side-effect profile has not been fully characterized. In addition to ocular adverse effects, a growing number of studies have reported face and neck erythema after starting dupilumab. Although the cause is unclear, one theory is that the inhibition of IL-4/IL-13 leads to TH1/TH17 polarization, thereby worsening ACD caused by allergens that activate a TH1-predominant response.21 Finally, not all cases of ACD respond to dupilumab.22
Patch Testing While on Dupilumab
Diagnosing ACD is a challenging process. An accurate history and physical examination are critical, and patch testing remains the gold standard when it comes to identifying the source of the contact allergen(s).
There is ongoing debate among contact dermatitis experts regarding the diagnostic accuracy of patch testing for those on immunomodulators or immunosuppressants, as these medications can dampen positive results and increase the risk for false-negative readings.23 Consequently, some have questioned whether patch testing on dupilumab is accurate or feasible.24 Contact dermatitis experts have examined patch testing results before and after initiation of dupilumab to further investigate. Puza and Atwater25 established that patients are able to mount a positive patch test reaction while on dupilumab. Moreover, a retrospective review by Raffi et al26 found that out of 125 before therapy/on therapy patch test pairs, only 13 were lost after administration of dupilumab. Although this would suggest that dupilumab has little impact on patch testing, Jo et al27 found in a systematic review that patch test reactions may remain positive, change to negative, or become newly positive after dupilumab initiation.
This inconsistency in results may relate to the allergen-specific pathogenesis of ACD—one allergen may have a different response to the mechanism of dupilumab than another.28,29 More recently, de Wijs et al30 reported a series of 20 patients in whom more than two-thirds of prior positive patch test reactions were lost after retesting on dupilumab; there were no clear trends according to the immune polarity of the allergens. This finding suggests that patient-specific factors also should be considered, as this too could have an impact on the reliability of patch test findings after starting dupilumab.29
Final Interpretation
Given its overall excellent safety profile, dupilumab may be a feasible off-label option for patients with ACD that does not respond to allergen avoidance or for those who experience adverse effects from traditional therapies; however, it remains difficult to obtain through insurance because it is not yet FDA approved for ACD. Likewise, its impact on the accuracy of patch testing is not yet well defined. Further investigations are needed to elucidate the pathophysiology of ACD and to guide further use of dupilumab in its treatment.
- Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy. 2020;50:5-14. doi:10.1111/cea.13491
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Chan CX, Zug KA. Diagnosis and management of dermatitis, including atopic, contact, and hand eczemas. Med Clin North Am. 2021;105:611-626. doi:10.1016/j.mcna.2021.04.003
- Simons JR, Bohnen IJ, van der Valk PG. A left-right comparison of UVB phototherapy and topical photochemotherapy in bilateral chronic hand dermatitis after 6 weeks’ treatment. Clin Exp Dermatol. 1997;22:7-10. doi:10.1046/j.1365-2230.1997.1640585.x
- Bhatia J, Sarin A, Wollina U, et al. Review of biologics in allergic contact dermatitis. Contact Dermatitis. 2020;83:179-181. doi:10.1111/cod.13584
- Todberg T, Zachariae C, Krustrup D, et al. The effect of anti-IL-17 treatment on the reaction to a nickel patch test in patients with allergic contact dermatitis. Int J Dermatol. 2019;58:E58-E61. doi:10.1111/ijd.14347
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:E12701. doi:10.1111/dth.12701
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
- Machler BC, Sung CT, Darwin E, et al. Dupilumab use in allergic contact dermatitis. J Am Acad Dermatol. 2019;80:280-281.e1. doi:10.1016/j.jaad.2018.07.043
- Chipalkatti N, Lee N, Zancanaro P, et al. A retrospective review of dupilumab for atopic dermatitis patients with allergic contact dermatitis. J Am Acad Dermatol. 2019;80:1166-1167. doi:10.1016/j.jaad.2018.12.048
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020;83:137-139. doi:10.1111/cod.13545
- Wilson B, Balogh E, Rayhan D, et al. Chromate-induced allergic contact dermatitis treated with dupilumab. J Drugs Dermatol. 2021;20:1340-1342. doi:10.36849/jdd.6246
- Jo CE, Finstad A, Georgakopoulos JR, et al. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84:1339-1347. doi:10.1016/j.jaad.2021.01.012
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Levian B, Chan J, DeLeo VA, et al. Patch testing and immunosuppression: a comprehensive review. Curr Derm Rep. 2021;10:128-139.
- Shah P, Milam EC, Lo Sicco KI, et al. Dupilumab for allergic contact dermatitis and implications for patch testing: irreconcilable differences. J Am Acad Dermatol. 2020;83:E215-E216. doi:10.1016/j.jaad.2020.05.036
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Jo CE, Mufti A, Sachdeva M, et al. Effect of dupilumab on allergic contact dermatitis and patch testing. J Am Acad Dermatol. 2021;84:1772-1776. doi:10.1016/j.jaad.2021.02.044
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Ludwig CM, Krase JM, Shi VY. T helper 2 inhibitors in allergic contact dermatitis. Dermatitis. 2021;32:15-18. doi: 10.1097/DER.0000000000000616
- de Wijs LEM, van der Waa JD, Nijsten T, et al. Effects of dupilumab treatment on patch test reactions: a retrospective evaluation. Clin Exp Allergy. 2021;51:959-967. doi:10.1111/cea.13892
Dupilumab is a humanized monoclonal antibody approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe atopic dermatitis. Through inhibition of the IL-4R α subunit, it prevents activation of the IL-4/IL-13 signaling cascade. This dampens the T H 2 inflammatory response, thereby improving the symptoms associated with atopic dermatitis. 1,2 Recent literature suggests that dupilumab may be useful in the treatment of other chronic dermatologic conditions, including allergic contact dermatitis (ACD) refractory to allergen avoidance and other treatments. Herein, we provide an overview of ACD, the role that dupilumab may play in its management, and its impact on patch testing results.
Pathogenesis of ACD
Allergic contact dermatitis is a cell-mediated type IV hypersensitivity reaction that develops through 2 distinct stages. In the sensitization phase, an allergen penetrates the skin and subsequently is engulfed by a cutaneous antigen-presenting cell. The allergen is then combined with a peptide to form a complex that is presented to naïve T lymphocytes in regional lymph nodes. The result is clonal expansion of a T-cell population that recognizes the allergen. In the elicitation phase, repeat exposure to the allergen leads to the recruitment of primed T cells to the skin, followed by cytokine release, inflammation, and resultant dermatitis.3
Historically, ACD was thought to be primarily driven by the TH1 inflammatory response; however, it is now known that TH2, TH9, TH17, and TH22 also may play a role in its pathogenesis.4,5 Another key finding is that the immune response in ACD appears to be at least partially allergen specific. Molecular profiling has revealed that nickel primarily induces a TH1/TH17 response, while allergens such as fragrance and rubber primarily induce a TH2 response.4
Management of ACD
Allergen avoidance is the mainstay of ACD treatment; however, in some patients, this approach does not always improve symptoms. In addition, eliminating the source of the allergen may not be possible in those with certain occupational, environmental, or medical exposures.
There are no FDA-approved treatments for ACD. When allergen avoidance alone is insufficient, first-line pharmacologic therapy typically includes topical or oral corticosteroids, the choice of which depends on the extent and severity of the dermatitis; however, a steroid-sparing agent often is preferred to avoid the unfavorable effects of long-term steroid use. Other systemic treatments for ACD include methotrexate, cyclosporine, mycophenolate mofetil, and azathioprine.6 These agents are used for severe ACD and typically are chosen as a last resort due to their immunosuppressive activity.
Phototherapy is another option, often as an adjunct to other therapies. Narrowband UVB and psoralen plus UVA have both been used. Psoralen plus UVA tends to have more side effects; therefore, narrowband UVB often is preferred.7,8
Use of Dupilumab in ACD
Biologics are unique, as they can target a single step in the immune response to improve a wide variety of symptoms. Research investigating their role as a treatment modality for ACD is still evolving alongside our increasing knowledge of its pathophysiology.9 Of note, studies examining the anti–IL-17 biologic secukinumab revealed it to be ineffective against ACD,10,11 which suggests that targeting specific immune components may not always result in improvement of ACD symptoms, likely because its pathophysiology involves several pathways.
There have been multiple reports demonstrating the effectiveness of dupilumab in the treatment of ACD (eTable).12-20 The findings from these studies show that dupilumab can improve recalcitrant dermatitis caused by a broad range of contact allergens, including nickel. This highlights its ability to improve ACD caused by allergens with a TH1 bias, despite its primarily TH2-dampening effects. Notably, several studies have reported successful use of dupilumab for systemic ACD.12,18 In addition, dupilumab may be able to improve symptoms of ACD in as little as 1 to 4 weeks. Unlike some systemic therapies for ACD, dupilumab also benefits from its lack of notable immunosuppressive effects.9 A phase 4 clinical trial at Brigham and Women’s Hospital (Boston, Massachusetts) is recruiting participants, with a primary goal of investigating dupilumab’s impact on ACD in patients who have not improved despite allergen avoidance (ClinicalTrials.gov identifier NCT03935971).
There are a few potential disadvantages to dupilumab. Because it is not yet FDA approved for the treatment of ACD, insurance companies may deny coverage, making it likely to be unaffordable for most patients. Furthermore, the side-effect profile has not been fully characterized. In addition to ocular adverse effects, a growing number of studies have reported face and neck erythema after starting dupilumab. Although the cause is unclear, one theory is that the inhibition of IL-4/IL-13 leads to TH1/TH17 polarization, thereby worsening ACD caused by allergens that activate a TH1-predominant response.21 Finally, not all cases of ACD respond to dupilumab.22
Patch Testing While on Dupilumab
Diagnosing ACD is a challenging process. An accurate history and physical examination are critical, and patch testing remains the gold standard when it comes to identifying the source of the contact allergen(s).
There is ongoing debate among contact dermatitis experts regarding the diagnostic accuracy of patch testing for those on immunomodulators or immunosuppressants, as these medications can dampen positive results and increase the risk for false-negative readings.23 Consequently, some have questioned whether patch testing on dupilumab is accurate or feasible.24 Contact dermatitis experts have examined patch testing results before and after initiation of dupilumab to further investigate. Puza and Atwater25 established that patients are able to mount a positive patch test reaction while on dupilumab. Moreover, a retrospective review by Raffi et al26 found that out of 125 before therapy/on therapy patch test pairs, only 13 were lost after administration of dupilumab. Although this would suggest that dupilumab has little impact on patch testing, Jo et al27 found in a systematic review that patch test reactions may remain positive, change to negative, or become newly positive after dupilumab initiation.
This inconsistency in results may relate to the allergen-specific pathogenesis of ACD—one allergen may have a different response to the mechanism of dupilumab than another.28,29 More recently, de Wijs et al30 reported a series of 20 patients in whom more than two-thirds of prior positive patch test reactions were lost after retesting on dupilumab; there were no clear trends according to the immune polarity of the allergens. This finding suggests that patient-specific factors also should be considered, as this too could have an impact on the reliability of patch test findings after starting dupilumab.29
Final Interpretation
Given its overall excellent safety profile, dupilumab may be a feasible off-label option for patients with ACD that does not respond to allergen avoidance or for those who experience adverse effects from traditional therapies; however, it remains difficult to obtain through insurance because it is not yet FDA approved for ACD. Likewise, its impact on the accuracy of patch testing is not yet well defined. Further investigations are needed to elucidate the pathophysiology of ACD and to guide further use of dupilumab in its treatment.
Dupilumab is a humanized monoclonal antibody approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe atopic dermatitis. Through inhibition of the IL-4R α subunit, it prevents activation of the IL-4/IL-13 signaling cascade. This dampens the T H 2 inflammatory response, thereby improving the symptoms associated with atopic dermatitis. 1,2 Recent literature suggests that dupilumab may be useful in the treatment of other chronic dermatologic conditions, including allergic contact dermatitis (ACD) refractory to allergen avoidance and other treatments. Herein, we provide an overview of ACD, the role that dupilumab may play in its management, and its impact on patch testing results.
Pathogenesis of ACD
Allergic contact dermatitis is a cell-mediated type IV hypersensitivity reaction that develops through 2 distinct stages. In the sensitization phase, an allergen penetrates the skin and subsequently is engulfed by a cutaneous antigen-presenting cell. The allergen is then combined with a peptide to form a complex that is presented to naïve T lymphocytes in regional lymph nodes. The result is clonal expansion of a T-cell population that recognizes the allergen. In the elicitation phase, repeat exposure to the allergen leads to the recruitment of primed T cells to the skin, followed by cytokine release, inflammation, and resultant dermatitis.3
Historically, ACD was thought to be primarily driven by the TH1 inflammatory response; however, it is now known that TH2, TH9, TH17, and TH22 also may play a role in its pathogenesis.4,5 Another key finding is that the immune response in ACD appears to be at least partially allergen specific. Molecular profiling has revealed that nickel primarily induces a TH1/TH17 response, while allergens such as fragrance and rubber primarily induce a TH2 response.4
Management of ACD
Allergen avoidance is the mainstay of ACD treatment; however, in some patients, this approach does not always improve symptoms. In addition, eliminating the source of the allergen may not be possible in those with certain occupational, environmental, or medical exposures.
There are no FDA-approved treatments for ACD. When allergen avoidance alone is insufficient, first-line pharmacologic therapy typically includes topical or oral corticosteroids, the choice of which depends on the extent and severity of the dermatitis; however, a steroid-sparing agent often is preferred to avoid the unfavorable effects of long-term steroid use. Other systemic treatments for ACD include methotrexate, cyclosporine, mycophenolate mofetil, and azathioprine.6 These agents are used for severe ACD and typically are chosen as a last resort due to their immunosuppressive activity.
Phototherapy is another option, often as an adjunct to other therapies. Narrowband UVB and psoralen plus UVA have both been used. Psoralen plus UVA tends to have more side effects; therefore, narrowband UVB often is preferred.7,8
Use of Dupilumab in ACD
Biologics are unique, as they can target a single step in the immune response to improve a wide variety of symptoms. Research investigating their role as a treatment modality for ACD is still evolving alongside our increasing knowledge of its pathophysiology.9 Of note, studies examining the anti–IL-17 biologic secukinumab revealed it to be ineffective against ACD,10,11 which suggests that targeting specific immune components may not always result in improvement of ACD symptoms, likely because its pathophysiology involves several pathways.
There have been multiple reports demonstrating the effectiveness of dupilumab in the treatment of ACD (eTable).12-20 The findings from these studies show that dupilumab can improve recalcitrant dermatitis caused by a broad range of contact allergens, including nickel. This highlights its ability to improve ACD caused by allergens with a TH1 bias, despite its primarily TH2-dampening effects. Notably, several studies have reported successful use of dupilumab for systemic ACD.12,18 In addition, dupilumab may be able to improve symptoms of ACD in as little as 1 to 4 weeks. Unlike some systemic therapies for ACD, dupilumab also benefits from its lack of notable immunosuppressive effects.9 A phase 4 clinical trial at Brigham and Women’s Hospital (Boston, Massachusetts) is recruiting participants, with a primary goal of investigating dupilumab’s impact on ACD in patients who have not improved despite allergen avoidance (ClinicalTrials.gov identifier NCT03935971).
There are a few potential disadvantages to dupilumab. Because it is not yet FDA approved for the treatment of ACD, insurance companies may deny coverage, making it likely to be unaffordable for most patients. Furthermore, the side-effect profile has not been fully characterized. In addition to ocular adverse effects, a growing number of studies have reported face and neck erythema after starting dupilumab. Although the cause is unclear, one theory is that the inhibition of IL-4/IL-13 leads to TH1/TH17 polarization, thereby worsening ACD caused by allergens that activate a TH1-predominant response.21 Finally, not all cases of ACD respond to dupilumab.22
Patch Testing While on Dupilumab
Diagnosing ACD is a challenging process. An accurate history and physical examination are critical, and patch testing remains the gold standard when it comes to identifying the source of the contact allergen(s).
There is ongoing debate among contact dermatitis experts regarding the diagnostic accuracy of patch testing for those on immunomodulators or immunosuppressants, as these medications can dampen positive results and increase the risk for false-negative readings.23 Consequently, some have questioned whether patch testing on dupilumab is accurate or feasible.24 Contact dermatitis experts have examined patch testing results before and after initiation of dupilumab to further investigate. Puza and Atwater25 established that patients are able to mount a positive patch test reaction while on dupilumab. Moreover, a retrospective review by Raffi et al26 found that out of 125 before therapy/on therapy patch test pairs, only 13 were lost after administration of dupilumab. Although this would suggest that dupilumab has little impact on patch testing, Jo et al27 found in a systematic review that patch test reactions may remain positive, change to negative, or become newly positive after dupilumab initiation.
This inconsistency in results may relate to the allergen-specific pathogenesis of ACD—one allergen may have a different response to the mechanism of dupilumab than another.28,29 More recently, de Wijs et al30 reported a series of 20 patients in whom more than two-thirds of prior positive patch test reactions were lost after retesting on dupilumab; there were no clear trends according to the immune polarity of the allergens. This finding suggests that patient-specific factors also should be considered, as this too could have an impact on the reliability of patch test findings after starting dupilumab.29
Final Interpretation
Given its overall excellent safety profile, dupilumab may be a feasible off-label option for patients with ACD that does not respond to allergen avoidance or for those who experience adverse effects from traditional therapies; however, it remains difficult to obtain through insurance because it is not yet FDA approved for ACD. Likewise, its impact on the accuracy of patch testing is not yet well defined. Further investigations are needed to elucidate the pathophysiology of ACD and to guide further use of dupilumab in its treatment.
- Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy. 2020;50:5-14. doi:10.1111/cea.13491
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Chan CX, Zug KA. Diagnosis and management of dermatitis, including atopic, contact, and hand eczemas. Med Clin North Am. 2021;105:611-626. doi:10.1016/j.mcna.2021.04.003
- Simons JR, Bohnen IJ, van der Valk PG. A left-right comparison of UVB phototherapy and topical photochemotherapy in bilateral chronic hand dermatitis after 6 weeks’ treatment. Clin Exp Dermatol. 1997;22:7-10. doi:10.1046/j.1365-2230.1997.1640585.x
- Bhatia J, Sarin A, Wollina U, et al. Review of biologics in allergic contact dermatitis. Contact Dermatitis. 2020;83:179-181. doi:10.1111/cod.13584
- Todberg T, Zachariae C, Krustrup D, et al. The effect of anti-IL-17 treatment on the reaction to a nickel patch test in patients with allergic contact dermatitis. Int J Dermatol. 2019;58:E58-E61. doi:10.1111/ijd.14347
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:E12701. doi:10.1111/dth.12701
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
- Machler BC, Sung CT, Darwin E, et al. Dupilumab use in allergic contact dermatitis. J Am Acad Dermatol. 2019;80:280-281.e1. doi:10.1016/j.jaad.2018.07.043
- Chipalkatti N, Lee N, Zancanaro P, et al. A retrospective review of dupilumab for atopic dermatitis patients with allergic contact dermatitis. J Am Acad Dermatol. 2019;80:1166-1167. doi:10.1016/j.jaad.2018.12.048
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020;83:137-139. doi:10.1111/cod.13545
- Wilson B, Balogh E, Rayhan D, et al. Chromate-induced allergic contact dermatitis treated with dupilumab. J Drugs Dermatol. 2021;20:1340-1342. doi:10.36849/jdd.6246
- Jo CE, Finstad A, Georgakopoulos JR, et al. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84:1339-1347. doi:10.1016/j.jaad.2021.01.012
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Levian B, Chan J, DeLeo VA, et al. Patch testing and immunosuppression: a comprehensive review. Curr Derm Rep. 2021;10:128-139.
- Shah P, Milam EC, Lo Sicco KI, et al. Dupilumab for allergic contact dermatitis and implications for patch testing: irreconcilable differences. J Am Acad Dermatol. 2020;83:E215-E216. doi:10.1016/j.jaad.2020.05.036
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Jo CE, Mufti A, Sachdeva M, et al. Effect of dupilumab on allergic contact dermatitis and patch testing. J Am Acad Dermatol. 2021;84:1772-1776. doi:10.1016/j.jaad.2021.02.044
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Ludwig CM, Krase JM, Shi VY. T helper 2 inhibitors in allergic contact dermatitis. Dermatitis. 2021;32:15-18. doi: 10.1097/DER.0000000000000616
- de Wijs LEM, van der Waa JD, Nijsten T, et al. Effects of dupilumab treatment on patch test reactions: a retrospective evaluation. Clin Exp Allergy. 2021;51:959-967. doi:10.1111/cea.13892
- Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy. 2020;50:5-14. doi:10.1111/cea.13491
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Chan CX, Zug KA. Diagnosis and management of dermatitis, including atopic, contact, and hand eczemas. Med Clin North Am. 2021;105:611-626. doi:10.1016/j.mcna.2021.04.003
- Simons JR, Bohnen IJ, van der Valk PG. A left-right comparison of UVB phototherapy and topical photochemotherapy in bilateral chronic hand dermatitis after 6 weeks’ treatment. Clin Exp Dermatol. 1997;22:7-10. doi:10.1046/j.1365-2230.1997.1640585.x
- Bhatia J, Sarin A, Wollina U, et al. Review of biologics in allergic contact dermatitis. Contact Dermatitis. 2020;83:179-181. doi:10.1111/cod.13584
- Todberg T, Zachariae C, Krustrup D, et al. The effect of anti-IL-17 treatment on the reaction to a nickel patch test in patients with allergic contact dermatitis. Int J Dermatol. 2019;58:E58-E61. doi:10.1111/ijd.14347
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:E12701. doi:10.1111/dth.12701
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
- Machler BC, Sung CT, Darwin E, et al. Dupilumab use in allergic contact dermatitis. J Am Acad Dermatol. 2019;80:280-281.e1. doi:10.1016/j.jaad.2018.07.043
- Chipalkatti N, Lee N, Zancanaro P, et al. A retrospective review of dupilumab for atopic dermatitis patients with allergic contact dermatitis. J Am Acad Dermatol. 2019;80:1166-1167. doi:10.1016/j.jaad.2018.12.048
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020;83:137-139. doi:10.1111/cod.13545
- Wilson B, Balogh E, Rayhan D, et al. Chromate-induced allergic contact dermatitis treated with dupilumab. J Drugs Dermatol. 2021;20:1340-1342. doi:10.36849/jdd.6246
- Jo CE, Finstad A, Georgakopoulos JR, et al. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84:1339-1347. doi:10.1016/j.jaad.2021.01.012
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Levian B, Chan J, DeLeo VA, et al. Patch testing and immunosuppression: a comprehensive review. Curr Derm Rep. 2021;10:128-139.
- Shah P, Milam EC, Lo Sicco KI, et al. Dupilumab for allergic contact dermatitis and implications for patch testing: irreconcilable differences. J Am Acad Dermatol. 2020;83:E215-E216. doi:10.1016/j.jaad.2020.05.036
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Jo CE, Mufti A, Sachdeva M, et al. Effect of dupilumab on allergic contact dermatitis and patch testing. J Am Acad Dermatol. 2021;84:1772-1776. doi:10.1016/j.jaad.2021.02.044
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Ludwig CM, Krase JM, Shi VY. T helper 2 inhibitors in allergic contact dermatitis. Dermatitis. 2021;32:15-18. doi: 10.1097/DER.0000000000000616
- de Wijs LEM, van der Waa JD, Nijsten T, et al. Effects of dupilumab treatment on patch test reactions: a retrospective evaluation. Clin Exp Allergy. 2021;51:959-967. doi:10.1111/cea.13892
Practice Points
- Dupilumab is approved by the US Food and Drug Administration for the treatment of moderate to severe atopic dermatitis.
- Multiple reports have suggested that dupilumab may be effective in the treatment of allergic contact dermatitis, and a phase 4 clinical trial is ongoing.
- The accuracy of patch testing after dupilumab initiation is unclear, as reactions may remain positive, change to negative, or become newly positive after its administration.